News Release. Merck KGaA, Darmstadt, Germany, Presents New Osteoarthritis Data at OARSI 2018 World Congress. April 17, 2018
|
|
|
- Natalie Ryan
- 5 years ago
- Views:
Transcription
1 News Release Your Contact Brenda Mulligan +1 (978) April 17, 2018 Merck KGaA, Darmstadt, Germany, Presents New Osteoarthritis Data at OARSI 2018 World Congress Company to present 16 abstracts highlighting the momentum of its progress in osteoarthritis (OA) research and showcasing the company s leading OA pipeline Oral presentations on sprifermin offer further insights supporting its dose-response structural effect in patients with knee OA, observed in earlier studies Darmstadt, Germany, April 16, 2018 Merck KGaA, Darmstadt Germany, a leading science and technology company which operates its healthcare business in the U.S. and Canada as EMD Serono, today announced 16 abstracts, including two oral presentations, will be presented at the Osteoarthritis Research Society International (OARSI) 2018 World Congress, held April 26-29, 2018 in Liverpool, United Kingdom. The presence of Merck KGaA, Darmstadt, Germany at OARSI reflects the company s dedication to helping optimize outcomes for patients living with chronic progressive diseases, with the goal of developing novel disease-modifying therapies for osteoarthritis (OA). Data of note includes an oral presentation of the three-year analysis of FORWARD, a five-year, multicenter Phase II study of sprifermin in OA of the knee. Results were consistent with the two-year results, which showed a statistically significant dosedependent increase in cartilage thickness in total, lateral and medial femorotibial compartments as compared to placebo treatment, based on quantitative magnetic resonance imaging (qmri). At year three, which was eighteen months after the last treatment cycle, cartilage thickness declined in all treatment arms as compared to year two. However, the difference observed at year two with sprifermin at the Page 1 of 5 Frankfurter Strasse Darmstadt Germany Hotline Head Media Relations Spokesperson: / / Fax media.relations@emdgroup.com
2 highest dose and frequency versus placebo was maintained at year three. The safety profile at year three was consistent with results observed at year two, where treatment emergent adverse events were balanced between groups and musculoskeletal and connective tissue disorders the most common. These data suggest the structural benefit of sprifermin at the highest dose was maintained in the third year and its long-term potential as a disease-modifying treatment for osteoarthritis will continue to be explored," said Dr. Marc C. Hochberg, primary investigator of the FORWARD study and Division Head, Rheumatology and Clinical Immunology, University of Maryland School of Medicine. "Osteoarthritis impacts an estimated 10 percent of the world s population over the age of 60 1 and represents an area of high unmet need for disease-modifying treatment options. A second oral presentation features the results of an ex vivo study that showed sprifermin induced extra-cellular matrix remodelling and cartilage regeneration. In the study, long-term treatment with sprifermin continuously increased metabolic activity and type II collagen formation in human OA articular cartilage compared with placebo. We are committed to helping patients with osteoarthritis by elevating our understanding of the disease and continuing to invest in highly-targeted therapies, said Luciano Rossetti, Executive Vice President, Global Head of Research & Development at the biopharma business of Merck KGaA, Darmstadt Germany. Our intent is to provide true advancement to the field of osteoarthritis by developing therapeutic options with disease-modifying potential. Additional presentations include: pre-clinical data for M6495, an ADAMTS-5 inhibiting nanobody moving into Phase I clinical development for OA; pre-clinical data for M1673, a GDF5 mutant for the potential treatment of OA; and research related to improving measures and patient recruitment in OA studies. Accepted abstracts at the OARSI 2018 World Congress include: Title Sprifermin Efficacy and Safety of Intra- Articular Sprifermin in Presenting Author Abstract Number Presentation Date/Time M Hochberg 32 Friday, April 27, 2:30 PM 4:00 PM Session Type/Title Concurrent Session 3 Page 2 of 5
3 Symptomatic Radiographic Knee Osteoarthritis: Pre- Specified Analysis of 3-Year Data From a 5-Year Randomized, Placebo- Controlled, Phase II Study with a 2 Year Treatment Phase Articular Cartilage from OA Patients Show Extracellular Matrix Remodelling Over the Course of Treatment with Sprifermin (Recombinant Human Fibroblast Growth Factor 18) Intra-Articular Sprifermin Reduces Cartilage Loss in Addition to Increasing Cartilage Gain Independent of Femorotibial Location: A Post Hoc Analysis of a Randomized, Placebo-Controlled Phase II Clinical Trial M6495 (ADAMTS-5) In Vitro Characterization of the ADAMTS-5 Specific Nanobody M6495 Structural and Symptomatic Benefit of a Half-Live Extended Systemically Applied Anti- ADAMTS-5 Inhibitor M6495 Pharmacokinetic and Pharmacodynamic Modelling of the Novel Anti-ADAMTS-5 Nanobody M6495 Using the Neo-Epitope Args as a Biomarker The Anti-ADAMTS-5 Nanobody, M6495, Protects Against Cartilage Breakdown in Cartilage and Synovial Joint Tissue Explant Models Study Design of a Phase I, Placebo-Controlled, First-In- Human Trial To Assess Safety and Tolerability, A Bay-Jensen 65 10:45 AM 12:15 PM F Eckstein 551 Friday, April 27, D Werkmann 346 Friday, April 27, C Brenneis 563 Friday, April 27, J Pereira 343 Friday, April 27, A Siebuhr 363 Friday, April 27, A Bihlet 522 Friday, April 27, OA Clinical Trials and Treatment (Oral) Plenary Session 5 Growth Factors in OA: Opportunities for Intervention (Oral) Page 3 of 5
4 Immunogenecity, and Pharmacokinetics and Pharmacodynamics of Single Ascending Doses of the Anti- ADAMTS-5 Nanobody, M6495, in Healthy Male Subjects M1673 (GDF5 mutant) M1673 (GDF5 mutant) Increases Matrix Production in Primary Porcine and Human Osteoarthritic Chondrocytes A GDF5 Mutant Induces Chondrogenesis in Mesenchymal Stem Cells Similarly to GDF5 Wildtype But Shows a Decreased Osteogenic Potential Osteoarthritis Research C1M, C2M, C3M, PRO-C2, and CRPM in Serum Reflect Different Potential Pathogenetic Domains of Osteoarthritis, Data from Check Recruitment Procedure Maximising Inclusion of Progressors in OA Clinical Studies Based on Existing Cohorts: Approach-Consortium Data Analysis Data Harmonisation for Machine Learning Model in APPROACH-consortium: Development to Predict Osteoarthritis Progression in Patients across Populations "APPROACH Study: A 2-Year, European, Cohort Study to Describe, Validate and Predict Phenotypes of Knee Osteoarthritis By Use Of Clinical, Imaging and Biochemical Markers Two Year Tibiofemoral Joint Cartilage Loss is Weakly Correlated With Increased Pain Among Knees With Lower Baseline Cartilage Thickness T Mang 138 Friday, April 27, T Mang 125 Friday, April 27, A Bay-Jensen 349 Friday, April 27, P Widera 521 Friday, April 27, P Widera 519 Friday, April 27, E van Helvoort 515 Friday, April 27, C Kwoh 474 Friday, April 27, Page 4 of 5
5 Pain Medication Reporting and Patient-Reported Outcomes in the Years Prior to Knee Replacement: Challenges to Assessing Symptomatic Experiences C Kwoh 455 Friday, April 27, For more information about the data to be presented, please access the OARSI app. Also, visit the Merck KGaA, Darmstadt Germany booth at this year s Congress to learn more about the company s commitment to advancing innovation in OA. About Sprifermin Sprifermin is in clinical development to investigate its potential as a treatment for osteoarthritis (OA) of the knee. It is a truncated recombinant human FGF-18 protein thought to induce chondrocyte proliferation and increased extra-cellular matrix (ECM) production, with the potential of promoting cartilage growth and repair. Sprifermin is currently in Phase II studies. About M6495 M6495 is in clinical development to investigate its potential as a treatment for osteoarthritis (OA). Administered subcutaneously, M6495 is a selective nanobody thought to inhibit a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5), a metalloproteinase crucial for cartilage matrix destruction as an early and key event in developing OA, with the potential for providing structural improvement and rapid pain relief for all OA joints. M6495 is currently being evaluated in a first-in-man Phase I study in healthy subjects. All Merck KGaA, Darmstadt, Germany, press releases are distributed by at the same time they become available on the EMD Group Website. In case you are a resident of the USA or Canada please go to to register again for your online subscription of this service as our newly introduced geo-targeting requires new links in the . You may later change your selection or discontinue this service. About Merck KGaA, Darmstadt, Germany Merck KGaA, Darmstadt, Germany, is a leading science and technology company in healthcare, life science and performance materials. More than 52,000 employees work to further develop technologies that improve and enhance life from biopharmaceutical therapies to treat cancer or multiple sclerosis, cutting-edge systems for scientific research and production, to liquid crystals for smartphones and LCD televisions. In 2017, Merck KGaA, Darmstadt, Germany, generated sales of 15.3 billion in 66 countries. Founded in 1668, Merck KGaA, Darmstadt, Germany, is the world's oldest pharmaceutical and chemical company. The founding family remains the majority owner of the publicly listed corporate group. Merck KGaA, Darmstadt, Germany, holds the global rights to the Merck name and brand. The only exceptions are the United States and Canada, where the company operates as EMD Serono, MilliporeSigma and EMD Performance Materials. 1 Arthritis Facts & Figures. Arthritis Foundation. Arthritis/arthritis-facts-stats-figures.pdf. Accessed 12 March Page 5 of 5
Pursuing Novel Pathways in Immunology: Merck KGaA, Darmstadt, Germany Presents New Clinical Data at 2017 ACR/ARHP Annual Meeting
 News Release Your Contact Brenda Mulligan +1 (978) 821-5345 October 20, 2017 ACR Abstract # Atacicept: 889, 2585, 2586, 2610; Sprifermin: 1207, 2183, 1L; Abituzumab: 774; Evobrutinib: 2565; Discovery Products:
News Release Your Contact Brenda Mulligan +1 (978) 821-5345 October 20, 2017 ACR Abstract # Atacicept: 889, 2585, 2586, 2610; Sprifermin: 1207, 2183, 1L; Abituzumab: 774; Evobrutinib: 2565; Discovery Products:
Pursuing Novel Pathways in Immunology: Merck Presents New Clinical Data at 2017 ACR/ARHP Annual Meeting
 Your Contact Brenda Mulligan +1 (978) 821-5345 October 20, 2017 ACR Abstract # Atacicept: 889, 2585, 2586, 2610; Sprifermin: 1207, 2183, 1L; Abituzumab: 774; Evobrutinib: 2565; Discovery Products: 25,
Your Contact Brenda Mulligan +1 (978) 821-5345 October 20, 2017 ACR Abstract # Atacicept: 889, 2585, 2586, 2610; Sprifermin: 1207, 2183, 1L; Abituzumab: 774; Evobrutinib: 2565; Discovery Products: 25,
News Release. March 29, 2019
 Your Contact Alice McGrail +1 781 738 8791 Investor Relations +49 6151 72 3321 March 29, 2019 FDA Approves MAVENCLAD (Cladribine) Tablets as First and Only Short-Course Oral Treatment for Relapsing-Remitting
Your Contact Alice McGrail +1 781 738 8791 Investor Relations +49 6151 72 3321 March 29, 2019 FDA Approves MAVENCLAD (Cladribine) Tablets as First and Only Short-Course Oral Treatment for Relapsing-Remitting
Merck KGaA, Darmstadt, Germany, Receives EU-Approval to Extend Kuvan Use to Children with PKU Below 4 Years of Age
 Your Contact News Release Gangolf Schrimpf +49 6151 72-9591 Investor Relations +49 6151 72-3321 July 20, 2015 Merck KGaA, Darmstadt, Germany, Receives EU-Approval to Extend Kuvan Use to Children with PKU
Your Contact News Release Gangolf Schrimpf +49 6151 72-9591 Investor Relations +49 6151 72-3321 July 20, 2015 Merck KGaA, Darmstadt, Germany, Receives EU-Approval to Extend Kuvan Use to Children with PKU
European Commission Grants Approval for Mavenclad (Cladribine Tablets)
 Your Contact Friederike Segeberg +49 6151 72-6328 Investor Relations +49 6151 72-3321 August 25, 2017 European Commission Grants Approval for Mavenclad (Cladribine Tablets) First oral short-course treatment
Your Contact Friederike Segeberg +49 6151 72-6328 Investor Relations +49 6151 72-3321 August 25, 2017 European Commission Grants Approval for Mavenclad (Cladribine Tablets) First oral short-course treatment
Data on Investigational Cladribine Tablets and Rebif (interferon beta-1a) in Relapsing Forms of Multiple Sclerosis (MS) to be Presented at EAN 2018
 Your Contact Erin-Marie Beals +1 781-681-2850 June 14, 2018 Data on Investigational Cladribine Tablets and Rebif (interferon beta-1a) in Relapsing Forms of Multiple Sclerosis (MS) to be Presented at EAN
Your Contact Erin-Marie Beals +1 781-681-2850 June 14, 2018 Data on Investigational Cladribine Tablets and Rebif (interferon beta-1a) in Relapsing Forms of Multiple Sclerosis (MS) to be Presented at EAN
Your Contacts. EMA ODD is an important regulatory milestone for avelumab in metastatic Merkel cell carcinoma (MCC)
 Your Contacts News Release Merck KGaA, Darmstadt, Germany Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 Pfizer Inc., New York, USA Media: Lisa O Neill +44 1737 331536 Investor
Your Contacts News Release Merck KGaA, Darmstadt, Germany Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 Pfizer Inc., New York, USA Media: Lisa O Neill +44 1737 331536 Investor
News Release. December 9, Not intended for UK-based media
 Your Contacts News Release Merck Media: Gangolf Schrimpf +49 6151 72-9591 Investor Relations: +49 6151 72-3321 Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160
Your Contacts News Release Merck Media: Gangolf Schrimpf +49 6151 72-9591 Investor Relations: +49 6151 72-3321 Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160
Merck KGaA, Darmstadt, Germany, and Pfizer to Present Avelumab Data in Seven Different Cancers at ASCO Annual Meeting
 News Release Merck KGaA, Darmstadt, Germany Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 Pfizer Inc., New York, USA Media: Sally Beatty +1 212 733 6566 Investor Relations:
News Release Merck KGaA, Darmstadt, Germany Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 Pfizer Inc., New York, USA Media: Sally Beatty +1 212 733 6566 Investor Relations:
ASCO Abstract # Avelumab: 4009, TPS4134, TPS4135, 9036, TPS9105, 3055, TPS3106, 8503, 4514, 4516, 5533, TPS5600, TPS4580, 9508; tepotinib: 4072
 Your Contact Gangolf Schrimpf +49 6151 72-9591 ASCO Abstract Avelumab: 4009, TPS4134, TPS4135, 9036, TPS9105, 3055, TPS3106, 8503, 4514, 4516, 5533, TPS5600, TPS4580, 9508; tepotinib: 4072 May 18, 2016
Your Contact Gangolf Schrimpf +49 6151 72-9591 ASCO Abstract Avelumab: 4009, TPS4134, TPS4135, 9036, TPS9105, 3055, TPS3106, 8503, 4514, 4516, 5533, TPS5600, TPS4580, 9508; tepotinib: 4072 May 18, 2016
News Release. Merck and Pfizer Advance Clinical Development Program with Two Additional Phase III Trials of Avelumab.
 Your Contacts News Release Merck Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160
Your Contacts News Release Merck Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160
News Release. November 4, 2016
 Your Contact Grace Mukasa +254 711 794 081 November 4, 2016 Merck More than a Mother addresses infertility challenges and solutions in Africa in partnership with International Federation Fertility Societies
Your Contact Grace Mukasa +254 711 794 081 November 4, 2016 Merck More than a Mother addresses infertility challenges and solutions in Africa in partnership with International Federation Fertility Societies
Not intended for UK based media. Merck Announces Detailed 26-Week Results from Phase IIIb Study with Kuvan in children with PKU below 4 years of age
 Your Contact News Release Bettina Frank Phone +49 6151 72-4660 Not intended for UK based media Merck Announces Detailed 26-Week Results from Phase IIIb Study with Kuvan in children with PKU below 4 years
Your Contact News Release Bettina Frank Phone +49 6151 72-4660 Not intended for UK based media Merck Announces Detailed 26-Week Results from Phase IIIb Study with Kuvan in children with PKU below 4 years
Your contact. Merck and Pfizer to Present Data at ASCO for Avelumab, an Investigational Anti-PD-L1 Antibody
 Your contact Merck Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 News Release Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160 May
Your contact Merck Media: Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 News Release Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160 May
Discovery of a Small Molecule Inhibitor of the Wnt Pathway (SM04690) as a Potential Disease Modifying Treatment for Knee Osteoarthritis
 Discovery of a Small Molecule Inhibitor of the Wnt Pathway (SM469) as a Potential Disease Modifying Treatment for Knee Osteoarthritis Vishal Deshmukh, Ph.D., Charlene Barroga, Ph.D., Yong Hu, Ph.D., John
Discovery of a Small Molecule Inhibitor of the Wnt Pathway (SM469) as a Potential Disease Modifying Treatment for Knee Osteoarthritis Vishal Deshmukh, Ph.D., Charlene Barroga, Ph.D., Yong Hu, Ph.D., John
Global Strategic Partners Merck and Pfizer Finalize Agreement to Co-Promote XALKORI (crizotinib)
 Merck Media: Markus Talanow +49 6151 72 7144 Investor Relations: +49 6151 72 3321 Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160 News Release April 7, 2015 Not
Merck Media: Markus Talanow +49 6151 72 7144 Investor Relations: +49 6151 72 3321 Pfizer Media: Sally Beatty +1 212 733 6566 Investor Relations: Ryan Crowe +1 212 733 8160 News Release April 7, 2015 Not
News Release. February 15, Not intended for UK-based media
 Your Contacts Merck Media Friederike Segeberg +49 6151 72 6328 Investor Relations +49 6151 72 3321 Pfizer Media (US) Sally Beatty +1 212 733 6566 Investor Relations Ryan Crowe +1 212 733 8160 February
Your Contacts Merck Media Friederike Segeberg +49 6151 72 6328 Investor Relations +49 6151 72 3321 Pfizer Media (US) Sally Beatty +1 212 733 6566 Investor Relations Ryan Crowe +1 212 733 8160 February
Merck KGaA, Darmstadt, Germany: Driving Innovation in Cancer Care at ESMO 2017 With New Data in Hard-to-Treat Cancers
 News Release Your Contact Martina Brunner +49 6151 724 3959 Not intended for UK-based media Embargoed until 00:05 CEST, August 31, 2017 ESMO 2017 abstract # Erbitux : 576P, 593P, 1068P, 1579P; avelumab:
News Release Your Contact Martina Brunner +49 6151 724 3959 Not intended for UK-based media Embargoed until 00:05 CEST, August 31, 2017 ESMO 2017 abstract # Erbitux : 576P, 593P, 1068P, 1579P; avelumab:
News Release. Merck: Driving Innovation in Cancer Care at ESMO 2017 With New Data in Hard-to-Treat Cancers. August 31, 2017
 Your Contact Martina Brunner +49 6151 724 3959 August 31, 2017 Not intended for U.K. or U.S. based media ESMO 2017 abstract # Erbitux : 576P, 593P, 1068P, 1579P; avelumab: 1227P, 913P, 1377TiP, 882P, 856P;
Your Contact Martina Brunner +49 6151 724 3959 August 31, 2017 Not intended for U.K. or U.S. based media ESMO 2017 abstract # Erbitux : 576P, 593P, 1068P, 1579P; avelumab: 1227P, 913P, 1377TiP, 882P, 856P;
Discovery of a Small Molecule Inhibitor of the Wnt Pathway as a Potential Disease Modifying Treatment for Knee Osteoarthritis
 Discovery of a Small Molecule Inhibitor of the Wnt Pathway as a Potential Disease Modifying Treatment for Knee Osteoarthritis Charlene Barroga, Ph.D., Yong Hu, Ph.D., Vishal Deshmukh, Ph.D., and John Hood,
Discovery of a Small Molecule Inhibitor of the Wnt Pathway as a Potential Disease Modifying Treatment for Knee Osteoarthritis Charlene Barroga, Ph.D., Yong Hu, Ph.D., Vishal Deshmukh, Ph.D., and John Hood,
14 abstracts to be presented, further characterizing the complementary profiles of Mavenclad (cladribine tablets) and Rebif (interferon beta-1a)
 Your Contact Erin-Marie Beals +49 151 1454 2694 June 14, 2018 Not intended for U.S. or U.K. based media Merck to present data on Mavenclad and Rebif in relapsing forms of Multiple Sclerosis at EAN 14 abstracts
Your Contact Erin-Marie Beals +49 151 1454 2694 June 14, 2018 Not intended for U.S. or U.K. based media Merck to present data on Mavenclad and Rebif in relapsing forms of Multiple Sclerosis at EAN 14 abstracts
Your Contacts. Pfizer Media (US) Media (EU)
 Your Contacts Merck KGaA, Darmstadt, Germany Media Gangolf Schrimpf +49 6151 72 9591 Investor Relations +49 6151 72 3321 Pfizer Media (US) Media (EU) Sally Beatty Lisa O Neill +1 212 733 6566 +44 1737
Your Contacts Merck KGaA, Darmstadt, Germany Media Gangolf Schrimpf +49 6151 72 9591 Investor Relations +49 6151 72 3321 Pfizer Media (US) Media (EU) Sally Beatty Lisa O Neill +1 212 733 6566 +44 1737
Medivir Q Conference call 18 February, 2016
 Medivir Q4-2015 Conference call 18 February, 2016 Niklas Prager CEO Ola Burmark CFO Richard Bethell EVP R&D A research-based pharmaceutical company with focus on infectious diseases and oncology Q4 Highlights
Medivir Q4-2015 Conference call 18 February, 2016 Niklas Prager CEO Ola Burmark CFO Richard Bethell EVP R&D A research-based pharmaceutical company with focus on infectious diseases and oncology Q4 Highlights
Flexion Therapeutics Reports First-Quarter 2018 Financial Results and Recent Business Highlights
 May 8, 2018 Flexion Therapeutics Reports First-Quarter 2018 Financial Results and Recent Business Highlights Company booked net ZILRETTA sales of $2.2 million in Q1 Positive developments in Medicare reimbursement
May 8, 2018 Flexion Therapeutics Reports First-Quarter 2018 Financial Results and Recent Business Highlights Company booked net ZILRETTA sales of $2.2 million in Q1 Positive developments in Medicare reimbursement
SM04690: Potential first-in-class disease modifying treatment for knee osteoarthritis. Nancy Lane, MD
 SM04690: Potential first-in-class disease modifying treatment for knee osteoarthritis Nancy Lane, MD 1 Disclosures Yusuf Yazici Timothy McAlindon Allan Gibofsky Nancy Lane Daniel Clauw Christopher Swearingen
SM04690: Potential first-in-class disease modifying treatment for knee osteoarthritis Nancy Lane, MD 1 Disclosures Yusuf Yazici Timothy McAlindon Allan Gibofsky Nancy Lane Daniel Clauw Christopher Swearingen
Merck Serono Expands Cilengitide Development Program
 Your contact Dr. Raphaela Farrenkopf Phone +49 6151-72 2274 March 16, 2009, 9 a.m. CET Merck Serono Expands Cilengitide Development Program Study combining cilengitide and Erbitux in 1 st - line non-small
Your contact Dr. Raphaela Farrenkopf Phone +49 6151-72 2274 March 16, 2009, 9 a.m. CET Merck Serono Expands Cilengitide Development Program Study combining cilengitide and Erbitux in 1 st - line non-small
Discovery of a Small Molecule Wnt Pathway Inhibitor (SM04690) as a Potential Disease Modifying Treatment for Knee Osteoarthritis
 Discovery of a Small Molecule Wnt Pathway Inhibitor (SM469) as a Potential Disease Modifying Treatment for Knee Osteoarthritis Vishal Deshmukh PhD, Charlene Barroga PhD, Carine Bossard PhD, Sunil KC PhD,
Discovery of a Small Molecule Wnt Pathway Inhibitor (SM469) as a Potential Disease Modifying Treatment for Knee Osteoarthritis Vishal Deshmukh PhD, Charlene Barroga PhD, Carine Bossard PhD, Sunil KC PhD,
A WORLD WHERE EVERYONE CAN LEAD A HEALTHY AND FULFILLING LIFE
 A WORLD WHERE EVERYONE CAN LEAD A HEALTHY AND FULFILLING LIFE Chairman of Executive Board and Family Board of E. Merck KG Chairman of Board of Trustees of Merck Foundation Prof. Dr. Frank Stangenberg-
A WORLD WHERE EVERYONE CAN LEAD A HEALTHY AND FULFILLING LIFE Chairman of Executive Board and Family Board of E. Merck KG Chairman of Board of Trustees of Merck Foundation Prof. Dr. Frank Stangenberg-
DISCLOSURES. T. McAlindon: Samumed, grant/research support; Astellas, Flexion, Pfizer, Regeneron, Samumed,and Seikugaku, consulting
 Radiographic Outcomes from a Randomized, Double- Blind, Placebo-Controlled, Phase 2 Study of a Novel, Intra-Articular, Wnt Pathway Inhibitor (SM04690) for the Treatment of Osteoarthritis of the Knee: Week
Radiographic Outcomes from a Randomized, Double- Blind, Placebo-Controlled, Phase 2 Study of a Novel, Intra-Articular, Wnt Pathway Inhibitor (SM04690) for the Treatment of Osteoarthritis of the Knee: Week
News Release. Merck KGaA, Darmstadt, Germany data at ASCO 2018 to showcase progress and further optionality of oncology pipeline.
 News Release Your Contact Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 May 16, 2018 Not intended for UK-based media ASCO Abstract # Avelumab: 9507, 9537, 9090, 9008, 8563, 3057,
News Release Your Contact Gangolf Schrimpf +49 6151 72 9591 Investor Relations: +49 6151 72 3321 May 16, 2018 Not intended for UK-based media ASCO Abstract # Avelumab: 9507, 9537, 9090, 9008, 8563, 3057,
Medivir announces positive topline results from phase IIa osteoarthritis study, showing disease-modifying benefit of MIV-711 on joint structure
 Investor call Q1 2017 Medivir announces positive topline results from phase IIa osteoarthritis study, showing disease-modifying benefit of MIV-711 on joint structure Christine Lind, CEO; John Öhd, CMO;
Investor call Q1 2017 Medivir announces positive topline results from phase IIa osteoarthritis study, showing disease-modifying benefit of MIV-711 on joint structure Christine Lind, CEO; John Öhd, CMO;
Osteoarthritis. Dr Anthony Feher. With special thanks to Dr. Tim Williams and Dr. Bhatia for allowing me to use some of their slides
 Osteoarthritis Dr Anthony Feher With special thanks to Dr. Tim Williams and Dr. Bhatia for allowing me to use some of their slides No Financial Disclosures Number one chronic disability in the United States
Osteoarthritis Dr Anthony Feher With special thanks to Dr. Tim Williams and Dr. Bhatia for allowing me to use some of their slides No Financial Disclosures Number one chronic disability in the United States
About X-Linked Hypophosphatemia (XLH)
 Ultragenyx and Kyowa Kirin Announce Publication of Phase 2 Study Results Demonstrating that Crysvita (burosumab) Improved Outcomes in Children with X-linked Hypophosphatemia in the New England Journal
Ultragenyx and Kyowa Kirin Announce Publication of Phase 2 Study Results Demonstrating that Crysvita (burosumab) Improved Outcomes in Children with X-linked Hypophosphatemia in the New England Journal
Anti-inflammatory properties of SM04690, a small molecule inhibitor of the Wnt pathway as a potential treatment for knee osteoarthritis
 Anti-inflammatory properties of SM04690, a small molecule inhibitor of the Wnt pathway as a potential treatment for knee osteoarthritis V. Deshmukh 1, T. Seo 1, C. Swearingen 1, Y. Yazici 1 1 Samumed,
Anti-inflammatory properties of SM04690, a small molecule inhibitor of the Wnt pathway as a potential treatment for knee osteoarthritis V. Deshmukh 1, T. Seo 1, C. Swearingen 1, Y. Yazici 1 1 Samumed,
Case 27 Clinical Presentation
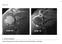 53 Case 27 Clinical Presentation 40-year-old man presents with acute shoulder pain and normal findings on radiographs. 54 RadCases Musculoskeletal Radiology Imaging Findings (,) Coronal images of the shoulder
53 Case 27 Clinical Presentation 40-year-old man presents with acute shoulder pain and normal findings on radiographs. 54 RadCases Musculoskeletal Radiology Imaging Findings (,) Coronal images of the shoulder
Results Confirm and Extend 40-Week Findings that Treatment with Crysvita is Superior to Conventional Therapy
 Ultragenyx and Kyowa Kirin Announce Positive 64-Week Results for Crysvita (burosumab) from Phase 3 Study in Children with X-linked Hypophosphatemia (XLH) Results Confirm and Extend 40-Week Findings that
Ultragenyx and Kyowa Kirin Announce Positive 64-Week Results for Crysvita (burosumab) from Phase 3 Study in Children with X-linked Hypophosphatemia (XLH) Results Confirm and Extend 40-Week Findings that
Inflammation is Not the Enemy
 6/22/2017 Inflammation is Not the Enemy Sean Mulvaney, MD 1 6/22/2017 2 6/22/2017 Lascaux 7.4 Billion 3 This image cannot currently be displayed. 6/22/2017 Goals 4 ANTI INFLAMMATORY THERAPIES NSAIDS 5
6/22/2017 Inflammation is Not the Enemy Sean Mulvaney, MD 1 6/22/2017 2 6/22/2017 Lascaux 7.4 Billion 3 This image cannot currently be displayed. 6/22/2017 Goals 4 ANTI INFLAMMATORY THERAPIES NSAIDS 5
Life. Uncompromised. The KineSpring Knee Implant System Surgeon Handout
 Life Uncompromised The KineSpring Knee Implant System Surgeon Handout 2 Patient Selection Criteria Patient Selection Criteria Medial compartment degeneration must be confirmed radiographically or arthroscopically
Life Uncompromised The KineSpring Knee Implant System Surgeon Handout 2 Patient Selection Criteria Patient Selection Criteria Medial compartment degeneration must be confirmed radiographically or arthroscopically
Results from a 52-Week, Phase 2A Study of an Intra-Articular, Wnt Pathway Inhibitor, SM04690, for Knee Osteoarthritis
 Results from a 52-Week, Phase 2A Study of an Intra-Articular, Wnt Pathway Inhibitor, SM04690, for Knee Osteoarthritis Yusuf Yazici 1, Timothy McAlindon 2, Allan Gibofsky 3, Nancy Lane 4, Daniel Clauw 5,
Results from a 52-Week, Phase 2A Study of an Intra-Articular, Wnt Pathway Inhibitor, SM04690, for Knee Osteoarthritis Yusuf Yazici 1, Timothy McAlindon 2, Allan Gibofsky 3, Nancy Lane 4, Daniel Clauw 5,
MERCK KGAA, DARMSTADT, GERMANY
 MERCK KGAA, DARMSTADT, GERMANY 37TH ANNUAL J.P. MORGAN HEALTHCARE CONFERENCE Belén Garijo, CEO Healthcare Udit Batra, CEO Life Science San Francisco January 7, 2019 Disclaimer Publication of Merck KGaA,
MERCK KGAA, DARMSTADT, GERMANY 37TH ANNUAL J.P. MORGAN HEALTHCARE CONFERENCE Belén Garijo, CEO Healthcare Udit Batra, CEO Life Science San Francisco January 7, 2019 Disclaimer Publication of Merck KGaA,
Effectiveness of True Acupuncture as an Adjunct to Standard Care or Electro-Physiotherapy in Osteoarthritis of the Knee
 Cronicon OPEN ACCESS ORTHOPAEDICS Research article Effectiveness of True Acupuncture as an Adjunct to Standard Care or Electro-Physiotherapy in Osteoarthritis of Dimitar Tonev 1 *, Stoyka Radeva 2 and
Cronicon OPEN ACCESS ORTHOPAEDICS Research article Effectiveness of True Acupuncture as an Adjunct to Standard Care or Electro-Physiotherapy in Osteoarthritis of Dimitar Tonev 1 *, Stoyka Radeva 2 and
Bayer s Rivaroxaban Demonstrated Superior Protection Against Recurrent Venous Thromboembolism Compared with Aspirin in EINSTEIN CHOICE Study
 News Release Not intended for U.S. and UK Media Bayer AG Communications and Public Affairs 51368 Leverkusen Germany Tel. +49 214 30-0 www.news.bayer.com New Late-Breaking Study Data Presented at ACC.17:
News Release Not intended for U.S. and UK Media Bayer AG Communications and Public Affairs 51368 Leverkusen Germany Tel. +49 214 30-0 www.news.bayer.com New Late-Breaking Study Data Presented at ACC.17:
4 2 Osteoarthritis 1
 Osteoarthritis 1 Osteoarthritis ( OA) Osteoarthritis is a chronic disease and the most common of all rheumatological disorders. It particularly affects individuals over the age of 65 years. The prevalence
Osteoarthritis 1 Osteoarthritis ( OA) Osteoarthritis is a chronic disease and the most common of all rheumatological disorders. It particularly affects individuals over the age of 65 years. The prevalence
Bayer to Present New Data on Advancing Oncology Portfolio. New Preclinical and Early Clinical Data Evaluating Innovative Compounds in Drug Development
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com 105 th Annual Meeting American Association for Cancer Research Bayer to Present
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com 105 th Annual Meeting American Association for Cancer Research Bayer to Present
CARTILAGE REPAIR INTRODUCTION. M. BERRUTO and G.M. PERETTI 1,2. Received January 6, Accepted January 8, 2013
 CARTILAGE REPAIR INTRODUCTION M. BERRUTO and G.M. PERETTI 1,2 Gaetano Pini Orthopedic Institute, Milan; 1 Department of Biomedical Sciences for Health, University of Milan, 2 IRCCS Galeazzi Orthopedic
CARTILAGE REPAIR INTRODUCTION M. BERRUTO and G.M. PERETTI 1,2 Gaetano Pini Orthopedic Institute, Milan; 1 Department of Biomedical Sciences for Health, University of Milan, 2 IRCCS Galeazzi Orthopedic
For more information about how to cite these materials visit
 Author(s): Seetha Monrad, M.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Noncommercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
Author(s): Seetha Monrad, M.D., 2009 License: Unless otherwise noted, this material is made available under the terms of the Creative Commons Attribution Noncommercial Share Alike 3.0 License: http://creativecommons.org/licenses/by-nc-sa/3.0/
NBQX, An AMPA/Kainate Glutamate Receptor Antagonist, Alleviates Joint Disease In Models Of Inflammatory- And Osteo- Arthritis.
 NBQX, An AMPA/Kainate Glutamate Receptor Antagonist, Alleviates Joint Disease In Models Of Inflammatory- And Osteo- Arthritis. Cleo S. Bonnet, PhD 1, Anwen S. Williams, PhD 1, Sophie J. Gilbert, PhD 1,
NBQX, An AMPA/Kainate Glutamate Receptor Antagonist, Alleviates Joint Disease In Models Of Inflammatory- And Osteo- Arthritis. Cleo S. Bonnet, PhD 1, Anwen S. Williams, PhD 1, Sophie J. Gilbert, PhD 1,
FORTIGEL for sustainable mobility
 FORTIGEL for sustainable mobility Scientifically studied to regenerate joint cartilage Stimulates the body s own mechanisms for maintaining healthy joints and optimum mobility Promotes joint health naturally
FORTIGEL for sustainable mobility Scientifically studied to regenerate joint cartilage Stimulates the body s own mechanisms for maintaining healthy joints and optimum mobility Promotes joint health naturally
Ricardo E. Colberg, MD, RMSK. PM&R Sports Medicine Physician Andrews Sports Medicine and Orthopedic Center American Sports Medicine Institute
 Ricardo E. Colberg, MD, RMSK PM&R Sports Medicine Physician Andrews Sports Medicine and Orthopedic Center American Sports Medicine Institute Pathophysiology of chronic orthopedic injuries Definition of
Ricardo E. Colberg, MD, RMSK PM&R Sports Medicine Physician Andrews Sports Medicine and Orthopedic Center American Sports Medicine Institute Pathophysiology of chronic orthopedic injuries Definition of
H. Lundbeck AS (LUN) - Financial and Strategic SWOT Analysis Review
 H. Lundbeck AS (LUN) - Financial and Strategic SWOT Analysis Review H. Lundbeck AS (LUN) - Financial and Strategic SWOT Analysis Review BioPortfolio has been marketing business and market research reports
H. Lundbeck AS (LUN) - Financial and Strategic SWOT Analysis Review H. Lundbeck AS (LUN) - Financial and Strategic SWOT Analysis Review BioPortfolio has been marketing business and market research reports
PIONEER AF-PCI Study with Bayer s Xarelto Accepted as Late- Breaking Clinical Trial Presentation at AHA 2016
 News Release Not intended for U.S. and UK Media Bayer AG Communications, Government Relations & Corporate Brand 51368 Leverkusen Germany Tel. +49 214 30-0 www.news.bayer.com American Heart Association
News Release Not intended for U.S. and UK Media Bayer AG Communications, Government Relations & Corporate Brand 51368 Leverkusen Germany Tel. +49 214 30-0 www.news.bayer.com American Heart Association
Evaluation and Treatment of Knee Arthritis Classification of Knee Arthritis Osteoarthritis Osteoarthritis Osteoarthritis of Knee
 1 2 Evaluation and Treatment of Knee Arthritis John Zebrack, MD Reno Orthopaedic Clinic Classification of Knee Arthritis Non-inflammatory Osteoarthritis Primary Secondary Post-traumatic, dysplasia, neuropathic,
1 2 Evaluation and Treatment of Knee Arthritis John Zebrack, MD Reno Orthopaedic Clinic Classification of Knee Arthritis Non-inflammatory Osteoarthritis Primary Secondary Post-traumatic, dysplasia, neuropathic,
Regulation of the IGF axis by TGF-b during periosteal chondrogenesis: implications for articular cartilage repair
 Regulation of the IGF axis by TGF-b during periosteal chondrogenesis: implications for articular cartilage repair Chapter 04 Boek 1_Gie.indb 55 21-05-2007 12:27:33 Chapter 04 Abstract Goal: TGF-b and IGF-I
Regulation of the IGF axis by TGF-b during periosteal chondrogenesis: implications for articular cartilage repair Chapter 04 Boek 1_Gie.indb 55 21-05-2007 12:27:33 Chapter 04 Abstract Goal: TGF-b and IGF-I
Conventional therapy n=32. RGI-C Score (Primary Endpoint) LS Mean* (0.83, 1.45) P< Substantial healing, % patients (RGI-C +2.
 Ultragenyx and Kyowa Kirin Announce Topline Phase 3 Study Results Demonstrating Superiority of Crysvita (burosumab) Treatment to Oral Phosphate and Active Vitamin D in Children with X-Linked Hypophosphatemia
Ultragenyx and Kyowa Kirin Announce Topline Phase 3 Study Results Demonstrating Superiority of Crysvita (burosumab) Treatment to Oral Phosphate and Active Vitamin D in Children with X-Linked Hypophosphatemia
Advances in Osteoarthritis Since the Founding of OARSI
 Advances in Osteoarthritis Since the Founding of OARSI Marc C. Hochberg, MD, MPH, MACP, MACR Professor of Medicine and Epidemiology and Public Health Head, Division of Rheumatology and Clinical Immunology
Advances in Osteoarthritis Since the Founding of OARSI Marc C. Hochberg, MD, MPH, MACP, MACR Professor of Medicine and Epidemiology and Public Health Head, Division of Rheumatology and Clinical Immunology
Intent to Consider the Appropriate Classification of Hyaluronic Acid Intra-articular Products
 This document is scheduled to be published in the Federal Register on 12/18/2018 and available online at https://federalregister.gov/d/2018-27351, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
This document is scheduled to be published in the Federal Register on 12/18/2018 and available online at https://federalregister.gov/d/2018-27351, and on govinfo.gov 4164-01-P DEPARTMENT OF HEALTH AND
Osteoarthritis. RA Hughes
 Osteoarthritis RA Hughes Osteoarthritis (OA) OA is the most common form of arthritis and the most common joint disease Most of the people who have OA are older than age 45, and women are more commonly
Osteoarthritis RA Hughes Osteoarthritis (OA) OA is the most common form of arthritis and the most common joint disease Most of the people who have OA are older than age 45, and women are more commonly
Tackling Osteoarthritis: Matriptase Inhibitor as a Disease Modifying Therapy Kevin Buchan
 Tackling Osteoarthritis: Matriptase Inhibitor as a Disease Modifying Therapy Kevin Buchan Business Development Manager, LifeArc Tackling Osteoarthritis Osteoarthritis (OA) is the most common joint disorder
Tackling Osteoarthritis: Matriptase Inhibitor as a Disease Modifying Therapy Kevin Buchan Business Development Manager, LifeArc Tackling Osteoarthritis Osteoarthritis (OA) is the most common joint disorder
EMA s CHMP Issues Positive Opinion for Avelumab for the Treatment of Metastatic Merkel Cell Carcinoma
 Your Contacts Merck KGaA, Darmstadt, Germany Media Friederike Segeberg +49 6151 72 6328 Investor Relations +49 6151 72 3321 Pfizer Media (US) Media (EU) Sally Beatty Dervila Keane +1 212 733 6566 +353
Your Contacts Merck KGaA, Darmstadt, Germany Media Friederike Segeberg +49 6151 72 6328 Investor Relations +49 6151 72 3321 Pfizer Media (US) Media (EU) Sally Beatty Dervila Keane +1 212 733 6566 +353
UCB announces first presentation of primary data from latest Phase 3 study evaluating brivaracetam
 UCB announces first presentation of primary data from latest Phase 3 study evaluating brivaracetam as adjunctive treatment of partial-onset seizures in epilepsy Primary efficacy and safety data from the
UCB announces first presentation of primary data from latest Phase 3 study evaluating brivaracetam as adjunctive treatment of partial-onset seizures in epilepsy Primary efficacy and safety data from the
New Directions in Osteoarthritis Research
 New Directions in Osteoarthritis Research Kananaskis October 22, 2015 Nick Mohtadi MD MSc FRCSC No conflicts of interest related to this presentation 1 Osteoarthritis: Disease? Fact of Life? Strong family
New Directions in Osteoarthritis Research Kananaskis October 22, 2015 Nick Mohtadi MD MSc FRCSC No conflicts of interest related to this presentation 1 Osteoarthritis: Disease? Fact of Life? Strong family
ASSOCIATE MEMBERSHIP SCHEME
 ASSOCIATE MEMBERSHIP SCHEME INTRODUCTION Dear colleague, I m delighted to launch the NHS Providers Associate Membership Scheme, our brand new offer to commercial suppliers in the health sector. While visiting
ASSOCIATE MEMBERSHIP SCHEME INTRODUCTION Dear colleague, I m delighted to launch the NHS Providers Associate Membership Scheme, our brand new offer to commercial suppliers in the health sector. While visiting
Bayer Launches Phase III Clinical Study of Trasylol in Elective Spinal Fusion Surgery
 Investor News Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer Launches Phase III Clinical Study of Trasylol in Elective Spinal Fusion Surgery Study Examines Effects of
Investor News Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer Launches Phase III Clinical Study of Trasylol in Elective Spinal Fusion Surgery Study Examines Effects of
o~ r;'c' - OSTEOARTHRITIS
 Osteoarthritis and Cartilage (2001) 9, Supplement A, S102-S108 2001 OsteoArthritis Research Society International doi:10.1053/joca.2001.0451, available online at http://www.idealibrary.com on IDE~l Osteoarthritis
Osteoarthritis and Cartilage (2001) 9, Supplement A, S102-S108 2001 OsteoArthritis Research Society International doi:10.1053/joca.2001.0451, available online at http://www.idealibrary.com on IDE~l Osteoarthritis
Private sector commitment to an initiative that links health and prosperity for women
 Private sector commitment to an initiative that links health and prosperity for women Jocelyn Ulrich, Senior Director, Global Healthcare Government and Public Affairs July 10 th, 2018 Healthy women, Healthy
Private sector commitment to an initiative that links health and prosperity for women Jocelyn Ulrich, Senior Director, Global Healthcare Government and Public Affairs July 10 th, 2018 Healthy women, Healthy
Results from Hokusai-VTE presented during ESC Congress 2013 Hot Line session and published in the New England Journal of Medicine
 Press Release Daiichi Sankyo s Once-Daily Edoxaban Shows Comparable Efficacy and Superiority for the Principal Safety Endpoint Compared to Warfarin in a Phase 3 Study for the Treatment of Symptomatic VTE
Press Release Daiichi Sankyo s Once-Daily Edoxaban Shows Comparable Efficacy and Superiority for the Principal Safety Endpoint Compared to Warfarin in a Phase 3 Study for the Treatment of Symptomatic VTE
The cellfree matrix for autoregeneration of articular cartilage defects
 The cellfree matrix for autoregeneration of articular cartilage defects What is Amedrix GmbH? Amedrix GmbH, located in Esslingen near Stuttgart, is a medical biotech company which develops highly innovative
The cellfree matrix for autoregeneration of articular cartilage defects What is Amedrix GmbH? Amedrix GmbH, located in Esslingen near Stuttgart, is a medical biotech company which develops highly innovative
Cohort A. Number of patients
 MyoKardia Announces Positive Results from Low- Dose Cohort of Phase 2 PIONEER-HCM Study of Mavacamten in Symptomatic, Obstructive Hypertrophic Cardiomyopathy Patients Met Primary Endpoint and Key Secondary
MyoKardia Announces Positive Results from Low- Dose Cohort of Phase 2 PIONEER-HCM Study of Mavacamten in Symptomatic, Obstructive Hypertrophic Cardiomyopathy Patients Met Primary Endpoint and Key Secondary
Bayer to showcase latest Ophthalmology research at EURETINA 2017
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com 17 th European Society of Retina Specialists (EURETINA) Congress Bayer to showcase
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com 17 th European Society of Retina Specialists (EURETINA) Congress Bayer to showcase
Arthritis. Canada s most common chronic health condition. About the Arthritis Society
 About the Arthritis Society The Arthritis Society is a national health charity, fueled by donors and volunteers, with a vision to live in a world where people are free from the devastating effects that
About the Arthritis Society The Arthritis Society is a national health charity, fueled by donors and volunteers, with a vision to live in a world where people are free from the devastating effects that
Ampion TM. Management Presentation 2018
 TM Management Presentation 2018 2 Forward-Looking Statement These slides and materials, including any accompanying oral presentation, contain forward-looking statements about our business. You should not
TM Management Presentation 2018 2 Forward-Looking Statement These slides and materials, including any accompanying oral presentation, contain forward-looking statements about our business. You should not
Castle Creek Pharmaceuticals
 Castle Creek Pharmaceuticals Dermatology Summit Entrepreneurial Showcase 2018 Michael Derby, CEO January 7, 2018 Castle Creek Pharma Driving innovation in dermatology We are a high-growth biotech company
Castle Creek Pharmaceuticals Dermatology Summit Entrepreneurial Showcase 2018 Michael Derby, CEO January 7, 2018 Castle Creek Pharma Driving innovation in dermatology We are a high-growth biotech company
TETEC the cartilage makers
 Powered by Website address: https://www.gesundheitsindustriebw.de/en/article/news/tetec-the-cartilage-makers/ TETEC the cartilage makers What you need is patient cells. The certified laboratories of TETEC
Powered by Website address: https://www.gesundheitsindustriebw.de/en/article/news/tetec-the-cartilage-makers/ TETEC the cartilage makers What you need is patient cells. The certified laboratories of TETEC
Is Viscosupplementation Effective in Reducing Osteoarthritis Knee Pain?
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2016 Is Viscosupplementation Effective in
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2016 Is Viscosupplementation Effective in
Non-Operative Options for Articular Cartilage Issues in the Athlete s Knee
 Non-Operative Options for Articular Cartilage Issues in the Athlete s Knee Sourav Poddar, MD Associate Professor Director, Primary Care Sports Medicine University of Colorado School of Medicine OBJECTIVES
Non-Operative Options for Articular Cartilage Issues in the Athlete s Knee Sourav Poddar, MD Associate Professor Director, Primary Care Sports Medicine University of Colorado School of Medicine OBJECTIVES
Details of UNCOVER-2 and UNCOVER-3 Study Results Published in The Lancet
 June 10, 2015 Eli Lilly and Company Lilly Corporate Center Indianapolis, Indiana 46285 U.S.A. +1.317.276.2000 www.lilly.com For Release: Refer to: Immediately Tim Coulom; tim.coulom@lilly.com; 317-771-2241
June 10, 2015 Eli Lilly and Company Lilly Corporate Center Indianapolis, Indiana 46285 U.S.A. +1.317.276.2000 www.lilly.com For Release: Refer to: Immediately Tim Coulom; tim.coulom@lilly.com; 317-771-2241
Decision follows a recommendation from Independent Data Monitoring Committee (IDMC)
 News Release Intended for U.S. Media Phase III trial of radium Ra 223 dichloride in combination with abiraterone acetate and prednisone/prednisolone for patients with metastatic castration-resistant prostate
News Release Intended for U.S. Media Phase III trial of radium Ra 223 dichloride in combination with abiraterone acetate and prednisone/prednisolone for patients with metastatic castration-resistant prostate
Personalisierte Medizin in der Praxis. Dr. Bernhard Kirschbaum DVFA Life Science Conference Frankfurt, 17. Juni 2009
 Personalisierte Medizin in der Praxis Dr. Bernhard Kirschbaum DVFA Life Science Conference Frankfurt, 17. Juni 2009 Merck Serono The largest division of the Merck Group Merck Group Revenues FY2008: 7,558m
Personalisierte Medizin in der Praxis Dr. Bernhard Kirschbaum DVFA Life Science Conference Frankfurt, 17. Juni 2009 Merck Serono The largest division of the Merck Group Merck Group Revenues FY2008: 7,558m
Omega-3 Fatty Acids Mitigate Obesity-induced Osteoarthritis And Accelerate Wound Repair
 Omega-3 Fatty Acids Mitigate Obesity-induced Osteoarthritis And Accelerate Wound Repair Chia-Lung Wu, MS, Deeptee Jain, MD, Jenna McNeill, BS, Dianne Little, BVSc, PhD, John Anderson, MD, Janet Huebner,
Omega-3 Fatty Acids Mitigate Obesity-induced Osteoarthritis And Accelerate Wound Repair Chia-Lung Wu, MS, Deeptee Jain, MD, Jenna McNeill, BS, Dianne Little, BVSc, PhD, John Anderson, MD, Janet Huebner,
Media Contacts: Kelley Dougherty Investor Contact: Graeme Bell (215) (908)
 News Release Media Contacts: Kelley Dougherty Investor Contact: Graeme Bell (215) 652-0059 (908) 423-5185 Janet Skidmore (267) 305-7715 Merck Launches National Advertising Campaign for GARDASIL, Merck's
News Release Media Contacts: Kelley Dougherty Investor Contact: Graeme Bell (215) 652-0059 (908) 423-5185 Janet Skidmore (267) 305-7715 Merck Launches National Advertising Campaign for GARDASIL, Merck's
Fast Facts. Fast Facts: Osteoarthritis. Philip G Conaghan and Leena Sharma Health Press Ltd.
 Fast Facts Fast Facts: Osteoarthritis Philip G Conaghan and Leena Sharma Fast Facts Fast Facts: Osteoarthritis Philip G Conaghan MB BS PhD FRACP FRCP Professor of Musculoskeletal Medicine University of
Fast Facts Fast Facts: Osteoarthritis Philip G Conaghan and Leena Sharma Fast Facts Fast Facts: Osteoarthritis Philip G Conaghan MB BS PhD FRACP FRCP Professor of Musculoskeletal Medicine University of
Study on the clinical value of mirna98b as a potential biomarker for osteoarthritis.
 Biomedical Research 2018; 29 (3): 549-553 ISSN 0970-938X www.biomedres.info Study on the clinical value of mirna98b as a potential biomarker for osteoarthritis. Qian Wang 1, Xiaoqing Ma 2, Zhongyang Xu
Biomedical Research 2018; 29 (3): 549-553 ISSN 0970-938X www.biomedres.info Study on the clinical value of mirna98b as a potential biomarker for osteoarthritis. Qian Wang 1, Xiaoqing Ma 2, Zhongyang Xu
PROCHONDRIX CARTILAGE RESTORATION MATRIX CONTAINS GROWTH FACTORS NECESSARY FOR HYALINE CARTILAGE REGENERATION
 A L L O S O U R C E PROCHONDRIX CARTILAGE RESTORATION MATRIX CONTAINS GROWTH FACTORS NECESSARY FOR HYALINE CARTILAGE REGENERATION Ryan Delaney MS; Carolyn Barrett BS, MBA; Peter Stevens PhD, MBA AlloSource,
A L L O S O U R C E PROCHONDRIX CARTILAGE RESTORATION MATRIX CONTAINS GROWTH FACTORS NECESSARY FOR HYALINE CARTILAGE REGENERATION Ryan Delaney MS; Carolyn Barrett BS, MBA; Peter Stevens PhD, MBA AlloSource,
VeriStrat Poor Patients Show Encouraging Overall Survival and Progression Free Survival Signal; Confirmatory Phase 2 Study Planned by Year-End
 AVEO and Biodesix Announce Exploratory Analysis of VeriStrat-Selected Patients with Non-Small Cell Lung Cancer in Phase 2 Study of Ficlatuzumab Presented at ESMO 2014 Congress VeriStrat Poor Patients Show
AVEO and Biodesix Announce Exploratory Analysis of VeriStrat-Selected Patients with Non-Small Cell Lung Cancer in Phase 2 Study of Ficlatuzumab Presented at ESMO 2014 Congress VeriStrat Poor Patients Show
Joint Space Width Criteria Can Reduce Knee Osteoarthritis Trial Heterogeneity: Phase 2 Post-Hoc Data from Wnt Pathway Inhibitor, SM04690
 Joint Space Width Criteria Can Reduce Knee Osteoarthritis Trial Heterogeneity: Phase 2 Post-Hoc Data from Wnt Pathway Inhibitor, SM04690 Jeyanesh R.S. Tambiah 1, Christian Lattermann 2, Christopher J.
Joint Space Width Criteria Can Reduce Knee Osteoarthritis Trial Heterogeneity: Phase 2 Post-Hoc Data from Wnt Pathway Inhibitor, SM04690 Jeyanesh R.S. Tambiah 1, Christian Lattermann 2, Christopher J.
U.S. Insomnia Market by Non-Pharmacological Therapy (CBTI, Hypnotherapy), Prescription Sleep Aids (Benzodiazepines, Non-Benzodiazepines (Zaleplon),
 U.S. Insomnia Market by Non-Pharmacological Therapy (CBTI, Hypnotherapy), Prescription Sleep Aids (Benzodiazepines, Non-Benzodiazepines (Zaleplon), Orexin Antagonist) & OTC Treatment (Antihistamine, Melatonin,
U.S. Insomnia Market by Non-Pharmacological Therapy (CBTI, Hypnotherapy), Prescription Sleep Aids (Benzodiazepines, Non-Benzodiazepines (Zaleplon), Orexin Antagonist) & OTC Treatment (Antihistamine, Melatonin,
Christopher J. Swearingen, Sarah Kennedy, Jeyanesh R.S. Tambiah. Samumed LLC, San Diego, CA, USA
 Radiographic Outcomes Were Concordant with Pain and Function Response: Post-Hoc Analysis from a Phase 2 Study of SM04690, a Wnt Pathway Inhibitor for Knee Osteoarthritis Treatment Christopher J. Swearingen,
Radiographic Outcomes Were Concordant with Pain and Function Response: Post-Hoc Analysis from a Phase 2 Study of SM04690, a Wnt Pathway Inhibitor for Knee Osteoarthritis Treatment Christopher J. Swearingen,
Table of Contents. 621 Lake Avenue, Unit 3A, Asbury Park, NJ Phone: Fax: gomohealth.com
 Table of Contents 1.0 Cartilage Injury of the Knee Clinical Trial NCT03275064...2 1.1 Joint Cartilage Injury Information...4 1.1.1 Joint Cartilage Injury FAQs...6 1.1.2 Cartilage Injury of the Knee Study
Table of Contents 1.0 Cartilage Injury of the Knee Clinical Trial NCT03275064...2 1.1 Joint Cartilage Injury Information...4 1.1.1 Joint Cartilage Injury FAQs...6 1.1.2 Cartilage Injury of the Knee Study
- Plans Commercial Launch of three dosing options for Excellagen in pre-filled syringes
 Generex Biotechnology Subsidiary Olaregen Therapeutix Inc. Plans Launch of FDA Cleared Excellagen Wound Conforming Gel Matrix with Three Dosage Options - Plans Commercial Launch of three dosing options
Generex Biotechnology Subsidiary Olaregen Therapeutix Inc. Plans Launch of FDA Cleared Excellagen Wound Conforming Gel Matrix with Three Dosage Options - Plans Commercial Launch of three dosing options
Shockwave Therapy. Leading Rehabilitation Technology
 Excellence in Shockwave Therapy Leading Rehabilitation Technology Since its foundation in 2003, REMED has tried to care for the patients suffering from different types of physical pains all over the world
Excellence in Shockwave Therapy Leading Rehabilitation Technology Since its foundation in 2003, REMED has tried to care for the patients suffering from different types of physical pains all over the world
Wnt7a Inhibits Cartilage Matrix Degradation in a Mouse In Vivo Osteoarthritis Model
 Wnt7a Inhibits Cartilage Matrix Degradation in a Mouse In Vivo Osteoarthritis Model Averi Leahy, Andrea Foote, Tomoya Uchimura, Li Zeng, PhD. Tufts University, Boston, MA, USA. Disclosures: A. Leahy: None.
Wnt7a Inhibits Cartilage Matrix Degradation in a Mouse In Vivo Osteoarthritis Model Averi Leahy, Andrea Foote, Tomoya Uchimura, Li Zeng, PhD. Tufts University, Boston, MA, USA. Disclosures: A. Leahy: None.
Innovative Nuclear Receptor Modulation Medicine
 Innovative Nuclear Receptor Modulation Medicine Dale C Leitman, M.D., Ph.D. Isaac Cohen, O.M.D., Ph.D. October 2015 Corporate Mission Iaterion, Inc. is Developing Novel Pharmaceutical Nuclear Receptor
Innovative Nuclear Receptor Modulation Medicine Dale C Leitman, M.D., Ph.D. Isaac Cohen, O.M.D., Ph.D. October 2015 Corporate Mission Iaterion, Inc. is Developing Novel Pharmaceutical Nuclear Receptor
THAT S PROVEN FOR THE LONG RUN
 A GUIDE FOR PATIENTS KNEE PAIN RELIEF THAT S PROVEN FOR THE LONG RUN TREATMENT OPTIONS FOR OA KNEE PAIN There are several things you can do to help reduce knee pain due to OA. Lifestyle changes Losing
A GUIDE FOR PATIENTS KNEE PAIN RELIEF THAT S PROVEN FOR THE LONG RUN TREATMENT OPTIONS FOR OA KNEE PAIN There are several things you can do to help reduce knee pain due to OA. Lifestyle changes Losing
News Release - 1/5 - Bayer Yakuhin, Ltd. Santen Pharmaceutical Co., Ltd.
 News Release Bayer Yakuhin, Ltd. Santen Pharmaceutical Co., Ltd. Intravitreal VEGF Inhibitor EYLEA (aflibercept) Solution for Intravitreal Injection Released on the Market for the Treatment of Wet Age-Related
News Release Bayer Yakuhin, Ltd. Santen Pharmaceutical Co., Ltd. Intravitreal VEGF Inhibitor EYLEA (aflibercept) Solution for Intravitreal Injection Released on the Market for the Treatment of Wet Age-Related
CLINICAL OUTCOME & MORPHOMETRY OVER 2 & 5 YEARS -
 CLINICAL OUTCOME & MORPHOMETRY OVER 2 & 5 YEARS - data from a treatment RCT on acute ACL injury R Frobell 1, W Wirth 2, LS Lohmander 1, M Hudelmaier 2, F Eckstein 2 1 Orthopedics, Clinical Sciences Lund,
CLINICAL OUTCOME & MORPHOMETRY OVER 2 & 5 YEARS - data from a treatment RCT on acute ACL injury R Frobell 1, W Wirth 2, LS Lohmander 1, M Hudelmaier 2, F Eckstein 2 1 Orthopedics, Clinical Sciences Lund,
Media Release. Basel, 10 November 2017
 Media Release Basel, 10 November 2017 Roche s OCREVUS (ocrelizumab) gains positive CHMP opinion for relapsing forms of multiple sclerosis and primary progressive multiple sclerosis If approved, OCREVUS
Media Release Basel, 10 November 2017 Roche s OCREVUS (ocrelizumab) gains positive CHMP opinion for relapsing forms of multiple sclerosis and primary progressive multiple sclerosis If approved, OCREVUS
Synovial fluid concentrations of the C-propeptide of type II collagen correlate with body mass index in primary knee osteoarthritis
 Synovial fluid concentrations of the C-propeptide of type II collagen correlate with body mass index in primary knee osteoarthritis Kobayashi, Tatsuo; Yoshihara, Yasuo; Samura, Atsuyoshi; Yamada, Harumoto;
Synovial fluid concentrations of the C-propeptide of type II collagen correlate with body mass index in primary knee osteoarthritis Kobayashi, Tatsuo; Yoshihara, Yasuo; Samura, Atsuyoshi; Yamada, Harumoto;
A GUIDE FOR PATIENTS KNEE PAIN RELIEF THAT JUST CAN T WAIT
 A GUIDE FOR PATIENTS KNEE PAIN RELIEF THAT JUST CAN T WAIT TREATMENT OPTIONS FOR OA KNEE PAIN There are several things you can do to help reduce knee pain due to OA. Lifestyle changes Losing weight, if
A GUIDE FOR PATIENTS KNEE PAIN RELIEF THAT JUST CAN T WAIT TREATMENT OPTIONS FOR OA KNEE PAIN There are several things you can do to help reduce knee pain due to OA. Lifestyle changes Losing weight, if
