The laboratory preparation for a new study a pragmatic approach. Compiled by Ken Clarke and Lindiwe Nhlangulela October 2011
|
|
|
- Bertram Osborne
- 5 years ago
- Views:
Transcription
1 The laboratory preparation for a new study a pragmatic approach Compiled by Ken Clarke and Lindiwe Nhlangulela October 2011
2 We are the Aurum Institute and our laboratory is situated in Klerksdorp in the Northwest Province and is centrally situated in the business area of the city The greater city area of Klerksdorp incorporates the town of Orkney, Stilfontein, Kanana, Khuma, Hartbeesfontein and Tigane and has a population of approximately 350,000 inhabitants. Klerksdorp is considered to be the hub of the gold mining industry
3
4
5
6
7
8 Our present staff complement is : Laboratory Manager Medical Technologist Medical Technician (BSc graduate)
9
10 In most instances the laboratory manager is informed by the site director of the new study Site inspections (including the laboratory) is carried out prior to the implementation of the study by elected persons of the sponsor and any deficiencies or requirements are discussed and implemented Staff informed of new study implementation
11
12 Laboratory manager will obtain a copy of the protocol and will give an overview to the relevant laboratory staff Staff are encouraged to familiarise themselves with the study especially areas associated with Site specific procedures Laboratory manual Specimen management
13
14
15 Local testing Validation and EQA(IQC & EQC) for all rapid tests carried out on site as per sponsors requirements Referred testing Tests that are to be referred to a preferred service provider usually of the sponsors choice Laboratory manager will contact the preferred provider to ensure compliance with testing procedures, TAT s and reference ranges Completion of lab activation summary,protocol analyte lists, analytical plan
16 All new equipment if acquired to be validated and current validation and maintenance certificates of existing equipment to be available
17 Laboratory manager together with operations manager will assess whether present staff complement is adequate or whether further staff is required If further staff is required then adequate budgeting, advertising of the posts, interviews and appointments will be carried out as per company policy
18
19 Laboratory manager will estimate the required stocks necessary for the completion of the study Quotations for the necessary reagents consumables and collection kits are obtained and orders are placed as per company policy
20
21 Relevant visit requisitions, worksheets and logs are devised in conjunction with the Quality department All files necessary for the filing of documents are prepared Shipping documents and shipping timetable is made available which will include export and import permits if required and courier contracts
22 LDMS storage guide is made available to assist with specimen management and storage
23 Training is arranged by the study coordinator prior to the implementation of the study Training is usually carried out by representatives of the sponsor which will incorporate all aspects of the protocol including laboratory training New and current staff should be GCP,GCLP and IATA compliant as well as to have passed PBMC proficiency testing and be familiar with the laboratory LIS system(ldms)
24 Weekly meetings between the laboratory and clinic staff are arranged in the initial stages of the study Problems are reported, identified and resolved Laboratory staff compile own visit and specimen type schedules and workflow charts to assist when receiving specimens
25
26 VISIT TYPE OF TUBE TEST BARC KOSH SCREENING 1 x URINE BhCG X ENROLLMENT SDA X 1 x 5ML SST ALT, AST, TBIL, CREAT,ALP X 1 x 5ML SST RPR X 1 x 4ML EDTA FBC/DIFF X 1 x 4ML EDTA CD4 X 1 x 4ML EDTA HIV RNA (VL) X 5 x 10ML EDTA PBMC & PLASMA STORAGE X 1 x VAGINAL SWAB RAPID FOR BV* X 1 x VAGINAL SWAB WET MOUNT FOR KOH* X 1 x VAGINAL SWAB TRICHOMONAS X 2 x VAGINAL SWAB STORAGE X 1 x LBC PAP SMEAR X 1 x CVL STORAGE X 1 X 5ML SST SERUM STORAGE X Month 1 POST 1 x 4ML EDTA CD4 X SEROCONVERSION 1 x 4ML EDTA HIV RNA (VL) X 5 x 10ML EDTA PBMC & PLASMA STORAGE X 2 x VAGINAL SWAB STORAGE X 1 x CVL STORAGE X *ONLY IF CLINICALLY INDICATED Month 3, POST 1 x 4ML EDTA CD4 X SEROCONVERSION 1 x 4ML EDTA HIV RNA (VL) X 1 x 4ML EDTA FBC/DIFF 5 x 10ML EDTA PBMC & PLASMA STORAGE X 2 x VAGINAL SWAB STORAGE X 1 x CVL STORAGE X MONTH 6, Q6 1 x 5ML SST ALT, AST, TBIL, CREAT, ALP X POST SEROCONVERSION1 x 4ML EDTA FBC/DIFF X 1 x 4ML EDTA CD4 X 1 x 4ML EDTA HIV RNA (VL) X 5 x 10ML EDTA PBMC & PLASMA STORAGE X 2 x VAGINAL SWAB STORAGE X 1 x CVL STORAGE X ANNUALLY 1 X URINE SDA X POST SERO 1 x 5ML SST ALT, AST, TBIL, CREAT, ALP X 1 x 5ML SST RPR X 1 x 4ML EDTA FBC/DIFF X 1 x 4ML EDTA CD4 X 1 x 4ML EDTA HIV RNA (VL) X 5 x 10ML EDTA PBMC & PLASMA STORAGE X 1 x VAGINAL SWAB RAPID BV* X 1 x VAGINAL SWAB WET MOUNT FOR KOH X 1 x VAGINAL SWAB TRICHOMONAS X 2 x VAGINAL SWAB STORAGE X 1 x LBC PAP SMEAR X
27 Thorough preparation by the laboratory staff is essential prior to the start of the study A broad overview of the protocol especially areas related to the laboratory requirements is necessary Equally important is a good relationship with other role players such as the study coordinator, clinic staff,quality department and referral laboratory amongst others
28 Lindiwe Nhlangulela for her valuable inputs Aurum Institute for still allowing me to practise my profession as a Medical Technologist
29 THANK YOU
MTN 015 SPECIMEN REVIEW. Gabriel Banda Core Lab Supervisor UNC Project, Lilongwe
 MTN 015 SPECIMEN REVIEW Gabriel Banda Core Lab Supervisor UNC Project, Lilongwe Review of MTN 015 Study MTN 015 is a multi-site observational cohort study whose study population are women who seroconverted
MTN 015 SPECIMEN REVIEW Gabriel Banda Core Lab Supervisor UNC Project, Lilongwe Review of MTN 015 Study MTN 015 is a multi-site observational cohort study whose study population are women who seroconverted
Specimen Management PHRU LAB
 Specimen Management PHRU LAB Amanda Mohlala, MT(HPCSA) Perinatal HIV Research Unit University of the Witwatersrand Chris Hani Baragwanath Hospital Johannesburg, South Africa MTN Regional Meeting 2010 PHRU
Specimen Management PHRU LAB Amanda Mohlala, MT(HPCSA) Perinatal HIV Research Unit University of the Witwatersrand Chris Hani Baragwanath Hospital Johannesburg, South Africa MTN Regional Meeting 2010 PHRU
Quicker, Better, Faster Optimising Turn Around Time of Laboratory Results
 Quicker, Better, Faster Optimising Turn Around Time of Laboratory Results Pranitha Ramchuran Regulatory Compliance Manager MTN 003 Regional Meeting Cape Town, South Africa October 2011 Outline Organogram
Quicker, Better, Faster Optimising Turn Around Time of Laboratory Results Pranitha Ramchuran Regulatory Compliance Manager MTN 003 Regional Meeting Cape Town, South Africa October 2011 Outline Organogram
HPTN 035. Laboratory Overview
 HPTN 035 Laboratory Overview SSP Laboratory Procedures As the transmission of HIV can occur through contact with contaminated needles, blood, blood product, and vaginal secretions, appropriate blood and
HPTN 035 Laboratory Overview SSP Laboratory Procedures As the transmission of HIV can occur through contact with contaminated needles, blood, blood product, and vaginal secretions, appropriate blood and
Overview of Wet Preps and Gram stains. Lorna Rabe Central Lab Magee-Womens Research Institute Pittsburgh, Pa
 Overview of Wet Preps and Gram stains Lorna Rabe Central Lab Magee-Womens Research Institute Pittsburgh, Pa Vaginal Flora A secondary objective of the 035 study is to assess the effectiveness of BufferGel
Overview of Wet Preps and Gram stains Lorna Rabe Central Lab Magee-Womens Research Institute Pittsburgh, Pa Vaginal Flora A secondary objective of the 035 study is to assess the effectiveness of BufferGel
SCREENING AND ENROLLMENT CONSIDERATIONS MTN-038 STUDY-SPECIFIC TRAINING
 SCREENING AND ENROLLMENT CONSIDERATIONS MTN-038 STUDY-SPECIFIC TRAINING SCREENING AND ENROLLMENT VISITS Screening/ Visit 1 Eligibility Criteria initially assessed Multiple visits, if needed (Split visit)
SCREENING AND ENROLLMENT CONSIDERATIONS MTN-038 STUDY-SPECIFIC TRAINING SCREENING AND ENROLLMENT VISITS Screening/ Visit 1 Eligibility Criteria initially assessed Multiple visits, if needed (Split visit)
MTN-038 Laboratory Training. May Beamer, Michele Austin MTN Laboratory Center Magee-Womens Research Institute Pittsburgh, PA
 MTN-038 Laboratory Training May Beamer, Michele Austin MTN Laboratory Center Magee-Womens Research Institute Pittsburgh, PA Objectives Resources Specimen Management Overview of Lab testing Supplies Q&A
MTN-038 Laboratory Training May Beamer, Michele Austin MTN Laboratory Center Magee-Womens Research Institute Pittsburgh, PA Objectives Resources Specimen Management Overview of Lab testing Supplies Q&A
Prior to site activation the following events were lined up
 Pardon Gomani CALENDAR OF EVENTS Prior to site activation the following events were lined up October 2008 Operational walk through in JHB 9 to 17 July study specific training Harare 27 August 09 site specific
Pardon Gomani CALENDAR OF EVENTS Prior to site activation the following events were lined up October 2008 Operational walk through in JHB 9 to 17 July study specific training Harare 27 August 09 site specific
QA Processes That Work: Minimizing Errors During On-site Rapid HIV Testing
 QA Processes That Work: Minimizing Errors During On-site Rapid HIV Testing Rashika Maharaj MRC HPRU Laboratory Manager MTN Regional Meeting 09 September 2008 HIV and Study Endpoints In most cases, clinical
QA Processes That Work: Minimizing Errors During On-site Rapid HIV Testing Rashika Maharaj MRC HPRU Laboratory Manager MTN Regional Meeting 09 September 2008 HIV and Study Endpoints In most cases, clinical
Section 8. Clinical Considerations
 Section 8. Clinical Considerations This section presents information on the clinical procedures performed in MTN-015. Further considerations related to participant safety monitoring and reporting are provided
Section 8. Clinical Considerations This section presents information on the clinical procedures performed in MTN-015. Further considerations related to participant safety monitoring and reporting are provided
VOICE (MTN-003) IT S ALL ABOUT THE ENDPOINTS. Edward Livant MT (ASCP), MPH University of Pittsburgh Medical Center MTN Network Laboratory
 VOICE (MTN-003) IT S ALL ABOUT THE ENDPOINTS Edward Livant MT (ASCP), MPH University of Pittsburgh Medical Center MTN Network Laboratory Review of Sample 2 Testing starts here with rapid test (s). Negative
VOICE (MTN-003) IT S ALL ABOUT THE ENDPOINTS Edward Livant MT (ASCP), MPH University of Pittsburgh Medical Center MTN Network Laboratory Review of Sample 2 Testing starts here with rapid test (s). Negative
WOMEN'S INTERAGENCY HIV STUDY
 WOMEN'S INTERAGENCY HIV STUDY SECTION 6: OVERVIEW OF THE BASELINE VISIT FOR NEW RECRUITS I. 2001/2002 RECRUITS This section provides an overview of the components of the baseline visit for 2001/2002 new
WOMEN'S INTERAGENCY HIV STUDY SECTION 6: OVERVIEW OF THE BASELINE VISIT FOR NEW RECRUITS I. 2001/2002 RECRUITS This section provides an overview of the components of the baseline visit for 2001/2002 new
MTN 005 Laboratory Training. Lorna Rabe MTN Network Laboratory Magee-Womens Research Institute Pittsburgh, PA
 MTN 005 Laboratory Training Lorna Rabe MTN Network Laboratory Magee-Womens Research Institute Pittsburgh, PA Objectives Overview of Lab testing HIV Testing Algorithm Collection and processing of IVR Specimen
MTN 005 Laboratory Training Lorna Rabe MTN Network Laboratory Magee-Womens Research Institute Pittsburgh, PA Objectives Overview of Lab testing HIV Testing Algorithm Collection and processing of IVR Specimen
GUIDANCE DOCUMENTS CONTAINING THE COMMON PROVISIONS ON THE CONDUCT OF GCP INSPECTIONS BY COMPETENT AUTHORITIES OF THE DIFFERENT MEMBER STATES
 EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Brussels, 5 November 2008 ENTR/F/2/SF D(2008) 34957 GUIDANCE DOCUMENTS CONTAINING THE COMMON PROVISIONS ON
EUROPEAN COMMISSION ENTERPRISE AND INDUSTRY DIRECTORATE-GENERAL Consumer goods Pharmaceuticals Brussels, 5 November 2008 ENTR/F/2/SF D(2008) 34957 GUIDANCE DOCUMENTS CONTAINING THE COMMON PROVISIONS ON
WOMEN S INTERAGENCY HIV STUDY MANUAL OF OPERATIONS: TABLE OF CONTENTS
 SECTION 1: STUDY PURPOSE PRIMARY RESEARCH AREAS... 1 A. WIHS V AIMS AND HYPOTHESES... 1 B. WIHS IV CORE AIMS AND HYPOTHESES... 10 C. WIHS III CORE AIMS AND HYPOTHESES... 18 D. WIHS II CORE RESEARCH QUESTIONS...
SECTION 1: STUDY PURPOSE PRIMARY RESEARCH AREAS... 1 A. WIHS V AIMS AND HYPOTHESES... 1 B. WIHS IV CORE AIMS AND HYPOTHESES... 10 C. WIHS III CORE AIMS AND HYPOTHESES... 18 D. WIHS II CORE RESEARCH QUESTIONS...
Section 13. Laboratory Considerations
 Section 13. Laboratory Considerations Table of Contents 13.1 Overview and General Guidance 13.2 Specimen Labeling 13.3 Procedures for Specimens That Cannot Be Evaluated 13.4 Use of LDMS 13.5 Documentation
Section 13. Laboratory Considerations Table of Contents 13.1 Overview and General Guidance 13.2 Specimen Labeling 13.3 Procedures for Specimens That Cannot Be Evaluated 13.4 Use of LDMS 13.5 Documentation
WOMEN S INTERAGENCY HIV STUDY MANUAL OF OPERATIONS: TABLE OF CONTENTS
 SECTION 1: STUDY PURPOSE PRIMARY RESEARCH AREAS... 1 A. WIHS V AIMS AND HYPOTHESES... 1 B. WIHS IV CORE AIMS AND HYPOTHESES... 10 C. WIHS III CORE AIMS AND HYPOTHESES... 18 D. WIHS II CORE RESEARCH QUESTIONS...
SECTION 1: STUDY PURPOSE PRIMARY RESEARCH AREAS... 1 A. WIHS V AIMS AND HYPOTHESES... 1 B. WIHS IV CORE AIMS AND HYPOTHESES... 10 C. WIHS III CORE AIMS AND HYPOTHESES... 18 D. WIHS II CORE RESEARCH QUESTIONS...
VOICE Screening Part 1 Visit. Operational Walkthrough Johannesburg, South Africa November 2008
 VOICE Screening Part 1 Visit Operational Walkthrough Johannesburg, South Africa November 2008 Protocol Requirements Administrative, Behavioral, and Regulatory Procedures Informed consent for screening
VOICE Screening Part 1 Visit Operational Walkthrough Johannesburg, South Africa November 2008 Protocol Requirements Administrative, Behavioral, and Regulatory Procedures Informed consent for screening
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT. Product: OraQuick HCV Rapid Antibody Test Kit WHO reference number: PQDx
 WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: OraQuick HCV Rapid Antibody Test Kit WHO reference number: PQDx 0244-055-00 OraQuick HCV Rapid Antibody Test Kit with product codes 1001-0270
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: OraQuick HCV Rapid Antibody Test Kit WHO reference number: PQDx 0244-055-00 OraQuick HCV Rapid Antibody Test Kit with product codes 1001-0270
Darwin Marine Supply Base HSEQ Quality Management Plan
 Darwin Marine Supply Base HSEQ Quality Management Plan REVISION SUMMARY Revision Date Comment Authorised 0 29.9.13 Initial input JC 1 12.1.15 General Review JC 2 3 4 5 6 7 8 9 Revision Log Revision No
Darwin Marine Supply Base HSEQ Quality Management Plan REVISION SUMMARY Revision Date Comment Authorised 0 29.9.13 Initial input JC 1 12.1.15 General Review JC 2 3 4 5 6 7 8 9 Revision Log Revision No
9.1 Overview and General Guidance This section contains information on the laboratory procedures performed in MTN-035.
 Section 9. Laboratory Considerations 9. Introduction... 9-1 9.1 Overview and General Guidance... 9-1 9.2 Specimen Labeling... 9-4 9.3 Procedures for Specimens that cannot be Evaluated... 9-4 9.4 Use of
Section 9. Laboratory Considerations 9. Introduction... 9-1 9.1 Overview and General Guidance... 9-1 9.2 Specimen Labeling... 9-4 9.3 Procedures for Specimens that cannot be Evaluated... 9-4 9.4 Use of
ENHANCING LAB PERFORMANCE
 ENHANCING LAB PERFORMANCE HPTN Regional Meeting October 19, 2017 Yaw Agyei, MPH, MT (ASCP) Lebah Lugalia, MT (ASCP) HPTN Laboratory Center Johns Hopkins University Hospital Introduction HPTN Lab QA/QC
ENHANCING LAB PERFORMANCE HPTN Regional Meeting October 19, 2017 Yaw Agyei, MPH, MT (ASCP) Lebah Lugalia, MT (ASCP) HPTN Laboratory Center Johns Hopkins University Hospital Introduction HPTN Lab QA/QC
Section 12. Laboratory Considerations
 Section 12. Laboratory Considerations 12.1 Overview and General Guidance This section contains information on to the laboratory procedures performed in HPTN 035. Some laboratory procedures will be performed
Section 12. Laboratory Considerations 12.1 Overview and General Guidance This section contains information on to the laboratory procedures performed in HPTN 035. Some laboratory procedures will be performed
ADOPTED REGULATION OF THE STATE BOARD OF HEALTH. LCB File No. R Effective December 3, 2003
 ADOPTED REGULATION OF THE STATE BOARD OF HEALTH LCB File No. R119-03 Effective December 3, 2003 EXPLANATION Matter in italics is new; matter in brackets omitted material is material to be omitted. AUTHORITY:
ADOPTED REGULATION OF THE STATE BOARD OF HEALTH LCB File No. R119-03 Effective December 3, 2003 EXPLANATION Matter in italics is new; matter in brackets omitted material is material to be omitted. AUTHORITY:
Challenges and Access to Viral Load Testing in Africa: Example of Cote d Ivoire Christiane Adje-Toure, PhD Lab Branch Chief CDC-COTE D IVOIRE
 Challenges and Access to Viral Load Testing in Africa: Example of Cote d Ivoire Christiane Adje-Toure, PhD Lab Branch Chief CDC-COTE D IVOIRE Outline 1. Introduction 2. Barriers to Scaling up Viral Load
Challenges and Access to Viral Load Testing in Africa: Example of Cote d Ivoire Christiane Adje-Toure, PhD Lab Branch Chief CDC-COTE D IVOIRE Outline 1. Introduction 2. Barriers to Scaling up Viral Load
Health Provider Partnerships for OSA Management in Transportation. Paul S. Valentine Chief Executive Officer Sleep HealthCenters LLC
 Health Provider Partnerships for OSA Management in Transportation Paul S. Valentine Chief Executive Officer Sleep HealthCenters LLC Sleep Apnea & Multi-Modal Transportation Conference November 2011 Programs
Health Provider Partnerships for OSA Management in Transportation Paul S. Valentine Chief Executive Officer Sleep HealthCenters LLC Sleep Apnea & Multi-Modal Transportation Conference November 2011 Programs
MTN 009 Laboratory Logistics
 MEDICAL RESEARCH COUNCIL OF SA HIV PREVENTION RESEARCH UNIT MTN 009 Laboratory Logistics RASHIKA MAHARAJ HPRU LABORATORY MANAGER Testing Menu Finger stick Blood Samples HIV Rapid Tests* Venous Blood Samples
MEDICAL RESEARCH COUNCIL OF SA HIV PREVENTION RESEARCH UNIT MTN 009 Laboratory Logistics RASHIKA MAHARAJ HPRU LABORATORY MANAGER Testing Menu Finger stick Blood Samples HIV Rapid Tests* Venous Blood Samples
Section 6. Participant Follow-up
 Section 6. Participant Follow-up This section provides information on requirements and procedures for participant follow-up. 6.1 Study Follow-up Plan and Participant Retention Targets Each enrolled participant
Section 6. Participant Follow-up This section provides information on requirements and procedures for participant follow-up. 6.1 Study Follow-up Plan and Participant Retention Targets Each enrolled participant
Section 9. Laboratory Considerations
 Section 9. Laboratory Considerations 9.1 Overview and General Guidance This section contains information on the laboratory procedures performed in MTN-012/IPM 010. As transmission of HIV and other infectious
Section 9. Laboratory Considerations 9.1 Overview and General Guidance This section contains information on the laboratory procedures performed in MTN-012/IPM 010. As transmission of HIV and other infectious
NJ RAPID HIV TESTING SUPPORT PROGRAM
 PROGRAM OVERVIEW Program Description: Point of Care testing may seem, on its face, simple and 'fool proof', but in a study conducted by HCFA in Colorado and Ohio, quality problems were identified in more
PROGRAM OVERVIEW Program Description: Point of Care testing may seem, on its face, simple and 'fool proof', but in a study conducted by HCFA in Colorado and Ohio, quality problems were identified in more
Basic Standards for Fellowship Training in Sleep Medicine
 Basic Standards for Fellowship Training in Sleep Medicine American Osteopathic Association and American College of Osteopathic Neurologists and Psychiatrists and American College of Osteopathic Internists
Basic Standards for Fellowship Training in Sleep Medicine American Osteopathic Association and American College of Osteopathic Neurologists and Psychiatrists and American College of Osteopathic Internists
WHO Prequalification of Diagnostics Programme PUBLIC REPORT. Product: Alere Determine HIV-1/2 Number: PQDx Abstract
 WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: Alere Determine HIV-1/2 Number: PQDx 0033-013-00 Abstract The Alere Determine HIV-1/2 with product codes 1 7D2342, 7D2343 and 7D2243
WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: Alere Determine HIV-1/2 Number: PQDx 0033-013-00 Abstract The Alere Determine HIV-1/2 with product codes 1 7D2342, 7D2343 and 7D2243
Product Overview CELL-FREE DNA BCT CELL-FREE RNA BCT CELL-FREE DNA URINE PRESERVE CYTO-CHEX BCT STRECK CELL PRESERVATIVE
 FIXED ON INTEGRITY Product Overview CELL-FREE DNA BCT A blood collection tube for stabilization of cell-free plasma DNA, CTCs and cellular DNA. The preservative in Cell-Free DNA BCT stabilizes nucleated
FIXED ON INTEGRITY Product Overview CELL-FREE DNA BCT A blood collection tube for stabilization of cell-free plasma DNA, CTCs and cellular DNA. The preservative in Cell-Free DNA BCT stabilizes nucleated
WHO Prequalification of Diagnostics Programme PUBLIC REPORT
 WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: COBAS AmpliPrep/COBAS TaqMan HIV-1 Qualitative Test, version 2.0 (TaqMan 48) Number: PQDx 0221-046-00 Abstract COBAS AmpliPrep/COBAS
WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: COBAS AmpliPrep/COBAS TaqMan HIV-1 Qualitative Test, version 2.0 (TaqMan 48) Number: PQDx 0221-046-00 Abstract COBAS AmpliPrep/COBAS
WHO Prequalification of Diagnostics Programme PUBLIC REPORT
 WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: COBAS AmpliPrep/COBAS TaqMan HIV-1 Qualitative Test, version 2.0 (TaqMan 96) Number: PQDx 0200-046-00 Abstract COBAS AmpliPrep/COBAS
WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: COBAS AmpliPrep/COBAS TaqMan HIV-1 Qualitative Test, version 2.0 (TaqMan 96) Number: PQDx 0200-046-00 Abstract COBAS AmpliPrep/COBAS
A Successful HIV Testing Quality Assurance Program: New York State Experience
 A Successful HIV Testing Quality Assurance Program: New York State Experience Mara San Antonio-Gaddy, RN, MSN NYS DOH AIDS Institute Division of HIV Prevention 2010 HIV Diagnostics Conference March 24,
A Successful HIV Testing Quality Assurance Program: New York State Experience Mara San Antonio-Gaddy, RN, MSN NYS DOH AIDS Institute Division of HIV Prevention 2010 HIV Diagnostics Conference March 24,
New Entrants Safety Education Seminar for Georgia Motor Carriers CHAPTER 11
 New Entrants Safety Education Seminar for Georgia Motor Carriers CHAPTER 11 Chapter 11 CONTROLLED SUBSTANCES AND ALCOHOL USE TESTING REGULATIONS OVERVIEW This chapter informs employers on FMCSA controlled
New Entrants Safety Education Seminar for Georgia Motor Carriers CHAPTER 11 Chapter 11 CONTROLLED SUBSTANCES AND ALCOHOL USE TESTING REGULATIONS OVERVIEW This chapter informs employers on FMCSA controlled
WHO Prequalification of Diagnostics Programme PUBLIC REPORT
 WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, version 2.0 (TaqMan 48) Number: PQDx 0126-046-00 Abstract The COBAS AmpliPrep/COBAS TaqMan
WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, version 2.0 (TaqMan 48) Number: PQDx 0126-046-00 Abstract The COBAS AmpliPrep/COBAS TaqMan
Genetic testing for hereditary cancer. An overview for healthcare providers
 Genetic testing for hereditary cancer An overview for healthcare providers Specimen requirements Whole blood Two 4.5 ml EDTA tubes (lavender top) Please wait at least 2 weeks after a packed cell/platelet
Genetic testing for hereditary cancer An overview for healthcare providers Specimen requirements Whole blood Two 4.5 ml EDTA tubes (lavender top) Please wait at least 2 weeks after a packed cell/platelet
NGS Gateway Lab Services
 TM NGS Gateway Lab Services Accelerating Precision Medicine Design a Complete Genomic Testing Portfolio with Turnkey Assays About NGS Gateway Lab Services TM Designed to provide a gateway to your own in-house
TM NGS Gateway Lab Services Accelerating Precision Medicine Design a Complete Genomic Testing Portfolio with Turnkey Assays About NGS Gateway Lab Services TM Designed to provide a gateway to your own in-house
IU Health Pathology Laboratory. DOS Updates
 IU Health Pathology Laboratory News Bulletin February 23, 2012 DOS Updates Apolipoprotein B QN Ship refrigerated plasma instead of frozen; Frozen plasma is acceptable however. Myoglobin, Serum Discontinued
IU Health Pathology Laboratory News Bulletin February 23, 2012 DOS Updates Apolipoprotein B QN Ship refrigerated plasma instead of frozen; Frozen plasma is acceptable however. Myoglobin, Serum Discontinued
WHO Prequalification of In Vitro Diagnostics Programme PUBLIC REPORT. Product: First Response HIV Card Test Number: PQDx
 WHO Prequalification of In Vitro Diagnostics Programme PUBLIC REPORT Product: First Response HIV 1-2-0 Card Test Number: PQDx 0018-010-00 Abstract First Response HIV 1-2-0 Card Test with product codes
WHO Prequalification of In Vitro Diagnostics Programme PUBLIC REPORT Product: First Response HIV 1-2-0 Card Test Number: PQDx 0018-010-00 Abstract First Response HIV 1-2-0 Card Test with product codes
TERMS AND CONDITIONS NATIONAL GOOD LABORATORY PRACTICE (GLP) COMPLIANCE MONITORING AUTHORITY
 TERMS AND CONDITIONS OF NATIONAL GOOD LABORATORY PRACTICE (GLP) COMPLIANCE MONITORING AUTHORITY FOR OBTAINING AND MAINTAINING ITS GLP CERTIFICATION BY A TEST FACILITY Document No.GLP-101 Version/Issue
TERMS AND CONDITIONS OF NATIONAL GOOD LABORATORY PRACTICE (GLP) COMPLIANCE MONITORING AUTHORITY FOR OBTAINING AND MAINTAINING ITS GLP CERTIFICATION BY A TEST FACILITY Document No.GLP-101 Version/Issue
Section 5. Study Procedures
 Section 5. Study Procedures 5.1 Visit Locations... 1 5.2 Eligibility Determination SOP... 1 5.3 Screening Visit... 1 5.3.1 Screening and Enrollment Timeframe... 2 5.3.2 Screening Visit Procedures... 2
Section 5. Study Procedures 5.1 Visit Locations... 1 5.2 Eligibility Determination SOP... 1 5.3 Screening Visit... 1 5.3.1 Screening and Enrollment Timeframe... 2 5.3.2 Screening Visit Procedures... 2
Section 5. Study Procedures
 Section 5. Study Procedures 5. Introduction 5-1 5.1 Visit Locations... 5-1 5.2 Eligibility Determination SOP... 5-1 5.3 Screening Visit... 5-2 5.3.1 Screening and Enrollment Timeframe... 5-2 5.3.2 Screening
Section 5. Study Procedures 5. Introduction 5-1 5.1 Visit Locations... 5-1 5.2 Eligibility Determination SOP... 5-1 5.3 Screening Visit... 5-2 5.3.1 Screening and Enrollment Timeframe... 5-2 5.3.2 Screening
JOB PROFILE. Project Administrator: Skills Development and Training. Skills, Development and Training
 1. POSITION DETAIL CURRENT JOB TITLE Project Administrator: Skills Development and Training JOB GRADE B5 PROPOSED JOB TITLE JOB CODE DEPARTMENT Skills, Development and Training DATE REVIEWED 14.02.2017
1. POSITION DETAIL CURRENT JOB TITLE Project Administrator: Skills Development and Training JOB GRADE B5 PROPOSED JOB TITLE JOB CODE DEPARTMENT Skills, Development and Training DATE REVIEWED 14.02.2017
Section 9 Laboratory Consideration
 Section 9 Laboratory Consideration 9. Introduction... 9-1 9.1 Overview and General Guidance... 9-2 Table 9-1: Overview of Laboratory Testing Locations, Specimens, and Methods for MTN-034... 9-3 Table 9-2:
Section 9 Laboratory Consideration 9. Introduction... 9-1 9.1 Overview and General Guidance... 9-2 Table 9-1: Overview of Laboratory Testing Locations, Specimens, and Methods for MTN-034... 9-3 Table 9-2:
PROFICIENCY TESTING POLICY
 Supersedes Prepared by: APPROVALS in this section Approved by: Date: Laboratory Director RECORD OF REVIEWS Date Signature Title Procedural Changes/Review VERSION HISTORY Revision # 0 Section #/Changes
Supersedes Prepared by: APPROVALS in this section Approved by: Date: Laboratory Director RECORD OF REVIEWS Date Signature Title Procedural Changes/Review VERSION HISTORY Revision # 0 Section #/Changes
SPRINGFIELD PUBLIC SCHOOLS COACH HANDBOOK INJURY MANAGEMENT PROTOCOL
 SPRINGFIELD PUBLIC SCHOOLS COACH HANDBOOK INJURY MANAGEMENT PROTOCOL The health and safety of the athletes at Springfield Public Schools is of the utmost importance. We are committed to providing a safe
SPRINGFIELD PUBLIC SCHOOLS COACH HANDBOOK INJURY MANAGEMENT PROTOCOL The health and safety of the athletes at Springfield Public Schools is of the utmost importance. We are committed to providing a safe
Goal of site data management 12/2/2009. Ultimate goal: reliable and valid clinical trial to improve health
 RC-4 Overview of Clinical Site Operations and Management Unit 4: Overview of Data Quality and Monitoring Principal Investigators Lynda Wilson, PhD, RN Marti Rice, PhD, RN Program Manager TBA Course Instructors:
RC-4 Overview of Clinical Site Operations and Management Unit 4: Overview of Data Quality and Monitoring Principal Investigators Lynda Wilson, PhD, RN Marti Rice, PhD, RN Program Manager TBA Course Instructors:
Sample Selection, Collection, Transport: Issues & Challenges
 Sample Selection, Collection, Transport: Issues & Challenges Jim Dunn, PhD, D(ABMM) Director, Medical Microbiology and Virology Texas Children s Hospital LEARNING OBJECTIVES 1. Identify factors to consider
Sample Selection, Collection, Transport: Issues & Challenges Jim Dunn, PhD, D(ABMM) Director, Medical Microbiology and Virology Texas Children s Hospital LEARNING OBJECTIVES 1. Identify factors to consider
VQA Control SOP Version 4.0 Roche Amplicor HIV-1 DNA Test, v August 2007
 1. PRINCIPLE 1.1. The Virology Quality Assurance (VQA) Laboratory provides external cell pellet controls for use in the validation of assays that detect HIV proviral DNA. 1.2. HIV seronegative peripheral
1. PRINCIPLE 1.1. The Virology Quality Assurance (VQA) Laboratory provides external cell pellet controls for use in the validation of assays that detect HIV proviral DNA. 1.2. HIV seronegative peripheral
INITIAL PRACTICE PERIOD FORMS
 INITIAL PRACTICE PERIOD FORMS 5060-3080 Yonge Street, Box 71 Toronto, Ontario M4N 3N1 416-975-5347 1-800-993-9459 www.caslpo.com Revised: May 2017 TABLE OF CONTENTS 1 MENTORSHIP GUIDANCE CONTRACT 3 2 1
INITIAL PRACTICE PERIOD FORMS 5060-3080 Yonge Street, Box 71 Toronto, Ontario M4N 3N1 416-975-5347 1-800-993-9459 www.caslpo.com Revised: May 2017 TABLE OF CONTENTS 1 MENTORSHIP GUIDANCE CONTRACT 3 2 1
The analytical phase
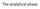 The analytical phase Result interpretation Test request Result Sampling Black box: the lab ANALYTICAL PHASE The CASE Uncle Pete, 67 years old Marked abdominal pain 8 pm, ED Acute abdomen? Assessment (+
The analytical phase Result interpretation Test request Result Sampling Black box: the lab ANALYTICAL PHASE The CASE Uncle Pete, 67 years old Marked abdominal pain 8 pm, ED Acute abdomen? Assessment (+
Economic Value Lab Efficiencies Safety Clinical Outcomes. Visit BD Booth AACC/ASCLS 2009 Annual Meeting
 Economic Value Lab Efficiencies Safety Clinical Outcomes Visit BD Booth 1225 AACC/ASCLS 2009 Annual Meeting BD Events & Co-Promotions Join us this year at booth #1225 to discover the full spectrum of solutions
Economic Value Lab Efficiencies Safety Clinical Outcomes Visit BD Booth 1225 AACC/ASCLS 2009 Annual Meeting BD Events & Co-Promotions Join us this year at booth #1225 to discover the full spectrum of solutions
Is focused on the local Members needs, while making its activities available to Chapter membership.
 CITY CENTER HANDBOOK WHAT IS A CITY CENTER? City Centers are groups of Members in a particular city who organize and deliver programs of interest to local Members, as frequently or infrequently as they
CITY CENTER HANDBOOK WHAT IS A CITY CENTER? City Centers are groups of Members in a particular city who organize and deliver programs of interest to local Members, as frequently or infrequently as they
WHO Prequalification of Diagnostics Programme PUBLIC REPORT. Product: Abbott RealTime HIV-1 (m2000sp) Number: PQDx
 WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: Abbott RealTime HIV-1 (m2000sp) Number: PQDx 0145-027-00 Abstract The Abbott RealTime HIV-1 (m2000sp) assay with product code 2G31 (which
WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: Abbott RealTime HIV-1 (m2000sp) Number: PQDx 0145-027-00 Abstract The Abbott RealTime HIV-1 (m2000sp) assay with product code 2G31 (which
Basic Standards for Osteopathic Fellowship Training in Sleep Medicine
 Basic Standards for Osteopathic Fellowship Training in Sleep Medicine American Osteopathic Association and the American College of Osteopathic Neurologists and Psychiatrists and the American College of
Basic Standards for Osteopathic Fellowship Training in Sleep Medicine American Osteopathic Association and the American College of Osteopathic Neurologists and Psychiatrists and the American College of
PAEDIATRIC PROVIDER INITIATED TESTING AND COUNSELLING
 PAEDIATRIC PROVIDER INITIATED TESTING AND COUNSELLING This checklist is offered as an aid to support supervision and quality assurance for paediatric PITC services. Through observation, interviews and
PAEDIATRIC PROVIDER INITIATED TESTING AND COUNSELLING This checklist is offered as an aid to support supervision and quality assurance for paediatric PITC services. Through observation, interviews and
Technical Bulletin No. 161
 CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 161 cobas 6800 HIV-1 Viral Load Assay - New Platform - June 1, 2017 Contact: Heather Habig, MLS (ASCP) CM, MB CM, 717-851-1422 Operations
CPAL Central Pennsylvania Alliance Laboratory Technical Bulletin No. 161 cobas 6800 HIV-1 Viral Load Assay - New Platform - June 1, 2017 Contact: Heather Habig, MLS (ASCP) CM, MB CM, 717-851-1422 Operations
HIV Viral Load Quality Assessment Program Summary for Panel HIVVL 2017Oct27
 1 The National Laboratory for HIV Reference Services is Accredited to ISO 15189 and ISO 17043 National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology
1 The National Laboratory for HIV Reference Services is Accredited to ISO 15189 and ISO 17043 National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology
DENTISTRY SCIENTIFIC AND PROFESSIONAL CATEGORY
 CLASSIFICATION STANDARD DENTISTRY SCIENTIFIC AND PROFESSIONAL CATEGORY O Ministre des Approvisionnements et Services Canada 1986 Dentistry CONTENTS PAGE INTRODUCTION 1 CATEGORY DEFINITION 2 CROUP DEFINITION
CLASSIFICATION STANDARD DENTISTRY SCIENTIFIC AND PROFESSIONAL CATEGORY O Ministre des Approvisionnements et Services Canada 1986 Dentistry CONTENTS PAGE INTRODUCTION 1 CATEGORY DEFINITION 2 CROUP DEFINITION
January Billing and compliance. Chemistry. Help us help you. Referral testing. Additional CPT code changes announced for 2015
 January 2015 Billing and compliance Additional CPT code changes announced for 2015 Chemistry CA 27.29 conversion to CA 15-3 nearing completion Vitamin B12 and LD, Total specimen change Timed Urine Protein
January 2015 Billing and compliance Additional CPT code changes announced for 2015 Chemistry CA 27.29 conversion to CA 15-3 nearing completion Vitamin B12 and LD, Total specimen change Timed Urine Protein
Specimen Collection Requirements. Test Name Specimen Type Storage Time Storage Conditions
 1 Specimen Collection Requirements PURPOSE/PRINCIPLE: To outline proper specimen collection for molecular HLA testing, including tube type, minimum specimen amount, proper tube and requisition labeling,
1 Specimen Collection Requirements PURPOSE/PRINCIPLE: To outline proper specimen collection for molecular HLA testing, including tube type, minimum specimen amount, proper tube and requisition labeling,
The IFI has developed strategic objectives around three core activities:
 Job Description Title: IFI Library and Special Collections Manager (LSCM) Overview The Irish Film Institute (IFI) is Ireland s national cultural institution for film. At the IFI s historic home in Eustace
Job Description Title: IFI Library and Special Collections Manager (LSCM) Overview The Irish Film Institute (IFI) is Ireland s national cultural institution for film. At the IFI s historic home in Eustace
WHO Prequalification of Diagnostics Programme PUBLIC REPORT. Product: HIV 1/2 STAT-PAK Number: PQDx Abstract
 WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: HIV 1/2 STAT-PAK Number: PQDx 0007-006-00 Abstract The HIV 1/2 STAT-PAK with product code HIV101, manufactured by Chembio Diagnostic
WHO Prequalification of Diagnostics Programme PUBLIC REPORT Product: HIV 1/2 STAT-PAK Number: PQDx 0007-006-00 Abstract The HIV 1/2 STAT-PAK with product code HIV101, manufactured by Chembio Diagnostic
Role of Core Lab in Clinical Trial M. Therese Tupas-Habib, RDCS Manager, Cardiovascular Core Lab MedStar Health Research Institute
 Role of Core Lab in Clinical Trial M. Therese Tupas-Habib, RDCS Manager, Cardiovascular Core Lab MedStar Health Research Institute ASE Echo in Clinical Trials JASE Oct 2004 Why Echo? - non-invasive diagnostic
Role of Core Lab in Clinical Trial M. Therese Tupas-Habib, RDCS Manager, Cardiovascular Core Lab MedStar Health Research Institute ASE Echo in Clinical Trials JASE Oct 2004 Why Echo? - non-invasive diagnostic
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT. Product: Alere HIV Combo WHO reference number: PQDx
 WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: Alere HIV Combo WHO reference number: PQDx 0243-013-00 Alere HIV Combo with product codes 7D2842, 7D2843, 7D2843SET manufactured by Alere
WHO Prequalification of In Vitro Diagnostics PUBLIC REPORT Product: Alere HIV Combo WHO reference number: PQDx 0243-013-00 Alere HIV Combo with product codes 7D2842, 7D2843, 7D2843SET manufactured by Alere
Phlebotomy Skills and Experience
 Western Technical College 10513107 Phlebotomy Skills and Experience Course Outcome Summary Course Information Description Career Cluster Instructional Level Total Credits 3 Total Hours 117 The phlebotomy
Western Technical College 10513107 Phlebotomy Skills and Experience Course Outcome Summary Course Information Description Career Cluster Instructional Level Total Credits 3 Total Hours 117 The phlebotomy
ADVANCED MANUFACTURING CAREER CLUSTER
 ADVANCED MANUFACTURING CAREER CLUSTER QUALITY ASSURANCE/ CONTROL PRODUCTION Industrial Production Managers MAINTENANCE AND REPAIR Research & Development/ Materials Industrial and Manufacturing Mechanical
ADVANCED MANUFACTURING CAREER CLUSTER QUALITY ASSURANCE/ CONTROL PRODUCTION Industrial Production Managers MAINTENANCE AND REPAIR Research & Development/ Materials Industrial and Manufacturing Mechanical
HIV Viral Load Quality Assessment Program Summary for Panel HIVVL 2018Oct26
 1 National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology Laboratory Public Health Agency of Canada HIV Viral Load Quality Assessment Program Summary
1 National Laboratory for HIV Reference Services National HIV and Retrovirology Laboratories National Microbiology Laboratory Public Health Agency of Canada HIV Viral Load Quality Assessment Program Summary
Pathology Specimen Handling Requirements
 CONWAY REGIONAL HEALTH SYSTEM CLINICAL LABORATORY Pathology Specimen Handling Requirements POLICY: Tissue or body fluids, etc. removed or collected during any procedure for purposes of Pathologist examination
CONWAY REGIONAL HEALTH SYSTEM CLINICAL LABORATORY Pathology Specimen Handling Requirements POLICY: Tissue or body fluids, etc. removed or collected during any procedure for purposes of Pathologist examination
Strengthening Laboratory Systems for HIV Differentiated Service Delivery
 ADVANCING THE LABORATORY PROFESSION AND NETWORKS IN AFRICA Strengthening Laboratory Systems for HIV Differentiated Service Delivery Anafi Mataka African Society for Laboratory Medicine AIDS 2018 Outline
ADVANCING THE LABORATORY PROFESSION AND NETWORKS IN AFRICA Strengthening Laboratory Systems for HIV Differentiated Service Delivery Anafi Mataka African Society for Laboratory Medicine AIDS 2018 Outline
Follow-up Visit Procedures MTN-009
 Follow-up Visit Procedures MTN-009 General Overview MTN-009 follow-up visits are only required for participants who are found to be HIV infected These participants will have a minimum of two follow-up
Follow-up Visit Procedures MTN-009 General Overview MTN-009 follow-up visits are only required for participants who are found to be HIV infected These participants will have a minimum of two follow-up
TRAINING COORDINATOR TRAINING
 TRAINING COORDINATOR TRAINING 1. Role of Training Coordinator: Responsible for all sports training for athletes and coaches in the local program. As a sports organization, our main focus should be to assure
TRAINING COORDINATOR TRAINING 1. Role of Training Coordinator: Responsible for all sports training for athletes and coaches in the local program. As a sports organization, our main focus should be to assure
2.T7. Explain the phlebotomy procedure to be performed to the patient. 1.C. Review the requisition for testing
 2011 to 2016 CPT Test Plan Crosswalk Crosswalk Section: The following bridges tasks on the 2011 CPT test plan with task statements on the 2016 CPT test plan. 2011 NHA Test TASK DESCRIPTION 2016 NHA TEST
2011 to 2016 CPT Test Plan Crosswalk Crosswalk Section: The following bridges tasks on the 2011 CPT test plan with task statements on the 2016 CPT test plan. 2011 NHA Test TASK DESCRIPTION 2016 NHA TEST
ISBN ISBN Title Information Events Management: a practical guide
 ISBN 10 0-9554126-0-9 ISBN 13 978-0-9554126-0-8 Title Information Events Management: a practical guide Copyright EventScotland 2006 Published in September 2006 by EventScotland This publication is also
ISBN 10 0-9554126-0-9 ISBN 13 978-0-9554126-0-8 Title Information Events Management: a practical guide Copyright EventScotland 2006 Published in September 2006 by EventScotland This publication is also
Financial Administration and Control of Research and Special Funds
 1 2 3 Financial Administration and Control of Research and Special Funds 4 Financial Administration and Control of Research and Special Funds Presenter Name Colin Nicolson Presenter Title Assistant Manager
1 2 3 Financial Administration and Control of Research and Special Funds 4 Financial Administration and Control of Research and Special Funds Presenter Name Colin Nicolson Presenter Title Assistant Manager
Panther has new prey
 Raising the Bar for Performance Testing Panther has new prey The Aptima HIV-1 Quant Dx assay leads the hunt for HIV-1 diagnosis and viral load monitoring. Freedom to work the way you choose Run what assays
Raising the Bar for Performance Testing Panther has new prey The Aptima HIV-1 Quant Dx assay leads the hunt for HIV-1 diagnosis and viral load monitoring. Freedom to work the way you choose Run what assays
TEST LIST SAMPLE REQUIREMENT. 1 ml serum None
 ALBUMIN TEST NAME ALKALINE PHOSPHATASE ALLERGY PROFILE, FOOD 30 allergens ALLERGY PROFILE, INHALANT 30 Allergens ALT AMYLASE ANA ANTI- TG ANTI-GLIADIN IGG ANTI-GLIADIN IGA ANTI-HBS ANTI-HCV ANTI-TPO APOLIPOPROTEIN
ALBUMIN TEST NAME ALKALINE PHOSPHATASE ALLERGY PROFILE, FOOD 30 allergens ALLERGY PROFILE, INHALANT 30 Allergens ALT AMYLASE ANA ANTI- TG ANTI-GLIADIN IGG ANTI-GLIADIN IGA ANTI-HBS ANTI-HCV ANTI-TPO APOLIPOPROTEIN
M.D.IPA, M.D.IPA Preferred, Optimum Choice and Optimum Choice Preferred STAT Laboratory List Revised Jan. 5, 2017
 M.D.IPA, M.D.IPA Preferred, Optimum Choice and Optimum Choice Preferred STAT Laboratory List Revised Jan. 5, 2017 If laboratory results are required on a STAT basis, the designated commercial medical laboratory
M.D.IPA, M.D.IPA Preferred, Optimum Choice and Optimum Choice Preferred STAT Laboratory List Revised Jan. 5, 2017 If laboratory results are required on a STAT basis, the designated commercial medical laboratory
Alabama Department of Public Health County Health Department Protocol
 Alabama Department of Public Health County Health Department Protocol BREAST AND CERVICAL CANCER TABLE OF CONTENTS ABCCEDP Overview and Purpose... 1 Clinical Guidelines... 1 Patient Enrollment... 1 Resource
Alabama Department of Public Health County Health Department Protocol BREAST AND CERVICAL CANCER TABLE OF CONTENTS ABCCEDP Overview and Purpose... 1 Clinical Guidelines... 1 Patient Enrollment... 1 Resource
The Virtues and Pitfalls of Implementing a New Test
 The Virtues and Pitfalls of Implementing a New Test James H. Nichols, Ph.D., DABCC, FACB Professor of Clinical Pathology, Microbiology and Immunology Associate Medical Director for Clinical Operations
The Virtues and Pitfalls of Implementing a New Test James H. Nichols, Ph.D., DABCC, FACB Professor of Clinical Pathology, Microbiology and Immunology Associate Medical Director for Clinical Operations
CDL Drivers Controlled Substance and Alcohol Policy
 CDL Drivers Controlled Substance and Alcohol Policy Section 1. General. It is the purpose of this policy to encourage an enlightened viewpoint toward alcoholism and other drug dependencies as behavioral/medical
CDL Drivers Controlled Substance and Alcohol Policy Section 1. General. It is the purpose of this policy to encourage an enlightened viewpoint toward alcoholism and other drug dependencies as behavioral/medical
Standard Operating Procedure
 Standard Operating Procedure 1. Definitions Section: Laboratory Version No. 1.2 Initials: AD Title: 2.0 Testing Algorithm Revision Date: 01 Sep 2011 1.1 SOP: Standard Operating Procedure 1.2 CRF: Case
Standard Operating Procedure 1. Definitions Section: Laboratory Version No. 1.2 Initials: AD Title: 2.0 Testing Algorithm Revision Date: 01 Sep 2011 1.1 SOP: Standard Operating Procedure 1.2 CRF: Case
Sonography Program Application and Information Packet. Lackawanna College Sonography Programs Technical Standards
 Sonography Program Application and Information Packet The ultrasound profession is subdivided into nine specialties. The specialties offered at Lackawanna College are General Diagnostic Medical Sonography,
Sonography Program Application and Information Packet The ultrasound profession is subdivided into nine specialties. The specialties offered at Lackawanna College are General Diagnostic Medical Sonography,
Clinical Experience 2
 Western Technical College 10513152 Clinical Experience 2 Course Outcome Summary Course Information Description Career Cluster Instructional Level Total Credits 4 Total Hours 288 This clinical provides
Western Technical College 10513152 Clinical Experience 2 Course Outcome Summary Course Information Description Career Cluster Instructional Level Total Credits 4 Total Hours 288 This clinical provides
DRIVING UNDER THE INFLUENCE
 AOM CHAPTER O 302 DRIVING UNDER THE INFLUENCE [61.1.11] Table of Contents I. INTRODUCTORY DISCUSSION II. ALCOHOL ENFORCEMENT TRAINING PROGRAM [61.1.10] III. SPECIALIZED DETAILS & ASSIGNMENTS [61.1.10]
AOM CHAPTER O 302 DRIVING UNDER THE INFLUENCE [61.1.11] Table of Contents I. INTRODUCTORY DISCUSSION II. ALCOHOL ENFORCEMENT TRAINING PROGRAM [61.1.10] III. SPECIALIZED DETAILS & ASSIGNMENTS [61.1.10]
New Haven/Fairfield Counties Ryan White Part A Program Oral Health Standard of Care
 DENTAL/ORAL HEALTH I. DEFINITION OF SERVICE Support for Oral Health Services including diagnostic, preventive, and therapeutic dental care that is in compliance with state dental practice laws, includes
DENTAL/ORAL HEALTH I. DEFINITION OF SERVICE Support for Oral Health Services including diagnostic, preventive, and therapeutic dental care that is in compliance with state dental practice laws, includes
GSK , 2.0, 18, 2014, DAIDS
 Letter of Amendment # 1 to: HPTN 077: A Phase IIa Study to Evaluate the, Tolerability and Pharmacokinetics of the Investigational Injectable HIV Integrase Inhibitor, GSK1265744, in HIV-uninfected Men and
Letter of Amendment # 1 to: HPTN 077: A Phase IIa Study to Evaluate the, Tolerability and Pharmacokinetics of the Investigational Injectable HIV Integrase Inhibitor, GSK1265744, in HIV-uninfected Men and
Fertility Center and Sperm Bank Manual for the Fertile Hope Program for Men
 Fertility Center and Sperm Bank Manual for the Fertile Hope Program for Men Table of Contents I. Fertile Hope Program for Men: An Overview...3 II. Referring to the Fertile Hope Program in Your Publications.3
Fertility Center and Sperm Bank Manual for the Fertile Hope Program for Men Table of Contents I. Fertile Hope Program for Men: An Overview...3 II. Referring to the Fertile Hope Program in Your Publications.3
4. Laboratory Diagnosis of Viral Haemorrhagic Fever Viruses
 4. Laboratory Diagnosis of Viral Haemorrhagic Fever Viruses 4.1 Introduction Early infection with VHF is characterised by a short non-specific viral prodrome with an extensive differential diagnosis. The
4. Laboratory Diagnosis of Viral Haemorrhagic Fever Viruses 4.1 Introduction Early infection with VHF is characterised by a short non-specific viral prodrome with an extensive differential diagnosis. The
CONTINUING CARE ADMINISTRATIVE MANUAL POLICY
 CONTINUING CARE ADMINISTRATIVE MANUAL POLICY NUMBER III-10 DATE Draft June 8, 2010 PAGE 1 OF 3 APPROVED BY: SITE: CATEGORY TITLE: Vice President & Senior Operating Officer, Rehab & Continuing Care Edmonton
CONTINUING CARE ADMINISTRATIVE MANUAL POLICY NUMBER III-10 DATE Draft June 8, 2010 PAGE 1 OF 3 APPROVED BY: SITE: CATEGORY TITLE: Vice President & Senior Operating Officer, Rehab & Continuing Care Edmonton
Level 3 Diploma in Sports Massage Therapy
 Qualification Guidance Syllabus Level 3 Diploma in Sports Massage Therapy Qualification Accreditation Number: 601/4930/9 Version AIQ005043 Active IQ wishes to emphasise that whilst every effort is made
Qualification Guidance Syllabus Level 3 Diploma in Sports Massage Therapy Qualification Accreditation Number: 601/4930/9 Version AIQ005043 Active IQ wishes to emphasise that whilst every effort is made
Sustainability of Canadian biorepositories: Status, needs and national initiatives
 Sustainability of Canadian biorepositories: Status, needs and national initiatives Dr. Brent Schacter Principal Investigator, CTRNet Professor, Department of Medicine, University of Manitoba/CancerCare
Sustainability of Canadian biorepositories: Status, needs and national initiatives Dr. Brent Schacter Principal Investigator, CTRNet Professor, Department of Medicine, University of Manitoba/CancerCare
Section 9. Laboratory Considerations
 Section 9. Laboratory Considerations This section contains information on the laboratory procedures performed in MTN-027. Table of Contents The page numbers are hyperlinked to the contents below 9.1 Overview
Section 9. Laboratory Considerations This section contains information on the laboratory procedures performed in MTN-027. Table of Contents The page numbers are hyperlinked to the contents below 9.1 Overview
idc 20 AUTOMATED LIQUID-BASED CYTOLOGY SLIDE PREPARATION SYSTEM
 idc 20 AUTOMATED LIQUID-BASED CYTOLOGY SLIDE PREPARATION SYSTEM ilsa : 16 rue de la Comtesse - 25640 MARCHAUX - FRANCE Phone : +33 (0) 381 579 034 Web : www.ilsa-france.com Email : contact@ilsa-france.com
idc 20 AUTOMATED LIQUID-BASED CYTOLOGY SLIDE PREPARATION SYSTEM ilsa : 16 rue de la Comtesse - 25640 MARCHAUX - FRANCE Phone : +33 (0) 381 579 034 Web : www.ilsa-france.com Email : contact@ilsa-france.com
Integrated Screening Guide How to plan a successful integrated screening clinic with a mobile mammography unit. January 2016
 Integrated Screening Guide How to plan a successful integrated screening clinic with a mobile mammography unit January 2016 Development of this Guide has been made possible through funding from the Public
Integrated Screening Guide How to plan a successful integrated screening clinic with a mobile mammography unit January 2016 Development of this Guide has been made possible through funding from the Public
Course Outline. Code: SPX121 Title: Exercise Prescription and Programming I
 Course Outline Code: SPX121 Title: Exercise Prescription and Programming I Faculty: Science, Health, Education and Engineering School: Health & Sport Science Teaching Session: Semester 2 Year: 2018 Course
Course Outline Code: SPX121 Title: Exercise Prescription and Programming I Faculty: Science, Health, Education and Engineering School: Health & Sport Science Teaching Session: Semester 2 Year: 2018 Course
