NICE-approved drugs available for use within the Trust
|
|
|
- Vernon Bryan Griffith
- 5 years ago
- Views:
Transcription
1 NICE-approved drugs available for use within the Trust The following drugs have been recommended by NICE as treatment options for the indications noted below. They are listed in the Trust Medicines Formulary but are not routinely stocked in pharmacy. These drugs will be ordered if authorised by the Trust DTC Chairman/High Cost Drugs process for use within the specific criteria set by NICE. Drug NICE-approved use NICE TA link Abatacept Abatacept (Orencia), adalimumab (Humira), etanercept (Enbrel) tocilizumab (RoActemra) are recommended as possible treatments for people with *Shared Care on behalf of Specialist polyarticular juvenile idiopathic arthritis. Paediatric Rheumatology Centres Only* Adalimumab etanercept are recommended as possible treatments for people with enthesitis-related juvenile idiopathic arthritis. Adalimumab Adalimumab Atezolizumab Etanercept is recommended as a possible treatment for people with psoriatic juvenile idiopathic arthritis. Infliximab (also known as Remicade, Inflectra or Remsima), adalimumab (Humira) golimumab (Simponi) are recommended. They are possible treatments for adults with moderate to severe ulcerative colitis if conventional therapy hasn t worked or isn t suitable. Infliximab is also recommended as a possible treatment for children or young people aged 6 17 years with severe ulcerative colitis, if conventional therapy hasn t worked or isn t suitable. People should be able to have the treatment for at least 12 months, unless it stops working well enough. Their condition should be assessed at least every 12 months. Their doctor should discuss with them the benefits risks of continuing or stopping treatment. If treatment is stopped the ulcerative colitis gets worse, people should be able to start treatment again. Abatacept (Orencia), adalimumab (Humira), etanercept (Enbrel) tocilizumab (RoActemra) are recommended as possible treatments for people with polyarticular juvenile idiopathic arthritis. Adalimumab etanercept are recommended as possible treatments for people with enthesitis-related juvenile idiopathic arthritis. Etanercept is recommended as a possible treatment for people with psoriatic juvenile idiopathic arthritis. 1.1 Atezolizumab is recommended as an option for treating locally advanced or metastatic non-small-cell lung cancer (NSCLC) in adults who have had chemotherapy ( targeted treatment if they have an EGFR- or ALK positive tumour), only if: *Shared Care on behalf of Specialist Paediatric Rheumatology Centres Only* atezolizumab is stopped at 2 years of uninterrupted treatment or earlier if the disease progresses
2 the company provides atezolizumab with the discount agreed in the patient access scheme. Azacitidine Baricitinib 1.2 This recommendation is not intended to affect treatment with atezolizumab that was started in the NHS before this guidance was published. People having treatment outside this recommendation may continue without change to the funding arrangements in place for them before this guidance was published, until they their NHS clinician consider it Please Note: this drug would be restricted to Royal Surrey County Hospital Oncology Patients treated at ASPH Only Azacitidine is recommended as a treatment option for adults who are not eligible for haematopoietic stem cell transplantation have: intermediate-2 high-risk myelodysplastic syndromes according to the International Prognostic Scoring System (IPSS) or chronic myelomonocytic leukaemia with 10 29% marrow blasts without myeloproliferative disorder or acute myeloid leukaemia with 20 30% blasts multilineage dysplasia, according to the World Health Organization classification if the manufacturer provides azacitidine with the discount agreed as part of the patient access scheme. 1.1 Baricitinib, with methotrexate, is recommended as an option for treating active rheumatoid arthritis in adults whose disease has responded inadequately to intensive therapy with a combination of conventional disease-modifying antirheumatic drugs (DMARDs), only if: disease is severe (a disease activity score [DAS28] of more than 5.1) the company provides baricitinib with the discount agreed in the patient access scheme. 1.2 Baricitinib, with methotrexate, is recommended as an option for treating active rheumatoid arthritis in adults whose disease has responded inadequately to or who cannot have other DMARDs, including at least 1 biological DMARD, only if: disease is severe (a DAS28 of more than 5.1) they cannot have rituximab the company provides baricitinib with the discount agreed in the patient access scheme. 1.3 Baricitinib can be used as monotherapy for people who cannot take methotrexate because it is contraindicated or because of intolerance, when the criteria in sections are met. 1.4 Continue treatment only if there is a moderate response measured using European League Against
3 Bivalirudin Canagliflozin Canagliflozin Ceritinib Rheumatism (EULAR) criteria at 6 months after starting therapy. After an initial response within 6 months, withdraw treatment if at least a moderate EULAR response is not maintained. 1.5 These recommendations are not intended to affect treatment with baricitinib that was started in the NHS before this guidance was published. People having treatment outside these recommendations may continue without change to the funding arrangements in place for them before this guidance was published, until they their NHS clinician consider it Bivalirudin in combination with aspirin clopidogrel is recommended for the treatment of adults with STsegment-elevation myocardial infarction undergoing primary percutaneous coronary intervention. If a person needs to take 2 antidiabetic drugs, canagliflozin is recommended as a possible treatment for people with type 2 diabetes when taken with a drug called metformin, only if the person: cannot take a type of drug called a sulfonylurea or is at significant risk of hypoglycaemia or its consequences. If a person needs to take 3 antidiabetic drugs, canagliflozin is recommended as a possible treatment when taken with either metformin a sulfonylurea, or metformin a type of drug called a thiazolidinedione. Canagliflozin is recommended as a possible treatment taken with insulin, with or without other antidiabetic drugs. Canagliflozin, dapagliflozin empagliflozin as monotherapies are recommended as options for treating type 2 diabetes in adults for whom metformin is contraindicated or not tolerated when diet exercise alone do not provide adequate glycaemic control, only if: a dipeptidyl peptidase 4 (DPP 4) inhibitor would otherwise be prescribed a sulfonylurea or pioglitazone is not appropriate. Adults whose treatment with canagliflozin, dapagliflozin or empagliflozin as monotherapy is not recommended in this NICE guidance, but was started within the NHS before this guidance was published, should be able to continue treatment until they their NHS clinician consider it Ceritinib is recommended, within its marketing authorisation, as an option for treating advanced anaplastic lymphoma kinase positive non small cell lung cancer in adults who have previously had crizotinib. The drug is recommended only if the company provides it with the discount agreed in the patient access scheme
4 Ciclosporin Cinacalcet Daclizumab Dronedarone Please Note: this drug would be restricted to Royal Surrey County Hospital Oncology Patients treated at ASPH Only Ciclosporin (Ikervis) is recommended as a possible treatment for people with dry eye disease that has not improved despite treatment with artificial tears. Cinacalcet is not recommended for the routine treatment of secondary hyperparathyroidism in patients with end-stage renal disease on maintenance dialysis therapy. Cinacalcet is recommended for the treatment of refractory secondary hyperparathyroidism in patients with end-stage renal disease (including those with calciphylaxis) only in those: who have 'very uncontrolled' plasma levels of intact parathyroid hormone (defined as greater than 85 pmol/litre [800 pg/ml]) that are refractory to stard therapy, a normal or high adjusted serum calcium level in whom surgical parathyroidectomy is contraindicated, in that the risks of surgery are considered to outweigh the benefits. Response to treatment should be monitored regularly treatment should be continued only if a reduction in the plasma levels of intact parathyroid hormone of 30% or more is seen within 4 months of treatment, including dose escalation as appropriate. 1.1 Daclizumab is recommended as an option for treating multiple sclerosis in adults, only if: the person has active relapsing remitting multiple sclerosis previously treated with disease-modifying therapy, or rapidly evolving severe relapsing remitting multiple sclerosis (that is, at least 2 relapses in the previous year at least 1 gadolinium-enhancing lesion at baseline MRI) alemtuzumab is contraindicated or otherwise unsuitable the company provides the drug with the discount agreed in the patient access scheme. 1.2 This guidance is not intended to affect the position of patients whose treatment with daclizumab was started within the NHS before this guidance was published. Treatment of those patients may continue without change to whatever funding arrangements were in place for them before this guidance was published until they their NHS clinician consider it Dronedarone is recommended as an option for the treatment of non-permanent atrial fibrillation only in people: whose atrial fibrillation is not controlled by first-line therapy (usually including beta-blockers), that is, as a second-line treatment option
5 Eluxadoline who have at least one of the following cardiovascular risk factors: hypertension requiring drugs of at least two different classes diabetes mellitus previous transient ischaemic attack, stroke or systemic embolism left atrial diameter of 50 mm or greater left ventricular ejection fraction less than 40% (noting that the summary of product characteristics [SPC] does not recommend dronedarone for people with left ventricular ejection fraction less than 35% because of limited experience of using it in this group) or age 70 years or older who do not have unstable New York Heart Association (NYHA) class III or IV heart failure. People who do not meet the above criteria who are currently receiving dronedarone should have the option to continue treatment until they their clinicians consider it 1.1 Eluxadoline is recommended as an option for treating irritable bowel syndrome with diarrhoea in adults, only if: the condition has not responded to other pharmacological treatments (for example, antimotility agents, antispasmodics, tricyclic antidepressants) or pharmacological treatments are contraindicated or not tolerated, it is started in secondary care Stop eluxadoline at 4 weeks if there is inadequate relief of the symptoms of irritable bowel syndrome with diarrhoea. Eptifibatide 1.3 These recommendations are not intended to affect treatment with eluxadoline that was started in the NHS before this guidance was published. Adults having treatment outside these recommendations may continue without change to the funding arrangements in place for them before this guidance was published, until they their NHS clinician consider it This guidance replaces Glycoprotein IIb/IIIa inhibitors in the treatment of acute coronary syndromes (Technology Appraisal Guidance No 12) issued in September This guidance has been partially updated by Unstable angina NSTEMI (NICE clinical guideline 94).
6 Etanercept Febuxostat Golimumab Ixekizumab It is recommended that a GP IIb/IIIa inhibitor is considered as an adjunct to PCI for all patients with diabetes undergoing elective PCI, for those patients undergoing complex procedures (for example, multi-vessel PCI, insertion of multiple stents, vein graft PCI or PCI for bifurcation lesions); currently only abciximab is licensed as an adjunct to PCI. In procedurally uncomplicated, elective PCI, where the risk of adverse sequelae is low, use of a GP IIb/IIIa inhibitor is not recommended unless unexpected immediate complications occur. GP IIb/IIIa inhibitors are not currently licensed in the UK for use as an adjunct to thrombolytic therapy in STsegment-elevation MI. Abatacept (Orencia), adalimumab (Humira), etanercept (Enbrel) tocilizumab (RoActemra) are recommended as possible treatments for people with polyarticular juvenile idiopathic arthritis. Adalimumab etanercept are recommended as possible treatments for people with enthesitis-related juvenile idiopathic arthritis. Etanercept is recommended as a possible treatment for people with psoriatic juvenile idiopathic arthritis. Febuxostat is recommended as a possible treatment for chronic hyperuricaemia in people with gout only if: they can t take the medicine allopurinol for medical reasons or the side effects of allopurinol are so bad that the person either has to stop taking it or can t be given the most effective dose. People who were already taking febuxostat when the guidance was issued should be able to carry on taking it until they their healthcare professional(s) decide that it is the right time to stop treatment. Infliximab (also known as Remicade, Inflectra or Remsima), adalimumab (Humira) golimumab (Simponi) are recommended. They are possible treatments for adults with moderate to severe ulcerative colitis if conventional therapy hasn t worked or isn t suitable. Infliximab is also recommended as a possible treatment for children or young people aged 6 17 years with severe ulcerative colitis, if conventional therapy hasn t worked or isn t suitable. People should be able to have the treatment for at least 12 months, unless it stops working well enough. Their condition should be assessed at least every 12 months. Their doctor should discuss with them the benefits risks of continuing or stopping treatment. If treatment is stopped the ulcerative colitis gets worse, people should be able to start treatment again. 1.1 Ixekizumab alone, or with methotrexate, is *Shared Care on behalf of Specialist Paediatric Rheumatology Centres Only*
7 recommended as an option for treating active psoriatic arthritis in adults, only if: it is used as described in NICE's technology appraisal guidance on etanercept, infliximab adalimumab for the treatment of psoriatic arthritis (recommendations ) or the person has had a tumour necrosis factor (TNF)-alpha inhibitor but their disease has not responded within the first 12 weeks or has stopped responding after the first 12 weeks or TNF-alpha inhibitors are contraindicated but would otherwise be considered (as described in NICE's technology appraisal guidance on etanercept, infliximab adalimumab for the treatment of psoriatic arthritis). Ixekizumab is only recommended if the company provides it according to the commercial arrangement. 1.2 Assess the response to ixekizumab after 16 weeks of treatment. Only continue treatment if there is clear evidence of response, defined as an improvement in at least 2 of the 4 Psoriatic Arthritis Response Criteria (PsARC), 1 of which must be joint tenderness or swelling score, with no worsening in any of the 4 criteria. People whose disease has a Psoriasis Area Severity Index (PASI) 75 response but whose PsARC response does not justify continuing treatment should be assessed by a dermatologist, to determine whether continuing treatment is appropriate based on skin response (as described in NICE's technology appraisal guidance on etanercept, infliximab adalimumab for the treatment of psoriatic arthritis, recommendation 1.3). 1.3 When using the PsARC, healthcare professionals should take into account any physical, sensory or learning disabilities or communication difficulties that
8 could affect a person's responses to components of the PsARC make any adjustments they consider appropriate. 1.4 When using the PASI, healthcare professionals should take into account skin colour how this could affect the PASI score, make the clinical adjustments they consider appropriate. 1.5 These recommendations are not intended to affect treatment with ixekizumab that was started in the NHS before this guidance was published. People having treatment outside these recommendations may continue without change to the funding arrangements in place for them before this guidance was published, until they their NHS clinician consider it Lubiprostone Mannitol Naloxeogol Lubiprostone is recommended as a possible treatment for people with chronic idiopathic constipation: who have previously been treated with 2 different types of laxatives at the highest possible recommended dose, for at least 6 months, but these haven't worked well enough, when invasive treatment is being considered. Review if treatment with lubiprostone has not worked after 2 weeks. Lubiprostone should only be prescribed by a doctor who is experienced in treating chronic idiopathic constipation, after they have carefully considered previous treatments. Mannitol dry powder for inhalation is recommended as an option for treating cystic fibrosis in adults: who cannot use rhdnase because of ineligibility, intolerance or inadequate response to rhdnase whose lung function is rapidly declining (forced expiratory volume in 1 second [FEV1] decline greater than 2% annually) for whom other osmotic agents are not considered appropriate. People currently receiving mannitol whose cystic fibrosis does not meet the above criteria should be able to continue treatment until they their clinician consider it Naloxegol (Moventig) is recommended as a possible treatment for people with opioid induced constipation that has had an inadequate response to laxatives
9 Nintedanib Obeticholic acid Ocrelizumab Pirfenidone Pixantrone Nintedanib is recommended as an option for treating idiopathic pulmonary fibrosis, only if: the person has a forced vital capacity (FVC) between 50% 80% of predicted the company provides nintedanib with the discount agreed in the patient access scheme treatment is stopped if disease progresses (a confirmed decline in percent predicted FVC of 10% or more) in any 12 month period. 1.1 Obeticholic acid is recommended, within its marketing authorisation, as an option for treating primary biliary cholangitis in combination with ursodeoxycholic acid for people whose disease has responded inadequately to ursodeoxycholic acid or as monotherapy for people who cannot tolerate ursodeoxycholic acid. Obeticholic acid is recommended only if the company provides it with the discount agreed in the patient access scheme. 1.2 Assess the response to obeticholic acid after 12 months. Only continue if there is evidence of clinical benefit. 1.1 Ocrelizumab is recommended as an option for treating relapsing remitting multiple sclerosis in adults with active disease defined by clinical or imaging features, only if: alemtuzumab is contraindicated or otherwise unsuitable the company provides ocrelizumab according to the commercial arrangement. 1.2 This recommendation is not intended to affect treatment with ocrelizumab that was started in the NHS before this guidance was published. People having treatment outside this recommendation may continue without change to the funding arrangements in place for them before this guidance was published, until they their NHS clinician consider it 1.1 Pirfenidone is recommended as an option for treating idiopathic pulmonary fibrosis in adults only if: the person has a forced vital capacity (FVC) between 50% 80% predicted the company provides pirfenidone with the discount agreed in the patient access scheme treatment is stopped if there is evidence of disease progression (an absolute decline of 10% or more in predicted FVC within any 12 month period). 1.2 This recommendation is not intended to affect treatment with pirfenidone that was started in the NHS before this guidance was published. People having treatment outside this recommendation may continue without change to the funding arrangements in place for them before this guidance was published, until they their NHS clinician consider it Pixantrone monotherapy is recommended as a possible treatment for adults with multiply relapsed or refractory aggressive non-hodgkin's B cell lymphoma if:
10 they have previously been treated with rituximab they are having third- or fourth-line treatment. Patients who have non-hodgkin s B-cell lymphoma should be able to have Pixantrone on the NHS if their doctor thinks that it is the right treatment People currently receiving Pixantrone that is not recommended according to the above criteria should have the option to continue treatment until they their clinician consider it Prucalopride Pertuzumab with trastuzumab docetaxel Reteplase Prucalopride is recommended as an option for the treatment of chronic constipation only in women for whom treatment with at least two laxatives from different classes, at the highest tolerated recommended doses for at least 6 months, has failed to provide adequate relief invasive treatment for constipation is being considered. If treatment with prucalopride is not effective after 4 weeks, the woman should be re-examined the benefit of continuing treatment reconsidered. Prucalopride should only be prescribed by a clinician with experience of treating chronic constipation, who has carefully reviewed the woman s previous courses of laxative treatments specified above. For Royal Surrey County Hospital Outreach patients only. Pertuzumab, in combination with trastuzumab docetaxel, is recommended, within its marketing authorisation, for treating HER2 positive metastatic or locally recurrent unresectable breast cancer, in adults who have not had previous anti HER2 therapy or chemotherapy for their metastatic disease, only if the company provides pertuzumab within the agreed commercial access arrangement. This guidance provides recommendations on the selection of thrombolytic drugs in patients with acute myocardial infarction (AMI). Recommendations are made in relation to the use of the drugs in hospital pre-hospital settings. The guidance does not compare hospital pre-hospital models of delivering thrombolysis. It is recommended that, in hospital, the choice of thrombolytic drug (alteplase, reteplase, streptokinase or tenecteplase) should take account of: the likely balance of benefit harm (for example, stroke) to which each of the thrombolytic agents would expose the individual patient. current UK clinical practice, in which it is accepted that patients who have previously received streptokinase should not be treated with it again. the hospital s arrangements for reducing delays in the administration of thrombolysis
11 Rituximab Roflumilast Where pre-hospital delivery of thrombolytic drugs is considered a beneficial approach as part of an emergency-care pathway for AMI (for example, because of population geography or the accessibility of acute hospital facilities), the practicalities of administering thrombolytic drugs in pre-hospital settings mean that the bolus drugs (reteplase or tenecteplase) are recommended as the preferred option. Rituximab taken with glucocorticoids is recommended as a possible treatment for people with anti-neutrophil cytoplasmic antibody-associated vasculitis (that is, severely active granulomatosis with polyangiitis [also known as Wegener's granulomatosis] microscopic polyangiitis) if: more treatment with cyclophosphamide would exceed the maximum amount of cyclophosphamide they can have or cyclophosphamide is not suitable for them or they cannot take it or they want to have children treatment with cyclophosphamide may affect their fertility or the disease has stayed active or got worse after a course of cyclophosphamide lasting 3 6 months or the person has had cancer affecting the lining of the bladder other parts of the urinary system. Patients already receiving Rituximab who do not meet the criteria above should have the option to continue treatment until they their clinicians consider it Roflumilast is recommended only in the context of research as part of a clinical trial for adults with severe chronic obstructive pulmonary disease (COPD) (for the purposes of this guidance defined as forced expiratory volume in 1 second [FEV 1 ] post-bronchodilator less than 50% predicted) associated with chronic bronchitis with a history of frequent exacerbations as an add-on to bronchodilator treatment. Such research should be designed to generate robust evidence about the benefits of roflumilast as an add-on to long-acting muscarinic antagonists (LAMA) plus long-acting beta 2 agonists (LABA) plus inhaled corticosteroids (ICS), or LAMA plus LABA for people who are intolerant to ICS. Patients receiving Roflumilast should have the option to continue treatment until they their clinicians consider it Streptokinase This guidance provides recommendations on the selection of thrombolytic drugs in patients with acute myocardial infarction (AMI). Recommendations are made in relation to the use of the drugs in hospital pre-hospital settings. The guidance does not compare hospital pre-hospital models of delivering thrombolysis.
12 It is recommended that, in hospital, the choice of thrombolytic drug (alteplase, reteplase, streptokinase or tenecteplase) should take account of: the likely balance of benefit harm (for example, stroke) to which each of the thrombolytic agents would expose the individual patient. current UK clinical practice, in which it is accepted that patients who have previously received streptokinase should not be treated with it again. the hospital s arrangements for reducing delays in the administration of thrombolysis. Where pre-hospital delivery of thrombolytic drugs is considered a beneficial approach as part of an emergency-care pathway for AMI (for example, because of population geography or the accessibility of acute hospital facilities), the practicalities of administering thrombolytic drugs in pre-hospital settings mean that the bolus drugs (reteplase or tenecteplase) are recommended as the preferred option. Teriparatide This guidance relates only to treatments for the secondary prevention of fragility fractures in postmenopausal women who have osteoporosis have sustained a clinically apparent osteoporotic fragility fracture. Osteoporosis is defined by a T-score [1] of 2.5 stard deviations (SD) or below on dualenergy X-ray absorptiometry (DXA) scanning. However, the diagnosis may be assumed in women aged 75 years or older if the responsible clinician considers a DXA scan to be clinically inappropriate or unfeasible. This guidance assumes that women who receive treatment have an adequate calcium intake are vitamin D replete. Unless clinicians are confident that women who receive treatment meet these criteria, calcium /or vitamin D supplementation should be considered. This guidance does not cover the following: The use of alendronate, etidronate, risedronate, raloxifene, strontium ranelate or teriparatide for the secondary prevention of osteoporotic fragility fractures in women with normal bone mineral density (BMD) or osteopenia (that is, women with a T-score between SD below peak BMD). The use of these drugs for the secondary prevention of osteoporotic fragility fractures in women who are on long-term systemic corticosteroid treatment. Teriparatide is recommended as an alternative treatment option for the secondary prevention of osteoporotic fragility fractures in postmenopausal women: who are unable to take alendronate either risedronate or etidronate, or have a contraindication to or are intolerant of alendronate either risedronate or etidronate, or
13 Tocilizumab who have a contraindication to, or are intolerant of strontium ranelate or who have had an unsatisfactory response to treatment with alendronate, risedronate or etidronate who are 65 years or older have a T-score of 4.0 SD or below, or a T-score of 3.5 SD or below plus more than two fractures, or who are aged years have a T-score of 4 SD or below plus more than two fractures. Women who are currently receiving treatment with one of the drugs covered by this guidance, but for whom treatment would not have been recommended according to the above, should have the option to continue treatment until they their clinicians consider it [1] T-score relates to the measurement of bone mineral density (BMD) using central (hip /or spine) DXA scanning, is expressed as the number of stard deviations (SD) from peak BMD. Abatacept (Orencia), adalimumab (Humira), etanercept (Enbrel) tocilizumab (RoActemra) are recommended as possible treatments for people with polyarticular juvenile idiopathic arthritis. Adalimumab etanercept are recommended as possible treatments for people with enthesitis-related juvenile idiopathic arthritis. *Shared Care on behalf of Specialist Paediatric Rheumatology Centres Only* Etanercept is recommended as a possible treatment for people with psoriatic juvenile idiopathic arthritis. Tofacitinib Moderate to severe rheumatoid arthritis Tofacitinib Active psoriatic arthritis after inadequate response to DMARDs Tolvaptan Tolvaptan (Jinarc) is recommended as a possible treatment for people with autosomal dominant polycystic kidney disease if: they have chronic kidney disease stage 2 or 3 at the start of treatment there is evidence of rapidly progressing disease. Varenicline Varenicline is recommended within its licensed indications as an option for smokers who have expressed a desire to quit smoking. Varenicline should normally be prescribed only as part of a programme of behavioural support.
Pegylated liposomal irinotecan for treating pancreatic cancer after gemcitabine TA440
 This spreadsheet is updated monthly and enables self-audit of a medicines for All guidelines refer to adults unless indicated. No copyright is asserted on this Technology appraisal (TA) Titles are hyperlinks
This spreadsheet is updated monthly and enables self-audit of a medicines for All guidelines refer to adults unless indicated. No copyright is asserted on this Technology appraisal (TA) Titles are hyperlinks
Costing statement: Denosumab for the prevention of osteoporotic fractures in postmenopausal women
 Costing statement: Denosumab for the prevention of osteoporotic fractures in postmenopausal women Resource impact The guidance Denosumab for the prevention of osteoporotic fractures in postmenopausal women
Costing statement: Denosumab for the prevention of osteoporotic fractures in postmenopausal women Resource impact The guidance Denosumab for the prevention of osteoporotic fractures in postmenopausal women
Technology appraisal guidance Published: 11 October 2017 nice.org.uk/guidance/ta480
 Tofacitinib for moderate to severeere rheumatoid arthritis Technology appraisal guidance Published: 11 October 2017 nice.org.uk/guidance/ta480 NICE 2018. All rights reserved. Subject to Notice of rights
Tofacitinib for moderate to severeere rheumatoid arthritis Technology appraisal guidance Published: 11 October 2017 nice.org.uk/guidance/ta480 NICE 2018. All rights reserved. Subject to Notice of rights
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE)
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA47 Glycoprotein IIb/IIIa inhibitors in the treatment of acute coronary syndromes This guidance was issued in September,
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE GUIDANCE EXECUTIVE (GE) Review of TA47 Glycoprotein IIb/IIIa inhibitors in the treatment of acute coronary syndromes This guidance was issued in September,
29 August 2016 Page 1 of 7. How does the NHS board decide which new medicines to make available for patients?
 NHS Greater Glasgow and Clyde: New Medicines Decisions In Scotland, a newly licensed medicine is routinely available for use in an NHS board only after it has been: accepted for use in the NHSScotland
NHS Greater Glasgow and Clyde: New Medicines Decisions In Scotland, a newly licensed medicine is routinely available for use in an NHS board only after it has been: accepted for use in the NHSScotland
NICE TECHNOLOGY APPRAISAL MEDICINES REPORT 2017
 NICE TECHNOLOGY PPRISL MEDICINES REPORT St George s Healthcare NHS Trust key for medicine-related NICE Technology ppraisals. has been approved by the Drugs and Therapeutics Committee as recommended within
NICE TECHNOLOGY PPRISL MEDICINES REPORT St George s Healthcare NHS Trust key for medicine-related NICE Technology ppraisals. has been approved by the Drugs and Therapeutics Committee as recommended within
Regulatory Status FDA-approved indication: Orencia is a selective T cell costimulation modulator indicated for: (1)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.18 Subject: Orencia Page: 1 of 8 Last Review Date: March 16, 2018 Orencia Description Orencia (abatacept)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.18 Subject: Orencia Page: 1 of 8 Last Review Date: March 16, 2018 Orencia Description Orencia (abatacept)
1 P a g e. Systemic Juvenile Idiopathic Arthritis (SJIA) (1.3) Patients 2 years of age and older with active systemic juvenile idiopathic arthritis.
 LENGTH OF AUTHORIZATION: Initial: 3 months for Crohn s or Ulcerative Colitis; 1 year for all other indications. Renewal: 1 year dependent upon medical records supporting response to therapy and review
LENGTH OF AUTHORIZATION: Initial: 3 months for Crohn s or Ulcerative Colitis; 1 year for all other indications. Renewal: 1 year dependent upon medical records supporting response to therapy and review
Prior treatment with non-biologic Disease- Modifying Antirheumatic. Not to be used in combination with another biologic DMARD
 Abatacept (Orencia) 1, 2, 7, 11, 13, 14, 18, 24, 31, 44, 48, 49, 51, 53, 55, 57 J0129 Alpha 1 - Proteinase inhibitor (Prolastin-C) 5, 6, 10, 12, 40 Medically Necessary (if all the following criteria apply):
Abatacept (Orencia) 1, 2, 7, 11, 13, 14, 18, 24, 31, 44, 48, 49, 51, 53, 55, 57 J0129 Alpha 1 - Proteinase inhibitor (Prolastin-C) 5, 6, 10, 12, 40 Medically Necessary (if all the following criteria apply):
Horizon Scanning Technology Summary. Adalimumab (Humira) for juvenile idiopathic arthritis. National Horizon Scanning Centre.
 Horizon Scanning Technology Summary National Horizon Scanning Centre Adalimumab (Humira) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Horizon Scanning Technology Summary National Horizon Scanning Centre Adalimumab (Humira) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Quality and Outcomes Framework Programme NICE cost impact statement July Indicator area: Osteoporosis - fragility fracture
 Quality and Outcomes Framework Programme NICE cost impact statement July 2011 Indicator area: Osteoporosis - fragility fracture Indicators NM29: The practice can produce a register of patients: 1. Aged
Quality and Outcomes Framework Programme NICE cost impact statement July 2011 Indicator area: Osteoporosis - fragility fracture Indicators NM29: The practice can produce a register of patients: 1. Aged
Osteoporosis Clinical Guideline. Rheumatology January 2017
 Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
Osteoporosis Clinical Guideline Rheumatology January 2017 Introduction Osteoporosis is a condition of low bone mass leading to an increased risk of low trauma fractures. The prevalence of osteoporosis
Horizon Scanning Technology Summary. Abatacept (Orencia) for juvenile idiopathic arthritis. National Horizon Scanning Centre.
 Horizon Scanning Technology Summary National Horizon Scanning Centre Abatacept (Orencia) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Horizon Scanning Technology Summary National Horizon Scanning Centre Abatacept (Orencia) for juvenile idiopathic arthritis June 2007 This technology summary is based on information available at the time
Regulatory Status FDA- approved indication: Simponi and Simponi ARIA are tumor necrosis factor (TNF) blockers indicated for the treatment of: (2-3)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 9 Last Review Date: March 16, 2018 Simponi / Simponi
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 9 Last Review Date: March 16, 2018 Simponi / Simponi
Drug Class Prior Authorization Criteria Therapeutic Agents in Rheumatic and Inflammatory Diseases
 Drug Class Prior Authorization Criteria Therapeutic Agents in Rheumatic and Inflammatory Diseases Line of Business: Medicaid P & T Approval Date: August 16, 2017 Effective Date: August 16, 2017 This policy
Drug Class Prior Authorization Criteria Therapeutic Agents in Rheumatic and Inflammatory Diseases Line of Business: Medicaid P & T Approval Date: August 16, 2017 Effective Date: August 16, 2017 This policy
Cyltezo (adalimumab-adbm) CG-DRUG-64, CG-DRUG-65
 Market DC Cyltezo (adalimumab-adbm) CG-DRUG-64, CG-DRUG-65 Override(s) Prior Authorization Quantity Limit Medications Cyltezo (adalimumab-adbm) 40 mg/0.8 ml prefilled syringe #* ^ Approval Duration 1 year
Market DC Cyltezo (adalimumab-adbm) CG-DRUG-64, CG-DRUG-65 Override(s) Prior Authorization Quantity Limit Medications Cyltezo (adalimumab-adbm) 40 mg/0.8 ml prefilled syringe #* ^ Approval Duration 1 year
2014 Date of Guidance Title of Guidance Summary of Recommendations Ratified at Croydon Time to Publication Number Prescribing Committee
 NICE Technology Appraisals - Time to Implementation Report - 18 December 2014 Produced by Tracy Steadman, Croydon Public Health Intelligence Team (C-PHIT), Croydon Council Date of Guidance Title of Guidance
NICE Technology Appraisals - Time to Implementation Report - 18 December 2014 Produced by Tracy Steadman, Croydon Public Health Intelligence Team (C-PHIT), Croydon Council Date of Guidance Title of Guidance
Volume 10; Number 4 February 2016
 Arden and Greater East Midlands Commissioning Support Unit in association with Lincolnshire Clinical Commissioning Groups, Lincolnshire Community Health Services, United Lincolnshire Hospitals Trust and
Arden and Greater East Midlands Commissioning Support Unit in association with Lincolnshire Clinical Commissioning Groups, Lincolnshire Community Health Services, United Lincolnshire Hospitals Trust and
Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta466
 Baricitinib for moderate to severeere rheumatoid arthritis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta466 NICE 2017. All rights reserved. Subject to Notice of rights
Baricitinib for moderate to severeere rheumatoid arthritis Technology appraisal guidance Published: 9 August 2017 nice.org.uk/guidance/ta466 NICE 2017. All rights reserved. Subject to Notice of rights
Cimzia. Cimzia (certolizumab pegol) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Section: Prescription Drugs Effective Date: April 1, 2018 Subject: Cimzia Page: 1 of 5 Last Review
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Section: Prescription Drugs Effective Date: April 1, 2018 Subject: Cimzia Page: 1 of 5 Last Review
Chelsea and Westminster Hospital NHS Foundation Trust Trust Medicines Group
 Summary of Main Points from the Meeting held on Monday 11 th July 2016 2. Minutes and Summary Notes from last meeting The Minutes and Summary notes from the June 2016 Medicines Group meeting were approved
Summary of Main Points from the Meeting held on Monday 11 th July 2016 2. Minutes and Summary Notes from last meeting The Minutes and Summary notes from the June 2016 Medicines Group meeting were approved
10 Musculoskeletal and Joint Diseases
 Recommendations from the Lothian Formulary Committee (FC) following Scottish Medicines Consortium (SMC) advice, NICE MTA advice, (FAF3) unlicensed and off-label medicines and (FAF2) medicines not considered
Recommendations from the Lothian Formulary Committee (FC) following Scottish Medicines Consortium (SMC) advice, NICE MTA advice, (FAF3) unlicensed and off-label medicines and (FAF2) medicines not considered
Infliximab/Infliximab-dyyb DRUG.00002
 Infliximab/Infliximab-dyyb DRUG.00002 Override(s) Prior Authorization Step Therapy Medications Remicade (infliximab) Inflectra (inflectra-dyyb) Approval Duration 1 year Comment Intravenous administration
Infliximab/Infliximab-dyyb DRUG.00002 Override(s) Prior Authorization Step Therapy Medications Remicade (infliximab) Inflectra (inflectra-dyyb) Approval Duration 1 year Comment Intravenous administration
Regulatory Status FDA-approved indication: Orencia is a selective T cell co-stimulation modulator indicated for: (1)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Orencia Page: 1 of 9 Last Review Date: September 20, 2018 Orencia Description Orencia (abatacept)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Orencia Page: 1 of 9 Last Review Date: September 20, 2018 Orencia Description Orencia (abatacept)
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women
 S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
S H A R E D C A R E G U I D E L I N E Drug: Denosumab 60mg injection Indication: treatment of osteoporosis in postmenopausal women Introduction Indication: Denosumab (Prolia ) is recommended in NICE TA204
Abatacept (Orencia) for active rheumatoid arthritis. August 2009
 Abatacept (Orencia) for active rheumatoid arthritis August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Abatacept (Orencia) for active rheumatoid arthritis August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Dumfries and Galloway. Treatment Protocol for Osteoporosis
 Dumfries and Galloway Treatment Protocol for Osteoporosis DIAGNOSIS OF OSTEOPOROSIS 2 Diagnostic Criteria 2 REFERRAL CRITERIA FOR DEXA 3 TREATMENT 4 Non-Drug Therapy : for all 4 Non-Drug Therapy : in the
Dumfries and Galloway Treatment Protocol for Osteoporosis DIAGNOSIS OF OSTEOPOROSIS 2 Diagnostic Criteria 2 REFERRAL CRITERIA FOR DEXA 3 TREATMENT 4 Non-Drug Therapy : for all 4 Non-Drug Therapy : in the
RHEUMATOID ARTHRITIS DRUGS
 Rheumatology Biologics Criteria from the Exceptional Access Program RHEUMATOID ARTHRITIS DRUGS DRUG NAME BRS REIMBURSED DOSAGE FORM/ STRENGTH Adalimumab Humira 40 mg/0.8 syringe and 40mg/0.8 pen for Anakinra
Rheumatology Biologics Criteria from the Exceptional Access Program RHEUMATOID ARTHRITIS DRUGS DRUG NAME BRS REIMBURSED DOSAGE FORM/ STRENGTH Adalimumab Humira 40 mg/0.8 syringe and 40mg/0.8 pen for Anakinra
Chronic obstructive pulmonary disease
 0 Chronic obstructive pulmonary disease Implementing NICE guidance June 2010 NICE clinical guideline 101 What this presentation covers Background Scope Key priorities for implementation Discussion Find
0 Chronic obstructive pulmonary disease Implementing NICE guidance June 2010 NICE clinical guideline 101 What this presentation covers Background Scope Key priorities for implementation Discussion Find
Evidence review for Surrey Prescribing Clinical Network SUMMARY
 East Surrey CCG, Guildford & Waverley CCG, North West Surrey CCG, Surrey Downs CCG, Surrey Heath CCG, Crawley CCG, Horsham & Mid-Sussex CCG Evidence review for Surrey Prescribing Clinical Network Medicine
East Surrey CCG, Guildford & Waverley CCG, North West Surrey CCG, Surrey Downs CCG, Surrey Heath CCG, Crawley CCG, Horsham & Mid-Sussex CCG Evidence review for Surrey Prescribing Clinical Network Medicine
Biologics for Autoimmune Diseases
 Biologics for Autoimmune Diseases Goal(s): Restrict use of biologics to OHP funded conditions and according to OHP guidelines for use. Promote use that is consistent with national clinical practice guidelines
Biologics for Autoimmune Diseases Goal(s): Restrict use of biologics to OHP funded conditions and according to OHP guidelines for use. Promote use that is consistent with national clinical practice guidelines
Guideline for the investigation and management of osteoporosis. for hospitals and General Practice
 Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
Guideline for the investigation and management of osteoporosis for hospitals and General Practice Background Low bone density is an important risk factor for fracture. The aim of assessing bone density
Corporate Medical Policy
 Corporate Medical Policy Rituximab for the Treatment of Rheumatoid Arthritis File Name: Origination: Last CAP Review: Next CAP Review: Last Review: rituximab_for_the_treatment_of_rheumatoid_arthritis 4/2008
Corporate Medical Policy Rituximab for the Treatment of Rheumatoid Arthritis File Name: Origination: Last CAP Review: Next CAP Review: Last Review: rituximab_for_the_treatment_of_rheumatoid_arthritis 4/2008
The contractor establishes and maintains a register of patients with AF
 Atrial Fibrillation The contractor establishes and maintains a register of patients with AF G5731 Those patients with AF in whom there is a record of CHADS2 score of 1, the % of patients who are currently
Atrial Fibrillation The contractor establishes and maintains a register of patients with AF G5731 Those patients with AF in whom there is a record of CHADS2 score of 1, the % of patients who are currently
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: abatacept_orencia 4/2008 2/2018 2/2019 2/2018 Description of Procedure or Service Abatacept (Orencia ), a
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: abatacept_orencia 4/2008 2/2018 2/2019 2/2018 Description of Procedure or Service Abatacept (Orencia ), a
Technology appraisal guidance Published: 26 January 2016 nice.org.uk/guidance/ta375
 Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed Technology
Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed Technology
National Institute for Health and Care Excellence. Single Technology Appraisal (STA) Empagliflozin combination therapy for treating type 2 diabetes
 National Institute for Health and Care Excellence Comment 1: the draft remit Single Technology Appraisal (STA) Empagliflozin combination therapy for treating type 2 diabetes Response to consultee and commentator
National Institute for Health and Care Excellence Comment 1: the draft remit Single Technology Appraisal (STA) Empagliflozin combination therapy for treating type 2 diabetes Response to consultee and commentator
Orencia (abatacept) DRUG.00040
 Market DC Orencia (abatacept) DRUG.00040 Override(s) Prior Authorization Quantity Limit Approval Duration 1 year Medications Comments Quantity Limit Orencia (abatacept) - AGP, VA MCD only 4 vials per 28
Market DC Orencia (abatacept) DRUG.00040 Override(s) Prior Authorization Quantity Limit Approval Duration 1 year Medications Comments Quantity Limit Orencia (abatacept) - AGP, VA MCD only 4 vials per 28
Technology appraisal guidance Published: 4 June 2015 nice.org.uk/guidance/ta340
 Ustekinumab for treating active psoriatic arthritis Technology appraisal guidance Published: 4 June 2015 nice.org.uk/guidance/ta340 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Ustekinumab for treating active psoriatic arthritis Technology appraisal guidance Published: 4 June 2015 nice.org.uk/guidance/ta340 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Immune Modulating Drugs Prior Authorization Request Form
 Patient: HPHC member ID #: Requesting provider: Phone: Servicing provider: Diagnosis: Contact for questions (name and phone #): Projected start and end date for requested Requesting provider NPI: Fax:
Patient: HPHC member ID #: Requesting provider: Phone: Servicing provider: Diagnosis: Contact for questions (name and phone #): Projected start and end date for requested Requesting provider NPI: Fax:
04 September 2017 Page 1 of 6
 NHS Greater Glasgow and Clyde: New Medicines Decisions In Scotland, a newly licensed medicine is routinely available in a health board only after it has been: accepted for use in by the Scottish Medicines
NHS Greater Glasgow and Clyde: New Medicines Decisions In Scotland, a newly licensed medicine is routinely available in a health board only after it has been: accepted for use in by the Scottish Medicines
Remicade (infliximab) DRUG.00002
 Applicability/Effective Date *- Florida Healthy Kids Remicade (infliximab) DRUG.00002 Override(s) Prior Authorization Step Therapy Medications Remicade (infliximab) Approval Duration 1 year Comment Intravenous
Applicability/Effective Date *- Florida Healthy Kids Remicade (infliximab) DRUG.00002 Override(s) Prior Authorization Step Therapy Medications Remicade (infliximab) Approval Duration 1 year Comment Intravenous
Inflectra (infliximab-dyyb), Remicade (infliximab), Renflexis (infliximab-abda) DRUG CG-DRUG-64
 Inflectra (infliximab-dyyb), Remicade (infliximab), Renflexis (infliximab-abda) DRUG.00002 CG-DRUG-64 Override(s) Prior Authorization *Washington Medicaid See State Specific Mandates Medications Inflectra
Inflectra (infliximab-dyyb), Remicade (infliximab), Renflexis (infliximab-abda) DRUG.00002 CG-DRUG-64 Override(s) Prior Authorization *Washington Medicaid See State Specific Mandates Medications Inflectra
Pathway from Fracture or Risk Factor to Treatment
 Appendix 6A - Guidance on Diagnosis and Management of Osteoporosis Pathway from Fracture or Risk Factor to Treatment Fragility Fracture = fracture sustained from a low energy fall from standing height
Appendix 6A - Guidance on Diagnosis and Management of Osteoporosis Pathway from Fracture or Risk Factor to Treatment Fragility Fracture = fracture sustained from a low energy fall from standing height
Technology appraisal guidance Published: 16 December 2015 nice.org.uk/guidance/ta373
 Abatacept, adalimumab, etanercept and tocilizumab for treating juvenile idiopathic arthritis Technology appraisal guidance Published: 16 December 2015 nice.org.uk/guidance/ta373 NICE 2017. All rights reserved.
Abatacept, adalimumab, etanercept and tocilizumab for treating juvenile idiopathic arthritis Technology appraisal guidance Published: 16 December 2015 nice.org.uk/guidance/ta373 NICE 2017. All rights reserved.
Xeljanz. Xeljanz, Xeljanz XR (tofacitinib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.24 Subject: Xeljanz Page: 1 of 6 Last Review Date: March 16, 2018 Xeljanz Description Xeljanz, Xeljanz
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.24 Subject: Xeljanz Page: 1 of 6 Last Review Date: March 16, 2018 Xeljanz Description Xeljanz, Xeljanz
UnitedHealthcare Pharmacy Clinical Pharmacy Programs
 Program Number 2017 P 3041-8 Program Step Therapy Medications UnitedHealthcare Pharmacy Clinical Pharmacy Programs *Orencia (abatacept) *This step criteria refers to the subcutaneous formulation of abatacept
Program Number 2017 P 3041-8 Program Step Therapy Medications UnitedHealthcare Pharmacy Clinical Pharmacy Programs *Orencia (abatacept) *This step criteria refers to the subcutaneous formulation of abatacept
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 : assessing the risk of fragility fracture bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new
: assessing the risk of fragility fracture bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new
Ixekizumab for treating moderate to severe plaque psoriasis [ID904]
![Ixekizumab for treating moderate to severe plaque psoriasis [ID904] Ixekizumab for treating moderate to severe plaque psoriasis [ID904]](/thumbs/86/94899473.jpg) Thank you for agreeing to make a submission on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Thank you for agreeing to make a submission on your organisation s view of the technology and the way it should be used in the NHS. Healthcare professionals can provide a unique perspective on the technology
Xeljanz (tofacitinib), Xeljanz XR (tofacitinib extended-release)
 Market DC Xeljanz (tofacitinib), Xeljanz XR (tofacitinib extended-release) Override(s) Prior Authorization Quantity Limit Medications Xeljanz (tofacitinib) Approval Duration 1 year Quantity Limit May be
Market DC Xeljanz (tofacitinib), Xeljanz XR (tofacitinib extended-release) Override(s) Prior Authorization Quantity Limit Medications Xeljanz (tofacitinib) Approval Duration 1 year Quantity Limit May be
NEW MEDICINE APPLICATIONS BROUGHT TO THE BORDERS FORMULARY COMMITTEE (BFC) AND AREA DRUG & THERAPEUTICS COMMITTEE (ADTC) 2016/17
 New Medicine Product/Device Endorsement Categories NEW MEDICINE APPLICATIONS BROUGHT TO THE BORDERS FORMULARY COMMITTEE (BFC) AND AREA DRUG & THERAPEUTICS COMMITTEE (ADTC) 2016/17 A B C D E F recommended
New Medicine Product/Device Endorsement Categories NEW MEDICINE APPLICATIONS BROUGHT TO THE BORDERS FORMULARY COMMITTEE (BFC) AND AREA DRUG & THERAPEUTICS COMMITTEE (ADTC) 2016/17 A B C D E F recommended
Case Finding and Risk Assessment for Osteoporosis
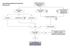 Case Finding and Risk Assessment for Osteoporosis Patient may present as a fragility fracture or risk fracture Fragility fracture age 50 Clinical risk factors aged 50 Very strong clinical risk factors
Case Finding and Risk Assessment for Osteoporosis Patient may present as a fragility fracture or risk fracture Fragility fracture age 50 Clinical risk factors aged 50 Very strong clinical risk factors
Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures
 APper apc15-0avgfh7 Shared Care Guideline Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures For the latest information on interactions and adverse effects,
APper apc15-0avgfh7 Shared Care Guideline Denosumab for the treatment of osteoporosis in postmenopausal women at increased risk of fractures For the latest information on interactions and adverse effects,
Summary. Background. Diagnosis
 March 2009 Management of post-menopausal osteoporosis This bulletin focuses on the pharmacological management of patients with post-menopausal osteoporosis both those with clinically evident disease (e.g.
March 2009 Management of post-menopausal osteoporosis This bulletin focuses on the pharmacological management of patients with post-menopausal osteoporosis both those with clinically evident disease (e.g.
Clinical guideline Published: 10 October 2012 nice.org.uk/guidance/cg152
 Crohn's disease: management Clinical guideline Published: 10 October 2012 nice.org.uk/guidance/cg152 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Crohn's disease: management Clinical guideline Published: 10 October 2012 nice.org.uk/guidance/cg152 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Understanding NICE guidance. NICE technology appraisal guidance advises on when and how drugs and other treatments should be used in the NHS.
 Understanding NICE guidance Information for people who use NHS services Alendronate, etidronate, risedronate, strontium ranelate and raloxifene for preventing bone fractures in postmenopausal women with
Understanding NICE guidance Information for people who use NHS services Alendronate, etidronate, risedronate, strontium ranelate and raloxifene for preventing bone fractures in postmenopausal women with
DERBYSHIRE JOINT AREA PRESCRIBING COMMITTEE (JAPC)
 DERBYSHIRE JOINT AREA PRERIBING COMMITTEE (JAPC) Derbyshire commissioning guidance on biologic drugs f the treatment of Rheumatoid arthritis with methotrexate This algithm is a tool to aid the implementation
DERBYSHIRE JOINT AREA PRERIBING COMMITTEE (JAPC) Derbyshire commissioning guidance on biologic drugs f the treatment of Rheumatoid arthritis with methotrexate This algithm is a tool to aid the implementation
NB Drug Plans Formulary Update
 Bulletin # 993 February 27, 2019 NB Drug Plans Formulary Update This update to the New Brunswick Drug Plans Formulary is effective February 27, 2019. Included in this bulletin: Special Authorization Benefit
Bulletin # 993 February 27, 2019 NB Drug Plans Formulary Update This update to the New Brunswick Drug Plans Formulary is effective February 27, 2019. Included in this bulletin: Special Authorization Benefit
Certolizumab pegol (Cimzia) for psoriatic arthritis second line
 Certolizumab pegol (Cimzia) for psoriatic arthritis second line This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Certolizumab pegol (Cimzia) for psoriatic arthritis second line This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Unstable angina and NSTEMI
 Issue date: March 2010 Unstable angina and NSTEMI The early management of unstable angina and non-st-segment-elevation myocardial infarction This guideline updates and replaces recommendations for the
Issue date: March 2010 Unstable angina and NSTEMI The early management of unstable angina and non-st-segment-elevation myocardial infarction This guideline updates and replaces recommendations for the
nogg Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK
 nogg NATIONAL OSTEOPOROSIS GUIDELINE GROUP Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK Produced by J Compston, A Cooper,
nogg NATIONAL OSTEOPOROSIS GUIDELINE GROUP Guideline for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK Produced by J Compston, A Cooper,
Technology appraisal guidance Published: 25 August 2010 nice.org.uk/guidance/ta195
 Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a TNF inhibitor Technology appraisal guidance Published: 25 August 2010 nice.org.uk/guidance/ta195
Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a TNF inhibitor Technology appraisal guidance Published: 25 August 2010 nice.org.uk/guidance/ta195
Otezla. Otezla (apremilast) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Otezla Page: 1 of 5 Last Review Date: March 16, 2018 Otezla Description Otezla (apremilast) Background
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Otezla Page: 1 of 5 Last Review Date: March 16, 2018 Otezla Description Otezla (apremilast) Background
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Certolizumab pegol (Cimzia) for the treatment of ankylosing spondylitis second or third line
 Certolizumab pegol (Cimzia) for the treatment of ankylosing spondylitis second or third line August 2011 This technology summary is based on information available at the time of research and a limited
Certolizumab pegol (Cimzia) for the treatment of ankylosing spondylitis second or third line August 2011 This technology summary is based on information available at the time of research and a limited
14/15 Threshold 15/16 Points 15/16. Points. Retired Replaced by NM82/AF007. Replacement NO CHANGE
 SUMMARY OF CHANGES TO QOF 2015/1 - ENGLAND KEY No change Retired/replaced Wording and/or change Point or threshold change Indicator ID change 14/15 QOF ID 15/1 QOF ID NICE ID Indicator wording Changes
SUMMARY OF CHANGES TO QOF 2015/1 - ENGLAND KEY No change Retired/replaced Wording and/or change Point or threshold change Indicator ID change 14/15 QOF ID 15/1 QOF ID NICE ID Indicator wording Changes
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Proposed Health Technology Appraisal Dapagliflozin in combination therapy for the Final scope Remit/appraisal objective To appraise the clinical and
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Proposed Health Technology Appraisal Dapagliflozin in combination therapy for the Final scope Remit/appraisal objective To appraise the clinical and
HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) DABIGATRAN RECOMMENDED What it is Indications Date decision last revised
 Name: generic (trade) Dabigatran etexilate (Pradaxa ) HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) DABIGATRAN RECOMMENDED What it is Indications Date decision last revised Direct thrombin inhibitor
Name: generic (trade) Dabigatran etexilate (Pradaxa ) HERTFORDSHIRE MEDICINES MANAGEMENT COMMITTEE (HMMC) DABIGATRAN RECOMMENDED What it is Indications Date decision last revised Direct thrombin inhibitor
Regulatory Status FDA-approved indication: Orencia is a selective T cell costimulation modulator indicated for: (1)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.18 Subject: Orencia Page: 1 of 6 Last Review Date: December 8, 2017 Orencia Description Orencia (abatacept)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.18 Subject: Orencia Page: 1 of 6 Last Review Date: December 8, 2017 Orencia Description Orencia (abatacept)
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December
 Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
Commissioning policies agreed by PCTs in Yorkshire and the Humber at Board meeting of YH SCG on December 17 2010. 32/10 Imatinib for gastrointestinal stromal tumours (unresectable/metastatic) (update on
DENOSUMAB SHARED CARE GUIDLINES
 DENOSUMAB LICENSING Denosumab (PROLIA ) is licensed for the treatment of osteoporosis in postmenopausal women at increased risk of fractures and for bone loss associated with hormone ablation in men with
DENOSUMAB LICENSING Denosumab (PROLIA ) is licensed for the treatment of osteoporosis in postmenopausal women at increased risk of fractures and for bone loss associated with hormone ablation in men with
Horizon Scanning Technology Briefing. Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal. National Horizon Scanning Centre
 Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
Horizon Scanning Technology Briefing National Horizon Scanning Centre Zoledronic Acid (Aclasta) once yearly treatment for postmenopausal osteoporosis December 2006 This technology summary is based on information
Simponi / Simponi ARIA (golimumab)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 6 Last Review Date: September 15, 2016 Simponi / Simponi
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 6 Last Review Date: September 15, 2016 Simponi / Simponi
Cosentyx. Cosentyx (secukinumab) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.11 Subject: Cosentyx Page: 1 of 7 Last Review Date: September 20, 2018 Cosentyx Description Cosentyx
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.90.11 Subject: Cosentyx Page: 1 of 7 Last Review Date: September 20, 2018 Cosentyx Description Cosentyx
Date of Guidance Title of Guidance Summary of Recommendations Ratified at Croydon Time to Publication Number Prescribing Committee Implementation
 NICE Technology Appraisals - Time to Implementation Report - 3 rd August 2015 Produced by Tracy Steadman, Croydon Public Health Intelligence Team, Croydon Council Date of Guidance Title of Guidance Summary
NICE Technology Appraisals - Time to Implementation Report - 3 rd August 2015 Produced by Tracy Steadman, Croydon Public Health Intelligence Team, Croydon Council Date of Guidance Title of Guidance Summary
Coverage Criteria: Express Scripts, Inc. monograph dated 12/15/ months or as otherwise noted by indication
 BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Kineret (anakinra subcutaneous injection) Commercial HMO/PPO/CDHP
BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Kineret (anakinra subcutaneous injection) Commercial HMO/PPO/CDHP
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Secukinumab for treating ankylosing spondylitis after inadequate response to non-steroidal anti-inflammatory drugs
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Secukinumab for treating ankylosing spondylitis after inadequate response to non-steroidal anti-inflammatory drugs
Cimzia. Cimzia (certolizumab pegol) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Subject: Cimzia Page: 1 of 5 Last Review Date: December 8, 2017 Cimzia Description Cimzia (certolizumab
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Subject: Cimzia Page: 1 of 5 Last Review Date: December 8, 2017 Cimzia Description Cimzia (certolizumab
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Proposed Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Vorapaxar for the secondary prevention of atherothrombotic events after myocardial infarction Draft scope (pre-referral)
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Proposed Health Technology Appraisal Vorapaxar for the secondary prevention of atherothrombotic events after myocardial infarction Draft scope (pre-referral)
Area Drug and Therapeutics Committee Prescribing Supplement No 59 July 2012
 Area Drug and Therapeutics Committee Prescribing Supplement No 59 In this issue Drugs reviewed by the SMC in June 2012 ADTC UPDATES ON DRUGS REVIEWED BY THE SMC The following new drugs have been reviewed
Area Drug and Therapeutics Committee Prescribing Supplement No 59 In this issue Drugs reviewed by the SMC in June 2012 ADTC UPDATES ON DRUGS REVIEWED BY THE SMC The following new drugs have been reviewed
Lincolnshire Prescribing and Clinical Effectiveness Bulletin
 S Lincolnshire Prescribing and Clinical Effectiveness Bulletin Volume 11 No 12, September 2017 Optum in association with Lincolnshire Clinical Commissioning Groups, Lincolnshire Community Health Services,
S Lincolnshire Prescribing and Clinical Effectiveness Bulletin Volume 11 No 12, September 2017 Optum in association with Lincolnshire Clinical Commissioning Groups, Lincolnshire Community Health Services,
PRESCRIPTION PAD. The Newsletter of the Cumbria Area Prescribing Committee. February 2012 No. 18. Click here to find more. News from the MHRA
 PRESCRIPTION PAD The Newsletter of the Cumbria Area Prescribing Committee February 2012 No. 18 Click here to find more Clinical policy and Formulary news Lothian changes Targeted Medication Use Reviews
PRESCRIPTION PAD The Newsletter of the Cumbria Area Prescribing Committee February 2012 No. 18 Click here to find more Clinical policy and Formulary news Lothian changes Targeted Medication Use Reviews
April May For adults for the treatment of visual impairment due to macular oedema secondary to central retinal vein occlusion.
 April May 2014 Recommended for use within NHS Scotland April May 2014 aflibercept intravitreal (Eylea ) 954/14 For adults for the treatment of visual impairment due to macular oedema secondary to central
April May 2014 Recommended for use within NHS Scotland April May 2014 aflibercept intravitreal (Eylea ) 954/14 For adults for the treatment of visual impairment due to macular oedema secondary to central
INFLIXIMAB Remicade (infliximab), Inflectra (infliximab-dyyb), Ixifi* (infliximabqbtx), Renflexis (infliximab-abda)
 Pre - PA Allowance None Prior-Approval Requirements Diagnoses Patient must have ONE of the following: 6 years of age or older 1. Moderate to severe Crohn s disease (CD) a. Patient has fistulizing disease
Pre - PA Allowance None Prior-Approval Requirements Diagnoses Patient must have ONE of the following: 6 years of age or older 1. Moderate to severe Crohn s disease (CD) a. Patient has fistulizing disease
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Single Technology Appraisal. Canagliflozin in combination therapy for treating type 2 diabetes
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal Canagliflozin in combination therapy for Final scope Remit/appraisal objective To appraise the clinical and cost effectiveness
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal Canagliflozin in combination therapy for Final scope Remit/appraisal objective To appraise the clinical and cost effectiveness
Osteoporosis/Fracture Prevention
 Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
Osteoporosis/Fracture Prevention NATIONAL GUIDELINE SUMMARY This guideline was developed using an evidence-based methodology by the KP National Osteoporosis/Fracture Prevention Guideline Development Team
NEW MEDICINE APPLICATIONS BROUGHT TO THE BORDERS FORMULARY COMMITTEE (BFC) AND AREA DRUG & THERAPEUTICS COMMITTEE (ADTC) 2015/16
 New Medicine Product/Device Endorsement Categories NEW MEDICINE APPLICATIONS BROUGHT TO THE BORDERS FORMULARY COMMITTEE (BFC) AND AREA DRUG & THERAPEUTICS COMMITTEE (ADTC) 2015/16 A B C D E F recommended
New Medicine Product/Device Endorsement Categories NEW MEDICINE APPLICATIONS BROUGHT TO THE BORDERS FORMULARY COMMITTEE (BFC) AND AREA DRUG & THERAPEUTICS COMMITTEE (ADTC) 2015/16 A B C D E F recommended
Remicade. Remicade (infliximab), Inflectra (infliximab-dyyb) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Remicade Page: 1 of 9 Last Review Date: June 22, 2017 Remicade Description Remicade (infliximab),
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Remicade Page: 1 of 9 Last Review Date: June 22, 2017 Remicade Description Remicade (infliximab),
Psoriatic Arthritis- Second Line Treatments
 Psoriatic Arthritis- Second Line Treatments Second line treatments for Psoriatic Arthritis (PsA) are usually prescribed by a Rheumatologist, Dermatologist, or in a combined clinic where both the Dermatologist
Psoriatic Arthritis- Second Line Treatments Second line treatments for Psoriatic Arthritis (PsA) are usually prescribed by a Rheumatologist, Dermatologist, or in a combined clinic where both the Dermatologist
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Myocardial infarction: secondary prevention in primary and secondary care for patients following a myocardial infarction 1.1
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Myocardial infarction: secondary prevention in primary and secondary care for patients following a myocardial infarction 1.1
SWL Drug Pathway Ulcerative Colitis Version 3 (Oct 2018) (based on NICE ulcerative colitis commissioning algorithm - with local adaptation)
 Adult with active ulcerative colitis Does the adult have moderately to severely active ulcerative colitis managed in outpatients with no need for hospitalisation/surgery? Moderately to severely active
Adult with active ulcerative colitis Does the adult have moderately to severely active ulcerative colitis managed in outpatients with no need for hospitalisation/surgery? Moderately to severely active
ORENCIA (ABATACEPT) INJECTION FOR INTRAVENOUS INFUSION
 UnitedHealthcare Community Plan Medical Benefit Drug Policy ORENCIA (ABATACEPT) INJECTION FOR INTRAVENOUS INFUSION Policy Number: CS2018D0039J Effective Date: March 1, 2018 Table of Contents Page INSTRUCTIONS
UnitedHealthcare Community Plan Medical Benefit Drug Policy ORENCIA (ABATACEPT) INJECTION FOR INTRAVENOUS INFUSION Policy Number: CS2018D0039J Effective Date: March 1, 2018 Table of Contents Page INSTRUCTIONS
Remicade. Remicade (infliximab), Inflectra (infliximab-dyyb) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.02 Subsection: Gastrointestinal nts Original Policy Date: May 20, 2011 Subject: Remicade Page: 1 of
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.02 Subsection: Gastrointestinal nts Original Policy Date: May 20, 2011 Subject: Remicade Page: 1 of
Empagliflozin (Jardiance ) for the treatment of type 2 diabetes mellitus, the EMPA REG OUTCOME study
 Empagliflozin (Jardiance ) for the treatment of type 2 diabetes mellitus, the EMPA REG OUTCOME study POSITION STATEMENT: Clinicians should continue to follow MHRA advice and NICE technology appraisal guidance
Empagliflozin (Jardiance ) for the treatment of type 2 diabetes mellitus, the EMPA REG OUTCOME study POSITION STATEMENT: Clinicians should continue to follow MHRA advice and NICE technology appraisal guidance
Carelirst.+.V Family of health care plans
 Carelirst.+.V Family of health care plans CVS Caremark POLICY Document for ACTEMRA The overall objective of this policy is to support the appropriate and cost effective use of the medication, specific
Carelirst.+.V Family of health care plans CVS Caremark POLICY Document for ACTEMRA The overall objective of this policy is to support the appropriate and cost effective use of the medication, specific
Osteoporosis challenges
 Osteoporosis challenges Osteoporosis challenges Who should have a fracture risk assessment? Who to treat? Drugs, holidays and unusual adverse effects Fracture liaison service? The size of the problem 1
Osteoporosis challenges Osteoporosis challenges Who should have a fracture risk assessment? Who to treat? Drugs, holidays and unusual adverse effects Fracture liaison service? The size of the problem 1
SUMMARY OF CHANGES TO QOF 2017/18 - ENGLAND CLINICAL
 SUMMARY OF CHANGES TO QOF 2017/18 - ENGLAND KEY No change Retired/replaced Wording and/or timeframe change Point or threshold change Indicator ID change 1/17 QOF ID 17/18 QOF ID NICE ID Indicator wording
SUMMARY OF CHANGES TO QOF 2017/18 - ENGLAND KEY No change Retired/replaced Wording and/or timeframe change Point or threshold change Indicator ID change 1/17 QOF ID 17/18 QOF ID NICE ID Indicator wording
Technology appraisal guidance Published: 23 November 2016 nice.org.uk/guidance/ta418
 Dapagliflozin in triple therapy for treating type 2 diabetes Technology appraisal guidance Published: 23 November 2016 nice.org.uk/guidance/ta418 NICE 2018. All rights reserved. Subject to Notice of rights
Dapagliflozin in triple therapy for treating type 2 diabetes Technology appraisal guidance Published: 23 November 2016 nice.org.uk/guidance/ta418 NICE 2018. All rights reserved. Subject to Notice of rights
Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis. Date: Date:
 Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis Section 1: Shared care arrangements and responsibilities Section 1.1 Agreement for transfer of prescribing to
Denosumab (Prolia 60 mg) Effective Shared Care Agreement For the treatment of Osteoporosis Section 1: Shared care arrangements and responsibilities Section 1.1 Agreement for transfer of prescribing to
