Frequency, Specificity, and Sites of. Pulmonary Influenza Virus Infection. Christopher W. Lawrence, Rebecca M. Ream and Thomas J.
|
|
|
- Hillary Reed
- 5 years ago
- Views:
Transcription
1 This information is current as of November 12, Frequency, Specificity, and Sites of Expansion of CD8 + T Cells during Primary Pulmonary Influenza Virus Infection Christopher W. Lawrence, Rebecca M. Ream and Thomas J. Braciale J Immunol 2005; 174: ; ; doi: /jimmunol References Subscription Permissions Alerts This article cites 39 articles, 23 of which you can access for free at: Why The JI? Submit online. Rapid Reviews! 30 days* from submission to initial decision No Triage! Every submission reviewed by practicing scientists Fast Publication! 4 weeks from acceptance to publication *average Information about subscribing to The Journal of Immunology is online at: Submit copyright permission requests at: Receive free -alerts when new articles cite this article. Sign up at: Downloaded from by guest on November 12, 2018 The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD Copyright 2005 by The American Association of Immunologists All rights reserved. Print ISSN: Online ISSN:
2 The Journal of Immunology Frequency, Specificity, and Sites of Expansion of CD8 T Cells during Primary Pulmonary Influenza Virus Infection 1 Christopher W. Lawrence,* Rebecca M. Ream,* and Thomas J. Braciale 2 * We have used intracellular cytokine staining and MHC class I tetramer binding in conjunction with granzyme B protease expression and in vivo BrdU uptake to characterize the primary murine CD8 T cell response to pulmonary influenza virus infection. We have observed that the majority (>90%) of the CD8 T cell response to the A/Japan/305/57 virus in the lung at the peak of the response (days 9 11) is directed to four epitopes (three dominant and one subdominant). Using induction of granzyme B as a surrogate to identify specific activated CD8 T cells, we found that an unexpectedly large fraction ( 70%) of lunginfiltrating CD8 T cells expressed granzyme B on day 6 of infection when estimates by MHC tetramer/intracellular cytokine staining yielded substantially lower frequencies ( 30%). In addition, by using intranasal administration of BrdU during infection, we obtained evidence for proliferative expansion of activated CD8 T cells in the infected lung early (days 5 7) in the primary response. These results suggest that the frequency and number of specific CTL present in the lung early in infection may be underestimated by standard detection methods, and primary CD8 T cell expansion may occur in both secondary lymphoid organs and the infected lung. The Journal of Immunology, 2005, 174: A significant amount of our current understanding of specific CD8 T cell responses to viral epitopes has been garnered through the study of experimental influenza virus infection of mice. For example, extensive investigations into the basis of immunodominance of CD8 T cell responses to multiple epitopes have been performed in both BALB/c (H-2 d ) and C57BL/6 (H-2 b ) mice after i.p. inoculation (1, 2). Furthermore, because influenza virus is a respiratory tract pathogen, much of our current knowledge about the specific CD8 T cell response to influenza virus has come from analyzing the specificity of the pulmonary CD8 T cell response to intranasal (i.n.) 3 challenge (3 5). These important studies have detailed the immunodominance hierarchy to several CD8 T cell epitopes from the HKx31 (H3N2) and PR/8 (H1N1) influenza viruses in H-2 b mice. The most dominant epitopes from these viruses are polymerase A and nucleoprotein (NP) , which, along with other minor epitopes, are targets for the 40% of pulmonary CD8 T cells that are identifiable as virus-specific during the primary immune response (3 5). By contrast, other respiratory tract viruses have been shown to induce an even more potent specific CD8 T cell response in mice. For example, i.n. Sendai virus infection elicits a highly restricted CD8 T cell population in which 70% of pulmonary CD8 T *Beirne B. Carter Center for Immunology Research and Departments of Microbiology and Pathology, University of Virginia Health Sciences Center, Charlottesville, VA Received for publication October 13, Accepted for publication February 18, The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. 1 This work was supported by U.S. Public Health Service Grants AI15608, HL33391, and HL71875 and U.S. Public Health Service Training Grant AI Address correspondence and reprint requests to Dr. Thomas J. Braciale, Beirne B. Carter Center for Immunology Research, University of Virginia Health Sciences Center, MR-4 Building, HSC Box , Charlottesville, VA address: tjb2r@virginia.edu 3 Abbreviations used in this paper: i.n., intranasal; BAL, bronchoalveolar lavage; HA, hemagglutinin; ICCS, intracellular cytokine staining; MLN, mediastinal lymph node; NP, nucleoprotein; TCID 50, half-maximal tissue culture infectious dose. cells are directed against a single immunodominant epitope (6). Similarly, 50 60% of CD8 T cells responding to a primary i.n. respiratory syncytial virus infection are directed against two epitopes, the vast majority of which are specific to a single epitope as well (7). Therefore, the frequency of CD8 T cells responding to respiratory viruses probably parallels the high frequencies of pathogen-specific CD8 T cells that are prevalent during the response to a potent, systemic viral infection such as acute lymphocytic choriomeningitis virus challenge (8, 9). To examine whether a similarly high frequency of virus-specific CD8 T cells may be directed against influenza virus, we analyzed the CD8 T cell response to primary i.n. infection of BALB/c mice with a mouseadapted A/Japan/305/57 (Japan/57) virus. CD8 T cells responding to Japan/57 virus in BALB/c mice recognize four known epitopes restricted by H-2K d (Table I). Three of these viral epitopes, hemagglutinin (HA) , HA (10, 11), and HA (12), are processed from the HA protein, whereas a fourth, NP (13), is derived from the viral NP. Previous studies have demonstrated that bulk, polyclonal cultures prepared from the splenocytes of mice that were immunized i.p. with Japan/57 possessed high cytolytic activity against the HA ,HA , and NP epitopes, whereas the cytolytic activity against HA was much weaker (14, 15). However, the frequency of virus-specific CD8 T cells responding in vivo to i.n. Japan/57 virus infection had not been analyzed. In this report we have characterized the magnitude and immunodominance hierarchy of the CD8 T cell response against the four previously described epitopes during pulmonary Japan/57 influenza virus infection. We demonstrate that the frequency of CD8 T cells that are specific to these influenza virus epitopes is indeed high during the peak of the pulmonary response, as determined by intracellular cytokine staining (ICCS) and MHC class I tetramer labeling. Activated CD8 T cells directed to these four epitopes represent 90% of the total responding CD8 T cells in the lungs on day 9 of infection by ICCS using cytokine synthesis by lung-infiltrating CD8 T cells to influenza virus-infected cells as a measure of total virus-specific CD8 T cells. The immunodominance hierarchy of the response to these epitopes in vivo followed the predicted pattern based on previous in vitro studies. Copyright 2005 by The American Association of Immunologists, Inc /05/$02.00
3 The Journal of Immunology 5333 Table I. Amino acid sequences of CD8 T cell epitopes from A/Japan/ 305/57 influenza virus In addition, we explored the use of granzyme B protease expression by activated CD8 T cells as a surrogate marker to identify specific CD8 T cells. We obtained evidence that granzyme B expression may identify virus-specific CD8 T cells that have migrated to the lungs early after infection, i.e., days 5 7, but are not efficiently detected by ICCS or MHC tetramer binding. Finally, we evaluated the kinetics of CD8 T cell proliferation in vivo during infection by systemic (i.p.) or local (i.n.) administration of the thymidine analog BrdU. Using this approach, we obtained evidence suggesting the idea that activated virus-specific CD8 T cells may undergo proliferative expansion in both secondary lymphoid organs (draining lymph nodes) and infected lungs early after infection. The significance of these results for the induction of CD8 T cell responses to respiratory virus infection is discussed. Materials and Methods Mice and experimental infection Female BALB/c mice (H-2 d ) were purchased from Taconic Farms and maintained under specific pathogen-free conditions. Mice that were 8 11 wk of age were lightly anesthetized by Metofane (Janssen) inhalation and sublethally infected by i.n. inoculation (in 50 l) with 3953 half-maximal tissue culture infectious doses (TCID 50 ) of a mouse-adapted A/Japan/ 305/57 (H2N2) influenza virus. Preparation of tissue lymphocytes At multiple days after influenza virus infection, mice were killed by cervical dislocation. Mouse tracheas were then surgically exposed to perform bronchoalveolar lavages (BAL). Briefly, the tracheas were cannulated with a syringe, and five separate 1-ml aliquots of IMEM were used to rinse the lower respiratory tract to collect inflammatory cells from the air spaces. Lavaged lungs were then perfused via the right ventricle of the heart with 5 10 ml of PBS containing 10 U/ml heparin (Sigma-Aldrich) to remove blood lymphocytes from the lung vasculature. The lungs were minced and passed through a steel screen. Tissue debris was removed by centrifugation at 300 g. Cells were counted and resuspended at appropriate concentrations for each particular experiment. Peptides CD8 T Cell Epitope Influenza virus peptides HA ,HA ,HA , and NP (Table I) were synthesized by University of Virginia Biomolecular Research Facility. Purity was confirmed by HPLC analysis. Ab labeling and flow cytometry Designation Amino Acid Sequence Hemagglutinin HA LYQNVGTYV Hemagglutinin HA TYVSVGTST Hemagglutinin HA IYATVAGSL Nucleoprotein NP TYQRTRALV For intracellular cytokine staining experiments, harvested cells were incubated for 6 h in 96-well, round-bottom plates in IMEM supplemented with 10% FBS, 10 U/ml penicillin G, 10 g/ml streptomycin sulfate, 100 U/ml human IL-2, 2 mm L-glutamine, 0.05% 2-ME, and 1 g/ml brefeldin A (Sigma-Aldrich). This relatively high concentration of human IL-2 was used to maximize the synthesis and accumulation of cytokines achieved in the ICCS. This high IL-2 concentration did not affect the frequency of cytokine-secreting cells (data not shown). Stimulatory conditions included 1 M of a given synthetic peptide and Japan/57 influenza virus-infected P815 (H-2 d ) mastocytoma cells. After incubation, cells were washed and incubated for 10 min in FcBlock (BD Biosciences). The allophycocyaninconjugated anti-cd8 mab (clone ; BD Biosciences) was used to label cells for 30 min at 4 C. After washing with FACS buffer (PBS/1% FBS), cells were fixed, and erythrocytes were simultaneously lysed using FACS Lysing Solution (BD Biosciences). The cells were labeled at room temperature with anti-ifn- (clone XMG1.2) or rat IgG1 (clone R3-34; BD Biosciences) in permeabilization buffer (FACS buffer containing 0.5% saponin). The percentage of background cytokine staining from the appropriate population of unstimulated cells was subtracted from the percentage of cytokine-positive cells under each stimulation condition before reporting. For intracellular granzyme B staining, cell suspensions were prepared from mediastinal lymph nodes (MLN) and lungs at multiple days after influenza virus infection. Cells were first surface-labeled with anti-cd8 (clone ; BD Biosciences) for 30 min, then fixed and permeabilized as described above. Permeabilized cells were labeled with anti-granzyme B (clone GB12; Caltag Laboratories) or mouse IgG1 (BD Biosciences) for 45 min at room temperature. Flow cytometry data were acquired for each of the experiments using a BD FACSCalibur (BD Immunocytometry Systems) and were analyzed using CellQuest software (BD Biosciences). Cell surface marker expression Lung cell suspensions (see Preparation of tissue lymphocytes above) were prepared from the lungs of four or five mice on day 6 after infection. Lung-infiltrating CD8 T cells were purified from pooled lung cell suspensions by CD8-conjugated magnetic bead positive selection (Miltenyi Biotec) according to the manufacturer s instructions. Surface CD107a mobilization was examined using a modification of the protocol described by Bells et al. (16). Briefly lung CD8 T cells (either in total lung cell homogenates or after purification) were cultured for 6 h at 37 C with P815 cells, which were either untreated or treated with a mixture of the four viral peptide epitopes in the presence of monensin (1 g/ml) and PE-conjugated anti-cd107 Ab (ID4B; Santa Cruz Biotechnology) at a final concentration of 5 g/ml throughout the assay period. Cells were then fixed and stained for CD69 and CD8 expression (as above). MHC class I tetramers MHC class I tetramers were produced according to published techniques (17). DNA plasmids encoding human 2 -microglobulin and the extracellular domain of the murine H-2K d H chain were provided by J. Altman (Emory University, Atlanta, GA) and E. Pamer (Sloan-Kettering Institute, New York, NY), respectively. MHC class I tetramers were prepared using each of the following influenza virus peptides: HA , HA , HA , and NP Tetramer labeling of harvested cells was performed for 45 min at 4 C. Virus titers Mice were i.n. infected with influenza Japan/57 virus and were killed by cervical dislocation at multiple time points after infection. Excised mouse lungs were weighed and homogenized. Multiple dilutions of clarified homogenates were plated on Mardin-Darby canine kidney cells in 96-well, flat-bottom plates. After 3 days in a 37 C humidified 7% CO 2 incubator, 50 l of supernatant was harvested from each well and mixed with 50 l of fresh 1% chicken erythrocyte solution in round-bottom, 96-well plates. After 1 h at room temperature, hemagglutination results were determined and used to calculate TCID 50 titers. BrdU labeling On multiple days after pulmonary influenza virus infection, mice were lightly anesthetized by Metofane (Janssen) inhalation and then were administered 50 l of 16 mg/ml BrdU (0.8 mg total/mouse) by the i.n. route or 250 l of 8 mg/ml BrdU (2 mg total/mouse) by i.p. injection. Each mouse was killed 24 h after BrdU treatment. Tissue cells were prepared for cell surface Ab labeling as described above. Anti-CD8 -labeled cells were fixed and permeabilized before BrdU staining was performed using the BD FastImmune BrdU kit according to the manufacturer s instructions (BD Biosciences). Results Frequency and magnitude of the pulmonary CD8 T cell response to defined epitopes after influenza virus infection We have analyzed the frequency of pulmonary CD8 T cells directed to four previously identified MHC class I-restricted epitopes of the Japan/57 (H2N2) virus in H-2 d haplotype BALB/c mice (Table I) (10 13) in response to sublethal i.n. Japan/57 infection by ICCS for IFN- and by MHC tetramer binding. Three of these H-2K d -restricted epitopes, HA , HA , and NP , were characterized in vitro as immunodominant, whereas the fourth epitope, HA , also K d -restricted, was
4 5334 CD8 T CELL RESPONSE TO PULMONARY INFLUENZA VIRUS classified as subdominant based on the magnitude of the in vitro CTL response (14, 15). Analysis of the kinetics of accumulation of IFN- -secreting CD8 T cells in BAL fluid revealed that Japan/57-specific, IFN- secreting CD8 T cells were first detected on day 5 postinfection (Fig. 1A). The frequency of IFN- -secreting T cells to each of the defined epitopes was always 5% at this time, with 10% of BAL CD8 T cells in aggregate identified as virus-specific by this criterion. As previously reported in other influenza virus infection models (3 5), over the next 2 5 days the frequency (percentage) of epitope-specific, IFN- -producing CD8 T cells increased significantly and reached a maximum level by days 9 10 of infection, with subsequent contraction of the response during the following week (Fig. 1A). Analysis of the IFN- response of CD8 T cells FIGURE 1. Kinetics of influenza virus-specific pulmonary CD8 T cells, as determined by intracellular cytokine and tetramer staining analyses. BALB/c mice were infected with 3953 TCID 50 of Japan/57 influenza virus. BAL (A) and lung parenchyma (B) cells were harvested at multiple days after infection and analyzed in 6-h intracellular cytokine assays. C, BAL cells were collected at multiple days after infection and labeled with specific tetramer. The percentage of specific IFN- expression or tetramer labeling is shown for CD8 gated T cells at the indicated number of days postinfection. Data are representative of three independent kinetic analyses with pooled tissue (samples) from three to five mice per time point. Values are the mean SEM for three or four replicate samples for each time point. infiltrating the lung parenchyma (Fig. 1B) revealed a comparable kinetics of expansion and contraction, although the magnitude of the response, i.e., the frequency of epitope-specific CD8 T cells in the lung parenchyma, was slightly reduced compared with that in BAL. A companion kinetic analysis of the CD8 T cell response in BAL by MHC class I tetramer staining demonstrated, as expected, a close correspondence in both the kinetics of accumulation and the frequency of specific T cells between MHC tetramer straining (Fig. 1C) and the ICCS assay (Fig. 1A). We did, however, note a modest delay in the contraction phase of the BAL CD8 T cell response, as defined by the frequency of epitope-specific MHC class I tetramer staining. As revealed above (Fig. 1), the hierarchy of immunodominance for the four epitopes, initially suggested by in vitro studies of immune T cell populations, was confirmed by the analysis of epitopespecific responses during the in vivo CD8 T cell response to primary infection in the respiratory tract. Thus, CD8 T cells directed to the NP ,HA , and HA epitopes dominated the response in vivo, with the weaker (subdominant) response to HA likewise demonstrable in the lungs after primary infection. When the kinetics of the total CD8 T cell response to each epitope was calculated by either tetramer staining (Fig. 2A) or ICCS (Fig. 2B), the rates of expansion and contraction of the response to the three dominant epitopes followed a predictable pattern (3 5) based on the frequency of responding T cells. Contribution of epitope-specific CD8 T cells to the overall pulmonary CD8 T cell response The findings described above suggested that a robust primary pulmonary CD8 T cell response is mounted to these four epitopes after infection with Japan/57 virus. Although weak/subdominant responses to sites on several viral gene products from various influenza virus strains have been reported (1, 2, 5), previous analyses from our laboratory (14, 15) (unpublished observations) suggested that the response to the Japan/57 virus in H-2 d haplotype mice is focused predominately, if not exclusively, on the four described epitopes. We therefore wished to determine the contribution to the overall pulmonary Japan/57-specific CD8 T cell response by CD8 T cells that were directed against these four epitopes in vivo after i.n. infection. To this end, we analyzed the frequency of IFN- -secreting CD8 T cells responding to each of the four peptide epitopes in BAL and lung parenchyma by ICCS at the peak of the primary response. In parallel, we also analyzed the IFN- response of pulmonary CD8 T cells to Japan/57-infected P815 cells (H-2 d ) as a source of naturally processed viral Ags. The virus-infected cells would probably process and present all potential H-2 d -restricted MHC class I epitopes, including, but not limited to, the four epitopes previously identified and analyzed in this study. We then calculated the sum total of the individual CD8 T cell IFN- responses to each of the four Japan/57 epitopes and compared this value to stimulation by the virus-infected cells (which was normalized to 100%). Strikingly, we observed a very high correlation (93% in lung, 92% in BAL) of IFN- expression when comparing CD8 T cell stimulation by virus-infected cells to the cumulative frequency of individual peptide-stimulated CD8 T cells (Fig. 3). These data demonstrate that the specific CD8 T cell response against influenza virus Japan/57 in H-2 d mice is nearly entirely directed against the four viral epitopes presently studied. In this regard, it should be noted that at the peak of the pulmonary CD8 T cell response (day 10), between 60 and 70% of the total pulmonary CD8 T cells secreted IFN- in response to infected P815 cells. We also determined that the total complement of lung CD8 T cells capable of secreting IFN- after nonspecific stimulation
5 The Journal of Immunology 5335 FIGURE 2. Quantitation of influenza virus-specific CD8 T cell numbers in the lung airways and parenchyma. A, BAL cells were harvested from mice at the indicated number of days after Japan/57 infection and analyzed by tetramer staining. B, Lung parenchyma cells were harvested at multiple days after infection and analyzed using the intracellular cytokine assay. The numbers of individual Ag-specific CD8 T cells were calculated using total cell count and IFN- (A) or tetramer (B) frequency data and are displayed in the left graphs. The total number of virus-specific CD8 T cells at each time point is inset in the right graphs. Data are representative of three independent analyses with values obtained from pools obtained from three to five donor mice per time point. with PMA/ionomycin was 70%. Thus, by these criteria, the vast majority of lung CD8 T cells were virus specific. Lung virus replication and the early CD8 T cell response Our previously discussed kinetic analysis of influenza virus-specific CD8 T cell accumulation in the lungs during primary i.n. infection revealed that specific CD8 T cells (defined by tetramer staining and ICCS) were first detectable on days 5 6 of infection. Furthermore, the response appeared to be directed primarily, if not exclusively, to the four described epitopes. With this information in hand, we next evaluated the time course of virus replication in the lungs to relate the tempo of virus clearance to the kinetics of virus-specific CD8 T cell trafficking to the lungs. As Fig. 4 demonstrates, virus titers rapidly peaked ( TCID 50 /g tissue) by 24 h postinfection and were sustained at or near peak titers through day 5 of infection. Between days 5 and 7, virus titers dropped precipitously ( 1000-fold), and infectious virus was no longer detectable in the lungs by day 9 of infection. This precipitous fall in pulmonary Japan/57 virus titer over days 5 7 of infection is in agreement with previously published results in the murine model using other influenza virus strains (18 21) and correlates with the FIGURE 3. The total influenza virus-specific CD8 T cell response is nearly entirely directed against four Japan/57 epitopes. Cell suspensions were prepared from the BAL and lung parenchyma on day 10 postinfection. Cells were stimulated for 6 h with each of the synthetic peptides and Japan/57-infected P815 cells. The frequency of IFN- expression by CD8 T cells after stimulation by virus-infected cells was set at 100% for comparison with the cumulative response to each of the individual viral epitopes. The data are representative of three independent analyses using data pooled from four mice for infectious virus and individual peptides. Percentages of the total influenza response (reflected in the sum of individual epitopes) ranged from 89 to 95%. early infiltration of specific CD8 T cells into the lung, i.e., days 5 6 (Fig. 1) (3). These kinetic observations implicate the early CD8 T cell response in the infected lungs as critical in controlling virus replication and clearance. However, it is also evident from this kinetic analysis that the major control of virus replication and clearance occurred on days 5 7, which is before the peak of pulmonary CD8 T cell accumulation in the lungs, i.e., days 9 11 (Fig. 2). Early accumulation of virus-specific CD8 T cells within the infected lungs The trafficking and accumulation of activated virus-specific T cells into the infected lungs are believed to be preceded by the activation and proliferative expansion of naive virus-specific CD8 T cell precursors in organized lymphoid tissues, most notably the MLN draining the infected lung (22, 23), and with subsequent migration of activated effector CD8 T cells from the lymph nodes to the infected lungs. However, analyses of the frequencies of FIGURE 4. Kinetics of Japan/57 virus clearance from infected mouse lungs. Mice were i.n. inoculated on day 0 with 3953 TCID 50 of Japan/57 influenza virus (inoculum dose denoted by asterisk). At several days after infection, mouse lungs were harvested, weighed, and homogenized. Clarified homogenates were plated on Mardin-Darby canine kidney cells. The presence of infectious influenza virus was analyzed 3 days later by hemagglutination assay to determine TCID 50 titers.
6 5336 CD8 T CELL RESPONSE TO PULMONARY INFLUENZA VIRUS epitope-specific CD8 T cells detected in draining MLN by tetramer staining and/or ICCS in the early inductive phase of the CD8 T cell response to primary i.n. influenza virus infection (i.e., up to day 4 postinfection) have demonstrated epitope-specific CD8 T cell frequencies of 2% (4, 24, 25). In the case of the four CD8 T cell epitopes examined in this study, the frequencies of specific CD8 T cells enumerated by tetramer staining in the draining lymph nodes on day 4 postinfection have a cumulative total of 3 5% (data not shown). Although the inability to detect significant proliferative expansion of CD8 T cell precursors in the draining lymph nodes before T cell accumulation in the infected lungs would most likely reflect the inherent low frequency of naive specific CD8 T cell precursors (22) and the presumed rapid egress of effector CD8 T cells out of the lymph nodes after proliferative expansion, several recent findings from our laboratory have modified our view of the early events in CD8 T cell activation in the draining lymph nodes and lungs. First, we have recently demonstrated that early in the activation of influenza virus-specific CD8 T cells within draining lymph nodes during pulmonary influenza virus infection, activated proliferating CD8 T cells transiently lose the ability to bind specific tetramers without any loss of cell surface TCR expression. 4 This transient loss of tetramer binding by activated T cells (up to days 5 6 postinfection) is associated with a transient diminished ability of the T cells to secrete IFN- in the ICCS assay, but without any deficit in Ag-driven cell proliferation. 4 Importantly, a significant fraction ( 60%) of activated CD8 T cells that have migrated to the infected lung early during the T cell response to infection (days 5 7) retain the diminished capacity to bind specific tetramer and secrete IFN- in the ICCS assay. Full tetramer staining and cytokine secretion are, however, restored in CD8 T cells infiltrating the lung by day 8 postinfection. 4 Second, we have recently demonstrated that h after naive CD8 T cell activation and the onset of T cell proliferation in draining MLN in response to pulmonary influenza virus infection, activated virusspecific CD8 T cells within the draining lymph nodes express the granule-associated lytic molecule granzyme B, and that granzyme B expression is uniformly retained by activated T cells in the lymph nodes and after T cell migration to the infected lungs (23). Taken together, these previously discussed observations suggested that the frequency of virus-specific CD8 T cell present in the lungs early in infection (i.e., days 5 7) may be underestimated by tetramer staining and/or ICCS, and that granzyme B expression may serve as a surrogate marker for the detection and enumeration of activated specific CD8 T cells at early time points during infection. To examine these possibilities, we studied the kinetics of granzyme B expression by CD8 T cells within draining MLN and lungs after i.n. influenza virus infection using a flow cytometrybased assay (26, 27). As Fig. 5 demonstrates, granzyme B CD8 T cells were identified at a detectable frequency on day 3 postinfection. The percentage of granzyme B T cells in the MLN increased 10-fold (3%) by day 4, doubled in frequency over the ensuing 24 h (to 6%), and was maintained at this level up to day 9 after infection. Analysis of granzyme B CD8 T cells infiltrating the lungs (Fig. 5) was noteworthy in several respects. First, there was minimal accumulation of granzyme B T cells in the infected lungs up to day 4 of infection. Thus, despite the high lung virus titer over this period, there was neither significant nonspecific (inflammation induced) induction of granzyme B expression by lung-resident 4 D. R. Drake, R. M. Ream, C. W. Lawrence, and T. J. Braciale. Transient loss of MHC class I tetramer binding after CD8 T cell activation reflects T cell effector function. Submitted for publication. FIGURE 5. High frequencies of granzyme B-expressing CD8 T cells are present in the draining lymph nodes and lungs during influenza virus infection. The lungs and MLN were harvested from BALB/c mice at multiple days after Japan/57 virus infection. The presence of granzyme B was detected by intracellular staining with specific Ab. The percentage of granzyme B expression by CD8 gated T cells is shown on the left y-axis ( ). The absolute numbers of granzyme B CD8 T cells are shown on the right y-axis (f). CD8 T cells nor significant recruitment of specific (or Ag-nonspecific) CD8 T cells to the infected lungs with an activated granzyme B phenotype. Second, by day 5 of infection, 30% of lung-resident CD8 T cells were granzyme B. The tempo of granzyme B CD8 T cell accumulation in the lungs directly paralleled our recent observations (22) on the time course of activated granzyme B T cell migration from the draining lymph nodes to the infected lungs in a TCR transgenic CD8 T cell adoptive transfer model. More importantly, this frequency of granzyme B T cells in the lungs was well above the predicted total frequency of epitope-specific CD8 T cells detected by ICCS/tetramer staining, i.e., 10% (Fig. 1A), and suggested that early in the CD8 T cell response to infection in the lungs, the frequency of virus-specific CD8 T cells may be substantially higher than that detected by the ICCS/tetramer labeling methods. Third, by day 6 postinfection, when pulmonary virus titers began to drop precipitously (Fig. 4), 70% of lung CD8 T cells were granzyme B. This T cell frequency was two to three times higher than the cumulative frequency of Japan/57 epitope-specific cells determined by ICCS and/or tetramer staining, again suggesting that the frequency of specific T cells infiltrating the lungs was substantially higher than that estimated by recognition of specific epitopes. Furthermore, cell surface marker analyses of the day 6 CD8 T cells demonstrated that at least 60% of these cells expressed activation marker phenotypes (CD62L, CD69, LFA-1 high, and VLA-4 high ) consistent with recent activation (data not shown). The high frequency of granzyme B CD8 T cells ( 70%) in the infected lungs was also sustained up to day 9. At this point in time, the frequency (percentage) of granzyme B CD8 T cells closely approximated the frequency estimates of virus-specific CD8 T cells in the
7 The Journal of Immunology 5337 lungs, defined in the ICCS assay using specific peptides or infected P815 cells to elicit IFN- synthesis (Fig. 3). To further explore the Ag responsiveness of the lung-infiltrating granzyme B CD8 T cells during the early phase of the adaptive response (i.e., day 6 postinfection), we evaluated the expression (up-regulation) of CD107a (liposome-associated membrane proteins-1a) and CD69 on that cell population after short term in vitro stimulation with a synthetic peptide mixture corresponding to the four A/JAPAN/57 epitopes (see Materials and Methods). We observed that only a small percentage ( 10%) of the lung-infiltrating granzyme B CD8 T cells up-regulated CD107a expression in response to antigenic stimulation. This finding is consistent with the low frequency of day 6 lung CD8 T cells responding to peptide stimulation in the ICCS assay. By contrast, 95% of the lunginfiltrating granzyme B CD8 T cells up-regulated cell surface CD69 expression in response to in vitro stimulation with the viral peptide mixture (data not shown). The latter finding suggests that these lung-infiltrating, granzyme B CD8 T cells, which exhibit diminished tetramer staining and responsiveness in effector assays such as ICCS and CD107a up-regulation (8), represent a population of virus-specific T cells that are responsive to TCR engagement and specific antigenic stimulation. Proliferation of lung-resident CD8 T cells If, as we believe, granzyme B expression is a useful marker for the activated Ag-specific responding CD8 T cells, then substantial numbers of specific CD8 T cells should have accumulated in the draining MLN on days 5 6 of infection. Although we did detect a significant expansion of granzyme B CD8 T cells in the draining MLN before (day 4) and during (days 5 6) CD8 T cell accumulation in the lungs, we could not readily account for the elevated numbers of T cells in the lungs based on our calculations of total MLN-resident granzyme B CD8 T cells (Fig. 5). One potential explanation is that specific CD8 T cells were activated in and recruited from other lymphoid tissues (e.g., the spleen) to the lungs. For reasons discussed later (see Discussion) we do not believe that this is the case. Alternatively, either the frequency of granzyme B T cells underestimated the true frequency of proliferating CD8 T cells in the draining MLN, or there was significant proliferation of the responding virus-specific CD8 T cells in the infected lungs. To directly estimate the frequency of proliferating CD8 T cells responding to i.n. infection, we used the strategy of in vivo uptake of the thymidine analog BrdU with a flow cytometry-based analysis to identify BrdU proliferating T cells. In a previous report (23), up to 80% of CD8 T cells infiltrating the lung airways were BrdU on day 8 of infection when a continuous in vivo labeling method was used to supply BrdU in the drinking water throughout infection. Our initial attempts to continuously label responding cells by adding BrdU to the drinking water of infected animals were unsuccessful, because mice became moribund shortly after i.n. infection and exhibited a marked decrease in water consumption. To circumvent this problem, we used the strategy of i.p. injection of BrdU at different time points after virus infection and harvested the MLN and infected lungs 24 h later to enumerate BrdU CD8 T cells in these sites. This strategy would also allow us to identify the approximate time point of maximum T cell proliferation in the MLN. As Fig. 6A demonstrates, proliferating (BrdU ) CD8 T cells were not detectable in the draining MLN at a significant frequency before the 24-h interval between days 3 4 of infection. The rate of CD8 T cell proliferation reached a maximum in the MLN during the day 5 6 interval, and proliferating T cells were detected at a similar frequency through day 8 before decreasing in frequency FIGURE 6. BrdU incorporation by proliferating CD8 T cells in MLN and lungs during influenza virus infection. BrdU was either injected i.p. (A) or administered via the i.n. route (B) to detect cells that had synthesized DNA in the previous 24 h. The percentage of BrdU expression is shown for CD8 gated T cells at the indicated number of days postinfection. later in infection. The frequency of BrdU T cells in the draining MLN at the peak of proliferation ( 12% on day 6) was slightly higher than the corresponding frequency of granzyme B cells in the lymph nodes at the same time period ( 7 8%; Fig. 5). We anticipated that the kinetics of BrdU T cell accumulation in the lungs might be slightly delayed compared with those in the MLN, and that the frequency of BrdU T cells within the infected lungs might be higher during the day 6 8 interval, reflecting the migration of activated/proliferating CD8 T cells from the MLN (or other lymphoid tissues) to the lungs over the 24-h labeling interval. As demonstrated in Fig. 6A, this was not the case. BrdU CD8 T cells were detected in the lungs at an overall frequency comparable to that in the draining MLN. The kinetics of BrdU T cell accumulation in the lungs, as determined by 24-h pulse BrdU labeling, was consistent with the concept that activated virus-specific CD8 T cells proliferated primarily, if not exclusively, in lymphoid tissues (e.g., draining MLN) before migration to the infected lungs. However, the frequency of BrdU CD8 T cells in the lungs on days 5 6 ( 12%) was well below the frequency of granzyme B T cells (30% on day 5 and 70% on day 6) detected in the lungs at this time (Fig. 5). Although the division time of activated CD8 T cells has been reported to be as short as 6 h (28), it is possible that this discrepancy in the frequency of specific CD8 T cells simply reflected our inability to accurately assess the extent of T cell proliferation in the MLN using the relatively short 24-h interval of BrdU labeling in vivo. An alternative explanation was suggested by the possibility that during the inflammatory response in a site such as the lungs (e.g., during pulmonary influenza virus infection), there is a local
8 5338 CD8 T CELL RESPONSE TO PULMONARY INFLUENZA VIRUS accumulation of nucleotide precursors resulting from DNA degradation and the release of precursor pools locally from dead and dying cells (29). This effect could result in dilution of the thymidine analog available for uptake by proliferating CD8 T cells in the lungs and a decrease in the efficiency of BrdU labeling in the lungs. We decided to explore this possibility by locally introducing BrdU in the respiratory tract by i.n. administration during virus infection at 24-h intervals before harvesting lungs and MLN for analysis of BrdU uptake in a manner analogous to the i.p. BrdU administration. We anticipated that this i.n. BrdU labeling strategy might result in a decreased efficiency of labeling of T cells in the draining MLN. As shown in Fig. 6B, this was indeed the case. The frequency of BrdU cells in the MLN was lower at any time point sampled, but the overall kinetics of BrdU labeling in the lymph nodes after i.n. BrdU administration were similar to the time course determined by i.p. BrdU administration (Fig. 6). In marked contrast, i.n. BrdU administration revealed a substantially higher frequency of BrdU T cells in the infected lungs, particularly during the 24-h interval between days 6 7 of infection when 30% of the lung-infiltrating CD8 T cells were BrdU. This finding suggests that in addition to undergoing proliferative expansion in the draining lymph nodes, activated T cells may continue to undergo one or more divisions upon entering infected lungs. Discussion In this report we examined the contribution of a set of defined MHC class I-restricted CD8 T cell epitopes to the overall pulmonary CD8 T cell response during experimental murine type A influenza virus infection (30). We used the complementary techniques of MHC class I tetramer labeling and Ag-dependent cytokine synthesis in the ICCS assay to identify epitope-specific T cells based on structural (TCR binding) and functional criteria. Using this approach, we were able to define the tempo and magnitude of the pulmonary CD8 T cell response during primary infection and demonstrate that 90% of the total influenza virus-specific pulmonary CD8 T cell response at its peak (i.e., days 9 11) was directed to four defined epitopes. Based on this information, we went on to examine the early CD8 T cell response in the draining lymph nodes and lungs using the expression of granzyme B by activated CD8 T cells as a surrogate marker for specific T cell activation. We observed that early in the primary CD8 T cell response to infection (i.e., days 4 7), the conventional tetramer/ intracellular cytokine methods for enumerating specific T cells appeared to underestimate the frequency of specific CD8 T cells in the lungs. In addition, we obtained evidence for proliferation of activated CD8 T cells in the lungs during this early phase of infection by using intranasal administration of the thymidine analog BrdU. The cumulative frequency of influenza virus epitope-specific CD8 T cells detected in our study is higher than that previously reported for a primary pulmonary influenza virus infection (3, 4, 25), most likely because the four epitopes represent the vast majority of specific CD8 T cells directed to this virus strain in H-2 d haplotype mice (Fig. 3). However, these frequencies are comparable to the high frequencies of virus-specific CD8 T cells detectable in the lungs during other respiratory virus infections (6, 7). The kinetics of specific CD8 T cell appearance, peak accumulation, and contraction in this report also closely parallel previously reported observations of the primary pulmonary CD8 T cell response to influenza virus infection using different mouse and virus strains (3 5). This suggests that our findings are not unique to a particular virus/mouse strain combination. CD8 T cells have been documented to play an important role in virus clearance during primary influenza virus infection (30). Consistent with previous reports (3, 25, 31), we observed that influenza virus-specific CD8 T cells were first detected in the BAL and lung parenchyma on days 5 6 postinfection by cytokine synthesis and/or tetramer binding (Fig. 1). This corresponded to the same time frame as the onset of virus clearance from the infected lungs (Fig. 4). However, the drop in lung virus titer was much more dramatic on days 5 7 of infection than might be expected from the relatively low frequency of CD8 T cells detected in lungs at this time. Furthermore, both the frequency and absolute number of virus-specific lung CD8 T cells on days 5 7 (as determined by cytokine synthesis/tetramer binding) represented only a small fraction of the specific T cells detected at the peak of the response 4 5 days later when infectious virus was no longer detectable in the lungs. This discrepancy between virus clearance and CD8 T cell frequency early in infection raised the possibility that techniques to evaluate cytokine synthesis/tcr binding underestimated the actual frequency/number of influenza virus-specific CD8 T cells in the lungs. This possibility was reinforced by our recent findings in a TCR transgenic model of in vivo CD8 T cell responses to pulmonary influenza virus infection. 4 Using this model, we demonstrated that shortly (1 2 days) after in vivo activation, naive influenza virus-specific CD8 T cells transiently lost the capacity to bind specific MHC class I tetramers and exhibited diminished cytokine (IFN- ) synthesis in the ICCS assay. This loss of tetramer binding/cytokine synthesis was the most pronounced on days 3 4 of infection in the draining lymph nodes and on days 4 6 in the infected lungs, with full restoration of tetramer binding/cytokine synthesis by days 8 10 postinfection. 4 Based on these considerations, we explored the possibility of using the expression of the cytolytic effector molecule granzyme B as a surrogate marker to identify virus-specific activated CD8 T cells responding in the lungs to infection. Granzyme B is not expressed by naive T cells, but is produced and stored in intracellular granules after CD8 T cell activation (27, 32). In this connection, we have recently reported (23) that granzyme B expression is rapidly induced in the majority of activated virus-specific CD8 T cells in response to respiratory influenza virus infection shortly after the onset of cell division in draining lymph nodes. Granzyme B expression is also sustained at high levels in lung-infiltrating effector CD8 T cells after migration from lymph nodes to infected lungs. The frequency of virus-specific CD8 T cells responding in the draining lymph nodes early (days 5 6) after pulmonary infection has been reported to be extremely low and at the limits of detection by tetramer staining (our unpublished observations) (4, 24, 25). However, we were able to reproducibly detect granzyme B CD8 T cells as early as day 3 of infection in the draining MLN, with a maximum frequency of granzyme B CD8 T cells of 7% in the draining lymph nodes 3 4 days later. The companion analysis of CD8 T cells in the infected lungs revealed that 30% of lung-infiltrating CD8 T cells expressed granzyme B on day 5 of infection, and 70% of the CD8 T cells in the lungs were granzyme B on day 6 postinfection. This frequency was 3 times higher than that of virus epitope-specific CD8 T cells detected by tetramer/cytokine at the corresponding day 6 point. It is also noteworthy that granzyme B CD8 T cells were detected at a similar frequency ( 70%) on day 9 of infection when the overall frequency of influenza-specific CD8 T cells in infected lungs was determined by ICCS using infected P815 cells to likewise correspond to 70%. These findings support the view that effector CD8 T cells accumulate in the lungs at significant frequencies and in substantial numbers early in the course of infection, i.e., days 5 6, when substantial virus clearance occurs.
9 The Journal of Immunology However, because the majority of these lung-infiltrating granzyme B CD8 T cells present early (i.e., days 5 6) in the host response do not bind specific tetramer and do not respond in either the ICCS or CD107a mobilization assay, we cannot directly document that these T cells are influenza specific. Because granzyme B expression merely marks CD8 T cells as having been previously activated (and therefore not necessarily virus specific), it is possible that the high frequency of granzyme B CD8 T cells infiltrating infected lungs early after infection (days 5 6) could reflect nonspecific recruitment of irrelevant CD8 T cells to a site of inflammation. Indeed, Ag-nonspecific memory CD8 T cells have been shown to migrate to the lungs after respiratory infection with RSV, influenza virus, and Sendai virus (33 35). However, nonspecific recruitment of irrelevant memory CD8 T cells to the inflamed lungs is an unlikely explanation for our findings. This phenomenon has been reported to peak early after infection, i.e., days 4 5 (33, 36) and to not occur at the sustained high frequency exhibited by the lung-infiltrating granzyme B cells demonstrated in this study. Furthermore, the level of granzyme B protein expression in memory cells has been previously demonstrated to be very low (26, 27, 36), whereas we detected a uniformly high level of granzyme B in the CD8 T cells infiltrating the lungs on days 5 6 after infection (data not shown). In this regard, it is noteworthy that differential granzyme B gene expression has been previously used in a reporter-based transgenic strategy to distinguish effector T cells from memory T cells in vivo (37). Finally, the extremely close correspondence between the frequency of granzyme B CD8 T cells and the predicted frequency of influenza virus-specific CD8 T cells on day 9 of infection supports the view that granzyme B may serve as a useful marker in identifying recently activated Ag-specific CD8 T cells throughout the course of infection. Our analysis of the frequency and total number of granzyme B CD8 T cells in draining MLN and infected lungs suggested that the observed numbers of granzyme B cells detected in the lungs early in infection could not readily be accounted for by the accumulation of migrant CD8 T cells derived from the draining lymph nodes. We believe this is unlikely to be due to the recruitment of activated virus-specific T cells from other secondary lymphoid organs (e.g., spleen or nondraining lymph nodes). The reasons for this are 2-fold. First, during influenza virus infection, Ag-bearing respiratory dendritic cells were found to preferentially, if not exclusively, migrate to the lung-draining lymph nodes (38). Second, in a recent analysis of CD8 T cell activation in vivo in response to influenza infection, initial/early CD8 T cell activation was restricted to those cells present in draining MLN (23). We therefore investigated the possibility that after migration from draining lymph nodes, activated CD8 T cells proliferated in the lungs and thereby contributed to the elevated T cell numbers detected. We were able to demonstrate proliferation of activated CD8 T cells directly in situ in the infected lungs by i.n. administration of the thymidine analog BrdU. Delivery of BrdU by the i.n. route proved superior to i.p. BrdU administration in the detection of proliferating CD8 T cells in the lungs. It is unlikely that the efficient labeling of lung CD8 T cells with BrdU after i.n. administration was due to transport of the nucleoside analog to the draining lymph nodes and labeling of proliferating T cells there before their migration to the infected lungs. In fact, the efficiency of BrdU uptake by CD8 T cells in the draining lymph nodes after i.n. administration was lower than the efficiency of labeling after i.p. administration. We believe that the high local concentration of DNA nucleotide/nucleoside at sites of inflammation and/or associated cell death (39), such as influenza virus-infected lungs, results in dilution of BrdU after parenteral administration and short term (24-h) in vivo labeling. Because in vivo BrdU uptake by T cells after i.n. administration was monitored at 24-h intervals, and transfer of BrdU from the respiratory tract to the draining MLN was readily detected in our analysis, it remains a formal possibility that the high frequency of BrdU CD8 T cells detected in the lung resulted from uptake of a label by cells at a distant sites (e.g., the secondary lymphoid organs). As yet, we cannot rigorously exclude this possibility. However, the finding that i.p. BrdU administration does not result in the preferential accumulation of BrdU CD8 T cells in the infected lungs does not readily support this view. In conclusion, we have characterized the CD8 T cell responses to a series of defined virus-specific CD8 T cell epitopes. These sites account for 90% of the overall CD8 T cell response directed against influenza virus in the lungs. With this information available, we were able to explore the use of a surrogate marker, i.e., granzyme B expression, to demonstrate that this protein may be useful in identifying specifically activated effector CD8 T cells. By using granzyme B expression, we demonstrated that virus-specific CD8 T cells may accumulate in the lungs at higher frequencies earlier in the course of the antiviral CD8 T cell response than predicted by the use of conventional tetramer/intracellular cytokine methods. Finally, we provided evidence for the first time that activated effector CD8 T cells may continue to undergo one or more rounds of proliferation in situ in the infected lungs after migrating from the draining lymph nodes. Thus, in situ CD8 T cell proliferation at a site of infection/inflammation such as the lungs as well as the proliferative expansion of naive CD8 T cells in secondary lymphoid organs such as the draining MLN contribute to the overall magnitude of the CD8 T cell response during pulmonary influenza virus infection. Acknowledgments We thank Dr. Donald R. Drake III for advice and assistance with the design of experiments. Disclosures The authors have no financial conflict of interest References 1. Chen, W., L. C. Anton, J. R. Bennink, and J. W. Yewdell Dissecting the multifactorial causes of immunodominance in class I-restricted T cell responses to viruses. Immunity 12: Chen, W., J. R. Bennink, P. A. Morton, and J. W. Yewdell Mice deficient in perforin, CD4 T cells, or CD28-mediated signaling maintain the typical immunodominance hierarchies of CD8 T-cell responses to influenza virus. J. Virol. 76: Flynn, K. J., G. T. Belz, J. D. Altman, R. Ahmed, D. L. Woodland, and P. C. Doherty Virus-specific CD8 T cells in primary and secondary influenza pneumonia. Immunity 8: Belz, G. T., W. Xie, J. D. Altman, and P. C. Doherty A previously unrecognized H-2D b -restricted peptide prominent in the primary influenza A virusspecific CD8 T-cell response is much less apparent following secondary challenge. J. Virol. 74: Belz, G. T., W. Xie, and P. C. Doherty Diversity of epitope and cytokine profiles for primary and secondary influenza a virus-specific CD8 T cell responses. J. Immunol. 166: Usherwood, E. J., R. J. Hogan, G. Crowther, S. L. Surman, T. L. Hogg, J. D. Altman, and D. L. Woodland Functionally heterogeneous CD8 T-cell memory is induced by Sendai virus infection of mice. J. Virol. 73: Chang, J., A. Srikiatkhachorn, and T. J. Braciale Visualization and characterization of respiratory syncytial virus F-specific CD8 T cells during experimental virus infection. J. Immunol. 167: Butz, E. A., and M. J. Bevan Massive expansion of antigen-specific CD8 T cells during an acute virus infection. Immunity 8: Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8: Sweetser, M. T., V. L. Braciale, and T. J. Braciale Class I major histocompatibility complex-restricted T lymphocyte recognition of the influenza hemagglutinin: overlap between class I cytotoxic T lymphocytes and antibody sites. J. Exp. Med. 170:1357.
SUPPLEMENTARY INFORMATION
 Complete but curtailed T-cell response to very-low-affinity antigen Dietmar Zehn, Sarah Y. Lee & Michael J. Bevan Supp. Fig. 1: TCR chain usage among endogenous K b /Ova reactive T cells. C57BL/6 mice
Complete but curtailed T-cell response to very-low-affinity antigen Dietmar Zehn, Sarah Y. Lee & Michael J. Bevan Supp. Fig. 1: TCR chain usage among endogenous K b /Ova reactive T cells. C57BL/6 mice
Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD-
 Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Supplementary Figures
 Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Supporting Information
 Supporting Information Valkenburg et al. 10.1073/pnas.1403684111 SI Materials and Methods ELISA and Microneutralization. Sera were treated with Receptor Destroying Enzyme II (RDE II, Accurate) before ELISA
Supporting Information Valkenburg et al. 10.1073/pnas.1403684111 SI Materials and Methods ELISA and Microneutralization. Sera were treated with Receptor Destroying Enzyme II (RDE II, Accurate) before ELISA
Supporting Information
 Supporting Information Aldridge et al. 10.1073/pnas.0900655106 Fig. S1. Flow diagram of sublethal (a) and lethal (b) influenza virus infections. (a) Infection of lung epithelial cells by influenza virus
Supporting Information Aldridge et al. 10.1073/pnas.0900655106 Fig. S1. Flow diagram of sublethal (a) and lethal (b) influenza virus infections. (a) Infection of lung epithelial cells by influenza virus
TITLE: MODULATION OF T CELL TOLERANCE IN A MURINE MODEL FOR IMMUNOTHERAPY OF PROSTATIC ADENOCARCINOMA
 AD Award Number: DAMD17-01-1-0085 TITLE: MODULATION OF T CELL TOLERANCE IN A MURINE MODEL FOR IMMUNOTHERAPY OF PROSTATIC ADENOCARCINOMA PRINCIPAL INVESTIGATOR: ARTHUR A HURWITZ, Ph.d. CONTRACTING ORGANIZATION:
AD Award Number: DAMD17-01-1-0085 TITLE: MODULATION OF T CELL TOLERANCE IN A MURINE MODEL FOR IMMUNOTHERAPY OF PROSTATIC ADENOCARCINOMA PRINCIPAL INVESTIGATOR: ARTHUR A HURWITZ, Ph.d. CONTRACTING ORGANIZATION:
Differential Response of Respiratory Dendritic Cell Subsets to Influenza Virus Infection
 JOURNAL OF VIROLOGY, May 2008, p. 4908 4919 Vol. 82, No. 10 0022-538X/08/$08.00 0 doi:10.1128/jvi.02367-07 Copyright 2008, American Society for Microbiology. All Rights Reserved. Differential Response
JOURNAL OF VIROLOGY, May 2008, p. 4908 4919 Vol. 82, No. 10 0022-538X/08/$08.00 0 doi:10.1128/jvi.02367-07 Copyright 2008, American Society for Microbiology. All Rights Reserved. Differential Response
L-selectin Is Essential for Delivery of Activated CD8 + T Cells to Virus-Infected Organs for Protective Immunity
 Cell Reports Supplemental Information L-selectin Is Essential for Delivery of Activated CD8 + T Cells to Virus-Infected Organs for Protective Immunity Rebar N. Mohammed, H. Angharad Watson, Miriam Vigar,
Cell Reports Supplemental Information L-selectin Is Essential for Delivery of Activated CD8 + T Cells to Virus-Infected Organs for Protective Immunity Rebar N. Mohammed, H. Angharad Watson, Miriam Vigar,
Draining Lymph Nodes in Response to Pulmonary Virus Infection
 This information is current as of November 18, 2018. Sequential Activation of CD8 + T Cells in the Draining Lymph Nodes in Response to Pulmonary Virus Infection Heesik Yoon, Kevin L. Legge, Sun-sang J.
This information is current as of November 18, 2018. Sequential Activation of CD8 + T Cells in the Draining Lymph Nodes in Response to Pulmonary Virus Infection Heesik Yoon, Kevin L. Legge, Sun-sang J.
Supplementary Materials for
 immunology.sciencemag.org/cgi/content/full/2/16/eaan6049/dc1 Supplementary Materials for Enzymatic synthesis of core 2 O-glycans governs the tissue-trafficking potential of memory CD8 + T cells Jossef
immunology.sciencemag.org/cgi/content/full/2/16/eaan6049/dc1 Supplementary Materials for Enzymatic synthesis of core 2 O-glycans governs the tissue-trafficking potential of memory CD8 + T cells Jossef
Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to
 Class II tetramer staining Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to PE were combined with dominant HIV epitopes (DRB1*0101-DRFYKTLRAEQASQEV, DRB1*0301- PEKEVLVWKFDSRLAFHH,
Class II tetramer staining Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to PE were combined with dominant HIV epitopes (DRB1*0101-DRFYKTLRAEQASQEV, DRB1*0301- PEKEVLVWKFDSRLAFHH,
Micro 204. Cytotoxic T Lymphocytes (CTL) Lewis Lanier
 Micro 204 Cytotoxic T Lymphocytes (CTL) Lewis Lanier Lewis.Lanier@ucsf.edu Lymphocyte-mediated Cytotoxicity CD8 + αβ-tcr + T cells CD4 + αβ-tcr + T cells γδ-tcr + T cells Natural Killer cells CD8 + αβ-tcr
Micro 204 Cytotoxic T Lymphocytes (CTL) Lewis Lanier Lewis.Lanier@ucsf.edu Lymphocyte-mediated Cytotoxicity CD8 + αβ-tcr + T cells CD4 + αβ-tcr + T cells γδ-tcr + T cells Natural Killer cells CD8 + αβ-tcr
Shenghua Zhou, Rong Ou, Lei Huang, and Demetrius Moskophidis*
 JOURNAL OF VIROLOGY, Jan. 2002, p. 829 840 Vol. 76, No. 2 0022-538X/02/$04.00 0 DOI: 10.1128/JVI.76.2.829 840.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved. Critical Role
JOURNAL OF VIROLOGY, Jan. 2002, p. 829 840 Vol. 76, No. 2 0022-538X/02/$04.00 0 DOI: 10.1128/JVI.76.2.829 840.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved. Critical Role
Detailed step-by-step operating procedures for NK cell and CTL degranulation assays
 Supplemental methods Detailed step-by-step operating procedures for NK cell and CTL degranulation assays Materials PBMC isolated from patients, relatives and healthy donors as control K562 cells (ATCC,
Supplemental methods Detailed step-by-step operating procedures for NK cell and CTL degranulation assays Materials PBMC isolated from patients, relatives and healthy donors as control K562 cells (ATCC,
Medical Virology Immunology. Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University
 Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Supplemental Information. T Cells Enhance Autoimmunity by Restraining Regulatory T Cell Responses via an Interleukin-23-Dependent Mechanism
 Immunity, Volume 33 Supplemental Information T Cells Enhance Autoimmunity by Restraining Regulatory T Cell Responses via an Interleukin-23-Dependent Mechanism Franziska Petermann, Veit Rothhammer, Malte
Immunity, Volume 33 Supplemental Information T Cells Enhance Autoimmunity by Restraining Regulatory T Cell Responses via an Interleukin-23-Dependent Mechanism Franziska Petermann, Veit Rothhammer, Malte
Cytotoxicity assays. Rory D. de Vries, PhD 1. Viroscience lab, Erasmus MC, Rotterdam, the Netherlands
 Cytotoxicity assays Rory D. de Vries, PhD 1 1 Viroscience lab, Erasmus MC, Rotterdam, the Netherlands Anti-influenza immunity Humoral / CD4+ / CD8+ / NK? Function of CTL Elimination of virus-infected cells?
Cytotoxicity assays Rory D. de Vries, PhD 1 1 Viroscience lab, Erasmus MC, Rotterdam, the Netherlands Anti-influenza immunity Humoral / CD4+ / CD8+ / NK? Function of CTL Elimination of virus-infected cells?
NK cell flow cytometric assay In vivo DC viability and migration assay
 NK cell flow cytometric assay 6 NK cells were purified, by negative selection with the NK Cell Isolation Kit (Miltenyi iotec), from spleen and lymph nodes of 6 RAG1KO mice, injected the day before with
NK cell flow cytometric assay 6 NK cells were purified, by negative selection with the NK Cell Isolation Kit (Miltenyi iotec), from spleen and lymph nodes of 6 RAG1KO mice, injected the day before with
Cationic Liposome-DNA Complexes as Immunomodulators and Vaccine Adjuvants
 Cationic Liposome-DNA Complexes as Immunomodulators and Vaccine Adjuvants September 29, 28 Platform Technology Proprietary Cationic Lipid-DNA Complexes Cationic/Neutral Lipid + DNA = JVRS-1 JVRS-1 + Antigen
Cationic Liposome-DNA Complexes as Immunomodulators and Vaccine Adjuvants September 29, 28 Platform Technology Proprietary Cationic Lipid-DNA Complexes Cationic/Neutral Lipid + DNA = JVRS-1 JVRS-1 + Antigen
The Cell Cycle Time of CD8 + T Cells Responding In Vivo Is Controlled by the Type of Antigenic Stimulus
 The Cell Cycle Time of CD8 + T Cells Responding In Vivo Is Controlled by the Type of Antigenic Stimulus Heesik Yoon 1, Taeg S. Kim 1,3, Thomas J. Braciale 1,2,3 * 1 The Carter Immunology Center, University
The Cell Cycle Time of CD8 + T Cells Responding In Vivo Is Controlled by the Type of Antigenic Stimulus Heesik Yoon 1, Taeg S. Kim 1,3, Thomas J. Braciale 1,2,3 * 1 The Carter Immunology Center, University
Viral Persistence Alters CD8 T-Cell Immunodominance and Tissue Distribution and Results in Distinct Stages of Functional Impairment
 JOURNAL OF VIROLOGY, Apr. 2003, p. 4911 4927 Vol. 77, No. 8 0022-538X/03/$08.00 0 DOI: 10.1128/JVI.77.8.4911 4927.2003 Copyright 2003, American Society for Microbiology. All Rights Reserved. Viral Persistence
JOURNAL OF VIROLOGY, Apr. 2003, p. 4911 4927 Vol. 77, No. 8 0022-538X/03/$08.00 0 DOI: 10.1128/JVI.77.8.4911 4927.2003 Copyright 2003, American Society for Microbiology. All Rights Reserved. Viral Persistence
Direct ex vivo characterization of human antigen-specific CD154 + CD4 + T cells Rapid antigen-reactive T cell enrichment (Rapid ARTE)
 Direct ex vivo characterization of human antigen-specific CD154 + CD4 + T cells Rapid antigen-reactive T cell enrichment (Rapid ARTE) Introduction Workflow Antigen (ag)-specific T cells play a central
Direct ex vivo characterization of human antigen-specific CD154 + CD4 + T cells Rapid antigen-reactive T cell enrichment (Rapid ARTE) Introduction Workflow Antigen (ag)-specific T cells play a central
TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer
 AD Award Number: W8-XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke, Ph.D. CONTRACTING ORGANIZATION: Johns Hopkins
AD Award Number: W8-XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke, Ph.D. CONTRACTING ORGANIZATION: Johns Hopkins
RAISON D ETRE OF THE IMMUNE SYSTEM:
 RAISON D ETRE OF THE IMMUNE SYSTEM: To Distinguish Self from Non-Self Thereby Protecting Us From Our Hostile Environment. Innate Immunity Acquired Immunity Innate immunity: (Antigen nonspecific) defense
RAISON D ETRE OF THE IMMUNE SYSTEM: To Distinguish Self from Non-Self Thereby Protecting Us From Our Hostile Environment. Innate Immunity Acquired Immunity Innate immunity: (Antigen nonspecific) defense
Critical Role for Alpha/Beta and Gamma Interferons in Persistence of Lymphocytic Choriomeningitis Virus by Clonal Exhaustion of Cytotoxic T Cells
 JOURNAL OF VIROLOGY, Sept. 2001, p. 8407 8423 Vol. 75, No. 18 0022-538X/01/$04.00 0 DOI: 10.1128/JVI.75.18.8407 8423.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved. Critical
JOURNAL OF VIROLOGY, Sept. 2001, p. 8407 8423 Vol. 75, No. 18 0022-538X/01/$04.00 0 DOI: 10.1128/JVI.75.18.8407 8423.2001 Copyright 2001, American Society for Microbiology. All Rights Reserved. Critical
Interferon γ regulates idiopathic pneumonia syndrome, a. Th17 + CD4 + T-cell-mediated GvH disease
 Interferon γ regulates idiopathic pneumonia syndrome, a Th17 + CD4 + T-cell-mediated GvH disease Nora Mauermann, Julia Burian, Christophe von Garnier, Stefan Dirnhofer, Davide Germano, Christine Schuett,
Interferon γ regulates idiopathic pneumonia syndrome, a Th17 + CD4 + T-cell-mediated GvH disease Nora Mauermann, Julia Burian, Christophe von Garnier, Stefan Dirnhofer, Davide Germano, Christine Schuett,
TCR, MHC and coreceptors
 Cooperation In Immune Responses Antigen processing how peptides get into MHC Antigen processing involves the intracellular proteolytic generation of MHC binding proteins Protein antigens may be processed
Cooperation In Immune Responses Antigen processing how peptides get into MHC Antigen processing involves the intracellular proteolytic generation of MHC binding proteins Protein antigens may be processed
TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer
 AD Award Number: W8-XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke,. CONTRACTING ORGANIZATION: Johns Hopkins
AD Award Number: W8-XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke,. CONTRACTING ORGANIZATION: Johns Hopkins
CD4 + T Cell Priming Accelerates the. T Cell Memory. Weimin Zhong, Dana Marshall, Christopher Coleclough and David L. Woodland
 This information is current as of April 7, 2018. CD4 + T Cell Priming Accelerates the Clearance of Sendai Virus Negative Effect on CD8 + in Mice, but Has a T Cell Memory Weimin Zhong, Dana Marshall, Christopher
This information is current as of April 7, 2018. CD4 + T Cell Priming Accelerates the Clearance of Sendai Virus Negative Effect on CD8 + in Mice, but Has a T Cell Memory Weimin Zhong, Dana Marshall, Christopher
The Adaptive Immune Responses
 The Adaptive Immune Responses The two arms of the immune responses are; 1) the cell mediated, and 2) the humoral responses. In this chapter we will discuss the two responses in detail and we will start
The Adaptive Immune Responses The two arms of the immune responses are; 1) the cell mediated, and 2) the humoral responses. In this chapter we will discuss the two responses in detail and we will start
Supplementary Figures
 Supplementary Figures Supplementary Fig. 1. Surface thiol groups and reduction of activated T cells. (a) Activated CD8 + T-cells have high expression levels of free thiol groups on cell surface proteins.
Supplementary Figures Supplementary Fig. 1. Surface thiol groups and reduction of activated T cells. (a) Activated CD8 + T-cells have high expression levels of free thiol groups on cell surface proteins.
Supplementary Figure 1. Enhanced detection of CTLA-4 on the surface of HIV-specific
 SUPPLEMENTARY FIGURE LEGEND Supplementary Figure 1. Enhanced detection of CTLA-4 on the surface of HIV-specific CD4 + T cells correlates with intracellular CTLA-4 levels. (a) Comparative CTLA-4 levels
SUPPLEMENTARY FIGURE LEGEND Supplementary Figure 1. Enhanced detection of CTLA-4 on the surface of HIV-specific CD4 + T cells correlates with intracellular CTLA-4 levels. (a) Comparative CTLA-4 levels
MATERIALS AND METHODS. Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All
 MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
Immunodomination during Peripheral Vaccinia Virus Infection
 Immunodomination during Peripheral Vaccinia Virus Infection Leon C. W. Lin, Inge E. A. Flesch, David C. Tscharke* Division of Biomedical Science and Biochemistry, Research School of Biology, The Australian
Immunodomination during Peripheral Vaccinia Virus Infection Leon C. W. Lin, Inge E. A. Flesch, David C. Tscharke* Division of Biomedical Science and Biochemistry, Research School of Biology, The Australian
Antigen Presentation and T Lymphocyte Activation. Abul K. Abbas UCSF. FOCiS
 1 Antigen Presentation and T Lymphocyte Activation Abul K. Abbas UCSF FOCiS 2 Lecture outline Dendritic cells and antigen presentation The role of the MHC T cell activation Costimulation, the B7:CD28 family
1 Antigen Presentation and T Lymphocyte Activation Abul K. Abbas UCSF FOCiS 2 Lecture outline Dendritic cells and antigen presentation The role of the MHC T cell activation Costimulation, the B7:CD28 family
Rapid antigen-specific T cell enrichment (Rapid ARTE)
 Direct ex vivo characterization of human antigen-specific CD154+CD4+ T cell Rapid antigen-specific T cell enrichment (Rapid ARTE) Introduction Workflow Antigen (ag)-specific T cells play a central role
Direct ex vivo characterization of human antigen-specific CD154+CD4+ T cell Rapid antigen-specific T cell enrichment (Rapid ARTE) Introduction Workflow Antigen (ag)-specific T cells play a central role
NKTR-255: Accessing The Immunotherapeutic Potential Of IL-15 for NK Cell Therapies
 NKTR-255: Accessing The Immunotherapeutic Potential Of IL-15 for NK Cell Therapies Saul Kivimäe Senior Scientist, Research Biology Nektar Therapeutics NK Cell-Based Cancer Immunotherapy, September 26-27,
NKTR-255: Accessing The Immunotherapeutic Potential Of IL-15 for NK Cell Therapies Saul Kivimäe Senior Scientist, Research Biology Nektar Therapeutics NK Cell-Based Cancer Immunotherapy, September 26-27,
The Adaptive Immune Response. B-cells
 The Adaptive Immune Response B-cells The innate immune system provides immediate protection. The adaptive response takes time to develop and is antigen specific. Activation of B and T lymphocytes Naive
The Adaptive Immune Response B-cells The innate immune system provides immediate protection. The adaptive response takes time to develop and is antigen specific. Activation of B and T lymphocytes Naive
Scott Abrams, Ph.D. Professor of Oncology, x4375 Kuby Immunology SEVENTH EDITION
 Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 13 Effector Responses: Cell- and Antibody-Mediated Immunity Copyright 2013 by W. H.
Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 13 Effector Responses: Cell- and Antibody-Mediated Immunity Copyright 2013 by W. H.
Loss of function in virus-specific lung effector T cells is independent of infection
 Loss of function in virus-specific lung effector T cells is independent of infection Subhashini Arimilli, Ellen M. Palmer, and Martha A. Alexander-Miller 1 Department of Microbiology and Immunology, Wake
Loss of function in virus-specific lung effector T cells is independent of infection Subhashini Arimilli, Ellen M. Palmer, and Martha A. Alexander-Miller 1 Department of Microbiology and Immunology, Wake
Lineage relationship and protective immunity of memory CD8 T cell subsets
 23 Nature Publishing Group http://www.nature.com/natureimmunology Lineage relationship and protective immunity of memory CD8 T cell subsets E. John Wherry 1 *,Volker Teichgräber 1 *,Todd C. Becker 1,David
23 Nature Publishing Group http://www.nature.com/natureimmunology Lineage relationship and protective immunity of memory CD8 T cell subsets E. John Wherry 1 *,Volker Teichgräber 1 *,Todd C. Becker 1,David
Adaptive Immunity: Humoral Immune Responses
 MICR2209 Adaptive Immunity: Humoral Immune Responses Dr Allison Imrie 1 Synopsis: In this lecture we will review the different mechanisms which constitute the humoral immune response, and examine the antibody
MICR2209 Adaptive Immunity: Humoral Immune Responses Dr Allison Imrie 1 Synopsis: In this lecture we will review the different mechanisms which constitute the humoral immune response, and examine the antibody
General Overview of Immunology. Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center
 General Overview of Immunology Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center Objectives Describe differences between innate and adaptive immune responses
General Overview of Immunology Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center Objectives Describe differences between innate and adaptive immune responses
NKTR-255: Accessing IL-15 Therapeutic Potential through Robust and Sustained Engagement of Innate and Adaptive Immunity
 NKTR-255: Accessing IL-15 Therapeutic Potential through Robust and Sustained Engagement of Innate and Adaptive Immunity Peiwen Kuo Scientist, Research Biology Nektar Therapeutics August 31 st, 2018 Emerging
NKTR-255: Accessing IL-15 Therapeutic Potential through Robust and Sustained Engagement of Innate and Adaptive Immunity Peiwen Kuo Scientist, Research Biology Nektar Therapeutics August 31 st, 2018 Emerging
Immunology - Lecture 2 Adaptive Immune System 1
 Immunology - Lecture 2 Adaptive Immune System 1 Book chapters: Molecules of the Adaptive Immunity 6 Adaptive Cells and Organs 7 Generation of Immune Diversity Lymphocyte Antigen Receptors - 8 CD markers
Immunology - Lecture 2 Adaptive Immune System 1 Book chapters: Molecules of the Adaptive Immunity 6 Adaptive Cells and Organs 7 Generation of Immune Diversity Lymphocyte Antigen Receptors - 8 CD markers
TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer
 AD Award Number: W8XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke Ph.D. CONTRACTING ORGANIZATION: Johns Hopkins
AD Award Number: W8XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke Ph.D. CONTRACTING ORGANIZATION: Johns Hopkins
Cell Responses in Lymph Nodes
 This information is current as of April 18, 2013. Supplementary Material Subscriptions Permissions Email Alerts Inoculation Dose of Mycobacterium tuberculosis Does Not Influence Priming of T Cell Responses
This information is current as of April 18, 2013. Supplementary Material Subscriptions Permissions Email Alerts Inoculation Dose of Mycobacterium tuberculosis Does Not Influence Priming of T Cell Responses
Supplementary Figure 1.
 Supplementary Figure 1. Female Pro-ins2 -/- mice at 5-6 weeks of age were either inoculated i.p. with a single dose of CVB4 (1x10 5 PFU/mouse) or PBS and treated with αgalcer or control vehicle. On day
Supplementary Figure 1. Female Pro-ins2 -/- mice at 5-6 weeks of age were either inoculated i.p. with a single dose of CVB4 (1x10 5 PFU/mouse) or PBS and treated with αgalcer or control vehicle. On day
Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells
 ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
Supplementary Figure 1. Characterization of basophils after reconstitution of SCID mice
 Supplementary figure legends Supplementary Figure 1. Characterization of after reconstitution of SCID mice with CD4 + CD62L + T cells. (A-C) SCID mice (n = 6 / group) were reconstituted with 2 x 1 6 CD4
Supplementary figure legends Supplementary Figure 1. Characterization of after reconstitution of SCID mice with CD4 + CD62L + T cells. (A-C) SCID mice (n = 6 / group) were reconstituted with 2 x 1 6 CD4
Principles of Adaptive Immunity
 Principles of Adaptive Immunity Chapter 3 Parham Hans de Haard 17 th of May 2010 Agenda Recognition molecules of adaptive immune system Features adaptive immune system Immunoglobulins and T-cell receptors
Principles of Adaptive Immunity Chapter 3 Parham Hans de Haard 17 th of May 2010 Agenda Recognition molecules of adaptive immune system Features adaptive immune system Immunoglobulins and T-cell receptors
Determinants of Immunogenicity and Tolerance. Abul K. Abbas, MD Department of Pathology University of California San Francisco
 Determinants of Immunogenicity and Tolerance Abul K. Abbas, MD Department of Pathology University of California San Francisco EIP Symposium Feb 2016 Why do some people respond to therapeutic proteins?
Determinants of Immunogenicity and Tolerance Abul K. Abbas, MD Department of Pathology University of California San Francisco EIP Symposium Feb 2016 Why do some people respond to therapeutic proteins?
Immune response. This overview figure summarizes simply how our body responds to foreign molecules that enter to it.
 Immune response This overview figure summarizes simply how our body responds to foreign molecules that enter to it. It s highly recommended to watch Dr Najeeb s lecture that s titled T Helper cells and
Immune response This overview figure summarizes simply how our body responds to foreign molecules that enter to it. It s highly recommended to watch Dr Najeeb s lecture that s titled T Helper cells and
General information. Cell mediated immunity. 455 LSA, Tuesday 11 to noon. Anytime after class.
 General information Cell mediated immunity 455 LSA, Tuesday 11 to noon Anytime after class T-cell precursors Thymus Naive T-cells (CD8 or CD4) email: lcoscoy@berkeley.edu edu Use MCB150 as subject line
General information Cell mediated immunity 455 LSA, Tuesday 11 to noon Anytime after class T-cell precursors Thymus Naive T-cells (CD8 or CD4) email: lcoscoy@berkeley.edu edu Use MCB150 as subject line
(From the Department of Epidemiology and Virus Laboratory, School of Pubbic Health, University of Michigan, Ann Arbor) Methods
 Published Online: 1 November, 1948 Supp Info: http://doi.org/1.184/jem.88.5.515 Downloaded from jem.rupress.org on May 3, 218 THE RELATION OF INFECTIOUS AND HEMAGGLUTINATION TITERS TO THE ADAPTATION OF
Published Online: 1 November, 1948 Supp Info: http://doi.org/1.184/jem.88.5.515 Downloaded from jem.rupress.org on May 3, 218 THE RELATION OF INFECTIOUS AND HEMAGGLUTINATION TITERS TO THE ADAPTATION OF
Adaptive (acquired) immunity. Professor Peter Delves University College London
 Adaptive (acquired) immunity Professor Peter Delves University College London p.delves@ucl.ac.uk Haematopoiesis Haematopoiesis Lymphocytes = adaptive response Recognition of pathogens by adaptive cells,
Adaptive (acquired) immunity Professor Peter Delves University College London p.delves@ucl.ac.uk Haematopoiesis Haematopoiesis Lymphocytes = adaptive response Recognition of pathogens by adaptive cells,
There are 2 major lines of defense: Non-specific (Innate Immunity) and. Specific. (Adaptive Immunity) Photo of macrophage cell
 There are 2 major lines of defense: Non-specific (Innate Immunity) and Specific (Adaptive Immunity) Photo of macrophage cell Development of the Immune System ery pl neu mφ nk CD8 + CTL CD4 + thy TH1 mye
There are 2 major lines of defense: Non-specific (Innate Immunity) and Specific (Adaptive Immunity) Photo of macrophage cell Development of the Immune System ery pl neu mφ nk CD8 + CTL CD4 + thy TH1 mye
Recombinant Vaccinia Virus-Induced T-Cell Immunity: Quantitation of the Response to the Virus Vector and the Foreign Epitope
 JOURNAL OF VIROLOGY, Apr. 2002, p. 3329 3337 Vol. 76, No. 7 0022-538X/02/$04.00 0 DOI: 10.1128/JVI.76.7.3329 3337.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved. Recombinant
JOURNAL OF VIROLOGY, Apr. 2002, p. 3329 3337 Vol. 76, No. 7 0022-538X/02/$04.00 0 DOI: 10.1128/JVI.76.7.3329 3337.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved. Recombinant
I. Lines of Defense Pathogen: Table 1: Types of Immune Mechanisms. Table 2: Innate Immunity: First Lines of Defense
 I. Lines of Defense Pathogen: Table 1: Types of Immune Mechanisms Table 2: Innate Immunity: First Lines of Defense Innate Immunity involves nonspecific physical & chemical barriers that are adapted for
I. Lines of Defense Pathogen: Table 1: Types of Immune Mechanisms Table 2: Innate Immunity: First Lines of Defense Innate Immunity involves nonspecific physical & chemical barriers that are adapted for
Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell?
 Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
ADCC Assay Protocol Vikram Srivastava 1, Zheng Yang 1, Ivan Fan Ngai Hung 2, Jianqing Xu 3, Bojian Zheng 3 and Mei- Yun Zhang 3*
 ADCC Assay Protocol Vikram Srivastava 1, Zheng Yang 1, Ivan Fan Ngai Hung 2, Jianqing Xu 3, Bojian Zheng 3 and Mei- Yun Zhang 3* 1 Department of Microbiology, Li Ka Shing Faculty of Medicine, University
ADCC Assay Protocol Vikram Srivastava 1, Zheng Yang 1, Ivan Fan Ngai Hung 2, Jianqing Xu 3, Bojian Zheng 3 and Mei- Yun Zhang 3* 1 Department of Microbiology, Li Ka Shing Faculty of Medicine, University
Control of Virus-Specific CD8 T-Cell Exhaustion and Immune-Mediated Pathology by E3 Ubiquitin Ligase Cbl-b during Chronic Viral Infection
 JOURNAL OF VIROLOGY, Apr. 2008, p. 3353 3368 Vol. 82, No. 7 0022-538X/08/$08.00 0 doi:10.1128/jvi.01350-07 Copyright 2008, American Society for Microbiology. All Rights Reserved. Control of Virus-Specific
JOURNAL OF VIROLOGY, Apr. 2008, p. 3353 3368 Vol. 82, No. 7 0022-538X/08/$08.00 0 doi:10.1128/jvi.01350-07 Copyright 2008, American Society for Microbiology. All Rights Reserved. Control of Virus-Specific
Tracking total polyclonal CD8 T cell responses in inbred and outbred hosts after infection
 University of Iowa Iowa Research Online Theses and Dissertations 2010 Tracking total polyclonal CD8 T cell responses in inbred and outbred hosts after infection Deepa Kumari Rai University of Iowa Copyright
University of Iowa Iowa Research Online Theses and Dissertations 2010 Tracking total polyclonal CD8 T cell responses in inbred and outbred hosts after infection Deepa Kumari Rai University of Iowa Copyright
Brief Definitive Report
 Brief Definitive Report HEMAGGLUTININ-SPECIFIC CYTOTOXIC T-CELL RESPONSE DURING INFLUENZA INFECTION BY FRANCIS A. ENNIS, W. JOHN MARTIN, ANY MARTHA W. VERBONITZ (From the Department of Health, Education
Brief Definitive Report HEMAGGLUTININ-SPECIFIC CYTOTOXIC T-CELL RESPONSE DURING INFLUENZA INFECTION BY FRANCIS A. ENNIS, W. JOHN MARTIN, ANY MARTHA W. VERBONITZ (From the Department of Health, Education
Third line of Defense
 Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
Role for CD40 and CD40L Expression in Generating CD8 T Cell Response to Minor Histcompatibility Antigen, H60
 Role for CD40 and CD40L Expression in Generating CD8 T Cell Response to Minor Histcompatibility Antigen, H60 Kyoung Min Jung and Eun Young Choi Graduate Program of Immunology, Seoul National University
Role for CD40 and CD40L Expression in Generating CD8 T Cell Response to Minor Histcompatibility Antigen, H60 Kyoung Min Jung and Eun Young Choi Graduate Program of Immunology, Seoul National University
Therapeutic PD L1 and LAG 3 blockade rapidly clears established blood stage Plasmodium infection
 Supplementary Information Therapeutic PD L1 and LAG 3 blockade rapidly clears established blood stage Plasmodium infection Noah S. Butler, Jacqueline Moebius, Lecia L. Pewe, Boubacar Traore, Ogobara K.
Supplementary Information Therapeutic PD L1 and LAG 3 blockade rapidly clears established blood stage Plasmodium infection Noah S. Butler, Jacqueline Moebius, Lecia L. Pewe, Boubacar Traore, Ogobara K.
Supplemental Methods. CD107a assay
 Supplemental Methods CD107a assay For each T cell culture that was tested, two tubes were prepared. One tube contained BCMA-K562 cells, and the other tube contained NGFR-K562 cells. Both tubes contained
Supplemental Methods CD107a assay For each T cell culture that was tested, two tubes were prepared. One tube contained BCMA-K562 cells, and the other tube contained NGFR-K562 cells. Both tubes contained
chapter 17: specific/adaptable defenses of the host: the immune response
 chapter 17: specific/adaptable defenses of the host: the immune response defense against infection & illness body defenses innate/ non-specific adaptable/ specific epithelium, fever, inflammation, complement,
chapter 17: specific/adaptable defenses of the host: the immune response defense against infection & illness body defenses innate/ non-specific adaptable/ specific epithelium, fever, inflammation, complement,
The Adaptive Immune Response: T lymphocytes and Their Functional Types *
 OpenStax-CNX module: m46560 1 The Adaptive Immune Response: T lymphocytes and Their Functional Types * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
OpenStax-CNX module: m46560 1 The Adaptive Immune Response: T lymphocytes and Their Functional Types * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution
Supplementary Data 1. Alanine substitutions and position variants of APNCYGNIPL. Applied in
 Supplementary Data 1. Alanine substitutions and position variants of APNCYGNIPL. Applied in Supplementary Fig. 2 Substitution Sequence Position variant Sequence original APNCYGNIPL original APNCYGNIPL
Supplementary Data 1. Alanine substitutions and position variants of APNCYGNIPL. Applied in Supplementary Fig. 2 Substitution Sequence Position variant Sequence original APNCYGNIPL original APNCYGNIPL
Heat-killed Lactobacillus casei
 Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection Yu-Jin Jung, Young-Tae Lee,
Heat-killed Lactobacillus casei confers broad protection against influenza A virus primary infection and develops heterosubtypic immunity against future secondary infection Yu-Jin Jung, Young-Tae Lee,
Chapter 22: The Lymphatic System and Immunity
 Bio40C schedule Lecture Immune system Lab Quiz 2 this week; bring a scantron! Study guide on my website (see lab assignments) Extra credit Critical thinking questions at end of chapters 5 pts/chapter Due
Bio40C schedule Lecture Immune system Lab Quiz 2 this week; bring a scantron! Study guide on my website (see lab assignments) Extra credit Critical thinking questions at end of chapters 5 pts/chapter Due
Optimizing Intracellular Flow Cytometry:
 Optimizing Intracellular Flow Cytometry: Simultaneous Detection of Cytokines and Transcription Factors Presented by Jurg Rohrer, PhD, BD Biosciences 23-10780-00 Outline Introduction Cytokines Transcription
Optimizing Intracellular Flow Cytometry: Simultaneous Detection of Cytokines and Transcription Factors Presented by Jurg Rohrer, PhD, BD Biosciences 23-10780-00 Outline Introduction Cytokines Transcription
Lecture 11. Immunology and disease: parasite antigenic diversity
 Lecture 11 Immunology and disease: parasite antigenic diversity RNAi interference video and tutorial (you are responsible for this material, so check it out.) http://www.pbs.org/wgbh/nova/sciencenow/3210/02.html
Lecture 11 Immunology and disease: parasite antigenic diversity RNAi interference video and tutorial (you are responsible for this material, so check it out.) http://www.pbs.org/wgbh/nova/sciencenow/3210/02.html
Naive and memory CD8 T cell responses after antigen stimulation in vivo
 University of Iowa Iowa Research Online Theses and Dissertations Summer 2011 Naive and memory CD8 T cell responses after antigen stimulation in vivo Matthew David Martin University of Iowa Copyright 2011
University of Iowa Iowa Research Online Theses and Dissertations Summer 2011 Naive and memory CD8 T cell responses after antigen stimulation in vivo Matthew David Martin University of Iowa Copyright 2011
IOM Immunization Safety Review 11/12/2001. Immunological Competition and the Infant Immune Response to Vaccines
 IOM Immunization Safety Review 11/12/2001 Immunological Competition and the Infant Immune Response to Vaccines Richard Insel University of Rochester Goals Neonatal and Infant Immune System Broad Effects
IOM Immunization Safety Review 11/12/2001 Immunological Competition and the Infant Immune Response to Vaccines Richard Insel University of Rochester Goals Neonatal and Infant Immune System Broad Effects
Adaptive immune responses: T cell-mediated immunity
 MICR2209 Adaptive immune responses: T cell-mediated immunity Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will discuss the T-cell mediated immune response, how it is activated,
MICR2209 Adaptive immune responses: T cell-mediated immunity Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will discuss the T-cell mediated immune response, how it is activated,
ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY
 ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY The recognition of specific antigen by naïve T cell induces its own activation and effector phases. T helper cells recognize peptide antigens through
ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY The recognition of specific antigen by naïve T cell induces its own activation and effector phases. T helper cells recognize peptide antigens through
Optimizing Intracellular Flow Cytometry:
 Optimizing Intracellular Flow Cytometry: Simultaneous Detection of Cytokines and Transcription Factors An encore presentation by Jurg Rohrer, PhD, BD Biosciences 10.26.10 Outline Introduction Cytokines
Optimizing Intracellular Flow Cytometry: Simultaneous Detection of Cytokines and Transcription Factors An encore presentation by Jurg Rohrer, PhD, BD Biosciences 10.26.10 Outline Introduction Cytokines
The Immune System: Innate and Adaptive Body Defenses Outline PART 1: INNATE DEFENSES 21.1 Surface barriers act as the first line of defense to keep
 The Immune System: Innate and Adaptive Body Defenses Outline PART 1: INNATE DEFENSES 21.1 Surface barriers act as the first line of defense to keep invaders out of the body (pp. 772 773; Fig. 21.1; Table
The Immune System: Innate and Adaptive Body Defenses Outline PART 1: INNATE DEFENSES 21.1 Surface barriers act as the first line of defense to keep invaders out of the body (pp. 772 773; Fig. 21.1; Table
staining and flow cytometry
 Detection of influenza virus-specific T cell responses by intracellular by cytokine intracellular staining cytokine staining and flow cytometry Detection of influenza virus-specific T cell responses and
Detection of influenza virus-specific T cell responses by intracellular by cytokine intracellular staining cytokine staining and flow cytometry Detection of influenza virus-specific T cell responses and
CONTRACTING ORGANIZATION: Johns Hopkins University School of Medicine Baltimore, MD 21205
 AD Award Number: DAMD7---7 TITLE: Development of Artificial Antigen Presenting Cells for Prostate Cancer Immunotherapy PRINCIPAL INVESTIGATOR: Jonathan P. Schneck, M.D., Ph.D. Mathias Oelke, Ph.D. CONTRACTING
AD Award Number: DAMD7---7 TITLE: Development of Artificial Antigen Presenting Cells for Prostate Cancer Immunotherapy PRINCIPAL INVESTIGATOR: Jonathan P. Schneck, M.D., Ph.D. Mathias Oelke, Ph.D. CONTRACTING
Memory CD4 T Cells Enhance Primary CD8 T-Cell Responses
 INFECTION AND IMMUNITY, July 2007, p. 3556 3560 Vol. 75, No. 7 0019-9567/07/$08.00 0 doi:10.1128/iai.00086-07 Copyright 2007, American Society for Microbiology. All Rights Reserved. Memory CD4 T Cells
INFECTION AND IMMUNITY, July 2007, p. 3556 3560 Vol. 75, No. 7 0019-9567/07/$08.00 0 doi:10.1128/iai.00086-07 Copyright 2007, American Society for Microbiology. All Rights Reserved. Memory CD4 T Cells
Comparison of Immunoprotection of Simultaneous to Individual Vaccinations in a Murine Model
 MQP-BIO-DSA-8444 Comparison of Immunoprotection of Simultaneous to Individual Vaccinations in a Murine Model A Major Qualifying Project Report Submitted to the Faculty of the WORCESTER POLYTECHNIC INSTITUTE
MQP-BIO-DSA-8444 Comparison of Immunoprotection of Simultaneous to Individual Vaccinations in a Murine Model A Major Qualifying Project Report Submitted to the Faculty of the WORCESTER POLYTECHNIC INSTITUTE
Ex vivo Human Antigen-specific T Cell Proliferation and Degranulation Willemijn Hobo 1, Wieger Norde 1 and Harry Dolstra 2*
 Ex vivo Human Antigen-specific T Cell Proliferation and Degranulation Willemijn Hobo 1, Wieger Norde 1 and Harry Dolstra 2* 1 Department of Laboratory Medicine - Laboratory of Hematology, Radboud University
Ex vivo Human Antigen-specific T Cell Proliferation and Degranulation Willemijn Hobo 1, Wieger Norde 1 and Harry Dolstra 2* 1 Department of Laboratory Medicine - Laboratory of Hematology, Radboud University
Out-of-sequence signal 3 as a mechanism for virusinduced immune suppression of CD8 T cell responses
 University of Massachusetts Medical School escholarship@umms Open Access Articles Open Access Publications by UMMS Authors 9-25-2014 Out-of-sequence signal 3 as a mechanism for virusinduced immune suppression
University of Massachusetts Medical School escholarship@umms Open Access Articles Open Access Publications by UMMS Authors 9-25-2014 Out-of-sequence signal 3 as a mechanism for virusinduced immune suppression
RAISON D ETRE OF THE IMMUNE SYSTEM:
 RAISON D ETRE OF THE IMMUNE SYSTEM: To Distinguish Self from Non-Self Thereby Protecting Us From Our Hostile Environment. Innate Immunity Adaptive Immunity Innate immunity: (Antigen - nonspecific) defense
RAISON D ETRE OF THE IMMUNE SYSTEM: To Distinguish Self from Non-Self Thereby Protecting Us From Our Hostile Environment. Innate Immunity Adaptive Immunity Innate immunity: (Antigen - nonspecific) defense
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/23854 holds various files of this Leiden University dissertation. Author: Marel, Sander van der Title: Gene and cell therapy based treatment strategies
Cover Page The handle http://hdl.handle.net/1887/23854 holds various files of this Leiden University dissertation. Author: Marel, Sander van der Title: Gene and cell therapy based treatment strategies
Resolution of a chronic viral infection after interleukin-10 receptor blockade
 ARTICLE Resolution of a chronic viral infection after interleukin-10 receptor blockade Mette Ejrnaes, 1 Christophe M. Filippi, 1 Marianne M. Martinic, 1 Eleanor M. Ling, 1 Lisa M. Togher, 1 Shane Crotty,
ARTICLE Resolution of a chronic viral infection after interleukin-10 receptor blockade Mette Ejrnaes, 1 Christophe M. Filippi, 1 Marianne M. Martinic, 1 Eleanor M. Ling, 1 Lisa M. Togher, 1 Shane Crotty,
Chapter 10 (pages ): Differentiation and Functions of CD4+ Effector T Cells Prepared by Kristen Dazy, MD, Scripps Clinic Medical Group
 FIT Board Review Corner September 2015 Welcome to the FIT Board Review Corner, prepared by Andrew Nickels, MD, and Sarah Spriet, DO, senior and junior representatives of ACAAI's Fellows-In-Training (FITs)
FIT Board Review Corner September 2015 Welcome to the FIT Board Review Corner, prepared by Andrew Nickels, MD, and Sarah Spriet, DO, senior and junior representatives of ACAAI's Fellows-In-Training (FITs)
Dynamics of Memory T Cell Proliferation Under Conditions of Heterologous Immunity and Bystander Stimulation
 This information is current as of September 16, 2018. Dynamics of Memory T Cell Proliferation Under Conditions of Heterologous Immunity and Bystander Stimulation Sung-Kwon Kim, Michael A. Brehm, Raymond
This information is current as of September 16, 2018. Dynamics of Memory T Cell Proliferation Under Conditions of Heterologous Immunity and Bystander Stimulation Sung-Kwon Kim, Michael A. Brehm, Raymond
Zheng, BJ; Du, LY; Zhao, GY; Lin, YP; Sui, HY; Chan, C; Ma, S; Guan, Y; Yuen, KY. Citation Hong Kong Medical Journal, 2008, v. 14 suppl. 4, p.
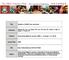 Title Studies of SARS virus vaccines Author(s) Zheng, BJ; Du, LY; Zhao, GY; Lin, YP; Sui, HY; Chan, C; Ma, S; Guan, Y; Yuen, KY Citation Hong Kong Medical Journal, 2008, v. 14 suppl. 4, p. 39-43 Issued
Title Studies of SARS virus vaccines Author(s) Zheng, BJ; Du, LY; Zhao, GY; Lin, YP; Sui, HY; Chan, C; Ma, S; Guan, Y; Yuen, KY Citation Hong Kong Medical Journal, 2008, v. 14 suppl. 4, p. 39-43 Issued
Viral acute lower respiratory infections impair CD8 + T cells through PD-1
 Research article Viral acute lower respiratory infections impair CD8 + T cells through PD-1 John J. Erickson, 1 Pavlo Gilchuk, 1 Andrew K. Hastings, 1 Sharon J. Tollefson, 2 Monika Johnson, 1 Melissa B.
Research article Viral acute lower respiratory infections impair CD8 + T cells through PD-1 John J. Erickson, 1 Pavlo Gilchuk, 1 Andrew K. Hastings, 1 Sharon J. Tollefson, 2 Monika Johnson, 1 Melissa B.
Supplementary Fig. 1 p38 MAPK negatively regulates DC differentiation. (a) Western blot analysis of p38 isoform expression in BM cells, immature DCs
 Supplementary Fig. 1 p38 MAPK negatively regulates DC differentiation. (a) Western blot analysis of p38 isoform expression in BM cells, immature DCs (idcs) and mature DCs (mdcs). A myeloma cell line expressing
Supplementary Fig. 1 p38 MAPK negatively regulates DC differentiation. (a) Western blot analysis of p38 isoform expression in BM cells, immature DCs (idcs) and mature DCs (mdcs). A myeloma cell line expressing
Excerpt from MACS&more Vol 17 1/2016. Kenji Miki¹, Koji Nagaoka¹, Hermann Bohnenkamp², Takayuki Yoshimoto³, Ryuji Maekawa¹*, and Takashi Kamigaki⁴
 Excerpt from MCS&more Vol 17 1/2016 Dendritic cells pulsed with induce both OV-specific CD4 + and CD8 + T cells and cause antitumor effects in a mouse model of lymphoma Kenji Miki¹, Koji Nagaoka¹, Hermann
Excerpt from MCS&more Vol 17 1/2016 Dendritic cells pulsed with induce both OV-specific CD4 + and CD8 + T cells and cause antitumor effects in a mouse model of lymphoma Kenji Miki¹, Koji Nagaoka¹, Hermann
T Cell Activation, Costimulation and Regulation
 1 T Cell Activation, Costimulation and Regulation Abul K. Abbas, MD University of California San Francisco 2 Lecture outline T cell antigen recognition and activation Costimulation, the B7:CD28 family
1 T Cell Activation, Costimulation and Regulation Abul K. Abbas, MD University of California San Francisco 2 Lecture outline T cell antigen recognition and activation Costimulation, the B7:CD28 family
Bioassays for Quality Control of Cell & Gene Therapy Products
 Bioassays for Quality Control of Cell & Gene Therapy Products Erik Rutjens, Cell & Gene Therapy, Novartis Pharma AG CASSS Bioassays, Silver Spring, March2015 CTL019 Introduction CARTs = Chimeric Antigen
Bioassays for Quality Control of Cell & Gene Therapy Products Erik Rutjens, Cell & Gene Therapy, Novartis Pharma AG CASSS Bioassays, Silver Spring, March2015 CTL019 Introduction CARTs = Chimeric Antigen
C. Incorrect! MHC class I molecules are not involved in the process of bridging in ADCC.
 Immunology - Problem Drill 13: T- Cell Mediated Immunity Question No. 1 of 10 1. During Antibody-dependent cell mediated cytotoxicity (ADCC), the antibody acts like a bridge between the specific antigen
Immunology - Problem Drill 13: T- Cell Mediated Immunity Question No. 1 of 10 1. During Antibody-dependent cell mediated cytotoxicity (ADCC), the antibody acts like a bridge between the specific antigen
Lecture 6. Burr BIO 4353/6345 HIV/AIDS. Tetramer staining of T cells (CTL s) Andrew McMichael seminar: Background
 Lecture 6 Burr BIO 4353/6345 HIV/AIDS Andrew McMichael seminar: Background Tetramer staining of T cells (CTL s) 1. Vβ 19: There are 52 T cell receptor (TCR) Vβ gene segments in germ line DNA (See following
Lecture 6 Burr BIO 4353/6345 HIV/AIDS Andrew McMichael seminar: Background Tetramer staining of T cells (CTL s) 1. Vβ 19: There are 52 T cell receptor (TCR) Vβ gene segments in germ line DNA (See following
