LOCAL CLINICAL GUIDELINE: METARAMINOL
|
|
|
- Frederica Cobb
- 5 years ago
- Views:
Transcription
1 LOCAL CLINICAL GUIDELINE: METARAMINOL CLINICAL GUIDELINE TITLE Metaraminol peripheral infusion 1)SUMMARY Metaraminol is an intravenous vasopressor used to increase systolic and diastolic blood pressures. This guideline is to allow the use of metaraminol infusions in theatre recovery and on Charles Pannett HDU for the short term management of hypotension secondary to epidural anaesthesia. This will avoid patients, who are otherwise well, requiring a central line in order to be given noradrenaline. 2) INTRODUCTION Metaraminol is commonly used by anaesthetists to counteract the drop in systemic vascular resistance induced by epidural and spinal anaesthetics and by general anaesthetics.it is given as a peripheral infusion and can be bolused. However if the hypotension persistsafter theatre the metaraminol is often changed to norepinephrine (noradrenaline) which must be given through a central line. Metaraminol can be given peripherally so removes the need for central access. Metaraminol infusion is only to be prescribed after consultation with an anaesthetic consultant or senior registrar (ST 5-7). It can only be given if the cause of the hypotension is clearly secondary to the epidural anaesthetic. 3) DEFINITIONS Metaraminol is a synthetic sympathomimetic amine which predominantly acts as an agonist at alpha 1 adrenoceptors. It also has some weak indirect and beta receptor activity which is not noticeable clinically. Activation of alpha1 receptors results in near-instant peripheral vasoconstriction and consequently a rise in arterial blood pressure. Unlike ephedrine it does not exhibit tachyphylaxis (i.e. dose increase to maintain the same effect). PHARMCODYNAMICS EFFECTS CLINICAL SIGNIFICANCE CIRCULATION Increases both systolic and diastolic Rise in blood pressure blood pressure Reflex bradycardia especially if Increases pulmonary vascular bolused resistance Can trigger pulmonary oedema Reduces heart rate Can reduce cardiac output DISABILITY OTHER Reduces cerebral bloodflow Increases cerebral perfusion pressure Can reduce renal bloodflow Reduces insulin secretion Can increase body temperature Negligible Often used safely in neurosurgical operations Be careful if renal perfusion is already compromised May increase blood glucose Page 1 of 11
2 Physical characteristics Metaraminol is presented as a clear, colourless solution in 1ml ampoules. Each ampoule contains 10mg of metaraminol tartrate. The standard dilution is to produce a final solution containing 0.5mg/ml (i.e. one ampoule in 20mls 0.9% sodium chloride or two ampoules in 40mls of 0.9% sodium chloride). The reconstituted drug is stable for up to 24hrs. Differences with Noradrenaline METARAMINOL NORADRENALINE Less potent Can be bolused safely May cause vasospasm Can be given peripherally Routine syringe change More potent Cannot be bolused No evidence of vasospasm Must be given centrally Must be 'double pumped' 4) SCOPE: On the St Mary s site this will only be used in the following locations for patients with hypotension secondary to epidural anaesthetic: Charles Pannett HDU QEQM theatre recovery Surgical Innovation Centre (ISIC) theatre recovery Page 2 of 11
3 5) QUICK GUIDELINE Indication: for hypotension secondary to epidural infusion if no central line is in situ Draw up drug Add 2 ampoules of neat metarminol (20mg) to 38ml of 0.9% sodium chloride Label syringe and line, attach to pump and dedicated cannula Start infusion at prescribed rate Consider ending infusion IF Patient becomes unwell YES NO Assess VIP score and record obs after 10-15min inform doctor on HDU and anaesthetist on bleep 1201 Is rate 1-2mls/hr? Is rate more than 20mls/hr or higher than prescribed maximum? YES NO Continue infusion at current rate Inform doctor on HDU and anaesthetist on bleep 1201 Target BP achieved? NO Increase or decrease infusion rate by 1-2mls as appropriate YES Continue current rate Reassess every 30min Page 3 of 11
4 FULL GUIDELINE Indications: Contra-indications: Hypotension secondary to epidural anaesthetic infusion If the cause of the hypotension is not clearly due to the epidural. It is important that the metaraminol infusion does not mask the following: Sepsis Hypovolaemia Cardiogenic shock Hypotension secondary to arrhythmias Severe ischaemic heart disease or cardiomyopathy And other potentially fatal causes of hypotension Caution: Contra-indications: Patient on anti-arrhythmics especially beta blockers and digoxin Patient on monoamine oxidase inhibitors or tricyclic antidepressants Hypersensitivity to metaraminol, including sulphites (more prevalent in patients with asthma). Patient on the antibiotic linezolid which is a reversible MAOI. Metaraminol infusion should only be prescribed and continued under the instruction of a consultant anaesthetist or senior registrar (ST5-7) either responsible for the patient or on call. The prescription must be reviewed every 24 hours by the consultant anaesthetist on call or the consultant anaesthetist responsible for the patient. At St Mary s the anaesthetist carrying bleep 1201 will be the point of contact for this. Dr Alison Knaggs, consultant anaesthetist(mobile via switchboard),must be informed that this infusion is being used so that this usage can be audited. The prescription should include the following details: Date Drug name and dose Diluent and volume Target mean arterial or systolic blood pressure Starting infusion rate (if not already running) Range of acceptable infusion rates For example: Metaraminol 20mg in 40mls of sodium chloride 0.9% Target mean arterial blood pressure of 80mmHg Start at 6 mls/hr Maximum infusion rate 20mls/hr Page 4 of 11
5 Preparing the infusion 1. Check patient details and prescription 2. Prepare equipment using ANTT: drug, diluent, needles, labels, 50ml syringe, IV extension line, pump 3. Draw up 38mls of 0.9% sodium chloride into the 50ml syringe 4. Add 2 ampoules (20mg) of metaraminol to make a total of 40mls 5. Label the syringe appropriately, connect & prime the line, load onto pump 6. Peripheral cannula: a. a dedicated cannula must be used b. cannula must not be sited in a very small, threadlike vein. 7. Final check before connecting to patient and starting at prescribed rate 8. Patient must have an arterial line in situ for first 24 hours of infusion or for longer if blood pressure is very labile or the nursing staff request it. Monitoring a metaraminol infusion Titration to target blood pressure The infusion rate of metaraminol can be increased or decreased every 15min by 2-3ml/hr to achieve the target blood pressure specified on the prescription. Changes to the rate should be documented (in mls/hr) on the HDU chart the same as for noradrenaline. Sudden changes to dose requirements should be reported both to the doctor in charge of the patient and to the anaesthetist carrying Bolusing metaraminol Unlike noradrenaline, metaraminol can be bolused by a doctor experienced in its use. Anaesthetists will typically bolus about 1ml (0.5 mg) depending on the patient s physiology and then increase the infusion rate to maintain the effect. Outside theatres metaraminol must not be bolused by anyone except an anaesthetist or consultant familiar with the drug as it can cause marked hypertension and bradycardia. Changing the syringe Metaraminol does not need to be 'double pumped' like noradrenaline - a short period of disconnection will not cause a big drop in blood pressure. The fresh syringe can be prepared in advance, the old one disconnected and the new one replaced within the space of 3-4min with no problems. Patient monitoring Blood pressure monitoring must initially be invasive. Patient should have an arterial line in situ for first 24 hours of infusion or for longer if blood pressure is very labile or the nursing staff request it. ECG, peripheral oxygen saturations and urine output must be monitored as per HDU and recovery protocols. Page 5 of 11
6 Extravasation: In most cases the treatment of extravasation is non-pharmacologic in nature; the efficacy of any specific approach has not been demonstrated in controlled studies. The recommended approach to the treatment of extravasation includes the following steps: Stop the infusion immediately if the patient admits to a burning sensation or complains of pain. Call anaesthetist carrying bleep 1201 to review patient within 30 minutes. The cannula should not be removed immediately, but should be left in place to attempt aspiration of fluid from the extravasated area. Aspiration of the drug and surrounding fluid should be attempted. Infiltrate the area with phentolamine 5 to 10 mg diluted in 10 to 15 ml of sodium chloride 0.9% with a fine hypodermic syringe. Phentolamine should be given within an hour. There is minimal evidence that phentolamine will help. If it cannot be found infiltrate the area with 10 to 15 mls of Plasmalyte 148 instead. Remove the cannula. Elevate the affected limb to minimize swelling. Page 6 of 11
7 Discontinuing a metaraminol Infusion Planned discontinuation When target blood pressure is consistently achieved with only 1-2mls/hour of metaraminol, the infusion can be safely discontinued. The primed line should be left connected on the pump in case the infusion needs to be restarted in the next hour, after which time the line can be disconnected and the drug discarded. The cannula should then be flushed to remove traces of residual drug. Unexpected discontinuation Immediately notify the doctors looking after the patient and anaesthetist carrying bleep 1201 in the event of severe hypotension. When to change to noradrenaline Certain situations will necessitate the insertion of a central venous line and starting administration of noradrenaline. If the need for vasopressors is going to exceed 48 hours If dose requirement is high and increasing, needing frequent syringe changes and high volumes If a central line is inserted for other reasons, metaraminol should be switched to noradrenaline 6) IMPLEMENTATION Training required for staff If yes, who will provide training: Please give name/post When will training be provided? Please give date Date for implementation of guideline: (after the process of ratification) Yes Dr Alison Knaggs, Consultant anaesthetistat St Mary s Dr Daniel Horner, Service Director at Charing Cross Dr Philippa Borra, Service Director at Hammersmith On the SMH site at CPA HDU nurse meetings and to recovery staff. Training on the other two sites will be implemented should a need for the infusion be identified. Currently there is no requirement known. 7) MONITORING / AUDIT When will this guideline be audited? Please give approximate date Who will be responsible for auditing this guideline? Please give name/post Continually for the first year. Dr Alison Knaggs Consultant anaesthetist, St Mary s Hospital Dr Daniel Horner, Service Director at Charing Cross Dr Philippa Borra, Service Director at Hammersmith Page 7 of 11
8 Are there any other specific recommendations for audit? 8) REVIEW Frequency of review All cases must be identified to ensure that guidelines cover all eventualities and that the use of this infusion does not mask sick patients. Please indicate frequency of review: Every 2 years Person and post responsible for the review: Dr A Knaggs 9) REFERENCES Drugs in Anaesthesia& Intensive Care, 3 rd edition, M Sasada& S Smith, OUP 2008 Pharmacology for Anaesthesia& Intensive Care, 3 rd edition, TE Peck et al, CUP ) GUIDELINE DETAIL Start Date: (date of final approval by Division) Approval Dates Have all relevant stakeholdersbeen included in the development of this guideline? (Trust sites, Divisions and Directorates) Who will you be notifying of the existence of this guidance? Related documents (if applicable) Authors Document review history (If applicable version number, dates of previous reviews) THIS GUIDELINE REPLACES: (list the title of the replaced guideline, its archive location and previous versions where known) 11) INTRANET HOUSEKEEPING Key words Enter name of Divisional group:sccs PPRM (Q&S Committee) Date of ratification:3 rd December 2014 Enter name of Directorate group: Date of ratification: Please list all (name and role): Please give names/depts: Nursing staff on Charles Pannett HDU Nursing staff in theatre recoveries (QEQM and SIC) None Dr Alison Knaggs (consultant anaesthetist), Dr Pete Williams (anaesthetic senior registrar), Dr Helgi Johannsson (chief of service for anaesthetics and ITU), Ms Depal Patel (Senior Lead Pharmacist, Surgery) Division: surgery, cancer and cardiovascular Site: St Mary s Hospital Contact: Mobile via switchboard Trust address:alison.knaggs@imperial.nhs.uk Next review due: December 2016 NA Metaraminol Page 8 of 11
9 Which Division/Directorate category does this belong to? Which specialty should this belong to when appearing on The Source? Surgery, cancer and cardiovascular division Anaesthetics 12) EQUALITY IMPACT OF GUIDELINE Is this guideline anticipated to have any significant equality-related impact on patients, carers or staff? No. Appendix One: Clinical Guidelines Guidance Notes for Authors These guidelines are for staff based within Imperial College Healthcare NHS Trust. Differences between POLICY and GUIDELINE POLICY is expected to reflect that the content is mandatory in nature, whereas a GUIDELINE, though evidence based and agreed by peers, is intended as advisory, applicable in most cases but open to deviation should the specifics of a particular clinical situation demand it. If you are writing a Policy document, use the separate Policy template which is available on The Source within the document Process for the Development and Management of Procedural Documents Policy. Model specification for a clinical guideline The title of the guideline should be succinct and precise, reflecting the content so that title searches on the Intranet have maximum chance of succeeding. Avoid starting titles with Guideline for or Management of or similar phrases of introduction. It is good practice to include an introduction to your guideline. This can also be used to define the target audience (e.g. a junior doctor who needs to deal with a clinical problem for a few hours). The applicability of the guideline should be clearly stated in the introduction. It should be as concise and easy to use as possible and outline a step-wise approach to management, emphasizing things that are essential. If high quality national guidelines on a topic exist, it will generally be appropriate to adopt or adapt these. Material differences from recommendations in national guidelines should be explicitly justified. References to drugs should be precise and both clinically and economically optimal. A summary should be included if this is likely to help the user. The date of the final draft and a scheduled review date should be stated. Abbreviations should first be stated in full on their initial usage in the document References, if cited, should be in the form: Oxman AD et al. Users' guides to the medical literature, VI How to use an overview. JAMA 1994; 272: How to publish a guideline on The Source Each service is encouraged to develop its own guidelines (and indeed many have already done this). Each Division can work independently on their portfolio of guidelines. Before finalising a guideline authors should seek comments on a draft from any professional group or specialty that may have an interest, e.g. Pathology Imaging Pharmacy Page 9 of 11
10 Therapies (physiotherapy, dietetics, speech, occupational therapy) Nursing Divisional management Other Divisions A & E The guideline should also be approved at Divisional level. Appendix Two: Equality Impact Assessment Screening Tool Title of Clinical Guideline: Metaraminol Division and Directorate: Surgery, cancer and cardiovascular Name of Person Responsible for this Equality Impact Dr Alison Knaggs Assessment: Date of Completion: 26 th November 2014 Aims and purposes of this Clinical Guideline: Insert a summary of the available evidence for each strand, including statistical such as percentages, as well as qualitative data, such as survey results, in the blank field in each category row. Indicate whether there is (or is likely to be) any significant impact on anyone or any group in relation to the following Equality Strands, and whether or not it is justified. Select from the following options: IMPACT JUSTIFICATION NO there is no significant impact Ethnicity/Race Disability Gender/Sex Religion/Belief Sexual Orientation Page 10 of 11
11 Age Deprivation If further evidence is required to complete this screening tool, take steps to obtain it before proceeding with the assessment. If the review of evidence indicates that there is a significant unjustified impact in at least one category, a Full Equality Impact Assessment must be carried out. Page 11 of 11
Clinical Competency Name: Post-operative use of Metaraminol in Recovery
 Clinical Competency Name: Post-operative use of Metaraminol in Recovery Trainee name: Title: Ward or department: Method of assessment: Clinical assessor: Name: Title: Professional assessor: Name: Title:
Clinical Competency Name: Post-operative use of Metaraminol in Recovery Trainee name: Title: Ward or department: Method of assessment: Clinical assessor: Name: Title: Professional assessor: Name: Title:
DRUG GUIDELINE. HYDRALAZINE (Intravenous severe hypertension in pregnancy)
 DRUG GUIDELINE HYDRALAZINE (Intravenous severe hypertension SCOPE (Area): FOR USE IN: Labour Ward, HDU, Theatre and ED EXCLUSIONS: Paediatrics (seek Paediatrician advice) and other general wards. SCOPE
DRUG GUIDELINE HYDRALAZINE (Intravenous severe hypertension SCOPE (Area): FOR USE IN: Labour Ward, HDU, Theatre and ED EXCLUSIONS: Paediatrics (seek Paediatrician advice) and other general wards. SCOPE
Titrating Critical Care Medications
 Titrating Critical Care Medications Chad Johnson, MSN (NED), RN, CNCC(C), CNS-cc Clinical Nurse Specialist: Critical Care and Neurosurgical Services E-mail: johnsoc@tbh.net Copyright 2017 1 Learning Objectives
Titrating Critical Care Medications Chad Johnson, MSN (NED), RN, CNCC(C), CNS-cc Clinical Nurse Specialist: Critical Care and Neurosurgical Services E-mail: johnsoc@tbh.net Copyright 2017 1 Learning Objectives
A Clinical Guideline for the use of Intravenous Aminophylline in Acute Severe Asthma in Children
 For Use in: By: For: Division responsible for document: Key words: Name and job titles of document author: Name and job title of document author s Line Manager: Supported by: Assessed and approved by the:
For Use in: By: For: Division responsible for document: Key words: Name and job titles of document author: Name and job title of document author s Line Manager: Supported by: Assessed and approved by the:
INTRAVENOUS HYDRALAZINE POLICY
 PURPOSE INTRAVENOUS HYDRALAZINE POLICY The purpose of this policy is to: provide safe and effective care for women establish a local approach to care that is evidence based and consistent inform good decision
PURPOSE INTRAVENOUS HYDRALAZINE POLICY The purpose of this policy is to: provide safe and effective care for women establish a local approach to care that is evidence based and consistent inform good decision
Guideline for Management of Severe or Fulminating Pre-Eclampsia
 Guideline for Management of Severe or Fulminating Pre-Eclampsia Originator: Labour Ward Forum, Maternity Services Date Approved: September 2011 Approved by: W&CH Quality & Safety Group Reviewed and ratified
Guideline for Management of Severe or Fulminating Pre-Eclampsia Originator: Labour Ward Forum, Maternity Services Date Approved: September 2011 Approved by: W&CH Quality & Safety Group Reviewed and ratified
SUMMARY OF PRODUCT CHARACTERISTICS. Each ml solution for injection contains phenylephrine hydrochloride corresponding to 0.1 mg phenylephrine.
 1. NAME OF THE MEDICINAL PRODUCT Fenylefrin Abcur 0.05 mg/ml, solution for injection Fenylefrin Abcur 0.1 mg/ml, solution for injection SUMMARY OF PRODUCT CHARACTERISTICS 2. QUALITATIVE AND QUANTITATIVE
1. NAME OF THE MEDICINAL PRODUCT Fenylefrin Abcur 0.05 mg/ml, solution for injection Fenylefrin Abcur 0.1 mg/ml, solution for injection SUMMARY OF PRODUCT CHARACTERISTICS 2. QUALITATIVE AND QUANTITATIVE
Clinical Guideline for Intravenous Opioids for Adults in Recovery Areas The Recovery Protocol
 Clinical Guideline for Intravenous Opioids for Adults in Recovery Areas The Recovery Protocol 1. Aim/Purpose of this Guideline 1.1. To Provide safe and efficient administration of Opioids in Recovery.
Clinical Guideline for Intravenous Opioids for Adults in Recovery Areas The Recovery Protocol 1. Aim/Purpose of this Guideline 1.1. To Provide safe and efficient administration of Opioids in Recovery.
Index No: MMG11/1. Version: 1. Date ratified: 12 th November 2013
 Index No: Intravenous fluid prescription in children For previously well children aged one month to 16 years (excluding renal, cardiac, diabetic ketoacidosis and acute burns patients) Version: 1 Date ratified:
Index No: Intravenous fluid prescription in children For previously well children aged one month to 16 years (excluding renal, cardiac, diabetic ketoacidosis and acute burns patients) Version: 1 Date ratified:
Paravertebral policy. The Acute pain Management Dept, UCLH
 UCLH PARAVERTEBRAL BLOCK (ADULTS) POLICY Paravertebral policy. The Acute pain Management Dept, UCLH DEFINITION A Paravertebral block is a method of providing effective analgesia using a local anaesthetic.
UCLH PARAVERTEBRAL BLOCK (ADULTS) POLICY Paravertebral policy. The Acute pain Management Dept, UCLH DEFINITION A Paravertebral block is a method of providing effective analgesia using a local anaesthetic.
Neosynephrine. Name of the Medicine
 Name of the Medicine Neosynephrine Phenylephrine hydrochloride 1% injection Neosynephrine Presentation Neosynephrine is a clear, colourless, aqueous solution, free from visible particulates, in sterile
Name of the Medicine Neosynephrine Phenylephrine hydrochloride 1% injection Neosynephrine Presentation Neosynephrine is a clear, colourless, aqueous solution, free from visible particulates, in sterile
Reducing the Door to Needle Time for Antibiotics in Suspected Neutropenic Sepsis using a Dedicated Clinical Pathway
 Reducing the Door to Needle Time for Antibiotics in Suspected Neutropenic Sepsis using a Dedicated Clinical Pathway Dr Alex Williams, Oncology Specialty Doctor. Cheltenham General Hospital Oncology Centre
Reducing the Door to Needle Time for Antibiotics in Suspected Neutropenic Sepsis using a Dedicated Clinical Pathway Dr Alex Williams, Oncology Specialty Doctor. Cheltenham General Hospital Oncology Centre
PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker
 PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
PACKAGE INSERT Pr PHENTOLAMINE MESYLATE INJECTION SANDOZ STANDARD 5 mg/ ml THERAPEUTIC CLASSIFICATION Alpha-adrenoreceptor Blocker ACTIONS AND CLINICAL PHARMACOLOGY Phentolamine produces an alpha-adrenergic
DOCUMENT CONTROL PAGE
 DOCUMENT CONTROL PAGE Title Title: UNDERGOING SPINAL DEFORMITY SURGERY Version: 2 Reference Number: Supersedes Supersedes: all other versions Description of Amendment(s): Revision of analgesia requirements
DOCUMENT CONTROL PAGE Title Title: UNDERGOING SPINAL DEFORMITY SURGERY Version: 2 Reference Number: Supersedes Supersedes: all other versions Description of Amendment(s): Revision of analgesia requirements
I. Subject: Continuous Aerosolization of Bronchodilators
 I. Subject: Continuous Aerosolization of Bronchodilators II. Indications: A. Acute airflow obstruction in which treatment with an aerosolized bronchodilator is desired for an extended period of time, i.e.
I. Subject: Continuous Aerosolization of Bronchodilators II. Indications: A. Acute airflow obstruction in which treatment with an aerosolized bronchodilator is desired for an extended period of time, i.e.
Guidance for the Use of Subcutaneous Furosemide by Continuous Infusion for Heart Failure in Community Settings
 Guidance for the Use of Subcutaneous Furosemide by Continuous Infusion for Heart Failure in Community Settings NHS Highland Authorised by: Planning For Fairness: Yes/No (Formerly EQIA) Distribution Consultant
Guidance for the Use of Subcutaneous Furosemide by Continuous Infusion for Heart Failure in Community Settings NHS Highland Authorised by: Planning For Fairness: Yes/No (Formerly EQIA) Distribution Consultant
Guideline for the Management of Continuous IV Vancomycin Infusion in Neonates on NICU A Clinical Guideline recommended for use
 Guideline for the Management of Continuous IV Vancomycin Infusion in A Clinical Guideline recommended for use For Use in: By: For: Division responsible for document: Key words: Name and job title of document
Guideline for the Management of Continuous IV Vancomycin Infusion in A Clinical Guideline recommended for use For Use in: By: For: Division responsible for document: Key words: Name and job title of document
Norepinephrine (Levophed )
 Norepinephrine (Levophed ) Scope C3IFT CCT Generic Name: Norepinephrine Trade Name: Levophed Chemical Class: Therapeutic Class: Actions: Pharmacokinetics: Vasopressor Vasopressor Mechanism of Action: Norepinephrine
Norepinephrine (Levophed ) Scope C3IFT CCT Generic Name: Norepinephrine Trade Name: Levophed Chemical Class: Therapeutic Class: Actions: Pharmacokinetics: Vasopressor Vasopressor Mechanism of Action: Norepinephrine
Guideline for the use of Clonidine for Sedation in Adult Intensive Care
 Guideline for the use of Clonidine for Sedation in Adult Intensive Care This guidance does not override the individual responsibility of health professionals to make appropriate decision according to the
Guideline for the use of Clonidine for Sedation in Adult Intensive Care This guidance does not override the individual responsibility of health professionals to make appropriate decision according to the
Initiation of Clozapine Treatment Community Patients
 Initiation of Clozapine Treatment Community Patients Who Should Read This Policy Target Audience All clinical staff working in the community N/A N/A Initiation of Clozapine Treatment for Patients in the
Initiation of Clozapine Treatment Community Patients Who Should Read This Policy Target Audience All clinical staff working in the community N/A N/A Initiation of Clozapine Treatment for Patients in the
Cardiac Catheter Labs Intravenous Drug Therapy Guide
 Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Contact Name and Job Title (author) Cardiac Catheter Lab IV Medicines Guideline Helen Buxton ( Senior Cath Lab
Title of Guideline (must include the word Guideline (not protocol, policy, procedure etc) Contact Name and Job Title (author) Cardiac Catheter Lab IV Medicines Guideline Helen Buxton ( Senior Cath Lab
Trust Guideline for the Management of Patient Controlled Analgesia (PCA) in Adults
 Patient Controlled Analgesia (PCA) in Adults A clinical guideline recommended for use For Use in: In all Clinical Areas By: Anaesthetists, Ward Nurses, Recovery Staff Acute Pain Service Staff For: Adult
Patient Controlled Analgesia (PCA) in Adults A clinical guideline recommended for use For Use in: In all Clinical Areas By: Anaesthetists, Ward Nurses, Recovery Staff Acute Pain Service Staff For: Adult
SOP for the Prescribing and Administration of Milrinone on The Children s Heart Centre
 SOP for the Prescribing and Administration of Milrinone on The Children s Heart Centre Version Number 2 Date of Issue July 2017 Reference Number PAMCHC-07-2017-MHPLMLAGOFSSDS-V2 Review Interval Approved
SOP for the Prescribing and Administration of Milrinone on The Children s Heart Centre Version Number 2 Date of Issue July 2017 Reference Number PAMCHC-07-2017-MHPLMLAGOFSSDS-V2 Review Interval Approved
PREOPERATIVE ANAEMIA PATHWAY
 PREOPERATIVE ANAEMIA PATHWAY Surname: Patient ID No. Forename: DOB: / / Age: NHS Number: Likes to be called: Address: Tel. No. Religion/Spirituality: Next of Kin: Name GP Name: GP Practice: Planned Operation:
PREOPERATIVE ANAEMIA PATHWAY Surname: Patient ID No. Forename: DOB: / / Age: NHS Number: Likes to be called: Address: Tel. No. Religion/Spirituality: Next of Kin: Name GP Name: GP Practice: Planned Operation:
Policy REVISED: 6/30/2016 3:30 PM. Applies To: ObGyn Responsible Department: ObGyn Revised: June 30, 2016
 Title: Antihypertensive Treatment for Severe Hypertension During Pregnancy Applies To: ObGyn Responsible Department: ObGyn Revised: June 30, 2016 Policy POLICY STATEMENT: Pregnant or postpartum patients
Title: Antihypertensive Treatment for Severe Hypertension During Pregnancy Applies To: ObGyn Responsible Department: ObGyn Revised: June 30, 2016 Policy POLICY STATEMENT: Pregnant or postpartum patients
PALIPERIDONE LONG ACTING INJECTION PRESCRIBING GUIDELINE. Chief Pharmacist. Chief Pharmacist
 REFERENCE NUMBER: PALIPERIDONE LONG ACTING INJECTION PRESCRIBING GUIDELINE AREA: NAME OF RESPONSIBLE COMMITTEE / INDIVIDUAL NAME OF ORIGINATOR / AUTHOR Trust-wide Chief Pharmacist Chief Pharmacist DATE
REFERENCE NUMBER: PALIPERIDONE LONG ACTING INJECTION PRESCRIBING GUIDELINE AREA: NAME OF RESPONSIBLE COMMITTEE / INDIVIDUAL NAME OF ORIGINATOR / AUTHOR Trust-wide Chief Pharmacist Chief Pharmacist DATE
concentrate intravenous solution and other strong potassium solutions
 Policy for the use of potassium chloride concentrate intravenous solution and other strong potassium solutions CLINICAL GUIDELINES ID TAG Policy for the use of potassium chloride Title: concentrate intravenous
Policy for the use of potassium chloride concentrate intravenous solution and other strong potassium solutions CLINICAL GUIDELINES ID TAG Policy for the use of potassium chloride Title: concentrate intravenous
PHARMACOLOGY AND PHARMACOKINETICS
 DRUG GUIDELINE Insulin, human neutral (Actrapid ) Intravenous Infusion for SCOPE (Area): FOR USE IN: Critical Care Unit, Emergency Department and Operating Suite EXCLUSIONS: Paediatrics (seek Paediatrician
DRUG GUIDELINE Insulin, human neutral (Actrapid ) Intravenous Infusion for SCOPE (Area): FOR USE IN: Critical Care Unit, Emergency Department and Operating Suite EXCLUSIONS: Paediatrics (seek Paediatrician
PAEDIATRIC NERVE BLOCK / WOUND INFILTRATION
 PAEDIATRIC NERVE BLOCK / WOUND INFILTRATION Addendum to the MULTIDISCIPLINARY GUIDELINES FOR ACUTE PAIN MANAGEMENT IN CHILDREN AND YOUNG PEOPLE Policy Owner: Approved by: Ratified by: ABMU HB Pain Management
PAEDIATRIC NERVE BLOCK / WOUND INFILTRATION Addendum to the MULTIDISCIPLINARY GUIDELINES FOR ACUTE PAIN MANAGEMENT IN CHILDREN AND YOUNG PEOPLE Policy Owner: Approved by: Ratified by: ABMU HB Pain Management
Please inform the Diabetes Nurse Specialist that this patient has been admitted within 24hrs of admission.
 Adult Diabetic Ketoacidosis Care Bundle (V1. Issued October 2014 Review October 2015) Improving patient care This pack includes: DKA Management Guideline Name: (Patient Addressograph) DOB: Hospital No:
Adult Diabetic Ketoacidosis Care Bundle (V1. Issued October 2014 Review October 2015) Improving patient care This pack includes: DKA Management Guideline Name: (Patient Addressograph) DOB: Hospital No:
PACKAGE LEAFLET: INFORMATION FOR THE USER. Cisatracurium 2 mg/ml solution for injection/infusion Cisatracurium 5 mg/ml solution for injection/infusion
 PACKAGE LEAFLET: INFORMATION FOR THE USER Cisatracurium 2 mg/ml solution for injection/infusion Cisatracurium 5 mg/ml solution for injection/infusion Cisatracurium Read all of this leaflet carefully before
PACKAGE LEAFLET: INFORMATION FOR THE USER Cisatracurium 2 mg/ml solution for injection/infusion Cisatracurium 5 mg/ml solution for injection/infusion Cisatracurium Read all of this leaflet carefully before
Acutely Painful testes
 2.0 FINAL Guideline adopted from the Bedside Clinical Guideline Partnership EQUALITY IMPACT The Trust strives to ensure equality of opportunity for all both as a major employer and as a provider of health
2.0 FINAL Guideline adopted from the Bedside Clinical Guideline Partnership EQUALITY IMPACT The Trust strives to ensure equality of opportunity for all both as a major employer and as a provider of health
PACKAGE LEAFLET: INFORMATION FOR THE USER. Cisatracurium 2 mg/ml Solution for Injection/Infusion Cisatracurium 5 mg/ml Solution for Injection/Infusion
 PACKAGE LEAFLET: INFORMATION FOR THE USER Cisatracurium 2 mg/ml Solution for Injection/Infusion Cisatracurium 5 mg/ml Solution for Injection/Infusion Cisatracurium Read all of this leaflet carefully before
PACKAGE LEAFLET: INFORMATION FOR THE USER Cisatracurium 2 mg/ml Solution for Injection/Infusion Cisatracurium 5 mg/ml Solution for Injection/Infusion Cisatracurium Read all of this leaflet carefully before
PREOPERATIVE ANAEMIA PATHWAY
 PREOPERATIVE ANAEMIA PATHWAY Surname: Unit No. Forename: DOB: / / Age: NHS Number: Likes to be called: Address: Tel. No. Religion/Spirituality: GP Name: GP Practice: Planned Operation: Postcode: Mobile
PREOPERATIVE ANAEMIA PATHWAY Surname: Unit No. Forename: DOB: / / Age: NHS Number: Likes to be called: Address: Tel. No. Religion/Spirituality: GP Name: GP Practice: Planned Operation: Postcode: Mobile
DRUG GUIDELINE SODIUM NITROPRUSSIDE
 DRUG GUIDELINE SODIUM NITROPRUSSIDE SCOPE (Area): FOR USE IN: Critical Care Unit, ED and Theatre EXCLUSIONS: Paediatrics (seek Paediatrician advice) and General Wards SCOPE (Staff): Medical, Nursing and
DRUG GUIDELINE SODIUM NITROPRUSSIDE SCOPE (Area): FOR USE IN: Critical Care Unit, ED and Theatre EXCLUSIONS: Paediatrics (seek Paediatrician advice) and General Wards SCOPE (Staff): Medical, Nursing and
Controlled Document Number: Version Number: 1. Controlled Document Sponsor: Controlled Document Lead (Author): On: July Review Date: July 2020
 Guidelines for the Use of Naloxone in Palliative Care in Adult Patients CONTROLLED DOCUMENT CATEGORY: CLASSIFICATION: Controlled Document Number: Version Number: 1 Controlled Document Sponsor: Controlled
Guidelines for the Use of Naloxone in Palliative Care in Adult Patients CONTROLLED DOCUMENT CATEGORY: CLASSIFICATION: Controlled Document Number: Version Number: 1 Controlled Document Sponsor: Controlled
SPAGG. Coversheet for Specialist Palliative Audit and Guideline Group Agreed Documentation
 SPAGG Coversheet for Specialist Palliative Audit and Guideline Group Agreed Documentation This sheet is to accompany all documentation agreed by SPAGG. This will assist maintenance of the guidelines as
SPAGG Coversheet for Specialist Palliative Audit and Guideline Group Agreed Documentation This sheet is to accompany all documentation agreed by SPAGG. This will assist maintenance of the guidelines as
1. Intrathecal must never be abbreviated to IT on a prescription form True / False
 Appendix 1: Intrathecal SACT Assessment Questions Core General Questions must be completed by all staff. 1. Intrathecal must never be abbreviated to IT on a prescription form 2. Intrathecal SACT must be
Appendix 1: Intrathecal SACT Assessment Questions Core General Questions must be completed by all staff. 1. Intrathecal must never be abbreviated to IT on a prescription form 2. Intrathecal SACT must be
Patient Group Directions Policy
 Patient Group Directions Policy Category: Summary: Equality Analysis undertaken: Valid From: Date of Next Review: Approval Date/ Via: Distribution: Related Documents: Author(s): Further Information: This
Patient Group Directions Policy Category: Summary: Equality Analysis undertaken: Valid From: Date of Next Review: Approval Date/ Via: Distribution: Related Documents: Author(s): Further Information: This
LRI Children s Hospital
 LRI Children s Hospital Guideline for the Care of Neonates, Children and Young People Requiring Epidural Analgesia Staff relevant to: All Health Professionals who care for neonates, children and young
LRI Children s Hospital Guideline for the Care of Neonates, Children and Young People Requiring Epidural Analgesia Staff relevant to: All Health Professionals who care for neonates, children and young
CLINICAL GUIDELINE FOR INTRAVENOUS FLUID THERAPY FOR ADULTS IN HOSPITAL 1. Aim/Purpose of this Guideline
 CLINICAL GUIDELINE FOR INTRAVENOUS FLUID THERAPY FOR ADULTS IN HOSPITAL 1. Aim/Purpose of this Guideline 1.1. This guideline contains recommendations about general principles for managing intravenous (IV)
CLINICAL GUIDELINE FOR INTRAVENOUS FLUID THERAPY FOR ADULTS IN HOSPITAL 1. Aim/Purpose of this Guideline 1.1. This guideline contains recommendations about general principles for managing intravenous (IV)
September 2014 V0.17. Paediatric Daily Fluid Prescription & Balance Chart
 September 14 V0.17 Aims and outcomes of session. Aim: To provide guidance on correctly completing the paediatric daily fluid prescription & balance chart. Outcomes: Demonstrate the ability to: calculate
September 14 V0.17 Aims and outcomes of session. Aim: To provide guidance on correctly completing the paediatric daily fluid prescription & balance chart. Outcomes: Demonstrate the ability to: calculate
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Medical technologies guidance SCOPE CardioQ-ODM oesophageal Doppler monitor for patients undergoing major or high-risk surgery and patients in critical
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE Medical technologies guidance SCOPE CardioQ-ODM oesophageal Doppler monitor for patients undergoing major or high-risk surgery and patients in critical
Developed By Name Signature Date
 Patient Group Direction 2155 version 2.0 Administration / Supply of Inhaled Salbutamol in Asthma by Registered Practitioners employed by Torbay and South Devon NHS Foundation Trust Date of Introduction:
Patient Group Direction 2155 version 2.0 Administration / Supply of Inhaled Salbutamol in Asthma by Registered Practitioners employed by Torbay and South Devon NHS Foundation Trust Date of Introduction:
CLINICAL GUIDELINE FOR USE OF A PATIENT CONTROLLED ANALGESIA OR INTRAVENOUS OPIATE INFUSION IN CHILD HEALTH. 1. Aim/Purpose of this Guideline
 CLINICAL GUIDELINE FOR USE OF A PATIENT CONTROLLED ANALGESIA OR INTRAVENOUS OPIATE INFUSION IN CHILD HEALTH. 1. Aim/Purpose of this Guideline Guideline for children with a Patient Controlled Analgesia
CLINICAL GUIDELINE FOR USE OF A PATIENT CONTROLLED ANALGESIA OR INTRAVENOUS OPIATE INFUSION IN CHILD HEALTH. 1. Aim/Purpose of this Guideline Guideline for children with a Patient Controlled Analgesia
patient group direction
 CYCLIZINE v01 1/7 CYCLIZINE PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner (Nurse)
CYCLIZINE v01 1/7 CYCLIZINE PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner (Nurse)
Sussex Trauma Network Guidelines for: The Management of Compartment Syndrome
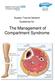 Sussex Trauma Network Guidelines for: The Management of Compartment Syndrome Management of Compartment Syndrome Control Page Version: 1 Category number: Approved by: Date approved: Name of author: and
Sussex Trauma Network Guidelines for: The Management of Compartment Syndrome Management of Compartment Syndrome Control Page Version: 1 Category number: Approved by: Date approved: Name of author: and
ADULT DRUG REFERENCE Drug Indication Adult Dosage Precautions / Comments
 ADENOSINE Paroxysmal SVT 1 st Dose 6 mg rapid IV 2 nd & 3 rd Doses 12 mg rapid IV push Follow each dose with rapid bolus of 20 ml NS May cause transient heart block or asystole. Side effects include chest
ADENOSINE Paroxysmal SVT 1 st Dose 6 mg rapid IV 2 nd & 3 rd Doses 12 mg rapid IV push Follow each dose with rapid bolus of 20 ml NS May cause transient heart block or asystole. Side effects include chest
Core Safety Profile. Pharmaceutical form(s)/strength: Film-coated tablets 1.25 mg, 2.5 mg, 3.75 mg, 5 mg, 7.5 mg and 10 mg. Date of FAR:
 Core Safety Profile Active substance: Bisoprolol Pharmaceutical form(s)/strength: Film-coated tablets 1.25 mg, 2.5 mg, 3.75 mg, 5 mg, 7.5 mg and 10 mg P - RMS: FI/H/PSUR/0002/002 Date of FAR: 13.12.2011
Core Safety Profile Active substance: Bisoprolol Pharmaceutical form(s)/strength: Film-coated tablets 1.25 mg, 2.5 mg, 3.75 mg, 5 mg, 7.5 mg and 10 mg P - RMS: FI/H/PSUR/0002/002 Date of FAR: 13.12.2011
Epidural Infusions for Pain Relief Including Discharge Advice
 Royal Manchester Children s Hospital Epidural Infusions for Pain Relief Including Discharge Advice Children s Pain Team- Information For Parents and Carers This leaflet aims to provide information for
Royal Manchester Children s Hospital Epidural Infusions for Pain Relief Including Discharge Advice Children s Pain Team- Information For Parents and Carers This leaflet aims to provide information for
Bolus (Push Dose) Pressors: Good Idea or a bit Much?
 Bolus (Push Dose) Pressors: Good Idea or a bit Much? Robert Katzer MD MBA FACEP FAEMS Associate Professor, Emergency Medicine University of California, Irvine Pressors: Which Ones Are Out There, What Do
Bolus (Push Dose) Pressors: Good Idea or a bit Much? Robert Katzer MD MBA FACEP FAEMS Associate Professor, Emergency Medicine University of California, Irvine Pressors: Which Ones Are Out There, What Do
PACKAGE LEAFLET: INFORMATION FOR THE USER. Active substance: enoximone
 PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfan Injection 100 mg/20 ml Concentrate for Solution for Injection Active substance: enoximone Read all of this leaflet carefully before you
PACKAGE LEAFLET 1 PACKAGE LEAFLET: INFORMATION FOR THE USER Perfan Injection 100 mg/20 ml Concentrate for Solution for Injection Active substance: enoximone Read all of this leaflet carefully before you
SUMMARY OF PRODUCT CHARACTERISTICS
 Page 1 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Sinora 1 mg/ml concentrate for solution for infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml concentrate for solution
Page 1 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Sinora 1 mg/ml concentrate for solution for infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml concentrate for solution
Pain and Itch Assessment and Management for the Burns Patient (Adults) Type: Clinical guideline Register No: Status: Public
 Pain and Itch Assessment and Management for the Burns Patient (Adults) Type: Clinical guideline Register No: 15025 Status: Public Developed in response to: Best Practice Contributes to CQC Regulation 9,11
Pain and Itch Assessment and Management for the Burns Patient (Adults) Type: Clinical guideline Register No: 15025 Status: Public Developed in response to: Best Practice Contributes to CQC Regulation 9,11
CLINICAL GUIDELINE FOR THE MANAGEMENT OF HYPOKALAEMIA
 POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
POLICY UNDER REVIEW Please note that this policy is under review. It does, however, remain current Trust policy subject to any recent legislative changes, national policy instruction (NHS or Department
Resuscitation Fluids
 Resuscitation Fluids Acceptable Fluids (also known as): Sodium Chloride Hartmann s Solution (Ringer-Lactate Solution, Compound Sodium Lactate) 4.5% Albumin Solution (PPS) Gelofusine 20ml/kg Bolus Can be
Resuscitation Fluids Acceptable Fluids (also known as): Sodium Chloride Hartmann s Solution (Ringer-Lactate Solution, Compound Sodium Lactate) 4.5% Albumin Solution (PPS) Gelofusine 20ml/kg Bolus Can be
Practical considerations in the administration of intravenous vasoactive drugs in the critical care setting Part II How safe is our practice?
 Intensive and Critical Care Nursing (2004) 20, 183 189 ORIGINAL ARTICLE Practical considerations in the administration of intravenous vasoactive drugs in the critical care setting Part II How safe is our
Intensive and Critical Care Nursing (2004) 20, 183 189 ORIGINAL ARTICLE Practical considerations in the administration of intravenous vasoactive drugs in the critical care setting Part II How safe is our
Southern Trust Home IV Service. Guidelines for the administration of IV antibiotics
 Southern Trust Home IV Service Guidelines for the administration of IV antibiotics Title: Author: CLINICAL GUIDELINES ID TAG Antibiotic guidelines - Southern Trust Home IV service guidelines for the administration
Southern Trust Home IV Service Guidelines for the administration of IV antibiotics Title: Author: CLINICAL GUIDELINES ID TAG Antibiotic guidelines - Southern Trust Home IV service guidelines for the administration
Trust Protocol for the Administration of Intravenous Methylprednisolone for Thyroid Eye Disease A Protocol. The Clinical Investigation Unit (CIU)
 A Protocol For Use in: By: For: Division responsible for document: Key words: Name of document author: Job title of document author: Name and job title of document author s Line Manager: Supported by:
A Protocol For Use in: By: For: Division responsible for document: Key words: Name of document author: Job title of document author: Name and job title of document author s Line Manager: Supported by:
Guideline for the Post Operative Management of Women who have received Intrathecal or Epidural Opioid Analgesia for Caesarean Section
 Guideline for the Post Operative Management of Women who have received Intrathecal or Epidural Opioid Analgesia for Caesarean Section Speciality: Maternity Approval Body: Labour Ward Forum Approval Date:
Guideline for the Post Operative Management of Women who have received Intrathecal or Epidural Opioid Analgesia for Caesarean Section Speciality: Maternity Approval Body: Labour Ward Forum Approval Date:
NHS Grampian Staff Guidance For The Management Of Hypomagnesaemia In Adults. Consultation Group: See Page 4. Review Date: June 2021
 NHS...... Grampian NHS Grampian Staff Guidance For The Management Of Hypomagnesaemia In Adults Co-ordinators: Consultation Group: Approver:. Senior Medicines Information Pharmacist See Page 4 Medicine
NHS...... Grampian NHS Grampian Staff Guidance For The Management Of Hypomagnesaemia In Adults Co-ordinators: Consultation Group: Approver:. Senior Medicines Information Pharmacist See Page 4 Medicine
The Newcastle upon Tyne Hospitals NHS Foundation Trust. Pre-filled Patient Controlled Analgesia (PCA) syringes
 The Newcastle upon Tyne Hospitals NHS Foundation Trust Pre-filled Patient Controlled Analgesia (PCA) syringes Version.: 2.2 Effective From: 1 June 2016 Expiry Date: 1 June 2019 Date Ratified: 20 April
The Newcastle upon Tyne Hospitals NHS Foundation Trust Pre-filled Patient Controlled Analgesia (PCA) syringes Version.: 2.2 Effective From: 1 June 2016 Expiry Date: 1 June 2019 Date Ratified: 20 April
Adrenaline 1mg in 10mL (1:10,000) Pre-filled syringe 3 Amiodarone 300mg/10mL Pre-filled syringe 5
 Quick Reference Guide for: Cardiac Arrest Medicines Box (BLUE) Please Note: Any medicines given must form part of an Airway, Breathing, Circulation, Disability and Exposure (ABCDE) Assessment (9)999 must
Quick Reference Guide for: Cardiac Arrest Medicines Box (BLUE) Please Note: Any medicines given must form part of an Airway, Breathing, Circulation, Disability and Exposure (ABCDE) Assessment (9)999 must
Children & Young People s Directorate Paediatric-Neonatal Guidelines Checklist & Version Control Sheet
 1 Children & Young People s Directorate Paediatric-Neonatal Guidelines Checklist & Version Control Sheet 1 Name of Guideline / Policy/ Procedure MANAGEMENT OF ACUTE PAEDIATRIC ASTHMA Purpose of Procedure/
1 Children & Young People s Directorate Paediatric-Neonatal Guidelines Checklist & Version Control Sheet 1 Name of Guideline / Policy/ Procedure MANAGEMENT OF ACUTE PAEDIATRIC ASTHMA Purpose of Procedure/
DIAGNOSIS AND MANAGEMENT OF ACUTE HEART FAILURE
 DIAGNOSIS AND MANAGEMENT OF ACUTE HEART FAILURE Mefri Yanni, MD Bagian Kardiologi dan Kedokteran Vaskular RS.DR.M.Djamil Padang The 3rd Symcard Padang, Mei 2013 Outline Diagnosis Diagnosis Treatment options
DIAGNOSIS AND MANAGEMENT OF ACUTE HEART FAILURE Mefri Yanni, MD Bagian Kardiologi dan Kedokteran Vaskular RS.DR.M.Djamil Padang The 3rd Symcard Padang, Mei 2013 Outline Diagnosis Diagnosis Treatment options
Drugs used in obstetrics
 Drugs used in obstetrics Drugs used in obstetrics Drugs may be used to modify uterine contractions. These include oxytocic drugs used to stimulate uterine contractions both in induction of labour and to
Drugs used in obstetrics Drugs used in obstetrics Drugs may be used to modify uterine contractions. These include oxytocic drugs used to stimulate uterine contractions both in induction of labour and to
Specialist Palliative Care Audit and Guidelines Group (SPAGG)
 Specialist Palliative Care Audit and Guidelines Group (SPAGG) Clinical Guideline for the Prescribing and Administration of Furosemide via continuous subcutaneous infusion (CSCI) for Heart Failure Patients
Specialist Palliative Care Audit and Guidelines Group (SPAGG) Clinical Guideline for the Prescribing and Administration of Furosemide via continuous subcutaneous infusion (CSCI) for Heart Failure Patients
Protocol for the Management of Neutropenic Sepsis in Adult Patients P
 Protocol for the Management of Neutropenic Sepsis in Adult Patients P Version 2 Date April 2015 Review Date April 2016 Author Rachel Onions Change Approver Acute Oncology Team UHMBT. Version 2. CHANGE
Protocol for the Management of Neutropenic Sepsis in Adult Patients P Version 2 Date April 2015 Review Date April 2016 Author Rachel Onions Change Approver Acute Oncology Team UHMBT. Version 2. CHANGE
DATA SHEET. Product Summary. 1. Trade Name of Medicinal Product. Protamine Sulphate Injection BP. 2. Qualitative and Quantitative Composition
 DATA SHEET Product Summary 1. Trade Name of Medicinal Product Protamine Sulphate Injection BP 2. Qualitative and Quantitative Composition Protamine Sulphate 10mg/ml 3. Pharmaceutical Form Solution for
DATA SHEET Product Summary 1. Trade Name of Medicinal Product Protamine Sulphate Injection BP 2. Qualitative and Quantitative Composition Protamine Sulphate 10mg/ml 3. Pharmaceutical Form Solution for
Package leaflet: Information for the user
 Package leaflet: Information for the user Cisatracurium 2 mg/ml Solution for injection/ infusion Cisatracurium 5 mg/ml Solution for injection/ infusion Cisatracurium The name of your medicine are Cisatracurium
Package leaflet: Information for the user Cisatracurium 2 mg/ml Solution for injection/ infusion Cisatracurium 5 mg/ml Solution for injection/ infusion Cisatracurium The name of your medicine are Cisatracurium
MAKING SENSE OF IT ALL AUGUST 17
 MAKING SENSE OF IT ALL AUGUST 17 @SepsisUK Dr Ron Daniels B.E.M. CEO, UK Sepsis Trust CEO, Global Sepsis Alliance Special Adviser to WHO SCALE AND BURDEN @sepsisuk Dr Ron Daniels B.E.M. CEO, UK Sepsis
MAKING SENSE OF IT ALL AUGUST 17 @SepsisUK Dr Ron Daniels B.E.M. CEO, UK Sepsis Trust CEO, Global Sepsis Alliance Special Adviser to WHO SCALE AND BURDEN @sepsisuk Dr Ron Daniels B.E.M. CEO, UK Sepsis
Introduction. Invasive Hemodynamic Monitoring. Determinants of Cardiovascular Function. Cardiovascular System. Hemodynamic Monitoring
 Introduction Invasive Hemodynamic Monitoring Audis Bethea, Pharm.D. Assistant Professor Therapeutics IV January 21, 2004 Hemodynamic monitoring is necessary to assess and manage shock Information obtained
Introduction Invasive Hemodynamic Monitoring Audis Bethea, Pharm.D. Assistant Professor Therapeutics IV January 21, 2004 Hemodynamic monitoring is necessary to assess and manage shock Information obtained
A step-by-step preparation guide
 A step-by-step preparation guide For needle and needle-free systems This guide provides detailed instructions on the reconstitution, dilution, and storage of VELETRI. It is intended to be used after your
A step-by-step preparation guide For needle and needle-free systems This guide provides detailed instructions on the reconstitution, dilution, and storage of VELETRI. It is intended to be used after your
LEVOPHED TM 1:1000. Name of medicine. Presentation LEVOPHED TM 1:1000 is a sterile noradrenaline concentrated solution for injection.
 LEVOPHED TM 1:1000 Name of medicine LEVOPHED TM 1:1000 Presentation LEVOPHED TM 1:1000 is a sterile noradrenaline concentrated solution for injection. Each ampoule contains noradrenaline 2 mg in 2 ml (1:1000),
LEVOPHED TM 1:1000 Name of medicine LEVOPHED TM 1:1000 Presentation LEVOPHED TM 1:1000 is a sterile noradrenaline concentrated solution for injection. Each ampoule contains noradrenaline 2 mg in 2 ml (1:1000),
- Arterial line safety Learning from a serious untoward incident on the Intensive Care Unit.
 - Arterial line safety Learning from a serious untoward incident on the Intensive Care Unit. Margi Jenkins Ex-Senior Sister Intensive care unit Royal United Hospital Bath Specialist Nurse Organ Donation
- Arterial line safety Learning from a serious untoward incident on the Intensive Care Unit. Margi Jenkins Ex-Senior Sister Intensive care unit Royal United Hospital Bath Specialist Nurse Organ Donation
Patient Controlled Analgesia/Intravenous Opiate Infusion in Child Health Clinical Guideline V4.0 October 2018
 Patient Controlled Analgesia/Intravenous Opiate Infusion in Child Health Clinical Guideline V4.0 October 2018 Page 1 of 12 1. Aim/Purpose of this Guideline Guideline for children with a Patient Controlled
Patient Controlled Analgesia/Intravenous Opiate Infusion in Child Health Clinical Guideline V4.0 October 2018 Page 1 of 12 1. Aim/Purpose of this Guideline Guideline for children with a Patient Controlled
IV Referral Standards for OPAT Services
 pecialist harmacy ervice Medicines Use and afety IV Referral tandards for OAT ervices andra Wolper Associate Director Medicines Use and afety Division pecialist harmacy ervices pecialist harmacy ervice
pecialist harmacy ervice Medicines Use and afety IV Referral tandards for OAT ervices andra Wolper Associate Director Medicines Use and afety Division pecialist harmacy ervices pecialist harmacy ervice
NHS Grampian Staff Guidance For The Management Of Hypomagnesaemia In Adults
 NHS Grampian Staff Guidance For The Management Of Hypomagnesaemia In Adults Co-ordinators: Sarah Gethings Medicine Information Pharmacist Consultation Group: See Page 4 Approver: Medicine Guidelines and
NHS Grampian Staff Guidance For The Management Of Hypomagnesaemia In Adults Co-ordinators: Sarah Gethings Medicine Information Pharmacist Consultation Group: See Page 4 Approver: Medicine Guidelines and
patient group direction
 PRESERVATIVE-FREE LIDOCAINE v1.0 1/9 PRESERVATIVE-FREE LIDOCAINE PGD Details Version 1.0 Legal category Staff grades Approved by POM Registered Paramedic Registered Nurse Medicines Management Group Date
PRESERVATIVE-FREE LIDOCAINE v1.0 1/9 PRESERVATIVE-FREE LIDOCAINE PGD Details Version 1.0 Legal category Staff grades Approved by POM Registered Paramedic Registered Nurse Medicines Management Group Date
ADVERSE REACTIONS Most common adverse reactions during treatment: nausea, vomiting, and tachycardia. (6)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AKOVAZ safely and effectively. See full prescribing information for AKOVAZ. AKOVAZ (ephedrine sulfate
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AKOVAZ safely and effectively. See full prescribing information for AKOVAZ. AKOVAZ (ephedrine sulfate
A step-by-step preparation guide
 A step-by-step preparation guide This guide provides detailed instruction on the reconstitution, dilution, and storage of Veletri (epoprostenol) for Injection. It is intended to be used after your healthcare
A step-by-step preparation guide This guide provides detailed instruction on the reconstitution, dilution, and storage of Veletri (epoprostenol) for Injection. It is intended to be used after your healthcare
NECN CHEMOTHERAPY HANDBOOK PROTOCOL
 DRUG ADMINISTRATION SCHEDULE Day Drug Dose Route Diluent Rate 1* to 5 Prednisolone 40mg/m 2 Oral Once Daily For 5 days 1 Paracetamol 1gram Oral Once Only Chlorphenamine 10mg IV bolus Ondansetron 8mg IV
DRUG ADMINISTRATION SCHEDULE Day Drug Dose Route Diluent Rate 1* to 5 Prednisolone 40mg/m 2 Oral Once Daily For 5 days 1 Paracetamol 1gram Oral Once Only Chlorphenamine 10mg IV bolus Ondansetron 8mg IV
Staff at the Nottingham Children s Hospital. Guidelines process.
 Diabetes and Surgery Title of Guideline Contact Name and Job Title (author) Guideline for the management of children and young people with diabetes aged 18 or under requiring surgery Dr Priyha Santhanam,
Diabetes and Surgery Title of Guideline Contact Name and Job Title (author) Guideline for the management of children and young people with diabetes aged 18 or under requiring surgery Dr Priyha Santhanam,
POLICY DOCUMENT. Pharmacy MMG/MPG. Approved By and Date Medicines Management roup March March 2016
 POLICY DOCUMENT Document Title High dose and combination antipsychotic guidance Reference Number n/a Policy Type Prescribing and Treatment Guideline Electronic File/Location Clinical Resources/Pharmacy/Prescribing
POLICY DOCUMENT Document Title High dose and combination antipsychotic guidance Reference Number n/a Policy Type Prescribing and Treatment Guideline Electronic File/Location Clinical Resources/Pharmacy/Prescribing
Users of this standard will need to ensure that practice reflects up-to-date information and policies.
 About this Unit This standard relates specifically to the insertion of intravenous cannula to facilitate access to the blood system for treatment or diagnostic purposes. Access may be required for serial
About this Unit This standard relates specifically to the insertion of intravenous cannula to facilitate access to the blood system for treatment or diagnostic purposes. Access may be required for serial
GUIDELINES FOR WEIGHT-BASED DOSING AND INFUSION
 GUIDELINES FOR WEIGHT-BASED DOSING AND INFUSION Includes Example dose calculation wheel Preparation and administration information for healthcare professionals Please see enclosed full Prescribing Information,
GUIDELINES FOR WEIGHT-BASED DOSING AND INFUSION Includes Example dose calculation wheel Preparation and administration information for healthcare professionals Please see enclosed full Prescribing Information,
Package leaflet: Information for the user Efedrin Stragen 3mg/ml, solution for injection Ephedrine hydrochloride
 Package leaflet: Information for the user Efedrin Stragen 3mg/ml, solution for injection Ephedrine hydrochloride Read all of this leaflet carefully before you start using this medicine because it contains
Package leaflet: Information for the user Efedrin Stragen 3mg/ml, solution for injection Ephedrine hydrochloride Read all of this leaflet carefully before you start using this medicine because it contains
GUIDELINE FOR RECOVERY ROOM MANAGEMENT OF PATIENTS AFTER CAROTID ENDARTERECTOMY
 GUIDELINE FOR RECOVERY ROOM MANAGEMENT OF PATIENTS AFTER CAROTID ENDARTERECTOMY Full Title of Guideline: Author (include email and role): Guideline for Recovery Room Management of Patients after Carotid
GUIDELINE FOR RECOVERY ROOM MANAGEMENT OF PATIENTS AFTER CAROTID ENDARTERECTOMY Full Title of Guideline: Author (include email and role): Guideline for Recovery Room Management of Patients after Carotid
Standard Operating Procedure (SOP) Management of intervention group patients SOP 001
 ` Standard Operating Procedure (SOP) Management of intervention group patients SOP 001 Authors: Mark Edwards & Rupert Pearse Authorisation: Rupert Pearse (Chief Investigator) Scope To provide guidance
` Standard Operating Procedure (SOP) Management of intervention group patients SOP 001 Authors: Mark Edwards & Rupert Pearse Authorisation: Rupert Pearse (Chief Investigator) Scope To provide guidance
Shared Care Guideline
 Shared Care Guideline Midodrine for Orthostatic hypotension and neurocardiogenic syncope Executive Summary Update of Guideline following licencing of drug. The responsibility for initiating midodrine will
Shared Care Guideline Midodrine for Orthostatic hypotension and neurocardiogenic syncope Executive Summary Update of Guideline following licencing of drug. The responsibility for initiating midodrine will
Troubleshooting Audio
 Welcome! Audio for this event is available via ReadyTalk Internet Streaming. No telephone line is required. Computer speakers or headphones are necessary to listen to streaming audio. Limited dial-in lines
Welcome! Audio for this event is available via ReadyTalk Internet Streaming. No telephone line is required. Computer speakers or headphones are necessary to listen to streaming audio. Limited dial-in lines
SALBUTAMOL INHALER PATIENT GROUP DIRECTION CHILD HEALTH 1. Aim/Purpose of this Guideline
 SALBUTAMOL INHALER PATIENT GROUP DIRECTION CHILD HEALTH 1. Aim/Purpose of this Guideline 1.1. This Patient Group Direction (PGD) applies to all nursing and clinical staff in the Child Health Department
SALBUTAMOL INHALER PATIENT GROUP DIRECTION CHILD HEALTH 1. Aim/Purpose of this Guideline 1.1. This Patient Group Direction (PGD) applies to all nursing and clinical staff in the Child Health Department
Intravenous Iloprost Guidelines. November 2020
 Index No: MMG31 (Formerly MM31) Intravenous Iloprost Guidelines Version: 3.0 Date ratified: Ratified by: (Name of Committee) Name of originator/author, job title and department: November 2017 Medicines
Index No: MMG31 (Formerly MM31) Intravenous Iloprost Guidelines Version: 3.0 Date ratified: Ratified by: (Name of Committee) Name of originator/author, job title and department: November 2017 Medicines
GUIDELINE FOR THE POST OPERATIVE MANAGEMENT OF WOMEN WHO HAVE RECEIVED INTRATHECAL OR EPIDURAL OPIOID ANALGESIA FOR CAESAREAN SECTION
 GUIDELINE FOR THE POST OPERATIVE MANAGEMENT OF WOMEN WHO HAVE RECEIVED INTRATHECAL OR EPIDURAL OPIOID ANALGESIA FOR CAESAREAN SECTION Originator: Maternity Services & Anaesthetics Dept Date Approved: January
GUIDELINE FOR THE POST OPERATIVE MANAGEMENT OF WOMEN WHO HAVE RECEIVED INTRATHECAL OR EPIDURAL OPIOID ANALGESIA FOR CAESAREAN SECTION Originator: Maternity Services & Anaesthetics Dept Date Approved: January
Guideline for the Management of patients with Regular Narrow-Complex Tachyarrhythmia
 Guideline for the Management of patients with Regular Narrow-Complex Tachyarrhythmia This guidance does not override the individual responsibility of health professionals to make appropriate decision according
Guideline for the Management of patients with Regular Narrow-Complex Tachyarrhythmia This guidance does not override the individual responsibility of health professionals to make appropriate decision according
Public Assessment Report Scientific discussion. Nepipe Junior, Nepipe (former Adrenalin MEDA) (adrenaline) SE/H/1287/01-02/DC
 Public Assessment Report Scientific discussion Nepipe Junior, Nepipe (former Adrenalin MEDA) (adrenaline) SE/H/1287/01-02/DC This module reflects the scientific discussion for the approval of Nepipe Junior
Public Assessment Report Scientific discussion Nepipe Junior, Nepipe (former Adrenalin MEDA) (adrenaline) SE/H/1287/01-02/DC This module reflects the scientific discussion for the approval of Nepipe Junior
1. NAME OF THE MEDICINAL PRODUCT. Vicks Sinex, 0.5 mg/ml, nasal spray solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 1. NAME OF THE MEDICINAL PRODUCT Vicks Sinex, 0.5 mg/ml, nasal spray solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Oxymetazoline hydrochloride 0.5 mg/ml 1 spray (50 l) contains approximately 25
1. NAME OF THE MEDICINAL PRODUCT Vicks Sinex, 0.5 mg/ml, nasal spray solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Oxymetazoline hydrochloride 0.5 mg/ml 1 spray (50 l) contains approximately 25
Acute Stroke with Alteplase Administration Order Set
 Review Due Date: 2017 October PATIENT CARE DERS Weight: Adverse Reactions or Intolerances Drug No Yes (list) Food No Yes (list) _ Latex No Yes Admission Admit to Neurology service: Dr. Critical Care Diagnosis:
Review Due Date: 2017 October PATIENT CARE DERS Weight: Adverse Reactions or Intolerances Drug No Yes (list) Food No Yes (list) _ Latex No Yes Admission Admit to Neurology service: Dr. Critical Care Diagnosis:
PACKAGE LEAFLET: INFORMATION FOR THE USER. Dopamine 40 mg/ml Sterile Concentrate
 PACKAGE LEAFLET: INFORMATION FOR THE USER Dopamine 40 mg/ml Sterile Concentrate Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need to read it again.
PACKAGE LEAFLET: INFORMATION FOR THE USER Dopamine 40 mg/ml Sterile Concentrate Read all of this leaflet carefully before you start using this medicine. Keep this leaflet. You may need to read it again.
Medical management of Hypercyanotic spells in neonates, infants with Tetralogy of Fallot
 Medical management of Hypercyanotic spells in neonates, infants with Tetralogy of Fallot Version: 3 Approval Committee (e.g. Clinical network): Date of Approval: 13 June 2018 Signature of approving Group
Medical management of Hypercyanotic spells in neonates, infants with Tetralogy of Fallot Version: 3 Approval Committee (e.g. Clinical network): Date of Approval: 13 June 2018 Signature of approving Group
