Lot Testing of malaria RDTs: achievements and lessons learned from a 7 year-long experience
|
|
|
- Andrea Chandler
- 6 years ago
- Views:
Transcription
1 Lot Testing of malaria RDTs: achievements and lessons learned from a 7 year-long experience Incardona S., Luchavez J., Nhem S., Barnwell J., Champouillon N., Chiodini P., Luna C., Ménard D., Meth R., Rees-Channer R., Sornillo J., Bell D., Cunningham J., González IJ. ASTMH meeting October 28 th, 205 Implementation, testing, and development of malaria diagnostics
2 WHO-FIND malaria RDT evaluation programme Manufacture -Product development -Availability of common reference standards Supply chain management CDC (USA) Stage : Product testing Evaluate product performance Stage 2: Lot testing Confirm product quality on arrival in country before distribution in the field Transport and storage IPC (Cambodia) RITM (Philippines) Stage 3: QC at point of use (positive control wells) Ensure that RDTs have maintained accuracy through transport and storage End users -Appropriate training and instructions -Management of positive and negative results -Monitoring of commodity supply and disease rates PCWs evaluated in Uganda and Lao PDR Before purchase Before distribution Before use ASTMH meeting Philadelphia October 28th, 205 2
3 Specimen bank for product and lot testing and network of partner organizations Sample type Details Product testing Lot testing Manufacturers panel Culture P. falciparum Several strains at parasites/µl and 0 parasites/µl X X Patient derived P. falciparum p/µl and 0 p/µl from Africa, Asia, South America X X Patient derived P. vivax p/µl and 0 p/µl from Asia and South America X X Negative samples ANA; rheumatoid factor/ Clean negative / Other tropical diseases X X ASTMH meeting 3
4 Product testing results Rounds 2 to 5 - P. falciparum Evaluation of 70 unique products from 8 to 203 ASTMH meeting Philadelphia October 28th, 205 4
5 Evaluation of lots of malaria RDTs Objective: To control quality of RDT lots directly after purchase (pre-shipment) or before distribution in the field (post-shipment) Methods: Two laboratories (IPC in Cambodia and IRTM in Philippines) closely supervised, with annual EQA assessments, refreshing workshops, and 6- monthly proficiency testing Formal request to FIND from procurement agencies, RDT manufacturers, national programmes Shipment and testing of RDTs with turnover of 5 days between RDTs receipt and issue of report ASTMH meeting Philadelphia October 28th, 205 4
6 Volumes and quality of lots of RDTs tested since RDT lots submitted for routine Lot Testing, RDTs submitted for Lot Testing are increasingly of high quality; this reflects adherence of major RDT procurers to WHO recommendations for RDT procurement. 800 Number of RDT lots Year 204 testing volume was equivalent to 20 million RDTs (around 66% of RDT sales) ASTMH meeting Philadelphia October 28th, 205 6
7 Users of the RDT lot testing programme 7 to 204 Lot Testing is requested early in the procurement process by the RDT manufacturers (often on behalf of procurers) or by the procurers themselves (agencies, NGOs, IOs). Countries 4% Manufacturers* 40% NGOs/IO 34% Procurement agencies 22% Lot-tested RDTs were distributed in 48 countries, with 88% in sub-saharan Africa ASTMH meeting Philadelphia October 28th, 205 7
8 Other issues identified with the observation guide Issues of buffer evaporation in single use buffer vials were observed on 57 lots from 0 products of 3 manufacturers, up until end of > Notice of concern issued by WHO, procurement of single-use RDT kits on hold, and corrective actions underway with the manufacturers. ASTMH meeting Philadelphia October 28th, 205 8
9 Transition to a decentralized sustainable lot testing of malaria RDTs Evaluation of RDTs with panels of recombinant proteins: Cheaper and simpler than sample collection Standard commercially available reference materials Cost covered by users (country budgets) to avoid dependence on donor funding Piloting lot testing in 2 countries: Capacity building in reference laboratories Testing after shipment and before distribution Confirmatory testing of lots failing in the field ASTMH meeting Philadelphia October 28th, 205 8
10 Overall structure of a global sustainable QA programme for malaria RDTs Internal QC Manufacturer QC Lab by manufacturer. PRODUCT TESTING directly overseen by central oversight agrncy (COA) Central Reference Lab 2. LOT TESTING directly overseen by MoH [+ TECHNICAL SUPPORT from COA] 3. Field QC [PCW] directly overseen by MoH [+TECHNICAL SUPPORT from COA] National Ref. Lab National Ref. Lab National Ref. Lab RDT lot failure RDT failure with PCWs ASTMH meeting Philadelphia October 28th, 205 9
11 Conclusions Quality control and quality assurance of malaria RDTs are necessary to ensure access of patients to adequate diagnosis and treatment Implementation of a QC/QA programme for malaria RDTs has positively affected the market: procurement agencies are selecting good products and manufacturers are improving their RDTs Every country using malaria RDTs should have the capacity to ensure quality of lots being deployed in their endemic settings Malaria RDTs should have adequate positive controls in order to facilitate QC after distribution and to increase confidence of endusers in RDT results ASTMH meeting Philadelphia October 28th, 205 9
12 Acknowledgments FIND Geneva: Sandra Incardona Nora Champouillon (David Bell) FIND Uganda: Daniel Kyabayinze Global Malaria Programme (WHO/GMP): Jane Cunningham Andrea Bosman Centers for Disease Control (CDC): Jeffrey Glenn John Barnwell Hospital for Tropical Diseases (HTD) in London: Roxanne Rees-Channer Peter L. Chiodini University of Queensland, Australia: Michelle Gatton Australian Army Malaria Institute: Qin Cheng Institut Pasteur du Cambodge: Sina Nhem Didier Ménard Research Institute for Tropical Medicine (RITM), Philippines: Jennifer Luchavez University of Lagos, Nigeria: Wellington Oyibo Universidad Peruana Cayetano de Heredia, Peru: Dionicia Gamboa Katherine Torres 2
13 Backup slides ASTMH meeting - Philadelphia October 28th, 205 3
14 Interactive guide for selection of RDTs ASTMH meeting Philadelphia October 28th, 205 4
15 Long term testing Initial Testing Initial QC testing (Combination tests)* Pf A P. falciparum P. vivax Pf B Pf C Pf D Pv E Pv F Pv G Pv H Negative I J K L M N O P Q R * Initial QC testing : Use 48 RDTs, and use QC samples from 4 different Pf cases (A, B, C, D), 4 different Pv cases (E, F, G, H) and 0 different malaria parasite negative cases (I-R). Long-term QC testing (Combination tests) P. falciparum P. vivax Pf Pf Pv Pv Negative A B E F Long term QC testing : Use only 8 RDTs, and use QC samples from 2 different Pf cases (A, B), 2 different Pv cases (E, F) and 2 malaria parasite negative cases (I,J) used in the initial QC testing (if possible). 5
Malaria Rapid Diagnostic Test Performance. Results of WHO product testing of malaria RDTs: round 8 ( )
 Malaria Rapid Diagnostic Test Performance Results of WHO product testing of malaria RDTs: round 8 (2016 2018) Malaria Rapid Diagnostic Test Performance Results of WHO product testing of malaria RDTs:
Malaria Rapid Diagnostic Test Performance Results of WHO product testing of malaria RDTs: round 8 (2016 2018) Malaria Rapid Diagnostic Test Performance Results of WHO product testing of malaria RDTs:
Characteristics of evaluation panel used for Round 4 of WHO Malaria RDT Product Testing at U.S. CDC,
 Programme for Research and Training in Tropical Diseases (TDR) Malaria ranch, Division of Parasitic Diseases Centers for Characteristics of evaluation panel used for Round 4 of WHO Malaria RDT Product
Programme for Research and Training in Tropical Diseases (TDR) Malaria ranch, Division of Parasitic Diseases Centers for Characteristics of evaluation panel used for Round 4 of WHO Malaria RDT Product
Characteristics of evaluation panel used for Round 2 of WHO malaria RDT product testing at U.S. CDC, 2009 WHO-FIND malaria RDT evaluation programme
 (FIND) Special Programme for Research and Training in Tropical Diseases (TDR) Malaria ranch, Division of Parasitic Characteristics of evaluation panel used for Round 2 of WHO malaria RDT product testing
(FIND) Special Programme for Research and Training in Tropical Diseases (TDR) Malaria ranch, Division of Parasitic Characteristics of evaluation panel used for Round 2 of WHO malaria RDT product testing
Malaria Rapid Diagnostic Test Performance. Results of WHO product testing of malaria RDTs: round 7 ( )
 Malaria Rapid Diagnostic Test Performance Results of WHO product testing of malaria RDTs: round 7 (2015 2016) Malaria Rapid Diagnostic Test Performance Results of WHO product testing of malaria RDTs:
Malaria Rapid Diagnostic Test Performance Results of WHO product testing of malaria RDTs: round 7 (2015 2016) Malaria Rapid Diagnostic Test Performance Results of WHO product testing of malaria RDTs:
Initiative for Quality Assurance of Malaria Rapid Diagnostic Tests
 Initiative for Quality Assurance of Malaria Rapid Diagnostic Tests Outline of product testing and associated protocols World Health Organization (WHO) Regional Office for the Western Pacific UNICEF / UNDP
Initiative for Quality Assurance of Malaria Rapid Diagnostic Tests Outline of product testing and associated protocols World Health Organization (WHO) Regional Office for the Western Pacific UNICEF / UNDP
Downloaded from:
 Gatton, ML; Ciketic, S; Barnwell, JW; Cheng, Q; Chiodini, PL; Incardona, S; Bell, D; Cunningham, J; Gonzlez, IJ (2018) An assessment of false positive rates for malaria rapid diagnostic tests caused by
Gatton, ML; Ciketic, S; Barnwell, JW; Cheng, Q; Chiodini, PL; Incardona, S; Bell, D; Cunningham, J; Gonzlez, IJ (2018) An assessment of false positive rates for malaria rapid diagnostic tests caused by
Testing for G6PD deficiency for safe use of primaquine in radical cure of P. vivax and P. ovale
 Testing for G6PD deficiency for safe use of primaquine in radical cure of P. vivax and P. ovale Webinar presentation to support the dissemination of the policy brief Silvia Schwarte, WHO/GMP e-mail: schwartes@who.int
Testing for G6PD deficiency for safe use of primaquine in radical cure of P. vivax and P. ovale Webinar presentation to support the dissemination of the policy brief Silvia Schwarte, WHO/GMP e-mail: schwartes@who.int
Proposed WHO Technical Consultation on external competence assessment for malaria microscopy
 Proposed WHO Technical Consultation on external competence assessment for malaria microscopy Malaria Policy Advisory Committee Meeting 17-19 October 2018, Executive Board meeting room World Health Organization,
Proposed WHO Technical Consultation on external competence assessment for malaria microscopy Malaria Policy Advisory Committee Meeting 17-19 October 2018, Executive Board meeting room World Health Organization,
Malaria Rapid Diagnostic Test Performance. Summary results of WHO product testing of malaria RDTs: round 1-8 ( )
 Malaria Rapid Diagnostic Test Performance Summary results of WHO product testing of malaria RDTs: round 1-8 (2008 2018) Malaria Rapid Diagnostic Test Performance Summary results of WHO product testing
Malaria Rapid Diagnostic Test Performance Summary results of WHO product testing of malaria RDTs: round 1-8 (2008 2018) Malaria Rapid Diagnostic Test Performance Summary results of WHO product testing
Malaria Rapid Diagnostic Test Performance. Summary results of WHO product testing of malaria RDTs: round 1-7 ( )
 Malaria Rapid Diagnostic Test Performance Summary results of WHO product testing of malaria RDTs: round 1-7 (2008 2016) Malaria Rapid Diagnostic Test Performance Summary results of WHO product testing
Malaria Rapid Diagnostic Test Performance Summary results of WHO product testing of malaria RDTs: round 1-7 (2008 2016) Malaria Rapid Diagnostic Test Performance Summary results of WHO product testing
Rapid diagnostic tests failing to detect Plasmodium falciparum infections in Eritrea: an investigation of reported false negative RDT results
 DOI 10.1186/s12936-017-1752-9 Malaria Journal RESEARCH Open Access Rapid diagnostic tests failing to detect Plasmodium falciparum infections in Eritrea: an investigation of reported false negative RDT
DOI 10.1186/s12936-017-1752-9 Malaria Journal RESEARCH Open Access Rapid diagnostic tests failing to detect Plasmodium falciparum infections in Eritrea: an investigation of reported false negative RDT
Molecular Profile and Survey of HRP2 and HRP3 Genetic Deletions in South America: An Update
 Molecular Profile and Survey of HRP2 and HRP3 Genetic Deletions in South America: An Update John W. Barnwell and Kumar Udhayakumar Malaria Research & Development Laboratory Unit Malaria Branch, DPDM, CGH
Molecular Profile and Survey of HRP2 and HRP3 Genetic Deletions in South America: An Update John W. Barnwell and Kumar Udhayakumar Malaria Research & Development Laboratory Unit Malaria Branch, DPDM, CGH
WHO Prequalification of In Vitro Diagnostics Programme
 WHO Prequalification of In Vitro Diagnostics Programme International HIV/Viral Hepatitis Co-Infection Satellite Meeting 19 July 2014, Melbourne Anita Sands Prequalification Diagnostics Team Department
WHO Prequalification of In Vitro Diagnostics Programme International HIV/Viral Hepatitis Co-Infection Satellite Meeting 19 July 2014, Melbourne Anita Sands Prequalification Diagnostics Team Department
Targeting HIV Settings Through PEPFAR. Bill Coggin, OGAC PEPFAR Laboratory Technical Working Group
 Targeting HIV Settings Through PEPFAR Bill Coggin, OGAC PEPFAR Laboratory Technical Working Group PEPFAR Laboratory Program is a Critical part of Health Systems Strengthening Mission: To support countries
Targeting HIV Settings Through PEPFAR Bill Coggin, OGAC PEPFAR Laboratory Technical Working Group PEPFAR Laboratory Program is a Critical part of Health Systems Strengthening Mission: To support countries
Vaccine Banks. Susanne Munstermann, OIE Scientific and Technical Department
 Vaccine Banks Susanne Munstermann, OIE Scientific and Technical Department Rift Valley fever: New Options for Trade, Prevention and Control Djibouti, 21 23 April 2015 Establishing a Regional Vaccine Bank
Vaccine Banks Susanne Munstermann, OIE Scientific and Technical Department Rift Valley fever: New Options for Trade, Prevention and Control Djibouti, 21 23 April 2015 Establishing a Regional Vaccine Bank
Summary World Malaria Report 2010
 Summary The summarizes information received from 106 malaria-endemic countries and other partners and updates the analyses presented in the 2009 Report. It highlights continued progress made towards meeting
Summary The summarizes information received from 106 malaria-endemic countries and other partners and updates the analyses presented in the 2009 Report. It highlights continued progress made towards meeting
GREATER MEKONG SUB-REGION ELIMINATION OF MALARIA (GEMS) PRIVATE SECTOR MALARIA SURVEILLANCE
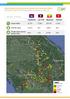 GREATER MEKONG SUB-REGION ELIMINATION OF MALARIA (GEMS) PRIVATE SECTOR MALARIA SURVEILLANCE JANUARY JUNE 218 Cambodia Lao PDR Myanmar Vietnam Cases tested 51,71 17,162 291,317 4,334 Positive cases 6,43
GREATER MEKONG SUB-REGION ELIMINATION OF MALARIA (GEMS) PRIVATE SECTOR MALARIA SURVEILLANCE JANUARY JUNE 218 Cambodia Lao PDR Myanmar Vietnam Cases tested 51,71 17,162 291,317 4,334 Positive cases 6,43
26/06/ NIMR 2018 Conference - Malaria - a reality
 Malaria Elimination: Reality or Myth? Wellington A. Oyibo ANDI CENTRE OF EXCELLENCE FOR MALARIA DIAGNOSIS WHO/FIND Malaria Specimen Collection Site The International Center for Malaria Microscopy and Malaria
Malaria Elimination: Reality or Myth? Wellington A. Oyibo ANDI CENTRE OF EXCELLENCE FOR MALARIA DIAGNOSIS WHO/FIND Malaria Specimen Collection Site The International Center for Malaria Microscopy and Malaria
HUMASIS MALARIA ANTIGEN TEST HIGH SENSITIVE DIFFERENTIAL DIAGNOSIS OF MALARIA INFECTION
 HUMASIS MALARIA ANTIGEN TEST HIGH SENSITIVE DIFFERENTIAL DIAGNOSIS OF MALARIA INFECTION References 1) World Malaria Report 2010, WHO 2) Rapid diagnostic tests for malaria parasites, Clin. Microbiol. Rev.
HUMASIS MALARIA ANTIGEN TEST HIGH SENSITIVE DIFFERENTIAL DIAGNOSIS OF MALARIA INFECTION References 1) World Malaria Report 2010, WHO 2) Rapid diagnostic tests for malaria parasites, Clin. Microbiol. Rev.
VOLUNTARY MEDICAL MALE CIRCUMCISION KITS THE JOURNEY SO FAR AND THE PATH AHEAD
 1 VOLUNTARY MEDICAL MALE CIRCUMCISION KITS THE JOURNEY SO FAR AND THE PATH AHEAD SESSION GOAL To provide an update of the progress, targets, and future trends in USG VMMC programs and explore ways to improve
1 VOLUNTARY MEDICAL MALE CIRCUMCISION KITS THE JOURNEY SO FAR AND THE PATH AHEAD SESSION GOAL To provide an update of the progress, targets, and future trends in USG VMMC programs and explore ways to improve
Rice Fortification: Making Rice More Nutritious Post Harvesting
 Rice Fortification: Making Rice More Nutritious Post Harvesting GRAPAS Asia Conference in Bangkok, April 8 th 2014 Judith Smit Rice Fortification Manager WFP Regional Bureau for Asia World Food Programme:
Rice Fortification: Making Rice More Nutritious Post Harvesting GRAPAS Asia Conference in Bangkok, April 8 th 2014 Judith Smit Rice Fortification Manager WFP Regional Bureau for Asia World Food Programme:
Malaria Rapid Diagnostic Tests: role and place in the diagnosis of malaria
 Malaria Rapid Diagnostic Tests: role and place in the diagnosis of malaria 1 Plasmodium falciparum Malaria : an overview 2 Plasmodium vivax 3 Plasmodium ovale Most serious Jan Jacobs Institute of Tropical
Malaria Rapid Diagnostic Tests: role and place in the diagnosis of malaria 1 Plasmodium falciparum Malaria : an overview 2 Plasmodium vivax 3 Plasmodium ovale Most serious Jan Jacobs Institute of Tropical
Proposal for a Workshop
 Proposal for a Workshop Pre-vaccination screening for the use of dengue vaccines with differential performance dependent on serostatus: rapid diagnostic tests and implementation strategies Background:
Proposal for a Workshop Pre-vaccination screening for the use of dengue vaccines with differential performance dependent on serostatus: rapid diagnostic tests and implementation strategies Background:
TECHNICAL REPORT SECOND SLIDE PANEL EXTERNAL QUALITY ASSURANCE PROGRAM FOR MALARIA MICROSCOPY DIAGNOSIS
 TECHNICAL REPORT SECOND SLIDE PANEL 2012-2013 EXTERNAL QUALITY ASSURANCE PROGRAM FOR MALARIA MICROSCOPY DIAGNOSIS REGIONAL MALARIA PROGRAM NEGLECTED, TROPICAL AND VECTOR-BORNE DISEASES COMMUNICABLE DISEASES
TECHNICAL REPORT SECOND SLIDE PANEL 2012-2013 EXTERNAL QUALITY ASSURANCE PROGRAM FOR MALARIA MICROSCOPY DIAGNOSIS REGIONAL MALARIA PROGRAM NEGLECTED, TROPICAL AND VECTOR-BORNE DISEASES COMMUNICABLE DISEASES
HIV Testing and Systems for Managing HIV Implementation in Resource Limited Settings
 HIV Testing and Systems for Managing HIV Implementation in Resource Limited Settings Elizabeth M. Dax with the staff of the National Serology Reference Laboratory, Australia My Background Worked in HIV
HIV Testing and Systems for Managing HIV Implementation in Resource Limited Settings Elizabeth M. Dax with the staff of the National Serology Reference Laboratory, Australia My Background Worked in HIV
UNITAID. Dr Philippe Duneton Deputy Executive Director Copenhagen September 2012
 UNITAID Dr Philippe Duneton Deputy Executive Director Copenhagen September 2012 Challenges Achievements WHO Prequalification UNITAID support for prequalification of medicines Since 2007 UNITAID support
UNITAID Dr Philippe Duneton Deputy Executive Director Copenhagen September 2012 Challenges Achievements WHO Prequalification UNITAID support for prequalification of medicines Since 2007 UNITAID support
Quality Assurance Policy. for the. Procurement of HIV Point-of-Care Technology. under the UNITAID Grant
 Quality Assurance Policy for the Procurement of HIV Point-of-Care Technology under the UNITAID Grant UNICEF Supply Division Quality Assurance Centre and Health Technology Centre Revisions Version Date
Quality Assurance Policy for the Procurement of HIV Point-of-Care Technology under the UNITAID Grant UNICEF Supply Division Quality Assurance Centre and Health Technology Centre Revisions Version Date
Global Fund Quality Assurance Policy for Diagnostics products : collaboration with WHO DLT
 Global Fund Quality Assurance Policy for Diagnostics products : collaboration with WHO DLT Pharmaceutical Management Unit Quality Assurance and Data Management Team QA for health products financed by Global
Global Fund Quality Assurance Policy for Diagnostics products : collaboration with WHO DLT Pharmaceutical Management Unit Quality Assurance and Data Management Team QA for health products financed by Global
Spotting Malaria reliably
 Spotting Malaria reliably Track down infections easily with highly sensitive Malaria-LAMP even in low-prevalent settings Molecular DX Microscopy and RDT s are not able to track down parasites in low-transmission
Spotting Malaria reliably Track down infections easily with highly sensitive Malaria-LAMP even in low-prevalent settings Molecular DX Microscopy and RDT s are not able to track down parasites in low-transmission
T he diagnosis of malaria in clinical laboratories in the UK
 862 ORIGINAL ARTICLE Use of rapid diagnostic tests for diagnosis of malaria in the UK D Chilton, A N J Malik, M Armstrong, M Kettelhut, J Parker-Williams, P L Chiodini... See end of article for authors
862 ORIGINAL ARTICLE Use of rapid diagnostic tests for diagnosis of malaria in the UK D Chilton, A N J Malik, M Armstrong, M Kettelhut, J Parker-Williams, P L Chiodini... See end of article for authors
Procurement policies and requirements
 Procurement policies and requirements JOINT UNICEF, UNFPA & WHO meeting with manufacturers, Copenhagen 22-24 Sep 2014 ELODIE JAMBERT International Pharmacist Coordinator Médecins Sans Frontières (MSF)
Procurement policies and requirements JOINT UNICEF, UNFPA & WHO meeting with manufacturers, Copenhagen 22-24 Sep 2014 ELODIE JAMBERT International Pharmacist Coordinator Médecins Sans Frontières (MSF)
The impact of SLMTA on Proficiency Testing (PT)/ External Quality Assessment (EQA) participation and performance
 The impact of SLMTA on Proficiency Testing (PT)/ External Quality Assessment (EQA) participation and performance SLMTA Symposium, ASLM Conference 2016, 3 rd to 4 th December 2016 Presentation Outline Introduction
The impact of SLMTA on Proficiency Testing (PT)/ External Quality Assessment (EQA) participation and performance SLMTA Symposium, ASLM Conference 2016, 3 rd to 4 th December 2016 Presentation Outline Introduction
Health Disparities: Time for Action
 Health Disparities: Time for Action Patricia J. García MD MPH PhD Former Minister of Health, Peru Professor, School of Public Health Cayetano Heredia University- Lima-Peru Latin America 19% more unequal
Health Disparities: Time for Action Patricia J. García MD MPH PhD Former Minister of Health, Peru Professor, School of Public Health Cayetano Heredia University- Lima-Peru Latin America 19% more unequal
Global Fund Financing of Contraceptives for Reproductive Health Commodity Security
 Global Fund Financing of Contraceptives for Reproductive Health Commodity Security International Conference on Family Planning Kampala, Uganda November 15-18, 2009 Dr. Fidele NGABO Dr NDAHINYUKA Jovith
Global Fund Financing of Contraceptives for Reproductive Health Commodity Security International Conference on Family Planning Kampala, Uganda November 15-18, 2009 Dr. Fidele NGABO Dr NDAHINYUKA Jovith
Repellent Soap. The Jojoo Mosquito. Africa s innovative solution to Malaria prevention. Sapphire Trading Company Ltd
 The Jojoo Mosquito Repellent Soap Africa s innovative solution to Malaria prevention Sapphire Trading Company Ltd P.O.Box: 45938-00100 Nairobi, Kenya. Tel: +254 735 397 267 +254 733 540 868 +254 700 550
The Jojoo Mosquito Repellent Soap Africa s innovative solution to Malaria prevention Sapphire Trading Company Ltd P.O.Box: 45938-00100 Nairobi, Kenya. Tel: +254 735 397 267 +254 733 540 868 +254 700 550
Dried Plasmodium falciparum-infected samples as positive controls for malaria rapid diagnostic tests
 Aidoo et al. Malaria Journal 2012, 11:239 METHODOLOGY Open Access Dried Plasmodium falciparum-infected samples as positive controls for malaria rapid diagnostic tests Michael Aidoo *, Jaymin C Patel and
Aidoo et al. Malaria Journal 2012, 11:239 METHODOLOGY Open Access Dried Plasmodium falciparum-infected samples as positive controls for malaria rapid diagnostic tests Michael Aidoo *, Jaymin C Patel and
Essential Medicines. WHO
 12 Health Technology and Pharmaceuticals Participants to the Workshop on Pharmaceutical Policies and Access to Good Quality Essential Medicines for Pacific Island Countries observing pharmaceutical services
12 Health Technology and Pharmaceuticals Participants to the Workshop on Pharmaceutical Policies and Access to Good Quality Essential Medicines for Pacific Island Countries observing pharmaceutical services
How best to structure a laboratory network with new technologies
 How best to structure a laboratory network with new technologies Cristina Gutierrez, MD, PhD Uniting to scale up TB care in Central Asia 14 and 15 April 2011 Tashkent, Uzbekistan New laboratory diagnostics
How best to structure a laboratory network with new technologies Cristina Gutierrez, MD, PhD Uniting to scale up TB care in Central Asia 14 and 15 April 2011 Tashkent, Uzbekistan New laboratory diagnostics
Supporting Accelerate to Zero: The BMGF Malaria Vector Control
 Supporting Accelerate to Zero: The BMGF Malaria Vector Control Research Agenda NCAR-CDC Climate and Health Workshop Boulder CO 14 Jul 2015 Mike Reddy PhD MPH Dan Strickman MS PhD Malaria, (Prevent Transmission
Supporting Accelerate to Zero: The BMGF Malaria Vector Control Research Agenda NCAR-CDC Climate and Health Workshop Boulder CO 14 Jul 2015 Mike Reddy PhD MPH Dan Strickman MS PhD Malaria, (Prevent Transmission
10 Years of Laboratory Capacity Building. September 6-11 th, 2015 Les Pensieres, Veyrier-du-Lac, France
 10 Years of Laboratory Capacity Building 6 th Advanced Course on Diagnostics September 6-11 th, 2015 Les Pensieres, Veyrier-du-Lac, France John N. Nkengasong, Ph.D Associate Director for Laboratory & Chief,
10 Years of Laboratory Capacity Building 6 th Advanced Course on Diagnostics September 6-11 th, 2015 Les Pensieres, Veyrier-du-Lac, France John N. Nkengasong, Ph.D Associate Director for Laboratory & Chief,
APHL Global Health TB-related Activities
 APHL Global Health TB-related Activities 5th National TB Conference 11-13 August 2008 San Diego, CA Travis Jobe APHL Global Health Projects Haiti Strategic planning TA & training Barbados WHO Twinning
APHL Global Health TB-related Activities 5th National TB Conference 11-13 August 2008 San Diego, CA Travis Jobe APHL Global Health Projects Haiti Strategic planning TA & training Barbados WHO Twinning
Malaria in London: Review of imported malaria cases. Data from 2000 to 2012
 : Review of imported malaria cases Data from 2000 to 2012 About Public Health England We work with national and local government, industry and the NHS to protect and improve the nation's health and support
: Review of imported malaria cases Data from 2000 to 2012 About Public Health England We work with national and local government, industry and the NHS to protect and improve the nation's health and support
Good practices for selecting and procuring
 WHO Global Malaria Programme Good practices for selecting and procuring rapid diagnostic tests for malaria Good practices for selecting and procuring rapid diagnostic tests for malaria Good practices for
WHO Global Malaria Programme Good practices for selecting and procuring rapid diagnostic tests for malaria Good practices for selecting and procuring rapid diagnostic tests for malaria Good practices for
Aboubacar Kampo Chief of Health UNICEF Nigeria
 Aboubacar Kampo Chief of Health UNICEF Nigeria Many thanks to UNICEF colleagues in Supply Division-Copenhagen and NY for contributing to this presentation Thirty-five countries are responsible for 98%
Aboubacar Kampo Chief of Health UNICEF Nigeria Many thanks to UNICEF colleagues in Supply Division-Copenhagen and NY for contributing to this presentation Thirty-five countries are responsible for 98%
GLOBAL TB IMPACT MEASUREMENT
 GLOBAL TB IMPACT MEASUREMENT What is it? Why is it important? How can it be done? What will it cost? 1. What is global TB impact measurement and why is it important? Global TB Impact Measurement is the
GLOBAL TB IMPACT MEASUREMENT What is it? Why is it important? How can it be done? What will it cost? 1. What is global TB impact measurement and why is it important? Global TB Impact Measurement is the
Workshop on Proficiency Testing for Medical Laboratories. Daniel W. Tholen, M.S. March 13, 2013
 Workshop on Proficiency Testing for Medical Laboratories Daniel W. Tholen, M.S. March 13, 2013 Workshop Schedule It s a Workshop! not a lecture Discussion expected My background in PT for medical laboratories
Workshop on Proficiency Testing for Medical Laboratories Daniel W. Tholen, M.S. March 13, 2013 Workshop Schedule It s a Workshop! not a lecture Discussion expected My background in PT for medical laboratories
Projected Demand for HIV Diagnostic Tests
 Projected Demand for HIV Diagnostic Tests John Stover, Yu Teng, Adebiyi Adesina, Eline Korenromp On behalf of the Diagnostics Forecasting Technical Working Group (CDC, CHAI, GFATM, PfSCM, UNAIDS, UNICEF,
Projected Demand for HIV Diagnostic Tests John Stover, Yu Teng, Adebiyi Adesina, Eline Korenromp On behalf of the Diagnostics Forecasting Technical Working Group (CDC, CHAI, GFATM, PfSCM, UNAIDS, UNICEF,
The Three Millennium Development Goal Fund A guide for the selection of Malaria Rapid Diagnostic Test kits purchased with 3MDG grants
 The Three Millennium Development Goal Fund A guide for the selection of Malaria Rapid Diagnostic Test kits purchased with 3MDG grants Version 4.0 February 2014 1. Contents A GUIDE FOR THE SELECTION OF
The Three Millennium Development Goal Fund A guide for the selection of Malaria Rapid Diagnostic Test kits purchased with 3MDG grants Version 4.0 February 2014 1. Contents A GUIDE FOR THE SELECTION OF
Balancing investment in point of care diagnostics versus laboratory testing in low resource settings. June 28, 2011
 Balancing investment in point of care diagnostics versus laboratory testing in low resource settings Workshop on TB and HIV Diagnostics Workshop on TB and HIV Diagnostics June 28, 2011 Complexity Delivery
Balancing investment in point of care diagnostics versus laboratory testing in low resource settings Workshop on TB and HIV Diagnostics Workshop on TB and HIV Diagnostics June 28, 2011 Complexity Delivery
Report of the Executive Director
 Report of the Executive Director Context for the 19 th Board meeting Financial and economic crisis 2008 Ambitious demand and disbursement targets met Strong results and progress towards MDGs ( Green Report
Report of the Executive Director Context for the 19 th Board meeting Financial and economic crisis 2008 Ambitious demand and disbursement targets met Strong results and progress towards MDGs ( Green Report
The Pediatric Dengue Vaccine Initiative (PDVI) Thailand, 2007
 The Pediatric Dengue Vaccine Initiative (PDVI) Thailand, 2007 The Vaccine Enterprise-the the disease Clinical data Surveillance data Laboratory data/diagnostics Input, analysis and reporting The Vaccine
The Pediatric Dengue Vaccine Initiative (PDVI) Thailand, 2007 The Vaccine Enterprise-the the disease Clinical data Surveillance data Laboratory data/diagnostics Input, analysis and reporting The Vaccine
Microscopy Studies. Evaluation Study design
 Ensuring quality in diagnostic trials: Microscopy Studies C N Paramasivan Evaluation Study design Evaluation of Clinical Performance*: Sensitivity: Direct & conc. smears in culture pos. cases compared
Ensuring quality in diagnostic trials: Microscopy Studies C N Paramasivan Evaluation Study design Evaluation of Clinical Performance*: Sensitivity: Direct & conc. smears in culture pos. cases compared
CRS Report for Congress
 Order Code RL33485 CRS Report for Congress Received through the CRS Web U.S. International HIV/AIDS, Tuberculosis, and Malaria Spending: FY2004-FY2008 Updated September 11, 2007 Tiaji Salaam-Blyther Specialist
Order Code RL33485 CRS Report for Congress Received through the CRS Web U.S. International HIV/AIDS, Tuberculosis, and Malaria Spending: FY2004-FY2008 Updated September 11, 2007 Tiaji Salaam-Blyther Specialist
Why is Medicine Quality Important?
 Why is Medicine Quality Important? March 3, 2015 Boston University School of Public Health Pharmaceutical Track Spring Symposium Laura Krech, MPH Consultant, based in Boston Promoting the Quality of Medicines
Why is Medicine Quality Important? March 3, 2015 Boston University School of Public Health Pharmaceutical Track Spring Symposium Laura Krech, MPH Consultant, based in Boston Promoting the Quality of Medicines
Strengthening Veterinary Services in Asia
 Strengthening Veterinary Services in Asia 4 th Steering Committee Highly Pathogenic Emerging and Re-Emerging Diseases (HPED) Programme Session 3 Progress on the OIE component Tokyo (Japan) 16 July 2013
Strengthening Veterinary Services in Asia 4 th Steering Committee Highly Pathogenic Emerging and Re-Emerging Diseases (HPED) Programme Session 3 Progress on the OIE component Tokyo (Japan) 16 July 2013
World Health Organization Global Fund concept note development WHO POLICY BRIEF
 World Health Organization 2014 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization,
World Health Organization 2014 All rights reserved. Publications of the World Health Organization are available on the WHO website (www.who.int) or can be purchased from WHO Press, World Health Organization,
Global call for action to ensure universal access to malaria diagnosis and treatment
 Global call for action to ensure universal access to malaria diagnosis and treatment Joint activity of SEE Team and PDT Unit Andrea Bosman and Richard Cibulskis Outline of the presentation Background Objectives
Global call for action to ensure universal access to malaria diagnosis and treatment Joint activity of SEE Team and PDT Unit Andrea Bosman and Richard Cibulskis Outline of the presentation Background Objectives
Working for an International Organization in Public-Private Partnership : The Global Fund to Fight AIDS, Tuberculosis and Malaria
 Working for an International Organization in Public-Private Partnership : The Global Fund to Fight AIDS, Tuberculosis and Malaria Birgit Poniatowski Acting Manager, Board Relations Overview The Global
Working for an International Organization in Public-Private Partnership : The Global Fund to Fight AIDS, Tuberculosis and Malaria Birgit Poniatowski Acting Manager, Board Relations Overview The Global
OIE Regional initiatives for rabies control
 OIE Regional initiatives for rabies control OIE Regional Commission for Asia, the Far East and Oceania Cebu, Philippines, 18-22/11/2013 Dr Agnes POIRIER Dr Mary-Joy GORDONCILLO OIE SRR-SEA OUTLINE OIE
OIE Regional initiatives for rabies control OIE Regional Commission for Asia, the Far East and Oceania Cebu, Philippines, 18-22/11/2013 Dr Agnes POIRIER Dr Mary-Joy GORDONCILLO OIE SRR-SEA OUTLINE OIE
Safe Injection Equipment
 Safe Injection Equipment Industry Consultation 28 th 29 th March 2017 Overview of UNICEF procurement of Safe Injection Equipment Rob Matthews, HTC Objective of Session Provide an overview of UNICEFs engagement
Safe Injection Equipment Industry Consultation 28 th 29 th March 2017 Overview of UNICEF procurement of Safe Injection Equipment Rob Matthews, HTC Objective of Session Provide an overview of UNICEFs engagement
Fighting Harder and Smarter Against Malaria. Dr.Bernard Nahlen Deputy US Global Malaria Coordinator University of Georgia, February 23, 2010
 Fighting Harder and Smarter Against Malaria Dr.Bernard Nahlen Deputy US Global Malaria Coordinator University of Georgia, February 23, 2010 Outline Burden of malaria Global support for rolling back malaria
Fighting Harder and Smarter Against Malaria Dr.Bernard Nahlen Deputy US Global Malaria Coordinator University of Georgia, February 23, 2010 Outline Burden of malaria Global support for rolling back malaria
UNITAID investments to innovate and scale up access to HIV diagnostics
 UNITAID investments to innovate and scale up access to HIV diagnostics WHO Annual meeting with Diagnostic Manufacturers and Stakeholders 10 March 2016 Smiljka de Lussigny (HIV Diagnostics Programme Manager,
UNITAID investments to innovate and scale up access to HIV diagnostics WHO Annual meeting with Diagnostic Manufacturers and Stakeholders 10 March 2016 Smiljka de Lussigny (HIV Diagnostics Programme Manager,
Rapid Diagnostics CHAI Experience. 6 th Moving Forward in Diagnostics Forum Les Pensieres November 7, 2012
 Rapid Diagnostics CHAI Experience 6 th Moving Forward in Diagnostics Forum Les Pensieres November 7, 2012 CHAI is working in 12 countries on HIV POC test implementation Kenya Tanzania Ethiopia Malawi Mozambique
Rapid Diagnostics CHAI Experience 6 th Moving Forward in Diagnostics Forum Les Pensieres November 7, 2012 CHAI is working in 12 countries on HIV POC test implementation Kenya Tanzania Ethiopia Malawi Mozambique
MALARIA P. FALCIPARUM / P. VIVAX
 MALARIA P. FALCIPARUM / P. VIVAX 1. EXPLANATION OF THE TEST: Malaria is a serious, sometimes fatal, parasitic disease characterized by fever, chills, and anemia and is caused by a parasite that is transmitted
MALARIA P. FALCIPARUM / P. VIVAX 1. EXPLANATION OF THE TEST: Malaria is a serious, sometimes fatal, parasitic disease characterized by fever, chills, and anemia and is caused by a parasite that is transmitted
Cross-cutting HSS (Health Systems Strengthening): Experience from WPRO
 Cross-cutting HSS (Health Systems Strengthening): Experience from WPRO Momoe Takeuchi WHO WPRO/Health Services Development Kunhee Park WHO Lao PDR/MCH INTEGRATING RMNCH CONSULTANTS TRAINING FOR GLOBAL
Cross-cutting HSS (Health Systems Strengthening): Experience from WPRO Momoe Takeuchi WHO WPRO/Health Services Development Kunhee Park WHO Lao PDR/MCH INTEGRATING RMNCH CONSULTANTS TRAINING FOR GLOBAL
BD/PEPFAR Laboratory Strengthening Program IOM MEETING 13 APRIL 2010 PRETORIA SOUTH AFRICA DANNI RAMDUTH
 BD/PEPFAR Laboratory Strengthening Program IOM MEETING 13 APRIL 2010 PRETORIA SOUTH AFRICA DANNI RAMDUTH Background Global CD4 GLP (Good Laboratory Practices) Training Volunteer Service Trip Program Public
BD/PEPFAR Laboratory Strengthening Program IOM MEETING 13 APRIL 2010 PRETORIA SOUTH AFRICA DANNI RAMDUTH Background Global CD4 GLP (Good Laboratory Practices) Training Volunteer Service Trip Program Public
TB Disease Prevalence Survey - Progress Report
 TB Disease Prevalence Survey - Progress Report 30 Oct 2011, Lille Ikushi Onozaki MD, MPH Team Leader, TB Prevalence Survey WHO Global Task Force on TB Impact Measurement Stop TB Department, WHO onozakii@who.int
TB Disease Prevalence Survey - Progress Report 30 Oct 2011, Lille Ikushi Onozaki MD, MPH Team Leader, TB Prevalence Survey WHO Global Task Force on TB Impact Measurement Stop TB Department, WHO onozakii@who.int
The Laboratories Role in Global Health
 The Laboratories Role in Global Health Larry Westerman, Ph.D. International Laboratory Branch Division of Global HIV/AIDS Centers for Disease Control and Prevention Center for Global Health International
The Laboratories Role in Global Health Larry Westerman, Ph.D. International Laboratory Branch Division of Global HIV/AIDS Centers for Disease Control and Prevention Center for Global Health International
Comparison of light microscopy and nested PCR assay in detecting of malaria mixed species infections in an endemic area of Iran
 Comparison of light microscopy and nested PCR assay in detecting of malaria mixed species infections in an endemic area of Iran Aliehsan Heidari, Manizheh Nourian, Hossein Keshavarz Associate Prof. Dept.
Comparison of light microscopy and nested PCR assay in detecting of malaria mixed species infections in an endemic area of Iran Aliehsan Heidari, Manizheh Nourian, Hossein Keshavarz Associate Prof. Dept.
MALARIA PARASITE COUNTING
 VERSION 1 EFFECTIVE DATE: 01/01/2016 MALARIA PARASITE COUNTING MALARIA MICROSCOPY STANDARD OPERATING PROCEDURE MM-SOP-09 1. PURPOSE AND SCOPE To describe the procedure for counting malaria parasites on
VERSION 1 EFFECTIVE DATE: 01/01/2016 MALARIA PARASITE COUNTING MALARIA MICROSCOPY STANDARD OPERATING PROCEDURE MM-SOP-09 1. PURPOSE AND SCOPE To describe the procedure for counting malaria parasites on
World Health Organization Emerging and other Communicable Diseases, Surveillance and Control
 WHO/EMC/ DIS/ICG/97.8 Response to epidemic meningitis in Africa, 1997. Report by IFRC-MSF-UNICEF-WHO to the International Coordinating Group (ICG) World Health Organization Emerging and other Communicable
WHO/EMC/ DIS/ICG/97.8 Response to epidemic meningitis in Africa, 1997. Report by IFRC-MSF-UNICEF-WHO to the International Coordinating Group (ICG) World Health Organization Emerging and other Communicable
GUATEMALA S FAMILY PLANNING TRANSITION Successes, Challenges, and Lessons Learned for Transitioning Countries
 GUATEMALA S FAMILY PLANNING TRANSITION Successes, Challenges, and Lessons Learned for Transitioning Countries ATTAINING SUSTAINABLE FINANCING FOR FAMILY PLANNING IN SUB-SAHARAN AFRICA ACCRA, JANUARY 2018
GUATEMALA S FAMILY PLANNING TRANSITION Successes, Challenges, and Lessons Learned for Transitioning Countries ATTAINING SUSTAINABLE FINANCING FOR FAMILY PLANNING IN SUB-SAHARAN AFRICA ACCRA, JANUARY 2018
Target Product Profile: Point-of-Care Malaria Plasmodium falciparum Highly Sensitive Rapid Diagnostic Test
 1 Target Product Profile: Point-of-Care Malaria Plasmodium falciparum Highly Sensitive Rapid Diagnostic Test For rapid detection of low-density, malaria infections Updated November 2015 2 Context Defining
1 Target Product Profile: Point-of-Care Malaria Plasmodium falciparum Highly Sensitive Rapid Diagnostic Test For rapid detection of low-density, malaria infections Updated November 2015 2 Context Defining
Past, present & future for HIV self-testing
 Past, present & future for HIV self-testing Cheryl Johnson WHO HIV Dept. Geneva 19 July 2014 No conflict of interest Why talk about HIVST? UNAIDS 90-90-90 10% 5% 49% 90% 95% 51% Current coverage 2020 Goal
Past, present & future for HIV self-testing Cheryl Johnson WHO HIV Dept. Geneva 19 July 2014 No conflict of interest Why talk about HIVST? UNAIDS 90-90-90 10% 5% 49% 90% 95% 51% Current coverage 2020 Goal
IAEA Support to Medical Physics in Africa and Latin America: achievements & challenges
 IAEA Support to Medical Physics in Africa and Latin America: achievements & challenges Ahmed Meghzifene Head, Dosimetry & Medical Radiation Physics Presentation objectives To learn about: 1. IAEA activities
IAEA Support to Medical Physics in Africa and Latin America: achievements & challenges Ahmed Meghzifene Head, Dosimetry & Medical Radiation Physics Presentation objectives To learn about: 1. IAEA activities
Common EQA Mistakes - A provider Perspective. Experience from East African Regional External Quality Assessment Scheme (EA-REQAS)
 Common EQA Mistakes - A provider Perspective Experience from East African Regional External Quality Assessment Scheme (EA-REQAS) Overview of Amref Health Africa Leading health development INGO in Africa
Common EQA Mistakes - A provider Perspective Experience from East African Regional External Quality Assessment Scheme (EA-REQAS) Overview of Amref Health Africa Leading health development INGO in Africa
TB Disease Prevalence Survey - Overview, why and how
 TB Disease Prevalence Survey - Overview, why and how Ikushi Onozaki MD, MPH Team Leader, TB Prevalence Survey WHO Global Task Force on TB Impact Measurement Stop TB Department, WHO onozakii@who.int 3 strategic
TB Disease Prevalence Survey - Overview, why and how Ikushi Onozaki MD, MPH Team Leader, TB Prevalence Survey WHO Global Task Force on TB Impact Measurement Stop TB Department, WHO onozakii@who.int 3 strategic
SUMMARY MEETING REPORT. Annual stakeholders and partners meeting AIDS Medicines and Diagnostics Service. Geneva, 7 8 May 2013
 SUMMARY MEETING REPORT Annual stakeholders and partners meeting AIDS Medicines and Diagnostics Service Geneva, 7 8 May 2013 Department of HIV/AIDS HIV Technologies and Commodities May 2013 WHO/HIV/2013.6
SUMMARY MEETING REPORT Annual stakeholders and partners meeting AIDS Medicines and Diagnostics Service Geneva, 7 8 May 2013 Department of HIV/AIDS HIV Technologies and Commodities May 2013 WHO/HIV/2013.6
PARASITOLOGY CASE HISTORY #14 (BLOOD PARASITES) (Lynne S. Garcia)
 PARASITOLOGY CASE HISTORY #14 (BLOOD PARASITES) (Lynne S. Garcia) A 37-year-old woman, who had traveled to New Guinea for several weeks, presented to the medical clinic with fever, chills, and rigors within
PARASITOLOGY CASE HISTORY #14 (BLOOD PARASITES) (Lynne S. Garcia) A 37-year-old woman, who had traveled to New Guinea for several weeks, presented to the medical clinic with fever, chills, and rigors within
PROFICIENCY TESTING POLICY
 Supersedes Prepared by: APPROVALS in this section Approved by: Date: Laboratory Director RECORD OF REVIEWS Date Signature Title Procedural Changes/Review VERSION HISTORY Revision # 0 Section #/Changes
Supersedes Prepared by: APPROVALS in this section Approved by: Date: Laboratory Director RECORD OF REVIEWS Date Signature Title Procedural Changes/Review VERSION HISTORY Revision # 0 Section #/Changes
DRAFT UNICEF PROCUREMENT OF HIV/AIDS-RELATED SUPPLIES AND SERVICES
 DRAFT UNICEF PROCUREMENT OF HIV/AIDS-RELATED SUPPLIES AND SERVICES April 2005 Summary: Millions of children in developing countries are affected by the HIV/AIDS pandemic. Despite significant international
DRAFT UNICEF PROCUREMENT OF HIV/AIDS-RELATED SUPPLIES AND SERVICES April 2005 Summary: Millions of children in developing countries are affected by the HIV/AIDS pandemic. Despite significant international
WHO Global Malaria Programme. February 2009
 WHO Global Malaria Programme February 2009 Table of Contents 1. The world malaria situation 2. The critical role of WHO's Global Malaria Programme 3. Our programme of work explained 4. Situation analysis
WHO Global Malaria Programme February 2009 Table of Contents 1. The world malaria situation 2. The critical role of WHO's Global Malaria Programme 3. Our programme of work explained 4. Situation analysis
What is this document and who is it for?
 Measles and Rubella Initiative s Standard Operating Procedures for Accessing Support for Measles and Rubella Supplementary Immunization Activities During 2016 In the context of measles and rubella elimination
Measles and Rubella Initiative s Standard Operating Procedures for Accessing Support for Measles and Rubella Supplementary Immunization Activities During 2016 In the context of measles and rubella elimination
Estimating the parasitaemia of Plasmodium falciparum: experience from a national EQA scheme
 Manser et al. Malaria Journal 2013, 12:428 RESEARCH Open Access Estimating the of Plasmodium falciparum: experience from a national EQA scheme Monika Manser 1, Catherine Olufsen 1, Nick Andrews 2 and Peter
Manser et al. Malaria Journal 2013, 12:428 RESEARCH Open Access Estimating the of Plasmodium falciparum: experience from a national EQA scheme Monika Manser 1, Catherine Olufsen 1, Nick Andrews 2 and Peter
Reducing malaria in Solomon Islands: lessons for effective aid
 Reducing malaria in Solomon Islands: lessons for effective aid Executive Summary Camilla Burkot and Katherine Gilbert The burden of malaria in Solomon Islands, a small island state of approximately 653,500
Reducing malaria in Solomon Islands: lessons for effective aid Executive Summary Camilla Burkot and Katherine Gilbert The burden of malaria in Solomon Islands, a small island state of approximately 653,500
The role of market intelligence in access to anti-malarial medicines: funding, procurement, and policy
 The role of market intelligence in access to anti-malarial medicines: funding, procurement, and policy Brenda Waning Boston University School of Medicine November 20, 2009 American Society of Tropical
The role of market intelligence in access to anti-malarial medicines: funding, procurement, and policy Brenda Waning Boston University School of Medicine November 20, 2009 American Society of Tropical
IDA and the concept of essential drugs
 International Journal of Risk & Safety in Medicine 12 (1999) 75 77 75 IOS Press IDA and the concept of essential drugs Hans V. Hogerzeil Medical Officer, WHO Department of Essential Drugs and Other Medicines,
International Journal of Risk & Safety in Medicine 12 (1999) 75 77 75 IOS Press IDA and the concept of essential drugs Hans V. Hogerzeil Medical Officer, WHO Department of Essential Drugs and Other Medicines,
Global Diagnostics Working Group
 Global Diagnostics Working Group AMDS Meeting with Stakeholders and Manufacturers, 2016 Anita SANDS, on behalf of the GDWG Why a GDWG? In the wake of a global product alert for a specific product all agencies
Global Diagnostics Working Group AMDS Meeting with Stakeholders and Manufacturers, 2016 Anita SANDS, on behalf of the GDWG Why a GDWG? In the wake of a global product alert for a specific product all agencies
HTLV Serology Quality Assessment Program Summary for Panel HTLVSER 2017Apr19
 National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology Laboratory HTLV Serology Quality Assessment Program Summary for Panel HTLVSER 2017Apr19
National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology Laboratory HTLV Serology Quality Assessment Program Summary for Panel HTLVSER 2017Apr19
Aidspan Review of a Study on the Costs and Health Impact of Continued Global Fund Support for Antiretroviral Therapy
 An independent watchdog of the Global Fund, and publisher of Global Fund Observer P.O. Box 66869-00800, Nairobi, Kenya Web: www.aidspan.org Email: info@aidspan.org Phone: +254-20-418-0149 Fax: +254-20-418-0156
An independent watchdog of the Global Fund, and publisher of Global Fund Observer P.O. Box 66869-00800, Nairobi, Kenya Web: www.aidspan.org Email: info@aidspan.org Phone: +254-20-418-0149 Fax: +254-20-418-0156
Fill the Nutrient Gap Analysis: An introduction. Giulia Baldi, MPH WFP, Nutrition Division
 Fill the Nutrient Gap Analysis: An introduction Giulia Baldi, MPH WFP, Nutrition Division 2 Meeting nutrient requirements is a prerequisite for preventing malnutrition MALNUTRITION Inadequate dietary intake
Fill the Nutrient Gap Analysis: An introduction Giulia Baldi, MPH WFP, Nutrition Division 2 Meeting nutrient requirements is a prerequisite for preventing malnutrition MALNUTRITION Inadequate dietary intake
The global context for financing delivery innovations in fever case management and malaria treatment. Ramanan Laxminarayan
 The global context for financing delivery innovations in fever case management and malaria treatment Ramanan Laxminarayan Changed environment 1. Greater challenges in continued funding for global health
The global context for financing delivery innovations in fever case management and malaria treatment Ramanan Laxminarayan Changed environment 1. Greater challenges in continued funding for global health
TB Disease Prevalence Survey - Overview and Introduction of the TF
 TB Disease Prevalence Survey - Overview and Introduction of the TF Ikushi Onozaki MD, MPH Team Leader, TB Prevalence Survey WHO Global Task Force on TB Impact Measurement Stop TB Department, WHO onozakii@who.int
TB Disease Prevalence Survey - Overview and Introduction of the TF Ikushi Onozaki MD, MPH Team Leader, TB Prevalence Survey WHO Global Task Force on TB Impact Measurement Stop TB Department, WHO onozakii@who.int
AMDS Partners and Stakeholders Meeting CHAI HIV Diagnostics Forecasting Overview th September, 2014
 AMDS Partners and Stakeholders Meeting CHAI HIV Diagnostics Forecasting Overview 29-30 th September, 2014 Agenda Introduction Overview of Global Diagnostics Forecasting Overview of Country Forecasting
AMDS Partners and Stakeholders Meeting CHAI HIV Diagnostics Forecasting Overview 29-30 th September, 2014 Agenda Introduction Overview of Global Diagnostics Forecasting Overview of Country Forecasting
Ethiopia Malaria Financial Landscape
 Ethiopia Malaria Financial Landscape PATH MACEPA DECEMBER 2015 MALARIA FUNDING IN ETHIOPIA AT A GLANCE The total estimated cost to implement Ethiopia s National Malaria Strategic Plan (NMSP) for 2015 2017
Ethiopia Malaria Financial Landscape PATH MACEPA DECEMBER 2015 MALARIA FUNDING IN ETHIOPIA AT A GLANCE The total estimated cost to implement Ethiopia s National Malaria Strategic Plan (NMSP) for 2015 2017
International Coordinating Group for vaccine provision. Emergency Vaccine Stockpiles
 International Coordinating Group for vaccine provision Emergency Vaccine Stockpiles Existing Vaccine Stockpiles Vaccine Year Use Qty million doses Smallpox 1980 Epidemic response 35 WHO/ countries Storage
International Coordinating Group for vaccine provision Emergency Vaccine Stockpiles Existing Vaccine Stockpiles Vaccine Year Use Qty million doses Smallpox 1980 Epidemic response 35 WHO/ countries Storage
Vaccines: (inter)national regulation and quality in resource limited settings
 Vaccines: (inter)national regulation and quality in resource limited settings Raffaella Ravinetto QUAMED Research & Networking Institute of Tropical Medicine Antwerp 12th July 2017 Based on a presentation
Vaccines: (inter)national regulation and quality in resource limited settings Raffaella Ravinetto QUAMED Research & Networking Institute of Tropical Medicine Antwerp 12th July 2017 Based on a presentation
Investing for Impact Prioritizing HIV Programs for GF Concept Notes. Lisa Nelson, WHO Iris Semini, UNAIDS
 Investing for Impact Prioritizing HIV Programs for GF Concept Notes Lisa Nelson, WHO Iris Semini, UNAIDS Top 5 Lessons Learned 1 2 3 4 5 Prioritize within the allocation amount Separate above allocation
Investing for Impact Prioritizing HIV Programs for GF Concept Notes Lisa Nelson, WHO Iris Semini, UNAIDS Top 5 Lessons Learned 1 2 3 4 5 Prioritize within the allocation amount Separate above allocation
Copyright by Population Services International and ACTwatch 2016.
 Malaria markets in the Greater Mekong Sub-Region: 2015-2016 1 Copyright by Population Services International and ACTwatch 2016. Suggested Citation: Malaria Markets in the Greater Mekong Sub-Region: 2015-2016.
Malaria markets in the Greater Mekong Sub-Region: 2015-2016 1 Copyright by Population Services International and ACTwatch 2016. Suggested Citation: Malaria Markets in the Greater Mekong Sub-Region: 2015-2016.
Michel BAIJOT Vice President, WW Business Development & Strategic Alliances
 Technology Transfer and Vaccines: The GSK Experience WHO Workshop on Technology Transfer for Local Manufacturing Capacity of Vaccines Geneva, November 30th, 2010 Michel BAIJOT Vice President, WW Business
Technology Transfer and Vaccines: The GSK Experience WHO Workshop on Technology Transfer for Local Manufacturing Capacity of Vaccines Geneva, November 30th, 2010 Michel BAIJOT Vice President, WW Business
