Anticancer cellular immunotherapies derived from umbilical cord blood
|
|
|
- Nelson Phillips
- 5 years ago
- Views:
Transcription
1 Expert Opinion on Biological Therapy ISSN: (Print) (Online) Journal homepage: Anticancer cellular immunotherapies derived from umbilical cord blood Katalin Balassa & Vanderson Rocha To cite this article: Katalin Balassa & Vanderson Rocha (2017): Anticancer cellular immunotherapies derived from umbilical cord blood, Expert Opinion on Biological Therapy, DOI: / To link to this article: Accepted author version posted online: 06 Nov Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at Download by: [Cornell University Library] Date: 07 November 2017, At: 12:12
2 Publisher: Taylor & Francis Journal: Expert Opinion on Biological Therapy DOI: / Anticancer cellular immunotherapies derived from umbilical cord blood Katalin Balassa 1,2, Vanderson Rocha 1,2, 3 Affiliations: 1 Oxford University Hospitals NHS Foundation Trust, Department of Clinical Haematology, Cancer and Haematology Centre, Churchill Hospital, Old Road, Headington, Oxford, OX3 7LE, UK 2 NHS Blood and Transplant, Oxford, UK 3 Department of Haematology, University of Sao Paulo, Sao Paulo, Brazil Correspondence: Katalin Balassa MD, Oxford University Hospitals NHS Foundation Trust, Department of Clinical Haematology, Cancer and Haematology Centre, Churchill Hospital, Old Road, Headington, Oxford, OX3 7LE, UK Telephone: Fax number: katalin.balassa@ouh.nhs.uk Abstract Introduction The lack of highly effective drugs in many malignancies has prompted scientific interest in the development of alternative treatment strategies. Cellular immunotherapy involving the
3 adoptive transfer of immune cells that potently recognize and eliminate malignantly transformed cells has become a promising new tool in the anticancer armory. Studies suggest that the unique biological properties of umbilical cord blood (UCB) cells could precipitate enhanced anticancer activity; hence, UCB could be an optimal source for immunotherapy with the potential to provide products with off-the-shelf availability. Areas covered In this review, the authors summarize data on the transfer of naturally occurring or genetically modified UCB cells to treat cancer. The focus within is on the phenotypic and functional differences compared to other sources, the alloreactive and anticancer properties, and manufacturing of these products. Therapies utilizing cytokine-induced killer (CIK) cells, natural killer (NK) cells and chimeric antigen receptor (CAR) T-cells, are also discussed. Expert opinion The cellular immunotherapy field has become a growing, exciting area that has generated much enthusiasm. There is evidence that anticancer immunotherapy with UCB-derived products is feasible and safe; however, considering the limited number of clinical trials using UCB-derived products, further studies are warranted to facilitate translation into clinical practice. Keywords: Anticancer immunotherapy, umbilical cord blood, cytokine-induced killer (CIK) cells, natural killer (NK) cells, chimeric antigen receptor (CAR) T cells. Article highlights: The rationale behind anticancer cellular immunotherapy is the ability of immune cells to recognize and eliminate malignantly transformed cells.
4 Umbilical cord blood (UCB) is widely used as an alternative stem cell source for allogeneic hematopoietic stem cell transplantation. Its abundance of progenitor cells with anticancer potential makes UCB attractive for immunotherapy development. Techniques have been established to overcome the limited number and immaturity of UCB cells. Data suggest that activated UCB-derived cells might mediate superior tumor-killing activity compared with other sources. Cytokine-induced killer (CIK) cells are a heterogeneous population of cells originating from T-lymphocytes that exhibit anticancer activity in a non-major histocompatibility complex (MHC)-restricted manner. Experience with UCB-derived CIK cells in a limited number of cancer patients confirms some clinical effectiveness. Natural killer (NK) cells are CD56+/CD3- lymphocytes of the innate immune system that play a fundamental role in cancer surveillance. Studies utilizing UCB-derived NK cells confirm the feasibility and safety of the modality, and further studies are under way to investigate its efficacy. Patients with acute lymphoblastic leukemia have achieved excellent complete remission rates with genetically engineered T-cell therapy. The modality has been intensively tested for other diseases. UCB-derived T-cells can be successfully modified to express chimeric antigen receptors, and results from clinical trials are awaited.
5 Universal, Good Manufacturing Practice-compliant, off-the-shelf immunotherapy products might become available in the near future, enabling wider access to cellular immunotherapy.
6 1. Introduction Umbilical cord blood transplantation (UCBT) has been successfully used to cure malignant and non-malignant diseases for decades; and due to the lower immunogenicity of the graft, a higher degree of human leucocyte antigen (HLA)-mismatch between the recipient and donor can be tolerated [1]. Although one could hypothesize that the lower graft-versus-host disease (GVHD) incidence could be accompanied with less effective graft-versus-leukemia (GVL) responses and a higher relapse rate, studies confirm that the transplanted umbilical cord blood (UCB) cells are capable of inducing potent antitumor reactions [2, 3]. To overcome delayed engraftment following UCBT secondary to limited cell dose, expansion of UCB-derived hematopoietic stem and progenitor cells and cellular engineering have been safely employed [4, 5, 6, 7]. Ex-vivo expanded UCB progenitor cells have not only been used to promote engraftment, but also to accelerate hematopoietic recovery in patients undergoing myelosuppressive chemotherapy [8]. Experiences with UCBT prompted further research outside the field of allogeneic hematopoietic stem cell transplantation (allo-hsct), and it has been proposed that UCBderived cells could be beneficial resources for anticancer immunotherapy. UCB is a rich source of many progenitor and mature cells with significant therapeutic potential as demonstrated in Figure 1 [9, 10, 11, 12, 13, 14, 15]. Cellular immunotherapy includes the transfer of natural killer (NK) cells, T-cells, or other cells with natural or genetically transferred tumor-killing ability [16, 17]. Although the nucleated cell dose in each UCB unit is limited, techniques have advanced to enable a greater than 500-fold expansion ex-vivo in compliance with Good Manufacturing Practice (GMP) [4, 5, 18]. UCB cells exhibit inferior baseline cytotoxicity compared with other sources; but this can be overcome by cytokine stimulation, which significantly increases the tumor-eradicating potential. In fact, murine studies suggest that UCB T-cells mediate enhanced tumor rejection compared with peripheral
7 blood (PB) T-cells [19]. By cryopreservation of ex-vivo expanded UCB cells, off-the-shelf products can be manufactured, allowing immediate availability for immunotherapy. UCB provides additional benefits for immunotherapy purposes, such as no risk of donor attrition or collection failure, decreased risk of viral transmission, lower alloreactivity, and rapid availability. The advantages and disadvantages of UCB-derived cellular immunotherapy are listed in Table 1 [5, 13, 19, 20, 21]. This review discusses the characteristics of cytokine-induced killer (CIK) cells (section 2.), natural killer (NK) cells (section 3.) and chimeric antigen receptor (CAR) T-cells (section 4.) with special view to products derived from UCB in comparison with other sources. Following a short introduction in the first subsection of section 2. and 3., the ex-vivo expansion techniques to overcome the small volume and limited cell numbers and the activation techniques to deal with immaturity of umbilical cord blood cells are discussed. In the second and third parts of the sections authors describe the anti-cancer activity and the allogeneic host reactions delivered by the cells. In the last subsections clinical applications and results are detailed. Although UCB-derived CAR T-cells have not been used in clinical trials yet, given their anticipated great future potential, authors briefly discuss the development and clinical experience with CAR T-cells in the last section (section 4.) of the review. The detailed discussion of all UCB-derived cellular products has been beyond the scope of this review. Hence, cell-based cancer vaccine therapies, dendritic cell, mesenchymal stem cell and non- CAR T-cell immunotherapies are not described in this paper. 2. Cytokine-induced killer cells Cytokine-induced killer (CIK) cells are a heterogeneous population of cells that originate from T-lymphocytes and exhibit strong anticancer activity in a non-major histocompatibility complex (MHC)-restricted fashion [22, 23, 24]. Their precursors can be obtained from various
8 sources, including peripheral blood (PB), bone marrow and UCB [24, 25, 26]. CIK cells contain three main subpopulations of cells with T/NK phenotype, the CD3+/CD56+, CD3+/CD56- and CD3-/CD56+ cells [26]. Following in-vitro culture, the majority of cells belong to the unique subpopulation of CD3+/CD56+ cells co-expressing NK and T markers that possess the strongest killing potential among all CIK cell types [24]. CIK cells have been increasingly used in the clinical setting due to their easy availability, wide efficacy against several tumor types and low toxicity as reviewed previously in this journal [27] In-vitro expansion and activation of CIK cells The Schmidt-Wolf group that first documented the potent anticancer activity of CIK cells successfully expanded cells derived from PB mononuclear cells by 754-fold using a cocktail of anti-cd3 monoclonal antibody, interleukin (IL)-1, IL-2, and interferon-gamma (IFNγ) [22]. Anti-CD3 promotes proliferation by providing mitogenic stimulus, which is then sustained by other cytokines [28]. IFNγ activates monocytes that relay signals for expansion, activation, and differentiation of CIK cells, leading to augmented cytotoxicity [28, 29]. The majority of in-vitro expanded CIK cells belong to the CD3+/CD56+ population that originates from CD3+/CD56- cells [24]. The above protocol has become the classical one for the expansion of CIK cells; and subsequent application has achieved variable, up to more than a 1000-fold expansion of CD3+/CD56+ cells following 2-3 weeks incubation [25, 30]. Expansion of CIK cells from cancer patients yields less robust results, and the NK-type CD3- /CD56+ cells expand poorly [24, 26, 31]. This protocol was also satisfyingly implemented for expansion of UCB-derived cells, but some groups introduced modifications [21, 25, 32, 33]. IL-15 has recently been used as an additive instead of IL-2, given that CIK cells can be generated more quickly with stronger cytotoxic potential by using this method [34]. An additional advantage of IL-15 is the
9 reduction of regulatory T-cells that inhibit the antitumor activity of CIK cells [35, 36]. IL-7 similarly increases cytotoxicity [37]. In co-culture of stem cell factor, FLT3 ligand, IL-2, IL- 7, and IL-15 Li et al. expanded the CD3+/CD56+ population by 796-fold and the CD3- /CD56+ population by 37-fold from UCB [38]. Introna et al. proved that the washouts of UCB unit bags used for UCB transplantation provided sufficient numbers of CIK cells endowed with strong cytotoxic activity against various tumor targets [25]. Starting from a very small percentage of total nucleated cells of the UCB unit ( %), which would have been wasted otherwise, a mean number of 473x10 6 CIK cells were obtained by a 1485-fold expansion [25] Tumor-eradicating ability of CIK cells The first report on the antitumor activity of CIK cells was published in 1991, followed by numerous studies confirming the efficacy and safety of the approach in-vitro and in-vivo for multiple types of malignancies [22, 39, 40]. The cytotoxic potential is associated with the CD56+ fraction that consists of predominantly CD3 and 56 double-positive cells [24, 41]. The cytotoxicity of CIK cells is not restricted to MHC, and the exact mechanism is yet to be revealed; however, several potential pathways have been suggested. The natural killer group 2 member D (NKG2D) molecule expressed at high levels on the membrane of CIK cells plays a fundamental role in cytotoxicity [42]. NKG2D can potently recognize ligands on tumor cells, such as the MHC class I polypeptide-related sequences A and B (MIC A and B) and UL16- binding proteins (ULBP) 1-4 [42]. Many tumor cells co-express, or even overexpress these ligands, thus making themselves ideal targets for CIK cell therapy [43, 44]. The involvement of the NKG2D receptor in cytotoxicity has been validated by experiments confirming impaired killing potential following NKG2D blockade [42, 45, 46]. Other molecules and pathways, such as the DNAX accessory molecule 1 (DNAM-1), natural killer p30 (NKp30), and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contribute to the killing
10 activity [46, 47, 48]. The implemented pathways induce perforin and granzyme-mediated lysis and apoptosis of their corresponding tumor cells [42, 49]. Utilizing mainly allogeneic tumor targets, Durrieu et al. have confirmed that various hematological and solid tumor cell lines are vulnerable to CIK cell induced killing [47]. As with other sources, the antitumor activity of UCB-derived CIK cells has been confirmed in-vitro [21, 25, 38, 47, 50]. In one of the studies, the authors had the opportunity to compare the activity of two UCB-derived CIK cell products with the patient s own PB-derived CIK cells [25]. The UCB-derived cells showed efficacy comparable to that of autologous ex-vivoexpanded CIK cells. In a further study, UCB-CIK cells exhibited increased proliferation and higher antitumor efficacy compared with PB-originated cells [21]. Testing the cytotoxic activity of PB and UCB-derived CIK cells against B-cell acute lymphoblastic leukemia (B- ALL) cell lines, Durrieu et al. reported that CIK cells from different sources did not use the same pathway for the elimination of cancer cells [47]. The same group proved that human IFN-alpha augmented the cytotoxic effect of UCB-derived CIK cells [50]. Further in-vitro studies supported the synergistic effects of combining CIK cell therapy with other agents. Immunomodulatory drugs (IMiDs) are widely used for the treatment of multiple myeloma (MM), and the combination of CIK cells with IMiDs proved to be superior to IMiDs alone [51] Alloreactivity of CIK cells The initial studies utilizing CIK cell therapy administered autologous PB-derived CIK cells, therefore alloreactivity was not an issue. However, the studies encountered difficulties in that there was poor response and that a sufficient number of cells was not always collectable and expandable from patients with advanced cancer. Animal models demonstrated that adoptive transfer of allogeneic CIK cells induced minimal graft-versus-host disease (GVHD) [40, 52,
11 53]. In animal studies the application of in-vivo bioluminescent imaging that monitors CIK cell trafficking confirmed that CIK cells retained strong tumor-killing activity and homing capacity to tumor growth for a prolonged period after bone marrow transplantation; however, they caused less GVHD due to reduced proliferation capacity [53]. These studies have proposed that CIK cells could be alternatives to donor lymphocyte infusions (DLI) for the prevention of relapse after allo-hsct and that higher numbers of allogeneic CIK cells might be tolerated without causing GVHD. Depletion of the CD3+/CD56- cells might further reduce the risk of GVHD without compromising the antitumor activity considering that alloreactivity is delivered by the CD3+/CD56- cell fraction [41]. Although murine studies anticipated low or minimal risk of GVHD, the pilot allogeneic human study revealed a nonnegligible GVHD incidence. Reassuringly, the GVHD severity was mild to moderate; four of eleven patients developed grade I or II acute GVHD, and two of these patients progressed to chronic GVHD [54]. Subsequent studies reported lower incidences [55, 56]. Following UCBT, the incidence of GVHD is lower compared to other sources; therefore, it is speculated that the GVHD risk might be attenuated by the implementation of UCB-derived CIK cells [57]. The human studies are confirmatory to this suggestion (Table 2), and only two cases of acute GVHD were reported among 40 patients (5%) [32, 58, 59] Clinical application of CIK cells for cancer treatment Given the easy availability, feasibility, efficacy, and safety of CIK cell immunotherapy, its clinical implementation has rapidly expanded. This modality has become especially popular in China [60]. The International Registry on CIK Cells (IRCC) was established in 2010 with the aim of collecting clinical data, introducing standards of reporting, and furthermore, establishing recommendations for clinical practice. The IRCC published two reports, the first in 2011, the second in 2015, including 11 and 45 clinical trials, respectively [39, 61]. In the
12 2015 report, the treatment of 2729 patients with 22 different entities was reviewed [39]. The majority of studies utilized fresh, autologous CIK cells and five studies used allogeneic cells. (One of five used UCB-derived cells, and the four others used allogeneic stem cell donorderived cells). The malignancies consisted of various hematological and solid tumors. The mean overall response rate (ORR) was 39%. Nineteen trials reported survival data; in 15 of these, progression-free survival (PFS) and overall survival (OS) were significantly prolonged in patients treated with CIK cells compared to the control groups. Side effects were mild, including short-lived fever, nausea, vomiting, and headache. In the allogeneic CIK cell trials, GVHD was reported in seven of 52 individuals (13%). The results of the largest allogeneic trial by Niu et al. that used UCB-derived CIK cells in combination with second-line chemotherapy for the treatment of advanced solid tumors are summarized in Table 2 [32]. The combination approach resulted in significantly better ORR, PFS, and OS compared with chemotherapy alone. In another Chinese cohort the responses of 15 patients with solid tumors were retrospectively analyzed (Table 2.) following the administration of UCB-derived CIK cells with or without chemotherapy [58]. Four patients achieved a complete or partial response, and the reported toxicity was minimal. As mentioned above in section 2.1., Introna and colleagues demonstrated the feasibility of expansion of UCB-CIK cells recovered from the washouts of UCB unit bags applying GMP conditions with the intention to treat UCBT patients at relapse [25]. The group published the pilot results - as demonstrated in Table 2 - of the first five patients treated with UCB-CIK cells for acute leukemia relapse in 2010 [59]. The treatment was associated with low toxicity; but only one patient showed a partial response. This patient at the same time developed grade III acute GVHD, whereas none of the nonresponders had GVHD.
13 As illustrated in Table 2, besides occasional case reports there have been only three clinical studies with available outcome data that employed UCB-originated CIK cells, of which none was a randomized controlled trial. Although data suggest that UCB-derived CIK cells can probably be used safely with some clinical activity, further phase 1-2 trials are warranted. 3. Natural killer cells NK cells are a unique population of innate lymphocytes with a natural capability of detecting and killing cells with missing-self or altered-self, such as infected or malignantly transformed cells, without prior sensitization [62]. Immature NK cells undergo a process called licensing, whereby they become competent to sustain tolerance against self via encountering class I self-human leucocyte antigen (HLA) molecules [63, 64]. NK cells are characterized by CD56 expression without co-expression of the T-cell marker CD3 [65]. Based on their CD16 and CD56 expression, two main subsets can be distinguished, of which the CD16+CD56 dim subset conducts the cytotoxic functions, whereas the CD16-CD56 bright cells possess immunoregulatory properties [65]. The effector function of NK cells is regulated by various signals received through their activating and inhibitory receptors and can be significantly augmented by cytokines [66]. The complex role of NK cells has been established in infection control, tumor surveillance, allogeneic HSCT reactions, and reproduction [66]. The ability of NK cells to kill various cancer subtypes without prior sensitization or stimulation makes them attractive targets for anticancer immunotherapy development [20] In-vitro expansion and activation of NK cells Similarly to CIK cells, NK cells can be obtained from different sources, including UCB that in fact contains a higher proportion of NK cells compared to PB (up to 30% vs. 15%) [20, 65]. Although the relative frequency of NK cells is higher in UCB, the small volume of UCB units
14 limits the availability of cells. To overcome this impediment, many different approaches for large-scale ex-vivo expansion have been developed. Cytokine-only culture conditions with IL-2, IL-12, IL-15, IL-18, or FLT3 ligand can boost the NK cell pool only modestly; however, some researchers achieved satisfactory yield by optimization of the culture [18, 38, 67]. The expansion of NK cells can be dramatically multiplied by the addition of feeder cells, such as monocytes, allogeneic irradiated PB mononuclear cells, cell lines (e.g., EBVtransformed lymphoblastoid cell lines, K562 cells), and genetically modified cells engineered to express ligands for additional stimulation (e.g., K562 cells co-expressing membrane-bound forms of IL-15, IL-21, or 4-1BB ligands) [18, 68, 69, 70]. Besides expansion, feeder cell techniques also enhance cytotoxicity [71]. Appropriate expansion of UCB-derived NK cells has been documented by several groups [72, 73, 74]. Shah et al., utilizing artificial antigen presenting feeder cells in a gas-permeable culture achieved an impressive 1848-fold expansion of UCB-derived NK cells from fresh UCB and a 2389-fold expansion from cryopreserved UCB with high purity (>95%) of NK cells containing only minimal (<1%) CD3+ cell contamination [74]. Besides feeder cells, gaspermeable devices are increasingly implemented due to additional advantages provided by gas exchange at the base of the culture, which allows higher volumes of medium per unit of culture, enhanced expansion, and decreased cell death [75]. Using the other thriving technique, differentiation of UCB NK cells from CD34+ progenitor cells, frozen cells similarly proved to be better expandable than fresh cells [76]. Although UCB cells are known to have relative immaturity, the above study demonstrated that ex-vivo expanded and activated NK cells were endowed with high tumor-killing activity against MM in-vivo, suggesting that UCB-derived NK cells become functionally fully competent during activation [74].
15 As described above, by up-to-date ex-vivo expansion and activation techniques, both major limitations of UCB-derived NK cells their limited numbers and relative phenotypic and functional immaturity can be auspiciously overcome. Manufacturing has advanced to provide GMP-compliant, clinical-grade NK cell products [18, 75] Tumor-eradicating ability of NK cells CD16+/CD56 dim cells are the main effectors of cytotoxicity [65]. According to earlier reports the two main subsets of NK cells can be found in similar proportions in PB and UCB; however, more recent experiments suggest that UCB may contain more CD16-/CD56 bright cells than PB does [71, 77, 78]. Concerning the tumor-eradicating ability of UCB-derived NK cells, research has revealed that non-manipulated UCB-NK cells display lower cytotoxicity compared to PB. Their function, however, is significantly enhanced following stimulation with cytokines resulting in a killing potential comparable to that of PB-derived cells [67, 71, 77, 78]. The functional differences might be related to the lower expression of certain adhesion molecules, such as CD2, CD11a, CD18, and CD54; but these phenotypic features can be rapidly increased by cytokine stimulation [71, 77, 79]. The expression of important mediators of killing, including granzyme B and perforin seems to be reduced in UCB-NK cells, although not all studies identified a difference [67, 78, 80]. Similarly, a higher expression of inhibitory receptors (KIRs and CD94/NKG2A) provides an explanation for the baseline functional inferiority of UCB-NK cells [80]. NK cells express many receptors that upon binding to their ligands transmit activating or inhibitory signals [62]. Many types of activating receptors, including natural cytotoxic receptors (NKp30, 44, 46), C-type lectin receptors (CD94/NKG2C, NKG2D and F), and killer cell immunoglobulin-like receptors (KIR2DS) have been distinguished, whereas other C-type
16 lectin (CD94/NKG2A or B) or KIR receptors (KIR2DL) act as inhibitory receptors [66]. KIR ligands are specific epitopes of class I HLA molecules on target cells, and their extracellular domain determines their specificity [62]. Activating receptors are capable of recognizing nonself molecules not expressed by the host and stress-induced modified-self molecules, the so called stress ligands on malignantly transformed cells [66]. Their activation leads to increased NK cell function and cytotoxicity. On the other hand, the identification of the cognate ligands of the inhibitory receptors results in tolerance or reduced cytotoxicity against the target, but the absence of their appropriate ligand generates enhanced cytotoxicity [17]. This latter phenomenon is defined as the missing self hypothesis, referring to the process whereby inhibitory KIRs do not relay inhibitory signals if their ligand is missing on their target, which allows the deliverance of NK cell-mediated killing toward the corresponding cell [17, 81]. Under stress conditions, such as malignant transformation, cells downregulate class I HLA expression, leading to the elimination of cells [66]. Similarly, other activating or inhibitory receptor/ligand encounters augment or attenuate NK cell function toward the target [62]. Equally, upregulation of stress signals by tumor cells and downregulation of self both lead to NK cell-mediated killing. Unfortunately, tumor cells can escape eradication through various mechanisms, such as the release of soluble NK cell ligands around the tumor cells, e.g., MIC ligands or downregulation of stress ligands, like NKG2D ligands on tumor cells [82, 83]. Upon activation, NK cells secrete many cytokines, which in turn lead to further stimulation and enhanced cytotoxicity. IFNγ has crucial significance among cytokines, given its role in the upregulation of stress ligands on cancer cells, making them more susceptible to NK cell killing [62]. Cytokines also serve as adaptive immune response inducers [62, 66]. Cytotoxicity is carried out via several mechanisms, such as direct cytotoxicity by the release of cytoplasmic granules containing perforin and granzyme, antibody-dependent cellular cytotoxicity, and activation of apoptotic pathways (TRAIL, Fas) [66].
17 3.3. Alloreactivity of NK cells NK cell alloreactivity has become a hot topic in the allo-hsct field following reports on improved disease-free survival (DFS), OS, and reduced relapse in acute myeloid leukemia (AML) patients after transplant with KIR ligand-mismatched donors (donor cells expressing KIR receptors specific to HLA molecules absent in the recipient) [84, 85]. The observation that NK cells unable to find their cognate ligand on cells deliver cytotoxicity could explain the reduced relapse risk described by several groups following KIR-mismatched transplants. However, studies show inconsistencies; and a recent meta-analysis failed to detect a difference in relapse, but confirmed favorable OS for KIR-mismatched unrelated transplants [86]. Studies investigating the role of KIR-mismatch in UCBT provided even less robust evidence [87, 88]. The first two studies showed conflicting results, the Eurocord study proved a favorable effect of KIR-mismatch on relapse incidence and leukemia-free survival, but the Minneapolis group detected no such associations [89, 90]. The impact of KIR-matching on UCBT outcomes has been recently revised in a study of a large series of UCBT recipients with AML that confirmed no beneficial impact of KIR-mismatch on the outcome [91]. Beside the beneficial GVL effect alloreactivity of NK cells could also induce GVHD in the host. Clinical experience suggests that this risk is low. In a recent clinical trial no GVHD was detected in patients treated with partially HLA-matched and in most cases KIR-mismatched NK cells, similarly GVHD was not observed in another cohort [92, 93] Clinical application of NK cells for cancer treatment The first studies investigating the effectiveness of NK cell immunotherapy applied autologous sources; however, this approach served no meaningful clinical benefit, as NK cells recognizing self-mhc transmit inhibitory signals and induce tolerance [66]. Attempts were made to enhance tumor-killing activity of NK cells via ex-vivo or in-vivo cytokine
18 stimulation, but the systemic administration of cytokines, such as IL-2 induces considerable toxicity with modest efficacy [62, 94]. Prompted by accumulating evidence on the favorable effect of KIR-mismatch in the haploidentical HSCT setting, the number of studies utilizing allogeneic NK cells rapidly grew. Studies have proved that haploidentical, related-donor NKcell infusions can be safely administered and that in-vivo expansion and persistence can be achieved following lymphodepleting chemotherapy regimens [95, 96]. In the first studies, patients with various malignancies like melanoma, renal cell carcinoma, lung cancer, and AML were included; and clinical responses were noted, despite advanced disease stages without high risk of GVHD. By now, numerous trials for various tumor types have been conducted that tested allogeneic NK cell therapy alone or in combination with chemotherapy, radiotherapy, monoclonal antibodies, immunomodulatory drugs or checkpoint inhibitors with limited clinical efficacy [66, 94]. UCB-derived NK cells are the focus of significant scientific interest, and several clinical trials are being currently conducted. The completed studies are shown in Table 2, and prove the feasibility and safety of the approach. In an early phase study published in 2017, UCBderived, ex-vivo expanded NK cells were used along with chemotherapy and autologous HSCT in MM patients [93]. Due to the combination nature of the therapy, it is challenging to judge the efficacy of NK cell immunotherapy, but most importantly, the study has confirmed persistence of cells in-vivo and low toxicity with no GVHD. In 2017 Dolstra et al. published the first-in-human phase 1 study utilizing UCB-derived NK cells generated from CD34+ hematopoietic stem and progenitor cells to treat older patients with AML not eligible for allo- HSCT [92]. Following lymphodepleting chemotherapy to prevent immediate rejection of allogeneic NK cells, successful donor chimerism was detectable in all patients, followed by transient persistence, homing to the bone marrow and in-vivo maturation. The treatment was
19 well tolerated without induction of GVHD, but as expected hematologic toxicity was significant secondary to chemotherapy. The currently ongoing trials testing the efficacy and toxicity of ex-vivo expanded UCB-NK cells are summarized in Table 3, including one study utilizing CAR-engineered NK cells. Many questions remain unanswered, such as the maximum tolerable dose, cellular alloreactivity (and whether HLA-, and KIR-matching matter), and the optimal conditioning and combination therapy to allow NK cell persistence and enhance in-vivo function. The ongoing trials will provide further evidence for the efficacy and tolerability of UCB-derived NK cell therapy. Nonetheless, further research is required before their therapeutic potential can be fully harnessed. 4. Adoptive T-cell anticancer immunotherapy Adoptive T-cell therapy has become an emerging cancer treatment with great promise due to the ability of T-lymphocytes to recognize and specifically kill tumor targets [16]. Research suggests that UCB-T-cells exhibit augmented anticancer activity compared with PB cells, and despite the immaturity of UCB cells, naïve T-cells rapidly undergo memory effector differentiation within the tumor microenvironment [19]. Four main ways of adoptive T-cell therapy can be distinguished: (i) performing allogeneic HSCT; (ii) isolation of tumorinfiltrating lymphocytes from tumors, followed by ex-vivo expansion and reinfusion; (iii) using genetically modified T-cells to express a chimeric antigen receptor (CAR) or a tumorspecific T-cell receptor; and (iv) ex-vivo expansion of tumor-specific T-cells in the presence of tumor antigens [97]. Given the revolutionary effect of genetically modified T-cells as an addition to the anticancer arsenal, in this part of the review we highlight the attributes of chimeric antigen receptor T- cell therapy.
20 4.1. Generation of chimeric antigen receptor T-cells Although the use of CAR T-cell therapy in routine clinical practice is awaited; its development has been one of the greatest achievements of cancer management lately. CAR T- cells are genetically engineered cells that express fusion proteins constituting of tumor antigen-targeted and T-cell activation domains [98]. Following ligand binding to a CAR, intracellular signaling is activated, this promotes MHC-unrestricted T-cell activation and proliferation, which leads to eradication of targeted cancer cells [99, 100]. For effective application of this approach, several crucial and labor-intensive steps are involved. First, a sufficient number of cells must be collected, and the target-specific new genetic material must be transferred into the cells ex-vivo [101]. For gene transfer, most trials have utilized viral vectors (retroviral, lentiviral) or transposon systems (Sleeping Beauty, PiggyBac) [100]. A further step includes the ex-vivo expansion and stimulation of the modified T-cells. Systems similar to those described above regarding NK cell expansion are applied by using artificial antigen presenting feeder cells with cytokines and co-stimulatory molecules [101]. Following infusion into the patient, T-cells must be capable of trafficking to tumor sites where they can engage with their antigen, triggering further in-vivo proliferation and persistence until a meaningful anticancer cytotoxicity is achieved [101]. Clinical studies have confirmed that the depletion of endogenous lymphocytes by chemotherapy or radiotherapy before the infusion of CAR T-cells augments in-vivo T-cell activity and that the application of more intensive lymphodepletion seems to be beneficial [98, 102, 103]. Several generations of CAR domains have been developed. First generation CARs containing a single intracellular signaling domain displayed only modest proliferative and anticancer activity; therefore, second generation CARs including a costimulatory cytoplasmic signaling domain (e.g., CD27, CD28, OX40, 4-1BB) were developed [100]. The incorporation of more
21 than one additional co-stimulatory molecule in third generation CARs further improved the tumor-killing potential [100]. Fourth generation CARs are armored with cytokine and costimulatory ligand-encoding genes for the enhancement of immune response upon activation, via production of cytokines and expression of co-stimulatory molecules on their cell surface [100]. The ideal CAR T-cell target is a tumor-specific antigen residing on the cell surface, which albeit is not found on normal cells in order to minimize toxicity by cross-reactivity with normal structures. The application of gene-engineered T-cell therapy offers many advantages, including selection of tumor-specific targets, involvement of additional genes that improve stimulation, prevent apoptosis and promote T-cell homing to tumor sites [16]. Drawbacks include monoclonal specificity, potential for tumor escape by downregulation of target antigens, and unexpected toxicities due to mechanism of action and cross-reactivity with normal tissues [16] Clinical application of CAR T-cells The clinical achievements delivered by CAR T-cell immunotherapy are extraordinary. Many clinical trials utilizing CAR T-cell therapy have been conducted or are in progress for the treatment of solid and hematological malignancies, as reviewed by Gill, Geyer, Kochenderfer and co-authors [98, 101, 102]. CD19-targeted CAR T-cells have been in the forefront of research, and this novel approach has delivered immense hope for patients with acute lymphoblastic leukemia (ALL). Excellent responses with up to 90% complete remission (CR) rates were noted in clinical trials in this otherwise extremely poor prognostic group with relapsed/refractory ALL [104, 105, 106]. Of the first five patients with relapsed ALL treated by the Brentjens group all achieved CR with CD19-targeted CAR T-cells [105]. In 2016 the group presented updated figures and confirmed an impressive 82% CR rate in 50 ALL
22 patients with many minimal residual disease (MRD)-negative responses (85% of CRs) [102]. In a predominantly pediatric cohort, 27 out of 30 patients (90%) with relapsed/refractory ALL, half of whom previously underwent allo-hsct, achieved CR with autologous T-cells transduced with a CD19-directed CAR lentiviral vector [106]. The six-month event-free survival rate was 67%, and the probability for CAR T-cell persistence at six months was 68%. The most frequently reported acute complications of CAR T-cell therapy are cytokine release syndrome, which affects patients almost universally, and neurotoxicity. Observations suggest that high CAR T-cell doses and tumor burden might be associated with increased risk [103]. CD19 is an ideal target for CAR T-cell manufacturing, given its expression on malignant B- cells but not on normal tissues [98]. CAR T-cell therapies with specificity to many other targets, such as CD30, CD33, CD123, NKG2D, kappa light chain, CD138, HER2, EGFR, etc., have been investigated [101]. It often proves to be a challenge to identify suitable targets not expressed on normal cells, and any new targets should be carefully evaluated before clinical introduction due to unpredictable side effects [101]. For instance, a fatal complication with sudden-onset respiratory failure was reported following the administration of ERBB2- specific CAR T-cells to a colon cancer patient; this effect was likely secondary to a cytokine storm triggered by the recognition of low levels of ERBB2 on lung epithelial cells [107]. The majority of CAR T-cell trials used autologous sources for manufacturing, but studies have also confirmed that allogeneic CAR T-cells can be administered safely [108, 109]. In 2013 it was reported for the first time that in patients following allo-hsct, donor-derived allogeneic anti-cd19-car T-cells can induce regression of B-cell malignancies that were resistant to standard DLI [108]. Despite the study design patients did not receive preconditioning prior to the CAR T-cell infusions no GVHD was observed. Grupp et al. reported the treatment of two children with ALL, one of whom relapsed post UCBT; this
23 patient's UCB donor-originated mononuclear cells were the main source for the CAR T-cells [110]. Complete remission was achieved; but two months later a CD19-negative relapse occurred, providing evidence for tumor escape by loss of the target CD19 antigen. CAR T-cell development has been effectively established from UCB [111, 112, 113]. By the genetic manipulation and stimulation, the formerly naïve UCB T-cell population promptly differentiates to an effector phenotype [111]. In an immunodeficient CD19+ leukemia/lymphoma murine model, the use of UCB T-cells transduced with lentiviral vectors expressing a CAR for CD19 confirmed a synergistic role of 4-1BB and CD28 co-stimulation [114]. The lack of DLI is a drawback to using UCBT, given that there is no possibility of returning to the donor to collect T-cells following transplantation for the treatment of relapse. However, newer techniques might allow the generation of sufficient number of T-cells from small volumes of UCB units [115]. Pegram et al. cultured UCB-derived T-cells with IL-12 and IL-15, which led to an over 150-fold expansion and acquisition of a central memory/effector phenotype [99]. Moreover, the cells were engineered to express CD-19 specific CARs and secrete IL-12, and the accordingly modified cells exhibited enhanced antitumor efficacy in-vitro and in-vivo. The authors concluded that CAR-modified UCB T- cells could improve GVL response in B-ALL patients undergoing UCBT. CD123 is expressed on the majority of AML cells, and therefore CD123-targeted CARs are the focus of studies addressing their efficacy for the treatment of AML [101, 116, 117]. Research groups have effectively generated CD123-engager T-cells from UCB stem and progenitor cells [118, 119]. However, the persistence of CD123-CARs could lead to severe myelosuppression, as they also target normal hematopoietic precursors. To mitigate this downside, Bonifant el al. developed a CAR incorporating a CD20 suicide gene, which
24 allowed anti-cd20 antibody-mediated CAR T-cell elimination in a xenograft model [118]. The myelosuppressive and anti-leukemic potential of CD123-specific CAR T-cells could, however, be beneficially combined in myeloablative conditioning HSCT for AML [120]. CAR T-cell persistence in-vivo remains crucial for the success of CAR T-cell treatment. It has been proved feasible to design UCB-derived CAR T-cells with specificity to cancer and virusspecific antigens with the advantage that constant activation by latent viral antigens could lead to prolonged persistence of CAR T-cells [121]. In a recent study, Ebstein-Barr-virus-directed vaccination enhanced the persistence of CAR T-cells utilizing Ebstein-Barr-virus-specific cytotoxic T-cells redirected with a CAR. Furthermore, the study used centrally manufactured products across multiple centers, proving the feasibility of central manufacture and distribution of immunotherapy products for future use [122]. Due to thriving technological developments, not only centrally manufactured, but also the desired universal, off-the-shelf CAR T-cell products could become available soon [123, 124]. In a 2017 publication, authors reported that in two infants with relapsed B-ALL, molecular remission was achieved after treatment with TALEN-engineered cells with lentivirus transduction [125]. The authors generated a bank of non-hla-matched, universal, CAR T-cells from a healthy female donor complying with GMP conditions. Gene-editing strategy could spread widely in the future, but safety concerns, such as carriage of genetic changes by edited cells need further exploration before routine use. The few CAR T-cell studies using allogeneic CAR T-cells advocate for low risk of alloreactivity. Although the risk of GVHD induced by UCB-derived CAR T-cells in humans is yet to be investigated, the above studies utilizing gene-editing technology suggest that this theoretical impediment could be efficiently eliminated.
25 5. Conclusion Immunotherapy represents a breakthrough approach for the management of cancer that is resistant to conventional modalities. UCB is an increasingly appreciated, readily available, rich source of cells with distinct anticancer potential. Although cellular immunotherapy holds great promise, its translation to the clinic has not yet resulted in outstanding success. Studies suggesting higher anticancer activity of UCB cells compared with other sources provide a rationale for the application of UCB-derived immunotherapy. However, the limited data from clinical trials makes the positioning of this approach challenging. As our understanding of anticancer biology is rapidly expanding, the advances might soon lead to the development of the desired off-the-self or even universal immunotherapeutic products generated from UCB. To harness the full potential of this modality and justify the routine clinical use, further clinical trials are warranted. 6. Expert opinion Umbilical cord blood has been an increasingly investigated but still underutilized source for cellular immunotherapy. The failure of significant clinical responses following treatment with autologous, non-engineered immune cells has prompted research in allogeneic cellular immunotherapy. Considering the rather limited success rates of most cellular immunotherapy modalities, including allogeneic ones, it has been speculated that better outcome could be achieved with the use of UCB as an allogeneic source. Results from in-vitro and animal studies indicate that UCB cells could possess higher anticancer activity compared with older cells from other sources [19]. Germeris and Karanikas have suggested that the poor response rates to immunotherapy might at least in part be associated with immunosenescence, whereby the aging lymphoid cells tackle the cancer cells less efficiently
26 [126]. Immunosenescence refers to a complex process involving the deterioration of innate and adaptive immune functions of the aging host at many levels [127]. Evidence supports the distinct phenotypic and functional properties of UCB cells. At baseline, UCB immune cells exhibit lower cytotoxicity against targets and have a naïve phenotype. Following priming and differentiation from stem and progenitor cells or ex-vivo expansion and activation, UCB cells rapidly become fully competent to carry out cytotoxicity against malignantly transformed cells. Many thousands of UCB units have been stored in cord blood banks with immediate availability for immunotherapy purposes. Although newborn babies are non-voluntary donors of UCB, the collection of placental blood is safe not harming the baby or the mother, therefore the collection and use of UCB for the treatment of patients following informed consent of the mother has been ethically approved [128]. Modern techniques allow manufacturing of immunotherapy products in accordance with Good Manufacturing Practice (GMP). Recent studies suggest that the adoptive transfer of off-the-shelf UCB-derived immunotherapy might become available soon. A major obstacle to cellular immunotherapy to be addressed by future studies is the effective prevention of rapid rejection of allogeneic immune cells by the host s immune system. NK and CAR T-cell studies provide evidence for transient or sustained persistence of cells following lymphodepleting chemotherapy. Similarly, data from CAR T-cell studies suggest that vaccination and gene-editing strategies could improve persistence. The ideal protocol is yet to be elucidated, however. Given that persistence correlates with clinical responses, relevant research is of high importance.
27 Clinical investigations are in progress to address the role of combination treatment with immunomodulatory or other agents and genetic modification of UCB cells that could possibly augment the function of the transferred immune cells in-vivo and break tumor resistance [66, 94]. With regard to toxicity, the UCB source is associated with lower GVHD risk in the allo- HSCT setting, and data is suggestive that this could apply to UCB-derived immunotherapy. Current evidence indicates low toxicity associated with CIK and NK cell immunotherapies; however, more studies are needed to assess GVHD risk in the allogeneic setting, including the use of non-hla-matched immunotherapy products. The safe use of immunotherapy across HLA barriers could facilitate the generation of universal therapies. CAR T-cell therapy is undoubtedly among the most quickly expanding areas of immunotherapy that can reshape the future landscape of cancer. The modality is associated with significant toxicity, but methods have been developed or are being investigated to manage and prevent side effects. CAR T-cells have been efficiently engineered using UCBderived T-cells; and although no results from human studies are available yet, the approach seems feasible. Preliminary results are promising; however, further studies are warranted to investigate the efficacy and safety of universal, non-hla-matched, off-the-shelf CAR T- cell products, which could significantly broaden the availability of CAR T-cell treatment. Many biological and clinical issues have yet to be addressed in order to translate our knowledge on UCB-derived immunotherapy into clinical practice; however, the unique biological properties and ready availability of UCB cells make this approach very attractive.
28 Funding: This work was supported by NHS Blood and Transplant (NHSBT). Declaration of Interest: K Balassa receives a fellowship from NHS Blood and Transplant while V Rocha is a principal investigator for NHS Blood and Transplant. The authors have no n other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial f conflict with the subject matter or materials discussed in thee manuscript apart from those disclosed. Figure 1. Clinical implication and potential use of various umbilical cord blood-derived cells References 1. Rocha V, Gluckman E, Eurocord Netcord R, et al. Improving outcomes of cord blood transplantation: HLA matching, cell dose and other graft- andd transplantation-related factors. British Journal of Haematology y Oct;147(2): doi: /j x. PubMed PMID: WOS:
Sleeping Beauty: Current applications and future strategies. CAR-TCR Summit 2017 Partow Kebriaei, MD
 Sleeping Beauty: Current applications and future strategies CAR-TCR Summit 2017 Partow Kebriaei, MD Outline Chimeric antigen receptor (CAR) technology Viral versus nonviral vectors Results of current clinical
Sleeping Beauty: Current applications and future strategies CAR-TCR Summit 2017 Partow Kebriaei, MD Outline Chimeric antigen receptor (CAR) technology Viral versus nonviral vectors Results of current clinical
The future of HSCT. John Barrett, MD, NHBLI, NIH Bethesda MD
 The future of HSCT John Barrett, MD, NHBLI, NIH Bethesda MD Transplants today Current approaches to improve SCT outcome Optimize stem cell dose and source BMT? PBSCT? Adjusting post transplant I/S to minimize
The future of HSCT John Barrett, MD, NHBLI, NIH Bethesda MD Transplants today Current approaches to improve SCT outcome Optimize stem cell dose and source BMT? PBSCT? Adjusting post transplant I/S to minimize
M.Sc. III Semester Biotechnology End Semester Examination, 2013 Model Answer LBTM: 302 Advanced Immunology
 Code : AS-2246 M.Sc. III Semester Biotechnology End Semester Examination, 2013 Model Answer LBTM: 302 Advanced Immunology A. Select one correct option for each of the following questions:- 2X10=10 1. (b)
Code : AS-2246 M.Sc. III Semester Biotechnology End Semester Examination, 2013 Model Answer LBTM: 302 Advanced Immunology A. Select one correct option for each of the following questions:- 2X10=10 1. (b)
One Day BMT Course by Thai Society of Hematology. Management of Graft Failure and Relapsed Diseases
 One Day BMT Course by Thai Society of Hematology Management of Graft Failure and Relapsed Diseases Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital
One Day BMT Course by Thai Society of Hematology Management of Graft Failure and Relapsed Diseases Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital
Natural Killer Cells: Development, Diversity, and Applications to Human Disease Dr. Michael A. Caligiuri
 Natural Killer Cells: Development, Diversity, November 26, 2008 The Ohio State University Comprehensive Cancer Center The James Cancer Hospital and Solove Research Institute Columbus, Ohio, USA 1 Human
Natural Killer Cells: Development, Diversity, November 26, 2008 The Ohio State University Comprehensive Cancer Center The James Cancer Hospital and Solove Research Institute Columbus, Ohio, USA 1 Human
Exploring Immunotherapies: Beyond Checkpoint Inhibitors
 Exploring Immunotherapies: Beyond Checkpoint Inhibitors Authored by: Jennifer Dolan Fox, PhD VirtualScopics (Now part of BioTelemetry Research) jennifer_fox@virtualscopics.com +1 585 249 6231 Introduction
Exploring Immunotherapies: Beyond Checkpoint Inhibitors Authored by: Jennifer Dolan Fox, PhD VirtualScopics (Now part of BioTelemetry Research) jennifer_fox@virtualscopics.com +1 585 249 6231 Introduction
COURSE: Medical Microbiology, PAMB 650/720 - Fall 2008 Lecture 16
 COURSE: Medical Microbiology, PAMB 650/720 - Fall 2008 Lecture 16 Tumor Immunology M. Nagarkatti Teaching Objectives: Introduction to Cancer Immunology Know the antigens expressed by cancer cells Understand
COURSE: Medical Microbiology, PAMB 650/720 - Fall 2008 Lecture 16 Tumor Immunology M. Nagarkatti Teaching Objectives: Introduction to Cancer Immunology Know the antigens expressed by cancer cells Understand
IMMUNOTHERAPY FOR CANCER A NEW HORIZON. Ekaterini Boleti MD, PhD, FRCP Consultant in Medical Oncology Royal Free London NHS Foundation Trust
 IMMUNOTHERAPY FOR CANCER A NEW HORIZON Ekaterini Boleti MD, PhD, FRCP Consultant in Medical Oncology Royal Free London NHS Foundation Trust ASCO Names Advance of the Year: Cancer Immunotherapy No recent
IMMUNOTHERAPY FOR CANCER A NEW HORIZON Ekaterini Boleti MD, PhD, FRCP Consultant in Medical Oncology Royal Free London NHS Foundation Trust ASCO Names Advance of the Year: Cancer Immunotherapy No recent
Medical Virology Immunology. Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University
 Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
Medical Virology Immunology Dr. Sameer Naji, MB, BCh, PhD (UK) Head of Basic Medical Sciences Dept. Faculty of Medicine The Hashemite University Human blood cells Phases of immune responses Microbe Naïve
T cell manipulation of the graft: Yes
 T cell manipulation of the graft: Yes J.H. Frederik Falkenburg Department of Hematology L M U C Allogeneic Hematopoietic Stem Cell Transplantation (SCT) for non-malignant disorders: no need for anti-tumor
T cell manipulation of the graft: Yes J.H. Frederik Falkenburg Department of Hematology L M U C Allogeneic Hematopoietic Stem Cell Transplantation (SCT) for non-malignant disorders: no need for anti-tumor
Haplo vs Cord vs URD Debate
 3rd Annual ASBMT Regional Conference for NPs, PAs and Fellows Haplo vs Cord vs URD Debate Claudio G. Brunstein Associate Professor University of Minnesota Medical School Take home message Finding a donor
3rd Annual ASBMT Regional Conference for NPs, PAs and Fellows Haplo vs Cord vs URD Debate Claudio G. Brunstein Associate Professor University of Minnesota Medical School Take home message Finding a donor
Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features
 Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features Loretta Gammaitoni, Lidia Giraudo, Valeria Leuci, et al. Clin Cancer Res
Effective activity of cytokine-induced killer cells against autologous metastatic melanoma including cells with stemness features Loretta Gammaitoni, Lidia Giraudo, Valeria Leuci, et al. Clin Cancer Res
Children s Hospital of Philadelphia Annual Progress Report: 2011 Formula Grant
 Children s Hospital of Philadelphia Annual Progress Report: 2011 Formula Grant Reporting Period January 1, 2012 June 30, 2012 Formula Grant Overview The Children s Hospital of Philadelphia received $3,521,179
Children s Hospital of Philadelphia Annual Progress Report: 2011 Formula Grant Reporting Period January 1, 2012 June 30, 2012 Formula Grant Overview The Children s Hospital of Philadelphia received $3,521,179
Immunotherapy on the Horizon: Adoptive Cell Therapy
 Immunotherapy on the Horizon: Adoptive Cell Therapy Joseph I. Clark, MD, FACP Professor of Medicine Loyola University Chicago Stritch School of Medicine Maywood, IL June 23, 2016 Conflicts of Interest
Immunotherapy on the Horizon: Adoptive Cell Therapy Joseph I. Clark, MD, FACP Professor of Medicine Loyola University Chicago Stritch School of Medicine Maywood, IL June 23, 2016 Conflicts of Interest
CAR T-CELLS: ENGINEERING IMMUNE CELLS TO TREAT CANCER. Roman GALETTO, PhD 17 th Club Phase 1 Annual Meeting April 5 th Paris
 CAR T-CELLS: ENGINEERING IMMUNE CELLS TO TREAT CANCER Roman GALETTO, PhD 17 th Club Phase 1 Annual Meeting April 5 th 2018 - Paris Cellectis, 05-APR-2018 2 FORWARD-LOOKING STATEMENTS THIS PRESENTATION
CAR T-CELLS: ENGINEERING IMMUNE CELLS TO TREAT CANCER Roman GALETTO, PhD 17 th Club Phase 1 Annual Meeting April 5 th 2018 - Paris Cellectis, 05-APR-2018 2 FORWARD-LOOKING STATEMENTS THIS PRESENTATION
Transplantation - Challenges for the future. Dr Gordon Cook S t James s Institute of Oncology, Leeds Teaching Hospitals Trust
 Transplantation - Challenges for the future Dr Gordon Cook S t James s Institute of Oncology, Leeds Teaching Hospitals Trust Bone Marrow Transplantation Timeline, 1957-2006 Appelbaum F. N Engl J Med 2007;357:1472-1475
Transplantation - Challenges for the future Dr Gordon Cook S t James s Institute of Oncology, Leeds Teaching Hospitals Trust Bone Marrow Transplantation Timeline, 1957-2006 Appelbaum F. N Engl J Med 2007;357:1472-1475
Donor Lymphocyte Infusion for Malignancies Treated with an Allogeneic Hematopoietic Stem-Cell Transplant
 Last Review Status/Date: September 2014 Page: 1 of 8 Malignancies Treated with an Allogeneic Description Donor lymphocyte infusion (DLI), also called donor leukocyte or buffy-coat infusion is a type of
Last Review Status/Date: September 2014 Page: 1 of 8 Malignancies Treated with an Allogeneic Description Donor lymphocyte infusion (DLI), also called donor leukocyte or buffy-coat infusion is a type of
08/02/59. Tumor Immunotherapy. Development of Tumor Vaccines. Types of Tumor Vaccines. Immunotherapy w/ Cytokine Gene-Transfected Tumor Cells
 Tumor Immunotherapy Autologous virus Inactivation Inactivated virus Lymphopheresis Culture? Monocyte s Dendritic cells Immunization Autologous vaccine Development of Tumor Vaccines Types of Tumor Vaccines
Tumor Immunotherapy Autologous virus Inactivation Inactivated virus Lymphopheresis Culture? Monocyte s Dendritic cells Immunization Autologous vaccine Development of Tumor Vaccines Types of Tumor Vaccines
What s a Transplant? What s not?
 What s a Transplant? What s not? How to report the difference? Daniel Weisdorf MD University of Minnesota Anti-cancer effects of BMT or PBSCT [HSCT] Kill the cancer Save the patient Restore immunocompetence
What s a Transplant? What s not? How to report the difference? Daniel Weisdorf MD University of Minnesota Anti-cancer effects of BMT or PBSCT [HSCT] Kill the cancer Save the patient Restore immunocompetence
An Introduction to Bone Marrow Transplant
 Introduction to Blood Cancers An Introduction to Bone Marrow Transplant Rushang Patel, MD, PhD, FACP Florida Hospital Medical Group S My RBC Plt Gran Polycythemia Vera Essential Thrombocythemia AML, CML,
Introduction to Blood Cancers An Introduction to Bone Marrow Transplant Rushang Patel, MD, PhD, FACP Florida Hospital Medical Group S My RBC Plt Gran Polycythemia Vera Essential Thrombocythemia AML, CML,
Haploidentical Transplantation: The Answer to our Donor Problems? Mary M. Horowitz, MD, MS CIBMTR, Medical College of Wisconsin January 2017
 Haploidentical Transplantation: The Answer to our Donor Problems? Mary M. Horowitz, MD, MS CIBMTR, Medical College of Wisconsin January 2017 Allogeneic Transplant Recipients in the US, by Donor Type 9000
Haploidentical Transplantation: The Answer to our Donor Problems? Mary M. Horowitz, MD, MS CIBMTR, Medical College of Wisconsin January 2017 Allogeneic Transplant Recipients in the US, by Donor Type 9000
Third line of Defense
 Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
Chapter 15 Specific Immunity and Immunization Topics -3 rd of Defense - B cells - T cells - Specific Immunities Third line of Defense Specific immunity is a complex interaction of immune cells (leukocytes)
Shiv Pillai Ragon Institute, Massachusetts General Hospital Harvard Medical School
 CTLs, Natural Killers and NKTs 1 Shiv Pillai Ragon Institute, Massachusetts General Hospital Harvard Medical School CTL inducing tumor apoptosis 3 Lecture outline CD8 + Cytotoxic T lymphocytes (CTL) Activation/differentiation
CTLs, Natural Killers and NKTs 1 Shiv Pillai Ragon Institute, Massachusetts General Hospital Harvard Medical School CTL inducing tumor apoptosis 3 Lecture outline CD8 + Cytotoxic T lymphocytes (CTL) Activation/differentiation
Chapter 7 Conclusions
 VII-1 Chapter 7 Conclusions VII-2 The development of cell-based therapies ranging from well-established practices such as bone marrow transplant to next-generation strategies such as adoptive T-cell therapy
VII-1 Chapter 7 Conclusions VII-2 The development of cell-based therapies ranging from well-established practices such as bone marrow transplant to next-generation strategies such as adoptive T-cell therapy
Adaptive immune responses: T cell-mediated immunity
 MICR2209 Adaptive immune responses: T cell-mediated immunity Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will discuss the T-cell mediated immune response, how it is activated,
MICR2209 Adaptive immune responses: T cell-mediated immunity Dr Allison Imrie allison.imrie@uwa.edu.au 1 Synopsis: In this lecture we will discuss the T-cell mediated immune response, how it is activated,
Reduced-intensity Conditioning Transplantation
 Reduced-intensity Conditioning Transplantation Current Role and Future Prospect He Huang M.D., Ph.D. Bone Marrow Transplantation Center The First Affiliated Hospital Zhejiang University School of Medicine,
Reduced-intensity Conditioning Transplantation Current Role and Future Prospect He Huang M.D., Ph.D. Bone Marrow Transplantation Center The First Affiliated Hospital Zhejiang University School of Medicine,
Clinical Policy: Donor Lymphocyte Infusion
 Clinical Policy: Reference Number: PA.CP.MP.101 Effective Date: 01/18 Last Review Date: 11/16 Coding Implications Revision Log This policy describes the medical necessity requirements for a donor lymphocyte
Clinical Policy: Reference Number: PA.CP.MP.101 Effective Date: 01/18 Last Review Date: 11/16 Coding Implications Revision Log This policy describes the medical necessity requirements for a donor lymphocyte
Manipulation of T Cells in the Thnsplant Inoculum
 International Journal of Cell Cloning 4: 122-126 Suppl 1 (1986) Manipulation of T Cells in the Thnsplant Inoculum J. Kersey Bone Marrow Transplantation Program, University of Minnesota, Minneapolis, MN,
International Journal of Cell Cloning 4: 122-126 Suppl 1 (1986) Manipulation of T Cells in the Thnsplant Inoculum J. Kersey Bone Marrow Transplantation Program, University of Minnesota, Minneapolis, MN,
CARs vs. BiTE in ALL. David L Porter, MD Jodi Fisher Horowitz Professor University of Pennsylvania Health System Abramson Cancer Center
 CARs vs. BiTE in ALL David L Porter, MD Jodi Fisher Horowitz Professor University of Pennsylvania Health System Abramson Cancer Center Disclosure Information David L Porter Speaker and members of study
CARs vs. BiTE in ALL David L Porter, MD Jodi Fisher Horowitz Professor University of Pennsylvania Health System Abramson Cancer Center Disclosure Information David L Porter Speaker and members of study
TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer
 AD Award Number: W8-XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke, Ph.D. CONTRACTING ORGANIZATION: Johns Hopkins
AD Award Number: W8-XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke, Ph.D. CONTRACTING ORGANIZATION: Johns Hopkins
The National Marrow Donor Program. Graft Sources for Hematopoietic Cell Transplantation. Simon Bostic, URD Transplant Recipient
 1988 199 1992 1994 1996 1998 2 22 24 26 28 21 212 214 216 218 Adult Donors Cord Blood Units The National Donor Program Graft Sources for Hematopoietic Cell Transplantation Dennis L. Confer, MD Chief Medical
1988 199 1992 1994 1996 1998 2 22 24 26 28 21 212 214 216 218 Adult Donors Cord Blood Units The National Donor Program Graft Sources for Hematopoietic Cell Transplantation Dennis L. Confer, MD Chief Medical
Personalized medicine - cancer immunotherapy
 Personalized medicine - cancer immunotherapy Özcan Met, PhD Senior Staff Scientist, Cell Therapy Director Center for Cancer Immune Therapy Department of Hematology Department of Oncology University Hospital
Personalized medicine - cancer immunotherapy Özcan Met, PhD Senior Staff Scientist, Cell Therapy Director Center for Cancer Immune Therapy Department of Hematology Department of Oncology University Hospital
Stem Cell Sources 2/22/13. Cellular Therapy Today and Tomorrow. Cellular Therapy in HCT. Bone Marrow
 2/22/13 Cellular Therapy Today and Tomorrow Robert S. Negrin, MD Division Chief, Stanford Bone and Marrow Transplant Program Professor of Medicine Cellular Therapy in Clinical Medicine Established Hematopoietic
2/22/13 Cellular Therapy Today and Tomorrow Robert S. Negrin, MD Division Chief, Stanford Bone and Marrow Transplant Program Professor of Medicine Cellular Therapy in Clinical Medicine Established Hematopoietic
Immunology Lecture 4. Clinical Relevance of the Immune System
 Immunology Lecture 4 The Well Patient: How innate and adaptive immune responses maintain health - 13, pg 169-181, 191-195. Immune Deficiency - 15 Autoimmunity - 16 Transplantation - 17, pg 260-270 Tumor
Immunology Lecture 4 The Well Patient: How innate and adaptive immune responses maintain health - 13, pg 169-181, 191-195. Immune Deficiency - 15 Autoimmunity - 16 Transplantation - 17, pg 260-270 Tumor
Bioassays for Quality Control of Cell & Gene Therapy Products
 Bioassays for Quality Control of Cell & Gene Therapy Products Erik Rutjens, Cell & Gene Therapy, Novartis Pharma AG CASSS Bioassays, Silver Spring, March2015 CTL019 Introduction CARTs = Chimeric Antigen
Bioassays for Quality Control of Cell & Gene Therapy Products Erik Rutjens, Cell & Gene Therapy, Novartis Pharma AG CASSS Bioassays, Silver Spring, March2015 CTL019 Introduction CARTs = Chimeric Antigen
The Lymphatic System and Body Defenses
 PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Lymphatic System and Body Defenses 12PART B Adaptive Defense System: Third Line of Defense Immune
PowerPoint Lecture Slide Presentation by Patty Bostwick-Taylor, Florence-Darlington Technical College The Lymphatic System and Body Defenses 12PART B Adaptive Defense System: Third Line of Defense Immune
Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell?
 Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Abbas Chapter 2: Sarah Spriet February 8, 2015 Question 1. Kupffer cells, microglial cells and osteoclasts are all examples of what type of immune system cell? a. Dendritic cells b. Macrophages c. Monocytes
Bone Marrow Transplantation and the Potential Role of Iomab-B
 Bone Marrow Transplantation and the Potential Role of Iomab-B Hillard M. Lazarus, MD, FACP Professor of Medicine, Director of Novel Cell Therapy Case Western Reserve University 1 Hematopoietic Cell Transplantation
Bone Marrow Transplantation and the Potential Role of Iomab-B Hillard M. Lazarus, MD, FACP Professor of Medicine, Director of Novel Cell Therapy Case Western Reserve University 1 Hematopoietic Cell Transplantation
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_cll_and_sll 2/2001
Corporate Medical Policy Hematopoietic Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_cll_and_sll 2/2001
Virological Surveillance in Paediatric HSCT Recipients
 Virological Surveillance in Paediatric HSCT Recipients Dr Pamela Lee Clinical Assistant Professor Department of Paediatrics & Adolescent Medicine Queen Mary Hospital LKS Faculty of Medicine, The University
Virological Surveillance in Paediatric HSCT Recipients Dr Pamela Lee Clinical Assistant Professor Department of Paediatrics & Adolescent Medicine Queen Mary Hospital LKS Faculty of Medicine, The University
Focus on Immunotherapy as a Targeted Therapy. Brad Nelson, PhD BC Cancer, Victoria, Canada FPON, Oct
 Focus on Immunotherapy as a Targeted Therapy Brad Nelson, PhD BC Cancer, Victoria, Canada FPON, Oct 18 2018 Disclosures I have nothing to disclose that is relevant to this presentation. Immunology @ Deeley
Focus on Immunotherapy as a Targeted Therapy Brad Nelson, PhD BC Cancer, Victoria, Canada FPON, Oct 18 2018 Disclosures I have nothing to disclose that is relevant to this presentation. Immunology @ Deeley
NATURAL KILLER T CELLS EBOOK
 08 April, 2018 NATURAL KILLER T CELLS EBOOK Document Filetype: PDF 90.41 KB 0 NATURAL KILLER T CELLS EBOOK Natural killer T cells (NK T cells) are a type of lymphocyte, or white blood cell. Natural killer
08 April, 2018 NATURAL KILLER T CELLS EBOOK Document Filetype: PDF 90.41 KB 0 NATURAL KILLER T CELLS EBOOK Natural killer T cells (NK T cells) are a type of lymphocyte, or white blood cell. Natural killer
Determinants of Immunogenicity and Tolerance. Abul K. Abbas, MD Department of Pathology University of California San Francisco
 Determinants of Immunogenicity and Tolerance Abul K. Abbas, MD Department of Pathology University of California San Francisco EIP Symposium Feb 2016 Why do some people respond to therapeutic proteins?
Determinants of Immunogenicity and Tolerance Abul K. Abbas, MD Department of Pathology University of California San Francisco EIP Symposium Feb 2016 Why do some people respond to therapeutic proteins?
Cover Page. The handle holds various files of this Leiden University dissertation.
 Cover Page The handle http://hdl.handle.net/1887/20522 holds various files of this Leiden University dissertation. Author: Stevanović, Sanja Title: Exploiting HLA-class II disparity for anti-tumor immunity
Cover Page The handle http://hdl.handle.net/1887/20522 holds various files of this Leiden University dissertation. Author: Stevanović, Sanja Title: Exploiting HLA-class II disparity for anti-tumor immunity
RXi Pharmaceuticals. Immuno-Oncology World Frontiers Conference. January 23, 2018 NASDAQ: RXII. Property of RXi Pharmaceuticals
 RXi Pharmaceuticals Immuno-Oncology World Frontiers Conference January 23, 2018 NASDAQ: RXII Forward Looking Statements This presentation contains forward-looking statements within the meaning of the Private
RXi Pharmaceuticals Immuno-Oncology World Frontiers Conference January 23, 2018 NASDAQ: RXII Forward Looking Statements This presentation contains forward-looking statements within the meaning of the Private
CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow
 White Paper September 2016 CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow Lily C. Trajman, PhD Introduction: Hematopoietic Stem Cells (HSCs)
White Paper September 2016 CD34+ Cells: A Comparison of Stem and Progenitor Cells in Cord Blood, Peripheral Blood, and the Bone Marrow Lily C. Trajman, PhD Introduction: Hematopoietic Stem Cells (HSCs)
C. Incorrect! MHC class I molecules are not involved in the process of bridging in ADCC.
 Immunology - Problem Drill 13: T- Cell Mediated Immunity Question No. 1 of 10 1. During Antibody-dependent cell mediated cytotoxicity (ADCC), the antibody acts like a bridge between the specific antigen
Immunology - Problem Drill 13: T- Cell Mediated Immunity Question No. 1 of 10 1. During Antibody-dependent cell mediated cytotoxicity (ADCC), the antibody acts like a bridge between the specific antigen
Hematopoietic Stem Cells, Stem Cell Processing, and Transplantation
 Hematopoietic Stem Cells, Stem Cell Processing, and Joseph (Yossi) Schwartz, M irector, Hemotherapy and Stem Cell Processing Facility Bone Marrow Can Cure: Leukemia Lymphoma Multiple Myeloma Genetic iseases:
Hematopoietic Stem Cells, Stem Cell Processing, and Joseph (Yossi) Schwartz, M irector, Hemotherapy and Stem Cell Processing Facility Bone Marrow Can Cure: Leukemia Lymphoma Multiple Myeloma Genetic iseases:
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
T-cell activation T cells migrate to secondary lymphoid tissues where they interact with antigen, antigen-presenting cells, and other lymphocytes:
 Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Interactions between innate immunity & adaptive immunity What happens to T cells after they leave the thymus? Naïve T cells exit the thymus and enter the bloodstream. If they remain in the bloodstream,
Understanding the role of ex vivo T cell depletion
 Prevention of graftversus-host disease (GVHD) Understanding the role of ex vivo T cell depletion Information for patients undergoing allogeneic stem cell transplantation in AML and their families 2 This
Prevention of graftversus-host disease (GVHD) Understanding the role of ex vivo T cell depletion Information for patients undergoing allogeneic stem cell transplantation in AML and their families 2 This
Is in vitro T-cell depletion necessary for Haploidentical TransplantationTitle of Presentation. Disclosure of Interest: Nothing to Disclose
 Rupert Handgretinger Children s University Hospital, Tübingen, Germany Is in vitro T-cell depletion necessary for Haploidentical TransplantationTitle of Presentation Disclosure of Interest: Nothing to
Rupert Handgretinger Children s University Hospital, Tübingen, Germany Is in vitro T-cell depletion necessary for Haploidentical TransplantationTitle of Presentation Disclosure of Interest: Nothing to
Revista Cubana de Hematología, Inmunología y Hemoterapia. 2017; 36 (Suplemento).
 Depletion of TCR alpha/beta+ T-lymphocytes from grafts for haplo haematopoietic CELL transplantation (HCT) in children Heilmann C, Ifversen M, Haastrup E, Fischer-Nielsen A. Haematopoietic Cell Transplantation
Depletion of TCR alpha/beta+ T-lymphocytes from grafts for haplo haematopoietic CELL transplantation (HCT) in children Heilmann C, Ifversen M, Haastrup E, Fischer-Nielsen A. Haematopoietic Cell Transplantation
Umbilical Cord Blood Transplantation
 Umbilical Cord Blood Transplantation Current Results John E. Wagner, M.D. Blood and Marrow Transplant Program and Stem Cell Institute University of Minnesota Donor Choices Unrelated Marrow/PBSC Results
Umbilical Cord Blood Transplantation Current Results John E. Wagner, M.D. Blood and Marrow Transplant Program and Stem Cell Institute University of Minnesota Donor Choices Unrelated Marrow/PBSC Results
Trends in Hematopoietic Cell Transplantation. AAMAC Patient Education Day Oct 2014
 Trends in Hematopoietic Cell Transplantation AAMAC Patient Education Day Oct 2014 Objectives Review the principles behind allogeneic stem cell transplantation Outline the process of transplant, some of
Trends in Hematopoietic Cell Transplantation AAMAC Patient Education Day Oct 2014 Objectives Review the principles behind allogeneic stem cell transplantation Outline the process of transplant, some of
ASH 2011 aktualijos: MSC TPŠL gydyme. Mindaugas Stoškus VULSK HOTC MRMS
 ASH 2011 aktualijos: MSC TPŠL gydyme Mindaugas Stoškus VULSK HOTC MRMS #3042. Yukiyasu Ozawa et al. Mesenchymal Stem Cells As a Treatment for Steroid-Resistant Acute Graft Versus Host Disease (agvhd);
ASH 2011 aktualijos: MSC TPŠL gydyme Mindaugas Stoškus VULSK HOTC MRMS #3042. Yukiyasu Ozawa et al. Mesenchymal Stem Cells As a Treatment for Steroid-Resistant Acute Graft Versus Host Disease (agvhd);
T cells III: Cytotoxic T lymphocytes and natural killer cells
 T cells III: Cytotoxic T lymphocytes and natural killer cells Margrit Wiesendanger Division of Rheumatology, CUMC September 17, 2008 Killer cells: CD8 + T cells (adaptive) vs. natural killer (innate) Shared
T cells III: Cytotoxic T lymphocytes and natural killer cells Margrit Wiesendanger Division of Rheumatology, CUMC September 17, 2008 Killer cells: CD8 + T cells (adaptive) vs. natural killer (innate) Shared
Tumor Immunology. Wirsma Arif Harahap Surgical Oncology Consultant
 Tumor Immunology Wirsma Arif Harahap Surgical Oncology Consultant 1) Immune responses that develop to cancer cells 2) Escape of cancer cells 3) Therapies: clinical and experimental Cancer cells can be
Tumor Immunology Wirsma Arif Harahap Surgical Oncology Consultant 1) Immune responses that develop to cancer cells 2) Escape of cancer cells 3) Therapies: clinical and experimental Cancer cells can be
Tumor Immunology: A Primer
 Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Transcript Details This is a transcript of a continuing medical education (CME) activity accessible on the ReachMD network. Additional media formats for the activity and full activity details (including
Cord Blood Transplant. E. Gluckman Eurocord ESH-EBMT training course Vienna 2014
 Cord Blood Transplant E. Gluckman Eurocord ESH-EBMT training course Vienna 2014 Background Since 1988, umbilical cord blood (CB) has been successfully used to treat children and adults needing stem cell
Cord Blood Transplant E. Gluckman Eurocord ESH-EBMT training course Vienna 2014 Background Since 1988, umbilical cord blood (CB) has been successfully used to treat children and adults needing stem cell
Immune Checkpoints. PD Dr med. Alessandra Curioni-Fontecedro Department of Hematology and Oncology Cancer Center Zurich University Hospital Zurich
 Immune Checkpoints PD Dr med. Alessandra Curioni-Fontecedro Department of Hematology and Oncology Cancer Center Zurich University Hospital Zurich Activation of T cells requires co-stimulation Science 3
Immune Checkpoints PD Dr med. Alessandra Curioni-Fontecedro Department of Hematology and Oncology Cancer Center Zurich University Hospital Zurich Activation of T cells requires co-stimulation Science 3
Additional file 6. Summary of experiments characterizing development of adaptive immune response
 Additional file 6. Summary of experiments characterizing development of adaptive immune response Tests performed to evaluate the development of adaptive immunity in patients are summarized in Table 1.
Additional file 6. Summary of experiments characterizing development of adaptive immune response Tests performed to evaluate the development of adaptive immunity in patients are summarized in Table 1.
Important new concerns or changes to the current ones will be included in updates of YESCARTA s RMP.
 PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN Summary of risk management plan for YESCARTA (axicabtagene ciloleucel) This is a summary of the risk management plan (RMP) for YESCARTA. The RMP details important
PART VI: SUMMARY OF THE RISK MANAGEMENT PLAN Summary of risk management plan for YESCARTA (axicabtagene ciloleucel) This is a summary of the risk management plan (RMP) for YESCARTA. The RMP details important
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplant_for_acute_myeloid_leukemia
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplant_for_acute_myeloid_leukemia
Darwinian selection and Newtonian physics wrapped up in systems biology
 Darwinian selection and Newtonian physics wrapped up in systems biology Concept published in 1957* by Macfarland Burnet (1960 Nobel Laureate for the theory of induced immune tolerance, leading to solid
Darwinian selection and Newtonian physics wrapped up in systems biology Concept published in 1957* by Macfarland Burnet (1960 Nobel Laureate for the theory of induced immune tolerance, leading to solid
THERAPEUTIC IMPLICATIONS OF PREPARING AND ADMINISTERING INNATE IMMUNE CELLS. 9:40 am to 10:10 pm Laurence Cooper
 THERAPEUTIC IMPLICATIONS OF PREPARING AND ADMINISTERING INNATE IMMUNE CELLS 9:40 am to 10:10 pm Laurence Cooper ljncooper@ziopharm.com 05-16-2016 Forward-looking statements This presentation contains certain
THERAPEUTIC IMPLICATIONS OF PREPARING AND ADMINISTERING INNATE IMMUNE CELLS 9:40 am to 10:10 pm Laurence Cooper ljncooper@ziopharm.com 05-16-2016 Forward-looking statements This presentation contains certain
Bases for Immunotherapy in Multiple Myeloma
 Bases for Immunotherapy in Multiple Myeloma Paola Neri, MD, PhD Associate Professor of Medicine University of Calgary, Arnie Charbonneau Cancer Institute Disclosures Paola Neri MD, PhD Grants/research
Bases for Immunotherapy in Multiple Myeloma Paola Neri, MD, PhD Associate Professor of Medicine University of Calgary, Arnie Charbonneau Cancer Institute Disclosures Paola Neri MD, PhD Grants/research
Ex-Vivo heat shock protein 70-peptide-activated, autologous natural killer cells adoptive therapy: from the bench to the clinic
 Ex-Vivo heat shock protein 70-peptide-activated, autologous natural killer cells adoptive therapy: from the bench to the clinic isbtc 10-13 November 2005 Valeria Milani, MD, PhD Munich Agenda 1. NK ligands
Ex-Vivo heat shock protein 70-peptide-activated, autologous natural killer cells adoptive therapy: from the bench to the clinic isbtc 10-13 November 2005 Valeria Milani, MD, PhD Munich Agenda 1. NK ligands
Scott Abrams, Ph.D. Professor of Oncology, x4375 Kuby Immunology SEVENTH EDITION
 Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 13 Effector Responses: Cell- and Antibody-Mediated Immunity Copyright 2013 by W. H.
Scott Abrams, Ph.D. Professor of Oncology, x4375 scott.abrams@roswellpark.org Kuby Immunology SEVENTH EDITION CHAPTER 13 Effector Responses: Cell- and Antibody-Mediated Immunity Copyright 2013 by W. H.
Abstract #163 Michael Kalos, PhD
 LONG TERM FUNCTIONAL PERSISTENCE, B CELL APLASIA AND ANTI LEUKEMIA EFFICACY IN REFRACTORY B CELL MALIGNANCIES FOLLOWING T CELL IMMUNOTHERAPY USING CAR REDIRECTED REDIRECTED T CELLS TARGETING CD19 Abstract
LONG TERM FUNCTIONAL PERSISTENCE, B CELL APLASIA AND ANTI LEUKEMIA EFFICACY IN REFRACTORY B CELL MALIGNANCIES FOLLOWING T CELL IMMUNOTHERAPY USING CAR REDIRECTED REDIRECTED T CELLS TARGETING CD19 Abstract
1. Overview of Adaptive Immunity
 Chapter 17A: Adaptive Immunity Part I 1. Overview of Adaptive Immunity 2. T and B Cell Production 3. Antigens & Antigen Presentation 4. Helper T cells 1. Overview of Adaptive Immunity The Nature of Adaptive
Chapter 17A: Adaptive Immunity Part I 1. Overview of Adaptive Immunity 2. T and B Cell Production 3. Antigens & Antigen Presentation 4. Helper T cells 1. Overview of Adaptive Immunity The Nature of Adaptive
Graft Versus Tumour Effect
 Graft Versus Tumour Effect Mairéad NíChonghaile 12 Abstract The treatment of relapsed disease remains challenging, and it is well accepted that concept of allogeneic HSCT relies upon both the conditioning
Graft Versus Tumour Effect Mairéad NíChonghaile 12 Abstract The treatment of relapsed disease remains challenging, and it is well accepted that concept of allogeneic HSCT relies upon both the conditioning
TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer
 AD Award Number: W8-XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke,. CONTRACTING ORGANIZATION: Johns Hopkins
AD Award Number: W8-XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke,. CONTRACTING ORGANIZATION: Johns Hopkins
The Immune System. Innate. Adaptive. - skin, mucosal barriers - complement - neutrophils, NK cells, mast cells, basophils, eosinophils
 Objectives - explain the rationale behind cellular adoptive immunotherapy - describe methods of improving cellular adoptive immunotherapy - identify mechanisms of tumor escape from cellular adoptive immunotherapy
Objectives - explain the rationale behind cellular adoptive immunotherapy - describe methods of improving cellular adoptive immunotherapy - identify mechanisms of tumor escape from cellular adoptive immunotherapy
Third line of Defense. Topic 8 Specific Immunity (adaptive) (18) 3 rd Line = Prophylaxis via Immunization!
 Topic 8 Specific Immunity (adaptive) (18) Topics - 3 rd Line of Defense - B cells - T cells - Specific Immunities 1 3 rd Line = Prophylaxis via Immunization! (a) A painting of Edward Jenner depicts a cow
Topic 8 Specific Immunity (adaptive) (18) Topics - 3 rd Line of Defense - B cells - T cells - Specific Immunities 1 3 rd Line = Prophylaxis via Immunization! (a) A painting of Edward Jenner depicts a cow
2/16/2018. The Immune System and Cancer. Fatal Melanoma Transferred in a Donated Kidney 16 years after Melanoma Surgery
 C007: Immunology of Melanoma: Mechanisms of Immune Therapies Delphine J. Lee, MD, PhD Chief and Program Director, Dermatology, Harbor UCLA Medical Center Principal Investigator, Los Angeles Biomedical
C007: Immunology of Melanoma: Mechanisms of Immune Therapies Delphine J. Lee, MD, PhD Chief and Program Director, Dermatology, Harbor UCLA Medical Center Principal Investigator, Los Angeles Biomedical
Children's Hospital of Pittsburgh Annual Progress Report: 2011 Formula Grant
 Children's Hospital of Pittsburgh Annual Progress Report: 2011 Formula Grant Reporting Period July 1, 2012 June 30, 2013 Formula Grant Overview The Children's Hospital of Pittsburgh received $228,401 in
Children's Hospital of Pittsburgh Annual Progress Report: 2011 Formula Grant Reporting Period July 1, 2012 June 30, 2013 Formula Grant Overview The Children's Hospital of Pittsburgh received $228,401 in
The Adaptive Immune Responses
 The Adaptive Immune Responses The two arms of the immune responses are; 1) the cell mediated, and 2) the humoral responses. In this chapter we will discuss the two responses in detail and we will start
The Adaptive Immune Responses The two arms of the immune responses are; 1) the cell mediated, and 2) the humoral responses. In this chapter we will discuss the two responses in detail and we will start
Immune Reconstitution Following Hematopoietic Cell Transplant
 Immune Reconstitution Following Hematopoietic Cell Transplant Patrick J. Kiel, PharmD, BCPS, BCOP Clinical Pharmacy Specialist Indiana University Simon Cancer Center Conflicts of Interest Speaker Bureau
Immune Reconstitution Following Hematopoietic Cell Transplant Patrick J. Kiel, PharmD, BCPS, BCOP Clinical Pharmacy Specialist Indiana University Simon Cancer Center Conflicts of Interest Speaker Bureau
The role of HLA in Allogeneic Hematopoietic Stem Cell Transplantation and Platelet Refractoriness.
 The role of HLA in Allogeneic Hematopoietic Stem Cell Transplantation and Platelet Refractoriness. Robert Liwski, MD, PhD, FRCPC Medical Director HLA Typing Laboratory Department of Pathology Dalhousie
The role of HLA in Allogeneic Hematopoietic Stem Cell Transplantation and Platelet Refractoriness. Robert Liwski, MD, PhD, FRCPC Medical Director HLA Typing Laboratory Department of Pathology Dalhousie
Summary of Changes BMT CTN 1101 Version 7.0 to 8.0 Dated: January 18, Original text: Changed to: Rationale
 BMT CTN 1101 RIC ducb vs. Haplo Page 1 of 10 Date: January 20, 2017 Summary of Changes BMT CTN 1101 Version 7.0 to 8.0 Dated: January 18, 2017 A Multi-Center, Phase III, Randomized Trial of Reduced Intensity(RIC)
BMT CTN 1101 RIC ducb vs. Haplo Page 1 of 10 Date: January 20, 2017 Summary of Changes BMT CTN 1101 Version 7.0 to 8.0 Dated: January 18, 2017 A Multi-Center, Phase III, Randomized Trial of Reduced Intensity(RIC)
Transplantation. Immunology Unit College of Medicine King Saud University
 Transplantation Immunology Unit College of Medicine King Saud University Objectives To understand the diversity among human leukocyte antigens (HLA) or major histocompatibility complex (MHC) To know the
Transplantation Immunology Unit College of Medicine King Saud University Objectives To understand the diversity among human leukocyte antigens (HLA) or major histocompatibility complex (MHC) To know the
Immune Checkpoint Inhibitors: The New Breakout Stars in Cancer Treatment
 Immune Checkpoint Inhibitors: The New Breakout Stars in Cancer Treatment 1 Introductions Peter Langecker, MD, PhD Executive Medical Director, Global Oncology Clinipace Worldwide Mark Shapiro Vice President
Immune Checkpoint Inhibitors: The New Breakout Stars in Cancer Treatment 1 Introductions Peter Langecker, MD, PhD Executive Medical Director, Global Oncology Clinipace Worldwide Mark Shapiro Vice President
Guidelines for Gamma Irradiation of Blood Components
 AUSTRALIAN & NEW ZEALAND SOCIETY OF BLOOD TRANSFUSION INC. AUSTRALIAN RED CROSS BLOOD SERVICE NEW ZEALAND BLOOD SERVICE Guidelines for Gamma Irradiation of Blood Components Revised 2003 AN ZS B T Australian
AUSTRALIAN & NEW ZEALAND SOCIETY OF BLOOD TRANSFUSION INC. AUSTRALIAN RED CROSS BLOOD SERVICE NEW ZEALAND BLOOD SERVICE Guidelines for Gamma Irradiation of Blood Components Revised 2003 AN ZS B T Australian
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_ transplantation_for_primary_amyloidosis 2/2001 11/2018 11/2019 11/2018 Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_ transplantation_for_primary_amyloidosis 2/2001 11/2018 11/2019 11/2018 Description
The Immune System: Innate and Adaptive Body Defenses Outline PART 1: INNATE DEFENSES 21.1 Surface barriers act as the first line of defense to keep
 The Immune System: Innate and Adaptive Body Defenses Outline PART 1: INNATE DEFENSES 21.1 Surface barriers act as the first line of defense to keep invaders out of the body (pp. 772 773; Fig. 21.1; Table
The Immune System: Innate and Adaptive Body Defenses Outline PART 1: INNATE DEFENSES 21.1 Surface barriers act as the first line of defense to keep invaders out of the body (pp. 772 773; Fig. 21.1; Table
Mon, Wed, Fri 11:00 AM-12:00 PM. Owen, Judy, Jenni Punt, and Sharon Stranford Kuby-Immunology, 7th. Edition. W.H. Freeman and Co., New York.
 Course Title: Course Number: Immunology Biol-341/541 Semester: Fall 2013 Location: HS 268 Time: Instructor: 8:00-9:30 AM Tue/Thur Dr. Colleen M. McDermott Office: Nursing Ed 101 (424-1217) E-mail*: mcdermot@uwosh.edu
Course Title: Course Number: Immunology Biol-341/541 Semester: Fall 2013 Location: HS 268 Time: Instructor: 8:00-9:30 AM Tue/Thur Dr. Colleen M. McDermott Office: Nursing Ed 101 (424-1217) E-mail*: mcdermot@uwosh.edu
Melanoma Bridge Meeting
 Melanoma Bridge Meeting Improving Adoptive Immune Therapy with Genetically Engineered T cells David Stroncek, MD Chief, Cell Processing Section, DTM, CC, NIH 3 December 2015 Adoptive T Cell Therapy: Dose
Melanoma Bridge Meeting Improving Adoptive Immune Therapy with Genetically Engineered T cells David Stroncek, MD Chief, Cell Processing Section, DTM, CC, NIH 3 December 2015 Adoptive T Cell Therapy: Dose
TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer
 AD Award Number: W8XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke Ph.D. CONTRACTING ORGANIZATION: Johns Hopkins
AD Award Number: W8XWH-5-- TITLE: Development of Antigen Presenting Cells for adoptive immunotherapy in prostate cancer PRINCIPAL INVESTIGATOR: Mathias Oelke Ph.D. CONTRACTING ORGANIZATION: Johns Hopkins
ACTR (Antibody Coupled T-cell Receptor): A universal approach to T-cell therapy
 ACTR (Antibody Coupled T-cell Receptor): A universal approach to T-cell therapy European Medicines Agency Workshop on Scientific and Regulatory Challenges of Genetically Modified Cell-based Cancer Immunotherapy
ACTR (Antibody Coupled T-cell Receptor): A universal approach to T-cell therapy European Medicines Agency Workshop on Scientific and Regulatory Challenges of Genetically Modified Cell-based Cancer Immunotherapy
Dedicated to Gordon. Stem Cell Transplantation: The Journey
 Dedicated to Gordon Stem Cell Transplantation: The Journey 1949 Jacobson et al: Radioprotection by lead shielding of the spleen of a lethally irradiated animal 1951 Lorenz et al: Radiation protection
Dedicated to Gordon Stem Cell Transplantation: The Journey 1949 Jacobson et al: Radioprotection by lead shielding of the spleen of a lethally irradiated animal 1951 Lorenz et al: Radiation protection
UPDATE Autologous Stem Cell Transplantation for Lymphoma and Myeloma
 UPDATE Autologous Stem Cell Transplantation for Lymphoma and Myeloma Supported by a grant from Supported by a grant from UPDATE Autologous Stem Cell Transplantation for Lymphoma and Myeloma Jonathan W.
UPDATE Autologous Stem Cell Transplantation for Lymphoma and Myeloma Supported by a grant from Supported by a grant from UPDATE Autologous Stem Cell Transplantation for Lymphoma and Myeloma Jonathan W.
Adoptive Immunotherapy
 Adoptive Immunotherapy Policy Number: 8.01.01 Last Review: 6/2018 Origination: 12/2015 Next Review: 12/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Adoptive
Adoptive Immunotherapy Policy Number: 8.01.01 Last Review: 6/2018 Origination: 12/2015 Next Review: 12/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Adoptive
6/7/17. Immune cells. Co-evolution of innate and adaptive immunity. Importance of NK cells. Cells of innate(?) immune response
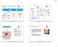 Immune cells Co-evolution of innate and adaptive immunity 1 2 Importance of NK cells Cells of innate(?) immune response Patients with NK cell deficiency may lead to fatal infections and have an increased
Immune cells Co-evolution of innate and adaptive immunity 1 2 Importance of NK cells Cells of innate(?) immune response Patients with NK cell deficiency may lead to fatal infections and have an increased
The question is not whether or not to deplete T-cells, but how to deplete which T-cells
 The question is not whether or not to deplete T-cells, but how to deplete which T-cells CD34+ positive selection Negative Depletion of: CD3/CD19 TcRαβ/CD19 T-cell depletion: positive selection versus negative
The question is not whether or not to deplete T-cells, but how to deplete which T-cells CD34+ positive selection Negative Depletion of: CD3/CD19 TcRαβ/CD19 T-cell depletion: positive selection versus negative
CHAPTER 3 LABORATORY PROCEDURES
 CHAPTER 3 LABORATORY PROCEDURES CHAPTER 3 LABORATORY PROCEDURES 3.1 HLA TYPING Molecular HLA typing will be performed for all donor cord blood units and patients in the three reference laboratories identified
CHAPTER 3 LABORATORY PROCEDURES CHAPTER 3 LABORATORY PROCEDURES 3.1 HLA TYPING Molecular HLA typing will be performed for all donor cord blood units and patients in the three reference laboratories identified
Transplant Booklet D Page 1
 Booklet D Pretest Correct Answers 4. (A) is correct. Technically, performing a hematopoietic stem cell transplant is one of the simplest transplantation procedures. The hematopoietic stem cells are infused
Booklet D Pretest Correct Answers 4. (A) is correct. Technically, performing a hematopoietic stem cell transplant is one of the simplest transplantation procedures. The hematopoietic stem cells are infused
Fluid movement in capillaries. Not all fluid is reclaimed at the venous end of the capillaries; that is the job of the lymphatic system
 Capillary exchange Fluid movement in capillaries Not all fluid is reclaimed at the venous end of the capillaries; that is the job of the lymphatic system Lymphatic vessels Lymphatic capillaries permeate
Capillary exchange Fluid movement in capillaries Not all fluid is reclaimed at the venous end of the capillaries; that is the job of the lymphatic system Lymphatic vessels Lymphatic capillaries permeate
Adoptive Immunotherapy
 Adoptive Immunotherapy Policy Number: 8.01.01 Last Review: 10/2018 Origination: 12/2015 Next Review: 12/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Adoptive
Adoptive Immunotherapy Policy Number: 8.01.01 Last Review: 10/2018 Origination: 12/2015 Next Review: 12/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC) will provide coverage for Adoptive
Synergistic combinations of targeted immunotherapy to combat cancer
 Synergistic combinations of targeted immunotherapy to combat cancer Myung Ah Lee, M.D., Ph. D Division of Medical Oncology, Hepato-biliary pancreatic cancer center Seoul St. Mary s hospital, The Catholic
Synergistic combinations of targeted immunotherapy to combat cancer Myung Ah Lee, M.D., Ph. D Division of Medical Oncology, Hepato-biliary pancreatic cancer center Seoul St. Mary s hospital, The Catholic
