INTERNAL ONLY STANDING ORDER EMERGENCY DEPARTMENTS SALBUTAMOL SULFATE Administration by Accredited Emergency Nurses for symptom relief of asthma
|
|
|
- Caren Clarke
- 6 years ago
- Views:
Transcription
1 POLICY STATEMENT This order may only be activated under the specific circumstances set out in the section Indications and provided there are no contraindications present. The administering nurse must be accredited to administer the drug and record the administration in ink on the once only section of the medication chart. This order must be checked and signed by a medical officer within 24 hours, and preferably within 4 hours, of activation of the standing order. This standing order is only valid until the date noted by the Drug and Quality Use of Medicines Committee under the heading "Effective To:" at the end of this document. 1. NURSING ACCREDITATION REQUIREMENTS Accredited Registered Nurses employed within SESLHD Emergency Departments (ED) who are working in a extended practice nurse or Advanced Clinical Nurse (ACN) capacity must have at least a minimum of two (2) years emergency / critical care experience and must be able to work at a minimum of resuscitation level or above (i.e. triage / clinical initiatives nurse) and/or as approved by the ED Nurse Manager. 2. INDICATIONS Adults and paediatric patients (1 year of age and over) who present to SESLHD Emergency Department (ED) with mild to moderate asthma and: are able to tolerate inhaled medications have a known history of asthma present with audible wheeze OR wheeze on auscultation rated as mild to moderate as per asthma severity scale (section 5) 3. CONTRAINDICATIONS Less than 1 year of age Haemodynamically unstable (meets PACE / Between The Flag criteria 2 ) Hypersensitivity to salbutamol sulphate or other sympathomimetics 4. PRECAUTIONS 1 Cardiac disease Cardiac arrhythmias Hypokalaemia Diabetes mellitus. (monitor hypoglycaemic affect) Hyperthyroidism. Caution in thyrotoxicosis Use in pregnancy (Category A) Use in lactation Treatment with other sympathomimetic amines REV 1 February 2015 Page 1 of 5
2 5. ACTIONS/MONITORING REQUIRED Pre administration: Assessment of previous treatments utilised prior to arrival to the emergency department, and time that last salbutamol sulfate was given/taken Complete respiratory assessment - auscultate chest A full set of vitals (heart rate, blood pressure, respiration rate, and temperature) must be taken prior to administration. Obtain spirometry or peak-flow prior to bronchodilator administration Assess severity 5 : Assessed Severity Mild Moderate Severe Life-threatening Altered consciousness No No Agitated Agitated, Confused, Drowsy Accessory muscle use No Minimal Moderate Severe Oximetry in air >94% 90-94% <90% <90% Talks in Sentences Phrases Words Words Pulsus paradoxus Not present May be palpable Palpable Palpable Pulse rate Normal Mild-mod tachycardia Mod-marked tachycardia Marked tachycardia or bradycardia Central cyanosis No No Likely to be present Likely to be present Wheeze on Variable Moderate-loud Often quiet Often quiet auscultation Physical exhaustion No No Yes Yes If patient meets severe asthma criteria seek immediate medical review. Documentation: Document patient observations including the patient s pain score on the ED Standard Adult General Observation (SAGO) Chart and/or electronically within Firstnet. The administering nurse must record the administration in ink on the once-only section of the National Inpatient Medication Chart (NIMC) or Paediatric National Inpatient Medication Chart (PNIMC) as Emergency Department Standing Order (i.e. ED SO ) plus print and sign their name. Date, time, drug, dose, and route must be completed. The EDSO drug order must be countersigned by the medical officer that subsequently assesses and treats the patient within 4 hours. The administering nurse must record in the patient s progress notes the administration and effect of the medication. Drugs must be checked and ordered according to hospital policy and adhering to the Ministry of Health Policy Medication Handling in NSW Public Health Facilities PD2013_043. Post administration: Evaluate and document therapeutic response Complete a respiratory assessment auscultate chest and a complete a full set of vitals (heart rate, blood pressure, respiration rate and temperature) Re-assessment within 20 minutes of administration If severe asthma initiate salbutamol sulfate and immediately notify an ED senior medical officer Notify a medical officer if patient meets PACE / Between The Flag criteria 1 Monitor for side effects and consider anti-emetics if nausea/vomiting develops REV 1 February 2015 Page 2 of 5
3 6. PROTOCOL/ADMINISTRATION GUIDELINES: Caution: CHECK for allergies and/or contraindications Drug Dose Route Frequency Salbutamol sulfate in Mild / Moderate asthma severity 1 to 5 years or less than 20 kg: 6 puffs (600 microg) 6 years or 20 kg and over: 12 puffs (1,200 microg) Inhaled via spacer Once only Salbutamol sulfate in Severe asthma severity* 1 to 5 years or less than 20 kg: 6 puffs (600 microg), or 2.5 mg via nebuliser Inhaled via 6 years or 20 kg and over: 12 puffs (1,200 microg), or 5 mg via nebuliser spacer OR Nebulised Once only Seek senior MO review* Nebulisers: Administer nebuliser, or via wall oxygen at flow rate 6-8 L/min. Dilute to 2-3 ml with normal saline, nebulise until entire solution consumed. Spacers: Patients with poor inhaler technique will benefit from the consistent use of a spacer device with their metered aerosol. Use of a spacer will also decrease the amount of drug deposited in the mouth and back of the throat, and therefore reduce the incidence of local irritation in susceptible patients. *Note: The most senior clinician should assess a child with life-threatening or persistent severe asthma, discuss management with local specialists and transfer the child to a Children s Hospital (NETS ) For further information on the management of Acute Asthma refer to Appendix 1: Algorithm Assessment & Initial Management of Acute Asthma 7. POTENTIAL ADVERSE EFFECTS/INTERACTIONS 1 : Most common: headache, inhalation site sensation, nervousness Uncommon: cough, tremor, dizziness, hyperkinesia, nausea and vomiting, tachycardia, palpitations, increased respiratory symptoms, pharyngitis, bronchospasm, chest pain, hyperglycaemia, cramps, hyperactivity, insomnia, hypokalaemia, hyperactivity in children Interactions: Beta-blocking drugs, other sympathomimetics, imipramine, chlordiazepoxide, chlorpromazine REV 1 February 2015 Page 3 of 5
4 8. REFERENCES: 1. MIMS Online. Salbutamol sulfate [cited 25/8/14] Available from: &searchkeyword=salbutamol&previouspage=~/search/quicksearch.aspx&searchtype=&id= _2 2. SESLHD. Patient with Acute Condition for Escalation (PACE): Management of the Deteriorating Adult and Maternity Inpatient (SESLHD/PR283) [cited 2014 March]; Available from: nts/seslhdpr283-pace-mgtofthedeterioratingadultmaternityinpatient.pdf. 3. Clinical Emergency Response System (CERS) for Paediatric Inpatients: Management of the Deteriorating Paediatric Inpatient. SESLHDPR/ nts/seslhdpr284-clinicalemergencyresponsesystemcers- MgtDeterioratingPaediatricInpatient.pdf 4. Ministry of Health Policy Medication Handling in NSW Public Health Facilities PD2013_ Ministry of Health Policy PD2012_056 Infants and Children: Acute Management of Asthma Clinical Practice Guideline. Authorised by: Name Designation Signature Date Endorsed by: Name Designation Signature Date Endorsed Effective To: REV 1 February 2015 Page 4 of 5
5 Appendix 1: Algorithm: Assessment and initial management of acute asthma 7 REV 1 February 2015 Page 5 of 5
Management of acute asthma in children in emergency department. Moderate asthma
 152 Moderate asthma SpO2 92% No clinical features of severe asthma NB: If a patient has signs and symptoms across categories, always treat according to their most severe features agonist 2-10 puffs via
152 Moderate asthma SpO2 92% No clinical features of severe asthma NB: If a patient has signs and symptoms across categories, always treat according to their most severe features agonist 2-10 puffs via
patient group direction
 SALBUTAMOL v01 1/12 SALBUTAMOL PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
SALBUTAMOL v01 1/12 SALBUTAMOL PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner
Patient Group Direction for SALBUTAMOL INHALER (Version 02) Valid From 1 October September 2019
 Version Control This PGD has been agreed by the following organisations FCMS PDS Medical Doncaster CCG Lancashire CCGs including East Lancashire, Fylde and Wyre and North Lancashire CCGs Change history
Version Control This PGD has been agreed by the following organisations FCMS PDS Medical Doncaster CCG Lancashire CCGs including East Lancashire, Fylde and Wyre and North Lancashire CCGs Change history
BRICANYL INJECTION. terbutaline sulfate PRODUCT INFORMATION
 BRICANYL INJECTION terbutaline sulfate PRODUCT INFORMATION NAME OF THE MEDICINE Terbutaline sulfate, 2-(tert-butylamino)-1-(3,5-dihydroxyphenyl) ethanol sulfate, a sympathomimetic bronchodilator with a
BRICANYL INJECTION terbutaline sulfate PRODUCT INFORMATION NAME OF THE MEDICINE Terbutaline sulfate, 2-(tert-butylamino)-1-(3,5-dihydroxyphenyl) ethanol sulfate, a sympathomimetic bronchodilator with a
PRODUCT INFORMATION VENTOLIN DISKS
 PRODUCT INFORMATION VENTOLIN DISKS APPROVED NAME: Salbutamol sulphate B.P. PHYSICAL AND CHEMICAL CHARACTERISTICS: Salbutamol sulphate is a white or almost white odourless powder. It is soluble in 4 parts
PRODUCT INFORMATION VENTOLIN DISKS APPROVED NAME: Salbutamol sulphate B.P. PHYSICAL AND CHEMICAL CHARACTERISTICS: Salbutamol sulphate is a white or almost white odourless powder. It is soluble in 4 parts
Composition Each ml of Ventol solution for inhalation contains 5 mg Salbutamol (as sulphate).
 VENTOL Composition Each ml of Ventol solution for inhalation contains 5 mg Salbutamol (as sulphate). Respiratory Solution Action Salbutamol is a short-acting, relatively selective beta2-adrenoceptor agonist.
VENTOL Composition Each ml of Ventol solution for inhalation contains 5 mg Salbutamol (as sulphate). Respiratory Solution Action Salbutamol is a short-acting, relatively selective beta2-adrenoceptor agonist.
VENTOLIN RESPIRATOR SOLUTION
 VENTOLIN RESPIRATOR SOLUTION Salbutamol QUALITATIVE AND QUANTITATIVE COMPOSITION VENTOLIN Respirator Solution contains 5mg salbutamol, as sulphate, per ml of solution and is supplied in 10 ml bottles.
VENTOLIN RESPIRATOR SOLUTION Salbutamol QUALITATIVE AND QUANTITATIVE COMPOSITION VENTOLIN Respirator Solution contains 5mg salbutamol, as sulphate, per ml of solution and is supplied in 10 ml bottles.
PRODUCT INFORMATION VENTOLIN ROTACAPS
 PRODUCT INFORMATION VENTOLIN ROTACAPS APPROVED NAME: Salbutamol sulphate B.P. PHYSICAL AND CHEMICAL CHARACTERISTICS: Salbutamol sulphate is a white or almost white odourless powder. It is soluble in 4
PRODUCT INFORMATION VENTOLIN ROTACAPS APPROVED NAME: Salbutamol sulphate B.P. PHYSICAL AND CHEMICAL CHARACTERISTICS: Salbutamol sulphate is a white or almost white odourless powder. It is soluble in 4
ASTHALIN Respirator Solution (Salbutamol sulphate)
 Published on: 28 Jan 2016 ASTHALIN Respirator Solution (Salbutamol sulphate) Composition Each 1 ml contains: Salbutamol Sulphate IP equivalent to Salbutamol IP.. 5 mg Dosage Form Solution for inhalation
Published on: 28 Jan 2016 ASTHALIN Respirator Solution (Salbutamol sulphate) Composition Each 1 ml contains: Salbutamol Sulphate IP equivalent to Salbutamol IP.. 5 mg Dosage Form Solution for inhalation
Developed By Name Signature Date
 Patient Group Direction 2155 version 2.0 Administration / Supply of Inhaled Salbutamol in Asthma by Registered Practitioners employed by Torbay and South Devon NHS Foundation Trust Date of Introduction:
Patient Group Direction 2155 version 2.0 Administration / Supply of Inhaled Salbutamol in Asthma by Registered Practitioners employed by Torbay and South Devon NHS Foundation Trust Date of Introduction:
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP
 PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
PACKAGE INSERT TEMPLATE FOR SALBUTAMOL TABLET & SALBUTAMOL SYRUP Brand or Product Name [Product name] Tablet 2mg [Product name] Tablet 4mg [Product name] Syrup 2mg/5ml Name and Strength of Active Substance(s)
PRODUCT INFORMATION VENTOLIN ROTACAPS. The chemical name of salbutamol sulfate is 1-(4-hydroxy-3-hydroxymethylphenyl)-2-(tertbutylamino)ethanol
 PRODUCT INFORMATION VENTOLIN ROTACAPS NAME OF THE MEDICINE: Ventolin Rotacaps Ventolin Rotacaps contain salbutamol (as sulfate) B.P. The chemical name of salbutamol sulfate is 1-(4-hydroxy-3-hydroxymethylphenyl)-2-(tertbutylamino)ethanol
PRODUCT INFORMATION VENTOLIN ROTACAPS NAME OF THE MEDICINE: Ventolin Rotacaps Ventolin Rotacaps contain salbutamol (as sulfate) B.P. The chemical name of salbutamol sulfate is 1-(4-hydroxy-3-hydroxymethylphenyl)-2-(tertbutylamino)ethanol
Document Details. Patient Group Direction
 Document Details Title Patient Group Direction (PGD) Salbutamol Aerosol Inhaler and salbutamol Nebulised Solution Trust Ref No 1569-34313 Local Ref (optional) Main points the document Treatment of acute
Document Details Title Patient Group Direction (PGD) Salbutamol Aerosol Inhaler and salbutamol Nebulised Solution Trust Ref No 1569-34313 Local Ref (optional) Main points the document Treatment of acute
A Clinical Guideline for the use of Intravenous Aminophylline in Acute Severe Asthma in Children
 For Use in: By: For: Division responsible for document: Key words: Name and job titles of document author: Name and job title of document author s Line Manager: Supported by: Assessed and approved by the:
For Use in: By: For: Division responsible for document: Key words: Name and job titles of document author: Name and job title of document author s Line Manager: Supported by: Assessed and approved by the:
3 RESPIRATORY SYSTEM
 3 RESPIRATORY SYSTEM 3.01 ANTIASTHMATICS 3.01a BETA 2 AGONIST ASTHMA, CHRONIC BRONCHITIS & EMPHYSEMA Salbutamol Inhaler 100ug/dose [Albuterol] (Ventolin) Salbutamol Sulphate Respirator Solution 0.5% (5mg/ml),
3 RESPIRATORY SYSTEM 3.01 ANTIASTHMATICS 3.01a BETA 2 AGONIST ASTHMA, CHRONIC BRONCHITIS & EMPHYSEMA Salbutamol Inhaler 100ug/dose [Albuterol] (Ventolin) Salbutamol Sulphate Respirator Solution 0.5% (5mg/ml),
Type: Clinical Guideline Register No: Status: Public MANAGEMENT OF ACUTE ASTHMA IN CHILDREN MORE THAN 2 YEARS IN HOSPITAL
 MANAGEMENT OF ACUTE ASTHMA IN CHILDREN MORE THAN 2 YEARS IN HOSPITAL Type: Clinical Guideline Register No: 09055 Status: Public Developed in Response to: Best practice CQC Fundamental Standard: 9, 12,
MANAGEMENT OF ACUTE ASTHMA IN CHILDREN MORE THAN 2 YEARS IN HOSPITAL Type: Clinical Guideline Register No: 09055 Status: Public Developed in Response to: Best practice CQC Fundamental Standard: 9, 12,
Title Protocol for the Management of Asthma
 Document Control Title Protocol for the Management of Asthma Author Author s job title Professional Lead, Minor Injuries Unit Directorate Emergency Services, Logistics and Resilience Department Version
Document Control Title Protocol for the Management of Asthma Author Author s job title Professional Lead, Minor Injuries Unit Directorate Emergency Services, Logistics and Resilience Department Version
NEW ZEALAND DATA SHEET SEREVENT Accuhaler
 NEW ZEALAND DATA SHEET SEREVENT Accuhaler Salmeterol xinafoate (50 mcg per inhalation) Presentation SEREVENT Accuhaler is a moulded plastic device containing a foil strip with 60 regularly placed blisters
NEW ZEALAND DATA SHEET SEREVENT Accuhaler Salmeterol xinafoate (50 mcg per inhalation) Presentation SEREVENT Accuhaler is a moulded plastic device containing a foil strip with 60 regularly placed blisters
ASTHALIN Respules (Salbutamol sulphate)
 Published on: 28 Jan 2016 ASTHALIN Respules (Salbutamol sulphate) Composition Each 2.5 ml respule contains: Salbutamol Sulphate IP equivalent to Salbutamol IP. 2.5 mg Normal saline solution.q.s. Dosage
Published on: 28 Jan 2016 ASTHALIN Respules (Salbutamol sulphate) Composition Each 2.5 ml respule contains: Salbutamol Sulphate IP equivalent to Salbutamol IP. 2.5 mg Normal saline solution.q.s. Dosage
Management of acute severe asthma in adults in general practice. Moderate asthma Acute severe asthma Life-threatening asthma INITIAL ASSESSMENT
 British guideline on the management of asthma Annex 2 Management of acute severe asthma in adults in general practice Many deaths from asthma are preventable. Delay can be fatal. Factors leading to poor
British guideline on the management of asthma Annex 2 Management of acute severe asthma in adults in general practice Many deaths from asthma are preventable. Delay can be fatal. Factors leading to poor
Asthma/wheeze management plan
 Asthma/wheeze management plan Name of Patient Date of Birth NHS Number GP surgery Telephone Next appointment Children s Assessment unit/ward telephone Out of hours call 111 Open access Y/N Until date Some
Asthma/wheeze management plan Name of Patient Date of Birth NHS Number GP surgery Telephone Next appointment Children s Assessment unit/ward telephone Out of hours call 111 Open access Y/N Until date Some
Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup.
 Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
Salapin Salbutamol Syrup 2mg/5mL Qualitative and quantitative composition Salapin: Salbutamol BP 2mg as sulphate in each 5mL of a raspberry cola flavoured, sugar free syrup. Clinical particulars Therapeutic
Summary of Product Characteristics
 1 NAME OF THE MEDICINAL PRODUCT Salbuvent 5 mg/2.5 ml Nebuliser Solution Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ampoule contains 5mg salbutamol (as sulphate).
1 NAME OF THE MEDICINAL PRODUCT Salbuvent 5 mg/2.5 ml Nebuliser Solution Summary of Product Characteristics 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Each ampoule contains 5mg salbutamol (as sulphate).
Asmol CFC-free inhaler Salbutamol (sulfate)
 Asmol CFC-free inhaler Salbutamol (sulfate) PRODUCT INFORMATION Name of the Medicine The active ingredient of Asmol CFC-free inhaler is Salbutamol sulfate B.P. The chemical name for Salbutamol sulfate
Asmol CFC-free inhaler Salbutamol (sulfate) PRODUCT INFORMATION Name of the Medicine The active ingredient of Asmol CFC-free inhaler is Salbutamol sulfate B.P. The chemical name for Salbutamol sulfate
Asmol uni-dose Salbutamol (sulfate) PRODUCT INFORMATION
 Asmol uni-dose Salbutamol (sulfate) PRODUCT INFORMATION NAME OF MEDICINE The active ingredient of Asmol uni-dose is salbutamol (as sulfate). The chemical name for salbutamol sulfate is: 1-(4-hydroxy-3-hydroxymethylphenyl)-2-(t-butylamino)ethanol
Asmol uni-dose Salbutamol (sulfate) PRODUCT INFORMATION NAME OF MEDICINE The active ingredient of Asmol uni-dose is salbutamol (as sulfate). The chemical name for salbutamol sulfate is: 1-(4-hydroxy-3-hydroxymethylphenyl)-2-(t-butylamino)ethanol
I. Subject: Continuous Aerosolization of Bronchodilators
 I. Subject: Continuous Aerosolization of Bronchodilators II. Indications: A. Acute airflow obstruction in which treatment with an aerosolized bronchodilator is desired for an extended period of time, i.e.
I. Subject: Continuous Aerosolization of Bronchodilators II. Indications: A. Acute airflow obstruction in which treatment with an aerosolized bronchodilator is desired for an extended period of time, i.e.
Clinical guideline for acute wheeze & asthma in children 5 years and over Hospital care
 Clinical guideline for acute wheeze & asthma in children years and over Hospital care Airedale NHS Trust Bradford Teaching Hospitals NHS Foundation Trust NHS Bradford and Airedale DOB: A&E/Hospital : Weight:
Clinical guideline for acute wheeze & asthma in children years and over Hospital care Airedale NHS Trust Bradford Teaching Hospitals NHS Foundation Trust NHS Bradford and Airedale DOB: A&E/Hospital : Weight:
PRODUCT INFORMATION VENTOLIN RESPIRATOR SOLUTION AND NEBULES
 PRODUCT INFORMATION VENTOLIN RESPIRATOR SOLUTION AND NEBULES APPROVED NAME: Salbutamol Sulphate BP PHYSICAL AND CHEMICAL CHARACTERISTICS: Salbutamol sulphate is a white or almost white odourless powder.
PRODUCT INFORMATION VENTOLIN RESPIRATOR SOLUTION AND NEBULES APPROVED NAME: Salbutamol Sulphate BP PHYSICAL AND CHEMICAL CHARACTERISTICS: Salbutamol sulphate is a white or almost white odourless powder.
Nursing Process Focus: Patients Receiving Salmeterol (Serevent)
 Prior to administration: Assess for presence/history of chronic asthma, exercise induced asthma, acute asthma attacks, and acute upper airway obstruction. Assess respiratory rate and lung sounds, pulse
Prior to administration: Assess for presence/history of chronic asthma, exercise induced asthma, acute asthma attacks, and acute upper airway obstruction. Assess respiratory rate and lung sounds, pulse
Drugs used in obstetrics
 Drugs used in obstetrics Drugs used in obstetrics Drugs may be used to modify uterine contractions. These include oxytocic drugs used to stimulate uterine contractions both in induction of labour and to
Drugs used in obstetrics Drugs used in obstetrics Drugs may be used to modify uterine contractions. These include oxytocic drugs used to stimulate uterine contractions both in induction of labour and to
Developed By Name Signature Date
 Patient Group Direction 2156 version 2.0 Administration of Ipratropium 250mcg/ml Nebuliser Solution in Acute Asthma by Registered Practitioners employed by Torbay and South Devon NHS Foundation Date of
Patient Group Direction 2156 version 2.0 Administration of Ipratropium 250mcg/ml Nebuliser Solution in Acute Asthma by Registered Practitioners employed by Torbay and South Devon NHS Foundation Date of
New Zealand Datasheet
 New Zealand Datasheet 1 PRODUCT NAME Asthalin 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Salbultamol respiratory solution 3 PHARMACEUTICAL FORM Asthalin is a plastic ampoule presentation containing a sterile,
New Zealand Datasheet 1 PRODUCT NAME Asthalin 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Salbultamol respiratory solution 3 PHARMACEUTICAL FORM Asthalin is a plastic ampoule presentation containing a sterile,
VENTOLIN. About your Ventolin Respirator Solution. Read all of this leaflet carefully before you use your medicine
 Patient Information VENTOLIN About your Ventolin Respirator Solution Read all of this leaflet carefully before you use your medicine This leaflet does not have the complete information about your medicine.
Patient Information VENTOLIN About your Ventolin Respirator Solution Read all of this leaflet carefully before you use your medicine This leaflet does not have the complete information about your medicine.
Chemical name: di[(rs)-2-(1,1-dimethyl)ethylamino-1-[4-hydroxy-3- (hydroxymethyl)phenyl]ethanol] sulphate
![Chemical name: di[(rs)-2-(1,1-dimethyl)ethylamino-1-[4-hydroxy-3- (hydroxymethyl)phenyl]ethanol] sulphate Chemical name: di[(rs)-2-(1,1-dimethyl)ethylamino-1-[4-hydroxy-3- (hydroxymethyl)phenyl]ethanol] sulphate](/thumbs/78/76910401.jpg) Product Information Page 1 PRODUCT INFORMATION SALBUTAMOL SANDOZ 2.5MG/2.5ML, 5MG/2.5ML INHALATION AMPOULES NAME OF THE MEDICINE Generic name: Salbutamol sulphate BP Chemical name: di[(rs)-2-(1,1-dimethyl)ethylamino-1-[4-hydroxy-3-
Product Information Page 1 PRODUCT INFORMATION SALBUTAMOL SANDOZ 2.5MG/2.5ML, 5MG/2.5ML INHALATION AMPOULES NAME OF THE MEDICINE Generic name: Salbutamol sulphate BP Chemical name: di[(rs)-2-(1,1-dimethyl)ethylamino-1-[4-hydroxy-3-
NEW ZEALAND DATA SHEET. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each metered dose contains terbutaline sulphate 250 µg.
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME BRICANYL TURBUHALER, Inhalation powder, 250 µg/dose 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each metered dose contains terbutaline sulphate 250 µg. For a full
NEW ZEALAND DATA SHEET 1. PRODUCT NAME BRICANYL TURBUHALER, Inhalation powder, 250 µg/dose 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each metered dose contains terbutaline sulphate 250 µg. For a full
GenRx SALBUTAMOL INHALATION AMPOULE
 GenRx SALBUTAMOL INHALATION AMPOULE NAME OF THE MEDICINE Salbutamol sulfate. Chemical Name: di[(rs)-2-(1,1-dimethyl)ethylamino-1-[4-hydroxy-3- (hydroxymethyl)phenyl]ethanol] sulfate. Structural Formula:
GenRx SALBUTAMOL INHALATION AMPOULE NAME OF THE MEDICINE Salbutamol sulfate. Chemical Name: di[(rs)-2-(1,1-dimethyl)ethylamino-1-[4-hydroxy-3- (hydroxymethyl)phenyl]ethanol] sulfate. Structural Formula:
Antiallergics and drugs used in anaphylaxis
 Antiallergics and drugs used in anaphylaxis Antiallergics and drugs used in anaphylaxis The H 1 -receptor antagonists are generally referred to as antihistamines. They inhibit the wheal, pruritus, sneezing
Antiallergics and drugs used in anaphylaxis Antiallergics and drugs used in anaphylaxis The H 1 -receptor antagonists are generally referred to as antihistamines. They inhibit the wheal, pruritus, sneezing
Administration of Short-Acting Beta-agonists for Acute Episodes of Moderate or Severe Asthma by Practice Nurses
 1. Clinical Condition or situation to which this Patient Group Direction applies Definition of clinical condition/situation Adults and children aged 2 years and above presenting with increasing symptoms
1. Clinical Condition or situation to which this Patient Group Direction applies Definition of clinical condition/situation Adults and children aged 2 years and above presenting with increasing symptoms
Posology For optimum results in most patients Salamol Easi Breathe CFC Free Inhaler should be used as required.
 Salamol Easi Breathe Summary of Product Characteristics Updated 11 Apr 2016 Teva UK Limited 1. Name of the medicinal product Salamol Easi Breathe CFC Free Inhaler 100 micrograms Pressurised Inhalation,
Salamol Easi Breathe Summary of Product Characteristics Updated 11 Apr 2016 Teva UK Limited 1. Name of the medicinal product Salamol Easi Breathe CFC Free Inhaler 100 micrograms Pressurised Inhalation,
Emergency Department Protocol Initiative
 Emergency Department Protocol Initiative ACUTE ASTHMA MANAGEMENT TOOLKIT March 2006 Provincial Emergency Services Project PHYSICIAN ORDER TEMPLATE FOR CTAS LEVEL 1 ASTHMA ADULT PEDIATRIC Date: Site: Arrival
Emergency Department Protocol Initiative ACUTE ASTHMA MANAGEMENT TOOLKIT March 2006 Provincial Emergency Services Project PHYSICIAN ORDER TEMPLATE FOR CTAS LEVEL 1 ASTHMA ADULT PEDIATRIC Date: Site: Arrival
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET NAME OF MEDICINE OXIS TURBUHALER Eformoterol fumarate dihydrate (6 µg per inhalation) PRESENTATION OXIS TURBUHALER is a multidose, inspiratory flow driven, metered dose, dry powder
NEW ZEALAND DATA SHEET NAME OF MEDICINE OXIS TURBUHALER Eformoterol fumarate dihydrate (6 µg per inhalation) PRESENTATION OXIS TURBUHALER is a multidose, inspiratory flow driven, metered dose, dry powder
PRODUCT INFORMATION. 1-(4-hydroxy-3-hydroxymethylphenyl)-2-(t-butylamino)ethanol Sulphate.
 PRODUCT INFORMATION VENTOLIN RESPIRATOR SOLUTION AND NEBULES NAME OF THE MEDICINE: Salbutamol Sulphate BP Chemical Name: 1-(4-hydroxy-3-hydroxymethylphenyl)-2-(t-butylamino)ethanol Sulphate. Structure:
PRODUCT INFORMATION VENTOLIN RESPIRATOR SOLUTION AND NEBULES NAME OF THE MEDICINE: Salbutamol Sulphate BP Chemical Name: 1-(4-hydroxy-3-hydroxymethylphenyl)-2-(t-butylamino)ethanol Sulphate. Structure:
Clinician Responsible for Training and Review: Emergency Department Consultant
 Patient Group Direction for the Supply of Salbutamol Inhaler to patients receiving treatment from NHS Borders Emergency Department/Out of Hours/Minor Injury Units This document authorises the supply or
Patient Group Direction for the Supply of Salbutamol Inhaler to patients receiving treatment from NHS Borders Emergency Department/Out of Hours/Minor Injury Units This document authorises the supply or
 β 2004 2004 o β β β β 2004 β β β β β β β β β 2004 2004 2004 2004 2004 β 2004 2004 2004 2004 β β 2004 β β β β β β β 2004 β β β β β β 2004 β β β β STEP 5: CONTINUOUS OR FREQUENT USE OF ORAL
β 2004 2004 o β β β β 2004 β β β β β β β β β 2004 2004 2004 2004 2004 β 2004 2004 2004 2004 β β 2004 β β β β β β β 2004 β β β β β β 2004 β β β β STEP 5: CONTINUOUS OR FREQUENT USE OF ORAL
NEW ZEALAND DATA SHEET
 NEW ZEALAND DATA SHEET 1. PRODUCT NAME Ventolin Inhaler (CFC-Free) 100 micrograms 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Ventolin Inhaler (CFC-Free) is a pressurised metered-dose inhaler which delivers
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Ventolin Inhaler (CFC-Free) 100 micrograms 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Ventolin Inhaler (CFC-Free) is a pressurised metered-dose inhaler which delivers
PRODUCT INFORMATION. di[(rs)-2-(1,1-dimethylethyl)amino-1-(4-hydroxy-3-hydroxymethylphenyl)ethanol]sulfate.
![PRODUCT INFORMATION. di[(rs)-2-(1,1-dimethylethyl)amino-1-(4-hydroxy-3-hydroxymethylphenyl)ethanol]sulfate. PRODUCT INFORMATION. di[(rs)-2-(1,1-dimethylethyl)amino-1-(4-hydroxy-3-hydroxymethylphenyl)ethanol]sulfate.](/thumbs/77/75431816.jpg) PRODUCT INFORMATION VENTOLIN CFC-FREE INHALER NAME OF THE MEDICINE: Salbutamol Sulfate B.P. Chemical name: di[(rs)-2-(1,1-dimethylethyl)amino-1-(4-hydroxy-3-hydroxymethylphenyl)ethanol]sulfate. Structure:
PRODUCT INFORMATION VENTOLIN CFC-FREE INHALER NAME OF THE MEDICINE: Salbutamol Sulfate B.P. Chemical name: di[(rs)-2-(1,1-dimethylethyl)amino-1-(4-hydroxy-3-hydroxymethylphenyl)ethanol]sulfate. Structure:
Emergency Asthma Care
 Emergency Asthma Care Contents Introduction 3 Why good emergency asthma care is important 4 Access to emergency asthma care 5 How to improve emergency asthma care 6 Developing emergency asthma treatment
Emergency Asthma Care Contents Introduction 3 Why good emergency asthma care is important 4 Access to emergency asthma care 5 How to improve emergency asthma care 6 Developing emergency asthma treatment
(PLACE PATIENT LABEL HERE) Date: Time: Assessment nurse: Sign: STOP!
 ASTHMA BEST CARE BUNDLE P A T H W A Y ADULT ASTHMA Date: Time: Assessment nurse: Sign: INCLUSION CRITERIA Known asthmatic Shortness of breath and / or wheeze EXCLUSION CRITERIA Chronic lung disease other
ASTHMA BEST CARE BUNDLE P A T H W A Y ADULT ASTHMA Date: Time: Assessment nurse: Sign: INCLUSION CRITERIA Known asthmatic Shortness of breath and / or wheeze EXCLUSION CRITERIA Chronic lung disease other
Australian Asthma Handbook. Key table and figures Version 1.2
 Australian Asthma Handbook Key table and figures Version 1.2 DIAGNOSIS OF ASTHMA Figure. Steps in the diagnosis of asthma in adults Table. Findings that increase or decrease the probability of asthma in
Australian Asthma Handbook Key table and figures Version 1.2 DIAGNOSIS OF ASTHMA Figure. Steps in the diagnosis of asthma in adults Table. Findings that increase or decrease the probability of asthma in
VENTOLIN EVOHALER. Salbutamol
 VENTOLIN EVOHALER CFC FREE Salbutamol QUALITATIVE AND QUANTITATIVE COMPOSITION VENTOLIN EVOHALER is a pressurised metered-dose inhaler which delivers 100 mcg salbutamol (as sulphate) per actuation, into
VENTOLIN EVOHALER CFC FREE Salbutamol QUALITATIVE AND QUANTITATIVE COMPOSITION VENTOLIN EVOHALER is a pressurised metered-dose inhaler which delivers 100 mcg salbutamol (as sulphate) per actuation, into
Medical Directive. Activation Date: April 24, 2013 Review due by: December 1, Medical Director: Date: December 1, 2017
 Medical Directive Pre and Post Bronchodilator Spirometry Testing and Treatment Initiation Assigned Number: Activation Date: April 24, 2013 Review due by: December 1, 2019 23 Approval Signature & Date Medical
Medical Directive Pre and Post Bronchodilator Spirometry Testing and Treatment Initiation Assigned Number: Activation Date: April 24, 2013 Review due by: December 1, 2019 23 Approval Signature & Date Medical
PRODUCT INFORMATION VENTOLIN. SUGAR FREE SYRUP and INJECTION. 1-(4-hydroxy-3-hydroxymethylphenyl)-2-(t-butylamino)ethanol Sulphate.
 PRODUCT INFORMATION VENTOLIN SUGAR FREE SYRUP and INJECTION NAME OF THE MEDICINE: Salbutamol Sulphate BP Chemical Name 1-(4-hydroxy-3-hydroxymethylphenyl)-2-(t-butylamino)ethanol Sulphate. Structure HOH
PRODUCT INFORMATION VENTOLIN SUGAR FREE SYRUP and INJECTION NAME OF THE MEDICINE: Salbutamol Sulphate BP Chemical Name 1-(4-hydroxy-3-hydroxymethylphenyl)-2-(t-butylamino)ethanol Sulphate. Structure HOH
EMT OPTIONAL SKILL. Cell Phones and Pagers. Epinephrine Auto-injector. Course Outline 9/2017
 EMT OPTIONAL SKILL Epinephrine Auto-injector Cell Phones and Pagers Be courteous to your classmates! Please set your cell phones and/or pagers to silent or turn them off. Course Outline Introduction and
EMT OPTIONAL SKILL Epinephrine Auto-injector Cell Phones and Pagers Be courteous to your classmates! Please set your cell phones and/or pagers to silent or turn them off. Course Outline Introduction and
3. Identify the importance in the prehospital setting for the administration of nebulized bronchodilator.
 TERMINAL OBJECTIVE At the end of this lesson, the EMT-Basic will be able to utilize the assessment findings to formulate a field impression of bronchospasm and understand the administration of nebulized
TERMINAL OBJECTIVE At the end of this lesson, the EMT-Basic will be able to utilize the assessment findings to formulate a field impression of bronchospasm and understand the administration of nebulized
PRODUCT INFORMATION. SUDAFED Sinus 12 Hour Relief Tablets
 PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
PRODUCT INFORMATION SUDAFED Sinus 12 Hour Relief Tablets NAME OF THE MEDICINE Pseudoephedrine Hydrochloride CAS 2 Registry Number: 345-78-8 DESCRIPTION SUDAFED Sinus 12 Hour Relief prolonged-release tablets
PRODUCT INFORMATION VENTOLIN CFC-FREE INHALER. Chemical name: di[(rs)-2-(1,1-dimethylethyl)amino-1-(4-hydroxy-3-hydroxymethylphenyl)ethanol]sulfate.
![PRODUCT INFORMATION VENTOLIN CFC-FREE INHALER. Chemical name: di[(rs)-2-(1,1-dimethylethyl)amino-1-(4-hydroxy-3-hydroxymethylphenyl)ethanol]sulfate. PRODUCT INFORMATION VENTOLIN CFC-FREE INHALER. Chemical name: di[(rs)-2-(1,1-dimethylethyl)amino-1-(4-hydroxy-3-hydroxymethylphenyl)ethanol]sulfate.](/thumbs/79/80085632.jpg) PRODUCT INFORMATION VENTOLIN CFC-FREE INHALER NAME OF THE MEDICINE: Salbutamol Sulfate B.P. Chemical name: di[(rs)-2-(1,1-dimethylethyl)amino-1-(4-hydroxy-3-hydroxymethylphenyl)ethanol]sulfate. Structure:
PRODUCT INFORMATION VENTOLIN CFC-FREE INHALER NAME OF THE MEDICINE: Salbutamol Sulfate B.P. Chemical name: di[(rs)-2-(1,1-dimethylethyl)amino-1-(4-hydroxy-3-hydroxymethylphenyl)ethanol]sulfate. Structure:
SEROBID Inhaler (Salmeterol xinafoate)
 Published on: 10 Jul 2014 SEROBID Inhaler (Salmeterol xinafoate) Composition Each actuation delivers: Salmeterol (as Salmeterol Xinafoate IP) 25 mcg Suspended in propellant HFA 134a.....q.s. Dosage Form
Published on: 10 Jul 2014 SEROBID Inhaler (Salmeterol xinafoate) Composition Each actuation delivers: Salmeterol (as Salmeterol Xinafoate IP) 25 mcg Suspended in propellant HFA 134a.....q.s. Dosage Form
Core Safety Profile. Pharmaceutical form(s)/strength: Dry Powder Inhaler, Nebuliser Suspension, pressurised Metered Dose Inhaler (pmdi)
 Core Safety Profile Active substance: Budesonide Pharmaceutical form(s)/strength: Dry Powder Inhaler, Nebuliser Suspension, pressurised Metered Dose Inhaler (pmdi) P - RMS: DK/H/PSUR/0041/001 Date of FAR:
Core Safety Profile Active substance: Budesonide Pharmaceutical form(s)/strength: Dry Powder Inhaler, Nebuliser Suspension, pressurised Metered Dose Inhaler (pmdi) P - RMS: DK/H/PSUR/0041/001 Date of FAR:
Title Protocol for the Management of Asthma in the Minor Injuries Units
 Document Control Title Protocol for the Management of Asthma in the Minor Injuries Units Author Karen Watts Author s job title Professional Lead, Minor Injuries Unit Directorate Emergency Services, Logistics
Document Control Title Protocol for the Management of Asthma in the Minor Injuries Units Author Karen Watts Author s job title Professional Lead, Minor Injuries Unit Directorate Emergency Services, Logistics
PRODUCT INFORMATION VENTOLIN. SUGAR FREE SYRUP and INJECTION. 1-(4-hydroxy-3-hydroxymethylphenyl)-2-(t-butylamino)ethanol Sulphate.
 PRODUCT INFORMATION VENTOLIN SUGAR FREE SYRUP and INJECTION NAME OF THE MEDICINE: Salbutamol Sulphate BP Chemical Name 1-(4-hydroxy-3-hydroxymethylphenyl)-2-(t-butylamino)ethanol Sulphate. Structure HOH
PRODUCT INFORMATION VENTOLIN SUGAR FREE SYRUP and INJECTION NAME OF THE MEDICINE: Salbutamol Sulphate BP Chemical Name 1-(4-hydroxy-3-hydroxymethylphenyl)-2-(t-butylamino)ethanol Sulphate. Structure HOH
Drugs acting on the respiratory tract
 Drugs acting on the respiratory tract Antiasthmatic drugs Asthma Asthma is a chronic inflammatory disease characterized by episodes of reversible airways obstruction due to bronchial hyperresponsiveness;
Drugs acting on the respiratory tract Antiasthmatic drugs Asthma Asthma is a chronic inflammatory disease characterized by episodes of reversible airways obstruction due to bronchial hyperresponsiveness;
New Zealand Data Sheet
 New Zealand Data Sheet 1 PRODUCT NAME Salair 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Pressurised metered-dose inhaler (pmdi) containing Salbutamol sulphate (equivalent to 100mcg salbutamol base per
New Zealand Data Sheet 1 PRODUCT NAME Salair 2 QUALITATIVE AND QUANTITATIVE COMPOSITION Pressurised metered-dose inhaler (pmdi) containing Salbutamol sulphate (equivalent to 100mcg salbutamol base per
Asthma Care in the Emergency Department Clinical Practice Guideline
 Asthma Care in the Emergency Department Clinical Practice Guideline Inclusion: 1) Children 2 years of age or older with a prior history of wheezing, and 2) Children less than 2 years of age with likely
Asthma Care in the Emergency Department Clinical Practice Guideline Inclusion: 1) Children 2 years of age or older with a prior history of wheezing, and 2) Children less than 2 years of age with likely
Resuscitation Fluids
 Resuscitation Fluids Acceptable Fluids (also known as): Sodium Chloride Hartmann s Solution (Ringer-Lactate Solution, Compound Sodium Lactate) 4.5% Albumin Solution (PPS) Gelofusine 20ml/kg Bolus Can be
Resuscitation Fluids Acceptable Fluids (also known as): Sodium Chloride Hartmann s Solution (Ringer-Lactate Solution, Compound Sodium Lactate) 4.5% Albumin Solution (PPS) Gelofusine 20ml/kg Bolus Can be
COPD. Breathing Made Easier
 COPD Breathing Made Easier Catherine E. Cooke, PharmD, BCPS, PAHM Independent Consultant, PosiHleath Clinical Associate Professor, University of Maryland School of Pharmacy This program has been brought
COPD Breathing Made Easier Catherine E. Cooke, PharmD, BCPS, PAHM Independent Consultant, PosiHleath Clinical Associate Professor, University of Maryland School of Pharmacy This program has been brought
Chapter 55. Changes in the Airway With COPD. Manifestations of Severe COPD. Drugs Used to Treat Obstructive Pulmonary Disorders
 Chapter 55 Drugs Used to Treat Obstructive Pulmonary Disorders Changes in the Airway With COPD Manifestations of Severe COPD Air is trapped in the lower respiratory tract The alveoli degenerate and fuse
Chapter 55 Drugs Used to Treat Obstructive Pulmonary Disorders Changes in the Airway With COPD Manifestations of Severe COPD Air is trapped in the lower respiratory tract The alveoli degenerate and fuse
IPRAVENT Respules/Respirator solution (Ipratropium bromide)
 Published on: 19 Sep 2014 IPRAVENT Respules/Respirator solution (Ipratropium bromide) Composition IPRAVENT Respules Each 2 ml contains: Ipratropium Bromide BP equivalent to Ipratropium Bromide (anhydrous)
Published on: 19 Sep 2014 IPRAVENT Respules/Respirator solution (Ipratropium bromide) Composition IPRAVENT Respules Each 2 ml contains: Ipratropium Bromide BP equivalent to Ipratropium Bromide (anhydrous)
Platelet aggregation inhibitor. Cardiac chest pain or suspected Myocardial Infarction.
 s Aspirin Platelet aggregation inhibitor. Anti-inflammatory agent and an inhibitor of platelet function. Useful agent in the treatment of various thromboembolic diseases such as acute myocardial infarction.
s Aspirin Platelet aggregation inhibitor. Anti-inflammatory agent and an inhibitor of platelet function. Useful agent in the treatment of various thromboembolic diseases such as acute myocardial infarction.
1. NAME OF THE MEDICINAL PRODUCT. Vicks Sinex, 0.5 mg/ml, nasal spray solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION
 1. NAME OF THE MEDICINAL PRODUCT Vicks Sinex, 0.5 mg/ml, nasal spray solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Oxymetazoline hydrochloride 0.5 mg/ml 1 spray (50 l) contains approximately 25
1. NAME OF THE MEDICINAL PRODUCT Vicks Sinex, 0.5 mg/ml, nasal spray solution 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Oxymetazoline hydrochloride 0.5 mg/ml 1 spray (50 l) contains approximately 25
VENTORLIN INHALER. Salbutamol Inhalation IP. Salbutamol Sulphate IP equivalent to 100 mcg Salbutamol suspended in CFC-free propellant HFA 134a.
 For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory VENTORLIN INHALER Salbutamol Inhalation IP QUALITATIVE AND QUANTITATIVE COMPOSITION Each metered actuation provides:
For the use only of a Registered Medical Practitioner or a Hospital or a Laboratory VENTORLIN INHALER Salbutamol Inhalation IP QUALITATIVE AND QUANTITATIVE COMPOSITION Each metered actuation provides:
Asthma training. Mike Levin Division of Asthma and Allergy Red Cross Hospital
 Asthma training Mike Levin Division of Asthma and Allergy Red Cross Hospital Introduction Physiology Diagnosis Severity Treatment Control Stage 3 of guidelines Acute asthma Drug delivery Conclusion Overview
Asthma training Mike Levin Division of Asthma and Allergy Red Cross Hospital Introduction Physiology Diagnosis Severity Treatment Control Stage 3 of guidelines Acute asthma Drug delivery Conclusion Overview
Ventolin TM Inhaler CFC Free Salbutamol
 Ventolin TM Inhaler CFC Free Salbutamol 1. QUALITATIVE AND QUANTITATIVE COMPOSITION Ventolin Inhaler is a pressurised metered-dose inhaler which delivers 100 micrograms salbutamol (as sulphate) per actuation,
Ventolin TM Inhaler CFC Free Salbutamol 1. QUALITATIVE AND QUANTITATIVE COMPOSITION Ventolin Inhaler is a pressurised metered-dose inhaler which delivers 100 micrograms salbutamol (as sulphate) per actuation,
Recognize Anaphylaxis Symptoms
 Recognize Anaphylaxis Symptoms File: JHCD-F1 Recognize the Common Anaphylaxis Symptoms Sudden difficulty breathing, wheezing Hives, generalized flushing, itching, or redness of the skin, Swelling of the
Recognize Anaphylaxis Symptoms File: JHCD-F1 Recognize the Common Anaphylaxis Symptoms Sudden difficulty breathing, wheezing Hives, generalized flushing, itching, or redness of the skin, Swelling of the
NEW ZEALAND DATA SHEET. Each pressurised metered-dose aerosol inhaler contains 100 micrograms of salbutamol (as sulfate)
 NEW ZEALAND DATA SHEET RESPIGEN TM 1. Product Name Respigen TM 100 micrograms per actuation inhalation aerosol 2. Qualitative and Quantitative Composition Each pressurised metered-dose aerosol inhaler
NEW ZEALAND DATA SHEET RESPIGEN TM 1. Product Name Respigen TM 100 micrograms per actuation inhalation aerosol 2. Qualitative and Quantitative Composition Each pressurised metered-dose aerosol inhaler
Expiry Date: July 2009 Template Version: Page 1 of 7
 Salbutamol nebules YOU MUST BE AUTHORISED BY NAME, UNDER THE CURRENT VERSION OF THIS PGD BEFORE YOU ATTEMPT TO WORK ACCORDING TO IT NPGD2007/355 (supersedes 2004/177) Clinical Condition Indication: Inclusion
Salbutamol nebules YOU MUST BE AUTHORISED BY NAME, UNDER THE CURRENT VERSION OF THIS PGD BEFORE YOU ATTEMPT TO WORK ACCORDING TO IT NPGD2007/355 (supersedes 2004/177) Clinical Condition Indication: Inclusion
Atrovent Administration
 Atrovent Administration ICEMA Training 2007 Sherri Shimshy RN OBJECTIVES Describe the pharmacology of Atrovent Identify the indications for use of Atrovent in the Adult Population Identify the indications
Atrovent Administration ICEMA Training 2007 Sherri Shimshy RN OBJECTIVES Describe the pharmacology of Atrovent Identify the indications for use of Atrovent in the Adult Population Identify the indications
SCVMC RESPIRATORY CARE PROCEDURE
 Page 1 of 8 Rev. - 11/99, 11/05, 4/11 R-NC - 08/99,08/00, 04/03,10/08,04/09, 07/11, 6/12 B7180-43 OBJECTIVE Continuous Nebulization allows for continuous, controlled drug delivery to the lung, avoiding
Page 1 of 8 Rev. - 11/99, 11/05, 4/11 R-NC - 08/99,08/00, 04/03,10/08,04/09, 07/11, 6/12 B7180-43 OBJECTIVE Continuous Nebulization allows for continuous, controlled drug delivery to the lung, avoiding
Drug Profiles Professional Responder
 Entonox Classification Medical Gas Entonox (50% oxygen 50% nitrous oxide) Effects Potent analgesic, weak anesthetic Onset Rapid Peak Immediate Indications Relief of moderate to severe pain Cardiac-related
Entonox Classification Medical Gas Entonox (50% oxygen 50% nitrous oxide) Effects Potent analgesic, weak anesthetic Onset Rapid Peak Immediate Indications Relief of moderate to severe pain Cardiac-related
TREATMENT OF ANAPHYLACTIC REACTION WITH EPINEPHRINE
 TREATMENT OF ANAPHYLACTIC REACTION WITH EPINEPHRINE FILE: JGCDC Background: The Bibb County School System recognizes the growing concern with severe life-threatening allergic reactions to food items, latex,
TREATMENT OF ANAPHYLACTIC REACTION WITH EPINEPHRINE FILE: JGCDC Background: The Bibb County School System recognizes the growing concern with severe life-threatening allergic reactions to food items, latex,
Bronchospasm & SOB. Kim Kilmurray Senior Clinical Teaching Fellow
 Bronchospasm & SOB Kim Kilmurray Senior Clinical Teaching Fellow LEARNING OBJECTIVES Perform a comprehensive respiratory examination & link clinical signs to underlying pathology Identify the spectrum
Bronchospasm & SOB Kim Kilmurray Senior Clinical Teaching Fellow LEARNING OBJECTIVES Perform a comprehensive respiratory examination & link clinical signs to underlying pathology Identify the spectrum
OBSERVATION UNIT ASTHMA PATHWAY OUTLINE Westmoreland Hospital PAGE 1 OF 5
 PAGE 1 OF 5 Exclusion Criteria: (Reason to admit to hospital) A. New EKG changes except sinus tachycardia B. Respiratory Rate > 40 C. Signs/symptoms of Heart Failure D. Impending respiratory failure or
PAGE 1 OF 5 Exclusion Criteria: (Reason to admit to hospital) A. New EKG changes except sinus tachycardia B. Respiratory Rate > 40 C. Signs/symptoms of Heart Failure D. Impending respiratory failure or
Asthma. chapter 7. Overview
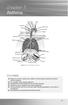 chapter 7 Asthma Sinus Sinus Sinus Right lung Adenoids Tonsils Pharynx Epiglottis Oesophagus Right bronchus Nasal cavity Oral cavity Tongue Larynx Trachea Ribs Left bronchus Diaphragm Bronchiole Pleura
chapter 7 Asthma Sinus Sinus Sinus Right lung Adenoids Tonsils Pharynx Epiglottis Oesophagus Right bronchus Nasal cavity Oral cavity Tongue Larynx Trachea Ribs Left bronchus Diaphragm Bronchiole Pleura
Management of Acute Asthma Exacerbations in Children 2012 Update. Sharon Kling Dept Paediatrics & Child Health University of Stellenbosch
 Management of Acute Asthma Exacerbations in Children 2012 Update Sharon Kling Dept Paediatrics & Child Health University of Stellenbosch Acknowledgements BTS/SIGN guidelines GINA guidelines NAEPP guidelines
Management of Acute Asthma Exacerbations in Children 2012 Update Sharon Kling Dept Paediatrics & Child Health University of Stellenbosch Acknowledgements BTS/SIGN guidelines GINA guidelines NAEPP guidelines
CARDIOLOGY QUESTIONS FOR THE FACEM EXAM TIME ALLOWED: 70 mins
 CARDIOLOGY QUESTIONS FOR THE FACEM EXAM 2015-2016 TIME ALLOWED: 70 mins QUESTION 1 A 71-year-old man presents to the emergency department with a history of chest pain and palpitations. His vital signs
CARDIOLOGY QUESTIONS FOR THE FACEM EXAM 2015-2016 TIME ALLOWED: 70 mins QUESTION 1 A 71-year-old man presents to the emergency department with a history of chest pain and palpitations. His vital signs
OXIS TURBUHALER 4.5 µg/dose and 9 µg/dose ASTRAZENECA
 10-14 OXIS TURBUHALER 4.5 µg/dose and 9 µg/dose ASTRAZENECA formoterol fumarate dihydrate Inhalation powder Composition Each delivered dose (i.e. the dose leaving the mouthpiece) from Oxis Turbuhaler contains
10-14 OXIS TURBUHALER 4.5 µg/dose and 9 µg/dose ASTRAZENECA formoterol fumarate dihydrate Inhalation powder Composition Each delivered dose (i.e. the dose leaving the mouthpiece) from Oxis Turbuhaler contains
DRUG GUIDELINE. HYDRALAZINE (Intravenous severe hypertension in pregnancy)
 DRUG GUIDELINE HYDRALAZINE (Intravenous severe hypertension SCOPE (Area): FOR USE IN: Labour Ward, HDU, Theatre and ED EXCLUSIONS: Paediatrics (seek Paediatrician advice) and other general wards. SCOPE
DRUG GUIDELINE HYDRALAZINE (Intravenous severe hypertension SCOPE (Area): FOR USE IN: Labour Ward, HDU, Theatre and ED EXCLUSIONS: Paediatrics (seek Paediatrician advice) and other general wards. SCOPE
Ventolin Injection 500 micrograms (0.5mg), salbutamol, as sulphate, in 1ml (500 micrograms/ml).
 Ventolin parenteral preparations Salbutamol QUALITATIVE AND QUANTITATIVE COMPOSITION Ventolin Injection 500 micrograms (0.5mg), salbutamol, as sulphate, in 1ml (500 micrograms/ml). Ventolin Solution for
Ventolin parenteral preparations Salbutamol QUALITATIVE AND QUANTITATIVE COMPOSITION Ventolin Injection 500 micrograms (0.5mg), salbutamol, as sulphate, in 1ml (500 micrograms/ml). Ventolin Solution for
ADASUVE (LOXAPINE) INHALATION POWDER. EDUCATION PROGRAM for HEALTHCARE PROFESSIONALS
 ADASUVE (LOXAPINE) INHALATION POWDER EDUCATION PROGRAM for HEALTHCARE PROFESSIONALS August2017 December 2012 PMR-JUN-2017-0017 ADASUVE Risk Evaluation and Mitigation Strategy (REMS) Education Program Content
ADASUVE (LOXAPINE) INHALATION POWDER EDUCATION PROGRAM for HEALTHCARE PROFESSIONALS August2017 December 2012 PMR-JUN-2017-0017 ADASUVE Risk Evaluation and Mitigation Strategy (REMS) Education Program Content
AEROCORT Inhaler (Beclomethasone dipropionate + Levosalbutamol)
 Published on: 28 Jan 2016 AEROCORT Inhaler ( + ) Composition Each actuation delivers: IP. 50 mcg sulphate IP equivalent to levosalbutamol..50 mcg Suspended in propellant HFA-134a.. q.s. Dosage Form Inhalation
Published on: 28 Jan 2016 AEROCORT Inhaler ( + ) Composition Each actuation delivers: IP. 50 mcg sulphate IP equivalent to levosalbutamol..50 mcg Suspended in propellant HFA-134a.. q.s. Dosage Form Inhalation
COMBIVENT Metered Aerosol Boehringer
 COMBIVENT Metered Aerosol Boehringer Composition 1 metered dose contains: (8r)-3*-Hydroxy-8-isopropyl-1*,5*-tropanium bromide (±)-tropate monohydrate (= ipratropium bromide monohydrate) 21 mcg equivalent
COMBIVENT Metered Aerosol Boehringer Composition 1 metered dose contains: (8r)-3*-Hydroxy-8-isopropyl-1*,5*-tropanium bromide (±)-tropate monohydrate (= ipratropium bromide monohydrate) 21 mcg equivalent
patient group direction
 CYCLIZINE v01 1/7 CYCLIZINE PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner (Nurse)
CYCLIZINE v01 1/7 CYCLIZINE PGD Details Version 1.0 Legal category Staff grades Approved by POM Paramedic (Non-ECP) Nurse (Non-ECP) Emergency Care Practitioner (Paramedic) Emergency Care Practitioner (Nurse)
Primary Care practice clinics within the Edmonton Southside Primary Care Network.
 Allergy/Immunotherapy Injections Last Review: November 2016 Intervention(s) and/or Procedure: Administration of allergen injections throughout immunotherapy treatment. Immunotherapy for allergic disease
Allergy/Immunotherapy Injections Last Review: November 2016 Intervention(s) and/or Procedure: Administration of allergen injections throughout immunotherapy treatment. Immunotherapy for allergic disease
WESTCHESTER REGIONAL EMERGENCY MEDICAL ADVISORY COMMITTEE
 WESTCHESTER REGIONAL EMERGENCY MEDICAL ADVISORY COMMITTEE POLICY STATEMENT Supercedes/Updates: New No. 04-02 Date: April 19, 2004 Re: EMT-B Administration of Nebulized Albuterol Pages: 3 Administration
WESTCHESTER REGIONAL EMERGENCY MEDICAL ADVISORY COMMITTEE POLICY STATEMENT Supercedes/Updates: New No. 04-02 Date: April 19, 2004 Re: EMT-B Administration of Nebulized Albuterol Pages: 3 Administration
Title: Management of Allergic Reactions after IV Contrast in Magnetic Resonance Imaging
 ABSTRACT FOR SPS POSTER CASE PRESENTATION K Singer Title: Management of Allergic Reactions after IV Contrast in Magnetic Resonance Imaging Introduction: Children undergoing radiologic imaging frequently
ABSTRACT FOR SPS POSTER CASE PRESENTATION K Singer Title: Management of Allergic Reactions after IV Contrast in Magnetic Resonance Imaging Introduction: Children undergoing radiologic imaging frequently
BRONCHIOLITIS. See also the PSNZ guideline - Wheeze & Chest Infections in infants under 1 year (www.paediatrics.org.nz)
 Definition What is Bronchiolitis? Assessment Management Flow Chart Admission Guidelines Investigations Management Use of Bronchodilators Other treatments Discharge Planning Bronchiolitis & Asthma References
Definition What is Bronchiolitis? Assessment Management Flow Chart Admission Guidelines Investigations Management Use of Bronchodilators Other treatments Discharge Planning Bronchiolitis & Asthma References
PRODUCT INFOMATION APO-IPRATROPIUM SOLUTION
 PRODUCT INFOMATION APO-IPRATROPIUM SOLUTION NAME OF THE MEDICINE Ipratropium bromide. Chemical Name: (1R,3r,5S,8r)-3-[[(2RS)-3-Hydroxy-2-phenylpropanoyl]oxy]-8-methyl-8-(1- methylethyl)-8-azoniabicyclo[3.2.1]octane
PRODUCT INFOMATION APO-IPRATROPIUM SOLUTION NAME OF THE MEDICINE Ipratropium bromide. Chemical Name: (1R,3r,5S,8r)-3-[[(2RS)-3-Hydroxy-2-phenylpropanoyl]oxy]-8-methyl-8-(1- methylethyl)-8-azoniabicyclo[3.2.1]octane
Printed copies of this document may not be up to date, obtain the most recent version from
 Children s Acute Transport Service Clinical Guidelines Acute Severe Asthma Document Control Information Author E Randle Author Position CATS Consultant Document Owner E Polke Document Owner Position Co-ordinator
Children s Acute Transport Service Clinical Guidelines Acute Severe Asthma Document Control Information Author E Randle Author Position CATS Consultant Document Owner E Polke Document Owner Position Co-ordinator
Clinical Practice Guidelines Edition (Updated February 2018)
 The Formulary is published by the Pre-Hospital Emergency Care Council (PHECC) to support Emergency First Responders to be competent in the use of medications permitted under Clinical Practice Guidelines
The Formulary is published by the Pre-Hospital Emergency Care Council (PHECC) to support Emergency First Responders to be competent in the use of medications permitted under Clinical Practice Guidelines
The dose for paediatric patients may never exceed the adult dose.
 The medication formulary is published by the Pre-Hospital Emergency Care Council (PHECC) to support Emergency First Responders to be competent in the use of medications permitted under Clinical Practice
The medication formulary is published by the Pre-Hospital Emergency Care Council (PHECC) to support Emergency First Responders to be competent in the use of medications permitted under Clinical Practice
Hypertonic Saline (7%) Administration Guideline (adults)
 Hypertonic Saline (7%) Administration Guideline (adults) Full Title of Guideline: Author (include email and role): Hypertonic Saline (7%) Administration Guideline for Practice (Adults) Clair Martin, Senior
Hypertonic Saline (7%) Administration Guideline (adults) Full Title of Guideline: Author (include email and role): Hypertonic Saline (7%) Administration Guideline for Practice (Adults) Clair Martin, Senior
