CV safety in COPD? Impact of Guidances and Whitepaper Regulatory position in Europe?
|
|
|
- Paul Evans
- 5 years ago
- Views:
Transcription
1 CV safety in COPD? Impact of Guidances and Whitepaper Regulatory position in Europe? K Prasad, MD FRCPE Manager, CV Unit, LD, MHRA, Member, CVS Working Group, of CHMP, EMA London, UK
2 Disclaimer: The views and opinions expressed in the following slides are those of the individual presenter and should not be attributed to any of the organisations the presenter is affiliated with (MHRA, EMA/CHMP or NHS organizations). Disclosures. I have no Financial interests to declare, only a risk averse attitude (necessary evil). I make predictions only in retrospect (and so am rarely wrong) 2
3 Available Guidances Current regulatory Guidances ICH Clinical Safety guidelines. ( E1 and E2A-F) QT ICH E-14 ICH- Safety pharmacology guidelines White papers on cardiac conduction General cardiac safety evaluation of BP in Drug development WP on Heart rate. Few clinical guidances?!! Specific reference to COPD is not a feature of these.. DOES it need to be?! 3
4 Background There is considerable of overlap of COPD and CVD as comorbidities There is evidence that patients with COPD are at increased CV risk. 1-2 Epidemiological evidence that COPD is an independent risk factor for CV morbidity Chest 2012; 142(5): Thorax, 2010;65: International J of COPD. 2009;4:
5 Background Treatments for COPD associated 3 with o Increased heart rate, Arrhythmias o Effect on QT (??) o Heart failure, o High blood pressure o CV death and myocardial infarction Lung Health study 4 - CV death with ipratropium. Meta-analysis-tiotropium & ipratropium 5 17 RCTs anticholinergics increase MACE in COPD. Meta-analysis tiotropium Respimat trials 6 (2011) raised request for withdrawal from the market. 3. International J of COPD. 2009;4: AJRRCM 2002;166: JAMA, 2008; 300: BMJ, 2011; 342: d3215
6 Where do we stand? Inconsistency/ Variability with the evidence RCTS; UPLIFT(2009) and Tiospir (2013) but are they generalisable? Meta-analysis. Other safety concerns resulted in guidances / WPs- but were consistent and related to MOAs/ off target effects QT and E-14 Diabetes and CV outcome trials. Review of NSAIDS & withdrawal of Cox-2 inhibitors.
7 What if we apply same guidances? There is need for clarity and definition of risk. MOA: with LAMA Heart rate/ Arrhythmia; HR associated with mortality Apply to COPD treatments? Question is what magnitude of change; 5 Bpm or 10 Bpm Time scale for evaluation? Do we apply the same to LABA? No Data on blood pressure? Should BP measurement be a requirement? What magnitude of change? 2-3 mmhg or > 5mm Hg 7. AHJ, 1987; 113: EHJ, 2008; 29: EHJ, 2010; 31: PLoS ONE 6(8): e
8 Questions Exposure Do events relate to exposure and if so for inhaled products with very low concentrations a dose response will need to be established. Is it time dependent? i.e., to sed risk of HR and BP limited to long term treatment and if so, how long? There is need to balance this risk with benefit related to the MOA? 8
9 QTc evaluation in COPD; ICH E-14 guidance is established. However, heart correction issue is debatable. There is a white paper on heart rate corrections, but it is unclear if this is regularly applied. Given the low exposure of inhaled products, there is inconsistency in the corrections applied thus far. There is also inconsistent assessment of effect on heart rate it self. CVCT-Paris,
10 European Guidances in COPD Identifier Guidance title CV safety? EMA/CHMP/483572/2012 CPMP/EWP/562/98 CPMP/EWP/4151/00 Rev. 1 Clinical investigation of medicinal products in the treatment of chronic obstructive pulmonary disease (COPD) Points to Consider on Clinical Investigation of Medicinal Products in the Treatment of Patients with Chronic Obstructive Pulmonary Disease (COPD) Requirements for clinical documentation for orally inhaled products (OIP) ( COPD and Asthma) General safety HR increase as a general measure NI or equivalence relating to safety expected but not CV safety. CPMP/180/95 PMS Studies for Metered Dose Inhalers with New Propellants CHMP/EWP/311890/07 Clinical investigation of medicinal products for cardiovascular disease prevention Not specific to COPD, but CV prevention. 10
11 CV safety & COPD Rx in Europe!. Recognised as a significant and controversial issue. MHRA issued a drug safety update in Tiotropium: safety studies of Spiriva Respimat : Identified the issues and the variability of data. CHMP; Is consistently seeking evaluation of CV safety aspects for LAMA or LAMA/LABA combinations. most Pharmacovigilance and Risk assessment Committee; Regular review of safety and advice to CHMP. Includes LAMAs, LABAs and even SABAs in certain clinical situations. 11
12 Requirements for CV safety for recent compounds!. LAMA or LAMA+LABA Need Post authorisation studies for CV safety-mostly cohort studies Drug utilisation studies in those with CV co-morbidity in the post authorisation period. Risk management Plans Need to identify CV safety and mortality as a safety concern. Includes HR, Myocardial infarction, cardiac arrhythmia, stroke and heart failure. RCT requirement is not applied consistently. 12
13 Summary/ Conclusions Current paradigm Few Guidances specific to CV risk in COPD. Need careful consideration of need for evaluation of CV risk. Are outcome studies the answer and do we need them for all LAMAs as a class? If, so when? Any new paradigm Need to know the direction Need evaluation of impact--
Tiotropium: Do the findings of a CV safety signal in the meta-analyses have implications for all drugs to treat COPD?
 Tiotropium: Do the findings of a CV safety signal in the meta-analyses have implications for all drugs to treat COPD? Lorcan McGarvey MD FRCP Queen s University Belfast CSRC Outcomes Think Tank meeting:
Tiotropium: Do the findings of a CV safety signal in the meta-analyses have implications for all drugs to treat COPD? Lorcan McGarvey MD FRCP Queen s University Belfast CSRC Outcomes Think Tank meeting:
Anoro Ellipta Case Study: Characterizing and Communicating Uncertainty in the Assessment of Benefit/Risk. IOM Workshop February 12, 2014
 Anoro Ellipta Case Study: Characterizing and Communicating Uncertainty in the Assessment of Benefit/Risk IOM Workshop February 12, 2014 Jennifer Rodriguez Pippins, MD, MPH Clinical Reviewer Division of
Anoro Ellipta Case Study: Characterizing and Communicating Uncertainty in the Assessment of Benefit/Risk IOM Workshop February 12, 2014 Jennifer Rodriguez Pippins, MD, MPH Clinical Reviewer Division of
Algorithm for the use of inhaled therapies in COPD
 Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Berkshire West Integrated Care System Representing Berkshire West Clinical Commisioning Group Royal Berkshire NHS Foundation Trust Berkshire Healthcare NHS Foundation Trust Berkshire West Primary Care
Summary of risk management plan for Ulunar Breezhaler (Indacaterol/glycopyrronium)
 Part VI: Summary of the risk management plan Summary of risk management plan for Ulunar Breezhaler (Indacaterol/glycopyrronium) This is a summary of the RMP for Ulunar Breezhaler. The RMP details important
Part VI: Summary of the risk management plan Summary of risk management plan for Ulunar Breezhaler (Indacaterol/glycopyrronium) This is a summary of the RMP for Ulunar Breezhaler. The RMP details important
Direct and indirect CV effects of current drugs and those in development
 Direct and indirect CV effects of current drugs and those in development Heribert Staudinger CSRC MARCH 201 Cardiac Manifestations of COPD Cardiovascular Disease is probably the most frequent and most
Direct and indirect CV effects of current drugs and those in development Heribert Staudinger CSRC MARCH 201 Cardiac Manifestations of COPD Cardiovascular Disease is probably the most frequent and most
Algorithm for the use of inhaled therapies in COPD Version 2 May 2017
 Algorithm for the use of inhaled therapies in COPD This document has been revised by the Berkshire West Respiratory Network to support clinicians in selecting the most appropriate, cost effective treatments
Algorithm for the use of inhaled therapies in COPD This document has been revised by the Berkshire West Respiratory Network to support clinicians in selecting the most appropriate, cost effective treatments
Reflection paper on assessment of cardiovascular safety profile of medicinal products
 25 February 2016 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) Reflection paper on assessment of cardiovascular safety profile of medicinal products Draft agreed by Cardiovascular
25 February 2016 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) Reflection paper on assessment of cardiovascular safety profile of medicinal products Draft agreed by Cardiovascular
Dr Stephen Child. General Physician Auckland. 14:20-14:40 Secondary Care Perspective
 Dr Stephen Child General Physician Auckland 14:20-14:40 Secondary Care Perspective Wheeze Witchery Stephen Child MD, FRACP, FRCPC General Physician Respiratory Interest Director of Clinical Training Auckland
Dr Stephen Child General Physician Auckland 14:20-14:40 Secondary Care Perspective Wheeze Witchery Stephen Child MD, FRACP, FRCPC General Physician Respiratory Interest Director of Clinical Training Auckland
Summary of the risk management plan (RMP) for Duaklir Genuair (aclidinium / formoterol fumarate dihydrate)
 EMA/605453/2014 Summary of the risk management plan (RMP) for Duaklir Genuair (aclidinium / formoterol fumarate dihydrate) This is a summary of the risk management plan (RMP) for Duaklir Genuair, which
EMA/605453/2014 Summary of the risk management plan (RMP) for Duaklir Genuair (aclidinium / formoterol fumarate dihydrate) This is a summary of the risk management plan (RMP) for Duaklir Genuair, which
DATE: 09 December 2009 CONTEXT AND POLICY ISSUES:
 TITLE: Tiotropium Compared with Ipratropium for Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease: A Review of the Clinical Effectiveness DATE: 09 December 2009 CONTEXT AND POLICY
TITLE: Tiotropium Compared with Ipratropium for Patients with Moderate to Severe Chronic Obstructive Pulmonary Disease: A Review of the Clinical Effectiveness DATE: 09 December 2009 CONTEXT AND POLICY
WORK PLAN FOR THE EFFICACY WORKING PARTY (EWP) CHAIRPERSON: Barbara van Zwieten-Boot
 European Medicines Agency London, 17 December 2009 EMA/CHMP/EWP/248088/2009 Rev. 1 WORK PLAN FOR THE EFFICACY WORKING PARTY (EWP) 2010 CHAIRPERSON: Barbara van Zwieten-Boot 1. MEETINGS SCHEDULED FOR 2010
European Medicines Agency London, 17 December 2009 EMA/CHMP/EWP/248088/2009 Rev. 1 WORK PLAN FOR THE EFFICACY WORKING PARTY (EWP) 2010 CHAIRPERSON: Barbara van Zwieten-Boot 1. MEETINGS SCHEDULED FOR 2010
Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984]
![Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984]](/thumbs/85/91889043.jpg) Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] 1 st Appraisal Committee meeting Background & Clinical Effectiveness John McMurray 11 th January 2016 For
Lead team presentation: Roflumilast for treating chronic obstructive pulmonary disease [ID984] 1 st Appraisal Committee meeting Background & Clinical Effectiveness John McMurray 11 th January 2016 For
Pharmacotherapy for COPD
 10/3/2017 Topics to be covered Pharmacotherapy for chronic treatment Pharmacotherapy for COPD Dr. W C Yu 3rd September 2017 Commonly used drugs Guidelines for their use Inhaled corticosteroids (ICS) in
10/3/2017 Topics to be covered Pharmacotherapy for chronic treatment Pharmacotherapy for COPD Dr. W C Yu 3rd September 2017 Commonly used drugs Guidelines for their use Inhaled corticosteroids (ICS) in
Surveillance report Published: 6 April 2016 nice.org.uk. NICE All rights reserved.
 Surveillance report 2016 Chronic obstructive pulmonary disease in over 16s: diagnosis and management (2010) NICE guideline CG101 Surveillance report Published: 6 April 2016 nice.org.uk NICE 2016. All rights
Surveillance report 2016 Chronic obstructive pulmonary disease in over 16s: diagnosis and management (2010) NICE guideline CG101 Surveillance report Published: 6 April 2016 nice.org.uk NICE 2016. All rights
Changing Landscapes in COPD New Zealand Respiratory Conference
 Changing Landscapes in COPD New Zealand Respiratory Conference Dr Robert Young BMedSc MBChB DPhil (Oxon) FRACP FRCP Associate Professor Consultant Physician Changing Landscapes in COPD: Summary 1. Overview
Changing Landscapes in COPD New Zealand Respiratory Conference Dr Robert Young BMedSc MBChB DPhil (Oxon) FRACP FRCP Associate Professor Consultant Physician Changing Landscapes in COPD: Summary 1. Overview
CSRC COPD Initiative Think Tank meeting Washington DC March 6, 2014
 What is the role of outcome studies in pulmonary drug development and what data can be collected during development to sufficiently characterize an agent so that an outcome study not necessary? CSRC COPD
What is the role of outcome studies in pulmonary drug development and what data can be collected during development to sufficiently characterize an agent so that an outcome study not necessary? CSRC COPD
Reflection paper on assessment of cardiovascular risk of medicinal products for the treatment of cardiovascular and metabolic diseases Draft
 1 2 3 21 May 2015 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on assessment of cardiovascular risk of medicinal products for the treatment of cardiovascular
1 2 3 21 May 2015 EMA/CHMP/50549/2015 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on assessment of cardiovascular risk of medicinal products for the treatment of cardiovascular
Disclosures (2013 to the present)
 What level and types of CV safety data acquisition should be considered to sufficiently characterize a drug s benefit/risk assessment by the end of Phase 3 development? Cardiac Safety Research Consortium
What level and types of CV safety data acquisition should be considered to sufficiently characterize a drug s benefit/risk assessment by the end of Phase 3 development? Cardiac Safety Research Consortium
aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A.
 aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A. 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
aclidinium 322 micrograms inhalation powder (Eklira Genuair ) SMC No. (810/12) Almirall S.A. 05 October 2012 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product and
SABA: VENTOLIN EVOHALER (SALBUTAMOL) SAMA: ATROVENT IPRATROPIUM. Offer LAMA (discontinue SAMA) OR LABA
 COPD GUIDELINES DIAGNOSIS >35 years of age Symptoms of cough, breathlessness, sputum, wheeze, Risk factor (SMOKING) Spirometry (post bronchodilator) FEV1/FVC = 0.7 ENCOURAGE PATIENTS TO BRING INHALERS
COPD GUIDELINES DIAGNOSIS >35 years of age Symptoms of cough, breathlessness, sputum, wheeze, Risk factor (SMOKING) Spirometry (post bronchodilator) FEV1/FVC = 0.7 ENCOURAGE PATIENTS TO BRING INHALERS
Supplementary appendix
 Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Calverley P M A, Anzueto A R, Carter K, et
Supplementary appendix This appendix formed part of the original submission and has been peer reviewed. We post it as supplied by the authors. Supplement to: Calverley P M A, Anzueto A R, Carter K, et
DECLARATION OF CONFLICT OF INTEREST
 DECLARATION OF CONFLICT OF INTEREST Is there a mortality risk associated with aspirin use in heart failure? Results from a large community based cohort Margaret Bermingham, Mary-Kate Shanahan, Saki Miwa,
DECLARATION OF CONFLICT OF INTEREST Is there a mortality risk associated with aspirin use in heart failure? Results from a large community based cohort Margaret Bermingham, Mary-Kate Shanahan, Saki Miwa,
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the
umeclidinium, 55 micrograms, powder for inhalation (Incruse ) SMC No. (1004/14) GlaxoSmithKline
 umeclidinium, 55 micrograms, powder for inhalation (Incruse ) SMC No. (1004/14) GlaxoSmithKline 07 November 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
umeclidinium, 55 micrograms, powder for inhalation (Incruse ) SMC No. (1004/14) GlaxoSmithKline 07 November 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Meeting the Quality Challenge for Orally Inhaled Drug Products. Review Impact of Emerging EMA/MHRA Guidelines and Standards
 IPAC-RS/RDD 2016 SYMPOSIUM Meeting the Quality Challenge for Orally Inhaled Drug Products Review Impact of Emerging EMA/MHRA Guidelines and Standards Joseph Lim (PhD) Senior Pharmaceutical Assessor (MHRA)
IPAC-RS/RDD 2016 SYMPOSIUM Meeting the Quality Challenge for Orally Inhaled Drug Products Review Impact of Emerging EMA/MHRA Guidelines and Standards Joseph Lim (PhD) Senior Pharmaceutical Assessor (MHRA)
Blue, Pink and everything in between: an update on COPD. Tara Lohmann MD FRCPC Division of Respirology University of Calgary
 Blue, Pink and everything in between: an update on COPD Tara Lohmann MD FRCPC Division of Respirology University of Calgary Disclosures I have eaten lunches provided by many pharmaceutical companies (GSK,
Blue, Pink and everything in between: an update on COPD Tara Lohmann MD FRCPC Division of Respirology University of Calgary Disclosures I have eaten lunches provided by many pharmaceutical companies (GSK,
Regulatory Experience in Reviewing CV Safety for Diabetes
 Regulatory Experience in Reviewing CV Safety for Diabetes Drugs Kristina Dunder MD, PhD, Alternate CHMP member MPA, Sweden Disclaimer The views and opinions expressed in the following PowerPoint slides
Regulatory Experience in Reviewing CV Safety for Diabetes Drugs Kristina Dunder MD, PhD, Alternate CHMP member MPA, Sweden Disclaimer The views and opinions expressed in the following PowerPoint slides
Presented by UIC College of Nursing
 Presented by UIC College of Nursing Describe COPD. Identify red flags for a COPD exacerbation. Identify COPD triggers or risk factors. Differentiate between long-acting inhalers and emergency use inhalers.
Presented by UIC College of Nursing Describe COPD. Identify red flags for a COPD exacerbation. Identify COPD triggers or risk factors. Differentiate between long-acting inhalers and emergency use inhalers.
Chronic Obstructive Pulmonary Disease 1/18/2018
 Presented by UIC College of Nursing Describe COPD. Identify red flags for a COPD exacerbation. Identify COPD triggers or risk factors. Differentiate between long acting inhalers and emergency use inhalers.
Presented by UIC College of Nursing Describe COPD. Identify red flags for a COPD exacerbation. Identify COPD triggers or risk factors. Differentiate between long acting inhalers and emergency use inhalers.
THE COPD PRESCRIBING TOOL
 THE COPD PRESCRIBING TOOL Revised edition, 2017 www.bpac.org.nz/copd CLASSIFICATION The COPD prescribing tool This tool provides pharmacological treatment options for patients with COPD based on their
THE COPD PRESCRIBING TOOL Revised edition, 2017 www.bpac.org.nz/copd CLASSIFICATION The COPD prescribing tool This tool provides pharmacological treatment options for patients with COPD based on their
7.2 Part VI.2 Elements for a Public Summary
 7.2 Part VI.2 Elements for a Public Summary 7.2.1 Part VI.2.1 Overview of disease epidemiology Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation that is usually
7.2 Part VI.2 Elements for a Public Summary 7.2.1 Part VI.2.1 Overview of disease epidemiology Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation that is usually
Summary of the risk management plan (RMP) for Laventair (umeclidinium bromide and vilanterol)
 EMA/258177/2014 Summary of the risk management plan (RMP) for Laventair (umeclidinium bromide and vilanterol) This is a summary of the risk management plan (RMP) for Laventair, which details the measures
EMA/258177/2014 Summary of the risk management plan (RMP) for Laventair (umeclidinium bromide and vilanterol) This is a summary of the risk management plan (RMP) for Laventair, which details the measures
April 10 th, Bond Street, Toronto ON, M5B 1W8
 Comprehensive Research Plan: Inhaled long-acting muscarinic antagonists (LAMAs; long-acting anticholinergics) for the treatment of chronic obstructive pulmonary disease (COPD) April 10 th, 2014 30 Bond
Comprehensive Research Plan: Inhaled long-acting muscarinic antagonists (LAMAs; long-acting anticholinergics) for the treatment of chronic obstructive pulmonary disease (COPD) April 10 th, 2014 30 Bond
PFT s / 2017 Pulmonary Update. Eric S. Papierniak, DO University of Florida NF/SG VHA
 PFT s / 2017 Pulmonary Update Eric S. Papierniak, DO University of Florida NF/SG VHA Outline Overview of pulmonary function testing Uses/indications/limitations Technical aspects Basics of interpretation
PFT s / 2017 Pulmonary Update Eric S. Papierniak, DO University of Florida NF/SG VHA Outline Overview of pulmonary function testing Uses/indications/limitations Technical aspects Basics of interpretation
Outcomes: Initially, our primary definitions of pneumonia was severe pneumonia, where the subject was hospitalized
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
What is COPD? COPD Pharmacotherapy. COPD Mortality Is Increasing
 COPD Pharmacotherapy Chronic Bronchitis What is COPD? 75% 17.5% Emphysema Laura C. Feemster, MD, MS Assistant Professor University of Washington Division of Pulmonary & Critical Care April 23,2015 COPD
COPD Pharmacotherapy Chronic Bronchitis What is COPD? 75% 17.5% Emphysema Laura C. Feemster, MD, MS Assistant Professor University of Washington Division of Pulmonary & Critical Care April 23,2015 COPD
Asthma in Day to Day Practice
 Asthma in Day to Day Practice VIJAY.K.VANAM Financial relationships: Disclosures Employed at Mercy Medical Center, Mason City. Nonfinancial relationships: I receive no financial gain from any pharmaceutical
Asthma in Day to Day Practice VIJAY.K.VANAM Financial relationships: Disclosures Employed at Mercy Medical Center, Mason City. Nonfinancial relationships: I receive no financial gain from any pharmaceutical
Safeguarding public health Subgroup Analyses: Important, Infuriating and Intractable
 Safeguarding public health Subgroup Analyses: Important, Infuriating and Intractable The views expressed herein are not necessarily those of MHRA, EMA, EMA committees or their working parties. Rob Hemmings
Safeguarding public health Subgroup Analyses: Important, Infuriating and Intractable The views expressed herein are not necessarily those of MHRA, EMA, EMA committees or their working parties. Rob Hemmings
CHRONIC OBSTRUCTIVE PULMONARY DISEASE
 A F O C A l p O I N T l E A r N I N g p r O g r A m m E CHRONIC OBSTRUCTIVE PULMONARY DISEASE S E C O N D E D I T I O N BOOK 1 September 2013 FP120/1 CENTRE FOR PHARMACY POSTGRADUATE EDUCATION About CPPE
A F O C A l p O I N T l E A r N I N g p r O g r A m m E CHRONIC OBSTRUCTIVE PULMONARY DISEASE S E C O N D E D I T I O N BOOK 1 September 2013 FP120/1 CENTRE FOR PHARMACY POSTGRADUATE EDUCATION About CPPE
Year in review. Vit Perlik Director of Regulatory Science and Clinical Development
 Year in review Vit Perlik Director of Regulatory Science and Clinical Development Content Year in review Covering September 2013 to September 2014 Where the regulation goes selection of events for illustration
Year in review Vit Perlik Director of Regulatory Science and Clinical Development Content Year in review Covering September 2013 to September 2014 Where the regulation goes selection of events for illustration
Hypertension Update Clinical Controversies Regarding Age and Race
 Hypertension Update Clinical Controversies Regarding Age and Race Allison Helmer, PharmD, BCACP Assistant Clinical Professor Auburn University Harrison School of Pharmacy July 22, 2017 DISCLOSURE/CONFLICT
Hypertension Update Clinical Controversies Regarding Age and Race Allison Helmer, PharmD, BCACP Assistant Clinical Professor Auburn University Harrison School of Pharmacy July 22, 2017 DISCLOSURE/CONFLICT
glycopyrronium 44 micrograms hard capsules of inhalation powder (Seebri Breezhaler ) SMC No. (829/12) Novartis Pharmaceuticals Ltd.
 glycopyrronium 44 micrograms hard capsules of inhalation powder (Seebri Breezhaler ) SMC No. (829/12) Novartis Pharmaceuticals Ltd. 07 December 2012 The Scottish Medicines Consortium (SMC) has completed
glycopyrronium 44 micrograms hard capsules of inhalation powder (Seebri Breezhaler ) SMC No. (829/12) Novartis Pharmaceuticals Ltd. 07 December 2012 The Scottish Medicines Consortium (SMC) has completed
THE BEST I HAVE READ THIS YEAR (IN PULMONARY AND CRITICAL CARE)
 23 March 2018 Boca Raton, Florida THE BEST I HAVE READ THIS YEAR (IN PULMONARY AND Johnson.margaret2@mayo.edu No disclosures or off label uses CRITICAL CARE) Margaret M. Johnson, MD Chair, Division of
23 March 2018 Boca Raton, Florida THE BEST I HAVE READ THIS YEAR (IN PULMONARY AND Johnson.margaret2@mayo.edu No disclosures or off label uses CRITICAL CARE) Margaret M. Johnson, MD Chair, Division of
Evidence Review for Prescribing Clinical Network
 Summary page Evidence Review for Prescribing Clinical Network Treatment: LABA/LAMA Combination devices in COPD Anoro Ellipta, Ultibro and Duaklir Genuair Prepared by: Noreen Devanney Topic Submitted by:
Summary page Evidence Review for Prescribing Clinical Network Treatment: LABA/LAMA Combination devices in COPD Anoro Ellipta, Ultibro and Duaklir Genuair Prepared by: Noreen Devanney Topic Submitted by:
APSR RESPIRATORY UPDATES
 APSR RESPIRATORY UPDATES Volume 6, Issue 12 Newsletter Date: December 2014 APSR EDUCATION PUBLICATION Inside this issue: COPD Simvastatin for the prevention of exacerbations in moderate-to severe COPD
APSR RESPIRATORY UPDATES Volume 6, Issue 12 Newsletter Date: December 2014 APSR EDUCATION PUBLICATION Inside this issue: COPD Simvastatin for the prevention of exacerbations in moderate-to severe COPD
CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) TREATMENT GUIDELINES
 CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) TREATMENT GUIDELINES Document Description Document Type Service Application Version Guidelines All healthcare professionals(hcps) caring for patients with asthma
CHRONIC OBSTRUCTIVE PULMONARY DISEASE (COPD) TREATMENT GUIDELINES Document Description Document Type Service Application Version Guidelines All healthcare professionals(hcps) caring for patients with asthma
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
abcd Clinical Study Synopsis for Public Disclosure This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data. The synopsis
Summary of Lothian Joint Formulary Amendments
 Summary of Lothian Joint Formulary Amendments The purpose of this summary is to detail the main changes to the LJF sections and provide additional information on the reasons for some of the changes. The
Summary of Lothian Joint Formulary Amendments The purpose of this summary is to detail the main changes to the LJF sections and provide additional information on the reasons for some of the changes. The
Address Comorbidities
 Greater Manchester COPD Management Plan Non-pharmacological management for ALL patients Smoking Cessation Annual Flu Vaccination Pulmonary Rehabilitation Increase daily activity Inhaler Technique Measure
Greater Manchester COPD Management Plan Non-pharmacological management for ALL patients Smoking Cessation Annual Flu Vaccination Pulmonary Rehabilitation Increase daily activity Inhaler Technique Measure
Heart Failure and COPD: Common Partners, Common Problems. Nat Hawkins Liverpool Heart and Chest Hospital
 Heart Failure and COPD: Common Partners, Common Problems Nat Hawkins Liverpool Heart and Chest Hospital Disclosures: No conflicts of interest Common partners, common problems COPD in HF common partners
Heart Failure and COPD: Common Partners, Common Problems Nat Hawkins Liverpool Heart and Chest Hospital Disclosures: No conflicts of interest Common partners, common problems COPD in HF common partners
Wirral COPD Prescribing Guidelines
 Wirral COPD Prescribing Guidelines (To be read in conjunction with the Wirral COPD Supplementary Information) STEP 1: Assess symptoms COPD Assessment Test (CAT) [Link for CAT-test Online] is a patient-completed
Wirral COPD Prescribing Guidelines (To be read in conjunction with the Wirral COPD Supplementary Information) STEP 1: Assess symptoms COPD Assessment Test (CAT) [Link for CAT-test Online] is a patient-completed
Annex. Scientific conclusions and grounds for refusal presented by the European Medicines Agency
 Annex Scientific conclusions and grounds for refusal presented by the European Medicines Agency Scientific conclusions and grounds for refusal presented by the European Medicines Agency Overall summary
Annex Scientific conclusions and grounds for refusal presented by the European Medicines Agency Scientific conclusions and grounds for refusal presented by the European Medicines Agency Overall summary
ANONYMOUS v PFIZER AND NOVARTIS
 CASE AUTH/2928/1/17 and AUTH/2929/1/17 ANONYMOUS v PFIZER AND NOVARTIS Pharmacovigilance compliance, promotion of an unlicensed indication and breach of undertaking An anonymous, non-contactable complainant,
CASE AUTH/2928/1/17 and AUTH/2929/1/17 ANONYMOUS v PFIZER AND NOVARTIS Pharmacovigilance compliance, promotion of an unlicensed indication and breach of undertaking An anonymous, non-contactable complainant,
Who can get most benefit
 Who can get most benefit from tiotropium in asthma? Y-M. Oh Asan Medical Center Univ. of Ulsan College of Medicine Seoul, Korea Tiotripium for Asthma 1 New in GINA 2015 Add-on tiotropium by soft-mist inhaler
Who can get most benefit from tiotropium in asthma? Y-M. Oh Asan Medical Center Univ. of Ulsan College of Medicine Seoul, Korea Tiotripium for Asthma 1 New in GINA 2015 Add-on tiotropium by soft-mist inhaler
Bioequivalence Requirements: USA and EU
 Bioequivalence Requirements: USA and EU Dr. Nicholas Cappuccino Chair, IGPA Science Committee Global Head of Quality, Dr. Reddy s Laboratories Ltd. 15 th Annual IGPA Conference Kyoto, Japan December 6,
Bioequivalence Requirements: USA and EU Dr. Nicholas Cappuccino Chair, IGPA Science Committee Global Head of Quality, Dr. Reddy s Laboratories Ltd. 15 th Annual IGPA Conference Kyoto, Japan December 6,
umeclidinium/vilanterol, 55/22 micrograms, inhalation powder (Anoro ) SMC No. (978/14) GlaxoSmithKline
 umeclidinium/vilanterol, 55/22 micrograms, inhalation powder (Anoro ) SMC No. (978/14) GlaxoSmithKline 04 July 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
umeclidinium/vilanterol, 55/22 micrograms, inhalation powder (Anoro ) SMC No. (978/14) GlaxoSmithKline 04 July 2014 The Scottish Medicines Consortium (SMC) has completed its assessment of the above product
Annex I. Scientific conclusions and grounds for refusal presented by the European Medicines Agency
 Annex I Scientific conclusions and grounds for refusal presented by the European Medicines Agency Scientific conclusions and grounds for refusal presented by the European Medicines Agency Overall summary
Annex I Scientific conclusions and grounds for refusal presented by the European Medicines Agency Scientific conclusions and grounds for refusal presented by the European Medicines Agency Overall summary
Safeguarding public health CHMP's view on multiplicity; through assessment, advice and guidelines
 Safeguarding public health CHMP's view on multiplicity; through assessment, advice and guidelines Rob Hemmings Statistics Unit Manager, MHRA CHMP member Chair, CHMP Scientific Advice Working Party Biostatistics
Safeguarding public health CHMP's view on multiplicity; through assessment, advice and guidelines Rob Hemmings Statistics Unit Manager, MHRA CHMP member Chair, CHMP Scientific Advice Working Party Biostatistics
COPD: Current Medical Therapy
 COPD: Current Medical Therapy Angela Golden, DNP, FNP-C, FAANP Owner, NP from Home, LLC Outcomes As a result of this activity, learners will be able to: 1. List the appropriate classes of medications for
COPD: Current Medical Therapy Angela Golden, DNP, FNP-C, FAANP Owner, NP from Home, LLC Outcomes As a result of this activity, learners will be able to: 1. List the appropriate classes of medications for
AECOPD: Management and Prevention
 Neil MacIntyre MD Duke University Medical Center Durham NC Professor P.J. Barnes, MD, National Heart and Lung Institute, London UK Professor Peter J. Barnes, MD National Heart and Lung Institute, London
Neil MacIntyre MD Duke University Medical Center Durham NC Professor P.J. Barnes, MD, National Heart and Lung Institute, London UK Professor Peter J. Barnes, MD National Heart and Lung Institute, London
Chronic Obstructive Pulmonary Disease (COPD) KAREN ALLEN MD PULMONARY & CRITICAL CARE MEDICINE VA HOSPITAL OKC / OUHSC
 Chronic Obstructive Pulmonary Disease (COPD) KAREN ALLEN MD PULMONARY & CRITICAL CARE MEDICINE VA HOSPITAL OKC / OUHSC I have no financial disclosures Definition COPD is a preventable and treatable disease
Chronic Obstructive Pulmonary Disease (COPD) KAREN ALLEN MD PULMONARY & CRITICAL CARE MEDICINE VA HOSPITAL OKC / OUHSC I have no financial disclosures Definition COPD is a preventable and treatable disease
Suffolk PCT Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice)
 Suffolk PCT Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice) This drug has been reviewed because it is a product that may be prescribed in primary
Suffolk PCT Drug & Therapeutics Committee New Medicine Report (Adopted by the CCG until review and further notice) This drug has been reviewed because it is a product that may be prescribed in primary
NHS Dumfries & Galloway Triple therapy in COPD patients over 16 years
 Title of Project: NHS Dumfries & Galloway Triple therapy in COPD patients over 16 years 1 Reason for the review Respiratory prescribing is long term and can be costly. Appropriate choice and use of inhaled
Title of Project: NHS Dumfries & Galloway Triple therapy in COPD patients over 16 years 1 Reason for the review Respiratory prescribing is long term and can be costly. Appropriate choice and use of inhaled
SUMMARY OF THE RMP. Boehringer Ingelheim International GmbH, Ingelheim, Germany 2.2 ELEMENTS FOR SUMMARY TABLES IN THE EPAR
 PART VI SUMMARY OF THE RMP Active substance Product concerned Name of Marketing Authorisation Holder or Applicant Olodaterol Striverdi Respimat Data lock point for this module 31 Jan 2014 Version number
PART VI SUMMARY OF THE RMP Active substance Product concerned Name of Marketing Authorisation Holder or Applicant Olodaterol Striverdi Respimat Data lock point for this module 31 Jan 2014 Version number
2017 GOLD Report. Is it worth its weight in GOLD??? CSHP-NB Fall Education Day September 30, 2017
 2017 GOLD Report Is it worth its weight in GOLD??? CSHP-NB Fall Education Day September 30, 2017 Lauren Munro; BSc(Pharm) Amanda Burns; BSc(Pharm) Pharmacy Residents The Moncton Hospital Objectives Explain
2017 GOLD Report Is it worth its weight in GOLD??? CSHP-NB Fall Education Day September 30, 2017 Lauren Munro; BSc(Pharm) Amanda Burns; BSc(Pharm) Pharmacy Residents The Moncton Hospital Objectives Explain
This is the publisher s version. This version is defined in the NISO recommended practice RP
 Journal Article Version This is the publisher s version. This version is defined in the NISO recommended practice RP-8-2008 http://www.niso.org/publications/rp/ Suggested Reference Chong, J., Karner, C.,
Journal Article Version This is the publisher s version. This version is defined in the NISO recommended practice RP-8-2008 http://www.niso.org/publications/rp/ Suggested Reference Chong, J., Karner, C.,
Hypertension. Does it Matter What Medications We Use? Nishant K. Sekaran, M.D. M.Sc. Intermountain Heart Institute
 Hypertension Does it Matter What Medications We Use? Nishant K. Sekaran, M.D. M.Sc. Intermountain Heart Institute Hypertension 2017 Classification BP Category Systolic Diastolic Normal 120 and 80 Elevated
Hypertension Does it Matter What Medications We Use? Nishant K. Sekaran, M.D. M.Sc. Intermountain Heart Institute Hypertension 2017 Classification BP Category Systolic Diastolic Normal 120 and 80 Elevated
Work plan for the joint CHMP/ CVMP Quality Working Party (QWP) for 2018
 4 December 2017 EMA/CHMP/CVMP/QWP/504882/2017 Committee for Medicinal Products for Human Use (CHMP) Committee for Medicinal Products for Veterinary Use (CVMP) Work plan for the joint CHMP/ CVMP Quality
4 December 2017 EMA/CHMP/CVMP/QWP/504882/2017 Committee for Medicinal Products for Human Use (CHMP) Committee for Medicinal Products for Veterinary Use (CVMP) Work plan for the joint CHMP/ CVMP Quality
The Committee for Medicinal Products for Human Use
 www.eurordis.org The Committee for Medicinal Products for Human Use Patrick Salmon HPRA Barcelona, 4th June, 2015 2 CHMP 3 CHMP Move 4 The New CHMP 5 Where it all happens! 6 CHMP... What is it? CHMP What
www.eurordis.org The Committee for Medicinal Products for Human Use Patrick Salmon HPRA Barcelona, 4th June, 2015 2 CHMP 3 CHMP Move 4 The New CHMP 5 Where it all happens! 6 CHMP... What is it? CHMP What
European Medicines Agency decision
 EMA/285017/2012 European Medicines Agency decision P/0105/2012 Of 4 June 2012 on the acceptance of a modification of an agreed paediatric investigation plan for tiotropium bromide (monohydrate) (Spiriva
EMA/285017/2012 European Medicines Agency decision P/0105/2012 Of 4 June 2012 on the acceptance of a modification of an agreed paediatric investigation plan for tiotropium bromide (monohydrate) (Spiriva
Responsible Respiratory Prescribing
 Responsible Respiratory Prescribing Dr Vincent Mak Consultant Physician in Respiratory Integrated Care Imperial College Healthcare and Central London Community Healthcare NHS Trust Respiratory Clinical
Responsible Respiratory Prescribing Dr Vincent Mak Consultant Physician in Respiratory Integrated Care Imperial College Healthcare and Central London Community Healthcare NHS Trust Respiratory Clinical
Interested parties (organisations or individuals) that commented on the draft document as released for consultation.
 23 March 2009 EMEA/CHMP/EWP/187653/2009 CPMP/EWP/4151/00 draft 1 Overview of comments received on 'Guideline on the Requirements for clinical documentation for orally inhaled products (OIP) including the
23 March 2009 EMEA/CHMP/EWP/187653/2009 CPMP/EWP/4151/00 draft 1 Overview of comments received on 'Guideline on the Requirements for clinical documentation for orally inhaled products (OIP) including the
Medical Directive. Activation Date: April 24, 2013 Review due by: December 1, Medical Director: Date: December 1, 2017
 Medical Directive Pre and Post Bronchodilator Spirometry Testing and Treatment Initiation Assigned Number: Activation Date: April 24, 2013 Review due by: December 1, 2019 23 Approval Signature & Date Medical
Medical Directive Pre and Post Bronchodilator Spirometry Testing and Treatment Initiation Assigned Number: Activation Date: April 24, 2013 Review due by: December 1, 2019 23 Approval Signature & Date Medical
Safety and efficacy of tiotropium in patients switching from HandiHaler to Respimat in the TIOSPIR trial
 To cite: Dahl R, Calverley PMA, Anzueto A, et al. Safety and efficacy of tiotropium in patients switching from HandiHaler to in the TIOSPIR trial. BMJ Open 2015;5: e009015. doi:10.1136/ bmjopen-2015-009015
To cite: Dahl R, Calverley PMA, Anzueto A, et al. Safety and efficacy of tiotropium in patients switching from HandiHaler to in the TIOSPIR trial. BMJ Open 2015;5: e009015. doi:10.1136/ bmjopen-2015-009015
3. Respiratory System
 1 3. Respiratory System Also see Appendix 3A Guidance on Management of Also see Appendix 3B Preferred Inhaler Devices for Adults Also see Appendix 3C Preferred Inhaler Devices for Adolescents (12-18 years)
1 3. Respiratory System Also see Appendix 3A Guidance on Management of Also see Appendix 3B Preferred Inhaler Devices for Adults Also see Appendix 3C Preferred Inhaler Devices for Adolescents (12-18 years)
Objectives. Describe results and implications of recent landmark hypertension trials
 Hypertension Update Daniel Schwartz, MD Assistant Professor of Medicine Associate Medical Director of Heart Transplantation Temple University School of Medicine Disclosures I currently have no relationships
Hypertension Update Daniel Schwartz, MD Assistant Professor of Medicine Associate Medical Director of Heart Transplantation Temple University School of Medicine Disclosures I currently have no relationships
Preoperative tests (update)
 National Institute for Health and Care Excellence. Preoperative tests (update) Routine preoperative tests for elective surgery NICE guideline NG45 Appendix C: April 2016 Developed by the National Guideline
National Institute for Health and Care Excellence. Preoperative tests (update) Routine preoperative tests for elective surgery NICE guideline NG45 Appendix C: April 2016 Developed by the National Guideline
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Pre-Authorisation Evaluation of Medicines for Human Use London, 19 February 2009 Doc. Ref. EMEA/CHMP/EWP/8197/2009 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) CONCEPT
European Medicines Agency Pre-Authorisation Evaluation of Medicines for Human Use London, 19 February 2009 Doc. Ref. EMEA/CHMP/EWP/8197/2009 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) CONCEPT
An Update in COPD John Hurst PhD FRCP
 An Update in COPD John Hurst PhD FRCP Reader in Respiratory Medicine / Honorary Consultant University College London / Royal Free London NHS Foundation Trust j.hurst@ucl.ac.uk What s new in COPD papers
An Update in COPD John Hurst PhD FRCP Reader in Respiratory Medicine / Honorary Consultant University College London / Royal Free London NHS Foundation Trust j.hurst@ucl.ac.uk What s new in COPD papers
Cardiac Safety Signals: Case Examples
 Cardiac Safety Signals: Case Examples Testosterone Peripherally-acting mu-receptor antagonist PAMORAs Dabigatran (Pradaxa) 2 Testosterone Testosterone replacement therapy is approved for men with low levels
Cardiac Safety Signals: Case Examples Testosterone Peripherally-acting mu-receptor antagonist PAMORAs Dabigatran (Pradaxa) 2 Testosterone Testosterone replacement therapy is approved for men with low levels
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 DRAFT NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary
DRAFT NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 25 May 2011
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 25 May 2011 Examination of the file for the proprietary medicinal product included for a period of 5 years by the
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 25 May 2011 Examination of the file for the proprietary medicinal product included for a period of 5 years by the
A multitude of devices
 A multitude of devices Dr Andrew Scroop Respiratory Consultants 15 th September 2018 STEPWISE PHARMACOLOGICAL MANAGEMENT OF STABLE COPD COPD Inhalers MILD FEV 1 60 80% predicted few symptoms breathless
A multitude of devices Dr Andrew Scroop Respiratory Consultants 15 th September 2018 STEPWISE PHARMACOLOGICAL MANAGEMENT OF STABLE COPD COPD Inhalers MILD FEV 1 60 80% predicted few symptoms breathless
Supplementary materials
 Supplementary materials Table S1 Patient comorbidities by diagnosis Total Asthma n (%) 5348 (148.4) COPD n (%) 4563 (143.4) ACOS n (%) 469 (197.9) No other comorbidities 95 (26.4) 614 (19.3) 27 (11.4)
Supplementary materials Table S1 Patient comorbidities by diagnosis Total Asthma n (%) 5348 (148.4) COPD n (%) 4563 (143.4) ACOS n (%) 469 (197.9) No other comorbidities 95 (26.4) 614 (19.3) 27 (11.4)
Implications of Drug-related Increases in Blood Pressure
 Implications of Drug-related Increases in Blood Pressure Preston M. Dunnmon, MD, FACP, FACC Division of Cardiovascular and Renal Products US Food and Drug Administration July 18, 2012 Disclaimer The findings
Implications of Drug-related Increases in Blood Pressure Preston M. Dunnmon, MD, FACP, FACC Division of Cardiovascular and Renal Products US Food and Drug Administration July 18, 2012 Disclaimer The findings
The Committee for Medicinal Products for Human Use
 www.eurordis.org The Committee for Medicinal Products for Human Use Patrick Salmon IMB Barcelona, 20 June, 2013 2 EURORDIS SUMMER SCHOOL 2013 CHMP CHMP... What is it? 3 EURORDIS SUMMER SCHOOL 2013 CHMP
www.eurordis.org The Committee for Medicinal Products for Human Use Patrick Salmon IMB Barcelona, 20 June, 2013 2 EURORDIS SUMMER SCHOOL 2013 CHMP CHMP... What is it? 3 EURORDIS SUMMER SCHOOL 2013 CHMP
Regulatory Hurdles for Drug Approvals
 Regulatory Hurdles for Drug Approvals William R. Hiatt, MD Professor of Medicine/Cardiology University of Colorado School of Medicine President, CPC Clinical Research 25 min Conflicts CPC Clinical Research
Regulatory Hurdles for Drug Approvals William R. Hiatt, MD Professor of Medicine/Cardiology University of Colorado School of Medicine President, CPC Clinical Research 25 min Conflicts CPC Clinical Research
UPDATE IN CHRONIC OBSTRUCTIVE PULMONARY DISEASE
 UPDATE IN CHRONIC OBSTRUCTIVE PULMONARY DISEASE Radhika Shah, MD Erlanger Health System University of Tennessee College of Medicine Chattanooga Respiratory, Critical Care, and Sleep medicine No disclosures
UPDATE IN CHRONIC OBSTRUCTIVE PULMONARY DISEASE Radhika Shah, MD Erlanger Health System University of Tennessee College of Medicine Chattanooga Respiratory, Critical Care, and Sleep medicine No disclosures
COPD: A Renewed Focus. Disclosures
 COPD: A Renewed Focus Heath Latham, MD Assistant Professor Division of Pulmonary and Critical Care Medicine Disclosures No Business Interests No Consulting No Speakers Bureau No Off Label Use to Discuss
COPD: A Renewed Focus Heath Latham, MD Assistant Professor Division of Pulmonary and Critical Care Medicine Disclosures No Business Interests No Consulting No Speakers Bureau No Off Label Use to Discuss
Optum Research Database Proportion of asthma patients with prescription fills for rescue and controller medications by year,
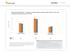 ASTHMA CHART 1 Optum Research Database Proportion of asthma patients with prescription fills for rescue and controller medications by year, 215-216 7 6 5 4 3 2 1 1 Controller medication 1 Rescue medication
ASTHMA CHART 1 Optum Research Database Proportion of asthma patients with prescription fills for rescue and controller medications by year, 215-216 7 6 5 4 3 2 1 1 Controller medication 1 Rescue medication
Potential recommendations for CT coronary angiography in athletes
 Potential recommendations for CT coronary angiography in athletes B.K. Velthuis Dept. of Radiology UMC Utrecht, the Netherlands EuroPRevent 15 April 2011 Declaration of interest Philips Medical Systems
Potential recommendations for CT coronary angiography in athletes B.K. Velthuis Dept. of Radiology UMC Utrecht, the Netherlands EuroPRevent 15 April 2011 Declaration of interest Philips Medical Systems
Roflumilast (Daxas) for chronic obstructive pulmonary disease
 Roflumilast (Daxas) for chronic obstructive pulmonary disease August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Roflumilast (Daxas) for chronic obstructive pulmonary disease August 2009 This technology summary is based on information available at the time of research and a limited literature search. It is not intended
Bulletin Independent prescribing information for NHS Wales
 Bulletin Independent prescribing information for NHS Wales Respiratory disease September 2014 Respiratory diseases account for one in seven of all deaths in Wales the third largest cause of mortality for
Bulletin Independent prescribing information for NHS Wales Respiratory disease September 2014 Respiratory diseases account for one in seven of all deaths in Wales the third largest cause of mortality for
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary and secondary
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary and secondary
Advancing COPD treatment strategies with evidencebased. 17:15 19:15 Monday 11 September 2017 ERS 2017, Milan, Italy
 Advancing COPD treatment strategies with evidencebased approaches 17:15 19:15 Monday 11 September 2017 ERS 2017, Milan, Italy Increasing understanding of COPD and the effect on guideline evolution. GOLD
Advancing COPD treatment strategies with evidencebased approaches 17:15 19:15 Monday 11 September 2017 ERS 2017, Milan, Italy Increasing understanding of COPD and the effect on guideline evolution. GOLD
USE OF UNLICENSED MEDICINES AND OFF-LABEL MEDICINES WHERE A LICENSED MEDICINE IS AVAILABLE
 NHS Scotland Directors of Pharmacy and Scottish Association of Medical Directors USE OF UNLICENSED MEDICINES AND OFF-LABEL MEDICINES WHERE A LICENSED MEDICINE IS AVAILABLE CONSENSUS STATEMENT This consensus
NHS Scotland Directors of Pharmacy and Scottish Association of Medical Directors USE OF UNLICENSED MEDICINES AND OFF-LABEL MEDICINES WHERE A LICENSED MEDICINE IS AVAILABLE CONSENSUS STATEMENT This consensus
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE
 BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE December 2014 Review Date: December 2017 Bulletin 206 : DuoResp Spiromax 160 / 4.5 and 320 / 9 budesonide & formoterol dry powder inhaler JPC Recommendations
BEDFORDSHIRE AND LUTON JOINT PRESCRIBING COMMITTEE December 2014 Review Date: December 2017 Bulletin 206 : DuoResp Spiromax 160 / 4.5 and 320 / 9 budesonide & formoterol dry powder inhaler JPC Recommendations
A European Regulator s perspective on Fixed Dose Combinations
 A European Regulator s perspective on Fixed Dose Combinations Peter Mol -Head assessor CBG-MEB, the Netherlands -SAWP member, EMA -Assistant professor, Clinical Pharmacology, University Medical Center
A European Regulator s perspective on Fixed Dose Combinations Peter Mol -Head assessor CBG-MEB, the Netherlands -SAWP member, EMA -Assistant professor, Clinical Pharmacology, University Medical Center
Responsible Respiratory Prescribing
 Responsible Respiratory Prescribing Dr Vincent Mak Consultant Physician in Respiratory Integrated Care Imperial College Healthcare and Central London Community Healthcare NHS Trust NHS England (London)
Responsible Respiratory Prescribing Dr Vincent Mak Consultant Physician in Respiratory Integrated Care Imperial College Healthcare and Central London Community Healthcare NHS Trust NHS England (London)
Kirthi Gunasekera MD Respiratory Physician National Hospital of Sri Lanka Colombo,
 Kirthi Gunasekera MD Respiratory Physician National Hospital of Sri Lanka Colombo, BRONCHODILATORS: Beta Adrenoreceptor Agonists Actions Adrenoreceptor agonists have many of the same actions as epinephrine/adrenaline,
Kirthi Gunasekera MD Respiratory Physician National Hospital of Sri Lanka Colombo, BRONCHODILATORS: Beta Adrenoreceptor Agonists Actions Adrenoreceptor agonists have many of the same actions as epinephrine/adrenaline,
