data indicating a 31% efficacy in northwest Colombia. 6
|
|
|
- Giles Harrington
- 6 years ago
- Views:
Transcription
1 Am. J. Trop. Med. Hyg., 64(3, 4), 2001, pp Copyright 2001 by The American Society of Tropical Medicine and Hygiene TREATMENT FAILURE IN CHILDREN IN A RANDOMIZED CLINICAL TRIAL WITH 10 AND 20 DAYS OF MEGLUMINE ANTIMONATE FOR CUTANEOUS LEISHMANIASIS DUE TO LEISHMANIA VIANNIA SPECIES RICARDO PALACIOS, LYDA ELENA OSORIO, LUIS FERNANDO GRAJALES, AND MARÍA TERESA OCHOA Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM), Cali, Colombia Abstract. Clinical response to supervised treatment of Colombian patients with cutaneous leishmaniasis was evaluated in a randomized controlled trial comparing 10 days versus 20 days of treatment with meglumine antimonate (20 mg Sb/kg/day). Masked examiners evaluated clinical response defined as 100% re-epithelialization of all lesions at 13 weeks and no relapses during 52 weeks of follow-up. The efficacy of meglumine antimonate for 10 days treatment was 61% (28 of 46) compared to 67% (24 of 36) for 20 days. There was a significantly lower clinical response for children 5 years in both 10-day (11%) and 20-day (25%) groups compared to patients aged 5 14 years (67% and 75%, respectively) and 15 years or more (81% and 83%, respectively). Overall efficacy of treatment schedules was comparable, but lower than expected, mainly because of low efficacy in children. Pathogenicity of infection and pharmacokinetics may affect the treatment response in children. New therapeutic alternatives should be evaluated in trials that include children and women. INTRODUCTION 187 In the New World, more than thirty-nine million people are at risk for cutaneous leishmaniasis and the estimated annual number of cases of this vector-borne disease is approximately 60, Most of these cases are due to species of the Leishmania Viannia subgenus. Systemic treatment is recommended for cutaneous leishmaniasis caused by Leishmania of this subgenus because of the risk of metastasis and mucosal involvement. Pentavalent antimonial compounds, pentamidine isethionate, and amphotericin B, are the currently available drugs with proven efficacy. Pentavalent antimonials have been the first-line therapy for cutaneous leishmaniasis since 1947, 2 in part because of the risk of more serious or irreversible toxicity with amphotericin B and pentamidine. Nevertheless, frequent adverse effects, parenteral route of administration (IV or IM), and the expense of the drug contribute to a high frequency of non-adherence to the current recommended treatment of 20 mg Sb/kg/d for 20 days. Daily doses less than 20 mg Sb/kg/d for days have shown a lower efficacy in previous clinical trials. 3 The logistic and economic impossibility of universal availability of the recommended dose for all patients in disease endemic countries and evidence of differing sensitivities of Leishmania species have motivated the evaluation of alternative regimens. 4 Reduction in the daily dose with a longer period of administration has been evaluated with success in Brazil. 5 However, a reduction in the duration of treatment, rather than in the daily dose, could decrease adverse effects, as well as the number of contacts with medical personnel, thereby improving adherence and lowering costs. In this regard, Arana and others reported 90% efficacy using ten days of antimonial therapy in Guatemala for lesions caused by Leishmania (Viannia) braziliensis in young male soldiers. 4 In addition, in a pilot study conducted by our group in the Colombian Pacific coast area, a 100% cure rate was achieved in 10 adult patients infected in areas where Leishmania (Viannia) panamensis predominates (Ochoa MT, unpublished data). However, Soto and others cited a report of historical data indicating a 31% efficacy in northwest Colombia. 6 The present investigation sought to determine whether 10 days of treatment with 20 mg Sb/kg/d had an efficacy comparable to that of 20 days with the same dosage (i.e., no greater than 25% reduction in efficacy) for the supervised treatment of patients infected in endemic areas for cutaneous leishmaniasis where L. (V.) panamensis is the predominant species. In order to address this question taking into consideration the patient profile of endemic areas, women and children were included in the trial and supervised treatment was achieved with the participation and training of health volunteers recruited from the affected communities. MATERIALS AND METHODS Patients. Patients consulting the clinical facilities of the Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM) in Cali and Tumaco from April 1996 to March 1997 and who had a parasitological diagnosis of cutaneous leishmaniasis were invited to participate in the study. Tumaco is an endemic area on the Colombian Pacific Coast where most of recruited patients live in rural areas. Cali is the largest city in the southwestern region, and patients residing in non-endemic areas are often referred to Cali for diagnostic tests and medical attention. Patients who had been treated previously with antimonials, ketoconazole, or another imidazole, amphotericin B or pentamidine, as well as those with mucosal leishmaniasis, severe cardiovascular, renal, hepatic, or pancreatic disease, and pregnant or nursing women were excluded from the trial. After explaining the purpose, potential risks and benefits and voluntary nature of participation, signed consent was obtained from each patient or from the guardian in the case of children less than 18 years of age. All protocols were reviewed and approved by the Institutional Review Board of CIDEIM in accordance with the guidelines of the United States Federal Policy for Protection of Human Subjects (Title 45, Code of Federal Regulations, Part 46) and the Colombian Ministry of Health.
2 188 PALACIOS AND OTHERS Direct smear, culture of aspirates, and a skin biopsy of the skin lesions, histopathology, and hamster inoculation were used for parasitological diagnosis and parasite isolation as described elsewhere. 7 The area of each lesion was calculated by multiplying its longest horizontal and perpendicular axes. 8 Parasites were identified by species using monoclonal antibodies 9 and/or isoenzyme analysis. 10 After medical examination, patients who were eligible and agreed to participate in the study were randomized to 10 or 20 days of treatment. The assignment process was performed using permuted block randomization. 11 Meglumine antimonate was prescribed according to the current recommendations of the Pan-American Health Organization/World Health Organization (PAHO/WHO), and the Ministry of Health of Colombia at a dosage of 20 mg Sb/Kg/day with no upper limit on the daily dose. 12 Treatment was applied intramuscularly, once a day during 10 or 20 days depending on the study group. The Ministry of Health of Colombia provided the meglumine antimonate (Glucantime, Rhone Poulenc-Rorer, France) and only two lots of drugs (312-2 and 312-3) were utilized. Due to the ethical considerations of applying a placebo by intramuscular injection over 10 days, the administration of treatment was not masked. Before the trial began, the research team trained health volunteers from the community of Tumaco in the administration of intramuscular injection. Thereafter, these volunteers applied the treatment to patients in their communities. Supervision of treatment was achieved by regular periodic visits by research personnel and a record of the daily dose administered to each patient signed by the corresponding volunteer. Each registry was reviewed at the end of the treatment and compared with the number of ampoules (empty or full) that were returned. In Cali, the treatment was administered by the physician in charge of the clinic and supervised by the use of a signed registry as in Tumaco. In order to define clinical response, all patients were clinically evaluated at 13, 26, and 52 weeks after initiation of treatment by a physician other than the one prescribing treatment and who was masked with respect to which study group the patient belonged. The response was classified at 13 weeks after the beginning of therapy as initial clinical response if the patient had complete re-epithelialization and absence of inflammatory signs of all lesions. Those patients with initial clinical response and no relapses of lesions between 13 and 52 weeks of follow-up were classified as having a final clinical response. Otherwise, the response was considered as a clinical failure. The adverse effects of antimonials were monitored over the treatment period using a structured questionnaire. Study personnel or health volunteers questioned patients about the presence of malaise, headache, myalgias, arthralgias, and anorexia. No laboratory tests were performed to evaluate toxicity of the treatments. Patients in either group who were classified as a clinical failure received a new treatment with meglumine antimonate at the same dose for 20 days. Two cultures of aspirates from the scars (initial clinical response) or active lesions (clinical failure) were performed at week 13 of follow-up for the evaluation of parasitological response. The sample size to detect a 25% or greater difference in efficacy between the 10- and 20-day treatment groups with a 95% confidence level and a beta error of 20% was 42 patients per group. A 25% difference was considered clinically significant, taking into account the potential benefits of a 10-day treatment as opposed to 20 days, the high efficacy of pentavalent antimonials reported in the literature for the treatment of New World cutaneous leishmaniasis (88 100%) 5,8,13 16 and the 100% cure we obtained in a previous pilot study involving 10 adult patients treated with meglumine antimonate for 10 days (Ochoa MT, unpublished data). The number of participants in the total study population was increased to 136 to compensate for the expected loss to follow-up. If the amount of drug that a patient received was 10% above or 10% below the target dosage of 20 mg Sb/kg/d, the dose was considered inadequate. Patients who received doses in a period longer than 12 days in the 10-dose group or 25 days in the 20-dose group, were considered non-adherent to treatment. Patients who received an inadequate dose or who were non-adherent to treatment as described above, or who missed the 13 th week follow-up (analysis of initial response), and/or the 52-week follow-up (final response) were excluded from the efficacy analysis. A cutaneous lesion was considered new if located in a site different from the initial lesion and distant from its lymphatic drainage. 17 Patients presenting a new parasitologically-confirmed cutaneous leishmaniasis lesion during the follow-up were also excluded. Non-adherent patients and those who missed follow-up examinations were, however, included as clinical failures in our analysis of minimal effectiveness. Data analysis was performed using SPSS 7.5 for Windows 1996 (SPSS Inc., Chicago, IL). The proportion of therapeutic response and its 95% confidence intervals were compared between groups. A chi-square test and a Fisher exact test were used for the comparison of categorical data. Comparison of two groups by no normally distributed continuous data was performed using non-parametric test (Mann-Whitney U test). For all the tests, P values less than 0.05 were considered statistically significant. RESULTS One hundred and thirty-six patients of 191 eligible individuals were enrolled in the study. Nine patients were recruited in Cali (7%) and 127 in Tumaco (93%). Reasons for ineligibility and the process of enrollment and follow-up of patients are shown in Figure 1. Sixty-eight patients were randomized to each study group. The two groups were comparable in sociodemographic and principal clinical characteristics, although patients randomized to receive 20 days of treatment bore lesions with median areas that were significantly larger and had less frequent regional adenopathies than patients who received 10 days of treatment. The 136 patients had a total of 321 cutaneous lesions (Table 1). Of the 136 patients randomized initially, 56 (41%) were not included in the efficacy analysis: six received an inadequate dose, 32 were non-adherent to treatment, 13 did not return for the 13-week follow-up examination, and three did not return for the 52-week evaluation (Figure 1). A total of 46 patients in the 10-day group and 36 in the 20-day group were analyzed for final response. Patients not included in the
3 LOW RESPONSE TO MEGLUMINE ANTIMONATE IN CHILDREN 189 FIGURE 1. Trial profile efficacy analysis did not differ in sociodemographic and principal clinical characteristics from those analyzed. The efficacy of meglumine antimonate for the 10-day treatment regimen was 62% (29 of 47) at the 13 th week evaluation and 61% (28 of 46) at the 52 nd week of follow-up. The initial response was 71% for the 20-day treatment regimen (27 of 38) and the final response 67% (24 of 36). There was not a significant difference between groups in the efficacy analysis at the initial or final response. The 95% confidence interval of the difference between the final clinical response of the 10-day and 20-day groups includes zero ( to 0.134). As shown in Figure 2, confidence intervals of response to treatment in both groups were wide and overlapped. When we included those patients who were lost to followup or non-adherent with the medication, the calculated minimum effectiveness for 10 days of meglumine antimonate was classified as initial clinical response in 56% (38 of 68) of patients and as final clinical response in 46% (31 of 68). In the 20-day group the initial clinical response was observed in 65% (44 of 68) of patients, but decreased to 57% (39 of 68) in the evaluation of final response. In order to identify possible risk factors for treatment failure, comparisons between patients who failed and those who responded to treatment were performed in the 10-day and 20-day groups. A significant difference in final clinical response rates was observed between children under 5 years for both 10 days (11%, n 9) and 20 days (25%, n 8) of treatment as compared with children from 5 to 15 years TABLE 1 Demographic and clinical characteristic of the randomized patients 10-day course n 68 (%) 20-day course n 68 (%) P value (10 versus 20 days) Gender Male 40 (58.8) 36 (52.9) 0.49 Female 28 (41.2) 32 (47.1) Race Black Indigenous Mestizo White 42 (61.8) 17 (25.0) 9 (13.2) 0 45 (66.2) 15 (22.1) 6 (8.8) 2 (2.9) Weight (kg) Age (years) Median Median (22.2) 29 (42.6) 13 (19.1) 8 (11.8) 3 (4.4) (25.0) 25 (36.8) 16 (23.5) 5 (7.4) 4 (5.9) Residence in endemic area Age of lesion (months) Yes Median (91.2) 2 38 (56) 21 (31) 3 (4) 6 (9) 58 (85.3) 2 44 (65) 17 (25) 4 (6) 3 (4) Lesions Total Number per patient (median) Lesion characteristics Ulcer Plaque Nodule Papule Regional adenopathy Satellite lesion Area (median) 119 (71) 29 (17) 10 (6) 8 (5) 35 (29) 39 (23) mm (77) 21 (14) 9 (5) 5 (4) 27 (17) 45 (29) mm * * Meglumine antimonate prescribed dose Median (mg Sb/kg/day) * P 0.05
4 190 PALACIOS AND OTHERS FIGURE 2. Initial and final clinical response to treatment with meglumine antimonate in the ten- and twenty-day treatment groups. FIGURE 3. Clinical response to treatment with ten and twenty days of meglumine antimonate by age groups. (67%, n 21, P for 10 days and 75%, n 16, P for 20 days) and with patients older than 15 years (81%, n 16, P for 10 days and 83%, n 12, P for 20 days group) (Figure 3). This difference was also found using the minimum effectiveness calculated as described above in the 10-day group ( 5 years; 7% versus 5 to 14 years: 53%, P and versus 15 years or more 61%, P ) and the 20-day group ( 5 years: 24% versus 5 to 14 years: 68%, P and versus 15 years or more 69%, P 0.004). Statistically significant differences were not detected in the number of lesions (1 or 2 versus 3 or more), time of evolution (0 3 months and 3 months), localization, total area of lesions, lot of drug, or gender. Lesions of all the patients with treatment failure at the week 13 evaluation had substantially improved and healed after the second course of treatment schedule with 20 days of meglumine antimonate. Parasite isolates obtained from 88 cultures were identified. Most cases (84) were due to L. (V.) panamensis. Leishmania (V.) braziliensis was identified in 4 cases. Of 38 who were not completely healed at 13 weeks, 8 (21%) had a positive culture of the aspirate obtained at 13 weeks. One patient was culture-positive among 68 who were completely healed by 13 weeks and did not have a cutaneous or mucosal recurrence by 52 weeks of follow-up and who had their scars aspirated. The most frequent adverse reactions in both the 10-day and 20-day groups were anorexia (25%), headache (25%), myalgias (24%), and malaise (23%) without statistically significant differences between groups. In contrast, arthralgias were significantly more frequent (P 0.021) in patients who were treated for 20 days with meglumine antimonate (25%) than those who were treated for 10 days (9%). No patient stopped treatment because of adverse effects (Figure 4). DISCUSSION It is logistically challenging to conduct clinical trials in endemic areas for leishmaniasis that take into account the facilities available to the community and health system, as FIGURE 4. Rate of adverse effects with meglumine antimonate in patients with adherence to treatment for 10- and 20-dose groups. The lines represent the 95% confidence interval.
5 LOW RESPONSE TO MEGLUMINE ANTIMONATE IN CHILDREN 191 well as the gender and age composition of the patient population. Ambulatory treatment is the rule in endemic areas and non-adherence is a high risk both outside and within the framework of a clinical trial. The results of this study underscore the advantage of conducting clinical trials in the endemic communities with the affected population. The overall low efficacy of pentavalent antimonials in this population and the relationship of age with poor clinical response would not have been detected in the adult male populations usually recruited for efficacy studies. 5,8,13,15,16 This study was designed as a two-arm active-medicine clinical trial because the use of placebo was considered unethical in view of the risk of mucosal or cutaneous relapses reported previously in the area. 17 The results did not reveal significant differences in efficacy between 10 and 20 days of supervised treatment with 20 mg Sb/kg/d. However, this does not imply that both treatment schedules have the same effect because there is no test to establish that a statistically significant similarity exists. 11 Clinical response is considered to be the main indicator of the therapeutic response in New World cutaneous leishmaniasis because of the lack of concordance between the parasitological and clinical response to treatment in this trial and other studies in which viable parasites have been isolated from healed lesions. 14,15,18 The role of these parasites in the reactivation of lesions or metastasis 17 and as a source of infection in a putative anthroponotic cycle of the Leishmania Viannia 19 are issues that need to be elucidated through experiments to demonstrate the availability of the parasite in infected humans. Comparing our data with other trials conducted in patients with New World cutaneous leishmaniasis, the response rate observed in this study for ten days of treatment with meglumine antimonate was higher (i.e., 31%, noted by Soto and others in Urabá, in northwest Colombia n 26, 95% CI, 15 52%); however, the sociodemographic characteristics of the patients included in that study were not described. 6 On the other hand, the unexpectedly low response to the recommended dose and duration of therapy using pentavalent antimonials in our study merits special attention. Published efficacy trials of pentavalent antimonial drugs using a regimen of 20 mg Sb/kg/d for 20 days in Colombia and the Americas, have documented an efficacy of 88 to 100% for the treatment of cutaneous leishmaniasis caused by species of the Leishmania Viannia subgenus. 5,8,13 16,20 One group in the Colombian southwest and close to our study area, reported efficacy of meglumine antimonate with a 15-day regimen, to be 40%, 21,22 and close to the 36 37% efficacy of placebo reported by other investigators in other areas of Colombia. 8,20 All of these trials have involved young-adult populations primarily. Our study, in contrast, included a high proportion of children (39.7% aged 5 to 14 years, 23.5% 5 years). The age distribution of the study participants reflects the high incidence (28.7 cases/10,000 population 5 years versus 13.7 cases/10,000 general population in 1996, Palacios R, unpublished data) of clinically apparent infection in this age group during this period in the study site, the municipality of Tumaco. A previous study in this area showed that younger age is associated with higher pathogenicity. 23 Children younger than 5 years of age had a particularly poor treatment response (10 days: 11%, 20 days: 25%), while the adults responded within the low end of the range reported in other studies. The high proportion of children in this study clearly influenced the observed efficacy, and we believe that this finding merits further investigation. The factors that account for these results may be related to the drug, the parasite, and the host. In relation to the drug, all the patients received either of two lots of meglumine antimonate (Glucantime, Rhone Poulenc-Rorer, Paris, France, lots and 312-3). With respect to the parasites, all the patients contracted their infections in areas where L. panamensis predominates and L. braziliensis is locally transmitted. 10 The identification by species of parasites isolated from the participating patients substantiates the predominance of L. panamensis and comparable distribution of the two species among the age and treatment groups of participants. Therefore, neither differences in the drug received nor the infecting species of Leishmania explain the disparity in efficacy between adults and children or between the treatment groups. Worthy of note, the clinical-failure treatment was not absolute; healing was achieved by a second course of treatment with 20 mg Sb/kg/d for 20 days. However, we cannot rule out differences in the sensitivity to Sb of the Leishmania strains circulating in the study area. Widely divergent sensitivity of Leishmania to pentavalent antimonials has been reported, 24,25 and could have been a factor in the response to treatment. 26 The mechanisms of experimentallyselected resistance strains are currently being studied. 27 In relation to the host factors that affect the response to treatment, the immune response, either innate or acquired, may differ in adults and children, especially if adults with a long history of residence in the endemic area had previously experienced infection. Prior disease evidenced by a typical scar has been shown to be associated with a more marked delayed-type hypersensitivity response to leishmanin and a lower number of parasites in subsequent lesions. 28 In this study, most of the adults that participated had lived more than ten years in the endemic area. Another possibility is that the pharmacokinetics of pentavalent antimonial compounds differ in children and adults. Preliminary data show that the four children aged 7 years or less who were included in a pharmacokinetic study conducted by the US Centers for Disease Control and Prevention (CDC) and its collaborators had approximately a 3 5-fold higher volume of distribution (when normalized by body weight) for pentavalent antimony and approximately 2-fold higher clearance of antimony than adults (Herwaldt and others, unpublished data). Therefore, the serum levels achieved in children receiving the recommended dose were lower than those in adults. If this observation is confirmed, the distinct pharmacokinetic behavior of Sb in children could explain the low efficacy in this age group. Patients younger than 5 years of age with cutaneous leishmaniasis due to Leishmania Viannia have not previously been included in a controlled clinical trial of efficacy of pentavalent antimonials, despite the importance of this age group in the endemic areas Given the low response rate in children, the power to detect differences in adults between the 10- and 20-day treatment groups was limited because of the reduction of the sample size in the stratified analysis by age groups and the relatively high rate of non-adherence to the treatment. Nevertheless, our data do not provide compelling evidence for
6 192 PALACIOS AND OTHERS the superiority of 20 days of treatment compared with 10 days, especially given the increased rate of adverse effects, particularly arthralgias, and higher costs for the patients and the health system and the risk of non-adherence with the 20- day regimen. Therefore, we consider the use of a 10-day regimen in patients older than 5 years of age with uncomplicated cutaneous leishmaniasis along with an obligatory clinical evaluation 13 weeks after the treatment began to identify patients requiring re-treatment, to represent a practical and achievable therapeutic alternative in endemic areas of the Colombian Pacific coast. The overall efficacy of both treatment schedules was lower than expected, and this was particularly dramatic in young children. These findings underscore the need for alternatives to the currently available treatments for cutaneous leishmaniasis due to Leishmania Viannia. The trials evaluating these alternatives should include women and children groups with high prevalence and vulnerability to leishmaniasis in endemic areas. Acknowledgments: The authors wish to thank Thomas R. Navin for the assessment in the design and critical review of the manuscript, and Barbara L. Herwaldt and Nancy Gore Saravia for their valuable contributions to this manuscript. The participation in this trial of the health volunteers from the affected communities and the logistic support of the Hospital San Andrés and the Departamento de Control de Patologías Tropicales in Tumaco is much appreciated. The technical services of the personnel of the Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM), particularly of José Rafael Tovar (statistical assistance); of Aura García, Andrés Dajome, and of Wilson Cortés (field assistance); and of Jaime Muñoz, Carmen Castillo, Marta Chica, Rubén Varela, and Hector Hernández (diagnosis and clinical evaluations), are gratefully acknowledged. Financial support: This work was supported by the Ministry of Health of Colombia and by a Young Investigator Studentship Grant from Colciencias to Ricardo Palacios. Authors addresses: Ricardo Palacios, Lyda Elena Osorio, Luis Fernando Grajales and María Teresa Ochoa, CIDEIM, Avenida 1 Norte Phone (57 2) Fax (57 2) Cali, Colombia. clinica@cideim.org.co Reprint requests: Ricardo Palacios, M.D. Clinical and Community Research Unit, CIDEIM, Avenida 1 Norte Phone: (57 2) Fax: (57 2) Cali, Colombia. clinica@cideim.org.co REFERENCES 1. Ashford RW, Desjeux P, deraadt P, Estimation of population at risk of infection and number of cases of leishmaniasis. Parasitology Today 8: Berman J, Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis 24: Herwaldt B, Berman J, Recommendations for treating leishmaniasis with sodium stibogluconate (Pentostam ) and review of pertinent clinical studies. Am J Trop Med Hyg 46: Arana B, Navin T, Arana F, Berman J, Rosenkaimer F, Efficacy of a short course (10 days) of high-dose meglumine antimonate with or without interferon- in treating cutaneous leishmaniasis in Guatemala. Clin Infect Dis 18: Oliveira-Neto MP, Schubach A, Mattos M, Goncalves-Costa SC, Pirmez C, A low-dose antimony treatment in 159 patients with American cutaneous leishmaniasis: extensive follow-up studies (up to 10 years). Am J Trop Med Hyg 57: Soto J, Hernández N, Mejia H, Grogl M, Berman J, Successful treatment of New World cutaneous of topical paramomycin/methilbenzethonium chloride and injectable meglumine antimonate. Clin Infect Dis 20: Weigle K, de Dávalos M, Heredia P, Molineros R, Saravia N, D Alessandro A, Diagnosis of cutaneous and mucocutaneous leishmaniasis in Colombia: comparison of seven methods. Am J Trop Med Hyg 36: Soto-Mancipe J, Grogl M, Berman J, Evaluation of Pentamidine for the treatment of cutaneous leishmaniasis in Colombia. Clin Infect Dis 16: McMahon-Pratt D, David JR, Monoclonal antibodies that distinguish between New World species of Leishmania. Nature 291: Saravia N, Segura I, Holguin A, Santrich C, Valderrama C, Ocampo C, Epidemiologic, genetic and clinical associations among phenotypically distinct populations of Leishmania (Viannia) in Colombia. Am J Trop Med Hyg 59: Spilker B, Guide to Clinical Trials. New York: Raven Press, Ministry of Health of Colombia, Leishmaniasis: Plan Nacional de Control. Bogotá: The Ministry of Health. 13. Ballou R, Gordon D, Andujar J, McClain B, Shanks D, Berman J, Chulay J, Safety and efficacy of high-dose sodium stibogluconate therapy of American cutaneous leishmaniasis. Lancet 2(8549): Guderian RH, Chico ME, Rogers MD, Pattishall KM, Grogl M, Berman JD, Placebo controlled treatment of Ecuadorian cutaneous leishmaniasis. Am J Trop Med Hyg 45: Navin T, Arana B, Arana F, Berman J, Chajón J, Placebocontrolled clinical trial of stibogluconate (Pentostam ) versus ketoconazole for treating cutaneous leishmaniasis in Guatemala. J Infect Dis 165: Hepburn N, Tidman M, Hunter J, Aminosidine (Paromomycin) versus sodium stibogluconate for the treatment of American cutaneous leishmaniasis. Trans R Soc Trop Med Hyg 88: Saravia NG, Weigle K, Segura I, Giannini SH, Pacheco R, Labrada LA, Goncalves A, Recurrent lesions in human Leishmania braziliensis infection reactivation or reinfection? Lancet 336: Schubach A, Marzochi MC, Cuzzi-Maya T, Olivira AV, Araujo ML, Oliveira AL, Pacheco RS, Momen H, Conceicao-Silva F, Coutinho SG, Marzochi KB, Cutaneous scars in American tegumentary leishmaniasis patients: a site of Leishmania (Viannia) braziliensis and viability eleven years after antimonial therapy and clinical cure. Am J Trop Med Hyg 58: Montoya-Lerma J, Palacios R, Osorio L, Jaramillo C, Cadena H, Further evidence of humans as source of Leishmania Viannia for sandflies. Mem Inst Oswaldo Cruz 93: Velez I, Agudelo S, Hendrockx E, Puerta J, Grogl M, Modabber F, Berman J, Inefficacy of allopurinol as monotherapy for Colombian cutaneous leishmaniasis. A randomized controlled trial. Ann Intern Med 126: Martinez S, Marr J, Allopurinol in the treatment of American cutaneous leishmaniasis. N Eng J Med 326: Martinez S, González M, Vernaza ME, Treatment of cutaneous leishmaniasis with allopurinol and stibogluconate. Clin Infect Dis 24: Weigle KA, Santrich C, Martinez F, Valderrama L, Saravia NG, Epidemiology of cutaneous leishmaniasis in Colombia: environmental and behavioral risk factors for infection, clinical manifestations, and pathogenicity. J Infect Dis 168: Grogl M, Thomason TN, Frankee ED, Drug resistance in leishmaniasis: its implications in systemic chemotherapy of cutaneous and mucocutaneous disease. Am J Trop Med Hyg 47: Robledo SM, Valencia AZ, Saravia NG, Sensitivity to Glucantime of Leishmania Viannia isolated from patients prior to treatment. J Parasitol 85: Lucumi A, Robledo R, Gama V, Saravia NG, Sensitivity
7 LOW RESPONSE TO MEGLUMINE ANTIMONATE IN CHILDREN 193 of Leishmania Viannia panamensis to pentavalent antimony is correlated with the formation of cleavable DNA-protein complexes. Antimicrob Ag Chemoth 42: Papadopoulou B, Kündig C, Singh A, Ouelette M, Drug resistance in Leishmania: similarities and differences to other organisms. Drug Resist Updates 1: Gutierrez Y, Salinas GH, Palma G, Valderrama LB, Santrich CV, Saravia NG, Correlation between histopathology, immune response, clinical presentation, and evolution in Leishmania braziliensis infection. Am J Trop Med Hyg 45: Weigle KA, Saravia NG, de Davalos M, Moreno LH, D Alessandro A, Leishmania braziliensis from the Pacific Coast region of Colombia: foci of transmission, clinical spectrum and isoenzyme phenotypes. Am J Trop Med Hyg 35: Alcais A, Abel L, David C, Torrez ME, Flandre P, Dedet JP, Risk factors for onset of cutaneous and mucocutaneous leishmaniasis in Bolivia. Am J Trop Med Hyg 57: Armijos RX, Weigel MM, Izurieta R, Racines J, Zurita C, Herrera W, Vega M, The epidemiology of cutaneous leishmaniasis in subtropical Ecuador. Trop Med Int Health 2: Costa JM, Balby IT, Rocha EJ, da Silva AR, Rebelo JM, Ferreira LA, Gama ME, Branco M dos R, Burattini MN, Soares N, 1998.Comparative study of American tegumentary leishmaniasis between childhood and teenagers from the endemic areas Buriticupu, Maranhão and Corte de Pedra, Bahía, Brazil. Rev Soc Bras Med Trop 31:
Miltefosine for New World Cutaneous Leishmaniasis
 MAJOR ARTICLE for New World Cutaneous sis J. Soto, 1 B. A. Arana, 4 J. Toledo, 1 N. Rizzo, 4 J. C. Vega, 2 A. Diaz, 4 M. Luz, 1 P. Gutierrez, 1 M. Arboleda, 3 J. D. Berman, 6,a K. Junge, 5 J. Engel, 5
MAJOR ARTICLE for New World Cutaneous sis J. Soto, 1 B. A. Arana, 4 J. Toledo, 1 N. Rizzo, 4 J. C. Vega, 2 A. Diaz, 4 M. Luz, 1 P. Gutierrez, 1 M. Arboleda, 3 J. D. Berman, 6,a K. Junge, 5 J. Engel, 5
Treatment of Cutaneous Leishmaniasis with Allopurinol and Stibogluconate
 165 Treatment of Cutaneous Leishmaniasis with Allopurinol and S. Martinez, M. Gonzalez, and M. E. Vernaza From the Department of Internal Medicine, University of Cauca, Popayan, and the University Hospital
165 Treatment of Cutaneous Leishmaniasis with Allopurinol and S. Martinez, M. Gonzalez, and M. E. Vernaza From the Department of Internal Medicine, University of Cauca, Popayan, and the University Hospital
Intralesional Infiltration with Meglumine Antimoniate for the Treatment of Leishmaniasis Recidiva Cutis in Ecuador
 In order to provide our readers with timely access to new content, papers accepted by the American Journal of Tropical Medicine and Hygiene are posted online ahead of print publication. Papers that have
In order to provide our readers with timely access to new content, papers accepted by the American Journal of Tropical Medicine and Hygiene are posted online ahead of print publication. Papers that have
Evidence for Leishmania (Viannia) Parasites in the Skin and Blood of Patients Before and After Treatment
 MAJOR ARTICLE Evidence for Leishmania (Viannia) Parasites in the Skin and Blood of Patients Before and After Treatment Carolina Vergel, Ricardo Palacios, a Horacio Cadena, Claudia Jimena Posso, Liliana
MAJOR ARTICLE Evidence for Leishmania (Viannia) Parasites in the Skin and Blood of Patients Before and After Treatment Carolina Vergel, Ricardo Palacios, a Horacio Cadena, Claudia Jimena Posso, Liliana
Intralesional meglumine antimoniate for the treatment of localised cutaneous leishmaniasis: a retrospective review of a Brazilian referral centre
 512 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 111(8): 512-516, August 2016 Intralesional meglumine antimoniate for the treatment of localised cutaneous leishmaniasis: a retrospective review of a Brazilian
512 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 111(8): 512-516, August 2016 Intralesional meglumine antimoniate for the treatment of localised cutaneous leishmaniasis: a retrospective review of a Brazilian
Treatment of cutaneous leishmaniasis among travellers
 Journal of Antimicrobial Chemotherapy (2004) 53, 158 166 DOI: 10.1093/jac/dkh058 Advance Access publication 16 January 2004 Treatment of cutaneous leishmaniasis among travellers J. Blum 1 *, P. Desjeux
Journal of Antimicrobial Chemotherapy (2004) 53, 158 166 DOI: 10.1093/jac/dkh058 Advance Access publication 16 January 2004 Treatment of cutaneous leishmaniasis among travellers J. Blum 1 *, P. Desjeux
SUMMARY. Cutaneous leishmaniasis with only skin involvement: single to multiple skin ulcers, satellite lesions and nodular lymphangitis.
 SUMMARY Leishmaniasis is a disease affecting predominantly people in the developing countries; 350 million people worldwide are at risk and yearly more than 2 million new cases occur. Leishmaniasis is
SUMMARY Leishmaniasis is a disease affecting predominantly people in the developing countries; 350 million people worldwide are at risk and yearly more than 2 million new cases occur. Leishmaniasis is
Acta Dermatovenerol Croat 2008;16(2):60-64 CLINICAL ARTICLE
 2008;16(2):60-64 CLINICAL ARTICLE Clinical Efficacy of Intramuscular Meglumine Antimoniate Alone and in Combination with Intralesional Meglumine Antimoniate in the Treatment of Old World Cutaneous Leishmaniasis
2008;16(2):60-64 CLINICAL ARTICLE Clinical Efficacy of Intramuscular Meglumine Antimoniate Alone and in Combination with Intralesional Meglumine Antimoniate in the Treatment of Old World Cutaneous Leishmaniasis
COMPARISON OF MEGLUMINE ANTIMONIATE AND PENTAMIDINE FOR PERUVIAN CUTANEOUS LEISHMANIASIS
 Am. J. Trop. Med. Hyg., 72(2), 2005, pp. 133 137 Copyright 2005 by The American Society of Tropical Medicine and Hygiene COMPARISON OF MEGLUMINE ANTIMONIATE AND PENTAMIDINE FOR PERUVIAN CUTANEOUS LEISHMANIASIS
Am. J. Trop. Med. Hyg., 72(2), 2005, pp. 133 137 Copyright 2005 by The American Society of Tropical Medicine and Hygiene COMPARISON OF MEGLUMINE ANTIMONIATE AND PENTAMIDINE FOR PERUVIAN CUTANEOUS LEISHMANIASIS
Original Article Comparison of fine needle aspiration with biopsy in the diagnosis of cutaneous leishmaniasis Ikramullah Khan, Rifat Yasmin
 Original Article Comparison of fine needle aspiration with biopsy in the diagnosis of cutaneous leishmaniasis Ikramullah Khan, Rifat Yasmin Department of Dermatology, Pakistan Institute of Medical Sciences,
Original Article Comparison of fine needle aspiration with biopsy in the diagnosis of cutaneous leishmaniasis Ikramullah Khan, Rifat Yasmin Department of Dermatology, Pakistan Institute of Medical Sciences,
Oral miltefosine for Indian post-kala-azar dermal leishmaniasis: a randomised trial
 Tropical Medicine and International Health doi:10.1111/tmi.12015 volume 18 no 1 pp 96 100 january 2013 Oral miltefosine for Indian post-kala-azar dermal leishmaniasis: a randomised trial Shyam Sundar 1,
Tropical Medicine and International Health doi:10.1111/tmi.12015 volume 18 no 1 pp 96 100 january 2013 Oral miltefosine for Indian post-kala-azar dermal leishmaniasis: a randomised trial Shyam Sundar 1,
Original Article Chloroquine in cutaneous leishmaniasis
 Original Article Chloroquine in cutaneous leishmaniasis Ikramullah Khan, Rifat Yasmin, Iqbal Sidiqui Department of Dermatology, Pakistan Institute of Medical Sciences, Islamabad Abstract Background Chloroquine
Original Article Chloroquine in cutaneous leishmaniasis Ikramullah Khan, Rifat Yasmin, Iqbal Sidiqui Department of Dermatology, Pakistan Institute of Medical Sciences, Islamabad Abstract Background Chloroquine
Randomized, Double-Blinded, Phase 2 Trial of WR 279,396 (Paromomycin and Gentamicin) for Cutaneous Leishmaniasis in Panama
 Am. J. Trop. Med. Hyg., 89(3), 2013, pp. 557 563 doi:10.4269/ajtmh.12-0736 Copyright 2013 by The American Society of Tropical Medicine and Hygiene Randomized, Double-Blinded, Phase 2 Trial of WR 279,396
Am. J. Trop. Med. Hyg., 89(3), 2013, pp. 557 563 doi:10.4269/ajtmh.12-0736 Copyright 2013 by The American Society of Tropical Medicine and Hygiene Randomized, Double-Blinded, Phase 2 Trial of WR 279,396
Impavido. (miltefosine) New Product Slideshow
 Impavido (miltefosine) New Product Slideshow Introduction Brand name: Impavido Generic name: Miltefosine Pharmacological class: Antileishmanial agent Strength and Formulation: 50mg; hard gel capsules Manufacturer:
Impavido (miltefosine) New Product Slideshow Introduction Brand name: Impavido Generic name: Miltefosine Pharmacological class: Antileishmanial agent Strength and Formulation: 50mg; hard gel capsules Manufacturer:
Detection of Leishmania in Unaffected Mucosal Tissues of Patients with Cutaneous Leishmaniasis Caused by Leishmania (Viannia) Species
 MAJOR ARTICLE Detection of Leishmania in Unaffected Mucosal Tissues of Patients with Cutaneous Leishmaniasis Caused by Leishmania (Viannia) Species Roger Adrian Figueroa, Leyder Elena Lozano, Ibeth Cristina
MAJOR ARTICLE Detection of Leishmania in Unaffected Mucosal Tissues of Patients with Cutaneous Leishmaniasis Caused by Leishmania (Viannia) Species Roger Adrian Figueroa, Leyder Elena Lozano, Ibeth Cristina
Cutaneous Leishmaniasis in a Chinese man returning from the Amazon forest in Brazil
 Hong Kong J. Dermatol. Venereol. (2013) 21, 79-83 Case Report Cutaneous Leishmaniasis in a Chinese man returning from the Amazon forest in Brazil JTHT Yu and SY Wong A 60-year-old man presented with ulcerated
Hong Kong J. Dermatol. Venereol. (2013) 21, 79-83 Case Report Cutaneous Leishmaniasis in a Chinese man returning from the Amazon forest in Brazil JTHT Yu and SY Wong A 60-year-old man presented with ulcerated
Evaluation of Thermotherapy for the Treatment of Cutaneous Leishmaniasis in Kabul, Afghanistan: A Randomized Controlled Trial
 MILITARY MEDICINE, 177, 3:345, 2012 Evaluation of Thermotherapy for the Treatment of Cutaneous Leishmaniasis in Kabul, Afghanistan: A Randomized Controlled Trial Najibullah Safi, MD*; COL Gary D. Davis,
MILITARY MEDICINE, 177, 3:345, 2012 Evaluation of Thermotherapy for the Treatment of Cutaneous Leishmaniasis in Kabul, Afghanistan: A Randomized Controlled Trial Najibullah Safi, MD*; COL Gary D. Davis,
Control of leishmaniasis
 SIXTIETH WORLD HEALTH ASSEMBLY A60/10 Provisional agenda item 12.3 22 March 2007 Control of leishmaniasis Report by the Secretariat BACKGROUND 1. Leishmaniasis is endemic in 88 countries in the world and
SIXTIETH WORLD HEALTH ASSEMBLY A60/10 Provisional agenda item 12.3 22 March 2007 Control of leishmaniasis Report by the Secretariat BACKGROUND 1. Leishmaniasis is endemic in 88 countries in the world and
Therapeutic Options for Visceral Leishmaniasis
 Drugs (2013) 73:1863 1888 DOI 10.1007/s40265-013-0133-0 REVIEW ARTICLE Therapeutic Options for Visceral Leishmaniasis Begoña Monge-Maillo Rogelio López-Vélez Published online: 30 October 2013 Ó The Author(s)
Drugs (2013) 73:1863 1888 DOI 10.1007/s40265-013-0133-0 REVIEW ARTICLE Therapeutic Options for Visceral Leishmaniasis Begoña Monge-Maillo Rogelio López-Vélez Published online: 30 October 2013 Ó The Author(s)
Biomédica ISSN: Instituto Nacional de Salud Colombia
 Biomédica ISSN: 0120-4157 biomedica@ins.gov.co Instituto Nacional de Salud Colombia Travi, Bruno L.; Tabares, Carlos Javier; Cadena, Horacio Leishmania ( Viannia) braziliensis infection in two Colombian
Biomédica ISSN: 0120-4157 biomedica@ins.gov.co Instituto Nacional de Salud Colombia Travi, Bruno L.; Tabares, Carlos Javier; Cadena, Horacio Leishmania ( Viannia) braziliensis infection in two Colombian
References for Application for inclusion of paromomycin in the WHO Model List of Essential Medicines
 6 (a) Visceral leishmaniasis disease burden. Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 2004:27:305-18. Sundar S, Murray HW. Availability of miltefosine
6 (a) Visceral leishmaniasis disease burden. Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 2004:27:305-18. Sundar S, Murray HW. Availability of miltefosine
Tropical medicine rounds
 Oxford, IJD International 0011-9059 Blackwell 41 UK Science, Journal Ltd 2002 of Dermatology Tropical medicine rounds Cutaneous André Báfica, Leishmaniasis Fabiano Oliveira, Luiz A. R. Freitas, Eliane
Oxford, IJD International 0011-9059 Blackwell 41 UK Science, Journal Ltd 2002 of Dermatology Tropical medicine rounds Cutaneous André Báfica, Leishmaniasis Fabiano Oliveira, Luiz A. R. Freitas, Eliane
Sodium Stibogluconate treatment for cutaneous leishmaniasis: A clinical study of 43 cases from the north of Jordan
 Sodium Stibogluconate treatment for cutaneous leishmaniasis: A clinical study of 43 cases from the north of Jordan Mamoun Mohammad Al-Athamneh Hiathem Qasem Abu Al-haija Ra ed Smadi Ayman S. Qaqaa Heba
Sodium Stibogluconate treatment for cutaneous leishmaniasis: A clinical study of 43 cases from the north of Jordan Mamoun Mohammad Al-Athamneh Hiathem Qasem Abu Al-haija Ra ed Smadi Ayman S. Qaqaa Heba
Interleukin-10 and Interferon-γ Levels in Patients with Cutaneous Leishmaniasis Treated with Cryotherapy
 IJMS Vol 42, No 5, September 2017 Brief Report Interleukin-10 and Interferon-γ Levels in Patients with Cutaneous Leishmaniasis Treated with Cryotherapy Farhad Handjani 1,2, MD; Saeed Reza Yousef 1,2, MD;
IJMS Vol 42, No 5, September 2017 Brief Report Interleukin-10 and Interferon-γ Levels in Patients with Cutaneous Leishmaniasis Treated with Cryotherapy Farhad Handjani 1,2, MD; Saeed Reza Yousef 1,2, MD;
Case Report Development of Cutaneous Leishmaniasis after Leishmania Skin Test
 Case Reports in Medicine Volume 2011, Article ID 631079, 4 pages doi:10.1155/2011/631079 Case Report Development of Cutaneous Leishmaniasis after Leishmania Skin Test Paulo R. Machado, 1, 2 Augusto M.
Case Reports in Medicine Volume 2011, Article ID 631079, 4 pages doi:10.1155/2011/631079 Case Report Development of Cutaneous Leishmaniasis after Leishmania Skin Test Paulo R. Machado, 1, 2 Augusto M.
FAMILIAL AGGREGATION OF MUCOSAL LEISHMANIASIS IN NORTHEAST BRAZIL
 Am. J. Trop. Med. Hyg., 73(1), 2005, pp. 69 73 Copyright 2005 by The American Society of Tropical Medicine and Hygiene FAMILIAL AGGREGATION OF MUCOSAL LEISHMANIASIS IN NORTHEAST BRAZIL LÉA CASTELLUCCI,*
Am. J. Trop. Med. Hyg., 73(1), 2005, pp. 69 73 Copyright 2005 by The American Society of Tropical Medicine and Hygiene FAMILIAL AGGREGATION OF MUCOSAL LEISHMANIASIS IN NORTHEAST BRAZIL LÉA CASTELLUCCI,*
CONTROLE DAS LEISHMANIOSES O QUE FALTA FAZER? Centro de Convenções de Reboças Red Room 17: 00h
 CONTROLE DAS LEISHMANIOSES O QUE FALTA FAZER? Centro de Convenções de Reboças 08.04.2014 Red Room 17: 00h Leishmaniasis - a Global Problem Visceral 2012 300 000 cases 20,000 deaths (6.7%) 310 million at
CONTROLE DAS LEISHMANIOSES O QUE FALTA FAZER? Centro de Convenções de Reboças 08.04.2014 Red Room 17: 00h Leishmaniasis - a Global Problem Visceral 2012 300 000 cases 20,000 deaths (6.7%) 310 million at
Downloaded from:
 Wise, ES; Armstrong, MS; Watson, J; Lockwood, DN (2012) Monitoring toxicity associated with parenteral sodium stibogluconate in the day-case management of returned travellers with New World cutaneous leishmaniasis
Wise, ES; Armstrong, MS; Watson, J; Lockwood, DN (2012) Monitoring toxicity associated with parenteral sodium stibogluconate in the day-case management of returned travellers with New World cutaneous leishmaniasis
Authors Chappuis, François; Alirol, Emilie; Worku, Dagemlidet T; Mueller, Yolanda; Ritmeijer, Koert
 MSF Field Research High mortality among older patients treated with pentavalent antimonials for visceral leishmaniasis in East Africa and rationale for switch to liposomal amphotericin B Item type Article
MSF Field Research High mortality among older patients treated with pentavalent antimonials for visceral leishmaniasis in East Africa and rationale for switch to liposomal amphotericin B Item type Article
Lesion aspirate culture for the diagnosis and isolation of Leishmania spp. from patients with cutaneous leishmaniasis
 62 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 104(1): 62-66, February 2009 Lesion aspirate culture for the diagnosis and isolation of Leishmania spp. from patients with cutaneous leishmaniasis Zélia Maria
62 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 104(1): 62-66, February 2009 Lesion aspirate culture for the diagnosis and isolation of Leishmania spp. from patients with cutaneous leishmaniasis Zélia Maria
Recommendations for Coping with Leishmaniasis: A Review of Control Strategies. Centro de Convenções de Reboças Red Room 17: 00h
 Recommendations for Coping with Leishmaniasis: A Review of Control Strategies Centro de Convenções de Reboças 08.04.2014 Red Room 17: 00h Leishmaniasis - a Global Problem Visceral 2012 300 000 cases 20,000
Recommendations for Coping with Leishmaniasis: A Review of Control Strategies Centro de Convenções de Reboças 08.04.2014 Red Room 17: 00h Leishmaniasis - a Global Problem Visceral 2012 300 000 cases 20,000
Efficacy of pentavalent antimoniate intralesional infiltration therapy for cutaneous leishmaniasis: A systematic review
 COLLECTION REVIEW Efficacy of pentavalent antimoniate intralesional infiltration therapy for cutaneous leishmaniasis: A systematic review Nayara Castelano Brito, Ana Rabello, Gláucia Fernandes Cota* Pesquisas
COLLECTION REVIEW Efficacy of pentavalent antimoniate intralesional infiltration therapy for cutaneous leishmaniasis: A systematic review Nayara Castelano Brito, Ana Rabello, Gláucia Fernandes Cota* Pesquisas
Current therapeutics for visceral leischmaniasis. F.Amri
 Current therapeutics for visceral leischmaniasis F.Amri Department of pediatrics Kairouan Intoduction The visceral leischmaniasis is an anthropozoonose caused by a protozoar, the Leishmania infantum The
Current therapeutics for visceral leischmaniasis F.Amri Department of pediatrics Kairouan Intoduction The visceral leischmaniasis is an anthropozoonose caused by a protozoar, the Leishmania infantum The
Persistence of Leishmania Parasites in Scars after Clinical Cure of American Cutaneous Leishmaniasis: Is There a Sterile Cure?
 MAJOR ARTICLE Persistence of Leishmania Parasites in Scars after Clinical Cure of American Cutaneous Leishmaniasis: Is There a Sterile Cure? Mitzi G. Mendonça, 1,a Maria E. F de Brito, 2 Eduardo H. G.
MAJOR ARTICLE Persistence of Leishmania Parasites in Scars after Clinical Cure of American Cutaneous Leishmaniasis: Is There a Sterile Cure? Mitzi G. Mendonça, 1,a Maria E. F de Brito, 2 Eduardo H. G.
Leishmaniasis: A forgotten disease among neglected people
 ISPUB.COM The Internet Journal of Health Volume 11 Number 2 Leishmaniasis: A forgotten disease among neglected people Y Homsi, G Makdisi Citation Y Homsi, G Makdisi. Leishmaniasis: A forgotten disease
ISPUB.COM The Internet Journal of Health Volume 11 Number 2 Leishmaniasis: A forgotten disease among neglected people Y Homsi, G Makdisi Citation Y Homsi, G Makdisi. Leishmaniasis: A forgotten disease
EVALUATION OF MILTEFOSINE AGAINST LEISHMANIA MAJOR (MRHO/IR/75/ER): IN VITRO AND IN VIVO STUDIES
 ORIGINAL REPORT EVALUATION OF MILTEFOSINE AGAINST LEISHMANIA MAJOR (MRHO/IR/75/ER): IN VITRO AND IN VIVO STUDIES J. Esmaeili 1, M. Mohebali 1*, G. H. Edrissian 1, S. M. Rezayat 2, M. Ghazi-Khansari 2 and
ORIGINAL REPORT EVALUATION OF MILTEFOSINE AGAINST LEISHMANIA MAJOR (MRHO/IR/75/ER): IN VITRO AND IN VIVO STUDIES J. Esmaeili 1, M. Mohebali 1*, G. H. Edrissian 1, S. M. Rezayat 2, M. Ghazi-Khansari 2 and
Control of leishmaniasis
 EXECUTIVE BOARD EB118/4 118th Session 11 May 2006 Provisional agenda item 5.1 Control of leishmaniasis Report by the Secretariat BACKGROUND 1. Leishmaniasis is endemic in 88 countries in the world and
EXECUTIVE BOARD EB118/4 118th Session 11 May 2006 Provisional agenda item 5.1 Control of leishmaniasis Report by the Secretariat BACKGROUND 1. Leishmaniasis is endemic in 88 countries in the world and
WMLN Case Study. Necrotizing Palate Biopsy Specimen. Youngmi Kim Sr. Microbiologist TB Lab & Parasitology Lab MS, M(ASCP)
 WMLN Case Study Necrotizing Palate Biopsy Specimen Youngmi Kim Sr. Microbiologist TB Lab & Parasitology Lab MS, M(ASCP) 44 year old male from Honduras Immigrated to US 12 years ago to WI 4 years ago Seen
WMLN Case Study Necrotizing Palate Biopsy Specimen Youngmi Kim Sr. Microbiologist TB Lab & Parasitology Lab MS, M(ASCP) 44 year old male from Honduras Immigrated to US 12 years ago to WI 4 years ago Seen
Mucosal leishmaniasis: the experience of a Brazilian referral center
 Rev Soc Bras Med Trop 5(3):38-33, May-June, 08 doi: 0.590/0037-88-0478-07 Major Article Mucosal leishmaniasis: the experience of a Brazilian referral center Mariana Junqueira Pedras [],[], Janaína de Pina
Rev Soc Bras Med Trop 5(3):38-33, May-June, 08 doi: 0.590/0037-88-0478-07 Major Article Mucosal leishmaniasis: the experience of a Brazilian referral center Mariana Junqueira Pedras [],[], Janaína de Pina
Etiologic Agent of an Epidemic of Cutaneous Leishmaniasis in Tolima, Colombia
 Am. J. Trop. Med. Hyg., 78(2), 2008, pp. 276 282 Copyright 2008 by The American Society of Tropical Medicine and Hygiene Etiologic Agent of an Epidemic of Cutaneous Leishmaniasis in Tolima, Colombia Isabel
Am. J. Trop. Med. Hyg., 78(2), 2008, pp. 276 282 Copyright 2008 by The American Society of Tropical Medicine and Hygiene Etiologic Agent of an Epidemic of Cutaneous Leishmaniasis in Tolima, Colombia Isabel
Cutaneous leishmaniasis: advances in disease pathogenesis, diagnostics and therapeutics
 Clinical dermatology Review article CED Clinical and Experimental Dermatology Cutaneous leishmaniasis: advances in disease pathogenesis, diagnostics and therapeutics M. Ameen Royal Free Hospital, Royal
Clinical dermatology Review article CED Clinical and Experimental Dermatology Cutaneous leishmaniasis: advances in disease pathogenesis, diagnostics and therapeutics M. Ameen Royal Free Hospital, Royal
Single-Dose Liposomal Amphotericin B in the Treatment of Visceral Leishmaniasis in India: A Multicenter Study
 MAJOR ARTICLE Single-Dose Liposomal Amphotericin B in the Treatment of Visceral Leishmaniasis in India: A Multicenter Study S. Sundar, 1 T. K. Jha, 2 C. P. Thakur, 3 M. Mishra, 4 V. P. Singh, 1 and R.
MAJOR ARTICLE Single-Dose Liposomal Amphotericin B in the Treatment of Visceral Leishmaniasis in India: A Multicenter Study S. Sundar, 1 T. K. Jha, 2 C. P. Thakur, 3 M. Mishra, 4 V. P. Singh, 1 and R.
Short-Course Paromomycin Treatment of Visceral Leishmaniasis in India: 14-Day vs 21-Day Treatment
 MAJOR ARTICLE Short-Course Paromomycin Treatment of Visceral Leishmaniasis in India: 14-Day vs 21-Day Treatment Shyam Sundar, Neha Agrawal, Rakesh Arora, Dipti Agarwal, Madhukar Rai, and Jaya Chakravarty
MAJOR ARTICLE Short-Course Paromomycin Treatment of Visceral Leishmaniasis in India: 14-Day vs 21-Day Treatment Shyam Sundar, Neha Agrawal, Rakesh Arora, Dipti Agarwal, Madhukar Rai, and Jaya Chakravarty
ISPUB.COM. Cutaneous Leishmaniasis in Iran. C Yazdi, M Narmani, B Sadri INTRODUCTION CASE 1 CASE 2
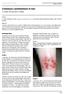 ISPUB.COM The Internet Journal of Infectious Diseases Volume 3 Number 1 Cutaneous Leishmaniasis in Iran C Yazdi, M Narmani, B Sadri Citation C Yazdi, M Narmani, B Sadri. Cutaneous Leishmaniasis in Iran.
ISPUB.COM The Internet Journal of Infectious Diseases Volume 3 Number 1 Cutaneous Leishmaniasis in Iran C Yazdi, M Narmani, B Sadri Citation C Yazdi, M Narmani, B Sadri. Cutaneous Leishmaniasis in Iran.
HAEMOFLAGELLATES. Dr. Anuluck Junkum Department of Parasitology Faculty of Medicine
 HAEMOFLAGELLATES Dr. Anuluck Junkum Department of Parasitology Faculty of Medicine Objective Can describe the morphology, life cycle, pathology, diagnosis and prevention of Leishmania spp. and Trypanosoma
HAEMOFLAGELLATES Dr. Anuluck Junkum Department of Parasitology Faculty of Medicine Objective Can describe the morphology, life cycle, pathology, diagnosis and prevention of Leishmania spp. and Trypanosoma
Welcome to the Jungle! Dr Aileen Oon, 2017 Microbiology Registrar
 Welcome to the Jungle! Dr Aileen Oon, 2017 Microbiology Registrar AA 55M presented with sores on left olecranon and umbilical area Umbilical sores present for 3 weeks Left olecranon lesions for 1 week
Welcome to the Jungle! Dr Aileen Oon, 2017 Microbiology Registrar AA 55M presented with sores on left olecranon and umbilical area Umbilical sores present for 3 weeks Left olecranon lesions for 1 week
Treatment of Disseminated Leishmaniasis With Liposomal Amphotericin B
 MAJOR ARTICLE Treatment of Disseminated Leishmaniasis With Liposomal Amphotericin B Paulo R. L. Machado, 1,2 Maria Elisa A. Rosa, 1 Luís H. Guimarães, 1,2 Fernanda V. O. Prates, 1 Adriano Queiroz, 1,2
MAJOR ARTICLE Treatment of Disseminated Leishmaniasis With Liposomal Amphotericin B Paulo R. L. Machado, 1,2 Maria Elisa A. Rosa, 1 Luís H. Guimarães, 1,2 Fernanda V. O. Prates, 1 Adriano Queiroz, 1,2
Statistical Analysis Plan (SAP)
 Statistical Analysis Plan (SAP) Comparison of artemether-lumefantrine and chloroquine with and without primaquine for the treatment of Plasmodium vivax in Ethiopia: a randomized controlled trial Contents
Statistical Analysis Plan (SAP) Comparison of artemether-lumefantrine and chloroquine with and without primaquine for the treatment of Plasmodium vivax in Ethiopia: a randomized controlled trial Contents
Visceral childhood leishmaniasis in southern Turkey: experience of twenty years
 The Turkish Journal of Pediatrics 2009; 51: 1-5 Original Visceral childhood leishmaniasis in southern Turkey: experience of twenty years Oğuz Dursun 1, Seyhan Erişir 2, Akif Yeşilipek 3 Divisions of 1
The Turkish Journal of Pediatrics 2009; 51: 1-5 Original Visceral childhood leishmaniasis in southern Turkey: experience of twenty years Oğuz Dursun 1, Seyhan Erişir 2, Akif Yeşilipek 3 Divisions of 1
NIH Public Access Author Manuscript Trans R Soc Trop Med Hyg. Author manuscript; available in PMC 2013 June 01.
 NIH Public Access Author Manuscript Published in final edited form as: Trans R Soc Trop Med Hyg. 2012 June ; 106(6): 376 381. doi:10.1016/j.trstmh.2012.03.007. A proposed new clinical staging system for
NIH Public Access Author Manuscript Published in final edited form as: Trans R Soc Trop Med Hyg. 2012 June ; 106(6): 376 381. doi:10.1016/j.trstmh.2012.03.007. A proposed new clinical staging system for
LeishMan Recommendations for Treatment of Cutaneous and Mucosal Leishmaniasis in Travelers, 2014
 I S T M 116 REVIEW LeishMan Recommendations for Treatment of Cutaneous and Mucosal Leishmaniasis in Travelers, 2014 Johannes Blum, MD, Pierre Buffet, MD, Leo Visser, MD, # Gundel Harms, MD, Mark S. Bailey,
I S T M 116 REVIEW LeishMan Recommendations for Treatment of Cutaneous and Mucosal Leishmaniasis in Travelers, 2014 Johannes Blum, MD, Pierre Buffet, MD, Leo Visser, MD, # Gundel Harms, MD, Mark S. Bailey,
Clinical and laboratory profiles of patients with early spontaneous healing in cutaneous localized leishmaniasis: a historical cohort study
 Oliveira-Ribeiro et al. BMC Infectious Diseases (2017) 17:559 DOI 10.1186/s12879-017-2658-4 RESEARCH ARTICLE Open Access Clinical and laboratory profiles of patients with early spontaneous healing in cutaneous
Oliveira-Ribeiro et al. BMC Infectious Diseases (2017) 17:559 DOI 10.1186/s12879-017-2658-4 RESEARCH ARTICLE Open Access Clinical and laboratory profiles of patients with early spontaneous healing in cutaneous
Cutaneous Leishmaniasis : Global overview
 Cutaneous Leishmaniasis : Global overview Meeting of stakeholders for selected Health R&D Demostration Projects, 7-10 May 2014, WHO, Geneva Dr. Daniel Argaw Dagne, NTD/WHO CSR - DDC AFRO Leishmaniasis
Cutaneous Leishmaniasis : Global overview Meeting of stakeholders for selected Health R&D Demostration Projects, 7-10 May 2014, WHO, Geneva Dr. Daniel Argaw Dagne, NTD/WHO CSR - DDC AFRO Leishmaniasis
Leishmaniasis. CDR R.L. Gutierrez Oct 2014
 Leishmaniasis CDR R.L. Gutierrez Oct 2014 Overview Protozoan parasite(s) of tissue and WBCs Many species / Many Syndromes (Cutaneous / Visceral) Pathogen: Location - Old World vs. New World Host: Immune
Leishmaniasis CDR R.L. Gutierrez Oct 2014 Overview Protozoan parasite(s) of tissue and WBCs Many species / Many Syndromes (Cutaneous / Visceral) Pathogen: Location - Old World vs. New World Host: Immune
Cutaneous leishmaniasis in an overseas Filipino worker who responded favorably to oral itraconazole
 CSE REPORT Cutaneous leishmaniasis in an overseas Filipino worker who responded favorably to oral itraconazole udi, MD 1, ilza ellamy R. Limcangco, MD, FPDS 2, Johannes F, Dayrit, MD, FPDS 2,3 Introduction:
CSE REPORT Cutaneous leishmaniasis in an overseas Filipino worker who responded favorably to oral itraconazole udi, MD 1, ilza ellamy R. Limcangco, MD, FPDS 2, Johannes F, Dayrit, MD, FPDS 2,3 Introduction:
Title: Treatment failure and miltefosine susceptibility in dermal leishmaniasis
 AAC Accepts, published online ahead of print on 21 October 2013 Antimicrob. Agents Chemother. doi:10.1128/aac.01023-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 2 Title:
AAC Accepts, published online ahead of print on 21 October 2013 Antimicrob. Agents Chemother. doi:10.1128/aac.01023-13 Copyright 2013, American Society for Microbiology. All Rights Reserved. 1 2 Title:
2.0 Synopsis. ABT-333 M Clinical Study Report R&D/09/956
 2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: ABT-333 Volume: Name of Active Ingredient: Page: Sodium N-{6-[3-tert-butyl-5-(2,4-
2.0 Synopsis Abbott Laboratories Individual Study Table Referring to Part of Dossier: (For National Authority Use Only) Name of Study Drug: ABT-333 Volume: Name of Active Ingredient: Page: Sodium N-{6-[3-tert-butyl-5-(2,4-
Iranian J Parasitol: Vol. 8, No.3, July -Sep 2013, pp Iranian J Parasitol. Open access Journal at ijpa.tums.ac.ir
 Iranian J Parasitol: Vol. 8, No.3, July -Sep 2013, pp.396-401 Iranian J Parasitol Tehran University of Medical Sciences Publication http:// tums.ac.ir Open access Journal at http:// ijpa.tums.ac.ir Iranian
Iranian J Parasitol: Vol. 8, No.3, July -Sep 2013, pp.396-401 Iranian J Parasitol Tehran University of Medical Sciences Publication http:// tums.ac.ir Open access Journal at http:// ijpa.tums.ac.ir Iranian
Leishmaniasis WRAIR- GEIS 'Operational Clinical Infectious Disease' Course
 Leishmaniasis WRAIR- GEIS 'Operational Clinical Infectious Disease' Course UNCLASSIFIED Acknowledgments LTC James E. Moon, MD Chief, Sleep Trials Branch Walter Reed Army Institute of Research CDR Ramiro
Leishmaniasis WRAIR- GEIS 'Operational Clinical Infectious Disease' Course UNCLASSIFIED Acknowledgments LTC James E. Moon, MD Chief, Sleep Trials Branch Walter Reed Army Institute of Research CDR Ramiro
Case Report Occupationally Acquired American Cutaneous Leishmaniasis
 Case Reports in Dermatological Medicine Volume 2012, Article ID 279517, 4 pages doi:10.1155/2012/279517 Case Report Occupationally Acquired American Cutaneous Leishmaniasis Maria Edileuza Felinto de Brito,
Case Reports in Dermatological Medicine Volume 2012, Article ID 279517, 4 pages doi:10.1155/2012/279517 Case Report Occupationally Acquired American Cutaneous Leishmaniasis Maria Edileuza Felinto de Brito,
Country Focus of study (Objectives) Methods Participants Main findings. Longitudinal follow up study. analysis. study
 Table S1: Summary of magnitude of HIV-VL co-infection Author(s) Country Focus of (Objectives) Methods Participants Main findings year of publication Rachel ter Horst et al., 2008 To assess its impact and
Table S1: Summary of magnitude of HIV-VL co-infection Author(s) Country Focus of (Objectives) Methods Participants Main findings year of publication Rachel ter Horst et al., 2008 To assess its impact and
Meglumine antimoniate intralesional infiltration for localised cutaneous leishmaniasis: a single arm, open label, phase II clinical trial
 ORIGINAL ARTICLE Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 113(9): e180200, 2018 1 8 Meglumine antimoniate intralesional infiltration for localised cutaneous leishmaniasis: a single arm, open label,
ORIGINAL ARTICLE Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 113(9): e180200, 2018 1 8 Meglumine antimoniate intralesional infiltration for localised cutaneous leishmaniasis: a single arm, open label,
Susceptibility of Spiny Rats (Proechimys semispinosus) to Leishmania (Viannia) panamensis and Leishmania (Leishmania) chagasi
 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 97(6): 887-892, September 2002 887 Susceptibility of Spiny Rats (Proechimys semispinosus) to Leishmania (Viannia) panamensis and Leishmania (Leishmania) chagasi
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 97(6): 887-892, September 2002 887 Susceptibility of Spiny Rats (Proechimys semispinosus) to Leishmania (Viannia) panamensis and Leishmania (Leishmania) chagasi
Leishmania major Infection
 Immunotherapy with Imiquimod Increases the Efficacy of Glucantime Therapy of Leishmania major Infection Ghader Khalili 1, Faramarz Dobakhti 2, Hamid Mahmoudzadeh Niknam 1, Vahid Khaze 1, Fatemeh Partovi
Immunotherapy with Imiquimod Increases the Efficacy of Glucantime Therapy of Leishmania major Infection Ghader Khalili 1, Faramarz Dobakhti 2, Hamid Mahmoudzadeh Niknam 1, Vahid Khaze 1, Fatemeh Partovi
Heat therapy for cutaneous leishmaniasis elicits a systemic cytokine response similar to that of antimonial (Glucantime) therapy
 Transactions of the Royal Society of Tropical Medicine and Hygiene (2006) 100, 642 649 available at www.sciencedirect.com journal homepage: www.elsevierhealth.com/journals/trst Heat therapy for cutaneous
Transactions of the Royal Society of Tropical Medicine and Hygiene (2006) 100, 642 649 available at www.sciencedirect.com journal homepage: www.elsevierhealth.com/journals/trst Heat therapy for cutaneous
Nanosilver in the treatment of localized cutaneous leishmaniasis caused by Leishmania major (MRHO/IR/75/ER): an in vitro and in vivo study
 DARU Vol. 17, No. 4 2009 285 Nanosilver in the treatment of localized cutaneous leishmaniasis caused by Leishmania major (MRHO/IR/75/ER): an in vitro and in vivo study *1 Mohebali M., 2 Rezayat M.M., 3
DARU Vol. 17, No. 4 2009 285 Nanosilver in the treatment of localized cutaneous leishmaniasis caused by Leishmania major (MRHO/IR/75/ER): an in vitro and in vivo study *1 Mohebali M., 2 Rezayat M.M., 3
Kala-Azar- Treatment Update
 JOURNAL OF ADVANCES IN MEDICINE (JAM) DOI: 10.5958/2319-4324.2016.00002.X REVIEW PAPER Kala-Azar- Treatment Update Anup Singh Associate Professor, Department of Medicine, Institute of Medical Sciences,
JOURNAL OF ADVANCES IN MEDICINE (JAM) DOI: 10.5958/2319-4324.2016.00002.X REVIEW PAPER Kala-Azar- Treatment Update Anup Singh Associate Professor, Department of Medicine, Institute of Medical Sciences,
Leishmaniasis. By Joseph Knight, PA-C. 2. Explain the differences in the reasons leishmaniasis is spreading in Afghanistan and India.
 Leishmaniasis By Joseph Knight, PA-C Objectives 1. Identify the two types of leishmaniasis. 2. Explain the differences in the reasons leishmaniasis is spreading in Afghanistan and India. 3. Discuss how
Leishmaniasis By Joseph Knight, PA-C Objectives 1. Identify the two types of leishmaniasis. 2. Explain the differences in the reasons leishmaniasis is spreading in Afghanistan and India. 3. Discuss how
Research Article Efficacy and Safety of Paromomycin in Treatment of Post-Kala-Azar Dermal Leishmaniasis
 ISRN Parasitology, Article ID 548010, 4 pages http://dx.doi.org/10.1155/2014/548010 Research Article Efficacy and Safety of Paromomycin in Treatment of Post-Kala-Azar Dermal Leishmaniasis Shyam Sundar,
ISRN Parasitology, Article ID 548010, 4 pages http://dx.doi.org/10.1155/2014/548010 Research Article Efficacy and Safety of Paromomycin in Treatment of Post-Kala-Azar Dermal Leishmaniasis Shyam Sundar,
Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry
 doi: 10.1111/j.1742-1241.2008.01824.x ORIGINAL PAPER Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry W. J. Martone, K. C. Lindfield, D. E. Katz OnlineOpen: This
doi: 10.1111/j.1742-1241.2008.01824.x ORIGINAL PAPER Outpatient parenteral antibiotic therapy with daptomycin: insights from a patient registry W. J. Martone, K. C. Lindfield, D. E. Katz OnlineOpen: This
The New England Journal of Medicine
 The New England Journal of Medicine Copyright 22 by the Massachusetts Medical Society VOLUME 347 N OVEMBER 28, 22 NUMBER 22 ORAL MILTEFOSINE FOR INDIAN VISCERAL LEISHMANIASIS SHYAM SUNDAR, M.D., T.K. JHA,
The New England Journal of Medicine Copyright 22 by the Massachusetts Medical Society VOLUME 347 N OVEMBER 28, 22 NUMBER 22 ORAL MILTEFOSINE FOR INDIAN VISCERAL LEISHMANIASIS SHYAM SUNDAR, M.D., T.K. JHA,
! " ! 5 #$% & .TL5 ],$:< #$% &'( 8 )!U O0 8D)$5: "#$% &% "'% (%) "'*.
![! ! 5 #$% & .TL5 ],$:< #$% &'( 8 )!U O0 8D)$5: #$% &% '% (%) '*. ! ! 5 #$% & .TL5 ],$:< #$% &'( 8 )!U O0 8D)$5: #$% &% '% (%) '*.](/thumbs/87/96629261.jpg) 1394/1/24: 1394/4/5: 1394 "!/338 /! " 3 2 1! 5 4 #) * #$ %&' #$% & 5.* 34 - )*+,-. /0 1 * (!) #$% &'( #' :.* A< B$C 8? & * @! #& 1: 1 #' ; ' < =/ 8 / >+ 77! 89 )' O0
1394/1/24: 1394/4/5: 1394 "!/338 /! " 3 2 1! 5 4 #) * #$ %&' #$% & 5.* 34 - )*+,-. /0 1 * (!) #$% &'( #' :.* A< B$C 8? & * @! #& 1: 1 #' ; ' < =/ 8 / >+ 77! 89 )' O0
Leishmanial Antigens in the Diagnosis of Active Lesions and Ancient Scars of American Tegumentary Leishmaniasis Patients
 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 96(7): 987-996, October 2001 Leishmanial Antigens in the Diagnosis of Active Lesions and Ancient Scars of American Tegumentary Leishmaniasis Patients Armando
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 96(7): 987-996, October 2001 Leishmanial Antigens in the Diagnosis of Active Lesions and Ancient Scars of American Tegumentary Leishmaniasis Patients Armando
I nfectious diseases steal the headlines on a
 649 REVIEW Leishmaniasis Tonio V Piscopo, Charles Mallia Azzopardi... Epidemiology, disease patterns, immunology, diagnosis, treatment and control measures of are described. Various issues relating to
649 REVIEW Leishmaniasis Tonio V Piscopo, Charles Mallia Azzopardi... Epidemiology, disease patterns, immunology, diagnosis, treatment and control measures of are described. Various issues relating to
Leishmaniasis. MAJ Kris Paolino September 2014
 Leishmaniasis MAJ Kris Paolino September 2014 Thanks to COL (Ret) Kent Kester MAJ Leyi Lin http://www.niaid.nih.gov/topics/leishmaniasis History Sir William Boog Leishman (1865-1926) Matriculated at the
Leishmaniasis MAJ Kris Paolino September 2014 Thanks to COL (Ret) Kent Kester MAJ Leyi Lin http://www.niaid.nih.gov/topics/leishmaniasis History Sir William Boog Leishman (1865-1926) Matriculated at the
Parental Attitudes toward Human Papilloma Virus Vaccine Participation of Adolescent Daughters in a Rural Population
 Diversity and Equality in Health and Care (2018) 15(4): 164-168 2018 Insight Medical Publishing Group Research Article Parental Attitudes toward Human Papilloma Virus Vaccine Participation of Adolescent
Diversity and Equality in Health and Care (2018) 15(4): 164-168 2018 Insight Medical Publishing Group Research Article Parental Attitudes toward Human Papilloma Virus Vaccine Participation of Adolescent
PANCREATIC TOXICITY AS AN ADVERSE EFFECT INDUCED BY MEGLUMINE ANTIMONIATE THERAPY IN A CLINICAL TRIAL FOR CUTANEOUS LEISHMANIASIS
 Rev. Inst. Med. Trop. Sao Paulo 2016;58:68 http://dx.doi.org/10.1590/s1678-9946201658068 ORIGINAL ARTICLE PANCREATIC TOXICITY AS AN ADVERSE EFFECT INDUCED BY MEGLUMINE ANTIMONIATE THERAPY IN A CLINICAL
Rev. Inst. Med. Trop. Sao Paulo 2016;58:68 http://dx.doi.org/10.1590/s1678-9946201658068 ORIGINAL ARTICLE PANCREATIC TOXICITY AS AN ADVERSE EFFECT INDUCED BY MEGLUMINE ANTIMONIATE THERAPY IN A CLINICAL
CUTANEOUS LEISHMANIASIS
 CUTANEOUS LEISHMANIASIS Why are you neglecting me? A WHO initiative to control Cutaneous Leishmaniasis in selected Old World areas This document has been produced as the result of a WHO Informal Consultative
CUTANEOUS LEISHMANIASIS Why are you neglecting me? A WHO initiative to control Cutaneous Leishmaniasis in selected Old World areas This document has been produced as the result of a WHO Informal Consultative
Biomédica Journal of the Instituto Nacional de Salud Volume 34, 2014
 Biomédica Journal of the Volume 34, 2014 EDITORIAL BOARD Luis Alberto Gómez Carlos Arturo Hernández Rubén Santiago Nicholls Emeritus Researcher Pan American Health Organization Brasilia, Brazil Enrique
Biomédica Journal of the Volume 34, 2014 EDITORIAL BOARD Luis Alberto Gómez Carlos Arturo Hernández Rubén Santiago Nicholls Emeritus Researcher Pan American Health Organization Brasilia, Brazil Enrique
World Health Organization Department of Communicable Disease Surveillance and Response
 WHO Report on Global Surveillance of Epidemic-prone Infectious Diseases World Health Organization Department of Communicable Disease Surveillance and Response This document has been downloaded from the
WHO Report on Global Surveillance of Epidemic-prone Infectious Diseases World Health Organization Department of Communicable Disease Surveillance and Response This document has been downloaded from the
THERMOTHERAPY EFFECTIVE AND SAFER THAN MILTEFOSINE IN THE TREATMENT OF CUTANEOUS LEISHMANIASIS IN COLOMBIA
 Rev. Inst. Med. Trop. Sao Paulo 55(3):197-204, May-June, 2013 doi: 10.1590/S0036-46652013000300011 THERMOTHERAPY EFFECTIVE AND SAFER THAN MILTEFOSINE IN THE TREATMENT OF CUTANEOUS LEISHMANIASIS IN COLOMBIA
Rev. Inst. Med. Trop. Sao Paulo 55(3):197-204, May-June, 2013 doi: 10.1590/S0036-46652013000300011 THERMOTHERAPY EFFECTIVE AND SAFER THAN MILTEFOSINE IN THE TREATMENT OF CUTANEOUS LEISHMANIASIS IN COLOMBIA
TOWARD AN APPROACH FOR CUTANEOUS LEISHMANIA TREATMENT. Mohammed Wael Daboul
 Original Articles DOI: 10.7241/ourd.20131.09 Source of Support: Nil Competing Interests: None TOWARD AN APPROACH FOR CUTANEOUS LEISHMANIA TREATMENT Mohammed Wael Daboul Laboratory Medicine Specialist,
Original Articles DOI: 10.7241/ourd.20131.09 Source of Support: Nil Competing Interests: None TOWARD AN APPROACH FOR CUTANEOUS LEISHMANIA TREATMENT Mohammed Wael Daboul Laboratory Medicine Specialist,
NOTES. Th1/Th2 Cytokine Profile in Patients Coinfected with HIV and Leishmania in Brazil
 CLINICAL AND VACCINE IMMUNOLOGY, Oct. 2011, p. 1765 1769 Vol. 18, No. 10 1556-6811/11/$12.00 doi:10.1128/cvi.00076-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. NOTES Th1/Th2
CLINICAL AND VACCINE IMMUNOLOGY, Oct. 2011, p. 1765 1769 Vol. 18, No. 10 1556-6811/11/$12.00 doi:10.1128/cvi.00076-11 Copyright 2011, American Society for Microbiology. All Rights Reserved. NOTES Th1/Th2
Epidemiology of hepatitis E infection in Hong Kong
 RESEARCH FUND FOR THE CONTROL OF INFECTIOUS DISEASES Epidemiology of hepatitis E infection in Hong Kong DPC Chan *, KCK Lee, SS Lee K e y M e s s a g e s 1. The overall anti hepatitis E virus (HEV) seropositivity
RESEARCH FUND FOR THE CONTROL OF INFECTIOUS DISEASES Epidemiology of hepatitis E infection in Hong Kong DPC Chan *, KCK Lee, SS Lee K e y M e s s a g e s 1. The overall anti hepatitis E virus (HEV) seropositivity
PFIZER INC. THERAPEUTIC AREA AND FDA APPROVED INDICATIONS: See USPI
 PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
PFIZER INC. These results are supplied for informational purposes only. Prescribing decisions should be made based on the approved package insert. For publications based on this study, see associated bibliography.
Citation for the original published paper (version of record): N.B. When citing this work, cite the original published paper.
 http://www.diva-portal.org This is the published version of a paper published in Tropical Medicine and Health. Citation for the original published paper (version of record): Eid, D., Guzman-Rivero, M.,
http://www.diva-portal.org This is the published version of a paper published in Tropical Medicine and Health. Citation for the original published paper (version of record): Eid, D., Guzman-Rivero, M.,
Fluconazole in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania braziliensis: A Randomized Controlled Trial
 Clinical Infectious Diseases MAJOR ARTICLE Fluconazole in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania braziliensis: A Randomized Controlled Trial Fernanda V. de O. Prates, 1 Mayra E.
Clinical Infectious Diseases MAJOR ARTICLE Fluconazole in the Treatment of Cutaneous Leishmaniasis Caused by Leishmania braziliensis: A Randomized Controlled Trial Fernanda V. de O. Prates, 1 Mayra E.
Major Article. Rev Soc Bras Med Trop 51(6): , Nov-Dec, 2018 doi: /
 Rev Soc Bras Med Trop 51(6):769-780, Nov-Dec, 2018 doi: 10.1590/0037-8682-0464-2017 Major Article Favorable responses to treatment with 5 mg Sb v /kg/day meglumine antimoniate in patients with American
Rev Soc Bras Med Trop 51(6):769-780, Nov-Dec, 2018 doi: 10.1590/0037-8682-0464-2017 Major Article Favorable responses to treatment with 5 mg Sb v /kg/day meglumine antimoniate in patients with American
DOWNLOAD OR READ : TROPICAL INFECTIOUS DISEASES SECOND EDITION PDF EBOOK EPUB MOBI
 DOWNLOAD OR READ : TROPICAL INFECTIOUS DISEASES SECOND EDITION PDF EBOOK EPUB MOBI Page 1 Page 2 tropical infectious diseases second edition tropical infectious diseases second pdf tropical infectious
DOWNLOAD OR READ : TROPICAL INFECTIOUS DISEASES SECOND EDITION PDF EBOOK EPUB MOBI Page 1 Page 2 tropical infectious diseases second edition tropical infectious diseases second pdf tropical infectious
Research Methods and Analysis
 Research Methods and Analysis Lecture 9 Analytic Experimental Studies Randomized Study Design Dr. Sayed Bahawaddin Hashemi, MD, MPH, ASCP Experimental Randomized Quasi Randomized Controlled Trial A study
Research Methods and Analysis Lecture 9 Analytic Experimental Studies Randomized Study Design Dr. Sayed Bahawaddin Hashemi, MD, MPH, ASCP Experimental Randomized Quasi Randomized Controlled Trial A study
DOI /j x
 THERAPEUTICS DOI 10.1111/j.1365-2133.2007.07872.x Diffuse cutaneous leishmaniasis responds to miltefosine but then relapses O. Zerpa, M. Ulrich, B. Blanco, M. Polegre, A. Avila, N. Matos,* I. Mendoza,
THERAPEUTICS DOI 10.1111/j.1365-2133.2007.07872.x Diffuse cutaneous leishmaniasis responds to miltefosine but then relapses O. Zerpa, M. Ulrich, B. Blanco, M. Polegre, A. Avila, N. Matos,* I. Mendoza,
T-cell responses associated with resistance to Leishmania infection in individuals from endemic areas for Leishmania (Viannia) braziliensis
 Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 102(5): 625-630, August 2007 625 T-cell responses associated with resistance to Leishmania infection in individuals from endemic areas for Leishmania (Viannia)
Mem Inst Oswaldo Cruz, Rio de Janeiro, Vol. 102(5): 625-630, August 2007 625 T-cell responses associated with resistance to Leishmania infection in individuals from endemic areas for Leishmania (Viannia)
VISERAL LEISHMANIASI S (KALA-AZAR)
 VISERAL LEISHMANIASI S (KALA-AZAR) :OUTLINES DEFINITION. EPIDEMIOLOGY. PARASITE & VECTOR. PATHOLOGY CLINICAL & LIFE CYCLE. PICTURE. COMPLICATIONS. DIAGNOSIS. INVESTIGATIONS. MANAGEMENT TREATMENT S CONTROL.
VISERAL LEISHMANIASI S (KALA-AZAR) :OUTLINES DEFINITION. EPIDEMIOLOGY. PARASITE & VECTOR. PATHOLOGY CLINICAL & LIFE CYCLE. PICTURE. COMPLICATIONS. DIAGNOSIS. INVESTIGATIONS. MANAGEMENT TREATMENT S CONTROL.
Efficacy of Miltefosine in the Treatment of Visceral Leishmaniasis in India After a Decade of Use
 MAJOR ARTICLE Efficacy of Miltefosine in the Treatment of Visceral Leishmaniasis in India After a Decade of Use Shyam Sundar, 1,a Anup Singh, 1,a Madhukar Rai, 1 Vijay K. Prajapati, 1 Avinash K. Singh,
MAJOR ARTICLE Efficacy of Miltefosine in the Treatment of Visceral Leishmaniasis in India After a Decade of Use Shyam Sundar, 1,a Anup Singh, 1,a Madhukar Rai, 1 Vijay K. Prajapati, 1 Avinash K. Singh,
BIO Parasitology Spring 2009
 BIO 475 - Parasitology Spring 2009 Stephen M. Shuster Northern Arizona University http://www4.nau.edu/isopod Lecture 5 Discovery of the Disease In 1924 the Kala-Azar Commission noted that the distribution
BIO 475 - Parasitology Spring 2009 Stephen M. Shuster Northern Arizona University http://www4.nau.edu/isopod Lecture 5 Discovery of the Disease In 1924 the Kala-Azar Commission noted that the distribution
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centers: Indication: Treatment: Objective: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Regulatory T Cells in the Pathogenesis and Healing of Chronic Human Dermal Leishmaniasis Caused by Leishmania (Viannia) Species
 Regulatory T Cells in the Pathogenesis and Healing of Chronic Human Dermal Leishmaniasis Caused by Leishmania (Viannia) Species Daniel Rodriguez-Pinto 1 *, Adriana Navas 1,Víctor Manuel Blanco 1, Lady
Regulatory T Cells in the Pathogenesis and Healing of Chronic Human Dermal Leishmaniasis Caused by Leishmania (Viannia) Species Daniel Rodriguez-Pinto 1 *, Adriana Navas 1,Víctor Manuel Blanco 1, Lady
Downloaded from:
 Campbell-Lendrum, D; Dujardin, JP; Martinez, E; Feliciangeli, MD; Perez, JE; de Silans, L; Desjeux, P (2001) Domestic and peridomestic transmission of American cutaneous leishmaniasis: Changing epidemiological
Campbell-Lendrum, D; Dujardin, JP; Martinez, E; Feliciangeli, MD; Perez, JE; de Silans, L; Desjeux, P (2001) Domestic and peridomestic transmission of American cutaneous leishmaniasis: Changing epidemiological
Seventy percent of people who are candidates for allogeneic hematopoietic
 274 7th Biennial Symposium on Minorities, the Medically Underserved and Cancer Supplement to Cancer The National Marrow Donor Program Meeting the Needs of the Medically Underserved Dennis L. Confer, M.D.
274 7th Biennial Symposium on Minorities, the Medically Underserved and Cancer Supplement to Cancer The National Marrow Donor Program Meeting the Needs of the Medically Underserved Dennis L. Confer, M.D.
