Enforcement Policy Statement on Marketing Claims for OTC Homeopathic Drugs
|
|
|
- Alexandra Lindsey
- 6 years ago
- Views:
Transcription
1 This document is scheduled to be published in the Federal Register on 12/13/2016 and available online at and on FDsys.gov [BILLING CODE: S] FEDERAL TRADE COMMISSION Enforcement Policy Statement on Marketing Claims for OTC Homeopathic Drugs AGENCY: Federal Trade Commission. ACTION: Commission policy statement. SUMMARY: The Federal Trade Commission has issued an Enforcement Policy Statement on Marketing Claims for OTC Homeopathic Drugs. The Statement describes the level of substantiation that the Commission expects for marketing claims for over-the-counter (OTC) homeopathic drugs. It also recognizes that marketing claims for OTC homeopathic products for an unsubstantiated indication might be made non-deceptive by the inclusion of additional explanatory information that effectively communicates to consumers that there is no scientific evidence that the product works and that the product s claims are based only on theories of homeopathy from the 1700s that are not accepted by most modern medical experts. DATES: The Commission announced the issuance of the Statement on November 15, FOR FURTHER INFORMATION CONTACT: Michael Ostheimer ( ) or Richard Cleland ( ), Bureau of Consumer Protection, 600 Pennsylvania Avenue, NW, Washington, DC SUPPLEMENTARY INFORMATION: Enforcement Policy Statement on Marketing Claims for OTC Homeopathic Drugs The Federal Trade Commission (FTC) is issuing this Policy Statement to provide guidance regarding its enforcement policy with respect to marketing claims for over-the-counter (OTC) homeopathic drugs. It applies only to OTC products intended solely for self-limiting disease
2 conditions 1 amenable to self-diagnosis of symptoms and treatment. 2 The Commission believes this Policy Statement is appropriate in light of the burgeoning mainstream marketing of OTC homeopathic products alongside other OTC drugs. The FTC s authority over disease and other health-related claims comes from Sections 5 and 12 of the FTC Act. Section 5, which applies to both advertising and labeling, prohibits unfair or deceptive acts or practices in or affecting commerce, such as the deceptive advertising or labeling of OTC drugs. 3 Section 12 prohibits the dissemination of false advertisements in or affecting commerce of food, drugs, devices, services, or cosmetics. 4 Under these provisions, companies must have a reasonable basis for making objective product claims, including claims that a product can treat specific conditions, before those claims are made. 5 Homeopathy, which dates back to the late-eighteenth century, is based on the view that disease symptoms can be treated by minute doses of substances that produce similar symptoms when provided in larger doses to healthy people. Many homeopathic products are diluted to such an extent that they no longer contain detectable levels of the initial substance. In general, homeopathic product claims are not based on modern scientific methods and are not accepted by modern medical experts, but homeopathy nevertheless has many adherents. 6 1 A self-limiting disease condition is one that resolves spontaneously with or without specific treatment. 2 This Policy Statement does not apply to the practice of medicine. 3 Federal Trade Commission Act, 15 U.S.C. 45(a)(2). 4 Federal Trade Commission Act, 15 U.S.C See Advertising Substantiation Policy Statement, appended to Thompson Medical Co., 104 F.T.C. 648, 839 (1984), aff d, 791 F.2d 189 (D.C. Cir. 1986) ( Advertising Substantiation Policy Statement ). 6 FTC Staff Report on the Homeopathic Medicine & Advertising Workshop (Nov. 2016). 2
3 In 1988, the Food & Drug Administration (FDA) issued a Compliance Policy Guide (CPG) entitled Conditions Under Which Homeopathic Drugs May be Marketed, which permitted marketers to distribute OTC homeopathic products without demonstrating their efficacy. 7 Under the CPG, only homeopathic products intended solely for self-limiting disease conditions amenable to self-diagnosis of symptoms and treatment may be marketed OTC. The CPG requires that OTC homeopathic drugs be labeled as homeopathic and that their labeling display at least one major OTC indication for use. The FTC Act does not exempt homeopathic products from the general requirement that objective product claims be truthful and substantiated. 8 Nevertheless, in the decades since the Commission announced in 1972 that objective product claims must be substantiated, 9 the FTC has rarely challenged misleading claims for products that were homeopathic or purportedly homeopathic See CPG Sec Conditions Under Which Homeopathic Drugs May be Marketed (revised Mar. 1995), 8 [A] product that contemporary technology does not understand must establish that this magic actually works. Proof is what separates an effect new to science from a swindle.... [I]f a condition responds to treatment, then selling a placebo as if it had therapeutic effect directly injures the consumer. FTC v. QT, Inc., 512 F.3d 858, (7th Cir. 2008). 9 See Pfizer, Inc., 81 F.T.C. 23, (1972). 10 See, e.g., Complaint, FTC v. HCG Diet Direct, LLC, No. 2:14-cv NVW (D. Ariz. Jan. 7, 2014) (stipulated judgment) (challenging weight-loss claims for purported homeopathic products); Complaint, FTC v. Iovate Health Scis. USA, Inc., No. 10-CV-587 (W.D.N.Y. July 14, 2010) (stipulated judgment) (challenging claims that purported allergy-relieving product was homeopathic and effective); Quigley Corp., No. C-3926, 2000 FTC LEXIS 24 (Feb. 10, 2000) (consent order) (challenging cold treatment and prevention claims for homeopathic products); Levey, 116 F.T.C. 885 (1993) (consent order) (challenging weight-loss and impotency treatment claims for purported homeopathic products). 3
4 Efficacy and safety claims for homeopathic drugs are held to the same standards as similar claims for non-homeopathic drugs. As articulated in the Advertising Substantiation Policy Statement, advertisers must have at least the advertised level of substantiation. Absent express or implied reference to a particular level of support, the Commission, in evaluating the types of evidence necessary to substantiate a claim, considers the type of claim, the product, the consequences of a false claim, the benefits of a truthful claim, the cost of developing substantiation for the claim, and the amount of substantiation experts believe is reasonable. 11 For health, safety, or efficacy claims, the FTC has generally required that advertisers possess competent and reliable scientific evidence, defined as tests, analyses, research, or studies that have been conducted and evaluated in an objective manner by qualified persons and [that] are generally accepted in the profession to yield accurate and reliable results. 12 In general, for health benefit claims, particularly claims that a product can treat or prevent a disease or its symptoms, the substantiation required has been well-designed human clinical testing Advertising Substantiation Policy Statement, 104 F.T.C. at 840. These factors are known as the Pfizer factors, after the 1972 case, supra note 9, in which they were first enunciated. 12 See, e.g., POM Wonderful LLC, 155 F.T.C. 56, 193 (2013), aff d in part, 777 F.3d 478, (D.C. Cir. 2015), cert. denied, No , 2016 U.S. LEXIS 2991 (May 2, 2016); Telebrands Corp., 140 F.T.C. 278, 347 (2005), aff d, 457 F.3d 354 (4th Cir. 2006); Novartis Corp., 127 F.T.C. 580, 725 (1999), aff d, 223 F.3d 783 (D.C. Cir. 2000); Brake Guard Prods., Inc., 125 F.T.C. 138, 256 (1998). 13 See, e.g., POM Wonderful LLC, 155 F.T.C. at 5-6 (requiring well-designed, well-conducted, double-blind, randomized controlled clinical testing to substantiate heart disease, prostate cancer, and erectile dysfunction prevention and treatment claims; also imposing such a requirement for all future disease claims), aff d in part, 777 F.3d at (affirming Commission holding that competent and reliable scientific evidence consisting of randomized, well-controlled human clinical testing is needed for disease-related claims but finding fencing-in order requirement of two such tests was not justified in this instance); see also FTC v. Nat l Urological Group, Inc., 645 F. Supp. 2d 1167, (N.D. Ga. 2008) (accepting undisputed expert testimony that erectile dysfunction claims require well-designed, placebo-controlled, randomized, double-blind clinical trials for substantiation); FTC v. Direct Mktg. Concepts, Inc., 569 F. Supp. 2d 285, 303 4
5 For the vast majority of OTC homeopathic drugs, the case for efficacy is based solely on traditional homeopathic theories and there are no valid studies using current scientific methods showing the product s efficacy. Accordingly, marketing claims that such homeopathic products have a therapeutic effect lack a reasonable basis and are likely misleading in violation of Sections 5 and 12 of the FTC Act. 14 However, the FTC has long recognized that marketing claims may include additional explanatory information in order to prevent the claims from being misleading. Accordingly, the promotion of an OTC homeopathic product for an indication that is not substantiated by competent and reliable scientific evidence may not be deceptive if that promotion effectively communicates to consumers that: (1) there is no scientific evidence that the product works and (2) the product s claims are based only on theories of homeopathy from the 1700s that are not accepted by most modern medical experts. 15 To be non-misleading, the (D. Mass. 2008), aff d, 624 F.3d 1 (1st Cir. 2010) ( it seems well-accepted that double-blind, placebo-controlled studies are necessary to substantiate health-related efficacy claims ); Removatron Int l Corp., 111 F.T.C. 206 (1988), aff d, 884 F.2d 1489 (1st Cir. 1989) (requiring adequate and well-controlled clinical testing to substantiate claims for hair removal product); Thompson Med. Co., 104 F.T.C. at 826 (requiring well-controlled clinical studies to substantiate certain analgesic drug claims). The Commission has also accepted numerous settlements that required randomized controlled clinical testing for disease treatment and prevention claims. See, e.g., Brown, 152 F.T.C. 466, (2011) (consent order); Nestlé HealthCare Nutrition, Inc., 151 F.T.C. 1, 13 (2011) (consent order); Viral Response Sys., Inc., 115 F.T.C. 676, 691 (1992) (consent order). 14 Although this Policy Statement is limited to OTC homeopathic products for the treatment of self-limiting disease conditions (ones that resolve spontaneously with or without specific treatment) amenable to self-diagnosis, marketing claims about the efficacy of homeopathic products not covered by this Policy Statement also are subject to the requirements to Sections 5 and A statement that a product is based on traditional homeopathic theories might put some consumers on notice as to the basis of the product s efficacy claims. However, because many consumers do not understand what homeopathy is, the Commission does not believe that such a statement alone would adequately put consumers on notice that a product s efficacy claims are not backed by scientific evidence, and could, in fact, enhance the perceived credibility of the claim. Similarly, the Commission believes that a statement that a product s efficacy has not 5
6 product and the claims must also comply with requirements for homeopathic products and traditional homeopathic principles. Of course, adequately substantiated claims for homeopathic products would not require additional explanation. Perfunctory disclaimers are unlikely to successfully communicate the information necessary to make claims for OTC homeopathic drugs non-misleading. The Commission notes: Any disclosure should stand out and be in close proximity to the efficacy message; to be effective, it may actually need to be incorporated into the efficacy message. Marketers should not undercut such qualifications with additional positive statements or consumer endorsements reinforcing a product s efficacy. In light of the inherent contradiction in asserting that a product is effective and also disclosing that there is no scientific evidence for such an assertion, it is possible that depending on how they are presented many of these disclosures will be insufficient to prevent consumer deception. Marketers are advised to develop extrinsic evidence, such as consumer surveys, to determine the net impressions communicated by their marketing materials. The Commission will carefully scrutinize the net impression of OTC homeopathic advertising or other marketing employing disclosures to ensure that it adequately conveys the extremely limited nature of the health claim being asserted. If, despite a marketer s been evaluated by the Food and Drug Administration does not adequately address the potential lack of substantiation for a product s efficacy claims; dietary supplements bear a similar disclosure but FDA does require that dietary supplement label claims be supported by competent and reliable scientific evidence. Finally, the Commission believes that a simple statement that a product s efficacy is not supported by scientific evidence does not convey the truly limited basis for the efficacy claim and that, to avoid deceiving consumers, it is likely necessary to explain that it is not accepted by modern medicine. 6
7 disclosures, an ad conveys more substantiation than the marketer has, the marketer will be in violation of the FTC Act. In summary, there is no basis under the FTC Act to treat OTC homeopathic drugs differently than other health products. Accordingly, unqualified disease claims made for homeopathic drugs must be substantiated by competent and reliable scientific evidence. Nevertheless, truthful, nonmisleading, effective disclosure of the basis for an efficacy claim may be possible. The approach outlined in this Policy Statement is therefore consistent with the First Amendment, and neither limits consumer access to OTC homeopathic products nor conflicts with the FDA s regulatory scheme. It would allow a marketer to include an indication for use that is not supported by scientific evidence so long as the marketer effectively communicates the limited basis for the claim in the manner discussed above. By direction of the Commission. Donald S. Clark, Secretary. [FR Doc Filed: 12/12/2016 8:45 am; Publication Date: 12/13/2016] 7
Theoriginalhcgdrops.com 11/28/11
 Theoriginalhcgdrops.com 11/28/11 UNITED STATES OF AMERICA FEDERAL TRADE COMMISSION BUREAU OF CONSUMER PROTECTION WASHINGTON, D.C. 20580 DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION
Theoriginalhcgdrops.com 11/28/11 UNITED STATES OF AMERICA FEDERAL TRADE COMMISSION BUREAU OF CONSUMER PROTECTION WASHINGTON, D.C. 20580 DEPARTMENT OF HEALTH AND HUMAN SERVICES FOOD AND DRUG ADMINISTRATION
Substantiation of Health Claims in Advertising. Richard L. Cleland Division of Advertising Practices Federal Trade Commission
 Substantiation of Health Claims in Advertising Richard L. Cleland Division of Advertising Practices Federal Trade Commission The FTC Act prohibits: Background unfair or deceptive acts or practices in or
Substantiation of Health Claims in Advertising Richard L. Cleland Division of Advertising Practices Federal Trade Commission The FTC Act prohibits: Background unfair or deceptive acts or practices in or
The opinions expressed are the speaker s and not his company s.
 May 13, 2010 James Brooks, Ph.D. Vice President, Science & Technology Christine Burdick-Bell, J.D. Vice President, Legal & Regulatory Affairs Pharmavite LLC The opinions expressed are the speaker s and
May 13, 2010 James Brooks, Ph.D. Vice President, Science & Technology Christine Burdick-Bell, J.D. Vice President, Legal & Regulatory Affairs Pharmavite LLC The opinions expressed are the speaker s and
Is POM Wonderful Really Wonderful? How the FTC, the FDA and Private Litigation Address Deceptive Food Advertising
 Is POM Wonderful Really Wonderful? How the FTC, the FDA and Private Litigation Address Deceptive Food Advertising Dee Pridgen October 9, 2014 14 th Consumer Issues Conference Pom Wonderful achieve immortality?
Is POM Wonderful Really Wonderful? How the FTC, the FDA and Private Litigation Address Deceptive Food Advertising Dee Pridgen October 9, 2014 14 th Consumer Issues Conference Pom Wonderful achieve immortality?
AC O M P E T I T O R I N T R O D U C E S A
 Antitrust, Vol. 25, No. 1, Fall 2010. 2010 by the American Bar Association. Reproduced with permission. All rights reserved. This information or any portion thereof may not be copied or disseminated in
Antitrust, Vol. 25, No. 1, Fall 2010. 2010 by the American Bar Association. Reproduced with permission. All rights reserved. This information or any portion thereof may not be copied or disseminated in
Agency Information Collection Activities; Submission for Office of Management and
 This document is scheduled to be published in the Federal Register on 02/19/2019 and available online at https://federalregister.gov/d/2019-02596, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
This document is scheduled to be published in the Federal Register on 02/19/2019 and available online at https://federalregister.gov/d/2019-02596, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
Advertising: The Federal Trade Commission and Private Rights of Action Venable LLP
 Advertising: The Federal Trade Commission and Private Rights of Action 2012 Venable LLP 1 Agenda FTC s Role and Authority Claim Substantiation Private Rights of Action 2 FTC s Role and Authority FTC Authority
Advertising: The Federal Trade Commission and Private Rights of Action 2012 Venable LLP 1 Agenda FTC s Role and Authority Claim Substantiation Private Rights of Action 2 FTC s Role and Authority FTC Authority
Presentation: Philip G. Hampton, II Haynes and Boone, LLP (202) September 14, 2017
 Presentation: Philip G. Hampton, II Haynes and Boone, LLP (202) 654-4533 September 14, 2017 What is a Trademark? A trademark is any word, product name, symbol or device that identifies the goods or services
Presentation: Philip G. Hampton, II Haynes and Boone, LLP (202) 654-4533 September 14, 2017 What is a Trademark? A trademark is any word, product name, symbol or device that identifies the goods or services
Opiate Freedom Center 1/11/18
 Opiate Freedom Center 1/11/18 UNITED STATES OF AMERICA DEPARTMENT OF HEALTH FEDERAL TRADE COMMISSION AND HUMAN SERVICES BUREAU OF CONSUMER FOOD AND DRUG ADMINISTRATION PROTECTION SILVER SPRING, MD 20993
Opiate Freedom Center 1/11/18 UNITED STATES OF AMERICA DEPARTMENT OF HEALTH FEDERAL TRADE COMMISSION AND HUMAN SERVICES BUREAU OF CONSUMER FOOD AND DRUG ADMINISTRATION PROTECTION SILVER SPRING, MD 20993
STATEMENT OF THE AMERICAN DENTAL ASSOCIATION ON REGULATION BY STATE BOARDS OF DENTISTRY OF MISLEADING DENTAL SPECIALTY CLAIMS.
 STATEMENT OF THE AMERICAN DENTAL ASSOCIATION ON REGULATION BY STATE BOARDS OF DENTISTRY OF MISLEADING DENTAL SPECIALTY CLAIMS August 10, 2018 From time to time, general dentists who are not adequately
STATEMENT OF THE AMERICAN DENTAL ASSOCIATION ON REGULATION BY STATE BOARDS OF DENTISTRY OF MISLEADING DENTAL SPECIALTY CLAIMS August 10, 2018 From time to time, general dentists who are not adequately
Dietary Supplement Health and Education Act of 1994 Public Law rd Congress
 Dietary Supplement Health and Education Act of 1994 Public Law 103-417 103rd Congress An Act To amend the Federal Food, Drug, and Cosmetic Act to establish standards with respect to dietary supplements,
Dietary Supplement Health and Education Act of 1994 Public Law 103-417 103rd Congress An Act To amend the Federal Food, Drug, and Cosmetic Act to establish standards with respect to dietary supplements,
Claims That Can Get You in Trouble
 Claims That Can Get You in Trouble What s New in Advertising Claims for Dietary Supplements Steve Mister President & CEO, CRN FTC Requirements for Advertising Claims must be truthful, not misleading and
Claims That Can Get You in Trouble What s New in Advertising Claims for Dietary Supplements Steve Mister President & CEO, CRN FTC Requirements for Advertising Claims must be truthful, not misleading and
December 4, 2017 VIA ELECTRONIC SUBMISSION
 VIA ELECTRONIC SUBMISSION December 4, 2017 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Development of a List of pre-dietary Supplement
VIA ELECTRONIC SUBMISSION December 4, 2017 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Development of a List of pre-dietary Supplement
Re: Bayer s false and deceptive marketing for its Men s Multis for prevention of cancer
 June 18, 2009 VIA REGULAR MAIL AND FAX TO 973-254-4853 Gary S. Balkema, President Consumer Care Division Bayer HealthCare LLC 36 Columbia Rd Morristown, NJ 07962-1910 Re: Bayer s false and deceptive marketing
June 18, 2009 VIA REGULAR MAIL AND FAX TO 973-254-4853 Gary S. Balkema, President Consumer Care Division Bayer HealthCare LLC 36 Columbia Rd Morristown, NJ 07962-1910 Re: Bayer s false and deceptive marketing
Respondent brews beer. In 1987, respondent applied to the Bureau of Alcohol, Tobacco
 ORubin v. Coors Brewing Company 514 U.S. 476 (1995) Justice Thomas: Respondent brews beer. In 1987, respondent applied to the Bureau of Alcohol, Tobacco and Firearms (BATF), an agency of the Department
ORubin v. Coors Brewing Company 514 U.S. 476 (1995) Justice Thomas: Respondent brews beer. In 1987, respondent applied to the Bureau of Alcohol, Tobacco and Firearms (BATF), an agency of the Department
New England Compounding Center 04-Dec-06
 New England Compounding Center 04-Dec-06 Department of Health and Human Services Public Health Service Food and Drug Administration New England District One Montvale Avenue Stoneham, Massachusetts 02180
New England Compounding Center 04-Dec-06 Department of Health and Human Services Public Health Service Food and Drug Administration New England District One Montvale Avenue Stoneham, Massachusetts 02180
October 31, Draft Guidance for Industry, Frequently Asked Questions About Medical Foods; Second Edition
 October 31, 2013 VIA ELECTRONIC SUBMISSION Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane Rockville, MD 20852 Re: Docket No. FDA-2013-D-0880 Draft Guidance for
October 31, 2013 VIA ELECTRONIC SUBMISSION Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane Rockville, MD 20852 Re: Docket No. FDA-2013-D-0880 Draft Guidance for
MEMORANDUM. David L. Thomas, Chief Executive Officer, American Dairy Products Institute
 KELLER AND HECKMAN LLP Serving Business through Law and Science MEMORANDUM TO: FROM: David L. Thomas, Chief Executive Officer, American Dairy Products Institute Richard F. Mann Evangelia C. Pelonis DATE:
KELLER AND HECKMAN LLP Serving Business through Law and Science MEMORANDUM TO: FROM: David L. Thomas, Chief Executive Officer, American Dairy Products Institute Richard F. Mann Evangelia C. Pelonis DATE:
Re: Docket No. FDA D Presenting Risk Information in Prescription Drug and Medical Device Promotion
 1201 Maryland Avenue SW, Suite 900, Washington, DC 20024 202-962-9200, www.bio.org August 25, 2009 Dockets Management Branch (HFA-305) Food and Drug Administration 5600 Fishers Lane, Rm. 1061 Rockville,
1201 Maryland Avenue SW, Suite 900, Washington, DC 20024 202-962-9200, www.bio.org August 25, 2009 Dockets Management Branch (HFA-305) Food and Drug Administration 5600 Fishers Lane, Rm. 1061 Rockville,
The proposed rule is significant, and the requirements and exceptions are complex. Key provisions of the proposal are described below.
 ADVISORY Food & Drug FDA ISSUES PROPOSED RULE TO ESTABLISH A UNIQUE DEVICE IDENTIFICATION SYSTEM FOR MEDICAL DEVICES July 16, 2012 On July 11, 2012, the Food and Drug Administration (FDA) published in
ADVISORY Food & Drug FDA ISSUES PROPOSED RULE TO ESTABLISH A UNIQUE DEVICE IDENTIFICATION SYSTEM FOR MEDICAL DEVICES July 16, 2012 On July 11, 2012, the Food and Drug Administration (FDA) published in
Nutrition Labeling Laws for Food and Supplements Timeline
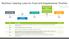 Federal Food, Drug, and Cosmetic Act (FDCA) Pure Food and Drug Act (PFDA) Wheeler-Lea Act st Multivitamin 920 938 Food Additives Amendment 906 st Dietary 934 958 Supplements Year Description Reference
Federal Food, Drug, and Cosmetic Act (FDCA) Pure Food and Drug Act (PFDA) Wheeler-Lea Act st Multivitamin 920 938 Food Additives Amendment 906 st Dietary 934 958 Supplements Year Description Reference
ACTION: Notification; declaratory order; extension of compliance date.
 This document is scheduled to be published in the Federal Register on 05/21/2018 and available online at https://federalregister.gov/d/2018-10714, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
This document is scheduled to be published in the Federal Register on 05/21/2018 and available online at https://federalregister.gov/d/2018-10714, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
5.I.1. GENERAL PRACTITIONER ANNOUNCEMENT OF CREDENTIALS IN NON-SPECIALTY INTEREST AREAS
 Report of the Council on Ethics, Bylaws and Judicial Affairs on Advisory Opinion 5.I.1. GENERAL PRACTITIONER ANNOUNCEMENT OF CREDENTIALS IN NON-SPECIALTY INTEREST AREAS Ethical Advertising under ADA Code:
Report of the Council on Ethics, Bylaws and Judicial Affairs on Advisory Opinion 5.I.1. GENERAL PRACTITIONER ANNOUNCEMENT OF CREDENTIALS IN NON-SPECIALTY INTEREST AREAS Ethical Advertising under ADA Code:
Advertising by Orthopaedic Surgeons
 Opinion on Ethics and Professionalism Advertising by Orthopaedic Surgeons An AAOS Opinion on Ethics and Professionalism is an official AAOS statement dealing with an ethical issue, which offers aspirational
Opinion on Ethics and Professionalism Advertising by Orthopaedic Surgeons An AAOS Opinion on Ethics and Professionalism is an official AAOS statement dealing with an ethical issue, which offers aspirational
Three-Month Extension of Certain Tobacco Product Compliance Deadlines Related to the
 This document is scheduled to be published in the Federal Register on 05/15/2017 and available online at https://federalregister.gov/d/2017-09754, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 05/15/2017 and available online at https://federalregister.gov/d/2017-09754, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Health Claims and FTC Advertising Law
 Health Claims and FTC Advertising Law Institute of Medicine Food Forum Workshop The Human Microbiome, Diet, and Health Michelle Rusk, Attorney* Division of Advertising Practices *Staff presentation does
Health Claims and FTC Advertising Law Institute of Medicine Food Forum Workshop The Human Microbiome, Diet, and Health Michelle Rusk, Attorney* Division of Advertising Practices *Staff presentation does
United States District Court
 Case :-cv-0-wha Document Filed 0// Page of IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF CALIFORNIA 0 0 SHANA BECERRA, individually and on behalf of a class of similarly situated persons,
Case :-cv-0-wha Document Filed 0// Page of IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF CALIFORNIA 0 0 SHANA BECERRA, individually and on behalf of a class of similarly situated persons,
Use of Light, Mild, Low, or Similar Descriptors in the Label, Labeling, or Advertising of Tobacco Products
 Guidance for Industry and FDA Staff Use of Light, Mild, Low, or Similar Descriptors in the Label, Labeling, or Advertising of Tobacco Products June 2010 For questions regarding this guidance, contact the
Guidance for Industry and FDA Staff Use of Light, Mild, Low, or Similar Descriptors in the Label, Labeling, or Advertising of Tobacco Products June 2010 For questions regarding this guidance, contact the
Plant Based Milk Labeling
 NatAgLaw@uark.edu (479) 575-7646 www.nationalaglawcenter.org An Agricultural Law Research Publication Plant Based Milk Labeling by Cash Barker Research Fellow, National Agricultural Law Center & Rusty
NatAgLaw@uark.edu (479) 575-7646 www.nationalaglawcenter.org An Agricultural Law Research Publication Plant Based Milk Labeling by Cash Barker Research Fellow, National Agricultural Law Center & Rusty
Raritan Pharmaceuticals, Inc. 6/20/17
 Raritan Pharmaceuticals, Inc. 6/20/17 U.S. Food & Drug Administration Division of Pharmaceutical Quality Operations I New Jersey District 10 Waterview Boulevard, 3rd Floor Parsippany, NJ 07054 June 20,
Raritan Pharmaceuticals, Inc. 6/20/17 U.S. Food & Drug Administration Division of Pharmaceutical Quality Operations I New Jersey District 10 Waterview Boulevard, 3rd Floor Parsippany, NJ 07054 June 20,
TOBACCO PRODUCT OR MEDICAL PRODUCT?
 TOBACCO PRODUCT OR MEDICAL PRODUCT? Priscilla Callahan-Lyon, MD Deputy Director Division of Individual Health Science Office of Science, CTP Grail Sipes, JD Director Office of Regulatory Policy, CDER Disclaimer:
TOBACCO PRODUCT OR MEDICAL PRODUCT? Priscilla Callahan-Lyon, MD Deputy Director Division of Individual Health Science Office of Science, CTP Grail Sipes, JD Director Office of Regulatory Policy, CDER Disclaimer:
Use of Standards in Substantial Equivalence Determinations
 Guidance for Industry and for FDA Staff Use of Standards in Substantial Equivalence Determinations Document issued on: March 12, 2000 U.S. Department Of Health And Human Services Food and Drug Administration
Guidance for Industry and for FDA Staff Use of Standards in Substantial Equivalence Determinations Document issued on: March 12, 2000 U.S. Department Of Health And Human Services Food and Drug Administration
GUIDELINES FOR MAKING PRODUCT CLAIMS
 GUIDELINES FOR MAKING PRODUCT CLAIMS This practical guide to making claims provides valuable information for Independent Business Owners (IBOS) to use in their everyday operations. It describes various
GUIDELINES FOR MAKING PRODUCT CLAIMS This practical guide to making claims provides valuable information for Independent Business Owners (IBOS) to use in their everyday operations. It describes various
Withdrawal of Proposed Rule on Supplemental Applications Proposing Labeling Changes for
 This document is scheduled to be published in the Federal Register on 12/14/2018 and available online at https://federalregister.gov/d/2018-27098, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
This document is scheduled to be published in the Federal Register on 12/14/2018 and available online at https://federalregister.gov/d/2018-27098, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
DEPARTMENT OF TRANSPORTATION. National Highway Traffic Safety Administration. [Docket Number: NHTSA ]
![DEPARTMENT OF TRANSPORTATION. National Highway Traffic Safety Administration. [Docket Number: NHTSA ] DEPARTMENT OF TRANSPORTATION. National Highway Traffic Safety Administration. [Docket Number: NHTSA ]](/thumbs/96/128041132.jpg) This document is scheduled to be published in the Federal Register on 10/04/2018 and available online at https://federalregister.gov/d/2018-21623, and on govinfo.gov DEPARTMENT OF TRANSPORTATION National
This document is scheduled to be published in the Federal Register on 10/04/2018 and available online at https://federalregister.gov/d/2018-21623, and on govinfo.gov DEPARTMENT OF TRANSPORTATION National
SUMMARY: The Food and Drug Administration (FDA) is requesting public input on updated
 This document is scheduled to be published in the Federal Register on 11/22/2017 and available online at https://federalregister.gov/d/2017-25245, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 11/22/2017 and available online at https://federalregister.gov/d/2017-25245, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
RE: Permits under the Arizona Pharmacy Act should not be required for dietary supplements
 Board Members Arizona State Board of Pharmacy 1616 W. Adams St., Suite 120 Phoenix, AZ 85007 c/o Kam Gandhi, PharmD Executive Director Arizona State Board of Pharmacy Via email: kgandhi@azpharmacy.gov
Board Members Arizona State Board of Pharmacy 1616 W. Adams St., Suite 120 Phoenix, AZ 85007 c/o Kam Gandhi, PharmD Executive Director Arizona State Board of Pharmacy Via email: kgandhi@azpharmacy.gov
NARB PANEL #225. Appeal of the NAD Final Decision Regarding Advertising Claims for Neurocore, LLC Neurocore Brain Performance Centers.
 NARB PANEL #225 Appeal of the NAD Final Decision Regarding Advertising Claims for Neurocore, LLC Neurocore Brain Performance Centers Panel Members Jef Richards (Chair) Chair and Professor, Advertising
NARB PANEL #225 Appeal of the NAD Final Decision Regarding Advertising Claims for Neurocore, LLC Neurocore Brain Performance Centers Panel Members Jef Richards (Chair) Chair and Professor, Advertising
Highly Concentrated Caffeine in Dietary Supplements; Guidance for Industry; Availability
 This document is scheduled to be published in the Federal Register on 04/16/2018 and available online at https://federalregister.gov/d/2018-07836, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 04/16/2018 and available online at https://federalregister.gov/d/2018-07836, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Comments in Response to the April 18 Workshop: Now Hear This: Competition, Innovation, and Consumer Protection Issues in Hearing Health Care
 Federal Trade Commission Office of the Secretary 600 Pennsylvania Avenue Washington, DC 20580 RE: Comments in Response to the April 18 Workshop: Now Hear This: Competition, Innovation, and Consumer Protection
Federal Trade Commission Office of the Secretary 600 Pennsylvania Avenue Washington, DC 20580 RE: Comments in Response to the April 18 Workshop: Now Hear This: Competition, Innovation, and Consumer Protection
DEPARTMENT OF VETERANS AFFAIRS SUMMARY: The Department of Veterans Affairs (VA) is amending its medical
 This document is scheduled to be published in the Federal Register on 03/23/2017 and available online at https://federalregister.gov/d/2017-05799, and on FDsys.gov DEPARTMENT OF VETERANS AFFAIRS 8320-01
This document is scheduled to be published in the Federal Register on 03/23/2017 and available online at https://federalregister.gov/d/2017-05799, and on FDsys.gov DEPARTMENT OF VETERANS AFFAIRS 8320-01
TSDR Pharmacy Inc. dba brandmd Skin Care 11/9/17
 TSDR Pharmacy Inc. dba brandmd Skin Care 11/9/17 Division of Pharmaceutical Quality Operations IV 19701 Fairchild, Irvine, CA 92612-2506 Telephone: 949-608-2900 Fax: 949-608-4417 WARNING LETTER VIA SIGNATURE
TSDR Pharmacy Inc. dba brandmd Skin Care 11/9/17 Division of Pharmaceutical Quality Operations IV 19701 Fairchild, Irvine, CA 92612-2506 Telephone: 949-608-2900 Fax: 949-608-4417 WARNING LETTER VIA SIGNATURE
Getting Your Ingredient to Market: Understanding Your Regulatory Options Venable LLP
 Getting Your Ingredient to Market: Understanding Your Regulatory Options 2013 Venable LLP 1 Overview of the Governing Laws 1. The Federal Food, Drug, and Cosmetic Act ("FD&C Act"): Requires foods, cosmetics,
Getting Your Ingredient to Market: Understanding Your Regulatory Options 2013 Venable LLP 1 Overview of the Governing Laws 1. The Federal Food, Drug, and Cosmetic Act ("FD&C Act"): Requires foods, cosmetics,
National Vaccine Injury Compensation Program: Adding the Category of Vaccines. AGENCY: Health Resources and Services Administration (HRSA), HHS.
 This document is scheduled to be published in the Federal Register on 04/04/2018 and available online at https://federalregister.gov/d/2018-06770, and on FDsys.gov Billing Code: 4165-15 DEPARTMENT OF HEALTH
This document is scheduled to be published in the Federal Register on 04/04/2018 and available online at https://federalregister.gov/d/2018-06770, and on FDsys.gov Billing Code: 4165-15 DEPARTMENT OF HEALTH
Health (Tobacco, Nicotine etc. and Care)(Scotland) Bill. Japan Tobacco International (JTI)
 Health (Tobacco, Nicotine etc. and Care)(Scotland) Bill Organisation name Japan Tobacco International (JTI) Japan Tobacco International (JTI) is part of the Japan Tobacco group (JT Group) of companies,
Health (Tobacco, Nicotine etc. and Care)(Scotland) Bill Organisation name Japan Tobacco International (JTI) Japan Tobacco International (JTI) is part of the Japan Tobacco group (JT Group) of companies,
Agency Information Collection Activities; Submission for Office of Management and Budget
 This document is scheduled to be published in the Federal Register on 01/28/2016 and available online at http://federalregister.gov/a/2016-01690, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 01/28/2016 and available online at http://federalregister.gov/a/2016-01690, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Case 1:09-cv WWC -MCC Document 607 Filed 06/11/12 Page 1 of 9 IN THE UNITED STATES DISTRICT COURT FOR THE MIDDLE DISTRICT OF PENNSYLVANIA
 Case 1:09-cv-01685-WWC -MCC Document 607 Filed 06/11/12 Page 1 of 9 IN THE UNITED STATES DISTRICT COURT FOR THE MIDDLE DISTRICT OF PENNSYLVANIA KIMBERLY-CLARK WORLDWIDE, INC., : Plaintiff : v. CIVIL NO.
Case 1:09-cv-01685-WWC -MCC Document 607 Filed 06/11/12 Page 1 of 9 IN THE UNITED STATES DISTRICT COURT FOR THE MIDDLE DISTRICT OF PENNSYLVANIA KIMBERLY-CLARK WORLDWIDE, INC., : Plaintiff : v. CIVIL NO.
Issue: March 2016 PROFESSIONAL STANDARDS AND GUIDANCE FOR ADVERTISING MEDICINES AND PROFESSIONAL SERVICES
 Issue: March 2016 PROFESSIONAL STANDARDS AND GUIDANCE FOR ADVERTISING MEDICINES AND PROFESSIONAL SERVICES PROFESSIONAL STANDARDS AND GUIDANCE FOR ADVERTISING MEDICINES AND PROFESSIONAL SERVICES CONTENTS
Issue: March 2016 PROFESSIONAL STANDARDS AND GUIDANCE FOR ADVERTISING MEDICINES AND PROFESSIONAL SERVICES PROFESSIONAL STANDARDS AND GUIDANCE FOR ADVERTISING MEDICINES AND PROFESSIONAL SERVICES CONTENTS
Guidance for Industry DRAFT GUIDANCE. This guidance document is being distributed for comment purposes only.
 Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry DRAFT GUIDANCE This guidance
MEDICINES, MEDICAL DEVICES, TREATMENTS AND HEALTH
 11 MEDICINES, MEDICAL DEVICES, TREATMENTS AND HEALTH Background The rules in this section are designed to ensure that advertisements that include health claims (please see Section 13 for health claims
11 MEDICINES, MEDICAL DEVICES, TREATMENTS AND HEALTH Background The rules in this section are designed to ensure that advertisements that include health claims (please see Section 13 for health claims
WARNING LETTER. Robert Essner Chairman and Chief Executive Officer Wyeth Pharmaceuticals Inc. P.O. Box 8299 Philadelphia, PA
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Robert Essner Chairman and Chief Executive Officer P.O. Box 8299 Philadelphia,
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Robert Essner Chairman and Chief Executive Officer P.O. Box 8299 Philadelphia,
Case 2:07-cv JLL-JAD Document 196 Filed 09/24/15 Page 1 of 38 PageID: 5812 UNITED STATES DISTRICT COURT DISTRICT OF NEW JERSEY
 Case 2:07-cv-00001-JLL-JAD Document 196 Filed 09/24/15 Page 1 of 38 PageID: 5812 NOT FOR PUBLICATION UNITED STATES DISTRICT COURT DISTRICT OF NEW JERSEY UNITED STATES OF AMERICA, v. Plaintiff, Civil Action
Case 2:07-cv-00001-JLL-JAD Document 196 Filed 09/24/15 Page 1 of 38 PageID: 5812 NOT FOR PUBLICATION UNITED STATES DISTRICT COURT DISTRICT OF NEW JERSEY UNITED STATES OF AMERICA, v. Plaintiff, Civil Action
FDA REGULATION OF DIETARY SUPPLEMENTS AND FOODS
 FDA REGULATION OF DIETARY SUPPLEMENTS AND FOODS Presented by W. Patrick Noonan And Chris Noonan For NUTRITION INDUSTRY ASSOCIATION W. Patrick Noonan, P.C. 21800 Oxnard Street, Suite 840 Woodland Hills,
FDA REGULATION OF DIETARY SUPPLEMENTS AND FOODS Presented by W. Patrick Noonan And Chris Noonan For NUTRITION INDUSTRY ASSOCIATION W. Patrick Noonan, P.C. 21800 Oxnard Street, Suite 840 Woodland Hills,
Mark M. Yacura. Partner
 Mark M. Yacura Partner Mark M. Yacura focuses his practice primarily on FDA legal and regulatory matters. He has practiced in this area for more than 30 years. He represents his clients before administrative
Mark M. Yacura Partner Mark M. Yacura focuses his practice primarily on FDA legal and regulatory matters. He has practiced in this area for more than 30 years. He represents his clients before administrative
Food Labeling: Revision of the Nutrition and Supplement Facts Labels and Serving Sizes of
 This document is scheduled to be published in the Federal Register on 10/02/2017 and available online at https://federalregister.gov/d/2017-21019, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 10/02/2017 and available online at https://federalregister.gov/d/2017-21019, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Problems with the 1906 Act
 Problems with the 1906 Act failed to provide clear-cut meanings and specific means for enforcement insufficient funding for enforcement USDA was responsible for testing, but no standards for foods were
Problems with the 1906 Act failed to provide clear-cut meanings and specific means for enforcement insufficient funding for enforcement USDA was responsible for testing, but no standards for foods were
Town and Country Compounding and Consultation Services, LLC 10/17/17
 Town and Country Compounding and Consultation Services, LLC 10/17/17 Division of Pharmaceutical Quality Operations I 10 Waterview Blvd, 3rd FL Parsippany, NJ 07054 Telephone: (973) 331-4900 FAX: (973)
Town and Country Compounding and Consultation Services, LLC 10/17/17 Division of Pharmaceutical Quality Operations I 10 Waterview Blvd, 3rd FL Parsippany, NJ 07054 Telephone: (973) 331-4900 FAX: (973)
Claims about health in ads for e-cigarettes. CAP and BCAP s regulatory statement
 Claims about health in ads for e-cigarettes CAP and BCAP s regulatory statement Contents 1. Executive Summary... 2 2. Policy background and the decision to consult... 4 3. Decisions... 6 4. Consequence
Claims about health in ads for e-cigarettes CAP and BCAP s regulatory statement Contents 1. Executive Summary... 2 2. Policy background and the decision to consult... 4 3. Decisions... 6 4. Consequence
FDA s Evidence-Based Review System
 Food & Drug January 28, 2009 FDA Releases Final Guidance for Evidence-Based Review System for the Scientific Evaluation of Health Claims On January 16, 2009, the Food and Drug Administration (FDA) announced
Food & Drug January 28, 2009 FDA Releases Final Guidance for Evidence-Based Review System for the Scientific Evaluation of Health Claims On January 16, 2009, the Food and Drug Administration (FDA) announced
Medical Devices. BARBADOS Clarke Gittens Farmer
 Medical Devices BARBADOS Clarke Gittens Farmer CONTACT INFORMATION Danielle Maycock Clarke Gittens Farmer Parker House, Wildey Business Park Wildey Road, St. Michael, Barbados Tel. 246.436.6287 rsm@clarkes.com.bb
Medical Devices BARBADOS Clarke Gittens Farmer CONTACT INFORMATION Danielle Maycock Clarke Gittens Farmer Parker House, Wildey Business Park Wildey Road, St. Michael, Barbados Tel. 246.436.6287 rsm@clarkes.com.bb
HILLSBOROUGH COUNTY AVIATION AUTHORITY AIRPORT BOARD OF ADJUSTMENT RULES OF PROCEDURE
 HILLSBOROUGH COUNTY AVIATION AUTHORITY AIRPORT BOARD OF ADJUSTMENT RULES OF PROCEDURE PURPOSE AND AUTHORITY Adopted May 6, 2010 Revised June 2, 2016 The Hillsborough County Aviation Authority Airport Board
HILLSBOROUGH COUNTY AVIATION AUTHORITY AIRPORT BOARD OF ADJUSTMENT RULES OF PROCEDURE PURPOSE AND AUTHORITY Adopted May 6, 2010 Revised June 2, 2016 The Hillsborough County Aviation Authority Airport Board
perpetuate -- and perhaps even intensify -- that controversy. 1 On July 18th, the Fifth Circuit affirmed FDA s longstanding position that
 Food & Drug July 29, 2008 Fifth Circuit Rules that FDA May Regulate Compounded Drugs as New Drugs Update on Medical Center Pharmacy v. Mukasey For decades, the pharmacy compounding industry has disputed
Food & Drug July 29, 2008 Fifth Circuit Rules that FDA May Regulate Compounded Drugs as New Drugs Update on Medical Center Pharmacy v. Mukasey For decades, the pharmacy compounding industry has disputed
Nestle Infant Nutrition 10/31/14
 U.S. Food and Drug Administration Protecting and Promoting Your Health Nestle Infant Nutrition 10/31/14 OCT 31, 2014 Department of Health and Human Services Public Health Service Food and Drug Administration
U.S. Food and Drug Administration Protecting and Promoting Your Health Nestle Infant Nutrition 10/31/14 OCT 31, 2014 Department of Health and Human Services Public Health Service Food and Drug Administration
Food Additives Permitted for Direct Addition to Food for Human Consumption; Vitamin D 2
 This document is scheduled to be published in the Federal Register on 03/11/2014 and available online at http://federalregister.gov/a/2014-05060, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 03/11/2014 and available online at http://federalregister.gov/a/2014-05060, and on FDsys.gov 4160-01-P DEPARTMENT OF HEALTH AND HUMAN
Inspections, Compliance, Enforcement, and Criminal Investigations
 Page 1 of 6 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations A Nelson & Co.,
Page 1 of 6 Home Inspections, Compliance, Enforcement, and Criminal Investigations Enforcement Actions Warning Letters Inspections, Compliance, Enforcement, and Criminal Investigations A Nelson & Co.,
One generally, applicable definition is "the use of living organisrns to make new products."
 1. What is Biotechnology'? A B One generally, applicable definition is "the use of living organisrns to make new products." Biotechnology is not new. For thousands of years, people have been using, living
1. What is Biotechnology'? A B One generally, applicable definition is "the use of living organisrns to make new products." Biotechnology is not new. For thousands of years, people have been using, living
Structure and Practices of the Video Relay Service Program; Telecommunications Relay Services and
 This document is scheduled to be published in the Federal Register on 03/21/2016 and available online at http://federalregister.gov/a/2016-06305, and on FDsys.gov 6712-01 FEDERAL COMMUNICATIONS COMMISSION
This document is scheduled to be published in the Federal Register on 03/21/2016 and available online at http://federalregister.gov/a/2016-06305, and on FDsys.gov 6712-01 FEDERAL COMMUNICATIONS COMMISSION
Food. [[Page 999]] Part IV. Department of Health and Human Services. Food and Drug Administration. 21 CFR Part 101
![Food. [[Page 999]] Part IV. Department of Health and Human Services. Food and Drug Administration. 21 CFR Part 101 Food. [[Page 999]] Part IV. Department of Health and Human Services. Food and Drug Administration. 21 CFR Part 101](/thumbs/95/126080208.jpg) 1 of 96 12/15/2009 2:32 PM Food Federal Register 65 FR 999 January 6, 1999 -- Regulations on Statements Made for Dietary Supplements Concerning the Effect of the Product on the Structure or Function of
1 of 96 12/15/2009 2:32 PM Food Federal Register 65 FR 999 January 6, 1999 -- Regulations on Statements Made for Dietary Supplements Concerning the Effect of the Product on the Structure or Function of
OUR COMMITMENT OPEN DIALOGUE & DEBATE COMMITMENT TO EDUCATION
 Everyday we make choices and take actions that ensure the scientific-basis and quality of Nature Made vitamins and supplements. As a vitamin and supplement company, for Nature Made transparency means:
Everyday we make choices and take actions that ensure the scientific-basis and quality of Nature Made vitamins and supplements. As a vitamin and supplement company, for Nature Made transparency means:
December 17, 2007 VIA OVERNIGHT DELIVERY & FAX TO
 December 17, 2007 VIA OVERNIGHT DELIVERY & FAX TO 630-598-8663 Ms. Brenda C. Barnes Chairman and Chief Executive Officer Sara Lee Corporation 3500 Lacey Road Downers Grove IL 60515-5424 Dear Chairman Barnes:
December 17, 2007 VIA OVERNIGHT DELIVERY & FAX TO 630-598-8663 Ms. Brenda C. Barnes Chairman and Chief Executive Officer Sara Lee Corporation 3500 Lacey Road Downers Grove IL 60515-5424 Dear Chairman Barnes:
Schedules of Controlled Substances: Temporary Placement of Furanyl Fentanyl. AGENCY: Drug Enforcement Administration, Department of Justice
 This document is scheduled to be published in the Federal Register on 09/27/2016 and available online at https://federalregister.gov/d/2016-23183, and on FDsys.gov Billing Code 4410-09-P DEPARTMENT OF
This document is scheduled to be published in the Federal Register on 09/27/2016 and available online at https://federalregister.gov/d/2016-23183, and on FDsys.gov Billing Code 4410-09-P DEPARTMENT OF
ENFORCEMENT ISSUE IN THIS ISSUE. Food and Drug Law Institute FDLI MEMB ER MAGAZI NE W W W.FDLI.OR G N OV E M B E R/DE C E M B E R 2016
 MEMB ER MAGAZI NE W W W..OR G N OV E M B E R/DE C E M B E R 2016 Food and Drug Law Institute ENFORCEMENT ISSUE IN THIS ISSUE FDCA Strict Liability A Brave New Food Enforcement World New Antitrust Risks
MEMB ER MAGAZI NE W W W..OR G N OV E M B E R/DE C E M B E R 2016 Food and Drug Law Institute ENFORCEMENT ISSUE IN THIS ISSUE FDCA Strict Liability A Brave New Food Enforcement World New Antitrust Risks
United States Court of Appeals
 In the United States Court of Appeals For the Seventh Circuit No. 10-3267 CAROLYN TUREK, v. Plaintiff-Appellant, GENERAL MILLS, INC. and KELLOGG CO., Defendants-Appellees. Appeal from the United States
In the United States Court of Appeals For the Seventh Circuit No. 10-3267 CAROLYN TUREK, v. Plaintiff-Appellant, GENERAL MILLS, INC. and KELLOGG CO., Defendants-Appellees. Appeal from the United States
What Regulators and Other Attorneys Will Not Tell You About FDA, FTC, and Class Action Lawsuits Venable LLP
 What Regulators and Other Attorneys Will Not Tell You About FDA, FTC, and Class Action Lawsuits 2012 Venable LLP 1 Agenda Current Enforcement Trends What is a Claim? Substantiation Hot Topic Claims FDA
What Regulators and Other Attorneys Will Not Tell You About FDA, FTC, and Class Action Lawsuits 2012 Venable LLP 1 Agenda Current Enforcement Trends What is a Claim? Substantiation Hot Topic Claims FDA
Federal Enforcement Regarding Marketing Claims in Light of the 2009 H1N1 Pandemic
 Federal Enforcement Regarding Marketing Claims in Light of the 2009 H1N1 Pandemic James Chen, Jennifer Schlosser, and Jennifer Knight, Crowell & Moring LLP In the wake of the worldwide 2009 H1N1 pandemic,
Federal Enforcement Regarding Marketing Claims in Light of the 2009 H1N1 Pandemic James Chen, Jennifer Schlosser, and Jennifer Knight, Crowell & Moring LLP In the wake of the worldwide 2009 H1N1 pandemic,
Microbiology Devices; Reclassification of Influenza Virus Antigen Detection Test Systems
 This document is scheduled to be published in the Federal Register on 01/12/2017 and available online at https://federalregister.gov/d/2017-00199, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
This document is scheduled to be published in the Federal Register on 01/12/2017 and available online at https://federalregister.gov/d/2017-00199, and on FDsys.gov 4164-01-P DEPARTMENT OF HEALTH AND HUMAN
Subtitle E--National Bioengineered Food Disclosure Standard
 GMO Labeling Bill S.764 as of July 14, 2016* [Congressional Bills 114th Congress] [From the U.S. Government Publishing Office]* [S. 764 Enrolled Bill (ENR)] S.764 One Hundred Fourteenth Congress of the
GMO Labeling Bill S.764 as of July 14, 2016* [Congressional Bills 114th Congress] [From the U.S. Government Publishing Office]* [S. 764 Enrolled Bill (ENR)] S.764 One Hundred Fourteenth Congress of the
Duragesic has the potential for abuse. The Drug Abuse and Dependence section of the PI states, in pertinent part:
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Ajit Shetty, M.D. CEO Janssen Pharmaceutics, Inc. 1125 Trenton-Harbourton
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Rockville, MD 20857 TRANSMITTED BY FACSIMILE Ajit Shetty, M.D. CEO Janssen Pharmaceutics, Inc. 1125 Trenton-Harbourton
MEALEY S TM California Section Report. A commentary article reprinted from the March 2014 issue of Mealey s California Section Report
 MEALEY S TM California Section 17200 Report Pom Wonderful Brings Food Labeling Dispute To The U.S. Supreme Court: When Are Claims Based On Allegedly Improper Product Labeling Barred By The Food, Drug,
MEALEY S TM California Section 17200 Report Pom Wonderful Brings Food Labeling Dispute To The U.S. Supreme Court: When Are Claims Based On Allegedly Improper Product Labeling Barred By The Food, Drug,
WARNING LETTER DEPARTMENT OF HEALTH & HUMAN SERVICES
 DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE David E.I. Pyott Chairman of the Board and Chief Executive Officer
DEPARTMENT OF HEALTH & HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 TRANSMITTED BY FACSIMILE David E.I. Pyott Chairman of the Board and Chief Executive Officer
Guideline for the Sale and Supply of Practitioner Only Products
 Guideline for the Sale and Supply of Practitioner Only Products Edition 1, December 2011 This document provides guidance to the complementary medicine industry on the sale and supply of practitioner only
Guideline for the Sale and Supply of Practitioner Only Products Edition 1, December 2011 This document provides guidance to the complementary medicine industry on the sale and supply of practitioner only
Listing of Color Additives Exempt from Certification; Synthetic Iron Oxide
 This document is scheduled to be published in the Federal Register on 03/20/2015 and available online at http://federalregister.gov/a/2015-06418, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
This document is scheduled to be published in the Federal Register on 03/20/2015 and available online at http://federalregister.gov/a/2015-06418, and on FDsys.gov DEPARTMENT OF HEALTH AND HUMAN SERVICES
UNITED STATES COURT OF APPEALS FOR THE NINTH CIRCUIT
 Case: 17-16242, 01/10/2019, ID: 11147808, DktEntry: 33-1, Page 1 of 14 FOR PUBLICATION UNITED STATES COURT OF APPEALS FOR THE NINTH CIRCUIT PAUL DACHAUER, On Behalf of Himself and All Others Similarly
Case: 17-16242, 01/10/2019, ID: 11147808, DktEntry: 33-1, Page 1 of 14 FOR PUBLICATION UNITED STATES COURT OF APPEALS FOR THE NINTH CIRCUIT PAUL DACHAUER, On Behalf of Himself and All Others Similarly
Re: National Bioengineered Food Disclosure Standard; Proposed Rule; Request for Comments, 83 Fed. Reg (May 4, 2018), Docket No.
 VIA ELECTRONIC SUBMISSION July 3, 2018 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: National Bioengineered Food Disclosure Standard;
VIA ELECTRONIC SUBMISSION July 3, 2018 Dockets Management Staff (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: National Bioengineered Food Disclosure Standard;
21 March JCI Reuse of Single-Use Devices White Paper. Dear and :
 21 March 2018 RE: JCI Reuse of Single-Use Devices White Paper Dear and : I write regarding the Joint Commission International White Paper, Reuse of Single-Use Devices: Understanding Risks and Strategies
21 March 2018 RE: JCI Reuse of Single-Use Devices White Paper Dear and : I write regarding the Joint Commission International White Paper, Reuse of Single-Use Devices: Understanding Risks and Strategies
May 7, Dear Mr. Landa:
 Ralph A. Simmons 202 429 6459 rsimmons@steptoe.com 1330 Connecticut Avenue, NW Washington, DC 20036-1795 202 429 3000 main www.steptoe.com May 7, 2013 Michael M. Landa Director, Center for Food Safety
Ralph A. Simmons 202 429 6459 rsimmons@steptoe.com 1330 Connecticut Avenue, NW Washington, DC 20036-1795 202 429 3000 main www.steptoe.com May 7, 2013 Michael M. Landa Director, Center for Food Safety
PLAN TO REGULATE THE COMPLEMENTARY MEDICINES INDUSTRY IN SOUTH AFRICA
 PLAN TO REGULATE THE COMPLEMENTARY MEDICINES INDUSTRY IN SOUTH AFRICA René Doms FPS Healthcare Regulatory Consultant Dip Pharm Adv Dip (B&A) BIuris LLB South African Registered Pharmacist Fellow of the
PLAN TO REGULATE THE COMPLEMENTARY MEDICINES INDUSTRY IN SOUTH AFRICA René Doms FPS Healthcare Regulatory Consultant Dip Pharm Adv Dip (B&A) BIuris LLB South African Registered Pharmacist Fellow of the
Historical and Contemporary Role of Consumer in the Regulation of Food Labeling. Michael T. Roberts Executive Director
 Historical and Contemporary Role of Consumer in the Regulation of Food Labeling Michael T. Roberts Executive Director UCLA Program Inception: August 1, 2013 Mission: Seeks legal and policy solutions for
Historical and Contemporary Role of Consumer in the Regulation of Food Labeling Michael T. Roberts Executive Director UCLA Program Inception: August 1, 2013 Mission: Seeks legal and policy solutions for
Food Labeling: Policy Rationale IFT Food Policy Impact, 2011
 Food Labeling: Policy Rationale IFT Food Policy Impact, 2011 Barbara O. Schneeman, Ph.D. Office of Nutrition, Labeling and Dietary Supplements CFSAN-FDA General Labeling Provisions FDA s authority to regulate
Food Labeling: Policy Rationale IFT Food Policy Impact, 2011 Barbara O. Schneeman, Ph.D. Office of Nutrition, Labeling and Dietary Supplements CFSAN-FDA General Labeling Provisions FDA s authority to regulate
Centers for Disease Control and Prevention. Final Revised Vaccine Information Materials for MMR
 This document is scheduled to be published in the Federal Register on 03/15/2018 and available online at https://federalregister.gov/d/2018-05299, and on FDsys.gov BILLING CODE: 4163-18-P DEPARTMENT OF
This document is scheduled to be published in the Federal Register on 03/15/2018 and available online at https://federalregister.gov/d/2018-05299, and on FDsys.gov BILLING CODE: 4163-18-P DEPARTMENT OF
Protein Quality and Labeling: Defending Attacks from Regulators, Attorneys and Competitors
 Protein Quality and Labeling: Defending Attacks from Regulators, Attorneys and Competitors May 23, 2018 Justin J. Prochnow prochnowjj@gtlaw.com (303) 572-6562 GREENBERG TRAURIG, LLP ATTORNEYS AT LAW WWW.GTLAW.COM
Protein Quality and Labeling: Defending Attacks from Regulators, Attorneys and Competitors May 23, 2018 Justin J. Prochnow prochnowjj@gtlaw.com (303) 572-6562 GREENBERG TRAURIG, LLP ATTORNEYS AT LAW WWW.GTLAW.COM
Establishment of a New Drug Code for Marihuana Extract. AGENCY: Drug Enforcement Administration, Department of Justice.
 This document is scheduled to be published in the Federal Register on 12/14/2016 and available online at https://federalregister.gov/d/2016-29941, and on FDsys.gov Billing Code 4410-09-P DEPARTMENT OF
This document is scheduled to be published in the Federal Register on 12/14/2016 and available online at https://federalregister.gov/d/2016-29941, and on FDsys.gov Billing Code 4410-09-P DEPARTMENT OF
Welcome to the Supply Network Market Quickstart Program
 Welcome to the Supply Network Market Quickstart Program Exhibitor & Advertising Standards Unique & Free New Hope is the only media company in the natural products industry that has a dedicated Exhibitor
Welcome to the Supply Network Market Quickstart Program Exhibitor & Advertising Standards Unique & Free New Hope is the only media company in the natural products industry that has a dedicated Exhibitor
STATEMENT OF JOSHUA M. SHARFSTEIN, M.D. PRINCIPAL DEPUTY COMMISSIONER U.S. FOOD AND DRUG ADMINISTRATION DEPARTMENT OF HEALTH AND HUMAN SERVICES
 DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 STATEMENT OF JOSHUA M. SHARFSTEIN, M.D. PRINCIPAL DEPUTY COMMISSIONER U.S. FOOD AND DRUG
DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Food and Drug Administration Silver Spring, MD 20993 STATEMENT OF JOSHUA M. SHARFSTEIN, M.D. PRINCIPAL DEPUTY COMMISSIONER U.S. FOOD AND DRUG
51ST LEGISLATURE - STATE OF NEW MEXICO - FIRST SESSION, 2013
 SENATE BILL ST LEGISLATURE - STATE OF NEW MEXICO - FIRST SESSION, 0 INTRODUCED BY Peter Wirth 0 AN ACT RELATING TO COMMERCE; AMENDING AND ENACTING SECTIONS OF THE NEW MEXICO FOOD ACT AND THE COMMERCIAL
SENATE BILL ST LEGISLATURE - STATE OF NEW MEXICO - FIRST SESSION, 0 INTRODUCED BY Peter Wirth 0 AN ACT RELATING TO COMMERCE; AMENDING AND ENACTING SECTIONS OF THE NEW MEXICO FOOD ACT AND THE COMMERCIAL
MEMBERSHIP OF THE COMMISSION ON DIETARY SUPPLEMENT LABELS
 COMMISSION ON DIETARY SUPPLEMENT LABELS November 1997 MEMBERSHIP OF THE COMMISSION ON DIETARY SUPPLEMENT LABELS Chair Malden C. Nesheim, Ph.D. Provost Emeritus Professor of Nutrition Cornell University
COMMISSION ON DIETARY SUPPLEMENT LABELS November 1997 MEMBERSHIP OF THE COMMISSION ON DIETARY SUPPLEMENT LABELS Chair Malden C. Nesheim, Ph.D. Provost Emeritus Professor of Nutrition Cornell University
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION MEMORANDUM OPINION AND ORDER
 Allergan, Inc. v. Teva Pharmaceuticals USA, Inc. et al Doc. 251 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION ALLERGAN, INC., Plaintiff, v. TEVA PHARMACEUTICALS
Allergan, Inc. v. Teva Pharmaceuticals USA, Inc. et al Doc. 251 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF TEXAS MARSHALL DIVISION ALLERGAN, INC., Plaintiff, v. TEVA PHARMACEUTICALS
PROPOSED REGULATION OF THE BOARD OF HEARING AID SPECIALISTS. LCB File No. R July 6, 2001
 PROPOSED REGULATION OF THE BOARD OF HEARING AID SPECIALISTS LCB File No. R062-01 July 6, 2001 EXPLANATION Matter in italics is new; matter in brackets [omitted material] is material to be omitted. AUTHORITY:
PROPOSED REGULATION OF THE BOARD OF HEARING AID SPECIALISTS LCB File No. R062-01 July 6, 2001 EXPLANATION Matter in italics is new; matter in brackets [omitted material] is material to be omitted. AUTHORITY:
FDLI ANNUAL CONFERENCE May 4, 2018
 FDLI ANNUAL CONFERENCE May 4, 2018 DIETARY SUPPLEMENTS RETAILER ISSUES AND LIABILITY Jean Frydman, Partner, Chair FDA Practice Fox Rothschild, LLP FDA Concern is Safety Only DHSEA Act of 1994 Know what
FDLI ANNUAL CONFERENCE May 4, 2018 DIETARY SUPPLEMENTS RETAILER ISSUES AND LIABILITY Jean Frydman, Partner, Chair FDA Practice Fox Rothschild, LLP FDA Concern is Safety Only DHSEA Act of 1994 Know what
