Protocol for the Examination of Specimens from Patients with Carcinoma of the Anus
|
|
|
- Kelly Clark
- 5 years ago
- Views:
Transcription
1 Protocol for the Examination of Specimens from Patients with Carcinoma of the Anus Protocol applies to all invasive carcinomas of the anal canal. Based on AJCC/UICC TNM, 7th edition Protocol web posting date: October 2009 Procedures Excisional Biopsy Local Excision (Transanal Disk Incision) Abdominoperineal Resection Authors Kay Washington, MD, PhD, FCAP* Department of Pathology, Vanderbilt University Medical Center, Nashville, TN Jordan Berlin, MD Department of Medicine, Vanderbilt University Medical Center, Nashville, TN Philip Branton, MD, FCAP Department of Pathology, Inova Fairfax Hospital, Falls Church, VA Lawrence J. Burgart, MD, FCAP Allina Laboratories, Abbott Northwestern Hospital, Minneapolis, MN David K. Carter, MD, FCAP Department of Pathology, St. Mary s/duluth Clinic Health System, Duluth, MN Patrick Fitzgibbons, MD, FCAP Department of Pathology, St. Jude Medical Center, Fullerton, CA Wendy Frankel, MD, FCAP Department of Pathology, Ohio State University Medical Center, Columbus, OH John Jessup, MD Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, MD Sanjay Kakar, MD, FCAP Department of Pathology, University of California San Francisco and the Veterans Affairs Medical Center, San Francisco, CA Bruce Minsky, MD Department of Radiation Oncology, University of Chicago, Chicago, IL Raouf Nakhleh, MD, FCAP Department of Pathology, Mayo Clinic, Jacksonville, FL Carolyn C. Compton, MD, PhD, FCAP Office of Biorepositories and Biospecimen Research, National Cancer Institute, Bethesda, MD For the Members of the Cancer Committee, College of American Pathologists *denotes primary author. denotes senior author. All other contributing authors are listed alphabetically. Previous contributor: R. R. Rickert, MD
2 2009 College of American Pathologists (CAP). All rights reserved. The College does not permit reproduction of any substantial portion of these protocols without its written authorization. The College hereby authorizes use of these protocols by physicians and other health care providers in reporting on surgical specimens, in teaching, and in carrying out medical research for nonprofit purposes. This authorization does not extend to reproduction or other use of any substantial portion of these protocols for commercial purposes without the written consent of the College. The CAP also authorizes physicians and other health care practitioners to make modified versions of the Protocols solely for their individual use in reporting on surgical specimens for individual patients, teaching, and carrying out medical research for non-profit purposes. The CAP further authorizes the following uses by physicians and other health care practitioners, in reporting on surgical specimens for individual patients, in teaching, and in carrying out medical research for non-profit purposes: (1) Dictation from the original or modified protocols for the purposes of creating a text-based patient record on paper, or in a word processing document; (2) Copying from the original or modified protocols into a text-based patient record on paper, or in a word processing document; (3) The use of a computerized system for items (1) and (2), provided that the Protocol data is stored intact as a single text-based document, and is not stored as multiple discrete data fields. Other than uses (1), (2), and (3) above, the CAP does not authorize any use of the Protocols in electronic medical records systems, pathology informatics systems, cancer registry computer systems, computerized databases, mappings between coding works, or any computerized system without a written license from CAP. Applications for such a license should be addressed to the SNOMED Terminology Solutions division of the CAP. Any public dissemination of the original or modified Protocols is prohibited without a written license from the CAP. The College of American Pathologists offers these protocols to assist pathologists in providing clinically useful and relevant information when reporting results of surgical specimen examinations of surgical specimens. The College regards the reporting elements in the Surgical Pathology Cancer Case Summary (Checklist) portion of the protocols as essential elements of the pathology report. However, the manner in which these elements are reported is at the discretion of each specific pathologist, taking into account clinician preferences, institutional policies, and individual practice. The College developed these protocols as an educational tool to assist pathologists in the useful reporting of relevant information. It did not issue the protocols for use in litigation, reimbursement, or other contexts. Nevertheless, the College recognizes that the protocols might be used by hospitals, attorneys, payers, and others. Indeed, effective January 1, 2004, the Commission on Cancer of the American College of Surgeons mandated the use of the checklist elements of the protocols as part of its Cancer Program Standards for Approved Cancer Programs. Therefore, it becomes even more important for pathologists to familiarize themselves with these documents. At the same time, the College cautions that use of the protocols other than for their intended educational purpose may involve additional considerations that are beyond the scope of this document. The inclusion of a product name or service in a CAP publication should not be construed as an endorsement of such product or service, nor is failure to include the name of a product of service to be construed as disapproval. 2
3 CAP Approved Surgical Pathology Cancer Case Summary (Checklist) Protocol web posting date: October 2009 ANUS: Excisional Biopsy or Local Excision (Transanal Disk Excision) Select a single response unless otherwise indicated. Specimen (select all that apply) Anal canal Anorectal junction Rectum Perianal skin Other (specify): Not specified Procedure Excisional biopsy (polypectomy) Local excision (transanal disk excision) Other (specify): Not specified Specimen Integrity (Note A) Intact Fragmented *Number of pieces in fragmented specimens: Other (specify): Tumor Site (Note B) Anal canal Anorectal junction Anal margin Anus, not otherwise specified Unknown Other (specify): Tumor Size Greatest dimension: cm *Additional dimensions: x cm Cannot be determined (see Comment) Histologic Type (Note C) Squamous cell carcinoma Adenocarcinoma Mucinous adenocarcinoma Small cell carcinoma Undifferentiated carcinoma Paget disease Other (specify): * Data elements with asterisks are not required. However, these elements may be clinically important but are not yet validated or regularly used in patient management. 3
4 CAP Approved Histologic Grade (Note D) Not applicable GX: Cannot be assessed G1: Well differentiated G2: Moderately differentiated G3: Poorly differentiated G4: Undifferentiated Other (specify): Microscopic Tumor Extension Cannot be assessed No evidence of primary tumor Carcinoma in situ Tumor invades lamina propria Tumor invades muscularis mucosae Tumor invades submucosa Tumor invades sphincter muscle Tumor invades perianal skin Margins (select all that apply) Cannot be assessed Margins uninvolved by invasive carcinoma Distance of invasive carcinoma from closest margin: mm Specify margin (if possible): Carcinoma in situ absent at mucosal margin Carcinoma in situ present at mucosal margin Margin(s) involved by invasive carcinoma Specify margin (if possible): Not applicable (specify reason): Treatment Effect (applicable to carcinomas treated with neoadjuvant therapy) (Note E) No prior treatment Present * Complete response (no viable tumor cells, grade 0) * Moderate response (single cells or small groups of tumor cells, grade 1) * Minimal response (residual tumor outgrown by fibrosis, grade 2) No definite response identified (grade 3, poor or no response; extensive residual tumor) Not known *Lymph-Vascular Invasion * Not identified * Present * Indeterminate *Perineural Invasion * Not identified * Present * Indeterminate * Data elements with asterisks are not required. However, these elements may be clinically important but are not yet validated or regularly used in patient management. 4
5 CAP Approved Pathologic Staging (ptnm) (Note F) TNM Descriptors (required only if applicable) (select all that apply) m (multiple primary tumors) r (recurrent) y (post-treatment) Primary Tumor (pt) ptx: Cannot be assessed pt0: No evidence of primary tumor ptis: Carcinoma in situ pt1: Tumor 2 cm or less in greatest dimension pt2: Tumor more than 2 cm but not more than 5 cm in greatest dimension pt3: Tumor more than 5 cm in greatest dimension pt4: Tumor of any size with invasion of adjacent organ(s); eg, vagina, urethra, bladder (involvement of sphincter muscles alone is not classified as T4). *Additional Pathologic Findings (select all that apply) (Note G) * None identified * Crohn disease * Condyloma accuminatum * Dysplasia * Associated rectal carcinoma (Paget disease) * Other (specify): *Ancillary Studies (Note H) *Specify: * Not performed *Clinical History (select all that apply) (Note I) * Solid organ transplantation * HIV/AIDS * Human papilloma virus infection * Crohn disease * Neoadjuvant therapy (specify type, if known: ) * Other (specify): * Not known *Comment(s) * Data elements with asterisks are not required. However, these elements may be clinically important but are not yet validated or regularly used in patient management. 5
6 CAP Approved Surgical Pathology Cancer Case Summary (Checklist) Protocol web posting date: October 2009 ANUS: Abdominoperineal Resection Select a single response unless otherwise indicated. Specimen (select all that apply) Anal canal Anorectal junction Rectum Perianal skin Other (specify): Not specified Procedure Abdominoperineal resection Other (specify): Not specified Tumor Site (select all that apply) (Note B) Anal canal Anorectal junction Anal margin Anus, not otherwise specified Unknown Other (specify): Tumor Size Greatest dimension: cm *Additional dimensions: x cm Cannot be determined (see Comment) Histologic Type (Note C) Squamous cell carcinoma Adenocarcinoma Mucinous adenocarcinoma Small cell carcinoma Undifferentiated carcinoma Paget disease Other (specify): Carcinoma, type cannot be determined * Data elements with asterisks are not required. However, these elements may be clinically important but are not yet validated or regularly used in patient management. 6
7 CAP Approved Histologic Grade (Note D) Not applicable GX: Cannot be assessed G1: Well differentiated G2: Moderately differentiated G3: Poorly differentiated G4: Undifferentiated Other (specify): Microscopic Tumor Extension Cannot be assessed No evidence of primary tumor Carcinoma in situ Tumor invades lamina propria Tumor invades muscularis mucosae Tumor invades submucosa Tumor invades into but not through sphincter muscle Tumor invades into but not through muscularis propria of rectum Tumor invades through sphincter muscle into perianal or perirectal soft tissue without involvement of adjacent structures Tumor directly invades adjacent structures (specify): Tumor invades perianal skin Margins (select all that apply) Proximal Margin Cannot be assessed Uninvolved by invasive carcinoma Carcinoma in situ absent at mucosal margin Carcinoma in situ present at mucosal margin Involved by invasive carcinoma Distal Margin Cannot be assessed Uninvolved by invasive carcinoma Carcinoma in situ absent at mucosal margin Carcinoma in situ present at mucosal margin Involved by invasive carcinoma Circumferential (Radial) Margin Cannot be assessed Uninvolved by invasive carcinoma Involved by invasive carcinoma If all margins uninvolved by invasive carcinoma: Distance of invasive carcinoma from closest margin: mm Specify margin: * Data elements with asterisks are not required. However, these elements may be clinically important but are not yet validated or regularly used in patient management. 7
8 CAP Approved Treatment Effect (applicable to carcinomas treated with neoadjuvant therapy) (select all that apply) (Note E) No prior treatment Present * Complete response (no viable tumor cells, grade 0) * Moderate response (single cells or small groups of tumor cells, grade 1) * Minimal response (residual tumor outgrown by fibrosis, grade 2) No definite response identified (grade 3, poor or no response; extensive residual tumor) Not known *Lymph-Vascular Invasion * Not identified * Present * Indeterminate *Perineural Invasion * Not identified * Present * Indeterminate Pathologic Staging (ptnm) (Note F) TNM Descriptors (required only if applicable) (select all that apply) m (multiple primary tumors) r (recurrent) y (post-treatment) Primary Tumor (pt) ptx: Cannot be assessed pt0: No evidence of primary tumor ptis: Carcinoma in situ pt1: Tumor 2 cm or less in greatest dimension pt2: Tumor more than 2 cm but not more than 5 cm in greatest dimension pt3: Tumor more than 5 cm in greatest dimension pt4: Tumor of any size with invasion of adjacent organ(s); eg, vagina, urethra, bladder (involvement of sphincter muscles alone is not classified as T4). Regional Lymph Nodes (pn) pnx: Cannot be assessed pn0: No regional lymph node metastasis pn1: Metastasis in perirectal lymph nodes pn2: Metastasis in unilateral internal iliac and/or inguinal lymph node(s) pn3: Metastasis in perirectal and inguinal lymph nodes and/or bilateral internal iliac and/or inguinal lymph nodes Specify: Number examined: Number involved: * Data elements with asterisks are not required. However, these elements may be clinically important but are not yet validated or regularly used in patient management. 8
9 CAP Approved Distant Metastasis (pm) Not applicable pm1: Distant metastasis *Specify site(s), if known: *Additional Pathologic Findings (select all that apply) (Note G) * None identified * Crohn disease * Condyloma accuminatum * Dysplasia * Associated rectal carcinoma (Paget disease) * Other (specify): *Ancillary Studies (Note H) *Specify: *Clinical History (select all that apply) (Note I) * Solid organ transplantation * HIV/AIDS * Human papilloma virus infection * Crohn disease * Neoadjuvant therapy (specify type if known) * Other (specify): * Not known *Comment(s) * Data elements with asterisks are not required. However, these elements may be clinically important but are not yet validated or regularly used in patient management. 9
10 Explanatory Notes A. Specimen Integrity and Handling For specimens from local excision procedures, all relevant margins, including the deep resection margin, should be inked. Evaluation of margins and invasion is facilitated if the specimen is pinned before fixation in formalin. B. Location Documentation of tumor location within the anal canal is important for purposes of stage assignment. Because of possible differences in staging and regional lymph nodes at risk of metastasis among cancers of the anal canal, the rectum, and the perianal skin, it is essential to assure that the anatomic site of the tumor is the anal canal. For the pathologist, however, the documentation of location may be problematic. Currently, most anal canal carcinomas are managed successfully without surgery, using combination chemotherapy and radiation therapy; 1 and resection specimens of anal tumors are seen only infrequently (primarily for small anal margin lesions or after failure of other treatment modalities). Although histological diagnosis is almost always performed on small biopsies, determination of the primary tumor location from biopsy specimens may be difficult or impossible. Therefore, documentation of anatomic site often requires clinical correlation. A major problem complicating determination of anatomic site clinically or pathologically is the controversy over the anatomic definition of the anal canal itself. The surgical definition of the anal canal is the one most widely accepted for practical reasons and is the preferred definition of the American Joint Committee on Cancer (AJCC). 2 However, it is based on clinically identifiable landmarks that are difficult or impossible for the pathologist to locate. By this definition, the anal canal begins at the point where the rectum enters the puborectalis sling at the apex of the anal sphincter complex, a landmark that is palpable in vivo on digital exam as the anorectal ring. The termination of the anal canal is defined as the squamous mucocutaneous junction (ie, the junction of the distal squamous mucosa of the anal canal with the perianal hair-bearing skin). 2 Thus defined, the anal canal (Figure 1) contains three epithelial zones: a proximal narrow zone (approximately 1 to 2 cm) of rectal-type glandular mucosa, an anal transition zone of variable length interposed between colorectal mucosa and squamous epithelium, and a squamous epithelial zone lacking skin appendages. The squamous zone gradually merges into the perianal skin, which contains hair follicles, sweat glands, and sebaceous glands. The anal transition zone may contain a variety of epithelial types, including multilayered transitional mucosa resembling squamous metaplasia or urothelium, which is often present at the dentate line. Anal glands may be found subjacent to the mucosa extending across the internal sphincter in the region of the dentate line. 10
11 Figure 1. Anatomy of the anal canal. From: Greene FL, Compton, CC, Fritz AG, et al, eds. AJCC Cancer Staging Atlas. New York: Springer; Copyright American Joint Committee on Cancer. Used with permission. Tumors involving the anorectal junction should be classified as rectal cancers if the epicenter is more than 2 cm proximal to the dentate line and as anal cancers if the epicenter is 2 cm or less from the dentate line. 2 Cancers that arise in the perianal skin are termed perianal cancers and are biologically similar to other skin tumors. They are staged according to the classification for cancers of the skin 2 (see CAP protocols for skin). C. Histologic Type For consistency in reporting, the histologic classification proposed by the World Health Organization (WHO) is recommended. 3 However, this protocol does not preclude the use of other systems of classification or histologic types. The great majority of carcinomas of the anus are squamous cell carcinomas. 4 The previous edition of the WHO classification included 3 subtypes of squamous cell carcinoma (SCC): large cell keratinizing, large cell nonkeratinizing, and basaloid. However, because most SCCs of the anal canal show more than one subtype, the diagnostic reproducibility of these subtypes has been low. Furthermore, no significant prognostic differences between subtypes have consistently been established, although the basaloid subtype of squamous cell carcinoma may be associated with a higher risk of distant metastasis. 5 Therefore, the WHO now recommends that the generic diagnostic term squamous cell carcinoma be used for all squamous malignancies of the anal canal. However, additional descriptive comment regarding specific histologic features, such as predominant cell size, basaloid features, degree of keratinization, or adjacent intraepithelial neoplasia, is encouraged. Prominent basaloid features and small tumor cell size are related to infection with high-risk human papilloma virus. 3 SCC with a predominantly basaloid differentiation pattern was formerly known as cloacogenic carcinoma, but this term is now considered obsolete. 11
12 Two variants of SCC of the anal canal deserve note because they differ in prognosis from typical squamous tumors. One is verrucous carcinoma (also known as giant condyloma or Buschke-Lowenstein tumor), which resembles a condyloma macroscopically but is larger and fails to respond to conservative therapy. These lesions are regarded as biologic intermediates between condylomas and SCCs, with a better prognosis than SCC. However, nearly half of these lesions undergo malignant transformation. Another important variant is SCC with mucinous microcysts (well-formed cystic spaces containing Alcian blue- or PAS-stainable mucin). This entity has an unfavorable prognosis as compared with that of SCC. 3 Finally, two rare types of anal canal carcinoma, anaplastic carcinoma and small cell carcinoma (high-grade neuroendocrine carcinoma), are tumors with aggressive biologic behavior and an unfavorable prognosis when compared with typical SCC. Tumors of the more distal anal canal and especially anal margin (mucocutaneous junction) are generally purely squamous in type and show fewer basaloid or glandular features. WHO Classification of Carcinoma of the Anal Canal 3 Intraepithelial neoplasia Squamous or transitional epithelium Glandular Paget disease Carcinoma Squamous cell carcinoma Adenocarcinoma Mucinous adenocarcinoma Small cell carcinoma # Undifferentiated carcinoma # Others # By convention, these histologic types are assigned grade 4. The term carcinoma, NOS (not otherwise specified) is not part of the WHO classification. D. Histologic Grade Histologic grades for anal canal squamous carcinoma are as follows: Grade X Grade 1 Grade 2 Grade 3 Grade cannot be assessed Well differentiated Moderately differentiated Poorly differentiated If there are variations in the differentiation within the tumor, the highest (least favorable) grade is recorded as the overall grade. Histologic grades for adenocarcinoma of the anal canal based on the proportion of gland formation by the tumor are suggested as follows: Grade X Grade 1 Grade cannot be assessed Well differentiated (greater than 95% of tumor composed of glands) 12
13 Grade 2 Grade 3 Moderately differentiated (50% to 95% of tumor composed of glands) Poorly differentiated (less than 50% of tumor composed of glands) Small cell carcinomas and tumors with no differentiation or minimal differentiation that is discernible only in rare, tiny foci (undifferentiated carcinomas by WHO classification) are categorized as grade 4. E. Treatment Effect Response of tumor to previous chemotherapy or radiation therapy should be reported. Although grading systems for tumor response have not been established, three-category systems generally provide good interobserver reproducibility. 6 The following system is suggested: Tumor Regression Grade Description No viable cancer cells Single cells or small groups of cancer cells Residual cancer outgrown by fibrosis Minimal or no tumor kill; extensive residual cancer Tumor Regression Grade 0 (Complete response) 1 (Moderate response) 2 (Minimal response) 3 (Poor response) F. TNM and Anatomic Stage/Prognostic Groupings The TNM staging system for anal carcinoma of the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) is recommended by the protocol and shown below. 2 The primary tumor is staged according to its size and local extension, as determined by clinical or pathologic examination. For most histologic types of anal canal cancer, the diameter of the tumor correlates with the depth of penetration. The staging system applies to all carcinomas arising in the anal canal, including carcinomas that arise within anorectal fistulas and anal glands, but excluding melanomas, low-grade neuroendocrine tumors (carcinoid tumors), and sarcomas. By AJCC/UICC convention, the designation T refers to a primary tumor that has not been previously treated. The symbol p refers to the pathologic classification of the TNM, as opposed to the clinical classification, and is based on gross and microscopic examination. pt entails a resection of the primary tumor or biopsy adequate to evaluate the highest pt category, pn entails removal of nodes adequate to validate lymph node metastasis, and pm implies microscopic examination of distant lesions. Clinical classification (ctnm) is usually carried out by the referring physician before treatment during initial evaluation of the patient or when pathologic classification is not possible. Pathologic staging is usually performed after surgical resection of the primary tumor. Pathologic staging depends on pathologic documentation of the anatomic extent of disease, whether or not the primary tumor has been completely removed. If a biopsied tumor is not resected for any reason (eg, when technically infeasible) and if the highest T and N categories or the M1 category of the tumor can be confirmed microscopically, the criteria for pathologic classification and staging have been satisfied without total removal of the primary cancer. 13
14 TNM Descriptors For identification of special cases of TNM or ptnm classifications, the m suffix and y, r, and a prefixes are used. Although they do not affect the stage grouping, they indicate cases needing separate analysis. The m suffix indicates the presence of multiple primary tumors in a single site and is recorded in parentheses: pt(m)nm. The y prefix indicates those cases in which classification is performed during or after initial multimodality therapy (ie, neoadjuvant chemotherapy, radiation therapy, or both chemotherapy and radiation therapy). The ctnm or ptnm category is identified by a y prefix. The yctnm or yptnm categorizes the extent of tumor actually present at the time of that examination. The y categorization is not an estimate of tumor before multimodality therapy (ie, before initiation of neoadjuvant therapy). The r prefix indicates a recurrent tumor when staged after a documented disease-free interval and is identified by the r prefix: rtnm. The a prefix designates the stage determined at autopsy: atnm. T Category Considerations T categories for anal canal cancer are illustrated in Figures 2 through 5. Figure 2. T1 is defined as tumor 2 cm or less in greatest dimension. From: Greene FL, Compton, CC, Fritz AG, et al, eds. AJCC Cancer Staging Atlas. New York: Springer; Copyright American Joint Committee on Cancer. Used with permission. 14
15 Figure 3. T2 is defined as tumor measuring more than 2 cm but 5 cm or less in greatest dimension. From: Greene FL, Compton, CC, Fritz AG, et al, eds. AJCC Cancer Staging Atlas. New York: Springer; Copyright American Joint Committee on Cancer. Used with permission. Figure 4. T3 is defined as tumor measuring more than 5 cm in greatest dimension. From: Greene FL, Compton, CC, Fritz AG, et al, eds. AJCC Cancer Staging Atlas. New York: Springer; Copyright American Joint Committee on Cancer. Used with permission. 15
16 Figure 5. T4 is defined as tumor of any size invading adjacent organs such as vagina (illustrated), urethra, or bladder. From: Greene FL, Compton, CC, Fritz AG, et al, eds. AJCC Cancer Staging Atlas. New York: Springer; Copyright American Joint Committee on Cancer. Used with permission. N Category Considerations Regional lymph nodes (N) (Figure 6) consist of the perirectal (anorectal, perirectal, and lateral sacral), the internal iliac (hypogastric), and the inguinal (superficial and deep femoral). 2 All other nodal groups represent sites of distant metastasis (M). The sites of regional node involvement correspond to the local lymphatic drainage, above to the rectal ampulla and below to the perineum. Tumors that arise in the anal canal usually spread initially to the anorectal and perirectal nodes, and those that arise at the anal margin spread to the superficial inguinal nodes. Figure 6. Regional lymph nodes of the anal canal. From: Greene FL, Compton, CC, Fritz AG, et al, eds. AJCC Cancer Staging Atlas. New York: Springer; Copyright American Joint Committee on Cancer. Used with permission. 16
17 Primary Tumor (T) TX Primary tumor cannot be assessed T0 No evidence of primary tumor Tis Carcinoma in situ T1 Tumor 2 cm or less in greatest dimension T2 Tumor more than 2 cm but not more than 5 cm in greatest dimension T3 Tumor more than 5 cm in greatest dimension T4 Tumor of any size invades adjacent organ(s), eg, vagina, urethra, bladder # # Direct invasion of the rectal wall, perianal skin, subcutaneous tissue, or the sphincter muscle is not classified as T4. Regional Lymph Nodes (N) NX Regional lymph nodes cannot be assessed N0 No regional lymph node metastasis # N1 Metastasis in perirectal lymph node(s) N2 Metastasis in unilateral internal iliac and/or inguinal lymph node(s) N3 Metastasis in perirectal and inguinal lymph nodes and/or bilateral internal iliac and/or inguinal lymph nodes Distant Metastasis (M) M0 No distant metastasis M1 Distant metastasis Stage Groupings Stage 0 Tis N0 M0 Stage I T1 N0 M0 Stage II T2 N0 M0 T3 N0 M0 Stage IIIA T1 N1 M0 T2 N1 M0 T3 N1 M0 T4 N0 M0 Stage IIIB T4 N1 M0 Any T N2 M0 Any T N3 M0 Stage IV Any T Any N M1 Vessel Invasion By AJCC/UICC convention, vessel invasion (lymphatic or venous) does not affect the T category indicating local extent of tumor unless specifically included in the definition of a T category. G. Additional Findings Predisposing conditions to anal canal carcinoma that may be found in the pathologic specimen include condyloma accuminatum associated with human papilloma virus infection. 7 Squamous intraepithelial neoplasia is recognized as a precursor lesion for squamous cell carcinoma of the anal canal, 8 and its presence should be reported. Both adenocarcinomas and squamous cell carcinomas have been reported in the setting of chronic anorectal fistulae arising in long-standing Crohn disease, 9 although the association of benign inflammatory lesions and anal cancer remains controversial. 10,11 17
18 H. Ancillary Studies Immunohistochemistry may be helpful in establishing tumor type for poorly differentiated carcinomas; squamous cell carcinomas of the anal canal express cytokeratin (CK) 7, CK5/6, p53, 12 and p63, 13 but are negative for CK20. In contrast, anal gland carcinomas are mucin positive and express CK 20 and CK7, but are negative for CK5/6 and p63. 12,14 Immunohistochemical studies may also aid in distinguishing primary anal Paget disease from secondary Paget disease of the perianal area, which is associated with colorectal and anal canal carcinoma. CK7 expression is a sensitive method for detection of both primary and secondary Paget cells within involved anal and perianal epithelium. In addition, however, the specific immunophenotype of Paget cells has been shown to correlate with pathogenesis and may be important in patient management. Demonstration of CK20 expression has been shown to identify Paget disease that is likely to be associated with underlying rectal adenocarcinoma (presenting either synchronously or metachronously). In contrast, Paget cells that do not express CK20 but instead are positive for gross cystic disease fluid protein (GCDFP), a marker for apocrine differentiation, are likely to represent primary cutaneous intraepithelial malignancy. 3,8,15 I. Clinical History Predisposing conditions for anal canal carcinomas include immunosuppression, most commonly from solid organ transplantation, or HIV/AIDs infection. 16 The association between human papilloma virus infection and anal cancer has been firmly established. 7 References 1. Engstrom PF, Benson AB 3rd, Chen Y-J, et al. Anal canal cancer clinical practice guidelines in oncology. J Natl Compr Cancer Netw. Jul 2005;3(4): Edge SB, Byrd DR, Carducci MA, Compton CC, eds. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; Fenger C, Frisch M, Marti MC, Parc R. Tumours of the anal canal. In: Hamilton SR, Aaltonen LA, eds. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Digestive System. Lyon, France: IARC Press; 2000: Hatzaras I, Abir F, Kozol R, Sullivan P, Longo WE. The demographics, histopathology and patterns of treatment of anal cancer in Connecticut: Conn Med. 2005;69(5): Das P, Bhatia S, Eng C, et al. Predictors and patterns of recurrence after definitive chemoradiation for anal cancer. Int J Radiat Oncol Biol Phys. 2007;68(3): Ryan R, Gibbons D, Hyland JMP, et al. Pathological response following longcourse neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47: Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer. 2004;101(2): Shepherd NA. Anal intraepithelial neoplasia and other neoplastic precursor lesions of the anal canal and perianal region. Gastroenterol Clin North Am. 2007;36(4): Ky A, Sohn H, Weinstein MA, Korelitz BI. Carcinoma arising in anorectal fistulas of Crohn's disease. Dis Colon Rectum. 1998;41: Frisch M, Olsen JH, Bautz A, Melbye M. Benign anal lesions and the risk of anal cancer. New Engl J Med. 1994;331:
19 11. Nordenvall C, Nyren O, Ye W. Elevated anal squamous cell carcinoma risk associated with benign inflammatory anal lesions. Gut. May 2006;55(5): Balachandra B, Marcus V, Jass JR. Poorly differentiated tumours of the anal canal: a diagnostic strategy for the surgical pathologist. Histopathology. Jan 2007;50(1): Owens SR, Greenson JK. Immunohistochemical staining for p63 is useful in the diagnosis of anal squamous cell carcinomas. Am J Surg Pathol. 2007;31(2): Lisovsky M, Patel K, Cymes K, Chase D, Bhuiya T, Morgenstern N. Immunophenotypic characterization of anal gland carcinoma: loss of p63 and cytokeratin 5/6. Arch Pathol Lab Med. 2007;131(8): Goldblum JR, Hart WR. Perianal Paget's disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22: Uronis HE, Bendell JC. Anal cancer: an overview. Oncologist. May 2007;12(5):
Protocol for the Examination of Specimens From Patients With Carcinoma of the Anus
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Anus Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual For accreditation
Protocol for the Examination of Specimens From Patients With Carcinoma of the Anus Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual For accreditation
Colon and Rectum. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6th edition
 Colon and Rectum Protocol applies to all invasive carcinomas of the colon and rectum. Carcinoid tumors, lymphomas, sarcomas, and tumors of the vermiform appendix are excluded. Protocol revision date: January
Colon and Rectum Protocol applies to all invasive carcinomas of the colon and rectum. Carcinoid tumors, lymphomas, sarcomas, and tumors of the vermiform appendix are excluded. Protocol revision date: January
Small Intestine. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition
 Small Intestine Protocol applies to all invasive carcinomas of the small intestine, including those with focal endocrine differentiation. Excludes carcinoid tumors, lymphomas, and stromal tumors (sarcomas).
Small Intestine Protocol applies to all invasive carcinomas of the small intestine, including those with focal endocrine differentiation. Excludes carcinoid tumors, lymphomas, and stromal tumors (sarcomas).
Uterine Cervix. Protocol applies to all invasive carcinomas of the cervix.
 Uterine Cervix Protocol applies to all invasive carcinomas of the cervix. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
Uterine Cervix Protocol applies to all invasive carcinomas of the cervix. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
Pancreas (Exocrine) Protocol applies to all carcinomas of the exocrine pancreas.
 Pancreas (Exocrine) Protocol applies to all carcinomas of the exocrine pancreas. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist)
Pancreas (Exocrine) Protocol applies to all carcinomas of the exocrine pancreas. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist)
Protocol for the Examination of Specimens From Patients With Primary Malignant Tumors of the Heart
 Protocol for the Examination of Specimens From Patients With Primary Malignant Tumors of the Heart Protocol applies to primary malignant cardiac tumors. Hematolymphoid neoplasms are not included. No AJCC/UICC
Protocol for the Examination of Specimens From Patients With Primary Malignant Tumors of the Heart Protocol applies to primary malignant cardiac tumors. Hematolymphoid neoplasms are not included. No AJCC/UICC
Protocol for the Examination of Specimens from Patients with Carcinoma of the Ampulla of Vater
 Protocol for the Examination of Specimens from Patients with Carcinoma of the Ampulla of Vater Protocol applies to all intra-ampullary, peri-ampullary, and mixed intra- and peri-ampullary carcinomas. Welldifferentiated
Protocol for the Examination of Specimens from Patients with Carcinoma of the Ampulla of Vater Protocol applies to all intra-ampullary, peri-ampullary, and mixed intra- and peri-ampullary carcinomas. Welldifferentiated
Urinary Bladder, Ureter, and Renal Pelvis
 Urinary Bladder, Ureter, and Renal Pelvis Protocol applies to all carcinomas of the urinary bladder, ureter, and renal pelvis. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6th edition Procedures
Urinary Bladder, Ureter, and Renal Pelvis Protocol applies to all carcinomas of the urinary bladder, ureter, and renal pelvis. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6th edition Procedures
Protocol applies to melanoma of cutaneous surfaces only.
 Melanoma of the Skin Protocol applies to melanoma of cutaneous surfaces only. Procedures Biopsy (No Accompanying Checklist) Excision Re-excision Protocol revision date: January 2005 Based on AJCC/UICC
Melanoma of the Skin Protocol applies to melanoma of cutaneous surfaces only. Procedures Biopsy (No Accompanying Checklist) Excision Re-excision Protocol revision date: January 2005 Based on AJCC/UICC
The College of American Pathologists offers these
 CAP Laboratory Improvement Programs Protocol for the Examination of Specimens From Patients With Carcinoma of the Distal Extrahepatic Bile Ducts Mary Kay Washington, MD, PhD; Jordan Berlin, MD; Philip
CAP Laboratory Improvement Programs Protocol for the Examination of Specimens From Patients With Carcinoma of the Distal Extrahepatic Bile Ducts Mary Kay Washington, MD, PhD; Jordan Berlin, MD; Philip
Protocol for the Examination of Specimens From Patients With Carcinoma of the Distal Extrahepatic Bile Ducts
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Distal Extrahepatic Bile Ducts Protocol applies to all invasive carcinomas of the distal extrahepatic bile ducts. Well-differentiated
Protocol for the Examination of Specimens From Patients With Carcinoma of the Distal Extrahepatic Bile Ducts Protocol applies to all invasive carcinomas of the distal extrahepatic bile ducts. Well-differentiated
Protocol for the Examination of Specimens From Patients With Carcinoma of the Ampulla of Vater
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Ampulla of Vater Protocol applies to all intra-ampullary, peri-ampullary, and mixed intra- and peri-ampullary carcinomas. Well-differentiated
Protocol for the Examination of Specimens From Patients With Carcinoma of the Ampulla of Vater Protocol applies to all intra-ampullary, peri-ampullary, and mixed intra- and peri-ampullary carcinomas. Well-differentiated
Tumours of the Oesophagus & Gastro-Oesophageal Junction Histopathology Reporting Proforma
 Tumours of the Oesophagus & Gastro-Oesophageal Junction Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given
Tumours of the Oesophagus & Gastro-Oesophageal Junction Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given
The College of American Pathologists offers these
 CAP Laboratory Improvement Programs Protocol for the Examination of Specimens From Patients With Carcinoma of the Intrahepatic Bile Ducts Mary Kay Washington, MD, PhD; Jordan Berlin, MD; Philip A. Branton,
CAP Laboratory Improvement Programs Protocol for the Examination of Specimens From Patients With Carcinoma of the Intrahepatic Bile Ducts Mary Kay Washington, MD, PhD; Jordan Berlin, MD; Philip A. Branton,
Protocol for the Examination of Specimens From Patients With Carcinoma of the Esophagus
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Esophagus Protocol applies to all carcinomas of the esophagus, including esophagogastric junction carcinomas. Well-differentiated
Protocol for the Examination of Specimens From Patients With Carcinoma of the Esophagus Protocol applies to all carcinomas of the esophagus, including esophagogastric junction carcinomas. Well-differentiated
Procedures Needle Biopsy Transurethral Prostatic Resection Suprapubic or Retropubic Enucleation (Subtotal Prostatectomy) Radical Prostatectomy
 Prostate Gland Protocol applies to invasive carcinomas of the prostate gland. Protocol web posting date: July 2006 Protocol effective date: April 2007 Based on AJCC/UICC TNM, 6 th edition Procedures Needle
Prostate Gland Protocol applies to invasive carcinomas of the prostate gland. Protocol web posting date: July 2006 Protocol effective date: April 2007 Based on AJCC/UICC TNM, 6 th edition Procedures Needle
Colorectal Cancer Structured Pathology Reporting Proforma DD MM YYYY
 Colorectal Cancer Structured Pathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.03). Family name Given name(s) Date of birth DD MM YYYY S1.02 Clinical details
Colorectal Cancer Structured Pathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.03). Family name Given name(s) Date of birth DD MM YYYY S1.02 Clinical details
Protocol for the Examination of Specimens From Patients With Carcinoma of the Distal Extrahepatic Bile Ducts
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Distal Extrahepatic Bile Ducts Protocol applies to all invasive carcinomas of the distal extrahepatic bile ducts. Low-grade
Protocol for the Examination of Specimens From Patients With Carcinoma of the Distal Extrahepatic Bile Ducts Protocol applies to all invasive carcinomas of the distal extrahepatic bile ducts. Low-grade
Protocol for the Examination of Specimens From Patients With Carcinoma of the Intrahepatic Bile Ducts
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Intrahepatic Bile Ducts Protocol applies to carcinomas of the intrahepatic bile ducts and mixed hepatocellular-cholangiocarcinoma.
Protocol for the Examination of Specimens From Patients With Carcinoma of the Intrahepatic Bile Ducts Protocol applies to carcinomas of the intrahepatic bile ducts and mixed hepatocellular-cholangiocarcinoma.
NUMERATOR: Reports that include the pt category, the pn category and the histologic grade
 Quality ID #100 (NQF 0392): Colorectal Cancer Resection Pathology Reporting: pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes) with Histologic Grade National Quality Strategy Domain: Effective
Quality ID #100 (NQF 0392): Colorectal Cancer Resection Pathology Reporting: pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes) with Histologic Grade National Quality Strategy Domain: Effective
Protocol for the Examination of Specimens from Patients with Neuroendocrine Tumors (Carcinoid Tumors) of the Appendix
 Protocol for the Examination of Specimens from Patients with Neuroendocrine Tumors (Carcinoid Tumors) of the Appendix Protocol applies to well-differentiated neuroendocrine tumors of the appendix. Goblet
Protocol for the Examination of Specimens from Patients with Neuroendocrine Tumors (Carcinoid Tumors) of the Appendix Protocol applies to well-differentiated neuroendocrine tumors of the appendix. Goblet
NUMERATOR: Reports that include the pt category, the pn category and the histologic grade
 Quality ID #100 (NQF 0392): Colorectal Cancer Resection Pathology Reporting: pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes) with Histologic Grade National Quality Strategy Domain: Effective
Quality ID #100 (NQF 0392): Colorectal Cancer Resection Pathology Reporting: pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes) with Histologic Grade National Quality Strategy Domain: Effective
Thyroid Gland. Protocol applies to all malignant tumors of the thyroid gland, except lymphomas.
 Thyroid Gland Protocol applies to all malignant tumors of the thyroid gland, except lymphomas. Procedures Cytology (No Accompanying Checklist) Partial Thyroidectomy Total Thyroidectomy With/Without Lymph
Thyroid Gland Protocol applies to all malignant tumors of the thyroid gland, except lymphomas. Procedures Cytology (No Accompanying Checklist) Partial Thyroidectomy Total Thyroidectomy With/Without Lymph
Gastric Cancer Histopathology Reporting Proforma
 Gastric Cancer Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given name(s) Date of birth Sex Male Female Intersex/indeterminate
Gastric Cancer Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given name(s) Date of birth Sex Male Female Intersex/indeterminate
LOINC. Clinical information. RCPA code. Record if different to report header Operating surgeon name and contact details. Absent.
 Complete as narrative or use the structured format below 55752-0 17.02.28593 Clinical information 22027-7 17.02.30001 Record if different to report header Operating surgeon name and contact details 52101004
Complete as narrative or use the structured format below 55752-0 17.02.28593 Clinical information 22027-7 17.02.30001 Record if different to report header Operating surgeon name and contact details 52101004
Case history: Figure 1. H&E, 5x. Figure 2. H&E, 20x.
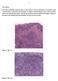 1 Case history: A 49 year-old female presented with a 5 year history of chronic anal fissure. The patient s past medical history is otherwise unremarkable. On digital rectal examination there was a very
1 Case history: A 49 year-old female presented with a 5 year history of chronic anal fissure. The patient s past medical history is otherwise unremarkable. On digital rectal examination there was a very
8. The polyp in the illustration can be described as (circle all that apply) a. Exophytic b. Pedunculated c. Sessile d. Frank
 Quiz 1 Overview 1. Beginning with the cecum, which is the correct sequence of colon subsites? a. Cecum, ascending, splenic flexure, transverse, hepatic flexure, descending, sigmoid. b. Cecum, ascending,
Quiz 1 Overview 1. Beginning with the cecum, which is the correct sequence of colon subsites? a. Cecum, ascending, splenic flexure, transverse, hepatic flexure, descending, sigmoid. b. Cecum, ascending,
STAGE CATEGORY DEFINITIONS
 CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX Tis Tis (DCIS) Tis (LCIS) Tis (Paget s) T1 T1mi T1a T1b T1c a b c
CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX Tis Tis (DCIS) Tis (LCIS) Tis (Paget s) T1 T1mi T1a T1b T1c a b c
Descriptor Definition Author s notes TNM descriptors Required only if applicable; select all that apply multiple foci of invasive carcinoma
 S5.01 The tumour stage and stage grouping must be recorded to the extent possible, based on the AJCC Cancer Staging Manual (7 th Edition). 11 (See Tables S5.01a and S5.01b below.) Table S5.01a AJCC breast
S5.01 The tumour stage and stage grouping must be recorded to the extent possible, based on the AJCC Cancer Staging Manual (7 th Edition). 11 (See Tables S5.01a and S5.01b below.) Table S5.01a AJCC breast
C ORPUS UTERI C ARCINOMA STAGING FORM (Carcinosarcomas should be staged as carcinomas)
 CLINICAL C ORPUS UTERI C ARCINOMA STAGING FORM PATHOLOGIC Extent of disease before S TAGE C ATEGORY D EFINITIONS Extent of disease through any treatment completion of definitive surgery y clinical staging
CLINICAL C ORPUS UTERI C ARCINOMA STAGING FORM PATHOLOGIC Extent of disease before S TAGE C ATEGORY D EFINITIONS Extent of disease through any treatment completion of definitive surgery y clinical staging
Carcinoma of the Renal Pelvis and Ureter Histopathology
 Carcinoma of the Renal Pelvis and Ureter Histopathology Reporting Proforma (NEPHROURETERECTOMY AND URETERECTOMY) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
Carcinoma of the Renal Pelvis and Ureter Histopathology Reporting Proforma (NEPHROURETERECTOMY AND URETERECTOMY) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
Protocol for the Examination of Specimens From Patients With Squamous Cell Carcinoma of the Skin
 Protocol for the Examination of Specimens From Patients With Squamous Cell Carcinoma of the Skin Protocol applies to invasive squamous cell carcinomas of the skin. Squamous cell carcinomas of the eyelid,
Protocol for the Examination of Specimens From Patients With Squamous Cell Carcinoma of the Skin Protocol applies to invasive squamous cell carcinomas of the skin. Squamous cell carcinomas of the eyelid,
Definition of Synoptic Reporting
 Definition of Synoptic Reporting The CAP has developed this list of specific features that define synoptic reporting formatting: 1. All required cancer data from an applicable cancer protocol that are
Definition of Synoptic Reporting The CAP has developed this list of specific features that define synoptic reporting formatting: 1. All required cancer data from an applicable cancer protocol that are
L ARYNX S TAGING F ORM
 CLI N I CA L Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX T0 Tis a b L ARYNX S TAGING F ORM LATERALITY: TUMOR SIZE: left
CLI N I CA L Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX T0 Tis a b L ARYNX S TAGING F ORM LATERALITY: TUMOR SIZE: left
Update on staging colorectal carcinoma, the 8 th edition AJCC. General overview of staging. When is staging required? 11/1/2017
 Update on staging colorectal carcinoma, the 8 th edition AJCC Dale C. Snover, MD November 3, 2017 General overview of staging Reason for uniform staging Requirements to use AJCC manual and/or CAP protocols
Update on staging colorectal carcinoma, the 8 th edition AJCC Dale C. Snover, MD November 3, 2017 General overview of staging Reason for uniform staging Requirements to use AJCC manual and/or CAP protocols
Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Colon and Rectum
 Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Colon and Rectum Well-differentiated neuroendocrine neoplasms (carcinoid tumors) are not included. Based on AJCC/UICC
Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Colon and Rectum Well-differentiated neuroendocrine neoplasms (carcinoid tumors) are not included. Based on AJCC/UICC
Carcinoma of the Urinary Bladder Histopathology
 Carcinoma of the Urinary Bladder Histopathology Reporting Proforma (Radical & Partial Cystectomy, Cystoprostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
Carcinoma of the Urinary Bladder Histopathology Reporting Proforma (Radical & Partial Cystectomy, Cystoprostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
Protocol for the Examination of Specimens from Patients with Carcinoma of the Gallbladder
 Protocol for the Examination of Specimens from Patients with Carcinoma of the Gallbladder Protocol applies to all invasive carcinomas of the gallbladder and cystic duct, including those showing focal endocrine
Protocol for the Examination of Specimens from Patients with Carcinoma of the Gallbladder Protocol applies to all invasive carcinomas of the gallbladder and cystic duct, including those showing focal endocrine
Extrahepatic Bile Ducts
 Extrahepatic Bile Ducts Protocol applies to all invasive carcinomas of the extrahepatic bile ducts. Sarcomas and carcinoid tumors are excluded. Protocol revision date: January 2005 Based on AJCC/UICC TNM,
Extrahepatic Bile Ducts Protocol applies to all invasive carcinomas of the extrahepatic bile ducts. Sarcomas and carcinoid tumors are excluded. Protocol revision date: January 2005 Based on AJCC/UICC TNM,
Uveal Melanoma. Protocol applies to malignant melanoma of the uvea.
 Uveal Melanoma Protocol applies to malignant melanoma of the uvea. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist) Biopsy (No Accompanying
Uveal Melanoma Protocol applies to malignant melanoma of the uvea. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist) Biopsy (No Accompanying
Protocol for the Examination of Specimens From Patients With Neuroendocrine Tumors (Carcinoid Tumors) of the Stomach
 Protocol for the Examination of Specimens From Patients With Neuroendocrine Tumors (Carcinoid Tumors) of the Stomach Protocol applies to well-differentiated neuroendocrine tumors of the stomach. Carcinomas
Protocol for the Examination of Specimens From Patients With Neuroendocrine Tumors (Carcinoid Tumors) of the Stomach Protocol applies to well-differentiated neuroendocrine tumors of the stomach. Carcinomas
Protocol for the Examination of Specimens from Patients with Carcinoma of the Vulva
 Protocol for the Examination of Specimens from Patients with Carcinoma of the Vulva Protocol applies to all invasive carcinomas of the vulva. Based on AJCC/UICC TNM, 7th edition, and FIGO 2008 Annual Report
Protocol for the Examination of Specimens from Patients with Carcinoma of the Vulva Protocol applies to all invasive carcinomas of the vulva. Based on AJCC/UICC TNM, 7th edition, and FIGO 2008 Annual Report
Protocol applies to specimens from patients with Wilms tumor (nephroblastoma) or other renal tumors of childhood.
 Wilms Tumor Protocol applies to specimens from patients with Wilms tumor (nephroblastoma) or other renal tumors of childhood. Procedures Cytology (No Accompanying Checklist) Incisional Biopsy (Needle or
Wilms Tumor Protocol applies to specimens from patients with Wilms tumor (nephroblastoma) or other renal tumors of childhood. Procedures Cytology (No Accompanying Checklist) Incisional Biopsy (Needle or
Protocol for the Examination of Specimens From Patients With Carcinoma of the Gallbladder
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Gallbladder Protocol applies to all invasive carcinomas of the gallbladder and cystic duct, including those showing focal endocrine
Protocol for the Examination of Specimens From Patients With Carcinoma of the Gallbladder Protocol applies to all invasive carcinomas of the gallbladder and cystic duct, including those showing focal endocrine
Protocol for the Examination of Specimens From Patients With Carcinoma of the Perihilar Bile Ducts
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Perihilar Bile Ducts Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging
Protocol for the Examination of Specimens From Patients With Carcinoma of the Perihilar Bile Ducts Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging
Question: If in a particular case, there is doubt about the correct T, N or M category, what do you do?
 Exercise 1 Question: If in a particular case, there is doubt about the correct T, N or M category, what do you do? : 1. I mention both categories that are in consideration, e.g. pt1-2 2. I classify as
Exercise 1 Question: If in a particular case, there is doubt about the correct T, N or M category, what do you do? : 1. I mention both categories that are in consideration, e.g. pt1-2 2. I classify as
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GYNECOLOGIC CANCER VULVAR
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GYNECOLOGIC CANCER VULVAR Last Revision Date July 2015 1 Site Group: Gynecologic Cancer Vulvar Author: Dr. Stephane Laframboise 1. INTRODUCTION
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GYNECOLOGIC CANCER VULVAR Last Revision Date July 2015 1 Site Group: Gynecologic Cancer Vulvar Author: Dr. Stephane Laframboise 1. INTRODUCTION
This form may provide more data elements than required for collection by standard setters such as NCI SEER, CDC NPCR, and CoC NCDB.
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
VULVAR CARCINOMA. Page 1 of 5
 VULVAR CARCINOMA EXAMPLE OF A VULVAR CARCINOMA USING PROPOSED TEMPLATE Case: Invasive squamous cell carcinoma arising in D-VIN Tumor in left labia major Left partial vaginectomy and sentinel lymph node
VULVAR CARCINOMA EXAMPLE OF A VULVAR CARCINOMA USING PROPOSED TEMPLATE Case: Invasive squamous cell carcinoma arising in D-VIN Tumor in left labia major Left partial vaginectomy and sentinel lymph node
Retinoblastoma. Protocol applies to retinoblastoma only.
 Retinoblastoma Protocol applies to retinoblastoma only. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist) Biopsy (No Accompanying
Retinoblastoma Protocol applies to retinoblastoma only. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist) Biopsy (No Accompanying
Staging Challenges in Lower GI Cancers. Disclosure of Relevant Financial Relationships. AJCC 8 th edition and CAP protocol updates
 Staging Challenges in Lower GI Cancers Sanjay Kakar, MD University of California, San Francisco March 05, 2017 Disclosure of Relevant Financial Relationships USCAP requires that all planners (Education
Staging Challenges in Lower GI Cancers Sanjay Kakar, MD University of California, San Francisco March 05, 2017 Disclosure of Relevant Financial Relationships USCAP requires that all planners (Education
Small Intestine. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition
 Small Intestine Protocol applies to all invasive carcinomas of the small intestine, including those with focal endocrine differentiation. Excludes carcinoid tumors, lymphomas, and stromal tumors (sarcomas).
Small Intestine Protocol applies to all invasive carcinomas of the small intestine, including those with focal endocrine differentiation. Excludes carcinoid tumors, lymphomas, and stromal tumors (sarcomas).
This form may provide more data elements than required for collection by standard setters such as NCI SEER, CDC NPCR, and CoC NCDB.
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
Protocol for the Examination of Specimens From Patients With Carcinoma of the Esophagus
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Esophagus Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual For
Protocol for the Examination of Specimens From Patients With Carcinoma of the Esophagus Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual For
AJCC Cancer Staging 8 th Edition
 AJCC Cancer Staging 8 th Edition Colon and Rectal Cancer Staging Update Webinar George J Chang, MD, MS Deputy Chair, Department of Surgical Oncology Chief, Colon and Rectal Surgery Professor of Surgical
AJCC Cancer Staging 8 th Edition Colon and Rectal Cancer Staging Update Webinar George J Chang, MD, MS Deputy Chair, Department of Surgical Oncology Chief, Colon and Rectal Surgery Professor of Surgical
Testis. Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies.
 Testis Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies. Protocol revision date: January 2005 Based on AJCC/UICC
Testis Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies. Protocol revision date: January 2005 Based on AJCC/UICC
Protocol for the Examination of Specimens from Patients with Primary Carcinoma of the Colon and Rectum
 Protocol for the Examination of Specimens from Patients with Primary Carcinoma of the Colon and Rectum Well-differentiated neuroendocrine neoplasms (carcinoid tumors) are not included. This modified NB
Protocol for the Examination of Specimens from Patients with Primary Carcinoma of the Colon and Rectum Well-differentiated neuroendocrine neoplasms (carcinoid tumors) are not included. This modified NB
Greater Manchester & Cheshire Guidelines for Pathology Reporting for Oesophageal and Gastric Malignancy
 Greater Manchester & Cheshire Guidelines for Pathology Reporting for Oesophageal and Gastric Malignancy Authors: Dr Gordon Armstrong, Dr Sue Pritchard 1. General Comments 1.1 Cancer reporting: Biopsies
Greater Manchester & Cheshire Guidelines for Pathology Reporting for Oesophageal and Gastric Malignancy Authors: Dr Gordon Armstrong, Dr Sue Pritchard 1. General Comments 1.1 Cancer reporting: Biopsies
M ALIGNANT MELANOMA OF THE UVEA STAGING FORM
 CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX T0 a a a d b c d a TUMOR SIZE: S TAGE C ATEGORY D EFINITIONS LATERALITY:
CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX T0 a a a d b c d a TUMOR SIZE: S TAGE C ATEGORY D EFINITIONS LATERALITY:
6. Cervical Lymph Nodes and Unknown Primary Tumors of the Head and Neck
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
Protocol for the Examination of Specimens from Patients with Carcinoma of the Appendix
 Protocol for the Examination of Specimens from Patients with Carcinoma of the Appendix Protocol applies to all carcinomas arising in the vermiform appendix, including goblet cell carcinoid tumors. Other
Protocol for the Examination of Specimens from Patients with Carcinoma of the Appendix Protocol applies to all carcinomas arising in the vermiform appendix, including goblet cell carcinoid tumors. Other
Protocol for the Examination of Specimens From Patients With Carcinoma of the Urethra and Periurethral Glands
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Urethra and Periurethral Glands Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition,
Protocol for the Examination of Specimens From Patients With Carcinoma of the Urethra and Periurethral Glands Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition,
Protocol for the Examination of Specimens from Patients With Carcinoma of the Distal Extrahepatic Bile Ducts
 Protocol for the Examination of Specimens from Patients With Carcinoma of the Distal Extrahepatic Bile Ducts Version: DistalExtrahepaticBileDucts 4.0.0.1 Protocol Posting Date: June 2017 Includes ptnm
Protocol for the Examination of Specimens from Patients With Carcinoma of the Distal Extrahepatic Bile Ducts Version: DistalExtrahepaticBileDucts 4.0.0.1 Protocol Posting Date: June 2017 Includes ptnm
Non-Hodgkin Lymphoma. Protocol applies to non-hodgkin lymphoma involving any organ system except the gastrointestinal tract.
 Non-Hodgkin Lymphoma Protocol applies to non-hodgkin lymphoma involving any organ system except the gastrointestinal tract. Protocol revision date: January 2005 No AJCC/UICC staging system Procedures Cytology
Non-Hodgkin Lymphoma Protocol applies to non-hodgkin lymphoma involving any organ system except the gastrointestinal tract. Protocol revision date: January 2005 No AJCC/UICC staging system Procedures Cytology
S1.04 Principal clinician. G1.01 Comments. G2.01 *Specimen dimensions (prostate) S2.02 *Seminal vesicles
 Prostate Cancer Histopathology Reporting Proforma (Radical Prostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family name Given name(s) Date of birth Sex Male
Prostate Cancer Histopathology Reporting Proforma (Radical Prostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family name Given name(s) Date of birth Sex Male
Protocol for the Examination of Specimens From Patients With Carcinoma of the Vagina
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Vagina Protocol applies to all invasive carcinomas of the vagina. Based on AJCC/UICC TNM, 7th edition, and FIGO 2006 Annual
Protocol for the Examination of Specimens From Patients With Carcinoma of the Vagina Protocol applies to all invasive carcinomas of the vagina. Based on AJCC/UICC TNM, 7th edition, and FIGO 2006 Annual
11/21/13 CEA: 1.7 WNL
 Case Scenario 1 A 70 year-old white male presented to his primary care physician with a recent history of rectal bleeding. He was referred for imaging and a colonoscopy and was found to have adenocarcinoma.
Case Scenario 1 A 70 year-old white male presented to his primary care physician with a recent history of rectal bleeding. He was referred for imaging and a colonoscopy and was found to have adenocarcinoma.
Protocol for the Examination of Specimens from Patients with Neuroendocrine Tumors (Carcinoid Tumors) of the Small Intestine and Ampulla
 Protocol for the Examination of Specimens from Patients with Neuroendocrine Tumors (Carcinoid Tumors) of the Small Intestine and Ampulla Protocol applies to well-differentiated neuroendocrine tumors of
Protocol for the Examination of Specimens from Patients with Neuroendocrine Tumors (Carcinoid Tumors) of the Small Intestine and Ampulla Protocol applies to well-differentiated neuroendocrine tumors of
Staging for Residents, Nurses, and Multidisciplinary Health Care Team
 Staging for Residents, Nurses, and Multidisciplinary Health Care Team Donna M. Gress, RHIT, CTR Validating science. Improving patient care. Learning Objectives Introduce the concept and history of stage
Staging for Residents, Nurses, and Multidisciplinary Health Care Team Donna M. Gress, RHIT, CTR Validating science. Improving patient care. Learning Objectives Introduce the concept and history of stage
Disclosures. Outline. What IS tumor budding?? Tumor Budding in Colorectal Carcinoma: What, Why, and How. I have nothing to disclose
 Tumor Budding in Colorectal Carcinoma: What, Why, and How Disclosures I have nothing to disclose Soo-Jin Cho, MD, PhD Assistant Professor UCSF Dept of Pathology Current Issues in Anatomic Pathology 2017
Tumor Budding in Colorectal Carcinoma: What, Why, and How Disclosures I have nothing to disclose Soo-Jin Cho, MD, PhD Assistant Professor UCSF Dept of Pathology Current Issues in Anatomic Pathology 2017
Protocol for the Examination of Specimens From Patients With Carcinoma of the Stomach
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Stomach Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual For
Protocol for the Examination of Specimens From Patients With Carcinoma of the Stomach Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual For
Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Colon and Rectum
 Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Colon and Rectum Well-differentiated neuroendocrine neoplasms (carcinoid tumors) are not included. Based on AJCC/UICC
Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Colon and Rectum Well-differentiated neuroendocrine neoplasms (carcinoid tumors) are not included. Based on AJCC/UICC
C ORPUS UTERI C ARCINOMA STAGING FORM (Carcinosarcomas should be staged as carcinomas)
 C ORPUS UTERI C ARCINOMA STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery Tis * T1 I T1a IA NX N0 N1 N2
C ORPUS UTERI C ARCINOMA STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery Tis * T1 I T1a IA NX N0 N1 N2
Vulvar Carcinoma. Definition: Cases should be classified as carsinoma of the vulva when the primary site growth is in the vulva Malignant melanoma sho
 Carcinoma Vulva & Vagina Subdivisi Onkologi Ginekologi Bagian Obgin FK USU Vulvar Carcinoma. Definition: Cases should be classified as carsinoma of the vulva when the primary site growth is in the vulva
Carcinoma Vulva & Vagina Subdivisi Onkologi Ginekologi Bagian Obgin FK USU Vulvar Carcinoma. Definition: Cases should be classified as carsinoma of the vulva when the primary site growth is in the vulva
Protocol for the Examination of Specimens from Patients with Primary Carcinomas of the Colon and Rectum
 Protocol for the Examination of Specimens from Patients with Primary Carcinomas of the Colon and Rectum Well differentiated neuroendocrine neoplasms (carcinoid tumors) are not included. Based on AJCC/UICC
Protocol for the Examination of Specimens from Patients with Primary Carcinomas of the Colon and Rectum Well differentiated neuroendocrine neoplasms (carcinoid tumors) are not included. Based on AJCC/UICC
Protocol for the Examination of Specimens From Patients With Gastrointestinal Stromal Tumor (GIST)
 Protocol for the Examination of Specimens From Patients With Gastrointestinal Stromal Tumor (GIST) Based on AJCC/UICC TNM, 7 th edition Protocol web posting date: December 2014 Procedures Biopsy Resection
Protocol for the Examination of Specimens From Patients With Gastrointestinal Stromal Tumor (GIST) Based on AJCC/UICC TNM, 7 th edition Protocol web posting date: December 2014 Procedures Biopsy Resection
S1.04 PRINCIPAL CLINICIAN G1.01 COMMENTS S2.01 SPECIMEN LABELLED AS G2.01 *SPECIMEN DIMENSIONS (PROSTATE) S2.03 *SEMINAL VESICLES
 Prostate Cancer Histopathology Reporting Proforma (Radical Prostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family name Given name(s) Date of birth Indigenous
Prostate Cancer Histopathology Reporting Proforma (Radical Prostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family name Given name(s) Date of birth Indigenous
3/23/2017. Disclaimer. Common Changes in TNM Staging. Overview. Overview. Understanding Terminology. Overview
 Common Changes in TNM Staging Understanding the General Rules of Cancer Staging Thomas P. Baker, MD FCAP March 5, 2017 Disclaimer The identification of specific products or scientific instrumentation is
Common Changes in TNM Staging Understanding the General Rules of Cancer Staging Thomas P. Baker, MD FCAP March 5, 2017 Disclaimer The identification of specific products or scientific instrumentation is
INTRODUCTION TO CANCER STAGING
 INTRODUCTION TO CANCER STAGING Patravoot Vatanasapt, MD Dept. Otorhinolaryngology Khon Kaen Cancer Registry Faculty of Medicine Khon Kaen University THAILAND Staging is the attempt to assess the size
INTRODUCTION TO CANCER STAGING Patravoot Vatanasapt, MD Dept. Otorhinolaryngology Khon Kaen Cancer Registry Faculty of Medicine Khon Kaen University THAILAND Staging is the attempt to assess the size
Colon and Rectum. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6th edition
 Colon and Rectum Protocol applies to all invasive carcinomas of the colon and rectum. Carcinoid tumors, lymphomas, sarcomas, and tumors of the vermiform appendix are excluded. Protocol revision date: January
Colon and Rectum Protocol applies to all invasive carcinomas of the colon and rectum. Carcinoid tumors, lymphomas, sarcomas, and tumors of the vermiform appendix are excluded. Protocol revision date: January
46. Merkel Cell Carcinoma
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
Stage: The Language of Cancer
 Stage: The Language of Cancer American Joint Committee on Cancer American College of Surgeons Chicago, IL Validating science. Improving patient care. No materials in this presentation may be repurposed
Stage: The Language of Cancer American Joint Committee on Cancer American College of Surgeons Chicago, IL Validating science. Improving patient care. No materials in this presentation may be repurposed
Protocol for the Examination of Specimens From Patients With Merkel Cell Carcinoma of the Skin
 Protocol for the Examination of Specimens From Patients With Merkel Cell Carcinoma of the Skin Protocol applies to Merkel cell carcinoma of cutaneous surfaces only. Based on AJCC/UICC TNM, 7th edition
Protocol for the Examination of Specimens From Patients With Merkel Cell Carcinoma of the Skin Protocol applies to Merkel cell carcinoma of cutaneous surfaces only. Based on AJCC/UICC TNM, 7th edition
CODING STAGE: TNM AND OTHER STAGING SYSTEMS. Liesbet Van Eycken Otto Visser
 CODING STAGE: TNM AND OTHER STAGING SYSTEMS Liesbet Van Eycken Otto Visser OVERVIEW PART I Introduction What is stage? Why stage? History and publications of TNM Classification Clinical and pathologic
CODING STAGE: TNM AND OTHER STAGING SYSTEMS Liesbet Van Eycken Otto Visser OVERVIEW PART I Introduction What is stage? Why stage? History and publications of TNM Classification Clinical and pathologic
Urinary Bladder, Ureter, and Renal Pelvis
 Urinary Bladder, Ureter, and Renal Pelvis Protocol applies to all carcinomas of the urinary bladder, ureter, and renal pelvis. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6th edition Procedures
Urinary Bladder, Ureter, and Renal Pelvis Protocol applies to all carcinomas of the urinary bladder, ureter, and renal pelvis. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6th edition Procedures
Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Vagina
 Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Vagina Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual,
Protocol for the Examination of Specimens From Patients With Primary Carcinoma of the Vagina Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual,
Histopathology of Endoscopic Resection Specimens from Barrett's Esophagus
 Histopathology of Endoscopic Resection Specimens from Barrett's Esophagus Br J Surg 38 oct. 1950 Definition of Barrett's esophagus A change in the esophageal epithelium of any length that can be recognized
Histopathology of Endoscopic Resection Specimens from Barrett's Esophagus Br J Surg 38 oct. 1950 Definition of Barrett's esophagus A change in the esophageal epithelium of any length that can be recognized
Protocol for the Examination of Specimens From Patients With Carcinoma of the Appendix
 Protocol for the Examination of Specimens From Patients With Carcinoma of the Appendix Protocol applies to all carcinomas arising in the vermiform appendix, including goblet cell carcinoid tumors. Other
Protocol for the Examination of Specimens From Patients With Carcinoma of the Appendix Protocol applies to all carcinomas arising in the vermiform appendix, including goblet cell carcinoid tumors. Other
Purpose. Encourage standard exchange of data between two key public health partners
 Reporting Pathology Protocols for Colorectal Cancer 2005 NAACCR Conference: June 9, 2005 Ken Gerlach: CDC-NPCR Bette Smith: Ohio Cancer Registry Kathleen Davidson-Allen: PHI/California Cancer Registry
Reporting Pathology Protocols for Colorectal Cancer 2005 NAACCR Conference: June 9, 2005 Ken Gerlach: CDC-NPCR Bette Smith: Ohio Cancer Registry Kathleen Davidson-Allen: PHI/California Cancer Registry
Protocol for the Examination of Specimens From Patients With Thymoma and Thymic Carcinoma
 Protocol for the Examination of Specimens From Patients With Thymoma and Thymic Carcinoma Protocol applies to thymic epithelial tumors located in any area of the mediastinum. No AJCC/UICC TNM Staging System
Protocol for the Examination of Specimens From Patients With Thymoma and Thymic Carcinoma Protocol applies to thymic epithelial tumors located in any area of the mediastinum. No AJCC/UICC TNM Staging System
A916: rectum: adenocarcinoma
 General facts of colorectal cancer The colon has cecum, ascending, transverse, descending and sigmoid colon sections. Cancer can start in any of the r sections or in the rectum. The wall of each of these
General facts of colorectal cancer The colon has cecum, ascending, transverse, descending and sigmoid colon sections. Cancer can start in any of the r sections or in the rectum. The wall of each of these
Endometrium. Protocol applies to all carcinomas of the endometrium. Procedures Cytology (No Accompanying Checklist) Biopsy Curettage Hysterectomy
 Endometrium Protocol applies to all carcinomas of the endometrium. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
Endometrium Protocol applies to all carcinomas of the endometrium. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
Rectal Cancer Cookbook Update. A. JOURET-MOURIN with the collaboration of A Hoorens,P Demetter, G De Hertogh,C Cuvelier and C Sempoux
 Rectal Cancer Cookbook Update A. JOURET-MOURIN with the collaboration of A Hoorens,P Demetter, G De Hertogh,C Cuvelier and C Sempoux Prof Dr A Jouret-Mourin, Department of Pathology, UCL, St Luc, Brussels
Rectal Cancer Cookbook Update A. JOURET-MOURIN with the collaboration of A Hoorens,P Demetter, G De Hertogh,C Cuvelier and C Sempoux Prof Dr A Jouret-Mourin, Department of Pathology, UCL, St Luc, Brussels
10. HPV-Mediated (p16+) Oropharyngeal Cancer
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
Standards and datasets for reporting cancers Dataset for histopathological reporting of anal cancer March 2018
 Standards and datasets for reporting cancers Dataset for histopathological reporting of anal cancer March 2018 Authors: Dr Morgan Moorghen, St Mark s Hospital, London North West Healthcare NHS Trust Dr
Standards and datasets for reporting cancers Dataset for histopathological reporting of anal cancer March 2018 Authors: Dr Morgan Moorghen, St Mark s Hospital, London North West Healthcare NHS Trust Dr
