BECAUSE of recent advances in the treatment of metastatic
|
|
|
- Ariel Austin
- 6 years ago
- Views:
Transcription
1 36 Seminars in Oncology Nursing, Vol 23, No 1 (February), 2007: pp OBJECTIVES: To illustrate the multiple factors that can influence the treatment continuum approach. DATA SOURCES: Actual cases drawn from the authors clinical experience, scientific studies, and review articles. CONCLUSION: Patients treated for metastatic colorectal cancer (mcrc) commonly experience toxicities that oncology nurses must be aware of and ready to manage with appropriate interventions. IMPLICATIONS FOR NURSING PRACTICE: By becoming familiar with mcrc, oncology nurses will be better able to address toxicities that arise from the treatments applied. By serving as mentors and guides, oncology nurses can aid patients in self-care management and problem solving to optimize quality of life and potentially extend life. Yvonne Lassere, RN, OCN : Manager, Clinical Protocol Administration, The University of Texas M.D. Anderson Cancer Center, Houston, TX. Robin Sommers, MS, APRN, BC, AOCNP: Oncology Nurse Practitioner, Department of Gastrointestinal Oncology, Dana-Farber Cancer Institute, Boston, MA. Pamela Hallquist Viale, RN, MS, APRN, BC, AOCNP: Oncology Nurse Practitioner, Sunnyvale, CA; Assistant Clinical Professor, Department of Physiological Nursing, University of California, San Francisco. Carol S. Viele, RN, MS: Clinical Nurse Specialist, Hematology-Oncology-Bone Marrow Transplant, University of California San Francisco Medical Center. Rita Wickham, PhD, RN, AOCN, Oncology & Palliative Care Consultant, Associate Professor, College of Nursing, Rush University Medical Center, Chicago, IL. Address correspondence to Yvonne Lassere, RN, OCN, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX Elsevier Inc. All rights reserved /07/2301-$30.00/0 doi: /j.soncn CASE STUDIES IN METASTATIC COLORECTAL CANCER YVONNE LASSERE, ROBIN SOMMERS, PAMELA HALLQUIST VIALE, CAROL S. VIELE, AND RITA WICKHAM BECAUSE of recent advances in the treatment of metastatic colorectal cancer (mcrc), patients have more choices in the initial treatment of this disease. These newer therapies have extended survival from approximately 7 months with best supportive care to as many as 24 months. Patients are gratified to learn of the multiple treatment options available to them; however, the mode of administration as well as the side-effect profile of the therapy chosen may be influenced by the patient s specific desires or comorbidities. Along with the patient s preference, the oncology nurse s assessment will contribute to the initial choice of therapy. These real-life case studies illustrate the multiple factors that can influence the treatment continuum approach, including individualized side-effect management. CASE STUDY #1: SELECTING THERAPY FOR MCRC: FOLFOX OR FOLFIRI PLUS BEVACIZUMAB Mrs B, a healthy 50-year-old African-American, began to have vague gastrointestinal symptoms, which included constipation, bloating, and occasional abdominal pain/cramping that she attributed to heartburn. Over the next several months her symptoms worsened, and she saw her primary care physician in February of She reported losing 15 pounds in the past year, which she attributed to decreased appetite and gastrointestinal symptoms. Laboratory evaluation showed grade 2 anemia (hemoglobin 9.4 g/dl) and a carcinoembryonic antigen (CEA) level of 200 ng/ml. A colonoscopy confirmed a 6-cm mass in the sigmoid colon. Subsequent chest and abdominal computed tomography (CT) scans
2 CASE STUDIES IN METASTATIC COLORECTAL CANCER 37 ruled out lung metastases but detected multiple liver lesions. The patient underwent left colectomy and was found to have a palpable tumor in the left lobe of the liver during surgery. The primary tumor had invaded the muscularis propria, extending into the subserosa, and 6 of 18 resected lymph nodes were positive for tumor. Mrs B was deemed to have T3, N2, M1 (stage IV) colon cancer. Findings included: Past medical history: diabetes, mild hypertension, and hyperlipidemia Past surgical history: wisdom teeth extracted in 1976 Family history: negative for cancer; inflammatory bowel disease, Gilbert s syndrome Social history: married, lives in a single-family home with her husband; three children, age 20, 23, and 26; college graduate, works full time as an administrative secretary; has close family and support systems in place Current medications: glyburide, 5 mg orally daily; lisinopril, 5 mg daily; atorvastin calcium, 10 mg daily No known drug, food, or environmental allergies Baseline vital signs: temperature, 98; pulse, 72; respirations, 18; blood pressure (BP), 122/60 Current laboratory values: complete blood count with differential; chemistries, including electrolytes and renal function tests, within normal limits (WNL); fasting blood glucose, 140; albumin and protein levels, WNL; alanine aminotransferase (ALT), or SGPT, 37; aspartate aminotransferase, or SGOT, 34; alkaline phosphatase, 150 (institutional parameters, ); total bilirubin, 0.3; baseline urine dipstick for protein, negative. Eastern Cooperative Oncology Group (ECOG) performance status: 1 Treatment Options and Interventions For Mrs B, the best evidence would support treatment with a regimen of 5-fluorouracil/leucovorin (5-FU/LV) plus oxaliplatin (Eloxatin; Sanofi-Aventis, Bridgewater, NJ) (the FOLFOX regimen) or 5-FU/LV plus irinotecan (Camptosar; Pfizer Inc, New York, NY) (the FOLFIRI regimen) in combination with bevacizumab (Avastin; Genentech, Inc, South San Francisco, CA). Considerations regarding treatment recommendations include: comorbid conditions (positive for diabetes, hypertension, and hyperlipidemia; negative for neuropathy, inflammatory bowel disease, liver dysfunction, or Gilbert s syndrome); physical and mental abilities needed to manage therapy (good performance status, able to manipulate infusional pump, highly educated); financial issues (has health insurance from a health maintenance organization, owns home, on medical leave); adequate social support (family willing to drive patient to appointments); current occupation (administrative secretary); and patient preference. Because she had diabetes, which may increase risk for neuropathy and because worsening neuropathy could affect functioning in her occupation as a secretary, FOLFIRI with bevacizumab was recommended to palliate metastatic disease and prolong survival. Mrs B agreed to receive four cycles of therapy and then undergo restaging. She had a double-lumen tunneled catheter surgically placed before treatment. Blood pressure and urine dipsticks were to be monitored before each treatment because of the risks of hypertension and proteinuria with bevacizumab. Five weeks following surgery her incision was healed, and Mrs B started FOLFIRI and bevacizumab every 2 weeks. Her nurse instructed her about associated side effects of each agent, and the patient verbalized that she understood. Written diarrhea management and loperamide dosing sheets were also reviewed. Mrs B developed mild abdominal cramping during the initial irinotecan infusion that resolved with atropine, 0.4 mg intravenously, and prophylactic atropine was given during subsequent cycles of the combination regimen. On follow-up 2 weeks later, she reported mild diarrhea (grade 2) on day 4 after treatment, which resolved within 24 hours use of loperamide as well as other diarrhea management recommendations (eating a bland diet consisting of such foods as plain pasta, toast, rice, and bananas; avoiding products containing lactose; and drinking eight glasses of clear liquids a day [Gatorade, Chicago, IL]). Mrs B gradually resumed her regular diet. She also developed grade 1 nausea that was controlled by ondansetron (Zofran; GlaxoSmithKline, Research Triangle Park, North Carolina), a prescribed antiemetic but reported no mucositis, fevers, weight loss, hair loss, or vomiting. After four cycles of FOLFIRI with bevacizumab, restaging CT scans demonstrated regression of liver metastases and the patient s CEA level was now 60. She continued therapy and on day 7 following the fifth cycle of combination therapy
3 38 LASSERE ET AL developed grade 3 diarrhea that did not resolve despite aggressive loperamide and other self-care measures. Mrs B was admitted to the hospital for aggressive intravenous hydration and electrolyte replacement and monitoring of glucose, electrolytes, stool cultures, and complete blood cell count. She was started on subcutaneous octreotide, 150 g 3 times daily, given for severe diarrhea, as well as on a fluoroquinolone antibiotic. 1 Her symptoms resolved within 24 hours, and she was discharged on day 3. The sixth cycle of FOLFIRI plus bevacizumab was delayed 1 week and then resumed with a 25% reduction in the dose of irinotecan. Discussion Tournigand et al 2 found that a regimen of FOLFOX followed by FOLFIRI had a similar median survival rate and efficacy in patients with advanced CRC as FOLFIRI followed by FOLFOX, a finding that informed the clinicians choice of therapy in this case. When the oncology team reviewed treatment options with Mrs B and her husband, several factors in the patient s history guided the clinical recommendations, including her functional status, comorbid conditions, preferences, occupation, socioeconomic status, and the social support systems available to her. Vigilant assessment is critical throughout the treatment continuum. Nurses must accurately assess baseline bowel habits and reassess before each subsequent treatment. Patient and family education about early recognition of toxicities are key to successful management, and pharmacologic and nondrug measures may be helpful in successfully managing diarrhea. Early recognition of adverse events may also minimize the effect on the patient s quality of life while helping to prevent lifethreatening sequelae. Summary of Key Points Consideration should be given to the patient s underlying coexisting conditions when determining the optimal sequence of chemotherapy regimens. Oncology nurses play a crucial role in performing a comprehensive assessment that takes into account the patient s functional status, comorbidities, social supports, and cognitive, emotional, and financial status. Patient and family education are key to early recognition and successful management of diarrhea symptoms. CASE STUDY #2: FRONTLINE THERAPY FOR METASTATIC COLORECTAL CARCINOMA: FOLFOX WITH BEVACIZUMAB OR FOLFOX ALONE Mrs K, a 46-year-old, had a 6-month history of bright red blood per rectum when she sought medical treatment after experiencing severe cramping and bloody diarrhea. She underwent a colonoscopy, which demonstrated an obstructive lesion in her sigmoid colon at 19 cm. Her CEA level was normal (1.1 ng/ml), as were her liver and renal function tests, but a CT scan of the abdomen demonstrated an 8-cm lesion in the liver that was deemed unresectable. The patient was admitted to the hospital for a laparoscopic sigmoid colectomy. Pathology demonstrated an infiltrating and poorly differentiated adenocarcinoma. The tumor penetrated through the muscularis propria into the pericolonic adipose tissue. Fifteen of 15 lymph nodes were positive for tumor, and Mrs K s cancer was diagnosed as stage IV disease (T4, N2, M1). Findings included: Past medical history: rosacea Past surgical history: appendectomy at age 24 Family history: father died at age 62 with cancer of unknown origin Social history: married with 2 children, ages 22 and 20; good family support Current medications: metronidazole gel (Metro- Gel; 3M Healthcare, St Paul, MN), 0.075% daily No known drug, food, or environmental allergies Current laboratory values: all WNL ECOG performance status: 0 Treatment Options and Interventions Chemotherapeutic options were discussed in detail. These included FOLFOX with or without bevacizumab, FOLFIRI with or without bevacizumab, capecitabine (Xeloda; Roche Laboratories, Nutley, NJ) alone, or entering a clinical trial. The patient was reluctant to receive any chemotherapy. Only after much discussion with her family and a delay of 2.5 months did she begin therapy with FOLFOX plus bevacizumab. Mrs K was instructed on possible toxicities, including neuropathy (acute and chronic), neutropenia, thrombo-
4 CASE STUDIES IN METASTATIC COLORECTAL CANCER 39 cytopenia, pharyngolaryngeal dysesthesia, and diarrhea. 3 A central venous access device was placed to facilitate chemotherapy administration. After her first cycle of therapy in clinic, Mrs K drank a cold beverage and immediately had the sensation of not being able to breathe. The feeling resolved fairly quickly and without intervention. She was assured by her primary oncology nurse that this was a common event often triggered by a cold beverage and that it was not life-threatening. Patient education by the clinical staff reinforced the necessity of avoiding cold liquids or foods for several days after receiving oxaliplatin. After four cycles of therapy, the patient presented with mild peripheral neuropathy and an elevation in ALT of 122. The most likely cause of the elevation was thought to be the oxaliplatin, but the clinicians also considered whether the increased level of ALT signified progression of disease in the liver. In addition, Mrs K was questioned about the use of herbal medications and other supplements. She admitted using several alternative medicines and was asked to discontinue them. The decision was made to stop oxaliplatin, continue with 5-FU/LV and bevacizumab, and monitor the patient s liver enzymes. Two weeks later her ALT level was 85, and was returned to normal in 4 weeks. The regimen of FOLFOX plus bevacizumab was then resumed. Restaging scans showed a 50% decrease in her liver lesion, but the lesion was still considered unresectable. Mrs K was encouraged by the scan results, and chemotherapy continued. A neuropathy assessment was performed before each dose of oxaliplatin was given. Until about the ninth cycle, the patient had experienced only minimal peripheral neuropathy for a few days after each dose of oxaliplatin. Grade 3 thrombocytopenia was noted after cycle 9, and the oxaliplatin dose was reduced. Before cycle 12, Mrs K reported having great difficulty buttoning her clothing and a lack of sensation on the bottoms of her feet, which represents grade 3 neuropathy. With these symptoms of neuropathy now more persistent and interfering with the patient s activities of daily living, oxaliplatin was discontinued. Four months later, the liver lesion remained stable and therapy with 5-FU/LV plus bevacizumab continued. Mrs K s neuropathy was downgraded to grade 1 and, at that point, her clinicians were considering the resumption of oxaliplatin. Discussion Many options are now available for the treatment of mcrc. 4 Mrs K was given a thorough explanation of several chemotherapy regimens, including information on efficacy and possible toxicities. 5 Mrs K s decision to receive FOLFOX plus bevacizumab was based in part on the regimen s toxicity profile and her family encouragement and support. Although she responded well to FOLFOX plus bevacizumab, her solitary liver lesion remained unresectable. Her oncology team performed neuropathy assessments before each dose of oxaliplatin. Persistent neuropathy precluded Mrs K from receiving further oxaliplatin. Given that her neuropathy was minimal and the liver lesion did not progress while she received oxaliplatin, the option remained open for her to resume oxaliplatin dosing at some point in the course of therapy. Summary of Key Points Elevation of liver enzymes is a known toxicity of oxaliplatin. Patient education should be emphasized throughout therapy. Approximately 30% of all CRCs do not produce abnormally high levels of CEA. Thorough assessment of neuropathy should occur before each dose of oxaliplatin given. Dose reductions may be necessary for thrombocytopenia and are required when thrombocytopenia is greater than grade 2. For a majority of patients, chronic neuropathy caused by oxaliplatin resolves or decreases in intensity over a period of many months once the drug is discontinued. CASE STUDY #3: CONSIDERATIONS FOR THERAPY: XELOX WITH BEVACIZUMAB VERSUS FOLFIRI/FOLFOX WITH BEVACIZUMAB While traveling to Hawaii for a vacation, Ms W, 48 years old and newly divorced, experienced sharp and cramping abdominal pain along with bright red blood per rectum. She sought medical attention and after evaluation was admitted to the hospital. An emergent colonoscopy found a partially obstructive, 5-cm lesion in her descending colon and a CT scan revealed
5 40 LASSERE ET AL two lesions in her liver. The rest of her metastatic workup was negative. Her baseline preoperative CEA level was 35.6 ng/ml. Therapy options were discussed, and the ultimate decision was to return home for a colectomy followed by neoadjuvant chemotherapy plus bevacizumab in hope that her liver lesions would become resectable. Findings included: Past medical history: mild hypertension, depression, mild peripheral vascular disease Past surgical history: hysterectomy because of uterine bleeding in 2001 Family history: mother diagnosed with stage I colon cancer at age 62, alive and well; father died of a myocardial infarction at age 71 Social history: divorced, no children, minimal family and social support in the home Current medications: sertraline, 75 mg daily; lisinopril, 10 mg daily; aspirin, 325 mg daily No known drug, food, or environmental allergies Current laboratory values: all WNL ECOG performance status: 1 Treatment Options and Interventions After surgery, chemotherapy options suggested to Ms W included bevacizumab plus either FOLFIRI or FOLFOX. The patient was adamant about not having a long-term venous access catheter placed and opted to receive the oral fluoropyrimidine capecitabine with oxaliplatin (the combination regimen XELOX) daily for 2 weeks plus infusional bevacizumab, even though she was informed that data were not yet available regarding the efficacy of XELOX in combination with bevacizumab. Patient education focused on potential toxicities of therapy, including oxaliplatin-associated neurotoxicity, hand-foot syndrome (HFS), neutropenia, the possibility of nosebleeds or other bleeding, and the need to monitor her hypertension. Ms W s nurse stressed the importance of taking all of her oral capecitabine and providing timely communication to the treatment team about any side effects to reduce the potential for drug toxicity. After two cycles of XELOX, the patient returned to the office for evaluation. Assessment focused on manifestations of HFS and peripheral neurotoxicity. She reported mild and short-lived cold sensory symptoms and mild redness and tenderness of her hands. She stated her feet were fine and declined to remove her shoes. After the nurse noted the patient s gait showed a small hesitation while walking, she again asked the patient to remove her shoes and found Ms W had grade 2 HFS of her feet. In further conversation about this the patient became tearful, stating she was afraid to report her symptoms because her therapy might be changed. Further discussion of side effects and efficacy relieved some of the patient s anxiety about treatment. Capecitabine was held until symptoms of HFS resolved (to grade 0) and then was resumed at 100% of her original dose. After 3 months of chemotherapy, a repeat staging CT scan showed that one liver lesion had disappeared completely and the other could now be considered resectable. Her CEA level was 7.5. The remaining liver lesion was successfully removed, and after surgery the patient s CEA dropped to 3.0 ng/ml. At the time of this writing, Ms W was completing her planned treatment with XELOX plus bevacizumab for a total of 6 months of therapy. A subsequent increase in HFS symptoms after cycle 9 prompted a reduction of capecitabine to 75% of the original dose. She continued to tolerate this dose without worsening of HFS symptoms and was to be followed with surveillance visits, serial CEA measurements, and periodic scans to assess for recurrent disease. Discussion There have been exciting advances in the treatment of mcrc, and with five new agents approved in the treatment of this common disease, initial choices in therapy may be influenced by patient desires as well as considerations of treatment efficacy. Ms W was very hesitant to have an implanted device placed for the infusion of continuous 5-FU therapy. Therefore, substituting an oral fluoropyrimidine is a rational choice for this patient. The combination of capecitabine with oxaliplatin was studied in 96 patients and found to be an effective first-line therapy for mcrc, as well as a more convenient regimen. 6,7 The addition of bevacizumab to XELOX is currently under study, and initial data reported at the 2006 annual meeting of the American Society of Clinical Oncology (Atlanta, GA) have been promising. 8 Offering the combination to this patient met her needs for primarily oral therapy. Current research has shown that the combination is potentially efficacious for this disease.
6 CASE STUDIES IN METASTATIC COLORECTAL CANCER 41 Summary of Key Points All protocol options must be discussed with patients and their understanding of the information should be evaluated. Reported efficacy of potential regimens should be explained as well as the method of drug administration. If the patient opts for an alternative therapy, the plan should be thoroughly discussed. The potential substitution of oral capecitabine for infusional 5-FU has been shown to be a reasonable option, but larger studies are needed to confirm the equivalence of XELOX to FOLFOX. 9 A current trial is recruiting patients to answer this question, but for the time being patients should know that there may be possible differences in efficacy when they choose oral capecitabine over intravenous fluoropyrimidines. If primary oral therapy is chosen, adherence to the regimen must be stressed and assessed if possible (ie, by having the patient bring in the capecitabine bottle for a pill count). Patients must verbalize their understanding of key toxicities as well as self-care strategies and conditions they should be reporting. Nurses and clinicians must be vigilant in patient assessment, particularly with regard to physical symptoms not readily apparent. pain, anorexia, and obstipation for the past 5 days. Findings included: Past medical history: hyperlipidemia, hypertension, and hypothyroidism Past surgical history: none before colon resection Family history: mother died at age 54 of breast cancer, father died at 90 of cardiac disease Social history: patient is divorced and lives alone in an apartment. He has two children who live nearby and help him get to appointments and who are becoming more involved in his care. He is as yet unwilling to accept his daughter s invitation to live with her because he values his independence. Current medications: losartin, 50 mg daily; lovastatin, 40 mg daily; levothyroxin, 0.75 mg daily; pantoprazole, 40 mg twice daily; lactulose, 30 cc twice daily; morphine, 15 mg every 4 hours; and senna at night and docusate 3 times daily Allergies: oxycodone (caused vomiting); oxaliplatin (induced shaking, chills, and severe back pain) Current laboratory values: electrolytes, WNL (sodium, 134; potassium, 5.0; chloride, 104; and carbon dioxide, 17); creatinine, 1.6 ECOG performance status: 2 CASE STUDY #4: PATIENT UNDERGOING THERAPY FOR MCRC: CETUXIMAB, BEVACIZUMAB, AND IRINOTECAN Mr J, age 56, was initially diagnosed with colon cancer by colonoscopy in November 2004 and underwent surgery to remove the bulk of the tumor. He was treated with six cycles of FOLFOX plus bevacizumab, and in July 2005 he was told he was cancer-free. However, at his 3-month follow-up, a positron-emission tomography (PET)/ CT scan confirmed tumor recurrence, this time in the stomach. The patient was treated with FOLFIRI for 6 months when a CT scan showed progression to the liver. A PET scan demonstrated extensive liver disease (multiple liver lesions, a mass in the porta hepatis, left portal vein occlusion, and biliary ductal dilatation), peritoneal carcinomatosis, ascites, and numerous suspicious pulmonary nodules. At this point Mr J also reported a 6-week history of left-sided abdominal Treatment Options and Interventions In considering therapeutic options for Mr J it was noted he had already received oxaliplatin, which led to severe back pain, so this agent was not considered again. His performance status was worsening, but he felt his quality of life was good enough to try another round of different chemotherapy and his children were supportive of his decision. Therapy was changed to cetuximab (Erbitux; ImClone Systems Inc and Bristol Myers Squibb Co, New York, NY), bevacizumab, and irinotecan (CBI). 10 There were several factors to consider in planning treatment and monitoring for Mr J. His BP before starting therapy was 136/74. Because his treatment regimen was to include bevacizumab, the presence of comorbid hypertension would require frequent monitoring. He was already testing his urine for protein by dipstick, which had thus far been negative; but the decision was made that the nursing staff would collect a 24-hour urine specimen for the detection of protein and would
7 42 LASSERE ET AL closely follow the patient during treatment with bevacizumab. Mr J previously received irinotecan without experiencing diarrhea and became somewhat constipated from the morphine. Close monitoring during cetuximab infusions was also indicated because his reaction to oxaliplatin could potentially increase his susceptibility to cetuximab reactions. 11 He had no history of skin diseases, but the nursing staff was to monitor him closely for skin rash along with his electrolytes, creatinine, and fluid balance. The patient had a dual-lumen implanted port in place for therapy. Mr J received his initial cycle of CBI as an inpatient after his obstipation was relieved. He was premedicated with 50 mg of diphenhydramine and 650 mg of acetaminophen, tolerated all three agents in the CBI regimen well and without evidence of hypersensitivity, and was discharged home. Mr J is to be evaluated for tumor response by PET/CT after two cycles of CBI. If he has further progression at that time, a palliative care consult will be initiated to explore possible admission to hospice. Discussion All members of the health care team, along with the patient and his children, discussed current options, including best supportive/palliative care and entering a limited clinical trial to evaluate possible benefits of CBI therapy. His children were supportive of his decision to pursue therapy and available to ensure close follow-up, particularly because of his relative debilitation and risks for fluid, electrolyte problems, and drug toxicities (eg, rash, increased BP, and positive urine protein). Mr J was to be seen weekly to determine if he was feeling better and whether his performance status was improving and to closely follow any symptoms. He was to continue CBI as long as it enhanced his functioning and quality of life. Summary of Key Points All avenues of therapy have been discussed. Patient is nearing end of continuum; if this fails, best supportive care would be an option. Laboratory data need to be monitored closely as this patient is already debilitated and at significant risk of toxicity. Both the patient and his family are aware of the patient s current disease status and prognosis. Close monitoring is needed to ensure that the patient will not experience significant toxicity from therapy and have his quality of life worsened or survival cut short. CONCLUSION In summary, we have presented four typical cases with the diagnosis of mcrc. The authors have described significant toxicities that patients commonly experience and then have discussed appropriate anticipatory management and interventions for each toxicity. It is incumbent on all practicing oncology nurses to be aware of the side-effect profile of the therapeutic agents used in each case. We hope these examples will be useful in aiding oncology nurses to better deal with these common toxicities in practice. Patients who have CRC look to their oncology nurses to be mentors and guides along the treatment continuum, to aid them in self-care management and problem-solving to optimize their quality of life and potentially extend life. REFERENCES 1. Benson A, Ajain A, Catalano R, et al. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J Clin Oncol 2004;22: Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX 6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol 2004;22: Eloxatin prescribing information. Bridgewater, NJ: Sanofi-Aventis; April Available at: (accessed July 9, 2006). 4. Wilkes G. Therapeutic options in the management of colon cancer: 2005 update. J Clin Oncol 2005;9: Yamamoto D, Viale P, Roessner K, et al. The clinical use of tumor markers in select cancers: Are you confident enough to discuss them with your patients? Oncol Nurs Forum 2005;32: Borner MM, Dietrich D, Stupp R, et al. Phase II study of capecitabine and oxaliplatin in first- and second-line treatment of advanced or metastatic colorectal cancer. J Clin Oncol 2002;20: Cassidy J, Tabernero J, Twelves C, et al. XELOX (capecitabine plus oxaliplatin): Active first-line therapy for patients with metastatic colorectal cancer. J Clin Oncol 2004;22:
8 CASE STUDIES IN METASTATIC COLORECTAL CANCER Berry SR, Cunningham D, Michael M, et al. Preliminary safety of bevacizumab with first-line Folfox, Capox, Folfiri and capecitabine for mcrc First B.E.A.Trial [abstr 3534]. Proc Am Soc Clin Oncol 2006;24:154s. 9. Kopetz S, Hoff PM. Cytotoxic chemotherapy for advanced colorectal cancer. Recent advances in management. Oncology 2005;19(suppl 6): Saltz LB, Lenz H, Hochster H, et al. Randomized phase II trial of cetuximab/bevacizumab/irinotecan (CBI) versus cetuximab/bevacizumab (CB) in irinotecan-refractory colorectal cancer [abstr 3508]. Proc Am Soc Clin Oncol 2005;23:348s. 11. Cunningham D, Humblet Y, Sienna S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecanrefractory metastatic colorectal cancer. N Engl J Med 2004; 351:
CASE STUDIES IN COLORECTAL CANCER: A ROUNDTABLE DISCUSSION
 CASE STUDIES IN COLORECTAL CANCER: A ROUNDTABLE DISCUSSION PROVIDED AS AN EDUCATIONAL SERVICE BY THE INSTITUTE FOR CONTINUING HEALTHCARE EDUCATION SUPPORTED BY AN EDUCATIONAL GRANT FROM GENENTECH LEARNING
CASE STUDIES IN COLORECTAL CANCER: A ROUNDTABLE DISCUSSION PROVIDED AS AN EDUCATIONAL SERVICE BY THE INSTITUTE FOR CONTINUING HEALTHCARE EDUCATION SUPPORTED BY AN EDUCATIONAL GRANT FROM GENENTECH LEARNING
Management of Advanced Colorectal Cancer in Older Patients
 Review Article [1] April 15, 2005 By Stuart M. Lichtman, MD, FACP [2] Many elderly individuals have substantial life expectancy, even in the setting of significant illness. There is evidence to indicate
Review Article [1] April 15, 2005 By Stuart M. Lichtman, MD, FACP [2] Many elderly individuals have substantial life expectancy, even in the setting of significant illness. There is evidence to indicate
Case Report Management of a Patient with Metastatic Colorectal Cancer and Liver Metastases
 Case Reports in Oncological Medicine, Article ID 790192, 4 pages http://dx.doi.org/10.1155/2014/790192 Case Report Management of a Patient with Metastatic Colorectal Cancer and Liver Metastases Muhammad
Case Reports in Oncological Medicine, Article ID 790192, 4 pages http://dx.doi.org/10.1155/2014/790192 Case Report Management of a Patient with Metastatic Colorectal Cancer and Liver Metastases Muhammad
Case 1 Metastatic Pancreatic Adenocarcinoma: What Therapy Should I Select First?
 Case 1 Metastatic Pancreatic Adenocarcinoma: What Therapy Should I Select First? Marc Peeters, MD, PhD Head of the Oncology Department Antwerp University Hospital Antwerp, Belgium marc.peeters@uza.be 71-year-old
Case 1 Metastatic Pancreatic Adenocarcinoma: What Therapy Should I Select First? Marc Peeters, MD, PhD Head of the Oncology Department Antwerp University Hospital Antwerp, Belgium marc.peeters@uza.be 71-year-old
Disclosures. Colorectal Cancer Update GAFP November Risk Assessment. Colon and Rectal Cancer The Challenge. Issues in Colon and Rectal Cancer
 Disclosures Colorectal Cancer Update GAFP November 2006 Robert C. Hermann, MD Georgia Center for Oncology Research and Education Northwest Georgia Oncology Centers, PC WellStar Health System Marietta,
Disclosures Colorectal Cancer Update GAFP November 2006 Robert C. Hermann, MD Georgia Center for Oncology Research and Education Northwest Georgia Oncology Centers, PC WellStar Health System Marietta,
COLORECTAL CANCER 44
 COLORECTAL CANCER 44 Colorectal Cancer Highlights from the 2009 Annual Meeting of the American Society of Clinical Oncology Edited by Stuart M. Lichtman, MD Memorial Sloan-Kettering Cancer Center Commack,
COLORECTAL CANCER 44 Colorectal Cancer Highlights from the 2009 Annual Meeting of the American Society of Clinical Oncology Edited by Stuart M. Lichtman, MD Memorial Sloan-Kettering Cancer Center Commack,
COLORECTAL CANCER CASES
 COLORECTAL CANCER CASES Case #1 Case #2 Colorectal Cancer Case 1 A 52 year-old female attends her family physician for her yearly complete physical examination. Her past medical history is significant
COLORECTAL CANCER CASES Case #1 Case #2 Colorectal Cancer Case 1 A 52 year-old female attends her family physician for her yearly complete physical examination. Her past medical history is significant
Diagnosed with Metastatic Colorectal Cancer?
 ESSENTIALS Metastatic Colorectal Cancer Diagnosed with Metastatic Colorectal Cancer? It can be frightening to learn you or a loved one has been diagnosed with metastatic colorectal cancer. It is important
ESSENTIALS Metastatic Colorectal Cancer Diagnosed with Metastatic Colorectal Cancer? It can be frightening to learn you or a loved one has been diagnosed with metastatic colorectal cancer. It is important
Targeted Therapies in Metastatic Colorectal Cancer: An Update
 Targeted Therapies in Metastatic Colorectal Cancer: An Update ASCO 2007: Targeted Therapies in Metastatic Colorectal Cancer: An Update Bevacizumab is effective in combination with XELOX or FOLFOX-4 Bevacizumab
Targeted Therapies in Metastatic Colorectal Cancer: An Update ASCO 2007: Targeted Therapies in Metastatic Colorectal Cancer: An Update Bevacizumab is effective in combination with XELOX or FOLFOX-4 Bevacizumab
Chemotherapy of colon cancers
 Chemotherapy of colon cancers Stage distribution Stage I : 15% T 1,2 NO Stage IV: 20 25% M+ Stage II : 20 30% T3,4 NO Stage III N+: 30 40% clinical stages I, II, or III colon cancer are at risk for having
Chemotherapy of colon cancers Stage distribution Stage I : 15% T 1,2 NO Stage IV: 20 25% M+ Stage II : 20 30% T3,4 NO Stage III N+: 30 40% clinical stages I, II, or III colon cancer are at risk for having
Colon, or Colorectal, Cancer Information
 Colon, or Colorectal, Cancer Information Definition Colon, or colorectal, cancer is cancer that starts in the large intestine (colon) or the rectum (end of the colon). Other types of cancer can affect
Colon, or Colorectal, Cancer Information Definition Colon, or colorectal, cancer is cancer that starts in the large intestine (colon) or the rectum (end of the colon). Other types of cancer can affect
MEET ROY*: A PATIENT WITH LIVER-LIMITED mcrc
 MEET ROY*: A PATIENT WITH LIVER-LIMITED mcrc * A hypothetical case study of a patient eligible for first-line mcrc therapy. mcrc = metastatic colorectal cancer. WHAT CLINICAL CHARACTERISTICS AFFECT YOUR
MEET ROY*: A PATIENT WITH LIVER-LIMITED mcrc * A hypothetical case study of a patient eligible for first-line mcrc therapy. mcrc = metastatic colorectal cancer. WHAT CLINICAL CHARACTERISTICS AFFECT YOUR
CASE-BASED SMALL GROUP DISCUSSION MHD II
 MHD II, Session 11, Student Copy Page 1 CASE-BASED SMALL GROUP DISCUSSION MHD II Session 11 April 11, 2016 STUDENT COPY MHD II, Session 11, Student Copy Page 2 CASE HISTORY 1 Chief complaint: Our baby
MHD II, Session 11, Student Copy Page 1 CASE-BASED SMALL GROUP DISCUSSION MHD II Session 11 April 11, 2016 STUDENT COPY MHD II, Session 11, Student Copy Page 2 CASE HISTORY 1 Chief complaint: Our baby
Genentech Statement on Counterfeit Drug Labeled as Avastin (bevacizumab) in the United States
 Genentech Statement on Counterfeit Drug Labeled as Avastin (bevacizumab) in the United States SOUTH SAN FRANCISCO, Calif. February 14, 2012 Roche and Genentech have been informed that a counterfeit product,
Genentech Statement on Counterfeit Drug Labeled as Avastin (bevacizumab) in the United States SOUTH SAN FRANCISCO, Calif. February 14, 2012 Roche and Genentech have been informed that a counterfeit product,
ITS MATCH MAY HAVE MET YOUR METASTATIC COLORECTAL CANCER. Important Safety Information. Indication and Limitation of Use. Your Doctor Discussion Guide
 YOUR METASTATIC COLORECTAL CANCER MAY HAVE MET ITS MATCH There are different types of metastatic colorectal cancer (mcrc). If a RAS test shows your mcrc is wild-type RAS, Vectibix may help you live longer.
YOUR METASTATIC COLORECTAL CANCER MAY HAVE MET ITS MATCH There are different types of metastatic colorectal cancer (mcrc). If a RAS test shows your mcrc is wild-type RAS, Vectibix may help you live longer.
BC Cancer Protocol Summary for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and Vinorelbine
 BC Cancer Protocol Summary for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and Vinorelbine Protocol Code Tumour Group Contact Physician UGOOVBEVV Gynecologic Oncology Dr.
BC Cancer Protocol Summary for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and Vinorelbine Protocol Code Tumour Group Contact Physician UGOOVBEVV Gynecologic Oncology Dr.
Colorectal Cancer Therapy and Associated Toxicity
 Colorectal Cancer Therapy and Associated Toxicity Mountain States Cancer Conference November 6, 2010 Colin D. Weekes, M.D., Ph.D Assistant Professor University of Colorado GI Cancers Are Common 2009 Estimated
Colorectal Cancer Therapy and Associated Toxicity Mountain States Cancer Conference November 6, 2010 Colin D. Weekes, M.D., Ph.D Assistant Professor University of Colorado GI Cancers Are Common 2009 Estimated
Capecitabine Oxaliplatin 21 day cycle (CAPOX)
 Systemic Anti Cancer Treatment Protocol Oxaliplatin 21 day cycle (CAPOX) PROTOCOL REF: MPHACAPOX (Version No: 1.0) Approved for use in: Adjuvant colorectal cancer stage 3 or high risk stage 2 Advanced
Systemic Anti Cancer Treatment Protocol Oxaliplatin 21 day cycle (CAPOX) PROTOCOL REF: MPHACAPOX (Version No: 1.0) Approved for use in: Adjuvant colorectal cancer stage 3 or high risk stage 2 Advanced
Association of Canada. Learning about. Colorectal Cancer. A Personalized Treatment Guide for Patients
 Colorectal Cancer Association of Canada Learning about Colorectal Cancer A Personalized Treatment Guide for Patients Avastin is a trademark of Genentech, Inc., Used under licence. Camptosar is a registered
Colorectal Cancer Association of Canada Learning about Colorectal Cancer A Personalized Treatment Guide for Patients Avastin is a trademark of Genentech, Inc., Used under licence. Camptosar is a registered
Doctor Discussion Guide
 Talking to your healthcare provider about LONSURF (trifluridine and tipiracil) tablets If you have colon or rectal cancer that has spread to other parts of your body and have previously been treated with
Talking to your healthcare provider about LONSURF (trifluridine and tipiracil) tablets If you have colon or rectal cancer that has spread to other parts of your body and have previously been treated with
11/21/13 CEA: 1.7 WNL
 Case Scenario 1 A 70 year-old white male presented to his primary care physician with a recent history of rectal bleeding. He was referred for imaging and a colonoscopy and was found to have adenocarcinoma.
Case Scenario 1 A 70 year-old white male presented to his primary care physician with a recent history of rectal bleeding. He was referred for imaging and a colonoscopy and was found to have adenocarcinoma.
OVERALL CLINICAL BENEFIT
 cetuximab plus FOLFIRI to convert unresectable liver metastatses to resectable, perc confirmed that neither the FIRE-3 study nor the CRYSTAL study were designed to assess resectability and, in the absence
cetuximab plus FOLFIRI to convert unresectable liver metastatses to resectable, perc confirmed that neither the FIRE-3 study nor the CRYSTAL study were designed to assess resectability and, in the absence
DALLA CAPECITABINA AL TAS 102
 DALLA CAPECITABINA AL TAS 102 Milano 29 settembre 2016 LE PROSPETTIVE NELLA RICERCA Armando Santoro Humanitas Cancer Center THE 1,2.AND 3 LINE CHEMOTHERAPY IN CRC M BEVACIZUMAB AFLIBERCET RAS wt RAS mu
DALLA CAPECITABINA AL TAS 102 Milano 29 settembre 2016 LE PROSPETTIVE NELLA RICERCA Armando Santoro Humanitas Cancer Center THE 1,2.AND 3 LINE CHEMOTHERAPY IN CRC M BEVACIZUMAB AFLIBERCET RAS wt RAS mu
TESTS AND MONITORING:
 BC Cancer Protocol Summary for Palliative Combination Chemotherapy for Metastatic Colorectal Cancer Using Irinotecan, Fluorouracil, Leucovorin, and Bevacizumab Protocol Code GIFFIRB Tumour Group Contact
BC Cancer Protocol Summary for Palliative Combination Chemotherapy for Metastatic Colorectal Cancer Using Irinotecan, Fluorouracil, Leucovorin, and Bevacizumab Protocol Code GIFFIRB Tumour Group Contact
PREMEDICATIONS: Antiemetic protocol for highly emetogenic chemotherapy. May not need any antiemetic with
 BCCA Protocol Summary for Palliative Therapy of Metastatic or Locally Advanced Gastric, Gastroesophageal Junction Adenocarcinoma, Esophageal Squamous Cell Carcinoma, or Anal Squamous Cell Carcinoma using
BCCA Protocol Summary for Palliative Therapy of Metastatic or Locally Advanced Gastric, Gastroesophageal Junction Adenocarcinoma, Esophageal Squamous Cell Carcinoma, or Anal Squamous Cell Carcinoma using
CLINICAL INVESTIGATION
 Research Article CLINICAL INVESTIGATION Research on the treatment of metastatic colon cancer patients treated by FOLFOXIRI: Efficacy and toxicity of first-line treatment in stage IV metastatic colorectal
Research Article CLINICAL INVESTIGATION Research on the treatment of metastatic colon cancer patients treated by FOLFOXIRI: Efficacy and toxicity of first-line treatment in stage IV metastatic colorectal
TESTS AND MONITORING:
 BCCA Protocol Summary for Palliative Combination Chemotherapy for Metastatic Colorectal Cancer Using Irinotecan, Fluorouracil, Leucovorin, and Bevacizumab Protocol Code Tumour Group Contact Physician GIFFIRB
BCCA Protocol Summary for Palliative Combination Chemotherapy for Metastatic Colorectal Cancer Using Irinotecan, Fluorouracil, Leucovorin, and Bevacizumab Protocol Code Tumour Group Contact Physician GIFFIRB
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
abcd Clinical Study for Public Disclosure This clinical study synopsis is provided in line with s Policy on Transparency and Publication of Clinical Study Data. The synopsis which is part of the clinical
Current Status of Adjuvant Therapy for Colorectal Cancer
 Review Article [1] May 01, 2004 By Michael J. O connell, MD [2] Adjuvant therapy with chemotherapy and/or radiation therapy in addition to surgery improves outcome for patients with high-risk carcinomas
Review Article [1] May 01, 2004 By Michael J. O connell, MD [2] Adjuvant therapy with chemotherapy and/or radiation therapy in addition to surgery improves outcome for patients with high-risk carcinomas
EXCLUSIONS: Suspected dihydropyrimidine dehydrogenase (DPD) deficiency (see Precautions)
 BC Cancer Protocol Summary for Palliative Combination Chemotherapy for Metastatic Colorectal Cancer Using Fluorouracil Injection and Infusion and Leucovorin Infusion Protocol Code: Tumour Group: Contact
BC Cancer Protocol Summary for Palliative Combination Chemotherapy for Metastatic Colorectal Cancer Using Fluorouracil Injection and Infusion and Leucovorin Infusion Protocol Code: Tumour Group: Contact
Bevacizumab 5mg/kg Therapy 14 days
 INDICATIONS FOR USE: Bevacizumab 5mg/kg Therapy 14 days Regimen Code 00211a *Reimbursement status Hospital INDICATION ICD10 In combination with fluoropyrimidine-based chemotherapy C18 for treatment of
INDICATIONS FOR USE: Bevacizumab 5mg/kg Therapy 14 days Regimen Code 00211a *Reimbursement status Hospital INDICATION ICD10 In combination with fluoropyrimidine-based chemotherapy C18 for treatment of
By: Tania Cortas, MD Arizona Oncology 03/10/2015
 By: Tania Cortas, MD Arizona Oncology 03/10/2015 Epidemiology In the United States, CRC incidence rates have declined about 2 to 3 percent per year over the last 15 years Death rates from CRC have declined
By: Tania Cortas, MD Arizona Oncology 03/10/2015 Epidemiology In the United States, CRC incidence rates have declined about 2 to 3 percent per year over the last 15 years Death rates from CRC have declined
Capecitabine Oxaliplatin 21 day cycle (XELOX)
 Systemic Anti Cancer Treatment Protocol Capecitabine Oxaliplatin 21 day cycle (XELOX) PROTOCOL REF: MPHAXELOX (Version No: 1.0) Approved for use in: Adjuvant colorectal cancer stage 3 or high risk stage
Systemic Anti Cancer Treatment Protocol Capecitabine Oxaliplatin 21 day cycle (XELOX) PROTOCOL REF: MPHAXELOX (Version No: 1.0) Approved for use in: Adjuvant colorectal cancer stage 3 or high risk stage
Chemotherapy must not be started unless the following drugs have been given:
 BC Cancer Protocol Summary for Second-Line Therapy for Metastatic or Locally Advanced Gastric or Gastroesophageal Junction Cancer Using Weekly PACLitaxel and Ramucirumab Protocol Code: Tumour Group: Contact
BC Cancer Protocol Summary for Second-Line Therapy for Metastatic or Locally Advanced Gastric or Gastroesophageal Junction Cancer Using Weekly PACLitaxel and Ramucirumab Protocol Code: Tumour Group: Contact
Navigators Lead the Way
 RN Navigators Their Role in patients with Cancers of the GI tract Navigators Lead the Way Nurse Navigator Defined Nurse Navigator A clinically trained individual responsible for the identification and
RN Navigators Their Role in patients with Cancers of the GI tract Navigators Lead the Way Nurse Navigator Defined Nurse Navigator A clinically trained individual responsible for the identification and
Retrospective analysis of the effect of CAPOX and mfolfox6 dose intensity on survival in colorectal patients in the adjuvant setting
 ORIGINAL ARTICLE CAPOX AND mfolfox6 DOSE INTENSITY AND CLINICAL OUTCOMES IN STAGE III CRC, Mamo et al. Retrospective analysis of the effect of CAPOX and mfolfox6 dose intensity on survival in colorectal
ORIGINAL ARTICLE CAPOX AND mfolfox6 DOSE INTENSITY AND CLINICAL OUTCOMES IN STAGE III CRC, Mamo et al. Retrospective analysis of the effect of CAPOX and mfolfox6 dose intensity on survival in colorectal
Case Scenario 1. Discharge Summary
 Case Scenario 1 Discharge Summary A 69-year-old woman was on vacation and noted that she was becoming jaundiced. Two months prior to leaving on that trip, she had had a workup that included an abdominal
Case Scenario 1 Discharge Summary A 69-year-old woman was on vacation and noted that she was becoming jaundiced. Two months prior to leaving on that trip, she had had a workup that included an abdominal
Eloxatin Oxaliplatin concentrated solution for injection
 Eloxatin Oxaliplatin concentrated solution for injection Consumer Medicine Information Please read this leaflet before you are given this medicine. What is in this leaflet This leaflet answers some common
Eloxatin Oxaliplatin concentrated solution for injection Consumer Medicine Information Please read this leaflet before you are given this medicine. What is in this leaflet This leaflet answers some common
NCCP Chemotherapy Regimen. Modified FOLFOX-6 Therapy-14 day
 INDICATIONS FOR USE: Modified FOLFOX-6 Therapy-14 day INDICATION ICD10 Regimen Code Adjuvant treatment of stage II or III colon cancer after C18 00209a complete resection of primary tumour Metastatic colorectal
INDICATIONS FOR USE: Modified FOLFOX-6 Therapy-14 day INDICATION ICD10 Regimen Code Adjuvant treatment of stage II or III colon cancer after C18 00209a complete resection of primary tumour Metastatic colorectal
Third Line and Beyond: Management of Refractory Colorectal Cancer
 Third Line and Beyond: Management of Refractory Colorectal Cancer George A. Fisher MD PhD Stanford University 1 Overview Defining the chemo refractory and intolerant Agents approved in 3 rd line setting
Third Line and Beyond: Management of Refractory Colorectal Cancer George A. Fisher MD PhD Stanford University 1 Overview Defining the chemo refractory and intolerant Agents approved in 3 rd line setting
NECN CHEMOTHERAPY HANDBOOK PROTOCOL
 DRUG ADMINISTRATION SCHEDULE First Cycle: Day Drug Daily Dose Route Diluent & Rate 1 Chlorphenamine 10mg IV bolus 1 Paracetamol 1000mg ORAL 1 Ranitidine 150mg ORAL 1 Dexamethasone 8mg IV bolus 1 Cetuximab
DRUG ADMINISTRATION SCHEDULE First Cycle: Day Drug Daily Dose Route Diluent & Rate 1 Chlorphenamine 10mg IV bolus 1 Paracetamol 1000mg ORAL 1 Ranitidine 150mg ORAL 1 Dexamethasone 8mg IV bolus 1 Cetuximab
RECTAL CANCER CLINICAL CASE PRESENTATION
 RECTAL CANCER CLINICAL CASE PRESENTATION Francesco Sclafani Medical Oncologist, Clinical Research Fellow The Royal Marsden NHS Foundation Trust, London, UK esmo.org Disclosure I have nothing to declare
RECTAL CANCER CLINICAL CASE PRESENTATION Francesco Sclafani Medical Oncologist, Clinical Research Fellow The Royal Marsden NHS Foundation Trust, London, UK esmo.org Disclosure I have nothing to declare
BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel
 BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel Protocol Code Tumour Group Contact Physician UGOOVBEVP Gynecologic Oncology Dr. Anna Tinker
BC Cancer Protocol for Treatment of Platinum Resistant Epithelial Ovarian Cancer with Bevacizumab and PACLitaxel Protocol Code Tumour Group Contact Physician UGOOVBEVP Gynecologic Oncology Dr. Anna Tinker
IMPORTANT AND COMMON SIDE EFFECTS OF TRIFLURIDINE/TIPIRACIL
 IMPORTANT AND COMMON SIDE EFFECTS OF TRIFLURIDINE/TIPIRACIL Trifluridine/Tipiracil (brand name: Lonsurf ) is a prescription medicine. One way it is used is to treat colon or rectal cancer (CRC) that has
IMPORTANT AND COMMON SIDE EFFECTS OF TRIFLURIDINE/TIPIRACIL Trifluridine/Tipiracil (brand name: Lonsurf ) is a prescription medicine. One way it is used is to treat colon or rectal cancer (CRC) that has
ITS MATCH MAY HAVE MET YOUR METASTATIC COLORECTAL CANCER. What to expect during treatment with Vectibix. Important Safety Information.
 YOUR METASTATIC COLORECTAL CANCER MAY HAVE MET ITS MATCH In a clinical study of wild-type RAS metastatic colorectal cancer, Vectibix + FOLFOX helped patients live longer than FOLFOX alone (25.8 months
YOUR METASTATIC COLORECTAL CANCER MAY HAVE MET ITS MATCH In a clinical study of wild-type RAS metastatic colorectal cancer, Vectibix + FOLFOX helped patients live longer than FOLFOX alone (25.8 months
EVIDENCE IN BRIEF OVERALL CLINICAL BENEFIT
 of the clinical trial data for this outcome. Therefore, perc considered that the cost-effectiveness of cetuximab plus FOLFIRI would be at the higher end of the EGP s range of best estimates. Therefore,
of the clinical trial data for this outcome. Therefore, perc considered that the cost-effectiveness of cetuximab plus FOLFIRI would be at the higher end of the EGP s range of best estimates. Therefore,
725 Jesse Jewell Pkwy, Suite 390 Gainesville, GA (770) (770) (facsimile)
 Charles Nash, III, M.D., F.A.C.P. Richard J. LoCicero, M.D. Anup K. Lahiry, M.D. Timothy M. Carey, M.D. Andrew Johnson, M.D. 725 Jesse Jewell Pkwy, Suite 390 Gainesville, GA 30501 (770) 297-5700 (770)
Charles Nash, III, M.D., F.A.C.P. Richard J. LoCicero, M.D. Anup K. Lahiry, M.D. Timothy M. Carey, M.D. Andrew Johnson, M.D. 725 Jesse Jewell Pkwy, Suite 390 Gainesville, GA 30501 (770) 297-5700 (770)
Case Scenario year-old white male presented to personal physician with dyspepsia with reflux.
 Case Scenario 1 57-year-old white male presented to personal physician with dyspepsia with reflux. 7/12 EGD: In the gastroesophageal junction we found an exophytic tumor. The tumor occupies approximately
Case Scenario 1 57-year-old white male presented to personal physician with dyspepsia with reflux. 7/12 EGD: In the gastroesophageal junction we found an exophytic tumor. The tumor occupies approximately
Jonathan Dickinson, LCL Xeloda
 Xeloda A blockbuster in the making Jonathan Dickinson, LCL Xeloda Xeloda unique tumor-activated mechanism Delivering more cancer-killing agent straight into cancer Highly effective comparable efficacy
Xeloda A blockbuster in the making Jonathan Dickinson, LCL Xeloda Xeloda unique tumor-activated mechanism Delivering more cancer-killing agent straight into cancer Highly effective comparable efficacy
Surgical Management of Advanced Stage Colon Cancer. Nathan Huber, MD 6/11/14
 Surgical Management of Advanced Stage Colon Cancer Nathan Huber, MD 6/11/14 Colon Cancer Overview Approximately 50,000 attributable deaths per year Colorectal cancer is the 3 rd most common cause of cancer-related
Surgical Management of Advanced Stage Colon Cancer Nathan Huber, MD 6/11/14 Colon Cancer Overview Approximately 50,000 attributable deaths per year Colorectal cancer is the 3 rd most common cause of cancer-related
NECN CHEMOTHERAPY HANDBOOK PROTOCOL
 DRUG ADMINISTRATION SCHEDULE First Cycle: Day Drug Daily Dose Route Diluent & Rate 1 Chlorphenamine 10mg IV bolus 1 Paracetamol 1000mg ORAL 1 Ranitidine 150mg ORAL 1 Dexamethasone 8mg IV bolus 1 Cetuximab
DRUG ADMINISTRATION SCHEDULE First Cycle: Day Drug Daily Dose Route Diluent & Rate 1 Chlorphenamine 10mg IV bolus 1 Paracetamol 1000mg ORAL 1 Ranitidine 150mg ORAL 1 Dexamethasone 8mg IV bolus 1 Cetuximab
Discover the facts about
 Avastin is approved to treat metastatic colorectal cancer (mcrc) for: First- or second-line treatment in combination with intravenous 5-fluorouracil based chemotherapy Second-line treatment when used with
Avastin is approved to treat metastatic colorectal cancer (mcrc) for: First- or second-line treatment in combination with intravenous 5-fluorouracil based chemotherapy Second-line treatment when used with
Colorectal Cancer Care
 Colorectal Cancer Care A Cancer Care Map for Patients Understanding the process of care that a patient goes through in the diagnosis and treatment of colorectal cancer in BC. ROUND2.3_CRCa_20pages_5.5x8.5_FINAL.indd
Colorectal Cancer Care A Cancer Care Map for Patients Understanding the process of care that a patient goes through in the diagnosis and treatment of colorectal cancer in BC. ROUND2.3_CRCa_20pages_5.5x8.5_FINAL.indd
NCCP Chemotherapy Regimen. Bevacizumab 5mg/kg and FOLFIRI Therapy 14 days
 Bevacizumab 5mg/kg and FOLFIRI Therapy 14 days INDICATIONS FOR USE: Regimen Code 00449a INDICATION ICD10 Treatment of adult patients with metastatic carcinoma of the colon or C18 rectum. C19 C20 *If the
Bevacizumab 5mg/kg and FOLFIRI Therapy 14 days INDICATIONS FOR USE: Regimen Code 00449a INDICATION ICD10 Treatment of adult patients with metastatic carcinoma of the colon or C18 rectum. C19 C20 *If the
Adjuvant Chemotherapy for Rectal Cancer: Are we making progress?
 Adjuvant Chemotherapy for Rectal Cancer: Are we making progress? Hagen Kennecke, MD, MHA, FRCPC Division Of Medical Oncology British Columbia Cancer Agency October 25, 2008 Objectives Review milestones
Adjuvant Chemotherapy for Rectal Cancer: Are we making progress? Hagen Kennecke, MD, MHA, FRCPC Division Of Medical Oncology British Columbia Cancer Agency October 25, 2008 Objectives Review milestones
BC Cancer Protocol Summary for Adjuvant Therapy of Colon Cancer using Fluorouracil Injection and Infusion and Leucovorin Infusion
 BC Cancer Protocol Summary for Adjuvant Therapy of Colon Cancer using Fluorouracil Injection and Infusion and Leucovorin Infusion Protocol Code: Tumour Group: Contact Physician: GIAJFL Gastrointestinal
BC Cancer Protocol Summary for Adjuvant Therapy of Colon Cancer using Fluorouracil Injection and Infusion and Leucovorin Infusion Protocol Code: Tumour Group: Contact Physician: GIAJFL Gastrointestinal
Please list all medications you are currently taking (include aspirin, vitamins, hormones), Dosage, and Frequency.
 GENERAL SURGICAL ASSOCIATES A practice of Lehigh Valley Physician Group Suite 208 ~ 1240 S. Cedar Crest Boulevard ~ Allentown, PA 18103 Phone: (610) 402-9780 PLEASE COMPLETE THIS FORM PRIOR TO YOUR VISIST
GENERAL SURGICAL ASSOCIATES A practice of Lehigh Valley Physician Group Suite 208 ~ 1240 S. Cedar Crest Boulevard ~ Allentown, PA 18103 Phone: (610) 402-9780 PLEASE COMPLETE THIS FORM PRIOR TO YOUR VISIST
Small Bowel and Colon Surgery
 Small Bowel and Colon Surgery Why Do I Need a Small Bowel Resection? A variety of conditions can damage your small bowel. In severe cases, your doctor may recommend removing part of your small bowel. Conditions
Small Bowel and Colon Surgery Why Do I Need a Small Bowel Resection? A variety of conditions can damage your small bowel. In severe cases, your doctor may recommend removing part of your small bowel. Conditions
Cytotoxic Chemotherapy for Advanced Colorectal Cancer
 Review Article [1] November 02, 2005 By Scott Kopetz, MD [2] and Paulo M. Hoff, MD, FACP [3] Several developments in the past few years have incrementally progressed the field and provided additional insights
Review Article [1] November 02, 2005 By Scott Kopetz, MD [2] and Paulo M. Hoff, MD, FACP [3] Several developments in the past few years have incrementally progressed the field and provided additional insights
Discharge Summary-Page 1
 Discharge Summary-Page 1 Admission diagnosis: 1. Gastritis. 2. Alcoholic cirrhosis, ascites, grade 1 esophageal varices. 3. Recent left knee arthroplasty. 4. Osteoporosis naqmq : 1. Three chest X-rays
Discharge Summary-Page 1 Admission diagnosis: 1. Gastritis. 2. Alcoholic cirrhosis, ascites, grade 1 esophageal varices. 3. Recent left knee arthroplasty. 4. Osteoporosis naqmq : 1. Three chest X-rays
Docetaxel. Class: Antineoplastic agent, Antimicrotubular, Taxane derivative.
 Docetaxel Class: Antineoplastic agent, Antimicrotubular, Taxane derivative. Indications: -Breast cancer: -Non small cell lung cancer -Prostate cancer -Gastric adenocarcinoma _Head and neck cancer Unlabeled
Docetaxel Class: Antineoplastic agent, Antimicrotubular, Taxane derivative. Indications: -Breast cancer: -Non small cell lung cancer -Prostate cancer -Gastric adenocarcinoma _Head and neck cancer Unlabeled
Conflicts of Interest GI Malignancies: An Update on Current Treatment Options
 Conflicts of Interest GI Malignancies: An Update on Current Treatment Options Nothing to disclose Trevor McKibbin, PharmD, MS, BCOP Clinical Specialist, Hematology/Oncology Winship Cancer Institute of
Conflicts of Interest GI Malignancies: An Update on Current Treatment Options Nothing to disclose Trevor McKibbin, PharmD, MS, BCOP Clinical Specialist, Hematology/Oncology Winship Cancer Institute of
Randomized Phase II Study of Irinotecan and Cetuximab with or without Vemurafenib in BRAF Mutant Metastatic Colorectal Cancer
 Randomized Phase II Study of Irinotecan and Cetuximab with or without Vemurafenib in BRAF Mutant Metastatic Colorectal Cancer This is a two-arm, randomized phase II trial for patients with BRAF mutant
Randomized Phase II Study of Irinotecan and Cetuximab with or without Vemurafenib in BRAF Mutant Metastatic Colorectal Cancer This is a two-arm, randomized phase II trial for patients with BRAF mutant
BOWEL CANCER. Causes of bowel cancer
 A cancer is an abnormality in an organ that grows without control. The growth is often quite slow, but will continue unabated until it is detected. It can cause symptoms by its presence in the organ or
A cancer is an abnormality in an organ that grows without control. The growth is often quite slow, but will continue unabated until it is detected. It can cause symptoms by its presence in the organ or
BCCA Protocol Summary for Combined Modality Adjuvant Therapy for High Risk Rectal Carcinoma using Capecitabine and Radiation Therapy
 BCCA Protocol Summary for Combined Modality Adjuvant Therapy for High Risk Rectal Carcinoma using Capecitabine and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GIRCRT Gastrointestinal
BCCA Protocol Summary for Combined Modality Adjuvant Therapy for High Risk Rectal Carcinoma using Capecitabine and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GIRCRT Gastrointestinal
What s New in Colon Cancer? Therapy over the last decade
 What s New in Colon Cancer? 9/19/2014 Michael McNamara, MD Therapy over the last decade Cytotoxic chemotherapy - 5FU ( Mayo, Roswell, Infusional) - Xeloda (01 ) - Oxaliplatin (02 ) - Irinotecan (96 ) Anti-
What s New in Colon Cancer? 9/19/2014 Michael McNamara, MD Therapy over the last decade Cytotoxic chemotherapy - 5FU ( Mayo, Roswell, Infusional) - Xeloda (01 ) - Oxaliplatin (02 ) - Irinotecan (96 ) Anti-
LONSURF (trifluridine-tipiracil) oral tablet
 LONSURF (trifluridine-tipiracil) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This
LONSURF (trifluridine-tipiracil) oral tablet Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This
Panitumumab + FOLFIRI for the 1 st line Treatment of Metastatic Colorectal Cancer
 DRUG ADMINISTRATION SCHEDULE Day Drug Daily Dose Route Diluent and rate Day 1 Panitumumab 6mg/kg Intravenous 100ml Sodium Chloride 0.9% at variable rate (see administration notes) Glucose 5% 500ml Infusion
DRUG ADMINISTRATION SCHEDULE Day Drug Daily Dose Route Diluent and rate Day 1 Panitumumab 6mg/kg Intravenous 100ml Sodium Chloride 0.9% at variable rate (see administration notes) Glucose 5% 500ml Infusion
Antiemetic protocol for low-moderately emetogenic chemotherapy (see SCNAUSEA)
 BC Cancer Protocol Summary for First Line Treatment of Locally Advanced Metastatic Pancreatic Cancer with Gemcitabine Protocol Code Tumour Group Contact Physician GIPGEMABR Gastrointestinal GI Systemic
BC Cancer Protocol Summary for First Line Treatment of Locally Advanced Metastatic Pancreatic Cancer with Gemcitabine Protocol Code Tumour Group Contact Physician GIPGEMABR Gastrointestinal GI Systemic
Pembrozulimab Induced Collagenous Colitis. Mokshya Sharma 1, MD, Santhosh Ambika 2, MD University of Nevada, Reno SOM
 Pembrozulimab Induced Collagenous Colitis Mokshya Sharma 1, MD, Santhosh Ambika 2, MD University of Nevada, Reno SOM Background Immune modulating therapy that targets PD1 pathway such as pembrozulimab
Pembrozulimab Induced Collagenous Colitis Mokshya Sharma 1, MD, Santhosh Ambika 2, MD University of Nevada, Reno SOM Background Immune modulating therapy that targets PD1 pathway such as pembrozulimab
CHEMOTHERAPY FOR COLON CANCER OUTLINE OF TODAY S TALK. Colon Cancer Epidemiology 11/6/2012 GATRA/GCCR FALL CONFERENCE NOVEMBER 14 16, 2012
 CHEMOTHERAPY FOR COLON CANCER JONATHAN C. BENDER,MD MEDICAL DIRECTOR OF PIEDMONT FAYETTE CANCER CENTER OUTLINE OF TODAY S TALK 1. Overview of Colon Cancer in the US 2. Colon Cancer staging and risks of
CHEMOTHERAPY FOR COLON CANCER JONATHAN C. BENDER,MD MEDICAL DIRECTOR OF PIEDMONT FAYETTE CANCER CENTER OUTLINE OF TODAY S TALK 1. Overview of Colon Cancer in the US 2. Colon Cancer staging and risks of
Current Treatment of Colorectal Metastases. Dr. Thavanathan Surgical Grand Rounds February 1, 2005
 Current Treatment of Colorectal Metastases Dr. Thavanathan Surgical Grand Rounds February 1, 2005 25% will have metastases at initial presentation 25-50% 50% will develop metastases later 40% of potentially
Current Treatment of Colorectal Metastases Dr. Thavanathan Surgical Grand Rounds February 1, 2005 25% will have metastases at initial presentation 25-50% 50% will develop metastases later 40% of potentially
Advances in Chemotherapy of Colorectal Cancer
 Advances in Chemotherapy of Colorectal Cancer Richard M. Goldberg Lineberger Comprehensive Cancer Center University of North Carolina at Chapel Hill Disease Settings Adjuvant Therapy MOSAIC, FOLFOX Andre
Advances in Chemotherapy of Colorectal Cancer Richard M. Goldberg Lineberger Comprehensive Cancer Center University of North Carolina at Chapel Hill Disease Settings Adjuvant Therapy MOSAIC, FOLFOX Andre
COME HOME Innovative Oncology Business Solutions, Inc.
 COME HOME Rectal Cancer Pathway V8, April 2015 Diagnostic Workup: Bethesda Criteria: Pathology Review All patients H&P All patients Biopsy All patients Colonoscopy All patients CEA All Patients Chest/Abdominal/Pelvic
COME HOME Rectal Cancer Pathway V8, April 2015 Diagnostic Workup: Bethesda Criteria: Pathology Review All patients H&P All patients Biopsy All patients Colonoscopy All patients CEA All Patients Chest/Abdominal/Pelvic
Adverse Effects of Chemotherapy Regimens Used in Colorectal Cancer Patients in a Referral Cancer Center in North of Iran,
 2016 jpc.tums.ac.ir Adverse Effects of Chemotherapy Regimens Used in Colorectal Cancer Patients in a Referral Cancer Center in North of Iran, 2008-2014 Ebrahim Salehifar 1*, Shayeste Gheibi 2, Ghasem Janbabaei
2016 jpc.tums.ac.ir Adverse Effects of Chemotherapy Regimens Used in Colorectal Cancer Patients in a Referral Cancer Center in North of Iran, 2008-2014 Ebrahim Salehifar 1*, Shayeste Gheibi 2, Ghasem Janbabaei
BCCA Protocol Summary for Adjuvant Therapy of Colon Cancer using
 BCCA Protocol Summary for Adjuvant Therapy of Colon Cancer using Capecitabine Protocol Code Tumour Group Contact Physician GIAJCAP Gastrointestinal GI Systemic Therapy ELIGIBILITY: Resected Stage III or
BCCA Protocol Summary for Adjuvant Therapy of Colon Cancer using Capecitabine Protocol Code Tumour Group Contact Physician GIAJCAP Gastrointestinal GI Systemic Therapy ELIGIBILITY: Resected Stage III or
Learning More Can Make a Difference
 When your advanced or metastatic HER2+ breast cancer progresses on an anthracycline, a taxane, and Herceptin (trastuzumab) T U R N T O T Y K E R B + C A P E C I T A B I N E Learning More Can Make a Difference
When your advanced or metastatic HER2+ breast cancer progresses on an anthracycline, a taxane, and Herceptin (trastuzumab) T U R N T O T Y K E R B + C A P E C I T A B I N E Learning More Can Make a Difference
Subject ID: I N D # # U A * Consent Date: Day Month Year
 IND Study # Eligibility Checklist Pg 1 of 15 Instructions: Check the appropriate box for each Inclusion and Exclusion Criterion below. Each criterion must be marked and all protocol criteria have to be
IND Study # Eligibility Checklist Pg 1 of 15 Instructions: Check the appropriate box for each Inclusion and Exclusion Criterion below. Each criterion must be marked and all protocol criteria have to be
Irinotecan. Class:Camptothecin. Indications : _Cervical cancer. _CNS tumor. _Esophageal cancer. _Ewing s sarcoma. _Gastric cancer
 Irinotecan Class:Camptothecin Indications : _Cervical cancer _CNS tumor _Esophageal cancer _Ewing s sarcoma _Gastric cancer _Nonsmall cell lung cancer _Pancreatic cancer _Small cell lung cancer _Colorectal
Irinotecan Class:Camptothecin Indications : _Cervical cancer _CNS tumor _Esophageal cancer _Ewing s sarcoma _Gastric cancer _Nonsmall cell lung cancer _Pancreatic cancer _Small cell lung cancer _Colorectal
BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, Capecitabine and Radiation Therapy
 BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GICART Gastrointestinal
BCCA Protocol Summary for Curative Combined Modality Therapy for Carcinoma of the Anal Canal Using Mitomycin, and Radiation Therapy Protocol Code: Tumour Group: Contact Physician: GICART Gastrointestinal
NCCP Chemotherapy Regimen. FOLFOX-4 Therapy-14 day
 INDICATIONS FOR USE: FOLFOX-4 Therapy-14 day INDICATION ICD10 Regimen Code Adjuvant treatment of stage II or III colon cancer after C18 00210a complete resection of primary tumour Metastatic colorectal
INDICATIONS FOR USE: FOLFOX-4 Therapy-14 day INDICATION ICD10 Regimen Code Adjuvant treatment of stage II or III colon cancer after C18 00210a complete resection of primary tumour Metastatic colorectal
Bevacizumab 10mg/kg 14 days
 INDICATIONS FOR USE: Bevacizumab 10mg/kg 14 days Regimen Code 00212a *Reimbursement status Hospital INDICATION ICD10 In combination with fluoropyrimidine-based chemotherapy C18 for treatment of adult patients
INDICATIONS FOR USE: Bevacizumab 10mg/kg 14 days Regimen Code 00212a *Reimbursement status Hospital INDICATION ICD10 In combination with fluoropyrimidine-based chemotherapy C18 for treatment of adult patients
Cycle 1 PERTuzumab (day 1) and trastuzumab (day 2) loading doses: Drug Dose BC Cancer Administration Guideline
 BC Cancer Protocol Summary for Palliative Therapy for Metastatic Breast Cancer Using PERTuzumab, Trastuzumab (HERCEPTIN), and PACLItaxel as First-Line Treatment for Advanced Breast Cancer Protocol Code:
BC Cancer Protocol Summary for Palliative Therapy for Metastatic Breast Cancer Using PERTuzumab, Trastuzumab (HERCEPTIN), and PACLItaxel as First-Line Treatment for Advanced Breast Cancer Protocol Code:
Ghosts in the Machine: Jonathan B. Koea MD; FRACS. Department of Surgery Auckland Hospital Auckland New Zealand
 Ghosts in the Machine: Patient Journeys Through Cancer Treatment Jonathan B. Koea MD; FRACS. Department of Surgery Auckland Hospital Auckland New Zealand Age-Standardised Cancer Incidence (100,000 population)
Ghosts in the Machine: Patient Journeys Through Cancer Treatment Jonathan B. Koea MD; FRACS. Department of Surgery Auckland Hospital Auckland New Zealand Age-Standardised Cancer Incidence (100,000 population)
PRODUCT INFORMATION 1 ABOUT THIS GUIDE DOSAGE FORM AND STRENGTH STORAGE AND HANDLING
 DOSING GUIDE INDICATION ONIVYDE (irinotecan liposome injection) is indicated, in combination with fluorouracil (5-FU) and leucovorin (LV), for the treatment of patients with metastatic adenocarcinoma of
DOSING GUIDE INDICATION ONIVYDE (irinotecan liposome injection) is indicated, in combination with fluorouracil (5-FU) and leucovorin (LV), for the treatment of patients with metastatic adenocarcinoma of
BCCA Protocol Summary for Palliative Combination Chemotherapy for Metastatic Colorectal Cancer Using Oxaliplatin, and Capecitabine
 BCCA Protocol Summary for Palliative Combination Chemotherapy for Metastatic Colorectal Cancer Using Oxaliplatin, and Capecitabine Protocol Code: Tumour Group: Contact Physician: GICAPOX Gastrointestinal
BCCA Protocol Summary for Palliative Combination Chemotherapy for Metastatic Colorectal Cancer Using Oxaliplatin, and Capecitabine Protocol Code: Tumour Group: Contact Physician: GICAPOX Gastrointestinal
GASTROENTEROLOGY PATIENT QUESTIONNAIRE - PLEASE PRINT
 GASTROENTEROLOGY PATIENT QUESTIONNAIRE - PLEASE PRINT Full name: Date: Telephone Number: Age: Address: Email address: CHIEF COMPLAINTS(List the problems about which you came to see the doctor) 1) 2) 3)
GASTROENTEROLOGY PATIENT QUESTIONNAIRE - PLEASE PRINT Full name: Date: Telephone Number: Age: Address: Email address: CHIEF COMPLAINTS(List the problems about which you came to see the doctor) 1) 2) 3)
Intraperitoneal Chemotherapy
 Intraperitoneal Chemotherapy What is Intraperitoneal (IP) Chemotherapy? Intraperitoneal (IP) chemotherapy is a way to put some of your chemotherapy into your abdomen (also called the peritoneal cavity)
Intraperitoneal Chemotherapy What is Intraperitoneal (IP) Chemotherapy? Intraperitoneal (IP) chemotherapy is a way to put some of your chemotherapy into your abdomen (also called the peritoneal cavity)
Irinotecan Capecitabine (14 day regimen) (I-Cap)
 Systemic Anti Cancer Treatment Protocol Irinotecan (14 day regimen) (I-Cap) PROTOCOL REF: MPHAICAP (Version No: 1.0) Approved for use in: Advanced colorectal cancer first line Advanced colorectal cancer
Systemic Anti Cancer Treatment Protocol Irinotecan (14 day regimen) (I-Cap) PROTOCOL REF: MPHAICAP (Version No: 1.0) Approved for use in: Advanced colorectal cancer first line Advanced colorectal cancer
State of the Art: Colorectal Cancer Liver Metastasis Dr. Iain Tan
 State of the Art: Colorectal Cancer Liver Metastasis Dr. Iain Tan Consultant GI Medical Oncologist National Cancer Centre Singapore Clinician Scientist, Genome Institute of Singapore OS (%) Overall survival
State of the Art: Colorectal Cancer Liver Metastasis Dr. Iain Tan Consultant GI Medical Oncologist National Cancer Centre Singapore Clinician Scientist, Genome Institute of Singapore OS (%) Overall survival
Avastin Sample Coding
 First- and Second-line Metastatic Colorectal Cancer C18.0 Malignant neoplasm of the cecum C18.1 Malignant neoplasm of appendix C18.2-C18.9 C19 C20 C21.8 Malignant neoplasm of the colon, various sites Malignant
First- and Second-line Metastatic Colorectal Cancer C18.0 Malignant neoplasm of the cecum C18.1 Malignant neoplasm of appendix C18.2-C18.9 C19 C20 C21.8 Malignant neoplasm of the colon, various sites Malignant
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES
 PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GASTROINTESTINAL RECTAL CANCER GI Site Group Rectal Cancer Authors: Dr. Jennifer Knox, Dr. Mairead McNamara 1. INTRODUCTION 3 2. SCREENING AND
PRINCESS MARGARET CANCER CENTRE CLINICAL PRACTICE GUIDELINES GASTROINTESTINAL RECTAL CANCER GI Site Group Rectal Cancer Authors: Dr. Jennifer Knox, Dr. Mairead McNamara 1. INTRODUCTION 3 2. SCREENING AND
2/20/14& Medical Management of Colon and Rectal Cancer: An Overview. Outline / Learning Objectives. How common is colon cancer?
 Medical Management of Colon and Rectal Cancer: An Overview Jonathan Grim, MD, PhD VA Puget Sound Health Care System Fred Hutchinson Cancer Research Center UW Medicine Outline / Learning Objectives Epidemiology
Medical Management of Colon and Rectal Cancer: An Overview Jonathan Grim, MD, PhD VA Puget Sound Health Care System Fred Hutchinson Cancer Research Center UW Medicine Outline / Learning Objectives Epidemiology
Effective Panitumumab Treatment in Patients with Heavily Pre-treated Metastatic Colorectal Cancer: A Case Series
 Effective Panitumumab Treatment in Patients with Heavily Pre-treated Metastatic Colorectal Cancer: A Case Series ALEXANDROS A. TZOVARAS, ATHANASSIOS KARAGIANNIS, CHARALAMBIA MARGARI, GEORGIA BARLA and
Effective Panitumumab Treatment in Patients with Heavily Pre-treated Metastatic Colorectal Cancer: A Case Series ALEXANDROS A. TZOVARAS, ATHANASSIOS KARAGIANNIS, CHARALAMBIA MARGARI, GEORGIA BARLA and
Colon Cancer , The Patient Education Institute, Inc. oc Last reviewed: 05/17/2017 1
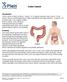 Colon Cancer Introduction Colon cancer is fairly common. About 1 in 15 people develop colon cancer. Colon cancer can be a life threatening condition that affects the large intestine. However, if it is
Colon Cancer Introduction Colon cancer is fairly common. About 1 in 15 people develop colon cancer. Colon cancer can be a life threatening condition that affects the large intestine. However, if it is
Adjuvant therapies for large bowel cancer Wasantha Rathnayake, MD
 LEADING ARTICLE Adjuvant therapies for large bowel cancer Wasantha Rathnayake, MD Consultant Clinical Oncologist, National Cancer Institute, Maharagama, Sri Lanka. Key words: Large bowel; Cancer; Adjuvant
LEADING ARTICLE Adjuvant therapies for large bowel cancer Wasantha Rathnayake, MD Consultant Clinical Oncologist, National Cancer Institute, Maharagama, Sri Lanka. Key words: Large bowel; Cancer; Adjuvant
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE. Health Technology Appraisal
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health Technology Appraisal Nivolumab for previously treated metastatic colorectal cancer with high microsatellite instability or mismatch repair deficiency
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Health Technology Appraisal Nivolumab for previously treated metastatic colorectal cancer with high microsatellite instability or mismatch repair deficiency
BC Cancer Protocol Summary for Combined Cetuximab and Radiation Treatment for Locally Advanced Squamous Cell Carcinoma of the Head and Neck
 BC Cancer Protocol Summary for Combined Cetuximab and Radiation Treatment for Locally Advanced Squamous Cell Carcinoma of the Head and Neck Protocol Code Tumour Group Contact Physician HNLACETRT Head and
BC Cancer Protocol Summary for Combined Cetuximab and Radiation Treatment for Locally Advanced Squamous Cell Carcinoma of the Head and Neck Protocol Code Tumour Group Contact Physician HNLACETRT Head and
Locally Advanced Colon Cancer. Feiran Lou MD. MS. Richmond University Medical Center Department of Surgery
 Locally Advanced Colon Cancer Feiran Lou MD. MS. Richmond University Medical Center Department of Surgery Case 34 yo man presented with severe RLQ abdominal pain X 24 hrs. No nausea/vomiting/fever. + flatus.
Locally Advanced Colon Cancer Feiran Lou MD. MS. Richmond University Medical Center Department of Surgery Case 34 yo man presented with severe RLQ abdominal pain X 24 hrs. No nausea/vomiting/fever. + flatus.
