Contents Page No. 1. Outline of Procedure 2. Area of Application 3. Objective 4. Stages of the Process 5. Responsibilities
|
|
|
- Ashlynn Boone
- 5 years ago
- Views:
Transcription
1
2 Contents Page No. 1. Outline of Procedure 3 2. Area of Application 3 3. Objective 3 4. Stages of the Process 3 5. Responsibilities 8 6. Other useful information 8 7. References 8 Page 2 of 10
3 Clinical Policy for Tumour Lysis Syndrome Management 1. Outline of Procedure To provide information to Haematology and Oncology staff on the Prevention and management of tumour lysis syndrome. 2. Area of Application This SOP applies to all adult SACT services across the NOSCAN region, excepting for the administrative areas of Argyll and Bute in NHS Highland which are linked to the WOSCAN CEL (2012) 30 governance framework. 3. Objective This document covers the prevention and treatment of tumour lysis syndrome (TLS) in adults. Please refer to the appropriate guidelines for metabolic derangement or renal failure related to other causes. 4. Stages of the Process Tumour lysis syndrome (TLS) is an emergency that is caused by massive tumour cell lysis, with the release of large amounts of potassium, phosphate and nucleic acids in to the systemic circulation. Catabolism of nucleic acids to uric acid leads to hyperuricaemia, resulting in the precipitation of uric acid in the renal tubules. Hyperphosphataemia can lead to the deposition of calcium phosphate deposition in the renal tubules and can also cause acute kidney injury. Symptoms and signs of tumour lysis syndrome include nausea, vomiting, diarrhoea, anorexia, lethargy, oedema, fluid overload, haematuria, congestive heart failure, cardiac dysrhythmias, seizures, muscle cramps, tetany, syncope and possible sudden death. Tumour lysis can occur spontaneously but generally occurs 12 to 72 hours after initiation of cytoreductive therapy. Care should be taken to avoid potentially nephrotoxic substances, including intravenous contrast and NSAIDs in patients at risk of tumour lysis syndrome. The cornerstones of management are recognition of the high risk patient and appropriate pre-emptive treatment. Diagnosis of tumour lysis syndrome There is no universally accepted system for grading TLS. It may be classified according to clinical or laboratory features. A laboratory screen for TLS is given below: Table 1: laboratory screen for tumour lysis syndrome Uric acid* 476 µmol/l or 25% increase Potassium* 6.0 mmol/l or 25% increase Phosphate* 1.45 mmol/l or 25% increase Corrected calcium* 1.75 mmol/l or 25% decrease LDH >2 x upper limit of normal Serum creatinine 1.5 x upper limit of normal Page 3 of 10
4 * 2 of these features between 3 days pre- and 7 days post-administration of chemotherapy meets the Cairo-Bishop laboratory definition of tumour lysis syndrome. A clinical diagnosis of tumour lysis syndrome can be made if acute renal failure, cardiac arrhythmia or seizures occur in conjunction with the Cairo-Bishop laboratory features listed above. Risk assessment and stratification pre-chemotherapy Prior to starting the first cycle of chemotherapy (including steroid monotherapy), a risk assessment for TLS must be carried out in all patients with malignancies being treated in Haematology and Oncology. This assessment must take into account disease type, white cell count and additional risk factors (Table 2). The individual should be assessed for the presence of renal dysfunction or renal involvement by the malignancy (e.g. oliguria, preexisting renal failure or hyperuricaemia) and for signs of TLS. Where possible, the risk group should be recorded at the MDT. Individuals with evidence of TLS at presentation should be treated as for high risk disease. An individual s risk of tumour lysis should be upgraded (low to intermediate risk or intermediate to high risk) if any one of the following is present (with the exception of individuals with solid tumours or myeloma): Pre-existing nephropathy/renal impairment Dehydration Acidosis Hypotension Prior exposure to a nephrotoxic agent Renal involvement by underlying disease process Page 4 of 10
5 Table 2 Low risk disease (<1%) Most solid tumours Intermediate risk disease (1-5%) High risk disease (>5%) Rarely highly chemotherapy-sensitive tumours e.g. germ cell tumour, small cell lung cancer with bulky or advanced stage disease Bulky metastatic germ cell tumours prior to chemotherapy Myeloma Plasma cell leukaemia N/A CML N/A N/A Indolent NHL N/A FL treated with PI3K inhibitors Hodgkin lymphoma N/A N/A CLL and white cell count (WCC) <50 x 10 9 /L, treated only with alkylating agents AML and WCC <50 x 10 9 /L and LDH <2 x ULN Adult intermediate grade NHL and LDH within normal limits Adult Anaplastic Large Cell Lymphoma (ALCL) N/A N/A CLL treated with fludarabine, rituximab or lenalidomide, and/or those with high WCC 50 x 10 9 /L AML with WCC x 10 9 /L; AML and WCC <25 x 10 9 /L and LDH 2 x ULN Adult T-cell leukaemia/lymphoma, diffuse large B-cell lymphoma, transformed high grade lymphoma, and mantle cell lymphomas with LDH >ULN but <2 x ULN, non bulky Childhood ALCL stage III or IV Childhood intermediate grade NHL stage III/IV with LDH <2 x ULN ALL and WCC <100 x 10 9 /L and LDH <2 x ULN CLL treated with PI3K inhibitors AML and WCC 100 x 10 9 /L Adult T-cell leukaemia/lymphoma, diffuse large B-cell lymphoma, transformed high grade lymphoma, and mantle cell lymphomas with bulky disease and LDH 2 x ULN prior to radiotherapy or chemotherapy N/A Stage III/IV childhood diffuse large B- cell lymphoma with LDH 2 x ULN Burkitt s leukaemia; Other ALL and WCC 100 x 10 9 /L and/or LDH 2 x ULN N/A Burkitt lymphoma and LDH <2 x ULN Burkitt lymphoma stage III/IV and/or LDH 2 x ULN N/A Lymphoblastic lymphoma stage I/II and LDH <2 x ULN Lymphoblastic lymphoma stage III/IV and/or LDH 2 x ULN N/A N/A Intermediate risk disease (IRD) with renal dysfunction and/or renal involvement; IRD with uric acid, potassium and/or phosphate >ULN Prophylaxis recommendations Monitoring Monitoring Monitoring Hydration Hydration Hydration ± allopurinol Allopurinol Rasburicase* *Rasburicase is contraindicated in patients with a history consistent with glucose-6 phosphate dehydrogenase deficiency. In these patients, rasburicase should be substituted with allopurinol. Page 5 of 10
6 Pre-emptive treatment of tumour lysis syndrome Hydration: High or intermediate risk of TLS: aggressive fluid hydration (2 3 L/m 2 daily) should be started at least 24 hours prior to chemotherapy, aiming for a urine output of at least ml/m 2 /hr (no added potassium unless specifically advised by consultant). If there is no evidence of acute obstructive uropathy and/or hypovolaemia, a loop diuretic may be used to maintain the urine output, although evidence for the benefits of this approach in humans with TLS is limited. Urinary alkalinisation is not recommended. Hypouricaemic agents Risk of TLS High risk Intermediate risk Low risk Treatment Rasburicase (0 2 mg/kg) for one dose and start allopurinol treatment. Monitor uric acid levels closely* and give additional doses of rasburicase given when and if hyperuricaemia recurs If uric acid levels are <476 µmol/l, give allopurinol mg orally 8 hourly***. Monitor for TLS and its complications. If the uric acid level is 476 µmol/l, give a single dose of rasburicase (0.2 mg/kg, 3 or 6 mg depending on body weight) and monitor uric acid levels closely. Watch and wait approach hydration and close monitoring, rather than prophylactic allopurinol or rasburicase. * Blood samples for uric acid should be collected in a pre-chilled tube, immediately placed on ice, and the assay completed within 4 hours, if possible. In patients with a history of glucose- 6-phosphate dehydrogenase (G6PD) deficiency, rasburicase is contraindicated and allopurinol should be utilized instead of rasburicase. Where possible, consider testing patients at risk (including those from African, Mediterranean, or Southeast Asian descent) of G6PD deficiency before administering rasburicase. **In the absence of established clinical or laboratory TLS, an alternative approach would be to use a single fixed dose of 3 mg rasburicase, with repeat dosing to be guided by careful monitoring of clinical and biochemical parameters. ***allopurinol and renal impairment: GFR ml/min: allopurinol dose mg daily GFR ml/min: allopurinol dose mg daily GFR <10 ml/min: allopurinol dose 100 mg daily or 100 mg on alternate days For patients receiving dialysis, see renal handbook. DOSE IN PATIENTS UNDERGOIN Post treatment monitoring Patients at high risk for TLS require intensive supportive care, continuous cardiac monitoring, close monitoring of urine output and fluid balance and frequent serial measurement of electrolytes, creatinine and uric acid. Page 6 of 10
7 For patients at intermediate or high risk of developing TLS, monitor the serum uric acid, phosphate, potassium, creatinine, corrected calcium and LDH 4 6 hours after the initial administration of chemotherapy, and every 6 12 hours thereafter. Delays in specimen transport and/or processing should be avoided especially in patients with high white counts to prevent spurious elevations in potassium. Immediate therapeutic intervention is required if evidence of TLS or rising level of uric acid. Adult patients at intermediate risk not receiving rasburicase should have electrolytes checked 8 hours after chemotherapy and should be monitored for at least 24 hours after completion of the first cycle of chemotherapy. Management of established tumour lysis syndrome Electrolyte abnormalities: monitor electrolytes, creatinine and uric acid every 4 6 hours and treat as shown in table 3. Table 3. Electrolyte abnormality Hyperphosphataemia (>2.1 mmol/l) Moderate Severe Hypocalcaemia (albumin corrected calcium 1.75 mmol/l) Asymptomatic Symptomatic Hyperkalaemia (see local guidelines*) Moderate ( 6.0 mmol/l) Severe ( 7.0 mmol/l) Renal failure (uraemia) Hyperuricaemia ( 476 µmol/l) Management Aluminum hydroxide (oral) mg/kg day in 4 divided doses (1 2 days) as phosphate binder if the patient is unsuitable for dialysis. Haemodialysis if unresponsive to conservative measures No therapy Calcium gluconate 10% ml iv as slow infusion over 10 minutes** Avoid potassium supplements ECG: if cardiotoxicity present give iv calcium gluconate, insulin and dextrose iv infusion and undertake haemodialysis if conservative management fails Haemodialysis Fluid, electrolyte and blood pressure management. Dose-adjust nephrotoxic drugs. Haemodialysis if conservative management fails Rasburicase for established hyperuricaemia and TLS *see acute medicine resource centre/electrolytes/hyperkalaemia on NHSG clinical guidance intranet ** Calcium gluconate will reverse the clinical effects of hypocalcaemia in the short term, but will further increase the deposition of calcium phosphate in the renal tubules, potentially worsening acute kidney injury Indications for renal replacement therapy: Severe oliguria or anuria Persistent hyperkalaemia Hyperphosphataemia-induced symptomatic hypocalcaemia Page 7 of 10
8 5. Responsibilities Clinical Haematology and Oncology staff. 6. Other useful information Overview of medications Detailed information relating to dosages, administration, toxicities and drug interactions can be found in the SPC on Allopurinol: this suppresses the formation of uric acid by inhibiting xanthine oxidase in the purine metabolic pathway. Skin rashes are common ( 1% and <10%) and, if they occur, allopurinol should be stopped and rasburicase started in its place. Hypersensitivity reactions are uncommon ( 0.1% and <1%). There are potential interactions with mercaptopurine, azathioprine, ciclosporin and thiazide diuretics. There may be no benefits to combining allopurinol with rasburicase in the prophylaxis or management of TLS. Rasburicase: this is a recombinant urate oxidase and converts uric acid into allantoin, which is readily soluble and easily excreted. The most significant drug-related adverse events are common allergic reactions, mainly rashes and urticaria. Cases of hypotension (<1%), bronchospasm (<1%), rhinitis (<0.1%) and severe hypersensitivity reactions (<1%), including anaphylaxis (<0.1%) have also been attributed to rasburicase. Rasburicase is contraindicated in glucose-6-phosphate (G6PD) deficiency. Oral phosphate binders: e.g. aluminium hydroxide. These are slow to act and poorly tolerated by ill patients. They should seldom be used, except if the patient is unfit for dialysis or as a temporary measure where immediate access to renal dialysis is not available. Diuretic therapy: this should only be given on the advice of a Nephrologist. It is important to be certain that the patient is not volume-depleted before considering diuretic therapy. Diuretics may encourage the precipitation of urate in renal tubules if administered to a patient who is not fluid replete 7. References Bose P, Qubaiah O. (2011) A review of tumour lysis syndrome with targeted therapies and role of rasburicase. J Clin Pharm Ther 2011; 36(3): Cairo MS, Coiffier B, Reiter A, Younes A; TLS Expert Panel (2010) Recommendation for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: An Expert TLS panel consensus. Br J Haematol 2010; 149(4): Coiffier B, Altman A, Pui CH, Younes A, Cairo MS (2008) Guidelines for the management of paediatric and adult tumour lysis syndrome: an evidence-based review. J Clin Oncol 2008; 26: Letter: ibid Author reply: ibid: Coutsouvelis J, Wiseman M, Hui L, Poole, S, Dooley M, Patil S, Avery S, Wei A, Spencer A. (2012) Effectiveness of a single fixed dose of rasburicase 3mg in the management of tumour lysis syndrome. Br J Clin Pharmacol 2012; 75(2): Page 8 of 10
9 Darmon M, Guichard I, Vincent F (2011) Rasburicase and tumour lysis syndrome: lower dosage, consideration of indications, and hyperhydrations. J Clin Oncol 2011; 29(3): e67-e68 Reply: Cortes J ibid e69 Darmon M, Vincent F, Camous L et al. (2013) Tumour lysis syndrome and acute kidney injury in highrisk haematology patients in the rasburicase era. A prospective multicentre study. Br J Haematol 2013; 162(4): Eaddy M et al (2009) The economic implications of rasburicase treatment in adult tumour lysis syndrome patients. J Clin Oncol Suppl abstr e17503 (Abstract only) Feng X, Dong K, Pham D, Pence S, Inciardi J, Bhutada NS. (2013) Efficacy and cost of single-dose rasburicase in prevention and treatment of adult tumour lysis syndrome: a meta-analysis. J Clin Pharm Ther 2013; 38(4): McBride A, Westervelt P. (2012) Recognising and managing the expanded risk of tumour lysis syndrome in haematologic and solid malignancies. J Hematol Oncol 2012; 5: 75 Sanofi (2012) FASTURTEC [Rasburicase] Summary of product characteristics. [acessed via medicines.org.uk, last updated on emc 23/11/12] Vadhan-Raj S, Fayad LE, Fanale MA et al. (2012) A randomized trial of a single-dose rasburicase versus five-daily doses in patients at risk for tumour lysis syndrome. Ann Oncol 2012; 23(6): Will A, Tholouli E. (2011) The clinical management of tumour lysis syndrome in haematological malignancies. Br J Haematol 2011; 154(1): 3-13 Wilson FP, Berns JS. (2012) Onco-nephrology: Tumour lysis syndrome. Clin J Am Soc Nephrol 2012; 7(10): UK Renal Association (Apr 2013) Clinical Practice Guidelines: Treatment of Acute Hyperkalaemia in Adults. Page 9 of 10
10 Replaces: Lead Author/Co-ordinator: Responsibilities of the Lead Author/Co-ordinator Key word(s): Document application: Purpose/description: (detail previous unique identifier if applicable) (as per front cover) Ensuring registration of this document on Document and Information Silo Disseminating document as per distribution list Retaining the master copy of this document Reviewing document in advance of review date (to help with the document search on intranet home page) NOSCAN (purpose of document) Policy statement: It is the responsibility of all staff to ensure that they are working to the most up to date and relevant clinical process documents. Responsibilities for implementation: Organisational: Operational Management Team and Chief Executive Sector General Managers, Medical Leads and Nursing Leads Departmental: Clinical Leads Area: Line Manager Review frequency and date of next review: (Include a statement that indicates that in the absence of any obvious changes review should occur every 2 years) Revision History: Revision Date Previous Revision Date Summary of Changes (Descriptive summary of the changes made) Changes Marked (Identify page numbers and section heading )
GUIDELINE FOR THE MANAGEMENT AND PREVENTION OF ACUTE TUMOUR LYSIS SYNDROME IN HAEMATOLOGICAL MALIGNANCIES
 GUIDELINE FOR THE MANAGEMENT AND PREVENTION OF ACUTE TUMOUR LYSIS SYNDROME IN HAEMATOLOGICAL MALIGNANCIES Full Title of Guideline: Author (include email and role): Division & Speciality: Scope (Target
GUIDELINE FOR THE MANAGEMENT AND PREVENTION OF ACUTE TUMOUR LYSIS SYNDROME IN HAEMATOLOGICAL MALIGNANCIES Full Title of Guideline: Author (include email and role): Division & Speciality: Scope (Target
Tumour Lysis Syndrome (TLS)
 (TLS) Overview: Tumour lysis syndrome refers to a number of metabolic disturbances (hyperuricaemia, hyperphosphataemia, hyperkalaemia and hypocalcaemia) that occur as the result of rapid cell lysis. This
(TLS) Overview: Tumour lysis syndrome refers to a number of metabolic disturbances (hyperuricaemia, hyperphosphataemia, hyperkalaemia and hypocalcaemia) that occur as the result of rapid cell lysis. This
Analysis of the incidence of tumor lysis syndrome in patients with hematological malignancies treated with rasburicase
 MOLECULAR AND CLINICAL ONCOLOGY 6: 955-959, 2017 Analysis of the incidence of tumor lysis syndrome in patients with hematological malignancies treated with rasburicase EISEKI USAMI 1, MICHIO KIMURA 1,
MOLECULAR AND CLINICAL ONCOLOGY 6: 955-959, 2017 Analysis of the incidence of tumor lysis syndrome in patients with hematological malignancies treated with rasburicase EISEKI USAMI 1, MICHIO KIMURA 1,
2.1 mmol/l or 25% increase from baseline mmol/l or 25% decrease from baseline
 22.1 Hyperleukocytosis and tumour lysis syndrome The guideline is addressed for ALL patients with hyperleukocytosis (WBC 100 x109/l) only and should not be used without modifications in case of other diseases
22.1 Hyperleukocytosis and tumour lysis syndrome The guideline is addressed for ALL patients with hyperleukocytosis (WBC 100 x109/l) only and should not be used without modifications in case of other diseases
Guidelines for the Prevention of Tumour Lysis Syndrome
 1.0 Introduction For the purposes of this guideline, tumour lysis syndrome (TLS) is defined as metabolic derangement resulting from abrupt and massive breakdown of malignant cells and the release of intracellular
1.0 Introduction For the purposes of this guideline, tumour lysis syndrome (TLS) is defined as metabolic derangement resulting from abrupt and massive breakdown of malignant cells and the release of intracellular
Tumor Lysis Syndrome Nephrology Grand Rounds Tuesday, July 27 th, 2010 Aditya Mattoo
 Tumor Lysis Syndrome Nephrology Grand Rounds Tuesday, July 27 th, 2010 Aditya Mattoo Outline Background/Definition Epidemiology/Risk Stratification Pathophysiology Treatment Renal Replacement Therapy Background/Definition
Tumor Lysis Syndrome Nephrology Grand Rounds Tuesday, July 27 th, 2010 Aditya Mattoo Outline Background/Definition Epidemiology/Risk Stratification Pathophysiology Treatment Renal Replacement Therapy Background/Definition
Guidelines for use of RASBURICASE in adult Haematology and Oncology patients
 Network Guidance Document Guidelines for use of RASBURICASE in adult Haematology and Oncology patients Status: Expiry Date: Version Number: Publication Date: Final September 2013 6 September 2011 Page
Network Guidance Document Guidelines for use of RASBURICASE in adult Haematology and Oncology patients Status: Expiry Date: Version Number: Publication Date: Final September 2013 6 September 2011 Page
Guidelines for the Management of Tumour Lysis Syndrome in Haematological and Solid Tumour Malignancies
 Guidelines for the Management of Tumour Lysis Syndrome in Haematological and Solid Tumour Malignancies CSPM 02 Date to be reviewed: September 2017 No of pages: 7 Author(s): Dr Yvonne Jones Author(s) title:
Guidelines for the Management of Tumour Lysis Syndrome in Haematological and Solid Tumour Malignancies CSPM 02 Date to be reviewed: September 2017 No of pages: 7 Author(s): Dr Yvonne Jones Author(s) title:
Oncology Emergency Essentials: Addressing Tumor Lysis Syndrome in Your Practice
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Subject: Rasburicase (Elitek )
 09-J2000-43 Original Effective Date: 10/15/15 Reviewed: 08/01/18 Revised: 09/15/18 Subject: Rasburicase (Elitek ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION OF
09-J2000-43 Original Effective Date: 10/15/15 Reviewed: 08/01/18 Revised: 09/15/18 Subject: Rasburicase (Elitek ) THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION OF
Announcements. Learning Objectives. Introduction. Prevention and Treatment of an Oncologic Emergency: Focus on Tumor Lysis Syndrome
 Prevention and Treatment of an Oncologic Emergency: Focus on Tumor Lysis Syndrome Kamakshi V. Rao, Pharm.D., BCOP Oncology Clinical Pharmacy Specialist University of North Carolina Hospitals and Clinics
Prevention and Treatment of an Oncologic Emergency: Focus on Tumor Lysis Syndrome Kamakshi V. Rao, Pharm.D., BCOP Oncology Clinical Pharmacy Specialist University of North Carolina Hospitals and Clinics
DERBY-BURTON LOCAL CANCER NETWORK FILENAME R-CODOX-M.DOC CONTROLLED DOC NO: HCCPG B115 CSIS Regimen Name: R-CODOXM. Rituximab + CODOX-M
 Rituximab + CODOX-M Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Burkitt
Rituximab + CODOX-M Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Burkitt
Outcome of Tumor Lysis Syndrome with Hydration and Alkalinization in Children with Acute Lymphoblastic Leukemia
 Bangladesh Journal of Medical Science Vol. 11 No. 04 Oct 12 Original article Outcome of Tumor Lysis Syndrome with Hydration and Alkalinization in Children with Acute Lymphoblastic Leukemia Sultana A 1,
Bangladesh Journal of Medical Science Vol. 11 No. 04 Oct 12 Original article Outcome of Tumor Lysis Syndrome with Hydration and Alkalinization in Children with Acute Lymphoblastic Leukemia Sultana A 1,
Spontaneous Tumor Lysis Syndrome in Small Cell Lung Cancer
 Open Access Case Report DOI: 10.7759/cureus.1017 Spontaneous Tumor Lysis Syndrome in Small Cell Lung Cancer Venkatkiran Kanchustambham 1, Swetha Saladi 2, Setu Patolia 2, David Stoeckel 2 1. Pulmonary
Open Access Case Report DOI: 10.7759/cureus.1017 Spontaneous Tumor Lysis Syndrome in Small Cell Lung Cancer Venkatkiran Kanchustambham 1, Swetha Saladi 2, Setu Patolia 2, David Stoeckel 2 1. Pulmonary
Avoiding Dialysis in Tumour Lysis Syndrome: Is Urate Oxidase Effective? A Case Report and Review of Literature
 Case Report 679 Avoiding Dialysis in Tumour Lysis Syndrome: Is Urate Oxidase Effective? A Case Report and Review of Literature Wan-Yee Teo, 1 MBBS, MRCPCH (Lond), Tsee-Foong Loh, 2 MBBS, MMed (Paeds),
Case Report 679 Avoiding Dialysis in Tumour Lysis Syndrome: Is Urate Oxidase Effective? A Case Report and Review of Literature Wan-Yee Teo, 1 MBBS, MRCPCH (Lond), Tsee-Foong Loh, 2 MBBS, MMed (Paeds),
NHS Grampian Staff Guideline For The Management Of Acute Hypophosphataemia In Adults
 NHS Grampian Staff Guideline For The Management Of Acute Hypophosphataemia In Adults Co-ordinators: Medicine Information Pharmacist Consultation Group: See Page 5 Approver: Medicine Guidelines and Policies
NHS Grampian Staff Guideline For The Management Of Acute Hypophosphataemia In Adults Co-ordinators: Medicine Information Pharmacist Consultation Group: See Page 5 Approver: Medicine Guidelines and Policies
NHS Grampian Staff Guidance For The Management Of Hypomagnesaemia In Adults. Consultation Group: See Page 4. Review Date: June 2021
 NHS...... Grampian NHS Grampian Staff Guidance For The Management Of Hypomagnesaemia In Adults Co-ordinators: Consultation Group: Approver:. Senior Medicines Information Pharmacist See Page 4 Medicine
NHS...... Grampian NHS Grampian Staff Guidance For The Management Of Hypomagnesaemia In Adults Co-ordinators: Consultation Group: Approver:. Senior Medicines Information Pharmacist See Page 4 Medicine
The pattern of metabolic derangements known as tumor. Tumor Lysis Syndrome. Resident Short Reviews
 Resident Short Reviews Tumor Lysis Syndrome Shelly M. Williams, MD; Anthony A. Killeen, MB, BCh, PhD Tumor lysis syndrome (TLS) is an acute, life-threatening disease among adults and children that is associated
Resident Short Reviews Tumor Lysis Syndrome Shelly M. Williams, MD; Anthony A. Killeen, MB, BCh, PhD Tumor lysis syndrome (TLS) is an acute, life-threatening disease among adults and children that is associated
Managing patients with bulky cancers
 SIOP PODC Supportive Care Education (ICON 2016) Presentation Date: 23 rd January 2016 Recording Link at www.cure4kids.org: https://www.cure4kids.org/ums/home/conference_rooms/enter.php?room=p2pjfjp8nha
SIOP PODC Supportive Care Education (ICON 2016) Presentation Date: 23 rd January 2016 Recording Link at www.cure4kids.org: https://www.cure4kids.org/ums/home/conference_rooms/enter.php?room=p2pjfjp8nha
Fasturtec 1.5 mg/ml powder and solvent for concentrate for solution for infusion.
 1. NAME OF THE MEDICINAL PRODUCT Fasturtec 1.5 mg/ml powder and solvent for concentrate for solution for infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION After reconstitution, 1 ml of Fasturtec concentrate
1. NAME OF THE MEDICINAL PRODUCT Fasturtec 1.5 mg/ml powder and solvent for concentrate for solution for infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION After reconstitution, 1 ml of Fasturtec concentrate
Haematological Emergencies (Part 1) Ray Mun Koo Haematology Advanced Trainee Canberra Hospital
 Haematological Emergencies (Part 1) Ray Mun Koo Haematology Advanced Trainee Canberra Hospital Case Number 1 43 year old male presenting with fevers, abdominal distension and weight gain over 2 weeks.
Haematological Emergencies (Part 1) Ray Mun Koo Haematology Advanced Trainee Canberra Hospital Case Number 1 43 year old male presenting with fevers, abdominal distension and weight gain over 2 weeks.
tumor lysis syndrome
 2.0 ANCC CONTACT HOURS Oncology Emergency series Recognizing and preventing tumor lysis syndrome By Roberta Kaplow, PhD, APRN-CCNS, AOCNS, CCRN, and Karen Iyere, MSN, RN TUMOR LYSIS SYNDROME (TLS) is one
2.0 ANCC CONTACT HOURS Oncology Emergency series Recognizing and preventing tumor lysis syndrome By Roberta Kaplow, PhD, APRN-CCNS, AOCNS, CCRN, and Karen Iyere, MSN, RN TUMOR LYSIS SYNDROME (TLS) is one
DERBY-BURTON CANCER NETWORK CONTROLLED DOC NO:
 OBINUTUZUMAB+CHLORAMBUCIL Regimen RDH; Day 1 and 2 Dose to be given on Ward Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community
OBINUTUZUMAB+CHLORAMBUCIL Regimen RDH; Day 1 and 2 Dose to be given on Ward Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community
Non-Hodgkin s Lymphomas Version
 NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Non-Hodgkin s Lymphomas Version 2.2015 NCCN.org Continue Supportive Care for NHL Tumor Lysis Syndrome (TLS) Laboratory hallmarks of TLS:
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Non-Hodgkin s Lymphomas Version 2.2015 NCCN.org Continue Supportive Care for NHL Tumor Lysis Syndrome (TLS) Laboratory hallmarks of TLS:
Note: There are other bendamustine protocols, ensure this is the correct one for a given patient.
 INDICATIONS 1 st line treatment for follicular lymphoma with FLIPI score 2 or higher: (NICE TA513- BLUETEQ required) Rituximab refractory follicular lymphoma (progression on R-chemo, R-maintenance or within
INDICATIONS 1 st line treatment for follicular lymphoma with FLIPI score 2 or higher: (NICE TA513- BLUETEQ required) Rituximab refractory follicular lymphoma (progression on R-chemo, R-maintenance or within
Iranian Journal of Blood & Cancer. The Efficacy of Single Dose Rasburicase in Prevention or Treatment of Tumor Lysis Syndrome in Children
 IJBC 2016; 8(2): 33-37 Iranian Journal of Blood & Cancer Journal Home Page: www.ijbc.ir Original Article The Efficacy of Single Dose Rasburicase in Prevention or Treatment of Tumor Lysis Syndrome in Children
IJBC 2016; 8(2): 33-37 Iranian Journal of Blood & Cancer Journal Home Page: www.ijbc.ir Original Article The Efficacy of Single Dose Rasburicase in Prevention or Treatment of Tumor Lysis Syndrome in Children
Burkitt s Lymphoma or DLBCL with adverse features PATIENTS WITH GOOD PERFORMANCE STATUS
 Regimen R-CODOX M Indication Burkitt s Lymphoma or DLBCL with adverse features Therapeutic Intent Radical/Curative PATIENTS WITH GOOD PERFORMANCE STATUS Day Medication Dose Route Administration Details
Regimen R-CODOX M Indication Burkitt s Lymphoma or DLBCL with adverse features Therapeutic Intent Radical/Curative PATIENTS WITH GOOD PERFORMANCE STATUS Day Medication Dose Route Administration Details
TIP Paclitaxel, Ifosfamide and Cisplatin
 Systemic Anti Cancer Treatment Protocol TIP Paclitaxel, Ifosfamide and Cisplatin PROTOCOL REF: MPHATIPGC (Version No: 1.0) Approved for use in: Second line treatment of germ cell tumours Dosage: Drug Dosage
Systemic Anti Cancer Treatment Protocol TIP Paclitaxel, Ifosfamide and Cisplatin PROTOCOL REF: MPHATIPGC (Version No: 1.0) Approved for use in: Second line treatment of germ cell tumours Dosage: Drug Dosage
Obinutuzumab+Bendamustine followed by Obinutuzumab Maintenance Burton in-patient Derby in-patient Burton day-case Derby day-case
 Obinutuzumab+Bendamustine followed by Obinutuzumab Maintenance Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Available
Obinutuzumab+Bendamustine followed by Obinutuzumab Maintenance Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Available
Rasburicase 1.5 mg/ml powder and solvent for concentrate for solution for infusion. FASTURTEC
 For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory This package insert is continually updated. Please read carefully before using a new pack. Rasburicase 1.5 mg/ml powder
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory This package insert is continually updated. Please read carefully before using a new pack. Rasburicase 1.5 mg/ml powder
The clinical management of tumour lysis syndrome in
 state of the art review The clinical management of tumour lysis syndrome in haematological malignancies Andrew Will 1 and Eleni Tholouli 2 1 Department of Paediatric Haematology, Royal Manchester Children
state of the art review The clinical management of tumour lysis syndrome in haematological malignancies Andrew Will 1 and Eleni Tholouli 2 1 Department of Paediatric Haematology, Royal Manchester Children
PRODUCT INFORMATION FASTURTEC
 PRODUCT INFORMATION FASTURTEC NAME OF THE MEDICINE NON-PROPRIETARY NAME Rasburicase rys for injection CAS REGISTRY NUMBER 134774-45-1 DESCRIPTION Fasturtec is a sterile powder supplied in a stoppered clear
PRODUCT INFORMATION FASTURTEC NAME OF THE MEDICINE NON-PROPRIETARY NAME Rasburicase rys for injection CAS REGISTRY NUMBER 134774-45-1 DESCRIPTION Fasturtec is a sterile powder supplied in a stoppered clear
Standard Operating Procedure for the Prevention and Treatment of Oral Mucositis
 the Prevention and Treatment of Oral Mucositis Lead Author/Co-ordinator: Lisa MacLeod Specialist Clinical Pharmacist Haem/Onc UK Oral Mucositis in Cancer Group Signature: Reviewers: NCAG Signature: Approver:
the Prevention and Treatment of Oral Mucositis Lead Author/Co-ordinator: Lisa MacLeod Specialist Clinical Pharmacist Haem/Onc UK Oral Mucositis in Cancer Group Signature: Reviewers: NCAG Signature: Approver:
keyword: diuretics Drug monitoring Monitoring diuretics in primary care 2 March 2009 best tests
 www.bpac.org.nz keyword: diuretics Drug monitoring Monitoring diuretics in primary care 2 March 2009 best tests Why do we monitor patients taking diuretics and what do we monitor? Monitoring a person on
www.bpac.org.nz keyword: diuretics Drug monitoring Monitoring diuretics in primary care 2 March 2009 best tests Why do we monitor patients taking diuretics and what do we monitor? Monitoring a person on
Lung Pathway Group Cisplatin & PO Vinorelbine in Non- Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Cisplatin & PO Vinorelbine in Non- Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in advanced/metastatic
Lung Pathway Group Cisplatin & PO Vinorelbine in Non- Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in advanced/metastatic
NHS Grampian Staff Guidance For The Management Of Hypomagnesaemia In Adults
 NHS Grampian Staff Guidance For The Management Of Hypomagnesaemia In Adults Co-ordinators: Sarah Gethings Medicine Information Pharmacist Consultation Group: See Page 4 Approver: Medicine Guidelines and
NHS Grampian Staff Guidance For The Management Of Hypomagnesaemia In Adults Co-ordinators: Sarah Gethings Medicine Information Pharmacist Consultation Group: See Page 4 Approver: Medicine Guidelines and
Mr PA. Clinical assessment of hydration. Poor urine output Sunken eyes Moistness of mucosa Cool peripheries Reduction in weight Postural hypotension
 X Anthony Warrens Mr PA 54 years old Previously well Went to Thailand Developed serious diarrhoea and vomiting two days before coming home 24 hours after return, still unwell GP found: urea 24 mmol/l creatinine
X Anthony Warrens Mr PA 54 years old Previously well Went to Thailand Developed serious diarrhoea and vomiting two days before coming home 24 hours after return, still unwell GP found: urea 24 mmol/l creatinine
Acute Renal Failure. Dr Kawa Ahmad
 62 Acute Renal Failure Dr Kawa Ahmad Acute Renal Failure It is characterised by an abrupt reduction (usually within a 48- h period) in kidney function. This results in an accumulation of nitrogenous waste
62 Acute Renal Failure Dr Kawa Ahmad Acute Renal Failure It is characterised by an abrupt reduction (usually within a 48- h period) in kidney function. This results in an accumulation of nitrogenous waste
Lung Pathway Group Cisplatin & IV Vinorelbine in Non- Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Cisplatin & IV Vinorelbine in Non- Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in advanced/metastatic
Lung Pathway Group Cisplatin & IV Vinorelbine in Non- Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in advanced/metastatic
Urate oxidase for the prevention and treatment of tumour lysis syndrome in children with cancer. Title. Cheuk, DKL; Chiang, AKS; Chan, GCF; Ha, SY
 Title Urate oxidase for the prevention and treatment of tumour lysis syndrome in children with cancer Author(s) Cheuk, DKL; Chiang, AKS; Chan, GCF; Ha, SY Citation Cochrane Database of Systematic Reviews,
Title Urate oxidase for the prevention and treatment of tumour lysis syndrome in children with cancer Author(s) Cheuk, DKL; Chiang, AKS; Chan, GCF; Ha, SY Citation Cochrane Database of Systematic Reviews,
R-BAC-500 (Rituximab, Bendamustine, Cytarabine) for Mantle Cell Lymphoma
 R-BAC-500 (Rituximab, Bendamustine, Cytarabine) for Mantle Cell Lymphoma Not routinely commissioned, each case requires prior documented approval before offering & commencing therapy from NHS England Cancer
R-BAC-500 (Rituximab, Bendamustine, Cytarabine) for Mantle Cell Lymphoma Not routinely commissioned, each case requires prior documented approval before offering & commencing therapy from NHS England Cancer
Acute tumor lysis syndrome (ATLS), although
 ACUTE TUMOR LYSIS SYNDROME Michele Cohen, DVM, DACVIM (Oncology) College of Veterinary Medicine Auburn University Auburn, Alabama Acute tumor lysis syndrome (ATLS), although uncommon, can be a serious
ACUTE TUMOR LYSIS SYNDROME Michele Cohen, DVM, DACVIM (Oncology) College of Veterinary Medicine Auburn University Auburn, Alabama Acute tumor lysis syndrome (ATLS), although uncommon, can be a serious
This is a controlled document and therefore must not be changed or photocopied L.80 - R-CHOP-21 / CHOP-21
 R- / INDICATION Lymphoma Histiocytosis Omit rituximab if CD20-negative. TREATMENT INTENT Disease modification or curative depending on clinical circumstances PRE-ASSESSMENT 1. Ensure histology is confirmed
R- / INDICATION Lymphoma Histiocytosis Omit rituximab if CD20-negative. TREATMENT INTENT Disease modification or curative depending on clinical circumstances PRE-ASSESSMENT 1. Ensure histology is confirmed
NHS Grampian Staff Guideline for the Management of Acute Hypokalaemia in Adults
 NHS Grampian Staff Guideline for the Management of Acute Hypokalaemia in Adults Co-ordinators: Medicines Information Pharmacist Consultation Group: See relevant page in guidance Approver: Medicine Guidelines
NHS Grampian Staff Guideline for the Management of Acute Hypokalaemia in Adults Co-ordinators: Medicines Information Pharmacist Consultation Group: See relevant page in guidance Approver: Medicine Guidelines
Development of tumor lysis syndrome (TLS): A potential risk factor in cancer patients receiving anticancer therapy
 www.bioinformation.net Hypothesis Volume 10(11) Development of tumor lysis syndrome (TLS): A potential risk factor in cancer patients receiving anticancer therapy Mahmood Rasool 1 *, Arif Malik 2, Muhammad
www.bioinformation.net Hypothesis Volume 10(11) Development of tumor lysis syndrome (TLS): A potential risk factor in cancer patients receiving anticancer therapy Mahmood Rasool 1 *, Arif Malik 2, Muhammad
StRs and CT doctors in haematology. September Folinic acid dose modified.
 High dose Methotrexate and folinic acid rescue Full Title of Guideline: Author (include email and role): Division & Speciality: Clinical Guideline Review Date September 2018 GUIDELINE FOR THE USE OF HIGH
High dose Methotrexate and folinic acid rescue Full Title of Guideline: Author (include email and role): Division & Speciality: Clinical Guideline Review Date September 2018 GUIDELINE FOR THE USE OF HIGH
Lung Pathway Group Carboplatin & PO Vinorelbine in Non-Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Carboplatin & PO Vinorelbine in Non-Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in
Lung Pathway Group Carboplatin & PO Vinorelbine in Non-Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in
Management of the patient with established AKI. Kelly Wright Lead Nurse for AKI King s College Hospital
 Management of the patient with established AKI Kelly Wright Lead Nurse for AKI King s College Hospital Medical management Medical management Respiratory- pulmonary oedema, repositioning- upright, oxygen
Management of the patient with established AKI Kelly Wright Lead Nurse for AKI King s College Hospital Medical management Medical management Respiratory- pulmonary oedema, repositioning- upright, oxygen
BEVACIZUMAB (AVASTIN ) & Paclitaxel PROTOCOL
 Bevacizumab (Avastin ) & Paclitaxel The treatment of Advanced Breast Cancer DRUG ADMINISTRATION Da Drug Daily Dose Route Diluent & Rate y 250mls Sodium Day 1,15 Bevacizumab 10 mg/kg Infusion Chloride 0.9%*
Bevacizumab (Avastin ) & Paclitaxel The treatment of Advanced Breast Cancer DRUG ADMINISTRATION Da Drug Daily Dose Route Diluent & Rate y 250mls Sodium Day 1,15 Bevacizumab 10 mg/kg Infusion Chloride 0.9%*
MabThera. SC. The wait is over. MabThera delivered in just 5 minutes. SC= subcutaneous injection
 MabThera SC. The wait is over. MabThera delivered in just 5 minutes Abbreviated Prescribing Information MabThera 1400 mg solution for subcutaneous (SC) injection (Rituximab) Indications: Indicated in adults
MabThera SC. The wait is over. MabThera delivered in just 5 minutes Abbreviated Prescribing Information MabThera 1400 mg solution for subcutaneous (SC) injection (Rituximab) Indications: Indicated in adults
Acute Kidney Injury. Eleanor Haskey BSc(hons) RVN VTS(ECC) VPAC A1
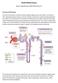 Acute Kidney Injury Eleanor Haskey BSc(hons) RVN VTS(ECC) VPAC A1 Anatomy and Physiology The role of the kidneys is to filter the blood through the glomerulus to form filtrate. The filtrate is then reabsorbed
Acute Kidney Injury Eleanor Haskey BSc(hons) RVN VTS(ECC) VPAC A1 Anatomy and Physiology The role of the kidneys is to filter the blood through the glomerulus to form filtrate. The filtrate is then reabsorbed
NECN CHEMOTHERAPY HANDBOOK PROTOCOL
 DRUG ADMINISTRATION SCHEDULE Day Drug Dose Route Diluent Rate 1* to 5 Prednisolone 40mg/m 2 Oral Once Daily For 5 days 1 Paracetamol 1gram Oral Once Only Chlorphenamine 10mg IV bolus Ondansetron 8mg IV
DRUG ADMINISTRATION SCHEDULE Day Drug Dose Route Diluent Rate 1* to 5 Prednisolone 40mg/m 2 Oral Once Daily For 5 days 1 Paracetamol 1gram Oral Once Only Chlorphenamine 10mg IV bolus Ondansetron 8mg IV
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Elements for a Public Summary
 VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Chronic lymphocytic leukaemia 1 Chronic lymphocytic leukemia (CLL) is a condition characterized by a progressive accumulation
VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Chronic lymphocytic leukaemia 1 Chronic lymphocytic leukemia (CLL) is a condition characterized by a progressive accumulation
BCCA Protocol Summary Guidelines for the Diagnosis and Management of Malignancy Related Hypercalcemia
 BCCA Protocol Summary Guidelines for the Diagnosis and Management of Malignancy Related Hypercalcemia Protocol Code Tumour Group Supportive Care Group Contacts SCHYPCAL Supportive Care Lisa Wanbon (VIC)
BCCA Protocol Summary Guidelines for the Diagnosis and Management of Malignancy Related Hypercalcemia Protocol Code Tumour Group Supportive Care Group Contacts SCHYPCAL Supportive Care Lisa Wanbon (VIC)
Doncaster & Bassetlaw. AKI guidelines for primary care
 Doncaster & Bassetlaw AKI guidelines for primary care Contents: FLOW DIAGRAM: MANAGEMENT OF PATIENTS WITH AKI DETECTED IN PRIMARY CARE... 2 FLOW DIAGRAM: MANAGEMENT OF HYPERKALAEMIA.... 3 FLOW DIAGRAM:
Doncaster & Bassetlaw AKI guidelines for primary care Contents: FLOW DIAGRAM: MANAGEMENT OF PATIENTS WITH AKI DETECTED IN PRIMARY CARE... 2 FLOW DIAGRAM: MANAGEMENT OF HYPERKALAEMIA.... 3 FLOW DIAGRAM:
RiTUXimab 375 mg/m 2 Therapy-7 day
 RiTUXimab 375 mg/m 2 Therapy-7 day This regimen supercedes NCCP Regimen 00208 rituximab 375mg/m2 therapy-follicular lymphoma as of February 2019 INDICATIONS FOR USE: Regimen *Reimbursement INDICATION ICD10
RiTUXimab 375 mg/m 2 Therapy-7 day This regimen supercedes NCCP Regimen 00208 rituximab 375mg/m2 therapy-follicular lymphoma as of February 2019 INDICATIONS FOR USE: Regimen *Reimbursement INDICATION ICD10
SPONTANEOUS TUMOR LYSIS SYNDROME: A case report and review of literature
 Case Report SPONTANEOUS TUMOR LYSIS SYNDROME: A case report and review of literature Ashraf Ahmed Hussein Hashem 1, Tarek Abdel Hameed Mostafa Dowod 2, Mohsen Mahmooud Abdelmajeed 3 ABSTRACT We report
Case Report SPONTANEOUS TUMOR LYSIS SYNDROME: A case report and review of literature Ashraf Ahmed Hussein Hashem 1, Tarek Abdel Hameed Mostafa Dowod 2, Mohsen Mahmooud Abdelmajeed 3 ABSTRACT We report
R-IDARAM. Dexamethasone is administered as an IV infusion in 100mL sodium chloride 0.9% over 30 minutes.
 R-IDARAM Indication Secondary CNS lymphoma ICD-10 codes Codes with a prefix C85 Regimen details Day Drug Dose Route 1 Rituximab 375mg/m 2 IV infusion 1 Methotrexate 12.5mg Intrathecal 1 Cytarabine 70mg
R-IDARAM Indication Secondary CNS lymphoma ICD-10 codes Codes with a prefix C85 Regimen details Day Drug Dose Route 1 Rituximab 375mg/m 2 IV infusion 1 Methotrexate 12.5mg Intrathecal 1 Cytarabine 70mg
Carboplatin + Paclitaxel Cancer of the Cervix
 Carboplatin + Paclitaxel Cancer of the Cervix Background: Topotecan in combination with cisplatin is recommended as a treatment option for women with recurrent or stage IVB cervical cancer only if they
Carboplatin + Paclitaxel Cancer of the Cervix Background: Topotecan in combination with cisplatin is recommended as a treatment option for women with recurrent or stage IVB cervical cancer only if they
Cisplatin and Gemcitabine (bladder)
 Cisplatin and Gemcitabine (bladder) Indication Palliative therapy for locally advanced or metastatic bladder cancer in patients with good renal function. Palliative therapy for urothelial transitional
Cisplatin and Gemcitabine (bladder) Indication Palliative therapy for locally advanced or metastatic bladder cancer in patients with good renal function. Palliative therapy for urothelial transitional
Frequency of Tumor Lysis Syndrome in Aggressive and Slow Introduction Chemotherapy in Children with ALL
 Original Article Frequency of Tumor Lysis Syndrome in Aggressive and Slow Introduction Chemotherapy in Children with ALL Downloaded from ijpho.ssu.ac.ir at 1:59 IRDT on Friday July 1th 2018 Hashemi A 1,
Original Article Frequency of Tumor Lysis Syndrome in Aggressive and Slow Introduction Chemotherapy in Children with ALL Downloaded from ijpho.ssu.ac.ir at 1:59 IRDT on Friday July 1th 2018 Hashemi A 1,
Tumour lysis syndrome: new therapeutic strategies and classification
 review Tumour lysis syndrome: new therapeutic strategies and classification Mitchell S. Cairo 1 and Michael Bishop 2 1 Department of Pediatrics, Children s Hospital of New York Presbyterian, Columbia University,
review Tumour lysis syndrome: new therapeutic strategies and classification Mitchell S. Cairo 1 and Michael Bishop 2 1 Department of Pediatrics, Children s Hospital of New York Presbyterian, Columbia University,
CD20-positive high-grade non-hodgkin Lymphoma in patients in which R-CHOP is not indicated
 INDICATION CD20-positive high-grade non-hodgkin Lymphoma in patients in which R-CHOP is not indicated TREATMENT INTENT Curative or Disease Modification. PRE-ASSESSMENT 1. Ensure histology is confirmed
INDICATION CD20-positive high-grade non-hodgkin Lymphoma in patients in which R-CHOP is not indicated TREATMENT INTENT Curative or Disease Modification. PRE-ASSESSMENT 1. Ensure histology is confirmed
Definition of high risk disease for diffuse large B-cell lymphoma is score 4 or 5 based on the following risk factors:
 INDICATION High grade lymphoma with high risk of CNS involvement Definition of high risk disease for diffuse large B-cell lymphoma is score 4 or 5 based on the following risk factors: Age > 60 Raised serum
INDICATION High grade lymphoma with high risk of CNS involvement Definition of high risk disease for diffuse large B-cell lymphoma is score 4 or 5 based on the following risk factors: Age > 60 Raised serum
RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University
 RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University OBJECTIVES By the end of this lecture each student should be able to: Define acute & chronic kidney disease(ckd)
RENAL FAILURE IN CHILDREN Dr. Mai Mohamed Elhassan Assistant Professor Jazan University OBJECTIVES By the end of this lecture each student should be able to: Define acute & chronic kidney disease(ckd)
CISPLATIN Chemo-radiation regimen Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol CISPLATIN Chemo-radiation regimen Gynaecological Cancer PROCTOCOL REF: MPHAGYNCIX (Version No: 1.0) Approved for use in: Locally advanced cervical cancer (adjuvant/curative)
Systemic Anti Cancer Treatment Protocol CISPLATIN Chemo-radiation regimen Gynaecological Cancer PROCTOCOL REF: MPHAGYNCIX (Version No: 1.0) Approved for use in: Locally advanced cervical cancer (adjuvant/curative)
guideline Recommendations
 guideline s for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology Gail L Jones, 1 Andrew Will,
guideline s for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology Gail L Jones, 1 Andrew Will,
ELECTROLYTES RENAL SHO TEACHING
 ELECTROLYTES RENAL SHO TEACHING Metabolic Alkalosis 2 factors are responsible for generation and maintenance of metabolic alkalosis this includes a process that raises serum bicarbonate and a process that
ELECTROLYTES RENAL SHO TEACHING Metabolic Alkalosis 2 factors are responsible for generation and maintenance of metabolic alkalosis this includes a process that raises serum bicarbonate and a process that
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION
 DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
DBL MAGNESIUM SULFATE CONCENTRATED INJECTION NAME OF MEDICINE Magnesium Sulfate BP DESCRIPTION DBL Magnesium Sulfate Concentrated Injection is a clear, colourless, sterile solution. Each ampoule contains
R-GDP: Rituximab, Gemcitabine, Dexamethasone &Cisplatin
 : Rituximab, Gemcitabine, Dexamethasone &Cisplatin INDICATION Relapsed or refractory Hodgkin and non-hodgkin lymphoma. Omit Rituximab for patients with Hodgkin Lymphoma or high grade T cell non-hodgkin
: Rituximab, Gemcitabine, Dexamethasone &Cisplatin INDICATION Relapsed or refractory Hodgkin and non-hodgkin lymphoma. Omit Rituximab for patients with Hodgkin Lymphoma or high grade T cell non-hodgkin
R-GemOx. Lymphoma group INDICATION. Relapsed or Refractory Lymphoma, for patients unsuitable for R-GDP regimen. Omit rituximab if CD20- negative
 R-GemOx INDICATION Relapsed or Refractory Lymphoma, for patients unsuitable for R-GDP regimen. Omit rituximab if CD20- negative TREATMENT INTENT Disease modification PRE-ASSESSMENT 1. Ensure histology
R-GemOx INDICATION Relapsed or Refractory Lymphoma, for patients unsuitable for R-GDP regimen. Omit rituximab if CD20- negative TREATMENT INTENT Disease modification PRE-ASSESSMENT 1. Ensure histology
NCCP Chemotherapy Regimen. Obinutuzumab and Chlorambucil Therapy
 INDICATIONS FOR USE: Obinutuzumab INDICATION ICD10 Regimen Code *Reimbursement Indicator Treatment of adult patients with previously untreated chronic lymphocytic leukaemia (CLL) and with comorbidities
INDICATIONS FOR USE: Obinutuzumab INDICATION ICD10 Regimen Code *Reimbursement Indicator Treatment of adult patients with previously untreated chronic lymphocytic leukaemia (CLL) and with comorbidities
PRODUCT INFORMATION RESONIUM A. Na m
 PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
Oxaliplatin and Gemcitabine
 Oxaliplatin and Gemcitabine Indication Palliative treatment for relapsed metastatic seminoma, non seminoma or combined tumours. ICD-10 codes Codes pre-fixed with C38, C48, C56, C62, C63, C75.3. Regimen
Oxaliplatin and Gemcitabine Indication Palliative treatment for relapsed metastatic seminoma, non seminoma or combined tumours. ICD-10 codes Codes pre-fixed with C38, C48, C56, C62, C63, C75.3. Regimen
Cisplatin and Pemetrexed (NSCLC, mesothelioma)
 Cisplatin and Pemetrexed (NSCLC, mesothelioma) Indication First-line treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) if the histology of the tumour has been confirmed as
Cisplatin and Pemetrexed (NSCLC, mesothelioma) Indication First-line treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) if the histology of the tumour has been confirmed as
Zerlinda (MRP DK/H/2265/001)
 Zerlinda (MRP DK/H/2265/001) VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prevention of bone complications, e.g. fractures, in adult patients with bone metastases (spread
Zerlinda (MRP DK/H/2265/001) VI.2 Elements for a Public Summary VI.2.1 Overview of disease epidemiology Prevention of bone complications, e.g. fractures, in adult patients with bone metastases (spread
Hypouricemic effect and safety of febuxostat used for prevention of tumor lysis syndrome
 Maie et al. SpringerPlus 2014, 3:501 a SpringerOpen Journal RESEARCH Hypouricemic effect and safety of febuxostat used for prevention of tumor lysis syndrome Open Access Koichiro Maie 1, Yasuhisa Yokoyama
Maie et al. SpringerPlus 2014, 3:501 a SpringerOpen Journal RESEARCH Hypouricemic effect and safety of febuxostat used for prevention of tumor lysis syndrome Open Access Koichiro Maie 1, Yasuhisa Yokoyama
FLAG-Ida + Gemtuzumab Ozogamicin Regimen (Also known as FLAG-Ida + GO3x2) (AML19 Trial Course 1)
 FLAG-Ida + Gemtuzumab Ozogamicin Regimen (Also known as FLAG-Ida + GO3x2) (AML19 Trial Course 1) AML19 Adults with Acute Myeloid Leukaemia or High-Risk Myelodysplastic Syndrome ***Refer to trial protocol
FLAG-Ida + Gemtuzumab Ozogamicin Regimen (Also known as FLAG-Ida + GO3x2) (AML19 Trial Course 1) AML19 Adults with Acute Myeloid Leukaemia or High-Risk Myelodysplastic Syndrome ***Refer to trial protocol
4/20/2015. Chemotherapy in the Infusion Clinic: Patient Electrolyte Imbalance Considerations. Learning Objectives. Ambulatory Oncology Infusion Center
 Chemotherapy in the Infusion Clinic: Patient Electrolyte Imbalance Considerations Nichole Korpi-Steiner, PhD, DABCC, FACB University of North Carolina Chapel Hill, NC Learning Objectives Explore the benefits
Chemotherapy in the Infusion Clinic: Patient Electrolyte Imbalance Considerations Nichole Korpi-Steiner, PhD, DABCC, FACB University of North Carolina Chapel Hill, NC Learning Objectives Explore the benefits
TCHP Docetaxel, Carboplatin, Trastuzumab, Pertuzumab Neoadjuvant Protocol
 Systemic Anti Cancer Treatment Protocol TCHP Docetaxel, Carboplatin, Trastuzumab, Pertuzumab Neoadjuvant Protocol PROTOCOL REF: MPHATCHP (Version No: 1.0) Approved for use in: Neoadjuvant breast: The neoadjuvant
Systemic Anti Cancer Treatment Protocol TCHP Docetaxel, Carboplatin, Trastuzumab, Pertuzumab Neoadjuvant Protocol PROTOCOL REF: MPHATCHP (Version No: 1.0) Approved for use in: Neoadjuvant breast: The neoadjuvant
WEEK. MPharm Programme. Acute Kidney Injury. Alan M. Green MPHM13: Acute Kidney Injury. Slide 1 of 47
 MPharm Programme Acute Kidney Injury Alan M. Green 2017 Slide 1 of 47 Overview Renal Function What is it? Why does it matter? What causes it? Who is at risk? What can we (Pharmacists) do? How do you recognise
MPharm Programme Acute Kidney Injury Alan M. Green 2017 Slide 1 of 47 Overview Renal Function What is it? Why does it matter? What causes it? Who is at risk? What can we (Pharmacists) do? How do you recognise
CLINICAL GUIDELINES ID TAG
 CLINICAL GUIDELINES ID TAG Title: Treatment of Hypomagnesaemia in adults Author: Speciality / Division: Directorate: Dr Peter Sharpe, Dr Neal Morgan, Jillian Redpath Chemical Pathology/Nephrology/Pharmacy
CLINICAL GUIDELINES ID TAG Title: Treatment of Hypomagnesaemia in adults Author: Speciality / Division: Directorate: Dr Peter Sharpe, Dr Neal Morgan, Jillian Redpath Chemical Pathology/Nephrology/Pharmacy
PACKAGE LEAFLET: INFORMATION FOR THE USER. SODIPHOS 22mEq / 10ml Concentrate for solution for infusion. Disodium phosphate dihydrate
 PACKAGE LEAFLET: INFORMATION FOR THE USER SODIPHOS 22mEq / 10ml Concentrate for solution for infusion Disodium phosphate dihydrate Read all of this leaflet carefully before you start using this medicine.
PACKAGE LEAFLET: INFORMATION FOR THE USER SODIPHOS 22mEq / 10ml Concentrate for solution for infusion Disodium phosphate dihydrate Read all of this leaflet carefully before you start using this medicine.
Single-Dose Rasburicase 6 mg in the Management of Tumor Lysis Syndrome in Adults
 Single-Dose Rasburicase 6 mg in the Management of Tumor Lysis Syndrome in Adults Anne M. McDonnell, Pharm.D., Kristi L. Lenz, Pharm.D., Debra A. Frei-Lahr, M.D., John Hayslip, M.D., and Philip D. Hall,
Single-Dose Rasburicase 6 mg in the Management of Tumor Lysis Syndrome in Adults Anne M. McDonnell, Pharm.D., Kristi L. Lenz, Pharm.D., Debra A. Frei-Lahr, M.D., John Hayslip, M.D., and Philip D. Hall,
NCCP Chemotherapy Protocol. Maintenance therapy for the treatment of follicular CD20 positive, B-cell NHL patients responding to induction therapy.
 RiTUXimab 375mg/m 2 Therapy-Follicular Lymphoma INDICATIONS FOR USE: INDICATION Maintenance therapy for the treatment of follicular CD20 positive, B-cell NHL patients responding to induction therapy. Monotherapy
RiTUXimab 375mg/m 2 Therapy-Follicular Lymphoma INDICATIONS FOR USE: INDICATION Maintenance therapy for the treatment of follicular CD20 positive, B-cell NHL patients responding to induction therapy. Monotherapy
A case of severe hyperkalaemia presenting with cardiac arrythmias: An uncommon initial manifestation of chronic kidney disease
 Case Report A case of severe hyperkalaemia presenting with cardiac arrythmias: An uncommon initial manifestation of chronic kidney disease D H Sudusinghe 1, J indrakumar 2 1 Department of Physiology, Faculty
Case Report A case of severe hyperkalaemia presenting with cardiac arrythmias: An uncommon initial manifestation of chronic kidney disease D H Sudusinghe 1, J indrakumar 2 1 Department of Physiology, Faculty
Policy for Central Nervous System [CNS] Prophylaxis in Lymphoid Malignancies
![Policy for Central Nervous System [CNS] Prophylaxis in Lymphoid Malignancies Policy for Central Nervous System [CNS] Prophylaxis in Lymphoid Malignancies](/thumbs/90/104309591.jpg) Policy for Central Nervous System [CNS] Prophylaxis in Lymphoid Malignancies UNCONTROLLED WHEN PRINTED Note: NOSCAN Haematology MCN has approved the information contained within this document to guide
Policy for Central Nervous System [CNS] Prophylaxis in Lymphoid Malignancies UNCONTROLLED WHEN PRINTED Note: NOSCAN Haematology MCN has approved the information contained within this document to guide
CHEMOTHERAPY PROTOCOL FOR ADMINISTRATION OF VENETOCLAX
 CHEMOTHERAPY PROTOCOL FOR ADMINISTRATION OF VENETOCLAX Therapeutic Indications: Venetoclax monotherapy is indicated for the treatment of chronic lymphocytic leukaemia (CLL) in the presence of 17p deletion
CHEMOTHERAPY PROTOCOL FOR ADMINISTRATION OF VENETOCLAX Therapeutic Indications: Venetoclax monotherapy is indicated for the treatment of chronic lymphocytic leukaemia (CLL) in the presence of 17p deletion
Lung Pathway Group Docetaxel & Carboplatin in Non- Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Docetaxel & Carboplatin in Non- Small Cell Lung Cancer (NSCLC) Indication: First line palliative therapy for previously untreated Stage IIIB or IV NSCLC patients Regimen details: Docetaxel
Lung Pathway Group Docetaxel & Carboplatin in Non- Small Cell Lung Cancer (NSCLC) Indication: First line palliative therapy for previously untreated Stage IIIB or IV NSCLC patients Regimen details: Docetaxel
Summary of the risk management plan (RMP) for Gazyvaro (obinutuzumab)
 EMA/319729/2014 Summary of the risk management plan (RMP) for Gazyvaro (obinutuzumab) This is a summary of the risk management plan (RMP) for Gazyvaro, which details the measures to be taken in order to
EMA/319729/2014 Summary of the risk management plan (RMP) for Gazyvaro (obinutuzumab) This is a summary of the risk management plan (RMP) for Gazyvaro, which details the measures to be taken in order to
Start. What is the serum phosphate concentration? Moderate Hypophosphataemia mmol/l. Replace using oral. phosphate. (See section 3.
 CLINICAL GUIDELINE FOR THE MANAGEMENT OF HYPOPHOSPHATAEMIA IN ADULTS Summary. Key: General Notes GP/SWASFT ED/MAU/SRU/Acute GP/Amb-Care In-patient wards Start What is the serum concentration? Mild Hypophosphataemia
CLINICAL GUIDELINE FOR THE MANAGEMENT OF HYPOPHOSPHATAEMIA IN ADULTS Summary. Key: General Notes GP/SWASFT ED/MAU/SRU/Acute GP/Amb-Care In-patient wards Start What is the serum concentration? Mild Hypophosphataemia
Tumor lysis syndrome (TLS) is a group of PROCEEDINGS CURRENT AND EMERGING TREATMENT OPTIONS FOR PATIENTS WITH TUMOR LYSIS SYNDROME * Sima Jeha, MD
 CURRENT AND EMERGING TREATMENT OPTIONS FOR PATIENTS WITH TUMOR LYSIS SYNDROME * Sima Jeha, MD ABSTRACT Tumor lysis syndrome (TLS) is a life-threatening complication of cancer chemotherapy in which hyperuricemia
CURRENT AND EMERGING TREATMENT OPTIONS FOR PATIENTS WITH TUMOR LYSIS SYNDROME * Sima Jeha, MD ABSTRACT Tumor lysis syndrome (TLS) is a life-threatening complication of cancer chemotherapy in which hyperuricemia
Krystexxa. Krystexxa (pegloticase) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.14 Subject: Krystexxa Page: 1 of 5 Last Review Date: March 16, 2018 Krystexxa Description Krystexxa
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.70.14 Subject: Krystexxa Page: 1 of 5 Last Review Date: March 16, 2018 Krystexxa Description Krystexxa
Cisplatin / Paclitaxel Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol Cisplatin / Paclitaxel Gynaecological Cancer PROCTOCOL REF: MPHAGYNCIP (Version No: 1.0) Approved for use in: First line treatment for stage Ib-IV with minimal residual
Systemic Anti Cancer Treatment Protocol Cisplatin / Paclitaxel Gynaecological Cancer PROCTOCOL REF: MPHAGYNCIP (Version No: 1.0) Approved for use in: First line treatment for stage Ib-IV with minimal residual
(R) CODOX M / (R) IVAC
 (R) CODOX M / (R) IVAC Indication Burkitt's or Burkitt's-like lymphoma, especially those with 1 or more of the following poor risk criteria: - Raised LDH level - WHO performance status 2-4 - Ann Arbor
(R) CODOX M / (R) IVAC Indication Burkitt's or Burkitt's-like lymphoma, especially those with 1 or more of the following poor risk criteria: - Raised LDH level - WHO performance status 2-4 - Ann Arbor
Carboplatin and Fluorouracil
 Carboplatin and Fluorouracil Indication Palliative chemotherapy for recurrent or metastatic head and neck squamous cell cancer for patients where cisplatin and / or cetuximab are not appropriate. Performance
Carboplatin and Fluorouracil Indication Palliative chemotherapy for recurrent or metastatic head and neck squamous cell cancer for patients where cisplatin and / or cetuximab are not appropriate. Performance
Guidelines for the administration of Rituximab
 the administration of 1. Introduction Administration of the anti-cd20 monoclonal antibody is associated with severe hypersensitivity reactions, potentially life threatening cytokine release syndrome, and
the administration of 1. Introduction Administration of the anti-cd20 monoclonal antibody is associated with severe hypersensitivity reactions, potentially life threatening cytokine release syndrome, and
CHRONIC KIDNEY DISEASE (CKD)
 CHRONIC KIDNEY DISEASE (CKD) CKD implies longstanding (more than 3 months), and usually progressive, impairment in renal function. In many instances, no effective means are available to reverse the primary
CHRONIC KIDNEY DISEASE (CKD) CKD implies longstanding (more than 3 months), and usually progressive, impairment in renal function. In many instances, no effective means are available to reverse the primary
ACUTE KIDNEY INJURY FOCUS ON OBSTETRICS DONNA HIGGINS, CLINICAL NURSE EDUCATOR, NORTHERN LINCOLNSHIRE HOSPITALS NHS FOUNDATION TRUST
 ACUTE KIDNEY INJURY FOCUS ON OBSTETRICS DONNA HIGGINS, CLINICAL NURSE EDUCATOR, NORTHERN LINCOLNSHIRE HOSPITALS NHS FOUNDATION TRUST AIMS & OBJECTIVES Review the functions of the kidney Identify renal
ACUTE KIDNEY INJURY FOCUS ON OBSTETRICS DONNA HIGGINS, CLINICAL NURSE EDUCATOR, NORTHERN LINCOLNSHIRE HOSPITALS NHS FOUNDATION TRUST AIMS & OBJECTIVES Review the functions of the kidney Identify renal
