Program for the ricerca corrente * of the IRCCS 2013 Institute for Advanced Technologies and Healthcare Protocols in Oncology-Research Lines
|
|
|
- Rosaline Baker
- 6 years ago
- Views:
Transcription
1 Program for the ricerca corrente * of the IRCCS 2013 Institute for Advanced Technologies and Healthcare Protocols in Oncology-Research Lines
2 Program for the ricerca corrente * of the IRCCS 2013 Institute for Advanced Technologies and Healthcare Protocols in Oncology Research Lines *Rircerca corrente means a specific funding program of Italian Ministry of Health that gives economic support to each Research Hospital-IRCCS- on the basis of actual data furnished by the Scientific Directorate to the Ministry on annual basis regarding clinical and research activities. The IRCCS obtained formal recognition with the M.D.12/4/2011, published in the general series Official Bulletin No.119 of 24 May 2011, and the subsequent period saw its consolidation in agreement with the plan and the research lines adopted in the process of recognition. In the three-year period, , the IRCCS will continues the activities of hospitalisation and care and basic, clinical and translational research, consistent with its area of recognition (Advanced Technologies and Healthcare Protocols in Oncology) and with the National Plan of Healthcare Research, through the three Research Lines already presented during the Ministerial Site Visit and re-defined as follows: 1. Complex Oncological Pathology, 2. Advanced Diagnostic and Therapeutic Technologies, 3. Healthcare Protocols and Oncological Pathways In addition to these three Lines, there will be a fourth line, focussed on translational research, in particular, on the subject: 4. Targets and innovative therapeutic strategies in Oncology and Oncohematology: microenvironment, inflammation, angiogenesis, immunity. Given below are the main research and healthcare activities to be carried out by the IRCCS in the coming three-year period: a) in the context of the re-organisation of the entire Hospital on the principles of Intensity of Care, a new hospitalisation ward and department for clinical research in Oncological Medicine will be activated (early in 2013) which will house the clinics for Oncological emergencies and Palliative Care; b) Inter-departmental and inter-hospital units shall be consolidated and integrated in the current Diagnostic-Therapeutic Care paths (PDTA - Percorso Diagnostico Terapeutico Assistenziale [Diagnostic Therapeutic Care Path]): Thyroid Centre, Breast Unit, Skin Cancer Unit and PDTA for lung, colon-rectal, mesothelioma and lymphoma, as well as a new Palliative Care centre ( ); c) The new Onco-Hematological centre will be opened, which will house the hospitalisation wards and laboratories (end of 2014, start of 2015); d) The network activities at the hospital (ASMN), provincial (Local Health Authority- AUSL), regional (with Regional Social and Health Agency and University) and international level (by participation in collaborative European projects) will increase. As regards the last point, the IRCCS will increase interaction with the ASMN (with the consolidation of the intra-hospital Oncological Network and with a research training path
3 aimed at health professionals so that the results of basic research can be easily translated into clinics and the other way around), with the AUSL (through research projects in the field of primary and secondary prevention) and with international networks (on Personalised Medicine). In this regard, the new laboratories forming part of the Translational Research Department belonging to the Research and Statistics Infrastructure Department will be completed (last part of 2013). Lastly, the ability to operate on the network will be increased with the creation of actions for consultation and coordination with the Region, University and other IRCCS. These objectives shall be achieved not only through greater interaction at the level of individual researchers, but also by the establishment of specific offices and the creation of coordination and consultation panels, coordinated by the Institute and/or by the Regional Health Agency. In order to promote research by the IRCCS and ensure its integration in the context of the ASMN multidisciplinary hospital where it is housed, two different tools will be used: - Consolidation of the Department Infrastructure Research and Statistics which, from 2012 has been re-organised and extended, with the aim of supporting all the research activities of the Hospital, both as regards scientific as well as healthcare activities, so that the IRCCS becomes the reference point for the entire Hospital, while at the same time making sure the Hospital is available to the IRCCS; - Creation of a Research Fund, mainly supported with the funding of Ricerca Corrente * also in order to support non-profit research activities. The funds shall be allocated to research teams by means of a transparent and meritocratic mechanism based on explicit standardised criteria. Ricerca Corrente * means means the Annual Research Program financed by the Italian Ministry of Health to the Italian IRCCS network. Research Line No.1: Complex oncological pathology Person-in-charge: dr.corrado Boni (Director of Oncology) Description: The capacity to translate into practice scientific discoveries in the field of genetics, molecular biology and oncology is one of the most innovative and important focal points for a research centre that deals with cancer patients. "Each patient is different from another and every tumour is different from another". The complexity of the cancer patient and the need to face these complexities by developing pathways which are modelled to suit the needs and clinical, biological, genetic and personal characteristics of the patient, is today the objective that clinicians and researches have prefixed to be able to treat the patients in the most specific and effective manner. Therefore, in this context, the term "complex" carries more than one meaning, but, as a whole, identifies a special case to be treated with special care. On the other hand, in Italian, the word has at least two meanings: difficult and/or having a number of aspects. We can therefore use this clue (complexity as synonym of difficulty and multiplicity) as the starting point for a reflection on its meaning when used in the context of healthcare. The interest in the complexity in medicine and healthcare derives from the need to be able to distinguish (identify and classify) the various cases (individuals) on the basis of their intrinsic difficulty/multiplicity since it is from these that greater difficulty/multiplicity may ensue in terms of treatment and also a different quoad vitam o valitudinem prognosis. A complex case will, in fact, require a different, more intense and, sometimes, more expensive treatment. At the same time, as regards research, a complex topic/case
4 deserves special attention and therefore a dedicated line of research. We therefore recognise at least two aspects that can create/increase the complexity of a case: the rarity (peculiarity) of the problem which needs to be dealt with by means of specific approaches not listed in the usual pathways of research and healthcare meant for frequent cases and the presence of certain non-biological conditions (sometimes referred to as welfare or social) which, although known and normal from the genetic and biological point of view, make the case particularly complex from the healthcare point of view. Translational research, which acts as a bridge between basic research and clinical research is the best way to transfer the findings of researchers in the clinical context and to provide insights for new laboratory research deriving from clinical practice and observation of patients. Thanks to an integrated approach between clinic and research, the ability to look at the world of personalised medicine and pharmacogenomics, makes it possible to classify the disease not only on the basis of the area affected, but according to its specific characteristics and to identify the biological and molecular parameters which sometimes make it possible to predict tumour response to treatment or to report specific risk factors in the patient for the development of toxicity. And it is precisely in the context of toxicity, thanks to a multidisciplinary integrated approach with the various professionals of the Institute, that the IRCCS of the S. Maria Nuova Hospital of Reggio Emilia aims to evaluate the impact of chemotherapy, new target therapy and the association of some of the most widely used anti-cancer drugs in terms of adverse reactions. Cardiotoxicity and cerebrovascular toxicity, in particular, are today one of the factors that most limit the use of anti-cancer drugs with a strong impact on the quality of life of the patient. Our research in this area has the aim of studying the mechanisms of cardiovascular toxicity and cerebrovascular toxicity of anticancer drugs and checking the feasibility of using diagnostic and chemo-preventive approaches to reduce the damage to the cardiovascular and cerebral damage. In parallel to clinical observation, our studies focus on analysis of the cellular and molecular mechanisms involved in the damage induced by drugs in order to understand the effect in vitro of chemotherapeutic agents on cell populations, as well as to identify biomarkers capable of defining a score risk that predicts the risk of toxicity. Objectives: The overall objective is expressed through four specific types of research, listed below: 1) complexity and testing of new drugs and new combinations of antineoplastic drugs; 2) Complexity of the patient due to a genetic-biological-molecular peculiarity of the tumour, such as affiliation to a specific sub-group with rare or little known genetic-biologicalmolecular profile and 3) Complexity of the cancer patient as a carrier of other specific conditions/diseases, such as the presence of a number of concurrent tumours, the presence of special metastatic areas or multiple non oncological diseases or the presence of multidimensional clinical-healthcare conditions, such as old age and fragility 4) Complexity of the patient due to cultural, social and psychological factors; 5) study of toxicities developed by the complex cancer patient treated with antineoplastic drugs. With regard to the specific objectives described above, it is proposed to "study" the patient under the various biological, genetic and clinical aspects, in the context of translational research, taking into consideration not only the patient s specific conditions correlated to
5 the disease, but also the patient s needs related to the family, social and economic contexts. The main objective of clinical and translational research in oncology by this IRCCS is to implement the knowhow that has until now made it possible to determine how each tumour has specific molecular characteristics which, like finger prints, distinguish and diversify it, modulating and modifying the patient s response to treatment as well as the toxicity of the treatment. In fact, with the progressive and increasing advent of new treatments targeting molecules, there is a need to gain a better and deeper understanding of the short-term as well as long-term toxicity these treatments can induce. Therefore, the understanding of the molecular mechanism underlying the action of new drugs could result in a more detailed understanding and prediction of risk of toxicity for the patient. The pharmacokinetic or pharmacogenomic approach, also through the use of new technologies and cutting-edge molecular biology approaches, will allow professionals of this Institute to balance research and clinic to offer the patient customised ad hoc treatment, with great advantage in terms of clinical and staff management for the patient, taking into consideration also an advantage in terms of quality of the service and savings on costs and resources. Indicator: - Articles in magazines (position, first name and surname; co-authors of publications with other Institutions). - Presentations at international conferences. - Patents. - Product IF. - H index for Researcher. - Chapters of Books. - Ability to acquire funds for research. - Collaboration with other institutions. - Organisation of training Courses and conferences related to the research lines. - Multidisciplinary procedures for the integrated management of the complex cancer patient. Result Indicator: - Number of publications per Research Line= Medium normalised IF per Line for the Year 2013= 5. - Projects under way funded by external Institutions =5. - Number of presentations at conferences =90. - Patents=1. - Chapters of Books= Collaborations with other institutions = 5. - Multidisciplinary procedures for the integrated management of the complex cancer patient = 7. Result described: The results we propose to obtain will emerge from the publications and research projects related to the complexity and investigation of new drugs and new associations of anticancer drugs, the genetic-biological-molecular evaluation of the tumour, study of the toxicity correlated to cancer treatments.
6 Moreover, we plan to evaluate from multiple points of view the complexity of the cancer patient as a possible carrier of other comorbidities, a number of concurrent tumours, multidimensional clinical-care conditions such as old age and fragility and cultural, social and psychological factors, which are at times difficult to manage. As regards translational research we propose to "study" the patient under the various biological, genetic and clinical aspects to understand how each tumour has precise molecular characteristics which, like finger prints, distinguish and diversify it, modulating and modifying the patient s response to treatment as well as the toxicity of the treatment. Research Line No.2: Advanced diagnostic and therapeutic technologies Person in charge: dr. Annibale Versari (Director of Nuclear Medicine) Description: Projects for the study/evaluation of Health Technologies (drugs, diagnostics, devices, algorithms, classification systems) characterised by innovativeness (recent/new, promising but not validated, in use but not studied, promising but devoid of formal evaluation of clinical utility). Type of projects/studies: 1) diagnostics (imaging-modulated and advanced technologies) and 2) innovative treatments (pharmacological and nonpharmacological). Objectives: - Development and validation of advanced technologies in "in vivo", cellular and molecular diagnostics. - Study of new methods (algorithms, models, etc.) for the quantitative and semiquantitative evaluation of biological processes. - Integration of molecular biology and imaging methods (molecular and morphological) for the development of the most customised oncological treatments. - Development and validation of new therapeutic techniques for the treatment of oncological diseases. - Research Effectiveness/Outcome studies to produce additional evidences of the clinical efficacy of diagnostic and therapeutic procedures. - Study of procedures for the early evaluation of the response to chemotherapy-radiotherapy treatments. - Promotion of early phase clinical trials for the verification/validation of diagnostictherapeutic procedures through phase II-III trials. Indicator: - Articles in magazines (position first name and surname, co-authors of publications with other Institutions). - Product IF. - Presentations at international congresses. Indicator result: - Number of publication per Research Line: Medium normalised IF per Line per Year: 4. - Number of presentations at congresses (national and international; oral and poster): 60.
7 Result described: Taking into account the objectives of the line of research, i.e.: - Development and validation of advanced technologies in "in vivo", cellular and molecular diagnostics. - Study of new methods (algorithms, models, etc.) for the quantitative and semiquantitative evaluation of biological processes. - Integration of molecular biology and imaging methods (molecular and morphological) for the development of more customised oncological treatments. - Development and validation of new therapeutic techniques for the treatment of oncological diseases. - Effectiveness/Outcome Research studies to produce additional evidences of clinical efficacy of diagnostic and therapeutic procedures. - Study of procedures for the early evaluation of response to chemotherapy radio therapy treatments. - Promotion of early phase clinical trials for the verification/validation of diagnostictherapeutic procedures by means of phase II and III studies.
8 Research Line No.3: Healthcare protocols and oncological pathways Person in charge: dr. Lucia Mangone (Director of Statistics and Quality of Clinical Trials) Description: Healthcare Research is the type of Translational Research conducted within the NHS with the aim of identifying bio-medical and healthcare information obligation through scientific research to fill cognitive gaps and check to see how much their application and implementation is reflected in terms of economically sustainable improvement. Its systematic application would make it possible to produce evidence documenting the real value and potential impact of these interventions in healthcare before and after their actual implementation. The projects and studies are focused on the development, evaluation and validation of complex clinical-organisational-healthcare interventions. Type of projects/studies: Projects for developing/validating new clinical pathways (PDTA) and epidemiological/evaluative and experimental studies for checking the impact, efficacy/effectiveness of complex interventions (new clinical-care strategies) for tumour of the breast, lung, colon-rectum and lymphoma. Projects for verifying the organisational and communicative aspects of healthcare pathways and attention also to aspects concerning the quality of life of the patients followed in the pathways. New pathways will also be implemented for tumour of the thyroid, melanoma, endometrium, ovaries and mesothelioma and projects which concern the overall quality of life of the cancer patient: age, gender, palliative treatment, fertility, application of integrated approaches of treatment (physiotherapy). Finally two crucial aspects of care will be implemented: one concerns the communication and relation among peers and with the patient and the other concerns the promotion of female personnel in the field of research and healthcare. Objectives: Implement the pathways in a coordinated manner integrating these with aspects of healthcare research and methods of evaluation of the knowledge available (systematic reviews, health technology assessment, appraisal) in order to be able to evaluate each path in terms of efficacy, safety and sustainability; Start up research programs to document the actual level of application in the context of healthcare, using clinical, "medical humanities" and economic type of outcomes and end-points. The efficacy/effectiveness indicators shall be evaluated for the clinical pathways (PDTA) of tumours of the breast, lung, colon-rectum and lymphomas. Pathways will also be implemented for tumour of the thyroid, melanoma and mesothelioma. The aspects concerning communication with the patient and the impact of the training activities on healthcare personnel will also be evaluated. Indicator: - Articles in magazines (position, name and surname, co-authors of publications with other institutions). - Product IF. - Presentations at national and international congresses. - Training and dissemination of the results concerning the Research Line. - Production of clinical pathways (PDTA) of the main tumor sites. - Divulgation of good Health Literacy practices.
9 Indicator result: - Number of publications per Research Line 3: Medium normalised IF per Line per Year: Presentations (oral and poster) at national and international congresses: Organisation of Training courses and Seminars concerning the Research Line: Production of clinical pathways (PDTA) of the main tumour sites (breast, lung, colon-rectum, lymphomas). - Number of days dedicated to the training on Health Literacy: 10. Result described: The objectives of the Line are: 1) Implementing the standardised methods of evaluation of the knowledge available in order to be able to evaluate the actual cognitive level of each healthcare pathway/protocol model in terms of efficacy, safety and sustainability; 2) Activate clinical and healthcare research programs in such a way as to document the level of actual application and the performance in the field of healthcare for 4 specific diagnostic-therapeutic pathways for tumours of the breast, lung, colon, rectum and lymphomas. The results will also be published on the pathways launched for tumours of the thyroid, endometrium, ovaries and mesotheliomas and on the aspects concerning the overall quality of life of cancer patients: age, gender, palliative treatments, fertility, application of integrated approaches of treatment (physiotherapy). Finally two aspects will be implemented concerning oncological pathways (Health Literacy project on correct communication/relation with the patient) and the healthcare protocols (preparation of a pathway to favour research among young researchers, during the period of pregnancy and maternity).
10 Research Line No.4: Targets and innovative therapeutic strategies in Oncology and Oncohematology: microenvironment, inflammation, angiogenesis, immunity Person in charge: dr. Francesco Merli (Director of Haematology) Description: Projects and activities aimed at the study/evaluation of protocols focussed on microenvironments in oncology and oncohematology. In vivo, the growth of the tumour is influenced in a decisive manner by the cells of the microenvironment (cells of the vascular and lymphatic network in angiogenesis, cells of the innate and adaptive immune system in inflammation and immune response defects, fibroblasts in the malignant stroma) and by components of the extracellular matrix (collagens, fibronectin, laminins and other components of the stroma, proteoglycans, protease). In recent years there has been a great diffusion of the so-called "biological" drugs which have action mechanisms and targets different from traditional chemotherapy and which, in some cases, have radically modified the prognosis of certain diseases (e.g. Rituximab in Non Hodgkin B lymphocytes Lymphoma, inhibitors of the thyrosin-kinase in Chronic Myeloid Leukemia, Bortezomib in Multiple Myeloma). In many cases, against the excellent results produced during the course of clinical trials, these new molecules have multiple action mechanisms which are not very well known. For example, the antiangiogenic effect of Lenalidomide is known, but its immunomodulation activity, which seems to be that mainly responsible for its efficacy in the treatment of multiple myeloma is not quite clear. The interactions between the tumour cells as well as the inflammatory cells have also been studied. In certain cases, the latter can become pharmacological targets with the aim of negatively conditioning the growth of the neoplastic component (along the same line the therapeutic experiences with Rituximab in Hodgkin s lymphoma is the preventive use of anti-inflammatory drugs in tumours of the colon-rectum). In this regard, in fact, the cells of the immune system can, on the one hand, obstruct the development of tumours while on the other hand they can favour it. The two apparently opposite functions are actually mainly linked to the polarisation of immune cells towards a pro- or anti-tumoral phenotype, to the modulation of the tumoral microenvironment and to immuno-suppression. For example, in case of macrophages, the cellular polarization has been widely studied and is linked to the variation of the factors which facilitate growth of the tumour and its nourishment by means of angiogenesis. The study of the immune components and their action on the microenvironment will therefore be one of the topics of the investigation. The study of the immunological pathways of diseases that are exclusively inflammatory, which has for some time been the object of interest of the Laboratory of Immunology, Laboratory of Molecular Biology and Laboratory of Translational Research of the ASMN- IRCCS of Reggio Emilia, may help towards better understanding of the mechanisms of inflammation which support the growth of neoplastic clones. Another field of study which is included in this line of research is the study of the mechanisms of chemo- and angio-prevention, i.e. the pharmacological prevention of cancer and angiogenesis. The latter, in fact, favours the tumoral growth by providing nourishment to the neoplastic cells. The identification and evaluation of markers useful for the diagnosis, ongoing monitoring of follow up and the prognostic definition of neoplastic and inflammatory diseases, possibly
11 through investigations that can be easily replicated in clinical practice are always current issues. Finally, the analysis of the relation between the metabolism and development/progression of the disease and response to treatments has been of great interest in recent times and will therefore be the subject of study. Objectives: Projects and studies a) on the role of the components of the microenvironment: leucocytes, lymphocytes, endothelial cells, stromal fibroblasts, proteins and enzymes of the matrix in translation and clinical research studies; b) markers derived from the microenvironment; c) control of inflammation and angiogenesis, stimulation of the immune system, permeabilization of the stroma to treatment by new drugs and technologies. Clinical studies on new markers and new drugs in phase I/II and II are included in this program. Indicator: - Articles in magazines (position first name and surname; co-authors of publications with other Organisations). - Presentations at international congresses. - Product IF. - Ability to acquire funds for research. - Collaboration with other institutions. Indicator result: - Number of publications per Research Line = Medium normalised IF per Line per Year = 5. - Projects under way funded by external institutions = 1. - Number of presentations at congresses = Collaboration with other institutions = 5. Result described: The results shall be illustrated by publications and the research projects concerning: - the study of cellular mechanisms involved in response to active molecules against tumours by means of interactions with the microenvironment of the tumour (e.g. anti-angiogenic); - the identification and study of diagnostic and prognostic markers of inflammatory and tumoral diseases; - the study of stem cells in tumours and enzymes involved in the formation of the tumour.
Bordeaux Integrated Oncology Research (BRIO) Part of the French National Cancer Institute (INCa) Integrated Research Sites Network (SIRICs)
 Bordeaux Integrated Oncology Research (BRIO) Part of the French National Cancer Institute (INCa) Integrated Research Sites Network (SIRICs) 1 Labellised between 2011 & 2012 8 Integrated Cancer Research
Bordeaux Integrated Oncology Research (BRIO) Part of the French National Cancer Institute (INCa) Integrated Research Sites Network (SIRICs) 1 Labellised between 2011 & 2012 8 Integrated Cancer Research
2 Diagnosis and Staging of Cancer 2.1 Pathophysiology of cancer 2.2 Classification and staging 2.3 Diagnostic measures for specific cancer types
 Oncology Nursing Sub-Specialty Module Reference: Gobel B. M., Triest-Robertson S. & Vogel W.H. (Eds). (205). Advanced Oncology Nursing Certificate Review and Resources Manual. Pittsburgh: Oncology Nursing
Oncology Nursing Sub-Specialty Module Reference: Gobel B. M., Triest-Robertson S. & Vogel W.H. (Eds). (205). Advanced Oncology Nursing Certificate Review and Resources Manual. Pittsburgh: Oncology Nursing
Macmillan Publications
 S1 S2 S3 S3 S3 S4 S5 S6 S7 S8 S8 S9 S10 S11 S11 S12 S13 S14 S15 S17 S18 S19 Bladder Cancer: Non-Invasive, Invasive and Advanced Bone Cancer: Primary, Secondary Colon Cancer, Anal Cancer, Rectal Cancer
S1 S2 S3 S3 S3 S4 S5 S6 S7 S8 S8 S9 S10 S11 S11 S12 S13 S14 S15 S17 S18 S19 Bladder Cancer: Non-Invasive, Invasive and Advanced Bone Cancer: Primary, Secondary Colon Cancer, Anal Cancer, Rectal Cancer
PRESS RELEASE FOR IMMEDIATE RELEASE SINGAPORE INSTITUTES COLLABORATE WITH SAMSUNG MEDICAL CENTER TO IMPROVE TREATMENT OF LIVER CANCER
 PRESS RELEASE FOR IMMEDIATE RELEASE SINGAPORE INSTITUTES COLLABORATE WITH SAMSUNG MEDICAL CENTER TO IMPROVE TREATMENT OF LIVER CANCER This collaboration creates the world s first clinically reliable and
PRESS RELEASE FOR IMMEDIATE RELEASE SINGAPORE INSTITUTES COLLABORATE WITH SAMSUNG MEDICAL CENTER TO IMPROVE TREATMENT OF LIVER CANCER This collaboration creates the world s first clinically reliable and
Genomics Up Close And Personal: What Are The Implications For Cancer Nursing? Candy Cooley Head of Education
 Genomics Up Close And Personal: What Are The Implications For Cancer Nursing? Candy Cooley Head of Education Aims of this session Understand how genomic information will change your clinical practice Remind
Genomics Up Close And Personal: What Are The Implications For Cancer Nursing? Candy Cooley Head of Education Aims of this session Understand how genomic information will change your clinical practice Remind
Summary of Research and Writing Activities in Oncology
 Summary of Research and Writing Activities in Oncology Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Manuscripts, Posters,
Summary of Research and Writing Activities in Oncology Carole Alison Chrvala, PhD 919.545.2149 (Work) 919.951.5230 (Mobile) cchrvala@centurylink.net www.healthmattersmedwriting.com 1 Manuscripts, Posters,
SUBSPECIALIST TRAINING PROGRAMME
 EUROPEAN BOARD AND COLLEGE OF OBSTETRICIANS AND GYNAECOLOGISTS (EBCOG) AND EUROPEAN SOCIETY OF GYNAECOLOGICAL ONCOLOGY (ESGO) SUBSPECIALIST TRAINING PROGRAMME IN GYNAECOLOGICAL ONCOLOGY This Programme
EUROPEAN BOARD AND COLLEGE OF OBSTETRICIANS AND GYNAECOLOGISTS (EBCOG) AND EUROPEAN SOCIETY OF GYNAECOLOGICAL ONCOLOGY (ESGO) SUBSPECIALIST TRAINING PROGRAMME IN GYNAECOLOGICAL ONCOLOGY This Programme
CoQ10 and Cancer Treatment
 http://www.lef.org/ Life Extension Magazine September 2009 CoQ10 and Cancer Treatment By William Faloon For more than a decade, Life Extension has reported on small clinical studies that demonstrate beneficial
http://www.lef.org/ Life Extension Magazine September 2009 CoQ10 and Cancer Treatment By William Faloon For more than a decade, Life Extension has reported on small clinical studies that demonstrate beneficial
BY Mrs. K.SHAILAJA., M. PHARM., LECTURER DEPT OF PHARMACY PRACTICE, SRM COLLEGE OF PHARMACY
 BY Mrs. K.SHAILAJA., M. PHARM., LECTURER DEPT OF PHARMACY PRACTICE, SRM COLLEGE OF PHARMACY Cancer is a group of more than 100 different diseases that are characterized by uncontrolled cellular growth,
BY Mrs. K.SHAILAJA., M. PHARM., LECTURER DEPT OF PHARMACY PRACTICE, SRM COLLEGE OF PHARMACY Cancer is a group of more than 100 different diseases that are characterized by uncontrolled cellular growth,
Identifying and counting people living with treatable but not curable cancer
 Identifying and counting people living with treatable but not curable cancer Rachel White Joanna Pethick, Archie Macnair, Gregory Fallica, Jennifer Than and Jane Maher September 2018 Who are the people
Identifying and counting people living with treatable but not curable cancer Rachel White Joanna Pethick, Archie Macnair, Gregory Fallica, Jennifer Than and Jane Maher September 2018 Who are the people
BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES. Overview and Mechanism of Action Dr.
 BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES Overview and Mechanism of Action Dr. Leah Klapper, CSO 88 BL-8040: Novel CXCR4 Antagonist For Hematological Cancers Indications:
BL-8040: BEST-IN-CLASS CXCR4 ANTAGONIST FOR TREATMENT OF ONCOLOGICAL MALIGNANCIES Overview and Mechanism of Action Dr. Leah Klapper, CSO 88 BL-8040: Novel CXCR4 Antagonist For Hematological Cancers Indications:
Blood Cancers. Blood Cells. Blood Cancers: Progress and Promise. Bone Marrow and Blood. Lymph Nodes and Spleen
 Blood Cancers: Progress and Promise Mike Barnett & Khaled Ramadan Division of Hematology Department of Medicine Providence Health Care & UBC Blood Cancers Significant health problem Arise from normal cells
Blood Cancers: Progress and Promise Mike Barnett & Khaled Ramadan Division of Hematology Department of Medicine Providence Health Care & UBC Blood Cancers Significant health problem Arise from normal cells
Haemato-oncology Clinical Forum. 20 th June 2013
 Haemato-oncology Clinical Forum 20 th June 2013 Welcome Dr Majid Kazmi, LCA Haemato-oncology Pathway Group Chair Purpose of today Provide an update on progress of the LCA to date Identify priorities for
Haemato-oncology Clinical Forum 20 th June 2013 Welcome Dr Majid Kazmi, LCA Haemato-oncology Pathway Group Chair Purpose of today Provide an update on progress of the LCA to date Identify priorities for
Progress Update June 2017 Lay Summary Funding: $6,000,000 Grant Funded: July 2015 Dream Team Members Dream Team Leader:
 SU2C -Ovarian Cancer Research Fund Alliance-National Ovarian Cancer Coalition Dream Team Translational Research Grant: DNA Repair Therapies for Ovarian Cancer AND SU2C Catalyst Merck-Supported Supplemental
SU2C -Ovarian Cancer Research Fund Alliance-National Ovarian Cancer Coalition Dream Team Translational Research Grant: DNA Repair Therapies for Ovarian Cancer AND SU2C Catalyst Merck-Supported Supplemental
R e s e a r c h S t r a t e g y
 Sheffield Teaching Hospitals NHS Foundation Trust Academic Directorate of Specialised Cancer R e s e a r c h S t r a t e g y Academic Directorate of Specialised Cancer Research Strategy Executive Summary
Sheffield Teaching Hospitals NHS Foundation Trust Academic Directorate of Specialised Cancer R e s e a r c h S t r a t e g y Academic Directorate of Specialised Cancer Research Strategy Executive Summary
Irish Experts Launch Global Report and Call for Increased Focus on Metastatic Breast Cancer
 Irish Experts Launch Global Report and Call for Increased Focus on Metastatic Breast Cancer Early detection does not help survival for metastatic breast cancer patients - average survival for women with
Irish Experts Launch Global Report and Call for Increased Focus on Metastatic Breast Cancer Early detection does not help survival for metastatic breast cancer patients - average survival for women with
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES CENTRAL NERVOUS SYSTEM BRAIN METASTASES CNS Site Group Brain Metastases Author: Dr. Norm Laperriere Date: February 20, 2018 1. INTRODUCTION
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES CENTRAL NERVOUS SYSTEM BRAIN METASTASES CNS Site Group Brain Metastases Author: Dr. Norm Laperriere Date: February 20, 2018 1. INTRODUCTION
Patient Leader Education Summit. Precision Medicine: Today and Tomorrow March 31, 2017
 Patient Leader Education Summit Precision Medicine: Today and Tomorrow March 31, 2017 Precision Medicine: Presentation Outline Agenda What is a Precision Medicine What is its clinical value Overview of
Patient Leader Education Summit Precision Medicine: Today and Tomorrow March 31, 2017 Precision Medicine: Presentation Outline Agenda What is a Precision Medicine What is its clinical value Overview of
 REPORT TO CONGRESS Multi-Disciplinary Brain Research and Data Sharing Efforts September 2013 The estimated cost of report or study for the Department of Defense is approximately $2,540 for the 2013 Fiscal
REPORT TO CONGRESS Multi-Disciplinary Brain Research and Data Sharing Efforts September 2013 The estimated cost of report or study for the Department of Defense is approximately $2,540 for the 2013 Fiscal
Developing effective ctdna testing services for lung cancer. Executive summary
 Developing effective ctdna testing services for lung cancer Executive summary September 2017 Authors: Laura Blackburn, Leila Luheshi, Sandi Deans, Mark Kroese, Hilary Burton Acknowledgements: The PHG Foundation
Developing effective ctdna testing services for lung cancer Executive summary September 2017 Authors: Laura Blackburn, Leila Luheshi, Sandi Deans, Mark Kroese, Hilary Burton Acknowledgements: The PHG Foundation
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: P
 Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170054P 1 NHS England INFORMATION READER BOX Directorate Medical
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170054P 1 NHS England INFORMATION READER BOX Directorate Medical
Curriculum: Goals and Objectives Department of Medicine Harbor-UCLA Medical Center
 MEDICAL ONCOLOGY AND HEMATOLOGY (R2, R3) A. The PURPOSE of this rotation is to afford medical residents a broad clinical and training experience in the clinical diagnosis and management of common adult
MEDICAL ONCOLOGY AND HEMATOLOGY (R2, R3) A. The PURPOSE of this rotation is to afford medical residents a broad clinical and training experience in the clinical diagnosis and management of common adult
SIBLINGs, cancer's multifunctional weapons
 SIBLINGs, cancer's multifunctional weapons 6/18/08 Akeila Bellahcène and Vincent Castronovo of the Metastasis Research laboratory of the University of Liège are among the first researchers to have discovered
SIBLINGs, cancer's multifunctional weapons 6/18/08 Akeila Bellahcène and Vincent Castronovo of the Metastasis Research laboratory of the University of Liège are among the first researchers to have discovered
More cancer patients are being treated with immunotherapy, but
 Bristol-Myers Squibb and Five Prime Present Phase 1a/1b Data Evaluating Cabiralizumab (anti-csf-1 receptor antibody) with Opdivo (nivolumab) in Patients with Advanced Solid Tumors PRINCETON, N.J. & SOUTH
Bristol-Myers Squibb and Five Prime Present Phase 1a/1b Data Evaluating Cabiralizumab (anti-csf-1 receptor antibody) with Opdivo (nivolumab) in Patients with Advanced Solid Tumors PRINCETON, N.J. & SOUTH
Allied Healthcare Professionals Module
 Allied Healthcare Professionals Module Allied health professionals (AHPs) are key members of today s multidisciplinary healthcare team. They work in partnership with health and social care colleagues across
Allied Healthcare Professionals Module Allied health professionals (AHPs) are key members of today s multidisciplinary healthcare team. They work in partnership with health and social care colleagues across
Fighting Cancer with Data: Perspectives on MD Anderson Moon Shot Program
 Fighting Cancer with Data: Perspectives on MD Anderson Moon Shot Program Joxel Garcia, MD, MBA Executive Director, Cancer Prevention and Control Platform Member, Moon Shots Program Leadership Team The
Fighting Cancer with Data: Perspectives on MD Anderson Moon Shot Program Joxel Garcia, MD, MBA Executive Director, Cancer Prevention and Control Platform Member, Moon Shots Program Leadership Team The
Fit4RareFit4All Conference Cluj - Napoca 3-4 September 2017
 Fit4RareFit4All Conference Cluj - Napoca 3-4 September 2017 Rare diseases registries as key tools in research and public health Domenica Taruscio Director, National Centre for Rare Diseases Istituto Superiore
Fit4RareFit4All Conference Cluj - Napoca 3-4 September 2017 Rare diseases registries as key tools in research and public health Domenica Taruscio Director, National Centre for Rare Diseases Istituto Superiore
Annual Report. Cape Cod Hospital and Falmouth Hospital Regional Cancer Network Expert physicians. Quality hospitals. Superior care.
 Annual Report Cape Cod Hospital and Falmouth Hospital Regional Cancer Network 2013 Expert physicians. Quality hospitals. Superior care. Cape Cod Hospital s Davenport- Mugar Hematology/Oncology Center and
Annual Report Cape Cod Hospital and Falmouth Hospital Regional Cancer Network 2013 Expert physicians. Quality hospitals. Superior care. Cape Cod Hospital s Davenport- Mugar Hematology/Oncology Center and
NEWS FROM. Roswell Park s LYMPHOMA AND MYELOMA SERVICE
 NEWS FROM Roswell Park s LYMPHOMA AND MYELOMA SERVICE MEET THE LYMPHOMA & MYELOMA TEAM The Lymphoma and Myeloma Service at Roswell Park Cancer Institute (RPCI) is dedicated to providing outstanding clinical
NEWS FROM Roswell Park s LYMPHOMA AND MYELOMA SERVICE MEET THE LYMPHOMA & MYELOMA TEAM The Lymphoma and Myeloma Service at Roswell Park Cancer Institute (RPCI) is dedicated to providing outstanding clinical
Urgent Clinical Commissioning Policy Statement: Pembrolizumab for drug-resistant gestational trophoblastic neoplasia. NHS England Reference: P
 Urgent Clinical Commissioning Policy Statement: Pembrolizumab for drug-resistant gestational trophoblastic neoplasia NHS England Reference: 170027P 1 Equality statement Promoting equality and addressing
Urgent Clinical Commissioning Policy Statement: Pembrolizumab for drug-resistant gestational trophoblastic neoplasia NHS England Reference: 170027P 1 Equality statement Promoting equality and addressing
National Cancer Registration and Analysis Service Short Report: Chemotherapy, Radiotherapy and Surgical Tumour Resections in England: (V2)
 National Cancer Registration and Analysis Service Short Report: Chemotherapy, Radiotherapy and Surgical Tumour Resections in England: 13-14 (V2) Produced as part of the Cancer Research UK - Public Health
National Cancer Registration and Analysis Service Short Report: Chemotherapy, Radiotherapy and Surgical Tumour Resections in England: 13-14 (V2) Produced as part of the Cancer Research UK - Public Health
Trends and disparities in cancer in Aotearoa/ NZ
 Trends and disparities in cancer in Aotearoa/ NZ Professor Diana Sarfati #cancercrossroads @DiSarfati Why cancer? Estimated number of incident cases from 2018 to 2040 in New Zealand, all cancers, both
Trends and disparities in cancer in Aotearoa/ NZ Professor Diana Sarfati #cancercrossroads @DiSarfati Why cancer? Estimated number of incident cases from 2018 to 2040 in New Zealand, all cancers, both
Nivolumab and AVD in Early-stage Unfavorable Classical Hodgkin Lymphoma (NIVAHL)
 This site became the new ClinicalTrials.gov on June 19th. Learn more. We will be updating this site in phases. This allows us to move faster and to deliver better services. Show less IMPORTANT: Listing
This site became the new ClinicalTrials.gov on June 19th. Learn more. We will be updating this site in phases. This allows us to move faster and to deliver better services. Show less IMPORTANT: Listing
Roche s subcutaneous formulation of MabThera approved in Switzerland for the treatment of common types of non-hodgkin lymphoma
 Media Release Basel, 12 January 2017 Roche s subcutaneous formulation of MabThera approved in Switzerland for the treatment of common types of non-hodgkin lymphoma Administration time significantly reduced
Media Release Basel, 12 January 2017 Roche s subcutaneous formulation of MabThera approved in Switzerland for the treatment of common types of non-hodgkin lymphoma Administration time significantly reduced
VeriStrat Poor Patients Show Encouraging Overall Survival and Progression Free Survival Signal; Confirmatory Phase 2 Study Planned by Year-End
 AVEO and Biodesix Announce Exploratory Analysis of VeriStrat-Selected Patients with Non-Small Cell Lung Cancer in Phase 2 Study of Ficlatuzumab Presented at ESMO 2014 Congress VeriStrat Poor Patients Show
AVEO and Biodesix Announce Exploratory Analysis of VeriStrat-Selected Patients with Non-Small Cell Lung Cancer in Phase 2 Study of Ficlatuzumab Presented at ESMO 2014 Congress VeriStrat Poor Patients Show
Letter to Editor Tissue micro arrays for immunohistochemical detection of inflammatory infiltrates in renal cell carcinoma
 Int J Clin Exp Med 2014;7(4):1175-1179 www.ijcem.com /ISSN:1940-5901/IJCEM0000102 Letter to Editor Tissue micro arrays for immunohistochemical detection of inflammatory infiltrates in renal cell carcinoma
Int J Clin Exp Med 2014;7(4):1175-1179 www.ijcem.com /ISSN:1940-5901/IJCEM0000102 Letter to Editor Tissue micro arrays for immunohistochemical detection of inflammatory infiltrates in renal cell carcinoma
ABBVIE VENTURES VISION. Drive strategic returns to AbbVie as a key constituent of overall corporate strategy
 ABBVIE VENTURES ABBVIE VENTURES VISION Drive strategic returns to AbbVie as a key constituent of overall corporate strategy Early access to external innovation with a long-term horizon to generate meaningful
ABBVIE VENTURES ABBVIE VENTURES VISION Drive strategic returns to AbbVie as a key constituent of overall corporate strategy Early access to external innovation with a long-term horizon to generate meaningful
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages)
 Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170029P 1 NHS England INFORMATION READER BOX Directorate Medical
Clinical Commissioning Policy: Bendamustine with rituximab for relapsed and refractory mantle cell lymphoma (all ages) NHS England Reference: 170029P 1 NHS England INFORMATION READER BOX Directorate Medical
PRELIMINARY PROGRAM MAY 31 - JUNE 4, 2019
 MONDAY, JUNE 3, 2019 7:30 AM - 9:15 AM HIGHLIGHTS OF THE DAY 8:00 AM - 9:00 AM S 8:00 AM - 9:15 AM S S 8:00 AM - 11:00 AM S Highlights of the Day Session II Bringing New and Innovative Glioblastoma Treatments
MONDAY, JUNE 3, 2019 7:30 AM - 9:15 AM HIGHLIGHTS OF THE DAY 8:00 AM - 9:00 AM S 8:00 AM - 9:15 AM S S 8:00 AM - 11:00 AM S Highlights of the Day Session II Bringing New and Innovative Glioblastoma Treatments
HEALTHCARE CLAIMS TRACKER A FOCUS ON ONCOLOGY CLAIMS. September 2018 For the period January 2017 December 2017
 HEALTHCARE A FOCUS ON CLAIMS September 208 For the period January 207 December 207 INTRODUCTION Cancer is one of the leading causes of death both globally and in South Africa. Worldwide, one in seven deaths
HEALTHCARE A FOCUS ON CLAIMS September 208 For the period January 207 December 207 INTRODUCTION Cancer is one of the leading causes of death both globally and in South Africa. Worldwide, one in seven deaths
Technology appraisal guidance Published: 8 November 2017 nice.org.uk/guidance/ta487
 Venetoclax for treating chronic lymphocytic leukaemia Technology appraisal guidance Published: 8 November 2017 nice.org.uk/guidance/ta487 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Venetoclax for treating chronic lymphocytic leukaemia Technology appraisal guidance Published: 8 November 2017 nice.org.uk/guidance/ta487 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
ESMO 2020 VISION. esmo.org
 ESMO 2020 VISION esmo.org CARING FOR PEOPLE WITH CANCER Cancer patients and their needs are at the centre of all that we do: our profession is driven by our determination, individually and collectively,
ESMO 2020 VISION esmo.org CARING FOR PEOPLE WITH CANCER Cancer patients and their needs are at the centre of all that we do: our profession is driven by our determination, individually and collectively,
Cancer of Unknown Primary (CUP) Protocol
 1 Department of Oncology. Cancer of Unknown Primary (CUP) Protocol Version: Document type: Document sponsor Designation Document author [ s] Designation[s] Approving committee / Group Ratified by: Date
1 Department of Oncology. Cancer of Unknown Primary (CUP) Protocol Version: Document type: Document sponsor Designation Document author [ s] Designation[s] Approving committee / Group Ratified by: Date
Novel Diagnostics and Biomarker Opportunities
 1 Novel Diagnostics and Biomarker Opportunities LYMPHOMA EYE HEART BREAST COLON BLADDER CERVIX PROSTATE From research to business Inven2 transforms science and technology into useful and profitable products
1 Novel Diagnostics and Biomarker Opportunities LYMPHOMA EYE HEART BREAST COLON BLADDER CERVIX PROSTATE From research to business Inven2 transforms science and technology into useful and profitable products
CTA Strengths. Organisational Structure Board. CTA Board CEO. Background to Cancer Trials Australia What we do well An increasing struggle Conclusions
 Models for Clinical Research: Cancer Trials Australia Professor Mark Rosenthal Chairman; Cancer Trials Australia and Director of Medical Oncology, Royal Melbourne Hospital For discussion: Background to
Models for Clinical Research: Cancer Trials Australia Professor Mark Rosenthal Chairman; Cancer Trials Australia and Director of Medical Oncology, Royal Melbourne Hospital For discussion: Background to
Non communicable diseases
 Non communicable diseases Information Day, Brussels 8 July 2016 K. Berkouk, PhD Deputy Head of Unit, Non-Communicable Diseases and the challenge of Healthy Ageing, European Commission NCD A Global challenge
Non communicable diseases Information Day, Brussels 8 July 2016 K. Berkouk, PhD Deputy Head of Unit, Non-Communicable Diseases and the challenge of Healthy Ageing, European Commission NCD A Global challenge
Checkpoint Regulators Cancer Immunotherapy takes centre stage. Dr Oliver Klein Department of Medical Oncology 02 May 2015
 Checkpoint Regulators Cancer Immunotherapy takes centre stage Dr Oliver Klein Department of Medical Oncology 02 May 2015 Adjuvant chemotherapy improves outcome in early breast cancer FDA approval of Imatinib
Checkpoint Regulators Cancer Immunotherapy takes centre stage Dr Oliver Klein Department of Medical Oncology 02 May 2015 Adjuvant chemotherapy improves outcome in early breast cancer FDA approval of Imatinib
Research For the Future. Len Lichtenfeld, MD, MACP Interim Chief Medical and Scientific Officer
 Research For the Future Len Lichtenfeld, MD, MACP Interim Chief Medical and Scientific Officer 1 2 ATTACKING CANCER FROM EVERY ANGLE 3 CANCER PREVENTION STUDIES (CPS) Intramural Research Smoking Obesity
Research For the Future Len Lichtenfeld, MD, MACP Interim Chief Medical and Scientific Officer 1 2 ATTACKING CANCER FROM EVERY ANGLE 3 CANCER PREVENTION STUDIES (CPS) Intramural Research Smoking Obesity
Corporate Presentation May Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers
 Corporate Presentation May 2016 Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers Forward-looking statements / safe harbor This presentation and the accompanying oral commentary contain
Corporate Presentation May 2016 Transforming Immuno-Oncology Using Next-Generation Immune Cell Engagers Forward-looking statements / safe harbor This presentation and the accompanying oral commentary contain
Clinical Commissioning Policy: Chemotherapy Algorithms for Adults and Children. January 2013 Reference: NHS England XXX/X/X.
 Clinical Commissioning Policy: Chemotherapy Algorithms for Adults and Children January 2013 Reference: NHS England XXX/X/X England 1 NHS England Clinical Commissioning Policy: Chemotherapy Algorithms for
Clinical Commissioning Policy: Chemotherapy Algorithms for Adults and Children January 2013 Reference: NHS England XXX/X/X England 1 NHS England Clinical Commissioning Policy: Chemotherapy Algorithms for
SCIENTIFIC TIMETABLE SIOP 2015
 SCIENTIFIC TIMETABLE SIOP 2015 September, 2015 WEDNESDAY, 7 TH OCTOBER 2015 / DAY 2 1.43 09:30 SIOP Committee 12:00 SIOP Essential Drugs Working 13:00 SIOP Work (Open) 14:00 Nurse Researchers 16:00 17:00
SCIENTIFIC TIMETABLE SIOP 2015 September, 2015 WEDNESDAY, 7 TH OCTOBER 2015 / DAY 2 1.43 09:30 SIOP Committee 12:00 SIOP Essential Drugs Working 13:00 SIOP Work (Open) 14:00 Nurse Researchers 16:00 17:00
Cancer survival by stage at diagnosis in Wales,
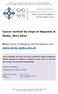 Cancer survival by stage at diagnosis in Wales, 2011-2014 Welsh Cancer Intelligence and Surveillance Unit www.wcisu.wales.nhs.uk Latest available one-year net cancer survival by stage at diagnosis official
Cancer survival by stage at diagnosis in Wales, 2011-2014 Welsh Cancer Intelligence and Surveillance Unit www.wcisu.wales.nhs.uk Latest available one-year net cancer survival by stage at diagnosis official
The Heart of the Matter: Issues in Cardio-Oncology Research
 The Heart of the Matter: Issues in Cardio-Oncology Research Edith Pituskin RN MN Nurse Practitioner, Radiation Oncology, Cross Cancer Institute PhD (c), Faculty of Rehabilitation Medicine, University of
The Heart of the Matter: Issues in Cardio-Oncology Research Edith Pituskin RN MN Nurse Practitioner, Radiation Oncology, Cross Cancer Institute PhD (c), Faculty of Rehabilitation Medicine, University of
CANCER 1.7 M 609,000 26% 15.5 M 73% JUST THE FACTS. More Than 1,100 Cancer Treatments in Clinical Testing Offer Hope to Patients
 CANCER MEDICINES IN DEVELOPMENT 2018 REPORT JUST THE FACTS MORE THAN 1.7 M ESTIMATED NEW CASES OF CANCER IN 2018 IN THE UNITED STATES MORE THAN 609,000 U.S. CANCER DEATHS ARE EXPECTED IN 2018 SINCE PEAKING
CANCER MEDICINES IN DEVELOPMENT 2018 REPORT JUST THE FACTS MORE THAN 1.7 M ESTIMATED NEW CASES OF CANCER IN 2018 IN THE UNITED STATES MORE THAN 609,000 U.S. CANCER DEATHS ARE EXPECTED IN 2018 SINCE PEAKING
Changing the prevention paradigm for the future what Europe can do
 November 3rd, 2014 Honorable Beatrice Lorenzin, Minister of Health of Italy Italian Presidency of the EU Council Conference/Meeting The State of Health of Vaccination in the EU: where do we stand, where
November 3rd, 2014 Honorable Beatrice Lorenzin, Minister of Health of Italy Italian Presidency of the EU Council Conference/Meeting The State of Health of Vaccination in the EU: where do we stand, where
OHSU Knight Cancer Institute Overview and Update
 OHSU Knight Cancer Institute Overview and Update for Senate Health Care and Human Services Committee Steve Stadum, COO, OHSU Knight Cancer Institute September 15, 2014 Presentation Brief Overview of OHSU
OHSU Knight Cancer Institute Overview and Update for Senate Health Care and Human Services Committee Steve Stadum, COO, OHSU Knight Cancer Institute September 15, 2014 Presentation Brief Overview of OHSU
VISION» FOCUS DISCOVERY» CREATION
 The Women s Health Research Institute at Northwestern University is turning possibility into reality. The possibility that we can improve women s health by requiring that all research studies put greater
The Women s Health Research Institute at Northwestern University is turning possibility into reality. The possibility that we can improve women s health by requiring that all research studies put greater
A Summary of the studies conducted with CV247
 A Summary of the studies conducted with CV247 Introduction Several epidemiological studies have shown that people deficient in trace elements such as selenium, copper, manganese, and vitamins, mainly C,D
A Summary of the studies conducted with CV247 Introduction Several epidemiological studies have shown that people deficient in trace elements such as selenium, copper, manganese, and vitamins, mainly C,D
Radical treatment for leukemia under way 17 January 2011, By Fumihiko Ishikawa
 Radical treatment for leukemia under way 17 January 2011, By Fumihiko Ishikawa Figure 1: Major blood cells that differentiate from hematopoietic stem cells. Hematopoietic stem cells differentiate into
Radical treatment for leukemia under way 17 January 2011, By Fumihiko Ishikawa Figure 1: Major blood cells that differentiate from hematopoietic stem cells. Hematopoietic stem cells differentiate into
PRESAGE CIVO: A NOVEL PLATFORM FOR PRECISION ONCOLOGY & DRUG DEVELOPMENT
 PRESAGE : A NOVEL PLATFORM FOR PRECISION ONCOLOGY & DRUG DEVELOPMENT DECEMBER 2017 Presage Biosciences, Inc. 2017. All rights reserved. Presage Proprietary Biosciences, information. Inc. 2017. All rights
PRESAGE : A NOVEL PLATFORM FOR PRECISION ONCOLOGY & DRUG DEVELOPMENT DECEMBER 2017 Presage Biosciences, Inc. 2017. All rights reserved. Presage Proprietary Biosciences, information. Inc. 2017. All rights
Translating Duke Health. Accelerating discovery and its translation
 Translating Duke Health Accelerating discovery and its translation Translating Duke Health Biomedical discovery is accelerating innovative and revolutionary advancements in our understanding of human biology,
Translating Duke Health Accelerating discovery and its translation Translating Duke Health Biomedical discovery is accelerating innovative and revolutionary advancements in our understanding of human biology,
National Policy on Traditional / Complementary Medicine, Malaysia Ministry of Health Malaysia August 2002
 National Policy on Traditional / Complementary Medicine, Malaysia Ministry of Health Malaysia August 2002 Contents Page 1 Introduction 2 2. Policy Statement 3 3. Definitions 3 4. Vision for T/CM 5 5. Mission
National Policy on Traditional / Complementary Medicine, Malaysia Ministry of Health Malaysia August 2002 Contents Page 1 Introduction 2 2. Policy Statement 3 3. Definitions 3 4. Vision for T/CM 5 5. Mission
Europass Curriculum Vitae
 Europass Curriculum Vitae Personal information First name(s) / Surname(s) Address Via Gattamelata 64 35128 Padova Telephone(s) +39 049.8215953/5910 E-mail(s) vittorina.zagonel@iov.veneto.it Nationality
Europass Curriculum Vitae Personal information First name(s) / Surname(s) Address Via Gattamelata 64 35128 Padova Telephone(s) +39 049.8215953/5910 E-mail(s) vittorina.zagonel@iov.veneto.it Nationality
Program Priorities 2018
 Program Priorities 2018 Blank Page 2 CONTENTS: About CPRIT Program Priorities Project...Page 5 Process to Develop Program Priorities...Page 6 Scope of Program Priorities Project...Page 6 CPRIT s Long-Term
Program Priorities 2018 Blank Page 2 CONTENTS: About CPRIT Program Priorities Project...Page 5 Process to Develop Program Priorities...Page 6 Scope of Program Priorities Project...Page 6 CPRIT s Long-Term
Using Histoindex Genesis 200 imaging technology to analyse the Pattern of ECM Deposition in Human Breast Cancer Tissue
 Using Histoindex Genesis 200 imaging technology to analyse the Pattern of ECM Deposition in Human Breast Cancer Tissue Introduction to technology High Resolution Detects fine collagen fibers up to 0.1µm
Using Histoindex Genesis 200 imaging technology to analyse the Pattern of ECM Deposition in Human Breast Cancer Tissue Introduction to technology High Resolution Detects fine collagen fibers up to 0.1µm
Translational Clinical Research, the Key to Personalised Healthcare in Practice
 New Models of Collaborations with Academia to foster Translational Clinical Research, the Key to ersonalised Healthcare in ractice Exploratory Clinical Development World Europe Conference 2012 London,
New Models of Collaborations with Academia to foster Translational Clinical Research, the Key to ersonalised Healthcare in ractice Exploratory Clinical Development World Europe Conference 2012 London,
IMMUNOTHERAPY FOR LUNG CANCER
 IMMUNOTHERAPY FOR LUNG CANCER Patient and Caregiver Guide IMMUNOTHERAPY FOR LUNG CANCER Patient and Caregiver Guide TABLE OF CONTENTS Lung Cancer Basics... 2 Immunotherapy... 3 FDA Approved Immunotherapies...
IMMUNOTHERAPY FOR LUNG CANCER Patient and Caregiver Guide IMMUNOTHERAPY FOR LUNG CANCER Patient and Caregiver Guide TABLE OF CONTENTS Lung Cancer Basics... 2 Immunotherapy... 3 FDA Approved Immunotherapies...
Education and Training Strategy
 ECN Pharmacy Group Education and Training Strategy Name of person presenting document: Reason for document development: Names of development team: Specify groups of staff to whom the document relates:
ECN Pharmacy Group Education and Training Strategy Name of person presenting document: Reason for document development: Names of development team: Specify groups of staff to whom the document relates:
One Palliative Care Annual Report
 One 203 Palliative Care Annual Report One In 202, ASCO released a provisional clinical opinion stating that concurrent palliative care should be considered early in the course of advanced or metastatic
One 203 Palliative Care Annual Report One In 202, ASCO released a provisional clinical opinion stating that concurrent palliative care should be considered early in the course of advanced or metastatic
Center for Cell Therapy and Regenerative Medicine (CCRG)
 Center for Cell Therapy and Regenerative Medicine (CCRG) What is CCRG? CCRG is a multidisciplinary platform for new types of cellular therapy and tissue therapy. It is located on the campus of the Antwerp
Center for Cell Therapy and Regenerative Medicine (CCRG) What is CCRG? CCRG is a multidisciplinary platform for new types of cellular therapy and tissue therapy. It is located on the campus of the Antwerp
The role of appropriateness criteria in the planning of health services: the case of FDG-PET in oncology
 V Annual Meeting of Health Tecnology Assessment International (HTAi) MONTREAL 8 july 2008 The role of appropriateness criteria in the planning of health services: the case of FDG-PET in oncology L. BALLINI
V Annual Meeting of Health Tecnology Assessment International (HTAi) MONTREAL 8 july 2008 The role of appropriateness criteria in the planning of health services: the case of FDG-PET in oncology L. BALLINI
PRIMARY THROMBOPROPHYLAXIS IN AMBULATORY CANCER PATIENTS: CURRENT GUIDELINES
 PRIMARY THROMBOPROPHYLAXIS IN AMBULATORY CANCER PATIENTS: CURRENT GUIDELINES Mario Mandalà, MD Unit of Clinical Research Department of Oncology and Haematology Papa Giovanni XXIII Hospital Cancer Center
PRIMARY THROMBOPROPHYLAXIS IN AMBULATORY CANCER PATIENTS: CURRENT GUIDELINES Mario Mandalà, MD Unit of Clinical Research Department of Oncology and Haematology Papa Giovanni XXIII Hospital Cancer Center
POSITION PAPER PALLIATIVE CARE, PEDIATRIC PALLIATIVE CARE AND PAIN THERAPY: HUMANIZATION OF CARE
 POSITION PAPER 1 PALLIATIVE CARE, PEDIATRIC PALLIATIVE CARE AND PAIN THERAPY: HUMANIZATION OF CARE GROUP LEADER: Basilicata Region OTHER PARTNERS: Calabria; Campania; Lazio; Marche; Piemonte; Prov. Aut.
POSITION PAPER 1 PALLIATIVE CARE, PEDIATRIC PALLIATIVE CARE AND PAIN THERAPY: HUMANIZATION OF CARE GROUP LEADER: Basilicata Region OTHER PARTNERS: Calabria; Campania; Lazio; Marche; Piemonte; Prov. Aut.
(generic name: ipilimumab) Injection 50 mg ( Yervoy ), a human anti-human CTLA-4 monoclonal. August 21, 2018
 August 21, 2018 Opdivo Approved for Supplemental Applications for Expanded Indications of Malignant Pleural Mesothelioma and Adjuvant Treatment of Melanoma, Change in Dosage and Administration (D&A) of
August 21, 2018 Opdivo Approved for Supplemental Applications for Expanded Indications of Malignant Pleural Mesothelioma and Adjuvant Treatment of Melanoma, Change in Dosage and Administration (D&A) of
Consensus statement between CM-Path, CRUK and the PHG Foundation following on from the Liquid Biopsy workshop on the 8th March 2018
 Consensus statement between CM-Path, CRUK and the PHG Foundation following on from the Liquid Biopsy workshop on the 8th March 2018 Summary: This document follows on from the findings of the CM-Path The
Consensus statement between CM-Path, CRUK and the PHG Foundation following on from the Liquid Biopsy workshop on the 8th March 2018 Summary: This document follows on from the findings of the CM-Path The
NATIONAL CANCER CONTROL PROGRAMME NATIONAL STRATEGY FOR THE SAFE ADMINISTATION OF CANCER DRUGS
 NATIONAL CANCER CONTROL PROGRAMME NATIONAL STRATEGY FOR THE SAFE ADMINISTATION OF CANCER DRUGS Dr Susan O Reilly MB, FRCPC, FRCPI National Director National Cancer Control Programme IMSN Networking for
NATIONAL CANCER CONTROL PROGRAMME NATIONAL STRATEGY FOR THE SAFE ADMINISTATION OF CANCER DRUGS Dr Susan O Reilly MB, FRCPC, FRCPI National Director National Cancer Control Programme IMSN Networking for
INTRATHECAL APPLICATION OF MONOCLONAL ANTIBODIES. Samo Rožman Institute of Oncology Ljubljana Slovenia
 INTRATHECAL APPLICATION OF MONOCLONAL ANTIBODIES Samo Rožman Institute of Oncology Ljubljana Slovenia AGENDA 1. INTRATHECAL APPLICATION 2. MONOCLONAL ANTIBODIES 3. MALIGNANT CARCINOMATOSIS 4. INTRATHECAL
INTRATHECAL APPLICATION OF MONOCLONAL ANTIBODIES Samo Rožman Institute of Oncology Ljubljana Slovenia AGENDA 1. INTRATHECAL APPLICATION 2. MONOCLONAL ANTIBODIES 3. MALIGNANT CARCINOMATOSIS 4. INTRATHECAL
SKCC Protocol Review Committee New Study Application
 Instructions: Submit the following documents to prc@jefferson.edu - Completed New Study Application (aka MCSF) - Protocol - Protocol Facilitation Committee Approval (if applicable) - MDG Priority Score
Instructions: Submit the following documents to prc@jefferson.edu - Completed New Study Application (aka MCSF) - Protocol - Protocol Facilitation Committee Approval (if applicable) - MDG Priority Score
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES CENTRAL NERVOUS SYSTEM LOW GRADE GLIOMAS CNS Site Group Low Grade Gliomas Author: Dr. Norm Laperriere 1. INTRODUCTION 3 2. PREVENTION 3 3. SCREENING
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES CENTRAL NERVOUS SYSTEM LOW GRADE GLIOMAS CNS Site Group Low Grade Gliomas Author: Dr. Norm Laperriere 1. INTRODUCTION 3 2. PREVENTION 3 3. SCREENING
CENTRO DI RIFERIMENTO ONCOLOGICO. I.R.C.C.S. CRO Aviano. 27 settembre 2018
 CENTRO DI RIFERIMENTO ONCOLOGICO I.R.C.C.S. CRO Aviano NATIONAL CANCER INSTITUTE OF RESEARCH AND CARE Clinical and experimental activity based on research lines: 1. Tumor genetics and biology (basic and
CENTRO DI RIFERIMENTO ONCOLOGICO I.R.C.C.S. CRO Aviano NATIONAL CANCER INSTITUTE OF RESEARCH AND CARE Clinical and experimental activity based on research lines: 1. Tumor genetics and biology (basic and
CLINICAL RESEARCH IN GEORGIA UPDATE. Nancy M. Paris, MS, FACHE President, Georgia CORE September 6, 2014
 CLINICAL RESEARCH IN GEORGIA UPDATE Nancy M. Paris, MS, FACHE President, Georgia CORE September 6, 2014 Georgia CORE is a public-private partnership dedicated to generating collaborative resources for
CLINICAL RESEARCH IN GEORGIA UPDATE Nancy M. Paris, MS, FACHE President, Georgia CORE September 6, 2014 Georgia CORE is a public-private partnership dedicated to generating collaborative resources for
National Surgical Adjuvant Breast and Bowel Project (NSABP) Foundation Annual Progress Report: 2009 Formula Grant
 National Surgical Adjuvant Breast and Bowel Project (NSABP) Foundation Annual Progress Report: 2009 Formula Grant Reporting Period July 1, 2012 June 30, 2013 Formula Grant Overview The National Surgical
National Surgical Adjuvant Breast and Bowel Project (NSABP) Foundation Annual Progress Report: 2009 Formula Grant Reporting Period July 1, 2012 June 30, 2013 Formula Grant Overview The National Surgical
24-Hour Helpline: BLK Cancer Centre. BLK Super Speciality Hospital. Pusa Road, New Delhi (India).
 24-Hour Helpline: 011-30403040 BLK Super Speciality Hospital Pusa Road, New Delhi - 110005 (India). www.blkhospital.com BLK Cancer Centre BLK Cancer Centre is one of the leading centres in the country,
24-Hour Helpline: 011-30403040 BLK Super Speciality Hospital Pusa Road, New Delhi - 110005 (India). www.blkhospital.com BLK Cancer Centre BLK Cancer Centre is one of the leading centres in the country,
Funding of patient-oriented clinical research by the German Federal Ministry of Education and Research (BMBF)
 Funding of patient-oriented clinical research by the German Federal Ministry of Education and Research (BMBF) Dr. Steinmüller/ Dr. Jacobs July 8th, 2011, Cologne Overview Health research programme of the
Funding of patient-oriented clinical research by the German Federal Ministry of Education and Research (BMBF) Dr. Steinmüller/ Dr. Jacobs July 8th, 2011, Cologne Overview Health research programme of the
Your Excellency, Mr Paul Madden, British High Commissioner, Singapore. It is a great pleasure to welcome all of you to the Biopolis for this two-day
 OPENING SPEECH BY MR LIM CHUAN POH, CHAIRMAN, AGENCY FOR SCIENCE, TECHNOLOGY AND RESEARCH, AT THE UK-SINGAPORE SYMPOSIUM ON P53: THE NEXT 30 YEARS, 25 NOVEMBER 2009 @ 0900HRS @ BREAKTHROUGH THEATRETTE,
OPENING SPEECH BY MR LIM CHUAN POH, CHAIRMAN, AGENCY FOR SCIENCE, TECHNOLOGY AND RESEARCH, AT THE UK-SINGAPORE SYMPOSIUM ON P53: THE NEXT 30 YEARS, 25 NOVEMBER 2009 @ 0900HRS @ BREAKTHROUGH THEATRETTE,
MOVEMBER FOUNDATION DANISH PROSTATE CANCER PROJECT REQUEST FOR APPLICATIONS
 MOVEMBER FOUNDATION CANCER PROJECT REQUEST FOR APPLICATIONS CONTENTS ABOUT THE MOVEMBER FOUNDATION... 3 DESCRIPTION... 4 ELIGIBILITY... 5 ALLOWABLE COSTS... 6 INELIGIBLE COSTS... 6 COMMUNICATION REQUIREMENTS...
MOVEMBER FOUNDATION CANCER PROJECT REQUEST FOR APPLICATIONS CONTENTS ABOUT THE MOVEMBER FOUNDATION... 3 DESCRIPTION... 4 ELIGIBILITY... 5 ALLOWABLE COSTS... 6 INELIGIBLE COSTS... 6 COMMUNICATION REQUIREMENTS...
1.5. Research Areas Treatment Selection
 1.5. Research Areas Cancer biomarker research embraces many areas of study, including tumorigenesis, metastasis, clinical trial and surrogate endpoints, cell isolation, target identification, drug resistance,
1.5. Research Areas Cancer biomarker research embraces many areas of study, including tumorigenesis, metastasis, clinical trial and surrogate endpoints, cell isolation, target identification, drug resistance,
CANCER IMMUNOPATHOLOGY. Eryati Darwin Faculty of Medicine Andalas University
 CANCER IMMUNOPATHOLOGY Eryati Darwin Faculty of Medicine Andalas University Padang 18 Mei 2013 INTRODUCTION Tumor: cells that continue to replicate, fail to differentiate into specialized cells, and become
CANCER IMMUNOPATHOLOGY Eryati Darwin Faculty of Medicine Andalas University Padang 18 Mei 2013 INTRODUCTION Tumor: cells that continue to replicate, fail to differentiate into specialized cells, and become
Revlimid. Revlimid (lenalidomide) Description. Section: Prescription Drugs Effective Date: July 1, 2015
 Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.04.47 Subject: Revlimid Page: 1 of 6 Last Review Date: June 19, 2015 Revlimid Description Revlimid (lenalidomide)
Federal Employee Program 1310 G Street, N.W. Washington, D.C. 20005 202.942.1000 Fax 202.942.1125 5.04.47 Subject: Revlimid Page: 1 of 6 Last Review Date: June 19, 2015 Revlimid Description Revlimid (lenalidomide)
Volunteering in NHSScotland Developing and Sustaining Volunteering in NHSScotland
 NG11-07 ing in NHSScotland Developing and Sustaining ing in NHSScotland Outcomes The National Group for ing in NHS Scotland agreed the outcomes below which formed the basis of the programme to develop
NG11-07 ing in NHSScotland Developing and Sustaining ing in NHSScotland Outcomes The National Group for ing in NHS Scotland agreed the outcomes below which formed the basis of the programme to develop
Media Release. Basel, 10 December 2017
 Media Release Basel, 10 December 2017 Phase II data showed Roche s investigational polatuzumab vedotin plus bendamustine and MabThera/Rituxan (BR) increased complete response rates compared to BR alone
Media Release Basel, 10 December 2017 Phase II data showed Roche s investigational polatuzumab vedotin plus bendamustine and MabThera/Rituxan (BR) increased complete response rates compared to BR alone
Integration of palliative care into oncology
 1 Integration of palliative care into oncology Stein Kaasa European Palliative Care Research Centre, Faculty of Medicine, NTNU and Department of Oncology, St. Olavs Hospital, Trondheim University Hospital
1 Integration of palliative care into oncology Stein Kaasa European Palliative Care Research Centre, Faculty of Medicine, NTNU and Department of Oncology, St. Olavs Hospital, Trondheim University Hospital
Rare cancers Medical oncologist Point of View
 Rare cancers Medical oncologist Point of View Isabelle Ray-Coquard, MD PhD Centre Léon Bérard GINECO & Université Claude Bernard Lyon I Identified factors to explain medical practices Initial Medical school
Rare cancers Medical oncologist Point of View Isabelle Ray-Coquard, MD PhD Centre Léon Bérard GINECO & Université Claude Bernard Lyon I Identified factors to explain medical practices Initial Medical school
In vitro scratch assay: method for analysis of cell migration in vitro labeled fluorodeoxyglucose (FDG)
 In vitro scratch assay: method for analysis of cell migration in vitro labeled fluorodeoxyglucose (FDG) 1 Dr Saeb Aliwaini 13/11/2015 Migration in vivo Primary tumors are responsible for only about 10%
In vitro scratch assay: method for analysis of cell migration in vitro labeled fluorodeoxyglucose (FDG) 1 Dr Saeb Aliwaini 13/11/2015 Migration in vivo Primary tumors are responsible for only about 10%
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION. 16 December 2009
 The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 16 December 2009 MOZOBIL 20 mg/ml, solution for injection Box containing 1 vial (CIP: 397 153-7) Applicant: GENZYME
The legally binding text is the original French version TRANSPARENCY COMMITTEE OPINION 16 December 2009 MOZOBIL 20 mg/ml, solution for injection Box containing 1 vial (CIP: 397 153-7) Applicant: GENZYME
UICC Members Regional Meeting. North America & Global Collaborations
 UICC Members Regional Meeting North America & Global Collaborations Hosted by Supported by Agenda Introduction Presentation of 3 collaborations case studies How can UICC support Discussion Conclusion 2
UICC Members Regional Meeting North America & Global Collaborations Hosted by Supported by Agenda Introduction Presentation of 3 collaborations case studies How can UICC support Discussion Conclusion 2
North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO)
 North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO) UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Original Prepared by NMcL April 2016
North of Scotland Cancer Network Clinical Management Guideline for Metastatic Malignancy of Undefined Primary Origin (MUO) UNCONTROLLED WHEN PRINTED DOCUMENT CONTROL Original Prepared by NMcL April 2016
Cancer prevention and control
 92 FIFTY-EIGHTH WORLD HEALTH ASSEMBLY Extracted from World Health Assembly 58 (2005) Official Records WHA58.22 Cancer prevention and control The Fifty-eighth World Health Assembly, Having examined the
92 FIFTY-EIGHTH WORLD HEALTH ASSEMBLY Extracted from World Health Assembly 58 (2005) Official Records WHA58.22 Cancer prevention and control The Fifty-eighth World Health Assembly, Having examined the
SINGAPORE SCIENTISTS DISCOVER NEW PATHWAYS LEADING TO CANCER PROGRESSION
 MEDIA RELEASE FOR IMMEDIATE RELEASE 18 JULY 2016 SINGAPORE SCIENTISTS DISCOVER NEW PATHWAYS LEADING TO CANCER PROGRESSION SINGAPORE Scientists from A*STAR s Genome Institute of Singapore (GIS) and the
MEDIA RELEASE FOR IMMEDIATE RELEASE 18 JULY 2016 SINGAPORE SCIENTISTS DISCOVER NEW PATHWAYS LEADING TO CANCER PROGRESSION SINGAPORE Scientists from A*STAR s Genome Institute of Singapore (GIS) and the
