Template for Reporting Results of Biomarker Testing of Specimens From Patients With Gastrointestinal Stromal Tumors
|
|
|
- Scarlett Simon
- 6 years ago
- Views:
Transcription
1 Template for Reporting Results of Biomarker Testing of Specimens From Patients With Gastrointestinal Stromal Tumors Template web posting date: February 2015 Authors Meera Hameed, MD, FCAP Department of Pathology, Memorial Sloan-Kettering Cancer Center, New York, NY Christopher L. Corless, MD, PhD Department of Pathology, Oregon Health & Science University, Portland, OR Suzanne George, MD Center for Sarcoma and Bone Oncology, Dan-Farber Cancer Institute, Boston, MA Jason L. Hornick MD, PhD, FCAP Department of Pathology, Brigham and Women's Hospital, Boston, MA Sanjay Kakar, MD, FCAP Department of Pathology, University of California, San Francisco and the Veterans Affairs Medical Center, San Francisco, CA Alex Lazar, MD, PhD, FCAP Department of Pathology, Sarcoma Research Center, The University of Texas MD Anderson Cancer Center, Houston, Texas Laura Tang, MD, FCAP Department of Pathology, Memorial Sloan-Kettering Cancer Center, New York, NY For the Members of the Cancer Biomarker Reporting Committee, College of American Pathologists
2 2015 College of American Pathologists (CAP). All rights reserved. The College does not permit reproduction of any substantial portion of these templates without its written authorization. The College hereby authorizes use of these templates by physicians and other health care providers in reporting results of biomarker testing on patient specimens, in teaching, and in carrying out medical research for nonprofit purposes. This authorization does not extend to reproduction or other use of any substantial portion of these templates for commercial purposes without the written consent of the College. The CAP also authorizes physicians and other health care practitioners to make modified versions of the templates solely for their individual use in reporting results of biomarker testing for individual patients, teaching, and carrying out medical research for non-profit purposes. The CAP further authorizes the following uses by physicians and other health care practitioners, in reporting on surgical specimens for individual patients, in teaching, and in carrying out medical research for non-profit purposes: (1) Dictation from the original or modified templates for the purposes of creating a text-based patient record on paper, or in a word processing document; (2) Copying from the original or modified templates into a text-based patient record on paper, or in a word processing document; (3) The use of a computerized system for items (1) and (2), provided that the template data is stored intact as a single text-based document, and is not stored as multiple discrete data fields. Other than uses (1), (2), and (3) above, the CAP does not authorize any use of the templates in electronic medical records systems, pathology informatics systems, cancer registry computer systems, computerized databases, mappings between coding works, or any computerized system without a written license from the CAP. Any public dissemination of the original or modified templates is prohibited without a written license from the CAP. The College of American Pathologists offers these templates to assist pathologists in providing clinically useful and relevant information when reporting results of biomarker testing. The College regards the reporting elements in the templates as important elements of the biomarker test report, but the manner in which these elements are reported is at the discretion of each specific pathologist, taking into account clinician preferences, institutional policies, and individual practice. The College developed these templates as educational tools to assist pathologists in the useful reporting of relevant information. It did not issue them for use in litigation, reimbursement, or other contexts. Nevertheless, the College recognizes that the templates might be used by hospitals, attorneys, payers, and others. The College cautions that use of the templates other than for their intended educational purpose may involve additional considerations that are beyond the scope of this document. The inclusion of a product name or service in a CAP publication should not be construed as an endorsement of such product or service, nor is failure to include the name of a product or service to be construed as disapproval. 2
3 CAP Gastrointestinal Stromal Tumor Biomarker Template Revision History Version Code The definition of the version code can be found at Version: Summary of Changes The following changes were made since the December 2014 version: Testing Methods NGS was spelled out: Next-generation sequencing URL was updated: Human Genome Variation Society: 3
4 CAP Approved GIST Biomarker Reporting Template Template web posting date: February 2015 Completion of the template is the responsibility of the laboratory performing the biomarker testing and/or providing the interpretation. When both testing and interpretation are performed elsewhere (eg, a reference laboratory), synoptic reporting of the results by the laboratory submitting the tissue for testing is also encouraged to ensure that all information is included in the patient s medical record and thus readily available to the treating clinical team. GASTROINTESTINAL STROMAL TUMOR (GIST) Select a single response unless otherwise indicated. Note: Use of this template is optional. + RESULTS + Immunohistochemical Studies (Note A) + KIT (CD117) # + Positive + Negative + DOG1 (ANO1) # + Positive + Negative + SDHB + Intact + Deficient + SDHA + Intact + Deficient + Other (specify): + Positive + Negative # Note: Duplicate testing/reporting of KIT (CD117) and DOG is not required if previously performed. + Molecular Genetic Studies (eg, KIT, PDGFRA, BRAF, SDHA/B/C/D, or NF1 mutational analysis) + Submitted for analysis; results pending + Performed, see separate report: + Performed + Specify method(s) and results: + Not performed + KIT Mutational Analysis (Note B) + No mutation detected + Mutation identified (specify:) + Cannot be determined (explain): + Data elements preceded by this symbol are not required. 4
5 CAP Approved + PDGFRA Mutational Analysis (Note C) + No mutation detected + Mutation identified (specify): + Cannot be determined (explain): + BRAF Mutational Analysis (Note D) + No BRAF mutation detected + BRAF V600E (c.1799t>a) mutation + Other BRAF mutation (specify): + Cannot be determined (explain): + SDHA/B/C/D Mutational Analysis (Note E) + No mutation detected + Mutation identified (specify): + Cannot be determined (explain): + NF1 Mutational Analysis (Note F) + No mutation detected + Mutation identified (specify): + Cannot be determined (explain): + METHODS + Dissection Method(s) (select all that apply) (Note G) + Laser capture microdissection + Manual under microscopic observation + Manual without microscopic observation + Cored from block + Whole tissue section (no tumor enrichment procedure employed) + KIT Mutational Analysis + Exons Assessed (select all that apply) + Exon 9 + Exon 11 + Exon 13 + Exon 14 + Exon 17 + Other (specify): + Testing Method(s) # + Specify name of method used and exons tested: # Please specify if different testing methods are used for different exons. + PDGFRA Mutational Analysis + Exons Assessed (select all that apply) + Exon 12 + Exon 14 + Exon 18 + Other (specify): + Data elements preceded by this symbol are not required. 5
6 CAP Approved + Testing Method(s) # + Specify name of method used and exons tested: # Please specify if different testing methods are used for different exons. + BRAF Mutational Analysis (Note D) + Exons Assessed + Exon 15 + Other (specify): + Testing Method(s) + Specify name of method used and exons tested: + SDH A/B/C/D Mutational Analysis (Note E) + Exons assessed (specify): + Testing Method(s) # + Specify name of method used and exons tested: # Please specify if different testing methods are used for different exons. + NF1 Mutational Analysis (Note F) + Exons assessed (specify): + Testing Method(s) # + Sanger + Next-generation sequencing (NGS) + Other (specify): + Specify name of method used: # Please specify if different testing methods are used for different exons. + COMMENT(S) Note: Fixative type, time to fixation (cold ischemia time), and time of fixation should be reported if applicable in this template or in the original pathology report. Gene names should follow recommendations of The Human Genome Organisation (HUGO) Nomenclature Committee ( accessed February 16, 2015). All reported gene sequence variations should be identified following the recommendations of the Human Genome Variation Society ( accessed February 16, 2015). + Data elements preceded by this symbol are not required. 6
7 Background Documentation Explanatory Notes A. Immunohistochemical Analysis Because of the advent of small-molecule kinase inhibitor therapy for the treatment of GIST (see the following), it has become imperative to distinguish GIST from its histologic mimics, mainly leiomyoma, leiomyosarcoma, schwannoma, and desmoid fibromatosis. 1,2 Immunohistochemistry is instrumental in the workup of GIST. Approximately 95% of GISTs are immunoreactive for KIT (CD117). 3 Most KIT-negative GISTs are gastric or omental tumors that harbor mutations in platelet-derived growth factor receptor A (PDGFRA). 4 KIT immunoreactivity is usually strong and diffuse but can be more limited in extent in some cases (Figure 1, A and B). It is not unusual for GISTs to exhibit dot-like perinuclear staining (Figure 1, C), while less commonly some cases exhibit membranous staining (Figure 1, D). These patterns do not clearly correlate with mutation type or response to therapy. DOG1 is another highly sensitive and specific marker for GIST, which was discovered by gene expression profiling. 5,6 DOG1 (also known as anoctamin 1, ANO1) is particularly useful for KIT-negative tumors and those with limited KIT expression; DOG1 is more sensitive than KIT for gastric epithelioid GISTs. 7 Approximately 70% of GISTs are positive for CD34, 30% to 40% are positive for smooth muscle actin, 5% are positive for S100 (usually focal), 5% are positive for desmin (usually focal), and 1% to 2% are positive for keratin (weak/focal). 8 Figure 1. Patterns of KIT staining in gastrointestinal stromal tumor (GIST). A, Diffuse and strong immunoreactivity in a typical GIST. B, Focal and weak pattern in an epithelioid gastric GIST with a PDGFRA mutation. C, Dot-like perinuclear staining. D, Membranous pattern. (Original magnification X400.) Approximately 8% of gastric GISTs are characterized by dysfunction of the mitochondrial succinate dehydrogenase (SDH) complex, known as SDH-deficient GISTs. 9 This clinically and pathologically distinctive subset of GISTs, which can be recognized by multinodular/plexiform architecture, has a predilection for children and young adults, is usually dominated by epithelioid cytomorphology, often metastasizes to lymph nodes (an exceeding rare occurrence in conventional GIST), and pursues a relatively indolent clinical course when metastatic. 10 Approximately 50% of SDH-deficient GISTs have mutations in one of the SDH subunit genes (see the following). The diagnosis of SDH-deficient GIST can be confirmed by demonstrating loss of expression of SDHB by immunohistochemistry, which is observed irrespective of the presence of an identifiable SDH mutation 7
8 Background Documentation (or the particular mutation type). Other genetic groups of GIST (eg, those with mutations in KIT or PDGFRA) show granular cytoplasmic staining for SDHB. 11 Mutations in SDHA are detected in 30% of SDH-deficient GISTs; SDHA is the most commonly mutated gene in this class of tumors (see below). Loss of expression of SDHA specifically identifies tumors with SDHA mutations 12,13 ; other SDH-deficient GISTs show normal (intact) cytoplasmic staining for SDHA. Immunohistochemistry for SDHB and SDHA can therefore be used to triage patients for genetic testing. Immunohistochemistry for SDHB/SDHA need not be performed on all GISTs, but only to confirm the diagnosis in a resection of a gastric GIST with multinodular architecture, and to screen small biopsies of gastric GISTs with epithelioid cytomorphology (particularly in younger patients). Molecular Analysis Most GISTs are driven by oncogenic mutations in one of two receptor tyrosine kinases, KIT (75%) and PDGFRA (10%). 14,15 These mutations result in constitutive ligand independent activation of full-length proteins. Mutations cluster within hot spots exons 9, 11, 13, 17 in KIT and exons 12, 14, 18 in PDGFRA (Figure 2). KIT and PDGFRA mutations are mutually exclusive. Multiple phase I, II, and international phase III trials have established the efficacy of tyrosine kinase inhibitors such as imatinib, sunitinib, and regorafinib in metastatic tumors and in the adjuvant setting Imatinib was originally granted accelerated approval for the treatment of advanced or metastatic GIST in In 2012, the Food and Drug Administration (FDA) approved the use of imatinib for GIST in the adjuvant setting. The most recent National Comprehensive Cancer Network (NCCN) task force on GIST strongly encourages that KIT and PDGFRA mutational analysis be performed if imatinib therapy is begun for unresectable or metastatic disease and that mutational analysis to be considered for patients with primary disease, particularly those with high-risk tumors. In the setting of long-term imatinib therapy, secondary or acquired mutations occur in KIT exons 13, 14, and 17 and PDGFRA exon * Refers to exons involved most frequently by secondary/acquired mutations. Figure 2. Locations and frequency of activating KIT and PDGFRA mutations in GIST. Adapted with permission from Heinrich et al. 14 Copyright 2003 by the American Society of Clinical Oncology. All rights reserved. B. KIT Mutational Analysis The most common mutations affect the juxta membrane domain encoded by exon 11 (two-thirds of GIST). These mutations include in-frame deletions, substitutions, and insertions. Deletions (in particular codon 557 and/or 558) are associated with shorter progression free and overall survival About 7% to 10% of the tumors harbor mutations in the extracellular domain encoded by exon 9 (most commonly insay ). 26 Primary mutations in the activation loop (exon 17) and ATP binding region (exon 13) are uncommon (1%). Majority of these mutations are substitutions. 27 KIT exon 8 mutations are extremely rare (0.15%). 28 Secondary or resistance mutations occur 8
9 Background Documentation commonly in tumors harboring primary exon 11 mutations. The newly acquired secondary mutations are always located in exons encoding tyrosine kinase domain (exons 13, 14, 17). 29 C. PDGFRA Mutational Analysis More than 80% of KIT-negative GISTS have PDGFRA mutations. Activation of PDGFRA is seen in GISTs harboring mutations in juxta membranous domain (exon 12), the ATP binding domain (exon 14), or the activation loop (exon 18). 30 Mutations include substitutions and deletions. Primary resistance to imatinib is seen with the most common PDGFRA exon 18 D842V mutation. D. BRAF Mutational Analysis Activating mutations of BRAF (V600E) has been identified in a small subset (7%) of KIT/PDGFRA wild-type GISTs. These tumors show a predilection for small bowel location. 31 E. SDH A/B/C/D Mutational Analysis The succinate dehydrogenase (SDH) complex (mitochondrial complex II) participates in both the Krebs cycle and the electron transport chain of oxidative phosphorylation. About 8% of gastric GISTs (all lacking mutations in KIT and PDGFRA) are caused by dysfunction of the SDH complex ("SDH-deficient GISTs"). Around 50% of patients affected by such tumors harbor germline mutations in one of the SDH subunit genes (SDHA/B/C or D). SDHAinactivating mutations are most common, detected in about 30% of SDH-deficient GISTs. Mutations involve exons 2, 3, 5, 6, 7, 8, 9, 10, 11, 13, 14 of SDHA; exons 1, 2, 3, 4, 6, 7 of SDHB; exons 1, 4, 5 of SDHC; and exons 4 and 6 of SDHD. While the majority of the mutations are substitutions, deletions, splice-site mutations, frame shift, and duplications have also been reported. 9,11,13,32 F. Neurofibromatosis Type 1 (NF1) Mutational Analysis NF1 is an inherited, autosomal dominant disease characterized by multiple café au lait spots, Lisch nodules, freckling, and development of neurofibromas. GISTs in NF1 patients arise predominantly from the small intestine and can be multicentric and lack KIT and PDGFRA mutations. Until now, no specific genetic alterations have been found in NF1-related GIST. 32 G. Dissection Method: While in majority of cases GIST samples show tumor percentage (%) well above the analytical sensitivity of Sanger sequencing (>50% neoplastic cell percentage/20% to 25% mutant allele percentage), in cases of mutation analysis of treated samples, careful macro/microdissection may be necessary to avoid false negative results. H. Reporting Nomenclature Consistent gene mutation nomenclature is essential for efficient and accurate reporting. 33 Following are examples as recommended by Human Genome Variation Society (HGVS) for description of variant changes. 34 It is also preferred that protein alterations are mentioned in the report in addition to genomic coordinates. Examples of DNA, RNA, and Protein Nomenclature DNA: A, G, C, T (example: c.957a>t) RNA: a, g, c, u (example: r.957 a>u) Protein: 3-letter amino acid code, X= Stop codon (example: p. Glu78Gln) Examples of Nomenclatures for Types of Sequence Variants Types of Variation Examples Substitution c.123a>g Deletion c.123dela, c.586_591deltggtca or c.586_591del6 Duplication c.123dupa, c.586_591duptggtca or c.586_591dup6 Insertion c.123_124insc, c.1086_1087insgcgtga Frame shift p. Arg83 fs or p. Arg83Ser fsx15 Deletion/insertions indel c.112_117delaggtcainstg References 1. Hornick JL, Fletcher CD. Immunohistochemical staining for KIT (CD117) in soft tissue sarcomas is very limited in distribution. Am J Clin Pathol. 2002;117(2):
10 Background Documentation 2. Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol. 2000;13(10): Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol. 1998;11(8): Medeiros F, Corless CL, Duensing A, et al. KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol. 2004;28(7): West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004;165(1): Espinosa I, Lee CH, Kim MK, et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008;32(2): Miettinen M, Wang ZF, Lasota J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am J Surg Pathol. 2009;33(9): Miettinen M, Wang ZF, Sarlomo-Rikala M, et al. Succinate dehydrogenase-deficient GISTs: a clinicopathologic, immunohistochemical, and molecular genetic study of 66 gastric GISTs with predilection to young age. Am J Surg Pathol. 2011;35(11): Doyle LA, Hornick JL. Gastrointestinal stromal tumours: from KIT to succinate dehydrogenase. Histopathology. 2014;64(1): Doyle LA, Nelson D, Heinrich MC, et al. Loss of succinate dehydrogenase subunit B (SDHB) expression is limited to a distinctive subset of gastric wild-type gastrointestinal stromal tumours: a comprehensive genotype-phenotype correlation study. Histopathology. 2012;61(5): Wagner AJ, Remillard SP, Zhang YX, et al. Loss of expression of SDHA predicts SDHA mutations in gastrointestinal stromal tumors. Mod Pathol. 2013;26(2): Dwight T, Benn DE, Clarkson A, et al. Loss of SDHA expression identifies SDHA mutations in succinate dehydrogenase-deficient gastrointestinal stromal tumors. Am J Surg Pathol. 2013;37(2): Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002; 33(5): Heinrich MC, Corless CL, Demetri GD, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21(23): Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607): Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350): Joensuu H, Roberts PJ, Sarlomo-Rikala M, et al. Effect of the tyrosine kinase inhibitor ST1571 in a patient with metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344(14): Van Oosterom AT, Judson I, Verweij J, et al. ST1571, an active drug in metastatic gastrointestinal stromal tumors (GIST) an EORTC phase 1 study (abstract). Proc Am Soc Clin Oncol. 2001;20:1a. 19. Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7): Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26(4): Verweiji J, Casali PG, Zaleberg J, et al. Progression free survival in gastrointestinal stromal tumors with high-dose imatinib: randomized trial. Lancet. 2004;364(9440): Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumor: a meta-analysis of 1640 patients. J Clin Oncol. 2010;28(7): Heinrich MC, Corless CL, Blanke CD, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24(29): Andersson J, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors with KIT exon 11 deletions are associated with poor prognosis. Gastroenterology. 2006;130(6): Liu XH, Bai CG, Xie Q, et al. Prognostic value of KIT mutation in gastrointestinal stromal tumors. World J Gastroenterol. 2005;11(25): Wardelmann E, Losen I, Hans V, et al. Deletion of Trp-557 and Lys-558 in the juxtamembrane domain of the c-kit protooncogene is associated with metastatic behavior of gastrointestinal stromal tumors. Int J Cancer. 2003;106(6):
11 Background Documentation 27. Lux ML, Rubin BP, Biase TL, et al. KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol. 2000;156(3): Huss S, Künstlinger H, Wardelmann E, et al. A subset of gastrointestinal stromal tumors previously regarded as wild-type tumors carries somatic activating mutations in KIT exon 8 (p. D419del). Mod Pathol. 2013;26(7): Lasota J, Corless CL, Heinrich MC, et al. Clinicopathologic profile of gastrointestinal stromal tumors (GISTs) with primary KIT exon 13 or 17 mutations: a multicenter study of 54 cases. Mod Pathol. 2008;21(4): LaCosta J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumors. Histopathology. 2008;53(3): Agaram NP, Wong GC, Guo T, et al. Novel V600E BRAF mutations in imatinib-naive and imatinib-resistant gastrointestinal stromal tumors. Genes Chromosomes Cancer. 2008;47(10): Nannini, M, Biasco B, Astolfi A, et al. An overview on molecular biology of KIT/PDGFRA wild type (WT) gastrointestinal stromal tumours (GIST). J Med Genet. 2013;50(10): Ogino S, Gulley M, den Dunneb JT, et al. Standard mutation nomenclature in molecular diagnostics: practical and educational challenges. J Mol Diagn. 2007;9(1): den Dunnen JT, Antonarakis SE. Mutation nomenclature. Curr Protoc Hum Genet. 2003;Chapter 7, Unit Accessed October 29,
Case: The patient is a 24 year- old female who was found to have multiple mural nodules within the antrum. Solid and cystic components were noted on
 Case: The patient is a 24 year- old female who was found to have multiple mural nodules within the antrum. Solid and cystic components were noted on imaging. There is no significant past medical history.
Case: The patient is a 24 year- old female who was found to have multiple mural nodules within the antrum. Solid and cystic components were noted on imaging. There is no significant past medical history.
Protocol for the Examination of Specimens From Patients With Gastrointestinal Stromal Tumor (GIST)
 Protocol for the Examination of Specimens From Patients With Gastrointestinal Stromal Tumor (GIST) Based on AJCC/UICC TNM, 7 th edition Protocol web posting date: December 2014 Procedures Biopsy Resection
Protocol for the Examination of Specimens From Patients With Gastrointestinal Stromal Tumor (GIST) Based on AJCC/UICC TNM, 7 th edition Protocol web posting date: December 2014 Procedures Biopsy Resection
Protocol for the Examination of Specimens From Patients With Gastrointestinal Stromal Tumor (GIST)
 Protocol for the Examination of Specimens From Patients With Gastrointestinal Stromal Tumor (GIST) Based on AJCC/UICC TNM, 7 th edition Protocol web posting date: June 2012 Procedures Biopsy Resection
Protocol for the Examination of Specimens From Patients With Gastrointestinal Stromal Tumor (GIST) Based on AJCC/UICC TNM, 7 th edition Protocol web posting date: June 2012 Procedures Biopsy Resection
Protocol for the Examination of Specimens From Patients With Gastrointestinal Stromal Tumor (GIST)
 Protocol for the Examination of Specimens From Patients With Gastrointestinal Stromal Tumor (GIST) Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging
Protocol for the Examination of Specimens From Patients With Gastrointestinal Stromal Tumor (GIST) Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging
Evaluating and Reporting Gastrointestinal Stromal Tumors after Imatinib Mesylate Treatment
 The Open Pathology Journal, 2009, 3, 53-57 53 Open Access Evaluating and Reporting Gastrointestinal Stromal Tumors after Imatinib Mesylate Treatment Katie L. Dennis * and Ivan Damjanov Department of Pathology
The Open Pathology Journal, 2009, 3, 53-57 53 Open Access Evaluating and Reporting Gastrointestinal Stromal Tumors after Imatinib Mesylate Treatment Katie L. Dennis * and Ivan Damjanov Department of Pathology
Protocol for the Examination of Specimens From Patients With Primary Malignant Tumors of the Heart
 Protocol for the Examination of Specimens From Patients With Primary Malignant Tumors of the Heart Protocol applies to primary malignant cardiac tumors. Hematolymphoid neoplasms are not included. No AJCC/UICC
Protocol for the Examination of Specimens From Patients With Primary Malignant Tumors of the Heart Protocol applies to primary malignant cardiac tumors. Hematolymphoid neoplasms are not included. No AJCC/UICC
Template for Reporting Results of Monitoring Tests for Patients With Chronic Myelogenous Leukemia (BCR-ABL1+)
 Template for Reporting Results of Monitoring Tests for Patients With Chronic Myelogenous Leukemia (BCR-ABL1+) Template web posting date: July 2015 Authors Todd W. Kelley, MD, FCAP University of Utah and
Template for Reporting Results of Monitoring Tests for Patients With Chronic Myelogenous Leukemia (BCR-ABL1+) Template web posting date: July 2015 Authors Todd W. Kelley, MD, FCAP University of Utah and
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW. Update on high dose imatinib for gastrointestinal stromal tumour (GIST) harbouring KIT exon 9 mutations
 LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Update on high dose imatinib for gastrointestinal stromal tumour (GIST) harbouring KIT exon 9 mutations Summary Date prepared: May 2012 Imatinib was the first
LONDON CANCER NEW DRUGS GROUP RAPID REVIEW Update on high dose imatinib for gastrointestinal stromal tumour (GIST) harbouring KIT exon 9 mutations Summary Date prepared: May 2012 Imatinib was the first
Editorial The Significance of KIT (CD117) in Gastrointestinal Stromal Tumors
 International Journal of Surgical Pathology 12(2):93 97, 2004 Editorial The Significance of KIT (CD117) in Gastrointestinal Stromal Tumors Jason L. Hornick, MD, PhD, and Christopher D. M. Fletcher, MD,
International Journal of Surgical Pathology 12(2):93 97, 2004 Editorial The Significance of KIT (CD117) in Gastrointestinal Stromal Tumors Jason L. Hornick, MD, PhD, and Christopher D. M. Fletcher, MD,
Template for Reporting Results of Monitoring Tests for Patients With Chronic Myelogenous Leukemia (BCR-ABL1+)
 Template for Reporting Results of Monitoring Tests for Patients With Chronic Myelogenous Leukemia (BCR-ABL1+) Version: CMLBiomarkers 1.0.0.2 Protocol Posting Date: June 2017 This biomarker template is
Template for Reporting Results of Monitoring Tests for Patients With Chronic Myelogenous Leukemia (BCR-ABL1+) Version: CMLBiomarkers 1.0.0.2 Protocol Posting Date: June 2017 This biomarker template is
Therapeutic strategies for wild-type gastrointestinal stromal tumor: is it different from KIT or PDGFRA-mutated GISTs?
 Editorial Therapeutic strategies for wild-type gastrointestinal stromal tumor: is it different from KIT or PDGFRA-mutated GISTs? Toshirou Nishida Department of Surgery, National Cancer Center Hospital,
Editorial Therapeutic strategies for wild-type gastrointestinal stromal tumor: is it different from KIT or PDGFRA-mutated GISTs? Toshirou Nishida Department of Surgery, National Cancer Center Hospital,
Mesenchymal neoplasms of the gastrointestinal tract what s new? Newton ACS Wong Department of Histopathology Bristol Royal Infirmary
 Mesenchymal neoplasms of the gastrointestinal tract what s new? Newton ACS Wong Department of Histopathology Bristol Royal Infirmary Talk plan Summary from 2010 talk. What s happened since 2010. GISTs
Mesenchymal neoplasms of the gastrointestinal tract what s new? Newton ACS Wong Department of Histopathology Bristol Royal Infirmary Talk plan Summary from 2010 talk. What s happened since 2010. GISTs
The Clinical Approach to Wild Type GIST. Margaret von Mehren, MD Professor and Director of Sarcoma Oncology Fox Chase Cancer Center
 The Clinical Approach to Wild Type GIST Margaret von Mehren, MD Professor and Director of Sarcoma Oncology Fox Chase Cancer Center Disclosure slide Scientific Advisor to Novartis, Pfizer, Merck Research
The Clinical Approach to Wild Type GIST Margaret von Mehren, MD Professor and Director of Sarcoma Oncology Fox Chase Cancer Center Disclosure slide Scientific Advisor to Novartis, Pfizer, Merck Research
Template for Reporting Results of Biomarker Testing for Myeloproliferative Neoplasms
 Template for Reporting Results of Biomarker Testing for Myeloproliferative Neoplasms Template web posting date: December 2014 Authors Todd W. Kelley, MD, FCAP University of Utah and ARUP Laboratories,
Template for Reporting Results of Biomarker Testing for Myeloproliferative Neoplasms Template web posting date: December 2014 Authors Todd W. Kelley, MD, FCAP University of Utah and ARUP Laboratories,
Gastrointestinal stromal tumor with KIT mutation in neurofibromatosis type 1
 J Korean Surg Soc 2011;81:276-280 http://dx.doi.org/10.4174/jkss.2011.81.4.276 CASE REPORT JKSS Journal of the Korean Surgical Society pissn 2233-7903 ㆍ eissn 2093-0488 Gastrointestinal stromal tumor with
J Korean Surg Soc 2011;81:276-280 http://dx.doi.org/10.4174/jkss.2011.81.4.276 CASE REPORT JKSS Journal of the Korean Surgical Society pissn 2233-7903 ㆍ eissn 2093-0488 Gastrointestinal stromal tumor with
Original Article. Keywords: Gastrointestinal stromal tumors (GIST); KIT; tyrosine kinase inhibitors (TKIs); immunohistochemical staining
 Original Article Treatment of non-resectable and metastatic gastrointestinal stromal tumors: experience with the use of tyrosine kinase inhibitors in a third level hospital in Mexico Abdel Karim Dip Borunda,
Original Article Treatment of non-resectable and metastatic gastrointestinal stromal tumors: experience with the use of tyrosine kinase inhibitors in a third level hospital in Mexico Abdel Karim Dip Borunda,
C-Kit-Negative Gastrointestinal Stromal Tumor in the Stomach
 pissn : 2093-582X, eissn : 2093-5641 J Gastric Cancer 2015;15(4):290-294 http://dx.doi.org/10.5230/jgc.2015.15.4.290 Case Report C-Kit-Negative Gastrointestinal Stromal Tumor in the Stomach Ho Seok Seo,
pissn : 2093-582X, eissn : 2093-5641 J Gastric Cancer 2015;15(4):290-294 http://dx.doi.org/10.5230/jgc.2015.15.4.290 Case Report C-Kit-Negative Gastrointestinal Stromal Tumor in the Stomach Ho Seok Seo,
Glivec en KIT: immuunhistochemie en mutatie analyse in gastro-intestinale stromacel tumoren. Judith V.M.G. Bovee Patholoog LUMC
 Glivec en KIT: immuunhistochemie en mutatie analyse in gastro-intestinale stromacel tumoren Judith V.M.G. Bovee Patholoog LUMC Case 60 year old female Pain and discomfort upper abdomen MRI: tumor small
Glivec en KIT: immuunhistochemie en mutatie analyse in gastro-intestinale stromacel tumoren Judith V.M.G. Bovee Patholoog LUMC Case 60 year old female Pain and discomfort upper abdomen MRI: tumor small
Long Term Results in GIST Treatment
 Long Term Results in GIST Treatment Dr. Laurentia Gales Prof. Dr. Rodica Anghel, Dr. Xenia Bacinschi Institute of Oncology Prof Dr Al Trestioreanu Bucharest 25 th RSRMO October 15-17 Sibiu Background Gastrointestinal
Long Term Results in GIST Treatment Dr. Laurentia Gales Prof. Dr. Rodica Anghel, Dr. Xenia Bacinschi Institute of Oncology Prof Dr Al Trestioreanu Bucharest 25 th RSRMO October 15-17 Sibiu Background Gastrointestinal
Abstract. Anatomic Pathology / c-kit and PDGFRA Mutations
 Anatomic Pathology / c-kit and PDGFRA Mutations Detection of c-kit and PDGFRA Gene Mutations in Gastrointestinal Stromal Tumors Comparison of DHPLC and DNA Sequencing Methods Using a Single Population-Based
Anatomic Pathology / c-kit and PDGFRA Mutations Detection of c-kit and PDGFRA Gene Mutations in Gastrointestinal Stromal Tumors Comparison of DHPLC and DNA Sequencing Methods Using a Single Population-Based
IMATINIB MESYLATE THERAPY IN ADVANCED GASTROINTESTINAL STROMAL TUMORS: EXPERIENCE FROM A SINGLE INSTITUTE
 IMATINIB MESYLATE THERAPY IN ADVANCED GASTRINTESTINAL STRMAL TUMRS: EXPERIENCE FRM A SINGLE INSTITUTE Hui-Hua Hsiao, 1,2 Yi-Chang Liu, 2 Hui-Jen Tsai, 2 Li-Tzong Chen, 1,2 Ching-Ping Lee, 2 Chieh-Han Chuan,
IMATINIB MESYLATE THERAPY IN ADVANCED GASTRINTESTINAL STRMAL TUMRS: EXPERIENCE FRM A SINGLE INSTITUTE Hui-Hua Hsiao, 1,2 Yi-Chang Liu, 2 Hui-Jen Tsai, 2 Li-Tzong Chen, 1,2 Ching-Ping Lee, 2 Chieh-Han Chuan,
Clinicopathologic Spectrum of Gastrointestinal Stromal Tumours; Six Years Experience at King Hussein Medical Center
 Clinicopathologic Spectrum of Gastrointestinal Stromal Tumours; Six Years Experience at King Hussein Medical Center Sahem T. Alqusous MD*, Osama J. Rabadi MD, MRCS*, Ala D. Al Omari MD*, Nabeeha N.Abbasi
Clinicopathologic Spectrum of Gastrointestinal Stromal Tumours; Six Years Experience at King Hussein Medical Center Sahem T. Alqusous MD*, Osama J. Rabadi MD, MRCS*, Ala D. Al Omari MD*, Nabeeha N.Abbasi
Extended Adjuvant Therapy with Imatinib in Patients with Gastrointestinal Stromal Tumors
 Mol Diagn Ther (2013) 17:9 19 DOI 10.1007/s40291-013-0018-7 CURRENT OPINION Extended Adjuvant Therapy with Imatinib in Patients with Gastrointestinal Stromal Tumors Recommendations for Patient Selection,
Mol Diagn Ther (2013) 17:9 19 DOI 10.1007/s40291-013-0018-7 CURRENT OPINION Extended Adjuvant Therapy with Imatinib in Patients with Gastrointestinal Stromal Tumors Recommendations for Patient Selection,
Reference No: Author(s) NICaN Drugs and Therapeutics Committee. Approval date: 12/05/16. January Operational Date: Review:
 Reference No: Title: Author(s) Systemic Anti-Cancer Therapy (SACT) Guidelines for Gastro- Intestinal Stromal Tumours Dr Martin Eatock, Consultant Medical Oncologist & on behalf of the GI Oncologists Group,
Reference No: Title: Author(s) Systemic Anti-Cancer Therapy (SACT) Guidelines for Gastro- Intestinal Stromal Tumours Dr Martin Eatock, Consultant Medical Oncologist & on behalf of the GI Oncologists Group,
Immunohistochemical Staining for KIT (CD117) in Soft Tissue Sarcomas Is Very Limited in Distribution
 Anatomic Pathology / KIT IN SOFT TISSUE SARCOMAS Immunohistochemical Staining for KIT (CD117) in Soft Tissue Sarcomas Is Very Limited in Distribution Jason L. Hornick, MD, PhD, and Christopher D.M. Fletcher,
Anatomic Pathology / KIT IN SOFT TISSUE SARCOMAS Immunohistochemical Staining for KIT (CD117) in Soft Tissue Sarcomas Is Very Limited in Distribution Jason L. Hornick, MD, PhD, and Christopher D.M. Fletcher,
NET and GIST Molecular and Ancillary Studies
 NET and GIST Molecular and Ancillary Studies Dr Gill has no conflicts of interest to disclose Anthony Gill MD FRCPA Pathologist PaLMS, Royal North Shore Hospital & Professor of Surgical Pathology University
NET and GIST Molecular and Ancillary Studies Dr Gill has no conflicts of interest to disclose Anthony Gill MD FRCPA Pathologist PaLMS, Royal North Shore Hospital & Professor of Surgical Pathology University
ANTICANCER RESEARCH 28: (2008)
 Comparative Analysis of Four Histopathological Classification Systems to Discriminate Benign and Malignant Behaviour in Gastrointestinal Stromal Tumors D. VALLBÖHMER 1, H.E. MARCUS 1, S.E. BALDUS 2, J.
Comparative Analysis of Four Histopathological Classification Systems to Discriminate Benign and Malignant Behaviour in Gastrointestinal Stromal Tumors D. VALLBÖHMER 1, H.E. MARCUS 1, S.E. BALDUS 2, J.
Template for Reporting Results of Biomarker Testing of Specimens From Patients With Melanoma
 Template for Reporting Results of Biomarker Testing of Specimens From Patients With Melanoma Template web posting date: February 2015 Authors Lynette M. Sholl, MD, FCAP* Department of Pathology, Brigham
Template for Reporting Results of Biomarker Testing of Specimens From Patients With Melanoma Template web posting date: February 2015 Authors Lynette M. Sholl, MD, FCAP* Department of Pathology, Brigham
Supplementary Online Content
 Supplementary Online Content Joensuu H, Wardelmann E, Sihto H, et al. Effect of KIT and PDGFRA mutations on survival in patients with gastrointestinal stromal tumors treated with adjuvant imatinib: an
Supplementary Online Content Joensuu H, Wardelmann E, Sihto H, et al. Effect of KIT and PDGFRA mutations on survival in patients with gastrointestinal stromal tumors treated with adjuvant imatinib: an
Disclosures 3/27/2017. Case 5. Clinical History. Disclosure of Relevant Financial Relationships
 Hereditary Cancer Predisposition in Children Case 5 Cristina R. Antonescu, MD Disclosure of Relevant Financial Relationships USCAP requires that all planners (Education Committee) in a position to influence
Hereditary Cancer Predisposition in Children Case 5 Cristina R. Antonescu, MD Disclosure of Relevant Financial Relationships USCAP requires that all planners (Education Committee) in a position to influence
Template for Reporting Results of Biomarker Testing of Specimens From Patients With Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma
 Template for Reporting Results of Biomarker Testing of Specimens From Patients With Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Template web posting date: December 2014 Authors Eric Duncavage,
Template for Reporting Results of Biomarker Testing of Specimens From Patients With Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Template web posting date: December 2014 Authors Eric Duncavage,
From micro GIST to GIST: How can it happen? (Why doesn t it happen?)
 Brigham and Women s Hospital, Harvard Medical School From micro GIST to GIST: How can it happen? (Why doesn t it happen?) Jonathan A. Fletcher, M.D. Dept of Pathology Brigham and Women s Hospital Dana-Farber
Brigham and Women s Hospital, Harvard Medical School From micro GIST to GIST: How can it happen? (Why doesn t it happen?) Jonathan A. Fletcher, M.D. Dept of Pathology Brigham and Women s Hospital Dana-Farber
Template for Reporting Results of Biomarker Testing for Myeloproliferative Neoplasms
 Template for Reporting Results of Biomarker Testing for Myeloproliferative Neoplasms Version: MPNBiomarkers 1.0.0.2 Protocol Posting Date: June 2017 This biomarker template is NOT required for accreditation
Template for Reporting Results of Biomarker Testing for Myeloproliferative Neoplasms Version: MPNBiomarkers 1.0.0.2 Protocol Posting Date: June 2017 This biomarker template is NOT required for accreditation
Correlation of Imatinib Resistance with the Mutational Status of KIT and PDGFRA Genes in Gastrointestinal Stromal Tumors: a Meta-analysis
 ORIGINAL PAPER Correlation of Imatinib Resistance with the Mutational Status of KIT and PDGFRA Genes in Gastrointestinal Stromal Tumors: a Meta-analysis Ju Han Lee,* Younghye Kim,* Jung Woo Choi, Young
ORIGINAL PAPER Correlation of Imatinib Resistance with the Mutational Status of KIT and PDGFRA Genes in Gastrointestinal Stromal Tumors: a Meta-analysis Ju Han Lee,* Younghye Kim,* Jung Woo Choi, Young
Utility of BRAF V600E Mutation-Specific Immunohistochemistry in Detecting BRAF V600E-Mutated Gastrointestinal Stromal Tumors
 AJCP / Original Article Utility of BRAF V600E -Specific Immunohistochemistry in Detecting BRAF V600E-Mutated Gastrointestinal Stromal Tumors Deepa T. Patil, MD, 1 Shuang Ma, PhD, 1 Mai Konishi, 2 Paula
AJCP / Original Article Utility of BRAF V600E -Specific Immunohistochemistry in Detecting BRAF V600E-Mutated Gastrointestinal Stromal Tumors Deepa T. Patil, MD, 1 Shuang Ma, PhD, 1 Mai Konishi, 2 Paula
International Journal of Scientific & Engineering Research, Volume 7, Issue 5, May ISSN Case Report Rare Case Of Small Bowel GIST
 International Journal of Scientific & Engineering Research, Volume 7, Issue 5, May-2016 282 Case Report Rare Case Of Small Bowel GIST Shahaji G. Chavan, Sagar R. Ambre, Vinayak kshirsagar, Ashish Vashistha
International Journal of Scientific & Engineering Research, Volume 7, Issue 5, May-2016 282 Case Report Rare Case Of Small Bowel GIST Shahaji G. Chavan, Sagar R. Ambre, Vinayak kshirsagar, Ashish Vashistha
Gastrointestinal Stromal Tumors: challenges in diagnosis and treatment
 Gastrointestinal Stromal Tumors: challenges in diagnosis and treatment Waleed Hammam Mosa (1) Abdelmoiem Mourad (2) Reem Ashery (2) Ibrahim Abdelkader Salama (3) (1) Department of Clinical Oncology, Faculty
Gastrointestinal Stromal Tumors: challenges in diagnosis and treatment Waleed Hammam Mosa (1) Abdelmoiem Mourad (2) Reem Ashery (2) Ibrahim Abdelkader Salama (3) (1) Department of Clinical Oncology, Faculty
Analysis of Prognostic Factors Impacting Oncologic Outcomes After Neoadjuvant Tyrosine Kinase Inhibitor Therapy for Gastrointestinal Stromal Tumors
 Ann Surg Oncol DOI 10.1245/s10434-014-3632-7 ORIGINAL ARTICLE BONE AND SOFT TISSUE SARCOMAS Analysis of Prognostic Factors Impacting Oncologic Outcomes After Neoadjuvant Tyrosine Kinase Inhibitor Therapy
Ann Surg Oncol DOI 10.1245/s10434-014-3632-7 ORIGINAL ARTICLE BONE AND SOFT TISSUE SARCOMAS Analysis of Prognostic Factors Impacting Oncologic Outcomes After Neoadjuvant Tyrosine Kinase Inhibitor Therapy
Clinical Trials. Phase II Studies. Connective Tissue Oncology Society. Jon Trent, MD, PhD
 Clinical Trials Phase II Studies Jon Trent, MD, PhD Associate Professor Dept. of Sarcoma Medical Oncology The University of Texas, M. D. Anderson Cancer Center Connective Tissue Oncology Society GIST Overview
Clinical Trials Phase II Studies Jon Trent, MD, PhD Associate Professor Dept. of Sarcoma Medical Oncology The University of Texas, M. D. Anderson Cancer Center Connective Tissue Oncology Society GIST Overview
Gastrointestinal Stromal Tumor Causes, Risk Factors, and Prevention
 Gastrointestinal Stromal Tumor Causes, Risk Factors, and Prevention Risk Factors A risk factor is anything that affects your chance of getting a disease such as cancer. Learn more about the risk factors
Gastrointestinal Stromal Tumor Causes, Risk Factors, and Prevention Risk Factors A risk factor is anything that affects your chance of getting a disease such as cancer. Learn more about the risk factors
Response to sunitinib of a gastrointestinal stromal tumor with a rare exon 12 PDGFRA mutation
 DOI 10.1186/s13569-015-0036-9 CLINICAL SARCOMA RESEARCH CASE REPORT Response to sunitinib of a gastrointestinal stromal tumor with a rare exon 12 PDGFRA mutation Andrew S. Brohl 1*, Elizabeth G. Demicco
DOI 10.1186/s13569-015-0036-9 CLINICAL SARCOMA RESEARCH CASE REPORT Response to sunitinib of a gastrointestinal stromal tumor with a rare exon 12 PDGFRA mutation Andrew S. Brohl 1*, Elizabeth G. Demicco
Template for Reporting Results of Biomarker Testing of Specimens From Patients With Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma
 Template for Reporting Results of Biomarker Testing of Specimens From Patients With Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Version: CLLBiomarkers 1.0.0.2 Protocol Posting Date: June 2017
Template for Reporting Results of Biomarker Testing of Specimens From Patients With Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Version: CLLBiomarkers 1.0.0.2 Protocol Posting Date: June 2017
Giant gastrointestinal stromal tumor of the jejunum resected after treatment with imatinib
 Int Canc Conf J (2012) 1:41 46 DOI 10.1007/s13691-011-0007-9 CASE REPORT Giant gastrointestinal stromal tumor of the jejunum resected after treatment with imatinib Kaori Shigemitsu Takako Yamada Shinichiro
Int Canc Conf J (2012) 1:41 46 DOI 10.1007/s13691-011-0007-9 CASE REPORT Giant gastrointestinal stromal tumor of the jejunum resected after treatment with imatinib Kaori Shigemitsu Takako Yamada Shinichiro
Circulating Tumor DNA in GIST and its Implications on Treatment
 Circulating Tumor DNA in GIST and its Implications on Treatment October 2 nd 2017 Dr. Ciara Kelly Assistant Attending Physician Sarcoma Medical Oncology Service Objectives Background Liquid biopsy & ctdna
Circulating Tumor DNA in GIST and its Implications on Treatment October 2 nd 2017 Dr. Ciara Kelly Assistant Attending Physician Sarcoma Medical Oncology Service Objectives Background Liquid biopsy & ctdna
Gastrointestinal Stromal Tumor Surgery and Adjuvant Therapy
 Gastrointestinal Stromal Tumor Surgery and Adjuvant Therapy Valerie P. Grignol, MD a, Paula M. Termuhlen, MD b, * KEYWORDS GIST Gastrointestinal stromal tumor KIT PDGFRA Imatinib mesylate Gastrointestinal
Gastrointestinal Stromal Tumor Surgery and Adjuvant Therapy Valerie P. Grignol, MD a, Paula M. Termuhlen, MD b, * KEYWORDS GIST Gastrointestinal stromal tumor KIT PDGFRA Imatinib mesylate Gastrointestinal
RESEARCH COMMUNICATION. Expression of DOG1, CD117 and PDGFRA in Gastrointestinal Stromal Tumors and Correlations with Clinicopathology
 DOI:http://dx.doi.org/10.7314/APJCP.2012.13.4.1389 RESEARCH COMMUNICATION Expression of DOG1, CD117 and PDGFRA in Gastrointestinal Stromal Tumors and Correlations with Clinicopathology Xiu-Wei Sun & *,
DOI:http://dx.doi.org/10.7314/APJCP.2012.13.4.1389 RESEARCH COMMUNICATION Expression of DOG1, CD117 and PDGFRA in Gastrointestinal Stromal Tumors and Correlations with Clinicopathology Xiu-Wei Sun & *,
A) PUBLIC HEALTH B) PRESENTATION & DIAGNOSIS
 GIST (Gastrointestinal stromal tumor) Updated MARCH 2017 by Dr. Doreen Ezeife, PGY-5 Resident, University of Calgary Reviewed by Dr. Jan-Willem Henning (Staff Medical Oncologist, University of Calgary)
GIST (Gastrointestinal stromal tumor) Updated MARCH 2017 by Dr. Doreen Ezeife, PGY-5 Resident, University of Calgary Reviewed by Dr. Jan-Willem Henning (Staff Medical Oncologist, University of Calgary)
Introduction. XXIII, Institute of General Pathology, Catholic University, Rome, Italy
 Histopathology 2005, 46, 522 531. DOI: 10.1111/j.1365-2559.2005.02128.x PDGFR expression in differential diagnosis between KIT-negative gastrointestinal stromal tumours and other primary soft-tissue tumours
Histopathology 2005, 46, 522 531. DOI: 10.1111/j.1365-2559.2005.02128.x PDGFR expression in differential diagnosis between KIT-negative gastrointestinal stromal tumours and other primary soft-tissue tumours
Imatinib treatment for gastrointestinal stromal tumour (GIST)
 Personalized Medicine Review Series J. Cell. Mol. Med. Vol 14, No 1-2, 2010 pp. 42-50 Guest Editors: R. Osamura & A. Gown Imatinib treatment for gastrointestinal stromal tumour (GIST) Lisandro F. Lopes,
Personalized Medicine Review Series J. Cell. Mol. Med. Vol 14, No 1-2, 2010 pp. 42-50 Guest Editors: R. Osamura & A. Gown Imatinib treatment for gastrointestinal stromal tumour (GIST) Lisandro F. Lopes,
Advanced or Metastatic Gastrointestinal Stromal Tumors: Systemic Treatment Options
 2011;104:888 895 Advanced or Metastatic Gastrointestinal Stromal Tumors: Systemic Treatment Options MEGAN V. CARAM, MD AND SCOTT M. SCHUETZE, MD, PhD* Division of Hematology/Oncology, Department of Internal
2011;104:888 895 Advanced or Metastatic Gastrointestinal Stromal Tumors: Systemic Treatment Options MEGAN V. CARAM, MD AND SCOTT M. SCHUETZE, MD, PhD* Division of Hematology/Oncology, Department of Internal
Oncologist. The. Sarcomas. Kinase Mutations and Efficacy of Imatinib in Korean Patients with Advanced Gastrointestinal Stromal Tumors
 The Oncologist Sarcomas Kinase Mutations and Efficacy of Imatinib in Korean Patients with Advanced Gastrointestinal Stromal Tumors TAE WON KIM, a MIN-HEE RYU, a HEUNGNAM LEE, b SUN JIN SYM, b JAE-LYUN
The Oncologist Sarcomas Kinase Mutations and Efficacy of Imatinib in Korean Patients with Advanced Gastrointestinal Stromal Tumors TAE WON KIM, a MIN-HEE RYU, a HEUNGNAM LEE, b SUN JIN SYM, b JAE-LYUN
Gastrointestinal Stromal Tumors: Epidemiology and Treatment Outcomes
 Gastrointestinal Stromal Tumors: Epidemiology and Treatment Outcomes Original Article Peyvandi H 1, Haji Nasrollah E 2, Mousavian SA 3, Daryaii P 4, Yeganeh R 2, Davati A 5, Ghanei GR 6 Abstract Introduction:
Gastrointestinal Stromal Tumors: Epidemiology and Treatment Outcomes Original Article Peyvandi H 1, Haji Nasrollah E 2, Mousavian SA 3, Daryaii P 4, Yeganeh R 2, Davati A 5, Ghanei GR 6 Abstract Introduction:
Case Report Massive Intra-Abdominal Imatinib-Resistant Gastrointestinal Stromal Tumor in a 21-Year-Old Male
 Case Reports in Medicine Volume 2013, Article ID 373981, 5 pages http://dx.doi.org/10.1155/2013/373981 Case Report Massive Intra-Abdominal Imatinib-Resistant Gastrointestinal Stromal Tumor in a 21-Year-Old
Case Reports in Medicine Volume 2013, Article ID 373981, 5 pages http://dx.doi.org/10.1155/2013/373981 Case Report Massive Intra-Abdominal Imatinib-Resistant Gastrointestinal Stromal Tumor in a 21-Year-Old
Gastrointestinal Stromal Tumors. Review on Morphology, Molecular Pathology, Prognosis, and Differential Diagnosis
 Gastrointestinal Stromal Tumors Review on Morphology, Molecular Pathology, Prognosis, and Differential Diagnosis Markku Miettinen, MD; Jerzy Lasota, MD This review summarizes the definition, histogenesis,
Gastrointestinal Stromal Tumors Review on Morphology, Molecular Pathology, Prognosis, and Differential Diagnosis Markku Miettinen, MD; Jerzy Lasota, MD This review summarizes the definition, histogenesis,
Objective To determine morphologic spectrum and risk category of gastrointestinal stromal tumour (GIST) and compare with overall patient survival.
 MORPHOLOGIC SPECTRUM AND CLINICO-PATHOLOGICAL CORRELATION OF GASTROINTESTINAL STROMAL TUMOURS (GIST): AN EXPERIENCE OF SIX YEARS AT A TERTIARY CARE HOSPITAL Asim Qureshi, Hina Tariq, Zafar Ali, Nadira
MORPHOLOGIC SPECTRUM AND CLINICO-PATHOLOGICAL CORRELATION OF GASTROINTESTINAL STROMAL TUMOURS (GIST): AN EXPERIENCE OF SIX YEARS AT A TERTIARY CARE HOSPITAL Asim Qureshi, Hina Tariq, Zafar Ali, Nadira
Affiliazione autori0. Riccardo Ricci Journal Club GIPAD, settore GIST Anatomia Patologica, Università Cattolica, Roma
 GIST Manifesting as a Retroperitoneal Tumor: Clinicopathologic Immunohistochemical, and Molecular Genetic Study of 112 Cases American Journal of Surgical Pathology, 2017, 41:577-585 Miettinen M*; Felisiak-Golabek
GIST Manifesting as a Retroperitoneal Tumor: Clinicopathologic Immunohistochemical, and Molecular Genetic Study of 112 Cases American Journal of Surgical Pathology, 2017, 41:577-585 Miettinen M*; Felisiak-Golabek
Clinical practice guidelines for patients with gastrointestinal stromal tumor in Taiwan
 Yeh et al. World Journal of Surgical Oncology 2012, 10:246 WORLD JOURNAL OF SURGICAL ONCOLOGY REVIEW Clinical practice guidelines for patients with gastrointestinal stromal tumor in Taiwan Open Access
Yeh et al. World Journal of Surgical Oncology 2012, 10:246 WORLD JOURNAL OF SURGICAL ONCOLOGY REVIEW Clinical practice guidelines for patients with gastrointestinal stromal tumor in Taiwan Open Access
A 58 year old man with multiple medical problems (ulcerative colitis, alcohol abuse, hypertension, cardiovascular disease) presented with dizziness,
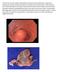 A 58 year old man with multiple medical problems (ulcerative colitis, alcohol abuse, hypertension, cardiovascular disease) presented with dizziness, hematemesis and hematochezia. An EGD showed a 5.0 cm
A 58 year old man with multiple medical problems (ulcerative colitis, alcohol abuse, hypertension, cardiovascular disease) presented with dizziness, hematemesis and hematochezia. An EGD showed a 5.0 cm
Perioperative Treatment of Gastrointestinal Stromal Tumors
 Review Article [1] January 01, 2009 By Gustavo Dos Santos Fernandes, MD [2], Charles D. Blanke, MD, FACP [3], Daniela Freitas, MD [4], Rodrigo Guedes, MD [5], and Paulo M. Hoff, MD, FACP [6] Gastrointestinal
Review Article [1] January 01, 2009 By Gustavo Dos Santos Fernandes, MD [2], Charles D. Blanke, MD, FACP [3], Daniela Freitas, MD [4], Rodrigo Guedes, MD [5], and Paulo M. Hoff, MD, FACP [6] Gastrointestinal
evaluation of a Portuguese series
 1 Life and Health Sciences Research Institute (ICVS), School of Health Sciences, University of Minho, Braga, Portugal; 2 IPATIMUP, Institute of Molecular Pathology and Immunology of the University of Porto,
1 Life and Health Sciences Research Institute (ICVS), School of Health Sciences, University of Minho, Braga, Portugal; 2 IPATIMUP, Institute of Molecular Pathology and Immunology of the University of Porto,
Case Report Unusual Appearance of a Pendulated Gastric Tumor: Always Think of GIST
 Case Reports in Surgery Volume 2012, Article ID 815941, 4 pages doi:10.1155/2012/815941 Case Report Unusual Appearance of a Pendulated Gastric Tumor: Always Think of GIST Kristel De Vogelaere, 1 Vanessa
Case Reports in Surgery Volume 2012, Article ID 815941, 4 pages doi:10.1155/2012/815941 Case Report Unusual Appearance of a Pendulated Gastric Tumor: Always Think of GIST Kristel De Vogelaere, 1 Vanessa
Images In Gastroenterology
 Images In Gastroenterology Thong-Ngam D, et al. THAI J GASTROENTEROL 2005 Vol. 6 No. 2 May - Aug. 2005 105 Imaging of Gastrointestinal Stromal Tumors Pornpim Fuangtharnthip, M.D. Narumol Hargroove, M.D.
Images In Gastroenterology Thong-Ngam D, et al. THAI J GASTROENTEROL 2005 Vol. 6 No. 2 May - Aug. 2005 105 Imaging of Gastrointestinal Stromal Tumors Pornpim Fuangtharnthip, M.D. Narumol Hargroove, M.D.
G astrointestinal stromal tumours (GISTs) are the most
 634 ORIGINAL ARTICLE The location of KIT and PDGFRA gene mutations in gastrointestinal stromal tumours is site and phenotype associated R Penzel, S Aulmann, M Moock, M Schwarzbach, R J Rieker, G Mechtersheimer...
634 ORIGINAL ARTICLE The location of KIT and PDGFRA gene mutations in gastrointestinal stromal tumours is site and phenotype associated R Penzel, S Aulmann, M Moock, M Schwarzbach, R J Rieker, G Mechtersheimer...
Yasumasa MONOBE 1), Yoshio NAOMOTO 2), Jiro HAYASHI 2), Tomoki YAMATSUJI 2), Yoshito SADAHIRA 3)
 Kawasaki Medical Journal 43(1):5 12,2017 doi:10.11482/kmj-e43(1)5 5 Case Report A case of gastrointestinal stromal tumor (GIST) with peritoneal dissemination - Imatinib re-challenged case - Yasumasa MONOBE
Kawasaki Medical Journal 43(1):5 12,2017 doi:10.11482/kmj-e43(1)5 5 Case Report A case of gastrointestinal stromal tumor (GIST) with peritoneal dissemination - Imatinib re-challenged case - Yasumasa MONOBE
Distinctive features of gastrointestinal stromal tumors arising from the colon and rectum
 Original Article Distinctive features of gastrointestinal stromal tumors arising from the colon and rectum Rebecca Zhu 1 *, Fangfang Liu 2 *, Gabriella Grisotti 1, Javier Pérez-Irizarry 3, Charles H. Cha
Original Article Distinctive features of gastrointestinal stromal tumors arising from the colon and rectum Rebecca Zhu 1 *, Fangfang Liu 2 *, Gabriella Grisotti 1, Javier Pérez-Irizarry 3, Charles H. Cha
Neoadjuvant, adjuvant and palliative treatment of gastrointestinal stromal tumours (GIST) with imatinib: a centre-based study of 17 patients
 British Journal of Cancer (2003) 89, 460 464 All rights reserved 0007 0920/03 $25.00 www.bjcancer.com Short Communication Neoadjuvant, adjuvant and palliative treatment of gastrointestinal stromal tumours
British Journal of Cancer (2003) 89, 460 464 All rights reserved 0007 0920/03 $25.00 www.bjcancer.com Short Communication Neoadjuvant, adjuvant and palliative treatment of gastrointestinal stromal tumours
3/24/2017 DENDRITIC CELL NEOPLASMS: HISTOLOGY, IMMUNOHISTOCHEMISTRY, AND MOLECULAR GENETICS. Disclosure of Relevant Financial Relationships
 DENDRITIC CELL NEOPLASMS: HISTOLOGY, IMMUNOHISTOCHEMISTRY, AND MOLECULAR GENETICS Jason L. Hornick, M.D., Ph.D. Director of Surgical Pathology and Immunohistochemistry Brigham and Women s Hospital Professor
DENDRITIC CELL NEOPLASMS: HISTOLOGY, IMMUNOHISTOCHEMISTRY, AND MOLECULAR GENETICS Jason L. Hornick, M.D., Ph.D. Director of Surgical Pathology and Immunohistochemistry Brigham and Women s Hospital Professor
Int J Clin Exp Pathol 2013;6(9): /ISSN: /IJCEP
 Int J Clin Exp Pathol 2013;6(9):1839-1846 www.ijcep.com /ISSN:1936-2625/IJCEP1307017 Original Article Value of epithelioid morphology and PDGFRA immunostaining pattern for prediction of PDGFRA mutated
Int J Clin Exp Pathol 2013;6(9):1839-1846 www.ijcep.com /ISSN:1936-2625/IJCEP1307017 Original Article Value of epithelioid morphology and PDGFRA immunostaining pattern for prediction of PDGFRA mutated
Corporate Medical Policy
 Corporate Medical Policy BRAF Gene Mutation Testing to Select Melanoma or Glioma Patients File Name: Origination: Last CAP Review: Next CAP Review: Last Review: braf_gene_mutation_testing_to_select_melanoma_or_glioma_patients_for_targeted_
Corporate Medical Policy BRAF Gene Mutation Testing to Select Melanoma or Glioma Patients File Name: Origination: Last CAP Review: Next CAP Review: Last Review: braf_gene_mutation_testing_to_select_melanoma_or_glioma_patients_for_targeted_
Understanding Your GIST Pathology Report
 Gastrointestinal Stromal Tumor Understanding Your GIST Pathology Report Jason L. Hornick, MD PhD Harvard Medical School Brigham and Women's Hospital Alexander J.F. Lazar, MD PhD Sarcoma Research Center
Gastrointestinal Stromal Tumor Understanding Your GIST Pathology Report Jason L. Hornick, MD PhD Harvard Medical School Brigham and Women's Hospital Alexander J.F. Lazar, MD PhD Sarcoma Research Center
MEDICAL GENOMICS LABORATORY. Next-Gen Sequencing and Deletion/Duplication Analysis of NF1 Only (NF1-NG)
 Next-Gen Sequencing and Deletion/Duplication Analysis of NF1 Only (NF1-NG) Ordering Information Acceptable specimen types: Fresh blood sample (3-6 ml EDTA; no time limitations associated with receipt)
Next-Gen Sequencing and Deletion/Duplication Analysis of NF1 Only (NF1-NG) Ordering Information Acceptable specimen types: Fresh blood sample (3-6 ml EDTA; no time limitations associated with receipt)
Original Article Relationship between gene mutations and protein expressions of PDGFR α and C-kit in gastrointestinal stromal tumors
 Int J Clin Exp Med 2015;8(5):7592-7598 www.ijcem.com /ISSN:1940-5901/IJCEM0006162 Original Article Relationship between gene mutations and protein expressions of PDGFR α and C-kit in gastrointestinal stromal
Int J Clin Exp Med 2015;8(5):7592-7598 www.ijcem.com /ISSN:1940-5901/IJCEM0006162 Original Article Relationship between gene mutations and protein expressions of PDGFR α and C-kit in gastrointestinal stromal
Management of gastrointestinal stromal tumors: Five years period, Tanta experience.
 Management of gastrointestinal stromal tumors: Five years period, Tanta experience Mohamed El-Shebiney 1, Alaa Maria 1 and Emad Sadaka 1 and Ayman El-Namer 2 1 Clinical Oncology department, Faculty of
Management of gastrointestinal stromal tumors: Five years period, Tanta experience Mohamed El-Shebiney 1, Alaa Maria 1 and Emad Sadaka 1 and Ayman El-Namer 2 1 Clinical Oncology department, Faculty of
Most gastrointestinal stromal tumors (GISTs) carry activating mutations of the KIT gene encoding the
 Journal of Molecular Diagnostics, Vol. 6, No. 3, August 2004 Copyright American Society for Investigative Pathology and the Association for Molecular Pathology Association of Platelet-Derived Growth Factor
Journal of Molecular Diagnostics, Vol. 6, No. 3, August 2004 Copyright American Society for Investigative Pathology and the Association for Molecular Pathology Association of Platelet-Derived Growth Factor
Department of Surgery, Division of Frontier Medical Science, Programs for Biomedical
 Case Report: Gastrointestinal Stromal Tumors of the Small Intestine in Pediatric Populations: A Case Report and Literature Review Manabu Shimomura 1,2, MD, Satoshi Ikeda 2, MD, Yuji Takakura 1, MD, Yasuo
Case Report: Gastrointestinal Stromal Tumors of the Small Intestine in Pediatric Populations: A Case Report and Literature Review Manabu Shimomura 1,2, MD, Satoshi Ikeda 2, MD, Yuji Takakura 1, MD, Yasuo
Downsizing Treatment with Tyrosine Kinase Inhibitors in Patients with Advanced Gastrointestinal Stromal Tumors Improved Resectability
 World J Surg (2010) 34:2090 2097 DOI 10.1007/s00268-010-0639-5 Downsizing Treatment with Tyrosine Kinase Inhibitors in Patients with Advanced Gastrointestinal Stromal Tumors Improved Resectability Katarina
World J Surg (2010) 34:2090 2097 DOI 10.1007/s00268-010-0639-5 Downsizing Treatment with Tyrosine Kinase Inhibitors in Patients with Advanced Gastrointestinal Stromal Tumors Improved Resectability Katarina
Intracranial Metastasis from an Esophageal Gastrointestinal Stromal Tumor
 CASE REPORT Intracranial Metastasis from an Esophageal Gastrointestinal Stromal Tumor Satoshi Hamada 1, Atsushi Itami 2, Go Watanabe 2, Shinya Nakayama 2, Eiji Tanaka 2, Masato Hojo 3, Akihiko Yoshizawa
CASE REPORT Intracranial Metastasis from an Esophageal Gastrointestinal Stromal Tumor Satoshi Hamada 1, Atsushi Itami 2, Go Watanabe 2, Shinya Nakayama 2, Eiji Tanaka 2, Masato Hojo 3, Akihiko Yoshizawa
CCR FOCUS. Resistance to Tyrosine Kinase Inhibitors in Gastrointestinal Stromal Tumors
 Resistance to Tyrosine Kinase Inhibitors in Gastrointestinal Stromal Tumors Ann W. Gramza, 1,2 Christopher L. Corless, 1,2 and Michael C. Heinrich 1,2 Abstract Gastrointestinal stromal tumors (GIST) are
Resistance to Tyrosine Kinase Inhibitors in Gastrointestinal Stromal Tumors Ann W. Gramza, 1,2 Christopher L. Corless, 1,2 and Michael C. Heinrich 1,2 Abstract Gastrointestinal stromal tumors (GIST) are
Title: KIT codon 558 insertions in gastrointestinal stromal tumors (GISTs). Analysis of 17 rare KIT mutants.*
 Elsevier Editorial System(tm) for Human Pathology Manuscript Draft Manuscript Number: YHUPA-D-07-00544R1 Title: KIT codon 558 insertions in gastrointestinal stromal tumors (GISTs). Analysis of 17 rare
Elsevier Editorial System(tm) for Human Pathology Manuscript Draft Manuscript Number: YHUPA-D-07-00544R1 Title: KIT codon 558 insertions in gastrointestinal stromal tumors (GISTs). Analysis of 17 rare
Toward a more individualised treatment of patients with gastrointestinal stromal tumour
 Toward a more individualised treatment of patients with gastrointestinal stromal tumour PhD thesis by Ivar Hompland Cand. Med. Faculty of Medicine University of Oslo 2018 Department of Oncology Norwegian
Toward a more individualised treatment of patients with gastrointestinal stromal tumour PhD thesis by Ivar Hompland Cand. Med. Faculty of Medicine University of Oslo 2018 Department of Oncology Norwegian
Florence Duffaud 1-3, Axel Le Cesne 4
 REVIEW Recent advances in managing gastrointestinal stromal tumor [version 1; referees: 2 approved] Florence Duffaud 1-3, Axel Le Cesne 4 1Service d Oncologie Médicale, CHU La Timone, Marseille, France
REVIEW Recent advances in managing gastrointestinal stromal tumor [version 1; referees: 2 approved] Florence Duffaud 1-3, Axel Le Cesne 4 1Service d Oncologie Médicale, CHU La Timone, Marseille, France
This is an author produced version of an article that appears in:
 This is an author produced version of an article that appears in: SARCOMA The internet address for this paper is: https://publications.icr.ac.uk/763/ Published text: I Judson, M Leahy, J Whelan, P Lorigan,
This is an author produced version of an article that appears in: SARCOMA The internet address for this paper is: https://publications.icr.ac.uk/763/ Published text: I Judson, M Leahy, J Whelan, P Lorigan,
Key Words. Imatinib Gleevec KIT CD117 Gastrointestinal stromal tumors
 The Oncologist Regulatory Issues: FDA The Oncologist CME Program is located online at http://cme.theoncologist.com/. To take the CME activity related to this article, you must be a registered user. Approval
The Oncologist Regulatory Issues: FDA The Oncologist CME Program is located online at http://cme.theoncologist.com/. To take the CME activity related to this article, you must be a registered user. Approval
Leiomyosarcoma Of The Intestine With Osseous Differentiation- A Rare Presentation
 International Journal Of Medical Science And Clinical Inventions Volume 2 issue 04 2015 page no. 866-871 ISSN: 2348-991X Available Online At: http://valleyinternational.net/index.php/our-jou/ijmsci Leiomyosarcoma
International Journal Of Medical Science And Clinical Inventions Volume 2 issue 04 2015 page no. 866-871 ISSN: 2348-991X Available Online At: http://valleyinternational.net/index.php/our-jou/ijmsci Leiomyosarcoma
Managing adult soft tissue sarcomas and gastrointestinal stromal tumours
 Managing adult soft tissue sarcomas and gastrointestinal stromal tumours Sarcomas and gastrointestinal stromal tumours include a wide variety of biologically diverse cancers, many of them very rare. Paolo
Managing adult soft tissue sarcomas and gastrointestinal stromal tumours Sarcomas and gastrointestinal stromal tumours include a wide variety of biologically diverse cancers, many of them very rare. Paolo
Corporate Medical Policy
 Corporate Medical Policy Molecular Analysis for Targeted Therapy for Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_analysis_for_targeted_therapy_for_non_small_cell_lung_cancer
Corporate Medical Policy Molecular Analysis for Targeted Therapy for Non-Small Cell Lung File Name: Origination: Last CAP Review: Next CAP Review: Last Review: molecular_analysis_for_targeted_therapy_for_non_small_cell_lung_cancer
NGS in tissue and liquid biopsy
 NGS in tissue and liquid biopsy Ana Vivancos, PhD Referencias So, why NGS in the clinics? 2000 Sanger Sequencing (1977-) 2016 NGS (2006-) ABIPrism (Applied Biosystems) Up to 2304 per day (96 sequences
NGS in tissue and liquid biopsy Ana Vivancos, PhD Referencias So, why NGS in the clinics? 2000 Sanger Sequencing (1977-) 2016 NGS (2006-) ABIPrism (Applied Biosystems) Up to 2304 per day (96 sequences
Morphological Features of Metastatic Gastrointestinal Stromal Tumors after Gleevec Treatment
 The Korean Journal of Pathology 2009; 43: 368-73 DOI: 10.4132/KoreanJPathol.2009.43.4.368 Morphological Features of Metastatic Gastrointestinal Stromal Tumors after Gleevec Treatment - Two Cases Report
The Korean Journal of Pathology 2009; 43: 368-73 DOI: 10.4132/KoreanJPathol.2009.43.4.368 Morphological Features of Metastatic Gastrointestinal Stromal Tumors after Gleevec Treatment - Two Cases Report
Corporate Medical Policy
 Corporate Medical Policy BRAF Gene Variant Testing to Select Melanoma or Glioma Patients File Name: Origination: Last CAP Review: Next CAP Review: Last Review: braf_gene_variant_testing_to_select_melanoma_or_glioma_patients_for_targeted_
Corporate Medical Policy BRAF Gene Variant Testing to Select Melanoma or Glioma Patients File Name: Origination: Last CAP Review: Next CAP Review: Last Review: braf_gene_variant_testing_to_select_melanoma_or_glioma_patients_for_targeted_
Description of Procedure or Service. Policy. Benefits Application
 Corporate Medical Policy KRAS, NRAS, BRAF Mutation Analysis and Related File Name: Origination: Last CAP Review: Next CAP Review: Last Review: kras_nras_braf_mutation_analysis_and_related_treatment_in_metastatic_colorectal_cancer
Corporate Medical Policy KRAS, NRAS, BRAF Mutation Analysis and Related File Name: Origination: Last CAP Review: Next CAP Review: Last Review: kras_nras_braf_mutation_analysis_and_related_treatment_in_metastatic_colorectal_cancer
Gastrointestinal Stromal Tumors in Northeastern Iran: 46 Cases During
 Original Article 161 Gastrointestinal Stromal Tumors in Northeastern Iran: 46 Cases During 2003-2012 asoumeh Salari 1, itra Ahadi 2, Seyed ousalreza Hoseini 2, Elham okhtari 3, Kamran Gafarzadehgan 4,
Original Article 161 Gastrointestinal Stromal Tumors in Northeastern Iran: 46 Cases During 2003-2012 asoumeh Salari 1, itra Ahadi 2, Seyed ousalreza Hoseini 2, Elham okhtari 3, Kamran Gafarzadehgan 4,
Clinical Analysis of Diagnosis and Treatment of Gastrointestinal Stromal Tumors (Report of 96 Cases)
 121 Clinical Analysis of Diagnosis and Treatment of Gastrointestinal Stromal Tumors (Report of 96 Cases) Yueliang Lou Xieliang Zhang Hua Chen Zhongli Zhan Cancer Hospital of Tianjin Medical University,
121 Clinical Analysis of Diagnosis and Treatment of Gastrointestinal Stromal Tumors (Report of 96 Cases) Yueliang Lou Xieliang Zhang Hua Chen Zhongli Zhan Cancer Hospital of Tianjin Medical University,
59 yo male with past medical history of prostate carcinoma, presented with upper abdominal pain
 December 2016 59 yo male with past medical history of prostate carcinoma, presented with upper abdominal pain Contributed by: Divya Sharma, MD. Fellow, Gastrointestinal Pathology, Department of Pathology
December 2016 59 yo male with past medical history of prostate carcinoma, presented with upper abdominal pain Contributed by: Divya Sharma, MD. Fellow, Gastrointestinal Pathology, Department of Pathology
Paraganglioma & Pheochromocytoma Syndromes: Genetic Risk Assessment
 Paraganglioma & Pheochromocytoma Syndromes: Genetic Risk Assessment 60 th Annual Spring Symposium for Houston Society of Clinical Pathologists Houston, TX April 6 th, 2019 Samuel Hyde, MMSc, CGC Certified
Paraganglioma & Pheochromocytoma Syndromes: Genetic Risk Assessment 60 th Annual Spring Symposium for Houston Society of Clinical Pathologists Houston, TX April 6 th, 2019 Samuel Hyde, MMSc, CGC Certified
Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial
 Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial Ronald P DeMatteo, Karla V Ballman, Cristina R Antonescu,
Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial Ronald P DeMatteo, Karla V Ballman, Cristina R Antonescu,
New and Emerging Therapies for Soft Tissue Sarcomas and GIST: Recent Clinical Data and Expert Recommendations
 New and Emerging Therapies for Soft Tissue Sarcomas and GIST: Recent Clinical Data and Expert Recommendations George D. Demetri, M.D. Director, Center for Sarcoma and Bone Oncology Dana-Farber Cancer Institute
New and Emerging Therapies for Soft Tissue Sarcomas and GIST: Recent Clinical Data and Expert Recommendations George D. Demetri, M.D. Director, Center for Sarcoma and Bone Oncology Dana-Farber Cancer Institute
GIST Exon gesteuerte Therapie adjuvant/palliativ
 GIST Exon gesteuerte Therapie adjuvant/palliativ Peter Reichardt HELIOS Klinikum Bad Saarow / Sarkomzentrum Berlin-Brandenburg Offenlegung potentieller Interessenkonflikte 1. Anstellungsverhältnis oder
GIST Exon gesteuerte Therapie adjuvant/palliativ Peter Reichardt HELIOS Klinikum Bad Saarow / Sarkomzentrum Berlin-Brandenburg Offenlegung potentieller Interessenkonflikte 1. Anstellungsverhältnis oder
Supplementary Tables. Supplementary Figures
 Supplementary Files for Zehir, Benayed et al. Mutational Landscape of Metastatic Cancer Revealed from Prospective Clinical Sequencing of 10,000 Patients Supplementary Tables Supplementary Table 1: Sample
Supplementary Files for Zehir, Benayed et al. Mutational Landscape of Metastatic Cancer Revealed from Prospective Clinical Sequencing of 10,000 Patients Supplementary Tables Supplementary Table 1: Sample
Pediatric gastrointestinal stromal tumors a review of diagnostic modalities
 Review Article Pediatric gastrointestinal stromal tumors a review of diagnostic modalities Hallie J. Quiroz 1, Brent A.Willobee 1, Matthew S. Sussman 1, Bradley R. Fox 2, Chad M. Thorson 1, Juan E. Sola
Review Article Pediatric gastrointestinal stromal tumors a review of diagnostic modalities Hallie J. Quiroz 1, Brent A.Willobee 1, Matthew S. Sussman 1, Bradley R. Fox 2, Chad M. Thorson 1, Juan E. Sola
