Low Prevalence of High-dose Methotrexate Nephropathy in Patients With Malignancy
|
|
|
- Kristin Robertson
- 5 years ago
- Views:
Transcription
1 Kidney Diseases Low Prevalence of High-dose Methotrexate Nephropathy in Patients With Malignancy Mohammad Ali Mashhadi, 1 Mahmoud Ali Kaykhaei, 2 Houshang Sanadgol 3 1 Division of Oncology and Hematology, Department of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran 2 Division of Endocrinology, Department of Medicine, Zahedan University of Medical 3 Division of Nephrology, Department of Medicine, Zahedan University of Medical Keywords. methotrexate, drug toxicity, acute kidney injury Introduction. Methotrexate is an antifolate medication frequently used in the treatment of malignant and nonmalignant diseases. The usage of high-dose methotrexate was limited to patients with osteosarcoma, Ewing sarcoma, lymphoma, and acute lymphoblastic leukemia. One of the major side effects of high-dose methotrexate is nephropathy. The aim of present study was to determine the renal side effects of high-dose methotrexate in patients with malignancies. Materials and Methods. In a study of 102 patients with osteosarcoma (n = 72), Ewing sarcoma (n = 15), and lymphoma (n = 15), treated with high-dose methotrexate, clinical and laboratory data including kidney function tests were recorded at baseline and during followup visits. The mean duration of follow-up was 6 months. Results. The mean age of the patients was 19.5 years (range, 5 to 80 years). The total courses of methotrexate therapy were 273 (median, 2.67 per patient). The mean creatinine level was 0.82 mg/dl. Of the 102 patients, 3 (2.9%) developed acute kidney injury with an at risk phase. Another patient (1.0%) developed acute kidney injury and its phase was injury according to the RIFLE criteria. None of the cases were failed and acute kidney injury was alleviated in all of the affected patients. Conclusions. Our data revealed a low prevalence of acute kidney injury with high-dose methotrexate therapy. In addition, these toxicities were limited to the first and second phases of the RIFLE classification, all of which resolved spontaneously. Original Paper IJKD 2012;6: INTRODUCTION Methotrexate is an antifolate medication used in the treatment of malignant and nonmalignant diseases. 1-3 Administration of methotrexate at a dose higher than 1 g/m 2 is generally referred to as high-dose methotrexate therapy. 4 One of the most important toxicities due to high-dose methotrexate is kidney failure. 5,6 Causes of kidney function impairment due to high-dose methotrexate have not been elucidated although precipitation of methotrexate or its metabolites in renal tubules or direct tubular effects may be the culprit. 7 Other factors such as abnormality in preglomerular resistance and direct damage to glomeruli are also considered. 6,8-10 Good hydration and alkalinization significantly reduce methotrexate nephrotoxicity. 11 Acute kidney failure, which is the most common high-dose methotrexate renal toxicity, seen in 2% to 10% of patients receiving methotrexate, 12,13 is usually reversible with complete recovery, and treatment could be continued without dose reduction. 14 A number of past studies have reported prevalence of methotrexate nephrotoxicity, but there is substantial 105
2 controversy. The aim of the present study was to determine the prevalence of high-dose methotrexate nephrotoxicity in patients with various malignancies in a medical center in Zahedan, Iran. MATERIALS AND METHODS In a prospective longitudinal study from 2007 to 2009, a total of 102 patients with osteosarcoma, Ewing sarcoma, and lymphoma were followed up during their treatment with high-dose methotrexate. The study was approved by regional ethical committee. The inclusion criteria were a neutrophil count greater than /L, a platelet count greater than /L, normal kidney function (evaluated by urinalysis and serum electrolytes, creatinine, and blood urea nitrogen), and normal liver and heart function. Patients with alcohol abuse, pregnancy, hepatitis, and heart disease, and those at risk of kidney dysfunction (such as infection and aminoglycoside therapy) were excluded. Complete blood count, blood urea nitrogen; serum creatinine, calcium, sodium, potassium, and uric acid; and urinalysis were measured before the start of treatment. The patients were assessed for toxicity before and 1 week after each cycle of treatment. Methotrexate therapy was administered every 21 days for osteogenic and Ewing sarcoma and every 2 weeks for lymphoma. Other drugs administered were vincristine and doxorubicin, which do not have any major renal toxicity. After establishing an appropriate intravenous line, maintenance fluid therapy and granisetron, 3 mg, were initiated 30 minutes before initiation of methotrexate therapy. The dose of methotrexate was 8 g/m 2 to 10 g/m 2 per cycle (for adult and pediatric patients, respectively), which was infused in 500 ml of isotonic saline within 4 hours. Leucovorin rescue was administered after methotrexate therapy (30 mg per 6 hours for at least 20 doses). Pre highdose methotrexate therapies were maintenance intravenous fluid therapy in 1000 ml of isotonic saline within 2 hours plus 1 vial of bicarbonate. All of the patients had a urine ph equal or higher than 7 and then started therapy. After 1 week and then before each cycle of methotrexate therapy, baseline electrolytes measurement and urnialysis were repeated. In children, glomerular filtration rate (GFR) was estimated as 0.41 height/ serum creatinine, and in adults, it was calculated according to the Cockroft-Gault formula. In case of any abnormality in urine output or a rise in serum creatinine level, other specific and sensitive methods were used for measurement of kidney injury, and the risk, injury, and failure (RIFLE) criteria were applied to determine the grade and phase of kidney injury. 15,16 RESULTS A total of 102 eligible patients were enrolled, of whom 60 (58.8%) were males and 42 (41.2%) were females. The mean age of the patients was 19.5 years old (range, 5 to 80 years). Twenty-three patients were in children aged younger than 15 years (12 males and 11 females), 71 were 15 to 30 years old (43 males and 28 females), and 8 were older than 30 years old (5 males and 3 females). Seventy-two patients had osteosarcoma (38 males and 34 females), 15 had Ewing sarcoma (11 males and 4 females), and 15 had lymphoma (11 males and 4 females). Table 1 summarizes demographic and clinical baseline data of the patients. The total courses of methotrexate therapy were 273 (mean, 2.67 courses per patient). Patients with osteosarcoma, Ewing sarcoma, and lymphoma received 204, 34, and 35 courses, respectively. All of the patients received at least 2 courses of high-dose methotrexate. Prior to treatment, all of the patients had normal kidney function tests, including blood urea nitrogen and serum creatinine level and urinalysis. After the first course, only 1 patient developed elevated creatinine, and in the second course, 2 other patients experienced a rise in creatinine level (Table 2). All of the Table 1. Baseline Characteristics of Patients* Characteristic Value Number of patients 102 Mean age, y 19.5 (5 to 80) Gender Male 60 (58.8) Female 42 (41.2) Primary disease Osteogenic sarcoma 72 (70.6) Ewing sarcoma 15 (14.7) Lymphoma 15 (14.7) Methotrexate Total cycles 273 Mean cycles per patient 2.67 Baseline creatinine, mg/dl 0.82 Mean body mass index, kg/m (10 to 32) *Values in parentheses are ranges for reports of mean values and percentages for categorical data. 106
3 Table 2. Changes in Serum Creatinine Level Number of Patients Serum Creatinine, mg/dl Cycle Total Acute Kidney Injury Injury Phase Baseline Follow-up First Second and and 1.5 Third Fourth 15 0 Fifth 15 0 patients, according to the RIFLE criteria, were at-risk groups. These patients had a normal urine output, but they had a rising in creatinine level and the calculated GFR showed a risk phase of kidney injury. Forty-eight patients received a third course of methotrexate, and among whom 1 patient developed abnormal creatinine level. The nephrotoxicity, according to the RIFLE criteria, was within the injury group. This patient had a normal urine output. Fifteen patients received a fourth course and 6 received a fifth course of methotrexate therapy, and none of them developed a rising of creatinine compared to the pretreatment levels. At the end of the study, 4.9% of all of the patients had developed a rising in creatinine level (2.94% with phase 1 kidney injury and 1.0% with phase 2 kidney injury). Failure, loss, or end-stage renal disease phases of acute kidney injury were not detected (Table 2). All of the patients with abnormal creatinine levels were in the adult group. All toxicities resolved spontaneously during the follow-up. No electrolyte imbalance, proteinuria, and glucosuria were detected in this cohort of patients. DISCUSSION Classically, high-dose methotrexate has been defined as administration of methotrexate more than 1000 mg/m 2, although the use of methotrexate more than 500 mg/m 2 may also be considered as high-dose methotrexate. 4,17 Results of the present study showed a low prevalence of renal toxicity due to high-dose methotrexate, and all toxicities were limited to phase 1 and 2 kidney injury. Different studies reported various frequencies of methotrexate nephropathy. In one study, nephrotoxicity was reported in 91% of 23 patients (defined as any elevation of serum creatinine from grade 1 to 4), 14 but another study in sarcoma patients showed nephrotoxicity in only 1.8%. 7 The former study by Green and coworkers evaluated 23 patients (12 males and 11 females) with a median age of 72 years old with primary central nervous system lymphoma. Rising of creatinine level more than 15% occurred in 91% of patients in the first cycle of treatment. 14 Fifteen patients experienced grade 2, 3 had grade 3, and 3 had grade 4 nephrotoxicity. Total courses of highdose methotrexate administration were 161 cycles, of which 122 cycles (76%) were associated with rising in creatinine level when compared to the baseline. All male patients experienced rising in creatinine level in at least 1 course of the treatment. In 4-month follow-up, 18 patients had permanent kidney injury and creatinine level was the same as that at the end of high-dose methotrexate therapy. 14 In contrast, Hempel and coworkers did not find any significant kidney dysfunction. In this study, transient decrease in GFR occurred, but all spontaneously recovered without medication. 18 Gronroos and coworkers evaluated kidney function in a long-term follow-up of 30 patients who received high-dose methotrexate. 21 Thirty-nine percent of the patients had normal GFR at the end of the treatment, 50% had moderate decrease in GFR (90 ml/min/1.73 m 2 to 114 ml/min/1.73 m 2 ), and 11% had a more significant decrease in GFR (< 90 ml/min/1.73 m 2 ). Twenty-nine percent of the patients developed significant albuminuria. Serum electrolytes such as potassium and sodium were in normal range without any abnormality and glycosuria was not detected. In another study, de Miguel and coworkers 20 enrolled 31 patients with a total of 158 courses of high-dose methotrexate. The dose of methotrexate was 2 g/m 2 to 3 g/m 2 per cycle and 6.4% of the patients developed acute kidney failure with serious complications which needed specific management. Our previous study showed no kidney dysfunction in patients with choriocarcinoma and acute lymphoblastic leukemia who received 500 mg/m 2 to 1500 mg/m 2 of methotrexate per 107
4 cycle, and toxicities were limited to nonrenal side effects. 17 In this study on 98 patients with acute lymphoblastic leukemia and choriocarcinoma, the maximum dose of methotrexate was 1500 mg/ m 2 in leukemic patients for a single course and 500 mg/m 2 in choriocarcinoma patients (dosage of methotrexate was much lower than that in the present study), and toxicities were other than kidney injury and the major toxicity was limited to liver damage. 17 In contrast to studies conducted by Green and colleagues 14 and Gronroos and coworkers, 19 in this large-scale study we found a low prevalence of methotrexate nephrotoxicity (about 4%) which may be due to variation in susceptibility to drug side effects in different populations. Gronroos and coworkers study 19 showed not only a high prevalence of methotrexate nephrotoxicity but also higher-grade kidney dysfunction plus proteinuria. However, Widemann and associates 7 also reported a low prevalence of methotrexate nephrotoxicity, but with higher grades than our study. Although our study showed a lower incidence of kidney dysfunction and reversible abnormality, we observed different results with some other drugs. 21,22 This finding revealed the lower susceptibility of our patients to drug toxicities and adverse effect. Another interesting finding in our study was the kidney injury independent of dose and cycle, as all of the patients in every cycle had complete renal recovery prior to next cycle, and in the following cycle, they did not have any evidence of kidney injury. Finally, the injuries were not linked with gender. The limitations of our study were the wide range of age, different disease settings, and different courses of treatment protocols. Another possible cause of bias is that we did not have many candidates for high-dose methotrexate therapy during the period of this study. CONCLUSIONS Our study revealed not only a low prevalence of renal toxicity due to very high-dose methotrexate therapy, but also toxicities which were only limited to the early phase of acute kidney injury. We observed toxicity episodes in the initial courses of treatment, usually occurred in the first or second course of methotrexate therapy, which were nondose dependent. CONFLICT OF INTEREST None declared. REFERENCES 1. Jolivet J, Cowan KH, Curt GA, Clendeninn NJ, Chabner BA. The pharmacology and clinical use of methotrexate. N Engl J Med. 1983;309: Oguz A, Hasan Oglu A, Azgu FS, Timlioglu O, Biberoglu G, Uluoglu C. Methotrexate related hepatotoxicity. Gazi Med J. 2002;13: Hersh EM, Wong VG, Henderson ES, Freireich EJ. Hepatotoxic effect of methotrexate. Cancer. 1966;4: Treon SP, Chabner BA. Concepts in use of high-dose methotrexate therapy. Clin Chem. 1996;42: Lawrenz-Wolf B, Wolfrom C, Frickel C, Fengler R, Wehinger H, Henze G. [Severe renal impairment of methotrexate elimination after high dose therapy]. Klin Padiatr. 1994;206: Maiche AG, Lappalainen K, Teerenhovi L. Renal insufficiency in patients treated with high dose methotrexate. Acta Oncol. 1988;27: Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11: Fuskevag OM, Kristiansen C, Olsen R, Aarbakke J, Lindal S. Microvascular perturbations in rats receiving the maximum tolerated dose of methotrexate or its major metabolite 7-hydroxymethotrexate. Ultrastruct Pathol. 2000;24: Abelson HT, Fosburg MT, Beardsley GP, et al. Methotrexate-induced renal impairment: clinical studies and rescue from systemic toxicity with high-dose leucovorin and thymidine. J Clin Oncol. 1983;1: Kovacs GT, Paal C, Somlo P, Koos R, Schuler D, Borsi JD. Proteinuria due to suboptimal hydration with high-dose methotrexate therapy. Cancer Chemother Pharmacol. 1993;33: Relling MV, Fairclough D, Ayers D, et al. Patient characteristics associated with high-risk methotrexate concentrations and toxicity. J Clin Oncol. 1994;12: Widemann BC, Adamson PC. Understanding and managing methotrexate nephrotoxicity. Oncologist. 2006;11: Chan H, Evans WE, Pratt CB. Recovery from toxicity associated with high-dose methotrexate: prognostic factors. Cancer Treat Rep. 1977;61: Green MR, Chamberlain MC. Renal dysfunction during and after high-dose methotrexate. Cancer Chemother Pharmacol. 2009;63: Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008;73: [No author listed]. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S Mashhadi MA. The effect of high dose MTX in patients 108
5 with neoplastic disease: a prospective study. Iran Red Crescent Med J. 2008;10: Hempel L, Misselwitz J, Fleck C, et al. Influence of highdose methotrexate therapy (HD-MTX) on glomerular and tubular kidney function. Med Pediatr Oncol. 2003;40: Gronroos MH, Jahnukainen T, Mottonen M, Perkkio M, Irjala K, Salmi TT. Long-term follow-up of renal function after high-dose methotrexate treatment in children. Pediatr Blood Cancer. 2008;51: de Miguel D, Garcia-Suarez J, Martin Y, Gil-Fernandez JJ, Burgaleta C. Severe acute renal failure following highdose methotrexate therapy in adults with haematological malignancies: a significant number result from unrecognized co-administration of several drugs. Nephrol Dial Transplant. 2008;23: Mashhadi MA. Renal side effects of ifosfamide in patients admitted for chemotherapy. J Res Med Sci. 2008;13: Mashhadi MA, Sanadgol H, Keikhaei M. Ifosfamide nephropathy in patients with sarcoma. Iran J Kidney Dis. 2011;5: Correspondence to: Mohammad Ali Mashhadi, MD Department of Medicine, Zahedan University of Medical Fax: dralimashhadi@yahoo.com Received October 2011 Revised December 2011 Accepted January
Doxorubicin and Ifosfamide Sarcoma
 Systemic Anti Cancer Treatment Protocol Doxorubicin and Ifosfamide Sarcoma PROTOCOL REF: MPHADOXIFO (Version No:.0) Approved for use in: Soft tissue sarcoma Dosage: Drug Dosage Route Frequency Doxorubicin
Systemic Anti Cancer Treatment Protocol Doxorubicin and Ifosfamide Sarcoma PROTOCOL REF: MPHADOXIFO (Version No:.0) Approved for use in: Soft tissue sarcoma Dosage: Drug Dosage Route Frequency Doxorubicin
1 Acute Lymphoblastic Leukaemia
 1 Acute Lymphoblastic Leukaemia 1.05 Intensification - Philadelphia Negative Patients Indication ALL Philadelphia negative patients Pre-treatment Evaluation The intensification module begins two weeks
1 Acute Lymphoblastic Leukaemia 1.05 Intensification - Philadelphia Negative Patients Indication ALL Philadelphia negative patients Pre-treatment Evaluation The intensification module begins two weeks
Vincristine Ifosfamide Doxorubicin Etoposide (VIDE) Sarcoma
 Systemic Anti Cancer Treatment Protocol Vincristine Ifosfamide Doxorubicin Etoposide (VIDE) Sarcoma PROTOCOL REF: MPHAVIDE (Version No: 1.0) Approved for use in: Ewings sarcoma Desmoplastic small round
Systemic Anti Cancer Treatment Protocol Vincristine Ifosfamide Doxorubicin Etoposide (VIDE) Sarcoma PROTOCOL REF: MPHAVIDE (Version No: 1.0) Approved for use in: Ewings sarcoma Desmoplastic small round
Editorial Review. Dunia de Miguel, Julio García-Suárez, Yolanda Martín, Juan José Gil-Fernández and Carmen Burgaleta. Methods.
 Nephrol Dial Transplant (2008) 23: 3762 3766 doi: 10.1093/ndt/gfn503 Advance Access publication 8 September 2008 Editorial Review Severe acute renal failure following high-dose methotrexate therapy in
Nephrol Dial Transplant (2008) 23: 3762 3766 doi: 10.1093/ndt/gfn503 Advance Access publication 8 September 2008 Editorial Review Severe acute renal failure following high-dose methotrexate therapy in
StRs and CT doctors in haematology. September Folinic acid dose modified.
 High dose Methotrexate and folinic acid rescue Full Title of Guideline: Author (include email and role): Division & Speciality: Clinical Guideline Review Date September 2018 GUIDELINE FOR THE USE OF HIGH
High dose Methotrexate and folinic acid rescue Full Title of Guideline: Author (include email and role): Division & Speciality: Clinical Guideline Review Date September 2018 GUIDELINE FOR THE USE OF HIGH
Leucovorin Calcium Tablets USP, 5 mg, 10 mg, 15 mg, and 25 mg
 Leucovorin Calcium Tablets USP, 5 mg, 10 mg, 15 mg, and 25 mg Rx only DESCRIPTION Leucovorin Calcium Tablets USP contain either 5 mg, 10 mg, 15 mg or 25 mg leucovorin as the calcium salt of N-[4-[[(2-amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl]
Leucovorin Calcium Tablets USP, 5 mg, 10 mg, 15 mg, and 25 mg Rx only DESCRIPTION Leucovorin Calcium Tablets USP contain either 5 mg, 10 mg, 15 mg or 25 mg leucovorin as the calcium salt of N-[4-[[(2-amino-5-formyl-1,4,5,6,7,8-hexahydro-4-oxo-6-pteridinyl)methyl]
Renal Function and Associated Laboratory Tests
 Renal Function and Associated Laboratory Tests Contents Glomerular Filtration Rate (GFR)... 2 Cockroft-Gault Calculation of Creatinine Clearance... 3 Blood Urea Nitrogen (BUN) to Serum Creatinine (SCr)
Renal Function and Associated Laboratory Tests Contents Glomerular Filtration Rate (GFR)... 2 Cockroft-Gault Calculation of Creatinine Clearance... 3 Blood Urea Nitrogen (BUN) to Serum Creatinine (SCr)
KHAPZORY (levoleucovorin) for injection, for intravenous use Initial U.S. Approval: 1952 (d,l-leucovorin)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use KHAPZORY safely and effectively. See full prescribing information for KHAPZORY. KHAPZORY (levoleucovorin)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use KHAPZORY safely and effectively. See full prescribing information for KHAPZORY. KHAPZORY (levoleucovorin)
Monitoring and Treatment of Acute Kidney Injury in Children with Acute Lymphoblastic Leukemia After High Dose Methotrexate Chemotherapy
 Iranian Journal of Pharmaceutical Research (2016), 15 (4): 957-961 Received: Feb 2015 Accepted: Jun 2016 Copyright 2016 by School of Pharmacy Shaheed Beheshti University of Medical Sciences and Health
Iranian Journal of Pharmaceutical Research (2016), 15 (4): 957-961 Received: Feb 2015 Accepted: Jun 2016 Copyright 2016 by School of Pharmacy Shaheed Beheshti University of Medical Sciences and Health
Lung Pathway Group Cisplatin & PO Vinorelbine in Non- Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Cisplatin & PO Vinorelbine in Non- Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in advanced/metastatic
Lung Pathway Group Cisplatin & PO Vinorelbine in Non- Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in advanced/metastatic
Burkitt s Lymphoma or DLBCL with adverse features PATIENTS WITH GOOD PERFORMANCE STATUS
 Regimen R-CODOX M Indication Burkitt s Lymphoma or DLBCL with adverse features Therapeutic Intent Radical/Curative PATIENTS WITH GOOD PERFORMANCE STATUS Day Medication Dose Route Administration Details
Regimen R-CODOX M Indication Burkitt s Lymphoma or DLBCL with adverse features Therapeutic Intent Radical/Curative PATIENTS WITH GOOD PERFORMANCE STATUS Day Medication Dose Route Administration Details
DERBY-BURTON LOCAL CANCER NETWORK FILENAME R-CODOX-M.DOC CONTROLLED DOC NO: HCCPG B115 CSIS Regimen Name: R-CODOXM. Rituximab + CODOX-M
 Rituximab + CODOX-M Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Burkitt
Rituximab + CODOX-M Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Burkitt
High-dose methotrexate toxicity in elderly patients with primary central nervous system lymphoma
 Original article Annals of Oncology 16: 445 449, 2005 doi:10.1093/annonc/mdi075 Published online 14 January 2005 High-dose methotrexate toxicity in elderly patients with primary central nervous system
Original article Annals of Oncology 16: 445 449, 2005 doi:10.1093/annonc/mdi075 Published online 14 January 2005 High-dose methotrexate toxicity in elderly patients with primary central nervous system
Evaluation of the Cockroft Gault, Jelliffe and Wright formulae in estimating renal function in elderly cancer patients
 Original article Annals of Oncology 15: 291 295, 2004 DOI: 10.1093/annonc/mdh079 Evaluation of the Cockroft Gault, Jelliffe and Wright formulae in estimating renal function in elderly cancer patients G.
Original article Annals of Oncology 15: 291 295, 2004 DOI: 10.1093/annonc/mdh079 Evaluation of the Cockroft Gault, Jelliffe and Wright formulae in estimating renal function in elderly cancer patients G.
HUGE EQUATION ACCURACY FOR SCREENING CHRONIC KIDNEY DISEASE: A PROSPECTIVE STUDY
 Journal of Aging Research & Clinical Practice Volume 6, 2017 J Aging Res Clin Practice 2017;inpress Published online in press HUGE EQUATION ACCURACY FOR SCREENING CHRONIC KIDNEY DISEASE: A PROSPECTIVE
Journal of Aging Research & Clinical Practice Volume 6, 2017 J Aging Res Clin Practice 2017;inpress Published online in press HUGE EQUATION ACCURACY FOR SCREENING CHRONIC KIDNEY DISEASE: A PROSPECTIVE
Definition of high risk disease for diffuse large B-cell lymphoma is score 4 or 5 based on the following risk factors:
 INDICATION High grade lymphoma with high risk of CNS involvement Definition of high risk disease for diffuse large B-cell lymphoma is score 4 or 5 based on the following risk factors: Age > 60 Raised serum
INDICATION High grade lymphoma with high risk of CNS involvement Definition of high risk disease for diffuse large B-cell lymphoma is score 4 or 5 based on the following risk factors: Age > 60 Raised serum
Objectives. Pre-dialysis CKD: The Problem. Pre-dialysis CKD: The Problem. Objectives
 The Role of the Primary Physician and the Nephrologist in the Management of Chronic Kidney Disease () By Brian Young, M.D. Assistant Clinical Professor of Medicine David Geffen School of Medicine at UCLA
The Role of the Primary Physician and the Nephrologist in the Management of Chronic Kidney Disease () By Brian Young, M.D. Assistant Clinical Professor of Medicine David Geffen School of Medicine at UCLA
Lung Pathway Group Cisplatin & IV Vinorelbine in Non- Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Cisplatin & IV Vinorelbine in Non- Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in advanced/metastatic
Lung Pathway Group Cisplatin & IV Vinorelbine in Non- Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in advanced/metastatic
Cisplatin Vinorelbine (Oral) therapy +/- radiotherapy
 1 REGIMEN TITLE: Cisplatin Vinorelbine (Oral) therapy +/- radiotherapy Page 1 of 5 Indication: First line in Radical/ Induction, Adjuvant and Advanced & Palliative treatment of Non-small cell lung cancer
1 REGIMEN TITLE: Cisplatin Vinorelbine (Oral) therapy +/- radiotherapy Page 1 of 5 Indication: First line in Radical/ Induction, Adjuvant and Advanced & Palliative treatment of Non-small cell lung cancer
Comparison of Serum Cystatin C and Creatinine Levels to Evaluate Early Renal Function after Kidney Transplantation
 IJMS Vol 34, No 2, June 2009 Original Article Comparison of Serum Cystatin C and Creatinine Levels to Evaluate Early Renal Function after Kidney Transplantation Reza Hekmat, Hamid Eshraghi Abstract Background:
IJMS Vol 34, No 2, June 2009 Original Article Comparison of Serum Cystatin C and Creatinine Levels to Evaluate Early Renal Function after Kidney Transplantation Reza Hekmat, Hamid Eshraghi Abstract Background:
Effects of infusion duration of high-dose methotrexate on cerebrospinal fluid drug levels in lymphoma patients
 [Chinese Journal of Cancer 27:10, 363-367; October 2008]; 2008 Sun Yat-Sen University Cancer Center Clinical Research Paper Effects of infusion duration of high-dose methotrexate on cerebrospinal fluid
[Chinese Journal of Cancer 27:10, 363-367; October 2008]; 2008 Sun Yat-Sen University Cancer Center Clinical Research Paper Effects of infusion duration of high-dose methotrexate on cerebrospinal fluid
Carboplatin / Gemcitabine Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol Carboplatin / Gemcitabine Gynaecological Cancer PROCTOCOL REF: MPHAGYNCAG (Version No: 1.0) Approved for use in: Recurrent/metastatic endometrial carcinoma Previously
Systemic Anti Cancer Treatment Protocol Carboplatin / Gemcitabine Gynaecological Cancer PROCTOCOL REF: MPHAGYNCAG (Version No: 1.0) Approved for use in: Recurrent/metastatic endometrial carcinoma Previously
Cisplatin and Gemcitabine (bladder)
 Cisplatin and Gemcitabine (bladder) Indication Palliative therapy for locally advanced or metastatic bladder cancer in patients with good renal function. Palliative therapy for urothelial transitional
Cisplatin and Gemcitabine (bladder) Indication Palliative therapy for locally advanced or metastatic bladder cancer in patients with good renal function. Palliative therapy for urothelial transitional
SAALT3W Sarcoma Dr. Meg Knowling. Protocol Code Tumour Group Contact Physician
 BCCA Protocol Summary for Etoposide, Ifosfamide-Mesna (SAIME) Alternating with vincristine, DOXOrubicin and Cyclophosphamide (with or without Mesna)(SAVAC or SAVACM) with Filgrastim Support at a THREE
BCCA Protocol Summary for Etoposide, Ifosfamide-Mesna (SAIME) Alternating with vincristine, DOXOrubicin and Cyclophosphamide (with or without Mesna)(SAVAC or SAVACM) with Filgrastim Support at a THREE
Cisplatin and Gemcitabine Bladder Cancer: Full and split dose
 Systemic Anti Cancer Treatment Protocol Cisplatin and Gemcitabine Bladder Cancer: Full and split dose PROCTOCOL REF: MPHAUROCIG (Version No: 1.0) Approved for use in: Neoadjuvant and palliative indications
Systemic Anti Cancer Treatment Protocol Cisplatin and Gemcitabine Bladder Cancer: Full and split dose PROCTOCOL REF: MPHAUROCIG (Version No: 1.0) Approved for use in: Neoadjuvant and palliative indications
Cisplatin Doxorubicin Sarcoma
 Systemic Anti Cancer Treatment Protocol Cisplatin Doxorubicin Sarcoma PROCEDURE REF: MPHACISDOX (Version No. _1.0) Approved for use in: Osteosarcoma Palliative / advanced disease Not suitable for PAM schedule
Systemic Anti Cancer Treatment Protocol Cisplatin Doxorubicin Sarcoma PROCEDURE REF: MPHACISDOX (Version No. _1.0) Approved for use in: Osteosarcoma Palliative / advanced disease Not suitable for PAM schedule
Gemcitabine + Cisplatin Regimen
 Gemcitabine + Cisplatin Regimen Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication
Gemcitabine + Cisplatin Regimen Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication
Cisplatin100 plus Radiotherapy for locally Advanced Squamous Cell Carcinoma Head and Neck
 Cisplatin100 plus Radiotherapy for locally Advanced Squamous Cell Carcinoma Head and Neck Indication: 1) Concomitant chemo-radiotherapy for locally advanced squamous cell carcinoma head and neck 2) Post-operative
Cisplatin100 plus Radiotherapy for locally Advanced Squamous Cell Carcinoma Head and Neck Indication: 1) Concomitant chemo-radiotherapy for locally advanced squamous cell carcinoma head and neck 2) Post-operative
TIP Paclitaxel, Ifosfamide and Cisplatin
 Systemic Anti Cancer Treatment Protocol TIP Paclitaxel, Ifosfamide and Cisplatin PROTOCOL REF: MPHATIPGC (Version No: 1.0) Approved for use in: Second line treatment of germ cell tumours Dosage: Drug Dosage
Systemic Anti Cancer Treatment Protocol TIP Paclitaxel, Ifosfamide and Cisplatin PROTOCOL REF: MPHATIPGC (Version No: 1.0) Approved for use in: Second line treatment of germ cell tumours Dosage: Drug Dosage
High Dose Cytarabine plus high dose Methotrexate for CNS Lymphoma
 High Dose Cytarabine plus high dose Methotrexate for CNS Lymphoma Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton
High Dose Cytarabine plus high dose Methotrexate for CNS Lymphoma Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton
The Effect of Residual Renal Function at the Initiation of Dialysis on Patient Survival
 ORIGINAL ARTICLE DOI: 10.3904/kjim.2009.24.1.55 The Effect of Residual Renal Function at the Initiation of Dialysis on Patient Survival Seoung Gu Kim 1 and Nam Ho Kim 2 Department of Internal Medicine,
ORIGINAL ARTICLE DOI: 10.3904/kjim.2009.24.1.55 The Effect of Residual Renal Function at the Initiation of Dialysis on Patient Survival Seoung Gu Kim 1 and Nam Ho Kim 2 Department of Internal Medicine,
Tumor Lysis Syndrome Nephrology Grand Rounds Tuesday, July 27 th, 2010 Aditya Mattoo
 Tumor Lysis Syndrome Nephrology Grand Rounds Tuesday, July 27 th, 2010 Aditya Mattoo Outline Background/Definition Epidemiology/Risk Stratification Pathophysiology Treatment Renal Replacement Therapy Background/Definition
Tumor Lysis Syndrome Nephrology Grand Rounds Tuesday, July 27 th, 2010 Aditya Mattoo Outline Background/Definition Epidemiology/Risk Stratification Pathophysiology Treatment Renal Replacement Therapy Background/Definition
BC Cancer Protocol Summary for Therapy of Acute Myeloid Leukemia Using azacitidine and SORAfenib
 BC Cancer Protocol Summary for Therapy of Acute Myeloid Leukemia Using azacitidine and SORAfenib Protocol Code Tumour Group Contact Physician ULKAMLAS Leukemia/BMT Dr. Donna Hogge ELIGIBILITY: Acute myeloid
BC Cancer Protocol Summary for Therapy of Acute Myeloid Leukemia Using azacitidine and SORAfenib Protocol Code Tumour Group Contact Physician ULKAMLAS Leukemia/BMT Dr. Donna Hogge ELIGIBILITY: Acute myeloid
Introduction to Clinical Diagnosis Nephrology
 Introduction to Clinical Diagnosis Nephrology I. David Weiner, M.D. C. Craig and Audrae Tisher Chair in Nephrology Professor of Medicine and Physiology and Functional Genomics University of Florida College
Introduction to Clinical Diagnosis Nephrology I. David Weiner, M.D. C. Craig and Audrae Tisher Chair in Nephrology Professor of Medicine and Physiology and Functional Genomics University of Florida College
Special Challenges and Co-Morbidities
 Special Challenges and Co-Morbidities Renal Disease/ Hypertension/ Diabetes in African-Americans M. Keith Rawlings, MD Medical Director Peabody Health Center AIDS Arms, Inc Dallas, TX Chair, Internal Medicine
Special Challenges and Co-Morbidities Renal Disease/ Hypertension/ Diabetes in African-Americans M. Keith Rawlings, MD Medical Director Peabody Health Center AIDS Arms, Inc Dallas, TX Chair, Internal Medicine
R-IDARAM. Dexamethasone is administered as an IV infusion in 100mL sodium chloride 0.9% over 30 minutes.
 R-IDARAM Indication Secondary CNS lymphoma ICD-10 codes Codes with a prefix C85 Regimen details Day Drug Dose Route 1 Rituximab 375mg/m 2 IV infusion 1 Methotrexate 12.5mg Intrathecal 1 Cytarabine 70mg
R-IDARAM Indication Secondary CNS lymphoma ICD-10 codes Codes with a prefix C85 Regimen details Day Drug Dose Route 1 Rituximab 375mg/m 2 IV infusion 1 Methotrexate 12.5mg Intrathecal 1 Cytarabine 70mg
Cisplatin and Pemetrexed (NSCLC, mesothelioma)
 Cisplatin and Pemetrexed (NSCLC, mesothelioma) Indication First-line treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) if the histology of the tumour has been confirmed as
Cisplatin and Pemetrexed (NSCLC, mesothelioma) Indication First-line treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) if the histology of the tumour has been confirmed as
Understanding and Managing Methotrexate Nephrotoxicity
 This material is protected by U.S. Copyright law. Unauthorized reproduction is prohibited. For reprints contact: Reprints@AlphaMedPress.com Pediatric Oncology Understanding and Managing Methotrexate Nephrotoxicity
This material is protected by U.S. Copyright law. Unauthorized reproduction is prohibited. For reprints contact: Reprints@AlphaMedPress.com Pediatric Oncology Understanding and Managing Methotrexate Nephrotoxicity
Acute Kidney Injury. Eleanor Haskey BSc(hons) RVN VTS(ECC) VPAC A1
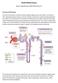 Acute Kidney Injury Eleanor Haskey BSc(hons) RVN VTS(ECC) VPAC A1 Anatomy and Physiology The role of the kidneys is to filter the blood through the glomerulus to form filtrate. The filtrate is then reabsorbed
Acute Kidney Injury Eleanor Haskey BSc(hons) RVN VTS(ECC) VPAC A1 Anatomy and Physiology The role of the kidneys is to filter the blood through the glomerulus to form filtrate. The filtrate is then reabsorbed
VORAXAZE (glucarpidase) For Injection, for intravenous use Initial U.S. Approval: 2012
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VORAXAZE safely and effectively. See full prescribing information for VORAXAZE. VORAXAZE (glucarpidase)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VORAXAZE safely and effectively. See full prescribing information for VORAXAZE. VORAXAZE (glucarpidase)
Recurrent Idiopathic Membranous Glomerulonephritis After Kidney Transplantation and Successful Treatment With Rituximab
 TRANSPLANTATION Recurrent Idiopathic Membranous Glomerulonephritis After Kidney Transplantation and Successful Treatment With Rituximab Khadijeh Makhdoomi, 1,2 Saeed Abkhiz, 1,2 Farahnaz Noroozinia, 1,3
TRANSPLANTATION Recurrent Idiopathic Membranous Glomerulonephritis After Kidney Transplantation and Successful Treatment With Rituximab Khadijeh Makhdoomi, 1,2 Saeed Abkhiz, 1,2 Farahnaz Noroozinia, 1,3
(R) CODOX M / (R) IVAC
 (R) CODOX M / (R) IVAC Indication Burkitt's or Burkitt's-like lymphoma, especially those with 1 or more of the following poor risk criteria: - Raised LDH level - WHO performance status 2-4 - Ann Arbor
(R) CODOX M / (R) IVAC Indication Burkitt's or Burkitt's-like lymphoma, especially those with 1 or more of the following poor risk criteria: - Raised LDH level - WHO performance status 2-4 - Ann Arbor
Acute Kidney Injury for the General Surgeon
 Acute Kidney Injury for the General Surgeon UCSF Postgraduate Course in General Surgery Maui, HI March 20, 2011 Epidemiology & Definition Pathophysiology Clinical Studies Management Summary Hobart W. Harris,
Acute Kidney Injury for the General Surgeon UCSF Postgraduate Course in General Surgery Maui, HI March 20, 2011 Epidemiology & Definition Pathophysiology Clinical Studies Management Summary Hobart W. Harris,
Theophylline for Prevention of Kidney Dysfunction in Neonates With Severe Asphyxia
 kidney diseases Theophylline for Prevention of Kidney Dysfunction in Neonates With Severe Asphyxia Zia Eslami, 1 Ahmad Shajari, 2 Maryam Kheirandish, 3 Azam Heidary 4 Original Paper 1 Division of Neonates
kidney diseases Theophylline for Prevention of Kidney Dysfunction in Neonates With Severe Asphyxia Zia Eslami, 1 Ahmad Shajari, 2 Maryam Kheirandish, 3 Azam Heidary 4 Original Paper 1 Division of Neonates
Assessment of glomerular filtration rate in healthy subjects and normoalbuminuric diabetic patients: validity of a new (MDRD) prediction equation
 Nephrol Dial Transplant (2002) 17: 1909 1913 Original Article Assessment of glomerular filtration rate in healthy subjects and normoalbuminuric diabetic patients: validity of a new () prediction equation
Nephrol Dial Transplant (2002) 17: 1909 1913 Original Article Assessment of glomerular filtration rate in healthy subjects and normoalbuminuric diabetic patients: validity of a new () prediction equation
Foo Koon Mian Pharmacy Resident National University of Singapore Hematology / Oncology Pharmacy Residency Program. 4th APOPC November 2012
 Foo Koon Mian Pharmacy Resident National University of Singapore Hematology / Oncology Pharmacy Residency Program 4th APOPC 2012 1-3 November 2012 1 Outline Use of BSA in chemotherapy dosing Chemotherapy
Foo Koon Mian Pharmacy Resident National University of Singapore Hematology / Oncology Pharmacy Residency Program 4th APOPC 2012 1-3 November 2012 1 Outline Use of BSA in chemotherapy dosing Chemotherapy
Docetaxel. Class: Antineoplastic agent, Antimicrotubular, Taxane derivative.
 Docetaxel Class: Antineoplastic agent, Antimicrotubular, Taxane derivative. Indications: -Breast cancer: -Non small cell lung cancer -Prostate cancer -Gastric adenocarcinoma _Head and neck cancer Unlabeled
Docetaxel Class: Antineoplastic agent, Antimicrotubular, Taxane derivative. Indications: -Breast cancer: -Non small cell lung cancer -Prostate cancer -Gastric adenocarcinoma _Head and neck cancer Unlabeled
Management of early chronic kidney disease
 Management of early chronic kidney disease GREENLANE SUMMER GP SYMPOSIUM 2018 Jonathan Hsiao Renal and General Physician Introduction A growing public health problem in NZ and throughout the world. Unknown
Management of early chronic kidney disease GREENLANE SUMMER GP SYMPOSIUM 2018 Jonathan Hsiao Renal and General Physician Introduction A growing public health problem in NZ and throughout the world. Unknown
Validation of El-Minia Equation for Estimation of Glomerular Filtration Rate in Different Stages of Chronic Kidney Disease
 Kidney Diseases Validation of El-Minia Equation for Estimation of Glomerular Filtration Rate in Different Stages of Chronic Kidney Disease Osama El Minshawy, 1 Eman El-Bassuoni 2 Original Paper 1 Department
Kidney Diseases Validation of El-Minia Equation for Estimation of Glomerular Filtration Rate in Different Stages of Chronic Kidney Disease Osama El Minshawy, 1 Eman El-Bassuoni 2 Original Paper 1 Department
Screening for chronic kidney disease racial implications. Not everybody that pees has healthy kidneys!
 Screening for chronic kidney disease racial implications Not everybody that pees has healthy kidneys! Screening for chronic kidney disease racial implications 1) Definition of CKD 2) Why should we screen
Screening for chronic kidney disease racial implications Not everybody that pees has healthy kidneys! Screening for chronic kidney disease racial implications 1) Definition of CKD 2) Why should we screen
BCCA Protocol Summary for Treatment of Primary Intracerebral Lymphoma with High Dose Methotrexate
 BCCA Protocol Summary for Treatment of Primary Intracerebral Lymphoma with High Dose Methotrexate Protocol Code Tumour Group Contact Physician LYHDMTXP Lymphoma Dr. Diego Villa ELIGIBILITY: 1. Age: 16
BCCA Protocol Summary for Treatment of Primary Intracerebral Lymphoma with High Dose Methotrexate Protocol Code Tumour Group Contact Physician LYHDMTXP Lymphoma Dr. Diego Villa ELIGIBILITY: 1. Age: 16
Gemcitabine & Cisplatin
 Gemcitabine & Cisplatin Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Advanced
Gemcitabine & Cisplatin Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Advanced
VIP (Etoposide, Ifosfamide and Cisplatin)
 VIP (Etoposide, Ifosfamide and Cisplatin) Indication First line treatment for metastatic seminoma, non seminoma or combined tumours where bleomycin is contra-indicated. Usually used for patients with intermediate
VIP (Etoposide, Ifosfamide and Cisplatin) Indication First line treatment for metastatic seminoma, non seminoma or combined tumours where bleomycin is contra-indicated. Usually used for patients with intermediate
MATRIX (Methotrexate, Cytarabine, Thiotepa and Rituximab)
 MATRIX (Methotrexate, Cytarabine, Thiotepa and Rituximab) Indication First line treatment of primary CNS lymphoma. ICD-10 codes Codes with a prefix C85 Regimen details Day Drug Dose Route 1 and 6 Rituximab
MATRIX (Methotrexate, Cytarabine, Thiotepa and Rituximab) Indication First line treatment of primary CNS lymphoma. ICD-10 codes Codes with a prefix C85 Regimen details Day Drug Dose Route 1 and 6 Rituximab
Cisplatin / Capecitabine (+ Trastuzumab) in Gastric Cancer
 Cisplatin / Capecitabine (+ Trastuzumab) in Gastric Cancer Page 1 of 5 Indication: Confirmed HER2-positive (3+ or FISH+) metastatic adenocarcinoma of the stomach or gastrooesophageal junction. Patient
Cisplatin / Capecitabine (+ Trastuzumab) in Gastric Cancer Page 1 of 5 Indication: Confirmed HER2-positive (3+ or FISH+) metastatic adenocarcinoma of the stomach or gastrooesophageal junction. Patient
Primary Care Physicians and Clinicians. XXX on behalf of the Upper Midwest Fistula First Coalition. Chronic Kidney Disease (CKD) Resources
 August 10, 2007 To: From: RE: Primary Care Physicians and Clinicians XXX on behalf of the Upper Midwest Fistula First Coalition Chronic Kidney Disease (CKD) Resources Caring for patients with chronic kidney
August 10, 2007 To: From: RE: Primary Care Physicians and Clinicians XXX on behalf of the Upper Midwest Fistula First Coalition Chronic Kidney Disease (CKD) Resources Caring for patients with chronic kidney
1.28 Protocol Name: CODOX-M/IVAC
 1.28 Protocol Name: CODOX-M/IVAC Indication Burkitt's or Burkitt's-like lymphoma - especially those with 1 of the following poor risk criteria: Lymphoblastic lymphoma - especially B subtype Acute Myeloid
1.28 Protocol Name: CODOX-M/IVAC Indication Burkitt's or Burkitt's-like lymphoma - especially those with 1 of the following poor risk criteria: Lymphoblastic lymphoma - especially B subtype Acute Myeloid
Gemcitabine, Dexamethasone and Cisplatin GDP Regimen
 Gemcitabine, Dexamethasone and Cisplatin GDP Regimen Available for Routine Use in Burton in-patient N/A Derby in-patient Burton day-case Derby day-case Burton outreach chemotherapy clinic N/A Derby outreach
Gemcitabine, Dexamethasone and Cisplatin GDP Regimen Available for Routine Use in Burton in-patient N/A Derby in-patient Burton day-case Derby day-case Burton outreach chemotherapy clinic N/A Derby outreach
Carboplatin / Liposomal Doxorubicin CARBO/CAELYX Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol Carboplatin / CARBO/CAELYX Gynaecological Cancer PROCTOCOL REF: MPHAGYNCCX (Version No: 1.0) Approved for use in: Advanced ovarian cancer in women who have progressed
Systemic Anti Cancer Treatment Protocol Carboplatin / CARBO/CAELYX Gynaecological Cancer PROCTOCOL REF: MPHAGYNCCX (Version No: 1.0) Approved for use in: Advanced ovarian cancer in women who have progressed
Policy for Central Nervous System [CNS] Prophylaxis in Lymphoid Malignancies
![Policy for Central Nervous System [CNS] Prophylaxis in Lymphoid Malignancies Policy for Central Nervous System [CNS] Prophylaxis in Lymphoid Malignancies](/thumbs/90/104309591.jpg) Policy for Central Nervous System [CNS] Prophylaxis in Lymphoid Malignancies UNCONTROLLED WHEN PRINTED Note: NOSCAN Haematology MCN has approved the information contained within this document to guide
Policy for Central Nervous System [CNS] Prophylaxis in Lymphoid Malignancies UNCONTROLLED WHEN PRINTED Note: NOSCAN Haematology MCN has approved the information contained within this document to guide
Pediatric Nephrology Consult and Referral Guidelines
 Pediatric Nephrology Consult and Referral Guidelines Introduction We see children and teens from birth to 21 years. The most common reasons patients are referred to pediatric nephrology services include:
Pediatric Nephrology Consult and Referral Guidelines Introduction We see children and teens from birth to 21 years. The most common reasons patients are referred to pediatric nephrology services include:
Lung Pathway Group Carboplatin & PO Vinorelbine in Non-Small Cell Lung Cancer (NSCLC)
 Lung Pathway Group Carboplatin & PO Vinorelbine in Non-Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in
Lung Pathway Group Carboplatin & PO Vinorelbine in Non-Small Cell Lung Cancer (NSCLC) Indication: First line in radical/induction treatment in locally advanced NSCLC First line palliative treatment in
This is a controlled document and therefore must not be changed or photocopied L.80 - R-CHOP-21 / CHOP-21
 R- / INDICATION Lymphoma Histiocytosis Omit rituximab if CD20-negative. TREATMENT INTENT Disease modification or curative depending on clinical circumstances PRE-ASSESSMENT 1. Ensure histology is confirmed
R- / INDICATION Lymphoma Histiocytosis Omit rituximab if CD20-negative. TREATMENT INTENT Disease modification or curative depending on clinical circumstances PRE-ASSESSMENT 1. Ensure histology is confirmed
ECX. Anti-emetics: Day 1: highly emetogenic Days 2 21: mildly emetogenic
 Page 1 of 5 As an alternative to ECF: For locally advanced (inoperable) or metastatic oesophageal or gastric cancer; peri-operative use in oesophageal or gastric cancer; adenocarcinoma of unknown primary
Page 1 of 5 As an alternative to ECF: For locally advanced (inoperable) or metastatic oesophageal or gastric cancer; peri-operative use in oesophageal or gastric cancer; adenocarcinoma of unknown primary
BASELINE CHARACTERISTICS OF THE STUDY POPULATION
 COMPARISON OF TREATING METABOLIC ACIDOSIS IN CKD STAGE 4 HYPERTENSIVE KIDNEY DISEASE WITH FRUITS & VEGETABLES OR SODIUM BICARBONATE This was a 1-year, single-center, prospective, randomized, interventional
COMPARISON OF TREATING METABOLIC ACIDOSIS IN CKD STAGE 4 HYPERTENSIVE KIDNEY DISEASE WITH FRUITS & VEGETABLES OR SODIUM BICARBONATE This was a 1-year, single-center, prospective, randomized, interventional
DBL LEUCOVORIN CALCIUM INJECTION USP AND TABLETS
 DBL LEUCOVORI CALCIUM IJECTIO USP AD TABLETS ame of medicine Calcium folinate Presentation DBL Leucovorin Calcium Injection USP is a sterile solution of folinic acid (as calcium salt) in water for injections.
DBL LEUCOVORI CALCIUM IJECTIO USP AD TABLETS ame of medicine Calcium folinate Presentation DBL Leucovorin Calcium Injection USP is a sterile solution of folinic acid (as calcium salt) in water for injections.
Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix
 Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix Indication: Neoadjuvant chemotherapy followed by Chemo-radiotherapy in Small Cell Carcinoma
Cisplatin + Etoposide IV / Oral therapy followed by Chemo-radiotherapy in Small Cell Carcinoma of the Cervix Indication: Neoadjuvant chemotherapy followed by Chemo-radiotherapy in Small Cell Carcinoma
Comparison of Meropenem with Ceftazidime as Monotherapy of Cancer Patients with Chemotherapy induced Febrile Neutropenia
 Comparison of Meropenem with Ceftazidime as Monotherapy of Cancer Patients with Chemotherapy induced Febrile Neutropenia I. Malik ( National Cancer lnsititute, Karachi ) Shaharyar (, Department of Radiotherapy
Comparison of Meropenem with Ceftazidime as Monotherapy of Cancer Patients with Chemotherapy induced Febrile Neutropenia I. Malik ( National Cancer lnsititute, Karachi ) Shaharyar (, Department of Radiotherapy
Contrast Induced Nephropathy
 Contrast Induced Nephropathy O CIAKI refers to an abrupt deterioration in renal function associated with the administration of iodinated contrast media O CIAKI is characterized by an acute (within 48 hours)
Contrast Induced Nephropathy O CIAKI refers to an abrupt deterioration in renal function associated with the administration of iodinated contrast media O CIAKI is characterized by an acute (within 48 hours)
Dr. Mehmet Kanbay Department of Medicine Division of Nephrology Istanbul Medeniyet University School of Medicine Istanbul, Turkey.
 The uric acid dilemma: causal risk factor for hypertension and CKD or mere bystander? Mehmet Kanbay, Istanbul, Turkey Chairs: Anton H. van den Meiracker, Rotterdam, The Netherlands Claudia R.C. Van Roeyen,
The uric acid dilemma: causal risk factor for hypertension and CKD or mere bystander? Mehmet Kanbay, Istanbul, Turkey Chairs: Anton H. van den Meiracker, Rotterdam, The Netherlands Claudia R.C. Van Roeyen,
MEGA DOSES OF METHOTREXATE IN THE TREATMENT OF OSTEOSARCOMA
 MEGA DOSES OF METHOTREXATE IN THE TREATMENT OF OSTEOSARCOMA Pages with reference to book, From 248 To 251 M. Perwaiz Iqbal ( Departments of Biochemistry, The Aga Khan University Medical College, Karachi.
MEGA DOSES OF METHOTREXATE IN THE TREATMENT OF OSTEOSARCOMA Pages with reference to book, From 248 To 251 M. Perwaiz Iqbal ( Departments of Biochemistry, The Aga Khan University Medical College, Karachi.
BCCA Protocol Summary for Treatment of Advanced Squamous Cell Carcinoma of the Head and Neck Cancer Using Fluorouracil and Platinum
 BCCA Protocol Summary for Treatment of Advanced Squamous Cell Carcinoma of the Head and Neck Cancer Using Fluorouracil and Platinum Protocol Code: Tumour Group: Contact Physician: HNAVFUP Head and Neck
BCCA Protocol Summary for Treatment of Advanced Squamous Cell Carcinoma of the Head and Neck Cancer Using Fluorouracil and Platinum Protocol Code: Tumour Group: Contact Physician: HNAVFUP Head and Neck
Oxaliplatin and Gemcitabine
 Oxaliplatin and Gemcitabine Indication Palliative treatment for relapsed metastatic seminoma, non seminoma or combined tumours. ICD-10 codes Codes pre-fixed with C38, C48, C56, C62, C63, C75.3. Regimen
Oxaliplatin and Gemcitabine Indication Palliative treatment for relapsed metastatic seminoma, non seminoma or combined tumours. ICD-10 codes Codes pre-fixed with C38, C48, C56, C62, C63, C75.3. Regimen
Dr.Nahid Osman Ahmed 1
 1 ILOS By the end of the lecture you should be able to Identify : Functions of the kidney and nephrons Signs and symptoms of AKI Risk factors to AKI Treatment alternatives 2 Acute kidney injury (AKI),
1 ILOS By the end of the lecture you should be able to Identify : Functions of the kidney and nephrons Signs and symptoms of AKI Risk factors to AKI Treatment alternatives 2 Acute kidney injury (AKI),
Estimation of Serum Creatinine, Urine Creatinine and Creatinine Clearance. BCH472 [Practical] 1
![Estimation of Serum Creatinine, Urine Creatinine and Creatinine Clearance. BCH472 [Practical] 1 Estimation of Serum Creatinine, Urine Creatinine and Creatinine Clearance. BCH472 [Practical] 1](/thumbs/73/68933269.jpg) Estimation of Serum Creatinine, Urine Creatinine and Creatinine Clearance BCH472 [Practical] 1 -Kidney functions: - The kidneys serve three essential functions: 1. They function as filters, removing metabolic
Estimation of Serum Creatinine, Urine Creatinine and Creatinine Clearance BCH472 [Practical] 1 -Kidney functions: - The kidneys serve three essential functions: 1. They function as filters, removing metabolic
Cisplatin and Fluorouracil
 Cisplatin and Fluorouracil Indication Neo-adjuvant treatment of nasopharyngeal head and neck cancer (stage II-IV) or bulky disease at other head and neck sites. Performance Status 0-1 ICD-10 codes Codes
Cisplatin and Fluorouracil Indication Neo-adjuvant treatment of nasopharyngeal head and neck cancer (stage II-IV) or bulky disease at other head and neck sites. Performance Status 0-1 ICD-10 codes Codes
Cisplatin and Fluorouracil (palliative)
 Cisplatin and Fluorouracil (palliative) Indication Palliative chemotherapy for recurrent or metastatic head and neck squamous cell cancer where combination treatment with cetuximab is not indicated. PS0-1
Cisplatin and Fluorouracil (palliative) Indication Palliative chemotherapy for recurrent or metastatic head and neck squamous cell cancer where combination treatment with cetuximab is not indicated. PS0-1
Methotrexate Pharmacokinetics and Survival in Osteosarcomat
 Methotrexate Pharmacokinetics and Survival in Osteosarcomat Irene Aquerreta, PharmD, PhD, 1 * Azucena Aldaz, PharmD, PhD, 1 Joaquín Giráldez, PharmD, PhD, 1 and Luis Sierrasesúmaga, MD, PhD 2 1 Department
Methotrexate Pharmacokinetics and Survival in Osteosarcomat Irene Aquerreta, PharmD, PhD, 1 * Azucena Aldaz, PharmD, PhD, 1 Joaquín Giráldez, PharmD, PhD, 1 and Luis Sierrasesúmaga, MD, PhD 2 1 Department
Cisplatin and Fluorouracil (head and neck)
 Cisplatin and Fluorouracil (head and neck) Indication Palliative chemotherapy for recurrent or metastatic head and neck squamous cell cancer where combination treatment with cetuximab is not indicated.
Cisplatin and Fluorouracil (head and neck) Indication Palliative chemotherapy for recurrent or metastatic head and neck squamous cell cancer where combination treatment with cetuximab is not indicated.
Severity and Outcome of Acute Kidney Injury According to Rifle Criteria in the Intensive Care Unit
 BANTAO Journal 2010; 8 (1): 35-39 BJ BANTAO Journal Original Article Severity and Outcome of Acute Kidney Injury According to Rifle Criteria in the Intensive Care Unit Albana Gjyzari 1, Elizana Petrela
BANTAO Journal 2010; 8 (1): 35-39 BJ BANTAO Journal Original Article Severity and Outcome of Acute Kidney Injury According to Rifle Criteria in the Intensive Care Unit Albana Gjyzari 1, Elizana Petrela
THE CLATTERBRIDGE CANCER CENTRE NHS FOUNDATION TRUST. Systemic Anti Cancer Treatment Protocol. EDP + mitotane
 Systemic Anti Cancer Treatment Protocol EDP + mitotane PROCEDURE REF: MPHAHANEDP (Version No: 1.0) Approved for use in: Symptomatic treatment for advanced (unresectable, metastatic or relapsed) adrenocortical
Systemic Anti Cancer Treatment Protocol EDP + mitotane PROCEDURE REF: MPHAHANEDP (Version No: 1.0) Approved for use in: Symptomatic treatment for advanced (unresectable, metastatic or relapsed) adrenocortical
Doreen P. Foley MS RN ANP-C Doctor of Nursing Practice Program Chamberlain College of Nursing
 Doreen P. Foley MS RN ANP-C Doctor of Nursing Practice Program Chamberlain College of Nursing This program has been developed solely for the purposes of describing the level of nurse practitioner (NP)
Doreen P. Foley MS RN ANP-C Doctor of Nursing Practice Program Chamberlain College of Nursing This program has been developed solely for the purposes of describing the level of nurse practitioner (NP)
Cisplatin + Etoposide + Thoracic Radiotherapy (TRT) INDICATIONS FOR USE:
 Cisplatin + Etoposide + Thoracic Radiotherapy (TRT) INDICATIONS FOR USE: Protocol INDICATION ICD10 Code Small cell lung cancer (SCLC) limited disease C34 00279a ELIGIBILTY: Indications as above ECOG 0-2
Cisplatin + Etoposide + Thoracic Radiotherapy (TRT) INDICATIONS FOR USE: Protocol INDICATION ICD10 Code Small cell lung cancer (SCLC) limited disease C34 00279a ELIGIBILTY: Indications as above ECOG 0-2
MYBORPRE. Protocol Code. Lymphoma, Leukemia/BMT. Tumour Group. Dr. Kevin Song. Contact Physician
 BC Cancer Protocol Summary for the Treatment of Multiple Myeloma Using Bortezomib, Dexamethasone With or Without Cyclophosphamide as Induction Pre-Stem Cell Transplant Protocol Code Tumour Group Contact
BC Cancer Protocol Summary for the Treatment of Multiple Myeloma Using Bortezomib, Dexamethasone With or Without Cyclophosphamide as Induction Pre-Stem Cell Transplant Protocol Code Tumour Group Contact
Faculty/Presenter Disclosure
 CSI for CKD Unravelling the myths surrounding chronic kidney disease Practical Evidence for Informed Practice Oct 21 2016 Dr. Scott Klarenbach University of Alberta Slide 1: Option B (Presenter with NO
CSI for CKD Unravelling the myths surrounding chronic kidney disease Practical Evidence for Informed Practice Oct 21 2016 Dr. Scott Klarenbach University of Alberta Slide 1: Option B (Presenter with NO
Cisplatin and Vinorelbine and radiotherapy (NSCLC)
 Cisplatin and Vinorelbine and radiotherapy (NSCLC) Indication First-line chemotherapy for use with concomitant radical radiotherapy for early or locally advanced non-small cell carcinoma (NSCLC) ICD-10
Cisplatin and Vinorelbine and radiotherapy (NSCLC) Indication First-line chemotherapy for use with concomitant radical radiotherapy for early or locally advanced non-small cell carcinoma (NSCLC) ICD-10
Proceedings of the 34th World Small Animal Veterinary Congress WSAVA 2009
 www.ivis.org Proceedings of the 34th World Small Animal Veterinary Congress WSAVA 2009 São Paulo, Brazil - 2009 Next WSAVA Congress : Reprinted in IVIS with the permission of the Congress Organizers HOW
www.ivis.org Proceedings of the 34th World Small Animal Veterinary Congress WSAVA 2009 São Paulo, Brazil - 2009 Next WSAVA Congress : Reprinted in IVIS with the permission of the Congress Organizers HOW
OST Course Schedule
 OST 572 2017 Course Schedule Updated 03/06/2017 (aes) Week 1 Monday March 13, 2017 7:30-7:45 Course Syllabus /Schedule and announcements (posted on D2L) Kaufman Self Study Tuesday, March 14, 2017 1 Course
OST 572 2017 Course Schedule Updated 03/06/2017 (aes) Week 1 Monday March 13, 2017 7:30-7:45 Course Syllabus /Schedule and announcements (posted on D2L) Kaufman Self Study Tuesday, March 14, 2017 1 Course
DERBY-BURTON LOCAL CANCER NETWORK FILENAME SMILE.DOC CONTROLLED DOC NO: HCCPG B57 CSIS Regimen Name: SMILE. SMILE chemotherapy
 SMILE chemotherapy Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Extranodal
SMILE chemotherapy Available for Routine Use in Burton in-patient Derby in-patient Burton day-case Derby day-case Burton community Derby community Burton out-patient Derby out-patient Indication Extranodal
2.07 Protocol Name: CHOP & Rituximab
 2.07 Protocol Name: CHOP & Rituximab Indication Intermediate and high grade, B-cell non-hodgkins lymphoma expressing CD20. Second or third line therapy for low grade, B cell non- Hodgkins lymphoma expressing
2.07 Protocol Name: CHOP & Rituximab Indication Intermediate and high grade, B-cell non-hodgkins lymphoma expressing CD20. Second or third line therapy for low grade, B cell non- Hodgkins lymphoma expressing
Cisplatin and Vinorelbine and radiotherapy (NSCLC)
 Cisplatin and Vinorelbine and radiotherapy (NSCLC) Indication First-line chemotherapy for use with concomitant radical radiotherapy for early or locally advanced non-small cell carcinoma (NSCLC) ICD-10
Cisplatin and Vinorelbine and radiotherapy (NSCLC) Indication First-line chemotherapy for use with concomitant radical radiotherapy for early or locally advanced non-small cell carcinoma (NSCLC) ICD-10
GUIDELINE FOR THE MANAGEMENT AND PREVENTION OF ACUTE TUMOUR LYSIS SYNDROME IN HAEMATOLOGICAL MALIGNANCIES
 GUIDELINE FOR THE MANAGEMENT AND PREVENTION OF ACUTE TUMOUR LYSIS SYNDROME IN HAEMATOLOGICAL MALIGNANCIES Full Title of Guideline: Author (include email and role): Division & Speciality: Scope (Target
GUIDELINE FOR THE MANAGEMENT AND PREVENTION OF ACUTE TUMOUR LYSIS SYNDROME IN HAEMATOLOGICAL MALIGNANCIES Full Title of Guideline: Author (include email and role): Division & Speciality: Scope (Target
Carboplatin Time to Drop the Curtain on the Dosing Debate
 Carboplatin Time to Drop the Curtain on the Dosing Debate Jon Herrington, Pharm.D., BCPS, BCOP Judith Smith, Pharm.D., BCOP, CPHQ, FCCP, FISOPP Scott Soefje, Pharm.D., MBA, BCOP Heimberg J, et al. N Engl
Carboplatin Time to Drop the Curtain on the Dosing Debate Jon Herrington, Pharm.D., BCPS, BCOP Judith Smith, Pharm.D., BCOP, CPHQ, FCCP, FISOPP Scott Soefje, Pharm.D., MBA, BCOP Heimberg J, et al. N Engl
Management of Acute Kidney Injury in the Neonate. Carolyn Abitbol, M.D. University of Miami Miller School of Medicine / Holtz Children s Hospital
 Management of Acute Kidney Injury in the Neonate Carolyn Abitbol, M.D. University of Miami Miller School of Medicine / Holtz Children s Hospital Objectives Summarize the dilemmas in diagnosing & recognizing
Management of Acute Kidney Injury in the Neonate Carolyn Abitbol, M.D. University of Miami Miller School of Medicine / Holtz Children s Hospital Objectives Summarize the dilemmas in diagnosing & recognizing
Renal Transporters- pathophysiology of drug - induced renal disorders. Lisa Harris, Pharmacist, John Hunter Hospital, Newcastle, 2015 November
 Renal Transporters- pathophysiology of drug - induced renal disorders Lisa Harris, Pharmacist, John Hunter Hospital, Newcastle, 2015 November Renal Failure Up to 25% of acute renal failure is drug induced
Renal Transporters- pathophysiology of drug - induced renal disorders Lisa Harris, Pharmacist, John Hunter Hospital, Newcastle, 2015 November Renal Failure Up to 25% of acute renal failure is drug induced
TIP: Paclitaxel / Ifosfamide / Cisplatin in Relapsed Germ Cell Tumour
 TIP: Paclitaxel / Ifosfamide / Cisplatin in Relapsed Germ Cell Tumour Indication: Second line therapy in Relapsed Germ Cell Tumours Regimen details: Paclitaxel 175mg/m 2 IV Cisplatin 20mg/m 2 IV - D5 Ifosfamide
TIP: Paclitaxel / Ifosfamide / Cisplatin in Relapsed Germ Cell Tumour Indication: Second line therapy in Relapsed Germ Cell Tumours Regimen details: Paclitaxel 175mg/m 2 IV Cisplatin 20mg/m 2 IV - D5 Ifosfamide
Jo Abraham MD Division of Nephrology University of Utah
 Jo Abraham MD Division of Nephrology University of Utah 68 year old male presented 3 weeks ago with a 3 month history of increasing fatigue He reported a 1 week history of increasing dyspnea with a productive
Jo Abraham MD Division of Nephrology University of Utah 68 year old male presented 3 weeks ago with a 3 month history of increasing fatigue He reported a 1 week history of increasing dyspnea with a productive
Irish Practice Nurses Association Annual Conference Tullamore Court Hotel OCTOBER 6 th 2012
 Irish Practice Nurses Association Annual Conference Tullamore Court Hotel OCTOBER 6 th 2012 Susan McKenna Renal Clinical Nurse Specialist Cavan General Hospital Renal patient population ACUTE RENAL FAILURE
Irish Practice Nurses Association Annual Conference Tullamore Court Hotel OCTOBER 6 th 2012 Susan McKenna Renal Clinical Nurse Specialist Cavan General Hospital Renal patient population ACUTE RENAL FAILURE
CISPLATIN Chemo-radiation regimen Gynaecological Cancer
 Systemic Anti Cancer Treatment Protocol CISPLATIN Chemo-radiation regimen Gynaecological Cancer PROCTOCOL REF: MPHAGYNCIX (Version No: 1.0) Approved for use in: Locally advanced cervical cancer (adjuvant/curative)
Systemic Anti Cancer Treatment Protocol CISPLATIN Chemo-radiation regimen Gynaecological Cancer PROCTOCOL REF: MPHAGYNCIX (Version No: 1.0) Approved for use in: Locally advanced cervical cancer (adjuvant/curative)
Allopurinol Against Progression of Chronic Kidney Disease
 KIDNEY DISEASES Allopurinol Against Progression of Chronic Kidney Disease Sima Golmohammadi, 1 Afshin Almasi, 2 M Manouchehri, 1 Hamid Reza Omrani, 1 Mohammad Reza Zandkarimi 3 Original Paper 1 Department
KIDNEY DISEASES Allopurinol Against Progression of Chronic Kidney Disease Sima Golmohammadi, 1 Afshin Almasi, 2 M Manouchehri, 1 Hamid Reza Omrani, 1 Mohammad Reza Zandkarimi 3 Original Paper 1 Department
