RECIST 1.1 Criteria Handout. Medical Imaging. ICONplc.com/imaging
|
|
|
- Baldric McKenzie
- 5 years ago
- Views:
Transcription
1 RECIST 1.1 Criteria Handout Medical Imaging ICONplc.com/imaging
2 2 Contents Basic Paradigm 3 3 Image Acquisition 44 Measurable Lesions 55 Non-Measurable Lesions. 66 Special Lesion Types 77 Baseline Lesion Burden 88 Target Lesions 99 Baseline Documentation 10 Lesions with Prior Local Treatment 11 Evaluating Response at Each Timepoint 12 Target Lesion Evaluation Missing Assessments and Non-evaluable Designation 15 Non-Target Lesion Evaluation 16 New Lesions FDG-PET Recurrence of Lesions Evaluation of Overall Timepoint Response for Patients with 20 Measurable Disease at Baseline 20 Evaluation of Overall Timepoint Response for Patients without Measurable Disease at Baseline Confirmation What Has Changed: RECIST 1.0 RECIST Modifications and Variants 24 References 25
3 3 Basic Paradigm Assess at baseline Look for measurable lesions Select target and non-target lesions Measure target lesions The sum of the target lesions longest diameters represents the tumor burden. Treat patient Follow-up evaluation Measure target lesions Assess non-target lesions and look for new lesions Calculate timepoint response
4 4 Image Acquisition CT Slice thickness 5mm if possible, contiguous IV and oral contrast used (3-phase liver if appropriate) Field of view adjusted to body habitus (include the whole body, out to the skin) MRI Axial T1 and T2, axial T1 post contrast 5mm contiguous slices if possible Use the same machine for all timepoints PET/CT Not required, but may be useful for assessment of new lesions on future timepoints (see page 18) CT portion of PET/CT is usually of lower quality, and should not be used instead of dedicated diagnostic CT. If the CT is of high quality, with oral and IV contrast. Additional information from PET may bias CT assessment Use the same machine for all timepoints Calipers - Reproducibility difficult Include ruler in photograph for skin lesions Chest x-ray (CXR) Use in place of CT (When CT is not available) Ultrasound Not reproducible
5 5 Measurable Lesions Tumor 10 mm in longest diameter (LD) on an axial image on CT or MRI with 5 mm reconstruction interval If slice thickness >5 mm, LD must be at least 2 times the thickness Tumor 20 mm LD by chest x-ray (if clearly defined and surrounded by aerated lung); CT is preferred (even without contrast) Tumor 10 mm LD on clinical evaluation (photo) with electronic calipers; skin photos should include ruler Lesions which cannot be accurately measured with calipers should be recorded as nonmeasurable Lymph nodes 15 mm in short axis on CT (CT slice thickness recommended to be no more than 5 mm) Ultrasound cannot be used to measure lesions
6 6 Non-Measurable Lesions All other definite tumor lesions Masses <10 mm Lymph nodes mm in short axis Leptomeningeal disease Ascites, pleural or pericardial effusion Inflammatory breast disease Lymphangitic involvement of skin or lung Abdominal masses or organomegaly identified by physical exam which cannot be measured by reproducible imaging techniques Benign findings are NEVER included. Also, do not include equivocal ( cannot exclude ) findings
7 7 Special Lesion Types Bone Lesions NMBS, PET scans & plain films can be used to confirm the presence or disappearance of bone lesions, but NOT for measurement. These should be confirmed by CT Bone lesions with identifiable soft tissue components seen on CT or MR can be measurable if the soft tissue component meets the definition above Blastic bone lesions are unmeasurable Cystic Lesions Simple cysts are not included as lesions Cystic metastases may be selected, but prefer to use non-cystic lesions as target Clarify with sponsor as to their acceptability before study start
8 8 Baseline Lesion Burden Lesions Measurable Non-measurable Measurable lesions not selected as target Target Followed qualitatively Non-target Followed qualitatively
9 9 Target Lesions Choose up to 5 lesions in total Up to 2 per organ Add up longest diameters (LD) of non-nodal lesions (axial plane) Add short axis diameters of nodes This is the sum of diameters
10 10 Baseline Documentation Only patients with measurable disease at baseline should be included in protocols where objective tumor response is the primary endpoint. Target Lesions A maximum of five (5) target lesions in total (up to two (2) per organ) Select largest reproducibly measurable lesions If the largest lesion cannot be measured reproducibly, select the next largest lesion which can be Non-Target Lesions It is possible to record multiple non-target lesions involving the same organ as a single item on the ecrf (e.g. multiple enlarged pelvic lymph nodes or multiple liver metastases )
11 1 11 Lesions with Prior Local Treatment Lesions in previously irradiated areas (or areas treated with local therapy) should not be selected as target lesions Conditions in which these lesions may be considered target lesions should be defined in study protocols
12 12 Evaluating Response at Each Timepoint 1. Measure previously chosen target lesions Even if they are no longer the largest 2. Evaluate all previously identified non-target lesions 3. Look for new definite neoplastic lesions 4. Combine lesion responses into timepoint response using appropriate table
13 13 Target Lesion Evaluation Measure LD (axial plane) for each non-nodal target lesion Measure short axis for target lymph nodes Add these measurements to get the sum of the longest diameters If too small to measure, a default value of 5 mm is assigned. If the lesion disappears completely, the measurement is recorded as 0 mm. If an accurate measurement can be obtained, use actual measurement even if smaller than 5 mm as long as the measurement is greater than the slice thickness Splitting or coalescent lesions If a target lesion fragments into multiple smaller lesions, the LDs of all fragmented portions are added to the sum If target lesions merge, the LD of the resulting merged lesion is added to the sum
14 14 Target Lesion Evaluation Response Complete Response (CR) Definition Disappearance of all extranodal target lesions. All pathological lymph nodes must have decreased to <10 mm in short axis Partial Response (PR) At least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum of diameters Progressive Disease (PD) Sum of diameters increased by at least 20% from the smallest value on study (including baseline, if that is the smallest) The sum of diameters must also demonstrate an absolute increase of at least 5mm. (Two lesions increasing from 2 mm to 3 mm, for example, does not qualify.) Stable Disease (SD) Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD
15 15 Missing Assessments and Non-evaluable Designation If all lesions cannot be evaluated due to missing data or poor image quality the patient is inevaluable (NE) at that time point If only a subset of lesions can be evaluated at an assessment, the visit is also considered NE, unless a convincing argument can be made that the contribution of the individual missing lesion(s) would not change the assigned time point response E.g. PD based on other findings
16 16 Non-Target Lesion Evaluation Response Complete Response (CR) Definition Disappearance of all extranodal non-target lesions. All lymph nodes must be non-pathological in size (<10 mm short axis.) Normalization of tumor marker level Non CR/Non PD Persistence of one or more non-target lesion(s) and/or maintenance of tumor marker level above the normal limits Progressive Disease (PD) Unequivocal progression of existing non-target lesions. (Subjective judgement by experienced reader)* *Be cautious when calling progression based on non-target lesions. If target lesions are also present, there must be substantial worsening in nontarget disease overall such that, even in presence of SD or PR in target disease, the disease burden has increased enough to merit discontinuation of therapy. A modest increase in non-target lesions is usually not sufficient to qualify for unequivocal progression status.
17 17 New Lesions Should be unequivocal and not attributable to differences in scanning technique or findings which may not be a tumor Does not have to meet criteria to be measurable If a new lesion is equivocal, continue to next timepoint. If confirmed then, PD is assessed at the date when the lesion was first seen Lesions identified in anatomic locations not scanned at baseline are considered new New lesions on US should be confirmed on CT/MRI
18 18 FDG-PET FDG-PET can be used to help detect progression A positive FDG-PET scan lesion means one with uptake greater than twice that of the surrounding tissue on the attenuation corrected image Negative PET at baseline and positive PET at follow-up: Progressive Disease (PD) based on a new lesion No PET acquired at baseline and positive PET at follow-up: If there is a corresponding new or growing lesion on CT, this is PD. If a later CT confirms appearance or growth of a lesion, the date of PD is the date of the initial positive PET scan No PET acquired at baseline and positive PET at follow-up: If there is a corresponding preexisting lesion on CT that is stable, this is not PD
19 19 19 Recurrence of Lesions For a patient with Stable Disease (SD)/Partial Response (PR), a target lesion which disappears and then reappears will continue to be measured and added to the sum Response will depend on the status of the other lesions For a patient with Complete Response (CR), reappearance of a target lesion would be considered Progressive Disease (PD)
20 20 Evaluation of Overall Timepoint Response for Patients with Measurable Disease at Baseline Target Lesions Non-Target Lesions New Lesions Overall Response CR CR No CR CR Non-CR/Non-PD No PR CR NE No PR PR Non-PD or NE* No PR SD Non-PD or NE* No SD Not all evaluated Non-PD No NE PD Any Yes or No PD Any PD Yes or No PD Any Any Yes PD CR = Complete Response, PR = Partial Response, SD = Stable Disease, PD = Progressive Disease, NE = Inevaluable *When target lesions show SD/PR and some subset of non-target lesions is inevaluable, a careful decision must be made whether to call the overall response at this timepoint SD/PR or NE. This is based on whether the inevaluable lesions, if they showed growth, could cause an overall response of PD in the context of the other lesion responses seen. If the inevaluable non-target lesions comprise a significant proportion of the overall disease burden, the appropriate timepoint response is NE.
21 21 Evaluation of Overall Timepoint Response for Patients without Measurable Disease at Baseline Non-Target New Overall Response CR No CR Non-CR/Non-PD No Non-CR/Non-PD Not all evaluated No NE Unequivocal Progression Yes or No PD Any Yes PD CR = Complete Response PD = Progressive Disease NE = Inevaluable
22 22 Confirmation Confirmation of Partial Response (PR)/Complete Response (CR) is only required for non-randomized trials where response is the primary endpoint In these trials, subsequent confirmation of PR with one interim time point of Inevaluable (NE) is acceptable
23 23 What Has Changed: RECIST 1.0 RECIST 1.1 RECIST 1.0 RECIST 1.1 Tumor Burden 10 Targets (5 per organ) 5 Targets (2 per organ) Lymph Nodes Measure like any other lesion Measure short axis Defined normal size PD Definition 20% increase in SLD 20% increase in sum of diameters 5 mm absolute increase Non-measurable PD Unequivocal More details and examples Confirmation Required for PR and CR Required in non-randomized trial with response as 1º endpoint New Lesions Section on FDG-PET Inevaluable Non-target Lesions Forces timepoint NE Does not force timepoint NE
24 24 Modifications & Variants RECIST is not set in stone Modifications for specific disease processes have been documented Modifications may be made to meet the needs of individual trials
25 25 References Print Version: Response assessment in solid tumours (RECIST): Version 1.1 and Supporting Papers European Journal of Cancer, Volume 45, Issue 2 Jan. 2009: Print. Online Version:
26 To learn more about how Medical Imaging can bring benefits to your clinical developments, contact us: ICON plc Corporate Headquarters South County Business Park Leopardstown, Dublin 18 Ireland T: (IRL) T: (US) F: US Headquarters 2100 Pennbrook Parkway, North Wales, PA 19454, USA Tel: +1 (215) Fax: +1 (215) ICONplc.com/imaging About ICON ICON plc is a global provider of outsourced development solutions and services to the pharmaceutical, biotechnology and medical device industries. The company specialises in the strategic development, management and analysis of programmes that support clinical development. With headquarters in Dublin, Ireland, ICON currently operates from 97 locations in 38 countries and has approximately 13,250 employees. Further information is available at ICONplc.com ICON plc.all rights reserved.
Welcome to the RECIST 1.1 Quick Reference
 Welcome to the RECIST 1.1 Quick Reference *Eisenhauer, E. A., et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. Subject Eligibility
Welcome to the RECIST 1.1 Quick Reference *Eisenhauer, E. A., et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. Subject Eligibility
Radiographic Assessment of Response An Overview of RECIST v1.1
 Radiographic Assessment of Response An Overview of RECIST v1.1 Stephen Liu, MD Georgetown University May 15 th, 2015 Presentation Objectives To understand the purpose of RECIST guidelines To describe the
Radiographic Assessment of Response An Overview of RECIST v1.1 Stephen Liu, MD Georgetown University May 15 th, 2015 Presentation Objectives To understand the purpose of RECIST guidelines To describe the
MEASUREMENT OF EFFECT SOLID TUMOR EXAMPLES
 MEASUREMENT OF EFFECT SOLID TUMOR EXAMPLES Although response is not the primary endpoint of this trial, subjects with measurable disease will be assessed by standard criteria. For the purposes of this
MEASUREMENT OF EFFECT SOLID TUMOR EXAMPLES Although response is not the primary endpoint of this trial, subjects with measurable disease will be assessed by standard criteria. For the purposes of this
Radiologic assessment of response of tumors to treatment. Copyright 2008 TIMC, Matthew A. Barish M.D. All rights reserved. 1
 Radiologic assessment of response of tumors to treatment Copyright 2008 TIMC, Matthew A. Barish M.D. All rights reserved. 1 Objective response assessment is important to describe the treatment effect of
Radiologic assessment of response of tumors to treatment Copyright 2008 TIMC, Matthew A. Barish M.D. All rights reserved. 1 Objective response assessment is important to describe the treatment effect of
Update on RECIST and Staging of Common Pediatric Tumors Ethan A. Smith, MD
 Update on RECIST and Staging of Common Pediatric Tumors Ethan A. Smith, MD Section of Pediatric Radiology C.S. Mott Children s Hospital University of Michigan ethans@med.umich.edu Disclosures No relevant
Update on RECIST and Staging of Common Pediatric Tumors Ethan A. Smith, MD Section of Pediatric Radiology C.S. Mott Children s Hospital University of Michigan ethans@med.umich.edu Disclosures No relevant
SWOG ONCOLOGY RESEARCH PROFESSIONAL (ORP) MANUAL RESPONSE ASSESSMENT LYMPHOMA CHAPTER 11B REVISED: SEPTEMBER 2016
 LYMPHOMA Definitions of Response According to Non Hodgkin s Lymphoma (NHL) Criteria Listed below is the new NCI Lymphoma criteria for evaluation and endpoint definitions for Non Hodgkin s Lymphoma response
LYMPHOMA Definitions of Response According to Non Hodgkin s Lymphoma (NHL) Criteria Listed below is the new NCI Lymphoma criteria for evaluation and endpoint definitions for Non Hodgkin s Lymphoma response
PharmaSUG 2018 Paper AD-02
 PharmaSUG 2018 Paper AD-02 Derivations of Response Status from SDTM Domains using RECIST 1.1 Christine Teng, Merck Research Laboratories, Merck & Co., Inc., Rahway, NJ USA Pang Lei, Merck Research Laboratories,
PharmaSUG 2018 Paper AD-02 Derivations of Response Status from SDTM Domains using RECIST 1.1 Christine Teng, Merck Research Laboratories, Merck & Co., Inc., Rahway, NJ USA Pang Lei, Merck Research Laboratories,
RECIST 1.1 and SWOG Protocol Section 10
 RECIST 1.1 and SWOG Protocol Section 10 Louise Highleyman, Data Coordinator SWOG Statistics and Data Management Center Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 2009: Revised RECIST guideline
RECIST 1.1 and SWOG Protocol Section 10 Louise Highleyman, Data Coordinator SWOG Statistics and Data Management Center Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 2009: Revised RECIST guideline
Update in Lymphoma Imaging
 Update in Lymphoma Imaging Victorine V. Muse, MD Lymphoma Update in Lymphoma Imaging Victorine V Muse, MD Heterogeneous group of lymphoid neoplasms divided into two broad histological categories Hodgkin
Update in Lymphoma Imaging Victorine V. Muse, MD Lymphoma Update in Lymphoma Imaging Victorine V Muse, MD Heterogeneous group of lymphoid neoplasms divided into two broad histological categories Hodgkin
Radiological staging of lung cancer. Shukri Loutfi,MD,FRCR Consultant Thoracic Radiologist KAMC-Riyadh
 Radiological staging of lung cancer Shukri Loutfi,MD,FRCR Consultant Thoracic Radiologist KAMC-Riyadh Bronchogenic Carcinoma Accounts for 14% of new cancer diagnoses in 2012. Estimated to kill ~150,000
Radiological staging of lung cancer Shukri Loutfi,MD,FRCR Consultant Thoracic Radiologist KAMC-Riyadh Bronchogenic Carcinoma Accounts for 14% of new cancer diagnoses in 2012. Estimated to kill ~150,000
How to evaluate tumor response? Yonsei University College of Medicine Kim, Beom Kyung
 How to evaluate tumor response? Yonsei University College of Medicine Kim, Beom Kyung End points in research for solid cancers Overall survival (OS) The most ideal one, but requires long follow-up duration
How to evaluate tumor response? Yonsei University College of Medicine Kim, Beom Kyung End points in research for solid cancers Overall survival (OS) The most ideal one, but requires long follow-up duration
Radiological evaluation, with RECIST criteria, of treatment response of non-microcytic lung cancer. Routine follow-up.
 Original article Anales de Radiología México 2015;14:31-42. Radiological evaluation, with RECIST criteria, of treatment response of non-microcytic lung cancer. Routine follow-up. Cuituny-Romero AK 1, Onofre-Castillo
Original article Anales de Radiología México 2015;14:31-42. Radiological evaluation, with RECIST criteria, of treatment response of non-microcytic lung cancer. Routine follow-up. Cuituny-Romero AK 1, Onofre-Castillo
ROLE OF PET-CT IN BREAST CANCER, GUIDELINES AND BEYOND. Prof Jamshed B. Bomanji Institute of Nuclear Medicine UCL Hospitals London
 ROLE OF PET-CT IN BREAST CANCER, GUIDELINES AND BEYOND Prof Jamshed B. Bomanji Institute of Nuclear Medicine UCL Hospitals London CANCER Key facts Estimated 15.2 million new cases per year in 2015 worldwide
ROLE OF PET-CT IN BREAST CANCER, GUIDELINES AND BEYOND Prof Jamshed B. Bomanji Institute of Nuclear Medicine UCL Hospitals London CANCER Key facts Estimated 15.2 million new cases per year in 2015 worldwide
CA 125 definitions agreed by GCIG November 2005
 CA 125 definitions agreed by GCIG November 2005 The GCIG has agreed criteria for defining response and progression of ovarian carcinoma which use the serum marker CA 125, and the situations where these
CA 125 definitions agreed by GCIG November 2005 The GCIG has agreed criteria for defining response and progression of ovarian carcinoma which use the serum marker CA 125, and the situations where these
FDG PET/CT STAGING OF LUNG CANCER. Dr Shakher Ramdave
 FDG PET/CT STAGING OF LUNG CANCER Dr Shakher Ramdave FDG PET/CT STAGING OF LUNG CANCER FDG PET/CT is used in all patients with lung cancer who are considered for curative treatment to exclude occult disease.
FDG PET/CT STAGING OF LUNG CANCER Dr Shakher Ramdave FDG PET/CT STAGING OF LUNG CANCER FDG PET/CT is used in all patients with lung cancer who are considered for curative treatment to exclude occult disease.
Paradigm shift - from "curing cancer" to making cancer a "chronic disease"
 Current Clinical Practice of Tumor Response Assessment David M. Panicek, MD Department of Radiology Memorial Sloan-Kettering Cancer Center, New York, NY Learning Objectives Review various response assessment
Current Clinical Practice of Tumor Response Assessment David M. Panicek, MD Department of Radiology Memorial Sloan-Kettering Cancer Center, New York, NY Learning Objectives Review various response assessment
Learning Objectives. 1. Identify which patients meet criteria for annual lung cancer screening
 Disclosure I, Taylor Rowlett, DO NOT have a financial interest /arrangement or affiliation with one or more organizations that could be perceived as a real or apparent conflict of interest in the context
Disclosure I, Taylor Rowlett, DO NOT have a financial interest /arrangement or affiliation with one or more organizations that could be perceived as a real or apparent conflict of interest in the context
Cancer will soon become the most common cause of
 From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors Richard L. Wahl 1,2, Heather Jacene 1, Yvette Kasamon 2, and Martin A. Lodge 1 1 Division of Nuclear Medicine,
From RECIST to PERCIST: Evolving Considerations for PET Response Criteria in Solid Tumors Richard L. Wahl 1,2, Heather Jacene 1, Yvette Kasamon 2, and Martin A. Lodge 1 1 Division of Nuclear Medicine,
PET/CT Frequently Asked Questions
 PET/CT Frequently Asked Questions General Q: Is FDG PET specific for cancer? A: No, it is a marker of metabolism. In general, any disease that causes increased metabolism can result in increased FDG uptake
PET/CT Frequently Asked Questions General Q: Is FDG PET specific for cancer? A: No, it is a marker of metabolism. In general, any disease that causes increased metabolism can result in increased FDG uptake
Exercise 15: CSv2 Data Item Coding Instructions ANSWERS
 Exercise 15: CSv2 Data Item Coding Instructions ANSWERS CS Tumor Size Tumor size is the diameter of the tumor, not the depth or thickness of the tumor. Chest x-ray shows 3.5 cm mass; the pathology report
Exercise 15: CSv2 Data Item Coding Instructions ANSWERS CS Tumor Size Tumor size is the diameter of the tumor, not the depth or thickness of the tumor. Chest x-ray shows 3.5 cm mass; the pathology report
Evaluation of Lung Cancer Response: Current Practice and Advances
 Evaluation of Lung Cancer Response: Current Practice and Advances Jeremy J. Erasmus I have no financial relationships, arrangements or affiliations and this presentation will not include discussion of
Evaluation of Lung Cancer Response: Current Practice and Advances Jeremy J. Erasmus I have no financial relationships, arrangements or affiliations and this presentation will not include discussion of
Instructions for Chronic Lymphocytic Leukemia Post-HSCT Data (Form 2113)
 Instructions for Chronic Lymphocytic Leukemia Post-HSCT Data (Form 2113) This section of the CIBMTR Forms Instruction Manual is intended to be a resource for completing the CLL Post-HSCT Data Form. E-mail
Instructions for Chronic Lymphocytic Leukemia Post-HSCT Data (Form 2113) This section of the CIBMTR Forms Instruction Manual is intended to be a resource for completing the CLL Post-HSCT Data Form. E-mail
Soft Tissue Tumour & Sarcoma Imaging Guidelines 2012
 Soft Tissue Tumour & Sarcoma Imaging Guidelines 2012 Version Control This is a controlled document please destroy all previous versions on receipt of a new version. Date Approved: March 2011 reissued April
Soft Tissue Tumour & Sarcoma Imaging Guidelines 2012 Version Control This is a controlled document please destroy all previous versions on receipt of a new version. Date Approved: March 2011 reissued April
Applying the New irecist Guidelines Radiologic and Clinical Trial Considerations
 Applying the New irecist Guidelines Radiologic and Clinical Trial Considerations Author: K. Luby, Mint Medical www.irecist.com irecist 1 : Guidelines for response criteria for use in trials testing immunotherapeutics
Applying the New irecist Guidelines Radiologic and Clinical Trial Considerations Author: K. Luby, Mint Medical www.irecist.com irecist 1 : Guidelines for response criteria for use in trials testing immunotherapeutics
Assessment of Efficacy and Immune Related RECIST criteria
 Assessment of Efficacy and Immune Related RECIST criteria Dr Kenneth O Byrne Princess Alexandra Hospital and Queensland University of Technology, Brisbane, Australia & Trinity College, Dublin, Ireland
Assessment of Efficacy and Immune Related RECIST criteria Dr Kenneth O Byrne Princess Alexandra Hospital and Queensland University of Technology, Brisbane, Australia & Trinity College, Dublin, Ireland
Recommendations for cross-sectional imaging in cancer management, Second edition
 www.rcr.ac.uk Recommendations for cross-sectional imaging in cancer management, Second edition Carcinoma of unknown primary origin (CUP) Faculty of Clinical Radiology www.rcr.ac.uk Contents Carcinoma of
www.rcr.ac.uk Recommendations for cross-sectional imaging in cancer management, Second edition Carcinoma of unknown primary origin (CUP) Faculty of Clinical Radiology www.rcr.ac.uk Contents Carcinoma of
Staging Colorectal Cancer
 Staging Colorectal Cancer CT is recommended as the initial staging scan for colorectal cancer to assess local extent of the disease and to look for metastases to the liver and/or lung Further imaging for
Staging Colorectal Cancer CT is recommended as the initial staging scan for colorectal cancer to assess local extent of the disease and to look for metastases to the liver and/or lung Further imaging for
Measure #405: Appropriate Follow-up Imaging for Incidental Abdominal Lesions National Quality Strategy Domain: Effective Clinical Care
 Measure #405: Appropriate Follow-up Imaging for Incidental Abdominal Lesions National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS, REGISTRY DESCRIPTION:
Measure #405: Appropriate Follow-up Imaging for Incidental Abdominal Lesions National Quality Strategy Domain: Effective Clinical Care 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: CLAIMS, REGISTRY DESCRIPTION:
performed to help sway the clinician in what the appropriate diagnosis is, which can substantially alter the treatment of management.
 Hello, I am Maura Polansky at the University of Texas MD Anderson Cancer Center. I am a Physician Assistant in the Department of Gastrointestinal Medical Oncology and the Program Director for Physician
Hello, I am Maura Polansky at the University of Texas MD Anderson Cancer Center. I am a Physician Assistant in the Department of Gastrointestinal Medical Oncology and the Program Director for Physician
CDISC Journey in Solid Tumor using RECIST 1.1 Kevin Lee PhUSE conference Oct 14th, 2013
 CDISC Journey in Solid Tumor using RECIST 1.1 Kevin Lee PhUSE conference Oct 14th, 2013 Disclaimer Any views or opinions presented in this presentation are solely those of the author and do not necessarily
CDISC Journey in Solid Tumor using RECIST 1.1 Kevin Lee PhUSE conference Oct 14th, 2013 Disclaimer Any views or opinions presented in this presentation are solely those of the author and do not necessarily
Comparison of RECIST version 1.0 and 1.1 in assessment of tumor response by computed tomography in advanced gastric cancer
 Original Article Comparison of RECIST version 1.0 and 1.1 in assessment of tumor response by computed tomography in advanced gastric cancer Gil-Su Jang 1 *, Min-Jeong Kim 2 *, Hong-Il Ha 2, Jung Han Kim
Original Article Comparison of RECIST version 1.0 and 1.1 in assessment of tumor response by computed tomography in advanced gastric cancer Gil-Su Jang 1 *, Min-Jeong Kim 2 *, Hong-Il Ha 2, Jung Han Kim
Revised RECIST Guideline Version 1.1: What Oncologists Want to Know and What Radiologists Need to Know
 Special rticle Pictorial Essay Nishino et al. Revised REIST Guideline Special rticle Pictorial Essay Downloaded from www.ajronline.org by 46.3.203.167 on 11/20/17 from IP address 46.3.203.167. opyright
Special rticle Pictorial Essay Nishino et al. Revised REIST Guideline Special rticle Pictorial Essay Downloaded from www.ajronline.org by 46.3.203.167 on 11/20/17 from IP address 46.3.203.167. opyright
Radiological assessment of neoadjuvent chemotherapy for breast cancer
 XV th Balkan Congress of Radiology Budapest, Hungary, October 12 15, 2017 Radiological assessment of neoadjuvent chemotherapy for breast cancer V. Bešlagić C l i n i c o f R a d i o l o g y, U n i v e
XV th Balkan Congress of Radiology Budapest, Hungary, October 12 15, 2017 Radiological assessment of neoadjuvent chemotherapy for breast cancer V. Bešlagić C l i n i c o f R a d i o l o g y, U n i v e
10 most frequently made mistakes with RECIST 1.1: how Radiologist can fail - and how to avoid them
 10 most frequently made mistakes with RECIST 1.1: how Radiologist can fail - and how to avoid them Poster No.: C-1689 Congress: ECR 2014 Type: Educational Exhibit Authors: M. Kekelidze, P. Lodise, M. Tozakidou,
10 most frequently made mistakes with RECIST 1.1: how Radiologist can fail - and how to avoid them Poster No.: C-1689 Congress: ECR 2014 Type: Educational Exhibit Authors: M. Kekelidze, P. Lodise, M. Tozakidou,
Analysis of Oncology Studies for Programmers and Statisticians
 PharmaSUG 2018 DS06 Analysis of Oncology Studies for Programmers and Statisticians Kevin Lee, Clindata Insight, Moraga, CA ABSTRACT Compared to other therapeutic studies, oncology studies are generally
PharmaSUG 2018 DS06 Analysis of Oncology Studies for Programmers and Statisticians Kevin Lee, Clindata Insight, Moraga, CA ABSTRACT Compared to other therapeutic studies, oncology studies are generally
FAST FACTS Eligibility Reviewed and Verified By MD/DO/RN/LPN/CRA Date MD/DO/RN/LPN/CRA Date Consent Version Dated
 Page 1 of 5 COG-AEWS1221: Randomized Phase 3 Trial Evaluating the Addition of the IGF-1R Monoclonal Antibody Ganitumab (AMG 479, NSC# 750008, IND# 120449) to Multiagent Chemotherapy for Patients with Newly
Page 1 of 5 COG-AEWS1221: Randomized Phase 3 Trial Evaluating the Addition of the IGF-1R Monoclonal Antibody Ganitumab (AMG 479, NSC# 750008, IND# 120449) to Multiagent Chemotherapy for Patients with Newly
Integrating Imaging Criteria Into Trial Endpoints
 Integrating Imaging Criteria Into Trial Endpoints Gregory Goldmacher, MD, PhD, MBA Sr. Director, Translational Biomarkers Merck Research Laboratories CBI Imaging In Clinical Trials 2017 Preview Purpose
Integrating Imaging Criteria Into Trial Endpoints Gregory Goldmacher, MD, PhD, MBA Sr. Director, Translational Biomarkers Merck Research Laboratories CBI Imaging In Clinical Trials 2017 Preview Purpose
The oncology specific domains TU, TR and RS: What to know as a statistical analyst
 Your statistical health consultancy CHM staying healthy CRO getting healthy TEMP WORK team health The oncology specific domains TU, TR and RS: What to know as a statistical analyst PhUSE EU Connect 2018,
Your statistical health consultancy CHM staying healthy CRO getting healthy TEMP WORK team health The oncology specific domains TU, TR and RS: What to know as a statistical analyst PhUSE EU Connect 2018,
Prof. Dr. NAGUI M. ABDELWAHAB,M.D.; MARYSE Y. AWADALLAH, M.D. AYA M. BASSAM, Ms.C.
 Role of Whole-body Diffusion MR in Detection of Metastatic lesions Prof. Dr. NAGUI M. ABDELWAHAB,M.D.; MARYSE Y. AWADALLAH, M.D. AYA M. BASSAM, Ms.C. Cancer is a potentially life-threatening disease,
Role of Whole-body Diffusion MR in Detection of Metastatic lesions Prof. Dr. NAGUI M. ABDELWAHAB,M.D.; MARYSE Y. AWADALLAH, M.D. AYA M. BASSAM, Ms.C. Cancer is a potentially life-threatening disease,
Programming LYRIC Response in Immunomodulatory Therapy Trials Yang Wang, Seattle Genetics, Inc., Bothell, WA
 PharmaSUG 2017 Paper BB12 Programming LYRIC Response in Immunomodulatory Therapy Trials Yang Wang, Seattle Genetics, Inc., Bothell, WA ABSTRACT The LYmphoma Response to Immunomodulatory therapy Criteria
PharmaSUG 2017 Paper BB12 Programming LYRIC Response in Immunomodulatory Therapy Trials Yang Wang, Seattle Genetics, Inc., Bothell, WA ABSTRACT The LYmphoma Response to Immunomodulatory therapy Criteria
8/10/2016. PET/CT Radiomics for Tumor. Anatomic Tumor Response Assessment in CT or MRI. Metabolic Tumor Response Assessment in FDG-PET
 PET/CT Radiomics for Tumor Response Evaluation August 1, 2016 Wei Lu, PhD Department of Medical Physics www.mskcc.org Department of Radiation Oncology www.umaryland.edu Anatomic Tumor Response Assessment
PET/CT Radiomics for Tumor Response Evaluation August 1, 2016 Wei Lu, PhD Department of Medical Physics www.mskcc.org Department of Radiation Oncology www.umaryland.edu Anatomic Tumor Response Assessment
ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015. ClinicalTrials.gov ID: NCT
 ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015 ClinicalTrials.gov ID: NCT01378962 Study Identification Unique Protocol ID: ML25514 Brief Title: A Study
ClinicalTrials.gov Protocol and Results Registration System (PRS) Receipt Release Date: 09/30/2015 ClinicalTrials.gov ID: NCT01378962 Study Identification Unique Protocol ID: ML25514 Brief Title: A Study
GUIDELINES FOR CANCER IMAGING Lung Cancer
 GUIDELINES FOR CANCER IMAGING Lung Cancer Greater Manchester and Cheshire Cancer Network Cancer Imaging Cross-Cutting Group April 2010 1 INTRODUCTION This document is intended as a ready reference for
GUIDELINES FOR CANCER IMAGING Lung Cancer Greater Manchester and Cheshire Cancer Network Cancer Imaging Cross-Cutting Group April 2010 1 INTRODUCTION This document is intended as a ready reference for
Phase II Cancer Trials: When and How
 Phase II Cancer Trials: When and How Course for New Investigators August 9-12, 2011 Learning Objectives At the end of the session the participant should be able to Define the objectives of screening vs.
Phase II Cancer Trials: When and How Course for New Investigators August 9-12, 2011 Learning Objectives At the end of the session the participant should be able to Define the objectives of screening vs.
Phase II Cancer Trials: When and How
 Phase II Cancer Trials: When and How Course for New Investigators August 21-23, 2013 Acknowledgment Elizabeth Eisenhauer for some slides! Learning Objectives At the end of the session the participant should
Phase II Cancer Trials: When and How Course for New Investigators August 21-23, 2013 Acknowledgment Elizabeth Eisenhauer for some slides! Learning Objectives At the end of the session the participant should
Dr Sneha Shah Tata Memorial Hospital, Mumbai.
 Dr Sneha Shah Tata Memorial Hospital, Mumbai. Topics covered Lymphomas including Burkitts Pediatric solid tumors (non CNS) Musculoskeletal Ewings & osteosarcoma. Neuroblastomas Nasopharyngeal carcinomas
Dr Sneha Shah Tata Memorial Hospital, Mumbai. Topics covered Lymphomas including Burkitts Pediatric solid tumors (non CNS) Musculoskeletal Ewings & osteosarcoma. Neuroblastomas Nasopharyngeal carcinomas
Breast Cancer. What is breast cancer?
 Scan for mobile link. Breast Cancer Breast cancer is a malignant tumor in or around breast tissue. It usually begins as a lump or calcium deposit that develops from abnormal cell growth. Most breast lumps
Scan for mobile link. Breast Cancer Breast cancer is a malignant tumor in or around breast tissue. It usually begins as a lump or calcium deposit that develops from abnormal cell growth. Most breast lumps
Form 2023 R2.0: Ovarian Cancer Pre-HSCT Data
 Key Fields Sequence Number Date Received: - - CIBMTR Center Number: CIBMTR Recipient ID: Today's Date: - - Date of HSCT for which this form is being completed: - - HSCT type: (check all that apply) Autologous
Key Fields Sequence Number Date Received: - - CIBMTR Center Number: CIBMTR Recipient ID: Today's Date: - - Date of HSCT for which this form is being completed: - - HSCT type: (check all that apply) Autologous
Breast Cancer. What is breast cancer?
 Scan for mobile link. Breast Cancer Breast cancer is a malignant tumor in or around breast tissue. It usually begins as a lump or calcium deposit that develops from abnormal cell growth. Most breast lumps
Scan for mobile link. Breast Cancer Breast cancer is a malignant tumor in or around breast tissue. It usually begins as a lump or calcium deposit that develops from abnormal cell growth. Most breast lumps
I8 COMPLETION INSTRUCTIONS
 The I8 Form is completed for each screening exam at T0, T1, and T2. At T0 (baseline), the I8 Form documents comparison review of the baseline screen (DR Form) with any historical images available. At T1
The I8 Form is completed for each screening exam at T0, T1, and T2. At T0 (baseline), the I8 Form documents comparison review of the baseline screen (DR Form) with any historical images available. At T1
Maria João Cardoso, MD, PhD
 Locally Advanced Breast Cancer Specific Issues in LocorregionalTreatment Surgery, MD, PhD Head Breast Surgeon Breast Unit, Champalimaud Foundation Lisbon, Portugal 1 Conflict of Interest Disclosure No
Locally Advanced Breast Cancer Specific Issues in LocorregionalTreatment Surgery, MD, PhD Head Breast Surgeon Breast Unit, Champalimaud Foundation Lisbon, Portugal 1 Conflict of Interest Disclosure No
PET/CT in Gynaecological Cancers. Stroobants Sigrid, MD, PhD Departement of Nuclear Medicine University Hospital,Antwerp
 PET/CT in Gynaecological Cancers Stroobants Sigrid, MD, PhD Departement of Nuclear Medicine University Hospital,Antwerp Cervix cancer Outline of this talk Initial staging Treatment monitoring/guidance
PET/CT in Gynaecological Cancers Stroobants Sigrid, MD, PhD Departement of Nuclear Medicine University Hospital,Antwerp Cervix cancer Outline of this talk Initial staging Treatment monitoring/guidance
Disclosure. Acknowledgement. What is the Best Workup for Rectal Cancer Staging: US/MRI/PET? Rectal cancer imaging. None
 What is the Best Workup for Rectal Cancer Staging: US/MRI/PET? Zhen Jane Wang, MD Assistant Professor in Residence UC SF Department of Radiology Disclosure None Acknowledgement Hueylan Chern, MD, Department
What is the Best Workup for Rectal Cancer Staging: US/MRI/PET? Zhen Jane Wang, MD Assistant Professor in Residence UC SF Department of Radiology Disclosure None Acknowledgement Hueylan Chern, MD, Department
Pediatric Lymphoma Update from the Children s Oncology Group
 Pediatric Lymphoma Update from the Children s Oncology Group Stephan D. Voss, MD, PhD Department of Radiology Boston Children s Hospital Harvard Medical School Staging Assessment Disclosures None Acknowledgements:
Pediatric Lymphoma Update from the Children s Oncology Group Stephan D. Voss, MD, PhD Department of Radiology Boston Children s Hospital Harvard Medical School Staging Assessment Disclosures None Acknowledgements:
Setting the Scene in Immuno-oncology
 Applying lessons learnt ICONplc.com/oncology Contents General Aspects of Immuno-oncology 3 Immuno-oncology trials for the last 5 years 3 Lessons Learned from I-O Trials 4 Pseudo-Progression and I-O Therapy
Applying lessons learnt ICONplc.com/oncology Contents General Aspects of Immuno-oncology 3 Immuno-oncology trials for the last 5 years 3 Lessons Learned from I-O Trials 4 Pseudo-Progression and I-O Therapy
Breast Cancer PET/CT Imaging Protocol
 Breast Cancer PET/CT Imaging Protocol Scanning Protocol: Patients are scanned from the top of the neck through the pelvis. Arms-up position is used to avoid beam-hardening artifact in the chest and abdomen.
Breast Cancer PET/CT Imaging Protocol Scanning Protocol: Patients are scanned from the top of the neck through the pelvis. Arms-up position is used to avoid beam-hardening artifact in the chest and abdomen.
PLACE LABEL HERE. Radiation Therapy Oncology Group Phase II - SBRT - Medically Inoperable I /II NSCLC Follow-up Form. RTOG Study No.
 Radiation Therapy Oncology Group Phase II - SBRT - Medically Inoperable I /II NSCLC Follow-up Form RTOG Study No. 0813 Case # Name RTOG Patient ID INSTRUCTIONS: Submit this form at the appropriate followup
Radiation Therapy Oncology Group Phase II - SBRT - Medically Inoperable I /II NSCLC Follow-up Form RTOG Study No. 0813 Case # Name RTOG Patient ID INSTRUCTIONS: Submit this form at the appropriate followup
Introduction Pediatric malignancies Changing trends & Radiation burden Radiation exposure from PET/CT Image gently PET & CT modification - PET/CT
 Introduction Pediatric malignancies Changing trends & Radiation burden Radiation exposure from PET/CT Image gently PET & CT modification - PET/CT protocols Tips Leukaemia / lymphoma: ~ 35% acute lymphoblastic
Introduction Pediatric malignancies Changing trends & Radiation burden Radiation exposure from PET/CT Image gently PET & CT modification - PET/CT protocols Tips Leukaemia / lymphoma: ~ 35% acute lymphoblastic
The OUTBACK Trial. Specific CRF Completion Guidelines
 The OUTBACK Trial A Phase III trial of adjuvant chemotherapy following chemoradiation as primary treatment for locally advanced cervical cancer compared to chemoradiation alone (ANZGOG 0902, GOG 0274,
The OUTBACK Trial A Phase III trial of adjuvant chemotherapy following chemoradiation as primary treatment for locally advanced cervical cancer compared to chemoradiation alone (ANZGOG 0902, GOG 0274,
Lung Cancer Staging: The Revised TNM Classification
 Norwegian Society of Thoracic Imaging Oslo, October 2011 Lung Cancer Staging: The Revised TNM Classification Sujal R Desai King s College Hospital, London Lung Cancer The Scale of the Problem Leading cause
Norwegian Society of Thoracic Imaging Oslo, October 2011 Lung Cancer Staging: The Revised TNM Classification Sujal R Desai King s College Hospital, London Lung Cancer The Scale of the Problem Leading cause
Supplementary Appendix
 Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Jänne PA, Yang JC-H, Kim D-W, et al. AZD9291 in EGFR inhibitor
Supplementary Appendix This appendix has been provided by the authors to give readers additional information about their work. Supplement to: Jänne PA, Yang JC-H, Kim D-W, et al. AZD9291 in EGFR inhibitor
8/10/2016. PET/CT for Tumor Response. Staging and restaging Early treatment response evaluation Guiding biopsy
 PET/CT for Tumor Response Evaluation August 4, 2016 Wei Lu, PhD Department of Medical Physics www.mskcc.org Department of Radiation Oncology www.umaryland.edu FDG PET/CT for Cancer Imaging Staging and
PET/CT for Tumor Response Evaluation August 4, 2016 Wei Lu, PhD Department of Medical Physics www.mskcc.org Department of Radiation Oncology www.umaryland.edu FDG PET/CT for Cancer Imaging Staging and
I9 COMPLETION INSTRUCTIONS
 The I9 Form is completed for each screening exam at T0, T1, and T2. At T0 (baseline), the I9 documents comparison review of the baseline screen (C2 Form) with any historical images available. At T1 and
The I9 Form is completed for each screening exam at T0, T1, and T2. At T0 (baseline), the I9 documents comparison review of the baseline screen (C2 Form) with any historical images available. At T1 and
Dr Claire Smith, Consultant Radiologist St James University Hospital Leeds
 Dr Claire Smith, Consultant Radiologist St James University Hospital Leeds Imaging in jaundice and 2ww pathway Image protocol Staging Limitations Pancreatic cancer 1.2.4 Refer people using a suspected
Dr Claire Smith, Consultant Radiologist St James University Hospital Leeds Imaging in jaundice and 2ww pathway Image protocol Staging Limitations Pancreatic cancer 1.2.4 Refer people using a suspected
Imaging in gastric cancer
 Imaging in gastric cancer Gastric cancer remains a deadly disease because of late diagnosis. Adenocarcinoma represents 90% of malignant tumors. Diagnosis is based on endoscopic examination with biopsies.
Imaging in gastric cancer Gastric cancer remains a deadly disease because of late diagnosis. Adenocarcinoma represents 90% of malignant tumors. Diagnosis is based on endoscopic examination with biopsies.
Denominator Criteria (Eligible Cases): Patient encounter during the performance period (CPT): 78300, 78305, 78306, 78315, 78320
 Quality ID #147: Nuclear Medicine: Correlation with Existing Imaging Studies for All Patients Undergoing Bone Scintigraphy National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS
Quality ID #147: Nuclear Medicine: Correlation with Existing Imaging Studies for All Patients Undergoing Bone Scintigraphy National Quality Strategy Domain: Communication and Care Coordination 2018 OPTIONS
Imaging Work-Up of a Neck Mass - Adults & Children
 Disclosures Imaging Work-Up of a Neck Mass - Adults & Children I have nothing to disclose Christine M Glastonbury MBBS Professor of Radiology & Biomedical Imaging Otolaryngology-Head & Neck Surgery and
Disclosures Imaging Work-Up of a Neck Mass - Adults & Children I have nothing to disclose Christine M Glastonbury MBBS Professor of Radiology & Biomedical Imaging Otolaryngology-Head & Neck Surgery and
Streamlined workflow for review and analysis of oncology patients
 Streamlined workflow for review and analysis of oncology patients Philips IntelliSpace Portal Multi-modality Tumor Tracking application (MMTT) Ekta Dharaiya, MS, Philips Healthcare, Cleveland, OH Cancer
Streamlined workflow for review and analysis of oncology patients Philips IntelliSpace Portal Multi-modality Tumor Tracking application (MMTT) Ekta Dharaiya, MS, Philips Healthcare, Cleveland, OH Cancer
بسم هللا الرحمن الرحيم. Prof soha Talaat
 بسم هللا الرحمن الرحيم Ovarian tumors The leading indication for gynecologic surgery. Preoperative characterization of complex solid and cystic adnexal masses is crucial for informing patients about possible
بسم هللا الرحمن الرحيم Ovarian tumors The leading indication for gynecologic surgery. Preoperative characterization of complex solid and cystic adnexal masses is crucial for informing patients about possible
Whole Body MRI. Dr. Nina Tunariu. Prostate Cancer recurrence, progression and restaging
 Whole Body MRI Prostate Cancer recurrence, progression and restaging Dr. Nina Tunariu Consultant Radiology Drug Development Unit and Prostate Targeted Therapies Group 12-13 Janeiro 2018 Evolving Treatment
Whole Body MRI Prostate Cancer recurrence, progression and restaging Dr. Nina Tunariu Consultant Radiology Drug Development Unit and Prostate Targeted Therapies Group 12-13 Janeiro 2018 Evolving Treatment
Imaging Tissue Response to Therapeutic Radiation
 1 Imaging Tissue Response to Therapeutic Radiation Sean P. Frigo, Ph.D. Assistant Professor Department of Human Oncology School of Medicine and Public Health University of Wisconsin Madison Slide 1 1 Based
1 Imaging Tissue Response to Therapeutic Radiation Sean P. Frigo, Ph.D. Assistant Professor Department of Human Oncology School of Medicine and Public Health University of Wisconsin Madison Slide 1 1 Based
PET IMAGING (POSITRON EMISSION TOMOGRAPY) FACT SHEET
 Positron Emission Tomography (PET) When calling Anthem (1-800-533-1120) or using the Point of Care authorization system for a Health Service Review, the following clinical information may be needed to
Positron Emission Tomography (PET) When calling Anthem (1-800-533-1120) or using the Point of Care authorization system for a Health Service Review, the following clinical information may be needed to
Sectional Anatomy Quiz II
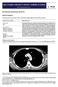 Sectional Anatomy II Rashid Hashmi Rural Clinical School, University of New South Wales, Wagga Wagga, New South Wales, Australia A R T I C L E I N F O Article type: Article history: Received: 3 Aug 2017
Sectional Anatomy II Rashid Hashmi Rural Clinical School, University of New South Wales, Wagga Wagga, New South Wales, Australia A R T I C L E I N F O Article type: Article history: Received: 3 Aug 2017
GROUP 1: Peripheral tumour with normal hilar and mediastinum on staging CT with no disant metastases. Including: Excluding:
 GROUP 1: Including: Excluding: Peripheral tumour with normal hilar and mediastinum on staging CT with no disant metastases Solid pulmonary nodules 8mm diameter / 300mm3 volume and BROCK risk of malignancy
GROUP 1: Including: Excluding: Peripheral tumour with normal hilar and mediastinum on staging CT with no disant metastases Solid pulmonary nodules 8mm diameter / 300mm3 volume and BROCK risk of malignancy
objectives Pitfalls and Pearls in PET/CT imaging Kevin Robinson, DO Assistant Professor Department of Radiology Michigan State University
 objectives Pitfalls and Pearls in PET/CT imaging Kevin Robinson, DO Assistant Professor Department of Radiology Michigan State University To determine the regions of physiologic activity To understand
objectives Pitfalls and Pearls in PET/CT imaging Kevin Robinson, DO Assistant Professor Department of Radiology Michigan State University To determine the regions of physiologic activity To understand
Appendix 5. EFSUMB Newsletter. Gastroenterological Ultrasound
 EFSUMB Newsletter 87 Examinations should encompass the full range of pathological conditions listed below A log book listing the types of examinations undertaken should be kept Training should usually
EFSUMB Newsletter 87 Examinations should encompass the full range of pathological conditions listed below A log book listing the types of examinations undertaken should be kept Training should usually
Full ultrasound breast volumes. Faster scans. Streamlined workflow. ACUSON S2000 Automated Breast Volume Scanner. Answers for life.
 Full ultrasound breast volumes. Faster scans. Streamlined workflow. ACUSON S2000 Automated Breast Volume Scanner Answers for life. 1 ACQUIRE An automated whole breast solution. Reduced acquisition time.
Full ultrasound breast volumes. Faster scans. Streamlined workflow. ACUSON S2000 Automated Breast Volume Scanner Answers for life. 1 ACQUIRE An automated whole breast solution. Reduced acquisition time.
NIH Public Access Author Manuscript AJR Am J Roentgenol. Author manuscript; available in PMC 2011 July 6.
 NIH Public Access Author Manuscript Published in final edited form as: AJR Am J Roentgenol. 2010 September ; 195(3): W221 W228. doi:10.2214/ajr.09.3928. New Response Evaluation Criteria in Solid Tumors
NIH Public Access Author Manuscript Published in final edited form as: AJR Am J Roentgenol. 2010 September ; 195(3): W221 W228. doi:10.2214/ajr.09.3928. New Response Evaluation Criteria in Solid Tumors
Follow-up investigations. Omgo E. Nieweg
 Follow-up investigations Omgo E. Nieweg Systematic literature review follow-up investigations identify few patients with recurrence frequent false positive findings impact on survival unclear Nieweg, Surg
Follow-up investigations Omgo E. Nieweg Systematic literature review follow-up investigations identify few patients with recurrence frequent false positive findings impact on survival unclear Nieweg, Surg
Ovarian Lesion Benign vs Malignant?
 Ovarian Lesion Benign vs Malignant? Michele Keenan 1,2 Bernice Dunne 2 Mary Moran 1 Therese Herlihy 1 1. Radiography and Diagnostic Imaging, School of Medicine, University College Dublin, Ireland 2. Midland
Ovarian Lesion Benign vs Malignant? Michele Keenan 1,2 Bernice Dunne 2 Mary Moran 1 Therese Herlihy 1 1. Radiography and Diagnostic Imaging, School of Medicine, University College Dublin, Ireland 2. Midland
Breast Cancer Diagnosis, Treatment and Follow-up
 Breast Cancer Diagnosis, Treatment and Follow-up What is breast cancer? Each of the body s organs, including the breast, is made up of many types of cells. Normally, healthy cells grow and divide to produce
Breast Cancer Diagnosis, Treatment and Follow-up What is breast cancer? Each of the body s organs, including the breast, is made up of many types of cells. Normally, healthy cells grow and divide to produce
Radiology. General radiology department. X-ray
 The radiology directorate provides a diagnostic, interventional and therapeutic service for its local population, and a tertiary service for the region. It also provides support to some national work such
The radiology directorate provides a diagnostic, interventional and therapeutic service for its local population, and a tertiary service for the region. It also provides support to some national work such
Challenges and Opportunities for In Vivo Imaging in Oncology
 Challenges and Opportunities for In Vivo Imaging in Oncology Daniel C. Sullivan, M.D. Unraveling of the genome and proteome, and advances in biomedical technology are changing the practice of medicine
Challenges and Opportunities for In Vivo Imaging in Oncology Daniel C. Sullivan, M.D. Unraveling of the genome and proteome, and advances in biomedical technology are changing the practice of medicine
Molecular Imaging and Cancer
 Molecular Imaging and Cancer Cancer causes one in every four deaths in the United States, second only to heart disease. According to the U.S. Department of Health and Human Services, more than 512,000
Molecular Imaging and Cancer Cancer causes one in every four deaths in the United States, second only to heart disease. According to the U.S. Department of Health and Human Services, more than 512,000
Recommendations for cross-sectional imaging in cancer management, Second edition
 www.rcr.ac.uk Recommendations for cross-sectional imaging in cancer management, Second edition Renal and adrenal tumours Faculty of Clinical Radiology www.rcr.ac.uk Contents Renal cell carcinoma 3 Clinical
www.rcr.ac.uk Recommendations for cross-sectional imaging in cancer management, Second edition Renal and adrenal tumours Faculty of Clinical Radiology www.rcr.ac.uk Contents Renal cell carcinoma 3 Clinical
Author's response to reviews
 Author's response to reviews Title: Intensive post-operative follow-up of breast cancer patients with tumour markers: CEA, TPA or CA15.3 vs MCA and MCA-CA15.3 vs CEA-TPA-CA15.3 panel in the early detection
Author's response to reviews Title: Intensive post-operative follow-up of breast cancer patients with tumour markers: CEA, TPA or CA15.3 vs MCA and MCA-CA15.3 vs CEA-TPA-CA15.3 panel in the early detection
Association office, Department of Digestive Surgery, Kyoto Prefectural University of Medicine, Kawaramachi, Kamigyo-ku, Kyoto , Japan
 Gastric Cancer (2001) 4: 1 8 Special article 2001 by International and Japanese Gastric Cancer Associations Japanese Classification of Gastric Carcinoma 2nd English Edition Response assessment of chemotherapy
Gastric Cancer (2001) 4: 1 8 Special article 2001 by International and Japanese Gastric Cancer Associations Japanese Classification of Gastric Carcinoma 2nd English Edition Response assessment of chemotherapy
Measuring Response in Solid Tumors: Comparison of RECIST and WHO Response Criteria
 Jpn J Clin Oncol 2003;33(10)533 537 Measuring Response in Solid Tumors: Comparison of RECIST and WHO Response Criteria Joon Oh Park 1, Soon Il Lee 1, Seo Young Song 1, Kihyun Kim 1, Won Seog Kim 1, Chul
Jpn J Clin Oncol 2003;33(10)533 537 Measuring Response in Solid Tumors: Comparison of RECIST and WHO Response Criteria Joon Oh Park 1, Soon Il Lee 1, Seo Young Song 1, Kihyun Kim 1, Won Seog Kim 1, Chul
Radiation exposure from a CT scan A guide for parents
 Diagnostic Imaging Radiation exposure from a CT scan A guide for parents Welcome to McMaster Children s Hospital. This handout will help to answer your questions about CT scans for your child. If you have
Diagnostic Imaging Radiation exposure from a CT scan A guide for parents Welcome to McMaster Children s Hospital. This handout will help to answer your questions about CT scans for your child. If you have
C2 COMPLETION INSTRUCTIONS
 The C2 Form is completed for each screening exam at T0, T1, and T2. The C2 Form is to be completed by each of the following ACRIN-NLST study staff: the research associate (study coordinator), CT technologist,
The C2 Form is completed for each screening exam at T0, T1, and T2. The C2 Form is to be completed by each of the following ACRIN-NLST study staff: the research associate (study coordinator), CT technologist,
Clinical Management Guideline for Small Cell Lung Cancer
 Diagnosis and Staging: Key Points 1. Ensure a CT scan that is
Diagnosis and Staging: Key Points 1. Ensure a CT scan that is
Pre-operative assessment of patients for cytoreduction and HIPEC
 Pre-operative assessment of patients for cytoreduction and HIPEC Washington Hospital Center Washington, DC, USA Ovarian Cancer Surgery New Strategies Bergamo, Italy May 5, 2011 Background Cytoreductive
Pre-operative assessment of patients for cytoreduction and HIPEC Washington Hospital Center Washington, DC, USA Ovarian Cancer Surgery New Strategies Bergamo, Italy May 5, 2011 Background Cytoreductive
Anatomical and Functional MRI of the Pancreas
 Anatomical and Functional MRI of the Pancreas MA Bali, MD, T Metens, PhD Erasme Hospital Free University of Brussels Belgium mbali@ulb.ac.be Introduction The use of MRI to investigate the pancreas has
Anatomical and Functional MRI of the Pancreas MA Bali, MD, T Metens, PhD Erasme Hospital Free University of Brussels Belgium mbali@ulb.ac.be Introduction The use of MRI to investigate the pancreas has
PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES. U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease
 PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease Refer back to original requester with this paperwork and review previous
PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease Refer back to original requester with this paperwork and review previous
Thoracic Imaging: A Case of Metastatic Adenocarcinoma of Unknown Primary
 January 28, 2009 Thoracic Imaging: A Case of Metastatic Adenocarcinoma of Unknown Primary Kristina Mirabeau-Beale, Harvard Medical School Year III Gillian Lieberman, MD Agenda Introduce Patient RS Discuss
January 28, 2009 Thoracic Imaging: A Case of Metastatic Adenocarcinoma of Unknown Primary Kristina Mirabeau-Beale, Harvard Medical School Year III Gillian Lieberman, MD Agenda Introduce Patient RS Discuss
Imaging Decisions Start Here SM
 Owing to its high resolution and wide anatomic coverage, dynamic first-pass perfusion 320-detector-row CT outperforms PET/CT for distinguishing benign from malignant lung nodules, researchers from Japan
Owing to its high resolution and wide anatomic coverage, dynamic first-pass perfusion 320-detector-row CT outperforms PET/CT for distinguishing benign from malignant lung nodules, researchers from Japan
Chapter 6. Hester Gietema Cornelia Schaefer-Prokop Willem Mali Gerard Groenewegen Mathias Prokop. Accepted for publication in Radiology
 Chapter 6 Interscan variability of semiautomated volume measurements in intraparenchymal pulmonary nodules using multidetector-row computed tomography: Influence of inspirational level, nodule size and
Chapter 6 Interscan variability of semiautomated volume measurements in intraparenchymal pulmonary nodules using multidetector-row computed tomography: Influence of inspirational level, nodule size and
Radiological Manifestations of Metastatic Melanoma
 Radiological Manifestations of Metastatic Melanoma Lia Hojman, Universidad de Chile Year VII Our patient: Clinical Presentation A 24 year old male. Former smoker. 8 months ago, he suffered a burn in his
Radiological Manifestations of Metastatic Melanoma Lia Hojman, Universidad de Chile Year VII Our patient: Clinical Presentation A 24 year old male. Former smoker. 8 months ago, he suffered a burn in his
PET/CT in Breast Cancer
 PET/CT in Breast Cancer Rodolfo Núñez Miller, M.D. Nuclear Medicine and Diagnostic Imaging Section Division of Human Health International Atomic Energy Agency Vienna, Austria Overview Introduction Locorregional
PET/CT in Breast Cancer Rodolfo Núñez Miller, M.D. Nuclear Medicine and Diagnostic Imaging Section Division of Human Health International Atomic Energy Agency Vienna, Austria Overview Introduction Locorregional
