Arizona Society of Pathologists
|
|
|
- Miles Cole
- 5 years ago
- Views:
Transcription
1 Arizona Society of Pathologists Using in laboratory medicine to improve operations and outcomes April 2, 2016 Ron B. Schifman, MD Southern Arizona VA Healthcare System Department of Veterans Affairs 1
2 Big Data A buzz word that describes massive amounts of structured and unstructured data, or data that expands or changes rapidly. Technology (tools and processes) that organization uses to handle and analyze large amounts of data. Department of Veterans Affairs 2
3 Big Data advertisers - monitoring social media to learn about customer behavior, preferences, or responses to campaigns financial services - predict risk hospitals predict readmission Department of Veterans Affairs 3
4 Big Data Healthcare Analytics Startups Department of Veterans Affairs 4
5 Department of Veterans Affairs 5
6 Big Data Healthcare Analytics Department of Veterans Affairs 6
7 Big Data Healthcare Analytics Department of Veterans Affairs 7
8 Big Data Healthcare Analytics Department of Veterans Affairs 8
9 Big Data Performance and Value Metrics Department of Veterans Affairs 9
10 Big Data Population Health Department of Veterans Affairs 10
11 Big Data Laboratory Medicine Department of Veterans Affairs 11
12 Big Data Laboratory Medicine Pat Hanrahan CANON Professor of Computer Science and Electrical Engineering at Stanford University. Department of Veterans Affairs 12
13 Tableau Healthcare Analytics Department of Veterans Affairs 13
14 Extract, Transform, Load VHA Corporate Data Warehouse VistA HDR NPCD DSS ADR VA DoD CMS Source Systems VHA c VHA a Other Metadata Repository Research Data Marts Conformed Dimensions Data Warehouse Region Warehouses Diabetes OEF/OIF Prog Office Data Marts Outpatient Encounters Pharmacy Benefits Management Value Added Data Common Query, Reporting, Analysis, and Data Mining Tools Acquire Populate Create Access Data Warehouse Marts Information VHA c VHA clinical systems VHA a VHA administrative and financial systems Closed Loop Information System Program Offices Pharmacy Benefits Prosthetics Dental HDR Health Data Repository NPCD National Patient Care Database DSS Decision Support System ADR Administrative Data Repository DoD Dept. of Defense CMS - Centers for Medicare & Medicaid Services Department of Veterans Affairs 14
15 Big Data Test performance Hemolysis (preanalytical) Germline and phenotype testing (analytical) Evidence-based laboratory practices Myoglobinuria Protein C Poplulation health High and low risk (birth cohort) HCV screening Utilization Germline mutation and phenotype testing Department of Veterans Affairs 15
16 Benchmarking Test performance (preanalytical) Hemolysis Department of Veterans Affairs 16
17 Benchmarking Test performance (preanalytical) Hemolysis 16 facilities (November 2014) 220,596 serum potassium results of which 22,442 (10.2%) from ED. Median hemolysis rate: 3.5% ED 0.7% Other Department of Veterans Affairs 17
18 Effect of vitamin K deficiency (INR) on protein C activity and antigen values Protein C and INR 102 VA laboratories and 25,461 cases Protein C testing with elevated INR results The incidence of abnormal protein C results with normal (<1.1) INR levels was 2.9% and accounted for 48.9% of all protein C tests. Protein C was frequently tested when INR results were elevated; 17.2% and 10.9% of cases with INRs of >1.5 and >2.0 were tested for protein C respectively. Department of Veterans Affairs 18
19 Protein C Method Selection Protein C Functional vs Antigenic Testing 73.6% of protein C results are performed by functional methods. However, the relative percent varied by facility; 18 laboratories reported only antigenic protein C results while 30 reported only functional protein C results Distribution among 102 VA laboratories of all protein C tests as % measured by functional assay Department of Veterans Affairs 19
20 Evidence Based Best Practices Myoglobinuria Performance of urine dipstick blood test for detecting myoglobinuria 81 facilities 7,579 cases Department of Veterans Affairs 20
21 Evidence Based Best Practices Department of Veterans Affairs 21
22 Population Health Department of Veterans Affairs 22
23 Patient Results Letters Department of Veterans Affairs 23
24 Automated Patient Letters Test result notification TIU notes are entered automatically via a rules based system, and printing and mailing is automated. Department of Veterans Affairs 24
25 Viral Hepatitis C VHA HIV, Hepatitis & Public Health Pathogens Program Department of Veterans Affairs 25
26 Patient Result Letters Initiative Hepatitis C birth-cohort screening Department of Veterans Affairs 26
27 Hepatitis C Birth Cohort Screening Department of Veterans Affairs 27
28 Viral Hepatitis C High Risk Screening Use of automated algorithm and HCV registry to screen for occult HCV infection among high risk population at risk for HCV infection (ICD-9 code).no history of anti-hcv testing.not in HCV registry.blood specimen in lab Department of Veterans Affairs 28
29 Viral Hepatitis C High risk vs low risk screening Department of Veterans Affairs 29
30 Duplicate Germline Genetic & Phenotype Testing Reliability of Test Results Test Utilization Department of Veterans Affairs 30
31 HLA-B *5701 Phenotype (Abacavir Hypersensitivity) HLA B5701 HLA-B*5701 W/RFL HLA-B HIGH (HLA B*5701.HLA-B*5701 GENOTY ABACAVIR SENS ABACAVIR HYPERSENSITIVITY ABACAVIR HYPERSENSITIVITY (006926) B*5701-HLA B5701 HLA B 5701 HLA B*5701 HLA B*5701 LC HLA B*5701 TYPING HLA B5701 HLA B5701 (D/C 8/1/13) HLA B5701 (LABCORP) HLA B5701 (LC006926)QQQ HLA B5701 (OUTPUT) HLA B5701 (V2) HLA B5701 ANTIGEN HLA B5701(PRE 8/1/2013) HLA B5701* HLA B5701/SEND OUT HLA-B 5701 HLA-B 5701 ( ) HLA-B 5701 TYPING HLA-B 5701 TYPING RT HLA-B 5701 TYPING(STL) HLA-B*5701 HLA-B*5701 HLA-B* HLA-B*5701 (Dcd ) HLA-B*5701 (dc'd 5/6/14) HLA-B*5701 (PCR) HLA-B*5701 Buccal Swab HLA-B*5701 REPORT,blood HLA-B*5701 Test HLA-B*5701 TYPING HLA-B*5701 TYPING (Q) HLA-B*5701 TYPING (QU) HLA-B*5701 TYPING HLA-B*5701 TYPING SPL HLA-B*5701 TYPING(Q) HLA-B*5701 TYPING,blood HLA-B*5701(DC'D 10/24/14) HLA-B*5701. HLA-B5701 HLA-B5701 (19774) HLA-B5701 (REF LAB) HLA-B5701 TEST HLA-B5701 TEST (LC) HLA-B5701 TYPING HLA-B5701(Mayo91833) HLA-B5701(o) HLA-B5701, ABACAVIR HYPERSENSITIVITY ID***HLA-B5701 zzhla B*5701 TYPING ZZ-HLA-B*5701 ZZHLA-B5701 TYPING ZZZHLA-B*5701 Department of Veterans Affairs 31
32 Factor V Leiden testing 120 facilities Department of Veterans Affairs 32
33 Repeat factor V Leiden testing 120 facilities Department of Veterans Affairs 33
34 Repeat factor V Leiden testing 120 facilities 20% retested at different facility Department of Veterans Affairs 34
35 Effect of genotype/phenotype on repeat testing Department of Veterans Affairs 35
36 Genetic Test Patient Registry Reliability of Test Results Department of Veterans Affairs 36
37 Duplicate Germline Genetic Test Utilization Program Process for detection and notification of laboratories about new orders for duplicate germline and phenotype orders Factor V Leiden Prothrombin gene mutation Hemochromatosis IL28B genotype HLA B*5701 HLA B27 Department of Veterans Affairs 37
38 Improving utilization of genetic tests National automated notification system Department of Veterans Affairs 38
39 Duplicate Genetic Testing Program February 2015 January 2016 Intervention Control 22 facilities 101 facilities Previous testing performed at a different facility in 87 (37.5%) cases 232 duplicates 949 duplicates Previous testing performed at a different facility in 280 (29.5%) of cases 142 (61.2%) cancellations 32 (3.4%) cancellations Department of Veterans Affairs 39
40 Duplicate Germline Genetic Test Utilization Program Department of Veterans Affairs 40
41 Duplicate Germline Genetic Test Utilization Program Department of Veterans Affairs 41
42 Department of Veterans Affairs 42
43 Big Data Team Department of Veterans Affairs 43
44 Department of Veterans Affairs
Big Data Phenomics in the VA. Outline
 Big Phenomics in the VA Mary Whooley MD Director, VA Measurement Science QUERI San Francisco VA Health Care System University of California, San Francisco Kelly Cho PhD MPH Phenomics Lead, Million Veteran
Big Phenomics in the VA Mary Whooley MD Director, VA Measurement Science QUERI San Francisco VA Health Care System University of California, San Francisco Kelly Cho PhD MPH Phenomics Lead, Million Veteran
Department of Veterans Affairs
 Department of Veterans Affairs HEPATITIS C TREATMENT : REQUEST FOR CHOICE PROGRAM FLEXIBILITY TO ADDRESS $500 MILLION SHORTFALL IN FY 2015 7/14/15 1 Veteran Hepatitis-C Treatment: Summary VA is changing
Department of Veterans Affairs HEPATITIS C TREATMENT : REQUEST FOR CHOICE PROGRAM FLEXIBILITY TO ADDRESS $500 MILLION SHORTFALL IN FY 2015 7/14/15 1 Veteran Hepatitis-C Treatment: Summary VA is changing
Monitoring the HCV care cascade to inform public health action
 Monitoring the HCV care cascade to inform public health action Susan Hariri, PhD Division of Viral Hepatitis Centers for Disease Control and Prevention NASTAD Hepatitis Technical Assistance Meeting Washington
Monitoring the HCV care cascade to inform public health action Susan Hariri, PhD Division of Viral Hepatitis Centers for Disease Control and Prevention NASTAD Hepatitis Technical Assistance Meeting Washington
HCV in Veterans Administration (VA)
 HCV in Veterans Administration (VA) Timothy Morgan, MD, Rachel Gonzalez, MPH, Angela Park, PharmD, Pam S. Belperio, PharmD, Bill Lukesh, Kristine Desotto, Tim Schmoke, Maggie Chartier, PhD, David Ross,
HCV in Veterans Administration (VA) Timothy Morgan, MD, Rachel Gonzalez, MPH, Angela Park, PharmD, Pam S. Belperio, PharmD, Bill Lukesh, Kristine Desotto, Tim Schmoke, Maggie Chartier, PhD, David Ross,
The Short-Term Incidence of Hepatocellular Carcinoma Is Not Increased After Hepatitis C Treatment with Direct-Acting Antivirals: An ERCHIVES Study
 The Short-Term Incidence of Hepatocellular Carcinoma Is Not Increased After Hepatitis C Treatment with Direct-Acting Antivirals: An ERCHIVES Study DK Li, YJ Ren, DS Fierer, S Rutledge, OS Shaikh, V Lo
The Short-Term Incidence of Hepatocellular Carcinoma Is Not Increased After Hepatitis C Treatment with Direct-Acting Antivirals: An ERCHIVES Study DK Li, YJ Ren, DS Fierer, S Rutledge, OS Shaikh, V Lo
From Population Health to Precision Health. William J, Kassler, MD, MPH Deputy Chief Health Officer March 28, 2017
 From Population Health to Precision Health William J, Kassler, MD, MPH Deputy Chief Health Officer March 28, 2017 2 The current health system faces serious challenges. 3 A New Era of Personalized Healthcare
From Population Health to Precision Health William J, Kassler, MD, MPH Deputy Chief Health Officer March 28, 2017 2 The current health system faces serious challenges. 3 A New Era of Personalized Healthcare
HCV Testing Challenges & Systems-based Solutions
 HCV Testing Challenges & Systems-based Solutions Webcast 2.3 Presented By: Denise Stinson, MN, RN Tacoma-Pierce County Health Department Communicable Disease Control Program Manager Webcast Overview 1
HCV Testing Challenges & Systems-based Solutions Webcast 2.3 Presented By: Denise Stinson, MN, RN Tacoma-Pierce County Health Department Communicable Disease Control Program Manager Webcast Overview 1
Validation of Clinical Outcomes in Electronic Data Sources
 Validation of Clinical Outcomes in Electronic Data Sources Vincent Lo Re, MD, MSCE Assistant Professor of Medicine and Epidemiology Center for Clinical Epidemiology and Biostatistics Center for Pharmacoepidemiology
Validation of Clinical Outcomes in Electronic Data Sources Vincent Lo Re, MD, MSCE Assistant Professor of Medicine and Epidemiology Center for Clinical Epidemiology and Biostatistics Center for Pharmacoepidemiology
Cancer Care in the Veterans Health Administration
 Cancer Care in the Veterans Health Administration Michael J Kelley, MD National Program Director for Oncology Department of Veterans Affairs Professor of Medicine Duke University Medical Center Chief,
Cancer Care in the Veterans Health Administration Michael J Kelley, MD National Program Director for Oncology Department of Veterans Affairs Professor of Medicine Duke University Medical Center Chief,
Veterans Health Administration Lung Cancer Screening Demonstration Project: Results & Lessons Learned
 Veterans Health Administration Lung Cancer Screening Demonstration Project: Results & Lessons Learned Jane Kim, MD, MPH Acting Chief Consultant for Preventive Medicine National Center for Health Promotion
Veterans Health Administration Lung Cancer Screening Demonstration Project: Results & Lessons Learned Jane Kim, MD, MPH Acting Chief Consultant for Preventive Medicine National Center for Health Promotion
Table 2.1. Cohort Studies of HCV and Hepatocellular Carcinoma
 Americas Guiltinan et al. (2008) USA Retrospective cohort of 10259 anti- HCV-positive allogeneic blood donors (6627 men, 3632 women) and 10259 anti-hcv-negative donors (6627 men, 3632 women) matched to
Americas Guiltinan et al. (2008) USA Retrospective cohort of 10259 anti- HCV-positive allogeneic blood donors (6627 men, 3632 women) and 10259 anti-hcv-negative donors (6627 men, 3632 women) matched to
Department of Veterans Affairs (VA): Program in Rehabilitation Research
 Department of Veterans Affairs (VA): Program in Rehabilitation Research Overview Funding Opportunities Christopher A. Moore, Ph.D., Dean College of Health and Rehabilitation Sciences: Sargent College Boston
Department of Veterans Affairs (VA): Program in Rehabilitation Research Overview Funding Opportunities Christopher A. Moore, Ph.D., Dean College of Health and Rehabilitation Sciences: Sargent College Boston
A Case Study on Visual Analytics for Optimizing Drug Duplicate Alerts in a Medication Clinical Decision Support System
 A Case Study on Visual Analytics for Optimizing Drug Duplicate Alerts in a Medication Clinical Decision Support System Jaehoon Lee, PhD Wendi L. Record, PharmD Nathan C. Hulse, PhD Intermountain Healthcare
A Case Study on Visual Analytics for Optimizing Drug Duplicate Alerts in a Medication Clinical Decision Support System Jaehoon Lee, PhD Wendi L. Record, PharmD Nathan C. Hulse, PhD Intermountain Healthcare
How we monitor the Programme
 How we monitor the Programme Andrew Boulle, 1,2 SA HIV Clinicians Society Conference, 13-16 April 2016 1) Health Impact Assessment, Department of Health, Western Cape Province of South Africa 2) School
How we monitor the Programme Andrew Boulle, 1,2 SA HIV Clinicians Society Conference, 13-16 April 2016 1) Health Impact Assessment, Department of Health, Western Cape Province of South Africa 2) School
CAP Laboratory Improvement Programs
 CAP Laboratory Improvement Programs A Q-Probes Study Involving Utilization of Free Prostate-Specific Antigen, Factor V Leiden, and Hepatitis A Serology Tests Ron B. Schifman, MD; Peter L. Perrotta, MD;
CAP Laboratory Improvement Programs A Q-Probes Study Involving Utilization of Free Prostate-Specific Antigen, Factor V Leiden, and Hepatitis A Serology Tests Ron B. Schifman, MD; Peter L. Perrotta, MD;
Retention in HIV care predicts subsequent retention and predicts survival well after the first year of care: a national study of US Veterans
 Retention in HIV care predicts subsequent retention and predicts survival well after the first year of care: a national study of US Veterans Thomas P. Giordano, MD, MPH, Jessica A. Davila, PhD, Christine
Retention in HIV care predicts subsequent retention and predicts survival well after the first year of care: a national study of US Veterans Thomas P. Giordano, MD, MPH, Jessica A. Davila, PhD, Christine
Reach Out to Patients for Better Disease Management
 CASE STUDY Reach Out to Patients for Better Disease Management Logansport Memorial Hospital How automated reminders led to a higher compliance rate for overdue labs by diabetic patients. Quick Summary
CASE STUDY Reach Out to Patients for Better Disease Management Logansport Memorial Hospital How automated reminders led to a higher compliance rate for overdue labs by diabetic patients. Quick Summary
The Architecture of Performance Measurement
 The Architecture of Performance Measurement Jason Oliveira Managing Director, Health Systems Consulting September 19 th, 2012 Copyright 2010 Recombinant Data Corp. All rights reserved. 1 Today s speaker
The Architecture of Performance Measurement Jason Oliveira Managing Director, Health Systems Consulting September 19 th, 2012 Copyright 2010 Recombinant Data Corp. All rights reserved. 1 Today s speaker
How to Develop an Effective Utilization Management Program. My background. Learning Objectives
 How to Develop an Effective Utilization Management Program Robert L Schmidt, MD, PhD, MBA Department of Pathology & ARUP Laboratories, University of Utah Director, Center for Evidence-Based Testing Medical
How to Develop an Effective Utilization Management Program Robert L Schmidt, MD, PhD, MBA Department of Pathology & ARUP Laboratories, University of Utah Director, Center for Evidence-Based Testing Medical
EMR Big Data and Clinical Research. -Distributed Research Network and Common Data Model-
 2016 Healthcare Innovation Forum EMR Big Data and Clinical Research -Distributed Research Network and Common Data Model- Rae Woong Park, MD, PhD Department of Biomedical Informatics Ajou University School
2016 Healthcare Innovation Forum EMR Big Data and Clinical Research -Distributed Research Network and Common Data Model- Rae Woong Park, MD, PhD Department of Biomedical Informatics Ajou University School
on October 4, 2018 by guest
 JCM Accepts, published online ahead of print on 3 July 2012 J. Clin. Microbiol. doi:10.1128/jcm.01249-12 Copyright 2012, American Society for Microbiology. All Rights Reserved. 1 Title: Performance of
JCM Accepts, published online ahead of print on 3 July 2012 J. Clin. Microbiol. doi:10.1128/jcm.01249-12 Copyright 2012, American Society for Microbiology. All Rights Reserved. 1 Title: Performance of
Core 3: Epidemiology and Risk Analysis
 Core 3: Epidemiology and Risk Analysis Aron J. Hall, DVM, MSPH, DACVPM CDC Viral Gastroenteritis Team NoroCORE Full Collaborative Meeting, Atlanta, GA November 7, 2012 Core 3: Purpose and Personnel * Purpose:
Core 3: Epidemiology and Risk Analysis Aron J. Hall, DVM, MSPH, DACVPM CDC Viral Gastroenteritis Team NoroCORE Full Collaborative Meeting, Atlanta, GA November 7, 2012 Core 3: Purpose and Personnel * Purpose:
Commercial Health Insurance Claims Data. for Studying HIV/AIDS Care. Senior Scientist, Innovus Epidemiology. David D.
 Commercial Health Insurance Claims Data for Studying HIV/AIDS Care David D. Dore, PharmD, PhD Senior Scientist, Innovus Epidemiology Adjunct Assistant Professor, Alpert Medical School, Brown University
Commercial Health Insurance Claims Data for Studying HIV/AIDS Care David D. Dore, PharmD, PhD Senior Scientist, Innovus Epidemiology Adjunct Assistant Professor, Alpert Medical School, Brown University
ECHO-Antibiotic Stewardship Program
 ECHO-Antibiotic Stewardship Program More Interesting Recent Literature Updates April 20, 2017 Charles Krasner, M.D. University of NV, Reno School of Medicine Sierra NV Veterans Affairs Medical Center Antibiotic
ECHO-Antibiotic Stewardship Program More Interesting Recent Literature Updates April 20, 2017 Charles Krasner, M.D. University of NV, Reno School of Medicine Sierra NV Veterans Affairs Medical Center Antibiotic
The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases
 Alimentary Pharmacology & Therapeutics The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases J. R. KRAMER*,, J.A.DAVILA*,, E.D.MILLER*,, P.RICHARDSON*,,
Alimentary Pharmacology & Therapeutics The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases J. R. KRAMER*,, J.A.DAVILA*,, E.D.MILLER*,, P.RICHARDSON*,,
POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK)
 POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK) Dated: January 1, 2018 Supersedes: Blood and Body Fluid Exposure (Needle stick) dated July 1, 2015 I. POLICY It is the policy of New York Medical
POLICY ON BLOOD AND BODY FLUID EXPOSURE (NEEDLE STICK) Dated: January 1, 2018 Supersedes: Blood and Body Fluid Exposure (Needle stick) dated July 1, 2015 I. POLICY It is the policy of New York Medical
Opioid Safety Initiative. Thomas Emmendorfer, PharmD Deputy Chief Consultant Pharmacy Benefits Management (PBM) Services
 Opioid Safety Initiative Thomas Emmendorfer, PharmD Deputy Chief Consultant Pharmacy Benefits Management (PBM) Services 1 2 Opioid Safety Initiative (OSI) Overdose Education and Naloxone Distribution (OEND):
Opioid Safety Initiative Thomas Emmendorfer, PharmD Deputy Chief Consultant Pharmacy Benefits Management (PBM) Services 1 2 Opioid Safety Initiative (OSI) Overdose Education and Naloxone Distribution (OEND):
ARIZONA INFLUENZA SUMMARY
 ARIZONA INFLUENZA SUMMARY Week 4 (1/21/2018 1/27/2018) Synopsis: Influenza activity is elevated. Arizona reported Widespread Activity for week 4. Subscribe to the Flu & RSV report at azhealth.gov/email.
ARIZONA INFLUENZA SUMMARY Week 4 (1/21/2018 1/27/2018) Synopsis: Influenza activity is elevated. Arizona reported Widespread Activity for week 4. Subscribe to the Flu & RSV report at azhealth.gov/email.
Supplementary Material*
 Supplementary Material* Park LS, Tate JP, Sigel K, Brown ST, Crothers K, Gibert C, et al. Association of Viral Suppression With Lower AIDS-Defining and Non AIDS-Defining Cancer Incidence in HIV-Infected
Supplementary Material* Park LS, Tate JP, Sigel K, Brown ST, Crothers K, Gibert C, et al. Association of Viral Suppression With Lower AIDS-Defining and Non AIDS-Defining Cancer Incidence in HIV-Infected
HIV Drug Resistance South Africa, How to address the increasing need? 14 Apr. 2016
 HIV Drug Resistance South Africa, How to address the increasing need? 14 Apr. 2016 1 Thus the HIV DR needs to focus on prevention and then diagnostic capacity to 1 st provide VL monitoring for early &
HIV Drug Resistance South Africa, How to address the increasing need? 14 Apr. 2016 1 Thus the HIV DR needs to focus on prevention and then diagnostic capacity to 1 st provide VL monitoring for early &
MEETING THE STANDARDS: FDA MANDATORY RAPID INFLUENZA DETECTION TEST (RIDTs) RECLASSIFICATION
 MEETING THE STANDARDS: FDA MANDATORY RAPID INFLUENZA DETECTION TEST (RIDTs) RECLASSIFICATION NOVEMBER 6, 2017 SALLY A. HOJVAT M.Sc., Ph.D. Retired as Director of FDA Division of Microbiology Devices, CDRH
MEETING THE STANDARDS: FDA MANDATORY RAPID INFLUENZA DETECTION TEST (RIDTs) RECLASSIFICATION NOVEMBER 6, 2017 SALLY A. HOJVAT M.Sc., Ph.D. Retired as Director of FDA Division of Microbiology Devices, CDRH
Faculty. W4: Identifying Patients with Rare Disorders Using Administrative Data. O Dan Malone, RPh, PhD University of Arizona
 W4: Identifying Patients with Rare Disorders Using Administrative Data Faculty O Dan Malone, RPh, PhD University of Arizona O Danny Yeh, PhD Biogen O Ed Armstrong Strategic Therapeutics, LLC O Eric Bell,
W4: Identifying Patients with Rare Disorders Using Administrative Data Faculty O Dan Malone, RPh, PhD University of Arizona O Danny Yeh, PhD Biogen O Ed Armstrong Strategic Therapeutics, LLC O Eric Bell,
Quality Improvement through HIT
 Quality Improvement through HIT What is quality in healthcare? Safe Effective Patientcentered Timely Efficient Equitable Overview Reinforce a vision for using HIT to improve quality Share our approach
Quality Improvement through HIT What is quality in healthcare? Safe Effective Patientcentered Timely Efficient Equitable Overview Reinforce a vision for using HIT to improve quality Share our approach
Tracking, Trending and Auditing Across a Multi-State Health System
 Tracking, Trending and Auditing Across a Multi-State Health System Fifth National Medicare RAC Summit March 10, 2011 Susan Shiflett, RHIA, CHC Director Corporate Responsibility Catholic Health Initiatives
Tracking, Trending and Auditing Across a Multi-State Health System Fifth National Medicare RAC Summit March 10, 2011 Susan Shiflett, RHIA, CHC Director Corporate Responsibility Catholic Health Initiatives
Global strategy on viral hepatitis and regional action plan: monitoring framework and 10 core indicators
 Global strategy on viral hepatitis and regional action plan: monitoring framework and 10 core indicators Antons Mozalevskis WHO Regional Office for Europe EMCDDA DRID National Expert Meeting Lisbon, 6
Global strategy on viral hepatitis and regional action plan: monitoring framework and 10 core indicators Antons Mozalevskis WHO Regional Office for Europe EMCDDA DRID National Expert Meeting Lisbon, 6
Table 2.6. Cohort studies of HCV and lymphoid malignancies
 HIV-negative subjects Ohsawa et al. (1999) Japan 2162 patients with HCVrelated chronic hepatitis (1398 men, 834 women), admitted to 3 medical institutions in Osaka between 1957 and 1997; age range: 18
HIV-negative subjects Ohsawa et al. (1999) Japan 2162 patients with HCVrelated chronic hepatitis (1398 men, 834 women), admitted to 3 medical institutions in Osaka between 1957 and 1997; age range: 18
Epidemiology and Screening for Hepatitis C Infection
 Epidemiology and Screening for Hepatitis C Infection Atif Zaman, MD MPH Oregon Health & Science University Professor of Medicine Division of Gastroenterology and Hepatology Epidemiology/Screening for Hepatitis
Epidemiology and Screening for Hepatitis C Infection Atif Zaman, MD MPH Oregon Health & Science University Professor of Medicine Division of Gastroenterology and Hepatology Epidemiology/Screening for Hepatitis
Homeless Incidence and Risk Factors for Becoming Homeless in Veterans
 Homeless Incidence and Risk Factors for Becoming Homeless in Veterans (http://www.va.gov/oig/pubs/vaoig-11-03428-173.pdf) Lin Clegg, Ph.D., M.S Lin.Clegg@VA.gov John Daigh, M.D., CPA Office of Inspector
Homeless Incidence and Risk Factors for Becoming Homeless in Veterans (http://www.va.gov/oig/pubs/vaoig-11-03428-173.pdf) Lin Clegg, Ph.D., M.S Lin.Clegg@VA.gov John Daigh, M.D., CPA Office of Inspector
RECENT HIV DATA TO CARE (D2C) EXPERIENCES MARYLAND
 RECENT HIV DATA TO CARE (D2C) EXPERIENCES MARYLAND HIV HEALTH IMPROVEMENT AFFINITY GROUP DECEMBER 6, 2016 Colin Flynn, Chief HIV Surveillance, Epidemiology and Evaluation Maryland Department of Health
RECENT HIV DATA TO CARE (D2C) EXPERIENCES MARYLAND HIV HEALTH IMPROVEMENT AFFINITY GROUP DECEMBER 6, 2016 Colin Flynn, Chief HIV Surveillance, Epidemiology and Evaluation Maryland Department of Health
Trends in molecular diagnostics
 Trends in molecular diagnostics Detection of target genes of interest Quantification Infectious diseases HIV Hepatitis C & B TB / MAC Cytomegalovirus Herpes simplex Varicella zoster CT/GC HPV Profiling
Trends in molecular diagnostics Detection of target genes of interest Quantification Infectious diseases HIV Hepatitis C & B TB / MAC Cytomegalovirus Herpes simplex Varicella zoster CT/GC HPV Profiling
Negative Hepatitis C Reporting and Linkage to Care Outreach
 Negative Hepatitis C Reporting and Linkage to Care Outreach NASTAD 7 th National Hepatitis Technical Assistance Meeting November 28-30, 2017 Angelica Bocour, MPH Director of Viral Hepatitis Surveillance
Negative Hepatitis C Reporting and Linkage to Care Outreach NASTAD 7 th National Hepatitis Technical Assistance Meeting November 28-30, 2017 Angelica Bocour, MPH Director of Viral Hepatitis Surveillance
Anti-Infective Clinical Trials
 Anti-Infective Clinical Trials The extensive clinical training and experience of our infectious disease staff places us in a unique position to fully appreciate the requirements of our clients conducting
Anti-Infective Clinical Trials The extensive clinical training and experience of our infectious disease staff places us in a unique position to fully appreciate the requirements of our clients conducting
NRL EQAS for NAT: Assessing the variability and performance of molecular assays for clinical pathogens
 NRL EQAS for NAT: Assessing the variability and performance of molecular assays for clinical pathogens Vincini GA*, Cabuang LM, Land S, Best SJ NRL London, 12 January 2011 NRL EQAS for NAT HCV Genotyping
NRL EQAS for NAT: Assessing the variability and performance of molecular assays for clinical pathogens Vincini GA*, Cabuang LM, Land S, Best SJ NRL London, 12 January 2011 NRL EQAS for NAT HCV Genotyping
Kelly M. Harrington, Ph.D. for the CSP 590 Study Group. Society for Clinical Trials Meeting May 19, 2015
 1 Kelly M. Harrington, Ph.D. for the CSP 590 Study Group Society for Clinical Trials Meeting May 19, 2015 2 Background Thoughtful pre trial planning determine the feasibility and costs necessary to balance
1 Kelly M. Harrington, Ph.D. for the CSP 590 Study Group Society for Clinical Trials Meeting May 19, 2015 2 Background Thoughtful pre trial planning determine the feasibility and costs necessary to balance
Surveillance and SEER Where are we going? NAACCR Meeting June 23, 2017 Lynne Penberthy MD, MPH
 Surveillance and SEER Where are we going? NAACCR Meeting June 23, 2017 Lynne Penberthy MD, MPH Presentation Objectives Describe Future Role of Registries & Registrars Challenges to cancer surveillance
Surveillance and SEER Where are we going? NAACCR Meeting June 23, 2017 Lynne Penberthy MD, MPH Presentation Objectives Describe Future Role of Registries & Registrars Challenges to cancer surveillance
at Kaiser Permanente, Southern California April 2017
 Complete Care at Kaiser Permanente, Southern California April 2017 Tim Ho, MD, MPH Regional Assistant Medical Director, Quality & Complete Care Southern California Permanente Medical Group Session Objectives
Complete Care at Kaiser Permanente, Southern California April 2017 Tim Ho, MD, MPH Regional Assistant Medical Director, Quality & Complete Care Southern California Permanente Medical Group Session Objectives
Risk Classification Modeling to Combat Opioid Abuse
 Risk Classification Modeling to Combat Opioid Abuse Improve Data Sharing to Identify High-Risk Prescribers of Opioids Christopher Sterling Chief Statistician NCI, Inc. www.nciinc.com 11730 Plaza America
Risk Classification Modeling to Combat Opioid Abuse Improve Data Sharing to Identify High-Risk Prescribers of Opioids Christopher Sterling Chief Statistician NCI, Inc. www.nciinc.com 11730 Plaza America
Chapter 2: Identification and Care of Patients With CKD
 Chapter 2: Identification and Care of Patients With CKD Over half of patients in the Medicare 5% sample (aged 65 and older) had at least one of three diagnosed chronic conditions chronic kidney disease
Chapter 2: Identification and Care of Patients With CKD Over half of patients in the Medicare 5% sample (aged 65 and older) had at least one of three diagnosed chronic conditions chronic kidney disease
Population Health in an Integrated Care Delivery System
 Population Health in an Integrated Care Delivery System Michael McNamara, MD Chief Medical Information Officer Northwest Permanente Medical Group Kaiser Permanente Agenda KPNW Overview of Population Health
Population Health in an Integrated Care Delivery System Michael McNamara, MD Chief Medical Information Officer Northwest Permanente Medical Group Kaiser Permanente Agenda KPNW Overview of Population Health
Laboratory diagnostics CH/HIV/0052/17/10/2017
 Laboratory diagnostics CH/HIV/0052/17/10/2017 This slide set was created by ViiV Healthcare GmbH with great care in order to provide balanced information about ViiV products and / or application areas.
Laboratory diagnostics CH/HIV/0052/17/10/2017 This slide set was created by ViiV Healthcare GmbH with great care in order to provide balanced information about ViiV products and / or application areas.
Icd 10 cm code for positive urine drug screen
 Icd 10 cm code for positive urine drug screen Free, official coding info for 2018 ICD- 10-CM R82.5 - includes detailed rules, notes,. Elevated urine levels of drugs, medicaments and biological substances.
Icd 10 cm code for positive urine drug screen Free, official coding info for 2018 ICD- 10-CM R82.5 - includes detailed rules, notes,. Elevated urine levels of drugs, medicaments and biological substances.
Chapter 2: Identification and Care of Patients with CKD
 Chapter 2: Identification and Care of Patients with CKD Over half of patients in the Medicare 5% sample (aged 65 and older) had at least one of three diagnosed chronic conditions chronic kidney disease
Chapter 2: Identification and Care of Patients with CKD Over half of patients in the Medicare 5% sample (aged 65 and older) had at least one of three diagnosed chronic conditions chronic kidney disease
AUSTRALIAN IMMUNISATION REGISTER - ENHANCEMENTS
 AUSTRALIAN IMMUNISATION REGISTER - ENHANCEMENTS Updates September 2016 June 2018... 1 AIR Enhancements - June 2018... 2 AIR Enhancements planned for release September 2018... 3 Using the AIR Online Secure
AUSTRALIAN IMMUNISATION REGISTER - ENHANCEMENTS Updates September 2016 June 2018... 1 AIR Enhancements - June 2018... 2 AIR Enhancements planned for release September 2018... 3 Using the AIR Online Secure
Transfusion Medicine (Comprehensive, Limited) J, J1
 www.cap.org Transfusion Medicine Analytes/procedures in bold type are regulated for proficiency testing by the Centers for Medicare & Medicaid Services (CMS). Transfusion Medicine (Comprehensive, Limited)
www.cap.org Transfusion Medicine Analytes/procedures in bold type are regulated for proficiency testing by the Centers for Medicare & Medicaid Services (CMS). Transfusion Medicine (Comprehensive, Limited)
REACH VET and the Possible Impact on Integrated Healthcare
 REACH VET and the Possible Impact on Integrated Healthcare Dr. Kaily Cannizzaro Rocky Mountain MIRECC for Suicide Prevention U.S. Department of Veterans Affairs REACH VET Based on the finding that, although
REACH VET and the Possible Impact on Integrated Healthcare Dr. Kaily Cannizzaro Rocky Mountain MIRECC for Suicide Prevention U.S. Department of Veterans Affairs REACH VET Based on the finding that, although
Centers for Disease Control and Prevention (CDC) FY 2009 Budget Request Summary
 Centers for Disease Control and Prevention (CDC) FY 2009 Budget Request Summary The President s FY 2009 Budget Request for the Centers for Disease Control and Prevention (CDC) discretionary funding is
Centers for Disease Control and Prevention (CDC) FY 2009 Budget Request Summary The President s FY 2009 Budget Request for the Centers for Disease Control and Prevention (CDC) discretionary funding is
Molecular Diagnostics Overview JAN A. NOWAK, PHD, MD PATHOLOGY AND LABORATORY MEDICINE MOLECULAR DIAGNOSTICS LABORATORY FEBRUARY 15, 2018
 Molecular Diagnostics Overview JAN A. NOWAK, PHD, MD PATHOLOGY AND LABORATORY MEDICINE MOLECULAR DIAGNOSTICS LABORATORY FEBRUARY 15, 2018 Some Key Points Molecular Testing has applications in every section
Molecular Diagnostics Overview JAN A. NOWAK, PHD, MD PATHOLOGY AND LABORATORY MEDICINE MOLECULAR DIAGNOSTICS LABORATORY FEBRUARY 15, 2018 Some Key Points Molecular Testing has applications in every section
COMPLIANCE. Individual Highlights: Advance Beneficiary Notices Medical Necessity IL Requisition Reflex Test List Panel Test. September 2018.
 September 2018 COMPLIANCE Dear Physician: Inova Laboratories (IL) is proud to serve the Northern Virginia community as the only full-service reference laboratory. Each year we disclose information about
September 2018 COMPLIANCE Dear Physician: Inova Laboratories (IL) is proud to serve the Northern Virginia community as the only full-service reference laboratory. Each year we disclose information about
TO: Physicians, other Healthcare Providers, and Laboratories. Please distribute a copy of this information to each provider in your organization.
 HEALTH ADVISORY TO: Physicians, other Healthcare Providers, and Laboratories Please distribute a copy of this information to each provider in your organization. Questions regarding this information may
HEALTH ADVISORY TO: Physicians, other Healthcare Providers, and Laboratories Please distribute a copy of this information to each provider in your organization. Questions regarding this information may
CPT Codes for Pharmacogenomic Tests
 CPT s for Pharmacogenomic Tests The table below lists CPT codes and lab fee information for pharmacogenomic tests as established by the Centers for Medicare and Medicaid Services. It was compiled by the
CPT s for Pharmacogenomic Tests The table below lists CPT codes and lab fee information for pharmacogenomic tests as established by the Centers for Medicare and Medicaid Services. It was compiled by the
Following the health of half a million participants
 Following the health of half a million participants Cathie Sudlow UK Biobank Scientific Conference London, June 2018 Follow-up of participants in very large prospective cohorts Aim: identify a wide range
Following the health of half a million participants Cathie Sudlow UK Biobank Scientific Conference London, June 2018 Follow-up of participants in very large prospective cohorts Aim: identify a wide range
Hepatitis C Best Practice Guidelines For Local Health Departments
 Hepatitis C Best Practice Guidelines For Local Health Departments LHDs are responsible for investigating and reporting all physician reported cases of acute hepatitis C (HCV). For clients known to have
Hepatitis C Best Practice Guidelines For Local Health Departments LHDs are responsible for investigating and reporting all physician reported cases of acute hepatitis C (HCV). For clients known to have
Weekly Influenza & Respiratory Activity: Statistics Summary
 Weekly Influenza & Respiratory Activity: Statistics Summary 2011-12 updated 7/12/12 Influenza Activity in Minnesota Summary of the 2011-12 Season Since the start of the influenza season, 552 people were
Weekly Influenza & Respiratory Activity: Statistics Summary 2011-12 updated 7/12/12 Influenza Activity in Minnesota Summary of the 2011-12 Season Since the start of the influenza season, 552 people were
Predictive Analytics for Retention in HIV Care
 Predictive Analytics for Retention in HIV Care Jessica Ridgway, MD, MS; Arthi Ramachandran, PhD; Hannes Koenig, MS; Avishek Kumar, PhD; Joseph Walsh, PhD; Christina Sung; Rayid Ghani, MS; John A. Schneider,
Predictive Analytics for Retention in HIV Care Jessica Ridgway, MD, MS; Arthi Ramachandran, PhD; Hannes Koenig, MS; Avishek Kumar, PhD; Joseph Walsh, PhD; Christina Sung; Rayid Ghani, MS; John A. Schneider,
Weekly Influenza Activity: Statistics Summary
 Weekly Influenza Activity: Statistics Summary 2010-11 updated 9/9/11 Summary of the 2010-11 Influenza Season Since the start of the influenza season, 215 schools reported outbreaks of ILI. Influenza Activity
Weekly Influenza Activity: Statistics Summary 2010-11 updated 9/9/11 Summary of the 2010-11 Influenza Season Since the start of the influenza season, 215 schools reported outbreaks of ILI. Influenza Activity
The future of healthcare is data.
 The future of healthcare is data. Experience Real Engagement. Real Data. In Real Time. TracPatch will provide the healthcare market with evidence-based care, predictive analytics, and remote monitoring.
The future of healthcare is data. Experience Real Engagement. Real Data. In Real Time. TracPatch will provide the healthcare market with evidence-based care, predictive analytics, and remote monitoring.
Using IBM Unified Data Model for Healthcare to Maximize the Value of Unstructured Data in a Population Healthcare Management Program
 IBM Software White paper Information Management Using IBM Unified Data Model for Healthcare to Maximize the Value of Unstructured Data in a Population Healthcare Management Program 2 Using IBM UDMH for
IBM Software White paper Information Management Using IBM Unified Data Model for Healthcare to Maximize the Value of Unstructured Data in a Population Healthcare Management Program 2 Using IBM UDMH for
Balancing Glycemic Overtreatment and Undertreatment for Seniors: An Out of Range (OOR) Population Health Safety Measure
 Balancing Glycemic Overtreatment and Undertreatment for Seniors: An Out of Range (OOR) Population Health Safety Measure Leonard Pogach, 1,2 Orysya Soroka, 1 Chin Lin Tseng, 1,2 Miriam Maney, 1 and David
Balancing Glycemic Overtreatment and Undertreatment for Seniors: An Out of Range (OOR) Population Health Safety Measure Leonard Pogach, 1,2 Orysya Soroka, 1 Chin Lin Tseng, 1,2 Miriam Maney, 1 and David
Predictive Models for Making Patient Screening Decisions
 Predictive Models for Making Patient Screening Decisions MICHAEL HAHSLER 1, VISHAL AHUJA 1, MICHAEL BOWEN 2, AND FARZAD KAMALZADEH 1 1 Southern Methodist University, 2 UT Southwestern Medical Center and
Predictive Models for Making Patient Screening Decisions MICHAEL HAHSLER 1, VISHAL AHUJA 1, MICHAEL BOWEN 2, AND FARZAD KAMALZADEH 1 1 Southern Methodist University, 2 UT Southwestern Medical Center and
July Billing and Compliance. Chemistry. Help Us Help You. Referral Testing
 July 2018 Billing and Compliance New Medicare card mailings have begun for Minnesota and Wisconsin residents Chemistry Beta hydroxybutyrate, whole blood specimen requirement change Help Us Help You Preventing
July 2018 Billing and Compliance New Medicare card mailings have begun for Minnesota and Wisconsin residents Chemistry Beta hydroxybutyrate, whole blood specimen requirement change Help Us Help You Preventing
MHSPHP Metrics Forum. ACG and Health Services
 MHSPHP Metrics Forum ACG and Health Services Overview What is ACG ACG RUB and ACG IBI Understanding PHDR reports How to use the PHDR reports with population management How to use the Health Services report
MHSPHP Metrics Forum ACG and Health Services Overview What is ACG ACG RUB and ACG IBI Understanding PHDR reports How to use the PHDR reports with population management How to use the Health Services report
Recommendations for Celiac Disease Testing. IgA & ttg IgA
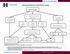 Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
Recommendations for Celiac Disease Testing IgA & ttg IgA IgA Normal ttg IgA Negative IgA >10 mg/dl, but less than age matched range IgA
Fran Cunningham, Pharm.D. Director, Center for Medication Safety PSCI Program Manager Pharmacoepidemiologic/ Outcomes Assessment Department of
 Fran Cunningham, Pharm.D. Director, Center for Medication Safety PSCI Program Manager Pharmacoepidemiologic/ Outcomes Assessment Department of Veterans Affairs PBM Services Overview of Pharmacovigilance
Fran Cunningham, Pharm.D. Director, Center for Medication Safety PSCI Program Manager Pharmacoepidemiologic/ Outcomes Assessment Department of Veterans Affairs PBM Services Overview of Pharmacovigilance
Amy Groom, MPH IHS Immunization Program Manager/CDC Public Health Advisor
 Amy Groom, MPH IHS Immunization Program Manager/CDC Public Health Advisor Jane Kim, MD, MPH Deputy Chief Consultant for Preventive Medicine VHA National Center for Health Promotion and Disease Prevention
Amy Groom, MPH IHS Immunization Program Manager/CDC Public Health Advisor Jane Kim, MD, MPH Deputy Chief Consultant for Preventive Medicine VHA National Center for Health Promotion and Disease Prevention
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT. Product: OraQuick HIV 1/2 Rapid Antibody Test WHO reference number: PQDx
 WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: OraQuick HIV 1/2 Rapid Antibody Test WHO reference number: PQDx 0159-055-00 OraQuick HIV 1/2 Rapid Antibody Test with product codes 5x4-0010
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: OraQuick HIV 1/2 Rapid Antibody Test WHO reference number: PQDx 0159-055-00 OraQuick HIV 1/2 Rapid Antibody Test with product codes 5x4-0010
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT. Product: OraQuick HIV 1/2 Rapid Antibody Test WHO reference number: PQDx
 WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: OraQuick HIV 1/2 Rapid Antibody Test WHO reference number: PQDx 0159-055-00 OraQuick HIV 1/2 Rapid Antibody Test with product codes 5x4-0010
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: OraQuick HIV 1/2 Rapid Antibody Test WHO reference number: PQDx 0159-055-00 OraQuick HIV 1/2 Rapid Antibody Test with product codes 5x4-0010
IP Lab Webinar 8/23/2012
 2 What Infection Preventionists need to know about the Laboratory Anne Maher, MS, M(ASCP), CIC Richard VanEnk PhD, CIC 1 Objectives Describe what the laboratory can do for you; common laboratory tests
2 What Infection Preventionists need to know about the Laboratory Anne Maher, MS, M(ASCP), CIC Richard VanEnk PhD, CIC 1 Objectives Describe what the laboratory can do for you; common laboratory tests
Large Scale Predictive Analytics for Chronic Illness using FuzzyLogix s In-Database Analytics Technology on Netezza
 Large Scale Predictive Analytics for Chronic Illness using FuzzyLogix s In-Database Analytics Technology on Netezza Christopher Hane, Vijay Nori, Partha Sen, Bill Zanine Fuzzy Logix Contact: Cyrus Golkar,
Large Scale Predictive Analytics for Chronic Illness using FuzzyLogix s In-Database Analytics Technology on Netezza Christopher Hane, Vijay Nori, Partha Sen, Bill Zanine Fuzzy Logix Contact: Cyrus Golkar,
INTERPRETING HEPATITIS B SEROLOGY
 INTERPRETING HEPATITIS B SEROLOGY RECOMMENDED WORDING FOR NATIONAL LABORATORIES TO REPORT HEPATITIS B DIAGNOSTIC TEST RESULTS THIS DOCUMENT HAS BEEN ENDORSED BY: Australasian Society for HIV Medicine,
INTERPRETING HEPATITIS B SEROLOGY RECOMMENDED WORDING FOR NATIONAL LABORATORIES TO REPORT HEPATITIS B DIAGNOSTIC TEST RESULTS THIS DOCUMENT HAS BEEN ENDORSED BY: Australasian Society for HIV Medicine,
November 13, 2009 Licensure, Evaluation, and Adverse Event Monitoring of the 2009 H1N1 Influenza Vaccine By Matthew Watson and Jennifer Nuzzo
 www.upmc-biosecurity.org www.upmc-cbn.org November 13, 2009 Licensure, Evaluation, and Adverse Event Monitoring of the 2009 H1N1 Influenza Vaccine By Matthew Watson and Jennifer Nuzzo In response to the
www.upmc-biosecurity.org www.upmc-cbn.org November 13, 2009 Licensure, Evaluation, and Adverse Event Monitoring of the 2009 H1N1 Influenza Vaccine By Matthew Watson and Jennifer Nuzzo In response to the
Potential disruption from private exchanges and narrow networks. In 2011, less than 10% of companies used High Performing Networks (narrow networks)
 1 3 2 Potential disruption from private exchanges and narrow networks. In 2011, less than 10% of companies used High Performing Networks (narrow networks) and in 2014 estimated to be 40%. By 2018, that
1 3 2 Potential disruption from private exchanges and narrow networks. In 2011, less than 10% of companies used High Performing Networks (narrow networks) and in 2014 estimated to be 40%. By 2018, that
Innovative Risk and Quality Solutions for Value-Based Care. Company Overview
 Innovative Risk and Quality Solutions for Value-Based Care Company Overview Meet Talix Talix provides risk and quality solutions to help providers, payers and accountable care organizations address the
Innovative Risk and Quality Solutions for Value-Based Care Company Overview Meet Talix Talix provides risk and quality solutions to help providers, payers and accountable care organizations address the
HEPATITIS C, ACUTE CRUDE DATA. Number of Cases 5 Annual Incidence a LA County 0.05 California b 0.10 United States b 0.68 Age at Diagnosis Mean 38
 2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
2016 Annual Morbidity Report HEPATITIS C, ACUTE a Rates calculated based on less than 19 cases or events are considered unreliable b Calculated from: CDC. Notice to Readers: Final 2016 Reports of Nationally
Workshop Overview. The Problem. National Milestones. The Registry Solution. Benefits of Registry/Managed Care Collaboration
 Using Immunization Registry Data to Support Medicaid Managed Care Services Noam H. Arzt, PhD HLN Consulting, LLC arzt@hln.com The New York City Experience Martha Rome, BSN, MPH Medical and Health Research
Using Immunization Registry Data to Support Medicaid Managed Care Services Noam H. Arzt, PhD HLN Consulting, LLC arzt@hln.com The New York City Experience Martha Rome, BSN, MPH Medical and Health Research
BENEFITS OF FUNDING CHRONIC HEPATITIS B NYC S EXPERIENCE AND C SURVEILLANCE:
 BENEFITS OF FUNDING CHRONIC HEPATITIS B AND C SURVEILLANCE: NYC S EXPERIENCE Katherine Bornschlegel, MPH New York City Department of Health and Mental Hygiene OUTLINE Background on chronic hep B and C
BENEFITS OF FUNDING CHRONIC HEPATITIS B AND C SURVEILLANCE: NYC S EXPERIENCE Katherine Bornschlegel, MPH New York City Department of Health and Mental Hygiene OUTLINE Background on chronic hep B and C
Title: Fecal Occult Blood Test Number: TCFHT-MD01 Activation Date: 01-January-2014 Review Date: 08-October-2017 Next Review Date: 08-October-2018
 MEDICAL DIRECTIVE Title: Fecal Occult Blood Test Number: TCFHT-MD01 Activation Date: 01-January-2014 Review Date: 08-October-2017 Next Review Date: 08-October-2018 Sponsoring/Contact Person(s) (name, position,
MEDICAL DIRECTIVE Title: Fecal Occult Blood Test Number: TCFHT-MD01 Activation Date: 01-January-2014 Review Date: 08-October-2017 Next Review Date: 08-October-2018 Sponsoring/Contact Person(s) (name, position,
Realigning Lab Focus Beyond Operational Efficiency to Laboratory Medicine that is Tightly Integrated into Patient Care and Clinician Support
 Realigning Lab Focus Beyond Operational Efficiency to Laboratory Medicine that is Tightly Integrated into Patient Care and Clinician Support Dr. Kevin Breuel, PhD East Tennessee State University Curt Johnson,
Realigning Lab Focus Beyond Operational Efficiency to Laboratory Medicine that is Tightly Integrated into Patient Care and Clinician Support Dr. Kevin Breuel, PhD East Tennessee State University Curt Johnson,
WHO recommendations. Diagnostic testing in infants
 WHO recommendations Diagnostic testing in infants Recommendations 2- testing in infants Intervention Reco QoE Comment At or around 6 weeks of age is most efficient time at which to perform a viral test
WHO recommendations Diagnostic testing in infants Recommendations 2- testing in infants Intervention Reco QoE Comment At or around 6 weeks of age is most efficient time at which to perform a viral test
Predictive Models: Current and Future. November 9, Steve Wickstrom Vice President, Research and Methods OptumInsight
 Predictive Models: Current and Future November 9, 2011 Steve Wickstrom Vice President, Research and Methods OptumInsight Steven.wickstrom@optum.com OptumInsight Predictive Solutions: Overview OptumInsight
Predictive Models: Current and Future November 9, 2011 Steve Wickstrom Vice President, Research and Methods OptumInsight Steven.wickstrom@optum.com OptumInsight Predictive Solutions: Overview OptumInsight
The Hepatitis B-e antigen-positive
 The Hepatitis B-e antigen-positive Dental Student Developing an Equitable Policy Hepatitis B Virus Serology 1 HBsAg A protein on the surface of HBV; it can be detected in high levels in serum during acute
The Hepatitis B-e antigen-positive Dental Student Developing an Equitable Policy Hepatitis B Virus Serology 1 HBsAg A protein on the surface of HBV; it can be detected in high levels in serum during acute
Project SUCCEED Overview. Project Scaling-Up Co-Infection Care to End Ethnic Disparities Brooklyn Hep C Task Force February 6, 2018
 Project SUCCEED Overview Project Scaling-Up Co-Infection Care to End Ethnic Disparities Brooklyn Hep C Task Force February 6, 2018 2 Goals and Objectives Goal: to eliminate hepatitis C (HCV) amongst people
Project SUCCEED Overview Project Scaling-Up Co-Infection Care to End Ethnic Disparities Brooklyn Hep C Task Force February 6, 2018 2 Goals and Objectives Goal: to eliminate hepatitis C (HCV) amongst people
Joined in 2016 Previously IT Manager at RSNWO in Northwest Ohio AAS in Computer Programming A+ Certification in 2012 Microsoft Certified Professional
 Joined in 2016 Previously IT Manager at RSNWO in Northwest Ohio AAS in Computer Programming A+ Certification in 2012 Microsoft Certified Professional in SQL Server 2012/2014 Overview The material in this
Joined in 2016 Previously IT Manager at RSNWO in Northwest Ohio AAS in Computer Programming A+ Certification in 2012 Microsoft Certified Professional in SQL Server 2012/2014 Overview The material in this
Technical Bulletin No. 104b
 CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 104b June 22, 2016 Update to Guidelines for Diagnosing HIV Infection CPAL s Testing Algorithm for Diagnostic HIV Testing HIV Geenius
CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 104b June 22, 2016 Update to Guidelines for Diagnosing HIV Infection CPAL s Testing Algorithm for Diagnostic HIV Testing HIV Geenius
FDA Sentinel Initiative: Status Update and Examples of Contributions to Regulatory Decisions
 FDA Sentinel Initiative: Status Update and Examples of Contributions to Regulatory Decisions Marsha E. Reichman, Ph.D. Scientific Lead for Surveillance Programs CDER Lead for the Sentinel Initiative OPE/OSE/CDER/FDA
FDA Sentinel Initiative: Status Update and Examples of Contributions to Regulatory Decisions Marsha E. Reichman, Ph.D. Scientific Lead for Surveillance Programs CDER Lead for the Sentinel Initiative OPE/OSE/CDER/FDA
a guide to Reimbursement of Intermittent Catheters Know your options M2116N 04.08
 a guide to Reimbursement of Intermittent Catheters 1 Know your options Coloplast Corp. Minneapolis, MN 55411 1.800.533.0464 usmedweb@coloplast.com www.us.coloplast.com is a registered trademark of Coloplast
a guide to Reimbursement of Intermittent Catheters 1 Know your options Coloplast Corp. Minneapolis, MN 55411 1.800.533.0464 usmedweb@coloplast.com www.us.coloplast.com is a registered trademark of Coloplast
Minnesota Influenza Geographic Spread
 Weekly Influenza & Respiratory Illness Activity Report A summary of influenza surveillance indicators prepared by the Division of Infectious Disease Epidemiology Prevention & Control Week Ending February
Weekly Influenza & Respiratory Illness Activity Report A summary of influenza surveillance indicators prepared by the Division of Infectious Disease Epidemiology Prevention & Control Week Ending February
Minnesota Influenza Geographic Spread
 Weekly Influenza & Respiratory Illness Activity Report A summary of influenza surveillance indicators prepared by the Division of Infectious Disease Epidemiology Prevention & Control Week Ending March
Weekly Influenza & Respiratory Illness Activity Report A summary of influenza surveillance indicators prepared by the Division of Infectious Disease Epidemiology Prevention & Control Week Ending March
The most current laboratory testing information can be obtained at
 About Adventist Lab Partners Reference Laboratory Adventist Lab Partners (ALPs) Reference Laboratory is affiliated with Adventist Health System. Adventist Lab Partners has been a hospital-based laboratory
About Adventist Lab Partners Reference Laboratory Adventist Lab Partners (ALPs) Reference Laboratory is affiliated with Adventist Health System. Adventist Lab Partners has been a hospital-based laboratory
Pre-Assessment Review: Microbiology, Part 2: Virology. Dr. David Hillyard
 Pre-Assessment Review: Microbiology, Part 2: Virology Dr. David Hillyard 1. Mutations or deletions within which gene are associated with the hyper-toxin producing strain of Clostridium difficile? a. tcda
Pre-Assessment Review: Microbiology, Part 2: Virology Dr. David Hillyard 1. Mutations or deletions within which gene are associated with the hyper-toxin producing strain of Clostridium difficile? a. tcda
Opportunities Created by Diagnostic HCV and HIV Nucleic Acid Tests
 Opportunities Created by Diagnostic HCV and HIV Nucleic Acid Tests Ann Winters, MD Medical Director, Viral Hepatitis Program, Bureau of Communicable Disease, New York City Department of Health and Mental
Opportunities Created by Diagnostic HCV and HIV Nucleic Acid Tests Ann Winters, MD Medical Director, Viral Hepatitis Program, Bureau of Communicable Disease, New York City Department of Health and Mental
