REVIEW. Prevention of toxoplasmosis in transplant patients F. Derouin 1 and H. Pelloux 2, on behalf of the ESCMID Study Group on Clinical Parasitology
|
|
|
- Emil Dawson
- 6 years ago
- Views:
Transcription
1 REVIEW /j x Prevention of toxoplasmosis in transplant patients F. Derouin 1 and H. Pelloux 2, on behalf of the ESCMID Study Group on Clinical Parasitology 1 Laboratory of Parasitology and Mycology, University Paris and Hôpital Saint-Louis, Assistance Publique-Hôpitaux de Paris, and 2 Parasitology-Mycology laboratory, A Michallon teaching Hospital, and UMR CNRS-UJF 5163, Grenoble, France ABSTRACT Toxoplasmosis is a life-threatening opportunistic infection that affects haematopoietic stem cell transplant (HSCT) and solid organ transplant (SOT) recipients. Its incidence in these patients is closely related to the prevalence of toxoplasmosis in the general population, which is high in Europe. In SOT recipients, toxoplasmosis results mainly from transmission of the parasite with the transplanted organ from a Toxoplasma-seropositive donor to a Toxoplasma-seronegative recipient. This risk is high in cases of transplantation of organs that are recognized sites of encystation of the parasite, e.g. the heart, and is markedly lower in other SOT recipients. Clinical symptoms usually occur within the first 3 months after transplantation, sometimes as early as 2 weeks post transplant, and involve febrile myocarditis, encephalitis or pneumonitis. In HSCT recipients, the major risk of toxoplasmosis results from the reactivation of a pre-transplant latent infection in seropositive recipients. The median point of disease onset is estimated at 2 months post transplant, with <10% of cases occurring before 30 days and 15 20% later than day 100. Toxoplasmosis usually manifests as encephalitis or pneumonitis, and frequently disseminates with multiple organ involvement. Diagnosis of toxoplasmosis is based on the demonstration of parasites or parasitic DNA in blood, bone marrow, cerebrospinal fluid, bronchoalveolar lavage fluid or biopsy specimens, and serological tests do not often contribute to the diagnosis. For prevention of toxoplasmosis, serological screening of donors and recipients before transplantation allows the identification of patients at higher risk of toxoplasmosis, i.e. seropositive HSCT recipients and mismatched (seropositive donor seronegative recipients) SOT recipients. Preventing toxoplasmosis disease in those patients presently relies on prophylaxis via prescription of co-trimoxazole. Keywords Prevention, Toxoplasma gondii, toxoplasmosis, transplantation Accepted: 16 August 2008 Clin Microbiol Infect 2008; 14: INTRODUCTION Toxoplasma gondii is a protozoan parasite with a worldwide prevalence in the general population that exceeds 50% in some countries. Human infection is acquired mainly by ingestion of undercooked infected meat containing Toxoplasma cysts or by ingestion of oocysts from faecally contaminated foods or via poor hand hygiene. In immunocompetent individuals, acute infection is usually self-limited and rarely symptomatic, Corresponding author and reprint requests: Francis Derouin, Laboratoire de Parasitologie-Mycologie, Hôpital Saint-Louis, 1 avenue Claude Vellefaux, Paris Cedex 10, France francis.derouin@sls.aphp.fr although some cases of severe infection due to unusual Toxoplasma genotypes have been reported in South and Central America [1]. The acute phase of infection is followed by a latent chronic phase that is characterized by the life-long persistence of cysts in tissues. The containment of cysts by specific immunity is a determiming factor for this latency, as it has been shown experimentally that depleting cellular immunity or cytokine mediators of macrophage activation results in the reactivation of chronic toxoplasmosis [2]. In humans, the relationship between immunosuppression and occurrence of severe toxoplasmosis is well recognized. In human immunodeficiency virus (HIV)-infected patients, the incidence of toxoplasmic encephalitis is Journal Compilation Ó 2008 European Society of Clinical Microbiology and Infectious Diseases
2 1090 Clinical Microbiology and Infection, Volume 14 Number 12, December 2008 closely related to the progression of immunodeficiency and a decrease in CD4 counts [3,4], whereas restoration of immunity following highly active antiretroviral therapy markedly decreases this incidence [5]. This relationship supports the rationale for preventing toxoplasmosis in HIV-infected patients via specific prophylaxis in patients with CD counts <100 mm 3 and by initiating highly active antiretroviral therapy to restore cellular immunity [6,7]. In non-aids immunocompromised patients, the risk of severe toxoplasmosis is also well recognized. In an extensive review of the literature, Israelki and Remington [8] identified 212 cases between 1953 and 1993, highlighting the high frequency of life-threatening toxoplasmosis in organ transplant patients. Since then, the practice of solid organ and bone marrow transplantation has markedly progressed, and the conditions of transplantation, as well as measures undertaken for prevention of infection, have improved. Taking into consideration what has been learned from the management of toxoplasmosis in AIDS patients, and considering the new methods for diagnosis of toxoplasmosis, the objective of this review is to consider the specific features of toxoplasmosis in transplant patients, focusing on practical approaches to diagnosis and prevention. PATHOGENESIS OF TOXOPLASMOSIS IN TRANSPLANT RECIPIENTS In transplant recipients, toxoplasmosis can result either from the transmission of the parasite with the transplanted organ from a Toxoplasma-seropositive donor (D + )toatoxoplasma-seronegative recipient (R ) ) or from the reactivation of a pretransplant latent infection in a seropositive recipient (R + ). Transmission of T. gondii via organ transplantation from a seropositive donor to a seropositive recipient seems to be possible. Toxoplasma transmission by blood transfusion is extremely rare. Graft-transmitted toxoplasmosis A clear differentiation should be made between solid organ transplantation (SOT), during which the risk of transmission is potentially high, and allogeneic haematopoietic stem cell transplantation (HSCT), during which this risk is more theoretical than real (Table 1). Transmission of T. gondii from a recently infected donor to a seronegative recipient. This risk of transmission is related to the possible presence of T. gondii in the blood and tissue of a donor who had been infected by T. gondii several days or weeks before blood tissue collection. In that case, Table 1. Summary of risk factors for toxoplasmosis in transplant patients Type of risk Clinical disease and prevention Transmission Reactivation Onset of symptoms Efficient prophylaxis Haematologous stem cell transplantation Autologous No risk Very low in R days ND Allogenic a Possible but no case reported Solid organ transplantation Heart heart lung b Liver c Kidney c Intestine c Other (eye, bone, artery) High in R +, regardless of donor s serology. Increased risk in cord blood SCT Median: 62 days. 80% of cases in the second and third months post-hcst CTX for pneumocystosis and toxoplasmosis. Alternatively, pyrimethamine sulphadoxine. Given from day 30 to day 180 after SCT. Prolonged or restarted in cases of GVHD or sustained immunosuppression High in mismatched D + R ) Low in R days CTX given for pneumocystosis Low in mismatched Low in R + Median: 24 days in CTX given for pneumocystosis D + R ) D + R ) ; up to 6 years in R + Low in mismatched Low in R + Median: 19 days in ND D + R ) D + R ), up to 2 months in R + Low in mismatched ND 1 case, month 3 ND D + R ) No risk No risk D, donor; R, recipient; +, positive pretransplant Toxoplasma serology; ), negative pretransplant Toxoplasma serology; ND, not documented; CTX, co-trimoxazole; SCT, stem cell transplant; HCST, haematopoietic stem cell transplant; GVHD, graft-versus-host disease. a Adapted from references [12,42,45 47]. b Adapted from reference [25]. c Adapted from reference [32].
3 Derouin and Pelloux Toxoplasmosis in transplant patients 1091 tachyzoites of T. gondii (i.e. the rapidly dividing stage of the parasite) may be present in the blood, bone marrow and in various organs, as the early phase of infection is characterized by a brief parasitaemia. Several cases of transmission of T. gondii to SOT patients who received organs from a single donor whose Toxoplasma serology was indicative of a recently acquired toxoplasmosis have been reported [9,10]. In HSCT, there is a potential risk of transmission if the donor is parasitaemic at the time of bone marrow collection, but, to our knowledge, it has never been confirmed, although it was suspected in several cases occurring in pre-transplant seronegative recipients [11,12]. The duration of the potential risk of transmission after an acquired infection of the donor is not known. In animal models of toxoplasmosis, parasitaemia can be detected up to 3 weeks following oral contamination by T. gondii cysts [13]. In humans, the precise duration of parasitaemia is not known. One study reported isolation of T. gondii by mouse inoculation 2 and 14 months after onset of toxoplasmosis [14]. In a more recent study using nested PCR, 53% of the patients with acute toxoplasmic lymphadenopathies had a detectable parasitaemia 5 weeks after the onset of symptoms [15]. Transmission from a chronically infected donor to a seronegative recipient. At the chronic stage of toxoplasmosis, tissue cysts are formed in the neural and muscular tissues, including the brain, eyes, skeletal muscles and cardiac muscles, but may also develop in visceral organs, including the lungs, liver and kidneys [16,17]. Their size range between lm in diameter, and they contain hundreds to thousands of bradyzoites (i.e. the slowly dividing stage of the parasite). It is believed that tissue cysts can occasionally rupture without any clinical symptoms in immunocompetent hosts, the released bradyzoites being destroyed by the specific anti-toxoplasma immunity [18]. Therefore, some organs taken from a donor who has been previously infected by T. gondii, even years before, probably contain cysts. Without specific immunity to T. gondii (seronegative recipient) and in the context of immunosuppressive therapy for transplant engraftment and tolerance, cysts present in the transplanted organ may be uncontrolled and may rupture, leading to an active infection. This risk is predominant in cases of transplantation of organs that are recognized sites of encystation, such as the heart (striated muscle), and is markedly lower when other organs are involved. It is estimated that there is no risk of cyst transmission with haematopoietic stem cells. Heart and heart lung transplantation Since the initial reports in the 1970s [19,20], most cases of toxoplasmosis in heart and heart lung transplantation have been reported in seronegative recipients receiving a heart transplant from a seropositive donor [8,21 27]. Clinical symptoms usually occur in the first 3 months after transplantation (sometimes as early as 2 weeks post transplant) and consist of febrile myocarditis, encephalitis or pneumonitis [8,22,25]. The true incidence of toxoplasmosis in cases of mismatch (D + R ) ) is not precisely known but can reach 25 75% in the absence of prophylaxis (Table 2). Administration of co-trimoxazole for prophylaxis of pneumocystosis proved efficient in preventing toxoplasmosis in cases of mismatch patients [28,29]. Non-cardiac SOT Cases of organ-transmitted toxoplasmosis are much less frequent with organs other than the heart [8,30,31]. In a recent review of the literature covering 40 years, Cambpell et al. [32] identified 52 cases of toxoplasmosis in non-cardiac SOT recipients (kidney, 34; liver, 12; pancreas, one; multivisceral, four; small bowel, one), i.e. a very low risk of toxoplasmosis in light of the number of such transplantations performed each year throughout the world. In that review, the serological status of donors and recipients was available for patients, with a D + R ) mismatch in 16 cases. In these patients, toxoplasmosis manifested early after transplantation, with a median time from transplant to symptoms of 21 days (range days). In most cases, serological data were insufficient to determine whether transmission from a recently infected donor could have occurred. However, it is estimated that the risk of transmission of T. gondii cysts with liver, kidney, pancreas or intestine from a chronically infected donor is very low, because T. gondii is not believed to form persistent cysts in these organs [32,33].
4 1092 Clinical Microbiology and Infection, Volume 14 Number 12, December 2008 Table 2. Reported prevalence of organ-transmitted and reactivated toxoplasmosis in heart and heart lung transplant recipients in various countries Transmission in seronegative recipients Reactivation in seropositive recipients Country Number of seronegative recipients Number of D + R ) mismatches Clinical toxoplasmosis (prevalence) Number of seropositive recipients Serological reactivations (prevalence) Clinical toxoplasmosis Reference USA 509 (84%) 32 4 (12.5%; 25% in the 98 (16%) ND 0 98 Montoya et al. [27] 16 patients without prophylaxis) USA 31 (62%) 4 3 (75%) 19 (38%) (52.6%) 0 Luft et al. [21] Switzerland 52 (43%) 18 3 (16.7%) 69 (57%) 5 69 (7.2%) 2 5 Gallino et al. [24] UK 175 (70%) 21 6 (28.5%; 57% in the 75 (30%) 3 75 (4%) 2 3 Wregitt et al. [22] four of seven patients without prophylaxis) France 23 (36%) ND 0 42 (64%) 10 (23.8%) 0 Lavarde et al. [59] D +, seropositive donor; R ), seronegative recipient; ND, not documented. Transmission of T. gondii from a seropositive donor to a seropositive recipient. Cases of severe toxoplasmosis have also been observed in seropositive recipients receiving a heart transplant from a seropositive donor [34], but this is far less frequent than in cases of mismatch (D + R ) ), supporting the notion that recipient immunity is protective against the transmission of T. gondii. In this case, graft transmission is difficult to confirm and to differentiate from a reactivation of latent infection in the recipient. However, the hypothesis that a seropositive recipient could be reinfected by a transplant from a seropositive donor has been suggested by Robert-Gangneux et al. [35]. In this study, western blot analysis of posttransplant sera of seropositive recipients showed neosynthesized IgG that could be related to the recognition of a new parasite strain, possibly acquired via the transplanted organ from a Toxoplasma-seropositive donor. The only means of proving this re-infection would be to identify the infecting strain(s), by serotyping or genotyping [36,37]. Reactivation of a latent toxoplasmosis The reactivation of a previously acquired and chronic infection is the main cause of toxoplasmic encephalitis in AIDS patients, and it has been closely related to the severity and progression of immunosuppression. The precise factors that trigger tissue cyst rupture and reactivation are largely unknown, but the risk of occurrence of toxoplasmic encephalitis sharply increases when the blood CD4 count is below 200 CD4 mm 3. In transplant recipients, this risk of reactivation also exists for recipients who are seropositive for Toxoplasma. It is closely related to the degree and duration of immunodeficiency and, therefore, markedly differs according to the type of transplantation (Table 1). Moreover, the incidence rate of Toxoplasma reactivation in transplant patients varies according to countries, as it is closely related to the prevalence of toxoplasmosis in the general population. HSCT recipients. Allogeneic HSCT recipients are at very high risk, whereas toxoplasmosis is a rare event in autologous HSCT recipients [38 41]. Allogeneic HSCT recipients are also at much higher risk than SOT recipients, because of the severity of duration of immunosuppression experienced by these patients. The main risk factors for toxoplasmosis in HSCT recipients, identified in a recent prospective study, were: cord blood transplantation (as opposed to other sources of stem cells), advanced underlying disease, antithymocyte globulin during a conditioning regimen, receipt of an organ from a seronegative donor, and lack of appropriate prophylaxis. In multivariate analysis, only cord blood transplantation, which is associated with a very severe and protracted immunocompromised status, retained statistical significance [42]. From several reviews and case reports [12,42 48], the median time to disease onset can be estimated at 2 months post-hsct, with fewer than 10% of cases occurring before 30 days and 15 20% later than day 100 (Fig. 1). Therefore, the risk of Toxoplasma reactivation in seropositive recipients is particularly high during the 2 4 months post transplant. It can be prolonged to 6 months, and is even longer in cases of delayed immune reconstitution and occurrence of graft-versus-host disease.
5 Derouin and Pelloux Toxoplasmosis in transplant patients Number of cases Fig. 1. Onset of toxoplasmosis in 81 allogeneic stem cell transplant (SCT) recipients; adapted from references [12,42,45 47] >26 Weeks post-sct The extreme severity of the disease is highlighted in most studies and case reports, and the mortality rate is high. Toxoplasmic encephalitis presenting with brain abscesses is the most frequent initial presentation [48,49], but diffuse brain lesions and meningitis have also been reported [45,50,51]. Pneumonitis, including acute distress syndrome and disseminated toxoplasmosis with multiple organ involvement, is frequently reported in autopsies [12,52,53], and has been associated with a haemophagocytic syndrome in some cases [54]. The true incidence of toxoplasmosis in HSCT recipients is difficult to estimate, because diagnosis is difficult, and autopsy records indicate that cases might have been misdiagnosed. The reported incidence markedly varies from 0.2% to 4% according to HSCT centre, with higher rates of incidence being seen in countries where the prevalence of toxoplasmosis is high in the general population. However, if only the seropositive recipients are considered, the range of incidences among countries is more narrow ( %) (Table 3) [40,42,44 47,55,56]. It has been suggested that the serological status of the donor could influence the risk of occurrence of reactivation, as approximately 80% of reported cases of toxoplasmosis are observed in patients whose donor was seronegative for T. gondii (D ) R + ) [42,43,45]. However, the protective function of the grafted cell from a seropositive donor is very uncertain, as the immune reconstitution of the recipient by the donor s cell is delayed and incomplete. Thus, even if a D ) R + mismatch represents a potentially greater risk factor, any seropositive recipient should be considered to be at high risk of reactivation. SOT recipients. Reactivation of latent Toxoplasma infections occurs in SOT seropositive recipients, but is far less frequent and less severe than in HSCT recipients [57]. In cases of heart and heart lung transplantation, the rates of reactivation markedly vary according to country and transplant centre; reactivation is mainly serological, with clinical symptoms of toxoplasmosis manifesting occasionally [21,22,24,27,58,59] (Table 2). Table 3. Reported prevalence of toxoplasmosis in haematopoietic stem cell transplant recipients in various countries Country Number of patients Toxoplasma seroprevalence (number of seropositive patients) Clinical toxoplasmosis Number of cases Prevalence (%) global/in seropositive patients Reference Japan % a (93) Matsuo et al. [40] USA % b (570) Slavin et al. [46] Brazil % b (472) De Meddeiros et al. [47] France % b (201) Derouin et al. [45] Germany 75 71% (53) Janitschke et al. [56] France % (106) c 6 ND 5.7 Martino et al. [42] ND, not documented. a Estimated prevalence in Japan provided in [40]. b Estimated from a fraction of patients. c Study restricted to Toxoplasma gondi-seropositive patients.
6 1094 Clinical Microbiology and Infection, Volume 14 Number 12, December 2008 In non-cardiac SOT recipients, clinical toxoplasmosis is a rare event. In a recent extensive review of the literature, Campbell et al. recorded 34 cases of clinical toxoplasmosis in kidney transplant recipients, of which eight could be related to reactivation [32]. Among liver transplant recipients, three cases of toxoplasmosis related to reactivation have been reported. Asymptomatic serological reactivations might be much more frequent, as observed in seven of 49 seropositive renal transplant recipients and eight of 25 liver transplant recipients in two French centres [60,61]. Considering the number of SOTs performed throughout the world, the number of reported cases of clinical toxoplasmosis is very low. However, clinicians should be aware of the risk in seropositive recipients, especially those receiving aggressive immunosuppressive therapy. In these patients, the risk of re-infection has also been hypothesized but needs to be further assessed by strain genotyping. DIAGNOSIS The diagnosis of toxoplasmosis in transplant recipients is often difficult. From a clinical point of view, the features of reactivated or transmitted toxoplasmosis are not specific, with fever being the main sign, associated with various other signs that are dependent on the dissemination of the parasite. Imaging by computed tomography, or preferably by magnetic resonance imaging, provides strong support for a diagnosis of toxoplasmic encephalitis. Focal necrosis is the most common presentation, with single or multiple contrast-enhancing lesions with hypodensities, surrounded by oedema; a mass effect may be present, with displacement of the ventricle (Fig. 2). However, as stated earlier, lesions may be non-focal (diffuse encephalitis) or atypical. Pulmonary toxoplasmosis usually manifests as interstitial pneumonitis with alveolar condensations, and is frequently associated with signs of dissemination and with multiple organ involvement or failure. The serological diagnosis of toxoplasmosis has important limitations, as the underlying immunosuppression alters antibody production and its kinetics. The demonstration of parasites in blood, body fluids and tissues is the mainstay of diagnosis, providing definitive proof of the disease. Fig. 2. Source: CD-ROM ANOFEL 3 and courtesy of Professor J. Frija, Service de Radiologie, Hôpital Saint- Louis, Paris, France. One must not forget that the demonstration of tachyzoites by microscopic examination of Giemsa-stained smears of bone marrow aspirates or bronchoalveolar lavage fluid remains the simplest and most rapid means of diagnosis of disseminated toxoplasmosis. Diagnosis can also rely on the demonstration of tachyzoites and cysts in haematoxylin- or Giemsa-stained tissue biopsy specimens, preferentially using immunohistochemistry [62,63]. Because of the low sensitivity of direct microscopic methods, enrichment methods should be used in association with microscopy. Mouse inoculation is the reference technique, allowing strain isolation and possible genotyping, but a definitive result can only be obtained 4 weeks post inoculation. Cell culture offers the possibility of reducing this delay, but is no longer used for diagnosis, since the development of DNA-based diagnostic methods. PCR techniques, the most sensitive of which involve the repeated B1 and AF gene targets, have markedly changed the strategy for diagnosis and treatment of transplant recipients [42,64 68]. Moreover, the use of real-time PCR avoids the risk of false positivity due to DNA carryover [65], and allows quantification of T. gondii DNA to monitor treatment efficacy [50]. In 2000, the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation defined probable toxoplasmosis as the observation of clinical and radiological evidence suggestive of organ involvement plus at least one positive PCR result from blood, CSF, or BAL, but no histologic confirmation and absence of another pathogen that may explain the
7 Derouin and Pelloux Toxoplasmosis in transplant patients 1095 findings [49]. The existence of a positive PCR result from blood, without evidence of organ involvement, should be considered as only a possible diagnosis of toxoplasmosis. A negative PCR result has a good negative predictive value, as demonstrated by the results of the large prospective study by Martino et al. [42], in which none of the stem cell transplant recipients for whom PCR from blood was negative developed toxoplasmosis. However, a negative PCR result cannot rule out the diagnosis of toxoplasmosis, as cases of toxoplasmosis have also been reported with negative PCR results from blood [63,65]. The negative predictive value of PCR results from blood might also be dependent on the timing and the repetition of blood sampling. Diagnosis of organ-transmitted infection. Organtransmitted infection can be suspected in cases of serological mismatch (D + R ) ). A seroconversion occurring early after transplant, with demonstration of IgM and IgG antibodies, and eventually IgA and IgE [69], is a strong indication of an acquired (and probably transmitted) infection with subsequent risk of disease. In cases of profound immunosuppression, the antibody response might be lacking or atypical, and diagnosis can be based only on the demonstration of parasites or parasite DNA in blood, body fluids or biopsy specimens, guided by clinical symptoms [67]. In HSCT recipients, seroconversion with the appearance of IgG and IgM antibodies has rarely been reported, whereas a transient increase of IgG antibodies at a low titre (<100 IU ml and without IgM) is more frequent and probably related to passive antibody transmission via the graft or blood transfusions [55]. Diagnosis of reactivation. A reactivation should be suspected upon observation of a rise in specific antibody titres, usually IgG antibodies without IgM or IgA antibodies [70]. IgG antibodies produced during reactivation have a high avidity index, indicating an immune recall rather than a primary immune response [71]. The appearance of IgM antibodies is occasionally observed in HSCT recipients, possibly as a primary immune response of grafted cells from seronegative donors. However, there is no clear relationship between serological reactivation in a seropositive recipient and clinical disease. Among cardiac transplant recipients, clinical symptoms indicative of toxoplasmosis have been reported at the time of antibody increase in two of five patients by Gallino et al. [24] and two of four patients by Wregitt et al. [22], but were not reported in other studies in which high rates of serological reactivation have been observed [21,59] (Table 2). Among renal transplant recipients, an antibody increase was observed in 5 10% of patients and was not associated with, or followed by, clinical symptoms indicative of toxoplasmosis [60,72]. Among HSCT recipients, serological reactivations that occur late after transplantation (10 18 months post-hsct) are frequent and usually asymptomatic [55]. The diagnosis of reactivation is even more difficult in patients who develop clinical toxoplasmosis when antibody titres remain stable after transplant. This is the most frequent situation in HSCT recipients. In these cases, no conclusion can be drawn from the results of serological tests, and diagnosis must be based on imaging and the demonstration of parasites or parasitic DNA. As stated earlier, the proof of infection is provided only by the demonstration of tachyzoites in blood, bone marrow, bronchoalveolar lavage fluid, cerebrospinal fluid or tissue biopsy specimens using microscopy, mouse inoculation or PCR techniques. Indeed, a positive PCR result is indicative of reactivation, but should be interpreted in the context of clinical symptoms and with the understanding that asymptomatic transient positive reactions can be observed after HSCT [64]. PREVENTION AND PROPHYLAXIS OF TOXOPLASMOSIS IN TRANSPLANT RECIPIENTS Toxoplasmosis represents a major infectious complication in transplant recipients that should be prevented, and three complementary approaches to prevention can be proposed. Serological screening of donor and recipients The determination of the Toxoplasma serological status of donors and recipients provides critical information concerning the potential risk of toxoplasmosis following transplantation. This test is inexpensive, and allows discrimination among three categories of risk: (i) the potential risk of
8 1096 Clinical Microbiology and Infection, Volume 14 Number 12, December 2008 transmission in SOT recipients in cases of mismatch (D + R ) ) and in seronegative HSCT recipients in cases of recently acquired infection of the donor; (ii) the risk of reactivation for seropositive HSCT and SOT recipients (independent of the serology of the donor); and (iii) the low risk of toxoplasmosis if both donor and recipient are seronegative. In the latter case, the only risk is contamination via ingestion of cysts with undercooked meat or oocysts with insufficiently washed foods, or via poor hand hygiene. In Europe, Toxoplasma serology is undertaken according to the practice of the respective transplant center (ESGP information). In some countries, e.g. France, the test for Toxoplasma is included among the serological tests legally required for every organ donor and is strongly recommended for recipients prior to transplantation. Among 29 countries participating in the Eurotransplant network, Toxoplasma serology is routinely undertaken for SOT donors in 11 and is undertaken according to the characteristics of the donor in ten [73]. In the USA, serological screening of donors is not mandatory, and routine screening of donors and recipients largely depends on the transplant centre (J. S. Remington, personal communication). Recent publications indicate that routine screening may be unnecessary because, for years, no case of toxoplasmosis has been observed among heart transplant recipients treated prophylactically with co-trimoxazole for prevention of pneumocystosis [29,74]. Indeed, the remarkable prophylactic efficacy of co-trimoxazole must be acknowledged, but there is a potential risk of toxoplasmosis if co-trimoxazole prophylaxis is suspended or replaced by pentaminide prophylaxis, which has no effect on T. gondii. Owing to the low cost of the serological test and the benefit provided in terms of risk management, we propose that a serological test should be performed in all transplant donors and recipients. In cases of HSCT, the serological status of both donor and recipient should be determined before transplantation, because of the early and high risk of toxoplasmosis following transplantation. In cases of SOT, which involves a lower risk of toxoplasmosis, a possible alternative would be to store both donor and recipient pre-transplant sera and perform serological testing only in cases of clinical suspicion of toxoplasmosis [74]. Serological and PCR follow-up of patients after transplant A serological follow-up is informative in cases of SOT mismatched recipients (D + R ) ), as the observation of a seroconversion clearly indicates transmission of T. gondii. In other SOT and HSCT recipients, such follow-up has little benefit, as an antibody increase does not reliably indicate a progressive disease. A PCR follow-up of patients at risk might be more informative, as a positive PCR result can be an early sign of disease [42,64,75]. However, because of the high cost of PCR testing, the indications and the timing of PCR follow-up should probably be restricted to defined high-risk periods and to patients who do not receive prophylaxis. This follow-up recommended in Toxoplasma-seropositive HSCT recipients who do not tolerate co-trimoxazole (or other anti-toxoplasma drugs) and thus receive pentamidine for prevention of pneumocystosis, and recipients of unrelated cord blood transplants, because of their high risk of reactivating toxoplasmosis. Chemoprophylaxis Considering the severity and rapid progression of toxoplasmosis in SOT and HSCT recipients, administration of primary chemoprophylaxis to high-risk patients should be considered. The ideal drug for prophylaxis would be able to eradicate cysts, would be preferentially parasiticidal against the different parasitic stages, would be well distributed in the main sites of infection, and would be well tolerated. No such drug exists. Presently, there is no drug with proven efficacy against tissue cysts. Atovaquone has been found to reduce the number of brain cysts in chronically infected mice [76], but this effect has not been (and probably cannot be) confirmed in humans. Other drugs are effective only on the tachyzoite, which is the highly replicative stage of the parasite that is present at the acute phase of infection and during reactivation. Among these drugs, folate inhibitors (dihydrofolate reductase inhibitors, sulphonamides) as well as atovaquone are strongly parasiticidal and are rapidly effective against tachyzoites in vitro and in vivo. Macrolides (spiramycine, clarithromycin, roxithromycin, azithromycin) and related drugs
9 Derouin and Pelloux Toxoplasmosis in transplant patients 1097 (clindamycin) have a delayed antiparasitic effect and are not adapted for the containment of an active infection [77]. The possible innate resistance of some genotypes of T. gondii and the risk of selecting drug-resistant parasites via long-term prophylaxis have been suggested for T. gondii, as for Pneumocystis and Plasmodium. Drug-resistant isolates of T. gondii can be obtained in vitro by mutagenesis, but there is no documented proof of such selection in humans. Recently, the study of 17 strains (15 of human origin) belonging to various genotypes of T. gondii revealed similar susceptibilities to pyrimethamine and atovaquone, whereas three strains were resistant to sulphadiazine, without evidence of mutation on the target dyhidropteroate synthase (DHPS) gene, or a relationship with the parasite genotype [78]. Presently, the combination of a dihydrofolate reductase inhibitor and a sulphonamide is the most efficient prophylaxis for toxoplasmosis, as both compounds are highly synergistic against T. gondii. Pyrimethamine alone has been proposed for prophylaxis for toxoplasmosis in heart transplant recipients [22,26], but this drug is at least ten-fold less efficient alone than in combination with a sulphonamide. Two drug combinations proved efficient in preventing toxoplasmosis in transplant recipients: trimethoprim sulphamethoxazole (co-trimoxazole) [29,74,79,80] and pyrimethamine sulphadoxine [80]. Both combinations have undesirable side effects, related to sulphonamides (skin rashes, nephrotoxicity and, rarely, Lyell syndrome with pyrimethamine sulphadoxine) and related to the folate inhibitor (myelotoxicity). Myelotoxicity can usually be prevented by concomitant administration of folinic acid (leucovorin) and adaptation of the dosage to the renal clearance. Atovaquone alone proved efficient for secondary prophylaxis of toxoplasmosis in AIDS patients [81], and proved to be both efficient and well tolerated in autologous HSCT recipients for prevention of pneumocystosis [82]. However, its use as primary prophylaxis for toxoplasmosis is limited by its poor intestinal absorption and risk of failure under therapy [83]. Prophylaxis in HSCT recipients. Prophylaxis prior to transplantation is recommended for all recipients who are seropositive for Toxoplasma. Co-trimoxazole is the recommended drug in the USA [84,85] and the most widely used drug in European HSCT centres [44]. Pyrimethamine sulphadoxine is preferred in some centres [80], as it can be administered weekly rather than oncedaily or two or three times weekly, which is the regimen for co-trimoxazole. For patients who are intolerant to co-trimoxazole, a combination of clindamycin, pyrimethamine and leucovorin has been proposed [84], but this combination exposes the patient to myelosupression. Atovaquone could be a safe alternative, but its efficacy has not been evaluated. There is no consensus concerning initiation and duration of prophylaxis. Starting prophylaxis immediately after HSCT exposes the patient to some degree of toxicity with regard to grafted cells and delayed engraftment, whereas delaying prophylaxis places the patient at risk for toxoplasmosis. A delay of 30 days after HSCT before initiation of prophylaxis seems reasonable, as 90% of the cases of toxoplasmosis occur 30 days post-hsct. If delayed prophylaxis is preferred, a weekly follow-up of the recipient using PCR with peripheral blood is recommended for high-risk patients during the entire period without prophylaxis. With regard to the incidence rate of toxoplasmosis following HSCT, prophylaxis should be maintained for 6 months post-hsct. It should be prolonged in cases of graft-versus-host disease, prolonged neutropenia and prolonged administration of corticosteroids. If prophylaxis must be suspended because of drug intolerance, a close follow-up by PCR should be instituted. HSCT recipients who are seronegative are at very low risk of acquiring toxoplasmosis, but they should be advised of the risk of acquiring toxoplasmosis via ingestion of undercooked meat (possibly containing cysts) or raw vegetables (possibly contaminated by oocysts). A low but potential risk of transmission via the stem cell graft from a recently infected donor should be taken into account if the donor s serology shows a pattern of recently acquired infection, i.e. presence of IgM, low avidity of IgG antibodies, and increasing IgG titre. In this case, the proposed procedure is: (i) to treat the donor, if possible, using a short course of drugs that rapidly eradicate parasitaemia, e.g. pyrimethamine combined with sulphadiazine; (ii) to assess the collected haematopoietic stem cells by PCR; and (iii) to
10 1098 Clinical Microbiology and Infection, Volume 14 Number 12, December 2008 initiate prophylaxis in the HSCT recipient following transplantation [86]. Prophylaxis in SOT recipients. The risk of transmission of T. gondii is almost restricted to mismatched patients, i.e. D + R, and is limited to a several-month period post transplantation. Co-trimoxazole is the drug of choice for prophylaxis. Its efficacy has been established in studies in which it was used for prophylaxis of pneumocystosis [29,74]. In cases of intolerance to sulphonamides, a possible alternative is the use of pyrimethamine alone [22,26]. The risk of reactivation of a latent toxoplasmosis is very low in SOT seropositive recipients, and does not justify a specific prophylaxis regimen in addition to that for pneumocystosis. However, the potential risk of reactivation of toxoplasmosis should not be ignored in patients who are no longer receiving prophylaxis for pneumocystosis. CONCLUSION Toxoplasmosis remains one of the most severe opportunistic infections occurring after transplantation, with a high mortality rate in cases of delayed diagnosis. It should be stressed that at least 75% of the cases occur in patients who have not received prophylaxis, emphasizing the need to make available updated information concerning the pathogenesis of toxoplasmosis, the procedures for identification of risk factors and the measures for prevention. The combination of serological screening of donors and recipients, chemoprophylaxis in patients susceptible to reactivation or exposed to organ transmission of T. gondii and PCR follow-up of high-risk patients should be effective in reducing the occurrence of toxoplasmosis in transplant recipients. ACKNOWLEDGEMENTS We thank the members of the executive committee of the ESCMID Study Group on Clinical Parasitology (B. Evengård, E. Petersen, F. Peyron, A. Mathis, T. van Gool, P. Chiodini, M. Junie, L. Kortbeek) for their support in preparing this article. We thank J. S. Remington for providing information on serological screening of donors and recipients in the USA, P. Ribaud, J. P. Brion and H. Talabani for critical review of the manuscript, A. Sulahian for English revision of the manuscript, and M. F. Le Gall for helpful secretarial assistance. We are indebted to the Association Française des Enseignants de Parasitologie et Mycologie Médicales (ANOFEL) for providing the cerebral toxoplasmosis magnetic resonance image (Fig. 1), extracted from the educational CD-ROM ANOFEL 3. This work was presented in part during the ESGT Educational Workshop at the 17th ECCMID 25th ICC 2007, 31 March to 3 April 2007, Munich, Germany. TRANSPARENCY DECLARATION The authors have no relationship that may constitute a conflicting or dual interest. REFERENCES 1. Demar M, Ajzenberg D, Maubon D et al. Fatal outbreak of human toxoplasmosis along the Maroni river: epidemiological, clinical, and parasitological aspects. Clin Infect Dis 2007; 45: e88 e Hunter CA, Remington JS. Immunopathogenesis of toxoplasmic encephalitis. J Infect Dis 1994; 170: Raffi F, Aboulker JP, Michelet C et al. A prospective study of criteria for the diagnosis of toxoplasmic encephalitis in 186 AIDS patients. The BIOTOXO Study Group. AIDS 1997; 11: Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis 1992; 15: Abgrall S, Rabaud C, Costagliola D. Clinical Epidemiology Group of the French Hospital Database on HIV. Incidence and risk factors for toxoplasmic encephalitis in human immunodeficiency virus-infected patients before and during the highly active antiretroviral therapy era. Clin Infect Dis 2001; 33: Kaplan JE, Masur H, Holmes KK, USPHS; Infectious Disease Society of America. Guidelines for preventing opportunistic infections among HIV-infected persons Recommendations of the US Public Health Service and the Infectious Diseases Society of America. MMWR Recomm Rep 2002; 51: Yeni P. Prise en charge médicale des personnes infectées par le VIH. Rapport Recommandations du groupe d experts.. Paris: Flammarion, 2006; Israelski DM, Remington JS. Toxoplasmosis in the non-aids immunocompromised host. In: Remington J, Swartz M, eds. Current Clinical Topics in Infectious Diseases. London: Blackwell Scientific Publications, 1993; Rogers NM, Peh CA, Faull R, Pannell M, Cooper J, Russ GR. Transmission of toxoplasmosis in two renal allograft recipients receiving an organ from the same donor. Transpl Infect Dis 2008; 10: Renoult E, Biava MF, Hulin C, Frimat L, Hestin D, Kessler M. Transmission of toxoplasmosis by renal transplant: a report of four cases. Transplant Proc 1996; 28: Jurges E, Young Y, Eltumi M et al. Transmission of toxoplasmosis by bone marrow transplant associated with Campath-1G. Bone Marrow Transplant 1992; 9: Chandrasekar PH, Momin F. Disseminated toxoplasmosis in marrow recipients: a report of three cases and a review of the literature. Bone Marrow Transplant Team. Bone Marrow Transplant 1997; 19: Paugam A, Dupouy-Camet J, Sumuyen MH, Romand S, Lamoril J, Derouin F. Detection of Toxoplasma gondii parasitemia by polymerase chain reaction in perorally infected mice. Parasite 1995; 2:
11 Derouin and Pelloux Toxoplasmosis in transplant patients Miller MJ, Aronson WJ, Remington JS. Late parasitemia in asymptomatic acquired toxoplasmosis. Ann Intern Med 1969; 71: Guy EC, Joynson DH. Potential of the polymerase chain reaction in the diagnosis of active Toxoplasma infection by detection of parasite in blood. J Infect Dis 1995; 172: Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev 1998; 11: Remington JS, Cavanaugh EN. Isolation of the encysted form of Toxoplasma gondii from human skeletal muscle and brain. N Engl J Med 1965; 273: Frenkel JK, Nelson BM, Arias-Stella J. Immunosuppression and toxoplasmic encephalitis: clinical and experimental aspects. Hum Pathol 1975; 6: Ryning FW, McLeod R, Maddox JC, Hunt S, Remington JS. Probable transmission of Toxoplasma gondii by organ transplantation. Ann Intern Med 1979; 90: Stinson EB, Bieber CP, Griepp RB, Clark DA, Shumway NE, Remington JS. Infectious complications after cardiac transplantation in man. Ann Intern Med 1971; 74: Luft BJ, Naot Y, Araujo FG, Stinson EB, Remington JS. Primary and reactivated Toxoplasma infection in patients with cardiac transplants. Clinical spectrum and problems in diagnosis in a defined population. Ann Intern Med 1983; 99: Wreghitt TG, Hakim M, Gray JJ et al. Toxoplasmosis in heart and heart and lung transplant recipients. J Clin Pathol 1989; 42: Speirs GE, Hakim M, Calne RY, Wreghitt TG. Relative risk of donor-acquired Toxoplasma gondii in heart, liver and kidney transplant recipients. Clin Transpl 1988; 2: Gallino A, Maggiorini M, Kiowski W et al. Toxoplasmosis in heart transplant recipients. Eur J Clin Microbiol Infect Dis 1996; 15: Michaels MG, Wald ER, Fricker FJ, del Nido PJ, Armitage J. Toxoplasmosis in pediatric recipients of heart transplants. Clin Infect Dis 1992; 14: Hakim M, Esmore D, Wallwork J, English TA, Wreghitt T. Toxoplasmosis in cardiac transplantation. BMJ (Clin Res Ed) 1986; 292: Montoya JG, Giraldo LF, Efron B et al. Infectious complications among 620 consecutive heart transplant patients at Stanford University Medical Center. Clin Infect Dis 2001; 33: Orr KE, Gould FK, Short G et al. Outcome of Toxoplasma gondii mismatches in heart transplant recipients over a period of 8 years. J Infect 1994; 29: Baran DA, Alwarshetty MM, Alvi S et al. Is toxoplasmosis prophylaxis necessary in cardiac transplantation? Longterm follow-up at two transplant centers. J Heart Lung Transplant 2006; 25: Garbino J, Romand JA, Pittet D, Giostra E, Mentha G, Suter P. Infection and rejection in liver transplant patients: a 10- year Swiss single-centre experience. Swiss Med Wkly 2005; 135: Assi MA, Rosenblatt JE, Marshall WF. Donor-transmitted toxoplasmosis in liver transplant recipients: a case report and literature review. Transpl Infect Dis 2007; 9: Campbell AL, Goldberg CL, Magid MS, Gondolesi G, Rumbo C, Herold BC. First case of toxoplasmosis following small bowel transplantation and systematic review of tissue-invasive toxoplasmosis following noncardiac solid organ transplantation. Transplantation 2006; 81: Hommann M, Schotte U, Voigt R et al. Cerebral toxoplasmosis after combined liver pancreas kidney and liver pancreas transplantation. Transplant Proc 2002; 34: Castagnini M, Bernazzali S, Ginanneschi C et al. Fatal disseminated toxoplasmosis in a cardiac transplantation with seropositive match for Toxoplasma: should prophylaxis be extended? Transpl Immunol 2007; 18: Robert-Gangneux F, Amrein C, Lavarde V, Botterel F, Dupouy-Camet J. Neosynthesized IgG detected by western blotting in Toxoplasma-seropositive heart or lung transplant recipients. Transpl Int 2000; 13: Morisset S, Peyron F, Lobry JR et al. Serotyping of Toxoplasma gondii: striking homogeneous pattern between symptomatic and asymptomatic infections within Europe and South America. Microbes Infect, 2008; 10: Dardé ML, Aubert D, Derouin F, Dumètre A, Pelloux H, Villena I. Toxoplasma gondii. In: Khan N, ed. Emerging protozoan pathogens. London: Taylor and Francis, 2007; Geissmann F, Derouin F, Marolleau JP, Gisselbrecht C, Brice P. Disseminated toxoplasmosis following autologous bone marrow transplantation. Clin Infect Dis 1994; 19: López-Duarte M, Insunza A, Conde E, Iriondo A, Mazorra F, Zubizarreta A. Cerebral toxoplasmosis after autologous peripheral blood stem cell transplantation. Eur J Clin Microbiol Infect Dis 2003; 22: Matsuo Y, Takeishi S, Miyamoto T et al. Toxoplasmosis encephalitis following severe graft-vs.-host disease after allogeneic hematopoietic stem cell transplantation: 17 yr experience in Fukuoka BMT group. Eur J Haematol 2007; 79: Peacock JE Jr, Greven CM, Cruz JM, Hurd DD. Reactivation toxoplasmic retinochoroiditis in patients undergoing bone marrow transplantation: is there a role for chemoprophylaxis? Bone Marrow Transplant 1995; 15: Martino R, Bretagne S, Einsele H et al. Early detection of Toxoplasma infection by molecular monitoring of Toxoplasma gondii in peripheral blood samples after allogeneic stem cell transplantation. Clin Infect Dis 2005; 40: Small TN, Leung L, Stiles J et al. Disseminated toxoplasmosis following T cell-depleted related and unrelated bone marrow transplantation. Bone Marrow Transplant 2000; 25: Martino R, Bretagne S, Rovira M et al. Toxoplasmosis after hematopoietic stem transplantation. Report of a 5-year survey from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2000; 25: Derouin F, Devergie A, Auber P et al. Toxoplasmosis in bone marrow-transplant recipients: report of seven cases and review. Clin Infect Dis 1992; 15: Slavin MA, Meyers JD, Remington JS, Hackman RC. Toxoplasma gondii infection in marrow transplant recipients: a 20 year experience. Bone Marrow Transplant 1994; 13:
12 1100 Clinical Microbiology and Infection, Volume 14 Number 12, December de Medeiros BC, de Medeiros CR, Werner B, Loddo G, Pasquini R, Bleggi-Torres LF. Disseminated toxoplasmosis after bone marrow transplantation: report of 9 cases. Transpl Infect Dis 2001; 3: Mele A, Paterson PJ, Prentice HG, Leoni P, Kibbler CC. Toxoplasmosis in bone marrow transplantation: a report of two cases and systematic review of the literature. Bone Marrow Transplant 2002; 29: Martino R, Maertens J, Bretagne S et al. Toxoplasmosis after hematopoietic stem cell transplantation. Clin Infect Dis 2000; 31: Menotti J, Vilela G, Romand S et al. Comparison of PCRenzyme-linked immunosorbent assay and real-time PCR assay for diagnosis of an unusual case of cerebral toxoplasmosis in a stem cell transplant recipient. J Clin Microbiol 2003; 41: Mueller-Mang C, Mang TG, Kalhs P, Thurnher MM. Imaging characteristics of toxoplasmosis encephalitis after bone marrow transplantation: report of two cases and review of the literature. Neuroradiology 2006; 48: Sing A, Leitritz L, Roggenkamp A et al. Pulmonary toxoplasmosis in bone marrow transplant recipients: report of two cases and review. Clin Infect Dis 1999; 29: Ortonne N, Ribaud P, Meignin V et al. Toxoplasmic pneumonitis leading to fatal acute respiratory distress syndrome after engraftment in three bone marrow transplant recipients. Transplantation 2001; 72: Duband S, Cornillon J, Tavernier E, Dumollard J-M, Guyotat D, Peoc h M. Toxoplasmosis with hemophagocytic syndrome after bone marrow transplantation: diagnosis at autopsy. Transpl Infect Dis 2008; 10: Derouin F, Gluckman E, Beauvais B et al. Toxoplasma infection after human allogeneic bone marrow transplantation: clinical and serological study of 80 patients. Bone Marrow Transplant 1986; 1: Janitschke K, Held T, Krüiger D, Schwerdtfeger R, Schlier G, Liesenfeld O. Diagnostic value of tests for Toxoplasma gondii-specific antibodies in patients undergoing bone marrow transplantation. Clin Lab 2003; 49: Kotton CN. Zoonoses in solid-organ and hematopoietic stem cell transplant recipients. Clin Infect Dis 2007; 44: Peleg AY, Husain S, Kwak EJ et al. Opportunistic infections in 547 organ transplant recipients receiving alemtuzumab, a humanized monoclonal CD-52 antibody. Clin Infect Dis 2007; 44: Lavarde V, Heyer F, Guillemain R, Amrein C, Vulser C, Dreyfus G. Toxoplasmose et transplantation cardiaque. Intérêt et limites de la sérologie. Med Mal Infect 1990; 20: Derouin F, Debure A, Godeaut E, Lariviere M, Kreis H. Toxoplasma antibody titers in renal transplant recipients. Pretransplant evaluation and posttransplant follow-up of 73 patients. Transplantation 1987; 44: Bourrée P, Romand S, Majoube A. Toxoplasmose et transplantation hépatique: surveillance de 34 patients par deux techniques sérologiques. Bull Soc Fr Parasitol 1995; 13: Conley FK, Jenkins KA, Remington JS. Toxoplasma gondii infection of the central nervous system. Use of the peroxidase antiperoxidase method to demonstrate Toxoplasma in formalin fixed, paraffin embedded tissue sections. Hum Pathol 1981; 12: Held TK, Krüger D, Switala AR et al. Diagnosis of toxoplasmosis in bone marrow transplant recipients: comparison of PCR-based results and immunohistochemistry. Bone Marrow Transplant 2000; 25: Bretagne S, Costa JM, Foulet F, Jabot-Lestang L, Baud- Camus F, Cordonnier C. Prospective study of Toxoplasma reactivation by polymerase chain reaction in allogeneic stem-cell transplant recipients. Transpl Infect Dis 2000; 2: Bretagne S. Molecular diagnostics in clinical parasitology and mycology: limits of the current polymerase chain reaction (PCR) assays and interest of the real-time PCR assays. Clin Microbiol Infect 2003; 9: Laibe S, Ranque S, Curtillet C, Faraut F, Dumon H, Franck J. Timely diagnosis of disseminated toxoplasmosis by sputum examination. J Clin Microbiol 2006; 44: Botterel F, Ichai P, Feray C et al. Disseminated toxoplasmosis, resulting from infection of allograft, after orthotopic liver transplantation: usefulness of quantitative PCR. J Clin Microbiol 2002; 40: Fricker-Hidalgo H, Brion JP, Durand M, Chavanon O, Brenier-Pinchart MP, Pelloux H. Disseminated toxoplasmosis with pulmonary involvement after heart transplantation. Transpl Infect Dis 2005; 7: Aubert D, Foudrinier F, Villena I, Pinon JM, Biava MF, Renoult E. PCR for diagnosis and follow-up of two cases of disseminated toxoplasmosis after kidney grafting. J Clin Microbiol 1996; 34: Sulahian A, Nugues C, Garin YJF et al. Serodiagnosis of toxoplasmosis in patients with acquired or reactivating toxoplasmosis and analysis of the specific IgA antibody responses by ELISA, agglutination and immunoblotting. Immunol Infect Dis 1993; 3: Mechain B, Garin YJ, Robert-Gangneux F, Dupouy-Camet J, Derouin F. Lack of utility of specific immunoglobulin G antibody avidity for serodiagnosis of reactivated toxoplasmosis in immunocompromised patients. Clin Diagn Lab Immunol 2000; 7: Renoult E, Biava MF, Aimone-Gastin I et al. Evolution and significance of Toxoplasma gondii antibody titers in kidney transplant recipients. Transplant Proc 1992; 24: European Commission. Directorate. Human organ transplantation in Europe; an overview. Report from the General Health and Consumer Protection Public Health and Risk Assessment Directorate; 2003; Available at: ec.europa.eu/health/ph_threats/human_substance/ documents/organ_survey.pdf. 74. Gourishankar S, Doucette K, Fenton J, Purych D, Kowalewska-Grochowska K, Preiksaitis J. The use of donor and recipient screening for Toxoplasma in the era of universal trimethoprim sulfamethoxazole prophylaxis. Transplantation 2008; 85: Edvinsson B, Lundquist J, Ljungman P, Ringden O, Evengard B. A prospective study of diagnosis of Toxoplasma gondii infection after bone marrow transplantation. APMIS ; 116: Araujo FG, Huskinson J, Remington JS. Remarkable in vitro and in vivo activities of the hydroxynaphthoquinone 566C80 against tachyzoites and tissue cysts of Toxoplasma gondii. Antimicrob Agents Chemother 1991; 35:
Lecture-7- Hazem Al-Khafaji 2016
 TOXOPLASMOSIS Lecture-7- Hazem Al-Khafaji 2016 TOXOPLASMOSIS It is a disease caused by Toxoplasma gondii which is a protozoan parasite that is infects a variety of mammals and birds throughout the world.
TOXOPLASMOSIS Lecture-7- Hazem Al-Khafaji 2016 TOXOPLASMOSIS It is a disease caused by Toxoplasma gondii which is a protozoan parasite that is infects a variety of mammals and birds throughout the world.
Toxoplasma gondii. Definitive Host adult forms sexual reproduction. Intermediate Host immature forms asexual reproduction
 Toxoplasma gondii cosmopolitan distribution seropositive prevalence rates vary generally 20-75% generally causes very benign disease in immunocompetent adults tissue cyst forming coccidia predator-prey
Toxoplasma gondii cosmopolitan distribution seropositive prevalence rates vary generally 20-75% generally causes very benign disease in immunocompetent adults tissue cyst forming coccidia predator-prey
Toxoplasma gondii. Jarmila Kliescikova, MD 1. LF UK
 Toxoplasma gondii Jarmila Kliescikova, MD 1. LF UK Toxoplasma gondii Apicomplexa, Koccidia Obligate intracellular parasite Distribution: cosmopolite Transmission: alimentary transplacentary (transfusions,
Toxoplasma gondii Jarmila Kliescikova, MD 1. LF UK Toxoplasma gondii Apicomplexa, Koccidia Obligate intracellular parasite Distribution: cosmopolite Transmission: alimentary transplacentary (transfusions,
CASE REPORT. Abstract. Introduction. Case Report
 CASE REPORT Severe Pulmonary Toxoplasmosis Mimicking Viral Pneumonitis after a Third Allogeneic Stem Cell Transplantation in a Man with Acute Lymphoblastic Leukemia Masahiko Sumi 1, Satoru Hata 2, Keijiro
CASE REPORT Severe Pulmonary Toxoplasmosis Mimicking Viral Pneumonitis after a Third Allogeneic Stem Cell Transplantation in a Man with Acute Lymphoblastic Leukemia Masahiko Sumi 1, Satoru Hata 2, Keijiro
Coccidia. Eucoccidioside
 Coccidia Kingdom Sub-Kingdom Phylum Class Order Family Genus Species Protista Protozoa Apicomplexa Sporozoasida Eucoccidioside Sarcocystidae Toxoplasma gondii 1 Toxoplasma gondii (life cycle) Sexual cycle
Coccidia Kingdom Sub-Kingdom Phylum Class Order Family Genus Species Protista Protozoa Apicomplexa Sporozoasida Eucoccidioside Sarcocystidae Toxoplasma gondii 1 Toxoplasma gondii (life cycle) Sexual cycle
GUIDELINE FOR THE MANAGEMENT OF TOXOPLASMOSIS ENCEPHALITIS
 GUIDELINE FOR THE MANAGEMENT OF TOXOPLASMOSIS ENCEPHALITIS Full title of guideline Guideline for the management of toxoplasmosis encephalitis Author Dr P Venkatesan (ID consultant) Division and specialty
GUIDELINE FOR THE MANAGEMENT OF TOXOPLASMOSIS ENCEPHALITIS Full title of guideline Guideline for the management of toxoplasmosis encephalitis Author Dr P Venkatesan (ID consultant) Division and specialty
PUO in the Immunocompromised Host: CMV and beyond
 PUO in the Immunocompromised Host: CMV and beyond PUO in the immunocompromised host: role of viral infections Nature of host defect T cell defects Underlying disease Treatment Nature of clinical presentation
PUO in the Immunocompromised Host: CMV and beyond PUO in the immunocompromised host: role of viral infections Nature of host defect T cell defects Underlying disease Treatment Nature of clinical presentation
Clinical Aspect and Application of Laboratory Test in Herpes Virus Infection. Masoud Mardani M.D,FIDSA
 Clinical Aspect and Application of Laboratory Test in Herpes Virus Infection Masoud Mardani M.D,FIDSA Shahidhid Bh BeheshtiMdi Medical lui Universityit Cytomegalovirus (CMV), Epstein Barr Virus(EBV), Herpes
Clinical Aspect and Application of Laboratory Test in Herpes Virus Infection Masoud Mardani M.D,FIDSA Shahidhid Bh BeheshtiMdi Medical lui Universityit Cytomegalovirus (CMV), Epstein Barr Virus(EBV), Herpes
Toxoplasmosis in heart and heart and lung transplant recipients
 J Clin Pathol 1989;42:194-199 Toxoplasmosis in heart and heart and lung transplant recipients T G WREGHITT,* M HAKIM,* J J GRAY,* A H BALFOUR,t P G I STOVIN,4 SUSAN STEWART,: J SCOTT, T A H ENGLISH,II
J Clin Pathol 1989;42:194-199 Toxoplasmosis in heart and heart and lung transplant recipients T G WREGHITT,* M HAKIM,* J J GRAY,* A H BALFOUR,t P G I STOVIN,4 SUSAN STEWART,: J SCOTT, T A H ENGLISH,II
Cerebral Toxoplasmosis in HIV-Infected Patients. Ahmed Saad,MD,FACP
 Cerebral Toxoplasmosis in HIV-Infected Patients Ahmed Saad,MD,FACP Introduction Toxoplasmosis: Caused by the intracellular protozoan, Toxoplasma gondii. Immunocompetent persons with primary infection
Cerebral Toxoplasmosis in HIV-Infected Patients Ahmed Saad,MD,FACP Introduction Toxoplasmosis: Caused by the intracellular protozoan, Toxoplasma gondii. Immunocompetent persons with primary infection
Ocular Toxoplasmosis Uveitis course Antalya Miles Stanford Medical Eye Unit St Thomas Hospital London
 Ocular Toxoplasmosis Uveitis course Antalya 2013 Miles Stanford Medical Eye Unit St Thomas Hospital London Toxoplasma gondii Obligate, intracellular, apicomplexan protozoan Infects > 1/3 world population
Ocular Toxoplasmosis Uveitis course Antalya 2013 Miles Stanford Medical Eye Unit St Thomas Hospital London Toxoplasma gondii Obligate, intracellular, apicomplexan protozoan Infects > 1/3 world population
Correlation of Parasite Load Determined by Quantitative PCR to Clinical Outcome in a Heart Transplant Patient with Disseminated Toxoplasmosis
 JOURNAL OF CLINICAL MICROBIOLOGY, July 2010, p. 2541 2545 Vol. 48, No. 7 0095-1137/10/$12.00 doi:10.1128/jcm.00252-10 Copyright 2010, American Society for Microbiology. All Rights Reserved. Correlation
JOURNAL OF CLINICAL MICROBIOLOGY, July 2010, p. 2541 2545 Vol. 48, No. 7 0095-1137/10/$12.00 doi:10.1128/jcm.00252-10 Copyright 2010, American Society for Microbiology. All Rights Reserved. Correlation
Recommendations for VZV management in. Dan Engelhard, Pierre Reusser, Rafael de la Camara, Hermann Einsele, Jan Styczynski, Kate Ward, Per Ljungman
 Recommendations for VZV management in patients Cas cliniques with leukemia Dan Engelhard, Pierre Reusser, Rafael de la Camara, Hermann Einsele, Jan Styczynski, Kate Ward, Per Ljungman Introduction Acute
Recommendations for VZV management in patients Cas cliniques with leukemia Dan Engelhard, Pierre Reusser, Rafael de la Camara, Hermann Einsele, Jan Styczynski, Kate Ward, Per Ljungman Introduction Acute
Toxoplasmosis Objective :
 Toxoplasmosis Objective : Describe the Life cycle Mention the Infective stages Define Congenital Toxoplasmosis List the Lab.Diagnosis Illustrate the Immunity to Toxoplasmosis Show the relationship between
Toxoplasmosis Objective : Describe the Life cycle Mention the Infective stages Define Congenital Toxoplasmosis List the Lab.Diagnosis Illustrate the Immunity to Toxoplasmosis Show the relationship between
Short Video. shows/monsters-inside- me/videos/toxoplasma-parasite/
 The word Toxoplasma Originated from the Greek word toxon, which meant "bow." The Latin word toxicum, which meant "poison." The original Greek meaning is the one used for the word Toxoplasma, meaning "bow
The word Toxoplasma Originated from the Greek word toxon, which meant "bow." The Latin word toxicum, which meant "poison." The original Greek meaning is the one used for the word Toxoplasma, meaning "bow
Opportunistic infections
 Opportunistic infections Opportunistic infections Decrease in number of CD4 lymphocytes is condition for development of opportunistic infections Risk is started, when number of CD4 lymphocytes drops to
Opportunistic infections Opportunistic infections Decrease in number of CD4 lymphocytes is condition for development of opportunistic infections Risk is started, when number of CD4 lymphocytes drops to
Toxoplasmosis Seroepidemiology in Serum of Suspected Patients Attending Medical Lab, in 2013
 Journal of Community Health Research. 2015;4(1):47-54. Original Article Toxoplasmosis Seroepidemiology in Serum of Suspected Patients Attending Medical Lab, in 2013 Ali Fattahi Bafghi 1, Roya Anvari 2,
Journal of Community Health Research. 2015;4(1):47-54. Original Article Toxoplasmosis Seroepidemiology in Serum of Suspected Patients Attending Medical Lab, in 2013 Ali Fattahi Bafghi 1, Roya Anvari 2,
Toxoplasmosis in immunocompetent and immunocompromised population of Constanta, Romania
 3rd International Conference on Clinical Microbiology & Microbial Genomics September 24-26, 2014 Valencia, Spain Toxoplasmosis in immunocompetent and immunocompromised population of Constanta, Romania
3rd International Conference on Clinical Microbiology & Microbial Genomics September 24-26, 2014 Valencia, Spain Toxoplasmosis in immunocompetent and immunocompromised population of Constanta, Romania
A 39 years old HIV-positive black African woman with previously treated cerebral
 1 Abstract A 39 years old HIV-positive black African woman with previously treated cerebral toxoplasmosis experienced a foetal intra-uterine death due to congenital toxoplasmosis. This case demonstrates
1 Abstract A 39 years old HIV-positive black African woman with previously treated cerebral toxoplasmosis experienced a foetal intra-uterine death due to congenital toxoplasmosis. This case demonstrates
Pneumocystis. Pneumocystis BIOL Summer Introduction. Mycology. Introduction (cont.) Introduction (cont.)
 Introduction Pneumocystis Disclaimer: This lecture slide presentation is intended solely for educational purposes. Many of the images contained herein are the property of the original owner, as indicated
Introduction Pneumocystis Disclaimer: This lecture slide presentation is intended solely for educational purposes. Many of the images contained herein are the property of the original owner, as indicated
Toxoplasmosis. Life cycle Infective stages Congenital Toxoplasmosis Lab.Diagnosis Immunity to Toxoplasmosis Toxoplasmosis & Pregnancy
 Toxoplasmosis Life cycle Infective stages Congenital Toxoplasmosis Lab.Diagnosis Immunity to Toxoplasmosis Toxoplasmosis & Pregnancy Human Toxoplasmosis Toxoplasmosis is a zoonotic disease Caused by Coccidian
Toxoplasmosis Life cycle Infective stages Congenital Toxoplasmosis Lab.Diagnosis Immunity to Toxoplasmosis Toxoplasmosis & Pregnancy Human Toxoplasmosis Toxoplasmosis is a zoonotic disease Caused by Coccidian
Performance of the BioPlex 2200 flow immunoassay (Bio- Rad) in critical cases of serodiagnosis of toxoplasmosis
 CVI Accepts, published online ahead of print on 29 January 2014 Clin. Vaccine Immunol. doi:10.1128/cvi.00624-13 Copyright 2014, American Society for Microbiology. All Rights Reserved. 1 2 3 Performance
CVI Accepts, published online ahead of print on 29 January 2014 Clin. Vaccine Immunol. doi:10.1128/cvi.00624-13 Copyright 2014, American Society for Microbiology. All Rights Reserved. 1 2 3 Performance
Human toxoplasmosis in Europe. Parasitology, Paris Descartes University, Cochin Hospital, Paris, France
 Human toxoplasmosis in Europe Prof Jean Dupouy-Camet Parasitology, Paris Descartes University, Cochin Hospital, Paris, France Toxoplasma gondii, discovered in 1908, by Nicolle at Institut Pasteur de Tunis,
Human toxoplasmosis in Europe Prof Jean Dupouy-Camet Parasitology, Paris Descartes University, Cochin Hospital, Paris, France Toxoplasma gondii, discovered in 1908, by Nicolle at Institut Pasteur de Tunis,
Diagnosis of CMV infection UPDATE ECIL
 UPDATE ECIL-4 2011 Recommendations for CMV and HHV-6 management in patients with hematological diseases Per Ljungman, Rafael de la Camara, Hermann Einsele, Dan Engelhard, Pierre Reusser, Jan Styczynski,
UPDATE ECIL-4 2011 Recommendations for CMV and HHV-6 management in patients with hematological diseases Per Ljungman, Rafael de la Camara, Hermann Einsele, Dan Engelhard, Pierre Reusser, Jan Styczynski,
Immunological Aspects of Parasitic Diseases in Immunocompromised Individuals. Taniawati Supali. Department of Parasitology
 Immunological Aspects of Parasitic Diseases in Immunocompromised Individuals Taniawati Supali Department of Parasitology 1 Defense mechanism in human Th17 (? ) Acute Chronic Th1 Th 2 Intracellular Treg
Immunological Aspects of Parasitic Diseases in Immunocompromised Individuals Taniawati Supali Department of Parasitology 1 Defense mechanism in human Th17 (? ) Acute Chronic Th1 Th 2 Intracellular Treg
toxoplasmosis Laboratory techniques in the investigation of immunocompromised. Cancer patients, (PCR). Clinical and radiological findings
 Genitourin Med 1992;68:55-59 Public Health Laboratory Service, Toxoplasma Reference Laboratory, St George's Hospital, Blackshaw Road, London, SW17 OQT, UK K F Barker R E Holliman Address correspondence
Genitourin Med 1992;68:55-59 Public Health Laboratory Service, Toxoplasma Reference Laboratory, St George's Hospital, Blackshaw Road, London, SW17 OQT, UK K F Barker R E Holliman Address correspondence
Comparison of the efficiency of two commercial kits ELFA and Western blot in estimating the phase of Toxoplasma gondii infection in pregnant women
 Annals of Agricultural and Environmental Medicine 2016, Vol 23, No 4, 570 575 www.aaem.pl ORIGINAL ARTICLE Comparison of the efficiency of two commercial kits ELFA and Western blot in estimating the phase
Annals of Agricultural and Environmental Medicine 2016, Vol 23, No 4, 570 575 www.aaem.pl ORIGINAL ARTICLE Comparison of the efficiency of two commercial kits ELFA and Western blot in estimating the phase
Seroprevalence of Toxoplasmosis in High School Girls in Fasa District, Iran
 Seroprevalence of Toxoplasmosis in High School Girls in Fasa District, Iran Gholamreza Hatam 1*, Azra Shamseddin 2, Farhoud Nikouee 3 1 Department of Parasitology and Mycology, School of Medicine, Shiraz
Seroprevalence of Toxoplasmosis in High School Girls in Fasa District, Iran Gholamreza Hatam 1*, Azra Shamseddin 2, Farhoud Nikouee 3 1 Department of Parasitology and Mycology, School of Medicine, Shiraz
Ocular Toxoplasmosis - A Laboratory View. Chatterton JMW* Evans R. Ho-Yen DO
 Ocular Toxoplasmosis - A Laboratory View Chatterton JMW* Evans R Ho-Yen DO Scottish Toxoplasma Reference Laboratory Microbiology Department Raigmore Hospital Inverness IV UJ * corresponding author Introduction
Ocular Toxoplasmosis - A Laboratory View Chatterton JMW* Evans R Ho-Yen DO Scottish Toxoplasma Reference Laboratory Microbiology Department Raigmore Hospital Inverness IV UJ * corresponding author Introduction
AIDS at 25. Epidemiology and Clinical Management MID 37
 AIDS at 25 Epidemiology and Clinical Management Blood HIV Transmission transfusion injection drug use Sexual Intercourse heterosexual male to male Perinatal intrapartum breast feeding Regional HIV and
AIDS at 25 Epidemiology and Clinical Management Blood HIV Transmission transfusion injection drug use Sexual Intercourse heterosexual male to male Perinatal intrapartum breast feeding Regional HIV and
Toxoplasmosis. Seminar. For personal use. Only reproduce with permission from The Lancet Publishing Group.
 Seminar Toxoplasmosis J G Montoya, O Liesenfeld Toxoplasma gondii is a protozoan parasite that infects up to a third of the world s population. Infection is mainly acquired by ingestion of food or water
Seminar Toxoplasmosis J G Montoya, O Liesenfeld Toxoplasma gondii is a protozoan parasite that infects up to a third of the world s population. Infection is mainly acquired by ingestion of food or water
New recommendations for immunocompromised patients
 New recommendations for immunocompromised patients Hepatitis E Virus (HEV): Transmission, incidence and presentation Emerging evidence regarding HEV transmission from blood components and dietary consumption
New recommendations for immunocompromised patients Hepatitis E Virus (HEV): Transmission, incidence and presentation Emerging evidence regarding HEV transmission from blood components and dietary consumption
PARASITOLOGY CASE HISTORY 10 (HISTOLOGY) (Lynne S. Garcia)
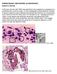 PARASITOLOGY CASE HISTORY 10 (HISTOLOGY) (Lynne S. Garcia) A 46-year-old man with AIDS was admitted to the hospital for complaints of a persisting fever and dry cough. A chest radiograph showed bilateral
PARASITOLOGY CASE HISTORY 10 (HISTOLOGY) (Lynne S. Garcia) A 46-year-old man with AIDS was admitted to the hospital for complaints of a persisting fever and dry cough. A chest radiograph showed bilateral
Indirect Enzyme-Linked Immunosorbent Assay for Immunoglobulin G and Four Immunoassays for Immunoglobulin M
 JOURNAL OF CLINICAL MICROBIOLOGY, Mar. 1989, p. 529-535 0095-1137/89/030529-07$02.00/0 Copyright 1989, American Society for Microbiology Vol. 27, No. 3 Indirect Enzyme-Linked Immunosorbent Assay for Immunoglobulin
JOURNAL OF CLINICAL MICROBIOLOGY, Mar. 1989, p. 529-535 0095-1137/89/030529-07$02.00/0 Copyright 1989, American Society for Microbiology Vol. 27, No. 3 Indirect Enzyme-Linked Immunosorbent Assay for Immunoglobulin
AIDS at 30 Epidemiology and Clinical Epidemiology and Management MID 37
 AIDS at 30 Epidemiology and Clinical Management Blood HIV Transmission transfusion injection drug use Sexual Intercourse heterosexual male to male Perinatal intrapartum breast feeding Adults and children
AIDS at 30 Epidemiology and Clinical Management Blood HIV Transmission transfusion injection drug use Sexual Intercourse heterosexual male to male Perinatal intrapartum breast feeding Adults and children
Complications after HSCT. ICU Fellowship Training Radboudumc
 Complications after HSCT ICU Fellowship Training Radboudumc Type of HSCT HSCT Improved outcome due to better HLA matching, conditioning regimens, post transplant supportive care Over one-third have pulmonary
Complications after HSCT ICU Fellowship Training Radboudumc Type of HSCT HSCT Improved outcome due to better HLA matching, conditioning regimens, post transplant supportive care Over one-third have pulmonary
Clinical Policy: Pyrimethamine (Daraprim) Reference Number: ERX.NPA.44 Effective Date:
 Clinical Policy: (Daraprim) Reference Number: ERX.NPA.44 Effective Date: 12.01.15 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Daraprim) Reference Number: ERX.NPA.44 Effective Date: 12.01.15 Last Review Date: 08.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Synergistic Activity of Azithromycin and Pyrimethamine or
 ANTMCROBAL AGNTS AND CHMOTHRAPY, May 199, p. 997-11 66-44/9/5997-5$./ Copyright 199, American Society for Microbiology Vol. 36, No. 5 Synergistic Activity of Azithromycin and Pyrimethamine or Sulfadiazine
ANTMCROBAL AGNTS AND CHMOTHRAPY, May 199, p. 997-11 66-44/9/5997-5$./ Copyright 199, American Society for Microbiology Vol. 36, No. 5 Synergistic Activity of Azithromycin and Pyrimethamine or Sulfadiazine
Extra-intestinal coccidians
 Extra-intestinal coccidians Apicomplexa Coccidia Gregarinea Piroplasmida Eimeriida Haemosporida -Theileriidae - Babesiidae - Eimeriidae - Cryptosporidiidae - Sarcocystidae (Sarcocystis) (Toxoplasma) -Haemosporiidae
Extra-intestinal coccidians Apicomplexa Coccidia Gregarinea Piroplasmida Eimeriida Haemosporida -Theileriidae - Babesiidae - Eimeriidae - Cryptosporidiidae - Sarcocystidae (Sarcocystis) (Toxoplasma) -Haemosporiidae
PREDICTIVE VALUE OF LATEX AGGLUTINATION TEST IN SEROLOGICAL SCREENING FOR TOXOPLASMA GONDII
 PREDICTIVE VALUE OF LATEX AGGLUTINATION TEST IN SEROLOGICAL SCREENING FOR TOXOPLASMA GONDII Yaowalark Sukthana, Thaiyooth Chintana, Waraporn Supatanapong, Chutatip Siripan, Amorn Lekkla and Rachatawan
PREDICTIVE VALUE OF LATEX AGGLUTINATION TEST IN SEROLOGICAL SCREENING FOR TOXOPLASMA GONDII Yaowalark Sukthana, Thaiyooth Chintana, Waraporn Supatanapong, Chutatip Siripan, Amorn Lekkla and Rachatawan
Evaluation of the Immunoglobulin G Avidity Test for Diagnosis of Toxoplasmic Lymphadenopathy
 JOURNAL OF CLINICAL MICROBIOLOGY, Oct. 2004, p. 4627 4631 Vol. 42, No. 10 0095-1137/04/$08.00 0 DOI: 10.1128/JCM.42.10.4627 4631.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved.
JOURNAL OF CLINICAL MICROBIOLOGY, Oct. 2004, p. 4627 4631 Vol. 42, No. 10 0095-1137/04/$08.00 0 DOI: 10.1128/JCM.42.10.4627 4631.2004 Copyright 2004, American Society for Microbiology. All Rights Reserved.
Significance of a positive Toxoplasma Immunoglobulin M test result. in the United States
 JCM Accepted Manuscript Posted Online 9 September 2015 J. Clin. Microbiol. doi:10.1128/jcm.01663-15 Copyright 2015, American Society for Microbiology. All Rights Reserved. 1 2 Significance of a positive
JCM Accepted Manuscript Posted Online 9 September 2015 J. Clin. Microbiol. doi:10.1128/jcm.01663-15 Copyright 2015, American Society for Microbiology. All Rights Reserved. 1 2 Significance of a positive
Received 8 April 1996/Returned for modification 19 June 1996/Accepted 15 July 1996
 JOURNAL OF CLINICAL MICROBIOLOGY, Oct. 1996, p. 2526 2530 Vol. 34, No. 10 0095-1137/96/$04.00 0 Copyright 1996, American Society for Microbiology Study of Abbott Toxo IMx System for Detection of Immunoglobulin
JOURNAL OF CLINICAL MICROBIOLOGY, Oct. 1996, p. 2526 2530 Vol. 34, No. 10 0095-1137/96/$04.00 0 Copyright 1996, American Society for Microbiology Study of Abbott Toxo IMx System for Detection of Immunoglobulin
Only one take home point for the talk 9/26/2018. Infectious Diseases and Donor Derived Infections. Don t forget about donor-derived infections
 Shane Colombo 1993 2018 Infectious Diseases and Donor Derived Infections Peter Chin-Hong, MD Division of Infectious Diseases UCSF Only one take home point for the talk Don t forget about donor-derived
Shane Colombo 1993 2018 Infectious Diseases and Donor Derived Infections Peter Chin-Hong, MD Division of Infectious Diseases UCSF Only one take home point for the talk Don t forget about donor-derived
Utility of Immunoblotting for Early Diagnosis of Toxoplasmosis Seroconversion in Pregnant Women
 CLINICAL AND VACCINE IMMUNOLOGY, Nov. 2011, p. 1908 1912 Vol. 18, No. 11 1556-6811/11/$12.00 doi:10.1128/cvi.05303-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. Utility of
CLINICAL AND VACCINE IMMUNOLOGY, Nov. 2011, p. 1908 1912 Vol. 18, No. 11 1556-6811/11/$12.00 doi:10.1128/cvi.05303-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. Utility of
EBV in HSCT 2015 update of ECIL guidelines
 ECIL-6 EBV in HSCT 2015 update of ECIL guidelines Jan Styczynski (Poland, chair), Walter van der Velden (Netherlands), Christopher Fox (United Kingdom), Dan Engelhard (Israel), Rafael de la Camara (Spain),
ECIL-6 EBV in HSCT 2015 update of ECIL guidelines Jan Styczynski (Poland, chair), Walter van der Velden (Netherlands), Christopher Fox (United Kingdom), Dan Engelhard (Israel), Rafael de la Camara (Spain),
Research Article Seroprevalence of Toxoplasma gondii Infection in Patients of Intensive Care Unit in China: A Hospital Based Study
 BioMed Research International Volume 2015, Article ID 908217, 4 pages http://dx.doi.org/10.1155/2015/908217 Research Article Seroprevalence of Toxoplasma gondii Infection in Patients of Intensive Care
BioMed Research International Volume 2015, Article ID 908217, 4 pages http://dx.doi.org/10.1155/2015/908217 Research Article Seroprevalence of Toxoplasma gondii Infection in Patients of Intensive Care
Laboratory Diagnosis of Toxoplasma gondii Infection and Toxoplasmosis
 S73 Laboratory Diagnosis of Toxoplasma gondii Infection and Toxoplasmosis Jose G. Montoya Department of Immunology and Infectious Diseases, Research Institute, Palo Alto Medical Foundation, Palo Alto,
S73 Laboratory Diagnosis of Toxoplasma gondii Infection and Toxoplasmosis Jose G. Montoya Department of Immunology and Infectious Diseases, Research Institute, Palo Alto Medical Foundation, Palo Alto,
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Daraprim) Reference Number: CP.PMN.44 Effective Date: 11.01.15 Last Review Date: 08.18 Line of Business: Commercial, HIM, Medicaid Revision Log See Important Reminder at the end of this
Clinical Policy: (Daraprim) Reference Number: CP.PMN.44 Effective Date: 11.01.15 Last Review Date: 08.18 Line of Business: Commercial, HIM, Medicaid Revision Log See Important Reminder at the end of this
International Conference on Parasitology August 24-26, 2015 Philadelphia, Pennsylvania, USA
 International Conference on Parasitology August 24-26, 2015 Philadelphia, Pennsylvania, USA SYMPOSIA THE CHALLENGE OF PARASITES AND IMMUNOSUPRESSION: FROM DIAGNOSIS TO TREATMENT from the bench to the bed
International Conference on Parasitology August 24-26, 2015 Philadelphia, Pennsylvania, USA SYMPOSIA THE CHALLENGE OF PARASITES AND IMMUNOSUPRESSION: FROM DIAGNOSIS TO TREATMENT from the bench to the bed
Disclosures. CMV and EBV Infection in Pediatric Transplantation. Goals. Common Aspects CMV (Cytomegalovirus) and EBV (Epstein-Barr virus)
 Disclosures I have financial relationships with the following companies: CMV and EBV Infection in Pediatric Transplantation Elekta Inc Lucence Diagnostics Spouse employed Spouse employed I will not discuss
Disclosures I have financial relationships with the following companies: CMV and EBV Infection in Pediatric Transplantation Elekta Inc Lucence Diagnostics Spouse employed Spouse employed I will not discuss
Relationship between pre-existing anti-varicella zoster virus ACCEPTED. (VZV) antibody and clinical VZV reactivation in hematopoietic
 JCM Accepts, published online ahead of print on 11 October 2006 J. Clin. Microbiol. doi:10.1128/jcm.01312-06 Copyright 2006, American Society for Microbiology and/or the Listed Authors/Institutions. All
JCM Accepts, published online ahead of print on 11 October 2006 J. Clin. Microbiol. doi:10.1128/jcm.01312-06 Copyright 2006, American Society for Microbiology and/or the Listed Authors/Institutions. All
Le infezioni fungine nel trapianto di cellule staminali emopoietiche. Claudio Viscoli Professor of Infectious Disease University of Genova, Italy
 Le infezioni fungine nel trapianto di cellule staminali emopoietiche Claudio Viscoli Professor of Infectious Disease University of Genova, Italy Potential conflicts of interest Received grants as speaker/moderator
Le infezioni fungine nel trapianto di cellule staminali emopoietiche Claudio Viscoli Professor of Infectious Disease University of Genova, Italy Potential conflicts of interest Received grants as speaker/moderator
Michael Grimley 1, Vinod Prasad 2, Joanne Kurtzberg 2, Roy Chemaly 3, Thomas Brundage 4, Chad Wilson 4, Herve Mommeja-Marin 4
 Twice-weekly Brincidofovir (BCV, CMX1) Shows Promising Antiviral Activity in Immunocompromised Transplant Patients with Asymptomatic Adenovirus Viremia Michael Grimley 1, Vinod Prasad, Joanne Kurtzberg,
Twice-weekly Brincidofovir (BCV, CMX1) Shows Promising Antiviral Activity in Immunocompromised Transplant Patients with Asymptomatic Adenovirus Viremia Michael Grimley 1, Vinod Prasad, Joanne Kurtzberg,
SURVEILLANCE OF TOXOPLASMOSIS IN DIFFERENT GROUPS
 SURVEILLANCE OF TOXOPLASMOSIS IN DIFFERENT GROUPS Pages with reference to book, From 183 To 186 Mughisuddin Ahmed ( Department of Pathology, Dow Medical College and Basic Medical Sciences Institute, Jinnah
SURVEILLANCE OF TOXOPLASMOSIS IN DIFFERENT GROUPS Pages with reference to book, From 183 To 186 Mughisuddin Ahmed ( Department of Pathology, Dow Medical College and Basic Medical Sciences Institute, Jinnah
EMERGING FUNGAL INFECTIONS IN IMMUNOCOMPROMISED PATIENTS
 EMERGING FUNGAL INFECTIONS IN IMMUNOCOMPROMISED PATIENTS DR LOW CHIAN YONG MBBS, MRCP(UK), MMed(Int Med), FAMS Consultant, Dept of Infectious Diseases, SGH Introduction The incidence of invasive fungal
EMERGING FUNGAL INFECTIONS IN IMMUNOCOMPROMISED PATIENTS DR LOW CHIAN YONG MBBS, MRCP(UK), MMed(Int Med), FAMS Consultant, Dept of Infectious Diseases, SGH Introduction The incidence of invasive fungal
This article was downloaded by: Publisher: KKG Publications Registered office: 18, Jalan Kenanga SD 9/7 Bandar Sri Damansara, Malaysia
 This article was downloaded by: Publisher: KKG Publications Registered office: 18, Jalan Kenanga SD 9/7 Bandar Sri Damansara, 52200 Malaysia Key Knowledge Generation Publication details, including instructions
This article was downloaded by: Publisher: KKG Publications Registered office: 18, Jalan Kenanga SD 9/7 Bandar Sri Damansara, 52200 Malaysia Key Knowledge Generation Publication details, including instructions
Infection and Immune Reconstitution: The NEW Forms
 Infection and Immune Reconstitution: The NEW Forms Marcie Tomblyn, MD, MS Assistant Professor, UMN Assistant Scientific Director, CIBMTR Minneapolis February 16, 2006 Why the changes? Infection data not
Infection and Immune Reconstitution: The NEW Forms Marcie Tomblyn, MD, MS Assistant Professor, UMN Assistant Scientific Director, CIBMTR Minneapolis February 16, 2006 Why the changes? Infection data not
Regulatory Status FDA-approved indications: Valcyte is a deoxynucleoside analogue cytomegalovirus (CMV) DNA polymerase inhibitor indicated for: (1)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.01.22 Subject: Valcyte Page: 1 of 5 Last Review Date: December 8, 2017 Valcyte Description Valcyte (valganciclovir)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.01.22 Subject: Valcyte Page: 1 of 5 Last Review Date: December 8, 2017 Valcyte Description Valcyte (valganciclovir)
CTYOMEGALOVIRUS (CMV) - BACKGROUND
 CTYOMEGALOVIRUS (CMV) - BACKGROUND PURPOSE The flowing information provides guidance on the use of CMV negative blood components provided by the blood bank at the Royal Children s Hospital (RCH) including
CTYOMEGALOVIRUS (CMV) - BACKGROUND PURPOSE The flowing information provides guidance on the use of CMV negative blood components provided by the blood bank at the Royal Children s Hospital (RCH) including
Wales Neonatal Network Guideline
 Congenital infection: Diagnosis and management Overview: Infections transmitted and acquired in utero. Most as a result of primary infection of mother during pregnancy, some organisms such as Cytomegalovirus
Congenital infection: Diagnosis and management Overview: Infections transmitted and acquired in utero. Most as a result of primary infection of mother during pregnancy, some organisms such as Cytomegalovirus
Appendix E1. Epidemiology
 Appendix E1 Epidemiology Viruses are the most frequent cause of human infectious diseases and are responsible for a spectrum of illnesses ranging from trivial colds to fatal immunoimpairment caused by
Appendix E1 Epidemiology Viruses are the most frequent cause of human infectious diseases and are responsible for a spectrum of illnesses ranging from trivial colds to fatal immunoimpairment caused by
Congenital CMV infection. Infectious and Tropical Pediatric Division Department of Child Health Medical Faculty, University of Sumatera Utara
 Congenital CMV infection Infectious and Tropical Pediatric Division Department of Child Health Medical Faculty, University of Sumatera Utara Congenital CMV infection Approximately 0.15 2% of live births
Congenital CMV infection Infectious and Tropical Pediatric Division Department of Child Health Medical Faculty, University of Sumatera Utara Congenital CMV infection Approximately 0.15 2% of live births
TELEFAX. Toxoplasma Serology Laboratory (TSL) DATE: TO: FAX: FROM:
 TELEFAX DATE: TO: FAX: FROM: Toxoplasma Serology Laboratory RE: SPECIMEN REQUIREMENTS AND TESTING INFORMATION (effective 1/15/16) THIS TRANSMISSION CONSISTS OF 16 PAGES INCLUDING THIS COVER PAGE. IF THERE
TELEFAX DATE: TO: FAX: FROM: Toxoplasma Serology Laboratory RE: SPECIMEN REQUIREMENTS AND TESTING INFORMATION (effective 1/15/16) THIS TRANSMISSION CONSISTS OF 16 PAGES INCLUDING THIS COVER PAGE. IF THERE
INTERNATIONAL JOURNAL CEUTICAL RESEARCH AND
 Research Article CODEN: IJPRNK B. V. Ramana, IJPRBS, 2013; Volume 2(4): 253-258 ISSN: 2277-8713 IJPRBS INTERNATIONAL JOURNAL OF PHARMAC CEUTICAL RESEARCH AND BIO-SCIENCE SEROPREVALENCE OF ACUTE TOXOPLASMOSIS
Research Article CODEN: IJPRNK B. V. Ramana, IJPRBS, 2013; Volume 2(4): 253-258 ISSN: 2277-8713 IJPRBS INTERNATIONAL JOURNAL OF PHARMAC CEUTICAL RESEARCH AND BIO-SCIENCE SEROPREVALENCE OF ACUTE TOXOPLASMOSIS
VIDAS Test for Avidity of Toxoplasma-Specific Immunoglobulin G for Confirmatory Testing of Pregnant Women
 JOURNAL OF CLINICAL MICROBIOLOGY, July 2002, p. 2504 2508 Vol. 40, No. 7 0095-1137/02/$04.00 0 DOI: 10.1128/JCM.40.7.2504 2508.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved.
JOURNAL OF CLINICAL MICROBIOLOGY, July 2002, p. 2504 2508 Vol. 40, No. 7 0095-1137/02/$04.00 0 DOI: 10.1128/JCM.40.7.2504 2508.2002 Copyright 2002, American Society for Microbiology. All Rights Reserved.
Innovation in Diagnostics. ToRCH. A complete line of kits for an accurate diagnosis INFECTIOUS ID DISEASES
 Innovation in Diagnostics ToRCH A complete line of kits for an accurate diagnosis INFECTIOUS ID DISEASES EN TOXOPLASMOSIS Toxoplasmosis is a parasitic disease caused by with the obligate intracellular
Innovation in Diagnostics ToRCH A complete line of kits for an accurate diagnosis INFECTIOUS ID DISEASES EN TOXOPLASMOSIS Toxoplasmosis is a parasitic disease caused by with the obligate intracellular
valganciclovir, 450mg tablets, 50mg/ml powder for oral solution (Valcyte ) SMC No. (662/10) Roche Products Ltd
 valganciclovir, 450mg tablets, 50mg/ml powder for oral solution (Valcyte ) SMC No. (662/10) Roche Products Ltd 17 December 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of the
valganciclovir, 450mg tablets, 50mg/ml powder for oral solution (Valcyte ) SMC No. (662/10) Roche Products Ltd 17 December 2010 The Scottish Medicines Consortium (SMC) has completed its assessment of the
J07 Titer dynamics, complement fixation test and neutralization tests
 avllm0421c (spring 2017) J07 Titer dynamics, complement fixation test and neutralization tests Outline titer, antibody titer dynamics complement, complement fixation reaction neutralization tests 2/35
avllm0421c (spring 2017) J07 Titer dynamics, complement fixation test and neutralization tests Outline titer, antibody titer dynamics complement, complement fixation reaction neutralization tests 2/35
2046: Fungal Infection Pre-Infusion Data
 2046: Fungal Infection Pre-Infusion Data Fungal infections are significant opportunistic infections affecting transplant patients. Because these infections are quite serious, it is important to collect
2046: Fungal Infection Pre-Infusion Data Fungal infections are significant opportunistic infections affecting transplant patients. Because these infections are quite serious, it is important to collect
Contents. The ACMSF s approach to its work Human cases: prevalence Burden of disease
 Contents Summary 1-6 1. Background 1.1-1.5 The ACMSF s approach to its work 1.4-1.5 2. Introduction 2.1-2.4 3. The organism 3.1-3.9 4. Human toxoplasmosis: clinical disease 4.1-4.4 5. Human cases: prevalence
Contents Summary 1-6 1. Background 1.1-1.5 The ACMSF s approach to its work 1.4-1.5 2. Introduction 2.1-2.4 3. The organism 3.1-3.9 4. Human toxoplasmosis: clinical disease 4.1-4.4 5. Human cases: prevalence
Preventing CMV Transmission through Leukodepletion
 Preventing CMV Transmission through Leukodepletion Possibility & Facts Prof.S.B.Rajadhyaksha, MD,DTM,PGDMLS Head, Dept. of Transfusion Medicine Tata Memorial Hospital, Mumbai 1 Donor Leukocytes Linked
Preventing CMV Transmission through Leukodepletion Possibility & Facts Prof.S.B.Rajadhyaksha, MD,DTM,PGDMLS Head, Dept. of Transfusion Medicine Tata Memorial Hospital, Mumbai 1 Donor Leukocytes Linked
Important new concerns or changes to the current ones will be included in updates of YESCARTA s RMP.
 PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN Summary of risk management plan for YESCARTA (axicabtagene ciloleucel) This is a summary of the risk management plan (RMP) for YESCARTA. The RMP details important
PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN Summary of risk management plan for YESCARTA (axicabtagene ciloleucel) This is a summary of the risk management plan (RMP) for YESCARTA. The RMP details important
Laboratory diagnosis of congenital infections
 Laboratory diagnosis of congenital infections Laboratory diagnosis of HSV Direct staining Tzanck test Immunostaining HSV isolation Serology PCR Tzanck test Cell scrape from base of the lesion smear on
Laboratory diagnosis of congenital infections Laboratory diagnosis of HSV Direct staining Tzanck test Immunostaining HSV isolation Serology PCR Tzanck test Cell scrape from base of the lesion smear on
Late complications after hematopoietic stem cell transplant in adult patients
 Late complications after hematopoietic stem cell transplant in adult patients Gérard Socié, MD, PhD Hematology/Transplantation, Hospital Saint Louis, Paris, France Synopsis H S C T Allogeneic HSCT activity
Late complications after hematopoietic stem cell transplant in adult patients Gérard Socié, MD, PhD Hematology/Transplantation, Hospital Saint Louis, Paris, France Synopsis H S C T Allogeneic HSCT activity
JOURNAL OF INTERNATIONAL ACADEMIC RESEARCH FOR MULTIDISCIPLINARY Impact Factor 1.393, ISSN: , Volume 2, Issue 2, March 2014
 PRELIMINARY EVALUATION OF CHORUS SYSTEM IN COMPARISON WITH COBAS 6000 SYSTEM FOR DETECTION OF ANTI-TOXOPLASMA-IGM ANTIBODIES BLERTA LAZE* ANILA MITRE** *Dept. of Biology, University Ismail Qemali, Vlora,
PRELIMINARY EVALUATION OF CHORUS SYSTEM IN COMPARISON WITH COBAS 6000 SYSTEM FOR DETECTION OF ANTI-TOXOPLASMA-IGM ANTIBODIES BLERTA LAZE* ANILA MITRE** *Dept. of Biology, University Ismail Qemali, Vlora,
New proposals for WHO International Standards for Human Herpesvirus 6 and Adenovirus
 New proposals for WHO International Standards for Human Herpesvirus 6 and Adenovirus Jacqueline Fryer Division of Virology, NIBSC National Institute for Biological Standards and Control Assuring the quality
New proposals for WHO International Standards for Human Herpesvirus 6 and Adenovirus Jacqueline Fryer Division of Virology, NIBSC National Institute for Biological Standards and Control Assuring the quality
Immunodeficiencies HIV/AIDS
 Immunodeficiencies HIV/AIDS Immunodeficiencies Due to impaired function of one or more components of the immune or inflammatory responses. Problem may be with: B cells T cells phagocytes or complement
Immunodeficiencies HIV/AIDS Immunodeficiencies Due to impaired function of one or more components of the immune or inflammatory responses. Problem may be with: B cells T cells phagocytes or complement
10/19/2012. Serologic Testing for Syphilis. Disclosures. Comparison of the Traditional and Reverse Screening Algorithms. Outline.
 Serologic Testing for Syphilis Comparison of the Traditional and Reverse Screening Algorithms Disclosures Elli S. Theel, Ph.D. Director, Infectious Diseases Serology Laboratory Assistant Professor of Laboratory
Serologic Testing for Syphilis Comparison of the Traditional and Reverse Screening Algorithms Disclosures Elli S. Theel, Ph.D. Director, Infectious Diseases Serology Laboratory Assistant Professor of Laboratory
Recognition of tissue cyst-specific antigens in reactivating toxoplasmosis
 J. Med. Microbiol. - Vol. 46 (1997), 587-595 0 1997 The Pathological Society of Great Britain and Ireland SE RO LOG I CAL AND M 0 LECU LAR D IAG N 0s IS Recognition of tissue cyst-specific antigens in
J. Med. Microbiol. - Vol. 46 (1997), 587-595 0 1997 The Pathological Society of Great Britain and Ireland SE RO LOG I CAL AND M 0 LECU LAR D IAG N 0s IS Recognition of tissue cyst-specific antigens in
8/11/16. Kevin Letz DNP, MSN, MBA, RN, CEN, CNE, FNP-C, PCPNP-BC, ANP-BC, FAANP
 Kevin Letz DNP, MSN, MBA, RN, CEN, CNE, FNP-C, PCPNP-BC, ANP-BC, FAANP Eosinophilia Eosinophilia refers to an absolute eosinophil count in the peripheral blood of 500 eosinophils/microl; this is considered
Kevin Letz DNP, MSN, MBA, RN, CEN, CNE, FNP-C, PCPNP-BC, ANP-BC, FAANP Eosinophilia Eosinophilia refers to an absolute eosinophil count in the peripheral blood of 500 eosinophils/microl; this is considered
Transplantation. Immunology Unit College of Medicine King Saud University
 Transplantation Immunology Unit College of Medicine King Saud University Objectives To understand the diversity among human leukocyte antigens (HLA) or major histocompatibility complex (MHC) To know the
Transplantation Immunology Unit College of Medicine King Saud University Objectives To understand the diversity among human leukocyte antigens (HLA) or major histocompatibility complex (MHC) To know the
Specific Requirements
 Specific Requirements AIMS Specific requirements your patients have for transfusion and how this is managed Classify which patients require: Irradiated components CMV negative components Washed components
Specific Requirements AIMS Specific requirements your patients have for transfusion and how this is managed Classify which patients require: Irradiated components CMV negative components Washed components
Hepatitis E and the English blood supply
 Hepatitis E and the English blood supply Mhairi Webster Microbiology Senior Scientist National Transfusion Microbiology Reference Laboratory With thanks to Dr Alan Kitchen Hepatitis E virus Small, non-enveloped
Hepatitis E and the English blood supply Mhairi Webster Microbiology Senior Scientist National Transfusion Microbiology Reference Laboratory With thanks to Dr Alan Kitchen Hepatitis E virus Small, non-enveloped
Reliable screening for early diagnosis
 Elecsys TORCH panel Reliable screening for early diagnosis Toxoplasmosis Rubella HSV CMV Toxoplasmosis The safe and sure approach to Toxo screening Ultrasensitive Toxo IgM optimized to detect all potential
Elecsys TORCH panel Reliable screening for early diagnosis Toxoplasmosis Rubella HSV CMV Toxoplasmosis The safe and sure approach to Toxo screening Ultrasensitive Toxo IgM optimized to detect all potential
Cytomegalovirus (CMV) Viral Load in Bronchoalveolar Lavage Fluid (BALF) and Plasma to Diagnose Lung Transplant Associated CMV Pneumonia
 Cytomegalovirus (CMV) Viral Load in Bronchoalveolar Lavage Fluid (BALF) and Plasma to Diagnose Lung Transplant Associated CMV Pneumonia I.P. Lodding 1,2, H.H. Schultz 3, J.U. Jensen 1,5, C. Andersen 4,
Cytomegalovirus (CMV) Viral Load in Bronchoalveolar Lavage Fluid (BALF) and Plasma to Diagnose Lung Transplant Associated CMV Pneumonia I.P. Lodding 1,2, H.H. Schultz 3, J.U. Jensen 1,5, C. Andersen 4,
Toxoplasmosis FACT SHEET. What is toxoplasmosis? Symptoms CONTACT US. Published 2016
 Toxoplasmosis What is toxoplasmosis? Toxoplasmosis is an infection caused by the parasite Toxoplasma gondii (T. gondii). The parasite is transmitted to people through eating undercooked meat, especially
Toxoplasmosis What is toxoplasmosis? Toxoplasmosis is an infection caused by the parasite Toxoplasma gondii (T. gondii). The parasite is transmitted to people through eating undercooked meat, especially
Epidemiology, diagnosis and control of Toxoplasma gondii in animals and food stuff
 Epidemiology, diagnosis and control of Toxoplasma gondii in animals and food stuff Aize Kijlstra Rome 2009 Toxoplasmosis is a neglected disease entity Disease burden is similar to salmonellosis and campylobacteriosis
Epidemiology, diagnosis and control of Toxoplasma gondii in animals and food stuff Aize Kijlstra Rome 2009 Toxoplasmosis is a neglected disease entity Disease burden is similar to salmonellosis and campylobacteriosis
11/19/2012. The spectrum of pulmonary diseases in HIV-infected persons is broad.
 The spectrum of pulmonary diseases in HIV-infected persons is broad. HIV-associated Opportunistic infections Neoplasms Miscellaneous conditions Non HIV-associated Antiretroviral therapy (ART)-associated
The spectrum of pulmonary diseases in HIV-infected persons is broad. HIV-associated Opportunistic infections Neoplasms Miscellaneous conditions Non HIV-associated Antiretroviral therapy (ART)-associated
ECMM Excellence Centers Quality Audit
 ECMM Excellence Centers Quality Audit Person in charge: Department: Head of Department: Laboratory is accredited according to ISO 15189 (Medical Laboratories Requirements for quality and competence) Inspected
ECMM Excellence Centers Quality Audit Person in charge: Department: Head of Department: Laboratory is accredited according to ISO 15189 (Medical Laboratories Requirements for quality and competence) Inspected
2017 CST-Astellas Canadian Transplant Fellows Symposium. EBV Post Transplantation Implications and Approach to Management
 2017 CST-Astellas Canadian Transplant Fellows Symposium EBV Post Transplantation Implications and Approach to Management Atul Humar, MD Atul Humar is a Professor in the Department of Medicine, University
2017 CST-Astellas Canadian Transplant Fellows Symposium EBV Post Transplantation Implications and Approach to Management Atul Humar, MD Atul Humar is a Professor in the Department of Medicine, University
Prophylaxis, Empirical, Pre-emptive Therapy of Aspergillosis in Hematological Patients: Which Strategy?
 TIMM-4 18-21 October 2009 Athens, Greece Prophylaxis, Empirical, Pre-emptive Therapy of Aspergillosis in Hematological Patients: Which Strategy? www.ichs.org Georg Maschmeyer Dept. of Hematology, Oncology
TIMM-4 18-21 October 2009 Athens, Greece Prophylaxis, Empirical, Pre-emptive Therapy of Aspergillosis in Hematological Patients: Which Strategy? www.ichs.org Georg Maschmeyer Dept. of Hematology, Oncology
Itraconazole vs. fluconazole for antifungal prophylaxis in allogeneic stem-cell transplant patients D. J. Winston
 REVIEW Itraconazole vs. fluconazole for antifungal prophylaxis in allogeneic stem-cell transplant patients D. J. Winston Division of Hematology-Oncology, Department of Medicine, UCLA Medical Center, Los
REVIEW Itraconazole vs. fluconazole for antifungal prophylaxis in allogeneic stem-cell transplant patients D. J. Winston Division of Hematology-Oncology, Department of Medicine, UCLA Medical Center, Los
Virological Surveillance in Paediatric HSCT Recipients
 Virological Surveillance in Paediatric HSCT Recipients Dr Pamela Lee Clinical Assistant Professor Department of Paediatrics & Adolescent Medicine Queen Mary Hospital LKS Faculty of Medicine, The University
Virological Surveillance in Paediatric HSCT Recipients Dr Pamela Lee Clinical Assistant Professor Department of Paediatrics & Adolescent Medicine Queen Mary Hospital LKS Faculty of Medicine, The University
HIV and Parasite Load. Keith Keller
 HIV and Parasite Load Keith Keller HIV: Human Immunodeficiency Virus Retrovirus that infects CD4+ T Cells Uses host cell s mechanics to reproduce, by means of reverse transcriptase and integrase which
HIV and Parasite Load Keith Keller HIV: Human Immunodeficiency Virus Retrovirus that infects CD4+ T Cells Uses host cell s mechanics to reproduce, by means of reverse transcriptase and integrase which
Pneumocystis jirovecii (Pj) quantitative PCR to differentiate Pj pneumonia from Pj colonization in immunocompromised patients
 DOI 10.1007/s10096-013-1960-3 ARTICLE Pneumocystis jirovecii (Pj) quantitative PCR to differentiate Pj pneumonia from Pj colonization in immunocompromised patients M. Maillet & D. Maubon & J. P. Brion
DOI 10.1007/s10096-013-1960-3 ARTICLE Pneumocystis jirovecii (Pj) quantitative PCR to differentiate Pj pneumonia from Pj colonization in immunocompromised patients M. Maillet & D. Maubon & J. P. Brion
BRINCIDOFOVIR WAS USED TO SUCCESSFULLY TREAT ADENOVIRUS INFECTIONS IN SOLID ORGAN TRANSPLANT RECIPIENTS AND OTHER IMMUNOCOMPROMISED PATIENTS
 BRINCIDOFOVIR WAS USED TO SUCCESSFULLY TREAT ADENOVIRUS INFECTIONS IN SOLID ORGAN TRANSPLANT RECIPIENTS AND OTHER IMMUNOCOMPROMISED PATIENTS Diana F. Florescu, MD 1, Michael S. Grimley, MD 2, Genovefa
BRINCIDOFOVIR WAS USED TO SUCCESSFULLY TREAT ADENOVIRUS INFECTIONS IN SOLID ORGAN TRANSPLANT RECIPIENTS AND OTHER IMMUNOCOMPROMISED PATIENTS Diana F. Florescu, MD 1, Michael S. Grimley, MD 2, Genovefa
Title: Increased incidence of traffic accidents in RhD-negative, Toxoplasma gondii-infected military drivers revealed by a prospective cohort study
 Author's response to reviews Title: Increased incidence of traffic accidents in RhD-negative, Toxoplasma gondii-infected military drivers revealed by a prospective cohort study Authors: Jaroslav Flegr
Author's response to reviews Title: Increased incidence of traffic accidents in RhD-negative, Toxoplasma gondii-infected military drivers revealed by a prospective cohort study Authors: Jaroslav Flegr
3.1 Clinical safety of chimeric or humanized anti-cd25 (ch/anti-cd25)
 3 Results 3.1 Clinical safety of chimeric or humanized anti-cd25 (ch/anti-cd25) Five infusions of monoclonal IL-2 receptor antibody (anti-cd25) were planned according to protocol between day 0 and day
3 Results 3.1 Clinical safety of chimeric or humanized anti-cd25 (ch/anti-cd25) Five infusions of monoclonal IL-2 receptor antibody (anti-cd25) were planned according to protocol between day 0 and day
Gastroenteritis and viral infections
 Gastroenteritis and viral infections A Large number of viruses are found in the human gut; these include some that are associated with gastroenteritis Rotaviruses Adenoviruses 40/41 Caliciviruses Norwalk-like
Gastroenteritis and viral infections A Large number of viruses are found in the human gut; these include some that are associated with gastroenteritis Rotaviruses Adenoviruses 40/41 Caliciviruses Norwalk-like
