Articles. Funding National Institute of Allergy and Infectious Diseases and the Bill & Melinda Gates Foundation.
|
|
|
- Christal Cox
- 6 years ago
- Views:
Transcription
1 HIV pre-exposure prophylaxis in men who have sex with men and transgender women: a secondary analysis of a phase 3 randomised controlled efficacy trial Susan P Buchbinder, David V Glidden, Albert Y Liu, Vanessa McMahan, Juan V Guanira, Kenneth H Mayer, Pedro Goicochea, Robert M Grant Lancet Infect Dis 2014; 14: Published Online March 7, S (14) See Comment page 443 Bridge HIV, San Francisco Department of Public Health, San Francisco, CA, USA (S P Buchbinder MD, A Y Liu MD); Department of Medicine (S P Buchbinder, A Y Liu), Department of Epidemiology and Biostatistics (S P Buchbinder, Prof D V Glidden PhD), University of California, San Francisco, CA, USA (Prof R M Grant MD); Gladstone Institute of Virology and Immunology, San Francisco, CA, USA (V McMahan BSc, P Goicochea MSc, Prof R M Grant); Investigaciones Médicas en Salud, Lima, Peru (J V Guanira MD); Fenway Community Health, Boston, MA, USA (Prof K H Mayer MD); Department of Medicine, Beth Israel Deaconess Hospital, Boston, MA, USA (Prof K H Mayer); and Department of Medicine, Harvard Medical School, Boston, MA, USA (Prof K H Mayer) Correspondence to: Dr Susan Buchbinder, Bridge HIV, San Francisco Department of Public Health, San Francisco, CA 94102, USA susan.buchbinder@sfdph.org Summary Background For maximum effect pre-exposure prophylaxis should be targeted to the subpopulations that account for the largest proportion of infections (population-attributable fraction [PAF]) and for whom the number needed to treat (NNT) to prevent infection is lowest. We aimed to estimate the PAF and NNT of participants in the iprex (Pre-Exposure Prophylaxis Initiative) trial. Methods The iprex study was a randomised controlled efficacy trial of pre-exposure prophylaxis with coformulated tenofovir disoproxil fumarate and emtricitabine in 2499 men who have sex with men (MSM) and transgender women. Participants aged 18 years or older who were male at birth were enrolled from 11 trial sites in Brazil, Ecuador, Peru, South Africa, Thailand, and the USA. Participants were randomly assigned (1:1) to receive either a pill with active preexposure prophylaxis or placebo, taken daily. We calculated the association between demographic and risk behaviour during screening and subsequent seroconversion among placebo recipients using a Poisson model, and we calculated the PAF and NNT for risk behaviour subgroups. The iprex trial is registered with ClinicalTrials.gov, NCT Findings Patients were enrolled between July 10, 2007, and Dec 17, 2009, and were followed up until Nov 21, Of the 2499 MSM and transgender women in the iprex trial, 1251 were assigned to pre-exposure prophylaxis and 1248 to placebo. 83 of 1248 patients in the placebo group became infected with HIV during follow-up. Participants reporting receptive anal intercourse without a condom seroconverted significantly more often than those reporting no anal sex without a condom (adjusted hazard ratio [AHR] 5 11, 95% CI ). The overall PAF for MSM and transgender women reporting receptive anal intercourse without a condom was 64% (prevalence 60%). Most of this risk came from receptive anal intercourse without a condom with partners with unknown serostatus (PAF 53%, prevalence 54%, AHR 4 76, 95% CI ); by contrast, the PAF for receptive anal intercourse without a condom with an HIV-positive partner was 1% (prevalence 1%, AHR 7 11, 95% CI ). The overall NNT per year for the cohort was 62 (95% CI ). NNTs were lowest for MSM and transgender women self-reporting receptive anal intercourse without a condom (NNT 36), cocaine use (12), or a sexually transmitted infection (41). Having one partner and insertive anal sex without a condom had the highest NNTs (100 and 77, respectively). Interpretation Pre-exposure prophylaxis may be most effective at a population level if targeted toward MSM and transgender women who report receptive anal intercourse without a condom, even if they perceive their partners to be HIV negative. Substance use history and testing for STIs should also inform individual decisions to start pre-exposure prophylaxis. Consideration of the PAF and NNT can aid in discussion of the benefits and risks of pre-exposure prophylaxis with MSM and transgender women. Funding National Institute of Allergy and Infectious Diseases and the Bill & Melinda Gates Foundation. Introduction Men who have sex with men (MSM) and transgender women make up the largest proportion of new HIV infections throughout North and South America, 1,2 western Europe, 3 Asia, 2 and Australia. 4 Despite increases in frequency of HIV testing, knowledge of HIV serostatus, and access to antiretroviral therapy, infection rates among MSM and transgender women are stable or rising. 2,5 So far, the only biomedical intervention proven to protect against HIV acquisition in MSM and transgender women in a randomised controlled trial is pre-exposure prophylaxis; 6 postexposure prophylaxis for HIV uninfected and treatment for HIV-positive MSM and transgender women probably also decrease the risk of HIV acquisition and transmission, respectively, although neither has been formally assessed in this population. Condom use is another biomedical intervention, although data on its effectiveness are limited to analyses of observational data. 7 Findings from the iprex (Pre-Exposure Prophylaxis Initiative) trial, 6,8 a randomised, placebo-controlled efficacy trial of daily coformulated tenofovir disoproxil fumarate and emtricitabine in HIV uninfected MSM and transgender women, showed a 42% reduction in new infections in participants assigned to the active treatment group when follow-up of the masked phase was complete. Comparison of drug concentrations in the iprex trial with findings from studies of directly observed dosing Vol 14 June 2014
2 showed that none of the seroconverters had drug concentrations consistent with daily dosing at the time their infection was detected. 9 In July 2012, the US Food and Drug Administration approved daily tenofovir disoproxil fumarate and emtricitabine for use as preexposure prophylaxis against sexually acquired HIV infection in high-risk uninfected adults. The Centers for Disease Control and Prevention (CDC) interim pre-exposure prophylaxis guidance document recom mended pre-exposure prophylaxis for MSM at substantial, ongoing, high risk for acquiring HIV, 10 and WHO recommended pre-exposure prophylaxis for MSM and transgender women where HIV transmission occurs and additional HIV prevention choices for them are need ed. 11 However, many health-care providers have difficulty assessing risk, 12 and neither the CDC nor WHO has yet provided specific behavioural criteria for preexposure prophylaxis. Some surveys have found that providers prioritise pre-exposure prophylaxis for known serodiscordant couples. 13,14 Findings from cost-effectiveness model ling suggest that the cost per infection averted is lowest if pre-exposure prophylaxis is used by the highest risk populations, 15 with an annual HIV incidence greater than 2 per 100 person-years. 16 However, each set of models uses different behavioural eligibility criteria for MSM and trans gender women receiving pre-exposure prophylaxis, and effectiveness is assumed to be uniform across risk groups. 15,17 19 Two epidemiological constructs, the populationattributable fraction (PAF) and the number needed to treat (NNT), are complementary strategies for identifying populations who may derive the most benefit from pre-exposure prophylaxis. The PAF combines the relative risk of a characteristic with its prevalence in a population to identify the proportion of infections associated with or attributable to that factor. Although the PAF has been estimated for populations of MSM in Australia, 20 estimates in the USA come from studies done 10 years or more ago, 21,22 and none exist for transgender women or MSM and transgender women in other parts of the world. Identification of subgroups of MSM and transgender women who have a high PAF could help to target pre-exposure prophylaxis to have the greatest effect in reducing HIV infections at a population level. NNT refers to the number of MSM and transgender women who would need to take daily tenofovir disoproxil fumarate and emtricitabine for 1 year to prevent one HIV infection. This measure is based on both the underlying HIV incidence and the effectiveness of pre-exposure prophylaxis within a population. The NNT has not been calculated for subsets of MSM and transgender women, and cost-effectiveness estimates for MSM and transgender women published so far assume a uniform effectiveness across subgroups. 15 Factors associated with a low NNT can be helpful in informing doctors and patients decisions regarding pre-exposure prophylaxis. In this study, we aimed to estimate the PAF and NNT of participants in the iprex study to identify subpopulations of people for whom pre-exposure prophylaxis may have the largest effect. Methods Study design We did a secondary analysis of study data from the iprex trial, a phase 3 randomised controlled trial of tenofovir disoproxil fumarate and emtricitabine pre-exposure prophylaxis, which is described in detail elsewhere. 6 Briefly, 2499 MSM and transgender women were enrolled from Total (n=1248) Infected (n=83) Uninfected (n=1165) Incidence per 100 person-years (95% CI) Gender Male 1086 (87%) 73 (88%) 1013 (87%) 4 0 ( ) Transgender male to female 162 (13%) 10 (12%) 152 (13%) 3 6 ( ) Age (years) (53%) 47 (57%) 614 (53%) 4 3 ( ) (19%) 15 (18%) 227 (19%) 3 7 ( ) (18%) 19 (23%) 205 (18%) 4 8 ( ) (10%) 2 (2%) 119 (10%) 1 0 ( ) Education* Below secondary 72/1236 (6%) 2/83 (2%) 70/1153 (6%) 4 0 ( ) Secondary 625/1236 (51%) 40/83 (48%) 585/1153 (51%) 3 3 ( ) Beyond secondary 539/1236 (44%) 41/83 (49%) 498/1153 (43%) 4 6 ( ) Country Peru 700 (56%) 49 (59%) 651 (56%) 3 5 ( ) Ecuador 150 (12%) 17 (20%) 133 (11%) 6 5 ( ) Brazil 184 (15%) 10 (12%) 174 (15%) 5 0 ( ) USA 114 (9%) 2 (2%) 112 (10%) 1 3 ( ) South Africa 43 (3%) 2 (2%) 41 (4%) 4 7 ( ) Thailand 57 (5%) 3 (4%) 54 (5%) 5 2 ( ) Ethnic origin White 209 (17%) 9 (11%) 200 (17%) 4 0 ( ) Black or African-American 97 (8%) 6 (7%) 91 (8%) 3 0 ( ) Mixed or other 874 (70%) 65 (78%) 809 (69%) 4 1 ( ) Asian 68 (5%) 3 (4%) 65 (6%) 3 5 ( ) Alcohol in past month None 184/1216 (15%) 10/83 (12%) 174/1133 (15%) 3 5 ( ) 1 4 drinks per day 345/1216 (28%) 29/83 (35%) 316/1133 (28%) 5 0 ( ) 5 drinks per day 687/1216 (56%) 44/83 (53%) 643/1133 (57%) 3 7 ( ) Cocaine use in past month None 1194 (96%) 76 (92%) 1118 (96%) 3 7 ( ) Any 54 (4%) 7 (8%) 47 (4%) 9 5 ( ) HIV-positive sex partner in past 3 months None 1139 (91%) 76 (92%) 1063 (91%) 3 9 ( ) Any 109 (9%) 7 (8%) 102 (9%) 4 4 ( ) Sex without a condom None 178 (14%) 3 (4%) 175 (15%) 1 2 ( ) Insertive only 317 (25%) 8 (10%) 309 (27%) 1 5 ( ) Any receptive 753 (60%) 72 (87%) 681 (58%) 5 4 ( ) (Table 1 continues on next page) Vol 14 June
3 Total (n=1248) Infected (n=83) Uninfected (n=1165) Incidence per 100 person-years (95% CI) (Continued from previous page) Receptive anal intercourse without a condom by partner serostatus None 495 (40%) 11 (13%) 484 (42%) 1 4 ( ) HIV negative only 67 (5%) 9 (11%) 58 (5%) 8 9 ( ) Unknown serostatus 669 (54%) 62 (75%) 607 (52%) 5 2 ( ) Any HIV positive 17 (1%) 1 (1%) 16 (1%) 4 3 ( ) Number of male sex partners 1 99 (8%) 5 (6%) 94 (8%) 3 4 ( ) (37%) 26 (31%) 436 (37%) 3 4 ( ) >5 687 (55%) 52 (63%) 635 (55%) 4 3 ( ) Transactional sex None 738 (59%) 49 (59%) 689 (59%) 4 1 ( ) Any 510 (41%) 34 (41%) 476 (41%) 3 6 ( ) STI by self-report None 932 (75%) 54 (65%) 878 (75%) 3 6 ( ) Any 316 (25%) 29 (35%) 287 (25%) 4 9 ( ) Data are number (%) or n/n (%), unless otherwise stated. Some percentages do not total 100% because of rounding. STI=sexually transmitted infection. *Data missing for 12 uninfected participants. Data missing for 32 participants, none of whom became infected. From computer self-administered data collection. In the previous 3 months according to an interviewer-administered questionnaire. In the previous 6 months according to an interviewer-administered questionnaire. Table 1: Baseline demographic and risk variables associated with HIV infection among placebo recipients in iprex 11 trial sites in Brazil, Ecuador, Peru, South Africa, Thailand, and the USA. HIV seronegative individuals aged 18 years or older who were male at birth (irrespective of present gender identity), were without medical contraindications for trial participation, and met behavioural risk criteria in the 6 months before screening were eligible for participation. Behavioural risk factors included anal sex with at least four (or six, depending on the study site) male partners, diagnosis of a sexually transmitted infection (STI), engaging in transactional sex, or anal sex without a condom with an HIV-positive or unknown-serostatus partner. Participants were randomly assigned (1:1) to receive a pill with coformulated tenofovir disoproxil fumarate and emtricitabine or a placebo pill, to be taken on a daily basis. We followed up participants on a monthly basis with HIV antibody testing and medical assessments. All participants were provided with free condoms and lubricant, given regular risk reduction counselling, and provided linkage to appropriate community and medical services. The study protocol was approved by national government public health authorities in Peru, Ecuador, South Africa, Brazil, Thailand, and the USA, and by the ethics committee at each site. Participants provided written informed consent at screening and enrolment. Procedures We collected baseline behavioural risk data at screening by interviewer-administered or computer self-administered data collection, using questions adapted from previous studies in these populations. 23 The total number of male sex partners with whom the participant had had oral or anal sex and the number of male partners with whom they had engaged in specific sexual practices in the previous 3 months were recorded, stratified by perceived HIV serostatus. Questions about exchange of sex for money, drugs, or services and self-reported STIs covered the previous 6 months. Interviewers asked questions about transactional sex, self-reported STIs, and sexual risk variables. Through computer self-administered data collection, participants answered questions about HIV-positive partners in the past 3 months, and drug and alcohol use in the previous month. Study staff did monthly HIV antibody testing with point-of-care rapid blood tests. All sites used two rapid HIV antibody tests; all reactive tests were confirmed with western blot or RNA tests. Statistical analysis Models for seroconversion were based on HIV infections through the study treatment period ending Nov 21, Because our goal was to identify subgroups of MSM and transgender women who might benefit most from preexposure prophylaxis in the future, analyses used baseline rather than time-dependent measures of sexual risk and drug use. Subgroup effectiveness and hazard ratios (HRs) were estimated from a Poisson model with a log link and offset for follow-up time. Adjusted HRs were adjusted for any of the other significant variables in the model and study site. Variables with a p value of less than 0 20 in univariate analyses were included in the multivariate model. The PAF for a variable was estimated as follows: RR 1 P e 1 + P e (RR 1) Where P e is the prevalence of the exposure and RR is the rate ratio for the factor analyses estimated from a Poisson model for HIV infections estimated from the placebo arm. For a variable with more than two categories, the PAF for the jth category was estimated 24 as follows: p j (RR j 1) / k Σ k=1 p k RR k The NNT was estimated 25 as follows: E k [exp( λ0k (1 )) exp ( λ 0k )] Where E k is the percentage modified intention-to-treat efficacy due to study treatment and λ 0k is the annual rate of HIV infections on the placebo in the kth stratum Vol 14 June 2014
4 We calculated efficacy by a Cox model stratified by site with terms for treatment, subgroup, and their interaction. The treatment effects were given by the appropriate linear contrast. The iprex trial is registered with ClinicalTrials.gov, NCT Role of the funding source The sponsors of the study approved the study design, but were not involved in data collection, data analysis, data interpretation, writing of the report, or the decision to submit for publication. The corresponding author had HR (95% CI) Adjusted HR (95% CI) Age (years) ( ) ( ) ( ) Education Below secondary Secondary 1 92 ( ) Beyond secondary 2 54 ( ) Ethnic origin White Black or African-American 1 39 ( ) Mixed or other 1 27 ( ) Asian 0 00 ( ) Alcohol in past month None 1 4 drinks per day 1 69 ( ) 5 drinks per day 1 10 ( ) Any cocaine use in past month 2 24 ( ) 1 85 ( ) Any HIV-positive sex partner* 1 62 ( ) Sex without a condom* None Insertive only 1 56 ( ) 1 68 ( ) Any receptive 5 17 ( ) 5 11 ( ) Receptive anal intercourse without a condom by partner serostatus* None Only HIV negative 6 69 ( ) 8 87 ( ) Unknown serostatus 3 56 ( ) 4 76 ( ) Any HIV positive 5 21 ( ) 7 11 ( ) Number of male sex partners* ( ) > ( ) Any transactional sex 0 96 ( ) Any self-reported STI 1 62 ( ) 1 27 ( ) Seropositive for syphilis at baseline 1 58 ( ) 1 30 ( ) HR=hazard ratio. STI=sexually transmitted infection. *In the 3 months before screening. In the 6 months before screening. Table 2: Univariate and multivariate risk of HIV seroconversion by baseline demographic and risk behaviours full access to all the data in the study and had final responsibility for the decision to submit for publication. Results Patients were enrolled between July 10, 2007, and Dec 17, 2009, and were followed up until Nov 21, Of the 2499 MSM and transgender women in the iprex trial, 1251 were randomly assigned to receive tenofovir disoproxil fumarate and emtricitabine and 1248 to placebo. Table 1 compares HIV incidence by baseline demographic and behavioural risk characteristics among the placebo group. This cohort was young (median age younger than 25 years) and mostly recruited from the South American countries, where enrolment began. More than half of the participants reported that they had consumed five or more alcoholic drinks per day of drinking in the past month, reported six or more sex partners, or had receptive anal intercourse without a condom with a partner of unknown HIV serostatus in the previous 3 months. Overall HIV incidence was 3 9 per 100 person-years in the placebo group (95% CI ). Only 1% of participants reported use of amphetamine or poppers (amyl nitrite or related compounds) in the past month. Participants reporting any receptive anal intercourse without a condom in the previous 3 months were more than five times as likely to acquire HIV as those reporting no sex without a condom (table 2). Among participants reporting receptive anal intercourse without a condom, the hazard was greatest among those reporting this activity with partners believed to be HIV negative, although the risk was also significantly increased for those with partners of unknown serostatus. Only 17 (1%) of 1248 participants reported receptive anal intercourse without a condom with known HIV-positive partners; Prevalence Efficacy PAF NNT Overall 100% 42% NA 62 Any cocaine use in the past month 4% 87% 6% 12 Any anal sex with an HIV-positive partner* 9% 63% 1% 43 Receptive anal intercourse without a condom by HIV serostatus* Only negative 5% 60% 10% 15 Unknown serostatus 54% 49% 53% 41 HIV positive 1% 100% 1% 24 Number of partners* 1 8% 36% 0% % 49% 0% 60 >5 55% 42% 13% 58 Any self-reported STI in the past 6 months 25% 50% 9% 41 NA=not applicable. NNT=number needed to treat. PAF=population-attributable fraction. STI=sexually transmitted infection. *In the 3 months before screening. Table 3: Prevalence, efficacy, population-attributable fraction, and number needed to treat for subgroups of iprex participants, stratified by baseline risk Vol 14 June
5 Number needed to treat partner Insertive anal intercourse without a condom 2 5 partners >5 partners HIV-positive partner Self-reported STI Syphilis Receptive anal intercourse without a condom with a partner of unknown serostatus Receptive anal intercourse without a condom with an HIV-positive partner Receptive anal intercourse without a condom (combined) Receptive anal intercourse without a condom with an HIV-negative partner Cocaine Population-attributable fraction (%) Figure: Population-attributable fraction by the number needed to treat per year to prevent one infection in iprex The dashed line shows the mean number needed to treat. The point estimate of the population-attributable fraction for insertive anal sex without a condom is negative because those who report this risk are at slightly lower risk than those who don t report it. STI=sexually transmitted infection. thus there was limited power to assess their risk of HIV acquisition. 317 (25%) of 1248 participants reported only insertive anal intercourse without a condom, but this risk factor was not associated with increased HIV acquisition in either univariate or multivariate analyses (table 2). Two risk behaviours were significantly associated with HIV acquisition on univariate but not multivariate analysis: cocaine use in the past month and self-reported STI in the past 6 months (table 2). The PAF combines data on both the prevalence of risk behaviours and the strength of their association with HIV acquisition to apportion new infections to that risk factor. Overall, receptive anal intercourse without a condom accounted for 64% of new infections, with a PAF for receptive anal intercourse without a condom with partners of unknown serostatus of 53% and with HIVnegative partners of 10% (table 3). By contrast, the PAF of receptive anal intercourse without a condom with HIV-positive partners was only 1%. The overall NNT is lowest when both the prevalence and intervention effectiveness are high for a particular subgroup. The overall NNT was 62 (95% CI ). The figure shows the PAF against the NNT for various subgroups; optimum characteristics would be a high PAF with a low NNT (bottom right corner of the plot), whereas less favourable characteristics would be a low PAF with a high NNT (upper left corner of the plot). Two risk factors stand out as possessing the desirable qualities of a high PAF and low NNT: participants reporting any receptive anal intercourse without a condom and specifically those reporting receptive anal intercourse without a condom with partners of unknown HIV serostatus. Two other factors stand out as having a low PAF and a higher NNT than the mean: participants reporting only one partner and those reporting only insertive anal intercourse without a condom, without receptive anal intercourse without a condom. Having receptive anal intercourse without a condom with an HIV-negative partner, an HIV-positive partner, a self-reported STI, more than one partner, and past substance use had a low PAF but also a low NNT. Discussion Based on the results of this analysis, the subgroup of MSM and transgender women most likely to benefit from pre-exposure prophylaxis is those reporting receptive anal intercourse without a condom, irrespective of partner serostatus (panel). The simplest and perhaps most effective strategy for identifying MSM and transgender women who may benefit most from preexposure prophylaxis would be to ask them two questions. In the past 3 months, have you had sex with men, women, or both? And in the past 3 months, have you had receptive anal sex without a condom? In the present study, receptive anal intercourse without a condom accounted for nearly two-thirds of new HIV infections an estimate similar to that reported in a 2011 study of MSM in Australia (69%). 20 Earlier studies of MSM in the USA also found a substantial PAF of receptive anal intercourse without a condom with partners of unknown serostatus 21,22 and with partners believed to be HIV negative. 22 Findings from several observational studies 23,27 support the notion that sex with HIV seronegative people without a condom (also known as condom serosorting) increases the risk of HIV acquisition compared with consistent condom use. The only exception to the risk associated with receptive anal intercourse without a condom may be for people in monogamous seroconcordant relationships; in this setting, the risk of HIV acquisition is low, even lower than in MSM who report always using condoms but who have several partners. 23 Conversely, participants who did not report having anal sex without a condom, or who only had insertive anal sex without a condom, had significantly lower rates of HIV acquisition (1 2 and 1 5 per 100 person-years, respectively) than did those who reported having receptive sex without a condom. These infection rates, although not negligible, are substantially lower than the 2 per 100 person-years incidence threshold recommended in some cost-effectiveness modelling exercises. 16 Findings from other studies suggest small-to-moderate PAFs for insertive anal intercourse without a condom (4 20%), 20,28 with a substantially lower per-contact risk from insertive than from receptive anal intercourse without a condom Vol 14 June 2014
6 Findings from a recent model of HIV transmission dynamics among MSM in Peru and the USA suggest that nearly 40% of new infections among MSM occur within primary relationships, although only two-thirds of these occur in known serodiscordant relationships. 28 By contrast, in our study, having a known HIV-positive partner had a PAF of only 1%. This difference is probably in part a result of the low prevalence of participants with this risk behaviour who entered the study. Another possible explanation is that the previous models were based on older data, when HIV-positive men may have been less likely to receive effective antiretroviral therapy, which may, in turn, reduce their infectiousness. 30 Although no direct data exist on the effectiveness of treatment as prevention for MSM and transgender women in serodiscordant partnerships, providers should prioritise provision of treatment to HIV-positive members of couples, both for the patients own health and to reduce the risk of transmission to uninfected partners. Pre-exposure prophylaxis can also be offered to the HIV uninfected partner, particularly if the HIVpositive partner is not virally suppressed, has STIs, or if the couple engages in sex without a condom. Because both cocaine use and self-reporting an STI had a low NNT in the iprex study, taking a substance-use history and regular STI screening in at-risk MSM and transgender women will also identify individuals who may benefit from pre-exposure prophylaxis. In other MSM cohort studies, amyl nitrite 21 and amphetamines 22 were independently associated with HIV acquisition, with PAFs of 28% and 16%, respectively. Use of both substances was low in the iprex study, precluding our ability to assess these risks. Similarly, we did not ask about alcohol or substance use before sex another risk factor with a substantial PAF in other cohorts. 22 The imprecision of self-reported versus diagnosed STIs might also reduce the usefulness of the former in identifying people at increased risk of HIV acquisition. Nonetheless, self-reported substance use or STIs should alert the provider to probe more explicitly about sexual risk and to consider pre-exposure prophylaxis as part of a larger screening and risk-reduction strategy. These examples show the challenges providers face in deciding who should receive pre-exposure prophylaxis. Clinicians must go beyond considerations of public health benefit to weigh the relative risks against potential benefits for their patients in their individual settings. Fortunately, tenofovir disoproxil fumarate and emtricitabine pre-exposure prophylaxis has caused few serious adverse effects in clinical trials, 6,31,32 although longer follow-up of larger cohorts is needed to detect rare serious events. Renal toxicity was uncommon in HIV uninfected people and seemed to be reversible if drugs were stopped during routine monitoring of creatinine. 6,31,32 Tenofovir disoproxil fumarate and emtricitabine seems to cause a small but significant decrease in bone mineral density, 33 but the clinical significance of this decrease is not clear. People with chronic hepatitis B infection might rebound when tenofovir disoproxil fumarate and emtricitabine pre-exposure prophylaxis is stopped and should be monitored, although this rebound has not been reported in trials that enrolled people with chronic hepatitis B infection. 6,34 People with undiagnosed HIV infection who start pre-exposure prophylaxis will probably develop antiretroviral resistance, 6,31,32 which could reduce their treatment options. This factor emphasises the importance of regular HIV testing for patients who receive pre-exposure prophylaxis and the need to counsel patients not to restart pre-exposure prophylaxis without first being tested for HIV. There is no explicit threshold for PAF or NNT to guide clinicians in choosing to whom they should offer preexposure prophylaxis. In addition to consideration of the NNT, clinicians must weigh the benefit of avoiding HIV infection against the dangers of tenofovir disoproxil fumarate and emtricitabine pre-exposure prophylaxis for each patient. 35,36 Condoms remain one partially effective Panel: Research in context Systematic review We searched PubMed (from inception to Jan 31, 2014) for studies published in English of population-attributable fraction for HIV infection among and guidance for offering pre-exposure prophylaxis to men who have sex with men (MSM) and transgender women using the following search terms: HIV, men who have sex with men, MSM, gay, transgender, population attributable fraction, population-attributable risk, pre-exposure prophylaxis, preexposure prophylaxis, eligibility, guidelines, guidance, recommendations, providers, physicians, clinicians, number needed to treat, and NNT. We found 168 potentially relevant articles from the search and found additional articles through references from relevant articles and our own files. The studies showed that the major risk factors for HIV acquisition among MSM and transgender women were number of sex partners, receptive anal sex without a condom with partners of any serostatus, insertive anal sex without a condom with an HIV-positive partner, sexually transmitted infections, substance use (primarily amyl nitrate, amphetamines, or substance use with sexual activity), depression, and young age. Black MSM in the USA, Canada, and UK are at disproportionate risk, which is associated with structural factors (low income or education, or incarceration) and partner characteristics (age and race), whereas individual behavioral risk was lower in black MSM than in white MSM in most reports. We found no studies that calculated the number needed to treat for MSM; for heterosexuals, the number needed to treat in one study ranged from 26 to 78, 26 depending on the subgroup. Interpretation In this study, we assessed clinical trial data to make recommendations about which MSM and transgender women should be offered pre-exposure prophylaxis. Present Centers for Disease Control and Prevention 10 and WHO 11 guidance is not explicit about risk criteria for pre-exposure prophylaxis for MSM and transgender women. We combined information about the risk behaviours contributing to new HIV infections and the number of patients per year who would have to be given pre-exposure prophylaxis to avert one infection. Receptive anal sex without a condom with partners of unknown serostatus contributed to more than half of all new HIV infections; similar results have been reported in cohorts from the USA. 21,22 We suggest that providers ask a few screening questions of their male and transgender patients and consider offering pre-exposure prophylaxis to patients with sexual or substance use risk, irrespective of knowledge of partner serostatus. Vol 14 June
7 strategy for reducing the risk of HIV acquisition; 7 preexposure prophylaxis offers additional protection that is controlled by the r eceptive partner. The benefits and risks of pre-exposure prophylaxis should be explicitly discussed with potential candidates in the context of other available HIV prevention methods. Potential pre-exposure prophylaxis users might also factor cost into decisions. At a societal level, discussion might also occur about prioritisation of pre-exposure prophylaxis over other health-care needs, including provision of antiretroviral therapy for HIV-infected people. 11 Additional costeffectiveness analyses will be helpful to prioritise how best to reduce new HIV infections in different target populations. This analysis has several limitations. Although findings from observational data and models suggest similar risk factors for infection among MSM in Peru and the USA, 28 most participants in iprex came from the Andean region of South America, and results might not be generalisable to other regions or people outside of randomised controlled trials. This analysis also does not apply to preexposure prophylaxis for heterosexual people, although efficacy has also been shown in this population. 31 The iprex trial enrolled few black or African-American MSM in the USA (34 of 227) or transgender women two populations at particularly high risk of HIV acquisition. The PAFs in this study, although similar in most cases to those from other studies, might have been affected by the behavioural eligibility criteria for iprex. Having anal sex without a condom with a known HIV-positive partner, although one of the behavioural inclusion criteria, might be substantially less common in geographical regions in which serostatus is often not discussed. 95% CIs for the PAF and NNT are likely to be large for small subgroups, lending some uncertainty to the estimates. Risk practices are self-reported and might be inaccurate because of social desirability, faulty recall, or desire to meet study eligibility criteria. Effectiveness of pre-exposure prophylaxis in clinical settings, and therefore the NNT, could be reduced if pre-exposure prophylaxis adherence is poor a common weakness among several pre-exposure prophylaxis trials. 32 Conversely, if high levels of adherence are achieved, such as those reported in the US sites, 37 the NNT will decrease even further. Demonstration projects and studies of innovative, scalable adherence interventions are underway. 38 Findings from this analysis suggest that MSM and transgender women can be screened for potential eligibility for pre-exposure prophylaxis even in busy clinical practices by focusing on receptive anal intercourse without a condom. By adding a few more questions about number and serostatus of sex partners, sexual practices, substance use, and risk reduction strategies, clinicians can gain a broad understanding of patients needs and formulate a comprehensive HIV and STI screening and prevention plan. Pre-exposure prophylaxis offers promise for reducing the spread of HIV worldwide, but clinicians will need to screen patients and provide pre-exposure prophylaxis to at-risk MSM and transgender women for it to achieve its promise. Contributors All authors contributed to the study design, data analysis, and interpretation. SPB, AYL, JVG, and KHM contributed to the data collection. SPB and DVG did the data analysis. SPB wrote the manuscript and all other authors provided comments leading to revisions. Declaration of interests SPB and AYL have led trials in which study drug was donated by Gilead Sciences and have received personal fees from Clinical Care Options. KHM has received unrestricted research and educational grants from Gilead Sciences and an unrestricted research grant from Merck. PG received an unrestricted grant from Gilead Sciences to develop an educational video related to pre-exposure prophylaxis. RMG has led trials in which study drug was donated by Gilead Sciences. Gilead Sciences also provided unrestricted travel grants that partially supported annual iprex investigator meetings. RMG has also received personal fees from Siemens Healthcare, University of Pennsylvania, ViiV Healthcare, Clinical Care Options, the Kirby Institute (Sydney), and Medscape Education. DVG, VMM, and JVG declare that they have no competing interests. Acknowledgments The study drug was donated by Gilead Sciences. References 1 Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, PLoS One 2011; 6: e Beyrer C, Baral SD, van Griensven F, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet 2012; 380: Phillips AN, Cambiano V, Nakagawa F, et al. Increased HIV incidence in men who have sex with men despite high levels of ART-induced viral suppression: analysis of an extensively documented epidemic. PLoS One 2013; 8: e Feigin A, El-Hayek C, Hellard M, et al. Increases in newly acquired HIV infections in Victoria, Australia: epidemiological evidence of successful prevention? Sex Health 2013; 10: Birrell PJ, Gill ON, Delpech VC, et al. HIV incidence in men who have sex with men in England and Wales : a nationwide population study. Lancet Infect Dis 2013; 13: Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363: Smith D, Herbst JH, Zhang X, Rose C. Condom efficacy by consistency of use among MSM. Conference on Retroviruses and Opportunistic Infections; Atlanta, GA, USA; March 3 6, Abstract Grant R, McMahan V, Liu A, et al. Completed observation of the randomized placebo-controlled phase of iprex: daily oral FTC/TDF preexposure HIV prophylaxis among men and trans women who have sex with men. 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Rome, Italy; July 17 20, Abstract WELBC04. 9 Anderson PL, Glidden DV, Liu A, et al. Emtricitabine tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med 2012; 4: 151ra Centers for Disease Control and Prevention (CDC). Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep 2011; 60: WHO. Guidance on pre-exposure oral prophylaxis (PrEP) for serodiscordant couples, men and transgender women who have sex with men at high risk of HIV: recommendations for use in the context of demonstration projects. Geneva: World Health Organization, Krakower D, Mayer KH. Engaging healthcare providers to implement HIV pre-exposure prophylaxis. Curr Opin HIV AIDS 2012; 7: Tellalian D, Maznavi K, Bredeek UF, Hardy WD. Pre-exposure prophylaxis (PrEP) for HIV infection: results of a survey of HIV healthcare providers evaluating their knowledge, attitudes, and prescribing practices. AIDS Patient Care STDS 2013; 27: Vol 14 June 2014
8 14 Arnold EA, Hazelton P, Lane T, et al. A qualitative study of provider thoughts on implementing pre-exposure prophylaxis (PrEP) in clinical settings to prevent HIV infection. PLoS One 2012; 7: e Gomez GB, Borquez A, Case KK, Wheelock A, Vassall A, Hankins C. The cost and impact of scaling up pre-exposure prophylaxis for HIV prevention: a systematic review of costeffectiveness modelling studies. PLoS Med 2013; 10: e Schackman BR, Eggman AA. Cost-effectiveness of pre-exposure prophylaxis for HIV: a review. Curr Opin HIV AIDS 2012; 7: Paltiel AD, Freedberg KA, Scott CA, et al. HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clin Infect Dis 2009; 48: Desai K, Sansom SL, Ackers ML, et al. Modeling the impact of HIV chemoprophylaxis strategies among men who have sex with men in the United States: HIV infections prevented and cost-effectiveness. AIDS 2008; 22: Juusola JL, Brandeau ML, Owens DK, Bendavid E. The costeffectiveness of preexposure prophylaxis for HIV prevention in the United States in men who have sex with men. Ann Intern Med 2012; 156: Guy RJ, Wand H, Wilson DP, et al. Using population attributable risk to choose HIV prevention strategies in men who have sex with men. BMC Public Health 2011; 11: Buchbinder SP, Vittinghoff E, Heagerty PJ, et al. Sexual risk, nitrite inhalant use, and lack of circumcision associated with HIV seroconversion in men who have sex with men in the United States. J Acquir Immune Defic Syndr 2005; 39: Koblin BA, Husnik MJ, Colfax G, et al. Risk factors for HIV infection among men who have sex with men. AIDS 2006; 20: Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health 1998; 88: Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 1999; 319: Vallabhaneni S, Li X, Vittinghoff E, Donnell D, Pilcher CD, Buchbinder SP. Seroadaptive practices: association with HIV acquisition among HIV-negative men who have sex with men. PLoS One 2012; 7: e Murnane PM, Celum C, Mugo N, et al, for the Partners PrEP Study Team. Efficacy of preexposure prophylaxis for HIV-1 prevention among high-risk heterosexuals: subgroup analyses from a randomized trial. AIDS 2013; 27: Golden MR, Stekler J, Hughes JP, Wood RW. HIV serosorting in men who have sex with men: is it safe? J Acquir Immune Defic Syndr 2008; 49: Goodreau SM, Carnegie NB, Vittinghoff E, et al. What drives the US and Peruvian HIV epidemics in men who have sex with men (MSM)? PLoS One 2012; 7: e Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol 2010; 39: Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365: Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367: Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367: Liu AY, Vittinghoff E, Sellmeyer DE, et al. Bone mineral density in HIV-negative men participating in a tenofovir pre-exposure prophylaxis randomized clinical trial in San Francisco. PLoS One 2011; 6: e Peterson L, Taylor D, Roddy R, et al. Tenofovir disoproxil fumarate for prevention of HIV infection in women: a phase 2, double-blind, randomized, placebo-controlled trial. PLoS Clin Trials 2007; 2: e Guyatt GH, Oxman AD, Kunz R, et al. What is quality of evidence and why is it important to clinicians? BMJ 2008; 336: Venter F, Allais L, Richter M. Exposure ethics: does HIV preexposure prophylaxis raise ethical problems for the health care provider and policy maker? Bioethics 2013; published online June 24. DOI: /bioe Gilmore HJ, Liu A, Koester KA, et al. Participant experiences and facilitators and barriers to pill use among men who have sex with men in the iprex pre-exposure prophylaxis trial in San Francisco. AIDS Patient Care STDS 2013; 27: AVAC. Global Advocacy for HIV Prevention. Ongoing PrEP trials. (accessed Jan 31, 2014). Vol 14 June
PrEP and Behavioral Strategies for HIV Prevention. Douglas Krakower, MD January 30, 2014
 PrEP and Behavioral Strategies for HIV Prevention Douglas Krakower, MD January 30, 2014 Potential Competing Interests Dr. Krakower: investigator-initiated research regarding HIV prevention National Institutes
PrEP and Behavioral Strategies for HIV Prevention Douglas Krakower, MD January 30, 2014 Potential Competing Interests Dr. Krakower: investigator-initiated research regarding HIV prevention National Institutes
Clinical Policy Title: Pre-exposure prophylaxis for HIV prevention
 Clinical Policy Title: Pre-exposure prophylaxis for HIV prevention Clinical Policy Number: 17.02.03 Effective Date: May 1, 2016 Initial Review Date: February 17, 2016 Most Recent Review Date: November
Clinical Policy Title: Pre-exposure prophylaxis for HIV prevention Clinical Policy Number: 17.02.03 Effective Date: May 1, 2016 Initial Review Date: February 17, 2016 Most Recent Review Date: November
Disclosure. Learning Objectives. Epidemiology. Transmission. Risk of Transmission PRE-EXPOSURE PROPHYLAXIS (PREP) FOR HIV PREVENTION 50,000.
 Disclosure PRE-EXPOSURE PROPHYLAXIS (PREP) FOR HIV PREVENTION I have no financial interest in and/or affiliation with any external organizations in relation to this CE program. DaleMarie Vaughan, PharmD
Disclosure PRE-EXPOSURE PROPHYLAXIS (PREP) FOR HIV PREVENTION I have no financial interest in and/or affiliation with any external organizations in relation to this CE program. DaleMarie Vaughan, PharmD
South African Guidelines for the Safe Use of. Dr. Oscar Radebe
 South African Guidelines for the Safe Use of PrEP in MSM Dr. Oscar Radebe Background Globally MSM(men who have sex with men) have been disproportionately at high risk of HIV transmission. Biological &
South African Guidelines for the Safe Use of PrEP in MSM Dr. Oscar Radebe Background Globally MSM(men who have sex with men) have been disproportionately at high risk of HIV transmission. Biological &
Pre-exposure Prophylaxis. Robert M Grant October 2014
 Pre-exposure Prophylaxis Robert M Grant October 2014 The HIV Pandemic 2.3 Million New HIV Infections in 2012 39% in Young People (ages 15-24) The HIV Pandemic 1.6 Million Started Therapy in 2012 1.4 New
Pre-exposure Prophylaxis Robert M Grant October 2014 The HIV Pandemic 2.3 Million New HIV Infections in 2012 39% in Young People (ages 15-24) The HIV Pandemic 1.6 Million Started Therapy in 2012 1.4 New
Case Studies in PrEP Management. Kevin L. Ard, MD, MPH Massachusetts General Hospital, National LGBT Health Education Center April 15, 2016
 Case Studies in PrEP Management Kevin L. Ard, MD, MPH Massachusetts General Hospital, National LGBT Health Education Center April 15, 2016 Continuing Medical Education Disclosure Program Faculty: Kevin
Case Studies in PrEP Management Kevin L. Ard, MD, MPH Massachusetts General Hospital, National LGBT Health Education Center April 15, 2016 Continuing Medical Education Disclosure Program Faculty: Kevin
Patterns and Correlates of PrEP Drug Detection among MSM and Transgender Women in the Global iprex Study
 Patterns and Correlates of PrEP Drug Detection among MSM and Transgender Women in the Global iprex Study A Liu, D Glidden, P Anderson, K.R. Amico, V McMahan, M Mehrotra, J Lama, J MacRae, JC Hinojosa,
Patterns and Correlates of PrEP Drug Detection among MSM and Transgender Women in the Global iprex Study A Liu, D Glidden, P Anderson, K.R. Amico, V McMahan, M Mehrotra, J Lama, J MacRae, JC Hinojosa,
Pre-Exposure Prophylaxis (PrEP) and the Governor s Plan to End AIDS: What Every Clinician Needs to Know
 Pre-Exposure Prophylaxis (PrEP) and the Governor s Plan to End AIDS: What Every Clinician Needs to Know ANTONIO E. URBINA, MD Medical Director Associate Professor of Medicine Mt. Sinai Hospital Disclosure
Pre-Exposure Prophylaxis (PrEP) and the Governor s Plan to End AIDS: What Every Clinician Needs to Know ANTONIO E. URBINA, MD Medical Director Associate Professor of Medicine Mt. Sinai Hospital Disclosure
Find both sets of guidelines on nyc.gov by searching HIV PrEP and PEP. Per CDC Guidelines, PrEP may be appropriate for the following populations:
 PrEP Provider FAQs 1. What is PrEP? PrEP is short for pre-exposure prophylaxis. It is the use of antiretroviral medication to prevent acquisition of HIV infection. PrEP is used by HIV uninfected people
PrEP Provider FAQs 1. What is PrEP? PrEP is short for pre-exposure prophylaxis. It is the use of antiretroviral medication to prevent acquisition of HIV infection. PrEP is used by HIV uninfected people
Drug development in relation to PrEP and the PROUD study
 Drug development in relation to PrEP and the PROUD study David Dolling Medical Statistician MRC Clinical Trials Unit 18 th October 2012 What is PrEP? - Pre-exposure Prophylaxis A strategy that uses antiretrovirals
Drug development in relation to PrEP and the PROUD study David Dolling Medical Statistician MRC Clinical Trials Unit 18 th October 2012 What is PrEP? - Pre-exposure Prophylaxis A strategy that uses antiretrovirals
Roy M. Gulick, MD, MPH Chief, Division of Infectious Diseases Professor of Medicine Weill Medical College of Cornell University New York City
 PrEP 2013 Roy M. Gulick, MD, MPH Chief, Division of Infectious Diseases Professor of Medicine Weill Medical College of Cornell University New York City Slide #2 U.S.: New HIV Infections Per Year ~50,000
PrEP 2013 Roy M. Gulick, MD, MPH Chief, Division of Infectious Diseases Professor of Medicine Weill Medical College of Cornell University New York City Slide #2 U.S.: New HIV Infections Per Year ~50,000
HIV Prevention Strategies HIV Pre-exposure prophylaxis
 HIV Prevention Strategies HIV Pre-exposure prophylaxis Michael Martin, MD, MPH Director HIV Research Program Thailand MOPH U.S. CDC Collaboration The findings and conclusions in this presentation are those
HIV Prevention Strategies HIV Pre-exposure prophylaxis Michael Martin, MD, MPH Director HIV Research Program Thailand MOPH U.S. CDC Collaboration The findings and conclusions in this presentation are those
Moving beyond condoms to prevent HIV transmission. Are you Prepared for HIV PrEP?
 Moving beyond condoms to prevent HIV transmission Are you Prepared for HIV PrEP? The Issues New HIV infections in the US continue Vast majority of infections are sexually acquired Condoms work but are
Moving beyond condoms to prevent HIV transmission Are you Prepared for HIV PrEP? The Issues New HIV infections in the US continue Vast majority of infections are sexually acquired Condoms work but are
Understanding the Results of VOICE
 CONTACT: Lisa Rossi +1-412- 916-3315 (mobile) or +27-(0)73-323-0087 (through 7 March) rossil@upmc.edu About VOICE Understanding the Results of VOICE VOICE Vaginal and Oral Interventions to Control the
CONTACT: Lisa Rossi +1-412- 916-3315 (mobile) or +27-(0)73-323-0087 (through 7 March) rossil@upmc.edu About VOICE Understanding the Results of VOICE VOICE Vaginal and Oral Interventions to Control the
PrEP efficacy the evidence
 PrEP efficacy the evidence Dr Michael Brady Consultant, HIV and Sexual Health King s College Hospital, London Medical Director Terrence Higgins Trust PrEP Pre-exposure prophylaxis Tenofovir and emtricitabine
PrEP efficacy the evidence Dr Michael Brady Consultant, HIV and Sexual Health King s College Hospital, London Medical Director Terrence Higgins Trust PrEP Pre-exposure prophylaxis Tenofovir and emtricitabine
HIV: Pregnancy in Serodiscordant Couple. Dr Chow TS ID Clinic HPP
 HIV: Pregnancy in Serodiscordant Couple Dr Chow TS ID Clinic HPP Sexual Reproductive Health and Rights The recognition of the sexual and reproductive health and rights (SRHR) of all individuals and couples
HIV: Pregnancy in Serodiscordant Couple Dr Chow TS ID Clinic HPP Sexual Reproductive Health and Rights The recognition of the sexual and reproductive health and rights (SRHR) of all individuals and couples
HIV Prevention among Women
 HIV Prevention among Women Assistant Professor of Medicine Division of Infectious Diseases Baylor College of Medicine Disclosures: Gilead Sciences - Scientific Advisory Board; Investigatorinitiated research
HIV Prevention among Women Assistant Professor of Medicine Division of Infectious Diseases Baylor College of Medicine Disclosures: Gilead Sciences - Scientific Advisory Board; Investigatorinitiated research
Receptive Anal Intercourse and HIV Infection
 World Journal of AIDS, 2017, 7, 269-278 http://www.scirp.org/journal/wja ISSN Online: 2160-8822 ISSN Print: 2160-8814 Receptive Anal Intercourse and HIV Infection Gilbert R. Lavoie 1, John F. Fisher 2
World Journal of AIDS, 2017, 7, 269-278 http://www.scirp.org/journal/wja ISSN Online: 2160-8822 ISSN Print: 2160-8814 Receptive Anal Intercourse and HIV Infection Gilbert R. Lavoie 1, John F. Fisher 2
PrEP for Women: HIV Prevention in Family Planning Settings
 National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention PrEP for Women: HIV Prevention in Family Planning Settings Dawn K. Smith, MD, MS, MPH Division of HIV/AIDS Prevention dsmith1@cdc.gov
National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention PrEP for Women: HIV Prevention in Family Planning Settings Dawn K. Smith, MD, MS, MPH Division of HIV/AIDS Prevention dsmith1@cdc.gov
HIV Pre-Exposure Prophylaxis (PrEP)
 HIV Pre-Exposure Prophylaxis (PrEP) A Crash Course For General Practitioners Vincent Cornelisse BSc(Hons) MBBS FRACGP FAChSHM(RACP) Sexual Health Physician Prahran Market Clinic & Melbourne Sexual Health
HIV Pre-Exposure Prophylaxis (PrEP) A Crash Course For General Practitioners Vincent Cornelisse BSc(Hons) MBBS FRACGP FAChSHM(RACP) Sexual Health Physician Prahran Market Clinic & Melbourne Sexual Health
HIV PREP THE NEWEST TOOL IN THE BOX
 HIV PREP THE NEWEST TOOL IN THE BOX Infectious Disease Update November 7, 2014 Andrew Petroll, MD, MS Department of Medicine, Division of Infectious Diseases Department of Psychiatry and Behavioral Medicine,
HIV PREP THE NEWEST TOOL IN THE BOX Infectious Disease Update November 7, 2014 Andrew Petroll, MD, MS Department of Medicine, Division of Infectious Diseases Department of Psychiatry and Behavioral Medicine,
Recent Breakthroughs in HIV Prevention for Men who Have Sex with Men and Transgender Populations
 Recent Breakthroughs in HIV Prevention for Men who Have Sex with Men and Transgender Populations Kevin Ard, MD, MPH Brigham and Women s Hospital The Fenway Institute Boston, MA Funding: The New England
Recent Breakthroughs in HIV Prevention for Men who Have Sex with Men and Transgender Populations Kevin Ard, MD, MPH Brigham and Women s Hospital The Fenway Institute Boston, MA Funding: The New England
Pre-Exposure Prophylaxis for HIV: The Basics and Beyond
 Pre-Exposure Prophylaxis for HIV: The Basics and Beyond Kevin L. Ard, MD, MPH National LGBT Health Education Center, Fenway Institute Infectious Disease Division, Massachusetts General Hospital Disclosure
Pre-Exposure Prophylaxis for HIV: The Basics and Beyond Kevin L. Ard, MD, MPH National LGBT Health Education Center, Fenway Institute Infectious Disease Division, Massachusetts General Hospital Disclosure
Pre exposure Prophylaxis (PrEP): Stepping Up HIV Prevention
 Pre exposure Prophylaxis (PrEP): Stepping Up HIV Prevention Dawn K. Smith, MD, MS, MPH Centers for Disease Control and Prevention The findings and conclusions in this presentation have not been formally
Pre exposure Prophylaxis (PrEP): Stepping Up HIV Prevention Dawn K. Smith, MD, MS, MPH Centers for Disease Control and Prevention The findings and conclusions in this presentation have not been formally
Tuesday, March 1, 2011; 12:15 p.m. EST Pedro Goicochea: (415)
 EMBARGOED FOR RELEASE MEDIA CONTACT Tuesday, March 1, 2011; 12:15 p.m. EST Pedro Goicochea: (415) 490-8350 pgoicochea@gladstone.ucsf.edu NEW DATA SHOW THE PROTECTIVE BENEFIT OF PRE-EXPOSURE PROPHYLAXIS
EMBARGOED FOR RELEASE MEDIA CONTACT Tuesday, March 1, 2011; 12:15 p.m. EST Pedro Goicochea: (415) 490-8350 pgoicochea@gladstone.ucsf.edu NEW DATA SHOW THE PROTECTIVE BENEFIT OF PRE-EXPOSURE PROPHYLAXIS
PrEP in the Real World: Clinical Case Studies
 PrEP in the Real World: Clinical Case Studies Kevin L. Ard, MD, MPH April 30, 2015 Massachusetts General Hospital, National LGBT Health Education Center Continuing Medical Education Disclosure Program
PrEP in the Real World: Clinical Case Studies Kevin L. Ard, MD, MPH April 30, 2015 Massachusetts General Hospital, National LGBT Health Education Center Continuing Medical Education Disclosure Program
Using anti-hiv drugs for prevention
 Using anti-hiv drugs for prevention Highlights from AIDS 2012 (and 2 nd International Workshops on Treatment as Prevention) Tim Rogers, trogers@catie.ca Using anti-hiv Drugs for Prevention Outline Treatment
Using anti-hiv drugs for prevention Highlights from AIDS 2012 (and 2 nd International Workshops on Treatment as Prevention) Tim Rogers, trogers@catie.ca Using anti-hiv Drugs for Prevention Outline Treatment
emtricitabine/tenofovir disoproxil 200mg/245mg film-coated tablets (Truvada ) SMC No. (1225/17) Gilead Sciences Ltd
 emtricitabine/tenofovir disoproxil 200mg/245mg film-coated tablets (Truvada ) SMC No. (1225/17) Gilead Sciences Ltd 10 March 2017 The Scottish Medicines Consortium (SMC) has completed its assessment of
emtricitabine/tenofovir disoproxil 200mg/245mg film-coated tablets (Truvada ) SMC No. (1225/17) Gilead Sciences Ltd 10 March 2017 The Scottish Medicines Consortium (SMC) has completed its assessment of
Biomedical Prevention Update Thomas C. Quinn, M.D.
 Biomedical Prevention Update Thomas C. Quinn, M.D. Associate Director of International Research National Institute of Allergy and Infectious Diseases Director, Johns Hopkins Center for Global Health Global
Biomedical Prevention Update Thomas C. Quinn, M.D. Associate Director of International Research National Institute of Allergy and Infectious Diseases Director, Johns Hopkins Center for Global Health Global
during conception, pregnancy and lactation at 2 U.S. medical centers
 Use of HIV preexposure prophylaxis during conception, pregnancy and lactation at 2 U.S. medical centers Dominika Seidman, MD Shannon Weber, Maria Teresa Timoney, Karishma Oza, Elizabeth Mullins, Rodney
Use of HIV preexposure prophylaxis during conception, pregnancy and lactation at 2 U.S. medical centers Dominika Seidman, MD Shannon Weber, Maria Teresa Timoney, Karishma Oza, Elizabeth Mullins, Rodney
Antiretroviral Drugs for HIV Seronegative People: It works in trials, what about the real world?
 Antiretroviral Drugs for HIV Seronegative People: It works in trials, what about the real world? Lut Van Damme 11 Oct 2012 1 Disclaimer Gilead donated the study product for the FEM-PrEP trial I participated
Antiretroviral Drugs for HIV Seronegative People: It works in trials, what about the real world? Lut Van Damme 11 Oct 2012 1 Disclaimer Gilead donated the study product for the FEM-PrEP trial I participated
PRE-EXPOSURE PROPHYLAXIS FOR HIV: EVIDENCE AND GENDER CONSIDERATIONS. Jean R. Anderson M.D. Director, Johns Hopkins HIV Women s Health Program
 PRE-EXPOSURE PROPHYLAXIS FOR HIV: EVIDENCE AND GENDER CONSIDERATIONS Jean R. Anderson M.D. Director, Johns Hopkins HIV Women s Health Program Disclosures None Objectives Review the evidence regarding the
PRE-EXPOSURE PROPHYLAXIS FOR HIV: EVIDENCE AND GENDER CONSIDERATIONS Jean R. Anderson M.D. Director, Johns Hopkins HIV Women s Health Program Disclosures None Objectives Review the evidence regarding the
HIV Pre-Exposure Prophylaxis (HIV PrEP) in Scotland. An update for registered practitioners September 2017
 HIV Pre-Exposure Prophylaxis (HIV PrEP) in Scotland An update for registered practitioners September 2017 Key Messages HIV is a major public health challenge for Scotland with an annual average of 359
HIV Pre-Exposure Prophylaxis (HIV PrEP) in Scotland An update for registered practitioners September 2017 Key Messages HIV is a major public health challenge for Scotland with an annual average of 359
Pre-exposure prophylaxis (PrEP)
 FACTSHEET Pre-exposure prophylaxis (PrEP) Summary Pre-exposure prophylaxis, or PrEP, is a way for an HIV-negative person who is at risk of HIV infection to reduce their risk of becoming infected with HIV.
FACTSHEET Pre-exposure prophylaxis (PrEP) Summary Pre-exposure prophylaxis, or PrEP, is a way for an HIV-negative person who is at risk of HIV infection to reduce their risk of becoming infected with HIV.
Clinical Education Initiative PRE-EXPOSURE PROPHYLAXIS. Speaker: Antonia Urbina, MD
 Clinical Education Initiative Support@ceitraining.org PRE-EXPOSURE PROPHYLAXIS Speaker: Antonia Urbina, MD 9/6/2017 Pre-Exposure Prophylaxis [video transcript] 1 00:00:07,480 --> 00:00:09,139 I mean we're
Clinical Education Initiative Support@ceitraining.org PRE-EXPOSURE PROPHYLAXIS Speaker: Antonia Urbina, MD 9/6/2017 Pre-Exposure Prophylaxis [video transcript] 1 00:00:07,480 --> 00:00:09,139 I mean we're
The role of Integrase Inhibitors during HIV prevention
 The role of Integrase Inhibitors during HIV prevention Pep Coll AIDS Research Institute-IrsiCaixa Fight AIDS Foundation BCN Checkpoint 2nd Global HIV Clinical Forum: Integrase Inhibitors Paris July 22th
The role of Integrase Inhibitors during HIV prevention Pep Coll AIDS Research Institute-IrsiCaixa Fight AIDS Foundation BCN Checkpoint 2nd Global HIV Clinical Forum: Integrase Inhibitors Paris July 22th
ACCEPTED. Objective: We derived an estimate of male condom effectiveness during anal sex among men
 JAIDS Journal of Acquired Immune Deficiency Syndromes Publish Ahead of Print DOI: 10.1097/QAI.0000000000000461 1 Title: Condom Effectiveness for HIV Prevention by Consistency of Use among Men Who Have
JAIDS Journal of Acquired Immune Deficiency Syndromes Publish Ahead of Print DOI: 10.1097/QAI.0000000000000461 1 Title: Condom Effectiveness for HIV Prevention by Consistency of Use among Men Who Have
Ready, set, PrEP! Renee-Claude Mercier PharmD, PhC, BCPS-AQ ID, FCCP Professor of Pharmacy and Medicine University of New Mexico
 Ready, set, PrEP! Renee-Claude Mercier PharmD, PhC, BCPS-AQ ID, FCCP Professor of Pharmacy and Medicine University of New Mexico Objectives At the end of the talk the audience should be able to: Understand
Ready, set, PrEP! Renee-Claude Mercier PharmD, PhC, BCPS-AQ ID, FCCP Professor of Pharmacy and Medicine University of New Mexico Objectives At the end of the talk the audience should be able to: Understand
Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) Training Guide for Healthcare Providers
 Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) Training Guide for Healthcare Providers About emtricitabine/tenofovir disoproxil fumarate for HIV-1 PrEP
Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) Training Guide for Healthcare Providers About emtricitabine/tenofovir disoproxil fumarate for HIV-1 PrEP
Challenges for HIV Pre-Exposure Prophylaxis among Men Who Have Sex with Men in the United States
 Challenges for HIV Pre-Exposure Prophylaxis among Men Who Have Sex with Men in the United States Gordon Mansergh, Centers for Disease Control and Prevention Beryl A. Koblin, New York Blood Center Patrick
Challenges for HIV Pre-Exposure Prophylaxis among Men Who Have Sex with Men in the United States Gordon Mansergh, Centers for Disease Control and Prevention Beryl A. Koblin, New York Blood Center Patrick
Pre-exposure Prophylaxis (PrEP)
 Pre-exposure Prophylaxis (PrEP) Kenneth Mayer, MD Fenway Health Beth Israel Deaconess Medical Center Harvard Medical School and School of Public Health Treatment As Prevention in Africa Workshop May 2
Pre-exposure Prophylaxis (PrEP) Kenneth Mayer, MD Fenway Health Beth Israel Deaconess Medical Center Harvard Medical School and School of Public Health Treatment As Prevention in Africa Workshop May 2
PrEP: Pre Exposure Prophylaxis
 PrEP: Pre Exposure Prophylaxis Lyn Stevens, NP, MS, ACRN Deputy Director Office of the Medical Director NYS Department of Health, AIDS Institute Faculty Disclosure Lyn Stevens No relationships to disclose
PrEP: Pre Exposure Prophylaxis Lyn Stevens, NP, MS, ACRN Deputy Director Office of the Medical Director NYS Department of Health, AIDS Institute Faculty Disclosure Lyn Stevens No relationships to disclose
WHO s early release guidelines on PrEP: implications for emtct. Dominika Seidman, MD October 13, 2015
 WHO s early release guidelines on PrEP: implications for emtct Dominika Seidman, MD October 13, 2015 Outline Evidence behind WHO early release guidelines on PrEP PrEP eligibility according to the WHO Rationale
WHO s early release guidelines on PrEP: implications for emtct Dominika Seidman, MD October 13, 2015 Outline Evidence behind WHO early release guidelines on PrEP PrEP eligibility according to the WHO Rationale
Clinical Commissioning Policy Proposition: Pre-exposure prophylaxis (PrEP) to prevent the acquisition of HIV in adults
 Clinical Commissioning Policy Proposition: Pre-exposure prophylaxis (PrEP) to prevent the acquisition of HIV in adults Reference: NHS England F03X06 First published: TBC Prepared by NHS England Specialised
Clinical Commissioning Policy Proposition: Pre-exposure prophylaxis (PrEP) to prevent the acquisition of HIV in adults Reference: NHS England F03X06 First published: TBC Prepared by NHS England Specialised
CONSOLIDATED GUIDELINES ON HIV PREVENTION, DIAGNOSIS, TREATMENT AND CARE FOR KEY POPULATIONS
 Consolidated Guidelines on HIV prevention, diagnosis, treatment and care for key populations. Updated version, July 2016 CONSOLIDATED GUIDELINES ON HIV PREVENTION, DIAGNOSIS, TREATMENT AND CARE FOR KEY
Consolidated Guidelines on HIV prevention, diagnosis, treatment and care for key populations. Updated version, July 2016 CONSOLIDATED GUIDELINES ON HIV PREVENTION, DIAGNOSIS, TREATMENT AND CARE FOR KEY
Assessing the Cost Effectiveness of Pre-Exposure Prophylaxis for HIV Prevention in the US
 PharmacoEconomics (2013) 31:1091 1104 DOI 10.1007/s40273-013-0111-0 CURRENT OPINION Assessing the Cost Effectiveness of Pre-Exposure Prophylaxis for HIV Prevention in the US Fred J. Hellinger Published
PharmacoEconomics (2013) 31:1091 1104 DOI 10.1007/s40273-013-0111-0 CURRENT OPINION Assessing the Cost Effectiveness of Pre-Exposure Prophylaxis for HIV Prevention in the US Fred J. Hellinger Published
HPTN 061: The Brothers Study
 HPTN 061: The Brothers Study PRESENTED BY: RISHA IRVIN, MD/MPH, FORMER HPTN SCHOLAR (SAN FRANCISCO DEPARTMENT OF PUBLIC HEALTH) AND CURRENT MEMBER OF THE JOHNS HOPKINS HPTN SITE ON BEHALF OF HPTN 061 Background
HPTN 061: The Brothers Study PRESENTED BY: RISHA IRVIN, MD/MPH, FORMER HPTN SCHOLAR (SAN FRANCISCO DEPARTMENT OF PUBLIC HEALTH) AND CURRENT MEMBER OF THE JOHNS HOPKINS HPTN SITE ON BEHALF OF HPTN 061 Background
The Science behind Preexposure Prophylaxis (PrEP) Yunus Moosa Department of Infectious Diseases UKZN
 The Science behind Preexposure Prophylaxis (PrEP) Yunus Moosa Department of Infectious Diseases UKZN 1 Ongoing HIV transmission despite expanding access to ART SA 18 16 14 12 10 8 6 4 2 0 Treatment exposure
The Science behind Preexposure Prophylaxis (PrEP) Yunus Moosa Department of Infectious Diseases UKZN 1 Ongoing HIV transmission despite expanding access to ART SA 18 16 14 12 10 8 6 4 2 0 Treatment exposure
HIV PREVENTION. ETHICAL CONSIDERATIONS IN DEVELOPING AN EVIDENCE BASE FOR PrEP IN PREGNANT WOMEN
 HIV PREVENTION ETHICAL CONSIDERATIONS IN DEVELOPING AN EVIDENCE BASE FOR PrEP IN PREGNANT WOMEN Kristen Sullivan, PhD, MSW Margaret Little, PhD Anne D. Lyerly, MD HIV & pregnancy Approximately 17.8 million
HIV PREVENTION ETHICAL CONSIDERATIONS IN DEVELOPING AN EVIDENCE BASE FOR PrEP IN PREGNANT WOMEN Kristen Sullivan, PhD, MSW Margaret Little, PhD Anne D. Lyerly, MD HIV & pregnancy Approximately 17.8 million
Using PrEP as Harm Reduction. Iman Little, MPH Team Lead, Preventative Services Chicago Center for HIV Elimination
 Be PrEPared! Using PrEP as Harm Reduction Iman Little, MPH Team Lead, Preventative Services Chicago Center for HIV Elimination Ricky Hill, MA, PhD candidate Team Lead, Community Partnerships and Engagement
Be PrEPared! Using PrEP as Harm Reduction Iman Little, MPH Team Lead, Preventative Services Chicago Center for HIV Elimination Ricky Hill, MA, PhD candidate Team Lead, Community Partnerships and Engagement
HIV. The Role of Pre-Exposure Prophylaxis (PrEP) for the Prevention of HIV. Brief History of HIV AIDS. Global HIV Infection.
 The Role of Pre-Exposure Prophylaxis (PrEP) for the Prevention of HIV HIV Sarah Kemink, PharmD, AAHIVP WMSHP Spring Seminar 5/05/2015 AIDS CD4 less than 200 +/- AIDS-defining illness Most common: Candidiasis
The Role of Pre-Exposure Prophylaxis (PrEP) for the Prevention of HIV HIV Sarah Kemink, PharmD, AAHIVP WMSHP Spring Seminar 5/05/2015 AIDS CD4 less than 200 +/- AIDS-defining illness Most common: Candidiasis
Pre-Exposure Prophylaxis (PrEP) Stefanie La Manna, PhD, MPH, APRN, FNP-C, AGACNP-BC October 12, 2018
 Pre-Exposure Prophylaxis (PrEP) Stefanie La Manna, PhD, MPH, APRN, FNP-C, AGACNP-BC October 12, 2018 Disclosures I have no financial disclosures to report Objectives Identify the need for HIV prevention
Pre-Exposure Prophylaxis (PrEP) Stefanie La Manna, PhD, MPH, APRN, FNP-C, AGACNP-BC October 12, 2018 Disclosures I have no financial disclosures to report Objectives Identify the need for HIV prevention
PrEP Initiative / Iniciativa PrEx
 PrEP Initiative / Iniciativa PrEx Sponsored by NIH/NIAID/DAIDS with co-funding by the Bill & Melinda Gates Foundation and drug donated by Gilead Sciences iprex: Global Prevention Initiative Enrolled 2,499
PrEP Initiative / Iniciativa PrEx Sponsored by NIH/NIAID/DAIDS with co-funding by the Bill & Melinda Gates Foundation and drug donated by Gilead Sciences iprex: Global Prevention Initiative Enrolled 2,499
ART and Transmission. Martin Fisher. Brighton and Sussex University Hospitals, UK
 ART and Transmission Martin Fisher Brighton and Sussex University Hospitals, UK 11th Advanced HIV Course Aix-en-Provence 2013 New HIV diagnoses by exposure group: United Kingdom, 2002 2011 1 Predicted
ART and Transmission Martin Fisher Brighton and Sussex University Hospitals, UK 11th Advanced HIV Course Aix-en-Provence 2013 New HIV diagnoses by exposure group: United Kingdom, 2002 2011 1 Predicted
A Sex-informed Framework for HIV Prevention. Robert M. Grant, M.D., M.P.H. Betty Jean and Hiro Ogawa Endowed Investigator
 A Sex-informed Framework for HIV Prevention Robert M. Grant, M.D., M.P.H. Betty Jean and Hiro Ogawa Endowed Investigator Disclosures Gilead Sciences donated study medication to US NIH for trials that I
A Sex-informed Framework for HIV Prevention Robert M. Grant, M.D., M.P.H. Betty Jean and Hiro Ogawa Endowed Investigator Disclosures Gilead Sciences donated study medication to US NIH for trials that I
About FEM-PrEP. FEM-PrEP is also studying various behaviors, clinical measures, and health outcomes among the trial s participants.
 Fact Sheet About FEM-PrEP What is the FEM-PrEP clinical trial? FEM-PrEP is a Phase III randomized, placebo-controlled, clinical trial designed to assess the safety and effectiveness of a daily oral dose
Fact Sheet About FEM-PrEP What is the FEM-PrEP clinical trial? FEM-PrEP is a Phase III randomized, placebo-controlled, clinical trial designed to assess the safety and effectiveness of a daily oral dose
PrEP for HIV prevention. Pep Coll AIDS Research Institute-IrsiCaixa Fight AIDS Foundation BCN Checkpoint
 PrEP for HIV prevention Pep Coll AIDS Research Institute-IrsiCaixa Fight AIDS Foundation BCN Checkpoint DISCLOSURES I have received a research grant from Gilead Sciences awarded to my institution I have
PrEP for HIV prevention Pep Coll AIDS Research Institute-IrsiCaixa Fight AIDS Foundation BCN Checkpoint DISCLOSURES I have received a research grant from Gilead Sciences awarded to my institution I have
Important Safety Information About Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP)
 Important Safety Information About Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) For Healthcare Providers About Emtricitabine/Tenofovir Disoproxil
Important Safety Information About Emtricitabine/Tenofovir Disoproxil Fumarate 200 mg/300 mg for HIV-1 Pre-exposure Prophylaxis (PrEP) For Healthcare Providers About Emtricitabine/Tenofovir Disoproxil
HIV Clinical Update- HIV prevention
 HIV Clinical Update- HIV prevention 18 th Australian and New Zealand Conference on Haemophilia and Rare Bleeding Disorders, Melbourne October 13 th 2017 Edwina Wright Disclosures None in the past 12 months
HIV Clinical Update- HIV prevention 18 th Australian and New Zealand Conference on Haemophilia and Rare Bleeding Disorders, Melbourne October 13 th 2017 Edwina Wright Disclosures None in the past 12 months
The Latest on HIV Testing. Dominika Seidman, MD MAS
 The Latest on HIV Testing Dominika Seidman, MD MAS Disclosures none 2 Learning objectives At the conclusion of this session, participants should be able to Define the window periods for various HIV tests
The Latest on HIV Testing Dominika Seidman, MD MAS Disclosures none 2 Learning objectives At the conclusion of this session, participants should be able to Define the window periods for various HIV tests
CATIE STATEMENT. on the use of oral pre-exposure prophylaxis (PrEP) as a highly effective strategy to prevent the sexual transmission of HIV
 CATIE STATEMENT on the use of oral pre-exposure prophylaxis (PrEP) as a highly effective strategy to prevent the sexual transmission of HIV The consistent and correct use of oral Truvada as pre-exposure
CATIE STATEMENT on the use of oral pre-exposure prophylaxis (PrEP) as a highly effective strategy to prevent the sexual transmission of HIV The consistent and correct use of oral Truvada as pre-exposure
The Open Label Trial Past and Present
 The Open Label Trial Past and Present Jared Baeten, M.D., Ph.D. University of Washington Beyond Phase III: Seeking civil society perspectives on next steps with the dapivirine ring for HIV prevention in
The Open Label Trial Past and Present Jared Baeten, M.D., Ph.D. University of Washington Beyond Phase III: Seeking civil society perspectives on next steps with the dapivirine ring for HIV prevention in
EPIDEMIOLOGY AND PREVENTION
 EPIDEMIOLOGY AND PREVENTION Age, Race/Ethnicity, and Behavioral Risk Factors Associated With Per Contact Risk of HIV Infection Among Men Who Have Sex With Men in the United States Hyman M. Scott, MD, MPH,*
EPIDEMIOLOGY AND PREVENTION Age, Race/Ethnicity, and Behavioral Risk Factors Associated With Per Contact Risk of HIV Infection Among Men Who Have Sex With Men in the United States Hyman M. Scott, MD, MPH,*
OR: Steps you can take in the clinic to prevent HIV infections
 Implementing Changes to Reduce HIV Incidence: Synergies between Public Health and Primary Care Kevin Ard, MD, MPH Brigham and Women s Hospital, Massachusetts General Hospital, and the Fenway Institute
Implementing Changes to Reduce HIV Incidence: Synergies between Public Health and Primary Care Kevin Ard, MD, MPH Brigham and Women s Hospital, Massachusetts General Hospital, and the Fenway Institute
Multiple choice questions: ANSWERS
 Multiple choice questions: ANSWERS Chapter 1. Diagnosis and promotion of serostatus awareness in sub-saharan Africa 1. Antiretroviral therapy reduces HIV transmission from a HIV- positive person to a susceptible
Multiple choice questions: ANSWERS Chapter 1. Diagnosis and promotion of serostatus awareness in sub-saharan Africa 1. Antiretroviral therapy reduces HIV transmission from a HIV- positive person to a susceptible
Fertility Desires/Management of Serodiscordant HIV + Couples
 Fertility Desires/Management of Serodiscordant HIV + Couples William R. Short, MD, MPH Assistant Professor of Medicine Division Of Infectious Diseases Jefferson Medical College of Thomas Jefferson University
Fertility Desires/Management of Serodiscordant HIV + Couples William R. Short, MD, MPH Assistant Professor of Medicine Division Of Infectious Diseases Jefferson Medical College of Thomas Jefferson University
Getting Prepped for PrEP. Ken Ho, MD, MPH World AIDS Day
 Getting Prepped for PrEP Ken Ho, MD, MPH World AIDS Day Objectives HIV epidemiology What is PrEP? Does it Work? Who gets PrEP? How do I prescribe PrEP What to do at the first visit? What to do at follow
Getting Prepped for PrEP Ken Ho, MD, MPH World AIDS Day Objectives HIV epidemiology What is PrEP? Does it Work? Who gets PrEP? How do I prescribe PrEP What to do at the first visit? What to do at follow
CROI 2015: HIV Prevention Updates
 NORTHWEST AIDS EDUCATION AND TRAINING CENTER CROI 2015: HIV Prevention Updates Ruanne V Barnabas, MBChB Dphil Global Health and Medicine University of Washington a bevy of new studies quelled most remaining
NORTHWEST AIDS EDUCATION AND TRAINING CENTER CROI 2015: HIV Prevention Updates Ruanne V Barnabas, MBChB Dphil Global Health and Medicine University of Washington a bevy of new studies quelled most remaining
PrEP and npep for HIV Prevention. Harry Rosado-Santos MD, FACP Associate Professor UU School of Medicine
 PrEP and npep for HIV Prevention Harry Rosado-Santos MD, FACP Associate Professor UU School of Medicine Case Study A 26 y/o M presents for routine asymptomatic screening for sexually transmitted infections
PrEP and npep for HIV Prevention Harry Rosado-Santos MD, FACP Associate Professor UU School of Medicine Case Study A 26 y/o M presents for routine asymptomatic screening for sexually transmitted infections
Pre-Exposure Prophylaxis: The New Frontier of Prophylaxis Against HIV Infection
 Pre-Exposure Prophylaxis: The New Frontier of Prophylaxis Against HIV Infection William F. Ryan Community Health Network PrEP Network Coordinator: Trevor Hedberg, MPH, CPH Supervising Health Educator:
Pre-Exposure Prophylaxis: The New Frontier of Prophylaxis Against HIV Infection William F. Ryan Community Health Network PrEP Network Coordinator: Trevor Hedberg, MPH, CPH Supervising Health Educator:
Sexually Transmitted Infection Treatment and HIV Prevention
 Sexually Transmitted Infection Treatment and HIV Prevention Toye Brewer, MD Co-Director, Fogarty International Training Program University of Miami Miller School of Medicine STI Treatment and HIV Prevention.
Sexually Transmitted Infection Treatment and HIV Prevention Toye Brewer, MD Co-Director, Fogarty International Training Program University of Miami Miller School of Medicine STI Treatment and HIV Prevention.
HIV Prevention in Clinical Care Settings
 NORTHWEST AIDS EDUCATION AND TRAINING CENTER HIV Prevention in Clinical Care Settings Jeanne Marrazzo, MD, MPH Professor, Division of Allergy and Infectious Diseases University of Washington HIV Prevention
NORTHWEST AIDS EDUCATION AND TRAINING CENTER HIV Prevention in Clinical Care Settings Jeanne Marrazzo, MD, MPH Professor, Division of Allergy and Infectious Diseases University of Washington HIV Prevention
Ending the Epidemic: What Clinicians Need to Know
 Ending the Epidemic: What Clinicians Need to Know Mary Goodspeed, RN, BS Coordinator, HIV Clinical Education Erie County Medical Center 716-898-4713 LEARNING OBJECTIVES: 1. List the three points of the
Ending the Epidemic: What Clinicians Need to Know Mary Goodspeed, RN, BS Coordinator, HIV Clinical Education Erie County Medical Center 716-898-4713 LEARNING OBJECTIVES: 1. List the three points of the
PrEP for HIV Prevention. Adult Clinical Guideline from the New York State Department of Health AIDS Institute
 PrEP for HIV Prevention Adult Clinical Guideline from the New York State Department of Health AIDS Institute www.hivguidelines.org Purpose of the PrEP Guideline Raise awareness of PrEP among healthcare
PrEP for HIV Prevention Adult Clinical Guideline from the New York State Department of Health AIDS Institute www.hivguidelines.org Purpose of the PrEP Guideline Raise awareness of PrEP among healthcare
HIV Reproductive Health: Conception Options in the Era of PrEP
 HIV Reproductive Health: Conception Options in the Era of PrEP Meg Sullivan, MD Director, HIV Clinical Programs, Section of Infectious Diseases Boston Medical Center Boston University School of Medicine
HIV Reproductive Health: Conception Options in the Era of PrEP Meg Sullivan, MD Director, HIV Clinical Programs, Section of Infectious Diseases Boston Medical Center Boston University School of Medicine
Understanding Adherence to Daily and Intermittent Regimens of Oral HIV Pre-exposure Prophylaxis Among Men Who Have Sex with Men in Kenya
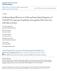 Portland State University PDXScholar OHSU-PSU Joint School of Public Health Faculty Publications and Presentations OHSU-PSU Joint School of Public Health 11-2014 Understanding Adherence to Daily and Intermittent
Portland State University PDXScholar OHSU-PSU Joint School of Public Health Faculty Publications and Presentations OHSU-PSU Joint School of Public Health 11-2014 Understanding Adherence to Daily and Intermittent
HIV Pre- Exposure Prophylaxis
 HIV Pre- Exposure Prophylaxis KNOWLEDGE AND ATTITUDES IN NORTH QUEENSLAND GENERAL PRACTITIONERS Principle Investigator: William Lane Co-Supervisor 1: Professor Clare Heal Co-Supervisor 2: Dr Jennifer Banks
HIV Pre- Exposure Prophylaxis KNOWLEDGE AND ATTITUDES IN NORTH QUEENSLAND GENERAL PRACTITIONERS Principle Investigator: William Lane Co-Supervisor 1: Professor Clare Heal Co-Supervisor 2: Dr Jennifer Banks
Pre-Exposure Topical Microbicides and Oral Prophylaxis Trials:
 Pre-Exposure Topical Microbicides and Oral Prophylaxis Trials: Rationale, Designs & Issues Connie Celum, MD, MPH Patrick Ndase, MBChB, MPH May 2007 Regional MTN Meeting Importance of HIV prevention Antiretroviral
Pre-Exposure Topical Microbicides and Oral Prophylaxis Trials: Rationale, Designs & Issues Connie Celum, MD, MPH Patrick Ndase, MBChB, MPH May 2007 Regional MTN Meeting Importance of HIV prevention Antiretroviral
The Open Label Trial Past and Present
 The Open Label Trial Past and Present Patrick Ndase, MBChB, M.P.H University of Washington Beyond Phase III: Seeking civil society perspectives on next steps with the dapivirine ring for HIV prevention
The Open Label Trial Past and Present Patrick Ndase, MBChB, M.P.H University of Washington Beyond Phase III: Seeking civil society perspectives on next steps with the dapivirine ring for HIV prevention
Treatment as prevention (TasP) Can treatment reduce the transmission of HIV: Experience from the UK
 Treatment as prevention (TasP) Can treatment reduce the transmission of HIV: Experience from the UK Dr Valerie Delpech Health Protection Agency, London (Public Health England) Outline! Brief overview Science,
Treatment as prevention (TasP) Can treatment reduce the transmission of HIV: Experience from the UK Dr Valerie Delpech Health Protection Agency, London (Public Health England) Outline! Brief overview Science,
Important Safety Information About TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication. For Healthcare Providers
 Important Safety Information About TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication For Healthcare Providers About TRUVADA for a PrEP Indication INDICATION AND PRESCRIBING CONSIDERATIONS TRUVADA,
Important Safety Information About TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication For Healthcare Providers About TRUVADA for a PrEP Indication INDICATION AND PRESCRIBING CONSIDERATIONS TRUVADA,
Update on PrEP progress: WHO/UNAIDS challenges and actions
 Update on PrEP progress: WHO/UNAIDS challenges and actions Kevin R. O'Reilly Prevention in the Health Sector, HIV/AIDS Department, WHO HQ Outline Review current status of PrEP Planning for PrEP Pre-exposure
Update on PrEP progress: WHO/UNAIDS challenges and actions Kevin R. O'Reilly Prevention in the Health Sector, HIV/AIDS Department, WHO HQ Outline Review current status of PrEP Planning for PrEP Pre-exposure
REVIEW OF PREP GUIDELINES: A PRIMER FOR THE PRIMARY CARE PRACTITIONER ANTONIO E. URBINA, MD. PrEP Webinar Series
 REVIEW OF PREP GUIDELINES: A PRIMER FOR THE PRIMARY CARE PRACTITIONER ANTONIO E. URBINA, MD PrEP Webinar Series Disclosure Speaker s Bureau: Gilead, VIIV, BMS, Merck, Serono LEARNING OBJECTIVES: 1. Review
REVIEW OF PREP GUIDELINES: A PRIMER FOR THE PRIMARY CARE PRACTITIONER ANTONIO E. URBINA, MD PrEP Webinar Series Disclosure Speaker s Bureau: Gilead, VIIV, BMS, Merck, Serono LEARNING OBJECTIVES: 1. Review
TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication. Training Guide for Healthcare Providers
 TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication Training Guide for Healthcare Providers About TRUVADA for a PrEP indication to reduce the risk of sexually acquired HIV-1 infection in high-risk
TRUVADA for a Pre-exposure Prophylaxis (PrEP) Indication Training Guide for Healthcare Providers About TRUVADA for a PrEP indication to reduce the risk of sexually acquired HIV-1 infection in high-risk
HIV & Condomless Sex - What is the Risk? Why Not? Alan J. Taege, MD Assistant Professor of Medicine Department of Infectious Disease Cleveland Clinic
 HIV & Condomless Sex - What is the Risk? Why Not? Alan J. Taege, MD Assistant Professor of Medicine Department of Infectious Disease Cleveland Clinic Yes No What s Going on Out There? Condomless Sex among
HIV & Condomless Sex - What is the Risk? Why Not? Alan J. Taege, MD Assistant Professor of Medicine Department of Infectious Disease Cleveland Clinic Yes No What s Going on Out There? Condomless Sex among
Development of a Clinical Screening Index Predictive of Incident HIV Infection Among Men Who Have Sex With Men in the United States
 EPIDEMIOLOGY AND PREVENTION Development of a Clinical Screening Index Predictive of Incident HIV Infection Among Men Who Have Sex With Men in the United States Dawn K. Smith, MD, MS, MPH, Sherri L. Pals,
EPIDEMIOLOGY AND PREVENTION Development of a Clinical Screening Index Predictive of Incident HIV Infection Among Men Who Have Sex With Men in the United States Dawn K. Smith, MD, MS, MPH, Sherri L. Pals,
Pre-exposure Prophylaxis and Primary Care
 Pre-exposure Prophylaxis and Primary Care National Latino HIV and Hepatitis C Conference June 7 th, 2016 Allison Finkenbinder, MSN, WHNP-BC Denver Prevention Training Center Who s in the audience? Disclosures
Pre-exposure Prophylaxis and Primary Care National Latino HIV and Hepatitis C Conference June 7 th, 2016 Allison Finkenbinder, MSN, WHNP-BC Denver Prevention Training Center Who s in the audience? Disclosures
Modernization of North Carolina s HIV control measures
 Modernization of North Carolina s HIV control measures North Carolina Division of Public Health Victoria Mobley, MD MPH Evelyn Foust, MPH CPM Agenda Introduction Summary of scientific evidence used to
Modernization of North Carolina s HIV control measures North Carolina Division of Public Health Victoria Mobley, MD MPH Evelyn Foust, MPH CPM Agenda Introduction Summary of scientific evidence used to
PrEP FAQs for Providers
 PrEP FAQs for Providers 1. WHAT IS PrEP? PrEP stands for pre-exposure prophylaxis. It is the use of antiretroviral medication to prevent acquisition of HIV infection. PrEP is used by HIV uninfected people
PrEP FAQs for Providers 1. WHAT IS PrEP? PrEP stands for pre-exposure prophylaxis. It is the use of antiretroviral medication to prevent acquisition of HIV infection. PrEP is used by HIV uninfected people
IAPAC Summit Daily And Intermittent PrEP
 IAPAC Summit 2012 Daily And Intermittent PrEP Six Clinical Trial Sites Nyanga District Population: 500 000 Unemployment: 70% Shack dwelling 50% Background The Global iprex Study Design Double blind,
IAPAC Summit 2012 Daily And Intermittent PrEP Six Clinical Trial Sites Nyanga District Population: 500 000 Unemployment: 70% Shack dwelling 50% Background The Global iprex Study Design Double blind,
Strategic use of antiretroviral drugs to prevent HIV transmission
 Strategic use of antiretroviral drugs to prevent HIV transmission 22th Tunisian Congress of Infectious Diseases 2nd Congress of Federation of Arab Societies of Clinical Microbiology and Infectious Diseases
Strategic use of antiretroviral drugs to prevent HIV transmission 22th Tunisian Congress of Infectious Diseases 2nd Congress of Federation of Arab Societies of Clinical Microbiology and Infectious Diseases
Pre-Exposure Prophylaxis (PrEP) for HIV Infection
 Pre-Exposure Prophylaxis (PrEP) for HIV Infection Jeffrey D. Klausner, MD, MPH Professor of Medicine and Public Health University of California Los Angeles Attending Physician UCLA Center AIDS Research
Pre-Exposure Prophylaxis (PrEP) for HIV Infection Jeffrey D. Klausner, MD, MPH Professor of Medicine and Public Health University of California Los Angeles Attending Physician UCLA Center AIDS Research
PrEP: Preexposure Prophylaxis to prevent HIV Lisa Pietrusza, BSN, RN FNP-DNP student, University of Pittsburgh Nov. 3, 2017.
 PrEP: Preexposure Prophylaxis to prevent HIV Lisa Pietrusza, BSN, RN FNP-DNP student, University of Pittsburgh Nov. 3, 2017 Disclosures The views expressed heroine do not necessarily reflect the official
PrEP: Preexposure Prophylaxis to prevent HIV Lisa Pietrusza, BSN, RN FNP-DNP student, University of Pittsburgh Nov. 3, 2017 Disclosures The views expressed heroine do not necessarily reflect the official
Open Forum Infectious Diseases MAJOR ARTICLE
 Open Forum Infectious Diseases MAJOR ARTICLE Is Emtricitabine-Tenofovir Disoproxil Fumarate Preexposure Prophylaxis for the Prevention of Human Immunodeficiency Virus Infection Safer Than Aspirin? Noah
Open Forum Infectious Diseases MAJOR ARTICLE Is Emtricitabine-Tenofovir Disoproxil Fumarate Preexposure Prophylaxis for the Prevention of Human Immunodeficiency Virus Infection Safer Than Aspirin? Noah
HIV Prevention: 2010
 Antiretrovirals for Prevention: Opportunities and Challenges for TREAT Asia KENNETH H. MAYER, M.D. BROWN UNIVERSITY/MIRIAM HOSPITAL FENWAY HEALTH HIV Prevention: 2010 DECREASE SOURCE OF INFECTION Barrier
Antiretrovirals for Prevention: Opportunities and Challenges for TREAT Asia KENNETH H. MAYER, M.D. BROWN UNIVERSITY/MIRIAM HOSPITAL FENWAY HEALTH HIV Prevention: 2010 DECREASE SOURCE OF INFECTION Barrier
ETHICS IN HIV PREVENTION RESEARCH IN THE NEW ERA OF
 ETHICS IN HIV PREVENTION RESEARCH IN THE NEW ERA OF PrEP MTN Regional Meeting Cape Town 20 September 2017 Dhevium Govender South African Medical Research Council HIV Prevention Research Unit Quality/Regulatory
ETHICS IN HIV PREVENTION RESEARCH IN THE NEW ERA OF PrEP MTN Regional Meeting Cape Town 20 September 2017 Dhevium Govender South African Medical Research Council HIV Prevention Research Unit Quality/Regulatory
QUESTIONS AND ANSWERS ABOUT PARTNERS PrEP AND VOICE
 CONTACT: Lisa Rossi +1-412-641-8940 +1-412- 916-3315 (mobile) rossil@upmc.edu QUESTIONS AND ANSWERS ABOUT PARTNERS PrEP AND VOICE 1. What is the Partners PrEP study? The Partners PrEP Study is a double-blind,
CONTACT: Lisa Rossi +1-412-641-8940 +1-412- 916-3315 (mobile) rossil@upmc.edu QUESTIONS AND ANSWERS ABOUT PARTNERS PrEP AND VOICE 1. What is the Partners PrEP study? The Partners PrEP Study is a double-blind,
Estimates of New HIV Infections in the United States
 Estimates of New HIV Infections in the United States CDC HIV/AIDS FactS A u g u s t 28 Accurately tracking the HIV epidemic is essential to the nation s HIV prevention efforts. Yet monitoring trends in
Estimates of New HIV Infections in the United States CDC HIV/AIDS FactS A u g u s t 28 Accurately tracking the HIV epidemic is essential to the nation s HIV prevention efforts. Yet monitoring trends in
HHS Public Access Author manuscript J Acquir Immune Defic Syndr. Author manuscript; available in PMC 2017 August 01.
 PrEP adherence patterns strongly impact individual HIV risk and observed efficacy in randomized clinical trials Dobromir T. DIMITROV, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research
PrEP adherence patterns strongly impact individual HIV risk and observed efficacy in randomized clinical trials Dobromir T. DIMITROV, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Research
