MALARIA SUPPLY CHAIN IN THE GREATER MEKONG SUBREGION
|
|
|
- Constance Whitehead
- 6 years ago
- Views:
Transcription
1 MALARIA SUPPLY CHAIN IN THE GREATER MEKONG SUBREGION
2
3 MALARIA SUPPLY CHAIN IN THE GREATER MEKONG SUBREGION
4 World Health Organization 2017 ISBN Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization ( Suggested citation. Malaria supply chain in the Greater Mekong Subregion Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. 1. Malaria prevention and control. 2. Mekong valley. I. World Health Organization Regional Office for the Western Pacific. (NLM Classification: WC765) Sales, rights and licensing. To purchase WHO publications, see To submit requests for commercial use and queries on rights and licensing, see For WHO Western Pacific Regional Publications, request for permission to reproduce should be addressed to Publications Office, World Health Organization, Regional Office for the Western Pacific, P.O. Box 2932, 1000, Manila, Philippines, Fax. No. (632) , wpropuballstaff@who.int Third-party materials. If you wish to reuse material from this work that is attributed to a third party, such as tables, figures or images, it is your responsibility to determine whether permission is needed for that reuse and to obtain permission from the copyright holder. The risk of claims resulting from infringement of any third-party-owned component in the work rests solely with the user. General disclaimers. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of WHO concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers products does not imply that they are endorsed or recommended by WHO in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by WHO to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall WHO be liable for damages arising from its use. For inquiries and request for WHO Western Pacific Regional Publications, please contact the Publications Office, World Health Organization, Regional Office for the Western Pacific, P.O. Box 2932, 1000, Manila, Philippines, Fax. No. (632) , wpropuballstaff@who.int
5 CONTENTS Abbreviations... iv Executive summary... vii Introduction... 1 Cambodia... 5 Lao People s Democratic Republic... 8 Myanmar Thailand Viet Nam Conclusion Country profiles References... 34
6 ABBREVIATIONS ACT AMTR AS-MQ BVBD CHAI CMPE CMS CMSD CMSSD CNM DDF DHA-PPQ DID EDAT FDA FDD GMS GP GSP IMPE IDA IRS ITNs JICA JSI LLIHN LLIN LMIS MC MIS MMA artemisinin-based combination therapy artemisinin monotherapy replacement artesunate-mefloquine Bureau of Vector-Borne Disease, Thailand Clinton Health Access Initiative Center of Malariology, Parasitology and Entomology, Lao People s Democratic Republic Central Medical Store, Cambodia Central Medical Store Depot, Myanmar Mandalay Central Medical Store Sub-Depot, Myanmar National Center for Parasitology, Entomology and Malaria Control, Cambodia Department of Drugs and Food, Cambodia dihydroartemisinin-piperaquine drug inventory database early diagnosis and treatment Food and Drug Administration Food and Drug Department, Lao People s Democratic Republic Greater Mekong Subregion general practitioner good storage practice Institute of Malariology, Parasitology and Entomology, Viet Nam International Dispensary Association, Thailand indoor residual spraying insecticide-treated bed nets Japan International Cooperation Agency John Snow Inc. long-lasting insecticidal hammock net long-lasting insecticidal net logistics management information system malaria clinic malaria information system Myanmar Medical Association iv
7 MMW MPF MPSC MP MSF NHQC NIMPE NMCP AMT OD ODK ODPC PAMS P.f. PHD PHO PMI PPM PSC PSI PSK PSM P.v. QA QC RAI RDT RSCS SOP UNOPS URC USAID USP VBDC VBDU VHV VMW WHO mobile malaria worker Myanmar Pharmaceutical Factory Medical Products Supply Center, Lao People s Democratic Republic malaria post Médecins Sans Frontières National Health Products Quality Control Center, Cambodia National Institute of Malariology, Parasitology and Entomology, Viet Nam National Malaria Control Programme oral artemisinin-based monotherapy operational district Open Data Kit Regional Office of Disease Prevention and Control Provincial Anti-Malaria Station, Lao People s Democratic Republic plasmodium falciparum provincial health department provincial health office The United States President s Malaria Initiative Public Private Mix procurement and supply chain Population Services International Population Services Khmer, Cambodia procurement and supply management plasmodium vivax quality assurance quality control Regional Artemisinin-resistance Initiative rapid diagnostic test Regional Supply Chain Strengthening standard operating procedure United Nations Office for Project Services University Research Council United States Agency for International Development U.S. Pharmacopeial Convention Vector-Borne Disease Control Programme, Myanmar vector-borne disease unit village health volunteer village malaria worker World Health Organization v
8 vi
9 EXECUTIVE SUMMARY Despite the considerable progress in malaria control across the Greater Mekong Subregion (GMS), malaria remains a significant public health concern, with growing resistance to antimalarial drugs threatening to undermine recent gains. The GMS countries of Cambodia, the Lao People s Democratic Republic, Myanmar, Thailand and Viet Nam will continue to require appropriate interventions for prevention, diagnosis and treatment. Effective procurement and supply chain management systems are crucial to ensure the timely availability of commodities to address malaria prevention and treatment. Across GMS, procurement and supply chain systems have evolved into complex mechanisms and processes that involve various stakeholders. These developments reflect significant efforts by countries to expand vector-control interventions, as well as efforts to improve the quality and availability of diagnosis and treatment, including focus on community-based services and improved integration with the private sector. Additionally, due to the continued presence of donor funding in particular the Global Fund to Fight AIDS, Tuberculosis and Malaria many external partners work directly in the procurement and supply chain process, alongside domestic agencies and organizations. While all countries have developed systems that aim to procure quality-assured commodities and distribute them throughout the health system and on to patients, there are ongoing challenges with workforce capacity, storage conditions, delivery, quality assurance, and the collection and use of timely data. Additionally, the decreasing number of malaria cases across the subregion calls for efforts to sustain funding, resources and engagement. GMS countries can continue to improve their supply chains through increased communication and collaboration among key stakeholders, greater clarity of roles and responsibilities, formalization of procedures and processes, and wider availability of data to support decision-making. vii
10 viii
11 INTRODUCTION While there has been significant progress in malaria control in the Greater Mekong Subregion (GMS) in recent years, malaria continues to be a major concern, primarily due to the development and possible spread of resistance to artemisinin and other antimalarial drugs. In order to achieve the GMS goal to eliminate Plasmodium falciparum by 2025 and all malaria by 2030 (1), the region requires strong services and systems for prevention, diagnosis and treatment, as well as effective supply chains to distribute commodities to carry out these activities. Table 1. Malaria Prevention, Diagnosis and Treatment in the GMS Cambodia Lao People s Democratic Republic Myanmar Thailand LLIN Yes Yes Yes Yes Yes IRS use Yes No Yes Yes Yes Viet Nam RDT type P.f. + P.v. specific P.f. + P.v. specific P.f. + all species P.f. + all species P.f. + P.v. specific P.f. treatment AS+MQ; DHA-PPQ+PQ AL AL; AS+MQ; DHA-PPQ+PQ DHA-PPQ DHA-PPQ+PQ P.v. treatment DHA-PPQ+PQ AL+PQ CQ+PQ CQ+PQ CQ+PQ Year of most recent treatment guidelines (to be updated in 2016) Procured antimalarials WHO-PQ WHO-PQ WHO-PQ Domestic Domestic Village malaria workers Yes Yes Yes Yes Yes LLIN = long-lasting insecticidal nets; IRS = indoor residual spraying; RDT = rapid diagnostic test; P.f. = Plasmodium falciparum; P.v. = Plasmodium vivax; AS+MQ = artesunate-mefloquine; dha-ppq = dihydroartemisinin-piperaquine; PQ = primaquine; AL = artemetherlumefantrine; CQ = chloroquine; WHO-PQ = World Health Organization prequalified. Sources: WHO (2); President s Malaria Initiative (3); interview notes. 1
12 The five countries of the GMS Cambodia, the Lao People s Democratic Republic, Myanmar, Thailand and Viet Nam are distinctly different, but they also share a number of similarities. All countries support free mass distribution of long-lasting insecticidal nets (LLINs) and targeted indoor residual spraying (IRS) for the prevention of malaria, except for the Lao People s Democratic Republic, where IRS is not a priority for prevention. Confirmatory testing with microscopy or rapid diagnostic tests (RDTs) is required in all countries before treatment is prescribed; microscopy is available at higher-level health facilities, while multispecies RDTs are used at the community level. All countries also recommend artemisinin-based combination therapies (ACTs) for first-line treatment of Plasmodium falciparum, although specific treatment regimens and drug choice differ by country. In the case of Plasmodium vivax, first-line treatment is chloroquine in most countries, with the exception of Cambodia and the Lao People s Democratic Republic, where ACT is used as the first-line treatment. All five countries have introduced village malaria workers (VMWs) and village health volunteers (VHVs) to provide community-based prevention, diagnosis and treatment, and extend the reach of services to remote and migrant populations. Additionally, the private sector can be a key source of services, providing a substantial amount of treatment in Cambodia and Myanmar, although it is less prominent in the Lao People s Democratic Republic, Thailand and Viet Nam. Regardless of the size of the private sector, all countries except Viet Nam have introduced public private partnerships to better integrate the private sector into national efforts to address malaria. However, in Viet Nam, there have been a number of successful public private partnerships in other areas of health, such as family planning and tuberculosis, which can serve as foundations for improved collaboration in malaria. Table 2. Private Sector and External Funding in the GMS Private sector share of antimalarial market Public-private partnerships Cambodia Lao People s Myanmar Thailand Viet Nam Democratic Republic 60% 35% 65% Minimal XX% Yes Yes Yes Yes No Main source of funding PMI/USAID GFATM GFATM GFATM GFATM Other key sources of funding GFATM WHO PMI/USAID and WHO PMI/USAID Other bilaterals PMI/USAID WHO PMI = President s Malaria Initiative; USAID = United States Agency for International Development; WHO = World Health Organization. Sources: WHO (2); ACTwatch Group and PSK (4); ACTwatch Group and PSI/PSI Lao PDR (5); PSI/Myanmar (6); interview notes. While all GMS countries have developed procurement and supply chain systems to ensure the availability of commodities at health facilities and within the community, these have evolved differently, with various stakeholders and processes. Due to the presence of donor funding in particular the Global Fund to Fight AIDS, Tuberculosis and Malaria across the GMS, many external partners participate in the supply chain management. For instance, the United Nations Office for Project Services (UNOPS) is heavily involved in procurement in a number of countries. 2
13 All countries have made efforts to improve data collection and analysis, including the development of logistics management information systems (LMIS), although in all cases there is room for further development. A comprehensive LMIS providing real-time information on stock levels does not currently exist in any of the GMS countries. Table 3. Agencies Responsible for Procurement and Supply Chain Management in the GMS Cambodia Lao People s Democratic Republic Myanmar Thailand Viet Nam Forecasting CNM CMPE NMCP BVBD NMCP Product selection CNM CMPE NMCP BVBD NMCP Procurement UNOPS PMU (MOH) UNOPS ODPC/BVBD NMCP Warehousing CMS MPSC CMSD BVBD NIMPE LMIS DDF/CMS CMPE MOH MOPH NIMPE QA/QC DDF/UNOPS FDD/CMPE FDA BVBD NIDQC/ NIMPE LMIS = logistics management information system; QA = quality assurance; QC = quality control; CNM = National Center for Parasitology, Entomology and Malaria Control; UNOPS = United Nations Office for Project Services; CMS = Central Medical Store; DDF = Department of Drugs and Food; CMPE = Center for Malaria, Parasitology and Entomology; PMU = Project Management Unit (established by Ministry of Health); MPSC = Medical Products Supply Center; FDD = Food and Drug Department; NMCP = National Malaria Control Program; CMSD = Central Medical Store Depot; MOH = Ministry of Health; FDA = Food and Drug Administration; BVBD = Bureau of Vector-Borne Disease; ODPC = Office of Disease Prevention and Control; MOPH = Ministry of Public Health; NIMPE = National Institute of Malariology, Parasitology and Entomology; NIDQC = National Institute of Drug Quality Control. Sources: Cambodia Ministry of Health (7); USAID (8); National Center for Malaria, Parasitology Entomology (9); President s Malaria Initiative (3); Center for Malaria, Parasitology and Entomology (10); Vectorborne Diseases Associates LLC (11); The Office of the Inspector General, Global Fund to Fight AIDS, Tuberculosis and Malaria (12); Myanmar Ministry of Health (13); Partnership for Supply Chain Management (14); Regional Artemisinin-resistance Initiative (RAI) (15). The following sections provide more detailed descriptions of prevention, diagnosis and treatment strategies in each of the five GMS countries, as well as procurement and supply chain management. These summaries are intended for a diverse audience to provide an overview of the current situation in each country. Each section also ends with a list of recommendations that may be particularly helpful for policy-makers, as well as other stakeholders involved in malaria funding, technical assistance and implementation, as they work to strengthen national procurement and supply chain systems. 3
14 4
15 CAMBODIA Background The number of malaria cases has halved over the last decade in Cambodia, which reported cases and 10 deaths in 2015 (2). Nevertheless, malaria remains a significant public health concern and is currently endemic in 21 out of 25 provinces (7), with approximately half of the population (7.5 million people), at high risk (2). The north-eastern part of the country is particularly affected, accounting for 70% of the malaria burden, especially along the forested borders of the Lao People s Democratic Republic, Thailand and Viet Nam (7). Cambodia was the first country to identify drug-resistant strains of Plasmodium falciparum in the GMS; resistance has now reached alarming levels, with drug failure increasing along the Cambodia Thailand border (7). Prevention, diagnosis and treatment The National Strategic Plan for Elimination of Malaria in the Kingdom of Cambodia calls for universal coverage and access to preventive measures among target populations (3). Mass distribution of LLINs and long-lasting insecticidal hammock nets (LLIHNs) has been implemented, particularly in western areas of the country with artemisinin resistance. The national strategy calls for one LLIN per person and one LLIHN per family provided to those living in villages at risk, as well as the retreatment of existing nets with long-lasting insecticide (7). IRS is used in targeted low-endemic areas, and may also be deployed in response to outbreaks in high-endemic areas. Malaria diagnosis is free-of-charge in the public sector (2). While microscopy is the preferred diagnostic tool, RDTs are used when microscopy is unavailable (3). At the community level, VMWs are trained to use RDTs to diagnose malaria and guide treatment. There has been a marked shift from malaria microscopy to RDTs in recent years, particularly with the scale up of VMWs. Due to growing artemisinin resistance, the Ministry of Health adopted new treatment guidelines in 2015 which identify different first-line regimens by province (3). The new policy recommends the reintroduction of the ACT artesunate-mefloquine (AS-MQ) in areas where treatment failures to dihydroartemisinin-piperaquine (DHA-PPQ) and atovaquone-proguanil have been identified, and continuation of DHA-PPQ in areas where treatment failures have not been identified. In regards to atovaquone-proguanil, Malarone will be registered in Cambodia for use in special situations with permission from the National Center for Parasitology, Entomology and Malaria Control (CNM), in combination with another antimalarial drug, such as artesunate. The Ministry of Health has banned the sale of oral artemisinin-based monotherapies (oamts) since 2008 (2). Malaria treatment and referral services are provided by trained VMWs and mobile malaria workers (MMWs), health facilities, military medical services, and select border checkpoints and mobile clinics (7). Some 2350 villages are covered by VMWs who provide treatment and refer severe cases to health facilities (3). Particularly in rural areas, VMWs and VHVs (who, unlike VMWs, do not have malariaspecific training but cover a range of conditions) often serve as the primary source of health information and services. VMWs report to health centres that provide basic primary health care. These centres report to one of 79 operational district (OD) health offices, which each manage a referral hospital with microscopy services. While the public health sector provides free access to malaria diagnosis and treatment, a substantial share of health-care services is provided through the private sector, which includes registered health outlets (pharmacies), non-registered health outlets (drugstores), and non-health outlets (grocery stores). Unlicensed outlets have decreased in recent years but continue to provide services, particularly in rural areas. In partnership with Population Services International (PSI) and University Research Council (URC), CNM provides malaria services in collaboration with private providers (9). Under the Public Private Mix (PPM) Programme, private providers are trained on early diagnosis and treatment according to national guidelines, provided with RDTs and ACTs, and incorporated into the national malaria surveillance system (7). CNM also collaborates with private enterprises, including mining and construction, with a focus on providing onsite malaria services for migrant workers. 5
16 CAMBODIA Procurement and supply chain management Various partners across Cambodia have developed quantifications of malaria commodity needs separately from, and in addition to, those supported by CNM (8). These quantifications, leveraging different datasets and with different objectives, approaches, assumptions and tools, have produced different results. A standardized approach and quantification methodology is required to ensure a continuous and coordinated supply of commodities. In 2015, the Ministry of Health, with technical assistance from the USAID DELIVER PROJECT, conducted a national Malaria Commodities Quantification Workshop to prepare a two-year forecast and supply plan of the total commodity and funding needs for 2016 and The selected method of forecasting used reported malaria cases from the national malaria information system (MIS), as well as central-level stock on hand from the public sector and from Population Services Khmer (PSK), PSI s Cambodian affiliate, to represent the private sector. Malaria commodities are selected by the CNM Technical Bureau and Pharmacy Unit in line with the World Health Organization (WHO) and Cambodia treatment guidelines. The Ministry of Health s Department of Drugs and Food (DDF) is involved in the revision of the Essential Medicines List to ensure inclusion of CNM-suggested medicines. Going forward, RDT brands will be selected from among WHO-prequalified suppliers, and CNM will submit RDTs for lot testing upon arrival in country by sending samples to Institute Pasteur in Cambodia (7). UNOPS previously carried out postshipment testing of all antimalarials at a WHO prequalification lab in Nepal, but this is no longer required by the Global Fund because procured products are sourced from WHO-prequalified sources. Currently, UNOPS only performs quality control (QC) at a national level through DDF; samples are collected from the private sector and tested in the national QC lab. Multiple partners are involved in the procurement of malaria commodities for the national malaria control programme (8). UNOPS, the Global Fund Principal Recipient, is the primary source for procurement and manages the entire procurement process for the Global Fund in regards to antimalarials, RDTs, LLINs, and other goods and services. The Cambodian Ministry of Health is involved in procuring second-line treatments, as well as treatment for severe malaria (8). The United States of America President s Malaria Initiative (PMI) has regional and country funds that are used to fill commodity gaps when required. Additional partners including Médecins Sans Frontières (MSF) and WHO may also be involved in procuring goods. In the private sector, PSK, which operates a nationwide social marketing programme of RDTs and ACTs, is responsible for procurement, with support from UNOPS. The importation of pharmaceutical products is regulated by the DDF, and only drugs registered with DDF can be legally imported in Cambodia. DDF requires importers to obtain an import permit for each shipment, and make the required payment to clear customs (16). Logistic management functions for the malaria programme are integrated into the overall Ministry of Health system supply chain for all commodities (9). These flow from the Central Medical Store (CMS) to the provincial health departments (PHDs) and OD health offices, then to health centres or referral hospitals and finally to VMWs. CMS is responsible for distributing essential medicines and commodities to ODs on a quarterly basis. The supplies that arrive at the OD stores are stored in the OD warehouses and then distributed to health facilities based on a pull system, in which the OD provides medical commodities after receiving requests from health centres. If the OD store is severely overstocked with a particular commodity, some OD stores will switch to a push system to rebalance their inventories (3). CMS will also resort to push policies if it has commodities that are nearing expiry. Oversupply and undersupply occur at the health facility and community levels. Storage capacity is currently inadequate, as products are frequently moved from one storage area to another due to a lack of space. There are no standard operating procedures (SOPs) for reception and storage, and the programme suffers from a lack of sufficient workforce dedicated to handling supplies that arrive at warehouses. The Drug Inventory Database (DID), which is maintained by DDF and CMS, contains data on 6
17 CAMBODIA stocks of medical supplies including commodities for malaria (9). At the health centre level staff use paper forms, while at the provincial hospital and OD levels data are entered into automated Drug Inventory Databases that feed into the national database. However, information cannot be accessed at the national level, which makes effective use of data for policy-making challenging. For example, while malaria commodity stock-on-hand and consumption data are entered into the DID at each level of the system, CNM only has access to quarterly data aggregated by ODs, hindering efforts to identify and mitigate shortages and stock-outs of malaria commodities for end users. CNM has plans to implement a mhealth-based stock management system at health centres across malaria-endemic ODs to monitor stock availability and ensure no commodity stock-outs occur (7). DDF, the National Health Products Quality Control Center (NHQC), and CNM are involved in quality assurance (QA) and QC in Cambodia for the private sector. Since 2003, the U.S. Pharmacopeial Convention (USP) has been involved in strengthening medicine QA/QC systems, with financial support from the United States Agency for International Development (USAID) and PMI (17). To address the issue of counterfeit goods, in 2014 the Cambodian Government established a national Counterfeit Committee, which is a cross-sectoral task force comprised of representatives from the ministries of interior, justice, health, finance, commerce and defense (3). In 2015, the committee facilitated the development of an action plan to perform inspections in private markets to detect and combat counterfeit medicines and to regulate private pharmacies and providers to ensure the delivery of quality medicines. Recommendations The following recommendations are made to strengthen malaria procurement and supply chain in Cambodia: Develop a consistent process for forecasting and planning. Align all partners around a standardized approach and quantification methodology to ensure a continuous and coordinated supply of commodities. There should be an agreed use of specific datasets, as well a shared understanding of objectives, approaches, assumptions and tools. Improve storage conditions at CMS by developing SOPs following good storage practice (GSP) and good distribution practice, building capacity for planning and stock management, and strengthening the capacity of CMS. Increase qualified human resources at CMS and other levels of procurement and the supply chain system. Develop a workforce plan and increase the number and capacity of staff involved in malaria procurement and the supply chain. Support CNM to enhance its LMIS system by ensuring greater accessibility to data at all levels of the supply chain. There is a need to streamline the DID, as current differences between the management and support of the DID at the national level and DID at the OD and hospital levels result in poor integrity control for both data and systems. Additionally, there is a need to ensure the availability of trained information technology (IT) staff in both CMS and DDF to support all electronic systems. 7
18 LAO PEOPLE S DEMOCRATIC REPUBLIC Background The number of reported malaria cases in the Lao People s Democratic Republic has fluctuated over the past decade, with outbreaks occurring in recent years (18), and in 2015 the figure stood at (2). Malaria mortality has fallen dramatically, with only two deaths reported in 2015 (2). An estimated 2.1 million people, or approximately one third of the population, are at high risk of malaria (2); ethnic minority groups, forest-fringe inhabitants, migrants and new forest settlers are particularly affected (19). Artemisinin resistance has been detected in southern areas of the country bordering Cambodia and Thailand and is an ongoing concern (3). Prevention, diagnosis and treatment Vector control in the Lao People s Democratic Republic is at present mainly based on insecticidetreated bed nets (ITNs), although LLINs have been introduced and will gradually replace all ITNs (19). IRS is mostly limited to outbreak response andfocal control. Active case detection of febrile cases in the community has been taking place since RDTs are available at village and health centre levels, as well as at district and provincial hospitals, although emphasis is placed on microscopic diagnosis at hospital facilities (3). Since 2011, the Center of Malariology, Parasitology, and Entomology (CMPE), within the Ministry of Health, has taken a more targeted approach to the distribution of malaria control measures, directing RDTs, as well as ACTs and ITNs, towards villages with the highest burden of malaria. National malaria treatment guidelines, issued by CMPE, were significantly modified in 2005 after reports of increasing resistance to chloroquine and sulphadoxine-pyrimethamine, resulting in current guidelines specifying the use of ACTs (20). There has been a ban on the sale of oamts since 2005 (2). Malaria activities are centralized at CMPE, which oversees 18 Provincial Anti-Malaria Stations (PAMS) (3). Under the PAMS, there are 140 district and provincial hospitals, and approximately 850 health centres that cover nearly 2000 malariaendemic villages. A national village-based early diagnosis and treatment (EDAT) strategy promotes the diagnosis and treatment at the village and health centre levels. VMWs provide care for patients within the community and use RDTs, prescribe ACTs and refer patients to health facilities, if necessary (3). In villages where there is little or no malaria, VHVs are available, although these multipurpose health workers are not usually trained to diagnose and treat malaria. Artesunate injectables are available at district and provincial hospitals for severe malaria (11). The public health system is predominant, although an alternative private sector is growing (19). There are around 1865 private pharmacies and 254 private clinics, mainly located in urban areas. A Public Private Mix (PPM) programme was piloted in 2008, targeting eight districts and providing commodities to private pharmacies and clinics (3). This approach has now been expanded to 22 districts in eight provinces. Cases detected and treated through these private pharmacies and clinics have continued to rise, indicating that this may be an effective approach to reach populations that seek care from the private sector. Procurement and supply chain management CMPE is responsible for carrying out quantification, demand forecasting and supply planning of commodities (3). National procurement projections are based on historical needs and some consumption estimates, which do not take into account the effect of stock-outs (10). CMPE conducts product selection in line with its national treatment guidelines (11). Twice each year, CMPE creates a distribution plan delineating the quantity of each commodity to be sent to each PAMS, central hospital and regional/provincial warehouse. A request letter is also received from each PAMS for products to supplement those included in the distribution plan. The majority of the case management commodities are procured through the country Global Fund 8
19 LAO PEOPLE S DEMOCRATIC REPUBLIC grants (10). Generally, the Procurement Unit within Project Management Unit established by the Ministry of Health to act as the implementing body of Global Fund-supported programmes procures malaria commodities based on CMPE s forecast. Commodities are stored with the Medical Products Supply Center (MPSC) within the Ministry of Health (3). Once these are delivered to a warehouse in the capital, the supplies are distributed to the provinces. The provinces subsequently supply the districts, which supply the health centres that ultimately provide commodities to VHVs. Transport mechanisms for commodities within the supply chain have not been formalized, with an ad hoc arrangement triggered by a tendering process that often results in supplies being transferred via public transportation networks (3). A bus system is often used and small quantities are transported using vehicles from PAMS to the districts and health centres. With support from partners, CMPE has developed a LMIS to strengthen reporting of stocks of malaria commodities (10). Once a month, CMPE receives paper-based monthly summary reports from PAMS containing consumption information for each PAMS, as well as aggregated consumption data from all districts, hospitals, health centres and village health/malaria workers in the province. In addition, CMPE receives stock-on-hand data every other week from PAMS via an electronic web form, using the Open Data Kit (ODK) platform. This information can be downloaded into a dashboard that includes worksheets for analysing logistics data at the national, provincial, and district levels. Efforts are being made to improve data use for forecasting of commodities and to identify and address bottlenecks in data and logistics management at the provincial and district levels. There is also a focus on ensuring that LMIS tools are harmonized with other national information systems. MPSC has been working with the Clinton Health Access Initiative (CHAI) to pilot and scale up msupply software in selected central and regional warehouses (3). At the time of the assessment, it was understood that while msupply was being used more regularly at sites with higher volumes of stock, there remains duplication of efforts in the supply chain management of health products and that other processes such as distribution planning and distribution reporting, monitoring and supervision still needs to be strengthened concurrently. There is no formalized system for postshipment QA, and there is no QC laboratory that is WHO prequalified (12). Before issuing products, staff at CMPE conduct a visual inspection to ensure that no products are damaged, expired or otherwise unusable (11). If damaged or expired products are found, these are sent for incineration to the Food and Drug Department (FDD). FDD has received Global Fund support to strengthen its QC of antimalarial drugs in recent years. No quality checks are performed at the intermediate warehouse level or dispensing level. Recommendations The following recommendations are made to strengthen malaria procurement and the supply chain in the Lao People s Democratic Republic. Agree on purpose and functionality of ODK as a data aggregation tool for end-users (district level and below) to report data on epidemiology and stock status. Agree on purpose and functionality of msupply as a warehouse management tool for store managers (central and provincial) to manage stock, while at the same time, establishing master databases on standardizing information and coding on all Ministry of Health items, health facilities, suppliers and storage facilities. Ensure procurement and supply chain systems and SOPs support field operations and programme needs in the southern and northern regions differently, rather than adhering to a one-size-fits-all approach. Epidemiologically, the country is divided into north and south zones that should have different strategies. In the north, transmission is already low and thus this region should be moving towards malaria elimination, with consideration of the possibility of scattered focal outbreaks. The south should focus on aggressive malaria control and outbreak response. 9
20 LAO PEOPLE S DEMOCRATIC REPUBLIC Review malaria procurement and supply chain management indicators consider changing no stock-out at community/village level to no stock-out at health centre (given lowest level of the health system). Conduct logistic network analysis optimize storage facilities and distribution plan. Develop costed scale-up plan of msupply. Focus on deployment of msupply in 18 provinces. Building a national picture will increase confidence and enhance the value of both the system and the data collected. Consider ODK (data aggregator tool) feeding data into msupply (supply chain management tool), which can then feed into DHIS2 (national data repository and dashboard tool). Develop costed human resources plan and operational plan for implementation of the Five- Year Strategic Plan for Drug Medical Product Supply and Medical Technology Management ( ). Organize a MPSC-led National Supply Chain Design Workshop to achieve consensus on one master plan. Develop a formalized system for QA/QC: There is a need to put in place a formalized system for postshipment QA. Improve storage conditions by developing national guidance on storage and the effective management of commodities. Although the Global Fund has provided support to renovate part of the MPSC building, significant quantities of expired commodities requiring disposal have been observed. There is lack of clear guidance about how to manage expired commodities. Strengthen MPSC distribution system: The distribution system could be improved to rely less on ad hoc transportation mechanisms. This could potentially help to address issues with persistent stock-outs, and ensure the safe and regular delivery of commodities. Improve data collection and sharing by aligning and harmonizing all national information systems. While the Lao People s Democratic Republic has made significant efforts to develop and improve its LMIS, poor data flow and reporting are ongoing challenges. Support the MPSC Luang Prabang project. Integrate stock at warehouses and stores, while strengthening data-driven decision-making of programme units. 10
21 MYANMAR Background Myanmar has the highest malaria burden in the GMS and reported cases and 37 deaths in 2015 (2), although this figure may only reflect 25 40% of the actual burden (3). Some 8.5 million people, or 16% of the population, are at high risk of malaria (2), which occurs mainly in or near forests, particularly among remote populations, ethnic minorities and migrants (13). The Myanmar Thailand border is associated with the development of artemisinin resistance, although recent studies indicate that resistance now extends across the country (21). Prevention, diagnosis and treatment Rapid scale-up of bed nets is planned in malaria-risk areas, with the National Malaria Control Programme (NMCP) aiming to achieve a 90% target of the total population at risk; the target is 100% for the population living in risk areas based on the risk stratification (13). Going forward, NMCP has chosen LLINs for distribution through mass campaigns and a continuous channel. With evidence from 90% of communities using conventional bed nets, treatment of nets with insecticide tablets is also a clear strategy. IRS is focused on outbreak areas, as well as development projects in endemic areas and new settlements, and is also recommended in areas affected by artemisinin resistance. The national policy calls for confirmatory testing with either microscopy or RDT before treatment is prescribed (13). In hospitals and higher-level facilities, microscopy is the preferred diagnostic method. However, although there are approximately 700 malaria microscopy centres nationwide, only around 60% are estimated to be fully functional (3). RDTs are being scaled up in lower-level facilities and at the community level through VHVs, and NMCP is now one of the biggest users of RDTs in the Myanmar. Private medical practitioners are also encouraged to use RDTs. drugs, and updated in line with WHO malaria treatment guidelines that place RDTs and ACTs as the main pillars of diagnosis and treatment, respectively. Standby treatment is recommended among migrants in areas where diagnostic facilities are not available (13). While oamt use has been banned, its ongoing presence in the market is a critical issue, with increasing availability undermining the gains achieved in recent years: although availability decreased from 67% in 2012 to 10% in 2014, this figure rose to 27% in Public health services, including malaria treatment, are predominantly delivered to communities through the township health department (13). Each department manages a township hospital, a station hospital, approximately five rural health centres, and approximately five subrural health centres per station hospitals. VHVs complement health staff in the delivery of services for malaria prevention and control in high- and moderate-risk villages, hard-to-reach areas and in areas with a high concentration of migrant workers where access to health facilities is difficult. Although diagnosis and treatment of malaria is free in the public sector, an estimated 60 70% of suspected malaria patients receive treatment from the private sector (3). Hospitals and clinics in the formal private sector, which is regulated by the Ministry of Health, are mainly located in urban areas. Informal private sector providers include unlicensed drugstores and pharmacies, retail shops and mobile drug vendors. PSI supports a social franchise of licensed private general practitioners (GPs) located across the country (3). PSI is also implementing the artemisinin monotherapy replacement (AMTR) project that provides quality-assured ACTs to the private sector. Similarly, the Myanmar Medical Association (MMA) supports a network of private clinics, providing GPs with training, as well as quality-assured diagnostics and treatment. Growing support for malaria control activities is also provided by the corporate private sector, including a partnership between approximately 30 companies and the Myanmar Health and Development Consortium. The national treatment policy, which was developed in 2002, was first updated in 2008 with further revisions in 2011, 2014 and 2015 (3). The policy is based on data on parasite resistance to antimalarial 11
22 MYANMAR Procurement and supply chain management NMCP is the Government body responsible for forecasting antimalarial medicines and other supplies based on epidemiological data and past consumption (13). Annual forecasting workshops are led by UNOPS, the Global Fund s Principal Recipient, which receives input from NMCP, WHO and other Global Fund sub-recipients, including nongovernmental organizations. However, due to limitations in the availability of data, forecasts do not take into account the total number of reported malaria cases. The national strategic plan states that only WHO-prequalified combination RDTs, and ACTs recommended as per the national treatment policy, should be procured (13). Additionally, pesticides recommended by the WHO Pesticide Evaluation Scheme (WHOPES) are selected. UNOPS is responsible for procurement under the Global Fund, and is responsible for ACTs, RDTs and other malaria medicines for 291 malaria-endemic townships. Commodities are received by Global Fund subrecipients, including NMCP, for distribution in their respective supply chains to the central and regional warehouses, subdepots, hospitals and health centres (22). Health commodities may also be procured by the Vector-borne Disease Control Programme (VBDC), as well as by the Central Medical Store Depot (CMSD) (3). Other agencies involved in procurement and supply chain include PMI/USAID, Chemonics, John Snow Inc. (JSI), PSI, MSF and the Japan International Cooperation Agency (JICA), with a myriad of nongovernmental organizations involved in storage and distribution. The range of players participating in procurement and supply chain management has created a complicated landscape, with limited coordination. Government- and donor-procured products are primarily cleared by the CMSD Customs Clearance Department (14). A formal customs management process is in place, although this process is lengthy and manual. Three levels of ministry approval (Ministry of Commerce, Ministry of Finance and Revenue, and the Ministry of Health) are required to obtain a letter of exemption, which can take a month. Clearance for shipping is reported to take seven to 10 days, and clearance for air takes two to three days. The first point of warehousing can be in one of several locations including CMSD, Myanmar Pharmaceutical Factory (MPF), suppliers, vendor warehouses, the central cold room, VBDC central warehouse and nongovernmental organization central warehouses (14). The VBDC central warehouse in Yangon consists of two buildings one for pesticides, and one for antimalarials, RDTs and other commodities. Warehouses and storerooms are generally overcrowded buildings with ageing infrastructure and intermittent power supply, although there are some exceptions including the Mandalay Central Medical Store Sub- Depot (CMSSD) and nongovernmental organization facilities, which are either new or refurbished. In the public sector, health commodities are distributed through VBDC, within the Department of Public Health, and through CMSD, within Medical Care Services of the Department of Health (3). State and regional VBDC teams obtain RDTs, antimalarials and other commodities by either collecting the items themselves when traveling to Yangon for meetings, or by hiring transport. Township VBDC staff then visit the state and regional offices to collect commodities, and a similar process of retrieval is followed down to the lower levels of the health system. Transportation thus consists of a largely informal network of independent operators (14). There is no official fleet within the public supply chain, and the public passenger transportation system is a key transport mechanism. A NMCP database using Microsoft Access is currently in place to capture data on diagnosis and treatment. Training on its use has been provided in over 100 townships, allowing staff to report locally. A LMIS is being developed, aimed at improving distribution processes and capacity at all levels (14). SOPs were jointly published by the Ministry of Health and UNOPS in However, while a portion of the facilities conducting procurement have some basic procedures in place to capture data to inform purchasing, limited access to computers and software constrains data capture and analysis. Stock recording remains largely paper based, with most facilities using stock ledgers and stock cards. Where computerized tools are present, Excel spreadsheets are often used for reporting but not for managing inventory. CHAI is currently developing an electronic LMIS based on the software msupply. The system is being piloted 12
23 MYANMAR in several states, with plans to scale this up by the end of 2017 to 69 sites, including all state-level VBDC warehouses and subdepots. Postmarketing surveillance and testing are conducted by UNOPS for antimalarials procured under the Global Fund grant. The Department of Food and Drug Administration (FDA) is responsible for QA and QC of antimalarial products under the Regional Artemisinin-resistance Initiative (RAI) grant, although the number of samples currently being tested is low. In a move to improve the QA of drugs and prevent the sale of oamt, RAI is funding support to strengthen FDA s capacity to conduct drug quality monitoring and regulation (15). Efforts are also being made to ensure that national pharmaceutical reference laboratories are able to conduct the necessary laboratory analyses for postmarketing surveillance of drug quality. Since 2013, USP has collaborated with the FDA to perform quality control of medicines including antimalarials (13). Some findings can be included from the malaria external programme review concluded in March Recommendations The following recommendations are made to strengthen malaria procurement and the supply chain in Myanmar. A caveat is made in this country given that this review mission had an overlap with the Global Fund grant negotiation mission, which resulted in a reduced amount of face time with key stakeholders. Limited observations were made based on meetings with NMCP, CHAI, USAID and WHO partners only. Encourage greater coordination of stakeholders as various partners currently undertake separate processes for procurement and the supply chain in malaria, with continued fragmentation. (See the Partner and Donor Mapping section of Myanmar Country Profile.) Improved communication is required to align all stakeholders and streamline processes. Support current scale-up of the NMCP Access database on case management. Local Access databases allow staff to report locally and synchronize to a central server where Internet access is reliable. However, the system is not yet integrated with logistic reporting and DHIS2 synchronization is being considered. There should be greater emphasis on ensuring that the various information systems are linked. Increase awareness and mutual recognition of efforts made by partners (Chemonics, CHAI, JSI and UNOPS) in strengthening the overall supply chain system of the Ministry of Health is important for sustainability and efficiency. There are clear opportunities for synergies to be realized: o The Ministry of Health national master databases for products, public health facilities and suppliers (developed by CHAI and Supply Chain Management System (SCMS)) is currently being used for the msupply LMIS system, which supports the HIV, tuberculosis (TB) and malaria supply chains and Mandalay CMSSD warehouse. These databases could be also leveraged for the JSI/United Nations Population Fund- supported reproductive health supply chain, UNOPS-supported HIV, TB and malaria supply chains, Management Sciences for Health (MSH)/Three Millennium Development Goal Fund (3MDG) supported the Regional Supply Chain Strengthening (RSCS) Project in three states. This will allow standardized coding and naming of products and health facilities regardless of LMIS software or paper-based systems across different supply chains. o msupply has the potential to support the Ministry of Health integrated national supply chain future state. There should be a concerted effort by partners to jointly put in place a costed transition plan to address issues related to financial sustainability, Ministry of Health staff capacity (central, regional and township levels), IT equipment and infrastructure investments, training and msupply developers technical support costs. The future of the msupply system should be a joint investment by donors and partners for the best interests of the Ministry of Health in a sustainable manner. o Coordination with next Global Fund New Funding Model and other donor grants in the areas of procurement, and 13
24 MYANMAR supply chain management strengthening activities should also be a priority. Mainly, investments should focus on ensuring uninterrupted availability of HIV, TB and malaria commodities but at the same time, there should be consideration for prioritizing interventions that also benefit the entire Ministry of Health supply chain system, in line with the National Supply Chain Strategy recommendations and action plans. 14
25 THAILAND Background Thailand reported malaria cases in 2015, the lowest number across the GMS. However, with 33 reported deaths, mortality was high relative to the number of cases (2). Some 5.4 million people, accounting for 8% of the total population, are at high risk (2), and malaria is now confined to international forest borders, particularly the Thailand Myanmar border (23). Artemisinin resistance is also evident along border areas, and on the Thailand Cambodia border Plasmodium falciparum has become resistant to almost all available antimalarial medicines (24). Prevention, diagnosis and treatment Bed nets are distributed during routine mass campaigns across Thailand, with the Bureau of Vector-Borne Disease (BVBD) targeting one LLIN per person in malaria-endemic villages. LLIHNs are distributed in specific locations where LLINs cannot be used, for instance among migrants in forest areas (3). IRS is used for vector control in endemic and receptive areas (25). Malaria diagnosis is free-of-charge in the public sector, and diagnostic services are predominantly provided by BVBD s network of malaria clinics (MCs) and malaria posts (MPs) in endemic areas (3). MCs are equipped with light microscopes and trained microscopists, while MPs, which are staffed by VMWs, utilize RDTs for diagnosis. Active case detection using microscopy and/or RDTs is carried out in high-risk villages and towns and in the artemisinin-resistance containment zones. The malaria case management guidelines from 2015 call for the use of antimalarial first-line drugs to treat uncomplicated malaria and specify second-line drugs to be used in case of failure with first-line drugs (26). In 2015, dihydroartemisininpiperaquine (DHA-PPQ) became the first-line treatment for Plasmodium falciparum, and its efficacy is currently being evaluated (27). The sale of oamts has been banned since 1995 (2). Treatment for uncomplicated malaria can be provided at MCs, MPs and hospitals, as well as nongovernmental organization malaria units (26). In particular, MCs and MPs play a significant role in providing treatment in malaria-endemic areas and along international borders (3). Management of severe malaria, including the use of injectable artesunate, takes place at sufficiently equipped hospitals. Most treatment is available in the public sector, although private hospitals offer care for malaria patients (3). Antimalarial drugs are not permitted to be sold in pharmacies or private clinics. BVBD currently aims to better integrate the private sector by organizing a cooperative network of public and private sector organizations to coordinate national malaria control activities. Programmes such as the USAID Control and Prevention of Malaria Project (CAP Malaria) involve working with the Government to build partnerships between private providers and companies operating in malaria-endemic areas (28). Procurement and supply chain management BVBD, which falls under the Department of Disease Control within the Ministry of Public Health, uses a consumption-based forecasting method for malaria drugs, which incorporates a 20% buffer. The method takes into account the previous year s consumption levels, number of malaria cases and a minimum usage amount for each province. The Global Fund Principal Recipient (Ministry of Public Health) is responsible for RDT forecasting, which is also based on the previous year s consumption, with a 20% buffer. Malaria commodities are selected based on whether they are recommended by the WHO and are FIND-prequalified (for RDTs). BVBD procures the newly selected first-line ACT (DHA-PPQ), and also procures RDTs under the Global Fund. For insecticide, BVBD receives requests from regional staff and, once approved, regional staff locally procure insecticide using the standard Government procurement policy. LLINs are procured with Government funding, as well as with Global Fund support. There are currently considerable delays in the procurement of malaria commodities, both through the Government and the Global Fund. 15
26 THAILAND For foreign imports, companies manage customs clearance themselves based on the hazardous substances trade procedures. For LLINs procured through the Government budget, national rules are followed. For LLINs procured through donor funding, the Global Fund Principal Recipient office (Ministry of Public Health) will send an official letter to the Department of Customs. Once approved, a letter is sent to International Dispensary Association (IDA) (the procurement agent) to process the order. The Principal Recipient office is responsible for customs clearance and receipt of goods. BVBD is responsible for storing commodities at its central warehouse. Regional offices of disease prevention and control (ODPCs) also have warehouses, but in some cases, the private rental of warehouses is necessary for temporary storage due to a lack of space at the regional level. The National Malaria Control Programme (NMCP) within BVBD manages the delivery of commodities to facilities, particularly to MCs and MPs (3). Medicines are delivered to the vector-borne disease centres (VBDCs) twice a year, while distribution from the VBDCs to the vector-borne disease units (VBDUs) and from the VBDUs to the MCs occurs on a monthly basis at regularly scheduled meetings where medicines are collected. In some cases, suppliers deliver directly to provinces. When there are problems with stock availability, antimalarial drugs are exchanged between facilities and districts. Although the principle of first expired, first out is applied, nearly expired drugs and nearly expired RDTs have been found in border areas. Stockpiles of medicines and diagnostics for potential epidemics are also limited. There is currently no system in place that reports regularly on commodities, although once a year data on stock status, consumption and need are consolidated to decide on procurement levels for the following year. An online disease surveillance system for malaria gathers data on case rates, vector control and RDTs, and this system is used to verify requests from regional ODPCs and provincial health offices (PHO) to BVBD for malaria commodities. Excel spreadsheet files are also used to track stock status of RDTs every quarter, which are ed from the PHOs to BVBD and then reported to the Global Fund Principal Recipient office (Ministry of Public Health). These figures are cross-checked with cases reported via the online surveillance system. The Bureau of Drug and Narcotic, under the Department of Medical Sciences, conducts QA and QC on behalf of BVBD. Once a year, random sampling of antimalarials is conducted in the field, although this is not formalized through guidelines. Recommendations The following recommendations are made to strengthen malaria procurement and supply chain in Thailand. Identify and rectify bottlenecks in procurement to address currently delays in procurement funded by both domestic government and donors. Efforts to address bottlenecks may be of particular importance during the transition of first-line treatment for Plasmodium falciparum, and the effective roll out the newly selected drug (DHA-PPQ). Barriers to ensuring the timely delivery of appropriate commodities at the correct locations will hinder efforts towards malaria elimination. Improve data collection and monitoring to ensure effective monitoring and planning. Data on procurement and supply chain are not currently covered under the national malaria online system. Additionally, the database on insecticide use is incomplete, with large data gaps relating to procurement that result in the inability to track what is procured and at what quantities, and their usage. Develop an operational plan to transition to the new first-line regimen of antimalarials for Plasmodium falciparum, including: o establish and disseminate clear timelines and updated road map for the switch from national to provincial hospitals; o identify and support registration of additional suppliers for DHA-PQ; o provide clarity of BVBD support of drugs beyond first year to hospitals; 16
27 THAILAND o engage with hospitals on budget planning, procurement, training and clinician support of a new regimen change based on scientific evidence; o provide guidance on usage of DHA-PQ and management of existing AS-MQ stock for mutual understanding and willingness/ confidence of new BVBD guidance; o consider putting in place system of resupply and reordering for DHA-PQ given only one supplier at this stage, as compared to existing system of drug sales representatives topping up AS-MQ stock at hospitals given multiple suppliers of AS-MQ; o consider the need for the provincial level to have a buffer stock to respond to unexpected outbreaks as currently at least one week is required for emergency stock which is not ideal for outbreaks; and o support advocacy and the provision of countrywide information on the switch to DHA-PQ as the first-line treatment for uncomplicated malaria Consider sustainable solutions to fund the future procurement of LLIN by Government. Using Government funds may be a challenge due to high cost in current market conditions. The Ministry of Public Health may consider the use of international procurement agents such as IDA or the Global Fund Pooled Procurement Mechanism to avoid the low-volume, high-price barrier that the Government will face if it runs its own tenders. Another possible solution is to leverage the Global Fund E-Marketplace and Ministry of Public Health E-Marketplace. This will be increasingly important given the need to maximize Government resources and health spending efficiency after Global Fund grant support ends. Ensure advanced planning to respond to upcoming regulatory changes and prevent shortages of WHO-prequalified RDTs. Currently, RDTs are the lowest risk category for general medical devices, resulting in importers only needing basic certifications of safety. However, based on the future implementation of the Association of Southeast Asian Nations Medical Device Directive, RDTs will be reclassified as class 2 or 3. In this case, a change in Thai FDA regulations would be necessary. The key implication of this likely change in regulations would mean that existing and future suppliers/manufacturers would need to submit samples for FDA evaluation. Invest in human resources for procurement and supply chain management as currently there is no pharmacist in BVBD. There is also a need to review level of training for provincial and district stock management so as to build decentralized capacity to manage supply chains. 17
28 VIET NAM Background The number of reported malaria cases has consistently declined in Viet Nam over the past decade, with cases reported in 2015 the second lowest figure in the GMS after Thailand (2). Only three malaria deaths were reported in Malaria is highly focal and mainly occurs in remote forest areas, disproportionately affecting migrant settlers, mobile populations and ethnic minorities (29). Some 6.4 million people, or 7% of the population, are at high risk of malaria (2). Artemisinin resistance is an ongoing concern, particularly in southern Viet Nam (3). Prevention, diagnosis and treatment ITNs are distributed free-of-charge to all age groups in moderate- and high-endemic areas, and during suspected outbreaks (2). The National Malaria Control Programme (NMCP) treats existing bed nets each year with insecticide and in recent years has introduced LLINs, particularly in hardto-reach areas (3). The programme also uses IRS to cover the population residing in high endemic areas where ITN use is low, primarily concentrated in border areas with Cambodia and the Lao People s Democratic Republic. Case detection and investigation may be conducted for imported cases, indigenous cases in low-endemic areas and when cases increase in high-endemic areas. Viet Nam favours microscopy over RDTs, with plans to diagnose 95% of malaria cases in endemic areas using slide microscopy by 2020 (29). Communal microscopy centres for early diagnosis will be established and sustained, particularly in areas with artemisinin resistance. RDTs are provided in select remote villages. Current treatment guidelines call for antimalarial drugs to be provided free-of-charge at public facilities (30). Mass screening and treatment are applied in epidemic settings only. New treatment guidelines are expected in late 2016 to update dosing requirements of ACT regimens and include new treatment options for treatment failure. The Ministry of Health has banned the manufacture and use of oamts since Malaria focal points are designated at district and commune levels (29). District teams run intercommunal microscopy points, and malaria centres are also available at the provincial level. Commune health stations employ specialized malaria staff and serve as microscopy points. Village health workers (VHWs) work across all primary health-care programmes following basic training and provide community-based care; for malaria patients, they may prepare blood films, make referrals for severe cases, treat with antimalarials, manage cases discharged from higher levels, and assist with spraying and net impregnation (31). Standby treatment is provided for those entering endemic areas where medical services are not easily accessible. The public sector provides most institutional and specialty care in the country, although the private sector delivers over 60% of outpatient care, particularly for young children (32). In the area of malaria, the private sector is relatively insignificant. In 2014, the availability of oamt had reduced to less than 1% of private pharmacies. Despite limited public private partnerships in malaria to date and poor integration of the private sector, Viet Nam has a number of successful partnerships in other areas such as family planning and TB that can potentially serve as a foundation for improved collaboration in malaria (32). PSI is currently using social franchising strategies in other priority public health areas, and has been in discussions with NMCP. Procurement and supply chain management Malaria focal points are designated at district and commune levels (29). District teams run intercommunal microscopy points, and malaria centres are also available at the provincial level. Commune health stations employ specialized malaria staff and serve as microscopy points. Village health workers (VHWs) work across all primary health-care programmes following basic training and provide community-based care; for malaria patients, they may prepare blood films, make referrals for severe cases, treat with antimalarials, manage cases discharged from higher levels, and assist with spraying and net impregnation (31). Standby treatment is provided for those entering endemic 18
29 VIET NAM areas where medical services are not easily accessible. The public sector provides most institutional and specialty care in the country, although the private sector delivers over 60% of outpatient care, particularly for young children (32). In the area of malaria, the private sector is relatively insignificant. In 2014, the availability of oamt had reduced to less than 1% of private pharmacies. Despite limited public private partnerships in malaria to date and poor integration of the private sector, Viet Nam has a number of successful partnerships in other areas such as family planning and TB that can potentially serve as a foundation for improved collaboration in malaria (32). PSI is currently using social franchising strategies in other priority public health areas, and has been in discussions with NMCP. Recommendations The following recommendations are made to strengthen malaria procurement and the supply chain in Viet Nam. A caveat has to be made for this review mission given insufficient time to travel to lower levels of the supply chain system and meetings were only held with NIMPE in Hanoi. Consider conducting an in-depth analysis of lead times given that current times are long. While delays are in part due to the strict and lengthy process for procurement based on Government regulations and procurement law, the full system for procurement and the supply chain should be reviewed to identify how lead times can be reduced most effectively. Challenges may be due to low volumes, which is further split by NIMPE and IMPE requirements. There is also an overall decreasing need given reduction in malaria cases. Explore the feasibility of a consolidated national order for antimalarials as it would be more efficient in theory, but in practice there are other factors such as financial accounting and administration that may hinder the consolidation of needs across NIMPE and the regional IMPEs. It may help to identify clear benefits such as reduced pricing and reduced lead times. 19
30 20
31 CONCLUSION In order to continue to achieve progress towards malaria elimination, countries in the GMS will require high coverage with appropriate interventions for prevention, diagnostics and treatment. As a result, all countries are making significant efforts to expand vectorcontrol interventions such as LLIN distribution, as well as efforts to improve the quality and availability of diagnosis and treatment, including a focus on community-based services and improved integration with the private sector. Supply chains which have become increasingly complex due to the expansion of malaria programmes and an increase in the range and volume of products and stakeholders are a critical component of such efforts to address malaria in the GMS. While all countries have developed supply chains that aim to procure quality-assured commodities and distribute these throughout the health system to the patient level, there are ongoing challenges with workforce capacity, storage conditions, delivery, quality assurance, and the collection and use of timely data. Additionally, the decreasing number of malaria cases across the GMS calls for efforts to sustain funding, resources and engagement, particularly with the emergence of artemisinin resistance, which threatens to undermine recent achievements. Supply chain management of malaria products must be effective in providing the right commodities to the right recipients in the right locations at the right times. All GMS countries can improve their supply chains through effective communication and collaboration among key stakeholders, greater clarity of roles and responsibilities, and formalization of procedures and processes, as well as increased availability of data to provide a seamless link between demand and supply. 21
32 22
33 COUNTRY PROFILES CAMBODIA LAO PEOPLE S DEMOCRATIC REPUBLIC MYANMAR THAILAND VIET NAM 23
34 MALARIA SUPPLY CHAIN SYSTEMS CAMBODIA Population GDP per capita Total health expenditure per capita Government expenditure on health per capita US$ 1159 US$ 61 US$ 14 Source: World Bank Databank (January 2017) and Global Health Expenditure Database (January 2017) MALARIA DISTRIBUTION IN CAMBODIA MALARIA BURDEN (2015) (1) Population at high risk of malaria Malaria deaths 10 Confirmed malaria cases Proportion of falciparum vs vivax cases The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement. Source: Cambodia country profile, World Malaria Report % 39% FINANCIAL CONTRIBUTIONS (2015) (1) MARKET SHARE (2014) (2) Government PMII/USAID Global Fund US$ US$ WHO US$ US$ Relative market volume of antimalarials Public 40% Private 60% NATIONAL DOCUMENTS PREVENTION, DIAGNOSIS AND TREATMENT RDTS Brand names SD Bioline G6DP RDT National strategic plan National treatment guidelines REGISTERED MEDICINES The Malaria Elimination Action Framework (January 2016) National Treatment Guidelines for Malaria (December 2014) First-line treatment of P.falciparum AS-MQ; DHA- PPQ+PQ Treatment failure of P.falciparum QN+T Treatment of severe malaria AM; AS; QN Treatment of P.vivax DHA-PPQ+PQ Types of RDT P.f. + P.v. specific (combo) Registered WHO prequalified or stringent regulatory authority (SRA) approved medicines Antimalarial Dose Brand name Dihydroartemisinin + Piperaquine - FDC 40mg + 320mg Blister-3 Dihydroartemisinin + Piperaquine - FDC 40mg + 320mg Blister-6 Dihydroartemisinin + Piperaquine - FDC 40mg + 320mg Blister-9 Primaquine 15mg - Bottle-100 Artesunate Injection 60mg - Vial-5 + dilutent Artesunate + Mefloquine FDC 100mg + 200mg Blister-3 ORAL ARTEMISININ-BASED MONOTHERAPIES Year of ban (1) Availability in market (2) 2008 No 24 Prevention efforts (3) RDT policy (1) Treatment guidelines (4) Types of treatment facilities (4) Community case management (5) Public vs. private sector (3) Public private partnerships (3) The national strategy calls for one long-lasting insecticidal net (LLIN) per person and one longlasting insecticidal hammock net (LLIHN) per family provided to those living in villages at risk, as well as the retreatment of existing nets with long-lasting insecticide. Indoor residual spraying (IRS) is used in targeted low-endemic areas, and may also be deployed in response to outbreaks in high-endemic areas. Malaria diagnosis is free-of-charge in the public sector. While microscopy is the preferred diagnostic tool, RDTs are used when microscopy is unavailable, particularly at the community level among village malaria workers (VMWs). Due to growing artemisinin resistance, the Ministry of Health adopted new treatment guidelines in 2015 which identify different first-line regimens by province. The sale of oral artemisinin-based monotherapies (oamts) has been banned since There are 2350 villages covered by VMWs who provide community-based malaria diagnosis and treatment. VMWs report to health centres which in turn report to one of 79 operational district (OD) health offices, which each manage a referral hospital that provides microscopy services. VMWs distribute LLINs, use rapid diagnostic tests (RDTs) and provide malaria treatment, and refer severe malaria cases to health facilities. The current target is to place at least one VMW in every village including cross-border sites considered at risk for transmission or importation of multidrug resistance. While the public health sector provides free access to malaria diagnosis and treatment, a substantial share of health-care services is provided through the private sector, which includes registered health outlets, non-registered health outlets, and nonhealth outlets. Under the Public-Private Mix (PPM) programme, private providers are trained on early diagnosis and treatment according to national guidelines, provided with artemisinin-based combination therapies (ACTs) and RDTs, and incorporated into the national malaria surveillance system.
35 PROCUREMENT AND SUPPLY CHAIN MANAGEMENT Product selection (3) Products are selected by National Center for Parasitology, Entomology and Malaria Control s (CNM) Technical Bureau and Pharmacy Unit in line with WHO and Cambodia treatment guidelines. Going forward, RDTs and antimalarials will be selected from among WHO-prequalified suppliers. LLINs and LLIHNs will be procured from suppliers approved by the Global Fund to Fight AIDS, Tuberculosis and Malaria. Procurement (6) Quality assurance and control The United Nations Office for Project Services (UNOPS) is the primary source for procurement and manages the entire procurement process for the Global Fund for antimalarials, RDTs, LLINs, and other goods and services. The procurement cycle is based on the length of Global Fund grants. CNM provides the needs for commodities at the beginning of each year. The Department of Drugs and Food (DDF), the National Health Products Quality Control Center (NHQC), and CNM are involved in quality assurance and control (QA/QC). UNOPS carries out QC testing of private procured pharmaceuticals in collaboration with DDF. Forecasting and quantification (6) Logistics management information system (7) Customs clearance While various partners across Cambodia have developed quantifications of malaria commodity needs, the Ministry of Health prepared a two-year commodity forecast in 2015 with support from JSI/USAID, using reported malaria cases, as well as stock data from the public and private sectors. To strengthen procurement and supply management, CNM aims to develop annual forecasts of all malaria commodities and submit procurement plans to the procurement partner. The logistics management database, which is maintained by DDF and the Ministry of Health-operated Central Medical Store (CMS), contains data on stocks of medical supplies. At the health centre level, staff use paper forms while at higher levels, data are entered into drug databases. UNOPS and the particular manufacturer are in charge of the customs clearance. All products are then stored at CMS warehouse facilities in Phnom Penh. Central warehousing facilities (3) Public sector procured commodities are received and stored at CMS. Commodities are then distributed and stored at provincial and district levels. Distribution system (7) Commodities flow from CMS to the provincial health departments (PHDs) and OD health offices, then to health centres or referral hospitals and finally to VMWs. CMS is responsible for distributing essential medicines and commodities to ODs on a quarterly basis. Distribution is based on a pull system. PUBLIC SECTOR MALARIA SUPPLY AND DISTRIBUTION SYSTEM The Global Fund PSK (Private procurement Private sector PARTNER AND DONOR MAPPING Funding source Procurement HosDID Product flow Information flow E-medicine use report UNOPS (Public procurement National hospitals (7) + Kunteak Bopha HosDID Provincial Referral Hospitals OD Referral Hospitals GFATM UNOPS, CNM National Malaria Program (CNM) Central Medical Stores (CMS) Phnom Penh Provincial Health Departments (25) Operational Districts (92) Health Centres PMI/USAID PMI Ministry of Health Department of Drugs and Food (DDF) NatDID ProDID OD DID Health Post BMGF PSI REFERENCES 1. World malaria report Geneva: World Health Organization; ACTwatch Group, Population Services Khmer. ACTwatch study reference document: Cambodia outlet survey Washington (DC): Population Services International; Cambodia malaria elimination action framework Cambodia: Ministry of Health; President s Malaria Initiative: Greater Mekong Sub-region malaria operational plan FY2016: Washington (DC): United States Agency for International Development; 2015 ( fy16/fy-2016-greater-mekong-subregionmalaria-operational-plan.pdf?sfvrsn=4, accessed 10 January 2017). 5. Malaria control in Cambodia: building a community-based response. Washington (DC): United States Agency for International Development (USAID); Akhlaghi L, Egharevba M. Technical Report: Cambodia: malaria commodities quantification Arlington (VA): USAID Deliver Project Task Orders 7; ( JSIInternet/Inc/Common/_download_pub. cfm?id=16865&lid=3, accessed 10 January 2017 ). 7. Chuor C. Benefits to national malaria programs from regional support: the Cambodia case. Cambodia: National Center for Parasitology, Entomology and Malaria Control; 2014 ABBREVIATIONS Implementing agency Storage and distribution UNOPS, CNM, NGOs CMS, NGOs PMI PSI PSI AM= artemether AS-MQ = artesunate mefloquine DHA-PPQ=dihydroartemisinin-piperaquine PQ=primaquine; P.f.= plasmodium falciparum; P.v.= plasmodium vivax QN+T= quinine + tetracycline BMGF = Bill and Melinda Gates Foundation; CMS = Central Malaria Store; CNM = National Center for Malariology, Parasitology, and Entomology; GFATM = Global Fund to Fight AIDS, Tuberculosis and Malaria; NGOs = non-governmental organizations; PMI = US President s Malaria Initiative ; PSI = Population Services International; UNOPS = United Nations Office for Project Services; USAID = US Agency for International Development 25
36 MALARIA SUPPLY CHAIN SYSTEMS LAO PEOPLE S DEMOCRATIC REPUBLIC Population GDP per capita Total health expenditure per capita Government expenditure on health per capita US$ 1818 US$ 33 US$ 16 Source: World Bank Databank (January 2017) and Global Health Expenditure Database (January 2017) MALARIA DISTRIBUTION IN LAO PEOPLE'S DEMOCRATIC REPUBLIC MALARIA BURDEN (2015) (1) Population at high risk of malaria Malaria deaths 4 Confirmed malaria cases Proportion of falciparum vs vivax cases 42% 58% The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement. Source: Lao People's Democratic Republic country profile, World Malaria Report 2015 FINANCIAL CONTRIBUTIONS (2015) (1) MARKET SHARE (2015) (2) Government PMII/USAID Global Fund US$ US$ WHO US$ US$ Relative market volume of antimalarials Public 65% Private 35% NATIONAL DOCUMENTS PREVENTION, DIAGNOSIS AND TREATMENT National strategic plan National treatment guidelines National Strategy Plan Malaria Treatment Guidelines for Provincial and District Hospitals (October 2011) Prevention efforts (3) Vector control is mainly based on insecticide-treated bed nets (ITNs), although long-lasting insecticidal nets (LLINs) have been introduced and will gradually replace all ITNs. Indoor residual spraying (IRS) is mostly limited to outbreak response and focal control. REGISTERED MEDICINES First-line treatment of P.falciparum AL Treatment failure of P.falciparum QN+D Treatment of severe malaria AS+AL Treatment of P.vivax AL+PQ Types of RDT P.f. + P.v. specific (combo) Registered WHO prequalified or stringent regulatory authority (SRA) approved medicines There is only one antimalarial (AML) manufactured and registered in the Lao People s Democratic Republic (chloroquine phosphate 250mg). Other AMLs procured by malaria programme are not registered but imported through waivers. RDT policy (4) Treatment guidelines (5) Types of treatment facilities (4) Rapid diagnostic tests (RDTs) are available at village and health centre levels, as well as at district and provincial hospitals, although emphasis is placed on microscopic diagnosis at hospital facilities. National malaria treatment guidelines were significantly modified in 2005 after reports of increasing drug resistance, resulting in current guidelines specifying the use of artemisinin-based combination therapies (ACTs). There has been a ban on the sale of oral artemisinin-based monotherapies (oamts) since Malaria activities are centralized at Center for Malaria, Parasitology and Entomology (CMPE), which oversees 17 Provincial Anti-Malaria Stations (PAMS). Under the PAMS, there are 140 district and provincial hospitals, and approximately 850 health centres that cover nearly 2000 malaria-endemic villages. RDTS ORAL ARTEMISININ-BASED MONOTHERAPIES Community case management (6) A national village-based early diagnosis and treatment (EDAT) strategy promotes the diagnosis and treatment at village and health-centre levels. Village malaria workers (VMWs) provide care for patients within the community and use RDTs, prescribe ACTs and refer patients to health facilities if necessary. Brand names No available information Year of ban (1) Availability in market (2) 2005 No Public vs private sector (4) The public health system is predominant, although an alternative private sector is growing. There are around 1865 private pharmacies and 254 private clinics, mainly located in urban areas. Public private partnerships (4) A public private mix strategy was piloted in 2008, targeting eight districts and providing commodities to private pharmacies and clinics. This approach has now been expanded to 22 districts in eight provinces. 26
37 PROCUREMENT AND SUPPLY CHAIN MANAGEMENT Product selection (7) CMPE conducts product selection in line with its national treatment guidelines. Procurement (4) Generally, the Procurement Unit within the Project Management Unit established by the Ministry of Health to act as the implementing body for programmes supported by the Global Fund to Fight AIDS, Tuberculosis and Malaria procures commodities. Quality assurance and control (6) There is no formalized system for postshipment quality assurance, and there is no quality control laboratory that is WHO prequalified. Before issuing products, staff at CMPE conduct a visual inspection and if damaged or expired products are found, these are sent to the Food and Drug Department (FDD). Forecasting and quantification (6) CMPE is responsible for carrying out quantification, demand forecasting and supply planning of commodities. National procurement projections are based on historical needs and some consumption estimates, which do not take into account the effect of stock-outs. Logistics management information system (6, 7) CMPE receives paper-based monthly summary reports from PAMS, as well as stock on hand data every other week via an electronic web form. The Medical Products Supply Center (MPSC) is piloting the software msupply in select warehouses. Customs clearance FDD and the particular manufacturer are in charge of the customs clearance. All products are then stored at CMS warehouse facilities in Vientiane. Central warehousing facilities (4) All commodities including drugs and RDTs are stored with MPSC within the Ministry of Health. Although the Global Fund has provided support to renovate part of the MPSC building, there continue to be significant quantities of expired commodities that require disposal. Distribution system (4, 6) PAMs and the Provincial Food and Drug Division supply districts and health centres with malaria commodities, which in turn, provide supplies to VMWs. Arrangements for the transport of commodities within the supply chain have not been formalized, resulting in an ad hoc arrangement using public transportation networks. PUBLIC SECTOR MALARIA SUPPLY AND DISTRIBUTION SYSTEM CHs CMPE MOH Procurement Unit Suppliers PARTNER AND DONOR MAPPING Funding source PHOs DHOs Flow of information Flow of commodities PMI/USAID MPSC PAMS DAMPS CH = central hospital; CMPE = Center for Malaria, Parasitology and Entomology; DAM = District Anti-malaria Station; DH = district hospital; DHO = District Health Office; HC = health centre; MOH = Ministry of Health; MPSC = Medical Products Supply Center; PAM = Provincial Anti-malaria Station; PH = provincial hospital; PHO = Provincial Health Office; PPM = Public-Private Mix program; VHV = village health volunteer HCs VHVs GFATM PHs DHs FPM REFERENCES 1. World malaria report Geneva: World Health Organization; ACTwatch Group, Lao PDR Population Services International. ACTwatch study reference document: Lao PDR outlet survey Washington (DC): Population Services International; National malaria strategy for malaria control and pre-elimination Lao People s Democratic Republic: Ministry of Health; President s Malaria Initiative: Greater Mekong Sub-region malaria operational plan FY2016: Washington (DC): United States Agency for International Development; 2015 ( fy16/fy-2016-greater-mekong-subregionmalaria-operational-plan.pdf?sfvrsn=4, accessed 10 January 2017 ). 5. Malaria treatment guidelines for provincial and district hospitals. Lao People s Democratic Republic: Center for Malariology, Parasitology and Entomology; Lao PDR malaria programme review. Hawaii: Vectorborne Diseases Associates LLC; Logistics management information system (LMIS) for malaria commodities: standard operating procedures. Lao People s Democratic Republic: Center for Malaria, Parasitology and Entomology; ABBREVIATIONS Procurement Implementing agency PMI MOH CMPE PSI AL= artemether-lumefantrine AS= artesunate DHA-PPQ=dihydroartemisinin-piperaquine PQ=primaquine P.f.= plasmodium falciparum P.v.= plasmodium vivax QN+D= quinine + doxycycline Storage and distribution MPSC PSI CMPE = Center for Malaria, Parasitology and Entomology; GFATM = Global Fund to Fight AIDS, Tuberculosis and Malaria; MPSC = Medical Products Supply Center; PMI = US President s Malaria Initiative ; PSI = Population Services International 27
38 MALARIA SUPPLY CHAIN SYSTEMS MYANMAR Population GDP per capita Total health expenditure per capita Government expenditure on health per capita US$ 1161 US$ 20 US$ 9 Source: World Bank Databank (January 2017) and Global Health Expenditure Database (January 2017) MALARIA DISTRIBUTION IN MYANMAR MALARIA BURDEN (2015) (1) Population at high risk of malaria Malaria deaths 92 Confirmed malaria cases Proportion of falciparum vs vivax cases 28% The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement. Source: Myanmar country profile, World Malaria Report % FINANCIAL CONTRIBUTIONS (2015) (1) MARKET SHARE (2012) (2) Government PMII/USAID Global Fund US$ US$ WHO US$ US$ Relative market volume of antimalarials Public 35% Private 65% NATIONAL DOCUMENTS PREVENTION, DIAGNOSIS AND TREATMENT RDTS Brand names No available information National strategic plan National treatment guidelines REGISTERED MEDICINES ORAL ARTEMISININ-BASED MONOTHERAPIES Year of ban (1) National Strategic Plan for Malaria Control in Myanmar, (2016) Guidelines for Malaria Diagnosis and Treatment in Myanmar (2015) First-line treatment of P.falciparum AL; AM; S+MQ; DHA-PPQ +PQ Treatment failure of P.falciparum AS-MQ; DHA- PPQ +PQ (within 28 days) or AL+PQ (after 28 days) Treatment of severe malaria AM; AS; QN Treatment of P.vivax CQ+PQ(14D) Types of RDT P.f. + all species (Combo) Registered WHO prequalified or stringent regulatory authority (SRA) approved medicines No available information 2012 Yes Availability in market 28 Prevention efforts (2) RDT policy (3) Treatment guidelines (4) Types of treatment facilities (3) Community case management (3) Public vs private sector (4) Public private partnerships (4) Rapid scale-up of long-lasting insecticidal nets (LLINs) is planned in malaria-risk areas, with a target of 100% coverage for the population living in artemisininresistance containment areas. Indoor residual spraying (IRS) is focused on outbreak areas, as well as development projects in endemic areas and new settlements, and artemisinin-resistance affected areas. The national policy calls for confirmatory testing with either microscopy or rapid diagnostic test (RDT) before treatment is prescribed. In hospitals and higher-level facilities, microscopy is the preferred diagnostic method. RDTs are being scaled up in lower-level facilities and at the community level through village health volunteers (VHVs). The national treatment policy is based on data on parasite resistance to antimalarial drugs, and updated in line with international standards that place RDTs and artemisinin-based combination therapies (ACTs) as the main pillars of diagnosis and treatment. Oral artemisininbased monotherapy (oamt) use has been banned, although oversight of the private sector is limited. Public health services, including malaria treatment, are predominantly delivered to communities through the township health department. Each department manages a township hospital, a station hospital, approximately five rural health centres, and approximately five subrural health centres per station hospital and rural health centre. VHVs complement health staff in the delivery of services for malaria prevention and control in highand moderate-risk villages and in areas with a high concentration of migrant workers where access to health facilities is difficult. Although diagnosis and treatment of malaria is free in the public sector, approximately 60 70% of suspected malaria patients receive treatment from the private sector. Hospitals and clinics in the formal private sector, which is regulated by the Ministry of Health, are mainly located in urban areas. Population Services International (PSI) supports a social franchise of licensed private general practitioners (GPs) and is also implementing the Artemisinin Monotherapy Replacement (AMTR) Project which provides qualityassured ACTs to the private sector. The Myanmar Medical Association (MMA) also supports a network of private clinics.
39 PROCUREMENT AND SUPPLY CHAIN MANAGEMENT Product selection (3, 5) Procurement (4, 6) The National Strategic Plan states that only WHO-prequalified RDTs and ACTs, recommended as per the national treatment policy, should be procured. The United Nations Office for Project Services (UNOPS) is the primary source of procurement under the Global Fund, and malaria commodities are received by subrecipients (including the National Malaria Control Programme NMCP). For donors other than UNOPS, like USAID/PMI the procurement is done by other organizations (Chemonics). Quality assurance and control (4, 7) Forecasting and quantification (3) Logistics management information system (5) Customs clearance (5) The Department of Food and Drug Administration (FDA) is responsible for quality assurance and control (QA/QC) of antimalarial products. To prevent the sale of artemisinin monotherapy, the Regional Artemisinin-resistance Initiative (RAI) grant is funding support to strengthen FDA s capacity to conduct drug-quality monitoring. NMCP is responsible for forecasting commodities based on epidemiological data and past consumption. Annual forecasting workshops are led by UNOPS with input from NMCP, WHO and other Global Fund subrecipients (including nongovernmental organizations). A logistics management information system (LMIS) has been developed, although only a portion of the facilities conducting procurement have some basic procedures in place to capture data. Stock recording remains largely paperbased. An electronic LMIS based on the software msupply is also being piloted. Government- and donor-procured products are primarily cleared by the Central Medical Store Depot (CMSD) Customs Clearance Department. Three levels of ministry approval (Ministry of Commerce, Ministry of Finance and Revenue, and the Ministry of Health) are required to obtain a letter of exemption, which can take a month. Central warehousing facilities (5) The first point of warehousing can be in one of several locations including CMSD, Myanmar Pharmaceutical Factory (MPF), suppliers, vendor warehouses, VBDC central warehouse and nongovernmental organization central warehouses. Distribution system (4,5) Health commodities are distributed through VBDC and through CMSD. A pull system is in place in which staff from lower-level facilities travel to retrieve commodities from higher levels of the health system. Transportation consists of a largely informal network of independent operators. PUBLIC SECTOR MALARIA SUPPLY AND DISTRIBUTION SYSTEM Current major supply chains in the Myanmar health system (8) Programme TB Malaria HIV General RH/Nutrition/ Medicine CH RH EPI Store/ Central Warehouse NTBP store State/ Region/ Division Township/ Hospital/ District RHIC/ Station Hospital Funding source Procurement Lower Store Upper Store Gates Fdtn VBDC DFID HIV Central Warehouse CMSD Transit Camp Subcentre DOTS Subcentre Provider CMSD = Central Medical Stores Depot; DOTS = TB Directly Observed Treatment, Short Course; NTBP = National TB program store; VBDC = Vector-Borne Disease Control Programme PARTNER AND DONOR MAPPING 3MDG (multiple donors) PMI/ USAID CMSD Sub- Depot State/Division/Regional Store Township RHIC, Urban Clinic, Station Hospital Global JICA Fund DANIDA MOH USAID DELIVER PSI 3MDG JICA UNOPS SAVE-PR PROJECT Consumables Equipment SAVE COLD EPI Central Cold Store State Cold Store S/R Leprosy Leprosy Center MOH MSF REFERENCES 1. World malaria report Geneva: World Health Organization; Outlet survey: baseline study - The Republic of the Union of Myanmar 2012 survey report. PSI/Myanmar; National strategic plan for malaria prevention and control Myanmar: Ministry of Health; President s Malaria Initiative: Greater Mekong Sub-region malaria operational plan FY2016: Washington (DC): United States Agency for International Development; 2015 ( fy16/fy-2016-greater-mekong-subregionmalaria-operational-plan.pdf?sfvrsn=4, accessed 10 January 2017 ). 5. Tolliver J, Bartram K. Burma national supply chain: baseline results. Arlington (VA): Partnership for Supply Chain Management; Global fund grants to Myanmar (audit report). Geneva: Office of the Inspector General, The Global Fund to Fight AIDS, Tuberculosis and Malaria; Support to quality assurance of malaria drugs in Myanmar [website]. Regional Artemisininresistance Initiative (RAI). ( raifund.org/en/news/support-qualityassurance-malaria-drugs-myanmar, accessed 3 November 2016). 8. Hike MMT. Reducing fragmentation in supply chain management [presentation]. Myanmar: Ministry of Health; 2016). Implementing agency PSI NGOs CAP- Malaria NMCP/UNOPS/ NGOs SAVE-PR (Int l NGOs) NMCP MSF ABBREVIATIONS Storage and distribution PSI NGOs CAP CMSD CAP- SAVE NMCP Central Warehouse Stores NGOs SAVE NGOs S/R, Township Private Providers Health Facilities/VMWs Private Facilities Patients/End-users/Beneficiaries 3MDG = Three Millennium Development Goal Fund; CAP = Control and Prevention of Malaria Project; CMSD = Central Medical Store Depot; DANIDA = Danish International Development Agency; DFID = Department for International Development; JICA = Japan International Cooperation Agency; MOH = Ministry of Health; MSF = Médecins Sans Frontières; NMCP = National Malaria Control Programme; PMI = US President s Malaria Initiative; PSI = Population Services International; UNOPS = United Nations Office for Projects and Services; USAID = United States Agency for International Development 29 MSF AL= artemether-lumefantrine AM= artemether AS+MQ= artesunate+mefloquine AS= artesunate CQ= chloroquine DHA-PPQ=dihydroartemisinin-piperaquine PQ=primaquine P.f.= plasmodium falciparum P.v.= plasmodium vivax QN= quinine
40 MALARIA SUPPLY CHAIN SYSTEMS THAILAND Population GDP per capita Total health expenditure per capita Government expenditure on health per capita US$ 5815 US$ 228 US$ 177 Source: World Bank Databank (January 2017) and Global Health Expenditure Database (January 2017) MALARIA DISTRIBUTION IN THAILAND MALARIA BURDEN (2015) (1) Population at high risk of malaria Malaria deaths 33 Confirmed malaria cases Proportion of falciparum vs vivax cases 19% The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement. Source: Thailand country profile, World Malaria Report % FINANCIAL CONTRIBUTIONS (2015) (1) Government PMII/USAID Global Fund US$ US$ US$ WHO None MARKET SHARE Relative market volume of antimalarials Public Majority Antimalarial drugs are not permitted to be sold in pharmacies or private clinics. Private Minimal NATIONAL DOCUMENTS RDTS Brand names No available information National strategic plan National treatment guidelines REGISTERED MEDICINES ORAL ARTEMISININ-BASED MONOTHERAPIES Year of ban (1) National Strategic Plan for Malaria Control in Myanmar, (2016) National Malaria Treatment Guidelines (April 2015) First-line treatment of P.falciparum DHA+PPQ Treatment failure of P.falciparum QN+D Treatment of severe malaria QN+D Treatment of P.vivax CQ+PQ(14D) Types of RDT P.f. + all species (Combo) Registered WHO prequalified or stringent regulatory authority (SRA) approved medicines No available information 1995 No Availability in market PREVENTION, DIAGNOSIS AND TREATMENT Prevention efforts (2) RDT policy (2) Treatment guidelines (3) Types of treatment facilities (3) Community case management (4) Public vs private sector (2) Public private partnerships (4, 5) Bed nets are distributed during routine mass campaigns with a target of one long-lasting insecticidal net (LLIN) per person in malaria-endemic villages. Long-lasting insecticidal hammock nets (LLIHNs) are distributed in specific locations where LLINs cannot be used, for instance among migrants in forest areas. Indoor residual spraying (IRS) is used for vector control in endemic and receptive areas. Malaria diagnosis is free-of-charge in the public sector, and diagnostic services are predominantly provided by a network of malaria clinics (MCs) and malaria posts (MPs) in endemic areas. MCs are equipped with light microscopes and trained microscopists, while MPs, which are staffed by village malaria workers (VMWs), utilize RDTs. Malaria treatment guidelines were updated in 2015 with a change in first-line treatment of Plasmodium falciparum to dihydroartemisinin-piperaquine (DHA- PPQ). The sale of oral artemisinin-based monotherapies (oamts) has been banned since Treatment for uncomplicated malaria can be provided at MCs, MPs and hospitals, as well as nongovernmental organization malaria units. Current national efforts aim to integrate malaria services into local primary health services. Management of severe malaria should take place at sufficiently equipped hospitals. Community-based MCs and MPs have been introduced to serve malaria endemic areas and populations along international borders and remote areas, and have effectively increased access to malaria early diagnosis and treatment. Most treatment is available in the public sector, although private hospitals offer care for malaria patients. Antimalarial drugs are not permitted to be sold in pharmacies or private clinics. Government efforts aim to better integrate the private sector by organizing a cooperative network of public and private sector organizations to coordinate national malaria control activities. Partnerships are also being established with private providers and companies operating in malaria endemic areas. 30
41 PROCUREMENT AND SUPPLY CHAIN MANAGEMENT Product selection Procurement Responsible agency BVBD Malaria commodities are selected based on whether they are recommended by the WHO and FIND-prequalified (for RDTs). Responsible agency BVBD The Bureau of Vector-Borne Disease (BVBD) procures the newly selected first-line artemisinin-based combination therapy (ACT) and also procures RDTs under the Global Fund. Regional staff locally procure insecticide using standard government procurement policy, with approval from BVBD. LLINs are procured through GFATM. Procurement occurs on an annual basis. Quality assurance and control Forecasting and quantification Logistics management information system Customs clearance Central warehousing facilities Responsible agency BVBD/BDN Quality assurance and control (QA/QC) is led by BVBD. Once a year, random sampling of antimalarials is conducted in the field, although this is not formalized through guidelines. The Bureau of Drug and Narcotics (BDN), under the Department of Medical Sciences, conducts testing on behalf of BVBD. Responsible agency BVBD BVBD conducts forecasting annually, and uses a consumption-based method for malaria drugs, which incorporates a 20% buffer. The Global Fund Principal Recipient, the Ministry of Public Health, is responsible for RDT forecasting, which is also based on the previous year s consumption, with a 20% buffer. Responsible agency MOPH There is currently no system that reports regularly on commodities, although once a year, data on stock status, consumption and need are consolidated to decide on procurement levels for the following year. Excel spreadsheet files are used to track stock status of RDTs every quarter. Responsible agency MOPH Companies manage customs clearance themselves based on the hazardous substances trade procedures. Responsible agency BVBD BVBD is responsible for storing commodities at its central warehouse. Regional offices also have warehouses, but in some cases, private rental of warehouses may be necessary for temporary storage if there is a lack of space at the regional level. Distribution system (2) Responsible agency NMCP The National Malaria Control Program (NMCP) within BVBD manages the delivery of commodities. Medicines are delivered to the Vector-Borne Disease Centers (VBDCs) twice a year, and distribution to the Vector-Borne Disease Units (VBDUs) and to MCs occurs monthly at scheduled meetings where medicines are collected. PUBLIC SECTOR MALARIA SUPPLY AND DISTRIBUTION SYSTEM Suppliers Flow of information Flow of commodities PHOs BVBD ODPCs VBDCs VBDUs BVBD = Bureau of Vector-Borne Disease; NMPC = National Malaria Control Program (within BVBD); ODPC = Offices of Disease Prevention and Control (regional level); PHO = Provincial Health Office; MC = malaria clinic; MP = malaria post; VBDC = Vector-Borne Disease Center (provincial level); VBDU = Vector-Borne Disease Unit (district level) MPs MCs REFERENCES 1. World malaria report Geneva: World Health Organization; President s Malaria Initiative: Greater Mekong Sub-region malaria operational plan FY2016: Washington (DC): United States Agency for International Development; 2015 ( fy16/fy-2016-greater-mekong-subregionmalaria-operational-plan.pdf?sfvrsn=4, accessed 10 January 2017 ). 3. Malaria diagnosis and case management guidelines. Thailand 2015: Thailand Department of Disease Control Ministry of Health; National Strategic Plan for Malaria Control and Elimination in Thailand Bureau of vector-bone disease control. Department of disease control; Building on shared value to develop public private partnerships for malaria control. Maryland: University Research Co. LLC; ABBREVIATIONS PARTNER AND DONOR MAPPING Funding source GFATM PMI/USAID CQ= chloroquine DHA-PPQ=dihydroartemisinin-piperaquine PQ=primaquine P.f.= plasmodium falciparum P.v.= plasmodium vivax QN+D= quinine + doxycycline Procurement BVBD/MOH PR CAP-Malaria Implementing agency BVBD Storage and distribution BVBD GFATM = Global Fund to Fight AIDS, Tuberculosis and Malaria; MOH = Ministry of Health 31
42 MALARIA SUPPLY CHAIN SYSTEMS VIET NAM Population GDP per capita Total health expenditure per capita Government expenditure on health per capita US$ 2111 US$ 142 US$ 77 Source: World Bank Databank (January 2017) and Global Health Expenditure Database (January 2017) MALARIA DISTRIBUTION IN VIET NAM MALARIA BURDEN (2015) (1) Population at high risk of malaria Malaria deaths 3 Confirmed malaria cases Proportion of falciparum vs vivax cases 49% 51% The boundaries and names shown and the designations used on this map do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted and dashed lines on maps represent approximate border lines for which there may not yet be full agreement. Source: Viet Nam country profile, World Malaria Report 2015 FINANCIAL CONTRIBUTIONS (2015) (1) Government PMII/USAID Global Fund US$ US$ WHO None US$ MARKET SHARE Relative market volume of antimalarials Public No data available Private NATIONAL DOCUMENTS PREVENTION, DIAGNOSIS AND TREATMENT RDTS Brand names SD Bioline Malaria Ag P.f./P.v. National strategic plan National treatment guidelines REGISTERED MEDICINES ORAL ARTEMISININ-BASED MONOTHERAPIES Year of ban (1) National Strategy for Malaria Control and Elimination in the Period and Orientation to 2030 (November 2011) Guideline for Malaria Diagnosis, Treatment and Prevention in Viet Nam (updated August 2013, to be updated in 2016) First-line treatment of unconfirmed malaria DHA-PPQ First-line treatment of P.falciparum DHA-PPQ Treatment failure of P.falciparum QN+CL; QN+D Treatment of severe malaria AS; QN Treatment of P.vivax CQ+PQ (14D) Types of RDT P.f. + P.v. specific (Combo) Registered WHO prequalified or stringent regulatory authority (SRA) approved medicines No available information 2013 No Availability in market 32 Prevention efforts (1, 2) RDT policy (3) Treatment guidelines (4) Types of treatment facilities (4) Community case management (5) Public vs private sector (6) Public private partnerships (6) Insecticide treated bed nets (ITNs) are distributed free-of-charge to all age groups in moderateand high- endemic areas, and during suspected outbreaks; long-lasting insecticidal nets (LLINs) are also increasingly used, particularly in hard-to-reach areas. Indoor residual spraying (IRS) is targeted at the population residing in hyper-endemic areas where ITN use is low, particularly border areas. Viet Nam favours microscopy over rapid diagnostic tests (RDTs), with plans to diagnose 95% of malaria cases in endemic areas using slide microscopy by Communal microscopy centres for early diagnosis will be established and sustained, particularly in areas with artemisinin resistance. RDTs are provided in select remote villages. New treatment guidelines are expected in late 2016 to update dosing requirements and include new options for treatment failure. A ban on oral artemisinin-based monotherapy (oamt) has been enforced since Malaria focal points are designated at district and commune levels. District teams run intercommunal microscopy points, and malaria centres are also available at the provincial level. Commune health stations employ specialized malaria staff and serve as microscopy points. Village health workers (VHWs) work across all primary health-care programmes and provide communitybased care; for malaria patients, they may prepare blood films, make referrals for severe cases, treat with antimalarials, manage cases discharged from facilities, and assist with spraying and net impregnation. The public sector provides most institutional and specialty care in the country, and in the area of malaria the private sector is relatively insignificant. Despite limited public private partnerships in malaria to date and poor integration of the private sector, Viet Nam has a number of successful partnerships in other areas such as family planning and TB that can potentially serve as a foundation for improved collaboration in malaria.
43 PROCUREMENT AND SUPPLY CHAIN MANAGEMENT Product selection The National Malaria Control Programme (NMCP) oversees product selection, and its policy development body is comprised of multiple stakeholders. Products are listed in the national treatment guidelines. Procurement NMCP is responsible for the tender annually. Current lead times are long, taking approximately six months from submission of forecast to receiving goods in Viet Nam at the National Institute of Malariology, Parasitology and Entomology s (NIMPE) warehouses. Quality assurance and control NIMPE is responsible for random sampling of drugs, and there are 17 mini-laboratories for quality assurance (QA) testing. Suspected samples are sent to the National Institute for Drug Quality Control. Forecasting and quantification NMCP leads annual forecasting, with support from NIMPE for technical inputs, and requests from provinces. Consumption data, epidemiological factors, and requests from provinces are used to develop forecasts that are consolidated nationally by NMCP. Logistics management information system Stock reports are part of the routine reporting system as a quarterly reporting form. While an electronic malaria information system is in use from district level and above, reporting and usage rates are low. Hard copy data are thus still in use, and are compiled in an Excel spreadsheet at the central level overseen by NIMPE. Customs clearance Antimalarials are procured locally and shipped to NIMPE s warehouses. The Global Fund Project Management Unit conducts custom clearance for internationally procured RDTs. Central warehousing facilities NIMPE is responsible for receipt and storage of procured goods. Two warehouses are available in central NIMPE for drugs, equipment and insecticides. Each regional Institute of Malariology, Parasitology and Entomology (IMPE) also has its own warehouse. Distribution system The system of distribution is a needs-based pull system. Once a year, provincial staff visit their respective NIMPE/ IMPE and collect their commodities for the year. District-level distribution takes place at meetings and involves the hand carry of commodities. PUBLIC SECTOR MALARIA SUPPLY AND DISTRIBUTION SYSTEM Flow of information Flow of commodities NIMPE = National Institute of Malariology, Parasitology and Entomology; NMCP = National Malaria Control Program; IMPE = regional Institute of Malariology, Parasitology and Entomology; VHWs = Village Health Workers PARTNER AND DONOR MAPPING Funding source GOVT. NPMC NIMPE IMPEs Provinces Districts Communes GFATM Suppliers REFERENCES 1. World malaria report Geneva: World Health Organization; President s Malaria Initiative: Greater Mekong Sub-region malaria operational plan FY2016: Washington (DC): United States Agency for International Development; 2015 ( fy16/fy-2016-greater-mekong-subregionmalaria-operational-plan.pdf?sfvrsn=4, accessed 10 January 2017). 3. Strategy and intervention matrix. Vietnam: Asia Pacific Malaria Elimination Network (APMEN); 2014 ( com/static/f/471029/ / / APMEN_Matrix_Vietnam_ 2013.pdf?token=SJrCcFuxGdDLD2ra3oSEJQ ockts%3d, accessed 10 January 2017). 4. Malaria fact sheet. In: Health topics [website]. Geneva: World Health Organization; 2014 ( malaria/factsheet/en/, accessed 7 November 2016). 5. Morrow M, Nguyen QA, Caruana S, Biggs BA, Doan NH, Nong TT. Pathways to malaria persistence in remote central Vietnam: a mixed-method study of health care and the community. BMC Public Health; Mar 23;9:85. doi: / The private sector s role in malaria surveillance. California: UCSF Global Health Sciences; Procurement Implementing agency Storage and distribution MOH/NMPC NIMPE NIMPE ABBREVIATIONS AS= artesunate CQ= chloroquine DHA-PPQ=dihydroartemisinin-piperaquine PQ=primaquine P.f.= plasmodium falciparum P.v.= plasmodium vivax QN+CL= quinine+ clindamycin QN+D= quinine + doxycycline GFATM = Global Fund to Fight AIDS, Tuberculosis and Malaria; MOH = Ministry of Health 33
44 REFERENCES 1. Strategy for malaria elimination in the Greater Mekong Subregion Geneva: World Health Organization; World malaria report Geneva: World Health Organization; Greater Mekong Sub-region malaria operational plan FY2016: President s Malaria Initiative; ACTwatch Group and PSK: ACTwatch study reference document: Cambodia outlet survey Washington (DC): PSI; ACTwatch Group and Population Services International/Lao PDR (PSI/PSI Lao PDR). ACTwatch study reference document: Lao PDR outlet survey Washington (DC): PSI; Household survey: baseline. The Republic of the Union of Myanmar. AMTR Survey Report: PSI/Myanmar; Cambodia malaria elimination action framework : Cambodia Ministry of Health; Akhlaghi L, Egharevba M. Technical report: Cambodia: malaria commodities quantification Arlington (VA): USAID DELIVER PROJECT; Chuor CM. Benefits to national malaria programs from regional support: the Cambodia case: National Center for Malaria, Parasitology Entomology; Logistics management information system (LMIS) for malaria commodities: standard operating procedures (SOP). Center for Malaria, Parasitology and Entomology; Lao PDR malaria programme review. Vectorborne Diseases Associates LLC; Audit of Global Fund grants to the Lao People s Democratic Republic: Office of the Inspector General. The Global Fund to Fight AIDS, Tuberculosis and Malaria; National strategic plan for malaria prevention and control : Myanmar Ministry of Health; Tolliver J, Bartram K. Burma national supply chain: baseline results. Arlington (VA): Partnership for supply chain management - PFSCM; Support to quality assurance of malaria drugs in Myanmar [website]. Regional Artemisininresistance Initiative (RAI). ( acessed 3 November 2016). 16. Patouillard E, Palafox B, Tougher S, Goodman C, Hanson K, Sochea P, et al. A qualitative assessment of the private sector antimalarial distribution chain in Cambodia, Nairobi: Population Services International; Krech LA, Lane-Barlow C, Siv L, Phanouvong S, Yuan WE, Heng B, et al. Cambodian Ministry of Health Takes Decisive Actions in the Fight against Substandard and Counterfeit Medicines. Trop Med Surg. 2014;2(166). 18. Lao PDR: Malaria outbreaks remain worrisome but progress is being made in response and control. World Health Organization; 2014 ( mekong/lao-pdr-outbreaks-remain-worrisome/en/, accessed 15 November 2016). 19. National malaria strategy for malaria control and pre-elimination : Lao PDR Ministry of Health;
45 20. Malaria treatment guidelines for provincial and district hospitals: Lao PDR Center of Malariology, Parasitology, and Entomology; Tun KM, Imwong M, Lwin KM, Win AA, Hlaing TM, Hlaing T, et al. Spread of artemisininresistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. Lancet. 2015;15(4): Global Fund grants to Myanmar (audit report): The Office of the Inspector General. The Global Fund to Fight AIDS, Tuberculosis and Malaria; Programmatic review of the national malaria programme in Thailand: 31st August 11th September World Health Organization; Q&A on artemisinin resistance. World Health Organization; 2013 (updated March 2017) ( accessed 14 November 2016). 25. Strategy and intervention matrix: Thailand. Asia Pacific Malaria Elimination Network (APMEN); Malaria diagnosis and case management guidelines. Thailand 2015: Thailand Department of Disease Control Ministry of Health; Malaria Policy Advisory Committee to the WHO: conclusions and recommendations of eighth biannual meeting (September 2015). Malaria Journal. 2016;15(1): Technical brief: building on shared value to develop public private partnerships for malaria control. University Research Co., LLC; Strategy and intervention matrix: Vietnam. Asia Pacific Malaria Elimination Network (APMEN); Malaria: fact sheet. World Health Organization; 2014 ( topics/malaria/factsheet/en/, accessed 7 November 2016). 31. Morrow M, Nguyen QA, Caruana S, Biggs BA, Doan NH, Nong TT. Pathways to malaria persistence in remote central Vietnam: a mixed-method study of health care and the community. BMC Public Health; p The private sector s role in malaria surveillance. UCSF Global Health Sciences;
46
47
48
BEST PRACTICES FOR PLANNING A VACCINATION CAMPAIGN FOR AN ENTIRE POPULATION
 PLANNING A VACCINATION CAMPAIGN FOR AN ENTIRE POPULATION THIS DOCUMENT IS A SUPPLEMENT TO BEST PRACTICES IN MICROPLANNING FOR POLIO ERADICATION. ACKNOWLEDGEMENTS These best practices documents for polio
PLANNING A VACCINATION CAMPAIGN FOR AN ENTIRE POPULATION THIS DOCUMENT IS A SUPPLEMENT TO BEST PRACTICES IN MICROPLANNING FOR POLIO ERADICATION. ACKNOWLEDGEMENTS These best practices documents for polio
BEST PRACTICES IN MICROPLANNING FOR CHILDREN OUT OF THE HOUSEHOLD: AN EXAMPLE FROM NORTHERN NIGERIA
 BEST PRACTICES IN MICROPLANNING FOR CHILDREN OUT OF THE HOUSEHOLD: AN EXAMPLE FROM NORTHERN NIGERIA THIS DOCUMENT IS A SUPPLEMENT TO BEST PRACTICES IN MICROPLANNING FOR POLIO ERADICATION. ACKNOWLEDGEMENTS
BEST PRACTICES IN MICROPLANNING FOR CHILDREN OUT OF THE HOUSEHOLD: AN EXAMPLE FROM NORTHERN NIGERIA THIS DOCUMENT IS A SUPPLEMENT TO BEST PRACTICES IN MICROPLANNING FOR POLIO ERADICATION. ACKNOWLEDGEMENTS
GUIDANCE FOR SAMPLING ART CLINICS IN COUNTRIES COMBINING SURVEILLANCE OF PRE-TREATMENT HIV DRUG RESISTANCE AND ACQUIRED HIV DRUG RESISTANCE AT 12 AND
 GUIDANCE FOR SAMPLING ART CLINICS IN COUNTRIES COMBINING SURVEILLANCE OF PRE-TREATMENT HIV DRUG RESISTANCE AND ACQUIRED HIV DRUG RESISTANCE AT 12 AND 48+ MONTHS DECEMBER 2017 Guidance For Sampling ART
GUIDANCE FOR SAMPLING ART CLINICS IN COUNTRIES COMBINING SURVEILLANCE OF PRE-TREATMENT HIV DRUG RESISTANCE AND ACQUIRED HIV DRUG RESISTANCE AT 12 AND 48+ MONTHS DECEMBER 2017 Guidance For Sampling ART
Raising tobacco taxes in Bangladesh in FY : An opportunity for development
 Raising tobacco taxes in Bangladesh in FY 2018-2019: An opportunity for development Raising tobacco taxes would: Generate extra revenues between BDT 75 billion and 100 billion. Reduce the number of current
Raising tobacco taxes in Bangladesh in FY 2018-2019: An opportunity for development Raising tobacco taxes would: Generate extra revenues between BDT 75 billion and 100 billion. Reduce the number of current
Progress on the Containment of Artemisinin Tolerant Malaria Parasites in South-East Asia (ARCE) Initiative
 Progress on the Containment of Artemisinin Tolerant Malaria Parasites in South-East Asia (ARCE) Initiative I. Background For many years, the border area between Cambodia and Thailand has been the source
Progress on the Containment of Artemisinin Tolerant Malaria Parasites in South-East Asia (ARCE) Initiative I. Background For many years, the border area between Cambodia and Thailand has been the source
Latent tuberculosis infection
 Latent tuberculosis infection Updated and consolidated guidelines for programmatic management ANNEX 3 Values and preferences for the management of latent tuberculosis infection: survey of populations affected
Latent tuberculosis infection Updated and consolidated guidelines for programmatic management ANNEX 3 Values and preferences for the management of latent tuberculosis infection: survey of populations affected
Summary report on the WHO-EM/WRH/104/E
 Summary report on the Training of trainers course for national gynaecology and obstetrics societies and midwifery associations on evidence-based guidelines for strengthening family planning services WHO-EM/WRH/104/E
Summary report on the Training of trainers course for national gynaecology and obstetrics societies and midwifery associations on evidence-based guidelines for strengthening family planning services WHO-EM/WRH/104/E
Intercountry meeting of national malaria programme managers from countries of HANMAT and PIAM-Net
 WHO-EM/MAL/367/E Summary report on the Intercountry meeting of national malaria programme managers from countries of HANMAT and PIAM-Net Muscat, Oman 22 24 September 2011 World Health Organization 2012
WHO-EM/MAL/367/E Summary report on the Intercountry meeting of national malaria programme managers from countries of HANMAT and PIAM-Net Muscat, Oman 22 24 September 2011 World Health Organization 2012
54% WHO s emergency response to artemisinin resistance SHARP DECLINE IN MALARIA CASES AND DEATHS SEEN ACROSS THE GMS BULLETIN #5 SEPTEMBER 2016
 BULLETIN #5 WHO s emergency response to artemisinin resistance SEPTEMBER 2016 SHARP DECLINE IN MALARIA CASES AND DEATHS SEEN ACROSS THE GMS 54% REDUCTION IN MALARIA CASES OVER 3 YEARS Increased access
BULLETIN #5 WHO s emergency response to artemisinin resistance SEPTEMBER 2016 SHARP DECLINE IN MALARIA CASES AND DEATHS SEEN ACROSS THE GMS 54% REDUCTION IN MALARIA CASES OVER 3 YEARS Increased access
Eighth intercountry meeting of national malaria programme managers from HANMAT and PIAM-Net countries
 Summary report on the Eighth intercountry meeting of national malaria programme managers from HANMAT and PIAM-Net countries WHO-EM/MAL/384/E Islamabad, Pakistan 12 14 December 2016 Summary report on the
Summary report on the Eighth intercountry meeting of national malaria programme managers from HANMAT and PIAM-Net countries WHO-EM/MAL/384/E Islamabad, Pakistan 12 14 December 2016 Summary report on the
WHO REPORT ON THE GLOBAL TOBACCO EPIDEMIC,
 WHO/NMH/PND/7.4 WHO REPORT ON THE GLOBAL TOBACCO EPIDEMIC, 207 Monitoring tobacco use and prevention policies Executive summary fresh and alive World Health Organization 207 Some rights reserved. This
WHO/NMH/PND/7.4 WHO REPORT ON THE GLOBAL TOBACCO EPIDEMIC, 207 Monitoring tobacco use and prevention policies Executive summary fresh and alive World Health Organization 207 Some rights reserved. This
Strategy to move from accelerated burden reduction to malaria elimination in the GMS by 2030
 Strategy to move from accelerated burden reduction to malaria elimination in the GMS by 2030 Dr Walter M Kazadi Coordinator Regional Hub Emergency Response to Artemisinin Resistance Regional Hub GMS MMV
Strategy to move from accelerated burden reduction to malaria elimination in the GMS by 2030 Dr Walter M Kazadi Coordinator Regional Hub Emergency Response to Artemisinin Resistance Regional Hub GMS MMV
Interpretation of the World Malaria Report Country Profile
 Interpretation of the World Malaria Report Country Profile Acknowledgements This presentation was developed to help explain the components of the World Malaria Report Country Profile. The 2017 World Malaria
Interpretation of the World Malaria Report Country Profile Acknowledgements This presentation was developed to help explain the components of the World Malaria Report Country Profile. The 2017 World Malaria
WHO Global Malaria Programme. February 2009
 WHO Global Malaria Programme February 2009 Table of Contents 1. The world malaria situation 2. The critical role of WHO's Global Malaria Programme 3. Our programme of work explained 4. Situation analysis
WHO Global Malaria Programme February 2009 Table of Contents 1. The world malaria situation 2. The critical role of WHO's Global Malaria Programme 3. Our programme of work explained 4. Situation analysis
DRAFT UNICEF PROCUREMENT OF HIV/AIDS-RELATED SUPPLIES AND SERVICES
 DRAFT UNICEF PROCUREMENT OF HIV/AIDS-RELATED SUPPLIES AND SERVICES April 2005 Summary: Millions of children in developing countries are affected by the HIV/AIDS pandemic. Despite significant international
DRAFT UNICEF PROCUREMENT OF HIV/AIDS-RELATED SUPPLIES AND SERVICES April 2005 Summary: Millions of children in developing countries are affected by the HIV/AIDS pandemic. Despite significant international
This Malaria Operational Plan has been approved by the U.S. Global Malaria Coordinator and reflects collaborative discussions with the national
 This Malaria Operational Plan has been approved by the U.S. Global Malaria Coordinator and reflects collaborative discussions with the national malaria control programs and partners in country. The final
This Malaria Operational Plan has been approved by the U.S. Global Malaria Coordinator and reflects collaborative discussions with the national malaria control programs and partners in country. The final
Summary report on the WHO-EM/CSR/124/E
 Summary report on the Consultative workshop to define an appropriate surveillance strategy for detection of clusters of Zika virus infection and other arboviral diseases using both syndromic- and event-based
Summary report on the Consultative workshop to define an appropriate surveillance strategy for detection of clusters of Zika virus infection and other arboviral diseases using both syndromic- and event-based
Ending Malaria in Nigeria: The WHO Agenda
 Nigeria Institute of Medical Research 2016 World Malaria Day Lecture 27 April, 2016 Ending Malaria in Nigeria: The WHO Agenda Dr Tolu Arowolo Malaria Containment Programme, WHO, Nigeria arowolot@who.int
Nigeria Institute of Medical Research 2016 World Malaria Day Lecture 27 April, 2016 Ending Malaria in Nigeria: The WHO Agenda Dr Tolu Arowolo Malaria Containment Programme, WHO, Nigeria arowolot@who.int
Ethiopia Malaria Financial Landscape
 Ethiopia Malaria Financial Landscape PATH MACEPA DECEMBER 2015 MALARIA FUNDING IN ETHIOPIA AT A GLANCE The total estimated cost to implement Ethiopia s National Malaria Strategic Plan (NMSP) for 2015 2017
Ethiopia Malaria Financial Landscape PATH MACEPA DECEMBER 2015 MALARIA FUNDING IN ETHIOPIA AT A GLANCE The total estimated cost to implement Ethiopia s National Malaria Strategic Plan (NMSP) for 2015 2017
WEB ANNEX I. REPORT ON COST-EFFECTIVENESS OF IMPLEMENTING AN INDETERMINATE RANGE FOR EARLY INFANT DIAGNOSIS OF HIV
 WEB ANNEX I. REPORT ON COST-EFFECTIVENESS OF IMPLEMENTING AN INDETERMINATE RANGE FOR EARLY INFANT DIAGNOSIS OF HIV In: Updated recommendations on first-line and second-line antiretroviral regimens and
WEB ANNEX I. REPORT ON COST-EFFECTIVENESS OF IMPLEMENTING AN INDETERMINATE RANGE FOR EARLY INFANT DIAGNOSIS OF HIV In: Updated recommendations on first-line and second-line antiretroviral regimens and
Summary World Malaria Report 2010
 Summary The summarizes information received from 106 malaria-endemic countries and other partners and updates the analyses presented in the 2009 Report. It highlights continued progress made towards meeting
Summary The summarizes information received from 106 malaria-endemic countries and other partners and updates the analyses presented in the 2009 Report. It highlights continued progress made towards meeting
Regional workshop on updating national strategic plans for the prevention of re-establishment of local malaria transmission in malaria-free countries
 Summary report on the Regional workshop on updating national strategic plans for the prevention of re-establishment of local malaria transmission in malaria-free countries Casablanca, Morocco 18 20 October
Summary report on the Regional workshop on updating national strategic plans for the prevention of re-establishment of local malaria transmission in malaria-free countries Casablanca, Morocco 18 20 October
Ninth intercountry meeting of national malaria programme managers from HANMAT and PIAM-Net countries
 Summary report on the Ninth intercountry meeting of national malaria programme managers from HANMAT and PIAM-Net countries WHO-EM/MAL/387/E Cairo, Egypt 24 26 October 2017 Summary report on the Ninth intercountry
Summary report on the Ninth intercountry meeting of national malaria programme managers from HANMAT and PIAM-Net countries WHO-EM/MAL/387/E Cairo, Egypt 24 26 October 2017 Summary report on the Ninth intercountry
Malaria Symposia Myanmar Medical Association. Dr Aung Thi National Malaria Control Program 04 March 2017
 Malaria Symposia Myanmar Medical Association Dr Aung Thi National Malaria Control Program 04 March 2017 OUTLINE OF THE PRESENTATION Country profile Programmatic achievements Challenges Lessons learnt Way
Malaria Symposia Myanmar Medical Association Dr Aung Thi National Malaria Control Program 04 March 2017 OUTLINE OF THE PRESENTATION Country profile Programmatic achievements Challenges Lessons learnt Way
Copyright by Population Services International and ACTwatch 2016.
 Malaria markets in the Greater Mekong Sub-Region: 2015-2016 1 Copyright by Population Services International and ACTwatch 2016. Suggested Citation: Malaria Markets in the Greater Mekong Sub-Region: 2015-2016.
Malaria markets in the Greater Mekong Sub-Region: 2015-2016 1 Copyright by Population Services International and ACTwatch 2016. Suggested Citation: Malaria Markets in the Greater Mekong Sub-Region: 2015-2016.
Latent tuberculosis infection
 Latent tuberculosis infection Updated and consolidated guidelines for programmatic management ANNEX 2 Evidence-to-Decision and GRADE tables Latent tuberculosis infection Updated and consolidated guidelines
Latent tuberculosis infection Updated and consolidated guidelines for programmatic management ANNEX 2 Evidence-to-Decision and GRADE tables Latent tuberculosis infection Updated and consolidated guidelines
preventing suicide Regional strategy on
 Over 800 000 people die due to suicide every year and there are many more who attempt suicide. Suicide was the second leading cause of death among 15-29 year olds globally in 2012. 75% of global suicide
Over 800 000 people die due to suicide every year and there are many more who attempt suicide. Suicide was the second leading cause of death among 15-29 year olds globally in 2012. 75% of global suicide
Key Messages for World Malaria Day 2009
 INFORMATION RBM/WG/2009/INF.12 10 APR 2009 Draft document General distribution English Only Key Messages for World Malaria Day 2009 Counting Malaria Out to Reaching the 2010 Targets On the occasion of
INFORMATION RBM/WG/2009/INF.12 10 APR 2009 Draft document General distribution English Only Key Messages for World Malaria Day 2009 Counting Malaria Out to Reaching the 2010 Targets On the occasion of
Antimalarial drug resistance
 Antimalarial drug resistance Md Mushfiqur Rahman*, Leonard Ortega**, R M Rastogi* and Krongthong Thimasarn* Abstract Antimalarial drug resistance is of great concern in the WHO South-East Asia (SEA) Region.
Antimalarial drug resistance Md Mushfiqur Rahman*, Leonard Ortega**, R M Rastogi* and Krongthong Thimasarn* Abstract Antimalarial drug resistance is of great concern in the WHO South-East Asia (SEA) Region.
WHO Consultation on universal access to core malaria interventions in high burden countries: main conclusions and recommendations
 WHO Consultation on universal access to core malaria interventions in high burden countries: main conclusions and recommendations 12-15 February 2018 Salle XI, ILO Building, Geneva, Switzerland Country
WHO Consultation on universal access to core malaria interventions in high burden countries: main conclusions and recommendations 12-15 February 2018 Salle XI, ILO Building, Geneva, Switzerland Country
Malaria Initiative: Access
 Novartis Social Business Malaria Initiative: Access Improving affordability and availability of medicines Over the past decade, the Novartis Malaria Initiative has pioneered the pharmaceutical response
Novartis Social Business Malaria Initiative: Access Improving affordability and availability of medicines Over the past decade, the Novartis Malaria Initiative has pioneered the pharmaceutical response
Essential Medicines. WHO
 12 Health Technology and Pharmaceuticals Participants to the Workshop on Pharmaceutical Policies and Access to Good Quality Essential Medicines for Pacific Island Countries observing pharmaceutical services
12 Health Technology and Pharmaceuticals Participants to the Workshop on Pharmaceutical Policies and Access to Good Quality Essential Medicines for Pacific Island Countries observing pharmaceutical services
SECTOR ASSESMENT (SUMMARY): HEALTH
 Greater Mekong Subregion Health Security Project RRP REG-48118-002 SECTOR ASSESMENT (SUMMARY): HEALTH A. Sector Performance, Problems, and Opportunities 1. The governments of Cambodia, the Lao PDR, Myanmar,
Greater Mekong Subregion Health Security Project RRP REG-48118-002 SECTOR ASSESMENT (SUMMARY): HEALTH A. Sector Performance, Problems, and Opportunities 1. The governments of Cambodia, the Lao PDR, Myanmar,
PURPOSE The purpose of the Malaria Control Strategic Plan 2005/ /10 is to provide a common platform and detailed description of interventions
 PURPOSE The purpose of the Malaria Control Strategic Plan 2005/06-2009/10 is to provide a common platform and detailed description of interventions for all RBM partners and sectors of society. It encourages
PURPOSE The purpose of the Malaria Control Strategic Plan 2005/06-2009/10 is to provide a common platform and detailed description of interventions for all RBM partners and sectors of society. It encourages
Cronfa - Swansea University Open Access Repository
 Cronfa - Swansea University Open Access Repository This is an author produced version of a paper published in: Malaria Journal Cronfa URL for this paper: http://cronfa.swan.ac.uk/record/cronfa40379 Paper:
Cronfa - Swansea University Open Access Repository This is an author produced version of a paper published in: Malaria Journal Cronfa URL for this paper: http://cronfa.swan.ac.uk/record/cronfa40379 Paper:
Annex A: Impact, Outcome and Coverage Indicators (including Glossary of Terms)
 IMPACT INDICATORS (INDICATORS PER GOAL) HIV/AIDS TUBERCULOSIS MALARIA Reduced HIV prevalence among sexually active population Reduced HIV prevalence in specific groups (sex workers, clients of sex workers,
IMPACT INDICATORS (INDICATORS PER GOAL) HIV/AIDS TUBERCULOSIS MALARIA Reduced HIV prevalence among sexually active population Reduced HIV prevalence in specific groups (sex workers, clients of sex workers,
Trans-border malaria programme
 In Foc us Mobile malaria worker, Sal Svan, visiting mobile forest workers in Lom Thmey village Trans-border malaria programme Fine-scale mapping of high risk populations and targeting of hotspots with
In Foc us Mobile malaria worker, Sal Svan, visiting mobile forest workers in Lom Thmey village Trans-border malaria programme Fine-scale mapping of high risk populations and targeting of hotspots with
NATIONAL COORDINATION MECHANISM FOR TOBACCO CONTROL A Model for the African Region
 NATIONAL COORDINATION MECHANISM FOR TOBACCO CONTROL A Model for the African Region I. World Health Organization. Regional Office for Africa II.Title ISBN 978-929023293-3 NLM Classification: WM 290) WHO
NATIONAL COORDINATION MECHANISM FOR TOBACCO CONTROL A Model for the African Region I. World Health Organization. Regional Office for Africa II.Title ISBN 978-929023293-3 NLM Classification: WM 290) WHO
NATIONAL MALARIA ELIMINATION ACTION PLAN Ministry of Health Belize
 NATIONAL MALARIA ELIMINATION ACTION PLAN 2015-2020 Ministry of Health Belize October, 2015 Table of Content Contextual Analysis 1 Situation Analysis of Malaria in Belize 2 Malaria Program in Belize 3 Vision
NATIONAL MALARIA ELIMINATION ACTION PLAN 2015-2020 Ministry of Health Belize October, 2015 Table of Content Contextual Analysis 1 Situation Analysis of Malaria in Belize 2 Malaria Program in Belize 3 Vision
26/06/ NIMR 2018 Conference - Malaria - a reality
 Malaria Elimination: Reality or Myth? Wellington A. Oyibo ANDI CENTRE OF EXCELLENCE FOR MALARIA DIAGNOSIS WHO/FIND Malaria Specimen Collection Site The International Center for Malaria Microscopy and Malaria
Malaria Elimination: Reality or Myth? Wellington A. Oyibo ANDI CENTRE OF EXCELLENCE FOR MALARIA DIAGNOSIS WHO/FIND Malaria Specimen Collection Site The International Center for Malaria Microscopy and Malaria
Copenhagen, Denmark, September August Malaria
 Regional Committee for Europe 64th session EUR/RC64/Inf.Doc./5 Copenhagen, Denmark, 15 18 September 2014 21 August 2014 140602 Provisional agenda item 3 ORIGINAL: ENGLISH Malaria Following the support
Regional Committee for Europe 64th session EUR/RC64/Inf.Doc./5 Copenhagen, Denmark, 15 18 September 2014 21 August 2014 140602 Provisional agenda item 3 ORIGINAL: ENGLISH Malaria Following the support
2016 FP2020 ANNUAL COMMITMENT UPDATE QUESTIONNAIRE RESPONSE
 MYANMAR HTTP://WWW.FAMILYPLANNING2020.ORG/MYANMAR In July 2016, the government of Myanmar shared the following update on progress toward achieving its Family Planning 2020 commitment during the 2015-2016
MYANMAR HTTP://WWW.FAMILYPLANNING2020.ORG/MYANMAR In July 2016, the government of Myanmar shared the following update on progress toward achieving its Family Planning 2020 commitment during the 2015-2016
Seminars for drug management and reporting system - Japanese consultant and local consultant conducted the seminars as below
 Ex-Post Evaluation for Grant Aid Project Country Name Republic of the Union of Myanmar I. Project Outline Background Objective of the Project Outputs of the Project conducted by Akiko Okitsu, Michiko Tajima,
Ex-Post Evaluation for Grant Aid Project Country Name Republic of the Union of Myanmar I. Project Outline Background Objective of the Project Outputs of the Project conducted by Akiko Okitsu, Michiko Tajima,
HIV PREVENTION, DIAGNOSIS, TREATMENT AND CARE FOR KEY POPULATIONS
 POLICY BRIEF HIV PREVENTION, DIAGNOSIS, TREATMENT AND CARE FOR KEY POPULATIONS CONSOLIDATED GUIDELINES 2016 UPDATE Policy brief: Consolidated guidelines on HIV prevention, diagnosis, treatment and care
POLICY BRIEF HIV PREVENTION, DIAGNOSIS, TREATMENT AND CARE FOR KEY POPULATIONS CONSOLIDATED GUIDELINES 2016 UPDATE Policy brief: Consolidated guidelines on HIV prevention, diagnosis, treatment and care
128th Session 25 November 2010 Provisional agenda item Malaria. Prevention and control: sustaining the gains and reducing transmission
 EXECUTIVE BOARD 128th Session 25 November 2010 Provisional agenda item 4.11 Malaria Prevention and control: sustaining the gains and reducing transmission Report by the Secretariat 1. Millennium Development
EXECUTIVE BOARD 128th Session 25 November 2010 Provisional agenda item 4.11 Malaria Prevention and control: sustaining the gains and reducing transmission Report by the Secretariat 1. Millennium Development
Issue 6: January - June 2016 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana
 Issue 6: January - June 2016 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana Contents Page Editorial and Report Highlights Page 1 Malaria Burden Page 2 Key Activities undertaken
Issue 6: January - June 2016 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana Contents Page Editorial and Report Highlights Page 1 Malaria Burden Page 2 Key Activities undertaken
THE ROAD TO 2020: MOBILSING THE PRIVATE SECTOR IN NIGERIA S FIGHT AGAINST MALARIA- THE LAGOS STATE APPROACH.
 THE ROAD TO 2020: MOBILSING THE PRIVATE SECTOR IN NIGERIA S FIGHT AGAINST MALARIA- THE LAGOS STATE APPROACH. A PAPER PRESENTED AT THE 2015 CAMA ANNUAL TECHNICAL FORUM. Dr Modele Osunkiyesi Permanent Secretary
THE ROAD TO 2020: MOBILSING THE PRIVATE SECTOR IN NIGERIA S FIGHT AGAINST MALARIA- THE LAGOS STATE APPROACH. A PAPER PRESENTED AT THE 2015 CAMA ANNUAL TECHNICAL FORUM. Dr Modele Osunkiyesi Permanent Secretary
UNICEF AND MALARIA MEDICINES. Supply Division October 2006
 UNICEF AND MALARIA MEDICINES Supply Division October 2006 PROCUREMENT SERVICES SUPPORTING CHILD RIGHTS UNICEF PROVIDES PROCUREMENT SERVICES AS PART OF ITS MANDATE Helping partners access resources for
UNICEF AND MALARIA MEDICINES Supply Division October 2006 PROCUREMENT SERVICES SUPPORTING CHILD RIGHTS UNICEF PROVIDES PROCUREMENT SERVICES AS PART OF ITS MANDATE Helping partners access resources for
Issue 9: January March, 2017 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana
 Issue 9: January March, 2017 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana Contents Page Editorial and Report Highlights 1 Malaria Burden 2 Key activities undertaken in First
Issue 9: January March, 2017 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana Contents Page Editorial and Report Highlights 1 Malaria Burden 2 Key activities undertaken in First
BMGF MALARIA STRATEGY TO 2020
 BMGF MALARIA STRATEGY TO 2020 Supporting the Drive to Elimination in Mesoamerica & Hispaniola Diana Measham, DrPH, MSc Lead Eliminate Initiative September 25, 2014 The world has three potential future
BMGF MALARIA STRATEGY TO 2020 Supporting the Drive to Elimination in Mesoamerica & Hispaniola Diana Measham, DrPH, MSc Lead Eliminate Initiative September 25, 2014 The world has three potential future
Global call for action to ensure universal access to malaria diagnosis and treatment
 Global call for action to ensure universal access to malaria diagnosis and treatment Joint activity of SEE Team and PDT Unit Andrea Bosman and Richard Cibulskis Outline of the presentation Background Objectives
Global call for action to ensure universal access to malaria diagnosis and treatment Joint activity of SEE Team and PDT Unit Andrea Bosman and Richard Cibulskis Outline of the presentation Background Objectives
SIAPS Cameroon Key Achievements
 SIAPS Cameroon Key Achievements 2012-2016 August 2016 Background USAID and the US Centers for Disease Control (CDC) are implementing the US President s Emergency Plan for AIDS Relief (PEPFAR) program
SIAPS Cameroon Key Achievements 2012-2016 August 2016 Background USAID and the US Centers for Disease Control (CDC) are implementing the US President s Emergency Plan for AIDS Relief (PEPFAR) program
DECLARATION ON ACCELERATION OF HIV PREVENTION EFFORTS IN THE AFRICAN REGION
 DECLARATION ON ACCELERATION OF HIV PREVENTION EFFORTS IN THE AFRICAN REGION AFRO Library Cataloguing-in-Publication Data Declaration on Acceleration of HIV Prevention efforts in the African Region 1. Acquired
DECLARATION ON ACCELERATION OF HIV PREVENTION EFFORTS IN THE AFRICAN REGION AFRO Library Cataloguing-in-Publication Data Declaration on Acceleration of HIV Prevention efforts in the African Region 1. Acquired
SUMMARY MEETING REPORT. Annual stakeholders and partners meeting AIDS Medicines and Diagnostics Service. Geneva, 7 8 May 2013
 SUMMARY MEETING REPORT Annual stakeholders and partners meeting AIDS Medicines and Diagnostics Service Geneva, 7 8 May 2013 Department of HIV/AIDS HIV Technologies and Commodities May 2013 WHO/HIV/2013.6
SUMMARY MEETING REPORT Annual stakeholders and partners meeting AIDS Medicines and Diagnostics Service Geneva, 7 8 May 2013 Department of HIV/AIDS HIV Technologies and Commodities May 2013 WHO/HIV/2013.6
Resolution adopted by the General Assembly. [without reference to a Main Committee (A/62/L.39 and Add.1)]
![Resolution adopted by the General Assembly. [without reference to a Main Committee (A/62/L.39 and Add.1)] Resolution adopted by the General Assembly. [without reference to a Main Committee (A/62/L.39 and Add.1)]](/thumbs/96/127242235.jpg) United Nations General Assembly Distr.: General 7 March 2008 Sixty-second session Agenda item 47 Resolution adopted by the General Assembly [without reference to a Main Committee (A/62/L.39 and Add.1)]
United Nations General Assembly Distr.: General 7 March 2008 Sixty-second session Agenda item 47 Resolution adopted by the General Assembly [without reference to a Main Committee (A/62/L.39 and Add.1)]
MALARIA LABORATORY DIAGNOSIS AND MONITORING PROJECT
 MALARIA LABORATORY DIAGNOSIS AND MONITORING PROJECT Nine Years of Action for Improving Quality of Malaria Laboratory Diagnosis and Case Management Services in Ethiopia Accurate early diagnosis and prompt
MALARIA LABORATORY DIAGNOSIS AND MONITORING PROJECT Nine Years of Action for Improving Quality of Malaria Laboratory Diagnosis and Case Management Services in Ethiopia Accurate early diagnosis and prompt
MONITORING THE BUILDING BLOCKS OF HEALTH SYSTEMS: A HANDBOOK OF INDICATORS AND THEIR MEASUREMENT STRATEGIES
 MONITORING THE BUILDING BLOCKS OF HEALTH SYSTEMS: A HANDBOOK OF INDICATORS AND THEIR MEASUREMENT STRATEGIES A WHO Library Cataloguing-in-Publication Data Monitoring the building blocks of health systems:
MONITORING THE BUILDING BLOCKS OF HEALTH SYSTEMS: A HANDBOOK OF INDICATORS AND THEIR MEASUREMENT STRATEGIES A WHO Library Cataloguing-in-Publication Data Monitoring the building blocks of health systems:
Strategic Plan to Stop TB in the Western Pacific
 Strategic Plan to Stop TB in the Western Pacific 26 21 1% PREVALENCE 5% MORTALITY % 2 21 TB Strategic Plan to STOP TB in the Western Pacific 26 21 Prepared by the Stop TB Unit in the WHO Regional Office
Strategic Plan to Stop TB in the Western Pacific 26 21 1% PREVALENCE 5% MORTALITY % 2 21 TB Strategic Plan to STOP TB in the Western Pacific 26 21 Prepared by the Stop TB Unit in the WHO Regional Office
UNITAID. Dr Philippe Duneton Deputy Executive Director Copenhagen September 2012
 UNITAID Dr Philippe Duneton Deputy Executive Director Copenhagen September 2012 Challenges Achievements WHO Prequalification UNITAID support for prequalification of medicines Since 2007 UNITAID support
UNITAID Dr Philippe Duneton Deputy Executive Director Copenhagen September 2012 Challenges Achievements WHO Prequalification UNITAID support for prequalification of medicines Since 2007 UNITAID support
AMDS Partners and Stakeholders Meeting CHAI HIV Diagnostics Forecasting Overview th September, 2014
 AMDS Partners and Stakeholders Meeting CHAI HIV Diagnostics Forecasting Overview 29-30 th September, 2014 Agenda Introduction Overview of Global Diagnostics Forecasting Overview of Country Forecasting
AMDS Partners and Stakeholders Meeting CHAI HIV Diagnostics Forecasting Overview 29-30 th September, 2014 Agenda Introduction Overview of Global Diagnostics Forecasting Overview of Country Forecasting
REGIONAL ALLIANCE FOR NATIONAL REGULATORY AUTHORITIES FOR VACCINES IN THE WESTERN PACIFIC. second edition
 REGIONAL ALLIANCE FOR NATIONAL REGULATORY AUTHORITIES FOR VACCINES IN THE WESTERN PACIFIC second edition World Health Organization 2014 The designations employed and the presentation of the material in
REGIONAL ALLIANCE FOR NATIONAL REGULATORY AUTHORITIES FOR VACCINES IN THE WESTERN PACIFIC second edition World Health Organization 2014 The designations employed and the presentation of the material in
Media centre Malaria. Key facts. Symptoms
 Media centre Malaria Fact sheet Updated November 2017 Key facts Malaria is a life-threatening disease caused by parasites that are transmitted to people through the bites of infected female Anopheles mosquitoes.
Media centre Malaria Fact sheet Updated November 2017 Key facts Malaria is a life-threatening disease caused by parasites that are transmitted to people through the bites of infected female Anopheles mosquitoes.
The Western Pacific Region faces significant
 COMBATING COMMUNICABLE DISEASES A medical technician draws blood for HIV screening in Manila. AFP elimination of mother-to-child transmission of HIV and congenital syphilis was piloted in Malaysia and
COMBATING COMMUNICABLE DISEASES A medical technician draws blood for HIV screening in Manila. AFP elimination of mother-to-child transmission of HIV and congenital syphilis was piloted in Malaysia and
COUNTRY PROFILE: ZAMBIA ZAMBIA COMMUNITY HEALTH PROGRAMS DECEMBER 2013
 COUNTRY PROFILE: ZAMBIA DECEMBER 2013 Advancing Partners & Communities Advancing Partners & Communities (APC) is a five-year cooperative agreement funded by the U.S. Agency for International Development
COUNTRY PROFILE: ZAMBIA DECEMBER 2013 Advancing Partners & Communities Advancing Partners & Communities (APC) is a five-year cooperative agreement funded by the U.S. Agency for International Development
Quality Assurance Policy. for the. Procurement of HIV Point-of-Care Technology. under the UNITAID Grant
 Quality Assurance Policy for the Procurement of HIV Point-of-Care Technology under the UNITAID Grant UNICEF Supply Division Quality Assurance Centre and Health Technology Centre Revisions Version Date
Quality Assurance Policy for the Procurement of HIV Point-of-Care Technology under the UNITAID Grant UNICEF Supply Division Quality Assurance Centre and Health Technology Centre Revisions Version Date
Evidence Review Group on MDA, MSAT and FSAT Malaria Policy Advisory Committee Geneva, Switzerland September 2015
 Evidence Review Group on MDA, MSAT and FSAT Malaria Policy Advisory Committee Geneva, Switzerland 16-18 September 2015 Kevin Marsh Chairperson of the Evidence Review Group 1 Outline of the Presentation
Evidence Review Group on MDA, MSAT and FSAT Malaria Policy Advisory Committee Geneva, Switzerland 16-18 September 2015 Kevin Marsh Chairperson of the Evidence Review Group 1 Outline of the Presentation
Second intercountry meeting on the Eastern Mediterranean Acute Respiratory Infection Surveillance (EMARIS) network
 Summary report on the Second intercountry meeting on the Eastern Mediterranean Acute Respiratory Infection Surveillance (EMARIS) network WHO-EM/CSR/070/E Sharm el-sheikh, Egypt 24 27 November 2013 Summary
Summary report on the Second intercountry meeting on the Eastern Mediterranean Acute Respiratory Infection Surveillance (EMARIS) network WHO-EM/CSR/070/E Sharm el-sheikh, Egypt 24 27 November 2013 Summary
Using Data from Electronic HIV Case Management Systems to Improve HIV Services in Central Asia
 Using Data from Electronic HIV Case Management Systems to Improve HIV Services in Central Asia Background HIV incidence continues to rise in Central Asia and Eastern Europe. Between 2010 and 2015, there
Using Data from Electronic HIV Case Management Systems to Improve HIV Services in Central Asia Background HIV incidence continues to rise in Central Asia and Eastern Europe. Between 2010 and 2015, there
Guide on Access to the Yellow Fever ICG Stockpile. Emergency Campaigns. International Coordinating Group on Vaccine. Geneva, Switzerland 2012
 Guide on Access to the Yellow Fever ICG Stockpile Emergency Campaigns Geneva, Switzerland 2012 International Coordinating Group on Vaccine Provision for Yellow Fever Control TABLE OF CONTENTS 1. Description
Guide on Access to the Yellow Fever ICG Stockpile Emergency Campaigns Geneva, Switzerland 2012 International Coordinating Group on Vaccine Provision for Yellow Fever Control TABLE OF CONTENTS 1. Description
Guidelines for scaling-up the 100% condom use programme:
 Guidelines for scaling-up the 100% condom use programme: Experience from Cambodia World Health Organization Regional Office for the Western Pacific Guidelines for scaling-up the 100% condom use programme:
Guidelines for scaling-up the 100% condom use programme: Experience from Cambodia World Health Organization Regional Office for the Western Pacific Guidelines for scaling-up the 100% condom use programme:
APPENDIX 1 DATA COLLECTION AND DISSEMINATION STEPS
 APPENDIX 1 DATA COLLECTION AND DISSEMINATION STEPS 97 Country/ area APPENDIX 2a DRUG REGIMENS SEA REGION, 2001 (dosage for adults) : P. falciparum Lab confirmed Treatment failure Severe malaria Pregnancy
APPENDIX 1 DATA COLLECTION AND DISSEMINATION STEPS 97 Country/ area APPENDIX 2a DRUG REGIMENS SEA REGION, 2001 (dosage for adults) : P. falciparum Lab confirmed Treatment failure Severe malaria Pregnancy
Using Routine Health Information to Improve Voluntary Counseling and Testing in Cote d Ivoire
 Using Routine Health Information to Improve Voluntary Counseling and Testing in Cote d Ivoire Data Demand and Information Use Case Study Series MEASURE Evaluation www.cpc.unc.edu/measure Data Demand and
Using Routine Health Information to Improve Voluntary Counseling and Testing in Cote d Ivoire Data Demand and Information Use Case Study Series MEASURE Evaluation www.cpc.unc.edu/measure Data Demand and
A New Class of Malaria Drugs: The Coartem Breakthrough from Novartis
 A New Class of Malaria Drugs: The Coartem Breakthrough from Novartis and its Chinese Partners Hans Rietveld, Director, Global Access and Marketing, Malaria Initiative, Novartis Pharma AG Workshop on Access
A New Class of Malaria Drugs: The Coartem Breakthrough from Novartis and its Chinese Partners Hans Rietveld, Director, Global Access and Marketing, Malaria Initiative, Novartis Pharma AG Workshop on Access
GREATER MEKONG SUB-REGION ELIMINATION OF MALARIA (GEMS) PRIVATE SECTOR MALARIA SURVEILLANCE
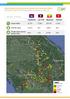 GREATER MEKONG SUB-REGION ELIMINATION OF MALARIA (GEMS) PRIVATE SECTOR MALARIA SURVEILLANCE JANUARY JUNE 218 Cambodia Lao PDR Myanmar Vietnam Cases tested 51,71 17,162 291,317 4,334 Positive cases 6,43
GREATER MEKONG SUB-REGION ELIMINATION OF MALARIA (GEMS) PRIVATE SECTOR MALARIA SURVEILLANCE JANUARY JUNE 218 Cambodia Lao PDR Myanmar Vietnam Cases tested 51,71 17,162 291,317 4,334 Positive cases 6,43
Public Health Laboratories for Alert and Response. A WHO Guidance Document
 Public Health Laboratories for Alert and Response A WHO Guidance Document WHO Library Cataloguing in Publication Data Public health laboratories for alert and response: a WHO guidance document 1. Laboratories
Public Health Laboratories for Alert and Response A WHO Guidance Document WHO Library Cataloguing in Publication Data Public health laboratories for alert and response: a WHO guidance document 1. Laboratories
Issue Paper: Monitoring a Rights based Approach: Key Issues and Suggested Approaches
 Issue Paper: Monitoring a Rights based Approach: Key Issues and Suggested Approaches Defining the Issue This paper explores issues and approaches relevant to the assessment of the application of a rights
Issue Paper: Monitoring a Rights based Approach: Key Issues and Suggested Approaches Defining the Issue This paper explores issues and approaches relevant to the assessment of the application of a rights
INTERVIEW GUIDE FOR THE EPI MANAGEMENT LEVEL GENERAL INFORMATION
 1 PAN AMERICAN HEALTH ORGANIZATION INTERVIEW GUIDE FOR THE EPI MANAGEMENT LEVEL GENERAL INFORMATION Date of interview: / / Interviewer s name: Management level where this interview was completed: National
1 PAN AMERICAN HEALTH ORGANIZATION INTERVIEW GUIDE FOR THE EPI MANAGEMENT LEVEL GENERAL INFORMATION Date of interview: / / Interviewer s name: Management level where this interview was completed: National
Multidrug-/ rifampicinresistant. (MDR/RR-TB): Update 2017
 Multidrug-/ rifampicinresistant TB (MDR/RR-TB): Update 2017 The global TB situation (1) Estimated incidence, 2016 Estimated number of deaths, 2016 All forms of TB HIV-associated TB Multidrug- / rifampicin-resistant
Multidrug-/ rifampicinresistant TB (MDR/RR-TB): Update 2017 The global TB situation (1) Estimated incidence, 2016 Estimated number of deaths, 2016 All forms of TB HIV-associated TB Multidrug- / rifampicin-resistant
TB Disease Prevalence Survey - Overview and Introduction of the TF
 TB Disease Prevalence Survey - Overview and Introduction of the TF Ikushi Onozaki MD, MPH Team Leader, TB Prevalence Survey WHO Global Task Force on TB Impact Measurement Stop TB Department, WHO onozakii@who.int
TB Disease Prevalence Survey - Overview and Introduction of the TF Ikushi Onozaki MD, MPH Team Leader, TB Prevalence Survey WHO Global Task Force on TB Impact Measurement Stop TB Department, WHO onozakii@who.int
NATIONAL AIDS PROGRAMME MANAGEMENT A SET OF TRAINING MODULES
 NATIONAL AIDS PROGRAMME MANAGEMENT A SET OF TRAINING MODULES WHO Library Cataloguing-in-Publication data World Health Organization, Regional Office for South-East Asia. National AIDS programme management:
NATIONAL AIDS PROGRAMME MANAGEMENT A SET OF TRAINING MODULES WHO Library Cataloguing-in-Publication data World Health Organization, Regional Office for South-East Asia. National AIDS programme management:
GOOD PHARMACOPOEIAL PRACTICES
 May 2013 RESTRICTED GOOD PHARMACOPOEIAL PRACTICES CONCEPT PAPER ON PURPOSE AND BENEFITS (MAY 2013) DRAFT FOR COMMENT Please address any comments on this proposal by 12 July 2013 to Dr S. Kopp, Medicines
May 2013 RESTRICTED GOOD PHARMACOPOEIAL PRACTICES CONCEPT PAPER ON PURPOSE AND BENEFITS (MAY 2013) DRAFT FOR COMMENT Please address any comments on this proposal by 12 July 2013 to Dr S. Kopp, Medicines
World Health Organization. A Sustainable Health Sector
 World Health Organization A Sustainable Health Sector Response to HIV Global Health Sector Strategy for HIV/AIDS 2011-2015 (DRAFT OUTLINE FOR CONSULTATION) Version 2.1 15 July 2010 15 July 2010 1 GLOBAL
World Health Organization A Sustainable Health Sector Response to HIV Global Health Sector Strategy for HIV/AIDS 2011-2015 (DRAFT OUTLINE FOR CONSULTATION) Version 2.1 15 July 2010 15 July 2010 1 GLOBAL
WESTERN PACIFIC REGIONAL ACTION PLAN for. Dengue Prevention and Control (2016)
 WESTERN PACIFIC REGIONAL ACTION PLAN for Dengue Prevention and Control (2016) WESTERN PACIFIC REGIONAL ACTION PLAN for Dengue Prevention and Control (2016) World Health Organization 2017 ISBN 978 92 9061
WESTERN PACIFIC REGIONAL ACTION PLAN for Dengue Prevention and Control (2016) WESTERN PACIFIC REGIONAL ACTION PLAN for Dengue Prevention and Control (2016) World Health Organization 2017 ISBN 978 92 9061
MALARIA CONTROL as a best practice Corporate Social Responsibility Programme
 GHANA MALARIA CONTROL as a best practice Corporate Social Responsibility Programme Steve Knowles Programme Director Steve Knowles (AngloGold Ashanti Malaria Programme Director) says: A Malaria Control
GHANA MALARIA CONTROL as a best practice Corporate Social Responsibility Programme Steve Knowles Programme Director Steve Knowles (AngloGold Ashanti Malaria Programme Director) says: A Malaria Control
Regional Response Plan for TB-HIV
 Regional Response Plan for TB-HIV 2017 2021 SEA-TB-370 Regional Response Plan for TB-HIV 2017 2021 Regional Response Plan for TB-HIV 2017 2021. World Health Organization 2017 Some rights reserved. This
Regional Response Plan for TB-HIV 2017 2021 SEA-TB-370 Regional Response Plan for TB-HIV 2017 2021 Regional Response Plan for TB-HIV 2017 2021. World Health Organization 2017 Some rights reserved. This
Ex post evaluation Tanzania
 Ex post evaluation Tanzania Sector: Health, family planning, HIV/AIDS (12250) Project: Promotion of national vaccination programme in cooperation with GAVI Alliance, Phase I and II (BMZ no. 2011 66 586
Ex post evaluation Tanzania Sector: Health, family planning, HIV/AIDS (12250) Project: Promotion of national vaccination programme in cooperation with GAVI Alliance, Phase I and II (BMZ no. 2011 66 586
Global Report on Antimalarial Drug. and the Global Plan for Artemisinin Resistance Containment (GPARC)
 Global Report on Antimalarial Drug Resistance and Drug Efficacy: 2-21 21 and the Global Plan for Artemisinin Resistance Containment (GPARC) MMV Stakeholders Meeting Dar es Salaam, Tanzania 2 June 211 Robert
Global Report on Antimalarial Drug Resistance and Drug Efficacy: 2-21 21 and the Global Plan for Artemisinin Resistance Containment (GPARC) MMV Stakeholders Meeting Dar es Salaam, Tanzania 2 June 211 Robert
Tanzania s Progress in Combating Malaria: Achievement and Challenges
 Tanzania s Progress in Combating Malaria: Achievement and Challenges DR RENATA A MANDIKE DEPUTY PROGRAMME MANAGER NATIONAL MALARIA CONTROL PROGRAMME, MINISTRY OF HEALTH, COMMUNITY DEVELOPMENT, GENDER,
Tanzania s Progress in Combating Malaria: Achievement and Challenges DR RENATA A MANDIKE DEPUTY PROGRAMME MANAGER NATIONAL MALARIA CONTROL PROGRAMME, MINISTRY OF HEALTH, COMMUNITY DEVELOPMENT, GENDER,
Module 9 Strategic information
 National AIDS Programme Management A Training Course Module 9 Strategic information 2007 WHO Library Cataloguing-in-Publication data World Health Organization, Regional Office for South-East Asia. National
National AIDS Programme Management A Training Course Module 9 Strategic information 2007 WHO Library Cataloguing-in-Publication data World Health Organization, Regional Office for South-East Asia. National
EPIDEMIOLOGICAL SURVEILLANCE REPORT Malaria in Greece, 2017, up to 17/08/2017
 Page 1 EPIDEMIOLOGICAL SURVEILLANCE REPORT Malaria in Greece, 2017, up to 17/08/2017 Introduction Greece was declared free from malaria in 1974, following an intense control program (1946-1960). Since
Page 1 EPIDEMIOLOGICAL SURVEILLANCE REPORT Malaria in Greece, 2017, up to 17/08/2017 Introduction Greece was declared free from malaria in 1974, following an intense control program (1946-1960). Since
The President s Malaria Initiative (PMI) Indoor Residual Spraying (IRS) in Motion: Malaria Stories from the Field
 The President s Malaria Initiative (PMI) Indoor Residual Spraying (IRS) in Motion: Malaria Stories from the Field Presenters: Allison Belemvire, Christen Fornadel & Kristen George Presentation Outline
The President s Malaria Initiative (PMI) Indoor Residual Spraying (IRS) in Motion: Malaria Stories from the Field Presenters: Allison Belemvire, Christen Fornadel & Kristen George Presentation Outline
UNITAID investments to innovate and scale up access to HIV diagnostics
 UNITAID investments to innovate and scale up access to HIV diagnostics WHO Annual meeting with Diagnostic Manufacturers and Stakeholders 10 March 2016 Smiljka de Lussigny (HIV Diagnostics Programme Manager,
UNITAID investments to innovate and scale up access to HIV diagnostics WHO Annual meeting with Diagnostic Manufacturers and Stakeholders 10 March 2016 Smiljka de Lussigny (HIV Diagnostics Programme Manager,
EBOLA VIRUS DISEASE. Democratic Republic of Congo. External Situation Report 2 Date of issue: 16 May Situation update
 EBOLA VIRUS DISEASE Democratic Republic of Congo External Situation Report 2 Date of issue: 16 May 2017 1. Situation update WHO continues to monitor the outbreak of Ebola virus disease (EVD) in Likati
EBOLA VIRUS DISEASE Democratic Republic of Congo External Situation Report 2 Date of issue: 16 May 2017 1. Situation update WHO continues to monitor the outbreak of Ebola virus disease (EVD) in Likati
EPIDEMIOLOGICAL SURVEILLANCE REPORT Malaria in Greece, 2018, up to 22/10/2018
 Page 1 EPIDEMIOLOGICAL SURVEILLANCE REPORT Malaria in Greece, 2018, up to 22/10/2018 Introduction Greece was declared free from malaria in 1974, following an intense control program (1946-1960). Since
Page 1 EPIDEMIOLOGICAL SURVEILLANCE REPORT Malaria in Greece, 2018, up to 22/10/2018 Introduction Greece was declared free from malaria in 1974, following an intense control program (1946-1960). Since
2. Treatment coverage: 3. Quality of care: 1. Access to diagnostic services:
 The theme for World TB Day 2014 is Reach the missed 3 million. Every year 3 million people who fall ill with TB are missed by health systems and do not always get the TB services that they need and deserve.
The theme for World TB Day 2014 is Reach the missed 3 million. Every year 3 million people who fall ill with TB are missed by health systems and do not always get the TB services that they need and deserve.
Implementing the Abuja Declaration and Plan of Action: the journey so far
 Implementing the Abuja Declaration and Plan of Action: the journey so far The Abuja Declaration African leaders who met on 25 April 2000 in Abuja, Nigeria, laid out the foundation for a sustained battle
Implementing the Abuja Declaration and Plan of Action: the journey so far The Abuja Declaration African leaders who met on 25 April 2000 in Abuja, Nigeria, laid out the foundation for a sustained battle
Action Plan of China Malaria Elimination ( ) Page 2
 http://www.gov.cn/gzdt/att/att/site1/20100526/001e3741a2cc0d67233801.doc Action Plan of China Malaria Elimination (2010 2020) Malaria is a major parasitic disease, which imposes serious threat to people
http://www.gov.cn/gzdt/att/att/site1/20100526/001e3741a2cc0d67233801.doc Action Plan of China Malaria Elimination (2010 2020) Malaria is a major parasitic disease, which imposes serious threat to people
RAPID DIAGNOSIS AND TREATMENT OF MDR-TB
 RAPID DIAGNOSIS AND TREATMENT OF MDR-TB FORMING PARTNERSHIPS TO STRENGTHEN THE GLOBAL RESPONSE TO MDR-TB - WHERE IT MATTERS MOST I am delighted that this initiative will improve both the technology needed
RAPID DIAGNOSIS AND TREATMENT OF MDR-TB FORMING PARTNERSHIPS TO STRENGTHEN THE GLOBAL RESPONSE TO MDR-TB - WHERE IT MATTERS MOST I am delighted that this initiative will improve both the technology needed
REGIONAL ACTION PLAN FOR MALARIA CONTROL AND ELIMINATION IN THE WESTERN PACIFIC ( )
 W O R L D H E A L T H ORGANIZATION ORGANISATION MONDIALE DE LA SANTE REGIONAL OFFICE FOR THE WESTERN PACIFIC BUREAU REGIONAL DU PACIFIQUE OCCIDENTAL REGIONAL COMMITTEE WPR/RC60/8 Sixtieth session 4 August
W O R L D H E A L T H ORGANIZATION ORGANISATION MONDIALE DE LA SANTE REGIONAL OFFICE FOR THE WESTERN PACIFIC BUREAU REGIONAL DU PACIFIQUE OCCIDENTAL REGIONAL COMMITTEE WPR/RC60/8 Sixtieth session 4 August
Issue 8: January December 2016 National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana
 Issue 8: January December National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana Contents Page Editorial and Report Highlights 1 Malaria Burden 2 Key Activities Undertaken in 2 Malaria
Issue 8: January December National Malaria Control Programme (NMCP) Box KB 493 Korle - Bu Accra Ghana Contents Page Editorial and Report Highlights 1 Malaria Burden 2 Key Activities Undertaken in 2 Malaria
