Supplementary material
|
|
|
- Neal Parks
- 6 years ago
- Views:
Transcription
1 Supplementary material Table S1: Dubois Guidelines for Diagnosis of DrugInduced Lupus 1. Continuous treatment with a known lupusinducing drug for 1month and usually much longer 2. Presenting symptoms: Common arthralgia, myalgias, malaise, fever, serositis, polyarthritis Rare rash or other associated dermatological problems, glomerulonephritis 3. Unrelated symptoms suggestive of SLE: Multisystem involvement especially neurological, renal and skin symptoms 4. Laboratory profile Common: ANA which is as a result of antihistone antibodies especially IgG anti[(h2ah2b)dna], leukopenia, thrombocytopenia and mild anaemia, increased erythrocyte sedimentation rate Rare: Antibodies to native DNA, Sm, RNP, SSA/Ro, SSB/La, hypocomplementemia 5. Improvement and permanent resolution of symptoms generally within days or weeks after discontinuation of therapy. Serological findings, especially autoantibody levels may take months to resolve. Reproduced with permission of the copyright owner. Dubois Lupus Erythematosus and Related Syndromes 8th edition ISBN: Patients were classified as having a lupuslike event for the purposes of this analysis if the met 2 of the above criteria. 1
2 Figure S1: Selection of patients for analysis All patients N=23, 699 Biologic naïve cohort (nbdmard) N=3774 Biologic cohort N=19,925 Physician diagnosis of RA N=3774 Physician diagnosis of RA N=18,144 Patient on antitnf drug N= 15,477 Returned at least 1 follow up N=3673* Returned at least 1 follow up N=14,897 Registered within 6 months of starting antitnf N=13,746 Patients on 1 st antitnf drug (excluding switchers) N= 12,937* Patients with no baseline history of systemic vasculitis N= 3,640** Patients with no baseline history of systemic vasculitis N= 12,745** *Denominator for lupuslike events analysis **Denominator for vasculitislike events and combined immune mediated adverse events analyses. Abbreviations: antitnf, antitumour necrosis factor; nbdmard, nonbiologic disease modifying antirheumatic drug; RA, rheumatoid arthritis. 2
3 Statistical methodology supplementary information Missing data Multiple imputations were performed in Stata version 13.1 using the ICE command. Missing data were present in the following baseline variables: disease duration, baseline HAQ, baseline HAQ score, smoking and ethnicity. The imputation model was constructed separately for the nbdmard and anti TNF cohorts. Age, gender, disease duration, baseline HAQ, separate components of baseline DAS28 score (swollen joint count, tender joint count, patient global visual analogue score and ESR), rheumatoid factor positivity, comorbidity, smoking status, ethnicity, entry year and baseline steroid exposure were included as predictors within the imputation model. Twenty imputation cycles were performed and final data analysed using Rubin s rule with the MIM command. Table S2: Missing baseline data Variable; n missing (%) nbdmard (n=3,673) TNFi (n=12,937) Disease duration 23 (1) 131 (1) HAQ score 729 (20) 805 (6) DAS28 score 55 (1) 102 (1) Smoking 18 (0) 128 (1) Ethnicity 648 (18) 2008 (16) Rheumatoid factor positive 3 (0) 118 (1) Abbreviations: HAQ, Health assessment questionnaire; DAS 28, Disease activity score of 28 joints Propensity Scores Confounders were adjusted using an inverse probability of treatment weighting score to assess drugspecific differences accurately. Logistic regression was used to generate a probability of treatment (propensity) score. The final propensity score for vasculitislike events included age, gender, disease duration, baseline HAQ scores, baseline DAS28 score, a composite measure for comorbidities, rheumatoid factor positive status, year of recruitment, baseline nbdmard use, baseline oral steroid. For the lupuslike events and combinedevent analysis, ethnicity (dichotomised as white/nonwhite) was also included. The inverse of the probability (1 minus the inverse of the probability in the nbdmard cohort) was used as the treatment weight in the analysis. Weights greater than 20 were truncated in the final combinedevents analysis to avoid destabilising the model. The balance of the variables was tested between the nbdmard and antitnf cohorts using standardised differences rather than estimated bias. 3
4 Table S3: Sensitivity analysis following exclusion of secondary causes leading to event Lupus like events (excluding all patients with known lupus inducing drugs) nbdmard (n=2,083) All TNFi (n=9,625) Etanercept (n=3,513) Adalimumab (n=3,038) Infliximab (n=2,658) Certolizumab (n=416) Total follow up time (patientyears) 10,070 39,521 16,580 11,844 10, Number Crude incidence rate of lupus like event per 10,000 personyears (95% CI) 2.8 (0.9, 8.8) 11.0 (8.2, 14.7) 11.3 (7.2, 17.7) 6.6 (3.3, 13.3) 16.1 (10.0, 25.8) Unadjusted hazard ratio (95%CI) Referent 3.71 (1.15,12.0)* 4.06 (1.20, 13.73) 2.20 (0.58, 8.30) Propensityadjusted hazard ratio (95%CI) Referent 2.33 (0.56, 9.71) 1.93 (0.48, 7.67) 2.62 (0.40, 17.11) 5.31 (1.55, 18.12)* 2.53 (0.62, 10.27) Vasculitis like events (excluding events with a possible secondary trigger) nbdmard (n=3,567) All TNFi (n=12,489) Etanercept (n=4,351) Adalimumab (n=4,233) Infliximab (n=3,216) Certolizumab (n=689) Total follow up time (patientyears) 20,148 51,530 20,936 16,886 12,
5 Number Crude incidence rate of lupus like event per 10,000 personyears (95% CI) 6.5 (3.7, 11.1) 13.2 (10.4, 16.7) (9.6, 20.0) 10.1 (6.3, 16.2) 17.4 (11.4, 26.3) Unadjusted hazard ratio (95%CI) Referent 1.92 (1.06, 3.47)* 2.17 (1.12, 4.18)* 1.41 (0.68, 2.90) Propensityadjusted hazard ratio (95%CI) Referent 1.05 (0.32, 3.45) 1.34 (0.40, 4.50) 0.66 (0.18, 2.36) 2.47 (1.24, 4.92)* 1.28 (0.37, 4.50) * p<0.05. Baseline use of the following drugs, lead to exclusion of patients in the LLE sensitivity analysis: quinidine, isoniazid, chlorpromazine, hydralazine, methylodopa, carbimazole, penicillin, propylthiouracil, phenobarbitone, phenytoin, diltiazem, lithium; baseline/current use of sulfasalazine, leflunomide, minocycline and penicillamine. Baseline use of the following drugs, lead to exclusion of patients in the vasculitis sensitivity analysis: propylthiouracil, carbamazepine, phenobarbitone, phenytoin, hydralazine, sodium valproate; baseline/current use of minocycline and penicillamine. In addition patients with a likely secondary cause of vasculitis (e.g. infection) and recorded nailfold vasculitis at baseline were also excluded. Abbreviations: CI, confidence interval; nbdmards, nonbiological diseasemodifying antirheumatic drugs; TNFi tumour necrosis factoralpha inhibitor. 5
6 Figure S2: Outcome of switching to biologics following lupuslike events 12 month outcome 5 events on 54 events on 1 st nbdmard, TNFi 1 switched to biologic Stopped TNFi permanently (n=17, 31%) Continued on same TNFi (n=11, 20%) Interrupted treatment due to event (n=12, 22%) Switched to another biologic (n=15, 27%) No recurrence by 12 months: n= 9 (81%). Recurrence of event within 12 months n=2 (18%) No recurrence by 12 months: n= 9 (75%). Recurrence of event within 12 months n=2 (17%); reported loss of effect n=1 (8%) Switched to another TNFi (n=10, 67%) Switched to Rituximab (n=5, 33%) No recurrence: n= 5 (50%). Recurrence of event n=5 (50%) No recurrence: n= 5 (100%). Recurrence of event n=0 Outcome following 12 months Switched to another biologic following 24 months of event n=18 (73%) Switched to another TNFi (n=8, 44%) Switched to Rituximab (n=9, 50%) Switched to Tocilizumab (n=1, 6%) No recurrence by 12 months following switch: n= 3 (37%). Recurrence of event by 12 months following switch n=5 (63%) No recurrence by 12 months following switch: n= 7 (78%). Recurrence of event by 12 months n=1 (11%). Treatment stopped due to inefficacy n=1 (11%) No recurrence by 12 months following switch: n= 1 (100%). Recurrence of event by 12 months following switch n=0 Switched 3 rd biologic due to event Rituximab; n= 4 (no recurrence 2; recurrence 1; inefficacy 1) 6
7 Of the 4 nbdmard patients who did not switch to a biologic, 1 patient switched to methotrexate from penicillamine, 1 patient continued with azathioprine for 2 years followed by death (unrelated to LLE), 1 patient continued with methotrexate and hydroxychloroquine and was deemed to have SLE following the initial event and 1 patient stopped their methotrexate for a few years post event and was restarted when the joint disease started to flare. Abbreviations: TNFi, tumour necrosisfactor inhibitor Table S4 MedDRA preferred terms used to search for Lupus Like Events and Vasculitis Like Events Lupus Like Events Cutaneous lupus erythematosus Systemic lupus erythematosus/ SLE Lupuslike syndrome Lupus nephritis Systemic lupus erythematosus rash Drug induced lupus Vasculitis Like Events Vasculitis Vasculopathy Purpuric vasculitic rash Vasculitic ulcers Vasculitis lesions Digital Vasculitis Drug induced vasculitis 104 and 217 events for LLE and VLE respectively were identified using MedDRA codes as listed above and a free text search within the adverse event reported fields that included the terms lupus and vasculitis like events. Cases were screened and excluded if they did not meet eligibility criteria outlined in the methods section. 7
Is it Autoimmune or NOT! Presented to AONP! October 2015!
 Is it Autoimmune or NOT! Presented to AONP! October 2015! Four main jobs of immune system Detects Contains and eliminates Self regulates Protects Innate Immune System! Epithelial cells, phagocytic cells
Is it Autoimmune or NOT! Presented to AONP! October 2015! Four main jobs of immune system Detects Contains and eliminates Self regulates Protects Innate Immune System! Epithelial cells, phagocytic cells
High Impact Rheumatology
 High Impact Rheumatology Systemic Lupus Erythematosus Bernard Rubin, DO MPH Case 1: History A 45-year-old woman presents with severe dyspnea and cough. She was in excellent health until 4 weeks ago when
High Impact Rheumatology Systemic Lupus Erythematosus Bernard Rubin, DO MPH Case 1: History A 45-year-old woman presents with severe dyspnea and cough. She was in excellent health until 4 weeks ago when
Perioperative Medicine:
 Perioperative Medicine: Management of rheumatologic agents Divya Gollapudi, MD May 2016 Medical Operative Consult Clinic Harborview Medical Center Your patient Ms. L is a 55 year-old F w/ h/o RA who presents
Perioperative Medicine: Management of rheumatologic agents Divya Gollapudi, MD May 2016 Medical Operative Consult Clinic Harborview Medical Center Your patient Ms. L is a 55 year-old F w/ h/o RA who presents
Development of SLE among Possible SLE Patients Seen in Consultation: Long-Term Follow-Up. Disclosures. Background. Evidence-Based Medicine.
 Development of SLE among Patients Seen in Consultation: Long-Term Follow-Up Abstract # 1699 May Al Daabil, MD Bonnie L. Bermas, MD Alexander Fine Hsun Tsao Patricia Ho Joseph F. Merola, MD Peter H. Schur,
Development of SLE among Patients Seen in Consultation: Long-Term Follow-Up Abstract # 1699 May Al Daabil, MD Bonnie L. Bermas, MD Alexander Fine Hsun Tsao Patricia Ho Joseph F. Merola, MD Peter H. Schur,
NICE DECISION SUPPORT UNIT
 SEQUENTIAL TNF-α INHIBITORS AND NON BIOLOGIC DMARDS ANALYSIS OF THE NATIONAL DATABANK FOR RHEUMATIC DISEASES. NICE DECISION SUPPORT UNIT Allan Wailoo School of Health and Related Research, University of
SEQUENTIAL TNF-α INHIBITORS AND NON BIOLOGIC DMARDS ANALYSIS OF THE NATIONAL DATABANK FOR RHEUMATIC DISEASES. NICE DECISION SUPPORT UNIT Allan Wailoo School of Health and Related Research, University of
Annual Rheumatology & Therapeutics Review for Organizations & Societies
 Annual Rheumatology & Therapeutics Review for Organizations & Societies Comparative Effectiveness Studies of Biologics Learning Objectives Understand the motivation for comparative effectiveness research
Annual Rheumatology & Therapeutics Review for Organizations & Societies Comparative Effectiveness Studies of Biologics Learning Objectives Understand the motivation for comparative effectiveness research
Infections and Biologics
 Overview Infections and Biologics James Galloway What is the risk of infection with biologics? Are some patients at greater risk? Are some drugs safer? Case scenario You recently commenced Judith, a 54
Overview Infections and Biologics James Galloway What is the risk of infection with biologics? Are some patients at greater risk? Are some drugs safer? Case scenario You recently commenced Judith, a 54
Rheumatoid arthritis
 Rheumatoid arthritis 1 Definition Rheumatoid arthritis is one of the most common inflammatory disorders affecting the population worldwide. It is a systemic inflammatory disease which affects not only
Rheumatoid arthritis 1 Definition Rheumatoid arthritis is one of the most common inflammatory disorders affecting the population worldwide. It is a systemic inflammatory disease which affects not only
2.0 Synopsis. Adalimumab DE019 OLE (5-year) Clinical Study Report Amendment 1 R&D/06/095. (For National Authority Use Only)
 2.0 Synopsis Abbott Laboratories Name of Study Drug: Humira Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title
2.0 Synopsis Abbott Laboratories Name of Study Drug: Humira Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only) Title
Rheumatology Primer: What Labs and When
 Rheumatology Primer: What Labs and When Irina Konon, MD Department of Internal Medicine Division of Rheumatology Medical College of Wisconsin Disclosures None 1 Objective Discuss principles of laboratory
Rheumatology Primer: What Labs and When Irina Konon, MD Department of Internal Medicine Division of Rheumatology Medical College of Wisconsin Disclosures None 1 Objective Discuss principles of laboratory
Coverage Criteria: Express Scripts, Inc. monograph dated 12/15/ months or as otherwise noted by indication
 BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Kineret (anakinra subcutaneous injection) Commercial HMO/PPO/CDHP
BENEFIT DESCRIPTION AND LIMITATIONS OF COVERAGE ITEM: PRODUCT LINES: COVERED UNDER: DESCRIPTION: CPT/HCPCS Code: Company Supplying: Setting: Kineret (anakinra subcutaneous injection) Commercial HMO/PPO/CDHP
New Evidence reports on presentations given at EULAR Tocilizumab for the Treatment of Rheumatoid Arthritis and Juvenile Idiopathic Arthritis
 New Evidence reports on presentations given at EULAR 2011 Tocilizumab for the Treatment of Rheumatoid Arthritis and Juvenile Idiopathic Arthritis Report on EULAR 2011 presentations Benefit of continuing
New Evidence reports on presentations given at EULAR 2011 Tocilizumab for the Treatment of Rheumatoid Arthritis and Juvenile Idiopathic Arthritis Report on EULAR 2011 presentations Benefit of continuing
Effective Health Care Program
 Comparative Effectiveness Review Number 28 Effective Health Care Program Disease-Modifying Antirheumatic Drugs (DMARDs) in Children With Juvenile Idiopathic Arthritis (JIA) Executive Summary Background
Comparative Effectiveness Review Number 28 Effective Health Care Program Disease-Modifying Antirheumatic Drugs (DMARDs) in Children With Juvenile Idiopathic Arthritis (JIA) Executive Summary Background
Abatacept (Orencia) for active rheumatoid arthritis. August 2009
 Abatacept (Orencia) for active rheumatoid arthritis August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
Abatacept (Orencia) for active rheumatoid arthritis August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended to
MMS Pharmacology Lecture 2. Antirheumatic drugs. Dr Sura Al Zoubi
 MMS Pharmacology Lecture 2 Antirheumatic drugs Dr Sura Al Zoubi Revision Rheumatoid Arthritis Definition (RA): is the most common systemic inflammatory disease characterized by symmetrical inflammation
MMS Pharmacology Lecture 2 Antirheumatic drugs Dr Sura Al Zoubi Revision Rheumatoid Arthritis Definition (RA): is the most common systemic inflammatory disease characterized by symmetrical inflammation
Study synopsis of the global non-interventional study SWITCH-RA
 Study synopsis of the global non-interventional study SWITCH-RA Protocol number: MA22401 Title of Study: A global multi-centre observational study in RA patients who are non-responders or intolerant to
Study synopsis of the global non-interventional study SWITCH-RA Protocol number: MA22401 Title of Study: A global multi-centre observational study in RA patients who are non-responders or intolerant to
Medical Management of Rheumatoid Arthritis (RA)
 Medical Management of Rheumatoid Arthritis (RA) Dr Lee-Suan Teh Rheumatologist Royal Blackburn Hospital Educational objectives ABC Appreciate the epidemiology of RA Be able to diagnosis of RA Competent
Medical Management of Rheumatoid Arthritis (RA) Dr Lee-Suan Teh Rheumatologist Royal Blackburn Hospital Educational objectives ABC Appreciate the epidemiology of RA Be able to diagnosis of RA Competent
Tocilizumab F. Hoffmann-La Roche Ltd. Protocol MA 27950, Version 3.0 1
 Protocol MA 27950, Version 3.0 1 Protocol MA 27950, Version 3.0 2 TABLE OF CONTENTS PROTOCOL ACCEPTANCE FORM... 8 PROTOCOL SYNOPSIS... 9 1. BACKGROUND... 15 1.1 Background on Rheumatoid Arthritis... 15
Protocol MA 27950, Version 3.0 1 Protocol MA 27950, Version 3.0 2 TABLE OF CONTENTS PROTOCOL ACCEPTANCE FORM... 8 PROTOCOL SYNOPSIS... 9 1. BACKGROUND... 15 1.1 Background on Rheumatoid Arthritis... 15
Rheumatoid arthritis 2010: Treatment and monitoring
 October 12, 2010 By Yusuf Yazici, MD [1] The significant changes in the way rheumatoid arthritis has been managed include earlier, more aggressive treatment with combination therapy. Significant changes
October 12, 2010 By Yusuf Yazici, MD [1] The significant changes in the way rheumatoid arthritis has been managed include earlier, more aggressive treatment with combination therapy. Significant changes
9/25/2013 SYSTEMIC LUPUS ERYTHEMATOSUS (SLE)
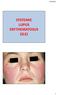 SYSTEMIC LUPUS ERYTHEMATOSUS (SLE) 1 Other Types of Lupus Discoid Lupus Erythematosus Lupus Pernio --- Sarcoidosis Lupus Vulgaris --- Tuberculosis of the face Manifestations of SLE Fever Rashes Arthritis
SYSTEMIC LUPUS ERYTHEMATOSUS (SLE) 1 Other Types of Lupus Discoid Lupus Erythematosus Lupus Pernio --- Sarcoidosis Lupus Vulgaris --- Tuberculosis of the face Manifestations of SLE Fever Rashes Arthritis
Pharmaco-epidemiological assessment of the clinical use of biologic therapies in the management of rheumatoid arthritis
 Pharmaco-epidemiological assessment of the clinical use of biologic therapies in the management of rheumatoid arthritis A thesis submitted to The University of Manchester for the degree of Doctor of Philosophy
Pharmaco-epidemiological assessment of the clinical use of biologic therapies in the management of rheumatoid arthritis A thesis submitted to The University of Manchester for the degree of Doctor of Philosophy
Risk of serious infections associated with use of immunosuppressive agents in pregnant women with autoimmune inflammatory conditions: cohor t study
 Risk of serious infections associated with use of immunosuppressive agents in pregnant women with autoimmune inflammatory conditions: cohor t study BMJ 2017; 356 doi: https://doi.org/10.1136/bmj.j895 (Published
Risk of serious infections associated with use of immunosuppressive agents in pregnant women with autoimmune inflammatory conditions: cohor t study BMJ 2017; 356 doi: https://doi.org/10.1136/bmj.j895 (Published
Benlysta (belimumab) Prior Authorization Criteria Program Summary
 Benlysta (belimumab) Prior Authorization Criteria Program Summary This prior authorization applies to Commercial, NetResults A series, NetResults F series and Health Insurance Marketplace formularies.
Benlysta (belimumab) Prior Authorization Criteria Program Summary This prior authorization applies to Commercial, NetResults A series, NetResults F series and Health Insurance Marketplace formularies.
ACTEMRA (TOCILIZUMAB) INJECTION FOR INTRAVENOUS INFUSION
 ACTEMRA (TOCILIZUMAB) INJECTION FOR INTRAVENOUS INFUSION UnitedHealthcare Commercial Medical Benefit Drug Policy Policy Number: 2018D0043I Effective Date: March 1, 2018 Table of Contents Page INSTRUCTIONS
ACTEMRA (TOCILIZUMAB) INJECTION FOR INTRAVENOUS INFUSION UnitedHealthcare Commercial Medical Benefit Drug Policy Policy Number: 2018D0043I Effective Date: March 1, 2018 Table of Contents Page INSTRUCTIONS
To help you with terms and abbreviations used in this document that may be unfamiliar to you, a glossary is provided on the last pages.
 ARTHRITIS CONSUMER EXPERTS 910B RICHARDS STREET VANCOUVER BC V6B 3C1 CANADA T: 604.974-1366 F: 604.974-1377 WWW.ARTHRITISCONSUMEREXPERTS.ORG Arthritis Consumer Experts In Health Care and Research Decision-making
ARTHRITIS CONSUMER EXPERTS 910B RICHARDS STREET VANCOUVER BC V6B 3C1 CANADA T: 604.974-1366 F: 604.974-1377 WWW.ARTHRITISCONSUMEREXPERTS.ORG Arthritis Consumer Experts In Health Care and Research Decision-making
Test Name Results Units Bio. Ref. Interval
 LL - LL-ROHINI (NATIONAL REFERENCE 135091593 Age 25 Years Gender Male 30/8/2017 91600AM 30/8/2017 93946AM 31/8/2017 84826AM Ref By Final COLLAGEN DISEASES ANTIBODY ANEL ANTI NUCLEAR ANTIBODY / FACTOR (ANA/ANF),
LL - LL-ROHINI (NATIONAL REFERENCE 135091593 Age 25 Years Gender Male 30/8/2017 91600AM 30/8/2017 93946AM 31/8/2017 84826AM Ref By Final COLLAGEN DISEASES ANTIBODY ANEL ANTI NUCLEAR ANTIBODY / FACTOR (ANA/ANF),
Otezla. Otezla (apremilast) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Otezla Page: 1 of 5 Last Review Date: March 16, 2018 Otezla Description Otezla (apremilast) Background
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Otezla Page: 1 of 5 Last Review Date: March 16, 2018 Otezla Description Otezla (apremilast) Background
Test Name Results Units Bio. Ref. Interval
 135091662 Age 45 Years Gender Male 29/8/2017 120000AM 29/8/2017 100215AM 29/8/2017 110825AM Ref By Final RHEUMATOID AUTOIMMUNE COMREHENSIVE ANEL ANTI NUCLEAR ANTIBODY / FACTOR (ANA/ANF), SERUM ----- 20-60
135091662 Age 45 Years Gender Male 29/8/2017 120000AM 29/8/2017 100215AM 29/8/2017 110825AM Ref By Final RHEUMATOID AUTOIMMUNE COMREHENSIVE ANEL ANTI NUCLEAR ANTIBODY / FACTOR (ANA/ANF), SERUM ----- 20-60
ORENCIA (ABATACEPT) INJECTION FOR INTRAVENOUS INFUSION
 UnitedHealthcare Community Plan Medical Benefit Drug Policy ORENCIA (ABATACEPT) INJECTION FOR INTRAVENOUS INFUSION Policy Number: CS2018D0039J Effective Date: March 1, 2018 Table of Contents Page INSTRUCTIONS
UnitedHealthcare Community Plan Medical Benefit Drug Policy ORENCIA (ABATACEPT) INJECTION FOR INTRAVENOUS INFUSION Policy Number: CS2018D0039J Effective Date: March 1, 2018 Table of Contents Page INSTRUCTIONS
Conflict of Interest. Systemic Lupus Erythematosus and the Antiphospholipid Syndrome Bonnie L. Bermas, MD Brigham and Women s Hospital.
 Systemic Lupus Erythematosus and the Antiphospholipid Syndrome Bonnie L. Bermas, MD Brigham and Women s Hospital Conflict of Interest Disclosures: None Overview Diagnostic Classification Criteria of SLE
Systemic Lupus Erythematosus and the Antiphospholipid Syndrome Bonnie L. Bermas, MD Brigham and Women s Hospital Conflict of Interest Disclosures: None Overview Diagnostic Classification Criteria of SLE
Accounting for treatment switching/discontinuation in comparative effectiveness studies
 Global Medical Affairs RWE and Digital Accounting for treatment switching/discontinuation in comparative effectiveness studies Melvin Skip Olson, PhD Global Head, Real World Data Strategy and Innovation
Global Medical Affairs RWE and Digital Accounting for treatment switching/discontinuation in comparative effectiveness studies Melvin Skip Olson, PhD Global Head, Real World Data Strategy and Innovation
ACTEMRA (TOCILIZUMAB) INJECTION FOR INTRAVENOUS INFUSION
 ACTEMRA (TOCILIZUMAB) INJECTION FOR INTRAVENOUS INFUSION UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 284.9 T2 Effective Date: June 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...
ACTEMRA (TOCILIZUMAB) INJECTION FOR INTRAVENOUS INFUSION UnitedHealthcare Oxford Clinical Policy Policy Number: PHARMACY 284.9 T2 Effective Date: June 1, 2018 Table of Contents Page INSTRUCTIONS FOR USE...
RHEUMATOLOGY OVERVIEW. Carmelita J. Colbert, MD Assistant Professor of Medicine Division of Rheumatology Loyola University Medical Center
 RHEUMATOLOGY OVERVIEW Carmelita J. Colbert, MD Assistant Professor of Medicine Division of Rheumatology Loyola University Medical Center What is Rheumatology? Medical science devoted to the rheumatic diseases
RHEUMATOLOGY OVERVIEW Carmelita J. Colbert, MD Assistant Professor of Medicine Division of Rheumatology Loyola University Medical Center What is Rheumatology? Medical science devoted to the rheumatic diseases
Undifferentiated Connective Tissue Disease and Overlap Syndromes. Mark S. Box, MD
 Undifferentiated Connective Tissue Disease and Overlap Syndromes Mark S. Box, MD Overlap Syndromes As many as 25% of patients with rheumatic diseases with systemic symptoms cannot be definitely diagnosed
Undifferentiated Connective Tissue Disease and Overlap Syndromes Mark S. Box, MD Overlap Syndromes As many as 25% of patients with rheumatic diseases with systemic symptoms cannot be definitely diagnosed
Table S1. Read and ICD 10 diagnosis codes for polymyalgia rheumatica and giant cell arteritis
 SUPPLEMENTARY MATERIAL TEXT Text S1. Multiple imputation TABLES Table S1. Read and ICD 10 diagnosis codes for polymyalgia rheumatica and giant cell arteritis Table S2. List of drugs included as immunosuppressant
SUPPLEMENTARY MATERIAL TEXT Text S1. Multiple imputation TABLES Table S1. Read and ICD 10 diagnosis codes for polymyalgia rheumatica and giant cell arteritis Table S2. List of drugs included as immunosuppressant
Supplemental Table 1. Key Inclusion Criteria Inclusion Criterion OPTIMA PREMIER 18 years old with RA (per 1987 revised American College of General
 Supplemental Table 1. Key Inclusion Criteria Inclusion Criterion OPTIMA PREMIER 18 years old with RA (per 1987 revised American College of General Rheumatology classification criteria) 34 ; erythrocyte
Supplemental Table 1. Key Inclusion Criteria Inclusion Criterion OPTIMA PREMIER 18 years old with RA (per 1987 revised American College of General Rheumatology classification criteria) 34 ; erythrocyte
SLE and the Antiphospholipid Syndrome
 SLE and the Antiphospholipid Syndrome Susan Y. Ritter MD, PhD Associate Physician Division of Rheumatology, Immunology and Allergy Department of Medicine Brigham and Women s Hospital Instructor in Medicine
SLE and the Antiphospholipid Syndrome Susan Y. Ritter MD, PhD Associate Physician Division of Rheumatology, Immunology and Allergy Department of Medicine Brigham and Women s Hospital Instructor in Medicine
Summary Article: Lupus (Systemic Lupus Erythematosus) from Harvard Medical School Health Topics A-Z
 Topic Page: Systemic Lupus Erythematosus Summary Article: Lupus (Systemic Lupus Erythematosus) from Harvard Medical School Health Topics A-Z What Is It? Lupus is thought to develop when the immune system
Topic Page: Systemic Lupus Erythematosus Summary Article: Lupus (Systemic Lupus Erythematosus) from Harvard Medical School Health Topics A-Z What Is It? Lupus is thought to develop when the immune system
Test Name Results Units Bio. Ref. Interval
 135091660 Age 44 Years Gender Male 29/8/2017 120000AM 29/8/2017 100219AM 29/8/2017 105510AM Ref By Final EXTRACTABLENUCLEAR ANTIGENS (ENA), QUANTITATIVE ROFILE CENTROMERE ANTIBODY, SERUM 20-30 Weak ositive
135091660 Age 44 Years Gender Male 29/8/2017 120000AM 29/8/2017 100219AM 29/8/2017 105510AM Ref By Final EXTRACTABLENUCLEAR ANTIGENS (ENA), QUANTITATIVE ROFILE CENTROMERE ANTIBODY, SERUM 20-30 Weak ositive
Proposed Retirement for HEDIS : Disease-Modifying Anti-Rheumatic Drug Therapy for Rheumatoid Arthritis (ART)
 Proposed Retirement for HEDIS 1 2020 2 : Disease-Modifying Anti-Rheumatic Drug Therapy for Rheumatoid Arthritis (ART) NCQA seeks public comment on the proposed retirement of the Disease-Modifying Anti-Rheumatic
Proposed Retirement for HEDIS 1 2020 2 : Disease-Modifying Anti-Rheumatic Drug Therapy for Rheumatoid Arthritis (ART) NCQA seeks public comment on the proposed retirement of the Disease-Modifying Anti-Rheumatic
1 P a g e. Systemic Juvenile Idiopathic Arthritis (SJIA) (1.3) Patients 2 years of age and older with active systemic juvenile idiopathic arthritis.
 LENGTH OF AUTHORIZATION: Initial: 3 months for Crohn s or Ulcerative Colitis; 1 year for all other indications. Renewal: 1 year dependent upon medical records supporting response to therapy and review
LENGTH OF AUTHORIZATION: Initial: 3 months for Crohn s or Ulcerative Colitis; 1 year for all other indications. Renewal: 1 year dependent upon medical records supporting response to therapy and review
Drug-Induced Lupus Erythematosus
 REVIEW ARTICLE Drug Saf 2011; 34 (5): 357-374 0114-5916/11/0005-0357/$49.95/0 ª 2011 Adis Data Information BV. All rights reserved. Drug-Induced Lupus Erythematosus Incidence, Management and Prevention
REVIEW ARTICLE Drug Saf 2011; 34 (5): 357-374 0114-5916/11/0005-0357/$49.95/0 ª 2011 Adis Data Information BV. All rights reserved. Drug-Induced Lupus Erythematosus Incidence, Management and Prevention
Biotechnologically produced drugs as second-line therapy for rheumatoid arthritis 1
 IQWiG Reports Commission No. A10-01 Biotechnologically produced drugs as second-line therapy for rheumatoid arthritis 1 Executive Summary 1 Translation of the executive summary of the final report Biotechnologisch
IQWiG Reports Commission No. A10-01 Biotechnologically produced drugs as second-line therapy for rheumatoid arthritis 1 Executive Summary 1 Translation of the executive summary of the final report Biotechnologisch
LUPUS CAN DO EVERYTHING, BUT NOT EVERYTHING IS LUPUS LUPUS 101 SLE SUBSETS AUTOIMMUNE DISEASE 11/4/2013 HOWARD HAUPTMAN, MD IDIOPATHIC DISCOID LUPUS
 LUPUS 101 LUPUS CAN DO EVERYTHING, BUT NOT EVERYTHING IS LUPUS HOWARD HAUPTMAN, MD IDIOPATHIC DISCOID LUPUS SLE SUBSETS SUBACUTE CUTANEOUS LUPUS DRUG INDUCED LUPUS NEONATAL LUPUS LATE ONSET LUPUS ANTI-PHOSPHOLIPID
LUPUS 101 LUPUS CAN DO EVERYTHING, BUT NOT EVERYTHING IS LUPUS HOWARD HAUPTMAN, MD IDIOPATHIC DISCOID LUPUS SLE SUBSETS SUBACUTE CUTANEOUS LUPUS DRUG INDUCED LUPUS NEONATAL LUPUS LATE ONSET LUPUS ANTI-PHOSPHOLIPID
Autoimmune (AI) Disorders
 Autoimmune (AI) Disorders Affect up to 50 million people in the U.S. 80 100 types, dozens more suspected #2 cause of chronic illness Women are more likely to be affected than men Symptoms overlap and are
Autoimmune (AI) Disorders Affect up to 50 million people in the U.S. 80 100 types, dozens more suspected #2 cause of chronic illness Women are more likely to be affected than men Symptoms overlap and are
New Evidence reports on presentations given at ACR Improving Radiographic, Clinical, and Patient-Reported Outcomes with Rituximab
 New Evidence reports on presentations given at ACR 2009 Improving Radiographic, Clinical, and Patient-Reported Outcomes with Rituximab From ACR 2009: Rituximab Rituximab in combination with methotrexate
New Evidence reports on presentations given at ACR 2009 Improving Radiographic, Clinical, and Patient-Reported Outcomes with Rituximab From ACR 2009: Rituximab Rituximab in combination with methotrexate
Index. Note: Page numbers of article titles are in boldface type.
 Index Note: Page numbers of article titles are in boldface type. A Abatacept, for rheumatoid arthritis, 789 Acetaminophen, for low back pain, 735 Acupuncture for fibromyalgia, 753 for low back pain, 738
Index Note: Page numbers of article titles are in boldface type. A Abatacept, for rheumatoid arthritis, 789 Acetaminophen, for low back pain, 735 Acupuncture for fibromyalgia, 753 for low back pain, 738
New Evidence reports on presentations given at EULAR Tocilizumab for the Treatment of Rheumatoid Arthritis
 New Evidence reports on presentations given at EULAR 2012 Tocilizumab for the Treatment of Rheumatoid Arthritis Report on EULAR 2012 presentations Tocilizumab monotherapy is superior to adalimumab monotherapy
New Evidence reports on presentations given at EULAR 2012 Tocilizumab for the Treatment of Rheumatoid Arthritis Report on EULAR 2012 presentations Tocilizumab monotherapy is superior to adalimumab monotherapy
Adult-onset Still s disease a polygenic autoinflammatory disease
 Adult-onset Still s disease a polygenic autoinflammatory disease Tom Pettersson, MD, PhD University of Helsinki and Helsinki University Central Hospital IL-1 mediated diseases past present and future Såstaholm
Adult-onset Still s disease a polygenic autoinflammatory disease Tom Pettersson, MD, PhD University of Helsinki and Helsinki University Central Hospital IL-1 mediated diseases past present and future Såstaholm
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: golimumab_simponi 8/2013 2/2018 2/2019 3/2018 Description of Procedure or Service Golimumab (Simponi and
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: golimumab_simponi 8/2013 2/2018 2/2019 3/2018 Description of Procedure or Service Golimumab (Simponi and
Predictors of arthritis in pediatric patients with lupus
 Sule et al. Pediatric Rheumatology (2015) 13:30 DOI 10.1186/s12969-015-0027-7 RESEARCH ARTICLE Open Access Predictors of arthritis in pediatric patients with lupus SD Sule 1*, DG Moodalbail 2, J Burnham
Sule et al. Pediatric Rheumatology (2015) 13:30 DOI 10.1186/s12969-015-0027-7 RESEARCH ARTICLE Open Access Predictors of arthritis in pediatric patients with lupus SD Sule 1*, DG Moodalbail 2, J Burnham
Regulatory Status FDA-approved indication: Orencia is a selective T cell co-stimulation modulator indicated for: (1)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Orencia Page: 1 of 9 Last Review Date: September 20, 2018 Orencia Description Orencia (abatacept)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Orencia Page: 1 of 9 Last Review Date: September 20, 2018 Orencia Description Orencia (abatacept)
THE DIAGNOSTIC VALUE OF ANTIHISTONE ANTIBODIES IN DRUG-INDUCED LUPUS ERYTHEMATOSUS
 158 THE DIAGNOSTIC VALUE OF ANTIHISTONE ANTIBODIES IN DRUG-INDUCED LUPUS ERYTHEMATOSUS ALAN EPSTEIN and PETER BARLAND This study was undertaken to see whether the presence of antihistone antibodies, measured
158 THE DIAGNOSTIC VALUE OF ANTIHISTONE ANTIBODIES IN DRUG-INDUCED LUPUS ERYTHEMATOSUS ALAN EPSTEIN and PETER BARLAND This study was undertaken to see whether the presence of antihistone antibodies, measured
Technology appraisal guidance Published: 11 October 2017 nice.org.uk/guidance/ta480
 Tofacitinib for moderate to severeere rheumatoid arthritis Technology appraisal guidance Published: 11 October 2017 nice.org.uk/guidance/ta480 NICE 2018. All rights reserved. Subject to Notice of rights
Tofacitinib for moderate to severeere rheumatoid arthritis Technology appraisal guidance Published: 11 October 2017 nice.org.uk/guidance/ta480 NICE 2018. All rights reserved. Subject to Notice of rights
Management of Coccidioidomycosis in Rheumatic Patients on Biologic Response Modifiers
 Management of Coccidioidomycosis in Rheumatic Patients on Biologic Response Modifiers Dominick George Sudano, MD Assistant Professor of Medicine Department of Rheumatology University of Arizona Objectives
Management of Coccidioidomycosis in Rheumatic Patients on Biologic Response Modifiers Dominick George Sudano, MD Assistant Professor of Medicine Department of Rheumatology University of Arizona Objectives
1 Executive summary. Background
 1 Executive summary Background Rheumatoid Arthritis (RA) is the most common inflammatory polyarthropathy in the UK affecting between.5% and 1% of the population. The mainstay of RA treatment interventions
1 Executive summary Background Rheumatoid Arthritis (RA) is the most common inflammatory polyarthropathy in the UK affecting between.5% and 1% of the population. The mainstay of RA treatment interventions
Budsakorn Darawankul, MD. Maharat Nakhon Ratchasima Hospital
 Budsakorn Darawankul, MD. Maharat Nakhon Ratchasima Hospital Outline What is ANA? How to detect ANA? Clinical application Common autoantibody in ANA diseases Outline What is ANA? How to detect ANA? Clinical
Budsakorn Darawankul, MD. Maharat Nakhon Ratchasima Hospital Outline What is ANA? How to detect ANA? Clinical application Common autoantibody in ANA diseases Outline What is ANA? How to detect ANA? Clinical
Rheumatoid Arthritis. By: Hadi Esmaily (PharmD., BCCP, MBA) Department of Clinical Pharmacy, Shahid Beheshti Medical University
 Rheumatoid Arthritis By: Hadi Esmaily (PharmD., BCCP, MBA) Department of Clinical Pharmacy, Shahid Beheshti Medical University Introduction RA is a Chronic, Systemic, Inflammatory disorder of unknown etiology
Rheumatoid Arthritis By: Hadi Esmaily (PharmD., BCCP, MBA) Department of Clinical Pharmacy, Shahid Beheshti Medical University Introduction RA is a Chronic, Systemic, Inflammatory disorder of unknown etiology
1. Background: Infliximab is administered parenterally; therefore, it is not covered under retail pharmacy benefits.
 Subject: Infliximab (Remicade ) Original Original Committee Approval: October 13, 2006 Revised Last Committee Approval: December 3, 2008 Last Review: October 19, 2007 1. Background: Infliximab is a genetically
Subject: Infliximab (Remicade ) Original Original Committee Approval: October 13, 2006 Revised Last Committee Approval: December 3, 2008 Last Review: October 19, 2007 1. Background: Infliximab is a genetically
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: abatacept_orencia 4/2008 2/2018 2/2019 2/2018 Description of Procedure or Service Abatacept (Orencia ), a
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: abatacept_orencia 4/2008 2/2018 2/2019 2/2018 Description of Procedure or Service Abatacept (Orencia ), a
Rheumatologic Laboratory Testing and Common Hematological Drugs. September 10, 2013 Dr. Jeffrey C. Hoschek, MD, FAAP
 Rheumatologic Laboratory Testing and Common Hematological Drugs September 10, 2013 Dr. Jeffrey C. Hoschek, MD, FAAP THERE ARE MULTIPLE TESTS USED TO BOTH SCREEN FOR AND MAKE THE DIAGNOSIS OF MANY RHEUMATOLOGICAL
Rheumatologic Laboratory Testing and Common Hematological Drugs September 10, 2013 Dr. Jeffrey C. Hoschek, MD, FAAP THERE ARE MULTIPLE TESTS USED TO BOTH SCREEN FOR AND MAKE THE DIAGNOSIS OF MANY RHEUMATOLOGICAL
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: belimumab_benlysta 6/2011 2/2018 2/2019 3/2018 Description of Procedure or Service Belimumab (Benlysta) is
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: belimumab_benlysta 6/2011 2/2018 2/2019 3/2018 Description of Procedure or Service Belimumab (Benlysta) is
Patient #1. Rheumatoid Arthritis. Rheumatoid Arthritis. 45 y/o female Morning stiffness in her joints >1 hour
 Patient #1 Rheumatoid Arthritis Essentials For The Family Medicine Physician 45 y/o female Morning stiffness in her joints >1 hour Hands, Wrists, Knees, Ankles, Feet Polyarticular, symmetrical swelling
Patient #1 Rheumatoid Arthritis Essentials For The Family Medicine Physician 45 y/o female Morning stiffness in her joints >1 hour Hands, Wrists, Knees, Ankles, Feet Polyarticular, symmetrical swelling
QUAN V. DOAN, PharmD; CHIUN-FANG CHIOU, PhD; and ROBERT W. DUBOIS, MD, PhD
 SUBJECT REVIEW Review of Eight Pharmacoeconomic Studies of the Value of Biologic DMARDs (Adalimumab, Etanercept, and Infliximab) in the Management of Rheumatoid Arthritis QUAN V. DOAN, PharmD; CHIUN-FANG
SUBJECT REVIEW Review of Eight Pharmacoeconomic Studies of the Value of Biologic DMARDs (Adalimumab, Etanercept, and Infliximab) in the Management of Rheumatoid Arthritis QUAN V. DOAN, PharmD; CHIUN-FANG
Xeljanz (tofacitinib), Xeljanz XR (tofacitinib extended-release)
 Market DC Xeljanz (tofacitinib), Xeljanz XR (tofacitinib extended-release) Override(s) Prior Authorization Quantity Limit Medications Xeljanz (tofacitinib) Approval Duration 1 year Quantity Limit May be
Market DC Xeljanz (tofacitinib), Xeljanz XR (tofacitinib extended-release) Override(s) Prior Authorization Quantity Limit Medications Xeljanz (tofacitinib) Approval Duration 1 year Quantity Limit May be
Clinical Policy: Certolizumab (Cimzia) Reference Number: PA.CP.PHAR.247 Effective Date: 01/18 Last Review Date: 08/17 Line of Business: Medicaid
 Clinical Policy: (Cimzia) Reference Number: PA.CP.PHAR.247 Effective Date: 01/18 Last Review Date: 08/17 Line of Business: Medicaid Coding Implications Revision Log Description (Cimzia ) is a tumor necrosis
Clinical Policy: (Cimzia) Reference Number: PA.CP.PHAR.247 Effective Date: 01/18 Last Review Date: 08/17 Line of Business: Medicaid Coding Implications Revision Log Description (Cimzia ) is a tumor necrosis
Definition Chronic autoimmune disease The body s immune system starts attacking itself Can affect most organs and tissues in the body Brain, lungs, he
 LIVING WITH SYSTEMIC LUPUS ERYTHEMATOSUS Stacy Kennedy, M.D.,M.B.A. Rowan Diagnostic Clinic Salisbury, N.C. May 11, 2013 Agenda What is lupus Who is affected Causes of lupus Symptoms and organ involvement
LIVING WITH SYSTEMIC LUPUS ERYTHEMATOSUS Stacy Kennedy, M.D.,M.B.A. Rowan Diagnostic Clinic Salisbury, N.C. May 11, 2013 Agenda What is lupus Who is affected Causes of lupus Symptoms and organ involvement
RHEUMATOID ARTHRITIS DRUGS
 Rheumatology Biologics Criteria from the Exceptional Access Program RHEUMATOID ARTHRITIS DRUGS DRUG NAME BRS REIMBURSED DOSAGE FORM/ STRENGTH Adalimumab Humira 40 mg/0.8 syringe and 40mg/0.8 pen for Anakinra
Rheumatology Biologics Criteria from the Exceptional Access Program RHEUMATOID ARTHRITIS DRUGS DRUG NAME BRS REIMBURSED DOSAGE FORM/ STRENGTH Adalimumab Humira 40 mg/0.8 syringe and 40mg/0.8 pen for Anakinra
ORENCIA (ABATACEPT) INJECTION FOR INTRAVENOUS INFUSION
 ORENCIA (ABATACEPT) INJECTION FOR INTRAVENOUS INFUSION UnitedHealthcare Commercial Medical Benefit Drug Policy Policy Number: 2018D0039J Effective Date: March 1, 2018 Table of Contents Page INSTRUCTIONS
ORENCIA (ABATACEPT) INJECTION FOR INTRAVENOUS INFUSION UnitedHealthcare Commercial Medical Benefit Drug Policy Policy Number: 2018D0039J Effective Date: March 1, 2018 Table of Contents Page INSTRUCTIONS
LUPUS (SLE) MEDICAL SOURCE STATEMENT
 LUPUS (SLE) MEDICAL SOURCE STATEMENT From: Re: (Name of Patient) (Social Security No.) Please answer the following questions concerning your patient s impairments. Attach relevant treatment notes, radiologist
LUPUS (SLE) MEDICAL SOURCE STATEMENT From: Re: (Name of Patient) (Social Security No.) Please answer the following questions concerning your patient s impairments. Attach relevant treatment notes, radiologist
Reiter s Syndrome: Clinical Features
 Reiter s Syndrome: Clinical Features Arthritis lower extremities Enthesitis Spondylitis Tenosynovitis Dactylitis Urethritis Uveitis Oral ulcers Keratoderma Balanitis HLA B-27 Positive (80%) Reactive Arthritis:
Reiter s Syndrome: Clinical Features Arthritis lower extremities Enthesitis Spondylitis Tenosynovitis Dactylitis Urethritis Uveitis Oral ulcers Keratoderma Balanitis HLA B-27 Positive (80%) Reactive Arthritis:
Regional Audit of Biologic Usage in Arthritis
 Regional Audit of Biologic Usage in Arthritis February 2014 Foreword The introduction of biologic agents, towards the end of the 1990 s, to the treatment of a number of conditions including rheumatoid
Regional Audit of Biologic Usage in Arthritis February 2014 Foreword The introduction of biologic agents, towards the end of the 1990 s, to the treatment of a number of conditions including rheumatoid
Azathioprine toxicity criteria and severity descriptors for the listing of biological agents for rheumatoid arthritis on the PBS
 Azathioprine toxicity criteria and severity descriptors for the listing of biological agents for rheumatoid arthritis on the PBS Only valid for adult patients Azathioprine must be at a dose of at least
Azathioprine toxicity criteria and severity descriptors for the listing of biological agents for rheumatoid arthritis on the PBS Only valid for adult patients Azathioprine must be at a dose of at least
Acute Emergencies in Rheumatology
 Acute Emergencies in Rheumatology Clare Higgens Northwick Park hospital and St George s Hospital London Acute Rheumatological Emergencies The Acute Hot joint Inflammatory back pain.. Systemic lupus erythematosus(sle)
Acute Emergencies in Rheumatology Clare Higgens Northwick Park hospital and St George s Hospital London Acute Rheumatological Emergencies The Acute Hot joint Inflammatory back pain.. Systemic lupus erythematosus(sle)
SHO Teaching. Dr. Amir Bhanji Consultant Nephrologist, Q.A hospital, Portsmouth
 SHO Teaching Vasculitis Renal medicine Dr. Amir Bhanji Consultant Nephrologist, Q.A hospital, Portsmouth OUTLINE What is vasculitis Causes Classification Brief look into ANCA Associated Vasculitis (AAV)
SHO Teaching Vasculitis Renal medicine Dr. Amir Bhanji Consultant Nephrologist, Q.A hospital, Portsmouth OUTLINE What is vasculitis Causes Classification Brief look into ANCA Associated Vasculitis (AAV)
CIMZIA (certolizumab pegol)
 Pre - PA Allowance None Prior-Approval Requirements Age Diagnoses 18 years of age or older Patient must have ONE of the following: 1. Moderate to severe Crohn s Disease (CD) a. Inadequate response, intolerance
Pre - PA Allowance None Prior-Approval Requirements Age Diagnoses 18 years of age or older Patient must have ONE of the following: 1. Moderate to severe Crohn s Disease (CD) a. Inadequate response, intolerance
A cost effectiveness analysis of treatment options for methotrexate-naive rheumatoid arthritis Choi H K, Seeger J D, Kuntz K M
 A cost effectiveness analysis of treatment options for methotrexate-naive rheumatoid arthritis Choi H K, Seeger J D, Kuntz K M Record Status This is a critical abstract of an economic evaluation that meets
A cost effectiveness analysis of treatment options for methotrexate-naive rheumatoid arthritis Choi H K, Seeger J D, Kuntz K M Record Status This is a critical abstract of an economic evaluation that meets
Editing file. Color code: Important in red Extra in blue. Autoimmune Diseases
 Editing file Color code: Important in red Extra in blue Autoimmune Diseases Objectives To know that the inflammatory processes in autoimmune diseases are mediated by hypersensitivity reactions (type II,
Editing file Color code: Important in red Extra in blue Autoimmune Diseases Objectives To know that the inflammatory processes in autoimmune diseases are mediated by hypersensitivity reactions (type II,
Ankylosing Spondylitis AS M45. Psoriatic Arthritis PSA L405, M070, M071, M073. Systemic Lupus Erythematosus SLE M320, M321, M328, M329
 Appendix 1. Diagnosis codes according to International Classification of Disease version 10 (ICD 10) used to define rheumatoid arthritis (RA) and related diseases Diagnosis Abbreviation ICD 10 Rheumatoid
Appendix 1. Diagnosis codes according to International Classification of Disease version 10 (ICD 10) used to define rheumatoid arthritis (RA) and related diseases Diagnosis Abbreviation ICD 10 Rheumatoid
Efficacy and Safety of Rituximab in the Treatment of Rheumatoid Arthritis and ANCA-associated Vasculitis
 New Evidence reports on presentations given at ACR/ARHP 2010 Efficacy and Safety of Rituximab in the Treatment of Rheumatoid Arthritis and ANCA-associated Vasculitis Report on ACR/ARHP 2010 presentations
New Evidence reports on presentations given at ACR/ARHP 2010 Efficacy and Safety of Rituximab in the Treatment of Rheumatoid Arthritis and ANCA-associated Vasculitis Report on ACR/ARHP 2010 presentations
The Hospital for Sick Children Technology Assessment at SickKids (TASK)
 The Hospital for Sick Children Technology Assessment at SickKids (TASK) THE USE OF BIOLOGIC RESPONSE MODIFIERS IN POLYARTICULAR-COURSE JUVENILE IDIOPATHIC ARTHRITIS Report No. 2010-01 Date: January 11,
The Hospital for Sick Children Technology Assessment at SickKids (TASK) THE USE OF BIOLOGIC RESPONSE MODIFIERS IN POLYARTICULAR-COURSE JUVENILE IDIOPATHIC ARTHRITIS Report No. 2010-01 Date: January 11,
Ask the Expert: Photosensitivity in Cutaneous Lupus
 Ask the Expert: Photosensitivity in Cutaneous Lupus Victoria P. Werth, MD Department of Dermatology & Medicine University of Pennsylvania ; Philadelphia VA Hospital Overview Definition Impact of photosensitivity
Ask the Expert: Photosensitivity in Cutaneous Lupus Victoria P. Werth, MD Department of Dermatology & Medicine University of Pennsylvania ; Philadelphia VA Hospital Overview Definition Impact of photosensitivity
Coccidioidomycosis in Rheumatologic Patients. Susan E. Hoover, MD
 Coccidioidomycosis in Rheumatologic Patients Susan E. Hoover, MD The problem Prevalence of rheumatoid arthritis is 1% Population of Arizona is 6 million At least 150,000 new cocci infections yearly DMARDs
Coccidioidomycosis in Rheumatologic Patients Susan E. Hoover, MD The problem Prevalence of rheumatoid arthritis is 1% Population of Arizona is 6 million At least 150,000 new cocci infections yearly DMARDs
Helpline No:
 ARTHRITIS FOUNDATION Registered Nonprofit Organisation - No. 002-847 NPO Helpline No: 0861 30 30 30 DRUGS AND ARTHRITIS This information leaflet is published by the Arthritis Foundation as part of our
ARTHRITIS FOUNDATION Registered Nonprofit Organisation - No. 002-847 NPO Helpline No: 0861 30 30 30 DRUGS AND ARTHRITIS This information leaflet is published by the Arthritis Foundation as part of our
Rheumatology Review Update in Internal Medicine COPYRIGHT. Robert H. Shmerling, M.D. Beth Israel Deaconess Medical Center.
 Rheumatology Review Update in Internal Medicine Robert H. Shmerling, M.D. Beth Israel Deaconess Medical Center Boston MA Case #1 True statement(s) regarding etanercept and leflunomide, for the treatment
Rheumatology Review Update in Internal Medicine Robert H. Shmerling, M.D. Beth Israel Deaconess Medical Center Boston MA Case #1 True statement(s) regarding etanercept and leflunomide, for the treatment
ONE of the following:
 Medical Coverage Policy Belimumab (Benlysta) EFFECTIVE DATE: 01 01 2012 POLICY LAST UPDATED: 11 21 2017 OVERVIEW Belimumab (Benlysta ) is indicated for the treatment of adult patients with active, autoantibody-positive,
Medical Coverage Policy Belimumab (Benlysta) EFFECTIVE DATE: 01 01 2012 POLICY LAST UPDATED: 11 21 2017 OVERVIEW Belimumab (Benlysta ) is indicated for the treatment of adult patients with active, autoantibody-positive,
BSR Biologics Register Rheumatoid Arthritis Clinical Baseline Form
 BSR Biologics Register Rheumatoid Arthritis Clinical Baseline Form Please complete the following PATIENT information ID For office use only Gender: Male Female Date of birth: D D M M Y Y Y Y Hospital Reg.
BSR Biologics Register Rheumatoid Arthritis Clinical Baseline Form Please complete the following PATIENT information ID For office use only Gender: Male Female Date of birth: D D M M Y Y Y Y Hospital Reg.
Cimzia. Cimzia (certolizumab pegol) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Section: Prescription Drugs Effective Date: April 1, 2018 Subject: Cimzia Page: 1 of 5 Last Review
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.50.11 Section: Prescription Drugs Effective Date: April 1, 2018 Subject: Cimzia Page: 1 of 5 Last Review
Systemic Lupus Erythematosus among Jordanians: A Single Rheumatology Unit Experience
 Systemic Lupus Erythematosus among Jordanians: A Single Rheumatology Unit Experience Ala M. AlHeresh MD* ABSTRACT Objectives: To study the characteristics of Systemic Lupus Erythematosus in Jordan and
Systemic Lupus Erythematosus among Jordanians: A Single Rheumatology Unit Experience Ala M. AlHeresh MD* ABSTRACT Objectives: To study the characteristics of Systemic Lupus Erythematosus in Jordan and
Drug selection in Rheumatoid Arthritis
 Drug selection in Rheumatoid Arthritis PROFESSOR KHAN ABUL KALAM AZAD PROFESSOR, DEPARTMENT OF MEDICINE DHAKA MEDICAL COLLEGE Rheumatoid arthritis Autoimmune disease Onset generally occurs between 30 and
Drug selection in Rheumatoid Arthritis PROFESSOR KHAN ABUL KALAM AZAD PROFESSOR, DEPARTMENT OF MEDICINE DHAKA MEDICAL COLLEGE Rheumatoid arthritis Autoimmune disease Onset generally occurs between 30 and
Challenges in Perioperative Medication Management. Learning Objectives. Case 1. » Appropriate use of beta-blockers. Management of diabetes drugs
 Challenges in Perioperative Medication Management Dimitriy Levin, MD Assistant Professor of Medicine University of Colorado Hospital Medicine Group Learning Objectives» Appropriate use of beta-blockers
Challenges in Perioperative Medication Management Dimitriy Levin, MD Assistant Professor of Medicine University of Colorado Hospital Medicine Group Learning Objectives» Appropriate use of beta-blockers
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists. International authors and editors
 We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,900 116,000 120M Open access books available International authors and editors Downloads Our
We are IntechOpen, the world s leading publisher of Open Access books Built by scientists, for scientists 3,900 116,000 120M Open access books available International authors and editors Downloads Our
Regulatory Status FDA- approved indication: Simponi and Simponi ARIA are tumor necrosis factor (TNF) blockers indicated for the treatment of: (2-3)
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 9 Last Review Date: March 16, 2018 Simponi / Simponi
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.51 Subject: Simponi / Simponi ARIA Page: 1 of 9 Last Review Date: March 16, 2018 Simponi / Simponi
2017 PERIOPERATIVE MEDICINE SYMPOSIUM Peri-operative use of immunosuppression in rheumatology patients
 2017 PERIOPERATIVE MEDICINE SYMPOSIUM Peri-operative use of immunosuppression in rheumatology patients Dr Alberta Hoi Rheumatologist MBBS, FRACP, PhD NEW ERA IN MUSCULOSKELETAL MEDICINE New drugs - Biologics,
2017 PERIOPERATIVE MEDICINE SYMPOSIUM Peri-operative use of immunosuppression in rheumatology patients Dr Alberta Hoi Rheumatologist MBBS, FRACP, PhD NEW ERA IN MUSCULOSKELETAL MEDICINE New drugs - Biologics,
2.0 Synopsis. Adalimumab (HUMIRA ) W Clinical Study Report R&D/15/0629. Individual Study Table Referring to Part of Dossier: Volume:
 2.0 Synopsis AbbVie Inc. Name of Study Drug: Adalimumab / HUMIRA Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only)
2.0 Synopsis AbbVie Inc. Name of Study Drug: Adalimumab / HUMIRA Name of Active Ingredient: Adalimumab Individual Study Table Referring to Part of Dossier: Volume: Page: (For National Authority Use Only)
Environmental factors including drugs. Jyoti Ranjan Parida
 Environmental factors including drugs Jyoti Ranjan Parida Scheme of presentation Pathogenesis of autoimmune disease Evidence for environmental influence Environmental factors Proposed -Role of Sunlight
Environmental factors including drugs Jyoti Ranjan Parida Scheme of presentation Pathogenesis of autoimmune disease Evidence for environmental influence Environmental factors Proposed -Role of Sunlight
Switching Between Biological Treatments in Psoriatic Arthritis: A Review of the Evidence
 Drugs R D (2017) 17:509 522 DOI 10.1007/s40268-017-0215-7 REVIEW ARTICLE Switching Between Biological Treatments in Psoriatic Arthritis: A Review of the Evidence Luisa Costa 1 Carlo Perricone 2 Maria Sole
Drugs R D (2017) 17:509 522 DOI 10.1007/s40268-017-0215-7 REVIEW ARTICLE Switching Between Biological Treatments in Psoriatic Arthritis: A Review of the Evidence Luisa Costa 1 Carlo Perricone 2 Maria Sole
Technology appraisal guidance Published: 26 January 2016 nice.org.uk/guidance/ta375
 Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed Technology
Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for rheumatoid arthritis not previously treated with DMARDs or after conventional DMARDs only have failed Technology
