Reviewers' comments: Reviewer #1 (Remarks to the Author):
|
|
|
- Julius Leonard
- 5 years ago
- Views:
Transcription
1 Reviewers' comments: Reviewer #1 (Remarks to the Author): In the manuscript Rational Combination of CXCL11-Expressing Oncolytic Virus and PD-L1 Blockade Works Synergistically to Enhance Therapeutic Efficacy Liu et al. described a rationalefor a new combination therapy with a CXCL-11 expressing oncolytic virus (VV) and anti PD-L1 antibody. The authors demonstrated an increase of PD-L1 expression after VV treatment in colon and ovarian cancer models. They also showed an increase in CD8+ T cell infiltration and cytokine expression in tumor after the combination therapy. This is a well written and fairly good manuscript, with interesting results that can be promising for future clinical application. However, the manuscript would gain in significance if an orthotopic model is used (Tseng et al. Jove 2007), and the overall quality of the data has to be substantially improved. Major points 1. The authors show an increase in PD-L1 expression by real-time PCR and flow cytometry after VV treatment. Although, in Figure 1a the real-time PCR shows significant (p<0.01) increase of PD-L1 expression, the fold-change difference is very small. Please explain this result. 2. In the paper Liu et al. consider CD45- as tumor cells. This is not correct, indeed multiple cells can be CD45-, i.e. endothelial cells, hematopoietic cells, fibroblasts 3. The markers used to discriminate MDSCs and TAMs are not enough to describe these populations of cells. In particular, Gr1 and F4/80 are not the best markers for flow cytometry. The authors should consider adding Ly6G and Ly6C to discriminate between monocytes, neutrophils and macrophages. In addition, the paper could gain in impact if DC and NK cells were also analyzed. 4. Flow cytometry analysis shows a down-regulation of PD-L1 (Figure 5) in the in vivo experiments. However, the same PD-L1 clone has been used for treatments and flow cytometry staining. This could potentially result in artefacts. 5. Despite the fact that the authors have evaluated CD8 and CD4/Treg infiltration, the activation of CD8+ T cells has not been analyzed. Moreover, given that authors are suggesting a combination therapy with checkpoint inhibitors, a deeper analysis of T cell activation/exhaustion status should be included. Minor points 1. Figure 1a - lack of axis labeling. 2. Figure 1b and c: specify whether it is a ratio between infected/mock or what else? 3. Add axis labeling to figure 3b and 3c. 4. due to the virus-induced direct oncolysis (page 7): in this case the authors are speculating, this sentence should be revised. 5. Which model did you used in figure 4a? 6. Figure 4 labels (a,b,c,d) are mixed. Please adjust that. Reviewer #2 (Remarks to the Author): This paper by Lin et al. represents an important contribution in the literature to define use of oncolytic viruses with the use of check point inhibitors in the treatment of cancer. The authors demonstrated: 1) up-regulation of t-regs with oncolytic viral therapy, 2) neutralization of this immunosuppressive activity with anti-pdl1 therapy, 3) synergism of oncolytic viral therapy and check-point inhibition on cancer killing, and 4) importance of the timing for combination therapy.
2 This paper is worthy of publication. Following are my queries and suggestions for improvement. 1. Is it the presence of viral antigens, replication, lysis or the combinations that lead to increase in t-regs in the area? Have the authors looked at inactivated viruses in their models? 2. What are the toxicities associated with administration of anti-pdl1 therapy? What are the toxicities locally at tumor site and distantly (colon, skin etc.)? 3. Have the authors attempted the treatments even earlier after tumor implantation, and does that produce cures in the animals? This would have implications as to use of such strategies in an adjuvant setting. 4. On figure four, are the survival curves for anti-pdl1 alone statistically significant compared to PBS? 5. What are the comparitive results using a vaccinia virus without the chemokine CXCR11? Is the combination of a purely oncolytic virus and checkpoint inhibition effective?
3 NCOMMS Specific Point-by-Points Responses to the Reviewers by the Authors: We thank both reviewers for very constructive comments which have led to a much improved manuscript. We have addressed the comments and suggestions by the reviewers in great detail. In the following, reviewers original comments are in black and authors responses are in blue. Reviewer #1 (Remarks to the Author): General comments: In the manuscript Rational Combination of CXCL11-Expressing Oncolytic Virus and PD-L1 Blockade Works Synergistically to Enhance Therapeutic Efficacy Liu et al. described a rationale for a new combination therapy with a CXCL-11 expressing oncolytic virus (VV) and anti PD-L1 antibody. The authors demonstrated an increase of PD-L1 expression after VV treatment in colon and ovarian cancer models. They also showed an increase in CD8+ T cell infiltration and cytokine expression in tumor after the combination therapy. This is a well written and fairly good manuscript, with interesting results that can be promising for future clinical application. However, the manuscript would gain in significance if an orthotopic model is used (Tseng et al. Jove 2007), and the overall quality of the data has to be substantially improved. We appreciate your positive comments. Your suggestion of using orthotopic model is a very good one. Our clinical practice is in the treatment of patients with peritoneal metastases from colon, ovarian, and mesothelioma. For our translational program, these models mimic our patients and are therefore relevant. In the future, it will be important to examine the impact these treatments can have on the primary tumors, using a true orthotopic model. Major points: 1. The authors show an increase in PD-L1 expression by real-time PCR and flow cytometry after VV treatment. Although, in Figure 1a the real-time PCR shows significant (p<0.01) increase of PD-L1 expression, the fold-change difference is very small. Please explain this result. Given the virus replication at the wound site, should the perioperative period be an exclusion criteria for injection on future trials? The authors should discuss why or why not. We have observed the induction of PD-L1, ranging from 2 to 16-fold, in human and murine cancer cells in vitro. It is important to point out that the fold of induction is sometimes misleading. For example, when the basal level of PD-L1 expression is high (such as MC38, at 22%), it is hard to induce to a few more fold (in this case, only to 35.4% cells). When the basal level is low (such as ID8 ovarian cancer cells, at 2.4%), it is easier to induce with high fold (at ~12-fold, but the absolute number of cells stand at 18% only). As a result, PD-L1 is positive in 35.4% MC38 cells and 18% ID8 cancer cells after VV treatment, even though the fold of induction was much higher in ID8 cancer cells than in MC38 cancer cells.
4 In the previous studies by other investigators, it has been shown that cytokines such as IFN-gamma can induce the expression of PD-L1 in vivo. Thus, the induction of PD-L1 in vitro and in vivo may be mediated through very different mechanisms. There is room for improvements in the future as two or more mechanisms of induction may work in synergy. Nevertheless, the induction we have observed so far, up to 16-fold, are significant both statistically and biologically. We agree that the perioperative period should be an exclusion criterion for injection on future clinical trials as the virus could replicate at the wound sites. The recovery of replicating virus in a healing wound suggests that systemic delivery is possible, and that the selective mutations of the vaccinia virus do not differentiate tumor tissue from healing or inflamed tissue. It has been previously noted that active psoriatic skin rashes supported vaccinia replication, and it is therefore a contraindication to smallpox vaccination. Based on clinical studies from us and others, while virus recovery was not associated with any significant toxicity, actively healing wounds and acute inflammatory conditions of the skin or oral mucosa in the perioperative period or pathological conditions should be an exclusion criterion in systemic delivery of WR strain-derived oncolytic virus. ( a few sentences are added in the Discussion). 2. In the paper Liu et al. consider CD45- as tumor cells. This is not correct, indeed multiple cells can be CD45-, i.e. endothelial cells, hematopoietic cells, fibroblasts You are correct. We have corrected the statement. 3. The markers used to discriminate MDSCs and TAMs are not enough to describe these populations of cells. In particular, Gr1 and F4/80 are not the best markers for flow cytometry. The authors should consider adding Ly6G and Ly6C to discriminate between monocytes, neutrophils and macrophages. In addition, the paper could gain in impact if DC and NK cells were also analyzed. Thank you for the great suggestion. We have used now more markers to classify the groups of MDSCs and TAMs. New data are presented in Figure 5 and supplementary figures 4-5. We have examined subgroups of MDSCs and TAMs: PD-L1 +.G-MDSC (defined as CD45 + CD11c - CD11b + Ly6G + Ly6c low PD-L1 + ), PD-L1 + M-MDSC (defined as CD45 + CD11c - CD11b + Ly6G - Ly6c hi PD-L1 + ), PD-L1 + TAM1 (defined as CD45 + CD11c - CD11b + Ly6G - F4/80 + CD206 - PD-L1 + ), PD-L1 + TAM2 (defined as CD45 + CD11c - CD11b + Ly6G - F4/80 + CD206 + PD-L1 + ). As you have suggested, we have also examined DC and NK cells. The data on DC are presented in Figure 5c, while data on NK cells are presented in Supplementary Figure 5g. 4. Flow cytometry analysis shows a down-regulation of PD-L1 (Figure 5) in the in vivo experiments. However, the same PD-L1 clone has been used for treatments and flow cytometry staining. This could potentially result in artefacts. Your point is well taken. For all the experiments where anti-pd-l1 antibody was needed for both treatment and later analysis, two different antibodies were used (Figures 5 and 6). In these studies, anti-pd-l1 antibody, clone 10F.9G2 was used for therapy, while clone
5 MHI 5 was used for analysis. Both clones of Mab were obtained from the company Bio X Cell, to the best of our knowledge, they recognize different epitopes of the PD-L1. 5. Despite the fact that the authors have evaluated CD8 and CD4/Treg infiltration, the activation of CD8+ T cells has not been analyzed. Moreover, given that authors are suggesting a combination therapy with checkpoint inhibitors, a deeper analysis of T cell activation/exhaustion status should be included. Another great suggestion. We have thus conducted additional experiments to answer this. New data are presented in figure 6 and supplementary figure 6. Minor points: 1. Figure 1a - lack of axis labeling: We have now added the axis labeling. 2. Figure 1b and c: specify whether it is a ratio between infected/mock or what else? The ratio is between infected versus mock-infected cancer cells. We have added the statement to the figure legend. 3. Add axis labeling to figure 3b and 3c. We have revised the figure and the issue has been resolved. 4. due to the virus-induced direct oncolysis (page 7): in this case the authors are speculating, this sentence should be revised. That phrase has been deleted. 5. Which model did you used in figure 4a? It was MC38-luc colon cancer model. We have clarified it in the figure legend. 6. Figure 4 labels (a,b,c,d) are mixed. Please adjust that. Thank you for pointing out the errors. We have now corrected that. Reviewer #2 (Remarks to the Author): General comments This paper by Liu et al. represents an important contribution in the literature to define use of oncolytic viruses with the use of check point inhibitors in the treatment of cancer. The authors demonstrated: 1) up-regulation of t-regs with oncolytic viral therapy, 2) neutralization of this immunosuppressive activity with anti-pdl1 therapy, 3) synergism of oncolytic viral therapy and check-point inhibition on cancer killing, and 4) importance of the timing for combination therapy. This paper is worthy of publication.
6 Thank you very much. Following are my queries and suggestions for improvement. 1. Is it the presence of viral antigens, replication, lysis or the combinations that lead to increase in T-regs in the area? Have the authors looked at inactivated viruses in their models? We did observe some enhanced Treg when the virus was used alone, but not with anti-pd-l1 antibody alone or the combination treatment (Fig. 6i). We have performed studies with inactivated virus in the past, which had no effect on tumor growth. While we have not included that control in these experiments, it is our impression that replication and both early and late gene expression is required for the complete effect of the virus. In the future, it would be interesting to study what specifically affects the T-reg infiltration. 2. What are the toxicities associated with administration of anti-pdl1 therapy? What are the toxicities locally at tumor site and distantly (colon, skin etc.)? Toxicities associated with anti-pd-1 and anti-pd-l1 antibodies have been documented in human clinical trials and are a result of autoimmune activity. In our current study with mice, we have not observed any severe toxicities or obvious toxicities under the conditions used. Nevertheless, it is an important issue and we will look into that in details in the combination setting in our future studies. We have added a few sentences to discuss this issue on the Discussion (page 17). 3. Have the authors attempted the treatments even earlier after tumor implantation, and does that produce cures in the animals? This would have implications as to use of such strategies in an adjuvant setting. In an earlier tumor model (MC38 colon), our OVs alone have achieved great therapeutic results, with complete tumor-regression and long term survival in a large fraction of mice. Thus, we have not tested this combination strategy in an earlier tumor model. We do feel that an adjuvant setting is ideal for this approach. A simple statement has been added to the Discussion (Page 17). 4. On figure four, are the survival curves for anti-pdl1 alone statistically significant compared to PBS? Yes it is. In both MC38-luc colon and ID8-luc ovarian tumor models, p 0.01 between PBS and anti-pd-l1 Ab. This is clarified in the Figure and legend. 5. What are the comparative results using a vaccinia virus without the chemokine CXCR11? Is the combination of a purely oncolytic virus and checkpoint inhibition effective?
7 We have done the experiment with MC38-luc tumor model. We have observed a trend of better results when the CXCL11-armed virus was combined with anti-pd-l1 antibody, as we noticed that three mice survived for at least 120 days in the group with CXCL11-expressing virus while in the other group, all of the mice were dead by day 72. The median survival was also extended from 42.5 days to 47 days. Yet the p value was not significant between the two groups. The data are as follows. For this reason, we have modified the title and discussion a little bit to de-emphasize the role of CXCL11. We have added the following sentence to the Results section in the manuscript (on page 7): It should be noted, however, that direct comparisons between vvdd + α-pd-l1 Ab and VV + α-pd-l1 Ab did demonstrate only a trend of better, yet not statistically significant difference in therapeutic efficacy for the CXCL11 virus (data not shown).
8 REVIEWERS' COMMENTS: Reviewer #1 (Remarks to the Author): authors have answered all our questions Reviewer #2 (Remarks to the Author): The Authors have addressed the reviews adequately. This reviewer is still not convinced by the presented data of the importance of CXCL11 as a chemokine in this model. Given that they have deemphasized this in the manuscript and title, their revisions are acceptable. NCOMMS A Specific Point-by-Points Responses to the Reviewers by the Authors: We thank both reviewers for very constructive comments in the first review which have led to a much improved manuscript. For the revised version of the manuscript, they did not raise any additional questions.
A Versatile Tool to Study Immune Checkpoint Therapeutics: Syngeneic Tumor Mouse Models in vivo
 The Solution Provider for Drug Discovery in Oncology A Versatile Tool to Study Immune Checkpoint Therapeutics: Syngeneic Tumor Mouse Models in vivo Holger Weber - 1 - Drug discovery platform for oncology
The Solution Provider for Drug Discovery in Oncology A Versatile Tool to Study Immune Checkpoint Therapeutics: Syngeneic Tumor Mouse Models in vivo Holger Weber - 1 - Drug discovery platform for oncology
L1 on PyMT tumor cells but Py117 cells are more responsive to IFN-γ. (A) Flow
 A MHCI B PD-L1 Fold expression 8 6 4 2 Fold expression 3 2 1 No tx 1Gy 2Gy IFN Py117 Py117 Supplementary Figure 1. Radiation and IFN-γ enhance MHCI expression and PD- L1 on PyMT tumor cells but Py117 cells
A MHCI B PD-L1 Fold expression 8 6 4 2 Fold expression 3 2 1 No tx 1Gy 2Gy IFN Py117 Py117 Supplementary Figure 1. Radiation and IFN-γ enhance MHCI expression and PD- L1 on PyMT tumor cells but Py117 cells
Supplementary Materials for
 www.sciencetranslationalmedicine.org/cgi/content/full/8/352/352ra110/dc1 Supplementary Materials for Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy
www.sciencetranslationalmedicine.org/cgi/content/full/8/352/352ra110/dc1 Supplementary Materials for Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy
Oncolytic Immunotherapy: A Local and Systemic Antitumor Approach
 Oncolytic Immunotherapy: A Local and Systemic Antitumor Approach Oncolytic immunotherapy Oncolytic immunotherapy the use of a genetically modified virus to attack tumors and induce a systemic immune response
Oncolytic Immunotherapy: A Local and Systemic Antitumor Approach Oncolytic immunotherapy Oncolytic immunotherapy the use of a genetically modified virus to attack tumors and induce a systemic immune response
Supplementary Figure 1 Chemokine and chemokine receptor expression during muscle regeneration (a) Analysis of CR3CR1 mrna expression by real time-pcr
 Supplementary Figure 1 Chemokine and chemokine receptor expression during muscle regeneration (a) Analysis of CR3CR1 mrna expression by real time-pcr at day 0, 1, 4, 10 and 21 post- muscle injury. (b)
Supplementary Figure 1 Chemokine and chemokine receptor expression during muscle regeneration (a) Analysis of CR3CR1 mrna expression by real time-pcr at day 0, 1, 4, 10 and 21 post- muscle injury. (b)
Supplementary Figure 1: TSLP receptor skin expression in dcssc. A: Healthy control (HC) skin with TSLP receptor expression in brown (10x
 Supplementary Figure 1: TSLP receptor skin expression in dcssc. A: Healthy control (HC) skin with TSLP receptor expression in brown (10x magnification). B: Second HC skin stained for TSLP receptor in brown
Supplementary Figure 1: TSLP receptor skin expression in dcssc. A: Healthy control (HC) skin with TSLP receptor expression in brown (10x magnification). B: Second HC skin stained for TSLP receptor in brown
4. Th1-related gene expression in infected versus mock-infected controls from Fig. 2 with gene annotation.
 List of supplemental information 1. Graph of mouse weight loss during course of infection- Line graphs showing mouse weight data during course of infection days 1 to 10 post-infections (p.i.). 2. Graph
List of supplemental information 1. Graph of mouse weight loss during course of infection- Line graphs showing mouse weight data during course of infection days 1 to 10 post-infections (p.i.). 2. Graph
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): This manuscript builds on the recently published observation by the same investigators that TNBC tumors with Ras/MAPK activation have decreased
Reviewers' comments: Reviewer #1 (Remarks to the Author): This manuscript builds on the recently published observation by the same investigators that TNBC tumors with Ras/MAPK activation have decreased
Supplementary Table 1 Clinicopathological characteristics of 35 patients with CRCs
 Supplementary Table Clinicopathological characteristics of 35 patients with CRCs Characteristics Type-A CRC Type-B CRC P value Sex Male / Female 9 / / 8.5 Age (years) Median (range) 6. (9 86) 6.5 (9 76).95
Supplementary Table Clinicopathological characteristics of 35 patients with CRCs Characteristics Type-A CRC Type-B CRC P value Sex Male / Female 9 / / 8.5 Age (years) Median (range) 6. (9 86) 6.5 (9 76).95
Supporting Information
 Supporting Information Aldridge et al. 10.1073/pnas.0900655106 Fig. S1. Flow diagram of sublethal (a) and lethal (b) influenza virus infections. (a) Infection of lung epithelial cells by influenza virus
Supporting Information Aldridge et al. 10.1073/pnas.0900655106 Fig. S1. Flow diagram of sublethal (a) and lethal (b) influenza virus infections. (a) Infection of lung epithelial cells by influenza virus
Immunologic Effects of Clinical Stage FAK & PI3K-Delta/Gamma Inhibitors
 1 Immunologic Effects of Clinical Stage FAK & PI3K-Delta/Gamma Inhibitors Jonathan Pachter, Ph.D - Chief Scientific Officer 3rd Annual Immuno-Oncology Congress, May 24, 2018 Disclosures 2 I am an employee
1 Immunologic Effects of Clinical Stage FAK & PI3K-Delta/Gamma Inhibitors Jonathan Pachter, Ph.D - Chief Scientific Officer 3rd Annual Immuno-Oncology Congress, May 24, 2018 Disclosures 2 I am an employee
Supplementary Information. Tissue-wide immunity against Leishmania. through collective production of nitric oxide
 Supplementary Information Tissue-wide immunity against Leishmania through collective production of nitric oxide Romain Olekhnovitch, Bernhard Ryffel, Andreas J. Müller and Philippe Bousso Supplementary
Supplementary Information Tissue-wide immunity against Leishmania through collective production of nitric oxide Romain Olekhnovitch, Bernhard Ryffel, Andreas J. Müller and Philippe Bousso Supplementary
Supplementary Table 1
 Supplementary Table 1 Flow Cytometry Antibodies Antibody Fluorochrome Clone Vendor CD45 PE-cyanine 7 30-F11 D ioscience CD3 Pacific lue 17A2 iolegend (San Diego, CA) CD11b APC M1/70 iolegend (San Diego,
Supplementary Table 1 Flow Cytometry Antibodies Antibody Fluorochrome Clone Vendor CD45 PE-cyanine 7 30-F11 D ioscience CD3 Pacific lue 17A2 iolegend (San Diego, CA) CD11b APC M1/70 iolegend (San Diego,
Oncolytic Virotherapy: Targeting Cancer Stem Cells
 Oncolytic Virotherapy: Targeting Cancer Stem Cells Cancer Stem Cells (CSCs) or Cancer Initiating Cells (CICs) A consensus of five defining criteria has been established to affirm the existence of CICs:
Oncolytic Virotherapy: Targeting Cancer Stem Cells Cancer Stem Cells (CSCs) or Cancer Initiating Cells (CICs) A consensus of five defining criteria has been established to affirm the existence of CICs:
Tumors arise from accumulated genetic mutations. Tumor Immunology (Cancer)
 Tumor Immunology (Cancer) Tumors arise from accumulated genetic mutations Robert Beatty MCB150 Mutations Usually have >6 mutations in both activation/growth factors and tumor suppressor genes. Types of
Tumor Immunology (Cancer) Tumors arise from accumulated genetic mutations Robert Beatty MCB150 Mutations Usually have >6 mutations in both activation/growth factors and tumor suppressor genes. Types of
SUPPLEMENTARY METHODS
 SUPPLEMENTARY METHODS Histological analysis. Colonic tissues were collected from 5 parts of the middle colon on day 7 after the start of DSS treatment, and then were cut into segments, fixed with 4% paraformaldehyde,
SUPPLEMENTARY METHODS Histological analysis. Colonic tissues were collected from 5 parts of the middle colon on day 7 after the start of DSS treatment, and then were cut into segments, fixed with 4% paraformaldehyde,
SUPPLEMENTARY INFORMATION
 Table S1: Combination Immunotherapy: Small Molecules that Boost Immune Response in Combination with Other Agents Target Compound (MOA) NLG919 and indoximod 1- MT Combination Model Observation Reference
Table S1: Combination Immunotherapy: Small Molecules that Boost Immune Response in Combination with Other Agents Target Compound (MOA) NLG919 and indoximod 1- MT Combination Model Observation Reference
sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5
 sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
sfigure 1 Styx mutant mice recapitulate the phenotype of SHIP -/- mice. (A) Analysis of the genomic sequences of a styx mutant reveals a T to A transversion in the donor splice site of intron 5 (GTAAC
Immuno-Oncology: Perspectives on Current Therapies & Future Developments
 Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/medical-industry-feature/immuno-oncology-perspectives-currenttherapies-future-developments/9502/
Transcript Details This is a transcript of an educational program accessible on the ReachMD network. Details about the program and additional media formats for the program are accessible by visiting: https://reachmd.com/programs/medical-industry-feature/immuno-oncology-perspectives-currenttherapies-future-developments/9502/
C. Data & methodology: validity of approach, quality of data, quality of presentation Excellent.
 Reviewers' comments: Reviewer #1 (Immunotherapy expert) (Remarks to the Author): A. Summary of the key results This study elegantly demonstrates the sequential effects of bevacizumab followed by bevacizumab
Reviewers' comments: Reviewer #1 (Immunotherapy expert) (Remarks to the Author): A. Summary of the key results This study elegantly demonstrates the sequential effects of bevacizumab followed by bevacizumab
Suppl Video: Tumor cells (green) and monocytes (white) are seeded on a confluent endothelial
 Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Supplementary Information Häuselmann et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade
Emerging Concepts of Cancer Immunotherapy
 Emerging Concepts of Cancer Immunotherapy Jeffrey Schlom, Ph.D. Laboratory of Tumor Immunology and Biology (LTIB) Center for Cancer Research National Cancer Institute, NIH Immune Cell Infiltrate in Primary
Emerging Concepts of Cancer Immunotherapy Jeffrey Schlom, Ph.D. Laboratory of Tumor Immunology and Biology (LTIB) Center for Cancer Research National Cancer Institute, NIH Immune Cell Infiltrate in Primary
Title: Oral administration of PPC enhances antigen-specific CD8+ T cell responses while reducing IgE levels in sensitized mice.
 Author's response to reviews Title: Oral administration of PPC enhances antigen-specific CD8+ T cell responses while reducing IgE levels in sensitized mice. Authors: Mike Burrows (mburrows@tampabayresearch.org)
Author's response to reviews Title: Oral administration of PPC enhances antigen-specific CD8+ T cell responses while reducing IgE levels in sensitized mice. Authors: Mike Burrows (mburrows@tampabayresearch.org)
2/16/2018. The Immune System and Cancer. Fatal Melanoma Transferred in a Donated Kidney 16 years after Melanoma Surgery
 C007: Immunology of Melanoma: Mechanisms of Immune Therapies Delphine J. Lee, MD, PhD Chief and Program Director, Dermatology, Harbor UCLA Medical Center Principal Investigator, Los Angeles Biomedical
C007: Immunology of Melanoma: Mechanisms of Immune Therapies Delphine J. Lee, MD, PhD Chief and Program Director, Dermatology, Harbor UCLA Medical Center Principal Investigator, Los Angeles Biomedical
Supplementary Figure 1. Immune profiles of untreated and PD-1 blockade resistant EGFR and Kras mouse lung tumors (a) Total lung weight of untreated
 1 Supplementary Figure 1. Immune profiles of untreated and PD-1 blockade resistant EGFR and Kras mouse lung tumors (a) Total lung weight of untreated (U) EGFR TL mice (n=7), Kras mice (n=7), PD-1 blockade
1 Supplementary Figure 1. Immune profiles of untreated and PD-1 blockade resistant EGFR and Kras mouse lung tumors (a) Total lung weight of untreated (U) EGFR TL mice (n=7), Kras mice (n=7), PD-1 blockade
Cancer immunotherapy with oncolytic viruses: more than just lysis
 Cancer immunotherapy with oncolytic viruses: more than just lysis Dmitriy Zamarin MD PhD Assistant Attending, Immune Therapeutics Center Memorial Sloan-Kettering Cancer Center New York, NY BCAN Think Tank
Cancer immunotherapy with oncolytic viruses: more than just lysis Dmitriy Zamarin MD PhD Assistant Attending, Immune Therapeutics Center Memorial Sloan-Kettering Cancer Center New York, NY BCAN Think Tank
NKTR-214 plus NKTR-262, a Scientifically-Guided Rational Combination Approach for Immune Oncology
 plus NKTR-262, a Scientifically-Guided Rational Combination Approach for Immune Oncology Jonathan Zalevsky SVP, Biology and Preclinical Development Nektar Therapeutics World Preclinical Congress, 2017
plus NKTR-262, a Scientifically-Guided Rational Combination Approach for Immune Oncology Jonathan Zalevsky SVP, Biology and Preclinical Development Nektar Therapeutics World Preclinical Congress, 2017
June IMMUNE DESIGN The in vivo generation of cytotoxic CD8 T cells (CTLs)
 June 2015 IMMUNE DESIGN The in vivo generation of cytotoxic CD8 T cells (CTLs) 1 Forward-looking Statements This presentation contains forward-looking statements with respect to, among other things, our
June 2015 IMMUNE DESIGN The in vivo generation of cytotoxic CD8 T cells (CTLs) 1 Forward-looking Statements This presentation contains forward-looking statements with respect to, among other things, our
David L. Bartlett, M.D. University of Pittsburgh
 A Phase I Dose-Escalation Trial of vvdd- CDSR (Double-Deleted Vaccinia Virus Plus CD/SMR) Administered by Intratumoral Injection in Patients with Superficial Injectable Tumors David L. Bartlett, M.D. University
A Phase I Dose-Escalation Trial of vvdd- CDSR (Double-Deleted Vaccinia Virus Plus CD/SMR) Administered by Intratumoral Injection in Patients with Superficial Injectable Tumors David L. Bartlett, M.D. University
Supplemental Materials. Stromal Modulation Reverses Primary Resistance to Immune Checkpoint Blockade in. Pancreatic Cancer.
 Supplemental Materials Stromal Modulation Reverses Primary Resistance to Immune Checkpoint Blockade in Pancreatic Cancer Jun Zhao 1, Zhilan Xiao 2, 3, Tingting Li 1, 4, Huiqin Chen 5, Ying Yuan 5, Alan
Supplemental Materials Stromal Modulation Reverses Primary Resistance to Immune Checkpoint Blockade in Pancreatic Cancer Jun Zhao 1, Zhilan Xiao 2, 3, Tingting Li 1, 4, Huiqin Chen 5, Ying Yuan 5, Alan
% of live splenocytes. STAT5 deletion. (open shapes) % ROSA + % floxed
 Supp. Figure 1. a 14 1 1 8 6 spleen cells (x1 6 ) 16 % of live splenocytes 5 4 3 1 % of live splenocytes 8 6 4 b 1 1 c % of CD11c + splenocytes (closed shapes) 8 6 4 8 6 4 % ROSA + (open shapes) % floxed
Supp. Figure 1. a 14 1 1 8 6 spleen cells (x1 6 ) 16 % of live splenocytes 5 4 3 1 % of live splenocytes 8 6 4 b 1 1 c % of CD11c + splenocytes (closed shapes) 8 6 4 8 6 4 % ROSA + (open shapes) % floxed
Supplementary Figures
 Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Inhibition of Pulmonary Anti Bacterial Defense by IFN γ During Recovery from Influenza Infection By Keer Sun and Dennis W. Metzger Supplementary Figures d a Ly6G Percentage survival f 1 75 5 1 25 1 5 1
Nature Immunology: doi: /ni Supplementary Figure 1. Production of cytokines and chemokines after vaginal HSV-2 infection.
 Supplementary Figure 1 Production of cytokines and chemokines after vaginal HSV-2 infection. C57BL/6 mice were (a) treated intravaginally with 20 µl of PBS or infected with 6.7x10 4 pfu of HSV-2 in the
Supplementary Figure 1 Production of cytokines and chemokines after vaginal HSV-2 infection. C57BL/6 mice were (a) treated intravaginally with 20 µl of PBS or infected with 6.7x10 4 pfu of HSV-2 in the
ONCOS-102 in mesothelioma. Dr Magnus Jaderberg Chief Medical Officer Targovax
 ONCOS-102 in mesothelioma Dr Magnus Jaderberg Chief Medical Officer Targovax ONCOS CLINICAL DEVELOPMENT STRATEGY 1 2 3 4 Path-to-market Mesothelioma Proof-of-concept CPI refractory Proof-of-concept New
ONCOS-102 in mesothelioma Dr Magnus Jaderberg Chief Medical Officer Targovax ONCOS CLINICAL DEVELOPMENT STRATEGY 1 2 3 4 Path-to-market Mesothelioma Proof-of-concept CPI refractory Proof-of-concept New
FcγRIIIA (CD16)-expressing monocytes mediate the depletion of tumor-infiltrating Tregs via ipilimumab-dependent ADCC in melanoma patients
 FcγRIIIA (CD16)-expressing monocytes mediate the depletion of tumor-infiltrating Tregs via ipilimumab-dependent ADCC in melanoma patients Emanuela Romano Department of Oncology University of Lausanne and
FcγRIIIA (CD16)-expressing monocytes mediate the depletion of tumor-infiltrating Tregs via ipilimumab-dependent ADCC in melanoma patients Emanuela Romano Department of Oncology University of Lausanne and
Supplementary Figure 1. NAFL enhanced immunity of other vaccines (a) An over-the-counter, hand-held non-ablative fractional laser (NAFL).
 Supplementary Figure 1. NAFL enhanced immunity of other vaccines (a) An over-the-counter, hand-held non-ablative fractional laser (NAFL). (b) Depiction of a MTZ array generated by NAFL. (c-e) IgG production
Supplementary Figure 1. NAFL enhanced immunity of other vaccines (a) An over-the-counter, hand-held non-ablative fractional laser (NAFL). (b) Depiction of a MTZ array generated by NAFL. (c-e) IgG production
Darwinian selection and Newtonian physics wrapped up in systems biology
 Darwinian selection and Newtonian physics wrapped up in systems biology Concept published in 1957* by Macfarland Burnet (1960 Nobel Laureate for the theory of induced immune tolerance, leading to solid
Darwinian selection and Newtonian physics wrapped up in systems biology Concept published in 1957* by Macfarland Burnet (1960 Nobel Laureate for the theory of induced immune tolerance, leading to solid
Optimized administration sequence and timing of active dendritic cell immunotherapy with a PD1 checkpoint inhibitor (CPI) resulted in strong synergy
 1 Optimized administration sequence and timing of active dendritic cell immunotherapy with a PD1 checkpoint inhibitor (CPI) resulted in strong synergy in a mouse model of renal cell carcinoma Mouse analog
1 Optimized administration sequence and timing of active dendritic cell immunotherapy with a PD1 checkpoint inhibitor (CPI) resulted in strong synergy in a mouse model of renal cell carcinoma Mouse analog
The Immune System: The Mind Body Connection. Presented by Margaret Kemeny, Ph.D. Department of Psychiatry, University of California, San Francisco
 The Immune System: The Mind Body Connection Presented by Margaret Kemeny, Ph.D. Department of Psychiatry, University of California, San Francisco Psychoneuroimmunology Investigation of the bidirectional
The Immune System: The Mind Body Connection Presented by Margaret Kemeny, Ph.D. Department of Psychiatry, University of California, San Francisco Psychoneuroimmunology Investigation of the bidirectional
Immunology and Immunotherapy 101 for the Non-Immunologist
 Immunology and Immunotherapy 101 for the Non-Immunologist Stephen P. Schoenberger, Ph.D La Jolla Institute for Allergy and Immunology & UCSD Moores Cancer Center Disclosures Human Longevity Inc: Salary
Immunology and Immunotherapy 101 for the Non-Immunologist Stephen P. Schoenberger, Ph.D La Jolla Institute for Allergy and Immunology & UCSD Moores Cancer Center Disclosures Human Longevity Inc: Salary
Identification of novel immune regulators of tumor growth using highthroughput
 Identification of novel immune regulators of tumor growth using highthroughput screening in vivo Tom Brennan 1 Five Prime s Unique Platform Tests Nearly Every Extracellular Protein to Identify Protein
Identification of novel immune regulators of tumor growth using highthroughput screening in vivo Tom Brennan 1 Five Prime s Unique Platform Tests Nearly Every Extracellular Protein to Identify Protein
Gene Vaccine Dr. Sina Soleimani
 Gene Vaccine Dr. Sina Soleimani Human Viral Vaccines Quality Control Laboratory (HVVQC) Titles 1. A short Introduction of Vaccine History 2. First Lineage of Vaccines 3. Second Lineage of Vaccines 3. New
Gene Vaccine Dr. Sina Soleimani Human Viral Vaccines Quality Control Laboratory (HVVQC) Titles 1. A short Introduction of Vaccine History 2. First Lineage of Vaccines 3. Second Lineage of Vaccines 3. New
Cancer immunity and immunotherapy. General principles
 1 Cancer immunity and immunotherapy Abul K. Abbas UCSF General principles 2 The immune system recognizes and reacts against cancers The immune response against tumors is often dominated by regulation or
1 Cancer immunity and immunotherapy Abul K. Abbas UCSF General principles 2 The immune system recognizes and reacts against cancers The immune response against tumors is often dominated by regulation or
Reviewers' Comments: Reviewer #1 (Remarks to the Author)
 Reviewers' Comments: Reviewer #1 (Remarks to the Author) The manuscript by Zhuang and colleagues characterizes in depth the phenotype, origin and role of mononuclear phagocytes infiltrating murine cardiac
Reviewers' Comments: Reviewer #1 (Remarks to the Author) The manuscript by Zhuang and colleagues characterizes in depth the phenotype, origin and role of mononuclear phagocytes infiltrating murine cardiac
Radiation Therapy as an Immunomodulator
 Radiation Therapy as an Immunomodulator Yvonne Mowery, MD, PhD February 20, 2017 Tumor/Immune System Balance Kalbasi, JCI 2013 UNC-Duke-NC State-Wake Forest Spring 2017 2 RT Can Shift Balance Toward Elimination
Radiation Therapy as an Immunomodulator Yvonne Mowery, MD, PhD February 20, 2017 Tumor/Immune System Balance Kalbasi, JCI 2013 UNC-Duke-NC State-Wake Forest Spring 2017 2 RT Can Shift Balance Toward Elimination
Reviewers' comments: Reviewer #1 (Remarks to the Author):
 Reviewers' comments: Reviewer #1 (Remarks to the Author): The manuscript by Sun et al., provide data showing that short-chain fatty acids produced by intestinal microbiota act through GPR43 to induced
Reviewers' comments: Reviewer #1 (Remarks to the Author): The manuscript by Sun et al., provide data showing that short-chain fatty acids produced by intestinal microbiota act through GPR43 to induced
Supplementary Figures
 Supplementary Figures Supplementary Figure 1. NKT ligand-loaded tumour antigen-presenting B cell- and monocyte-based vaccine induces NKT, NK and CD8 T cell responses. (A) The cytokine profiles of liver
Supplementary Figures Supplementary Figure 1. NKT ligand-loaded tumour antigen-presenting B cell- and monocyte-based vaccine induces NKT, NK and CD8 T cell responses. (A) The cytokine profiles of liver
Immunotherapy: The Newest Treatment Route
 Immunotherapy: The Newest Treatment Route IWMF Patient Forum, Phoenix, AZ Madhav Dhodapkar, MD Professor of Medicine and Immunobiology Chief, Section of Hematology Yale University or the Oldest William
Immunotherapy: The Newest Treatment Route IWMF Patient Forum, Phoenix, AZ Madhav Dhodapkar, MD Professor of Medicine and Immunobiology Chief, Section of Hematology Yale University or the Oldest William
Harnessing the Immune System to Prevent Cancer: Basic Immunologic Mechanisms
 Harnessing the Immune System to Prevent Cancer: Basic Immunologic Mechanisms (the language of immunology) Barbara K. Dunn NCI/Division of Cancer Prevention October 23, 2017 Harnessing the Immune System
Harnessing the Immune System to Prevent Cancer: Basic Immunologic Mechanisms (the language of immunology) Barbara K. Dunn NCI/Division of Cancer Prevention October 23, 2017 Harnessing the Immune System
Immunotherapy versus targeted treatments in metastatic renal cell carcinoma: The return game?
 Immunotherapy versus targeted treatments in metastatic renal cell carcinoma: The return game? Sylvie NEGRIER MD, PhD Centre Léon Bérard, Lyon Université Lyon I IMMUNOTHERAPY: A LONG AND WIDING ROAD! WHERE
Immunotherapy versus targeted treatments in metastatic renal cell carcinoma: The return game? Sylvie NEGRIER MD, PhD Centre Léon Bérard, Lyon Université Lyon I IMMUNOTHERAPY: A LONG AND WIDING ROAD! WHERE
Arming the Oncolytic Virus Enadenotucirev to Develop Tumor-Localized Combination Immunotherapeutics. Charles Q. Morris MBChB MRCP(UK)
 Arming the Oncolytic Virus Enadenotucirev to Develop Tumor-Localized Combination Immunotherapeutics Charles Q. Morris MBChB MRCP(UK) 1 Enadenotucirev (EnAd): Developed using directed evolution EnAd Start
Arming the Oncolytic Virus Enadenotucirev to Develop Tumor-Localized Combination Immunotherapeutics Charles Q. Morris MBChB MRCP(UK) 1 Enadenotucirev (EnAd): Developed using directed evolution EnAd Start
BAVARIAN NORDIC BIO DEUTSCHLAND PRESENTATION OCTOBER 2014 CSE/OMX:BAVA, OTC:BVNRY
 BAVARIAN NORDIC BIO DEUTSCHLAND PRESENTATION OCTOBER 2014 CSE/OMX:BAVA, OTC:BVNRY This presentation includes "forward-looking statements" that involve risks, uncertainties and other factors, many of which
BAVARIAN NORDIC BIO DEUTSCHLAND PRESENTATION OCTOBER 2014 CSE/OMX:BAVA, OTC:BVNRY This presentation includes "forward-looking statements" that involve risks, uncertainties and other factors, many of which
TOP-LINE DATA FROM THE RANDOMIZED PHASE 2 IMPULSE STUDY IN SMALL-CELL LUNG CANCER (SCLC): IMMUNOTHERAPEUTIC MAINTENANCE TREATMENT WITH LEFITOLIMOD
 Abstract #1527O TOP-LINE DATA FROM THE RANDOMIZED PHASE 2 IMPULSE STUDY IN SMALL-CELL LUNG CANCER (SCLC): IMMUNOTHERAPEUTIC MAINTENANCE TREATMENT WITH LEFITOLIMOD M. Thomas, S. Ponce-Aix, A. Navarro Mendivil,
Abstract #1527O TOP-LINE DATA FROM THE RANDOMIZED PHASE 2 IMPULSE STUDY IN SMALL-CELL LUNG CANCER (SCLC): IMMUNOTHERAPEUTIC MAINTENANCE TREATMENT WITH LEFITOLIMOD M. Thomas, S. Ponce-Aix, A. Navarro Mendivil,
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10134 Supplementary Figure 1. Anti-inflammatory activity of sfc. a, Autoantibody immune complexes crosslink activating Fc receptors, promoting activation of macrophages, and WWW.NATURE.COM/NATURE
doi:10.1038/nature10134 Supplementary Figure 1. Anti-inflammatory activity of sfc. a, Autoantibody immune complexes crosslink activating Fc receptors, promoting activation of macrophages, and WWW.NATURE.COM/NATURE
Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer
 Supplementary Information Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer Grit S. Herter-Sprie, Shohei Koyama, Houari Korideck, Josephine Hai, Jiehui Deng, Yvonne Y. Li, Kevin A. Buczkowski,
Supplementary Information Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer Grit S. Herter-Sprie, Shohei Koyama, Houari Korideck, Josephine Hai, Jiehui Deng, Yvonne Y. Li, Kevin A. Buczkowski,
DISCLOSURES. Roche/MSD-Merck/Celgene: Research Funding
 DISCLOSURES Roche/MSD-Merck/Celgene: Research Funding Roche/Celgene/AstraZeneca/Amgen/MSD/Novartis/Sanofi- Aventis/Pierre Fabré: Advisory Board or Consultant No conflict of interest with respect to this
DISCLOSURES Roche/MSD-Merck/Celgene: Research Funding Roche/Celgene/AstraZeneca/Amgen/MSD/Novartis/Sanofi- Aventis/Pierre Fabré: Advisory Board or Consultant No conflict of interest with respect to this
Immunotherapy of HNC: immune mechanisms and therapeutic targets
 Immunotherapy of HNC: immune mechanisms and therapeutic targets Ourania Tsitsilonis, MD, PhD Department of Biology National & Kapodistrian University of Athens What does the Immune System see in Cancer?
Immunotherapy of HNC: immune mechanisms and therapeutic targets Ourania Tsitsilonis, MD, PhD Department of Biology National & Kapodistrian University of Athens What does the Immune System see in Cancer?
An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy
 CORRECTION NOTICE Nat. Med. 9, 111 113 (13) An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy Alicia S Chung, Xiumin Wu, Guanglei Zhuang, Hai Ngu, Ian Kasman,
CORRECTION NOTICE Nat. Med. 9, 111 113 (13) An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy Alicia S Chung, Xiumin Wu, Guanglei Zhuang, Hai Ngu, Ian Kasman,
Copyright. Tocagen Inc. Lead Product Candidate: Toca 511 & Toca FC Preclinical Overview
 Lead Product Candidate: Toca 511 & Toca FC Preclinical Overview Toca 511, delivers CD prodrug activator gene selectively to cancer cells Regulatory genes Structural RRV genes CD gene Regulatory genes Toca
Lead Product Candidate: Toca 511 & Toca FC Preclinical Overview Toca 511, delivers CD prodrug activator gene selectively to cancer cells Regulatory genes Structural RRV genes CD gene Regulatory genes Toca
Evaluation of the Tumor Microenvironment. Mark Headley University of California, San Francisco SITC 2016
 Evaluation of the Tumor Microenvironment Mark Headley University of California, San Francisco SITC 2016 Presenter Disclosure Information Mark B. Headley, PhD In addition to appointment at UCSF Joint position
Evaluation of the Tumor Microenvironment Mark Headley University of California, San Francisco SITC 2016 Presenter Disclosure Information Mark B. Headley, PhD In addition to appointment at UCSF Joint position
Supplemental Table I.
 Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
Supplemental Table I Male / Mean ± SEM n Mean ± SEM n Body weight, g 29.2±0.4 17 29.7±0.5 17 Total cholesterol, mg/dl 534.0±30.8 17 561.6±26.1 17 HDL-cholesterol, mg/dl 9.6±0.8 17 10.1±0.7 17 Triglycerides,
CANCER VACCINES THE NEXT WAVE IN IMMUNO-ONCOLOGY
 CANCER VACCINES THE NEXT WAVE IN IMMUNO-ONCOLOGY Peter Skorpil VP Business Development peter.skorpil@targovax.com Cutting Edge Oslo 2016-10-18 www.targovax.com 1 Important notice and disclaimer This report
CANCER VACCINES THE NEXT WAVE IN IMMUNO-ONCOLOGY Peter Skorpil VP Business Development peter.skorpil@targovax.com Cutting Edge Oslo 2016-10-18 www.targovax.com 1 Important notice and disclaimer This report
IMMUNOTHERAPY FOR CANCER A NEW HORIZON. Ekaterini Boleti MD, PhD, FRCP Consultant in Medical Oncology Royal Free London NHS Foundation Trust
 IMMUNOTHERAPY FOR CANCER A NEW HORIZON Ekaterini Boleti MD, PhD, FRCP Consultant in Medical Oncology Royal Free London NHS Foundation Trust ASCO Names Advance of the Year: Cancer Immunotherapy No recent
IMMUNOTHERAPY FOR CANCER A NEW HORIZON Ekaterini Boleti MD, PhD, FRCP Consultant in Medical Oncology Royal Free London NHS Foundation Trust ASCO Names Advance of the Year: Cancer Immunotherapy No recent
pplementary Figur Supplementary Figure 1. a.
 pplementary Figur Supplementary Figure 1. a. Quantification by RT-qPCR of YFV-17D and YFV-17D pol- (+) RNA in the supernatant of cultured Huh7.5 cells following viral RNA electroporation of respective
pplementary Figur Supplementary Figure 1. a. Quantification by RT-qPCR of YFV-17D and YFV-17D pol- (+) RNA in the supernatant of cultured Huh7.5 cells following viral RNA electroporation of respective
Immunotherapy, an exciting era!!
 Immunotherapy, an exciting era!! Yousef Zakharia MD University of Iowa and Holden Comprehensive Cancer Center Alliance Meeting, Chicago November 2016 Presentation Objectives l General approach to immunotherapy
Immunotherapy, an exciting era!! Yousef Zakharia MD University of Iowa and Holden Comprehensive Cancer Center Alliance Meeting, Chicago November 2016 Presentation Objectives l General approach to immunotherapy
Immunotherapy in Lung Cancer - TLR9 as a therapeutic target -
 Immunotherapy in Lung Cancer - TLR9 as a therapeutic target - Wilfried Eberhardt,, MD Head of Outpatient Unit, Dept. of Internal Medicine (Cancer Research) West German Cancer Centre Essen University Hospital
Immunotherapy in Lung Cancer - TLR9 as a therapeutic target - Wilfried Eberhardt,, MD Head of Outpatient Unit, Dept. of Internal Medicine (Cancer Research) West German Cancer Centre Essen University Hospital
Basic Principles of Tumor Immunotherapy and Mechanisms of Tumor Immune Suppression. Bryon Johnson, PhD
 Basic Principles of Tumor Immunotherapy and Mechanisms of Tumor Immune Suppression Bryon Johnson, PhD Disclosures There will be discussion about the use of products for non-fda indications in this presentation.
Basic Principles of Tumor Immunotherapy and Mechanisms of Tumor Immune Suppression Bryon Johnson, PhD Disclosures There will be discussion about the use of products for non-fda indications in this presentation.
PD-L1 Expression and Signaling by Tumors and Macrophages: Comparative Studies in Mice and Dogs
 PD-L1 Expression and Signaling by Tumors and Macrophages: Comparative Studies in Mice and Dogs Steven Dow, DVM, PhD Department of Clinical Sciences, Animal Cancer Center Colorado State University Consortium
PD-L1 Expression and Signaling by Tumors and Macrophages: Comparative Studies in Mice and Dogs Steven Dow, DVM, PhD Department of Clinical Sciences, Animal Cancer Center Colorado State University Consortium
Personalized Cancer Neoantigen Vaccines
 Personalized Cancer Neoantigen Vaccines Turning the Immune System Against Your own Unique Tumour-Specific Antigens 3 rd Annual Advances in Immuno-Oncology Congress London, May 24, 2018 Agnete Fredriksen,
Personalized Cancer Neoantigen Vaccines Turning the Immune System Against Your own Unique Tumour-Specific Antigens 3 rd Annual Advances in Immuno-Oncology Congress London, May 24, 2018 Agnete Fredriksen,
Oncolytic Viruses as a Potential Approach to Eliminate the HIV Reservoir
 Oncolytic Viruses as a Potential Approach to Eliminate the HIV Reservoir Costiniuk CT, Côté SC, Al-Ghazawi FM, Carrasco-Medina L,Young CD, & Angel JB University of Ottawa, November 12 th, 2012 HIV Reservoirs
Oncolytic Viruses as a Potential Approach to Eliminate the HIV Reservoir Costiniuk CT, Côté SC, Al-Ghazawi FM, Carrasco-Medina L,Young CD, & Angel JB University of Ottawa, November 12 th, 2012 HIV Reservoirs
Immunity to Viruses. Patricia Fitzgerald-Bocarsly September 25, 2008
 Immunity to Viruses Patricia Fitzgerald-Bocarsly September 25, 2008 The Immune System Deals with a Huge Range of Pathogens Roitt, 2003 Immune Responses to Viruses Viruses are dependent on the host cell
Immunity to Viruses Patricia Fitzgerald-Bocarsly September 25, 2008 The Immune System Deals with a Huge Range of Pathogens Roitt, 2003 Immune Responses to Viruses Viruses are dependent on the host cell
FOCiS. Lecture outline. The immunological equilibrium: balancing lymphocyte activation and control. Immunological tolerance and immune regulation -- 1
 1 Immunological tolerance and immune regulation -- 1 Abul K. Abbas UCSF FOCiS 2 Lecture outline Principles of immune regulation Self-tolerance; mechanisms of central and peripheral tolerance Inhibitory
1 Immunological tolerance and immune regulation -- 1 Abul K. Abbas UCSF FOCiS 2 Lecture outline Principles of immune regulation Self-tolerance; mechanisms of central and peripheral tolerance Inhibitory
Bezzi et al., Supplementary Figure 1 *** Nature Medicine: doi: /nm Pten pc-/- ;Zbtb7a pc-/- Pten pc-/- ;Pml pc-/- Pten pc-/- ;Trp53 pc-/-
 Gr-1 Gr-1 Gr-1 Bezzi et al., Supplementary Figure 1 a Gr1-CD11b 3 months Spleen T cells 3 months Spleen B cells 3 months Spleen Macrophages 3 months Spleen 15 4 8 6 c CD11b+/Gr1+ cells [%] 1 5 b T cells
Gr-1 Gr-1 Gr-1 Bezzi et al., Supplementary Figure 1 a Gr1-CD11b 3 months Spleen T cells 3 months Spleen B cells 3 months Spleen Macrophages 3 months Spleen 15 4 8 6 c CD11b+/Gr1+ cells [%] 1 5 b T cells
Trim29 gene-targeting strategy. (a) Genotyping of wildtype mice (+/+), Trim29 heterozygous mice (+/ ) and homozygous mice ( / ).
 Supplementary Figure 1 Trim29 gene-targeting strategy. (a) Genotyping of wildtype mice (+/+), Trim29 heterozygous mice (+/ ) and homozygous mice ( / ). (b) Immunoblot analysis of TRIM29 in lung primary
Supplementary Figure 1 Trim29 gene-targeting strategy. (a) Genotyping of wildtype mice (+/+), Trim29 heterozygous mice (+/ ) and homozygous mice ( / ). (b) Immunoblot analysis of TRIM29 in lung primary
Supplementary figure 1. Systemic delivery of anti-cd47 antibody controls tumor growth in
 T u m o r v o lu m e (m m 3 ) P e rc e n t s u rv iv a l P e rc e n t s u rv iv a l Supplementary data a 1 8 6 4 2 5 1 1 5 2 2 5 3 3 5 4 T im e a fte r tu m o r in o c u la tio n (d ) b c 1 5 1 1 5 * *
T u m o r v o lu m e (m m 3 ) P e rc e n t s u rv iv a l P e rc e n t s u rv iv a l Supplementary data a 1 8 6 4 2 5 1 1 5 2 2 5 3 3 5 4 T im e a fte r tu m o r in o c u la tio n (d ) b c 1 5 1 1 5 * *
Basic Principles of Tumor Immunotherapy. Ryan J. Sullivan, M.D. Massachusetts General Hospital Cancer Center Boston, MA
 Basic Principles of Tumor Immunotherapy Ryan J. Sullivan, M.D. Massachusetts General Hospital Cancer Center Boston, MA Disclosures Consulting Fees: Biodesix, Novartis Pharmaceuticals Other: Boehringer
Basic Principles of Tumor Immunotherapy Ryan J. Sullivan, M.D. Massachusetts General Hospital Cancer Center Boston, MA Disclosures Consulting Fees: Biodesix, Novartis Pharmaceuticals Other: Boehringer
Immuno-Oncology. Axel Hoos, MD, PhD Senior Vice President, Oncology R&D. February 24, 2016
 Immuno-Oncology Axel Hoos, MD, PhD Senior Vice President, Oncology R&D February 24, 216 GSK Pipeline Oncology R&D strategy Focusing on 3 areas fundamental to oncology Cancer Epigenetics Long-Term Survival
Immuno-Oncology Axel Hoos, MD, PhD Senior Vice President, Oncology R&D February 24, 216 GSK Pipeline Oncology R&D strategy Focusing on 3 areas fundamental to oncology Cancer Epigenetics Long-Term Survival
Additional file 6. Summary of experiments characterizing development of adaptive immune response
 Additional file 6. Summary of experiments characterizing development of adaptive immune response Tests performed to evaluate the development of adaptive immunity in patients are summarized in Table 1.
Additional file 6. Summary of experiments characterizing development of adaptive immune response Tests performed to evaluate the development of adaptive immunity in patients are summarized in Table 1.
Supplementary Figure 1: Fn14 is upregulated in the epidermis and dermis of mice
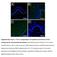 Supplementary Figure 1: Fn14 is upregulated in the epidermis and dermis of mice undergoing AD- and psoriasis-like disease. Immunofluorescence staining for Fn14 (green) and DAPI (blue) in skin of naïve
Supplementary Figure 1: Fn14 is upregulated in the epidermis and dermis of mice undergoing AD- and psoriasis-like disease. Immunofluorescence staining for Fn14 (green) and DAPI (blue) in skin of naïve
General Overview of Immunology. Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center
 General Overview of Immunology Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center Objectives Describe differences between innate and adaptive immune responses
General Overview of Immunology Kimberly S. Schluns, Ph.D. Associate Professor Department of Immunology UT MD Anderson Cancer Center Objectives Describe differences between innate and adaptive immune responses
Evidence for Systemic Effects Moves PV-10 Toward Further Clinical Trials
 PV-10 Moves Forward-1 Evidence for Systemic Effects Moves PV-10 Toward Further Clinical Trials At the same time that current research projects are solidifying and reinforcing the evidence for PV-10 s systemic
PV-10 Moves Forward-1 Evidence for Systemic Effects Moves PV-10 Toward Further Clinical Trials At the same time that current research projects are solidifying and reinforcing the evidence for PV-10 s systemic
CONTRACTING ORGANIZATION: Children s Hospital Los Angeles Los Angeles, CA 90027
 AD Award Number: TITLE: Studies of the Tumor Microenvironment in Pathogenesis of Neuroblastoma PRINCIPAL INVESTIGATOR: Shahab Asgharzadeh, M D CONTRACTING ORGANIZATION: Children s Hospital Los Angeles
AD Award Number: TITLE: Studies of the Tumor Microenvironment in Pathogenesis of Neuroblastoma PRINCIPAL INVESTIGATOR: Shahab Asgharzadeh, M D CONTRACTING ORGANIZATION: Children s Hospital Los Angeles
Supporting Information
 Supporting Information Valkenburg et al. 10.1073/pnas.1403684111 SI Materials and Methods ELISA and Microneutralization. Sera were treated with Receptor Destroying Enzyme II (RDE II, Accurate) before ELISA
Supporting Information Valkenburg et al. 10.1073/pnas.1403684111 SI Materials and Methods ELISA and Microneutralization. Sera were treated with Receptor Destroying Enzyme II (RDE II, Accurate) before ELISA
Measles virus vaccine induces oncolysis of tumor cells and activates immune responses.
 Measles virus vaccine induces oncolysis of tumor cells and activates immune responses. Marc Grégoire*, J.-F. Fonteneau, N. Boisgerault, J.-B. Guillerme and F. Tangy. * INSERM, Nantes, F-44, France. Pasteur
Measles virus vaccine induces oncolysis of tumor cells and activates immune responses. Marc Grégoire*, J.-F. Fonteneau, N. Boisgerault, J.-B. Guillerme and F. Tangy. * INSERM, Nantes, F-44, France. Pasteur
Nature Immunology: doi: /ni Supplementary Figure 1. Gene expression profile of CD4 + T cells and CTL responses in Bcl6-deficient mice.
 Supplementary Figure 1 Gene expression profile of CD4 + T cells and CTL responses in Bcl6-deficient mice. (a) Gene expression profile in the resting CD4 + T cells were analyzed by an Affymetrix microarray
Supplementary Figure 1 Gene expression profile of CD4 + T cells and CTL responses in Bcl6-deficient mice. (a) Gene expression profile in the resting CD4 + T cells were analyzed by an Affymetrix microarray
Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD-
 Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Supplementary Methods Blocking antibodies and peptides. Rat anti-mouse PD-1 (29F.1A12, rat IgG2a, k), PD- L1 (10F.9G2, rat IgG2b, k), and PD-L2 (3.2, mouse IgG1) have been described (24). Anti-CTLA-4 (clone
Oncolytic virus strategy
 Oncolytic viruses Oncolytic virus strategy normal tumor NO replication replication survival lysis Oncolytic virus strategy Mechanisms of tumor selectivity of several, some of them naturally, oncolytic
Oncolytic viruses Oncolytic virus strategy normal tumor NO replication replication survival lysis Oncolytic virus strategy Mechanisms of tumor selectivity of several, some of them naturally, oncolytic
Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell?
 Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Supplementary Figure 1. ETBF activate Stat3 in B6 and Min mice colons
 Supplementary Figure 1 ETBF activate Stat3 in B6 and Min mice colons a pstat3 controls Pos Neg ETBF 1 2 3 4 b pstat1 pstat2 pstat3 pstat4 pstat5 pstat6 Actin Figure Legend: (a) ETBF induce predominantly
Supplementary Figure 1 ETBF activate Stat3 in B6 and Min mice colons a pstat3 controls Pos Neg ETBF 1 2 3 4 b pstat1 pstat2 pstat3 pstat4 pstat5 pstat6 Actin Figure Legend: (a) ETBF induce predominantly
Daratumumab: Uncovering a novel mechanism of action for an approved antibody therapy
 Daratumumab: Uncovering a novel mechanism of action for an approved antibody therapy Dr. Kate Sasser, PhD Corporate VP, Translational Research December 14 th, 2017 CD38 is ubiquitously expressed in Myeloma
Daratumumab: Uncovering a novel mechanism of action for an approved antibody therapy Dr. Kate Sasser, PhD Corporate VP, Translational Research December 14 th, 2017 CD38 is ubiquitously expressed in Myeloma
Cytokines: Interferons, Interleukins and Beyond. Michael B. Atkins, MD Georgetown-Lombardi Comprehensive Cancer Center
 Cytokines: Interferons, Interleukins and Beyond Michael B. Atkins, MD Georgetown-Lombardi Comprehensive Cancer Center Disclosures Advisory Boards: Bristol-Myers Squibb,Amgen, Novartis, Alkermes, Infinity,
Cytokines: Interferons, Interleukins and Beyond Michael B. Atkins, MD Georgetown-Lombardi Comprehensive Cancer Center Disclosures Advisory Boards: Bristol-Myers Squibb,Amgen, Novartis, Alkermes, Infinity,
Supplementary Figure 1 IL-27 IL
 Tim-3 Supplementary Figure 1 Tc0 49.5 0.6 Tc1 63.5 0.84 Un 49.8 0.16 35.5 0.16 10 4 61.2 5.53 10 3 64.5 5.66 10 2 10 1 10 0 31 2.22 10 0 10 1 10 2 10 3 10 4 IL-10 28.2 1.69 IL-27 Supplementary Figure 1.
Tim-3 Supplementary Figure 1 Tc0 49.5 0.6 Tc1 63.5 0.84 Un 49.8 0.16 35.5 0.16 10 4 61.2 5.53 10 3 64.5 5.66 10 2 10 1 10 0 31 2.22 10 0 10 1 10 2 10 3 10 4 IL-10 28.2 1.69 IL-27 Supplementary Figure 1.
Nature Immunology: doi: /ni Supplementary Figure 1. Cellularity of leukocytes and their progenitors in naive wild-type and Spp1 / mice.
 Supplementary Figure 1 Cellularity of leukocytes and their progenitors in naive wild-type and Spp1 / mice. (a, b) Gating strategies for differentiated cells including PMN (CD11b + Ly6G hi and CD11b + Ly6G
Supplementary Figure 1 Cellularity of leukocytes and their progenitors in naive wild-type and Spp1 / mice. (a, b) Gating strategies for differentiated cells including PMN (CD11b + Ly6G hi and CD11b + Ly6G
LAG-3: Validation Of Next Generation Checkpoint Pathways
 LAG-3: Validation Of Next Generation Checkpoint Pathways Frédéric Triebel, CO/CMO Immune Checkpoint Modulation & Combination Therapies April 13, 2016 London, UK. 1 AX:PRR; NADAQ:PBMD Notice: Forward Looking
LAG-3: Validation Of Next Generation Checkpoint Pathways Frédéric Triebel, CO/CMO Immune Checkpoint Modulation & Combination Therapies April 13, 2016 London, UK. 1 AX:PRR; NADAQ:PBMD Notice: Forward Looking
Immunomax reinforces immunity defends against infections
 Immunomax reinforces immunity defends against infections INSTRUCTION on the Medical Use of Immunomax Registration number: P No.001919/02-2002 Commercial name: Immunomax Chemical name: acidic peptidoglycan
Immunomax reinforces immunity defends against infections INSTRUCTION on the Medical Use of Immunomax Registration number: P No.001919/02-2002 Commercial name: Immunomax Chemical name: acidic peptidoglycan
1. Engineering Foot-and-Mouth Disease Viruses with Improved
 Engineering Foot-and-Mouth Disease Virus with Improved Properties for the Development of Effective Vaccine Haixue Zheng, Fan Yang, Ye Jin, Jianhong Guo, Jijun He, Lvlv, Xuepeng Cai, Xiangtao Liu, Hong
Engineering Foot-and-Mouth Disease Virus with Improved Properties for the Development of Effective Vaccine Haixue Zheng, Fan Yang, Ye Jin, Jianhong Guo, Jijun He, Lvlv, Xuepeng Cai, Xiangtao Liu, Hong
SUPPLEMENTARY INFORMATION
 doi:1.138/nature1554 a TNF-α + in CD4 + cells [%] 1 GF SPF 6 b IL-1 + in CD4 + cells [%] 5 4 3 2 1 Supplementary Figure 1. Effect of microbiota on cytokine profiles of T cells in GALT. Frequencies of TNF-α
doi:1.138/nature1554 a TNF-α + in CD4 + cells [%] 1 GF SPF 6 b IL-1 + in CD4 + cells [%] 5 4 3 2 1 Supplementary Figure 1. Effect of microbiota on cytokine profiles of T cells in GALT. Frequencies of TNF-α
C. Incorrect! MHC class I molecules are not involved in the process of bridging in ADCC.
 Immunology - Problem Drill 13: T- Cell Mediated Immunity Question No. 1 of 10 1. During Antibody-dependent cell mediated cytotoxicity (ADCC), the antibody acts like a bridge between the specific antigen
Immunology - Problem Drill 13: T- Cell Mediated Immunity Question No. 1 of 10 1. During Antibody-dependent cell mediated cytotoxicity (ADCC), the antibody acts like a bridge between the specific antigen
Supplementary material page 1/10
 Supplementary Figure 1. Metoprolol administration during ongoing AMI reduces MVO in STEMI patients (a, b) Complete representative CMR exams (short-axis covering the entire left ventricle (LV) from base
Supplementary Figure 1. Metoprolol administration during ongoing AMI reduces MVO in STEMI patients (a, b) Complete representative CMR exams (short-axis covering the entire left ventricle (LV) from base
Immune surveillance hypothesis (Macfarlane Burnet, 1950s)
 TUMOR-IMMUNITÄT A.K. Abbas, A.H. Lichtman, S. Pillai (6th edition, 2007) Cellular and Molecular Immunology Saunders Elsevier Chapter 17, immunity to tumors Immune surveillance hypothesis (Macfarlane Burnet,
TUMOR-IMMUNITÄT A.K. Abbas, A.H. Lichtman, S. Pillai (6th edition, 2007) Cellular and Molecular Immunology Saunders Elsevier Chapter 17, immunity to tumors Immune surveillance hypothesis (Macfarlane Burnet,
