DESCRIPTION OF PROCEDURE/SERVICE/PHARMACEUTICAL
|
|
|
- Louisa Cooper
- 5 years ago
- Views:
Transcription
1 Subject: Hematopoietic Stem Cell Transplantation for Immunodeficiency Disorders Original Effective Date: 2/10/16 Policy Number: MCP-265 Revision Date(s): Review Date: 06/06/17, 05/01/18 DISCLAIMER This Molina Clinical Policy (MCP) is intended to facilitate the Utilization Management process. It expresses Molina's determination as to whether certain services or supplies are medically necessary, experimental, investigational, or cosmetic for purposes of determining appropriateness of payment. The conclusion that a particular service or supply is medically necessary does not constitute a representation or warranty that this service or supply is covered (i.e., will be paid for by Molina) for a particular member. The member's benefit plan determines coverage. Each benefit plan defines which services are covered, which are excluded, and which are subject to dollar caps or other limits. Members and their providers will need to consult the member's benefit plan to determine if there are any exclusion(s) or other benefit limitations applicable to this service or supply. If there is a discrepancy between this policy and a member's plan of benefits, the benefits plan will govern. In addition, coverage may be mandated by applicable legal requirements of a State, the Federal government or CMS for Medicare and Medicaid members. CMS's Coverage Database can be found on the CMS website. The coverage directive(s) and criteria from an existing National Coverage Determination (NCD) or Local Coverage Determination (LCD) will supersede the contents of this Molina Clinical Policy (MCP) document and provide the directive for all Medicare members. 1 DESCRIPTION OF PROCEDURE/SERVICE/PHARMACEUTICAL Immunodeficiency Disorders The primary immunodeficiencies are a genetically heterogeneous group of diseases that affect distinct components of the immune system. The most severe defects (collectively known as severe combined immunodeficiency or SCID) represents a group of rare, sometimes fatal, congenital disorders characterized by little or no immune response. The defining feature of SCID, commonly known as "bubble boy" disease, is a defect in the specialized white blood cells (B- and T-lymphocytes) that defend us from infection by viruses, bacteria and fungi. Without a functional immune system, SCID patients are susceptible to recurrent infections such as pneumonia, meningitis and chicken pox, and can die before the first year of life. Though invasive, new treatments such as bone marrow and stem-cell transplantation save as many as 80% of SCID patients. 32 Stem Cell Transplantation Stem-cell transplantation refers to transplantation of hematopoietic stem cells (HSCs) from a donor into a patient. HSCs are immature cells that can develop into any of the three types of blood cells (red cells, white cells or platelets). HSCs are created in the bone marrow and are found in the bone marrow and peripheral blood. There is also a high concentration of HSCs in umbilical-cord blood. Hematopoietic stem-cell transplantation (HSCT) can be either autologous (using the person s own stem cells) or allogeneic (using stem cells from a donor). In allogeneic HSCT, it is preferable for donors to have a human leukocyte antigen (HLA) type that is identical to the recipient. Matching is performed on the basis of variability at three of more loci of the HLA Page 1 of 10
2 gene (e.g., HLA-A, HLA-B, HLA-DRB1). As HLA variability increases, transplant-related morbidity and mortality, including graft rejection and graft-versus-host disease, also increase. RECOMMENDATION All transplants require prior authorization from the Corporate Transplant Department. Solid organ transplant requests will be reviewed by the Corporate Senior Medical Director or qualified clinical designee. All other transplants will be by the Corporate Senior Medical Director or covering Medical Director. If the criteria are met using appropriate NCD and/or LCD guidelines, state regulations and/or MCP policies the Corporate Senior Medical Director s designee can approve the requested transplant. Members must meet UNOS guidelines for transplantation and the diagnosis must be made by a Specialist in the Disease and or Transplant Surgeon. Pre-Transplant Evaluation: Criteria for transplant evaluation include all of the following: History and physical examination Psychosocial evaluation and clearance: o No behavioral health disorder by history or psychosocial issues: if history of behavioral health disorder, no severe psychosis or personality disorder mood/anxiety disorder must be excluded or treated member has understanding of surgical risk and post procedure compliance and follow-up required o Adequate family and social support EKG Chest x-ray Cardiac clearance in the presence of any of the following: o chronic smokers o > 50 years age o those with a clinical or family history of heart disease or diabetes Pulmonary clearance if evidence of pulmonary artery hypertension (PAH) or chronic pulmonary disease Neurological exam and clearance for transplant: [ONE] o Normal exam by H&P o Abnormal neurological exam with positive findings: [ONE] Lumbar puncture normal cytology Lumbar puncture with cytological exam abnormal: CNS disease treated prior to clearance Performance Status : [ONE] o Karnofsky score %; or o Eastern Cooperative Oncology Group (ECOG) grade 0-2 Lab studies: Page 2 of 10
3 o *Complete blood count, Kidney profile (blood urea nitrogen, creatinine), electrolytes, calcium, phosphorous, albumin, liver function tests, Coagulation profile (prothrombin time, and partial thromboplastin time) o *Serologic screening for HIV, Epstein Barr virus (EBV), Hepatitis virus B (HBV), and Hepatitis C(HCV), cytomegalovirus (CMV), RPR and/or FTA: If HIV positive all of the following are met: CD4 count >200 cells/mm-3 for >6 months HIV-1 RNA undetectable On stable anti-retroviral therapy >3 months No other complications from AIDS (e.g., opportunistic infection, including aspergillus, tuberculosis, coccidioides mycosis, resistant fungal infections, Kaposi s sarcoma, or other neoplasm) If abnormal serology need physician plan to address and/or treatment as indicated o UDS (urine drug screen) if patient is current or gives a history of past drug abuse *Colonoscopy (if indicated or if patient is 50 > older should have had an initial screening colonoscopy, after initial negative screening requires follow up colonoscopy every ten years) with complete workup and treatment of abnormal results as indicated *GYN examination with Pap smear for women > 21 to < 65 years of age or indicated (not indicated in women who have had a TAH or TVH) with in the last three year with complete workup and treatment of abnormal results as indicated Within the last 12 months: Dental examination or oral exam showing good dentition and oral care or no abnormality on panorex or plan for treatment of problems pre or post-transplant *Mammogram (if indicated or > age 40) with complete workup and treatment of abnormal results as indicated *PSA if history of prostate cancer or previously elevated PSA with complete workup and treatment of abnormal results as indicated *Participating Centers of Excellence may waive these criteria Criteria for Hematopoietic Allogeneic Stem Cell transplantation (HSCT) Transplantation: Hematopoietic Allogeneic stem-cell transplantation (HSCT) ablative or non-myeloablative from a human leukocyte antigen (HLA)-matched donor (i.e., at least six out of eight match of the HLA-A, HLA-B, HLA-C and HLA-DRB1 markers) or from cord blood when there are no matched sibling or unrelated donors (i.e. at least four out of six match of the HLA-A, HLA-B and HLA-DRB-1 markers) is considered medically necessary and may be authorized for the treatment of immunodeficiency disorders when ANY of the following criteria are met: [ALL] All pre-transplant criteria are met; and Diagnosis of one of the following immunodeficiency disorders (including but not limited to): [ONE] o Hemophagocytic Lymphohistiocytosis (HLH) o Severe Combined Immunodeficiency (SCID) o Wiskott-Aldrich Syndrome (WAS) o X-linked lymphoproliferative syndrome Page 3 of 10
4 o Chediak-Higashi syndrome o Primary granulocyte dysfunction o Chronic granulomatous disease o Omenn Syndrome o Leukocyte adhesion deficiency o DiGeorge Syndrome o Kostmann Syndrome AND The requesting transplant recipient should not have any of the following absolute contraindications: o Cardiac, pulmonary, and nervous system disease that cannot be corrected and is a prohibitive risk for surgery o Malignant neoplasm with a high risk for reoccurrence, non-curable malignancy (excluding localized skin cancer) o Systemic and/or uncontrolled infection o AIDS (CD4 count < 200cells/mm3) o Unwilling or unable to follow post-transplant regimen Documented history of non-compliance Inability to follow through with medication adherence or office follow-up o Chronic illness with one year or less life expectancy o Limited, irreversible rehabilitation potential o Active untreated substance abuse issues, requires documentation supporting free from addiction for minimally 6 months if previous addiction was present o No adequate social/family support The requesting transplant recipient should be evaluated carefully and potentially treated if the following relative contraindications are present: o Irreversible lung disease patients require consultation and clearance by a Pulmonologist prior to consideration of transplantation, this includes the following: o Smoking, documentation supporting free from smoking for 6 months o Active peptic ulcer disease o Active gastroesophageal reflux disease o CVA with long term impairment that is not amendable to rehabilitation or a patient with CVA/transient ischemic attack within past 6 months o Obesity with body mass index of >30 kg/m 2 may increase surgical risk o Chronic liver disease such as Hepatitis B/C/D, or cirrhosis which increases the risk of death from sepsis and hepatic failure requires consultation by a gastroenterologist or hepatologist o Gall bladder disease requires ultrasound of the gall bladder with treatment prior to transplantation Criteria for Subsequent Hematopoietic Stem Cell Transplantation: Page 4 of 10
5 2. Hematopoietic Allogeneic stem cell transplantation (ablative or non-myeloablative) may be authorized after the first prior stem cell transplantation has occurred only one time for members with mucopolysaccharidoses lycosomal storage disorders who meet all of the above criteria for transplant and have any of the following:[one] primary graft failure indicated by no signs of engraftment* by 42 days after the transplant; OR failure to engraft*; AND a suitable allogeneic donor has been identified if applicable *Note: Engraftment is defined as the first 3 consecutive days on which the absolute neutrophil count (ANC) exceeds 5 x 10 9 /L or > ANC500 at any time after transplantation. 26 CONTINUATION OF THERAPY When extension of a previously approved transplant authorization is requested, review using updated clinical information is appropriate. If Molina Healthcare has authorized prior requests for transplantation, the following information is required for medical review: [ALL] o Presence of no absolute contraindication as listed above; o History and physical within the last 12 months; o Kidney profile within the last 12 months; o Cardiac update if history of cardiac disease within two years (> 50 years of age); o Psychosocial evaluation or update within the last 12 months; o Per initial and updated history and physical, any other clinically indicated tests and/or scans as determined by transplant center physician or Molina Medical Director. If authorized prior requests for transplantation were obtained from another insurer, the following information is required for medical review: [ALL] o Authorization letter/documentation from previous insurer; o Presence of no absolute contraindication as listed above; o History and physical within the last 12 months; o Cardiac update if history of cardiac disease within two years (> 50 years of age); o Psychosocial evaluation or update within the last 12 months; o Per initial and updated history and physical, any other clinically indicated tests and/or scans as determined by transplant center physician or Molina Medical Director. COVERAGE EXCLUSIONS 1. Allogeneic (ablative or non-myeloablative) stem cell transplantation when the above criteria are not met 2. A second or repeat allogeneic (ablative or non-myeloablative) transplant due to persistent, progressive or relapsed disease 3. Autologous stem cell transplantation 4. A planned tandem allogeneic hematopoietic stem cell transplantation 5. Hematopoietic stem cell collection, storage and freezing for a future unplanned transplant Page 5 of 10
6 SUMMARY OF MEDICAL EVIDENCE The published medical evidence and outcomes for hematopoietic stem cell transplantation for immunodeficiency disorders in the United States consists of registry data obtained from transplant centers that perform adult and pediatric transplantation and is available from the United Network for Organ Sharing (UNOS) database. Registry data demonstrates graft survival rates and outcomes for stem cell transplantation based on demographic and clinical information. 2 There is a large amount of published literature regarding transplant outcomes in immunodeficiency disorders. Two recent studies that show graft survival rates and outcomes for stem cell transplantation are outlined below: A retrospective analysis by Rousso et al. was conducted of HSCT in children with PID in a tertiary medical center over the period of 1983 to Participants included 93 children with PID with a median follow-up of 3.6 years (range, 29 d to 21.2 y) after HSCT. The 2-year survival rates after HSCT for children with severe combined immune deficiency, hemophagocytic lymphohistiocytosis/lymphoproliferative disease, Wiskott- Aldrich syndrome, granulocyte defect, and undefined PID were 65.7%±6.8%, 80%±10.3%, 83.3%±15.2%, 75%±12.5%, and 25%±21.7%, respectively. Survival was associated with year of HSCT and matching. The hazard ratio (HR) (95% CI) for HSCT done in 1983 to 1999 compared with 2000 to 2012 and for matched (related and unrelated) compared with mismatched donor were 2.14 (0.99 to 4.653) and 3.07 (1.46 to 6.4), respectively. Survival was not associated with age, sex of the recipient, underlying PID, conditioning regimen, and presence of acute graft-versus-host disease. After adjustment to the underlying PID, donor and use of fludarabine-based conditioning, the HR (95% CI) for HSCT from the year 2000 was 4.69 (range, 1.4 to 15.45). Advances in HSCT over time have improved the survival of children with PID. 22 Gungor et al. performed a prospective study in 16 centers in 10 countries worldwide enrolled patients aged 0 to 40 years with CGD treated with RIC HSCT consisting of high-dose Flu, serotherapy or low-dose alemtuzumab, and low-dose (50% to 72% of myeloablative dose) or targeted busulfan administration. Unmanipulated bone marrow or peripheral blood stem cells from HLA-matched related-donors or HLA-9/10 or HLA-10/10 matched unrelateddonors were infused. The primary end points were OS and EFS, probabilities of OS and EFS at 2 years, incidence of acute and chronic GVHD, achievement of at least 90% myeloid donor chimerism, and incidence of graft failure after at least 6 months of follow-up. A total 56 patients (median age 12.7 years) with chronic granulomatous disease were enrolled; 42 patients (75%) had high-risk features (i.e., intractable infections and autoinflammation), 25 (45%) were adolescents and young adults (age years). Median time to engraftment was 19 days for neutrophils and 21 days for platelets. At median follow-up of 21 months, OS was 93% (52/56) and EFS was 89% (50/56). The 2-year probability of OS was 96% (95% confidence interval [CI], to 99.09) and of EFS was 91% (79.78 to 96.17). Graft-failure occurred in 5% (3/56) of patients. The cumulative incidence of acute GVHD of grade III to IV was 4% (2/56) and of chronic GVHD was 7% (4/56). Stable (>/=90%) myeloid donor chimerism was documented in 52 (93%) surviving patients. 11 Professional Society Guidelines: The National Marrow Donor Program: The NMDP recommends HCT at time of diagnosis for immunodeficiency disorders. 2 Page 6 of 10
7 CODING INFORMATION THE CODES LISTED IN THIS POLICY ARE FOR REFERENCE PURPOSES ONLY. LISTING OF A SERVICE OR DEVICE CODE IN THIS POLICY DOES NOT IMPLY THAT THE SERVICE DESCRIBED BY THIS CODE IS COVERED OR NON-COVERED. COVERAGE IS DETERMINED BY THE BENEFIT DOCUMENT. THIS LIST OF CODES MAY NOT BE ALL INCLUSIVE. CPT Description Collection Codes Blood-derived hematopoietic progenitor cell harvesting for transplantation, per collection; allogeneic Blood-derived hematopoietic progenitor cell harvesting for transplantation, per collection; autologous Bone marrow harvesting for transplantation; allogeneic Bone marrow harvesting for transplantation; autologous Cell Processing Services Transplant preparation of hematopoietic progenitor cells; cryopreservation and storage Transplant preparation of hematopoietic progenitor cells; thawing of previously frozen harvest, without washing Transplant preparation of hematopoietic progenitor cells; thawing of previously frozen harvest, with washing Transplant preparation of hematopoietic progenitor cells; specific cell depletion within harvest, T-cell depletion Transplant preparation of hematopoietic progenitor cells; tumor cell depletion Transplant preparation of hematopoietic progenitor cells; red blood cell removal Transplant preparation of hematopoietic progenitor cells; platelet depletion Transplant preparation of hematopoietic progenitor cells; plasma (volume) depletion Transplant preparation of hematopoietic progenitor cells; cell concentration in plasma, mononuclear, or buffy coat layer Cell infusion codes Bone marrow or blood-derived peripheral stem cell transplantation; allogeneic Bone marrow or blood-derived peripheral stem cell transplantation; autologous Bone marrow or blood-derived peripheral stem cell transplantation; allogeneic donor lymphocyte infusions Bone marrow or blood-derived peripheral stem cell transplantation; allogeneic hematopoietic cellular transplant boost HCPCS Description S2140 Cord blood harvesting for transplantation, allogeneic S2142 Cord blood derived stem-cell transplantation, allogeneic S2150 Bone marrow or blood-derived stem cells (peripheral or umbilical), allogeneic or autologous, harvesting, transplantation, and related complications; including pheresis and cell preparation/storage; marrow ablative therapy; drugs; supplies; hospitalization with outpatient followup; medical/surgical, diagnostic, emergency, and rehabilitative services; and the number of days of pre-and post-transplant care in the global definition ICD-9 Description: [For dates of service prior to 10/01/2015] Wiskott-Aldrich syndrome Combined immunity deficiency (severe combined immunodeficiency) Functional disorders of polymorphonuclear neutrophils (chronic granulomatous disease) Genetic anomalies of leukocytes (Chediak-Higashi) Page 7 of 10
8 ICD-10 Description: [For dates of service on or after 10/01/2015] D71 Functional disorders of polymorphonuclear neutrophils (chronic granulomatous disease) D76.1 Hemophagocytic lymphohistiocytosis D81.0-D81.9 Severe combined immunodeficiency (SCID) D82.0 Wiskott-Aldrich syndrome D82.3 Immunodeficiency following hereditary defective response to Epstein-Barr virus (X-linked lymphoproliferative disease) E Chediak-Higashi syndrome E X-linked adrenoleukodystrophy E RESOURCE REFERENCES Government Agency 1. Centers for Medicare & Medicaid Services. NCD for Stem Cell Transplantation ( ) Accessed at: 2. National Marrow Donor Program. Immune Deficiency Disorders Transplant Outcomes. Accessed at: Outcomes 3. National Bone Marrow Donor Program HLA Matching Requirements. Accessed at: Finding_the_Best_Don or_or_cord_blood_unit.aspx 4. Eastern Cooperative Oncology Group (ECOG) Performance Status. Accessed at: Peer Reviewed Publications 5. Blue Cross and Blue Shield Association. High-Dose Lymphoablative Therapy (HDLT) with or without Stem-Cell Rescue for Treatment of Severe Autoimmune Diseases. TEC Assessment. 2002; 16(14). 6. Boyle JM, Buckley RH. Population prevalence of diagnosed primary immunodeficiency diseases in the United States. J Clin Immunol. 2007; 27(5): Brown L, Xu-Bayford J, Allwood Z, et al. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: The case for newborn screening. Blood. 2011; 117(11): Filipovich, A. Hematopoietic cell transplantation for correction of primary immunodeficiencies. Bone Marrow Transplant Aug;42 Suppl 1:S49-S52. PMID: Filipovich, AH, Stone, JV, Tomany, SC, et al. Impact of donor type on outcome of bone marrow transplantation for Wiskott-Aldrich syndrome: collaborative study of the International Bone Marrow Transplant Registry and the National Marrow Donor Program. Blood Mar 15;97(6): PMID: Gennery AR, Cant AJ. Advances in hematopoietic stem cell transplantation for primary immunodeficiency. Immunol Allergy Clin North Am. 2008; 28(2): Gungor, T, Teira, P, Slatter, M, et al. Reduced-intensity conditioning and HLA-matched haemopoietic stemcell transplantation in patients with chronic granulomatous disease: a prospective multicentre study. Lancet Feb 1;383(9915): PMID: Page 8 of 10
9 12. Hassan, A, Booth, C, Brightwell, A, et al. Outcome of hematopoietic stem cell transplantation for adenosine deaminase-deficient severe combined immunodeficiency. Blood Oct 25;120(17): ; quiz 26. PMID: Henter JI, Samuelson-Horne A, Arico M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002; 100(7): Horwitz ME, Barrett AJ, Brown MR, et al. Treatment of chronic granulomatous disease with nonmyeloablative conditioning and T-cell-depleted hematopoietic allograft. N Engl J Med. 2001; 344(12): Joshi AY, Iyer VN, Hagan JB, St. Sauver JL, Boyce TG. Incidence and temporal trends of primary immunodeficiency: A population-based cohort study. Mayo Clin Proc. 2009; 84(1): Marsh, RA, Bleesing, JJ, Chandrakasan, S, Jordan, MB, Davies, SM, Filipovich, AH. Reduced-intensity conditioning hematopoietic cell transplantation is an effective treatment for patients with SLAMassociated protein deficiency/x-linked lymphoproliferative disease type 1. Biol Blood Marrow Transplant Oct;20(10): PMID: Moratto, D, Giliani, S, Bonfim, C, et al. Long-term outcome and lineage-specific chimerism in 194 patients with Wiskott-Aldrich syndrome treated by hematopoietic cell transplantation in the period : an international collaborative study. Blood Aug 11;118(6): PMID: Myers LA, Patel DD, Puck JM, Buckley RH. Hematopoietic stem cell transplantation for severe combined immunodeficiency in the neonatal period leads to superior thymic output and improved survival. Blood. 2002; 99(3): Ozsahin H, Cavazzana-Calvo M, Notarangelo LD, et al. Long-term outcome following hematopoietic stem-cell transplantation in Wiskott-Aldrich syndrome: collaborative study of the European Society for Immunodeficiencies and European Group for Blood and Marrow Transplantation. Blood. 2008; 111(1): Pai S-Y, Logan BR, Griffith LM, et al. Transplantation outcomes for severe combined immunodeficiency New Engl J Med. 2014; 371(5): Porta, F, Forino, C, De Martiis, D, et al. Stem cell transplantation for primary immunodeficiencies. Bone Marrow Transplant Jun;41 Suppl 2:S83-6. PMID: Rousso SZ, Shamriz O, Zilkha A. Hematopoietic Stem Cell Transplantations for Primary Immune Deficiencies: 3 Decades of Experience From a Tertiary Medical Center. J Pediatr Hematol Oncol Jul ;37(5):e doi: /MPH Smith, AR, Gross, TG, Baker, KS. Transplant outcomes for primary immunodeficiency disease. Semin Hematol Jan;47(1): PMID: Szabolcs, P, Cavazzana-Calvo, M, Fischer, A, Veys, P. Bone marrow transplantation for primary immunodeficiency diseases. Pediatr Clin North Am Feb;57(1): PMID: Professional Society Guidelines 25. National Marrow Donor Program (NMDP) and the American Society for Blood and Marrow Transplantation (ASBMT) 2016 referral guidelines: Recommended Timing for Transplant Consultation. Accessed at: Guidelines/ 26. National Marrow Donor Program (NMDP). Patient Eligibility for HCT. Accessed at: National Bone Marrow Donor Program. Measuring Engraftment. Accessed at: Joint Council of Allergy, Asthma, and Immunology (JCAAI) practice parameter on diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol 2005 May;94(5 Suppl 1):S1. Page 9 of 10
10 Other Resources 29. McKesson InterQual Criteria for Procedures: Adult 2015 InterQual Transplantation, Allogeneic Stem Cell; DynaMed Plus [Internet]. Ipswich (MA): EBSCO Information Services Severe combined immunodeficiency (SCID). Updated UpToDate: Holmberg L, Deeg H, Sandmaier B. Determining eligibility for autologous/allogenic hematopoietic cell transplantation Bonilla F. Severe combined immunodeficiency (SCID): An overview Bonilla F. Combined immunodeficiencies National Center for Biotechnology Information, U.S. National Library of Medicine. Diseases of the Immune System. Bethesda MD, USA Page 10 of 10
Subject: Hematopoietic Stem Cell Transplantation for Ewing s Sarcoma. Revision Date(s):
 Subject: Hematopoietic Stem Cell Transplantation for Ewing s Sarcoma Policy Number: MCP-272 Revision Date(s): Original Effective Date: 5/3/2016 Review Date: 6/22/2017, 3/8/2018 MCPC Approval Date: 3/8/2018
Subject: Hematopoietic Stem Cell Transplantation for Ewing s Sarcoma Policy Number: MCP-272 Revision Date(s): Original Effective Date: 5/3/2016 Review Date: 6/22/2017, 3/8/2018 MCPC Approval Date: 3/8/2018
Subject: Hematopoietic Stem Cell Transplantation for Chronic Myelogenous Leukemia (CML)
 Subject: Hematopoietic Stem Cell Transplantation for Chronic Myelogenous Leukemia (CML) Original Effective Date: 7/25/14 Policy Number: MCP-187 Revision Date(s): 6/2/15, 7/27/2017 Review Date: 12/16/15,
Subject: Hematopoietic Stem Cell Transplantation for Chronic Myelogenous Leukemia (CML) Original Effective Date: 7/25/14 Policy Number: MCP-187 Revision Date(s): 6/2/15, 7/27/2017 Review Date: 12/16/15,
Subject: Hematopoietic Stem Cell Transplantation for Germ Cell Tumors 9/17/14
 Subject: Hematopoietic Stem Cell Transplantation for Germ Cell Tumors Original Effective Date: 9/17/14 Policy Number: MCP-194 Revision Date(s): 6/2/15, 12/13/2017 Review Date: 12/16/15, 9/15/16 MCPC Approval
Subject: Hematopoietic Stem Cell Transplantation for Germ Cell Tumors Original Effective Date: 9/17/14 Policy Number: MCP-194 Revision Date(s): 6/2/15, 12/13/2017 Review Date: 12/16/15, 9/15/16 MCPC Approval
Subject: Hematopoietic Stem Cell Transplantation for Acute Myelogenous Leukemia (AML) Revision Date(s): 9/1/2015
 Subject: Hematopoietic Stem Cell Transplantation for Acute Myelogenous Leukemia (AML) Original Effective Date: 10/31/12 Policy Number: MCP-119 Revision Date(s): 9/1/2015 Review Date: 12/16/15, 12/14/16,
Subject: Hematopoietic Stem Cell Transplantation for Acute Myelogenous Leukemia (AML) Original Effective Date: 10/31/12 Policy Number: MCP-119 Revision Date(s): 9/1/2015 Review Date: 12/16/15, 12/14/16,
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_cll_and_sll 2/2001
Corporate Medical Policy Hematopoietic Cell Transplantation for CLL and SLL File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_cll_and_sll 2/2001
Subject: Hematopoietic Stem Cell Transplantation for Chronic Lymphoblastic Leukemia (CLL) and small lymphocytic lymphoma (SLL)
 Subject: Hematopoietic Stem Cell Transplantation for Chronic Lymphoblastic Leukemia (CLL) and small lymphocytic lymphoma (SLL) Original Effective Date: 7/29/14 Guidance Number: MCG-188 Revision Date(s):
Subject: Hematopoietic Stem Cell Transplantation for Chronic Lymphoblastic Leukemia (CLL) and small lymphocytic lymphoma (SLL) Original Effective Date: 7/29/14 Guidance Number: MCG-188 Revision Date(s):
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Waldenstrom Macroglobulinemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem_cell_transplantation_for_waldenstrom_macroglobulinemia
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Waldenstrom Macroglobulinemia File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem_cell_transplantation_for_waldenstrom_macroglobulinemia
Revision Date(s): 4/8/2015
 Subject: Heart Transplantation Original Effective Date: 9/24/2012 Policy Number: MCP-116 Review Date: 12/16/15, 9/15/16, 6/22/17 Revision Date(s): 4/8/2015 DISCLAIMER This imolina Clinical Policy (MCP)
Subject: Heart Transplantation Original Effective Date: 9/24/2012 Policy Number: MCP-116 Review Date: 12/16/15, 9/15/16, 6/22/17 Revision Date(s): 4/8/2015 DISCLAIMER This imolina Clinical Policy (MCP)
Rob Wynn RMCH & University of Manchester, UK. HCT in Children
 Rob Wynn RMCH & University of Manchester, UK HCT in Children Summary Indications for HCT in children Donor selection for Paediatric HCT Using cords Achieving engraftment in HCT Conditioning Immune action
Rob Wynn RMCH & University of Manchester, UK HCT in Children Summary Indications for HCT in children Donor selection for Paediatric HCT Using cords Achieving engraftment in HCT Conditioning Immune action
Regence. Next Review: 08/2019 Last Review: 08/2018 Medicare Link(s) Revised: 10/01/2018 IMPORTANT REMINDER
 Regence Medicare Advantage Policy Manual Policy ID: M-TRA45 Stem Cell / Bone Marrow Transplants Published: 10/01/2018 Next Review: 08/2019 Last Review: 08/2018 Medicare Link(s) Revised: 10/01/2018 IMPORTANT
Regence Medicare Advantage Policy Manual Policy ID: M-TRA45 Stem Cell / Bone Marrow Transplants Published: 10/01/2018 Next Review: 08/2019 Last Review: 08/2018 Medicare Link(s) Revised: 10/01/2018 IMPORTANT
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Autoimmune Diseases File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_autoimmune_diseases
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Autoimmune Diseases File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_for_autoimmune_diseases
STEM CELL TRANSPLANTATION FOR ACUTE MYELOID LEUKEMIA
 STEM CELL TRANSPLANTATION FOR ACUTE MYELOID LEUKEMIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
STEM CELL TRANSPLANTATION FOR ACUTE MYELOID LEUKEMIA Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer
 Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer Policy Number: 8.01.23 Last Review: 1/2018 Origination: 9/2002 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer Policy Number: 8.01.23 Last Review: 1/2018 Origination: 9/2002 Next Review: 1/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_ transplantation_for_primary_amyloidosis 2/2001 11/2018 11/2019 11/2018 Description
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_ transplantation_for_primary_amyloidosis 2/2001 11/2018 11/2019 11/2018 Description
HAEMATOPOIETIC STEM CELL TRANSPLANTATION
 PRIMARY IMMUNODEFICIENCIES HAEMATOPOIETIC STEM CELL TRANSPLANTATION HAEMATOPOIETIC STEM CELL TRANSPLANTATION 1 PRIMARY IMMUNODEFICIENCIES KEY ABBREVIATIONS CID GvHD HSCT IPOPI PID SCID BMT HSC Combined
PRIMARY IMMUNODEFICIENCIES HAEMATOPOIETIC STEM CELL TRANSPLANTATION HAEMATOPOIETIC STEM CELL TRANSPLANTATION 1 PRIMARY IMMUNODEFICIENCIES KEY ABBREVIATIONS CID GvHD HSCT IPOPI PID SCID BMT HSC Combined
Placental and Umbilical Cord Blood as a Source of Stem Cells
 Placental and Umbilical Cord Blood as a Source of Stem Cells Policy Number: 7.01.50 Last Review: 12/2018 Origination: 12/2001 Next Review: 12/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Placental and Umbilical Cord Blood as a Source of Stem Cells Policy Number: 7.01.50 Last Review: 12/2018 Origination: 12/2001 Next Review: 12/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Clinical Policy: Donor Lymphocyte Infusion Reference Number: CP.MP.101 Last Review Date: 11/17
 Clinical Policy: Reference Number: CP.MP.101 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description
Clinical Policy: Reference Number: CP.MP.101 Last Review Date: 11/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description
HEMATOPOIETIC CELL TRANSPLANTATION FOR HODGKIN LYMPHOMA
 HEMATOPOIETIC CELL TRANSPLANTATION FOR HODGKIN LYMPHOMA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services,
HEMATOPOIETIC CELL TRANSPLANTATION FOR HODGKIN LYMPHOMA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services,
Trends in Hematopoietic Cell Transplantation. AAMAC Patient Education Day Oct 2014
 Trends in Hematopoietic Cell Transplantation AAMAC Patient Education Day Oct 2014 Objectives Review the principles behind allogeneic stem cell transplantation Outline the process of transplant, some of
Trends in Hematopoietic Cell Transplantation AAMAC Patient Education Day Oct 2014 Objectives Review the principles behind allogeneic stem cell transplantation Outline the process of transplant, some of
MEDICAL POLICY SUBJECT: KIDNEY TRANSPLANT
 MEDICAL POLICY SUBJECT: KIDNEY TRANSPLANT PAGE: 1 OF: 6 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an
MEDICAL POLICY SUBJECT: KIDNEY TRANSPLANT PAGE: 1 OF: 6 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation in the Treatment of Germ File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_in_the_treatment_of_germ_cell_tumor
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation in the Treatment of Germ File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplantation_in_the_treatment_of_germ_cell_tumor
Medical Policy. MP Placental and Umbilical Cord Blood as a Source of Stem Cells
 Medical Policy MP 7.01.50 BCBSA Ref. Policy: 7.01.50 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section: Surgery Related Policies 8.01.20 Hematopoietic Cell Transplantation for Non- Hodgkin Lymphomas
Medical Policy MP 7.01.50 BCBSA Ref. Policy: 7.01.50 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section: Surgery Related Policies 8.01.20 Hematopoietic Cell Transplantation for Non- Hodgkin Lymphomas
Subject: Hematopoietic Stem Cell Transplantation for Neuroblastoma
 Subject: Hematopoietic Stem Cell Transplantation for Neuroblastoma Guidance Number: MCG-193 Revision Date(s): Original Effective Date: 7/17/14 PREFACE This Medical Guidance is intended to facilitate the
Subject: Hematopoietic Stem Cell Transplantation for Neuroblastoma Guidance Number: MCG-193 Revision Date(s): Original Effective Date: 7/17/14 PREFACE This Medical Guidance is intended to facilitate the
Yescarta (axicabtagene ciloleucel)
 Last Review Date: March 30, 2018 Effective Date: April 1, 2018 Number: MG.MM.PH.42 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider
Last Review Date: March 30, 2018 Effective Date: April 1, 2018 Number: MG.MM.PH.42 Medical Guideline Disclaimer Property of EmblemHealth. All rights reserved. The treating physician or primary care provider
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Cell Transplantation for Hodgkin Lymphoma File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_hodgkin_lymphoma
Corporate Medical Policy Hematopoietic Cell Transplantation for Hodgkin Lymphoma File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_cell_transplantation_for_hodgkin_lymphoma
Clinical Policy: Donor Lymphocyte Infusion
 Clinical Policy: Reference Number: PA.CP.MP.101 Effective Date: 01/18 Last Review Date: 11/16 Coding Implications Revision Log This policy describes the medical necessity requirements for a donor lymphocyte
Clinical Policy: Reference Number: PA.CP.MP.101 Effective Date: 01/18 Last Review Date: 11/16 Coding Implications Revision Log This policy describes the medical necessity requirements for a donor lymphocyte
Table of Contents. 1.0 Description of the Procedure, Product, or Service Definitions... 2
 Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Definitions... 2 2.0 Eligibility Requirements... 3 2.1 Provisions... 3 2.1.1 General... 3 2.1.2 Specific... 3 2.2 Special
Table of Contents 1.0 Description of the Procedure, Product, or Service... 1 1.1 Definitions... 2 2.0 Eligibility Requirements... 3 2.1 Provisions... 3 2.1.1 General... 3 2.1.2 Specific... 3 2.2 Special
Name of Policy: Hematopoietic Stem-Cell Transplantation for Epithelial Ovarian Cancer
 Name of Policy: Hematopoietic Stem-Cell Transplantation for Epithelial Ovarian Cancer Policy #: 401 Latest Review Date: November 2013 Category: Surgery Policy Grade: A Background/Definitions: As a general
Name of Policy: Hematopoietic Stem-Cell Transplantation for Epithelial Ovarian Cancer Policy #: 401 Latest Review Date: November 2013 Category: Surgery Policy Grade: A Background/Definitions: As a general
BMTCN REVIEW COURSE PRE-TRANSPLANT CARE
 BMTCN REVIEW COURSE PRE-TRANSPLANT CARE Jennifer Shamai MS, RN, AOCNS, BMTCN Professional Practice Leader Department of Clinical Practice And Professional Education Click How to edit the Master Experts
BMTCN REVIEW COURSE PRE-TRANSPLANT CARE Jennifer Shamai MS, RN, AOCNS, BMTCN Professional Practice Leader Department of Clinical Practice And Professional Education Click How to edit the Master Experts
Subject: Small Bowel Transplantation, Small Bowel and Liver Transplantation and Multivisceral Transplantation. Original Effective Date: 8/30/2012
 Subject: Small Bowel Transplantation, Small Bowel and Liver Transplantation and Multivisceral Transplantation Original Effective Date: 8/30/2012 Policy Number: MCP-117 Revision Date(s): 5/26/2015 Review
Subject: Small Bowel Transplantation, Small Bowel and Liver Transplantation and Multivisceral Transplantation Original Effective Date: 8/30/2012 Policy Number: MCP-117 Revision Date(s): 5/26/2015 Review
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplant_for_acute_myeloid_leukemia
Corporate Medical Policy Hematopoietic Stem-Cell Transplantation for Acute Myeloid File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplant_for_acute_myeloid_leukemia
HEMATOPOIETIC CELL TRANSPLANTATION FOR CHRONIC MYELOID LEUKEMIA
 LEUKEMIA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
LEUKEMIA Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are dependent
Corporate Medical Policy
 Corporate Medical Policy Pancreas Transplant File Name: Origination: Last CAP Review: Next CAP Review: Last Review: pancreas_transplant 1/2000 5/2017 5/2018 8/2017 Description of Procedure or Service Transplantation
Corporate Medical Policy Pancreas Transplant File Name: Origination: Last CAP Review: Next CAP Review: Last Review: pancreas_transplant 1/2000 5/2017 5/2018 8/2017 Description of Procedure or Service Transplantation
Corporate Medical Policy
 Corporate Medical Policy Small Bowel, Small Bowel with Liver, or Multivisceral Transplant File Name: Origination: Last CAP Review: Next CAP Last Review: small_bowel_liver_and_multivisceral_transplant 2/1996
Corporate Medical Policy Small Bowel, Small Bowel with Liver, or Multivisceral Transplant File Name: Origination: Last CAP Review: Next CAP Last Review: small_bowel_liver_and_multivisceral_transplant 2/1996
Hematopoietic Stem Cell Transplantation for Epithelial Ovarian Cancer
 Hematopoietic Stem Cell Transplantation for Epithelial Ovarian Cancer Policy Number: Original Effective Date: MM.07.014 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 04/22/2016 Section:
Hematopoietic Stem Cell Transplantation for Epithelial Ovarian Cancer Policy Number: Original Effective Date: MM.07.014 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 04/22/2016 Section:
Original Effective Date: 9/10/09
 Subject: Oral and Tube Fed Enteral Nutrition Policy Number: MCR-070 *(This MCR replaces and combines MCG-070 & 071) Original Effective Date: 9/10/09 Revision Date(s): 6/29/12, 8/7/14 This MCR is no longer
Subject: Oral and Tube Fed Enteral Nutrition Policy Number: MCR-070 *(This MCR replaces and combines MCG-070 & 071) Original Effective Date: 9/10/09 Revision Date(s): 6/29/12, 8/7/14 This MCR is no longer
Informed Consent for Liver Transplant Patients
 Informed Consent for Liver Transplant Patients Evaluation Process You will be evaluated with consultations, lab tests and various procedures to determine the medical appropriateness of liver transplant.
Informed Consent for Liver Transplant Patients Evaluation Process You will be evaluated with consultations, lab tests and various procedures to determine the medical appropriateness of liver transplant.
Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer
 Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer Policy Number: MM.07.014 Lines of Business: HMO; PPO Place of Service: Outpatient; Inpatient Current Effective Date: February 22, 2019 Original
Hematopoietic Cell Transplantation for Epithelial Ovarian Cancer Policy Number: MM.07.014 Lines of Business: HMO; PPO Place of Service: Outpatient; Inpatient Current Effective Date: February 22, 2019 Original
Subject: Kidney Transplantation Effective Date: Guidance Number: MCG-045 Revision Date(s): 10/26/11, 8/20/12, 1/14/13
 Subject: Kidney Transplantation Original Effective Date: Guidance Number: MCG-045 Revision Date(s): 10/26/11, 8/20/12, 1/14/13 2/28/08 Medical Coverage Guidance Approval Date: 10/26/11 8/23/12, 1/14/13
Subject: Kidney Transplantation Original Effective Date: Guidance Number: MCG-045 Revision Date(s): 10/26/11, 8/20/12, 1/14/13 2/28/08 Medical Coverage Guidance Approval Date: 10/26/11 8/23/12, 1/14/13
Protocol. Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis
 Protocol Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis (80142) Medical Benefit Effective Date: 04/01/13 Next Review Date: 07/15 Preauthorization Yes Review Dates: 04/07, 05/08, 01/10,
Protocol Hematopoietic Stem-Cell Transplantation for Primary Amyloidosis (80142) Medical Benefit Effective Date: 04/01/13 Next Review Date: 07/15 Preauthorization Yes Review Dates: 04/07, 05/08, 01/10,
. TCR Alpha, Beta and CD19+ Cell Depleted Haploidentical Transplant for Primary Immunodeficiency Disorders Feb 22 nd,2018
 . TCR Alpha, Beta and CD19+ Cell Depleted Haploidentical Transplant for Primary Immunodeficiency Disorders Feb 22 nd,2018 Neena Kapoor Professor of Pediatrics Children s Hospital Los Angeles Keck School
. TCR Alpha, Beta and CD19+ Cell Depleted Haploidentical Transplant for Primary Immunodeficiency Disorders Feb 22 nd,2018 Neena Kapoor Professor of Pediatrics Children s Hospital Los Angeles Keck School
One Day BMT Course by Thai Society of Hematology. Management of Graft Failure and Relapsed Diseases
 One Day BMT Course by Thai Society of Hematology Management of Graft Failure and Relapsed Diseases Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital
One Day BMT Course by Thai Society of Hematology Management of Graft Failure and Relapsed Diseases Piya Rujkijyanont, MD Division of Hematology-Oncology Department of Pediatrics Phramongkutklao Hospital
Reduced-intensity Conditioning Transplantation
 Reduced-intensity Conditioning Transplantation Current Role and Future Prospect He Huang M.D., Ph.D. Bone Marrow Transplantation Center The First Affiliated Hospital Zhejiang University School of Medicine,
Reduced-intensity Conditioning Transplantation Current Role and Future Prospect He Huang M.D., Ph.D. Bone Marrow Transplantation Center The First Affiliated Hospital Zhejiang University School of Medicine,
Hematopoietic Cell Transplantation for Breast Cancer
 Medical Policy Manual Transplant, Policy No. 45.29 Hematopoietic Cell Transplantation for Breast Cancer Next Review: January 2019 Last Review: February 2018 Effective: March 1, 2018 IMPORTANT REMINDER
Medical Policy Manual Transplant, Policy No. 45.29 Hematopoietic Cell Transplantation for Breast Cancer Next Review: January 2019 Last Review: February 2018 Effective: March 1, 2018 IMPORTANT REMINDER
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma
 Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Policy Number: 8.01.15 Last Review: 6/2017 Origination: 12/2001 Next Review: 6/2018 Policy Blue Cross and
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma Policy Number: 8.01.15 Last Review: 6/2017 Origination: 12/2001 Next Review: 6/2018 Policy Blue Cross and
Table of Contents. 1.0 Description of the Procedure, Product, or Service Definitions... 2
 Hematopoietic Stem-Cell & Bone Clinical Coverage Policy No: 11A-3 Marrow Transplantation for Chronic Amended Date: October 1, 2015 Myelogenous Leukemia Table of Contents 1.0 Description of the Procedure,
Hematopoietic Stem-Cell & Bone Clinical Coverage Policy No: 11A-3 Marrow Transplantation for Chronic Amended Date: October 1, 2015 Myelogenous Leukemia Table of Contents 1.0 Description of the Procedure,
Subject: Hematopoietic Stem Cell Transplantation for Neuroblastoma. Revision Date(s): 6/2/15, 6/12/17
 Subject: Hematopoietic Stem Cell Transplantation for Neuroblastoma Policy Number: MCP-193 Review Date: 6/15/16, 7/10/18 MCPC Approval Date: 6/22/17, 7/10/18 Revision Date(s): 6/2/15, 6/12/17 Original Effective
Subject: Hematopoietic Stem Cell Transplantation for Neuroblastoma Policy Number: MCP-193 Review Date: 6/15/16, 7/10/18 MCPC Approval Date: 6/22/17, 7/10/18 Revision Date(s): 6/2/15, 6/12/17 Original Effective
Allogeneic Hematopoietic Stem-Cell Transplantation for Myelodysplastic Syndromes and Myeloproliferative Neoplasms. Policy Specific Section:
 Medical Policy Allogeneic Hematopoietic Stem-Cell Transplantation for Myelodysplastic Syndromes and Myeloproliferative Type: Medical Necessity and Investigational / Experimental Policy Specific Section:
Medical Policy Allogeneic Hematopoietic Stem-Cell Transplantation for Myelodysplastic Syndromes and Myeloproliferative Type: Medical Necessity and Investigational / Experimental Policy Specific Section:
Donatore HLA identico di anni o MUD giovane?
 Donatore HLA identico di 60-70 anni o MUD giovane? Stella Santarone Dipartimento di Ematologia, Medicina Trasfusionale e Biotecnologie Pescara AGENDA 1. Stem Cell Donation: fatalities and severe events
Donatore HLA identico di 60-70 anni o MUD giovane? Stella Santarone Dipartimento di Ematologia, Medicina Trasfusionale e Biotecnologie Pescara AGENDA 1. Stem Cell Donation: fatalities and severe events
Populations Interventions Comparators Outcomes Individuals: With intestinal failure and evidence of impending end-stage liver failure
 Protocol Small Bowel/Liver and Multivisceral Transplant (70305) Medical Benefit Effective Date: 07/01/14 Next Review Date: 05/18 Preauthorization Yes Review Dates: 05/09, 05/10, 05/11, 05/12, 05/13, 07/13,
Protocol Small Bowel/Liver and Multivisceral Transplant (70305) Medical Benefit Effective Date: 07/01/14 Next Review Date: 05/18 Preauthorization Yes Review Dates: 05/09, 05/10, 05/11, 05/12, 05/13, 07/13,
Ontario s Referral and Listing Criteria for Adult Kidney Transplantation
 Ontario s Referral and Listing Criteria for Adult Kidney Transplantation Version 3.0 Trillium Gift of Life Network Adult Kidney Transplantation Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The
Ontario s Referral and Listing Criteria for Adult Kidney Transplantation Version 3.0 Trillium Gift of Life Network Adult Kidney Transplantation Referral & Listing Criteria PATIENT REFERRAL CRITERIA: The
Ontario s Referral and Listing Criteria for Adult Pancreas-After- Kidney Transplantation
 Ontario s Referral and Listing Criteria for Adult Pancreas-After- Kidney Transplantation Version 2.0 Trillium Gift of Life Network Adult Pancreas-After-Kidney Transplantation Referral & Listing Criteria
Ontario s Referral and Listing Criteria for Adult Pancreas-After- Kidney Transplantation Version 2.0 Trillium Gift of Life Network Adult Pancreas-After-Kidney Transplantation Referral & Listing Criteria
MEDICAL POLICY SUBJECT: HEART & HEART/LUNG TRANSPLANT
 MEDICAL POLICY SUBJECT: HEART & HEART/LUNG PAGE: 1 OF: 6 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an
MEDICAL POLICY SUBJECT: HEART & HEART/LUNG PAGE: 1 OF: 6 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an
What s a Transplant? What s not?
 What s a Transplant? What s not? How to report the difference? Daniel Weisdorf MD University of Minnesota Anti-cancer effects of BMT or PBSCT [HSCT] Kill the cancer Save the patient Restore immunocompetence
What s a Transplant? What s not? How to report the difference? Daniel Weisdorf MD University of Minnesota Anti-cancer effects of BMT or PBSCT [HSCT] Kill the cancer Save the patient Restore immunocompetence
The National Marrow Donor Program. Graft Sources for Hematopoietic Cell Transplantation. Simon Bostic, URD Transplant Recipient
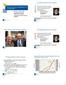 1988 199 1992 1994 1996 1998 2 22 24 26 28 21 212 214 216 218 Adult Donors Cord Blood Units The National Donor Program Graft Sources for Hematopoietic Cell Transplantation Dennis L. Confer, MD Chief Medical
1988 199 1992 1994 1996 1998 2 22 24 26 28 21 212 214 216 218 Adult Donors Cord Blood Units The National Donor Program Graft Sources for Hematopoietic Cell Transplantation Dennis L. Confer, MD Chief Medical
Clinical Policy Title: Bone marrow transplant for children with hyper IgM disorder
 Clinical Policy Title: Bone marrow transplant for children with hyper IgM disorder Clinical Policy Number: 17.02.01 Effective Date: July 1, 2016 Initial Review Date: April 20, 2016 Most Recent Review Date:
Clinical Policy Title: Bone marrow transplant for children with hyper IgM disorder Clinical Policy Number: 17.02.01 Effective Date: July 1, 2016 Initial Review Date: April 20, 2016 Most Recent Review Date:
Neutrophil Recovery: The. Posttransplant Recovery. Bus11_1.ppt
 Neutrophil Recovery: The First Step in Posttransplant Recovery No conflicts of interest to disclose Bus11_1.ppt Blood is Made in the Bone Marrow Blood Stem Cell Pre-B White cells B Lymphocyte T Lymphocyte
Neutrophil Recovery: The First Step in Posttransplant Recovery No conflicts of interest to disclose Bus11_1.ppt Blood is Made in the Bone Marrow Blood Stem Cell Pre-B White cells B Lymphocyte T Lymphocyte
MP Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma
 Medical Policy MP 8.01.15 Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma BCBSA Ref. Policy: 8.01.15 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section:
Medical Policy MP 8.01.15 Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma BCBSA Ref. Policy: 8.01.15 Last Review: 01/30/2018 Effective Date: 01/30/2018 Section:
Table of Contents. 1.0 Description of the Procedure, Product, or Service... 1
 Allogeneic Hematopoietic & Bone Clinical Coverage Policy No: 11A-5 Marrow Transplant for Genetic Amended Date: October 1, 2015 Diseases and Acquired Anemias Table of Contents 1.0 Description of the Procedure,
Allogeneic Hematopoietic & Bone Clinical Coverage Policy No: 11A-5 Marrow Transplant for Genetic Amended Date: October 1, 2015 Diseases and Acquired Anemias Table of Contents 1.0 Description of the Procedure,
Umbilical Cord Blood Transplantation
 Umbilical Cord Blood Transplantation Current Results John E. Wagner, M.D. Blood and Marrow Transplant Program and Stem Cell Institute University of Minnesota Donor Choices Unrelated Marrow/PBSC Results
Umbilical Cord Blood Transplantation Current Results John E. Wagner, M.D. Blood and Marrow Transplant Program and Stem Cell Institute University of Minnesota Donor Choices Unrelated Marrow/PBSC Results
Hematopoietic Stem-Cell Transplantation for Breast Cancer
 Hematopoietic Stem-Cell Transplantation for Breast Cancer Policy Number: Original Effective Date: MM.07.010 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 07/22/2011 Section: Transplants
Hematopoietic Stem-Cell Transplantation for Breast Cancer Policy Number: Original Effective Date: MM.07.010 04/01/2008 Line(s) of Business: Current Effective Date: HMO; PPO 07/22/2011 Section: Transplants
New Evidence reports on presentations given at EHA/ICML Bendamustine in the Treatment of Lymphoproliferative Disorders
 New Evidence reports on presentations given at EHA/ICML 2011 Bendamustine in the Treatment of Lymphoproliferative Disorders Report on EHA/ICML 2011 presentations Efficacy and safety of bendamustine plus
New Evidence reports on presentations given at EHA/ICML 2011 Bendamustine in the Treatment of Lymphoproliferative Disorders Report on EHA/ICML 2011 presentations Efficacy and safety of bendamustine plus
Bone Marrow Transplantation and the Potential Role of Iomab-B
 Bone Marrow Transplantation and the Potential Role of Iomab-B Hillard M. Lazarus, MD, FACP Professor of Medicine, Director of Novel Cell Therapy Case Western Reserve University 1 Hematopoietic Cell Transplantation
Bone Marrow Transplantation and the Potential Role of Iomab-B Hillard M. Lazarus, MD, FACP Professor of Medicine, Director of Novel Cell Therapy Case Western Reserve University 1 Hematopoietic Cell Transplantation
Clinical Policy Title: Bone marrow transplant for children with hyper IgM disorder
 Clinical Policy Title: Bone marrow transplant for children with hyper IgM disorder Clinical Policy Number: 17.02.01 Effective Date: July 1, 2016 Initial Review Date: April 20, 2016 Most Recent Review Date:
Clinical Policy Title: Bone marrow transplant for children with hyper IgM disorder Clinical Policy Number: 17.02.01 Effective Date: July 1, 2016 Initial Review Date: April 20, 2016 Most Recent Review Date:
HEMATOPOIETIC CELL TRANSPLANTATION FOR SOLID TUMORS OF CHILDHOOD
 CHILDHOOD Non-Discrimination Statement Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices drugs are dependent upon
CHILDHOOD Non-Discrimination Statement Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices drugs are dependent upon
HEMATOPOIETIC CELL TRANSPLANTATION FOR GENETIC DISEASES AND ACQUIRED ANEMIAS
 ACQUIRED ANEMIAS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are
ACQUIRED ANEMIAS Non-Discrimination Statement and Multi-Language Interpreter Services information are located at the end of this document. Coverage for services, procedures, medical devices and drugs are
Hematopoietic Stem Cell Therapy
 Hematopoietic Stem Cell Therapy Grace Totoe, MBChB, SBB CME August 2012 Accra-Ghana Hematopoietic Stem Cells Cells capable of self renewal and differentiation into all blood cell lineages Objectives Historical
Hematopoietic Stem Cell Therapy Grace Totoe, MBChB, SBB CME August 2012 Accra-Ghana Hematopoietic Stem Cells Cells capable of self renewal and differentiation into all blood cell lineages Objectives Historical
5/9/2018. Bone marrow failure diseases (aplastic anemia) can be cured by providing a source of new marrow
 5/9/2018 or Stem Cell Harvest Where we are now, and What s Coming AA MDS International Foundation Indianapolis IN Luke Akard MD May 19, 2018 Infusion Transplant Conditioning Treatment 2-7 days STEM CELL
5/9/2018 or Stem Cell Harvest Where we are now, and What s Coming AA MDS International Foundation Indianapolis IN Luke Akard MD May 19, 2018 Infusion Transplant Conditioning Treatment 2-7 days STEM CELL
PATIENT SELECTION FOR DECEASED DONOR KIDNEY ONLY TRANSPLANTATION
 PATIENT SELECTION FOR DECEASED DONOR KIDNEY ONLY TRANSPLANTATION This policy has been created by the Kidney Advisory Group on behalf of NHSBT. The policy has been considered and approved by the Organ Donation
PATIENT SELECTION FOR DECEASED DONOR KIDNEY ONLY TRANSPLANTATION This policy has been created by the Kidney Advisory Group on behalf of NHSBT. The policy has been considered and approved by the Organ Donation
Role of NMDP Repository in the Evolution of HLA Matching and Typing for Unrelated Donor HCT
 Role of NMDP Repository in the Evolution of HLA Matching and Typing for Unrelated Donor HCT Stephen Spellman, MBS Director, Immunobiology and Observational Research Assistant Scientific Director CIBMTR,
Role of NMDP Repository in the Evolution of HLA Matching and Typing for Unrelated Donor HCT Stephen Spellman, MBS Director, Immunobiology and Observational Research Assistant Scientific Director CIBMTR,
Hematopoietic Cell Transplantation for Acute Lymphoblastic Leukemia
 Hematopoietic Cell Transplantation for Acute Lymphoblastic Leukemia Policy Number: 8.01.32 Last Review: 7/2018 Origination: 7/2002 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Hematopoietic Cell Transplantation for Acute Lymphoblastic Leukemia Policy Number: 8.01.32 Last Review: 7/2018 Origination: 7/2002 Next Review: 7/2019 Policy Blue Cross and Blue Shield of Kansas City (Blue
Abstract 861. Stein AS, Topp MS, Kantarjian H, Gökbuget N, Bargou R, Litzow M, Rambaldi A, Ribera J-M, Zhang A, Zimmerman Z, Forman SJ
 Treatment with Anti-CD19 BiTE Blinatumomab in Adult Patients With Relapsed/Refractory B-Precursor Acute Lymphoblastic Leukemia (R/R ALL) Post-Allogeneic Hematopoietic Stem Cell Transplantation Abstract
Treatment with Anti-CD19 BiTE Blinatumomab in Adult Patients With Relapsed/Refractory B-Precursor Acute Lymphoblastic Leukemia (R/R ALL) Post-Allogeneic Hematopoietic Stem Cell Transplantation Abstract
An Overview of Blood and Marrow Transplantation
 An Overview of Blood and Marrow Transplantation October 24, 2009 Stephen Couban Department of Medicine Dalhousie University Objectives What are the types of blood and marrow transplantation? Who may benefit
An Overview of Blood and Marrow Transplantation October 24, 2009 Stephen Couban Department of Medicine Dalhousie University Objectives What are the types of blood and marrow transplantation? Who may benefit
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma
 Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma Policy Number: MM.07.011 Lines of Business: HMO; PPO Precertification: Required see section IV Current
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma Policy Number: MM.07.011 Lines of Business: HMO; PPO Precertification: Required see section IV Current
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma
 Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma Policy Number: Original Effective Date: MM.07.011 04/01/2008 Line(s) of Business: Current Effective Date:
Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia and Small Lymphocytic Lymphoma Policy Number: Original Effective Date: MM.07.011 04/01/2008 Line(s) of Business: Current Effective Date:
Iowa Methodist Medical Center Transplant Center. Informed Consent for Kidney Transplant Recipient
 Iowa Methodist Transplant Center Iowa Methodist Medical Center Transplant Center 1215 Pleasant Street, Suite 506 Des Moines, IA 50309 515-241-4044 Phone 515-241-4100 Fax Iowa Methodist Medical Center Transplant
Iowa Methodist Transplant Center Iowa Methodist Medical Center Transplant Center 1215 Pleasant Street, Suite 506 Des Moines, IA 50309 515-241-4044 Phone 515-241-4100 Fax Iowa Methodist Medical Center Transplant
Populations Interventions Comparators Outcomes Individuals: With primary amyloidosis
 Protocol Hematopoietic Cell Transplantation for Primary Amyloidosis (80142) (Formerly Hematopoietic Stem Cell Transplantation for Primary Amyloidosis) Medical Benefit Effective Date: 04/01/13 Next Review
Protocol Hematopoietic Cell Transplantation for Primary Amyloidosis (80142) (Formerly Hematopoietic Stem Cell Transplantation for Primary Amyloidosis) Medical Benefit Effective Date: 04/01/13 Next Review
Stem Cell Transplantation for Severe Aplastic Anemia
 Number of Transplants 10/24/2011 Stem Cell Transplantation for Severe Aplastic Anemia Claudio Anasetti, MD Professor of Oncology and Medicine Chair, Blood and Marrow Transplant Dpt Moffitt Cancer Center
Number of Transplants 10/24/2011 Stem Cell Transplantation for Severe Aplastic Anemia Claudio Anasetti, MD Professor of Oncology and Medicine Chair, Blood and Marrow Transplant Dpt Moffitt Cancer Center
Medical Benefit Effective Date: 07/01/12 Next Review Date: 05/13 Preauthorization* Yes Review Dates: 04/07, 05/08, 05/11, 05/12
 Hematopoietic Stem-Cell Transplantation for Chronic Lymphocytic (80115) Medical Benefit Effective Date: 07/01/12 Next Review Date: 05/13 Preauthorization* Yes Review Dates: 04/07, 05/08, 05/11, 05/12 The
Hematopoietic Stem-Cell Transplantation for Chronic Lymphocytic (80115) Medical Benefit Effective Date: 07/01/12 Next Review Date: 05/13 Preauthorization* Yes Review Dates: 04/07, 05/08, 05/11, 05/12 The
Subject: Hematopoietic Stem Cell Transplantation for Aplastic Anemia. Revision Date(s):
 Subject: Hematopoietic Stem Cell Transplantation for Aplastic Anemia Guidance Number: Medical Coverage Guidance Approval Date: 6/26/13 MCG-143 Revision Date(s): Original Effective Date:6/26/13 PREFACE
Subject: Hematopoietic Stem Cell Transplantation for Aplastic Anemia Guidance Number: Medical Coverage Guidance Approval Date: 6/26/13 MCG-143 Revision Date(s): Original Effective Date:6/26/13 PREFACE
European Risk Management Plan. Measures impairment. Retreatment after Discontinuation
 European Risk Management Plan Table 6.1.4-1: Safety Concern 55024.1 Summary of Risk Minimization Measures Routine Risk Minimization Measures Additional Risk Minimization Measures impairment. Retreatment
European Risk Management Plan Table 6.1.4-1: Safety Concern 55024.1 Summary of Risk Minimization Measures Routine Risk Minimization Measures Additional Risk Minimization Measures impairment. Retreatment
Corporate Medical Policy
 Corporate Medical Policy Hematopoietic Stem-Cell Transplant for Non-Hodgkin Lymphomas File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplant_for_non_hodgkin_lymphomas
Corporate Medical Policy Hematopoietic Stem-Cell Transplant for Non-Hodgkin Lymphomas File Name: Origination: Last CAP Review: Next CAP Review: Last Review: hematopoietic_stem-cell_transplant_for_non_hodgkin_lymphomas
Corporate Medical Policy
 Corporate Medical Policy Allogeneic Hematopoietic Transplant for Genetic Diseases and File Name: Origination: Last CAP Review: Next CAP Review: Last Review: allogeneic_hematopoietic_transplant_for_genetic_diseases
Corporate Medical Policy Allogeneic Hematopoietic Transplant for Genetic Diseases and File Name: Origination: Last CAP Review: Next CAP Review: Last Review: allogeneic_hematopoietic_transplant_for_genetic_diseases
MUD HSCT as first line Treatment in Idiopathic SAA. Dr Sujith Samarasinghe Great Ormond Street Hospital for Children, London, UK
 MUD HSCT as first line Treatment in Idiopathic SAA Dr Sujith Samarasinghe Great Ormond Street Hospital for Children, London, UK No Financial Disclosures Guidelines for management of aplastic anaemia British
MUD HSCT as first line Treatment in Idiopathic SAA Dr Sujith Samarasinghe Great Ormond Street Hospital for Children, London, UK No Financial Disclosures Guidelines for management of aplastic anaemia British
Hematopoietic Stem-Cell Clinical Coverage Policy No: 11A-7 & Bone Marrow Transplantation Amended Date: October 1, 2015 for Hodgkin Lymphoma
 Hematopoietic Stem-Cell Clinical Coverage Policy No: 11A-7 & Bone Marrow Transplantation Amended Date: October 1, 2015 for Hodgkin Lymphoma Table of Contents 1.0 Description of the Procedure, Product,
Hematopoietic Stem-Cell Clinical Coverage Policy No: 11A-7 & Bone Marrow Transplantation Amended Date: October 1, 2015 for Hodgkin Lymphoma Table of Contents 1.0 Description of the Procedure, Product,
Clinical Policy: Valganciclovir (Valcyte) Reference Number: CP.CPA.116 Effective Date: Last Review Date: Line of Business: Commercial
 Clinical Policy: (Valcyte) Reference Number: CP.CPA.116 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important
Clinical Policy: (Valcyte) Reference Number: CP.CPA.116 Effective Date: 11.16.16 Last Review Date: 11.17 Line of Business: Commercial Revision Log See Important Reminder at the end of this policy for important
Chapter 4 Section Combined Heart-Kidney Transplantation (CHKT)
 Surgery Chapter 4 Section 24.3 Issue Date: May 7, 1999 Authority: 32 CFR 199.4(e)(5) 1.0 POLICY 1.1 is a TRICARE benefit that requires preauthorization. 1.1.1 A TRICARE Prime enrollee must have a referral
Surgery Chapter 4 Section 24.3 Issue Date: May 7, 1999 Authority: 32 CFR 199.4(e)(5) 1.0 POLICY 1.1 is a TRICARE benefit that requires preauthorization. 1.1.1 A TRICARE Prime enrollee must have a referral
Corporate Medical Policy
 Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: renal_kidney_transplantation 4/1980 4/2017 4/2018 4/2017 Description of Procedure or Service A kidney transplant,
Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: renal_kidney_transplantation 4/1980 4/2017 4/2018 4/2017 Description of Procedure or Service A kidney transplant,
Corporate Medical Policy
 White Blood Cell Growth Factors Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: white_blood_cell_growth_factors 9/2016 4/2017 4/2018 6/2017 Description of
White Blood Cell Growth Factors Corporate Medical Policy File Name: Origination: Last CAP Review: Next CAP Review: Last Review: white_blood_cell_growth_factors 9/2016 4/2017 4/2018 6/2017 Description of
Disclosures. Newborn Screening for Severe Combined Immune Deficiency Syndromes (SCIDS): Why; why now? Learning objectives.
 Newborn Screening for Severe Combined Immune Deficiency Syndromes (SCIDS): Why; why now? Anthony J. Infante, MD, PhD Professor, Pediatrics and Microbiology & Immunology Chief, Division of Immunology &
Newborn Screening for Severe Combined Immune Deficiency Syndromes (SCIDS): Why; why now? Anthony J. Infante, MD, PhD Professor, Pediatrics and Microbiology & Immunology Chief, Division of Immunology &
Leukine. Leukine (sargramostim) Description
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Leukine Page: 1 of 6 Last Review Date: November 30, 2018 Leukine Description Leukine (sargramostim)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 Subject: Leukine Page: 1 of 6 Last Review Date: November 30, 2018 Leukine Description Leukine (sargramostim)
Michael Grimley 1, Vinod Prasad 2, Joanne Kurtzberg 2, Roy Chemaly 3, Thomas Brundage 4, Chad Wilson 4, Herve Mommeja-Marin 4
 Twice-weekly Brincidofovir (BCV, CMX1) Shows Promising Antiviral Activity in Immunocompromised Transplant Patients with Asymptomatic Adenovirus Viremia Michael Grimley 1, Vinod Prasad, Joanne Kurtzberg,
Twice-weekly Brincidofovir (BCV, CMX1) Shows Promising Antiviral Activity in Immunocompromised Transplant Patients with Asymptomatic Adenovirus Viremia Michael Grimley 1, Vinod Prasad, Joanne Kurtzberg,
Original Policy Date
 MP 7.03.02 Small Bowel/Liver and Multivisceral Transplant Medical Policy Section Surgery Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013 Return
MP 7.03.02 Small Bowel/Liver and Multivisceral Transplant Medical Policy Section Surgery Issue 12/2013 Original Policy Date 12/2013 Last Review Status/Date Reviewed with literature search/12/2013 Return
UNRELATED DONOR TRANSPLANTATION FOR SICKLE CELL DISEASE AN UPDATE
 UNRELATED DONOR TRANSPLANTATION FOR SICKLE CELL DISEASE AN UPDATE Naynesh Kamani, M.D. Children s National Medical Center GW University School of Medicine Washington, DC SCD scope of problem in USA Commonest
UNRELATED DONOR TRANSPLANTATION FOR SICKLE CELL DISEASE AN UPDATE Naynesh Kamani, M.D. Children s National Medical Center GW University School of Medicine Washington, DC SCD scope of problem in USA Commonest
Nonmyeloablative Allogeneic Transplants of Hematopoietic Stem Cells for Treatment of Malignancy Archived Medical Policy
 Nonmyeloablative Allogeneic Transplants of Hematopoietic Stem Cells for Treatment of Malignancy Codes associated with this policy will no longer pend for clinical review. Applies to all products administered
Nonmyeloablative Allogeneic Transplants of Hematopoietic Stem Cells for Treatment of Malignancy Codes associated with this policy will no longer pend for clinical review. Applies to all products administered
Workshop I: Patient Selection Current indication for HCT in adults. Shinichiro Okamoto MD, PhD Keio University, Tokyo, Japan
 Workshop I: Patient Selection Current indication for HCT in adults Shinichiro Okamoto MD, PhD Keio University, Tokyo, Japan Factors to Take into Account with Recommending HCT Patient & disease factors
Workshop I: Patient Selection Current indication for HCT in adults Shinichiro Okamoto MD, PhD Keio University, Tokyo, Japan Factors to Take into Account with Recommending HCT Patient & disease factors
Clinical Policy: Heart-Lung Transplant Reference Number: CP.MP.132
 Clinical Policy: Reference Number: CP.MP.132 Effective Date: 06/17 Last Review Date: 06/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
Clinical Policy: Reference Number: CP.MP.132 Effective Date: 06/17 Last Review Date: 06/17 Coding Implications Revision Log See Important Reminder at the end of this policy for important regulatory and
