A Novel, Versatile Valved Holding Chamber for Delivering Inhaled Medications to Neonates and Small Children: Laboratory Simulation of Delivery Options
|
|
|
- Estella Anabel Elliott
- 5 years ago
- Views:
Transcription
1 A Novel, Versatile Valved Holding Chamber for Delivering Inhaled Medications to Neonates and Small Children: Laboratory Simulation of Delivery Options Robert M DiBlasi RRT-NPS, Dominic P Coppolo RRT MBA FAARC, Mark W Nagel, Cathy C Doyle, Valentina I Avvakoumova, Rubina S Ali, and Jolyon P Mitchell PhD CChem CSci BACKGROUND: Delivery of bronchodilator to infants and small children from a pressurized metered-dose inhaler with valved holding chamber (pmdi-vhc) is limited by airway narrowness, short respiratory cycle time, and small tidal volume (V T ). There is a need for a versatile, efficient VHC, given the variety of treatment modalities. METHODS: We tested the AeroChamber Mini VHC (the internal geometry of which is optimized for aerosol delivery, and which accepts a pmdi canister that has a dose counter) in experiments to determine differences in the delivery of hydrofluoroalkane-propelled albuterol (90 g/actuation) during: mechanical ventilation via endotracheal tube (ETT); manual resuscitation via ETT; and spontaneous breathing via face mask. We tested 5 units of the AeroChamber Mini VHC per test. We simulated the tidal breathing of a premature neonate (V T 6 ml), a term neonate (V T 20 ml), and a child approximately 2 years old (V T 60 ml). We collected the aerosol on an electret filter and quantitatively assayed for albuterol. RESULTS: The total emitted mass of albuterol per actuation that exited the VHC was marginally greater during spontaneous breathing ( g) than during manual resuscitation ( g) (P.046). Albuterol delivery via mechanical ventilation, though comparable with the premature-neonate model ( g), the term-neonate model ( g), and the 2-y-old-child model ( g) (P.63), was significantly lower than in the spontaneousbreathing and manual-resuscitation models (P <.001). In the neonatal models the total emitted mass was similar with the spontaneous-breathing model ( g with the premature-neonate model, g with the term-neonate model) and the manual-resuscitation model ( g premature-neonate model, g term-neonate model) (P >.46 via one-way analysis of variance). CONCLUSION: The reduced delivery of albuterol during mechanical ventilation (compared to during spontaneous breathing and manual resuscitation via ETT) was probably associated with the saturated atmosphere in the breathing circuit (37 C, relative humidity > 99%), compared to the ambient air (22 1 C, 44 7% relative humidity). The AeroChamber Mini VHC may provide a versatile alternative to VHCs that are designed exclusively for one aerosol treatment modality. Key words: neonate; small child; inhaler; aerosol; mechanical ventilation; manual resuscitation; spontaneous breathing; simulation. [Respir Care 2010;55(4): Daedalus Enterprises] Robert M DiBlasi RRT-NPS is affiliated with the Center for Developmental Therapeutics, Seattle Children s Research Institute, and with the Department of Respiratory Care, Seattle Children s Hospital, Seattle, Washington. Dominic P Coppolo RRT MBA FAARC is affiliated with Monaghan Medical, Syracuse, New York. Mark W Nagel, Cathy C Doyle, Valentina I Avvakoumova, Rubina S Ali, and Jolyon P Mitchell PhD CChem CSci are affiliated with Trudell Medical International, London, Ontario, Canada. Mr DiBlasi has disclosed a relationship with Trudell Medical. Correspondence: Jolyon P Mitchell PhD CChem CSci, Trudell Medical International, 725 Third Street, London, Ontario, N5V 5G4, Canada. jmitchell@trudellmed.com. RESPIRATORY CARE APRIL 2010 VOL 55 NO 4 419
2 Introduction Inhaled bronchodilator therapy in pre-term and term infants may improve lung function among those who are developing chronic lung disease, at least in the short-term, despite the speculation that airway smooth muscle may not be clinically responsive in the acute treatment of pre-term infants. 1 The case for inhaled bronchodilator therapy is clearer in children of toddler age, as 2 agonists are routinely recommended for treatment of acute bronchoconstriction in this age group, 2 and widely administered via nebulizer or pressurized metered-dose inhaler (pmdi) plus valved holding chamber (VHC). 3 However, infants in particular have both small tidal volume (V T ) and low inspiratory/expiratory ratio, compared to adults, 4 resulting in short residence times for drug particle delivery to the airways. These factors combine to make it difficult to achieve optimum clinical benefit in terms of improving airway patency 5 via the 3 alternative aerosol-delivery modalities available in the acute setting: via endotracheal tube (ETT) during mechanical ventilation; via ETT during manual resuscitation; and via tight-fitting face mask during spontaneous breathing. In particular, the challenges of delivering aerosols via mechanical ventilation to neonates are substantial, and there is evidence that the precise configuration of the ventilator circuit, the ETT size, and the gas flow rate and pattern can each influence the outcome. 6 The situation is complicated further by the variety of aerosoldelivery devices available for mechanical ventilation, 5 manual resuscitation 7 and spontaneous breathing. 8 Given these circumstances, the desirability of an efficient small-volume VHC that accepts commonly prescribed pmdi bronchodilators and that is not bulky, so that it can be used in all of the aforementioned clinical situations, becomes selfevident. The objective of this in vitro investigation was to determine the differences in the delivery of inhaled 2 -agonist via a novel VHC between mechanical ventilation, manual resuscitation, and face-mask aerosol delivery. We simulated neonates and infants with normal lung mechanics. We evaluated a novel small-volume (110 ml) VHC (AeroChamber Mini, Monaghan Medical, Plattsburgh, New York) (Fig. 1), which is designed to be adaptable without modification for mechanical ventilation, manual resuscitation, and face-mask aerosol delivery with each patient category. The AeroChamber Mini VHC has a sensitive inhalation flap valve that opens rapidly at the onset of inhalation, even with neonates with low inspiratory flow/ time profiles. This device also has a valveless (offering near-zero resistance) exhalation channel separated from and below the inhalation pathway, which minimizes mixing with residual inhaled aerosol remaining in the upper part of the chamber. The AeroChamber Mini VHC accepts Fig. 1. AeroChamber Mini valved holding chamber. The receptacle for the pressurized metered-dose inhaler (pmdi) canister is above the inhalation valve, and there is a separate exhalation channel at the base of the device. the valve stem of most pmdi canisters, including the Glaxo SmithKline pmdi canister that has a dose counter, which we used in these tests. We hypothesized that there would be no differences in aerosol delivery between the various test scenarios. Methods Trudell Medical International funded the study and it was conducted at their aerosol laboratory. The first author (RMD) designed the study and the experimental setup, was present and assisted in all of the data collection, and was actively involved in the statistical analysis, data review, generation of figures and tables, and writing of the paper. Employees of Trudell Medical International interacted with the first author in the study design, data collection, data analysis, and writing of the paper. We evaluated 5 AeroChamber Mini VHCs per test. We tested the VHCs immediately out-of-package, with no prewashing. We used hydrofluoroalkane-propelled pmdi canisters of albuterol (Ventolin-HFA, GlaxoSmithKline, Research Triangle Park, North Carolina) that deliver 90 g 15% albuterol base equivalent from the actuator nozzle per actuation. We simulated aerosol delivery via ETT during mechanical ventilation (Fig. 2), via ETT during manual resuscitation (Fig. 3), and via face mask during spontaneous breathing (Fig. 4). In each scenario we used a pre-primed pmdi-vhc setup and delivered 4 actuations, at 30-s intervals. We shook the canister between actuations to ensure reproducibility of the actuations. We allocated a separate pmdi for each of the 3 aerosol-delivery routes. Each batch of tests was undertaken without disassembling the setup, and with the breath simulator operating continuously. 420 RESPIRATORY CARE APRIL 2010 VOL 55 NO 4
3 A NOVEL, VERSATILE VALVED HOLDING CHAMBER Fig. 2. Simulated aerosol delivery with the AeroChamber Mini valved holding chamber (VHC) during mechanical ventilation. A: Schematic. B: Setup with aerosol-collection filters at the distal end of the endotracheal tube (ETT). pmdi pressurized metered-dose inhaler. We collected the aerosol from the VHC with a bacterial/ viral electret filter (Respirgard-II, Vital Signs, Englewood, Colorado) after the fourth actuation. Preliminary methodvalidation studies confirmed that the deposited albuterol from 2 5 actuations was measureable (n 5 replicate measurements), using methanol (95% volume/volume) as solvent, and the range of recovery was ⱖ 98%. We quantified the albuterol with high-performance liquid chromatography (Star, Varian Canada, Mississauga, Ontario, Canada), under room ambient conditions. The mobile-phase proportions were 70/30 volume/volume methanol/5 mm sodium dodecyl-sulphate buffer (ph 2.5). The isocratic ternary pump (model 230) delivered mobile phase at a flow of 1.6 ml/min. We injected a 50- L aliquot from each albuterol sample onto a mm Allsphere octadecylsilane-2 5- m column (Grace Davison Discovery Sciences, Deerfield, Illinois) via the autosampler (ProStar, Varian Canada, Mississauga, Ontario, Canada). The ultraviolet-visible detector (model 325) was operated at a wavelength of 276 nm. Validation studies with standard solutions of analyte had correlation coefficients (r2 values) ⱖ The range of assay inter-day coefficients of variation with albuterol quality-control solutions was 0.5 RESPIRATORY CARE APRIL 2010 VOL 55 NO 4 Fig. 3. Simulated aerosol delivery with the AeroChamber Mini valved holding chamber (VHC) during manual resuscitation. A: Schematic. The servo ventilator drives the resuscitation bag in an airtight plethysmograph-type chamber to provide a consistent, reproducible breathing pattern via the VHC and endotracheal tube (ETT). B: Setup with airtight chamber for manual resuscitation bag. pmdi pressurized metered-dose inhaler. 1.3%. We converted absorbance units to micrograms of albuterol with the chromatography workstation s software (version 6.41). The simulations of aerosol-delivery via ETT during mechanical ventilation were all undertaken with a ventilator (Inspiration 1.5, event Medical, Galway, Ireland), with the target VT and frequency set shown in Table 1, and with positive end-expiratory pressure (PEEP) of 5 cm H2O. We used a 10-mm, flexible tube, universal, dual-heated, infant breathing circuit (model , Intersurgical, Syracuse, New York) in the premature-neonate model and the termneonate model. We used a 15-mm, flexible tube, universal, dual-heated, child circuit (model , Intersurgical, Syracuse, New York) in the 2-year-old-child model. In the premature-neonate model we used a 2.0-mm diameter inline suction catheter (Ballard Trach Care, Kimberley-Clark, Roswell, Georgia). In the term-neonate model and 2-yearold-child model we used a 2.6-mm diameter in-line suction catheter (Ballard Trach Care, Kimberley-Clark, Roswell, Georgia). In all the tests the breathing circuit was humidified (model MR290, Fisher & Paykel, Auckland, New Zealand) to achieve a humidity of 37 mg/l (saturated at 38 C), as in clinical use. Figure 2A schematically illustrates the setup for the mechanical-ventilation model, show- 421
4 A NOVEL, VERSATILE VALVED HOLDING CHAMBER Fig. 4. Simulated spontaneous breathing with the (A) infant and (B) small child face models, with the small face mask and medium face mask, respectively. ing the circuit humidification arrangement. Figure 2B shows the interface between the VHC-ETT and the test lung (ASL 5000, IngMar Medical, Pittsburgh, Pennsylvania), configured for appropriate age-specific breathing pattern and lung mechanics variables (see Table 1).4,9-11 We chose ETTs of inner diameter 2.5 mm, 3.5 mm, and 4.0 mm in the premature-neonate model, term-neonate model, and 2-yearold-child model, respectively. Aerosol available for inhalation was determined via filter collection, with the filter placed at the distal end of the ETT, adjacent to the test lung. Two additional similar filters were placed in series, to protect the airway to the test lung. The manual-resuscitation simulations (see Fig. 3A) were all undertaken with a Servo ventilator (SV900C, Siemens, Sweden), set to mimic manual resuscitation of either an infant (premature-neonate model and term-neonate model) via a 250-mL resuscitation bag (VBM, Sulz-am-Neckar, Germany) or child (2-year-old-child model) via a 500-mL capacity resuscitation bag (Mercury Medical, Clearwater, 422 Florida). Reproducible external pressure changes could be applied by the ventilator to the bag while the bag was in a plethysmograph-type chamber (see Fig. 3B). The exit from the resuscitation bag was connected directly to the proximal end of the VHC, near where the pmdi canister is inserted, with the distal end of the ETT attached to the test lung via the same type of aerosol-collection filter as in the mechanical-ventilation scenarios. The target pressures and frequency applied to the plethysmograph-type chamber to compress the manual-resuscitation bag were configured so that the VT, frequency, and inspiratory time delivered to the test lung conformed to the values in Table 1. All measurements were undertaken at ambient temperature and relative humidity (22 1 C and 44 7% relative humidity). The simulations of aerosol delivery via face mask during spontaneous breathing were undertaken with infant and small-child face models (ADAM-II, Trudell Medical International, London, Ontario, Canada) that have soft, responsive surfaces where they contact the face mask.12,13 The face mask was manually held against the face model, with sufficient force to ensure a seal between the mask and the face model; this was verified before conducting the breath simulations, by comparing the flow measurements between the model and the test lung to those made via thermal mass flow meter (model 4000, TSI, St Paul, Minnesota) located at the entry to the VHC, with a vacuum applied to the distal end of the model. The VHC was attached to the face mask (see Fig 4). The aerosol available for inhalation was captured on an electret filter square (Filtrete media type G, 3M, St Paul, Minnesota) located immediately behind the half-closed lips of the face model. The target airway resistance and compliance and bag setup were configured so that the VT and frequency delivered conformed to the values in Table 1. All the measurements were made at ambient conditions (22 1 C, 44 7% relative humidity). Statistical interpretation of the total emitted mass per actuation was via one-way analysis of variance (SigmaStat version 3.01, Aspire Software, Ashburn, Virginia). Differences were deemed significant when P.05. The uncertainty limits in Table 2 each represent 1 standard deviation about the mean value. Results In the preliminary validation studies, the mass of albuterol per actuation emitted from the pmdi without the VHC (benchmark data) was g (mean SD for 5 inhalers, one measurement per inhaler). The measurements of total emitted mass that exited the VHC with patient interface (ETT or mask) are summarized as absolute mass per actuation, and were also normalized to the RESPIRATORY CARE APRIL 2010 VOL 55 NO 4
5 Table 1. Breathing Model Variables Simulation Scenario Airway Resistance (cm H 2 O/L/s) Compliance (ml/cm H 2 O) Tidal Volume (ml) Respiratory Rate (breaths/min) Inhalation Time (s) Inspiratory/ Expiratory Ratio Premature neonate :3 Term neonate :3 2-y-old child :2 Table 2. Total Emitted Mass of Albuterol Simulation Scenario Premature Neonate Term Neonate 2-Year-Old Child Observed* % of Label Claim Observed* % of Label Claim Observed* % of Label Claim Mechanical ventilation Manual resuscitation Spontaneous breathing * Mean SD g/actuation. Mean SD percent of the manufacturer s label claim (90 g) albuterol base equivalent that exited the actuator mouthpiece of the pressurized metered-dose-inhaler canister. label-claim 90 g albuterol per actuation in each simulation (see Table 2). Albuterol delivery through the ETT during mechanical ventilation, though comparable in the premature-neonate model ( g), the term-neonate model ( g), and the 2-year-old-child model ( g) (P.63), was significantly lower than in the spontaneous-breathing and manual-resuscitation models (P.001). In the 2-year-old-child model the total emitted mass was marginally less in the manual resuscitation model ( g) than in the spontaneous-breathing model ( g) (P.046). In the neonatal models the total emitted mass was similar in the manual resuscitation scenario ( g in the premature-neonate model, g in the term-neonate model) and the spontaneous-breathing scenario ( g in the premature-neonate model, g in the term-neonate model) (P.46 via one-way analysis of variance). Discussion To our knowledge, our study is only the second systematic laboratory investigation of aerosol delivery via pmdi- VHC to neonates and infants via these 3 aerosol-delivery modalities. A few years ago, Cole et al 14 were the first to examine the problem in this way, but they used a previous generation of VHC prototypes that are not commercially available. Cole et al conducted the manual-resuscitation simulation by manually operating the resuscitation bag, based on previous clinical experience of one of the authors in a neonatal intensive care unit. We automated the operation of the resuscitation bag to make replication of these measurements easier and to reduce the breath-to-breath V T variability and inspiratory/expiratory-ratio variability of manual resuscitation. Our hypothesis that there would be no differences in medication delivery within each simulated patient group with these treatment modalities was disproven, and the total emitted mass measurements (see Table 2) were small in relation to the label-claim emitted dose (90 g). It should be noted that these small measures of total emitted mass should be interpreted in relation to the limited clinical data that exist for pmdi albuterol delivery to neonates and small children, rather than to older children or adults. On this basis, even the lowest values normalized to the labelclaim values during the mechanical ventilation simulations (range % of label-claim value per actuation [see Table 2]) compared well with similar data from Tal et al 15 with 15 spontaneously breathing small children (mean age 21 months, age range 3 60 months) who had airway obstruction from asthma, cystic fibrosis, or bronchopulmonary dysplasia, treated via a 145-mL holding chamber and face mask. Tal et al determined the mean chlorofluorocarbon-propelled pmdi albuterol deposition to be 4.36% of the label claim (1.97% in the lungs, 1.28% in the oropharynx, and 1.11% in the stomach). We used their value of 4.36% of label claim for total patient-delivered albuterol as a benchmark, because, to the best of our knowledge, their study is the only one that has used radiolabeling to assess pmdi albuterol delivery in that very young age range. Furthermore, treatment of spontaneously breathing patients via VHC and face mask probably represented the RESPIRATORY CARE APRIL 2010 VOL 55 NO 4 423
6 optimum modality, given the likelihood of decreased lung delivery via the narrow ETTs used with infants, compared to a tight-fitting and therefore probably leak-free face mask. The comparability we found between the values in our manual resuscitation models and spontaneous-breathing models is therefore somewhat surprising and probably indicates that the aerosol loss in the ETT is similar to the aerosol loss in the face mask. Taking the value of 4.36% of label claim from Tal et al 15 as a point of reference to the previous work, our values for the manual resuscitation and spontaneousbreathing scenarios (see Table 2) ranged from just under 140% (in the premature-neonate during manual resuscitation model) to about 300% (in the 2-year-old-child during spontaneous-breathing model). However, these apparent increases in drug delivery in our in vitro investigation need to be related to the fact that by not modeling either the upper airway or the effects of disease, we have almost certainly overestimated the mass of medication that would reach the lower respiratory tract in the patients we simulated. Our focus was to establish the mass of albuterol available for inhalation at the distal end of the ETT or face mask, as guidance for clinicians faced with providing treatment to infants and small children. We might have anticipated greater medication delivery if the inspiratory/expiratory ratios were lower than the values used in our simulations (see Table 1), by virtue of the greater amount of time spent inhaling the aerosol. Likewise, increasing the V T would probably be associated with improved medication delivery, as seen when comparing equivalent measures of total emitted mass between the premature-neonate model (V T 6 ml) and the term-neonate model (V T 20 ml) in the manual resuscitation and spontaneous breathing simulations (see Table 2). However, considering the same simulations, when V T was increased to 60 ml and the inspiratory time per respiratory cycle was increased (inspiratory/expiratory ratio 1:2) in the 2-yearold-child model, the anticipated improvement in total emitted mass was not apparent. We conjecture that increased inertial deposition of particles at the higher instantaneous flow velocities in the 2-year-old-child model might have offset V T /inspiratory-time-related effects on aerosol transport via the patient interfaces that we studied. However, further work is needed to investigate this behavior in a more systematic way before definitive conclusions can be made. During the mechanical ventilation and manual resuscitation scenarios, the additive imposed expiratory resistance caused by the PEEP valve (during manual ventilation) and the ventilator s exhalation valve may have applied some back-pressure when exhaling down to PEEP. However, these effects are not deemed likely to have impacted drug delivery, given that an air dam exists at the entry to the VHC, by virtue of the VHC inhalation valve closure dur- Table 3. Estimated Mean Mass of Albuterol Available for Inhalation, Divided by Body Weight* Premature Neonate Simulation Scenario Term Neonate 2-Year-Old Child Mechanical ventilation Manual resuscitation Spontaneous breathing * All values are g/kg. ing exhalation, thereby preventing mixing of the aerosol with exhaled air. It is interesting to interpret our data in the context of assessing the emitted mass of albuterol normalized by nominal patient weight (assuming 1 kg, 4 kg, and 12 kg in the premature neonate model, term-neonate model, and 2-yearold-child model, respectively) As a benchmark, an average value of 1.3 g/kg has been reported 13 for a spontaneously breathing 70-kg adult, based on the label-claim delivery of Ventolin-HFA without a VHC or face mask. 16 In the present study, the total emitted mass/kg in the spontaneous-breathing models ranged from 1.0 g/kg (in the 2-year-old-child model) to 6.0 g/kg (in the prematureneonate model) (Table 3), and those values straddle the benchmark value. However, closer agreement is evident when the results from the present investigation are compared to the equivalent measurements reported in the current United States prescribing instructions for Ventolin- HFA for infant and small child use (range g/ kg). 16 Those estimates also originated from in vitro measurements with Ventolin-HFA and the same infant and small-child face models we used, 13 albeit with sampling at constant flows of 4.9 L/min and 12.0 L/min, respectively, rather than mimicking tidal breathing as in the present study, and with slightly larger (149-mL) anti-static and non-conducting VHC and mask devices. Given that the AeroChamber Mini VHC is manufactured from an electrostatic-charge-dissipative polymer, it is germane to note that precautions were taken in the earlier study to eliminate electrostatic charge, via pre-treatment per manufacturer instructions (with the non-conducting VHC) and the use of charge-dissipative-materials VHCs. The greatest differences we observed between treatment modalities with the AeroChamber Mini VHC occurred in the mechanical-ventilation scenario, whereas aerosol delivery in the manual-resuscitation scenario and the spontaneous-breathing scenario was substantially equivalent (see Table 2). Apart from the fact that there were differences between the configurations in filter location with respect to the aerosol-generation source, which probably reduced aerosol delivery, particularly with the mechanical- 424 RESPIRATORY CARE APRIL 2010 VOL 55 NO 4
7 ventilation scenario (see Figs. 2A, 3A, and 4A), the significantly reduced aerosol delivery in these simulations was probably associated with the saturation of the circuit gas (37 C, 99% relative humidity). The circuit gas was much more humid than in the manual-resuscitation scenario or the spontaneous-breathing scenario, which were all at room ambient conditions (22 1 C, 44 7% relative humidity). It is well known that aerosol delivery is decreased by saturated or near-saturated conditions in adult mechanical ventilation circuits, 17,18 and medication delivery can be reduced by as much as 40%, compared to that with a non-humidified circuit at room ambient conditions. 19,20 However, although circuit humidity reduces drug delivery, bypassing the humidifier is not recommended for routine inhalation therapy in ventilator-supported patients. 21 It also follows that in mechanically ventilated patients lower-respiratory-tract aerosol deposition is generally lower with nebulizer than with pmdi, 20 which favors pmdi with VHC. Limitations First, our study was intended primarily to establish the mass of medication emitted from the distal end of the ETT or mask and thus available to be inhaled. Second, as in all in vitro models, we could not simulate the outcome of patient exhalation in any of the treatment scenarios, which would result in a small loss of particles that did not deposit in the airways during inspiration. 3,21 Third, we could not account for patient-related factors such as individual airway geometry, or type, location, or severity of pulmonary disease. 22 Fourth, we were not able to evaluate our data in relation to the clinical study with hydrofluoroalkane-propelled albuterol, of the type presented by Tal et al, 15 because such data do not yet exist. Fifth, we measured total emitted mass rather than fine-particle mass, which could be deemed more appropriate for assessing lung deposition. 23 Measuring the aerosol particle size distribution would have required use of a cascade impactor with the breathing simulator, which would have added substantially to the complexity of the measurement methodology 24 and therefore to the variability of each data set. 25 In defense of our choice to report just total emitted mass, the use of a VHC (with either ETT or face mask) would be expected to eliminate most, if not all, particles 5 m aerodynamic diameter, which deposit in the oropharynx rather than the lungs. 26 Finally, although in our simulations of spontaneous breathing we applied the mask to the model face with just sufficient force to achieve the necessary seal, 27 we were unable to ensure that the force exerted was constant. Instead, we relied on visual observation that the face mask was mildly compressed on the face, as would be the case in clinical practice. The bench-top studies by Shah et al suggest that it is possible that slightly increased total emitted mass might be possible with greater force applied to the mask, because the greater compression decreases the functional dead space between the mask and the face. 28 Conclusions Our in vitro study demonstrates that delivery of pmdi albuterol via VHC to pre-term and term neonates and small children with obstructive lung disease is possible, via ETT during mechanical ventilation or manual resuscitation, or via mask during spontaneous breathing, which are the most likely scenarios. On a weight-adjusted basis, our measurements of total emitted mass per actuation were comparable to previous in vitro measurements for pmdi albuterol to spontaneously breathing younger pediatric patients, and similar to or somewhat greater than the mean value for total patient-delivered albuterol reported in the benchmark clinical study, which involved chlorofluorocarbon-propelled albuterol to very young pediatric patients, 14 most likely because our study could not model either the exhalation portion of each breathing cycle or replicate patientspecific factors such as disease severity and location. As expected, aerosol delivery during mechanical ventilation via ETT was less than during manual resuscitation via ETT or spontaneous breathing, probably because the circuit gas was saturated in the mechanical-ventilation scenario. We conclude that the AeroChamber Mini VHC provides a versatile alternative to existing VHCs. As well as the need to evaluate its performance in the clinical setting, further laboratory investigations are warranted with other pmdi-based formulations that might be delivered to the classes of patients modeled. REFERENCES 1. Hintz S. Pharmacology review: bronchodilator therapy in the preterm infant. Neo Reviews 2003;4(9):e245-e American Association for Respiratory Care. AARC Clinical Practice Guideline. Selection of an aerosol delivery device for neonatal and pediatric patients. Respir Care 1995;40(12): Ferguson C, Gidwani S. Delivery of bronchodilators in acute asthma in children. Emerg Med. J 2006;23(6): Stocks J, Hislop AA. Structure and function of the respiratory system. In: Bisgaard H, O Callaghan C, Smaldone GC, editors. Drug delivery to the lung. New York: Marcel Dekker; 2002: Fink J. Aerosol delivery to ventilated infant and pediatric patients. Respir Care 2004;49(6): Silverman M. Aerosol therapy in the newborn. Arch Dis Child 1990; 65(8): Schleufe P, Reiffen HP, Piepenbrock S. Effective application of bronchodilator aerosols from metered-dose inhalers (MDI) via resuscitator-bag and adapter. Resuscitation 1998;39(3): Fink J, Dhand R. Bronchodilator resuscitation in the emergency department. Part 1 of 2: device selection. Respir Care 1999;44(11): RESPIRATORY CARE APRIL 2010 VOL 55 NO 4 425
8 9. Waugh JB, Deshpande VM, Hardwood RJ. Rapid interpretation of ventilator waveforms. Upper Saddle River, New Jersey: Prentice Hall; Bhutani VK, Sivieri EM. Pulmonary function and graphics. In: Goldsmith JP, Karotkin EH, editors. Assisted ventilation of the neonate. Philadelphia: WB Saunders; 2004: Lough MD. Pulmonary function. In: Chatburn RL, Lough MD, editors. Handbook of respiratory care. Chicago: Year Book Medical Publishers; 1990: Mitchell JP, Wiersema KJ, MacKay HA, Nagel MW, Pischel SM, Cripps AL. Novel approach for evaluating medication delivered via valved holding chambers (VHCs) with facemask using a face model for infants and small children. In: Dalby RN, Byron PR, Peart J, Suman JD, Farr SJ, Young PM, editors. Respiratory drug delivery. River Grove, IL: Davis Healthcare International; 2008: Cripps AL, Davis AM, Pischel SM, Wiersema KJ, Nagel MW, Mitchell JP. In-vitro performance of albuterol hfa formulation in two valved holding chambers at low inspiratory flow rates and extended delay times (abstract). J Allergy Clin Immunol 2008;121(2 Suppl 1):S Cole CH, Mitchell JP, Foley MP, Nagel MW. Hydrofluoroalkaneversus chlorofluorocarbon-beclomethasone delivery in neonatal models. Arch Dis Child Fetal Neonat Edn 2004;89(5):F417-F Tal A, Golan H, Grauer N, Aviram M, Albin D, Quastel MR. Deposition pattern of radiolabeled salbutamol inhaled from a metereddose inhaler by means of a spacer with mask in young children with airway obstruction. J Pediatr 1996;128(4): GlaxoSmithKline. Ventolin-HFA: prescribing information. Research Triangle Park, NC; tolin_hfa.pdf. Accessed February 3, Fink JB, Dhand R, Grychowski J, Fahey PJ, Tobin MJ. Reconciling in-vitro and in-vivo measurements of aerosol delivery from a metered-dose inhaler during mechanical ventilation, and defining efficiency enhancing factors. Am J Respir Crit Care Med 1999(1);159: Dhand R, Tobin MJ. Inhaled bronchodilator therapy in mechanically ventilated patients. Am J Respir Crit Care Med 1997;156(1): Miller DD, Amin MM, Palmer LB, Shah AR, Smaldone GC. Aerosol delivery and modern mechanical ventilation: in vitro/in vivo evaluation. Am J Respir Crit Care Med 2003;168(10): Dhand R, Mercier E. Effective inhaled drug administration to mechanically ventilated patients. Expert Opin Drug Deliv 2007;4(1): Dhand R. Aerosol delivery during mechanical ventilation: from basic techniques to new devices. J Aerosol Med Pulmon Deliv 2008;21(1): Newman SP. Therapeutic aerosols 1. Physical and practical considerations. Thorax 1983;38(12): Cole CH. Special problems in aerosol delivery: neonatal and pediatric considerations. Respir Care 2000;45(6): Mitchell JP, Nagel MW. Cascade impactors for the size characterization of aerosols from medical inhalers: their uses and limitations. J Aerosol Med 2003;16(4): Bonam M, Christopher D, Cipolla D, Donovan B, Goodwin D, Holmes S, et al. Minimizing variability of cascade impaction measurements in inhalers and nebulizers. AAPS PharmSciTech 2008;9(2): Bisgaard H. Delivery of inhaled medications in children. J Asthma 1997;34(6): Esposito-Festen JE, Ates B, Van Vliet FLM, Verbraak AFM, de Jongste JC, Tiddens HA. Effect of a facemask leak on aerosol delivery from a pmdi-spacer system. J Aerosol Med 2004;17(1): Shah SA, Berlinski A, Rubin BK. Force-dependent static dead space of face masks used with holding chambers. Respir Care 2006;51(2): RESPIRATORY CARE APRIL 2010 VOL 55 NO 4
Hydrofluoroalkane- vs Chlorofluorocarbon-Beclomethasone Delivery in Neonatal Models. Tufts University School of Medicine, Boston, MA, and
 Hydrofluoroalkane- vs Chlorofluorocarbon-Beclomethasone Delivery in Neonatal Models Cynthia H. Cole, MD, MPH 1, Jolyon P. Mitchell, PhD 2, Martin P. Foley 2, and Mark W. Nagel, HBSC 2 1 The Floating Hospital
Hydrofluoroalkane- vs Chlorofluorocarbon-Beclomethasone Delivery in Neonatal Models Cynthia H. Cole, MD, MPH 1, Jolyon P. Mitchell, PhD 2, Martin P. Foley 2, and Mark W. Nagel, HBSC 2 1 The Floating Hospital
Equivalence Evaluation of Valved Holding Chambers (VHCs) with Albuterol Pressurized Metered Dose Inhaler (pmdi)
 Respiratory Drug Delivery Europe 2017 Nagel and Suggett Equivalence Evaluation of Valved Holding Chambers (VHCs) with Albuterol Pressurized Metered Dose Inhaler (pmdi) Mark W. Nagel and Jason A. Suggett
Respiratory Drug Delivery Europe 2017 Nagel and Suggett Equivalence Evaluation of Valved Holding Chambers (VHCs) with Albuterol Pressurized Metered Dose Inhaler (pmdi) Mark W. Nagel and Jason A. Suggett
Original Contributions
 Original Contributions The Delivery of Chlorofluorocarbon-Propelled Versus Hydrofluoroalkane-Propelled Beclomethasone Dipropionate Aerosol to the Mechanically Ventilated Patient: A Laboratory Study Jolyon
Original Contributions The Delivery of Chlorofluorocarbon-Propelled Versus Hydrofluoroalkane-Propelled Beclomethasone Dipropionate Aerosol to the Mechanically Ventilated Patient: A Laboratory Study Jolyon
Delay Between Actuation and Shaking of a Hydrofluoroalkane Fluticasone Pressurized Metered-Dose Inhaler
 Delay Between Actuation and Shaking of a Hydrofluoroalkane Fluticasone Pressurized Metered-Dose Inhaler Ariel Berlinski MD, Dirk von Hollen, John N Pritchard PhD, and Ross HM Hatley PhD BACKGROUND: Inhaled
Delay Between Actuation and Shaking of a Hydrofluoroalkane Fluticasone Pressurized Metered-Dose Inhaler Ariel Berlinski MD, Dirk von Hollen, John N Pritchard PhD, and Ross HM Hatley PhD BACKGROUND: Inhaled
600 RESPIRATORY CARE MAY 2016 VOL 61 NO 5
 Quantifying Aerosol Delivery in Simulated Spontaneously Breathing Patients With Tracheostomy Using Different Humidification Systems With or Without Exhaled Humidity Arzu Ari PhD RRT PT CPFT FAARC, Robert
Quantifying Aerosol Delivery in Simulated Spontaneously Breathing Patients With Tracheostomy Using Different Humidification Systems With or Without Exhaled Humidity Arzu Ari PhD RRT PT CPFT FAARC, Robert
JAMES B. FINK, RAJIV DHAND, JERRY GRYCHOWSKI, PATRICK J. FAHEY, and MARTIN J. TOBIN
 Reconciling In Vitro and In Vivo Measurements of Aerosol Delivery from a Metered-Dose Inhaler during Mechanical Ventilation and Defining Efficiency-enhancing Factors JAMES B. FINK, RAJIV DHAND, JERRY GRYCHOWSKI,
Reconciling In Vitro and In Vivo Measurements of Aerosol Delivery from a Metered-Dose Inhaler during Mechanical Ventilation and Defining Efficiency-enhancing Factors JAMES B. FINK, RAJIV DHAND, JERRY GRYCHOWSKI,
Effect of Interval Between Actuations of Albuterol Hydrofluoroalkane Pressurized Metered-Dose Inhalers on Their Aerosol Characteristics
 Effect of Interval Between Actuations of Albuterol Hydrofluoroalkane Pressurized Metered-Dose Inhalers on Their Aerosol Characteristics Ariel Berlinski MD and David Pennington BACKGROUND: Albuterol hydrofluoroalkane
Effect of Interval Between Actuations of Albuterol Hydrofluoroalkane Pressurized Metered-Dose Inhalers on Their Aerosol Characteristics Ariel Berlinski MD and David Pennington BACKGROUND: Albuterol hydrofluoroalkane
Effects of Heat and Moisture Exchangers and Exhaled Humidity on Aerosol Deposition in a Simulated Ventilator-Dependent Adult Lung Model
 Effects of Heat and Moisture Exchangers and Exhaled Humidity on Aerosol Deposition in a Simulated Ventilator-Dependent Adult Lung Model Arzu Ari PhD RRT PT CPFT FAARC, Khalid S Alwadeai MSc RRT-NPS, and
Effects of Heat and Moisture Exchangers and Exhaled Humidity on Aerosol Deposition in a Simulated Ventilator-Dependent Adult Lung Model Arzu Ari PhD RRT PT CPFT FAARC, Khalid S Alwadeai MSc RRT-NPS, and
Pediatrics in mechanical ventilation
 Pediatrics Optimization Intitulé du cours of aerosol therapy in mechanical ventilation Thèmes donnés Ermindo Di Paolo, PhD Departments of Pharmacy and Pediatrics Lausanne University Hospital Switzerland
Pediatrics Optimization Intitulé du cours of aerosol therapy in mechanical ventilation Thèmes donnés Ermindo Di Paolo, PhD Departments of Pharmacy and Pediatrics Lausanne University Hospital Switzerland
Metered Dose Inhalers with Valved Holding Chamber: A Pediatric Hospital Experience
 Metered Dose Inhalers with Valved Holding Chamber: A Pediatric Hospital Experience 8th Annual North Regional Respiratory Care Conference Minnesota & Wisconsin Societies for Respiratory Care Mayo Civic
Metered Dose Inhalers with Valved Holding Chamber: A Pediatric Hospital Experience 8th Annual North Regional Respiratory Care Conference Minnesota & Wisconsin Societies for Respiratory Care Mayo Civic
I. Subject: Medication Delivery by Metered Dose Inhaler (MDI)
 I. Subject: Medication Delivery by Metered Dose Inhaler (MDI) II. Policy: Aerosol medication administration by metered dose inhaler will be performed upon a physician's order by Respiratory Therapy personnel.
I. Subject: Medication Delivery by Metered Dose Inhaler (MDI) II. Policy: Aerosol medication administration by metered dose inhaler will be performed upon a physician's order by Respiratory Therapy personnel.
Albuterol Delivery via Facial and Tracheostomy Route in a Model of a Spontaneously Breathing Child
 Albuterol Delivery via Facial and Tracheostomy Route in a Model of a Spontaneously Breathing Child Brandy Cooper and Ariel Berlinski MD BACKGROUND: Some pediatric patients receiving therapeutic aerosols
Albuterol Delivery via Facial and Tracheostomy Route in a Model of a Spontaneously Breathing Child Brandy Cooper and Ariel Berlinski MD BACKGROUND: Some pediatric patients receiving therapeutic aerosols
Aerosolized Antibiotics in Mechanically Ventilated Patients
 Aerosolized Antibiotics in Mechanically Ventilated Patients Gerald C Smaldone MD PhD Introduction Topical Delivery of Antibiotics to the Lung Tracheobronchitis Aerosolized Antibiotic Delivery in the Medical
Aerosolized Antibiotics in Mechanically Ventilated Patients Gerald C Smaldone MD PhD Introduction Topical Delivery of Antibiotics to the Lung Tracheobronchitis Aerosolized Antibiotic Delivery in the Medical
Perceptual reasons for resistance to change in the emergency department use of holding chambers for children with asthma
 Perceptual reasons for resistance to change in the emergency department use of holding chambers for children with asthma The need for sufficient human resources and deal with the perceived increase in
Perceptual reasons for resistance to change in the emergency department use of holding chambers for children with asthma The need for sufficient human resources and deal with the perceived increase in
In Vitro Evaluation of Positive Expiratory Pressure Devices Attached to Nebulizers
 In Vitro Evaluation of Positive Expiratory Pressure Devices Attached to Nebulizers Ariel Berlinski MD BACKGROUND: Patients with cystic fibrosis perform airway clearance techniques and receive nebulized
In Vitro Evaluation of Positive Expiratory Pressure Devices Attached to Nebulizers Ariel Berlinski MD BACKGROUND: Patients with cystic fibrosis perform airway clearance techniques and receive nebulized
Dolci U 1, Sidler-Moix AL 1, Di Paolo ER 1,Berger- Gryllaki 1 M, Pannatier A 1, Cotting J 2
 SALBUTAMOL DELIVERY IN AN IN VITRO PAEDIATRIC VENTILATOR-LUNG MODEL: COMPARISON OF JET, ULTRASONIC AND MESH NEBULISERS Dolci U 1, Sidler-Moix AL 1, Di Paolo ER 1,Berger- Gryllaki 1 M, Pannatier A 1, Cotting
SALBUTAMOL DELIVERY IN AN IN VITRO PAEDIATRIC VENTILATOR-LUNG MODEL: COMPARISON OF JET, ULTRASONIC AND MESH NEBULISERS Dolci U 1, Sidler-Moix AL 1, Di Paolo ER 1,Berger- Gryllaki 1 M, Pannatier A 1, Cotting
In Vitro Comparison of Aerosol Delivery Using Different Face Masks and Flow Rates With a High-Flow Humidity System
 In Vitro Comparison of Aerosol Delivery Using Different Face Masks and Flow Rates With a High-Flow Humidity System Hui-Ling Lin MSc RRT RN FAARC, Robert J Harwood MSA RRT FAARC, James B Fink PhD RRT FAARC,
In Vitro Comparison of Aerosol Delivery Using Different Face Masks and Flow Rates With a High-Flow Humidity System Hui-Ling Lin MSc RRT RN FAARC, Robert J Harwood MSA RRT FAARC, James B Fink PhD RRT FAARC,
Articles. The Advantages of Nebulization in the Treatment of Mechanically Ventilated Neonates. Kristin Smith, RRT-NPS
 Articles The Advantages of Nebulization in the Treatment of Mechanically Ventilated Neonates Kristin Smith, RRT-NPS A major goal in the care of premature babies is growth, and so all therapies are applied
Articles The Advantages of Nebulization in the Treatment of Mechanically Ventilated Neonates Kristin Smith, RRT-NPS A major goal in the care of premature babies is growth, and so all therapies are applied
Pediatric Aerosol Therapy
 Pediatric Aerosol Therapy Ariel Berlinski MD Introduction Delivery Device Selection for the Treatment of Acute Pediatric Asthma Transnasal Aerosol Delivery in Pediatric Patients Delivery Device Selection
Pediatric Aerosol Therapy Ariel Berlinski MD Introduction Delivery Device Selection for the Treatment of Acute Pediatric Asthma Transnasal Aerosol Delivery in Pediatric Patients Delivery Device Selection
ADDITIONAL TECHNOLOGIES FOR PRESSURIZED METERED DOSE INHALERS. Steve Newman Scientific Consultant Nottingham, UK
 THE PRESS-AND-BREATHE pmdi ADDITIONAL TECHNOLOGIES FOR PRESSURIZED METERED DOSE INHALERS Steve Newman Scientific Consultant Nottingham, UK steve.newman@physics.org Compact, portable, convenient Asthma
THE PRESS-AND-BREATHE pmdi ADDITIONAL TECHNOLOGIES FOR PRESSURIZED METERED DOSE INHALERS Steve Newman Scientific Consultant Nottingham, UK steve.newman@physics.org Compact, portable, convenient Asthma
Respiratory Care in PICU Aerosol Therapy ส พ ชชา ชา แสงโขต โรงพยาบาลสมเด จพระป นเกล า
 Respiratory Care in PICU Aerosol Therapy น.อ.หญ ง ส พ ชชา ชา แสงโขต โรงพยาบาลสมเด จพระป นเกล า Aerosol liquid droplets or solid particles suspended in a gas(air) visible like fog Aerosol vs Humidity Humidity
Respiratory Care in PICU Aerosol Therapy น.อ.หญ ง ส พ ชชา ชา แสงโขต โรงพยาบาลสมเด จพระป นเกล า Aerosol liquid droplets or solid particles suspended in a gas(air) visible like fog Aerosol vs Humidity Humidity
NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS
 NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS Douglas S. Gardenhire, Ed.D, RRT-NPS MODULE 1 Manipulate Small Volume Nebulizers by Order or Protocol 1 Objectives for Module 1 At the end of
NEBULIZERS, METERED DOSE INHALERS, AND DRY POWDER INHALERS Douglas S. Gardenhire, Ed.D, RRT-NPS MODULE 1 Manipulate Small Volume Nebulizers by Order or Protocol 1 Objectives for Module 1 At the end of
Misty Max 10 nebulizer
 AirLife brand Misty Max 10 nebulizer Purpose Introduction Delivery of nebulized medication to the lungs is a complex process dependant upon a variety of clinical and device-related variables. Patient breathing
AirLife brand Misty Max 10 nebulizer Purpose Introduction Delivery of nebulized medication to the lungs is a complex process dependant upon a variety of clinical and device-related variables. Patient breathing
Delivery of Iloprost Inhalation Solution With the HaloLite, Prodose, and I-neb Adaptive Aerosol Delivery Systems: An In Vitro Study
 Delivery of Iloprost Inhalation Solution With the HaloLite, Prodose, and I-neb Adaptive Aerosol Delivery Systems: An In Vitro Study Robert E Van Dyke MSc and Kurt Nikander BACKGROUND: Iloprost (Ventavis)
Delivery of Iloprost Inhalation Solution With the HaloLite, Prodose, and I-neb Adaptive Aerosol Delivery Systems: An In Vitro Study Robert E Van Dyke MSc and Kurt Nikander BACKGROUND: Iloprost (Ventavis)
Overcoming the Adverse Effect of Humidity in Aerosol Delivery via Pressurized Metered-Dose Inhalers during Mechanical Ventilation
 Overcoming the Adverse Effect of Humidity in Aerosol Delivery via Pressurized Metered-Dose Inhalers during Mechanical Ventilation CARLOS F. LANGE and WARREN H. FINLAY Aerosol Research Laboratory, Department
Overcoming the Adverse Effect of Humidity in Aerosol Delivery via Pressurized Metered-Dose Inhalers during Mechanical Ventilation CARLOS F. LANGE and WARREN H. FINLAY Aerosol Research Laboratory, Department
Delivering Aerosol Medication in ICU
 Delivering Aerosol Medication in ICU 18th Aug 2017 Lau Chee Lan Pharmacist HCTM PPUKM ASMIC 2017 Aerosol Therapy Part of the treatment for a variety of respiratory disease * asthma and chronic obstructive
Delivering Aerosol Medication in ICU 18th Aug 2017 Lau Chee Lan Pharmacist HCTM PPUKM ASMIC 2017 Aerosol Therapy Part of the treatment for a variety of respiratory disease * asthma and chronic obstructive
Characterizing the Performance of Metered Dose Inhalers with Add-On Devices: New Methods For Clinically Relevant Testing
 INSTRUMENTATION» Characterizing the Performance of Metered Dose Inhalers with Add-On Devices: New Methods For Clinically Relevant Testing Mark Copley Director Copley Scientific The recent introduction
INSTRUMENTATION» Characterizing the Performance of Metered Dose Inhalers with Add-On Devices: New Methods For Clinically Relevant Testing Mark Copley Director Copley Scientific The recent introduction
Small Volume Nebulizer Treatment (Hand-Held)
 Small Volume Aerosol Treatment Page 1 of 6 Purpose Policy Physician's Order Small Volume Nebulizer Treatment To standardize the delivery of inhalation aerosol drug therapy via small volume (hand-held)
Small Volume Aerosol Treatment Page 1 of 6 Purpose Policy Physician's Order Small Volume Nebulizer Treatment To standardize the delivery of inhalation aerosol drug therapy via small volume (hand-held)
NIV and Aerosoltherapy
 NIV and Aerosoltherapy Workshop Jean-Bernard Michotte (Lausanne-CH) Simone Gambazza (Milano-IT) Jean-Bernard Michotte Haute Ecole de Santé Vaud, 1011 Lausanne - Suisse Cliniques Universitaires Saint-Luc,
NIV and Aerosoltherapy Workshop Jean-Bernard Michotte (Lausanne-CH) Simone Gambazza (Milano-IT) Jean-Bernard Michotte Haute Ecole de Santé Vaud, 1011 Lausanne - Suisse Cliniques Universitaires Saint-Luc,
Metered Dose Inhalers (MDIs) In Vitro Measures to Confirm Patient Perceptions: HFA vs. CFC
 Metered Dose Inhalers (MDIs) In Vitro Measures to Confirm Patient Perceptions: HFA vs. CFC William H. Doub, Ph.D. Division of Pharmaceutical Analysis (DPA) US FDA/CDER/OPS/OTR The information presented
Metered Dose Inhalers (MDIs) In Vitro Measures to Confirm Patient Perceptions: HFA vs. CFC William H. Doub, Ph.D. Division of Pharmaceutical Analysis (DPA) US FDA/CDER/OPS/OTR The information presented
It is recommended that a mask and protective eyewear be worn when providing care to a patient with a cough
 UNIVERSITY HEALTH NETWORK POLICY #: PAGE 1 OF 7 POLICY AND PROCEDURE MANUAL: RESPIRATORY THERAPY DEPT PATIENT CARE SECTION ORIGINAL DATE: 04/03 ISSUED BY: SITE LEADER APPROVED BY: Infection Prevention
UNIVERSITY HEALTH NETWORK POLICY #: PAGE 1 OF 7 POLICY AND PROCEDURE MANUAL: RESPIRATORY THERAPY DEPT PATIENT CARE SECTION ORIGINAL DATE: 04/03 ISSUED BY: SITE LEADER APPROVED BY: Infection Prevention
AEROSOL THERAPY: THE PRACTICALITIES
 AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
AEROSOL THERAPY: THE PRACTICALITIES Lester I. Harrison, PhD Section Head, Clinical Pharmacokinetics, 3M Pharmaceuticals, 3M Center 270-3S-05, St. Paul, MN, USA 55144 liharrison@mmm.com Introduction: Horses,
Comparison of patient spirometry and ventilator spirometry
 GE Healthcare Comparison of patient spirometry and ventilator spirometry Test results are based on the Master s thesis, Comparison between patient spirometry and ventilator spirometry by Saana Jenu, 2011
GE Healthcare Comparison of patient spirometry and ventilator spirometry Test results are based on the Master s thesis, Comparison between patient spirometry and ventilator spirometry by Saana Jenu, 2011
Effect of Mask Dead Space and Occlusion of Mask Holes on Delivery of Nebulized Albuterol
 Effect of Mask Dead Space and Occlusion of Mask Holes on Delivery of Nebulized Albuterol Ariel Berlinski MD BACKGROUND: Infants and children with respiratory conditions are often prescribed bronchodilators.
Effect of Mask Dead Space and Occlusion of Mask Holes on Delivery of Nebulized Albuterol Ariel Berlinski MD BACKGROUND: Infants and children with respiratory conditions are often prescribed bronchodilators.
Special Problems in Aerosol Delivery: Neonatal and Pediatric Considerations
 Special Problems in Aerosol Delivery: Neonatal and Pediatric Considerations Cynthia H Cole MD MPH Introduction Patient Considerations Aerosol-System Considerations Choosing an Aerosol Delivery System Summary:
Special Problems in Aerosol Delivery: Neonatal and Pediatric Considerations Cynthia H Cole MD MPH Introduction Patient Considerations Aerosol-System Considerations Choosing an Aerosol Delivery System Summary:
Albuterol Delivery via Intrapulmonary Percussive Ventilator and Jet Nebulizer in a Pediatric Ventilator Model
 Albuterol Delivery via Intrapulmonary Percussive Ventilator and Jet Nebulizer in a Pediatric Ventilator Model Ariel Berlinski MD and J Randy Willis RRT-NPS BACKGROUND: In-line administration of bronchodilators
Albuterol Delivery via Intrapulmonary Percussive Ventilator and Jet Nebulizer in a Pediatric Ventilator Model Ariel Berlinski MD and J Randy Willis RRT-NPS BACKGROUND: In-line administration of bronchodilators
Factors affecting bronchodilator delivery in mechanically ventilated adults
 LITERATURE REVIEW Factors affecting bronchodilator delivery in mechanically ventilated adults Arzu Ari and James B Fink ABSTRACT Background: Bronchodilators are increasingly being used in patients undergoing
LITERATURE REVIEW Factors affecting bronchodilator delivery in mechanically ventilated adults Arzu Ari and James B Fink ABSTRACT Background: Bronchodilators are increasingly being used in patients undergoing
Vibrating Mesh Nebulizer Compared With Metered-Dose Inhaler in Mechanically Ventilated Subjects
 Vibrating Mesh Nebulizer Compared With Metered-Dose Inhaler in Mechanically Ventilated Subjects Meagan N Dubosky MSc RRT, Yi-Fan Chen PhD, Mary E Henriksen MSc RRT, and David L Vines MHS RRT FAARC BACKGROUND:
Vibrating Mesh Nebulizer Compared With Metered-Dose Inhaler in Mechanically Ventilated Subjects Meagan N Dubosky MSc RRT, Yi-Fan Chen PhD, Mary E Henriksen MSc RRT, and David L Vines MHS RRT FAARC BACKGROUND:
The abbreviated impactor measurement concept
 AS APPEARED IN Inhalation JUNE 2009 www.inhalationmag.com The abbreviated impactor measurement concept A potentially faster and more precise way to assess quality of inhaled drug products Jolyon P. Mitchell,
AS APPEARED IN Inhalation JUNE 2009 www.inhalationmag.com The abbreviated impactor measurement concept A potentially faster and more precise way to assess quality of inhaled drug products Jolyon P. Mitchell,
Invacare Aerosol Products. Stratos Aerosol Compressors Nebulizers Asthma Management
 Invacare Aerosol Products Stratos Aerosol Compressors Nebulizers Asthma Management Invacare Aerosol Products Invacare is proud to offer an updated aerosol product line that includes the Stratos Compact,
Invacare Aerosol Products Stratos Aerosol Compressors Nebulizers Asthma Management Invacare Aerosol Products Invacare is proud to offer an updated aerosol product line that includes the Stratos Compact,
Inhalation Therapy. Inhalation Therapy
 Matching Device and Patient Matching Patient to the Device Søren Pedersen University of Southern Denmark Kolding Hospital Anatomical factors (age) Training and education Delivered dose Psycomotor skills
Matching Device and Patient Matching Patient to the Device Søren Pedersen University of Southern Denmark Kolding Hospital Anatomical factors (age) Training and education Delivered dose Psycomotor skills
Assessing New Technologies: Patient-Device Interactions and Deposition
 Assessing New Technologies: Patient-Device Interactions and Deposition Gerald C Smaldone MD PhD Introduction: Principles The Inhaled Mass The Aerosol Deposition Measurement of Inhaled Mass Pediatric in
Assessing New Technologies: Patient-Device Interactions and Deposition Gerald C Smaldone MD PhD Introduction: Principles The Inhaled Mass The Aerosol Deposition Measurement of Inhaled Mass Pediatric in
In Vitro Evaluation of Radio-Labeled Aerosol Delivery Via a Variable-Flow Infant CPAP System
 In Vitro Evaluation of Radio-Labeled Aerosol Delivery Via a Variable-Flow Infant CPAP System Kimberly D Farney RRT, Brandon T Kuehne RRT MBA, Laurie A Gibson RT (N), Leif D Nelin MD, and Edward G Shepherd
In Vitro Evaluation of Radio-Labeled Aerosol Delivery Via a Variable-Flow Infant CPAP System Kimberly D Farney RRT, Brandon T Kuehne RRT MBA, Laurie A Gibson RT (N), Leif D Nelin MD, and Edward G Shepherd
An update on inhalation devices
 OPTIMIZING INHALED DRUG DELIVERY An update on inhalation devices Contents 1. Introduction...1 2. History of spacers... 1 3. Working of a spacer... 2 4. Advantages of spacer devices... 3 5. Who should
OPTIMIZING INHALED DRUG DELIVERY An update on inhalation devices Contents 1. Introduction...1 2. History of spacers... 1 3. Working of a spacer... 2 4. Advantages of spacer devices... 3 5. Who should
2008 IPAC-RS Conference Doing the Right Thing Science, Quality and Patient Focus
 2008 IPAC-RS Conference Doing the Right Thing Science, Quality and Patient Focus Patient Perspective Alpha-1 1 Foundation John W. Walsh Patient Focus Personal perspective Challenges and lessons Importance
2008 IPAC-RS Conference Doing the Right Thing Science, Quality and Patient Focus Patient Perspective Alpha-1 1 Foundation John W. Walsh Patient Focus Personal perspective Challenges and lessons Importance
University of Groningen. Technology in practice Lexmond, Anne
 University of Groningen Technology in practice Lexmond, Anne IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
University of Groningen Technology in practice Lexmond, Anne IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document
Latex Free. An affordable, easy to use, high density, small volume nebulizer with a breath enhanced design! Breath Enhanced High Density Jet Nebulizer
 Latex Free Breath Enhanced High Density Jet Nebulizer The NebuTech HDN nebulizer, a breath enhanced design, by Salter Labs is quickly becoming the product of choice for caregivers and patients alike. This
Latex Free Breath Enhanced High Density Jet Nebulizer The NebuTech HDN nebulizer, a breath enhanced design, by Salter Labs is quickly becoming the product of choice for caregivers and patients alike. This
Special Problems in Aerosol Delivery: Artificial Airways
 Special Problems in Aerosol Delivery: Artificial Airways Rajiv Dhand MD Introduction Factors Influencing Aerosol Delivery through Endotracheal Tubes Endotracheal Tube Size Endotracheal Tube Material/Design
Special Problems in Aerosol Delivery: Artificial Airways Rajiv Dhand MD Introduction Factors Influencing Aerosol Delivery through Endotracheal Tubes Endotracheal Tube Size Endotracheal Tube Material/Design
Response to Albuterol MDI Delivered Through an Anti-Static Chamber During Nocturnal Bronchospasm
 Response to Albuterol MDI Delivered Through an Anti-Static Chamber During Nocturnal Bronchospasm Sreekala Prabhakaran MD, Jonathan Shuster PhD, Sarah Chesrown MD PhD, and Leslie Hendeles PharmD BACKGROUND:
Response to Albuterol MDI Delivered Through an Anti-Static Chamber During Nocturnal Bronchospasm Sreekala Prabhakaran MD, Jonathan Shuster PhD, Sarah Chesrown MD PhD, and Leslie Hendeles PharmD BACKGROUND:
Understanding cascade impaction and its importance for inhaler testing
 Understanding cascade impaction and its importance for inhaler testing Mark Copley, Technical Sales Manager Inhalation product development is an important area of activity for the pharmaceutical sector.
Understanding cascade impaction and its importance for inhaler testing Mark Copley, Technical Sales Manager Inhalation product development is an important area of activity for the pharmaceutical sector.
Rush University, College of Health Sciences
 Rush University, College of Health Sciences An Evaluation of the Prototype OxyMulti Mask Prototype Compared to the Oxymask Aerosol, OxyMulti Mask and Airlife Aerosol Mask for Aerosol Delivery in Adults
Rush University, College of Health Sciences An Evaluation of the Prototype OxyMulti Mask Prototype Compared to the Oxymask Aerosol, OxyMulti Mask and Airlife Aerosol Mask for Aerosol Delivery in Adults
Nebulized Albuterol Delivery in a Model of Spontaneously Breathing Children With Tracheostomy
 Nebulized Albuterol Delivery in a Model of Spontaneously Breathing Children With Ariel Berlinski MD BACKGROUND: Nebulized therapy is commonly used in spontaneously breathing tracheostomized patients, despite
Nebulized Albuterol Delivery in a Model of Spontaneously Breathing Children With Ariel Berlinski MD BACKGROUND: Nebulized therapy is commonly used in spontaneously breathing tracheostomized patients, despite
Blow-by as Potential Therapy for Uncooperative Children: An In-Vitro Study. Mohamed Mohsen Mansour MD and Gerald C Smaldone MD PhD
 Original Research Blow-by as Potential Therapy for Uncooperative Children: An In-Vitro Study Mohamed Mohsen Mansour MD and Gerald C Smaldone MD PhD BACKGROUND: Blow-by, a common form of nebulizer therapy,
Original Research Blow-by as Potential Therapy for Uncooperative Children: An In-Vitro Study Mohamed Mohsen Mansour MD and Gerald C Smaldone MD PhD BACKGROUND: Blow-by, a common form of nebulizer therapy,
Albuterol Delivery From a Metered-Dose Inhaler With Spacer Is Reduced Following Short-Duration Manual Ventilation in a Neonatal Ventilator-Lung Model
 Albuterol Delivery From a Metered-Dose Inhaler With Spacer Is Reduced Following Short-Duration Manual Ventilation in a Neonatal Ventilator-Lung Model Ralph A Lugo PharmD and Julie Ballard RRT INTRODUCTION:
Albuterol Delivery From a Metered-Dose Inhaler With Spacer Is Reduced Following Short-Duration Manual Ventilation in a Neonatal Ventilator-Lung Model Ralph A Lugo PharmD and Julie Ballard RRT INTRODUCTION:
INTRODUCTION. size and total nozzle area decrease with stage number. Volumetric air flow rate through
 CASCADE I M P A C T I O N Optimizing Cascade Impactor Testing for Characterizing Orally Inhaled & Nasal Drug Products By: Mark Copley INTRODUCTION Cascade impaction is a core analytical technique for characterizing
CASCADE I M P A C T I O N Optimizing Cascade Impactor Testing for Characterizing Orally Inhaled & Nasal Drug Products By: Mark Copley INTRODUCTION Cascade impaction is a core analytical technique for characterizing
Vancouver Coastal Health Guidelines for the use of Respiratory Equipment for Patients on Airborne Precautions in Acute Care Facilities
 Vancouver Coastal Health Guidelines for the use of Respiratory Equipment for Patients on Airborne Precautions in Acute Care Facilities Goals 1. To meet respiratory care needs in patients who are on airborne
Vancouver Coastal Health Guidelines for the use of Respiratory Equipment for Patients on Airborne Precautions in Acute Care Facilities Goals 1. To meet respiratory care needs in patients who are on airborne
Basic Techniques for Aerosol Delivery During Mechanical Ventilation. Rajiv Dhand MD
 Symposium Papers Basic Techniques for Aerosol Delivery During Mechanical Ventilation Rajiv Dhand MD Introduction Factors That Influence Aerosol Delivery During Mechanical Ventilation Methods to Assess
Symposium Papers Basic Techniques for Aerosol Delivery During Mechanical Ventilation Rajiv Dhand MD Introduction Factors That Influence Aerosol Delivery During Mechanical Ventilation Methods to Assess
Aerosol Delivery and Modern Mechanical Ventilation In Vitro/In Vivo Evaluation
 Aerosol Delivery and Modern Mechanical Ventilation In Vitro/In Vivo Evaluation Dorisanne D. Miller, Mohammad M. Amin, Lucy B. Palmer, Akbar R. Shah, and Gerald C. Smaldone Department of Respiratory Care,
Aerosol Delivery and Modern Mechanical Ventilation In Vitro/In Vivo Evaluation Dorisanne D. Miller, Mohammad M. Amin, Lucy B. Palmer, Akbar R. Shah, and Gerald C. Smaldone Department of Respiratory Care,
Nebulizers. Small Volume Nebulizers
 Small Volume MICRO MIST Nebulizer The MICRO MIST small volume nebulizer is designed for performance and economy and includes the design features needed by today s respiratory care practitioners. It can
Small Volume MICRO MIST Nebulizer The MICRO MIST small volume nebulizer is designed for performance and economy and includes the design features needed by today s respiratory care practitioners. It can
Archives of Pulmonology and Respiratory Care
 v Clinical Group Archives of Pulmonology and Respiratory Care DOI CC By Marwa Mohsen 1, Ahmed A Elberry 2, Abeer Salah Eldin 3, Raghda RS Hussein 1 and Mohamed EA Abdelrahim 1,4 * Research Article Effects
v Clinical Group Archives of Pulmonology and Respiratory Care DOI CC By Marwa Mohsen 1, Ahmed A Elberry 2, Abeer Salah Eldin 3, Raghda RS Hussein 1 and Mohamed EA Abdelrahim 1,4 * Research Article Effects
Evaluation of a Nasal Cannula in Noninvasive Ventilation Using a Lung Simulator
 Evaluation of a Nasal in Noninvasive Ventilation Using a Lung Simulator Narayan P Iyer MD and Robert Chatburn MHHS RRT-NPS FAARC BACKGROUND: Nasal noninvasive ventilation (NIV) is a common form of noninvasive
Evaluation of a Nasal in Noninvasive Ventilation Using a Lung Simulator Narayan P Iyer MD and Robert Chatburn MHHS RRT-NPS FAARC BACKGROUND: Nasal noninvasive ventilation (NIV) is a common form of noninvasive
Continuous Aerosol Therapy
 PROCEDURE - : Page 1 of 5 Purpose Policy Physician's Order To standardize the administration of continuous aerosol therapy. Respiratory Care Services provides equipment and therapy according to physician
PROCEDURE - : Page 1 of 5 Purpose Policy Physician's Order To standardize the administration of continuous aerosol therapy. Respiratory Care Services provides equipment and therapy according to physician
A Method for Increasing Jet Nebulizer Delivery Efficiency for Aerosol Drug Delivery in Ventilated Newborns: An In Vitro Study
 A Method for Increasing Jet Nebulizer Delivery for Aerosol Drug Delivery in Ventilated Newborns: An In Vitro Study Michael C Quong, Bernard Thébaud PhD MD, and Warren H Finlay PhD PEng BACKGROUND: A substantial
A Method for Increasing Jet Nebulizer Delivery for Aerosol Drug Delivery in Ventilated Newborns: An In Vitro Study Michael C Quong, Bernard Thébaud PhD MD, and Warren H Finlay PhD PEng BACKGROUND: A substantial
Pulmonary deposition of inhaled drugs
 Pulmonary deposition of inhaled drugs Federico Lavorini Dept. Experimental and Clinical Medicine Careggi University Hospital Florence - Italy Presenter Disclosures F.L. has received in the last 5 years
Pulmonary deposition of inhaled drugs Federico Lavorini Dept. Experimental and Clinical Medicine Careggi University Hospital Florence - Italy Presenter Disclosures F.L. has received in the last 5 years
Paediatric pulmonary drug delivery: considerations in asthma treatment
 Review Paediatric pulmonary drug delivery: considerations in asthma treatment 1. Introduction 2. Guideline recommendations 3. Factors affecting drug delivery to the lung 4. Nebulisers 5. Metered-dose inhalers
Review Paediatric pulmonary drug delivery: considerations in asthma treatment 1. Introduction 2. Guideline recommendations 3. Factors affecting drug delivery to the lung 4. Nebulisers 5. Metered-dose inhalers
Aerosol Delivery Devices
 Aerosol Delivery Devices Judy Bielenda LRRT Respiratory Therapy Clinical Specialist Pediatric Asthma Education Coordinator University of Michigan C.S. Mott Children s and Von Voigtlander Women s Hospital
Aerosol Delivery Devices Judy Bielenda LRRT Respiratory Therapy Clinical Specialist Pediatric Asthma Education Coordinator University of Michigan C.S. Mott Children s and Von Voigtlander Women s Hospital
Valved holding chamber drug delivery is dependent on breathing pattern and device design
 ORIGINAL ARTICLE PAEDIATRIC PULMONOLOGY Valved holding chamber drug delivery is dependent on breathing pattern and device design Péter Csonka 1,2 and Lauri Lehtimäki 3,4 Affiliations: 1 Centre for Child
ORIGINAL ARTICLE PAEDIATRIC PULMONOLOGY Valved holding chamber drug delivery is dependent on breathing pattern and device design Péter Csonka 1,2 and Lauri Lehtimäki 3,4 Affiliations: 1 Centre for Child
Assessing Quality of Inhaled Products And Links to Efficacy and Safety
 Assessing Quality of Inhaled Products And Links to Efficacy and Safety Prasad Peri, PhD ONDQA 2011 IPAC-RS Conference Bringing Value To The Patient In A Changing World March 30, 2011 1 Outline of the Presentation
Assessing Quality of Inhaled Products And Links to Efficacy and Safety Prasad Peri, PhD ONDQA 2011 IPAC-RS Conference Bringing Value To The Patient In A Changing World March 30, 2011 1 Outline of the Presentation
InspiraChamber System From Salter. One system for all your patients NEW
 InspiraChamber System From Salter One system for all your patients NEW Collaborative Innovation Today, Salter Labs is building a bold new future focusing on superior patient care, reliable outcomes, and
InspiraChamber System From Salter One system for all your patients NEW Collaborative Innovation Today, Salter Labs is building a bold new future focusing on superior patient care, reliable outcomes, and
Patient. Device Clinician. Safety & efficacy
 Patient Device Clinician Formulation Safety & efficacy 1. Modified from Daley-Yates et al., Expert Opin. Drug Deliv. 2011: 8(10):1297-1308 2. Modified from Laube et al., Eur Respir J 2011; 37: 1308 1331
Patient Device Clinician Formulation Safety & efficacy 1. Modified from Daley-Yates et al., Expert Opin. Drug Deliv. 2011: 8(10):1297-1308 2. Modified from Laube et al., Eur Respir J 2011; 37: 1308 1331
High-Flow Nasal Cannula and Aerosolized Agonists for Rescue Therapy in Children With Bronchiolitis: A Case Series
 High-Flow Nasal Cannula and Aerosolized Agonists for Rescue Therapy in Children With Bronchiolitis: A Case Series Sherwin E Morgan RRT, Steve Mosakowski RRT, Patti Solano RRT, Jesse B Hall MD, and Avery
High-Flow Nasal Cannula and Aerosolized Agonists for Rescue Therapy in Children With Bronchiolitis: A Case Series Sherwin E Morgan RRT, Steve Mosakowski RRT, Patti Solano RRT, Jesse B Hall MD, and Avery
AeroChamber Mitchell, J., Coppolo, D.C., Nagel, M.W. Khan Y, Tang Y, Hochhaus G, Shuster JJ, Spencer T, Chesrown S, Hendeles L.
 AeroChamber Anti-Static Clinical Summary The following summaries have been extracted from various journals and resources to produce a comprehensive analysis of clinical data available. Mitchell, J., Coppolo,
AeroChamber Anti-Static Clinical Summary The following summaries have been extracted from various journals and resources to produce a comprehensive analysis of clinical data available. Mitchell, J., Coppolo,
Effect of particle size of bronchodilator aerosols on lung distribution and pulmonary function in patients
 Thorax 1987;42:457-461 Effect of particle size of bronchodilator aerosols on lung distribution and pulmonary function in patients with chronic asthma D M MITCHELL, M A SOLOMON, S E J TOLFREE, M SHORT,
Thorax 1987;42:457-461 Effect of particle size of bronchodilator aerosols on lung distribution and pulmonary function in patients with chronic asthma D M MITCHELL, M A SOLOMON, S E J TOLFREE, M SHORT,
Last updated: July 2011
 EFFICIENT DATA ANALYSIS, ABBREVIATED IMPACTOR MEASUREMENTS, AERODYNAMIC PARTICLE SIZE DISTRIBUTIONS: PUBLICATIONS AND PRESENTATIONS BY MEMBERS OF THE IPAC-RS CASCADE IMPACTION WORKING GROUP AND EPAG 2000-2011
EFFICIENT DATA ANALYSIS, ABBREVIATED IMPACTOR MEASUREMENTS, AERODYNAMIC PARTICLE SIZE DISTRIBUTIONS: PUBLICATIONS AND PRESENTATIONS BY MEMBERS OF THE IPAC-RS CASCADE IMPACTION WORKING GROUP AND EPAG 2000-2011
Revision 2. Improving Aerosol Drug Delivery During Invasive Mechanical Ventilation with Redesigned Components
 Revision 2 Improving Aerosol Drug Delivery During Invasive Mechanical Ventilation with Redesigned Components P. Worth Longest, Ph.D., 1,2* Mandana Azimi, Pharm.D., 2 Laleh Golshahi, Ph.D., 2 and Michael
Revision 2 Improving Aerosol Drug Delivery During Invasive Mechanical Ventilation with Redesigned Components P. Worth Longest, Ph.D., 1,2* Mandana Azimi, Pharm.D., 2 Laleh Golshahi, Ph.D., 2 and Michael
STATE OF OKLAHOMA 2014 EMERGENCY MEDICAL SERVICES PROTOCOLS
 3K NON-INVASIVE POSITIVE PRESSURE VENTILATION (NIPPV) ADULT EMT EMT-INTERMEDIATE 85 ADVANCED EMT PARAMEDIC Indications: 1. Dyspnea Uncertain Etiology Adult. 2. Dyspnea Asthma Adult. 3. Dyspnea Chronic
3K NON-INVASIVE POSITIVE PRESSURE VENTILATION (NIPPV) ADULT EMT EMT-INTERMEDIATE 85 ADVANCED EMT PARAMEDIC Indications: 1. Dyspnea Uncertain Etiology Adult. 2. Dyspnea Asthma Adult. 3. Dyspnea Chronic
Effective Aerosol Delivery in Acute Care
 Effective Aerosol Delivery in Acute Care Jim Fink, RRT, PhD, FAARC, FCCP Chief Science Officer, Aerogen Pharma Corp., San Mateo, CA USA Visiting Professor, Dept of Physical therapy, Universidade Federal
Effective Aerosol Delivery in Acute Care Jim Fink, RRT, PhD, FAARC, FCCP Chief Science Officer, Aerogen Pharma Corp., San Mateo, CA USA Visiting Professor, Dept of Physical therapy, Universidade Federal
Resistance of Colorimetric Carbon Dioxide Detectors Commonly Utilized in Neonates
 Resistance of Colorimetric Carbon Dioxide Detectors Commonly Utilized in Neonates Melissa K Brown RRT-NPS, Danielle V Lazarus RRT, Sarah R Gonzales RRT, Wade D Rich RRT-NPS CCRC, Madeline J Wozniak, Debra
Resistance of Colorimetric Carbon Dioxide Detectors Commonly Utilized in Neonates Melissa K Brown RRT-NPS, Danielle V Lazarus RRT, Sarah R Gonzales RRT, Wade D Rich RRT-NPS CCRC, Madeline J Wozniak, Debra
Effective Date: August 31, 2006 SUBJECT: CARE AND USE OF NEBULIZER AND INTERMITTENT POSITIVE PRESSURE BREATHING DEVICE
 COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION - Treatments POLICY NUMBER: 421 Effective Date: August 31, 2006 SUBJECT: CARE AND USE OF NEBULIZER AND INTERMITTENT POSITIVE PRESSURE
COALINGA STATE HOSPITAL NURSING POLICY AND PROCEDURE MANUAL SECTION - Treatments POLICY NUMBER: 421 Effective Date: August 31, 2006 SUBJECT: CARE AND USE OF NEBULIZER AND INTERMITTENT POSITIVE PRESSURE
Systems differ in their ability to deliver optimal humidification
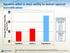 Average Absolute Humidity (mg H 2 O/L) Systems differ in their ability to deliver optimal humidification 45 Flows Tested 40 35 30 Optiflow Airvo 2 Vapotherm Vapotherm 5 L/min 10L/min 20L/min 30L/min 40L/min
Average Absolute Humidity (mg H 2 O/L) Systems differ in their ability to deliver optimal humidification 45 Flows Tested 40 35 30 Optiflow Airvo 2 Vapotherm Vapotherm 5 L/min 10L/min 20L/min 30L/min 40L/min
In-Vitro Comparison of Aerosol Drug Delivery in Pediatrics Using Pressurized Metered Dose Inhaler, Jet Nebulizer, and Vibrating Mesh Nebulizers
 Georgia State University ScholarWorks @ Georgia State University Respiratory Therapy Theses Department of Respiratory Therapy 7-31-2012 In-Vitro Comparison of Aerosol Drug Delivery in Pediatrics Using
Georgia State University ScholarWorks @ Georgia State University Respiratory Therapy Theses Department of Respiratory Therapy 7-31-2012 In-Vitro Comparison of Aerosol Drug Delivery in Pediatrics Using
Pharmacokinetics, pharmacodynamics, and the delivery of pediatric bronchodilator therapy
 Pharmacokinetics, pharmacodynamics, and the delivery of pediatric bronchodilator therapy David P. Skoner, MD Pittsburgh, Pa β 2 -adrenergic receptor agonists have long been used for the amelioration of
Pharmacokinetics, pharmacodynamics, and the delivery of pediatric bronchodilator therapy David P. Skoner, MD Pittsburgh, Pa β 2 -adrenergic receptor agonists have long been used for the amelioration of
ENDOTRACHEAL INTUBATION POLICY
 POLICY Indications: Ineffective ventilation with mask and t-piece, or mask and bag technique Inability to maintain a patent airway Need or anticipation of need for prolonged ventilation Need for endotracheal
POLICY Indications: Ineffective ventilation with mask and t-piece, or mask and bag technique Inability to maintain a patent airway Need or anticipation of need for prolonged ventilation Need for endotracheal
I. Subject: Continuous Positive Airway Pressure CPAP by Continuous Flow Device
 I. Subject: Continuous Positive Airway Pressure CPAP by Continuous Flow Device II. Policy: Continuous Positive Airway Pressure CPAP by the Down's system will be instituted by Respiratory Therapy personnel
I. Subject: Continuous Positive Airway Pressure CPAP by Continuous Flow Device II. Policy: Continuous Positive Airway Pressure CPAP by the Down's system will be instituted by Respiratory Therapy personnel
Assessing the role of breathing simulators in OIP testing
 As first AppeAred in Inhalation April 2014 www.inhalationmag.com Assessing the role of breathing simulators in OIP testing Exploring how the application of patient-representative inhalation profiles can
As first AppeAred in Inhalation April 2014 www.inhalationmag.com Assessing the role of breathing simulators in OIP testing Exploring how the application of patient-representative inhalation profiles can
Device Change Management for Inhaled Products. Loy Britto, Ph.D. GlaxoSmithKline ISAM Congress Munich 2015
 Device Change Management for Inhaled Products Loy Britto, Ph.D. GlaxoSmithKline ISAM Congress Munich 2015 Topics to be covered Update on ISO/TC 084/WG 15: Guidelines for development of drug products- Quality
Device Change Management for Inhaled Products Loy Britto, Ph.D. GlaxoSmithKline ISAM Congress Munich 2015 Topics to be covered Update on ISO/TC 084/WG 15: Guidelines for development of drug products- Quality
Combining imaging techniques and CFD to model lung deposition in various age classes of the paediatric population
 Combining imaging techniques and CFD to model lung deposition in various age classes of the paediatric population Contents: Motivation: Why modelling the paediatric dose to lung via in-vitro and in-silico
Combining imaging techniques and CFD to model lung deposition in various age classes of the paediatric population Contents: Motivation: Why modelling the paediatric dose to lung via in-vitro and in-silico
COMPARISON OF THE RESPIRABLE FRACTION FROM THREE DIFERENT DPI DEVICES
 COMPARISON OF THE RESPIRABLE FRACTION FROM THREE DIFERENT DPI DEVICES Miriam Sanz Cermeño and Helena Maria Cabral Marques UCTF, Faculdade de Farmácia, Universidade de Lisboa, PORTUGAL 1. Introduction Inhalation
COMPARISON OF THE RESPIRABLE FRACTION FROM THREE DIFERENT DPI DEVICES Miriam Sanz Cermeño and Helena Maria Cabral Marques UCTF, Faculdade de Farmácia, Universidade de Lisboa, PORTUGAL 1. Introduction Inhalation
Bronchodilator delivery by metered-dose inhaler in mechanically ventilated COPD patients: influence of flow pattern
 Eur Respir J 2; 16: 263±268 Printed in UK ± all rights reserved Copyright #ERS Journals Ltd 2 European Respiratory Journal ISSN 93-1936 Bronchodilator delivery by metered-dose inhaler in mechanically ventilated
Eur Respir J 2; 16: 263±268 Printed in UK ± all rights reserved Copyright #ERS Journals Ltd 2 European Respiratory Journal ISSN 93-1936 Bronchodilator delivery by metered-dose inhaler in mechanically ventilated
IVIVC in Pediatric OIPs
 IPAC-RS/UF Orlando Conference 2014 March 20, 2014 IVIVC in Pediatric OIPs Herbert Wachtel Declaration of Conflicts of Interest H. Wachtel is employee of Boehringer Ingelheim Pharma GmbH & Co. KG, Germany.
IPAC-RS/UF Orlando Conference 2014 March 20, 2014 IVIVC in Pediatric OIPs Herbert Wachtel Declaration of Conflicts of Interest H. Wachtel is employee of Boehringer Ingelheim Pharma GmbH & Co. KG, Germany.
Appendix M: Device Technique
 Nursing Care of Dyspnea: The 6th Vital Sign in Individuals with Chronic Obstructive Pulmonary Disease (COPD) Appendix M: Device Technique Medications: Inhalation Devices Medications come in many forms.
Nursing Care of Dyspnea: The 6th Vital Sign in Individuals with Chronic Obstructive Pulmonary Disease (COPD) Appendix M: Device Technique Medications: Inhalation Devices Medications come in many forms.
The Collapsible Chamber
 NEW The Collapsible Chamber DESIGNED FOR PERFORMANCE AND CONVENIENCE INDICATIONS FOR USE Flexichamber is an anti-static valved collapsible holding chamber intended to be used by patients under the care
NEW The Collapsible Chamber DESIGNED FOR PERFORMANCE AND CONVENIENCE INDICATIONS FOR USE Flexichamber is an anti-static valved collapsible holding chamber intended to be used by patients under the care
21/03/2011 AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY. Fundamentals of aerosols
 AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY Steve Newman Scientific Consultant Norfolk, UK steve.newman@physics.org
AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY AEROSOL DEPOSITION AND THE ASSESSMENT OF PULMONARY DRUG DELIVERY Steve Newman Scientific Consultant Norfolk, UK steve.newman@physics.org
Effect of mask dead space and occlusion of mask holes on delivery of nebulized albuterol. Ariel Berlinski, MD 1,2. Little Rock, Arkansas.
 Effect of mask dead space and occlusion of mask holes on delivery of nebulized albuterol Ariel Berlinski, MD 1,2 1 University of Arkansas for Medical Sciences, Department of Pediatrics, Pulmonology Section
Effect of mask dead space and occlusion of mask holes on delivery of nebulized albuterol Ariel Berlinski, MD 1,2 1 University of Arkansas for Medical Sciences, Department of Pediatrics, Pulmonology Section
The Effect of Different Interfaces on Aerosol Delivery in Simulated Spontaneously Breathing Adult with Tracheostomy
 Georgia State University ScholarWorks @ Georgia State University Respiratory Therapy Theses Department of Respiratory Therapy Fall 12-15-2010 The Effect of Different Interfaces on Aerosol Delivery in Simulated
Georgia State University ScholarWorks @ Georgia State University Respiratory Therapy Theses Department of Respiratory Therapy Fall 12-15-2010 The Effect of Different Interfaces on Aerosol Delivery in Simulated
How to Use Inhaled Medications for Asthma and COPD
 How to Use Inhaled Medications for Asthma and COPD This information is not intended to diagnose health problems or to take the place of medical advice or care you receive from your physician or other health
How to Use Inhaled Medications for Asthma and COPD This information is not intended to diagnose health problems or to take the place of medical advice or care you receive from your physician or other health
8/13/11. RSPT 1410 Humidity & Aerosol Therapy Part 3. Humidification Equipment. Aerosol Therapy
 1 RSPT 1410 Humidity & Aerosol Therapy Part 3 Wilkins: Chapter 35, p. 775-799 Cairo: Chapter 4, p. 88-143 2 Humidification Equipment A humidifier is a device that adds molecular liquid (e.g. water vapor)
1 RSPT 1410 Humidity & Aerosol Therapy Part 3 Wilkins: Chapter 35, p. 775-799 Cairo: Chapter 4, p. 88-143 2 Humidification Equipment A humidifier is a device that adds molecular liquid (e.g. water vapor)
I. Subject: Continuous Aerosolization of Bronchodilators
 I. Subject: Continuous Aerosolization of Bronchodilators II. Indications: A. Acute airflow obstruction in which treatment with an aerosolized bronchodilator is desired for an extended period of time, i.e.
I. Subject: Continuous Aerosolization of Bronchodilators II. Indications: A. Acute airflow obstruction in which treatment with an aerosolized bronchodilator is desired for an extended period of time, i.e.
Patricia KP Burnell Inhalation Product Development
 Patricia KP Burnell Inhalation Product Development Inhaled products: types, development The critical parameters In-vitro testing Ex-vivo testing What dose? Product Development: drug medicine Safety and
Patricia KP Burnell Inhalation Product Development Inhaled products: types, development The critical parameters In-vitro testing Ex-vivo testing What dose? Product Development: drug medicine Safety and
