Hematopoietic Stem-Cell & Clinical Coverage Policy No: 11A-11 Bone Marrow Transplantation Amended Date: March 1, 2017 For Non-Hodgkin s Lymphoma
|
|
|
- Catherine Greer
- 5 years ago
- Views:
Transcription
1 Hematopoietic Stem-Cell & Clinical Coverage Policy No: 11A-11 Bone Marrow Transplantation Amended Date: March 1, 2017 For Non-Hodgkin s Lymphoma Table of Contents 1.0 Description of the Procedure, Product, or Service Hematopoietic Stem-Cell Transplantation Conventional Preparative Conditioning for Hematopoietic SCT Reduced Intensity Conditioning for Allogeneic HSCT Non-Hodgkin s Lymphoma (NHL) Staging Definitions Eligibility Requirements Provisions General Specific Special Provisions EPSDT Special Provision: Exception to Policy Limitations for a Medicaid Beneficiary under 21 Years of Age EPSDT does not apply to NCHC beneficiaries Health Choice Special Provision for a Health Choice Beneficiary age 6 through 18 years of age When the Procedure, Product, or Service Is Covered General Criteria Covered Specific Criteria Covered Specific criteria covered by both Medicaid and NCHC Medicaid Additional Criteria Covered NCHC Additional Criteria Covered Policy Guidelines When the Procedure, Product, or Service Is Not Covered General Criteria Not Covered Specific Criteria Not Covered Specific Criteria Not Covered by both Medicaid and NCHC Medicaid Additional Criteria Not Covered NCHC Additional Criteria Not Covered Requirements for and Limitations on Coverage Prior Approval Prior Approval Requirements General Specific Specific Transplant Prior Approval Requirements Provider(s) Eligible to Bill for the Procedure, Product, or Service Provider Qualifications and Occupational Licensing Entity Regulations Provider Certifications B17 i
2 Hematopoietic Stem-Cell & Clinical Coverage Policy No: 11A-11 Bone Marrow Transplantation Amended Date: March 1, 2017 For Non-Hodgkin s Lymphoma 7.0 Additional Requirements Compliance Policy Implementation/Revision Information Attachment A: Claims-Related Information A. Claim Type B. International Classification of Diseases, Tenth Revisions, Clinical Modification (ICD-10- CM) and Procedural Coding System (PCS) C. Code(s) D. Modifiers E. Billing Units F. Place of Service G. Co-payments H. Reimbursement I. Billing for Donor Expenses B17 ii
3 Hematopoietic Stem-Cell & Clinical Coverage Policy No: 11A-11 Bone Marrow Transplantation Amended Date: March 1, 2017 For Non-Hodgkin s Lymphoma 1.0 Description of the Procedure, Product, or Service 1.1 Hematopoietic Stem-Cell Transplantation Hematopoietic stem cell transplantation (HSCT) refers to a procedure in which hematopoietic stem cells are infused to restore bone marrow function in cancer patients who receive bone-marrow-toxic doses of cytotoxic drugs, with or without whole-body radiation therapy. Hematopoietic stem cells may be obtained from the transplant recipient (i.e., autologous HSCT) or from a donor (i.e., allogeneic HSCT). They can be harvested from bone marrow, peripheral blood, or umbilical cord blood and placenta shortly after delivery of neonates. Although cord blood is an allogeneic source, the stem cells in it are antigenically naïve and thus are associated with a lower incidence of rejection or graftversus-host disease (GVHD). Immunologic compatibility between infused stem cells and the recipient is not an issue in autologous HSCT. However, immunologic compatibility between donor and patient is a critical factor for achieving a good outcome of allogeneic HSCT. Compatibility is established by typing human leukocyte antigens (HLA) using cellular, serologic, or molecular techniques. HLA refers to the tissue type expressed at the HLA A, B, and DR loci on each leg of chromosome 6. Depending on the disease being treated, an acceptable donor will match the patient at all or most of the HLA loci with the exception of umbilical cord blood). 1.2 Conventional Preparative Conditioning for Hematopoietic SCT The success of autologous HSCT is predicated on the ability of cytotoxic chemotherapy with or without radiation to eradicate cancerous cells from the blood and bone marrow. This permits subsequent engraftment and repopulation of bone marrow space with presumably normal hematopoietic stem cells obtained from the patient prior to undergoing bone marrow ablation. As a consequence, autologous HSCT is typically performed as consolidation therapy when the patient s disease is in complete remission. Patients who undergo autologous HSCT are susceptible to chemotherapy-related toxicities and opportunistic infections prior to engraftment, but not GVHD. The conventional ( classical ) practice of allogeneic HSCT involves administration of cytotoxic agents (e.g., cyclophosphamide, busulfan) with or without total-body irradiation at doses sufficient to destroy endogenous hematopoietic capability in the recipient. The beneficial treatment effect in this procedure is due to a combination of initial eradication of malignant cells and subsequent graft-versus-malignancy effect (GVM) effect mediated by nonself immunologic effector cells that develop after engraftment of allogeneic stem cells within the patient s bone marrow space. While the slower GVM effect is considered to be the potentially curative component, it may be CPT codes, descriptors, and other data only are copyright 2016 American Medical Association. All rights reserved. Applicable FARS/DFARS apply. 17B17 1
4 overwhelmed by extant disease without the use of pretransplant conditioning. However, intense conditioning regimens are limited to patients who are sufficiently fit medically to tolerate substantial adverse effects that include pre-engraftment opportunistic infections secondary to loss of endogenous bone marrow function and organ damage and failure caused by the cytotoxic drugs. Furthermore, in any allogeneic HSCT, immune suppressant drugs are required to minimize graft rejections and GVHD, which also increases susceptibility of the patient to opportunistic infections. 1.3 Reduced Intensity Conditioning for Allogeneic HSCT Reduced-intensity conditioning (RIC) refers to the pretransplant use of lower doses or less intense regimens of cytotoxic drugs or radiation than are used in traditional full-dose myeloablative conditioning treatments. The goal of RIC is to reduce disease burden but also to minimize as much as possible associated treatment-related morbidity and nonrelapse mortality (NRM) in the period during which the beneficial GVM effect of allogeneic transplantation develops. Although the definition of RIC remains arbitrary, with numerous versions employed, all seek to balance the competing effects of NRM and relapse due to residual disease. RIC regimens can be viewed as a continuum in effects, from nearly totally myeloablative to minimally myeloablative with lymphoablation, with intensity tailored to specific diseases and patient condition. Patients who undergo RIC with allogeneic HSCT initially demonstrate donor cell engraftment and bone marrow mixed chimerism. Most will subsequently convert to full-donor chimerism, which may be supplemented with donor lymphocyte infusions to eradicate residual malignant cells. For the purposes of the Policy, the term reduced-intensity conditioning will refer to all conditioning regimens intended to be nonmyeloablative, as opposed to fully myeloablative (traditional) regimens. 1.4 Non-Hodgkin s Lymphoma (NHL) A heterogeneous group of lymphoproliferative malignancies, NHL usually originates in lymphoid tissue. Historically, uniform treatment of patients with NHL was hampered by the lack of a uniform classification system. In 1982, the Working Formulation (WF) was developed to unify different classification systems into one. The WF divided NHL into low-, intermediate-, and high-grade, with subgroups based on histologic cell type. Since our understanding of NHL has improved, the diagnosis has become more sophisticated and includes the incorporation of new immunophenotyping and genetic techniques. As a result, the WF has become outdated. European and American pathologists proposed a new classification, the Revised European American Lymphoma (REAL) Classification, and an updated version of the REAL system, the new World Health Organization (WHO) classification. The WHO/REAL classification recognizes three (3) major categories of lymphoid malignancies based on morphology and cell lineage: B-cell neoplasms, T-cell/natural killer (NK)-cell neoplasms, and Hodgkin s lymphoma. The most recent lymphoma classification is the 2008 WHO Classification: Mature B-cell neoplasms a. Chronic lymphocytic leukemia/small lymphocytic lymphoma b. B-cell prolymphocytic leukemia 17B17 2
5 c. Splenic marginal zone lymphoma d. Hairy cell leukemia e. Splenic lymphoma/leukemia, unclassifiable 1. Splenic diffuse red pulp small B-cell lymphoma* 2. Hairy cell leukemia-variant* f. Lymphoplasmacytic lymphoma 1. Waldenstrom macroglobulinemia g. Heavy chain diseases 1. Alpha heavy chain disease 2. Gamma heavy chain disease 3. Mu heavy chain disease h. Plasma cell myeloma i. Solitary plasmacytoma of bone j. Extraosseous plasmacytoma k. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) l. Nodal marginal zone B-cell lymphoma (MZL) 1. Pediatric type nodal MZL m. Follicular lymphoma 1. Pediatric type follicular lymphoma n. Primary cutaneous follicle center lymphoma o. Mantle cell lymphoma p. Diffuse large B-cell lymphoma (DLBCL), not otherwise specified 1. T cell/histiocyte rich large B-cell lymphoma 2. DLBCL associated with chronic inflammation 3. Epstein-Barr virus (EBV)+ DLBCL of the elderly q. Lymphomatoid granulomatosis r. Primary mediastinal (thymic) large B-cell lymphoma s. Intravascular large B-cell lymphoma t. Primary cutaneous DLBCL, leg type u. ALK [anaplastic lymphoma kinase] + large B-cell lymphoma v. Plasmablastic lymphoma w. Primary effusion lymphoma x. Large B-cell lymphoma arising in HHV8-associated multicentric Castleman disease y. Burkitt lymphoma z. B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B- cell lymphoma and Burkitt lymphoma aa. B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B- cell lymphoma and classical Hodgkin s lymphoma *These represent provisional entities or provisional subtypes of other neoplasms. Diseases shown in italics are newly included in the 2008 WHO classification. Mature T-cell and NK-cell neoplasms a. T-cell prolymphocytic leukemia b. T-cell large granular lymphocytic leukemia c. Chronic lymphoproliferative disorder of NK-cells* d. Aggressive NK cell leukemia e. Systemic EBV [Epstein-Bar virus]+ T-cell lymphoproliferative disease of childhood 17B17 3
6 (associated with chronic active EBV infection) f. Hydroa vacciniforme-like lymphoma g. Adult T-cell leukemia/ lymphoma h. Extranodal NK/T cell lymphoma, nasal type i. Enteropathy-associated T-cell lymphoma j. Hepatosplenic T-cell lymphoma k. Subcutaneous panniculitis-like T-cell lymphoma l. Mycosis fungoides m. Sézary syndrome n. Primary cutaneous CD30+ T-cell lymphoproliferative disorder 1. Lymphomatoid papulosis 2. Primary cutaneous anaplastic large-cell lymphoma o. Primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma* p. Primary cutaneous gamma-delta T-cell lymphoma q. Primary cutaneous small/medium CD4+ T-cell lymphoma* r. Peripheral T-cell lymphoma, not otherwise specified s. Angioimmunoblastic T-cell lymphoma t. Anaplastic large cell lymphoma (ALCL), ALK+ u. Anaplastic large cell lymphoma (ALCL), ALK * *These represent provisional entities or provisional subtypes of other neoplasms. Diseases shown in italics are newly included in the 2008 WHO classification In the United States, B-cell lymphomas represent 80% 85% of cases of NHL, and T-cell lymphomas represent 15% 20%. NK lymphomas are relatively rare. In general, the NHL can be divided into two prognostic groups, indolent and aggressive. Indolent NHL has a relatively good prognosis, with a median survival of 10 years; however, it is not curable in advanced clinical stages. Early-stage indolent NHL [stage 1 or 2] may be effectively treated with radiation alone. Although indolent NHL is responsive to radiation and chemotherapy, a continuous rate of relapse is seen in advanced stages. These patients can often be re-treated, if their disease remains of the indolent type. Indolent NHL may transform into a more aggressive form, which is generally treated with regimens that are used for aggressive, recurrent NHL. Histologic transformation to higher grade lymphoma occurs in up to 70% of patients with low-grade lymphoma, and median survival with conventional chemotherapy is one year or less. Follicular lymphoma (FL) is the most common indolent NHL (70% 80% of cases), and often the terms indolent lymphoma and FL are used synonymously. Also included in the indolent NHL are small lymphocytic lymphoma/chronic lymphocytic leukemia (SLL/CLL), lymphoplasmactic lymphoma, marginal zone lymphomas, and cutaneous T- cell lymphoma. Aggressive NHL has a shorter natural history; however, 30% 60% of these patients can be cured with intensive combination chemotherapy regimens. Aggressive lymphomas include diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), peripheral T-cell lymphoma (PTCL), anaplastic large cell lymphoma, and Burkitt s lymphoma. Oncologists developed a clinical tool to aid in predicting the prognosis of patients with aggressive NHL (specifically DLBCL), referred to as the International Prognostic Index 17B17 4
7 (IPI). Prior to the development of IPI in 1993, prognosis was predominantly based on disease stage. Based on the number of risk factors present and adjusted for patient age, the IPI defines four risk groups: low, low intermediate, high intermediate and high risk, based on five significant risk factors prognostic of overall survival (OS): 1. Age older than 60 years 2. Elevated serum lactate dehydrogenase (LDH) level 3. Ann Arbor stage III or IV disease 4. Eastern Cooperative Oncology Group (ECOG) performance status of 2, 3, or 4 5. Involvement of more than one extranodal site Risk groups are stratified according to the number of adverse factors as follows: 0 or 1 is low risk, 2 is low intermediate, 3 is high intermediate, and 4 or 5 are high risk. Patients with two or more risk factors have a less than 50% chance of relapse-free survival and overall survival (OS) at five years. Age-adjusted (aaipi) and stage-adjusted modifications of this IPI are used for younger patients with localized disease. Adverse risk factors for age-adjusted IPI include stage III or IV disease, elevated LDH and ECOG performance status greater than 2, and can be calculated as follows: 0 is low risk, 1 is low intermediate, 2 is high intermediate, and 3 is high risk. With the success of the IPI, a separate prognostic index was developed for FL, which has multiple independent risk factors for relapse after a first complete remission. The proposed and validated Follicular Lymphoma International Prognostic Index (FLIPI) contains five adverse prognostic factors: 1. Age older than 60 years 2. Ann Arbor stage III-IV 3. Hemoglobin level less than 12.0 g/dl 4. More than four lymph node areas involved 5. Elevated serum lactate dehydrogenase (LDH) level These five factors are used to stratify patients into 3 categories of risk: low [0-1 risk factor], intermediate [2 risk factors], or poor [more than 3 risk factors]. a. Mantle Cell Lymphoma (MCL) Mantle cell lymphoma (MCL) comprises approximately 6% 8% of NHL, and has been recognized within the past 15 years as a unique lymphoma subtype with a particularly aggressive course. MCL is characterized by a chromosomal translocation t(11;14), and the term mantle cell lymphoma was proposed in 1992 by Banks et al. The number of therapeutic trials are not as numerous for MCL as for other NHL as it was not widely recognized until the REAL classification. MCL shows a strong predilection for elderly men, and the majority of cases (70%) present with disseminated [stage 4] disease and extranodal involvement is common. Localized MCL is quite rare. MCL has a median survival of approximately two four years, and although most patients achieve remission with first-line therapy, relapse inevitably occurs, often within months. MCL is rarely, if ever, cured with conventional therapy, and no standardized therapeutic approach to MCL is used. There had been no generally established prognostic index for patients with MCL. Application of the IPI or FLIPI system to patients with MCL showed serious limitations, which included no separation of some important risk groups. In addition, some of the individual IPI and FLIPI risk factors, including number of extranodal 17B17 5
8 sites and number of involved nodal areas showed no prognostic relevance, and hemoglobin showed no independent prognostic relevance in patients with MCL. Therefore, a new prognostic index for patients with MCL was developed, and should prove useful in comparing clinical trial results for MCL. 1. MCL international prognostic index (MIPI): A. Age B. ECOG performance status C. Serum LDH (calculated as a ratio of LDH to a laboratory s upper limit of normal) D. White blood cell count (WBC) (i) Zero points each are assigned for age younger than 50 years, ECOG performance 0 1, LDH ratio less than 0.67, WBC less than 6,700 (ii) One point each for age years, LDH ratio , WBC 6,700 9,999. (iii) Two points each for age years, ECOG 2 4, LDH ratio , WBC 10,000 14,999 (iv) Three points each for age 70 years or older, LDH ratio 1.5 or greater, WBC 15,000 or more 2. MIPI allows separation of three groups with significantly different prognoses: A. 0-3 points=low risk, 44% of patients, median OS not reached and a fiveyear OS rate of 60% B. 4-5 points=intermediate risk, 35% of patients, median OS 51 months C points=high risk, 21% of patients, median OS 29 months b. Peripheral T-Cell Lymphoma (PTCL) 1.5 Staging The majority of peripheral T-cell lymphomas are aggressive and fall into the category of PTCL, unspecified (PTCL-u) or not otherwise specified (PTCL-NOS), angioimmunoblastic or anaplastic large cell which, combined make up approximately 60 70% of T-cell lymphomas. PTCLs are less responsive to standard chemotherapy than DLBCLs and carry a worse prognosis than aggressive B-cell counterparts. Survival rates at 5 years with standard chemotherapy regimens range from 20-35%. The poor results with conventional chemotherapy have prompted exploration of the role of HSCT as therapy. The Ann Arbor staging classification is commonly used for the staging of lymphomas and is the scheme defined in the American Joint Committee on Cancer (AJCC) Manual for Staging Cancer. Originally developed for Hodgkin's disease, this staging scheme was later expanded to include non-hodgkin's lymphoma. Staging of Lymphoma: Ann Arbor Classification: a. Stage I: Involvement of a single lymph node region (I) or of a single extralymphatic organ or site (IE) b. Stage II: Involvement of 2 or more lymph node regions on the same side of the diaphragm (II) or localized involvement of extralymphatic organ or site and of one or more lymph node regions on the same side of the diaphragm (IIE). 17B17 6
9 c. Stage III: Involvement of lymph node regions on both sides of the diaphragm (III) which may also be accompanied by localized involvement of extralymphatic organ or site (IIIE) or by involvement of the spleen (IIIS) or both (IIISE) d. Stage IV: Diffuse or disseminated involvement of one or more extralymphatic organs or tissues with or without associated lymph node enlargement. 1.6 Definitions None Apply. 2.0 Eligibility Requirements 2.1 Provisions General (The term General found throughout this policy applies to all Medicaid and NCHC policies) a. An eligible beneficiary shall be enrolled in either: 1. the NC Medicaid Program (Medicaid is NC Medicaid program, unless context clearly indicates otherwise); or 2. the NC Health Choice (NCHC is NC Health Choice program, unless context clearly indicates otherwise) Program on the date of service and shall meet the criteria in Section 3.0 of this policy. b. Provider(s) shall verify each Medicaid or NCHC beneficiary s eligibility each time a service is rendered. c. The Medicaid beneficiary may have service restrictions due to their eligibility category that would make them ineligible for this service. d. Following is only one of the eligibility and other requirements for participation in the NCHC Program under GS 108A-70.21(a): Children must be between the ages of 6 through Specific (The term Specific found throughout this policy only applies to this policy) a. Medicaid None Apply. b. NCHC None Apply. 2.2 Special Provisions EPSDT Special Provision: Exception to Policy Limitations for a Medicaid Beneficiary under 21 Years of Age a. 42 U.S.C. 1396d(r) [1905(r) of the Social Security Act] Early and Periodic Screening, Diagnostic, and Treatment (EPSDT) is a federal Medicaid requirement that requires the state Medicaid agency to cover services, products, or procedures for Medicaid beneficiary under 21 years of age if the service is medically necessary health care to correct or ameliorate a defect, physical or mental illness, or a condition [health problem] identified through a screening examination (includes any evaluation by a physician or other licensed practitioner). 17B17 7
10 This means EPSDT covers most of the medical or remedial care a child needs to improve or maintain his or her health in the best condition possible, compensate for a health problem, prevent it from worsening, or prevent the development of additional health problems. Medically necessary services will be provided in the most economic mode, as long as the treatment made available is similarly efficacious to the service requested by the beneficiary s physician, therapist, or other licensed practitioner; the determination process does not delay the delivery of the needed service; and the determination does not limit the beneficiary s right to a free choice of providers. EPSDT does not require the state Medicaid agency to provide any service, product or procedure: 1. that is unsafe, ineffective, or experimental or investigational. 2. that is not medical in nature or not generally recognized as an accepted method of medical practice or treatment. Service limitations on scope, amount, duration, frequency, location of service, and other specific criteria described in clinical coverage policies may be exceeded or may not apply as long as the provider s documentation shows that the requested service is medically necessary to correct or ameliorate a defect, physical or mental illness, or a condition [health problem]; that is, provider documentation shows how the service, product, or procedure meets all EPSDT criteria, including to correct or improve or maintain the beneficiary s health in the best condition possible, compensate for a health problem, prevent it from worsening, or prevent the development of additional health problems. b. EPSDT and Prior Approval Requirements 1. If the service, product, or procedure requires prior approval, the fact that the beneficiary is under 21 years of age does NOT eliminate the requirement for prior approval. 2. IMPORTANT ADDITIONAL INFORMATION about EPSDT and prior approval is found in the NCTracks Provider Claims and Billing Assistance Guide, and on the EPSDT provider page. The Web addresses are specified below. NCTracks Provider Claims and Billing Assistance Guide: EPSDT provider page: EPSDT does not apply to NCHC beneficiaries Health Choice Special Provision for a Health Choice Beneficiary age 6 through 18 years of age The Division of Medical Assistance (DMA) shall deny the claim for coverage for an NCHC beneficiary who does not meet the criteria within Section 3.0 of this policy. Only services included under the NCHC State Plan and the DMA clinical 17B17 8
11 coverage policies, service definitions, or billing codes are covered for an NCHC beneficiary. 3.0 When the Procedure, Product, or Service Is Covered Note: Refer to Subsection regarding EPSDT Exception to Policy Limitations for Medicaid Beneficiaries under 21 Years of Age. 3.1 General Criteria Covered Medicaid and NCHC shall cover the procedure, product, or service related to this policy when medically necessary, and: a. the procedure, product, or service is individualized, specific, and consistent with symptoms or confirmed diagnosis of the illness or injury under treatment, and not in excess of the beneficiary s needs; b. the procedure, product, or service can be safely furnished, and no equally effective and more conservative or less costly treatment is available statewide; and c. the procedure, product, or service is furnished in a manner not primarily intended for the convenience of the beneficiary, the beneficiary s caretaker, or the provider. 3.2 Specific Criteria Covered Specific criteria covered by both Medicaid and NCHC Medicaid and NCHC shall cover hematopoietic stem cell and bone marrow transplantation for non-hodgkin s lymphoma in the following situations: a. For beneficiaries with non-hodgkin s lymphoma (NHL) B-cell subtypes considered aggressive (except mantle cell lymphoma), either allogeneic hematopoietic stem cell transplantation (HSCT) using a myeloablative conditioning regimen or autologous HSCT may be considered medically necessary: 1. as salvage therapy for beneficiaries who do not achieve a complete remission (CR) after first-line treatment (induction) with a full course of standard-dose chemotherapy; 2. to achieve or consolidate a CR for those in a chemosensitive first or subsequent relapse; or 3. to consolidate a first CR in beneficiaries with diffuse large B-cell lymphoma, with an age adjusted International Prognostic Index score that predicts a high- or high-intermediate risk of relapse. b. For patients with mantle cell lymphoma: 1. Autologous HSCT may be considered medically necessary to consolidate a first remission. 2. Allogeneic HSCT, myeloablative or reduced-intensity conditioning, may be considered medically necessary as salvage therapy. c. For patients with NHL B-cell subtypes considered indolent, either allogeneic HSCT using a myeloablative conditioning regimen or autologous HSCT may be considered medically necessary: 1. as salvage therapy for beneficiaries who do not achieve CR after firstline treatment (induction) with a full course of standard-dose chemotherapy; or 17B17 9
12 2. to achieve or consolidate CR for those in a first or subsequent chemosensitive relapse, whether or not their lymphoma has undergone transformation to a higher grade. d. Reduced-intensity conditioning allogeneic HSCT may be considered medically necessary as a treatment of NHL in beneficiaries who meet criteria for an allogeneic HSCT but who do not qualify for a myeloablative allogeneic HSCT (refer to Policy Guidelines). e. For beneficiaries with peripheral T-cell lymphoma: 1. Autologous HSCT may be considered medically necessary to consolidate a first complete remission in high-risk peripheral T-cell lymphoma. (refer to Policy Guidelines) 2. Autologous or allogeneic HSCT (myeloablative or reduced-intensity conditioning) may be considered medically necessary as salvage therapy Medicaid Additional Criteria Covered None Apply NCHC Additional Criteria Covered None Apply Policy Guidelines a. Reduced-intensity conditioning (RIC) would be considered an option in beneficiaries who meet criteria for an allogeneic hematopoietic stem-cell transplant (HSCT) but whose age (typically older than 55 years) or comorbidities (e.g., liver or kidney dysfunction, generalized debilitation, or prior intensive chemotherapy) preclude use of a standard conditioning regimen. b. In beneficiaries who qualify for a myeloablative allogeneic HSCT on the basis of overall health and disease status, allogeneic HSCT using either myeloablative or RIC may be considered. However, a myeloablative conditioning regimen with allogeneic HSCT may benefit younger beneficiaries with good performance status and minimal comorbidities more than allogeneic HSCT with RIC. c. The term salvage therapy describes chemotherapy given to beneficiaries who have either: 1) failed to achieve complete remission after initial treatment for newly diagnosed lymphoma; or 2) relapsed after an initial complete remission. d. A chemosensitive relapse is defined as relapsed non-hodgkin s lymphoma that does not progress during or immediately after standard-dose induction chemotherapy (i.e., achieves stable disease or a partial response.) e. Transformation describes a lymphoma whose histologic pattern has evolved to a higher grade lymphoma. Transformed lymphomas typically evolve from a nodular pattern to a diffuse pattern. f. Tandem transplants usually are defined as the planned administration of 2 successive cycles of high-dose myeloablative chemotherapy, each followed by infusion of autologous hematopoietic stem cells, whether or not there is evidence of persistent disease following the first treatment cycle. Sometimes, the second cycle may use non-myeloablative 17B17 10
13 immunosuppressive conditioning followed by infusion of allogeneic stem cells. 4.0 When the Procedure, Product, or Service Is Not Covered Note: Refer to Subsection regarding EPSDT Exception to Policy Limitations for Medicaid Beneficiaries under 21 Years of Age. 4.1 General Criteria Not Covered Medicaid and NCHC shall not cover the procedure, product, or service related to this policy when: a. the beneficiary does not meet the eligibility requirements listed in Section 2.0; b. the beneficiary does not meet the criteria listed in Section 3.0; c. the procedure, product, or service duplicates another provider s procedure, product, or service; or d. the procedure, product, or service is experimental, investigational, or part of a clinical trial. 4.2 Specific Criteria Not Covered Specific Criteria Not Covered by both Medicaid and NCHC Medicaid and NCHC shall not cover hematopoietic stem-cell or bone marrow transplantation for non-hodgkin s lymphoma in the following situations: a. For beneficiaries with mantle cell lymphoma: 1. Autologous HSCT is considered investigational as salvage therapy. 2. Allogeneic HSCT is considered investigational to consolidate a first remission. b. Either autologous HSCT or allogeneic HSCT is considered investigational: 1. as initial therapy (i.e., without a full course of standard-dose induction chemotherapy) for any NHL; 2. to consolidate a first complete remission (CR) for beneficiaries with diffuse large B-cell lymphoma and an International Prognostic Index score that predicts a low- or low-intermediate risk of relapse; 3. to consolidate a first complete remission (CR) for those with indolent NHL B-cell subtypes. c. Tandem transplants are considered investigational to treat beneficiaries with any stage, grade, or subtype of NHL. d. For beneficiaries with peripheral T-cell lymphoma, allogeneic HSCT is considered investigational to consolidate a first remission Medicaid Additional Criteria Not Covered None Apply NCHC Additional Criteria Not Covered a. NCGS 108A-70.21(b) Except as otherwise provided for eligibility, fees, deductibles, copayments, and other cost sharing charges, health benefits coverage provided to children eligible under the Program shall be equivalent to coverage provided for dependents under North Carolina Medicaid Program except for the following: 17B17 11
14 1. No services for long-term care. 2. No nonemergency medical transportation. 3. No EPSDT. 4. Dental services shall be provided on a restricted basis in accordance with criteria adopted by the Department to implement this subsection. 5.0 Requirements for and Limitations on Coverage Note: Refer to Subsection regarding EPSDT Exception to Policy Limitations for Medicaid Beneficiaries under 21 Years of Age. 5.1 Prior Approval Medicaid and NCHC shall require prior approval for hematopoietic stem cell and bone marrow transplantation for non-hodgkin s lymphoma. The provider shall obtain prior approval before rendering hematopoietic stem cell and bone marrow transplantation for non-hodgkin s lymphoma If prior approval has been given for stem cell transplant, DMA shall reimburse for the following donor transplant-related medical expenses: procuring, harvesting, short-term storage and all associated laboratory costs. 5.2 Prior Approval Requirements General The provider(s) shall submit to the Department of Health and Human Services (DHHS) Utilization Review Contractor the following: a. the prior approval request; and b. all health records and any other records that support the beneficiary has met the specific criteria in Subsection 3.2 of this policy Specific None Apply. 5.3 Specific Transplant Prior Approval Requirements The provider(s) shall submit the following to the DMA transplant nurse consultant: a. Letter of medical necessity signed by the attending transplant physician, which documents regimens and dates, the social history and the transplant evaluation; b. All health care records and any other records that support the beneficiary has met the specific criteria in Subsection 3.2 of this policy including: 1. Lab results (less than three months old) to include Complete Blood Count (CBC), complete electrolytes, liver enzymes, Prothrombin Time (PT), International Normalized Ratio (INR), glucose and A1C (Glycated Hemoglobin if Type I or Type II diabetic), and blood type; 2. Serologies: to include Human Immunodeficiency Virus (HIV), Hepatitis, Rapid Plasma Reagin (RPR), Epstein-Barr Virus (EBV), Cytomegalovirus (CMV), Varicella, Rubella, Herpes Simplex Virus (HSV) I/II, and toxoplasmosis. (Positive serology results may be reported that are greater than three months old); 17B17 12
15 3. Diagnostic studies (less than six months old) required in a complete packet include: A. Cardiac: Echocardiogram, Electrocardiogram (ECG), and/or cardiac catheterization as appropriate for beneficiary s clinical status; B. Pulmonary: Pulmonary Function Test if beneficiary has cardiac or pulmonary issues, or a history of smoking; and C. Chest x-ray for all transplant candidates; 4. Other diagnostic tests may be requested as appropriate; 5. Beneficiary s height and weight 6. All diagnostic and procedure results, including bone marrow aspiration (not more than six months old) c. Complete psychological and social evaluation to include: 1. beneficiary s medical compliance; 2. beneficiary s support network; 3. post-transplant care plan, with identification of primary and secondary care providers; and 4. history of mental health issues/substance use/legal issues d. Beneficiaries with a psychiatric history are required to have an evaluation by a psychiatrist with expertise in evaluating the specific psychiatric issues that relate to transplant candidates. 6.0 Provider(s) Eligible to Bill for the Procedure, Product, or Service To be eligible to bill for the procedure, product, or service related to this policy, the provider(s) shall: a. meet Medicaid or NCHC qualifications for participation; b. have a current and signed Department of Health and Human Services (DHHS) Provider Administrative Participation Agreement; and c. bill only for procedures, products, and services that are within the scope of their clinical practice, as defined by the appropriate licensing entity. 6.1 Provider Qualifications and Occupational Licensing Entity Regulations None Apply. 6.2 Provider Certifications None Apply. 17B17 13
16 7.0 Additional Requirements Note: Refer to Subsection regarding EPSDT Exception to Policy Limitations for Medicaid Beneficiaries under 21 Years of Age. 7.1 Compliance Provider(s) shall comply with the following in effect at the time the service is rendered: a. All applicable agreements, federal, state and local laws and regulations including the Health Insurance Portability and Accountability Act (HIPAA) and record retention requirements; and b. All DMA s clinical (medical) coverage policies, guidelines, policies, provider manuals, implementation updates, and bulletins published by the Centers for Medicare and Medicaid Services (CMS), DHHS, DHHS division(s) or fiscal contractor(s) c. FDA approved procedures, products, and devices for implantation must be utilized. d. A statement signed by the surgeon certifying all FDA requirements for the implants, products, and devices must be retained in the beneficiary s medical record and made available for review upon request. 17B17 14
17 8.0 Policy Implementation/Revision Information Original Effective Date: January 1, 1994 Revision Information: Date Section Revised Change 07/01/2005 Throughout Medicaid Policy was updated to include coverage criteria effective with approved date of State Plan amendment 4/1/05. 09/01/2005 Section 2.2 Medicaid: The special provision related to EPSDT was revised. 12/01/2005 Section 2.2 Medicaid The web address for DMA s EDPST policy instructions was added to this section. 12/01/2006 Sections 2.2 Medicaid: The special provision related to EPSDT was revised. 12/01/2006 Sections 3.0 and Medicaid: A note regarding EPSDT was added to these /01/2007 Sections 2 through 4 sections. Medicaid: EPSDT information was revised to clarify exceptions to policy limitations for recipients under 21 years of age. 05/01/2007 Attachment A Medicaid: Added the UB-04 as an accepted claims form. 07/01/2010 Throughout NCHC: Session Law , Section 10.31(a) Transition of NC Health Choice Program administrative oversight from the State Health Plan to the Division of Medical Assistance (DMA) in the NC Department of Health and Human Services. 03/01/2012 Throughout NCHC: To be equivalent where applicable to NC DMA s Clinical Coverage Policy # 11A-11 under Session Law , (b) 03/01/2012 Throughout Policy updated to reflect current community standards and changing transplant protocols 03/01/2012 Throughout Technical changes to merge Medicaid and NCHC current coverage into one policy. 10/01/2015 All Sections and Attachments 03/01/2017 Attachment A, Section B Updated policy template language and added ICD-10 codes to comply with federally mandated 10/1/2015 implementation where applicable. ICD-10 diagnosis codes updated. 17B17 15
18 Attachment A: Claims-Related Information Provider(s) shall comply with the, NCTracks Provider Claims and Billing Assistance Guide, Medicaid bulletins, fee schedules, DMA s clinical coverage policies and any other relevant documents for specific coverage and reimbursement for Medicaid and NCHC: A. Claim Type Professional (CMS-1500/837P transaction) B. International Classification of Diseases, Tenth Revisions, Clinical Modification (ICD-10-CM) and Procedural Coding System (PCS) Provider(s) shall report the ICD-10-CM and Procedural Coding System (PCS) to the highest level of specificity that supports medical necessity. Provider(s) shall use the current ICD-10 edition and any subsequent editions in effect at the time of service. Provider(s) shall refer to the applicable edition for code description, as it is no longer documented in the policy AZ 30230G G Y Y AZ 30233G G Y0 ICD-10 Procedure Code(s) 30233Y Y AZ 30243Y G G G G Y Y Y Y AZ 30253G G G G Y Y G G Y Y G G Y Y1 C. Code(s) Providers Provider(s) shall report the most specific billing code that accurately and completely describes the procedure, product or service provided. Provider(s) shall use the Current Procedural Terminology (CPT), Health Care Procedure Coding System (HCPCS), and UB-04 Data Specifications Manual (for a complete listing of valid revenue codes) and any subsequent editions in effect at the time of service. Provider(s) shall refer to the applicable edition for the code description, as it is no longer documented in the policy. If no such specific CPT or HCPCS code exists, then the provider(s) shall report the procedure, product or service using the appropriate unlisted procedure or service code. CPT Code(s) B17 16
19 HCPCS Code(s) S2150 Unlisted Procedure or Service CPT: The provider(s) shall refer to and comply with the Instructions for Use of the CPT Codebook, Unlisted Procedure or Service, and Special Report as documented in the current CPT in effect at the time of service. HCPCS: The provider(s) shall refer to and comply with the Instructions For Use of HCPCS National Level II codes, Unlisted Procedure or Service and Special Report as documented in the current HCPCS edition in effect at the time of service. D. Modifiers Providers shall follow applicable modifier guidelines. E. Billing Units Provider(s) shall report the appropriate code(s) used which determines the billing unit unit(s). F. Place of Service Inpatient hospital, Outpatient hospital G. Co-payments For Medicaid refer to Medicaid State Plan, Attachment 4.18-A, page 1, located at For NCHC refer to G.S. 108A-70.21(d), located at html. H. Reimbursement Provider(s) shall bill their usual and customary charges. For a schedule of rates, see: I. Billing for Donor Expenses 1. Billing for Donor Expenses for Medicaid Beneficiaries Donor transplant-related medical expenses are billed on the Medicaid beneficiary s transplant claim using the beneficiary s Medicaid identification number. Medicaid reimburses only for the actual donor s transplant-related medical expenses. Medicaid does not reimburse for unsuccessful donor searches. 2. Billing for Donor Expenses for NCHC Beneficiaries Donor transplant-related medical expenses donors are billed on the NCHC beneficiary s transplant claim. NCHC reimburses only for the actual donor s transplant-related medical expenses. NCHC does not reimburse for unsuccessful donor searches. 17B17 17
Table of Contents. 1.0 Description of the Procedure, Product, or Service Definitions... 2
 Hematopoietic Stem-Cell & Bone Clinical Coverage Policy No: 11A-3 Marrow Transplantation for Chronic Amended Date: October 1, 2015 Myelogenous Leukemia Table of Contents 1.0 Description of the Procedure,
Hematopoietic Stem-Cell & Bone Clinical Coverage Policy No: 11A-3 Marrow Transplantation for Chronic Amended Date: October 1, 2015 Myelogenous Leukemia Table of Contents 1.0 Description of the Procedure,
Table of Contents. 1.0 Description of the Procedure, Product, or Service Definitions... 2
 Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Definitions... 2 2.0 Eligibility Requirements... 3 2.1 Provisions... 3 2.1.1 General... 3 2.1.2 Specific... 3 2.2 Special
Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Definitions... 2 2.0 Eligibility Requirements... 3 2.1 Provisions... 3 2.1.1 General... 3 2.1.2 Specific... 3 2.2 Special
Hematopoietic Stem-Cell Clinical Coverage Policy No: 11A-7 & Bone Marrow Transplantation Amended Date: October 1, 2015 for Hodgkin Lymphoma
 Hematopoietic Stem-Cell Clinical Coverage Policy No: 11A-7 & Bone Marrow Transplantation Amended Date: October 1, 2015 for Hodgkin Lymphoma Table of Contents 1.0 Description of the Procedure, Product,
Hematopoietic Stem-Cell Clinical Coverage Policy No: 11A-7 & Bone Marrow Transplantation Amended Date: October 1, 2015 for Hodgkin Lymphoma Table of Contents 1.0 Description of the Procedure, Product,
Table of Contents. 1.0 Description of the Procedure, Product, or Service... 1
 Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 2.0 Eligibility Requirements... 3 2.1 Provisions... 3 2.1.1 General... 3 2.1.2 Specific... 3 2.2 Special Provisions... 4 2.2.1
Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 2.0 Eligibility Requirements... 3 2.1 Provisions... 3 2.1.1 General... 3 2.1.2 Specific... 3 2.2 Special Provisions... 4 2.2.1
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplant for Non-Hodgkin Lymphomas File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplant_for_non_hodgkin_lymphomas
Corporate Medical Policy Hematopoietic Stem-Cell Transplant for Non-Hodgkin Lymphomas File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplant_for_non_hodgkin_lymphomas
Table of Contents. 1.0 Description of the Procedure, Product, or Service Definitions... 2
 Hematopoietic Stem-Cell & Bone Clinical Coverage Policy No: 11A-10 Marrow Transplantation for Amended Date: October 1, 2015 Central Nervous System (CNS) Table of Contents 1.0 Description of the Procedure,
Hematopoietic Stem-Cell & Bone Clinical Coverage Policy No: 11A-10 Marrow Transplantation for Amended Date: October 1, 2015 Central Nervous System (CNS) Table of Contents 1.0 Description of the Procedure,
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_cll_and_sll 2/2001
Corporate Medical Policy Hematopoietic Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_cll_and_sll 2/2001
Pancreas Transplant Clinical Coverage Policy No: 11B-7 Amended Date: October 1, Table of Contents
 Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Definitions... 1 2.0 Eligible Beneficiaries... 1 2.1 Provisions... 1 2.1.1 General... 1 2.1.2 Specific... 2 2.2 Special
Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Definitions... 1 2.0 Eligible Beneficiaries... 1 2.1 Provisions... 1 2.1.1 General... 1 2.1.2 Specific... 2 2.2 Special
Hematopoietic Stem-Cell and Bone Clinical Coverage Policy No: 11A-2 Marrow Transplant for Amended Date: October 1, 2015 Acute Myeloid Leukemia (AML)
 Hematopoietic Stem-Cell and Bone Clinical Coverage Policy No: 11A-2 Marrow Transplant for Amended Date: October 1, 2015 Acute Myeloid Leukemia (AML) Table of Contents 1.0 Description of the Procedure,
Hematopoietic Stem-Cell and Bone Clinical Coverage Policy No: 11A-2 Marrow Transplant for Amended Date: October 1, 2015 Acute Myeloid Leukemia (AML) Table of Contents 1.0 Description of the Procedure,
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Waldenstrom Macroglobulinemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem_cell_transplantation_for_waldenstrom_macroglobulinemia
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Waldenstrom Macroglobulinemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem_cell_transplantation_for_waldenstrom_macroglobulinemia
Hematopoietic Stem-Cell Transplantation for Non-Hodgkin Lymphomas. Original Policy Date
 MP 7.03.13 Hematopoietic Stem-Cell Transplantation for Non-Hodgkin Lymphomas Medical Policy Section Therapy Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013
MP 7.03.13 Hematopoietic Stem-Cell Transplantation for Non-Hodgkin Lymphomas Medical Policy Section Therapy Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_ transplantation_for_primary_amyloidosis 2/2001 11/2018 11/2019 11/2018 Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_ transplantation_for_primary_amyloidosis 2/2001 11/2018 11/2019 11/2018 Description
Table of Contents. 1.0 Description of the Procedure, Product, or Service... 1
 Allogeneic Hematopoietic & Bone Clinical Coverage Policy No: 11A-5 Marrow Transplant for Genetic Amended Date: October 1, 2015 Diseases and Acquired Anemias Table of Contents 1.0 Description of the Procedure,
Allogeneic Hematopoietic & Bone Clinical Coverage Policy No: 11A-5 Marrow Transplant for Genetic Amended Date: October 1, 2015 Diseases and Acquired Anemias Table of Contents 1.0 Description of the Procedure,
Hematopoietic Stem-Cell Transplantation for Non-Hodgkin s Lymphomas
 Hematopoietic Stem-Cell Transplantation for Non-Hodgkin s Lymphomas Policy Number: 8.01.20 Last Review: 7/2014 Origination: 7/2002 Next Review: 7/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue
Hematopoietic Stem-Cell Transplantation for Non-Hodgkin s Lymphomas Policy Number: 8.01.20 Last Review: 7/2014 Origination: 7/2002 Next Review: 7/2015 Policy Blue Cross and Blue Shield of Kansas City (Blue
Hematopoietic Stem-Cell Clinical Coverage Policy No: 11A-15 Transplantation for Solid Amended Date: October 1, 2015 Tumors of Childhood
 Hematopoietic Stem-Cell Clinical Coverage Policy No: 11A-15 Transplantation for Solid Amended Date: October 1, 2015 Tumors of Childhood Table of Contents 1.0 Description of the Procedure, Product, or Service...
Hematopoietic Stem-Cell Clinical Coverage Policy No: 11A-15 Transplantation for Solid Amended Date: October 1, 2015 Tumors of Childhood Table of Contents 1.0 Description of the Procedure, Product, or Service...
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Cell Transplantation for Hodgkin Lymphoma File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_hodgkin_lymphoma
Corporate Medical Policy Hematopoietic Cell Transplantation for Hodgkin Lymphoma File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_hodgkin_lymphoma
Hematopoietic Cell Transplantation for Non-Hodgkin's Lymphomas
 Medical Policy Manual Transplant, Policy No. 45.23 Hematopoietic Cell Transplantation for Non-Hodgkin's Lymphomas Next Review: September 2018 Last Review: December 2017 Effective: January 1, 2018 IMPORTANT
Medical Policy Manual Transplant, Policy No. 45.23 Hematopoietic Cell Transplantation for Non-Hodgkin's Lymphomas Next Review: September 2018 Last Review: December 2017 Effective: January 1, 2018 IMPORTANT
Hematopoietic Stem-Cell Transplantation for Non-Hodgkin Lymphomas
 Hematopoietic Stem-Cell Transplantation for Non-Hodgkin Lymphomas Policy Number: Original Effective Date: MM.07.018 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 03/27/2015 Section:
Hematopoietic Stem-Cell Transplantation for Non-Hodgkin Lymphomas Policy Number: Original Effective Date: MM.07.018 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 03/27/2015 Section:
Lymphoma/CLL 101: Know your Subtype. Dr. David Macdonald Hematologist, The Ottawa Hospital
 Lymphoma/CLL 101: Know your Subtype Dr. David Macdonald Hematologist, The Ottawa Hospital Function of the Lymph System Lymph Node Lymphocytes B-cells develop in the bone marrow and influence the immune
Lymphoma/CLL 101: Know your Subtype Dr. David Macdonald Hematologist, The Ottawa Hospital Function of the Lymph System Lymph Node Lymphocytes B-cells develop in the bone marrow and influence the immune
Medical Policy Manual. Date of Origin: May Topic: Hematopoietic Stem-Cell Transplantation for Non- Hodgkin Lymphomas
 Medical Policy Manual Topic: Hematopoietic Stem-Cell Transplantation for Non- Hodgkin Lymphomas Section: Transplant Policy No: 45.23 Date of Origin: May 2010 Last Reviewed Date: September 2013 Effective
Medical Policy Manual Topic: Hematopoietic Stem-Cell Transplantation for Non- Hodgkin Lymphomas Section: Transplant Policy No: 45.23 Date of Origin: May 2010 Last Reviewed Date: September 2013 Effective
Protocol. Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis
 Protocol Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis (80142) Medical Benefit Effective Date: 04/01/13 Next Review Date: 07/15 Preauthorization Yes Review Dates: 04/07, 05/08, 01/10,
Protocol Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis (80142) Medical Benefit Effective Date: 04/01/13 Next Review Date: 07/15 Preauthorization Yes Review Dates: 04/07, 05/08, 01/10,
Non-Hodgkin Lymphoma. Protocol applies to non-hodgkin lymphoma involving any organ system except the gastrointestinal tract.
 Non-Hodgkin Lymphoma Protocol applies to non-hodgkin lymphoma involving any organ system except the gastrointestinal tract. Protocol revision date: January 2005 No AJCC/UICC staging system Procedures Cytology
Non-Hodgkin Lymphoma Protocol applies to non-hodgkin lymphoma involving any organ system except the gastrointestinal tract. Protocol revision date: January 2005 No AJCC/UICC staging system Procedures Cytology
Clinical Policy: Bendamustine (Bendeka, Treanda) Reference Number: PA.CP.PHAR.307
 Clinical Policy: (Bendeka, Treanda) Reference Number: PA.CP.PHAR.307 Effective Date: 01/18 Last Review Date: 11/17 Coding Implications Revision Log Description The intent of the criteria is to ensure that
Clinical Policy: (Bendeka, Treanda) Reference Number: PA.CP.PHAR.307 Effective Date: 01/18 Last Review Date: 11/17 Coding Implications Revision Log Description The intent of the criteria is to ensure that
During past decades, because of the lack of knowledge
 Staging and Classification of Lymphoma Ping Lu, MD In 2004, new cases of non-hodgkin s in the United States were estimated at 54,370, representing 4% of all cancers and resulting 4% of all cancer deaths,
Staging and Classification of Lymphoma Ping Lu, MD In 2004, new cases of non-hodgkin s in the United States were estimated at 54,370, representing 4% of all cancers and resulting 4% of all cancer deaths,
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplant_for_acute_myeloid_leukemia
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplant_for_acute_myeloid_leukemia
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation in the Treatment of Germ File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_in_the_treatment_of_germ_cell_tumor
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation in the Treatment of Germ File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_in_the_treatment_of_germ_cell_tumor
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Autoimmune Diseases File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_autoimmune_diseases
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Autoimmune Diseases File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_autoimmune_diseases
2010 Hematopoietic and Lymphoid ICD-O Codes - Alphabetical List THIS TABLE REPLACES ALL ICD-O-3 Codes
 Acute basophilic leukemia 9870/3 Acute biphenotypic leukemia [OBS] 9805/3 Acute erythroid leukemia 9840/3 Acute megakaryoblastic leukemia 9910/3 Acute monoblastic and monocytic leukemia 9891/3 Acute myeloid
Acute basophilic leukemia 9870/3 Acute biphenotypic leukemia [OBS] 9805/3 Acute erythroid leukemia 9840/3 Acute megakaryoblastic leukemia 9910/3 Acute monoblastic and monocytic leukemia 9891/3 Acute myeloid
2012 Hematopoietic and Lymphoid ICD-O Codes - Numerical List THIS TABLE REPLACES ALL ICD-O-3 Codes
 Malignant lymphoma, NOS 9590/3 Non-Hodgkin lymphoma, NOS 9591/3 B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma 9596/3 Primary
Malignant lymphoma, NOS 9590/3 Non-Hodgkin lymphoma, NOS 9591/3 B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma 9596/3 Primary
Medical Benefit Effective Date: 04/01/12 Next Review Date: 01/13 Preauthorization* Yes Review Dates: 04/07, 05/08, 01/10, 01/11, 01/12
 Protocol Hematopoietic Stem-Cell Transplantation for Hodgkin Lymphoma (80129) Medical Benefit Effective Date: 04/01/12 Next Review Date: 01/13 Preauthorization* Yes Review Dates: 04/07, 05/08, 01/10, 01/11,
Protocol Hematopoietic Stem-Cell Transplantation for Hodgkin Lymphoma (80129) Medical Benefit Effective Date: 04/01/12 Next Review Date: 01/13 Preauthorization* Yes Review Dates: 04/07, 05/08, 01/10, 01/11,
Large cell immunoblastic Diffuse histiocytic (DHL) Lymphoblastic lymphoma Diffuse lymphoblastic Small non cleaved cell Burkitt s Non- Burkitt s
 Non Hodgkin s Lymphoma Introduction 6th most common cause of cancer death in United States. Increasing in incidence and mortality. Since 1970, the incidence of has almost doubled. Overview The types of
Non Hodgkin s Lymphoma Introduction 6th most common cause of cancer death in United States. Increasing in incidence and mortality. Since 1970, the incidence of has almost doubled. Overview The types of
Medication Assisted Treatment: Buprenorphine Clinical Coverage Policy 8A-3 Amended Date: 10/1/2015 DRAFT Table of Contents
 Medication Assisted Treatment: Buprenorphine Clinical Coverage Policy 8A-3 10/1/2015 Table of Contents 1.0 Description of the Procedure, Product, or Service... 3 1.1 Definitions... 3 2.0 Eligibility Requirements...
Medication Assisted Treatment: Buprenorphine Clinical Coverage Policy 8A-3 10/1/2015 Table of Contents 1.0 Description of the Procedure, Product, or Service... 3 1.1 Definitions... 3 2.0 Eligibility Requirements...
Medical Benefit Effective Date: 07/01/12 Next Review Date: 05/13 Preauthorization* Yes Review Dates: 04/07, 05/08, 05/11, 05/12
 Hematopoietic Stem-Cell Transplantation for Chronic Lymphocytic (80115) Medical Benefit Effective Date: 07/01/12 Next Review Date: 05/13 Preauthorization* Yes Review Dates: 04/07, 05/08, 05/11, 05/12 The
Hematopoietic Stem-Cell Transplantation for Chronic Lymphocytic (80115) Medical Benefit Effective Date: 07/01/12 Next Review Date: 05/13 Preauthorization* Yes Review Dates: 04/07, 05/08, 05/11, 05/12 The
Bone Marrow. Procedures Blood Film Aspirate, Cell Block Trephine Biopsy, Touch Imprint
 Bone Marrow Protocol applies to acute leukemias, myelodysplastic syndromes, myeloproliferative disorders, chronic lymphoproliferative disorders, malignant lymphomas, plasma cell dyscrasias, histiocytic
Bone Marrow Protocol applies to acute leukemias, myelodysplastic syndromes, myeloproliferative disorders, chronic lymphoproliferative disorders, malignant lymphomas, plasma cell dyscrasias, histiocytic
Change Summary - Form 2018 (R3) 1 of 12
 Summary - Form 2018 (R3) 1 of 12 Form Question Number (r3) Type Description New Text Previous Text Today's date was removed 2018 N/A Today's Date Removed from Key Fields 2018 N/A HCT Type 2018 N/A Product
Summary - Form 2018 (R3) 1 of 12 Form Question Number (r3) Type Description New Text Previous Text Today's date was removed 2018 N/A Today's Date Removed from Key Fields 2018 N/A HCT Type 2018 N/A Product
WHO Classification. B-cell chronic lymphocytic leukemia/small T-cell granular lymphocytic leukemia
 Blood Malignancies-II Prof. Dr. Herman Hariman, a Ph.D, SpPK (KH). Prof. Dr. Adikoesoema Aman, SpPK (KH) Dept. of Clinical Pathology, School of Medicine, University of North Sumatra WHO classification
Blood Malignancies-II Prof. Dr. Herman Hariman, a Ph.D, SpPK (KH). Prof. Dr. Adikoesoema Aman, SpPK (KH) Dept. of Clinical Pathology, School of Medicine, University of North Sumatra WHO classification
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: (Istodax) Reference Number: CP.PHAR.314 Effective Date: 01.01.17 Last Review Date: 11.18 Line of Business: Medicaid, HIM-Medical Benefit Coding Implications Revision Log See Important
Clinical Policy: (Istodax) Reference Number: CP.PHAR.314 Effective Date: 01.01.17 Last Review Date: 11.18 Line of Business: Medicaid, HIM-Medical Benefit Coding Implications Revision Log See Important
Combinations of morphology codes of haematological malignancies (HM) referring to the same tumour or to a potential transformation
 Major subgroups according to the World Health Organisation (WHO) Classification Myeloproliferative neoplasms (MPN) Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB or
Major subgroups according to the World Health Organisation (WHO) Classification Myeloproliferative neoplasms (MPN) Myeloid and lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA, PDGFRB or
Clinical Policy: Bendamustine (Bendeka, Treanda) Reference Number: CP.PHAR.307
 Clinical Policy: (Bendeka, Treanda) Reference Number: CP.PHAR.307 Effective Date: 02/17 Last Review Date: 02/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Bendeka, Treanda) Reference Number: CP.PHAR.307 Effective Date: 02/17 Last Review Date: 02/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important
Aggressive Lymphomas - Current. Dr Kevin Imrie Physician-in-Chief, Sunnybrook Health Sciences Centre
 Aggressive Lymphomas - Current Dr Kevin Imrie Physician-in-Chief, Sunnybrook Health Sciences Centre Conflicts of interest I have no conflicts of interest to declare Outline What does aggressive lymphoma
Aggressive Lymphomas - Current Dr Kevin Imrie Physician-in-Chief, Sunnybrook Health Sciences Centre Conflicts of interest I have no conflicts of interest to declare Outline What does aggressive lymphoma
Hematopoietic Stem-Cell Transplantation for Non- Hodgkin Lymphomas
 Hematopoietic Stem-Cell Transplantation for Non- Hodgkin (80120) Medical Benefit Effective Date: 04/01/13 Next Review Date: 05/15 Preauthorization Yes Review Dates: 04/07, 05/08, 05/09, 05/10, 05/11, 05/12,
Hematopoietic Stem-Cell Transplantation for Non- Hodgkin (80120) Medical Benefit Effective Date: 04/01/13 Next Review Date: 05/15 Preauthorization Yes Review Dates: 04/07, 05/08, 05/09, 05/10, 05/11, 05/12,
POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT VARIATIONS DISCLAIMER CODING INFORMATION REFERENCES POLICY HISTORY
 Original Issue Date (Created): October 1, 2014 Most Recent Review Date (Revised): May 20, 2014 Effective Date: October 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Original Issue Date (Created): October 1, 2014 Most Recent Review Date (Revised): May 20, 2014 Effective Date: October 1, 2014 POLICY PRODUCT VARIATIONS DESCRIPTION/BACKGROUND RATIONALE DEFINITIONS BENEFIT
Hepatic Lymphoma Diagnosis An Algorithmic Approach
 Hepatic Lymphoma Diagnosis An Algorithmic Approach Ryan M. Gill, M.D., Ph.D. University of California, San Francisco PLEASE TURN OFF YOUR CELL PHONES Disclosure of Relevant Financial Relationships USCAP
Hepatic Lymphoma Diagnosis An Algorithmic Approach Ryan M. Gill, M.D., Ph.D. University of California, San Francisco PLEASE TURN OFF YOUR CELL PHONES Disclosure of Relevant Financial Relationships USCAP
Indolent Lymphomas. Dr. Melissa Toupin The Ottawa Hospital
 Indolent Lymphomas Dr. Melissa Toupin The Ottawa Hospital What does indolent mean? Slow growth Often asymptomatic Chronic disease with periods of relapse (long natural history possible) Incurable with
Indolent Lymphomas Dr. Melissa Toupin The Ottawa Hospital What does indolent mean? Slow growth Often asymptomatic Chronic disease with periods of relapse (long natural history possible) Incurable with
Electrocardiography, Clinical Coverage Policy No.: 1R-4 Echocardiography, and Amended Date: October 1, 2015 Intravascular Ultrasound
 Echocardiography, and Amended Date: October 1, 2015 Intravascular Ultrasound Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Electrocardiography... 1 1.1.1 Electrocardiogram...
Echocardiography, and Amended Date: October 1, 2015 Intravascular Ultrasound Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Electrocardiography... 1 1.1.1 Electrocardiogram...
Indolent Lymphomas: Current. Dr. Laurie Sehn
 Indolent Lymphomas: Current Dr. Laurie Sehn Why does indolent mean? Slow growth Often asymptomatic Chronic disease with periods of relapse (long natural history possible) Incurable with current standard
Indolent Lymphomas: Current Dr. Laurie Sehn Why does indolent mean? Slow growth Often asymptomatic Chronic disease with periods of relapse (long natural history possible) Incurable with current standard
Table of Contents. 1.0 Description of the Procedure, Product, or Service Safety and Provider Compliance... 1
 Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Safety and Provider Compliance... 1 2.0 Eligibility Requirements... 1 2.1 Provisions... 1 2.1.1 General... 1 2.1.2 Specific...
Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Safety and Provider Compliance... 1 2.0 Eligibility Requirements... 1 2.1 Provisions... 1 2.1.1 General... 1 2.1.2 Specific...
Aggressive NHL and Hodgkin Lymphoma. Dr. Carolyn Faught November 10, 2017
 Aggressive NHL and Hodgkin Lymphoma Dr. Carolyn Faught November 10, 2017 What does aggressive mean? Shorter duration of symptoms Generally need treatment at time of diagnosis Immediate, few days, few weeks
Aggressive NHL and Hodgkin Lymphoma Dr. Carolyn Faught November 10, 2017 What does aggressive mean? Shorter duration of symptoms Generally need treatment at time of diagnosis Immediate, few days, few weeks
Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 04/07, 05/08, 03/10, 03/11, 03/12
 Hematopoietic Stem-Cell Transplantation for Chronic Myelogenous (80130) Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 04/07, 05/08, 03/10, 03/11,
Hematopoietic Stem-Cell Transplantation for Chronic Myelogenous (80130) Medical Benefit Effective Date: 07/01/12 Next Review Date: 03/13 Preauthorization* Yes Review Dates: 04/07, 05/08, 03/10, 03/11,
Yescarta (axicabtagene ciloleucel)
 Last Review Date: March 30, 2018 Effective Date: April 1, 2018 Number: MG.MM.PH.42 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider
Last Review Date: March 30, 2018 Effective Date: April 1, 2018 Number: MG.MM.PH.42 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider
Populations Interventions Comparators Outcomes Individuals: With primary amyloidosis
 Protocol Hematopoietic Cell Transplantation for Primary Amyloidosis (80142) (Formerly Hematopoietic Stem Cell Transplantation for Primary Amyloidosis) Medical Benefit Effective Date: 04/01/13 Next Review
Protocol Hematopoietic Cell Transplantation for Primary Amyloidosis (80142) (Formerly Hematopoietic Stem Cell Transplantation for Primary Amyloidosis) Medical Benefit Effective Date: 04/01/13 Next Review
KANSAS MEDICAL ASSISTANCE PROGRAM. Fee-for-Service Provider Manual. Rehabilitative Therapy Services
 Fee-for-Service Provider Manual Rehabilitative Therapy Services Updated 12.2015 PART II (PHYSICAL THERAPY, OCCUPATIONAL THERAPY, SPEECH/LANGUAGE PATHOLOGY) Introduction Section BILLING INSTRUCTIONS Page
Fee-for-Service Provider Manual Rehabilitative Therapy Services Updated 12.2015 PART II (PHYSICAL THERAPY, OCCUPATIONAL THERAPY, SPEECH/LANGUAGE PATHOLOGY) Introduction Section BILLING INSTRUCTIONS Page
CLINICAL MEDICAL POLICY
 Policy Name: Policy Number: Approved By: CLINICAL MEDICAL POLICY ADCETRIS (Brentuximab Vedotin) MP-035-MD-DE Provider Notice Date: 11/1/2016 Original Effective Date: 12/1/2016 Medical Management Annual
Policy Name: Policy Number: Approved By: CLINICAL MEDICAL POLICY ADCETRIS (Brentuximab Vedotin) MP-035-MD-DE Provider Notice Date: 11/1/2016 Original Effective Date: 12/1/2016 Medical Management Annual
STEM CELL TRANSPLANTATION FOR ACUTE MYELOID LEUKEMIA
 STEM CELL TRANSPLANTATION FOR ACUTE MYELOID LEUKEMIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
STEM CELL TRANSPLANTATION FOR ACUTE MYELOID LEUKEMIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
HEMATOPOIETIC CELL TRANSPLANTATION FOR HODGKIN LYMPHOMA
 HEMATOPOIETIC CELL TRANSPLANTATION FOR HODGKIN LYMPHOMA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services,
HEMATOPOIETIC CELL TRANSPLANTATION FOR HODGKIN LYMPHOMA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services,
CLINICAL MEDICATION POLICY
 Policy Name: Policy Number: Approved By: CLINICAL MEDICATION POLICY Adcetris (Brentuximab Vedotin) MP-035-MD-WV Provider Notice Date: 07/03/2017 Original Effective Date: 08/03/2017 Annual Approval Date:
Policy Name: Policy Number: Approved By: CLINICAL MEDICATION POLICY Adcetris (Brentuximab Vedotin) MP-035-MD-WV Provider Notice Date: 07/03/2017 Original Effective Date: 08/03/2017 Annual Approval Date:
CLINICAL MEDICATION POLICY
 Policy Name: Policy Number: Approved By: CLINICAL MEDICATION POLICY Adcetris (brentuximab vedotin) MP-035-MD-DE Provider Notice Date: 08/01/2017 Original Effective Date: 09/01/2017 Annual Approval Date:
Policy Name: Policy Number: Approved By: CLINICAL MEDICATION POLICY Adcetris (brentuximab vedotin) MP-035-MD-DE Provider Notice Date: 08/01/2017 Original Effective Date: 09/01/2017 Annual Approval Date:
Rituxan Hycela (rituximab and hyaluronidase human) (Subcutaneous)
 Rituxan Hycela (rituximab and hyaluronidase human) (Subcutaneous) Document Number: IC-0322 Last Review Date: 02/06/2018 Date of Origin: 7/20/2010 Dates Reviewed: 09/2010, 12/2010, 02/2011, 03/2011, 05/2011,
Rituxan Hycela (rituximab and hyaluronidase human) (Subcutaneous) Document Number: IC-0322 Last Review Date: 02/06/2018 Date of Origin: 7/20/2010 Dates Reviewed: 09/2010, 12/2010, 02/2011, 03/2011, 05/2011,
Overview of Cutaneous Lymphomas: Diagnosis and Staging. Lauren C. Pinter-Brown MD, FACP Health Sciences Professor of Medicine and Dermatology
 Overview of Cutaneous Lymphomas: Diagnosis and Staging Lauren C. Pinter-Brown MD, FACP Health Sciences Professor of Medicine and Dermatology Definition of Lymphoma A cancer or malignancy that comes from
Overview of Cutaneous Lymphomas: Diagnosis and Staging Lauren C. Pinter-Brown MD, FACP Health Sciences Professor of Medicine and Dermatology Definition of Lymphoma A cancer or malignancy that comes from
See Important Reminder at the end of this policy for important regulatory and legal information.
 Clinical Policy: Vorinostat (Zolinza) Reference Number: CP.PHAR.83 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Revision Log See Important Reminder at the end
Clinical Policy: Vorinostat (Zolinza) Reference Number: CP.PHAR.83 Effective Date: 10.01.18 Last Review Date: 07.13.18 Line of Business: Oregon Health Plan Revision Log See Important Reminder at the end
Allogeneic Hematopoietic Stem-Cell Transplantation for Myelodysplastic Syndromes and Myeloproliferative Neoplasms. Policy Specific Section:
 Medical Policy Allogeneic Hematopoietic Stem-Cell Transplantation for Myelodysplastic Syndromes and Myeloproliferative Type: Medical Necessity and Investigational / Experimental Policy Specific Section:
Medical Policy Allogeneic Hematopoietic Stem-Cell Transplantation for Myelodysplastic Syndromes and Myeloproliferative Type: Medical Necessity and Investigational / Experimental Policy Specific Section:
SAMPLE. Laboratory Services. An essential coding, billing, and reimbursement resource for laboratory and pathology services ICD-10
 Coding and Payment Guide www.optumcoding.com Laboratory Services An essential coding, billing, and reimbursement resource for laboratory and pathology services 2017 ICD-10 A full suite of resources including
Coding and Payment Guide www.optumcoding.com Laboratory Services An essential coding, billing, and reimbursement resource for laboratory and pathology services 2017 ICD-10 A full suite of resources including
Lymphoma: The Basics. Dr. Douglas Stewart
 Lymphoma: The Basics Dr. Douglas Stewart Objectives What is lymphoma? How common is it? Why does it occur? How do you diagnose it? How do you manage it? How do you follow patients after treatment? What
Lymphoma: The Basics Dr. Douglas Stewart Objectives What is lymphoma? How common is it? Why does it occur? How do you diagnose it? How do you manage it? How do you follow patients after treatment? What
Hematopoietic Cell Transplantation for Hodgkin Lymphoma
 Hematopoietic Cell Transplantation for Hodgkin Lymphoma Policy Number: Original Effective Date: MM.07.015 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 05/26/2017 Section: Transplants
Hematopoietic Cell Transplantation for Hodgkin Lymphoma Policy Number: Original Effective Date: MM.07.015 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 05/26/2017 Section: Transplants
Corporate Medical Policy. Policy Effective February 23, 2018
 Corporate Medical Policy Genetic Testing for FLT3, NPM1 and CEBPA Mutations in Acute File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_flt3_npm1_and_cebpa_mutations_in_acute_myeloid_leukemia
Corporate Medical Policy Genetic Testing for FLT3, NPM1 and CEBPA Mutations in Acute File Name: Origination: Last CAP Review: Next CAP Review: Last Review: genetic_testing_for_flt3_npm1_and_cebpa_mutations_in_acute_myeloid_leukemia
Non-Hodgkin lymphomas (NHLs) Hodgkin lymphoma )HL)
 Non-Hodgkin lymphomas (NHLs) Hodgkin lymphoma )HL) Lymphoid Neoplasms: 1- non-hodgkin lymphomas (NHLs) 2- Hodgkin lymphoma 3- plasma cell neoplasms Non-Hodgkin lymphomas (NHLs) Acute Lymphoblastic Leukemia/Lymphoma
Non-Hodgkin lymphomas (NHLs) Hodgkin lymphoma )HL) Lymphoid Neoplasms: 1- non-hodgkin lymphomas (NHLs) 2- Hodgkin lymphoma 3- plasma cell neoplasms Non-Hodgkin lymphomas (NHLs) Acute Lymphoblastic Leukemia/Lymphoma
Special Ophthalmological Services Clinical Coverage Policy No: 1T-2 Amended Date: October 1, Table of Contents
 Special Ophthalmological Services Clinical Coverage Policy No: 1T-2 Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Computerized Corneal Topography... 1 1.2 Sensorimotor
Special Ophthalmological Services Clinical Coverage Policy No: 1T-2 Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Computerized Corneal Topography... 1 1.2 Sensorimotor
Hematopoietic Cell Transplantation for Acute Lymphoblastic Leukemia
 Hematopoietic Cell Transplantation for Acute Lymphoblastic Leukemia Policy Number: 8.01.32 Last Review: 7/2018 Origination: 7/2002 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Hematopoietic Cell Transplantation for Acute Lymphoblastic Leukemia Policy Number: 8.01.32 Last Review: 7/2018 Origination: 7/2002 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
T-cell Lymphomas Biology and Management
 T-cell Lymphomas Biology and Management March-27-2017 Outline Epidemiology Initial Work-up International Prognostic Index Treatment of Diffuse Large B-cell Lymphoma: -Limited Stage -Advanced Stage Frontline:
T-cell Lymphomas Biology and Management March-27-2017 Outline Epidemiology Initial Work-up International Prognostic Index Treatment of Diffuse Large B-cell Lymphoma: -Limited Stage -Advanced Stage Frontline:
Integrated Hematopathology. Morphology and FCI with IHC
 Integrated Hematopathology Morphology and FCI with IHC FrontMatter.indd i 9/6/2009 9:30:12 PM FrontMatter.indd ii 9/6/2009 9:30:18 PM Integrated Hematopathology Morphology and FCI with IHC Cherie H Dunphy,
Integrated Hematopathology Morphology and FCI with IHC FrontMatter.indd i 9/6/2009 9:30:12 PM FrontMatter.indd ii 9/6/2009 9:30:18 PM Integrated Hematopathology Morphology and FCI with IHC Cherie H Dunphy,
Clinical Policy: Donor Lymphocyte Infusion
 Clinical Policy: Reference Number: PA.CP.MP.101 Effective Date: 01/18 Last Review Date: 11/16 Coding Implications Revision Log This policy describes the medical necessity requirements for a donor lymphocyte
Clinical Policy: Reference Number: PA.CP.MP.101 Effective Date: 01/18 Last Review Date: 11/16 Coding Implications Revision Log This policy describes the medical necessity requirements for a donor lymphocyte
New Evidence reports on presentations given at EHA/ICML Bendamustine in the Treatment of Lymphoproliferative Disorders
 New Evidence reports on presentations given at EHA/ICML 2011 Bendamustine in the Treatment of Lymphoproliferative Disorders Report on EHA/ICML 2011 presentations Efficacy and safety of bendamustine plus
New Evidence reports on presentations given at EHA/ICML 2011 Bendamustine in the Treatment of Lymphoproliferative Disorders Report on EHA/ICML 2011 presentations Efficacy and safety of bendamustine plus
Hematopoietic Stem-Cell Transplantation for Breast Cancer
 Hematopoietic Stem-Cell Transplantation for Breast Cancer Policy Number: Original Effective Date: MM.07.010 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 07/22/2011 Section: Transplants
Hematopoietic Stem-Cell Transplantation for Breast Cancer Policy Number: Original Effective Date: MM.07.010 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 07/22/2011 Section: Transplants
Small B-cell (Histologically Low Grade) Lymphoma
 Frequency of Lymphoid Neoplasms Small B-cell (Histologically Low Grade) Lymphoma Stephen Hamilton-Dutoit Institute of Pathology Aarhus University Hospital B-cell neoplasms 88% Diffuse large B-cell lymphoma
Frequency of Lymphoid Neoplasms Small B-cell (Histologically Low Grade) Lymphoma Stephen Hamilton-Dutoit Institute of Pathology Aarhus University Hospital B-cell neoplasms 88% Diffuse large B-cell lymphoma
Corporate Medical Policy
 Corporate Medical Policy Monoclonal Antibodies for Non-Hodgkin Lymphoma and Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: monoclonal_antibodies_for_non_hodgkin_lymphoma_acute_myeloid_leukemia
Corporate Medical Policy Monoclonal Antibodies for Non-Hodgkin Lymphoma and Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: monoclonal_antibodies_for_non_hodgkin_lymphoma_acute_myeloid_leukemia
Clinical Policy: Bendamustine (Bendeka, Treanda) Reference Number: CP.PHAR.307 Effective Date: Last Review Date: 11.18
 Clinical Policy: (Bendeka, Treanda) Reference Number: CP.PHAR.307 Effective Date: 02.01.17 Last Review Date: 11.18 Coding Implications Revision Log Line of Business: Medicaid, HIM-Medical Benefit See Important
Clinical Policy: (Bendeka, Treanda) Reference Number: CP.PHAR.307 Effective Date: 02.01.17 Last Review Date: 11.18 Coding Implications Revision Log Line of Business: Medicaid, HIM-Medical Benefit See Important
Protocol. Hematopoietic Cell Transplantation for Hodgkin Lymphoma
 Protocol Hematopoietic Cell Transplantation for Hodgkin Lymphoma (80129) Medical Benefit Effective Date: 04/01/18 Next Review Date: 01/20 Preauthorization Yes Review Dates: 04/07, 05/08, 01/10, 01/11,
Protocol Hematopoietic Cell Transplantation for Hodgkin Lymphoma (80129) Medical Benefit Effective Date: 04/01/18 Next Review Date: 01/20 Preauthorization Yes Review Dates: 04/07, 05/08, 01/10, 01/11,
LYMPHOMA Joginder Singh, MD Medical Oncologist, Mercy Cancer Center
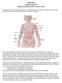 LYMPHOMA Joginder Singh, MD Medical Oncologist, Mercy Cancer Center Lymphoma is cancer of the lymphatic system. The lymphatic system is made up of organs all over the body that make up and store cells
LYMPHOMA Joginder Singh, MD Medical Oncologist, Mercy Cancer Center Lymphoma is cancer of the lymphatic system. The lymphatic system is made up of organs all over the body that make up and store cells
Policy for Central Nervous System [CNS] Prophylaxis in Lymphoid Malignancies
![Policy for Central Nervous System [CNS] Prophylaxis in Lymphoid Malignancies Policy for Central Nervous System [CNS] Prophylaxis in Lymphoid Malignancies](/thumbs/90/104309591.jpg) Policy for Central Nervous System [CNS] Prophylaxis in Lymphoid Malignancies UNCONTROLLED WHEN PRINTED Note: NOSCAN Haematology MCN has approved the information contained within this document to guide
Policy for Central Nervous System [CNS] Prophylaxis in Lymphoid Malignancies UNCONTROLLED WHEN PRINTED Note: NOSCAN Haematology MCN has approved the information contained within this document to guide
Protocol. Hematopoietic Cell Transplantation for Hodgkin Lymphoma
 Protocol Hematopoietic Cell Transplantation for Hodgkin Lymphoma (80129) (Formerly Hematopoietic Stem Cell Transplantation for Hodgkin Lymphoma) Medical Benefit Effective Date: 04/01/18 Next Review Date:
Protocol Hematopoietic Cell Transplantation for Hodgkin Lymphoma (80129) (Formerly Hematopoietic Stem Cell Transplantation for Hodgkin Lymphoma) Medical Benefit Effective Date: 04/01/18 Next Review Date:
Protocol. Hematopoietic Cell Transplantation for Non-Hodgkin Lymphomas
 Protocol Hematopoietic Cell Transplantation for Non-Hodgkin Lymphomas (80120) (Formerly Hematopoietic Stem Cell Transplantation for Non-Hodgkin Lymphomas) Medical Benefit Effective Date: 04/01/13 Next
Protocol Hematopoietic Cell Transplantation for Non-Hodgkin Lymphomas (80120) (Formerly Hematopoietic Stem Cell Transplantation for Non-Hodgkin Lymphomas) Medical Benefit Effective Date: 04/01/13 Next
Lymphoma: What You Need to Know. Richard van der Jagt MD, FRCPC
 Lymphoma: What You Need to Know Richard van der Jagt MD, FRCPC Overview Concepts, classification, biology Epidemiology Clinical presentation Diagnosis Staging Three important types of lymphoma Conceptualizing
Lymphoma: What You Need to Know Richard van der Jagt MD, FRCPC Overview Concepts, classification, biology Epidemiology Clinical presentation Diagnosis Staging Three important types of lymphoma Conceptualizing
Rituximab and Combination Chemotherapy in Treating Patients With Non- Hodgkin's Lymphoma
 Page 1 of 5 Home Search Study Topics Glossary Search Full Text View Tabular View No Study Results Posted Related Studies Rituximab and Combination Chemotherapy in Treating Patients With Non- Hodgkin's
Page 1 of 5 Home Search Study Topics Glossary Search Full Text View Tabular View No Study Results Posted Related Studies Rituximab and Combination Chemotherapy in Treating Patients With Non- Hodgkin's
Non-Hodgkin s Lymphomas Version
 NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Non-Hodgkin s Lymphomas Version 2.2015 NCCN.org Continue Use of Immunophenotyping/ Genetic Testing in Differential Diagnosis of Mature B-Cell
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) Non-Hodgkin s Lymphomas Version 2.2015 NCCN.org Continue Use of Immunophenotyping/ Genetic Testing in Differential Diagnosis of Mature B-Cell
Summary of Changes Page BMT CTN 1205 Protocol Amendment #4 (Version 5.0) Dated July 22, 2016
 Page 1 of 8 Date: July 22, 2016 Summary of Changes Page BMT CTN 1205 Protocol #4 Dated July 22, 2016 The following changes, and the rationale for the changes, were made to the attached protocol in this
Page 1 of 8 Date: July 22, 2016 Summary of Changes Page BMT CTN 1205 Protocol #4 Dated July 22, 2016 The following changes, and the rationale for the changes, were made to the attached protocol in this
NON HODGKINS LYMPHOMA: INDOLENT Updated June 2015 by Dr. Manna (PGY-5 Medical Oncology Resident, University of Calgary)
 NON HODGKINS LYMPHOMA: INDOLENT Updated June 2015 by Dr. Manna (PGY-5 Medical Oncology Resident, University of Calgary) Reviewed by Dr. Michelle Geddes (Staff Hematologist, University of Calgary) and Dr.
NON HODGKINS LYMPHOMA: INDOLENT Updated June 2015 by Dr. Manna (PGY-5 Medical Oncology Resident, University of Calgary) Reviewed by Dr. Michelle Geddes (Staff Hematologist, University of Calgary) and Dr.
UPDATE Autologous Stem Cell Transplantation for Lymphoma and Myeloma
 UPDATE Autologous Stem Cell Transplantation for Lymphoma and Myeloma Supported by a grant from Supported by a grant from UPDATE Autologous Stem Cell Transplantation for Lymphoma and Myeloma Jonathan W.
UPDATE Autologous Stem Cell Transplantation for Lymphoma and Myeloma Supported by a grant from Supported by a grant from UPDATE Autologous Stem Cell Transplantation for Lymphoma and Myeloma Jonathan W.
HEMATOPOIETIC CELL TRANSPLANTATION FOR CHRONIC MYELOID LEUKEMIA
 LEUKEMIA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
LEUKEMIA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
Bone Marrow Transplantation and the Potential Role of Iomab-B
 Bone Marrow Transplantation and the Potential Role of Iomab-B Hillard M. Lazarus, MD, FACP Professor of Medicine, Director of Novel Cell Therapy Case Western Reserve University 1 Hematopoietic Cell Transplantation
Bone Marrow Transplantation and the Potential Role of Iomab-B Hillard M. Lazarus, MD, FACP Professor of Medicine, Director of Novel Cell Therapy Case Western Reserve University 1 Hematopoietic Cell Transplantation
癌症診療準則與核心測量 - 淋巴瘤 彰化基督教醫院內科部血液腫瘤科張正雄. Agenda
 癌症診療準則與核心測量 - 淋巴瘤 彰化基督教醫院內科部血液腫瘤科張正雄 Agenda Lymphocyte differentiation Classification of lymphomas Disease definitions and symptoms Tests that identify specific lymphoid histologies Staging Cancer registry
癌症診療準則與核心測量 - 淋巴瘤 彰化基督教醫院內科部血液腫瘤科張正雄 Agenda Lymphocyte differentiation Classification of lymphomas Disease definitions and symptoms Tests that identify specific lymphoid histologies Staging Cancer registry
LYMPHOMAS an overview of some subtypes of NHLs
 One of the confusing aspects of the lymphoid neoplasms concerns the use of the descriptive terms "leukemia" and "lymphoma." LYMPHOMAS an overview of some subtypes of NHLs Leukemia is used for lymphoid
One of the confusing aspects of the lymphoid neoplasms concerns the use of the descriptive terms "leukemia" and "lymphoma." LYMPHOMAS an overview of some subtypes of NHLs Leukemia is used for lymphoid
Clinical Policy: Obinutuzumab (Gazyva) Reference Number: CP.PHAR.305 Effective Date: Last Review Date: Line of Business: Medicaid
 Clinical Policy: (Gazyva) Reference Number: CP.PHAR.305 Effective Date: 02.01.17 Last Review Date: 11.17 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of
Clinical Policy: (Gazyva) Reference Number: CP.PHAR.305 Effective Date: 02.01.17 Last Review Date: 11.17 Line of Business: Medicaid Coding Implications Revision Log See Important Reminder at the end of
Instructions for Chronic Lymphocytic Leukemia Post-HSCT Data (Form 2113)
 Instructions for Chronic Lymphocytic Leukemia Post-HSCT Data (Form 2113) This section of the CIBMTR Forms Instruction Manual is intended to be a resource for completing the CLL Post-HSCT Data Form. E-mail
Instructions for Chronic Lymphocytic Leukemia Post-HSCT Data (Form 2113) This section of the CIBMTR Forms Instruction Manual is intended to be a resource for completing the CLL Post-HSCT Data Form. E-mail
Supplementary Appendix to manuscript submitted by Trappe, R.U. et al:
 Supplementary Appendix to manuscript submitted by Trappe, R.U. et al: Response to rituximab induction is a predictive marker in B-cell post-transplant lymphoproliferative disorder and allows successful
Supplementary Appendix to manuscript submitted by Trappe, R.U. et al: Response to rituximab induction is a predictive marker in B-cell post-transplant lymphoproliferative disorder and allows successful
Lymphatic system component
 Introduction Lymphatic system component Statistics Overview Lymphoma Non Hodgkin s Lymphoma Non- Hodgkin's is a type of cancer that originates in the lymphatic system. It is estimated to be the sixth most
Introduction Lymphatic system component Statistics Overview Lymphoma Non Hodgkin s Lymphoma Non- Hodgkin's is a type of cancer that originates in the lymphatic system. It is estimated to be the sixth most
Hematopoietic Cell Transplantation for Hodgkin Lymphoma
 Hematopoietic Cell Transplantation for Hodgkin Lymphoma Policy Number: 8.01.29 Last Review: 1/2019 Origination: 4/2014 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
Hematopoietic Cell Transplantation for Hodgkin Lymphoma Policy Number: 8.01.29 Last Review: 1/2019 Origination: 4/2014 Next Review: 1/2020 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will
