Completeness Assessments for Type II API DMFs Under GDUFA Guidance for Industry
|
|
|
- Barrie Tucker
- 5 years ago
- Views:
Transcription
1 Completeness Assessments for Type II API DMFs Under GDUFA Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) February 2016 Pharmaceutical Quality/CMC/Generics
2 Completeness Assessments for Type II API DMFs Under GDUFA Guidance for Industry Additional copies are available from: Office of Communications, Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration New Hampshire Ave., Hillandale Bldg., 4 th Floor Silver Spring, MD Phone: or ; Fax: druginfo@fda.hhs.gov or Office of Communication, Outreach and Development Center for Biologics Evaluation and Research Food and Drug Administration New Hampshire Ave., Bldg. 71, Room 3128 Silver Spring, MD Phone: or ocod@fda.hhs.gov U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) February 2016 Pharmaceutical Quality/CMC/Generics
3 Contains nbinding Recommendations TABLE OF CONTENTS I. INTRODUCTION... 1 II. BACKGROUND... 2 III. COMPLETENESS ASSESSMENT... 2 A. Information Confirmed During the Completeness Assessment... 3 B. Check of Completeness Assessment Elements... 4 IV. COMPLETENESS ASSESSMENT OUTCOMES... 5 V. API INFORMATION INCLUDED IN A GENERIC DRUG SUBMISSION... 6 VI. SUMMARY... 6 DEFINITIONS... 7 APPENDIX i
4 Contains nbinding Recommendations Completeness Assessments for Type II API DMFs Under GDUFA Guidance for Industry 1 This guidance represents the current thinking of the Food and Drug Administration (FDA or Agency) on this topic. It does not establish any rights for any person and is not binding on FDA or the public. You can use an alternative approach if it satisfies the requirements of the applicable statutes and regulations. To discuss an alternative approach, contact the FDA office responsible for this guidance as listed on the title page. I. INTRODUCTION This guidance is intended for holders of Type II active pharmaceutical ingredient (API) drug master files (DMFs) that are or will be referenced in an abbreviated new drug application (ANDA), an amendment to an ANDA, a prior approval supplement (PAS) to an ANDA, or an amendment to a PAS (generic drug submissions). The guidance explains that, as of October 1, 2012, under the Generic Drug User Fee Amendments of 2012, commonly referred to as GDUFA: 2 DMF holders are required to pay a DMF fee when first authorizing the reference of their DMF in a generic application 3 Type II API DMFs must undergo an FDA completeness assessment (CA) The guidance makes recommendations about the information that should be included in the DMF to facilitate a GDUFA CA. The guidance does not apply to Type II API DMFs used to support new drug applications (NDAs), biologics license applications (BLAs), 4 other submissions that are not generic drug submissions, or any other types of DMFs. 5 In general, FDA s guidance documents do not establish legally enforceable responsibilities. Instead, guidances describe the Agency s current thinking on a topic and should be viewed only as recommendations, unless specific regulatory or statutory requirements are cited. The use of the word should in Agency guidances means that something is suggested or recommended, but not required. 1 This guidance has been prepared by the Center for Drug Evaluation and Research and the Center for Biologics Evaluation and Research at the Food and Drug Administration. 2 Public Law , Title III. 3 For these purposes, such authorization is deemed to have occurred when the DMF is referenced on or after October 1, 2012, in a generic drug submission by an initial letter of authorization, Section 744B(a)(2)(A) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) (21 U.S.C. 379j-42(a)(2)(A). 4 Type II API, API intermediate, and drug product DMFs are not used to support BLAs submitted pursuant to sections 351(a) and 351(k) of the Public Health Service Act (42 U.S.C. 262). 5 See section 744A(7) of the FD&C Act (21 U.S.C. 379j-41(7)). 1
5 Contains nbinding Recommendations II. BACKGROUND Under GDUFA, beginning October 1, 2012, the holder of a Type II API DMF must pay a onetime DMF fee when the DMF is first referenced in a generic drug submission submitted to FDA on the basis of a letter of authorization (LOA) from the DMF holder. 6 Also under GDUFA, holders of Type II API DMFs that were evaluated before October 1, 2012, must pay a one-time fee for the DMF when their DMF is first referenced in a new ANDA, an ANDA or PAS amendment, or an ANDA PAS on or after October 1, Only Type II API DMFs for use in generic drug submissions incur this one-time fee. Under GDUFA, Type II API DMFs intended for reference in a generic drug submission for which the fee is paid will undergo a CA. Section 744B(a)(2)(D)(iii) of the FD&C Act requires FDA to make publicly available on its Web site a list of DMF numbers that correspond to DMFs that, having successfully undergone a CA in accordance with criteria to be published by FDA, are available for reference. Although the requirement for a CA for Type II API DMFs is new, FDA has previously evaluated DMFs in accordance with the criteria set out in the GDUFA Completeness Assessment Checklist for Type II API DMFs (CA Checklist), attached to this guidance as Appendix 1. In order to ensure adequate time for the CA, FDA strongly encourages the DMF holder to submit a complete DMF and pay the DMF fee at least 6 months prior to the submission of an ANDA or PAS that will rely on the DMF. When submitting a DMF, the DMF holder should also submit Form FDA 3794, the Generic Drug User Fee Cover Sheet, which includes the minimum information necessary for FDA to determine whether a DMF holder has satisfied all relevant user fee obligations. 8 DMF holders are encouraged to submit their DMFs using the Electronic Common Technical Document (ectd) format. 9 More information is available on the ectd format on FDA s Web site. 10 III. COMPLETENESS ASSESSMENT 6 Section 744B(a)(2) of the FD&C Act (21 U.S.C. 379j-42(a)(2)). For discussion of LOAs, see 21 CFR (b) and (g)(1). 7 The fee amount will be announced in the Federal Register not later than 60 days before the start of the fiscal year (generally on or about August 1 of the previous fiscal year). 8 See 9 te that 24 months after the guidance for industry Providing Regulatory Submissions in Electronic Format Certain Human Pharmaceutical Product Applications and Related Submissions Using the ectd Specification is finalized (i.e., May 5, 2017), DMFs will be required to be submitted using the ectd format pursuant to the implementation timeline identified in the final guidance. 10 See information about electronic submissions at m htm. 2
6 Contains nbinding Recommendations FDA will perform a CA once a DMF holder files a Type II API DMF 11 with the Form FDA 3794 and there is an initial verification of the fee payment. The CA does not replace the full scientific review, which determines whether the information contained in the DMF is adequate to support an ANDA regulatory action. In brief, FDA will undertake a CA to determine the following: Is the DMF active? Has the fee been paid? Has the DMF been previously reviewed? Does the DMF pertain to a single API? Does the DMF contain certain administrative information? Does the DMF contain all the information necessary to enable a scientific review? 12 Is the DMF written in English? 13 FDA will conduct the CA by determining the answers to a series of questions listed in the CA Checklist, which is included in Appendix 1. DMFs for which the fee has been paid and which have been found complete in accordance with the criteria for a CA set out in the CA Checklist will be identified on FDA s public Web site as available for reference in support of a generic drug submission. For complex APIs, in addition to the recommendations in the CA Checklist, DMF holders should ensure the DMF provides the data necessary for the Agency to review the DMF with respect to active ingredient sameness. Information on active ingredient sameness is discussed in the product s specific bioequivalence (BE) guidance when it becomes available on FDA s Web site. 14 A. Information Confirmed During the Completeness Assessment FDA will use the CA Checklist to perform the CA. At the top of the cover page of the CA Checklist, FDA will fill in basic information about the DMF, including its name, number, receipt date, and whether the DMF was submitted in electronic or paper format. The FDA will also note whether the primary DMF the ANDA references refers to any other DMFs (subject DMFs). A primary DMF can reference subject DMFs, which provide additional 11 See FDA s Web site on Drug Master Files at efault.htm. 12 Id. 13 If any part of the application is in a foreign language, an accurate and complete English translation shall be appended to such part. See 21 CFR (d)(5). 14 For example, for enoxaparin sodium: listed sameness equivalence criteria in the draft guidance include mode of depolymerization, source material, physicochemical properties, disaccharide building blocks, fragment mapping, sequence of oligosaccharide species, and biological and biochemical assays. If the appropriate product-specific data is not in the DMF, the DMF will be deemed incomplete. 3
7 Contains nbinding Recommendations information needed to completely describe the manufacture of an API. 15 Before submitting its DMF, the primary DMF holder should check with the holders of any referenced subject DMFs to make sure the subject DMFs are filed with FDA and FDA still considers them active. 1. Is the DMF Active? Before assigning a DMF to a reviewer for a CA, FDA will confirm that the DMF is active. 16 the primary DMF or any referenced subject DMFs on file at FDA are inactive, FDA will consider the primary DMF incomplete and send a letter notifying the DMF holder. If 2. Has the DMF fee been paid? Before assigning a DMF to a reviewer for a CA, FDA will confirm that the DMF fee has been paid. If it has not, FDA will not assign the DMF for CA. ANDA applicants that reference a DMF for which a fee is due will be notified that the DMF holder has not paid the fee. If the DMF fee is not paid within 20 days after notification, FDA will refuse to receive the ANDA referencing the DMF. 3. Has the DMF been previously reviewed for chemistry, manufacturing, and controls (CMC) by FDA in the context of a review of a prior application? If FDA has reviewed the DMF for CMC after vember 30, 2007, the DMF will be considered to have passed the CA without further analysis. If the DMF was reviewed for CMC prior to vember 30, 2007, a CA assessment will need to be performed. For all DMFs that have not previously received a full CMC review, a CA assessment will need to be performed. If the DMF has not previously received this full review, it will be assigned to a reviewer for a CA. B. Check of Completeness Assessment Elements FDA will complete the administrative part of the CA Checklist (i.e., General Information ) during the CA. If the DMF is incomplete, FDA will send the DMF holder a GDUFA DMF Incomplete Letter. With certain exceptions specified in this guidance document, this letter will provide comments about each element that resulted in an incomplete designation for the CA. If an item is marked n/a and does not apply to the DMF, the element is treated the same as if it were marked yes. 1. Is the subject of the DMF a single API produced by one manufacturing process? The subject of a DMF should be limited to one API and one manufacturing process. If a DMF includes information on more than one API or more than one manufacturing process for an API, the DMF will be deemed incomplete. If the DMF describes multiple APIs, the DMF holder 15 For example, a subject DMF may describe the manufacture of a material used in producing the active ingredient. If a subject DMF does not meet the definition of a Type II API DMF, it will not incur a DMF fee. 16 Active is defined in the Definitions section of this guidance. 4
8 Contains nbinding Recommendations should file separate DMFs for each API. Similarly, if there are multiple manufacturing processes for an API, the DMF holder should file separate DMFs for each manufacturing process. 2. Does the DMF holder need to submit a complete update? If the DMF is in paper format and it has been five years or more since the DMF received a complete update, 17 or if there have been more than five amendments to the DMF, the DMF holder should provide a complete and comprehensive update to it. If such a DMF has not received an update, FDA will consider the DMF incomplete. The DMF holder must submit a complete update for FDA to determine whether it passes the CA. 18 FDA believes that the remainder of the CA Checklist is self-explanatory. IV. COMPLETENESS ASSESSMENT OUTCOMES Following the CA, FDA will find the DMF either complete or incomplete. If the DMF is found complete, FDA will post the DMF number on a publicly available list on FDA s Web site to indicate the DMF is available for reference by generic drug submission applicants. 19 If the DMF is found incomplete, the CA findings and comments will be compiled in a GDUFA DMF Incomplete Letter to the DMF holder that explains why the DMF was deemed incomplete. Information about the CA status of a DMF, other than the lack of a public listing on the FDA s Web site, will not be provided to anyone except the DMF holder and, as necessary, any generic drug submission applicant that the DMF holder has authorized to rely on the DMF. To remedy a GDUFA DMF Incomplete Letter and pass the CA, the DMF holder should submit an amendment to its DMF to correct the deficiencies identified in the Incomplete Letter, or, if FDA has determined that the DMF should undergo a complete update, the DMF holder should resubmit the DMF with that update. FDA will then assess the revised DMF s completeness. If there are no deficiencies at this time, FDA will declare the DMF to be complete and to have passed the CA. Although a DMF may be deemed incomplete upon its first CA, FDA will work with DMF holders to provide guidance on how to revise the DMF so it may be found complete 17 All changes must be reported as amendments. Annual reports are NOT to be used to report changes in the DMF. 18 The requirement for a complete update does not apply to the DMF if the entire DMF is in ectd format, which always presents the DMF in its current state. The Agency highly encourages DMF holders to convert the entire DMF into an ectd submission, which does not require reference to any previous paper submission. 19 For the public list of DMFs available for reference, see 5
9 Contains nbinding Recommendations after resubmission. Once the DMF passes the CA, FDA will make the DMF number publicly available on its Web site. 20 V. API INFORMATION INCLUDED IN A GENERIC DRUG SUBMISSION If a generic drug submission contains all the necessary API information and does not rely on information contained in a DMF, no CA will be performed. Instead, this information will be evaluated during the ANDA filing review. 21 However, because GDUFA requires collection of a one-time fee for API information included in a generic drug submission (i.e., an (a)(3)(f) fee), 22 the applicant submitting the generic drug submission containing the API information must pay this fee. VI. SUMMARY Once the DMF fee is received, FDA will evaluate the DMF to make sure it meets the CA criteria. If the DMF passes the CA, it will be found complete and the DMF number will be made publicly available on FDA s Web site. If the DMF fails the CA, FDA will send a GDUFA DMF Incomplete Letter describing to the DMF holder the missing elements in its DMF. If the DMF holder addresses all the deficiencies and amends the DMF or completely updates the DMF per the CA request, the FDA will complete its CA. If the DMF is then found complete, the DMF number will be made publicly available on FDA s Web site. If a generic drug submission contains all the necessary API information and does not reference a DMF, the generic drug submission applicant will be required to pay the (a)(3)(f) fee. CA will be conducted. 20 Under GDUFA, a condition for a Type II API DMF to be considered available for reference is that it has not failed an initial completeness assessment (Section 744B(a)(2)(D)(ii)(II) of the FD&C Act). FDA does not interpret that provision as disqualifying a DMF if it has ever failed a CA. Instead, FDA considers this condition to be satisfied when, after submission of an amendment or resubmission of the DMF to address deficiencies identified by FDA, FDA finds that the DMF has passed the CA. 21 See guidance for industry ANDA Submissions Refuse-to-Receive Standards. 22 Section 744B(a)(3)(F) of the FD&C Act. 6
10 Contains nbinding Recommendations DEFINITIONS Active pharmaceutical ingredient 23 (as defined by GDUFA): (A) A substance, or a mixture when the substance is unstable or cannot be transported on its own, intended (i) to be used as a component of a drug; and (ii) to furnish pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease, or to affect the structure or any function of the human body; or (B) a substance intended for final crystallization, purification, or salt formation, or any combination of those activities, to become a substance or mixture described in subparagraph (A). Active DMF: A drug master file (DMF) for which the FDA has made a determination that the DMF was acceptable for filing administratively and is up to date. DMF holder: 24 Designated owner of the DMF, which may be different from the U.S. agent listed as the contact. Generic drug submission: 25 An abbreviated new drug application (ANDA), an amendment to an ANDA, a prior approval supplement (PAS) to an ANDA, or an amendment to a PAS. Letter of authorization (LOA): 26 A written statement by the holder or designated U.S. agent or representative permitting FDA to refer to information in the DMF in support of another person s 27 generic drug submission. Type II Active Pharmaceutical Ingredient Drug Master Files: 28 The submission of API information to the FDA by the DMF holder who intends to authorize generic drug applicants to rely on the information to support submissions to the FDA without the holder having to disclose the information to the generic drug applicants. 23 Section 744A(2) of the FD&C Act. 24 See 21 CFR (a). 25 Section 744A(7) of the FD&C Act. 26 See FDA s Web site on Drug Master Files at: efault.htm. 27 The word person includes individual, partnership, corporation, and association (section 201(e) of the FD&C Act. 28 Section 744A(12) of the FD&C Act; also see 21 CFR (a)(2). 7
11 Contains nbinding Recommendations APPENDIX 1 GDUFA COMPLETENESS ASSESSMENT CHECKLIST FOR TYPE II API DMFs DMF NUMBER: DMF HOLDER: DRUG NAME (subject): SUBMIT DATE: RECEIVED DATE: Electronic or paper submission: DMF(s) referenced by the primary DMF being assessed, if applicable: EXPEDITED ASSESSMENT per REQUEST from FDA by: (requestor name here) Primary reviewer: Date: Review recommendation for completeness assessment: COMPLETE INCOMPLETE 1. Has the GDUFA fee been paid? Enter date paid: 2. Is the DMF active? If no, DMF is INCOMPLETE per policy. Issue Incomplete Letter to DMF holder. 3. Has the DMF been reviewed, after vember 30, 2007, for chemistry, manufacturing, and controls (CMC) by FDA in the context of a review of a prior application? If yes, the DMF is COMPLETE per policy. If no, review DMF with checklist. ADDITIONAL COMMENTS REGARDING THE DMF: 8
12 Contains nbinding Recommendations Checklist Review GENERAL INFORMATION NOTE(S) 1. Subject of the DMF is a single For #1: The DMF is limited to: (i) API produced by one one API, although multiple manufacturing process. manufacturing sites for a single API are permitted when the same process 2. For previously submitted is used in each of those sites; (ii) one DMFs, the DMF holder has manufacturing process, although submitted a complete update. certain process alternatives/changes may be permissible with sufficient 3. Provides current Good supportive information provided. Manufacturing Practice (cgmp) Examples include: validated Statement of Commitment. reprocess/rework procedures; micronization leading to different 4. Provides complete name, particle sizes (excluding nano address, and contact information particles); addition of a stabilizing for holder. agent for stability purposes; and minor process variation that leaves 5. Designates U.S. Agent for the chemical transformation the non-u.s. DMF holders, with n/a same, with little risk to the impurity appropriate designation letter. profile. 6. Contains Letters of A separate DMF should be filed for: Authorization for any DMFs n/a different salt form; different synthetic referenced to support this DMF. route; and significant process variation resulting in a different 7. All DMFs referenced in this impurity profile and requiring a DMF have been filed with the n/a different control strategy. Agency and are active. For the definition of API and the 8. Contains label with storage Agency s interpretation, refer to conditions and expiry/retest date. GDUFA Q&A guidance. 9. Contains bovine spongiform For #2: Five years or five amendments encephalopathy (BSE)/ are a good guide to determine the transmissible spongiform n/a necessity for submission of a complete encephalopathy (TSE) update, because a large number of certification, if animal-sourced. amendments with significant changes make it difficult to determine the current 10. Contains information on state of the information in the DMF. adventitious agents, if animal- n/a The complete update should reflect the sourced. current state of the process and not require reference to any previous 11. Contains information on submission for information. presence of pesticides, if plant- n/a sourced. The requirement for a complete update does not apply to the DMF if the entire DMF is in electronic common technical document (ectd) format, which always presents the DMF in its current state. We highly encourage DMF holders to convert 9
13 Contains nbinding Recommendations the entire DMF into an ectd submission, which does not require reference to any previous paper submission. MODULE 2: SUMMARIES 29 ectd Contains a Quality Overall Summary (QoS). NOTE(S) n/a For #12: If a QoS is provided, the Question-based Review (QbR) format is highly encouraged. MODULE 3: QUALITY 3.2 Body of Data 3.2.S API [name, manufacturer] ectd. NOTE(S) 3.2.S.1 General Information Contains complete General Information on the following: 13. menclature. 14. Structure. 15. General Properties: basic information regarding the general properties of the API. 29 DMF holders are highly encouraged to submit files in ectd format. See information about electronic submissions at m htm and ICH M4Q: R1_.pdf. te that 24 months after the guidance for industry Providing Regulatory Submissions in Electronic Format Certain Human Pharmaceutical Product Applications and Related Submissions Using the ectd Specification is finalized (i.e., May 5, 2017), DMFs will be submitted using the ectd format pursuant to the implementation timeline identified in the final guidance. 10
14 Contains nbinding Recommendations ectd. NOTE(S) Manufacture 3.2.S S.2.1 Contains complete Manufacturer Information on the following for each site: 16. Name and full address(es) of the manufacturing facility(ies), contact name of on-site individual, phone and fax numbers, and address. 3.2.S.2.2 Contains Description of Manufacturing Process and Process Controls addressing the following: 17. If the API is synthetic/semisynthetic, provides complete synthetic scheme from appropriately supported starting materials. Scheme includes structural representation with reagents, reaction conditions, molar ratio, etc. 18. Flow chart for every stage. n/a For #16: Separate facilities used for release testing of the API and for additional processing (e.g., micronization) should also be listed. Central File Number (CFN), Facility Establishment Identifier (FEI), and Data Universal Numbering System (DUNS) numbers should be provided if available. For #17: For a fermentation process, information pertaining to the quality and control of the microorganism, cell bank systems, and media components should be provided. 19. Description of the manufacturing process. 3.2.S.2.3 Contains information on the Control of Materials, as follows: Starting Material: 20. Starting material, clearly designated, with appropriate justification. 21. Name of each manufacturer. 22. Specification. 23. Analytical method. For #20: Justification for designation of each starting material should be in agreement with the general principle outlined in International Conference on Harmonisation (ICH) Q11. This can include information, if applicable, on: Name, address, and contact information of the manufacturer(s) of each proposed starting material. A flow diagram and description outlining the synthetic route and conditions of each proposed starting material. Discussion of the impurities (including residual solvents and inorganic impurities) arising from 11
15 Contains nbinding Recommendations ectd. NOTE(S) 24. Certificate of Analysis (CoA) from manufacturer. 25. CoAs from the DMF holder. Reagents/Solvents: 26. Specifications. 27. Representative CoAs. 3.2.S.2.4 Contains information for Controls of Critical Steps and Intermediates, as follows: 28. In-process controls and tests, described for each critical step. the manufacturing process of each proposed starting material. The ability of analytical procedures to detect impurities in the starting material. The fate and purge of those impurities and their derivatives in subsequent processing steps. How the proposed specification for each starting material will contribute to the control strategy. For #26: Specifications should clearly identify the test methods. For non-compendial methods, the method description should be provided. Intermediates: 29. Specifications for each identified intermediate. 30. Analytical methods for each intermediate. 3.2.S.2.5 Process Validation and/or Evaluation 31. Contains a summary of Process Validation and/or Evaluation information. 32. Provides sterility assurance data for sterile API. n/a n/a For #31: If the validation has been performed, the DMF holder should provide a validation summary, including batch descriptions with traceability among steps/stages, and analytical test results for the starting materials, intermediates, in-process controls, and final API, preferably in a tabular format. 3.2.S.2.6 Manufacturing Process Development 33. Contains a summary of Manufacturing Process Development. For #32: The full sterilization process validation and the method validation of sterility tests for the sterilized API should be provided, including but not limited to: description of the specific sterilization processes for the bulk drug substance and all associated manufacturing equipment, components, and containers/closures, description of sterile processing areas, list of all filling lines/rooms/suites, legible floor plans for sterile API manufacturers, description of environmental monitoring program for sterile 12
16 Contains nbinding Recommendations ectd. 3.2.S.3 Characterization NOTE(S) products, validation data for sterilization of bulk API, filter validation data if applicable, validation data for the sterilization and depyrogenation of the final container/closure system, validation data for the sterilization of all manufacturing equipment and components, description of process simulation/media fill program and associated data from media fill runs and container/closure integrity testing results. 3.2.S.3.1 Provides information to support the Elucidation of Structure and other Characteristics of the API as follows: 34. Characterization information appropriate for the material (e.g., nuclear magnetic resonance (NMR), infrared (IR), ultraviolet (UV), mass spectrometry (MS), elemental analysis, etc.). For #34: Data interpretation should be provided; spectroscopic comparison with authenticated material (e.g., United States Pharmacopeia (USP) Convention reference standard), if available, is encouraged to confirm the structure. 3.2.S.3.2 Provides information on Impurities, as follows: 35. A table including the name(s), structure(s), origin (degradant, process impurity) of observed/potential organic impurities. For #35: Potential genotoxic impurities can be included in this table or summarized in a separate table. 36. Information on potential inorganic impurities (inorganic) (e.g., metal catalysts, reagents). 37. Potential residual solvents. 3.2.S.4 Provides information to support the Control of the API, as follows: For 3.2.S.4: A summary of the control strategy, as illustrated in ICH Q11, is highly encouraged. 38. Full test specification. 39. Description of the analytical methods. For #40: Method verification reports 13
17 Contains nbinding Recommendations ectd. NOTE(S) 40. Method validation and/or method verification reports. 41. CoAs for representative batches (batch analysis). 42. Justification for each specification. should be provided for compendial methods, such as assay or related substance by high-performance liquid chromatography (HPLC); the method equivalency study should be provided when an in-house method is used in lieu of a compendial method. 3.2.S.5 Provides information to support the Reference Standards or Materials, as follows: API: 43. The source, lot #, CoA (for primary reference standard (RS) and working standard (WS)). 44. Qualification data on the drug substance (DS) RS. Impurities: 45. The source, lot #, and CoA for RS and WS for each identified impurity. For #44: Qualification data on inhouse primary RSs includes spectroscopic characterization and quantification by mass balance. When a compendial RS is used as the primary RS, the lot number and certificate are sufficient. WSs should be adequately qualified against the primary RS. Spectroscopic comparison with compendial reference standards, when available, is highly encouraged. Data can be crossreferenced to 3.2.S.3.2, if appropriate. 46. Qualification data on the impurity RS. For #46: See note for #44 above. 3.2.S.6 Provides information to support the Container/Closure System, as follows: 47. Description of container/ closure system (including contact material and secondary material). 3.2.S Certification statements for contact materials for use in food and drugs. 49. Manufacturer, specifications, and representative CoA for primary contact material and functional secondary packaging component. Provides information to support the Stability of the API, as follows: For #48: The certification statement from the supplier should state that each primary packaging material for the DS is safe to use in contact with food, with an appropriate reference to the indirect food additive regulations (21 CFR ). 14
18 Contains nbinding Recommendations ectd. NOTE(S) 3.2.S.7.1 Stability Summary and Conclusions 50. Indicates clearly the retest date or expiration date of API. 3.2.S.7.2 Postapproval Stability Protocol and Stability Commitment 51. Provides stability protocol. 52. Provides stability commitment. 3.2.S.7.3 Stability Data 53. Provides Stability Data. MODULE 3: 3.2.R REGIONAL INFORMATION (API) ectd. NOTE(S) 3.2.R Provides regional information, as follows: 3.2.R.1.S Executed Batch Records for API For #54: Yields, results of inprocess controls, and intermediate analysis should be provided where appropriate. 54. Provides representative executed batch records, with translation, where appropriate. 15
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry
 ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
ANDA Submissions Refuse to Receive for Lack of Proper Justification of Impurity Limits Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
Proper CMC Submission -API. Ramnarayan Randad, Ph.D. DMF Review Staff/OGD June 4, 2014 GPhA/FDA Bethesda, MD
 Proper CMC Submission -API Ramnarayan Randad, Ph.D. DMF Review Staff/OGD June 4, 2014 GPhA/FDA Bethesda, MD 1 Type II Drug Master Files (DMFs) and GDUFA Generic Drug User Fee Amendments (GDUFA) July 9,
Proper CMC Submission -API Ramnarayan Randad, Ph.D. DMF Review Staff/OGD June 4, 2014 GPhA/FDA Bethesda, MD 1 Type II Drug Master Files (DMFs) and GDUFA Generic Drug User Fee Amendments (GDUFA) July 9,
Regulatory Classification of Pharmaceutical Co-Crystals Guidance for Industry
 Regulatory Classification of Pharmaceutical Co-Crystals Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February
Regulatory Classification of Pharmaceutical Co-Crystals Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February
Guidance for Industry DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
ANDA Filing and Refuse to Receive Issues. Johnny Young, M.A.L.A.
 ANDA Filing and Refuse to Receive Issues Johnny Young, M.A.L.A. 1 Statistics 2 497 ANDAs Refused-for-Receipt between CYs 2009-2012: - 12% (2009) - 18% (2010) - 15.5% (2011) - 9.4% (2012) 3 For 2012, 100
ANDA Filing and Refuse to Receive Issues Johnny Young, M.A.L.A. 1 Statistics 2 497 ANDAs Refused-for-Receipt between CYs 2009-2012: - 12% (2009) - 18% (2010) - 15.5% (2011) - 9.4% (2012) 3 For 2012, 100
Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs 425 Privet Road Horsham, PA 19044
 DEPARTMENT OF HEALTH & HUMAN SERVICES ANDA 090783 Food and Drug Administration Silver Spring, MD 20993 Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs
DEPARTMENT OF HEALTH & HUMAN SERVICES ANDA 090783 Food and Drug Administration Silver Spring, MD 20993 Teva Pharmaceuticals USA Attention: Scott D. Tomsky Vice President, US Generics Regulatory Affairs
Guidance for Industry ANDA Submissions Refuse-to-Receive Standards
 Guidance for Industry ANDA Submissions Refuse-to-Receive Standards DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document
Guidance for Industry ANDA Submissions Refuse-to-Receive Standards DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft document
Status Update on the Review of DMFs
 Status Update on the Review of DMFs Presented by Dave Skanchy, Ph.D. Director DMF Review Staff GPhA/FDA CMC Workshop June 4, 2013 1 Outline Changes to the DMF Review Staff: Update on GDUFA hiring and the
Status Update on the Review of DMFs Presented by Dave Skanchy, Ph.D. Director DMF Review Staff GPhA/FDA CMC Workshop June 4, 2013 1 Outline Changes to the DMF Review Staff: Update on GDUFA hiring and the
Guidance for Industry
 Guidance for Industry Liposome Drug Products Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation DRAFT GUIDANCE This guidance document is being
Guidance for Industry Liposome Drug Products Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation DRAFT GUIDANCE This guidance document is being
Liposome Drug Products Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation
 Liposome Drug Products Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation Guidance for Industry U.S. Department of Health and Human Services
Liposome Drug Products Chemistry, Manufacturing, and Controls; Human Pharmacokinetics and Bioavailability; and Labeling Documentation Guidance for Industry U.S. Department of Health and Human Services
DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Inborn Errors of Metabolism That Use Dietary Management: Considerations for Optimizing and Standardizing Diet in Clinical Trials for Drug Product Development Guidance for Industry DRAFT GUIDANCE This guidance
Inborn Errors of Metabolism That Use Dietary Management: Considerations for Optimizing and Standardizing Diet in Clinical Trials for Drug Product Development Guidance for Industry DRAFT GUIDANCE This guidance
Generic Drug User Fee Amendments of 2012; Regulatory Science Initiatives; Public Hearing;
 4160-01-P DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Part 15 [Docket No. FDA-2013-N-0402] Generic Drug User Fee Amendments of 2012; Regulatory Science Initiatives; Public
4160-01-P DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration 21 CFR Part 15 [Docket No. FDA-2013-N-0402] Generic Drug User Fee Amendments of 2012; Regulatory Science Initiatives; Public
Compare Results. 153 Replacements 26 Insertions 344 Deletions. Total Changes. Styling and. Content. 0 Annotations. Old File: New File:
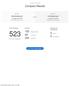 3/1/2019 4:12:31 PM Compare Results Old File: Draft Guidance.pdf 20 pages (438 KB) 3/21/2018 3:55:17 PM versus New File: Final Guidance.pdf 21 pages (323 KB) 2/28/2019 11:42:05 AM Total Changes 523 Text
3/1/2019 4:12:31 PM Compare Results Old File: Draft Guidance.pdf 20 pages (438 KB) 3/21/2018 3:55:17 PM versus New File: Final Guidance.pdf 21 pages (323 KB) 2/28/2019 11:42:05 AM Total Changes 523 Text
GDUFA II User Fees. Update on Implementation
 GDUFA II User Fees Update on Implementation Donal Parks, Director Division of User Fee Management and Budget Formulation OM CDER US FDA November 8, 2017 Outline Refresher on GDUFA II fee structure What
GDUFA II User Fees Update on Implementation Donal Parks, Director Division of User Fee Management and Budget Formulation OM CDER US FDA November 8, 2017 Outline Refresher on GDUFA II fee structure What
Inspections, Compliance, Enforcement, and Criminal Investigations
 Page 1 of 6 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations P.A. Benjamin Manufacturing
Page 1 of 6 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations P.A. Benjamin Manufacturing
Draft Guidance for Industry and FDA Staff
 Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
Draft Guidance for Industry and FDA Staff Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile DRAFT GUIDANCE This guidance document
GDUFA: 2 ½ years later Impact & Importance
 GDUFA: 2 ½ years later Impact & Importance Georgia Hizon Manager, Regulatory Publications Fresenius Kabi USA, LLC Disclaimer The views and opinions expressed in the following PowerPoint slides are those
GDUFA: 2 ½ years later Impact & Importance Georgia Hizon Manager, Regulatory Publications Fresenius Kabi USA, LLC Disclaimer The views and opinions expressed in the following PowerPoint slides are those
Hypertension: Developing Fixed- Dose Combination Drugs for Treatment Guidance for Industry
 Hypertension: Developing Fixed- Dose Combination Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions
Hypertension: Developing Fixed- Dose Combination Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions
Agenda. The current state of Pharmaceutical Excipients in Japan -JPE, JP, DMF, GAB (GMP Auditing Board)- Pharmaceutical Excipients
 The current state of Pharmaceutical Excipients in Japan -JPE, JP, DMF, GAB (GMP Auditing Board)- Dr. Keiji Kijima, Secretary General, IPEC Japan April 26, 2012 Agenda JPE (Japanese ) JP (Japanese Pharmacopoeia)
The current state of Pharmaceutical Excipients in Japan -JPE, JP, DMF, GAB (GMP Auditing Board)- Dr. Keiji Kijima, Secretary General, IPEC Japan April 26, 2012 Agenda JPE (Japanese ) JP (Japanese Pharmacopoeia)
ANDA Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY December 3, 2015
 DEPARTMENT OF HEALTH & HUMAN SERVICES Silver Spring, MD 20993 ANDA 060851 Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY 10970 Docket No. FDA-2011-P-0081
DEPARTMENT OF HEALTH & HUMAN SERVICES Silver Spring, MD 20993 ANDA 060851 Arthur P. Bedrosian, President Armenpharm, Ltd. 49 South Ridge Road P.O. Box D1400 Pomona, NY 10970 Docket No. FDA-2011-P-0081
Organic Impurities in Drug Substances and Drug Products. Antonio Hernandez-Cardoso, M.Sc. Senior Scientific Liaison September 8, 2017
 Organic Impurities in Drug Substances and Drug Products Antonio Hernandez-Cardoso, M.Sc. Senior Scientific Liaison September 8, 2017 Potential sources of drug impurities during development AAPS PharmSciTech,
Organic Impurities in Drug Substances and Drug Products Antonio Hernandez-Cardoso, M.Sc. Senior Scientific Liaison September 8, 2017 Potential sources of drug impurities during development AAPS PharmSciTech,
Citric Acid, Monohydrate
 Citric Acid, Monohydrate Product Regulatory Data Sheet Avantor Performance Materials, Inc. Section 1 Product Information Products Covered Brand Product Code Product Description J.T.Baker 0115 Citric Acid,
Citric Acid, Monohydrate Product Regulatory Data Sheet Avantor Performance Materials, Inc. Section 1 Product Information Products Covered Brand Product Code Product Description J.T.Baker 0115 Citric Acid,
Withdrawal of Proposed Rule on Supplemental Applications Proposing Labeling Changes for
 This document is scheduled to be published in the Federal Register on 12/14/2018 and available online at https://federalregister.gov/d/2018-27098, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
This document is scheduled to be published in the Federal Register on 12/14/2018 and available online at https://federalregister.gov/d/2018-27098, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
Residual Solvents: FDA/ Regulatory Perspective
 Rosa Motta Compliance Officer Residual Solvents: FDA/ Regulatory Perspective PDA/USP Residual Solvents Conference January 18-19, 2007 1 Outline Laws and regulations governing the compliance requirements
Rosa Motta Compliance Officer Residual Solvents: FDA/ Regulatory Perspective PDA/USP Residual Solvents Conference January 18-19, 2007 1 Outline Laws and regulations governing the compliance requirements
Agency Information Collection Activities; Submission for Office of Management and Budget
 This document is scheduled to be published in the Federal Register on 01/28/2016 and available online at http://federalregister.gov/a/2016-01690, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 01/28/2016 and available online at http://federalregister.gov/a/2016-01690, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
OFFICE OF PHARMACEUTICAL SCIENCES. COVER FORM FOR THE TECHNICAL REVIEW OF DRUG MASTER FILES (DMFs) CONTENTS
 MANUAL OF POLICIES AND PROCEDURES CENTER FOR DRUG EVALUATION AND RESEARCH MAPP 5015.3 OFFICE OF PHARMACEUTICAL SCIENCES COVER FORM FOR THE TECHNICAL REVIEW OF DRUG MASTER FILES (DMFs) CONTENTS PURPOSE
MANUAL OF POLICIES AND PROCEDURES CENTER FOR DRUG EVALUATION AND RESEARCH MAPP 5015.3 OFFICE OF PHARMACEUTICAL SCIENCES COVER FORM FOR THE TECHNICAL REVIEW OF DRUG MASTER FILES (DMFs) CONTENTS PURPOSE
Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen
 Reprinted from FDA s website by EAS Consulting Group, LLC Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed
Reprinted from FDA s website by EAS Consulting Group, LLC Over-the-Counter Pediatric Liquid Drug Products Containing Acetaminophen Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed
Raritan Pharmaceuticals, Inc. 6/20/17
 Raritan Pharmaceuticals, Inc. 6/20/17 U.S. Food & Drug Administration Division of Pharmaceutical Quality Operations I New Jersey District 10 Waterview Boulevard, 3rd Floor Parsippany, NJ 07054 June 20,
Raritan Pharmaceuticals, Inc. 6/20/17 U.S. Food & Drug Administration Division of Pharmaceutical Quality Operations I New Jersey District 10 Waterview Boulevard, 3rd Floor Parsippany, NJ 07054 June 20,
SUMMARY: The Food and Drug Administration (FDA) is requesting public input on updated
 This document is scheduled to be published in the Federal Register on 11/22/2017 and available online at https://federalregister.gov/d/2017-25245, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 11/22/2017 and available online at https://federalregister.gov/d/2017-25245, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Migraine: Developing Drugs for Acute Treatment Guidance for Industry
 Migraine: Developing Drugs for Acute Treatment Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2018
Migraine: Developing Drugs for Acute Treatment Guidance for Industry U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) February 2018
Amyotrophic Lateral Sclerosis: Developing Drugs for Treatment Guidance for Industry
 Amyotrophic Lateral Sclerosis: Developing Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Amyotrophic Lateral Sclerosis: Developing Drugs for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
TOBACCO PRODUCT OR MEDICAL PRODUCT?
 TOBACCO PRODUCT OR MEDICAL PRODUCT? Priscilla Callahan-Lyon, MD Deputy Director Division of Individual Health Science Office of Science, CTP Grail Sipes, JD Director Office of Regulatory Policy, CDER Disclaimer:
TOBACCO PRODUCT OR MEDICAL PRODUCT? Priscilla Callahan-Lyon, MD Deputy Director Division of Individual Health Science Office of Science, CTP Grail Sipes, JD Director Office of Regulatory Policy, CDER Disclaimer:
MEDICNES CONTROL AGENCY GUIDELINE FOR REGISTRATION OF HERBAL MEDICINAL PRODUCTS IN THE GAMBIA
 MEDICNES CONTROL AGENCY GUIDELINE FOR REGISTRATION OF HERBAL MEDICINAL PRODUCTS IN THE GAMBIA Document No.: MCA/HMPG/17/12/01 Date of Adoption: 20 th January 2017 Date of Issue: 25 th July 2017 Version
MEDICNES CONTROL AGENCY GUIDELINE FOR REGISTRATION OF HERBAL MEDICINAL PRODUCTS IN THE GAMBIA Document No.: MCA/HMPG/17/12/01 Date of Adoption: 20 th January 2017 Date of Issue: 25 th July 2017 Version
Guidance for Industry
 Reprinted from FDA s website by Guidance for Industry Bar Code Label Requirements Questions and Answers (Question 12 Update) DRAFT GUIDANCE This guidance document is for comment purposes only. Submit comments
Reprinted from FDA s website by Guidance for Industry Bar Code Label Requirements Questions and Answers (Question 12 Update) DRAFT GUIDANCE This guidance document is for comment purposes only. Submit comments
Three-Month Extension of Certain Tobacco Product Compliance Deadlines Related to the
 This document is scheduled to be published in the Federal Register on 05/15/2017 and available online at https://federalregister.gov/d/2017-09754, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 05/15/2017 and available online at https://federalregister.gov/d/2017-09754, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Gluten in Drug Products and Associated Labeling Recommendations Guidance for Industry
 Gluten in Drug Products and Associated Labeling Recommendations Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Gluten in Drug Products and Associated Labeling Recommendations Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Dr. Christian Zeine LGC Standards GmbH. Webinar Series 2013 July 2013
 The 7 truths of impurities and their reference standards FDA's and other regulators' viewpoints and further stories (Part 1) Dr. Christian Zeine LGC Standards GmbH Webinar Series 2013 July 2013 Quick guide
The 7 truths of impurities and their reference standards FDA's and other regulators' viewpoints and further stories (Part 1) Dr. Christian Zeine LGC Standards GmbH Webinar Series 2013 July 2013 Quick guide
Nonallergic Rhinitis: Developing Drug Products for Treatment Guidance for Industry
 Nonallergic Rhinitis: Developing Drug Products for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
Nonallergic Rhinitis: Developing Drug Products for Treatment Guidance for Industry DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding
General Concepts in the European Pharmacopoeia. Anne-Sophie Bouin European Pharmacopoeia Department, EDQM, Council of Europe
 General Concepts in the European Pharmacopoeia Anne-Sophie Bouin European Pharmacopoeia Department, EDQM, Council of Europe General notices Anne-Sophie Bouin, 28/10/09 2009 EDQM, Council of Europe, All
General Concepts in the European Pharmacopoeia Anne-Sophie Bouin European Pharmacopoeia Department, EDQM, Council of Europe General notices Anne-Sophie Bouin, 28/10/09 2009 EDQM, Council of Europe, All
Class II Special Controls Guidance Document: Intraoral Devices for Snoring and/or Obstructive Sleep Apnea; Guidance for Industry and FDA
 Class II Special Controls Guidance Document: Intraoral Devices for Snoring and/or Obstructive Sleep Apnea; Guidance for Industry and FDA Document issued on: November 12, 2002 This document supersedes the
Class II Special Controls Guidance Document: Intraoral Devices for Snoring and/or Obstructive Sleep Apnea; Guidance for Industry and FDA Document issued on: November 12, 2002 This document supersedes the
COMMENTS. Submitted by The International Pharmaceutical Aerosol Consortium
 COMMENTS on a draft Guidance for Industry Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products Chemistry, Manufacturing, and Controls Documentation (Docket No. 99D-1454) Submitted by
COMMENTS on a draft Guidance for Industry Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products Chemistry, Manufacturing, and Controls Documentation (Docket No. 99D-1454) Submitted by
510(k) submissions. Getting US FDA clearance for your device: Improving
 Getting US FDA clearance for your device: Improving 510(k) submissions Audrey Swearingen, RAC Director, Regulatory Affairs Telephone: +1 512.222.0263 Email: aswearingen@emergogroup.com Download this white
Getting US FDA clearance for your device: Improving 510(k) submissions Audrey Swearingen, RAC Director, Regulatory Affairs Telephone: +1 512.222.0263 Email: aswearingen@emergogroup.com Download this white
Overview of USP General Chapters <476> and <1086> Prescription/Non-Prescription Stakeholder Forum October 19, 2017
 Overview of USP General Chapters and Prescription/Non-Prescription Stakeholder Forum October 19, 2017 Introduction Periodic review of existing general chapters Typically an approximately 5
Overview of USP General Chapters and Prescription/Non-Prescription Stakeholder Forum October 19, 2017 Introduction Periodic review of existing general chapters Typically an approximately 5
FDA 510(k) 101 The Basics
 FDA 510(k) 101 The Basics Floyd G. Larson President, PaxMed International San Diego, CA OMTEC June 17, 2010 Chicago Agenda History of 510(k) process FDA s risk based approach FDA guidance and standards
FDA 510(k) 101 The Basics Floyd G. Larson President, PaxMed International San Diego, CA OMTEC June 17, 2010 Chicago Agenda History of 510(k) process FDA s risk based approach FDA guidance and standards
Guideline on the use of the CTD format in the preparation of a registration application for traditional herbal medicinal products 1
 5 April 2016 EMA/HMPC/71049/2007 Rev. 2 Committee on Herbal Medicinal Products (HMPC) Guideline on the use of the CTD format in the preparation of a registration application for traditional herbal medicinal
5 April 2016 EMA/HMPC/71049/2007 Rev. 2 Committee on Herbal Medicinal Products (HMPC) Guideline on the use of the CTD format in the preparation of a registration application for traditional herbal medicinal
Guideline on the use of the CTD format in the preparation of a registration application for traditional herbal medicinal products 1
 1 2 3 8 August 2012 EMA/HMPC/71049/2007 Rev. 1 Committee on Herbal Medicinal Products (HMPC) 4 5 6 7 Guideline on the use of the CTD format in the preparation of a registration application for traditional
1 2 3 8 August 2012 EMA/HMPC/71049/2007 Rev. 1 Committee on Herbal Medicinal Products (HMPC) 4 5 6 7 Guideline on the use of the CTD format in the preparation of a registration application for traditional
JAN MbTten Jurs Chief Executive Officer Pronova BioPharma Norge AS P.O. Box 420 NO-1327 Lysaker NORWAY. Docket No.
 DEPARTMENT OF HEALTH &. HUMAN SERVICES Food and Drug Administration Rockville MD 20857 JAN 28 2010 MbTten Jurs Chief Executive Officer Pronova BioPharma Norge AS P.O. Box 420 NO-1327 Lysaker NORWAY Re:
DEPARTMENT OF HEALTH &. HUMAN SERVICES Food and Drug Administration Rockville MD 20857 JAN 28 2010 MbTten Jurs Chief Executive Officer Pronova BioPharma Norge AS P.O. Box 420 NO-1327 Lysaker NORWAY Re:
USP Perspective on Atypical Actives November 29, 2017
 USP Perspective on Atypical Actives November 29, 2017 USP Excipients Stakeholder Forum USP Perspective on Atypical Actives Catherine Sheehan, M.S., M.S. Senior Director, Science Excipients Outline Role
USP Perspective on Atypical Actives November 29, 2017 USP Excipients Stakeholder Forum USP Perspective on Atypical Actives Catherine Sheehan, M.S., M.S. Senior Director, Science Excipients Outline Role
Use of Standards in Substantial Equivalence Determinations
 Guidance for Industry and for FDA Staff Use of Standards in Substantial Equivalence Determinations Document issued on: March 12, 2000 U.S. Department Of Health And Human Services Food and Drug Administration
Guidance for Industry and for FDA Staff Use of Standards in Substantial Equivalence Determinations Document issued on: March 12, 2000 U.S. Department Of Health And Human Services Food and Drug Administration
Practical guidance for applicants on the submission of applications on food additives, food enzymes and food flavourings
 Version 2 Updated on 29/11/2011 Practical guidance for applicants on the submission of applications on food additives, food enzymes and food flavourings Valid as of: 11 September 2011 Disclaimer: This
Version 2 Updated on 29/11/2011 Practical guidance for applicants on the submission of applications on food additives, food enzymes and food flavourings Valid as of: 11 September 2011 Disclaimer: This
TSDR Pharmacy Inc. dba brandmd Skin Care 11/9/17
 TSDR Pharmacy Inc. dba brandmd Skin Care 11/9/17 Division of Pharmaceutical Quality Operations IV 19701 Fairchild, Irvine, CA 92612-2506 Telephone: 949-608-2900 Fax: 949-608-4417 WARNING LETTER VIA SIGNATURE
TSDR Pharmacy Inc. dba brandmd Skin Care 11/9/17 Division of Pharmaceutical Quality Operations IV 19701 Fairchild, Irvine, CA 92612-2506 Telephone: 949-608-2900 Fax: 949-608-4417 WARNING LETTER VIA SIGNATURE
Requirements to the Registration of Medicinal products in the Republic of Armenia
 Requirements to the Registration of Medicinal products in the Republic of Armenia Yerevan 2010 Requirements to the Registration of Medicinal products in the Republic of Armenia Current requirements to
Requirements to the Registration of Medicinal products in the Republic of Armenia Yerevan 2010 Requirements to the Registration of Medicinal products in the Republic of Armenia Current requirements to
Sterility Assurance and Risk Management: A CDER Microbiologist s Perspective
 Sterility Assurance and Risk Management: A CDER Microbiologist s Perspective John W. Metcalfe, Ph.D. Senior Review Microbiologist FDA/CDER/OPQ/OPF/Division of Microbiology Assessment Center for Drug Evaluation
Sterility Assurance and Risk Management: A CDER Microbiologist s Perspective John W. Metcalfe, Ph.D. Senior Review Microbiologist FDA/CDER/OPQ/OPF/Division of Microbiology Assessment Center for Drug Evaluation
Agency Information Collection Activities; Submission for Office of Management and
 This document is scheduled to be published in the Federal Register on 02/19/2019 and available online at https://federalregister.gov/d/2019-02596, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
This document is scheduled to be published in the Federal Register on 02/19/2019 and available online at https://federalregister.gov/d/2019-02596, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
IPEC Europe Suggested Alternative (if none then original text is clear and needs no alteration) Purpose and Scope
 IPEC Europe Observations and Recommendations on Guidelines On The Formalised Risk Assessment For Ascertaining The Appropriate Good Manufacturing Practice For Excipients Of Medicinal Products For Human
IPEC Europe Observations and Recommendations on Guidelines On The Formalised Risk Assessment For Ascertaining The Appropriate Good Manufacturing Practice For Excipients Of Medicinal Products For Human
Lactose, Monohydrate
 Lactose, Monohydrate Product Regulatory Data Sheet Section 1 Product Information Products Covered Brand Product Code Product Description MOC Code J.T.Baker 2249 Lactose, Monohydrate, Powder N.F. R J.T.Baker
Lactose, Monohydrate Product Regulatory Data Sheet Section 1 Product Information Products Covered Brand Product Code Product Description MOC Code J.T.Baker 2249 Lactose, Monohydrate, Powder N.F. R J.T.Baker
NDA NDA APPROVAL
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 022200 NDA APPROVAL Amylin Pharmaceuticals, Inc. Orville Kolterman, M.D. Sr. Vice President, Research & Development
DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 022200 NDA APPROVAL Amylin Pharmaceuticals, Inc. Orville Kolterman, M.D. Sr. Vice President, Research & Development
Guidance for Industry Migraine: Developing Drugs for Acute Treatment
 Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Guidance for Industry Migraine: Developing Drugs for Acute Treatment DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this draft
Inspections, Compliance, Enforcement, and Criminal Investigations
 Page 1 of 6 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations A Nelson & Co.,
Page 1 of 6 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations A Nelson & Co.,
CENTER FOR DRUG EVALUATION AND RESEARCH. APPLICATION NUMBER: Orig1s000 APPROVAL LETTER
 CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 202107Orig1s000 APPROVAL LETTER DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 202107 NDA APPROVAL
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 202107Orig1s000 APPROVAL LETTER DEPARTMENT OF HEALTH AND HUMAN SERVICES Food and Drug Administration Silver Spring MD 20993 NDA 202107 NDA APPROVAL
Talon Compounding Pharmacy 10/3/17
 Talon Compounding Pharmacy 10/3/17 Office of Pharmaceutical Quality Operations, Division II 4040 N. Central Expressway, Suite 300 Dallas, Texas 75204 October 3, 2017 CMS Case # 522630 VIA UPS EXPRESS WARNING
Talon Compounding Pharmacy 10/3/17 Office of Pharmaceutical Quality Operations, Division II 4040 N. Central Expressway, Suite 300 Dallas, Texas 75204 October 3, 2017 CMS Case # 522630 VIA UPS EXPRESS WARNING
Alignment of FSMA with Existing Food Safety Programs International Citrus & Beverage Conference
 Alignment of FSMA with Existing Food Safety Programs International Citrus & Beverage Conference Donald Kautter US Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Food
Alignment of FSMA with Existing Food Safety Programs International Citrus & Beverage Conference Donald Kautter US Food and Drug Administration Center for Food Safety and Applied Nutrition Office of Food
THE NON PENICILLIN BETA LACTAM DRUG CROSS CONTAMINATION PREVENTION; USFDA PERSPECTIVE
 THE NON PENICILLIN BETA LACTAM DRUG CROSS CONTAMINATION PREVENTION; USFDA PERSPECTIVE An overview by Sarah Vugigi, M. Pharm, Elys Chemical Industries Ltd, Nairobi, Kenya INTRODUCTION This guidance describes
THE NON PENICILLIN BETA LACTAM DRUG CROSS CONTAMINATION PREVENTION; USFDA PERSPECTIVE An overview by Sarah Vugigi, M. Pharm, Elys Chemical Industries Ltd, Nairobi, Kenya INTRODUCTION This guidance describes
(Cochineal Extract and Carmine: Declaration by Name on the Label of All Foods and Cosmetic Products That Contain These Color Additives)
 ก ก 2553 ก : ก (Cochineal Extract and Carmine: Declaration by Name on the Label of All Foods and Cosmetic Products That Contain These Color Additives) : ก ก ก : ก ก ก : 2552 : 2552 1 ก 5 ก : ก (Cochineal
ก ก 2553 ก : ก (Cochineal Extract and Carmine: Declaration by Name on the Label of All Foods and Cosmetic Products That Contain These Color Additives) : ก ก ก : ก ก ก : 2552 : 2552 1 ก 5 ก : ก (Cochineal
Food Additives Program
 U.S. FDA s Food Additive Program: An Update on Resources and Challenges 18 th Food Packaging Law Seminar October 11, 2017 Arlington, VA 1 Food Additives Program Dennis Keefe, PhD Director, Office of Food
U.S. FDA s Food Additive Program: An Update on Resources and Challenges 18 th Food Packaging Law Seminar October 11, 2017 Arlington, VA 1 Food Additives Program Dennis Keefe, PhD Director, Office of Food
International Pharmaceutical Aerosol Consortium on Regulation and Science
 International Pharmaceutical Aerosol Consortium on Regulation and Science 1500 K Street NW Washington DC 20005 Telephone +1 202 230 5607 Fax +1 202 842 8465 Email info@ipacrs.org Web www.ipacrs.org Submitted
International Pharmaceutical Aerosol Consortium on Regulation and Science 1500 K Street NW Washington DC 20005 Telephone +1 202 230 5607 Fax +1 202 842 8465 Email info@ipacrs.org Web www.ipacrs.org Submitted
Please find enclosed Dosimetry Service Licence No which replaces Dosimetry Service Licence No
 Directorate of Nuclear Substance Regulation Telephone: 1 (888) 229-2672 April 12, 2017 Licence Number Christopher Passmore Landauer Inc. 2 Science Road Glenwood, IL 60425-1586 Subject: Dosimetry Service
Directorate of Nuclear Substance Regulation Telephone: 1 (888) 229-2672 April 12, 2017 Licence Number Christopher Passmore Landauer Inc. 2 Science Road Glenwood, IL 60425-1586 Subject: Dosimetry Service
Division of Registrations Gregory Ferland Interim Division Director. Corrected Notice of Proposed Rulemaking and Rulemaking Hearing
 Division of Registrations Gregory Ferland Interim Division Director State Physical Therapy Board Deann Conroy Program Director John W. H1ckenlooper Governor Barbara J. Kelley Executive Director Corrected
Division of Registrations Gregory Ferland Interim Division Director State Physical Therapy Board Deann Conroy Program Director John W. H1ckenlooper Governor Barbara J. Kelley Executive Director Corrected
Inspections, Compliance, Enforcement, and Criminal Investigations
 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations Burzynski Research Institute
Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations Burzynski Research Institute
Product Information Sheet
 Contents: 1. Supplier Information 2. Emergency Contacts 3. Product Information 4. Regulatory Status 5. Ingredient Statement 6. Quality Documents 7. Kosher/Halal Status 8. Allergen Status 9. Sulfur Dioxide
Contents: 1. Supplier Information 2. Emergency Contacts 3. Product Information 4. Regulatory Status 5. Ingredient Statement 6. Quality Documents 7. Kosher/Halal Status 8. Allergen Status 9. Sulfur Dioxide
Exhibit 2 RFQ Engagement Letter
 Exhibit 2 RFQ 17-25 Engagement Letter The attached includes the 6 page proposed engagement letter to be used by HCC. ENGAGEMENT LETTER Dear: [Lead Counsel/Partner] We are pleased to inform you that your
Exhibit 2 RFQ 17-25 Engagement Letter The attached includes the 6 page proposed engagement letter to be used by HCC. ENGAGEMENT LETTER Dear: [Lead Counsel/Partner] We are pleased to inform you that your
Stonegate Pharmacy LP 11/10/16
 Stonegate Pharmacy LP 11/10/16 November 10, 2016 2017 DAL WL 03 WARNING LETTER UPS Overnight Rene F. Garza, Pharm.D., Chief Executive Officer Stonegate Pharmacy, LP 2501 W. William Cannon Drive, Suite
Stonegate Pharmacy LP 11/10/16 November 10, 2016 2017 DAL WL 03 WARNING LETTER UPS Overnight Rene F. Garza, Pharm.D., Chief Executive Officer Stonegate Pharmacy, LP 2501 W. William Cannon Drive, Suite
Preparing a US FDA Medical Device 510(K) Submission
 Preparing a US FDA Medical Device 510(K) Submission If you want to introduce your medical device to the US market, you need to obtain clearance from the FDA. This clearance is obtained from the FDA via
Preparing a US FDA Medical Device 510(K) Submission If you want to introduce your medical device to the US market, you need to obtain clearance from the FDA. This clearance is obtained from the FDA via
Product Information Sheet MERIZET
 Contents: 1. Supplier Information 2. Emergency Contacts 3. Product Information 4. Regulatory Status 5. Ingredient Statement 6. Quality Documents 7. Kosher/Halal Status 8. Allergen Status 9. Sulfur Dioxide
Contents: 1. Supplier Information 2. Emergency Contacts 3. Product Information 4. Regulatory Status 5. Ingredient Statement 6. Quality Documents 7. Kosher/Halal Status 8. Allergen Status 9. Sulfur Dioxide
Guidance for Industry
 Guidance for Industry Revised Recommendations for Donor and Product Management Based on Screening Tests for Syphilis DRAFT GUIDANCE This document is being distributed for comment purposes only. Submit
Guidance for Industry Revised Recommendations for Donor and Product Management Based on Screening Tests for Syphilis DRAFT GUIDANCE This document is being distributed for comment purposes only. Submit
Annex. (Draft for Comments)
 Annex Announcement on the Matters related to filing and Joint Review & Approval of Active Pharmaceutical Ingredients, Pharmaceutical Excipients and Pharmaceutical Packaging Materials for Drug Products
Annex Announcement on the Matters related to filing and Joint Review & Approval of Active Pharmaceutical Ingredients, Pharmaceutical Excipients and Pharmaceutical Packaging Materials for Drug Products
Procedural advice on the submission of variations for annual update of human influenza inactivated vaccines applications in the centralised procedure
 1 2 3 4 5 6 7 8 9 14 April 2010 EMA/CHMP/BWP/99698/2007 Rev. 1 Committee for Medicinal Products for Human Use (CHMP) Procedural advice on the submission of variations for annual update of human influenza
1 2 3 4 5 6 7 8 9 14 April 2010 EMA/CHMP/BWP/99698/2007 Rev. 1 Committee for Medicinal Products for Human Use (CHMP) Procedural advice on the submission of variations for annual update of human influenza
247 CMR BOARD OF REGISTRATION IN PHARMACY
 247 CMR 18.00: NON-STERILE COMPOUNDING Section 18.01: Authority and Purpose 18.02: Non-Sterile Compounding Process 18.03: Non-Sterile Compounding Facility 18.04: Non-Sterile Compounding Equipment 18.05:
247 CMR 18.00: NON-STERILE COMPOUNDING Section 18.01: Authority and Purpose 18.02: Non-Sterile Compounding Process 18.03: Non-Sterile Compounding Facility 18.04: Non-Sterile Compounding Equipment 18.05:
Prof. Marina Heinonen University of Helsinki Member of the NDA Panel and EFSA s WG on Novel Foods
 Guidance on Novel Foods Composition, production process and specification Prof. Marina Heinonen University of Helsinki Member of the NDA Panel and EFSA s WG on Novel Foods Info-Session 06 March 2017 Parma
Guidance on Novel Foods Composition, production process and specification Prof. Marina Heinonen University of Helsinki Member of the NDA Panel and EFSA s WG on Novel Foods Info-Session 06 March 2017 Parma
Guidance for Industry
 Guidance for Industry Dosage Delivery Devices for OTC Liquid Drug Products DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this
Guidance for Industry Dosage Delivery Devices for OTC Liquid Drug Products DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments and suggestions regarding this
Hieber's Pharmacy 12/5/17
 Hieber's Pharmacy 12/5/17 Division of Pharmaceutical Quality Operations I 10 Waterview Blvd, 3rd FL Parsippany, NJ 07054 Telephone: (973) 331-4900 FAX: (973) 331-4969 CERTIFIED MAIL RETURN RECEIPT REQUESTED
Hieber's Pharmacy 12/5/17 Division of Pharmaceutical Quality Operations I 10 Waterview Blvd, 3rd FL Parsippany, NJ 07054 Telephone: (973) 331-4900 FAX: (973) 331-4969 CERTIFIED MAIL RETURN RECEIPT REQUESTED
This license is required for any businesses offering tobacco products for sale.
 Guidelines for City of Moorhead 500 Center Avenue, PO Box 779 Moorhead, MN 56560-0799 Phone: 218.299.5304 Fax: 218.299.5306 cityclerk@ci.moorhead.mn.us Moorhead City Code, 2-5A OVERVIEW This license is
Guidelines for City of Moorhead 500 Center Avenue, PO Box 779 Moorhead, MN 56560-0799 Phone: 218.299.5304 Fax: 218.299.5306 cityclerk@ci.moorhead.mn.us Moorhead City Code, 2-5A OVERVIEW This license is
Points to Consider CPhI Japan Briefing Session on Pharmaceutical Regulations in Japan
 2013 CPhI Japan Briefing Session on Pharmaceutical Regulations in Japan The Master File System and Points to Consider Kentaro Hashimoto, Master File Management Group, Division of Pharmacopoeia and Standards
2013 CPhI Japan Briefing Session on Pharmaceutical Regulations in Japan The Master File System and Points to Consider Kentaro Hashimoto, Master File Management Group, Division of Pharmacopoeia and Standards
4/26/2013. Libia Lugo Drug Specialist San Juan District Office Investigations Branch
 Libia Lugo Drug Specialist San Juan District Office Investigations Branch The Food and Drug Administration (FDA) responsibilities extend to the 50 United States, the District of Columbia, Puerto Rico,
Libia Lugo Drug Specialist San Juan District Office Investigations Branch The Food and Drug Administration (FDA) responsibilities extend to the 50 United States, the District of Columbia, Puerto Rico,
FDA s Food Additives Program
 FDA s Food Additives Program LaShonda T. Cureton, PhD Office of Food Additive Safety US Food and Drug Administration Food Additives: A Global Perspective on Safety Evaluation and Use Procedures for Approval
FDA s Food Additives Program LaShonda T. Cureton, PhD Office of Food Additive Safety US Food and Drug Administration Food Additives: A Global Perspective on Safety Evaluation and Use Procedures for Approval
Product Information Sheet MALTOSWEET
 Contents: 1. Supplier Information 2. Emergency Contacts 3. Product Information 4. Regulatory Status 5. Ingredient Statement 6. Quality Documents 7. Kosher/Halal Status 8. Allergen Status 9. Sulfur Dioxide
Contents: 1. Supplier Information 2. Emergency Contacts 3. Product Information 4. Regulatory Status 5. Ingredient Statement 6. Quality Documents 7. Kosher/Halal Status 8. Allergen Status 9. Sulfur Dioxide
Product Information Sheet RESISTAMYL
 Contents: 1. Supplier Information 2. Emergency Contacts 3. Product Information 4. Regulatory Status 5. Ingredient Statement 6. Quality Documents 7. Kosher/Halal Status 8. Allergen Status 9. Sulfur Dioxide
Contents: 1. Supplier Information 2. Emergency Contacts 3. Product Information 4. Regulatory Status 5. Ingredient Statement 6. Quality Documents 7. Kosher/Halal Status 8. Allergen Status 9. Sulfur Dioxide
GENERAL INFORMATION AND INSTRUCTIONS
 NON-PARTICIPATING MANUFACTURER CERTIFICATION FOR LISTING ON OREGON DIRECTORY GENERAL INFORMATION AND INSTRUCTIONS Who is required to file this Certification? Any tobacco product manufacturer who is a non-participating
NON-PARTICIPATING MANUFACTURER CERTIFICATION FOR LISTING ON OREGON DIRECTORY GENERAL INFORMATION AND INSTRUCTIONS Who is required to file this Certification? Any tobacco product manufacturer who is a non-participating
USP <232> and <233> Understanding Your Path to Compliance with the New Elemental Impurity Chapters. Steve Wall Agilent Technologies
 USP and Understanding Your Path to Compliance with the New Elemental Impurity Chapters Steve Wall Agilent Technologies Outline, USP - USP Chapter - Chapter Limits -
USP and Understanding Your Path to Compliance with the New Elemental Impurity Chapters Steve Wall Agilent Technologies Outline, USP - USP Chapter - Chapter Limits -
Product Information Sheet SODA-LO EXTRA FINE
 Contents: 1. Supplier Information 2. Emergency Contacts 3. Product Information 4. Regulatory Status 5. Ingredient Statement 6. Quality Documents 7. Kosher/Halal Status 8. Allergen Status 9. Sulfur Dioxide
Contents: 1. Supplier Information 2. Emergency Contacts 3. Product Information 4. Regulatory Status 5. Ingredient Statement 6. Quality Documents 7. Kosher/Halal Status 8. Allergen Status 9. Sulfur Dioxide
ASSEMBLY COMMITTEE ON HUMAN SERVICES Blanca Rubio, Chair AB 2702 (McCarty) As Amended April 2, 2018
 Page 1 Date of Hearing: April 24, 2018 SUBJECT: Trustline registry. ASSEMBLY COMMITTEE ON HUMAN SERVICES Blanca Rubio, Chair AB 2702 (McCarty) As Amended April 2, 2018 SUMMARY: Requires the Department
Page 1 Date of Hearing: April 24, 2018 SUBJECT: Trustline registry. ASSEMBLY COMMITTEE ON HUMAN SERVICES Blanca Rubio, Chair AB 2702 (McCarty) As Amended April 2, 2018 SUMMARY: Requires the Department
Product Information Sheet SODA-LO EXTRA FINE IODIZED
 Contents: 1. Supplier Information 2. Emergency Contacts 3. Product Information 4. Regulatory Status 5. Ingredient Statement 6. Quality Documents 7. Kosher/Halal Status 8. Allergen Status 9. Sulfur Dioxide
Contents: 1. Supplier Information 2. Emergency Contacts 3. Product Information 4. Regulatory Status 5. Ingredient Statement 6. Quality Documents 7. Kosher/Halal Status 8. Allergen Status 9. Sulfur Dioxide
Rules of Procedure for Screening and Hearing Meetings
 Page: 1 of 15 SYNOPSIS: The purpose of this document is to provide rules of procedure for Screening and Hearing meetings conducted pursuant to the City s Parking Administrative Monetary Penalties By-law
Page: 1 of 15 SYNOPSIS: The purpose of this document is to provide rules of procedure for Screening and Hearing meetings conducted pursuant to the City s Parking Administrative Monetary Penalties By-law
GUIDE FOR THE NEW ELECTRONIC PAYMENT SYSTEM. Version 1.0 Spanish Agency of Medicines and Medical Devices
 GUIDE FOR THE NEW ELECTRONIC PAYMENT SYSTEM Version 1.0 Spanish Agency of Medicines and Medical Devices NEW ELECTRONIC PAYMENT SYSTEM INTRODUCTION AND ENTRY TO THE SYSTEM DEPARTMENT OF MEDICINES FOR HUMAN
GUIDE FOR THE NEW ELECTRONIC PAYMENT SYSTEM Version 1.0 Spanish Agency of Medicines and Medical Devices NEW ELECTRONIC PAYMENT SYSTEM INTRODUCTION AND ENTRY TO THE SYSTEM DEPARTMENT OF MEDICINES FOR HUMAN
Product Information Sheet TASTEVA Stevia Sweetener
 Contents: 1. Supplier Information 2. Emergency Contacts 3. Product Information 4. Regulatory Status 5. Ingredient Statement 6. Quality Documents 7. Kosher/Halal Status 8. Allergen Status 9. Sulfur Dioxide
Contents: 1. Supplier Information 2. Emergency Contacts 3. Product Information 4. Regulatory Status 5. Ingredient Statement 6. Quality Documents 7. Kosher/Halal Status 8. Allergen Status 9. Sulfur Dioxide
Town and Country Compounding and Consultation Services, LLC 10/17/17
 Town and Country Compounding and Consultation Services, LLC 10/17/17 Division of Pharmaceutical Quality Operations I 10 Waterview Blvd, 3rd FL Parsippany, NJ 07054 Telephone: (973) 331-4900 FAX: (973)
Town and Country Compounding and Consultation Services, LLC 10/17/17 Division of Pharmaceutical Quality Operations I 10 Waterview Blvd, 3rd FL Parsippany, NJ 07054 Telephone: (973) 331-4900 FAX: (973)
Overview of Generic Drug Policy and Introduction of its Review Points/BE Guideline in Japan
 1 5 th Joint Conference of Taiwan and Japan on Medical Products Regulation Overview of Generic Drug Policy and Introduction of its Review Points/BE Guideline in Japan Mr. Yoshihiko Sano Deputy Director,
1 5 th Joint Conference of Taiwan and Japan on Medical Products Regulation Overview of Generic Drug Policy and Introduction of its Review Points/BE Guideline in Japan Mr. Yoshihiko Sano Deputy Director,
Analytical method validation. Presented by Debbie Parker 4 July, 2016
 Analytical method validation Presented by Debbie Parker 4 July, 2016 Introduction This session will cover: Guidance and references The types of test methods Validation requirements Summary Slide 2 PharmOut
Analytical method validation Presented by Debbie Parker 4 July, 2016 Introduction This session will cover: Guidance and references The types of test methods Validation requirements Summary Slide 2 PharmOut
