MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection
|
|
|
- Emory Lawson
- 6 years ago
- Views:
Transcription
1 MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection Anda Meierovics, Wei-Jen Chua Yankelevich, and Siobhán C. Cowley 1 Division of Bacterial, Parasitic, and Allergenic Products, Laboratory of Mycobacterial Diseases and Cellular Immunology, Center for Biologics Evaluation and Research, US Food and Drug Administration, Rockville, MD Edited by Rafi Ahmed, Emory University, Atlanta, GA, and approved July 3, 2013 (received for review February 12, 2013) Mucosa-associated invariant T (MAIT) cells are innate T cells that express an invariant T-cell receptor α-chain restricted by the nonclassical MHC class I molecule MHC-related protein 1 (MR1). A recent discovery that MR1 presents vitamin B metabolites, presumably from pathogenic and/or commensal bacteria, distinguishes MAIT cells from peptide- or lipid-recognizing αβ T cells in the immune system. MAIT cells are activated by a wide variety of bacterial strains in vitro, but their role in defense against infectious assaults in vivo remains largely unknown. To investigate how MAIT cells contribute to mucosal immunity in vivo, we used a murine model of pulmonary infection by using the live vaccine strain (LVS) of Francisella tularensis. In the early acute phase of infection, MAIT cells expanded robustly in the lungs, where they preferentially accumulated after reaching their peak expansion in the late phase of infection. Throughout the course of infection, MAIT cells produced the critical cytokines IFN-γ, TNF-α, and IL-17A. Mechanistic studies showed that MAIT cells required both MR1 and IL kda subunit (IL-12p40) signals from infected antigen presenting cells to control F. tularensis LVS intracellular growth. Importantly, pulmonary F. tularensis LVS infection of MR1-deficient (MR1 / ) mice, which lack MAIT cells, revealed defects in early mucosal cytokine production, timely recruitment of IFN-γ producing CD4 + and CD8 + T cells to the infected lungs, and control of pulmonary F. tularensis LVS growth. This study provides in vivo evidence demonstrating that MAIT cells are an important T-cell subset with activities that influence the innate and adaptive phases of mucosal immunity. tularemia respiratory infection For intracellular bacteria, T-cell mediated immune responses are paramount for control of primary infection and adaptive secondary responses. However, conventional T cells must rely upon the innate immune system to initially detect a pathogen, requiring time for activation and expansion before they can control pathogen growth. This lag in the generation of adaptive immune responses is a critical time for the pathogen and the host. Several unconventional T-cell subsets exist that can act during this critical lag time. These populations include certain types of γδ T cells, invariant natural killer (NK) T (inkt) cells, and M3- restricted T cells. Collectively termed innate T cells, they recognize molecular patterns and have the capacity to immediately express effector functions both features that allow them to mount responses earlier than conventional T cells (1). Correspondingly, both M3-restricted and inkt cells exhibit extremely rapid response kinetics during infections in vivo, peaking in numbers and elaborating effector functions before conventional T-cell responses (2 5). Mucosa-associated invariant T (MAIT) cells are a recently identified T-cell subset that also belongs to this class of innate T cells. MAIT cells express an evolutionarily conserved T-cell receptor (TCR) α-chain that is the product of a canonical Vα19- Jα33 rearrangement in mice and Vα7.2-Jα33 in humans. Biochemical and genetic studies have shown that MHC-related protein 1 (MR1) presents antigen for MAIT cell activation, and is necessary for their in vivo development (6 12). The strong evolutionary conservation of MR1 across mammalian species indicates that MAIT cells likely have an important physiological role in host immune responses (13, 14). Interestingly, MR1 possesses a unique antigen-binding cleft that presents vitamin B metabolites (15, 16). Because vitamin B biosynthesis pathways are unique to bacteria and yeast, MAIT cells sense infection through the recognition of a novel class of conserved microbial ligands. Several in vitro studies have demonstrated that MAIT cells have the capacity to respond to a wide variety of pathogens (12, 17 19), although studies examining the in vivo role of MAIT cells in microbial defense have thus far been limited (17, 19, 20). The rapid overgrowth of Klebsiella pneumoniae in MR1-deficient mice, which lack murine MAIT cells, suggests MAIT cells may have an early innate role in bacterial defense (20). In humans, MAIT cells were present in the lungs of patients infected with the pulmonary pathogen Mycobacterium tuberculosis, whereas MR1-deficient mice exhibited transiently elevated lung bacterial burdens following aerosol Mycobacterium bovis bacillus Calmette Guérin infection (17 19). These studies indicated that MAIT cells are likely important contributors to defense against respiratory infections. Despite a model favoring a critical, evolutionarily conserved role for MAIT cells in microbial immunity, critical questions remain regarding their activities and influence on the outcome of in vivo mucosal infections (21). Indeed, although MAIT cells are proposed to act as innate T cells with the potential to bridge innate and adaptive immune immunity, their in vivo response kinetics and role in facilitating adaptive immune responses are unknown. To address these questions in vivo, we used a murine model of pulmonary infection that used Francisella tularensis live vaccine strain (LVS). F. tularensis is a Gram-negative, facultative Significance Mucosa-associated invariant T (MAIT) cells are an innate T-cell subset uniquely activated by microbe-derived vitamin B metabolites. Thus far, little is known about MAIT cell contributions to defense against pathogens in vivo. Using murine respiratory tularemia as a model of mucosal infection, we show that MAIT cells are required for the prompt initiation of immune responses in the lungs. Surprisingly, MAIT cells also actively contributed to immunity throughout the later stages of infection. Thus, MAIT cells are a unique T-cell subset with wide-ranging activities that may be harnessed to improve future vaccines and adjuvants used at mucosal surfaces. Author contributions: S.C.C. designed research; A.M., W.-J.C.Y., and S.C.C. performed research; A.M., W.-J.C.Y., and S.C.C. analyzed data; and A.M., W.-J.C.Y., and S.C.C. wrote the paper. The authors declare no conflict of interest. This article is a PNAS Direct Submission. 1 To whom correspondence should be addressed. siobhan.cowley@fda.hhs.gov. This article contains supporting information online at /pnas /-/DCSupplemental. IMMUNOLOGY PNAS PLUS PNAS Published online July 29, 2013 E3119 E3128
2 intracellular bacterium and the causative agent of tularemia. Classified as a Category A bioterrorism agent, inhalation of virulent strains of F. tularensis rapidly progresses to acute lethal disease in as many as 60% of untreated patients (22). The F.tularensis LVS has shown potential as a protective vaccine in animal studies, and is currently an investigational product in the United States (23). Intranasal (i.n.) infection of mice with sublethal doses of F. tularensis LVS offers a convenient model to perform a detailed study of mucosal immune responses. Optimal defense against primary LVS pulmonary infection requires conventional CD4 + and/or CD8 + T cells for clearance of the bacterium, although a distinct lag time exists before these adaptive immune responses are operational. This lag in the generation of adaptive immunity represents a critical window when innate T cells, including MAIT cells, are proposed to bridge immune responses. Here we took advantage of the murine model of sublethal F. tularensis LVS pulmonary infection to investigate the kinetics and function of murine MAIT cell responses in the lungs in vivo. In the early acute phase of infection, MAIT cells expanded robustly in the lungs. After reaching their peak expansion in the late phase of infection, MAIT cells remained a prominent presence in the lungs. MAIT cells contributed to mucosal immunity by actively producing antibacterial cytokines throughout infection. The potency of MAIT cell activities was revealed by their ability to provide long-term control of an in vivo sublethal LVS pulmonary infection in the absence of adaptive CD4 + and CD8 + T cells. Moreover, comparative studies between WT C57BL/6 and MR1-deficient (MR1 / ) mice revealed a previously unrecognized role for MAIT cells in facilitating the early production of proinflammatory cytokines, and the timely recruitment of activated, IFN-γ producing CD4 + and CD8 + T cells to the lungs. Overall, this report provides evidence that MAIT cells act early to initiate immunity, but, unlike our current understanding of other innate TCRβ + T cells, MAIT cells actively contribute to pulmonary immune responses that extend into the later phases of infection. Results MAIT Cells Are a Large CD4 CD8 T-Cell Population in the Lungs During Primary Murine F. tularensis LVS i.n. Infection. Despite abundant in vitro evidence of MAIT cell activation by microbial infections, little is known about how MAIT cells respond to infections in vivo. For this reason, we sought to identify a murine mucosal infection model in which in vivo MAIT cell responses could be examined in detail. As the majority of murine MAIT cells are CD4 CD8 double negative (DN) T cells (10), and previous evidence has revealed a robust population of DN T cells in the lungs of WT C57BL/6 mice during primary sublethal LVS i.n. infection (24), we first examined this lung DN T cell population for the presence of MAIT cells. Currently there are no definitive antibodies that distinguish murine MAIT cells from other T cells, but MAIT cells can be identified by their expression of the Vα19-Jα33 invariant TCR α-chain. Therefore, we used a two-step approach that combined (i) FACS to purify the TCRβ + DN T cell population from the lungs of WT C57BL/6 mice during sublethal LVS i.n. infection and (ii) quantitative real time (RT)-PCR (qpcr) to assess the expression of MAIT cell Vα19-Jα33 invariant α-chain transcripts in the highly purified lung TCRβ + DN T cell population. Results showed that, in the early acute stage (day 7) of i.n. infection of LVS, TCRβ + DN T cells were a relatively small lung population (Fig. 1A), but Vα19-Jα33 transcripts were readily detected (Fig. 1B). Interestingly, in the late clearance phase (day 14) of infection, the lung TCRβ + DN T cell population had expanded significantly (Fig. 1A), and was highly enriched for Vα19-Jα33 transcripts (Fig. 1B). Because MAIT cells are known to preferentially associate with TCR β-chains containing Vβ6 or Vβ8 Fig. 1. MAIT cells expand in the lungs in a murine model of pulmonary F. tularensis LVS infection. WT C57BL/6 mice were infected i.n. with a sublethal dose of cfu LVS, and (A) total lung cells were harvested at the indicated time points after infection to evaluate the presence of TCRβ + DN T cells (circled). (Exclusion channel: anti-cd4, CD8, B220, γδtcr Abs.) Data are representative of four independent experiments. (B) TCRβ + DN T cells were purified from the lungs of LVS-infected mice at the indicated time points by FACS and evaluated for expression of MAIT cell invariant TCR Vα19-Jα33 transcripts by qpcr (relative units normalized to TCRα constant region mrna). Lung single cell suspensions were pooled from 20 mice for sorting; data are representative of three independent experiments. (C) Quantification of CD3 and Vβ6+8 expression by DN T cells in the lungs of mice on day 14 after LVS i.n. infection; in the flow cytometry dot plot shown, cells were first gated on TCRβ + DN T cells as indicated in A. Data are representative of three independent experiments. segments (10, 25), we further examined lung DN T cells for surface expression of these TCR β-chains. Approximately 79% of the lung CD3 + DN T cells on day 14 after LVS infection expressed either Vβ6 orvβ8 (Fig.1C), compared with 25% to 45% of conventional CD4 + and CD8 + T cells in the same mice. These data collectively demonstrate that TCRβ + DN T cells responding in the lungs of mice after LVS i.n. infection expanded to become a large population highly enriched for MAIT cells with characteristic expression of the invariant Vα19-Jα33 TCR α-chain and TCR Vβ6/Vβ8-chains. We next performed a detailed characterization of the temporal responses of MAIT cells in the lungs of mice during sublethal i.n. infection with LVS. TCRβ + DN T cells were present in only marginally detectable numbers in the lungs of naive mice, but began to expand during the early (day 8) and intermediate (day 10) stages of infection (Fig. 2 A and B). Growth of LVS in the lungs of WT mice following sublethal i.n. infection is provided in Fig. 2C for comparison; LVS numbers increased steadily in the lungs until approximately day 8 after inoculation, followed by a continuous reduction in bacterial numbers thereafter. Interestingly, MAIT cells reached peak expansion in the late clearance phase of infection (day 14), and remained a notable presence in the lungs even after clearance of the bacteria (day 18; Fig. 2 A and B). In contrast, only small numbers of TCRβ + DN T cells (<1%) were detected throughout infection in the lungs of MR1-deficient mice (MR1 / ), which lack MAIT cells (7, 8) (Fig. 2 A and B). Analyses of the levels of expression of Vα19-Jα33 transcripts in total lung cells harvested from WT mice after LVS i.n. infection confirmed the response kinetics of MAIT cells, whereas Vα19-Jα33 transcripts were undetectable in the lungs of LVS-infected MR1 / mice (Fig. 2D). These combined data demonstrate that the vast majority of TCRβ + DN T cells present in the lungs of mice after i.n. infection of LVS are MAIT cells, and further demonstrate that MAIT cells appear relatively E Meierovics et al.
3 Fig. 2. Kinetics of MAIT cell accumulation in the lungs during pulmonary F. tularensis LVS infection. (A) Flow cytometry analyses of TCRβ + DN T cell accumulation in the lungs of WT and MR1 / mice infected with a sublethal dose of cfu LVS i.n. (exclusion channel, anti-cd4, CD8, B220, γδtcr Abs); in the representative dot plots, total live lung cells from individual mice are shown. (B) Enumeration of the total number of TCRβ + DN T cells in the lungs of WT and MR1 / mice during sublethal LVS i.n. infection (n = 5 per group). Data are representative of three independent experiments, and are the mean ± SEM (*P < 0.01 vs. LVS-infected MR1 / mice at the same time point, analyzed by Student t test.) (C) Growth of LVS in the lungs of WT mice following sublethal LVS i.n. infection. Data are representative of three independent experiments, and values shown are the mean cfu count per lung ± SEM of viable bacteria for five mice per group at each time point. (D) qpcr analyses of expression of MAIT cell invariant TCR Vα19-Jα33 transcripts in total cells harvested from the lungs of WT mice at the indicated time points after a sublethal LVS i.n. infection (n = 5 per group). qpcr results are presented as relative units normalized to TCRα constant region mrna (*P < 0.01 vs. naive mice by Student t test). early during the course of infection and reach peak expansion in the late clearance stage of infection. MAIT Cells Preferentially Accumulate in the Lungs During i.n. LVS Infection. Because LVS pulmonary infection is initiated in the lungs but quickly spreads systemically to a variety of organs (including the liver and spleen), we next sought to determine whether MAIT cells circulate to other organs and lymph nodes during i.n. infection of LVS. We monitored the presence of the TCRβ + DN T cell population in the lungs, livers, spleens, mediastinal lymph nodes, and mesenteric lymph nodes in WT mice on day 14 after a sublethal i.n. infection of LVS, at a time when MAIT cells reached maximal numbers in the lungs of infected WT mice (Fig. 2 A and B). We included LVS-infected MR1 / mice in the analysis to differentiate MAIT cells from other T cell types that might be present in the TCRβ + DN T cell population. Total TCRβ + T cells were examined by flow cytometry in the aforementioned tissues and lymph nodes, and total single-cell suspensions harvested from these tissues were assessed for Vα19- Jα33 transcripts by qpcr (Fig. 3 A and B). As expected, although TCRβ + DN T cells could be detected at low frequencies in various organs and lymph nodes from MR1 / mice (Fig. 3A), they exhibited undetectable levels of Vα19-Jα33 transcripts, confirming that the low numbers of TCRβ + DN T cells present in MR1 / mice are not MAIT cells. Among the organs and lymph nodes examined in LVS-infected WT mice, the lungs displayed the highest abundance of MAIT cells, with a large TCRβ + DN T-cell population ( 21% of all CD45 + TCRβ + cells) that was highly reduced in MR1 / mice (Fig. 3A), and high levels of Vα19-Jα33 transcripts (Fig. 3B). Interestingly, livers from LVS-infected WT mice displayed the second highest abundance of MAIT cells, as determined by flow cytometry and Vα19- Jα33 qpcr (Fig. 3 A and B). In contrast, although detectable, MAIT cells represented a much smaller population in the Fig. 3. MAIT cells preferentially accumulate in the lungs during pulmonary F. tularensis LVS infection. (A) Flow cytometry analyses of total TCRβ + cells in the lungs, livers, spleens, mediastinal lymph nodes (Med LN), and mesenteric lymph nodes (Mes LN) of WT and MR1 / mice on day 14 after a sublethal ( cfu) LVS i.n. infection; in the representative dot plots shown, cells from different tissues were first gated on total live CD45 + cells, and then gated on TCRβ + cells. The red box denotes the TCRβ + DN T cell population in LVS-infected WT or MR1 / mice. (B) qpcr analyses of expression of MAIT cell invariant TCR Vα19-Jα33 transcripts in total cells harvested from the lungs, livers, spleens, mediastinal lymph nodes, and mesenteric lymph nodes of WT mice on day 14 after a sublethal LVS i.n. infection (n = 5 pooled mice per group). qpcr results are presented as units normalized to TCRα constant region mrna. Data are expressed as the mean relative units ± SEM, and are representative of three independent experiments. IMMUNOLOGY PNAS PLUS Meierovics et al. PNAS Published online July 29, 2013 E3121
4 spleens, mediastinal lymph nodes, and mesenteric lymph nodes on day 14 after LVS i.n. infection (Fig. 3 A and B). Further analyses of the large MAIT cell population present in the lungs on day 14 after infection revealed a cell surface phenotype indicative of trafficking under inflammatory conditions, including a high proportion of cells expressing CCR2, CXCR3, and CCR5, as well as the integrin α4β1, which has previously been shown to mediate T-cell infiltration into the lungs (Fig. S1) (26, 27). This MAIT cell phenotype was similar to conventional CD4 + and CD8 + T cells present in the lungs on day 14 (Table S1). Only a small proportion of αβtcr + DN T cells expressed the NK markers NK1.1, CD49b, and Ly49d (Fig. S1). However, consistent with human MAIT cells, a large proportion of lung murine MAIT cells were IL-18 receptor α-positive (91.1%) (8, 17). Overall, these data demonstrate that, despite the systemic spread of LVS pulmonary infection, MAIT cells preferentially accumulated at the initial site of infection, and expressed cell surface receptors in the lungs consistent with inflammatory recruitment. MAIT Cells Are Essential for Full Resistance to F. tularensis LVS i.n. Infection. To investigate the ability of MAIT cells to contribute to in vivo protective immune responses during mucosal infection, we compared the course of a primary pulmonary sublethal LVS i.n. infection in the lungs of WT and MR1 / mice (Fig. 4A). LVS-infected MR1 / mice exhibited significantly higher bacterial burdens in the lungs compared with WT mice, beginning on day 10 after infection (Fig. 4A). This difference in bacterial burden coincided with the substantial expansion of MAIT cells in the lungs of WT mice in the early/intermediate phase of infection (days 8 10; Fig. 2 A and B). The bacterial burden in the lungs of LVS-infected MR1 / mice remained significantly higher (on average greater than two log 10 cfus among experiments, with a maximal 580-fold higher cfu count in the MR1 / mice on day 13) than LVS-infected WT mice throughout the course of the infection, and clearance of the bacteria from the lungs of MR1 / mice was significantly delayed compared with WT mice (Fig. 4A). Interestingly, no significant defect was observed in bacterial clearance from the spleens and livers of LVS-infected MR1 / mice (Fig. S2). Thus, MAIT cells have a nonredundant role in controlling bacterial burdens in the lungs during LVS i.n. infection. To assess the role of MAIT cells in the generation of mucosal immune responses, we examined the expression of a range of critical antibacterial mediators in the lungs of WT and MR1 / mice during sublethal LVS i.n. infection. Specifically, we assessed production of IFN-γ, IL-17A, TNF, and inducible NO synthase (inos), which are essential for host defense against LVS infection (28 30). LVS-infected MR1 / mice exhibited impaired production of IFN-γ, IL-17A, TNF, and inos transcripts in their lungs compared with LVS-infected WT mice; peak production of all four immune mediators was delayed in MR1 / mice (day 14) compared with WT mice (day 8; Fig. 4 B E). Thus, in the absence of MAIT cells, MR1 / mice had a defect in production of four critical antimicrobial mediators during the early stages of infection. The impaired cytokine and inos responses in MR1 / mice corresponded with the observation that MR1 / mice exhibited higher bacterial burdens and delayed clearance compared with WT mice (Fig. 4A). Overall, these data indicate that, in the absence of MAIT cells, initiation of protective immune responses in the lungs of infected mice was delayed during LVS i.n. infection. MAIT Cells Produce Critical Antibacterial Cytokines in the Lungs During F. tularensis LVS i.n. Infection. We next assessed the ability of MAIT cells to directly contribute to the reduction of LVS bacterial burdens in the lungs by production of critical cytokines. Previous evidence has established that T-cell production of IFN-γ and Fig. 4. MR1 / mice display impaired antibacterial responses during pulmonary F. tularensis LVS infection. WT and MR1 / mice were infected i.n. with LVS at a sublethal dose of cfu. Bacterial burdens of LVS (in cfu) in the lungs were enumerated at the indicated time points (A). Values shown are the mean cfu count per lung ± SEM of viable bacteria for five mice per group at each time point. (*P < 0.01 vs. cfu count in LVS-infected WT mice, as analyzed by Student t test.) qpcr analyses of expression of IFN-γ (B), TNF (C), IL-17A (D), and inos (E) in the lungs of LVS-infected mice. Values shown are the mean expression ± SEM of the indicated transcript relative to GAPDH for five mice per group. (*P < 0.05 vs. LVS-infected WT mice at the same time point, as analyzed by Student t test.) Data are representative of three independent experiments. TNF limits LVS bacterial growth in macrophages (31, 32). Therefore, to evaluate the antibacterial capacity of MAIT cells in the lungs of mice during sublethal LVS i.n. infection, we analyzed their production of IFN-γ and TNF, as well as another important mucosal mediator, IL-17A. MAIT cells were purified from the lungs of LVS-infected WT mice by FACS as described in Fig. 1, and their cytokine production was assessed by qpcr analyses. The results show that, on day 8 after infection, MAIT cells robustly produced IFN-γ, TNF, and IL-17A (Fig. 5A). MAIT cells also contributed to the production of these three important cytokines during the intermediate phase of infection, as assessed by intracellular cytokine staining (day 10; Fig. 5B). In contrast, because of their lack of MAIT cells, LVS-infected MR1 / mice had only minimal numbers of cytokine-producing TCRβ + DN T cells in the lungs after i.n. infection of LVS (Fig. 5B). Interestingly, on day 14 after infection, when adaptive CD4 + and CD8 + T cells actively produced cytokines, MAIT cells remained strong producers of IFN-γ and TNF (Fig. 5C). These in vivo data demonstrate that MAIT cells are significant contributors to antimicrobial cytokine production in the lungs throughout the course of pulmonary LVS infection. E Meierovics et al.
5 Fig. 5. MAIT cells produce robust levels of critical cytokines in the lungs of WT mice at different stages of pulmonary infection of F. tularensis LVS. (A) TCRβ + DN, CD4 +, and CD8 + T cells were purified from the lungs of WT mice on day 8 after a sublethal ( cfu) i.n. infection of LVS by FACS, and evaluated for expression of IFN-γ, TNF, and IL-17A by qpcr (relative units normalized to GAPDH mrna). Lung cells were pooled from 10 mice; data include values ± SEM from three replicates, and are representative of two independent experiments. (B) Representative flow cytometry dot plots of IFN-γ, TNF, and IL-17A production by TCRβ + cells in the lungs of WT and MR1 / mice on day 10 after sublethal LVS i.n. infection. In the representative dot plots shown, cells were first gated on total live lung TCRβ + cells. Data are representative of three independent experiments. The red box denotes the cytokine-positive TCRβ + DN T cell population in WT and MR1 / mice. (C) TCRβ + DN, CD4 +, and CD8 + T cells were purified from mouse lungs on day 14 after sublethal LVS i.n. infection by FACS, and evaluated for expression of IFN-γ, TNF, and IL-17A by qpcr (relative units normalized to GAPDH mrna). Lung cells were pooled from 10 mice; data include values ± SEM from three replicates, and are representative of two independent experiments. To investigate the ability of MAIT cells to use these antimicrobial cytokines to control the intracellular growth of LVS, we used an in vitro macrophage intracellular growth assay. Bone marrow-derived macrophages (BMMØs) were infected with LVS and cocultured with MAIT cells. As a source of MAIT cells, we enriched total Thy1.2 + T cells from naive mice transgenic for the canonical MAIT cell Vα19-Jα33 invariant TCR α-chain (Vα19iTgMR1 +/+ mice), which have a high frequency of MAIT cells (25). Growth of LVS in the BMMØ monolayer was assessed after 3 d of coculture. Naive MAIT cells resulted in greater than 95% reduction of LVS growth in BMMØs compared with cultures lacking MAIT cells (Fig. 6A). Addition of a blocking anti- MR1 Ab to the cocultures completely reversed this control of growth (Fig. 6A). Thus, naive MAIT cells were capable of directly controlling LVS growth in macrophages in a manner that required interaction with their cognate antigen, MR1. Similar results were observed for enriched DN Vα19iTg-MR1 +/+ MAIT cells (Fig. S3A). Of note, naive T cells harvested from MR1- deficient Vα19iTg mice (Vα19iTgMR1 / ) had no impact on LVS growth in BMMØs (Fig. S3A), revealing that MR1 is required during in vivo development for the innate capacity of the Vα19-Jα33 expressing transgenic T cells to control LVS intracellular growth. To further investigate the mechanisms by which MAIT cells control LVS intracellular growth in macrophages, we assessed macrophage viability, cytokine production, and nitric oxide (NO) or nitrite levels in the cocultures. Macrophage viability in cocultures containing naive Vα19iTgMR1 +/+ MAIT cells was not significantly diminished compared with control uninfected macrophage cultures (Fig. S3B). In contrast, cocultures that exhibited unrestricted LVS intramacrophage growth (containing naive Vα19iTgMR1 / T cells or no T cells) displayed significant decreases in macrophage viability by the 72-h time point. Overall, these data indicate that MAIT cells do not significantly rely upon induction of macrophage cell death to limit LVS replication in this coculture system. Macrophage-activating cytokines such as IFN-γ and TNF were readily detectable in the 72-h coculture supernatants, in addition to IL-17A and nitrite; production of all these mediators was significantly inhibited by the presence of anti-mr1 Ab in the cocultures (Fig. 6 B E). Addition of neutralizing antibodies for IL kda subunit (IL-12p40), TNF, and IFN-γ to the cocultures completely reversed MAIT cell control of bacterial growth, whereas addition of a neutralizing anti IL-17A antibody had no effect on growth control of LVS by MAIT cells (Fig. 6A). The addition of the inos inhibitor N G -monomethyl arginine (NMMA) to the cultures also completely reversed MAIT control of LVS growth (Fig. 6A). In addition, blockade of IL-12p40 also inhibited production of IFN-γ, TNF, and NO, but not IL-17A, in the cocultures (Fig. 6). Further, coculture of LVS-infected IL-12p40 / BMMØs with MAIT cells revealed that macrophage IL-12p40 is required for MAIT cells to obtain the capacity to control LVS growth (Fig. S3C). Thus, naive MAIT cells required cognate MR1 and soluble IL-12p40 signals from infected macrophages to control LVS intramacrophage growth. Finally, MAIT cells used a mechanism of macrophage activation that involved IFN-γ, TNF, and NO production to effect LVS growth control. MAIT Cells Are Required for Timely Recruitment of Activated CD4 + and CD8 + T Cells to the Lungs During Pulmonary F. tularensis LVS Infection. Because CD4 + and CD8 + T cells are critical for the final clearance of in vivo LVS infection from the tissues (24, 33, 34), we hypothesized that the delayed bacterial clearance observed in MR1 / mice may be the result of impaired recruitment of adaptive immune responses in the lungs. Indeed, MAIT cells have been proposed to serve as early responders that influence the development of adaptive immune responses, although there is currently no direct supporting evidence. Therefore, we examined the recruitment of activated TCRβ + CD4 + and TCRβ + CD8 + T cells to the lungs of infected WT and MR1 / mice at days 8 to 14, time points surrounding the emergence of the defect in LVS growth control in MR1 / mice (day 10; Fig. 4A). On days 8 and 10 after LVS sublethal i.n. infection, the total numbers of TCRβ + CD4 + or CD8 + T cells in the lungs of MR1 / mice were significantly lower than those of WT mice (Fig. 7A). The diminished numbers of TCRβ + CD4 + and CD8 + T cells in MR1 / mouse lungs was not a consequence of some of them being MAIT cells, because TCRβ + CD4 + and CD8 + T cells IMMUNOLOGY PNAS PLUS Meierovics et al. PNAS Published online July 29, 2013 E3123
6 CD44 hi ), and IFN-γ producing CD4 + and CD8 + T cells had increased in MR1 / mice to either equal (in the case of CD8 + T cells) or exceed (in the case of CD4 + T cells) those of WT mice (Fig. 7 C and D). Thus, MR1 / mice exhibited delayed recruitment of activated, IFN-γ producing CD4 + and CD8 + T cells to the lungs during LVS pulmonary infection. Fig. 6. Naive transgenic MAIT cells release cytokines and control F. tularensis LVS intracellular growth in macrophages. BMMØs from WT mice were infected with LVS and cultured alone or with Thy1.2 + cells enriched from naive Vα19iTg-MR1 +/+ mice. In some cases, as indicated, neutralizing Abs or NMMA were added to the cultures at the time of addition of Vα19iTg-MR1 +/+ T cells. (A) Growth of LVS in the BMMØ monolayer was determined after 72 h coculture. Values shown are the mean cfu counts per milliliter ± SEM of triplicate cultures. (*P < 0.01 vs. Infected BMMØ alone by Student t test.) Supernatants were collected after 72 h of coculture and the amount of IFN-γ (B), TNF (C), IL-17A (D), and nitrite (E) was evaluated. Values shown are the mean pg/ml (cytokines) or μm (nitrite) ± SEM of triplicate cultures. (*P < 0.01 vs. Vα19iTg-MR1 +/+ T-cell cultures containing isotype control Ab.) Data are representative of three independent experiments of similar design. FACS-sorted from the lungs of WT mice on day 8 after LVS i.n. infection expressed only negligible levels of Vα19-Jα33 chain transcripts (Fig. S4). Importantly, further investigation of the quality of the TCRβ + CD4 + and CD8 + T cells in the lungs of MR1 / mice revealed that the number of activated CD4 + and CD8 + T cells (characterized by CD69 + CD44 hi ) was significantly diminished compared with WT mice at days 8 and 10 after infection (Fig. 7 B and C). This resulted in 80% and 60% reductions in activated CD4 + T cells present in total lung cells of MR1 / mice on days 8 and 10 after infection, respectively; and an 60% reduction in activated CD8 + T cells on both days. Similarly, diminished levels of effector CD62L lo TCRβ + CD4 + and CD8 + T cells were observed in the lungs of infected MR1 / mice (Fig. 7B). Notably, the numbers of activated CD4 + and CD8 + T cells present in the lungs of MR1 / mice on day 8 after infection were not significantly different from uninfected animals (Fig. 7C). Finally, the numbers of IFN-γ producing TCRβ + CD4 + cells and CD8 + T cells in the lungs of MR1 / mice were 10-fold lower than in WT mice at day 8 after infection, and approximately threefold lower than in WT mice at day 10 after infection (Fig. 7 B and E). To determine whether this defect in the recruitment of activated T cells was a permanent deficiency, we investigated the presence of activated CD4 + and CD8 + T cells in infected mice at a later time point (day 14). By day 14 after i.n. infection of LVS, the numbers of activated (CD69 + MAIT Cells Control Long-Term Chronic F. tularensis LVS i.n. Infection in Immunocompromised Mice. Because the aforementioned data demonstrated that, despite being an innate T-cell subset, MAIT cells expanded and produced important cytokines during the later stages of infection (day 14; Figs. 2B and 5C), we were prompted to more precisely measure the ability of MAIT cells to provide antibacterial functions throughout infection. Because the contributions of MAIT cells to LVS growth control in vivo may be masked by the potent antibacterial activities of conventional CD4 + and CD8 + T cells responding during the later stages of infection, we evaluated the capacity of MAIT cells to control sublethal i.n. infection of LVS in the absence of CD4 + and CD8 + T cells. Previous studies have shown that WT mice depleted of CD4 + and CD8 + T cells do not clear sublethal LVS infection, but instead develop a long-term chronic infection that requires another, unknown, αβ T-cell subset (33 35). To determine whether MAIT cells are responsible for this long-term control, we analyzed the progression of sublethal i.n. infection of LVS in WT and MR1 / mice simultaneously depleted of CD4 + and CD8 + T cells. On day 14 after infection, the DN TCRβ + subset in the lungs of CD4 + CD8 + -depleted WT mice had expanded to comprise 20% of total lung cells compared with 10.8% of lung cells in untreated WT control mice (Fig. 8A). DN TCRβ + cells comprised only 2.3% of lung cells in CD4 + CD8 + - depleted MR1 / mice. Consistent with previous studies, CD4 + CD8 + -depleted WT mice developed a chronic infection and remained alive after 2 mo of infection (Fig. 8 B and C). In striking contrast, CD4 + CD8 + -depleted MR1 / mice showed significantly higher burdens of LVS in the lungs than their WT counterparts, and ultimately died at the same time as LVSinfected TCRα / mice that lack all αβ T cells (Table 1 and Fig. 8C). Altogether, these results demonstrate that MAIT cells have the capacity to function long-term to sustain control of LVS growth in the absence of CD4 + and CD8 + T cells. Discussion The in vivo evidence presented in the present study demonstrated that MAIT cells actively contributed to immune responses throughout the innate and adaptive phases of primary pulmonary infection with F. tularensis LVS. During the early innate phase of infection, MAIT cells were required for prompt production of proinflammatory cytokines and timely recruitment of activated CD4 + and CD8 + T cells in the lungs. Later during infection, when conventional CD4 + and CD8 + T cells were active, MAIT cells continued to accumulate in the lungs of infected mice and produced important cytokines such as IFN-γ, suggesting an ongoing role for MAIT cells during the adaptive phase of infection. Here we found that MAIT cells were a small population in the lungs of naive mice that expanded 25-fold following LVS i.n. infection, ultimately comprising as much as 20% to 25% of all αβt cells in infected lungs. Interestingly, the kinetics of MAIT cell expansion appear different from other TCRαβ + innate T cell subsets; MAIT cell numbers increased gradually and peaked during the later phases of infection, when CD4 + and CD8 + T-cell responses were also maximal. These observations contrast with the rapid kinetics of other nonconventional TCRαβ + innate T cells. For instance, CD1d-restricted inkt cells expanded and contracted rapidly during M. bovis bacillus Calmette Guérin infection, before the peak of conventional T-cell responses (4). Similarly, the IFN-γ producing function of inkt cells was limited to the early phases of Streptococcus pneumoniae E Meierovics et al.
7 Fig. 7. Recruitment of activated CD4 + and CD8 + T cells to the lungs is delayed in MR1 / mice during F. tularensis LVS pulmonary infection. WT and MR1 / mice were infected i.n. with a sublethal dose of cfu LVS, and (A) total numbers of TCRβ + CD4 + and CD8 + T cells were enumerated in the lungs at the indicated time points by flow cytometry. Data are presented as mean ± SEM (n = 5 per group; *P < 0.05 vs. LVS-infected WT mice, as analyzed by Student t test). (B) Flow cytometry staining for activation marker expression and IFN-γ production by TCRβ + CD4 + and CD8 + T cells in the lungs of LVS-infected WT mice (gray line, gray shaded histograms) and LVS-infected MR1 / mice (black line, unshaded histograms) on day 8 after infection (in the IFN-γ plot, isotype control Ab staining is indicated by a dotted line). (C) Comparison of the total numbers of activated (positively stained for both CD69 and CD44) TCRβ + CD4 + or CD8 + T cells in the lungs of LVS-infected WT and MR1 / mice at the indicated time points. Data are presented as mean ± SEM [n = 5 per group; *P < 0.05 vs. LVSinfected WT mice; **P < 0.01 vs. naive mice (day 0) of the same genotype; ns, not significant, i.e., P > 0.05 vs. naive mice (day 0) of the same genotype, as analyzed by Student t test]. (D) Numbers of IFN-γ producing cells in TCRβ + CD4 + and CD8 + T cells in the lungs of LVS-infected WT and MR1 / mice at the indicated time points. Data are presented as mean ± SEM (n = 5 per group; *P < 0.05 vs. WT mice by Student t test). All data are representative of three independent experiments of similar design. infection in vivo, and H2-M3 restricted T cells were early effectors that peaked in numbers before the adaptive phase of Listeria monocytogenes infection (1, 5, 36). Notably, inkt cells exhibit an effector/memory phenotype in the periphery of humans and naive mice that requires up-regulation of the transcription factor PLZF during thymic selection (37). In contrast, MAIT cells do not acquire PLZF expression or an effector/ memory phenotype during thymic development (38, 39). Thus, MAIT and inkt cells undergo different developmental programs that may contribute to their differential patterns of expansion or recruitment to infection loci. Interestingly, MAIT cells and inkt cells appear to have different relationships with commensal microbes. Murine MAIT cells are not found in the periphery of germ-free mice, but expand after reconstitution with commensal bacterial strains (7, 8). Human MAIT cells acquire a memory phenotype in the periphery after birth, a developmental step thought to result from exposure to commensal microbes (38). In contrast, increased numbers of inkt cells accumulated in the colons and lungs of germ-free mice, a phenomenon that was reversed by reconstitution of commensal bacteria in neonates (40). Here, the characterization of MAIT cell expansion in pulmonary LVS infection provides evidence that MAIT cells may be programmed to have intrinsically different responses from other innate T cells, including inkt cells. The rapid contraction of inkt cells before the peak of conventional T-cell responses during microbial infections in mice has been attributed to apoptosis, a phenomenon proposed to result from sharing of survival cytokines between inkt cells and conventional T cells (4, 36). From the data presented here, it is clear that MAIT cell numbers in the lungs during LVS infection were not limited by the expansion and recruitment of classical MHC-restricted T cells, nor did MAIT cells lose functional capacity later in infection, as measured by their continued cytokine production. Interestingly, recent evidence indicates that the apoptotic propensity of human peripheral MAIT and inkt cells is partially dependent on the balance between expression of proapoptotic proteins (including PLZF) and the antiapoptotic factor X-linked inhibitor of apoptosis (XIAP) (41). In light of the results presented in our murine model, it is possible that MAIT and inkt cells possess different apoptotic sensitivities during infectious assaults. An enduring high number of MAIT cells at the initial site of infection could serve as an extended defense mechanism to augment adaptive immune responses and aid in prevention of further pathogen assaults. Of note, the kinetics of human MAIT and inkt cell responses during infectious diseases are not well defined as a result of the limitations of obtaining patient samples throughout infection. Although both cell types have been detected in lesions of chronically infected patients, their long-term functional capacities in these tissues remain uncertain (17, 18, 42). Although T-cell deficient mice are highly susceptible to LVS infection (33, 43), it has previously been noted that mice deficient IMMUNOLOGY PNAS PLUS Meierovics et al. PNAS Published online July 29, 2013 E3125
8 Fig. 8. MAIT cells maintain a long-term chronic F. tularensis LVS infection in the absence of CD4 + and CD8 + T cells. WT and MR1 / mice were depleted of CD4 + and CD8 + T cells before and throughout a sublethal ( cfu) LVS i.n. infection by administration of anti-cd4 and anti-cd8 Abs ( depleted ). (A) Flow cytometry analyses of TCRβ + DN T-cell accumulation (boxed) in the lungs of mice on day 14 after LVS i.n. infection (exclusion channel, anti-cd4, CD8, B220, γδtcr Abs); representative dot plots are shown of total lung cells from individual mice. (B) LVS cfu counts in the lungs of LVS-infected WT and MR1 / mice depleted of CD4 and CD8 cells at the indicated time points after sublethal LVS i.n. infection. Data are presented as mean ± SEM (n = 5 per group; *P < 0.01 vs. WT depleted mice by Student t test). (C) Survival of LVS-infected WT and MR1 / mice depleted of CD4 and CD8 cells, as well as of LVS-infected TCRα / mice, after sublethal LVS i.n. infection (n = 5 per group). Data are representative of three independent experiments. for either CD4 + or CD8 + T cells readily survived sublethal infection, a phenomenon that was attributed to the capacity of each T cell subset to compensate for the absence of the other. However, the data presented here indicated that MAIT cells are another T-cell subset that actively contributes to defense against infection. To assess the potency of MAIT cell activities during infection, we evaluated their ability to provide long-term control of an in vivo sublethal LVS pulmonary infection in the absence of CD4 + and CD8 + T cells. We found that MAIT cells are essential for survival of mice simultaneously depleted of CD4 + and CD8 + T cells during LVS pulmonary infection. Importantly, in the absence of CD4 + and CD8 + T cells, MAIT cell activities in the lungs were sufficient to control, but not clear, LVS infection. Because MAIT cells possessed the capacity to produce critical cytokines such as TNF, IFN-γ, and IL-17A throughout LVS infection, their inability to fully eradicate the bacteria presents an Table 1. Survival of mice depleted of CD4 + and CD8 + T cells during a sublethal i.n. infection of F. tularensis LVS Mouse strain Ab treatment Deaths/total Mean time to death (d) WT Anti-CD4 + CD8 0/5 CD1d / Anti-CD4 + CD8 0/5 MR1 / Anti-CD4 + CD8 5/ ± 8.9* TCRα / None 5/ ± 8.5 Mice were treated with anti-cd4 and anti-cd8 Abs twice weekly before, and throughout F. tularensis LVS i.n. infection. Mice were observed for a total of 75 d. This experiment is representative of three experiments of similar design. *P > 0.05 vs. LVS-infected TCRα / mice. intriguing question. MAIT cells may lack a critical, yet-undefined antimicrobial mechanism, or their tissue tropism may limit their capacity to eliminate a systemic infection. Regardless, our study provides in vivo evidence that MAIT cells can mediate long-term control of a pathogenic infection in the absence of conventional CD4 + and CD8 + T cells. Interestingly, MAIT cells in patients with chronic HIV infection became exhausted and depleted in the circulation, but were relatively preserved in the rectal mucosa (44, 45). Thus, it is possible that MAIT cells are retained in mucosal tissues as a first line of defense during HIV infection. Our observation that MAIT cells can control bacterial infection in the absence of CD4 + and CD8 + T cells may provide a basis for future studies on the capacity of MAIT cells to provide immunity in an immunocompromised host. Recent evidence has shown that human MAIT cells uniquely recognize vitamin B metabolites presented by MR1. In particular, intermediates of the vitamin B2 (i.e., riboflavin) biosynthetic pathway bind MR1 and activate human MAIT cells to produce IFN-γ and TNF (16). Thus far, microbes capable of activating MAIT cells possess the riboflavin biosynthesis pathway, whereas nonactivating microbes do not. In accordance with our in vitro data demonstrating MR1-dependent activation of murine MAIT cells by LVS, F. tularensis also possesses the enzymatic pathway that generates MR1-binding riboflavin precursors. Interestingly, although M. bovis bacillus Calmette Guérin similarly has the pathway for vitamin B synthesis, murine MAIT cells responded to bacillus Calmette Guérin-infected macrophages in an MR1- independent manner, instead relying on the noncognate signal IL-12p40 (19). Similar to inkt cells, it is possible that MR1 dependence is influenced by the relative strengths of the TCRmediated signal vs. the IL-12 signal (5, 46). Because LVS is a relatively fast-growing organism that replicates in the cytoplasm (47), whereas M. bovis bacillus Calmette Guérin is slowgrowing and interferes with endocytic trafficking to replicate in an early endosome (48), the different intracellular lifestyles of these two bacteria may impact the availability of ligands for loading onto MR1. Data presented in this study demonstrate that MAIT cells have an essential role in the timely initiation of adaptive immune responses during infection. Recruitment of activated IFN-γ producing CD4 + and CD8 + T cells was significantly delayed in the lungs of MR1 / mice; activated T cell numbers in MR1 / mice peaked 2 to 4 d later than WT mice and were accompanied by higher bacterial burdens in the lungs. We similarly observed a lag in the production of TNF, IFN-γ, IL-17A, and inos transcripts in the lungs of infected MR1 / mice compared with WT mice, providing further evidence that MAIT cells participate in early events that initiate protective immune responses. Consistent with our findings, previous evidence has demonstrated that MR1 / mice exhibited transiently higher bacterial burdens in the lungs in the early phase of M. bovis bacillus Calmette Guérin pulmonary infection compared with WT mice (19). Similarly, K. pneumoniae-infected MR1 / mice displayed transiently higher bacterial growth within 2 d of i.p. infection (20). Given the evidence provided here, it is likely that MR1-dependent initiation of immune responses during infection is a process that may be common to multiple bacterial pathogens. The precise mechanisms used by MAIT cells to initiate early adaptive immune responses remain undefined. For inkt cells, their extremely early production of IFN-γ and/or other cytokines is thought to influence other innate immune cells and the progression of adaptive immune responses (49 51). In vitro and in vivo evidence provided here demonstrate that MAIT cells produce critical cytokines such as IFN-γ, TNF, and IL-17A in response to LVS infection all important for initiating adaptive immunity (29, 52), and controlling LVS growth (53). The generation of optimal protective immunity to intracellular pathogens is dependent on the timely and efficient responses of E Meierovics et al.
9 CD4 + and CD8 + T cells. Here we show that MAIT cells are required for early cytokine production and prompt recruitment of activated CD4 + and CD8 + T cells to the lungs following pulmonary infection with the intracellular bacterial pathogen F. tularensis LVS. Further, we show that MAIT cells not only have an early role in the initiation of immune responses in the lung, but also actively produce important cytokines during the later phases of infection. Given the abundance of MAIT cells in humans, coupled with their capacity to function at mucosal surfaces during multiple stages of the immune response, the contribution of MAIT cells to immunity should be considered during the development of mucosal vaccines against human pathogens. Materials and Methods Bacteria. F. tularensis LVS (no ; American Type Culture Collection) was grown and frozen as previously described (31). Viable bacteria were quantified by plating serial dilutions on supplemented Mueller Hinton agar (MHA) plates. Animals, Infections, and Determination of Bacterial Organ Burdens. Male and female specific pathogen-free C57BL/6J mice were purchased from Jackson Laboratory. MR1-deficient mice (7) and Vα19iTgMR1 +/+ and Vα19iTgMR1 / transgenic mice (25) were obtained from Ted Hansen (Washington University in St. Louis, St. Louis, MO) and bred at the Center for Biologics Evaluation and Research (CBER) (US Food and Drug Administration). Animals were housed in a barrier environment at the Center for Biologics Evaluation and Research, and procedures were performed according to approved protocols under animal care and use committee guidelines (CBER Institutional Animal Care and Use Committee). i.n. infections were performed by delivering LVS cfu in a volume of 25 μl per naris to anesthetized mice. Bacteria were diluted in PBS solution (Cambrex) containing <0.01 ng/ml endotoxin. Numbers of cfus in organs from infected mice were determined using groups of five mice. The cfu counts in lungs of infected mice were assessed by plating organ homogenates on MHA plates containing an antibiotic mixture to suppress growth of adventitious agents (colistin sulfate salt, lincomycin hydrochloride, trimethoprim, and ampicillin). Preparation of Single-Cell Suspensions from Organs. To prepare lung cells for RNA extraction and/or flow cytometry, the lungs were excised following perfusion through the right heart ventricle with a solution of DMEM containing 2% (vol/vol) FBS, collagenase (0.72 mg/ml), and DNase (10 U/mL). Harvested lungs were subjected to pressure disruption, followed by incubation for 20 min at 37 C in 5% CO 2. Released cells were filtered through a Filtra-bag (Lab Plas), subjected to ammonium chloride/potassium lysis, and passed through a 40-μm filter. Liver cells were prepared by pressure disruption of whole livers followed by centrifugation for 20 min at 500 g on a 40%/80% (wt/vol) Percoll gradient to separate leukocytes from cell debris and epithelial cells. Spleen cells, mediastinal lymph nodes, and mesenteric lymph nodes were prepared by pressure disruption and ammonium chloride/ potassium lysis and passed through a 40-μm filter to remove debris. In all cases, live cells prepared from tissues were enumerated on a hemocytometer after dilution in trypan blue. Flow Cytometry Analyses and Intracellular Cytokine Staining. Single-cell suspensions from tissues were prepared as described earlier. Cells were stained for a panel of murine cell surface markers and analyzed by using a Becton Dickinson LSR II flow cytometer and FlowJo software. Ab clones used included RM4-5 (anti-cd4), (anti-cd8a), GL3 (anti-γ/δ TCR), NK1.1 (anti-nk1.1), (anti-thy1.2), H (anti TCR β-chain), 17A2 (anti-cd3), RA3-6B2 (anti-cd45/b220), and RR4-7 and MR5.2 (anti-vβ6 and Vβ8), all obtained from BD Pharmingen. In addition, H1.2F3 (anti-cd69), IM7 (anti-cd44), MP6- XT22 (anti-tnf), 30-F11 (anti-cd45), MEL-14 (anti-cd62l), XMG-6 (anti IFNγ), GL3 (anti-γ/δ TCR), M1/70 (anti-cd11b), 2G12 (anti-ccr4), HM-CCR5 (anti- CCR5), 29-2L17 (anti-ccr6), CXCR3-173 (anti-cxcr3), DATK32 (anti-α4β7), 2E7 (anti-cd103), FIB27 (anti-β7), 9C10(MFR4.B) (anti-α4), HMb1-1 (anti-β1), BG/IL18RA (anti IL-18Rα), 4E5 (anti-ly49d), HMa2 (anti-cd49b), PK136 (anti- NK1.1), and C7 (anti-nkg2d) were obtained from BioLegend. Unloaded and α-galcer loaded CD1d tetramers were obtained from National Institutes of Health Tetramer Core Facility (Atlanta, GA). Clones ebio17b7 (anti IL-17A) and SPRCL5 (anti-cxcr5) were obtained from ebioscience. Clone (anti-ccr2) was obtained from R&D Systems. Live/Dead Near IR stain (Molecular Probes) was included in all staining protocols. Optimal antibody concentrations were determined in separate experiments, and appropriate fluorochrome-labeled isotype control antibodies used throughout. In all cases, cells were first gated on singlets (forward scatter-width vs. forward scatter-area or -height) and live cells (Live/Dead Near IR negative) before further analyses. In some experiments, as indicated, cells were also gated on CD45 + leukocytes before further analyses. To monitor the expression of TNF, IFN-γ, and IL-17A, lung cells were incubated in complete Dulbecco s modified eagle medium (cdmem) containing 5 μg/ml Brefeldin A at 37 C in 5% CO 2 for 4 h (without additional exogenous stimulation, as cell preparations also contained live bacteria from the in vivo infection), and stained for cell surface markers. Intracellular staining was performed by using the BD Biosciences buffer system according to the manufacturer s instructions. For flow sorting experiments, lung cells from LVS-infected mice were stained with the appropriate cell surface markers, and sorted directly into Qiagen RNA Protect for RNA extraction and quantitative real-time RT-PCR. Sorting of DN T cells was performed by selecting cells negative for CD4, CD8, γδtcr, and B220, but positive for TCRβ. Cells positive for TCRβ and CD4, or TCRβ and CD8, were sorted for analyses of CD4 + and CD8 + T-cell gene expression. RNA Extraction and Quantitative Real-Time RT-PCR. Total RNA was prepared using RNeasy Mini Kit (Qiagen), and first-strand cdna was synthesized using the Retroscript Kit (Ambion) according to the manufacturer s instructions. Real-time PCR was performed by using the ABI Prism ViiA7 instrument (Applied Biosystems). Cytokine and inos transcripts were quantified by using TaqMan Predeveloped Assay Kits (Applied Biosystems) according to the manufacturer s instructions. Assessment of expression of Vα19-Jα33 and Vα14-Jα18 transcripts was performed by quantitative real-time PCR using previously described primer probe pairs (10), and the results were normalized to expression of the TCRα constant chain. Culture and Infection of BMMØ with Bacteria. Bone marrow macrophages were used as the target cells for the in vitro system. BMMØs were cultured as previously described (31). Cells were plated at in 96-well plates in cdmem supplemented with 50 μg/ml gentamycin (Life Technologies) and incubated at 37 C in 5% CO 2. After 1 d, the medium was replaced with antibiotic-free cdmem, and the cells were incubated for an additional 6 d at 37 C in 5% CO 2. The medium was replaced with fresh, gentamycin-free cdmem every 2 d during the 7-d incubation. Following the 7-d culture period, the BMMØ concentration was estimated to be cells per well. BMMØ were then infected with F. tularensis LVS at a multiplicity of infection of 1:10 (bacterium-to-bmmø ratio). LVS was coincubated with BMMØs at 37 C in 5% CO 2 for 2 h and then washed three times with PBS solution (Life Technologies). The monolayers were incubated for 1 h in cdmem supplemented with 50 μg/ml gentamicin to eliminate extracellular bacteria. The BMMØ were washed a further three times with PBS solution, followed by addition of cdmem and purified splenocytes at a ratio of one purified T cell per two macrophages. The cells were cocultured at 37 C in 5% CO 2 for 3 d before lysis to determine bacterial growth. Vα19iTgMR1 +/+ mice were used as source of naive splenic total Thy1.2 + T cells or DN MAIT cells for coculture with LVS-infected BMMØs. Total Thy1.2 + T cells were enriched from Vα19iTgMR1 +/+ mouse spleens by using a Thy1.2 + Dynabead cell enrichment column (Life Technologies), according to the manufacturer s recommendations. DN T cells were enriched by using a combination of in vivo depletion by antibody treatment and in vitro positive selection, as described previously (31). The enriched cells were routinely >90% TCRβ + CD4 CD8 NK1.1. Where indicated, anti-mr1 Ab clone 26.5 (Ted Hansen, Washington University, St. Louis, MO) was added to BMMØ cultures at a concentration of 25 μg/ml Abs (BD Pharmingen), and inhibitors were added at a concentration of 25 μg/ml (anti IFN-γ and anti-tnf), 100 μg/ ml (anti-il-12p40), and 1 mm NMMA (Sigma). In all cases, Abs and inhibitors were added at the time of addition of MAIT cells to the cultures, and remained present for the entire 3 d coculture period. In Vivo Depletion of Cell Subpopulations and Enrichment of T-Cell Subpopulations. Antibodies for in vivo depletion of CD4 + T cells (clone GK1.5), CD8 + Tcells (clone 2.43), and NK1.1 + cells (clone PK136), were obtained as purified antibodies from the National Cell Culture Center (Minneapolis, MN); all contained <10 EU/mL endotoxin. Antibodies were administered to mice intraperitoneally at a concentration of 0.5 mg per injection (GK1.5 and 2.43) or 0.25 mg per injection (PK136) twice weekly, and routinely reduced the depleted cell type to less than 0.5% detectable remaining cells as assessed by flow cytometry. IMMUNOLOGY PNAS PLUS Meierovics et al. PNAS Published online July 29, 2013 E3127
CD44
 MR1-5-OP-RU CD24 CD24 CD44 MAIT cells 2.78 11.2 WT RORγt- GFP reporter 1 5 1 4 1 3 2.28 1 5 1 4 1 3 4.8 1.6 8.1 1 5 1 4 1 3 1 5 1 4 1 3 3.7 3.21 8.5 61.7 1 2 1 3 1 4 1 5 TCRβ 2 1 1 3 1 4 1 5 CD44 1 2 GFP
MR1-5-OP-RU CD24 CD24 CD44 MAIT cells 2.78 11.2 WT RORγt- GFP reporter 1 5 1 4 1 3 2.28 1 5 1 4 1 3 4.8 1.6 8.1 1 5 1 4 1 3 1 5 1 4 1 3 3.7 3.21 8.5 61.7 1 2 1 3 1 4 1 5 TCRβ 2 1 1 3 1 4 1 5 CD44 1 2 GFP
Supplementary Figure 1. Characterization of basophils after reconstitution of SCID mice
 Supplementary figure legends Supplementary Figure 1. Characterization of after reconstitution of SCID mice with CD4 + CD62L + T cells. (A-C) SCID mice (n = 6 / group) were reconstituted with 2 x 1 6 CD4
Supplementary figure legends Supplementary Figure 1. Characterization of after reconstitution of SCID mice with CD4 + CD62L + T cells. (A-C) SCID mice (n = 6 / group) were reconstituted with 2 x 1 6 CD4
Supplementary Figures
 Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD-
 Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
SUPPLEMENTARY INFORMATION
 doi:1.138/nature1554 a TNF-α + in CD4 + cells [%] 1 GF SPF 6 b IL-1 + in CD4 + cells [%] 5 4 3 2 1 Supplementary Figure 1. Effect of microbiota on cytokine profiles of T cells in GALT. Frequencies of TNF-α
doi:1.138/nature1554 a TNF-α + in CD4 + cells [%] 1 GF SPF 6 b IL-1 + in CD4 + cells [%] 5 4 3 2 1 Supplementary Figure 1. Effect of microbiota on cytokine profiles of T cells in GALT. Frequencies of TNF-α
MATERIALS AND METHODS. Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All
 MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
MATERIALS AND METHODS Antibodies (Abs), flow cytometry analysis and cell lines Neutralizing antibodies specific to mouse Dll1, Dll4, J1 and J2 were prepared as described. 1,2 All other antibodies used
T Cells from Lungs and Livers of Francisella tularensis-immune Mice Control the Growth of Intracellular Bacteria
 INFECTION AND IMMUNITY, May 2009, p. 2010 2021 Vol. 77, No. 5 0019-9567/09/$08.00 0 doi:10.1128/iai.01322-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. T Cells from Lungs and
INFECTION AND IMMUNITY, May 2009, p. 2010 2021 Vol. 77, No. 5 0019-9567/09/$08.00 0 doi:10.1128/iai.01322-08 Copyright 2009, American Society for Microbiology. All Rights Reserved. T Cells from Lungs and
NK cell flow cytometric assay In vivo DC viability and migration assay
 NK cell flow cytometric assay 6 NK cells were purified, by negative selection with the NK Cell Isolation Kit (Miltenyi iotec), from spleen and lymph nodes of 6 RAG1KO mice, injected the day before with
NK cell flow cytometric assay 6 NK cells were purified, by negative selection with the NK Cell Isolation Kit (Miltenyi iotec), from spleen and lymph nodes of 6 RAG1KO mice, injected the day before with
Structure and Function of Antigen Recognition Molecules
 MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
MICR2209 Structure and Function of Antigen Recognition Molecules Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will examine the major receptors used by cells of the innate and
Supplemental Figure 1. Signature gene expression in in vitro differentiated Th0, Th1, Th2, Th17 and Treg cells. (A) Naïve CD4 + T cells were cultured
 Supplemental Figure 1. Signature gene expression in in vitro differentiated Th0, Th1, Th2, Th17 and Treg cells. (A) Naïve CD4 + T cells were cultured under Th0, Th1, Th2, Th17, and Treg conditions. mrna
Supplemental Figure 1. Signature gene expression in in vitro differentiated Th0, Th1, Th2, Th17 and Treg cells. (A) Naïve CD4 + T cells were cultured under Th0, Th1, Th2, Th17, and Treg conditions. mrna
Supplementary Information. Tissue-wide immunity against Leishmania. through collective production of nitric oxide
 Supplementary Information Tissue-wide immunity against Leishmania through collective production of nitric oxide Romain Olekhnovitch, Bernhard Ryffel, Andreas J. Müller and Philippe Bousso Supplementary
Supplementary Information Tissue-wide immunity against Leishmania through collective production of nitric oxide Romain Olekhnovitch, Bernhard Ryffel, Andreas J. Müller and Philippe Bousso Supplementary
/01/$ DOI: /IAI Received 21 July 2000/Returned for modification 26 September 2000/Accepted 13 October 2000
 INFECTION AND IMMUNITY, Jan. 2001, p. 194 203 Vol. 69, No. 1 0019-9567/01/$04.00 0 DOI: 10.1128/IAI.69.1.194 203.2001 Susceptibility to Secondary Francisella tularensis Live Vaccine Strain Infection in
INFECTION AND IMMUNITY, Jan. 2001, p. 194 203 Vol. 69, No. 1 0019-9567/01/$04.00 0 DOI: 10.1128/IAI.69.1.194 203.2001 Susceptibility to Secondary Francisella tularensis Live Vaccine Strain Infection in
Supplemental Information. CD4 + CD25 + Foxp3 + Regulatory T Cells Promote. Th17 Cells In Vitro and Enhance Host Resistance
 Immunity, Volume 34 Supplemental Information D4 + D25 + + Regulatory T ells Promote Th17 ells In Vitro and Enhance Host Resistance in Mouse andida albicans Th17 ell Infection Model Pushpa Pandiyan, Heather
Immunity, Volume 34 Supplemental Information D4 + D25 + + Regulatory T ells Promote Th17 ells In Vitro and Enhance Host Resistance in Mouse andida albicans Th17 ell Infection Model Pushpa Pandiyan, Heather
The Involvement of IL-17A in the Murine Response to Sub-Lethal Inhalational Infection with Francisella tularensis
 The Involvement of IL-17A in the Murine Response to Sub-Lethal Inhalational Infection with Francisella tularensis Gal Markel 1, Erez Bar-Haim 1, Eran Zahavy 2, Hila Cohen 1, Ofer Cohen 1, Avigdor Shafferman
The Involvement of IL-17A in the Murine Response to Sub-Lethal Inhalational Infection with Francisella tularensis Gal Markel 1, Erez Bar-Haim 1, Eran Zahavy 2, Hila Cohen 1, Ofer Cohen 1, Avigdor Shafferman
D CD8 T cell number (x10 6 )
 IFNγ Supplemental Figure 1. CD T cell number (x1 6 ) 18 15 1 9 6 3 CD CD T cells CD6L C CD5 CD T cells CD6L D CD8 T cell number (x1 6 ) 1 8 6 E CD CD8 T cells CD6L F Log(1)CFU/g Feces 1 8 6 p
IFNγ Supplemental Figure 1. CD T cell number (x1 6 ) 18 15 1 9 6 3 CD CD T cells CD6L C CD5 CD T cells CD6L D CD8 T cell number (x1 6 ) 1 8 6 E CD CD8 T cells CD6L F Log(1)CFU/g Feces 1 8 6 p
SUPPLEMENTARY FIGURES
 SUPPLEMENTARY FIGURES Supplementary Figure 1: Chemokine receptor expression profiles of CCR6 + and CCR6 - CD4 + IL-17A +/ex and Treg cells. Quantitative PCR analysis of chemokine receptor transcript abundance
SUPPLEMENTARY FIGURES Supplementary Figure 1: Chemokine receptor expression profiles of CCR6 + and CCR6 - CD4 + IL-17A +/ex and Treg cells. Quantitative PCR analysis of chemokine receptor transcript abundance
SUPPLEMENTARY INFORMATION
 Complete but curtailed T-cell response to very-low-affinity antigen Dietmar Zehn, Sarah Y. Lee & Michael J. Bevan Supp. Fig. 1: TCR chain usage among endogenous K b /Ova reactive T cells. C57BL/6 mice
Complete but curtailed T-cell response to very-low-affinity antigen Dietmar Zehn, Sarah Y. Lee & Michael J. Bevan Supp. Fig. 1: TCR chain usage among endogenous K b /Ova reactive T cells. C57BL/6 mice
Supporting Information
 Supporting Information Aldridge et al. 10.1073/pnas.0900655106 Fig. S1. Flow diagram of sublethal (a) and lethal (b) influenza virus infections. (a) Infection of lung epithelial cells by influenza virus
Supporting Information Aldridge et al. 10.1073/pnas.0900655106 Fig. S1. Flow diagram of sublethal (a) and lethal (b) influenza virus infections. (a) Infection of lung epithelial cells by influenza virus
T cell development October 28, Dan Stetson
 T cell development October 28, 2016 Dan Stetson stetson@uw.edu 441 Lecture #13 Slide 1 of 29 Three lectures on T cells (Chapters 8, 9) Part 1 (Today): T cell development in the thymus Chapter 8, pages
T cell development October 28, 2016 Dan Stetson stetson@uw.edu 441 Lecture #13 Slide 1 of 29 Three lectures on T cells (Chapters 8, 9) Part 1 (Today): T cell development in the thymus Chapter 8, pages
General Overview of Immunology. Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center
 General Overview of Immunology Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center Objectives Describe differences between innate and adaptive immune responses
General Overview of Immunology Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center Objectives Describe differences between innate and adaptive immune responses
L-selectin Is Essential for Delivery of Activated CD8 + T Cells to Virus-Infected Organs for Protective Immunity
 Cell Reports Supplemental Information L-selectin Is Essential for Delivery of Activated CD8 + T Cells to Virus-Infected Organs for Protective Immunity Rebar N. Mohammed, H. Angharad Watson, Miriam Vigar,
Cell Reports Supplemental Information L-selectin Is Essential for Delivery of Activated CD8 + T Cells to Virus-Infected Organs for Protective Immunity Rebar N. Mohammed, H. Angharad Watson, Miriam Vigar,
T cell maturation. T-cell Maturation. What allows T cell maturation?
 T-cell Maturation What allows T cell maturation? Direct contact with thymic epithelial cells Influence of thymic hormones Growth factors (cytokines, CSF) T cell maturation T cell progenitor DN DP SP 2ry
T-cell Maturation What allows T cell maturation? Direct contact with thymic epithelial cells Influence of thymic hormones Growth factors (cytokines, CSF) T cell maturation T cell progenitor DN DP SP 2ry
Increased IL-12 induced STAT-4 signaling in CD8 T cells. from aged mice
 Increased IL-2 induced STAT-4 signaling in CD8 T cells from aged mice Erin Rottinghaus * Abstract: Aging is associated with poor immune function leading to increased susceptibility to infectious diseases
Increased IL-2 induced STAT-4 signaling in CD8 T cells from aged mice Erin Rottinghaus * Abstract: Aging is associated with poor immune function leading to increased susceptibility to infectious diseases
Supplementary Figure S1. PTPN2 levels are not altered in proliferating CD8+ T cells. Lymph node (LN) CD8+ T cells from C57BL/6 mice were stained with
 Supplementary Figure S1. PTPN2 levels are not altered in proliferating CD8+ T cells. Lymph node (LN) CD8+ T cells from C57BL/6 mice were stained with CFSE and stimulated with plate-bound α-cd3ε (10µg/ml)
Supplementary Figure S1. PTPN2 levels are not altered in proliferating CD8+ T cells. Lymph node (LN) CD8+ T cells from C57BL/6 mice were stained with CFSE and stimulated with plate-bound α-cd3ε (10µg/ml)
Supplemental Information. T Cells Enhance Autoimmunity by Restraining Regulatory T Cell Responses via an Interleukin-23-Dependent Mechanism
 Immunity, Volume 33 Supplemental Information T Cells Enhance Autoimmunity by Restraining Regulatory T Cell Responses via an Interleukin-23-Dependent Mechanism Franziska Petermann, Veit Rothhammer, Malte
Immunity, Volume 33 Supplemental Information T Cells Enhance Autoimmunity by Restraining Regulatory T Cell Responses via an Interleukin-23-Dependent Mechanism Franziska Petermann, Veit Rothhammer, Malte
Supplemental Table I.
 Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
T Cell Differentiation
 T Cell Differentiation Ned Braunstein, MD MHC control of Immune Responsiveness: Concept Whether or not an individual makes an immune response to a particular antigen depends on what MHC alleles an individual
T Cell Differentiation Ned Braunstein, MD MHC control of Immune Responsiveness: Concept Whether or not an individual makes an immune response to a particular antigen depends on what MHC alleles an individual
Supporting Information
 Supporting Information lpek et al. 1.173/pnas.1121217 SI Materials and Methods Mice. cell knockout, inos / (Taconic arms), Rag1 /, INγR /, and IL-12p4 / mice (The Jackson Laboratory) were maintained and/or
Supporting Information lpek et al. 1.173/pnas.1121217 SI Materials and Methods Mice. cell knockout, inos / (Taconic arms), Rag1 /, INγR /, and IL-12p4 / mice (The Jackson Laboratory) were maintained and/or
SUPPLEMENTARY INFORMATION
 Supplemental Figure 1. Furin is efficiently deleted in CD4 + and CD8 + T cells. a, Western blot for furin and actin proteins in CD4cre-fur f/f and fur f/f Th1 cells. Wild-type and furin-deficient CD4 +
Supplemental Figure 1. Furin is efficiently deleted in CD4 + and CD8 + T cells. a, Western blot for furin and actin proteins in CD4cre-fur f/f and fur f/f Th1 cells. Wild-type and furin-deficient CD4 +
Nature Medicine: doi: /nm.2109
 HIV 1 Infects Multipotent Progenitor Cells Causing Cell Death and Establishing Latent Cellular Reservoirs Christoph C. Carter, Adewunmi Onafuwa Nuga, Lucy A. M c Namara, James Riddell IV, Dale Bixby, Michael
HIV 1 Infects Multipotent Progenitor Cells Causing Cell Death and Establishing Latent Cellular Reservoirs Christoph C. Carter, Adewunmi Onafuwa Nuga, Lucy A. M c Namara, James Riddell IV, Dale Bixby, Michael
Supplementary Materials for
 immunology.sciencemag.org/cgi/content/full/2/16/eaan6049/dc1 Supplementary Materials for Enzymatic synthesis of core 2 O-glycans governs the tissue-trafficking potential of memory CD8 + T cells Jossef
immunology.sciencemag.org/cgi/content/full/2/16/eaan6049/dc1 Supplementary Materials for Enzymatic synthesis of core 2 O-glycans governs the tissue-trafficking potential of memory CD8 + T cells Jossef
Supplementary Figure 1
 Supplementary Figure 1 Identification of IFN-γ-producing CD8 + and CD4 + T cells with naive phenotype by alternative gating and sample-processing strategies. a. Contour 5% probability plots show definition
Supplementary Figure 1 Identification of IFN-γ-producing CD8 + and CD4 + T cells with naive phenotype by alternative gating and sample-processing strategies. a. Contour 5% probability plots show definition
Scott Abrams, Ph.D. Professor of Oncology, x4375 Kuby Immunology SEVENTH EDITION
 Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 13 Effector Responses: Cell- and Antibody-Mediated Immunity Copyright 2013 by W. H.
Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 13 Effector Responses: Cell- and Antibody-Mediated Immunity Copyright 2013 by W. H.
Kerdiles et al - Figure S1
 Kerdiles et al - Figure S1 a b Homo sapiens T B ce ce l ls c l M ls ac r PM oph N ag es Mus musculus Foxo1 PLCγ Supplementary Figure 1 Foxo1 expression pattern is conserved between mouse and human. (a)
Kerdiles et al - Figure S1 a b Homo sapiens T B ce ce l ls c l M ls ac r PM oph N ag es Mus musculus Foxo1 PLCγ Supplementary Figure 1 Foxo1 expression pattern is conserved between mouse and human. (a)
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. NKT ligand-loaded tumour antigen-presenting B cell- and monocyte-based vaccine induces NKT, NK and CD8 T cell responses. (A) The cytokine profiles of liver
Supplementary Figures Supplementary Figure 1. NKT ligand-loaded tumour antigen-presenting B cell- and monocyte-based vaccine induces NKT, NK and CD8 T cell responses. (A) The cytokine profiles of liver
Supplementary Figure 1. Efficient DC depletion in CD11c.DOG transgenic mice
 Supplementary Figure 1. Efficient DC depletion in CD11c.DOG transgenic mice (a) CD11c.DOG transgenic mice (tg) were treated with 8 ng/g body weight (b.w.) diphtheria toxin (DT) i.p. on day -1 and every
Supplementary Figure 1. Efficient DC depletion in CD11c.DOG transgenic mice (a) CD11c.DOG transgenic mice (tg) were treated with 8 ng/g body weight (b.w.) diphtheria toxin (DT) i.p. on day -1 and every
Supplemental Materials for. Effects of sphingosine-1-phosphate receptor 1 phosphorylation in response to. FTY720 during neuroinflammation
 Supplemental Materials for Effects of sphingosine-1-phosphate receptor 1 phosphorylation in response to FTY7 during neuroinflammation This file includes: Supplemental Table 1. EAE clinical parameters of
Supplemental Materials for Effects of sphingosine-1-phosphate receptor 1 phosphorylation in response to FTY7 during neuroinflammation This file includes: Supplemental Table 1. EAE clinical parameters of
Adaptive Immunity to Bacteria. T cell subsets
 Adaptive Immunity to Bacteria Role of T cells in anti-bacterial host responses. Dr. C. Piccirillo Department of Microbiology & Immunology McGill University T cell subsets MHC I and II -restricted cells
Adaptive Immunity to Bacteria Role of T cells in anti-bacterial host responses. Dr. C. Piccirillo Department of Microbiology & Immunology McGill University T cell subsets MHC I and II -restricted cells
B220 CD4 CD8. Figure 1. Confocal Image of Sensitized HLN. Representative image of a sensitized HLN
 B220 CD4 CD8 Natarajan et al., unpublished data Figure 1. Confocal Image of Sensitized HLN. Representative image of a sensitized HLN showing B cell follicles and T cell areas. 20 µm thick. Image of magnification
B220 CD4 CD8 Natarajan et al., unpublished data Figure 1. Confocal Image of Sensitized HLN. Representative image of a sensitized HLN showing B cell follicles and T cell areas. 20 µm thick. Image of magnification
Chapter 10 (pages ): Differentiation and Functions of CD4+ Effector T Cells Prepared by Kristen Dazy, MD, Scripps Clinic Medical Group
 FIT Board Review Corner September 2015 Welcome to the FIT Board Review Corner, prepared by Andrew Nickels, MD, and Sarah Spriet, DO, senior and junior representatives of ACAAI's Fellows-In-Training (FITs)
FIT Board Review Corner September 2015 Welcome to the FIT Board Review Corner, prepared by Andrew Nickels, MD, and Sarah Spriet, DO, senior and junior representatives of ACAAI's Fellows-In-Training (FITs)
The Adaptive Immune Response. B-cells
 The Adaptive Immune Response B-cells The innate immune system provides immediate protection. The adaptive response takes time to develop and is antigen specific. Activation of B and T lymphocytes Naive
The Adaptive Immune Response B-cells The innate immune system provides immediate protection. The adaptive response takes time to develop and is antigen specific. Activation of B and T lymphocytes Naive
Supplementary Material
 Supplementary Material Supplementary Figure 1. NOS2 -/- mice develop an analogous Ghon complex after infection in the ear dermis and show dissemination of Mtb to the lung. (A) WT and NOS2 -/- mice were
Supplementary Material Supplementary Figure 1. NOS2 -/- mice develop an analogous Ghon complex after infection in the ear dermis and show dissemination of Mtb to the lung. (A) WT and NOS2 -/- mice were
Supplementary Materials for
 www.sciencemag.org/content/348/6241/aaa825/suppl/dc1 Supplementary Materials for A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells Georg Stary,* Andrew Olive,
www.sciencemag.org/content/348/6241/aaa825/suppl/dc1 Supplementary Materials for A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells Georg Stary,* Andrew Olive,
Nature Immunology: doi: /ni Supplementary Figure 1. Cytokine pattern in skin in response to urushiol.
 Supplementary Figure 1 Cytokine pattern in skin in response to urushiol. Wild-type (WT) and CD1a-tg mice (n = 3 per group) were sensitized and challenged with urushiol (uru) or vehicle (veh). Quantitative
Supplementary Figure 1 Cytokine pattern in skin in response to urushiol. Wild-type (WT) and CD1a-tg mice (n = 3 per group) were sensitized and challenged with urushiol (uru) or vehicle (veh). Quantitative
Supplementary Figure 1.
 Supplementary Figure 1. Female Pro-ins2 -/- mice at 5-6 weeks of age were either inoculated i.p. with a single dose of CVB4 (1x10 5 PFU/mouse) or PBS and treated with αgalcer or control vehicle. On day
Supplementary Figure 1. Female Pro-ins2 -/- mice at 5-6 weeks of age were either inoculated i.p. with a single dose of CVB4 (1x10 5 PFU/mouse) or PBS and treated with αgalcer or control vehicle. On day
TITLE: MODULATION OF T CELL TOLERANCE IN A MURINE MODEL FOR IMMUNOTHERAPY OF PROSTATIC ADENOCARCINOMA
 AD Award Number: DAMD17-01-1-0085 TITLE: MODULATION OF T CELL TOLERANCE IN A MURINE MODEL FOR IMMUNOTHERAPY OF PROSTATIC ADENOCARCINOMA PRINCIPAL INVESTIGATOR: ARTHUR A HURWITZ, Ph.d. CONTRACTING ORGANIZATION:
AD Award Number: DAMD17-01-1-0085 TITLE: MODULATION OF T CELL TOLERANCE IN A MURINE MODEL FOR IMMUNOTHERAPY OF PROSTATIC ADENOCARCINOMA PRINCIPAL INVESTIGATOR: ARTHUR A HURWITZ, Ph.d. CONTRACTING ORGANIZATION:
ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY
 ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY The recognition of specific antigen by naïve T cell induces its own activation and effector phases. T helper cells recognize peptide antigens through
ACTIVATION OF T LYMPHOCYTES AND CELL MEDIATED IMMUNITY The recognition of specific antigen by naïve T cell induces its own activation and effector phases. T helper cells recognize peptide antigens through
Supplemental Figure 1
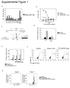 Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
Supplemental Figure 1 1a 1c PD-1 MFI fold change 6 5 4 3 2 1 IL-1α IL-2 IL-4 IL-6 IL-1 IL-12 IL-13 IL-15 IL-17 IL-18 IL-21 IL-23 IFN-α Mut Human PD-1 promoter SBE-D 5 -GTCTG- -1.2kb SBE-P -CAGAC- -1.kb
CD4 + T cells recovered in Rag2 / recipient ( 10 5 ) Heart Lung Pancreas
 a CD4 + T cells recovered in Rag2 / recipient ( 1 5 ) Heart Lung Pancreas.5 1 2 4 6 2 4 6 Ctla4 +/+ Ctla4 / Ctla4 / Lung Ctla4 / Pancreas b Heart Lung Pancreas Ctla4 +/+ Ctla4 / Ctla4 / Lung Ctla4 / Pancreas
a CD4 + T cells recovered in Rag2 / recipient ( 1 5 ) Heart Lung Pancreas.5 1 2 4 6 2 4 6 Ctla4 +/+ Ctla4 / Ctla4 / Lung Ctla4 / Pancreas b Heart Lung Pancreas Ctla4 +/+ Ctla4 / Ctla4 / Lung Ctla4 / Pancreas
Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to
 Class II tetramer staining Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to PE were combined with dominant HIV epitopes (DRB1*0101-DRFYKTLRAEQASQEV, DRB1*0301- PEKEVLVWKFDSRLAFHH,
Class II tetramer staining Commercially available HLA Class II tetramers (Beckman Coulter) conjugated to PE were combined with dominant HIV epitopes (DRB1*0101-DRFYKTLRAEQASQEV, DRB1*0301- PEKEVLVWKFDSRLAFHH,
Adaptive immune responses: T cell-mediated immunity
 MICR2209 Adaptive immune responses: T cell-mediated immunity Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will discuss the T-cell mediated immune response, how it is activated,
MICR2209 Adaptive immune responses: T cell-mediated immunity Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will discuss the T-cell mediated immune response, how it is activated,
General information. Cell mediated immunity. 455 LSA, Tuesday 11 to noon. Anytime after class.
 General information Cell mediated immunity 455 LSA, Tuesday 11 to noon Anytime after class T-cell precursors Thymus Naive T-cells (CD8 or CD4) email: lcoscoy@berkeley.edu edu Use MCB150 as subject line
General information Cell mediated immunity 455 LSA, Tuesday 11 to noon Anytime after class T-cell precursors Thymus Naive T-cells (CD8 or CD4) email: lcoscoy@berkeley.edu edu Use MCB150 as subject line
Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell?
 Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/3/114/ra23/dc1 Supplementary Materials for Regulation of Zap70 Expression During Thymocyte Development Enables Temporal Separation of CD4 and CD8 Repertoire Selection
www.sciencesignaling.org/cgi/content/full/3/114/ra23/dc1 Supplementary Materials for Regulation of Zap70 Expression During Thymocyte Development Enables Temporal Separation of CD4 and CD8 Repertoire Selection
Supplementary Figure 1. Deletion of Smad3 prevents B16F10 melanoma invasion and metastasis in a mouse s.c. tumor model.
 A B16F1 s.c. Lung LN Distant lymph nodes Colon B B16F1 s.c. Supplementary Figure 1. Deletion of Smad3 prevents B16F1 melanoma invasion and metastasis in a mouse s.c. tumor model. Highly invasive growth
A B16F1 s.c. Lung LN Distant lymph nodes Colon B B16F1 s.c. Supplementary Figure 1. Deletion of Smad3 prevents B16F1 melanoma invasion and metastasis in a mouse s.c. tumor model. Highly invasive growth
The Adaptive Immune Responses
 The Adaptive Immune Responses The two arms of the immune responses are; 1) the cell mediated, and 2) the humoral responses. In this chapter we will discuss the two responses in detail and we will start
The Adaptive Immune Responses The two arms of the immune responses are; 1) the cell mediated, and 2) the humoral responses. In this chapter we will discuss the two responses in detail and we will start
Supplementary Figure 1. ETBF activate Stat3 in B6 and Min mice colons
 Supplementary Figure 1 ETBF activate Stat3 in B6 and Min mice colons a pstat3 controls Pos Neg ETBF 1 2 3 4 b pstat1 pstat2 pstat3 pstat4 pstat5 pstat6 Actin Figure Legend: (a) ETBF induce predominantly
Supplementary Figure 1 ETBF activate Stat3 in B6 and Min mice colons a pstat3 controls Pos Neg ETBF 1 2 3 4 b pstat1 pstat2 pstat3 pstat4 pstat5 pstat6 Actin Figure Legend: (a) ETBF induce predominantly
for six pairs of mice. (b) Representative FACS analysis of absolute number of T cells (CD4 + and
 SUPPLEMENTARY DATA Supplementary Figure 1: Peripheral lymphoid organs of SMAR1 -/- mice have an effector memory phenotype. (a) Lymphocytes collected from MLNs and Peyer s patches (PPs) of WT and SMAR1
SUPPLEMENTARY DATA Supplementary Figure 1: Peripheral lymphoid organs of SMAR1 -/- mice have an effector memory phenotype. (a) Lymphocytes collected from MLNs and Peyer s patches (PPs) of WT and SMAR1
Cell Mediated Immunity CELL MEDIATED IMMUNITY. Basic Elements of Cell Mediated Immunity (CMI) Antibody-dependent cell-mediated cytotoxicity (ADCC)
 Chapter 16 CELL MEDIATED IMMUNITY Cell Mediated Immunity Also known as Cellular Immunity or CMI The effector phase T cells Specificity for immune recognition reactions TH provide cytokines CTLs do the
Chapter 16 CELL MEDIATED IMMUNITY Cell Mediated Immunity Also known as Cellular Immunity or CMI The effector phase T cells Specificity for immune recognition reactions TH provide cytokines CTLs do the
pplementary Figur Supplementary Figure 1. a.
 pplementary Figur Supplementary Figure 1. a. Quantification by RT-qPCR of YFV-17D and YFV-17D pol- (+) RNA in the supernatant of cultured Huh7.5 cells following viral RNA electroporation of respective
pplementary Figur Supplementary Figure 1. a. Quantification by RT-qPCR of YFV-17D and YFV-17D pol- (+) RNA in the supernatant of cultured Huh7.5 cells following viral RNA electroporation of respective
Supplementary Figure 1. Efficiency of Mll4 deletion and its effect on T cell populations in the periphery. Nature Immunology: doi: /ni.
 Supplementary Figure 1 Efficiency of Mll4 deletion and its effect on T cell populations in the periphery. Expression of Mll4 floxed alleles (16-19) in naive CD4 + T cells isolated from lymph nodes and
Supplementary Figure 1 Efficiency of Mll4 deletion and its effect on T cell populations in the periphery. Expression of Mll4 floxed alleles (16-19) in naive CD4 + T cells isolated from lymph nodes and
Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells
 ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
ICI Basic Immunology course Effector mechanisms of cell-mediated immunity: Properties of effector, memory and regulatory T cells Abul K. Abbas, MD UCSF Stages in the development of T cell responses: induction
Supporting Information
 Supporting Information Desnues et al. 10.1073/pnas.1314121111 SI Materials and Methods Mice. Toll-like receptor (TLR)8 / and TLR9 / mice were generated as described previously (1, 2). TLR9 / mice were
Supporting Information Desnues et al. 10.1073/pnas.1314121111 SI Materials and Methods Mice. Toll-like receptor (TLR)8 / and TLR9 / mice were generated as described previously (1, 2). TLR9 / mice were
A Fungal Glycosphingolipid Directly Activates Natural Killer T Cells and Rapidly Induces Airways Disease
 A Fungal Glycosphingolipid Directly Activates Natural Killer T Cells and Rapidly Induces Airways Disease Lee A. Albacker, Vinod Chaudhary, Ya-Jen Chang, Hye Young Kim, Ya-Ting Chuang, Muriel Pichavant,
A Fungal Glycosphingolipid Directly Activates Natural Killer T Cells and Rapidly Induces Airways Disease Lee A. Albacker, Vinod Chaudhary, Ya-Jen Chang, Hye Young Kim, Ya-Ting Chuang, Muriel Pichavant,
Supporting Information
 Supporting Information Valkenburg et al. 10.1073/pnas.1403684111 SI Materials and Methods ELISA and Microneutralization. Sera were treated with Receptor Destroying Enzyme II (RDE II, Accurate) before ELISA
Supporting Information Valkenburg et al. 10.1073/pnas.1403684111 SI Materials and Methods ELISA and Microneutralization. Sera were treated with Receptor Destroying Enzyme II (RDE II, Accurate) before ELISA
Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice
 Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
Supplementary Methods: Cell isolation. Spleen and lymph nodes (axillary, inguinal) were removed from mice and gently meshed in DMEM containing 10% FBS to prepare for single cell suspensions. CD4 + CD25
Distinct activation thresholds of human conventional and innate-like memory T cells
 Distinct activation thresholds of human conventional and innate-like memory T cells Chloe K. Slichter,, Raphael Gottardo, Martin Prlic JCI Insight. 2016;1(8):e86292. https://doi.org/10.1172/jci.insight.86292.
Distinct activation thresholds of human conventional and innate-like memory T cells Chloe K. Slichter,, Raphael Gottardo, Martin Prlic JCI Insight. 2016;1(8):e86292. https://doi.org/10.1172/jci.insight.86292.
Medical Virology Immunology. Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University
 Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Suppl Video: Tumor cells (green) and monocytes (white) are seeded on a confluent endothelial
 Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Therapeutic PD L1 and LAG 3 blockade rapidly clears established blood stage Plasmodium infection
 Supplementary Information Therapeutic PD L1 and LAG 3 blockade rapidly clears established blood stage Plasmodium infection Noah S. Butler, Jacqueline Moebius, Lecia L. Pewe, Boubacar Traore, Ogobara K.
Supplementary Information Therapeutic PD L1 and LAG 3 blockade rapidly clears established blood stage Plasmodium infection Noah S. Butler, Jacqueline Moebius, Lecia L. Pewe, Boubacar Traore, Ogobara K.
Supplemental Figure 1. Protein L
 Supplemental Figure 1 Protein L m19delta T m1928z T Suppl. Fig 1. Expression of CAR: B6-derived T cells were transduced with m19delta (left) and m1928z (right) to generate CAR T cells and transduction
Supplemental Figure 1 Protein L m19delta T m1928z T Suppl. Fig 1. Expression of CAR: B6-derived T cells were transduced with m19delta (left) and m1928z (right) to generate CAR T cells and transduction
Intracellular MHC class II molecules promote TLR-triggered innate. immune responses by maintaining Btk activation
 Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Intracellular MHC class II molecules promote TLR-triggered innate immune responses by maintaining Btk activation Xingguang Liu, Zhenzhen Zhan, Dong Li, Li Xu, Feng Ma, Peng Zhang, Hangping Yao and Xuetao
Putting it Together. Stephen Canfield Secondary Lymphoid System. Tonsil Anterior Cervical LN s
 Putting it Together Stephen Canfield smc12@columbia.edu Secondary Lymphoid System Tonsil Anterior Cervical LN s Axillary LN s Mediastinal/Retroperitoneal LN s Thoracic Duct Appendix Spleen Inguinal LN
Putting it Together Stephen Canfield smc12@columbia.edu Secondary Lymphoid System Tonsil Anterior Cervical LN s Axillary LN s Mediastinal/Retroperitoneal LN s Thoracic Duct Appendix Spleen Inguinal LN
HLA and antigen presentation. Department of Immunology Charles University, 2nd Medical School University Hospital Motol
 HLA and antigen presentation Department of Immunology Charles University, 2nd Medical School University Hospital Motol MHC in adaptive immunity Characteristics Specificity Innate For structures shared
HLA and antigen presentation Department of Immunology Charles University, 2nd Medical School University Hospital Motol MHC in adaptive immunity Characteristics Specificity Innate For structures shared
The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23- induced production of the cytokine GM-CSF
 CORRECTION NOTICE Nat.Immunol. 12, 568 575 (2011) The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23- induced production of the cytokine GM-CSF Mohamed El-Behi, Bogoljub Ciric, Hong
CORRECTION NOTICE Nat.Immunol. 12, 568 575 (2011) The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23- induced production of the cytokine GM-CSF Mohamed El-Behi, Bogoljub Ciric, Hong
Micro 204. Cytotoxic T Lymphocytes (CTL) Lewis Lanier
 Micro 204 Cytotoxic T Lymphocytes (CTL) Lewis Lanier Lewis.Lanier@ucsf.edu Lymphocyte-mediated Cytotoxicity CD8 + αβ-tcr + T cells CD4 + αβ-tcr + T cells γδ-tcr + T cells Natural Killer cells CD8 + αβ-tcr
Micro 204 Cytotoxic T Lymphocytes (CTL) Lewis Lanier Lewis.Lanier@ucsf.edu Lymphocyte-mediated Cytotoxicity CD8 + αβ-tcr + T cells CD4 + αβ-tcr + T cells γδ-tcr + T cells Natural Killer cells CD8 + αβ-tcr
CD40L TCR IL-12 TLR-L
 CD40L B cells plasmacells Neutrophils TCR inkt cells IL-12 Ab production Can inkt cells modulate the cytokine profile of neutrophils? TLR-L CD4+ and CD8+ T cell responses 1. The invariant TCR expressed
CD40L B cells plasmacells Neutrophils TCR inkt cells IL-12 Ab production Can inkt cells modulate the cytokine profile of neutrophils? TLR-L CD4+ and CD8+ T cell responses 1. The invariant TCR expressed
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table
 Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Peer Review File Description: Innate Scavenger Receptor-A regulates
Title of file for HTML: Supplementary Information Description: Supplementary Figures and Supplementary Table Title of file for HTML: Peer Review File Description: Innate Scavenger Receptor-A regulates
B6.SJL (Ly5.2) mice were obtained from Taconic Farms. Rag1-deficient mice were
 Supplementary Methods Mice. B6.SJL (Ly5.2) mice were obtained from Taconic Farms. Rag1-deficient mice were purchased from The Jackson Laboratory. Real-time PCR Total cellular RNA was extracted from the
Supplementary Methods Mice. B6.SJL (Ly5.2) mice were obtained from Taconic Farms. Rag1-deficient mice were purchased from The Jackson Laboratory. Real-time PCR Total cellular RNA was extracted from the
Antigen Presentation and T Lymphocyte Activation. Abul K. Abbas UCSF. FOCiS
 1 Antigen Presentation and T Lymphocyte Activation Abul K. Abbas UCSF FOCiS 2 Lecture outline Dendritic cells and antigen presentation The role of the MHC T cell activation Costimulation, the B7:CD28 family
1 Antigen Presentation and T Lymphocyte Activation Abul K. Abbas UCSF FOCiS 2 Lecture outline Dendritic cells and antigen presentation The role of the MHC T cell activation Costimulation, the B7:CD28 family
TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer
 AD Award Number: W8-XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke, Ph.D. CONTRACTING ORGANIZATION: Johns Hopkins
AD Award Number: W8-XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke, Ph.D. CONTRACTING ORGANIZATION: Johns Hopkins
Human and mouse T cell regulation mediated by soluble CD52 interaction with Siglec-10. Esther Bandala-Sanchez, Yuxia Zhang, Simone Reinwald,
 Human and mouse T cell regulation mediated by soluble CD52 interaction with Siglec-1 Esther Bandala-Sanchez, Yuxia Zhang, Simone Reinwald, James A. Dromey, Bo Han Lee, Junyan Qian, Ralph M Böhmer and Leonard
Human and mouse T cell regulation mediated by soluble CD52 interaction with Siglec-1 Esther Bandala-Sanchez, Yuxia Zhang, Simone Reinwald, James A. Dromey, Bo Han Lee, Junyan Qian, Ralph M Böhmer and Leonard
and follicular helper T cells is Egr2-dependent. (a) Diagrammatic representation of the
 Supplementary Figure 1. LAG3 + Treg-mediated regulation of germinal center B cells and follicular helper T cells is Egr2-dependent. (a) Diagrammatic representation of the experimental protocol for the
Supplementary Figure 1. LAG3 + Treg-mediated regulation of germinal center B cells and follicular helper T cells is Egr2-dependent. (a) Diagrammatic representation of the experimental protocol for the
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Supplemental Information. Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection. Cell Host & Microbe, Volume 15
 Cell Host & Microbe, Volume 15 Supplemental Information Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection Arya Khosravi, Alberto Yáñez, Jeremy G. Price, Andrew Chow, Miriam Merad, Helen
Cell Host & Microbe, Volume 15 Supplemental Information Gut Microbiota Promotes Hematopoiesis to Control Bacterial Infection Arya Khosravi, Alberto Yáñez, Jeremy G. Price, Andrew Chow, Miriam Merad, Helen
NKTR-255: Accessing The Immunotherapeutic Potential Of IL-15 for NK Cell Therapies
 NKTR-255: Accessing The Immunotherapeutic Potential Of IL-15 for NK Cell Therapies Saul Kivimäe Senior Scientist, Research Biology Nektar Therapeutics NK Cell-Based Cancer Immunotherapy, September 26-27,
NKTR-255: Accessing The Immunotherapeutic Potential Of IL-15 for NK Cell Therapies Saul Kivimäe Senior Scientist, Research Biology Nektar Therapeutics NK Cell-Based Cancer Immunotherapy, September 26-27,
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the T H 17 cell it reg cell balance Rajatava Basu 1,5, Sarah K.
SUPPLEMENTARY MATERIAL IL-1 signaling modulates activation of STAT transcription factors to antagonize retinoic acid signaling and control the T H 17 cell it reg cell balance Rajatava Basu 1,5, Sarah K.
The development of T cells in the thymus
 T cells rearrange their receptors in the thymus whereas B cells do so in the bone marrow. The development of T cells in the thymus The lobular/cellular organization of the thymus Immature cells are called
T cells rearrange their receptors in the thymus whereas B cells do so in the bone marrow. The development of T cells in the thymus The lobular/cellular organization of the thymus Immature cells are called
Test Bank for Basic Immunology Functions and Disorders of the Immune System 4th Edition by Abbas
 Test Bank for Basic Immunology Functions and Disorders of the Immune System 4th Edition by Abbas Chapter 04: Antigen Recognition in the Adaptive Immune System Test Bank MULTIPLE CHOICE 1. Most T lymphocytes
Test Bank for Basic Immunology Functions and Disorders of the Immune System 4th Edition by Abbas Chapter 04: Antigen Recognition in the Adaptive Immune System Test Bank MULTIPLE CHOICE 1. Most T lymphocytes
Supplementary Figure 1 Chemokine and chemokine receptor expression during muscle regeneration (a) Analysis of CR3CR1 mrna expression by real time-pcr
 Supplementary Figure 1 Chemokine and chemokine receptor expression during muscle regeneration (a) Analysis of CR3CR1 mrna expression by real time-pcr at day 0, 1, 4, 10 and 21 post- muscle injury. (b)
Supplementary Figure 1 Chemokine and chemokine receptor expression during muscle regeneration (a) Analysis of CR3CR1 mrna expression by real time-pcr at day 0, 1, 4, 10 and 21 post- muscle injury. (b)
NK1.1 þ CD8 þ T cells escape TGF-b control and contribute to early microbial pathogen response
 Received 24 Apr 214 Accepted 5 Sep 214 Published 6 Oct 214 DOI: 1.138/ncomms615 NK1.1 þ CD8 þ T cells escape TGF-b control and contribute to early microbial pathogen response Anne L. Ruiz 1,2,3,4, Saidi
Received 24 Apr 214 Accepted 5 Sep 214 Published 6 Oct 214 DOI: 1.138/ncomms615 NK1.1 þ CD8 þ T cells escape TGF-b control and contribute to early microbial pathogen response Anne L. Ruiz 1,2,3,4, Saidi
Natural Killer Cells: Development, Diversity, and Applications to Human Disease Dr. Michael A. Caligiuri
 Natural Killer Cells: Development, Diversity, November 26, 2008 The Ohio State University Comprehensive Cancer Center The James Cancer Hospital and Solove Research Institute Columbus, Ohio, USA 1 Human
Natural Killer Cells: Development, Diversity, November 26, 2008 The Ohio State University Comprehensive Cancer Center The James Cancer Hospital and Solove Research Institute Columbus, Ohio, USA 1 Human
Absence of mouse 2B4 promotes NK cell mediated killing of activated CD8 + T cells, leading to prolonged viral persistence and altered pathogenesis
 Research article Absence of mouse 2B4 promotes NK cell mediated killing of activated CD8 + T cells, leading to prolonged viral persistence and altered pathogenesis Stephen N. Waggoner, 1 Ruth T. Taniguchi,
Research article Absence of mouse 2B4 promotes NK cell mediated killing of activated CD8 + T cells, leading to prolonged viral persistence and altered pathogenesis Stephen N. Waggoner, 1 Ruth T. Taniguchi,
Nature Immunology: doi: /ni Supplementary Figure 1. Production of cytokines and chemokines after vaginal HSV-2 infection.
 Supplementary Figure 1 Production of cytokines and chemokines after vaginal HSV-2 infection. C57BL/6 mice were (a) treated intravaginally with 20 µl of PBS or infected with 6.7x10 4 pfu of HSV-2 in the
Supplementary Figure 1 Production of cytokines and chemokines after vaginal HSV-2 infection. C57BL/6 mice were (a) treated intravaginally with 20 µl of PBS or infected with 6.7x10 4 pfu of HSV-2 in the
Eosinophils are required. for the maintenance of plasma cells in the bone marrow
 Eosinophils are required for the maintenance of plasma cells in the bone marrow Van Trung Chu, Anja Fröhlich, Gudrun Steinhauser, Tobias Scheel, Toralf Roch, Simon Fillatreau, James J. Lee, Max Löhning
Eosinophils are required for the maintenance of plasma cells in the bone marrow Van Trung Chu, Anja Fröhlich, Gudrun Steinhauser, Tobias Scheel, Toralf Roch, Simon Fillatreau, James J. Lee, Max Löhning
Hua Tang, Weiping Cao, Sudhir Pai Kasturi, Rajesh Ravindran, Helder I Nakaya, Kousik
 SUPPLEMENTARY FIGURES 1-19 T H 2 response to cysteine-proteases requires dendritic cell-basophil cooperation via ROS mediated signaling Hua Tang, Weiping Cao, Sudhir Pai Kasturi, Rajesh Ravindran, Helder
SUPPLEMENTARY FIGURES 1-19 T H 2 response to cysteine-proteases requires dendritic cell-basophil cooperation via ROS mediated signaling Hua Tang, Weiping Cao, Sudhir Pai Kasturi, Rajesh Ravindran, Helder
Supplementary Figure 1. mrna expression of chitinase and chitinase-like protein in splenic immune cells. Each splenic immune cell population was
 Supplementary Figure 1. mrna expression of chitinase and chitinase-like protein in splenic immune cells. Each splenic immune cell population was sorted by FACS. Surface markers for sorting were CD11c +
Supplementary Figure 1. mrna expression of chitinase and chitinase-like protein in splenic immune cells. Each splenic immune cell population was sorted by FACS. Surface markers for sorting were CD11c +
Spleen. mlns. E Spleen 4.1. mlns. Spleen. mlns. Mock 17. Mock CD8 HIV-1 CD38 HLA-DR. Ki67. Spleen. Spleen. mlns. Cheng et al. Fig.
 C D E F Mock 17 Mock 4.1 CD38 57 CD8 23.7 HLA-DR Ki67 G H I Cheng et al. Fig.S1 Supplementary Figure 1. persistent infection leads to human T cell depletion and hyper-immune activation. Humanized mice
C D E F Mock 17 Mock 4.1 CD38 57 CD8 23.7 HLA-DR Ki67 G H I Cheng et al. Fig.S1 Supplementary Figure 1. persistent infection leads to human T cell depletion and hyper-immune activation. Humanized mice
5/1/13. The proportion of thymus that produces T cells decreases with age. The cellular organization of the thymus
 T cell precursors migrate from the bone marrow via the blood to the thymus to mature 1 2 The cellular organization of the thymus The proportion of thymus that produces T cells decreases with age 3 4 1
T cell precursors migrate from the bone marrow via the blood to the thymus to mature 1 2 The cellular organization of the thymus The proportion of thymus that produces T cells decreases with age 3 4 1
