FEP Medical Policy Manual
|
|
|
- Janice Sutton
- 6 years ago
- Views:
Transcription
1 FEP Medical Policy Manual Effective Date: July15, 2017 Related Policies: Recombinant and Autologous Platelet-Derived Growth Factors for Healing and Other Non Orthopedic Conditions Bioengineered Skin and Soft Tissue Substitutes Orthopedic Applications of Stem Cell Therapy Amniotic Membrane and Amniotic Fluid Injections Description Several commercially available forms of human amniotic membrane (HAM) and amniotic fluid can be administered by patches, topical application, or injection. Amniotic membrane and amniotic fluid are being evaluated for the treatment of a variety of conditions, including chronic full-thickness diabetic lower extremity ulcers, venous ulcers, knee osteoarthritis, plantar fasciitis, and ophthalmic conditions. FDA REGULATORY STATUS The U.S. Food and Drug Administration regulates human cells and tissues intended for implantation, transplantation, or infusion through the Center for Biologics Evaluation and Research, under Code of Federal Regulation (CFR) title 21, parts 1270 and Human amniotic membrane and amniotic fluid are included in these regulations. POLICY STATEMENT Treatment of nonhealing diabetic lower-extremity ulcers using the following human amniotic membrane products (AmnioBand Membrane, Biovance, Epifix, Grafix ) may be considered medically necessary. Amniotic membrane grafts that are fixated using sutures or glue fixation or secured under a bandage contact lens may be considered medically necessary for the treatment of the following ophthalmic indications: Neurotrophic keratitis Corneal ulcers and melts Pterygium repair Stevens-Johnson syndrome Persistent epithelial defects Amniotic membrane grafts using suture or glue fixation is considered investigational for the treatment of all other ophthalmic conditions including but not limited to dry eye syndrome, burns, corneal perforation, bullous keratopathy, limbus stem cell deficiency, and after photorefractive keratectomy. Self-contained or unfixated amniotic membrane products (eg, Prokera ) for ophthalmic indications are considered not medically necessary. Original Policy Date: January 2017 Page: 1
2 Effective Policy Date: July 15, 2017 Page: 2 of 6 Injection of micronized or particulated human amniotic membrane is considered investigational for all indications. Injection of human amniotic fluid is considered investigational for all indications. All other human amniotic membrane products and indications not listed above are considered investigational. POLICY GUIDELINES Nonhealing is defined as less than a 20% decrease in wound area with standard wound care for at least 2 weeks. Persistent epithelial defect has failed to close completely after 5 days of conservative treatment or has failed to demonstrate a decrease in size after 2 days of conservative treatment. Conservative treatment is defined as use of topical lubricants and/or topical antibiotics and/or therapeutic contact lens and/or patching. Failure of multiple modalities should not be required prior to moving to amniotic membrane grafts (AMGs). AMG requires less effort on the part of the patient to adhere to a treatment regimen and has a significant advantage in that regard over treatments that require multiple drops per day. BENEFIT APPLICATION Experimental or investigational procedures, treatments, drugs, or devices are not covered (See General Exclusion Section of brochure). RATIONALE Summary of Evidence Diabetic Lower-Extremity Ulcers For individuals who have nonhealing diabetic lower-extremity ulcers who receive patch or flowable formulation of human amniotic membrane (HAM; AmnioBand Membrane, Biovance, Epifix, Grafix), the evidence includes randomized controlled trials (RCTs). Relevant outcomes are symptoms, morbid events, functional outcomes, and quality of life. The evidence on amniotic and placental membrane products for the treatment of nonhealing (<20% healing with 2 weeks of standard care) diabetic lower-extremity ulcers includes several RCTs that compared HAM to standard care or to an established advanced wound care product. These industry-sponsored studies used wound closure as the primary outcome measure, and some used power analysis, blinded assessment of wound healing, and intention-to-treat analysis. For the HAM products that have been sufficiently evaluated (AmnioBand Membrane, Biovance, Epifix, Grafix), results have shown improved outcomes compared to standard care, and outcomes that are at least as good as an established advanced wound care product. Improved health outcomes in the RCTs are supported by multicenter registries. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. Lower-Extremity Ulcers due to Venous Insufficiency For individuals who have lower-extremity ulcers due to venous insufficiency who receive patch or flowable formulation of HAM, the evidence includes an RCT. Relevant outcomes are symptoms, morbid events, functional outcomes, and quality of life. In a randomized comparison of a cryopreserved HAM (c-ham) product to standard of care, there was no difference between the experimental and controls groups in complete wound closure at 4 weeks. Because HAM has not been shown to improve healing of venous ulcers in controlled studies, comparative studies on other HAM products are needed. The evidence is insufficient to determine the effects of the technology on health outcomes.
3 Effective Policy Date: July 15, 2017 Page: 3 of 6 Osteoarthritis For individuals who have knee osteoarthritis who receive injection of suspension or particulate formulation of human amniotic membrane or amniotic fluid, the evidence includes a feasibility study. Relevant outcomes are symptoms, functional outcomes, quality of life, and treatment-related morbidity. The pilot study was in preparation for a larger RCT of HAM injection. Additional trials, which will have a larger sample sizes and longer follow-up, are needed to permit conclusions on the effect of this treatment. The evidence is insufficient to determine the effects of the technology on health outcomes. Plantar Fasciitis For individuals who have plantar fasciitis who receive injection of suspension or particulate formulation of human amniotic membrane or amniotic fluid, the evidence includes 2 small RCTs. Relevant outcomes are symptoms, functional outcomes, quality of life, and treatment-related morbidity. Literature on HAM injections is at a very early stage. Evidence includes a small (N=23) double-blind comparison with corticosteroid and a patient-blinded (N=45) comparison of 2 different doses of dehydrated HAM with saline. Additional controlled trials with larger sample sizes and longer follow-up are needed to permit conclusions on the effect of this treatment on plantar fasciitis pain. Also needed are RCTs in humans to evaluate the efficacy of amniotic membrane and amniotic fluid injections for the treatment of other conditions, including but not limited to tendonitis. The evidence is insufficient to determine the effects of the technology on health outcomes. Ophthalmic Conditions For individuals who have neurotrophic keratitis, corneal ulcers and melts, pterygium repair, Stevens Johnson, or persistent epithelial defects who receive fixated (sutured, glued or secured under a bandage contact lens) human amniotic membrane graft the evidence incudes several RCTs and a technology assessment. Relevant outcomes are symptoms, morbid events, functional outcomes, and quality of life. The most widely studied condition with a technology assessment of RCT evidence is use of HAM following pterygium repair. The technology assessment concluded, based on 4 RCTs, that conjunctival or limbal autograft was more effective than HAM. An RCT on HAM for refractory neurotrophic corneal ulcers found that outcomes following HAM graft were similar to conventional therapy. One RCT has shown that application of c-ham in the early stages of Stevens Johnson leads to clinically significant improvement compared to medical therapy alone. Other indications have been studied only in case series. RCTs are needed to evaluate the efficacy of this procedure. The evidence is insufficient to determine the effects of the technology on health outcomes. For individuals who have ophthalmic disorders other than neurotrophic keratitis, corneal ulcers and melts, pterygium repair, Stevens Johnson, or persistent epithelial defects who receive fixated (sutured, glued or secured under a bandage contact lens) human amniotic membrane graft, the evidence includes 2 RCTs and a systematic review of 1 RCT. Relevant outcomes are symptoms, morbid events, functional outcomes, and quality of life. A 2012 Cochrane review found a single RCT on HAM graft for acute ocular burns. The study suggested a benefit on rate of healing for ocular burns but the study was considered to be at high or uncertain risk of bias due to unequal baseline scores and the lack of masking to treatment condition. A study on HAM for the treatment of bullous keratopathy reported that that there was no difference in clinical outcomes between HAM and stromal puncture. RCTs are needed for the benefit of HAM for these other indications. The evidence is insufficient to determine the effects of the technology on health outcomes. For individuals who have ophthalmic conditions who receive an self-retained or unfixated formulation of HAM, the evidence includes one within-subject comparative study and case series. Relevant outcomes
4 Effective Policy Date: July 15, 2017 Page: 4 of 6 are symptoms, morbid events, functional outcomes, and quality of life. Traditionally, amniotic membrane has been sutured onto the eye for a variety of severe ocular surface disorders. The Prokera device is novel by having a ring around the c-ham allograft that allows it to be inserted under topical anesthesia, similar to insertion of a contact lens, allowing for more widespread use. Use of Prokera has been reported for refractory ulcerative keratitis, neurotrophic keratitis, recurrent epithelial erosion, high-risk corneal grafts, acute chemical and thermal burns, acute Stevens-Johnson syndrome, necrotizing scleritis, and limbal stem cell deficiency. Current evidence on use of the Prokera device is limited. While the case series report generally positive effects, the prospective comparative trial found no benefit of HAM compared to a bandage contact lens when used for wound healing after PRK. RCTs are needed to determine whether HAM improves healing for the various ophthalmic disorders. The evidence is insufficient to determine the effects of the technology on health outcomes. SUPPLEMENTAL INFORMATION No guidelines or statements were identified. Practice Guidelines and Position Statements Not applicable. U.S. Preventive Services Task Force Recommendations Medicare National Coverage There is no national coverage determination (NCD). In the absence of an NCD, coverage decisions are left to the discretion of local Medicare carriers. REFERENCES 1. Parolini O, Soncini M, Evangelista M, et al. Amniotic membrane and amniotic fluid-derived cells: potential tools for regenerative medicine? Regen Med. Mar 2009;4(2): PMID Koob TJ, Rennert R, Zabek N, et al. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J. Oct 2013;10(5): PMID Shimberg M, Wadsworth K. The use of amniotic-fluid concentrate in orthopaedic conditions. J Bone Joint Surg. 1938;20(I): U.S. Food and Drug Administration. Guidance for industry: Chronic cutaneous ulcer and burn wounds. 2006; Accessed December 22, Gottrup F, Apelqvist J, Price P, et al. Outcomes in controlled and comparative studies on non-healing wounds: recommendations to improve the quality of evidence in wound management. J Wound Care. Jun 2010;19(6): PMID Sheehan P, Jones P, Caselli A, et al. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care. Jun 2003;26(6): PMID DiDomenico LA, Orgill DP, Galiano RD, et al. Aseptically processed placental membrane improves healing of diabetic foot ulcerations: prospective, randomized clinical trial. Plast Reconstr Surg Glob Open. Oct 2016;4(10):e1095. PMID Snyder RJ, Shimozaki K, Tallis A, et al. A prospective, randomized, multicenter, controlled evaluation of the use of dehydrated amniotic membrane allograft compared to standard of care for the closure of chronic diabetic foot ulcer. Wounds. Mar 2016;28(3): PMID Smiell JM, Treadwell T, Hahn HD, et al. Real-world experience with a decellularized dehydrated human amniotic membrane allograft. Wounds. Jun 2015;27(6): PMID Zelen CM, Serena TE, Denoziere G, et al. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. Oct 2013;10(5): PMID
5 Effective Policy Date: July 15, 2017 Page: 5 of Zelen CM, Serena TE, Fetterolf DE. Dehydrated human amnion/chorion membrane allografts in patients with chronic diabetic foot ulcers: a long term follow-up study. Wound Med. 2014;4: Zelen CM, Gould L, Serena TE, et al. A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J. Dec 2015;12(6): PMID Kirsner RS, Sabolinski ML, Parsons NB, et al. Comparative effectiveness of a bioengineered living cellular construct vs. a dehydrated human amniotic membrane allograft for the treatment of diabetic foot ulcers in a real world setting. Wound Repair Regen. Sep 2015;23(5): PMID Lavery LA, Fulmer J, Shebetka KA, et al. The efficacy and safety of Grafix((R)) for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J. Oct 2014;11(5): PMID Serena TE, Carter MJ, Le LT, et al. A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. multilayer compression therapy alone in the treatment of venous leg ulcers. Wound Repair and Regeneration. Nov-Dec 2014;22(6): PMID Serena TE, Yaakov R, DiMarco D, et al. Dehydrated human amnion/chorion membrane treatment of venous leg ulcers: correlation between 4-week and 24-week outcomes. J Wound Care. Nov 2015;24(11): PMID Vines JB, Aliprantis AO, Gomoll AH, et al. Cryopreserved amniotic suspension for the treatment of knee osteoarthritis. J Knee Surg. Aug 2016;29(6): PMID Tsikopoulos K, Vasiliadis HS, Mavridis D. Injection therapies for plantar fasciopathy ('plantar fasciitis'): a systematic review and network meta-analysis of 22 randomised controlled trials. Br J Sports Med. Nov 2016;50(22): PMID Hanselman AE, Tidwell JE, Santrock RD. Cryopreserved human amniotic membrane injection for plantar fasciitis: a randomized, controlled, double-blind pilot study. Foot Ankle Int. Feb 2015;36(2): PMID Zelen CM, Poka A, Andrews J. Prospective, randomized, blinded, comparative study of injectable micronized dehydrated amniotic/chorionic membrane allograft for plantar fasciitis--a feasibility study. Foot Ankle Int. Oct 2013;34(10): PMID Khokhar S, Natung T, Sony P, et al. Amniotic membrane transplantation in refractory neurotrophic corneal ulcers: a randomized, controlled clinical trial. Cornea. Aug 2005;24(6): PMID Kaufman SC, Jacobs DS, Lee WB, et al. Options and adjuvants in surgery for pterygium: a report by the American Academy of Ophthalmology. Ophthalmology. Jan 2013;120(1): PMID Sharma N, Thenarasun SA, Kaur M, et al. Adjuvant role of amniotic membrane transplantation in acute ocular stevens-johnson syndrome: a randomized control trial. Ophthalmology. Mar 2016;123(3): PMID Bouchard CS, John T. Amniotic membrane transplantation in the management of severe ocular surface disease: indications and outcomes. Ocul Surf. Jul 2004;2(3): PMID Clare G, Suleman H, Bunce C, et al. Amniotic membrane transplantation for acute ocular burns. Cochrane Database Syst Rev. 2012;9:CD PMID Paris Fdos S, Goncalves ED, Campos MS, et al. Amniotic membrane transplantation versus anterior stromal puncture in bullous keratopathy: a comparative study. Br J Ophthalmol. Aug 2013;97(8): PMID Cheng AM, Zhao D, Chen R, et al. Accelerated restoration of ocular surface health in dry eye disease by selfretained cryopreserved amniotic membrane. Ocul Surf. Jan 2016;14(1): PMID Vlasov A, Sia RK, Ryan DS, et al. Sutureless cryopreserved amniotic membrane graft and wound healing after photorefractive keratectomy. J Cataract Refract Surg. Mar 2016;42(3): PMID
6 Effective Policy Date: July 15, 2017 Page: 6 of 6 POLICY HISTORY Date Action Description January 2017 New Policy March 2017 Update Policy Policy updated with literature review through November 7, 2016; material on patch formulations of amniotic membrane moved from policy (Bioengineered Skin and Soft Tissue Substitutes); references 7-8, 15, 18, 20, and added. AmnioBand Membrane, Biovance, Epifix, Grafix considered medically necessary for diabetic foot ulcers; all other products and indications are investigational. June 2017 Revise Policy Policy updated with literature review through April 27, 2017; references added. Clinical input reviewed. Fixated amniotic membrane grafts considered medically necessary for neurotrophic keratitis, corneal ulcers and melts, following pterygium repair, Stevens Johnson, and persistent epithelial defects.
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: April 15, 2018 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors for Healing and Other Non Orthopedic Conditions 7.01.113 Bioengineered
FEP Medical Policy Manual Effective Date: April 15, 2018 Related Policies: 2.01.16 Recombinant and Autologous Platelet-Derived Growth Factors for Healing and Other Non Orthopedic Conditions 7.01.113 Bioengineered
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: POLICY LAST UPDATED:
 Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 05 01 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 05 01 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
medically necessary as the evidence is insufficient to determine the effects of the technology on health outcomes.
 Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 10 02 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
Medical Coverage Policy Amniotic Membrane and Amniotic Fluid EFFECTIVE DATE: 09 01 2018 POLICY LAST UPDATED: 10 02 2018 OVERVIEW Several commercially available forms of human amniotic membrane (HAM) and
Amniotic Membrane and Amniotic Fluid
 Amniotic Membrane and Amniotic Fluid Policy Number: 7.01.149 Last Review: 3/2019 Origination: 4/2016 Next Review: 3/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Amniotic Membrane and Amniotic Fluid Policy Number: 7.01.149 Last Review: 3/2019 Origination: 4/2016 Next Review: 3/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Clinical Policy: EpiFix Wound Treatment
 Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
Clinical Policy: Reference Number: PA.CP.MP.140 Effective Date: 03/18 Last Review Date: 04/18 Coding Implications Revision Log Description EpiFix (MiMedx Group) is dehydrated human amniotic tissue that
Medical Policy. MP Amniotic Membrane and Amniotic Fluid. Related Policies Experimental / Investigational Services
 Medical Policy BCBSA Ref. Policy: 7.01.149 Last Review: 02/26/2018 Effective Date: 02/26/2018 Section: Surgery Related Policies 9.01.502 Experimental / Investigational Services DISCLAIMER Our medical policies
Medical Policy BCBSA Ref. Policy: 7.01.149 Last Review: 02/26/2018 Effective Date: 02/26/2018 Section: Surgery Related Policies 9.01.502 Experimental / Investigational Services DISCLAIMER Our medical policies
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Amniotic Membrane and Amniotic Fluid Page 1 of 26 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Amniotic Membrane and Amniotic Fluid Bio-Engineered Skin
Amniotic Membrane and Amniotic Fluid Page 1 of 26 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Amniotic Membrane and Amniotic Fluid Bio-Engineered Skin
CLINICAL INPUT RESPONSES
 CLINICAL INPUT RESPONSES Additional Comments With regard to the 9 indications listed above, there was lower range confidence that there is adequate evidence demonstrating that sutureless fixation human
CLINICAL INPUT RESPONSES Additional Comments With regard to the 9 indications listed above, there was lower range confidence that there is adequate evidence demonstrating that sutureless fixation human
HUMAN AMNIOTIC MEMBRANE GRAFTS TO ENHANCE HEALING. Frank Burrows, MBA, EMT David Mason, MD
 HUMAN AMNIOTIC MEMBRANE GRAFTS TO ENHANCE HEALING Frank Burrows, MBA, EMT David Mason, MD Good Wound Care Treat the whole patient, not just the hole in the patient Ensure adequate perfusion to wound site
HUMAN AMNIOTIC MEMBRANE GRAFTS TO ENHANCE HEALING Frank Burrows, MBA, EMT David Mason, MD Good Wound Care Treat the whole patient, not just the hole in the patient Ensure adequate perfusion to wound site
Medical Affairs Policy
 Medical Affairs Policy Service: Corneal Treatments and Specialized Contact Lenses (Corneal remodeling, Corneal transplant, Corneal collagen crosslinking, Intrastromal Rings- INTACS, Keratoconus treatments,
Medical Affairs Policy Service: Corneal Treatments and Specialized Contact Lenses (Corneal remodeling, Corneal transplant, Corneal collagen crosslinking, Intrastromal Rings- INTACS, Keratoconus treatments,
Granulox Update on evidence and science
 Update on evidence and science New clinical data in DFU wounds Retrospective cohort evaluation Site: Southtees, UK Main Inclusion criteria: DFU, < wound size reduction in 4 weeks Verum Group: Standard
Update on evidence and science New clinical data in DFU wounds Retrospective cohort evaluation Site: Southtees, UK Main Inclusion criteria: DFU, < wound size reduction in 4 weeks Verum Group: Standard
NEW OPPORTUNITIES OF USING THERAPEUTICAL CONTACT LENSES IN OCULAR SURGERY
 NEW OPPORTUNITIES OF USING THERAPEUTICAL CONTACT LENSES IN OCULAR SURGERY Authors: Prof univ. dr. Adriana Stănilă, Dr. Elena Mihai, Dr. Adrian Teodoru, Dr. IonuŃ Costache The Clinical Department of Op
NEW OPPORTUNITIES OF USING THERAPEUTICAL CONTACT LENSES IN OCULAR SURGERY Authors: Prof univ. dr. Adriana Stănilă, Dr. Elena Mihai, Dr. Adrian Teodoru, Dr. IonuŃ Costache The Clinical Department of Op
Use of Placental Tissue for Orthopedic Injuries: Potentials and Pitfalls. Daniel Kuebler, PhD Franciscan University of Steubenville
 Use of Placental Tissue for Orthopedic Injuries: Potentials and Pitfalls Daniel Kuebler, PhD Franciscan University of Steubenville Benjamin D. Smith & Daniel A. Grande. The current state of scaffolds for
Use of Placental Tissue for Orthopedic Injuries: Potentials and Pitfalls Daniel Kuebler, PhD Franciscan University of Steubenville Benjamin D. Smith & Daniel A. Grande. The current state of scaffolds for
Subject Index. Atopic keratoconjunctivitis (AKC) management 16 overview 15
 Subject Index Acanthamoeba keratitis, see Infective keratitis Acute allergic conjunctivitis AKC, see Atopic keratoconjunctivitis Allergy acute allergic conjunctivitis 15 atopic keratoconjunctivitis 15
Subject Index Acanthamoeba keratitis, see Infective keratitis Acute allergic conjunctivitis AKC, see Atopic keratoconjunctivitis Allergy acute allergic conjunctivitis 15 atopic keratoconjunctivitis 15
Charles Zelen, DPM Attila Poka, MD James Andrews, MD
 A Prospective, Randomized, Blinded, Comparative Study of Injectable Dehydrated Human Amniotic/Chorionic Membrane (dhacm) in the Treatment of Recalcitrant Plantar Fasciitis Charles Zelen, DPM Attila Poka,
A Prospective, Randomized, Blinded, Comparative Study of Injectable Dehydrated Human Amniotic/Chorionic Membrane (dhacm) in the Treatment of Recalcitrant Plantar Fasciitis Charles Zelen, DPM Attila Poka,
FORWARD LOOKING STATEMENT
 June 2018 FORWARD LOOKING STATEMENT This presentation includes forward looking statements regarding: future incremental insurance coverage; future size of sales force; future approved indications for use
June 2018 FORWARD LOOKING STATEMENT This presentation includes forward looking statements regarding: future incremental insurance coverage; future size of sales force; future approved indications for use
Non-Contact Ultrasound Treatment for Wounds
 Non-Contact Ultrasound Treatment for Wounds Policy Number: 2.01.79 Last Review: 1/2018 Origination: 1/2008 Next Review: 7/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Non-Contact Ultrasound Treatment for Wounds Policy Number: 2.01.79 Last Review: 1/2018 Origination: 1/2008 Next Review: 7/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
FEP Medical Policy Manual
 FEP Medical Manual 9.03.05 Corneal Topography/Computer-Assisted Corneal Topography/ Photokeratoscopy Last Review: September 2016 Next Review: September 2017 Related Policies 9.03.28 Corneal Collagen Cross-linking
FEP Medical Manual 9.03.05 Corneal Topography/Computer-Assisted Corneal Topography/ Photokeratoscopy Last Review: September 2016 Next Review: September 2017 Related Policies 9.03.28 Corneal Collagen Cross-linking
10/8/14. Revision Date(s): Policy Number: MCP-207. Review Date: 12/16/15, 9/15/16
 Subject: Platelet-rich Plasma (PRP) Policy Number: MCP-207 Review Date: 12/16/15, 9/15/16 Revision Date(s): Original Effective Date: 10/8/14 DISCLAIMER This Molina Clinical Policy (MCP) is intended to
Subject: Platelet-rich Plasma (PRP) Policy Number: MCP-207 Review Date: 12/16/15, 9/15/16 Revision Date(s): Original Effective Date: 10/8/14 DISCLAIMER This Molina Clinical Policy (MCP) is intended to
Eyelid Thermal Pulsation for the Treatment of Dry Eye Syndrome
 Eyelid Thermal Pulsation for the Treatment of Dry Eye Syndrome Policy Number: 9.03.29 Last Review: 5/2018 Origination: 9/2013 Next Review: 11/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Eyelid Thermal Pulsation for the Treatment of Dry Eye Syndrome Policy Number: 9.03.29 Last Review: 5/2018 Origination: 9/2013 Next Review: 11/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
FEP Medical Policy Manual
 FEP Medical Policy Manual Last Review: September 2016 Next Review: September 2017 Related Policies 6.01.10 Stereotactic Radiosurgery and Stereotactic Body Radiotherapy 8.01.10 Charged-Particle (Proton
FEP Medical Policy Manual Last Review: September 2016 Next Review: September 2017 Related Policies 6.01.10 Stereotactic Radiosurgery and Stereotactic Body Radiotherapy 8.01.10 Charged-Particle (Proton
Clinical Policy: Refractive Surgery Reference Number: CP.MP. 391
 Clinical Policy: Refractive Surgery Reference Number: CP.MP. 391 Effective Date: November 2007 Last Review Date: January 2016 Coding Implications Revision Log See Important Reminder at the end of this
Clinical Policy: Refractive Surgery Reference Number: CP.MP. 391 Effective Date: November 2007 Last Review Date: January 2016 Coding Implications Revision Log See Important Reminder at the end of this
Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18
 Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18 See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Low-Frequency Ultrasound Therapy for Wound Management Reference Number: CP.MP.139 Last Review Date: 01/18 See Important Reminder at the end of this policy for important regulatory and
8/11/2017. Rob Allen, O.D. Rob Allen, Ph.D. Rob Allen, O.D. None. Rob Allen, Ph.D. Represents BioD/BioDOPTIX Amniotic Membrane
 Rob Allen, O.D. Rob Allen, Ph.D. VisionAmerica August 25, 2017 Rob Allen, O.D. None Rob Allen, Ph.D. Represents BioD/BioDOPTIX Amniotic Membrane Rob Allen, O.D. Rob Allen, Ph.D. 1 Rob Allen, O.D. Rob Allen,
Rob Allen, O.D. Rob Allen, Ph.D. VisionAmerica August 25, 2017 Rob Allen, O.D. None Rob Allen, Ph.D. Represents BioD/BioDOPTIX Amniotic Membrane Rob Allen, O.D. Rob Allen, Ph.D. 1 Rob Allen, O.D. Rob Allen,
rhngf for neurotrophic keratitis first line
 September 2015 Horizon Scanning Research & Intelligence Centre rhngf for neurotrophic keratitis first line LAY SUMMARY This briefing is based on information available at the time of research and a limited
September 2015 Horizon Scanning Research & Intelligence Centre rhngf for neurotrophic keratitis first line LAY SUMMARY This briefing is based on information available at the time of research and a limited
Corporate Presentation December Two Versatile Platforms Moving Towards Commercialization NASDAQ: EYEG
 Corporate Presentation December 2018 Two Versatile Platforms Moving Towards Commercialization NASDAQ: EYEG Forward Looking Statements Some of the matters discussed in this presentation contain forward-looking
Corporate Presentation December 2018 Two Versatile Platforms Moving Towards Commercialization NASDAQ: EYEG Forward Looking Statements Some of the matters discussed in this presentation contain forward-looking
Novel therapies for the treatment of persistent corneal epithelial defects
 Novel therapies for the treatment of persistent corneal epithelial defects Disclosures I have no financial interests in any of the techniques or products discussed. Bennie H. Jeng, M.D. Associate Professor
Novel therapies for the treatment of persistent corneal epithelial defects Disclosures I have no financial interests in any of the techniques or products discussed. Bennie H. Jeng, M.D. Associate Professor
Corporate Medical Policy Growth Factors in Wound Healing
 Corporate Medical Policy Growth Factors in Wound Healing File Name: Origination: Last CAP Review: Next CAP Review: Last Review: growth_factors_in_wound_healing 4/1993 11/2017 11/2018 11/2017 Description
Corporate Medical Policy Growth Factors in Wound Healing File Name: Origination: Last CAP Review: Next CAP Review: Last Review: growth_factors_in_wound_healing 4/1993 11/2017 11/2018 11/2017 Description
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: October 15, 2018 Related Policies: None Three-Dimensional Printed Orthopedic Implants Description This evidence review addresses orthopedic implants that are constructed
FEP Medical Policy Manual Effective Date: October 15, 2018 Related Policies: None Three-Dimensional Printed Orthopedic Implants Description This evidence review addresses orthopedic implants that are constructed
The Georgetown Team Approach to Diabetic Limb Salvage: 2013
 The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
The Georgetown Team Approach to Diabetic Limb Salvage: 2013 John S. Steinberg, DPM FACFAS Associate Professor, Department of Plastic Surgery Georgetown University School of Medicine Disclosures: None Need
Table 1. Characteristics of patients. Postoperative Comorbidity acuity band keratopathy. Visual Cause of. Case Age (Yr) Sex F/U (Month)
 착색양막을이용한띠각막병증의미용적치료 1459 Table 1. Characteristics of patients Case Age (Yr) Sex F/U (Month) Visual Cause of Postoperative Comorbidity acuity band keratopathy complications 1 19 M 13 NLP * PHPV Injection,
착색양막을이용한띠각막병증의미용적치료 1459 Table 1. Characteristics of patients Case Age (Yr) Sex F/U (Month) Visual Cause of Postoperative Comorbidity acuity band keratopathy complications 1 19 M 13 NLP * PHPV Injection,
Living Cells in DFU. Clinical Outcomes of Amnion Allografts with. Management. Alla Danilkovitch, PhD, RN
 Clinical Outcomes of Amnion Allografts with Living Cells in DFU Management Alla Danilkovitch, PhD, RN Chief Scientific Officer Osiris Therapeutics, Inc. Columbia, Maryland, USA Disclosures Stockholder:
Clinical Outcomes of Amnion Allografts with Living Cells in DFU Management Alla Danilkovitch, PhD, RN Chief Scientific Officer Osiris Therapeutics, Inc. Columbia, Maryland, USA Disclosures Stockholder:
Osteochondral allografting for all other joints is not covered as the evidence is insufficient to determine the
 Medical Coverage Policy Osteochondral Autologous Chrondrocyte Implantation for Focal Articular Cartilage Lesions EFFECTIVE DATE: 05 20 2008 POLICY LAST UPDATED: 10 16 2018 OVERVIEW A variety of procedures
Medical Coverage Policy Osteochondral Autologous Chrondrocyte Implantation for Focal Articular Cartilage Lesions EFFECTIVE DATE: 05 20 2008 POLICY LAST UPDATED: 10 16 2018 OVERVIEW A variety of procedures
Sclerokeratoplasty David S. Chu, M.D. Cases
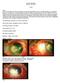 Sclerokeratoplasty David S. Chu, M.D. Cases Case 1 40 year-old female from Peru presented to our Service with inflamed OS for 2 months duration. Her symptoms began as red painful OS, which progressively
Sclerokeratoplasty David S. Chu, M.D. Cases Case 1 40 year-old female from Peru presented to our Service with inflamed OS for 2 months duration. Her symptoms began as red painful OS, which progressively
Clinical Policy Title: Therapeutic contact lenses
 Clinical Policy Title: Therapeutic contact lenses Clinical Policy Number: 10.02.02 Effective Date: June 1, 2014 Initial Review Date: December 18, 2013 Most Recent Review Date: January 11, 2018 Next Review
Clinical Policy Title: Therapeutic contact lenses Clinical Policy Number: 10.02.02 Effective Date: June 1, 2014 Initial Review Date: December 18, 2013 Most Recent Review Date: January 11, 2018 Next Review
Various therapies for ocular surface diseases
 Romanian Journal of Ophthalmology, Volume 62, Issue 1, January-March 2018. pp:83-87 CASE REPORT Various therapies for ocular surface diseases Gheorghe Alina*, Rosoga Ancuţa Teodora**, Mrini Fildys*, Vărgău
Romanian Journal of Ophthalmology, Volume 62, Issue 1, January-March 2018. pp:83-87 CASE REPORT Various therapies for ocular surface diseases Gheorghe Alina*, Rosoga Ancuţa Teodora**, Mrini Fildys*, Vărgău
WHS Abstract selected for the podium presentation and WHS Industrial Research & Development Poster
 Osiris Therapeutics to Present 29 Advanced Clinical and Scientific Abstracts, Including a Late-Breaking Abstract on its New Ambient Viable Tissue Preservation Technology Experts to host a variety of presentations
Osiris Therapeutics to Present 29 Advanced Clinical and Scientific Abstracts, Including a Late-Breaking Abstract on its New Ambient Viable Tissue Preservation Technology Experts to host a variety of presentations
WOUND CARE UPDATE. -Commonly Used Skin Substitute Products For Wound. -Total Contact Casting. Jack W. Hutter DPM, FACFAS, C. ped.
 WOUND CARE UPDATE -Commonly Used Skin Substitute Products For Wound Closure -Total Contact Casting Jack W. Hutter DPM, FACFAS, C. ped. Commonly Used Skin Substitute Products for Wound Closure why are they
WOUND CARE UPDATE -Commonly Used Skin Substitute Products For Wound Closure -Total Contact Casting Jack W. Hutter DPM, FACFAS, C. ped. Commonly Used Skin Substitute Products for Wound Closure why are they
Publication list of Dr. Alfred T S Leung
 Publication list of Dr. Alfred T S Leung No. of journal articles: 51 (as of January 2012) 1. Lam DS, Fan DS, Chan WM, Tam BS, Kwok AK, Leung AT, Parsons H. Prevalence and characteristics of peripheral
Publication list of Dr. Alfred T S Leung No. of journal articles: 51 (as of January 2012) 1. Lam DS, Fan DS, Chan WM, Tam BS, Kwok AK, Leung AT, Parsons H. Prevalence and characteristics of peripheral
Amniotic Membrane Transplantation In Ocular Surface Disorders
 Orginal Article Amniotic Membrane Transplantation In Ocular Surface Disorders Khalid Iqbal Talpur, Faiz Muhammad Halepota, Muhammad Pak J Ophthalmol 2005, Vol. 22 No. 3.................................................................................................
Orginal Article Amniotic Membrane Transplantation In Ocular Surface Disorders Khalid Iqbal Talpur, Faiz Muhammad Halepota, Muhammad Pak J Ophthalmol 2005, Vol. 22 No. 3.................................................................................................
Eyelid Thermal Pulsation for the Treatment of Dry Eye Syndrome
 Eyelid Thermal Pulsation for the Treatment of Dry Eye Syndrome Policy Number: 9.03.29 Last Review: 12/2017 Origination: 9/2013 Next Review: 5/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Eyelid Thermal Pulsation for the Treatment of Dry Eye Syndrome Policy Number: 9.03.29 Last Review: 12/2017 Origination: 9/2013 Next Review: 5/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Pachymetry Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Pachymetry Professional Institutional Original Effective Date: March 11, 2004 Original Effective
Pachymetry Page 1 of 8 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Pachymetry Professional Institutional Original Effective Date: March 11, 2004 Original Effective
THERAPEUTIC CONTACT LENSES
 THERAPEUTIC CONTACT LENSES Prof. Univ. Dr. Adriana Stanila Victor Papilian Faculty of Medicine Emergency Academic Hospital Sibiu Ocular Surface Research Center ROMANIA INTRODUCTION therapeuein greac =
THERAPEUTIC CONTACT LENSES Prof. Univ. Dr. Adriana Stanila Victor Papilian Faculty of Medicine Emergency Academic Hospital Sibiu Ocular Surface Research Center ROMANIA INTRODUCTION therapeuein greac =
Protocol. Endothelial Keratoplasty
 Protocol Endothelial Keratoplasty (90322) Medical Benefit Effective Date: 04/01/14 Next Review Date: 11/18 Preauthorization No Review Dates: 01/14, 11/14, 11/15, 11/16, 11/17 Preauthorization is not required.
Protocol Endothelial Keratoplasty (90322) Medical Benefit Effective Date: 04/01/14 Next Review Date: 11/18 Preauthorization No Review Dates: 01/14, 11/14, 11/15, 11/16, 11/17 Preauthorization is not required.
MEDICAL POLICY SUBJECT: CORNEAL ULTRASOUND PACHYMETRY. POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: CORNEAL ULTRASOUND,, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: CORNEAL ULTRASOUND,, PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
Non-Contact Ultrasound Treatments for Wounds
 Medical Policy Manual Medicine, Policy No. 131 Non-Contact Ultrasound Treatments for Wounds Next Review: February 2019 Last Review: February 2018 Effective: April 1, 2018 IMPORTANT REMINDER Medical Policies
Medical Policy Manual Medicine, Policy No. 131 Non-Contact Ultrasound Treatments for Wounds Next Review: February 2019 Last Review: February 2018 Effective: April 1, 2018 IMPORTANT REMINDER Medical Policies
The management of chronic
 Amniotic Membranes for Diabetic Foot Ulcerations Here s an update on their use. By Alison Migonis, DPM and John M. Giurini, DPM The management of chronic diabetic foot wounds poses a significant economic
Amniotic Membranes for Diabetic Foot Ulcerations Here s an update on their use. By Alison Migonis, DPM and John M. Giurini, DPM The management of chronic diabetic foot wounds poses a significant economic
Facet Arthroplasty. Policy Number: Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019
 Facet Arthroplasty Policy Number: 7.01.120 Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for total facet
Facet Arthroplasty Policy Number: 7.01.120 Last Review: 9/2018 Origination: 9/2009 Next Review: 3/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide coverage for total facet
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: endothelial_keratoplasty 9/2009 6/2018 6/2019 6/2018 Description of Procedure or Service Endothelial keratoplasty
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: endothelial_keratoplasty 9/2009 6/2018 6/2019 6/2018 Description of Procedure or Service Endothelial keratoplasty
MEDICAL POLICY SUBJECT: GAS PERMEABLE SCLERAL CONTACT LENS (E.G., BOSTON OCULAR SURFACE PROSTHESIS)
 MEDICAL POLICY SUBJECT: GAS PERMEABLE SCLERAL CONTACT PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: GAS PERMEABLE SCLERAL CONTACT PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
SMILE A Solution to Those who go in Harm s Way
 SMILE A Solution to Those who go in Harm s Way Steven Schallhorn, M.D. Chief Medical Officer November 12, 2016 Operational Basis for Involvement Commander of Naval Special Warfare identified deficiencies
SMILE A Solution to Those who go in Harm s Way Steven Schallhorn, M.D. Chief Medical Officer November 12, 2016 Operational Basis for Involvement Commander of Naval Special Warfare identified deficiencies
POLICIES AND PROCEDURE MANUAL
 POLICIES AND PROCEDURE MANUAL Policy: MP267 Section: Medical Benefit Policy Subject: Amniotic Membrane Transplant for Ocular Surface Defects I. Policy: Amniotic Membrane Transplant for Ocular Surface Defects
POLICIES AND PROCEDURE MANUAL Policy: MP267 Section: Medical Benefit Policy Subject: Amniotic Membrane Transplant for Ocular Surface Defects I. Policy: Amniotic Membrane Transplant for Ocular Surface Defects
CORNEAL CONDITIONS CORNEAL TRANSPLANTATION
 GENERAL INFORMATION CORNEAL CONDITIONS CORNEAL TRANSPLANTATION WHAT ARE CORNEAL CONDITIONS? The cornea is the clear outer layer of the eye. Shaped like a dome, it helps to protect the eye from foreign
GENERAL INFORMATION CORNEAL CONDITIONS CORNEAL TRANSPLANTATION WHAT ARE CORNEAL CONDITIONS? The cornea is the clear outer layer of the eye. Shaped like a dome, it helps to protect the eye from foreign
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: July 15, 2017 Related Policies: 1.01.05 Ultrasound Accelerated Fracture Healing Device 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton
FEP Medical Policy Manual Effective Date: July 15, 2017 Related Policies: 1.01.05 Ultrasound Accelerated Fracture Healing Device 7.01.07 Electrical Bone Growth Stimulation of the Appendicular Skeleton
Clinical Policy Title: Platelet rich plasma
 Clinical Policy Title: Platelet rich plasma Clinical Policy Number: 05.02.10 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2017 Next Review
Clinical Policy Title: Platelet rich plasma Clinical Policy Number: 05.02.10 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2017 Next Review
Clinical study of sutureless and glue free conjunctival autograft in pterygium surgery
 Original Article Clinical udy of sutureless and glue free conjunctival autograft in pterygium surgery Satish Desai 1*, Amol T Wanjari 2 1 Assiant Professor, PG. Student, Department of Ophthalmology, Government
Original Article Clinical udy of sutureless and glue free conjunctival autograft in pterygium surgery Satish Desai 1*, Amol T Wanjari 2 1 Assiant Professor, PG. Student, Department of Ophthalmology, Government
Peripheral Subcutaneous Field Stimulation. Description
 Subject: Peripheral Subcutaneous Field Stimulation Page: 1 of 6 Last Review Status/Date: June 2016 Peripheral Subcutaneous Field Stimulation Description Peripheral subcutaneous field stimulation (PSFS,
Subject: Peripheral Subcutaneous Field Stimulation Page: 1 of 6 Last Review Status/Date: June 2016 Peripheral Subcutaneous Field Stimulation Description Peripheral subcutaneous field stimulation (PSFS,
Original Research Article
 STUDY OF EPITHELIAL PHENOTYPE AFTER PTERYGIUM EXCISION BY USING CONJUNCTIVAL IMPRESSION CYTOLOGY. Dr. Sachin O. Agrawal*, Dr. Sudhir Pendke, Dr. Ravi Chauhan Department of Ophthalmology, Indira Gandhi
STUDY OF EPITHELIAL PHENOTYPE AFTER PTERYGIUM EXCISION BY USING CONJUNCTIVAL IMPRESSION CYTOLOGY. Dr. Sachin O. Agrawal*, Dr. Sudhir Pendke, Dr. Ravi Chauhan Department of Ophthalmology, Indira Gandhi
Photochemical corneal collagen cross-linkage using riboflavin and ultraviolet A for keratoconus and keratectasia
 Photochemical corneal collagen cross-linkage using riboflavin and ultraviolet A for keratoconus and keratectasia Issued: September 2013 guidance.nice.org.uk/ipg466 NICE has accredited the process used
Photochemical corneal collagen cross-linkage using riboflavin and ultraviolet A for keratoconus and keratectasia Issued: September 2013 guidance.nice.org.uk/ipg466 NICE has accredited the process used
Revitalization of the Anterior Segment: Corneal Transplantation and Secondary Lens Repair
 Revitalization of the Anterior Segment: Corneal Transplantation and Secondary Lens Repair CATHERINE REPPA, MD CORNEA SPECIALIST, ASSISTANT PROFESSOR TTUHSC DEPARTMENT OF OPHTHALMOLOGY AND VISUAL SCIENCES
Revitalization of the Anterior Segment: Corneal Transplantation and Secondary Lens Repair CATHERINE REPPA, MD CORNEA SPECIALIST, ASSISTANT PROFESSOR TTUHSC DEPARTMENT OF OPHTHALMOLOGY AND VISUAL SCIENCES
DESCRIPTION OF PROCEDURE/SERVICE/PHARMACEUTICAL
 Subject: Platelet-rich Plasma (PRP) Policy Number: MCP-207 Review Date: 12/16/15, 9/15/16, 9/19/17 Revision Date(s): Original Effective Date: 10/8/14 DISCLAIMER This Molina Clinical Policy (MCP) is intended
Subject: Platelet-rich Plasma (PRP) Policy Number: MCP-207 Review Date: 12/16/15, 9/15/16, 9/19/17 Revision Date(s): Original Effective Date: 10/8/14 DISCLAIMER This Molina Clinical Policy (MCP) is intended
Interventional procedures guidance Published: 25 September 2013 nice.org.uk/guidance/ipg466
 Photochemical corneal collagen cross-linkage using riboflavin and ultraviolet A for keratoconus and keratectasia Interventional procedures guidance Published: 25 September 2013 nice.org.uk/guidance/ipg466
Photochemical corneal collagen cross-linkage using riboflavin and ultraviolet A for keratoconus and keratectasia Interventional procedures guidance Published: 25 September 2013 nice.org.uk/guidance/ipg466
Some of the ophthalmic surgeries
 Some of the ophthalmic surgeries Some of the ophthalmic surgeries performed at the DMV Center. This document presents some types of the surgeries performed by the ophthalmology service at the DMV veterinary
Some of the ophthalmic surgeries Some of the ophthalmic surgeries performed at the DMV Center. This document presents some types of the surgeries performed by the ophthalmology service at the DMV veterinary
Protocol. Implantable Sinus Stents for Postoperative Use Following Endoscopic Sinus Surgery and for Recurrent Sinus Disease
 (701134) Medical Benefit Effective Date: 04/01/17 Next Review Date: 01/19 Preauthorization No Review Dates: 01/13, 01/14, 01/15, 01/16, 01/17, 01/18 This protocol considers this test or procedure investigational.
(701134) Medical Benefit Effective Date: 04/01/17 Next Review Date: 01/19 Preauthorization No Review Dates: 01/13, 01/14, 01/15, 01/16, 01/17, 01/18 This protocol considers this test or procedure investigational.
Description. Section: Medicine Effective Date: April 15, 2015 Subsection: Medicine Original Policy Date: September 13, 2012 Subject:
 Last Review Status/Date: March 2015 Description Page: 1 of 6 Low-frequency ultrasound (US) in the kilohertz (KHz) range may improve wound healing. Several noncontact devices have received regulatory approval
Last Review Status/Date: March 2015 Description Page: 1 of 6 Low-frequency ultrasound (US) in the kilohertz (KHz) range may improve wound healing. Several noncontact devices have received regulatory approval
Amniotic membrane transplantation (AMT) without the use of sutures/fibrin glue
 Original article Amniotic membrane transplantation (AMT) without the use of sutures/fibrin glue Ajai Agrawal 1, VB Pratap 2 1 Department of Ophthalmology, Kalpana Chawla Government Medical College, Karnal,
Original article Amniotic membrane transplantation (AMT) without the use of sutures/fibrin glue Ajai Agrawal 1, VB Pratap 2 1 Department of Ophthalmology, Kalpana Chawla Government Medical College, Karnal,
WPS Medicare Policy Primer
 WPS Medicare Policy Primer Medicare Jurisdiction (J5 & J8) NE, KS, IA, MO, IN, & MI Retired skin substitute LCD 3/2016 Indications Apligraf Non-infected partial and full-thickness skin ulcers due to VSU
WPS Medicare Policy Primer Medicare Jurisdiction (J5 & J8) NE, KS, IA, MO, IN, & MI Retired skin substitute LCD 3/2016 Indications Apligraf Non-infected partial and full-thickness skin ulcers due to VSU
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: January 15, 2019 Related Policies: 1.01.30 Artificial Pancreas Device Systems 7.03.12 Autologous Islet transplantation Allogeneic Pancreas Transplant Description
FEP Medical Policy Manual Effective Date: January 15, 2019 Related Policies: 1.01.30 Artificial Pancreas Device Systems 7.03.12 Autologous Islet transplantation Allogeneic Pancreas Transplant Description
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: October 15, 2018 Related Policies: 8.01.52 Orthopedic Applications of Stem Cell Therapy (Including Allografts and Bone Substitutes Used With Autologous Bone Marrow)
FEP Medical Policy Manual Effective Date: October 15, 2018 Related Policies: 8.01.52 Orthopedic Applications of Stem Cell Therapy (Including Allografts and Bone Substitutes Used With Autologous Bone Marrow)
Clinical Policy Title: Therapeutic contact lenses
 Clinical Policy Title: Therapeutic contact lenses Clinical Policy Number: 10.02.02 Effective Date: June 1, 2014 Initial Review Date: December 18, 2013 Most Recent Review Date: January 20, 2016 Next Review
Clinical Policy Title: Therapeutic contact lenses Clinical Policy Number: 10.02.02 Effective Date: June 1, 2014 Initial Review Date: December 18, 2013 Most Recent Review Date: January 20, 2016 Next Review
Fitting Keratoconus and Other Complicated Corneas
 Fitting Keratoconus and Other Complicated Corneas Christine W Sindt OD FAAO Professor, Clinical Ophthalmology Director, Contact Lens Service University of Iowa Disclosure Consultant: ALCON Vision Care
Fitting Keratoconus and Other Complicated Corneas Christine W Sindt OD FAAO Professor, Clinical Ophthalmology Director, Contact Lens Service University of Iowa Disclosure Consultant: ALCON Vision Care
Suture Less and Glue Free Limbal Conjunctival Autografting following Pterygium Excision
 ORIGINALARTICLE Suture Less and Glue Free Limbal Conjunctival Autografting following Pterygium Excision Ashok Sharma, Hans Raj, Amit Vikram Raina Abstract A prospective interventional self-control study
ORIGINALARTICLE Suture Less and Glue Free Limbal Conjunctival Autografting following Pterygium Excision Ashok Sharma, Hans Raj, Amit Vikram Raina Abstract A prospective interventional self-control study
Clinical Policy Title: Platelet rich plasma
 Clinical Policy Title: Platelet rich plasma Clinical Policy Number: 05.02.10 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2016 Next Review
Clinical Policy Title: Platelet rich plasma Clinical Policy Number: 05.02.10 Effective Date: February 1, 2017 Initial Review Date: November 16, 2016 Most Recent Review Date: November 16, 2016 Next Review
Peripheral Subcutaneous Field Stimulation
 Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 3/2018 Origination: 7/2013 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Peripheral Subcutaneous Field Stimulation Policy Number: 7.01.139 Last Review: 3/2018 Origination: 7/2013 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Corporate Presentation NASDAQ: EYEG
 Corporate Presentation NASDAQ: EYEG Forward Looking Statements Some of the matters discussed in this presentation contain forward-looking statements that involve significant risks and uncertainties, including
Corporate Presentation NASDAQ: EYEG Forward Looking Statements Some of the matters discussed in this presentation contain forward-looking statements that involve significant risks and uncertainties, including
P. P.2-7 P R A C T I C A L
 P R A T I A L O U R S E The use of hyaluronic acid derivatives in the treatment of long-term non-healing wounds Systematic review and meta-analysis of RSs (randomized controlled studies) J. Voigt and V.
P R A T I A L O U R S E The use of hyaluronic acid derivatives in the treatment of long-term non-healing wounds Systematic review and meta-analysis of RSs (randomized controlled studies) J. Voigt and V.
FUCH S DYSTROPHY & CATARACT SURGERY TREATMENT ALGORITHM
 FUCH S DYSTROPHY & CATARACT SURGERY TREATMENT ALGORITHM ΙΟΑΝΝΙS Α. MALLIAS, MD, PHD Director of the Dept. of Ophthalmology, Mediterraneo Hospital, Glyfada, Athens, Greece Clinical Fellow in Cornea and
FUCH S DYSTROPHY & CATARACT SURGERY TREATMENT ALGORITHM ΙΟΑΝΝΙS Α. MALLIAS, MD, PHD Director of the Dept. of Ophthalmology, Mediterraneo Hospital, Glyfada, Athens, Greece Clinical Fellow in Cornea and
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: October 15, 2018 Related Policies: 2.04.38 Cytochrome P450 Genotype-Guided Treatment Strategy Genotype-Guided Tamoxifen Treatment Description Tamoxifen is prescribed
FEP Medical Policy Manual Effective Date: October 15, 2018 Related Policies: 2.04.38 Cytochrome P450 Genotype-Guided Treatment Strategy Genotype-Guided Tamoxifen Treatment Description Tamoxifen is prescribed
FEP Medical Policy Manual
 FEP Medical Policy Manual Last Review: December 2016 Effective Date: January 15, 2017 Related Policies None Gene Expression Profiling for Uveal Melanoma Summary Uveal melanoma is associated with a high
FEP Medical Policy Manual Last Review: December 2016 Effective Date: January 15, 2017 Related Policies None Gene Expression Profiling for Uveal Melanoma Summary Uveal melanoma is associated with a high
Corporate Medical Policy
 Corporate Medical Policy Continuous Passive Motion in the Home Setting File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_passive_motion_in_the_home_setting 9/1993 6/2018
Corporate Medical Policy Continuous Passive Motion in the Home Setting File Name: Origination: Last CAP Review: Next CAP Review: Last Review: continuous_passive_motion_in_the_home_setting 9/1993 6/2018
Failure of amniotic membrane transplantation in the treatment of acute ocular burns
 Br J Ophthalmol 2001;85:1065 1069 1065 ORIGINAL ARTICLES Clinical science Failure of amniotic membrane transplantation in the treatment of acute ocular burns Annie Joseph, Harminder S Dua, Anthony J King
Br J Ophthalmol 2001;85:1065 1069 1065 ORIGINAL ARTICLES Clinical science Failure of amniotic membrane transplantation in the treatment of acute ocular burns Annie Joseph, Harminder S Dua, Anthony J King
Ocular Surface Reconstruction
 OCULAR SURFACE From Tissue Transplantation to Cell Therapy Abraham Solomon, MD Abstract: The most difficult part in ocular surface reconstruction for total limbal stem cell deficiency is restoring a healthy
OCULAR SURFACE From Tissue Transplantation to Cell Therapy Abraham Solomon, MD Abstract: The most difficult part in ocular surface reconstruction for total limbal stem cell deficiency is restoring a healthy
Is there an icd 10 code for smoking vapor
 Is there an icd 10 code for smoking vapor Thanks for your response, I'm just thinking that 305.1 is tobacco use and there is no tobacco in the e-cig. Just the nicotine and other chemicals. I have been
Is there an icd 10 code for smoking vapor Thanks for your response, I'm just thinking that 305.1 is tobacco use and there is no tobacco in the e-cig. Just the nicotine and other chemicals. I have been
TIME S UP ON HEEL PAIN OPTIONS FOR CHRONIC FASCIAL AND TENDON INJURIES
 TIME S UP ON HEEL PAIN OPTIONS FOR CHRONIC FASCIAL AND TENDON INJURIES ERIKA M. SCHWARTZ, DPM FOOT AND ANKLE SPECIALISTS OF THE MID -ATLANTIC WASHINGTON DC AND CHEVY CHASE, MD TENDON INJURY Tendon injuries
TIME S UP ON HEEL PAIN OPTIONS FOR CHRONIC FASCIAL AND TENDON INJURIES ERIKA M. SCHWARTZ, DPM FOOT AND ANKLE SPECIALISTS OF THE MID -ATLANTIC WASHINGTON DC AND CHEVY CHASE, MD TENDON INJURY Tendon injuries
Clinical Policy Title: Vacuum assisted closure in surgical wounds
 Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Topic: Percutaneous Axial Anterior Lumbar Fusion Date of Origin: June Section: Surgery Last Reviewed Date: June 2013
 Medical Policy Manual Topic: Percutaneous Axial Anterior Lumbar Fusion Date of Origin: June 2007 Section: Surgery Last Reviewed Date: June 2013 Policy No: 157 Effective Date: August 1, 2013 IMPORTANT REMINDER
Medical Policy Manual Topic: Percutaneous Axial Anterior Lumbar Fusion Date of Origin: June 2007 Section: Surgery Last Reviewed Date: June 2013 Policy No: 157 Effective Date: August 1, 2013 IMPORTANT REMINDER
Innovation In Ophthalmology
 Innovation In Ophthalmology INVELTYS TM Approval August 2018 Disclaimers and Notices This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform
Innovation In Ophthalmology INVELTYS TM Approval August 2018 Disclaimers and Notices This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform
Medical Services Protocol Updates
 Protocol Medical Services Protocol Updates Distribution Date: September 1, 2016 The following Medical Protocol update includes information on protocols that have undergone a review over the last several
Protocol Medical Services Protocol Updates Distribution Date: September 1, 2016 The following Medical Protocol update includes information on protocols that have undergone a review over the last several
Orthopedic Applications of Platelet- Rich Plasma
 Orthopedic Applications of Platelet- Rich Plasma Policy Number: 2.01.98 Last Review: 5/2018 Origination: 5/2016 Next Review: 5/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Orthopedic Applications of Platelet- Rich Plasma Policy Number: 2.01.98 Last Review: 5/2018 Origination: 5/2016 Next Review: 5/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will not provide
Update 1. Red Eye. Adenovirus Numbers 3/4/14. For Your Eyes Only 2014 Ophthalmology Update. TKorn, MD, FACS San Diego, California
 For Your Eyes Only 2014 Ophthalmology Update TKorn, MD, FACS San Diego, California Update 1 Red Eye Adenovirus Numbers 15.7 - Adults touch their face in an hour 1-5 weeks - Adenovirus infectivity period
For Your Eyes Only 2014 Ophthalmology Update TKorn, MD, FACS San Diego, California Update 1 Red Eye Adenovirus Numbers 15.7 - Adults touch their face in an hour 1-5 weeks - Adenovirus infectivity period
Update 1. Red Eye. Adenovirus Numbers 7/29/14. Adenovirus Conjunctivitis Outbreaks. Adenovirus Conjunctivitis Management
 For Your Eyes Only 2014 Ophthalmology Update Update 1 Red Eye TKorn, MD, FACS San Diego, California Adenovirus Numbers Adenovirus Conjunctivitis Outbreaks 15.7 - Adults touch their face in an hour 1-5
For Your Eyes Only 2014 Ophthalmology Update Update 1 Red Eye TKorn, MD, FACS San Diego, California Adenovirus Numbers Adenovirus Conjunctivitis Outbreaks 15.7 - Adults touch their face in an hour 1-5
Implantation of a corneal graft keratoprosthesis for severe corneal opacity in wet blinking eyes
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Implantation of a corneal graft keratoprosthesis for severe corneal opacity in wet blinking eyes The cornea
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Implantation of a corneal graft keratoprosthesis for severe corneal opacity in wet blinking eyes The cornea
MEDICAL POLICY SUBJECT: OCULAR PHOTOSCREENING. POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally recognized by the medical community.
MEDICAL POLICY Clinical criteria used to make utilization review decisions are based on credible scientific evidence published in peer reviewed medical literature generally recognized by the medical community.
Scleral Lens Essentials
 Scleral Lens Essentials Disclosures: Alcon Bausch + Lomb Contamac CooperVision Johnson & Johnson SpecialEyes Valley Contax Matthew J. Lampa, OD, FAAO lampa@pacificu.edu Scleral Lenses Which Lens Modality???
Scleral Lens Essentials Disclosures: Alcon Bausch + Lomb Contamac CooperVision Johnson & Johnson SpecialEyes Valley Contax Matthew J. Lampa, OD, FAAO lampa@pacificu.edu Scleral Lenses Which Lens Modality???
Transtympanic Micropressure Applications as a Treatment of Meniere s Disease
 Transtympanic Micropressure Applications as a Treatment of Meniere s Disease Policy Number: 1.01.23 Last Review: 8/2017 Origination: 2/2006 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas
Transtympanic Micropressure Applications as a Treatment of Meniere s Disease Policy Number: 1.01.23 Last Review: 8/2017 Origination: 2/2006 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Recombinant and Autologous Platelet-Derived Growth Factors Page 1 of 20 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Recombinant and Autologous Platelet-Derived
Recombinant and Autologous Platelet-Derived Growth Factors Page 1 of 20 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See Also: Recombinant and Autologous Platelet-Derived
SKIN AND SOFT TISSUE SUBSTITUTES
 SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Community Plan Medical Policy Policy Number: CS153.B Effective Date: November 1, 2018 Instructions for Use Table of Contents Page COVERAGE RATIONALE...
SKIN AND SOFT TISSUE SUBSTITUTES UnitedHealthcare Community Plan Medical Policy Policy Number: CS153.B Effective Date: November 1, 2018 Instructions for Use Table of Contents Page COVERAGE RATIONALE...
History. Examination. Diagnosis/Course
 History A 51 year-old female with a history of chronic dry eyes and photosensitivity was referred for evaluation. She reported a five year history of symptoms of frequent irritation and photophobia in
History A 51 year-old female with a history of chronic dry eyes and photosensitivity was referred for evaluation. She reported a five year history of symptoms of frequent irritation and photophobia in
Protocol. Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion Procedures
 Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion (70185) Medical Benefit Effective Date: 07/01/12 Next Review Date: 01/19 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10,
Electrical Stimulation of the Spine as an Adjunct to Spinal Fusion (70185) Medical Benefit Effective Date: 07/01/12 Next Review Date: 01/19 Preauthorization Yes Review Dates: 09/07, 09/08, 09/09, 05/10,
