Synoviocytes. Macrophage. B cell C H R O N I C. Neutrophil. Mast cell I N F L A M M A T I O N. Tissue cell. Endothelial cell. Th1/Th17 IL17 IL22
|
|
|
- Amelia Higgins
- 5 years ago
- Views:
Transcription
1
2
3
4 DC IL12, IL23 chemokines, ECM, Co-stimulation IL17, IL22 IFNγ ± Macrophage peptidoglycan lipopolysaccharide heat shock proteins Th1/Th17 IL17 IL22 Cell contact, co-stimulation immune complexes acute phase reactants complement TNFα IL1 IL15 IL18 IL6 IL20 IL32 IL33 RANKL GM-CSF IL10 IL1Ra IL18BP sil1r stnfr IL27 IL35 TGFβ Synoviocytes B cell Neutrophil Mast cell Tissue cell Endothelial cell C H R O N I C I N F L A M M A T I O N
5 Cytokines in the pathogenesis of RA In RA, abnormal interactions between immune cells lead to a cytokine-mediated inflammatory response 1-5 Several cytokines are active in synovial tissues, including pro- and anti-inflammatory cytokines 1-5 The cytokine network can be disrupted, leading to chronic inflammation and tissue damage 1-5 Joint damage Cytokines Key cytokines in the pathogenesis of RA 1 IFNα and IFNß IL-6 IL-7 IL-10 IL-12 IL-15 IL-23 IL-1 IL-17 IL-18 TGF-ß TNF 1. Brennan FM, McInnes IB. J Clin Invest 2008;118: McInnes IB, Schett G. Nat Rev Immunol 2007;7: Murray PJ. J Immunol 2007;178: Smolen JS, Steiner G. Nat Rev Drug Discov 2003;2: Arend WP. Arthritis Care Res 2001;45:
6 Antigenpresenting cells ACTIVATION T-cells Mediators & modulators of inflammation Anti-inflammatory IL-1R antagonist Soluble IL-1R Soluble TNFR IL-10 IL-4 Pro-inflammatory TNF IL-1 IL-1R = interleukin-1 receptor TNFR = tumour necrosis factor receptor Rheumatoid arthritis
7 Rationale for targeting TNF
8 Involvement of TNF in rheumatoid arthritis Activated macrophage TNF
9 S S S S S S S S S S S S C H 3 C H 2 Fc portion of human immunoglobulin G1 Human p75 TNF receptor
10
11
12 DC IL12, IL23 chemokines, ECM, Co-stimulation IL17, IL22 IFNγ ± Macrophage peptidoglycan lipopolysaccharide heat shock proteins Th1/Th17 IL17 IL22 Cell contact, co-stimulation immune complexes acute phase reactants complement TNFα IL1 IL15 IL18 IL6 IL20 IL32 IL33 RANKL GM-CSF IL10 IL1Ra IL18BP sil1r stnfr IL27 IL35 TGFβ Synoviocytes B cell Neutrophil Mast cell Tissue cell Endothelial cell C H R O N I C I N F L A M M A T I O N
13 Cytokines in the pathogenesis of RA In RA, abnormal interactions between immune cells lead to a cytokine-mediated inflammatory response 1-5 Several cytokines are active in synovial tissues, including pro- and anti-inflammatory cytokines 1-5 The cytokine network can be disrupted, leading to chronic inflammation and tissue damage 1-5 Joint damage Cytokines Key cytokines in the pathogenesis of RA 1 IFNα and IFNß IL-6 IL-7 IL-10 IL-12 IL-15 IL-23 IL-1 IL-17 IL-18 TGF-ß TNF 1. Brennan FM, McInnes IB. J Clin Invest 2008;118: McInnes IB, Schett G. Nat Rev Immunol 2007;7: Murray PJ. J Immunol 2007;178: Smolen JS, Steiner G. Nat Rev Drug Discov 2003;2: Arend WP. Arthritis Care Res 2001;45:
14 Antigenpresenting cells ACTIVATION T-cells Mediators & modulators of inflammation Anti-inflammatory IL-1R antagonist Soluble IL-1R Soluble TNFR IL-10 IL-4 Pro-inflammatory TNF IL-1 IL-1R = interleukin-1 receptor TNFR = tumour necrosis factor receptor Rheumatoid arthritis
15 Rationale for targeting TNF
16 Involvement of TNF in rheumatoid arthritis Activated macrophage TNF
17 S S S S S S S S S S S S C H 3 C H 2 Fc portion of human immunoglobulin G1 Human p75 TNF receptor
18
19
20 Subsequent entry biologics in RA: What s next? Health Canada defines a SEB as "a biologic product that is similar to and would enter the market subsequent to an approved innovator biologic product 1 Both the CRA and the ORA have released position statements on SEBs. Issues tackled include: 1,2 Cost and accessibility to safe and effective drugs Traceability and clear nomenclature No automatic interchangeability or substitution with innovator molecules Ongoing assessment and post-marketing surveillance Health Canada approved two infliximab SEBs in January, Ontario Rheumatology Association. Position paper on subsequent entry biologics in Canada. November Available at: 2. Canadian Rheumatology Association. Position paper on the establishment of a Common Drug Review Procedure and Process for reviewing subsequent entry biologics (SEBs). October 4, Available at: 3. GaBI. Subsequent entry biologics approved in Canada. Available at: 22
21 The PCP s Role in Ongoing Management 23
22 24
23
24 A woman on biologic therapy has a son who was exposed to chicken pox after attending a classmate s birthday party. What would you do?
25 Bombardier C, et al. J Rheumatol 2012;39: ; Canadian Immunization Guide (2014). Public Health Agency of Canada, Ottawa. 27
26 Bombardier C, et al. J Rheumatol 2012;39: ; Canadian Immunization Guide (2014). Public Health Agency of Canada, Ottawa; Keystone EC. J Rheumatol 2011;38:
27 British Society for Rheumatology. Vaccinations in the Immunocompromised Person: Guidelines for the Patient taking Immunosuppressants, Steroids and the New Biologic Therapies. January 28, 2002; Canadian Immunization Guide (2014). Public Health Agency of Canada, Ottawa; Keystone EC. J Rheumatol 2011;38:
28 Live vaccines Oral live polio vaccine (OPV) a Suggested alternative Inactivated polio vaccine (IPV) Measles, mumps, rubella (MMR) b Yellow fever Live typhoid vaccine Inactivated vaccine (but only 70% protective) Chickenpox/shingles (Varicella) Bacillus Calmette-Guérin (BCG) a Must not be given to patient OR household contacts b Must not be given to patients; household contacts OK If the patient is travelling to an endemic region, please consult your travel/id expert for further management information British Society for Rheumatology. Vaccinations in the Immunocompromised Person: Guidelines for the Patient taking Immunosuppressants, Steroids and the New Biologic Therapies. January 28, 2002; Canadian Immunization Guide (2014). Public Health Agency of Canada, Ottawa; van Assen S et al. Ann Rheum Dis 2011;70:
29 British Society for Rheumatology. Vaccinations in the Immunocompromised Person: Guidelines for the Patient taking Immunosuppressants, Steroids and the New Biologic Therapies. January 28, 2002; Canadian Immunization Guide (2014). Public Health Agency of Canada, Ottawa; van Assen S et al. Ann Rheum Dis 2011;70:
30 Key recommendations During immunosuppressive therapy 1,2 1. Canadian Immunization Guide (2014). Public Health Agency of Canada, Ottawa; 2. Bombardier C, et al. J Rheumatol 2012;39: ; 3. Lindsey S, et al. ACR Abstracts #1836; ACR 2014, Boston, MA; available at: 32
31 33
32
33 A man on biologic therapy presents with an upper respiratory tract infection. How would you manage his condition?
34 Patients should be screened for TB, hepatitis B virus, and hepatitis C virus prior to initiation of anti-tnf therapy 1. Steering committee, personal communication, September 2014; 2. Enbrel (Etanercept) product monograph. 3. Humira (adalimumab) product monograph. 4. Remicade (infliximab) product monograph. 5. Simponi (Golimumab) product monograph. 36
35
36 A man on biologic therapy is planning to have elective surgery for a bunion. How should his medications be managed?
37 Drug Treatment-free interval prior to surgery based on risk of intra-operative contamination 1 Low-risk surgery Very high-risk surgery Adalimumab (Humira ) ~ 4 weeks ~ 10 weeks Certolizumab (Cimzia ) ~ 4 weeks ~ 10 weeks Etanercept (Enbrel ) ~ 2 weeks ~ 4 weeks Infliximab (Remicade ) ~ 3 weeks ~ 8 weeks Golimumab (Simponi ) ~ 4 weeks ~ 10 weeks 1. Pham T, et al. Joint Bone Spine 2011;78(S1):S109-15; 2. Bombardier C, et al. J Rheumatol 2012;39:
38 Non-biologic DMARD No need to stop Prednisone Stress doses may be ordered perioperatively Also consider Biologic DMARD The type of surgery The risk for infection based on the surgical procedure The patient s general health and risk for infections The half-life of the biologic agent Should be stopped prior to surgery*; restart if no evidence of infection and wound healing is satisfactory *A recent meta-analysis showed that the increased rate of infection in surgical patients treated with anti- TNFs was not improved by stopping anti-tnf before surgery Bombardier C, et al. J Rheumatol 2012;39: ; 2. Saag KG, et al. Arthritis Rheum 2008;59:762-84; 3. Hall J, Schmidt G. Wood L. Principles of Critical Care (2005, 3rd ed). New York: McGraw-Hill Professional; 4. Mabille C, et al. ACR abstract #466; 2014 ACR, Boson, MA; available at: 40
39
40 A woman on biologic therapy would like to start a family. What would you tell her about RA and pregnancy?
41 With proper planning, women with RA can have a successful pregnancy 1 1. Ateka-Barrutia O, Nelson-Piercy C. In J Clin Rheumatol 2012;7:541-58; 2. Barnabe C et al. Int J Rheumatol 2011;345727; 3. Nørgaard M et al. J Int Med 2010;268:329-37; 4. Brouwer J et al. Ann Rheum Dis 2014 May 15 [Epub ahead of print]. 43
42 Drug Biologics FDA Drug Category Pregnancy Safety Breastfeeding Anti-TNF agents 1 * B Safe (1 st and 2 nd trimesters) Safe Certolizumab 1 B Probably safe Probably safe Abatacept 1 C Unknown Unknown (probably safe) Rituximab 1 C Avoid (consider in severe disease) Unknown (probably safe) Tocilizumab 1 C Unknown Unknown (probably safe) Non-biologics Sulfasalazine 1 B Safe Safe Hydroxychloroquine 1 C Safe Safe Tofacitinib 2 C Not listed Not listed Leflunomide 1 X Avoid Avoid Methotrexate 1 X Avoid Avoid *Adalimumab, etanercept, infliximab, golimumab; Supplement with folate 5 mg once daily preconception and throughout pregnancy; Had not received regulatory approval at time of article publication 1. Ateka-Barrutia O, Nelson-Piercy C. In J Clin Rheumatol 2012;7:541-58; 2. U.S. Prescribing Information for Xeljanz. 44
43
44
45 Keystone EC. J Rheumatol 2011;38:
46
Rheumatoid Arthritis and the Changing Canadian Landscape
 Rheumatoid Arthritis and the Changing Canadian Landscape This event is an Accredited Group Learning Activity (Section 1) as defined by the Maintenance of Certification program of The Royal College of Physicians
Rheumatoid Arthritis and the Changing Canadian Landscape This event is an Accredited Group Learning Activity (Section 1) as defined by the Maintenance of Certification program of The Royal College of Physicians
Perioperative Medicine:
 Perioperative Medicine: Management of rheumatologic agents Divya Gollapudi, MD May 2016 Medical Operative Consult Clinic Harborview Medical Center Your patient Ms. L is a 55 year-old F w/ h/o RA who presents
Perioperative Medicine: Management of rheumatologic agents Divya Gollapudi, MD May 2016 Medical Operative Consult Clinic Harborview Medical Center Your patient Ms. L is a 55 year-old F w/ h/o RA who presents
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: golimumab_simponi 8/2013 2/2018 2/2019 3/2018 Description of Procedure or Service Golimumab (Simponi and
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: golimumab_simponi 8/2013 2/2018 2/2019 3/2018 Description of Procedure or Service Golimumab (Simponi and
RHEUMATOID ARTHRITIS DRUGS
 Rheumatology Biologics Criteria from the Exceptional Access Program RHEUMATOID ARTHRITIS DRUGS DRUG NAME BRS REIMBURSED DOSAGE FORM/ STRENGTH Adalimumab Humira 40 mg/0.8 syringe and 40mg/0.8 pen for Anakinra
Rheumatology Biologics Criteria from the Exceptional Access Program RHEUMATOID ARTHRITIS DRUGS DRUG NAME BRS REIMBURSED DOSAGE FORM/ STRENGTH Adalimumab Humira 40 mg/0.8 syringe and 40mg/0.8 pen for Anakinra
Regulatory Status FDA-approved indication: Orencia is a selective T cell costimulation modulator indicated for: (1)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.18 Subject: Orencia Page: 1 of 8 Last Review Date: March 16, 2018 Orencia Description Orencia (abatacept)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.18 Subject: Orencia Page: 1 of 8 Last Review Date: March 16, 2018 Orencia Description Orencia (abatacept)
Cimzia. Cimzia (certolizumab pegol) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Section: Prescription Drugs Effective Date: April 1, 2018 Subject: Cimzia Page: 1 of 5 Last Review
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Section: Prescription Drugs Effective Date: April 1, 2018 Subject: Cimzia Page: 1 of 5 Last Review
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: abatacept_orencia 4/2008 2/2018 2/2019 2/2018 Description of Procedure or Service Abatacept (Orencia ), a
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: abatacept_orencia 4/2008 2/2018 2/2019 2/2018 Description of Procedure or Service Abatacept (Orencia ), a
Regulatory Status FDA- approved indication: Simponi and Simponi ARIA are tumor necrosis factor (TNF) blockers indicated for the treatment of: (2-3)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 9 Last Review Date: March 16, 2018 Simponi / Simponi
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 9 Last Review Date: March 16, 2018 Simponi / Simponi
Guidelines for rheumatology patients on biologic therapy. Rheumatology Department Patient Information Leaflet
 Guidelines for rheumatology patients on biologic therapy Rheumatology Department Patient Information Leaflet Introduction In general, people who have biologic therapy for their rheumatic condition (e.g.
Guidelines for rheumatology patients on biologic therapy Rheumatology Department Patient Information Leaflet Introduction In general, people who have biologic therapy for their rheumatic condition (e.g.
Regulatory Status FDA-approved indication: Orencia is a selective T cell co-stimulation modulator indicated for: (1)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Orencia Page: 1 of 9 Last Review Date: September 20, 2018 Orencia Description Orencia (abatacept)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Orencia Page: 1 of 9 Last Review Date: September 20, 2018 Orencia Description Orencia (abatacept)
Stelara. Stelara (ustekinumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.04 Subject: Stelara Page: 1 of 9 Last Review Date: September 20, 2018 Stelara Description Stelara
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.04 Subject: Stelara Page: 1 of 9 Last Review Date: September 20, 2018 Stelara Description Stelara
Fml Limits. Azathioprine (Imuran) 50mg, 75mg, 100mg - $26.85 Cyclosporine, 25mg, 100mg. $ Leflunomide (Arava) 10mg Tablet - $144.
 MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Rheumatoid Arthritis (RA) P&T DATE: 2/15/2017 CLASS: Rheumatology/Anti-inflammatory Disorders REVIEW HISTORY 2/16, 5/15,
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Rheumatoid Arthritis (RA) P&T DATE: 2/15/2017 CLASS: Rheumatology/Anti-inflammatory Disorders REVIEW HISTORY 2/16, 5/15,
Xeljanz. Xeljanz, Xeljanz XR (tofacitinib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.24 Subject: Xeljanz Page: 1 of 6 Last Review Date: March 16, 2018 Xeljanz Description Xeljanz, Xeljanz
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.24 Subject: Xeljanz Page: 1 of 6 Last Review Date: March 16, 2018 Xeljanz Description Xeljanz, Xeljanz
Cimzia. Cimzia (certolizumab pegol) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Subject: Cimzia Page: 1 of 5 Last Review Date: December 8, 2017 Cimzia Description Cimzia (certolizumab
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Subject: Cimzia Page: 1 of 5 Last Review Date: December 8, 2017 Cimzia Description Cimzia (certolizumab
MEDICATION GUIDE XELJANZ (ZEL JANS ) (tofacitinib)
 MEDICATION GUIDE XELJANZ (ZEL JANS ) (tofacitinib) Read this Medication Guide before you start taking XELJANZ and each time you get a refill. There may be new information. This Medication Guide does not
MEDICATION GUIDE XELJANZ (ZEL JANS ) (tofacitinib) Read this Medication Guide before you start taking XELJANZ and each time you get a refill. There may be new information. This Medication Guide does not
2017 PERIOPERATIVE MEDICINE SYMPOSIUM Peri-operative use of immunosuppression in rheumatology patients
 2017 PERIOPERATIVE MEDICINE SYMPOSIUM Peri-operative use of immunosuppression in rheumatology patients Dr Alberta Hoi Rheumatologist MBBS, FRACP, PhD NEW ERA IN MUSCULOSKELETAL MEDICINE New drugs - Biologics,
2017 PERIOPERATIVE MEDICINE SYMPOSIUM Peri-operative use of immunosuppression in rheumatology patients Dr Alberta Hoi Rheumatologist MBBS, FRACP, PhD NEW ERA IN MUSCULOSKELETAL MEDICINE New drugs - Biologics,
VACCINATIONS AND INFLAMMATORY BOWEL DISEASE
 VACCINATIONS AND INFLAMMATORY BOWEL DISEASE Bob Kizer MD Assistant Professor of Medicine Creighton University School of Medicine CONFLICTS OF INTEREST None 1 AN OPPORTUNITY FOR IMPROVEMENT IBD patients
VACCINATIONS AND INFLAMMATORY BOWEL DISEASE Bob Kizer MD Assistant Professor of Medicine Creighton University School of Medicine CONFLICTS OF INTEREST None 1 AN OPPORTUNITY FOR IMPROVEMENT IBD patients
Psoriatic Arthritis- Second Line Treatments
 Psoriatic Arthritis- Second Line Treatments Second line treatments for Psoriatic Arthritis (PsA) are usually prescribed by a Rheumatologist, Dermatologist, or in a combined clinic where both the Dermatologist
Psoriatic Arthritis- Second Line Treatments Second line treatments for Psoriatic Arthritis (PsA) are usually prescribed by a Rheumatologist, Dermatologist, or in a combined clinic where both the Dermatologist
Cosentyx. Cosentyx (secukinumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.11 Subject: Cosentyx Page: 1 of 7 Last Review Date: September 20, 2018 Cosentyx Description Cosentyx
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.11 Subject: Cosentyx Page: 1 of 7 Last Review Date: September 20, 2018 Cosentyx Description Cosentyx
Immunizations for Children and Teens with Suppressed Immune Systems
 Immunizations for Children and Teens with Suppressed Immune Systems Your child is starting treatment that will suppress the immune system. This will affect how your child s body responds to routine immunizations
Immunizations for Children and Teens with Suppressed Immune Systems Your child is starting treatment that will suppress the immune system. This will affect how your child s body responds to routine immunizations
Anti-Tumor Necrosis Factor (Anti-TNF)
 Anti-Tumor Necrosis Factor (Anti-TNF) 110465 Anti-TNF.indd 1 9/20/16 9:01 AM s About your medicine Anti-tumor necrosis factor (anti-tnf) is a type of medicine called biologic agent that targets substance
Anti-Tumor Necrosis Factor (Anti-TNF) 110465 Anti-TNF.indd 1 9/20/16 9:01 AM s About your medicine Anti-tumor necrosis factor (anti-tnf) is a type of medicine called biologic agent that targets substance
(tofacitinib) are met.
 Xeljanz (tofacitinib) Policy Number: 5.01. 560 Origination: 3/2014 Last Review: 3/2014 Next Review: 3/2015 Policy BCBSKC will provide coverage for Xeljanz (tofacitinib) when it is determined to be medically
Xeljanz (tofacitinib) Policy Number: 5.01. 560 Origination: 3/2014 Last Review: 3/2014 Next Review: 3/2015 Policy BCBSKC will provide coverage for Xeljanz (tofacitinib) when it is determined to be medically
Actemra. Actemra (tocilizumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.12 Subject: Actemra Page: 1 of 13 Last Review Date: September 20, 2018 Actemra Description Actemra
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.12 Subject: Actemra Page: 1 of 13 Last Review Date: September 20, 2018 Actemra Description Actemra
ACTEMRA (tocilizumab)
 Pre - PA Allowance None Prior-Approval Requirements Diagnoses Patient must have ONE of the following: 1. Active Polyarticular Juvenile Idiopathic Arthritis (PJIA) b. Patient has an intolerance or has experienced
Pre - PA Allowance None Prior-Approval Requirements Diagnoses Patient must have ONE of the following: 1. Active Polyarticular Juvenile Idiopathic Arthritis (PJIA) b. Patient has an intolerance or has experienced
Cimzia. Cimzia (certolizumab pegol) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Cimzia Page: 1 of 5 Last Review Date: March 17, 2017 Cimzia Description Cimzia (certolizumab pegol)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Cimzia Page: 1 of 5 Last Review Date: March 17, 2017 Cimzia Description Cimzia (certolizumab pegol)
Otezla. Otezla (apremilast) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Otezla Page: 1 of 5 Last Review Date: March 16, 2018 Otezla Description Otezla (apremilast) Background
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Otezla Page: 1 of 5 Last Review Date: March 16, 2018 Otezla Description Otezla (apremilast) Background
Cell-Mediated Immunity and T Lymphocytes
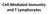 Cell-Mediated Immunity and T Lymphocytes T helper (Th) Cells Peripheral lymhoid tissue thymus Lymphoid stem cell CD8+ CD4+ CD4+ Treg CD8+ CTL + antigen Cytotoxic T lymphocyte CD4+ Th Helper T cell CD4+
Cell-Mediated Immunity and T Lymphocytes T helper (Th) Cells Peripheral lymhoid tissue thymus Lymphoid stem cell CD8+ CD4+ CD4+ Treg CD8+ CTL + antigen Cytotoxic T lymphocyte CD4+ Th Helper T cell CD4+
Psoriatic Arthritis- Secondary Care
 Psoriatic Arthritis- Secondary Care Our Psoriatic Arthritis: First Line Treatments information sheet gives information on the treatments that can be prescribed by a GP, or that might be prescribed if the
Psoriatic Arthritis- Secondary Care Our Psoriatic Arthritis: First Line Treatments information sheet gives information on the treatments that can be prescribed by a GP, or that might be prescribed if the
Regulatory Status FDA- approved indication: Simponi and Simponi ARIA are tumor necrosis factor (TNF) blockers indicated for the treatment of:
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 8 Last Review Date: March 17, 2017 Simponi / Simponi
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 8 Last Review Date: March 17, 2017 Simponi / Simponi
CIMZIA (certolizumab pegol)
 Pre - PA Allowance None Prior-Approval Requirements Age Diagnoses 18 years of age or older Patient must have ONE of the following: 1. Moderate to severe Crohn s Disease (CD) a. Inadequate response, intolerance
Pre - PA Allowance None Prior-Approval Requirements Age Diagnoses 18 years of age or older Patient must have ONE of the following: 1. Moderate to severe Crohn s Disease (CD) a. Inadequate response, intolerance
Rheumatoid Arthritis Update
 Rheumatoid Arthritis Update Beth Valashinas, DO, FACOI, FACR Disclosures Speaker for AbbVie Pharmaceuticals Learning Objectives Upon completion of this session, participants should be able to discuss:
Rheumatoid Arthritis Update Beth Valashinas, DO, FACOI, FACR Disclosures Speaker for AbbVie Pharmaceuticals Learning Objectives Upon completion of this session, participants should be able to discuss:
The GPs role with biologics for immune-mediated inflammatory diseases David Gardner Rheumatologist GPCME 2015
 The GPs role with biologics for immune-mediated inflammatory diseases David Gardner Rheumatologist GPCME 2015 The views and opinions expressed in the following presentation are those of the presenter and
The GPs role with biologics for immune-mediated inflammatory diseases David Gardner Rheumatologist GPCME 2015 The views and opinions expressed in the following presentation are those of the presenter and
MEDICATION GUIDE. (tocilizumab)
 MEDICATION GUIDE ACTEMRA (AC-TEM-RA) (tocilizumab) Read this Medication Guide before you start ACTEMRA and before each infusion. There may be new information. This Medication Guide does not take the place
MEDICATION GUIDE ACTEMRA (AC-TEM-RA) (tocilizumab) Read this Medication Guide before you start ACTEMRA and before each infusion. There may be new information. This Medication Guide does not take the place
Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC
 Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC Update on the Treatment of Rheumatoid Arthritis Sabrina Fallavollita MDCM McGill University Canadian Society of Internal Medicine
Canadian Society of Internal Medicine Annual Meeting 2016 Montreal, QC Update on the Treatment of Rheumatoid Arthritis Sabrina Fallavollita MDCM McGill University Canadian Society of Internal Medicine
Lecture: Rheumatology Update on DMARDs in Common Rheumatologic Diseases for the Family Physician. Sco Jay Harris, DO, FACFR
 Lecture: Rheumatology Update on DMARDs in Common Rheumatologic Diseases for the Family Physician Sco Jay Harris, DO, FACFR UPDATE ON DMARDS, BIOLOGICALS, CYTOKINE INHIBITORS/B CELL DEPLETIONS/CO-STIMULATION
Lecture: Rheumatology Update on DMARDs in Common Rheumatologic Diseases for the Family Physician Sco Jay Harris, DO, FACFR UPDATE ON DMARDS, BIOLOGICALS, CYTOKINE INHIBITORS/B CELL DEPLETIONS/CO-STIMULATION
Corporate Medical Policy
 Corporate Medical Policy Rituximab for the Treatment of Rheumatoid Arthritis File Name: Origination: Last CAP Review: Next CAP Review: Last Review: rituximab_for_the_treatment_of_rheumatoid_arthritis 4/2008
Corporate Medical Policy Rituximab for the Treatment of Rheumatoid Arthritis File Name: Origination: Last CAP Review: Next CAP Review: Last Review: rituximab_for_the_treatment_of_rheumatoid_arthritis 4/2008
Tuberculosis and Biologic Therapies: Risk and Prevention
 Tuberculosis and Biologic Therapies: Risk and Prevention Kevin L. Winthrop, MD, MPH Associate Professor, Divisions of Infectious Diseases, Public Health and Preventive Medicine Oregon Health & Science
Tuberculosis and Biologic Therapies: Risk and Prevention Kevin L. Winthrop, MD, MPH Associate Professor, Divisions of Infectious Diseases, Public Health and Preventive Medicine Oregon Health & Science
Practical RA Treatment: James R. O Dell, M.D. University of Nebraska Medical Center May 24, 2014
 Practical RA Treatment: 2014 James R. O Dell, M.D. University of Nebraska Medical Center May 24, 2014 Disclosures James R. O Dell PI of Multinational RA trial supported by VA and NIH (NIAMS) that receives
Practical RA Treatment: 2014 James R. O Dell, M.D. University of Nebraska Medical Center May 24, 2014 Disclosures James R. O Dell PI of Multinational RA trial supported by VA and NIH (NIAMS) that receives
Treating Rheumatologic Disease in Arizona: Good News, Bad News
 Treating Rheumatologic Disease in Arizona: Good News, Bad News Jeffrey R. Lisse, M.D. Ethel P. McChesney Bilby Professor of Medicine Chief, Section of Rheumatology University of Arizona School of Medicine
Treating Rheumatologic Disease in Arizona: Good News, Bad News Jeffrey R. Lisse, M.D. Ethel P. McChesney Bilby Professor of Medicine Chief, Section of Rheumatology University of Arizona School of Medicine
INFLIXIMAB Remicade (infliximab), Inflectra (infliximab-dyyb), Ixifi* (infliximabqbtx), Renflexis (infliximab-abda)
 Pre - PA Allowance None Prior-Approval Requirements Diagnoses Patient must have ONE of the following: 6 years of age or older 1. Moderate to severe Crohn s disease (CD) a. Patient has fistulizing disease
Pre - PA Allowance None Prior-Approval Requirements Diagnoses Patient must have ONE of the following: 6 years of age or older 1. Moderate to severe Crohn s disease (CD) a. Patient has fistulizing disease
1 P a g e. Systemic Juvenile Idiopathic Arthritis (SJIA) (1.3) Patients 2 years of age and older with active systemic juvenile idiopathic arthritis.
 LENGTH OF AUTHORIZATION: Initial: 3 months for Crohn s or Ulcerative Colitis; 1 year for all other indications. Renewal: 1 year dependent upon medical records supporting response to therapy and review
LENGTH OF AUTHORIZATION: Initial: 3 months for Crohn s or Ulcerative Colitis; 1 year for all other indications. Renewal: 1 year dependent upon medical records supporting response to therapy and review
The Medical Letter. on Drugs and Therapeutics
 The Medical Letter publications are protected by US and international copyright laws. Forwarding, copying or any other distribution of this material is strictly prohibited. For further information call:
The Medical Letter publications are protected by US and international copyright laws. Forwarding, copying or any other distribution of this material is strictly prohibited. For further information call:
Regulatory Status FDA-approved indication: Orencia is a selective T cell costimulation modulator indicated for: (1)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.18 Subject: Orencia Page: 1 of 6 Last Review Date: December 8, 2017 Orencia Description Orencia (abatacept)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.18 Subject: Orencia Page: 1 of 6 Last Review Date: December 8, 2017 Orencia Description Orencia (abatacept)
Simponi / Simponi ARIA (golimumab)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 6 Last Review Date: September 15, 2016 Simponi / Simponi
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 6 Last Review Date: September 15, 2016 Simponi / Simponi
A Clinical Context Report
 Rheumatoid Arthritis in Practice An Expert Commentary with Diane Horowitz, MD A Clinical Context Report Rheumatoid Arthritis in Practice Jointly Sponsored by: and Rheumatoid Arthritis in Practice Expert
Rheumatoid Arthritis in Practice An Expert Commentary with Diane Horowitz, MD A Clinical Context Report Rheumatoid Arthritis in Practice Jointly Sponsored by: and Rheumatoid Arthritis in Practice Expert
Regulatory Status FDA approved indication: Kineret is an interleukin-1 receptor antagonist indicated for: (1)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.50 Subject: Kineret Page: 1 of 5 Last Review Date: March 17, 2017 Kineret Description Kineret (anakinra)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.50 Subject: Kineret Page: 1 of 5 Last Review Date: March 17, 2017 Kineret Description Kineret (anakinra)
Remicade and Friends What You Need to Know Treating the Patient on TNF-alpha Inhibitors and Related Meds
 Remicade and Friends What You Need to Know Treating the Patient on TNF-alpha Inhibitors and Related Meds Scott Stienecker MD FACP FSHEA Medical Director for Epidemiology and Infection Prevention Parkview
Remicade and Friends What You Need to Know Treating the Patient on TNF-alpha Inhibitors and Related Meds Scott Stienecker MD FACP FSHEA Medical Director for Epidemiology and Infection Prevention Parkview
Remicade. Remicade (infliximab), Inflectra (infliximab-dyyb) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.02 Subsection: Gastrointestinal nts Original Policy Date: May 20, 2011 Subject: Remicade Page: 1 of
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.02 Subsection: Gastrointestinal nts Original Policy Date: May 20, 2011 Subject: Remicade Page: 1 of
Center for Evidence-based Policy
 P&T Committee Brief Targeted Immune Modulators: Comparative Drug Class Review Alison Little, MD Center for Evidence-based Policy Oregon Health & Science University 3455 SW US Veterans Hospital Road, SN-4N
P&T Committee Brief Targeted Immune Modulators: Comparative Drug Class Review Alison Little, MD Center for Evidence-based Policy Oregon Health & Science University 3455 SW US Veterans Hospital Road, SN-4N
TOFACITINIB PATIENT INFORMATION ON. (Brand name: Xeljanz ) What is tofacitinib? Important things to remember
 PATIENT INFORMATION ON TOFACITINIB (Brand name: Xeljanz ) This information sheet has been produced by the Australian Rheumatology Association to help you understand the medicine that has been prescribed
PATIENT INFORMATION ON TOFACITINIB (Brand name: Xeljanz ) This information sheet has been produced by the Australian Rheumatology Association to help you understand the medicine that has been prescribed
BACK FIGHT. It s time to. Take action today. Ask your rheumatologist about ORENCIA.
 For moderate to severe Rheumatoid Arthritis (RA) It s time to FIGHT BACK Model, not actual patient. Take action today. Ask your rheumatologist about ORENCIA. What is ORENCIA? ORENCIA (abatacept) is a prescription
For moderate to severe Rheumatoid Arthritis (RA) It s time to FIGHT BACK Model, not actual patient. Take action today. Ask your rheumatologist about ORENCIA. What is ORENCIA? ORENCIA (abatacept) is a prescription
Regulatory Status FDA-approved indications: Entyvio is an α4β7integrin receptor antagonist indicated for: (1)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.12 Subject: Entyvio Page: 1 of 7 Last Review Date: September 20, 2018 Entyvio Description Entyvio
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.12 Subject: Entyvio Page: 1 of 7 Last Review Date: September 20, 2018 Entyvio Description Entyvio
Certolizumab pegol (Cimzia) for psoriatic arthritis second line
 Certolizumab pegol (Cimzia) for psoriatic arthritis second line This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Certolizumab pegol (Cimzia) for psoriatic arthritis second line This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
First Name. Specialty: Fax. First Name DOB: Duration:
 Prescriber Information Last ame: First ame DEA/PI: Specialty: Phone - - Fax - - Member Information Last ame: First ame Member ID umber DOB: - - Medication Information: Drug ame and Strength: Diagnosis:
Prescriber Information Last ame: First ame DEA/PI: Specialty: Phone - - Fax - - Member Information Last ame: First ame Member ID umber DOB: - - Medication Information: Drug ame and Strength: Diagnosis:
Treatment of Rheumatoid Arthritis: The Past, the Present and the Future
 Treatment of Rheumatoid Arthritis: The Past, the Present and the Future Lai-Ling Winchow FCP(SA) Cert Rheum(SA) Chris Hani Baragwanath Academic Hospital University of the Witwatersrand Outline of presentation
Treatment of Rheumatoid Arthritis: The Past, the Present and the Future Lai-Ling Winchow FCP(SA) Cert Rheum(SA) Chris Hani Baragwanath Academic Hospital University of the Witwatersrand Outline of presentation
Pre-talk Questions. Infections and Lupus. Pre-talk Questions. Pre-talk Questions. 1) What is the best treatment for a cold?
 Infections and Lupus 1) What is the best treatment for a cold? a) Chicken soup b) Zinc losenges c) Echinacea d) Antibiotics (such as azithromycin Z-Pak) Donald Thomas, MD, FACP, FACR, CCD Arthritis and
Infections and Lupus 1) What is the best treatment for a cold? a) Chicken soup b) Zinc losenges c) Echinacea d) Antibiotics (such as azithromycin Z-Pak) Donald Thomas, MD, FACP, FACR, CCD Arthritis and
Remicade. Remicade (infliximab), Inflectra (infliximab-dyyb) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Remicade Page: 1 of 9 Last Review Date: June 22, 2017 Remicade Description Remicade (infliximab),
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Remicade Page: 1 of 9 Last Review Date: June 22, 2017 Remicade Description Remicade (infliximab),
Medication Policy Manual. Topic: Xeljanz, tofacitinib Date of Origin: January 21, 2013
 Medication Policy Manual Policy No: dru289 Topic: Xeljanz, tofacitinib Date of Origin: January 21, 2013 Committee Approval Date: January 19, 2015 Next Review Date: January 2016 Effective Date: April 1,
Medication Policy Manual Policy No: dru289 Topic: Xeljanz, tofacitinib Date of Origin: January 21, 2013 Committee Approval Date: January 19, 2015 Next Review Date: January 2016 Effective Date: April 1,
Clinical Policy: Anakinra (Kineret) Reference Number: ERX.SPA.135 Effective Date:
 Clinical Policy: (Kineret) Reference Number: ERX.SPA.135 Effective Date: 10.01.16 Last Review Date: 05.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Kineret) Reference Number: ERX.SPA.135 Effective Date: 10.01.16 Last Review Date: 05.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Rheumatology Therapeutics: Perioperative DMARD Management, Infection and Malignancy Risks, Vaccination Considerations. Heather Hansen, MD
 Rheumatology Therapeutics: Perioperative DMARD Management, Infection and Malignancy Risks, Vaccination Considerations Heather Hansen, MD Objectives Know Broad Categories of Rheumatoid Arthritis Medications
Rheumatology Therapeutics: Perioperative DMARD Management, Infection and Malignancy Risks, Vaccination Considerations Heather Hansen, MD Objectives Know Broad Categories of Rheumatoid Arthritis Medications
Steps TO HELP YOU TALK WITH YOUR DOCTOR ABOUT YOUR TREATMENT OPTIONS
 Moderate to Severe Rheumatoid Arthritis (RA) 3 Steps TO HELP YOU TALK WITH YOUR DOCTOR ABOUT YOUR TREATMENT OPTIONS INDICATION/USAGE Adult Rheumatoid Arthritis (RA): ORENCIA (abatacept) is a prescription
Moderate to Severe Rheumatoid Arthritis (RA) 3 Steps TO HELP YOU TALK WITH YOUR DOCTOR ABOUT YOUR TREATMENT OPTIONS INDICATION/USAGE Adult Rheumatoid Arthritis (RA): ORENCIA (abatacept) is a prescription
Get an Insurance Benefits Review for ORENCIA (abatacept)
 Get an Insurance Benefits Review for ORENCIA (abatacept) If you and your doctor decide that ORENCIA is right for you, you can call 1-800-ORENCIA (1-800-673-6242) to do an Insurance Benefits Review. Our
Get an Insurance Benefits Review for ORENCIA (abatacept) If you and your doctor decide that ORENCIA is right for you, you can call 1-800-ORENCIA (1-800-673-6242) to do an Insurance Benefits Review. Our
Rheumatology for the Internist 2017 Dr. Mark Matsos Associate Professor of Medicine Division of Rheumatology McMaster University
 Canadian Society of Internal Medicine Annual Meeting 2017 Toronto, ON Rheumatology for the Internist 2017 Dr. Mark Matsos Associate Professor of Medicine Division of Rheumatology McMaster University CSIM
Canadian Society of Internal Medicine Annual Meeting 2017 Toronto, ON Rheumatology for the Internist 2017 Dr. Mark Matsos Associate Professor of Medicine Division of Rheumatology McMaster University CSIM
Drug Class Prior Authorization Criteria Therapeutic Agents in Rheumatic and Inflammatory Diseases
 Drug Class Prior Authorization Criteria Therapeutic Agents in Rheumatic and Inflammatory Diseases Line of Business: Medicaid P & T Approval Date: August 16, 2017 Effective Date: August 16, 2017 This policy
Drug Class Prior Authorization Criteria Therapeutic Agents in Rheumatic and Inflammatory Diseases Line of Business: Medicaid P & T Approval Date: August 16, 2017 Effective Date: August 16, 2017 This policy
By: For: Division responsible for document: Key words: Name and job title of document author: Name and job title of document author s Line Manager:
 For Use in: By: For: Guidelines for the Management of Interruption of Biologic Therapies for Division responsible for document: Key words: Name and job title of document author: Name and job title of document
For Use in: By: For: Guidelines for the Management of Interruption of Biologic Therapies for Division responsible for document: Key words: Name and job title of document author: Name and job title of document
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2018 P 1017-7 Program Prior Authorization/Notification Medication Cimzia (certolizumab) P&T Approval Date 1/2007, 6/2008, 4/2009, 6/2009,
UnitedHealthcare Pharmacy Clinical Pharmacy Programs Program Number 2018 P 1017-7 Program Prior Authorization/Notification Medication Cimzia (certolizumab) P&T Approval Date 1/2007, 6/2008, 4/2009, 6/2009,
Clinical Policy: Certolizumab (Cimzia) Reference Number: PA.CP.PHAR.247 Effective Date: 01/18 Last Review Date: 08/17 Line of Business: Medicaid
 Clinical Policy: (Cimzia) Reference Number: PA.CP.PHAR.247 Effective Date: 01/18 Last Review Date: 08/17 Line of Business: Medicaid Coding Implications Revision Log Description (Cimzia ) is a tumor necrosis
Clinical Policy: (Cimzia) Reference Number: PA.CP.PHAR.247 Effective Date: 01/18 Last Review Date: 08/17 Line of Business: Medicaid Coding Implications Revision Log Description (Cimzia ) is a tumor necrosis
Actemra (tocilizumab) CG-DRUG-81
 Market DC Actemra (tocilizumab) CG-DRUG-81 Override(s) Prior Authorization Approval Duration 1 year Medications Line of Business Quantity Limit Actemra (tocilizumab) vials VA MCD and All L-AGP May be subject
Market DC Actemra (tocilizumab) CG-DRUG-81 Override(s) Prior Authorization Approval Duration 1 year Medications Line of Business Quantity Limit Actemra (tocilizumab) vials VA MCD and All L-AGP May be subject
Biologics for Autoimmune Diseases
 Biologics for Autoimmune Diseases Goal(s): Restrict use of biologics to OHP funded conditions and according to OHP guidelines for use. Promote use that is consistent with national clinical practice guidelines
Biologics for Autoimmune Diseases Goal(s): Restrict use of biologics to OHP funded conditions and according to OHP guidelines for use. Promote use that is consistent with national clinical practice guidelines
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 Program Number 2017 P 3041-8 Program Step Therapy Medications UnitedHealthcare Pharmacy Clinical Pharmacy Programs *Orencia (abatacept) *This step criteria refers to the subcutaneous formulation of abatacept
Program Number 2017 P 3041-8 Program Step Therapy Medications UnitedHealthcare Pharmacy Clinical Pharmacy Programs *Orencia (abatacept) *This step criteria refers to the subcutaneous formulation of abatacept
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other GOLIMUMAB SIMPONI 22533, 22536, 34697, 35001 ROUTE = SUBCUTANE. GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the request for a
Generic Brand HICL GCN Exception/Other GOLIMUMAB SIMPONI 22533, 22536, 34697, 35001 ROUTE = SUBCUTANE. GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the request for a
Carelirst.+.V Family of health care plans
 Carelirst.+.V Family of health care plans CVS Caremark POLICY Document for ACTEMRA The overall objective of this policy is to support the appropriate and cost effective use of the medication, specific
Carelirst.+.V Family of health care plans CVS Caremark POLICY Document for ACTEMRA The overall objective of this policy is to support the appropriate and cost effective use of the medication, specific
Stelara. Stelara (ustekinumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.04 Subject: Stelara Page: 1 of 6 Last Review Date: December 8, 2017 Stelara Description Stelara (ustekinumab)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.04 Subject: Stelara Page: 1 of 6 Last Review Date: December 8, 2017 Stelara Description Stelara (ustekinumab)
Immunizing Immunocompromised Adults
 Immunizing Immunocompromised Adults Deepali Kumar MD MSc FRCPC Associate Professor of Medicine University of Toronto / University Health Network, Toronto, Canada Disclosure Industry Funding: GSK, Roche
Immunizing Immunocompromised Adults Deepali Kumar MD MSc FRCPC Associate Professor of Medicine University of Toronto / University Health Network, Toronto, Canada Disclosure Industry Funding: GSK, Roche
INFLIXIMAB Remicade (infliximab), Inflectra (infliximab-dyyb), Renflexis (infliximab-abda)
 RATIONALE FOR INCLUSION IN PA PROGRAM Background Remicade, Renflexis and Inflectra are tumor necrosis factor (TNFα) blockers. Tumor necrosis factor is an endogenous protein that regulates a number of physiologic
RATIONALE FOR INCLUSION IN PA PROGRAM Background Remicade, Renflexis and Inflectra are tumor necrosis factor (TNFα) blockers. Tumor necrosis factor is an endogenous protein that regulates a number of physiologic
Preventive Care and Monitoring of the IBD Patient
 Preventive Care and Monitoring of the IBD Patient Francis A. Farraye, MD, MSc, FACG Clinical Director, Section of Gastroenterology Director, Inflammatory Bowel Disease Center Boston Medical Center Professor
Preventive Care and Monitoring of the IBD Patient Francis A. Farraye, MD, MSc, FACG Clinical Director, Section of Gastroenterology Director, Inflammatory Bowel Disease Center Boston Medical Center Professor
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Otezla (apremilast) Page 1 of 7 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Otezla (apremilast) Prime Therapeutics will review Prior Authorization requests Prior
Otezla (apremilast) Page 1 of 7 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Otezla (apremilast) Prime Therapeutics will review Prior Authorization requests Prior
Technology appraisal guidance Published: 11 October 2017 nice.org.uk/guidance/ta480
 Tofacitinib for moderate to severeere rheumatoid arthritis Technology appraisal guidance Published: 11 October 2017 nice.org.uk/guidance/ta480 NICE 2018. All rights reserved. Subject to Notice of rights
Tofacitinib for moderate to severeere rheumatoid arthritis Technology appraisal guidance Published: 11 October 2017 nice.org.uk/guidance/ta480 NICE 2018. All rights reserved. Subject to Notice of rights
Xeljanz (tofacitinib), Xeljanz XR (tofacitinib extended-release)
 Market DC Xeljanz (tofacitinib), Xeljanz XR (tofacitinib extended-release) Override(s) Prior Authorization Quantity Limit Medications Xeljanz (tofacitinib) Approval Duration 1 year Quantity Limit May be
Market DC Xeljanz (tofacitinib), Xeljanz XR (tofacitinib extended-release) Override(s) Prior Authorization Quantity Limit Medications Xeljanz (tofacitinib) Approval Duration 1 year Quantity Limit May be
Golimumab: In Combination with Methotrexate as Once Monthly Treatment for Moderate to Severe Rheumatoid Arthritis
 Clinical Medicine Reviews in Therapeutics Review Golimumab: In Combination with Methotrexate as Once Monthly Treatment for Moderate to Severe Rheumatoid Arthritis Lauren Keyser McCluggage 1 and Kelly Michelle
Clinical Medicine Reviews in Therapeutics Review Golimumab: In Combination with Methotrexate as Once Monthly Treatment for Moderate to Severe Rheumatoid Arthritis Lauren Keyser McCluggage 1 and Kelly Michelle
Autoimmune Diseases. Betsy Kirchner CNP The Cleveland Clinic
 Autoimmune Diseases Betsy Kirchner CNP The Cleveland Clinic Disclosures (financial) No relevant disclosures Learning Objectives Explain the pathophysiology of autoimmune disease Discuss safe administration
Autoimmune Diseases Betsy Kirchner CNP The Cleveland Clinic Disclosures (financial) No relevant disclosures Learning Objectives Explain the pathophysiology of autoimmune disease Discuss safe administration
KEEP UP THE FIGHT WITH. Little Victories
 FOR MODERATE TO SEVERE RHEUMATOID ARTHRITIS (RA) KEEP UP THE FIGHT WITH Little Victories Small improvements in your daily activities may be a sign that ORENCIA (abatacept) is working for you. Use this
FOR MODERATE TO SEVERE RHEUMATOID ARTHRITIS (RA) KEEP UP THE FIGHT WITH Little Victories Small improvements in your daily activities may be a sign that ORENCIA (abatacept) is working for you. Use this
Technology appraisal guidance Published: 26 January 2016 nice.org.uk/guidance/ta375
 Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed Technology
Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed Technology
Drugs and Applicable Coding: J-code: Enbrel-J1438; Humira-J0135; Remicade-J1745; Inflectra-Q5102; Cimzia-J0718; Simponi-J1602 Renflexis - pending
 Policy Subject: Anti-TNF Agents Policy Number: SHS PBD16 Category: Rheumatology & Autoimmune Policy Type: Medical Pharmacy Department: Pharmacy Product (check all that apply): Group HMO/POS Individual
Policy Subject: Anti-TNF Agents Policy Number: SHS PBD16 Category: Rheumatology & Autoimmune Policy Type: Medical Pharmacy Department: Pharmacy Product (check all that apply): Group HMO/POS Individual
Regulatory Status FDA-approved indication: Enbrel and Erelzi are tumor necrosis factor (TNF) blockers indicated for the treatment of: (2-3)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.27 Subject: Enbrel Page: 1 of 8 Last Review Date: March 16, 2018 Enbrel Description Enbrel (etanercept),
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.27 Subject: Enbrel Page: 1 of 8 Last Review Date: March 16, 2018 Enbrel Description Enbrel (etanercept),
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,700 108,500 1.7 M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,700 108,500 1.7 M Open access books available International authors and editors Downloads Our
Medication Policy Manual. Topic: Otezla, apremilast Date of Origin: May 9, 2014
 Medication Policy Manual Policy No: dru342 Topic: Otezla, apremilast Date of Origin: May 9, 2014 Committee Approval Date: January 19, 2015 Next Review Date: January 2016 Effective Date: April 1, 2015 IMPORTANT
Medication Policy Manual Policy No: dru342 Topic: Otezla, apremilast Date of Origin: May 9, 2014 Committee Approval Date: January 19, 2015 Next Review Date: January 2016 Effective Date: April 1, 2015 IMPORTANT
For Rheumatoid Arthritis
 For Rheumatoid Arthritis APRIL 2017 NOTICE: On April 14, 2017 the FDA issued a complete response letter for baricitinib indicating that the FDA is unable to approve the application in its current form
For Rheumatoid Arthritis APRIL 2017 NOTICE: On April 14, 2017 the FDA issued a complete response letter for baricitinib indicating that the FDA is unable to approve the application in its current form
Report on New Patented Drugs - Orencia
 Report on New Patented Drugs - Orencia Under its transparency initiative, the PMPRB publishes the results of the reviews of new patented drugs by Board Staff, for purposes of applying the Board s Excessive
Report on New Patented Drugs - Orencia Under its transparency initiative, the PMPRB publishes the results of the reviews of new patented drugs by Board Staff, for purposes of applying the Board s Excessive
Although this presentation includes information regarding pharmaceuticals (including products under development), the information is not intended as
 Although this presentation includes information regarding pharmaceuticals (including products under development), the information is not intended as any advertisement and/or medical advice. Forward-Looking
Although this presentation includes information regarding pharmaceuticals (including products under development), the information is not intended as any advertisement and/or medical advice. Forward-Looking
Dr. Lyubomir Marinchev Chief of Rheumatology Department, MHAT SOFIAMED, Sofia, Bulgaria
 Dr. Lyubomir Marinchev Chief of Rheumatology Department, MHAT SOFIAMED, Sofia, Bulgaria Inter-Balkan meeting Open the frontiers and exchange of experiences, 27 th April 2013, Rhodes, Greece Patients with
Dr. Lyubomir Marinchev Chief of Rheumatology Department, MHAT SOFIAMED, Sofia, Bulgaria Inter-Balkan meeting Open the frontiers and exchange of experiences, 27 th April 2013, Rhodes, Greece Patients with
Common Drug Review Pharmacoeconomic Review Report
 Common Drug Review Pharmacoeconomic Review Report August 2015 Drug tocilizumab (Actemra) (162 mg/0.9 ml solution for subcutaneous injection) Indication Adult patients with moderately to severely active
Common Drug Review Pharmacoeconomic Review Report August 2015 Drug tocilizumab (Actemra) (162 mg/0.9 ml solution for subcutaneous injection) Indication Adult patients with moderately to severely active
The ORENCIA (abatacept) JIA Observational Registry
 Moderate to Severe Juvenile Idiopathic Arthritis (JIA) Injection for Intravenous Use Injection for Subcutaneous Use The ORENCIA (abatacept) JIA Observational Registry Injection for Intravenous Use Injection
Moderate to Severe Juvenile Idiopathic Arthritis (JIA) Injection for Intravenous Use Injection for Subcutaneous Use The ORENCIA (abatacept) JIA Observational Registry Injection for Intravenous Use Injection
HARVARD PILGRIM HEALTH CARE RECOMMENDED MEDICATION REQUEST GUIDELINES
 Generic Brand HICL GCN Exception/Other CERTOLIZUMAB PEGOL CIMZIA 35554 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the request for a patient with a diagnosis of moderate
Generic Brand HICL GCN Exception/Other CERTOLIZUMAB PEGOL CIMZIA 35554 GUIDELINES FOR USE INITIAL CRITERIA (NOTE: FOR RENEWAL CRITERIA SEE BELOW) 1. Is the request for a patient with a diagnosis of moderate
We will review the need for continuing treatment with you after 3 months and again after one year.
 Patient Information Drugs for Inflammatory Bowel Disease Adalimumab (Humira) Clinical Nurse Specialist - Allyson Lewis Clinical Nurse Specialist Gareth Lloyd-Ford Clinical Nurse Specialist Lynsey Hook
Patient Information Drugs for Inflammatory Bowel Disease Adalimumab (Humira) Clinical Nurse Specialist - Allyson Lewis Clinical Nurse Specialist Gareth Lloyd-Ford Clinical Nurse Specialist Lynsey Hook
Traveling with Inflammatory Bowel Disease. Suresh Pola MD Inflammatory Bowel Disease Center UC San Diego Health System
 Traveling with Inflammatory Bowel Disease Suresh Pola MD Inflammatory Bowel Disease Center UC San Diego Health System Overview What You Need to Do Now Pre-Travel Preparation While You Are Away When You
Traveling with Inflammatory Bowel Disease Suresh Pola MD Inflammatory Bowel Disease Center UC San Diego Health System Overview What You Need to Do Now Pre-Travel Preparation While You Are Away When You
Regulatory Status FDA-approved indication: Humira and Amjevita are tumor necrosis factor (TNF) blockers indicated for the treatment of: (2-3)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.29 Subject: Humira Page: 1 of 10 Last Review Date: June 22, 2017 Humira Description Humira (adalimumab),
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.29 Subject: Humira Page: 1 of 10 Last Review Date: June 22, 2017 Humira Description Humira (adalimumab),
Remicade (infliximab), Inflectra (infliximab-dyyb), Renflexis (infliximababda)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.02 Subject: Infliximab Page: 1 of 13 Last Review Date: December 8, 2017 Infliximab Description Remicade
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.02 Subject: Infliximab Page: 1 of 13 Last Review Date: December 8, 2017 Infliximab Description Remicade
