PRESCRIBING INFORMATION. Hydrocortisone Acetate USP 10% Rectal Anti-Inflammatory Corticosteroid Foam
|
|
|
- Phyllis Willis
- 6 years ago
- Views:
Transcription
1 PRESCRIBING INFORMATION CORTIFOAM Hydrocortisone Acetate USP 10% Rectal Anti-Inflammatory Corticosteroid Foam Paladin Labs Inc. Date of Preparation: 6111 Royalmount, Suite 102 July 7, 2009 Montréal, Québec Version: 4.0 H4P 2T4 Control No:
2 PRESCRIBING INFORMATION NAME OF DRUG CORTIFOAM (Hydrocortisone Acetate USP 10%) THERAPEUTIC CLASSIFICATION Rectal Anti-Inflammatory Corticosteroid Foam ACTION AND CLINICAL PHARMACOLOGY The action of Cortifoam (hydrocortisone acetate USP 10%) appears to be due to a local anti-inflammatory effect of hydrocortisone acetate on the mucosa rather than as a result of a systemic effect due to absorption of hydrocortisone. INDICATIONS Cortifoam (hydrocortisone acetate USP 10%) is indicated as adjunctive therapy in the treatment of ulcerative colitis of the sigmoid colon, proctosigmoiditis, granular proctitis and ulcerative proctitis. CONTRAINDICATIONS Contraindications to the use of intrarectal steroids include obstruction, abscess, perforation, peritonitis, fresh intestinal anastomoses, extensive fistulas and sinus tracts. Tuberculosis (active, latent or questionably healed), ocular herpes simplex, varicella, vaccinia, and acute psychosis are usually considered contraindications to the use of corticosteroids. Other contraindications include active peptic ulcer, acute glomerulonephritis, myasthenia gravis, osteoporosis, diverticulitis, thrombophlebitis, psychic disturbances, pregnancy, diabetes, hyperthyroidism, acute coronary disease, hypertension, limited cardiac reserve, and local or systemic infections, including fungal or exanthematous diseases. Where these conditions exist, the expected benefits from steroid therapy must be weighed against the risks involved in its use. Cortifoam (hydrocortisone acetate USP 10%) is also contraindicated in systemic fungal infection and in the presence of hypersensitivity to any of its components. 2
3 WARNINGS CAUTION: Contents are flammable and the aerosol container may explode if heated. Do not insert any part of the aerosol container into the anus. Do not use in presence of an open flame or spark. Contents of the container are under pressure. Do not refrigerate. Do not place in hot water or near radiators, stoves or other sources of heat. Do not incinerate or puncture the aerosol container or store at temperatures over 50 C. Because Cortifoam (hydrocortisone acetate USP 10%) is not expelled, systemic hydrocortisone absorption may be greater from Cortifoam than from corticosteroid enema formulations. If there is no evidence of clinical or proctologic improvement within two or three weeks after starting Cortifoam therapy, or if the patient's condition worsens, discontinue the drug. Signs and symptoms of intestinal perforation and peritonitis may be difficult to detect during corticosteroid treatment. PRECAUTIONS General: A complete rectal examination to rule out serious pathology and extension of the disease process should be completed before instituting therapy. Do not use on infected lesions unless accompanied with anti-infective agents. Steroid therapy should be administered with caution in patients with severe ulcerative disease because these patients are predisposed to perforation of the bowel wall. Where surgery is imminent, it is hazardous to wait more than a few days for a satisfactory response to medical treatment. General precautions common to all corticosteroid therapy should be observed during treatment with Cortifoam (hydrocortisone acetate USP 10%). These include gradual withdrawal of therapy to allow for possible adrenal insufficiency and awareness of possible growth suppression in children. Patients should be kept under close observation, for as with all drugs, rare individuals may react unfavorably under certain conditions. If severe reactions or idiosyncrasies occur, steroids should be discontinued immediately and appropriate measures instituted. Do not employ in immediate or early postoperative period following ileorectostomy. Patients should be advised to inform subsequent physicians of the prior use of corticosteroids. Use in Pregnancy: Steroids should not be used during pregnancy since safety during pregnancy has not been fully established. 3
4 If corticosteroids must be administered during pregnancy, particularly during the third trimester, the newborn infant must be observed closely for signs of hypoadrenalism, and the appropriate therapy administered if needed. Nursing Mothers: Mothers using Cortifoam should be advised not to nurse. ADVERSE REACTIONS Corticosteroid therapy may produce side effects which include moon face, fluid retention, excessive appetite and weight gain, abnormal fat deposits, mental symptoms, hypertrichosis, acne, ecchymosis, increased sweating, pigmentation, dry scaly skin, thinning scalp hair, thrombophlebitis, decreased resistance to infection, negative nitrogen balance with delayed bone and wound healing, menstrual disorders, neuropathy, peptic ulcer, decreased glucose tolerance, hypokalemia, adrenal insufficiency, necrotizing angiitis, hypertension, pancreatitis and increased intraocular pressure. In children, suppression of growth may occur. Increased intracranial pressure may occur and possibly account for headache, insomnia and fatigue. Subcapsular cataracts may result from prolonged usage. Long-term use of all corticosteroids results in catabolic effects characterized by negative protein and calcium balance. Osteoporosis, spontaneous fractures and aseptic necrosis of the hip and humerus may occur as part of this catabolic phenomenon. Where hypokalemia and other symptoms associated with fluid and electrolyte imbalance call for potassium supplementation and salt poor or salt-free diets, these may be instituted and are compatible with diet requirements for ulcerative proctitis. Local effects of itching and burning have been reported following rectal use. Symptoms: SYMPTOMS AND TREATMENT OF OVERDOSAGE Acute toxicity, even with massive doses is not a clinical problem. Chronic toxicity involves manifestations of the physiologic effects described above and include Cushingoid appearance, muscle weakness, osteoporosis, posterior subcapsular cataracts, peptic ulcers, hypertension, psychosis, and growth suppression in children. Glaucoma, pancreatitis, reactivation of tuberculosis and poor wound healing may occur. Sodium and fluid retention with potassium loss occur to varying degrees, depending upon the mineralocorticoid effects of the particular corticosteroid. Treatment: Acute overdosage probably requires no treatment. Acute overdosage requires no tapering as in withdrawal of patients on long-term administration. If there is any question that other drugs have 4
5 been ingested simultaneously, then standard measures for those drugs should be followed as per instructions for their management. 1) Avoid chronic dosage for durations greater than 3 weeks when possible. 2) When chronic dosage for periods greater than 3 weeks is essential, attempts should be made to manage the underlying disease if possible with alternate day dosage using single daily doses on alternate mornings of shorter acting preparations such as prednisone, prednisolone or methylprednisolone. 3) Even with alternate day dosage of appropriate agents, continued attempts should be made to minimize dosage compatible with maintained control of the underlying disease. 4) The diet should have adequate protein content but caloric restrictions should be considered because of the apparent appetite stimulating properties of the corticosteroids. 5) The ultimate treatment of toxicity should be avoidance of inappropriate usage or if toxicity is already present, withdrawal of the corticosteroids and conventional management of those effects which are treatable such as peptic ulcers, cataracts and hypertension. DOSAGE AND ADMINISTRATION The usual dose is one applicator full once or twice daily for two or three weeks, and every second day thereafter, administered rectally. The patient direction insert describes how to use the aerosol container and applicator. Satisfactory response usually occurs within five to seven days marked by a decrease in symptoms. Symptomatic improvement should not be used as the sole criterion for evaluating efficacy. Sigmoidoscopy is also recommended to judge dosage adjustment, duration of therapy and rate of improvement. The hydrocortisone acetate is present at 10% in a foam vehicle, which is aerosolized in an aerosol container. A special applicator is provided which is designed to deliver approximately 6.5 ml (900 mg) of foam, 80 mg of hydrocortisone (90 mg hydrocortisone acetate). 5
6 PHARMACEUTICAL INFORMATION Drug Substance: Common Name: Hydrocortisone Acetate Chemical Name: Pregn-4-ene-3, 20-dione, 21-(acetyloxy)-11, 17-dihydroxy-, (11 )-. Molecular Weight: Structural Formula: Physical Form: white to practically white, odourless crystalline powder Solubility: insoluble in water, slightly soluble in alcohol and chloroform Melting point: 220 C. Composition: Contains hydrocortisone acetate USP 10% as the sole active ingredient in a foam containing propylene glycol, ethoxylated stearyl alcohol, polyoxyethylene-10 stearyl ether, cetyl alcohol, methylparaben, propylparaben, triethanolamine, water and inert propellants, isobutane and propane. AVAILABILITY OF DOSAGE FORMS Cortifoam (hydrocortisone acetate USP 10%) is supplied in an aerosol container with a special rectal applicator. Each applicatorful delivers approximately 900 mg of foam containing approximately 80 mg of hydrocortisone as 90 mg of hydrocortisone acetate. The 15 g aerosol container will deliver approximately 14 applications. Contents of the container are under pressure and are flammable. Do not use in the presence of an open flame or spark. 6
7 Do not puncture or incinerate the aerosol container. Store at room temperature, not over 50 o C. Clinical Pharmacology: PHARMACOLOGY 1 A bioavailability study was designed and conducted by William H. Barr, Pharm. D., PhD at the Department of Pharmacy and Pharmaceutics and the Department of Gastroenterology at Medical College of Virginia/Virginia Commonwealth University to assess the extent of absorption of biologically active hydrocortisone into the systemic circulation from the rectal route (from Cortifoam rectal foam) versus oral administration. The study was required to detect with a 95% confidence limit a 25% difference in the amount absorbed. To decrease the intra and intersubject variability in both absorption and first pass metabolism, a deuterated and a non-deuterated hydrocortisone were administered simultaneously. In the study, seven normal male student subjects received orally 50 mg of deuterated hydrocortisone alcohol initially dissolved in 10 ml 95% alcohol and diluted in 100 ml distilled water. The subjects immediately afterward received a self-administered dose of 50 mg non-deuterated hydrocortisone as the acetate in about 5 ml of the rectal foam. To suppress endogenous hydrocortisone each subject received a 0.75 mg tablet of dexamethasone the night before and again on the morning of the study. The subjects received the hydrocortisone after a 12-hour fast and ate a light fat-free breakfast and a light lunch. The subjects were ambulatory throughout the study. Blood samples were taken just prior to dosing to determine base levels of endogenous hydrocortisone and at 0, 10, 20, 40, 70, 100, 140, 180, 240, 360, 600 and 720 minutes. The blood was separated and the plasma frozen until assayed. The ratio of deuterated hydrocortisone to normal hydrocortisone was determined by mass spectrometer in conjunction with a gas chromatograph. The apparent bioavailabilities of rectal versus oral ranged from %. However, the endogenous hydrocortisone as evident from zero time, as the result of incomplete endogenous hydrocortisone suppression with dexamethasone, was applied to the bioavailability data and the actual bioavailabilities dropped to 1-10% with an average bioavailability 3% rectal compared with oral administration. The study concluded that the rectal absorption of hydrocortisone is very limited (of the order of 10% or less), and the beneficial results of Cortifoam appear to be due to local effects on the mucosa rather than a result of systemic effects due to absorbed hydrocortisone. Pharmacology: 1 This section is taken without change from the original product monograph dated February 9,
8 The effects of corticosteroids are numerous and widespread. They influence carbohydrate, protein, fat and purine metabolism; electrolyte and water balance; and the functions of the cardiovascular system, the kidney, skeletal muscle, the nervous system and other organs and tissues. Furthermore, the corticosteroids endow the organism with the capacity to resist many types of noxious stimuli and environmental change. In Cortifoam, the most significant pharmacological property of hydrocortisone is its anti-inflammatory property. Hydrocortisone and the synthetic analogs of hydrocortisone have the capacity to prevent or suppress the development of the local heat, redness, swelling and tenderness by which inflammation is recognized. At the microscopic level, they inhibit not only the early phenomena of the inflammatory process (edema, fibrin deposition, capillary dilatation, migration of leukocytes into the inflamed area, and phagocytic activity) but also the later manifestations (capillary proliferation, fibroblast proliferation, deposition of collagen, and, still later, cicatrization). Although understanding of these effects is unsatisfactory, many observations have been made that have therapeutic relevance and that must be taken into account in explanatory formulations. Perhaps the most important of these for the physician is that corticosteroids inhibit the inflammatory response whether the inciting agent is radiant, mechanical, chemical, infectious, or immunological. In clinical terms, the administration of corticosteroids for their anti-inflammatory effects is palliative therapy; the underlying cause of the disease remains; the inflammatory manifestations are merely suppressed. It is this suppression of inflammation and its consequences that has made the corticosteroids such valuable therapeutic agents - indeed, at times lifesaving. It is also this property that gives them a nearly unique potential for therapeutic disaster. The signs and symptoms of inflammation are expressions of the disease process that are often used by the physician in diagnosis and in evaluating the effectiveness of treatment. These may be missing in patients treated with glucocorticoids. Hydrocortisone and presumably other anti-inflammatory steroids are to be found in inflamed tissues, although they are not selectively concentrated there. Anti-inflammatory effects depend upon the direct local action of the steroids, since those that do not require metabolic modification for activity are very effective on topical application to skin or eye. Several discrete effects of the steroids relevant to their anti-inflammatory properties are beginning to be understood. For example, a considerable amount is known about the inhibitory effects of the glucocorticoids on fibroblasts, phenomena that are of undoubted importance in the suppression of later phases of inflammation. The mechanism by which glucocorticoids inhibit accumulation of macrophages is to block the effect of the migration inhibitory factor (MIF), a lymphokine produced by an antigen activated lymphocyte, on macrophages; that is, the movement of these cells is no longer impeded and the macrophages no longer accumulate. It has also been found that hydrocortisone, when added to a mixture of cultured lymphocytes and monocytes, causes the appearance of a factor in the culture medium that stimulates the migration of polymorphonuclear leukocytes. This may account for suppression of infiltration by polymorphonuclear cells. The effects of glucocorticoids on neutrophils have been reviewed by Mishler. Low concentrations of glucocorticoids inhibit the formation by macrophages of an 8
9 activator of plasminogen, an effect that may contribute to their anti-inflammatory properties. There is also substantial evidence that the glucocorticoids inhibit release of arachidonic acid from phospholipids and thereby decrease formation of prostaglandins and related compounds, such as prostaglandin endoperoxides and thromboxane, which may play an important role in inflammation. TOXICOLOGY 2 Acute toxicity, even with massive doses, is not a clinical problem. However, two categories of toxic effects are observed in the therapeutic use of adrenocorticosteroids: those resulting from withdrawal and those resulting from continued use of large doses. Acute adrenal insufficiency results from too rapid withdrawal of corticosteroids after prolonged therapy. In addition to pituitary-adrenal suppression, the principle complications resulting from prolonged therapy with corticosteroids are fluid and electrolyte disturbances; hyperglycemia and glycosuria; increased susceptibility to infections including tuberculosis; peptic ulcers, which may bleed or perforate; osteoporosis; a characteristic myopathy; behavioral disturbances; posterior subcapsular cataracts; arrest of growth and Cushing's habitus, consisting in "moon face", "buffalo hump", enlargement of supraclavicular fat pads, "central obesity", striae, ecchymoses, acne and hirsutism. Paladin Labs Inc Royalmount, Suite 102 Montréal, Québec H4P 2T4 2 This section is taken without change from the original product monograph dated February 9,
10 REFERENCES 3 1. Korelitz and Lindner. In: Goodman and Gilman's "The Pharmacological Basis of Therapeutics", 6th edition, page 1491, Farmer RG and Schumacher OP. Treatment of ulcertive colitis with hydrocortisone enemas. Dis Col & Rect 1970; 13: Kratzer GL. Treatment of u1cerative colitis with 10% hydrocortisone acetate foam (Preliminary Study). Presented at Northeastern Proctologic Conference. Bermuda. Oct. 19, Kratzer GL, Collmann IR, Kalser MH, McMahon WA, Rosser RG and Salvati EP. Conference on chronic ulcerative colitis and clinical experience with Cortifoam. AJCR 1970; 1: Kratzer GL, Panas PG and Onsanit T. Mucosal biopsy in ulcerative colitis for evaluating topical hydrocortisone therapy. Dis Col & Rect 1973; Scherl ND and Scherl BA. Adjunctive use of a steroid rectal foam in the treatment of ulcerative colitis. Dis Col & Rec 1973; Mar-Apr. 7. Rosser RG. Clinical investigation of a rectally administered hydrocortisone acetate foam. Clinical Medicine 1975; 82: Clark ML. Clinical Trials - A local foam aerosol in ulcerative colitis. The Practitioner 1977; 219: Hay DJ, Sharma H and Irving MH. Spread of steroid-containing foam after intrarectal administration. Brit Med Journal 1979; 1: Farthing MJG, Rutland MD and Clark ML. Retrograde spread of hydrocortisone containing foam given intrarectally in ulcerative colitis. Brit Med Journal 1979; 2: Ruddell WSJ, Dickinson RJ, Dixon MF and Axon ATR. Treatment of distal ulcerative colitis (proctosigmoiditis) in relapse: comparison of hydrocortisone enemas and rectal hydrocortisone foam. Gut 1980; 21: Barr WH, Kline B, Beightol L and Zfass A. Bioavailability of hydrocortisone acetate rectal foam (CORTIFOAM ). Research Report, Medical College of Virginia, Virginia Commonwealth University, October 25, This section was revised when the prescribing info was updated in References 13, 14 & 15 were added. 10
11 13. Somerville KW, Langman MJS, Kane SP, MacGilchrist AJ, Watkinson G and Salmon P. Effect of treatment on symptoms and quality of life in patients with ulcerative colitis: comparative trial of hydrocortisone acetate foam and prednisolone 21-phosphate enemas. BMJ 1985; 291: Mollmann H, Barth J, Mollmann C, Tunn S, Krieg M and Derendorf H. Pharmacokinetics and rectal bioavailability of hydrocortisone acetate. J Pharm Sciences 1991; 80: Goodacre RL and Shannon S. Cortifoam in distal ulcerative colitis. Data on File. Reed & Carnrick
PRESCRIBING INFORMATION
 PRESCRIBING INFORMATION Pr FLORINEF (fludrocortisone acetate) 0.1 mg Tablets Mineralocorticoid for adrenal insufficiency Paladin Labs Inc. Date of Preparation: 6111 Royalmount Avenue, Suite 102 May 1,
PRESCRIBING INFORMATION Pr FLORINEF (fludrocortisone acetate) 0.1 mg Tablets Mineralocorticoid for adrenal insufficiency Paladin Labs Inc. Date of Preparation: 6111 Royalmount Avenue, Suite 102 May 1,
Prescribing Information. Taro-Clobetasol. Taro-Clobetasol
 Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
Prescribing Information Pr Taro-Clobetasol Clobetasol Propionate Cream USP, 0.05% w/w Pr Taro-Clobetasol Clobetasol Propionate Ointment USP, 0.05% w/w Therapeutic Classification Topical corticosteroid
PRESCRIBING INFORMATION. (betamethasone sodium phoshate) Enema 5 mg/100 ml. Topical Corticosteroid
 PRESCRIBING INFORMATION Pr BETNESOL (betamethasone sodium phoshate) Enema 5 mg/100 ml Topical Corticosteroid Paladin Labs Inc. Date of revision: 100 Alexis Nihon Blvd, Suite 600 May 1, 2009 St Laurent,
PRESCRIBING INFORMATION Pr BETNESOL (betamethasone sodium phoshate) Enema 5 mg/100 ml Topical Corticosteroid Paladin Labs Inc. Date of revision: 100 Alexis Nihon Blvd, Suite 600 May 1, 2009 St Laurent,
Prescribing Information
 Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
Prescribing Information Pr DERMOVATE Cream (clobetasol propionate cream, USP) Pr DERMOVATE Ointment (clobetasol propionate ointment, USP) Topical corticosteroid TaroPharma Preparation Date: A Division
Cordran Cream and Cordran Ointment Flurandrenolide, USP
 Cordran Cream and Cordran Ointment Flurandrenolide, USP DESCRIPTION Cordran (flurandrenolide, USP) is a potent corticosteroid intended for topical use. Flurandrenolide occurs as white to off-white, fluffy,
Cordran Cream and Cordran Ointment Flurandrenolide, USP DESCRIPTION Cordran (flurandrenolide, USP) is a potent corticosteroid intended for topical use. Flurandrenolide occurs as white to off-white, fluffy,
RAYOS (prednisone tablet delayed release) oral tablet
 RAYOS (prednisone tablet delayed release) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
RAYOS (prednisone tablet delayed release) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
Pharmacology of Corticosteroids
 Pharmacology of Corticosteroids Dr. Aliah Alshanwani Dept. of Pharmacology College of Medicine, KSU Feb 2018 1 The Corticosteroids are steroid hormones produced by the adrenal cortex. They consist of two
Pharmacology of Corticosteroids Dr. Aliah Alshanwani Dept. of Pharmacology College of Medicine, KSU Feb 2018 1 The Corticosteroids are steroid hormones produced by the adrenal cortex. They consist of two
For topical use only. Not for oral, ophthalmic, or intravaginal use.
 DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
DESOXIMETASONE Ointment USP, 0.25% For topical use only. Not for oral, ophthalmic, or intravaginal use. Rx only DESCRIPTION Desoximetasone ointment USP, 0.25% contains the active synthetic corticosteroid
PRESCRIBING INFORMATION. ratio-topisone. Betamethasone dipropionate USP. 0.5 mg Cream, Ointment and Lotion. Topical corticosteroid
 PRESCRIBING INFORMATION ratio-topisone Betamethasone dipropionate USP 0.5 mg Cream, Ointment and Lotion Topical corticosteroid Teva Canada Limited Date of Revision 30 Novopharm Court January 21, 2013 Toronto,
PRESCRIBING INFORMATION ratio-topisone Betamethasone dipropionate USP 0.5 mg Cream, Ointment and Lotion Topical corticosteroid Teva Canada Limited Date of Revision 30 Novopharm Court January 21, 2013 Toronto,
Corticosteroids. Abdulmoein Al-Agha, FRCPCH Professor of Pediatric Endocrinology, King Abdulaziz University Hospital,
 Corticosteroids Abdulmoein Al-Agha, FRCPCH Professor of Pediatric Endocrinology, King Abdulaziz University Hospital, http://aagha.kau.edu.sa History 1855 Addison's disease 1856 Adrenal glands essential
Corticosteroids Abdulmoein Al-Agha, FRCPCH Professor of Pediatric Endocrinology, King Abdulaziz University Hospital, http://aagha.kau.edu.sa History 1855 Addison's disease 1856 Adrenal glands essential
INVELTYS (loteprednol etabonate ophthalmic suspension) 1%, for topical ophthalmic use Initial U.S. Approval: 1998
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use INVELTYS safely and effectively. See full prescribing information for INVELTYS. INVELTYS (loteprednol
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use INVELTYS safely and effectively. See full prescribing information for INVELTYS. INVELTYS (loteprednol
Chemically, clobetasol propionate is 21-chloro-9-fluoro,11β,17-dihydroxy-16βmethylpregna-1,4-diene-3,20-dione
 Clobetasol Propionate Cream USP, 0.05% FOR TOPICAL DERMATOLOGIC USE ONLY NOT FOR OPHTHALMIC, ORAL, OR INTRAVAGINAL USE. DESCRIPTION Clobetasol propionate cream USP, 0.05% contain the active compound clobetasol
Clobetasol Propionate Cream USP, 0.05% FOR TOPICAL DERMATOLOGIC USE ONLY NOT FOR OPHTHALMIC, ORAL, OR INTRAVAGINAL USE. DESCRIPTION Clobetasol propionate cream USP, 0.05% contain the active compound clobetasol
New Zealand Data Sheet
 New Zealand Data Sheet Prednisolone-AFT 1% Prednisolone acetate (Ph Eur) 1% w/v ophthalmic suspension Presentation Prednisolone-AFT 1% is a milky white suspension in an eyedropper bottle for ophthalmic
New Zealand Data Sheet Prednisolone-AFT 1% Prednisolone acetate (Ph Eur) 1% w/v ophthalmic suspension Presentation Prednisolone-AFT 1% is a milky white suspension in an eyedropper bottle for ophthalmic
Prednisolone Sodium Phosphate Ophthalmic Solution USP, 1% (Sterile) Rx only
 Prednisolone Sodium Phosphate Ophthalmic Solution USP, 1% (Sterile) Rx only DESCRIPTION Prednisolone Sodium Phosphate Ophthalmic Solution, 1%, is a sterile solution for ophthalmic administration having
Prednisolone Sodium Phosphate Ophthalmic Solution USP, 1% (Sterile) Rx only DESCRIPTION Prednisolone Sodium Phosphate Ophthalmic Solution, 1%, is a sterile solution for ophthalmic administration having
HYDROCORTISONE OINTMENT USP,
 HYDROCORTISONE- hydrocortisone ointment E. Fougera & Co. a division of Fougera Pharmaceuticals Inc. ---------- HYDROCORTISONE OINTMENT USP, 1% Rx only For External Us e Only Not For Ophthalmic Us e DESCRIPTION:
HYDROCORTISONE- hydrocortisone ointment E. Fougera & Co. a division of Fougera Pharmaceuticals Inc. ---------- HYDROCORTISONE OINTMENT USP, 1% Rx only For External Us e Only Not For Ophthalmic Us e DESCRIPTION:
D90 (27/10/2005) Final SmPC NL/H/653/01
 1/6 1. NAME OF THE MEDICINAL PRODUCT MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg of dexamethasone phosphate as dexamethasone
1/6 1. NAME OF THE MEDICINAL PRODUCT MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg of dexamethasone phosphate as dexamethasone
PRODUCT MONOGRAPH. (Fluorometholone 0.1% Ophthalmic Suspension), USP. Corticosteroid
 PRODUCT MONOGRAPH Pr Sandoz Fluorometholone (Fluorometholone 0.1% Ophthalmic Suspension), USP Corticosteroid Sandoz Canada Inc., Date of Revision: June 21, 2012 145 Jules-Léger Boucherville, QC, Canada
PRODUCT MONOGRAPH Pr Sandoz Fluorometholone (Fluorometholone 0.1% Ophthalmic Suspension), USP Corticosteroid Sandoz Canada Inc., Date of Revision: June 21, 2012 145 Jules-Léger Boucherville, QC, Canada
CONTRAINDICATIONS Active ocular infections (4).
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DEXTENZA safely and effectively. See full prescribing information for DEXTENZA. DEXTENZA (dexamethasone
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DEXTENZA safely and effectively. See full prescribing information for DEXTENZA. DEXTENZA (dexamethasone
Emflaza. (deflazacort) New Product Slideshow
 Emflaza (deflazacort) New Product Slideshow Introduction Brand name: Emflaza Generic name: Deflazacort Pharmacological class: Corticosteroid Strength and Formulation: 6mg, 18mg, 30mg, 36mg tablets; 22.75mg/mL
Emflaza (deflazacort) New Product Slideshow Introduction Brand name: Emflaza Generic name: Deflazacort Pharmacological class: Corticosteroid Strength and Formulation: 6mg, 18mg, 30mg, 36mg tablets; 22.75mg/mL
50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate).
 DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
DUPISOR Composition Gel 50 microgram/g Calcipotriol and 500 microgram/g betamethasone (as dipropionate). Action Calcipotriol is a non-steroidal antipsoriatic agent, derived from vitamin D. Calcipotriol
SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM
 Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML Liquifilm Sterile Eye Suspension COMPOSITION FML Liquifilm Sterile Eye Suspension contains: Fluorometholone 1,0 mg/ml Liquifilm
Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM FML Liquifilm Sterile Eye Suspension COMPOSITION FML Liquifilm Sterile Eye Suspension contains: Fluorometholone 1,0 mg/ml Liquifilm
For the list of inactive ingredients see section "Additional information".
 Patient leaflet in accordance with the Pharmacists' Regulations (Preparations) - 1986 This medicine is to be supplied by physician s prescription only Proctofoam HC Rectal foam Active ingredients: Hydrocortisone
Patient leaflet in accordance with the Pharmacists' Regulations (Preparations) - 1986 This medicine is to be supplied by physician s prescription only Proctofoam HC Rectal foam Active ingredients: Hydrocortisone
INDICATIONS For steroid responsive inflammation of the palpebral and bulbar conjunctiva, cornea, and anterior segment of the eye globe.
 Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM PRED FORTE Sterile Eye Suspension COMPOSITION PRED FORTE Sterile Eye Suspension contains: Prednisolone acetate 10 mg/ml Preservative:
Page 1 of 5 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME AND DOSAGE FORM PRED FORTE Sterile Eye Suspension COMPOSITION PRED FORTE Sterile Eye Suspension contains: Prednisolone acetate 10 mg/ml Preservative:
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION
 AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
AUSTRALIAN PRODUCT INFORMATION FLAREX (FLUOROMETHOLONE ACETATE) EYE DROPS SUSPENSION 1 NAME OF THE MEDICINE Fluorometholone acetate. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION The active ingredient in
Corticosteroids. Hawler Medical University College of Medicine Department of Pharmacology and Biophysics Dr.Susan Abdulkadir Farhadi MSc Pharmacology
 Corticosteroids Hawler Medical University College of Medicine Department of Pharmacology and Biophysics Dr.Susan Abdulkadir Farhadi MSc Pharmacology Objectives By the end of this lecture you should be
Corticosteroids Hawler Medical University College of Medicine Department of Pharmacology and Biophysics Dr.Susan Abdulkadir Farhadi MSc Pharmacology Objectives By the end of this lecture you should be
NEW ZEALAND DATA SHEET 1. PRODUCT NAME
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Flucon fluorometholone 0.1% Eye Drops Suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each ml of Flucon contains 1.0 mg of fluorometholone (0.1% w/v). Excipient
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME (strength pharmaceutical form) Hydrocortisone Cream 1% (PSM) Topical Cream 1% w/w 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Hydrocortisone Cream
1. PRODUCT NAME (strength pharmaceutical form) Hydrocortisone Cream 1% (PSM) Topical Cream 1% w/w 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Name and strength of the active substance Hydrocortisone Cream
ENTOFOAM Rectal Foam (Hydrocortisone acetate)
 Published on: 10 Jul 2014 ENTOFOAM Rectal Foam (Hydrocortisone acetate) Composition Each canister contains: Hydrocortisone Acetate, IP. 10% w/w Dosage Form Aerosol rectal foam Pharmacology Pharmacodynamics
Published on: 10 Jul 2014 ENTOFOAM Rectal Foam (Hydrocortisone acetate) Composition Each canister contains: Hydrocortisone Acetate, IP. 10% w/w Dosage Form Aerosol rectal foam Pharmacology Pharmacodynamics
CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP)
 CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) DESCRIPTION CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) is a
CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) DESCRIPTION CORTISPORIN Cream (neomycin and polymyxin B sulfates and hydrocortisone acetate cream, USP) is a
FERRING PHARMACEUTICALS. Enjoy The simple COR/939/2014/CH3
 Enjoy The simple pleasures of life COR/939/2014/CH3 Ulcerative colitis disrupts the simple pleasures in life Patients with ulcerative colitis may live with a considerable symptom burden despite medical
Enjoy The simple pleasures of life COR/939/2014/CH3 Ulcerative colitis disrupts the simple pleasures in life Patients with ulcerative colitis may live with a considerable symptom burden despite medical
Adrenocorticosteroids
 Adrenocorticosteroids Divided into those having: 1. Important effects on intermediary metabolism and immune function = glucocorticoids (cortisol). 2. Salt-retaining activity = mineralocorticoids (aldosterone).
Adrenocorticosteroids Divided into those having: 1. Important effects on intermediary metabolism and immune function = glucocorticoids (cortisol). 2. Salt-retaining activity = mineralocorticoids (aldosterone).
Adrenal Pharmacology
 Adrenal Pharmacology Pharmacology Team Naim Kittana, Suhaib Hattab, Ansam Sawalha, Adham Abu Taha, Waleed Sweileh, Ramzi Shawahneh Faculty of Medicine & Health Sciences An-Najah National University 1 Steroidal
Adrenal Pharmacology Pharmacology Team Naim Kittana, Suhaib Hattab, Ansam Sawalha, Adham Abu Taha, Waleed Sweileh, Ramzi Shawahneh Faculty of Medicine & Health Sciences An-Najah National University 1 Steroidal
Each gram of the ointment contains 0.25 mg Fluocinolone Acetonide in a base containing White Petrolatum.
 FLUOCINOLONE ACETONIDE- fluocinolone acetonide ointment E. Fougera & Co. a division of Fougera Pharmaceuticals Inc. ---------- FLUOCINOLONE ACETONIDE OINTMENT, USP, 0.025% For Topical Us e Only. Not for
FLUOCINOLONE ACETONIDE- fluocinolone acetonide ointment E. Fougera & Co. a division of Fougera Pharmaceuticals Inc. ---------- FLUOCINOLONE ACETONIDE OINTMENT, USP, 0.025% For Topical Us e Only. Not for
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 1 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION mg per tablet Active: Prednisolone 1.0 Other ingredients:
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE VETERINARY MEDICINAL PRODUCT Prednicare Tablets 1 mg 2. QUALITATIVE AND QUANTITATIVE COMPOSITION mg per tablet Active: Prednisolone 1.0 Other ingredients:
Corticosteroids. Veterinary Pharmacology Endocrine System. University of Tehran Faculty of Veterinary Medicine Academic Year
 Veterinary Pharmacology Endocrine System Corticosteroids University of Tehran Faculty of Veterinary Medicine Academic Year 2008-9 Goudarz Sadeghi, DVM, PhD, DSc Associate Professor of Pharmacology Introduction
Veterinary Pharmacology Endocrine System Corticosteroids University of Tehran Faculty of Veterinary Medicine Academic Year 2008-9 Goudarz Sadeghi, DVM, PhD, DSc Associate Professor of Pharmacology Introduction
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Synacthen 250 micrograms/ml solution for injection. Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active Substance 250 micrograms of Tetracosactide.
1 NAME OF THE MEDICINAL PRODUCT Synacthen 250 micrograms/ml solution for injection. Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Active Substance 250 micrograms of Tetracosactide.
NEW ZEALAND DATA SHEET. Each applicator full of Colifoam contains approximately 90 mg hydrocortisone acetate.
 NEW ZEALAND DATA SHEET COLIFOAM RECTAL 1. Product Name Colifoam Rectal, 10% w/w, foam. 2. Qualitative and Quantitative Composition One gram of foam contains 100 mg of hydrocortisone acetate. Each applicator
NEW ZEALAND DATA SHEET COLIFOAM RECTAL 1. Product Name Colifoam Rectal, 10% w/w, foam. 2. Qualitative and Quantitative Composition One gram of foam contains 100 mg of hydrocortisone acetate. Each applicator
DIPROLENE AF (augmented betamethasone dipropionate) Cream, 0.05% for topical use Initial U.S. Approval: 1983
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DIPROLENE AF Cream safely and effectively. See full prescribing information for DIPROLENE AF Cream.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DIPROLENE AF Cream safely and effectively. See full prescribing information for DIPROLENE AF Cream.
NEW ZEALAND DATA SHEET
 1. PRODUCT NAME KENACOMB ear drops NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g contains the following active ingredients: 0.025% gramicidin, 0.25% neomycin, 100,000 units nystatin,
1. PRODUCT NAME KENACOMB ear drops NEW ZEALAND DATA SHEET 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 g contains the following active ingredients: 0.025% gramicidin, 0.25% neomycin, 100,000 units nystatin,
Pharmacology Adrenal Steroids. Adrenal steroids. Dr. Ahmad Al-Zohyri. Dr. Ahmad Al-Zohyri Lec. 10
 Adrenal steroids Dr. Ahmad Al-Zohyri Lec. 10 Total Lectures No. 23 Adrenal steroids (corticosteroids & corticotrophins) They are hormones produced by the adrenal cortex, they include: hydrocortisone (cortisol),
Adrenal steroids Dr. Ahmad Al-Zohyri Lec. 10 Total Lectures No. 23 Adrenal steroids (corticosteroids & corticotrophins) They are hormones produced by the adrenal cortex, they include: hydrocortisone (cortisol),
Fluocinolone Acetonide 0.01% Topical Oil (Scalp Oil)
 Fluocinolone Acetonide 0.01% Topical Oil (Scalp Oil) For Topical Use Only- Not for Oral, Ophthalmic, or Intravaginal Use DESCRIPTION Fluocinolone Acetonide 0.01% Topical Oil contains fluocinolone acetonide
Fluocinolone Acetonide 0.01% Topical Oil (Scalp Oil) For Topical Use Only- Not for Oral, Ophthalmic, or Intravaginal Use DESCRIPTION Fluocinolone Acetonide 0.01% Topical Oil contains fluocinolone acetonide
SODIUM POLYSTYRENE SULFONATE Suspension, USP
 SODIUM POLYSTYRENE SULFONATE Suspension, USP DESCRIPTION Sodium Polystyrene Sulfonate Suspension, USP can be administered orally or in an enema. It is a cherryflavored suspension containing 15 grams of
SODIUM POLYSTYRENE SULFONATE Suspension, USP DESCRIPTION Sodium Polystyrene Sulfonate Suspension, USP can be administered orally or in an enema. It is a cherryflavored suspension containing 15 grams of
Revised: 03/2018. *Sections or subsections omitted from the full prescribing information are not listed.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DIPROLENE Ointment safely and effectively. See full prescribing information for DIPROLENE Ointment.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DIPROLENE Ointment safely and effectively. See full prescribing information for DIPROLENE Ointment.
PRESCRIBING INFORMATION. Cream 0.025% Topical Corticosteroid
 PRESCRIBING INFORMATION Pr PROPADERM (beclomethasone dipropionate) Cream 0.025% Topical Corticosteroid Valeant Canada LP 2150 St-Elzear Blvd. West, Laval, Quebec, Canada H7L 4A8 Date of preparation: October
PRESCRIBING INFORMATION Pr PROPADERM (beclomethasone dipropionate) Cream 0.025% Topical Corticosteroid Valeant Canada LP 2150 St-Elzear Blvd. West, Laval, Quebec, Canada H7L 4A8 Date of preparation: October
Agreed Core Safety Profile for Budesonide nasal spray suspension and Budesonide nasal powder
 CSP Drug Budesonide Substance Date 13 Oct 2011 rev 11Nov Supersedes 18 Aug 2011 Agreed Core Safety Profile for DK/H/PSUR/0041/001 TABLE OF CONTENTS PAGE TITLE PAGE... 1 TABLE OF CONTENTS... 2 Introduction...
CSP Drug Budesonide Substance Date 13 Oct 2011 rev 11Nov Supersedes 18 Aug 2011 Agreed Core Safety Profile for DK/H/PSUR/0041/001 TABLE OF CONTENTS PAGE TITLE PAGE... 1 TABLE OF CONTENTS... 2 Introduction...
TOPCORT Cream/Ointment (Mometasone furoate 0.1%)
 Published on: 10 Jul 2014 TOPCORT Cream/Ointment (Mometasone furoate 0.1%) Composition TOPCORT Cream Mometasone Furoate, IP... 0.1% w/w In a cream base... q.s. TOPCORT Ointment Mometasone Furoate, IP...
Published on: 10 Jul 2014 TOPCORT Cream/Ointment (Mometasone furoate 0.1%) Composition TOPCORT Cream Mometasone Furoate, IP... 0.1% w/w In a cream base... q.s. TOPCORT Ointment Mometasone Furoate, IP...
For a full list of excipients, see section 6.1. A sterile, clear, bright, colourless, aqueous solution.
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Sofradex Ear/Eye Drops, Solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each bottle contains 0.5% w/v of Framycetin Sulphate, Dexamethasone
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Sofradex Ear/Eye Drops, Solution 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each bottle contains 0.5% w/v of Framycetin Sulphate, Dexamethasone
SYNACTHEN DEPOT i.m. tetracosactide hexaacetate
 SYNACTHEN DEPOT i.m. tetracosactide hexaacetate 1 mg/ml suspension for injection New Zealand Consumer Medicine Information What is in this leaflet Read all of this leaflet carefully before you are given
SYNACTHEN DEPOT i.m. tetracosactide hexaacetate 1 mg/ml suspension for injection New Zealand Consumer Medicine Information What is in this leaflet Read all of this leaflet carefully before you are given
Ultravate (halobetasol propionate) Cream, 0.05% (halobetasol propionate) Ointment, 0.05% For Dermatological Use Only. Not for Ophthalmic Use.
 Ultravate (halobetasol propionate) Cream, 0.05% (halobetasol propionate) Ointment, 0.05% For Dermatological Use Only. Not for Ophthalmic Use. Rx only DESCRIPTION Ultravate contains halobetasol propionate,
Ultravate (halobetasol propionate) Cream, 0.05% (halobetasol propionate) Ointment, 0.05% For Dermatological Use Only. Not for Ophthalmic Use. Rx only DESCRIPTION Ultravate contains halobetasol propionate,
FLOMIST Aqueous Nasal Spray (Fluticasone propionate)
 Published on: 10 Jul 2014 FLOMIST Aqueous Nasal Spray (Fluticasone propionate) Composition FLOMIST Aqueous Nasal Spray Each spray delivers: Fluticasone Propionate BP...50 mcg Fluticasone Propionate BP...
Published on: 10 Jul 2014 FLOMIST Aqueous Nasal Spray (Fluticasone propionate) Composition FLOMIST Aqueous Nasal Spray Each spray delivers: Fluticasone Propionate BP...50 mcg Fluticasone Propionate BP...
See 17 for PATIENT COUNSELING INFORMATION. Revised: 03/2018
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DIPROLENE Lotion safely and effectively. See full prescribing information for DIPROLENE Lotion. DIPROLENE
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use DIPROLENE Lotion safely and effectively. See full prescribing information for DIPROLENE Lotion. DIPROLENE
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Dexafree 1 mg/ml, eye drops, solution in single-dose container 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Dexafree 1 mg/ml, eye drops, solution in single-dose container 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 1 ml solution contains 1 mg
INDICATIONS ACULAR 0,4% ophthalmic solution is indicated for the reduction of ocular pain and burning/stinging following corneal refractive surgery.
 Page 1 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME (and dosage form) ACULAR 0,4% COMPOSITION ACULAR 0,4% ophthalmic solution contains: Ketorolac tromethamine: 4 mg/ml Preservative: Benzalkonium chloride
Page 1 SCHEDULING STATUS Schedule 4 PROPRIETARY NAME (and dosage form) ACULAR 0,4% COMPOSITION ACULAR 0,4% ophthalmic solution contains: Ketorolac tromethamine: 4 mg/ml Preservative: Benzalkonium chloride
Package leaflet: Information for the patient. Dexametason Abcur 4 mg tablets. Dexamethasone
 Package leaflet: Information for the patient Dexametason Abcur 1 mg tablets Dexametason Abcur 4 mg tablets Dexamethasone Read all of this leaflet carefully before you start taking this medicine because
Package leaflet: Information for the patient Dexametason Abcur 1 mg tablets Dexametason Abcur 4 mg tablets Dexamethasone Read all of this leaflet carefully before you start taking this medicine because
HydroVal (Hydrocortisone Valerate)
 PRODUCT MONOGRAPH HydroVal (Hydrocortisone Valerate) Cream & Ointment 0.2 % Topical Corticosteroid TaroPharma Preparation Date: A Division of Taro Pharmaceuticals Inc. Sept 02, 2003 130 East Drive Brampton,
PRODUCT MONOGRAPH HydroVal (Hydrocortisone Valerate) Cream & Ointment 0.2 % Topical Corticosteroid TaroPharma Preparation Date: A Division of Taro Pharmaceuticals Inc. Sept 02, 2003 130 East Drive Brampton,
PRODUCT INFORMATION RESONIUM A. Na m
 PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
PRODUCT INFORMATION RESONIUM A NAME OF THE MEDICINE Non-proprietary Name Sodium polystyrene sulfonate Chemical Structure CH - 2 CH SO 3 Na + n CAS Number 28210-41-5 [9003-59-2] CH 2 CH SO - 3 m DESCRIPTION
PACKAGE LEAFLET: INFORMATION FOR THE USER. Flutarzole 0,05% w/w cream, Fluticasone propionate
 PACKAGE LEAFLET: INFORMATION FOR THE USER Flutarzole 0,05% w/w cream, Fluticasone propionate 1. IDENTIFICATION OF THE MEDICINAL PRODUCT 1.1. Trade name Flutarzole 1.2. Composition Active substance: Fluticasone
PACKAGE LEAFLET: INFORMATION FOR THE USER Flutarzole 0,05% w/w cream, Fluticasone propionate 1. IDENTIFICATION OF THE MEDICINAL PRODUCT 1.1. Trade name Flutarzole 1.2. Composition Active substance: Fluticasone
CORTISPORIN Ointment (neomycin and polymyxin B sulfates, bacitracin zinc, and. hydrocortisone ointment, USP)
 CORTISPORIN Ointment (neomycin and polymyxin B sulfates, bacitracin zinc, and hydrocortisone ointment, USP) DESCRIPTION CORTISPORIN Ointment (neomycin and polymyxin B sulfates, bacitracin zinc, and hydrocortisone
CORTISPORIN Ointment (neomycin and polymyxin B sulfates, bacitracin zinc, and hydrocortisone ointment, USP) DESCRIPTION CORTISPORIN Ointment (neomycin and polymyxin B sulfates, bacitracin zinc, and hydrocortisone
355 Lexington Avenue, 15th Floor New York, NY (Helpline) fax
 The MGFA mission is to facilitate the timely diagnosis and optimal care of individuals affected by myasthenia gravis and closely related disorders and to improve their lives through programs of patient
The MGFA mission is to facilitate the timely diagnosis and optimal care of individuals affected by myasthenia gravis and closely related disorders and to improve their lives through programs of patient
FURAMIST Nasal Spray (Fluticasone furoate )
 Published on: 21 Jan 2016 FURAMIST Nasal Spray (Fluticasone furoate ) Composition Each spray contains: Fluticasone furoate 27.5 mcg Dosage Form Aqueous intranasal spray Pharmacology Pharmacodynamics Fluticasone
Published on: 21 Jan 2016 FURAMIST Nasal Spray (Fluticasone furoate ) Composition Each spray contains: Fluticasone furoate 27.5 mcg Dosage Form Aqueous intranasal spray Pharmacology Pharmacodynamics Fluticasone
Abiraterone Acetate is an antiandrogen used in the treatment of Castration-Resistant Prostate Cancer(CRPC).
 For the use only of an Oncologist or a Hospital or a Laboratory ABIRATERONE ACETATE TABLETS Zabiteron-250 COMPOSITION Abiraterone Acetate Tablets 250mg Each uncoated tablets contains: Abiraterone Acetate
For the use only of an Oncologist or a Hospital or a Laboratory ABIRATERONE ACETATE TABLETS Zabiteron-250 COMPOSITION Abiraterone Acetate Tablets 250mg Each uncoated tablets contains: Abiraterone Acetate
The adrenal gland consists of the cortex & the medulla. Medulla secretes epinephrine, whereas cortex synthesizes & secretes two major classes of
 Adrenocorticosteroids Dr. Entisar Al-Mukhtar The adrenal gland consists of the cortex & the medulla. Medulla secretes epinephrine, whereas cortex synthesizes & secretes two major classes of steroid hormones:
Adrenocorticosteroids Dr. Entisar Al-Mukhtar The adrenal gland consists of the cortex & the medulla. Medulla secretes epinephrine, whereas cortex synthesizes & secretes two major classes of steroid hormones:
COLIFOAM Hydrocortisone acetate
 AUSTRALIAN PRODUCT INFORMATION COLIFOAM Hydrocortisone acetate 1 NAME OF THE MEDICINE Hydrocortisone acetate 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Hydrocortisone acetate occurs as white or almost
AUSTRALIAN PRODUCT INFORMATION COLIFOAM Hydrocortisone acetate 1 NAME OF THE MEDICINE Hydrocortisone acetate 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Hydrocortisone acetate occurs as white or almost
Distribution The in vitro protein binding for Mometasone furoate was reported to be 98% to 99% in concentra on range of 5 to 500 ng/ml.
 NOSATREX Composition Mometasone Furoate 0.05% w/w Spray Action Mechanism of Action Mometasone Nasal Spray 50 mcg is a cor costeroid demonstra ng potent an -inflammatory properties. The precise mechanism
NOSATREX Composition Mometasone Furoate 0.05% w/w Spray Action Mechanism of Action Mometasone Nasal Spray 50 mcg is a cor costeroid demonstra ng potent an -inflammatory properties. The precise mechanism
SUMMARY OF PRODUCT CHARACTERISTICS. Excipient with known effect Cetyl alcohol For the full list of excipients, see section 6.1.
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Haelan Ointment Fludroxycortide 0.0125% w/w Ointment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Fludroxycortide 0.0125% w/w. Excipient
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Haelan Ointment Fludroxycortide 0.0125% w/w Ointment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Fludroxycortide 0.0125% w/w. Excipient
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Haelan Tape Fludroxycortide 4 micrograms per square centimetre Tape 2. QUALITATIVE AND QUANTITATIVE COMPOSITION The tape is impregnated
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Haelan Tape Fludroxycortide 4 micrograms per square centimetre Tape 2. QUALITATIVE AND QUANTITATIVE COMPOSITION The tape is impregnated
OLUX Foam, 0.05% (clobetasol propionate)
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 OLUX Foam, 0.05% (clobetasol propionate) Rx Only For Dermatologic Use Only Not for Ophthalmic Use DESCRIPTION OLUX Foam contains clobetasol propionate, USP, a synthetic
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 OLUX Foam, 0.05% (clobetasol propionate) Rx Only For Dermatologic Use Only Not for Ophthalmic Use DESCRIPTION OLUX Foam contains clobetasol propionate, USP, a synthetic
Chemical Names: Prednisolone acetate: 11ß,17,21-Trihydroxypregna-1,4-diene-3,20-dione 21-acetate.
 PRED-G (gentamicin and prednisolone acetate ophthalmic suspension, USP) 0.3%/1% sterile DESCRIPTION PRED-G sterile ophthalmic suspension is a topical anti-inflammatory/anti-infective combination product
PRED-G (gentamicin and prednisolone acetate ophthalmic suspension, USP) 0.3%/1% sterile DESCRIPTION PRED-G sterile ophthalmic suspension is a topical anti-inflammatory/anti-infective combination product
FLUOCINOLONE ACETONIDE-
 FLUOCINOLONE ACETONIDE- fluocinolone acetonide cream E. & Co. a division of Pharmaceuticals Inc. ---------- FLUOCINOLONE ACETONIDE CREAM, USP 0.01%, 0.025% For Topical Us e Only. Not for Ophthalmic Us
FLUOCINOLONE ACETONIDE- fluocinolone acetonide cream E. & Co. a division of Pharmaceuticals Inc. ---------- FLUOCINOLONE ACETONIDE CREAM, USP 0.01%, 0.025% For Topical Us e Only. Not for Ophthalmic Us
SUMMARY OF PRODUCT CHARACTERISTICS. Each gram of ointment contains 1 mg of mometasone furoate and 50 mg of salicylic acid
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Elosalic 1 mg/g + 50 mg/g ointment. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram of ointment contains 1 mg of mometasone furoate
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Elosalic 1 mg/g + 50 mg/g ointment. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each gram of ointment contains 1 mg of mometasone furoate
Adrenal Gland Disorders
 1 Adrenal Gland Disorders Adrenal cortex steroid hormones (corticosteroids) 1. Glucocorticoids Regulate metabolism and blood glucose Critical to physiologic stress response 2. Mineralocorticoids Regulate
1 Adrenal Gland Disorders Adrenal cortex steroid hormones (corticosteroids) 1. Glucocorticoids Regulate metabolism and blood glucose Critical to physiologic stress response 2. Mineralocorticoids Regulate
Children Enteric coated tablet : 1-3 mg/kg per day in divided doses.
 Ultrafen Tablet/SR Tablet/Suppository/Gel Description Ultrafen is a preparation of Diclofenac is a non-steroidal antiinflammatory agent with marked analgesic, anti-inflammatory and antipyretic properties.
Ultrafen Tablet/SR Tablet/Suppository/Gel Description Ultrafen is a preparation of Diclofenac is a non-steroidal antiinflammatory agent with marked analgesic, anti-inflammatory and antipyretic properties.
Ulcerative Colitis. ulcerative colitis usually only affects the colon.
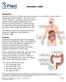 Ulcerative Colitis Introduction Ulcerative colitis is an inflammatory bowel disease. It is one of the 2 most common inflammatory bowel diseases. The other one is Crohn s disease. Ulcerative colitis and
Ulcerative Colitis Introduction Ulcerative colitis is an inflammatory bowel disease. It is one of the 2 most common inflammatory bowel diseases. The other one is Crohn s disease. Ulcerative colitis and
PRODUCT INFORMATION ELEUPHRAT CREAM, OINTMENT AND LOTION. Betamethasone dipropionate equivalent to betamethasone 0.5 mg/g (0.
 PRODUCT INFORMATION ELEUPHRAT CREAM, OINTMENT AND LOTION NAME OF THE MEDICINE Betamethasone dipropionate equivalent to betamethasone 0.5 mg/g (0.05% w/w) Chemical Structure: Betamethasone dipropionate
PRODUCT INFORMATION ELEUPHRAT CREAM, OINTMENT AND LOTION NAME OF THE MEDICINE Betamethasone dipropionate equivalent to betamethasone 0.5 mg/g (0.05% w/w) Chemical Structure: Betamethasone dipropionate
DERMATOP Ointment (prednicarbate ointment) 0.1% FOR DERMATOLOGIC USE ONLY. NOT FOR USE IN EYES.
 DERMATP intment (prednicarbate ointment) 0.1% FR DERMATLGIC USE NLY. NT FR USE IN EYES. DESCRIPTIN DERMATP intment (prednicarbate ointment) 0.1% contains the non-halogenated prednisolone derivative prednicarbate.
DERMATP intment (prednicarbate ointment) 0.1% FR DERMATLGIC USE NLY. NT FR USE IN EYES. DESCRIPTIN DERMATP intment (prednicarbate ointment) 0.1% contains the non-halogenated prednisolone derivative prednicarbate.
PRED-G (gentamicin and prednisolone acetate ophthalmic ointment, USP) 0.3%/0.6% sterile
 PRED-G (gentamicin and prednisolone acetate ophthalmic ointment, USP) 0.3%/0.6% sterile PRED-G sterile ophthalmic ointment is a topical anti-inflammatory/anti-infective combination product for ophthalmic
PRED-G (gentamicin and prednisolone acetate ophthalmic ointment, USP) 0.3%/0.6% sterile PRED-G sterile ophthalmic ointment is a topical anti-inflammatory/anti-infective combination product for ophthalmic
NEW ZEALAND DATA SHEET
 EUMOVATE Cream NEW ZEALAND DATA SHEET Name of each active ingredient Clobetasone 17-Butyrate BP 0.05% w/w Presentations Eumovate Cream is white in appearance and contains 0.05% w/w clobetasone butyrate.
EUMOVATE Cream NEW ZEALAND DATA SHEET Name of each active ingredient Clobetasone 17-Butyrate BP 0.05% w/w Presentations Eumovate Cream is white in appearance and contains 0.05% w/w clobetasone butyrate.
TOBAFLAM Eye Drops (Loteprednol etabonate 0.5% + Tobramycin 0.3%)
 Published on: 23 Sep 2014 TOBAFLAM Eye Drops (Loteprednol etabonate 0.5% + Tobramycin 0.3%) Composition Loteprednol Etabonate 5 mg (0.5% w/v) Tobramycin...3 mg (0.3% w/v) Benzalkonium Chloride.. 0.01%
Published on: 23 Sep 2014 TOBAFLAM Eye Drops (Loteprednol etabonate 0.5% + Tobramycin 0.3%) Composition Loteprednol Etabonate 5 mg (0.5% w/v) Tobramycin...3 mg (0.3% w/v) Benzalkonium Chloride.. 0.01%
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory BETNESOL - N
 For the use only of Registered Medical Practitioners or a Hospital or a Laboratory BETNESOL - N Betamethasone Sodium Phosphate and Neomycin Sulphate Eye/Ear Drops QUALITATIVE AND QUANTITATIVE COMPOSITION
For the use only of Registered Medical Practitioners or a Hospital or a Laboratory BETNESOL - N Betamethasone Sodium Phosphate and Neomycin Sulphate Eye/Ear Drops QUALITATIVE AND QUANTITATIVE COMPOSITION
MESALO Foam (Mesalazine)
 Published on: 10 Jul 2014 MESALO Foam (Mesalazine) Composition MESALO Foam Each actuation delivers: Mesalazine, BP.... 1.0 g White-grayish to slightly reddish-violet, creamy, firm foam. Dosage Form Rectal
Published on: 10 Jul 2014 MESALO Foam (Mesalazine) Composition MESALO Foam Each actuation delivers: Mesalazine, BP.... 1.0 g White-grayish to slightly reddish-violet, creamy, firm foam. Dosage Form Rectal
HIGHLIGHTS OF PRESCRIBING INFORMATION
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VERDESO Foam safely and effectively. See full prescribing information for VERDESO Foam. VERDESO (desonide)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use VERDESO Foam safely and effectively. See full prescribing information for VERDESO Foam. VERDESO (desonide)
PRODUCT INFORMATION. PREDNEFRIN FORTE Eye Drops NAME OF THE MEDICINE
 PRODUCT INFORMATION PREDNEFRIN FORTE Eye Drops NAME OF THE MEDICINE AUSTRALIAN APPROVED NAME The active constituents of PREDNEFRIN FORTE eye drops are prednisolone acetate and phenylephrine hydrochloride.
PRODUCT INFORMATION PREDNEFRIN FORTE Eye Drops NAME OF THE MEDICINE AUSTRALIAN APPROVED NAME The active constituents of PREDNEFRIN FORTE eye drops are prednisolone acetate and phenylephrine hydrochloride.
LESSON ASSIGNMENT. After completing this lesson, you should be able to:
 LESSON ASSIGNMENT LESSON 9 Adrenocortical Hormones. LESSON ASSIGNMENT Paragraphs 9-1 through 9-14. LESSON OBJECTIVES After completing this lesson, you should be able to: 9-1. From a list of names of hormones,
LESSON ASSIGNMENT LESSON 9 Adrenocortical Hormones. LESSON ASSIGNMENT Paragraphs 9-1 through 9-14. LESSON OBJECTIVES After completing this lesson, you should be able to: 9-1. From a list of names of hormones,
Package leaflet : Information for the patient. Synacthen Ampoules 250 micrograms Tetracosactide acetate
 Package leaflet : Information for the patient Synacthen Ampoules 250 micrograms Tetracosactide acetate Read all of this leaflet carefully before you are given this medicine because it contains important
Package leaflet : Information for the patient Synacthen Ampoules 250 micrograms Tetracosactide acetate Read all of this leaflet carefully before you are given this medicine because it contains important
The chemical name of acyclovir, USP is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6Hpurin-6-one; it has the following structural formula:
![The chemical name of acyclovir, USP is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6Hpurin-6-one; it has the following structural formula: The chemical name of acyclovir, USP is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6Hpurin-6-one; it has the following structural formula:](/thumbs/74/71271167.jpg) Acyclovir Ointment, USP 5% DESCRIPTION Acyclovir, USP, is a synthetic nucleoside analogue active against herpes viruses. Acyclovir ointment, USP 5% is a formulation for topical administration. Each gram
Acyclovir Ointment, USP 5% DESCRIPTION Acyclovir, USP, is a synthetic nucleoside analogue active against herpes viruses. Acyclovir ointment, USP 5% is a formulation for topical administration. Each gram
Proposed PIL NL/H/0653/001/IB/024/G. MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution in single-dose container Dexamethasone phosphate
 Proposed PIL NL/H/0653/001/IB/024/G PACKAGE LEAFLET: INFORMATION FOR THE USER MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution in single-dose container Dexamethasone phosphate Read all of this leaflet
Proposed PIL NL/H/0653/001/IB/024/G PACKAGE LEAFLET: INFORMATION FOR THE USER MONOFREE DEXAMETHASON 1 mg/ml, eye drops, solution in single-dose container Dexamethasone phosphate Read all of this leaflet
What is ulcerative colitis?
 What is ulcerative colitis? Ulcerative colitis is a disease that causes inflammation and sores, called ulcers, in the lining of the rectum and colon. Ulcers form where inflammation has killed the cells
What is ulcerative colitis? Ulcerative colitis is a disease that causes inflammation and sores, called ulcers, in the lining of the rectum and colon. Ulcers form where inflammation has killed the cells
U.S. 0.05% INDICATIONS AND USAGE
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OLUX-E Foam safely and effectively. See full prescribing information for OLUX-E Foam. OLUX-E (clobetasol
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use OLUX-E Foam safely and effectively. See full prescribing information for OLUX-E Foam. OLUX-E (clobetasol
Patients recovered more quickly and completely with ACTH gel compared to placebo during the 30-day treatment period* 1
 In one study of MS patients treated for optic neuritis Patients recovered more quickly and completely with ACTH gel compared to placebo during the 30-day treatment period* 1 PERCENTAGE OF PATIENTS WITH
In one study of MS patients treated for optic neuritis Patients recovered more quickly and completely with ACTH gel compared to placebo during the 30-day treatment period* 1 PERCENTAGE OF PATIENTS WITH
SUMMARY OF PRODUCT CHARACTERISTICS
 SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ovixan 1 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram cream contains 1 mg mometasone furoate Excipients with known
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ovixan 1 mg/g cream 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One gram cream contains 1 mg mometasone furoate Excipients with known
OLUX-E. (clobetasol propionate) Foam, 0.05%
 PRESCRIBING INFORMATION OLUX-E (clobetasol propionate) Foam, 0.05% HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Olux-E Foam safely and effectively.
PRESCRIBING INFORMATION OLUX-E (clobetasol propionate) Foam, 0.05% HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use Olux-E Foam safely and effectively.
DATA SHEET. Betamethasone dipropionate equivalent to betamethasone 0.5mg/g (0.05% w/w).
 DATA SHEET 1. DIPROSONE DIPROSONE (0.05% w/w) cream DIPROSONE (0.05% w/w) ointment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Betamethasone dipropionate equivalent to betamethasone 0.5mg/g (0.05% w/w).
DATA SHEET 1. DIPROSONE DIPROSONE (0.05% w/w) cream DIPROSONE (0.05% w/w) ointment 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Betamethasone dipropionate equivalent to betamethasone 0.5mg/g (0.05% w/w).
Summary of Product Characteristics
 Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Prednesol 5mg Tablets. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 5 mg of prednisolone as Prednisolone Sodium Phosphate.
Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Prednesol 5mg Tablets. 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 5 mg of prednisolone as Prednisolone Sodium Phosphate.
NOVASONE CREAM, OINTMENT AND LOTION PRODUCT INFORMATION
 NAME OF THE MEDICINE Mometasone furoate 0.1% (1 mg/g) Chemical structure: NOVASONE CREAM, OINTMENT AND LOTION PRODUCT INFORMATION Mometasone furoate is 9,21-dichloro-11ß,17-dihydroxy-16 -methylpregna-1,4-diene-
NAME OF THE MEDICINE Mometasone furoate 0.1% (1 mg/g) Chemical structure: NOVASONE CREAM, OINTMENT AND LOTION PRODUCT INFORMATION Mometasone furoate is 9,21-dichloro-11ß,17-dihydroxy-16 -methylpregna-1,4-diene-
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Hydrocortisone Cream 1% 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Cream containing 1% micronised hydrocortisone For excipients, see 6.1
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT Hydrocortisone Cream 1% 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Cream containing 1% micronised hydrocortisone For excipients, see 6.1
FULL PRESCRIBING INFORMATION
 IGLIGTS F PRESCRIBING INFRMATIN These highlights do not include all the information needed to use DESNATE Gel safely and effectively. See full prescribing information for DESNATE Gel. DESNATE (desonide)
IGLIGTS F PRESCRIBING INFRMATIN These highlights do not include all the information needed to use DESNATE Gel safely and effectively. See full prescribing information for DESNATE Gel. DESNATE (desonide)
Proceedings of the World Small Animal Veterinary Association Sydney, Australia 2007
 Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress USE AND MISUSE OF GLUCOCORTICOIDS IN VETERINARY NEUROLOGY/NEUROSURGERY Richard A. LeCouteur, BVSc, PhD, DIP ACVIM
Proceedings of the World Small Animal Sydney, Australia 2007 Hosted by: Next WSAVA Congress USE AND MISUSE OF GLUCOCORTICOIDS IN VETERINARY NEUROLOGY/NEUROSURGERY Richard A. LeCouteur, BVSc, PhD, DIP ACVIM
Cortisol levels. Naturally produced by the adrenal Cortisol
 1 + 2 Cortisol levels asleep awake Naturally produced by the adrenal Cortisol Man made tablets, injections, creams & inhalers Cortisone Hydrocortisone Prednisone Prednisolone Betamethasone Methylprednisolone
1 + 2 Cortisol levels asleep awake Naturally produced by the adrenal Cortisol Man made tablets, injections, creams & inhalers Cortisone Hydrocortisone Prednisone Prednisolone Betamethasone Methylprednisolone
PRODUCT NAME: SOLONE
 NAME OF THE MEDICINE Prednisolone Structural formula: PRODUCT INFORMATION PRODUCT NAME: SOLONE Chemical name: 11, 17,21-Trihydroxypregna 1,4-diene-3,20-dione Cas No. 50-24-8 (Anhydrous) DESCRIPTION Prednisolone
NAME OF THE MEDICINE Prednisolone Structural formula: PRODUCT INFORMATION PRODUCT NAME: SOLONE Chemical name: 11, 17,21-Trihydroxypregna 1,4-diene-3,20-dione Cas No. 50-24-8 (Anhydrous) DESCRIPTION Prednisolone
PRODUCT MONOGRAPH. Betamethasone dipropionate USP. 0.5 mg Cream, Ointment and Lotion. Topical corticosteroid
 PRODUCT MONOGRAPH Pr TEVA-TOPISONE Betamethasone dipropionate USP 0.5 mg Cream, Ointment and Lotion Topical corticosteroid Teva Canada Limited Date of Revision 30 Novopharm Court October 25, 2018 Toronto,
PRODUCT MONOGRAPH Pr TEVA-TOPISONE Betamethasone dipropionate USP 0.5 mg Cream, Ointment and Lotion Topical corticosteroid Teva Canada Limited Date of Revision 30 Novopharm Court October 25, 2018 Toronto,
