316 Cell 141, , April 16, 2010 ª2010 Elsevier Inc.
|
|
|
- Hugh Kennedy
- 6 years ago
- Views:
Transcription
1 Figure 1. In Vitro Reconstitution of the RIG-I Pathway and Regulation of RIG-I by RNA and Ubiquitination (A) Purification of RIG-I protein from Sendai virus-infected (+SeV) or untreated ( SeV) HEK293T cells stably expressing RIG-I containing a C-terminal Flag epitope. 316 Cell 141, , April 16, 2010 ª2010 Elsevier Inc.
2 To dissect the signaling mechanisms in the RIG-I pathway, we establish a cell-free system that mimics viral infection in intact cells. This in vitro reconstitution system contains recombinant RIG-I protein, mitochondria, cytosol, RNA, and ubiquitination enzymes including TRIM25. We demonstrate that 5 0 -ppprna and dsrna, which resemble viral RNA, can activate the entire RIG-I signaling cascade in vitro, leading to activation of IRF3 and NF-kB. Interestingly, we find that upon incubation with both RNA and unanchored K63-polyubiquitin (polyub) chains, RIG-I is fully activated to cause IRF3 dimerization. We show that the N terminus of RIG-I, termed RIG-I(N), which contains tandem CARDs, binds specifically to K63-polyUb chains, and that this binding is both necessary and sufficient to activate RIG-I(N). Furthermore, we devise a protocol to isolate endogenous ubiquitin chains associated with RIG-I in a human cell line and demonstrate that these are unanchored K63-polyUb chains with potent ability to activate RIG-I. Our results support a model in which RIG-I binds to two ligands, viral RNA and endogenous ubiquitin chains, in a sequential manner through the C-terminal RD and N-terminal CARDs, respectively. The binding of both ligands is required for full activation of RIG-I. RESULTS In Vitro Reconstitution of a RIG-I Signaling Cascade from RNA to IRF3 Activation To reconstitute the RIG-I pathway in vitro, we first examined whether RIG-I isolated from virus-infected cells could cause IRF3 activation in the presence of mitochondria, which contain MAVS, and cytosolic extracts, which contain TBK1 and many other components. A hallmark of IRF3 activation is its dimerization, which depends on its phosphorylation by TBK1 and can be measured by native gel electrophoresis (Yoneyama et al., 2002). To set up the assay, HEK293T cells stably expressing full-length RIG-I with a C-terminal Flag tag were infected with Sendai virus (SeV) or uninfected, then RIG-I was affinity purified (Figure 1A). Crude mitochondria (P5) and cytosolic extracts (S5) were prepared from uninfected HEK293T cells by differential centrifugation (Figure 1B), and 35 S-labeled IRF3 protein was synthesized by in vitro translation. As shown in Figure 1C, dimerization of IRF3 was observed when RIG-I from virus-infected cells was incubated with mitochondria (P5) and cytosolic extracts (S5) in the presence of ATP (lane 3). In contrast, RIG-I from mocktreated cells did not promote IRF3 dimerization (lane 2). The activation of IRF3 required both cytosol and mitochondria (lanes 5 and 6). Mitochondria isolated from cells depleted of MAVS by RNAi could not support IRF3 dimerization (Figure S1A available online), confirming that MAVS is essential for activating the downstream pathway in this in vitro assay. When RIG-I was isolated from cells depleted of TRIM25 by RNAi, its ability to promote IRF3 dimerization in the in vitro assay was greatly reduced (Figure S1B), supporting an important role of TRIM25 in RIG-I activation. As we have shown recently, mitochondria isolated from virus-infected cells activated IRF3 in the absence of RIG-I (Figure 1C, lane 1) (Zeng et al., 2009). Transfection of cells with synthetic RNAs, such as the dsrna analog poly(i:c) and 5 0 -ppprna, potently induces IRF3 dimerization. To test if RNA could activate the entire RIG-I pathway in vitro, we expressed and purified full-length RIG-I from insect cells (Sf9; Figure 1D, lower left) and incubated it with ATP and a 79 nucleotide (79 nt) 5 0 -ppprna (Figure S1C). This RNA strongly induced IFN-b when transfected into HEK293-IFN-b-luciferse reporter cells (Figure S1D). However, incubation of this RNA with the recombinant RIG-I protein did not cause IRF3 dimerization in the reconstituted system (Figure 1D, lane 1 in lower right panel). As ubiquitination has been shown to be important in the RIG-I pathway, we incubated RIG-I with E1, Ubc5c (E2), TRIM25 (E3), and ubiquitin together with ATP and RNA. Remarkably, this condition caused RIG-I to activate the entire pathway, resulting in IRF3 dimerization (Figure 1D, lane 3 in lower right panel). A K270A mutation in the ATPase domain of RIG-I, which abrogates the ability of RIG-I to induce interferons in vivo (Yoneyama et al., 2004), also abolished its ability to induce IRF3 dimerization in vitro. Poly(I:C) stimulated IRF3 dimerization in this reconstituted system in a manner dependent on ubiquitin, E1, Ubc5c, TRIM25, and RIG-I (Figure 1E). Activation of RIG-I was also observed with another 5 0 -ppprna containing 135 nucleotides (135 nt) (Figure 1F). Dephosphorylation of the 5 0 -ppprnas with shrimp alkaline phosphatase (SAP) destroyed their ability to activate IRF3 in vitro (Figure 1F) and IFN-b induction in HEK293T cells (Figure S1D). In contrast, treatment of poly(i:c) with SAP did not inhibit its activity, consistent with the previous report that this (B) Procedures for isolation of crude mitochondria (P5) and cytosol (S5) by differential centrifugation. (C) Virus-activated RIG-I induced IRF3 dimerization in vitro. The reconstitution reaction contained mitochondria (P5), RIG-I isolated from virus-infected or untreated cells, cytosolic extracts (S5) from uninfected cells, 35 S-IRF3, and ATP. Dimerization of IRF3 was analyzed by native gel electrophoresis. (D) In vitro activation of RIG-I by 5 0 -ppprna and ubiquitination. His 6 -tagged RIG-I (wild-type or WT) or its ATPase mutant (K270A) was purified from Sf9 cells (lower left panel), then incubated with 5 0 -ppprna (79 nucleotides), ATP, and ubiquitination enzymes as outlined in the diagram. After incubation, aliquots of the reaction mixtures were further incubated with mitochondria (P5) and cytosol (S5) from uninfected cells together with 35 S-IRF3 and ATP, and then IRF3 dimerization was analyzed by native gel electrophoresis. (E) In vitro activation of RIG-I by poly(i:c) and ubiquitination. Similar to (D), except that poly(i:c) was used and the dependency on ubiquitin and ubiquitination enzymes was tested. (F) The role of 5 0 -triphosphate for RNA to activate RIG-I in vitro. Similar to (D), except that the RNA was pretreated with or without shrimp alkaline phosphatase (SAP). (G) 5 0 -ppprna and viral RNA are potent activators of the RIG-I pathway. Total RNA was extracted from HEK293T cells from viral-infected or untreated HEK293T cells, then incubated with RIG-I as in (D), followed by IRF3 dimerization assay (lanes 1 3). To measure the potency of 5 0 -ppprna in RIG-I activation, increasing amounts of the RNA (135 nt) (0.07 to 70 nm, at 3-fold increments) were incubated with RIG-I in the presence (lanes 12 18) or absence of cellular RNA from uninfected cells (lanes 5 11), then IRF3 dimerization assay was performed. (H) IRF3 dimer shown in (G) was quantified with ImageQuant, then plotted against the concentration of 5 0 -ppprna. See also Figure S1. Cell 141, , April 16, 2010 ª2010 Elsevier Inc. 317
3 Figure 2. K63 Polyubiquitination Is Essential for RIG-I Activation (A) Ubc5 and Ubc13/Uev1a activate RIG-I in vitro. RIG-I was incubated with E1, different E2s as indicated, TRIM25, ubiquitin, RNA, and ATP, followed by IRF3 dimerization assay as described in Figure 1D. The E2 proteins (2 mg) were analyzed by Coomassie blue staining (lower panel). (B) Ubc5 and Ubc13 are required for viral activation of MAVS in the mitochondria. U2OS cells stably integrated with tetracycline-inducible shrna against Ubc5b/c and Ubc13 were treated with or without tetracycline (Tet). After viral infection for the indicated time, mitochondrial fraction (P5) was prepared and the MAVS activity was measured by IRF3 dimerization assay. 318 Cell 141, , April 16, 2010 ª2010 Elsevier Inc.
4 dsrna analog activates RIG-I and MDA5 in a manner independent of 5 0 -triphosphate (Kato et al., 2008). As expected, the DNA poly(di:dc) did not activate the RIG-I pathway in vitro or in cells (Figure 1F and Figure S1D). In vitro reconstitution of the RIG-I pathway also led to site-specific phosphorylation of IRF3 and IkBa (Extended Results and Figures S1E S1G). To determine if RIG-I could be activated by naturally occurring viral RNA, we incubated purified RIG-I protein with total RNA from HEK293T cells infected with Sendai virus. Indeed, RNA from virus-infected but not mock-treated cells stimulated RIG-I to activate IRF3 (Figure 1G, lanes 1 3). To estimate the sensitivity of 5 0 -ppprna detection by RIG-I, we incubated RIG-I with different amounts of 5 0 -ppprna in the presence or absence of HEK293T cellular RNA (Figure 1G, lanes 4 18). The half-maximal effective concentration (EC 50 ) of the 5 0 -ppprna was estimated at 1.2 nm and 0.4 nm in the absence and presence of the cellular RNA, respectively (Figure 1H). It is not clear how the cellular RNA enhances the potency of 5 0 -ppprna, but one possibility is that these RNAs reduce the nonspecific loss of very small amounts of 5 0 -ppprna in the reactions. In any case, the fact that the presence of a large excess of cellular RNA does not interfere with the specific recognition of 5 0 -ppprna by RIG-I underscores the remarkable specificity of viral RNA detection by RIG-I. Assuming that the cytoplasm of a human cell has a volume of 500 mm 3,we estimated that less than 20 molecules of viral RNA in a cell (equivalent to 0.07 nm) are sufficient to trigger detectable IRF3 dimerization. Thus, our in vitro reconstitution recapitulates the entire RIG-I pathway with exquisite sensitivity and specificity for 5 0 -ppprna (see Discussion). Ubc5 and Ubc13 Are Required for the Activation of RIG-I and MAVS Ubc5 is a family of E2s comprising highly homologous isoforms (Ubc5a, b, and c; putative Ubc5d in human) that catalyze the synthesis of polyub chains linked through various lysines of ubiquitin, including K63 (Xu et al., 2009). In contrast to Ubc5, the E2 complex consisting of Ubc13 and Uev1A is highly specific in synthesizing K63-polyUb chains (Deng et al., 2000; Hofmann and Pickart, 1999). To determine the E2 involved in RIG-I activation, we examined a panel of E2s for their ability to stimulate RIG-I in the presence of TRIM25 and RNA (Figure 2A). The Ubc5 isoforms (Ubc5a, b, and c) and Ubc13/Uev1A were capable of stimulating RIG-I to promote IRF3 dimerization. In contrast, Ubc3, Ubc7, and E2-25K had no activity (Figure 2A), despite the ability of these E2s to form thioesters with ubiquitin (data not shown; see also Zeng et al., 2009). To test if Ubc5 and/or Ubc13 are involved in the activation of MAVS by RIG-I, we established human osteosarcoma U2OS cell lines stably integrated with tetracycline-inducible shrna vectors targeting both Ubc13 and two isoforms of Ubc5 (Ubc5b and c) (Xu et al., 2009). As shown in Figure 2B, the mitochondria from the cells depleted of Ubc13, Ubc5b, and Ubc5c lost the ability to activate IRF3. These results suggest that Ubc5a and Ubc5d, which are not targeted by the Ubc5 shrnas, play a minor role in IRF3 activation in U2OS cells. RNAi of Ubc13 or Ubc5 alone partially inhibited the IRF3-stimulatory activity of the mitochondria from virus-infected cells (Figure S2A; Figure S3 in Zeng et al., 2009). These results suggest that both Ubc5 and Ubc13 are involved in the activation of MAVS in the mitochondria by RIG-I in response to viral infection. K63 Polyubiquitination Is Essential for the Activation of RIG-I and MAVS To determine if K63 of ubiquitin is required for RIG-I activation in the in vitro system, we incubated various ubiquitin lysine mutants with RIG-I in the presence of RNA, ATP, E1, TRIM25, and Ubc5c or Ubc13/Uev1A. The reaction mixture was then incubated with the mitochondrial fraction (P5) to activate MAVS. The activated mitochondria were isolated and then tested for their ability to stimulate IRF3 dimerization in the presence of cytosolic extracts (Figure 2C). The ubiquitin proteins containing a lysine at position 63 (wild-type, K48R and K63-only) were capable of activating RIG-I, whereas those containing a substitution at K63 (K63R, K48-only, KO, and methylated ubiquitin) had no activity (Figure 2C; see Figure S2C for an illustration of ubiquitin mutants). Interestingly, although Ubc5c and TRIM25 catalyze the synthesis of polyub chains from K63R (Figure S2D, lane 2), these chains did not stimulate RIG-I (Figure 2C, lane 2), indicating that K63-polyUb chain synthesis is specifically required for RIG-I activation in vitro. To investigate the role of K63 polyubiquitination in the activation of MAVS in cells, we used recently developed U2OS cell lines in which endogenous ubiquitin is replaced with wild-type or K63R mutant of ubiquitin through a tetracycline-inducible strategy (Xu et al., 2009). As shown in Figure 2D, depletion of endogenous ubiquitin severely impaired the ability of the mitochondria to promote IRF3 dimerization, but this activity was rescued by the expression of the wild-type ubiquitin transgene. In contrast, when the endogenous ubiquitin was replaced with the K63R mutant, the mitochondria isolated from these cells failed to activate IRF3 in the in vitro assay, strongly suggesting that K63 polyubiquitination is essential for viral activation of MAVS in the mitochondria. K63-Polyubiquitin Chains Activate RIG-I through Its N-terminal CARDs The N terminus of RIG-I contains tandem CARDs, which, when overexpressed in mammalian cells, constitutively activate IRF3 (Yoneyama et al., 2004). To determine if this N-terminal fragment (C) K63 of ubiquitin is essential for RIG-I activation in vitro. RIG-I and IRF3 activation assays were performed using Ubc13/Uev1a (upper panel) or Ubc5c (middle panel) as the E2 and various ubiquitin mutants as indicated. KO, lysine-less mutant; MeUb, methylated ubiquitin. (D) K63 polyubiquitination is essential for viral activation of MAVS in the mitochondria. U2OS cells stably integrated with tetracycline-inducible shrna against endogenous ubiquitin genes and a rescue expression vector for wild-type or K63R mutant of ubiquitin were grown in the presence or absence of tetracycline. After infection by Sendai virus, mitochondria (P5) were prepared to measure MAVS activation. The expression of MAVS and ubiquitin was analyzed by immunoblotting (lower panels). The HA antibody detects HA-Ub(WT) or HA-Ub(K63R) expressed from the transgene rescue vector. See also Figure S2. Cell 141, , April 16, 2010 ª2010 Elsevier Inc. 319
5 Figure 3. Activation of RIG-I N Terminus by K63-Polyubiquitin Chains (A) Depiction of RIG-I functional domains. (B) Purification ofrecombinantgst-rig-i(n) protein from E. coli. (C) Activation of RIG-I(N) by ubiquitination. GST or GST-RIG-I(N) was incubated with ubiquitination reaction (E1, Ubc5, TRIM25, and ubiquitin) and/or poly(i:c) as indicated, then an aliquot of the reaction was further incubated with mitochondria (P5) and cytosol (S5) to measure IRF3 dimerization. (D) K63-polyUb chain synthesis, but not ubiquitination of RIG-I(N), is required for RIG-I activation in vitro. Ubiquitination reactions containing different combinations of E2s and E3s as indicated were carried out before N-ethylmaleimide (NEM) was added to inactivate E1 and E2s. An aliquot of the reaction mixture was incubated with GST- RIG-I(N), then further incubated with mitochondria (P5) and cytosol (S5) to measure IRF3 dimerization. Another aliquot of the reaction was analyzed by immunoblotting with a ubiquitin antibody (lower panel). SCF b-trcp2 : an E3 complex containing Skp1, Cul1, Rbx1, and b-trcp2; LUBAC: an E3 complex containing HOIL-1L and HOIP. (E) Similar to (D), except that ubiquitination reactions were carried out in the presence of Ubc5c, TRIM25, and various ubiquitin mutants as indicated. See also Figure S3. [RIG-I(N), see Figure 3A] could activate IRF3 in vitro, we expressed the protein in E. coli and purified it to near homogeneity (Figure 3B). When the protein was incubated with mitochondria and cytosolic extracts, it did not promote IRF3 dimerization (Figure 3C, lane 2). However, when the incubation mixtures contained ubiquitination components, including E1, Ubc5c, TRIM25, and ubiquitin, robust IRF3 dimerization was detected even in the absence of RNA (lane 3). To determine if ubiquitination of RIG- I(N) is required for IRF3 activation in the in vitro system, we carried out a ubiquitination reaction in the presence of different pairs of E2 and E3 and then treated the reaction mixture with the chemical N-ethylmaleimide (NEM), which alkylates the active site cysteine of E1 and E2. The reaction mixtures were then incubated with GST-RIG-I(N) before further incubation with the mitochondrial and cytosolic fractions to measure IRF3 dimerization (Figure 3D). Under these conditions, RIG-I(N) was not ubiquitinated because E1 and E2 had been inactivated by NEM. Remarkably, when TRIM25 or another RING domain E3 TRAF6 was incubated with either Ubc13/Uev1A or Ubc5c, robust IRF3 dimerization was detected (lanes 2 5). When K48 polyub chains were synthesized by Ubc3 and an E3 complex consisting of Skp1, Cul-1, Rbx1, and btrcp2 (SCFbTrCP2), these chains did not activate the RIG-I pathway. Similarly, linear polyub chains generated by Ubc5c and an E3 complex consisting of HOIP-1 and HOIL-1L (termed LUBAC) did not lead to significant IRF3 dimerization. To further test if 320 Cell 141, , April 16, 2010 ª2010 Elsevier Inc.
6 ubiquitin chains of other linkages could support IRF3 dimerization, we used a panel of ubiquitin mutants harboring a single lysine (Figure 3E). Although all the ubiquitin mutants were capable of forming polyub chains in the presence of Ubc5c and TRIM25, the only mutants capable of supporting IRF3 activation were those containing a lysine at position 63 (K63 only and His 6 -K63 only). Collectively, these results clearly demonstrate that polyub chains containing the K63 linkage, but not other linkages, specifically activate the RIG-I pathway. We also found that unanchored polyub chains, but not ubiquitinated TRIM25, mediate RIG-I activation (Extended Results and Figure S3). Short, Unanchored, K63-Linked Ubiquitin Chains Activate RIG-I To determine if short ubiquitin chains are capable of activating RIG-I, we incubated GST-RIG-I(N) with a ubiquitin polymer containing four units of ubiquitin linked through K63 (K63-Ub4; Figure 4A). Strikingly, incubation of GST-RIG-I(N) with K63-Ub4 led to robust activation of IRF3 in this assay (lane 2), whereas GST-RIG-I(N) or K63-Ub4 alone had no activity (lanes 1 and 3). We then tested a panel of ubiquitin chains of different lengths and linkages for their ability to activate RIG-I(N) (Figure 4B). Interestingly, K63-Ub chains containing more than two ubiquitin moieties potently activated RIG-I(N) (Figure 4B, lanes 3 8). K63-Ub2 had very weak activity (lane 2), whereas monomeric ubiquitin and K48-linked ubiquitin chains were inactive (lane 1; lanes 11 14). Ub4 containing mixed linkages of K48 and K63 also had greatly reduced activity (lanes 9 10). We then carried out titration experiments to quantify the relative potency of different ubiquitin chains in the activation of RIG-I(N) (Figures 4C and 4E). Similar titration experiments were also carried out using full-length RIG-I in the presence of 5 0 -ppprna (Figures 4D and 4F). Among the ubiquitin chains tested, K63-Ub4 was the most potent activator, with an EC 50 of 25 nm for RIG-I(N) activation. The potency of K63-Ub3 was about 27-fold less (EC 50 z 680 nm), whereas K63-Ub2 was nearly inactive even at the highest concentration (1 mm). Ubiquitin, K48-Ub3, and linear-ub3 were inactive at all the concentrations tested. These results demonstrate that short, unanchored K63-ubiquitin chains containing at least three ubiquitin moieties are potent and specific activators of RIG-I. The Tandem CARDs of RIG-I Bind to K63-Ubiquitin Chains The potent activation of RIG-I(N) by K63-ubiquitin chains implies that the N terminus of RIG-I binds to these chains. Indeed, GST-RIG-I(N) was able to pull down K63-Ub3, but not K48-Ub3 or linear Ub3 (Figure 5A). Both CARDs are required for ubiquitin binding because the fragments containing residues or of RIG-I failed to bind K63-Ub4 or activate IRF3 (Figures 5B and 5C; see also Figures S5A and S5B). We also generated a RIG-I(N) protein containing a T55I mutation, which was previously shown to render RIG-I defective in interferon induction (Sumpter et al., 2005). Interestingly, T55I mutation severely impaired the ability of RIG-I(N) to bind K63-Ub4 and activate IRF3 (Figure 5C, lane 10), suggesting that the signaling defect of this mutant may be due to its failure to bind K63-ubiquitin chains. To determine if ubiquitin chain binding induces oligomerization of RIG-I(N), we incubated RIG-I(N) with K63-Ub4 and then performed gel filtration analysis using Superdex-200. K63-Ub4 and RIG-I(N) have predicted molecular masses of 30 and 24 kda, respectively; however, they formed an active complex with an apparent molecular mass of 200 kda (Figure 5D). When each protein alone was fractionated on the same column, K63-Ub4 and RIG-I(N) eluted at positions corresponding to 25 and 50 kda, respectively. These results suggest that the binding of K63-Ub4 to RIG-I(N) promotes the formation of an oligomeric complex (e.g., a tetramer). Full-length RIG-I Binds to K63-Ubiquitin Chains in a Manner that Depends on RNA and ATP As full-length RIG-I is regulated by RNA binding and ATP hydrolysis, we tested its binding to K63-ubiquitin chains in the presence or absence of RNA and ATP. Immunoprecipitation of His 8 -RIG-I-Flag led to coprecipitation of K63-Ub4 and long K63-polyUb chains in the presence of 5 0 -ppprna and ATP (Figure 5E, lanes 2 and 6). This binding was lost when the RNA was absent or when EDTA was added to chelate Mg 2+, which is required for ATP binding. Two different mutations that abrogate the ATPase activity of RIG-I, K270A and D372N, also disrupted the ability of RIG-I to bind K63 polyub (Figure 5F). To determine if RIG-I binds to RNA and K63 polyub in a sequential manner, we incubated RIG-I with 5 0 -ppprna or K63 polyub in reciprocal orders (Figure 5G). If RIG-I was preincubated with 5 0 -ppprna in the first step and then with K63 polyub in the second step, it was capable of activating IRF3 dimerization in the reconstitution assay (lane 4). In contrast, if RIG-I was preincubated with K63 polyub first, then immunoprecipitated to remove unbound polyub, its subsequent incubation with 5 0 -ppprna failed to activate it (lane 6). Thus, the binding of RIG-I to RNA precedes its binding to K63 polyub. Binding of K63-Ubiquitin Chains Is Necessary for RIG-I Activation Previous studies have shown that RIG-I is ubiquitinated at K172 and that a mutation of this residue (K172R) impairs its ability to induce IFN-b (Gack et al., 2007). Consistent with an important role of K172 in RIG-I activation, we found that GST-RIG-I(N) containing the K172R mutation failed to activate IRF3 in the in vitro reconstitution assay (Figure 6A, lanes 4 6). A GST-RIG-I(N) protein containing mutations at six lysines (K99, 169, 172, 181, 190, 193; herein referred to as 6KR) was also inactive (lanes 10 12), but this activity was rescued by keeping the lysine at position 172 (K172-only; lanes 7 9). Interestingly, the K172R, 6KR, and T55I mutants were greatly compromised in their ability to bind K63 polyub, whereas K172-only was fully capable of binding to these chains (Figure 6B). These results show that the IRF3-activating function of RIG-I correlates well with its ubiquitin-binding activity. To determine if ubiquitination or ubiquitin binding of RIG-I is important for its activation in cells, we expressed GST-RIG-I(N) and various mutants in HEK293-IFN-b-luciferase reporter cells (Figure 6C), then pulled down these proteins with glutathione Cell 141, , April 16, 2010 ª2010 Elsevier Inc. 321
7 Figure 4. Short, Unanchored K63-Ubiquitin Chains Potently Activate RIG-I (A) K63-Ub4 activates RIG-I(N). GST-RIG-I(N) (0.2 mm) was incubated with or without K63-Ub4 (0.3 mm), followed by IRF3 dimerization assay as outlined. (B) K63, but not K48, ubiquitin chains activate RIG-I(N). Ubiquitin chains of defined lengths and linkages were incubated with GST-RIG-I(N), then IRF3 dimerization assay was carried out as in (A). The quality of the ubiquitin chains was evaluated by immunoblotting (lower panel) or silver staining (see Figure S4). (C) K63-ubiquitin chains potently activate RIG-I(N) in a chain length- and linkage-dependent manner. Different concentrations of Ub chains ( mm) or ubiquitin ( mm) were tested for RIG-I(N) activation using the IRF3 dimerization assay. 322 Cell 141, , April 16, 2010 ª2010 Elsevier Inc.
8 Sepharose (Figure 6D). To distinguish ubiquitin chains covalently conjugated to RIG-I from those noncovalently associated with RIG-I, the Sepharose beads were washed with either phosphate-buffered saline (PBS) or a more stringent buffer containing 0.1% SDS and 1% deoxycholate (RIPA; Figure 6D). Immunoblotting with a GST antibody showed that whereas the wild-type GST-RIG-I(N) was conjugated by ubiquitin chains, no apparent ubiquitination of the mutants, including K172R and K172-only, was detected (Figure 6D, lower panel). Immunoblotting of the pull-down proteins with a ubiquitin antibody showed an interesting difference depending on the wash buffers (upper panel). Whereas GST-RIG-I(N) containing K172-only was able to pull down polyub chains from the cells when the beads were washed with PBS (lane 9), these chains were largely washed away with the RIPA buffer (lane 4), indicating that this mutant was defective in ubiquitination but retained the ability to bind polyub chains. Because GST-RIG-I(N)-K172-only was active in inducing IFN-b (Figure 6C), these results suggest that polyub binding by RIG-I is responsible for its function. The other RIG-I mutants, including K172R, 6KR, and T55I, were unable to bind ubiquitin chains in cells, consistent with their inability to induce IFN-b. To further evaluate whether RIG-I ubiquitination is required for its function, we treated GST-RIG-I(N) isolated from HEK293T cells with a deubiquitination enzyme (DUB), which contains the OTU domain of the Crimean Congo hemorrhagic fever virus (CCHFV) large (L) protein (Figure 6E) (Frias-Staheli et al., 2007; Xia et al., 2009). The viral OTU (votu) can cleave polyub chains from target proteins as well as unanchored polyub chains. In contrast, another DUB, isopeptidase T (IsoT), only cleaves unanchored polyub chains (Reyes-Turcu et al., 2006). Following treatment with votu or IsoT, GST-RIG-I(N) was tested for its ability to activate IRF3 in the in vitro reconstitution assay. Unlike GST-RIG-I(N) expressed in E. coli, which does not have the ubiquitin system, the same protein expressed in HEK293T cells was able to activate IRF3 without preincubation with ubiquitin chains (Figure 6E, bottom panel), probably because RIG-I(N) isolated from human cells is already bound to endogenous polyub chains (e.g., lane 9 in Figure 6D). Interestingly, although votu removed most of the ubiquitin chains from GST-RIG-I(N) (Figure 6E, lanes 6 and 9 in upper panel), the deubiquitinated RIG-I protein was as active in causing IRF3 dimerization as the protein that was mock treated (lanes 1 4 and 9 12, lower panel). These results suggest that ubiquitination of the RIG-I CARDs may be dispensable for its activity. Unanchored K63-Polyubiquitin Chains Isolated from Human Cells Are Potent Activators of RIG-I In Figure 6E, we noted that IsoT treatment did not remove much of the polyub chains from GST-RIG-I(N) (lane 5 in upper panel), indicating that RIG-I(N) protects the ubiquitin chains from degradation by IsoT (see Extended Results and Figure S6). The protection of polyub by RIG-I(N) offers an opportunity to detect the otherwise labile ubiquitin chains in cells but poses another challenge of how to release these chains while preserving their functions. We devised a protocol to isolate the RIG-I-bound ubiquitin chains from HEK293T cells by employing two strategies (Figure 7A). First, we lysed the cells in the presence of NEM to inactivate most of the deubiquitination enzymes. Ubiquitin contains no cysteine, therefore evading modification by NEM. Second, as ubiquitin is known to be relatively stable at high temperatures, we heated the RIG-I(N):polyUb complex at different temperatures to identify a condition that denatures RIG-I(N) but preserves the structure and function of ubiquitin chains. Following centrifugation that precipitates denatured protein aggregates, the supernatant is expected to contain polyub chains and can be tested for its ability to activate IRF3 in the presence of fresh RIG-I(N). Because GST-RIG-I(N)-K172- only appears to capture more polyub chains, we expressed this protein in HEK293T cells (Figure 7B). In control experiments, we incubated GST-RIG-I(N)-K172-only protein with K63-polyUb chains in vitro, carried out GST pull-down, and then heated the complex (lanes 1 6). Five minutes of heating at 70 C 80 C was sufficient to inactivate the RIG-I protein (lane 1, 3, and 5) and release polyub chains into the supernatant, which were capable of activating IRF3 when supplied with fresh GST-RIG- I(N) (lane 2, 4, and 6). When the GST-RIG-I(N) protein expressed in HEK293T cells was heated at 70 C, it was still capable of activating IRF3 in the absence of fresh RIG-I(N) (lane 7), indicating that the activated RIG-I(N) complex was resistant to NEM and 70 C treatment. However, raising the temperatures to 75 C and 80 C inactivated the GST-RIG-I(N) protein (lanes 9 and 11). Strikingly, the supernatant containing polyub chains released from the RIG-I(N) complex at the high temperatures were capable of activating IRF3 when supplied with fresh GST-RIG-I(N) (lanes 10 and 12). To determine whether the supernatant of the heated GST-RIG-I(N) complex contained unanchored polyub chains and whether these chains were responsible for activating the RIG-I pathway, we performed two sets of experiments. First, we incubated the supernatant with E1 to determine if the endogenous ubiquitin chains could form thioesters with E1. Indeed, in the presence of E1 and ATP, substantial fractions of both synthetic and endogenous ubiquitin chains formed thioesters that were sensitive to reduction by b-mercaptoethanol, indicating that they contained unanchored C termini (Figure S7A). Second, we incubated the endogenous ubiquitin chains with IsoT and then measured their activity in the IRF3 dimerization assay (Figure 7C). Importantly, the IsoT treatment completely abolished the ability of the supernatant to activate IRF3 in the presence of GST-RIG-I(N) (lane 8). However, if we reversed the order by incubating the supernatant with GST-RIG-I(N) first and then with IsoT, IRF3 dimerization was observed (lane 9). Parallel control experiments show that unanchored K63-polyUb chains were sensitive to IsoT treatment but became resistant after preincubation with GST-RIG-I(N) (lanes 2 5). To directly visualize unanchored polyub chains associated with the GST-RIG-I(N) complex isolated from HEK293T cells, the heated supernatants treated with and without IsoT were analyzed by immunoblotting with a ubiquitin antibody (Figure 7C, right panel). Several bands in the range of kda, most prominently the (D) Similar to (C), except that full-length His 6 -RIG-I and 5 0 -ppprna were used in lieu of RIG-I(N) in the reactions. (E and F) IRF3 dimers in (C) and (D), respectively, were quantified using ImageQuant, then plotted against the concentration of Ub or Ub chains. Cell 141, , April 16, 2010 ª2010 Elsevier Inc. 323
9 Figure 5. RIG-I CARDs Bind K63-Ubiquitin Chains, and This Binding in Full-Length RIG-I Is Regulated by RNA and ATP (A) RIG-I(N) binds specifically to K63-ubiquitin chains. GST or GST-RIG-I(N) was incubated with Ub3 containing K63, K48, or linear linkage, then pulled down with glutathione Sepharose, followed by immunoblotting. Input represents 10% of Ub3 used in the pull-down experiments. (B) Diagram of RIG-I N terminus containing the tandem CARDs and various deletion and point mutants. The table on the right summarizes the results in (C). (C) Both CARDsof RIG-I are required for polyub binding and IRF3 activation. GST-RIG-I(N) andvarious mutants were incubated withk63-ub4. One microliter aliquot of eachmixturewasused for IRF3 dimerization assay (upper panel), andtheremainder waspulled down withglutathionesepharose followed byimmunoblottingwith a Ub antibody (middle panel). The GST-RIG-I(N) and the mutant proteins (2 mg each) were analyzed by Coomassie blue staining (lower panel). See also Figure S5. (D) RIG-I(N) and K63-Ub4 form an active high-molecular-weight complex. RIG-I(N) was incubated with K63-Ub4 and then the mixture was fractionated on Superdex-200. Aliquots of the fractions were assayed for their ability to stimulate IRF3 dimerization, whereas other aliquots were subjected to immunoblotting with antibodies against RIG-I and ubiquitin. K63-Ub4 or RIG-I(N) alone was also analyzed by gel filtration on the same column (lower two panels). 324 Cell 141, , April 16, 2010 ª2010 Elsevier Inc.
10 40 kda band, disappeared in the IsoT-treated sample, indicating that these are unanchored ubiquitin polymers containing approximately 5 10 ubiquitin moieties. However, most of the high-molecular-weight bands remained resistant to IsoT treatment, suggesting that they represent ubiquitin chains conjugated to some protein targets. Because the IsoT treatment completely abolished the activity of the heat supernatant to promote IRF3 dimerization (Figure 7C, lane 8 on left panel), the high-molecular-weight ubiquitin conjugates remaining after IsoT treatment apparently did not contribute to RIG-I activation. To determine if the endogenous unanchored polyub chains are linked through K63 of ubiquitin, we treated the heat-resistant supernatant with the K63-specific deubiquitination enzyme CYLD. This treatment markedly diminished the ability of the supernatant to activate RIG-I (Figure 7D, left panel). CYLD also destroyed the ubiquitin chains with molecular masses in the range of kda, which could be detected with an antibody specific for the K63-ubiquitin linkage (Figure 7D, right panel). TRIM25 and CYLD have previously been shown to be important for the activation and inactivation of the RIG-I pathway, respectively (Friedman et al., 2008; Gack et al., 2007; Zhang et al., 2008). To determine if these enzymes are involved in the synthesis and disassembly of endogenous K63-ubiquitin chains, we used sirna to knock down the expression of TRIM25 or CYLD in HEK293T cells, then isolated the endogenous ubiquitin chains as outlined in Figure 7A. Immunoblotting experiments showed that depletion of TRIM25 diminished, whereas depletion of CYLD enhanced, the production of endogenous polyub chains, which in turn activated IRF3 (Figure 7E). To evaluate the potency of endogenous polyub chains in activating RIG-I, we carried out titration experiments using different amounts of heat-resistant supernatants isolated from HEK293T cells (Figure 7F). The amount of endogenous K63-Ub6 was estimated by comparing to synthetic K63-Ub6 using semiquantitative immunoblotting (Figure S7B). Because K63-Ub6 is the dominant species of endogenous polyub chains, its concentration was used to calculate the EC 50 for stimulating RIG-I. Remarkably, the EC 50 for the endogenous ubiquitin chains was estimated to be approximately 50 picomolar (pm), indicating that these ubiquitin chains are highly potent ligands of RIG-I. Finally, we examined whether viral infection induces the formation of polyub chains associated with full-length RIG-I in cells (Figure S7C). HEK293T cells expressing RIG-I-Flag were infected with Sendai virus or mock treated, and then the RIG-I complex was immunoprecipitated and heated at 76 C to release polyub chains. The heat supernatant prepared from the virusinfected cells, but not mock-treated cells, stimulated IRF3 dimerization in the presence of RIG-I(N). This activity was diminished by IsoT treatment (Figure S7D), indicating that the supernatant contained unanchored polyub chains. It should be noted that the activity associated with full-length RIG-I was much lower than that associated with RIG-I(N) because the activation of full-length RIG-I is limited by its accessibility to viral RNA (see Discussion). Nevertheless, given the potency of 5 0 -ppprna and unanchored polyub chains in activating RIG-I, small amounts of viral RNA and endogenous ubiquitin chains are likely to be sufficient to trigger the RIG-I signaling cascade. In sum, the results presented herein demonstrate that (1) the unanchored K63-ubiquitin chains are present in human cells; (2) they are bound and protected by RIG-I; and (2) they are potent activators of the RIG-I pathway. We propose that sequential binding of RIG-I to RNA and unanchored K63-polyUb chains leads to full activation of RIG-I, which in turn activates MAVS and downstream signaling cascades (Figure 7G). DISCUSSION In this report, we demonstrate that RNA with characteristics of viral RNA (5 0 -ppprna and dsrna) can activate the RIG-I pathway in vitro in a reconstitution system with exquisite specificity and sensitivity. To our knowledge, this is the first in vitro reconstitution of an immune signaling cascade from a microbial ligand (viral RNA) to activation of a transcription factor (IRF3). Our success in reconstituting the RIG-I pathway in vitro is beneficiary of at least three previous discoveries that (1) RNAs containing 5 0 -triphosphate are direct functional ligands of RIG-I (Hornung et al., 2006; Pichlmair et al., 2006); (2) mitochondria containing MAVS are essential for RIG-I signaling (Seth et al., 2005); and (3) ubiquitination is important for RIG-I activation (Gack et al., 2007). The in vitro reconstitution of the RIG-I pathway sets the stage for delineating the biochemical mechanism of RIG-I activation and subsequent steps of downstream signaling. Testimonial to the power of this in vitro system are the discoveries revealed in this study that the tandem CARDs of RIG-I represent a new class of ubiquitin-binding domain with specificity for K63-polyUb chains and that unanchored K63-polyUb chains are potent intracellular signaling molecules that activate the RIG-I pathway. Specificity and Sensitivity of Viral RNA Detection by RIG-I A major determinant that distinguishes viral RNA from the host RNA in mammalian cells is the presence of 5 0 -triphosphate in viral RNA but not in host RNA (Fujita, 2009) ppprna is generated in the course of RNA replication by viral RNA polymerases. However, RIG-I may have very limited access to viral 5 0 -ppprna for the following reasons: (1) viral RNA replication normally occurs within a membrane compartment (e.g., vesicles) that is sequestered from the cytosolic immune sensors such as RIG-I; (2) some viruses such as influenza replicate in the nucleus rather (E) Full-length RIG-I binds to ubiquitin chains in a manner that depends on 5 0 -ppprna and ATP. His 8 -RIG-I-Flag was incubated with K63-Ub4 or K63-polyUb chains in the presence or absence of 5 0 -ppprna (135 nt), ATP, or EDTA. Following immunoprecipitation with a Flag antibody, the precipitated proteins were detected with an antibody against Ub or RIG-I. The asterisk indicates a nonspecific band. (F) Similar to (E), except that RIG-I ATPase mutants (K270A and D372N) were also tested for binding to K63-polyUb chains. The asterisk indicates a nonspecific band. (G) Sequential binding of RIG-I to RNA and polyub leads to IRF3 activation. His 8 -RIG-I-Flag was incubated with 5 0 -ppprna or K63-polyUb in the first step, then immunopurified using a Flag antibody. The purified RIG-I was incubated with K63-polyUb or 5 0 -ppprna in the second step, followed by IRF3 dimerization assay. Cell 141, , April 16, 2010 ª2010 Elsevier Inc. 325
11 Figure 6. Polyubiquitin Binding Is Required for RIG-I Activation (A) Mutations at K172 and T55 impair RIG-I(N) activation in vitro. GST-RIG-I(N) and the indicated mutants were expressed and purified from E. coli, then incubated with K63-Ub4, followed by IRF3 dimerization assay (upper panel). Aliquots of the reaction mixtures were analyzed by immunoblotting with a GST antibody (lower panel). 6KR: GST-RIG-I(N) containing six K > R mutations, including K172R. K172-only: similar to 6KR except that K172 is not mutated. (B) Mutations at K172 and T55 impair polyub binding by RIG-I(N) in vitro. GST-RIG-I(N) and mutant proteins were incubated with K63-polyUb chains, then pulled down with glutathione Sepharose and analyzed by immunoblotting. (C) Mutations at K172 and T55 impair RIG-I(N) s ability to induce IFN-b in cells. Different amounts (30 ng and 100 ng) of mammalian expression vectors for GST- RIG-I(N) and mutants were transfected into HEK293-IFN-b-Luc cells, then luciferase activity was measured. Error bars represent the variation range of duplicate experiments. (D) GST-RIG-I(N) and mutant proteins as described in (C) were pulled down with glutathione Sepharose beads, washed with PBS or RIPA buffer, then analyzed by immunoblotting. (E) GST-RIG-I(N) was expressed in HEK293T cells, then purified using glutathione Sepharose. The purified protein was treated with isopeptidase T (IsoT) or viral OTU (votu) or mock-treated, then immunoblotted with an antibody against ubiquitin or GST (upper panel; N.S indicates a nonspecific band). Aliquots of the treated GST-RIG-I(N) were tested for their ability to promote IRF3 dimerization in the reconstitution assay (lower panel). See also Figure S Cell 141, , April 16, 2010 ª2010 Elsevier Inc.
12 than in the cytosol; (3) the majority of viral RNA is in complex with viral capsid proteins to form ribonucleoprotein particles (RNPs), and the 5 0 end of viral RNA can be blocked by viral proteins; (4) like their mammalian counterparts, most viral mrnas also contain 5 0 -cap or other 5 0 -modifications. Thus, RIG-I must be capable of detecting very tiny amounts of 5 0 -ppprna that may be inadvertently or transiently exposed during the course of viral replication and intracellular trafficking. By reconstituting RNA-mediated activation of the RIG-I cascade in vitro, we demonstrate that RIG-I can detect a few 5 0 -ppprna molecules (<20 in a cell) in the presence of abundant cellular RNA and trigger a signaling cascade leading to IRF3 activation. Thus, our reconstitution system recapitulates the specificity and sensitivity of viral RNA recognition by the RIG-I pathway in cells. RIG-I CARDs Represent a New Type of Ubiquitin Sensor CARDs are protein interaction domains that play key roles in many cellular processes including inflammation and apoptosis (Park et al., 2007). Unexpectedly, we found that the tandem CARDs of RIG-I bind specifically to K63-linked polyub chains. We also found that the tandem CARDs of MDA5, but not the CARDs of NOD2 or MAVS, bind to K63-polyUb chains (X.J. and Z.C., unpublished data; Figure S5C). Thus, a subset of CARDs appears to have the specialized ability to bind K63-poly- Ub chains. Importantly, mutations that disrupt ubiquitin binding of RIG-I also impair its ability to activate IRF3, indicating that polyub binding plays a key role in RIG-I activation. It will be of significant interest to identify other CARDs capable of ubiquitin binding and determine whether proteins harboring these CARDs require ubiquitin binding for their functions. Polyubiquitin Binding Is Required for RIG-I Activation An important role of RIG-I ubiquitination in its activation was suggested based on the observations that K172R mutation of RIG-I diminishes its ubiquitination and its ability to induce IFN-b and that the K172-only mutant can induce IFN-b (Gack et al., 2007). Further supporting this conclusion was the finding that the T55I mutation, which was known to abrogate IFN-b induction, also impairs RIG-I ubiquitination (Gack et al., 2008). However, closer examination of these mutants reveals that K172R and T55I mutations also impair the ability of RIG-I to bind K63-polyUb chains (Figure 6B). Moreover, the K172-only mutation reinstalls polyub binding by RIG-I, but not ubiquitination of RIG-I (Figure 6D), implying that polyub binding but not ubiquitin modification is required for RIG-I s function. In support of this notion, removal of ubiquitin chains from RIG-I with a DUB (votu) had virtually no effect on RIG-I s activity (Figure 6E). Most importantly, we demonstrated that unanchored K63-poly- Ub chains exist in human cells and that these endogenous chains are potent ligands of RIG-I (Figure 7). Interestingly, although the RIG-I complex isolated from HEK293T cells contains both IsoT-sensitive unanchored ubiquitin chains and IsoT-resistant ubiquitin conjugates, the IsoT treatment completely abolished the ability of these ubiquitin chains to activate RIG-I (Figure 7C), strongly suggesting that unanchored polyub chains are responsible for RIG-I activation. Although we cannot rule out the possibility that ubiquitination of some signaling proteins may contribute to RIG-I activation, our data show that ubiquitinated TRIM25 is incapable of activating RIG-I (Figure S3). Regulation of RIG-I by Endogenous Polyubiquitin Chains during Viral Infection We estimated that approximately 10 ng of endogenous K63-Ub6 could be isolated from 20 million HEK293T cells using our protocol (Figure S7B). This is equivalent to approximately 6000 molecules of K63-Ub6 per cell. These Ub chains activate RIG-I with an EC 50 of approximately 50 pm, which is equivalent to 15 molecules per cell. This estimate, although imprecise, suggests that there should be abundant endogenous Ub chains to activate RIG-I(N), explaining why overexpression of RIG-I(N) is sufficient to induce interferons in the absence of viral infection. However, in normal cells containing endogenous full-length RIG-I, which likely adopts an autoinhibited conformation, the ubiquitin chains cannot gain access to the CARDs of RIG-I and are rapidly disassembled by high levels of cellular DUB activity. Viral infection or introduction of 5 0 -ppprna into the cytosol presumably induces a conformational change of RIG-I that exposes the CARDs, which then capture and stabilize unanchored K63-polyUb chains synthesized by TRIM25. We speculate that local and transient accumulation of these ubiquitin chains in the vicinity of RNA-bound RIG-I allows the CARDs to bind these chains, resulting in full activation of RIG-I. However, as discussed above, only a very small fraction of RIG-I is activated by viral infection due to limited access to viral RNA in the cytosol. Nevertheless, we obtained evidence that Sendai virus infection led to association of IsoT-sensitive ubiquitin chains with full-length RIG-I (Figures S7C and S7D). Given the high potency of unanchored K63-ubiquitin chains in RIG-I activation, accumulation of just a few molecules of these chains in a cell is likely to activate endogenous RIG-I, provided that a few molecules of viral RNA are also present. Sequential Binding to RNA and Unanchored K63-Polyubiquitin Chains Leads to Full Activation of RIG-I Our study reveals unanchored K63-polyUb chains as the second ligand of RIG-I and demonstrates that full activation of RIG-I requires an orderly binding of viral RNA followed by polyub chains. Based on available evidence, we propose a multistep model of RIG-I activation (Figure 7G). After viral infection, RIG-I binds to viral 5 0 -ppprna through its C-terminal RD domain and may bind to the double-stranded segments of the RNA through the central helicase domain (Fujita, 2009). The RNA binding causes dimerization of RIG-I and activates its ATPase activity (Cui et al., 2008). The energy derived from ATP hydrolysis propels the translocation of RIG-I along the viral RNA (Myong et al., 2009). The translocation of RIG-I on viral RNA may help displace viral proteins from the RNA, facilitating further RIG-I binding. The translocating RIG-I dimer likely has an open conformation that exposes the N-terminal CARDs, which form a cluster consisting of four CARDs. This cluster of CARDs binds to K63-polyUb chains. This binding may cause additional conformational changes and/or oligomerization of RIG-I, forming a new signaling platform to activate MAVS. The dependency of RIG-I Cell 141, , April 16, 2010 ª2010 Elsevier Inc. 327
13 Figure 7. Endogenous Unanchored K63-Polyubiquitin Chains Activate the RIG-I Pathway (A) A protocol for isolating functional endogenous polyub chains in human cells. (B) Endogenous polyub chains bound to RIG-I can be released by heat treatment and remain functional. GST-RIG-I(N)-K172-only was expressed in HEK293T cells and isolated as described in (A), then heated for 5 min at the indicated temperatures. After centrifugation, the supernatant containing heat-resistant ubiquitin chains was incubated with GST-RIG-I(N) expressed and purified from E. coli (lanes 7 12). The activity of GST-RIG-I(N) was then measured by IRF3 dimerization assay. As positive controls, K63-polyUb chains were incubated with GST-RIG-I(N)-K172-only, which was then pulled down and heated in parallel experiments (lanes 1 6). Endo.polyUb: endogenous polyub. (C) Endogenous unanchored polyub chains activate RIG-I(N). PolyUb chains associated with GST-RIG-I(N)-K172-only were captured and released at 75 Casin (B). The heat-resistant supernatant was incubated with GST-RIG-I(N) followed by IsoT treatment (lane 9), or in reverse order (lane 8). As positive controls, 328 Cell 141, , April 16, 2010 ª2010 Elsevier Inc.
14 activation on binding to unanchored K63-ubiquitin chains may provide another level of regulation of antiviral immune responses. Unanchored K63-Polyubiquitin Chains Are Intracellular Signaling Molecules Our previous study has shown that unanchored polyub chains directly activate the protein kinases TAK1 and IKK by binding to the essential regulatory subunits TAB2 and NEMO, respectively (Xia et al., 2009). We now show that unanchored polyub chains bind and activate RIG-I, suggesting that these ubiquitin chains may have more general functions in cell signaling. However, unlike TAK1 and IKK activation, which requires very long polyub chains, RIG-I can be activated by short K63 chains consisting of only 3 or 4 ubiquitin moieties. Indeed, K63-Ub4 and endogenous Ub chains are very potent activators of RIG-I(N) (Figure 4E and Figure 7F). The clusters of multiple CARDs at the N termini of RIG-I may bind to K63-ubiquitin chains with strong affinity and high selectivity. This is in contrast to most ubiquitin-binding domains, which bind to ubiquitin chains with weak affinity and low selectivity (Hurley et al., 2006). Our finding that short ubiquitin chains associate with and potently activate RIG-I in cells strongly suggests that these are endogenous ligands of RIG-I. These findings present an unprecedented example of a biological function of short unanchored ubiquitin chains and deviate from the paradigm that ubiquitin functions through covalent conjugation of another protein. It will be of enormous interest to investigate whether unanchored polyub chains regulate other physiological functions besides the RIG-I and NF-kB pathways. EXPERIMENTAL PROCEDURES Biochemical Assay for RIG-I Activation In Vitro For the standard assay with full-length RIG-I, each ml mixture contained 10 ng His 6 -E1, 15 ng Ubc5c, 50 ng His 8 -TRIM25, 1 mg ubiquitin, indicated amounts of RNA (200 ng poly(i:c), 30 ng 5 0 -ppprna [79nt] or 50 ng 5 0 -ppprna [135nt]), and 50 to 100 ng His 8 -RIG-I-Flag in Buffer A (20 mm Tris-HCl [ph 7.5], 2 mm ATP, 5 mm MgCl 2, and 0.5 mm DTT). After incubation at 30 C for 1 hr, 1 ml of reaction mixture was directly used in IRF3 activation assay as described in the Extended Experimental Procedures (see also Zeng et al., 2009). For RIG-I activation assays containing mutant ubiquitin, 1 ml of reaction mixture was mixed with 10 mg of mitochondrial fraction (P5) in 10 ml Buffer B (20 mm HEPES-KOH [ph 7.0], 2 mm ATP, 5 mm MgCl 2, and 0.25 M D-mannitol) at 30 C for 30 min. P5 was then pelleted at 10,000 g for 10 min and washed twice with Buffer C (20 mm HEPES-KOH at ph 7.4, 0.5 mm EGTA, 0.25 M D-mannitol, and EDTA-free protease inhibitor cocktail) before it was used for the IRF3 activation assay. For the standard assay with RIG-I (N), each ml mixture contained 100 ng GST-RIG-I(N) and 50 to 100 ng ubiquitin chains in a buffer containing 20 mm HEPES-KOH (ph 7.0) and 10% (v/v) glycerol. After incubation at 4 C for 10 min or up to 1 hr, the mixture was directly used in the IRF3 activation assay. Ubiquitin-Binding Assay For ubiquitin binding by full-length RIG-I, His 8 -RIG-I-Flag (1 mg) or the ATPase mutant (K270A or D372N) was incubated with K63-Ub4 or K63-polyUb (1 mg), and 5 0 -ppprna (0.3 mg for 135nt RNA) in 10 ml Buffer A at 4 C for 1 hr. For reactions lacking ATP, EDTA (10 mm) was added. The mixture was then diluted 5-fold in Buffer D (20 mm Tris-HCl [ph 7.5], 100 mm NaCl, 0.5% [w/v] NP-40, and 10% [v/v] glycerol) and immunoprecipitated with anti-flag or anti-rig-i antibody. The bound proteins were eluted with Flag peptide or SDS sample buffer and subjected to immunoblotting analysis with antibodies specific for ubiquitin or RIG-I. For ubiquitin binding by RIG-I(N), 2 mg of wild-type or mutant GST-RIG-I(N) was incubated with 1 mg ubiquitin chains in 10 ml buffer containing 20 mm HEPES-KOH (ph 7.0) and 10% (v/v) glycerol at 4 C for 1 hr. The mixture was then diluted 5-fold in Buffer D before the GST proteins were pulled down with glutathione Sepharose. The bound proteins were analyzed by immunoblotting. To analyze RIG-I(N):K63-Ub4 complex, GST-RIG-I(N) was cleaved with Tev protease and then RIG-I(N) was purified on Q-Sepharose and Superdex-200 columns. Five micrograms each of purified RIG-I(N) and K63-Ub4 were incubated at 4 C for 60 min, and then the mixture was separated on Superdex-200 PC (3.2/30) using the SMART purification system. The elution buffer contained 20 mm Tris-HCl (ph7.5), 0.1 mm EGTA, 0.5 mm DTT, and 0.02% CHAPS. Aliquots of the fractions were analyzed in the in vitro IRF3 dimerization assay and also by immunoblotting using antibodies against RIG-I or ubiquitin. Isolation and Functional Analysis of Endogenous Polyubiquitin Chains HEK293T cells were transfected in 10 cm dishes with 5 mg of pef-gst-rig- I(N)-K172-only. The cells were lysed 48 hr later in Buffer E (20 mm Tris-HCl at ph 7.5, 150 mm NaCl, 10% [v/v] glycerol, 1% [w/v] Triton X-100, 20 mm b-glycerol phosphate, 1 mm Na 3 VO 4, 0.1 mm NaF, 0.1 mm phenylmethanesulfonyl fluoride, and EDTA-free protease inhibitor cocktail) supplemented with 10 mm NEM. After centrifugation at 20,000 g for 15 min, 5 mm DTT was added to the supernatant to quench NEM, then the GST proteins were isolated with glutathione Sepharose. The proteins on the beads were incubated in 30 ml Buffer F (20 mm HEPES-KOH at ph 7.0, 10% [v/v] glycerol, and 0.02% [w/v] CHAPS) at 70 C, 75 C, or 80 C for 5 min. Following a brief centrifugation, the supernatant containing the dissociated polyub chains was incubated with 100 ng of bacterially expressed GST-RIG-I(N) at 4 C for 1 hr, followed by IRF3 dimerization assay. For deubiquitination enzyme treatment, the heated supernatant was incubated with 10 ng/ml IsoT or CYLD at 30 C for 1 hr. The concentration of endogenous polyub chains was estimated by semiquantitative immunoblotting using synthetic K63-Ub6 (Boston Biochem) as the standard (Figure S7B). unanchored K63-polyUb chains were incubated with GST-RIG-I(N) and IsoT in sequential orders as indicated. In the right panel, the heat supernatant containing endogenous polyub from HEK293T cells was incubated with or without IsoT, then analyzed by immunoblotting with a ubiquitin antibody. The arrow denotes an 40 kda band that is likely K63-Ub6 (see Figure S7B). (D) Similar to (C), except that the supernatant containing endogenous polyub chains was treated with CYLD. The ubiquitin chains were detected with a ubiquitin antibody or another antibody specific for the K63 linkage of ubiquitin chains. (E) sirna oligos targeting GFP (control), TRIM25,or CYLD were transfected into HEK293T cells, which were subsequently transfected with an expression vector encoding GST-RIG-I(N)-K172-only. Endogenous polyub chains associated with the GST-RIG-I(N) protein were isolated as described in (A), then tested in IRF3 dimerization assay and visualized by immunoblotting with a ubiquitin antibody. The efficiency of RNAi was also confirmed by immunoblotting. (F) Potent activation of RIG-I by endogenous polyub chains. Different amounts of heat-resistant supernatant containing endogenous polyub were incubated with GST-RIG-I(N) to measure IRF3 dimerization. The concentration of the ubiquitin chains was estimated by semiquantitative immunoblotting (Figure S7B). Error bars represent the variation range of duplicate experiments. (G) A proposed mechanism of RIG-I activation by RNA and polyub (see Results and Discussion). Cell 141, , April 16, 2010 ª2010 Elsevier Inc. 329
15 SUPPLEMENTAL INFORMATION Supplemental Information includes Extended Results, Extended Experimental Procedures, and seven figures and can be found with this article online at doi: /j.cell ACKNOWLEDGMENTS We thank Francesco Melandri and Dr. Kal Lapan (Boston Biochem) for generously providing some of the ubiquitin chains and Dr. Jae Jung (University of Southern California) for the mammalian expression plasmids of GST-RIG- I(N), K172R, K172-only, and 6KR mutants. We also thank Brian Skaug for reading the manuscript. This work was supported by grants from NIH (RO1-AI09919 and RO1-GM63692) and the Welch Foundation (I-1389). Z.J.C. is an Investigator of Howard Hughes Medical Institute. Received: December 20, 2009 Revised: February 22, 2010 Accepted: March 23, 2010 Published: April 15, 2010 REFERENCES Akira, S., Uematsu, S., and Takeuchi, O. (2006). Pathogen recognition and innate immunity. Cell 124, Bhoj, V.G., and Chen, Z.J. (2009). Ubiquitylation in innate and adaptive immunity. Nature 458, Cui, S., Eisenacher, K., Kirchhofer, A., Brzozka, K., Lammens, A., Lammens, K., Fujita, T., Conzelmann, K.K., Krug, A., and Hopfner, K.P. (2008). The C-terminal regulatory domain is the RNA 5 0 -triphosphate sensor of RIG-I. Mol. Cell 29, Deng, L., Wang, C., Spencer, E., Yang, L., Braun, A., You, J., Slaughter, C., Pickart, C., and Chen, Z.J. (2000). Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103, Frias-Staheli, N., Giannakopoulos, N.V., Kikkert, M., Taylor, S.L., Bridgen, A., Paragas, J., Richt, J.A., Rowland, R.R., Schmaljohn, C.S., Lenschow, D.J., et al. (2007). Ovarian tumor domain-containing viral proteases evade ubiquitin- and ISG15-dependent innate immune responses. Cell Host Microbe 2, Friedman, C.S., O Donnell, M.A., Legarda-Addison, D., Ng, A., Cardenas, W.B., Yount, J.S., Moran, T.M., Basler, C.F., Komuro, A., Horvath, C.M., et al. (2008). The tumour suppressor CYLD is a negative regulator of RIG-I-mediated antiviral response. EMBO Rep. 9, Fujita, T. (2009). A nonself RNA pattern: tri-p to panhandle. Immunity 31, 4 5. Gack, M.U., Shin, Y.C., Joo, C.H., Urano, T., Liang, C., Sun, L., Takeuchi, O., Akira, S., Chen, Z., Inoue, S., et al. (2007). TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446, Gack, M.U., Kirchhofer, A., Shin, Y.C., Inn, K.S., Liang, C., Cui, S., Myong, S., Ha, T., Hopfner, K.P., and Jung, J.U. (2008). Roles of RIG-I N-terminal tandem CARD and splice variant in TRIM25-mediated antiviral signal transduction. Proc. Natl. Acad. Sci. USA 105, Hofmann, R.M., and Pickart, C.M. (1999). Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell 96, Hornung, V., Ellegast, J., Kim, S., Brzozka, K., Jung, A., Kato, H., Poeck, H., Akira, S., Conzelmann, K.K., Schlee, M., et al. (2006) Triphosphate RNA is the ligand for RIG-I. Science 314, Hurley, J.H., Lee, S., and Prag, G. (2006). Ubiquitin-binding domains. Biochem. J. 399, Kato, H., Takeuchi, O., Mikamo-Satoh, E., Hirai, R., Kawai, T., Matsushita, K., Hiiragi, A., Dermody, T.S., Fujita, T., and Akira, S. (2008). Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-i and melanoma differentiation-associated gene 5. J. Exp. Med. 205, Kawai, T., Takahashi, K., Sato, S., Coban, C., Kumar, H., Kato, H., Ishii, K.J., Takeuchi, O., and Akira, S. (2005). IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6, Meylan, E., Curran, J., Hofmann, K., Moradpour, D., Binder, M., Bartenschlager, R., and Tschopp, J. (2005). Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature 437, Myong, S., Cui, S., Cornish, P.V., Kirchhofer, A., Gack, M.U., Jung, J.U., Hopfner, K.P., and Ha, T. (2009). Cytosolic viral sensor RIG-I is a 5 0 -triphosphate-dependent translocase on double-stranded RNA. Science 323, Nakhaei, P., Genin, P., Civas, A., and Hiscott, J. (2009). RIG-I-like receptors: sensing and responding to RNA virus infection. Semin. Immunol. 21, Park, H.H., Lo, Y.C., Lin, S.C., Wang, L., Yang, J.K., and Wu, H. (2007). The death domain superfamily in intracellular signaling of apoptosis and inflammation. Annu. Rev. Immunol. 25, Pichlmair, A., Schulz, O., Tan, C.P., Naslund, T.I., Liljestrom, P., Weber, F., and Reis e Sousa, C. (2006). RIG-I-mediated antiviral responses to single-stranded RNA bearing 5 0 -phosphates. Science 314, Reyes-Turcu, F.E., Horton, J.R., Mullally, J.E., Heroux, A., Cheng, X., and Wilkinson, K.D. (2006). The ubiquitin binding domain ZnF UBP recognizes the C-terminal diglycine motif of unanchored ubiquitin. Cell 124, Seth, R.B., Sun, L., Ea, C.K., and Chen, Z.J. (2005). Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122, Sumpter, R., Jr., Loo, Y.M., Foy, E., Li, K., Yoneyama, M., Fujita, T., Lemon, S.M., and Gale, M., Jr. (2005). Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79, Xia, Z.P., Sun, L., Chen, X., Pineda, G., Jiang, X., Adhikari, A., Zeng, W., and Chen, Z.J. (2009). Direct activation of protein kinases by unanchored polyubiquitin chains. Nature 461, Xu, L.G., Wang, Y.Y., Han, K.J., Li, L.Y., Zhai, Z., and Shu, H.B. (2005). VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol. Cell 19, Xu, M., Skaug, B., Zeng, W., and Chen, Z.J. (2009). A Ubiquitin replacement strategy in human cells reveals distinct mechanisms of IKK activation by TNFalpha and IL-1beta. Mol. Cell 36, Yoneyama, M., Suhara, W., and Fujita, T. (2002). Control of IRF-3 activation by phosphorylation. J. Interferon Cytokine Res. 22, Yoneyama, M., Kikuchi, M., Natsukawa, T., Shinobu, N., Imaizumi, T., Miyagishi, M., Taira, K., Akira, S., and Fujita, T. (2004). The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5, Zeng, W., Xu, M., Liu, S., Sun, L., and Chen, Z.J. (2009). Key role of Ubc5 and lysine-63 polyubiquitination in viral activation of IRF3. Mol. Cell 36, Zhang, M., Wu, X., Lee, A.J., Jin, W., Chang, M., Wright, A., Imaizumi, T., and Sun, S.C. (2008). Regulation of IkappaB kinase-related kinases and antiviral responses by tumor suppressor CYLD. J. Biol. Chem. 283, Cell 141, , April 16, 2010 ª2010 Elsevier Inc.
16 NLRC5 Negatively Regulates the NF-kB and Type I Interferon Signaling Pathways Jun Cui, 1,2,5 Liang Zhu, 1,3,5 Xiaojun Xia, 1,5 Helen Y. Wang, 1 Xavier Legras, 1 Jun Hong, 1 Jiabing Ji, 1 Pingping Shen, 2 Shu Zheng, 3 Zhijian J. Chen, 4 and Rong-Fu Wang 1, * 1 Center for Cell and Gene Therapy, and Department of Pathology and Immunology, Baylor College of Medicine, Houston, Texas 77030, USA 2 The College of Life Science, Nanjing University, Nanjing, People s Republic of China 3 Cancer Institute, The Second Affiliated Hospital, Zhejiang University Medical School, Hangzhou, People s Republic of China 4 Howard Hughes Medical Institute, Department of Molecular Biology, University of Texas Southwestern Medical Center, Dallas, Texas 75390, USA 5 These authors contributed equally to this work *Correspondence: rongfuw@bcm.edu DOI /j.cell SUMMARY Stringent control of the NF-kB and type I interferon signaling pathways is critical to effective host immune responses, yet the molecular mechanisms that negatively regulate these pathways are poorly understood. Here, we show that NLRC5, a member of the highly conserved NOD-like protein family, can inhibit the IKK complex and RIG-I/MDA5 function. NLRC5 inhibited NF-kB-dependent responses by interacting with IKKa and IKKb and blocking their phosphorylation. It also interacted with RIG-I and MDA5, but not with MAVS, to inhibit RLR-mediated type I interferon responses. Consistent with these observations, NLRC5-specific sirna knockdown not only enhanced the activation of NF-kB and its responsive genes, TNF-a and IL-6, but also promoted type I interferon signaling and antiviral immunity. Our findings identify NLRC5 as a negative regulator that blocks two central components of the NF-kB and type I interferon signaling pathways and suggest an important role for NLRC5 in homeostatic control of innate immunity. INTRODUCTION The innate immune response, elicited through the detection of pathogen-associated molecular patterns (PAMPs), provides the first line of defense against invading microorganisms. PAMP recognition depends on several classes of pattern recognition receptors (PRRs), including Toll-like receptors (TLRs), NOD-like receptors (NLRs), and RIG-I-like receptors (RLRs) (Akira et al., 2006; Honda and Taniguchi, 2006; Inohara et al., 2005; Medzhitov, 2007; Meylan et al., 2006; Ting et al., 2006). Activation of most TLRs leads to the recruitment of a common adaptor, MyD88, and in turn to a series of downstream signaling events that culminate in NF-kB activation (Akira et al., 2006; Chen, 2005; Hayden and Ghosh, 2008). By contrast, activation of RLRs (RIG-I and MDA5) by double- and single-stranded RNAs or certain viruses (Hornung et al., 2006; Kato et al., 2006; Pichlmair et al., 2006) results in recruitment of the MAVS protein (mitochondrial antiviral signaling; also called VISA, IPS-1 and Cardif), which further activates the downstream signaling molecules TBK1/IKKi and IRF3 for type I interferon responses, as well as IKK molecules for NF-kB activation (Meylan et al., 2006). Besides their roles in innate immunity and inflammation, TLR-mediated signaling pathways have been shown to play an important role in the control of regulatory T cell function (Liu et al., 2006; Peng et al., 2005, 2007; Sutmuller et al., 2006). Because uncontrolled immune responses can be harmful, even fatal, to the host (Liew et al., 2005), NF-kB activation and type I interferon signaling must be tightly regulated to maintain immune balance in the organism. Despite the importance of the IKK complex as a central transducer of signaling from various stimuli, leading to the activation of the NF-kB pathway, and of RLRs as critical receptors in type I interferon signaling (Chen, 2005; Honda and Taniguchi, 2006), the molecular mechanisms responsible for IKK activation and RLR-mediated signaling remain poorly understood. NLRs represent a large family of intracellular PRRs that are characterized by a conserved nucleotide-binding and oligomerization domain (NOD) and a leucine-rich repeat (LRR) region, and are involved in the activation of diverse signaling pathways (Akira et al., 2006; Inohara et al., 2005; Meylan et al., 2006). Several NLRs, such as NOD1, NOD2 and NALP3, have been extensively studied and shown to activate signaling pathways once they encounter relevant PAMPs (Akira et al., 2006; Chen, 2005; Inohara et al., 2005; Meylan et al., 2006). NALP3 inflammasome, for example, functions as a crucial component in the adjuvant effect of aluminum and asbestos (Dostert et al., 2008; Eisenbarth et al., 2008). More recently, NLRX1 was demonstrated to function as a mitochondrial protein that interacts with the mitochondrial adaptor MAVS to inhibit the RIG-I-mediated signaling pathway and triggers the generation of reactive oxygen species as well (Moore et al., 2008; Tattoli et al., 2008). These studies suggest that understanding the function and mechanisms of these innate immune receptors or regulators may aid in developing more effective strategies for the immunological treatment Cell 141, , April 30, 2010 ª2010 Elsevier Inc. 483
17 Figure 1. Domain Organization, Expression, and Intracellular Localization of Human and Mouse NLRC5 (A) Domain organization of human and mouse NLRC5 proteins. CARD, caspase recruitment domain; NOD, nucleotide binding domain; LRR, leucine rich repeats. (B) Western blot analysis of HA-tagged NLRC5 and mnlrc5 protein expression in 293T cells. (C) Human and mouse NLRC5 mrnas were determined in different tissues determined by real-time PCR analysis. (D) Expression of mnlrc5 mrna in RAW264.7 cells was determined by real-time PCR analysis after LPS, intracellular poly (I:C) stimulation or VSV-eGFP infection. (E) Immunoblot analysis of mnlrc5 protein expression after LPS stimulation or VSV-eGFP infection (left panel). The relative protein levels of mnlrc5 were quantified by the band density scanning of western blot images and plotted against time (hr) after LPS treatment or VSV infection (right panel). (F) Both wild-type and MyD88-deficient macrophages were treated with LPS, and mnlrc mrna expression was determined by real-time PCR analysis. See also Figure S1. of inflammation-associated diseases (Karin et al., 2006; Wang et al., 2008). Given that the NLR protein family is involved in many biological processes and functions as proinflammatory receptors as well as negative regulators, we hypothesized that some NLR members may play a critical regulatory role in the control of NF-kB and type I interferon signaling. Here we report the identification of NLRC5 as a potent negative regulator of NF-kB and IRF3 activation. It strongly inhibits NF-kB-dependent responses by interacting with IKKa and IKKb and blocking their phosphorylation. It also interacts with RIG-I and MDA5, but not with MAVS, to potently inhibit RLR-mediated type I interferon responses. As a key negative regulator of NF-kB and type I interferon signaling, NLRC5 may serve as a useful target for manipulating immune responses against infectious or inflammation-associated diseases, including cancer. RESULTS Molecular Cloning and Characterization of NLRC5 As a member of the NLR protein family, NLRC5 contains a CARD-like domain, a central NOD domain and a large LRR region (Figure 1A), but its biological function remains unknown (Chen et al., 2009; Dowds et al., 2003). To determine the function of NLRC5, we cloned full-length human and murine NLRC5 complementary DNAs (cdnas) by rapid amplification of cdna ends (RACE) (Figure 1A). There was a 64% amino acid sequence identity between the human and murine proteins. Expression of both HA-tagged NLRC5 and mnlrc5 was demonstrated by western blotting with an anti-ha antibody (Figure 1B). Both human and murine NLRC5 mrnas were strongly expressed in spleen, thymus, and lung (Figure 1C), suggesting that this molecule is biologically conserved in these tissues. To further demonstrate the protein expression of NLRC5 in different cell types, we generated polyclonal antibodies against endogenous NLRC5 and mnlrc5 (Figures S1A S1E available online), which readily detected these proteins in human HEK293T (293T) cells, human THP1 cells or mouse RAW264.7 cells (Figure S1F). To determine whether NLRC5 expression could be regulated in response to TLR stimulation or virus infection, we treated RAW264.7 cells with LPS, intracellular poly(i:c), or infection with vesticular stomatitis virus-enhanced green fluorescent protein (VSV-eGFP), which are known to activate the NF-kB or type I IFN pathway (Yoneyama and Fujita, 2009). Real-time PCR and western blot analyses revealed strong upregulation of mnlrc5 at both the mrna and protein levels, which peaked at 6 hr after LPS treatment (Figures 1D and 1E and Figure S1G). In contrast, we observed only a weak increase of mnlrc5 mrna and little or no change in mnlrc5 protein after intracellular poly(i:c) treatment or VSV infection (Figures 1D and 484 Cell 141, , April 30, 2010 ª2010 Elsevier Inc.
18 Figure 2. NLRC5 Inhibits NF-kB Activation Induced by IL-1b, LPS, TNF-a, and Their Downstream Signaling Molecules (A) 293T cells were transfected with an NF-kB-luc reporter plasmid and TLR4 plasmid (for LPS treatment), together with an empty vector or NLRC5 construct, and analyzed for NF-kB-dependent luciferase activity (fold induction) after treatment with IL-1b, LPS, or TNF-a. (B) 293T cells were transfected with MyD88, TRAF6, IKKa, IKKb, orp65, along with NF-kB-luc. (C) 293T cells were transfected with NOD1 or NOD2, along with NF-kB-luc. (D) Detection of endogenous NF-kB DNA binding activity in a gel-mobility shift assay. Oct-1/DNAbinding complexes served as a loading control for nuclear extracts. (E) Human THP-1 cells and murine embryonic fibroblasts (MEF) were transfected with the NF-kB-luc reporter plasmid, together with (or without) NLRC5 or mnlrc5 plasmid, and then analyzed for NF-kB-dependent luciferase activity after LPS treatment (***p < 0.001). See also Figure S2. 1E), consistent with a previous study showing that VSV infection is a weak NF-kB inducer (Ishikawa and Barber, 2008). To further explore the mechanisms by which NLRC5 is regulated, we performed similar experiments with wild-type and MyD88 knockout (KO) macrophages and found that upregulation of mnlrc5 was completely abolished when MyD88 KO macrophages were stimulated with LPS (Figure 1F), indicating that the expression of mnlrc5 itself is controlled by the NF-kB signaling pathway. To determine the cellular localization of NLRC5, we transfected 293T cells with NLRC5-GFP fusion DNA and found that NLRC5-GFP was present in the cytoplasm, but not in the nucleus or mitochondria (Figures S1H S1I), suggesting that NLRC5 is a cytosolic protein. No change in the cellular localization of NLRC5 was observed after LPS treatment (Figures S1I S1K). NLRC5 Is a Potent Negative Regulator of NF-kB Activation Induced by TLR Ligands To determine whether NLRC5 is involved in TLR- and/or cytokine-mediated NF-kB activation, we transfected 293T or 293T/TLR4 cells with NF-kB-luc reporter DNA, with or without the NLRC5 plasmid, and then treated them with interleukin (IL)-1b, TNF-a or LPS. Results in Figure 2A show that NLRC5 potently inhibited NF-kB activation induced by IL-1b, TNF-a or LPS. We next sought to identify potential signaling molecules that activated the NFkB-luc reporter. Expression of MyD88, TRAF6, IKKa, or IKKb strongly induced NF-kB-luc activity, but such activity was inhibited when NLRC5 was cotransfected at increasing concentrations (Figure 2B). In contrast, NLRC5 did not inhibit p65- mediated NF-kB-luc activity, suggesting that it may interact with IKK signaling molecules upstream of p65 to block NF-kB activation (Figure 2B). The control plasmids NOD1 and NOD2 enhanced rather than inhibited NF-kB activity (Figure 2C), consistent with previous studies (Inohara et al., 1999; Ogura et al., 2001). Since LPS can also activate the type I IFN pathway through an adaptor TRIF molecule, we asked whether NLRC5 can inhibit LPS-induced IFN-b-luc activity (which requires cooperation between IRF3 and NF-kB activation) or interferon-stimulated response element (ISRE)-luc activity (which requires IRF3 activation only) (Zhong et al., 2008). Functional assays showed that NLRC5 inhibited LPS- or TRIF-induced NF-kB-luc and IFN-bluc activities, but had no effects on ISRE-luc activity (Figures S2A and S2B). Further experiments demonstrated that NLRC5 strongly inhibited R848-induced NF-kB-luc activity and weakly inhibited IFN-b-luc activity, but did not inhibit ISRE-luc activity (Figure S2C). Thus, NLRC5 strongly inhibits NF-kB activation induced by different TLR ligands and weakly blocks IFN-b activation mainly due to its requirement for NF-kB activity, but has no apparent effect on ISRE activity. Cell 141, , April 30, 2010 ª2010 Elsevier Inc. 485
19 To determine whether the observed NLRC5-mediated inhibition of NF-kB-luc activity is associated with endogenous NF-kB activity, we performed experiments with a gel-mobility shift assay. IKKb expression allowed endogenous NF-kB to bind to the biotin-hrp-labeled DNA with NF-kB binding sites, but this activity was completely inhibited when NLRC5 was cotransfected. However, NLRC5 failed to inhibit p65-mediated NF-kB activation (Figure 2D), consistent with results obtained with the NF-kB-luc reporter assay. To substantiate these findings, we found that like its human homolog, mnlrc5 strongly inhibited NF-kB activation by MyD88, TRAF6, IKKa and IKKb, but not by p65 (Figure S2D). Moreover, NLRC5- or mnlrc5-mediated NF-kB inhibition in 293T cells could be extended to THP-1 cells and murine embryonic fibroblasts (MEFs) (Figure 2E). Taken together, these results suggest that NLRC5 functions as a negative regulator of NF-kB activation induced by TNF-a, IL-1b, LPS or by their downstream signaling molecules, and that its biological function is conserved between humans and mice as well as among multiple cell types. NLRC5 Interacts with IKKa/IKKb in Unstimulated and Stimulated Cells Results presented in Figures 2B and 2D suggest that NLRC5 may directly interact with IKKa, IKKb or NEMO to inhibit NF-kB activation. To test this prediction, we transfected 293T cells with HA-tagged NLRC5 together with Flag-tagged IKKa, Flagtagged IKKb or Flag-tagged NEMO expression plasmids. Coimmunoprecipitation and western blot analyses revealed that NLRC5 interacted with IKKa and IKKb subunits, but not with NEMO, although the corresponding proteins were readily detected in whole-cell lysates (Figure 3A). Notably, NLRC5 did not interact with either TAK1 or MEKK3 upstream of IKK (Figure S3A). To further test whether NLRC5 can interact with endogenous IKKa/b, we immunoprecipitated 293T cell lysates with an isotype IgG or anti-nlrc5 antibody, followed by western blot analysis with the anti-ikka/b, anti-nemo or anti-nlrc5 antibody. As shown in Figure 3B, the IKKa/b, but not NEMO, could be detected in the anti-nlrc5 or anti-mnlrc5-immunoprecipitants. Such an interaction between NLRC5 and IKKa/b was also observed when anti-ikka/b and anti-nemo antibodies were used for immunoprecipitation (Figure S3B). These results suggest that NLRC5 can interact with IKKa/b, but not with NEMO, under physiological conditions. Since IKKa/b generally forms a complex with NEMO, a key question is whether there are two distinct complexes in unstimulated cells: IKKa/b/NEMO and IKKa/b/NLRC5. To address this issue, we used different antibodies (anti-ikka, anti-flag and anti-nemo) to immunoprecipitate the IKK complexes containing either NLRC5 or NEMO, and then determined the components of their complexes. We found NEMO and NLRC5 in the anti-ikka immunoprecipitants, NLRC5 and IKKa/b (but not NEMO) in the anti-flag-nlrc5 immunoprecipitants, and IKKa/b and NEMO (but not NLRC5) in the anti-nemo immunoprecipitants (Figure S3C), suggesting that the IKKa/b/NEMO and IKKa/b/ NLRC5 complexes coexist in unstimulated cells. To definitively demonstrate the presence of two distinct complexes, we fractionated cell extracts of RAW264.7 on a size-exclusion column (HiPrep 16/60 Sephacryl S-300 HR). Immunoblotting of fractions collected from chromatography showed that IKKa/b mainly coeluted with NEMO in fractions (molecular mass about 700 kda) (Figure 3C), consistent with a previous report (Rothwarf et al., 1998). In contrast, NLRC5 mainly eluted in fractions (molecular mass about 1100 kda), which also contained IKKa/ b but no NEMO (Figure 3C), suggesting that NLRC5 forms a larger complex with IKKa/b than previously reported for the IKKa/b/NEMO complex, thus confirming the coexistence of IKKa/b/NLRC5 and IKKa/b/NEMO complexes in unstimulated cells. To determine the dynamics of NLRC5 interaction with IKKa/ b after stimulation, we treated RAW264.7 cells with LPS, collected them at different time points and performed immunoprecipitation and western blot analyses. The interaction between NLRC5 and IKKa/b was reduced at 30 min, but was restored at 60 min after LPS stimulation (Figure S4A). This oscillating pattern of interaction correlated inversely with IKK phosphorylation (p-ikk) during the first 60 min posttreatment and then diminished or even disappeared after 2 hr of treatment (Figures S4A S4C), suggesting that the negative regulatory activity of NLRC5 is signal-dependent, but is not affected exclusively by protein concentration. NLRC5 Competes with NEMO for IKKa/IKKb and Inhibits Their Phosphorylation and Kinase Activity We next tested whether NLRC5 and NEMO compete each other for binding to IKKa/b. 293T cells were transfected with a fixed concentration of Flag-NLRC5 and HA-IKKb DNAs, together with increasing concentrations of HA-NEMO DNA. Coimmunoprecipitation and immunoblot revealed that the binding between NLRC5 and IKKb was markedly decreased with increasing concentrations of NEMO (Figure 3D). Although the phosphorylation and kinase activity of IKKa/b could be detected in the IKKa/b/NEMO complex after LPS stimulation, they decreased with increasing amounts of transfected NLRC5. There were no detectable phosphorylation and kinase activity of IKKa/b in the IKKa/b/NLRC5 complex (Figure 3E). Importantly, increasing amounts of NLRC5 had more pronouced effects on the phosphorylation and kinase activity of IKKa/b in the total and NEMO-immunoprecipitated IKKa/b fraction than on its changes at the protein level (Figure 3E). These results suggest that NLRC5 not only competes with NEMO for IKKa/b binding, but also inhibits IKKa/b phosphorylation and its ability to phosphorylate IkB or free IKKa/IKKb. To test this possibility, we performed experiments with constitutively active IKKa (SS176/180/EE) or IKKb (SS177/181/EE) mutants and found that NLRC5 interacted with constitutively active IKKa (EE) and IKKb (EE) (Figure S4D). Kinase assays revealed that expression of NLRC5 inhibited the ability of IKKa (EE) and IKKb (EE) to phosphorylate IkB (i.e., 32p-GST-IkBa), as well as their ability to autophosphorylate IKK (i.e., 32p-IKK) (Figure S4E). These observations were further supported by NF-kB-luc assays showing that NLRC5 can inhibit NF-kB activation by the constitutively active IKKa (EE) and IKKb (EE) (Figure S4F). These results suggest that NLRC5 can inhibit the ability of active IKKa/IKKb to phosphorylate IkBa or free IKKa/IKKb. To determine how NLRC5 inhibits the phosphorylation of IKK, we sought to identify the domain of IKKb responsible 486 Cell 141, , April 30, 2010 ª2010 Elsevier Inc.
20 Figure 3. NLRC5 Interacts with IKKa and IKKb to Inhibit Their Phosphorylation (A) 293T cells transfected with Flag-IKKa, Flag-IKKb, Flag-NEMO and HA-NLRC5. HA-tagged NLRC5 protein was immunoprecipitated with anti-ha beads, and blotted with anti-flag. (B) 293T and RAW264.7 cell extracts were immunoprecipitated with IgG, anti-nlrc5 or anti-mnlrc5 antibody, respectively, and then analyzed together with whole-cell extracts by western blot with an anti-ikka/b, anti-nemo, anti-nlrc5 or anti-mnlrc5 antibody. (C) Cell extracts of RAW264.7 cells were fractionated on a size-exclusion column (HiPrep 16/60 Sephacryl S-300 HR). The collected factions with an equal volume were used for western blot analysis with specific antibodies. The elution positions of calibration proteins with known molecular masses (kda) were used to determine the size of complexes. (D) 293T cells were transfected with the indicated doses of Flag-NLRC5, HA-IKKb and HA-NEMO. Whole-cell extracts were immunoprecipitated with anti-flag beads and blotted with anti-ha. (E) 293T/TLR4 cells were transfected with empty vector or different doses of HA-NLRC5 (0, 50 or 200 ng) DNA and then treated with LPS. Cell extracts were collected at 30 min poststimulation and prepared for immunoprecipitation with anti-ha and anti-nemo, followed by immunoblot (IB) with indicated antibodies or kinase assay (KA). (F) The domain structure of IKKb. Numbers in parentheses indicate amino acid position in construct. LZ, leucine zipper; HLH, helix-loop-helix. (G) 293T cells were transfected with HA-NLRC5 and Flag-IKKb or various Flag-IKKb mutants. Whole-cell extracts were immunoprecipitated with anti-flag beads, and blotted with anti-ha. (H and I) 293T cells transfected with IKKa, IKKb, JNK1, JNK2, and p38 with or without HA-NLRC5 were used to analyze the phosphorylation of IKKa/b, JNK and p38. (J) RAW264.7 cells were treated with LPS and collected at the indicated time points. Cell extracts were prepared for immunoprecipitation with anti-mnlrc5 or anti- NEMO, followed by immunoblot (IB) to determine the IKK phosphorylation with anti-p-ikk or anti-ikka/b antibody and kinase activity of IKK. See also Figure S3 and Figure S4. Cell 141, , April 30, 2010 ª2010 Elsevier Inc. 487
21 Figure 4. Interaction and Functional Analysis of NLRC5 Deletions in the Inhibition of NF-kB Activation (A) Four NLRC5 deletion constructs were generated. (B and C) 293T cells were transfected with NLRC5 and its mutations constructs, IKKb or kinase domain of IKKb and analyzed by coimmunoprecipitation and western blot. (D) 293T cells were transfected with NF-kB-luc reporter, together with an empty vector, or with full-length NLRC5 and its deletion constructs, and analyzed for luciferase activity (fold induction). (E) 293T cells were transfected with various expression plasmids and the phosphorylation of IKKa or IKKb was determined. See also Figure S4G. for interacting with NLRC5. Because NEMO is known to bind to the C terminus of IKKb (May et al., 2000), we generated deletion mutants encompassing the amino-terminal kinase domain (KD), leucine zipper domain (LZ) and a C-terminal helix-loophelix (HLH) domain of IKKb, and tested them for their ability to interact with NLRC5 in an immunoprecipitation assay (Figure 3F). Like the full-length IKKb, the IKKb-KD construct strongly interacted with NLRC5, while neither the IKKb-LZ nor the IKKb-HLH construct showed appreciable binding activity with NLRC5 (Figure 3G), indicating that NLRC5 specifically binds to the IKKb-KD domain. However, because of the large size of the NLRC5 protein, we considered that its binding to IKKb might physically block any binding of NEMO to IKK (Figures 3D and 3E). We next tested the specificity of NLRC5-mediated inhibition of IKK phosphorylation. As shown in Figures 3H and 3I, NLRC5 markedly inhibited the IKKa/b phosphorylation, but not p38 or JNK phosphorylation, consistent with the observation that NLRC5 did not interact with TAK1 or MEKK3 (Figure S3A). Finally, we further tested the status of the phosphorylation and kinase activity of IKKa/b in IKKa/b /NLRC5 and IKKa/b /NEMO complexes in RAW264.7 cells under physiological conditions. IKKa/b in the IKKa/IKKb/NLRC5 (NLRC5-IP) complex was not phosphorylated and showed no kinase activity during LPS stimulation. By contrast, IKKa/b in the IKKa/b /NEMO (NEMO-IP) complex was phosphorylated and exhibited a strong kinase activity after LPS stimulation (Figure 3J), suggesting that NLRC5 inhibits its phosphorylation and kinase activity in the IKKa/ IKKb/NLRC5 complex under physiological conditions. LRR Region Is Responsible for NLRC5-Mediated Inhibition of IKK Phosphorylation To identify the functional domains of NLRC5, we generated four deletion constructs: NLRC5-D1, containing the CARD and NOD domains (aa 1 517); NLRC5-D2, containing the LRR-R1 (aa ); NLRC5-D3, containing the linker region and LRR-R2 (aa ); and NLRC5- D4, containing the CARD domain and LRR-R3 (aa plus ) (Figure 4A). While all four NLRC5 deletion constructs could interact with the full-length IKKb protein as well as IKKb-KD (Figures 4B and 4C), NLRC5- D3, like the full-length NLRC5, strongly inhibited NF-kB-luc activity (Figure 4D). Other NLRC5 deletions showed either partial or no inhibitory effect on NF-kB-luc activity. These results suggest that NLRC5-D3 is required for the observed inhibition of NF-kB activity by NLRC5. To further investigate the molecular mechanism by which NLRC5-D3 inhibits NF-kB activity, we tested whether these deletions can inhibit the phosphorylation of IKKa/b. We found that NLRC5-D3 strongly inhibited IKKa and IKKb phosphorylation, while NLRC5-D1, -D2 and -D4 produced little or weak inhibition (Figure 4E). Notably, the inhibitory activity of these NLRC5 deletion mutants on the phosphorylation of IKKa and IKKb correlated with their ability to inhibit NF-kB activation (Figure 4D), suggesting that NLRC5-D3 inhibits NF-kB activation by blocking the phosphorylation of IKK complexes. Unlike the full-length NLRC5, however, NLRC5-D3 failed to compete with NEMO for binding to IKKa/IKKb (Figure S4G), suggesting that the size of full-length NLRC5 is critical for its ability to physically block NEMO from interacting with IKKa/b. Knockdown of NLRC5 Enhances NF-kB Activation as Well as the Inflammatory Response Since NLRC5 specifically interacts with IKKa/b and inhibits NF-kB activation, we reasoned that knockdown of NLRC5 would release IKKa/b for increased NF-kB activation under physiological conditions. To test this prediction, we first demonstrated the 488 Cell 141, , April 30, 2010 ª2010 Elsevier Inc.
22 Figure 5. Knockdown of NLRC5 Can Significantly Enhance NF-kB Activation and Inflammatory Responses (A) Specific knockdown of NLRC5 or mnlrc5 was evaluated in various types of cells transfected with NLRC5/mNLRC5 sirna or scrambled sirna. (B) RAW264.7 cells were transfected with mnlrc5 sirna or scrambled sirna, and then treated with LPS. The cell extracts were harvested at different time points and used for western blot of various kinases and signaling proteins. (C) The cell extracts of mnlrc5 knockdown and control RAW264.7 cells after LPS treatment were used for immunoprecipitation to obtain NEMOassociated IKK (IP-1) and NEMO-free IKK (free IKK and mnlrc5 associated IKK (IP-2). The phosphorylation of IKKa/b, the total amount of IKKa/b and NEMO proteins in different fractions were determined by anti-p-ikk, anti-ikka/b or anti- NEMO antibody. (D) NLRC5 or mnlrc5 was knocked down in 293T/TLR4 and RAW264.7 cells. NF-kB-luc activity was determined after LPS treatment. (E) The mnlrc5 knockdown and control AW264.7 cells were treated with LPS for 1 h; the nuclear proteins were harvested for NF-kB binding activity determined by EMSA. Oct-1 DNA-binding complexes served as a control. (F) Endogenous NLRC5 was knocked down in THP-1 and RAW264.7 cells and TNF-a and IL-6 production was measured after LPS treatment. Data in panels (D) and (F) are presented as means ± SEM. Asterisks indicate significant differences between groups (*p < 0.05, **p < 0.01, ***p < as determined by Student s t test analysis). See also Figure S5. knockdown efficiency and specificity of NLRC5 or mnlrc5 at both the mrna and protein levels in various cell types with at least two corresponding sirnas, but not scrambled sirnas (Figure 5A and Figures S5A S5C). We next tested the effect of mnlrc5 knockdown on the phosphorylation of IKK, IkB, and other kinases, including JNK, ERK, and p38. As shown in Figure 5B and Figure S5D, the phosphorylation of IKK (p-ikk) and IkB (p-ikb) in the mnlrc5 knockdown cells was at least 3-fold higher than that in the scrambled sirna-transfected (control) cells at 30 min after LPS treatment, although the total amounts of IKK and IkB proteins were comparable between mnlrc5 knockdown and control cells. More importantly, we did not observe any appreciable differences in p-jnk, p-erk and p-p38 between mnlrc5 knockdown and control cells, clearly indicating the specific inhibitory effect of mnlrc5 on the phosphorylation of IKK, but not on JNK, ERK, and p38 phosphorylation. Similar results were obtained with human THP-1 cells (Figure S5E). Collectively, these results suggest that specific knockdown of NLRC5 or mnlrc5 strongly enhances the phosphorylation of the IKK complexes, but does not affect the phosphorylation of JNK, ERK, and p38 after LPS stimulation. To further determine the molecular mechanisms by which NLRC5 knockdown affects NF-kB signaling, we performed two-step immunoprecipitations to obtain NEMO-associated IKK (IP-1) and NEMO-free IKK (i.e., free IKK and NLRC5-associated IKK, IP-2) (Figure 5C) and then compared the amounts of IKKa/ IKKb in each complex and their phosphorylation (p-ikk) in mnlrc5 knockdown versus control cells. We found that the total IKKa/b proteins in the NEMO-free IKK fraction (i.e., IP-2) of mnlrc5 knockdown cells was slightly lower than that of control cells, while IKKa/b in the IKKa/b/NEMO fraction (i.e., Cell 141, , April 30, 2010 ª2010 Elsevier Inc. 489
23 IP-1) in mnlrc5 knockdown cells was slightly higher than that in control cells, suggesting that the reduction of IKKa/b /NLRC5 complex in mnlrc5 knockdown cells decreases the IKKa/b proteins in the NEMO-free IKK fraction, but increases the IKKa/b proteins in the NEMO/IKK complex (Figure 5C). Consistent with this observation, we found that the phosphorylation of IKK (p-ikk) in the NEMO-containing IP fraction (IP-1) in the mnlrc5 knockdown cells was higher than that in control cells. Importantly, even though the total IKKa/b in the NEMO-free fraction (IP-2) of mnlrc5 knockdown cells was less than that of control cells, its phosphorylation (p-ikk) level in mnlrc5 knockdown cells was higher than that in control cells (Figure 5C), suggesting that mnlrc5 knockdown reduces the inhibition of phosphorylation of free IKKa/b. This notion agrees with data, showing that NLRC5 interacts with constitutively active IKKa (EE) and IKKb (EE) and inhibits their phosphorylation and kinase activity (Figures S4D and S4E). We next sought to determine whether the enhanced IKK phosphorylation seen with NLRC5 knockdown promotes NF-kB activation and NF-kB-dependent gene expression. Using the NF-kBluc reporter assay, we found that NLRC5 knockdown markedly increased NF-kB-luc activity in 293T/TLR4 and RAW264.7 cells after LPS treatment (Figure 5D). To directly demonstrate that mnlrc5 knockdown enhances endogenous NF-kB activity, we examined the DNA-binding activity of endogenous NF-kB. Results in Figure 5E show that endogenous NF-kB activity in mnlrc5 knockdown cells was at least 2-fold higher than that in control cells. Consistent with this observation, knockdown of NLRC5 or mnlrc5 resulted in markedly increased secretions of NF-kB-responsive cytokines, such as TNF-a and IL-6, in both mnlrc5 knockdown THP-1 and RAW264.7 cells (Figure 5F). Hence, knockdown of NLRC5 or mnlrc5 enhances the IKKa/b phosphorylation and NF-kB activity, thus increasing NF-kBdependent cytokine responses under physiological conditions. NLRC5 Negatively Regulates Type I Interferon Signaling by Interacting with RIG-I and MDA5 Recent studies show that NLRX1 inhibits the RIG-I-mediated signaling pathway by targeting MAVS (Moore et al., 2008). To determine whether NLRC5 might also be involved in the regulation of type I interferon signaling, we performed functional assays in TLR3-deficient 293T cells and found that intracellular poly(i:c) activated IFN-b signaling, although such activation was strongly inhibited by NLRC5 (Figure 6A), suggesting that NLRC5 functions as a negative regulator of this antiviral pathway. To determine the molecular mechanisms by which NLRC5 could inhibit the IFN-b response, we performed similar experiments with different signaling molecules and found that RIG-Iand MDA5-induced IFN-b-luc activities could be markedly inhibited by increasing concentrations of NLRC5. However, NLRC5 weakly inhibited MAVS- and TBK1-induced IFN-b-luc activity and did not inhibit IKKi-induced IFN-b luciferase activity (Figure 6A and Figure S6A). Furthermore, NLRC5 markedly inhibited NF-kB-luc activity induced by intracellular poly(i:c), RIG-I, MDA5, MAVS or TBK1 (Figure 6B). To determine whether the weak inhibition of MAVS- and TBK1-induced IFN-b-luc activity by NLRC5 might be due to the inhibitory effect of NLRC5 on IKK complexes, but not due to any direct effect on MAVS or TBK1, we transfected 293T cells with ISRE-luc, together with RIG-I, MDA5, MAVS, or TBK1 in the presence or absence of NLRC5. Both RIG-I- and MDA5-induced ISRE-luc activities were potently inhibited, while MAVS- and TBK1-induced ISREluc activities were not (Figure 6C), suggesting that NLRC5 inhibits IFN-b activation by directly interacting with RIG-I and MDA5, but not with MAVS or TBK1. Indeed, we found that NLRC5 was strongly associated with RIG-I and MDA5, but did not interact with MAVS, IKKi, TBK1, TRIF, TRAF3, or IRF3 (Figures 6D and 6E and Figure S6B). Thus, NLRC5 specifically binds to the RIG-I and MDA5 proteins to inhibit the IFN-b response. To further define the interaction between RIG-I and mnlrc5 under physiological conditions, we infected RAW264.7 cells with VSV-eGFP and performed immunoprecipitation with antimnlrc5 at different time points after VSV-eGFP infection. We detected only a weak RIG-I protein band in anti-mnlrc5 immunoprecipitants at 4 hr postinfection; however, it peaked at 6 hr and then became weak at 8 and 10 hr postinfection (Figure 6F). Notably, RIG-I protein expression was also increased at 6 hr postinfection (Figure 6F), consistent with previous observations (Honda and Taniguchi, 2006). Importantly, IRF3 phosporylation was observed at 4 hr and further increased at 6 hr postinfection, indicating that VSV-eGFP infection activated the IRF3-dependent signaling pathway. However, IKK phosphorylation and IkB degradation were weak after VSV-eGFP infection (Figure 6F). These results suggest that interaction between NLRC5 and RIG-I is inducible after VSV-eGFP infection. Because both the RIG-I protein level and IRF3 phosphorylation peaked at 6 hr postinfection, coincident with the peak interaction between mnlrc5 and RIG-I in RAW264.7 cells, we considered that this interaction might critically rely on activating signal. To test this possibility, we generated RIG-I-CARD and RIG-I-helicase constructs and tested their ability to interact with NLRC5. Interestingly, NLRC5 strongly bound to the CARD domain of RIG-I, but not to the helicase domain, while binding of NLRC5 to the CARD domain of RIG-I markedly exceeded that to the full-length RIG-I (Figure 6G), suggesting that the CARD domain becomes accessible only when RIG-I is activated by virusderived ligands. Furthermore, competition experiments revealed that NLRC5 strongly inhibited the ability of MAVS to bind to RIG-I (Figure 6H). Thus, it appears that the accessibility of the CARD domain of RIG-I is critical for a productive interaction between NLRC5 and RIG-I, and that such interaction may also be influenced by the relative concentrations of MAVS and NLRC5. To confirm the inhibitory effect of NLRC5 on the IRF3 phosphorylation, we assessed the phosphorylation states of IRF3 by overexpressing NLRC5 in 293T cells and found that NLRC5 potently blocked the phosphorylation of endogenous IRF3 induced by RIG-I, but not by MAVS (Figure 6I). In contrast, NLRC5 or mnlrc5 knockdown in THP-1 and RAW264.7 cells markedly increased IRF3 phosphorylation (Figure 6J). Thus, NLRC5 can inhibit IFN-b signaling by blocking the binding of MAVS to RIG-I and the IRF3 phosphorylation. Knockdown of NLRC5 Enhances Innate and Antiviral Immunity To further demonstrate the effects of NLRC5 knockdown on the expression of IFN-responsive genes, we knocked down 490 Cell 141, , April 30, 2010 ª2010 Elsevier Inc.
24 Figure 6. NLRC5 Negatively Regulates IFN-b Activation by Inhibiting RIG-I and MDA5 Function (A C) 293T cells were transfected with NF-kB-luc, INF-b-luc or ISRE-luc, NLRC5 plus poly(i:c)/lyovec, RIG-I, MDA5, MAVS, TBK1, or IKKi plasmids and analyzed for INF-b or ISRE luciferase activity. Values are means ± SEM of three independent experiments (*p < 0.05, **p < 0.01, ***p < 0.001). (D) 293T cells were transfected with HA-NLRC5 plus RIG-I, MAVS or IKKi. After immunoprecipitation with anti-ha beads, specific proteins were analyzed by western blot with anti-flag. (E) 293T cells were transfected with MDA5 with or without HA-NLRC5. After immunoprecipitation with anti-ha beads, specific proteins were analyzed by western blot with anti-mda5. (F) RAW264.7 cells were infected with VSV-eGFP, and cell extracts were harvested at different time points, immunoprecipitated with anti-mnlrc5 antibody and analyzed by western blot with anti-rig-i. (G) NLRC5 binds to the CARD domain of RIG-I. 293T cells were transfected with HA-NLRC5 plus Flag-RIG-I, Flag-RIG-I CARD domain and Helicase domain (HD). After immunoprecipitation with anti-flag beads, specific proteins were analyzed by western blot with anti-ha. (H) NLRC5 competitively binds to RIG-I with MAVS. HEK293T cells were transfected with Flag-MAVS-HA plus Flag-RIG-1,or Flag-NLRC5. After immunoprecipitation with anti-ha beads, specific proteins were analyzed by western blot with anti-flag. (I) 293T cells were transfected with Flag-RIG-I and Flag-MAVS, with or without HA-NLRC5, and used for western blot analysis with anti-phospho-irf3 and IRF3 antibodies. (J) RAW264.7 and THP-1 cells were transfected with NLRC5/mNLRC5-specific sirna or scrambled sirna, respectively, and then infected with VSV-eGFP. The cell extracts were harvested for western blot with anti-phospho-irf3 and IRF3 antibodies. See also Figure S6. Cell 141, , April 30, 2010 ª2010 Elsevier Inc. 491
25 Figure 7. Knockdown of NLRC5 Enhances Cytokine Response and Antiviral Immunity (A and B) RAW264.7 cells or THP-1 cells were transfected with mnlrc5/nlrc5-specific sirna or scrambled sirna, followed by poly(i:c)/lyovec treatment. ISG-54, ISG-56, IFN-b mrna and IFN-b protein were determined by real-time RT-PCR or ELISA. (C) NLRC5 or mnlrc5 knockdown and control cells were infected with VSV-eGFP. Cell supernatants were used to measure IFN-b protein secretion by ELISA. (D) RAW264.7 cells were transfected with mnlrc5 sirna or scrambled sirna for 36 hr and then treated with LPS, poly (I:C)/LyoVec (1 mg/ml) or VSV-eGFP infection. Total RNAs from the treated cells were harvested at different time points and used for real-time PCR analysis to determine the expression of TNF-a, IL-6, IFN-a, and IFN-b. The data in (A D) are reported as means + SEM of three independent experiments. Asterisks indicate significant differences between groups (*p < 0.05, **p < 0.01, ***p < determined by Student s t test analysis). 492 Cell 141, , April 30, 2010 ª2010 Elsevier Inc.
SUPPLEMENTARY INFORMATION
 Figure S1. Silver staining and immunoblotting of the purified TAK1 kinase complex. The TAK1 kinase complex was purified through tandem affinity methods (Protein A and FLAG), and aliquots of the purified
Figure S1. Silver staining and immunoblotting of the purified TAK1 kinase complex. The TAK1 kinase complex was purified through tandem affinity methods (Protein A and FLAG), and aliquots of the purified
RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh-
 1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
1 a b Supplementary Figure 1. Effects of GSK3b knockdown on poly I:C-induced cytokine production. RAW264.7 cells stably expressing control shrna (Con) or GSK3b-specific shrna (sh- GSK3b) were stimulated
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
SUPPLEMENTARY INFORMATION doi:1.138/nature9814 a A SHARPIN FL B SHARPIN ΔNZF C SHARPIN T38L, F39V b His-SHARPIN FL -1xUb -2xUb -4xUb α-his c Linear 4xUb -SHARPIN FL -SHARPIN TF_LV -SHARPINΔNZF -SHARPIN
Study of different types of ubiquitination
 Study of different types of ubiquitination Rudi Beyaert (rudi.beyaert@irc.vib-ugent.be) VIB UGent Center for Inflammation Research Ghent, Belgium VIB Training Novel Proteomics Tools: Identifying PTMs October
Study of different types of ubiquitination Rudi Beyaert (rudi.beyaert@irc.vib-ugent.be) VIB UGent Center for Inflammation Research Ghent, Belgium VIB Training Novel Proteomics Tools: Identifying PTMs October
Supplementary Figure 1
 Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
Supplementary Figure 1 YAP negatively regulates IFN- signaling. (a) Immunoblot analysis of Yap knockdown efficiency with sh-yap (#1 to #4 independent constructs) in Raw264.7 cells. (b) IFN- -Luc and PRDs
Cell Quality Control. Peter Takizawa Department of Cell Biology
 Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Cell Quality Control Peter Takizawa Department of Cell Biology Cellular quality control reduces production of defective proteins. Cells have many quality control systems to ensure that cell does not build
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
Reviewers' comments: Reviewer #1 (Remarks to the Author): In this manuscript, Song et al. identified FBXW7 as a new positive regulator for RIG-Itriggered type I IFN signaling pathway. The authors observed
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Innate Immunity & Inflammation
 Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
Innate Immunity & Inflammation The innate immune system is an evolutionally conserved mechanism that provides an early and effective response against invading microbial pathogens. It relies on a limited
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature05732 SUPPLEMENTARY INFORMATION Supplemental Data Supplement Figure Legends Figure S1. RIG-I 2CARD undergo robust ubiquitination a, (top) At 48 h posttransfection with a GST, GST-RIG-I-2CARD
doi: 10.1038/nature05732 SUPPLEMENTARY INFORMATION Supplemental Data Supplement Figure Legends Figure S1. RIG-I 2CARD undergo robust ubiquitination a, (top) At 48 h posttransfection with a GST, GST-RIG-I-2CARD
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer
 Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
Supplementary Information TRAF6 ubiquitinates TGFβ type I receptor to promote its cleavage and nuclear translocation in cancer Yabing Mu, Reshma Sundar, Noopur Thakur, Maria Ekman, Shyam Kumar Gudey, Mariya
SUPPLEMENTARY INFORMATION
 Supplementary Table 1. Cell sphingolipids and S1P bound to endogenous TRAF2. Sphingolipid Cell pmol/mg TRAF2 immunoprecipitate pmol/mg Sphingomyelin 4200 ± 250 Not detected Monohexosylceramide 311 ± 18
Supplementary Table 1. Cell sphingolipids and S1P bound to endogenous TRAF2. Sphingolipid Cell pmol/mg TRAF2 immunoprecipitate pmol/mg Sphingomyelin 4200 ± 250 Not detected Monohexosylceramide 311 ± 18
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Recombinant Protein Expression Retroviral system
 Recombinant Protein Expression Retroviral system Viruses Contains genome DNA or RNA Genome encased in a protein coat or capsid. Some viruses have membrane covering protein coat enveloped virus Ø Essential
Recombinant Protein Expression Retroviral system Viruses Contains genome DNA or RNA Genome encased in a protein coat or capsid. Some viruses have membrane covering protein coat enveloped virus Ø Essential
S1a S1b S1c. S1d. S1f S1g S1h SUPPLEMENTARY FIGURE 1. - si sc Il17rd Il17ra bp. rig/s IL-17RD (ng) -100 IL-17RD
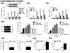 SUPPLEMENTARY FIGURE 1 0 20 50 80 100 IL-17RD (ng) S1a S1b S1c IL-17RD β-actin kda S1d - si sc Il17rd Il17ra rig/s15-574 - 458-361 bp S1f S1g S1h S1i S1j Supplementary Figure 1. Knockdown of IL-17RD enhances
SUPPLEMENTARY FIGURE 1 0 20 50 80 100 IL-17RD (ng) S1a S1b S1c IL-17RD β-actin kda S1d - si sc Il17rd Il17ra rig/s15-574 - 458-361 bp S1f S1g S1h S1i S1j Supplementary Figure 1. Knockdown of IL-17RD enhances
Intrinsic cellular defenses against virus infection
 Intrinsic cellular defenses against virus infection Detection of virus infection Host cell response to virus infection Interferons: structure and synthesis Induction of antiviral activity Viral defenses
Intrinsic cellular defenses against virus infection Detection of virus infection Host cell response to virus infection Interferons: structure and synthesis Induction of antiviral activity Viral defenses
NLRC5 Negatively Regulates the NF-kB and Type I Interferon Signaling Pathways
 NLRC5 Negatively Regulates the NF-kB and Type I Interferon Signaling Pathways Jun Cui, 1,2,5 Liang Zhu, 1,3,5 Xiaojun Xia, 1,5 Helen Y. Wang, 1 Xavier Legras, 1 Jun Hong, 1 Jiabing Ji, 1 Pingping Shen,
NLRC5 Negatively Regulates the NF-kB and Type I Interferon Signaling Pathways Jun Cui, 1,2,5 Liang Zhu, 1,3,5 Xiaojun Xia, 1,5 Helen Y. Wang, 1 Xavier Legras, 1 Jun Hong, 1 Jiabing Ji, 1 Pingping Shen,
Nature Methods: doi: /nmeth Supplementary Figure 1
 Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
Supplementary Figure 1 Subtiligase-catalyzed ligations with ubiquitin thioesters and 10-mer biotinylated peptides. (a) General scheme for ligations between ubiquitin thioesters and 10-mer, biotinylated
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
SUPPLEMENTAL FIGURE LEGENDS
 SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure S1: Endogenous interaction between RNF2 and H2AX: Whole cell extracts from 293T were subjected to immunoprecipitation with anti-rnf2 or anti-γ-h2ax antibodies
SUPPLEMENTAL FIGURE LEGENDS Supplemental Figure S1: Endogenous interaction between RNF2 and H2AX: Whole cell extracts from 293T were subjected to immunoprecipitation with anti-rnf2 or anti-γ-h2ax antibodies
Supplementary information. MARCH8 inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins
 Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Supplementary information inhibits HIV-1 infection by reducing virion incorporation of envelope glycoproteins Takuya Tada, Yanzhao Zhang, Takayoshi Koyama, Minoru Tobiume, Yasuko Tsunetsugu-Yokota, Shoji
Signal Transduction Cascades
 Signal Transduction Cascades Contents of this page: Kinases & phosphatases Protein Kinase A (camp-dependent protein kinase) G-protein signal cascade Structure of G-proteins Small GTP-binding proteins,
Signal Transduction Cascades Contents of this page: Kinases & phosphatases Protein Kinase A (camp-dependent protein kinase) G-protein signal cascade Structure of G-proteins Small GTP-binding proteins,
USP10 inhibits genotoxic NF-κB activation by MCPIP1- facilitated deubiquitination of NEMO
 Manuscript EMBO-2013-84573 USP10 inhibits genotoxic NF-κB activation by MCPIP1- facilitated deubiquitination of NEMO Jixiao Niu, Yuling Shi, Jingyan Xue, Ruidong Miao, Shengping Huang, Tianyi Wang, Jiong
Manuscript EMBO-2013-84573 USP10 inhibits genotoxic NF-κB activation by MCPIP1- facilitated deubiquitination of NEMO Jixiao Niu, Yuling Shi, Jingyan Xue, Ruidong Miao, Shengping Huang, Tianyi Wang, Jiong
Chapter 6. Antigen Presentation to T lymphocytes
 Chapter 6 Antigen Presentation to T lymphocytes Generation of T-cell Receptor Ligands T cells only recognize Ags displayed on cell surfaces These Ags may be derived from pathogens that replicate within
Chapter 6 Antigen Presentation to T lymphocytes Generation of T-cell Receptor Ligands T cells only recognize Ags displayed on cell surfaces These Ags may be derived from pathogens that replicate within
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS)
 Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
Supplementary Figure S1. Venn diagram analysis of mrna microarray data and mirna target analysis. (a) Western blot analysis of T lymphoblasts (CLS) and their exosomes (EXO) in resting (REST) and activated
The Ubiquitin E3 Ligase RAUL Negatively Regulates Type I Interferon through Ubiquitination of the Transcription Factors IRF7 and IRF3
 Article The Ubiquitin E3 Ligase Negatively Regulates Type I Interferon through Ubiquitination of the Transcription Factors and Yanxing Yu 1,2, * and Gary S. Hayward 1, * 1 Viral Oncology Program, The Sidney
Article The Ubiquitin E3 Ligase Negatively Regulates Type I Interferon through Ubiquitination of the Transcription Factors and Yanxing Yu 1,2, * and Gary S. Hayward 1, * 1 Viral Oncology Program, The Sidney
Ubiquitination and deubiquitination of NP protein regulates influenza A virus RNA replication
 Manuscript EMBO-2010-74756 Ubiquitination and deubiquitination of NP protein regulates influenza A virus RNA replication Tsai-Ling Liao, Chung-Yi Wu, Wen-Chi Su, King-Song Jeng and Michael Lai Corresponding
Manuscript EMBO-2010-74756 Ubiquitination and deubiquitination of NP protein regulates influenza A virus RNA replication Tsai-Ling Liao, Chung-Yi Wu, Wen-Chi Su, King-Song Jeng and Michael Lai Corresponding
Complexity DNA. Genome RNA. Transcriptome. Protein. Proteome. Metabolites. Metabolome
 DNA Genome Complexity RNA Transcriptome Systems Biology Linking all the components of a cell in a quantitative and temporal manner Protein Proteome Metabolites Metabolome Where are the functional elements?
DNA Genome Complexity RNA Transcriptome Systems Biology Linking all the components of a cell in a quantitative and temporal manner Protein Proteome Metabolites Metabolome Where are the functional elements?
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.10/nature10195 NCBI gene: Tagged Subunit(s: HA-Vpx; FLAG-Cul4 HA-DCAF1 FLAG-Cul4 HA-FLAG-Vpx Mock Vpx (SIVmac 100 (a ; 0.159 (b ; 0.05 DCAF1 DDB1 DDA1 Cul4A 1; 0.024591
SUPPLEMENTARY INFORMATION doi:10.10/nature10195 NCBI gene: Tagged Subunit(s: HA-Vpx; FLAG-Cul4 HA-DCAF1 FLAG-Cul4 HA-FLAG-Vpx Mock Vpx (SIVmac 100 (a ; 0.159 (b ; 0.05 DCAF1 DDB1 DDA1 Cul4A 1; 0.024591
The Nobel Prize in Chemistry 2004
 The Nobel Prize in Chemistry 2004 Ubiquitous Quality Control of Life C S Karigar and K R Siddalinga Murthy The Nobel Prize in Chemistry for 2004 is shared by Aaron Ciechanover, Avram Hershko and Irwin
The Nobel Prize in Chemistry 2004 Ubiquitous Quality Control of Life C S Karigar and K R Siddalinga Murthy The Nobel Prize in Chemistry for 2004 is shared by Aaron Ciechanover, Avram Hershko and Irwin
7.012 Quiz 3 Answers
 MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Friday 11/12/04 7.012 Quiz 3 Answers A > 85 B 72-84
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3076 Supplementary Figure 1 btrcp targets Cep68 for degradation during mitosis. a) Cep68 immunofluorescence in interphase and metaphase. U-2OS cells were transfected with control sirna
DOI: 10.1038/ncb3076 Supplementary Figure 1 btrcp targets Cep68 for degradation during mitosis. a) Cep68 immunofluorescence in interphase and metaphase. U-2OS cells were transfected with control sirna
The Immunoassay Guide to Successful Mass Spectrometry. Orr Sharpe Robinson Lab SUMS User Meeting October 29, 2013
 The Immunoassay Guide to Successful Mass Spectrometry Orr Sharpe Robinson Lab SUMS User Meeting October 29, 2013 What is it? Hey! Look at that! Something is reacting in here! I just wish I knew what it
The Immunoassay Guide to Successful Mass Spectrometry Orr Sharpe Robinson Lab SUMS User Meeting October 29, 2013 What is it? Hey! Look at that! Something is reacting in here! I just wish I knew what it
SUPPLEMENTARY INFORMATION
 Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
Figure S1 Treatment with both Sema6D and Plexin-A1 sirnas induces the phenotype essentially identical to that induced by treatment with Sema6D sirna alone or Plexin-A1 sirna alone. (a,b) The cardiac tube
The Coxsackievirus B 3C pro Protease Cleaves MAVS and TRIF to Attenuate Host Type I Interferon and Apoptotic Signaling
 The Coxsackievirus B 3C pro Protease Cleaves MAVS and TRIF to Attenuate Host Type I Interferon and Apoptotic Signaling Amitava Mukherjee 1, Stefanie A. Morosky 2, Elizabeth Delorme-Axford 1, Naomi Dybdahl-Sissoko
The Coxsackievirus B 3C pro Protease Cleaves MAVS and TRIF to Attenuate Host Type I Interferon and Apoptotic Signaling Amitava Mukherjee 1, Stefanie A. Morosky 2, Elizabeth Delorme-Axford 1, Naomi Dybdahl-Sissoko
File Name: Supplementary Information Description: Supplementary Figures and Supplementary Tables. File Name: Peer Review File Description:
 File Name: Supplementary Information Description: Supplementary Figures and Supplementary Tables File Name: Peer Review File Description: Primer Name Sequence (5'-3') AT ( C) RT-PCR USP21 F 5'-TTCCCATGGCTCCTTCCACATGAT-3'
File Name: Supplementary Information Description: Supplementary Figures and Supplementary Tables File Name: Peer Review File Description: Primer Name Sequence (5'-3') AT ( C) RT-PCR USP21 F 5'-TTCCCATGGCTCCTTCCACATGAT-3'
crossmark Ca V subunits interact with the voltage-gated calcium channel
 crossmark THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 291, NO. 39, pp. 20402 20416, September 23, 2016 Author s Choice 2016 by The American Society for Biochemistry and Molecular Biology, Inc. Published in
crossmark THE JOURNAL OF BIOLOGICAL CHEMISTRY VOL. 291, NO. 39, pp. 20402 20416, September 23, 2016 Author s Choice 2016 by The American Society for Biochemistry and Molecular Biology, Inc. Published in
Activation of IKK by TNFa Requires Site-Specific Ubiquitination of RIP1 and Polyubiquitin Binding by NEMO
 Molecular Cell 22, 245 257, April 21, 2006 ª2006 Elsevier Inc. DOI 10.1016/j.molcel.2006.03.026 Activation of IKK by TNFa Requires Site-Specific Ubiquitination of RIP1 and Polyubiquitin Binding by NEMO
Molecular Cell 22, 245 257, April 21, 2006 ª2006 Elsevier Inc. DOI 10.1016/j.molcel.2006.03.026 Activation of IKK by TNFa Requires Site-Specific Ubiquitination of RIP1 and Polyubiquitin Binding by NEMO
Supplemental Information. NRF2 Is a Major Target of ARF. in p53-independent Tumor Suppression
 Molecular Cell, Volume 68 Supplemental Information NRF2 Is a Major Target of ARF in p53-independent Tumor Suppression Delin Chen, Omid Tavana, Bo Chu, Luke Erber, Yue Chen, Richard Baer, and Wei Gu Figure
Molecular Cell, Volume 68 Supplemental Information NRF2 Is a Major Target of ARF in p53-independent Tumor Suppression Delin Chen, Omid Tavana, Bo Chu, Luke Erber, Yue Chen, Richard Baer, and Wei Gu Figure
Tel: ; Fax: ;
 Tel.: +98 216 696 9291; Fax: +98 216 696 9291; E-mail: mrasadeghi@pasteur.ac.ir Tel: +98 916 113 7679; Fax: +98 613 333 6380; E-mail: abakhshi_e@ajums.ac.ir A Soluble Chromatin-bound MOI 0 1 5 0 1 5 HDAC2
Tel.: +98 216 696 9291; Fax: +98 216 696 9291; E-mail: mrasadeghi@pasteur.ac.ir Tel: +98 916 113 7679; Fax: +98 613 333 6380; E-mail: abakhshi_e@ajums.ac.ir A Soluble Chromatin-bound MOI 0 1 5 0 1 5 HDAC2
TRIM5 requires Ube2W to anchor Lys63-linked ubiquitin chains and restrict reverse transcription
 Manuscript EMBO-2014-90361 TRIM5 requires Ube2W to anchor Lys63-linked ubiquitin chains and restrict reverse transcription Adam J Fletcher, Devin E Christensen, Chad Nelson, Choon Ping Tan, Torsten Schaller,
Manuscript EMBO-2014-90361 TRIM5 requires Ube2W to anchor Lys63-linked ubiquitin chains and restrict reverse transcription Adam J Fletcher, Devin E Christensen, Chad Nelson, Choon Ping Tan, Torsten Schaller,
Appendix. Table of Contents
 Appendix Table of Contents Appendix Figures Figure S1: Gp78 is not required for the degradation of mcherry-cl1 in Hela Cells. Figure S2: Indel formation in the MARCH6 sgrna targeted HeLa clones. Figure
Appendix Table of Contents Appendix Figures Figure S1: Gp78 is not required for the degradation of mcherry-cl1 in Hela Cells. Figure S2: Indel formation in the MARCH6 sgrna targeted HeLa clones. Figure
The K48-K63 Branched Ubiquitin Chain Regulates NF-kB Signaling
 Article The K48-K63 Branched Ubiquitin Chain Regulates NF-kB Signaling Graphical Abstract Authors Fumiaki Ohtake, Yasushi Saeki, Satoshi Ishido, Jun Kanno, Keiji Tanaka Correspondence otake-fm@igakuken.or.jp
Article The K48-K63 Branched Ubiquitin Chain Regulates NF-kB Signaling Graphical Abstract Authors Fumiaki Ohtake, Yasushi Saeki, Satoshi Ishido, Jun Kanno, Keiji Tanaka Correspondence otake-fm@igakuken.or.jp
Supplementary Figure 1
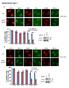 Supplementary Figure 1 a γ-h2ax MDC1 RNF8 FK2 BRCA1 U2OS Cells sgrna-1 ** 60 sgrna 40 20 0 % positive Cells (>5 foci per cell) b ** 80 sgrna sgrna γ-h2ax MDC1 γ-h2ax RNF8 FK2 MDC1 BRCA1 RNF8 FK2 BRCA1
Supplementary Figure 1 a γ-h2ax MDC1 RNF8 FK2 BRCA1 U2OS Cells sgrna-1 ** 60 sgrna 40 20 0 % positive Cells (>5 foci per cell) b ** 80 sgrna sgrna γ-h2ax MDC1 γ-h2ax RNF8 FK2 MDC1 BRCA1 RNF8 FK2 BRCA1
Supplementary Figure 1
 Supplementary Figure 1 Constitutive EGFR signaling does not activate canonical EGFR signals (a) U251EGFRInd cells with or without tetracycline exposure (24h, 1µg/ml) were treated with EGF for 15 minutes
Supplementary Figure 1 Constitutive EGFR signaling does not activate canonical EGFR signals (a) U251EGFRInd cells with or without tetracycline exposure (24h, 1µg/ml) were treated with EGF for 15 minutes
Cover Page. The following handle holds various files of this Leiden University dissertation:
 Cover Page The following handle holds various files of this Leiden University dissertation: http://hdl.handle.net/188/59466 Author: Oudshoorn, D. Title: Wrapping up : nidovirus membrane structures and
Cover Page The following handle holds various files of this Leiden University dissertation: http://hdl.handle.net/188/59466 Author: Oudshoorn, D. Title: Wrapping up : nidovirus membrane structures and
William C. Comb, Jessica E. Hutti, Patricia Cogswell, Lewis C. Cantley, and Albert S. Baldwin
 Molecular Cell, Volume 45 Supplemental Information p85 SH2 Domain Phosphorylation by IKK Promotes Feedback Inhibition of PI3K and Akt in Response to Cellular Starvation William C. Comb, Jessica E. Hutti,
Molecular Cell, Volume 45 Supplemental Information p85 SH2 Domain Phosphorylation by IKK Promotes Feedback Inhibition of PI3K and Akt in Response to Cellular Starvation William C. Comb, Jessica E. Hutti,
Species-Specific Inhibition of RIG-I Ubiquitination and IFN Induction by the Influenza A Virus NS1 Protein
 Species-Specific Inhibition of RIG-I Ubiquitination and IFN Induction by the Influenza A Virus NS1 Protein Ricardo Rajsbaum 1,2, Randy A. Albrecht 1,2, May K. Wang 3, Natalya P. Maharaj 3, Gijs A. Versteeg
Species-Specific Inhibition of RIG-I Ubiquitination and IFN Induction by the Influenza A Virus NS1 Protein Ricardo Rajsbaum 1,2, Randy A. Albrecht 1,2, May K. Wang 3, Natalya P. Maharaj 3, Gijs A. Versteeg
Mammalian Tissue Protein Extraction Reagent
 Mammalian Tissue Protein Extraction Reagent Catalog number: AR0101 Boster s Mammalian Tissue Protein Extraction Reagent is a ready-to-use Western blot related reagent solution used for efficient extraction
Mammalian Tissue Protein Extraction Reagent Catalog number: AR0101 Boster s Mammalian Tissue Protein Extraction Reagent is a ready-to-use Western blot related reagent solution used for efficient extraction
Oncolytic virus strategy
 Oncolytic viruses Oncolytic virus strategy normal tumor NO replication replication survival lysis Oncolytic virus strategy Mechanisms of tumor selectivity of several, some of them naturally, oncolytic
Oncolytic viruses Oncolytic virus strategy normal tumor NO replication replication survival lysis Oncolytic virus strategy Mechanisms of tumor selectivity of several, some of them naturally, oncolytic
supplementary information
 Figure S1 Nucleotide binding status of RagA mutants. Wild type and mutant forms of MycRagA was transfected into HEK293 cells and the transfected cells were labeled with 32 Pphosphate. MycRagA was immunoprecipitated
Figure S1 Nucleotide binding status of RagA mutants. Wild type and mutant forms of MycRagA was transfected into HEK293 cells and the transfected cells were labeled with 32 Pphosphate. MycRagA was immunoprecipitated
ARV Mode of Action. Mode of Action. Mode of Action NRTI. Immunopaedia.org.za
 ARV Mode of Action Mode of Action Mode of Action - NRTI Mode of Action - NNRTI Mode of Action - Protease Inhibitors Mode of Action - Integrase inhibitor Mode of Action - Entry Inhibitors Mode of Action
ARV Mode of Action Mode of Action Mode of Action - NRTI Mode of Action - NNRTI Mode of Action - Protease Inhibitors Mode of Action - Integrase inhibitor Mode of Action - Entry Inhibitors Mode of Action
Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid.
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid. HEK293T
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 Supplementary Figure 1. SC35M polymerase activity in the presence of Bat or SC35M NP encoded from the phw2000 rescue plasmid. HEK293T
Tbk1-TKO! DN cells (%)! 15! 10!
 a! T Cells! TKO! B Cells! TKO! b! CD4! 8.9 85.2 3.4 2.88 CD8! Tbk1-TKO! 1.1 84.8 2.51 2.54 c! DN cells (%)! 4 3 2 1 DP cells (%)! 9 8 7 6 CD4 + SP cells (%)! 5 4 3 2 1 5 TKO! TKO! TKO! TKO! 15 1 5 CD8
a! T Cells! TKO! B Cells! TKO! b! CD4! 8.9 85.2 3.4 2.88 CD8! Tbk1-TKO! 1.1 84.8 2.51 2.54 c! DN cells (%)! 4 3 2 1 DP cells (%)! 9 8 7 6 CD4 + SP cells (%)! 5 4 3 2 1 5 TKO! TKO! TKO! TKO! 15 1 5 CD8
Nature Immunology doi: /ni.3268
 Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
Supplementary Figure 1 Loss of Mst1 and Mst2 increases susceptibility to bacterial sepsis. (a) H&E staining of colon and kidney sections from wild type and Mst1 -/- Mst2 fl/fl Vav-Cre mice. Scale bar,
Received 28 September 2010/Returned for modification 19 October 2010/Accepted 27 April 2011
 MOLECULAR AND CELLULAR BIOLOGY, July 2011, p. 2774 2786 Vol. 31, No. 14 0270-7306/11/$12.00 doi:10.1128/mcb.01139-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. A Cytosolic
MOLECULAR AND CELLULAR BIOLOGY, July 2011, p. 2774 2786 Vol. 31, No. 14 0270-7306/11/$12.00 doi:10.1128/mcb.01139-10 Copyright 2011, American Society for Microbiology. All Rights Reserved. A Cytosolic
Supplementary Figure 1
 Supplementary Figure 1 Asymmetrical function of 5p and 3p arms of mir-181 and mir-30 families and mir-142 and mir-154. (a) Control experiments using mirna sensor vector and empty pri-mirna overexpression
Supplementary Figure 1 Asymmetrical function of 5p and 3p arms of mir-181 and mir-30 families and mir-142 and mir-154. (a) Control experiments using mirna sensor vector and empty pri-mirna overexpression
Life Sciences 1A Midterm Exam 2. November 13, 2006
 Name: TF: Section Time Life Sciences 1A Midterm Exam 2 November 13, 2006 Please write legibly in the space provided below each question. You may not use calculators on this exam. We prefer that you use
Name: TF: Section Time Life Sciences 1A Midterm Exam 2 November 13, 2006 Please write legibly in the space provided below each question. You may not use calculators on this exam. We prefer that you use
Protein Trafficking in the Secretory and Endocytic Pathways
 Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
Protein Trafficking in the Secretory and Endocytic Pathways The compartmentalization of eukaryotic cells has considerable functional advantages for the cell, but requires elaborate mechanisms to ensure
SUPPLEMENTARY INFORMATION
 Supplementary Discussion The cell cycle machinery and the DNA damage response network are highly interconnected and co-regulated in assuring faithful duplication and partition of genetic materials into
Supplementary Discussion The cell cycle machinery and the DNA damage response network are highly interconnected and co-regulated in assuring faithful duplication and partition of genetic materials into
Nature Immunology doi: /ni Supplementary Figure 1. Raf-1 inhibition does not affect TLR4-induced type I IFN responses.
 Supplementary Figure 1 Raf-1 inhibition does not affect TLR4-induced type I IFN responses. Real-time PCR analyses of IFNB, ISG15, TRIM5, TRIM22 and APOBEC3G mrna in modcs 6 h after stimulation with TLR4
Supplementary Figure 1 Raf-1 inhibition does not affect TLR4-induced type I IFN responses. Real-time PCR analyses of IFNB, ISG15, TRIM5, TRIM22 and APOBEC3G mrna in modcs 6 h after stimulation with TLR4
Supplementary Figure 1. Prevalence of U539C and G540A nucleotide and E172K amino acid substitutions among H9N2 viruses. Full-length H9N2 NS
 Supplementary Figure 1. Prevalence of U539C and G540A nucleotide and E172K amino acid substitutions among H9N2 viruses. Full-length H9N2 NS nucleotide sequences (a, b) or amino acid sequences (c) from
Supplementary Figure 1. Prevalence of U539C and G540A nucleotide and E172K amino acid substitutions among H9N2 viruses. Full-length H9N2 NS nucleotide sequences (a, b) or amino acid sequences (c) from
7.06 Cell Biology EXAM #3 April 24, 2003
 7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
7.06 Spring 2003 Exam 3 Name 1 of 8 7.06 Cell Biology EXAM #3 April 24, 2003 This is an open book exam, and you are allowed access to books and notes. Please write your answers to the questions in the
Supplementary Information
 Supplementary Information mediates STAT3 activation at retromer-positive structures to promote colitis and colitis-associated carcinogenesis Zhang et al. a b d e g h Rel. Luc. Act. Rel. mrna Rel. mrna
Supplementary Information mediates STAT3 activation at retromer-positive structures to promote colitis and colitis-associated carcinogenesis Zhang et al. a b d e g h Rel. Luc. Act. Rel. mrna Rel. mrna
The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein
 THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
THESIS BOOK The functional investigation of the interaction between TATA-associated factor 3 (TAF3) and p53 protein Orsolya Buzás-Bereczki Supervisors: Dr. Éva Bálint Dr. Imre Miklós Boros University of
Received 3 September 2002/Accepted 15 January 2003
 JOURNAL OF VIROLOGY, Apr. 2003, p. 4646 4657 Vol. 77, No. 8 0022-538X/03/$08.00 0 DOI: 10.1128/JVI.77.8.4646 4657.2003 Copyright 2003, American Society for Microbiology. All Rights Reserved. Ability of
JOURNAL OF VIROLOGY, Apr. 2003, p. 4646 4657 Vol. 77, No. 8 0022-538X/03/$08.00 0 DOI: 10.1128/JVI.77.8.4646 4657.2003 Copyright 2003, American Society for Microbiology. All Rights Reserved. Ability of
RAS Genes. The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes.
 ۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
۱ RAS Genes The ras superfamily of genes encodes small GTP binding proteins that are responsible for the regulation of many cellular processes. Oncogenic ras genes in human cells include H ras, N ras,
Supplementary Materials
 Supplementary Materials Supplementary Figure S1 Regulation of Ubl4A stability by its assembly partner A, The translation rate of Ubl4A is not affected in the absence of Bag6. Control, Bag6 and Ubl4A CRISPR
Supplementary Materials Supplementary Figure S1 Regulation of Ubl4A stability by its assembly partner A, The translation rate of Ubl4A is not affected in the absence of Bag6. Control, Bag6 and Ubl4A CRISPR
Table S1. Sequence of human and mouse primers used for RT-qPCR measurements.
 Table S1. Sequence of human and mouse primers used for RT-qPCR measurements. Ca9, carbonic anhydrase IX; Ndrg1, N-myc downstream regulated gene 1; L28, ribosomal protein L28; Hif1a, hypoxia inducible factor
Table S1. Sequence of human and mouse primers used for RT-qPCR measurements. Ca9, carbonic anhydrase IX; Ndrg1, N-myc downstream regulated gene 1; L28, ribosomal protein L28; Hif1a, hypoxia inducible factor
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
SUPPLEMENTARY INFORMATION
 a c e doi:10.1038/nature10407 b d f Supplementary Figure 1. SERCA2a complex analysis. (a) Two-dimensional SDS-PAGE gels of SERCA2a complexes. A silver-stained SDSPAGE gel is shown, which reveals a 12 kda
a c e doi:10.1038/nature10407 b d f Supplementary Figure 1. SERCA2a complex analysis. (a) Two-dimensional SDS-PAGE gels of SERCA2a complexes. A silver-stained SDSPAGE gel is shown, which reveals a 12 kda
Why do cellular proteins linked to K63-polyubiquitin chains not associate with proteasomes?
 The EMBO Journal (2013) 32, 552 565 www.embojournal.org Why do cellular proteins linked to K63-polyubiquitin chains not associate with proteasomes? THE EMBO JOURNAL James A Nathan 1,2, Hyoung Tae Kim 1,
The EMBO Journal (2013) 32, 552 565 www.embojournal.org Why do cellular proteins linked to K63-polyubiquitin chains not associate with proteasomes? THE EMBO JOURNAL James A Nathan 1,2, Hyoung Tae Kim 1,
Selective protection of an ARF1-GTP signaling axis by a bacterial scaffold induces bidirectional trafficking arrest.
 Selective protection of an ARF1-GTP signaling axis by a bacterial scaffold induces bidirectional trafficking arrest. Andrey S. Selyunin, L. Evan Reddick, Bethany A. Weigele, and Neal M. Alto Supplemental
Selective protection of an ARF1-GTP signaling axis by a bacterial scaffold induces bidirectional trafficking arrest. Andrey S. Selyunin, L. Evan Reddick, Bethany A. Weigele, and Neal M. Alto Supplemental
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
Newly Recognized Components of the Innate Immune System
 Newly Recognized Components of the Innate Immune System NOD Proteins: Intracellular Peptidoglycan Sensors NOD-1 NOD-2 Nod Protein LRR; Ligand Recognition CARD RICK I-κB p50 p65 NF-κB Polymorphisms in Nod-2
Newly Recognized Components of the Innate Immune System NOD Proteins: Intracellular Peptidoglycan Sensors NOD-1 NOD-2 Nod Protein LRR; Ligand Recognition CARD RICK I-κB p50 p65 NF-κB Polymorphisms in Nod-2
Materials and Methods , The two-hybrid principle.
 The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
The enzymatic activity of an unknown protein which cleaves the phosphodiester bond between the tyrosine residue of a viral protein and the 5 terminus of the picornavirus RNA Introduction Every day there
Western Immunoblotting Preparation of Samples:
 Western Immunoblotting Preparation of Samples: Total Protein Extraction from Culture Cells: Take off the medium Wash culture with 1 x PBS 1 ml hot Cell-lysis Solution into T75 flask Scrap out the cells
Western Immunoblotting Preparation of Samples: Total Protein Extraction from Culture Cells: Take off the medium Wash culture with 1 x PBS 1 ml hot Cell-lysis Solution into T75 flask Scrap out the cells
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/10/471/eaah5085/dc1 Supplementary Materials for Phosphorylation of the exocyst protein Exo84 by TBK1 promotes insulin-stimulated GLUT4 trafficking Maeran Uhm,
www.sciencesignaling.org/cgi/content/full/10/471/eaah5085/dc1 Supplementary Materials for Phosphorylation of the exocyst protein Exo84 by TBK1 promotes insulin-stimulated GLUT4 trafficking Maeran Uhm,
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures
 Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Type of file: PDF Title of file for HTML: Supplementary Information Description: Supplementary Figures Type of file: MOV Title of file for HTML: Supplementary Movie 1 Description: NLRP3 is moving along
Chromatin IP (Isw2) Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles.
 Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
Chromatin IP (Isw2) 7/01 Toshi last update: 06/15 Reagents Fix soln: 11% formaldehyde, 0.1 M NaCl, 1 mm EDTA, 50 mm Hepes-KOH ph 7.6. Freshly prepared. Do not store in glass bottles. 2.5 M glycine. TBS:
supplementary information
 DOI: 1.138/ncb1 Control Atg7 / NAC 1 1 1 1 (mm) Control Atg7 / NAC 1 1 1 1 (mm) Lamin B Gstm1 Figure S1 Neither the translocation of into the nucleus nor the induction of antioxidant proteins in autophagydeficient
DOI: 1.138/ncb1 Control Atg7 / NAC 1 1 1 1 (mm) Control Atg7 / NAC 1 1 1 1 (mm) Lamin B Gstm1 Figure S1 Neither the translocation of into the nucleus nor the induction of antioxidant proteins in autophagydeficient
Supporting Information
 Supporting Information Dauvillée et al. 10.1073/pnas.0907424106 Fig. S1. Iodine screening of the C. cohnii mutant bank. Each single colony was grown on rich-medium agar plates then vaporized with iodine.
Supporting Information Dauvillée et al. 10.1073/pnas.0907424106 Fig. S1. Iodine screening of the C. cohnii mutant bank. Each single colony was grown on rich-medium agar plates then vaporized with iodine.
Regulation of Gene Expression in Eukaryotes
 Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Ch. 19 Regulation of Gene Expression in Eukaryotes BIOL 222 Differential Gene Expression in Eukaryotes Signal Cells in a multicellular eukaryotic organism genetically identical differential gene expression
Toll-like Receptor Signaling
 Toll-like Receptor Signaling 1 Professor of Medicine University of Massachusetts Medical School, Worcester, MA, USA Why do we need innate immunity? Pathogens multiply very fast We literally swim in viruses
Toll-like Receptor Signaling 1 Professor of Medicine University of Massachusetts Medical School, Worcester, MA, USA Why do we need innate immunity? Pathogens multiply very fast We literally swim in viruses
condition. Left panel, the HCT-116 cells were lysed with RIPA buffer containing 0.1%
 FIGURE LEGENDS Supplementary Fig 1 (A) sumoylation pattern detected under denaturing condition. Left panel, the HCT-116 cells were lysed with RIPA buffer containing 0.1% SDS in the presence and absence
FIGURE LEGENDS Supplementary Fig 1 (A) sumoylation pattern detected under denaturing condition. Left panel, the HCT-116 cells were lysed with RIPA buffer containing 0.1% SDS in the presence and absence
Homework Hanson section MCB Course, Fall 2014
 Homework Hanson section MCB Course, Fall 2014 (1) Antitrypsin, which inhibits certain proteases, is normally secreted into the bloodstream by liver cells. Antitrypsin is absent from the bloodstream of
Homework Hanson section MCB Course, Fall 2014 (1) Antitrypsin, which inhibits certain proteases, is normally secreted into the bloodstream by liver cells. Antitrypsin is absent from the bloodstream of
Practice Exam 2 MCBII
 1. Which feature is true for signal sequences and for stop transfer transmembrane domains (4 pts)? A. They are both 20 hydrophobic amino acids long. B. They are both found at the N-terminus of the protein.
1. Which feature is true for signal sequences and for stop transfer transmembrane domains (4 pts)? A. They are both 20 hydrophobic amino acids long. B. They are both found at the N-terminus of the protein.
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
SUPPLEMENTARY INFORMATION doi:10.1038/nature11429 S1a 6 7 8 9 Nlrc4 allele S1b Nlrc4 +/+ Nlrc4 +/F Nlrc4 F/F 9 Targeting construct 422 bp 273 bp FRT-neo-gb-PGK-FRT 3x.STOP S1c Nlrc4 +/+ Nlrc4 F/F casp1
Supporting Online Material Material and Methods References Supplemental Figures S1, S2, and S3
 Supporting Online Material Material and Methods References Supplemental Figures S1, S2, and S3 Sarbassov et al. 1 Material and Methods Materials Reagents were obtained from the following sources: protein
Supporting Online Material Material and Methods References Supplemental Figures S1, S2, and S3 Sarbassov et al. 1 Material and Methods Materials Reagents were obtained from the following sources: protein
reads observed in trnas from the analysis of RNAs carrying a 5 -OH ends isolated from cells induced to express
 Supplementary Figure 1. VapC-mt4 cleaves trna Ala2 in E. coli. Histograms representing the fold change in reads observed in trnas from the analysis of RNAs carrying a 5 -OH ends isolated from cells induced
Supplementary Figure 1. VapC-mt4 cleaves trna Ala2 in E. coli. Histograms representing the fold change in reads observed in trnas from the analysis of RNAs carrying a 5 -OH ends isolated from cells induced
Brd4 Coactivates Transcriptional Activation of NF- B via Specific Binding to Acetylated RelA
 MOLECULAR AND CELLULAR BIOLOGY, Mar. 2009, p. 1375 1387 Vol. 29, No. 5 0270-7306/09/$08.00 0 doi:10.1128/mcb.01365-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Brd4 Coactivates
MOLECULAR AND CELLULAR BIOLOGY, Mar. 2009, p. 1375 1387 Vol. 29, No. 5 0270-7306/09/$08.00 0 doi:10.1128/mcb.01365-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. Brd4 Coactivates
PRODUCT INFORMATION & MANUAL
 PRODUCT INFORMATION & MANUAL 0.4 micron for Overall Exosome Isolation (Cell Media) NBP2-49826 For research use only. Not for diagnostic or therapeutic procedures. www.novusbio.com - P: 303.730.1950 - P:
PRODUCT INFORMATION & MANUAL 0.4 micron for Overall Exosome Isolation (Cell Media) NBP2-49826 For research use only. Not for diagnostic or therapeutic procedures. www.novusbio.com - P: 303.730.1950 - P:
MEK1 Assay Kit 1 Catalog # Lot # 16875
 MEK1 Assay Kit 1 Kit Components Assay Dilution Buffer (ADB), Catalog # 20-108. Three vials, each containing 1.0ml of assay dilution buffer (20mM MOPS, ph 7.2, 25mM ß-glycerol phosphate, 5mM EGTA, 1mM sodium
MEK1 Assay Kit 1 Kit Components Assay Dilution Buffer (ADB), Catalog # 20-108. Three vials, each containing 1.0ml of assay dilution buffer (20mM MOPS, ph 7.2, 25mM ß-glycerol phosphate, 5mM EGTA, 1mM sodium
otherwise known as Cytotoxic T lymphocytes (CTLs)
 MIT Biology Department 7.012: Introductory Biology - Fall 200 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel NAME TA SEC 7.012 Problem Set 5 FRIDAY October 29, 2004
MIT Biology Department 7.012: Introductory Biology - Fall 200 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel NAME TA SEC 7.012 Problem Set 5 FRIDAY October 29, 2004
Introduction retroposon
 17.1 - Introduction A retrovirus is an RNA virus able to convert its sequence into DNA by reverse transcription A retroposon (retrotransposon) is a transposon that mobilizes via an RNA form; the DNA element
17.1 - Introduction A retrovirus is an RNA virus able to convert its sequence into DNA by reverse transcription A retroposon (retrotransposon) is a transposon that mobilizes via an RNA form; the DNA element
Supplementary information
 Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
Supplementary information Human Cytomegalovirus MicroRNA mir-us4-1 Inhibits CD8 + T Cell Response by Targeting ERAP1 Sungchul Kim, Sanghyun Lee, Jinwook Shin, Youngkyun Kim, Irini Evnouchidou, Donghyun
SUPPLEMENTARY INFORMATION
 Complete but curtailed T-cell response to very-low-affinity antigen Dietmar Zehn, Sarah Y. Lee & Michael J. Bevan Supp. Fig. 1: TCR chain usage among endogenous K b /Ova reactive T cells. C57BL/6 mice
Complete but curtailed T-cell response to very-low-affinity antigen Dietmar Zehn, Sarah Y. Lee & Michael J. Bevan Supp. Fig. 1: TCR chain usage among endogenous K b /Ova reactive T cells. C57BL/6 mice
TRIM25 Is Required for the Antiviral Activity of Zinc-finger Antiviral Protein
 JVI Accepted Manuscript Posted Online 15 February 2017 J. Virol. doi:10.1128/jvi.00088-17 Copyright 2017 American Society for Microbiology. All Rights Reserved. 1 2 TRIM25 Is Required for the Antiviral
JVI Accepted Manuscript Posted Online 15 February 2017 J. Virol. doi:10.1128/jvi.00088-17 Copyright 2017 American Society for Microbiology. All Rights Reserved. 1 2 TRIM25 Is Required for the Antiviral
Part-4. Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death
 Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
