Skin-Derived Dendritic Cells Induce Potent CD8 + T Cell Immunity in Recombinant Lentivector-Mediated Genetic Immunization
|
|
|
- Tyrone Murphy
- 6 years ago
- Views:
Transcription
1 Immunity 24, , May 2006 ª2006 Elsevier Inc. DOI /j.immuni Skin-Derived Dendritic Cells Induce Potent CD8 + T Cell Immunity in Recombinant Lentivector-Mediated Genetic Immunization Yukai He, 1,2, * Jiying Zhang, 1 Cara Donahue, 1 and Louis D. Falo, Jr. 1, * 1 Department of Dermatology 2 Department of Immunology University of Pittsburgh School of Medicine Suite 145 Lothrop Hall 190 Lothrop Street Pittsburgh, Pennsylvania Summary The skin contains readily accessible dendritic cells (DCs) with potent antigen presentation function and functional plasticity enabling the integration of antigen specificity with environmentally responsive immune control. Recent studies challenge the established paradigm of cutaneous immune function by suggesting that lymph node-resident DCs, rather than skin-derived DCs (sdcs), are responsible for eliciting T cell immunity against cutaneous pathogens including viral vectors. We show that cutaneous delivery of lentivirus results in direct transfection of sdcs and potent and prolonged antigen presentation. Further, sdcs are the predominant antigen-presenting cells for the induction of potent and durable CD8 + T cell immunity. These results support the classical paradigm of cutaneous immune function and suggest that antigen presentation by sdcs contributes to the high potency of lentivector-mediated genetic immunization. Introduction *Correspondence: ykhe@pitt.edu (Y.H.); lof2@pitt.edu (L.D.F.) The skin is rich in readily accessible DCs and has long been regarded as a highly immunogenic target for vaccine delivery (Larrengina and Falo, 2005; Glenn et al., 2003). Recent observations support this notion by demonstrating that, in human subjects, administration of the common protein-based flu vaccine intradermally is significantly more immunogenic than intramuscular immunization (Belshe et al., 2004; Kenney et al., 2004; La Montagne and Fauci, 2004). Understanding the skin immune network and the mechanisms of cutaneous immunization is critically important for the future development of efficacious vaccines and immunotherapies against infectious agents and neoplastic diseases, and potentially for the development of suppressive immunotherapies to alleviate autoimmune disease. Skin contains both epidermal (Langerhans cells, LCs) and dermal DCs, and skin-draining lymph nodes (DLNs) contain both resident and skin-derived DC (sdc) populations. In the classical paradigm, antigens delivered to the skin are taken up by immature skin-resident DCs, which, in response to natural or adjuvant induced danger signals, migrate to the skin DLNs. These sdcs can then present antigen (signal 1) to lymph node (LN) resident T cells and provide both costimulatory signals (signal 2) necessary for T cell activation and memory induction, and integrated environmental stimuli (signal 3) that influence immune skewing responsive to conditions in the periphery (Banchereau and Steinman, 1998). In this way, sdcs integrate antigen specificity and environmentally responsive immunomodulation. However, recent studies have challenged this paradigm, particularly in regard to CD8 + T cell priming. Following viral infection, LN resident CD8 + DCs, rather than sdcs, have been implicated as key antigen-presenting cells (APCs) for priming CD8 + T cell responses against virally encoded antigens (Allan et al., 2003). This has been demonstrated for a number of viral vectors including those derived from vaccinia virus (VV), herpes simplex virus (HSV), and influenza A viruses (IAV) (Allan et al., 2003; Belz et al., 2004; Smith et al., 2003). Understanding the mechanism of immune induction against virally introduced antigens is of particular interest, given the considerable potential of viral-mediated genetic immunization (Yewdell and Haeryfar, 2005). In genetic immunization, antigen presentation can occur either by in vivo transfection of DCs, resulting in direct presentation of endogenously synthesized antigen (Condon et al., 1996; Porgador et al., 1998), or by in vivo transfection of non-apcs such as keratinocytes or muscle cells which synthesize large amounts of transgenic antigen that can be secreted or crosspresented as cell-associated antigen (Heath et al., 2004; Kurts et al., 2001). In addition, viral vectors and plasmid DNA can serve as potent adjuvants, enhancing and skewing immune responses (Ada and Ramshaw, 2003; Liu et al., 2004; Sasaki et al., 2003). Although in many cases recombinant viral vectors have been shown to be more potent than other genetic immunization approaches, infection by viral vectors can alter DC function, and immune responses against vector proteins can limit immunity directed against transgenic antigens (Larsson et al., 2004; Yewdell and Haeryfar, 2005). Recently, lentivirus has been explored as a vector for genetic immunization, and preliminary studies suggest that it is among the most potent vectors for immune induction (He et al., 2005; Esslinger et al., 2003; Esslinger et al., 2002; Palmowski et al., 2004). Importantly, we and others have shown that lentivectors efficiently and stably transfect DCs without apparent alteration of DC function (He et al., 2005; Breckpot et al., 2003). Lentivector-transfected DCs can process and present transgenic antigens through both the class I and class II restricted processing pathways and prime naive CD8 + and CD4 + T cells (He et al., 2005). Like conventional oncoretroviral vectors, lentivectors do not encode viral proteins, reducing the likelihood of antivector immunity. Here we address the role of sdcs in T cell activation, and the potency and mechanism of lentivector-mediated genetic immunization. Our studies show that a single cutaneous injection of lentivector induces potent antigen-specific lytic activity against model, viral, and tumor antigens, and therapeutic antitumor immunity. Lentivector-induced lytic responses persist for longer
2 Immunity 644 than 2 months after a single immunization. In addition, we find that preexposure to immunizing doses of lentivector does not inhibit the elicitation of an immune response against an encoded antigen by a second vaccination with the same vector, suggesting that vectorspecific immunity does not limit the immunogencity of lentivector vaccination or boosting at the tested doses. Importantly, our studies demonstrate that, following cutaneous lentivector immunization, the sdc population (CD11c + CD8 2/lo DEC205 + ) expresses transgenic antigens and is the predominant DC population capable of priming CD8 + T cells. These results suggest that, in contrast to previous reports obtained using HSV, IAV, and VV vectors, in lentivector-mediated immunization, sdcs may be the primary DC subset responsible for the induction of CD8 + T cell immunity. This supports the classical paradigm of cutaneous immune function based on the capture, transportation, and presentation of antigen by sdcs, enabling the integration of antigen specificity and environmentally responsive immunomodulation. The direct in vivo stable transfection of sdcs and their role in priming naive T cells likely contribute to the high potency of lentivector-mediated genetic immunization we observe. These results suggest that lentivirus may have unique advantages as a vector for genetic immunization. Results A Single Injection of Recombinant Lentivector Induces Potent Antigen-Specific Lytic Activity against Encoded Antigens Most plasmid DNA delivery strategies require priming followed by boosting immunizations with DNA or protein to achieve potent immune responses (Moore and Hill, 2004). Two of the more extensively studied genetic immunization approaches, intramuscular injection of naked DNA and cutaneous biolistic delivery of naked DNA, can elicit potent lytic responses but generally require boosting immunizations. To compare the potency of induced T cell immunity, we delivered a single immunizing dose of OVA expressing naked plasmid DNA intramuscularly or by gene gun bombardment and immunized other groups of animals by intradermal injection of OVA expressing recombinant adenoviral vector (OVA-Ad), lentivector (OVA-lvv), or vaccinia vector (OVA-vv). Eight days after immunization, we evaluated in vivo antigen-specific lytic activity using the welldescribed in vivo killing assay (Barchet et al., 2000; He et al., 2005). As expected, immunization with a single dose of plasmid DNA did not result in significant levels of in vivo killing (Figure 1A). In striking contrast, lentivector-mediated immunization (1 million transduction units, TU) induced strong antigen-specific in vivo lytic activity, comparable to that observed following immunization with highly potent adenoviral vector. Vaccinia vector immunization induced significant but lower levels of antigen-specific lytic activity. Consistent with this, both recombinant adenovirus- and lentivirus-immunized animals demonstrated significant numbers of antigenspecific IFN-g-producing CD8 + T cells by intracellular staining (Figure 1B). Antigen-specific IFN-g-producing CD8 + T cells were minimally detectable in animals immunized with plasmid DNA or vaccinia vector. Similarly potent immune responses against clinically relevant Hepatitis B virus S antigen (HBV S) (Figure 1C) and the murine melanoma self-antigen TRP2 were induced using recombinant lentivectors (Figure 1D). Furthermore, a single immunization with lentivector OVA-lvv induced tumor specific immunity sufficient to inhibit tumor growth and prolong survival of tumor bearing animals (Figures 1E and 1F). A major drawback of adenoviral vector vaccination is the development of antivector immunity that can limit the effectiveness of readministration of the vector in individuals with natural preexisting antivector immunity, or those who have been previously immunized (Rosenberg et al., 1998). To evaluate the limitations imposed by antivector immune responses, we pretreated mice with varying doses of recombinant lentivector expressing the EGFP gene (EGFP-lvv) and then immunized mice 12 days later with 1 million TU of OVA-lvv. Preimmunization with 10 million TU of lentivector encoding the irrelevant EGFP antigen did not inhibit antigen-specific immunity induced by the same lentivector encoding OVA (Figure 1G). Even a 10-fold increase in the dose of preimmunizing vector (100 million TU of EGFP-lvv) resulted in only partial inhibition of the OVA-specific lytic response. In contrast, even at low doses, preimmunization with adenoviral vector encoding EGFP (10 million pfu) significantly inhibited the subsequent development of an OVA-specific response. Higher preimmunization doses (100 million pfu) completely blocked induction of OVA-specific lytic responses in mice subsequently immunized with OVA-Ad. These data suggest that lentivector-specific immune responses are less likely to limit lentivector immunization and boosting, and consistent with this, we find that mice immunized with lentivector developed potent antigen-specific recall responses when reimmunized with the same vector (see Figure S2 in the Supplemental Data available with this article online). We sought to compare the immune response and mechanisms of immune induction following immunization with lentivector, a nonlytic virus without apparent inhibitory effects on DC function, to vaccinia vector, a well-characterized immunization vector representative of lytic vectors. To evaluate the magnitude and durability of the immune responses, we determined the kinetics of in vivo antigen-specific lytic activity induced by immunization. Following a single immunization with lentivector, in vivo killing activity reached a peak in 8 days and then began to gradually decline (Figure 2A). However, immunized mice maintained remarkably high killing activity (>50% of killing) for at least 50 days (Figure 2A, Figure S2). Even after 4 months, there was still detectable killing activity of 15% (Figure S2). In addition, reimmunization at this time with the same lentivector elicited lytic activity approaching 100% lysis. In contrast, vaccinia vector immunization resulted in substantial but significantly less initial lytic activity that also peaked at day 8 but then decreased rapidly to near-baseline levels (Figure 2A). Importantly, these differences in the kinetics of the immune response correlated with differences in the magnitude and duration of antigen presentation in vivo. The prolonged immune responses observed following lentivector immunization correlated with prolonged antigen presentation in vivo in the draining LNs (Figure 2B).
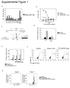
3 Immunization Targeting Skin Dendritic Cells 645 Figure 1. Immunization with Recombinant Lentivector Elicits Strong Antigen-Specific Lytic Activity In Vivo and Effective Antitumor Immunity (A D) Mice were immunized with 1 million TU of lentivector expressing OVA (A and B), HBV S antigen (C), or EGFP-TRP2 fusion protein (D). Immunization by intramuscular injection (100 mg) or gene gun bombardment (20 mg) of naked plasmid DNA encoding the same antigen was compared. Groups of mice immunized by injection of 1 million pfu of adenovector or vaccinia vector expressing OVA (OVA-Ad and OVA-VV) were also included in (A) and (B). Eight days later, antigen-specific in vivo killing was determined (4 hr assay for OVA or 20 hr assay for HBS and TRP2), and ex vivo intracellular staining for IFN-g was performed to evaluate the T cell responses in vivo and in vitro. Mean 6 SD of three mice is shown for each group, and the experiment was repeated at least three times with similar results. (E and F) C57BL/6 mice were inoculated with 0.1 million of B16-OVA cells and immunized or not (control) 3 days later with 1 million TU of OVA-lvv or EGFP-lvv encoding the irrelevant antigen EGFP. Tumor growth (mean 6 SD of ten mice per group) (E) and survival (F) were recorded. This experiment was repeated twice with similar results. (G) Mice were preimmunized with different doses of EGFP-lvv or EGFP-Ad and 12 days later immunized with 1 million pfu of OVA- Ad or 1 million TU of OVA-lvv. Another 8 days later, in vivo antigen-specific lytic activity was measured (20 hr assay). Mean 6 SD of three mice is indicated for each group, and the experiment was repeated three times with similar results. sdcs from Lentivector-Immunized Mice Prime Naive T Cells Previous reports suggest that LN resident CD8 + DCs, and not sdcs, are the critical APCs responsible for crosspriming CD8 + T cells after cutaneous delivery of viral vectors, calling into question the established paradigm of sdc function (Allan et al., 2003; Belz et al., 2004; Carbone et al., 2004; Heath et al., 2004; Smith et al., 2003). To investigate the role of sdcs in T cell priming following lentivector immunization, we utilized the same approaches used in those studies to isolate DC subsets and measured their ability to stimulate naive OVA-specific OT-I cells ex vivo (Belz et al., 2004; Smith et al., 2003). We first isolated and purified three DC subsets based on expression of CD11c, CD8, and B220 surface markers from mice injected via footpad with lentivector OVA-lvv (Figure S3) and determined their ability to stimulate naive OT-I T cells ex vivo. Sorted DC subsets were cocultured with CFSE-labelled OT-I cells isolated from TCR transgenic mice. We found that only CD8 2/lo DCs were able to stimulate CFSE-labelled OT-I cell proliferation manifested by progressive reduction of fluorescence intensity of CFSE (Figures 3A and 3B). Neither
4 Immunity 646 Figure 2. Comparison of the Kinetics of Antigen-Specific Lytic Activity and the Duration of Ag Presentation In Vivo Induced by Lentivector Versus Vaccinia Vector-Mediated Genetic Immunization (A) Forty mice were immunized with 0.5 million TU of OVA-lvv or 0.5 million pfu of OVA-VV. At each time point shown, three mice were used to determine in vivo lytic activity. In vivo killing was determined using the 20 hr assay at all time points. At the 8 and 15 day time points where maximum killing was observed in OVA-lvv immunized groups, 4 hr assays was also included (right panel). Mean 6 SD of three mice is shown for each time point. (B) Mice were immunized with 1 million TU of lentivector OVA-lvv or 1 million pfu of vaccinia vector OVA-VV. At the indicated time points, 10 million OT-I cells labelled with CFSE were administered through tail vein. Sixty-four hours after injection of OT-I cells, proliferation of OT-I cells in draining popliteal LNs was determined by progressive dilution of CFSE intensity determined by flow cytometry. M1, nonproliferating OT-I cells. M2, proliferating OT-I cells. CD8 + DCs nor pdcs induced measurable OT-I T cell proliferation. Similar results were obtained using the well-established 3 H-thymidine incorporation assay to evaluate the OT-I cell proliferation (Figure 3C). All DC populations stimulated strong OT-1 proliferation when pulsed with the class I restricted peptide SIINFEKL, verifying that the antigen presentation function of each subset was intact (Figure 3C). Cells within the CD8 2/lo DC subset that includes sdcs also express CD11b, while the CD8 + LN resident DC subset does not (Shortman and Liu, 2002). We used this difference to distinguish these DC subsets based on expression of the CD8 and CD11b markers. Both CD8 2/lo CD11b + and CD8 + CD11b 2 DCs were isolated to more than 96% purity (Figure S4). Isolated DC subsets were then cocultured with OT-I cells to measure the stimulation of OT-I cell activation using the 3 H-thymidine incorporation assay. Using these alternative markers to define these subsets, we confirmed that CD8 2/lo CD11b + DCs prime naive T cells in response to lentivector mediated genetic immunization. As expected from our previous results, CD8 + CD11b 2 DCs were unable to induce OT-I cell proliferation ex vivo (Figure 3E). To address effector function, we also evaluated cytokine secretion by proliferating OT-I cells elicited by different DC subsets. A substantial amount of IFN-g was detected in the supernatant of OT-I cells stimulated by CD8 2/lo CD11b + DCs, but not in supernatants of cells stimulated by CD8 + CD11b 2 DCs or CD8 2/lo CD11b 2 DCs (Figure 3F). The concentration of IFN-g in the supernatant of 150,000 OT-I cells reached 30 ng/ml in 72 hr. DC subsets alone did not produce significant levels of IFN-g (data not shown). In the skin-draining LNs, the CD8 2/lo DCs include LN resident CD8 2 DEC205 2 and skin-derived CD8 2/lo DEC205 + that include both LCs and dermal DC populations. Pure populations of CD8 2/lo DEC205 +, CD8 2/lo DEC205 2, CD8 + DEC205 2/+ DCs were isolated from DLNs by cell sorting (Figure S5A). Only CD8 2/lo DEC205 + DCs strongly stimulated OT-I cell proliferation (Figure 4A). CD8 + DEC205 2/+ DCs induce much lower levels of OT-I cell proliferation. To directly compare our results with those obtained using the well-described vaccinia model, we purified the same subsets from the skin
5 Immunization Targeting Skin Dendritic Cells 647 Figure 3. Stimulation of Naive CD8+ T Cells Ex Vivo by APC Subsets from Lentivector-Immunized Mice (A) Mice were immunized with 10 million TU of OVA-lvv. Two days later, DLN cells were prepared, and DC subsets were sorted based on expression of CD11c, B220, and CD8. (B and C) Twenty thousand DCs of each subsets were cocultured with 150,000 CFSE labelled OT-I cells for 3 days, and then OT-I cell division was determined by progressive dilution of CFSE intensity (B). Alternatively, DC subsets were cocultured with OT-I cells for 2 days, and OT-1 proliferation was determined by 3 H-thymidine incorporation ([C], left panel). Identically treated DC subsets pulsed with SIINFEKL peptide were included as controls ([C], right panel). Mean 6 SD of triplicate wells is shown. These experiments were repeated at least five times, and similar results were observed. (D) Mice were immunized with 10 million TU of OVA-lvv, and 2 days later DC subsets from DLNs were sorted based on CD11c, CD11b, and CD8. (E and F). Twenty thousand DCs of each subset were cocultured with 150,000 OT-I cells for 2 days, and then OT-1 proliferation was determined by 3 H-thymidine incorporation (E). Supernatants were assayed for the presence of IFN-g by ELISA (F). Mean 6 SD of triplicate wells is shown for groups in (E) and (F). These experiments were repeated at least five, times and similar results were observed. DLNs of mice immunized with recombinant VV expressing OVA (OVA-VV) (Figure S5B). Consistent with previous reports, we found that, following VV infection, the endogenous CD8 + DEC205 2/+ DC subset was the main DC subset capable of crosspriming naive CD8 + T cells ex vivo (Figure 4B). To further substantiate the role of sdcs in antigen presentation, we utilized the well-established technique of topical FITC application to identify DCs migrating from the skin (Douillard et al., 2005; Itano et al., 2003). Mice were immunized with lentivector, and then the injection site was painted with FITC. Two days later, DC subsets were isolated from the draining lymph node and analyzed. CD11c + FITC + and CD11c + FITC 2 populations could be identified and sorted to high purity (Figure 5). The CD11c + FITC + DCs predominantly expressed the CD8 2/lo DEC205 + phenotype consistent with sdcs. Consistent with previous reports, this CD11c + FITC +
6 Immunity 648 Figure 4. Stimulation of Naive CD8+ T Cells by sdcs after Immunization with Recombinant Lentivector Versus VV Mice were immunized with 10 million TU of OVA-lvv (A) or 10 million pfu of VV-OVA (B). Two days later, DC subsets from DLNs were sorted based on expression of CD11c, DEC205, and CD8. Twenty thousand DCs of each subset were cocultured with 150,000 OT-I cells for 2 days, and then OT-1 proliferation was determined by 3 H-thymidine incorporation. Mean 6 SD of triplicate wells is shown. This experiment was repeated at least three times with similar results. population decreased substantially in the LN when we codelivered pertusis toxin, a DC migration inhibitor (Douillard et al., 2005; Itano et al., 2003; and data not shown), further suggesting that these LN resident CD11c + FITC + cells migrated from the skin. Functionally, the CD11c + FITC + DC subset induced strong proliferation of OT-1 T cells, indicating that this is the predominant APC subset. Transduction of DC Subsets We sought to account for the pronounced difference in the mechanism of antigen presentation observed between lentivector and VV immunization. The striking difference in antigen presentation between DC subsets was of particular interest, given accumulating recent evidence suggesting that presentation by the CD8 + DEC205 2/+ DC subset, as exemplified by VV infection, is characteristic or even required for the generation of CD8+ T cell responses against viral- and cell-associated antigens. We hypothesized that, in the case of viral infection, DC dysfunction or apoptosis induced by infection limits the antigen presentation ability of infected cells, resulting in predominance of CD8 + DEC205 2/+ DC-mediated crosspresentation of secreted or cellassociated antigens released from dying cells. This is supported by previous studies suggesting that viral infection of DCs can limit APC function or induce apoptosis. (Engelmayer et al., 1999; Humlova et al., 2002; Larsson et al., 2001, 2004; Norbury et al., 2002). In contrast, lentivector infection results in stable expression of transgenic antigens by infected DCs without inhibition of their APC function or viability (He et al., 2005). To directly compare antigen expression in DLNs following infection with lentivector or VV, we isolated mrna from total DLN cells at 6 hr, 24 hr, and 72 hr after infection and determined OVA gene expression using semiquantitative RT-PCR. As shown in Figure 6A, OVA expression appeared early (6 hr) following VV infection, peaked at 24 hr, and was dramatically reduced by 72 hr. In contrast, following lentivector injection, OVA expression was first detectable at 24 hr but persisted and even increased at the 72 hr time point. These results are consistent with previously reported results for VV and with the interpretation that VV infection results in transgene expression followed closely by gene silencing or death of transfected cells that could provide an antigen source for crosspresentation by CD8 + DCs (Norbury et al., 2002). In contrast, lentivector administration results in sustained expression of transgenic antigen in the DLNs. To more specifically address this issue, we directly compared the kinetics of antigen expression in isolated DLN CD8 2/lo DEC205 + and CD8 + DEC205 2/+ DC subsets. Following vaccinia vector immunization, OVA mrna was detected in both DC subsets at 7 hr, peaked at 20 hr, and then declined substantially by the 46 hr time point (Figure 6B). In contrast, OVA mrna was undetectable 7 hr after lentivector immunization but was present at significant levels at 20 hr and only in the sdc subset. OVA mrna levels increased substantially by 46 hr and continued to be selectively expressed in the sdc subset. These results are consistent with the accumulation and persistence of migrating antigen-expressing sdcs in the draining lymph node. Finally, using a lentivectorexpressing luciferase (Luc-lvv), we consistently found
7 Immunization Targeting Skin Dendritic Cells 649 Figure 5. Migrating sdcs Prime Antigen-Specific Naive T Cells Ex Vivo (A) Mice were immunized with OVA-lvv in the shaved thigh skin, and injection sites were painted with FITC. Two days later, draining LNs were collected and DCs were enriched from single-cell suspensions. FITC+ and FITC2 DC populations were sorted and analyzed phenotypically. (B) Sorted CD11c + FITC + and CD11c + FITC 2 DCs were cocultured with OT-I cells in vitro to determine their T cell priming capability. OT-1 proliferation was determined by 3 H-thymidine incorporation. Mean 6 SD of triplicate wells is shown. Experiments were repeated twice with similar results. luciferase activity in the CD8 2/lo DCs (CD11c + CD8 2/lo B220 2 and CD11c + CD8 2/lo DEC205 + DCs) isolated form lentivector immunized mice (Figures 6C and 6D, and Figures S6 and S7). No luciferase activity was detected in CD8a + (CD11c + CD8 + B220 2 ) or plasmacytoid (CD11c + CD8 2/+ B220 + ) DC subsets (Figures 6C and 6D). These results are consistent with the mechanistic interpretation that lentivector immunization results in sustained expression and presentation of antigen by sdcs, while vaccinia vector immunization results in substantial but transient expression of antigen in the draining lymph nodes. We evaluated and compared DC apoptosis in lentivector versus vaccinia vector-transduced DC populations in vitro. Consistent with this interpretation, in vitro infection of DCs by vaccinia vector resulted in rapid and substantial apoptosis of DCs, while survival of lentivector-infected DCs was comparable to that of untransfected cells throughout the 3 day time course evaluated (Figure 7A). Furthermore, artificial induction of apoptosis (by UVB irradiation) of OVA-lvv-transfected DCs results in complete inhibition of their ability to stimulate antigen-specific OT-1 T cells in vitro and in vivo and inhibition of antigen-specific lytic activity in vivo (Figures 7B 7D). Thus, unlike vaccinia vector, lentivector transduction does not significantly induce apoptosis of transduced DCs in vitro, and induction of apoptosis of OVAlvv-transduced DCs eliminates their ability to directly present expressed antigens. Taken together, our data demonstrate that cutaneous injection of lentivector results in transduction and transgene expression in skin-derived CD8 2/lo DEC205 + DCs. Importantly, in contrast to previous studies using HSV, IAV, and VV, we demonstrate that, in lentivector immunization, sdcs can directly prime naive CD8 + T cells, demonstrating an important role for sdcs in the induction of potent and durable CD8 + T cell immunity. Discussion sdcs have long been recognized as potent APCs, capable of priming naive T cell responses (Green et al., 1980; Stingl et al., 1980a; Stingl et al., 1980b; Stingl et al., 1977; Valladeau and Saeland, 2005). Their critical role in immune regulation can be attributed to both this APC function and functional plasticity that enables them to shape the nature of the immune responses they induce in response to environmental conditions in the periphery. Because of their remarkable immunoregulatory capacity and unique accessibility, cutaneous DCs remain the focus of intense study for the purpose of vaccine development, immunotherapy, and efforts to regulate autoimmunity. Here, we sought to determine the role of sdcs in inducing CD8 + T cell immunity against cutaneously introduced antigens. The traditional paradigm of cutaneous immune function integrates antigen presentation with immunomodulation by proposing that sdcs both present antigen and serve as biosensors capable of modulating the nature of the immune response in response to environmental stimuli. In this integrative model, sdcs both sample their antigenic environment in the periphery and translate diverse environmental signals encountered there into
8 Immunity 650 Figure 6. Expression of Transgenic Antigen by sdc of Immunized Mice (A) Mice were injected with 10 million TUs of OVA-lvv or OVA-VV via footpad. RNA was isolated from total DLN cells at the indicated time points. OVA transgene expression was determined by RT-PCR. Titrations of varying amounts of total RNA were utilized to semiquantify the abundance of OVA-specific mrna. RT-PCR specific for S15 was determined as an internal control. Lv, RNA from OVA-lvv injected mice; VV, RNA from OVA- VV injected mice. (B) Mice were injected with 10 million TUs of OVA-lvv or OVA-VV via footpad. At the indicated time points, DC subsets from popliteal and inguinal LNs were sorted based on expression of CD11c, CD8a, and DEC205. Total RNA was isolated from sorted DC subsets that are >95% pure (data not shown). One hundred nanograms of total RNA was utilized for RT-PCR to detect OVA specific mrna. Primers specific for ribosomal protein S15 were used to measure S15 mrna as an internal control. (C and D) Mice were injected with 10 million TUs of Luc-lvv via footpad. Two days later, DC subsets from popliteal and inguinal LNs were sorted based on expression of CD11c, CD8, and B220 (C) or based on expression of CD11c, CD8a, and DEC205 (D). Luciferase activity was determined for each subset using luciferase detection reagent (Promega). Mean 6 SD of duplicate wells is shown. This experiment was repeated three times with similar results. a functional program characterized by patterns of expression of chemokines, cytokines, and cell-surface molecules that influence trafficking, immune skewing, and the balance between T cell activation or tolerance. By exhibiting functional plasticity, sdcs integrate environmentally imprinted functional programming with the presentation of antigens obtained from the same environment, linking environmentally responsive immunity with antigen specificity. Recent studies challenge this paradigm, particularly in regard to the induction of CD8 + T cell responses essential for immunity to viral infections, cancer, intracellular bacteria, and parasites. The definition of at least six distinct DC subsets based on partially overlapping
9 Immunization Targeting Skin Dendritic Cells 651 Figure 7. The Antigen Presentation Function of Live or Apoptotic Transduced DCs (A) DCs infected with vaccinia vector or lentivector were monitored for cell death by determining the number of surviving cells (left panel) or by identification of apoptosis in DC populations by flow cytometry using Annexin-V staining (right panel) at indicated time points. (B) OVA-lvv-transduced DCs were exposed to 1 J of UVB light to induce apoptosis. Twenty-five thousand live or irradiated lentivector-transduced DCs were cocultured with OT-I cells to determine their ability to stimulate naive CD8 T cells. Mean 6 SD of triplicate wells is shown. (C) Naive mice were immunized with 0.5 million of live or irradiated OVA-lvv-transduced DCs, and 8 days later antigen-specific in vivo killing was determined as previously described. Mean 6 SD of two mice is shown. (D) Naive mice were cutaneously immunized with 0.5 million live or irradiated OVA-lvv-transduced DCs, and the capacity of DCs to stimulate OT-I cell proliferation in vivo was determined by evaluating progressive dilution of CFSE intensity. M1, nonproliferating OT-I cells. M2, proliferating OT-I cells. phenotypic profiles, distinct patterns of expression of TLRs, and evolving functional correlations has raised the possibility that certain DC subsets may be specialized and restricted in their functional capacity (Carbone et al., 2004; Heath et al., 2004; Serbina and Pamer, 2003). Consistent with this, blood-derived splenic CD8a + DCs have been shown to play a critical role in crosspriming CD8 + T cell immunity against cell-associated antigens (den Haan et al., 2000). Furthermore, in direct contrast to the current paradigm of cutaneous immunity, new studies suggest that CD8 + T cell responses against virally encoded antigens are crossprimed by bloodderived, LN resident CD8 + DCs, and not sdcs (Allan et al., 2003; Belz et al., 2004; Smith et al., 2003). This has been demonstrated for VV, HSV, and IAV. In those studies, following viral delivery, sdcs from skin DLNs did not appear capable of directly presenting virally encoded antigens to CD8 + T cells, suggesting that sdcs were insufficient to prime CD8 + T cell responses. Subsequent studies suggest that splenic CD8 + DCs also play a dominant role in priming CD8 + T cell responses against lymphocytic choriomeningitis virus (LCMV) and Listeria monocytogenes (LM), although, in these systems, animals were challenged parenterally, bypassing the cutaneous immune system (Belz et al., 2005). Taken together, these observations have led to an alternative model of cutaneous immune function based on the idea that sdcs can transport antigens from the periphery and transfer them to other LN resident DCs for crosspresentation (Carbone et al., 2004; Heath et al., 2004).
10 Immunity 652 This collaborative model offers the alluring possibility that antigen transfer between DC populations could efficiently distribute antigen to a large network of LN resident DCs specialized for antigen presentation. It would, however, require both an efficient mechanism for the distribution of small quantities of antigen to multiple DCs and a mechanism to translate and transfer inflammatory or tolerogenic signals from the periphery into appropriate T cell responses. Early studies demonstrating the remarkable antigen presentation capacity of DCs suggest that CD8 2 DCs could prime CD8 + T cell immunity. We and others have demonstrated that cutaneous injection of CD8 2 bone marrow-derived DCs pulsed with peptide antigens induces potent antigen specific CD8 + T cell immunity (Celluzzi et al., 1996; Mayordomo et al., 1995; Paglia et al., 1996; Young and Inaba, 1996). Similarly, we have shown that CD8 2 bone marrow-derived DCs can efficiently internalize and crosspresent antigens from live cells to induce CD8 + T cell immunity (Celluzzi and Falo, 1998). Studies specifically evaluating the role of sdcs in T cell priming have shown that peptide-pulsed LCs isolated from epidermis also induce potent antigen-specific CD8 + T cell responses when injected intradermally (Celluzzi and Falo, 1997). In each of these examples, it is formally possible that the adoptively transferred DCs distribute antigen to collaborating resident DCs, though no direct evidence has been reported to support this interpretation. The relative contributions of direct priming versus crosspriming in DC adoptive transfer approaches remain controversial. Recent imaging studies strongly suggest that injected DCs do directly prime T cells, as evidenced by cognate interactions between labelled injected DCs and antigen-specific T cells, and associated T cell proliferation and activation (Bousso and Robey, 2003; Mempel et al., 2004; Stoll et al., 2002). Many of the models used to support the predominant role of LN-resident CD8a + DCs in CD8 + T cell cross priming rely on infection of skin and sdcs (Belz et al., 2004; Smith et al., 2003). Both the cytolytic nature of these viruses and their well-described mechanisms for immune evasion can directly or indirectly alter antigen presentation function (Bosnjak et al., 2005; Engelmayer et al., 1999; Larsson et al., 2004; Yewdell and Haeryfar, 2005). We sought to directly address the role of sdcs in CD8 + T cell priming by utilizing a viral vector known to efficiently transduce DCs without inhibiting the antigen processing and presentation function of infected cells. Very recently lentivirus has been explored as a vector for genetic immunization, and preliminary studies suggest that it is among the most potent vectors for immune induction (He et al., 2005; Esslinger et al., 2003). Importantly, we and others have shown that lentivectors efficiently and stably transfect DCs without apparent alteration of DC function (He et al., 2005; Breckpot et al., 2003). Lentivector-transfected bone marrow-derived CD8 2 DCs can process and present transgenic antigens through both the class I and class II restricted processing pathways and prime naive CD8 + and CD4 + T cells (He et al., 2005). Studies we present here demonstrate the remarkable potency of cutaneous immunization with lentivectors. We show that a single cutaneous injection of lentivector results in potent CD8 + T cell activation and antigen-specific lytic activity. Lentivector immunization results in potent immune responses against model (OVA), viral (HBS), and self (TRP-2) antigens, and a single immunization against a model tumor antigen results in inhibition of tumor growth and prolonged survival of mice bearing the highly aggressive B16 melanoma. Importantly, lentivector-based immunization results in prolonged effector function that correlates with prolonged presentation of transgenic antigens by DCs in the DLNs. While initial immune responses induced by lentivector are comparable in magnitude to those induced by adenovector, a gold standard for genetic immunization, lentivectors do not appear to induce significant levels of antivector immune responses. This obviates a major limitation of adenovirus and other viral vectors that become ineffective for boosting or reimmunization due to the presence of antivector immune responses. In the skin DLNs, sdcs are phenotypically characterized as CD11c + CD11b + CD8 2/lo DEC205 + B By isolating DC subsets from LNs draining the site of lentivector injection and evaluating their ability to stimulate antigen-specific CD8 + T cells ex vivo, we demonstrate that the CD8 + T cell-activating capacity is found predominantly in sdcs. Specifically, the ability to present lentiviral-encoded transgenic OVA to OVA-specific CD8 + T cells was present predominantly in DC populations characterized in separate experiments as CD11c + CD8 2/lo B220 2, CD11c + CD8 2/lo CD11b +, and CD11c + CD8 2/lo DEC205 +, all phenotypically consistent with sdcs. Although there are at least two recognized subsets of sdcs, LCs and dermal DCs, because they differ only by intermediate degrees of expression of these markers, they can be difficult to distinguish using these panels, and we did not attempt to do so (Itano et al., 2003). This panel of phenotypic markers is sufficient to clearly distinguish sdcs from the CD8 + DC subset (CD11c + CD8 + B220 2 ), the plasmacytoid DC subset (CD11c + CD8 2/lo B220 + ), and the LN-resident myeloid DC population (CD11c + CD8 2 DEC205 2 ) in skin DLNs. Importantly, the methods we used were similar to those used previously to demonstrate that sdcs do not present cutaneously delivered viral antigens (Allan et al., 2003; Belz et al., 2004; Smith et al., 2003). Using the same VV construct used in those previous studies, we verified that, after cutaneous immunization, CD8 + DCs and not sdcs presented vaccinia-encoded OVA to OVA-specific CD8 + T cells. Thus, our experimental approach is comparable to that used previously, supporting the interpretation that our distinctly different results are a consequence of the vector used for immunization and different mechanisms of immune induction. To further define the mechanism by which lentiviral vectors induce immune responses, and to investigate mechanistic differences between lentivectors and other well-described viral vectors, we evaluated and compared gene expression in vivo following lentivector and vaccinia vector immunization. We found considerable differences. VV immunization resulted in rapid but transient transgene expression in DLNs. This is consistent with previous reports and a mechanism based on expression of antigen in DCs or non-dcs, death of infected cells, and crosspresentation of released antigens by CD8 + DCs (Engelmayer et al., 1999; Humlova et al., 2002; Larsson et al., 2001, 2004; Norbury et al., 2002).
11 Immunization Targeting Skin Dendritic Cells 653 On the other hand, lentivirus immunization resulted in persistent high-level transgene expression in the DLNs and in DLN DCs. Importantly, transgenic mrna and protein expression was detectable exclusively in the sdc subset, as evidenced by separate experiments demonstrating transgenic mrna and luciferase exclusively in CD11c + CD8 2/lo B220 2 and CD11c + CD8 2/lo DEC205 + DC subsets. Our results suggest that sdcs were primarily transfected in the skin, though some transfection of sdcs by viral particles reaching the node through the afferent lymphatics may also occur. The time course of gene expression by sdcs is consistent with the accumulation and persistence of transfected DCs migrating from the skin. Furthermore, predominant presentation by a migration inhibitable, FITC+ sdc population subsequent to FITC skin painting strongly suggests that DCs transfected in the skin make important contributions to the induction of the immune response we observe. The identity of DC subset(s) that have access to substances in the afferent lymph remains controversial, with recent reports describing uptake of soluble antigens exclusively by sdcs (CD11c + CD8 2/lo DEC205 + )(Itano et al., 2003) or LN resident blood-derived myeloid DCs (CD11c + CD11b + DEC205 2 ) (Sixt et al., 2005), respectively. In the case of antigen uptake by sdc resident in LNs, it is noteworthy that antigen presentation by LN resident sdcs was insufficient to fully activate CD4 + T cells, which required subsequent presentation by sdcs arriving from antigen-exposed skin (Itano et al., 2003). Interestingly, our demonstration of sdc presentation of antigen to CD8 + T cells is consistent with recently published studies showing that sdcs present keratinocyte-derived antigens to activate naive CD8 + T cells (Mayerova et al., 2004). Our results are also consistent with recent studies showing that direct contact with invading microbes is necessary for DCs to acquire the ability to stimulate functional effector CD4 + T cells (Sporri and Reis e Sousa, 2005). These results demonstrate that sdcs can present virally encoded antigens to activate CD8 + T cells, and they support the traditional paradigm of cutaneous immune function in which antigen presentation and immunomodulation functions are integrated into the same APC subset, namely the sdcs. Differences in the nature of the vectors used could explain the differences between these results and previously published studies. Many pathogens have evolved mechanisms for immune evasion, and DCs infected by them, or exposed to signals from infected cells, could have altered antigen presentation function (Larsson et al., 2004; Yewdell and Haeryfar, 2005). In particular, cytolytic viruses whose antigens are presented by CD8 + DCs could drive antigen expression in infected sdcs that die after migration to the DLNs, facilitating uptake and presentation of apoptotic debris by LN-resident CD8 + DCs. This is supported by studies demonstrating effective internalization and processing of apoptotic debris by CD8 + DCs and enhanced immunity generated by cytopathic self-replicating RNAinduced apoptosis of adoptively transferred antigen expressing CD8 2 DCs (Racanelli et al., 2004). Alternatively, the discrepancy could be related to plasticity between CD8 2 DC and CD8 + DC subsets. Some studies suggest that CD8 2 DCs can subsequently become CD8 + (Moron et al., 2002), and it has been suggested that sdcs (and LCs in particular) can upregulate CD8 expression in the DLNs, albeit at low levels (Anjuere et al., 2000). Interestingly, recent studies demonstrate expression of langerin, thought to be an LC-specific marker, in LN-resident CD8 + DCs (Bennett et al., 2005; Kissenpfennig et al., 2005). On the other hand, a quantitative analysis of DC subsets argues against CD8 2 to CD8a + DC conversion, and radiation chimera experiments support the interpretation that, in some viral infections, bloodderived CD8 + DCs rather than sdcs from skin-resident radio-resistant sdc precursors are critical for CD8 + T cell activation (Allan et al., 2003; Naik et al., 2003). Given these observations, it remains possible that the collaborative paradigm of cutaneous immune function can be operable under certain specific conditions. The coexistence of integrative and collaborative mechanisms of immune induction further demonstrates the remarkable resiliency and plasticity of DCs and the cutaneous immune system. It is tempting to speculate that the potency and durability of immune responses induced by lentivector-mediated genetic immunization are related to the vectors capacity to efficiently transfect sdcs without adversely effecting their survival, plasticity, or antigen processing and presentation function. Further studies elucidating the role of DC subsets in the generation and control of immunity against cutaneously delivered antigens will be necessary to develop effective strategies to target and engineer the function of DC subsets in vivo. Experimental Procedures Mice C57BL/6 and OVA TCR transgenic mice OT-I were purchased from Jackson Laboratory (Bar Harbor, Maine). The mice were housed under SPF conditions in the central animal facility of the University of Pittsburgh. Handling of the mice was according to institutional guidelines of the University of Pittsburgh. Viral Vectors The third-generation lentivectors OVA-lvv and EGFP-lvv expressing OVA and EGFP were described as before (He et al., 2005). HBV S antigen was amplified by PCR from template plasmid prc/cmv- HBs(S) (Aldevron, Fargo, North Dakota). EGFP-TRP fusion gene was constructed as previously described (Zhang et al., 2004). Briefly, the TRP2 epitope of aa was fused next to the EGFP protein gene to form the EGFP-TRP2 fusion gene and then cloned into lentivector. Lentivector expressing the luciferase gene was also constructed. The resulting lentivectors were designated as HBS-lvv, TRP2-lvv, Luc-lvv, or EGFP-TRP2-lvv and shown in Figure S1. Lentivectors were prepared and concentrated as described (He et al., 2005). Adenoviral vectors were kindly provided by Dr. Robbins of the University of Pittsburgh. The vaccinia vector expressing OVA was kindly provided by Dr. Yewdell, National Institutes of Health. Handling of viral vectors was according to the guidelines of BSL-2+ laboratories established by the Recombinant DNA Committee of the University of Pittsburgh. Genetic Immunization For gene gun bombardment, plasmid DNA coated bullets were administered onto the abdomen of mice after the hair was shaved. Four shots with approximately 20 mg of DNA were administered. For intramuscular injection, mice were anesthetized with isoflurane, and then 100 mg of plasmid DNA in 100 ml of saline was injected into gluteal muscles after incision of the skin. Immunizations with recombinant viral vectors were conducted by footpad injection in 50 ml containing the indicated doses.
12 Immunity 654 OT-I Cell Proliferation Assay This assay was performed as described previously (He et al., 2005). Briefly, OT-I T cells were isolated from the spleen and LN of OVA TCR transgenic mice, and the purity of OT-I T cells was determined to be above 95%. OT-I cells or CFSE labelled OT-I cells (150,000) were cocultured with indicated number of DC subsets for 2 3 days. In experiments shown in Figure 7, bone marrow-derived DCs were generated and transduced with lentivector as described previously (He et al., 2005). Where indicated, DCs were exposed to 1J of UVB to induce apoptosis as described (Nicolo et al., 2001). Proliferation of OT-I cells was measured by reduction of CFSE intensity or by 3 H- thymidine incorporation. In some experiments, supernatant was saved to determine IFN-g cytokine production. In vivo antigen-specific lytic activity was measured by in vivo killing assay as described previously (Barchet et al., 2000; He et al., 2005). In Vivo Antigen Presentation Assay In vivo antigen presentation assay was performed as described (Stock et al., 2004). Briefly, mice were injected in the footpad with 1 million TU of lentivector (OVA-lvv) or 1 million pfu of vaccinia vector (OVA-VV). At the indicated time points, 10 million OT-I cells labelled with CFSE were administered through tail vein. Forty-eight hours after injection, draining popliteal LN and contralateral LN cells were collected and stained with anti-va2-pe and anti-vb biotin+ APC-labelled Streptavidin to identify OT-I cells, and proliferation of OT-I cells was measured by reduction of CFSE intensity. Flow Cytometry Analysis Cells were collected and washed with PBS. One half million cells were added to each staining tube. Anti-CD11c-FITC, anti-cd8- APC, anti-b220-pe, anti-cd11b-pe, anti-ifn-g-apc, anti-va2-pe, and anti-vb biotin antibodies were purchased from BD Pharmingen. APC-labelled Streptoavidin was used as secondary reagent at 1:1000 dilution. Anti-DEC205-PE was from Cedarlane (Hornby, Ontario, Canada). Cells were fixed, and 30,000 events were collected and analyzed. For DC subset isolation, CD11c+ DCs were enriched from draining LN cells by depleting B and T cells using magnetic beads. Enriched DCs were stained for CD11c, CD8, and either B220, CD11b, or DEC205, and subsets were gated and sorted as described. To evaluate intracellular expression of IFN-g, splenocytes were collected and stimulated in vitro for 4 hr by adding OVA class I peptide SIINFEKL in the presence of Golgistop to block the secretion of IFN-g. Cells were then collected and stained for CD8 and intracellular IFN-g using an intracellular staining kit according to the manufacturer (BD Pharmingen, San Diego, California). Tumor Growth and Immunization Mice were inoculated with 0.1 million B16-OVA cells in 50 ml of PBS s.c. Three days later, 1 million TU of lentivector OVA-lvv or EGFP-lvv was injected into the footpads of the mice. Tumor growth was monitored by measuring the perpendicular diameter of the tumor, and survival was recorded daily. RT-PCR Total RNA was isolated from total LN cells or sorted DC subsets using RNA isolation kit RNAqueous-4PCR or RNAqueous-Micro (Ambion, Austin, Texas). The indicated amount of RNA was utilized for each RT-PCR using Access RT-PCR system (Promega, Madison, Wisconsin) with OVA-specific primers (OVA-Forward, 5 0 -TAAGGG GAAACACAT-CTGCC-3 0 ; OVA-Reverse, 5 0 -CAGATCAAGCCAGAG AGCTCATC-3 0 ). Amplification of ribosomal S15 mrna was conducted as internal control using specific primers (S15-Forward, TTCCGCAAGTTCACCTACC-3 0 ; S15-Reverse, 5 0 -CGGGCCGGCCA TG-CTTTACG-3 0 ). DC Migration The hind legs of mice were shaved and injected intradermally with lentivector OVA-lvv with or without the DC migration inhibitor pertusis toxin (0.5 mg). The injected area was then painted with 0.5 mg/ml of FITC in acetone/dibutylphthalate solution. Two days later, draining LNs were harvested, and DCs were analyzed and purified by flow cytometery for functional analysis. Statistic Analysis Unpaired t test analysis (two-tailed) was used to determine whether the differences among the tumor size in OVA-lvv, EGFP-lvv, and control groups were significant. Supplemental Data Supplemental Data include seven figures and can be found with this article online at 643/DC1/. Acknowledgments We thank Dr. Zhongsheng Guo of the University of Pittsburgh Cancer Institute for helping us prepare the vaccinia vector OVA-VV, kindly provided by Dr. Yewdell of NIAID, NIH, and Jeffery Plowey for technical assistance. This work is supported by a K01 grant from NIH NIAMS to Y.H. and grants from NIAMS, NIAID, and the NCI to L.D.F. Received: October 5, 2005 Revised: February 16, 2006 Accepted: March 1, 2006 Published: May 23, 2006 References Ada, G., and Ramshaw, I. (2003). DNA vaccination. Expert Opin. Emerg. Drugs 8, Allan, R.S., Smith, C.M., Belz, G.T., van Lint, A.L., Wakim, L.M., Heath, W.R., and Carbone, F.R. (2003). Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science 301, Anjuere, F., Martinez del Hoyo, G., Martin, P., and Ardavin, C. (2000). Langerhans cells acquire a CD8+ dendritic cell phenotype on maturation by CD40 ligation. J. Leukoc. Biol. 67, Banchereau, J., and Steinman, R.M. (1998). Dendritic cells and the control of immunity. Nature 392, Barchet, W., Oehen, S., Klenerman, P., Wodarz, D., Bocharov, G., Lloyd, A.L., Nowak, M.A., Hengartner, H., Zinkernagel, R.M., and Ehl, S. (2000). Direct quantitation of rapid elimination of viral antigen-positive lymphocytes by antiviral CD8(+) T cells in vivo. Eur. J. Immunol. 30, Belshe, R.B., Newman, F.K., Cannon, J., Duane, C., Treanor, J., Van Hoecke, C., Howe, B.J., and Dubin, G. (2004). Serum antibody responses after intradermal vaccination against influenza. N. Engl. J. Med. 351, Belz, G.T., Smith, C.M., Eichner, D., Shortman, K., Karupiah, G., Carbone, F.R., and Heath, W.R. (2004). Cutting edge: conventional CD8 alpha+ dendritic cells are generally involved in priming CTL immunity to viruses. J. Immunol. 172, Belz, G.T., Shortman, K., Bevan, M.J., and Heath, W.R. (2005). CD8{alpha}+ dendritic cells selectively present MHC class I- restricted noncytolytic viral and intracellular bacterial antigens in vivo. J. Immunol. 175, Bennett, C.L., van Rijn, E., Jung, S., Inaba, K., Steinman, R.M., Kapsenberg, M.L., and Clausen, B.E. (2005). Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J. Cell Biol. 169, Bosnjak, L., Miranda-Saksena, M., Koelle, D.M., Boadle, R.A., Jones, C.A., and Cunningham, A.L. (2005). Herpes simplex virus infection of human dendritic cells induces apoptosis and allows crosspresentation via uninfected dendritic cells. J. Immunol. 174, Bousso, P., and Robey, E. (2003). Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat. Immunol. 4, Breckpot, K., Dullaers, M., Bonehill, A., van Meirvenne, S., Heirman, C., de Greef, C., van der Bruggen, P., and Thielemans, K. (2003). Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J. Gene Med. 5,
Institute t of Experimental Immunology University of Bonn, Germany
 How T cells recognize antigen Christian Kurts Institute t of Experimental Immunology University of Bonn, Germany When do T cells recognize antigen? Antigen uptake Activation phase 1st recognition Effector
How T cells recognize antigen Christian Kurts Institute t of Experimental Immunology University of Bonn, Germany When do T cells recognize antigen? Antigen uptake Activation phase 1st recognition Effector
SUPPLEMENTARY INFORMATION
 Complete but curtailed T-cell response to very-low-affinity antigen Dietmar Zehn, Sarah Y. Lee & Michael J. Bevan Supp. Fig. 1: TCR chain usage among endogenous K b /Ova reactive T cells. C57BL/6 mice
Complete but curtailed T-cell response to very-low-affinity antigen Dietmar Zehn, Sarah Y. Lee & Michael J. Bevan Supp. Fig. 1: TCR chain usage among endogenous K b /Ova reactive T cells. C57BL/6 mice
Dendritic cell subsets and CD4 T cell immunity in Melanoma. Ben Wylie 1 st year PhD Candidate
 Dendritic cell subsets and CD4 T cell immunity in Melanoma Ben Wylie 1 st year PhD Candidate Melanoma Melanoma is the 4 th most common cancer in Australia. Current treatment options are ineffective resulting
Dendritic cell subsets and CD4 T cell immunity in Melanoma Ben Wylie 1 st year PhD Candidate Melanoma Melanoma is the 4 th most common cancer in Australia. Current treatment options are ineffective resulting
Dendritic cells in cancer immunotherapy Aimin Jiang
 Dendritic cells in cancer immunotherapy Aimin Jiang Feb. 11, 2014 Dendritic cells at the interface of innate and adaptive immune responses Dendritic cells: initiators of adaptive immune responses Dendritic
Dendritic cells in cancer immunotherapy Aimin Jiang Feb. 11, 2014 Dendritic cells at the interface of innate and adaptive immune responses Dendritic cells: initiators of adaptive immune responses Dendritic
TITLE: MODULATION OF T CELL TOLERANCE IN A MURINE MODEL FOR IMMUNOTHERAPY OF PROSTATIC ADENOCARCINOMA
 AD Award Number: DAMD17-01-1-0085 TITLE: MODULATION OF T CELL TOLERANCE IN A MURINE MODEL FOR IMMUNOTHERAPY OF PROSTATIC ADENOCARCINOMA PRINCIPAL INVESTIGATOR: ARTHUR A HURWITZ, Ph.d. CONTRACTING ORGANIZATION:
AD Award Number: DAMD17-01-1-0085 TITLE: MODULATION OF T CELL TOLERANCE IN A MURINE MODEL FOR IMMUNOTHERAPY OF PROSTATIC ADENOCARCINOMA PRINCIPAL INVESTIGATOR: ARTHUR A HURWITZ, Ph.d. CONTRACTING ORGANIZATION:
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Dendritic cells and their role in immunity
 PART I Dendritic cells and their role in immunity CHAPTER 1 Subpopulations and differentiation of mouse dendritic cells Carlos Ardavín Universidad Autónoma 1.1 DENDRITIC CELL SUBPOPULATIONS Dendritic cells
PART I Dendritic cells and their role in immunity CHAPTER 1 Subpopulations and differentiation of mouse dendritic cells Carlos Ardavín Universidad Autónoma 1.1 DENDRITIC CELL SUBPOPULATIONS Dendritic cells
Hua Tang, Weiping Cao, Sudhir Pai Kasturi, Rajesh Ravindran, Helder I Nakaya, Kousik
 SUPPLEMENTARY FIGURES 1-19 T H 2 response to cysteine-proteases requires dendritic cell-basophil cooperation via ROS mediated signaling Hua Tang, Weiping Cao, Sudhir Pai Kasturi, Rajesh Ravindran, Helder
SUPPLEMENTARY FIGURES 1-19 T H 2 response to cysteine-proteases requires dendritic cell-basophil cooperation via ROS mediated signaling Hua Tang, Weiping Cao, Sudhir Pai Kasturi, Rajesh Ravindran, Helder
Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice
 Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
Micro 204. Cytotoxic T Lymphocytes (CTL) Lewis Lanier
 Micro 204 Cytotoxic T Lymphocytes (CTL) Lewis Lanier Lewis.Lanier@ucsf.edu Lymphocyte-mediated Cytotoxicity CD8 + αβ-tcr + T cells CD4 + αβ-tcr + T cells γδ-tcr + T cells Natural Killer cells CD8 + αβ-tcr
Micro 204 Cytotoxic T Lymphocytes (CTL) Lewis Lanier Lewis.Lanier@ucsf.edu Lymphocyte-mediated Cytotoxicity CD8 + αβ-tcr + T cells CD4 + αβ-tcr + T cells γδ-tcr + T cells Natural Killer cells CD8 + αβ-tcr
Supplementary Figure 1. Characterization of basophils after reconstitution of SCID mice
 Supplementary figure legends Supplementary Figure 1. Characterization of after reconstitution of SCID mice with CD4 + CD62L + T cells. (A-C) SCID mice (n = 6 / group) were reconstituted with 2 x 1 6 CD4
Supplementary figure legends Supplementary Figure 1. Characterization of after reconstitution of SCID mice with CD4 + CD62L + T cells. (A-C) SCID mice (n = 6 / group) were reconstituted with 2 x 1 6 CD4
1. Overview of Adaptive Immunity
 Chapter 17A: Adaptive Immunity Part I 1. Overview of Adaptive Immunity 2. T and B Cell Production 3. Antigens & Antigen Presentation 4. Helper T cells 1. Overview of Adaptive Immunity The Nature of Adaptive
Chapter 17A: Adaptive Immunity Part I 1. Overview of Adaptive Immunity 2. T and B Cell Production 3. Antigens & Antigen Presentation 4. Helper T cells 1. Overview of Adaptive Immunity The Nature of Adaptive
Cytotoxicity assays. Rory D. de Vries, PhD 1. Viroscience lab, Erasmus MC, Rotterdam, the Netherlands
 Cytotoxicity assays Rory D. de Vries, PhD 1 1 Viroscience lab, Erasmus MC, Rotterdam, the Netherlands Anti-influenza immunity Humoral / CD4+ / CD8+ / NK? Function of CTL Elimination of virus-infected cells?
Cytotoxicity assays Rory D. de Vries, PhD 1 1 Viroscience lab, Erasmus MC, Rotterdam, the Netherlands Anti-influenza immunity Humoral / CD4+ / CD8+ / NK? Function of CTL Elimination of virus-infected cells?
Regulation of anti-tumor immunity through migration of immune cell subsets within the tumor microenvironment Thomas F. Gajewski, M.D., Ph.D.
 Regulation of anti-tumor immunity through migration of immune cell subsets within the tumor microenvironment Thomas F. Gajewski, M.D., Ph.D. Professor, Departments of Pathology and Medicine Program Leader,
Regulation of anti-tumor immunity through migration of immune cell subsets within the tumor microenvironment Thomas F. Gajewski, M.D., Ph.D. Professor, Departments of Pathology and Medicine Program Leader,
Supplementary Fig. 1 p38 MAPK negatively regulates DC differentiation. (a) Western blot analysis of p38 isoform expression in BM cells, immature DCs
 Supplementary Fig. 1 p38 MAPK negatively regulates DC differentiation. (a) Western blot analysis of p38 isoform expression in BM cells, immature DCs (idcs) and mature DCs (mdcs). A myeloma cell line expressing
Supplementary Fig. 1 p38 MAPK negatively regulates DC differentiation. (a) Western blot analysis of p38 isoform expression in BM cells, immature DCs (idcs) and mature DCs (mdcs). A myeloma cell line expressing
Dynamic Interplay among Monocyte-Derived, Dermal, and Resident Lymph Node Dendritic Cells during the Generation of Vaccine Immunity to Fungi
 Article Dynamic Interplay among Monocyte-Derived, Dermal, and Resident Lymph Node Dendritic Cells during the Generation of Vaccine Immunity to Fungi Karen Ersland, 1 Marcel Wüthrich, 2 and Bruce S. Klein
Article Dynamic Interplay among Monocyte-Derived, Dermal, and Resident Lymph Node Dendritic Cells during the Generation of Vaccine Immunity to Fungi Karen Ersland, 1 Marcel Wüthrich, 2 and Bruce S. Klein
NK cell flow cytometric assay In vivo DC viability and migration assay
 NK cell flow cytometric assay 6 NK cells were purified, by negative selection with the NK Cell Isolation Kit (Miltenyi iotec), from spleen and lymph nodes of 6 RAG1KO mice, injected the day before with
NK cell flow cytometric assay 6 NK cells were purified, by negative selection with the NK Cell Isolation Kit (Miltenyi iotec), from spleen and lymph nodes of 6 RAG1KO mice, injected the day before with
ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1
 ZH, Li et al, page 1 ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1 Zhenhu Li 1,4,Yuan Zhang 1,4, Zhiduo Liu 1, Xiaodong Wu 1, Yuhan Zheng 1, Zhiyun Tao 1, Kairui Mao 1,
ZH, Li et al, page 1 ECM1 controls T H 2 cell egress from lymph nodes through re-expression of S1P 1 Zhenhu Li 1,4,Yuan Zhang 1,4, Zhiduo Liu 1, Xiaodong Wu 1, Yuhan Zheng 1, Zhiyun Tao 1, Kairui Mao 1,
Antigen Presentation and T Lymphocyte Activation. Abul K. Abbas UCSF. FOCiS
 1 Antigen Presentation and T Lymphocyte Activation Abul K. Abbas UCSF FOCiS 2 Lecture outline Dendritic cells and antigen presentation The role of the MHC T cell activation Costimulation, the B7:CD28 family
1 Antigen Presentation and T Lymphocyte Activation Abul K. Abbas UCSF FOCiS 2 Lecture outline Dendritic cells and antigen presentation The role of the MHC T cell activation Costimulation, the B7:CD28 family
Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD-
 Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
General Overview of Immunology. Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center
 General Overview of Immunology Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center Objectives Describe differences between innate and adaptive immune responses
General Overview of Immunology Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center Objectives Describe differences between innate and adaptive immune responses
Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells
 ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
% of live splenocytes. STAT5 deletion. (open shapes) % ROSA + % floxed
 Supp. Figure 1. a 14 1 1 8 6 spleen cells (x1 6 ) 16 % of live splenocytes 5 4 3 1 % of live splenocytes 8 6 4 b 1 1 c % of CD11c + splenocytes (closed shapes) 8 6 4 8 6 4 % ROSA + (open shapes) % floxed
Supp. Figure 1. a 14 1 1 8 6 spleen cells (x1 6 ) 16 % of live splenocytes 5 4 3 1 % of live splenocytes 8 6 4 b 1 1 c % of CD11c + splenocytes (closed shapes) 8 6 4 8 6 4 % ROSA + (open shapes) % floxed
M.Sc. III Semester Biotechnology End Semester Examination, 2013 Model Answer LBTM: 302 Advanced Immunology
 Code : AS-2246 M.Sc. III Semester Biotechnology End Semester Examination, 2013 Model Answer LBTM: 302 Advanced Immunology A. Select one correct option for each of the following questions:- 2X10=10 1. (b)
Code : AS-2246 M.Sc. III Semester Biotechnology End Semester Examination, 2013 Model Answer LBTM: 302 Advanced Immunology A. Select one correct option for each of the following questions:- 2X10=10 1. (b)
TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer
 AD Award Number: W8-XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke, Ph.D. CONTRACTING ORGANIZATION: Johns Hopkins
AD Award Number: W8-XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke, Ph.D. CONTRACTING ORGANIZATION: Johns Hopkins
Chapter 22: The Lymphatic System and Immunity
 Bio40C schedule Lecture Immune system Lab Quiz 2 this week; bring a scantron! Study guide on my website (see lab assignments) Extra credit Critical thinking questions at end of chapters 5 pts/chapter Due
Bio40C schedule Lecture Immune system Lab Quiz 2 this week; bring a scantron! Study guide on my website (see lab assignments) Extra credit Critical thinking questions at end of chapters 5 pts/chapter Due
Tumors arise from accumulated genetic mutations. Tumor Immunology (Cancer)
 Tumor Immunology (Cancer) Tumors arise from accumulated genetic mutations Robert Beatty MCB150 Mutations Usually have >6 mutations in both activation/growth factors and tumor suppressor genes. Types of
Tumor Immunology (Cancer) Tumors arise from accumulated genetic mutations Robert Beatty MCB150 Mutations Usually have >6 mutations in both activation/growth factors and tumor suppressor genes. Types of
LYMPHOCYTES & IMMUNOGLOBULINS. Dr Mere Kende, Lecturer SMHS
 LYMPHOCYTES & IMMUNOGLOBULINS Dr Mere Kende, Lecturer SMHS Immunity Immune- protection against dangers of non-self/invader eg organism 3 components of immune system 1 st line: skin/mucosa/cilia/hair/saliva/fatty
LYMPHOCYTES & IMMUNOGLOBULINS Dr Mere Kende, Lecturer SMHS Immunity Immune- protection against dangers of non-self/invader eg organism 3 components of immune system 1 st line: skin/mucosa/cilia/hair/saliva/fatty
Combined Rho-kinase inhibition and immunogenic cell death triggers and propagates immunity against cancer
 Supplementary Information Combined Rho-kinase inhibition and immunogenic cell death triggers and propagates immunity against cancer Gi-Hoon Nam, Eun-Jung Lee, Yoon Kyoung Kim, Yeonsun Hong, Yoonjeong Choi,
Supplementary Information Combined Rho-kinase inhibition and immunogenic cell death triggers and propagates immunity against cancer Gi-Hoon Nam, Eun-Jung Lee, Yoon Kyoung Kim, Yeonsun Hong, Yoonjeong Choi,
MATERIALS AND METHODS. Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All
 MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
Memory CD4 T Cells Enhance Primary CD8 T-Cell Responses
 INFECTION AND IMMUNITY, July 2007, p. 3556 3560 Vol. 75, No. 7 0019-9567/07/$08.00 0 doi:10.1128/iai.00086-07 Copyright 2007, American Society for Microbiology. All Rights Reserved. Memory CD4 T Cells
INFECTION AND IMMUNITY, July 2007, p. 3556 3560 Vol. 75, No. 7 0019-9567/07/$08.00 0 doi:10.1128/iai.00086-07 Copyright 2007, American Society for Microbiology. All Rights Reserved. Memory CD4 T Cells
Oncolytic Immunotherapy: A Local and Systemic Antitumor Approach
 Oncolytic Immunotherapy: A Local and Systemic Antitumor Approach Oncolytic immunotherapy Oncolytic immunotherapy the use of a genetically modified virus to attack tumors and induce a systemic immune response
Oncolytic Immunotherapy: A Local and Systemic Antitumor Approach Oncolytic immunotherapy Oncolytic immunotherapy the use of a genetically modified virus to attack tumors and induce a systemic immune response
RAISON D ETRE OF THE IMMUNE SYSTEM:
 RAISON D ETRE OF THE IMMUNE SYSTEM: To Distinguish Self from Non-Self Thereby Protecting Us From Our Hostile Environment. Innate Immunity Acquired Immunity Innate immunity: (Antigen nonspecific) defense
RAISON D ETRE OF THE IMMUNE SYSTEM: To Distinguish Self from Non-Self Thereby Protecting Us From Our Hostile Environment. Innate Immunity Acquired Immunity Innate immunity: (Antigen nonspecific) defense
Therapeutic PD L1 and LAG 3 blockade rapidly clears established blood stage Plasmodium infection
 Supplementary Information Therapeutic PD L1 and LAG 3 blockade rapidly clears established blood stage Plasmodium infection Noah S. Butler, Jacqueline Moebius, Lecia L. Pewe, Boubacar Traore, Ogobara K.
Supplementary Information Therapeutic PD L1 and LAG 3 blockade rapidly clears established blood stage Plasmodium infection Noah S. Butler, Jacqueline Moebius, Lecia L. Pewe, Boubacar Traore, Ogobara K.
Nature Immunology: doi: /ni Supplementary Figure 1. Gene expression profile of CD4 + T cells and CTL responses in Bcl6-deficient mice.
 Supplementary Figure 1 Gene expression profile of CD4 + T cells and CTL responses in Bcl6-deficient mice. (a) Gene expression profile in the resting CD4 + T cells were analyzed by an Affymetrix microarray
Supplementary Figure 1 Gene expression profile of CD4 + T cells and CTL responses in Bcl6-deficient mice. (a) Gene expression profile in the resting CD4 + T cells were analyzed by an Affymetrix microarray
Supplemental Figure 1
 Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
Trends in vaccinology
 Trends in vaccinology Mathieu Peeters, MD Joint Conference of European Human Pharmacological Societies and Joint Conference of European Human Pharmacological Societies and 20th Anniversary of AGAH March
Trends in vaccinology Mathieu Peeters, MD Joint Conference of European Human Pharmacological Societies and Joint Conference of European Human Pharmacological Societies and 20th Anniversary of AGAH March
Naive and memory CD8 T cell responses after antigen stimulation in vivo
 University of Iowa Iowa Research Online Theses and Dissertations Summer 2011 Naive and memory CD8 T cell responses after antigen stimulation in vivo Matthew David Martin University of Iowa Copyright 2011
University of Iowa Iowa Research Online Theses and Dissertations Summer 2011 Naive and memory CD8 T cell responses after antigen stimulation in vivo Matthew David Martin University of Iowa Copyright 2011
Cationic Liposome-DNA Complexes as Immunomodulators and Vaccine Adjuvants
 Cationic Liposome-DNA Complexes as Immunomodulators and Vaccine Adjuvants September 29, 28 Platform Technology Proprietary Cationic Lipid-DNA Complexes Cationic/Neutral Lipid + DNA = JVRS-1 JVRS-1 + Antigen
Cationic Liposome-DNA Complexes as Immunomodulators and Vaccine Adjuvants September 29, 28 Platform Technology Proprietary Cationic Lipid-DNA Complexes Cationic/Neutral Lipid + DNA = JVRS-1 JVRS-1 + Antigen
Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell?
 Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Determinants of Immunogenicity and Tolerance. Abul K. Abbas, MD Department of Pathology University of California San Francisco
 Determinants of Immunogenicity and Tolerance Abul K. Abbas, MD Department of Pathology University of California San Francisco EIP Symposium Feb 2016 Why do some people respond to therapeutic proteins?
Determinants of Immunogenicity and Tolerance Abul K. Abbas, MD Department of Pathology University of California San Francisco EIP Symposium Feb 2016 Why do some people respond to therapeutic proteins?
Supplementary Figures
 Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Supplementary Figure 1. Efficient DC depletion in CD11c.DOG transgenic mice
 Supplementary Figure 1. Efficient DC depletion in CD11c.DOG transgenic mice (a) CD11c.DOG transgenic mice (tg) were treated with 8 ng/g body weight (b.w.) diphtheria toxin (DT) i.p. on day -1 and every
Supplementary Figure 1. Efficient DC depletion in CD11c.DOG transgenic mice (a) CD11c.DOG transgenic mice (tg) were treated with 8 ng/g body weight (b.w.) diphtheria toxin (DT) i.p. on day -1 and every
The humoral immune responses to IBV proteins.
 The humoral immune responses to IBV proteins. E. Dan Heller and Rosa Meir The Hebrew University of Jerusalem, Israel COST FA1207 meeting WG2 + WG3, Budapest, Jan. 2015 1 IBV encodes four major structural
The humoral immune responses to IBV proteins. E. Dan Heller and Rosa Meir The Hebrew University of Jerusalem, Israel COST FA1207 meeting WG2 + WG3, Budapest, Jan. 2015 1 IBV encodes four major structural
Measuring Dendritic Cells
 Measuring Dendritic Cells A.D. Donnenberg, V.S. Donnenberg UNIVERSITY of PITTSBURGH CANCER INSTITUTE CCS Longbeach 10_04 Measuring DC Rare event detection The basics of DC measurement Applications Cancer
Measuring Dendritic Cells A.D. Donnenberg, V.S. Donnenberg UNIVERSITY of PITTSBURGH CANCER INSTITUTE CCS Longbeach 10_04 Measuring DC Rare event detection The basics of DC measurement Applications Cancer
COURSE: Medical Microbiology, MBIM 650/720 - Fall TOPIC: Antigen Processing, MHC Restriction, & Role of Thymus Lecture 12
 COURSE: Medical Microbiology, MBIM 650/720 - Fall 2008 TOPIC: Antigen Processing, MHC Restriction, & Role of Thymus Lecture 12 FACULTY: Dr. Mayer Office: Bldg. #1, Rm B32 Phone: 733-3281 Email: MAYER@MED.SC.EDU
COURSE: Medical Microbiology, MBIM 650/720 - Fall 2008 TOPIC: Antigen Processing, MHC Restriction, & Role of Thymus Lecture 12 FACULTY: Dr. Mayer Office: Bldg. #1, Rm B32 Phone: 733-3281 Email: MAYER@MED.SC.EDU
Adaptive immune responses: T cell-mediated immunity
 MICR2209 Adaptive immune responses: T cell-mediated immunity Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will discuss the T-cell mediated immune response, how it is activated,
MICR2209 Adaptive immune responses: T cell-mediated immunity Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will discuss the T-cell mediated immune response, how it is activated,
Lineage relationship and protective immunity of memory CD8 T cell subsets
 23 Nature Publishing Group http://www.nature.com/natureimmunology Lineage relationship and protective immunity of memory CD8 T cell subsets E. John Wherry 1 *,Volker Teichgräber 1 *,Todd C. Becker 1,David
23 Nature Publishing Group http://www.nature.com/natureimmunology Lineage relationship and protective immunity of memory CD8 T cell subsets E. John Wherry 1 *,Volker Teichgräber 1 *,Todd C. Becker 1,David
Supplemental Table I.
 Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
Supplementary Figure 1 Protease allergens induce IgE and IgG1 production. (a-c)
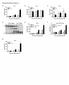 1 Supplementary Figure 1 Protease allergens induce IgE and IgG1 production. (a-c) Serum IgG1 (a), IgM (b) and IgG2 (c) concentrations in response to papain immediately before primary immunization (day
1 Supplementary Figure 1 Protease allergens induce IgE and IgG1 production. (a-c) Serum IgG1 (a), IgM (b) and IgG2 (c) concentrations in response to papain immediately before primary immunization (day
W/T Itgam -/- F4/80 CD115. F4/80 hi CD115 + F4/80 + CD115 +
 F4/8 % in the peritoneal lavage 6 4 2 p=.15 n.s p=.76 CD115 F4/8 hi CD115 + F4/8 + CD115 + F4/8 hi CD115 + F4/8 + CD115 + MHCII MHCII Supplementary Figure S1. CD11b deficiency affects the cellular responses
F4/8 % in the peritoneal lavage 6 4 2 p=.15 n.s p=.76 CD115 F4/8 hi CD115 + F4/8 + CD115 + F4/8 hi CD115 + F4/8 + CD115 + MHCII MHCII Supplementary Figure S1. CD11b deficiency affects the cellular responses
Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to
 Class II tetramer staining Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to PE were combined with dominant HIV epitopes (DRB1*0101-DRFYKTLRAEQASQEV, DRB1*0301- PEKEVLVWKFDSRLAFHH,
Class II tetramer staining Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to PE were combined with dominant HIV epitopes (DRB1*0101-DRFYKTLRAEQASQEV, DRB1*0301- PEKEVLVWKFDSRLAFHH,
Supplementary Figure 1. Example of gating strategy
 Supplementary Figure 1. Example of gating strategy Legend Supplementary Figure 1: First, gating is performed to include only single cells (singlets) (A) and CD3+ cells (B). After gating on the lymphocyte
Supplementary Figure 1. Example of gating strategy Legend Supplementary Figure 1: First, gating is performed to include only single cells (singlets) (A) and CD3+ cells (B). After gating on the lymphocyte
General information. Cell mediated immunity. 455 LSA, Tuesday 11 to noon. Anytime after class.
 General information Cell mediated immunity 455 LSA, Tuesday 11 to noon Anytime after class T-cell precursors Thymus Naive T-cells (CD8 or CD4) email: lcoscoy@berkeley.edu edu Use MCB150 as subject line
General information Cell mediated immunity 455 LSA, Tuesday 11 to noon Anytime after class T-cell precursors Thymus Naive T-cells (CD8 or CD4) email: lcoscoy@berkeley.edu edu Use MCB150 as subject line
The Adaptive Immune Responses
 The Adaptive Immune Responses The two arms of the immune responses are; 1) the cell mediated, and 2) the humoral responses. In this chapter we will discuss the two responses in detail and we will start
The Adaptive Immune Responses The two arms of the immune responses are; 1) the cell mediated, and 2) the humoral responses. In this chapter we will discuss the two responses in detail and we will start
Immune response. This overview figure summarizes simply how our body responds to foreign molecules that enter to it.
 Immune response This overview figure summarizes simply how our body responds to foreign molecules that enter to it. It s highly recommended to watch Dr Najeeb s lecture that s titled T Helper cells and
Immune response This overview figure summarizes simply how our body responds to foreign molecules that enter to it. It s highly recommended to watch Dr Najeeb s lecture that s titled T Helper cells and
1. The scavenger receptor, CD36, functions as a coreceptor for which TLR? a. TLR ½ b. TLR 3 c. TLR 4 d. TLR 2/6
 Allergy and Immunology Review Corner: Cellular and Molecular Immunology, 8th Edition By Abul K. Abbas, MBBS, Andrew H. H. Lichtman, MD, PhD and Shiv Pillai, MBBS, PhD. Chapter 4 (pages 62-74): Innate Immunity
Allergy and Immunology Review Corner: Cellular and Molecular Immunology, 8th Edition By Abul K. Abbas, MBBS, Andrew H. H. Lichtman, MD, PhD and Shiv Pillai, MBBS, PhD. Chapter 4 (pages 62-74): Innate Immunity
Immunological alterations in mice irradiated with low doses
 Immunological alterations in mice irradiated with low doses "Frédéric Joliot-Curie" National Research Institute for Radiobiology and Radiohygiene, Budapest, Hungary The structure of the immune system INNATE
Immunological alterations in mice irradiated with low doses "Frédéric Joliot-Curie" National Research Institute for Radiobiology and Radiohygiene, Budapest, Hungary The structure of the immune system INNATE
IOM Immunization Safety Review 11/12/2001. Immunological Competition and the Infant Immune Response to Vaccines
 IOM Immunization Safety Review 11/12/2001 Immunological Competition and the Infant Immune Response to Vaccines Richard Insel University of Rochester Goals Neonatal and Infant Immune System Broad Effects
IOM Immunization Safety Review 11/12/2001 Immunological Competition and the Infant Immune Response to Vaccines Richard Insel University of Rochester Goals Neonatal and Infant Immune System Broad Effects
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.
 Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Figure S1. Generation of inducible PTEN deficient mice and the BMMCs (A) B6.129 Pten loxp/loxp mice were mated with B6.129-Gt(ROSA)26Sor tm1(cre/ert2)tyj /J mice. To induce deletion of the Pten locus,
Supplementary Information:
 Supplementary Information: Follicular regulatory T cells with Bcl6 expression suppress germinal center reactions by Yeonseok Chung, Shinya Tanaka, Fuliang Chu, Roza Nurieva, Gustavo J. Martinez, Seema
Supplementary Information: Follicular regulatory T cells with Bcl6 expression suppress germinal center reactions by Yeonseok Chung, Shinya Tanaka, Fuliang Chu, Roza Nurieva, Gustavo J. Martinez, Seema
Third line of Defense
 Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
NKTR-255: Accessing The Immunotherapeutic Potential Of IL-15 for NK Cell Therapies
 NKTR-255: Accessing The Immunotherapeutic Potential Of IL-15 for NK Cell Therapies Saul Kivimäe Senior Scientist, Research Biology Nektar Therapeutics NK Cell-Based Cancer Immunotherapy, September 26-27,
NKTR-255: Accessing The Immunotherapeutic Potential Of IL-15 for NK Cell Therapies Saul Kivimäe Senior Scientist, Research Biology Nektar Therapeutics NK Cell-Based Cancer Immunotherapy, September 26-27,
A Multicistronic DNA Vaccine Induces Significant Protection against Tuberculosis in Mice and Offers Flexibility in the Expressed Antigen Repertoire.
 Company LOGO A Multicistronic DNA Vaccine Induces Significant Protection against Tuberculosis in Mice and Offers Flexibility in the Expressed Antigen Repertoire. Fayaz Ahmad Mir, Stefan H. E. Kaufmann,
Company LOGO A Multicistronic DNA Vaccine Induces Significant Protection against Tuberculosis in Mice and Offers Flexibility in the Expressed Antigen Repertoire. Fayaz Ahmad Mir, Stefan H. E. Kaufmann,
activation with anti-cd3/cd28 beads and 3d following transduction. Supplemental Figure 2 shows
 Supplemental Data Supplemental Figure 1 compares CXCR4 expression in untreated CD8 + T cells, following activation with anti-cd3/cd28 beads and 3d following transduction. Supplemental Figure 2 shows the
Supplemental Data Supplemental Figure 1 compares CXCR4 expression in untreated CD8 + T cells, following activation with anti-cd3/cd28 beads and 3d following transduction. Supplemental Figure 2 shows the
TITLE: Second-Generation Therapeutic DNA Lymphoma Vaccines. CONTRACTING ORGANIZATION: University of Texas MD Anderson Cancer Center Houston, TX 77030
 AD Award Number: W81XWH-07-1-0345 TITLE: Second-Generation Therapeutic DNA Lymphoma Vaccines PRINCIPAL INVESTIGATOR: Larry Kwak, M.D., Ph.D. CONTRACTING ORGANIZATION: University of Texas MD Anderson Cancer
AD Award Number: W81XWH-07-1-0345 TITLE: Second-Generation Therapeutic DNA Lymphoma Vaccines PRINCIPAL INVESTIGATOR: Larry Kwak, M.D., Ph.D. CONTRACTING ORGANIZATION: University of Texas MD Anderson Cancer
Nature Medicine: doi: /nm.3922
 Title: Glucocorticoid-induced tumor necrosis factor receptor-related protein co-stimulation facilitates tumor regression by inducing IL-9-producing helper T cells Authors: Il-Kyu Kim, Byung-Seok Kim, Choong-Hyun
Title: Glucocorticoid-induced tumor necrosis factor receptor-related protein co-stimulation facilitates tumor regression by inducing IL-9-producing helper T cells Authors: Il-Kyu Kim, Byung-Seok Kim, Choong-Hyun
Adenovirus engineered human dendritic cell vaccine induces natural killer cell chemotaxis
 Adenovirus engineered human dendritic cell vaccine induces natural killer cell chemotaxis via CXCL8/IL 8 and CXCL10/IP 10 chemokines Lazar Vujanović, Ph.D. Research Instructor P.I. Lisa H. Butterfield,
Adenovirus engineered human dendritic cell vaccine induces natural killer cell chemotaxis via CXCL8/IL 8 and CXCL10/IP 10 chemokines Lazar Vujanović, Ph.D. Research Instructor P.I. Lisa H. Butterfield,
ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY
 ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY The recognition of specific antigen by naïve T cell induces its own activation and effector phases. T helper cells recognize peptide antigens through
ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY The recognition of specific antigen by naïve T cell induces its own activation and effector phases. T helper cells recognize peptide antigens through
IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia
 Supplementary Figures IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia Yaming Wang, Kristy J. Szretter, William Vermi, Susan Gilfillan, Cristina
Supplementary Figures IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia Yaming Wang, Kristy J. Szretter, William Vermi, Susan Gilfillan, Cristina
D CD8 T cell number (x10 6 )
 IFNγ Supplemental Figure 1. CD T cell number (x1 6 ) 18 15 1 9 6 3 CD CD T cells CD6L C CD5 CD T cells CD6L D CD8 T cell number (x1 6 ) 1 8 6 E CD CD8 T cells CD6L F Log(1)CFU/g Feces 1 8 6 p
IFNγ Supplemental Figure 1. CD T cell number (x1 6 ) 18 15 1 9 6 3 CD CD T cells CD6L C CD5 CD T cells CD6L D CD8 T cell number (x1 6 ) 1 8 6 E CD CD8 T cells CD6L F Log(1)CFU/g Feces 1 8 6 p
Immunodomination during Peripheral Vaccinia Virus Infection
 Immunodomination during Peripheral Vaccinia Virus Infection Leon C. W. Lin, Inge E. A. Flesch, David C. Tscharke* Division of Biomedical Science and Biochemistry, Research School of Biology, The Australian
Immunodomination during Peripheral Vaccinia Virus Infection Leon C. W. Lin, Inge E. A. Flesch, David C. Tscharke* Division of Biomedical Science and Biochemistry, Research School of Biology, The Australian
Supplementary Figure 1. NAFL enhanced immunity of other vaccines (a) An over-the-counter, hand-held non-ablative fractional laser (NAFL).
 Supplementary Figure 1. NAFL enhanced immunity of other vaccines (a) An over-the-counter, hand-held non-ablative fractional laser (NAFL). (b) Depiction of a MTZ array generated by NAFL. (c-e) IgG production
Supplementary Figure 1. NAFL enhanced immunity of other vaccines (a) An over-the-counter, hand-held non-ablative fractional laser (NAFL). (b) Depiction of a MTZ array generated by NAFL. (c-e) IgG production
Any correspondence concerning this service should be sent to The Strathprints Administrator:
 Millington, Owain R. and Di Lorenzo, Caterina and Phillips, R. Stephen and Garside, Paul and Brewer, James M. (6) Suppression of adaptive immunity to heterologous antigens during Plasmodium infection through
Millington, Owain R. and Di Lorenzo, Caterina and Phillips, R. Stephen and Garside, Paul and Brewer, James M. (6) Suppression of adaptive immunity to heterologous antigens during Plasmodium infection through
Advances in Cancer Immunotherapy
 Advances in Cancer Immunotherapy Immunology 101 for the Non-Immunologist Arnold H. Zea, PhD azea@lsuhsc.edu Disclosures No relevant financial relationships to disclose This presentation does not contain
Advances in Cancer Immunotherapy Immunology 101 for the Non-Immunologist Arnold H. Zea, PhD azea@lsuhsc.edu Disclosures No relevant financial relationships to disclose This presentation does not contain
TCR, MHC and coreceptors
 Cooperation In Immune Responses Antigen processing how peptides get into MHC Antigen processing involves the intracellular proteolytic generation of MHC binding proteins Protein antigens may be processed
Cooperation In Immune Responses Antigen processing how peptides get into MHC Antigen processing involves the intracellular proteolytic generation of MHC binding proteins Protein antigens may be processed
Shiv Pillai Ragon Institute, Massachusetts General Hospital Harvard Medical School
 CTLs, Natural Killers and NKTs 1 Shiv Pillai Ragon Institute, Massachusetts General Hospital Harvard Medical School CTL inducing tumor apoptosis 3 Lecture outline CD8 + Cytotoxic T lymphocytes (CTL) Activation/differentiation
CTLs, Natural Killers and NKTs 1 Shiv Pillai Ragon Institute, Massachusetts General Hospital Harvard Medical School CTL inducing tumor apoptosis 3 Lecture outline CD8 + Cytotoxic T lymphocytes (CTL) Activation/differentiation
Chapter 17B: Adaptive Immunity Part II
 Chapter 17B: Adaptive Immunity Part II 1. Cell-Mediated Immune Response 2. Humoral Immune Response 3. Antibodies 1. The Cell-Mediated Immune Response Basic Steps of Cell-Mediated IR 1 2a CD4 + MHC cl.
Chapter 17B: Adaptive Immunity Part II 1. Cell-Mediated Immune Response 2. Humoral Immune Response 3. Antibodies 1. The Cell-Mediated Immune Response Basic Steps of Cell-Mediated IR 1 2a CD4 + MHC cl.
Supplementary Data Table of Contents:
 Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/1175194/dc1 Supporting Online Material for A Vital Role for Interleukin-21 in the Control of a Chronic Viral Infection John S. Yi, Ming Du, Allan J. Zajac* *To whom
www.sciencemag.org/cgi/content/full/1175194/dc1 Supporting Online Material for A Vital Role for Interleukin-21 in the Control of a Chronic Viral Infection John S. Yi, Ming Du, Allan J. Zajac* *To whom
T cell memory Friday, February 10, 17
 T cell memory 2-10-2017 expansion contraction memory recall EFFECTORS 10 6 pathogen load secondary challenge MEMORY 10 2 NAIVE 2 7-8 30 >1 year days post-infection In mouse, ~100 naïve, CD8s become ~3x10
T cell memory 2-10-2017 expansion contraction memory recall EFFECTORS 10 6 pathogen load secondary challenge MEMORY 10 2 NAIVE 2 7-8 30 >1 year days post-infection In mouse, ~100 naïve, CD8s become ~3x10
There are 2 major lines of defense: Non-specific (Innate Immunity) and. Specific. (Adaptive Immunity) Photo of macrophage cell
 There are 2 major lines of defense: Non-specific (Innate Immunity) and Specific (Adaptive Immunity) Photo of macrophage cell Development of the Immune System ery pl neu mφ nk CD8 + CTL CD4 + thy TH1 mye
There are 2 major lines of defense: Non-specific (Innate Immunity) and Specific (Adaptive Immunity) Photo of macrophage cell Development of the Immune System ery pl neu mφ nk CD8 + CTL CD4 + thy TH1 mye
All animals have innate immunity, a defense active immediately upon infection Vertebrates also have adaptive immunity
 1 2 3 4 5 6 7 8 9 The Immune System All animals have innate immunity, a defense active immediately upon infection Vertebrates also have adaptive immunity Figure 43.2 In innate immunity, recognition and
1 2 3 4 5 6 7 8 9 The Immune System All animals have innate immunity, a defense active immediately upon infection Vertebrates also have adaptive immunity Figure 43.2 In innate immunity, recognition and
SUPPLEMENTARY INFORMATION
 doi: 1.138/nature775 4 O.D. (595-655) 3 1 -ζ no antibody isotype ctrl Plated Soluble 1F6 397 7H11 Supplementary Figure 1 Soluble and plated anti- Abs induce -! signalling. B3Z cells stably expressing!
doi: 1.138/nature775 4 O.D. (595-655) 3 1 -ζ no antibody isotype ctrl Plated Soluble 1F6 397 7H11 Supplementary Figure 1 Soluble and plated anti- Abs induce -! signalling. B3Z cells stably expressing!
Cell Mediated Immunity CELL MEDIATED IMMUNITY. Basic Elements of Cell Mediated Immunity (CMI) Antibody-dependent cell-mediated cytotoxicity (ADCC)
 Chapter 16 CELL MEDIATED IMMUNITY Cell Mediated Immunity Also known as Cellular Immunity or CMI The effector phase T cells Specificity for immune recognition reactions TH provide cytokines CTLs do the
Chapter 16 CELL MEDIATED IMMUNITY Cell Mediated Immunity Also known as Cellular Immunity or CMI The effector phase T cells Specificity for immune recognition reactions TH provide cytokines CTLs do the
Hepatitis virus immunity. Mar 9, 2005 Rehermann and Nascimbeni review Crispe review
 Hepatitis virus immunity Mar 9, 2005 Rehermann and Nascimbeni review Crispe review HBV & HCV infection outcomes Both viruses cause immune-mediated active and chronic hepatitis HBV Vertical transmission
Hepatitis virus immunity Mar 9, 2005 Rehermann and Nascimbeni review Crispe review HBV & HCV infection outcomes Both viruses cause immune-mediated active and chronic hepatitis HBV Vertical transmission
Eosinophils are required. for the maintenance of plasma cells in the bone marrow
 Eosinophils are required for the maintenance of plasma cells in the bone marrow Van Trung Chu, Anja Fröhlich, Gudrun Steinhauser, Tobias Scheel, Toralf Roch, Simon Fillatreau, James J. Lee, Max Löhning
Eosinophils are required for the maintenance of plasma cells in the bone marrow Van Trung Chu, Anja Fröhlich, Gudrun Steinhauser, Tobias Scheel, Toralf Roch, Simon Fillatreau, James J. Lee, Max Löhning
Recombinant adeno-associated virus vectors induce functionally impaired transgene product specific CD8 + T cells in mice
 Recombinant adeno-associated virus vectors induce functionally impaired transgene product specific CD8 + T cells in mice Shih-Wen Lin,, Marcio O. Lasaro, Hildegund C.J. Ertl J Clin Invest. 2007;117(12):3958-3970.
Recombinant adeno-associated virus vectors induce functionally impaired transgene product specific CD8 + T cells in mice Shih-Wen Lin,, Marcio O. Lasaro, Hildegund C.J. Ertl J Clin Invest. 2007;117(12):3958-3970.
Kinetics of tumor-specific T-cell response development after active immunization in patients with HER-2/neu overexpressing cancers
 Clinical Immunology (2007) 125, 275 280 available at www.sciencedirect.com www.elsevier.com/locate/yclim Kinetics of tumor-specific T-cell response development after active immunization in patients with
Clinical Immunology (2007) 125, 275 280 available at www.sciencedirect.com www.elsevier.com/locate/yclim Kinetics of tumor-specific T-cell response development after active immunization in patients with
Effector Mechanisms of Cell-Mediated Immunity
 Effector Mechanisms of Cell-Mediated Immunity Dr. Julia Rempel Section of Hepatology 789-3825 jdrempel@cc.umanitoba.ca 804D JBRC Topics: I. Types of Cell-Mediated Immunity II. Migration of Effector T Lymphocytes
Effector Mechanisms of Cell-Mediated Immunity Dr. Julia Rempel Section of Hepatology 789-3825 jdrempel@cc.umanitoba.ca 804D JBRC Topics: I. Types of Cell-Mediated Immunity II. Migration of Effector T Lymphocytes
Immunology Lecture 4. Clinical Relevance of the Immune System
 Immunology Lecture 4 The Well Patient: How innate and adaptive immune responses maintain health - 13, pg 169-181, 191-195. Immune Deficiency - 15 Autoimmunity - 16 Transplantation - 17, pg 260-270 Tumor
Immunology Lecture 4 The Well Patient: How innate and adaptive immune responses maintain health - 13, pg 169-181, 191-195. Immune Deficiency - 15 Autoimmunity - 16 Transplantation - 17, pg 260-270 Tumor
TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer
 AD Award Number: W8XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke Ph.D. CONTRACTING ORGANIZATION: Johns Hopkins
AD Award Number: W8XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke Ph.D. CONTRACTING ORGANIZATION: Johns Hopkins
Nature Medicine doi: /nm.3957
 Supplementary Fig. 1. p38 alternative activation, IL-21 expression, and T helper cell transcription factors in PDAC tissue. (a) Tissue microarrays of pancreatic tissue from 192 patients with pancreatic
Supplementary Fig. 1. p38 alternative activation, IL-21 expression, and T helper cell transcription factors in PDAC tissue. (a) Tissue microarrays of pancreatic tissue from 192 patients with pancreatic
Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features
 Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features Loretta Gammaitoni, Lidia Giraudo, Valeria Leuci, et al. Clin Cancer Res
Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features Loretta Gammaitoni, Lidia Giraudo, Valeria Leuci, et al. Clin Cancer Res
TEPZZ 7ZZ7Z8A T EP A2 (19) (11) EP A2 (12) EUROPEAN PATENT APPLICATION. (51) Int Cl.: C12N 5/0784 ( )
 (19) TEPZZ 7ZZ7Z8A T (11) EP 2 700 708 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 26.02.2014 Bulletin 2014/09 (51) Int Cl.: C12N 5/0784 (2010.01) (21) Application number: 13181345.3
(19) TEPZZ 7ZZ7Z8A T (11) EP 2 700 708 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: 26.02.2014 Bulletin 2014/09 (51) Int Cl.: C12N 5/0784 (2010.01) (21) Application number: 13181345.3
B6/COLODR/SPL/11C/83/LAP/#2.006 B6/COLODR/SPL/11C/86/LAP/#2.016 CD11C B6/COLODR/SPL/11C/80/LAP/#2.011 CD11C
 CD3-specific antibody-induced immune tolerance and suppression of autoimmune encephalomyelitis involves TGF-β production through phagocytes digesting apoptotic T cells Sylvain Perruche 1,3, Pin Zhang 1,
CD3-specific antibody-induced immune tolerance and suppression of autoimmune encephalomyelitis involves TGF-β production through phagocytes digesting apoptotic T cells Sylvain Perruche 1,3, Pin Zhang 1,
NOTES: CH 43, part 1 The Immune System - Nonspecific & Specific Defenses ( )
 NOTES: CH 43, part 1 The Immune System - Nonspecific & Specific Defenses (43.1-43.2) The lymphatic system is closely associated with the cardiovascular system. LYMPHATIC PATHWAYS Lymphatic capillaries
NOTES: CH 43, part 1 The Immune System - Nonspecific & Specific Defenses (43.1-43.2) The lymphatic system is closely associated with the cardiovascular system. LYMPHATIC PATHWAYS Lymphatic capillaries
PBMC from each patient were suspended in AIM V medium (Invitrogen) with 5% human
 Anti-CD19-CAR transduced T-cell preparation PBMC from each patient were suspended in AIM V medium (Invitrogen) with 5% human AB serum (Gemini) and 300 international units/ml IL-2 (Novartis). T cell proliferation
Anti-CD19-CAR transduced T-cell preparation PBMC from each patient were suspended in AIM V medium (Invitrogen) with 5% human AB serum (Gemini) and 300 international units/ml IL-2 (Novartis). T cell proliferation
Medical Virology Immunology. Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University
 Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
