Cell-based immunotherapy products for the treatment of blood cancers and inherited blood disorders. Company Presentation June 2016
|
|
|
- Kathryn Summers
- 5 years ago
- Views:
Transcription
1 Cell-based immunotherapy products for the treatment of blood cancers and inherited blood disorders Company Presentation June 2016
2 Disclaimer These slides and the accompanying oral presentation contain forward-looking statements and information. Forwardlooking statements are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry s actual results, levels or activity, performance or achievements to be materially different from those anticipated by such statements. The use of words such as may, might, will, should, could, expect, plan, anticipate, believe, estimate, project, intend, future, potential or continue, and other similar expressions are intended to identify forward looking statements. For example, all statements we make regarding (i) the initiation, timing, cost, progress and results of our preclinical and clinical studies and our research and development programs, (ii) our ability to advance product candidates into, and successfully complete, clinical studies, (iii) the timing or likelihood of regulatory filings and approvals, (iv) our ability to develop, manufacture and commercialize our product candidates and to improve the manufacturing process, (v) the rate and degree of market acceptance of our product candidates, (vi) the size and growth potential of the markets for our product candidates and our ability to serve those markets, and (vii) our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates, are forward looking. All forward-looking statements are based on current estimates, assumptions and expectations by our management that, although we believe to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. Any forward-looking statement speaks only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. This presentation is not, and nothing in it should be construed as, an offer, invitation or recommendation in respect of our securities, or an offer, invitation or recommendation to sell, or a solicitation of an offer to buy, any of our securities in any jurisdiction. Neither this presentation nor anything in it shall form the basis of any contract or commitment. This presentation is not intended to be relied upon as advice to investors or potential investors and does not take into account the investment objectives, financial situation or needs of any investor. 2
3 Company highlights and introduction to ATIR 1 Lead product ATIR is an innovative immunotherapy which addresses the risks and limitations of current bone marrow and stem cell treatments (HSCT) 2 Strong clinical Phase I and Phase II data package No patient (0/42) experienced grade III/IV GVHD Significantly improved Overall Survival Excellent safety and efficacy profile demonstrated to date 3 Clear upcoming route to market and strong news flow catalysts Initiation of Phase III trial in 2H 2016 (ATIR101) Expected submission to EMA for conditional approval (ATIR101) Initiation of clinical trial in Thalassemia (ATIR201) 4 Experienced management team and international, diverse shareholder base 3
4 HSCT as curative treatment for blood cancer and inherited blood disorders Basic principle of HSCT 1. Destroy the diseased bone marrow 2. Create bone marrow space in the recipient for the donor cells to engraft 3. Replace it with healthy donor cells 4. Suppress the immune system or eliminate the recipient T-cells to minimize risks of rejection 4
5 Significant risks & limitations in HSCT Causes of death after MUD HSCT 1% 6% Risks Opportunistic infections Immunosuppressed patients are highly susceptible to pathogens 19% 17% 20% 37% Graft-versus-Host Disease (GVHD) GVHD occurs when donor T-cells recognize a patient s tissue as foreign. Incidence of grade III/IV GVHD is as high as 30% and often fatal Relapse GvHD Infections Other Organ Failure New Malignancy 20% CIBMTR-data includes centers located globally and is measured over Limitations Cancer relapse Ablated immune system has poor ability to fight residual leukemic cells Donor availability Approx. 75% of patients do not have a matched family donor and approx. 35% of patients eligible for HSCT will not find a matched donor in time 5
6 Haplo-HSCT with ATIR101: addressing risks and limitations of HSCT within standard hospital procedures ATIR101 allows for an immunosuppressant-free transplant regimen for haploidentical donor transplantation (i.e. from a family member) Adjunctive to (partially) T-cell depleted stem cell graft donor Donor CD34+ PBMC graft Selective photodepletion to eliminate GVHD-causing T-cells Conditioning Haplo HSCT Isolation ATIR101 infusion Myeloablative conditioning including ATG T-cell depleted stem cell graft from family member No prophylactic immunosuppression Donor lymphocyte infusion days post- HSCT 6
7 ATIR101 manufacturing removes GVHD-causing T-cells while retaining key immune cells 1. Immune cells collected and mixed ex vivo 2. Activation of donor T- cells 3. TH9402 addition 4. Exposure to light 5. ATIR101 infusion ATIR101 characteristics Selective removal of GVHD-causing T-cells Patient Key immune cells are retained to protect against infections T-cells directed against leukemic cells are Donor GVHD-causing donor T- cells are activated by patient cells TH9402 is added TH9402 is retained only in activated donor T-cells Light induced killing Cells formulated for infusion Infusion days after haplo HSCT retained Day 1-4 Day 5 Non-activated GVHDcausing donor T-cells Irradiated patient cells Activated GVHD-causing donor T-cells Graft-versus-Leukemia T-cells Activated GVHD-causing donor T-cells with TH9402 retained Other immune cells (e.g. virus specific memory and effector cells, naïve cells, NK cells) 7
8 ATIR101: alloreactive T-cells eliminated while T-cell response to allo viruses/bacteria/fungi Example from Phase II CR-AIR-007 release testing Typical example in CR-AIR-007 Donor ATIR Proliferation Index (PI) Donor ATIR : Cells from the donor/starting material : Final product manufactured from donor Donor Recipient 1.0 Donor Recipient 3rd party CD3/28 Selective depletion Retained functional cells 3rd party SAFETY POTENCY 8
9 ATIR101 contains virus-specific T-cells Virus-specific T-cells before and after manufacturing of ATIR101 Control EBV CMV Influenza Donor ATIR 9
10 Graft-versus-Leukemia T-cells have been detected in ATIR101 Figure 2: ATIR101 cells were thawed, recovered for two days in medium supplemented withil-2, IL-7 and IL-15 followed by stimulation with Myb628/HLA-B44 aapcs. Myb628 specific T cell expansion was assessed after one (upper panel) or two (lower panel) rounds of stimulation with a Myb628/HLA-B44 multimer. As control, cells were stained with an irrelevant HLA-B44 multimer. Collaboration with Prof. Angela Krackhardt, Medizinische Klinik III, Klinikum Rechts der Isar, TU Munich, Munich, Germany 10
11 Overview of ATIR101/201 clinical studies Trial ID # Patients Aim Phase Design Status CR-GVH- 001 CR-AIR Dose finding Phase I/II Open-label, dose- escalation 158 Control group Observational cohort trial Completed 5 year follow-up completed Completed Data analysis CR-AIR Safety and efficacy Phase II Open-label, multi-center trial using optimal ATIR101 dose On-going All patients enrolled CR-AIR Dose Optimization Phase II Open-label, exploratory trial using repeat dose administration On-going First patient enrolled and treated Planned Clinical Studies 2016 CR-AIR- 009 Approx. 200 Safety and efficacy Phase III Randomized clinical trial: Haplo + ATIR101 (KIADIS) vs. Haplo + Cyclophosphamide (Baltimore) CR-BD- 001 Approx. 10 Safety and efficacy Phase II Exploratory Phase II trial in pediatric patients suffering from β-thalassemia major 11
12 Trial characteristics & endpoints Phase II CR-AIR-007 Trial characteristics (EudraCT : US-IND#14255) Design: open-label, single arm, multi-center study Patient population: AML or ALL in first remission with high-risk features or in second or higher remission No suitable matched donor Haploidentical family Locations: CA, BE, DE, UK (8 sites in total) Primary endpoint: Transplant Related Mortality (TRM) at 6 months Secondary endpoints: Acute and chronic GVHD Immune reconstitution TRM, relapse, Overall Survival (OS), up to 24 months Patient follow-up (per 24 March 2016): Median 414 days (range ) Last patient reached primary endpoint on 24 March
13 Patient & donor characteristics Patient and donors N=23 patients (HSCT + ATIR101) Median patient age (range): 41 years (21 64) Gender: 13 female, 10 male Median donor age (range): 33 years (21-61 ) Diagnosis AML: n=16 (70%): 11 in CR1 5 in CR2 ALL: n =7 (30%): 4 in CR1 3 in CR2 Risk classification Cytogenetic risk profile 1 : Favorable 0 Intermediate 9 (39 %) Adverse 14 (61 %) Disease-risk index 2 : Low risk index 0 Intermediate risk index 10 (43 %) High risk index 13 (57 %) 1 Mrozek K, et al. JCO 2012, 30 (36): Armand P, et al. Blood 2014, 123 (23):
14 HSCT characteristics Myeloablative conditioning TBI (1200 cgy; n=11) or melphalan (120mg/m 2 ; n=12) Thiotepa (10 mg/kg), fludarabine (30 mg/m 2 x 5d) and ATG (2.5mg/kg x 4d) HSCT CliniMACS CD34 isolation system (Miltenyi Biotec) Target: 8-11x10 6 CD34+ cells/kg, with max. of 3x10 4 CD3+ cells/kg Prophylaxis No GVHD prophylaxis CMV/EBV monitoring Prophylactic use of ganciclovir/foscarnet (CMV + recipient/donor) ATIR101 infusion Day 28 post HSCT (median) 14
15 Top-line results: acute and chronic GVHD Acute GVHD No (0%) grade III/IV GVHD after ATIR101 infusion Only 3 cases of grade II GVHD Chronic GVHD Only 1 case of chronic GVHD (severe) has been reported (median of >400 days follow-up) Conclusions top-line results GVHD ATIR101 is safe Haplo HSCT without any grade III/IV GVHD Overall exposure to ATIR101 Phase I/II + Phase II combined: N=42 Grade III/IV acute GVHD: 0% 15
16 Top-line results: Transplant Related Mortality, relapse and Overall Survival Results at 100 days, 6 months and 12 months 1 Time after HSCT N = days 6 months 12 months Transplant Related 0 3 (13%) 29% Mortality Death due to relapse/disease progression 0 1 (4%) 10.5% Overall Survival 23 (100%) 19 (83%) 64% Primary endpoint: TRM 6-months = 3 / 23 (13%); all infection-related No mortality within first 100 days post-hsct Only two patients relapsed within 12 months post-hsct Relapse at day 61 and 90 post-hsct 1 based on Kaplan-Meier estimates 16
17 Comparison with control data from an observational cohort study 1 Transplant Related Mortality Overall Survival P=0.005 P= HSCT + ATIR101 HSCT + ATIR101 ATIR101 significantly reduces TRM and improves OS compared to CD34+ haplo-transplants 1 Data from cohort study, CR-AIR-006: poster S. Mielke, P442 EBMT
18 GRFS 1 Comparing transplant regimens on GRFS GVHD-free/relapse-free survival (GRFS) is survival without: Acute GVHD (grade III/IV) Chronic GVHD Cancer relapse Key advantage HSCT + ATIR , 3 No acute GVHD, limited chronic GVHD Low relapse rate Comparison to alternative donor source Improved GRFS compared to one-locus Mismatched Unrelated Donor transplants (MMUD) and to Umbilical Cord Blood (UCB) transplants 1. Haplo + ATIR101 vs MUD* (10/10) p= Haplo + ATIR101 vs MMUD (9/10) p= Haplo + ATIR101 vs UCB p= Holtan et al. 2015, Blood 125: * Matched Unrelated Donor 18
19 Comparison with literature on post-transplant cyclophosphamide (PTCy) PTCy (Baltimore protocol) Post-HSCT use of high doses of cyclophosphamide (PTCy) is an emerging standard for haploidentical stem cell transplantations Unmanipulated graft used Post-HSCT use of high dose cyclophosphamide to control GVHD Additional use of immunosuppressants (calcineurin inhibitors/mmf) for 6 months Outcome 1 PTCy 2 ATIR101 Grade II/IV agvhd 3 16% 4% Grade III/IV agvhd 3 7% 0 cgvhd 28% 4% NRM 12% 29% Relapse 41% 10.5% OS 65% 64% Patient characteristics: Disease-risk index (DRI) Low risk index 5% 0 Intermediate risk index 63% 43% High risk index 32% 57% 1 Data at 12-months post-hsct (Kaplan-Meier estimates) 2 AML patients, myeloablative conditioning (Ciurea 2015) 3 GVHD at day 90 2 Ciurea et al. 2015, Blood 126:
20 Conclusions Phase II (CR-AIR-007) ATIR101 significantly reduces TRM and improves OS ATIR101 is safe and does not cause grade III/IV GVHD Relapse rates on ATIR101 protocol are very low Improved clinical benefit over 9/10 MMUD or umbilical cord transplants on event-free survival (GRFS) ATIR101 provides a safe and effective, immunosuppressant-free regimen for haploidentical transplantation Substantially less relapse and GVHD on ATIR101 compared to posttransplant cyclophosphamide (PTCy) Second dose of ATIR101 might improve survival even further Kiadis is preparing a randomized Phase III trial comparing haploidentical HSCT with PTCy against a T-depleted HSCT + ATIR101 20
21 EMA FDA Regulatory strategy update Process of Marketing Authorization Application (MAA) submission started, Rapporteurs appointed Meetings held with Rapporteur and co-rapporteur Decision taken to prepare for MAA filing in 1Q 2017 (awaiting 1 year results CR- AIR-007, dossier writing, completion process validation) New ODD submitted: treatment in hematopoeitic stem cell transplantation (covers all indications) End-of-Phase II meeting held in June 2016 Health Canada Possibility to submit MAA to be discussed 2H
22 Phase III: CR-AIR-009 Phase III design (CR-AIR-009) Randomized, controlled trial Comparing outcome of two haplo-hsct protocols: 1) Kiadis Protocol: T-cell depletion (CD34+ selection) + ATIR101 2) Baltimore Protocol: post HSCT Cyclophosphamide (PTCy) Primary endpoint: GFRS Event-free-survival (EFS) defined as GVHD-free AND Relapse-Free Survival (GRFS) Secondary endpoints: GVHD, NRM, RRM, DFS, OS Estimated sample size: 200 patients total Assumption of EFS of 60% for HSCT+ATIR and 40-45% for control group (PTCy) Trial conduct Global trial: North-America: USA & Canada Europe: Belgium, NL, UK, Germany, France, Italy Estimated number of trial sites: End-of-Phase II meeting held with FDA (June 2016) Protocol will be finalized based on feedback Timelines Trial initiation 3Q 2016 First patient in 4Q 2016 Enrollment appr. 1.5 years 2Q 2018 Expected analysis 2Q
23 Route to market in EU & US for ATIR101 FDA Launch Data submission Analysis Phase Trial ID # Patients Observational cohort trial CR-AIR Establish control group for EMA II (Efficacy trial) CR-AIR Confirm and extend safety and effectiveness II (Repeat administration trial) CR-AIR Dosing study III (Pivotal trial) CR-AIR- 009 appr. 200 Pivotal trial randomized, controlled Past Ongoing/planned 23
24 Route to market in EU & US for ATIR101 Submission EMA and HC 1 Endpoint reported FDA Launch Data submission Analysis Phase Trial ID # Patients Observational cohort trial CR-AIR Establish control group for EMA II (Efficacy trial) CR-AIR Confirm and extend safety and effectiveness II (Repeat administration trial) CR-AIR Dosing study III (Pivotal trial) CR-AIR- 009 appr. 200 Pivotal trial randomized, controlled Past Ongoing/planne d 24
25 ATIR may be used in additional indications Growth options and market size ATIR Market size (in new patients p.a.) ~ 19,000 ~ 6,000 19,000 TBD Growth options Total potential market Haplo HSCT in blood cancers Thalassemia ATIR201 Cannibalization of cord blood and MUD based HSCT in blood cancers Expansion into: Additional cancer indications Inherited blood disorders Creating chimerism in solid organ transplantation Time 25
26 ATIR201 in β-thalassemia major: current therapeutic environment Current approaches in β-thalassemia major: Symptomatic approaches (lifelong): Standard of care is symptomatic: blood transfusions and iron chelators Drug support to improve hematopoiesis Curative approaches (by intent): Allogeneic bone marrow transplantation to replace the diseased system: Limited availability of matching donors or healthy sibling donors Significant/high risk of infectious complications and GVHD Gene-therapy based autologous approaches (e.g. Bluebird Bio): Unknown duration of gene expression Unknown level of gene expression Risk of proto-oncogenic insertion of gene into genome 26
27 Clinical program ATIR201 Collaboration with TIF Awareness on HSCT option (specifically haploidentical) Access to families and to HSCT CR-BD-001 Exploratory Phase I/II trial in pediatric patients with β-thalassemia major ATIR201 as adjuvant to a T-cell depleted haploidentical HSCT Optimize manufacturing for pediatric setting Expected centers: Regensburg (Germany), London, Manchester, Birmingham (UK) Protocol drafted, submissions planned Trial to be initiated in 2H 2016 First results to be expected in 2017, full read-out in
28 Anticipated Company milestones H 2016 Topline results announced CR-AIR-007 trial (ATIR101) Set-up US-based ATIR101 manufacturer for Phase III Opening US clinical sites and strengthening US footprint and presence 2H 2016 Read-out (safety) of CR-AIR-008 trial First patient to be enrolled in ATIR101 Phase III trial (CR-AIR-009) Initiation ATIR201 Phase I/II Thalassemia trial (CR-BD-001) Expected submission to EMA for (conditional) approval ATIR101 (Q1, 2017) 28
29 Financial information Financial highlights Shares listed via an initial public offering (IPO) on Euronext Amsterdam and Euronext Brussels on 2 July 2015 raising gross proceeds of EUR 34.7 million. The equity position of the Company improved significantly and increased to EUR 25.7 million at year-end 2015 compared to EUR 2.7 million at the end of With net proceeds of EUR 31.2 million raised in the IPO, the Company s cash position improved to EUR 28.7 million at year-end 2015, which is an increase of EUR 23 million compared to cash of EUR 5.7 million at the end of Operating loss increased to EUR 16 million in 2015 from a loss of EUR 6.2 million in Operating expenses for 2015 included non-cash share-based payments of EUR 7.8 million. Net loss increased from EUR 7.8 million in 2014 to EUR 16.5 million in
30 Financial information 30
31 Thank you
Annual Results 2017 & Business Update 13 April 2018
 Annual Results 2017 & Business Update 13 April 2018 1 Disclaimer These slides and the accompanying oral presentation contain forward-looking statements and information. Forward-looking statements are subject
Annual Results 2017 & Business Update 13 April 2018 1 Disclaimer These slides and the accompanying oral presentation contain forward-looking statements and information. Forward-looking statements are subject
Making hematopoietic stem cell transplantation (HSCT) safer and more effective
 Making hematopoietic stem cell transplantation (HSCT) safer and more effective Cell-based immunotherapy products for the treatment of blood cancers and inherited blood disorders Company presentation, April
Making hematopoietic stem cell transplantation (HSCT) safer and more effective Cell-based immunotherapy products for the treatment of blood cancers and inherited blood disorders Company presentation, April
Making bone marrow transplantations safer and more effective
 Making bone marrow transplantations safer and more effective Patient specific cell-based immunotherapy products, as adjunctive treatment to haploidentical hematopoietic stem cell transplantation (HSCT),
Making bone marrow transplantations safer and more effective Patient specific cell-based immunotherapy products, as adjunctive treatment to haploidentical hematopoietic stem cell transplantation (HSCT),
Haploidentical Transplantation: The Answer to our Donor Problems? Mary M. Horowitz, MD, MS CIBMTR, Medical College of Wisconsin January 2017
 Haploidentical Transplantation: The Answer to our Donor Problems? Mary M. Horowitz, MD, MS CIBMTR, Medical College of Wisconsin January 2017 Allogeneic Transplant Recipients in the US, by Donor Type 9000
Haploidentical Transplantation: The Answer to our Donor Problems? Mary M. Horowitz, MD, MS CIBMTR, Medical College of Wisconsin January 2017 Allogeneic Transplant Recipients in the US, by Donor Type 9000
Bone Marrow Transplantation and the Potential Role of Iomab-B
 Bone Marrow Transplantation and the Potential Role of Iomab-B Hillard M. Lazarus, MD, FACP Professor of Medicine, Director of Novel Cell Therapy Case Western Reserve University 1 Hematopoietic Cell Transplantation
Bone Marrow Transplantation and the Potential Role of Iomab-B Hillard M. Lazarus, MD, FACP Professor of Medicine, Director of Novel Cell Therapy Case Western Reserve University 1 Hematopoietic Cell Transplantation
The National Marrow Donor Program. Graft Sources for Hematopoietic Cell Transplantation. Simon Bostic, URD Transplant Recipient
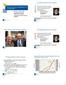 1988 199 1992 1994 1996 1998 2 22 24 26 28 21 212 214 216 218 Adult Donors Cord Blood Units The National Donor Program Graft Sources for Hematopoietic Cell Transplantation Dennis L. Confer, MD Chief Medical
1988 199 1992 1994 1996 1998 2 22 24 26 28 21 212 214 216 218 Adult Donors Cord Blood Units The National Donor Program Graft Sources for Hematopoietic Cell Transplantation Dennis L. Confer, MD Chief Medical
Reduced-intensity Conditioning Transplantation
 Reduced-intensity Conditioning Transplantation Current Role and Future Prospect He Huang M.D., Ph.D. Bone Marrow Transplantation Center The First Affiliated Hospital Zhejiang University School of Medicine,
Reduced-intensity Conditioning Transplantation Current Role and Future Prospect He Huang M.D., Ph.D. Bone Marrow Transplantation Center The First Affiliated Hospital Zhejiang University School of Medicine,
MUD SCT. Pimjai Niparuck Division of Hematology, Department of Medicine Ramathibodi Hospital, Mahidol University
 MUD SCT Pimjai Niparuck Division of Hematology, Department of Medicine Ramathibodi Hospital, Mahidol University Outlines Optimal match criteria for unrelated adult donors Role of ATG in MUD-SCT Post-transplant
MUD SCT Pimjai Niparuck Division of Hematology, Department of Medicine Ramathibodi Hospital, Mahidol University Outlines Optimal match criteria for unrelated adult donors Role of ATG in MUD-SCT Post-transplant
Haploidentical Transplantation today: and the alternatives
 Haploidentical Transplantation today: and the alternatives Daniel Weisdorf MD University of Minnesota February, 2013 No matched sib: where to look? URD donor requires close HLA matching and 3-12 weeks
Haploidentical Transplantation today: and the alternatives Daniel Weisdorf MD University of Minnesota February, 2013 No matched sib: where to look? URD donor requires close HLA matching and 3-12 weeks
One Day BMT Course by Thai Society of Hematology. Management of Graft Failure and Relapsed Diseases
 One Day BMT Course by Thai Society of Hematology Management of Graft Failure and Relapsed Diseases Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital
One Day BMT Course by Thai Society of Hematology Management of Graft Failure and Relapsed Diseases Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital
The future of HSCT. John Barrett, MD, NHBLI, NIH Bethesda MD
 The future of HSCT John Barrett, MD, NHBLI, NIH Bethesda MD Transplants today Current approaches to improve SCT outcome Optimize stem cell dose and source BMT? PBSCT? Adjusting post transplant I/S to minimize
The future of HSCT John Barrett, MD, NHBLI, NIH Bethesda MD Transplants today Current approaches to improve SCT outcome Optimize stem cell dose and source BMT? PBSCT? Adjusting post transplant I/S to minimize
Haplo vs Cord vs URD Debate
 3rd Annual ASBMT Regional Conference for NPs, PAs and Fellows Haplo vs Cord vs URD Debate Claudio G. Brunstein Associate Professor University of Minnesota Medical School Take home message Finding a donor
3rd Annual ASBMT Regional Conference for NPs, PAs and Fellows Haplo vs Cord vs URD Debate Claudio G. Brunstein Associate Professor University of Minnesota Medical School Take home message Finding a donor
Rob Wynn RMCH & University of Manchester, UK. HCT in Children
 Rob Wynn RMCH & University of Manchester, UK HCT in Children Summary Indications for HCT in children Donor selection for Paediatric HCT Using cords Achieving engraftment in HCT Conditioning Immune action
Rob Wynn RMCH & University of Manchester, UK HCT in Children Summary Indications for HCT in children Donor selection for Paediatric HCT Using cords Achieving engraftment in HCT Conditioning Immune action
Allogeneic Hematopoietic Stem Cell Transplantation: State of the Art in 2018 RICHARD W. CHILDS M.D. BETHESDA MD
 Allogeneic Hematopoietic Stem Cell Transplantation: State of the Art in 2018 RICHARD W. CHILDS M.D. BETHESDA MD Overview: Update on allogeneic transplantation for malignant and nonmalignant diseases: state
Allogeneic Hematopoietic Stem Cell Transplantation: State of the Art in 2018 RICHARD W. CHILDS M.D. BETHESDA MD Overview: Update on allogeneic transplantation for malignant and nonmalignant diseases: state
Revista Cubana de Hematología, Inmunología y Hemoterapia. 2017; 36 (Suplemento).
 Depletion of TCR alpha/beta+ T-lymphocytes from grafts for haplo haematopoietic CELL transplantation (HCT) in children Heilmann C, Ifversen M, Haastrup E, Fischer-Nielsen A. Haematopoietic Cell Transplantation
Depletion of TCR alpha/beta+ T-lymphocytes from grafts for haplo haematopoietic CELL transplantation (HCT) in children Heilmann C, Ifversen M, Haastrup E, Fischer-Nielsen A. Haematopoietic Cell Transplantation
Company Overview. January 2019
 Company Overview January 2019 1 Disclaimer This Presentation includes certain projections and forward-looking statements as of the date of this Presentation provided by Gamida Cell Ltd (the Company ).
Company Overview January 2019 1 Disclaimer This Presentation includes certain projections and forward-looking statements as of the date of this Presentation provided by Gamida Cell Ltd (the Company ).
Company Overview. October 2018
 Company Overview October 2018 1 Disclaimer This presentation, the information contained herein and the materials accompanying it (collectively, this Presentation ) does not purport to contain all of the
Company Overview October 2018 1 Disclaimer This presentation, the information contained herein and the materials accompanying it (collectively, this Presentation ) does not purport to contain all of the
Hematopoietic Stem Cell Transplant in Sickle Cell Disease- An update
 Hematopoietic Stem Cell Transplant in Sickle Cell Disease- An update Dr Chirag A Shah Diplomate American Board of Hematology and Medical Oncology Director, Dept of Hemato-Oncology and Stem Cell Transplant
Hematopoietic Stem Cell Transplant in Sickle Cell Disease- An update Dr Chirag A Shah Diplomate American Board of Hematology and Medical Oncology Director, Dept of Hemato-Oncology and Stem Cell Transplant
Current Status of Haploidentical Hematopoietic Stem Cell Transplantation
 Current Status of Haploidentical Hematopoietic Stem Cell Transplantation Annalisa Ruggeri, MD, PhD Hematology and BMT Unit Hôpital Saint Antoine, Paris, France #EBMTITC16 www.ebmt.org Hematopoietic SCT
Current Status of Haploidentical Hematopoietic Stem Cell Transplantation Annalisa Ruggeri, MD, PhD Hematology and BMT Unit Hôpital Saint Antoine, Paris, France #EBMTITC16 www.ebmt.org Hematopoietic SCT
High dose cyclophosphamide in HLAhaploidentical
 High dose cyclophosphamide in HLAhaploidentical stem cell transplantation Ephraim J. Fuchs, M.D., M.B.A. Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins fuchsep@jhmi.edu Alternative Donor Transplantation:
High dose cyclophosphamide in HLAhaploidentical stem cell transplantation Ephraim J. Fuchs, M.D., M.B.A. Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins fuchsep@jhmi.edu Alternative Donor Transplantation:
An Introduction to Bone Marrow Transplant
 Introduction to Blood Cancers An Introduction to Bone Marrow Transplant Rushang Patel, MD, PhD, FACP Florida Hospital Medical Group S My RBC Plt Gran Polycythemia Vera Essential Thrombocythemia AML, CML,
Introduction to Blood Cancers An Introduction to Bone Marrow Transplant Rushang Patel, MD, PhD, FACP Florida Hospital Medical Group S My RBC Plt Gran Polycythemia Vera Essential Thrombocythemia AML, CML,
What s a Transplant? What s not?
 What s a Transplant? What s not? How to report the difference? Daniel Weisdorf MD University of Minnesota Anti-cancer effects of BMT or PBSCT [HSCT] Kill the cancer Save the patient Restore immunocompetence
What s a Transplant? What s not? How to report the difference? Daniel Weisdorf MD University of Minnesota Anti-cancer effects of BMT or PBSCT [HSCT] Kill the cancer Save the patient Restore immunocompetence
Stem Cell Transplantation
 Stem Cell Transplantation Evelyne Willems Centre Hospitalier Universitaire, ULg, Liège Post-ASH meeting, January 11, 2012, Brussels Plan 1. Select the patient: validation of HCT-CI 2. Select the donor
Stem Cell Transplantation Evelyne Willems Centre Hospitalier Universitaire, ULg, Liège Post-ASH meeting, January 11, 2012, Brussels Plan 1. Select the patient: validation of HCT-CI 2. Select the donor
Myeloablative and Reduced Intensity Conditioning for HSCT Annalisa Ruggeri, MD, Hôpital Saint Antoine Eurocord- Hôpital Saint Louis, Paris
 Myeloablative and Reduced Intensity Conditioning for HSCT Annalisa Ruggeri, MD, Hôpital Saint Antoine Eurocord- Hôpital Saint Louis, Paris 18th ESH - EBMT Training Course on HSCT 8-10 May 2014, Vienna,
Myeloablative and Reduced Intensity Conditioning for HSCT Annalisa Ruggeri, MD, Hôpital Saint Antoine Eurocord- Hôpital Saint Louis, Paris 18th ESH - EBMT Training Course on HSCT 8-10 May 2014, Vienna,
Federica Galaverna, 1 Daria Pagliara, 1 Deepa Manwani, 2 Rajni Agarwal-Hashmi, 3 Melissa Aldinger, 4 Franco Locatelli 1
 Administration of Rivogenlecleucel (Rivo-cel, BPX-501) Following αβ T- and B-Cell Depleted Haplo-HSCT in Children With Transfusion-Dependent Thalassemia Federica Galaverna, 1 Daria Pagliara, 1 Deepa Manwani,
Administration of Rivogenlecleucel (Rivo-cel, BPX-501) Following αβ T- and B-Cell Depleted Haplo-HSCT in Children With Transfusion-Dependent Thalassemia Federica Galaverna, 1 Daria Pagliara, 1 Deepa Manwani,
The question is not whether or not to deplete T-cells, but how to deplete which T-cells
 The question is not whether or not to deplete T-cells, but how to deplete which T-cells CD34+ positive selection Negative Depletion of: CD3/CD19 TcRαβ/CD19 T-cell depletion: positive selection versus negative
The question is not whether or not to deplete T-cells, but how to deplete which T-cells CD34+ positive selection Negative Depletion of: CD3/CD19 TcRαβ/CD19 T-cell depletion: positive selection versus negative
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_cll_and_sll 2/2001
Corporate Medical Policy Hematopoietic Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_cll_and_sll 2/2001
Umbilical Cord Blood Transplantation
 Umbilical Cord Blood Transplantation Current Results John E. Wagner, M.D. Blood and Marrow Transplant Program and Stem Cell Institute University of Minnesota Donor Choices Unrelated Marrow/PBSC Results
Umbilical Cord Blood Transplantation Current Results John E. Wagner, M.D. Blood and Marrow Transplant Program and Stem Cell Institute University of Minnesota Donor Choices Unrelated Marrow/PBSC Results
MUD HSCT as first line Treatment in Idiopathic SAA. Dr Sujith Samarasinghe Great Ormond Street Hospital for Children, London, UK
 MUD HSCT as first line Treatment in Idiopathic SAA Dr Sujith Samarasinghe Great Ormond Street Hospital for Children, London, UK No Financial Disclosures Guidelines for management of aplastic anaemia British
MUD HSCT as first line Treatment in Idiopathic SAA Dr Sujith Samarasinghe Great Ormond Street Hospital for Children, London, UK No Financial Disclosures Guidelines for management of aplastic anaemia British
What s new in Blood and Marrow Transplant? Saar Gill, MD PhD Jan 22, 2016
 What s new in Blood and Marrow Transplant? Saar Gill, MD PhD Jan 22, 2016 Division of Hematology-Oncology University of Pennsylvania Perelman School of Medicine 1 Who should be transplanted and how? Updates
What s new in Blood and Marrow Transplant? Saar Gill, MD PhD Jan 22, 2016 Division of Hematology-Oncology University of Pennsylvania Perelman School of Medicine 1 Who should be transplanted and how? Updates
AIH, Marseille 30/09/06
 ALLOGENEIC STEM CELL TRANSPLANTATION FOR MYELOID MALIGNANCIES Transplant and Cellular Therapy Unit Institut Paoli Calmettes Inserm U599 Université de la Méditerranée ée Marseille, France AIH, Marseille
ALLOGENEIC STEM CELL TRANSPLANTATION FOR MYELOID MALIGNANCIES Transplant and Cellular Therapy Unit Institut Paoli Calmettes Inserm U599 Université de la Méditerranée ée Marseille, France AIH, Marseille
Haploidentical Transplants for Lymphoma. Andrea Bacigalupo Universita Cattolica Policlinico Gemelli Roma - Italy
 Haploidentical Transplants for Lymphoma Andrea Bacigalupo Universita Cattolica Policlinico Gemelli Roma - Italy HODGKIN NON HODGKIN Non Myelo Ablative Regimen Luznik L et al BBMT 2008 Comparison of Outcomes
Haploidentical Transplants for Lymphoma Andrea Bacigalupo Universita Cattolica Policlinico Gemelli Roma - Italy HODGKIN NON HODGKIN Non Myelo Ablative Regimen Luznik L et al BBMT 2008 Comparison of Outcomes
5/9/2018. Bone marrow failure diseases (aplastic anemia) can be cured by providing a source of new marrow
 5/9/2018 or Stem Cell Harvest Where we are now, and What s Coming AA MDS International Foundation Indianapolis IN Luke Akard MD May 19, 2018 Infusion Transplant Conditioning Treatment 2-7 days STEM CELL
5/9/2018 or Stem Cell Harvest Where we are now, and What s Coming AA MDS International Foundation Indianapolis IN Luke Akard MD May 19, 2018 Infusion Transplant Conditioning Treatment 2-7 days STEM CELL
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Waldenstrom Macroglobulinemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem_cell_transplantation_for_waldenstrom_macroglobulinemia
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Waldenstrom Macroglobulinemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem_cell_transplantation_for_waldenstrom_macroglobulinemia
Introduction to Clinical Hematopoietic Cell Transplantation (HCT) George Chen, MD Thursday, May 03, 2018
 Introduction to Clinical Hematopoietic Cell Transplantation (HCT) George Chen, MD Thursday, May 03, 2018 The transfer of hematopoietic progenitor and stem cells for therapeutic purposes Hematopoietic Cell
Introduction to Clinical Hematopoietic Cell Transplantation (HCT) George Chen, MD Thursday, May 03, 2018 The transfer of hematopoietic progenitor and stem cells for therapeutic purposes Hematopoietic Cell
Experience of patients transplanted with naïve T cell depleted stem cell graft in CMUH
 Experience of patients transplanted with naïve T cell depleted stem cell graft in CMUH Tzu-Ting Chen, Wen-Jyi Lo, Chiao-Lin Lin, Ching-Chan Lin, Li-Yuan Bai, Supeng Yeh, Chang-Fang Chiu Hematology and
Experience of patients transplanted with naïve T cell depleted stem cell graft in CMUH Tzu-Ting Chen, Wen-Jyi Lo, Chiao-Lin Lin, Ching-Chan Lin, Li-Yuan Bai, Supeng Yeh, Chang-Fang Chiu Hematology and
ASCO Analyst & Investor Webcast. June 1, 2018
 ASCO Analyst & Investor Webcast June 1, 2018 June 1, 2018 NASDAQ: BLUE Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information
ASCO Analyst & Investor Webcast June 1, 2018 June 1, 2018 NASDAQ: BLUE Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information
Trends in Hematopoietic Cell Transplantation. AAMAC Patient Education Day Oct 2014
 Trends in Hematopoietic Cell Transplantation AAMAC Patient Education Day Oct 2014 Objectives Review the principles behind allogeneic stem cell transplantation Outline the process of transplant, some of
Trends in Hematopoietic Cell Transplantation AAMAC Patient Education Day Oct 2014 Objectives Review the principles behind allogeneic stem cell transplantation Outline the process of transplant, some of
Related haploidentical donors versus matched unrelated donors
 Related haploidentical donors versus matched unrelated donors Bronwen Shaw, MD PhD Professor of Medicine, MCW Senior Scientific Director, CIBMTR Definition Matched Unrelated donor Refers to HLA matching
Related haploidentical donors versus matched unrelated donors Bronwen Shaw, MD PhD Professor of Medicine, MCW Senior Scientific Director, CIBMTR Definition Matched Unrelated donor Refers to HLA matching
Making Hope A Reality December 10, Nasdaq : BLUE
 Making Hope A Reality December 10, 2014 Nasdaq : BLUE Forward Looking Statement These slides and the accompanying oral presentation contain forward-looking statements and information. The use of words
Making Hope A Reality December 10, 2014 Nasdaq : BLUE Forward Looking Statement These slides and the accompanying oral presentation contain forward-looking statements and information. The use of words
Quarterly Update & ASH 2017 Abstract Conference Call
 Quarterly Update & ASH 2017 Abstract Conference Call November 1, 2017 Nasdaq : BLUE 1 Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and
Quarterly Update & ASH 2017 Abstract Conference Call November 1, 2017 Nasdaq : BLUE 1 Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_ transplantation_for_primary_amyloidosis 2/2001 11/2018 11/2019 11/2018 Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_ transplantation_for_primary_amyloidosis 2/2001 11/2018 11/2019 11/2018 Description
EBMT Complications and Quality of Life Working Party Educational Course
 EBMT Complications and Quality of Life Working Party Educational Course Organisers: R. Duarte, G. Basak 23-24 October 2014, Warsaw, Poland #EBMT2014 www.ebmt.org EBMT Complications and Quality of Life
EBMT Complications and Quality of Life Working Party Educational Course Organisers: R. Duarte, G. Basak 23-24 October 2014, Warsaw, Poland #EBMT2014 www.ebmt.org EBMT Complications and Quality of Life
Does NK cell alloreactivity prevent relapse? Yes!!! Andrea Velardi Bone Marrow Transplant Program University of Perugia
 Does NK cell alloreactivity prevent relapse? Yes!!! Andrea Velardi Bone Marrow Transplant Program University of Perugia Recognition of missing self HLA triggers lysis NK Inhibitory receptor Activating
Does NK cell alloreactivity prevent relapse? Yes!!! Andrea Velardi Bone Marrow Transplant Program University of Perugia Recognition of missing self HLA triggers lysis NK Inhibitory receptor Activating
March Corporate Presentation
 March 2017 Corporate Presentation Disclaimer This presentation contains forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements
March 2017 Corporate Presentation Disclaimer This presentation contains forward-looking statements, as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements
Alloreattività e Tolleranza nei Trapianti di Cellule Staminali Emopoietiche Allogeniche
 Alloreattività e Tolleranza nei Trapianti di Cellule Staminali Emopoietiche Allogeniche Massimo Fabrizio Martelli Ematologia ed Immunologia Clinica Università degli Studi di Perugia 41 Congresso Nazionale
Alloreattività e Tolleranza nei Trapianti di Cellule Staminali Emopoietiche Allogeniche Massimo Fabrizio Martelli Ematologia ed Immunologia Clinica Università degli Studi di Perugia 41 Congresso Nazionale
Dr. Joseph McGuirk Professor of Medicine, BMT Medical Director, Interim Director, Division of Hematology/Oncology
 Advances in Autologous and Allogeneic Stem Cell Transplantation Dr. Joseph McGuirk Professor of Medicine, BMT Medical Director, Interim Director, Division of Hematology/Oncology April 12, 2014 Disclosures
Advances in Autologous and Allogeneic Stem Cell Transplantation Dr. Joseph McGuirk Professor of Medicine, BMT Medical Director, Interim Director, Division of Hematology/Oncology April 12, 2014 Disclosures
UNRELATED DONOR TRANSPLANTATION FOR SICKLE CELL DISEASE AN UPDATE
 UNRELATED DONOR TRANSPLANTATION FOR SICKLE CELL DISEASE AN UPDATE Naynesh Kamani, M.D. Children s National Medical Center GW University School of Medicine Washington, DC SCD scope of problem in USA Commonest
UNRELATED DONOR TRANSPLANTATION FOR SICKLE CELL DISEASE AN UPDATE Naynesh Kamani, M.D. Children s National Medical Center GW University School of Medicine Washington, DC SCD scope of problem in USA Commonest
Bone Marrow Transplantation in Myelodysplastic Syndromes. An overview for the Myelodysplasia Support Group of Ottawa
 Bone Marrow Transplantation in Myelodysplastic Syndromes An overview for the Myelodysplasia Support Group of Ottawa Objectives Provide brief review of marrow failure Re emphasize the importance of predictions
Bone Marrow Transplantation in Myelodysplastic Syndromes An overview for the Myelodysplasia Support Group of Ottawa Objectives Provide brief review of marrow failure Re emphasize the importance of predictions
Patient-specific immunotherapy T-cell product ATIR
 Patient-specific immunotherapy T-cell product ATIR Unlock full potential of haploidentical hematopoietic stem cell transplantations (HSCT) Company presentation, July 20, 2018 Amsterdam, The Netherlands
Patient-specific immunotherapy T-cell product ATIR Unlock full potential of haploidentical hematopoietic stem cell transplantations (HSCT) Company presentation, July 20, 2018 Amsterdam, The Netherlands
ALLOGENEIC STEM CELL TRANSPLANTATION FOR ACUTE MYELOBLASTIC LEUKEMIAS
 ALLOGENEIC STEM CELL TRANSPLANTATION FOR ACUTE MYELOBLASTIC LEUKEMIAS Didier Blaise, MD Transplant and Cellular Therapy Unit (U2T) Department of Hematology Centre de Recherche en Cancérologie, Inserm U891
ALLOGENEIC STEM CELL TRANSPLANTATION FOR ACUTE MYELOBLASTIC LEUKEMIAS Didier Blaise, MD Transplant and Cellular Therapy Unit (U2T) Department of Hematology Centre de Recherche en Cancérologie, Inserm U891
Business Update & Financial Results for Q1 2018
 Business Update & Financial Results for Q1 2018 May 15, 2018 Disclaimer The statements made in this presentation may include forward-looking statements regarding the future operations of ERYTECH Pharma
Business Update & Financial Results for Q1 2018 May 15, 2018 Disclaimer The statements made in this presentation may include forward-looking statements regarding the future operations of ERYTECH Pharma
Transplantation - Challenges for the future. Dr Gordon Cook S t James s Institute of Oncology, Leeds Teaching Hospitals Trust
 Transplantation - Challenges for the future Dr Gordon Cook S t James s Institute of Oncology, Leeds Teaching Hospitals Trust Bone Marrow Transplantation Timeline, 1957-2006 Appelbaum F. N Engl J Med 2007;357:1472-1475
Transplantation - Challenges for the future Dr Gordon Cook S t James s Institute of Oncology, Leeds Teaching Hospitals Trust Bone Marrow Transplantation Timeline, 1957-2006 Appelbaum F. N Engl J Med 2007;357:1472-1475
Acknowledgements. Department of Hematological Malignancy and Cellular Therapy, University of Kansas Medical Center
 The Addition of Extracorporeal Photopheresis (ECP) to Tacrolimus and Methotrexate to Prevent Acute and Chronic Graft- Versus Host Disease in Myeloablative Hematopoietic Cell Transplant (HCT) Anthony Accurso,
The Addition of Extracorporeal Photopheresis (ECP) to Tacrolimus and Methotrexate to Prevent Acute and Chronic Graft- Versus Host Disease in Myeloablative Hematopoietic Cell Transplant (HCT) Anthony Accurso,
Le infezioni fungine nel trapianto di cellule staminali emopoietiche. Claudio Viscoli Professor of Infectious Disease University of Genova, Italy
 Le infezioni fungine nel trapianto di cellule staminali emopoietiche Claudio Viscoli Professor of Infectious Disease University of Genova, Italy Potential conflicts of interest Received grants as speaker/moderator
Le infezioni fungine nel trapianto di cellule staminali emopoietiche Claudio Viscoli Professor of Infectious Disease University of Genova, Italy Potential conflicts of interest Received grants as speaker/moderator
ZIOPHARM / Intrexon Graft-Versus-Host Disease Exclusive Channel Collaboration SEPTEMBER 28, 2015
 ZIOPHARM / Intrexon Graft-Versus-Host Disease Exclusive Channel Collaboration SEPTEMBER 28, 2015 1 Forward-looking Statements This presentation contains certain forward-looking information about ZIOPHARM
ZIOPHARM / Intrexon Graft-Versus-Host Disease Exclusive Channel Collaboration SEPTEMBER 28, 2015 1 Forward-looking Statements This presentation contains certain forward-looking information about ZIOPHARM
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplant_for_acute_myeloid_leukemia
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplant_for_acute_myeloid_leukemia
Post Transplant Management for Sickle Cell. Title
 Post Transplant Management for Sickle Cell Title Kimberly Kasow, DO October 14, 2016 Thank you for this opportunity to present this information I have no financial interests to disclose. Goal of Transplant
Post Transplant Management for Sickle Cell Title Kimberly Kasow, DO October 14, 2016 Thank you for this opportunity to present this information I have no financial interests to disclose. Goal of Transplant
NiCord Single Unit Expanded Umbilical Cord Blood Transplantation: Results of Phase I/II Trials
 NiCord Single Unit Expanded Umbilical Cord Blood Transplantation: Results of Phase I/II Trials Mitchell E. Horwitz, MD Duke University Medical Center Duke Cancer Institute Adult Umbilical Cord Blood Transplantation
NiCord Single Unit Expanded Umbilical Cord Blood Transplantation: Results of Phase I/II Trials Mitchell E. Horwitz, MD Duke University Medical Center Duke Cancer Institute Adult Umbilical Cord Blood Transplantation
Therapeutic Advances in Treatment of Aplastic Anemia. Seiji Kojima MD. PhD.
 Therapeutic Advances in Treatment of Aplastic Anemia Seiji Kojima MD. PhD. Department of Pediatrics Nagoya University Graduate School of Medicine Chairman of the Severe Aplastic Anemia Working Party Asia-Pacific
Therapeutic Advances in Treatment of Aplastic Anemia Seiji Kojima MD. PhD. Department of Pediatrics Nagoya University Graduate School of Medicine Chairman of the Severe Aplastic Anemia Working Party Asia-Pacific
Sickle Cell Diseasechronic. curable disease? Objectives. Why would a family ask about cure for SCD?
 Sickle Cell Diseasechronic illness or curable disease? Gregory M.T. Guilcher MD, FRCPC, FAAP Objectives To review the general principles of hematopoietic stem cell transplantation (HSCT), including risks
Sickle Cell Diseasechronic illness or curable disease? Gregory M.T. Guilcher MD, FRCPC, FAAP Objectives To review the general principles of hematopoietic stem cell transplantation (HSCT), including risks
Patient-specific immunotherapy designed to reduce the risk of GVHD and relapse in stem cell transplantations
 Patient-specific immunotherapy designed to reduce the risk of GVHD and relapse in stem cell transplantations Unlock full potential of haploidentical hematopoietic stem cell transplantations (HSCT), with
Patient-specific immunotherapy designed to reduce the risk of GVHD and relapse in stem cell transplantations Unlock full potential of haploidentical hematopoietic stem cell transplantations (HSCT), with
Sunesis Pharmaceuticals Reports Third Quarter 2014 Financial Results and Recent Highlights
 Sunesis Pharmaceuticals Reports Third Quarter 2014 Financial Results and Recent Highlights November 10, 2014 7:00 AM ET Announces Submission of Letter of Intent to File MAA for Vosaroxin in Relapsed or
Sunesis Pharmaceuticals Reports Third Quarter 2014 Financial Results and Recent Highlights November 10, 2014 7:00 AM ET Announces Submission of Letter of Intent to File MAA for Vosaroxin in Relapsed or
COHEM Barcellona 2012 Hemoglobinopathies debate
 COHEM Barcellona 2012 Hemoglobinopathies debate September 8, 2012: h. 10:30-12:00 Hall: A Is it justified to perform BMT in hemoglobinopathies using unrelated and/or partially mismatched donors? HSCT indication
COHEM Barcellona 2012 Hemoglobinopathies debate September 8, 2012: h. 10:30-12:00 Hall: A Is it justified to perform BMT in hemoglobinopathies using unrelated and/or partially mismatched donors? HSCT indication
AML:Transplant or ChemoTherapy?
 AML:Transplant or ChemoTherapy? 1960 s: Importance of HLA type in Animal Models Survival of Dogs Given 1000 RAD TBI and a Marrow Infusion from a Littermate Matched or Mismatched for Dog Leucocyte Antigens
AML:Transplant or ChemoTherapy? 1960 s: Importance of HLA type in Animal Models Survival of Dogs Given 1000 RAD TBI and a Marrow Infusion from a Littermate Matched or Mismatched for Dog Leucocyte Antigens
3.1 Clinical safety of chimeric or humanized anti-cd25 (ch/anti-cd25)
 3 Results 3.1 Clinical safety of chimeric or humanized anti-cd25 (ch/anti-cd25) Five infusions of monoclonal IL-2 receptor antibody (anti-cd25) were planned according to protocol between day 0 and day
3 Results 3.1 Clinical safety of chimeric or humanized anti-cd25 (ch/anti-cd25) Five infusions of monoclonal IL-2 receptor antibody (anti-cd25) were planned according to protocol between day 0 and day
Are We There Yet? Gene Therapy and BMT as Curative Therapies in Sickle Cell. Ann Haight, MD 9 Sept 2017
 Are We There Yet? Gene Therapy and BMT as Curative Therapies in Sickle Cell Ann Haight, MD 9 Sept 2017 Spoiler alert Yes (we have a cure) And No Work to do! 2 Sickle Cell Treatment Options Supportive Care
Are We There Yet? Gene Therapy and BMT as Curative Therapies in Sickle Cell Ann Haight, MD 9 Sept 2017 Spoiler alert Yes (we have a cure) And No Work to do! 2 Sickle Cell Treatment Options Supportive Care
Corporate Presentation. December, 2018
 Corporate Presentation December, 2018 Forward Looking Statement This presentation contains estimates, projections and other forward-looking statements, concerning, among other things: our research and
Corporate Presentation December, 2018 Forward Looking Statement This presentation contains estimates, projections and other forward-looking statements, concerning, among other things: our research and
Disclosures of: Emanuele Angelucci
 Company name Novartis Disclosures of: Emanuele Angelucci Research support Employee Consultant Stockholder Speakers bureau Advisory board Chair of TELESTO pro Other EBMT 2012 Educational Session Haemoglobinopathy
Company name Novartis Disclosures of: Emanuele Angelucci Research support Employee Consultant Stockholder Speakers bureau Advisory board Chair of TELESTO pro Other EBMT 2012 Educational Session Haemoglobinopathy
Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC
 Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC Forward-Looking Statements To the extent that statements contained in this presentation are not descriptions of historical facts
Corporate Overview June 2014 Jefferies Healthcare Conference NASDAQ: GLYC Forward-Looking Statements To the extent that statements contained in this presentation are not descriptions of historical facts
Programmed Cellular Immunotherapies
 Better Cells For Better Therapies Programmed Cellular Immunotherapies Corporate Overview August 2017-1 - Forward-Looking Statements This presentation contains "forward-looking statements" within the meaning
Better Cells For Better Therapies Programmed Cellular Immunotherapies Corporate Overview August 2017-1 - Forward-Looking Statements This presentation contains "forward-looking statements" within the meaning
Summary of Changes BMT CTN 1101 Version 7.0 to 8.0 Dated: January 18, Original text: Changed to: Rationale
 BMT CTN 1101 RIC ducb vs. Haplo Page 1 of 10 Date: January 20, 2017 Summary of Changes BMT CTN 1101 Version 7.0 to 8.0 Dated: January 18, 2017 A Multi-Center, Phase III, Randomized Trial of Reduced Intensity(RIC)
BMT CTN 1101 RIC ducb vs. Haplo Page 1 of 10 Date: January 20, 2017 Summary of Changes BMT CTN 1101 Version 7.0 to 8.0 Dated: January 18, 2017 A Multi-Center, Phase III, Randomized Trial of Reduced Intensity(RIC)
Understanding the role of ex vivo T cell depletion
 Prevention of graftversus-host disease (GVHD) Understanding the role of ex vivo T cell depletion Information for patients undergoing allogeneic stem cell transplantation in AML and their families 2 This
Prevention of graftversus-host disease (GVHD) Understanding the role of ex vivo T cell depletion Information for patients undergoing allogeneic stem cell transplantation in AML and their families 2 This
HLA-DR-matched Parental Donors for Allogeneic Hematopoietic Stem Cell Transplantation in Patients with High-risk Acute Leukemia
 BRIEF COMMUNICATION HLA-DR-matched Parental Donors for Allogeneic Hematopoietic Stem Cell Transplantation in Patients with High-risk Acute Leukemia Shang-Ju Wu, Ming Yao,* Jih-Luh Tang, Bo-Sheng Ko, Hwei-Fang
BRIEF COMMUNICATION HLA-DR-matched Parental Donors for Allogeneic Hematopoietic Stem Cell Transplantation in Patients with High-risk Acute Leukemia Shang-Ju Wu, Ming Yao,* Jih-Luh Tang, Bo-Sheng Ko, Hwei-Fang
Actinium Pharmaceuticals Highlights Analysis of Pivotal Iomab-B Phase 3 SIERRA Trial Presented in Oral Session at ASH Annual Meeting
 December 4, 2018 Actinium Pharmaceuticals Highlights Analysis of Pivotal Iomab-B Phase 3 SIERRA Trial Presented in Oral Session at ASH Annual Meeting - Key highlights include near universal engraftment
December 4, 2018 Actinium Pharmaceuticals Highlights Analysis of Pivotal Iomab-B Phase 3 SIERRA Trial Presented in Oral Session at ASH Annual Meeting - Key highlights include near universal engraftment
Il Trapianto da donatore MUD. Alessandro Rambaldi
 Il Trapianto da donatore MUD Alessandro Rambaldi Overview Comparison of outcomes of allo- HSCT from matched related and unrelated donors. We need evidence based results! Is the Dme needed to find an unrelated
Il Trapianto da donatore MUD Alessandro Rambaldi Overview Comparison of outcomes of allo- HSCT from matched related and unrelated donors. We need evidence based results! Is the Dme needed to find an unrelated
Cord Blood Transplant. E. Gluckman Eurocord ESH-EBMT training course Vienna 2014
 Cord Blood Transplant E. Gluckman Eurocord ESH-EBMT training course Vienna 2014 Background Since 1988, umbilical cord blood (CB) has been successfully used to treat children and adults needing stem cell
Cord Blood Transplant E. Gluckman Eurocord ESH-EBMT training course Vienna 2014 Background Since 1988, umbilical cord blood (CB) has been successfully used to treat children and adults needing stem cell
Summary of Changes Page BMT CTN 1205 Protocol Amendment #4 (Version 5.0) Dated July 22, 2016
 Page 1 of 8 Date: July 22, 2016 Summary of Changes Page BMT CTN 1205 Protocol #4 Dated July 22, 2016 The following changes, and the rationale for the changes, were made to the attached protocol in this
Page 1 of 8 Date: July 22, 2016 Summary of Changes Page BMT CTN 1205 Protocol #4 Dated July 22, 2016 The following changes, and the rationale for the changes, were made to the attached protocol in this
Factors Influencing Haematopoietic Progenitor cell transplant outcome Optimising donor selection
 Factors Influencing Haematopoietic Progenitor cell transplant outcome Optimising donor selection Alison Logan Transplantation Laboratory Manchester Royal Infirmary Haematopoietic progenitor cell transplants
Factors Influencing Haematopoietic Progenitor cell transplant outcome Optimising donor selection Alison Logan Transplantation Laboratory Manchester Royal Infirmary Haematopoietic progenitor cell transplants
Advancing Cell Therapies
 Take Control of Life Take Control of Life Jefferies 2016 Global Healthcare Conference Advancing Cell Therapies by giving physicians control over cells inside the body June 8, 2016 Forward Looking Statements
Take Control of Life Take Control of Life Jefferies 2016 Global Healthcare Conference Advancing Cell Therapies by giving physicians control over cells inside the body June 8, 2016 Forward Looking Statements
Help patients with immunotherapy designed to reduce risk of GVHD in stem cell transplantations
 Help patients with immunotherapy designed to reduce risk of GVHD in stem cell transplantations Unlock full potential of haploidentical hematopoietic stem cell transplantations (HSCT), with allo-depleted
Help patients with immunotherapy designed to reduce risk of GVHD in stem cell transplantations Unlock full potential of haploidentical hematopoietic stem cell transplantations (HSCT), with allo-depleted
Full Year 2017 Financial Results. February 14, 2018
 Full Year 2017 Financial Results February 14, 2018 Agios Conference Call Participants Prepared Remarks Introduction KENDRA ADAMS, Sr. Director, Investor Relations 2018 Vision & Key Milestones DAVID SCHENKEIN,
Full Year 2017 Financial Results February 14, 2018 Agios Conference Call Participants Prepared Remarks Introduction KENDRA ADAMS, Sr. Director, Investor Relations 2018 Vision & Key Milestones DAVID SCHENKEIN,
Bank of America Merrill Lynch 2016 Health Care Conference
 Bank of America Merrill Lynch 2016 Health Care Conference Dr. Steven Stein Chief Medical Officer David Gryska Chief Financial Officer May 11, 2016 Forward Looking Statements Except for the historical information
Bank of America Merrill Lynch 2016 Health Care Conference Dr. Steven Stein Chief Medical Officer David Gryska Chief Financial Officer May 11, 2016 Forward Looking Statements Except for the historical information
ISCT Workshop #7 Perspectives in Cell Selection Immunomagnetic Selection
 ISCT Workshop #7 Perspectives in Cell Selection Immunomagnetic Selection Carolyn A. Keever-Taylor, PhD Medical College of Wisconsin June 7, 2012 History of Available Devices CellPro CEPRATE Avidin/Biotin
ISCT Workshop #7 Perspectives in Cell Selection Immunomagnetic Selection Carolyn A. Keever-Taylor, PhD Medical College of Wisconsin June 7, 2012 History of Available Devices CellPro CEPRATE Avidin/Biotin
Multi-Virus-Specific T cell Therapy for Patients after HSC and CB Transplant
 Multi-Virus-Specific T cell Therapy for Patients after HSC and CB Transplant Hanley PJ, Krance BR, Brenner MK, Leen AM, Rooney CM, Heslop HE, Shpall EJ, Bollard CM Hematopoietic Stem Cell Transplantation
Multi-Virus-Specific T cell Therapy for Patients after HSC and CB Transplant Hanley PJ, Krance BR, Brenner MK, Leen AM, Rooney CM, Heslop HE, Shpall EJ, Bollard CM Hematopoietic Stem Cell Transplantation
PLEO-CMT Top-line Results. Presentation October 16, 2018
 PLEO-CMT Top-line Results Presentation October 16, 2018 Disclaimer References herein to this presentation (the Presentation ) shall mean and include this document, any oral presentation accompanying this
PLEO-CMT Top-line Results Presentation October 16, 2018 Disclaimer References herein to this presentation (the Presentation ) shall mean and include this document, any oral presentation accompanying this
Corporate Presentation May Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers
 Corporate Presentation May 2016 Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers Forward-looking statements / safe harbor This presentation and the accompanying oral commentary contain
Corporate Presentation May 2016 Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers Forward-looking statements / safe harbor This presentation and the accompanying oral commentary contain
Haploidentical Donor Transplants: Outcomes and Comparison to Other. Paul V. O Donnell BSBMT Education Day London 12 October 2011
 Haploidentical Donor Transplants: Outcomes and Comparison to Other Donor Types Paul V. O Donnell BSBMT Education Day London 12 October 2011 Clinical Problem: Identification of a Donor for Allogeneic Transplantation
Haploidentical Donor Transplants: Outcomes and Comparison to Other Donor Types Paul V. O Donnell BSBMT Education Day London 12 October 2011 Clinical Problem: Identification of a Donor for Allogeneic Transplantation
THE ROLE OF TBI IN STEM CELL TRANSPLANTATION. Dr. Biju George Professor Department of Haematology CMC Vellore
 THE ROLE OF TBI IN STEM CELL TRANSPLANTATION Dr. Biju George Professor Department of Haematology CMC Vellore Introduction Radiotherapy is the medical use of ionising radiation. TBI or Total Body Irradiation
THE ROLE OF TBI IN STEM CELL TRANSPLANTATION Dr. Biju George Professor Department of Haematology CMC Vellore Introduction Radiotherapy is the medical use of ionising radiation. TBI or Total Body Irradiation
Is in vitro T-cell depletion necessary for Haploidentical TransplantationTitle of Presentation. Disclosure of Interest: Nothing to Disclose
 Rupert Handgretinger Children s University Hospital, Tübingen, Germany Is in vitro T-cell depletion necessary for Haploidentical TransplantationTitle of Presentation Disclosure of Interest: Nothing to
Rupert Handgretinger Children s University Hospital, Tübingen, Germany Is in vitro T-cell depletion necessary for Haploidentical TransplantationTitle of Presentation Disclosure of Interest: Nothing to
February Company Overview. Curative Treatments for Cancer and Orphan Genetic Diseases
 February 2017 Company Overview Curative Treatments for Cancer and Orphan Genetic Diseases Curative Treatments for Orphan Indications NiCord - a bone marrow transplantation treatment for patients with high
February 2017 Company Overview Curative Treatments for Cancer and Orphan Genetic Diseases Curative Treatments for Orphan Indications NiCord - a bone marrow transplantation treatment for patients with high
Results of Nicord Phase I II Trials and Plans for Phase III Trial
 Results of Nicord Phase I II Trials and Plans for Phase III Trial Mitchell E. Horwitz, MD Duke Cancer Institute Duke University Medical Center June 10, 2016 Learning Objectives To provide an overview of
Results of Nicord Phase I II Trials and Plans for Phase III Trial Mitchell E. Horwitz, MD Duke Cancer Institute Duke University Medical Center June 10, 2016 Learning Objectives To provide an overview of
Back to the Future: The Resurgence of Bone Marrow??
 Back to the Future: The Resurgence of Bone Marrow?? Thomas Spitzer, MD Director. Bone Marrow Transplant Program Massachusetts General Hospital Professor of Medicine, Harvard Medical School Bone Marrow
Back to the Future: The Resurgence of Bone Marrow?? Thomas Spitzer, MD Director. Bone Marrow Transplant Program Massachusetts General Hospital Professor of Medicine, Harvard Medical School Bone Marrow
Company presentation. Claudio Bordignon, Chairman and CEO. Jefferies Global Healthcare Conference New York, 7 June 2012
 Company presentation Claudio Bordignon, Chairman and CEO Jefferies Global Healthcare Conference New York, 7 June 2012 Forward-looking statements The presentation contains certain forward-looking statements.
Company presentation Claudio Bordignon, Chairman and CEO Jefferies Global Healthcare Conference New York, 7 June 2012 Forward-looking statements The presentation contains certain forward-looking statements.
Q4 Report Webcast February 7, 2019 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO
 Q4 Report 2018 Webcast February 7, 2019 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation has been prepared by Calliditas Therapeutics AB
Q4 Report 2018 Webcast February 7, 2019 Presenters: Renée Aguiar-Lucander, CEO Fredrik Johansson, CFO Disclaimer Important information This presentation has been prepared by Calliditas Therapeutics AB
Virological Surveillance in Paediatric HSCT Recipients
 Virological Surveillance in Paediatric HSCT Recipients Dr Pamela Lee Clinical Assistant Professor Department of Paediatrics & Adolescent Medicine Queen Mary Hospital LKS Faculty of Medicine, The University
Virological Surveillance in Paediatric HSCT Recipients Dr Pamela Lee Clinical Assistant Professor Department of Paediatrics & Adolescent Medicine Queen Mary Hospital LKS Faculty of Medicine, The University
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Autoimmune Diseases File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_autoimmune_diseases
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Autoimmune Diseases File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_autoimmune_diseases
REATA PHARMACEUTICALS, INC. ANNOUNCES SECOND QUARTER 2018 FINANCIAL RESULTS AND AN UPDATE ON DEVELOPMENT PROGRAMS
 REATA PHARMACEUTICALS, INC. ANNOUNCES SECOND QUARTER 2018 FINANCIAL RESULTS AND AN UPDATE ON DEVELOPMENT PROGRAMS IRVING, Texas August 8, 2018 Reata Pharmaceuticals, Inc. (Nasdaq: RETA), a clinical-stage
REATA PHARMACEUTICALS, INC. ANNOUNCES SECOND QUARTER 2018 FINANCIAL RESULTS AND AN UPDATE ON DEVELOPMENT PROGRAMS IRVING, Texas August 8, 2018 Reata Pharmaceuticals, Inc. (Nasdaq: RETA), a clinical-stage
Neutrophil Recovery: The. Posttransplant Recovery. Bus11_1.ppt
 Neutrophil Recovery: The First Step in Posttransplant Recovery No conflicts of interest to disclose Bus11_1.ppt Blood is Made in the Bone Marrow Blood Stem Cell Pre-B White cells B Lymphocyte T Lymphocyte
Neutrophil Recovery: The First Step in Posttransplant Recovery No conflicts of interest to disclose Bus11_1.ppt Blood is Made in the Bone Marrow Blood Stem Cell Pre-B White cells B Lymphocyte T Lymphocyte
Haploidentical Stem Cell Transplantation with post transplantation Cyclophosphamide for the treatment of Fanconi Anemia
 Haploidentical Stem Cell Transplantation with post transplantation Cyclophosphamide for the treatment of Fanconi Anemia Carmem Bonfim Director Pediatric Blood and Marrow Transplantation Program HC Federal
Haploidentical Stem Cell Transplantation with post transplantation Cyclophosphamide for the treatment of Fanconi Anemia Carmem Bonfim Director Pediatric Blood and Marrow Transplantation Program HC Federal
