Clinical Commissioning Policy: Stereotactic radiosurgery/ radiotherapy for the treatment of pituitary adenomas (all ages)
|
|
|
- Maximilian Rodgers
- 6 years ago
- Views:
Transcription
1 Clinical Commissioning Policy: Stereotactic radiosurgery/ radiotherapy for the treatment of pituitary adenomas (all ages) NHS England Reference: P 1
2 NHS England INFORMATION READER BOX Directorate Medical Operations and Information Specialised Commissioning Nursing Trans. & Corp. Ops. Commissioning Strategy Finance Publications Gateway Reference: Document Purpose Document Name Author Publication Date Target Audience Policy Clinical Commissioning Policy: Stereotactic radiosurgery/ radiotherapy for the treatment of pituitary adenomas Specialised Commissioning Team 20 April 2018 CCG Clinical Leaders, Care Trust CEs, Foundation Trust CEs, Medical Directors, Directors of PH, Directors of Nursing, NHS England Regional Directors, NHS England Directors of Commissioning Operations, Directors of Finance, NHS Trust CEs Additional Circulation List #VALUE! Description Cross Reference Superseded Docs (if applicable) Action Required Routinely Commissioned - NHS England will routinely commission this specialised treatment in accordance with the criteria described in this policy Timing / Deadlines (if applicable) Contact Details for further information Document Status 0 By 00 January 1900 england.specialisedcommissioning@nhs.net 0 0 This is a controlled document. Whilst this document may be printed, the electronic version posted on the intranet is the controlled copy. Any printed copies of this document are not controlled. As a controlled document, this document should not be saved onto local or network drives but should always be accessed from the intranet. 2
3 Clinical Commissioning Policy: Stereotactic radiosurgery/ radiotherapy for the treatment of pituitary adenomas (all ages) First published: April 2018 Prepared by NHS England Specialised Services Clinical Reference Group for Radiotherapy and Specialised Cancer Surgery Published by NHS England, in electronic format only. 3
4 Contents... 3 Policy Statement... 5 Equality Statement... 5 Plain Language Summary Introduction Definitions Aims and Objectives Epidemiology and Needs Assessment Evidence Base Criteria for Commissioning Patient Pathway Governance Arrangements Mechanism for Funding Audit Requirements Documents which have informed this Policy Date of Review References
5 Policy Statement NHS England will commission stereotactic radiosurgery/radiotherapy for the treatment of pituitary adenomas in accordance with the criteria outlined in this document. In creating this policy NHS England has reviewed this clinical condition and the options for its treatment. It has considered the place of this treatment in current clinical practice, whether scientific research has shown the treatment to be of benefit to patients, (including how any benefit is balanced against possible risks) and whether its use represents the best use of NHS resources. This policy document outlines the arrangements for funding of this treatment for the population in England. Equality Statement Promoting equality and addressing health inequalities are at the heart of NHS England s values. Throughout the development of the policies and processes cited in this document, we have: given due regard to the need to eliminate discrimination, harassment and victimisation, to advance equality of opportunity, and to foster good relations between people who share a relevant protected characteristic (as cited under the Equality Act 2010) and those who do not share it; and given regard to the need to reduce inequalities between patients in access to, and outcomes from healthcare services and to ensure services are provided in an integrated way where this might reduce health inequalities. Plain Language Summary About pituitary adenomas The pituitary is a gland about the size of a pea that lies beneath the base of the brain. It releases hormones which affect growth, sexual development, reproduction 5
6 and metabolism. A pituitary adenoma is a benign (non-cancerous) slow growing tumour that arises within the pituitary gland. Most pituitary adenomas occur in the front of the pituitary gland which regulates hormones. There are two main types of pituitary adenoma: Functioning pituitary adenomas which are non-cancerous tumours in the pituitary gland that secrete hormones which cause symptoms. Non-functioning pituitary adenomas which are non-cancerous tumours in the pituitary gland that do not secrete hormones but cause effects as they grow and press onto nearby structures in the brain. The majority of pituitary adenomas will be first treated with surgery. However, sometimes it is not possible to remove the whole tumour with surgery, this means that some of the tumour cells are left behind which is called residual tumour. In addition, sometimes the tumour can return following surgical treatment, this is called recurrent tumour. Both residual and recurrent tumours can grow and continue to cause harmful effects and may require further treatment. This policy relates to the treatment of residual and recurrent pituitary adenomas following first (or primary ) treatment with surgery and where primary treatment with surgery is not medically possible. About current treatments There are currently three treatment options for patients with residual and recurrent pituitary adenomas: further surgery; conventional radiotherapy for larger or diffuse lesions; and SRS/SRT. In addition, there are medical treatments available for functioning pituitary adenomas. SRS/SRT is a current treatment option for some recurrent or residual pituitary adenomas. It can be used in certain highly selected relatively small tumours where primary surgery is not an option. 6
7 About the new treatment SRS/SRT are highly targeted radiation therapies that can be used to treat a wide range of conditions, including pituitary tumours. The benefit of this treatment over further surgery or conventional radiotherapy is that it is possible to give the tumour cells a high dose of radiation while better protecting the surrounding healthy tissue. Treatment with SRS/SRT can also be given in fewer visits to the hospital. What we have decided NHS England has carefully reviewed the evidence to treat pituitary tumours with SRS/SRT. We have concluded that there is enough evidence to make this treatment available. 7
8 1 Introduction This document describes the evidence that has been considered by NHS England in deciding to routinely commission stereotactic radiotherapy (SRT) or stereotactic radiosurgery (SRS) as a treatment option, for adults and children and young people presenting with an adult type tumour, with recurrent or residual pituitary adenomas following primary treatment with surgery and for the primary treatment of pituitary adenomas in cases where surgery is not medically possible. This document also describes the criteria for commissioning, governance arrangements and funding mechanisms. Clinical indication Pituitary adenomas tend to be benign and have a slow growth rate. There are two main types of pituitary adenomas, those that secrete hormones (functioning) causing clinical syndromes of hormone excess such as Cushing s disease, and those that do not secrete hormones (non-functioning). Surgery is the primary treatment for pituitary adenomas, except for prolactinomas, which are functioning pituitary adenomas and which are treated medically. However, residual tumour is common after surgery (Reddy et al 2011). Residual nonfunctioning tumours can start to grow, or grow and continue to secrete hormones in the case of functioning tumours. This may necessitate additional treatment. For patients with confirmed symptomatic residual disease further surgery is possible, but it has increased risk of complications and less favourable clinical outcomes than primary surgery alone. Intervention SRS and SRT will be commissioned by NHS England as a treatment option for the management of residual and recurrent pituitary adenomas (for adults and children presenting with an adult type tumour) following primary treatment with surgery and for those patients that are not able to have surgery as a primary treatment. 8
9 Both SRS/SRT are methods of delivering doses of precisely targeted cranial radiotherapy treatment. The basic principle of the treatment is the targeted elimination of tumour viability to improve local control. As the radiation is precisely focused on the target area this reduces potential toxicity in the surrounding tissues e.g. the optic apparatus. The use of SRS or SRT in the treatment of pituitary adenomas has demonstrated evidence of clinical effectiveness in both tumour and hormonal control. This effectiveness is demonstrated in patients who have had prior therapy as well as those that are treatment naïve. There is also potential to improve patient experience and overall service delivery, in that treatments are accomplished in a single setting or in fewer episodes than conventionally fractioned treatments. For the purposes of this policy, both SRS and SRT are defined as highly conformal radiotherapy treatment to a precisely delineated target volume, delivered using stereotactic localisation techniques. SRS treatment is delivered in a single fraction and SRT must be delivered in two to five fractions. SRT is sometimes referred to as intracranial hypofractionated SRT. 2 Definitions Functioning adenoma is a pituitary tumour that secretes a hormone in excess to cause a recognisable clinical condition (such as Cushing s disease, acromegaly, prolactinomas). Non-functioning adenoma is a pituitary tumour that does not secrete hormones. These pituitary adenomas create clinical symptoms by growing and putting pressure on adjacent structures such as the normal pituitary gland and the optic nerves. Hypofractionated radiation is a treatment in which the total dose of radiation is divided into relatively large doses and intracranial treatments are given in up to 5 fractions over 5 days or less (typically 3-5 fractions over 3-5 days). 9
10 Conventional fractionated radiotherapy - is a treatment in which the total dose of radiation is divided into relatively small doses and treatments are given in fractions daily (usually Monday to Friday). Intracranial - means within the cranial cavity (skull). Prolactinoma is a prolactin producing tumour of the pituitary gland Cushing s disease: a clinical syndrome caused by increased secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary gland (of which a pituitary adenoma is the most common cause) leading to over production of cortisol by the adrenal glands and resulting in Cushing s syndrome. Acromegaly is a disorder caused by excessive production of growth hormone (GH) by the pituitary gland and marked by progressive enlargement of hands, feet, and face. There is also increased risk of cancer. 3 Aims and Objectives This policy considers SRS/SRT as a treatment option for the management of patients with residual and recurrent pituitary adenomas, together with those who are medically unable to have surgery. The objectives were to establish, via an evidence review, the following information: Efficacy, safety and toxicity of the treatment; Identification of patient sub-groups (i.e., functioning and non-functioning tumours) and appropriate clinical criteria; and Cost effectiveness of the treatment. 4 Epidemiology and Needs Assessment Epidemiology Pituitary adenomas are usually benign and grow slowly to exert their harmful effects by pressure on surrounding structures or through hormone secretion. Autopsy studies suggest pituitary tumours are found in 10% of the population, but the clinically relevant incidence is much lower (Kontogeorgos, 1991). 10
11 Non-functioning pituitary adenomas have a prevalence rate of 22.2 per 100,000 (Fernandez, 2010) and are the second largest group of pituitary adenomas after prolactinomas, accounting for 70% of the relevant neurosurgical operations performed each year in the UK. Most non-functioning pituitary adenomas will require primary surgery and, potentially, may also need adjuvant treatment. There are three types of functioning pituitary adenomas; Prolactinomas, which have a prevalence rate of 44.4 per 100,000 (Fernandez, 2010) and secrete prolactin. These are usually small microadenomas, 80% of which are less than 1 cm in diameter; Growth hormone (GH) secreting pituitary adenomas, which cause a condition called Acromegaly and have a prevalence rate of 8.6 per 100,000 (Fernandez, 2010); and Adrenocorticotrophic hormone (ACTH) secreting pituitary adenomas, which cause a condition called Cushing s disease and have a prevalence rate of 1.2 per 100,000 (Fernandez, 2010). Both Cushing s Disease and Acromegaly are rarer conditions associated with higher morbidity and mortality. Needs Assessment Based on the epidemiology, it is estimated that approximately 400 patients per year may be suitable for SRS or SRT. 5 Evidence Base NHS England has concluded that there is sufficient evidence to support the routine commissioning of this treatment for the indication. Study design Fifty-six published studies were identified evaluating the effectiveness and safety of SRS or SRT for recurring and residual tumours. However they tended to be of low to moderate quality. The majority of the papers were retrospective case series ranging in size, baseline characteristics and treatment dosage. There were also a number of 11
12 retrospective non-controlled cohort studies comparing different interventions. There was no randomisation or blinding in any study including the comparison studies. One study evaluated health related quality of life. There were no cost effectiveness studies. Approximately a quarter of studies had more than 100 patients but over half included only 9-40 patients. Baseline characteristics The baseline characteristics of patients differed significantly in terms of tumour volume, tumour functional status and previous treatment. This an important limitation for the comparator studies as these characteristics have been shown to have an effect on both efficacy and safety outcomes. The majority of patients had recurrent or residual pituitary adenoma despite 1 prior treatment. Around 15% of patients were treatment naïve. The majority of these patients were inappropriate for surgery due to medical reasons or the site and size of their tumour. As the results of the studies were presented as whole patient cohorts their outcomes could not be analysed separately. Patients underwent SRS or fractionated SRT. The main outcomes measured were tumour growth/recurrence, control or remission of hormone secretion, progression- free survival and safety. As there are no internationally recognised standards of outcome reporting for pituitary adenomas, studies used different measures of effectiveness. While there are studies that have compared different radiation modalities, none directly compared SRS or SRT with repeat surgery and so judgements on efficacy and safety will be limited. The picture is further complicated by the fact that patients in most of the studies had a varied clinical history ranging from those with a primary presentation to those who have had multiple surgical interventions and previous fractionated radiotherapy. Most studies analysed these patients together and thus outcomes could not be split by baseline characteristics. Clinical effectiveness 12
13 In non-functioning tumours tumour control (a composite of complete response, tumour shrinkage and stable tumour) was reported as 93.4% at median 36 months in the largest case series (Sheehan et al 2013). Van den Burgh et al (2007) also demonstrated statistically significant (p<0.001) improved tumour control in 76 patients who had previously had surgery who were then treated with SRS. The comparator group (n=28) received no intervention. Tumour control ranged from 75 to 100% in all other reporting studies. In functioning tumours hormonal control (normalisation of hormone levels with or without medication) was reported as 67% in the largest (Lee et al 2014) study and ranged from 0 to 100% in all other reporting studies. An analysis of efficacy and safety by tumour type was undertaken but was limited by studies often pooling outcomes rather than reporting them by specific tumour type. Additionally sub-groups by tumour type were often small so led to a wide range of outcomes when comparing all case series. SRS appears to be effective in controlling the growth of recurrent/residual pituitary tumours and has a role in hormonal remission in the short to medium term. There is evidence that SRS is more effective in achieving desired outcomes in nonfunctioning than functioning tumours. This is mostly due to the need to control hormonal secretion as well as tumour size in functioning tumours. SRS also appears to have variable effectiveness depending on the functioning tumour type ACTHsecreting tumours had the best response followed by GH-secreting and PRLsecreting tumours. There were too few Nelson s and LH/FSH-secreting tumours reviewed to make a judgement on efficacy. Follow up The length of follow up also varied and ranged between 33 and 152 months. The studies with the shorter follow up may not have had sufficient time to record tumour response/recurrence, hormonal response/relapse or radiation-induced adverse events. Adverse events 13
14 The main adverse events identified were hypopituitarism (ranging between 0 to 39% in functional tumours and 0 to 38% in functioning tumours) and new/deteriorating visual dysfunction (ranging from 0% to 21% for non-functioning tumours and from 0% to 9% for functioning tumours). Other adverse events such as stroke, transient ischaemic attack (TIA), and hypothyroidism were infrequently reported. The data from the comparative studies is too limited to make any firm conclusions about efficacy relative to other treatment but suggests a reduced rate of adverse events in SRS/SRT compared to conventional fractionated radiotherapy. Additionally while SRS and SRT seem to have comparable efficacy, hypopituitarism may be higher in SRT as compared to SRS. However given the low numbers of patients, limited quality of these studies and lack of statistical testing this may not be a true difference. It is difficult to discern whether some of the adverse events reported are attributable to the disease or to SRS/SRT, or whether both contributed to some degree. For example both SRS/SRT (radiation-induced toxicity) and disease progression (pressure effects of tumour) can lead to new visual deficit. Conclusion The published evidence on SRS and SRT for treatment of residual/recurrent pituitary adenoma consists of retrospective case series, prospective cohort studies and nonrandomised/controlled comparative studies. The major drawback of these types of study is the difficulty in understanding the true efficacy of an intervention due to a lack of control over factors that influence the outcomes being measured. The evidence suggests a role for SRS in effective tumour control (non-functioning pituitary adenomas) and to a lesser degree, hormonal control (functioning pituitary adenomas). The evidence for SRT suggests that it only has a role in the management of non-functioning tumours. A lack of randomised control trials mean it is difficult to make direct comparisons with standard care. The evidence suggests lower rates of adverse events in SRS/SRT compared to conventional fractionated radiotherapy but a lack of randomised control trials mean it is difficult to make direct comparisons with standard care. 14
15 6 Criteria for Commissioning All patients being considered for SRS or SRT must undergo prior assessment by the local multidisciplinary pituitary multi-disciplinary team (MDT) (with a core membership as defined in the NICE Improving Outcomes Guidance and described within the SRS/SRT service specification) and the SRS/SRT MDT, sometimes these may be combined. In addition, where appropriate, patients should be discussed in Teenage and Young Adult (TYA) MDTs, as well as the SRS/SRT and pituitary MDTs. Inclusion criteria Treatment with SRS (a single fraction of stereotactic treatment) should be considered as a treatment option for the following groups: People with residual or recurrent non-functioning pituitary adenomas that continue to grow following surgical intervention and require further treatment; People with residual pituitary adenomas who are unsuitable for further surgery who have only a small gap to the optic apparatus where future growth would preclude use of SRS; People with functioning pituitary adenomas with raised hormone levels that have not adequately responded to medical and/or surgical treatment and where further treatment is indicated; People with non-functioning pituitary adenomas or functioning pituitary adenomas who are medically unable to undergo surgery and where medical treatment is not sufficiently effective and further treatment is indicated. Treatment with SRT (using 2-5 stereotactic radiotherapy treatments) should be considered as a treatment option in people with: Residual or recurrent non-functioning pituitary adenomas that continue to grow following surgical intervention; and Non-functioning pituitary adenomas who are medically unable to undergo surgery and treatment is indicated. Exclusion criteria for treatment with SRS or SRT 15
16 Non-functioning pituitary adenomas that haven t shown growth on serial imaging; Larger or diffuse lesions more effectively treated with conventional fractionated external beam radiotherapy; Lesions not sufficiently separated from the optic apparatus and brainstem to allow organ at risk preservation doses to be achieved using SRS or SRT. Exclusion criteria specifically for the use of SRT Functioning pituitary adenomas must not be treated with SRT. 7 Patient Pathway The Intracranial SRS/SRT service specification (NHS England, D05/S/a) describes the detail of the care pathways of SRS/SRT services. The service specification states that patients with pituitary adenomas should be referred to designated Tier 1/2 SRS/SRT service providers by local brain and CNS tumour MDTs (pituitary). The decision to accept referrals will be taken by the SRS/SRT MDT, in line with clinical eligibility and referral guidelines. Clinically complex cases, such as those where the adenoma is close to the optic chiasm, may need to be treated in designated services with particular expertise. 8 Governance Arrangements The Intracranial SRS/SRT service specification (NHS England, D05/S/a) describes the governance arrangements for this service. In particular, it is imperative that the radiotherapy service is compliant with the Ionising Radiation (Medical Exposure) Regulations (IR(ME)R) Mechanism for Funding 16
17 The clinical indication forms part of the scope of the intracranial SRS/SRT services which are commissioned from a small number of designated providers with agreed local prices. 10 Audit Requirements Audit requirements include the following data items for each patient: Karnofsky or World Health Organisation (WHO) Performance Status Functioning versus non-functioning Whether the tumour is primary, residual or recurrent Tumour volume (cc) Prescription isodose and dose prescription Fractionation Treatment outcome (tumour control, progression free survival and where relevant hormonal control 1,3 and 5 years) Side effects (including but not limited to hypopituitarism, visual defects, stroke or new malignancy) Visual acuity Quality of life and patient experience NHS England sub-regions will have access to this data for audit purposes, as required. 11 Documents which have informed this Policy The documents that have informed this policy are: NHS England Intracranial SRS/SRT service specification (D05/s/a). National Institute for Health and Care Excellence Clinical Guideline 10: Improving outcomes for people with brain and other CNS tumours. London NICE, NHS England Clinical Commissioning Policy Statement: Stereotactic Radiosurgery / Radiotherapy for Ocular Melanoma and Pituitary Adenoma (D05/PS/a),
18 NHS England Clinical Commissioning policies for the use of Intracranial Stereotactic Radiosurgery/ Stereotactic Radiotherapy (D05/P/e; D05/P/f). 12 Date of Review This document will be reviewed when information is received which indicates that the policy requires revision. 18
19 References Becker G, Kocher M, Kortmann RD, Paulsen F, Jeremic B, Muller RP, Bamberg M. Radiation therapy in the multimodal treatment approach of pituitary adenoma. Strathlenther Onkol Apr; 178(4): Diallo AM, Colin P, Litre CF, Diallo MM, Decoudier B, Bertoin F, Higel B, Patey M, Rousseaux P, Delemer B. Long-term results of fractionated stereotactic radiotherapy as third-line treatment in acromegaly. Endocrine 2015 Dec;50(3): Fernandez A., Karavitaki N., Wass J. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clinical Endocrinology (2010) 72, Gittoes, N., Bates, A., Tse, W., Bullivant, B., Sheppard, M., Clayton, R., & Stewart, P. (1998). Radiotherapy for non-functioning pituitary tumours. Clinical Endocrinology- Oxford-, 48, Heringer L, Oliveira MF, Rotta JM, and Botelho RV. Effect of repeated transsphenoidal surgery in recurrent or residual pituitary adenomas: A systematic review and meta-analysis. Surg Neurol Int. 2016; 7: 14. Jenkins, P. J., Bates, P., Carson, M. N., Stewart, P. M., & Wass, J. A. H. (2006). Conventional Pituitary Irradiation Is Effective in Lowering Serum Growth Hormone and Insulin-Like Growth Factor-I in Patients with Acromegaly. The Journal of Clinical Endocrinology and Metabolism, 91(4), Kong DS, Lee JI, Lim DH, Kim KW, Shin HJ, Nam DH, Park K, Kim JH. The efficacy of fractionated radiotherapy and stereotactic radiosurgery for pituitary adenomas: long-term results of 125 consecutive patients treated in a single institution. Cancer Aug 15;110(4):
20 Kontogeorgos G, Kovacs K, Horvath E, Scheithauer BW. Multiple adenomas of the anterior pituitary A retrospective autopsy study with clinical implications. J Neurosurg 1991; 74: Lee CC, Vance ML, Xu Z, Yen CP, Shlesinger D, Dodson B, Sheehan J. Stereotactic radiosurgery for acromegaly. J Clin Endocrinol Metab Apr; 99 (4): Leenstra JL, Tanaka S, Kline RW, Brown PD, Link MJ, Nippoldt TB, et al. Factors associated with endocrine deficits after stereotactic radiosurgery of pituitary adenomas. Neurosurgery. 2010;67: discussion 32-3 Marek J, Jezkova J, Hana V, Krsek M, Bandurova L, Pecen L, et al. Is it possible to avoid hypopituitarism after irradiation of pituitary adenomas by the Leksell gamma knife? Eur J Endocrinol. 2011;164: Minniti, G., Osti, M., Jaffrain-Rea, M. L., Esposito, V., Cantore, G., & Maurizi Enrici, R. (2007). Long-term follow-up results of postoperative radiation therapy for Cushing s disease. Journal of Neuro-Oncology, 84(1), Park KJ, Kano H, Parry PV, et al: Long-term outcomes after gamma knife stereotactic radiosurgery for nonfunctional pituitary adenomas. Neurosurgery 69: , 2011 Pollock BE, Cochran J, Natt N, et al: Gamma knife radiosurgery for patients with nonfunctioning pituitary adenomas: Results from a 15-year experience Int J Radiat Oncol Biol Phys 70: , 2008 Reddy, R., Cudlip, S., Byrne, J. V., Karavitaki, N., & Wass, J. A. H. (2011). Can we ever stop imaging in surgically treated and radiotherapy-naive patients with nonfunctioning pituitary adenoma? European Journal of Endocrinology / European Federation of Endocrine Societies, 165(5), Roberts BK, Ouyang DL, Lad SP, et al: Efficacy and safety of CyberKnife radiosurgery for acromegaly. Pituitary 10:19-25,
21 Russell EJ, Molitch ME. The pituitary incidentaloma. Ann Intern Med 1990; 112: Sheehan, J. P., Xu, Z., & Lobo, M. J. (2012). External Beam Radiation Therapy and Stereotactic Radiosurgery for Pituitary Adenomas. Neurosurgery Clinics of North America, 23(4), Sheehan JP, Starke RM, Mathieu D, et al: Gamma Knife radiosurgery for the management of non-functioning pituitary adenomas: A multicenter study. J Neurosurg 119: , 2013 Tinnel BA, Henderson MA, Witt TC, et al: Endocrine response after gamma knifebased stereotactic radiosurgery for secretory pituitary adenoma. Stereotact Funct Neurosurg 86: , 2008 Van den Bergh AC, van den Berg G, Schoorl MA, Sluiter WJ, van der Vliet AM, Hoving EW, et al. Immediate postoperative radiotherapy in residual nonfunctioning pituitary adenoma: Beneficial effect on local control without additional negative impact on pituitary function and life expectancy. Int J Radiat Oncol Biol Phys. 2007;67: Voges J, Kocher M, Runge M, Poggenborg J, Lehrke R, Lenartz D, et al. Linear accelerator radiosurgery for pituitary macroadenomas: A 7-year follow-up study. Cancer. 2006;107: Wan H, Chihiro O, Yuan S. MASEP gamma knife radiosurgery for secretory pituitary adenomas: Experience in 347 consecutive cases. J Exp Clin Cancer Res. 2009;28:36 Wilson PJ, De-Loyde KJ, Williams JR, et al: A single centre s experience of stereotactic radiosurgery and radiotherapy for non-functioning pituitary adenomas with the Linear Accelerator (Linac). J Clin Neurosci 19: ,
22 Zeiler FA, Bigder M, Kaufmann A, McDonald PJ, Fewer D, Butler J, Schroeder G, West M. Gamma Knife in the Treatment of Pituitary Adenomas: Results of a Single Center. Can J Neurol Sci. 2013; 40:
Clinical Commissioning Policy Proposition: Stereotactic radiosurgery/ radiotherapy for the treatment of pituitary adenomas [Adults]
![Clinical Commissioning Policy Proposition: Stereotactic radiosurgery/ radiotherapy for the treatment of pituitary adenomas [Adults] Clinical Commissioning Policy Proposition: Stereotactic radiosurgery/ radiotherapy for the treatment of pituitary adenomas [Adults]](/thumbs/90/101395775.jpg) Clinical Commissioning Policy Proposition: Stereotactic radiosurgery/ radiotherapy for the treatment of pituitary adenomas [Adults] Reference: NHS England 1603 First published: TBC Prepared by NHS England
Clinical Commissioning Policy Proposition: Stereotactic radiosurgery/ radiotherapy for the treatment of pituitary adenomas [Adults] Reference: NHS England 1603 First published: TBC Prepared by NHS England
Reference: NHS England: 16022/P
 Clinical Commissioning Policy: The use of Stereotactic Ablative Radiotherapy (SABR) as a treatment option for patients with Hepatocellular carcinoma or Cholangiocarcinoma Reference: NHS England: 16022/P
Clinical Commissioning Policy: The use of Stereotactic Ablative Radiotherapy (SABR) as a treatment option for patients with Hepatocellular carcinoma or Cholangiocarcinoma Reference: NHS England: 16022/P
Clinical Commissioning Policy: Gemcitabine and capecitabine following surgery for pancreatic cancer (all ages)
 Clinical Commissioning Policy: Gemcitabine and capecitabine following surgery for pancreatic cancer (all ages) NHS England Reference: 1711P 1 NHS England INFORMATION READER BOX Directorate Medical Operations
Clinical Commissioning Policy: Gemcitabine and capecitabine following surgery for pancreatic cancer (all ages) NHS England Reference: 1711P 1 NHS England INFORMATION READER BOX Directorate Medical Operations
Radioterapia degli adenomi ipofisari
 Radioterapia degli adenomi ipofisari G Minniti Radiation Oncology, Sant Andrea Hospital, University of Rome Sapienza, and IRCCS Neuromed, Pozzilli (IS) Roma 6-9 Novembre 14 ! Outline " Radiation techniques
Radioterapia degli adenomi ipofisari G Minniti Radiation Oncology, Sant Andrea Hospital, University of Rome Sapienza, and IRCCS Neuromed, Pozzilli (IS) Roma 6-9 Novembre 14 ! Outline " Radiation techniques
Clinical Commissioning Policy: Pegvisomant for acromegaly as a third-line treatment (adults)
 Clinical Commissioning Policy: Pegvisomant for acromegaly as a third-line treatment (adults) Reference: NHS England: 16050/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Pegvisomant for acromegaly as a third-line treatment (adults) Reference: NHS England: 16050/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Auditory brainstem implant with congenital abnormalities of the auditory nerves of cochleae
 Clinical Commissioning Policy: Auditory brainstem implant with congenital abnormalities of the auditory nerves of cochleae Reference: NHS England: 16062/P NHS England INFORMATION READER BOX Directorate
Clinical Commissioning Policy: Auditory brainstem implant with congenital abnormalities of the auditory nerves of cochleae Reference: NHS England: 16062/P NHS England INFORMATION READER BOX Directorate
Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy (all ages) NHS England Reference: P
 Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy (all ages) NHS England Reference: 1736P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy (all ages) NHS England Reference: 1736P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Specialised Services Policy: CP22. Stereotactic Radiosurgery
 Specialised Services Policy: CP22 Document Author: Assistant Director of Planning Executive Lead: Director of Planning ad Performance Approved by: Management Group Issue Date: 01 July 2015 Review Date:
Specialised Services Policy: CP22 Document Author: Assistant Director of Planning Executive Lead: Director of Planning ad Performance Approved by: Management Group Issue Date: 01 July 2015 Review Date:
Clinical Commissioning Policy: Immune Tolerance Induction (ITI) for haemophilia A (all ages)
 Clinical Commissioning Policy: Immune Tolerance Induction (ITI) for haemophilia A (all ages) Reference: NHS England: 16042/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Immune Tolerance Induction (ITI) for haemophilia A (all ages) Reference: NHS England: 16042/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy Proposition: Pasireotide: An injectable medical therapy for the treatment of Cushing's disease
 Clinical Commissioning Policy Proposition: Pasireotide: An injectable medical therapy for the treatment of Cushing's disease Information Reader Box (IRB) to be inserted on inside front cover for documents
Clinical Commissioning Policy Proposition: Pasireotide: An injectable medical therapy for the treatment of Cushing's disease Information Reader Box (IRB) to be inserted on inside front cover for documents
Clinical Commissioning Policy: The use of Stereotactic Ablative Radiotherapy (SABR) in the treatment of oligometastatic disease
 Clinical Commissioning Policy: The use of Stereotactic Ablative Radiotherapy (SABR) in the treatment of oligometastatic disease Reference: NHS England: 16032/P 2 NHS England INFORMATION READER BOX Directorate
Clinical Commissioning Policy: The use of Stereotactic Ablative Radiotherapy (SABR) in the treatment of oligometastatic disease Reference: NHS England: 16032/P 2 NHS England INFORMATION READER BOX Directorate
Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Skull Base Tumour Treatment NHS Overseas Programme (Adult)
 Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Skull Base Tumour Treatment NHS Overseas Programme (Adult) Reference: NHS England B01/P/d NHS England INFORMATION READER BOX Directorate
Clinical Commissioning Policy: Proton Beam Radiotherapy (High Energy) for Skull Base Tumour Treatment NHS Overseas Programme (Adult) Reference: NHS England B01/P/d NHS England INFORMATION READER BOX Directorate
RESEARCH ARTICLE. Abstract. Introduction. Materials and Methods
 DOI:http://dx.doi.org/10.7314/APJCP.2015.16.13.5279 Outcomes after Linac Based SRS/FSRT for Pituitary Adenomas RESEARCH ARTICLE Outcomes for Pituitary Adenoma Patients Treated with Linac- Based Stereotactic
DOI:http://dx.doi.org/10.7314/APJCP.2015.16.13.5279 Outcomes after Linac Based SRS/FSRT for Pituitary Adenomas RESEARCH ARTICLE Outcomes for Pituitary Adenoma Patients Treated with Linac- Based Stereotactic
Long-term results of gamma knife surgery for growth hormone producing pituitary adenoma: is the disease difficult to cure?
 J Neurosurg (Suppl) 102:119 123, 2005 Long-term results of gamma knife surgery for growth hormone producing pituitary adenoma: is the disease difficult to cure? TATSUYA KOBAYASHI, M.D., PH.D., YOSHIMASA
J Neurosurg (Suppl) 102:119 123, 2005 Long-term results of gamma knife surgery for growth hormone producing pituitary adenoma: is the disease difficult to cure? TATSUYA KOBAYASHI, M.D., PH.D., YOSHIMASA
Somatotroph Pituitary Adenomas (Acromegaly) The Diagnostic Pathway (11-2K-234)
 Somatotroph Pituitary Adenomas (Acromegaly) The Diagnostic Pathway (11-2K-234) Common presenting symptoms/clinical assessment: Pituitary adenomas are benign neoplasms of the pituitary gland. In patients
Somatotroph Pituitary Adenomas (Acromegaly) The Diagnostic Pathway (11-2K-234) Common presenting symptoms/clinical assessment: Pituitary adenomas are benign neoplasms of the pituitary gland. In patients
Clinical Commissioning Policy: Rituximab for the treatment of idiopathic membranous nephropathy in adults
 Clinical Commissioning Policy: Rituximab for the treatment of idiopathic membranous nephropathy in adults Reference: NHS England: 16047/P NHS England INFORMATION READER BOX Directorate Medical Operations
Clinical Commissioning Policy: Rituximab for the treatment of idiopathic membranous nephropathy in adults Reference: NHS England: 16047/P NHS England INFORMATION READER BOX Directorate Medical Operations
Stereotactic Radiosurgery/Fractionated Stereotactic Radiotherapy for Acoustic Neuroma (Vestibular Schwannomas)
 Strategic Commissioning Group West Midlands Commissioning Policy (WM/38) Stereotactic Radiosurgery/Fractionated Stereotactic Radiotherapy for Acoustic Neuroma (Vestibular Schwannomas) Version 1 July 2010
Strategic Commissioning Group West Midlands Commissioning Policy (WM/38) Stereotactic Radiosurgery/Fractionated Stereotactic Radiotherapy for Acoustic Neuroma (Vestibular Schwannomas) Version 1 July 2010
DRAFT FOR CONSULTATION. Clinical Commissioning Policy Proposition: Palliative radiotherapy for bone pain
 Clinical Commissioning Policy Proposition: Palliative radiotherapy for bone pain Information Reader Box (IRB) to be inserted on inside front cover for documents of 6 pages and over, with Publications Gateway
Clinical Commissioning Policy Proposition: Palliative radiotherapy for bone pain Information Reader Box (IRB) to be inserted on inside front cover for documents of 6 pages and over, with Publications Gateway
Clinical Commissioning Policy: Dornase alfa inhaled therapy for primary ciliary dyskinesia (all ages)
 Clinical Commissioning Policy: Dornase alfa inhaled therapy for primary ciliary dyskinesia (all ages) Reference: NHS England: 16029/P NHS England INFORMATION READER BOX Directorate Medical Operations and
Clinical Commissioning Policy: Dornase alfa inhaled therapy for primary ciliary dyskinesia (all ages) Reference: NHS England: 16029/P NHS England INFORMATION READER BOX Directorate Medical Operations and
Clinical Commissioning Policy: Selexipag in the treatment of Pulmonary Arterial Hypertension
 Clinical Commissioning Policy: Selexipag in the treatment of Pulmonary Arterial Hypertension Reference: NHS England: 16017/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Selexipag in the treatment of Pulmonary Arterial Hypertension Reference: NHS England: 16017/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy Proposition:
 Clinical Commissioning Policy Proposition: Stereotactic Radiosurgery (SRS) for adults with Parkinson's tremor and Familial Essential Tremor Version Number: NHS England B13X06/01 Information Reader Box
Clinical Commissioning Policy Proposition: Stereotactic Radiosurgery (SRS) for adults with Parkinson's tremor and Familial Essential Tremor Version Number: NHS England B13X06/01 Information Reader Box
Extracranial doses in stereotactic and conventional radiotherapy for pituitary adenomas
 JOURNAL OF APPLIED CLINICAL MEDICAL PHYSICS, VOLUME 7, NUMBER 2, SPRING 2006 Extracranial doses in stereotactic and conventional radiotherapy for pituitary adenomas Thomas Samuel Ram, a Paul B. Ravindran,
JOURNAL OF APPLIED CLINICAL MEDICAL PHYSICS, VOLUME 7, NUMBER 2, SPRING 2006 Extracranial doses in stereotactic and conventional radiotherapy for pituitary adenomas Thomas Samuel Ram, a Paul B. Ravindran,
NHS public health functions agreement
 NHS public health functions agreement 2016-17 Service specification No.14 Shingles immunisation programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning Operations
NHS public health functions agreement 2016-17 Service specification No.14 Shingles immunisation programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning Operations
Clinical Commissioning Policy Statement: Eculizumab for atypical haemolytic uraemic syndrome. September 2013 Reference: E03/PS(HSS)/a.
 Clinical Commissioning Policy Statement: Eculizumab for atypical haemolytic uraemic syndrome September 2013 Reference: E03/PS(HSS)/a England 1 NHS England INFORMATION READER BOX Directorate Medical Operations
Clinical Commissioning Policy Statement: Eculizumab for atypical haemolytic uraemic syndrome September 2013 Reference: E03/PS(HSS)/a England 1 NHS England INFORMATION READER BOX Directorate Medical Operations
Clinical Commissioning Policy: Stereotactic Radiosurgery / Radiotherapy For Cerebral Metastases. December Reference : NHSCB/D5/1
 Clinical Commissioning Policy: Stereotactic Radiosurgery / Radiotherapy For Cerebral Metastases December 2012 Reference : NHSCB/D5/1 NHS Commissioning Board Clinical Commissioning Policy: Stereotactic
Clinical Commissioning Policy: Stereotactic Radiosurgery / Radiotherapy For Cerebral Metastases December 2012 Reference : NHSCB/D5/1 NHS Commissioning Board Clinical Commissioning Policy: Stereotactic
Brain Tumors. Andrew J. Fabiano, MD FAANS. Associate Professor of Neurosurgery Roswell Park Cancer Institute SUNY at Buffalo School of Medicine
 Brain Tumors Andrew J. Fabiano, MD FAANS Associate Professor of Neurosurgery Roswell Park Cancer Institute SUNY at Buffalo School of Medicine Brain Tumors Brain Tumor Basics Types of Tumors Cases Brain
Brain Tumors Andrew J. Fabiano, MD FAANS Associate Professor of Neurosurgery Roswell Park Cancer Institute SUNY at Buffalo School of Medicine Brain Tumors Brain Tumor Basics Types of Tumors Cases Brain
Clinical Commissioning Policy: Pasireotide for acromegaly as thirdline treatment (adults)
 Clinical Commissioning Policy: Pasireotide for acromegaly as thirdline treatment (adults) Reference: NHS England: 16003/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy: Pasireotide for acromegaly as thirdline treatment (adults) Reference: NHS England: 16003/P NHS England INFORMATION READER BOX Directorate Medical Operations and Information
Clinical Commissioning Policy Proposition: Proton Beam Therapy for Cancer of the Prostate
 Clinical Commissioning Policy Proposition: Proton Beam Therapy for Cancer of the Prostate Reference: NHS England B01X09 First published: March 2016 Prepared by NHS England Specialised Services Clinical
Clinical Commissioning Policy Proposition: Proton Beam Therapy for Cancer of the Prostate Reference: NHS England B01X09 First published: March 2016 Prepared by NHS England Specialised Services Clinical
Radiotherapy approaches to pituitary tumors
 Disclosures No relevant disclosures Radiotherapy approaches to pituitary tumors Pituitary Disorders: Advances in Diagnosis and Management Steve Braunstein, MD, PhD UCSF Department of Radiation Oncology
Disclosures No relevant disclosures Radiotherapy approaches to pituitary tumors Pituitary Disorders: Advances in Diagnosis and Management Steve Braunstein, MD, PhD UCSF Department of Radiation Oncology
Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy
 Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy Reference: NHS England xxx/x/x 1 Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy First published:
Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy Reference: NHS England xxx/x/x 1 Clinical Commissioning Policy: Deep Brain Stimulation for Refractory Epilepsy First published:
Reference: NHS England B01X26
 Clinical Commissioning Policy Proposition: The use of Stereotactic Ablative Radiotherapy (SABR) as a treatment option for patients with Hepatocellular carcinoma or Cholangiocarcinoma Reference: NHS England
Clinical Commissioning Policy Proposition: The use of Stereotactic Ablative Radiotherapy (SABR) as a treatment option for patients with Hepatocellular carcinoma or Cholangiocarcinoma Reference: NHS England
Prolactin-Secreting Pituitary Adenomas (Prolactinomas) The Diagnostic Pathway (11-2K-234)
 Prolactin-Secreting Pituitary Adenomas (Prolactinomas) The Diagnostic Pathway (11-2K-234) Common presenting symptoms/clinical assessment: Pituitary adenomas are benign neoplasms of the pituitary gland.
Prolactin-Secreting Pituitary Adenomas (Prolactinomas) The Diagnostic Pathway (11-2K-234) Common presenting symptoms/clinical assessment: Pituitary adenomas are benign neoplasms of the pituitary gland.
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy
 Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 03/01/2013 Section:
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 03/01/2013 Section:
NANOS Patient Brochure
 NANOS Patient Brochure Pituitary Tumor Copyright 2015. North American Neuro-Ophthalmology Society. All rights reserved. These brochures are produced and made available as is without warranty and for informational
NANOS Patient Brochure Pituitary Tumor Copyright 2015. North American Neuro-Ophthalmology Society. All rights reserved. These brochures are produced and made available as is without warranty and for informational
Pituitary Tumors and Incidentalomas. Bijan Ahrari, MD, FACE, ECNU Palm Medical Group
 Pituitary Tumors and Incidentalomas Bijan Ahrari, MD, FACE, ECNU Palm Medical Group Background Pituitary incidentaloma: a previously unsuspected pituitary lesion that is discovered on an imaging study
Pituitary Tumors and Incidentalomas Bijan Ahrari, MD, FACE, ECNU Palm Medical Group Background Pituitary incidentaloma: a previously unsuspected pituitary lesion that is discovered on an imaging study
Insert heading depending line on length; please delete delete. length; please delete other cover options once
 Insert heading depending Insert on Interim Insert heading line length; Clinical please Commissioning depending on line on length; please delete delete other Policy: on line line cover Circumcision length;
Insert heading depending Insert on Interim Insert heading line length; Clinical please Commissioning depending on line on length; please delete delete other Policy: on line line cover Circumcision length;
NHS public health functions agreement
 NHS public health functions agreement 2016-17 Service specification No.1 Neonatal hepatitis B immunisation programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning
NHS public health functions agreement 2016-17 Service specification No.1 Neonatal hepatitis B immunisation programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages)
 Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170029P 1 NHS England INFORMATION READER BOX Directorate Medical
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170029P 1 NHS England INFORMATION READER BOX Directorate Medical
CCG data collection for people with severe mental illness receiving a full physical health check and follow-up interventions in primary care
 CCG data collection for people with severe mental illness receiving a full physical health check and follow-up interventions in primary care Technical guidance NHS England INFORMATION READER BOX Directorate
CCG data collection for people with severe mental illness receiving a full physical health check and follow-up interventions in primary care Technical guidance NHS England INFORMATION READER BOX Directorate
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy
 Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2015
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2015
NHS public health functions agreement
 NHS public health functions agreement 2016-17 Service specification No.7 Hib/MenC vaccination programme NHS England INFORMATION READER BOX Directorate Medical Commissioning Operations Patients and Information
NHS public health functions agreement 2016-17 Service specification No.7 Hib/MenC vaccination programme NHS England INFORMATION READER BOX Directorate Medical Commissioning Operations Patients and Information
Leksell Gamma Knife Icon. Treatment information
 Leksell Gamma Knife Icon Treatment information You may be feeling frightened or overwhelmed by your recent diagnosis. It can be confusing trying to process a diagnosis, understand a new and challenging
Leksell Gamma Knife Icon Treatment information You may be feeling frightened or overwhelmed by your recent diagnosis. It can be confusing trying to process a diagnosis, understand a new and challenging
Brain and Spine Tumors
 Brain and Spine Tumors Andrew J. Fabiano, MD FAANS Associate Professor of Neurosurgery Roswell Park Cancer Institute SUNY at Buffalo School of Medicine Brain Tumors Brain Tumor Basics Types of Tumors Cases
Brain and Spine Tumors Andrew J. Fabiano, MD FAANS Associate Professor of Neurosurgery Roswell Park Cancer Institute SUNY at Buffalo School of Medicine Brain Tumors Brain Tumor Basics Types of Tumors Cases
Otolaryngologist s Perspective of Stereotactic Radiosurgery
 Otolaryngologist s Perspective of Stereotactic Radiosurgery Douglas E. Mattox, M.D. 25 th Alexandria International Combined ORL Conference April 18-20, 2007 Acoustic Neuroma Benign tumor of the schwann
Otolaryngologist s Perspective of Stereotactic Radiosurgery Douglas E. Mattox, M.D. 25 th Alexandria International Combined ORL Conference April 18-20, 2007 Acoustic Neuroma Benign tumor of the schwann
Process / Evidence Class. Clinical Assessment / III
 Table 2: Endocrine Author Cozzi et al (2009) 1 Study Design: Prospectively followed case series. Fourteen patients had pre-op hypocortisolism. Patient Population: Seventy-two adult patients who underwent
Table 2: Endocrine Author Cozzi et al (2009) 1 Study Design: Prospectively followed case series. Fourteen patients had pre-op hypocortisolism. Patient Population: Seventy-two adult patients who underwent
Directorate Medical Operations Patients and Information Nursing Policy Commissioning Development. Clinical Reference Group Chairs
 Clinical Commissioning Policy: Stereotactic Radiosurgery/ Radiotherapy for Glomus Tumours (skull base paragangliomas, glomus jugulare tumours) September 2013 Reference: NHS ENGLAND D05/P/f England 1 NHS
Clinical Commissioning Policy: Stereotactic Radiosurgery/ Radiotherapy for Glomus Tumours (skull base paragangliomas, glomus jugulare tumours) September 2013 Reference: NHS ENGLAND D05/P/f England 1 NHS
NHS public health functions agreement
 NHS public health functions agreement 2016-17 Service specification No.4 Immunisation against diphtheria, tetanus, poliomyelitis, pertussis and Hib programme Classification: official NHS England INFORMATION
NHS public health functions agreement 2016-17 Service specification No.4 Immunisation against diphtheria, tetanus, poliomyelitis, pertussis and Hib programme Classification: official NHS England INFORMATION
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: P
 Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170054P 1 NHS England INFORMATION READER BOX Directorate Medical
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170054P 1 NHS England INFORMATION READER BOX Directorate Medical
England. Clinical Commissioning Policy: Radiosurgery/Radiotherapy for Cavernous Venous Malformations (Cavernomas)
 Clinical Commissioning Policy: Radiosurgery/Radiotherapy for Cavernous Venous Malformations (Cavernomas) September 2013 Reference: NHS ENGLAND D05/P/g England 1 NHS England INFORMATION READER BOX Directorate
Clinical Commissioning Policy: Radiosurgery/Radiotherapy for Cavernous Venous Malformations (Cavernomas) September 2013 Reference: NHS ENGLAND D05/P/g England 1 NHS England INFORMATION READER BOX Directorate
Gamma knife surgery in management of secretory pituitary adenoma Preliminary evaluation of role, efficacy and safety
 International Journal of Clinical Medicine Research 2014; 1(2): 48-56 Published online June 10, 2014 (http://www.aascit.org/journal/ijcmr) Gamma knife surgery in management of secretory pituitary adenoma
International Journal of Clinical Medicine Research 2014; 1(2): 48-56 Published online June 10, 2014 (http://www.aascit.org/journal/ijcmr) Gamma knife surgery in management of secretory pituitary adenoma
Skull Base Tumour Service. The Multi-Disciplinary Team (MDT) Explained. Jan 2018 v1
 Skull Base Tumour Service The Multi-Disciplinary Team (MDT) Explained Jan 2018 v1 Skull base tumours grow in the bones of the skull that form the bottom of the head and the body ridge between the nose
Skull Base Tumour Service The Multi-Disciplinary Team (MDT) Explained Jan 2018 v1 Skull base tumours grow in the bones of the skull that form the bottom of the head and the body ridge between the nose
MedStar Pituitary Center. Expert, Experienced Care for Pituitary Disorders
 MedStar Pituitary Center Expert, Experienced Care for Pituitary Disorders Pictured clockwise from the back left are Kenneth Burman, MD, Endocrinology; Edward Aulisi, MD, Neurosurgery; Stanley Chia, MD,
MedStar Pituitary Center Expert, Experienced Care for Pituitary Disorders Pictured clockwise from the back left are Kenneth Burman, MD, Endocrinology; Edward Aulisi, MD, Neurosurgery; Stanley Chia, MD,
FOR PUBLIC CONSULTATION ONLY STEREOTACTIC RADIOSURGERY/ STEROTACTIC RADIOTHERAPY FOR PILOCYTIC ASTROCYTOMA
 1 EVIDENCE SUMMARY REPORT FOR PUBLIC CONSULTATION ONLY STEREOTACTIC RADIOSURGERY/ STEROTACTIC RADIOTHERAPY FOR PILOCYTIC ASTROCYTOMA QUESTIONS TO BE ADDRESSED: SUMMARY 1. What is the evidence for the clinical
1 EVIDENCE SUMMARY REPORT FOR PUBLIC CONSULTATION ONLY STEREOTACTIC RADIOSURGERY/ STEROTACTIC RADIOTHERAPY FOR PILOCYTIC ASTROCYTOMA QUESTIONS TO BE ADDRESSED: SUMMARY 1. What is the evidence for the clinical
Gamma Knife Radiosurgery A tool for treating intracranial conditions. CNSA Annual Congress 2016 Radiation Oncology Pre-congress Workshop
 Gamma Knife Radiosurgery A tool for treating intracranial conditions CNSA Annual Congress 2016 Radiation Oncology Pre-congress Workshop ANGELA McBEAN Gamma Knife CNC State-wide Care Coordinator Gamma Knife
Gamma Knife Radiosurgery A tool for treating intracranial conditions CNSA Annual Congress 2016 Radiation Oncology Pre-congress Workshop ANGELA McBEAN Gamma Knife CNC State-wide Care Coordinator Gamma Knife
Surgical therapeutic strategy for giant pituitary adenomas.
 Biomedical Research 2017; 28 (19): 8284-8288 ISSN 0970-938X www.biomedres.info Surgical therapeutic strategy for giant pituitary adenomas. Han-Shun Deng, Zhi-Quan Ding, Sheng-fan Zhang, Zhi-Qiang Fa, Qing-Hua
Biomedical Research 2017; 28 (19): 8284-8288 ISSN 0970-938X www.biomedres.info Surgical therapeutic strategy for giant pituitary adenomas. Han-Shun Deng, Zhi-Quan Ding, Sheng-fan Zhang, Zhi-Qiang Fa, Qing-Hua
Clinical Commissioning Policy: Arteriovenous Malformations. December Reference : NHSCB/D5/4
 Clinical Commissioning Policy: Arteriovenous Malformations December 2012 Reference : NHSCB/D5/4 NHS Commissioning Board Clinical Commissioning Policy: Arteriovenous Malformations First published: December
Clinical Commissioning Policy: Arteriovenous Malformations December 2012 Reference : NHSCB/D5/4 NHS Commissioning Board Clinical Commissioning Policy: Arteriovenous Malformations First published: December
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy
 Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 11/20/2015
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 11/20/2015
Clinical Commissioning Policy: Levofloxacin nebuliser solution for chronic Pseudomonas lung infection in cystic fibrosis (all ages)
 Clinical Commissioning Policy: Levofloxacin nebuliser solution for chronic Pseudomonas lung infection in cystic fibrosis (all ages) NHS England Reference: 1732P NHS England INFORMATION READER BOX Directorate
Clinical Commissioning Policy: Levofloxacin nebuliser solution for chronic Pseudomonas lung infection in cystic fibrosis (all ages) NHS England Reference: 1732P NHS England INFORMATION READER BOX Directorate
Stereotactic Radiosurgery. Extracranial Stereotactic Radiosurgery. Linear accelerators. Basic technique. Indications of SRS
 Stereotactic Radiosurgery Extracranial Stereotactic Radiosurgery Annette Quinn, MSN, RN Program Manager, University of Pittsburgh Medical Center Using stereotactic techniques, give a lethal dose of ionizing
Stereotactic Radiosurgery Extracranial Stereotactic Radiosurgery Annette Quinn, MSN, RN Program Manager, University of Pittsburgh Medical Center Using stereotactic techniques, give a lethal dose of ionizing
NHS public health functions agreement
 NHS public health functions agreement 2016-17 Service specification No. 31 Meningococcal group B (MenB) programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning
NHS public health functions agreement 2016-17 Service specification No. 31 Meningococcal group B (MenB) programme Classification: official NHS England INFORMATION READER BOX Directorate Medical Commissioning
Clinical Commissioning Policy Proposition:
 Clinical Commissioning Policy Proposition: Chemosaturation for liver metastases from ocular melanomas Reference: NHS England A02X05/01 Information Reader Box (IRB) to be inserted on inside front cover
Clinical Commissioning Policy Proposition: Chemosaturation for liver metastases from ocular melanomas Reference: NHS England A02X05/01 Information Reader Box (IRB) to be inserted on inside front cover
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy
 Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 04/01/2014 Section:
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST 04/01/2014 Section:
SUCCESSFUL TREATMENT OF METASTATIC BRAIN TUMOR BY CYBERKNIFE: A CASE REPORT
 SUCCESSFUL TREATMENT OF METASTATIC BRAIN TUMOR BY CYBERKNIFE: A CASE REPORT Cheng-Ta Hsieh, 1 Cheng-Fu Chang, 1 Ming-Ying Liu, 1 Li-Ping Chang, 2 Dueng-Yuan Hueng, 3 Steven D. Chang, 4 and Da-Tong Ju 1
SUCCESSFUL TREATMENT OF METASTATIC BRAIN TUMOR BY CYBERKNIFE: A CASE REPORT Cheng-Ta Hsieh, 1 Cheng-Fu Chang, 1 Ming-Ying Liu, 1 Li-Ping Chang, 2 Dueng-Yuan Hueng, 3 Steven D. Chang, 4 and Da-Tong Ju 1
Background Principles and Technical Development
 Contents Part I Background Principles and Technical Development 1 Introduction and the Nature of Radiosurgery... 3 Definitions of Radiosurgery... 5 Consequences of Changing Definitions of Radiosurgery...
Contents Part I Background Principles and Technical Development 1 Introduction and the Nature of Radiosurgery... 3 Definitions of Radiosurgery... 5 Consequences of Changing Definitions of Radiosurgery...
FOR PUBLIC CONSULTATION ONLY STEREOTACTIC RADIOSURGERY/ STEROTACTIC RADIOTHERAPY FOR EPENDYMOMA
 1 EVIDENCE SUMMARY REPORT FOR PUBLIC CONSULTATION ONLY STEREOTACTIC RADIOSURGERY/ STEROTACTIC RADIOTHERAPY FOR EPENDYMOMA QUESTIONS TO BE ADDRESSED: 1. What is the evidence for the clinical effectiveness
1 EVIDENCE SUMMARY REPORT FOR PUBLIC CONSULTATION ONLY STEREOTACTIC RADIOSURGERY/ STEROTACTIC RADIOTHERAPY FOR EPENDYMOMA QUESTIONS TO BE ADDRESSED: 1. What is the evidence for the clinical effectiveness
Endocrinology. Endocrinology. Services. Anal Fissure
 Endocrinology Endocrinology Services Anal Fissure Conditions we treat Diabetes Mellitus Diabetes Mellitus (diabetes) is diagnosed when the body s blood glucose (sugar) level is high. This happens when
Endocrinology Endocrinology Services Anal Fissure Conditions we treat Diabetes Mellitus Diabetes Mellitus (diabetes) is diagnosed when the body s blood glucose (sugar) level is high. This happens when
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy
 Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2017
Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy Policy Number: Original Effective Date: MM.05.008 05/12/1999 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 04/01/2017
Original Date: April 2016 Page 1 of 7 FOR CMS (MEDICARE) MEMBERS ONLY
 National Imaging Associates, Inc. Clinical guidelines STEREOTACTIC RADIATION THERAPY: STEREO RADIOSURGERY (SRS) AND STEREOTACTIC BODY RADIATION THERAPY (SBRT) CPT4 Codes: Please refer to pages 5-6 LCD
National Imaging Associates, Inc. Clinical guidelines STEREOTACTIC RADIATION THERAPY: STEREO RADIOSURGERY (SRS) AND STEREOTACTIC BODY RADIATION THERAPY (SBRT) CPT4 Codes: Please refer to pages 5-6 LCD
Impact of Gamma Knife Radiosurgery on the neurosurgical management of skull-base lesions: The Combined Approach
 Radiosurgery as part of the neurosurgical armamentarium: Educational Symposium November 24 th 2011 Impact of Gamma Knife Radiosurgery on the neurosurgical management of skull-base lesions: The Combined
Radiosurgery as part of the neurosurgical armamentarium: Educational Symposium November 24 th 2011 Impact of Gamma Knife Radiosurgery on the neurosurgical management of skull-base lesions: The Combined
Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex
 Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex Reference: NHS England E09X04/01 Information Reader Box (IRB)
Clinical Commissioning Policy Proposition: Everolimus for subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex Reference: NHS England E09X04/01 Information Reader Box (IRB)
Imaging pituitary gland tumors
 November 2005 Imaging pituitary gland tumors Neel Varshney,, Harvard Medical School Year IV Two categories of presenting signs of a pituitary mass Functional tumors present with symptoms due to excess
November 2005 Imaging pituitary gland tumors Neel Varshney,, Harvard Medical School Year IV Two categories of presenting signs of a pituitary mass Functional tumors present with symptoms due to excess
Shared Care Protocol
 THE SHEFFIELD AREA PRESCRIBING COMMITTEE Shared Care Protocol for Acromegaly Devised by: The Endocrine Unit Sheffield Teaching Hospitals NHS Trust Date approved: April 2002 Review Date: On publication
THE SHEFFIELD AREA PRESCRIBING COMMITTEE Shared Care Protocol for Acromegaly Devised by: The Endocrine Unit Sheffield Teaching Hospitals NHS Trust Date approved: April 2002 Review Date: On publication
ANALYSIS OF TREATMENT OUTCOMES WITH LINAC BASED STEREOTACTIC RADIOSURGERY IN INTRACRANIAL ARTERIOVENOUS MALFORMATIONS
 ANALYSIS OF TREATMENT OUTCOMES WITH LINAC BASED STEREOTACTIC RADIOSURGERY IN INTRACRANIAL ARTERIOVENOUS MALFORMATIONS Dr. Maitri P Gandhi 1, Dr. Chandni P Shah 2 1 Junior resident, Gujarat Cancer & Research
ANALYSIS OF TREATMENT OUTCOMES WITH LINAC BASED STEREOTACTIC RADIOSURGERY IN INTRACRANIAL ARTERIOVENOUS MALFORMATIONS Dr. Maitri P Gandhi 1, Dr. Chandni P Shah 2 1 Junior resident, Gujarat Cancer & Research
STEREOTACTIC RADIATION THERAPY. Monique Blanchard ANUM Radiation Oncology Epworth HealthCare
 STEREOTACTIC RADIATION THERAPY Monique Blanchard ANUM Radiation Oncology Epworth HealthCare Overview Stereotactic radiation therapy at Epworth Healthcare What is stereotactic radiation therapy? Delivery
STEREOTACTIC RADIATION THERAPY Monique Blanchard ANUM Radiation Oncology Epworth HealthCare Overview Stereotactic radiation therapy at Epworth Healthcare What is stereotactic radiation therapy? Delivery
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES CENTRAL NERVOUS SYSTEM MENINGIOMA CNS Site Group Meningioma Author: Dr. Norm Laperriere Date: February 20, 2018 1. INTRODUCTION 3 2. PREVENTION
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES CENTRAL NERVOUS SYSTEM MENINGIOMA CNS Site Group Meningioma Author: Dr. Norm Laperriere Date: February 20, 2018 1. INTRODUCTION 3 2. PREVENTION
TREATMENT OF CUSHING S DISEASE
 TREATMENT OF CUSHING S DISEASE Surgery, Radiation, Medication Peter J Snyder, MD Professor of Medicine Disclosures Novartis Research grant Pfizer Consultant Ipsen Research grant Cortendo Research grant
TREATMENT OF CUSHING S DISEASE Surgery, Radiation, Medication Peter J Snyder, MD Professor of Medicine Disclosures Novartis Research grant Pfizer Consultant Ipsen Research grant Cortendo Research grant
Imaging The Turkish Saddle. Russell Goodman, HMS III Dr. Gillian Lieberman
 Imaging The Turkish Saddle Russell Goodman, HMS III Dr. Gillian Lieberman Learning Objectives Review the anatomy of the sellar region Discuss the differential diagnosis of sellar masses Discuss typical
Imaging The Turkish Saddle Russell Goodman, HMS III Dr. Gillian Lieberman Learning Objectives Review the anatomy of the sellar region Discuss the differential diagnosis of sellar masses Discuss typical
Clinical Commissioning Policy: Hypofractionated external beam radiotherapy in the treatment of localised prostate cancer (adults)
 Clinical Commissioning Policy: Hypofractionated external beam radiotherapy in the treatment of localised prostate cancer (adults) Reference: NHS England: 170021/P Standard NHS England INFORMATION READER
Clinical Commissioning Policy: Hypofractionated external beam radiotherapy in the treatment of localised prostate cancer (adults) Reference: NHS England: 170021/P Standard NHS England INFORMATION READER
NON MALIGNANT BRAIN TUMOURS Facilitator. Ros Taylor Advanced Neurosurgical Nurse Practitioner Southmead Hospital Bristol
 NON MALIGNANT BRAIN TUMOURS Facilitator Ros Taylor Advanced Neurosurgical Nurse Practitioner Southmead Hospital Bristol Neurosurgery What will be covered? Meningioma Vestibular schwannoma (acoustic neuroma)
NON MALIGNANT BRAIN TUMOURS Facilitator Ros Taylor Advanced Neurosurgical Nurse Practitioner Southmead Hospital Bristol Neurosurgery What will be covered? Meningioma Vestibular schwannoma (acoustic neuroma)
Clinical Commissioning Policy: Use of cobicistat as a booster in treatment of HIV positive adults and adolescents
 Clinical Commissioning Policy: Use of cobicistat as a booster in treatment of HIV positive adults and adolescents Reference: NHS England F03/P/b NHS England INFORMATION READER BOX Directorate Medical Commissioning
Clinical Commissioning Policy: Use of cobicistat as a booster in treatment of HIV positive adults and adolescents Reference: NHS England F03/P/b NHS England INFORMATION READER BOX Directorate Medical Commissioning
JMSCR Vol 05 Issue 01 Page January 2017
 MEN1, AIP, PRKAR1A and CDKN1B are familial pituitary syndromes found to be associated with four different genes. Pituitary gland is situated in hypophyseal fossa which is bounded supero-laterally by dural
MEN1, AIP, PRKAR1A and CDKN1B are familial pituitary syndromes found to be associated with four different genes. Pituitary gland is situated in hypophyseal fossa which is bounded supero-laterally by dural
Linear Accelerator Radiosurgery for Pituitary Macroadenomas. BACKGROUND. A prospective study was conducted to assess the efficacy and side
 1355 Linear Accelerator Radiosurgery for Pituitary Macroadenomas A 7-Year Follow-Up Study Juergen Voges, MD 1 Martin Kocher, MD 2 Matthias Runge, MD 1 Jorg Poggenborg, MD 3 Ralph Lehrke, MD 1 Doris Lenartz,
1355 Linear Accelerator Radiosurgery for Pituitary Macroadenomas A 7-Year Follow-Up Study Juergen Voges, MD 1 Martin Kocher, MD 2 Matthias Runge, MD 1 Jorg Poggenborg, MD 3 Ralph Lehrke, MD 1 Doris Lenartz,
High and Low GH: an update of diagnosis and management of GH disorders
 High and Low GH: an update of diagnosis and management of GH disorders Georgia Chapter-AACE 2017 Laurence Katznelson, MD Professor of Medicine and Neurosurgery Associate Dean of Graduate Medical Education
High and Low GH: an update of diagnosis and management of GH disorders Georgia Chapter-AACE 2017 Laurence Katznelson, MD Professor of Medicine and Neurosurgery Associate Dean of Graduate Medical Education
Stereotactic radiotherapy for meningiomas using CyberKnife
 Stereotactic radiotherapy for meningiomas using CyberKnife Delivering the best in care UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm
Stereotactic radiotherapy for meningiomas using CyberKnife Delivering the best in care UHB is a no smoking Trust To see all of our current patient information leaflets please visit www.uhb.nhs.uk/patient-information-leaflets.htm
Urgent Clinical Commissioning Policy Statement: Pembrolizumab for drug-resistant gestational trophoblastic neoplasia. NHS England Reference: P
 Urgent Clinical Commissioning Policy Statement: Pembrolizumab for drug-resistant gestational trophoblastic neoplasia NHS England Reference: 170027P 1 Equality statement Promoting equality and addressing
Urgent Clinical Commissioning Policy Statement: Pembrolizumab for drug-resistant gestational trophoblastic neoplasia NHS England Reference: 170027P 1 Equality statement Promoting equality and addressing
National Breast Cancer Audit next steps. Martin Lee
 National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
Disclosure SBRT. SBRT for Spinal Metastases 5/2/2010. No conflicts of interest. Overview
 Stereotactic Body Radiotherapy (SBRT) for Recurrent Spine Tumors Arjun Sahgal M.D., F.R.C.P.C. Assistant Professor Princess Margaret Hospital Sunnybrook Health Sciences Center University of Toronto Department
Stereotactic Body Radiotherapy (SBRT) for Recurrent Spine Tumors Arjun Sahgal M.D., F.R.C.P.C. Assistant Professor Princess Margaret Hospital Sunnybrook Health Sciences Center University of Toronto Department
Diseases of pituitary gland
 Diseases of pituitary gland A brief introduction Anterior lobe = adenohypophysis Posterior lobe = neurohypophysis The production of most pituitary hormones is controlled in large part by positively and
Diseases of pituitary gland A brief introduction Anterior lobe = adenohypophysis Posterior lobe = neurohypophysis The production of most pituitary hormones is controlled in large part by positively and
FOR PUBLIC CONSULTATION ONLY STEREOTACTIC RADIOSURGERY/ STEROTACTIC RADIOTHERAPY FOR HAEMANGIOBLASTOMA
 1 EVIDENCE SUMMARY REPORT FOR PUBLIC CONSULTATION ONLY STEREOTACTIC RADIOSURGERY/ STEROTACTIC RADIOTHERAPY FOR HAEMANGIOBLASTOMA QUESTIONS TO BE ADDRESSED: 1. What is the evidence for the clinical effectiveness
1 EVIDENCE SUMMARY REPORT FOR PUBLIC CONSULTATION ONLY STEREOTACTIC RADIOSURGERY/ STEROTACTIC RADIOTHERAPY FOR HAEMANGIOBLASTOMA QUESTIONS TO BE ADDRESSED: 1. What is the evidence for the clinical effectiveness
Collection of Recorded Radiotherapy Seminars
 IAEA Human Health Campus Collection of Recorded Radiotherapy Seminars http://humanhealth.iaea.org The Role of Radiosurgery in the Treatment of Gliomas Luis Souhami, MD Professor Department of Radiation
IAEA Human Health Campus Collection of Recorded Radiotherapy Seminars http://humanhealth.iaea.org The Role of Radiosurgery in the Treatment of Gliomas Luis Souhami, MD Professor Department of Radiation
Clinical Concerns about Recurrence of Non-Functioning Pituitary Adenoma
 ORIGINAL ARTICLE Brain Tumor Res Treat 2016;4(1):1-7 / pissn 2288-2405 / eissn 2288-2413 http://dx.doi.org/10.14791/btrt.2016.4.1.1 Clinical Concerns about Recurrence of Non-Functioning Pituitary Adenoma
ORIGINAL ARTICLE Brain Tumor Res Treat 2016;4(1):1-7 / pissn 2288-2405 / eissn 2288-2413 http://dx.doi.org/10.14791/btrt.2016.4.1.1 Clinical Concerns about Recurrence of Non-Functioning Pituitary Adenoma
(3) Pituitary tumours
 Hypopituitarism Diabetes Insipidus Pituitary tumours (2) Dr T Kemp - Endocrinology and Metabolism Unit - Steve Biko Academic Hospital (3) Pituitary tumours Pituitary microadenoma - intrasellar adenoma
Hypopituitarism Diabetes Insipidus Pituitary tumours (2) Dr T Kemp - Endocrinology and Metabolism Unit - Steve Biko Academic Hospital (3) Pituitary tumours Pituitary microadenoma - intrasellar adenoma
Proposed Neurosciences National Structure. Accountability Framework Neurological Alliance (Feedback Loop) National Advisory Group (Neurosciences)
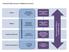 Accountability Framework Neurological Alliance (Feedback Loop) Proposed s National Structure National NHS England National Medical Director National Advisory Group (s) Regional NHS England Sub-Regional
Accountability Framework Neurological Alliance (Feedback Loop) Proposed s National Structure National NHS England National Medical Director National Advisory Group (s) Regional NHS England Sub-Regional
Imaging for suspected glioma
 Imaging for suspected glioma 1.1.1 Offer standard structural MRI (defined as T2 weighted, FLAIR, DWI series and T1 pre- and post-contrast volume) as the initial diagnostic test for suspected glioma, unless
Imaging for suspected glioma 1.1.1 Offer standard structural MRI (defined as T2 weighted, FLAIR, DWI series and T1 pre- and post-contrast volume) as the initial diagnostic test for suspected glioma, unless
The primary management of the majority of symptomatic
 J Neurosurg 116:1304 1310, 2012 Cranial nerve dysfunction following Gamma Knife surgery for pituitary adenomas: long-term incidence and risk factors Clinical article Christopher P. Cifarelli, M.D., Ph.D.,
J Neurosurg 116:1304 1310, 2012 Cranial nerve dysfunction following Gamma Knife surgery for pituitary adenomas: long-term incidence and risk factors Clinical article Christopher P. Cifarelli, M.D., Ph.D.,
Technology appraisal guidance Published: 31 January 2018 nice.org.uk/guidance/ta501
 Intrabeam radiotherapy system for adjuvant treatment of early breast cancer Technology appraisal guidance Published: 31 January 2018 nice.org.uk/guidance/ta501 NICE 2018. All rights reserved. Subject to
Intrabeam radiotherapy system for adjuvant treatment of early breast cancer Technology appraisal guidance Published: 31 January 2018 nice.org.uk/guidance/ta501 NICE 2018. All rights reserved. Subject to
Head and Neck Cancer surgeon level data - first report. Queen Victoria Hospital NHS Foundation Trust
 Head and Neck Cancer surgeon level data - first report Queen Victoria Hospital NHS Foundation Trust 1 of 8 Foreword This report for the first time presents data for individual surgeons relating to head
Head and Neck Cancer surgeon level data - first report Queen Victoria Hospital NHS Foundation Trust 1 of 8 Foreword This report for the first time presents data for individual surgeons relating to head
Abstract. Introduction
 Clinical Features and Outcome of Surgery in 30 Patients with Acromegaly A. Chandna, N. Islam, A. Jabbar, L. Zuberi, N. Haque Endocrinology Section, Department of Medicine, Aga Khan University Hospital,
Clinical Features and Outcome of Surgery in 30 Patients with Acromegaly A. Chandna, N. Islam, A. Jabbar, L. Zuberi, N. Haque Endocrinology Section, Department of Medicine, Aga Khan University Hospital,
OFFICIAL. Document Status. NHS England INFORMATION READER BOX
 NHS England INFORMATION READER BOX OFFICIAL Directorate Medical Operations and Information Specialised Commissioning Nursing Trans. & Corp. Ops. Strategy & Innovation Finance Publications Gateway Reference:
NHS England INFORMATION READER BOX OFFICIAL Directorate Medical Operations and Information Specialised Commissioning Nursing Trans. & Corp. Ops. Strategy & Innovation Finance Publications Gateway Reference:
AAPM WGSBRT NTCP Optic Apparatus (chiasm and nerve)
 AAPM WGSBRT NTCP Optic Apparatus (chiasm and nerve) Michael T. Milano, MD PhD Department of Radiation Oncology University of Rochester, Rochester, NY 07/16/15 AAPM WGSBRT Optic Apparatus NTCP Issam El
AAPM WGSBRT NTCP Optic Apparatus (chiasm and nerve) Michael T. Milano, MD PhD Department of Radiation Oncology University of Rochester, Rochester, NY 07/16/15 AAPM WGSBRT Optic Apparatus NTCP Issam El
