Aerospan (flunisolide)
|
|
|
- Jasmine West
- 5 years ago
- Views:
Transcription
1 STRENGTH DOSAGE FORM ROUTE GPID 80mcg/actuation HFA aerosol inhaler w/ Inhaled g/canister adapter MANUFACTURER Meda Pharmaceuticals INDICATION Aerospan Inhalation Aerosol is indicated for the maintenance treatment of asthma as prophylactic therapy in adult and pediatric patients 6 years of age and older. Aerospan Inhalation Aerosol is also indicated for asthma patients requiring oral corticosteroid therapy, where adding Aerospan Inhalation Aerosol may reduce or eliminate the need for oral corticosteroids. Aerospan Inhalation Aerosol is NOT indicated for the relief of acute bronchospasm. DRUG CLASS GLUCOCORTICOIDS, ORALLY INHALED PLACE IN THERAPY Aerospan HFA is a corticosteroid inhaler that includes a two-piece plastic purple actuator and gray spacer assembly with the canister. Each 8.9 g canister provides 120 metered actuations. Other corticosteroid inhalers available include: Asmanex (mometasone), Flovent (fluticasone), Pulmicort (budesonide), Qvar (beclomethasone) and Alvesco (ciclesonide). Aerospan HFA does not contain chlorofluorocarbons (CFCs). Airway inflammation in both large and small airways is an important component in the pathogenesis of asthma. Corticosteroids have been shown to have a wide range of anti-inflammatory effects, inhibiting both inflammatory cells and release of inflammatory mediators. It is presumed that these antiinflammatory actions play an important role in the efficacy of flunisolide in controlling symptoms and improving lung function in asthma. Inhaled flunisolide most likely acts topically at the site of deposition in the bronchial tree after inhalation. EFFICACY The efficacy of Aerospan has been studied in two double-blind, parallel, placebo-and active-controlled clinical studies of 12 weeks duration involving more than 1250 patients. Studies had a 2-week run-in period followed by a 12-week randomized treatment period. During the run-in period all patients received flunisolide CFC inhalation aerosol 500 mcg twice daily. Patients were then randomized to double-blind treatment with different doses of Aerospan Inhalation Aerosol or flunisolide CFC inhalation aerosol and monitored for lung function changes to see if they maintained, improved, or lost stability.
2 Baseline was assessed at the end of the run-in period. The primary endpoint was the change from baseline in percent predicted FEV1 after 12 weeks treatment. Adult and Adolescent Patients with Asthma Efficacy was evaluated in 669 asthma patients (which included 581 adults and 88 children who were years of age). The primary endpoint was change from baseline in percent predicted FEV1 at 12 weeks. In primary endpoint, patients in the placebo group deteriorated 4.3% from baseline after 12 weeks whereas patients treated with Aerospan Inhalation Aerosol 160 mcg or 320 mcg twice daily maintained FEV1 over the course of the study. Aerospan Inhalation Aerosol 160 and 320 mcg twice daily provided statistically significant results (see Figure below) over placebo. However, the 80 mcg dose provided no significant advantage when compared to placebo. Furthermore, Aerospan Inhalation Aerosol and flunisolide CFC inhalation aerosol gave comparable results. Pediatric Patients with Asthma Obtained from Aerospan Inhalation Aerosol package insert Although the study enrolled 583 pediatric asthma patients who were 4 to 11 years of age, the primary efficacy parameter only evaluated 513 patients who were ages 6 to 11 years. Patients were randomized to Aerospan Inhalation Aerosol 80 mcg or 160 mcg twice daily, flunisolide CFC inhalation aerosol 250 mcg or 500 mcg twice daily, or placebo. The primary endpoint was change from baseline in percent predicted FEV1 at 12 weeks in patients 6 years of age and older. In primary endpoint, patients in the placebo group deteriorated 4.0% from baseline after 12 weeks, whereas patients treated with Aerospan Inhalation Aerosol 80 mcg or 160 mcg twice daily maintained FEV1 over the course of the study. When compared to placebo, Aerospan Inhalation Aerosol 80 mcg and 160 mcg dosages provided statistically significant results. However, the 160 mcg BID dosage was not significantly better than the 80 mcg BID dose (see Figure below). Aerospan Inhalation Aerosol and flunisolide CFC inhalation aerosol gave comparable results in patients 6 years of age and older.
3 Obtained from Aerospan Inhalation Aerosol package insert SAFETY Aerospan is contraindicated in the primary treatment of status asthmaticus or other acute episodes of asthma where intensive measures are required. Safety and efficacy of Aerospan has not been studied in patients less than 4 years of age. In clinical trials, the adverse event profile observed in patients was similar between all age groups (4-5, 6-11, years and older).
4 Adverse Events with >3% incidence reported in controlled clinical studies with AEROSPAN Inhalation Aerosol (% of patients) ADVERSE EVENT PLACEBO (n = 220) 80 MCG (n = 189) AEROSPAN Inhalation Aerosol 160 MCG (n = 217) 320 MCG (n = 113) BODY AS A WHOLE Headache Fever Allergic Reaction Pain Accidental Injury Infection, Bacterial Back Pain DIGESTIVE SYSTEM Vomiting Dyspepsia RESPIRATORY SYSTEM Pharyngitis Rhinitis Cough Increased Sinusitis Epistaxis SKIN AND APPENDAGES Rash UROGENITAL SYSTEM Urinary Tract Infection DOSAGE Adults: The recommended adult dose is 2 actuations (160 mcg) inhaled PO twice daily; may titrate upward, not to exceed 4 actuations (320 mcg) twice daily. Pediatrics: For children <6 years, Aerospan Inhalation Aerosol is NOT indicated. For children 6-11 years: 1 actuation (80 mcg) inhaled PO twice daily initially; may titrate upward, not to exceed 2 actuations (160 mcg) twice daily. For children 12 years: 2 actuations (160 mcg) inhaled PO twice daily; may titrate upward, not to exceed 4 actuations (320 mcg) twice daily.
5 COST Drug Cost/unit Cost per 30 days* Aerospan (flunisolide) Inhalation Aerosol 80mcg $194 $388 Asmanex (mometasone) Twisthaler 110mcg, 220mcg $ $287 Flovent (fluticasone) Diskus 50mcg, 100mcg, 250mcg $144-$203 $407 Flovent (fluticasone) HFA 44mcg, 110mcg, 220mcg $152-$317 $317 Pulmicort (budesonide) Flexhaler 90mcg, 180mcg $144-$193 $385 Qvar (beclomethasone) inhaler 40mcg, 80mcg $87-$191 $384 Alvesco (ciclesonide) inhaler 80mcg, 160mcg $207 $417 *Based on maximum dosage FORMULARY PLACEMENT RECOMMENDATIONS Based on this initial assessment of available clinical and financial information, consider NOT ADDING Aerospan Inhalation Aerosol to the formulary pending complete review by the appropriate oversight committee for the plan. REFERENCES Aerospan [Prescribing Information]. Accessed on December 20, Available at
12/18/2017. Disclosures. Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath.
 Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath. AccuNeb inhalation 0.021% solution: 0.63mg/3mL 3-4 times solution
Inhaled bronchodilators relax constricted airways and treat the noisy part of asthma: coughing, wheezing, choking and shortness of breath. AccuNeb inhalation 0.021% solution: 0.63mg/3mL 3-4 times solution
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing
 Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Asthma Management Updates: A Focus on Long-acting Muscarinic Antagonists and Intermittent Inhaled Corticosteroid Dosing Diana M. Sobieraj, PharmD, BCPS Assistant Professor University of Connecticut School
Clinical Policy: Omalizumab (Xolair) Reference Number: ERX.SPA.141 Effective Date: Last Review Date: 08.17
 Clinical Policy: (Xolair) Reference Number: ERX.SPA.141 Effective Date: 03.01.14 Last Review Date: 08.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Xolair) Reference Number: ERX.SPA.141 Effective Date: 03.01.14 Last Review Date: 08.17 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Inhaled Corticosteroid Dose Comparison in Asthma
 This Clinical Resource gives subscribers additional insight related to the Recommendations published in April 2017 ~ Resource #330402 Inhaled Corticosteroid Dose Comparison in Asthma The chart below provides
This Clinical Resource gives subscribers additional insight related to the Recommendations published in April 2017 ~ Resource #330402 Inhaled Corticosteroid Dose Comparison in Asthma The chart below provides
Drug Class Monograph
 Drug Class Monograph Class: Inhaled Corticosteroids Drugs: Aerospan (flunisolide), Advair Diskus, Advair HFA (fluticasone/salmeterol), Alvesco (ciclesonide), Arnuity Ellipta (fluticasone furoate), Asmanex
Drug Class Monograph Class: Inhaled Corticosteroids Drugs: Aerospan (flunisolide), Advair Diskus, Advair HFA (fluticasone/salmeterol), Alvesco (ciclesonide), Arnuity Ellipta (fluticasone furoate), Asmanex
Diagnosis and Management of Asthma
 Supporting Evidence: Diagnosis and Management of Asthma The subdivision of this section is: Appendix B Tables Copyright 2016 by 1 Eleventh Edition/December 2016 Appendix B Asthma Summary Tables Class:
Supporting Evidence: Diagnosis and Management of Asthma The subdivision of this section is: Appendix B Tables Copyright 2016 by 1 Eleventh Edition/December 2016 Appendix B Asthma Summary Tables Class:
APPENDIX 1 Printable point-of-care tables Asthma Action Plan Yellow Zone Formulation Table Region: Europe
 APPENDIX 1 Printable point-of-care tables Asthma Action Plan Yellow Zone Formulation Table Region: Europe Instructions: Print on 8.5 x14 (216 x 279 mm) paper (Legal size) Medication in Green Zone Change
APPENDIX 1 Printable point-of-care tables Asthma Action Plan Yellow Zone Formulation Table Region: Europe Instructions: Print on 8.5 x14 (216 x 279 mm) paper (Legal size) Medication in Green Zone Change
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Fasenra) Reference Number: CP.PHAR.## Effective Date: 01.16.18 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Fasenra) Reference Number: CP.PHAR.## Effective Date: 01.16.18 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Revision Log See Important Reminder at the end of this policy
benralizumab (Fasenra )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
CLINICAL MEDICAL POLICY
 CLINICAL MEDICAL POLICY Policy Name: Xolair (omalizumab) Policy Number: MP-051-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
CLINICAL MEDICAL POLICY Policy Name: Xolair (omalizumab) Policy Number: MP-051-MD-DE Responsible Department(s): Medical Management; Clinical Pharmacy Provider Notice Date: 10/01/2017 Original Effective
Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 12 Years and Older
 Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 12 Years and Older The Canadian Thacic Society recommends a tempary, greater
Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 12 Years and Older The Canadian Thacic Society recommends a tempary, greater
Inhaled Corticosteroids Drug Class Prior Authorization Protocol
 Inhaled Corticosteroids Drug Class Prior Authorization Protocol Line of Business: Medi-Cal P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Inhaled Corticosteroids Drug Class Prior Authorization Protocol Line of Business: Medi-Cal P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Inhaled Corticosteroids Drug Class Prior Authorization Protocol
 Inhaled Corticosteroids Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
Inhaled Corticosteroids Drug Class Prior Authorization Protocol Line of Business: Medicaid P&T Approval Date: February 21, 2018 Effective Date: April 1, 2018 This policy has been developed through review
1.* Dosage. A. Adults
 Aerosolized Agents (Metered-Dose Inhalers) - Inhaled Anti-Inflammatory Drugs: Corticosteroids [Developed, January 1995; Revised, February, 1997; August 1997; March 1998; February 1999; January 2000; March
Aerosolized Agents (Metered-Dose Inhalers) - Inhaled Anti-Inflammatory Drugs: Corticosteroids [Developed, January 1995; Revised, February, 1997; August 1997; March 1998; February 1999; January 2000; March
Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 16 Years and Older
 Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 16 Years and Older The Canadian Thoracic Society and other international asthma
Adjustment of Inhaled Controller Therapy of Asthma in the Yellow Zone, Based on the Inhaler Product Used in the Green Zone Age 16 Years and Older The Canadian Thoracic Society and other international asthma
Key features and changes to these four components of asthma care include:
 Guidelines for the Diagnosis and Management of Asthma in Adults Clinical Practice Guideline MedStar Health These guidelines are provided to assist physicians and other clinicians in making decisions regarding
Guidelines for the Diagnosis and Management of Asthma in Adults Clinical Practice Guideline MedStar Health These guidelines are provided to assist physicians and other clinicians in making decisions regarding
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL
 DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
DOD PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. Uniform Formulary Review Process Under 10 U.S.C. 1074g, as implemented by 32
A Visual Approach to Simplifying Respiratory Drug Regimens
 A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP 3 Main Categories Inhaled Respiratory Drugs Binds to beta-2 receptors Relaxation of smooth muscles in the lung
A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP 3 Main Categories Inhaled Respiratory Drugs Binds to beta-2 receptors Relaxation of smooth muscles in the lung
Orally Inhaled Corticosteroids to 2022
 Greystone Research Associates 1+603-595-4340 April 2015 Orally Inhaled Corticosteroids to 2022 Drugs, Devices, Markets and Forecasts Contents A Comprehensive Market Analysis Report Scope & Overview 2 Table
Greystone Research Associates 1+603-595-4340 April 2015 Orally Inhaled Corticosteroids to 2022 Drugs, Devices, Markets and Forecasts Contents A Comprehensive Market Analysis Report Scope & Overview 2 Table
The Inflammatory Response. Inflammation in the Airway. The Inflammatory Response. Inflammation in the Airway. Inflammation in the Airway
 The Inflammatory Response RSPT 2217 Gardenhire Chapter 11 A general definition inflammation is the response of vascularized tissue to injury First described in the first century A.D. and revised in the
The Inflammatory Response RSPT 2217 Gardenhire Chapter 11 A general definition inflammation is the response of vascularized tissue to injury First described in the first century A.D. and revised in the
AT TRIAGE. Alberta Acute Childhood Asthma Pathway: Evidence based* recommendations For Emergency / Urgent Care
 1 1 Should the child be placed into the Pathway? Asthma Clinical Score (PRAM) Inclusion Children 1 year and 18 years of age who present with wheezing and respiratory distress, and have been diagnosed by
1 1 Should the child be placed into the Pathway? Asthma Clinical Score (PRAM) Inclusion Children 1 year and 18 years of age who present with wheezing and respiratory distress, and have been diagnosed by
TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder
 TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
TRELEGY ELLIPTA (fluticasone-umeclidinium-vilanterol) aerosol powder Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
Preschool Asthma What you need to know in 10 minutes
 Preschool Asthma What you need to know in 10 minutes Alan Kaplan MD CCFP(EM) FCFP Family Physician Airways Group of Canada Respiratory Medicine section CFPC Faculty/Presenter Disclosure Faculty: Alan Kaplan
Preschool Asthma What you need to know in 10 minutes Alan Kaplan MD CCFP(EM) FCFP Family Physician Airways Group of Canada Respiratory Medicine section CFPC Faculty/Presenter Disclosure Faculty: Alan Kaplan
Clinical Policy: Dupilumab (Dupixent) Reference Number: ERX.SPA.49 Effective Date:
 Clinical Policy: (Dupixent) Reference Number: ERX.SPA.49 Effective Date: 06.01.17 Last Review Date: 02.19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Dupixent) Reference Number: ERX.SPA.49 Effective Date: 06.01.17 Last Review Date: 02.19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Question I was one of the first dry power devices available in the US Flovent, Serevent and Advair are all available in this device
 What Device am I Class Side Effects History Potpourri Monitoring Tools 10 10 10 10 10 20 20 20 20 20 30 30 30 30 30 40 40 40 40 40 50 50 50 50 50 WHAT KIND OF DEVICE AM I? I was one of the first dry power
What Device am I Class Side Effects History Potpourri Monitoring Tools 10 10 10 10 10 20 20 20 20 20 30 30 30 30 30 40 40 40 40 40 50 50 50 50 50 WHAT KIND OF DEVICE AM I? I was one of the first dry power
Allergies and Asthma 5/21/2013. Objectives. Allergic Rhinitis (AR): Risk Factor for ASTHMA. Rhinitis and Asthma
 Allergies and Asthma Presented By: Dr. Fadwa Gillanders, Pharm.D Clinical Pharmacy Specialist May 2013 Objectives Understand the relationship between asthma and allergic rhinitis Understand what is going
Allergies and Asthma Presented By: Dr. Fadwa Gillanders, Pharm.D Clinical Pharmacy Specialist May 2013 Objectives Understand the relationship between asthma and allergic rhinitis Understand what is going
The Medical Letter. on Drugs and Therapeutics
 The Medical Letter publications are protected by US and international copyright laws. Forwarding, copying or any other distribution of this material is strictly prohibited. For further information call:
The Medical Letter publications are protected by US and international copyright laws. Forwarding, copying or any other distribution of this material is strictly prohibited. For further information call:
reslizumab (Cinqair )
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Clinical Policy: Mepolizumab (Nucala) Reference Number: ERX.SPA.214 Effective Date:
 Clinical Policy: (Nucala) Reference Number: ERX.SPA.214 Effective Date: 07.01.16 Last Review Date: 02.19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Nucala) Reference Number: ERX.SPA.214 Effective Date: 07.01.16 Last Review Date: 02.19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: Omalizumab (Xolair) Reference Number: ERX.SPA.141 Effective Date:
 Clinical Policy: (Xolair) Reference Number: ERX.SPA.141 Effective Date: 03.01.14 Last Review Date: 02.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Xolair) Reference Number: ERX.SPA.141 Effective Date: 03.01.14 Last Review Date: 02.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Nucala) Reference Number: CP.PHAR.200 Effective Date: 04.01.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at
Clinical Policy: (Nucala) Reference Number: CP.PHAR.200 Effective Date: 04.01.16 Last Review Date: 02.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at
Revised: 08/2013 FULL PRESCRIBING INFORMATION: CONTENTS*
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AEROSPAN TM safely and effectively. See full prescribing information for AEROSPAN TM. AEROSPAN TM
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use AEROSPAN TM safely and effectively. See full prescribing information for AEROSPAN TM. AEROSPAN TM
A Visual Approach to Simplifying Respiratory Drug Regimens
 Adverse Effects of Inhaled Medications A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP June 28, 2017 Drug Category Beta 2 agonists antagonists Adverse Effects
Adverse Effects of Inhaled Medications A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP June 28, 2017 Drug Category Beta 2 agonists antagonists Adverse Effects
INTRODUCTION YING FAN, 1 LIAN MA, 2 JENNIFER PIPPINS, 3 SUSAN LIMB, 3 YUN XU, 1 CHANDRAHAS G. SAHAJWALLA 1
 REVIEW Impact of Study Design on the Evaluation of Inhaled and Intranasal Corticosteroids Effect on Hypothalamic Pituitary Adrenal Axis Function, Part I: General Overview of HPA Axis Study Design YING
REVIEW Impact of Study Design on the Evaluation of Inhaled and Intranasal Corticosteroids Effect on Hypothalamic Pituitary Adrenal Axis Function, Part I: General Overview of HPA Axis Study Design YING
Nucala (mepolizumab injection for subcutaneous use)
 Nucala (mepolizumab injection for subcutaneous use) Policy Number: 5.01.612 Last Review: 01/2018 Origination: 02/2016 Next Review: 02/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Nucala (mepolizumab injection for subcutaneous use) Policy Number: 5.01.612 Last Review: 01/2018 Origination: 02/2016 Next Review: 02/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
AIRDUO RESPICLICK (fluticasone-salmeterol) aerosol DULERA (mometasone furoate and formoterol fumarate dihydrate) aerosol
 DULERA (mometasone furoate and formoterol fumarate dihydrate) aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
DULERA (mometasone furoate and formoterol fumarate dihydrate) aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific
Asthma By Mayo Clinic staff
 MayoClinic.com reprints This single copy is for your personal, noncommercial use only. For permission to reprint multiple copies or to order presentation-ready copies for distribution, use the reprints
MayoClinic.com reprints This single copy is for your personal, noncommercial use only. For permission to reprint multiple copies or to order presentation-ready copies for distribution, use the reprints
Cinqair (reslizumab injection for intravenous use)
 Cinqair (reslizumab injection for intravenous use) Policy Number: 5.02.522 Last Review: 04/2018 Origination: 04/2016 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Cinqair (reslizumab injection for intravenous use) Policy Number: 5.02.522 Last Review: 04/2018 Origination: 04/2016 Next Review: 04/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
A Visual Approach to Simplifying Respiratory Drug Regimens
 A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP October 23, 2017 Learning Objectives Be able to list at least 3 major adverse effects of inhaled medications
A Visual Approach to Simplifying Respiratory Drug Regimens Stephanie Cheng, PharmD, MPH, BCGP October 23, 2017 Learning Objectives Be able to list at least 3 major adverse effects of inhaled medications
Asthma. Definition. Symptoms
 Asthma Definition Asthma is a condition in which your airways narrow and swell and produce extra mucus. This can make breathing difficult and trigger coughing, wheezing and shortness of breath. For some
Asthma Definition Asthma is a condition in which your airways narrow and swell and produce extra mucus. This can make breathing difficult and trigger coughing, wheezing and shortness of breath. For some
First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida
 First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida Prasad Peri, Ph.D., Branch Chief, ONDQA, FDA March 19, 2014 1 Topics for discussion Introduction
First to Market or 505 (b)2 CMC Considerations IPAC-RS/UF Orlando Inhalation Conference Orlando, Florida Prasad Peri, Ph.D., Branch Chief, ONDQA, FDA March 19, 2014 1 Topics for discussion Introduction
SAMPLE. mg by mouth every day for day(s) Prednisolone. Other Medicine: Medicine Dose How long Directions
 Pediatric Asthma Discharge Prescription and Short-Term Plan The doctor will fill out this form before your child is discharged. Please follow this plan until you see your usual doctor in 3 to 7 days. Hospital
Pediatric Asthma Discharge Prescription and Short-Term Plan The doctor will fill out this form before your child is discharged. Please follow this plan until you see your usual doctor in 3 to 7 days. Hospital
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Nucala) Reference Number: CP.PHAR.200 Effective Date: 04.01.16 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at
Clinical Policy: (Nucala) Reference Number: CP.PHAR.200 Effective Date: 04.01.16 Last Review Date: 05.18 Line of Business: Commercial, Medicaid Coding Implications Revision Log See Important Reminder at
Alberta Childhood Asthma Pathway for Primary Care
 Asthma Diagnosis Box 1 Diagnosis: Based on symptom pattern, careful and thorough history of symptoms (wheeze, cough, night waking and activity limitations), and assessment of family history of asthma and
Asthma Diagnosis Box 1 Diagnosis: Based on symptom pattern, careful and thorough history of symptoms (wheeze, cough, night waking and activity limitations), and assessment of family history of asthma and
SYNOPSIS THIS IS A PRINTED COPY OF AN ELECTRONIC DOCUMENT. PLEASE CHECK ITS VALIDITY BEFORE USE.
 Drug product: Drug substance(s): Document No.: Edition No.: 1 Study code: Accolate Zafirlukast (ZD9188) 9188IL/0138 Date: 02 May 2007 SYNOPSIS A Multicenter, Randomized, Double-blind, -controlled, Parallel
Drug product: Drug substance(s): Document No.: Edition No.: 1 Study code: Accolate Zafirlukast (ZD9188) 9188IL/0138 Date: 02 May 2007 SYNOPSIS A Multicenter, Randomized, Double-blind, -controlled, Parallel
Asthma medications: Know your options - MayoClinic.com. Asthma medications: Know your options
 MayoClinic.com reprints This single copy is for your personal, noncommercial use only. For permission to reprint multiple copies or to order presentation-ready copies for distribution, use the reprints
MayoClinic.com reprints This single copy is for your personal, noncommercial use only. For permission to reprint multiple copies or to order presentation-ready copies for distribution, use the reprints
Select Inhaled Respiratory Agents
 Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
Applies to all products administered or underwritten by Blue Cross and Blue Shield of Louisiana and its subsidiary, HMO Louisiana, Inc.(collectively referred to as the Company ), unless otherwise provided
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Fluticasone/Salmeterol (Advair Diskus, Advair HFA) Reference Number: CP.PMN.31 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan See Important Reminder
Clinical Policy: Fluticasone/Salmeterol (Advair Diskus, Advair HFA) Reference Number: CP.PMN.31 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan See Important Reminder
2019 STEP THERAPY CRITERIA UCare Individual & Family Plans UCare Individual & Family Plans with Fairview
 2019 STEP THERAPY CRITERIA UCare Individual & Family Plans UCare Individual & Family Plans with Fairview In some cases, UCare requires you to first try certain drugs to treat your medical condition before
2019 STEP THERAPY CRITERIA UCare Individual & Family Plans UCare Individual & Family Plans with Fairview In some cases, UCare requires you to first try certain drugs to treat your medical condition before
Patient Information ALVESCO [ael- ves-koʊ] (ciclesonide) Inhalation Aerosol. Do not use your ALVESCO Inhalation Aerosol near heat or an open flame.
![Patient Information ALVESCO [ael- ves-koʊ] (ciclesonide) Inhalation Aerosol. Do not use your ALVESCO Inhalation Aerosol near heat or an open flame. Patient Information ALVESCO [ael- ves-koʊ] (ciclesonide) Inhalation Aerosol. Do not use your ALVESCO Inhalation Aerosol near heat or an open flame.](/thumbs/77/74496032.jpg) Note: For Oral Inhalation Only Patient Information ALVESCO [ael- ves-koʊ] (ciclesonide) Inhalation Aerosol Do not use your ALVESCO Inhalation Aerosol near heat or an open flame. Read this Patient Information
Note: For Oral Inhalation Only Patient Information ALVESCO [ael- ves-koʊ] (ciclesonide) Inhalation Aerosol Do not use your ALVESCO Inhalation Aerosol near heat or an open flame. Read this Patient Information
Foundations of Pharmacology
 Pharmacologic Management of Asthma Objectives: 1. Review the physiological basis for asthma therapy 2. Discuss the differences between SABA and LABA 3. Discuss the role of inhaled and oral systemic corticosteroids
Pharmacologic Management of Asthma Objectives: 1. Review the physiological basis for asthma therapy 2. Discuss the differences between SABA and LABA 3. Discuss the role of inhaled and oral systemic corticosteroids
STRIVERDI RESPIMAT (olodaterol hcl) aerosol
 STRIVERDI RESPIMAT (olodaterol hcl) aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
STRIVERDI RESPIMAT (olodaterol hcl) aerosol Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy
Disclosure. Case. Objectives. Case Continued. Inhalers. Asthma: A GINA Update to the NAEPP 2007 Guidelines 1/20/2015
 Disclosure Asthma: A GINA Update to the NAEPP 2007 Guidelines Robert (RC) Hellinga, Pharm.D. PGY 1 Pharmacy Resident Wolfson Children s Hospital/Baptist Health I do not have a vested interest in or affiliation
Disclosure Asthma: A GINA Update to the NAEPP 2007 Guidelines Robert (RC) Hellinga, Pharm.D. PGY 1 Pharmacy Resident Wolfson Children s Hospital/Baptist Health I do not have a vested interest in or affiliation
Medications Affecting The Respiratory System
 Medications Affecting The Respiratory System Overview Asthma is a chronic inflammatory disorder of the airways. It is an intermittent and reversible airflow obstruction that affects the bronchioles. The
Medications Affecting The Respiratory System Overview Asthma is a chronic inflammatory disorder of the airways. It is an intermittent and reversible airflow obstruction that affects the bronchioles. The
Patients with a known hypersensitivity to milk proteins or any ingredients of ASMANEX TWISTHALER. (4.2)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ASMANEX TWISTHALER safely and effectively. See full prescribing information for ASMANEX TWISTHALER.
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ASMANEX TWISTHALER safely and effectively. See full prescribing information for ASMANEX TWISTHALER.
MANAGING ASTHMA. Nancy Davis, RRT, AE-C
 MANAGING ASTHMA Nancy Davis, RRT, AE-C What is asthma? Asthma is a chronic respiratory disease characterized by episodes or attacks of inflammation and narrowing of small airways in response to asthma
MANAGING ASTHMA Nancy Davis, RRT, AE-C What is asthma? Asthma is a chronic respiratory disease characterized by episodes or attacks of inflammation and narrowing of small airways in response to asthma
Clinical Policy: Mepolizumab (Nucala) Reference Number: ERX.SPA.214 Effective Date:
 Clinical Policy: (Nucala) Reference Number: ERX.SPA.214 Effective Date: 07.01.16 Last Review Date: 05.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Nucala) Reference Number: ERX.SPA.214 Effective Date: 07.01.16 Last Review Date: 05.18 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
MEDICATION GUIDE. ADVAIR [ad vair] HFA 45/21 (fluticasone propionate 45 mcg and salmeterol 21 mcg) Inhalation Aerosol
![MEDICATION GUIDE. ADVAIR [ad vair] HFA 45/21 (fluticasone propionate 45 mcg and salmeterol 21 mcg) Inhalation Aerosol MEDICATION GUIDE. ADVAIR [ad vair] HFA 45/21 (fluticasone propionate 45 mcg and salmeterol 21 mcg) Inhalation Aerosol](/thumbs/78/76835983.jpg) MEDICATION GUIDE ADVAIR [ad vair] HFA 45/21 (fluticasone propionate 45 mcg and salmeterol 21 mcg) Inhalation Aerosol ADVAIR HFA 115/21 (fluticasone propionate 115 mcg and salmeterol 21 mcg) Inhalation
MEDICATION GUIDE ADVAIR [ad vair] HFA 45/21 (fluticasone propionate 45 mcg and salmeterol 21 mcg) Inhalation Aerosol ADVAIR HFA 115/21 (fluticasone propionate 115 mcg and salmeterol 21 mcg) Inhalation
What You Need to Know about Metered-Dose Inhalers and the HFA Propellant
 What You Need to Know about Metered- Inhalers and the HFA Propellant There are a number ways to deliver inhaled medication. They include: Metered-dose inhaler () Metered-dose inhaler with spacer/holding
What You Need to Know about Metered- Inhalers and the HFA Propellant There are a number ways to deliver inhaled medication. They include: Metered-dose inhaler () Metered-dose inhaler with spacer/holding
XOLAIR (omalizumab) Prior Authorization
 MP9309 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria below Yes as shown below May only be prescribed by Allergy, Pulmonary, Immunology or Dermatology specialists
MP9309 Covered Service: Prior Authorization Required: Additional Information: Yes when meets criteria below Yes as shown below May only be prescribed by Allergy, Pulmonary, Immunology or Dermatology specialists
Study designs and PD/Clinical endpoints to demonstrate therapeutic equivalence: European Views
 IPAC-RS/University of Florida Study designs and PD/Clinical endpoints to demonstrate therapeutic equivalence: European Views 20 th March 2014 Dr. Alfredo García - Arieta Head of the Service of Generic
IPAC-RS/University of Florida Study designs and PD/Clinical endpoints to demonstrate therapeutic equivalence: European Views 20 th March 2014 Dr. Alfredo García - Arieta Head of the Service of Generic
Study No.: SAM40012 Title: A multicentre, randomised, double-blind, double-dummy, parallel group comparison of three treatments : 1)
 Study No.: SAM40012 Title: A multicentre, randomised, double-blind, double-dummy, parallel group comparison of three treatments : 1) salmeterol/fluticasone propionate () (mcg strength) bd via DISKUS/ACCUHALER
Study No.: SAM40012 Title: A multicentre, randomised, double-blind, double-dummy, parallel group comparison of three treatments : 1) salmeterol/fluticasone propionate () (mcg strength) bd via DISKUS/ACCUHALER
Asthma in Pregnancy. Asthma. Chronic Airway Inflammation. Objective Measures of Airflow. Peak exp. flow rate (PEFR)
 Chronic Airway Inflammation Asthma in Pregnancy Robin Field, MD Maternal Fetal Medicine Kaiser Permanente San Francisco Asthma Chronic airway inflammation increased airway responsiveness to a variety of
Chronic Airway Inflammation Asthma in Pregnancy Robin Field, MD Maternal Fetal Medicine Kaiser Permanente San Francisco Asthma Chronic airway inflammation increased airway responsiveness to a variety of
MEDICAL COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 07/05/18 SECTION: DRUGS LAST REVIEW DATE: LAST CRITERIA REVISION DATE: ARCHIVE DATE:
 CINQAIR (reslizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
CINQAIR (reslizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
Clinical Policy: Dupilumab (Dupixent) Reference Number: ERX.SPA.49 Effective Date:
 Clinical Policy: (Dupixent) Reference Number: ERX.SPA.49 Effective Date: 06.01.17 Last Review Date: 02.19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
Clinical Policy: (Dupixent) Reference Number: ERX.SPA.49 Effective Date: 06.01.17 Last Review Date: 02.19 Revision Log See Important Reminder at the end of this policy for important regulatory and legal
*Sections or subsections omitted from the full prescribing information are not listed.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use QVAR safely and effectively. See full prescribing information for QVAR Inhalation Aerosol., for oral
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use QVAR safely and effectively. See full prescribing information for QVAR Inhalation Aerosol., for oral
Australian Asthma Handbook. Key table and figures Version 1.2
 Australian Asthma Handbook Key table and figures Version 1.2 DIAGNOSIS OF ASTHMA Figure. Steps in the diagnosis of asthma in adults Table. Findings that increase or decrease the probability of asthma in
Australian Asthma Handbook Key table and figures Version 1.2 DIAGNOSIS OF ASTHMA Figure. Steps in the diagnosis of asthma in adults Table. Findings that increase or decrease the probability of asthma in
Nancy Davis, RRT, AE-C
 Nancy Davis, RRT, AE-C Asthma Statistics 25.6 million Americans diagnosed with asthma 6.8 million are children 10.5 million missed school days per year 14.2 lost work days for adults Approximately 10%
Nancy Davis, RRT, AE-C Asthma Statistics 25.6 million Americans diagnosed with asthma 6.8 million are children 10.5 million missed school days per year 14.2 lost work days for adults Approximately 10%
Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence
 Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence Clinical and cost-effectiveness of QVAR for the treatment of chronic asthma in adults and children
Ivax Pharmaceuticals UK Sponsor Submission to the National Institute for Health and Clinical Excellence Clinical and cost-effectiveness of QVAR for the treatment of chronic asthma in adults and children
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Xolair) Reference Number: CP.CPA.298 Effective Date: 11.16.16 Last Review Date: 11.15.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
Clinical Policy: (Xolair) Reference Number: CP.CPA.298 Effective Date: 11.16.16 Last Review Date: 11.15.17 Line of Business: Medicaid Medi-Cal Revision Log See Important Reminder at the end of this policy
ANTINEOPLASTIC DRUGS CHAPTER 21. Antineoplastic drugs - designed to treat malignancies, now also used to treat diseases with inflammatory component
 ANTINEOPLASTIC DRUGS CHAPTER 21 Antineoplastic drugs - designed to treat malignancies, now also used to treat diseases with inflammatory component Tx of malignancies Antineoplastic drugs: methotrexate
ANTINEOPLASTIC DRUGS CHAPTER 21 Antineoplastic drugs - designed to treat malignancies, now also used to treat diseases with inflammatory component Tx of malignancies Antineoplastic drugs: methotrexate
FOR ORAL INHALATION ONLY (2)
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ALVESCO safely and effectively. See full prescribing information for ALVESCO. ALVESCO (ciclesonide)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use ALVESCO safely and effectively. See full prescribing information for ALVESCO. ALVESCO (ciclesonide)
Respiratory Medications and Devices Update 2/15
 Respiratory Medications and Devices Update 2/15 Dewey Hahlbohm, PA-C, AE-C Wendy Brown, Pharm.D., MPAS, PA-C, AE-C Objectives! Review mechanism of action for asthma pharmacologic agents! Describe key patient
Respiratory Medications and Devices Update 2/15 Dewey Hahlbohm, PA-C, AE-C Wendy Brown, Pharm.D., MPAS, PA-C, AE-C Objectives! Review mechanism of action for asthma pharmacologic agents! Describe key patient
Practical Approach to Managing Paediatric Asthma
 Practical Approach to Managing Paediatric Asthma Dr Andrew Tai FRACP, PhD Paediatric Respiratory and Sleep Specialist Women's and Children's Hospital, Adelaide Approaching the patient Check the diagnosis
Practical Approach to Managing Paediatric Asthma Dr Andrew Tai FRACP, PhD Paediatric Respiratory and Sleep Specialist Women's and Children's Hospital, Adelaide Approaching the patient Check the diagnosis
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome/Efficacy Variable:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
Sponsor. Generic drug name. Trial indication(s) Protocol number. Protocol title. Clinical trial phase. Study Start/End Dates.
 Sponsor Novartis Generic drug name Fluticasone propionate Trial indication(s) Moderate-severe bronchial asthma Protocol number CQAE397A2202 Protocol title A randomized open label study to assess the utility
Sponsor Novartis Generic drug name Fluticasone propionate Trial indication(s) Moderate-severe bronchial asthma Protocol number CQAE397A2202 Protocol title A randomized open label study to assess the utility
2014 Step Therapy Criteria (List of Step Therapy Criteria)
 Criteria Last Updated: November 1, 2014 2014 Step Therapy Criteria (List of Step Therapy Criteria) PLEASE READ CAREFULLY: IEHP MEDICARE DUALCHOICE (HMO SNP) REQUIRES YOU TO FIRST TRY CERTAIN DRUGS TO TREAT
Criteria Last Updated: November 1, 2014 2014 Step Therapy Criteria (List of Step Therapy Criteria) PLEASE READ CAREFULLY: IEHP MEDICARE DUALCHOICE (HMO SNP) REQUIRES YOU TO FIRST TRY CERTAIN DRUGS TO TREAT
Ferris State University College of Pharmacy MPA CE Symposium 2016 Paul Thill, PharmD, BCPS
 Ferris State University College of Pharmacy MPA CE Symposium 2016 Paul Thill, PharmD, BCPS Objectives Categorize the new asthma and COPD inhalers in to existing or newly created categories Discuss the
Ferris State University College of Pharmacy MPA CE Symposium 2016 Paul Thill, PharmD, BCPS Objectives Categorize the new asthma and COPD inhalers in to existing or newly created categories Discuss the
The Medical Letter. on Drugs and Therapeutics. Drug Some Formulations OTC/Rx Usual Dosage Comments Class Comments Cost 1
 The Medical Letter publications are protected by US and international copyright laws. Forwarding, copying or any other distribution of this material is strictly prohibited. For further information call:
The Medical Letter publications are protected by US and international copyright laws. Forwarding, copying or any other distribution of this material is strictly prohibited. For further information call:
Study No.: Title: Rationale: Phase: Study Period: Study Design: Centres: Indication: Treatment: Objectives: Primary Outcome:
 The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product.
International co-ordinating investigator Dr Dencho Osmanliev, St Sofia, Pulmonary Dept, 19 D Nestorov Str, Sofia, 1431, Bulgaria.
 Drug product: SYNOPSIS Drug substance(s): Budesonide/formoterol Document No.: SD-039-CR-0681 Referring to part Edition No.: 1 of the dossier Study code: SD-039-0681 Date: 24 Oktober, 2003 (For national
Drug product: SYNOPSIS Drug substance(s): Budesonide/formoterol Document No.: SD-039-CR-0681 Referring to part Edition No.: 1 of the dossier Study code: SD-039-0681 Date: 24 Oktober, 2003 (For national
*Sections or subsections omitted from the full prescribing information are not listed.
 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use QVAR REDIHALER safely and effectively. See full prescribing information for QVAR, for oral inhalation
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use QVAR REDIHALER safely and effectively. See full prescribing information for QVAR, for oral inhalation
Report on New Patented Drugs - Alvesco
 Report on New Patented Drugs - Alvesco Under its transparency initiative, the PMPRB publishes the results of the reviews of new patented drugs by Board Staff, for purposes of applying the Board s Excessive
Report on New Patented Drugs - Alvesco Under its transparency initiative, the PMPRB publishes the results of the reviews of new patented drugs by Board Staff, for purposes of applying the Board s Excessive
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma
 Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
Diagnosis, Assessment, Monitoring and Pharmacological Treatment of Asthma Magnitude of Asthma - India Delhi Childhood asthma: 10.9% Adults: 8% Other Cities 3 to 18% Chhabra SK et al Ann Allergy Asthma
Stepping down asthma treatment guidelines
 Stepping down asthma treatment guidelines The potential for inhaled corticosteroids (ICS) to cause dose-related side-effects has led to asthma management guidelines recommending a dose reduction once asthma
Stepping down asthma treatment guidelines The potential for inhaled corticosteroids (ICS) to cause dose-related side-effects has led to asthma management guidelines recommending a dose reduction once asthma
PRODUCT INFORMATION INTAL CFC-FREE INHALER AND INTAL FORTE CFC-FREE INHALER
 PRODUCT INFORMATION INTAL CFC-FREE INHALER AND INTAL FORTE CFC-FREE INHALER NAME OF MEDICINE Sodium Cromoglycate for Oral Inhalation. C 23 H 14 Na 2 O 11 Mol Wt. 512.3 CAS No. [15826-37-6] Sodium cromoglycate
PRODUCT INFORMATION INTAL CFC-FREE INHALER AND INTAL FORTE CFC-FREE INHALER NAME OF MEDICINE Sodium Cromoglycate for Oral Inhalation. C 23 H 14 Na 2 O 11 Mol Wt. 512.3 CAS No. [15826-37-6] Sodium cromoglycate
Updates in the Management of Asthma and COPD. Case 1
 Updates in the Management of Asthma and COPD Soraya Azari, MD Associate Clinical Professor of Medicine Case 1 40yo F with a history of asthma, allergic rhinitis, nicotine dependence, and obesity comes
Updates in the Management of Asthma and COPD Soraya Azari, MD Associate Clinical Professor of Medicine Case 1 40yo F with a history of asthma, allergic rhinitis, nicotine dependence, and obesity comes
PRODUCT MONOGRAPH ALVESCO
 PRODUCT MONOGRAPH ALVESCO ciclesonide inhalation aerosol 100 mcg and 200 mcg/ actuation (ex-valve) Corticosteroid for oral inhalation AstraZeneca Canada Inc. 1004 Middlegate Road Mississauga, Ontario L4Y
PRODUCT MONOGRAPH ALVESCO ciclesonide inhalation aerosol 100 mcg and 200 mcg/ actuation (ex-valve) Corticosteroid for oral inhalation AstraZeneca Canada Inc. 1004 Middlegate Road Mississauga, Ontario L4Y
NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS
 NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS Douglas S. Gardenhire, Ed.D, RRT-NPS MODULE 1 Manipulate Small Volume Nebulizers by Order or Protocol 1 Objectives for Module 1 At the end of
NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS Douglas S. Gardenhire, Ed.D, RRT-NPS MODULE 1 Manipulate Small Volume Nebulizers by Order or Protocol 1 Objectives for Module 1 At the end of
II. UF CLASS REVIEWS NASAL ALLERGY DRUGS
 DEPARTMENT OF DEFENSE PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. UNIFORM FORMULARY REVIEW PROCESS Under 10 United States Code
DEPARTMENT OF DEFENSE PHARMACY AND THERAPEUTICS COMMITTEE RECOMMENDATIONS INFORMATION FOR THE UNIFORM FORMULARY BENEFICIARY ADVISORY PANEL I. UNIFORM FORMULARY REVIEW PROCESS Under 10 United States Code
Annex II. Scientific conclusions
 Annex II Scientific conclusions 5 Scientific conclusions Beclometasone dipropionate (BDP) is a glucocorticoid and a prodrug of the active metabolite, beclometasone-17-monopropionate. Beclometasone dipropionate
Annex II Scientific conclusions 5 Scientific conclusions Beclometasone dipropionate (BDP) is a glucocorticoid and a prodrug of the active metabolite, beclometasone-17-monopropionate. Beclometasone dipropionate
Q: Should patients with mild asthma
 1-MINUTE CONSULT CME CREDIT EDUCATIONAL OBJECTIVE: Readers will consider prescribing inhaled corticosteroids to their patients who have mild persistent asthma brief answers to specific clinical questions
1-MINUTE CONSULT CME CREDIT EDUCATIONAL OBJECTIVE: Readers will consider prescribing inhaled corticosteroids to their patients who have mild persistent asthma brief answers to specific clinical questions
Pediatric Asthma: Pharmacotherapy. Joseph Spahn, MD Children s Hospital Colorado & University of Colorado Medical School Aurora, Colorado
 Pediatric Asthma: Pharmacotherapy Joseph Spahn, MD Children s Hospital Colorado & University of Colorado Medical School Aurora, Colorado Pediatric Asthma: Pharmacotherapy Disclosures/Conflicts of Interest:
Pediatric Asthma: Pharmacotherapy Joseph Spahn, MD Children s Hospital Colorado & University of Colorado Medical School Aurora, Colorado Pediatric Asthma: Pharmacotherapy Disclosures/Conflicts of Interest:
SYNOPSIS. Study center(s) This study was conducted in the United States (128 centers).
 Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
Drug product: Drug substance(s): Document No.: Edition No.: Study code: Date: SYMBICORT pmdi 160/4.5 µg Budesonide/formoterol SD-039-0725 17 February 2005 SYNOPSIS A Twelve-Week, Randomized, Double-blind,
MDI Bonanza. Dwayne Griffin, DO
 MDI Bonanza Dwayne Griffin, DO Bonanza 3. A MDI costing $200 - $500 per month SISYPHUS MDI Griffin Mountain Evolution of Deliver Systems for COPD in the US 2003 2009 2011 2013 2004 2012 2014 Prescribing
MDI Bonanza Dwayne Griffin, DO Bonanza 3. A MDI costing $200 - $500 per month SISYPHUS MDI Griffin Mountain Evolution of Deliver Systems for COPD in the US 2003 2009 2011 2013 2004 2012 2014 Prescribing
Script Notes. Treatment of Mild to Moderate Asthma. In This Issue: Script Notes Has a New Look!
 Script Notes Third Quarter 2006 The Pharmacy and Therapeutics Newsletter for Keystone Mercy Health Plan Participating Providers In This Issue: Treatment of Mild to Moderate Asthma........... 1 Formulary
Script Notes Third Quarter 2006 The Pharmacy and Therapeutics Newsletter for Keystone Mercy Health Plan Participating Providers In This Issue: Treatment of Mild to Moderate Asthma........... 1 Formulary
FASENRA (benralizumab)
 FASENRA (benralizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
FASENRA (benralizumab) Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs
10/18/2012. Penn State University Children s Hospital JODIE STABINSKI CRNP MSN AE-C
 Penn State University Children s Hospital JODIE STABINSKI CRNP MSN AE-C Daily: Long-Term Control Corticosteroids (inhaled and systemic) Long-acting beta 2 -agonists (Serevent, Foradil) Methylxanthines
Penn State University Children s Hospital JODIE STABINSKI CRNP MSN AE-C Daily: Long-Term Control Corticosteroids (inhaled and systemic) Long-acting beta 2 -agonists (Serevent, Foradil) Methylxanthines
Reference Guide for Caring for Pediatric Patients with Asthma
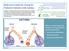 Reference Guide for Caring for Pediatric Patients with Asthma Co-Chair: Nancy Cantey Banasiak, DNP, PPCNP-BC, APRN Co-Chair: Deborah Hickman, DNP, APRN-CNP, CPNP-PC, NNP-BC Asthma and Allergy SIG Members
Reference Guide for Caring for Pediatric Patients with Asthma Co-Chair: Nancy Cantey Banasiak, DNP, PPCNP-BC, APRN Co-Chair: Deborah Hickman, DNP, APRN-CNP, CPNP-PC, NNP-BC Asthma and Allergy SIG Members
