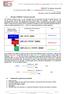
Manual. Transfer step. Figure 1. Workflow comparison of manual phenylalanine assay and automated GSP Neonatal Phenylalanine assay
|
|
|
- Ashlynn Oliver
- 6 years ago
- Views:
Transcription
1 TECHNICAL NOTE GSP Neonatal Phenylalanine kit / B GSP Neonatal Phenylalanine kit ( / B) is a fully Introduction automated enzymatic assay for the GSP system. Optimized for the quantitative determination of phenylalanine concentration in blood specimens dried on filter paper, the kit is an effective aid in screening newborns for phenylketonuria (Figure 1.). Analytical performance of the GSP Neonatal Phenylalanine kit was determined in verification studies conducted at PerkinElmer, Turku, Finland. In order to establish population distribution data and screening performance for the GSP Neonatal Phenylalanine kit two studies were conducted in newborn screening laboratories. In these studies GSP Phenylalanine kit was compared to manual Phenylalanine kit (NP-1000/-4000). Punch Reagent preparation Pipetting & incubation Transfer step Pipetting & incubation Measurement Result GSP Punch Reagent preparation Load Result Figure 1. Workflow comparison of manual phenylalanine assay and automated GSP Neonatal Phenylalanine assay Key benefits over existing manual assays: Automated assay Only two hands-on steps for reconstitution 24 hrs valid calibration curve Control step to detect missing sample disks Resorufin step added in the reaction to decrease the unspecific background fluorescence All reagents and QC material are bar-coded Simultaneous screening of any other GSP assays in any order Reagent on-board stability 4 days / 4 plates
2 Full selection of reagents in one kit Two different sizes of kits are available as 12 plates kit ( ) and a bulk kit for 60 plates [ B] Neonatal Phenylalanine Calibrators - 7 [15] dried blood spot cassettes each containing 1 set of dried blood spots Neonatal Phenylalanine Controls - 4 [20] dried blood spot cassettes each containing 3 sets of dried blood spots Neonatal Phenylalanine Substrate Reagent - 3 [15] vials, lyophilized Neonatal Phenylalanine Substrate Reagent - 3 [15] vials, lyophilized Neonatal Phenylalanine Assay Buffer - 3 [15] bottles, ready-for-use Neonatal Extraction Solution - 1 [5] bottles, ready-for-use Barcode labels for the plates Validated assay method The GSP Neonatal Phenylalanine assay is based on the fluorescent phenylalanine dehydrogenase method, which differs from the fluorescent ninhydrin method used in manual Neonatal Phenylalanine kit (NP-1000/NP-4000). In the first reaction phenylalanine dehydrogenase converts phenylalanine in sample to phenylpyruvate generating NADH. In the presence of NADH, resazurin dye is reduced to fluorescent resorufin, which is measured using an excitation wavelength of 505 nm and an emission wavelength of 580 nm (Figure 3.). Due to longer excitation wavelength than with manual phenylalanine assay, unspecific background fluorescence is reduced for improved performance. GSP Neonatal Phenylalanine kit assay protocol After punching of the samples and reconstitution of the Phenylalanine Substrate and Enzyme Reagents, the assay is fully automated from plate loading to completion. The assay time for one plate is 2 h 28 min and around 2-3 plates can be processed per hour after an initial 3 hour dwell time (Figure 4.). Dispensing Extraction Solution (20 µl/well) Incubation (1 h) Dispensing Assay Buffer (100 µl/well) Dispensing Substrate Reagent (5 µl/well) Dispensing Enzyme Reagent (5 µl/well) Incubation (1 h) Measurement (Phe concentration) Measurement (Elution Control to check the sample disk is present in a well) Figure 3. The assay principle of the GSP Neonatal Phenylalanine kit Figure 2. Content of the GSP Neonatal Phenylalanine kit for 12 plates ( ) Figure 4. Run scenario for GSP Neonatal Phenylalanine assay protocol. 2 3
3 Assay Performance Calibration The primary calibrator of the kit is the 3rd ISNS Reference preparation for Neonatal screening. The calibration unit for phenylalanine concentration is defined as µmol/l (or mg/dl) of whole blood. Conversion factor is 1 µmol/l blood= mg/dl blood (1 mg/dl=60.61 µmol/l). The kit calibrators cover the clinically relevant concentration range and the reagents include controls for low (150 µmol/l) and high (700 µmol/l) phenylalanine concentration. A typical calibration curve is shown in Figure 5. The calibration curve is valid for 24 hours. Precision The precision of the assay was determined in accordance with CLSI document EP05-A2 [1] using dried blood spot samples, 3 kit lots and 3 GSP instruments. An analysis of variance approach was used to calculate the variance components. The within-run, within-lot and total variation results for the GSP Neonatal Phenylalanine kit are presented in Table 1. Lower limits of detection, measuring range and linearity The analytical limits were determined in accordance with CLSI document EP17-A2 [2] and linearity was determined in accordance with CLSI document EP06-A [3]. Analytical limits, measuring range and linearity of the GSP Neonatal Phenylalanine kit are summarized in Table 2. The Limit of Blank (LoB) is defined as the 95th percentile of a distribution of blank samples (n=150), the Limit of Detection (LoD) is based on 216 determinations of low level samples and the Limit of Quantitation (LoQ) is defined as the lowest concentration with a total CV equal to or less than 24%. Table 2. Analytical limits, measuring range and linearity LoB LoD LoQ Measuring range Linearity Specimen stability The influence of storage time, temperature, and humidity on phenylalanine concentration was studied using several artificial samples spiked with phenylalanine. Phenylalanine concentrations were measured at different time points after different storage conditions. Storage under desiccated conditions is recommended (Figure 6.), [4] µmol/l (0.7 mg/dl) 68 µmol/l (1.1 mg/dl) 68 µmol/l (1.1 mg/dl) from 68 µmol/l to 1200 µmol/l (from 1.1 mg/dl to 19.8 mg/dl) from LoQ to CalF from 45 µmol/l to 1420 µmol/l (from 0.7 mg/dl to 23.4 mg/dl) Figure 5. A typical calibration curve for GSP Neonatal Phenylalanine assay. The approximate concentration values for controls are shown as red crosses and relevant clinical range is highlighted. Table 1. Variation of GSP Neonatal Phenylalanine kit using one calibration curve valid for 24 hours. The clinically relevant range is highlighted. Sample n phenylalanine concentration µmol/l (mg/dl) Within lot variation Between lot variation CV% CV% CV% (0.9) (1.1) (1.5) (2.2) (7.0) (10.7) (16.6) Total variation Figure 6. The change of phenylalanine concentration during storage at different temperatures and humidity conditions over time (% of reference without storage at the zero time point). 4 5
4 Distribution of results Internal and external studies were conducted to produce normal newborn population distribution data for the GSP Neonatal Phenylalanine kit, to assess the screening performance and to compare GSP Neonatal Phenylalanine assay to manual Phenylalanine assay (NP-1000/-4000). External study 1 Phenylalanine concentration of 2064 newborn screening specimens and 22 archived confirmed high phenylalanine specimens were measured using GSP Neonatal Phenylalanine kit in a European newborn screening laboratory. Population range, mean, median and cut-off values corresponding to 99th, 99.5th and 99.9th percentile were calculated. The frequency distributions of the newborn dried blood spot specimens of measured phenylalanine concentrations are visualised in Figure 7. and descriptive statistics in Table 3. The 22 positive samples consist of 7 PKU cases with classical phenylketonuria or deficient cofactor (PKU) and 15 hyperphenylalaninemia (HPA) cases. All of the 22 retrospective high phenylalanine specimens were classified as screening positive by the GSP Neonatal Phenylalanine kit by using the higher 99th percentile and 99.5th percentile cut-off values. Figure 7. Frequency distribution of the phenylalanine concentrations measured in external study site 1 with GSP Neonatal Phenylalanine kit. Table 3. Range, mean, median values and upper percentiles for the GSP Neonatal Phenylalanine kit ( /-B) carried out in an external study 1. Figure 9. Frequency distribution of the PKU concentrations measured in an internal study with GSP Neonatal Phenylalanine kit and manual Neonatal Phenylalanine kit (NP-1000/-4000). PKU data is shown using HPLC calibration. External Study 1 Sample Size Min Max Upper percentiles ( µmol/l blood) 99% 99.5% 99.9% Routine samples Retrospective positive * Internal study An internal study comprising of 2212 leftover routine newborn screening DBS samples and 13 confirmed PKU positive newborn specimens was conducted at PerkinElmer, Turku, Finland. Phenylalanine concentration was measured with both the GSP Neonatal Phenyalalanine kit ( /-B) and manual Neonatal Table 5. Range, mean and median values with upper percentiles for the routine and retrospective positive samples using both GSP Neonatal Phenylalanine kit and manual PKU in internal study (excluding confirmed positive PKU specimens). Phenylalanine kit (NP-1000/-4000). All the positive samples were classified as screening positive using higher 99.9% percentile. The frequency distribution for routine specimens (excluding confirmed PKU specimens) is shown in Figure 9. and Table 5. External study routine newborn screening dried blood spot specimens and 37 archived confirmed high phenylalanine specimens were included in an external study 2 conducted in China and the phenylalanine concentration was measured with GSP Neonatal Phenylalanine assay. The frequency distributions of the newborn specimens for PKU concentrations are visualised in Figure 8. and descriptive statistics in Table 4. Platform Routine samples (n=2212) Confirmed positive (n=13) Upper percentiles (µmol/l blood) Min Max Min Max 95% 99% 99.5% 99.9% GSP PKU * > PKU (HPLC calibration) PKU (ISNS Calibration) * > * > *This sample was drawn from an HPA patient. The lowest sample result with classical PKU or deficient cofactor was µmol/l. Table 4. Range, mean and median values with upper percentiles for the routine and retrospective positive samples using GSP Neonatal Phenylalanine kit of external study 2 Sample type Sample Size Min Fixure 8. Frequency distribution of the PKU concentrations measured in an external study 2 with GSP Neonatal Phenylalanine kit. The x-axis is truncated to 200 µmol/l. Max Upper percentiles (µmol/l blood) 95% 99% 99.5% 99.9% Routine samples * * Retrospective positive >1400 *Sample size is too small to calculate accurate cut-off value 6 7
5 Method comparison GSP Neonatal Phenylalanine kit is calibrated against ISNS Reference preparation for Neonatal Screening [5], whereas manual PKU kit is calibrated against HPLC calibration. The method comparison data from internal verification study shows that there is a level difference between GSP and manual Phenylalanine assays (Figure 10) and no conversion factor between the methods is available. At GSP Neonatal Phenylalanine concentration levels below 120 µmol/l (or <2 mg/dl) the manual PKU results are ~15 % higher results than the GSP method, whereas at GSP Neonatal Phenylalanine concent t are ~15 % lower results than the GSP method. Result 95% Confidence Iimit Intercept to Slope to 0.81 Figure 10. Deming regression analysis results between the GSP and manual Phenylalanine methods using GSP Neonatal Phenylalanine as a reference. References [1] CLSI (2004): Evaluation of Precision Performance of Quantitative Measurement Methods; Approved Guideline Second Edition; CLSI Document EP05-A2. Wayne, PA: Clinical and Laboratory Standards Institute. [2] CLSI (2012): Evaluation of Detection Capability for Clinical Laboratory Measurement procedures; Approved Guideline Second Edition; CLSI Document EP17-A2. Wayne, PA: Clinical and Laboratory Standards Institute. [3] CLSI (2003): Evaluation of the Linearity of Quantitative Measurement Procedures: A Statistical Approach; Approved Guideline. CLSI document EP06-A. Wayne, PA: Clinical and Laboratory Standards Institute. [4] Adam, B.W., et al. (2011): The stability of markers in dried-blood spots for recommended newborn screening disorders in the United States. Clin Biochem 44, [5] Dhondt J.L., et al. (1998): Preparation of the first European working standard for phenylalanine determination in dried blood spots. J Med Screen 5, The product is not available in the USA, Canada, Russia and some Asian and Latin American countries. For a complete listing of our global offices, visit Copyright 2015, PerkinElmer, Inc. All rights reserved. PerkinElmer is a registered trademark of PerkinElmer, Inc. All other trademarks are the property of their respective owners TCH GSP Neonatal Phenylalanine kit
Reliable. results. efficiently. for CH, galactosemia, CAH and CF screening
 GSP Neonatal assays for CH, galactosemia, CAH and CF screening Reliable results efficiently GSP Neonatal kits DELFIA or enzyme-based fluorescence assays GSP is the new, automated neonatal screening system
GSP Neonatal assays for CH, galactosemia, CAH and CF screening Reliable results efficiently GSP Neonatal kits DELFIA or enzyme-based fluorescence assays GSP is the new, automated neonatal screening system
USING THE ACCESS AMH ASSAY IN YOUR LABORATORY
 INFORMATION BULLETIN USING THE ACCESS AMH ASSAY IN YOUR LABORATORY ///////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////
INFORMATION BULLETIN USING THE ACCESS AMH ASSAY IN YOUR LABORATORY ///////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////////
PKU (Phenylketonuria) Serum HPLC Analysis Kit User Manual
 Page 1 / 20 PKU (Phenylketonuria) Serum HPLC Analysis Kit User Manual ZV-4003-0200-10 200 2-8 C Page 2 / 20 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. TEST PRINCIPLE... 3
Page 1 / 20 PKU (Phenylketonuria) Serum HPLC Analysis Kit User Manual ZV-4003-0200-10 200 2-8 C Page 2 / 20 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. TEST PRINCIPLE... 3
STELLUX C-peptide Chemiluminescence ELISA
 STELLUX C-peptide Chemiluminescence ELISA For the quantitative determination of Human C-peptide in Human Serum, Heparin Plasma, EDTA plasma, and Tissue Culture Supernatants. For In Vitro Diagnostic use
STELLUX C-peptide Chemiluminescence ELISA For the quantitative determination of Human C-peptide in Human Serum, Heparin Plasma, EDTA plasma, and Tissue Culture Supernatants. For In Vitro Diagnostic use
PKU (Phenylketonuria) Serum LC-MS/MS Analysis Kit User Manual
 Page 1 / 24 PKU (Phenylketonuria) Serum LC-MS/MS Analysis Kit User Manual ZV-3003-0200-10 200 2-8 C Page 2 / 24 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. TEST PRINCIPLE...
Page 1 / 24 PKU (Phenylketonuria) Serum LC-MS/MS Analysis Kit User Manual ZV-3003-0200-10 200 2-8 C Page 2 / 24 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. TEST PRINCIPLE...
retardation in infants. A wide variety of analytical methods for the analysis of
 IN THE NAME OF GOD Diagnosis and measurment of Phenylalanine in plasma and dried bilood spot (DBS) by a simple and sensitive HPLC method and compaire with Recipe Reference Health Laboratory Research Center,
IN THE NAME OF GOD Diagnosis and measurment of Phenylalanine in plasma and dried bilood spot (DBS) by a simple and sensitive HPLC method and compaire with Recipe Reference Health Laboratory Research Center,
Vedolizumab Drug Level ELISA
 Vedolizumab Drug Level ELISA For the quantitative determination of free Entyvio concentration in human serum and EDTA plasma. Please read carefully due to Critical Changes, e.g., Updates to specificity
Vedolizumab Drug Level ELISA For the quantitative determination of free Entyvio concentration in human serum and EDTA plasma. Please read carefully due to Critical Changes, e.g., Updates to specificity
Cholesterol/Cholesteryl Ester Detection Kit
 ab102515 Cholesterol/Cholesteryl Ester Detection Kit Instructions for Use For the rapid, sensitive and accurate measurement of free cholesterol, cholesteryl esters, or both in various samples. This product
ab102515 Cholesterol/Cholesteryl Ester Detection Kit Instructions for Use For the rapid, sensitive and accurate measurement of free cholesterol, cholesteryl esters, or both in various samples. This product
Mouse serum Insulin 200 tests
 Headquarters & Europe Office Cisbio Bioassays Phone: +33 (0)4 66 79 67 05 Fax: +33 (0)4 66 79 19 20 bioassays@cisbio.com cisbio_dd_pi_62in3pef-2µl USA Office Cisbio US, Inc. Phone: +1 888 963 4567 Fax:
Headquarters & Europe Office Cisbio Bioassays Phone: +33 (0)4 66 79 67 05 Fax: +33 (0)4 66 79 19 20 bioassays@cisbio.com cisbio_dd_pi_62in3pef-2µl USA Office Cisbio US, Inc. Phone: +1 888 963 4567 Fax:
Insulin (Porcine/Canine) ELISA
 Insulin (Porcine/Canine) ELISA For the quantitative measurement of insulin in Porcine/Canine serum and plasma (EDTA) For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSPO-E01
Insulin (Porcine/Canine) ELISA For the quantitative measurement of insulin in Porcine/Canine serum and plasma (EDTA) For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSPO-E01
Rat C-peptide ELISA. For the quantitative determination of C-peptide in rat serum
 Rat C-peptide ELISA For the quantitative determination of C-peptide in rat serum Please read carefully due to Critical Changes, e.g., see Calculation of Results. For Research Use Only. Not For Use In Diagnostic
Rat C-peptide ELISA For the quantitative determination of C-peptide in rat serum Please read carefully due to Critical Changes, e.g., see Calculation of Results. For Research Use Only. Not For Use In Diagnostic
RayBio Phenylalanine Fluorometric Assay Kit
 RayBio Phenylalanine Fluorometric Assay Kit User Manual Version 1.0 June 25, 2014 RayBio Phenylalanine Fluorometric (Cat#: 68-Phenyl-S100) RayBiotech, Inc. We Provide You With Excellent Support And Service
RayBio Phenylalanine Fluorometric Assay Kit User Manual Version 1.0 June 25, 2014 RayBio Phenylalanine Fluorometric (Cat#: 68-Phenyl-S100) RayBiotech, Inc. We Provide You With Excellent Support And Service
Rat Insulin ELISA. For the quantitative determination of insulin in rat serum and plasma. For Research Use Only. Not For Use In Diagnostic Procedures.
 Rat Insulin ELISA For the quantitative determination of insulin in rat serum and plasma For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: Size: 80-INSRT-E01, E10 96 wells, 10
Rat Insulin ELISA For the quantitative determination of insulin in rat serum and plasma For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: Size: 80-INSRT-E01, E10 96 wells, 10
Phenylalanine Assay Kit
 ab83376 Phenylalanine Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Phenylalanine levels in various samples This product is for research use only and is not intended
ab83376 Phenylalanine Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Phenylalanine levels in various samples This product is for research use only and is not intended
LSD EASY AND EFFICIENT SCREENING. NeoLSD MSMS kit
 EASY AND EFFICIENT LSD NeoLSD MSMS kit The first commercial IVD kit for screening of Pompe, MPS-I, Fabry, Gaucher, Niemann-Pick A/B and Krabbe disorders from a single DBS sample. A PIONEER IN EXPANDING
EASY AND EFFICIENT LSD NeoLSD MSMS kit The first commercial IVD kit for screening of Pompe, MPS-I, Fabry, Gaucher, Niemann-Pick A/B and Krabbe disorders from a single DBS sample. A PIONEER IN EXPANDING
Insulin ELISA. For the quantitative determination of insulin in serum and plasma.
 Insulin ELISA For the quantitative determination of insulin in serum and plasma. For In Vitro Diagnostic use within the United States of America. This product is for Research Use Only outside of the United
Insulin ELISA For the quantitative determination of insulin in serum and plasma. For In Vitro Diagnostic use within the United States of America. This product is for Research Use Only outside of the United
Bovine Insulin ELISA
 Bovine Insulin ELISA For quantitative determination of insulin in bovine serum and plasma. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSBO-E01 Size: 96 wells Version:
Bovine Insulin ELISA For quantitative determination of insulin in bovine serum and plasma. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-INSBO-E01 Size: 96 wells Version:
SERUM GLUCAGON KITS PROTOCOL
 SERUM GLUCAGON KITS PROTOCOL Part # 62SGLPEB & 62SGLPEF Test size#: 5 x 200 tests (62SGLPEB), 200 tests (62SGLPEF) - assay volume: 20 µl Revision: 02-Jan.2018 Store at: -60 C or below (62SGLPEB); -60 C
SERUM GLUCAGON KITS PROTOCOL Part # 62SGLPEB & 62SGLPEF Test size#: 5 x 200 tests (62SGLPEB), 200 tests (62SGLPEF) - assay volume: 20 µl Revision: 02-Jan.2018 Store at: -60 C or below (62SGLPEB); -60 C
Porcine/Canine Insulin ELISA
 Porcine/Canine Insulin ELISA For the quantitative determination of insulin in porcine or canine serum and plasma. Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research
Porcine/Canine Insulin ELISA For the quantitative determination of insulin in porcine or canine serum and plasma. Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research
Rat C-peptide ELISA. For the quantitative determination of C-peptide in rat serum. For Research Use Only. Not For Use In Diagnostic Procedures.
 Rat C-peptide ELISA For the quantitative determination of C-peptide in rat serum. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: Size: 80-CPTRT-E01 96 wells Version: May 26,
Rat C-peptide ELISA For the quantitative determination of C-peptide in rat serum. For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: Size: 80-CPTRT-E01 96 wells Version: May 26,
Mouse C-peptide ELISA
 Mouse C-peptide ELISA For the quantitative determination of C-peptide in mouse serum. For Research Use Only. Not for use in Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Preparation
Mouse C-peptide ELISA For the quantitative determination of C-peptide in mouse serum. For Research Use Only. Not for use in Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Preparation
INSULIN MOUSE SERUM KITS
 INSULIN MOUSE SERUM KITS PROTOCOL Part # 62IN3PEF & 62IN3PEB Test size#: 200 tests (62IN3PEF), 5 x 200 tests (62IN3PEB) - assay volume: 20 µl Revision: 03-Jan.2018 Store at: 2-8 C (62IN3PEF); 2-8 C (62IN3PEB)
INSULIN MOUSE SERUM KITS PROTOCOL Part # 62IN3PEF & 62IN3PEB Test size#: 200 tests (62IN3PEF), 5 x 200 tests (62IN3PEB) - assay volume: 20 µl Revision: 03-Jan.2018 Store at: 2-8 C (62IN3PEF); 2-8 C (62IN3PEB)
25OH Vitamin D Total ELISA
 25OH Vitamin D Total ELISA For the quantitative determination of 25-hydroxyvitamin D2 and D3 (25-OH D2 and 25-OH D3) in human serum For In Vitro Diagnostic use within the United States of America. This
25OH Vitamin D Total ELISA For the quantitative determination of 25-hydroxyvitamin D2 and D3 (25-OH D2 and 25-OH D3) in human serum For In Vitro Diagnostic use within the United States of America. This
Mouse C-peptide ELISA
 Mouse C-peptide ELISA For the quantitative determination of C-peptide in mouse serum. For Research Use Only. Not for use in Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Calculation
Mouse C-peptide ELISA For the quantitative determination of C-peptide in mouse serum. For Research Use Only. Not for use in Diagnostic Procedures. Please read carefully due to Critical Changes, e.g., Calculation
Insulin Chemiluminescence ELISA
 Insulin Chemiluminescence ELISA For the quantitative determination of insulin in human Serum, Heparin Plasma, and Tissue Culture Supernatants. For In Vitro Diagnostic use within the United States of America.
Insulin Chemiluminescence ELISA For the quantitative determination of insulin in human Serum, Heparin Plasma, and Tissue Culture Supernatants. For In Vitro Diagnostic use within the United States of America.
Insulin Rodent (Mouse/Rat) Chemiluminescence ELISA
 Insulin Rodent (Mouse/Rat) Chemiluminescence ELISA For the quantitative determination of insulin in rodent serum, plasma, and tissue culture supernatants. For Research use Only. Not For Use In Diagnostic
Insulin Rodent (Mouse/Rat) Chemiluminescence ELISA For the quantitative determination of insulin in rodent serum, plasma, and tissue culture supernatants. For Research use Only. Not For Use In Diagnostic
Gentian Canine CRP Immunoassay Application Note for Abbott Architect * c4000
 v2-jan214 Gentian Canine CRP Immunoassay Application Note for Abbott Architect * c4 Intended Use The canine CRP immunoassay on Abbott's Architect c4 is an in vitro diagnostic test for quantitative determination
v2-jan214 Gentian Canine CRP Immunoassay Application Note for Abbott Architect * c4 Intended Use The canine CRP immunoassay on Abbott's Architect c4 is an in vitro diagnostic test for quantitative determination
Mouse Ultrasensitive Insulin ELISA
 Mouse Ultrasensitive Insulin ELISA For the quantitative determination of insulin in mouse serum and plasma. Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research Use
Mouse Ultrasensitive Insulin ELISA For the quantitative determination of insulin in mouse serum and plasma. Please read carefully due to Critical Changes, e.g., Calculation of Results. For Research Use
Mouse C-Peptide ELISA Kit
 Mouse C-Peptide ELISA Kit Cat.No: DEIA4507 Lot. No. (See product label) Size 96T Intended Use The Mouse C-Peptide ELISA kit is for the quantitative determination of c-peptide in mouse serum, plasma, and
Mouse C-Peptide ELISA Kit Cat.No: DEIA4507 Lot. No. (See product label) Size 96T Intended Use The Mouse C-Peptide ELISA kit is for the quantitative determination of c-peptide in mouse serum, plasma, and
Glucose and Sucrose Assay Kit
 ab65334 Glucose and Sucrose Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Glucose and Sucrose levels in various samples This product is for research use only and is
ab65334 Glucose and Sucrose Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Glucose and Sucrose levels in various samples This product is for research use only and is
For the accurate measurement of glucose in cells and tissues, plasma, serum and other body fluids, growth media and food.
 ab169559 Picoprobe Glucose Assay Kit Instructions for Use For the accurate measurement of glucose in cells and tissues, plasma, serum and other body fluids, growth media and food. This product is for research
ab169559 Picoprobe Glucose Assay Kit Instructions for Use For the accurate measurement of glucose in cells and tissues, plasma, serum and other body fluids, growth media and food. This product is for research
Insulin ELISA. For the quantitative determination of insulin in serum and plasma
 Insulin ELISA For the quantitative determination of insulin in serum and plasma For In Vitro Diagnostic use within the United States of America. This product is for Research Use Only outside of the United
Insulin ELISA For the quantitative determination of insulin in serum and plasma For In Vitro Diagnostic use within the United States of America. This product is for Research Use Only outside of the United
ab Factor Xa Activity Assay Kit (Fluorometric)
 ab204711 Factor Xa Activity Assay Kit (Fluorometric) Instructions for Use For rapid, sensitive and accurate detection of Factor Xa activity. This product is for research use only and is not intended for
ab204711 Factor Xa Activity Assay Kit (Fluorometric) Instructions for Use For rapid, sensitive and accurate detection of Factor Xa activity. This product is for research use only and is not intended for
Branched Chain Amino Acid Assay Kit
 ab83374 Branched Chain Amino Acid Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Branched Chain Amino Acids in various samples This product is for research use only
ab83374 Branched Chain Amino Acid Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Branched Chain Amino Acids in various samples This product is for research use only
HbA1c (Human) ELISA Kit
 HbA1c (Human) ELISA Kit Cat. No.:DEIA3509 Pkg.Size:96T Intended use GHbA1c (Human) ELISA Kit is a sandwich enzyme immunoassay for the quantitative measurement of human GHbA1c. General Description vhemoglobin,
HbA1c (Human) ELISA Kit Cat. No.:DEIA3509 Pkg.Size:96T Intended use GHbA1c (Human) ELISA Kit is a sandwich enzyme immunoassay for the quantitative measurement of human GHbA1c. General Description vhemoglobin,
Glutamine Kit. For the determination of glutamine and glutamate in human EDTA plasma and serum. For research use only K 7732.
 Li StarFish S.r.l. Via Cavour, 35-20063 Cernusco S/N (MI), Italy Tel. +39-02-92150794 - Fax. +39-02-92157285 info@listarfish.it -www.listarfish.it Manual Kit For the determination of glutamine and glutamate
Li StarFish S.r.l. Via Cavour, 35-20063 Cernusco S/N (MI), Italy Tel. +39-02-92150794 - Fax. +39-02-92157285 info@listarfish.it -www.listarfish.it Manual Kit For the determination of glutamine and glutamate
ab Myeloperoxidase (MPO) Inhibitor Screening Assay Kit
 ab133080 Myeloperoxidase (MPO) Inhibitor Screening Assay Kit Instructions for Use The Myeloperoxidase (MPO) Inhibitor Screening Assay provides convenient fluorescence-based methods for screening inhibitors
ab133080 Myeloperoxidase (MPO) Inhibitor Screening Assay Kit Instructions for Use The Myeloperoxidase (MPO) Inhibitor Screening Assay provides convenient fluorescence-based methods for screening inhibitors
02006B 1 vial 02006B 1 vial Store at -20 C. Lyophilized recombinant IL-2
 For detection and measurement of human interleukin 2 Catalog #02006 Catalog #02007 2 Plates 10 Plates Product Description The Human Interleukin 2 (IL-2) ELISA Kit is designed for the quantitative detection
For detection and measurement of human interleukin 2 Catalog #02006 Catalog #02007 2 Plates 10 Plates Product Description The Human Interleukin 2 (IL-2) ELISA Kit is designed for the quantitative detection
G. Snodgrass, K. Ackles, A. Blanco and A. Versaggi Ortho Clinical Diagnostics, Rochester, NY 14626
 Performance of the EKF Diagnostics, Stanbio β-hydroxybutyrate LiquiColor Assay on the VITROS 4600 Chemistry System and the VITROS 5600 Integrated System. G. Snodgrass, K. Ackles, A. Blanco and A. Versaggi
Performance of the EKF Diagnostics, Stanbio β-hydroxybutyrate LiquiColor Assay on the VITROS 4600 Chemistry System and the VITROS 5600 Integrated System. G. Snodgrass, K. Ackles, A. Blanco and A. Versaggi
human Total Cathepsin B Catalog Number: DY2176
 human Total Cathepsin B Catalog Number: DY2176 This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human Total
human Total Cathepsin B Catalog Number: DY2176 This DuoSet ELISA Development kit contains the basic components required for the development of sandwich ELISAs to measure natural and recombinant human Total
ab HRP Substrate Instructions for Use
 ab155473 HRP Substrate Instructions for Use For detecting the activity of many oxidases, related enzymes/substrates or cofactors such as glucose, acetylcholine and cholesterol, L-glutamate, amino acids
ab155473 HRP Substrate Instructions for Use For detecting the activity of many oxidases, related enzymes/substrates or cofactors such as glucose, acetylcholine and cholesterol, L-glutamate, amino acids
Rat Proinsulin ELISA
 Rat Proinsulin ELISA For the quantitative determination of proinsulin in rat serum For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-PINRT-E01 Size: 96 wells Version: June
Rat Proinsulin ELISA For the quantitative determination of proinsulin in rat serum For Research Use Only. Not For Use In Diagnostic Procedures. Catalog Number: 80-PINRT-E01 Size: 96 wells Version: June
Gentian Canine CRP Immunoassay Application Note for scil VitroVet*
 v02-jan2014 Gentian Canine CRP Immunoassay Application Note for scil VitroVet* Intended Use The canine CRP immunoassay on scil VitroVet is an in vitro diagnostic test for quantitative determination of
v02-jan2014 Gentian Canine CRP Immunoassay Application Note for scil VitroVet* Intended Use The canine CRP immunoassay on scil VitroVet is an in vitro diagnostic test for quantitative determination of
Immunoenzymetric assay for the in vitro quantitative measurement of 25- hydroxyvitamin D 2 and D 3 (25OH-D 2 and 25OH-D 3 ) in serum.
 Immunoenzymetric assay for the in vitro quantitative measurement of 25- hydroxyvitamin D 2 and D 3 (25OH-D 2 and 25OH-D 3 ) in serum. SUMMARY MicroVue 25-OH Vitamin D EIA Page 1 of 14 INTENDED USE The
Immunoenzymetric assay for the in vitro quantitative measurement of 25- hydroxyvitamin D 2 and D 3 (25OH-D 2 and 25OH-D 3 ) in serum. SUMMARY MicroVue 25-OH Vitamin D EIA Page 1 of 14 INTENDED USE The
Lipase Detection Kit II (Colorimetric)
 ab102525 Lipase Detection Kit II (Colorimetric) Instructions for Use For the rapid, sensitive and accurate measurement of Lipase activity in various samples. This product is for research use only and is
ab102525 Lipase Detection Kit II (Colorimetric) Instructions for Use For the rapid, sensitive and accurate measurement of Lipase activity in various samples. This product is for research use only and is
For the rapid, sensitive and accurate measurement of Triglyceride in various samples.
 ab65336 Triglyceride Quantification Kit Instructions for Use For the rapid, sensitive and accurate measurement of Triglyceride in various samples. This product is for research use only and is not intended
ab65336 Triglyceride Quantification Kit Instructions for Use For the rapid, sensitive and accurate measurement of Triglyceride in various samples. This product is for research use only and is not intended
Cisbio Bioassays MAP-Tau assay is only intended for quantitative measurement of microtubule-associated protein tau (MAP-Tau) using HTRF technology.
 MAP-TAU KITS PROTOCOL Part # 63ADK008PEG & 63ADK008PEH Test size#: 500 tests (63ADK008PEG), 10,000 tests (63ADK008PEH) - assay volume: 20 µl Revision: 02-Jan.2018 Store at: -20 C or below (63ADK008PEG);
MAP-TAU KITS PROTOCOL Part # 63ADK008PEG & 63ADK008PEH Test size#: 500 tests (63ADK008PEG), 10,000 tests (63ADK008PEH) - assay volume: 20 µl Revision: 02-Jan.2018 Store at: -20 C or below (63ADK008PEG);
SERION ELISA classic CMV Avidity Reagent B109 AVID
 SERION ELISA classic CMV Avidity Reagent B109 AVID SERION ELISA classic Avidity Reagents are complementary components which, in combination with the corresponding SERION ELISA classic, enables the avidity
SERION ELISA classic CMV Avidity Reagent B109 AVID SERION ELISA classic Avidity Reagents are complementary components which, in combination with the corresponding SERION ELISA classic, enables the avidity
Tissue type Plasminogen Activator Human Chromogenic Activity Assay Kit
 ab108905 Tissue type Plasminogen Activator Human Chromogenic Activity Assay Kit Instructions for Use For the quantitative measurement of Human Tissue type Plasminogen Activator activity in cell culture
ab108905 Tissue type Plasminogen Activator Human Chromogenic Activity Assay Kit Instructions for Use For the quantitative measurement of Human Tissue type Plasminogen Activator activity in cell culture
ab HRV 3C Protease Inhibitor Screening Kit (Colorimetric)
 Version 2 Last updated 2 March 2017 ab211089 HRV 3C Protease Inhibitor Screening Kit (Colorimetric) For the rapid, sensitive and accurate screening of potential HRV 3C Protease inhibitors. This product
Version 2 Last updated 2 March 2017 ab211089 HRV 3C Protease Inhibitor Screening Kit (Colorimetric) For the rapid, sensitive and accurate screening of potential HRV 3C Protease inhibitors. This product
Free Fatty Acid Uptake Assay Kit (Fluorometric)
 ab176768 Free Fatty Acid Uptake Assay Kit (Fluorometric) Instructions for Use For measurement of fatty acid uptake in cells containing fatty acid transporters. This product is for research use only and
ab176768 Free Fatty Acid Uptake Assay Kit (Fluorometric) Instructions for Use For measurement of fatty acid uptake in cells containing fatty acid transporters. This product is for research use only and
QMS TACROLIMUS APPLICATION BECKMAN COULTER AU480 /AU680 /AU5800
 QMS TACROLIMUS APPLICATION BECKMAN COULTER AU480 /AU680 /AU5800 Beckman Coulter Reagent REF A53727 The QMS Tacrolimus Immunoassay is intended for the quantitative determination of tacrolimus in human whole
QMS TACROLIMUS APPLICATION BECKMAN COULTER AU480 /AU680 /AU5800 Beckman Coulter Reagent REF A53727 The QMS Tacrolimus Immunoassay is intended for the quantitative determination of tacrolimus in human whole
PD1/PD-L1 BINDING ASSAY KITS
 PD1/PD-L1 BINDING ASSAY KITS PROTOCOL Part # 64ICP01PEG & 64ICP01PEH Test size: 500 tests (64ICP01PEG), 10,000 tests (64ICP01PEH) - assay volume: 20 µl Revision: 02 (July 2017) Store at: -60 C or below
PD1/PD-L1 BINDING ASSAY KITS PROTOCOL Part # 64ICP01PEG & 64ICP01PEH Test size: 500 tests (64ICP01PEG), 10,000 tests (64ICP01PEH) - assay volume: 20 µl Revision: 02 (July 2017) Store at: -60 C or below
EliKine Free Thyroxine (ft4) ELISA Kit
 EliKine Free Thyroxine (ft4) ELISA Kit Booklet Item NO. KET0005 Product Name EliKine Free Thyroxine (ft4) ELISA Kit ATTENTION For laboratory research use only. Not for clinical or diagnostic use TABLE
EliKine Free Thyroxine (ft4) ELISA Kit Booklet Item NO. KET0005 Product Name EliKine Free Thyroxine (ft4) ELISA Kit ATTENTION For laboratory research use only. Not for clinical or diagnostic use TABLE
D-Mannitol Colorimetric Assay Kit
 ab155890 D-Mannitol Colorimetric Assay Kit Instructions for Use For the sensitive and accurate measurement of D-Mannitol/L-Arabitol in various plant tissues/cells and food products. This product is for
ab155890 D-Mannitol Colorimetric Assay Kit Instructions for Use For the sensitive and accurate measurement of D-Mannitol/L-Arabitol in various plant tissues/cells and food products. This product is for
Branched Chain Amino Acid (Leu, Ile, Val) Assay Kit
 Branched Chain Amino Acid (Leu, Ile, Val) Assay Kit Catalog Number KA0824 100 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General
Branched Chain Amino Acid (Leu, Ile, Val) Assay Kit Catalog Number KA0824 100 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General
For the rapid, sensitive and accurate measurement of Glucose in various samples
 ab65333 Glucose Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Glucose in various samples This product is for research use only and is not intended for diagnostic use.
ab65333 Glucose Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Glucose in various samples This product is for research use only and is not intended for diagnostic use.
Glucose 6 Phosphate Assay Kit (Colorimetric)
 ab83426 Glucose 6 Phosphate Assay Kit (Colorimetric) Instructions for Use For the rapid, sensitive and accurate measurement of Glucose 6 Phosphate levels in various samples This product is for research
ab83426 Glucose 6 Phosphate Assay Kit (Colorimetric) Instructions for Use For the rapid, sensitive and accurate measurement of Glucose 6 Phosphate levels in various samples This product is for research
For the rapid, sensitive and accurate measurement of Lactose levels in various samples
 ab83384 Lactose Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Lactose levels in various samples This product is for research use only and is not intended for diagnostic
ab83384 Lactose Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Lactose levels in various samples This product is for research use only and is not intended for diagnostic
Morinaga Mouse C-peptide ELISA Kit
 Morinaga Mouse C-peptide ELISA Kit For the quantitative determination of C-peptide in mouse serum, plasma, and fluid 96wells For Laboratory Use Only, not for use in diagnostic procedure Please read full
Morinaga Mouse C-peptide ELISA Kit For the quantitative determination of C-peptide in mouse serum, plasma, and fluid 96wells For Laboratory Use Only, not for use in diagnostic procedure Please read full
For the rapid, sensitive and accurate measurement of Maltose levels in various samples.
 ab83388 Maltose Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Maltose levels in various samples. This product is for research use only and is not intended for diagnostic
ab83388 Maltose Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Maltose levels in various samples. This product is for research use only and is not intended for diagnostic
Acetoacetate Assay Kit (Colorimetric)
 ab180875 Acetoacetate Assay Kit (Colorimetric) Instructions for Use For the rapid, sensitive and accurate measurement of endogenous Acetoacetate in various samples. This product is for research use only
ab180875 Acetoacetate Assay Kit (Colorimetric) Instructions for Use For the rapid, sensitive and accurate measurement of endogenous Acetoacetate in various samples. This product is for research use only
Blood Urea Nitrogen Enzymatic Kit Manual Catalog #:
 BIOO LIFE SCIENCE PRODUCTS Blood Urea Nitrogen Enzymatic Kit Manual Catalog #: 5602-01 BIOO Scientific Corp. 2010 TABLE OF CONTENTS GENERAL INFORMATION... 1 Product Description... 1 Procedure Overview...
BIOO LIFE SCIENCE PRODUCTS Blood Urea Nitrogen Enzymatic Kit Manual Catalog #: 5602-01 BIOO Scientific Corp. 2010 TABLE OF CONTENTS GENERAL INFORMATION... 1 Product Description... 1 Procedure Overview...
25-Hydroxyvitamin D2-D3 Serum VD-200 UHPLC Analysis Kit User Manual
 Page 1 / 12 25-Hydroxyvitamin D2-D3 Serum VD-200 UHPLC Analysis Kit User Manual ZV-4027-0500-10 500 2-8 C Page 2 / 12 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. IVD SYMBOLS...
Page 1 / 12 25-Hydroxyvitamin D2-D3 Serum VD-200 UHPLC Analysis Kit User Manual ZV-4027-0500-10 500 2-8 C Page 2 / 12 Table of Contents 1. INTENDED USE... 3 2. SUMMARY AND EXPLANATION... 3 3. IVD SYMBOLS...
METHOD VALIDATION CASE
 METHOD VALIDATION CASE METHOD VALIDATION PROTOCOL CLIA Regulation 493.1253 (2) 1.Accuracy (closeness to true/comparative method) 2.Precision (reproducibility) 3.Reference Interval 4.Reportable range (linearity,
METHOD VALIDATION CASE METHOD VALIDATION PROTOCOL CLIA Regulation 493.1253 (2) 1.Accuracy (closeness to true/comparative method) 2.Precision (reproducibility) 3.Reference Interval 4.Reportable range (linearity,
Glucose Detection Kit
 ab102517 Glucose Detection Kit Instructions for Use For the rapid, sensitive and accurate measurement of Glucose levels in various samples This product is for research use only and is not intended for
ab102517 Glucose Detection Kit Instructions for Use For the rapid, sensitive and accurate measurement of Glucose levels in various samples This product is for research use only and is not intended for
ab Creatinine Assay Kit (Colorimetric)
 ab204537 Creatinine Assay Kit (Colorimetric) Instructions for Use For the quantitative determination of Creatinine in urine samples. This product is for research use only and is not intended for diagnostic
ab204537 Creatinine Assay Kit (Colorimetric) Instructions for Use For the quantitative determination of Creatinine in urine samples. This product is for research use only and is not intended for diagnostic
ab83375 Sialic Acid (NANA) Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Sialic Acid (NANA) in various samples.
 ab83375 Sialic Acid (NANA) Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Sialic Acid (NANA) in various samples. Version 2 Last Updated 4 March 2015 This product is
ab83375 Sialic Acid (NANA) Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Sialic Acid (NANA) in various samples. Version 2 Last Updated 4 March 2015 This product is
Cotinine (Mouse/Rat) ELISA Kit
 Cotinine (Mouse/Rat) ELISA Kit Catalog Number KA2264 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Cotinine (Mouse/Rat) ELISA Kit Catalog Number KA2264 96 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle
Galactose and Lactose Assay Kit
 Galactose and Lactose Assay Kit Catalog Number KA0842 100 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials
Galactose and Lactose Assay Kit Catalog Number KA0842 100 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 General Information... 4 Materials
Fructose-6-Phosphate Colorimetric Assay Kit
 Fructose-6-Phosphate Colorimetric Assay Kit Catalog No. KM0060 Detection and Quantification of Fructose-6-Phosphate Concentrations in Biological Samples. Research Purposes Only. Not Intended for Diagnostic
Fructose-6-Phosphate Colorimetric Assay Kit Catalog No. KM0060 Detection and Quantification of Fructose-6-Phosphate Concentrations in Biological Samples. Research Purposes Only. Not Intended for Diagnostic
For the rapid, sensitive and accurate measurement of Aspartate aminotransferase activity in various samples
 ab105135 Aspartate Aminotransferase Activity Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Aspartate aminotransferase activity in various samples This product is for
ab105135 Aspartate Aminotransferase Activity Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Aspartate aminotransferase activity in various samples This product is for
Vitamin A-E Serum HPLC Analysis Kit
 Vitamin A-E Serum HPLC Analysis Kit ZV-4013-0200-10 200 2-8 C ZIVAK TEKNOLOJI SANAYI VE TICARET LIMITED SIRKETI Gursel Mh. Eski Besiktas Cd. No:44/1-2 KAGITHANE-ISTANBUL-TURKEY www.zivak.com info@zivak.com
Vitamin A-E Serum HPLC Analysis Kit ZV-4013-0200-10 200 2-8 C ZIVAK TEKNOLOJI SANAYI VE TICARET LIMITED SIRKETI Gursel Mh. Eski Besiktas Cd. No:44/1-2 KAGITHANE-ISTANBUL-TURKEY www.zivak.com info@zivak.com
beta Hydroxybutyrate (beta HB) Assay Kit
 ab83390 beta Hydroxybutyrate (beta HB) Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of beta Hydroxybutyrate in various samples. This product is for research use only
ab83390 beta Hydroxybutyrate (beta HB) Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of beta Hydroxybutyrate in various samples. This product is for research use only
L-Amino Acid Assay Kit
 ab65347 L-Amino Acid Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of L-Amino Acid levels in various samples. This product is for research use only and is not intended
ab65347 L-Amino Acid Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of L-Amino Acid levels in various samples. This product is for research use only and is not intended
Product information: Human Apolipoprotein B kit 10,000 tests 1. ASSAY DESCRIPTION AND INTENDED USE 2. PROTOCOL AT GLANCE
 Headquarters & Europe Office Cisbio Bioassays Phone: +33 ()4 66 79 67 5 Fax: +33 ()4 66 79 19 2 bioassays@cisbio.com USA Office Cisbio US, Inc. Phone: +1 888 963 4567 Fax: +1 781 687 15 htrfinfo@cisbio.us
Headquarters & Europe Office Cisbio Bioassays Phone: +33 ()4 66 79 67 5 Fax: +33 ()4 66 79 19 2 bioassays@cisbio.com USA Office Cisbio US, Inc. Phone: +1 888 963 4567 Fax: +1 781 687 15 htrfinfo@cisbio.us
Glucose 6 Phosphate Dehydrogenase Assay Kit (Fluorometric)
 ab176722 Glucose 6 Phosphate Dehydrogenase Assay Kit (Fluorometric) Instructions for Use For monitoring Glucose 6 Phosphate Dehydrogenase in a variety of biological samples. This product is for research
ab176722 Glucose 6 Phosphate Dehydrogenase Assay Kit (Fluorometric) Instructions for Use For monitoring Glucose 6 Phosphate Dehydrogenase in a variety of biological samples. This product is for research
Human Thyroid-Peroxidase Antibody, TPO-Ab ELISA Kit
 Human Thyroid-Peroxidase Antibody, TPO-Ab ELISA Kit Catalog No: E0442h 96 Tests Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Human Thyroid-Peroxidase Antibody, TPO-Ab ELISA Kit Catalog No: E0442h 96 Tests Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Thyroid Stimulating Hormone (TSH) ELISA Catalog No. GWB , legacy id (96 Tests)
 For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. TSH ELISA Kit is intended for the quantitative measurement of TSH in human serum or plasma. For research use only.
For Research Use Only. Not for use in Diagnostic Procedures. INTENDED USE The GenWay, Inc. TSH ELISA Kit is intended for the quantitative measurement of TSH in human serum or plasma. For research use only.
Glucose-6-phosphate Isomerase Activity Assay Kit (Colorimetric)
 ab155897 Glucose-6-phosphate Isomerase Activity Assay Kit (Colorimetric) Instructions for Use For the sensitive and accurate measurement of Glucose-6-phosphate Isomerase activity in various biological
ab155897 Glucose-6-phosphate Isomerase Activity Assay Kit (Colorimetric) Instructions for Use For the sensitive and accurate measurement of Glucose-6-phosphate Isomerase activity in various biological
Human Immunodeficiency Virus type-1 p24 protein (HIV p24) Kit
 TECHNICAL DATA SHEET AlphaLISA Research Reagents Caution: For Laboratory Use. A research chemical for research purposes only. Human Immunodeficiency Virus type-1 p24 protein (HIV p24) Kit Product No.:
TECHNICAL DATA SHEET AlphaLISA Research Reagents Caution: For Laboratory Use. A research chemical for research purposes only. Human Immunodeficiency Virus type-1 p24 protein (HIV p24) Kit Product No.:
Supplemental Data. Methods- Different concentrations of substrate solutions (final concentrations during incubation- 10, 3,
 Supplemental Data Michaelis-Menten Kinetics Methods- Different concentrations of substrate solutions (final concentrations during incubation- 10, 3, 1, 0.3 and 0.1 mmol/l) were used and enzymatic analysis
Supplemental Data Michaelis-Menten Kinetics Methods- Different concentrations of substrate solutions (final concentrations during incubation- 10, 3, 1, 0.3 and 0.1 mmol/l) were used and enzymatic analysis
DIHYDROSTREPTOMYCIN and STREPTOMYCIN First draft prepared by Lynn G. Friedlander, Rockville, MD, USA Rainer W. Stephany, Bilthoven, The Netherlands
 DIHYDROSTREPTOMYCIN and STREPTOMYCIN First draft prepared by Lynn G. Friedlander, Rockville, MD, USA Rainer W. Stephany, Bilthoven, The Netherlands ADDENDUM To the monograph and addenda prepared by the
DIHYDROSTREPTOMYCIN and STREPTOMYCIN First draft prepared by Lynn G. Friedlander, Rockville, MD, USA Rainer W. Stephany, Bilthoven, The Netherlands ADDENDUM To the monograph and addenda prepared by the
Blood Urea Nitrogen Enzymatic Kit Manual Catalog #:
 Blood Urea Nitrogen Enzymatic Kit Manual Catalog #: 5602-01 TABLE OF CONTENTS GENERAL INFORMATION... 2 Product Description... 2 Procedure Overview... 2 Kit Contents, Storage and Shelf Life... 3 Required
Blood Urea Nitrogen Enzymatic Kit Manual Catalog #: 5602-01 TABLE OF CONTENTS GENERAL INFORMATION... 2 Product Description... 2 Procedure Overview... 2 Kit Contents, Storage and Shelf Life... 3 Required
Ascorbic Acid Assay Kit
 ab65346 Ascorbic Acid Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Ascorbic Acid in various samples This product is for research use only and is not intended for
ab65346 Ascorbic Acid Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Ascorbic Acid in various samples This product is for research use only and is not intended for
ab HIV-1 Protease Inhibitor Screening Kit (Fluorometric)
 Version 1 Last updated 2 March 2017 ab211106 HIV-1 Protease Inhibitor Screening Kit (Fluorometric) For the rapid, sensitive and accurate screening of potential HIV-1 Protease inhibitors. This product is
Version 1 Last updated 2 March 2017 ab211106 HIV-1 Protease Inhibitor Screening Kit (Fluorometric) For the rapid, sensitive and accurate screening of potential HIV-1 Protease inhibitors. This product is
Lipase Detection Kit III (Fluorometric)
 ab118969 Lipase Detection Kit III (Fluorometric) Instructions for Use For the rapid, sensitive and accurate measurement of Lipase activity in various samples. This product is for research use only and
ab118969 Lipase Detection Kit III (Fluorometric) Instructions for Use For the rapid, sensitive and accurate measurement of Lipase activity in various samples. This product is for research use only and
Auto-absorbance of fish extract and co-factors. IMP + Hx + Ino. Absorbance Blank. Absorbance Blank
 PRECICE Freshness Assay Kit For rapid measurement of IMP, inosine and hypoxanthine in muscle tissues Ref: 0700-003 Key words: K-value, ATP nucleotide catabolites I. Principle of PRECICE Freshness Assay
PRECICE Freshness Assay Kit For rapid measurement of IMP, inosine and hypoxanthine in muscle tissues Ref: 0700-003 Key words: K-value, ATP nucleotide catabolites I. Principle of PRECICE Freshness Assay
Rat Mullerian Inhibiting Substance/Anti-Mullerian hormone, MIS/AMH ELISA kit
 Rat Mullerian Inhibiting Substance/Anti-Mullerian hormone, MIS/AMH ELISA kit Catalog No. E0228r (96 tests) Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS!
Rat Mullerian Inhibiting Substance/Anti-Mullerian hormone, MIS/AMH ELISA kit Catalog No. E0228r (96 tests) Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS!
SCIEX Vitamin D 200M Assay for the Topaz System
 The First FDA-Cleared LC-MS/MS Assay for Vitamin D SCIEX Vitamin D 200M Assay for the Topaz System The first FDA-cleared LC-MS/MS assay for Vitamin D Vitamin D is an important building block for human
The First FDA-Cleared LC-MS/MS Assay for Vitamin D SCIEX Vitamin D 200M Assay for the Topaz System The first FDA-cleared LC-MS/MS assay for Vitamin D Vitamin D is an important building block for human
Phenylalanine. in cases of hyperphenylalaninemia and tetrahydrobiopterin deficiency
 Evolving Methods for the Measurement of Phenylalanine A vital diagnostic marker and indicator for follow-up in cases of hyperphenylalaninemia and tetrahydrobiopterin deficiency Dr. Zoltan Lukacs Department
Evolving Methods for the Measurement of Phenylalanine A vital diagnostic marker and indicator for follow-up in cases of hyperphenylalaninemia and tetrahydrobiopterin deficiency Dr. Zoltan Lukacs Department
Canine Thyroid Stimulating Hormone, TSH ELISA Kit
 Canine Thyroid Stimulating Hormone, TSH ELISA Kit Catalog No: E0463c 96 Tests Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Canine Thyroid Stimulating Hormone, TSH ELISA Kit Catalog No: E0463c 96 Tests Operating instruction www.eiaab.com FOR RESEARCH USE ONLY; NOT FOR THERAPEUTIC OR DIAGNOSTIC APPLICATIONS! PLEASE READ THROUGH
Rat cholesterol ELISA Kit
 Rat cholesterol ELISA Kit Catalog No. CSB-E11706r (96T) This immunoassay kit allows for the in vitro quantitative determination of rat Cholesterol concentrations in serum, plasma and other biological fluids.
Rat cholesterol ELISA Kit Catalog No. CSB-E11706r (96T) This immunoassay kit allows for the in vitro quantitative determination of rat Cholesterol concentrations in serum, plasma and other biological fluids.
Galactose Colorimetric Detection Kit
 K-ASSAY Galactose Colorimetric Detection Kit For the quantitative determination of galactose in serum, plasma, buffers and TCM Cat. No. KT-724 For Research Use Only. 1 K-ASSAY PRODUCT INFORMATION Galactose
K-ASSAY Galactose Colorimetric Detection Kit For the quantitative determination of galactose in serum, plasma, buffers and TCM Cat. No. KT-724 For Research Use Only. 1 K-ASSAY PRODUCT INFORMATION Galactose
PicoProbe Acetyl CoA Assay Kit
 ab87546 PicoProbe Acetyl CoA Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Acetyl CoA levels in various samples. This product is for research use only and is not intended
ab87546 PicoProbe Acetyl CoA Assay Kit Instructions for Use For the rapid, sensitive and accurate measurement of Acetyl CoA levels in various samples. This product is for research use only and is not intended
For the rapid, sensitive and accurate detection of long-chain Free Fatty Acids in various samples.
 ab65341 Free Fatty Acid Quantification Kit Instructions for Use For the rapid, sensitive and accurate detection of long-chain Free Fatty Acids in various samples. This product is for research use only
ab65341 Free Fatty Acid Quantification Kit Instructions for Use For the rapid, sensitive and accurate detection of long-chain Free Fatty Acids in various samples. This product is for research use only
6-Phosphofructokinase Activity Assay Kit (Colorimetric)
 ab155898 6-Phosphofructokinase Activity Assay Kit (Colorimetric) Instructions for Use For the sensitive and accurate measurement of phosphofructokinase activity in animal tissue and cell culture samples
ab155898 6-Phosphofructokinase Activity Assay Kit (Colorimetric) Instructions for Use For the sensitive and accurate measurement of phosphofructokinase activity in animal tissue and cell culture samples
