JBC Papers in Press. Published on January 20, 2011 as Manuscript M Myosin phosphatase targeting subunit1 controls chromatid segregation*
|
|
|
- Jody Knight
- 6 years ago
- Views:
Transcription
1 JBC Papers in Press. Published on January 20, 2011 as Manuscript M The latest version is at Myosin phosphatase targeting subunit1 controls chromatid segregation* Fumio Matsumura 1, Yoshihiko Yamakita 1, and Shigeko Yamashiro 1 From the Department of Molecular Biology & Biochemistry 1, Rutgers Univ., Piscataway, NJ, Address correspondence to: Fumio Matsumura, Ph.D. Department of Molecular Biology & Biochemistry 1, Rutgers Univ., Piscataway, NJ, Fax: ; ; Matsumura@biology.rutgers.edu (F. M.) Myosin phosphatase is a heterotrimeric holoenzyme consisting of myosin phosphatase targeting subunit1 (MYPT1), a catalytic subunit of PP1Cβ and a 20kDa subunit of an unknown function. We have previously reported that myosin phosphatase also controls mitosis, apparently by antagonizing polo-like kinase1 (PLK1). Here we found that depletion of MYPT1 by sirna led to precocious chromatid segregation when HeLa cells were arrested at metaphase by a proteasome inhibitor, MG132, or by Cdc20 depletion. Consistently, cyclin B1 and securin were not degraded, indicating that the chromatid segregation is independent of the Anaphase Promoting Complex/Cyclosome. Precocious segregation induced by MYPT1 depletion requires PLK1 activity because a PLK1 inhibitor, BI-2536, blocked precocious segregation. Furthermore, the expression of an unphosphorylatable mutant of SA2 (SCC3 homologue 2), a subunit of the cohesin complex, prevented precocious chromatid segregation induced by MYPT1 depletion. It has been shown that SA2 at centromeres is protected from phosphorylation by PP2A phosphatase recruited by Shugoshin (Sgo1), whereas SA2 along chromosome arms is phosphorylated by PLK1, leading to SA2 dissociation at chromosome arms. Taken together, our results suggest that hyper-activation of PLK1 caused by MYPT1 reduction could override the counteracting PP2A phosphatase, resulting in precocious chromatid segregation. We propose that SA2 at the centromeres is protected by two phosphatases. One is PP2A directly dephosphorylating SA2 and the other is myosin phosphatase counteracting PLK1. It has become increasingly clear that Ser/Thr protein phosphatases play active roles in regulating protein phosphorylation during mitosis (1). Protein phosphatase1 (PP1) belongs to the PPP family of Ser/Thr protein phosphatases, and its catalytic subunits (PP1C) are known to bind to a variety of targeting subunits (2,3). The association of PP1C with its targeting subunits is physiologically important for temporal and spatial regulation of protein phosphorylation because such binding provides the limited number of PP1C a means to counteract a wide array of Ser/Thr kinases, either by targeting PP1C to a specific subcellular site or a particular substrate at a specific stage of cell cycle, and/or by allowing them to directly counteract a Ser/Thr kinase (2,3). For example, it has been demonstrated that Repo- Man, a novel PP1C targeting protein, is essential for recruiting PP1Cγ, one of three mammalian PP1C isoforms, to chromatin during anaphase. This targeting plays a critical role in the maintenance of chromosome architecture (4,5). However, judging from the complexity of cell cycle control, multiple targeting subunits must be involved in various mitotic events. Thus, major questions remaining to be addressed are how and which mitotic event each targeting subunit controls, and which kinase each targeting subunit counteracts. Myosin phosphatase targeting subunit1 (MYPT1, also called MBS or M130) is a known targeting subunit of PP1C (2,6). We have recently demonstrated that MYPT1 also controls mitosis, apparently by antagonizing polo-like kinase1 (PLK1) (7). MYPT1 binds to PP1Cβ at its N- terminus and to a 20kDa subunit of unknown function at its C-terminus, to form a hetrotrimeric holoenzyme. As the holoenzyme is known to dephosphorylate the regulatory light chains of myosin II and control actomyosin contractility in smooth muscle and nonmuscle cells, it is often called myosin phosphatase (MP) (2,6). The phenotype of mice lacking MYPT1, however, 1 Copyright 2011 by The American Society for Biochemistry and Molecular Biology, Inc.
2 indicates that MYPT1 plays a fundamental role in cell proliferation, beyond the regulation of actomyosin contractility. Deletion of MYPT1 is embryonic lethal and the mice die at a very early stage of development (7.5 dpc), and no MYPT1 null cells have been isolated so far (8). In contrast, mice lacking MLCK, a major kinase that phosphorylates the regulatory myosin light chains, are able to survive until birth though they show postnatal lethality (9). Our previous finding that MYPT1 controls mitosis by counteracting PLK1 (7) explains, at least in part, the lethal phenotype of MYPT1 KO mice. PLK1 is an essential mitotic kinase that controls a variety of critical mitotic events including the G2/M transition, centrosome maturation, bipolar mitotic spindle assembly, chromatid segregation, mitotic exit, and cytokinesis (10-15). We previously showed that depletion of MYPT1 resulted in increased phosphorylation of PLK1 at its activation site of Thr210, and consistently, simultaneous reduction of both MYPT1 and PLK1 levels by sirna rescued the mitotic arrest phenotype caused by single reduction of PLK1 levels. Due to this antagonism between MP and PLK1, loss of MYPT1 would be predicted to deleteriously activate PLK1 at incorrect times and/or places, disrupting one or more PLK1-mediated mitotic events. In this work, we examined the effects of MYPT1 depletion on mitotic exit, one of the PLK1-mediated mitotic events. We found that MYPT1 depletion resulted in premature chromatid segregation. Experimental Procedures Cells and antibodies- SW962 human vulva carcinoma (HTB-118, ATCC, Manassas, VA) and HeLa cells were maintained in Dulbecco s modified Eagle s medium (DME) containing 10 % fetal calf serum. HeLa Tet-on cells that were able to induce myc-tagged, wild type SA2 (called myc- SA2-wild), as well as an unphosphorylatable mutant of SA2 (called myc-sa2-12xa, mitosisspecific, twelve Ser and Thr sites replaced with Ala), were kindly provided by Dr. J-M. Peters (Research Institute of Molecular Pathology, Vienna, Austria) (16). Myc-tagged SA2 was induced by 2µg/ml Dox for 2 days. In some experiments, HeLa cells were synchronized by a double thymidine block. Treatment of MG132 (5µM) was performed for 1 to 5.5 hours. We confirmed that HeLa cells were kept arrested at mitosis during this time window of MG132 treatment: As live cell imaging (supplemental Fig. 1A) shows, HeLa cells stably expressing histone H2B-GFP were arrested at the metaphase during 6hr treatment of MG132 and the metaphase chromatid morphology appeared intact without any sign of mitotic exit or apoptosis (see supplemental movie1). Furthermore, Western blotting revealed that the levels of cyclinb1, as well as those of securin and phospho (S10) Histone H3, remained unaltered during 6hr of MG132 treatment (supplemental Fig. 1B). The following antibodies were used: rabbit polyclonal antibodies against MYPT1 (17); a mouse polyclonal antibody against Ser473- phopshorylated MYPT1 (7); a monoclonal against myc (9E10, Covance, Richmond, CA); human autoimmune serum against CREST (B. Brinkley, Baylor College of Medicine); a rabbit polyclonal against Shugoshin (Sgo1) (Y. Watanabe, Univ. of Tokyo); a rabbit polyclonal against cyclin B1 (BioLegend, San Diego, CA); a mouse monoclonal against cyclin B1 (GNS1, Santa Cruz Biotechnology); rabbit polyclonal against phospho (S10) histone H3 (Cell Signaling Technology, Boston MA), a rabbit polyclonal against Cdc20 (Bethyl, Montgomery TX); and a rabbit polyclonal against securin (Invitrogen, Carlsbad, CA). Immunofluorescence - Immunofluorescence was performed as described (17). Briefly, cells were fixed with 3.7% formaldehyde in the PHEM buffer (54mM Pipes, 22.5mM Hepes, 10mM EGTA and 8mM MgSO 4, ph 7.0) for 10 minutes, and permeabilized with 0.2% Triton X-100 in PHEM for 5 minutes. After blocking with normal goat serum (Jackson laboratory, Bar Harbor, ME), cells were labeled with primary antibodies. Chromosomes were detected by DAPI staining (Molecular Probes). Mitotic cells were obtained by the shake-off method, cytospun onto poly-dlysine coated coverslips, and analyzed by immunofluorescence as described above. Immunofluorescent images were taken as Z-stacks with a DeltaVision Image Restoration Microscope system (Applied Precision Instrument, LLC Issaquah, WA), deconvoluted with the SoftWoRx software (Applied Precision Instruments) or the Huygen software (SVI, The Netherland), and 2
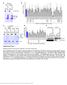
3 projected images were generated. Exposure times and settings for deconvolution were constant for all samples to be compared within any given experiment. Image contrast and brightness were adjusted by Photoshop (Adobe, San Jose, CA). Immunofluorescent images were also taken by a Nikon TE300 microscope with a x plan Apo lens (N. A. 1.40). Live cell imaging Live cell imaging was performed with HeLa cells or SW962 cells stably expressing histone H2B GFP as described previously (7). HeLa cells stably expressing histone H2B-GFP were kindly provided by Dr. G.M. Wahl (The Salk Institute, CA) (18) and SW962 cells stably expressing histone H2B-GFP were isolated by transfection with a pbos- H2BGFP vector (BD Biosciences). After transfection with sirnas for 72 hours, cells were imaged at 37 o C using a DeltaVision Microscope system with a Narishige temperature control system (MS-200D, Narishige International USA, East Meadow, NY). Five Z-section images were taken in 1min intervals, deconvoluted, and projected images were generated with the SoftWoRx software (Applied Precision Instruments). sirna treatment MYPT1 was depleted by sirna treatment as described (7), and effects of depletion were analyzed at either 48 or 72 hours post transfection. Sgo1 depletion was performed according to (19). Cdc20 depletion was performed using an sirna sequence described in (20), which has been demonstrated to deplete Cdc20 levels less than 5% and to arrest HeLa cells at mitosis for an average of 18h (20). Single-strand, sense RNA was used for a mock control. Generation of MYPT1-depleted, stable HeLa cells Lentiviral-based shrna constructs (Mission shrna, Sigma-Aldrich) were used to generate stable, MYPT1-depleted HeLa cells as described (21). Of three shrna constructs (TRCN ; ; ) we tested, an shrna construct (TRCN ) was found to show strongest reduction of MYPT1. The MYPT1 depleted, stable HeLa cells exhibited oblique chromatid segregation (data not shown), the phenotype characteristic to MYPT1 depleted HeLa cells by sirna transfection (7), indicating that the shrna-based depletion of MYPT1 replicated the phenotype observed with sirna-based, MYPT1 depletion. Control cells were isolated using a non-targeted shrna construct. Giemsa staining and immunofluorescence of chromosome spreads- Chromosome spreading was performed as described (22) with minor modifications. Briefly, mitotic cells were diluted 4 times with water for 7 minutes at room temperature. After centrifugation, swollen cells were fixed in Camoy s solution (75% methanol/25% acetic acid). After washing 3 times with the same solution, cells were dropped onto coverslips, dried and stained either with DAPI or 5% Giemsa solution. Seventy to hundred chromatids were analyzed to determine chromatid segregation and analyses were repeated three times. Immunofluorescence of chromosome spreads was performed as described (19) with slight modification. Briefly, cells swollen as described above were cytospun on a coverslip, fixed with 4% formaldehyde containing 0.2% Triton X-100, and stained with the anti-myc, anti-crest antibodies together with DAPI. Statistical analysis- Statistical analyses were performed with two-tailed student s t-test ( To determine statistical significance of precocious segregation, fractions of mostly detached and completely scattered chromatids (see Fig. 1 for definition) were combined and analyzed by student s t-test. Means and standard deviations for the analyses of precocious chromatid segregation are listed in supplemental Table 1. RESULTS MYPT1 is required to prevent premature chromatid segregation in the presence of nocodazole or taxol. Using Giemsa staining, we examined whether MYPT1 depletion affects chromatid segregation when cells are arrested overnight by nocodazole (0.25 µg/ml) or taxol (5 µm). We found that MYPT1 depletion resulted in premature chromatid segregation. To quantitatively determine the effects of MYPT1 depletion on chromatid segregation, we categorized chromatid morphology into the following five groups (Fig. 1A, a-e): (a), prophase chromatids; (b), metaphase chromatids; (c), partially detached chromatids (see arrowheads, 3
4 less than 50% detached at the centromere); (d), mostly detached chromatids (more than 50% sister detachment); (e) completely scattered chromatids with no identifiable pairs of sister chromatids representing completely and prematurely segregated chromatids. Whereas the categories a and b represent normal chromatids, the categories c-d represent the process of premature chromatid segregation. Figs 1B and C show quantitative analyses of chromatid segregation of SW962 and HeLa cells, respectively. In nocodazole-treated SW962 cells, MYPT1 depletion greatly increased the fraction of the category e (completely scattered chromatids) from 12% (control) to 35% (MYPT1-depleted). The fraction of mostly detached chromatids (category d) also increased from 17% to 34%. On the other hand, the percentage of sister chromatids attached at the centromeres (category b) was reduced from 43% (control) to 18% (MYPT1 depleted). Similar results were obtained when SW962 cells were treated with taxol. HeLa cells also showed premature segregation of sister chromatids when MYPT1 was depleted. After overnight treatment of nocodazole or taxol, MYPT1 depletion increased the category of completely scattered chromatids (from 3% to 25% by nocodazole treatment, and from 0% to 9% by taxol treatment). The category of mostly detached chromatids was also increased from 13% to 25% by nocodazole treatment, and from 15% to 35% by taxol treatment. These results indicate that MYPT1 depletion results in precocious chromatid segregation when cells are arrested at mitosis. Premature chromatid segregation occurs independently of the Anaphase Promoting Complex/Cyclosome (APC/C). One possibility for precocious chromatid segregation is premature activation of APC/C. To test this possibility, we examined whether the inhibition of APC/C by a proteasome inhibitor, MG132, can block the precocious chromatid segregation of MYPT1- depleted HeLa cells. MYPT1-depleted cells were treated with MG132 for 1hr and 3hr and chromatid segregation was examined by Giemsa staining. Because MG132-arrested mitotic cells had few prophase cells, chromatid morphology was categorized into the following four groups including (a) metaphase chromatids; (b), partially detached chromatids (less than 50% detached at the centromere); (c), mostly detached chromatids (more than 50% sister detachment); and (d) completely scattered chromatids. As Fig. 2A shows, control mock-transfected cells showed no obvious chromatid segregation at 1hr, whereas MYPT1-depleted cells exhibited slightly increased chromatid segregation. At 3hr of MG132 treatment, however, effects of MYPT1 depletion became obvious. About 19% of MYPT1-depleted cells exhibited completely scattered chromatids whereas only 6% of control cells showed such chromatid morphology. Western blots with MG132-treated cells confirmed that cyclin B1 was not degraded in both control and MYPT1-depleted cells (B), indicating that APC/C was effectively blocked by MG132. Precocious segregation in the presence of MG132 was confirmed by live cell imaging using HeLa cells stably expressing GFP-histone H2B (C). We found that about one-fifth of MYPT1-depleted cells (4 out of 20 cells) exhibited precocious chromatid segregation (the lower panel of Fig. 2C, see movie 1) whereas virtually all control cells were arrested at metaphase (the upper panel, see movie 2). Shugoshin (Sgo1) depletion has been demonstrated to cause precocious chromatid segregation (19,23). It was thus possible that MYPT1 depletion might cause dissociation of Sgo1 from the centromeres, leading to precocious segregation. To test this possibility, Sgo1 localization was examined in MYPT1-depleted cells. We found that Sgo1 localization was not affected by MYPT1 depletion (see supplemental Figure 2), indicating that precocious chromatid segregation by MYPT1 depletion is independent of Sgo1. In reciprocal experiments, we found that Sgo1 depletion did not affect MYPT1 localization. These results indicate that the localization of Sgo1 and MYPT1 is not inter-dependent. Double depletion of MYPT1 and Cdc20 resulted in increased premature chromatid segregation. Because MG132 is a general inhibitor of proteasomes, we asked whether MYPT1-depleted cells show precocious segregation when APC/C is specifically inhibited by depletion of Cdc20, an activator of APC/C. To this end, MYPT1- depleted, stable HeLa cells generated with a Lentiviral-based shrna construct were 4
5 transfected with sirna to deplete Cdc20. Eight hours after transfection, Control, as well as MYPT1-depleted, stable HeLa cells were treated with thymidine (2mM) for 24hr and released for synchronization. Cells entered mitosis at about 11hr after the release and stayed arrested at mitosis as reported (20,24,25). We collected mitotic cells at 14hr after thymidine block (at which time cells were arrested at mitosis for 4hr), and analyzed by Giemsa staining, as well as by Western blotting, to determine chromatid morphology and states of mitosis, respectively. Western blots (Fig. 3A) revealed that Cdc20 depletion effectively blocked APC/C in both control (lanes 1 & 2) and MYPT1-depleted (lanes 3 & 4), stable HeLa cells. The levels of cyclin B1 and securin (another substrate of APC/C) of Cdc20-depleted cells (lanes 2 & 4) were equivalent to those of MG132-arrested (for 2hr) mitotic cells (lanes 1 & 3). The levels of phospho(s10)-histone H3 are also similar, indicating that Cdc20-depleted cells were arrested at mitosis in this time window. We first checked whether shrna-based MYPT1 depletion replicated the precocious chromatid segregation phenotype observed with sirna-based MYPT1 depletion. Giemsa staining (B) revealed that MYPT1-depleted, stable HeLa cells, when arrested at mitosis for 2hr by MG132, showed precocious chromatid segregation: The fraction of completely scattered chromatids in MYPT1-depleted, stable HeLa cells was increased from 1.4% to 5%. The fraction of mostly detached chromatids also increased from 1.4% to 19%. The extent of precocious segregation is equivalent to that observed with sirna-based MYPT1 depletion (compare Fig. 3B with Fig. 2A). MYPT1 depletion also resulted in increased precocious segregation when cells were arrested at mitosis by Cdc20 depletion (C). While Cdc20- depleted cells exhibited precocious chromatid segregation to a certain extent, double depletion of both Cdc20 and MYPT1 greatly increased the fraction of completely scattered chromatids from 27% to 52%. These findings, together with the precocious segregation of MYPT1-depelted cells in the presence of MG132, support the notion that precocious chromatid segregation by MYPT1 depletion is APC/C independent. We do not know why Cdc20 depletion alone resulted in precocious segregation. Cdc20 has been reported to localize at kinetochores (25,26) and affect kinetochoremicrotubule attachments (25). It is thus possible that Cdc20 is involved in sister chromatid cohesion. Premature chromatid segregation requires PLK1 activity. Our previous results indicate that MYPT1 depletion increased PLK1 activity (7). If the precocious chromatid segregation is caused by hyper-activated PLK1, then inhibition of PLK1 should block the precocious segregation. To test this idea, we examined whether MYPT1-depleted cells showed precocious segregation in the presence of a PLK1 inhibitor of BI After short time (1.5hr) treatment with MG132, metaphase cells were isolated by the shake-off method from control, as well as MYPT1-depleted cells (in this short time treatment precocious chromatid segregation was minimum in both control and MYPT1-depleted cells, See Fig. 2). The metaphase cells were then transferred into MG132-containing medium with or without BI for another 4hr, and their chromosome morphology was categorized into the same four groups as described in Fig. 2A, i.e., (a) metaphase chromatids; (b), partially detached chromatids (less than 50% detached at the centromere); (c), mostly detached chromatids (more than 50% sister detachment); and (d) completely scattered chromatids. It was noted that PLK1 inhibition altered chromosome appearance. As Fig. 4A shows, chromosomes in the presence of BI-2536 are more condensed and shorter than those in the absence of the inhibitor. Nonetheless, we were able to categorize chromatid morphology of PLK1-inhibited cells into the above four groups (see panels a-d in Fig. 4A). Quantitative analyses (Fig. 4B) revealed that PLK1 inhibition blocked precocious segregation caused by MYPT1 depletion. In the absence of BI-2536, MYPT1 depletion causes precocious chromatid segregation: Forty-one percent of MYPT1-depleted cells exhibited either completely scattered chromatids or mostly detached chromatids while only 13% of control cells showed such detached chromatids (the extent of precocious segregation in this experimental setting was more severe than that observed in Fig. 2, which is probably due to the longer treatment with MG132 (5.5hr vs. 3hr)). PLK1 inhibition, however, greatly reduced the fractions of the 5
6 precociously segregated chromatids of MYPT1- depleted cells. While 20% of MYPT1-depleted cells showed completely scattered chromatids in the absence of BI-2536, PLK1 inhibition decreased this fraction to 8%. The fraction of mostly detached chromatids was also lessened from 21% to 9%. PLK1 inhibition in control cells also reduced the fraction of completely scattered chromatids from 8% to 5%. These findings indicate that PLK1 activity is required for the precocious dissociation of cohesin at the centromeres in MYPT1-depleted cells, and are consistent with our previous result that MP antagonizes PLK1 (7). PLK1 activity was also required for the precocious segregation in MYPT1-depleted cells that were arrested at mitosis by Cdc20 depletion. As Fig. 3D shows, mostly detached and completely scattered sister chromatids were virtually eliminated in the presence of BI-2536, further supporting the requirement of PLK1 for precocious segregation caused by MYPT1 depletion. An unphosphorylatable mutant of SA2 blocks premature chromatid segregation. One possible mechanism for the precocious segregation by MYPT1 depletion is via phosphorylation of a cohesin subunit of SA2. It has been reported that PLK1 phosphorylates SA2, leading to the dissociation of most cohesin complexes along chromosome arms during prometaphase. The cohesin complex at the centromeres, however, protected from phosphorylation by PP2A protein phosphatase recruited by Sgo1, preventing untimely chromatid segregation (1,16,27). We therefore hypothesized that excessive activation of PLK1 caused by MYPT1 depletion would override PP2A phosphatase, leading to SA2 phosphorylation at the centromeres, thereby dissociating the cohesin complex and causing precocious chromatid segregation. To test this hypothesis, we examined whether the unphosphorylatable mutant of SA2 could prevent precocious chromatid segregation of MYPT1-depleted cells. We used Tet-on HeLa cell lines developed by Hauf et al, who demonstrated that the SA2 unphosphorylatable mutant stayed associated with centromeres, as well as along chromosome arms (16). The Tet-on HeLa cells can induce myc-tagged, unphosphorylatable mutant (myc-sa2-12xa) or wild-type SA2 (see ref. (16)) upon addition of Dox. Because about 30-40% of cells of these cell lines lost expression of myc-tagged SA2, chromosome spreads were stained with an anti-myc antibody to identify cells expressing the myc-tagged SA2. They were counterstained with DAPI and an anti-crest antibody to determine chromatid morphology and kinetochore segregation, respectively. Fig. 5A shows typical immunofluorescence of chromosome spreads from mock-transfected, control cells expressing wild type SA2 (a), or mutant SA2 (c), as well as from MYPT1-depleted cells expressing wild type (b) or mutant SA2 (d). CREST staining of MYPT1-depleted cells expressing the mutant SA2 revealed that all kinetochores were present as closely spaced pairs (d), indicating that the unphosphorylatable mutant of SA2 blocked precocious segregation of chromatids. In contrast, MYPT1-depleted cells expressing wild type SA2 showed scattered CREST staining (b), indicating chromatid segregation. Quantitative measurement (B) confirmed the above conclusion. The unphosphorylatable mutant of SA2, but not wild type SA2, effectively protected MYPT1-depleted cells from precocious chromatid segregation. Without induction with Dox, both types of cells show precocious chromatid segregation to a similar extent when MYPT1 was depleted (Supplemental Fig. 3). Taken together, these results support our hypothesis that MP, by antagonizing PLK1, prevents SA2 phosphorylation at the centromeres. MYPT1-depleted SW962 cells exit mitosis prematurely under normal conditions. Our result that MYPT1 depletion causes premature chromatid segregation in mitotically arrested cells suggests that MYPT1 depletion could also affect mitotic exit under normal conditions. We found that this is the case with SW962 cells. Fig 6A shows phase-contrast (a & c) and DAPI staining (b & d) images of control (a & b) and MYPT1-depleted (c & d) mitotic cells isolated from asynchronous culture of SW962 cells. As expected from asynchronous culture, DAPI staining of control mitotic cells (panel b of A) exhibited varying stages of mitosis including prophase, prometaphase, metaphase, anaphase and telophase. In contrast, MYPT1-depleted 6
7 chromatids were mostly abnormal, showing few chromosomes at a metaphase, anaphase or telophase stage (panel d of A). They were clearly more spread than normal prometaphase chromatids and did not seem to represent normal prophase chromosomes. Quantitative analyses (B) revealed that about 90% of MYPT1-depleted chromatids (n=287) had such spread morphology. Box plot analyses of area measurements (supplemental Figure 4) revealed that the median value of the area of MYPT1-depleted chromatids was twice as large as that of control prophase chromatids (p<0.0001). This large increase of the area could not be accounted by cytokinesis failure alone because MYPT1 depletion resulted in only 16% increase in multinuclear cells (7). Importantly, these spread chromatids were often associated with orphan chromatids (see arrowheads in panel d of A), suggesting that chromatids are precociously segregated and/or incorrectly connected to kinetochore microtubules. Giemsa staining (Fig. 6C) confirmed that the chromatids of MYPT1-depleted SW962 cells were indeed precociously segregated without mitotic arrest. The morphology of mitotic chromatids was categorized into five groups according to the same criteria described for Fig. 1 (anaphase and telophase chromatids were excluded from the measurements). While more than 90% of control cells exhibited normal chromatids with either the prophase (category a), or prometaphase/metaphase (category b) morphology, only 33% of MYPT1- depleted chromatids showed such normal morphology. The fraction of the category e (completely scattered chromatids) in MYPT1- depleted mitotic cells was increased from 3% to 30% after 72 hours of MYPT1 depletion. In addition, partially (category c) and mostly (category d) detached chromatids were also increased from 3% to 16%, and from 3% to 30%, respectively. Precocious chromatids segregation was confirmed by live cell imaging with SW962 cells stably expressing GFP-tagged histone H2B. Fig. 7 exhibits representative still images of mitotic progression for control (A) and three typical examples of MYPT1-depleted (B-D) SW962 cells. In control cells (15/16), sister chromatids were able to align at the metaphase plate around minutes, and segregated at about 50- minutes after chromatid condensation (A, see movie 3). In contrast, most (30/32) MYPT1-depleted cells showed abnormal mitosis of three phenotypes (B- D). The largest fraction (53%, 16 out of 30) was unable to completely align the chromatids at the metaphase plate, and showed apparent premature segregation of chromatids (B, see movie 4). The precocious segregation resulted in spread chromatids with orphan chromosomes (see arrows in the image of B at 180 minutes), explaining the image obtained by DAPI staining of MYPT1- depleted chromatids (panel d of Fig. 6A). In the second phenotype (13%, 4/30), chromatids were unable to align, did not show apparent segregation (C, movie 5), and stayed condensed for a prolonged time (over 2 hours). The third phenotype (6.7%, 2/30) observed in MYPT1- depleted cells was oblique segregation of chromatids (D, movie 6). These results strongly suggest that the majority of MYPT1-depleted chromatids were precociously segregated. DISCUSSION We have shown that MYPT1 depletion induced precocious chromatid segregation in the absence of APC/C activity. This precocious segregation requires PLK1 activity (Fig. 4) and is blocked by expression of the unphosphorylatable mutant of SA2 (Fig. 5), suggesting that SA2 phosphorylation by PLK1 at the centromeres is likely to cause the precocious segregation. SA2 phosphorylation is controlled by a balance between kinases (including PLK1) and PP2A phosphatase. This enzymatic balance is inherently delicate and could be errorprone: If PLK1 is hyper-activated, it could override PP2A phosphatase. Such hyperactivation of PLK1 could happen, in particular, at the kinetochores and their vicinities because PLK1 activity needs to be kept active for the attachment between kinetochores and kinetochore microtubules, as well as stabilization of kinetochore microtubules (28,29). We propose that MP keeps PLK1 in check, thereby acting as a safeguard to protect the cohesin complexes at the centromeres from deleterious dissociation before the onset of the anaphase. Fig. 8 shows our model. Under normal conditions (A), the cohesin complexes at the centromeres are protected by two phosphatases: One is Sgo1-associated PP2A, directly dephosphorylating SA2, and the other is MP (MYPT1/PP1C), keeping PLK1 in check. In 7
8 MYPT1-depleted cells (B), PLK1 is hyperactivated, overriding dephosphorylation by PP2A, leading to precocious chromatid segregation. It has been reported that depletion of PLK1 or inhibition of AuroraB did not rescue precocious segregation of chromatids observed in Sgo1- depleted cells, suggesting that there is yet another kinase that phosphorylates SA2 (19). This result does not seem to be consistent with our result that inhibition of PLK1 blocked precocious segregation caused by MYPT1 depletion (Fig. 4). While we do not know the reason for the discrepancy, we speculate that double depletion of Sgo1 and PLK1 might activate some other kinases that could phosphorylate SA2. This speculation is based on the following reports: PLK1 depletion alone is known to block the dissociation of the cohesin complex along chromosome arms (16,30), indicating that PLK1 is mainly responsible for the dissociation of chromosome arms. However, cells depleted for both Sgo1 and PLK1 have been shown to exhibit chromatid morphology with open arms (19). Thus, it is possible that other kinases that are not usually activated in normal, as well as PLK1-single-depleted cells, might be activated in these double-depleted cells. In contrast to MYPT1-depleted SW962 cell, MYPT1-depleted HeLa cells showed no obvious precocious chromatid segregation under normal conditions. Instead, MYPT1-depleted HeLa cells frequently underwent oblique chromatid segregation (7). The reason for this difference in the phenotypes is not clear at this time. One reason could be due to lower levels of MYPT1 in SW962 cells: the MYPT1 level in SW962 cells is three-fold less than that in HeLa cells (data not shown), and thus, MYPT1 depletion in SW962 cells is more complete than that in HeLa cells. The correlation between more severe effects and more complete depletion of MYPT1 appears to be consistent with the embryonic lethal phenotype of MYPT1 KO mice (8). We and others have demonstrated that MYPT1 and PLK1 are associated in a mitosis-specific way (7,31). Such physical association would allow rapid regulation of PLK1. In addition, because MP is controlled by phosphorylation of MYPT1 with upstream kinases including ROCK (see for review 6), such upstream kinases could form a new regulatory pathway for PLK1. For example, because ROCK is present in centrosomes (32), it might be possible that ROCK negatively regulates MYPT1, allowing PLK1 more active at the centrosomes. Furthermore, PLK1 is known to phosphorylate and activate ROCK (31), possibly generating a positive feedback system for further activation of PLK1. Such activation of PLK1 may be necessary for the centrosomal functions including centrosome maturation. On the other hand, the apparent absence of ROCK at the kinetochores might keep MP active, controlling PLK1 for the protection of the centromeric cohesin. REFERENCES 1. Trinkle-Mulcahy, L., and Lamond, A. I. (2006) Curr Opin Cell Biol 18, Cohen, P. T. W. (2002) J Cell Sci 115, Ceulemans, H., and Bollen, M. (2004) Physiol Rev 84, Vagnarelli, P., Hudson, D. F., Ribeiro, S. A., Trinkle-Mulcahy, L., Spence, J. M., Lai, F., Farr, C. J., Lamond, A. I., and Earnshaw, W. C. (2006) Nat Cell Biol 8, Trinkle-Mulcahy, L., Andersen, J., Lam, Y. W., Moorhead, G., Mann, M., and Lamond, A. I. (2006) J Cell Biol 172, Hartshorne, D. J., Ito, M., and Erdodi, F. (2004) J Biol Chem 279, Yamashiro, S., Yamakita, Y., Totsukawa, G., Goto, H., Kaibuchi, K., Ito, M., Hartshorne, D. J., and Matsumura, F. (2008) Dev Cell 14, Okamoto, R., Ito, M., Suzuki, N., Kongo, M., Moriki, N., Saito, H., Tsumura, H., Imanaka- Yoshida, K., Kimura, K., Mizoguchi, A., Hartshorne, D. J., and Nakano, T. (2005) Transgenic Res 14, Somlyo, A. V., Wang, H., Choudhury, N., Khromov, A. S., Majesky, M., Owens, G. K., and Somlyo, A. P. (2004) J Muscle Res Cell Motil 25, Blagden, S. P., and Glover, D. M. (2003) Nat Cell Biol 5,
9 11. Barr, F. A., Sillje, H. H., and Nigg, E. A. (2004) Nat Rev Mol Cell Biol 5, Dai, W., and Cogswell, J. P. (2003) Prog Cell Cycle Res 5, Archambault, V., and Glover, D. M. (2009) Nat Rev Mol Cell Biol 10, Petronczki, M., Lenart, P., and Peters, J. M. (2008) Dev Cell 14, Lowery, D. M., Lim, D., and Yaffe, M. B. (2005) Oncogene 24, Hauf, S., Roitinger, E., Koch, B., Dittrich, C. M., Mechtler, K., and Peters, J. M. (2005) PLoS Biol 3, e Totsukawa, G., Yamakita, Y., Yamashiro, S., Hartshorne, D. J., Sasaki, Y., and Matsumura, F. (2000) J Cell Biol 150, Kanda, T., Sullivan, K. F., and Wahl, G. M. (1998) Curr Biol 8, McGuinness, B. E., Hirota, T., Kudo, N. R., Peters, J. M., and Nasmyth, K. (2005) PLoS Biol 3, e Huang, H. C., Shi, J., Orth, J. D., and Mitchison, T. J. (2009) Cancer Cell 16, Zagorska, A., Deak, M., Campbell, D. G., Banerjee, S., Hirano, M., Aizawa, S., Prescott, A. R., and Alessi, D. R. (2010) Sci Signal 3, ra Gimenez-Abian, J. F., Clarke, D. J., Mullinger, A. M., Downes, C. S., and Johnson, R. T. (1995) J Cell Biol 131, Salic, A., Waters, J. C., and Mitchison, T. J. (2004) Cell 118, Wolthuis, R., Clay-Farrace, L., van Zon, W., Yekezare, M., Koop, L., Ogink, J., Medema, R., and Pines, J. (2008) Mol Cell 30, Malureanu, L., Jeganathan, K. B., Jin, F., Baker, D. J., van Ree, J. H., Gullon, O., Chen, Z., Henley, J. R., and van Deursen, J. M. (2010) J Cell Biol 191, Kallio, M. J., Beardmore, V. A., Weinstein, J., and Gorbsky, G. J. (2002) J Cell Biol 158, Kitajima, T. S., Sakuno, T., Ishiguro, K., Iemura, S., Natsume, T., Kawashima, S. A., and Watanabe, Y. (2006) Nature 441, Lenart, P., Petronczki, M., Steegmaier, M., Di Fiore, B., Lipp, J. J., Hoffmann, M., Rettig, W. J., Kraut, N., and Peters, J. M. (2007) Curr Biol 17, Peters, U., Cherian, J., Kim, J. H., Kwok, B. H., and Kapoor, T. M. (2006) Nat Chem Biol 2, Gimenez-Abian, J. F., Sumara, I., Hirota, T., Hauf, S., Gerlich, D., de la Torre, C., Ellenberg, J., and Peters, J. M. (2004) Curr Biol 14, Lowery, D. M., Clauser, K. R., Hjerrild, M., Lim, D., Alexander, J., Kishi, K., Ong, S. E., Gammeltoft, S., Carr, S. A., and Yaffe, M. B. (2007) EMBO J. 26, Chevrier, V., Piel, M., Collomb, N., Saoudi, Y., Frank, R., Paintrand, M., Narumiya, S., Bornens, M., and Job, D. (2002) J Cell Biol 157, FOOTNOTES *We thank Dr. G.M. Wahl for HeLa cells stably expressing histone H2B-GFP, Dr. J-M. Peters for Tet-on HeLa cells expressing myc-tagged SA2 and SA2 mutant, Dr. B. Brinkley for the anti-crest antibody, Dr. Y. Watanabe for the anti-shugoshin antibody, and Drs Barth Grant and Frank Deis for their critical reading. This work was supported by the NCI grant, CA The abbreviations used are: MP, myosin phosphatase; MYPT1, myosin phosphatase targeting subunit1; PLK1, polo-like kinase1 Running Title: Premature mitotic exit by MYPT1 depletion FIGURE LEGENDS 9
10 Fig. 1. Precocious chromatid segregation by MYPT1 depletion in the presence of nocodazole or Taxol. A, Giemsa staining images representing five categories of the chromatid morphology. Arrowheads indicate seregated chromatids. B & C, MYPT1 depletion increases the fraction of segregated chromatids in the presence of either nocodazole or taxol. SW962 (B) or HeLa (C) cells were treated either with nocodazole or taxol overnight, then chromatid morphology was examined by Giemsa staining and categorized according to the groups shown in A. One asterisk, p<0.05; two asterisks; p<0.01; three asterisks, p< Fig. 2. Precocious chromatid segregation in the presence of MG132. A. Control (Cnt) and MYPT1- depleted (si) HeLa cells were treated with 5µM MG132 for 1hr or 3hr. Mitotic cells were isolated and chromatid morphology was categorized into the four groups shown below the figure. One asterisk, p<0.05; two asterisks, p<0.01. B. Western blot analyses of control (lane 1) and MYPT1-depleted (lane 2) mitotic cells to indicate that cyclin B1 was not degraded in the presence of MG132. C. Live cell imaging of control (upper panel) and MYPT1-depleted (lower panel), mitotic cells in the presence of MG132. Time-lapse imaging was started 1hr after the treatment. Examples of control and precocious segregation are shown. Bar, 10µm. Time in minutes. Fig. 3. Double depletion of MYPT1 and Cdc20 results in severe precocious chromatid segregation. A, Western blots showing that Cdc20 depletion arrested both control and MYPT1-depleted HeLa cells at mitosis. Control HeLa cells (lanes 1 & 2) or HeLa cells stably expressing shrna for MYPT1 depletion (lanes 3 & 4) were transfected with sirna to deplete Cdc20 (lanes 2 & 4). Both cyclin B1 and securin are not degraded, indicating that APC/C is kept inactive by depletion of Cdc20. Lanes 1 & 2, no Cdc20 depletion. B, Precocious segregation shown by MYPT1-depleted, stable HeLa cells in the presence of MG132 for 2hr. C, Severe precocious chromatid segregation by double depletion of MYPT1 and Cdc20. While Cdc 20 depletion alone resulted in precocious chromatid segregation of control HeLa cells to a certain extent, simultaneous depletion of MYPT1 and Cdc20 greatly increased the fraction of completely scattered chromatids. D, PLK1 inhibition blocked precocious chromatid segregation of Cdc20-depleted control or Cdc20-depleted, MYPT1-depleted HeLa cells. Two asterisks, p<0.01; three asterisks, p<0.001; N.S., statistically not significant. Fig. 4. Precocious chromatid segregation requires PLK1 activity. A. Representative images of chromatids in the presence of both MG132 and BI They are categorized into four groups including chromatids at metaphase (a), as well as partially separated chromatids (b), mostly separated chromatids (c) and completely scattered chromatids (d). Note that, while chromatids are shorter and more condensed, chromosome arms in (a) are clearly separated as expected from metaphase arrested cells. B. Quantitative measurements of chromatid morphologies of control (Cnt), and MYPT1-depleted (si) mitotic cells in the absence or presence of BI One asterisk, p<0.05; N.S., statistically not significant. Fig. 5. The unphosphorylatable SA2 mutant rescues precocious chromatid segregation. A. Representative immunofluorescence images of chromatid spreads isolated from control (a & c) and MYPT1-depleted (b & d), Tet-on HeLa cells expressing myc-tagged, wild type (a and b) or the unphosphorylatable mutant (c & d) of SA2. Merged images show CREST (green) and DAPI (blue) localization. Insets, myc staining. Bar, 10µm. B. Quantitative measurements of chromatid segregation of control (Cnt) or MYPT1-depleted (si), Tet-on HeLa cells expressing wild type or the mutant SA2. Two asterisks, p<0.01; N.S., statistically not significant. Fig. 6. MYPT1 depletion results in precocious chromatid segregation under normal conditions. A, MYPT1-depleted cells accumulate mitotic cells with spread chromatid morphology. Control (a, b) or MYPT1-depleted (c, d), mitotic cells were collected by the shake-off method and observed with phasecontrast (a, c) or fluorescence microscopy with DAPI staining (b & d). Arrowheads in d indicate orphan 10
11 chromosomes in MYPT1-depleted cells (inset, the enlarged image of the white rectangle showing an orphan chromosome). Bar, 20µm. Representative images from three independent experiments are shown. B, Distribution of prometaphase, metaphase, anaphase and telophase cells in mock-transfected (white bar) and MYPT1-depleted (black bar) mitotic cells. Chromatids with spread morphology of MYPT1-depleted cells are not clearly distinguished from prophase cells and thus are categorized into spread chromatids. C. Precocious chromatid segregation by MYPT1 depletion. Chromatids from control (Cnt) and MYPT1- depelted (si) mitotic cells (48 hours or 72 hours depletion) were Giemsa stained and categorized into the five groups according to the criteria shown in Fig. 1A. Two asterisks, p<0.01. Fig. 7. Live cell imaging of precocious chromatid segregation shown by of MYPT1-depleted SW962 cells. A, control. B-D, MYPT1-depleted SW962 cells stably expressing histone H2B-GFP. Examples of precocious segregation (B), prolonged mitosis (C) and oblique segregation (D) are shown. B, major phenotype of MYPT1-depleted cells; C & D, minor phenotype. Bar, 5µm. Time in minutes. Fig. 8. A working model for the role of MP in antagonizing PLK1 in the regulation of SA2 phosphorylation at the centromeres. SUPPLEMENTAL FIGURES: Supplemental Figure 1- A, Live cell imaging of HeLa cells in the presence of MG132. HeLa cells stably expressing Histone H2B-GFP were treated with 5µM MG132. Two hours later, cells were imaged every 10min by a Nikon TE300 microscope for the next 4hr. Both phase contrast and fluorescence images were shown. B, Six-hour MG132 treatment did not alter the levels of cyclin B1, as well as those of securin and phospho (S10) Histone H3. Mitotic cells were collected from HeLa cells that had been arrested for 2hr and 6hr with MG132 and total cell lysates were immunoblotted with indicated antibodies. Supplemental Figure 2-Localization of Sgo1 and MYPT1 at the kinetochores is independent. Control (ad), MYPT1-depleted (e-h) and Sgo1-depleted (i-l) were stained with the anti-sgo1 (a, e, i) and antiphosphomypt1 (b, f, j) antibodies and counterstained with DAPI (c, g, k). Merged images (d, h, l) show Sgo1 (green), Ser473-phosphorylated MYPT1 (red), and DNA (blue). Supplemental Figure 3 Chromatid segregation of Tet-on HeLa cells in the absence of Dox. Both cell lines show a similar extent of precocious segregation by MYPT1 depletion. Cnt, control; si, MYPT1 depletion. Supplemental Figure 4- Box plot analyses to compare the areas of prophase chromatids of control SW962 cells with those of chromatids with spread morphology from MYPT1-depleted SW962 cells. SUPPLEMENTAL TABLE Supplemental Table 1- Mean values and standard deviations of the stacked graphs in Figs 1B and 1C, Fig. 2A, Fig. 3B-D, Fig. 4B, Fig. 5B, and Fig. 6C are shown. SUPPLEMENTAL VIDEOS: Legends for movies 1 - Time-lapse sequences of MG132-arrested, control HeLa Cells stably expressing Histone H2B-GFP. One hour after the addition of MG132, mitotically arrested cells were imaged with a DeltaVision Image Restoration Microscope system. Five z-section images were taken at 1min intervals for about 180min. After deconvolution, projected images are shown as a movie. One second in a movie corresponds to 10 minutes elapsed time. Selected frames are shown in the upper panel of Fig. 2C. 11
12 Legends for movies 2 - Time-lapse sequences of MG132-arrested, MYPT1-depleted HeLa Cells stably expressing Histone H2B-GFP. The conditions are the same as the legend described in movie 1. Selected frames are shown in the lower panel of Fig. 2C. Legends for movies 3-6- Time-lapse sequences of mitotic SW962 Cells stably expressing Histone H2B- GFP. Both control (movie 3) and MYPT1-depleted (movie 4-6) HeLa cells without drug treatment were imaged with a DeltaVision Image Restoration Microscope system. Five z-section images were taken at 1min intervals for min. After deconvolution, projected images are shown as a movie. One second in a movie corresponds to 10 minutes elapsed time. Movie 3. Control SW962 mitotic cells. The time-lapse covers a period of about min. Selected frames are shown in Fig. 6A. Movie 4. MYPT1-depleted mitotic SW962 cells. Major phenotype. The time-lapse covers a period of about 180min. Selected frames are shown in Fig. 6B. Movie 5. MYPT1-depleted mitotic SW962 cells. Minor Phenotype. The time-lapse covers a period of about 120min. Selected frames are shown in Fig. 6C. Movie 6. MYPT1-depleted mitotic SW962 cells. Minor phenotype. The time-lapse covers a period of about 50min. Selected frames are shown in Fig. 6D. 12
13 Fig. 1 A a b c d e a b c d e tightly attached along chromosomes attached at the centromere partly detached--less than 50% mostly detached-more than 50% but pairs recognizable-- completely scattered B SW962 Nocodazole 100 ** 80 Taxol * C Nocodazole HeLa Taxol *** ** % % % % Control Si, MYPT1 0 Control Si, MYPT1 0 Control Si, MYPT1 0 Control Si, MYPT1 11
14 Fig. 2 A 100 * ** B 80 MG132 Cnt. Si % MYPT1 Cyclin B1 Actin C 0 metaphase, attached at the centromere partly detached--less than 50% Control Cnt, 1h si, 1h Cnt, 3h si, 3h 1 2 mostly detached but pairs recognizable-- -more than 50% completely scattered6 si
15 Fig. 3 A Cdc20, sirna + MYPT1, shrna - - MG MYPT1 B MG132 ** Cdc20 CyclinB1 Securin % 40 ps10his Actin 0 control shrna C Cdc20, sirna D Cdc20, sirna+plk1 inhibitor 100 *** N.S. 80 % % control shrna 0 control shrna tightly attached along chromosomes attached at the centromere partly detached--less than 50% mostly detached-more than 50% but pairs recognizable-- completely scattered
16 Fig. 4 A a +BI-2536 * b B 100% * N.S. 80% % c d 40% 20% 0% Cnt Cnt+BI si si+bi a b metaphase, attached at the centromere partly detached, less than 50% c mostly detached, more than 50% d completely scattered
17 Fig. 5 Control A si, MYPT1 a Myc Myc Myc-SA2-Wild, cnt Myc-SA2-Wild, si c d Myc Myc Myc-SA2-12xA, cnt B 100 Myc-SA2-Wild Myc-SA2-12xA ** N.S. 80 b Myc-SA2-12xA, si metaphase, attached at the centromere partly detached, less than 50% mostly detached, more than 50% 40 completely scattered % 20 0 Cnt si Cnt si
18 A a Fig. 6 Phase b DAPI C 100 ** ** 80 B Control c sirna d % Cnt si, 48hr si, 72hr tightly attached along chromosomes attached at the centromere partly detached--less than 50% mostly detached-more than 50% but pairs recognizable completely scattered
19 Fig. 7 A A. Control B B-D. MYPT1 depletion C D
20 Fig. 8 A. Normal condition B. MYPT1 depletion MYPT1/PP1C MYPT1/PP1C PLK PLK SA2 SA2-P SA2 SA2-P PP2A PP2A Shugoshin Shugoshin Precocious chromatid segregation
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3076 Supplementary Figure 1 btrcp targets Cep68 for degradation during mitosis. a) Cep68 immunofluorescence in interphase and metaphase. U-2OS cells were transfected with control sirna
DOI: 10.1038/ncb3076 Supplementary Figure 1 btrcp targets Cep68 for degradation during mitosis. a) Cep68 immunofluorescence in interphase and metaphase. U-2OS cells were transfected with control sirna
Nature Structural and Molecular Biology: doi: /nsmb Supplementary Figure 1
 Supplementary Figure 1 Mutational analysis of the SA2-Scc1 interaction in vitro and in human cells. (a) Autoradiograph (top) and Coomassie stained gel (bottom) of 35 S-labeled Myc-SA2 proteins (input)
Supplementary Figure 1 Mutational analysis of the SA2-Scc1 interaction in vitro and in human cells. (a) Autoradiograph (top) and Coomassie stained gel (bottom) of 35 S-labeled Myc-SA2 proteins (input)
Figure S1. HP1α localizes to centromeres in mitosis and interacts with INCENP. (A&B) HeLa
 SUPPLEMENTARY FIGURES Figure S1. HP1α localizes to centromeres in mitosis and interacts with INCENP. (A&B) HeLa tet-on cells that stably express HP1α-CFP, HP1β-CFP, or HP1γ-CFP were monitored with livecell
SUPPLEMENTARY FIGURES Figure S1. HP1α localizes to centromeres in mitosis and interacts with INCENP. (A&B) HeLa tet-on cells that stably express HP1α-CFP, HP1β-CFP, or HP1γ-CFP were monitored with livecell
Effects of UBL5 knockdown on cell cycle distribution and sister chromatid cohesion
 Supplementary Figure S1. Effects of UBL5 knockdown on cell cycle distribution and sister chromatid cohesion A. Representative examples of flow cytometry profiles of HeLa cells transfected with indicated
Supplementary Figure S1. Effects of UBL5 knockdown on cell cycle distribution and sister chromatid cohesion A. Representative examples of flow cytometry profiles of HeLa cells transfected with indicated
Regulators of Cell Cycle Progression
 Regulators of Cell Cycle Progression Studies of Cdk s and cyclins in genetically modified mice reveal a high level of plasticity, allowing different cyclins and Cdk s to compensate for the loss of one
Regulators of Cell Cycle Progression Studies of Cdk s and cyclins in genetically modified mice reveal a high level of plasticity, allowing different cyclins and Cdk s to compensate for the loss of one
Supplementary information. The Light Intermediate Chain 2 Subpopulation of Dynein Regulates Mitotic. Spindle Orientation
 Supplementary information The Light Intermediate Chain 2 Subpopulation of Dynein Regulates Mitotic Spindle Orientation Running title: Dynein LICs distribute mitotic functions. Sagar Mahale a, d, *, Megha
Supplementary information The Light Intermediate Chain 2 Subpopulation of Dynein Regulates Mitotic Spindle Orientation Running title: Dynein LICs distribute mitotic functions. Sagar Mahale a, d, *, Megha
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Posch et al., http://www.jcb.org/cgi/content/full/jcb.200912046/dc1 Figure S1. Biochemical characterization of the interaction between
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Posch et al., http://www.jcb.org/cgi/content/full/jcb.200912046/dc1 Figure S1. Biochemical characterization of the interaction between
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION
 Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
Supplementary Information POLO-LIKE KINASE 1 FACILITATES LOSS OF PTEN-INDUCED PROSTATE CANCER FORMATION X. Shawn Liu 1, 3, Bing Song 2, 3, Bennett D. Elzey 3, 4, Timothy L. Ratliff 3, 4, Stephen F. Konieczny
 a Control IgG Intestine c Testis b Thymus 1 3 2 S S 2 1 3 4 4 Figure S1 The wild-type mouse (C57BL/6J) organs (intestine, thymus and testis) were frozen in liquid nitrogen and sectioned at 5 µm on a cryostat.
a Control IgG Intestine c Testis b Thymus 1 3 2 S S 2 1 3 4 4 Figure S1 The wild-type mouse (C57BL/6J) organs (intestine, thymus and testis) were frozen in liquid nitrogen and sectioned at 5 µm on a cryostat.
Supplementary Figure 1. Mother centrioles can reduplicate while in the close association
 C1-GFP distance (nm) C1-GFP distance (nm) a arrested HeLa cell expressing C1-GFP and Plk1TD-RFP -3 s 1 2 3 4 5 6 7 8 9 11 12 13 14 16 17 18 19 2 21 22 23 24 26 27 28 29 3 b 9 8 7 6 5 4 3 2 arrested HeLa
C1-GFP distance (nm) C1-GFP distance (nm) a arrested HeLa cell expressing C1-GFP and Plk1TD-RFP -3 s 1 2 3 4 5 6 7 8 9 11 12 13 14 16 17 18 19 2 21 22 23 24 26 27 28 29 3 b 9 8 7 6 5 4 3 2 arrested HeLa
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Dunsch et al., http://www.jcb.org/cgi/content/full/jcb.201202112/dc1 Figure S1. Characterization of HMMR and CHICA antibodies. (A) HeLa
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Dunsch et al., http://www.jcb.org/cgi/content/full/jcb.201202112/dc1 Figure S1. Characterization of HMMR and CHICA antibodies. (A) HeLa
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Krenn et al., http://www.jcb.org/cgi/content/full/jcb.201110013/dc1 Figure S1. Levels of expressed proteins and demonstration that C-terminal
Cell Cycle, Mitosis, and Microtubules. LS1A Final Exam Review Friday 1/12/07. Processes occurring during cell cycle
 Cell Cycle, Mitosis, and Microtubules LS1A Final Exam Review Friday 1/12/07 Processes occurring during cell cycle Replicate chromosomes Segregate chromosomes Cell divides Cell grows Cell Growth 1 The standard
Cell Cycle, Mitosis, and Microtubules LS1A Final Exam Review Friday 1/12/07 Processes occurring during cell cycle Replicate chromosomes Segregate chromosomes Cell divides Cell grows Cell Growth 1 The standard
Regulation of Sister Chromatid Cohesion between Chromosome Arms
 Current Biology, Vol. 14, 1187 1193, July 13, 2004, 2004 Elsevier Ltd. All rights reserved. DOI 10.1016/j.cub.2004.06.052 Regulation of Sister Chromatid Cohesion between Chromosome Arms Juan F. Giménez-Abián,
Current Biology, Vol. 14, 1187 1193, July 13, 2004, 2004 Elsevier Ltd. All rights reserved. DOI 10.1016/j.cub.2004.06.052 Regulation of Sister Chromatid Cohesion between Chromosome Arms Juan F. Giménez-Abián,
Campbell Biology in Focus (Urry) Chapter 9 The Cell Cycle. 9.1 Multiple-Choice Questions
 Campbell Biology in Focus (Urry) Chapter 9 The Cell Cycle 9.1 Multiple-Choice Questions 1) Starting with a fertilized egg (zygote), a series of five cell divisions would produce an early embryo with how
Campbell Biology in Focus (Urry) Chapter 9 The Cell Cycle 9.1 Multiple-Choice Questions 1) Starting with a fertilized egg (zygote), a series of five cell divisions would produce an early embryo with how
(a) Reproduction. (b) Growth and development. (c) Tissue renewal
 100 µm 200 µm 20 µm (a) Reproduction (b) Growth and development (c) Tissue renewal 1 20 µm 2 0.5 µm Chromosomes DNA molecules Chromosome arm Centromere Chromosome duplication (including DNA synthesis)
100 µm 200 µm 20 µm (a) Reproduction (b) Growth and development (c) Tissue renewal 1 20 µm 2 0.5 µm Chromosomes DNA molecules Chromosome arm Centromere Chromosome duplication (including DNA synthesis)
Cell cycle and Apoptosis. Chalermchai Mitrpant
 Cell cycle and Apoptosis 2556 Chalermchai Mitrpant Overview of the cell cycle Outline Regulatory mechanisms controlling cell cycle Progression of the cell cycle Checkpoint of the cell cycle Phases of the
Cell cycle and Apoptosis 2556 Chalermchai Mitrpant Overview of the cell cycle Outline Regulatory mechanisms controlling cell cycle Progression of the cell cycle Checkpoint of the cell cycle Phases of the
Supplemental Materials. STK16 regulates actin dynamics to control Golgi organization and cell cycle
 Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
Supplemental Materials STK16 regulates actin dynamics to control Golgi organization and cell cycle Juanjuan Liu 1,2,3, Xingxing Yang 1,3, Binhua Li 1, Junjun Wang 1,2, Wenchao Wang 1, Jing Liu 1, Qingsong
To segregate sister chromatids evenly into daughter cells
 Human Bub1 protects centromeric sister-chromatid cohesion through Shugoshin during mitosis Zhanyun Tang*, Yuxiao Sun, Sara E. Harley*, Hui Zou, and Hongtao Yu* Departments of *Pharmacology and Molecular
Human Bub1 protects centromeric sister-chromatid cohesion through Shugoshin during mitosis Zhanyun Tang*, Yuxiao Sun, Sara E. Harley*, Hui Zou, and Hongtao Yu* Departments of *Pharmacology and Molecular
Supplemental Information. 3D-CLEM Reveals that a Major Portion. of Mitotic Chromosomes Is Not Chromatin
 Molecular Cell, Volume 64 Supplemental Information 3D-CLEM Reveals that a Major Portion of Mitotic Chromosomes Is Not Chromatin Daniel G. Booth, Alison J. Beckett, Oscar Molina, Itaru Samejima, Hiroshi
Molecular Cell, Volume 64 Supplemental Information 3D-CLEM Reveals that a Major Portion of Mitotic Chromosomes Is Not Chromatin Daniel G. Booth, Alison J. Beckett, Oscar Molina, Itaru Samejima, Hiroshi
Supplementary Figure S1
 Supplementary Figure S1 Supplementary Figure S1. PARP localization patterns using GFP-PARP and PARP-specific antibody libraries GFP-PARP localization in non-fixed (A) and formaldehyde fixed (B) GFP-PARPx
Supplementary Figure S1 Supplementary Figure S1. PARP localization patterns using GFP-PARP and PARP-specific antibody libraries GFP-PARP localization in non-fixed (A) and formaldehyde fixed (B) GFP-PARPx
How Cells Divide. Chapter 10
 How Cells Divide Chapter 10 Bacterial Cell Division Bacteria divide by binary fission. -the single, circular bacterial chromosome is replicated -replication begins at the origin of replication and proceeds
How Cells Divide Chapter 10 Bacterial Cell Division Bacteria divide by binary fission. -the single, circular bacterial chromosome is replicated -replication begins at the origin of replication and proceeds
SUPPLEMENTARY INFORMATION
 DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
DOI: 1.138/ncb222 / b. WB anti- WB anti- ulin Mitotic index (%) 14 1 6 2 T (h) 32 48-1 1 2 3 4 6-1 4 16 22 28 3 33 e. 6 4 2 Time (min) 1-6- 11-1 > 1 % cells Figure S1 depletion leads to mitotic defects
The Cell Cycle. Packet #9. Thursday, August 20, 2015
 1 The Cell Cycle Packet #9 2 Introduction Cell Cycle An ordered sequence of events in the life of a dividing eukaryotic cell and is a cellular asexual reproduction. The contents of the parent s cell nucleus
1 The Cell Cycle Packet #9 2 Introduction Cell Cycle An ordered sequence of events in the life of a dividing eukaryotic cell and is a cellular asexual reproduction. The contents of the parent s cell nucleus
Mitosis and the Cell Cycle
 Mitosis and the Cell Cycle Chapter 12 The Cell Cycle: Cell Growth & Cell Division Where it all began You started as a cell smaller than a period at the end of a sentence Getting from there to here Cell
Mitosis and the Cell Cycle Chapter 12 The Cell Cycle: Cell Growth & Cell Division Where it all began You started as a cell smaller than a period at the end of a sentence Getting from there to here Cell
The Cell Cycle. Dr. SARRAY Sameh, Ph.D
 The Cell Cycle Dr. SARRAY Sameh, Ph.D Overview When an organism requires additional cells (either for growth or replacement of lost cells), new cells are produced by cell division (mitosis) Somatic cells
The Cell Cycle Dr. SARRAY Sameh, Ph.D Overview When an organism requires additional cells (either for growth or replacement of lost cells), new cells are produced by cell division (mitosis) Somatic cells
Molecular Cell Biology - Problem Drill 22: The Mechanics of Cell Division
 Molecular Cell Biology - Problem Drill 22: The Mechanics of Cell Division Question No. 1 of 10 1. Which of the following statements about mitosis is correct? Question #1 (A) Mitosis involves the dividing
Molecular Cell Biology - Problem Drill 22: The Mechanics of Cell Division Question No. 1 of 10 1. Which of the following statements about mitosis is correct? Question #1 (A) Mitosis involves the dividing
University of Bristol - Explore Bristol Research
 Shandilya, J., Medler, K., & Roberts, S. G. E. (2016). Regulation of AURORA B function by mitotic checkpoint protein MAD2. Cell Cycle, 15(16), 2196-2201. https://doi.org/10.1080/15384101.2016.1200773 Peer
Shandilya, J., Medler, K., & Roberts, S. G. E. (2016). Regulation of AURORA B function by mitotic checkpoint protein MAD2. Cell Cycle, 15(16), 2196-2201. https://doi.org/10.1080/15384101.2016.1200773 Peer
Supplementary Information for. Shi and King, Chromosome Nondisjunction Yields Tetraploid Rather than Aneuploid Cells in Human Cell Lines.
 Supplementary Information for Shi and King, Chromosome Nondisjunction Yields Tetraploid Rather than Aneuploid Cells in Human Cell Lines Contains Supplementary Methods Supplementary Figures 1-7 Supplementary
Supplementary Information for Shi and King, Chromosome Nondisjunction Yields Tetraploid Rather than Aneuploid Cells in Human Cell Lines Contains Supplementary Methods Supplementary Figures 1-7 Supplementary
Bacterial cell. Origin of replication. Septum
 Bacterial cell Bacterial chromosome: Double-stranded DNA Origin of replication Septum 1 2 3 Chromosome Rosettes of Chromatin Loops Scaffold protein Chromatin Loop Solenoid Scaffold protein Chromatin loop
Bacterial cell Bacterial chromosome: Double-stranded DNA Origin of replication Septum 1 2 3 Chromosome Rosettes of Chromatin Loops Scaffold protein Chromatin Loop Solenoid Scaffold protein Chromatin loop
BIOLOGY - CLUTCH CH.12 - CELL DIVISION.
 !! www.clutchprep.com CONCEPT: CELL DIVISION Cell division is the process by which one cell splits into two or more daughter cells. Cell division generally requires that cells produce enough materials,
!! www.clutchprep.com CONCEPT: CELL DIVISION Cell division is the process by which one cell splits into two or more daughter cells. Cell division generally requires that cells produce enough materials,
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
基醫所. The Cell Cycle. Chi-Wu Chiang, Ph.D. IMM, NCKU
 基醫所 The Cell Cycle Chi-Wu Chiang, Ph.D. IMM, NCKU 1 1 Introduction to cell cycle and cell cycle checkpoints 2 2 Cell cycle A cell reproduces by performing an orderly sequence of events in which it duplicates
基醫所 The Cell Cycle Chi-Wu Chiang, Ph.D. IMM, NCKU 1 1 Introduction to cell cycle and cell cycle checkpoints 2 2 Cell cycle A cell reproduces by performing an orderly sequence of events in which it duplicates
Cell division functions in 1. reproduction, 2. growth, and 3. repair
 Cell division functions in 1. reproduction, 2. growth, and 3. repair What do you think you are looking at here??? Can something like you or I do this??? Fig. 12.1 How did you start out? How did you grow?
Cell division functions in 1. reproduction, 2. growth, and 3. repair What do you think you are looking at here??? Can something like you or I do this??? Fig. 12.1 How did you start out? How did you grow?
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Lu et al., http://www.jcb.org/cgi/content/full/jcb.201012063/dc1 Figure S1. Kinetics of nuclear envelope assembly, recruitment of Nup133
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Lu et al., http://www.jcb.org/cgi/content/full/jcb.201012063/dc1 Figure S1. Kinetics of nuclear envelope assembly, recruitment of Nup133
Origin of replication. Septum
 Bacterial cell Bacterial chromosome: Double-stranded DNA Origin of replication Septum 1 2 3 Chromosome Rosettes of Chromatin Loops Chromatin Loop Solenoid Scaffold protein Scaffold protein Chromatin loop
Bacterial cell Bacterial chromosome: Double-stranded DNA Origin of replication Septum 1 2 3 Chromosome Rosettes of Chromatin Loops Chromatin Loop Solenoid Scaffold protein Scaffold protein Chromatin loop
klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach
![klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach klp-18 (RNAi) Control. supplementary information. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach](/thumbs/91/104639484.jpg) DOI: 10.1038/ncb1891 A. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach embryos let hatch overnight transfer to RNAi plates; incubate 5 days at 15 C RNAi food L1 worms adult worms
DOI: 10.1038/ncb1891 A. starting strain: AV335 [emb-27(g48); GFP::histone; GFP::tubulin] bleach embryos let hatch overnight transfer to RNAi plates; incubate 5 days at 15 C RNAi food L1 worms adult worms
BIOLOGY. The Cell Cycle CAMPBELL. Reece Urry Cain Wasserman Minorsky Jackson. Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick
 CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 12 The Cell Cycle Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick The Key Roles of Cell Division The ability
CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 12 The Cell Cycle Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick The Key Roles of Cell Division The ability
The Cell Cycle. Chapter 12. Biology Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 12 The Cell Cycle PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Copyright
Chapter 12 The Cell Cycle PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Copyright
Mad2-independent spindle assembly checkpoint activation and controlled. metaphase-anaphase transition in Drosophila S2 cells
 Mad2-independent spindle assembly checkpoint activation and controlled metaphase-anaphase transition in Drosophila S2 cells Bernardo Orr 1, Hassan Bousbaa 2 and Claudio E. Sunkel 1,3,4 1) Instituto de
Mad2-independent spindle assembly checkpoint activation and controlled metaphase-anaphase transition in Drosophila S2 cells Bernardo Orr 1, Hassan Bousbaa 2 and Claudio E. Sunkel 1,3,4 1) Instituto de
Cell Division and Mitosis
 Chromatin-Uncoiled DNA during interphase Cell Division and Mitosis Chromosomes-Tightly coiled DNA Chromatid-One half of a duplicated chromosome. Each is identical and called sister chromatids Centromere-The
Chromatin-Uncoiled DNA during interphase Cell Division and Mitosis Chromosomes-Tightly coiled DNA Chromatid-One half of a duplicated chromosome. Each is identical and called sister chromatids Centromere-The
How deregulated cell cycles (might) trigger cancer. Dan Fisher. Friday 27th October Lucie Fisher, 3 Eddie Fisher, 6.
 How deregulated cell cycles (might) trigger cancer http://www.igmm.cnrs.fr Dan Fisher Friday 27th October 2017 Lucie Fisher, 3 Eddie Fisher, 6 1 transformed cells transformed cells Structure of this lecture
How deregulated cell cycles (might) trigger cancer http://www.igmm.cnrs.fr Dan Fisher Friday 27th October 2017 Lucie Fisher, 3 Eddie Fisher, 6 1 transformed cells transformed cells Structure of this lecture
Supplementary figures
 Supplementary figures Supplementary Figure 1. B cells stimulated with pokeweed mitogen display normal mitotic figures but not cells infected with B95-8. The figures show cells stimulated with pokeweed
Supplementary figures Supplementary Figure 1. B cells stimulated with pokeweed mitogen display normal mitotic figures but not cells infected with B95-8. The figures show cells stimulated with pokeweed
Outline Interphase Mitotic Stage Cell Cycle Control Apoptosis Mitosis Mitosis in Animal Cells Cytokinesis Cancer Prokaryotic Cell Division
 The Cell Cycle and Cellular Reproduction Chapter 9 Outline Interphase Mitotic Stage Cell Cycle Control Apoptosis Mitosis Mitosis in Animal Cells Cytokinesis Cancer Prokaryotic Cell Division 1 2 Interphase
The Cell Cycle and Cellular Reproduction Chapter 9 Outline Interphase Mitotic Stage Cell Cycle Control Apoptosis Mitosis Mitosis in Animal Cells Cytokinesis Cancer Prokaryotic Cell Division 1 2 Interphase
2014 Pearson Education, Inc.
 2 The Cell Cycle CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson The Key Roles of Cell Division The ability of organisms to produce more of their own kind best distinguishes living
2 The Cell Cycle CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson The Key Roles of Cell Division The ability of organisms to produce more of their own kind best distinguishes living
 Supplementary Figure Legends Supplementary Figure S1. Aurora-A is essential for SAC establishment in early mitosis. (a-c) RPE cells were treated with DMSO (a), MLN8237 (b) or BI2536 (c) for Two hours.
Supplementary Figure Legends Supplementary Figure S1. Aurora-A is essential for SAC establishment in early mitosis. (a-c) RPE cells were treated with DMSO (a), MLN8237 (b) or BI2536 (c) for Two hours.
The Cell Cycle CHAPTER 12
 The Cell Cycle CHAPTER 12 The Key Roles of Cell Division cell division = reproduction of cells All cells come from pre-exisiting cells Omnis cellula e cellula Unicellular organisms division of 1 cell reproduces
The Cell Cycle CHAPTER 12 The Key Roles of Cell Division cell division = reproduction of cells All cells come from pre-exisiting cells Omnis cellula e cellula Unicellular organisms division of 1 cell reproduces
Mitosis. AND Cell DiVISION
 Mitosis AND Cell DiVISION Cell Division Characteristic of living things: ability to reproduce their own kind. Cell division purpose: When unicellular organisms such as amoeba divide to form offspring reproduction
Mitosis AND Cell DiVISION Cell Division Characteristic of living things: ability to reproduce their own kind. Cell division purpose: When unicellular organisms such as amoeba divide to form offspring reproduction
CELL CYCLE INTRODUCTION PART I ANIMAL CELL CYCLE INTERPHASE
 CELL CYCLE INTRODUCTION The nuclei in cells of eukaryotic organisms contain chromosomes with clusters of genes, discrete units of hereditary information consisting of double-stranded DNA. Structural proteins
CELL CYCLE INTRODUCTION The nuclei in cells of eukaryotic organisms contain chromosomes with clusters of genes, discrete units of hereditary information consisting of double-stranded DNA. Structural proteins
Yan Ma 1.,XiYuan 1., William R. Wyatt 2, Joseph R. Pomerening 1 * Abstract. Introduction
 Expression of Constitutively Active CDK1 Stabilizes APC- Cdh1 Substrates and Potentiates Premature Spindle Assembly and Checkpoint Function in G1 Cells Yan Ma 1.,XiYuan 1., William R. Wyatt 2, Joseph R.
Expression of Constitutively Active CDK1 Stabilizes APC- Cdh1 Substrates and Potentiates Premature Spindle Assembly and Checkpoint Function in G1 Cells Yan Ma 1.,XiYuan 1., William R. Wyatt 2, Joseph R.
Mad2-independent Spindle Assembly Checkpoint Activation and Controlled Metaphase Anaphase Transition D V
 Molecular Biology of the Cell Vol. 18, 850 863, March 2007 Mad2-independent Spindle Assembly Checkpoint Activation and Controlled Metaphase Anaphase Transition D V in Drosophila S2 Cells Bernardo Orr,*
Molecular Biology of the Cell Vol. 18, 850 863, March 2007 Mad2-independent Spindle Assembly Checkpoint Activation and Controlled Metaphase Anaphase Transition D V in Drosophila S2 Cells Bernardo Orr,*
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis
 Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Chromosomes Chromosomes were first observed by the German embryologist Walther Fleming in 1882. Chromosome number varies among organisms most
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Chromosomes Chromosomes were first observed by the German embryologist Walther Fleming in 1882. Chromosome number varies among organisms most
Prolonged mitotic arrest induces a caspase-dependent DNA damage
 SUPPLEMENTARY INFORMATION Prolonged mitotic arrest induces a caspase-dependent DNA damage response at telomeres that determines cell survival Karolina O. Hain, Didier J. Colin, Shubhra Rastogi, Lindsey
SUPPLEMENTARY INFORMATION Prolonged mitotic arrest induces a caspase-dependent DNA damage response at telomeres that determines cell survival Karolina O. Hain, Didier J. Colin, Shubhra Rastogi, Lindsey
Chromosomes Can Congress To The Metaphase Plate Prior. To Bi-Orientation
 Chromosomes Can Congress To The Metaphase Plate Prior To Bi-Orientation Tarun M. Kapoor 1,2, Michael Lampson 1, Polla Hergert 3, Lisa Cameron 2,4, Daniela Cimini 4, E.D. Salmon 2,4, Bruce F. McEwen 3,
Chromosomes Can Congress To The Metaphase Plate Prior To Bi-Orientation Tarun M. Kapoor 1,2, Michael Lampson 1, Polla Hergert 3, Lisa Cameron 2,4, Daniela Cimini 4, E.D. Salmon 2,4, Bruce F. McEwen 3,
Chapter 8 The Cell Cycle
 What molecule stores your genetic information or determines everything about you? DNA a nucleic acid How are DNA molecules arranged in the nucleus? As you can see DNA is: Chapter 8 The Cell Cycle 1. Arranged
What molecule stores your genetic information or determines everything about you? DNA a nucleic acid How are DNA molecules arranged in the nucleus? As you can see DNA is: Chapter 8 The Cell Cycle 1. Arranged
Cellular Reproduction, Part 2: Meiosis Lecture 10 Fall 2008
 Mitosis & 1 Cellular Reproduction, Part 2: Lecture 10 Fall 2008 Mitosis Form of cell division that leads to identical daughter cells with the full complement of DNA Occurs in somatic cells Cells of body
Mitosis & 1 Cellular Reproduction, Part 2: Lecture 10 Fall 2008 Mitosis Form of cell division that leads to identical daughter cells with the full complement of DNA Occurs in somatic cells Cells of body
Why do cells divide? Cells divide in order to make more cells they multiply in order to create a larger surface to volume ratio!!!
 Why do cells divide? Cells divide in order to make more cells they multiply in order to create a larger surface to volume ratio!!! Chromosomes Are made of chromatin: a mass of genetic material composed
Why do cells divide? Cells divide in order to make more cells they multiply in order to create a larger surface to volume ratio!!! Chromosomes Are made of chromatin: a mass of genetic material composed
The Cell Cycle. Chapter 12. PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece
 Chapter 12 The Cell Cycle PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Overview:
Chapter 12 The Cell Cycle PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp Overview:
Cell cycle control (mammalian)
 Apr. 21, 2005 Cell cycle control (mammalian) Basic mechanisms & protein components Checkpoints Chap. 21, by Lodish et al., 5 th ed. 2004 Chap. 17, by Alberts et al., 4 th ed. 2002 鍾明怡 mychung@vghtpe.gov.tw
Apr. 21, 2005 Cell cycle control (mammalian) Basic mechanisms & protein components Checkpoints Chap. 21, by Lodish et al., 5 th ed. 2004 Chap. 17, by Alberts et al., 4 th ed. 2002 鍾明怡 mychung@vghtpe.gov.tw
SUPPLEMENT. Materials and methods
 SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
SUPPLEMENT Materials and methods Cell culture and reagents Cell media and reagents were from Invitrogen unless otherwise indicated. Antibiotics and Tet-certified serum were from Clontech. In experiments
Prof. R. V. Skibbens. BIOS 10 and BIOS 90: BioScience in the 21 st Century. Cell Cycle, Cell Division and intro to Cancer.
 Prof. R. V. Skibbens August 31, 2015 BIOS 10 and BIOS 90: BioScience in the 21 st Century Cell Cycle, Cell Division and intro to Cancer Cell Cycle Why a cell cycle? What is the goal? trauma growth development
Prof. R. V. Skibbens August 31, 2015 BIOS 10 and BIOS 90: BioScience in the 21 st Century Cell Cycle, Cell Division and intro to Cancer Cell Cycle Why a cell cycle? What is the goal? trauma growth development
Biology is the only subject in which multiplication is the same thing as division
 The Cell Cycle Biology is the only subject in which multiplication is the same thing as division Why do cells divide? For reproduction asexual reproduction For growth one-celled organisms from fertilized
The Cell Cycle Biology is the only subject in which multiplication is the same thing as division Why do cells divide? For reproduction asexual reproduction For growth one-celled organisms from fertilized
(a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable
 Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
Supplementary Figure 1. Frameshift (FS) mutation in UVRAG. (a) Schematic diagram of the FS mutation of UVRAG in exon 8 containing the highly instable A 10 DNA repeat, generating a premature stop codon
supplementary information
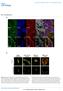 DOI: 10.1038/ncb2133 Figure S1 Actomyosin organisation in human squamous cell carcinoma. (a) Three examples of actomyosin organisation around the edges of squamous cell carcinoma biopsies are shown. Myosin
DOI: 10.1038/ncb2133 Figure S1 Actomyosin organisation in human squamous cell carcinoma. (a) Three examples of actomyosin organisation around the edges of squamous cell carcinoma biopsies are shown. Myosin
Chapter 10 Cell Growth and Division
 Chapter 10 Cell Growth and Division 10 1 Cell Growth 2 Limits to Cell Growth The larger a cell becomes, the more demands the cell places on its DNA. In addition, the cell has more trouble moving enough
Chapter 10 Cell Growth and Division 10 1 Cell Growth 2 Limits to Cell Growth The larger a cell becomes, the more demands the cell places on its DNA. In addition, the cell has more trouble moving enough
Ploidy and Human Cell Types. Cell Cycle and Mitosis. DNA and Chromosomes. Where It All Began 11/19/2014. Chapter 12 Pg
 Ploidy and Human Cell Types Cell Cycle and Mitosis Chapter 12 Pg. 228 245 Cell Types Somatic cells (body cells) have 46 chromosomes, which is the diploid chromosome number. A diploid cell is a cell with
Ploidy and Human Cell Types Cell Cycle and Mitosis Chapter 12 Pg. 228 245 Cell Types Somatic cells (body cells) have 46 chromosomes, which is the diploid chromosome number. A diploid cell is a cell with
Cell Cycle Phase. Interphase (G 1, S, G 2 ) Mitotic Phase (M phase) Prophase. Metaphase. Anaphase. Telophase
 Part I: The Cell Cycle Use your resources at hand and the Explore Student Guide to outline what occurs within the cell during each stage of the cell cycle. Record this information in Table 1 below. Cell
Part I: The Cell Cycle Use your resources at hand and the Explore Student Guide to outline what occurs within the cell during each stage of the cell cycle. Record this information in Table 1 below. Cell
MBoC ARTICLE. Sushama Sivakumar a,b, John R. Daum a, Aaron R. Tipton a, Susannah Rankin a,b, and Gary J. Gorbsky a,b a
 MBoC ARTICLE The spindle and kinetochore associated (Ska) complex enhances binding of the anaphasepromoting complex/cyclosome (APC/C) to chromosomes and promotes mitotic exit Sushama Sivakumar a,b, John
MBoC ARTICLE The spindle and kinetochore associated (Ska) complex enhances binding of the anaphasepromoting complex/cyclosome (APC/C) to chromosomes and promotes mitotic exit Sushama Sivakumar a,b, John
The Cell Cycle 4/10/12. Chapter 12. Overview: The Key Roles of Cell Division
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 12 The Cell Cycle Lectures by Erin
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 12 The Cell Cycle Lectures by Erin
LECTURE PRESENTATIONS
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 12 The Cell Cycle Lectures by Erin
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 12 The Cell Cycle Lectures by Erin
SUPPLEMENTARY INFORMATION
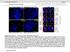 doi: 10.1038/nature05473 SUPPLEMENTARY FIGURES SUPPLEMENTARY INFORMATION Supplementary Figure 1: Association of Runx2 with mitotic chromosomes Mitotic chromosome spreads were prepared for a. Human Saos-2
doi: 10.1038/nature05473 SUPPLEMENTARY FIGURES SUPPLEMENTARY INFORMATION Supplementary Figure 1: Association of Runx2 with mitotic chromosomes Mitotic chromosome spreads were prepared for a. Human Saos-2
The Cell Cycle. Chapter 12. Biology Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 12 The Cell Cycle PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp 1
Chapter 12 The Cell Cycle PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions from Joan Sharp 1
BCHM3972 Human Molecular Cell Biology (Advanced) 2013 Course University of Sydney
 BCHM3972 Human Molecular Cell Biology (Advanced) 2013 Course University of Sydney Page 2: Immune Mechanisms & Molecular Biology of Host Defence (Prof Campbell) Page 45: Infection and Implications for Cell
BCHM3972 Human Molecular Cell Biology (Advanced) 2013 Course University of Sydney Page 2: Immune Mechanisms & Molecular Biology of Host Defence (Prof Campbell) Page 45: Infection and Implications for Cell
BIOLOGY 4/6/2015. Cell Cycle - Mitosis. Outline. Overview: The Key Roles of Cell Division. identical daughter cells. I. Overview II.
 2 Cell Cycle - Mitosis CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Overview II. Mitotic Phase I. Prophase II. III. Telophase IV. Cytokinesis III. Binary fission
2 Cell Cycle - Mitosis CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Overview II. Mitotic Phase I. Prophase II. III. Telophase IV. Cytokinesis III. Binary fission
The Cell Cycle and How Cells Divide
 The Cell Cycle and How Cells Divide 1 Phases of the Cell Cycle The cell cycle consists of Interphase normal cell activity The mitotic phase cell divsion INTERPHASE Growth G 1 (DNA synthesis) Growth G 2
The Cell Cycle and How Cells Divide 1 Phases of the Cell Cycle The cell cycle consists of Interphase normal cell activity The mitotic phase cell divsion INTERPHASE Growth G 1 (DNA synthesis) Growth G 2
Cell cycle and apoptosis
 Cell cycle and apoptosis Cell cycle Definition Stages and steps Cell cycle Interphase (G1/G0, S, and G2) Mitosis (prophase, metaphase, anaphase, telophase, karyokinesis, cytokinesis) Control checkpoints
Cell cycle and apoptosis Cell cycle Definition Stages and steps Cell cycle Interphase (G1/G0, S, and G2) Mitosis (prophase, metaphase, anaphase, telophase, karyokinesis, cytokinesis) Control checkpoints
SUPPLEMENTARY INFORMATION
 SUPPLEENTRY INFORTION DOI: 1.138/ncb2577 Early Telophase Late Telophase B icrotubules within the ICB (percent of total cells in telophase) D G ultinucleate cells (% total) 8 6 4 2 2 15 1 5 T without gaps
SUPPLEENTRY INFORTION DOI: 1.138/ncb2577 Early Telophase Late Telophase B icrotubules within the ICB (percent of total cells in telophase) D G ultinucleate cells (% total) 8 6 4 2 2 15 1 5 T without gaps
Stages of Mitosis. Introduction
 Name: Due: Stages of Mitosis Introduction Mitosis, also called karyokinesis, is division of the nucleus and its chromosomes. It is followed by division of the cytoplasm known as cytokinesis. Both mitosis
Name: Due: Stages of Mitosis Introduction Mitosis, also called karyokinesis, is division of the nucleus and its chromosomes. It is followed by division of the cytoplasm known as cytokinesis. Both mitosis
Histones- protein molecules that are used to fold and package DNA into chromosomes.
 Chromosome- a portion of the DNA in a cell, a chromosome is created when the DNA segment coils around histones then twists further to create a long twisted mass. Histones- protein molecules that are used
Chromosome- a portion of the DNA in a cell, a chromosome is created when the DNA segment coils around histones then twists further to create a long twisted mass. Histones- protein molecules that are used
LECTURE PRESENTATIONS
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 12 The Cell Cycle Lectures by Erin
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 12 The Cell Cycle Lectures by Erin
scientificreport The PITSLRE/CDK11 p58 protein kinase promotes centrosome maturation and bipolar spindle formation scientific report
 scientificreport The PITSLRE/ p58 protein kinase promotes centrosome maturation and bipolar spindle formation Clotilde Petretti 1, Matthew Savoian 2, Emilie Montembault 1,DavidM.Glover 2,ClaudePrigent
scientificreport The PITSLRE/ p58 protein kinase promotes centrosome maturation and bipolar spindle formation Clotilde Petretti 1, Matthew Savoian 2, Emilie Montembault 1,DavidM.Glover 2,ClaudePrigent
10-2 Cell Division. Slide 1 of 38. End Show. Copyright Pearson Prentice Hall
 1 of 38 Cell Division In eukaryotes, cell division occurs in two major stages. The first stage, division of the cell nucleus, is called mitosis. The second stage, division of the cell cytoplasm, is called
1 of 38 Cell Division In eukaryotes, cell division occurs in two major stages. The first stage, division of the cell nucleus, is called mitosis. The second stage, division of the cell cytoplasm, is called
Mislocalization of centromeric histone H3 variant CENP-A contributes to chromosomal instability (CIN) in human cells
 /, 2017, Vol. 8, (No. 29), pp: 46781-46800 Mislocalization of centromeric histone H3 variant CENP-A contributes to chromosomal instability (CIN) in human cells Roshan L. Shrestha 1, Grace S. Ahn 1, Mae
/, 2017, Vol. 8, (No. 29), pp: 46781-46800 Mislocalization of centromeric histone H3 variant CENP-A contributes to chromosomal instability (CIN) in human cells Roshan L. Shrestha 1, Grace S. Ahn 1, Mae
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division Ch. 10 Where it all began You started as a cell smaller than a period
Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division Ch. 10 Where it all began You started as a cell smaller than a period
Why do cells reproduce?
 Outline Cell Reproduction 1. Overview of Cell Reproduction 2. Cell Reproduction in Prokaryotes 3. Cell Reproduction in Eukaryotes 1. Chromosomes 2. Cell Cycle 3. Mitosis and Cytokinesis Examples of Cell
Outline Cell Reproduction 1. Overview of Cell Reproduction 2. Cell Reproduction in Prokaryotes 3. Cell Reproduction in Eukaryotes 1. Chromosomes 2. Cell Cycle 3. Mitosis and Cytokinesis Examples of Cell
BIOLOGY. Cell Cycle - Mitosis. Outline. Overview: The Key Roles of Cell Division. identical daughter cells. I. Overview II.
 2 Cell Cycle - Mitosis CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Overview II. Mitotic Phase I. Prophase II. III. Telophase IV. Cytokinesis III. Binary fission
2 Cell Cycle - Mitosis CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson Outline I. Overview II. Mitotic Phase I. Prophase II. III. Telophase IV. Cytokinesis III. Binary fission
Biology is the only subject in which multiplication is the same thing as division. AP Biology
 Biology is the only subject in which multiplication is the same thing as division Chapter 12. The Cell Cycle: Cell Growth, Cell Division Where it all began You started as a cell smaller than a period at
Biology is the only subject in which multiplication is the same thing as division Chapter 12. The Cell Cycle: Cell Growth, Cell Division Where it all began You started as a cell smaller than a period at
Name. A.P. Biology Chapter 12 The Cell Cycle
 A.P. Biology Chapter 12 The Cell Cycle Name Living species MUST possess the ability to r if they are to flourish. The Cell Cycle follows the life of a cell from its o until its d. The Key Roles Of Cell
A.P. Biology Chapter 12 The Cell Cycle Name Living species MUST possess the ability to r if they are to flourish. The Cell Cycle follows the life of a cell from its o until its d. The Key Roles Of Cell
Mitosis in Onion Root Tip Cells
 Mitosis in Onion Root Tip Cells A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules, or chromosomes.
Mitosis in Onion Root Tip Cells A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules, or chromosomes.
Chapter 12. The Cell Cycle
 Chapter 12 The Cell Cycle The Key Roles of Cell Division The ability of organisms to produce more of their own kind is the one characteristic that best distinguishes living things from nonliving things.
Chapter 12 The Cell Cycle The Key Roles of Cell Division The ability of organisms to produce more of their own kind is the one characteristic that best distinguishes living things from nonliving things.
10-2 Cell Division. Chromosomes
 Cell Division In eukaryotes, cell division occurs in two major stages. The first stage, division of the cell nucleus, is called mitosis. The second stage, division of the cell cytoplasm, is called cytokinesis.
Cell Division In eukaryotes, cell division occurs in two major stages. The first stage, division of the cell nucleus, is called mitosis. The second stage, division of the cell cytoplasm, is called cytokinesis.
The Cell Cycle. Chapter 12. Biology. Edited by Shawn Lester. Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 12 The Cell Cycle Edited by Shawn Lester PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 12 The Cell Cycle Edited by Shawn Lester PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis
 Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Prokaryotes Have a Simpler Cell Cycle Cell division in prokaryotes takes place in two stages, which together make up a simple cell cycle 1. Copy
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Prokaryotes Have a Simpler Cell Cycle Cell division in prokaryotes takes place in two stages, which together make up a simple cell cycle 1. Copy
Lecture 10. G1/S Regulation and Cell Cycle Checkpoints. G1/S regulation and growth control G2 repair checkpoint Spindle assembly or mitotic checkpoint
 Lecture 10 G1/S Regulation and Cell Cycle Checkpoints Outline: G1/S regulation and growth control G2 repair checkpoint Spindle assembly or mitotic checkpoint Paper: The roles of Fzy/Cdc20 and Fzr/Cdh1
Lecture 10 G1/S Regulation and Cell Cycle Checkpoints Outline: G1/S regulation and growth control G2 repair checkpoint Spindle assembly or mitotic checkpoint Paper: The roles of Fzy/Cdc20 and Fzr/Cdh1
-The cell s hereditary endowment of DNA -Usually packaged into chromosomes for manageability
 Binary Fission-Bacterial Cell Division -Asexual reproduction of prokaryotes -No mitosis -Circular DNA and organelles replicate, the copies migrate to opposite sides of the elongating cell, and the cell
Binary Fission-Bacterial Cell Division -Asexual reproduction of prokaryotes -No mitosis -Circular DNA and organelles replicate, the copies migrate to opposite sides of the elongating cell, and the cell
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
Chapter 8: Cellular Reproduction
 Chapter 8: Cellular Reproduction 1. The Cell Cycle 2. Mitosis 3. Meiosis 2 Types of Cell Division 2n 1n Mitosis: occurs in somatic cells (almost all cells of the body) generates cells identical to original
Chapter 8: Cellular Reproduction 1. The Cell Cycle 2. Mitosis 3. Meiosis 2 Types of Cell Division 2n 1n Mitosis: occurs in somatic cells (almost all cells of the body) generates cells identical to original
LECTURE PRESENTATIONS
 LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 12 The Cell Cycle Lectures by Erin
LECTURE PRESENTATIONS For CAMPBELL BIOLOGY, NINTH EDITION Jane B. Reece, Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Robert B. Jackson Chapter 12 The Cell Cycle Lectures by Erin
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
