Clinical Policy Title: Phototherapy and photochemotherapy (PUVA) for skin conditions
|
|
|
- Philip Steven Arnold
- 5 years ago
- Views:
Transcription
1 Clinical Policy Title: Phototherapy and photochemotherapy (PUVA) for skin conditions Clinical Policy Number: Effective Date: October 1, 2015 Initial Review Date: May 20, 2015 Most Recent Review Date: June 22, 2017 Next Review Date: June 2018 Policy contains: Phototherapy and photochemotherapy. Psoralen ultraviolet A (PUVA). Related policies: None. ABOUT THIS POLICY: Prestige Health Choice has developed clinical policies to assist with making coverage determinations. Prestige Health Choice s clinical policies are based on guidelines from established industry sources, such as the Centers for Medicare & Medicaid Services (CMS), state regulatory agencies, the American Medical Association (AMA), medical specialty professional societies, and peer-reviewed professional literature. These clinical policies along with other sources, such as plan benefits and state and federal laws and regulatory requirements, including any state- or plan-specific definition of medically necessary, and the specific facts of the particular situation are considered by Prestige Health Choice when making coverage determinations. In the event of conflict between this clinical policy and plan benefits and/or state or federal laws and/or regulatory requirements, the plan benefits and/or state and federal laws and/or regulatory requirements shall control. Prestige Health Choice s clinical policies are for informational purposes only and not intended as medical advice or to direct treatment. Physicians and other health care providers are solely responsible for the treatment decisions for their patients. Prestige Health Choice s clinical policies are reflective of evidence-based medicine at the time of review. As medical science evolves, Prestige Health Choice will update its clinical policies as necessary. Prestige Health Choice s clinical policies are not guarantees of payment. Coverage policy Prestige Health Choice considers the use of phototherapy and photochemotherapy (PUVA) to be clinically proven and, therefore, medically necessary for the following skin conditions after conventional therapies have failed: Severe refractory atopic dermatitis/eczema. Eosinophilic folliculitis and other pruritic eruptions of HIV infection. Mycosis fungoides/sezary syndrome (cutaneous T-cell lymphoma). Severe lichen planus. Photodermatoses. Pityriasis lichenoides. Pityriasis rosea. Prurigo nodularis. Psoriasis. 1
2 Vitiligo. Prestige Health Choice considers the use of phototherapy at home to be investigational and, therefore, not medically necessary. Limitations: Coverage determinations are subject to benefit limitations and exclusions as delineated by the state Medicaid authority. The Florida Medicaid website may be accesses at PUVA treatments for the above conditions are limited to two to three times per week for 23 weeks and one treatment every one to three weeks thereafter if the patient s condition improves. If there is not adequate improvement after two months, the treatment is not considered medically necessary thereafter. All other uses of PUVA are not medically necessary, including, but not limited to: Keratosis follicularis. Lichen amyloidosis. Lichen myxedematosus. Melasma. Low skin tolerance for sunlight. Alternative covered services: Biologic systemic agents, nonbiologic systemic agents, and phototherapy including broadband (BB-UVB) and narrowband (NB-UVB). Background Ultraviolet (UV) light a cause of sunburns, wrinkles and skin cancer can be used in a medical setting as therapy for certain hard-to-treat skin problems and other medical conditions. The main forms of ultraviolet light are ultraviolet A (UVA) and ultraviolet B (UVB). Psoralen ultraviolet A (PUVA) is a topical treatment of disease by exposure to light at a specific portion of the solar spectrum 320 to 400 nanometers in wavelength. Psoralens are chemicals found in plants that can absorb UV light. PUVA treatment for various skin conditions typically involves administration of an oral drug (e.g., methoxypsoralen) followed by the UV portion 45 to 60 minutes later. Other forms of PUVA include: Topical PUVA, with subsequent PUVA exposure. Bath PUVA, which is not approved and rarely used in the United States. 2
3 Paint PUVA, used locally on palms and plantar surfaces of the feet with 8-methoxypsoralen ointment or lotion applied directly to lesions. Soak PUVA in which the area is immersed in a basin of water containing 8-methoxypsoralen. Originally PUVA was developed for psoriasis, a relatively common skin disorder. It is also used for conditions such as vitiligo and mycosis fungoides (the most common type of T-cell lymphoma). While mild psoriasis can often be controlled by topical medications, severe cases often require treatments involving UV light exposures. Before initiating PUVA therapy, other types of treatment should be discussed with the patient. The potential for PUVA to increase the risk of skin cancer, especially when treating psoriasis, should also be discussed. Persons at elevated risk for skin cancer from PUVA include children and persons with a genetic predisposition, a history of skin cancer or a history of at least 150 prior PUVA treatments. Toxicity to PUVA includes etythema, pruritus, xerosis, irregular pigmentation and gastrointestinal symptoms. Most toxicity can be avoided by altering or dividing the dose. There is an elevated (and dosedependent) risk of nonmelanoma skin cancer from PUVA. Whether PUVA raises the risk of melanoma is controversial. When administered to pregnant women, PUVA has been associated with a rise in lowweight births, but not congenital anomalies. An expert panel concluded that PUVA is contraindicated for patients with lupus erythematosus, porphyria or xeroderma pigmentation (Menter, 2010). Caution should be exercised for patients with skin types I and II who tend to burn easily; with a history of arsenic intake; with a likelihood of requiring cyclosporin or methotrexate with previous ionizing radiation therapy; or with a history of melanoma or nonmelanoma skin cancer. Searches Prestige Health Choice searched PubMed and the databases of: UK National Health Services Centre for Reviews and Dissemination. Agency for Healthcare Research and Quality s National Guideline Clearinghouse and other evidence-based practice centers. The Centers for Medicare & Medicaid Services (CMS). We conducted searches on April 24, Search terms were: Phototherapy" (MeSH), "Photochemotherapy" (MeSH), PUVA therapy, UVA, UV-B, and PUVA therapy home. We included: Systematic reviews, which pool results from multiple studies to achieve larger sample sizes and greater precision of effect estimation than in smaller primary studies. Systematic reviews use predetermined transparent methods to minimize bias, effectively treating the review as a scientific endeavor, and are thus rated highest in evidence-grading hierarchies. Guidelines based on systematic reviews. 3
4 Economic analyses, such as cost-effectiveness, and benefit or utility studies (but not simple cost studies), reporting both costs and outcomes sometimes referred to as efficiency studies which also rank near the top of evidence hierarchies. Findings PUVA-related guidelines are often specific to a patient s condition, e.g.: A 2014 practice guideline by the American Academy of Dermatology on dermatitis treatment recommended phototherapy as a second-line treatment if emollients, topical steroid, and calcineurin inhibitors have failed, and that phototherapy may be considered for home use if patients are unable to receive the treatment in an office setting (Sidbury, 2014). A 2012 guideline on psoriasis from the National Institute for Health and Clinical Excellence (NICE) suggests offering NB-UVB phototherapy to psoriasis patients whose condition cannot be controlled with topical treatments alone, but recommends not using any type of phototherapy as maintenance therapy (NICE, 2012). A 2016 review of guidelines for psoriasis concludes that NB-UVB is an effective treatment option for psoriasis (Mehta, 2016). A 2012 guideline on alopecia areata from the British Association of Dermatologists recommends against PUVA use due to potentially serious side-effects and inadequate evidence of efficacy (Messenger, 2012). A 2016 guideline on mycosis fungoindes and Sezary syndrome, for which ultraviolet light is often used, suggests a more refined guideline based on patient stage, centers, and in combination with other agents in practice and clinical trials (Olsen, 2016). Psoriasis is the condition most studied for phototherapy outcomes. A systematic review of 29 articles (n=675) of persons with palmoplantar pustular psoriasis found that phototherapy, ciclosporin, and topical corticosteroids each controlled the disease, with PUVA having greater efficacy than UVB therapy ( Sevrain, 2014). Another meta-analysis of psoriasis (23 studies, n=765) also found PUVA to be more efficacious than non-larger targeted UV-B phototherapy, although both treatments had positive outcomes (Almutawa, 2015). PUVA s superiority to NB-UVB was also upheld in a 2012 meta-analysis of 29 trials (n=773), and accomplished these results in fewer sessions (Archier, 2012). A 2013 Cochrane review of 13 trials (n=662) found the PUVA-UVB comparison to be hampered by heterogenous evidence, and could not make a definitive conclusion on which was more effective (Chen, 2013). Phototherapy is generally found to work better as part of combination treatments, rather than monotherapy, in psoriasis patients (Bailey, 2012). Another systematic review of 41 trials (n=2416) found that PUVA was more effective than NB-UVB as a monotherapy, and NB-UVB worked better than BB-UVB and bath PUVA in treating adults with moderate to severe psoriasis (Almutawa, 2013). A systematic review of 21 randomized controlled trials (RCTs) including 961 patients determined that NB-UVB and UV-A1 phototherapy in moderate to severe dermatitis were helpful, but data on PUVA use 4
5 and phototherapy in children are scarce (Perez-Ferriols, 2015). Another systematic review of 19 studies (n=905) found that UVA1 and NB-UVB were the most effective treatments for reducing signs and symptoms in dermatitis (Garritsen, 2014). Findings from 19 systematic reviews have determined that NB-UVB can be used for chronic atopic eczema, and UVA used for acute eczema (Williams, 2008). A recent meta-analysis of 38 studies of persons with vitiligo compared NB-UVB phototherapy (n=1201) to PUVA phototherapy (n=227). After six and 12 months of treatment, the UV-B group had more at least mild responses (74.2 and 75.0 percent) than did the PUVA group (51.4 and 61.6 percent). Marked responses were more common in the face and neck (44.2 percent) compared to the trunk (26.1) and the extremities (17.3) after six months of UV-B phototherapy (Bae, 2017). A literature review found that combination therapies for vitiligo, compared to monotherapy, was more effective, especially when phototherapy was included (Bacigalupi, 2012). A systematic review determined NB-UVB had fewer side effects and was marginally better than PUVA for vitiligo, and that (along with topical corticosteroids), offers the greatest benefits of any vitiligo treatment (Whitton, 2015). Mycosis fungoides is the most common cutaneous T-cell lymphoma, for which conventional therapy is not always effective. A review of 20 papers documents that photodynamic therapy is a promising and well-tolerated option for treating localized lesions, with excellent cosmetic outcomes (Xue, 2017). PUVA and NB-UVB monotherapy were found to be effective first-line interventions for mycosis fungoides; the effectiveness for PUVA either as maintenance therapy or combined with drugs as first-line therapy is uncertain, but may be beneficial for relapse and late-stage disease (Dogra, 2015). PUVA is usually administered in the outpatient setting, but this treatment is also available for home use. The costs of home therapy are considerably lower, in part because a home light box costs $3,000 to $7,000, compared to $15,000 or more for clinical units (Hayes, 2012). Yertzer (2009) reported that firstyear costs of PUVA to patients were $2,590 compared to $3,040 for office use; per-treatment costs to insurers were $5 for home use and $76 for office use. Research has yet to demonstrate the efficacy of home phototherapy, which was used for years despite lack of a consensus on efficacy (Koek, 2006). Raipara (2010) found home NB-UVB was as safe, effective, and cost-effective as outpatient treatment and was more convenient and generated higher satisfaction (Raipara, 2010). One study of home-based phototherapy found NB-UVB to be safer than PUVA (Lapolla, 2011). Regular skin examinations by a dermatologist should be performed as PUVA home treatments are conducted. But a Cochrane review failed to support or refute home-based phototherapy for nonhemolytic jaundice in infants over 37 weeks gestation (Malwade, 2014). The issue of whether home phototherapy use is safe and effective remains unresolved. A review of 60 publications on psoriasis treatments was not able to generate a comparable costeffectiveness analysis of various methods, including phototherapy (Gutknecht, 2016), as did another analysis from 2015 (Hamilton, 2015). 5
6 Policy updates: A total of six guidelines/other and 12 peer-reviewed references were added to this policy in Summary of clinical evidence: Citation Bae (2017) Content, Methods, Recommendations Key points: Comparison of phototherapy modes for vitiligo Meta-analysis of 35 studies (n=1428) comparing narrow band UV-B (n=1201) and UVA (n=227) phototherapy for vitiligo. At 6 and 12 months after therapy, UV-B had higher percent of patients with at least a mild response (74.2 and 75.0), compared to UVA (51.4 and 61.6). Percent of patients with marked responses was 44.2% in the face and neck, 26.1% on the trunk, and 17.3% on the extremeties. Almutawa (2015) PUVA, UVB and photodynamic therapy (PDT) for psoriasis Dogra (2015) Key points: Systematic review and meta-analysis of six RCTs and 17 case series. The primary outcome was 75% reduction in severity score from baseline. Overall quality: low with high risk of bias. Small sample size, study heterogeneity. PUVA had a statistically nonsignificant (P = 0.183) advantage over targeted UVB. The pooled effect estimate of topical PUVA, targeted UVB and PDT were 77%, 61% and 22%, respectively (15-case series). Topical PUVA and targeted UVB phototherapy are effective in treating localized psoriasis. PDT has low efficacy and high percentage of side effects. Key points: Phototherapy for mycosis fungoides (MF) Synthesis of 107 systematic reviews, meta-analyses, national guidelines, RCTs, prospective open label studies and retrospective case series. For early stage MF (stage IA, IB, and IIA): o PUVA is a safe, effective and well-tolerated first-line therapy (Level of evidence [LOE] 1+, grade of recommendation B). o NB-UVB is comparable to PUVA but less robust evidence (LOE 2++, Grade B). o PUVA with methotrexate, bexarotene or interferon-alpha-2b has unclear advantage over monotherapy. o NB-UVB preferred in patients with patches and thin plaques. o PUVA preferred for thick plaques and relapse after initial NB-UVB therapy. For inducing remission, three treatment sessions per week of either PUVA phototherapy or NB-UVB phototherapy until complete remission. In cases of relapse, PUVA monotherapy or PUVA combined with adjuvants like methotrexate and interferon (LOE 2+, Grade B). For late stage MF, above combination therapy may be first-line treatment (LOE 3, grade C). No consensus regarding maintenance therapy with phototherapy once in remission. Routine maintenance PUVA therapy not recommended; reserved for early relapse after initial 6
7 Citation Hamilton (2015) Economic evaluations of psoriasis treatment Lukacs (2015) Generalized granuloma annulare (GGA) Whitton (2015) Cochrane review Interventions for vitiligo Content, Methods, Recommendations course of phototherapy (LOE 2+, Grade B). Bath-water PUVA similar efficacy to oral PUVA and may be alternative in case PUVA cannot be administered (LOE 2-, Grade C). In pediatric MF and in hypopigmented MF, NB-UVB and PUVA may be tried (LOE 3, grade D). Key points: Systematic review of 37 studies reporting 71 comparisons: systemic treatment (45 studies), topical (22), phototherapies (14) and combination (four). Cost effectiveness of all therapies unclear due to variation in settings, perspective and design. Economic evaluations limited by: lack of high-quality short- and long-term head-to-head comparisons of the effectiveness, safety and adherence of different interventions; comparisons of interventions to placebo, with implicit comparisons of different therapies; absence of patient preferences; and barriers/facilitators to treatment. Primary and secondary integrated clinical and economic research is needed. Key points: Systematic review of individual case reports and small, uncontrolled series. No RCTs. Multiple treatment modalities for GGA were reported including topical and systemic steroids, PUVA and drug therapy. Insufficient evidence. Well-designed RCTs needed. Key points: Systematic review of 96 RCTs (4,512 total participants) of all interventions; three RCTs comparing NB-UVB with PUVA eligible for meta-analysis. Overall quality: Low with high risk of bias. NB-UVB has fewer side effects and is marginally better than PUVA. Proportion of participants achieving > 75% repigmentation favored NB-UVB compared to PUVA. NB-UVB group reported less nausea in three studies (N = 156) and erythema in two studies (N = 106), but not itching in two studies (N = 106). Very few studies only assessed children or included segmental vitiligo. There is a need for follow-up studies to assess permanence of repigmentation and high-quality RCTs using standardized measures that address quality of life. References Professional society guidelines/other: Ling TC, Clayton TH, Crawley J, et al. British Association of Dermatologists and British Photodermatology Group guidelines for the safe and effective use of psoralen-ultraviolet A therapy 2015 (Ling, 2016). Menter A, Korman NJ, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Audiol. January 2010; 62(1): Messenger AG, McKillop J, Farrant P, McDonagh AJ, Sladden M. British Association of Dermatologists 7
8 guidelines for the management of alopecia areata Br J Dermatol. 2012;166(5): Mehta D, Lim HW. Ultraviolet B phototherapy for psoriasis: review of practical guidelines. Am J Clin Dermatol. 2016;17(2): Morton C, Szeimies RM, Sidoroff A, et al. European Dermatology Forum Guidelines on topical photodynamic therapy. Eur J Dermatol. 2015;25(4): National Institute for Health and Clinical Excellence (NICE). Psoriasis: the assessment and management of psoriasis. October 2012; 61 p. (NICE clinical guideline; no. 153). Accessed April 24, Olsen EA, Hodak E, Anderson T, et al. Guidelines for phototherapy of mycosis fungoides and Sezary syndrome: a consensus statement of the United States Cutaneous Lymphoma Consortium. J Am Acad Dermatol. 2016;74(1): Sidbury R, Davis DM, Cohen DE, et al. guidelines of care for the management of atopic dermatitis. Section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2): Peer-reviewed references: Almutawa F, Thalib L, Hekman D, et al. Efficacy of localized phototherapy and photodynamic therapy for psoriasis: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2015; 31(1): Almutawa F, Alnomair N, Wang Y, Hamzavi I, Lim HW. Systematic review of UV-based therapy for psoriasis. Am J Clin Dermatol. 2013;14(2): Archier E, Devaux S, Castela E, et al. Efficacy of psoralen UV-A therapy vs. narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2012;26 Suppl 3: Bacigalupi RM, Postolova A, Davis RS. Evidence-based, non-surgical treatments for vitiligo: a review. Am J Clin Dermatol. 2012;13(4): Doi: / Bae JM, Jung HM, Hong BY, et al. Phototherapy for vitiligo: a systematic review and meta-analysis. JAMA Dermatol. March 29, 2017 [Epub ahead of print]. Doi: /jamadermatol Bailey EE, Ference EH, Alikhan A, Hession MT, Armstrong AW. Combination treatments for psoriasis: a systematic review and meta-analysis. Arch Dermatol. 2012; 148(4):
9 Chen X, Yang M, Cheng Y, Liu GJ, Zhang M. Narrow-band ultraviolet B phototherapy versus broad-band ultraviolet B or psoralen-ultraviolet A photochemotherapy for psoriasis. Cochrane Database Syst Rev. 2013; 10: CD Dogra S, Mahajan R. Phototherapy for mycosis fungoides. Indian J Dermatol Venereol Leprol. 2015; 81(2): Garritsen FM, Brouwer MW, Limpens J, Spuls PI. Photo(chemo) therapy in the management of atopic dermatitis: an updated systematic review with implications for practice and research. Br J Dermatol. 2014;170(3): Gutknecht M, Krensel M, Augustin M. Health economic analyses of psoriasis management: a systematic literature search. Arch Dermatol Res. 2016;308(9): Hamilton MP, Ntais D, Griffiths CE, Davies LM. Psoriasis treatment and management - a systematic review of full economic evaluations. Br J Dermatol. 2015; 172(3): Hayes Inc., Hayes Medical Technology Report. Phototherapy for early-stage mycosis fungoides.. Lansdale, Pa. Hayes Inc.; January 30, 2012, annual review. January 16, Koek MB, Buskens E, Bruijnzeel-Koomen CA, Sigurdsson V. Home ultraviolet B phototherapy for psoriasis: discrepancy between literature, guidelines, general opinions and actual use. Results of a literature review, a web search, and a questionnaire among dermatologists. Br J Dermatol. 2006;154(4): Lapolla W, Yentzer BA, Bagel J, Halvorson CR, Feldman SR. A review of phototherapy protocols for psoriasis treatment. J Am Acad Dermatol. 2011; 64(5): Lukacs J, Schliemann S, Elsner P. Treatment of generalized granuloma annulare - a systematic review. J Eur Acad Dermatol Venereol. 2015; 29(8): Malwade US, Jardine LA. Home- versus hospital-based phototherapy for the treatment of nonhaemolytic jaundice I infants at more than 37 weeks gestation. Cochrane Database Syst Rev. 2014;(6):CD Perez-Ferriols A, Aranegui B, Pujol-Montcusi JA, et al. Phototherapy in atopic dermatitis: a systematic review of the literature. Actas Dermosifiliogr. 2015;106(5): Rajpara AN, O'Neill JL, Nolan BV, Yentzer BA, Feldman SR. Review of home phototherapy. Dermatol Online J. 2010; 16(12): 2. 9
10 Sevrain M, Richard MA, Bametche T, et al. Treatment for palmoplantar pustular psoriasis: systematic literature review, evidence-based recommendations and expert opinion. J Eur Acad Dermatol Venereol. 2014;28 Suppl 5: Trott J, Gerber W, Hammes S, Ockenfels HM. The effectiveness of PUVA treatment in severe psoriasis is significantly increased by additional UV 308-nm excimer laser sessions. Eur J Dermatol. 2008; 18(1): Weberschock T, Strametz R, Lorenz M, et al. Interventions for mycosis fungoides. Cochrane Database Syst Rev. 2012; 9: Cd Whitton ME, Pinart M, Batchelor J, et al. Interventions for vitiligo. Cochrane Database Syst Rev. 2015; 2: Cd Williams HC, Grindlay DJ. What s new in atopic eczema? An analysis of the clinical significance of systematic reviews on atopic eczema published in 2006 and Clin Exp Dermatol. 2008;33(6): Xue J, Liu C, Liu Y. Photodynamic therapy as an alternative treatment for relapsed or refractory mycosis fungoides: a systemic review. Photodiagnosis Photodyn Ther. 2017;17: Yertzer BA, Yelverton CB, et al. Paradoxical effects of cost reduction measures in managed care systems for treatment of severe psoriasis. Dermatol Online J. April 15, 2009; 15(4):1. CMS National Coverage Determinations (NCDs): Treatment of Psoriasis. CMS website. Accessed April 21, Local Coverage Determinations (LCDs): L34966 Benign Skin Lesions. CMS website. Accessed April 21, L33918 Laser Treatment for Psoriasis. CMS website. Accessed April 21, Commonly submitted codes Below are the most commonly submitted codes for the service(s)/item(s) subject to this policy. This is not an exhaustive list of codes. Providers are expected to consult the appropriate coding manuals and bill accordingly. 10
11 CPT Code Description Comment Photodynamic therapy by external application of light to destroy premalignant and/or malignant lesions of the skin and adjacent mucosa (eg, lip) by activation of photosensitive drug(s), each phototherapy exposure session Photochemotherapy; psoralens and ultraviolet A (PUVA) Photochemotherapy (Goeckerman and/or PUVA) for severe photoresponsive dermatoses requiring at least 4-8 hours of care under direct supervision of the physician (includes application of medication and dressings) ICD-10 Code Description Comment L20.82 Flexural eczema L20.84 Intrinsic (allergic) eczema L28.0 Lichen simplex chronicus L28.1 Prurigo nodularis L40.0 Psoriasis vulgaris L40.1 Generalized pustular psoriasis L40.2 Acrodermatitis continua L40.3 Pustulosis palmaris et plantaris L40.4 Guttate psoriasis L40.8 Other psoriasis L40.9 Psoriasis, unspecified L41.0 Pityriasis lichenoides et varioliformis acuta L41.1 Pityriasis lichenoides chronica L41.3 Small plaque parapsoriasis L41.4 Large plaque parapsoriasis L41.5 Retiform parapsoriasis L41.8 Other parapsoriasis L41.9 Parapsoriasis, L42 Pityriasis rosea L43.0 Hypertrophic lichen planus L43.1 Bullous lichen planus L43.2 Lichenoid drug reaction L43.3 Subacute (active) lichen planus L43.8 Other lichen planus L43.9 Lichen planus, unspecified L57.8 Other skin L66.1 Lichen planopilaris L66.3 Perifolliculitis capitis abscedens L70.1 Acne vulgaris L70.2 Acne L70.3 Acne tropica L70.4 Infantile acne L70.5 Acne excoriee des jeunes L70.8 Other acne L70.9 Acne, unspecified L73.0 Acne, keloid L73.8 Other specified follicular disorders L80 Vitiligo 11
12 HCPCS Level II Code N/A Description Comment 12
Clinical Policy Title: Phototherapy and photochemotherapy for skin conditions
 Clinical Policy Title: Phototherapy and photochemotherapy for skin conditions Clinical Policy Number: 16.02.04 Effective Date: October 1, 2015 Initial Review Date: May 20, 2015 Most Recent Review Date:
Clinical Policy Title: Phototherapy and photochemotherapy for skin conditions Clinical Policy Number: 16.02.04 Effective Date: October 1, 2015 Initial Review Date: May 20, 2015 Most Recent Review Date:
Photochemotherapy MM /09/2004. HMO; PPO; QUEST Integration June 1, 2016 Section: Medicine Place(s) of Service: Home; Office
 Photochemotherapy Policy Number: Original Effective Date: MM.02.015 11/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration June 1, 2016 Section: Medicine Place(s) of Service:
Photochemotherapy Policy Number: Original Effective Date: MM.02.015 11/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration June 1, 2016 Section: Medicine Place(s) of Service:
Photochemotherapy MM /09/2004. HMO; PPO; QUEST Integration 08/25/2017 Section: Medicine Place(s) of Service: Home; Office
 Photochemotherapy Policy Number: Original Effective Date: MM.02.015 11/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 08/25/2017 Section: Medicine Place(s) of Service:
Photochemotherapy Policy Number: Original Effective Date: MM.02.015 11/09/2004 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 08/25/2017 Section: Medicine Place(s) of Service:
Clinical Policy Title: Phototherapy and photochemotherapy (PUVA) for skin conditions
 Clinical Policy Title: Phototherapy and photochemotherapy (PUVA) for skin conditions Clinical Policy Number: 16.02.04 Effective Date: October 1, 2015 Initial Review Date: May 20, 2015 Most Recent Review
Clinical Policy Title: Phototherapy and photochemotherapy (PUVA) for skin conditions Clinical Policy Number: 16.02.04 Effective Date: October 1, 2015 Initial Review Date: May 20, 2015 Most Recent Review
Phototherapy and Photochemotherapy Treatment (Ultraviolet A [PUVA] and B [UBV])
![Phototherapy and Photochemotherapy Treatment (Ultraviolet A [PUVA] and B [UBV]) Phototherapy and Photochemotherapy Treatment (Ultraviolet A [PUVA] and B [UBV])](/thumbs/89/99382632.jpg) Origination: 09/27/07 Revised: 08/2/17 Annual Review: 11/2/17 Purpose: To provide Phototherapy and Photochemotherapy Treatment (PUVA and UBV) guidelines for the Medical Department staff to reference when
Origination: 09/27/07 Revised: 08/2/17 Annual Review: 11/2/17 Purpose: To provide Phototherapy and Photochemotherapy Treatment (PUVA and UBV) guidelines for the Medical Department staff to reference when
Original Policy Date
 MP 2.01.07 Psoralens with Ultraviolet A (PUVA) Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed by consensus/12:2013 Return to Medical Policy
MP 2.01.07 Psoralens with Ultraviolet A (PUVA) Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed by consensus/12:2013 Return to Medical Policy
Clinical Policy: Phototherapy and Photochemotherapy for Dermatological Conditions Reference Number: CP.MP. 441
 Clinical Policy: Phototherapy and Photochemotherapy for Dermatological Conditions Reference Number: CP.MP. 441 Effective Date: November 2008 Last Review Date: January 2017 See Important Reminder at the
Clinical Policy: Phototherapy and Photochemotherapy for Dermatological Conditions Reference Number: CP.MP. 441 Effective Date: November 2008 Last Review Date: January 2017 See Important Reminder at the
Corporate Medical Policy
 Corporate Medical Policy Ultraviolet Light Therapy in the Home Setting(UVB) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ultraviolet_light_therapy_in_the_home 3/1996 11/2017 11/2018
Corporate Medical Policy Ultraviolet Light Therapy in the Home Setting(UVB) File Name: Origination: Last CAP Review: Next CAP Review: Last Review: ultraviolet_light_therapy_in_the_home 3/1996 11/2017 11/2018
Phototherapy, Photochemotherapy and Photodynamic Therapy for Dermatologic Conditions
 Last Review Date: October 12, 2018 Number: MG.MM.ME.27j Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: October 12, 2018 Number: MG.MM.ME.27j Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Corporate Medical Policy
 Corporate Medical Policy Light Therapy for Dermatologic Conditions File Name: Origination: Last CAP Review: Next CAP Review: Last Review: light_therapy_for_dermatologic_conditions 5/2012 11/2017 11/2018
Corporate Medical Policy Light Therapy for Dermatologic Conditions File Name: Origination: Last CAP Review: Next CAP Review: Last Review: light_therapy_for_dermatologic_conditions 5/2012 11/2017 11/2018
Phototherapy, Photochemotherapy and Photodynamic Therapy for Dermatologic Conditions
 Last Review Date: September 21, 2017 Number: MG.MM.ME.27iv2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Last Review Date: September 21, 2017 Number: MG.MM.ME.27iv2 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider must submit to EmblemHealth
Light Therapy for Psoriasis Protocol Medical Benefit Effective Date Next Review Date Preauthorization Review Dates Preauthorization is required.
 Protocol Light Therapy for Psoriasis (20147) Medical Benefit Effective Date: 07/01/16 Next Review Date: 03/18 Preauthorization Yes Review Dates: 03/16, 03/17 Preauthorization is required. The following
Protocol Light Therapy for Psoriasis (20147) Medical Benefit Effective Date: 07/01/16 Next Review Date: 03/18 Preauthorization Yes Review Dates: 03/16, 03/17 Preauthorization is required. The following
PUVA: Shall we still use it for psoriasis in 2019?
 PUVA: Shall we still use it for psoriasis in 2019? Ben Stoff MD, MA Associate Professor Emory Department of Dermatology Phototherapy: F003 March 1, 2019 DISCLOSURE OF RELEVANT RELATIONSHIPS WITH INDUSTRY
PUVA: Shall we still use it for psoriasis in 2019? Ben Stoff MD, MA Associate Professor Emory Department of Dermatology Phototherapy: F003 March 1, 2019 DISCLOSURE OF RELEVANT RELATIONSHIPS WITH INDUSTRY
Clinical Policy: Laser Therapy for Skin Conditions Reference Number: CP.MP.123 Last Review Date: 08/17
 Clinical Policy: Laser Therapy for Skin Conditions Reference Number: CP.MP.123 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Clinical Policy: Laser Therapy for Skin Conditions Reference Number: CP.MP.123 Last Review Date: 08/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory
Original Policy Date
 MP 2.01.58 Light Therapy for Vitiligo Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Created with literature search/12:2013 Return to Medical Policy
MP 2.01.58 Light Therapy for Vitiligo Medical Policy Section Medicine Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Created with literature search/12:2013 Return to Medical Policy
Clinical Policy Title: Cardiac rehabilitation
 Clinical Policy Title: Cardiac rehabilitation Clinical Policy Number: 04.02.02 Effective Date: September 1, 2013 Initial Review Date: February 19, 2013 Most Recent Review Date: February 6, 2018 Next Review
Clinical Policy Title: Cardiac rehabilitation Clinical Policy Number: 04.02.02 Effective Date: September 1, 2013 Initial Review Date: February 19, 2013 Most Recent Review Date: February 6, 2018 Next Review
Narrow-band UVB PHOTOTHERAPY for Skin Diseases
 Narrow-band UVB PHOTOTHERAPY for Skin Diseases By Dr. Manal Bosseila Cairo University, Egypt HISTORICAL ASPECT In 1978: Irradiation cabin with broad band UVB tubes was introduced for psoriasis & uremic
Narrow-band UVB PHOTOTHERAPY for Skin Diseases By Dr. Manal Bosseila Cairo University, Egypt HISTORICAL ASPECT In 1978: Irradiation cabin with broad band UVB tubes was introduced for psoriasis & uremic
Laser, Light Therapy, and Cryotherapy for Acne Vulgaris Non-Pharmacologic Treatment of Rosacea
 2.01.47 Light Therapy for Psoriasis Section 2.0 Medicine Subsection Effective Date October 31, 2014 Original Policy Date June 13, 2001 Next Review Date October 2015 Description Plaque psoriasis, also called
2.01.47 Light Therapy for Psoriasis Section 2.0 Medicine Subsection Effective Date October 31, 2014 Original Policy Date June 13, 2001 Next Review Date October 2015 Description Plaque psoriasis, also called
A Retrospective Study on the Risk of Non-Melanoma Skin Cancer in PUVA and Narrowband UVB Treated Patients
 Volume 1, Issue 3 Research Article A Retrospective Study on the Risk of Non-Melanoma Skin Cancer in PUVA and Narrowband UVB Treated Patients Darukarnphut P, Rattanakaemakorn P *, Rajatanavin N Division
Volume 1, Issue 3 Research Article A Retrospective Study on the Risk of Non-Melanoma Skin Cancer in PUVA and Narrowband UVB Treated Patients Darukarnphut P, Rattanakaemakorn P *, Rajatanavin N Division
Light Therapy for Psoriasis. Description
 Subject: Light Therapy for Psoriasis Page: 1 of 11 Last Review Status/Date: June 2015 Light Therapy for Psoriasis Description Light therapy for psoriasis includes both targeted phototherapy and photochemotherapy
Subject: Light Therapy for Psoriasis Page: 1 of 11 Last Review Status/Date: June 2015 Light Therapy for Psoriasis Description Light therapy for psoriasis includes both targeted phototherapy and photochemotherapy
Clinical Policy Title: Psoriasis dermatology treatment
 Clinical Policy Title: Psoriasis dermatology treatment Clinical Policy Number: 16.02.06 Effective Date: October 1, 2016 Initial Review Date: September 17, 2016 Most Recent Review Date: September 21, 2017
Clinical Policy Title: Psoriasis dermatology treatment Clinical Policy Number: 16.02.06 Effective Date: October 1, 2016 Initial Review Date: September 17, 2016 Most Recent Review Date: September 21, 2017
Clinical Policy Title: Zoster (shingles) vaccine
 Clinical Policy Title: Zoster (shingles) vaccine Clinical Policy Number: 18.02.10 Effective Date: June 1, 2018 Initial Review Date: April 10, 2018 Most Recent Review Date: May 1, 2018 Next Review Date:
Clinical Policy Title: Zoster (shingles) vaccine Clinical Policy Number: 18.02.10 Effective Date: June 1, 2018 Initial Review Date: April 10, 2018 Most Recent Review Date: May 1, 2018 Next Review Date:
Light Therapy for Psoriasis and Eczema
 Light Therapy for Psoriasis and Eczema Policy Number: 2.01.47 Last Review: 5/2018 Origination: 5/2006 Next Review: 5/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
Light Therapy for Psoriasis and Eczema Policy Number: 2.01.47 Last Review: 5/2018 Origination: 5/2006 Next Review: 5/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage
MEDICAL POLICY SUBJECT: LIGHT AND LASER THERAPIES FOR DERMATOLOGIC CONDITIONS
 MEDICAL POLICY SUBJECT: LIGHT AND LASER THERAPIES FOR PAGE: 1 OF: 9 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product (including
MEDICAL POLICY SUBJECT: LIGHT AND LASER THERAPIES FOR PAGE: 1 OF: 9 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product (including
Clinical Policy Title: Vitiligo dermatology treatment
 Clinical Policy Title: Vitiligo dermatology treatment Clinical Policy Number: 16.02.08 Effective Date: June 1, 2017 Initial Review Date: April 19, 2017 Most Recent Review Date: April 10, 2018 Next Review
Clinical Policy Title: Vitiligo dermatology treatment Clinical Policy Number: 16.02.08 Effective Date: June 1, 2017 Initial Review Date: April 19, 2017 Most Recent Review Date: April 10, 2018 Next Review
A systematic review of treatments for severe psoriasis Griffiths C E, Clark C M, Chalmers R J, Li Wan Po A, Williams H C
 A systematic review of treatments for severe psoriasis Griffiths C E, Clark C M, Chalmers R J, Li Wan Po A, Williams H C Authors' objectives To compare the effectiveness of currently available treatments
A systematic review of treatments for severe psoriasis Griffiths C E, Clark C M, Chalmers R J, Li Wan Po A, Williams H C Authors' objectives To compare the effectiveness of currently available treatments
Comparison of the narrow band UVB versus systemic corticosteroids in the treatment of lichen planus: A randomized clinical trial
 Received: 10.7.2011 Accepted: 5.12.2011 Original Article Comparison of the narrow band UVB versus systemic corticosteroids in the treatment of lichen planus: A randomized clinical trial Fariba Iraji, 1
Received: 10.7.2011 Accepted: 5.12.2011 Original Article Comparison of the narrow band UVB versus systemic corticosteroids in the treatment of lichen planus: A randomized clinical trial Fariba Iraji, 1
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 Ultraviolet Light Therapy for Skin Conditions Page 1 of 17 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Ultraviolet Light Therapy for Skin Conditions Professional
Ultraviolet Light Therapy for Skin Conditions Page 1 of 17 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Ultraviolet Light Therapy for Skin Conditions Professional
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Original Issue Date (Created): July 1, 2002 Most Recent Review Date (Revised): March 25, 2014 Effective Date: June 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Clinical Policy Title: Intralesional steroid injection for acne
 Clinical Policy Title: Intralesional steroid injection for acne Clinical Policy Number: 16.02.07 Effective Date: June 1, 2017 Initial Review Date: April 19, 2017 Most Recent Review Date: May 19, 2017 Next
Clinical Policy Title: Intralesional steroid injection for acne Clinical Policy Number: 16.02.07 Effective Date: June 1, 2017 Initial Review Date: April 19, 2017 Most Recent Review Date: May 19, 2017 Next
Phototherapy for Psoriasis. Henry W. Lim, MD Chairman and C.S. Livingood Chair Department of Dermatology Henry Ford Hospital, Detroit, MI, USA
 Phototherapy for Psoriasis Henry W. Lim, MD Chairman and C.S. Livingood Chair Department of Dermatology Henry Ford Hospital, Detroit, MI, USA Disclosure Investigator: Clinuvel Estée Lauder Ferndale Incyte
Phototherapy for Psoriasis Henry W. Lim, MD Chairman and C.S. Livingood Chair Department of Dermatology Henry Ford Hospital, Detroit, MI, USA Disclosure Investigator: Clinuvel Estée Lauder Ferndale Incyte
Name of Policy: Phototherapy for the Treatment of Skin Disorders
 Name of Policy: Phototherapy for the Treatment of Skin Disorders Policy #: 301 Latest Review Date: April 2014 Category: Medical/DME Policy Grade: B Background: As a general rule, benefits are payable under
Name of Policy: Phototherapy for the Treatment of Skin Disorders Policy #: 301 Latest Review Date: April 2014 Category: Medical/DME Policy Grade: B Background: As a general rule, benefits are payable under
Medical Policy. MP Light Therapy for Psoriasis
 Medical Policy MP 2.01.47 BCBSA Ref. Policy: 2.01.47 Last Review: 12/27/2017 Effective Date: 12/27/2017 Section: Medicine Related Policies 2.01.44 Dermatologic Applications of Photodynamic Therapy 2.01.86
Medical Policy MP 2.01.47 BCBSA Ref. Policy: 2.01.47 Last Review: 12/27/2017 Effective Date: 12/27/2017 Section: Medicine Related Policies 2.01.44 Dermatologic Applications of Photodynamic Therapy 2.01.86
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Psoriasis: the management of psoriasis 1.1 Short title Psoriasis 2 The remit The Department of Health has asked NICE: 'to produce
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Psoriasis: the management of psoriasis 1.1 Short title Psoriasis 2 The remit The Department of Health has asked NICE: 'to produce
Clinical Policy Title: Strep testing
 Clinical Policy Title: Strep testing Clinical Policy Number: 07.01.09 Effective Date: December 1, 2017 Initial Review Date: October 19, 2017 Most Recent Review Date: November 16, 2017 Next Review Date:
Clinical Policy Title: Strep testing Clinical Policy Number: 07.01.09 Effective Date: December 1, 2017 Initial Review Date: October 19, 2017 Most Recent Review Date: November 16, 2017 Next Review Date:
Clinical Policy Title: Dermabrasion and chemical peels
 Clinical Policy Title: Dermabrasion and chemical peels Clinical Policy Number: 16.02.09 Effective Date: August 1, 2017 Initial Review Date: July 20, 2017 Most Recent Review Date: August 17, 2017 Next Review
Clinical Policy Title: Dermabrasion and chemical peels Clinical Policy Number: 16.02.09 Effective Date: August 1, 2017 Initial Review Date: July 20, 2017 Most Recent Review Date: August 17, 2017 Next Review
Clinical Policy Title: Measurement of serum antibodies to infliximab and adalimumab
 Clinical Policy Title: Measurement of serum antibodies to infliximab and adalimumab Clinical Policy Number: 01.01.03 Effective Date: January 1, 2016 Initial Review Date: September 16, 2015 Most Recent
Clinical Policy Title: Measurement of serum antibodies to infliximab and adalimumab Clinical Policy Number: 01.01.03 Effective Date: January 1, 2016 Initial Review Date: September 16, 2015 Most Recent
Clinical Policy Title: Genicular nerve block
 Clinical Policy Title: Genicular nerve block Clinical Policy Number: 14.01.10 Effective Date: October 1, 2017 Initial Review Date: September 21, 2017 Most Recent Review Date: October 19, 2017 Next Review
Clinical Policy Title: Genicular nerve block Clinical Policy Number: 14.01.10 Effective Date: October 1, 2017 Initial Review Date: September 21, 2017 Most Recent Review Date: October 19, 2017 Next Review
Clinical Policy Title: Computerized gait analysis
 Clinical Policy Title: Computerized gait analysis Clinical Policy Number: 15.01.01 Effective Date: October 1, 2014 Initial Review Date: May 21, 2014 Most Recent Review Date: May 1, 2018 Next Review Date:
Clinical Policy Title: Computerized gait analysis Clinical Policy Number: 15.01.01 Effective Date: October 1, 2014 Initial Review Date: May 21, 2014 Most Recent Review Date: May 1, 2018 Next Review Date:
Use of light for the treatment of skin diseases
 An Update on At-Home UVB Phototherapy At-home options increase accessibility to phototherapy, which is effective and generally safe for psoriasis management. By Joseph Bikowski, MD Use of light for the
An Update on At-Home UVB Phototherapy At-home options increase accessibility to phototherapy, which is effective and generally safe for psoriasis management. By Joseph Bikowski, MD Use of light for the
Table of Contents: Part 1 Medical Dermatology. Chapter 1 Acneiform Disorders. Acne. Acne Vulgaris. Pomade Acne. Steroid Acne
 Table of Contents: Part 1 Medical Dermatology Chapter 1 Acneiform Disorders Acne Acne Vulgaris Pomade Acne Steroid Acne Infantile Acne Pediatric Perspectives Neonatal Acne (Acne Neonatorum) Pediatric Perspectives
Table of Contents: Part 1 Medical Dermatology Chapter 1 Acneiform Disorders Acne Acne Vulgaris Pomade Acne Steroid Acne Infantile Acne Pediatric Perspectives Neonatal Acne (Acne Neonatorum) Pediatric Perspectives
SCIENTIFIC PAPER ABSTRACT
 SCIENTIFIC PAPER ABSTRACT Vitiligo Treatment with Monochromatic Excimer Light and Tacrolimus: Results of an Open Randomized Controlled Study Nistico` S., Chiricozzi A., M.D., Rosita Saraceno R., Schipani
SCIENTIFIC PAPER ABSTRACT Vitiligo Treatment with Monochromatic Excimer Light and Tacrolimus: Results of an Open Randomized Controlled Study Nistico` S., Chiricozzi A., M.D., Rosita Saraceno R., Schipani
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden
 Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
The utilization of phototherapy in the department of dermatology, Hospital Kuala Lumpur: A 5-year audit
 ORIGINAL ARTICLE The utilization of phototherapy in the department of dermatology, Hospital Kuala Lumpur: A 5-year audit Vaani Valerie Visuvanathan, AdvMDerm 1, Min Moon Tang, AdvMDerm 2, Li Lian Tan,
ORIGINAL ARTICLE The utilization of phototherapy in the department of dermatology, Hospital Kuala Lumpur: A 5-year audit Vaani Valerie Visuvanathan, AdvMDerm 1, Min Moon Tang, AdvMDerm 2, Li Lian Tan,
Clinical Policy Title: Ketamine for treatment-resistant depression
 Clinical Policy Title: Ketamine for treatment-resistant depression Clinical Policy Number: 00.02.13 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: January
Clinical Policy Title: Ketamine for treatment-resistant depression Clinical Policy Number: 00.02.13 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: January
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden
 Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Clinical Policy Title: Genetic testing for G1691A polymorphism factor V Leiden Clinical Policy Number: 05.01.03 Effective Date: January 1, 2016 Initial Review Date: July 15, 2015 Most Recent Review Date:
Follow this and additional works at: Part of the Skin and Connective Tissue Diseases Commons
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2016 Is Narrowband UVB Phototherapy in Combination
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2016 Is Narrowband UVB Phototherapy in Combination
Clinical Policy Title: Abdominal aortic aneurysm screening
 Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: June 5, 2018 Next
Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: June 5, 2018 Next
Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening
 Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening Clinical Policy Number: 01.01.02 Effective Date: April 1, 2015 Initial Review Date: January 21, 2015 Most Recent
Clinical Policy Title: Fluorescence in situ hybridization for cervical cancer screening Clinical Policy Number: 01.01.02 Effective Date: April 1, 2015 Initial Review Date: January 21, 2015 Most Recent
Clinical Policy Title: Breast cancer index genetic testing
 Clinical Policy Title: Breast cancer index genetic testing Clinical Policy Number: 02.01.22 Effective Date: January 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: October 19, 2016
Clinical Policy Title: Breast cancer index genetic testing Clinical Policy Number: 02.01.22 Effective Date: January 1, 2017 Initial Review Date: October 19, 2016 Most Recent Review Date: October 19, 2016
Name of Policy: Phototherapy for the Treatment of Skin Disorders
 Name of Policy: Phototherapy for the Treatment of Skin Disorders Policy #: 301 Latest Review Date: December 2017 Category: Medical/DME Policy Grade: B Background: As a general rule, benefits are payable
Name of Policy: Phototherapy for the Treatment of Skin Disorders Policy #: 301 Latest Review Date: December 2017 Category: Medical/DME Policy Grade: B Background: As a general rule, benefits are payable
Topical Immunomodulator Step Therapy Program
 Topical Immunomodulator Step Therapy Program Policy Number: 5.01.557 Last Review: 8/2017 Origination: 7/2013 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) BCBSKC will provide
Topical Immunomodulator Step Therapy Program Policy Number: 5.01.557 Last Review: 8/2017 Origination: 7/2013 Next Review: 8/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) BCBSKC will provide
Pediatric Use: Safety and effectiveness of Ustekinumab (STELARA ) in pediatric patients have not been evaluated.
 Original Issue Date (Created): January 1, 2010 Most Recent Review Date (Revised): January 28, 2014 Effective Date: April 1, 2014 I. POLICY Preauthorization Requirements for Ustekinumab (STELARA ) Note:
Original Issue Date (Created): January 1, 2010 Most Recent Review Date (Revised): January 28, 2014 Effective Date: April 1, 2014 I. POLICY Preauthorization Requirements for Ustekinumab (STELARA ) Note:
It is estimated that about 26,000 new cases of
 Focus on CME at Dalhousie University Set On Soothing Psoriasis A. H. Murray, MD, FRCP(C) Presented at the 76th Annual Dalhousie Refresher Course It is estimated that about 26,000 new cases of psoriasis
Focus on CME at Dalhousie University Set On Soothing Psoriasis A. H. Murray, MD, FRCP(C) Presented at the 76th Annual Dalhousie Refresher Course It is estimated that about 26,000 new cases of psoriasis
Clinical Policy Title: Altered auditory feedback devices for speech dysfluency (stuttering)
 Clinical Policy Title: Altered auditory feedback devices for speech dysfluency (stuttering) Clinical Policy Number: 17.02.02 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent
Clinical Policy Title: Altered auditory feedback devices for speech dysfluency (stuttering) Clinical Policy Number: 17.02.02 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent
The Natural History of Psoriasis and Treatment Goals
 The Natural History of Psoriasis and Treatment Goals Psoriasis Epidemiology Prevalence Affects 2 3% of adult population (>7 million in US) Caucasians: 25% 2.5% African Americans: 1.3% (more likely to have
The Natural History of Psoriasis and Treatment Goals Psoriasis Epidemiology Prevalence Affects 2 3% of adult population (>7 million in US) Caucasians: 25% 2.5% African Americans: 1.3% (more likely to have
Original article Comparative study of psoralen-uvb vs. UVB-alone therapy in the treatment of psoriasis
 Original article Comparative study of psoralen-uvb vs. UVB-alone therapy in the treatment of psoriasis Syed Shamsuddin, *Tahir Saeed Haroon Department of Dermatology, Bolan Medical Complex, Quetta * Department
Original article Comparative study of psoralen-uvb vs. UVB-alone therapy in the treatment of psoriasis Syed Shamsuddin, *Tahir Saeed Haroon Department of Dermatology, Bolan Medical Complex, Quetta * Department
Clinical Policy Title: Vacuum assisted closure in surgical wounds
 Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Clinical Policy Title: Vacuum assisted closure in surgical wounds Clinical Policy Number: 17.03.00 Effective Date: September 1, 2015 Initial Review Date: June 16, 2013 Most Recent Review Date: August 17,
Clinical Policy Title: Abdominal aortic aneurysm screening
 Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: July 20, 2017 Next
Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: July 20, 2017 Next
Ultraviolet phototherapy for cutaneous diseases: a concise review
 INVITED MEDICAL REVIEW : a concise review R Vangipuram 1, SR Feldman 2 (2015) doi:10.1111/odi.12366 2015 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd All rights reserved www.wiley.com 1 University
INVITED MEDICAL REVIEW : a concise review R Vangipuram 1, SR Feldman 2 (2015) doi:10.1111/odi.12366 2015 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd All rights reserved www.wiley.com 1 University
Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions
 Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions Policy Number: Original Effective Date: MM.02.016 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST
Photodynamic Therapy for the Treatment of Actinic Keratoses and Other Skin Lesions Policy Number: Original Effective Date: MM.02.016 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST
Clinical Policy Title: Computerized gait analysis
 Clinical Policy Title: Computerized gait analysis Clinical Policy Number: 15.01.01 Effective Date: October 1, 2014 Initial Review Date: May 21, 2014 Most Recent Review Date: June 22, 2017 Next Review Date:
Clinical Policy Title: Computerized gait analysis Clinical Policy Number: 15.01.01 Effective Date: October 1, 2014 Initial Review Date: May 21, 2014 Most Recent Review Date: June 22, 2017 Next Review Date:
Clinical Policy Title: Home phototherapy for hyperbilirubinemia
 Clinical Policy Title: Home phototherapy for hyperbilirubinemia Clinical Policy Number: 11.02.04 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: August 17,
Clinical Policy Title: Home phototherapy for hyperbilirubinemia Clinical Policy Number: 11.02.04 Effective Date: January 1, 2016 Initial Review Date: August 19, 2015 Most Recent Review Date: August 17,
Clinical Policy Title: Abdominal aortic aneurysm screening
 Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: June 5, 2018 Next
Clinical Policy Title: Abdominal aortic aneurysm screening Clinical Policy Number: 08.01.10 Effective Date: August 1, 2017 Initial Review Date: June 22, 2017 Most Recent Review Date: June 5, 2018 Next
Combination Topical PUVAsol with Methotrexate Versus Methotrexate in the Treatment of Palmoplantar Psoriasis Karn D, KC S
 Combination Topical PUVAsol with Methotrexate Versus Methotrexate in the Treatment of Palmoplantar Psoriasis Karn D, KC S ABSTRACT Background Department of Dermatology Dhulikhel Hospital, Kathmandu University
Combination Topical PUVAsol with Methotrexate Versus Methotrexate in the Treatment of Palmoplantar Psoriasis Karn D, KC S ABSTRACT Background Department of Dermatology Dhulikhel Hospital, Kathmandu University
Efficacy of Concomitant Use of PUVA and Methotrexate in Disease Clearance Time in Plaque Type Psoriasis
 Efficacy of Concomitant Use of PUVA and Methotrexate in Disease Clearance Time in Plaque Type Psoriasis T. Shehzad ( Departments of Dermatology Naval Hospital PNS Shifa, Karachi. ) N. R. Dar ( Departments
Efficacy of Concomitant Use of PUVA and Methotrexate in Disease Clearance Time in Plaque Type Psoriasis T. Shehzad ( Departments of Dermatology Naval Hospital PNS Shifa, Karachi. ) N. R. Dar ( Departments
Narrow band UVB (311 nm), psoralen UVB (311 nm) and PUVA therapy in the treatment of early-stage mycosis fungoides: a right left comparative study
 Photodermatol Photoimmunol Photomed 2005; 21: 281 286 Blackwell Munksgaard Copyright r Blackwell Munksgaard 2005 Narrow band UVB (311 nm), psoralen UVB (311 nm) and therapy in the treatment of early-stage
Photodermatol Photoimmunol Photomed 2005; 21: 281 286 Blackwell Munksgaard Copyright r Blackwell Munksgaard 2005 Narrow band UVB (311 nm), psoralen UVB (311 nm) and therapy in the treatment of early-stage
A Vitiligo Update for Pharmacists: Current Practices and Future Advances
 A Vitiligo Update for Pharmacists: Current Practices and Future Advances Dalal Hammoudi Halat, RPh, MSc, PhD Assistant Professor School of Pharmacy, Lebanese International University Disclosure Dalal Hammoudi
A Vitiligo Update for Pharmacists: Current Practices and Future Advances Dalal Hammoudi Halat, RPh, MSc, PhD Assistant Professor School of Pharmacy, Lebanese International University Disclosure Dalal Hammoudi
Clinical Policy Title: Frenectomy for ankyloglossia
 Clinical Policy Title: Frenectomy for ankyloglossia Clinical Policy Number: 11.03.03 Effective Date: October 1, 2014 Initial Review Date: April 16, 2014 Most Recent Review Date: May 18, 2016 Next Review
Clinical Policy Title: Frenectomy for ankyloglossia Clinical Policy Number: 11.03.03 Effective Date: October 1, 2014 Initial Review Date: April 16, 2014 Most Recent Review Date: May 18, 2016 Next Review
Comparison of PUVA and UVB therapy in moderate plaque psoriasis. Arfan ul Bari, Nadia Iftikhar*, Simeen ber Rahman*
 Comparison of PUVA and UVB therapy in moderate plaque psoriasis Arfan ul Bari et al. Arfan ul Bari, Nadia Iftikhar*, Simeen ber Rahman* Department of Dermatology, PAF Hospital, Sargodha. * Department of
Comparison of PUVA and UVB therapy in moderate plaque psoriasis Arfan ul Bari et al. Arfan ul Bari, Nadia Iftikhar*, Simeen ber Rahman* Department of Dermatology, PAF Hospital, Sargodha. * Department of
Therapeutic Management of Early Cutaneous Mycosis Fungoides
 Therapeutic Management of Early Cutaneous Mycosis Fungoides L Frank Glass, MD Cutaneous Lymphoma Programs H Lee Moffitt Cancer Center and Research Institute George Washington University Dermatology and
Therapeutic Management of Early Cutaneous Mycosis Fungoides L Frank Glass, MD Cutaneous Lymphoma Programs H Lee Moffitt Cancer Center and Research Institute George Washington University Dermatology and
Policy. Number: *Pleasesee amendment forpennsylvaniamedicaidattheend ofthis CPB.
 1 of 49 Number: 0205 Policy *Pleasesee amendment forpennsylvaniamedicaidattheend ofthis CPB. PUVA Aetna considers psoralens and ultraviolet A light (PUVA) treatments medically necessary for the following
1 of 49 Number: 0205 Policy *Pleasesee amendment forpennsylvaniamedicaidattheend ofthis CPB. PUVA Aetna considers psoralens and ultraviolet A light (PUVA) treatments medically necessary for the following
Summary. DOI /j x
 PHOTOBIOLOGY DOI 10.1111/j.1365-2133.2005.06533.x Comparison of the 308-nm excimer laser and a 308-nm excimer lamp with 311-nm narrowband ultraviolet B in the treatment of psoriasis K. Köllner, M.B. Wimmershoff,
PHOTOBIOLOGY DOI 10.1111/j.1365-2133.2005.06533.x Comparison of the 308-nm excimer laser and a 308-nm excimer lamp with 311-nm narrowband ultraviolet B in the treatment of psoriasis K. Köllner, M.B. Wimmershoff,
Maintenance Therapy of Adult Vitiligo with 0.1% Tacrolimus Ointment: A Randomized, Double Blind, Placebo Controlled Study
 ORIGINAL ARTICLE Maintenance Therapy of Adult Vitiligo with 0.1% Tacrolimus Ointment: A Randomized, Double Blind, Placebo Controlled Study Marine Cavalié 1, Khaled Ezzedine 2, Eric Fontas 3, Henri Montaudié
ORIGINAL ARTICLE Maintenance Therapy of Adult Vitiligo with 0.1% Tacrolimus Ointment: A Randomized, Double Blind, Placebo Controlled Study Marine Cavalié 1, Khaled Ezzedine 2, Eric Fontas 3, Henri Montaudié
Clinical Policy Title: Immediate post-concussion assessment and cognitive testing (ImPACT)
 Clinical Policy Title: Immediate post-concussion assessment and cognitive testing (ImPACT) Clinical Policy Number: 09.01.02 Effective Date: September 1, 2013 Initial Review Date: February 18, 2013 Most
Clinical Policy Title: Immediate post-concussion assessment and cognitive testing (ImPACT) Clinical Policy Number: 09.01.02 Effective Date: September 1, 2013 Initial Review Date: February 18, 2013 Most
Follow this and additional works at: Part of the Skin and Connective Tissue Diseases Commons
 Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2014 Is the Addition of a Topical Agent to
Philadelphia College of Osteopathic Medicine DigitalCommons@PCOM PCOM Physician Assistant Studies Student Scholarship Student Dissertations, Theses and Papers 2014 Is the Addition of a Topical Agent to
Review Article. Narrow band UVB phototherapy in dermatology
 Review Article Narrow band UVB phototherapy in dermatology Sunil Dogra, Amrinder Jit Kanwar Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education & Research,
Review Article Narrow band UVB phototherapy in dermatology Sunil Dogra, Amrinder Jit Kanwar Department of Dermatology, Venereology and Leprology, Postgraduate Institute of Medical Education & Research,
ICD 10 Codes. L82.1 Seborrheic Keratosis L82.0 Irritated Seborrheic Keratosis
 Leon H. Kircik M.D. Clinical Associate Professor of Dermatology Indiana University School of Medicine Mount Sinai Medical Center, New York, NY Physicians Skin Care, PLLC Louisville, KY 1 ICD 10 Codes L82.1
Leon H. Kircik M.D. Clinical Associate Professor of Dermatology Indiana University School of Medicine Mount Sinai Medical Center, New York, NY Physicians Skin Care, PLLC Louisville, KY 1 ICD 10 Codes L82.1
Acta Medica Marisiensis 2018;64(1):17-21
 Acta Medica Marisiensis 2018;64(1):17-21 DOI: 10.2478/amma-2018-0003 RESEARCH ARTICLE Clinical and Therapeutic Trial for the Efficacy of Narrow Band - UVB Phototherapy versus Systemic Therapy in Moderate
Acta Medica Marisiensis 2018;64(1):17-21 DOI: 10.2478/amma-2018-0003 RESEARCH ARTICLE Clinical and Therapeutic Trial for the Efficacy of Narrow Band - UVB Phototherapy versus Systemic Therapy in Moderate
Clinical Policy Title: Ear tubes (tympanostomy)
 Clinical Policy Title: Ear tubes (tympanostomy) Clinical Policy Number: 11.03.05 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: September 21, 2017 Next
Clinical Policy Title: Ear tubes (tympanostomy) Clinical Policy Number: 11.03.05 Effective Date: January 1, 2015 Initial Review Date: September 17, 2014 Most Recent Review Date: September 21, 2017 Next
Atopic dermatitis (AD) is a common chronic skin. Phototherapy in the management of atopic dermatitis: a systematic review.
 Photodermatol Photoimmunol Photomed 2007; 23: 106 112 Blackwell Munksgaard r 2007 The Authors Journal compilation r 2007 Blackwell Munksgaard Review article Phototherapy in the management of atopic dermatitis:
Photodermatol Photoimmunol Photomed 2007; 23: 106 112 Blackwell Munksgaard r 2007 The Authors Journal compilation r 2007 Blackwell Munksgaard Review article Phototherapy in the management of atopic dermatitis:
Index. derm.theclinics.com. Note: Page numbers of article titles are in boldface type.
 Note: Page numbers of article titles are in boldface type. A Abatacept for DLE, 493 for SLE, 497 Ablative therapies, localized, for cutaneous T-cell lymphoma, 502 506. See also Cutaneous T-cell lymphoma,
Note: Page numbers of article titles are in boldface type. A Abatacept for DLE, 493 for SLE, 497 Ablative therapies, localized, for cutaneous T-cell lymphoma, 502 506. See also Cutaneous T-cell lymphoma,
A Retrospective Study of 231 Japanese Vitiligo Patients with Special Reference to Phototherapy
 2014;22(1):13-18 CLINICAL ARTILCE A Retrospective Study of 231 Japanese Vitiligo Patients with Special Reference to Phototherapy Akiko Yoshida, Atsushi Takagi, Ayako Ikejima, Hiroshi Takenaka, Tatsuo Fukai,
2014;22(1):13-18 CLINICAL ARTILCE A Retrospective Study of 231 Japanese Vitiligo Patients with Special Reference to Phototherapy Akiko Yoshida, Atsushi Takagi, Ayako Ikejima, Hiroshi Takenaka, Tatsuo Fukai,
Clinical Policy Title: Subtalar arthroereisis (implant)
 Clinical Policy Title: Subtalar arthroereisis (implant) Clinical Policy Number: 14.03.05 Effective Date: April 1, 2017 Initial Review Date: August 17, 2016 Most Recent Review Date: September 21, 2017 Next
Clinical Policy Title: Subtalar arthroereisis (implant) Clinical Policy Number: 14.03.05 Effective Date: April 1, 2017 Initial Review Date: August 17, 2016 Most Recent Review Date: September 21, 2017 Next
11 PROTOCOL NO. 11: Psoracomb (UVB TL01) protocol PROTOCOL NO. 12: MPD protocol 23 Appendix 25
 Classification: Policy Lead Author: Tsui Ling Consultant Dermatologist, Clinical Additional author(s): N/A Authors Division: Dermatology Unique ID: GSCDerm02(13) Issue number: 3 Expiry Date: September
Classification: Policy Lead Author: Tsui Ling Consultant Dermatologist, Clinical Additional author(s): N/A Authors Division: Dermatology Unique ID: GSCDerm02(13) Issue number: 3 Expiry Date: September
Clinical Policy Title: Outpatient diabetes self-management training (DSMT)
 Clinical Policy Title: Outpatient diabetes self-management training (DSMT) Clinical Policy Number: 06.02.02 Effective Date: July 1, 2013 Initial Review Date: April 23, 2013 Most Recent Review Date: March
Clinical Policy Title: Outpatient diabetes self-management training (DSMT) Clinical Policy Number: 06.02.02 Effective Date: July 1, 2013 Initial Review Date: April 23, 2013 Most Recent Review Date: March
Clinical Policy Title: Vitiligo dermatology treatment
 Clinical Policy Title: Vitiligo dermatology treatment Clinical Policy Number: 16.02.08 Effective Date: June 1, 2017 Initial Review Date: April 19, 2017 Most Recent Review Date: May 19, 2017 Next Review
Clinical Policy Title: Vitiligo dermatology treatment Clinical Policy Number: 16.02.08 Effective Date: June 1, 2017 Initial Review Date: April 19, 2017 Most Recent Review Date: May 19, 2017 Next Review
This is a repository copy of Treatment of severe, chronic hand eczema. Results from a UK-wide survey..
 This is a repository copy of Treatment of severe, chronic hand eczema. Results from a UK-wide survey.. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/104211/ Version: Accepted
This is a repository copy of Treatment of severe, chronic hand eczema. Results from a UK-wide survey.. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/104211/ Version: Accepted
An otherwise healthy 12-year-old
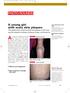 1105 Photo Rounds.finalREV 10/19/05 2:05 PM Page 947 A young girl with scaly skin plaques The patient had numerous thick red plaques on her back and the extensor surfaces of elbows, knees, and forearms
1105 Photo Rounds.finalREV 10/19/05 2:05 PM Page 947 A young girl with scaly skin plaques The patient had numerous thick red plaques on her back and the extensor surfaces of elbows, knees, and forearms
Treating your skin condition with Narrowband ultraviolet B radiation (NB-UVB)
 Treating your skin condition with Narrowband ultraviolet B radiation (NB-UVB) Introduction You have been referred to the Phototherapy department at Colchester General Hospital for a course of narrowband
Treating your skin condition with Narrowband ultraviolet B radiation (NB-UVB) Introduction You have been referred to the Phototherapy department at Colchester General Hospital for a course of narrowband
2. Does the patient have a diagnosis of Crohn s disease? Y N
 Pharmacy Prior Authorization MERC CARE PLA (MEDICAID) Stelara (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign and date. Fax
Pharmacy Prior Authorization MERC CARE PLA (MEDICAID) Stelara (Medicaid) This fax machine is located in a secure location as required by HIPAA regulations. Complete/review information, sign and date. Fax
