Low-dose YC-1 combined with glucose and insulin selectively induces apoptosis in hypoxic gastric carcinoma cells by inhibiting anaerobic glycolysis
|
|
|
- Wesley Matthews
- 6 years ago
- Views:
Transcription
1 Low-dose YC-1 combined with glucose and insulin selectively induces apoptosis in hypoxic gastric carcinoma cells by inhibiting anaerobic glycolysis The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters. Citation Published Version Accessed Citable Link Terms of Use Wakiyama, Kota, Yoshihiko Kitajima, Tomokazu Tanaka, Masao Kaneki, Kazuyoshi Yanagihara, Shinichi Aishima, Jun Nakamura, and Hirokazu Noshiro Low-dose YC-1 combined with glucose and insulin selectively induces apoptosis in hypoxic gastric carcinoma cells by inhibiting anaerobic glycolysis. Scientific Reports 7 (1): doi: /s doi: /s December 18, :01:04 AM EST This article was downloaded from Harvard University's DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at (Article begins on next page)
2 Received: 24 May 2017 Accepted: 23 August 2017 Published: xx xx xxxx OPEN Low-dose YC-1 combined with glucose and insulin selectively induces apoptosis in hypoxic gastric carcinoma cells by inhibiting anaerobic glycolysis Kota Wakiyama 1,2 1,2, Yoshihiko Kitajima, Tomokazu Tanaka 1,3, Masao Kaneki 3, Kazuyoshi Yanagihara 4, Shinichi Aishima 5, Jun Nakamura 1 & Hirokazu Noshiro 1 This study aimed to establish a therapeutic strategy targeting hypoxic cancer cells in gastric carcinoma (GC). YC-1 is a HIF-1α inhibitor, and we revealed that low-dose YC-1 (10 µm) suppressed HIF-1α expression, and induced hypoxia-dependent apoptosis in the GC cell line 58As9. This hypoxia-specific apoptosis induction by YC-1 involved excessive reactive oxygen species (ROS) generation. The apoptotic effect of 10 µm YC-1 was enhanced by additional glucose (G) and insulin (I) treatments. RT- PCR demonstrated that 10 µm YC-1 reduced hypoxia-induced expression of HIF-1α targets involved in anaerobic glycolysis. Metabolic analysis showed that YC-1 shifted glucose metabolism in hypoxic cells from anaerobic glycolysis to oxidative phosphorylation (OXPHOS). Additional GI accelerated membranous GLUT1 translocation, elevating glucose uptake, and increased acetyl-coa levels, leading to more ROS generation in hypoxic YC-1-treated cells. Finally, we evaluated the anti-cancer effect of low-dose YC-1 (1 mg/kg) + G (2 g/kg) and I (1 unit/3 g G) treatment in xenograft models. YC-1 + GI therapy strongly inhibited tumour growth. Immunohistochemical analysis demonstrated that YC-1 + GI reduced HIF-1α expression and pimonidazole accumulation in tumours. Conversely, YC-1 + GI increased intratumoral 8-OHdG and levels of apoptosis markers. Low-dose YC-1 + GI is a unique therapy targeting hypoxic GC cells that generates lethal ROS via forced activation of OXPHOS. Intratumoral hypoxia (low O 2 ) is a common characteristic of many solid tumours 1,2. HIF-α (HIF-1α or HIF-2α), a basic-helix-loop-helix transcription factor, functions as a master regulator of oxygen homeostasis. Under normoxia, prolyl hydroxylases (PHDs) use oxygen as a substrate to hydroxylate key proline residues within HIF-α, which is then degraded through the proteasomal pathway following pvhl-mediated ubiquitination. Under hypoxia, PHD activity is inhibited, and HIF-α is stabilized, forming an active complex with aryl hydrocarbon receptor nuclear translocator (ARNT), and upregulates hundreds of target genes through binding hypoxia-response elements (HREs) 3 5. HIF-α overexpression has been found in many human cancers and is associated with the induction of genes implicated in angiogenesis, tumour metabolism, invasion, metastasis and radio- and chemo-resistance 6 11, which contribute to poor patient survival 11. Therefore, inhibition of HIF-α is an attractive strategy for cancer therapy; however, no selective HIF-α inhibitor has been clinically approved Recently, we reported that HIF-1α knockdown (KD) by sirna induces apoptosis in the gastric carcinoma (GC) cell line 58As9 under hypoxia. This hypoxia-dependent apoptosis was induced by excessive production of reactive oxygen species (ROS), whereby HIF-1α KD inhibited hypoxic induction of genes involved in the ROS 1 Department of Surgery, Saga University Faculty of Medicine, Nabeshima, Saga, , Japan. 2 Department of Surgery, NHO Higashisaga Hospital,7324 Harakoga, Miyaki-cho, Miyaki-Gun, Saga, , Japan. 3 Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, USA. 4 Division of Biomarker Discovery, Exploratory Oncology Research & Clinical Trial Center, National Cancer Center, Kashiwanoha, Kashiwa, Chiba, , Japan. 5 Department of Pathology, Saga University Faculty of Medicine, Nabeshima, Saga, , Japan. Correspondence and requests for materials should be addressed to Y.K. ( kitajiy@hosp.go.jp) 1
3 Figure 1. YC-1 inhibited growth in the gastric cancer cell line 58As9. (a) Viability of YC-1-treated 58As9 cells under normoxia and hypoxia; viability of HIF-1α KD (KD) cells was evaluated in parallel. (b) Cell death rate in 58As9 cells with or without YC-1 (10 μm) under normoxia and hypoxia. (c) Western blot analysis of HIF-1α expression in 58As9 cells under normoxia and hypoxia with or without YC-1 (10 μm) treatment for 8 and 12 h. β-actin was equally expressed in all cells. ns: not significant, **p < control system including anaerobic glycolysis in 58As9 cells 16. This study further revealed that hypoxia-induced apoptosis was accelerated by additional glucose (G) and insulin (I) treatments in the KD cells, as higher ROS generated via increased glucose uptake 16. Based on this study, we attempted to establish a novel anti-cancer therapy using a specific HIF-1α inhibitor combined with GI to target hypoxic cancer cells in gastric tumours. ROS are mainly generated in the mitochondria by oxidative phosphorylation (OXPHOS), a process performed by the electron transport chain (ETC) Excessive ROS generation is known to cause ROS-mediated damage to nucleic acids, proteins and lipids, leading to cell death It has been reported that ROS are increased in hypoxic cancer cells, and HIF-1α induction plays an adaptive mechanism in controlling ROS generation via up-regulating genes involved in anaerobic glycolysis 3,15,16,19. In the anaerobic glycolysis pathway, HIF-1α first activates GLUT1 transcription to increase glucose uptake in cells 22. Glucose is then metabolized to pyruvate by the actions of glycolytic members including ALDOC 23. Under aerobic conditions, pyruvate is converted to acetyl-coa by pyruvate dehydrogenase (PDH) for entry into the tricarboxylic acid (TCA) cycle 18. Conversely, in cancer cells exposed to hypoxia, pyruvate is shunted away from the mitochondria by HIF-1α-mediated PDK1 upregulation, which inhibits PDH activity. Thereafter, LDHA alternatively converts pyruvate to lactate and MCT4 effluxes the lactate Together, these reports indicate that HIF-1α is a central molecule in suppressing excessive ROS production in hypoxic cells via up-regulating target genes involved in anaerobic glycolysis. YC-1 [3-(5 -hydroxymethyl-2 -furyl)-1-benzylindazole] was originally developed as a potential therapeutic agent for circulation disorders because of its inhibitory effect on platelet aggregation and vascular contraction 27. Recent studies have found that YC-1 blocked HIF-1α expression at the post-transcriptional level and consequently inhibits the transcription factor activity of HIF-1 in cancer cells under hypoxia However, no study has assessed the anti-cancer effect of YC-1 on cancer metabolism under hypoxia. In this study, we first determined the optimal dose of YC-1 that effectively inhibited HIF-1α expression and induced hypoxia-dependent apoptosis in GC cells. We next analyzed whether additional GI treatment enhanced this apoptotic effect. Metabolic analysis addressed the mechanism of YC-1 + GI-induced apoptosis in cells under hypoxia. Finally, we assessed whether this combination therapy selectively induced apoptosis in hypoxic cancer cells in vivo. Results Growth inhibition by YC-1 treatment in GC cells. The GC cell line 58As9 was treated with YC-1 at 1, 10 and 100 μm, and cell viability was evaluated by the MTS assay (Fig. 1a). The results showed that 1 μm YC-1 did not influence cell viability, while 10 μm YC-1-treated 58As9 and KD cells showed significantly decreased 2
4 Figure 2. Hypoxia-dependent apoptosis in 58As9 cells with YC-1 or YC-1 + GI treatment. (a) Cell viability in control and 10 μm YC-1-treated cells under normoxia or hypoxia was analyzed in the presence of G and/or I for 72 h. Fold change (FC) of hypoxia/normoxia is presented at the bottom. (b) Cell death rate in YC-1-, GI- and YC-1 + GI-treated cells under normoxia or hypoxia for 3 d. (c) Western blot analysis of cleaved-caspase3 and cleaved-parp in YC-1-, YC-1 + G-, YC-1 + I- and YC-1 + GI-treated cells under hypoxia for 24 h. β-actin was equally expressed in all cells. ns: not significant, *p < 0.05, **p < viability under hypoxia but not normoxia. At 100 μm, YC-1 decreased viability under both normoxia and hypoxia (Fig. 1a). YC-1 exhibited similar effects in another GC cell line, MKN74 (Supplemental Fig. 1). Cell death was next evaluated in 58As9 cells with or without 10 μm YC-1 (Fig. 1b). Under normoxia, there was no difference in the cell death rate between controls (no YC-1) and 10 μm YC-1. The cell death rate significantly increased under hypoxia with 10 μm YC-1, but not in controls (Fig. 1b). WB analysis showed that HIF-1α was elevated by hypoxia in control cells, while the hypoxic induction of HIF-1α was inhibited in YC-1-treated 58As9 and KD cells (Fig. 1c). YC-1 plus GI treatment induced apoptosis in 58As9 cells under hypoxia. We next evaluated the effect of additional GI treatment on hypoxia-dependent cell death in 10 μm YC-1-treated 58As9 cells. Cell viability was evaluated in control (no YC-1) and 10 μm YC-1-treated cells in the presence of glucose (G) and/or insulin (I) (Fig. 2a). In the control group, cell viability was unchanged between normoxia and hypoxia in control, G and I treatments, whereas viability decreased under hypoxia in GI treatment (fold change (FC) of hypoxia/normoxia: 0.9) (Fig. 2a). In the 10 μm YC-1 group, cell viability was more strongly inhibited under hypoxia than normoxia in all treatments (Fig. 2a). The lowest FC was found in the YC-1 + GI (FC: 0.5) treatment. Further, in the YC-1 group, cell viability under hypoxia was significantly lower in GI treatment than controls (Fig. 2a). The strong inhibitory effect of YC-1 + GI on cell viability was also observed in hypoxic MKN74 cells (Supplement Fig. 2). As shown in Fig. 2b, the cell death rate was not different among control, 10 μm YC-1, GI and YC-1 + GI under normoxia. However, the cell death rate was significantly increased in 10 μm YC-1 and YC-1 + GI compared with controls, and there was a higher death rate in YC-1 + GI than 10 μm YC-1 alone (Fig. 2b). WB analysis was used to evaluate apoptotic cell death in hypoxic 58As9 cells treated with YC-1 and/or GI (Fig. 2c). Expression of the apoptosis markers cleaved-caspase3 and cleaved-parp was increased in hypoxic 58As9 cells by the four treatments, but not controls (Fig. 2c). The highest expression of apoptosis markers was observed in YC-1 + GI-treated cells (Fig. 2c). Assessing ROS production after YC-1 + GI treatment under hypoxia. We analyzed ROS levels in 58As9 cells under hypoxia with or without 10 μm YC-1 (Fig. 3a). Compared with controls (no YC-1), 10 μm YC-1 more strongly elevated ROS production in a time-dependent manner in hypoxic 58As9 cells (Fig. 3a). ROS levels on day 3 were significantly higher in 10 μm YC-1-treated cells than control cells under hypoxia, while the highest ROS was produced in hypoxic KD cells. ROS levels in hypoxic 58As9 cells were significantly higher 3
5 Figure 3. Effect of YC-1 + GI on ROS generation under hypoxia. (a) ROS levels in 58As9 cells with or without 10 μm YC-1 and in KD cells were analyzed under hypoxia for 3 d. (b) ROS levels in YC-1- or YC-1 + GI-treated cells under hypoxia. (c) ROS levels in YC-1 + GI-treated cells with or without 5 μm NAC. (d) Cell death rate in YC-1 + GI-treated cells with or without NAC under normoxia and hypoxia. ns: not significant, *p < 0.05, **p < in YC-1 + GI than in YC-1 on day 3 (Fig. 3b). Moreover, elevated ROS was blocked by the antioxidant NAC in YC-1 + GI-treated cells under hypoxia (Fig. 3c). The cell death rate was assessed with or without NAC in YC-1 + GI-treated cells (Fig. 3d). Under normoxia, the cell death rate was not different between the NAC ( ) and NAC (+). In contrast, the cell death rate was significantly higher in the NAC ( ) than NAC (+) under hypoxia on day 3 (Fig. 3d). RT-qPCR analysis of genes involved in anaerobic glycolysis. RT-qPCR was used to evaluate hypoxia-induced expression of HIF-1α target genes in 58As9 cells in the control, GI, 10 μm YC-1 and YC-1 + GI groups (Fig. 4). The FC of hypoxia/normoxia was significantly decreased compared with controls by YC-1 and YC-1 + GI for all genes, except MCT4 in YC-1 + GI. However, KD cells showed the lowest FC for all five genes (Fig. 4). Levels of GLUT1, ALDOC, PDK1 and MCT4 expression under hypoxia were significantly decreased in YC-1 and YC-1 + GI to similar degrees, compared with controls. Expression of all five genes was most strongly suppressed in hypoxic KD cells (Fig. 4). Glucose uptake analysis. To assess glucose uptake, the 2DG uptake test was performed in 58As9 cells (Fig. 5a b). As shown in Fig. 5a, 2DG uptake was elevated by insulin in 58As9 cells with and without YC-1 under normoxia. Under hypoxia, 2DG uptake was strongly increased in control (no YC-1) cells, and additional insulin treatment further elevated uptake (Fig. 5b). In 10 μm YC-1-treated cells, 2DG uptake was also promoted by insulin, but to a lower degree than in control cells (Fig. 5b). We next analyzed membranous GLUT1 expression in control and 10 μm YC-1 cells under hypoxia, in combination with G and/or I treatments (Fig. 5c). In the control group, membranous GLUT1 was increased with G or I, and most strongly elevated by GI. In the 10 μm YC-1 group, membranous GLUT1 was entirely reduced, compared with controls (Fig. 5c). Among treatments, the highest GLUT1 expression was observed in GI (Fig. 5c). Alteration in glucose metabolism by YC-1 + GI treatment. OCR and ECAR were measured in absence or presence of the hypoxia-mimetic cobalt chloride (CoCl 2 ) (Fig. 6a). The OCR/ECAR ratio was unchanged between YC-1 ( ) and YC-1 (+) cells in the absence of CoCl 2. In contrast, the OCR/ECAR ratio was significantly higher in YC-1 (+) than YC-1 ( ) cells in the presence of CoCl 2 (Fig. 6a). We further measured glucose metabolites in YC-1 ( ) and YC-1 (+) cells with or without GI (Fig. 6b). In the YC-1 ( ) group, there was no significant difference in intracellular acetyl-coa between normoxia and hypoxia (Fig. 6b). However, in the 10 μm YC-1 group, intracellular acetyl-coa was significantly elevated under hypoxia 4
6 Figure 4. Expression of HIF-1α targets essential to anaerobic glycolysis. RT-qPCR analysis of five genes in control and GI-, YC-1- and YC-1 + GI-treated cells under normoxia and hypoxia; the expression levels are presented together with those in KD cells. FC of hypoxia/normoxia is presented at the bottom of each graph. mrna expression levels under hypoxia were compared between control and treatment groups for five genes. ns: not significant, *p < 0.05, **p < in GI ( ) cells with an FC of 1.4, and this hypoxic induction was further increased in GI (+) cells with an FC of 1.9 (Fig. 6b). In the 10 μm YC-1 group under hypoxia, acetyl-coa was significantly higher in GI (+) than GI ( ) (Fig. 6b). Extracellular lactate was next assessed (Fig. 6b); in the YC-1 ( ) group, lactate levels were increased by hypoxia in the GI (+) (FC: 1.3) (Fig. 6b). In the 10 μm YC-1 group, lactate levels were significantly decreased under hypoxia compared with normoxia in both the GI ( ) (FC: 0.7) and GI ( + ) (FC: 0.5). Furthermore, in the 10 μm YC-1 group under hypoxia, extracellular lactate was significantly lower in GI (+) than GI ( ) (Fig. 6b). YC-1 plus GI treatment suppressed xenograft tumour growth in nude mice. Finally, we evaluated the in vivo effect of YC-1 + GI treatment in tumour xenografts (Fig. 7). The four drugs were ip injected into mice from day 1 to day 14, as shown in Fig. 7a. On day 15, tumours were harvested and subjected to WB analysis. HIF-1α expression was observed in control and GI mice, while its expression was inhibited in YC-1 and YC-1 + GI (Fig. 7b). In contrast, cleaved-parp and cleaved-caspase3 were present in YC-1 and YC-1 + GI, and the levels were higher in YC-1 + GI than YC-1 (Fig. 7b). Figure 7c shows the growth curves of xenograft tumours that underwent the four treatments. There was no significant difference in size between control and GI tumors on day 15 (Fig. 7c). In contrast, tumour sizes of YC-1 or YC-1 + GI were significantly smaller than control, and tumour growth in YC-1 + GI was more strongly inhibited than in YC-1 (Fig. 7c). In the representative images of tumor-bearing mice, tumours appeared to be smaller in order of control, YC-1 and YC-1 + GI (Fig. 7d). In this model, no mice died in any treatment. Immunohistochemistry evaluated levels of HIF-1α, pimonidazole, cleaved-caspase3 and an oxidized base, 8-OHdG, in xenograft tumours that underwent control or YC-1 + GI treatments (Fig. 7e). HIF-1α and pimonidazole staining appeared to be stronger in control than YC-1 + GI-treated tumours, whereas cleaved-caspase3 and 8-OHdG staining were stronger in YC-1 + GI (Fig. 7e). Statistical analysis demonstrated that the number of positive HIF-1α and pimonidazole cells was significantly higher in control than YC-1 + GI, while cleaved-caspase3 and 8-OHdG were higher in YC-1 + GI (Fig. 7f). Discussion In this study, we first attempted to isolate a compound that inhibited cell viability in 58As9 cells specifically under hypoxia, similar to what we had previously shown for HIF-1α KD 58As9 cells 16. Many small molecules have been reported to be HIF-1α inhibitors We explored a desirable drug that reduced cell viability specifically in hypoxia, but not normoxia among the 15 known HIF-1α inhibitors The MTS assay determined that only 5
7 Figure 5. Glucose uptake in 58As9 cells with or without YC-1 + GI. (a,b) 2DG uptake levels in control and YC- 1-treated cells were analyzed under normoxia (a) and hypoxia (b) for 24 h in the presence or absence of insulin. (c) Western blot analysis of membranous GLUT1 (54 kda) levels in control and YC-1-treated cells that were cultured under hypoxia for 24 h in the presence or absence of G and/or I. β-actin was equally expressed in all cells. ns: not significant, **p < μm YC-1 decreased cell viability in 58As9 cells selectively in hypoxia and inhibited HIF-1α expression. These results suggest low-dose YC-1 (10 μm) is essential for hypoxia-dependent cell growth inhibition. Conversely, high-dose YC-1 (100 μm) decreased cell viability under both normoxia and hypoxia. The inhibitory effect of 100 μm YC-1 on cell viability under normoxia may be derived from unknown HIF-1α-independent mechanisms. In this study, we did not assess whether YC-1 treatment inhibits HIF-2α expression, or whether HIF-2α KD affects cell growth in hypoxic 58As9 cells. Further investigation may solve this question. We next demonstrated that additional GI to 10 μm YC-1 induced hypoxia-dependent apoptosis more strongly than YC-1 mono-treatment. Low-dose YC-1 or YC-1 + GI exhibited similar effects in another GC cell line, MKN74, indicating hypoxia-dependent cell death by these treatments was not restricted to one cell line. A previous study reported that 100 μm YC-1 inhibited HIF-1α expression and induced apoptosis in PC3 cells under hypoxia 29. However, this study did not evaluate YC-1 under normoxia. Another study reported that 1 μm YC-1 inhibited Hep3B proliferation under both normoxia and hypoxia 30. Therefore, we showed for the first time that hypoxia-dependent apoptosis is induced by 10 μm YC-1 in GC cells. Thereafter, we showed YC-1 or YC-1 + GI time-dependently accumulated ROS in hypoxic 58As9 cells, and higher ROS was generated in YC-1 + GI than YC-1. NAC reversed cell death by YC-1 + GI in hypoxic cells, indicating apoptosis was induced by excessive ROS generation. RT-qPCR analysis revealed hypoxic induction of the HIF-1α targets GLUT1, ALDOC, PDK1 and MCT4 was attenuated by YC-1 and YC-1 + GI to similar degrees. These results implied HIF-1α inhibition by YC-1 suppressed anaerobic glycolysis via reducing expression of these genes. However, the inhibitory effect of YC-1 or YC-1 + GI on HIF-1α targets was weaker than hypoxic KD cells. Additionally, hypoxic LDHA induction was not affected by 10 μm YC-1 treatment. Higher doses of YC-1 than 10 μm may be necessary for stronger HIF-1α inhibition, thereby hypoxic induction of the HIF-1α targets GLUT1, ALDOC, LDHA, PDK1 and MCT4 may be more strongly suppressed. We further investigated the biological effect of YC-1 + GI on glucose metabolism. To assess the effect of YC-1 or YC-1 + GI on glucose uptake, the 2DG uptake test was performed with or without YC-1 ± I. 2DG uptake was strongly accelerated by hypoxic stimuli in control cells, which was further elevated by insulin. The promotion of 2DG uptake by hypoxia was smaller in YC-1 (10 μm) compared with controls. WB analysis showed that membranous GLUT1 expression was elevated by GI treatment in control cells under hypoxia, while it was entirely reduced by YC-1 under hypoxia; however, membranous GLUT1 was increased by GI treatment. This suggested that the strong elevation of glucose uptake in hypoxic control cells was due to GLUT1 up-regulation via HIF-1α activation, and the uptake was further promoted by insulin, which enhanced GLUT1 membrane translocation. Insulin signalling may stimulate GLUT1 translocation from intracellular storage vesicles to the plasma membrane as was reported for GLUT4 in adipocytes 31. Conversely, the hypoxic stimulation of glucose uptake was smaller in 10 μm 6
8 Figure 6. Reprograming glucose metabolism by YC-1 or YC-1 + GI treatment. (a) Using a Seahorse XF extracellular flux analyzer, OCR and ECAR were measured with or without the hypoxia-mimetic CoCl2. (b) Intracellular acetyl-coa and extracellular lactate levels were measured in control, 10 μm YC-1 or KD cells under normoxia and 24 h hypoxia. In control and YC-1-treated cells, metabolite levels were further analyzed with or without GI treatment. FC values of hypoxia/normoxia are presented on the bottom of each graph. Acetyl-CoA and lactate levels under hypoxia were statistically compared as indicated in the graphs. ns: not significant, *p < 0.05, **p < YC-1 than controls. RT-qPCR showed that the hypoxic induction of GLUT1 mrna was attenuated by YC-1, which may lead to weaker stimulation of glucose uptake by hypoxia in YC-1-treated cells. However, additional GI sustained increased GLUT1 translocation, and may contribute to promotion of glucose uptake. Metabolic analysis showed that the OCR/ECAR ratio was significantly elevated by YC-1 in 58As9 cells under hypoxia-mimetic conditions. These results suggested that YC-1 switched glucose metabolism from anaerobic glycolysis to OXPHOS in hypoxic cells. Assessments of glucose metabolites further revealed that 10 μm YC-1 elevated intracellular acetyl-coa in hypoxic 58As9 cells, while the treatment decreased extracellular lactate. These results suggested that reduced PDK1 expression allows the conversion of pyruvate to acetyl-coa but not lactate in YC-1-treated cells under hypoxia, which results in the elevation of intracellular acetyl-coa instead of lactate; furthermore reduced MCT4 expression may decrease lactate efflux. This metabolic reprograming, derived from HIF-1α inhibition by YC-1, may generate excessive ROS and induce hypoxia-dependent apoptosis in YC-1-treated cells. Moreover, additional GI may elevate glucose uptake through membranous GLUT1 translocation in YC-1 treated cells under hypoxia. Thereafter, larger amounts of acetyl-coa may be produced through glycolysis, inducing further ROS production in the OXPHOS pathway, causing more apoptosis than YC-1 mono-treatment. Finally, we analyzed the in vivo effect of YC-1 + GI treatment using a tumour xenograft model. YC-1 is known to prevent intravascular thrombus formation by inhibiting platelet aggregation 27. A previous study reported that YC-1 ip injections (10, 30 mg/kg) prolonged tail bleeding-time in mice 27,28. Hence, we determined 1 mg/ kg of YC-1 as the optimal dose for preclinical study. Doses of GI at glucose (2 g/kg) and insulin (1 unit/3 g glucose) were determined, because higher doses of glucose (4 or 8 g/kg) resulted in serious hyperglycemia (supplement Fig. 3). The results demonstrated that YC-1 + GI strongly suppressed xenograft tumour growth, while YC-1 mono-treatment incompletely blocked growth. WB analysis showed HIF-1α expression in control 58As9 tumours, suggesting hypoxic regions persisted. In contrast, HIF-1α expression was inhibited in YC-1- or YC-1 + GI-treated tumours, but apoptosis markers were more strongly induced by YC-1 + GI than YC-1. Immunohistochemistry findings indicated that YC-1 + GI inhibited HIF-1α expression in xenograft tumours, and this combination coincidently decreased pimonidazole expression. Conversely, YC-1 + GI increased cleaved-caspase3 and 8-OHdG levels in tumours. Thus, YC-1 + GI treatment selectively inhibited hypoxic cancer cell growth in xenograft tumours. In these hypoxic cells, YC-1 inhibits anaerobic glycolysis via HIF-1α suppression, and additional GI promotes glucose uptake. These dual effects of YC-1 + GI synergistically elevate acetyl-coa through glycolysis, and induce lethal ROS production. 7
9 Figure 7. In vivo effect of YC-1 + GI treatment on tumour xenografts. (a) Experimental schedule of the YC-1 + GI treatment. The day 58As9 cells were subcutaneously injected is indicated by a triangle. The daily ip injections are shown by green arrows. Tumours were harvested on day 15, which is indicated by a reticulated triangle. Doses of drug treatments including control, glucose, YC-1 and YC-1 + GI are shown in the blue square. (b) Western blot analysis of HIF-1α, cleaved-caspase3 and cleaved-parp in tumour xenografts from control, GI, YC-1 and YC-1 + GI groups. (c) Tumours sizes in the control, GI, YC-1 and YC-1 + GI groups were estimated on the indicated days. Mean value ± SE of tumor volumes was calculated and plotted on the graph. (d) Pictures of tumours treated with YC-1 + GI, YC-1 or control. (e) Immunohistochemical analysis of HIF-1α, pimonidazole, cleaved-caspase3 and 8-OHdG expressions in xenograft tumours from control or YC-1 + GI mice. (f) Comparison of the number of positively-stained cells from HIF-1α, pimonidazole, cleaved-caspase3 and 8-OHdG immunohistochemistry between control and YC-1 + GI treatment. ns: not significant, *p < 0.05, **p <
10 Figure 8. In vitro apoptotic effect of low-dose YC-1 + GI treatment on hypoxic 58As9 cells. (a) HIF-1α controls ROS production in hypoxic 58As9 cells by up-regulating genes involved in anaerobic glycolysis such as GLUT1, ALDOC, LDHA, PDK1 and MCT4 (indicated by red letters). (b) Low-dose YC-1 + GI treatment induces hypoxia-dependent apoptosis in 58As9 cells. YC-1 treatment inhibits HIF-1α expression in hypoxic 58As9 cells and suppresses up-regulation of the HIF-1α targets GLUT1, ALDOC, PDK1 and MCT4 (indicated by gray letters). Attenuation of the expression of these genes causes a metabolic switch from anaerobic glycolysis to OXPHOS in hypoxic 58As9 cells, resulting in the elevated OCR/ECAR ratio. Additionally, GI treatment accelerates GLUT1 membrane translocation (indicated by red letters) via insulin signalling, promotes glucose uptake in hypoxic 58As9 cells, and contributes to increased AcCoA entry into the TCA cycle. These dual effects of low-dose YC-1 + GI treatment result in lethal ROS production through the ETC. CM: cell membrane, NC: nucleus, HRE: hypoxia responsive element, MC: mitochondria, GL: glycolysis, IR: insulin receptor. AcCoA: acetyl-coa, OCR: oxygen consumption rate, ECAR: extracellular acidification rate. In summary, in vitro apoptotic mechanism of low-dose YC-1 + GI treatment in hypoxic 58As9 cells is illustrated in Fig. 8. This study revealed that low-dose YC-1 + GI therapy targets hypoxic cancer cells, where a metabolic switch from anaerobic glycolysis to OXPHOS is induced under hypoxia, resulting in ROS-mediated apoptosis. Additionally, this treatment may supply glucose to normal cells that live under normoxic environments. Therefore, low-dose YC-1 + GI may be an attractive anti-cancer therapy because hypoxic cancer cells with malignant behaviors may be selectively killed, while normal cells will survive and receive energy from the GI treatment. Methods Cell culture conditions and reagents. The GC cell line 58As9 was established and kindly provided by Dr. K. Yanagihara (National Cancer Center, Chiba, Japan) 32. This cells line was further authenticated by the JCRB Cell Bank (Osaka, Japan). MKN74 cells were purchase from Cell Bank, RIKEN Bio Resource Center (Tsukuba, Japan). HIF-1α knockdown (KD) cells were established by transfecting plasmids harboring HIF-1α RNAi sequences into 58As9 and MKN74 cells, as previously described 11,16. The cells were cultured at 37 C in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% heat-inactivated fetal bovine serum and 100 μg/ ml kanamycin (Meiji, Tokyo, Japan). Cells were cultured under either normoxic (20% O 2 and 5% CO 2 in air) or hypoxic conditions (1% O 2, 5% CO 2 and 94% N 2 ) in a hypoxic chamber (ASTEC, Fukuoka, Japan). YC-1 (Sigma- Aldrich), NAC (Sigma-Aldrich) and Insulin (Wako, Osaka, Japan) were used at a final concentration of 10 µm, 5 mm and 500 ng/ml, respectively. High glucose medium was prepared using a 45% D-(+)-Glucose solution (Sigma-Aldrich) and the concentration was determined to be 10 g/l, 5 times higher than RPMI-1640 medium. Cell viability assay. Cell viability was assessed by the MTS assay using the CellTiter 96 AQueous One Solution Cell Proliferation Assay kit (Promega, Madison, WI, USA), as previously described 11. The cell death rate was estimated by trypan blue dye exclusion method, as previously described 16. These experiments were independently repeated at least three times. 9
11 Western blot (WB) analysis. Whole cell lysates from cultured cells and mouse tumors were prepared as previously described 16. Cell lysates from the cell membrane fraction were prepared using Plasma Membrane Protein Extraction Kit (BioVision Inc., Milpitas, CA, USA) according to the manufacturer s instructions. WB analysis was performed as previously described 16. using the following primary antibodies: anti-hif-1α (1:1000, clone54/hif1α, BD-Biosciences, Franklin Lakes, NJ, USA), anti-cleaved caspase3 (1:1000, Cell Signaling Technology, Danvers, MA, USA), anti-cleaved PARP (1:1000, Cell Signaling Technology), anti-glut1 (1:1000, Abcam, Cambridge, UK) and anti β-actin (1:10,000, Sigma-Aldrich). Detecting intracellular ROS by flow cytometry. Intracellular ROS levels were evaluated using the Total ROS Detection Kit (Enzo Life Sciences, Inc., Farmingdale, NY, USA) according to the manufacturer s instructions as previously described 16. ROS fluorescence was detected using a FACSCalibur flow cytometer (Becton-Dickinson, San Jose, CA, USA) and analyzed by the Cell Quest program to determine mean fluorescence. Total RNA extraction and real-time qpcr (RT-qPCR). Total RNA extraction, followed by cdna conversion was done as previously described 11. RT-qPCR was performed using the Light Cycler instrument system (Roche Diagnostics GmbH, Mannheim, Germany) as previously described 16. Five genes were analyzed by RT-qPCR: glucose transporter 1 (GLUT1), aldolase C (ALDOC), pyruvate dehydrogenase kinase 1 (PDK1), lactate dehydrogenase A (LDHA) and monocarboxylate transporter 4 (MCT4). Primers were designed according to the reported cdna sequences (GenBank, Bethesda, MD, USA), and the sequences are shown in previous study 16. All experiments were performed in triplicate, and mean values were calculated. Glucose uptake assay. Glucose uptake in cultured cells was determined using a 2-Deoxyglucose (2-DG) Uptake Measurement Kit (COSMO BIO Co. Ltd., Tokyo, Japan) as previously described 16. Briefly, cells were cultured under a serum-starved condition for 6 h, followed by further culture for 18 h in regular medium supplemented with 10% FBS. The cells were then incubated for 24 h under normoxia or hypoxia. Thereafter, the cells were treated with or without 500 ng/ml insulin for 18 h. Finally, the cells were treated with 2DG for 20 min and subjected to 2DG uptake measurements according to the manufacturer s instruction. All experiments were performed in triplicate, and mean values were calculated. Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) measurement. The mitochondrial OCR and ECAR were measured using Seahorse XFp Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA) as described previously 33. Briefly, cells were seeded in each well of Seahorse XFp Cell Culture Miniplates coated with poly-l-lysine solution (Sigma-Aldrich, St. Louis, MO) two days prior to the assay. The cells were exposed to cobalt chloride (CoCl2) (Sigma-Aldrich) at 100 μm final concentration for 16hr to create the mimic hypoxia. Subsequently, the cells were treated with or without YC-1 (10 μm) for 24 h. Before performing the glycolysis stress test, the culture medium was removed from each well, and then the cells were washed 2 times and filled by the assay medium for Seahorse adjusted the ph to 7.4 ( ± 0.02). Thereafter, the glycolysis stress test was employed by sequential injections of glucose (10 mm), oligomycin (2.5 μm) and 2-deoxy-glucose (2-DG) (50 mm). OCR and ECAR under basal and glucose-stimulated conditions were evaluated as means of values at the three time points before and after the addition of glucose, respectively. Evaluation of acetyl-coa and lactate levels. Intracellular acetyl-coa was measured using a Pico Probe Acetyl-CoA Fluorometric Assay Kit (BioVision Inc.). Extracellular lactate was measured using a Lactate Colorimetric/Fluorometric Assay Kit (BioVision Inc.). Animal experiments. All methods were performed in accordance with the relevant guidelines and regulations. Further, all animal protocols were approved by the Animal Care Committee of Saga University. Female athymic BALB/cA Jcl mice (nu/nu, 4-weeks-old) were obtained from Nihon Crea Co. (Osaka, Japan), kept under specific-pathogen-free conditions and given sterile food and autoclaved water. To establish the tumour models, As9 cells were subcutaneously injected into the back of the mice. One week after inoculation, xenografts became palpable. The 20 xenograft-bearing mice were divided into four treatment groups as follows: Control [phosphate-buffered saline (PBS)], GI (glucose: 4 g/kg/day, insulin: 1 unit per 3 g-glucose/day), YC-1 (1 mg/kg) and YC-1 + GI (above treatments combined). All drugs were intraperitoneally (ip) administered every 24 h from day 1 to day 14. During treatment, tumours sizes were measured along 2 perpendicular dimensions with a caliper every 4 d. Tumour size (T) was evaluated as the maximum cut area and determined by the formula: T = π/4 a b, where a (mm) is the shorter axis and b (mm) is the longer axis. Immunohistochemistry. After deparaffinization and rehydration, antigen retrieval was performed with high ph CC1 buffer at 99 C for 1 h (Ventana Medical Systems, Inc., Tucson, AZ, USA). For immunostaining, 4-μm sections were incubated with antibodies against HIF-1α (1:50), pimonidazole (1:100, clone Pab2627, rabbit polyclonal, COSMO BIO Co. Ltd., Tokyo, Japan), cleaved caspase-3 (1:50) and 8-hydroxy-2 -deoxyguanosine (8-OHdG) (1:50, N45.1, mouse monoclonal, Japan Institute for Control of Aging, Fukuroi, Japan). Slides were incubated with primary antibodies at 4 C overnight, and immunohistochemical staining was performed with the EnVision + system (Dako, Glostrup, Denmark). The sections were then treated with 3,30-diaminobenzidine and counterstained with hematoxylin. Evaluation of immunohistochemical staining. The proportion of positively-stained nuclei for HIF-1α and 8-OHdG, or cytoplasm for pimonidazole and cleaved caspase-3 were assessed in the central region of 10
12 tumours, and semi-quantitatively scored by a pathologist. The proportion of stained cells was evaluated in three fields of hot-spot areas at high power (200 ) and scored from 0 100%. Statistical Analysis. Data were analyzed by ANOVA using Prism 5 software (GraphPad Software, La Jolla, CA, USA). For comparisons between two groups, the differences in mean values were evaluated by Student s t-test and Mann Whitney U test. For comparisons among three or more groups, Bonferroni post-hoc tests were performed for One-way ANOVA. A value of p < 0.05 was considered statistically significant. All values are expressed as means ± SEM. References 1. Vaupel, P., Mayer, A. & Hockel, M. Tumor hypoxia and malignant progression. Methods Enzymol 381, (2004). 2. Harris, A. L. Hypoxia-a key regulatory factor in tumour growth. Nat Rev Cancer 2, (2002). 3. Kitajima, Y. & Miyazaki, K. The critical impact of HIF-1α on gastric cancer biology. Cancers (Basel) 5, (2013). 4. Majmundar, A. J., Wong, W. J. & Simon, M. C. Hypoxia inducible factors and the response to hypoxic stress. Mol Cell 40, (2010). 5. Rankin, E. B. & Giaccia, A. J. Hypoxic control of metastasis. Science 352, (2016). 6. Lu, X. & Kang, Y. Hypoxia and hypoxia-inducible factors (HIFs): master regulators of metastasis. Clin Canecr Res 16, (2010). 7. Rohwer, N. & Cramer, T. HIFs as central regulators of gastric cancer pathogenesis. Cancer Biology & Therapy 10, (2010). 8. Wang, Y. et al. HIF-1α and HIF-2α correlate with migration and invasion in gastric cancer. Cancer Biology & Therapy 10, (2010). 9. Miyake, S. et al. HIF-1α is a crucial factor in the development of peritoneal dissemination via natural metastatic routes in scirrhous gastric cancer. Int J Oncol 43, (2013). 10. Comerford, K. M. et al. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 62, (2002). 11. Nakamura, J. et al. HIF-1 alpha is an unfavorable determinant of relapse in gastric cancer patients who underwent curative surgery followed by adjuvant 5-FU chemotherapy. Int J Cancer 127, (2010). 12. Semenza, G. L. Targeting HIF-1 for cancer therapy. Nature Rev. Cancer 3, (2003). 13. Georgina, N. M. & Wei, L. HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharmaceutica Sinica B 5, (2015). 14. Nable, D. G. & Zhou, Y. D. Natural product-based inhibitors of hypoxia-inducible factor-1 (HIF-1). Curr Drug Targets 7, (2006). 15. Burroughs, S. et al. Hypoxia inducible factor pathway inhibitors as anticancer therapeutics. Future Med Chem 5, (2013). 16. Tanaka, T. et al. The apoptotic effect of HIF-1α inhibition combined with glucose plus insulin treatment on gastric cancer under hypoxic conditions. PLOS ONE. (2015). 17. Warburg, O. On the origin of cancer cells. Science 123, (1956). 18. Ray, P. D., Huang, B. W. & Tsuji, Y. Reaction oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24, (2012). 19. Semenza, G. L. Hypoxia-inducible factor 1: Regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochimica et Biophysica Acta 1813, (2011). 20. Semenza, G. L. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest 123, (2013). 21. Semenza, G. L. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev 51, 1 10 (2010). 22. Hayashi, M. et al. Induction of glucose transporter 1 expression through hypoxia-inducible factor 1α under hypoxic conditions in trophoblast-derived cells. J Endocrinol 183, (2004). 23. Jean, J. C., Rich, C. B. & Joyce-Brady, M. Hypoxia results in an HIF-1-dependent induction of brain-specific aldolase C in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 291, L950 L956 (2006). 24. Kim, J. W., Gao, P., Liu, Y. C., Semenza, G. L. & Dang, C. V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3, (2006). 25. Semenza, G. L. et al. Hypoxia response element in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 271, (1996). 26. Ullah, M. S., Davies, A. J. & Halestrap, A. P. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1α-dependent mechanism. J Biol Chem 281, (2006). 27. Teng, C. M., Wu, C. C., Ko, F. N., Lee, F. Y. & Kuo, S. C. Y. C.-1 a nitric oxide-independent activation of soluble guanylate cyclase, inhibits platelet-rich thrombosis in mice. Eur J Pharmacol 320, (1997). 28. Yeo, E. J., Chun, Y. S., Cho, Y. S., Kim, J. & Lee, J. C. YC-1: A potential anticancer drug targeting hypoxia-inducible factor 1. J. Natl Cancer Inst 95, (2003). 29. Sun, H. L. et al. YC-1 inhibits HIF-1 expression in prostate cancer cells: contribution of Akt/NF-κB signaling to HIF-1α accumulation during hypoxia. Oncogene 26, (2007). 30. Yeo, E. J. et al. YC-1 induces S cell cycle arrest and apoptosis by activating checkpoint kinases. Cancer Res 66, (2006). 31. Sano, H. et al. Rab10, a target of the AS160 Rab GAP is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab 5, (2007). 32. Yanagihara, K. et al. Development and biological analysis of peritoneal metastasis mouse models for human scirrhous stomach cancer. Cancer Sci 96, (2005). 33. Tanaka, T. et al. Low-dose farnesyltransferase inhibitor suppresses HIF-1α and snail expression in triple-negative breast cancer MDA-MB-231 cells in vitro. J Cell Physiol 232, (2017). Acknowledgements We would like to tank Mr. F Mutoh for his valuable contributions to the immunohistochemical studies. We also thank Edanz Group ( for editing a draft of this manuscript. This study was financially supported by JSPS KAKENHI Grants-in-Aid for Scientific Research (Research Project no ). Author Contributions Y.K. and K.W. conceived and designed the experiments. K.W., T.T. and S.A. performed the experiments. Y.K., K.W., T.T. and S.A. analyzed the data. M.K., K.Y. and J.N. contributed to reagents/materials/analysis tools. K.W., Y.K. and H.N. wrote the paper. All authors discussed the results and commented on the manuscript. 11
13 Additional Information Supplementary information accompanies this paper at Competing Interests: The authors declare that they have no competing interests. Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit The Author(s)
Identifying Metabolic Phenotype Switches in Cancer Cells Using the Agilent Seahorse XF Analyzer in an Hypoxic Environment
 Identifying Metabolic Phenotype Switches in Cancer Cells Using the Agilent Seahorse XF Analyzer in an Hypoxic Environment Application Note Introduction Decreased oxygen levels, or hypoxia, and hypoxic-mediated
Identifying Metabolic Phenotype Switches in Cancer Cells Using the Agilent Seahorse XF Analyzer in an Hypoxic Environment Application Note Introduction Decreased oxygen levels, or hypoxia, and hypoxic-mediated
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting. protein3) regulate autophagy and mitophagy in renal tubular cells in. acute kidney injury
 Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury by Masayuki Ishihara 1, Madoka Urushido 2, Kazu Hamada
Mechanisms of Gene Regulation and Signal! Transduction in Hypoxia!
 Mechanisms of Gene Regulation and Signal! Transduction in Hypoxia! Lorenz Poellinger! Dept. of Cell and Molecular Biology! Karolinska Institutet, Stockholm, Sweden! Normoxia - O 2 availability is in balance
Mechanisms of Gene Regulation and Signal! Transduction in Hypoxia! Lorenz Poellinger! Dept. of Cell and Molecular Biology! Karolinska Institutet, Stockholm, Sweden! Normoxia - O 2 availability is in balance
p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO
 Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supplementary Information p47 negatively regulates IKK activation by inducing the lysosomal degradation of polyubiquitinated NEMO Yuri Shibata, Masaaki Oyama, Hiroko Kozuka-Hata, Xiao Han, Yuetsu Tanaka,
Supporting Information. Non-thermal plasma with 2-deoxy-D-glucose synergistically induces cell death by targeting glycolysis in blood cancer cells
 Supporting Information Non-thermal plasma with 2-deoxy-D-glucose synergistically induces cell death by targeting glycolysis in blood cancer cells Neha Kaushik, 1 Su Jae Lee, 2 Tae Gyu Choi 3, Ku Youn Baik,
Supporting Information Non-thermal plasma with 2-deoxy-D-glucose synergistically induces cell death by targeting glycolysis in blood cancer cells Neha Kaushik, 1 Su Jae Lee, 2 Tae Gyu Choi 3, Ku Youn Baik,
SUPPLEMENTARY DATA. Supplementary Table 1. Primers used in qpcr
 Supplementary Table 1. Primers used in qpcr Gene forward primer (5'-3') reverse primer (5'-3') β-actin AGAGGGAAATCGTGCGTGAC CAATAGTGATGACCTGGCCGT Hif-p4h-2 CTGGGCAACTACAGGATAAAC GCGTCCCAGTCTTTATTTAGATA
Supplementary Table 1. Primers used in qpcr Gene forward primer (5'-3') reverse primer (5'-3') β-actin AGAGGGAAATCGTGCGTGAC CAATAGTGATGACCTGGCCGT Hif-p4h-2 CTGGGCAACTACAGGATAAAC GCGTCCCAGTCTTTATTTAGATA
Supplementary Materials
 Supplementary Materials Figure S1. MTT Cell viability assay. To measure the cytotoxic potential of the oxidative treatment, the MTT [3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl tetrazolium bromide] assay
Supplementary Materials Figure S1. MTT Cell viability assay. To measure the cytotoxic potential of the oxidative treatment, the MTT [3-(4,5-dimethylthiazol- 2-yl)-2,5-diphenyl tetrazolium bromide] assay
(A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and a
 Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
Supplementary figure legends Supplementary Figure 1. Expression of Shh signaling components in a panel of gastric cancer. (A) RT-PCR for components of the Shh/Gli pathway in normal fetus cell (MRC-5) and
Plasma exposure levels from individual mice 4 hours post IP administration at the
 Supplemental Figure Legends Figure S1. Plasma exposure levels of MKC-3946 in mice. Plasma exposure levels from individual mice 4 hours post IP administration at the indicated dose mg/kg. Data represent
Supplemental Figure Legends Figure S1. Plasma exposure levels of MKC-3946 in mice. Plasma exposure levels from individual mice 4 hours post IP administration at the indicated dose mg/kg. Data represent
Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsrna-induced retinal degeneration
 Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsrna-induced retinal degeneration The Harvard community has made this article openly available. Please
Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsrna-induced retinal degeneration The Harvard community has made this article openly available. Please
Supplementary Materials and Methods
 Supplementary Materials and Methods Immunoblotting Immunoblot analysis was performed as described previously (1). Due to high-molecular weight of MUC4 (~ 950 kda) and MUC1 (~ 250 kda) proteins, electrophoresis
Supplementary Materials and Methods Immunoblotting Immunoblot analysis was performed as described previously (1). Due to high-molecular weight of MUC4 (~ 950 kda) and MUC1 (~ 250 kda) proteins, electrophoresis
Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle.
 Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle. (a) mrna levels of Dj1 measured by quantitative RT-PCR in soleus, gastrocnemius (Gastroc.) and extensor digitorum longus
Supplementary Figure 1. DJ-1 modulates ROS concentration in mouse skeletal muscle. (a) mrna levels of Dj1 measured by quantitative RT-PCR in soleus, gastrocnemius (Gastroc.) and extensor digitorum longus
Supplementary Figure (OH) 22 nanoparticles did not affect cell viability and apoposis. MDA-MB-231, MCF-7, MCF-10A and BT549 cells were
 Supplementary Figure 1. Gd@C 82 (OH) 22 nanoparticles did not affect cell viability and apoposis. MDA-MB-231, MCF-7, MCF-10A and BT549 cells were treated with PBS, Gd@C 82 (OH) 22, C 60 (OH) 22 or GdCl
Supplementary Figure 1. Gd@C 82 (OH) 22 nanoparticles did not affect cell viability and apoposis. MDA-MB-231, MCF-7, MCF-10A and BT549 cells were treated with PBS, Gd@C 82 (OH) 22, C 60 (OH) 22 or GdCl
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells
 Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
Supplementary Figure 1.TRIM33 binds β-catenin in the nucleus. a & b, Co-IP of endogenous TRIM33 with β-catenin in HT-29 cells (a) and HEK 293T cells (b). TRIM33 was immunoprecipitated, and the amount of
General Laboratory methods Plasma analysis: Gene Expression Analysis: Immunoblot analysis: Immunohistochemistry:
 General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
General Laboratory methods Plasma analysis: Plasma insulin (Mercodia, Sweden), leptin (duoset, R&D Systems Europe, Abingdon, United Kingdom), IL-6, TNFα and adiponectin levels (Quantikine kits, R&D Systems
Supporting Information
 Supporting Information van der Windt et al. 10.1073/pnas.1221740110 SI Materials and Methods Mice and Reagents. C57BL/6 and major histocompatibility complex class I-restricted OVA-specific T-cell receptor
Supporting Information van der Windt et al. 10.1073/pnas.1221740110 SI Materials and Methods Mice and Reagents. C57BL/6 and major histocompatibility complex class I-restricted OVA-specific T-cell receptor
ANGPTL2 increases bone metastasis of breast cancer cells through. Tetsuro Masuda, Motoyoshi Endo, Yutaka Yamamoto, Haruki Odagiri, Tsuyoshi
 Masuda et al. Supplementary information for ANGPTL2 increases bone metastasis of breast cancer cells through enhancing CXCR4 signaling Tetsuro Masuda, Motoyoshi Endo, Yutaka Yamamoto, Haruki Odagiri, Tsuyoshi
Masuda et al. Supplementary information for ANGPTL2 increases bone metastasis of breast cancer cells through enhancing CXCR4 signaling Tetsuro Masuda, Motoyoshi Endo, Yutaka Yamamoto, Haruki Odagiri, Tsuyoshi
(a) Significant biological processes (upper panel) and disease biomarkers (lower panel)
 Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
Supplementary Figure 1. Functional enrichment analyses of secretomic proteins. (a) Significant biological processes (upper panel) and disease biomarkers (lower panel) 2 involved by hrab37-mediated secretory
supplementary information
 DOI: 10.1038/ncb1875 Figure S1 (a) The 79 surgical specimens from NSCLC patients were analysed by immunohistochemistry with an anti-p53 antibody and control serum (data not shown). The normal bronchi served
DOI: 10.1038/ncb1875 Figure S1 (a) The 79 surgical specimens from NSCLC patients were analysed by immunohistochemistry with an anti-p53 antibody and control serum (data not shown). The normal bronchi served
HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation
 SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
SUPPLEMENTARY INFORMATION Materials and Methods Human cell lines and culture conditions HCC1937 is the HCC1937-pcDNA3 cell line, which was derived from a breast cancer with a mutation in exon 20 of BRCA1
Post-translational modifications of proteins in gene regulation under hypoxic conditions
 203 Review Article Post-translational modifications of proteins in gene regulation under hypoxic conditions 1, 2) Olga S. Safronova 1) Department of Cellular Physiological Chemistry, Tokyo Medical and
203 Review Article Post-translational modifications of proteins in gene regulation under hypoxic conditions 1, 2) Olga S. Safronova 1) Department of Cellular Physiological Chemistry, Tokyo Medical and
Supplementary Figure 1
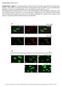 Supplementary Figure 1. (A) Representative confocal analysis of cardiac mesenchymal cells from nondiabetic (ND-CMSC) and diabetic (D-CMSC) immunostained with anti-h3k9ac and anti-h3k27me3 antibodies. Analysis
Supplementary Figure 1. (A) Representative confocal analysis of cardiac mesenchymal cells from nondiabetic (ND-CMSC) and diabetic (D-CMSC) immunostained with anti-h3k9ac and anti-h3k27me3 antibodies. Analysis
Oncolytic Adenovirus Complexes Coated with Lipids and Calcium Phosphate for Cancer Gene Therapy
 Oncolytic Adenovirus Complexes Coated with Lipids and Calcium Phosphate for Cancer Gene Therapy Jianhua Chen, Pei Gao, Sujing Yuan, Rongxin Li, Aimin Ni, Liang Chu, Li Ding, Ying Sun, Xin-Yuan Liu, Yourong
Oncolytic Adenovirus Complexes Coated with Lipids and Calcium Phosphate for Cancer Gene Therapy Jianhua Chen, Pei Gao, Sujing Yuan, Rongxin Li, Aimin Ni, Liang Chu, Li Ding, Ying Sun, Xin-Yuan Liu, Yourong
Aberrant Promoter CpG Methylation is a Mechanism for Lack of Hypoxic Induction of
 Aberrant Promoter CpG Methylation is a Mechanism for Lack of Hypoxic Induction of PHD3 in a Diverse Set of Malignant Cells Abstract The prolyl-hydroxylase domain family of enzymes (PHD1-3) plays an important
Aberrant Promoter CpG Methylation is a Mechanism for Lack of Hypoxic Induction of PHD3 in a Diverse Set of Malignant Cells Abstract The prolyl-hydroxylase domain family of enzymes (PHD1-3) plays an important
What is the Warburg Effect
 What is the Warburg Effect Roles nutrients play in the biochemistry of a cell Thus, proliferating cells must acquire more nutrients, convert them into biosynthetic building blocks, and coordinate the reactions
What is the Warburg Effect Roles nutrients play in the biochemistry of a cell Thus, proliferating cells must acquire more nutrients, convert them into biosynthetic building blocks, and coordinate the reactions
Islet viability assay and Glucose Stimulated Insulin Secretion assay RT-PCR and Western Blot
 Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Islet viability assay and Glucose Stimulated Insulin Secretion assay Islet cell viability was determined by colorimetric (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide assay using CellTiter
Supplementary Information
 Supplementary Information Supplementary Figure 1. Effect of mir mimics and anti-mirs on DTPs a, Representative fluorescence microscopy images of GFP vector control or mir mimicexpressing parental and DTP
Supplementary Information Supplementary Figure 1. Effect of mir mimics and anti-mirs on DTPs a, Representative fluorescence microscopy images of GFP vector control or mir mimicexpressing parental and DTP
Part-4. Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death
 Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Part-4 Cell cycle regulatory protein 5 (Cdk5) A novel target of ERK in Carb induced cell death 95 1. Introduction The process of replicating DNA and dividing cells can be described as a series of coordinated
Human recombinat MIF protein (hrmif), MW: Da. m/z. hrmif ( Da) + 4-IPP (282 Da) MWtot ~ Da. m/z.
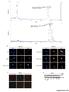 Intensity % Intensity % A Human recombinat MIF protein (hrmif), MW: 12428.31 Da m/z hrmif (12428.31 Da) + 4-IPP (282 Da) MWtot ~ 12715.21 Da m/z B HTC/C3 DAPI phistone-h3 Merge HTC/C3 DAPI phistone-h3
Intensity % Intensity % A Human recombinat MIF protein (hrmif), MW: 12428.31 Da m/z hrmif (12428.31 Da) + 4-IPP (282 Da) MWtot ~ 12715.21 Da m/z B HTC/C3 DAPI phistone-h3 Merge HTC/C3 DAPI phistone-h3
Cell Culture. The human thyroid follicular carcinoma cell lines FTC-238, FTC-236 and FTC-
 Supplemental material and methods Reagents. Hydralazine was purchased from Sigma-Aldrich. Cell Culture. The human thyroid follicular carcinoma cell lines FTC-238, FTC-236 and FTC- 133, human thyroid medullary
Supplemental material and methods Reagents. Hydralazine was purchased from Sigma-Aldrich. Cell Culture. The human thyroid follicular carcinoma cell lines FTC-238, FTC-236 and FTC- 133, human thyroid medullary
TITLE: Overcoming Resistance to Inhibitors of the Akt Protein Kinase by Modulation of the Pim Kinase Pathway
 AWARD NUMBER: W81XWH-12-1-0560 TITLE: Overcoming Resistance to Inhibitors of the Akt Protein Kinase by Modulation of the Pim Kinase Pathway PRINCIPAL INVESTIGATOR: Andrew S. Kraft, MD CONTRACTING ORGANIZATION:
AWARD NUMBER: W81XWH-12-1-0560 TITLE: Overcoming Resistance to Inhibitors of the Akt Protein Kinase by Modulation of the Pim Kinase Pathway PRINCIPAL INVESTIGATOR: Andrew S. Kraft, MD CONTRACTING ORGANIZATION:
RNA extraction, RT-PCR and real-time PCR. Total RNA were extracted using
 Supplementary Information Materials and Methods RNA extraction, RT-PCR and real-time PCR. Total RNA were extracted using Trizol reagent (Invitrogen,Carlsbad, CA) according to the manufacturer's instructions.
Supplementary Information Materials and Methods RNA extraction, RT-PCR and real-time PCR. Total RNA were extracted using Trizol reagent (Invitrogen,Carlsbad, CA) according to the manufacturer's instructions.
Additional methods appearing in the supplement are described in the Experimental Procedures section of the manuscript.
 Supplemental Materials: I. Supplemental Methods II. Supplemental Figure Legends III. Supplemental Figures Supplemental Methods Cell Culture and Transfections for Wild Type and JNK1-/-,JNK2-/- MEFs: The
Supplemental Materials: I. Supplemental Methods II. Supplemental Figure Legends III. Supplemental Figures Supplemental Methods Cell Culture and Transfections for Wild Type and JNK1-/-,JNK2-/- MEFs: The
AperTO - Archivio Istituzionale Open Access dell'università di Torino
 AperTO - Archivio Istituzionale Open Access dell'università di Torino From the nucleus to the mitochondria and backthe odyssey of a multitask STAT3 This is the author's manuscript Original Citation: From
AperTO - Archivio Istituzionale Open Access dell'università di Torino From the nucleus to the mitochondria and backthe odyssey of a multitask STAT3 This is the author's manuscript Original Citation: From
A549 and A549-fLuc cells were maintained in high glucose Dulbecco modified
 Cell culture and animal model A549 and A549-fLuc cells were maintained in high glucose Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum at 37 C in humidified atmosphere containing
Cell culture and animal model A549 and A549-fLuc cells were maintained in high glucose Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum at 37 C in humidified atmosphere containing
(A) PCR primers (arrows) designed to distinguish wild type (P1+P2), targeted (P1+P2) and excised (P1+P3)14-
 1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
1 Supplemental Figure Legends Figure S1. Mammary tumors of ErbB2 KI mice with 14-3-3σ ablation have elevated ErbB2 transcript levels and cell proliferation (A) PCR primers (arrows) designed to distinguish
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism SUPPLEMENTARY FIGURES, LEGENDS AND METHODS
 A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
A Hepatocyte Growth Factor Receptor (Met) Insulin Receptor hybrid governs hepatic glucose metabolism Arlee Fafalios, Jihong Ma, Xinping Tan, John Stoops, Jianhua Luo, Marie C. DeFrances and Reza Zarnegar
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/8/364/ra18/dc1 Supplementary Materials for The tyrosine phosphatase (Pez) inhibits metastasis by altering protein trafficking Leila Belle, Naveid Ali, Ana Lonic,
www.sciencesignaling.org/cgi/content/full/8/364/ra18/dc1 Supplementary Materials for The tyrosine phosphatase (Pez) inhibits metastasis by altering protein trafficking Leila Belle, Naveid Ali, Ana Lonic,
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk
 Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
Supplementary Figure 1. Normal T lymphocyte populations in Dapk -/- mice. (a) Normal thymic development in Dapk -/- mice. Thymocytes from WT and Dapk -/- mice were stained for expression of CD4 and CD8.
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
doi:10.1038/nature12652 Supplementary Figure 1. PRDM16 interacts with endogenous EHMT1 in brown adipocytes. Immunoprecipitation of PRDM16 complex by flag antibody (M2) followed by Western blot analysis
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 Page 1 09/10/2013 AD Award Number: W81XWH-12-1-0453 TITLE: The cytoplasm translocation of the androgen receptor cofactor p44 as a target for prostate cancer treatment PRINCIPAL INVESTIGATOR: Zhengxin Wang
Page 1 09/10/2013 AD Award Number: W81XWH-12-1-0453 TITLE: The cytoplasm translocation of the androgen receptor cofactor p44 as a target for prostate cancer treatment PRINCIPAL INVESTIGATOR: Zhengxin Wang
Argininosuccinate synthetase 1 suppression and arginine restriction inhibit cell
 Argininosuccinate synthetase 1 suppression and arginine restriction inhibit cell migration in gastric cancer cell lines Yan-Shen Shan 1, Hui-Ping Hsu 1, Ming-Derg Lai 2,3, Meng-Chi Yen 2,4, Wei-Ching Chen
Argininosuccinate synthetase 1 suppression and arginine restriction inhibit cell migration in gastric cancer cell lines Yan-Shen Shan 1, Hui-Ping Hsu 1, Ming-Derg Lai 2,3, Meng-Chi Yen 2,4, Wei-Ching Chen
In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic
 Glycolysis 1 In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic glycolysis. If this pyruvate is converted instead
Glycolysis 1 In glycolysis, glucose is converted to pyruvate. If the pyruvate is reduced to lactate, the pathway does not require O 2 and is called anaerobic glycolysis. If this pyruvate is converted instead
A. Generation and characterization of Ras-expressing autophagycompetent
 Supplemental Material Supplemental Figure Legends Fig. S1 A. Generation and characterization of Ras-expressing autophagycompetent and -deficient cell lines. HA-tagged H-ras V12 was stably expressed in
Supplemental Material Supplemental Figure Legends Fig. S1 A. Generation and characterization of Ras-expressing autophagycompetent and -deficient cell lines. HA-tagged H-ras V12 was stably expressed in
Life Sciences METABOLISM. Transform Your Metabolic Research
 METABOLISM Transform Your Metabolic Research COMPREHENSIVE TOOLS FOR ADVANCING YOUR METABOLIC RESEARCH Metabolism refers to a set of chemical reactions which are responsible for transforming carbohydrates,
METABOLISM Transform Your Metabolic Research COMPREHENSIVE TOOLS FOR ADVANCING YOUR METABOLIC RESEARCH Metabolism refers to a set of chemical reactions which are responsible for transforming carbohydrates,
Enterprise Interest None
 Enterprise Interest None Hypoxic Gene Signature of Primary and Metastatic Melanoma Cell Lines: Focusing on HIF1-Beta and NDRG-1 Mustafa Emre Ercin,MD Department of Pathology, Karadeniz Technical University
Enterprise Interest None Hypoxic Gene Signature of Primary and Metastatic Melanoma Cell Lines: Focusing on HIF1-Beta and NDRG-1 Mustafa Emre Ercin,MD Department of Pathology, Karadeniz Technical University
SHREE ET AL, SUPPLEMENTAL MATERIALS. (A) Workflow for tumor cell line derivation and orthotopic implantation.
 SHREE ET AL, SUPPLEMENTAL MATERIALS SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure 1. Derivation and characterization of TS1-TGL and TS2-TGL PyMT cell lines and development of an orthotopic
SHREE ET AL, SUPPLEMENTAL MATERIALS SUPPLEMENTAL FIGURE AND TABLE LEGENDS Supplemental Figure 1. Derivation and characterization of TS1-TGL and TS2-TGL PyMT cell lines and development of an orthotopic
NLRX1: 5 -GCTCCATGGCTTAGAGCATC-3 (forward) 5 -AACTCCTCCTCCGTCCTGAT-3 (reverse) β-actin
 NLRX1 β-actin 1 2 3 4 5 6 1 2 3 4 5 6 NLRX1 (667 bp) β-actin (523 bp) Supplementary Figure 1: Expression of NLRX1 in human cell lines. 1: HeLa, 2: HEK293T, 3: MCF-7, 4:Ramos, 5:Jurkat, 6: THP1. The following
NLRX1 β-actin 1 2 3 4 5 6 1 2 3 4 5 6 NLRX1 (667 bp) β-actin (523 bp) Supplementary Figure 1: Expression of NLRX1 in human cell lines. 1: HeLa, 2: HEK293T, 3: MCF-7, 4:Ramos, 5:Jurkat, 6: THP1. The following
Supplementary Figure 1: Digitoxin induces apoptosis in primary human melanoma cells but not in normal melanocytes, which express lower levels of the
 Supplementary Figure 1: Digitoxin induces apoptosis in primary human melanoma cells but not in normal melanocytes, which express lower levels of the cardiac glycoside target, ATP1A1. (a) The percentage
Supplementary Figure 1: Digitoxin induces apoptosis in primary human melanoma cells but not in normal melanocytes, which express lower levels of the cardiac glycoside target, ATP1A1. (a) The percentage
Supplemental Data. TGF-β-mediated mir-181a expression promotes breast cancer metastasis by targeting Bim.
 Supplemental Data TGF-β-mediated mir-181a expression promotes breast cancer metastasis by targeting Bim. Molly A. Taylor 1, Khalid Sossey-Alaoui 2, Cheryl L. Thompson 3, David Danielpour 4, and William
Supplemental Data TGF-β-mediated mir-181a expression promotes breast cancer metastasis by targeting Bim. Molly A. Taylor 1, Khalid Sossey-Alaoui 2, Cheryl L. Thompson 3, David Danielpour 4, and William
SUPPLEMENTARY INFORMATION
 Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
Supplementary Figures Supplementary Figure S1. Binding of full-length OGT and deletion mutants to PIP strips (Echelon Biosciences). Supplementary Figure S2. Binding of the OGT (919-1036) fragments with
New tools bring greater understanding to cellular metabolism research
 New tools bring greater understanding to cellular metabolism research Mourad Ferhat, Ph.D, 7 Juin 2017 FDSS Users Meeting, Hamamatsu mourad.ferhat@promega.com Today s talk : focus on new cell-based assays
New tools bring greater understanding to cellular metabolism research Mourad Ferhat, Ph.D, 7 Juin 2017 FDSS Users Meeting, Hamamatsu mourad.ferhat@promega.com Today s talk : focus on new cell-based assays
EPIGENETIC RE-EXPRESSION OF HIF-2α SUPPRESSES SOFT TISSUE SARCOMA GROWTH
 EPIGENETIC RE-EXPRESSION OF HIF-2α SUPPRESSES SOFT TISSUE SARCOMA GROWTH Supplementary Figure 1. Supplementary Figure 1. Characterization of KP and KPH2 autochthonous UPS tumors. a) Genotyping of KPH2
EPIGENETIC RE-EXPRESSION OF HIF-2α SUPPRESSES SOFT TISSUE SARCOMA GROWTH Supplementary Figure 1. Supplementary Figure 1. Characterization of KP and KPH2 autochthonous UPS tumors. a) Genotyping of KPH2
Supplementary Data Table of Contents:
 Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
Supplementary Data Table of Contents: - Supplementary Methods - Supplementary Figures S1(A-B) - Supplementary Figures S2 (A-B) - Supplementary Figures S3 - Supplementary Figures S4(A-B) - Supplementary
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained
 Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
Supplementary Figure 1. Confocal immunofluorescence showing mitochondrial translocation of Drp1. Cardiomyocytes treated with H 2 O 2 were prestained with MitoTracker (red), then were immunostained with
SUPPLEMENTARY INFORMATION
 DOI:.38/ncb3399 a b c d FSP DAPI 5mm mm 5mm 5mm e Correspond to melanoma in-situ Figure a DCT FSP- f MITF mm mm MlanaA melanoma in-situ DCT 5mm FSP- mm mm mm mm mm g melanoma in-situ MITF MlanaA mm mm
DOI:.38/ncb3399 a b c d FSP DAPI 5mm mm 5mm 5mm e Correspond to melanoma in-situ Figure a DCT FSP- f MITF mm mm MlanaA melanoma in-situ DCT 5mm FSP- mm mm mm mm mm g melanoma in-situ MITF MlanaA mm mm
Appendix Table of Contents. 1. Appendix Figure legends S1-S13 and Appendix Table S1 and S2. 2. Appendix Figures S1-S13
 Appendix Table of Contents. Appendix Figure legends S-S3 and Appendix Table S and S. Appendix Figures S-S3 . Appendix Figure legends S-S3 and Appendix Table S and S Appendix Figure S. Western blot analysis
Appendix Table of Contents. Appendix Figure legends S-S3 and Appendix Table S and S. Appendix Figures S-S3 . Appendix Figure legends S-S3 and Appendix Table S and S Appendix Figure S. Western blot analysis
SUPPLEMENTARY FIGURES AND TABLES
 SUPPLEMENTARY FIGURES AND TABLES Supplementary Figure S1: CaSR expression in neuroblastoma models. A. Proteins were isolated from three neuroblastoma cell lines and from the liver metastasis of a MYCN-non
SUPPLEMENTARY FIGURES AND TABLES Supplementary Figure S1: CaSR expression in neuroblastoma models. A. Proteins were isolated from three neuroblastoma cell lines and from the liver metastasis of a MYCN-non
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2607 Figure S1 Elf5 loss promotes EMT in mammary epithelium while Elf5 overexpression inhibits TGFβ induced EMT. (a, c) Different confocal slices through the Z stack image. (b, d) 3D rendering
DOI: 10.1038/ncb2607 Figure S1 Elf5 loss promotes EMT in mammary epithelium while Elf5 overexpression inhibits TGFβ induced EMT. (a, c) Different confocal slices through the Z stack image. (b, d) 3D rendering
Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice.
 Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
Supplementary Figures: Supplementary Figure S I: Effects of D4F on body weight and serum lipids in apoe -/- mice. Male apoe -/- mice were fed a high-fat diet for 8 weeks, and given PBS (model group) or
glucose glucose 6-phospho fructose 6-phosphate fructose 1,6-bisphosphate glyceraldehyde 3-phosphate 1,3-bisphosphoglycerate 3-phosphoglycerate
 Cells glucose glucose glucose 6-phospho glycogen pentose phosphate T 1-phosphate 6-phosphate gluconate CC T CC+PD-1 pathway Isobar: fructose 1,6-diphosphate; glucose 1,6-diphosphate DHAP lactate fructose
Cells glucose glucose glucose 6-phospho glycogen pentose phosphate T 1-phosphate 6-phosphate gluconate CC T CC+PD-1 pathway Isobar: fructose 1,6-diphosphate; glucose 1,6-diphosphate DHAP lactate fructose
Advances in Computer Science Research, volume 59 7th International Conference on Education, Management, Computer and Medicine (EMCM 2016)
 7th International Conference on Education, Management, Computer and Medicine (EMCM 2016) Expression of Beta-Adrenergic Receptor in Glioma LN229 Cells and Its Effect on Cell Proliferation Ping Wang1, Qingluan
7th International Conference on Education, Management, Computer and Medicine (EMCM 2016) Expression of Beta-Adrenergic Receptor in Glioma LN229 Cells and Its Effect on Cell Proliferation Ping Wang1, Qingluan
Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2)
 Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
Supplemental Methods Cells and reagents. Synaptopodin knockdown (1) and dynamin knockdown (2) podocytes were cultured as described previously. Staurosporine, angiotensin II and actinomycin D were all obtained
hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This
 SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
SUPPLEMENTAL FIGURE LEGEND Fig. S1. Generation and characterization of. (A) Coomassie staining of soluble hexahistidine tagged GRP78 devoid of the KDEL motif (GRP78-His) on SDS-PAGE. This protein was expressed
Supplemental information
 Carcinoemryonic antigen-related cell adhesion molecule 6 (CEACAM6) promotes EGF receptor signaling of oral squamous cell carcinoma metastasis via the complex N-glycosylation y Chiang et al. Supplemental
Carcinoemryonic antigen-related cell adhesion molecule 6 (CEACAM6) promotes EGF receptor signaling of oral squamous cell carcinoma metastasis via the complex N-glycosylation y Chiang et al. Supplemental
Impact factor: Reporter:4A1H0019 Chen Zi Hao 4A1H0023 Huang Wan ting 4A1H0039 Sue Yi Zhu 4A1H0070 Lin Guan cheng 4A1H0077 Chen Bo xuan
 Curcumin Protects Neonatal Rat Cardiomyocytes against High Glucose-Induced Apoptosis via PI3K/Akt Signalling Pathway Wei Yu,1,2 Wenliang Zha,1 Zhiqiang Ke,1 Qing Min,2 Cairong Li,1 Huirong Sun,3 and Chao
Curcumin Protects Neonatal Rat Cardiomyocytes against High Glucose-Induced Apoptosis via PI3K/Akt Signalling Pathway Wei Yu,1,2 Wenliang Zha,1 Zhiqiang Ke,1 Qing Min,2 Cairong Li,1 Huirong Sun,3 and Chao
MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands)
 Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Supplemental data Materials and Methods Cell culture MTC-TT and TPC-1 cell lines were cultured in RPMI medium (Gibco, Breda, The Netherlands) supplemented with 15% or 10% (for TPC-1) fetal bovine serum
Supplementary Table 1. The primers used for quantitative RT-PCR. Gene name Forward (5 > 3 ) Reverse (5 > 3 )
 770 771 Supplementary Table 1. The primers used for quantitative RT-PCR. Gene name Forward (5 > 3 ) Reverse (5 > 3 ) Human CXCL1 GCGCCCAAACCGAAGTCATA ATGGGGGATGCAGGATTGAG PF4 CCCCACTGCCCAACTGATAG TTCTTGTACAGCGGGGCTTG
770 771 Supplementary Table 1. The primers used for quantitative RT-PCR. Gene name Forward (5 > 3 ) Reverse (5 > 3 ) Human CXCL1 GCGCCCAAACCGAAGTCATA ATGGGGGATGCAGGATTGAG PF4 CCCCACTGCCCAACTGATAG TTCTTGTACAGCGGGGCTTG
Simultaneous blockade of PD-1 and VEGFR2 induces synergistic. Short title: Synergistic antitumour effect by dual blockade of PD-1 and VEGFR2
 carticle Simultaneous blockade of PD-1 and VEGFR2 induces synergistic antitumour effect in vivo 1 Short title: Synergistic antitumour effect by dual blockade of PD-1 and VEGFR2 S. Yasuda 1, M. Sho 1, I.
carticle Simultaneous blockade of PD-1 and VEGFR2 induces synergistic antitumour effect in vivo 1 Short title: Synergistic antitumour effect by dual blockade of PD-1 and VEGFR2 S. Yasuda 1, M. Sho 1, I.
T H E J O U R N A L O F C E L L B I O L O G Y
 Supplemental material Díaz et al., http://www.jcb.org/cgi/content/full/jcb.201209151/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Hypoxia induces invadopodia formation in different epithelial
Supplemental material Díaz et al., http://www.jcb.org/cgi/content/full/jcb.201209151/dc1 T H E J O U R N A L O F C E L L B I O L O G Y Figure S1. Hypoxia induces invadopodia formation in different epithelial
Supporting Information. Metabolic plasticity in CLL: Adaptation to the hypoxic niche
 Supporting Information Metabolic plasticity in CLL: Adaptation to the hypoxic niche Katarzyna M. Koczula a, Christian Ludwig a, Rachel Hayden b, Laura Cronin b, Guy Pratt a,d, Helen Parry a, Daniel Tennant
Supporting Information Metabolic plasticity in CLL: Adaptation to the hypoxic niche Katarzyna M. Koczula a, Christian Ludwig a, Rachel Hayden b, Laura Cronin b, Guy Pratt a,d, Helen Parry a, Daniel Tennant
Supplementary Figure 1. Deletion of Smad3 prevents B16F10 melanoma invasion and metastasis in a mouse s.c. tumor model.
 A B16F1 s.c. Lung LN Distant lymph nodes Colon B B16F1 s.c. Supplementary Figure 1. Deletion of Smad3 prevents B16F1 melanoma invasion and metastasis in a mouse s.c. tumor model. Highly invasive growth
A B16F1 s.c. Lung LN Distant lymph nodes Colon B B16F1 s.c. Supplementary Figure 1. Deletion of Smad3 prevents B16F1 melanoma invasion and metastasis in a mouse s.c. tumor model. Highly invasive growth
SUPPLEMENTARY INFORMATION. Supplementary Figures S1-S9. Supplementary Methods
 SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
SUPPLEMENTARY INFORMATION SUMO1 modification of PTEN regulates tumorigenesis by controlling its association with the plasma membrane Jian Huang 1,2#, Jie Yan 1,2#, Jian Zhang 3#, Shiguo Zhu 1, Yanli Wang
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation.
 Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
Supplementary Figure 1: si-craf but not si-braf sensitizes tumor cells to radiation. (a) Embryonic fibroblasts isolated from wildtype (WT), BRAF -/-, or CRAF -/- mice were irradiated (6 Gy) and DNA damage
p = formed with HCI-001 p = Relative # of blood vessels that formed with HCI-002 Control Bevacizumab + 17AAG Bevacizumab 17AAG
 A.. Relative # of ECs associated with HCI-001 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0.0 ol b p < 0.001 Relative # of blood vessels that formed with HCI-001 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0.0 l b p = 0.002 Control IHC:
A.. Relative # of ECs associated with HCI-001 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0.0 ol b p < 0.001 Relative # of blood vessels that formed with HCI-001 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0.0 l b p = 0.002 Control IHC:
http / / cjbmb. bjmu. edu. cn Chinese Journal of Biochemistry and Molecular Biology COX-2 NTera-2 NTera-2 RT-PCR FasL caspase-8 caspase-3 PARP.
 ISSN 1007-7626 CN 11-3870 / Q http / / cjbmb bjmu edu cn Chinese Journal of Biochemistry and Molecular Biology 2012 7 28 7 630 ~ 636 NTera-2 ** ** * 410081 COX-2 NTera-2 MTT NTera-2 NTera-2 Hoechest 33258
ISSN 1007-7626 CN 11-3870 / Q http / / cjbmb bjmu edu cn Chinese Journal of Biochemistry and Molecular Biology 2012 7 28 7 630 ~ 636 NTera-2 ** ** * 410081 COX-2 NTera-2 MTT NTera-2 NTera-2 Hoechest 33258
TIGAR's promiscuity Bolaños, Juan P.
 TIGAR's promiscuity Bolaños, Juan P. TIGAR [TP53 (tumour protein 53)-induced glycolysis and apoptosis regulator] is an important survival factor for cancer cells. The enzymatic activity supported by sequence
TIGAR's promiscuity Bolaños, Juan P. TIGAR [TP53 (tumour protein 53)-induced glycolysis and apoptosis regulator] is an important survival factor for cancer cells. The enzymatic activity supported by sequence
Supporting Information
 Supporting Information Identification of Novel ROS Inducers: Quinone Derivatives Tethered to Long Hydrocarbon Chains Yeonsun Hong,, Sandip Sengupta,, Wooyoung Hur, *, Taebo Sim,, * KU-KIST Graduate School
Supporting Information Identification of Novel ROS Inducers: Quinone Derivatives Tethered to Long Hydrocarbon Chains Yeonsun Hong,, Sandip Sengupta,, Wooyoung Hur, *, Taebo Sim,, * KU-KIST Graduate School
Supplementary Figure 1
 Supplementary Figure 1 Expression of apoptosis-related genes in tumor T reg cells. (a) Identification of FOXP3 T reg cells by FACS. CD45 + cells were gated as enriched lymphoid cell populations with low-granularity.
Supplementary Figure 1 Expression of apoptosis-related genes in tumor T reg cells. (a) Identification of FOXP3 T reg cells by FACS. CD45 + cells were gated as enriched lymphoid cell populations with low-granularity.
CUE domain-containing protein 2 promotes the Warburg effect and tumorigenesis
 Article CUE domain-containing protein 2 promotes the Warburg effect and tumorigenesis Xiuying Zhong 1,, Shengya Tian 1,, Xiang Zhang 1, Xinwei Diao 2, Fangting Dong 2, Jie Yang 2, Zhaoyong Li 1, Linchong
Article CUE domain-containing protein 2 promotes the Warburg effect and tumorigenesis Xiuying Zhong 1,, Shengya Tian 1,, Xiang Zhang 1, Xinwei Diao 2, Fangting Dong 2, Jie Yang 2, Zhaoyong Li 1, Linchong
Supplementary data Supplementary Figure 1 Supplementary Figure 2
 Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Supplementary data Supplementary Figure 1 SPHK1 sirna increases RANKL-induced osteoclastogenesis in RAW264.7 cell culture. (A) RAW264.7 cells were transfected with oligocassettes containing SPHK1 sirna
Cell Metabolism Assays. for. Immunology. Research
 the immunity-metabolism link Cell Metabolism Assays for Immunology Research GOLD STANDARD ASSAYS FOR MEASURING METABOLIC RePROGRAMMING Metabolic Signatures Cell activation, amplification, and effector
the immunity-metabolism link Cell Metabolism Assays for Immunology Research GOLD STANDARD ASSAYS FOR MEASURING METABOLIC RePROGRAMMING Metabolic Signatures Cell activation, amplification, and effector
Cancer as a Metabolic Disease
 Cancer as a Metabolic Disease On the Origin, Management and Prevention of Cancer Thomas N. Seyfried @WILEY "" Forword Preface xiii xv 1. Images of Cancer 1 How Cancer is Viewed 2 References 13 2. Confusion
Cancer as a Metabolic Disease On the Origin, Management and Prevention of Cancer Thomas N. Seyfried @WILEY "" Forword Preface xiii xv 1. Images of Cancer 1 How Cancer is Viewed 2 References 13 2. Confusion
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3021 Supplementary figure 1 Characterisation of TIMPless fibroblasts. a) Relative gene expression of TIMPs1-4 by real time quantitative PCR (RT-qPCR) in WT or ΔTimp fibroblasts (mean ±
DOI: 10.1038/ncb3021 Supplementary figure 1 Characterisation of TIMPless fibroblasts. a) Relative gene expression of TIMPs1-4 by real time quantitative PCR (RT-qPCR) in WT or ΔTimp fibroblasts (mean ±
Overexpression of Mcl-1 confers resistance to BRAFV600E inhibitors alone and in combination with MEK1/2 inhibitors in melanoma
 Overexpression of Mcl-1 confers resistance to BRAFV600E inhibitors alone and in combination with MEK1/2 inhibitors in melanoma The Harvard community has made this article openly available. Please share
Overexpression of Mcl-1 confers resistance to BRAFV600E inhibitors alone and in combination with MEK1/2 inhibitors in melanoma The Harvard community has made this article openly available. Please share
HEK293FT cells were transiently transfected with reporters, N3-ICD construct and
 Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Supplementary Information Luciferase reporter assay HEK293FT cells were transiently transfected with reporters, N3-ICD construct and increased amounts of wild type or kinase inactive EGFR. Transfections
Antibodies for Unfolded Protein Response
 Novus-lu-2945 Antibodies for Unfolded rotein Response Unfolded roteins ER lumen GR78 IRE-1 GR78 ERK Cytosol GR78 TRAF2 ASK1 JNK Activator Intron RIDD elf2α Degraded mrna XB1 mrna Translation XB1-S (p50)
Novus-lu-2945 Antibodies for Unfolded rotein Response Unfolded roteins ER lumen GR78 IRE-1 GR78 ERK Cytosol GR78 TRAF2 ASK1 JNK Activator Intron RIDD elf2α Degraded mrna XB1 mrna Translation XB1-S (p50)
Type of file: PDF Size of file: 0 KB Title of file for HTML: Supplementary Information Description: Supplementary Figures
 Type of file: PDF Size of file: 0 KB Title of file for HTML: Supplementary Information Description: Supplementary Figures Supplementary Figure 1 mir-128-3p is highly expressed in chemoresistant, metastatic
Type of file: PDF Size of file: 0 KB Title of file for HTML: Supplementary Information Description: Supplementary Figures Supplementary Figure 1 mir-128-3p is highly expressed in chemoresistant, metastatic
SUPPLEMENTARY INFORMATION
 DOI: 1.138/ncb3355 a S1A8 + cells/ total.1.8.6.4.2 b S1A8/?-Actin c % T-cell proliferation 3 25 2 15 1 5 T cells Supplementary Figure 1 Inter-tumoral heterogeneity of MDSC accumulation in mammary tumor
DOI: 1.138/ncb3355 a S1A8 + cells/ total.1.8.6.4.2 b S1A8/?-Actin c % T-cell proliferation 3 25 2 15 1 5 T cells Supplementary Figure 1 Inter-tumoral heterogeneity of MDSC accumulation in mammary tumor
Chemical Chaperones Mitigate Experimental Asthma By Attenuating Endoplasmic
 Chemical Chaperones Mitigate Experimental Asthma By Attenuating Endoplasmic Reticulum Stress Lokesh Makhija, BE, Veda Krishnan, MSc, Rakhshinda Rehman, MTech, Samarpana Chakraborty, MSc, Shuvadeep Maity,
Chemical Chaperones Mitigate Experimental Asthma By Attenuating Endoplasmic Reticulum Stress Lokesh Makhija, BE, Veda Krishnan, MSc, Rakhshinda Rehman, MTech, Samarpana Chakraborty, MSc, Shuvadeep Maity,
Expanded View Figures
 Shao-Ming Shen et al Role of I in MT of cancers MO reports xpanded View igures igure V1. nalysis of the expression of I isoforms in cancer cells and their interaction with PTN. RT PR detection of Ish and
Shao-Ming Shen et al Role of I in MT of cancers MO reports xpanded View igures igure V1. nalysis of the expression of I isoforms in cancer cells and their interaction with PTN. RT PR detection of Ish and
Figure S1. ERBB3 mrna levels are elevated in Luminal A breast cancers harboring ERBB3
 Supplemental Figure Legends. Figure S1. ERBB3 mrna levels are elevated in Luminal A breast cancers harboring ERBB3 ErbB3 gene copy number gain. Supplemental Figure S1. ERBB3 mrna levels are elevated in
Supplemental Figure Legends. Figure S1. ERBB3 mrna levels are elevated in Luminal A breast cancers harboring ERBB3 ErbB3 gene copy number gain. Supplemental Figure S1. ERBB3 mrna levels are elevated in
PD-L1 Analyte Control DR
 Quality in Control PD-L1 Analyte Control DR PD-L1_PI_v2 Product Codes: HCL019, HCL020 and HCL021 Contents PD-L1 Analyte Control DR 2 What is PD-L1? 3 The Role of PD-L1 in Cancer 3 PD-L1 Assessment 4 PD-L1
Quality in Control PD-L1 Analyte Control DR PD-L1_PI_v2 Product Codes: HCL019, HCL020 and HCL021 Contents PD-L1 Analyte Control DR 2 What is PD-L1? 3 The Role of PD-L1 in Cancer 3 PD-L1 Assessment 4 PD-L1
Rabbit Polyclonal antibody to NFkB p65 (v-rel reticuloendotheliosis viral oncogene homolog A (avian))
 Datasheet GeneTex, Inc : Toll Free 1-877-GeneTex (1-877-436-3839) Fax:1-949-309-2888 info@genetex.com GeneTex International Corporation : Tel:886-3-6208988 Fax:886-3-6208989 infoasia@genetex.com Date :
Datasheet GeneTex, Inc : Toll Free 1-877-GeneTex (1-877-436-3839) Fax:1-949-309-2888 info@genetex.com GeneTex International Corporation : Tel:886-3-6208988 Fax:886-3-6208989 infoasia@genetex.com Date :
Figure 1. Possible role of oncogene activation, receptor, G-protein mutation, or tumor
 Figures Part of introduction Figure 1. Possible role of oncogene activation, receptor, G-protein mutation, or tumor supressor gene deletion in the induction of thyroid carcinoma. ( by James A Fagin, M.D.)
Figures Part of introduction Figure 1. Possible role of oncogene activation, receptor, G-protein mutation, or tumor supressor gene deletion in the induction of thyroid carcinoma. ( by James A Fagin, M.D.)
Application Note. Introduction
 Simultaneously Measuring Oxidation of Exogenous and Endogenous Fatty Acids Using the XF Palmitate-BSA FAO Substrate with the Agilent Seahorse XF Cell Mito Stress Test Application Note Introduction The
Simultaneously Measuring Oxidation of Exogenous and Endogenous Fatty Acids Using the XF Palmitate-BSA FAO Substrate with the Agilent Seahorse XF Cell Mito Stress Test Application Note Introduction The
Supplementary Figures
 Supplementary Figures Figure S1. Validation of kinase regulators of ONC201 sensitivity. Validation and screen results for changes in cell viability associated with the combination of ONC201 treatment (1
Supplementary Figures Figure S1. Validation of kinase regulators of ONC201 sensitivity. Validation and screen results for changes in cell viability associated with the combination of ONC201 treatment (1
Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary Materials and Methods
 Silva et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary
Silva et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability Supplementary Table; Supplementary Figures and legends S1-S21; Supplementary
BIO360 Fall 2013 Quiz 1
 BIO360 Fall 2013 Quiz 1 1. Examine the diagram below. There are two homologous copies of chromosome one and the allele of YFG carried on the light gray chromosome has undergone a loss-of-function mutation.
BIO360 Fall 2013 Quiz 1 1. Examine the diagram below. There are two homologous copies of chromosome one and the allele of YFG carried on the light gray chromosome has undergone a loss-of-function mutation.
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 EGFR inhibition activates signaling pathways (a-b) EGFR inhibition activates signaling pathways (a) U251EGFR cells were treated with erlotinib (1µM) for the indicated times followed
Supplementary Figure 1 EGFR inhibition activates signaling pathways (a-b) EGFR inhibition activates signaling pathways (a) U251EGFR cells were treated with erlotinib (1µM) for the indicated times followed
