Adaptive Design of Affordable Clinical Trials Using Master Protocols in the Era of Precision Medicine
|
|
|
- Bruno Hodge
- 5 years ago
- Views:
Transcription
1 Adaptive Design of Affordable Clinical Trials Using Master Protocols in the Era of Precision Medicine Tze Leung Lai Dept. of Statistics, Biomedical Data Science, Computational & Mathematical Engineering; Center for Innovative Study Design; Comprehensive Cancer Institute; Stanford University Joint work with Shivaani Kummar, Ying Lu, Jing Miao, Teresa Purzner, Stanford University
2 Outline Outline Master protocols to study multiple therapies, multiple diseases, or both (Woodcock and LaVange, 2017): Mechanism-based, precision medicine trials affordable in cost, time, sample size. Examples of platform/umbrella and basket trials. Challenges in design and analyses of basket trials: adaptive design and innovations in statistical methodology, illustrated by one such trial currently planned at Stanford. Discussion
3 Table 2 of Woodcock and LaVange (2017 NEJM) Trial Description Design... B2225 Basket Phase 2... BRAF V600 Basket Early phase 2... NCI-Match Umbrella Exploratory... BATTLE-1 Umbrella Phase 2... I-SPY 2 Platform Phase 2... Lung-MAP Master protocol Phase Basket: Study single therapy for multiple disease(s)/subtypes Umbrella: Study multiple therapies for single disease Platform: Study multiple therapies that can exit and enter platform Lung-MAP: Three drugs in phase 3 for NSCLC, with four molecular targets initially and trimmed to three.
4 BRAF V600 Mutations BRAF V600 Mutations (Hyman et al., 2015) BRAF V600 mutations occur in about 50% of cutaneous melanomas and result in constitutive activation of downstream signaling through the MAPK (mitogen-activated protein kinase) pathway, based on articles in Nature (2002) and NEJM (2005). Vemurafenib (Roche/Genentech), a selective oral inhibitor of BRAF V600 kinase, is associated with response rate of 50% and improved survivals with BRAF V600E mutation-positive metastatic melanoma (NEJM 2011). Efforts by the Cancer Genome Atlas and other initiatives have identified BRAF V600 mutations in non-melanoma cancers (Lancet Oncology 2010, J Clin Oncology 2011, Nature Genetics 2013, JAMA 2014). The efficacy of vemurafenib in these non-melanoma cancers has not been systematically explored, despite its therapeutic potential.
5 BRAF V600 Basket Trial BRAF V600 Basket Trial The large number of tumor types, low frequency of BRAF V600 mutations, and the variety of some of the (non-melanoma) cancers make disease-specific studies difficult (unaffordable) to conduct. Phase 2 study of the drug for 6 baskets (NSCL, ovarian, colorectal, breast, multiple myeloma, cholangiocarcinoma) and a seventh ( all-others ) basket which permitted enrollment of patients with any other BRAF V600 mutation-positive cancer. The goal of this study was to identify promising signals of activity in individual tumor types that could then be definitively explored.
6 Trial Design and Results An adaptive Simon two-stage design was used for all tumor-specific cohorts in order to minimize the number of patients treated if vermurafenib was deemed ineffective for a specific tumor type. The primary efficacy endpoint was response rate at week 8. Kaplan-Meier methods were used to estimate progression-free and overall survival. No adjustments were made for multiple hypothesis testing that could result in positive findings. 122 adults received at least one dose of vemurafenib (20 for NSCLC, 37 for colorectal cancer, 5 for multiple myeloma, 8 for cholangocarcinoma, 18 for ECD or LCH, 34 for breast, ovarian, and other cancers). 89% of these patients had at least one previous line of therapy.
7 Trial Design and Results Vemurafenib showed (a) efficacy in BRAF V600 mutation-positive NSCLC compared to standard second-line docetexal in molecularly unselected patients, and (b) for ECD or LCH which are closely related orphan diseases with no approved therapies, the response rate was 43% and none of the patients had disease progression while receiving therapy, despite a median treatment duration of 5.9 months. One challenge in interpreting the results of basket studies is drawing inferences from small numbers of patients.
8 Challenges and How to Tackle Them Berry (2015) discusses other challenges in inference from basket trials: Even though patients have the same biomarker, different tumor sites and tumor types may have different response rates, and simply pooling trial results across tumor types may mislead interpretation. On the other hand, different tumor types may have similar response rates, and hierarchical Bayesian modeling can help borrow information across these types to compensate for the small sample sizes. Chen, Heyse and Lai (2018, Chapman & Hall/CRC) discuss the connections between frequentist error-controlling methods and Bayesian (in particular, empirical Bayes) methods to address multiplicity issues in the analysis of clinical trials data. Although they focus on evaluation of adverse outcomes of a new drug/medical product, the statistical problems are quite similar: adverse event types for the same body system-organ [genetic aberratings in organs under different basket /cancers], biological models of toxicity [mechanism-based background] of the treatment, etc. They also relate to the foundational works of Robbins, Stein and Efron in compound statistical decision theory for optimal balance between type I error and power and its relation to empirical Bayes approach.
9 Challenges and Opportunities Dr. Kummar recently collaborated with investigators at Loxo Oncology and other investigators at UCLA, USC, Harvard, Cornell, Vanderbilt, MD Anderson, and Sloan Kettering on designing and carrying out a basket trial involving seven specified cancer types and an eighth basket ( other cancers ) to evaluate the efficacy and safety of larotretinib, a highly selective TRK inhibitor produced by Lox Oncology in South San Francisco, in adults and children who had TRK fusion-positive cancers. A total of 55 patients were enrolled into one of three protocols and treated with larotretinib: a phase 1 study involving adults, a phase 1-2 study involving adults and children, and a phase 2 study involving adolescents and adults with TRK fusion-positive tumors. The phase 2 study uses the recommended dose of the drug twice daily. The dose-escalation phase 1 study and the phase 1 portion of the phase 1-2 study do not require the subjects to have TRK fusions although the combined analysis only includes patients with prospectively identified TRK fusions.
10 Challenges and Opportunities The primary endpoint for the combined analysis was the overall response assessed by an independent radiology committee. Secondary endpoints include duration of response, progression-free survival, and safety. At the data-cutoff date 7/17/2017, the overall response rate was 75%, and 7 of the patients had complete reponse and 34 had partial response (Drilon et al., 2018, NEJM). In the accompanying editorial of that issue of NEJM, André (2018) says that this study is an illustration of what is likely to be the future of drug development in rare genomic entities and that according to the Magnitude of Clinical Benefit Scale for single-arm trials recently developed by the European Society of Medical Oncology, studies that show rates of objective response of more than 60% and a median progression-free survival of more than 6 months, as the study conducted by Drilon et al. does, are considered to have the highest magnitude of clinical benefit in line with the pathway for single-arm trials of treatments of rare diseases with well-established natural histories to receive approval from regulatory agencies.
11 Challenges and Opportunities André (2018) also mentions that the study by Drilon et al. did not find any difference in efficacy among the 12 tumor histotypes (including those in the all-other basket), proving a successful trans-tumor approach in the case of TRK fusions with larotrectinib, but that some basket trials have not shown evidence of trans-tumor efficacy of targeted therapies, notably BRAF inhibitors. He points out the importance of developing statistical tools to support a claim that a drug works across tumor types and to provide a more in-depth understanding of the failure of some targets in a trans-tumor approach.
12 Adaptive Design of a Basket Trial in Drug Development Dr. Purzner has been working with a company that produces a potent and selective inhibitor of CK2 (casein kinase 2) for treatment of medulloblastoma (MB), which is a pediatric brain cancer. The drug is believed to be efficacious for other cancers that are associated with the hedgehog signaling pathway. Hence an adaptive design of a basket trial is used. The baskets includue (a) MB and rhabdomyosarcoma (RMS) for children, and (b) basel-cell carcinoma (BCC), colon, pancreatic cancers for adults, together with (c) a basket of other cancers. Because of the company s plan to proceed first with the MB and BCC indications, phase 2 trials are first initialized using novel adaptive designs that improve Simon s two-stage designs (Bartroff, Lai and Shih, 2013). If at least one of these trials does not end in futility, then proceed to the basket trial.
13 Adaptive Design of a Basket Trial in Drug Development The overall adaptive design uses a three-stage design (group sequential trial with 3 groups) as in Bartroff-Lai-Shih. It augments the theory of adaptive designs by also including baskets if the first stage ends with continuation. The theory of these 3-stage designs has been worked out in a related problem adaptive enrichment trials, an example of which is the DEFUSE 3 trial that recently appeared in NEJM (Albers et al, 2018).
14 Conclusion Woodcock and LaVange (2017) say: With multiple questions to address under a single protocol, usually in an area of unmet need, and an extensive infrastructure in place to handle data flow, master protocols are a natural environment for considering innovative trial designs. The flexibility to allow promising new therapies to enter and poor-performing therapies to discontinue usually requires some form of adaptive design, but the level of complexity of those adaptations can vary according to the objectives of the master protocol. Our recent work on adaptive design of a basket trial for a CK2 inhibitor illustrates both the new challenges and opportunities for adaptive design noted by Woodcock and LaVange, especially how the design should adapt to the objectives of the master protocol during the course of the trial.
15 Conclusion Woodcock and LaVange (2017) also point out that two types of innovation are hallmarks of master protocols: the use of a trial network with infrastructure in place to streamline trial logistics, improve data quality, and facilitate data collection and sharing; and the use of a common protocol that incorporates innovative statistical approaches to study design and data analysis, enabling a broader set of objectives to be met more effectively than would be possible in independent trials. Our medical colleagues Drs. Kummar and Purzner have shown us not only the importance of the trial network and infrastructure mentioned by Woodcock and LaVange but also the connection to biomedical companies producing these drugs, and funding the study and how they can be connected to academic networks and consortia such as PBTC (Pediatric Brain Tumor Consortium) that will run the MB phase 2 trial.
16 Reference G.W. Albers et al. (2018). Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. New England Journal of Medicine, 378: F. André (2018). Developing drugs in orphan molecular entities a paradigm under construction. New England Journal of Medicine, 378: J. Bartroff, T.L. Lai, M.C. Shih (2013). Sequential Experimentation in Clinical Trials. Springer. J. Chen, J. Heyse, T.L. Lai (2018). Medical Product Safety Evaluation: Biological Models and Statistical Methods. Chapman & Hall/CRC. A. Drilon, T.W. Laetsb, S. Kummar, D.M. Hyman et al. (2018). Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. New England Journal of Medicine, 378:
17 Reference D.M. Hyman et al. (2015). Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. New England Journal of Medicine, 373: J. Woodcock, and L.M. LaVange (2017). The Changing Face of Clinical Trials. New England Journal of Medicine, 377:
Basket and Umbrella Trial Designs in Oncology
 Basket and Umbrella Trial Designs in Oncology Eric Polley Biomedical Statistics and Informatics Mayo Clinic Polley.Eric@mayo.edu Dose Selection for Cancer Treatment Drugs Stanford Medicine May 2017 1 /
Basket and Umbrella Trial Designs in Oncology Eric Polley Biomedical Statistics and Informatics Mayo Clinic Polley.Eric@mayo.edu Dose Selection for Cancer Treatment Drugs Stanford Medicine May 2017 1 /
Basket Trials: Features, Examples, and Challenges
 : Features, s, and Challenges Lindsay A. Renfro, Ph.D. Associate Professor of Research Division of Biostatistics University of Southern California ASA Biopharm / Regulatory / Industry Statistics Workshop
: Features, s, and Challenges Lindsay A. Renfro, Ph.D. Associate Professor of Research Division of Biostatistics University of Southern California ASA Biopharm / Regulatory / Industry Statistics Workshop
Is there a Cookbook for Oncology Clinical Trials?
 Masterclass for Masters See beyond : An Oncology Brainstorm Ghent, 16th of September 2016 Is there a Cookbook for Oncology Clinical Trials? Dimitrios Zardavas MD Associate Scientific Director, Breast International
Masterclass for Masters See beyond : An Oncology Brainstorm Ghent, 16th of September 2016 Is there a Cookbook for Oncology Clinical Trials? Dimitrios Zardavas MD Associate Scientific Director, Breast International
Statistical Considerations for Novel Trial Designs: Biomarkers, Umbrellas and Baskets
 Statistical Considerations for Novel Trial Designs: Biomarkers, Umbrellas and Baskets Bibhas Chakraborty, PhD Centre for Quantitative Medicine, Duke-NUS March 29, 2015 Personalized or Precision Medicine
Statistical Considerations for Novel Trial Designs: Biomarkers, Umbrellas and Baskets Bibhas Chakraborty, PhD Centre for Quantitative Medicine, Duke-NUS March 29, 2015 Personalized or Precision Medicine
Evolution of Early Phase Trials: Clinical Trial Design in the Modern Era
 Evolution of Early Phase Trials: Clinical Trial Design in the Modern Era Shivaani Kummar, MD, FACP Professor of Medicine (Oncology) Director, Phase I Clinical Research Program Co-Director, Translational
Evolution of Early Phase Trials: Clinical Trial Design in the Modern Era Shivaani Kummar, MD, FACP Professor of Medicine (Oncology) Director, Phase I Clinical Research Program Co-Director, Translational
09,00 13,40. I Sessione. Moderatori: Giordano Beretta, Roberto Labianca
 09,00 13,40 I Sessione Moderatori: Giordano Beretta, Roberto Labianca 11,20-11,40 Gli aspetti metodologici in ambito di ricerca Valter Torri Gli aspetti metodologici in ambito di ricerca Valter Torri valter
09,00 13,40 I Sessione Moderatori: Giordano Beretta, Roberto Labianca 11,20-11,40 Gli aspetti metodologici in ambito di ricerca Valter Torri Gli aspetti metodologici in ambito di ricerca Valter Torri valter
PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective
 PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective Davy Chiodin, VP - Regulatory Science, QA and Compliance, Acerta Pharma (A Member of the AstraZeneca
PLENARY SESSION 1: CLINICAL TRIAL DESIGN IN AN ERA OF HORIZONTAL DRUG DEVELOPMENT Industry Perspective Davy Chiodin, VP - Regulatory Science, QA and Compliance, Acerta Pharma (A Member of the AstraZeneca
Efficacy of larotrectinib in adolescents and young adults with TRK fusion cancer
 Efficacy of larotrectinib in adolescents and young adults with TRK fusion cancer Soledad Gallego, 1 Valentina Boni, 2 Ulrik Lassen, 3 Anna Farago, 4 Wafik El-Deiry, 5 David Hong, 6 Blanca López-Ibor, 2
Efficacy of larotrectinib in adolescents and young adults with TRK fusion cancer Soledad Gallego, 1 Valentina Boni, 2 Ulrik Lassen, 3 Anna Farago, 4 Wafik El-Deiry, 5 David Hong, 6 Blanca López-Ibor, 2
Designs for Basket Clinical Trials and the Exploratory/Confirmatory Paradigm
 Designs for Basket Clinical Trials and the Exploratory/Confirmatory Paradigm Richard Simon, D.Sc. R Simon Consulting rmaceysimon@gmail.com http://rsimon.us Richard Simon, D.Sc. Formerly, Director Biometric
Designs for Basket Clinical Trials and the Exploratory/Confirmatory Paradigm Richard Simon, D.Sc. R Simon Consulting rmaceysimon@gmail.com http://rsimon.us Richard Simon, D.Sc. Formerly, Director Biometric
Design considerations for Phase II trials incorporating biomarkers
 Design considerations for Phase II trials incorporating biomarkers Sumithra J. Mandrekar Professor of Biostatistics, Mayo Clinic Pre-Meeting Workshop Enhancing the Design and Conduct of Phase II Studies
Design considerations for Phase II trials incorporating biomarkers Sumithra J. Mandrekar Professor of Biostatistics, Mayo Clinic Pre-Meeting Workshop Enhancing the Design and Conduct of Phase II Studies
Current Issues in Clinical Trials A Biostatistician s perspective
 Current Issues in Clinical Trials A Biostatistician s perspective Centra de Recerca Matematica CRM Seminar 10 September 2015 BARCELONA CATALUNYA Urania Dafni National and Kapodistrian University of Athens
Current Issues in Clinical Trials A Biostatistician s perspective Centra de Recerca Matematica CRM Seminar 10 September 2015 BARCELONA CATALUNYA Urania Dafni National and Kapodistrian University of Athens
Master Protocols FDA Oncology Experience
 Master Protocols FDA Oncology Experience Rajeshwari Sridhara, Ph.D. Director, Division of Biometrics V Center for Drug Evaluation and Research, USFDA Outline Regulations FDA Experience with Basket, Umbrella
Master Protocols FDA Oncology Experience Rajeshwari Sridhara, Ph.D. Director, Division of Biometrics V Center for Drug Evaluation and Research, USFDA Outline Regulations FDA Experience with Basket, Umbrella
Master Protocols for Immunotherapy Combinations
 Master Protocols for Immunotherapy Combinations Ahmad Tarhini, MD, PhD Professor of Medicine, CCLCM, CWRU Director, Melanoma and Skin Cancer Program Director, Center for Immuno-Oncology Research Cleveland
Master Protocols for Immunotherapy Combinations Ahmad Tarhini, MD, PhD Professor of Medicine, CCLCM, CWRU Director, Melanoma and Skin Cancer Program Director, Center for Immuno-Oncology Research Cleveland
Design for Targeted Therapies: Statistical Considerations
 Design for Targeted Therapies: Statistical Considerations J. Jack Lee, Ph.D. Department of Biostatistics University of Texas M. D. Anderson Cancer Center Outline Premise General Review of Statistical Designs
Design for Targeted Therapies: Statistical Considerations J. Jack Lee, Ph.D. Department of Biostatistics University of Texas M. D. Anderson Cancer Center Outline Premise General Review of Statistical Designs
Larotrectinib efficacy and safety in TRK fusion cancer: an expanded clinical dataset showing consistency in an age and tumor agnostic approach
 Larotrectinib efficacy and safety in TRK fusion cancer: an expanded clinical dataset showing consistency in an age and tumor agnostic approach Ulrik Lassen, 1 Catherine M. Albert, 2 Shivaani Kummar, 3
Larotrectinib efficacy and safety in TRK fusion cancer: an expanded clinical dataset showing consistency in an age and tumor agnostic approach Ulrik Lassen, 1 Catherine M. Albert, 2 Shivaani Kummar, 3
Accelerating Phase II-III Oncology Drug Development Through the Use of Adaptive Designs
 Accelerating Phase II-III Oncology Drug Development Through the Use of Adaptive Designs - Jonathan R. Smith, Ph.D. June 15th, 2004, DIA Annual Meeting, Washington Outline of Presentation Oncology Background
Accelerating Phase II-III Oncology Drug Development Through the Use of Adaptive Designs - Jonathan R. Smith, Ph.D. June 15th, 2004, DIA Annual Meeting, Washington Outline of Presentation Oncology Background
Innovations and Combinations for Novel Anticancer Strategies
 Innovations and Combinations for Novel Anticancer Strategies Axel-R. Hanauske, MD, Ph.D., MBA Senior Medical Fellow Oncology Early Phase Eli Lilly and Company 2017 Eli Lilly and Company Disclosure Information
Innovations and Combinations for Novel Anticancer Strategies Axel-R. Hanauske, MD, Ph.D., MBA Senior Medical Fellow Oncology Early Phase Eli Lilly and Company 2017 Eli Lilly and Company Disclosure Information
Next Generation Sequencing in Clinical Practice: Impact on Therapeutic Decision Making
 Next Generation Sequencing in Clinical Practice: Impact on Therapeutic Decision Making November 20, 2014 Capturing Value in Next Generation Sequencing Symposium Douglas Johnson MD, MSCI Vanderbilt-Ingram
Next Generation Sequencing in Clinical Practice: Impact on Therapeutic Decision Making November 20, 2014 Capturing Value in Next Generation Sequencing Symposium Douglas Johnson MD, MSCI Vanderbilt-Ingram
In 2014, the National Cancer Institute will launch a series of
 NCI S PRECISION MEDICINE INITIATIVES FOR THE NEW NCTN National Cancer Institute s Precision Medicine Initiatives for the New National Clinical Trials Network Jeffrey Abrams, MD, Barbara Conley, MD, Margaret
NCI S PRECISION MEDICINE INITIATIVES FOR THE NEW NCTN National Cancer Institute s Precision Medicine Initiatives for the New National Clinical Trials Network Jeffrey Abrams, MD, Barbara Conley, MD, Margaret
NCI Precision Medicine Trial Designs
 NCI Precision Medicine Trial Designs Shakun Malik, M.D. Head, Thoracic and Head & Neck Cancer Therapeutics Cancer Therapy Evaluation Program (CTEP) National Cancer institute/nih 1 Outline Background Current
NCI Precision Medicine Trial Designs Shakun Malik, M.D. Head, Thoracic and Head & Neck Cancer Therapeutics Cancer Therapy Evaluation Program (CTEP) National Cancer institute/nih 1 Outline Background Current
Lights and sheds of early approval of new drugs in clinical routine. Carmen Criscitiello, MD, PhD European Institute of Oncology Milan, Italy
 Lights and sheds of early approval of new drugs in clinical routine Carmen Criscitiello, MD, PhD European Institute of Oncology Milan, Italy RCT 5-10% pts «real» patients are here Clin. Pharm. Ther. 2012
Lights and sheds of early approval of new drugs in clinical routine Carmen Criscitiello, MD, PhD European Institute of Oncology Milan, Italy RCT 5-10% pts «real» patients are here Clin. Pharm. Ther. 2012
Bayesian hierarchical models for adaptive randomization in biomarker-driven studies: Umbrella and platform trials
 Bayesian hierarchical models for adaptive randomization in biomarker-driven studies: Umbrella and platform trials William T. Barry, PhD Nancy and Morris John Lurie Investigator Biostatistics and Computational
Bayesian hierarchical models for adaptive randomization in biomarker-driven studies: Umbrella and platform trials William T. Barry, PhD Nancy and Morris John Lurie Investigator Biostatistics and Computational
Trial design in the presence of non-exchangeable subpopulations
 Trial design in the presence of non-exchangeable subpopulations Brian P. Hobbs, PhD Cancer Biostatistics Section Head in The Taussig Cancer Institute Associate Staff, Department of Quantitative Health
Trial design in the presence of non-exchangeable subpopulations Brian P. Hobbs, PhD Cancer Biostatistics Section Head in The Taussig Cancer Institute Associate Staff, Department of Quantitative Health
Design Concept for a Confirmatory Basket Trial
 Design Concept for a Confirmatory Basket Trial Robert A. Beckman, MD 1 and Cong Chen, PhD 2 1 Professor of Oncology & of Biostatistics, Bioinformatics, and Biomathematics Lombardi Comprehensive Cancer
Design Concept for a Confirmatory Basket Trial Robert A. Beckman, MD 1 and Cong Chen, PhD 2 1 Professor of Oncology & of Biostatistics, Bioinformatics, and Biomathematics Lombardi Comprehensive Cancer
Oncology Drug Development Using Molecular Pathology
 Oncology Drug Development Using Molecular Pathology Introduction PRESENTERS Lee Schacter, PhD, MD, FACP Executive Medical Director, Oncology Clinipace Worldwide Martha Bonino Director, Strategic Accounts
Oncology Drug Development Using Molecular Pathology Introduction PRESENTERS Lee Schacter, PhD, MD, FACP Executive Medical Director, Oncology Clinipace Worldwide Martha Bonino Director, Strategic Accounts
Bayesian Latent Subgroup Design for Basket Trials
 Bayesian Latent Subgroup Design for Basket Trials Yiyi Chu Department of Biostatistics The University of Texas School of Public Health July 30, 2017 Outline Introduction Bayesian latent subgroup (BLAST)
Bayesian Latent Subgroup Design for Basket Trials Yiyi Chu Department of Biostatistics The University of Texas School of Public Health July 30, 2017 Outline Introduction Bayesian latent subgroup (BLAST)
CUP: Treatment by molecular profiling
 CUP: Treatment by molecular profiling George Pentheroudakis Professor of Oncology Medical School, University of Ioannina Greece Chair, ESMO Guidelines September 2018 Enterprise Interest No disclosures.
CUP: Treatment by molecular profiling George Pentheroudakis Professor of Oncology Medical School, University of Ioannina Greece Chair, ESMO Guidelines September 2018 Enterprise Interest No disclosures.
Delivering Value Through Personalized Medicine: An Industry Perspective
 Delivering Value Through Personalized Medicine: An Industry Perspective Josephine A. Sollano, Dr.PH Head, Global HEOR and Medical Communications Pfizer Oncology, NY, USA josephine.sollano@pfizer.com What
Delivering Value Through Personalized Medicine: An Industry Perspective Josephine A. Sollano, Dr.PH Head, Global HEOR and Medical Communications Pfizer Oncology, NY, USA josephine.sollano@pfizer.com What
Big data vs. the individual liver from a regulatory perspective
 Big data vs. the individual liver from a regulatory perspective Robert Schuck, Pharm.D., Ph.D. Genomics and Targeted Therapy Office of Clinical Pharmacology Center for Drug Evaluation and Research Food
Big data vs. the individual liver from a regulatory perspective Robert Schuck, Pharm.D., Ph.D. Genomics and Targeted Therapy Office of Clinical Pharmacology Center for Drug Evaluation and Research Food
New paradigms for treating metastatic melanoma
 New paradigms for treating metastatic melanoma Paul B. Chapman, MD Melanoma Clinical Director Melanoma and Immunotherapeutics Service Memorial Sloan Kettering Cancer Center, New York 20 th Century Overall
New paradigms for treating metastatic melanoma Paul B. Chapman, MD Melanoma Clinical Director Melanoma and Immunotherapeutics Service Memorial Sloan Kettering Cancer Center, New York 20 th Century Overall
Histology independent indications in oncology. The BRAF Story. Yibing Yan PhD Roche / Genentech
 Histology independent indications in oncology The BRAF Story Yibing Yan PhD Roche / Genentech 1 Introduction the discovery Case study in BRAF mutant melanoma heterogeneity within the tumour variability
Histology independent indications in oncology The BRAF Story Yibing Yan PhD Roche / Genentech 1 Introduction the discovery Case study in BRAF mutant melanoma heterogeneity within the tumour variability
Subgroup Mixable Inference for Targeted Therapies
 Subgroup Mixable Inference for Targeted Therapies Jason C. Hsu The Ohio State University Duke Industry Statistics Symposium September 2017 In collaboration with Hong Tian, Haiyan Xu, Hui-Min Lin, Ying
Subgroup Mixable Inference for Targeted Therapies Jason C. Hsu The Ohio State University Duke Industry Statistics Symposium September 2017 In collaboration with Hong Tian, Haiyan Xu, Hui-Min Lin, Ying
Use of Single-Arm Cohorts/Trials to Demonstrate Clinical Benefit for Breakthrough Therapies. Eric H. Rubin, MD Merck Research Laboratories
 Use of Single-Arm Cohorts/Trials to Demonstrate Clinical Benefit for Breakthrough Therapies Eric H. Rubin, MD Merck Research Laboratories Outline Pembrolizumab P001 study - example of multiple expansion
Use of Single-Arm Cohorts/Trials to Demonstrate Clinical Benefit for Breakthrough Therapies Eric H. Rubin, MD Merck Research Laboratories Outline Pembrolizumab P001 study - example of multiple expansion
Relevance of Innovative Trial Design Today. Basket of Baskets and other Innovative Clinical Trials
 Relevance of Innovative Trial Design Today Basket of Baskets and other Innovative Clinical Trials Outline Precision Medicine Clinical Trials Evolving Field Classic industry-sponsored design: single drug,
Relevance of Innovative Trial Design Today Basket of Baskets and other Innovative Clinical Trials Outline Precision Medicine Clinical Trials Evolving Field Classic industry-sponsored design: single drug,
Where We ve Been & Where We re Going SWOG FALL MEETING OCTOBER 5, 2018
 Celebrating 4 Years of Lung MAP Where We ve Been & Where We re Going SWOG FALL MEETING OCTOBER 5, 2018 Slide 1 Lung MAP study leadership Vali Papadimitrakopoulou, MD study chair Roy Herbst, MD PhD study
Celebrating 4 Years of Lung MAP Where We ve Been & Where We re Going SWOG FALL MEETING OCTOBER 5, 2018 Slide 1 Lung MAP study leadership Vali Papadimitrakopoulou, MD study chair Roy Herbst, MD PhD study
Should novel molecular therapies replace old knowledge of clinical tumor biology?
 Should novel molecular therapies replace old knowledge of clinical tumor biology? Danai Daliani, M.D. Director, 1 st Oncology Clinic Euroclinic of Athens Cancer Treatments Localized disease Surgery XRT
Should novel molecular therapies replace old knowledge of clinical tumor biology? Danai Daliani, M.D. Director, 1 st Oncology Clinic Euroclinic of Athens Cancer Treatments Localized disease Surgery XRT
High-quality evidence is what we use to guide medical practice.
 The new england journal of medicine Review Article The Changing Face of Clinical Trials Jeffrey M. Drazen, M.D., David P. Harrington, Ph.D., John J.V. McMurray, M.D., James H. Ware, Ph.D., and Janet Woodcock,
The new england journal of medicine Review Article The Changing Face of Clinical Trials Jeffrey M. Drazen, M.D., David P. Harrington, Ph.D., John J.V. McMurray, M.D., James H. Ware, Ph.D., and Janet Woodcock,
Regulatory Challenges in Reviewing Oncology Product Applications
 Regulatory Challenges in Reviewing Oncology Product Applications Kun He & Rajeshwari Sridhara Division of Biometrics V Office of Biostatistics Center for Drug Evaluation and Research U.S. Food and Drug
Regulatory Challenges in Reviewing Oncology Product Applications Kun He & Rajeshwari Sridhara Division of Biometrics V Office of Biostatistics Center for Drug Evaluation and Research U.S. Food and Drug
Bayer and Loxo Oncology to develop and commercialize two novel oncology therapies selectively targeting genetic drivers of cancer
 Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer and Loxo Oncology to develop and commercialize two novel oncology therapies
Investor News Not intended for U.S. and UK Media Bayer AG Investor Relations 51368 Leverkusen Germany www.investor.bayer.com Bayer and Loxo Oncology to develop and commercialize two novel oncology therapies
Case Studies in Bayesian Augmented Control Design. Nathan Enas Ji Lin Eli Lilly and Company
 Case Studies in Bayesian Augmented Control Design Nathan Enas Ji Lin Eli Lilly and Company Outline Drivers for innovation in Phase II designs Case Study #1 Pancreatic cancer Study design Analysis Learning
Case Studies in Bayesian Augmented Control Design Nathan Enas Ji Lin Eli Lilly and Company Outline Drivers for innovation in Phase II designs Case Study #1 Pancreatic cancer Study design Analysis Learning
Oncology Drug Development
 Oncology Drug Development Advantages and challenges of using stratified clinical studies Simon J Hollingsworth The 18th Anti-Tumour Drug Development Forum 21 February Japan Stratified Medicine In The Clinic
Oncology Drug Development Advantages and challenges of using stratified clinical studies Simon J Hollingsworth The 18th Anti-Tumour Drug Development Forum 21 February Japan Stratified Medicine In The Clinic
Patient Leader Education Summit. Precision Medicine: Today and Tomorrow March 31, 2017
 Patient Leader Education Summit Precision Medicine: Today and Tomorrow March 31, 2017 Precision Medicine: Presentation Outline Agenda What is a Precision Medicine What is its clinical value Overview of
Patient Leader Education Summit Precision Medicine: Today and Tomorrow March 31, 2017 Precision Medicine: Presentation Outline Agenda What is a Precision Medicine What is its clinical value Overview of
Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406)
 Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406) ASCO Annual Meeting 2017 Randomized trial of irinotecan and cetuximab with
Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406) ASCO Annual Meeting 2017 Randomized trial of irinotecan and cetuximab with
An introduction to the Essential Medicines concept: balancing innovation with public health priorities
 An introduction to the Essential Medicines concept: balancing innovation with public health priorities World Oncology Forum Lugano 20 October 2017 Nicola Magrini Secretary, WHO Expert Committee on the
An introduction to the Essential Medicines concept: balancing innovation with public health priorities World Oncology Forum Lugano 20 October 2017 Nicola Magrini Secretary, WHO Expert Committee on the
Subject: Cobimetinib (Cotellic ) Tablet
 09-J2000-53 Original Effective Date: 03/15/16 Reviewed: 11/14/18 Revised: 12/15/18 Subject: Cobimetinib (Cotellic ) Tablet THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
09-J2000-53 Original Effective Date: 03/15/16 Reviewed: 11/14/18 Revised: 12/15/18 Subject: Cobimetinib (Cotellic ) Tablet THIS MEDICAL COVERAGE GUIDELINE IS NOT AN AUTHORIZATION, CERTIFICATION, EXPLANATION
Oncology Therapeutics without Compromise APRIL 2011
 Oncology Therapeutics without Compromise APRIL 2011 Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties, including among other
Oncology Therapeutics without Compromise APRIL 2011 Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties, including among other
(Regulatory) views on Biomarker defined Subgroups
 (Regulatory) views on Biomarker defined Subgroups Norbert Benda Disclaimer: Views expressed in this presentation are the author's personal views and not necessarily the views of BfArM Biomarker defined
(Regulatory) views on Biomarker defined Subgroups Norbert Benda Disclaimer: Views expressed in this presentation are the author's personal views and not necessarily the views of BfArM Biomarker defined
My name is Dr. David Ilson, Professor of Medicine at Memorial Sloan Kettering Cancer Center and Weill Cornell Medical Center in New York, New York.
 Welcome to this CME/CE-certified activity entitled, Integrating the Latest Advances Into Clinical Experience: Data and Expert Insights From the 2016 Meeting on Gastrointestinal Cancers in San Francisco.
Welcome to this CME/CE-certified activity entitled, Integrating the Latest Advances Into Clinical Experience: Data and Expert Insights From the 2016 Meeting on Gastrointestinal Cancers in San Francisco.
Management of Brain Metastases Sanjiv S. Agarwala, MD
 Management of Brain Metastases Sanjiv S. Agarwala, MD Professor of Medicine Temple University School of Medicine Chief, Oncology & Hematology St. Luke s Cancer Center, Bethlehem, PA, USA Incidence (US):
Management of Brain Metastases Sanjiv S. Agarwala, MD Professor of Medicine Temple University School of Medicine Chief, Oncology & Hematology St. Luke s Cancer Center, Bethlehem, PA, USA Incidence (US):
Design Concept for a Confirmatory Basket Trial
 Design Concept for a Confirmatory Basket Trial Robert A. Beckman, MD 1 Professor of Oncology & of Biostatistics, Bioinformatics, and Biomathematics Lombardi Comprehensive Cancer Center and Innovation Center
Design Concept for a Confirmatory Basket Trial Robert A. Beckman, MD 1 Professor of Oncology & of Biostatistics, Bioinformatics, and Biomathematics Lombardi Comprehensive Cancer Center and Innovation Center
Looking Beyond the Standard-of- Care : The Clinical Trial Option
 1 Looking Beyond the Standard-of- Care : The Clinical Trial Option Terry Mamounas, M.D., M.P.H., F.A.C.S. Medical Director, Comprehensive Breast Program UF Health Cancer Center at Orlando Health Professor
1 Looking Beyond the Standard-of- Care : The Clinical Trial Option Terry Mamounas, M.D., M.P.H., F.A.C.S. Medical Director, Comprehensive Breast Program UF Health Cancer Center at Orlando Health Professor
BLU-554, A Novel, Potent and Selective Inhibitor of FGFR4 for the Treatment of Liver Cancer
 BLU-554, A Novel, Potent and Selective Inhibitor of FGFR4 for the Treatment of Liver Cancer Dr Klaus Hoeflich, PhD Director of Biology, Blueprint Medicines Disclosures Employee and shareholder of Blueprint
BLU-554, A Novel, Potent and Selective Inhibitor of FGFR4 for the Treatment of Liver Cancer Dr Klaus Hoeflich, PhD Director of Biology, Blueprint Medicines Disclosures Employee and shareholder of Blueprint
The questions are changing in early phase cancer clinical trials: Opportunities for novel statistical designs
 The questions are changing in early phase cancer clinical trials: Opportunities for novel statistical designs Elizabeth Garrett-Mayer, PhD Associate Professor Director of Biostatistics, Hollings Cancer
The questions are changing in early phase cancer clinical trials: Opportunities for novel statistical designs Elizabeth Garrett-Mayer, PhD Associate Professor Director of Biostatistics, Hollings Cancer
Corporate Presentation: Jefferies Global Healthcare Conference June 7, 2018
 Corporate Presentation: Jefferies Global Healthcare Conference June 7, 2018 2018 CytomX Therapeutics, Inc. 1 Forward Looking Statements Special Note Regarding Forward-Looking Statements This presentation
Corporate Presentation: Jefferies Global Healthcare Conference June 7, 2018 2018 CytomX Therapeutics, Inc. 1 Forward Looking Statements Special Note Regarding Forward-Looking Statements This presentation
Accelerating Innovation in Statistical Design
 Implementing a National Cancer Clinical Trials System for the 21 st Century, Workshop #2 Session #5: Accelerating Innovation Through Effective Partnerships Accelerating Innovation in Statistical Design
Implementing a National Cancer Clinical Trials System for the 21 st Century, Workshop #2 Session #5: Accelerating Innovation Through Effective Partnerships Accelerating Innovation in Statistical Design
Personalized medecine Biomarker
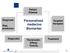 Patient Disease Diagnosis Risk prediction Personalized medecine Biomarker Targeted therapies Diagnostic Theranostic: Efficay Toxicity Treatment 1 THE CASE FOR PERSONALIZED MEDICINE IS COMPELLING.. Toxicity
Patient Disease Diagnosis Risk prediction Personalized medecine Biomarker Targeted therapies Diagnostic Theranostic: Efficay Toxicity Treatment 1 THE CASE FOR PERSONALIZED MEDICINE IS COMPELLING.. Toxicity
Personalised Healthcare (PHC) with Foundation Medicine (FMI) Fatma Elçin KINIKLI, FMI Turkey, Science Leader
 Personalised Healthcare (PHC) with Foundation Medicine (FMI) Fatma Elçin KINIKLI, FMI Turkey, Science Leader Agenda PHC Approach Provides Better Patient Outcome FMI offers Comprehensive Genomic Profiling,
Personalised Healthcare (PHC) with Foundation Medicine (FMI) Fatma Elçin KINIKLI, FMI Turkey, Science Leader Agenda PHC Approach Provides Better Patient Outcome FMI offers Comprehensive Genomic Profiling,
Update on Genetic Testing for Melanoma
 Update on Genetic Testing for Melanoma Emily Y. Chu, M.D., Ph.D. Assistant Professor of Dermatology & Pathology and Laboratory Medicine Hospital of the University of Pennsylvania February 18, 2018 AAD
Update on Genetic Testing for Melanoma Emily Y. Chu, M.D., Ph.D. Assistant Professor of Dermatology & Pathology and Laboratory Medicine Hospital of the University of Pennsylvania February 18, 2018 AAD
ArQule Jefferies Global Healthcare Conference June 2015
 ArQule Jefferies Global Healthcare Conference June 2015 1 ArQule Corporate Update Safe Harbor This presentation and other statements by ArQule may contain forward-looking statements within the meaning
ArQule Jefferies Global Healthcare Conference June 2015 1 ArQule Corporate Update Safe Harbor This presentation and other statements by ArQule may contain forward-looking statements within the meaning
The use of larotrectinib in the management of locally advanced pediatric NTRK-fusion sarcoma
 The use of larotrectinib in the management of locally advanced pediatric NTRK-fusion sarcoma DuBois SG, 1 Laetsch TW, 2 Federman N, 3 Albert CM, 4 Turpin B, 5 Nagasubramanian R, 6 Reynolds M, 7 Cruickshank
The use of larotrectinib in the management of locally advanced pediatric NTRK-fusion sarcoma DuBois SG, 1 Laetsch TW, 2 Federman N, 3 Albert CM, 4 Turpin B, 5 Nagasubramanian R, 6 Reynolds M, 7 Cruickshank
Identification of BRAF mutations in melanoma lesions with the Roche z480 instrument
 Identification of BRAF mutations in melanoma lesions with the Roche z480 instrument Márta Széll IAP, Siófok May 14, 2012 Multifactorial skin diseses: much more frequent then genodermatoses exhibit familial
Identification of BRAF mutations in melanoma lesions with the Roche z480 instrument Márta Széll IAP, Siófok May 14, 2012 Multifactorial skin diseses: much more frequent then genodermatoses exhibit familial
Summary... 2 TRANSLATIONAL RESEARCH Tumour gene expression used to direct clinical decision-making for patients with advanced cancers...
 ESMO 2016 Congress 7-11 October, 2016 Copenhagen, Denmark Table of Contents Summary... 2 TRANSLATIONAL RESEARCH... 3 Tumour gene expression used to direct clinical decision-making for patients with advanced
ESMO 2016 Congress 7-11 October, 2016 Copenhagen, Denmark Table of Contents Summary... 2 TRANSLATIONAL RESEARCH... 3 Tumour gene expression used to direct clinical decision-making for patients with advanced
Targeted therapies for advanced non-small cell lung cancer. Tom Stinchcombe Duke Cancer Insitute
 Targeted therapies for advanced non-small cell lung cancer Tom Stinchcombe Duke Cancer Insitute Topics ALK rearranged NSCLC ROS1 rearranged NSCLC EGFR mutation: exon 19/exon 21 L858R and uncommon mutations
Targeted therapies for advanced non-small cell lung cancer Tom Stinchcombe Duke Cancer Insitute Topics ALK rearranged NSCLC ROS1 rearranged NSCLC EGFR mutation: exon 19/exon 21 L858R and uncommon mutations
ArQule CorporateUpdate
 ArQule April 2015 1 ArQule CorporateUpdate Safe Harbor This presentation and other statements by ArQule may contain forwardlooking statements within the meaning of the Private Securities Litigation Reform
ArQule April 2015 1 ArQule CorporateUpdate Safe Harbor This presentation and other statements by ArQule may contain forwardlooking statements within the meaning of the Private Securities Litigation Reform
Bank of America Merrill Lynch 2018 Health Care Conference. Reinventing Therapeutic Antibodies for the Treatment of Cancer
 Bank of America Merrill Lynch 2018 Health Care Conference Reinventing Therapeutic Antibodies for the Treatment of Cancer May 17, 2018 1 Forward Looking Statements Special Note Regarding Forward-Looking
Bank of America Merrill Lynch 2018 Health Care Conference Reinventing Therapeutic Antibodies for the Treatment of Cancer May 17, 2018 1 Forward Looking Statements Special Note Regarding Forward-Looking
2015 EUROPEAN CANCER CONGRESS
 2015 EUROPEAN CANCER CONGRESS 25-29 September 2015 Vienna, Austria SUMMARY The European Cancer Congress (ECC 2015) combined the 40th European Society for Medical Oncology (ESMO) congress with the 18th
2015 EUROPEAN CANCER CONGRESS 25-29 September 2015 Vienna, Austria SUMMARY The European Cancer Congress (ECC 2015) combined the 40th European Society for Medical Oncology (ESMO) congress with the 18th
Pre-clinical PK/PD modelling of combination therapies in oncology: abemaciclib and vemurafenib
 Pre-clinical PK/PD modelling of combination therapies in oncology: abemaciclib and vemurafenib Combination Therapies in Oncology Combination therapies in oncology have been explored and used for many decades
Pre-clinical PK/PD modelling of combination therapies in oncology: abemaciclib and vemurafenib Combination Therapies in Oncology Combination therapies in oncology have been explored and used for many decades
Refining Prognosis of Early Stage Lung Cancer by Molecular Features (Part 2): Early Steps in Molecularly Defined Prognosis
 5/17/13 Refining Prognosis of Early Stage Lung Cancer by Molecular Features (Part 2): Early Steps in Molecularly Defined Prognosis Johannes Kratz, MD Post-doctoral Fellow, Thoracic Oncology Laboratory
5/17/13 Refining Prognosis of Early Stage Lung Cancer by Molecular Features (Part 2): Early Steps in Molecularly Defined Prognosis Johannes Kratz, MD Post-doctoral Fellow, Thoracic Oncology Laboratory
Precision Genetic Testing in Cancer Treatment and Prognosis
 Precision Genetic Testing in Cancer Treatment and Prognosis Deborah Cragun, PhD, MS, CGC Genetic Counseling Graduate Program Director University of South Florida Case #1 Diana is a 47 year old cancer patient
Precision Genetic Testing in Cancer Treatment and Prognosis Deborah Cragun, PhD, MS, CGC Genetic Counseling Graduate Program Director University of South Florida Case #1 Diana is a 47 year old cancer patient
The Development of Encorafenib (LGX818) and Binimetinib (MEK162) in Patients With Metastatic Melanoma
 The Development of Encorafenib (LGX818) and Binimetinib (MEK162) in Patients With Metastatic Melanoma Reinhard Dummer, 1 Keith Flaherty, 2 Richard Kefford, 3 Paolo A. Ascierto, 4 Laure Moutouh-de Parseval,
The Development of Encorafenib (LGX818) and Binimetinib (MEK162) in Patients With Metastatic Melanoma Reinhard Dummer, 1 Keith Flaherty, 2 Richard Kefford, 3 Paolo A. Ascierto, 4 Laure Moutouh-de Parseval,
UNC Cancer Epidemiology Seminar: Cancer Risk in New Users of Overactive Bladder Drugs
 February 19, 2016 UNC Cancer Epidemiology Seminar: Cancer Risk in New Users of Overactive Bladder Drugs James A. Kaye, MD, DrPH Senior Director, Epidemiology, RTI Health Solutions Collaborators: Andrea
February 19, 2016 UNC Cancer Epidemiology Seminar: Cancer Risk in New Users of Overactive Bladder Drugs James A. Kaye, MD, DrPH Senior Director, Epidemiology, RTI Health Solutions Collaborators: Andrea
Statistical controversies in clinical research: basket trials, umbrella trials, and other master protocols: a review and examples
 Annals of Oncology 28: 34 43, 2017 doi:10.1093/annonc/mdw413 Published online 11 October 2016 SPECIAL ARTICLE Statistical controversies in clinical research: basket trials, umbrella trials, and other master
Annals of Oncology 28: 34 43, 2017 doi:10.1093/annonc/mdw413 Published online 11 October 2016 SPECIAL ARTICLE Statistical controversies in clinical research: basket trials, umbrella trials, and other master
NGS IN ONCOLOGY: FDA S PERSPECTIVE
 NGS IN ONCOLOGY: FDA S PERSPECTIVE ASQ Biomed/Biotech SIG Event April 26, 2018 Gaithersburg, MD You Li, Ph.D. Division of Molecular Genetics and Pathology Food and Drug Administration (FDA) Center for
NGS IN ONCOLOGY: FDA S PERSPECTIVE ASQ Biomed/Biotech SIG Event April 26, 2018 Gaithersburg, MD You Li, Ph.D. Division of Molecular Genetics and Pathology Food and Drug Administration (FDA) Center for
PCI Biotech press release 24 th August Attachment
 PCI Biotech press release 24 th August 2018 Attachment 1 BILE DUCT CANCER CLINICAL PHASE I STUDY Cohort IV is selected dose for pivotal study limited but promising data (per Aug 2018) Parameters 1) Average
PCI Biotech press release 24 th August 2018 Attachment 1 BILE DUCT CANCER CLINICAL PHASE I STUDY Cohort IV is selected dose for pivotal study limited but promising data (per Aug 2018) Parameters 1) Average
Highlights from AACR 2015: The Emerging Potential of Immunotherapeutic Approaches in Non-Small Cell Lung Cancer
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
pan-canadian Oncology Drug Review Final Clinical Guidance Report Vemurafenib (Zelboraf) for Advanced Melanoma October 1, 2012
 pan-canadian Oncology Drug Review Final Clinical Guidance Report Vemurafenib (Zelboraf) for Advanced Melanoma October 1, 2012 DISCLAIMER Not a Substitute for Professional Advice This report is primarily
pan-canadian Oncology Drug Review Final Clinical Guidance Report Vemurafenib (Zelboraf) for Advanced Melanoma October 1, 2012 DISCLAIMER Not a Substitute for Professional Advice This report is primarily
Oncologic Imaging. Gaps & Challenges to High-Quality Cancer Diagnosis in Clinical Practice
 Oncologic Imaging Gaps & Challenges to High-Quality Cancer Diagnosis in Clinical Practice Hedvig Hricak, MD, PhD Chairman, Radiology Memorial Sloan Kettering Cancer Center National Academy of Science,
Oncologic Imaging Gaps & Challenges to High-Quality Cancer Diagnosis in Clinical Practice Hedvig Hricak, MD, PhD Chairman, Radiology Memorial Sloan Kettering Cancer Center National Academy of Science,
An update on your support of the Kaplan Cancer Research Fund Swedish Cancer Institute
 An update on your support of the Swedish Cancer Institute At Swedish, we re committed to offering our patients the newest, most innovative cancer treatment available. Through your support of the at the
An update on your support of the Swedish Cancer Institute At Swedish, we re committed to offering our patients the newest, most innovative cancer treatment available. Through your support of the at the
Corporate Presentation: 2018 Wedbush PacGrow Healthcare Conference August 14, 2018
 Corporate Presentation: 2018 Wedbush PacGrow Healthcare Conference August 14, 2018 2018 CytomX Therapeutics, Inc. 1 Forward Looking Statements Special Note Regarding Forward-Looking Statements This presentation
Corporate Presentation: 2018 Wedbush PacGrow Healthcare Conference August 14, 2018 2018 CytomX Therapeutics, Inc. 1 Forward Looking Statements Special Note Regarding Forward-Looking Statements This presentation
Supplementary Table 1: Summary of 33 Patients Treated with Abemaciclib in Dose Escalation
 Supplementary Table 1: Summary of 33 Patients Treated with Abemaciclib in Dose Escalation Cohort Dose and Frequency Age and Sex Tumor Type Number of Cycles Best Overall Response 4M Colorectal cancer -
Supplementary Table 1: Summary of 33 Patients Treated with Abemaciclib in Dose Escalation Cohort Dose and Frequency Age and Sex Tumor Type Number of Cycles Best Overall Response 4M Colorectal cancer -
7/6/2015. Cancer Related Deaths: United States. Management of NSCLC TODAY. Emerging mutations as predictive biomarkers in lung cancer: Overview
 Emerging mutations as predictive biomarkers in lung cancer: Overview Kirtee Raparia, MD Assistant Professor of Pathology Cancer Related Deaths: United States Men Lung and bronchus 28% Prostate 10% Colon
Emerging mutations as predictive biomarkers in lung cancer: Overview Kirtee Raparia, MD Assistant Professor of Pathology Cancer Related Deaths: United States Men Lung and bronchus 28% Prostate 10% Colon
Tarceva. Tarceva (erlotinib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.82 Subject: Tarceva Page: 1 of 5 Last Review Date: June 22, 2018 Tarceva Description Tarceva (erlotinib)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.82 Subject: Tarceva Page: 1 of 5 Last Review Date: June 22, 2018 Tarceva Description Tarceva (erlotinib)
ALK Fusion Oncogenes in Lung Adenocarcinoma
 ALK Fusion Oncogenes in Lung Adenocarcinoma Vincent A Miller, MD Associate Attending Physician, Thoracic Oncology Service Memorial Sloan-Kettering Cancer Center New York, New York The identification of
ALK Fusion Oncogenes in Lung Adenocarcinoma Vincent A Miller, MD Associate Attending Physician, Thoracic Oncology Service Memorial Sloan-Kettering Cancer Center New York, New York The identification of
RARE ONCOLOGY. Rapid Change, Real Promise: The Future of Rare Oncology Research ABSTRACT
 WHITE PAPER PRESENTED BY PREMIER RESEARCH Rapid Change, Real Promise: The Future of Rare Oncology Research ABSTRACT As our understanding of the genetic and molecular basis of cancer advances, rare oncology
WHITE PAPER PRESENTED BY PREMIER RESEARCH Rapid Change, Real Promise: The Future of Rare Oncology Research ABSTRACT As our understanding of the genetic and molecular basis of cancer advances, rare oncology
Phase 2b trial met key clinical objectives and is being discontinued early by the sponsor
 NEWS RELEASE SELLAS Life Sciences Provides Clinical Update on Phase 2b NeuVax (nelipepimut-s) Study in Combination with Trastuzumab in HER2 1+/2+ Breast Cancer Patients 6/1/2018 Phase 2b trial met key
NEWS RELEASE SELLAS Life Sciences Provides Clinical Update on Phase 2b NeuVax (nelipepimut-s) Study in Combination with Trastuzumab in HER2 1+/2+ Breast Cancer Patients 6/1/2018 Phase 2b trial met key
Opportunity and Challenge
 Opportunity and Challenge Implementation of Comparative Effectiveness Research: The Patient Protection and Affordable Care Act Dr. Ellen V. Sigal, Chair & Founder, Friends of Cancer Research Comparative
Opportunity and Challenge Implementation of Comparative Effectiveness Research: The Patient Protection and Affordable Care Act Dr. Ellen V. Sigal, Chair & Founder, Friends of Cancer Research Comparative
Identification and clinical validation of biomarkers for drug resistance.
 01/02/2017 1 Identification and clinical validation of biomarkers for drug resistance. Jan Stenvang & Nils Brünner, Institute of Drug Design and Pharmacology University of Copenhagen & Danish Cancer Society
01/02/2017 1 Identification and clinical validation of biomarkers for drug resistance. Jan Stenvang & Nils Brünner, Institute of Drug Design and Pharmacology University of Copenhagen & Danish Cancer Society
Paediatric Strategy Forum A new concept proposed by ACCELERATE
 A new concept proposed by ACCELERATE 1 2 s Objective - provide a unique opportunity for interaction between all stakeholders (regulators, pharmaceutical companies, clinical and researcher academics and
A new concept proposed by ACCELERATE 1 2 s Objective - provide a unique opportunity for interaction between all stakeholders (regulators, pharmaceutical companies, clinical and researcher academics and
Ovarian Cancers: Evolving Paradigms in Research and Care Report Release Wednesday, March 2, 2016
 Ovarian Cancers: Evolving Paradigms in Research and Care Report Release Wednesday, March 2, 2016 Committee Membership Jerome F. Strauss, III, (Chair), Virginia Commonwealth University School of Medicine
Ovarian Cancers: Evolving Paradigms in Research and Care Report Release Wednesday, March 2, 2016 Committee Membership Jerome F. Strauss, III, (Chair), Virginia Commonwealth University School of Medicine
Tafinlar. Tafinlar (dabrafenib) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.37 Subject: Tafinlar Page: 1 of 8 Last Review Date: September 20, 2018 Tafinlar Description Tafinlar
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.21.37 Subject: Tafinlar Page: 1 of 8 Last Review Date: September 20, 2018 Tafinlar Description Tafinlar
PLX7486 Background Information October Candidate for CRUK Combinations Alliance
 PLX7486 Background Information October 2015 Candidate for CRUK Combinations Alliance 1 Oct 2015 Plexxikon s Development Pipeline Compound Target Cancer Indication Stage of Development Pre- IND Ph1 Ph2
PLX7486 Background Information October 2015 Candidate for CRUK Combinations Alliance 1 Oct 2015 Plexxikon s Development Pipeline Compound Target Cancer Indication Stage of Development Pre- IND Ph1 Ph2
Population Enrichment Designs Case Study of a Large Multinational Trial
 Population Enrichment Designs Case Study of a Large Multinational Trial Harvard Schering-Plough Workshop Boston, 29 May 2009 Cyrus R. Mehta, Ph.D Cytel Corporation and Harvard School of Public Health email:
Population Enrichment Designs Case Study of a Large Multinational Trial Harvard Schering-Plough Workshop Boston, 29 May 2009 Cyrus R. Mehta, Ph.D Cytel Corporation and Harvard School of Public Health email:
Personalized Medicine in Oncology and the Implication for Clinical Development
 THE POWER OFx Experts. Experienc e. Execution. Personalized Medicine in Oncology and the Implication for Clinical Development Jamal Gasmi MD, PhD, Medical Director, Medpace Introduction The notable success
THE POWER OFx Experts. Experienc e. Execution. Personalized Medicine in Oncology and the Implication for Clinical Development Jamal Gasmi MD, PhD, Medical Director, Medpace Introduction The notable success
Bayesian Response-Adaptive Designs for Basket Trials. Dana-Farber Cancer Institute, Boston, Massachusetts 2
 Biometrics DOI: 0./biom. 0 0 0 0 Bayesian Response-Adaptive Designs for Basket Trials Steffen Ventz,,,* William T. Barry,, Giovanni Parmigiani,, and Lorenzo Trippa, Q Dana-Farber Cancer Institute, Boston,
Biometrics DOI: 0./biom. 0 0 0 0 Bayesian Response-Adaptive Designs for Basket Trials Steffen Ventz,,,* William T. Barry,, Giovanni Parmigiani,, and Lorenzo Trippa, Q Dana-Farber Cancer Institute, Boston,
Circulating Tumor DNA in GIST and its Implications on Treatment
 Circulating Tumor DNA in GIST and its Implications on Treatment October 2 nd 2017 Dr. Ciara Kelly Assistant Attending Physician Sarcoma Medical Oncology Service Objectives Background Liquid biopsy & ctdna
Circulating Tumor DNA in GIST and its Implications on Treatment October 2 nd 2017 Dr. Ciara Kelly Assistant Attending Physician Sarcoma Medical Oncology Service Objectives Background Liquid biopsy & ctdna
NGS ONCOPANELS: FDA S PERSPECTIVE
 NGS ONCOPANELS: FDA S PERSPECTIVE CBA Workshop: Biomarker and Application in Drug Development August 11, 2018 Rockville, MD You Li, Ph.D. Division of Molecular Genetics and Pathology Food and Drug Administration
NGS ONCOPANELS: FDA S PERSPECTIVE CBA Workshop: Biomarker and Application in Drug Development August 11, 2018 Rockville, MD You Li, Ph.D. Division of Molecular Genetics and Pathology Food and Drug Administration
Incorporating the direct assignment option into broader design frameworks
 Incorporating the direct assignment option into broader design frameworks Ming-Wen An Sumithra J. Mandrekar, Daniel J. Sargent Lucy Lu Martin J. Edelman Vassar College, Poughkeepsie NY Mayo Clinic, Rochester
Incorporating the direct assignment option into broader design frameworks Ming-Wen An Sumithra J. Mandrekar, Daniel J. Sargent Lucy Lu Martin J. Edelman Vassar College, Poughkeepsie NY Mayo Clinic, Rochester
COMPANY OVERVIEW. June CytomX Therapeutics, Inc.
 COMPANY OVERVIEW June 2016 2016 CytomX Therapeutics, Inc. Forward Looking Statements Special Note Regarding Forward-Looking Statements This presentation may contain projections and other forward-looking
COMPANY OVERVIEW June 2016 2016 CytomX Therapeutics, Inc. Forward Looking Statements Special Note Regarding Forward-Looking Statements This presentation may contain projections and other forward-looking
An adaptive phase II basket trial design
 Novartis Translation Clinical Oncology An adaptive phase II basket trial design Matt Whiley, Group Head, Early Development Biostatistics BBS / PSI One-day Event on Cancer Immunotherapy Basel 15 June 2017
Novartis Translation Clinical Oncology An adaptive phase II basket trial design Matt Whiley, Group Head, Early Development Biostatistics BBS / PSI One-day Event on Cancer Immunotherapy Basel 15 June 2017
Revolutionizing the Treatment of Cancer
 Revolutionizing the Treatment of Cancer June 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results may
Revolutionizing the Treatment of Cancer June 2014 Safe Harbor Statement The statements that follow (including projections and business trends) are forward looking statements. Rexahn's actual results may
