Notice of Decision Not to Designate Pneumocystis Pneumonia as a Tropical Disease
|
|
|
- Miles Price
- 5 years ago
- Views:
Transcription
1 This document is scheduled to be published in the Federal Register on 08/24/2018 and available online at and on govinfo.gov P DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration [Docket No. FDA-2008-N-0567] Notice of Decision Not to Designate Pneumocystis Pneumonia as a Tropical Disease AGENCY: Food and Drug Administration, HHS. ACTION: Notice. SUMMARY: The Food and Drug Administration (FDA or Agency), in response to suggestions submitted to Docket No. FDA-2008-N-0567, has analyzed whether Pneumocystis pneumonia (PCP) meets the statutory criteria for designation as a tropical disease for the purposes of obtaining a priority review voucher (PRV) under the Federal Food, Drug, and Cosmetic Act (FD&C Act), namely whether it primarily affects poor and marginalized populations and whether there is no significant market for drugs that prevent or treat PCP in developed countries. The Agency has determined that PCP does not meet the statutory criteria for designation as a tropical disease and declines to designate it as such. DATES: [INSERT DATE OF PUBLICATION IN THE FEDERAL REGISTER]. ADDRESSES: Submit electronic comments on additional diseases suggested for designation to Submit written comments on additional diseases suggested for designation to the Dockets Management Staff (HFA-305), Food and Drug Administration, 5630 Fishers Lane, Rm. 1061, Rockville, MD All comments should be identified with the docket number found in brackets in the heading of this document. FOR FURTHER INFORMATION CONTACT: Katherine Schumann, Center for Drug Evaluation and Research, Food and Drug Administration, New Hampshire Ave., Bldg. 22,
2 2 Rm. 6242, Silver Spring, MD , , or Office of Communication, Outreach and Development (OCOD), Center for Biologics Evaluation and Research, Food and Drug Administration, New Hampshire Ave., Silver Spring, MD , or , SUPPLEMENTARY INFORMATION: Table of Contents I. Background: Priority Review Voucher Program II. Decision Not to Designate Pneumocystis Pneumonia A. Significant Market in Developed Nations B. Disproportionately Affects Poor and Marginalized Populations C. FDA Determination III. Process for Requesting Additional Diseases to be Added to the List IV. Paperwork Reduction Act V. References I. Background: Priority Review Voucher Program Section 524 of the FD&C Act (21 U.S.C. 360n), which was added by section 1102 of the Food and Drug Administration Amendments Act of 2007 (FDAAA), uses a PRV incentive to encourage the development of new drugs, including biologics, for prevention and treatment of certain diseases that, in the aggregate, affect millions of people throughout the world. Further information about the tropical disease PRV program can be found in guidance for industry Tropical Disease Priority Review Vouchers (81 FR 69537, October 6, 2016, available at Additions to the
3 3 statutory list of tropical diseases published in the Federal Register can be accessed at m htm. In August 2015, FDA published a final order (80 FR 50559, August 20, 2015) (final order) designating Chagas disease and neurocysticercosis as tropical diseases. That final order also sets forth FDA s interpretation of the statutory criteria for tropical disease designation and expands the list of tropical diseases under section 524(a)(3)(R) of the FD&C Act, which authorizes the FDA to designate by order [a]ny other infectious disease for which there is no significant market in developed nations and that disproportionately affects poor and marginalized populations as a tropical disease. FDA has applied its August 2015 criteria as set forth in the final order to analyze whether PCP meets the statutory criteria for addition to the tropical disease list. As discussed below, the Agency has determined that PCP does not meet the statutory criteria for designation as a tropical disease and thus will not add it to the list of tropical diseases whose applications may be eligible for a priority review voucher. II. Decision Not to Designate Pneumocystis Pneumonia FDA has considered all diseases submitted to the public docket (FDA-2008-N-0567) between August 20, 2015, and June 20, 2018, as potential additions to the list of tropical diseases under section 524 of the FD&C Act, under the docket review process explained on the Agency s website (see m htm). Based on an assessment using the criteria from its final order, FDA has
4 4 determined that PCP will not be designated as a tropical disease under section 524 of the FD&C Act. Pneumocystis species are genetically distinct, host-specific opportunistic fungal pathogens widely found in nature. Pneumocystis jirovecii, found in humans, causes PCP in immunocompromised patients. Human immunodeficiency virus (HIV)-infected patients with a low CD4 count are at the highest risk of PCP. Others at substantial risk include hematopoietic cell and solid organ transplant recipients, those with cancer (particularly hematologic malignancies), and those receiving glucocorticoids, chemotherapeutic agents, and other immunosuppressive medications. Among patients with acquired immunodeficiency syndrome (AIDS) and PCP, the mortality rate is 10 to 20 percent during the initial infection, but the rate increases substantially when the patient requires mechanical ventilation. The mortality rate among patients with PCP in the absence of AIDS is 30 to 60 percent, depending on the population at risk, with a greater risk of death among patients with cancer than among patients undergoing transplantation or those with connective tissue disease (Ref. 1). A. Significant Market in Developed Nations In developed nations, a sizable market exists for PCP prophylaxis drugs. The prevalence of stage-3 AIDS by year end 2014 in the United States (i.e., with AIDS requiring PCP prophylaxis) was approximately 530,000 (Ref 2). In addition, approximately 30,000 solid organ transplantations (Ref. 3) and 19,000 hematopoietic stem cell transplants (Ref. 4) are performed annually in the United States. These patients receive PCP prophylaxis for 6 months to one year in the post-transplantation period. There were also about 6,590 new cases of acute lymphocytic leukemia (ALL) eligible for PCP prophylaxis in the United States in 2016 (Ref. 5).
5 5 Regarding treatment of PCP, the incidence of PCP has declined substantially with widespread use of PCP prophylaxis and anti-retroviral therapy (ART) (see, e.g., Refs. 6-9). In a prospective cohort study of 8070 participants at 12 HIV clinics across the United States, the incidence in was <1 case per 100 person-years (Ref. 10). PCP now mainly occurs in individuals who are unaware that they are HIV positive, lack access to medical care, or are noncompliant with medications. In contrast to HIV-positive patients, the incidence of PCP in non-hiv patients is rising in some areas (see, e.g., Refs. 8, 9, 11); however, the number of cases in non-hiv patients is still below the number of cases in HIV-positive patients (Ref. 12). In the United States, the incidence of PCP is estimated to be 9 percent among hospitalized HIV/AIDS patients and 1 percent among solid organ transplant recipients (Ref. 13). Current clinical guidelines recommend chemoprophylaxis against primary PCP for HIV infected persons with a CD4 cell count <200 cells/µl or a history of oral candidiasis (Ref. 14). As indicated above, the prevalence of stage-3 HIV infection (i.e., AIDS requiring PCP prophylaxis) by year end 2014 in the United States was approximately 530,000 patients, including 18,303 patients diagnosed with stage-3 HIV infection in 2015 (Ref. 2). These subjects were eligible for PCP prophylaxis. In summary, a sizable market in developed nations exists for drugs indicated for prevention of PCP. At present, FDA is unaware of any significant funding by military, the Biomedical Advanced Research and Development Authority (BARDA), or any other United States Government sources for drug development targeting treatment of or prophylaxis against PCP.
6 6 B. Disproportionately Affects Poor and Marginalized Populations Although no disability-adjusted life year (DALY) data were found to distinguish the disease burden of PCP in developing versus developed countries, it is noted that PCP occurs frequently among HIV-infected patients in many parts of the developing world. In addition, the prevalence of HIV-infected persons with PCP ranges from 24 percent (42/177) in Mexico (Ref. 15) to 55 percent in Thailand (Ref. 16). A Brazilian study found 55 percent (15/27) of HIVinfected persons with respiratory symptoms had PCP (Ref. 17). Studies estimating the burden of fungal infections in different countries suggest low total yearly number of PCP cases in Belgium (n = 120), in contrast, for example, to 9,600 cases among HIV-infected people in Tanzania in 2012 (Refs. 18 and 19). In Uganda, the frequency of PCP among HIV-infected patients hospitalized with suspected pneumonia who had negative sputum acid-fast bacilli smears and underwent bronchoscopy decreased from nearly 40 percent of bronchoscopies between 1999 and 2000 to less than ten percent between 2007 and 2008 (Ref. 20). However, it is estimated that there are approximately 800 HIV-positive adults with PCP annually and up to 42,000 children with PCP annually in Uganda (Ref. 21). In Vietnam, the prevalence of PCP was 608 cases in 2012, 1149 cases per year in Senegal and 990 cases yearly in Nepal (HIV positive individuals) (Refs. 22, 23, 24). In Ukraine, 13.5 per 100,000 individuals develop PCP annually (Ref. 25). High rates of anti-pneumocystis antibodies among African children suggest that exposure to the organism is common, and that Pneumocystis jirovecii is a common cause of pneumonia among children in sub-saharan Africa (Ref. 26). Furthermore, limited diagnostic resources and less commonly performed induced sputum and bronchoalveolar lavage with reliance on empiric
7 7 therapy may cause underestimation of the true incidence of PCP (Ref. 27). Several studies suggest that the incidence of PCP is increasing in Africa (Refs. 26, 28, 29). PCP has been reported to be a leading cause of death in HIV-infected infants, responsible for at least one quarter of all pneumonia deaths in HIV-infected infants (Ref. 30). A recent review found PCP to be one of the factors strongly associated with mortality from acute lower respiratory infections in children under five years of age in low-income economies, lowermiddle-income economies, and upper-middle-income economies (referred to as low- and middleincome countries (LMICs)), with odds ratio of 95 percent confidence interval of 4.79, (Ref. 31). However, the incidence of PCP in infants and children in developed countries has decreased because PCP prophylaxis has been initiated in all neonates born to HIV-positive mothers (Refs. 32 and 33). The HIV epidemic imposes a particular burden on women and children, specifically in sub-saharan Africa where women account for approximately 57 percent of all people living with HIV (Ref. 34). In 2012, there were an estimated 260,000 newly infected children in LMICs (id.). Children with HIV are more likely to face gaps in access to HIV treatment. In 2012, approximately 34 percent of children had access to HIV treatment versus approximately 64 percent for adults (id.). Since PCP is the most prevalent in persons infected with HIV (Ref. 1) and HIV disproportionately impacts women and children, it is reasonable to conclude that PCP also disproportionately affects these populations. PCP has not been designated by the World Health Organization (WHO) as a neglected tropical disease (Ref. 35).
8 8 C. FDA Determination In sum, although PCP disproportionately affects poor and marginalized populations, it is an infectious disease for which there is a significant market in developed nations for drugs indicated for prevention of PCP. Based on the information reviewed, FDA has determined that PCP does not meet the statutory criteria for a tropical disease in section 524 of the FD&C Act. III. Process for Requesting Additional Diseases to be Added to the List FDA s current determination regarding PCP does not preclude interested persons from requesting its consideration in the future. To facilitate the consideration of future additions to the list, FDA established a public docket (see Docket No. FDA N-0567) through which interested persons may submit requests for additional diseases to be added to the list. Such requests should be accompanied by information to document that the disease meets the criteria set forth in section 524(a)(3)(S) of the FD&C Act. FDA will periodically review these requests, and, when appropriate, expand the list. For further information, see m htm. IV. Paperwork Reduction Act This notice reiterates the open status of the previously established public docket through which interested persons may submit requests for additional diseases to be added to the list of tropical diseases that FDA has found to meet the criteria in section 524(a)(3)(S) of the FD&C Act. Such a request for information is exempt from Office of Management and Budget review under 5 CFR (h)(4) of the Paperwork Reduction Act of 1995 (44 U.S.C ). Specifically, [f]acts or opinions submitted in response to general solicitations of
9 9 comments from the public, published in the Federal Register or other publications, regardless of the form or format thereof are exempt, provided that no person is required to supply specific information pertaining to the commenter, other than that necessary for self-identification, as a condition of the Agency s full consideration of the comment. V. References The following references have been placed on display at the Dockets Management Staff (see ADDRESSES). They may be seen by interested persons between 9 a.m. and 4 p.m. Monday through Friday and are available electronically at (FDA has verified the website addresses, but FDA is not responsible for any subsequent changes to the websites after this document publishes in the Federal Register.) 1. Thomas, C. F., Jr. and A. H. Limper, Pneumocystis Pneumonia, The New England Journal of Medicine, 350(24): , June 10, Centers for Disease Control and Prevention (CDC), HIV Surveillance Report, 2015, vol. 27, November 2016, available at accessed January 19, United Network for Organ Sharing, Annual Reports, accessed December 16, 2016, available at 4. The U.S. Health Resources and Services Administration, Transplant Activity Report: Total Transplants by Year, accessed January 19, 2017, available at ndex.html.
10 10 5. The American Cancer Society, Key Statistics for Acute Lymphocytic Leukemia, accessed January 19, 2017, available at 6. Miller, R. F., L. Huang, and P. D. Walzer, Pneumocystis Pneumonia Associated with Human Immunodeficiency Virus, Clinics in Chest Medicine, 34(2): , June Morris, A., J. D. Lundgren, H. Masur, et al., Current Epidemiology of Pneumocystis Pneumonia, Emerging Infectious Diseases, 10(10): , October Azoulay, E., A. Bergeron, S. Chevret, N. Bele, et al., Polymerase Chain Reaction for Diagnosing Pneumocystis Pneumonia in Non-HIV Immunocompromised Patients with Pulmonary Infiltrates, Chest, 135(3): , March Gamaletsou, M. N., M. Drogari-Apiranthitou, D. W. Denning, et al., An Estimate of the Burden of Serious Fungal Diseases in Greece, European Journal of Clinical Microbiology & Infectious Diseases (official publication of the European Society of Clinical Microbiology), 35(7): , July Buchacz, K., R. K. Baker, F. J. Palella, Jr., et al., AIDS-Defining Opportunistic Illnesses in US patients, : A Cohort Study, AIDS, 24(10): , Jun 19, Maini, R., K. L. Henderson, E. A. Sheridan, et al., Increasing Pneumocystis Pneumonia, England, UK, , Emerging Infectious Diseases, 19(3): , March 2013.
11 Walzer, P. D. and A. G. Smulian, Pneumocystis species, in: Mandell, G. L., Bennett, J. E., Dolin, R., eds. Mandell, Douglas, and Bennett s Principles and Practice of Infectious Disease, 7th ed. PA: Elsevier; 2010, CDC, Fungal Diseases, Pneumocystis Pneumonia, February 21, 2018, available at accessed November 21, Kaplan, J. E., H. Masur, K. K. Holmes, et al., USPHS/IDSA Guidelines for the Prevention of Opportunistic Infections in Persons Infected with Human Immunodeficiency Virus: A Summary, USPHS/IDSA Prevention of Opportunistic Infections Working Group, Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 21(Suppl 1):S12-31, August Mohar, A., J. Romo, F. Salido, et al., The Spectrum of Clinical and Pathological Manifestations of AIDS in a Consecutive Series of Autopsied Patients in Mexico, AIDS, 6(5): , May Wannamethee, S. G., S. Sirivichayakul, A. N. Phillips, et al., Clinical and Immunological Features of Human Immunodeficiency Virus Infection in Patients from Bangkok, Thailand, International Journal of Epidemiology, 27(2): , April Weinberg, A. and M. I. Duarte, Respiratory Complications in Brazilian Patients Infected with Human Immunodeficiency Virus, Revista do Instituto de Medicina Tropical de Sao Paulo, 35(2): , March-April Lagrou, K., J. Maertens, E. Van Even, et al., Burden of Serious Fungal Infections in Belgium, Mycoses, 58(Suppl 5):1-5, October 2015.
12 Faini, D., W. Maokola, W., H. Furrer, et al., Burden of Serious Fungal Infections in Tanzania, Mycoses, 58(Suppl 5):70-79, October Worodria, W., J. L. Davis, A. Cattamanchi, et al., Bronchoscopy is Useful for Diagnosing Smear-Negative Tuberculosis in HIV-Infected Patients, The European Respiratory Journal, 36(2): , August Parkes Ratanshi, R., B. Achan, R. Kwizera, et al., Cryptococcal Disease and the Burden of Other Fungal Diseases in Uganda; Where are the Knowledge Gaps and How Can We Fill Them? Mycoses, 58(Suppl 5):85-93, October Beardsley, J., D. W. Denning, N. V. Chau, et al., Estimating the Burden of Fungal Disease in Vietnam, Mycoses, 58(Suppl 5): , October Badiane, A. S., D. Ndiaye, D. W. Denning, Burden of Fungal Infections in Senegal, Mycoses, 58(Suppl 5):63-69, October Khwakhali, U. S. and D. W. Denning, Burden of Serious Fungal Infections in Nepal, Mycoses, 58(Suppl 5):45-50, October Osmanov, A. and D. W. Denning, Burden of Serious Fungal Infections in Ukraine, Mycoses, 58(Suppl 5): , October Morrow, B. M., N. Y. Hsaio, M. Zampoli, et al., Pneumocystis Pneumonia in South African Children with and Without Human Immunodeficiency Virus Infection in the Era of Highly Active Antiretroviral Therapy, The Pediatric Infectious Disease Journal, 29(6): , June Stansell, J. D., D. H. Osmond, E. Charlebois, E., et al., Predictors of Pneumocystis carinii Pneumonia in HIV-Infected Persons. Pulmonary Complications of
13 13 HIV Infection Study Group, American Journal of Respiratory and Critical Care Medicine, 155(1):60-66, January Wasserman, S., M. E. Engel, M. Mendelson, Burden of Pneumocystis Pneumonia in HIV-Infected Adults in Sub-Saharan Africa: Protocol for a Systematic Review, Systematic Reviews, 2:112, December 12, Fisk, D. T., S. Meshnick, S., and P. H. Kazanjian, Pneumocystis carinii Pneumonia in Patients in the Developing World Who Have Acquired Immunodeficiency Syndrome, Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 36(1):70-78, January 1, de Boer, M. G., J. W. de Fijter, F. P. Kroon, Outbreaks and Clustering of Pneumocystis Pneumonia in Kidney Transplant Recipients: A Systematic Review, Medical Mycology, 49(7): , October Sonego, M., M. C. Pellegrin, G. Becker, et al., Risk Factors for Mortality from Acute Lower Respiratory Infections (ALRI) in Children under Five Years of Age in Low and Middle-Income Countries: A Systematic Review and Meta-Analysis of Observational Studies, PLOS One, 10(1):e , Morris, A., J. D. Lundgren, H. Masur, et al., Current Epidemiology of Pneumocystis Pneumonia, Emerging Infectious Diseases, 10(10): , October Avino, L. J., S. M. Naylor, A. M. Roecker, Pneumocystis jirovecii Pneumonia in the Non-HIV-Infected Population, The Annals of Pharmacotherapy, 50(8): , August 2016.
14 Joint United Nations Programme on HIV/AIDS (UNAIDS), Global Report: UNAIDS Report on the Global AIDS Epidemic 2013, accessed December 9, 2016, available at 13/UNAIDS_Global_Report_2013_en.pdf. 35. WHO, Neglected Tropical Diseases, accessed December 9, 2016, available at Dated: August 21, Leslie Kux, Associate Commissioner for Policy. [FR Doc Filed: 8/23/2018 8:45 am; Publication Date: 8/24/2018]
Withdrawal of Proposed Rule on Supplemental Applications Proposing Labeling Changes for
 This document is scheduled to be published in the Federal Register on 12/14/2018 and available online at https://federalregister.gov/d/2018-27098, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
This document is scheduled to be published in the Federal Register on 12/14/2018 and available online at https://federalregister.gov/d/2018-27098, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
SUMMARY: The Food and Drug Administration (FDA) is requesting public input on updated
 This document is scheduled to be published in the Federal Register on 11/22/2017 and available online at https://federalregister.gov/d/2017-25245, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 11/22/2017 and available online at https://federalregister.gov/d/2017-25245, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Three-Month Extension of Certain Tobacco Product Compliance Deadlines Related to the
 This document is scheduled to be published in the Federal Register on 05/15/2017 and available online at https://federalregister.gov/d/2017-09754, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 05/15/2017 and available online at https://federalregister.gov/d/2017-09754, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Agency Information Collection Activities; Submission for Office of Management and Budget
 This document is scheduled to be published in the Federal Register on 01/28/2016 and available online at http://federalregister.gov/a/2016-01690, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 01/28/2016 and available online at http://federalregister.gov/a/2016-01690, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Highly Concentrated Caffeine in Dietary Supplements; Guidance for Industry; Availability
 This document is scheduled to be published in the Federal Register on 04/16/2018 and available online at https://federalregister.gov/d/2018-07836, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 04/16/2018 and available online at https://federalregister.gov/d/2018-07836, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Agency Information Collection Activities; Submission for Office of Management and
 This document is scheduled to be published in the Federal Register on 02/19/2019 and available online at https://federalregister.gov/d/2019-02596, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
This document is scheduled to be published in the Federal Register on 02/19/2019 and available online at https://federalregister.gov/d/2019-02596, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
Agency Information Collection Activities; Submission for Office of Management and
 This document is scheduled to be published in the Federal Register on 03/04/2019 and available online at https://federalregister.gov/d/2019-03824, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
This document is scheduled to be published in the Federal Register on 03/04/2019 and available online at https://federalregister.gov/d/2019-03824, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
Generic Drug User Fee Amendments of 2012; Regulatory Science Initiatives; Public Hearing;
 4160-01-P DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Part 15 [Docket No. FDA-2013-N-0402] Generic Drug User Fee Amendments of 2012; Regulatory Science Initiatives; Public
4160-01-P DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Part 15 [Docket No. FDA-2013-N-0402] Generic Drug User Fee Amendments of 2012; Regulatory Science Initiatives; Public
Patient-Focused Drug Development Public Meeting on Chagas Disease
 This document is scheduled to be published in the Federal Register on 12/10/2014 and available online at http://federalregister.gov/a/2014-28828, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 12/10/2014 and available online at http://federalregister.gov/a/2014-28828, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Opioid Policy Steering Committee: Prescribing Intervention--Exploring a Strategy for
 This document is scheduled to be published in the Federal Register on 12/13/2017 and available online at https://federalregister.gov/d/2017-26785, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 12/13/2017 and available online at https://federalregister.gov/d/2017-26785, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
ANDA Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY December 3, 2015
 DEPARTMENT OF HEALTH & HUMAN SERVICES Silver Spring, MD 20993 ANDA 060851 Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY 10970 Docket No. FDA-2011-P-0081
DEPARTMENT OF HEALTH & HUMAN SERVICES Silver Spring, MD 20993 ANDA 060851 Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY 10970 Docket No. FDA-2011-P-0081
Food Labeling: Revision of the Nutrition and Supplement Facts Labels and Serving Sizes of
 This document is scheduled to be published in the Federal Register on 10/02/2017 and available online at https://federalregister.gov/d/2017-21019, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 10/02/2017 and available online at https://federalregister.gov/d/2017-21019, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Controlling the Progression of Myopia: Contact Lenses and Future Medical Devices; Public
 This document is scheduled to be published in the Federal Register on 07/11/2016 and available online at http://federalregister.gov/a/2016-16353, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 07/11/2016 and available online at http://federalregister.gov/a/2016-16353, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
ACTION: Notification; declaratory order; extension of compliance date.
 This document is scheduled to be published in the Federal Register on 05/21/2018 and available online at https://federalregister.gov/d/2018-10714, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
This document is scheduled to be published in the Federal Register on 05/21/2018 and available online at https://federalregister.gov/d/2018-10714, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
and the Working Group from the EMBO-AIDS Related Mycoses Workshop Institute of Infectious Disease and Molecular Medicine, University of Cape Town,
 1 AIDS-related mycoses: the way forward Gordon D. Brown 1,2*, Graeme Meintjes 1, Jay K. Kolls 3, Clive Gray 1, William Horsnell 1 and the Working Group from the EMBO-AIDS Related Mycoses Workshop 1 Institute
1 AIDS-related mycoses: the way forward Gordon D. Brown 1,2*, Graeme Meintjes 1, Jay K. Kolls 3, Clive Gray 1, William Horsnell 1 and the Working Group from the EMBO-AIDS Related Mycoses Workshop 1 Institute
Flavored Milk; Petition to Amend the Standard of Identity for Milk and 17 Additional Dairy
 This document is scheduled to be published in the Federal Register on 02/20/2013 and available online at http://federalregister.gov/a/2013-03835, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 02/20/2013 and available online at http://federalregister.gov/a/2013-03835, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
Intent to Consider the Appropriate Classification of Hyaluronic Acid Intra-articular Products
 This document is scheduled to be published in the Federal Register on 12/18/2018 and available online at https://federalregister.gov/d/2018-27351, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
This document is scheduled to be published in the Federal Register on 12/18/2018 and available online at https://federalregister.gov/d/2018-27351, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
Listing of Color Additives Exempt from Certification; Synthetic Iron Oxide
 This document is scheduled to be published in the Federal Register on 03/20/2015 and available online at http://federalregister.gov/a/2015-06418, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
This document is scheduled to be published in the Federal Register on 03/20/2015 and available online at http://federalregister.gov/a/2015-06418, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
Indirect Food Additives: Adhesives and Components of Coatings and Paper and Paperboard Components
 4 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Parts 175 and 176 [Docket No. 99F-09253 Indirect Food Additives: Adhesives and Components of Coatings and Paper and Paperboard
4 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Parts 175 and 176 [Docket No. 99F-09253 Indirect Food Additives: Adhesives and Components of Coatings and Paper and Paperboard
DATES: This rule is effective December 8, See section X for further information on the
 This document is scheduled to be published in the Federal Register on 11/07/2017 and available online at https://federalregister.gov/d/2017-24194, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 11/07/2017 and available online at https://federalregister.gov/d/2017-24194, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
The Food and Drug Administration's Approach to Evaluating Nicotine Replacement Therapies;
 This document is scheduled to be published in the Federal Register on 11/30/2017 and available online at https://federalregister.gov/d/2017-25671, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 11/30/2017 and available online at https://federalregister.gov/d/2017-25671, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Guidance for Industry
 Reprinted from FDA s website by Guidance for Industry Bar Code Label Requirements Questions and Answers (Question 12 Update) DRAFT GUIDANCE This guidance document is for comment purposes only. Submit comments
Reprinted from FDA s website by Guidance for Industry Bar Code Label Requirements Questions and Answers (Question 12 Update) DRAFT GUIDANCE This guidance document is for comment purposes only. Submit comments
SUMMARY: The Food and Drug Administration (FDA, the Agency, or we) is proposing to
 This document is scheduled to be published in the Federal Register on 10/26/2016 and available online at https://federalregister.gov/d/2016-25850, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 10/26/2016 and available online at https://federalregister.gov/d/2016-25850, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
SUMMARY: The Food and Drug Administration (FDA, the Agency, or we) is amending its
 This document is scheduled to be published in the Federal Register on 10/26/2016 and available online at https://federalregister.gov/d/2016-25851, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 10/26/2016 and available online at https://federalregister.gov/d/2016-25851, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Guidance for Industry DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
Food Additives Permitted for Direct Addition to Food for Human Consumption; Acacia (Gum
 This document is scheduled to be published in the Federal Register on 12/06/2013 and available online at http://federalregister.gov/a/2013-29073, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 12/06/2013 and available online at http://federalregister.gov/a/2013-29073, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
Clinical Chemistry and Clinical Toxicology Devices Panel of the Medical Devices Advisory
 This document is scheduled to be published in the Federal Register on 03/01/2018 and available online at https://federalregister.gov/d/2018-04167, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 03/01/2018 and available online at https://federalregister.gov/d/2018-04167, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Nonallergic Rhinitis: Developing Drug Products for Treatment Guidance for Industry
 Nonallergic Rhinitis: Developing Drug Products for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Nonallergic Rhinitis: Developing Drug Products for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
DEPARTMENT OF VETERANS AFFAIRS SUMMARY: The Department of Veterans Affairs (VA) is amending its medical
 This document is scheduled to be published in the Federal Register on 03/23/2017 and available online at https://federalregister.gov/d/2017-05799, and on FDsys.gov DEPARTMENT OF VETERANS AFFAIRS 8320-01
This document is scheduled to be published in the Federal Register on 03/23/2017 and available online at https://federalregister.gov/d/2017-05799, and on FDsys.gov DEPARTMENT OF VETERANS AFFAIRS 8320-01
Title: Role of Interferon-gamma Release Assays in the Diagnosis of Pulmonary Tuberculosis in Patients with Advanced HIV infection
 Author's response to reviews Title: Role of Interferon-gamma Release Assays in the Diagnosis of Pulmonary Tuberculosis in Patients with Advanced HIV infection Authors: Adithya Cattamanchi (acattamanchi@medsfgh.ucsf.edu)
Author's response to reviews Title: Role of Interferon-gamma Release Assays in the Diagnosis of Pulmonary Tuberculosis in Patients with Advanced HIV infection Authors: Adithya Cattamanchi (acattamanchi@medsfgh.ucsf.edu)
Food Additives Permitted for Direct Addition to Food for Human Consumption; Vitamin D 2
 This document is scheduled to be published in the Federal Register on 03/11/2014 and available online at http://federalregister.gov/a/2014-05060, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 03/11/2014 and available online at http://federalregister.gov/a/2014-05060, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
DEPARTMENT OF VETERANS AFFAIRS SUMMARY: The Department of Veterans Affairs (VA) proposes to amend its medical
 This document is scheduled to be published in the Federal Register on 08/05/2016 and available online at http://federalregister.gov/a/2016-18660, and on FDsys.gov DEPARTMENT OF VETERANS AFFAIRS 8320-01
This document is scheduled to be published in the Federal Register on 08/05/2016 and available online at http://federalregister.gov/a/2016-18660, and on FDsys.gov DEPARTMENT OF VETERANS AFFAIRS 8320-01
SUMMARY: The Food and Drug Administration (FDA or we) is amending the color additive
 This document is scheduled to be published in the Federal Register on 08/21/2015 and available online at http://federalregister.gov/a/2015-20676, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 08/21/2015 and available online at http://federalregister.gov/a/2015-20676, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Proposed Data Collection Submitted for Public Comment and. AGENCY: Centers for Disease Control and Prevention (CDC),
 This document is scheduled to be published in the Federal Register on 09/26/2017 and available online at https://federalregister.gov/d/2017-20511, and on FDsys.gov BILLING CODE 4163-18-P DEPARTMENT OF
This document is scheduled to be published in the Federal Register on 09/26/2017 and available online at https://federalregister.gov/d/2017-20511, and on FDsys.gov BILLING CODE 4163-18-P DEPARTMENT OF
Amyotrophic Lateral Sclerosis: Developing Drugs for Treatment Guidance for Industry
 Amyotrophic Lateral Sclerosis: Developing Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Amyotrophic Lateral Sclerosis: Developing Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Copyright 2011 Joint United Nations Programme on HIV/AIDS (UNAIDS) All rights reserved ISBN
 UNAIDS DATA TABLES 2011 Copyright 2011 Joint United Nations Programme on HIV/AIDS (UNAIDS) All rights reserved ISBN 978-92-9173-945-5 UNAIDS / JC2225E The designations employed and the presentation of
UNAIDS DATA TABLES 2011 Copyright 2011 Joint United Nations Programme on HIV/AIDS (UNAIDS) All rights reserved ISBN 978-92-9173-945-5 UNAIDS / JC2225E The designations employed and the presentation of
Outcomes of Moderate-to-Severe Pneumocystis Pneumonia Treated with Adjunctive Steroid in Non-HIV-Infected Patients
 ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Oct. 2011, p. 4613 4618 Vol. 55, No. 10 0066-4804/11/$12.00 doi:10.1128/aac.00669-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. Outcomes
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Oct. 2011, p. 4613 4618 Vol. 55, No. 10 0066-4804/11/$12.00 doi:10.1128/aac.00669-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. Outcomes
National Vaccine Injury Compensation Program: Adding the Category of Vaccines. AGENCY: Health Resources and Services Administration (HRSA), HHS.
 This document is scheduled to be published in the Federal Register on 04/04/2018 and available online at https://federalregister.gov/d/2018-06770, and on FDsys.gov Billing Code: 4165-15 DEPARTMENT OF HEALTH
This document is scheduled to be published in the Federal Register on 04/04/2018 and available online at https://federalregister.gov/d/2018-06770, and on FDsys.gov Billing Code: 4165-15 DEPARTMENT OF HEALTH
Proposed Data Collection Submitted for Public Comment and. AGENCY: Centers for Disease Control and Prevention (CDC),
 This document is scheduled to be published in the Federal Register on 11/15/2018 and available online at https://federalregister.gov/d/2018-24969, and on govinfo.gov BILLING CODE: 4163-18-P DEPARTMENT
This document is scheduled to be published in the Federal Register on 11/15/2018 and available online at https://federalregister.gov/d/2018-24969, and on govinfo.gov BILLING CODE: 4163-18-P DEPARTMENT
DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Inborn Errors of Metabolism That Use Dietary Management: Considerations for Optimizing and Standardizing Diet in Clinical Trials for Drug Product Development Guidance for Industry DRAFT GUIDANCE This guidance
Inborn Errors of Metabolism That Use Dietary Management: Considerations for Optimizing and Standardizing Diet in Clinical Trials for Drug Product Development Guidance for Industry DRAFT GUIDANCE This guidance
Hypertension: Developing Fixed- Dose Combination Drugs for Treatment Guidance for Industry
 Hypertension: Developing Fixed- Dose Combination Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions
Hypertension: Developing Fixed- Dose Combination Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions
Centers for Disease Control and Prevention
 This document is scheduled to be published in the Federal Register on 12/11/2018 and available online at https://federalregister.gov/d/2018-26708, and on govinfo.gov Billing Code: 4163-18-P DEPARTMENT
This document is scheduled to be published in the Federal Register on 12/11/2018 and available online at https://federalregister.gov/d/2018-26708, and on govinfo.gov Billing Code: 4163-18-P DEPARTMENT
DEPARTMENT OF TRANSPORTATION. National Highway Traffic Safety Administration. [Docket Number: NHTSA ]
![DEPARTMENT OF TRANSPORTATION. National Highway Traffic Safety Administration. [Docket Number: NHTSA ] DEPARTMENT OF TRANSPORTATION. National Highway Traffic Safety Administration. [Docket Number: NHTSA ]](/thumbs/96/128041132.jpg) This document is scheduled to be published in the Federal Register on 10/04/2018 and available online at https://federalregister.gov/d/2018-21623, and on govinfo.gov DEPARTMENT OF TRANSPORTATION National
This document is scheduled to be published in the Federal Register on 10/04/2018 and available online at https://federalregister.gov/d/2018-21623, and on govinfo.gov DEPARTMENT OF TRANSPORTATION National
Billing Code: P DEPARTMENT OF HEALTH AND HUMAN SERVICES. Centers for Disease Control and Prevention. [30Day-18-17AUZ]
![Billing Code: P DEPARTMENT OF HEALTH AND HUMAN SERVICES. Centers for Disease Control and Prevention. [30Day-18-17AUZ] Billing Code: P DEPARTMENT OF HEALTH AND HUMAN SERVICES. Centers for Disease Control and Prevention. [30Day-18-17AUZ]](/thumbs/89/98265456.jpg) This document is scheduled to be published in the Federal Register on 06/18/2018 and available online at https://federalregister.gov/d/2018-12971, and on FDsys.gov Billing Code: 4163-18-P DEPARTMENT OF
This document is scheduled to be published in the Federal Register on 06/18/2018 and available online at https://federalregister.gov/d/2018-12971, and on FDsys.gov Billing Code: 4163-18-P DEPARTMENT OF
Marc Moss, MD President, American Thoracic Society (202) th St, N.W. #300 Washington, DC 20036
 Marc Moss, MD President, American Thoracic Society Nmoore@thoracic.org; (202) 296. 9770 1150 18 th St, N.W. #300 Washington, DC 20036 STATEMENT OF THE AMERICAN THORACIC SOCIETY SUBMITTED TO THE HOUSE LABOR,
Marc Moss, MD President, American Thoracic Society Nmoore@thoracic.org; (202) 296. 9770 1150 18 th St, N.W. #300 Washington, DC 20036 STATEMENT OF THE AMERICAN THORACIC SOCIETY SUBMITTED TO THE HOUSE LABOR,
Objective: Specific Aims:
 Title: Retention to Care of HIV pregnant females in Kumasi, Ghana Brown Faculty: Aadia Rana, MD. Assistant Professor of Medicine in Division of Infectious Diseases, Awewura Kwara, Assistant Professor of
Title: Retention to Care of HIV pregnant females in Kumasi, Ghana Brown Faculty: Aadia Rana, MD. Assistant Professor of Medicine in Division of Infectious Diseases, Awewura Kwara, Assistant Professor of
A Review on Prevalence of TB and HIV Co-infection
 Human Journals Review Article May 2015 Vol.:1, Issue:1 All rights are reserved by Jyoti P. Waghmode et al. A Review on Prevalence of TB and HIV Co-infection Keywords: tuberculosis, HIV, co-infection, prevalence
Human Journals Review Article May 2015 Vol.:1, Issue:1 All rights are reserved by Jyoti P. Waghmode et al. A Review on Prevalence of TB and HIV Co-infection Keywords: tuberculosis, HIV, co-infection, prevalence
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry
 ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
HIV AND LUNG HEALTH. Stephen Aston Infectious Diseases SpR Royal Liverpool University Hospital
 HIV AND LUNG HEALTH Stephen Aston Infectious Diseases SpR Royal Liverpool University Hospital Introduction HIV infection exerts multiple effects on pulmonary immune responses: Generalised state of immune
HIV AND LUNG HEALTH Stephen Aston Infectious Diseases SpR Royal Liverpool University Hospital Introduction HIV infection exerts multiple effects on pulmonary immune responses: Generalised state of immune
AIDS at 25. Epidemiology and Clinical Management MID 37
 AIDS at 25 Epidemiology and Clinical Management Blood HIV Transmission transfusion injection drug use Sexual Intercourse heterosexual male to male Perinatal intrapartum breast feeding Regional HIV and
AIDS at 25 Epidemiology and Clinical Management Blood HIV Transmission transfusion injection drug use Sexual Intercourse heterosexual male to male Perinatal intrapartum breast feeding Regional HIV and
Schedules of Controlled Substances: Placement of Furanyl fentanyl, 4- Fluoroisobutyryl fentanyl, Acryl fentanyl, Tetrahydrofuranyl fentanyl, and
 This document is scheduled to be published in the Federal Register on 11/29/2018 and available online at https://federalregister.gov/d/2018-26045, and on govinfo.gov Billing Code 4410-09-P DEPARTMENT OF
This document is scheduled to be published in the Federal Register on 11/29/2018 and available online at https://federalregister.gov/d/2018-26045, and on govinfo.gov Billing Code 4410-09-P DEPARTMENT OF
C~ 6 D~~J ~ old ~ s rw~ o, - _ - 2 DEPARTMENT OF HEALTH AND HUMAN SERVICES. Food and Drug Administration. 21 CFR Part 530
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Part 530, s rw~ o, - _ - 2 [Docket No. 2006N-0106] New Animal Drugs ; Adamantane and Neuraminidase Inhibitor Anti-influenza Drugs
DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Part 530, s rw~ o, - _ - 2 [Docket No. 2006N-0106] New Animal Drugs ; Adamantane and Neuraminidase Inhibitor Anti-influenza Drugs
Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen
 Reprinted from FDA s website by EAS Consulting Group, LLC Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed
Reprinted from FDA s website by EAS Consulting Group, LLC Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed
Annual Tuberculosis Report Oregon 2007
 Annual Tuberculosis Report Oregon 7 Oregon Department of Human Services Public Health Division TB Program April 8 Page 2 Table of Contents Charts Chart 1 TB Incidence in the US and Oregon, 1985-7.. page
Annual Tuberculosis Report Oregon 7 Oregon Department of Human Services Public Health Division TB Program April 8 Page 2 Table of Contents Charts Chart 1 TB Incidence in the US and Oregon, 1985-7.. page
Eastern Mediterranean Health Journal, Vol. 14, No. 5,
 Eastern Mediterranean Health Journal, Vol. 14, No. 5, 2008 1217 Case report Inappropriate use of steroid and Pneumocystis jiroveci pneumonia: report of two cases P. Tabarsi, 1 M. Mirsaeidi, 1 M. Amiri,
Eastern Mediterranean Health Journal, Vol. 14, No. 5, 2008 1217 Case report Inappropriate use of steroid and Pneumocystis jiroveci pneumonia: report of two cases P. Tabarsi, 1 M. Mirsaeidi, 1 M. Amiri,
Summary of Key Points WHO Position Paper on BCG Vaccine, February 2018
 Summary of Key Points WHO Position Paper on BCG Vaccine, February 2018 1 Introduction This position paper replaces the 2004 WHO position paper on Bacille Calmette-Guérin (BCG) vaccine and the 2007 WHO
Summary of Key Points WHO Position Paper on BCG Vaccine, February 2018 1 Introduction This position paper replaces the 2004 WHO position paper on Bacille Calmette-Guérin (BCG) vaccine and the 2007 WHO
INSTRUCTOR S NOTE. Copyright 2018, The Johns Hopkins University and Teaching Vaccine Economics Everywhere.
 INSTRUCTOR S NOTE This case study can be used to motivate several learning objectives and can be tied to specific course objectives in the modules. In case studies the students are not spoon-fed. Instead,
INSTRUCTOR S NOTE This case study can be used to motivate several learning objectives and can be tied to specific course objectives in the modules. In case studies the students are not spoon-fed. Instead,
Proposed Data Collection Submitted for Public Comment and. AGENCY: Centers for Disease Control and Prevention (CDC),
 This document is scheduled to be published in the Federal Register on 03/13/2018 and available online at https://federalregister.gov/d/2018-05000, and on FDsys.gov BILLING CODE 4163-18-P DEPARTMENT OF
This document is scheduled to be published in the Federal Register on 03/13/2018 and available online at https://federalregister.gov/d/2018-05000, and on FDsys.gov BILLING CODE 4163-18-P DEPARTMENT OF
Scaling up priority HIV/AIDS interventions in the health sector
 TOWARDS UNIVERSAL ACCESS? Scaling up priority HIV/AIDS interventions in the health sector Yves Souteyrand, WHO October 2011 Towards universal access targets UN General Assembly High level Meeting June
TOWARDS UNIVERSAL ACCESS? Scaling up priority HIV/AIDS interventions in the health sector Yves Souteyrand, WHO October 2011 Towards universal access targets UN General Assembly High level Meeting June
Prevalent opportunistic infections associated with HIV-positive children 0-5 years in Benin city, Nigeria
 Malaysian Journal of Microbiology, Vol 4(2) 2008, pp. 11-14 http://dx.doi.org/10.21161/mjm.11508 Prevalent opportunistic infections associated with HIV-positive children 0-5 years in Benin city, Nigeria
Malaysian Journal of Microbiology, Vol 4(2) 2008, pp. 11-14 http://dx.doi.org/10.21161/mjm.11508 Prevalent opportunistic infections associated with HIV-positive children 0-5 years in Benin city, Nigeria
Original Article. Noparat Oniem, M.D., Somnuek Sungkanuparph, M.D.
 Original Article Vol. 29 No. 1 Primary prophylaxis for cryptococcosis with fluconazole:- Oniem N & Sungkanuparph S. 5 Primary prophylaxis for cryptococcosis with fluconazole among HIV-infected patients
Original Article Vol. 29 No. 1 Primary prophylaxis for cryptococcosis with fluconazole:- Oniem N & Sungkanuparph S. 5 Primary prophylaxis for cryptococcosis with fluconazole among HIV-infected patients
Proposed Data Collection Submitted for Public Comment and. AGENCY: Centers for Disease Control and Prevention (CDC),
 This document is scheduled to be published in the Federal Register on 03/06/2019 and available online at https://federalregister.gov/d/2019-04011, and on govinfo.gov BILLING CODE: 4163-18-P DEPARTMENT
This document is scheduled to be published in the Federal Register on 03/06/2019 and available online at https://federalregister.gov/d/2019-04011, and on govinfo.gov BILLING CODE: 4163-18-P DEPARTMENT
AIDS at 30 Epidemiology and Clinical Epidemiology and Management MID 37
 AIDS at 30 Epidemiology and Clinical Management Blood HIV Transmission transfusion injection drug use Sexual Intercourse heterosexual male to male Perinatal intrapartum breast feeding Adults and children
AIDS at 30 Epidemiology and Clinical Management Blood HIV Transmission transfusion injection drug use Sexual Intercourse heterosexual male to male Perinatal intrapartum breast feeding Adults and children
Renewing Momentum in the fight against HIV/AIDS
 2011 marks 30 years since the first cases of AIDS were documented and the world has made incredible progress in its efforts to understand, prevent and treat this pandemic. Progress has been particularly
2011 marks 30 years since the first cases of AIDS were documented and the world has made incredible progress in its efforts to understand, prevent and treat this pandemic. Progress has been particularly
April 25, 2014 Reference No. HAMB FDA Transparency Initiative Regulations Development
 Reference No. HAMB14001 Via Email Dr. Margaret Hamburg Commissioner U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 margaret.hamburg@fda.hhs.gov SUBJECT: FDA Transparency
Reference No. HAMB14001 Via Email Dr. Margaret Hamburg Commissioner U.S. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring, MD 20993 margaret.hamburg@fda.hhs.gov SUBJECT: FDA Transparency
Establishment of a New Drug Code for Marihuana Extract. AGENCY: Drug Enforcement Administration, Department of Justice.
 This document is scheduled to be published in the Federal Register on 12/14/2016 and available online at https://federalregister.gov/d/2016-29941, and on FDsys.gov Billing Code 4410-09-P DEPARTMENT OF
This document is scheduled to be published in the Federal Register on 12/14/2016 and available online at https://federalregister.gov/d/2016-29941, and on FDsys.gov Billing Code 4410-09-P DEPARTMENT OF
Centers for Disease Control and Prevention. Recommendations for Providers Counseling Male Patients and
 This document is scheduled to be published in the Federal Register on 12/02/2014 and available online at http://federalregister.gov/a/2014-27814, and on FDsys.gov BILLING CODE: 4163-18-P DEPARTMENT OF
This document is scheduled to be published in the Federal Register on 12/02/2014 and available online at http://federalregister.gov/a/2014-27814, and on FDsys.gov BILLING CODE: 4163-18-P DEPARTMENT OF
National Organic Program (NOP); Sunset 2017 Amendments to the National List
 This document is scheduled to be published in the Federal Register on 07/06/2017 and available online at https://federalregister.gov/d/2017-14006, and on FDsys.gov DEPARTMENT OF AGRICULTURE Agricultural
This document is scheduled to be published in the Federal Register on 07/06/2017 and available online at https://federalregister.gov/d/2017-14006, and on FDsys.gov DEPARTMENT OF AGRICULTURE Agricultural
SUMMARY: Before a Federal agency can collect certain information from the public, it must
 This document is scheduled to be published in the Federal Register on 08/08/2017 and available online at https://federalregister.gov/d/2017-16651, and on FDsys.gov 4910-59-P DEPARTMENT OF TRANSPORTATION
This document is scheduled to be published in the Federal Register on 08/08/2017 and available online at https://federalregister.gov/d/2017-16651, and on FDsys.gov 4910-59-P DEPARTMENT OF TRANSPORTATION
PARASITOLOGY CASE HISTORY 10 (HISTOLOGY) (Lynne S. Garcia)
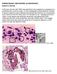 PARASITOLOGY CASE HISTORY 10 (HISTOLOGY) (Lynne S. Garcia) A 46-year-old man with AIDS was admitted to the hospital for complaints of a persisting fever and dry cough. A chest radiograph showed bilateral
PARASITOLOGY CASE HISTORY 10 (HISTOLOGY) (Lynne S. Garcia) A 46-year-old man with AIDS was admitted to the hospital for complaints of a persisting fever and dry cough. A chest radiograph showed bilateral
HIV/AIDS MEASURES GROUP OVERVIEW
 2014 PQRS OPTIONS F MEASURES GROUPS: HIV/AIDS MEASURES GROUP OVERVIEW 2014 PQRS MEASURES IN HIV/AIDS MEASURES GROUP: #159. HIV/AIDS: CD4+ Cell Count or CD4+ Percentage Performed #160. HIV/AIDS: Pneumocystis
2014 PQRS OPTIONS F MEASURES GROUPS: HIV/AIDS MEASURES GROUP OVERVIEW 2014 PQRS MEASURES IN HIV/AIDS MEASURES GROUP: #159. HIV/AIDS: CD4+ Cell Count or CD4+ Percentage Performed #160. HIV/AIDS: Pneumocystis
COHORT STUDY OF HIV POSITIVE AND HIV NEGATIVE TUBERCULOSIS in PENANG HOSPITAL: COMPARISON OF CLINICAL MANIFESTATIONS
 COHORT STUDY OF HIV POSITIVE AND HIV NEGATIVE TUBERCULOSIS in PENANG HOSPITAL: COMPARISON OF CLINICAL MANIFESTATIONS Ong CK 1, Tan WC 2, Leong KN 2, Abdul Razak M 1, Chow TS 2 1 Respiratory Unit, Penang
COHORT STUDY OF HIV POSITIVE AND HIV NEGATIVE TUBERCULOSIS in PENANG HOSPITAL: COMPARISON OF CLINICAL MANIFESTATIONS Ong CK 1, Tan WC 2, Leong KN 2, Abdul Razak M 1, Chow TS 2 1 Respiratory Unit, Penang
AIDS denialism: the pseudoscience that kills
 AIDS denialism: the pseudoscience that kills Guido Silvestri, MD Emory University School of Medicine Yerkes National Primate Research Center Emory Center for AIDS Research (CFAR) Emory Vaccine Center G.R.A.
AIDS denialism: the pseudoscience that kills Guido Silvestri, MD Emory University School of Medicine Yerkes National Primate Research Center Emory Center for AIDS Research (CFAR) Emory Vaccine Center G.R.A.
Human Immunodeficiency Virus
 Human Immunodeficiency Virus Isolated in 1983 by Luc Montagnier & Robert Gallo, separately. Genetic material is RNA Carries reverse transcriptase, an enzyme that makes DNA out of RNA in the host cell Cells
Human Immunodeficiency Virus Isolated in 1983 by Luc Montagnier & Robert Gallo, separately. Genetic material is RNA Carries reverse transcriptase, an enzyme that makes DNA out of RNA in the host cell Cells
DEPARTMENT OF VETERANS AFFAIRS Secondary Service Connection for Diagnosable Illnesses Associated With Traumatic
 This document is scheduled to be published in the Federal Register on 12/10/2012 and available online at http://federalregister.gov/a/2012-29709, and on FDsys.gov DEPARTMENT OF VETERANS AFFAIRS 8320-01
This document is scheduled to be published in the Federal Register on 12/10/2012 and available online at http://federalregister.gov/a/2012-29709, and on FDsys.gov DEPARTMENT OF VETERANS AFFAIRS 8320-01
Notice of Request for Extension of Approval of an Information Collection; Swine Health. AGENCY: Animal and Plant Health Inspection Service, USDA.
 This document is scheduled to be published in the Federal Register on 09/24/2013 and available online at http://federalregister.gov/a/2013-23194, and on FDsys.gov DEPARTMENT OF AGRICULTURE Animal and Plant
This document is scheduled to be published in the Federal Register on 09/24/2013 and available online at http://federalregister.gov/a/2013-23194, and on FDsys.gov DEPARTMENT OF AGRICULTURE Animal and Plant
Antiretroviral therapy for adults and adolescents KEY MESSAGES. HIV/AIDS Department BACKGROUND
 KEY MESSAGES New WHO Recommendations: Antiretroviral therapy for adults and adolescents The World Health Organization (WHO) is revising its guidelines on antiretroviral therapy (ART) for adults and adolescents.
KEY MESSAGES New WHO Recommendations: Antiretroviral therapy for adults and adolescents The World Health Organization (WHO) is revising its guidelines on antiretroviral therapy (ART) for adults and adolescents.
Pneumocystis. Pneumocystis BIOL Summer Introduction. Mycology. Introduction (cont.) Introduction (cont.)
 Introduction Pneumocystis Disclaimer: This lecture slide presentation is intended solely for educational purposes. Many of the images contained herein are the property of the original owner, as indicated
Introduction Pneumocystis Disclaimer: This lecture slide presentation is intended solely for educational purposes. Many of the images contained herein are the property of the original owner, as indicated
Guidance for Industry Migraine: Developing Drugs for Acute Treatment
 Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Strategic use of antiretroviral drugs to prevent HIV transmission
 Strategic use of antiretroviral drugs to prevent HIV transmission 22th Tunisian Congress of Infectious Diseases 2nd Congress of Federation of Arab Societies of Clinical Microbiology and Infectious Diseases
Strategic use of antiretroviral drugs to prevent HIV transmission 22th Tunisian Congress of Infectious Diseases 2nd Congress of Federation of Arab Societies of Clinical Microbiology and Infectious Diseases
OCCURRENCE OF PNEUMOCYSTIS PNEUMONIA IN HIV-INFECTED PATIENTS AND THE INTERFERENCE OF THE HIGHLY ACTIVE ANTIRETROVIRAL THERAPY
 Received: August 24, 2007 Accepted: December 6, 2007 Abstract published online: December 17, 2007 Full paper published online: March 8, 2008 J. Venom. Anim. Toxins incl. Trop. Dis. V.14, n.1, p.152-160,
Received: August 24, 2007 Accepted: December 6, 2007 Abstract published online: December 17, 2007 Full paper published online: March 8, 2008 J. Venom. Anim. Toxins incl. Trop. Dis. V.14, n.1, p.152-160,
On behalf of the Infectious Diseases Society of America (IDSA), I offer testimony in
 Testimony of the Infectious Diseases Society of America (IDSA) on the Fiscal Year 2018 Department of Health and Human Services Budget Prepared for the U.S. House of Representatives Subcommittee on Labor-HHS-Education
Testimony of the Infectious Diseases Society of America (IDSA) on the Fiscal Year 2018 Department of Health and Human Services Budget Prepared for the U.S. House of Representatives Subcommittee on Labor-HHS-Education
Submitted electronically to:
 Submitted electronically to: ODOARRFI19@nih.gov Maureen Goodenow, PhD Associate Director of AIDS Research, NIH Director, Office of AIDS Research (OAR) National Institutes of Health 5601 Fishers Lane. Room
Submitted electronically to: ODOARRFI19@nih.gov Maureen Goodenow, PhD Associate Director of AIDS Research, NIH Director, Office of AIDS Research (OAR) National Institutes of Health 5601 Fishers Lane. Room
Research priorities in medical mycology
 Research priorities in medical mycology David W. Denning National Aspergillosis Centre University Hospital of South Manchester The University of Manchester Agenda How many patients are there with serious
Research priorities in medical mycology David W. Denning National Aspergillosis Centre University Hospital of South Manchester The University of Manchester Agenda How many patients are there with serious
To provide you with the basic concepts of HIV prevention using HIV rapid tests combined with counselling.
 Module 2 Integration of HIV Rapid Testing in HIV Prevention and Treatment Programs Purpose Pre-requisite Modules Learning Objectives To provide you with the basic concepts of HIV prevention using HIV rapid
Module 2 Integration of HIV Rapid Testing in HIV Prevention and Treatment Programs Purpose Pre-requisite Modules Learning Objectives To provide you with the basic concepts of HIV prevention using HIV rapid
Projected Demand for HIV Diagnostic Tests
 Projected Demand for HIV Diagnostic Tests John Stover, Yu Teng, Adebiyi Adesina, Eline Korenromp On behalf of the Diagnostics Forecasting Technical Working Group (CDC, CHAI, GFATM, PfSCM, UNAIDS, UNICEF,
Projected Demand for HIV Diagnostic Tests John Stover, Yu Teng, Adebiyi Adesina, Eline Korenromp On behalf of the Diagnostics Forecasting Technical Working Group (CDC, CHAI, GFATM, PfSCM, UNAIDS, UNICEF,
Global and National trends of HIV/AIDS
 Research and Review Insights Research Article ISSN: 2515-2637 Global and National trends of HIV/AIDS Hikmat Bahadur Raya* Lecturer, Department of Population Studies, Tribhuvan University, Terhathum M.
Research and Review Insights Research Article ISSN: 2515-2637 Global and National trends of HIV/AIDS Hikmat Bahadur Raya* Lecturer, Department of Population Studies, Tribhuvan University, Terhathum M.
TB & HIV CO-INFECTION IN CHILDREN. Reené Naidoo Paediatric Infectious Diseases Broadreach Healthcare 19 April 2012
 TB & HIV CO-INFECTION IN CHILDREN Reené Naidoo Paediatric Infectious Diseases Broadreach Healthcare 19 April 2012 Introduction TB & HIV are two of the leading causes of morbidity & mortality in children
TB & HIV CO-INFECTION IN CHILDREN Reené Naidoo Paediatric Infectious Diseases Broadreach Healthcare 19 April 2012 Introduction TB & HIV are two of the leading causes of morbidity & mortality in children
Tuberculosis-related deaths in people living with HIV have fallen by 36% since 2004.
 F A C T S H E E T 2014 GLOBAL STATISTICS People living with HIV In 2013, there were 35 [33.2 37.2 ] people living with HIV. - Since the start of the epidemic, around 78 [71 87 ] people have become infected
F A C T S H E E T 2014 GLOBAL STATISTICS People living with HIV In 2013, there were 35 [33.2 37.2 ] people living with HIV. - Since the start of the epidemic, around 78 [71 87 ] people have become infected
Guidance for Industry
 Guidance for Industry Clinical Considerations for Therapeutic Cancer Vaccines DRAFT GUIDANCE This guidance document is for comment purposes only. Submit comments on this draft guidance by the date provided
Guidance for Industry Clinical Considerations for Therapeutic Cancer Vaccines DRAFT GUIDANCE This guidance document is for comment purposes only. Submit comments on this draft guidance by the date provided
PRIORITIES FOR HIV/AIDS PROCUREMENT AND PRODUCT DEVELOPMENT
 PRIORITIES FOR HIV/AIDS PROCUREMENT AND PRODUCT DEVELOPMENT Dr Chewe Luo MMed (Paeds), Mtrop Paed, PhD Senior Adviser and Team Leader Country Programme Scale up HIV Section Programme Division UNICEF, NY
PRIORITIES FOR HIV/AIDS PROCUREMENT AND PRODUCT DEVELOPMENT Dr Chewe Luo MMed (Paeds), Mtrop Paed, PhD Senior Adviser and Team Leader Country Programme Scale up HIV Section Programme Division UNICEF, NY
Sysmex Educational Enhancement and Development No
 SEED Haematology No 1 2015 Introduction to the basics of CD4 and HIV Viral Load Testing The purpose of this newsletter is to provide an introduction to the basics of the specific laboratory tests that
SEED Haematology No 1 2015 Introduction to the basics of CD4 and HIV Viral Load Testing The purpose of this newsletter is to provide an introduction to the basics of the specific laboratory tests that
Assessing the Impact of HIV/AIDS: Information for Policy Dialogue
 Assessing the Impact of HIV/AIDS: Information for Policy Dialogue Timothy B. Fowler International Programs Center Population Division U.S. Census Bureau For presentation at the International Expert Group
Assessing the Impact of HIV/AIDS: Information for Policy Dialogue Timothy B. Fowler International Programs Center Population Division U.S. Census Bureau For presentation at the International Expert Group
Number of people receiving ARV therapy in developing and transitional countries by region,
 Progress in numbers The last six months have seen dramatic progress toward the 3 by 5 target. Between June and, the number of people receiving antiretroviral (ARV) therapy in developing and transitional
Progress in numbers The last six months have seen dramatic progress toward the 3 by 5 target. Between June and, the number of people receiving antiretroviral (ARV) therapy in developing and transitional
EDMA HIV-AIDS TEAM Fact Sheet November 2007
 EDMA HIV-AIDS TEAM Fact Sheet November 2007 1. HIV Facts AIDS epidemic update UNAIDS Epidemic Update, November 2007 (1) 760,000 people to be living with HIV in Western and Central Europe in 2007. 31,000
EDMA HIV-AIDS TEAM Fact Sheet November 2007 1. HIV Facts AIDS epidemic update UNAIDS Epidemic Update, November 2007 (1) 760,000 people to be living with HIV in Western and Central Europe in 2007. 31,000
INTERNATIONAL SOCIETY FOR HEART AND LUNG TRANSPLANTATION a Society that includes Basic Science, the Failing Heart, and Advanced Lung Disease
 International Society of Heart and Lung Transplantation Advisory Statement on the Implications of Pandemic Influenza for Thoracic Organ Transplantation This advisory statement has been produced by the
International Society of Heart and Lung Transplantation Advisory Statement on the Implications of Pandemic Influenza for Thoracic Organ Transplantation This advisory statement has been produced by the
In 2013, around 12.9 million people living with HIV had access to antiretroviral therapy.
 F A C T S H E E T 2014 GLOBAL STATISTICS People living with HIV In 2013, there were 35 [33.2 37.2 ] people living with HIV. - Since the start of the epidemic, around 78 [71 87 ] people have become infected
F A C T S H E E T 2014 GLOBAL STATISTICS People living with HIV In 2013, there were 35 [33.2 37.2 ] people living with HIV. - Since the start of the epidemic, around 78 [71 87 ] people have become infected
DEPARTMENT OF HEALTH AND HUMAN SERVICES. Health Resources and Service Administration. Women's Preventive Services Guidelines
 This document is scheduled to be published in the Federal Register on 02/27/2018 and available online at https://federalregister.gov/d/2018-03840, and on FDsys.gov Billing Code 4165-15 DEPARTMENT OF HEALTH
This document is scheduled to be published in the Federal Register on 02/27/2018 and available online at https://federalregister.gov/d/2018-03840, and on FDsys.gov Billing Code 4165-15 DEPARTMENT OF HEALTH
Proposed Data Collection Submitted for Public Comment and. AGENCY: Centers for Disease Control and Prevention (CDC),
 This document is scheduled to be published in the Federal Register on 06/01/2018 and available online at https://federalregister.gov/d/2018-11789, and on FDsys.gov BILLING CODE: 4163-18-P DEPARTMENT OF
This document is scheduled to be published in the Federal Register on 06/01/2018 and available online at https://federalregister.gov/d/2018-11789, and on FDsys.gov BILLING CODE: 4163-18-P DEPARTMENT OF
E/2001/23 E/CN.4/2001/167. COMMISSION ON HUMAN RIGHTS REPORT ON THE FIFTY-SEVENTH SESSION (19 March - 27 April 2001) ECONOMIC AND SOCIAL COUNCIL
 E/2001/23 E/CN.4/2001/167 COMMISSION ON HUMAN RIGHTS REPORT ON THE FIFTY-SEVENTH SESSION (19 March - 27 April 2001) ECONOMIC AND SOCIAL COUNCIL OFFICIAL RECORDS, 2001 SUPPLEMENT No. 3 UNITED NATIONS 1
E/2001/23 E/CN.4/2001/167 COMMISSION ON HUMAN RIGHTS REPORT ON THE FIFTY-SEVENTH SESSION (19 March - 27 April 2001) ECONOMIC AND SOCIAL COUNCIL OFFICIAL RECORDS, 2001 SUPPLEMENT No. 3 UNITED NATIONS 1
