Package RImmPort. July 22, 2018
|
|
|
- Rebecca Burns
- 5 years ago
- Views:
Transcription
1 Type Package Package RImmPort July 22, 2018 Title RImmPort: Enabling Ready-for-analysis Immunology Research Data Version Author Ravi Shankar Maintainer Zicheng Hu Ravi Shankar URL The RImmPort package simplifies access to ImmPort data for analysis in the R environment. It provides a standards-based interface to the ImmPort study data that is in a proprietary format. License GPL-3 Imports plyr, dplyr, DBI, data.table, reshape2, methods, sqldf, tools, utils, RSQLite Suggests knitr biocviews BiomedicalInformatics, DataImport, DataRepresentation VignetteBuilder knitr RoxygenNote NeedsCompilation no git_url git_branch master git_last_commit bdda32b git_last_commit_date Date/Publication R topics documented: Adverse Events Domain AE APMH Associated Persons Medical History Domain buildnewsqlitedb Cellular Quantification Domain CM
2 2 R topics documented: Concomitant Medications Domain Demographics Domain DM DV Events-class EX Exposure Domain FA Findings About Domain Findings-class Genetics Findings Domain getassaydataofstudies getdomaincode getdomaindataofstudies getlistofassaytypes getlistofdomains getlistofstudies getstudieswithspecificassaydata getstudieswithspecificdomaindata getstudy Interventions-class Laboratory Test Results Domain LB loadserializedstudydata Medical History Domain mergedomainandsupplemental MH Nucleic Acid Quantification Domain PE PF Physical Examination Domain Protein Quantification Domain Protocol Deviations Domain QS Questionnaires Domain RImmPort serialzestudydata setimmportdatasource Skin Response Domain SpecialPurpose-class SR Study-class SU Subject Visits Domain Substance Use Domain SUPP SUPPDV SUPPFA SUPPLB SUPPMH SUPPPE SUPPPF
3 Adverse Events Domain 3 SUPPVS SUPPZA SUPPZB SUPPZC SUPPZD SV TA TI Titer Assay Results Domain Trial Arms Domain Trial Inclusion Exclusion Criteria Domain Trial Summary Domain Trial Visits Domain TrialDesign-class TS TV Vital Signs Domain VS ZA ZB ZC ZD Index 40 Adverse Events Domain Adverse Events Domain The Adverse Events data of an ImmPort study is reformated to the CDISC SDTM Adverse Events (AE) domain model, and is a list of 2 data frames containing 1) Adverse Events data AE and 2) any supplemental Adverse Events data SUPP AE Adverse Events Domain Variables AESEQ AESPID AETERM AEMODIFY AEBODYSYS AELOC AESEV Sponsor-Defined Identifier Reported Term for the Adverse Event Modified Reported Term Body System or Organ Class Location of Event Severity/Intensity
4 4 Associated Persons Medical History Domain AEACN AEACNOTH AEREL AERELNST AEOUT AESTDY AEENDY Action Taken with Study Treatment Other Action Taken Causality Relationship to Non-Study Treatment Outcome of Adverse Event Study Day of Start of Adverse Event Study Day of End of Adverse Event APMH Associated Persons Medical History Domain Variables APID MHSEQ RSUBJID SREL MHTERM MHCAT MHBODSYS MHDTC MHDY Associated Persons Identifier Related Subject Subject Relationship Reported Term for the Medical History Category for Medical History Body System or Organ Class Date/Time of History Collection RStudy Day of History Collection Associated Persons Medical History Domain Associated Persons Medical History Domain The Associated Persons Medical History data of an ImmPort study is reformated to the CDISC SDTM AAssociated Persons Medical History (APMH) domain model, and is a list of 2 data frames containing 1) Associated Persons Medical History data APMH and 2) any supplemental Associated Persons Medical History data SUPP
5 buildnewsqlitedb 5 buildnewsqlitedb buildnewsqlitedb Usage The function buildsqlitedb builds a sqlite db of ImmPort study data. It takes in as input the study files in the TSV (Tab) format. buildnewsqlitedb(data_dir, db_dir) Arguments data_dir db_dir File directory where the study TSV files are stored File directory where the sqlite database will be stored Value The SQLite database name Examples studies_dir <- system.file("extdata", "ImmPortStudies", package = "RImmPort") # set tab_dir to the folder where the zip files are located tab_dir <- file.path(studies_dir, "Tab") # set db_dir to the folder where the database file 'ImmPort.sqlite' should be stored db_dir <- file.path(studies_dir, "Db") # build a new ImmPort SQLite database with the data in the downloaded zip files # dbname <- buildnewsqlitedb(tab_dir, db_dir) Cellular Quantification Domain Cellular Quantification Domain The ImmPort study data generated from assays of types: Flow and ELISPOT are grouped into the Cellular Quantification Domain. The data is reformated to a custom Cellular Quantification domain model in CDISC SDTM standards, and is a list of 2 data frames containing 1) Cellular Quantification data ZB and 2) any supplemental Cellular Quantification data SUPP
6 6 DM CM Concomitant Medications Domain Variables CMSEQ CMTRT CMCAT CMDOSE CMDOSTXT CMDOSU CMDOSFREQ CMROUTE CMSTDTC CMENDTC CMSTDY CMENDY Reported Name of Drug, Med, or Therapy Category for Medication Dose per Administration Dose Dose Units Dosing Frequency per Interval Route of Administration Start Date/Time of Medication End Date/Time of Medication Study Day of Start of Medication Study Day of End of Medication Concomitant Medications Domain Concomitant Medications Domain The Concomitant Medications data of an ImmPort study is reformated to the CDISC SDTM Concomitant Medications (CM) domain model, and is a list of 2 data frames containing 1) Concomitant Medications data CM and 2) any supplemental Concomitant Medications data SUPP Demographics Domain Demographics Domain The Demographics data of an ImmPort study is reformated to the CDISC SDTM Demographics (DM) domain model, and is a list of 2 data frames containing 1) Demographics data DM and 2) any supplemental Demographics data SUPP DM Demographics Domain Variables
7 Events-class 7 AGE AGEU SEX RACE ETHNIC SPECIES STRAIN SBSTRAIN ARMCD ARM Age Age Units Sex Race (only for human data) Ethnicity (only for human data) Species (only for non-human data) Strain/Substrain (only for non-human data) Strain/Substrain Details (only for non-human data) Planned Arm Code of Planned Arm DV Protocol Deviations Domain Variables DVSEQ DVTERM Protocol Deviation Term Events-class Events class Events class Fields ae_l Adverse Events data AE and supplemental Adverse Events data SUPP dv_l Protocol Deviations data DV and supplemental Protocol Deviations data SUPPDV mh_l Medical History data MH and supplemental Medical History data SUPPMH apmh_l Associated Persons Medical History data APMH and supplemental Associated Persons Medical History data SUPP
8 8 FA Examples library(dbi) library(sqldf) studies_dir <- system.file("extdata", "ImmPortStudies", package = "RImmPort") db_dir <- file.path(studies_dir, "Db") sqlite_conn <- dbconnect(sqlite(), dbname=file.path(db_dir, "ImmPort.sqlite")) setimmportdatasource(sqlite_conn) sdy139 <- getstudy("sdy139") ae_df <- sdy139$events$ae_l$ae_df EX Exposure Domain Variables EXSEQ EXTRT EXCAT EXDOSE EXDOSTXT EXDOSU EXDOSFRQ EXROUTE EXSTDTC EXENDTC EXSTDY EXENDY Name of Treatment Category of Treatment Dose Dose Dose Units Dosing Frequency per Interval Route of Administration Start Date/Time of Treatment End Date/Time of Treatment Study Day of Start of Treatment Study Day of End of Treatment Exposure Domain Exposure Domain The Exposure data of an ImmPort study is reformated to the CDISC SDTM Exposure (EX) domain model, and is a list of 2 data frames containing 1) Exposure data EX and 2) any supplemental Exposure data SUPP FA Findings About Domain Variables
9 Findings About Domain 9 FASEQ FATEST FAOBJ FACAT FAORRES FAORRESU FALOC VISITNUM VISIT FADY Findings About Test Name Object of the Observation Category for Findings About Results or Findings in Original Units Original Units Location of the Finding About Visit Number Visit Name Study Day of Collection Findings About Domain Findings About Domain The Findings About data of an ImmPort study is reformated to the CDISC SDTM Findings About (FA) domain model, and is a list of 2 data frames containing 1) Findings About data FA and 2) any supplemental Findings About data SUPPFA Findings-class Findings class Findings class Fields lb_l Laboratory Test Results data LB and supplemental Laboratory Test Results data SUPPLB pe_l Physical Examination data PE and supplemental Physical Examination data SUPPPE vs_l Vital Signs data VS and supplemental Vital Signs data SUPPVS qs_l Questionnaires data QS and supplemental Questionnaires data SUPP fa_l Findings About data FA and supplemental Findings About data SUPPFA sr_l Skin Response data SR and supplemental Skin Response data SUPP pf_l Genetics Findings data PF and supplemental Genetics Findings data SUPPPF za_l Protein Quantification data ZA and supplemental Protein Quantification data SUPPZA zb_l Cellular Quantification data ZB and supplemental Cellular Quantification data SUPP zc_l Nucleic Acid Quantification data ZC and supplemental Nucleic Acid Quantification data SUPP zd_l Titer Assay Results data ZD and supplemental Titer Assay Results data SUPP
10 10 getassaydataofstudies Examples library(dbi) library(sqldf) studies_dir <- system.file("extdata", "ImmPortStudies", package = "RImmPort") db_dir <- file.path(studies_dir, "Db") sqlite_conn <- dbconnect(sqlite(), dbname=file.path(db_dir, "ImmPort.sqlite")) setimmportdatasource(sqlite_conn) sdy139 <- getstudy("sdy139") zb_df <- sdy139$findings$zb_l$zb_df Genetics Findings Domain Genetics Findings Domain The ImmPort study data generated from assays of types: HLA Typing and Array are grouped into the Genetics Findings Domain. The data is reformated to a PharmacoGenomics and Genetics Findings (PF) model in CDISC SDTM standards, and is a list of 2 data frames containing 1) Genetics Findings data PF and 2) any supplemental Genetics Findings data SUPP getassaydataofstudies Get specific assay data of one or more studies from the ImmPort database Get specific assay data of one or more studies from the ImmPort database Usage getassaydataofstudies(study_ids, assay_type) Arguments study_ids assay_type List of study indentifiers Assay Type Value a list of 1) domain data of specicifc assay technology and 2) any supplemental domain data of the studies Author(s) Ravi Shankar
11 getdomaincode 11 Examples library(dbi) library(sqldf) studies_dir <- system.file("extdata", "ImmPortStudies", package = "RImmPort") db_dir <- file.path(studies_dir, "Db") sqlite_conn <- dbconnect(sqlite(), dbname=file.path(db_dir, "ImmPort.sqlite")) setimmportdatasource(sqlite_conn) elispot_l <- getassaydataofstudies("sdy139", "ELISPOT") if (length(elispot_l) > 0) names(elispot_l) head(elispot_l$zb_df) getdomaincode Get code of a specific domain The function getlistofdomains returns the code of a specific domain Usage getdomaincode(domain) Arguments domain Name of a specific domain Value A list of of all domain names and codes Examples domain <- "Demographics" code <- getdomaincode(domain) getdomaindataofstudies Get specific domain data of one or more studies from the ImmPort database Get specific domain data of one or more studies from the ImmPort database Usage getdomaindataofstudies(domain, study_ids)
12 12 getlistofdomains Arguments domain study_ids Name of a specific domain List of study indentifiers Value a list of 1) domain data and 2) supplemental domain data of the studies Examples library(dbi) library(sqldf) studies_dir <- system.file("extdata", "ImmPortStudies", package = "RImmPort") db_dir <- file.path(studies_dir, "Db") sqlite_conn <- dbconnect(sqlite(), dbname=file.path(db_dir, "ImmPort.sqlite")) setimmportdatasource(sqlite_conn) dm_df <- getdomaindataofstudies("demographics", "SDY139") getlistofassaytypes Get a list of Assay Types The function getlistofassaytypes returns a list of assay types that ImmPort studies have employed in their experimetal assays Usage getlistofassaytypes() Value A list of assay types Examples at_l <- getlistofassaytypes() getlistofdomains Get names of all domains and their codes The function getlistofdomains returns a list of all domain names and codes Usage getlistofdomains()
13 getlistofstudies 13 Value A list of of all domain names and codes Examples domains_df <- getlistofdomains() getlistofstudies Get a list of all studies Get a list of all studies Usage getlistofstudies() Value List of study indentifiers Examples library(dbi) library(sqldf) studies_dir <- system.file("extdata", "ImmPortStudies", package = "RImmPort") db_dir <- file.path(studies_dir, "Db") sqlite_conn <- dbconnect(sqlite(), dbname=file.path(db_dir, "ImmPort.sqlite")) setimmportdatasource(sqlite_conn) study_ids <- getlistofstudies() getstudieswithspecificassaydata Get a list of studies that have specific assay type data Get a list of studies that have specific assay type data Usage getstudieswithspecificassaydata(assay_type, all_study_ids = c("all")) Arguments assay_type all_study_ids Assay Type List of study indentifiers to search on
14 14 getstudieswithspecificdomaindata Value List of study indentifiers Examples library(dbi) library(sqldf) studies_dir <- system.file("extdata", "ImmPortStudies", package = "RImmPort") db_dir <- file.path(studies_dir, "Db") sqlite_conn <- dbconnect(sqlite(), dbname=file.path(db_dir, "ImmPort.sqlite")) setimmportdatasource(sqlite_conn) study_ids <- getstudieswithspecificassaydata("elispot") getstudieswithspecificdomaindata Get a list of studies that have specific domain data Get a list of studies that have specific domain data Usage getstudieswithspecificdomaindata(domain, all_study_ids = c("all")) Arguments domain all_study_ids Name of a specific domain List of study indentifiers to search on Value List of study indentifiers Examples library(dbi) library(sqldf) studies_dir <- system.file("extdata", "ImmPortStudies", package = "RImmPort") db_dir <- file.path(studies_dir, "Db") sqlite_conn <- dbconnect(sqlite(), dbname=file.path(db_dir, "ImmPort.sqlite")) setimmportdatasource(sqlite_conn) study_ids <- getstudieswithspecificdomaindata("demographics")
15 getstudy 15 getstudy Get all data of a specific study from the ImmPort data source The function getstudy queries the ImmPort data source for data of a specific study in all domains. The data is then structured into Study as classes, domains, variables and values. Usage getstudy(study_id) Arguments study_id Identifier of a specific study Value A study data object where in all data are structured as classes, domains, variables and values (in CDISC format) Examples library(dbi) library(sqldf) studies_dir <- system.file("extdata", "ImmPortStudies", package = "RImmPort") db_dir <- file.path(studies_dir, "Db") sqlite_conn <- dbconnect(sqlite(), dbname=file.path(db_dir, "ImmPort.sqlite")) setimmportdatasource(sqlite_conn) sdy139 <- getstudy("sdy139") Interventions-class Interventions class Interventions class Fields cm_l Concomitant Medications data CM and supplemental Concomitant Medications data SUPP ex_l Exposure data EX and supplemental Exposure data SUPP su_l Substance Use data SU and supplemental Substance Use data SUPP
16 16 loadserializedstudydata Examples library(dbi) library(sqldf) studies_dir <- system.file("extdata", "ImmPortStudies", package = "RImmPort") db_dir <- file.path(studies_dir, "Db") sqlite_conn <- dbconnect(sqlite(), dbname=file.path(db_dir, "ImmPort.sqlite")) setimmportdatasource(sqlite_conn) sdy139 <- getstudy("sdy139") cm_df <- sdy139$interventions$cm_l$cm_df Laboratory Test Results Domain Laboratory Test Results Domain The Laboratory Test Results data of an ImmPort study is reformated to the CDISC SDTM Laboratory Test Results (LB) domain model, and is a list of 2 data frames containing 1) Laboratory Test Results data LB and 2) any supplemental Laboratory Test Results data SUPPLB LB Laboratory Test Results Domain Variables LBSEQ LBTEST LBCAT LBORRES LBORRESU LBSPEC LBREFID VISITNUM VISIT LBELTM LBTPTREF Lab Test or Examination Name Category for Lab Test Result or Finding in Original Units Original Units Specimen Type Specimen Identifier Visit Number Visit Name Planned Elapsed Time from Time Point Ref Time Point Reference loadserializedstudydata Load the Serialized Data of a Study
17 Medical History Domain 17 Load the serialzed data (.rds) file of a specific domain of a study study from the directory where the file is located Usage loadserializedstudydata(data_dir, study_id, domain) Arguments data_dir study_id domain Path to a file folder where the.rds study files reside Study indentifier Domain of interest Value A study data object where in all data are structured as classes, domains, variables and values (in CDISC format) Examples studies_dir <- system.file("extdata", "ImmPortStudies", package = "RImmPort") # the folder where the.rds files will be stored rds_dir <- file.path(studies_dir, "Rds") # load the serialized data of study `SDY208` loadserializedstudydata(rds_dir, 'SDY208', "Demographics") Medical History Domain Medical History Domain The Medical History data of an ImmPort study is reformated to the CDISC SDTM Medical History (MH) domain model, and is a list of 2 data frames containing 1) Medical History data MH and 2) any supplemental Medical History data SUPPMH mergedomainandsupplemental Merge the Domain dataframe and Supplemental dataframe (long form) The Domain data list comprises of the the Domain datafrome that is in wide form, and any Supplemental dataframe that is in long form. The function mergedomainandsupplemental transposes the Supplemental dataframe into a wide form, and merges it with the Domain dataframe.
18 18 Nucleic Acid Quantification Domain Usage mergedomainandsupplemental(data_list) Arguments data_list A list of 1) Domain dataframe and 2) any Supplemental dataframe Value The merged dataframe Examples library(dbi) library(sqldf) studies_dir <- system.file("extdata", "ImmPortStudies", package = "RImmPort") db_dir <- file.path(studies_dir, "Db") sqlite_conn <- dbconnect(sqlite(), dbname=file.path(db_dir, "ImmPort.sqlite")) setimmportdatasource(sqlite_conn) l <- getdomaindataofstudies("cellular Quantification", "SDY208") df <- mergedomainandsupplemental(l) MH Medical History Domain Variables MHSEQ MHTERM MHCAT MHBODSYS MHDY Reported Term for the Medical History Category for Medical History Body System or Organ Class Study Day of History Collection Nucleic Acid Quantification Domain Nucleic Acid Quantification Domain The ImmPort study data generated from assays of types: PCR are grouped into the Nucleic Acid Quantification Domain. The data is reformated to a custom Nucleic Acid Quantification domain model in CDISC SDTM standards, and is a list of 2 data frames containing 1) Nucleic Acid Quantification data ZC and 2) any supplemental Nucleic Acid Quantification data SUPP
19 PF 19 PE Physical Examination Domain Variables PESEQ PETEST PECAT PEBODSYS PEORRES PEORRESU PELOC VISITNUM VISIT PEDY Body System Examined Category for Examination Body System or Organ Class Verbatim Examination Finding Original Units Location of Physical Exam Finding Visit Number Visit Name Study Day of Examination PF Genetics Findings Domain Variables PFSEQ PFGRPID PFTEST PFCAT PFMETHOD PFGENRI PFORRES PFALLELC PFSPEC PFREFID VISITNUM VISIT PFELTM PFTPTREF PFXFN Group Identifier Test Name Category for Test Method of the Test Genetic Region of Interest Result or Finding in Original Units Allele (Chromosome) Identifier Specimen Type Reference ID (Specimen Identifier) Visit Number Visit Name Planned Elapsed Time from Time Point Ref Time Point Reference Raw Data File or Life Science Identifier
20 20 QS Physical Examination Domain Physical Examination Domain The Physical Examination data of an ImmPort study is reformated to the CDISC SDTM Physical Examination (PE) domain model, and is a list of 2 data frames containing 1) Physical Examination data PE and 2) any supplemental Physical Examination data SUPPPE Protein Quantification Domain Protein Quantification Domain The ImmPort study data generated from assays of types: ELISA and MBAA are grouped into the Cellular Quantification Domain. The data is reformated to a custom Protein Quantification domain model in CDISC SDTM standards, and is a list of 2 data frames containing 1) Protein Quantification data ZA and 2) any supplemental Protein Quantification data SUPP Protocol Deviations Domain Protocol Deviations Domain The Protocol Deviations data of an ImmPort study is reformated to the CDISC SDTM Protocol Deviations (DV) domain model, and is a list of 2 data frames containing 1) Protocol Deviations data DV and 2) any supplemental Protocol Deviations data SUPPDV QS Questionnaires Domain Variables QSSEQ QSTEST QSCAT QSORRES QSORRESU VISITNUM VISIT QSDY Questionnaires Test Name Category for Questionnaires Results or Findings in Original Units Original Units Visit Number Visit Name Study Day of Finding
21 Questionnaires Domain 21 Questionnaires Domain Questionnaires Domain The Questionnaires data of an ImmPort study is reformated to the CDISC SDTM Questionnaires (QS) domain model, and is a list of 2 data frames containing 1) Questionnaires data QS and 2) any supplemental Questionnaires data SUPP RImmPort RImmPort: Enabling ready-for-analysis immunology research data The RImmPort package simplifies access to ImmPort data for analysis, as the name implies, in the R statistical environment. It provides a standards-based interface to the ImmPort study data that is in a proprietary format. serialzestudydata Serialize the Study Data Load specific studies from the database and save it in.rds format in a local file directory Usage serialzestudydata(study_ids, data_dir) Arguments study_ids data_dir List of study indentifiers Path to a file folder where the.rds study files will be saved into Value List of study indentifiers that were serialized successfully Examples library(dbi) library(sqldf) studies_dir <- system.file("extdata", "ImmPortStudies", package = "RImmPort") db_dir <- file.path(studies_dir, "Db") sqlite_conn <- dbconnect(sqlite(), dbname=file.path(db_dir, "ImmPort.sqlite")) setimmportdatasource(sqlite_conn) # the folder where the.rds files will be stored rds_dir <- file.path(studies_dir, "Rds") study_ids <- c('sdy139', 'SDY208') serialzestudydata(study_ids, rds_dir)
22 22 Skin Response Domain setimmportdatasource Set ImmPort data ource The function setimmportdatasource sets the data source variable in RImmPort environment, to the connection handle to the MySQL or SQLite database, or to the file directory where the precreated RImmPort-formatted files are stored. Usage setimmportdatasource(data_src) Arguments data_src A connection handle to ImmPort (MySQL or SQLite) database instance or a directory handle to folder where study RImmPort-formatted (.rds) files located Value 1 if successful Examples library(dbi) library(sqldf) studies_dir <- system.file("extdata", "ImmPortStudies", package = "RImmPort") db_dir <- file.path(studies_dir, "Db") sqlite_conn <- dbconnect(sqlite(), dbname=file.path(db_dir, "ImmPort.sqlite")) setimmportdatasource(sqlite_conn) Skin Response Domain Skin Response Domain The Skin Response data of an ImmPort study is reformated to the CDISC SDTM Skin Response (SR) domain model, and is a list of 2 data frames containing 1) Skin Response data SR and 2) any supplemental Skin Response data SUPP
23 Study-class 23 SpecialPurpose-class Special Purpose class Fields Special Purpose class dm_l Demographics data DM and supplemental Demographics data SUPP Examples library(dbi) library(sqldf) studies_dir <- system.file("extdata", "ImmPortStudies", package = "RImmPort") db_dir <- file.path(studies_dir, "Db") sqlite_conn <- dbconnect(sqlite(), dbname=file.path(db_dir, "ImmPort.sqlite")) setimmportdatasource(sqlite_conn) sdy139 <- getstudy("sdy139") dm_df <- sdy139$special_purpose$dm_l$dm_df SR Skin Response Domain Variables SRSEQ SRTEST SROBJ SRCAT SRORRES SRORRESU SRLOC VISITNUM VISIT SRDY Skin Response Test or Examination Name Object of the Observation Category for Test Results or Findings in Original Units Original Units Location used for Measurement Visit Number Visit Name Study Day of Visit/Collection/Exam Study-class Study class
24 24 Subject Visits Domain Fields Study class special_purpose SpecialPurpose interventions Interventions events Events findings Findings trial_design TrialDesign Examples library(dbi) library(sqldf) studies_dir <- system.file("extdata", "ImmPortStudies", package = "RImmPort") db_dir <- file.path(studies_dir, "Db") sqlite_conn <- dbconnect(sqlite(), dbname=file.path(db_dir, "ImmPort.sqlite")) setimmportdatasource(sqlite_conn) sdy139 <- getstudy("sdy139") SU Substance Use Domain Variables SUSEQ SUTRT SUCAT SUDOSE SUDOSTXT SUDOSU SUDOSFRQ SUROUTE SUSTDTC SUENDTC SUSTDY SUENDY Reported Name of Substance Category of Substance Use Substance Use Consumption Substance Use Consumption Text Consumption Units Use Frequency per Interval Route of Administration Start Date/Time of Substance Use End Date/Time of Substance Use Study Day of Start of Substance Use Study Day of End of Substance Use Subject Visits Domain Subject Visits Domain
25 SUPPDV 25 Actual subject visits data of an ImmPort study is reformated to the CDISC SDTM Subject Visits (SV) domain model, and is a list of 2 data frames containing 1) Subject Visits data SV and 2) any supplemental Subject Visits data SUPP Substance Use Domain Substance Use Domain The Substance Use data of an ImmPort study is reformated to the CDISC SDTM Substance Use (SU) domain model, and is a list of 2 data frames containing 1) Substance Use data SU and 2) any supplemental Substance Use data SUPP SUPP Supplemental Variables R IDVAR IDVARVAL QVAL Related Identifying Variable Identifying Variable Value Qualifier Qualifier Data Value SUPPDV Protocol Deviations Domain Supplemental Variables R IDVAR IDVARVAL QVAL Related Identifying Variable Identifying Variable Value Qualifier Qualifier Data Value
26 26 SUPPLB Note The following table enumerates the values in and variables DVRELAE DVREASON DVRESOL DVCONT DVSTDY DVENDY Is Deviation Related to an Adverse Event? Reason for Deviation Resulotion of Deviation Did Subject continued in Study? Study Day of Start of Deviation Study Day of End of Deviation SUPPFA Findings About Domain Supplemental Variables R FASEQ IDVAR IDVARVAL QVAL Related Identifying Variable Identifying Variable Value Qualifier Qualifier Data Value Note The following table enumerates the values in and variables FATOD Time of Day of Collection SUPPLB Laboratory Test Results Domain Supplemental Variables
27 SUPPMH 27 R IDVAR IDVARVAL QVAL Related Identifying Variable Identifying Variable Value Qualifier Qualifier Data Value Note The following table enumerates the values in and variables LBSPECSB VISITMIN VISITMAX Specimen Subtype Planned Visit Minimum Start Day Planned Visit Maximum Start Day SUPPMH Medical History Domain Supplemental Variables R MHSEQ IDVAR IDVARVAL QVAL Related Identifying Variable Identifying Variable Value Qualifier Qualifier Data Value Note The following table enumerates the values in and variables MHAGE MHAGEU MHTOD Age at Onset Age at Onset Units Time of Day
28 28 SUPPPF SUPPPE Physical Examination Domain Supplemental Variables R PESEQ IDVAR IDVARVAL QVAL Related Identifying Variable Identifying Variable Value Qualifier Qualifier Data Value Note The following table enumerates the values in and variables PETOD Time of Day SUPPPF Genetics Findings Domain Supplemental Variables R IDVAR IDVARVAL QVAL Related Identifying Variable Identifying Variable Value Qualifier Qualifier Data Value Note The following table enumerates the values in and variables
29 SUPPZA 29 PFPOPAR PFSPECSB PFREFIDP VISITMIN VISITMAX PFSPTRT PFTRTAMV PFTRTAMU PFTRTDUV PFTRTDUU PFTRTTMV PFTRTTMU Geographic Area of the Population Specimen Subtype Source Specimen Identifier Planned Visit Minimum Start Day Planned Visit Maximum Start Day Specimen Treatment Specimen Treatment Amount Value Specimen Treatment Amount Unit Specimen Treatment Duration Value Specimen Treatment Duration Unit Specimen Treatment Temperature Value Specimen Treatment Temperature Unit SUPPVS Vital Signs Domain Supplemental Variables R VSSEQ IDVAR IDVARVAL QVAL Related Identifying Variable Identifying Variable Value Qualifier Qualifier Data Value Note The following table enumerates the values in and variables VSTOD Time of Day SUPPZA Protein Quantification Domain Supplemental Variables
30 30 SUPPZB R IDVAR IDVARVAL QVAL Related Identifying Variable Identifying Variable Value Qualifier Qualifier Data Value Note The following table enumerates the values in and variables ZAMFI ZAMFICRD ZASPECSB ZAREFIDP VISITMIN VISITMAX ZASPTRT ZATRTAMV ZATRTAMU ZATRTDUV ZATRTDUU ZATRTTMV ZATRTTMU MFI MFI Coordinate Specimen Subtype Source Specimen Identifier Planned Visit Minimum Start Day Planned Visit Maximum Start Day Specimen Treatment Specimen Treatment Amount Value Specimen Treatment Amount Unit Specimen Treatment Duration Value Specimen Treatment Duration Unit Specimen Treatment Temperature Value Specimen Treatment Temperature Unit SUPPZB Cellular Quantification Domain Supplemental Variables R IDVAR IDVARVAL QVAL Related Identifying Variable Identifying Variable Value Qualifier Qualifier Data Value
31 SUPPZC 31 Note The following table enumerates the values in and variables ZBSPECSB ZBREFIDP ZBFCF VISITMIN VISITMAX ZBSPTRT ZBTRTAMV ZBTRTAMU ZBTRTDUV ZBTRTDUU ZBTRTTMV ZBTRTTMU Specimen Subtype Source Specimen Identifier Control Files Names Planned Visit Minimum Start Day Planned Visit Maximum Start Day Specimen Treatment Specimen Treatment Amount Value Specimen Treatment Amount Unit Specimen Treatment Duration Value Specimen Treatment Duration Unit Specimen Treatment Temperature Value Specimen Treatment Temperature Unit SUPPZC Nucleic Acid Quantification Domain Supplemental Variables R IDVAR IDVARVAL QVAL Related Identifying Variable Identifying Variable Value Qualifier Qualifier Data Value Note The following table enumerates the values in and variables ZCSPECSB ZCREFIDP VISITMIN VISITMAX ZCSPTRT ZCTRTAMV ZCTRTAMU ZCTRTDUV ZCTRTDUU ZCTRTTMV Specimen Subtype Source Specimen Identifier Planned Visit Minimum Start Day Planned Visit Maximum Start Day Specimen Treatment Specimen Treatment Amount Value Specimen Treatment Amount Unit Specimen Treatment Duration Value Specimen Treatment Duration Unit Specimen Treatment Temperature Value
32 32 SV ZCTRTTMU Specimen Treatment Temperature Unit SUPPZD Titer Assay Results Domain Supplemental Variables R IDVAR IDVARVAL QVAL Related Identifying Variable Identifying Variable Value Qualifier Qualifier Data Value Note The following table enumerates the values in and variables ZDSPECSB ZDREFIDP VISITMIN VISITMAX ZDSPTRT ZDTRTAMV ZDTRTAMU ZDTRTDUV ZDTRTDUU ZDTRTTMV ZDTRTTMU Specimen Subtype Source Specimen Identifier Planned Visit Minimum Start Day Planned Visit Maximum Start Day Specimen Treatment Specimen Treatment Amount Value Specimen Treatment Amount Unit Specimen Treatment Duration Value Specimen Treatment Duration Unit Specimen Treatment Temperature Value Specimen Treatment Temperature Unit SV Subject Visits Domain Variables VISITNUM VISIT SVSTDY Visit Number Visit Name Study Day of Start of Visit
33 Titer Assay Results Domain 33 TA Trial Arms Domain Variables ARMCD ARM ARMDESC ARMRULE Planned Arm Code Name of Planned Arm of Planned Arm Population Selection Rule TI Trial Inclusion Exclusion Criteria Domain Variables IETEST IECAT Inclusion/Exclusion Criterion Inclusion/Exclusion Category Titer Assay Results Domain Titer Assay Results Domain The ImmPort study data generated from assays of types: HAI and Neut Ab Titer are grouped into the Titer Assay Results Domain. The data is reformated to a custom Titer Assay Results domain model in CDISC SDTM standards, and is a list of 2 data frames containing 1) Titer Assay Results data ZD and 2) any supplemental Titer Assay Results data SUPP
34 34 Trial Visits Domain Trial Arms Domain Trial Arms Domain The Trial Arms data of an ImmPort study is reformated to the CDISC SDTM Trial Arms (TA) domain model, and is a list of 2 data frames containing 1) Trial Arms data TA and 2) any supplemental Trial Arms data SUPP Trial Inclusion Exclusion Criteria Domain Trial Inclusion Exclusion Criteria Domain The Trial Inclusion Exclusion Criteria data of an ImmPort study is reformated to the CDISC SDTM Trial Inclusion Exclusion Criteria (TI) domain model, and is a list of 2 data frames containing 1) Trial Inclusion Exclusion Criteria data TI and 2) any supplemental Trial Inclusion Exclusion Criteria data SUPP Trial Summary Domain Trial Summary Domain The Trial Summary data of an ImmPort study is reformated to the CDISC SDTM Trial Summary (TS) domain model, and is a list of 2 data frames containing 1) Trial Summary data TS and 2) any supplemental Trial Summary data SUPP Trial Visits Domain Trial Visits Domain Information on the planned visits of an ImmPort study is reformated to the CDISC SDTM Trial Visits (TV) domain model, and is a list of 2 data frames containing 1) Trial Visits data TV and 2) any supplemental Trial Visits data SUPP
35 TS 35 TrialDesign-class Trial Design class Fields Trial Design class ta_l Trial Arms data TA and supplemental Trial Arms data SUPP tv_l Trial Visits data TA and supplemental Trial Visits data SUPP ti_l Trial Inclusion Exclusion Criteria data TI and supplemental Trial Inclusion Exclusion Criteria data SUPP ts_l Trial Summary data TS and supplemental Trial Summary data SUPP Examples library(dbi) library(sqldf) studies_dir <- system.file("extdata", "ImmPortStudies", package = "RImmPort") db_dir <- file.path(studies_dir, "Db") sqlite_conn <- dbconnect(sqlite(), dbname=file.path(db_dir, "ImmPort.sqlite")) setimmportdatasource(sqlite_conn) sdy139 <- getstudy("sdy139") ts_df <- sdy139$trial_design$ts_l$ts_df TS Trial Summary Domain Variables TSSEQ TSPARMCD TSPARM TSVAL Trial Summary Parameter Short Name Trial Summary Parameter Parameter Value Note The following table enumerates the values in TSPARMCD and TSPARM variables TSPARMCD TITLE DESCR TSPARM Trial Title Trial
36 36 VS INDIC TRT HYPOTHS SSTDTC SENDTC PLANSUB ACTSUB AGEMAX AGEMIN AGEU SEXPOP SPONSOR PUBRLDAT ISTRIAL RESFOCUS Trial Indication Investigational Therapy or Treatment Trial Hypotheses Study Start Date Study End Date Planned Number of Subjects Actual Number of Subjects Planned Maximum Age of Subjects Planned Minimum Age of Subjects Age Units Sex of Participants Clinical Study Sponsor Public Release Date Study Type Trial Research Focus TV Trial Visits Domain Variables VISITNUM VISIT ARMCD ARM TVSTRL TVENRL Visit Number Visit Name Planned Arm Code Name of Planned Arm Visit Start Rule Visit End Rule Vital Signs Domain Vital Signs Domain The Vital Signs data of an ImmPort study is reformated to the CDISC SDTM Vital Signs (VS) domain model, and is a list of 2 data frames containing 1) Vital Signs data VS and 2) any supplemental Vital Signs data SUPPVS VS Vital Signs Domain Variables
37 ZB 37 VSSEQ VSTEST VSCAT VSORRES VSORRESU VSLOC VISITNUM VISIT VSDY Vital Signs Test Name Category for Vital Signs Result or Finding in Original Units Original Units Location of Vital Signs Measurement Visit Number Visit Name Study Day of Vital Signs ZA Protein Quantification Domain Variables ZASEQ ZATEST ZACAT ZAMETHOD ZAANALYT ZAORRES ZAORRESU ZASPEC VISITNUM VISIT ZAELTM ZATPTREF ZAREFID ZAXFN Protein Quantification Test Name Category for Protein Quantification Measurement Technique Analyte Result or Finding in Original Units Original Units Specimen Type Visit Number Visit Name Planned Elapsed Time from Time Point Ref Time Point Reference Specimen Identifier Raw Data File or Life Science Identifier ZB Cellular Quantification Domain Variables
38 38 ZC ZBSEQ ZBTEST ZBCAT ZBMETHOD ZBPOPDEF ZBPOPNAM ZBORRES ZBORRESU ZBBASPOP ZBSPEC VISITNUM VISIT ZBELTM ZBTPTREF ZBREFID ZBXFN Cellular Quantification Test Name Category for Cellular Quantification Measurement Technique Cell Population Definition Cell Population Name Result or Finding in Original Units Original Units Base Parent Population Specimen Type Visit Number Visit Name Planned Elapsed Time from Time Point Ref Time Point Reference Specimen Identifier Raw Data File or Life Science Identifier ZC Nucleic Acid Quantification Domain Variables ZCSEQ ZCTEST ZCCAT ZCMETHOD ZCENTRZD ZCGENNAM ZCGENSYM ZCORRES ZCORRESU ZCSPEC ZCREFID VISITNUM VISIT ZCELTM ZCTPTREF Nucleic Acid Quantification Test Name Category for Nucleic Acid Quantification Measurement Technique Entrez Gene ID Gene Name Gene Symbol Result or Finding in Original Units Original Units Specimen Type Specimen Identifier Visit Number Visit Name Planned Elapsed Time from Time Point Ref Time Point Reference
39 ZD 39 ZD Titer Assay Results Domain Variables ZDSEQ ZDTEST ZDCAT ZDMETHOD ZDSTRAIN ZDORRES ZDORRESU ZDSPEC ZDREFID VISITNUM VISIT ZDELTM ZDTPTREF ZDXFN Titer Assay Results Test Name Category for Titer Assay Results Measurement Technique Virus Strain Result or Finding in Original Units Original Units Specimen Type Specimen Identifier Visit Number Visit Name Planned Elapsed Time from Time Point Ref Time Point Reference Raw Data File or Life Science Identifier
40 Index Adverse Events Domain, 3 AE, 3, 3, 7 APMH, 4, 4, 7 Associated Persons Medical History Domain, 4 buildnewsqlitedb, 5 Cellular Quantification Domain, 5 CM, 6, 6, 15 Concomitant Medications Domain, 6 Demographics Domain, 6 DM, 6, 6, 23 DV, 7, 7, 20 Events, 24 Events (Events-class), 7 Events-class, 7 EX, 8, 8, 15 Exposure Domain, 8 FA, 8, 9 Findings, 24 Findings (Findings-class), 9 Findings About Domain, 9 Findings-class, 9 Genetics Findings Domain, 10 getassaydataofstudies, 10 getdomaincode, 11 getdomaindataofstudies, 11 getlistofassaytypes, 12 getlistofdomains, 12 getlistofstudies, 13 getstudieswithspecificassaydata, 13 getstudieswithspecificdomaindata, 14 getstudy, 15 Interventions, 24 Interventions (Interventions-class), 15 Interventions-class, 15 Laboratory Test Results Domain, 16 LB, 9, 16, 16 loadserializedstudydata, 16 Medical History Domain, 17 mergedomainandsupplemental, 17 MH, 7, 17, 18 Nucleic Acid Quantification Domain, 18 PE, 9, 19, 20 PF, 9, 10, 19 Physical Examination Domain, 20 Protein Quantification Domain, 20 Protocol Deviations Domain, 20 QS, 9, 20, 21 Questionnaires Domain, 21 RImmPort, 21 RImmPort-package (RImmPort), 21 serialzestudydata, 21 setimmportdatasource, 22 Skin Response Domain, 22 SpecialPurpose, 24 SpecialPurpose (SpecialPurpose-class), 23 SpecialPurpose-class, 23 SR, 9, 22, 23 Study (Study-class), 23 Study-class, 23 SU, 15, 24, 25 Subject Visits Domain, 24 Substance Use Domain, 25 SUPP, 3 10, 15, 18, 20 23, 25, 25, SUPPDV, 7, 20, 25 SUPPFA, 9, 26 SUPPLB, 9, 16, 26 SUPPMH, 7, 17, 27 SUPPPE, 9, 20, 28 SUPPPF, 9, 28 SUPPVS, 9, 29, 36 SUPPZA, 9, 29 SUPPZB, 30 SUPPZC, 31 SUPPZD, 32 40
41 INDEX 41 SV, 25, 32 TA, 33, 34, 35 TI, 33, 34, 35 Titer Assay Results Domain, 33 Trial Arms Domain, 34 Trial Inclusion Exclusion Criteria Domain, 34 Trial Summary Domain, 34 Trial Visits Domain, 34 TrialDesign, 24 TrialDesign (TrialDesign-class), 35 TrialDesign-class, 35 TS, 34, 35, 35 TV, 34, 36 Vital Signs Domain, 36 VS, 9, 36, 36 ZA, 9, 20, 37 ZB, 5, 9, 37 ZC, 9, 18, 38 ZD, 9, 33, 39
PharmaSUG Paper DS04
 PharmaSUG2014 - Paper DS04 Considerations in the Submission of Exposure Data in SDTM-Based Datasets Fred Wood, Accenture Life Sciences, Wayne, PA Jerry Salyers, Accenture Life Sciences, Wayne, PA Richard
PharmaSUG2014 - Paper DS04 Considerations in the Submission of Exposure Data in SDTM-Based Datasets Fred Wood, Accenture Life Sciences, Wayne, PA Jerry Salyers, Accenture Life Sciences, Wayne, PA Richard
Pain Relief (PR) Notes to Readers
 Pain (PR) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC and Analgesic Clinical Trial Translations, Innovations, Opportunities,
Pain (PR) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC and Analgesic Clinical Trial Translations, Innovations, Opportunities,
SDTMIG v3.3 Batch 2 Upcoming Public Review. Presented by Fred Wood
 1 SDTMIG v3.3 Batch 2 Upcoming Public Review Presented by Fred Wood 2 SDTMIG v3.3 Batch 2? What This package constitutes Batch 2 for the planned SDTMIG v3.3. It is planned to be available for a public
1 SDTMIG v3.3 Batch 2 Upcoming Public Review Presented by Fred Wood 2 SDTMIG v3.3 Batch 2? What This package constitutes Batch 2 for the planned SDTMIG v3.3. It is planned to be available for a public
How to - Trial Summary Parameter. CJUG SDTM Team : LISaS
 How to - Trial Summary Parameter CJUG SDTM Team : LISaS 2013-11-08 Preconditions for the Discussion CDISC Controlled Terminology: As of 2013-10-04 SDTM IG: v3.1.3 or v3.1.2 CDISC Protocol Representation
How to - Trial Summary Parameter CJUG SDTM Team : LISaS 2013-11-08 Preconditions for the Discussion CDISC Controlled Terminology: As of 2013-10-04 SDTM IG: v3.1.3 or v3.1.2 CDISC Protocol Representation
Graph Display of Patient Profile Using SAS
 PharmaSUG 2018 - Paper DV-17 Graph Display of Patient Profile Using SAS Yanwei Han, Seqirus USA Inc., Cambridge, MA Hongyu Liu, Seqirus USA Inc., Cambridge, MA ABSTRACT We usually create patient profile
PharmaSUG 2018 - Paper DV-17 Graph Display of Patient Profile Using SAS Yanwei Han, Seqirus USA Inc., Cambridge, MA Hongyu Liu, Seqirus USA Inc., Cambridge, MA ABSTRACT We usually create patient profile
Trial Design Datasets
 Trial Design Datasets /////////// 26 th Meeting German CDISC User Network 20.02.2018 / Michael Schmitz / Agenda Hintergrund CDISC Study Design Workbook TS Trial Summary TE Trial Elements TA Trial Arms
Trial Design Datasets /////////// 26 th Meeting German CDISC User Network 20.02.2018 / Michael Schmitz / Agenda Hintergrund CDISC Study Design Workbook TS Trial Summary TE Trial Elements TA Trial Arms
Short-Form McGill Pain Questionniare-2 (SHORT-FORM MPQ-2)
 Short-Form McGill Pain Questionniare-2 ( ) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC and Analgesic Clinical Trial Translations,
Short-Form McGill Pain Questionniare-2 ( ) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC and Analgesic Clinical Trial Translations,
Neuropathic Pain Scale (NPS)
 Neuropathic Pain Scale (NPS) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by Business & Decision Life Sciences and the CDISC Questionnaire
Neuropathic Pain Scale (NPS) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by Business & Decision Life Sciences and the CDISC Questionnaire
Columbia-Suicide Severity Rating Scale Baseline (C-SSRS BASELINE)
 Columbia-Suicide Severity Rating Scale Baseline ( ) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC and Analgesic Clinical Trial
Columbia-Suicide Severity Rating Scale Baseline ( ) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC and Analgesic Clinical Trial
Disability Assessment for Dementia (DAD)
 Disability Assessment for Dementia (DAD) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CAMD AD v1.1 Team and the CDISC Questionnaire
Disability Assessment for Dementia (DAD) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CAMD AD v1.1 Team and the CDISC Questionnaire
Alzheimer s Disease-specific Therapeutic Area Supplement to the Study Data Tabulation Model User Guide
 CDISC Alzheimer s disease SDTM User Guide (Version 1.0) Alzheimer s Disease-specific Therapeutic Area Supplement to the Study Data Tabulation Model User Guide Prepared by the Coalition Against Major Diseases
CDISC Alzheimer s disease SDTM User Guide (Version 1.0) Alzheimer s Disease-specific Therapeutic Area Supplement to the Study Data Tabulation Model User Guide Prepared by the Coalition Against Major Diseases
Clinical Global Impression (CGI)
 Clinical Global Impression (CGI) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC and Analgesic Clinical Trial Translations,
Clinical Global Impression (CGI) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC and Analgesic Clinical Trial Translations,
What to Expect in SDTMIG v3.3 Fred Wood, Accenture Accelerated R&D Services, Berwyn, PA
 PharmaSUG2015 - Paper DS14 What to Expect in SDTMIG v3.3 Fred Wood, Accenture Accelerated R&D Services, Berwyn, PA ABSTRACT The next version of the SDTMIG (version 3.3) is expected in Q3 of this year.
PharmaSUG2015 - Paper DS14 What to Expect in SDTMIG v3.3 Fred Wood, Accenture Accelerated R&D Services, Berwyn, PA ABSTRACT The next version of the SDTMIG (version 3.3) is expected in Q3 of this year.
Columbia-Suicide Severity Rating Scale Baseline (C-SSRS BASELINE)
 Columbia-Suicide Severity Rating Scale Baseline ( ) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC and Analgesic Clinical Trial
Columbia-Suicide Severity Rating Scale Baseline ( ) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC and Analgesic Clinical Trial
The Standard for the Exchange of Nonclinical Data (SEND): History, Basics, and Comparisons with Clinical Data
 Life Sciences Accelerated R&D Services The Science of Getting Products to Patients Faster The Standard for the Exchange of Nonclinical Data (SEND): History, Basics, and Comparisons with Clinical Data Fred
Life Sciences Accelerated R&D Services The Science of Getting Products to Patients Faster The Standard for the Exchange of Nonclinical Data (SEND): History, Basics, and Comparisons with Clinical Data Fred
Epworth Sleepiness Scale (ESS)
 Epworth Sleepiness Scale (ESS) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC Questionnaires Sub-team Notes to Readers This
Epworth Sleepiness Scale (ESS) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC Questionnaires Sub-team Notes to Readers This
Modified Hachinski Ischemic Scale: NACC Version (MHIS-NACC) v.1.0
 Modified Hachinski Ischemic Scale: NACC Version (MHIS-NACC) v.1.0 Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CAMD AD v2.0 Team
Modified Hachinski Ischemic Scale: NACC Version (MHIS-NACC) v.1.0 Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CAMD AD v2.0 Team
Inclusion/Exclusion DOMAIN: IE. Subject ID USUBJID. STUDYID Visit Date. Phase Visit Week / / IEDTC EPOCH VISIT. Sequence Number
 DOMAIN: IE BUP-NX For Adolescent/Young Adult Opiate Dependency Protocol Number Node ID Site ID NIDA-CTN-0010 Visit Date Phase Visit Week / / IEDTC EPOCH VISIT For use with Phase Two Only Subject ID USUBJID
DOMAIN: IE BUP-NX For Adolescent/Young Adult Opiate Dependency Protocol Number Node ID Site ID NIDA-CTN-0010 Visit Date Phase Visit Week / / IEDTC EPOCH VISIT For use with Phase Two Only Subject ID USUBJID
Hospital Anxiety and Depression Scale (HADS)
 Hospital Anxiety and Depression Scale (HADS) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by Business & Decision Life Sciences and
Hospital Anxiety and Depression Scale (HADS) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by Business & Decision Life Sciences and
Package MSstatsTMT. February 26, Title Protein Significance Analysis in shotgun mass spectrometry-based
 Package MSstatsTMT February 26, 2019 Title Protein Significance Analysis in shotgun mass spectrometry-based proteomic experiments with tandem mass tag (TMT) labeling Version 1.1.2 Date 2019-02-25 Tools
Package MSstatsTMT February 26, 2019 Title Protein Significance Analysis in shotgun mass spectrometry-based proteomic experiments with tandem mass tag (TMT) labeling Version 1.1.2 Date 2019-02-25 Tools
Michigan Neuropathy Screening Instrument (MNSI)
 Michigan Neuropathy Screening Instrument () Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC and Analgesic Clinical Trial Translations,
Michigan Neuropathy Screening Instrument () Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC and Analgesic Clinical Trial Translations,
Barnes Akathisia Rating Scale (BARS)
 Barnes Akathisia Rating Scale () Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC Questionnaire Sub-team Notes to Readers This
Barnes Akathisia Rating Scale () Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC Questionnaire Sub-team Notes to Readers This
Brief Psychiatric Rating Scale-Anchored (BPRS-A)
 Brief Psychiatric Rating Scale-Anchored (BPRS-A) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC Questionnaire Sub-team Notes
Brief Psychiatric Rating Scale-Anchored (BPRS-A) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC Questionnaire Sub-team Notes
adsm TB Version July 25 th, 2016
 WHO central database for collection of safety data (AE and/or SAE) in the scope of adsm of anti-tb drugs Variables to be collected ID case number CONTR Case ID number Case identification number in national
WHO central database for collection of safety data (AE and/or SAE) in the scope of adsm of anti-tb drugs Variables to be collected ID case number CONTR Case ID number Case identification number in national
Columbia-Suicide Severity Rating Scale Baseline (C-SSRS BASELINE)
 Columbia-Suicide Severity Rating Scale Baseline ( ) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC and Analgesic Clinical Trial
Columbia-Suicide Severity Rating Scale Baseline ( ) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC and Analgesic Clinical Trial
Brief Psychiatric Rating Scale-Anchored (BPRS-A)
 Brief Psychiatric Rating Scale-Anchored (BPRS-A) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC Questionnaire Sub-team Notes
Brief Psychiatric Rating Scale-Anchored (BPRS-A) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC Questionnaire Sub-team Notes
Hamilton Depression Rating Scale 17-Item (HAMD 17)
 Hamilton Depression Rating Scale -Item ( ) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC and Analgesic Clinical Trial Translations,
Hamilton Depression Rating Scale -Item ( ) Questionnaire Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by CDISC and Analgesic Clinical Trial Translations,
Package msgbsr. January 17, 2019
 Type Package Package msgbsr January 17, 2019 Title msgbsr: methylation sensitive genotyping by sequencing (MS-GBS) R functions Version 1.6.1 Date 2017-04-24 Author Benjamin Mayne Maintainer Benjamin Mayne
Type Package Package msgbsr January 17, 2019 Title msgbsr: methylation sensitive genotyping by sequencing (MS-GBS) R functions Version 1.6.1 Date 2017-04-24 Author Benjamin Mayne Maintainer Benjamin Mayne
Programmatic Challenges of Dose Tapering Using SAS
 ABSTRACT: Paper 2036-2014 Programmatic Challenges of Dose Tapering Using SAS Iuliana Barbalau, Santen Inc., Emeryville, CA Chen Shi, Santen Inc., Emeryville, CA Yang Yang, Santen Inc., Emeryville, CA In
ABSTRACT: Paper 2036-2014 Programmatic Challenges of Dose Tapering Using SAS Iuliana Barbalau, Santen Inc., Emeryville, CA Chen Shi, Santen Inc., Emeryville, CA Yang Yang, Santen Inc., Emeryville, CA In
Package pepdat. R topics documented: March 7, 2015
 Package pepdat March 7, 2015 Type Package Title Peptide microarray data package Version 1.0.0 Author Renan Sauteraud, Raphael Gottardo Maintainer Renan Sauteraud Date 2012-08-14 Provides
Package pepdat March 7, 2015 Type Package Title Peptide microarray data package Version 1.0.0 Author Renan Sauteraud, Raphael Gottardo Maintainer Renan Sauteraud Date 2012-08-14 Provides
Package MethPed. September 1, 2018
 Type Package Version 1.8.0 Date 2016-01-01 Package MethPed September 1, 2018 Title A DNA methylation classifier tool for the identification of pediatric brain tumor subtypes Depends R (>= 3.0.0), Biobase
Type Package Version 1.8.0 Date 2016-01-01 Package MethPed September 1, 2018 Title A DNA methylation classifier tool for the identification of pediatric brain tumor subtypes Depends R (>= 3.0.0), Biobase
Intervention ML: Mapping of Meal data. Rahul Loharkar, inventiv Health Clinical, Pune, India Sandeep Sawant, inventiv Health Clinical, Mumbai, India
 Paper DS06 Intervention ML: Mapping of Meal data. Rahul Loharkar, inventiv Health Clinical, Pune, India Sandeep Sawant, inventiv Health Clinical, Mumbai, India ABSTRACT The focus of most bioavailability/bioequivalence
Paper DS06 Intervention ML: Mapping of Meal data. Rahul Loharkar, inventiv Health Clinical, Pune, India Sandeep Sawant, inventiv Health Clinical, Mumbai, India ABSTRACT The focus of most bioavailability/bioequivalence
CONSTRUCTING A DATA WAREHOUSE FOR NUTRITION CLINICAL STUDIES
 CONSTRUCTING A DATA WAREHOUSE FOR NUTRITION CLINICAL STUDIES Paul Vervuren PhUSE Barcelona October 10 th, 2016 CLINICAL DATA WAREHOUSE PROJECT PART OF DANONE FOUR DIVISIONS WATERS MEDICAL NUTRITION FRESH
CONSTRUCTING A DATA WAREHOUSE FOR NUTRITION CLINICAL STUDIES Paul Vervuren PhUSE Barcelona October 10 th, 2016 CLINICAL DATA WAREHOUSE PROJECT PART OF DANONE FOUR DIVISIONS WATERS MEDICAL NUTRITION FRESH
Using CDISC Models for the Analysis of Safety Data
 Paper RA02 Using CDISC Models for the Analysis of Safety Data Susan J. Kenny, Inspire Pharmaceuticals Inc. Research Triangle Park, NC, USA Edward D. Helton, SAS Institute, Cary, NC, USA ABSTRACT The collection
Paper RA02 Using CDISC Models for the Analysis of Safety Data Susan J. Kenny, Inspire Pharmaceuticals Inc. Research Triangle Park, NC, USA Edward D. Helton, SAS Institute, Cary, NC, USA ABSTRACT The collection
Innovation and Regulatory Review What is JumpStart?
 Innovation and Regulatory Review What is JumpStart? Alan M. Shapiro, MD, PhD, FAAP Office of Computational Science FDA/CDER/OTS May 17, 2017 Disclosure No financial or commercial conflict of interest The
Innovation and Regulatory Review What is JumpStart? Alan M. Shapiro, MD, PhD, FAAP Office of Computational Science FDA/CDER/OTS May 17, 2017 Disclosure No financial or commercial conflict of interest The
Package seqcombo. R topics documented: March 10, 2019
 Package seqcombo March 10, 2019 Title Visualization Tool for Sequence Recombination and Reassortment Version 1.5.1 Provides useful functions for visualizing sequence recombination and virus reassortment
Package seqcombo March 10, 2019 Title Visualization Tool for Sequence Recombination and Reassortment Version 1.5.1 Provides useful functions for visualizing sequence recombination and virus reassortment
Package medicalrisk. August 29, 2016
 Type Package Package medicalrisk August 29, 2016 Title Medical Risk and Comorbidity Tools for ICD-9-CM Data Version 1.2 Date 2016-01-23 Generates risk estimates and comorbidity flags from ICD-9-CM codes
Type Package Package medicalrisk August 29, 2016 Title Medical Risk and Comorbidity Tools for ICD-9-CM Data Version 1.2 Date 2016-01-23 Generates risk estimates and comorbidity flags from ICD-9-CM codes
Let s Create Standard Value Level Metadata
 PRA Health Sciences PhUSE US Connect 2019 Paper DS12 February 27, 2019 David Fielding Value Level Metadata Value Level Metadata is an important part of the Define-XML that allows us to explain our study
PRA Health Sciences PhUSE US Connect 2019 Paper DS12 February 27, 2019 David Fielding Value Level Metadata Value Level Metadata is an important part of the Define-XML that allows us to explain our study
Package citccmst. February 19, 2015
 Version 1.0.2 Date 2014-01-07 Package citccmst February 19, 2015 Title CIT Colon Cancer Molecular SubTypes Prediction Description This package implements the approach to assign tumor gene expression dataset
Version 1.0.2 Date 2014-01-07 Package citccmst February 19, 2015 Title CIT Colon Cancer Molecular SubTypes Prediction Description This package implements the approach to assign tumor gene expression dataset
SDTM, Implementation Guide, and User Guide Updates
 Accenture Life Sciences Rethink Reshape Restructure for better health outcomes. Octagon is Now Part of Accenture SDTM, Implementation Guide, and User Guide Updates Fred Wood July 2013 Agenda Model, IG,
Accenture Life Sciences Rethink Reshape Restructure for better health outcomes. Octagon is Now Part of Accenture SDTM, Implementation Guide, and User Guide Updates Fred Wood July 2013 Agenda Model, IG,
CDISC Controlled Terminology. Presented by Chris Gemma and Erin Muhlbradt, Ph.D.
 CDISC Controlled Terminology Presented by Chris Gemma and Erin Muhlbradt, Ph.D. Panelists Erin Muhlbradt, Clincal/Biomedical Information Specialist, MSC Chris Gemma, Project Manager, CDISC 2 Question &
CDISC Controlled Terminology Presented by Chris Gemma and Erin Muhlbradt, Ph.D. Panelists Erin Muhlbradt, Clincal/Biomedical Information Specialist, MSC Chris Gemma, Project Manager, CDISC 2 Question &
Package HAP.ROR. R topics documented: February 19, 2015
 Type Package Title Recursive Organizer (ROR) Version 1.0 Date 2013-03-23 Author Lue Ping Zhao and Xin Huang Package HAP.ROR February 19, 2015 Maintainer Xin Huang Depends R (>=
Type Package Title Recursive Organizer (ROR) Version 1.0 Date 2013-03-23 Author Lue Ping Zhao and Xin Huang Package HAP.ROR February 19, 2015 Maintainer Xin Huang Depends R (>=
Imputing Dose Levels for Adverse Events
 Paper HO03 Imputing Dose Levels for Adverse Events John R Gerlach & Igor Kolodezh CSG Inc., Raleigh, NC USA ABSTRACT Besides the standard reporting of adverse events in clinical trials, there is a growing
Paper HO03 Imputing Dose Levels for Adverse Events John R Gerlach & Igor Kolodezh CSG Inc., Raleigh, NC USA ABSTRACT Besides the standard reporting of adverse events in clinical trials, there is a growing
Package AbsFilterGSEA
 Type Package Package AbsFilterGSEA September 21, 2017 Title Improved False Positive Control of Gene-Permuting GSEA with Absolute Filtering Version 1.5.1 Author Sora Yoon Maintainer
Type Package Package AbsFilterGSEA September 21, 2017 Title Improved False Positive Control of Gene-Permuting GSEA with Absolute Filtering Version 1.5.1 Author Sora Yoon Maintainer
PROGRAMMER S SAFETY KIT: Important Points to Remember While Programming or Validating Safety Tables
 PharmaSUG 2012 - Paper IB09 PROGRAMMER S SAFETY KIT: Important Points to Remember While Programming or Validating Safety Tables Sneha Sarmukadam, Statistical Programmer, PharmaNet/i3, Pune, India Sandeep
PharmaSUG 2012 - Paper IB09 PROGRAMMER S SAFETY KIT: Important Points to Remember While Programming or Validating Safety Tables Sneha Sarmukadam, Statistical Programmer, PharmaNet/i3, Pune, India Sandeep
How to manage changes to CDISC standards at Novo Nordisk. PhUSE EU Connect 2018 Sune Dandanell and Mikkel Traun
 How to manage changes to CDISC standards at Novo Nordisk PhUSE EU Connect 2018 Sune Dandanell and Mikkel Traun How to manage changes to CDISC standards at Novo Nordisk 06-Nov-2018 2 How to manage changes
How to manage changes to CDISC standards at Novo Nordisk PhUSE EU Connect 2018 Sune Dandanell and Mikkel Traun How to manage changes to CDISC standards at Novo Nordisk 06-Nov-2018 2 How to manage changes
Therapeutic Area User Guide Cardiovascular V1.0 Public Review Webinar April 3, 2014
 Therapeutic Area User Guide Cardiovascular V1.0 Public Review Webinar April 3, 2014 1 Therapeutic Area User Guide CV V1.0 Public Review Webinar April 3, 2014 James Tcheng, MD, FACC, DCRI Steve Kopko, CDISC,
Therapeutic Area User Guide Cardiovascular V1.0 Public Review Webinar April 3, 2014 1 Therapeutic Area User Guide CV V1.0 Public Review Webinar April 3, 2014 James Tcheng, MD, FACC, DCRI Steve Kopko, CDISC,
Package pollen. October 7, 2018
 Type Package Title Analysis of Aerobiological Data Version 0.71.0 Package pollen October 7, 2018 Supports analysis of aerobiological data. Available features include determination of pollen season limits,
Type Package Title Analysis of Aerobiological Data Version 0.71.0 Package pollen October 7, 2018 Supports analysis of aerobiological data. Available features include determination of pollen season limits,
PharmaSUG Paper DS12
 PharmaSUG 2017 - Paper DS12 Considerations and Conventions in the Submission of the SDTM Tumor and Response Domains Jerry Salyers, Accenture Accelerated R&D Services, Berwyn, PA Fred Wood, Accenture Accelerated
PharmaSUG 2017 - Paper DS12 Considerations and Conventions in the Submission of the SDTM Tumor and Response Domains Jerry Salyers, Accenture Accelerated R&D Services, Berwyn, PA Fred Wood, Accenture Accelerated
Package pathifier. August 17, 2018
 Type Package Title Quantify deregulation of pathways in cancer Version 1.19.0 Date 2013-06-27 Author Yotam Drier Package pathifier August 17, 2018 Maintainer Assif Yitzhaky
Type Package Title Quantify deregulation of pathways in cancer Version 1.19.0 Date 2013-06-27 Author Yotam Drier Package pathifier August 17, 2018 Maintainer Assif Yitzhaky
Package PELVIS. R topics documented: December 18, Type Package
 Type Package Package PELVIS December 18, 2018 Title Probabilistic Sex Estimate using Logistic Regression, Based on VISual Traits of the Human Os Coxae Version 1.0.5 Date 2018-12-18 Depends R (>= 3.4.0),
Type Package Package PELVIS December 18, 2018 Title Probabilistic Sex Estimate using Logistic Regression, Based on VISual Traits of the Human Os Coxae Version 1.0.5 Date 2018-12-18 Depends R (>= 3.4.0),
Package leukemiaseset
 Package leukemiaseset August 14, 2018 Type Package Title Leukemia's microarray gene expression data (expressionset). Version 1.16.0 Date 2013-03-20 Author Sara Aibar, Celia Fontanillo and Javier De Las
Package leukemiaseset August 14, 2018 Type Package Title Leukemia's microarray gene expression data (expressionset). Version 1.16.0 Date 2013-03-20 Author Sara Aibar, Celia Fontanillo and Javier De Las
Therapeutic Area Data Standards User Guide for Cardiovascular Studies Version 1.0 (Provisional) Prepared by the CFAST Cardiovascular Team
 Therapeutic Area Data Standards User Guide for Cardiovascular Studies Version 1.0 (Provisional) Prepared by the CFAST Cardiovascular Team Notes to Readers This is the provisional version 1.0 of the Therapeutic
Therapeutic Area Data Standards User Guide for Cardiovascular Studies Version 1.0 (Provisional) Prepared by the CFAST Cardiovascular Team Notes to Readers This is the provisional version 1.0 of the Therapeutic
Research Compliance and Quality Assurance Program (RCQA): Audit Checklist Subject Specific
 Protocol Title / Code: Sponsor: PI Name: Auditor Name: Audit Date(s): Subject #: Key Dates: Date participant was identified: Date of Initial Consent: Date of Optional Consent: Date of Re-consent(s): Date
Protocol Title / Code: Sponsor: PI Name: Auditor Name: Audit Date(s): Subject #: Key Dates: Date participant was identified: Date of Initial Consent: Date of Optional Consent: Date of Re-consent(s): Date
CDISC CONTROLLED TERMINOLOGY. Presented by Chris Gemma and Erin Muhlbradt, Ph.D.
 1 CDISC CONTROLLED TERMINOLOGY Presented by Chris Gemma and Erin Muhlbradt, Ph.D. CDISC 2018 2 Question & Answer Panelist : Question OR Presentation : Question Examples: 1) What should be supported by
1 CDISC CONTROLLED TERMINOLOGY Presented by Chris Gemma and Erin Muhlbradt, Ph.D. CDISC 2018 2 Question & Answer Panelist : Question OR Presentation : Question Examples: 1) What should be supported by
Package cancer. July 10, 2018
 Type Package Package cancer July 10, 2018 Title A Graphical User Interface for accessing and modeling the Cancer Genomics Data of MSKCC. Version 1.14.0 Date 2018-04-16 Author Karim Mezhoud. Nuclear Safety
Type Package Package cancer July 10, 2018 Title A Graphical User Interface for accessing and modeling the Cancer Genomics Data of MSKCC. Version 1.14.0 Date 2018-04-16 Author Karim Mezhoud. Nuclear Safety
CDISC BrCa TAUG & Oncology Information Session
 1 15 September 2016 CDISC BrCa TAUG & Oncology Information Session Barrie Nelson @ DCDISC 2 24 February 2016 10:00-11:30am CST CDISC Oncology Information Session Rhonda Facile, Ann White, John Owen, Diane
1 15 September 2016 CDISC BrCa TAUG & Oncology Information Session Barrie Nelson @ DCDISC 2 24 February 2016 10:00-11:30am CST CDISC Oncology Information Session Rhonda Facile, Ann White, John Owen, Diane
Jerry Salyers, Accenture Accelerated R&D Services Fred Wood, Accenture Accelerated R&D Services. PhUSE 2017 Edinburgh, Scotland Paper #DS05
 Jerry Salyers, Accenture Accelerated R&D Services Fred Wood, Accenture Accelerated R&D Services PhUSE 2017 Paper #DS05 } Review of Tumor domains and representative CRFs Ø TU (Tumor Identification) Ø TR
Jerry Salyers, Accenture Accelerated R&D Services Fred Wood, Accenture Accelerated R&D Services PhUSE 2017 Paper #DS05 } Review of Tumor domains and representative CRFs Ø TU (Tumor Identification) Ø TR
Package inctools. January 3, 2018
 Encoding UTF-8 Type Package Title Incidence Estimation Tools Version 1.0.11 Date 2018-01-03 Package inctools January 3, 2018 Tools for estimating incidence from biomarker data in crosssectional surveys,
Encoding UTF-8 Type Package Title Incidence Estimation Tools Version 1.0.11 Date 2018-01-03 Package inctools January 3, 2018 Tools for estimating incidence from biomarker data in crosssectional surveys,
Data mining with Ensembl Biomart. Stéphanie Le Gras
 Data mining with Ensembl Biomart Stéphanie Le Gras (slegras@igbmc.fr) Guidelines Genome data Genome browsers Getting access to genomic data: Ensembl/BioMart 2 Genome Sequencing Example: Human genome 2000:
Data mining with Ensembl Biomart Stéphanie Le Gras (slegras@igbmc.fr) Guidelines Genome data Genome browsers Getting access to genomic data: Ensembl/BioMart 2 Genome Sequencing Example: Human genome 2000:
Review of Veterinary Epidemiologic Research by Dohoo, Martin, and Stryhn
 The Stata Journal (2004) 4, Number 1, pp. 89 92 Review of Veterinary Epidemiologic Research by Dohoo, Martin, and Stryhn Laurent Audigé AO Foundation laurent.audige@aofoundation.org Abstract. The new book
The Stata Journal (2004) 4, Number 1, pp. 89 92 Review of Veterinary Epidemiologic Research by Dohoo, Martin, and Stryhn Laurent Audigé AO Foundation laurent.audige@aofoundation.org Abstract. The new book
Package BCRA. April 25, 2018
 Type Package Title Breast Cancer Risk Assessment Version 2.0 Date 2018-04-25 Author Fanni Zhang Maintainer Fanni Zhang Package BCRA April 25, 2018 Functions provide risk projections
Type Package Title Breast Cancer Risk Assessment Version 2.0 Date 2018-04-25 Author Fanni Zhang Maintainer Fanni Zhang Package BCRA April 25, 2018 Functions provide risk projections
A SAS sy Study of ediary Data
 A SAS sy Study of ediary Data ABSTRACT PharmaSUG 2017 - Paper BB14 A SAS sy Study of ediary Data Amie Bissonett, inventiv Health Clinical, Minneapolis, MN Many sponsors are using electronic diaries (ediaries)
A SAS sy Study of ediary Data ABSTRACT PharmaSUG 2017 - Paper BB14 A SAS sy Study of ediary Data Amie Bissonett, inventiv Health Clinical, Minneapolis, MN Many sponsors are using electronic diaries (ediaries)
AE: An Essential Part of Safety Summary Table Creation. Rucha Landge, Inventiv International Pharma Services Pvt. Ltd.
 PharmaSUG 2016 - Paper IB11 AE: An Essential Part of Safety Summary Table Creation. Rucha Landge, Inventiv International Pharma Services Pvt. Ltd., Pune, India ABSTRACT Adverse event (AE) summary tables
PharmaSUG 2016 - Paper IB11 AE: An Essential Part of Safety Summary Table Creation. Rucha Landge, Inventiv International Pharma Services Pvt. Ltd., Pune, India ABSTRACT Adverse event (AE) summary tables
Standards for Analyzable Submission Datasets for Directed Safety Analyses
 Standards for Analyzable Submission Datasets for Directed Safety Analyses Michael Nessly Director, Clinical Biostatistics Merck Research Labs CDISC Analysis Dataset Models Team (ADaM) nesslym@merck.com
Standards for Analyzable Submission Datasets for Directed Safety Analyses Michael Nessly Director, Clinical Biostatistics Merck Research Labs CDISC Analysis Dataset Models Team (ADaM) nesslym@merck.com
Package CompareTests
 Type Package Package CompareTests February 6, 2017 Title Correct for Verification Bias in Diagnostic Accuracy & Agreement Version 1.2 Date 2016-2-6 Author Hormuzd A. Katki and David W. Edelstein Maintainer
Type Package Package CompareTests February 6, 2017 Title Correct for Verification Bias in Diagnostic Accuracy & Agreement Version 1.2 Date 2016-2-6 Author Hormuzd A. Katki and David W. Edelstein Maintainer
Package appnn. R topics documented: July 12, 2015
 Package appnn July 12, 2015 Type Package Title Amyloid Propensity Prediction Neural Network Version 1.0-0 Date 2015-07-11 Encoding UTF-8 Author Maintainer Carlos Família Amyloid
Package appnn July 12, 2015 Type Package Title Amyloid Propensity Prediction Neural Network Version 1.0-0 Date 2015-07-11 Encoding UTF-8 Author Maintainer Carlos Família Amyloid
User Guide. Association analysis. Input
 User Guide TFEA.ChIP is a tool to estimate transcription factor enrichment in a set of differentially expressed genes using data from ChIP-Seq experiments performed in different tissues and conditions.
User Guide TFEA.ChIP is a tool to estimate transcription factor enrichment in a set of differentially expressed genes using data from ChIP-Seq experiments performed in different tissues and conditions.
TRANSITIONING FROM PP EXTRACT TO A VENDOR NEUTRAL DATA EXTRACTION APPROACH R U T H J E N K I N S, P H D A U G U S T 2 2,
 TRANSITIONING FROM PP EXTRACT TO A VENDOR NEUTRAL DATA EXTRACTION APPROACH R U T H J E N K I N S, P H D A U G U S T 2 2, 2 0 1 4 AGENDA Why a new extract is needed Explain new extract process Data Extracted
TRANSITIONING FROM PP EXTRACT TO A VENDOR NEUTRAL DATA EXTRACTION APPROACH R U T H J E N K I N S, P H D A U G U S T 2 2, 2 0 1 4 AGENDA Why a new extract is needed Explain new extract process Data Extracted
HDClarity Data Dictionary
 HDClarity Data Dictionary Version 1.0 Table of Contents 1 Visit Plan 4 2 Form Demographics (Demog) 6 3 Form CAG Report (CAG) 7 4 Form Comorbid Conditions (Comorbid) 8 5 Form Pharmacotherapy (PharmacoTx)
HDClarity Data Dictionary Version 1.0 Table of Contents 1 Visit Plan 4 2 Form Demographics (Demog) 6 3 Form CAG Report (CAG) 7 4 Form Comorbid Conditions (Comorbid) 8 5 Form Pharmacotherapy (PharmacoTx)
Package biocancer. August 29, 2018
 Package biocancer August 29, 2018 Title Interactive Multi-Omics Cancers Data Visualization and Analysis Version 1.8.0 Date 2018-04-12 biocancer is a Shiny App to visualize and analyse interactively Multi-Assays
Package biocancer August 29, 2018 Title Interactive Multi-Omics Cancers Data Visualization and Analysis Version 1.8.0 Date 2018-04-12 biocancer is a Shiny App to visualize and analyse interactively Multi-Assays
Package AIMS. June 29, 2018
 Type Package Package AIMS June 29, 2018 Title AIMS : Absolute Assignment of Breast Cancer Intrinsic Molecular Subtype Version 1.12.0 Date 2014-06-25 Description This package contains the AIMS implementation.
Type Package Package AIMS June 29, 2018 Title AIMS : Absolute Assignment of Breast Cancer Intrinsic Molecular Subtype Version 1.12.0 Date 2014-06-25 Description This package contains the AIMS implementation.
Package ncar. July 20, 2018
 Version 0.4.1 Date 2018-06-20 KST Package ncar July 20, 2018 Title Noncompartmental Analysis for Pharmacokinetic Report Description Conduct a noncompartmental analysis as closely as possible to the most
Version 0.4.1 Date 2018-06-20 KST Package ncar July 20, 2018 Title Noncompartmental Analysis for Pharmacokinetic Report Description Conduct a noncompartmental analysis as closely as possible to the most
Package hei. August 17, 2017
 Type Package Title Calculate Healthy Eating Index (HEI) Scores Date 2017-08-05 Version 0.1.0 Package hei August 17, 2017 Maintainer Tim Folsom Calculates Healthy Eating Index
Type Package Title Calculate Healthy Eating Index (HEI) Scores Date 2017-08-05 Version 0.1.0 Package hei August 17, 2017 Maintainer Tim Folsom Calculates Healthy Eating Index
Timed Up and Go (TUG)
 Timed Up and Go (TUG) Functional Test Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by Multiple Sclerosis Outcomes Assessment Consortium and the
Timed Up and Go (TUG) Functional Test Supplement to the Study Data Tabulation Model Implementation Guide for Human Clinical Trials Prepared by Multiple Sclerosis Outcomes Assessment Consortium and the
Package diggitdata. April 11, 2019
 Type Package Title Example data for the diggit package Version 1.14.0 Date 2014-08-29 Author Mariano Javier Alvarez Package diggitdata April 11, 2019 Maintainer Mariano Javier Alvarez
Type Package Title Example data for the diggit package Version 1.14.0 Date 2014-08-29 Author Mariano Javier Alvarez Package diggitdata April 11, 2019 Maintainer Mariano Javier Alvarez
The Hospital Anxiety and Depression Scale Guidance and Information
 The Hospital Anxiety and Depression Scale Guidance and Information About Testwise Testwise is the powerful online testing platform developed by GL Assessment to host its digital tests. Many of GL Assessment
The Hospital Anxiety and Depression Scale Guidance and Information About Testwise Testwise is the powerful online testing platform developed by GL Assessment to host its digital tests. Many of GL Assessment
Package IMAS. March 10, 2019
 Type Package Package IMAS March 10, 2019 Title Integrative analysis of Multi-omics data for Alternative Splicing Version 1.6.1 Author Seonggyun Han, Younghee Lee Maintainer Seonggyun Han
Type Package Package IMAS March 10, 2019 Title Integrative analysis of Multi-omics data for Alternative Splicing Version 1.6.1 Author Seonggyun Han, Younghee Lee Maintainer Seonggyun Han
ADaM Tips for Exposure Summary
 ABSTRACT PharmaSUG China 2016 Paper 84 ADaM Tips for Exposure Summary Kriss Harris, SAS Specialists Limited, Hertfordshire, United Kingdom Have you ever created the Analysis Dataset for the Exposure Summary
ABSTRACT PharmaSUG China 2016 Paper 84 ADaM Tips for Exposure Summary Kriss Harris, SAS Specialists Limited, Hertfordshire, United Kingdom Have you ever created the Analysis Dataset for the Exposure Summary
a. From the grey navigation bar, mouse over Analyze & Visualize and click Annotate Nucleotide Sequences.
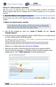 Section D. Custom sequence annotation After this exercise you should be able to use the annotation pipelines provided by the Influenza Research Database (IRD) and Virus Pathogen Resource (ViPR) to annotate
Section D. Custom sequence annotation After this exercise you should be able to use the annotation pipelines provided by the Influenza Research Database (IRD) and Virus Pathogen Resource (ViPR) to annotate
Package BUScorrect. September 16, 2018
 Type Package Package BUScorrect September 16, 2018 Title Batch Effects Correction with Unknown Subtypes Version 0.99.12 Date 2018-06-07 Author , Yingying Wei Maintainer
Type Package Package BUScorrect September 16, 2018 Title Batch Effects Correction with Unknown Subtypes Version 0.99.12 Date 2018-06-07 Author , Yingying Wei Maintainer
Package memapp. R topics documented: June 2, Version 2.10 Date
 Version 2.10 Date 2018-06-01 Package memapp June 2, 2018 Title The Moving Epidemic Method Web Application Description The Moving Epidemic Method, created by T Vega and JE Lozano (2012, 2015) ,
Version 2.10 Date 2018-06-01 Package memapp June 2, 2018 Title The Moving Epidemic Method Web Application Description The Moving Epidemic Method, created by T Vega and JE Lozano (2012, 2015) ,
CSCI/CMPE 3326 Spring Homework 3 Due
 Homework 3 Due Program 1: Make Carmax program. The program provides functions to manage customers and car inventory using MySQL. You can define any class, method, and database table if you need. (You may
Homework 3 Due Program 1: Make Carmax program. The program provides functions to manage customers and car inventory using MySQL. You can define any class, method, and database table if you need. (You may
Project Charter. Template v2 June 3, February 25, Name of Project: Prostate Cancer (PrCa) Therapeutic Area Data Standards
 Project Charter Template v2 June 3, 2015 Name of Project: Prostate Cancer (PrCa) Therapeutic Area Data Standards February 25, 2016 Project Manager: John Owen 1. Scope of Prostate Cancer Therapeutic Area
Project Charter Template v2 June 3, 2015 Name of Project: Prostate Cancer (PrCa) Therapeutic Area Data Standards February 25, 2016 Project Manager: John Owen 1. Scope of Prostate Cancer Therapeutic Area
Where Is the Link Broken Another Look at SDTM Oncology Tumor Packages
 PharmaSUG 2017 Paper DS07 Where Is the Link Broken Another Look at SDTM Oncology Tumor Packages ABSTRACT Hong WANG, Boehringer Ingelheim, Ridgefield, CT Ke XIAO, Boehringer Ingelheim, Ridgefield, CT CDISC
PharmaSUG 2017 Paper DS07 Where Is the Link Broken Another Look at SDTM Oncology Tumor Packages ABSTRACT Hong WANG, Boehringer Ingelheim, Ridgefield, CT Ke XIAO, Boehringer Ingelheim, Ridgefield, CT CDISC
LabVIEW PROFIBUS VISA Driver DP-Master
 LabVIEW PROFIBUS VISA Driver DP-Master Getting Started V1.35 27.04.2017 Project No.: 5303 Doc-ID.: LabVIEW PROFIBUS VISA Driver KUNBUS d:\project\5302_df_profi_ii\anwenderdoku\labview\version 1.35\gettingstarted_win_dp-master_e.doc
LabVIEW PROFIBUS VISA Driver DP-Master Getting Started V1.35 27.04.2017 Project No.: 5303 Doc-ID.: LabVIEW PROFIBUS VISA Driver KUNBUS d:\project\5302_df_profi_ii\anwenderdoku\labview\version 1.35\gettingstarted_win_dp-master_e.doc
Cortex Gateway 2.0. Administrator Guide. September Document Version C
 Cortex Gateway 2.0 Administrator Guide September 2015 Document Version C Version C of the Cortex Gateway 2.0 Administrator Guide had been updated with editing changes. Contents Preface... 1 About Cortex
Cortex Gateway 2.0 Administrator Guide September 2015 Document Version C Version C of the Cortex Gateway 2.0 Administrator Guide had been updated with editing changes. Contents Preface... 1 About Cortex
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Clinical Trials of Pandemic Vaccines: Key Issues. John Treanor University of Rochester Rochester, NY
 Clinical Trials of Pandemic Vaccines: Key Issues John Treanor University of Rochester Rochester, NY Inactivated vaccine approach Proven technology Used successfully in 1957 and 1968 Abundant efficacy data
Clinical Trials of Pandemic Vaccines: Key Issues John Treanor University of Rochester Rochester, NY Inactivated vaccine approach Proven technology Used successfully in 1957 and 1968 Abundant efficacy data
HL7 CDS Project Update
 HL7 CDS Project Update Virtual Medical Record (vmr) Clinical Genomics HL7 Phoenix May 2008 Robert Dunlop, MD Technical Lead: HL7 vmr & GELLO Projects InferMed UK Director, Clinical Development (M) +44-7291-7410
HL7 CDS Project Update Virtual Medical Record (vmr) Clinical Genomics HL7 Phoenix May 2008 Robert Dunlop, MD Technical Lead: HL7 vmr & GELLO Projects InferMed UK Director, Clinical Development (M) +44-7291-7410
Package ega. March 21, 2017
 Title Error Grid Analysis Version 2.0.0 Package ega March 21, 2017 Maintainer Daniel Schmolze Functions for assigning Clarke or Parkes (Consensus) error grid zones to blood glucose values,
Title Error Grid Analysis Version 2.0.0 Package ega March 21, 2017 Maintainer Daniel Schmolze Functions for assigning Clarke or Parkes (Consensus) error grid zones to blood glucose values,
Package SEERaBomb. May 11, 2018
 Package SEERaBomb May 11, 2018 Title SEER and Atomic Bomb Survivor Data Analysis Tools Version 2018.1 Date 2018-5-10 Author Tomas Radivoyevitch Creates SEER (Surveillance, Epidemiology and End Results)
Package SEERaBomb May 11, 2018 Title SEER and Atomic Bomb Survivor Data Analysis Tools Version 2018.1 Date 2018-5-10 Author Tomas Radivoyevitch Creates SEER (Surveillance, Epidemiology and End Results)
Analysis Datasets for Baseline Characteristics and Exposure Time for Multi-stage Clinical Trials
 Analysis Datasets for Baseline Characteristics and Exposure Time for Multi-stage Clinical Trials Kim Umans, Katherine Riester, Manjit McNeill, Wei Liu, and Lukasz Kniola PhUSE 2016, CD06, Barcelona Introduction
Analysis Datasets for Baseline Characteristics and Exposure Time for Multi-stage Clinical Trials Kim Umans, Katherine Riester, Manjit McNeill, Wei Liu, and Lukasz Kniola PhUSE 2016, CD06, Barcelona Introduction
Package singlecase. April 19, 2009
 Package singlecase April 19, 2009 Type Package Title statistical tests for single case studies in neuropsychology Version 0.1 Date 2008-05-07 Author Matthieu Dubois Maintainer Matthieu
Package singlecase April 19, 2009 Type Package Title statistical tests for single case studies in neuropsychology Version 0.1 Date 2008-05-07 Author Matthieu Dubois Maintainer Matthieu
Immunogenicity and Safety of GSK s FluLaval Quadrivalent Inactivated Influenza Vaccine in Children 6-35 Months of Age
 Immunogenicity and Safety of GSK s FluLaval Quadrivalent Inactivated Influenza Vaccine in Children 6-35 Months of Age Bruce L Innis, MD, FIDSA GSK Vaccines Inactivated Influenza Vaccines for 6-35 Months
Immunogenicity and Safety of GSK s FluLaval Quadrivalent Inactivated Influenza Vaccine in Children 6-35 Months of Age Bruce L Innis, MD, FIDSA GSK Vaccines Inactivated Influenza Vaccines for 6-35 Months
Centocor Ortho Biotech Services, LLC
 SYNOPSIS Issue Date: 17 June 2009 Name of Sponsor/Company Name of Finished Product PROCRIT Name of Active Ingredient(s) Protocol No.: PR04-15-001 Centocor Ortho Biotech Services, LLC Epoetin alfa Title
SYNOPSIS Issue Date: 17 June 2009 Name of Sponsor/Company Name of Finished Product PROCRIT Name of Active Ingredient(s) Protocol No.: PR04-15-001 Centocor Ortho Biotech Services, LLC Epoetin alfa Title
1. Automatically create Flu Shot encounters in AHLTA in 2 mouse clicks. 2. Ensure accurate DX and CPT codes used for every encounter, every time.
 In clinics around the MHS, upwards of 70% of all flu shot workload credit is lost because the encounters are not documented within AHLTA. Let the Immunization KAT s PASBA approved coding engine do the
In clinics around the MHS, upwards of 70% of all flu shot workload credit is lost because the encounters are not documented within AHLTA. Let the Immunization KAT s PASBA approved coding engine do the
ivlewbel: Uses heteroscedasticity to estimate mismeasured and endogenous regressor models
 ivlewbel: Uses heteroscedasticity to estimate mismeasured and endogenous regressor models Fernihough, A. ivlewbel: Uses heteroscedasticity to estimate mismeasured and endogenous regressor models Queen's
ivlewbel: Uses heteroscedasticity to estimate mismeasured and endogenous regressor models Fernihough, A. ivlewbel: Uses heteroscedasticity to estimate mismeasured and endogenous regressor models Queen's
MyWindFit Member Analytics Portal
 Member Analytics Portal The member section of is a private and personalized cloud database that contains the detailed records of all your activity. It also contains an analysis package that enables you
Member Analytics Portal The member section of is a private and personalized cloud database that contains the detailed records of all your activity. It also contains an analysis package that enables you
Package rvtdt. February 20, rvtdt... 1 rvtdt.example... 3 rvtdts... 3 TrendWeights Index 6. population control weighted rare-varaints TDT
 Type Package Package rvtdt February 20, 2015 Title population control weighted rare-variants TDT Version 1.0 Date 2014-04-07 Author Yu Jiang, Andrew S. Allen Maintainer Yu Jiang
Type Package Package rvtdt February 20, 2015 Title population control weighted rare-variants TDT Version 1.0 Date 2014-04-07 Author Yu Jiang, Andrew S. Allen Maintainer Yu Jiang
