About the treatment. Introduction
|
|
|
- Chastity Hopkins
- 6 years ago
- Views:
Transcription
1 Clinical Recommendations for The Use Of Omalizumab In Severe Asthma in Adults Clinical Recommendations for The Use Of Omalizumab In Severe Asthma in Adults... 1 About the treatment... 1 Introduction... 1 Mechanism of action... 1 Pharmacodynamic effects... 2 Clinical Benefit... 2 PBS eligibility for Omalizumab access... 2 Safety profile... 4 Dosage... 6 Storage... 7 Procedures... 7 Before the First Injection... 7 Injection day... 8 Administration... 9 Post administration Assessing response Protocol developed by: Resources used References About the treatment Introduction Omalizumab (Xolair) is indicated as an add-on therapy for severe allergic asthma. In children aged 6 to <12 years, omalizumab is indicated as an add-on therapy to improve asthma control in patients with severe allergic asthma, who have documented exacerbations despite daily high-dose ICS and with IgE levels in the recommended dose range. In adults and adolescents 12 years of age, omalizumab is indicated for the management of patients with moderate to severe allergic asthma, who are already being treated with maximal recommended asthma therapy, and who have serum IgE levels ( 30 IU/mL) and positive allergen-specific IgE Mechanism of action Omalizumab is a recombinant, DNA-derived, humanised, monoclonal IgG 1 antibody directed against immunoglobulin E (IgE). IgE is produced by the immune system and is involved in activation of immune responses during allergy. 1
2 Omalizumab binds to free human IgE in the blood and interstitial tissues, as well as to receptor-bound IgE on immune cells. The binding of omalizumab blocks cross-linking of IgE receptors on immune cells, and prevents allergic activation of these cells. This process reduces allergic responses and recruitment of immune cells into tissues. Pharmacodynamic effects Following single, subcutaneous administration, omalizumab reaches peak serum concentrations after around 6-10 days. In clinical trials, free IgE levels are reduced in a dosedependent manner within 2 hours of subcutaneous dosing. Average decreases were 84-99% of baseline. Serum total IgE levels increased an average of 4-fold post-dosing due to formation of omalizumab-ige binding complexes. Following discontinuation of omalizumab dosing, increases in total IgE and decreases in free IgE were reversible. [Content extracted from Xolair Production Information document] Clinical Benefit In a meta-analysis, exacerbation frequency was reduced, with an odds ratio of 0.55 (95% CI; )(1). The absolute reduction in risk of exacerbations fell from 26% to 16%. Hospitalisations were also reduced (OR: 0.16; 95%C CI: ). In addition, more patients were able to reduce ICS treatment. No significant reduction in OCS was observed. PBS eligibility for Omalizumab access In Australia, omalizumab can only be prescribed by a respiratory physician or allergist via S100 Authority prescription in accordance with specific application criteria. After discussing the risks, benefits, and alternatives to omalizumab, patients must complete the acknowledgement of understanding using the PBS initial application form. Patients require review of omalizumab efficacy and safety, by the prescribing respiratory physician, at 22 to 26 weeks after the first dose. For second and subsequent courses, the assessment should be undertaken after 18 to 22 weeks of treatment. Omalizumab is considered a high-risk medicine and must be administered with an independent second person check. Criteria for eligibility: CHECKLIST Age 12 years (see separate eligibility criteria for children 6 to <12 years - Asthma diagnosis confirmed by doctor and with at least a documented 1 year history Under the care of the same respiratory physician for 12 months 2
3 Confirmed variable airflow limitation (1 or more of the following): a. FEV1 reversibility 12% and 200mL at baseline within 30 minutes after administration of salbutamol ( g), OR b. Airway hyper-responsiveness (AHR) defined as >20% decline in FEV1 during a direct bronchial provocation test or >15% decline during an indirect test, OR c. Peak Expiratory Flow (PEF) variability of >15% between the 2 highest and 2 lowest peak expiratory flow rates during 14 days FEV1 80% predicted documented on one or more occasions in the last 12 months Past or current evidence of atopy, documented by skin prick testing or RAST Total serum human IgE greater than or equal to 30 IU/mL, no more than 12 months before application The patient has received optimised asthma therapy including adherence to high dose inhaled corticosteroid (ICS) and long-acting beta-2 agonist (LABA) for at least 12 months and treatment with oral corticosteroids (OCS) (either as daily OCS for at least 6 weeks or a cumulative dose of oral corticosteroids of 500mg prednisolone equivalent in the previous 12 months. The patient/guardian must sign the application indicating they understand and acknowledge that the PBS-subsidised treatment will cease if they do not meet a predetermined response criteria for ongoing PBS-subsidised treatment Failed to achieve adequate control with optimised asthma therapy, despite formal assessment of and adherence to correct inhaler technique, which as been documented Must not receive more than 28 weeks of treatment (further treatment requires continuation form submission see below) The treatment must not be used in combination with, or within 6 months of treatment with, PBS-subsidised Mepolizumab Ongoing uncontrolled asthma, as defined by: a. Asthma Control Questionnaire (ACQ-5) score 2 in the previous month AND b. One of the following experienced in the previous year: i. 1 admission to hospital for a severe asthma exacerbation, OR ii. 1 severe asthma exacerbation requiring documented use of systemic corticosteroids (OCS initiated or increased for at least 3 days, or parenteral corticosteroids) prescribed/supervised by a physician. 3
4 Safety profile Omalizumab therapy is generally well tolerated, but there are some safety considerations, the most important of which is the rare, but potentially life-threatening omalizumabassociated anaphylaxis. Despite these concerns, omalizumab has been safely administered in the community setting in Australia and internationally. Adverse reactions with omalizumab were observed (all studies with adult and adolescent patients 12 years of age and older) at a frequency of 6.6 % of patients, treated with active drug, during clinical trials [extracted from Xolair Product Information document]. The most commonly associated adverse drug reactions were injection site reactions, including injection site pain, swelling, itching and redness (1.7%) and headaches (1%). Other adverse reactions most frequently observed were weight increase (0.7%), urticaria (0.4%), fatigue, arm swelling, nausea, pharyngitis and skin rashes (all at 0.3%). Most of these events were mild or moderate in severity [extracted from Xolair Product Information document]. In clinical trials with patients 6 to <12 years of age, the most commonly reported adverse reactions were headache, pyrexia and upper abdominal pain. Most of the events were mild or moderate in severity. The incidence of anaphylaxis associated with post-marketing use of omalizumab may be as high as 0.2% though other researchers have found the incidence to be much lower at 0.09% (2). The anaphylaxis risk requires this medication to be administered in a clinical setting that has access to trained health professionals with equipment and medications to treat anaphylaxis and cardiac arrest. PRECAUTIONS [extracted from Xolair Product Information] Contraindications: Hypersensitivity to omalizumab or any other component of the formulation. Asthma-related adverse events or exacerbations may occur during treatment with omalizumab. Patients should be instructed to seek medical advice if their asthma remains uncontrolled or worsens after initiation of treatment with omalizumab. Abrupt discontinuation of asthma medication after initiation of omalizumab therapy is not recommended. Reductions in medication doses, if warranted, should be gradual and performed under the supervision of a physician. ADVERSE EFFECTS [extracted from Xolair Product Information] Allergic reactions: Local or systemic allergic reactions, including anaphylaxis, may occur. In post-marketing experience, anaphylaxis reactions have been reported following the first and subsequent omalizumab administrations. Although most of these reactions occurred with 2 hours after administration, some occurred beyond 2 or even 24 hours after injection. Medications for the treatment of anaphylactic reactions should always be available for immediate use 4
5 following omalizumab administration. Patients should be informed that such reactions are possible and prompt medical attention should be sought if allergic reactions do occur. Serum sickness and serum sickness-like reactions, which are delayed allergic type III reactions, have rarely been seen in patients treated with humanized monoclonal antibodies including omalizumab. The onset has typically been 1-5 days after administration of the first or subsequent injections, also after long duration of treatment. Symptoms suggestive of serum sickness include arthritis/arthralgia, rash (urticaria or other forms), fever and lymphadenopathy. Patients should be advised to report any suspected symptoms. Churg-Strauss syndrome and hypereosinophilic syndrome: Patients with severe asthma may rarely present systemic hypereosinophilic syndrome or allergic eosinophilic granulomatous vasculitis (Churg-Strauss syndrome), both of which are usually treated with systemic corticosteroids. In rare cases, patients on therapy with antiasthma agents, including omalizumab, may present or develop systemic eosinophilia and vasculitis. A causal association between omalizumab and these underlying conditions has not been established. These events are commonly associated with the reduction of oral corticosteroid therapy. In these patients, clinicians should be alert to the development of marked eosinophilia, vasculitic rash, worsening pulmonary symptoms, paranasal sinus abnormalities, cardiac complications, and/or neuropathy. Discontinuation of omalizumab should be considered in all severe cases with the above-mentioned immune system disorders. Other IgE-associated disorders: Omalizumab has not been studied in patients with anaphylaxis, hyperimmunoglobulin E syndrome, allergic bronchopulmonary aspergillosis, food allergy or atopic dermatitis. Parasitic infestation may also result in elevation of serum IgE concentrations. In a study of patients with asthma who had been treated for gut parasites, the level of reinfection did not differ significantly between omalizumab and placebo groups and there were no serious or severe infections (3). There is no current evidence to suggest that parasitic infections are predisposed to by omalizumab. Interpretation of serum IgE levels, skin patch and skin prick testing: Patients treated with omalizumab have a rapid reduction in free IgE in the serum but an overall increase in the total serum IgE (commonly measured in clinical laboratories), which reflects free IgE and IgE bound by omalizumab. IgE measured following treatment cannot be used to guide treatment or dosing decisions. Because omalizumab reduces free IgE in the serum and tissues, results of skin prick testing, patch testing, and RAST testing for hypersensitivity to potential allergens may be affected. A positive test to a potential allergen in a patient receiving omalizumab can be correctly interpreted as representing hypersensitivity to that allergen; however, a negative test in a patient receiving omalizumab may not be interpretable. Physicians are urged to use caution in interpreting such tests in patients receiving omalizumab. Thrombocytopenia: At serum concentrations in excess of maximum human exposure used in pivotal clinical trials, dose-related thrombocytopenia occurred in 2 out of 4 non-human primate species studied. The thrombocytopenia was more pronounced in juvenile animals. No omalizumabrelated thrombocytopenia has been observed in clinical trials, but it has been reported in the post-market setting. 5
6 Omalizumab should be used with caution in patients with thrombocytopenia and patients with a history of thrombocytopenia. It is recommended that patients have a platelet count before commencing therapy with omalizumab and then periodically during treatment with omalizumab. Pre-filled syringe, latex-sensitive individuals: The removable needle cap of omalizumab solution for injection in pre-filled syringe contains a derivative of natural rubber latex. Although no natural rubber latex is detected in the removable needle cap, the safe use of omalizumab solution for injection in pre-filled syringe in latex-sensitive individuals has not been studied. Use in Pregnancy (Category B1): No studies have been performed in pregnant or breast-feeding women. Studies in cynomolgus monkeys do not indicate direct or indirect harmful effects with respect to pregnancy, embryonic/fetal development, parturition or neonatal growth at weekly doses up to 75 mg/kg SC about 10 times the maximum anticipated clinical exposure in adult patients, based on serum AUC). Because immunoglobulins are known to cross the placenta and the potential for harm to the foetus is unknown, caution should be exercised when prescribing Xolair to pregnant women. Use in Lactation: It is not known whether omalizumab is excreted in human milk. Because human IgG is excreted in human milk, and because the potential for absorption and harm to the infant are unknown, caution should be exercised when omalizumab is administered to breast-feeding women. In order to assess the effect of omalizumab on late gestation, and to evaluate the placental transfer and milk excretion of omalizumab, doses of 75 mg/kg/week were administered subcutaneously to female cynomolgus monkeys. Transport of omalizumab into maternal milk was limited. The serum levels of omalizumab observed in dams, fetuses, and neonates are consistent with reported transport and distribution of IgG class immunoglobulins. Dosage Omalizumab dosing is determined based on a matrix of baseline IgE levels and body weight, administered by subcutaneous injection every two or four weeks. Dosing tables are available in the Xolair product information document, available at the following link (accessed 06 December 2017; document updated 30 March 2017): PI &d=
7 Figure 1: Dosing for patients aged 6 years (accessed 01 November 2017) Doses greater than 750 mg were not studied in the pivotal clinical trials. However, observational studies provide evidence for the effectiveness of a ceiling dose of omalizumab (750 mg) in individuals above the recommended dosing criteria (4). Storage Omalizumab is stored with the syringe sealed in its outer box in the refrigerator between 2 C and 8 C. DO NOT FREEZE. This medication should be stored in the refrigerator but needs to be administered at room temperature necessitating removal from refrigeration at least 20 minutes prior to injection. For pre-filled syringes, the product may be kept out of the refrigerator, for a total of 4 hours at 25 C. If necessary, the product may be returned to the refrigerator for later use, but this must not be done more than once. Procedures The patient should remain for two hours after the first 3 omalizumab injections, and 30 minutes thereafter, in an area under direct staff observation Omalizumab should be administered by a registered health care professional (physician or registered nurse). Monitoring of patients after administration of biological agents is recommended. Before the First Injection 1. Complete PBS omalizumab application including ACQ-5 calculation sheet (or ACQ-IA for patients between 6-12 years of age), including the date of assessment of the patients symptoms. An IgE pathology report must be provided for each initial application. A re-assessment of free IgE can only be made 12 months or more after the last dose of omalizumab was administered. Initial treatment forms for paediatric patients can be downloaded at: 7
8 Initial treatment forms for adolescent and adult patients can be downloaded at: 2. Complete an authority script. The most cost-effective syringe combination should be prescribed. Each strength must be written on a separate authority prescription. 3. Send the completed application form and all required supporting documentation to: Online through Health Professional Online Services (HPOS) at humanservices.gov.au/hpos OR Department of Human Services Complex Drugs Programs Reply Paid 9826 HOBART TAS Once approval is received invite the patient to attend the clinic for their first dose and arrange for the medication to be ordered and obtained according to your hospital s policy. Allow time for the patient to receive the script and the pharmacy to order stock. Injection day An assessment of the patient s current asthma and general health should be made before each injection to determine whether there were any recent health changes that might require withholding treatment. This assessment should include vital signs, exacerbation history and spirometry. Patients should be reminded to continue to take their other asthma medications unless the regimen is changed by their treating physician. Procedure can only take place in an area where there is access to emergency procedures and adequate medical support. Ensure rescue medication such as adrenaline, salbutamol MDI and spacer or nebuliser therapy, antihistamines and systemic corticosteroids are accessible. Ensure omalizumab has been ordered by the respiratory physician or allergist on an approved medication chart. Confirm that the patient has taken their usual asthma medications. Assess current asthma control and exacerbation status and manage as required. Perform baseline observations (HR, RR, BP, SpO2 and Temp). Spirometry assessment should be performed at baseline and continuation assessment. At other visits it may be performed according to the physician discretion. Record all information in patient s medical record. 8
9 Administration Figure 3: Xolair pre-filled syringe The needle cap of the syringe may contain dry rubber (latex), which should not be handled by persons sensitive to this substance. Do not use the syringe if either the seal on the outer box or the plastic wrapper is broken, as it may be not safe for use. Be careful not to touch the device activation clips (see first illustration) at any time. By touching them, the safety device may self-activate. Do not remove the needle cap until just before you give the injection. The syringe cannot be re-used. Dispose of the used syringe immediately after use. The syringe needs to be at room temperature at time of injection, this requires approximately 20 minutes out of the fridge. The cumulative time during which the syringe is kept at room temperature must not exceed 4 hours. The syringe can be returned to the fridge for use at a later time but this can only be done once. If the occurs the medication must be clearly labelled indication the date and time that this occurred. Process of administration Remove the plastic inner case from cardboard box, remove the peel-back lid. Wash or gel hands and don PPE. Inspect the syringe. DO NOT USE if it is broken or if the liquid looks cloudy or contains particles. In all these cases, return the entire product pack to the pharmacy or return to patient to return to the dispensing pharmacy. Omalizumab can be injected in either the upper outer thigh or the upper outer arm. If you need more than one injection at a time, repeat the injection in another location (e.g. the opposite thigh or arm). Clean the chosen injection site with an alcohol swab, starting at the centre and wiping outwards in a circular motion for approximately 5 cm diameter and allow to dry. Hold the syringe horizontally as shown in Figure 4 and look into the viewing window to check the dose (75 mg or 150 mg) and the expiration date printed on the label. Note: Rotate the internal syringe as shown below so the label can be read in the viewing window. 9
10 Figure 4: Dose & expiry window Hold the syringe vertically with the plunger uppermost and tap the side of the syringe against your finger to allow the air bubble to rise. Check to see if the liquid level is at or above the minimum fill line. If the liquid is below the fill line, return the entire pack to the pharmacy. See Figure 5. Figure 5: Fill line Holding the syringe with the needle pointing up, carefully pull off the needle cap from the syringe and discard it. Then, gently tap the syringe with your finger until the air bubble rises to the top of the syringe. Slowly push the plunger up to force the air bubble out of the syringe without inadvertently expelling solution. Pinch the skin up with the forefinger and thumb to ensure the medication is delivered subcutaneously. Needle is inserted at a 45 degree angle, continue to hold the syringe in place using the finger flange and inject the medication with a slow even pressure. After the complete dose is given, remove the needle from the skin while holding the plunger down. See Figure 6. Slowly release the plunger and allow the needle guard to automatically cover the exposed needle. See Figure 7. If the needle guard does not extend automatically, firmly push on the plunger. Then release the plunger and allow the guard to cover the needle. 10
11 Figure 6: Needle withdrawal Figure 7: Auto-shield activation Dispose syringe in sharps disposal container. Check injection site and cover with puncture-site, round dressing. Repeat steps if more than one prefilled syringe is required using other sites i.e. opposite arm or leg. Remove PPE and wash hands. Post administration The patient must be observed directly by a suitably qualified clinician administering the injection for at least two hours after each of the first 3 doses and 30 minutes thereafter, looking for adverse effects from the medication. Assessing response All applications for continuing treatment must include a measurement of response to the prior course of therapy. The assessment of the patient s response to an initial course of treatment must be made at around 22 to 26 weeks after the first dose. For second and subsequent treatment courses, the assessment must be made around 18 to 22 weeks of treatment. The same physician who initiated treatment with omalizumab should also complete the application for the first assessment. Continuing applications must include an ACQ5 calculation sheet. An adequate response to treatment is defined as: A reduction in ACQ5 scores of at least 0.5 from baseline OR Maintenance OCS dose reduced by at least 25% from baseline and no deterioration of ACQ5 score from baseline OR Reduction in time-adjusted exacerbation rates compared to the 12 months prior to baseline (this is only applicable to patients transitioning from paediatric to adolescent/adult restriction) This assessment, which will be used to determine eligibility for continuing treatment, must be submitted within 4 weeks of the date of assessment, and no later than 2 weeks prior to the patient completing their current treatment course, to avoid an interruption to supply. Continuation forms can be downloaded at: Adolescent and adult: Paediatric: 11
12 Protocol developed by: Professor Vanessa McDonald Centre of Excellence in Severe Asthma Professor Peter Gibson Centre of Excellence in Severe Asthma John Harrington - Respiratory Clinical Nurse Consultant John Hunter Hospital Jane Civitico - Respiratory Clinical Nurse Consultant RPAH Steven Maltby Research Academic Centre of Excellence in Severe Asthma John Hunter Hospital Clinical Guideline Administration and Monitoring of Omalizumab (Xolair) Resources used Xolair Production Information &d= (date accessed ) Severe allergic asthma adolescent and adult patient (Human Services Australia, PBS guidelines) (date accessed ) Paediatric severe allergic asthma Initial PBS authority application form - (date accessed ) Paediatric severe allergic asthma continuing PBS authority application form - (date accessed ) Adolescent and adult severe allergic asthma Initial PBS authority application form - (date accessed ) Adolescent and adult severe allergic asthma Continuing PBS authority application form - (date accessed ) 12
13 References 1. Normansell, R., S. Walker, S. J. Milan, E. H. Walters, and P. Nair Omalizumab for asthma in adults and children. Cochrane Database Syst Rev 1: CD Kim, H. L., R. Leigh, and A. Becker Omalizumab: Practical considerations regarding the risk of anaphylaxis. Allergy, Asthma, and Clinical Immunology : Official Journal of the Canadian Society of Allergy and Clinical Immunology 6: Cruz, A. A., F. Lima, E. Sarinho, G. Ayre, C. Martin, H. Fox, and P. J. Cooper Safety of anti-immunoglobulin E therapy with omalizumab in allergic patients at risk of geohelminth infection. Clinical and Experimental Allergy 37: Hew, M., A. Gillman, M. Sutherland, P. Wark, J. Bowden, M. Guo, H. K. Reddel, C. Jenkins, G. B. Marks, F. Thien, J. Rimmer, G. P. Katsoulotos, M. Cook, I. Yang, C. Katelaris, S. Bowler, D. Langton, C. Wright, M. Bint, V. Yozghatlian, S. Burgess, P. Sivakumaran, K. Y. Yan, V. Kritikos, M. Peters, M. Baraket, A. Aminazad, P. Robinson, A. Jaffe, H. Powell, J. W. Upham, V. M. McDonald, and P. G. Gibson Real-life effectiveness of omalizumab in severe allergic asthma above the recommended dosing range criteria. Clin Exp Allergy 46:
Local Omalizumab Treatment Protocol (For children 6 to <12 years of age)
 Local Omalizumab Treatment Protocol (For children 6 to
Local Omalizumab Treatment Protocol (For children 6 to
Introduction Mepolizumab (Nucala) is indicated as an add-on therapy for people with severe refractory eosinophilic asthma, aged 12 years.
 Clinical Recommendations For The Use Of Mepolizumab In Severe Asthma Clinical Recommendations For The Use Of Mepolizumab In Severe Asthma... 1 About the treatment... 1 Introduction... 1 Mechanism of action...
Clinical Recommendations For The Use Of Mepolizumab In Severe Asthma Clinical Recommendations For The Use Of Mepolizumab In Severe Asthma... 1 About the treatment... 1 Introduction... 1 Mechanism of action...
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Xolair 75 mg powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 75
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Xolair 75 mg powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 75
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS
 ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Xolair 75 mg powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 75
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Xolair 75 mg powder and solvent for solution for injection 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One vial contains 75
SELF-INJECTION GUIDE
 SELF-INJECTION GUIDE (asfotase alfa) 40 mg/ml solution for injection 18 mg/0.45 ml 28 mg/0.7 ml 40 mg/1 ml 100 mg/ml solution for injection 80 mg/0.8 ml asfotase alfa Introduction Contents This Self-Injection
SELF-INJECTION GUIDE (asfotase alfa) 40 mg/ml solution for injection 18 mg/0.45 ml 28 mg/0.7 ml 40 mg/1 ml 100 mg/ml solution for injection 80 mg/0.8 ml asfotase alfa Introduction Contents This Self-Injection
Preparation and Administration
 Preparation and Administration A guide for healthcare professionals XOLAIR IS INDICATED FOR: Adults and adolescents (aged 12 years) with moderate-to-severe persistent asthma who have a positive skin test
Preparation and Administration A guide for healthcare professionals XOLAIR IS INDICATED FOR: Adults and adolescents (aged 12 years) with moderate-to-severe persistent asthma who have a positive skin test
BOLSTRAN Injection (Omalizumab)
 Published on: 27 Feb 2017 BOLSTRAN Injection (Omalizumab) Composition Active Substance Omalizumab is a humanized monoclonal antibody manufactured from a mammalian cell line. One vial of BOLSTRAN 150 mg
Published on: 27 Feb 2017 BOLSTRAN Injection (Omalizumab) Composition Active Substance Omalizumab is a humanized monoclonal antibody manufactured from a mammalian cell line. One vial of BOLSTRAN 150 mg
INFORMATION FOR THE CONSUMER
 INFORMATION FOR THE CONSUMER This insert provides a summary of the information about CETROTIDE (cetrorelix for injection). If you have any questions or concerns, or want more information about CETROTIDE,
INFORMATION FOR THE CONSUMER This insert provides a summary of the information about CETROTIDE (cetrorelix for injection). If you have any questions or concerns, or want more information about CETROTIDE,
IMPORTANT: PLEASE READ. Don t
 PATIENT/CAREGIVER INSTRUCTIONS FOR USE - ORENCIA (ABATACEPT) PREFILLED SYRINGE WITH BD ULTRASAFE PASSIVE TM NEEDLE GUARD WITH FLANGE EXTENDERS Getting started with ORENCIA therapy Did you receive self-injection
PATIENT/CAREGIVER INSTRUCTIONS FOR USE - ORENCIA (ABATACEPT) PREFILLED SYRINGE WITH BD ULTRASAFE PASSIVE TM NEEDLE GUARD WITH FLANGE EXTENDERS Getting started with ORENCIA therapy Did you receive self-injection
PRODUCT MONOGRAPH XOLAIR. (omalizumab) Sterile powder for reconstitution, 150 mg vial. Solution for injection, 75 mg and 150mg pre-filled syringe
 PRODUCT MONOGRAPH Pr XOLAIR (omalizumab) Sterile powder for reconstitution, 150 mg vial Solution for injection, 75 mg and 150mg pre-filled syringe IgE-Neutralizing Antibody (Anti-IgE) Novartis Pharmaceuticals
PRODUCT MONOGRAPH Pr XOLAIR (omalizumab) Sterile powder for reconstitution, 150 mg vial Solution for injection, 75 mg and 150mg pre-filled syringe IgE-Neutralizing Antibody (Anti-IgE) Novartis Pharmaceuticals
PRODUCT MONOGRAPH XOLAIR. (omalizumab) Sterile powder for reconstitution, 150 mg vial. Solution for injection, 75 mg and 150mg pre-filled syringe
 PRODUCT MONOGRAPH Pr XOLAIR (omalizumab) Sterile powder for reconstitution, 150 mg vial Solution for injection, 75 mg and 150mg pre-filled syringe IgE-Neutralizing Antibody (Anti-IgE) Novartis Pharmaceuticals
PRODUCT MONOGRAPH Pr XOLAIR (omalizumab) Sterile powder for reconstitution, 150 mg vial Solution for injection, 75 mg and 150mg pre-filled syringe IgE-Neutralizing Antibody (Anti-IgE) Novartis Pharmaceuticals
Important Safety Information for Adlyxin (lixisenatide) injection
 A GLP-1 receptor agonist (RA) Adlyxin (lixisenatide) injection 20 mcg Information To Help Answer Patients Questions This booklet contains information about Adlyxin to help you answer patients questions
A GLP-1 receptor agonist (RA) Adlyxin (lixisenatide) injection 20 mcg Information To Help Answer Patients Questions This booklet contains information about Adlyxin to help you answer patients questions
EVOGAM. Information for patients Evogam NZ Patient Brochure Update FA3
 EVOGAM Information for patients 11179 Evogam NZ Patient Brochure Update FA3 Information for patients and caregivers about EVOGAM This booklet is designed to help you follow the training you will have been
EVOGAM Information for patients 11179 Evogam NZ Patient Brochure Update FA3 Information for patients and caregivers about EVOGAM This booklet is designed to help you follow the training you will have been
Omalizumab vs. Mepolizumab for Asthma Patients: How to Decide. Indications
 vs. for Asthma Patients: How to Decide Indications Asthma is indicated for patients 6 years of age and older with moderate to severe persistent asthma who have a positive skin test or in vitro reactivity
vs. for Asthma Patients: How to Decide Indications Asthma is indicated for patients 6 years of age and older with moderate to severe persistent asthma who have a positive skin test or in vitro reactivity
Normal Immunoglobulin (Human) 16%, solution for subcutaneous administration.
 Normal Immunoglobulin (Human) 16%, solution for subcutaneous administration. Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It does not contain
Normal Immunoglobulin (Human) 16%, solution for subcutaneous administration. Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It does not contain
Home Care Services HomeMed MedEQUIP Michigan Visiting Care Michigan Visiting Nurses Wheelchair Seating Service PROCEDURE
 UNIVERSITY OF MICHIGAN HOSPITALS AND HEALTH CENTERS UMHHC-HCS: 253.054 First Approved Date: 3/2010 Home Care Services HomeMed MedEQUIP Michigan Visiting Care Michigan Visiting Nurses Wheelchair Seating
UNIVERSITY OF MICHIGAN HOSPITALS AND HEALTH CENTERS UMHHC-HCS: 253.054 First Approved Date: 3/2010 Home Care Services HomeMed MedEQUIP Michigan Visiting Care Michigan Visiting Nurses Wheelchair Seating
GUIDELINES FOR WEIGHT-BASED DOSING AND INFUSION
 GUIDELINES FOR WEIGHT-BASED DOSING AND INFUSION Includes Example dose calculation wheel Preparation and administration information for healthcare professionals Please see enclosed full Prescribing Information,
GUIDELINES FOR WEIGHT-BASED DOSING AND INFUSION Includes Example dose calculation wheel Preparation and administration information for healthcare professionals Please see enclosed full Prescribing Information,
SELF-INJECTION TRAINING GUIDE
 SELF-INJECTION TRAINING GUIDE FOR THE PEGASYS PREFILLED SYRINGE INDICATIONS What is PEGASYS? PEGASYS (peginterferon alfa-2a) is a prescription medication that is: used alone or with COPEGUS (ribavirin,
SELF-INJECTION TRAINING GUIDE FOR THE PEGASYS PREFILLED SYRINGE INDICATIONS What is PEGASYS? PEGASYS (peginterferon alfa-2a) is a prescription medication that is: used alone or with COPEGUS (ribavirin,
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. AIM-oh-vig Pr AIMOVIG (erenumab injection)
 READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION AIM-oh-vig Pr AIMOVIG (erenumab injection) Single-Use Pre-filled SureClick Autoinjector Read this carefully before you
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION AIM-oh-vig Pr AIMOVIG (erenumab injection) Single-Use Pre-filled SureClick Autoinjector Read this carefully before you
PART III: CONSUMER INFORMATION XOLAIR (omalizumab)
 PART III: CONSUMER INFORMATION XOLAIR (omalizumab) This leaflet is part III of a three-part "Product Monograph" published when XOLAIR was approved for sale in Canada and is designed specifically for Consumers.
PART III: CONSUMER INFORMATION XOLAIR (omalizumab) This leaflet is part III of a three-part "Product Monograph" published when XOLAIR was approved for sale in Canada and is designed specifically for Consumers.
Package leaflet: Information for the user
 Package leaflet: Information for the user Aimovig 70 mg solution for injection in pre-filled pen erenumab This medicine is subject to additional monitoring. This will allow quick identification of new
Package leaflet: Information for the user Aimovig 70 mg solution for injection in pre-filled pen erenumab This medicine is subject to additional monitoring. This will allow quick identification of new
Instructions for Use. Welcome!
 Instructions for Use Welcome! The AMJEVITA SureClick autoinjector is a single-use prefilled autoinjector. Consult your doctor if you have any questions about your dose. Your doctor has prescribed AMJEVITA
Instructions for Use Welcome! The AMJEVITA SureClick autoinjector is a single-use prefilled autoinjector. Consult your doctor if you have any questions about your dose. Your doctor has prescribed AMJEVITA
Monocast Description Indications
 Monocast Tablet Description The active ingredient of Monocast tablet is Montelukast Sodium INN. Montelukast is a selective and orally active leukotriene receptor antagonist that inhibits the cysteinyl
Monocast Tablet Description The active ingredient of Monocast tablet is Montelukast Sodium INN. Montelukast is a selective and orally active leukotriene receptor antagonist that inhibits the cysteinyl
The only biologic approved to treat SLE: now with multiple delivery options
 The only biologic approved to treat SLE: now with multiple delivery options BENLYSTA (belimumab) Autoinjector SC Prefilled syringe IV Intravenous infusion Consider the options: visit Belimumab.com INDICATION
The only biologic approved to treat SLE: now with multiple delivery options BENLYSTA (belimumab) Autoinjector SC Prefilled syringe IV Intravenous infusion Consider the options: visit Belimumab.com INDICATION
XOLAIR Omalizumab (rch)
 XOLAIR Omalizumab (rch) NAME OF THE DRUG The active ingredient of Xolair is omalizumab. DESCRIPTION Omalizumab is a recombinant DNA-derived humanised monoclonal antibody produced in Chinese hamster ovary
XOLAIR Omalizumab (rch) NAME OF THE DRUG The active ingredient of Xolair is omalizumab. DESCRIPTION Omalizumab is a recombinant DNA-derived humanised monoclonal antibody produced in Chinese hamster ovary
INFUSING. ORENCIA (abatacept) IV INDICATION/USAGE. Your go-to guide for preparing and administering an ORENCIA intravenous (IV) infusion
 INFUSING ORENCIA (abatacept) IV Your go-to guide for preparing and administering an ORENCIA intravenous (IV) infusion INDICATION/USAGE Adult Rheumatoid Arthritis (RA): ORENCIA is indicated for reducing
INFUSING ORENCIA (abatacept) IV Your go-to guide for preparing and administering an ORENCIA intravenous (IV) infusion INDICATION/USAGE Adult Rheumatoid Arthritis (RA): ORENCIA is indicated for reducing
Instructions for Use. BASAGLAR KwikPen. insulin glargine injection (100 units/ml, 3 ml pen)
 Instructions for Use BASAGLAR KwikPen insulin glargine injection (100 units/ml, 3 ml pen) 1 Read the Instructions for Use before you start using BASAGLAR and each time you get another BASAGLAR KwikPen.
Instructions for Use BASAGLAR KwikPen insulin glargine injection (100 units/ml, 3 ml pen) 1 Read the Instructions for Use before you start using BASAGLAR and each time you get another BASAGLAR KwikPen.
PACKAGE LEAFLET: INFORMATION FOR THE USER. Xolair 75 mg solution for injection Xolair 150 mg solution for injection Omalizumab
 PACKAGE LEAFLET: INFORMATION FOR THE USER Xolair 75 mg solution for injection Xolair 150 mg solution for injection Omalizumab Read all of this leaflet carefully before you start using Xolair. - Keep this
PACKAGE LEAFLET: INFORMATION FOR THE USER Xolair 75 mg solution for injection Xolair 150 mg solution for injection Omalizumab Read all of this leaflet carefully before you start using Xolair. - Keep this
PLEASE READ THIS USER MANUAL BEFORE USE
 USER MANUAL Humalog 200 units/ml KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THIS USER MANUAL BEFORE USE USE ONLY IN THIS PEN, OR SEVERE OVERDOSE CAN RESULT Read the
USER MANUAL Humalog 200 units/ml KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THIS USER MANUAL BEFORE USE USE ONLY IN THIS PEN, OR SEVERE OVERDOSE CAN RESULT Read the
AUSTRALIAN PRODUCT INFORMATION 2 QUALITATIVE AND QUANTITATIVE COMPOSITION
 This medicinal product is subject to additional monitoring in Australia. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse
This medicinal product is subject to additional monitoring in Australia. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse
STEP-BY-STEP GUIDE TO SELF-INFUSION. Subcutaneous Administration of GAMMAGARD LIQUID
 STEP-BY-STEP GUIDE TO SELF-INFUSION Subcutaneous Administration of GAMMAGARD LIQUID This handy guide will help you manage your subcutaneous administration of GAMMAGARD LIQUID. If you have questions on
STEP-BY-STEP GUIDE TO SELF-INFUSION Subcutaneous Administration of GAMMAGARD LIQUID This handy guide will help you manage your subcutaneous administration of GAMMAGARD LIQUID. If you have questions on
Sharps Container (not included) 1 Gather and check supplies. Gather supplies
 Instructions for Use BENLYSTA (ben-list-ah) (belimumab) injection, for subcutaneous use Prefilled Syringe Read this Instructions for Use before you start to use BENLYSTA and each time you refill your prescription.
Instructions for Use BENLYSTA (ben-list-ah) (belimumab) injection, for subcutaneous use Prefilled Syringe Read this Instructions for Use before you start to use BENLYSTA and each time you refill your prescription.
Instructions for Use Enbrel (en-brel) (etanercept) for injection, for subcutaneous use Multiple-dose Vial
 Instructions for Use Enbrel (en-brel) (etanercept) for injection, for subcutaneous use Multiple-dose Vial How do I prepare and give an injection with Enbrel multiple-dose vial? A multiple-dose vial contains
Instructions for Use Enbrel (en-brel) (etanercept) for injection, for subcutaneous use Multiple-dose Vial How do I prepare and give an injection with Enbrel multiple-dose vial? A multiple-dose vial contains
Monoclonal antibody therapy for severe asthma
 INFORMATION PAPER FOR PRIMARY HEALTHCARE PROFESSIONALS MONOCLONAL ANTIBODY THERAPY Monoclonal antibody therapy for severe asthma KEY POINTS Benralizumab, mepolizumab and omalizumab are monoclonal antibody
INFORMATION PAPER FOR PRIMARY HEALTHCARE PROFESSIONALS MONOCLONAL ANTIBODY THERAPY Monoclonal antibody therapy for severe asthma KEY POINTS Benralizumab, mepolizumab and omalizumab are monoclonal antibody
TRANSCRIPT for Lantus SoloSTAR pen injection for your patients
 TRANSCRIPT for Lantus SoloSTAR pen injection for your patients SUPER: Prescription Lantus is a long-acting insulin used to treat adults with type 2 diabetes and adults and pediatric patients (children
TRANSCRIPT for Lantus SoloSTAR pen injection for your patients SUPER: Prescription Lantus is a long-acting insulin used to treat adults with type 2 diabetes and adults and pediatric patients (children
How to Use ENBREL : Vial Adapter Method
 How to Use ENBREL : Vial Adapter Method SETTING UP FOR AN INJECTION Select a clean, well-lit, flat working surface, such as a table. Take the ENBREL dose tray out of the refrigerator and place it on your
How to Use ENBREL : Vial Adapter Method SETTING UP FOR AN INJECTION Select a clean, well-lit, flat working surface, such as a table. Take the ENBREL dose tray out of the refrigerator and place it on your
Instructions for Use. HUMALOG KwikPen. insulin lispro injection (rdna origin) 100 units/ml, 3 ml pen
 1 Instructions for Use HUMALOG KwikPen insulin lispro injection (rdna origin) 100 units/ml, 3 ml pen Read the Instructions for Use before you start taking HUMALOG and each time you get another KwikPen.
1 Instructions for Use HUMALOG KwikPen insulin lispro injection (rdna origin) 100 units/ml, 3 ml pen Read the Instructions for Use before you start taking HUMALOG and each time you get another KwikPen.
Instructions for Use Enbrel (en-brel) (etanercept) injection, for subcutaneous use Single-dose Prefilled Syringe
 Instructions for Use Enbrel (en-brel) (etanercept) injection, for subcutaneous use Single-dose Prefilled Syringe How do I prepare and give an injection with Enbrel Single-dose Prefilled Syringe? There
Instructions for Use Enbrel (en-brel) (etanercept) injection, for subcutaneous use Single-dose Prefilled Syringe How do I prepare and give an injection with Enbrel Single-dose Prefilled Syringe? There
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
Inspection Window. Syringe Body
 Guide to Parts Before use Instructions for Use UDENYCA (yoo-den-i-kah) (pegfilgrastim-cbqv) Injection Single-Dose Prefilled Syringe Inspection Window Needle Cap Needle Label Plunger Plunger Head Needle
Guide to Parts Before use Instructions for Use UDENYCA (yoo-den-i-kah) (pegfilgrastim-cbqv) Injection Single-Dose Prefilled Syringe Inspection Window Needle Cap Needle Label Plunger Plunger Head Needle
GlaxoSmithKline. Renal impairment. Hepatic impairment
 RELENZA GlaxoSmithKline Zanamivir QUALITATIVE AND QUANTITATIVE COMPOSITION Each RELENZA ROTADISK consists of four regularly spaced double foil blisters each containing a white to off-white micronised powder
RELENZA GlaxoSmithKline Zanamivir QUALITATIVE AND QUANTITATIVE COMPOSITION Each RELENZA ROTADISK consists of four regularly spaced double foil blisters each containing a white to off-white micronised powder
Glatopa is prescription medicine used for the treatment of people with relapsing forms of multiple sclerosis (MS).
 PATIENT INFORMATION Glatopa (gluh-toh-puh) (Glatiramer Acetate Injection) for Subcutaneous Use Read this Patient Information before you start using Glatopa and each time you get a refill. There may be
PATIENT INFORMATION Glatopa (gluh-toh-puh) (Glatiramer Acetate Injection) for Subcutaneous Use Read this Patient Information before you start using Glatopa and each time you get a refill. There may be
Before you use it. When you must not use it
 Lipegfilgrastim (rbe) Consumer Medicine Information What is in this booklet This booklet answers some common questions about Lonquex. Please note that this booklet does not contain everything there is
Lipegfilgrastim (rbe) Consumer Medicine Information What is in this booklet This booklet answers some common questions about Lonquex. Please note that this booklet does not contain everything there is
Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Syringe (75 mg/ml)
 Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Syringe (75 mg/ml) Important Information The device is a single-dose pre-filled syringe.
Instructions For Use PRALUENT (PRAHL-u-ent) (alirocumab) Injection, for Subcutaneous Injection Single-Dose Pre-Filled Syringe (75 mg/ml) Important Information The device is a single-dose pre-filled syringe.
West of Scotland Difficult Asthma Group Statement of Practice
 West of Scotland Difficult Asthma Group Statement of Practice Member Health Boards: Ayrshire and Arran Dumfries and Galloway Forth Valley Lanarkshire Glasgow Omalizumab in Asthma Advice Note for Asthma
West of Scotland Difficult Asthma Group Statement of Practice Member Health Boards: Ayrshire and Arran Dumfries and Galloway Forth Valley Lanarkshire Glasgow Omalizumab in Asthma Advice Note for Asthma
Lilly. Disposable Insulin Delivery Device User Manual
 1 PV 3734 AMP Lilly Disposable Insulin Delivery Device User Manual Instructions for Use Read and follow all of these instructions carefully. If you do not follow these instructions completely, you may
1 PV 3734 AMP Lilly Disposable Insulin Delivery Device User Manual Instructions for Use Read and follow all of these instructions carefully. If you do not follow these instructions completely, you may
EVOGAM. Information for patients Evogam 2014 NZ Patient Brochure Update v11
 EVOGAM Information for patients 11881 Evogam 2014 NZ Patient Brochure Update v11 Information for patients and caregivers about EVOGAM This booklet is designed to help you follow the training you will have
EVOGAM Information for patients 11881 Evogam 2014 NZ Patient Brochure Update v11 Information for patients and caregivers about EVOGAM This booklet is designed to help you follow the training you will have
Before you take. Tell your doctor if you have any of the conditions mentioned below or if you have ever experienced any of these conditions.
 This medicine is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. You can report side effects to
This medicine is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. You can report side effects to
Instructions for Use. For use with. 10 mg vial
 Instructions for Use For use with 10 mg vial Table of Contents Parts of the ZOMA-Jet 10... 1 Supplies you will need to mix a ZOMACTON 10 mg Vial... 2 Mix a ZOMACTON 10 mg vial... 3 Reset the ZOMA-Jet 10...
Instructions for Use For use with 10 mg vial Table of Contents Parts of the ZOMA-Jet 10... 1 Supplies you will need to mix a ZOMACTON 10 mg Vial... 2 Mix a ZOMACTON 10 mg vial... 3 Reset the ZOMA-Jet 10...
Cap Clip Rubber Seal Plunger Pen Body Dose Window
 Instructions for Use Humalog 100 units/ml Junior KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THESE INSTRUCTIONS BEFORE USE Read the Instructions for Use before you start
Instructions for Use Humalog 100 units/ml Junior KwikPen, solution for injection in a pre-filled pen insulin lispro PLEASE READ THESE INSTRUCTIONS BEFORE USE Read the Instructions for Use before you start
IMPORTANT: PLEASE READ
 PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
PART III: CONSUMER INFORMATION ALPROLIX [pronounced all prō liks] Coagulation Factor IX (Recombinant), Fc Fusion Protein This leaflet is part III of a three-part "Product Monograph" published when ALPROLIX
Instructions for Use HEMLIBRA (hem-lee-bruh) (emicizumab-kxwh) injection, for subcutaneous use
 Instructions for Use HEMLIBRA (hem-lee-bruh) (emicizumab-kxwh) injection, for subcutaneous use Be sure that you read, understand, and follow the Instructions for Use before injecting HEMLIBRA. Your healthcare
Instructions for Use HEMLIBRA (hem-lee-bruh) (emicizumab-kxwh) injection, for subcutaneous use Be sure that you read, understand, and follow the Instructions for Use before injecting HEMLIBRA. Your healthcare
TRANSPARENCY COMMITTEE OPINION. 13 January 2010
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 13 January 2010 XOLAIR 150 mg, powder and solvent for solution for injection Box containing 1 x 150 mg vial + 1 x
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 13 January 2010 XOLAIR 150 mg, powder and solvent for solution for injection Box containing 1 x 150 mg vial + 1 x
PRODUCT INFORMATION INTAL CFC-FREE INHALER AND INTAL FORTE CFC-FREE INHALER
 PRODUCT INFORMATION INTAL CFC-FREE INHALER AND INTAL FORTE CFC-FREE INHALER NAME OF MEDICINE Sodium Cromoglycate for Oral Inhalation. C 23 H 14 Na 2 O 11 Mol Wt. 512.3 CAS No. [15826-37-6] Sodium cromoglycate
PRODUCT INFORMATION INTAL CFC-FREE INHALER AND INTAL FORTE CFC-FREE INHALER NAME OF MEDICINE Sodium Cromoglycate for Oral Inhalation. C 23 H 14 Na 2 O 11 Mol Wt. 512.3 CAS No. [15826-37-6] Sodium cromoglycate
MYALEPT (MAI-uh-lept) (metreleptin) for injection for subcutaneous use
 .3 mg per vial _ A healthcare provider should show you how to inject MYALEPT before you use it for the first time. A healthcare provider should also watch you inject your MYALEPT dose the first time you
.3 mg per vial _ A healthcare provider should show you how to inject MYALEPT before you use it for the first time. A healthcare provider should also watch you inject your MYALEPT dose the first time you
HUMULIN 70/30 KwikPen
 1 Instructions for Use HUMULIN 70/30 KwikPen (70% human insulin isophane suspension 30% human insulin injection [rdna origin]) 100 units/ml, 3 ml pen Read the Instructions for Use before you start taking
1 Instructions for Use HUMULIN 70/30 KwikPen (70% human insulin isophane suspension 30% human insulin injection [rdna origin]) 100 units/ml, 3 ml pen Read the Instructions for Use before you start taking
The Enbrel SureClick autoinjector is a single-dose prefilled autoinjector. It contains one 50 mg dose of Enbrel.
 Instructions for Use Welcome! The Enbrel SureClick autoinjector is a single-dose prefilled autoinjector. It contains one 50 mg dose of Enbrel. Your healthcare provider has prescribed Enbrel SureClick autoinjector
Instructions for Use Welcome! The Enbrel SureClick autoinjector is a single-dose prefilled autoinjector. It contains one 50 mg dose of Enbrel. Your healthcare provider has prescribed Enbrel SureClick autoinjector
Instructions for Use Neulasta (nu-las-tah) (pegfilgrastim) Injection, for subcutaneous use Single-Dose Prefilled Syringe. Plunger rod Used plunger rod
 Instructions for Use Neulasta (nu-las-tah) (pegfilgrastim) Injection, for subcutaneous use Single-Dose Prefilled Syringe Guide to parts Before use After use Plunger rod Used plunger rod Finger grip Label
Instructions for Use Neulasta (nu-las-tah) (pegfilgrastim) Injection, for subcutaneous use Single-Dose Prefilled Syringe Guide to parts Before use After use Plunger rod Used plunger rod Finger grip Label
DO NOT TRANSFER TO A SYRINGE SEVERE OVERDOSE CAN RESULT
 HP8824 Instructions for Use ENTUZITY KwikPen (insulin injection, human biosynthetic) 500 units/ml, 3 ml pen www.lilly.ca IMPORTANT Know your dose of ENTUZITY insulin. The Pen delivers your dose in insulin
HP8824 Instructions for Use ENTUZITY KwikPen (insulin injection, human biosynthetic) 500 units/ml, 3 ml pen www.lilly.ca IMPORTANT Know your dose of ENTUZITY insulin. The Pen delivers your dose in insulin
How to use your gonal-f pre-filled pen
 How to use your gonal-f pre-filled pen Information in this user guide is based on the European product information for Gonal-f. It is not country specific and may vary from country to country. Please always
How to use your gonal-f pre-filled pen Information in this user guide is based on the European product information for Gonal-f. It is not country specific and may vary from country to country. Please always
Step-by-step instructions for intravenous (iv) infusions for patients with:
 Step-by-step instructions for intravenous (iv) infusions for patients with: Rheumatoid Arthritis (RA) Systemic Juvenile Idiopathic Arthritis (sjia) Polyarticular Juvenile Idiopathic Arthritis (pjia) Please
Step-by-step instructions for intravenous (iv) infusions for patients with: Rheumatoid Arthritis (RA) Systemic Juvenile Idiopathic Arthritis (sjia) Polyarticular Juvenile Idiopathic Arthritis (pjia) Please
Neulasta pegfilgrastim (rbe) Consumer Medicine Information
 Neulasta pegfilgrastim (rbe) Consumer Medicine Information What is in this booklet This booklet answers some common questions about Neulasta. Please note that this booklet does not contain everything there
Neulasta pegfilgrastim (rbe) Consumer Medicine Information What is in this booklet This booklet answers some common questions about Neulasta. Please note that this booklet does not contain everything there
PATIENT INFORMATION LEAFLET MEDICINE TO TREAT: DIABETES. Insulin
 PATIENT INFORMATION LEAFLET MEDICINE TO TREAT: DIABETES Insulin This medicine has been proven to be safe and effective, but it can cause serious injury if a mistake happens while taking it. This means
PATIENT INFORMATION LEAFLET MEDICINE TO TREAT: DIABETES Insulin This medicine has been proven to be safe and effective, but it can cause serious injury if a mistake happens while taking it. This means
CIMZIA (certolizumab pegol)
 CIMZIA (certolizumab pegol) solution for subcutaneous use Prefilled syringe step-by-step instructions for use INSTRUCTIONS FOR USE Indications: CIMZIA is approved for the treatment of adults with moderate
CIMZIA (certolizumab pegol) solution for subcutaneous use Prefilled syringe step-by-step instructions for use INSTRUCTIONS FOR USE Indications: CIMZIA is approved for the treatment of adults with moderate
PLEASE READ THESE INSTRUCTIONS BEFORE USE
 Instructions for use KwikPen ABASAGLAR 100 units/ml solution for injection in a pre-filled pen Insulin glargine PLEASE READ THESE INSTRUCTIONS BEFORE USE Read the instructions for use before you start
Instructions for use KwikPen ABASAGLAR 100 units/ml solution for injection in a pre-filled pen Insulin glargine PLEASE READ THESE INSTRUCTIONS BEFORE USE Read the instructions for use before you start
Patient Information Publications Warren Grant Magnuson Clinical Center National Institutes of Health
 Warren Grant Magnuson Clinical Center National Institutes of Health What is a subcutaneous injection? A subcutaneous injection is given in the fatty layer of tissue just under the skin. A subcutaneous
Warren Grant Magnuson Clinical Center National Institutes of Health What is a subcutaneous injection? A subcutaneous injection is given in the fatty layer of tissue just under the skin. A subcutaneous
Drew University Health Service 36 Madison Avenue Madison, New Jersey Tel: Fax:
 Dear Student, Enclosed you will find our policies, procedures and student consent form for your allergy immunotherapy. We ask that you read them carefully, sign the consent form, and take the physician
Dear Student, Enclosed you will find our policies, procedures and student consent form for your allergy immunotherapy. We ask that you read them carefully, sign the consent form, and take the physician
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Xolair (omalizumab) Page 1 of 15 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Xolair (omalizumab) Prime Therapeutics will review Prior Authorization requests.
Xolair (omalizumab) Page 1 of 15 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Xolair (omalizumab) Prime Therapeutics will review Prior Authorization requests.
PEN USER MANUAL Byetta 5 micrograms solution for injection in pre-filled pen (exenatide)
 PEN USER MANUAL Byetta 5 micrograms solution for injection in pre-filled pen (exenatide) Section 1. WHAT YOU NEED TO KNOW ABOUT YOUR BYETTA PEN Read this section completely before you begin. Then, move
PEN USER MANUAL Byetta 5 micrograms solution for injection in pre-filled pen (exenatide) Section 1. WHAT YOU NEED TO KNOW ABOUT YOUR BYETTA PEN Read this section completely before you begin. Then, move
Reference ID:
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use XOLAIR safely and effectively. See full prescribing information for XOLAIR. XOLAIR (omalizumab) for
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use XOLAIR safely and effectively. See full prescribing information for XOLAIR. XOLAIR (omalizumab) for
PRODUCT MONOGRAPH XOLAIR. (omalizumab) Sterile powder for reconstitution. 150 mg vial. IgE-Neutralizing Antibody (Anti-IgE)
 PRODUCT MONOGRAPH Pr XOLAIR (omalizumab) Sterile powder for reconstitution 150 mg vial IgE-Neutralizing Antibody (Anti-IgE) Novartis Pharmaceuticals Canada Inc. Dorval, Quebec H9S 1A9 Date of Approval:
PRODUCT MONOGRAPH Pr XOLAIR (omalizumab) Sterile powder for reconstitution 150 mg vial IgE-Neutralizing Antibody (Anti-IgE) Novartis Pharmaceuticals Canada Inc. Dorval, Quebec H9S 1A9 Date of Approval:
Contraindication: CinnoVex is contraindicated in patients with a history of hypersensitivity to interferon beta, or
 Please read this leaflet carefully before you start taking CinnoVex.Since the information is regularly updated, make sure you read the information each time. For more information, contact your doctor or
Please read this leaflet carefully before you start taking CinnoVex.Since the information is regularly updated, make sure you read the information each time. For more information, contact your doctor or
Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup.
 Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
FIRAZYR Icatibant acetate
 Icatibant acetate Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It does not contain all the available information. It does not take the place of
Icatibant acetate Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It does not contain all the available information. It does not take the place of
GET TO KNOW YOUR PEN. Needle cap. Rubber seal. Insulin scale. Pen cap. Cartridge holder Plunger. Insulin name. Dose window Dose. pointer.
 GET TO KNOW YOUR PEN You ve taken an important step by adding Toujeo (insulin glargine injection) U-3 to your diabetes treatment plan. Toujeo is a long-acting insulin used to treat adults with type and
GET TO KNOW YOUR PEN You ve taken an important step by adding Toujeo (insulin glargine injection) U-3 to your diabetes treatment plan. Toujeo is a long-acting insulin used to treat adults with type and
Patient Information. EGRIFTA (eh-grif-tuh) (tesamorelin for injection) for subcutaneous use
 Patient Information EGRIFTA (eh-grif-tuh) (tesamorelin for injection) for subcutaneous use Read the Patient Information that comes with EGRIFTA before you start to take it and each time you get a refill.
Patient Information EGRIFTA (eh-grif-tuh) (tesamorelin for injection) for subcutaneous use Read the Patient Information that comes with EGRIFTA before you start to take it and each time you get a refill.
Human Normal Immunoglobulin 20% (20 g per 100 ml), solution for subcutaneous administration.
 Human Normal Immunoglobulin 20% (20 g per 100 ml), solution for subcutaneous administration. Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It does
Human Normal Immunoglobulin 20% (20 g per 100 ml), solution for subcutaneous administration. Consumer Medicine Information What is in this leaflet This leaflet answers some common questions about. It does
PATIENT INSTRUCTIONS FOR USE SINGLE- DOSE PREFILLED SYRINGE
 PATIENT INSTRUCTIONS FOR USE EXEMPTIA TM (ADALIMUMAB) SINGLE- DOSE PREFILLED SYRINGE Do not try to inject EXEMPTIA TM yourself until you have been shown the right way to give the injections and have read
PATIENT INSTRUCTIONS FOR USE EXEMPTIA TM (ADALIMUMAB) SINGLE- DOSE PREFILLED SYRINGE Do not try to inject EXEMPTIA TM yourself until you have been shown the right way to give the injections and have read
Package leaflet: Information for the user. Strensiq 100 mg/ml solution for injection (80 mg/0.8 ml) Asfotase alfa
 Package leaflet: Information for the user Strensiq 100 mg/ml solution for injection (80 mg/0.8 ml) Asfotase alfa This medicine is subject to additional monitoring. This will allow quick identification
Package leaflet: Information for the user Strensiq 100 mg/ml solution for injection (80 mg/0.8 ml) Asfotase alfa This medicine is subject to additional monitoring. This will allow quick identification
Acknowledgement goes to WHO for use of their hand washing technique
 How to administer third party insulin injections safely with a pen device, safety pen needle and syringe 1 Hand washing It takes at least 15 seconds to wash your hands. This is about how long it takes
How to administer third party insulin injections safely with a pen device, safety pen needle and syringe 1 Hand washing It takes at least 15 seconds to wash your hands. This is about how long it takes
SCIG INFUSIONS A PRACTICAL GUIDE FOR PATIENTS
 PRIMARY IMMUNODEFICIENCIES SCIG INFUSIONS: A PRACTICAL GUIDE FOR PATIENTS SCIG INFUSIONS A PRACTICAL GUIDE FOR PATIENTS 1 PRIMARY IMMUNODEFICIENCIES ABBREVIATIONS IG IVIG PID SCIG Immunoglobulin Intravenous
PRIMARY IMMUNODEFICIENCIES SCIG INFUSIONS: A PRACTICAL GUIDE FOR PATIENTS SCIG INFUSIONS A PRACTICAL GUIDE FOR PATIENTS 1 PRIMARY IMMUNODEFICIENCIES ABBREVIATIONS IG IVIG PID SCIG Immunoglobulin Intravenous
PATIENT INFORMATION. D iabetes. What You Need to Know About. Insulin
 PATIENT INFORMATION Medicine To Treat: D iabetes What You Need to Know About Insulin INTRODUCTION The insulin preparations currently available in Singapore are mostly from human origin; pork or bovine
PATIENT INFORMATION Medicine To Treat: D iabetes What You Need to Know About Insulin INTRODUCTION The insulin preparations currently available in Singapore are mostly from human origin; pork or bovine
Package leaflet: Information for the patient. Orgalutran 0.25 mg/0.5 ml solution for injection Ganirelix
 Package leaflet: Information for the patient Orgalutran 0.25 mg/0.5 ml solution for injection Ganirelix Read all of this leaflet carefully before you start using this medicine because it contains important
Package leaflet: Information for the patient Orgalutran 0.25 mg/0.5 ml solution for injection Ganirelix Read all of this leaflet carefully before you start using this medicine because it contains important
AIMOVIG TM solution for injection in pre-filled pen erenumab Consumer Medicine Information
 This medicine is subject to additional monitoring in Australia. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. You can report side
This medicine is subject to additional monitoring in Australia. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. You can report side
TREMFYA (guselkumab) Information for patients
 TREMFYA (guselkumab) Information for patients Pre-filled syringe Please read this booklet carefully as it contains important information about TREMFYA, the treatment that your doctor has prescribed to
TREMFYA (guselkumab) Information for patients Pre-filled syringe Please read this booklet carefully as it contains important information about TREMFYA, the treatment that your doctor has prescribed to
HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE
 1 HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. The color of your HumaPen
1 HumaPen LUXURA HD INSULIN DELIVERY DEVICE INSTRUCTIONS FOR USE For use only with Lilly 3 ml insulin cartridges (100 units/ml). Do not use other brands of insulin cartridges. The color of your HumaPen
How to use your Pergoveris pre-filled pen
 How to use your Pergoveris pre-filled pen Information in this user guide is based on the European product information for Pergoveris. It is not country specific and may vary from country to country. Please
How to use your Pergoveris pre-filled pen Information in this user guide is based on the European product information for Pergoveris. It is not country specific and may vary from country to country. Please
AIMOVIG solution for injection in pre-filled pen
 This medicine is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. You can report side effects to
This medicine is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. You can report side effects to
Omalizumab (Xolair ) ( Genentech, Inc., Novartis Pharmaceuticals Corp.) September Indication
 ( Genentech, Inc., Novartis Pharmaceuticals Corp.) September 2003 Indication The FDA recently approved Omalizumab on June 20, 2003 for adults and adolescents (12 years of age and above) with moderate to
( Genentech, Inc., Novartis Pharmaceuticals Corp.) September 2003 Indication The FDA recently approved Omalizumab on June 20, 2003 for adults and adolescents (12 years of age and above) with moderate to
Biologic Agents in the treatment of Severe Asthma
 Biologic Agents in the treatment of Severe Asthma Daniel L Maxwell, D.O., FACOI, FAASM Clinical Assistant Professor of Medicine Michigan State University College of Osteopathic Medicine College of Human
Biologic Agents in the treatment of Severe Asthma Daniel L Maxwell, D.O., FACOI, FAASM Clinical Assistant Professor of Medicine Michigan State University College of Osteopathic Medicine College of Human
PATIENT INFORMATION INCRELEX (EENK-RUH-LEX)
 PATIENT INFORMATION INCRELEX (EENK-RUH-LEX) (mecasermin) injection for subcutaneous use Read the Patient Information that comes with INCRELEX before your child starts receiving INCRELEX and each time your
PATIENT INFORMATION INCRELEX (EENK-RUH-LEX) (mecasermin) injection for subcutaneous use Read the Patient Information that comes with INCRELEX before your child starts receiving INCRELEX and each time your
Instructions for Use Enbrel (en-brel) (etanercept) Single-use Prefilled SureClick Autoinjector
 Instructions for Use Enbrel (en-brel) (etanercept) Single-use Prefilled SureClick Autoinjector How do I prepare and give an injection with Enbrel Single-use Prefilled SureClick Autoinjector? This section
Instructions for Use Enbrel (en-brel) (etanercept) Single-use Prefilled SureClick Autoinjector How do I prepare and give an injection with Enbrel Single-use Prefilled SureClick Autoinjector? This section
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]
![READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la] READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION. [new-ka la]](/thumbs/81/83994292.jpg) READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
READ THIS FOR SAFE AND EFFECTIVE USE OF YOUR MEDICINE PATIENT MEDICATION INFORMATION Pr NUCALA [new-ka la] mepolizumab lyophilized powder for subcutaneous injection Read this carefully before you start
Vial. A healthcare provider should show you how to inject MYALEPT before you use it for
 MYALEPT (metreleptin) for injection Instructions for Use MYALEPT (MAI-uh-lept) (metreleptin) for injection Vial A healthcare provider should show you how to inject MYALEPT before you use it for 8 Do not
MYALEPT (metreleptin) for injection Instructions for Use MYALEPT (MAI-uh-lept) (metreleptin) for injection Vial A healthcare provider should show you how to inject MYALEPT before you use it for 8 Do not
Mepolizumab is a humanised monoclonal antibody (IgG1, kappa), which targets human
 NUCALA PRODUCT INFORMATION N A M E OF THE MEDICINE The active ingredient of NUCALA is mepolizumab. Structure of mepolizumab: CAS number: 196078-29-2 DESCRIPTION Mepolizumab is a humanised monoclonal antibody
NUCALA PRODUCT INFORMATION N A M E OF THE MEDICINE The active ingredient of NUCALA is mepolizumab. Structure of mepolizumab: CAS number: 196078-29-2 DESCRIPTION Mepolizumab is a humanised monoclonal antibody
Things you need to know about injections
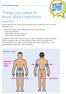 www.trend-uk.org Things you need to know about injections Injection sites: You can see from the picture below the best sites for injecting insulin or GLP-1 medications are: Back of the upper arms (difficult
www.trend-uk.org Things you need to know about injections Injection sites: You can see from the picture below the best sites for injecting insulin or GLP-1 medications are: Back of the upper arms (difficult
NUCALA is indicated as an add-on treatment for severe refractory eosinophilic asthma in adult patients
 QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains 100 mg mepolizumab. After reconstitution, each ml of solution contains 100 mg mepolizumab. Mepolizumab is a humanised monoclonal antibody (IgG1,
QUALITATIVE AND QUANTITATIVE COMPOSITION Each vial contains 100 mg mepolizumab. After reconstitution, each ml of solution contains 100 mg mepolizumab. Mepolizumab is a humanised monoclonal antibody (IgG1,
SymlinPen (SĬM-lĭnPen) 120 (pramlintide acetate) Pen-Injector
 SYMLIN (pramlintide acetate) injection for subcutaneous use 4 SymlinPen (SĬM-lĭnPen) 120 (pramlintide acetate) Pen-Injector Read the Medication Guide and these Instructions for Use before you start using
SYMLIN (pramlintide acetate) injection for subcutaneous use 4 SymlinPen (SĬM-lĭnPen) 120 (pramlintide acetate) Pen-Injector Read the Medication Guide and these Instructions for Use before you start using
