Protocol for the Examination of Specimens From Patients With Merkel Cell Carcinoma of the Skin
|
|
|
- Maria Williams
- 6 years ago
- Views:
Transcription
1 Protocol for the Examination of Specimens From Patients With Merkel Cell Carcinoma of the Skin Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual For accreditation purposes, this protocol should be used for the following procedures AND tumor types: Procedure Description Excision Tumor Type Description Merkel cell carcinoma This protocol is NOT required for accreditation purposes for the following: Procedure Biopsy Primary resection specimen with no residual cancer (eg, following previous excision) Cytologic specimens Authors Bruce Robert Smoller, MD*; Christopher Bichakjian, MD; J. Ahmad Brown, MD; Arthur N. Crowson, MD; Dimitrios Divaris, MD; David P. Frishberg, MD; Ling Gao, MD, PhD; Jeff Gershenwald, MD; Jennifer M. McNiff, MD; Paul Nghiem, MD, PhD; Victor G. Prieto, MD, PhD; Richard Scolyer, MD; Maria Angelica Selim, MD; Sara Shalin, MD, PhD; Janis Marie Taube, MD With guidance from the CAP Cancer and CAP Pathology Electronic Reporting Committees. * Denotes primary author. All other contributing authors are listed alphabetically College of American Pathologists (CAP). All rights reserved. For Terms of Use please visit
2 Accreditation Requirements This protocol can be utilized for a variety of procedures and tumor types for clinical care purposes. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. Core data elements are required in reports to adequately describe appropriate malignancies. For accreditation purposes, essential data elements must be reported in all instances, even if the response is not applicable or cannot be determined. Conditional data elements are only required to be reported if applicable as delineated in the protocol. For instance, the total number of lymph nodes examined must be reported, but only if nodes are present in the specimen. Optional data elements are identified with + and although not required for CAP accreditation purposes, may be considered for reporting as determined by local practice standards. The use of this protocol is not required for recurrent tumors or for metastatic tumors that are resected at a different time than the primary tumor. Use of this protocol is also not required for pathology reviews performed at a second institution (ie, secondary consultation, second opinion, or review of outside case at second institution). Synoptic Reporting All core and conditionally required data elements outlined on the surgical case summary from this cancer protocol must be displayed in synoptic report format. Synoptic format is defined as: Data element: followed by its answer (response), outline format without the paired "Data element: Response" format is NOT considered synoptic. The data element must be represented in the report as it is listed in the case summary. The response for any data element may be modified from those listed in the case summary, including Cannot be determined if appropriate. Each diagnostic parameter pair (Data element: Response) is listed on a separate line or in a tabular format to achieve visual separation. The following exceptions are allowed to be listed on one line: o Anatomic site or specimen, laterality, and procedure o Pathologic Stage Classification (ptnm) elements o Negative margins, as long as all negative margins are specifically enumerated where applicable The synoptic portion of the report can appear in the diagnosis section of the pathology report, at the end of the report or in a separate section, but all Data element: Responses must be listed together in one location Organizations and pathologists may choose to list the required elements in any order, use additional methods in order to enhance or achieve visual separation, or add optional items within the synoptic report. The report may have required elements in a summary format elsewhere in the report IN ADDITION TO but not as replacement for the synoptic report i.e. all required elements must be in the synoptic portion of the report in the format defined above. CAP Laboratory Accreditation Program Protocol Required Use Date: March 2018* * Beginning January 1, 2018, the 8th edition AJCC Staging Manual should be used for reporting ptnm. CAP Merkel Cell Carcinoma Protocol Summary of Changes The following data elements were modified: Pathologic Stage Classification (ptnm, AJCC 8th Edition) Mitotic Rate 2
3 CAP Approved Surgical Pathology Cancer Case Summary Protocol posting date: June 2017 MERKEL CELL CARCINOMA OF THE SKIN: Select a single response unless otherwise indicated. Procedure (select all that apply) Excision Re-excision Lymphadenectomy, sentinel node(s) Lymphadenectomy, regional nodes (specify): Other (specify): Not specified + Specimen Laterality + Right + Left + Midline + Not specified Tumor Site Specify (if known): Not specified Tumor Size Greatest dimension (centimeters): cm + Additional dimensions (centimeters): x cm Cannot be determined (explain): + Tumor Thickness (Note A) + Specify (millimeters): mm + At least (millimeters): mm (explain): Margins Peripheral Margins Cannot be assessed Uninvolved by carcinoma Distance of carcinoma from margin (millimeters): mm Specify location(s), if possible: Involved by carcinoma Specify location(s), if possible: Deep Margin Cannot be assessed Uninvolved by carcinoma Distance of carcinoma from margin (millimeters): mm Specify location(s), if possible: Involved by carcinoma Specify location(s), if possible: Lymphovascular Invasion Not identified + Data elements preceded by this symbol are not required for accreditation purposes. These optional elements may be clinically important but are not yet validated or regularly used in patient management. 3
4 CAP Approved Present Cannot be determined Tumor Extension (select all that apply) Not identified Tumor invades bone Tumor invades muscle Tumor invades fascia Tumor invades cartilage Other (specify): Cannot be assessed Not applicable + Mitotic Rate (Note B) + <1/mm 2 + 1/mm 2 (specify number): + Tumor-Infiltrating Lymphocytes (Note C) + Not identified + Present, nonbrisk + Present, brisk + Tumor Growth Pattern (Note D) + Nodular + Infiltrative + Presence of Second Malignancy (Note E) + Present (specify type): + Not identified Regional Lymph Nodes (Note F) No lymph nodes submitted or found Lymph Node Examination (required only if lymph nodes are present in the specimen) Number of Lymph Nodes Involved: Number cannot be determined (explain): + Size of Largest Metastatic Deposit (millimeters): mm + Extranodal Extension + Not identified + Present + Cannot be determined Number of Lymph Nodes Examined: Number cannot be determined (explain): Number of Sentinel Nodes Examined: Number cannot be determined (explain): Pathologic Stage Classification (ptnm, AJCC 8 th Edition) (Note G) Note: Reporting of pt, pn, and (when applicable) pm categories is based on information available to the pathologist at the time the report is issued. Only the applicable T, N, or M category is required for reporting; their definitions need not be included in the report. The categories (with modifiers when applicable) can be listed on 1 line or more than 1 line. + Data elements preceded by this symbol are not required for accreditation purposes. These optional elements may be clinically important but are not yet validated or regularly used in patient management. 4
5 CAP Approved TNM Descriptors (required only if applicable) (select all that apply) m (multiple) r (recurrent) y (posttreatment) Primary Tumor (pt) Note: If clinical tumor size is unavailable, gross or microscopic tumor measurement should be used for determining the T category. ptx: Primary tumor cannot be assessed (eg, curetted) pt0: No evidence of primary tumor ptis: In situ primary tumor pt1: Maximum clinical tumor diameter 2 cm pt2: Maximum clinical tumor diameter >2 but 5 cm pt3: Maximum clinical tumor diameter >5 cm pt4: Primary tumor invades bone, muscle, fascia, or cartilage Regional Lymph Nodes (pn) pnx: Regional lymph nodes cannot be assessed (eg, previously removed for another reason or not removed for pathological evaluation) pn0: No regional lymph node metastasis detected on pathological evaluation pn1: Metastasis in regional lymph node(s) pn1a(sn): Clinically occult regional lymph node metastasis identified only by sentinel lymph node biopsy pn1a: Clinically occult regional lymph node metastasis following lymph node dissection pn1b: Clinically and/or radiologically detected regional lymph node metastasis # pn2: In transit metastasis (discontinuous from primary tumor; located between primary tumor and draining regional nodal basin, or distal to the primary tumor) without lymph node metastasis pn3: In transit metastasis (discontinuous from primary tumor; located between primary tumor and draining regional nodal basin, or distal to the primary tumor) with lymph node metastasis # Note: The pn1b, subcategory is dependent on clinical information that may be unavailable to the pathologist. If this information is not available, the parent category (pn1) should be selected. Distant Metastasis (pm) (required only if confirmed pathologically in this case) pm1: Distant metastasis microscopically confirmed pm1a: Metastasis to distant skin, distant subcutaneous tissue, or distant lymph node(s), microscopically confirmed pm1b: Metastasis to lung, microscopically confirmed pm1c: Metastasis to all other distant sites, microscopically confirmed Specify site(s), if known: + Additional Pathologic Findings + Specify: + Comment(s) + Data elements preceded by this symbol are not required for accreditation purposes. These optional elements may be clinically important but are not yet validated or regularly used in patient management. 5
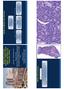
6 Background Documentation Explanatory Notes A. Tumor Thickness There are published 1 and unpublished data from 3 independent prospective cohorts of Merkel cell carcinoma (MCC) patients examining tumor thickness (measured in millimeters from the stratum granulosum to the deepest infiltrating tumor cells) as a prognostic indicator for outcome. 1,2 All 3 centers have data that find that tumor thickness is more predictive of outcome than maximum tumor diameter (a current staging parameter). In 2 of the studies, the outcome thus far examined was nodal metastasis; the 3rd study evaluated disease-specific survival. If the tumor is transected at the deep margin of the specimen, the depth may be indicated as at least mm with a comment explaining the limitation of thickness assessment. B. Mitotic Rate The presence of >10 mitotic figures/high-power field (HPF) has been shown to correlate with large tumor size as well as a poor prognosis. 3,4 The definition of what constitutes a high-power field was not specified in these reports; typically a 10X ocular and a 40X objective will yield a field area of approximately 0.15 mm 2, but this will differ from microscope to microscope and should be determined on an individual basis by direct measurement and calculation of the field or manufacturer s specifications. Reporting mitotic figures per square millimeter should have the advantage of greater reproducibility. The identification of no mitotic figures may be reported as <1/mm 2. Uniformly accepted thresholds for low- or high-risk mitotic counts are not established for either reporting method (number per HPF versus number per square millimeter), and this case summary item remains optional at this time. It has also been suggested that an MIB-1 proliferation index of greater than 50% is associated with a significantly worse prognosis. 4 C. Tumor-Infiltrating Lymphocytes Tumor-infiltrating lymphocytes (TILs) are defined as lymphocytes present at the interface of the tumor and the stroma. Some authors have suggested that the presence of TILs has been shown to portend a poor prognosis, especially when considered in concurrence with a tumor depth of >5 mm. 5 However, there are conflicting data on the subject. 4 In the absence of specific accepted guidelines for assessment of TILs, it is recommended in this protocol that, for purposes of uniformity, pathologists choosing to report TILs employ guidelines used for assessment of TILs as in cutaneous melanomas, given below: TILs not identified: No lymphocytes present, or lymphocytes present but do not infiltrate tumor at all. TILs nonbrisk: Lymphocytes infiltrate tumor only focally or not along the entire base of the vertical growth phase. TILs brisk: Lymphocytes diffusely infiltrate the entire base of the dermal tumor (Figure, A) or the entire invasive component of the tumor (Figure, B). 6
7 Background Documentation Brisk tumor-infiltrating lymphocytes. A, Lymphocytes diffusely infiltrate the entire base of the invasive tumor. B, Lymphocytes infiltrate the entire invasive component of the carcinoma. D. Tumor Growth Pattern In a series of 156 patients with MCC, nodular tumor growth pattern was found on both uni- and multivariate analysis to correlate with better survival. 1 Nodular pattern is defined as tumors with a relatively well-circumscribed interface with the surrounding tissue, typically composed of one or multiple nodules. 2 Infiltrative pattern is defined as tumors without a well-circumscribed interface with the surrounding tissue, composed of single cells, rows, trabeculae or strands of cells infiltrating through dermal collagen or deeper soft tissue. A tumor exhibiting both nodular and infiltrative patterns should be classified as infiltrative. E. Presence of Second Malignancy There is the occasional association of MCC and in situ SCC: primarily a histologic finding. 6 There is some question whether this is inversely correlated with merkel cell polyomavirus (MCPyV) detection. There is also an association of MCC with an immunosuppressed status, which may iatrogenic (transplant) or due an underlying malignancy that affects T cell immunity. The poor prognosis of MCC patients with underlying chronic lymphocytic leukemia (CLL) is therefore not necessarily due to the malignancy, but rather the associated immunosuppression. 7 F. Lymph Node Examination Clinical detection of nodal disease may be via inspection, palpation, and/or imaging. Micrometastases are defined by identification of metastasis on pathologic examination of sentinel or regional lymphadenectomy specimens. Macrometastases are defined as clinically detectable nodal metastases, confirmed by pathologic examination of therapeutic lymphadenectomy specimens. Because the pathologist may not have this clinical information, subdivision of N categories in the pathology report is optional. In-transit metastasis is defined as a tumor distinct from the primary lesion and located either (1) between the primary lesion and the draining node bed or (2) distal to the primary lesion. Metastatic MCC to the lymph node may be difficult to identify on routine hematoxylin-eosin (H&E)-stained sections. The use of immunostains has been shown to increase the sensitivity of identifying occult lymph node metastases. 8 It is strongly recommended that at least 1 immunostain be performed before designating a lymph node as negative. Depending on the experience or preference of the laboratory, stains may include but are not limited to AE1/AE3, CK116, Cam 5.2, CD56, CK20, synaptophysin, and/or chromogranin, many of which show a perinuclear dot-like staining pattern. All immunohistochemical results should be documented in the final pathology report. 7
8 Background Documentation Isolated tumor cells in a lymph node are classified as micrometastases (pn1a). G. TNM Staging An MCC-specific 4-tier staging system was first adopted by the American Joint Committee on Cancer (AJCC) in Recent analysis of more than 9300 patients with MCC was used to validate and revise the staging system for the 8 th edition of the AJCC Cancer Staging Manual published in Primary tumor dimension ( 2 cm versus >2 cm), nodal status, and stage at presentation remain the primary predictors of survival. 10 The most important changes in the updated 8 th edition staging system include: Separation of clinical and pathological stage groupings, consistent with other AJCC staging systems Elimination of stage I and II subgroups based on pathologic nodal status Inclusion of category pn1a(sn) into stage group IIIA for pathologically detected, clinically occult nodal metastasis identified only by sentinel lymph node biopsy without completion lymphadenectomy Inclusion of category T0 pn1b M0 in pathologic stage group IIIA, to identify patients with clinically detected nodal MCC metastases with unknown primary tumor Separation of patients with in-transit metastases into category pn2 without and pn3 with nodal metastases Those patients with MCC in whom the primary tumor cannot be assessed (eg, curetted) should be categorized as TX. Merkel cell carcinoma in situ (ie, completely limited to epidermis or adnexal epithelium) is categorized as Tis. The T category of MCC is classified primarily by measuring the maximum dimension of the tumor with a threshold of 2 cm (T1), >2 cm but 5 cm (T2), or >5 cm (T3). Extracutaneous invasion by the primary tumor into bone, muscle, fascia, or cartilage is classified as T4. Histologic measurement of tumor diameter is subject to underestimation due to shrinkage of formalin-fixed tissue and inaccuracy of measurement of the largest diameter of oval tumors. If clinical tumor size is unavailable, histopathologic gross or microscopic measurement should be used. 10 Regional metastases most commonly present in the regional lymph nodes. Nodal staging is primarily based on nodal tumor burden: microscopic versus macroscopic. Therefore, patients without clinical or radiologic evidence of lymph node metastases, but who have pathologically documented nodal metastases, are defined by convention as exhibiting microscopic or clinically occult nodal metastases. In contrast, MCC patients with both clinical evidence of nodal metastases and pathologic examination confirming nodal metastases are defined by convention as having macroscopic or clinically apparent nodal metastases. Distant metastases are defined as metastases that have spread beyond the draining lymph node basin, including cutaneous, nodal, and visceral sites. Stage Groupings Patients with primary Merkel cell carcinoma with no evidence of regional or distant metastases (either clinically or pathologically) are divided into 2 stages: stage I for primary tumors 2 cm in size and stage II for primary tumors >2 cm in size (IIA) or with extracutaneous invasion (IIB). Stage III is divided into stage groups IIIA for patients with microscopically positive and clinically occult nodes, and patients with clinically detected lymph node metastases with unknown primary tumor (T0), and IIIB for patients with clinically and/or radiologically detected regional lymph node and/or in-transit metastases. There are no subgroups of stage IV Merkel cell carcinoma. Clinical Stage Groups (ctnm) Stage 0 Tis N0 M0 Stage I T1 N0 M0 Stage IIA T2/T3 N0 M0 Stage IIB T4 N0 M0 Stage III T0-4 N1-3 M0 Stage IV T0-4 Any N M1 8
9 Background Documentation Pathological stage groups (ptnm) Stage 0 Tis N0 M0 Stage I T1 N0 M0 Stage IIA T2/3 N0 M0 Stage IIB T4 N0 M0 Stage IIIA T1-4 N1a(sn) or N1a M0 T0 N1b M0 Stage IIIB T1-4 N1b-3 M0 Stage IV T0-4 Any N M1 References 1. Smith FO Yue B, Marzban SS, et al. Both tumor depth and diameter are predictive of sentinel lymph node status and survival in Merkel cell carcinoma. Cancer. 2015;121(18): Andea AA, Coit DG, Amin B, Busam KJ. Merkel cell carcinoma: histologic features and prognosis. Cancer. 2008;113(9): Skelton HG, Smith KJ, Hitchcock CL, McCarthy WF, Lupton GP, Graham JH. Merkel cell carcinoma: analysis of clinical, histologic, and immunohistologic features of 132 cases with relation to survival. J Am Acad Dermatol. 1997;37(5 Pt 1): Llombart B, Monteagudo C, Lopez-Guerrero JA, et al. Clinicopathological and immunohistochemical analysis of 20 cases of Merkel cell carcinoma in search of prognostic markers. Histopathology. 2005;46(6): Mott RT, Smoller BR, Morgan MB. Merkel cell carcinoma: a clinicopathologic study with prognostic implications. J Cutan Pathol. 2004;31(3): Lai JH, Fleming KE, Ly TY, et al. Pure versus combined Merkel cell carcinonomas: immunohistochemical evaluation of cellular proteins (p53, bcl-2 and c-kit) reveals signification overexpression of p53 in combined tumors. Hum Pathol. 2015;46(9): Brewer JD, Shanafelt TD, Otley CC, et al. Chronic lymphocytic leukemia is associated with decreased survival of patients with malignnt melanoma and Merkel cell carcinoma in a SEER population-based study. J Clin Oncol. 2012;30(8): Allen PJ, Busam K, Hill AD, Stojadinovic A, Coit DG. Immunohistochemical analysis of sentinel lymph nodes from patients with Merkel cell carcinoma. Cancer. 2001;92(6): Harms KL, Healy MA, Nhgiem P, et al. Analysis of prognostic factors from 9387 merkel cell carcinoma cases forms the basis for the new 8th edition AJCC staging system. Ann Surg Oncol. 2016;23(11): Amin MB, Edge SB, Greene FL, et al, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer;
Protocol for the Examination of Specimens From Patients With Merkel Cell Carcinoma of the Skin
 Protocol for the Examination of Specimens From Patients With Merkel Cell Carcinoma of the Skin Protocol applies to Merkel cell carcinoma of cutaneous surfaces only. Based on AJCC/UICC TNM, 7th edition
Protocol for the Examination of Specimens From Patients With Merkel Cell Carcinoma of the Skin Protocol applies to Merkel cell carcinoma of cutaneous surfaces only. Based on AJCC/UICC TNM, 7th edition
46. Merkel Cell Carcinoma
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
Protocol applies to melanoma of cutaneous surfaces only.
 Melanoma of the Skin Protocol applies to melanoma of cutaneous surfaces only. Procedures Biopsy (No Accompanying Checklist) Excision Re-excision Protocol revision date: January 2005 Based on AJCC/UICC
Melanoma of the Skin Protocol applies to melanoma of cutaneous surfaces only. Procedures Biopsy (No Accompanying Checklist) Excision Re-excision Protocol revision date: January 2005 Based on AJCC/UICC
47. Melanoma of the Skin
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
Descriptor Definition Author s notes TNM descriptors Required only if applicable; select all that apply multiple foci of invasive carcinoma
 S5.01 The tumour stage and stage grouping must be recorded to the extent possible, based on the AJCC Cancer Staging Manual (7 th Edition). 11 (See Tables S5.01a and S5.01b below.) Table S5.01a AJCC breast
S5.01 The tumour stage and stage grouping must be recorded to the extent possible, based on the AJCC Cancer Staging Manual (7 th Edition). 11 (See Tables S5.01a and S5.01b below.) Table S5.01a AJCC breast
STAGE CATEGORY DEFINITIONS
 CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX Tis Tis (DCIS) Tis (LCIS) Tis (Paget s) T1 T1mi T1a T1b T1c a b c
CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX Tis Tis (DCIS) Tis (LCIS) Tis (Paget s) T1 T1mi T1a T1b T1c a b c
Melanoma Update: 8th Edition of AJCC Staging System
 Melanoma Update: 8th Edition of AJCC Staging System Rosalie Elenitsas, M.D. Professor of Dermatology Director, Dermatopathology University of Pennsylvania DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY None
Melanoma Update: 8th Edition of AJCC Staging System Rosalie Elenitsas, M.D. Professor of Dermatology Director, Dermatopathology University of Pennsylvania DISCLOSURE OF RELATIONSHIPS WITH INDUSTRY None
Translating Evidence into Practice: Primary Cutaneous Melanoma Guidelines. Sentinel Lymph Node Biopsy
 American Academy of Dermatology 2018 Annual Meeting San Diego, CA, February 17, 2018 Translating Evidence into Practice: Primary Cutaneous Melanoma Guidelines. Sentinel Lymph Node Biopsy Christopher Bichakjian,
American Academy of Dermatology 2018 Annual Meeting San Diego, CA, February 17, 2018 Translating Evidence into Practice: Primary Cutaneous Melanoma Guidelines. Sentinel Lymph Node Biopsy Christopher Bichakjian,
Collaborative Stage for TNM 7 - Revised 12/02/2009 [ Schema ]
![Collaborative Stage for TNM 7 - Revised 12/02/2009 [ Schema ] Collaborative Stage for TNM 7 - Revised 12/02/2009 [ Schema ]](/thumbs/82/86783199.jpg) CS Tumor Size Collaborative Stage for TNM 7 - Revised 12/02/2009 [ Schema ] Note: the specific tumor size as documented in the medical record. If the ONLY information regarding tumor size is the physician's
CS Tumor Size Collaborative Stage for TNM 7 - Revised 12/02/2009 [ Schema ] Note: the specific tumor size as documented in the medical record. If the ONLY information regarding tumor size is the physician's
Update on 8 th Edition Cutaneous AJCC Staging of Primary Cutaneous Melanoma. Michael T. Tetzlaff MD, PhD
 Update on 8 th Edition Cutaneous AJCC Staging of Primary Cutaneous Melanoma Michael T. Tetzlaff MD, PhD Associate Professor Departments of Pathology (Dermatopathology) and Translational and Molecular Pathology
Update on 8 th Edition Cutaneous AJCC Staging of Primary Cutaneous Melanoma Michael T. Tetzlaff MD, PhD Associate Professor Departments of Pathology (Dermatopathology) and Translational and Molecular Pathology
Chapter 2 Staging of Breast Cancer
 Chapter 2 Staging of Breast Cancer Zeynep Ozsaran and Senem Demirci Alanyalı 2.1 Introduction Five decades ago, Denoix et al. proposed classification system (tumor node metastasis [TNM]) based on the dissemination
Chapter 2 Staging of Breast Cancer Zeynep Ozsaran and Senem Demirci Alanyalı 2.1 Introduction Five decades ago, Denoix et al. proposed classification system (tumor node metastasis [TNM]) based on the dissemination
Protocol for the Examination of Specimens From Patients With Melanoma of the Skin
 Protocol for the Examination of Specimens From Patients With Melanoma of the Skin Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual For accreditation
Protocol for the Examination of Specimens From Patients With Melanoma of the Skin Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual For accreditation
Definition of Synoptic Reporting
 Definition of Synoptic Reporting The CAP has developed this list of specific features that define synoptic reporting formatting: 1. All required cancer data from an applicable cancer protocol that are
Definition of Synoptic Reporting The CAP has developed this list of specific features that define synoptic reporting formatting: 1. All required cancer data from an applicable cancer protocol that are
Clinicopathologic Features of Primary Merkel Cell Carcinoma: A Detailed Descriptive Analysis of a Large Contemporary Cohort
 Clinicopathologic Features of Primary Merkel Cell Carcinoma: A Detailed Descriptive Analysis of a Large Contemporary Cohort JENNIFER L. SCHWARTZ, MD,* CHRISTOPHER K. BICHAKJIAN, MD,* LORI LOWE, MD,* KENT
Clinicopathologic Features of Primary Merkel Cell Carcinoma: A Detailed Descriptive Analysis of a Large Contemporary Cohort JENNIFER L. SCHWARTZ, MD,* CHRISTOPHER K. BICHAKJIAN, MD,* LORI LOWE, MD,* KENT
Primary Cutaneous Melanoma Pathology Reporting Proforma DD MM YYYY. *Tumour site. *Specimen laterality. *Specimen type
 Primary Cutaneous Melanoma Pathology Reporting Proforma Includes the International Collaboration on Cancer reporting dataset denoted by * Family name Given name(s) Date of birth DD MM YYYY Sex Male Female
Primary Cutaneous Melanoma Pathology Reporting Proforma Includes the International Collaboration on Cancer reporting dataset denoted by * Family name Given name(s) Date of birth DD MM YYYY Sex Male Female
Michael T. Tetzlaff MD, PhD
 American Joint Cancer Committee (AJCC) staging system for primary cutaneous melanoma (8 th Edition) and principles of sentinel lymph node evaluation Emphasis on concise and accurate reporting of primary
American Joint Cancer Committee (AJCC) staging system for primary cutaneous melanoma (8 th Edition) and principles of sentinel lymph node evaluation Emphasis on concise and accurate reporting of primary
Talk to Your Doctor. Fact Sheet
 Talk to Your Doctor Hearing the words you have skin cancer is overwhelming and would leave anyone with a lot of questions. If you have been diagnosed with Stage I or II cutaneous melanoma with no apparent
Talk to Your Doctor Hearing the words you have skin cancer is overwhelming and would leave anyone with a lot of questions. If you have been diagnosed with Stage I or II cutaneous melanoma with no apparent
Michael T. Tetzlaff MD, PhD
 Update on American Joint Cancer Committee (AJCC) staging system for primary cutaneous melanoma Emphasis on concise and accurate reporting of primary and metastatic melanoma for effective risk stratification
Update on American Joint Cancer Committee (AJCC) staging system for primary cutaneous melanoma Emphasis on concise and accurate reporting of primary and metastatic melanoma for effective risk stratification
Update on Merkel cell carcinoma
 Merkel cell carcinoma: Diagnosis, staging, sentinel lymph node biopsy and prognostic markers Michael T. Tetzlaff MD, PhD Associate Professor Departments of Pathology (Dermatopathology) and Translational
Merkel cell carcinoma: Diagnosis, staging, sentinel lymph node biopsy and prognostic markers Michael T. Tetzlaff MD, PhD Associate Professor Departments of Pathology (Dermatopathology) and Translational
category cm0. Category will ensure it T1 melanoma. 68 Retinoblastoma
 AJCC 8 th Edition Chapter 1 Principles of Cancer Staging: Node Status Not Required in Rare Circumstances Clinical Staging, cn Category For some cancer sites in which lymph node involvement is rare, patients
AJCC 8 th Edition Chapter 1 Principles of Cancer Staging: Node Status Not Required in Rare Circumstances Clinical Staging, cn Category For some cancer sites in which lymph node involvement is rare, patients
Merkel cell carcinoma: Diagnosis, staging, sentinel lymph node biopsy and prognostic markers
 Merkel cell carcinoma: Diagnosis, staging, sentinel lymph node biopsy and prognostic markers Michael T. Tetzlaff MD, PhD Associate Professor Departments of Pathology (Dermatopathology) and Translational
Merkel cell carcinoma: Diagnosis, staging, sentinel lymph node biopsy and prognostic markers Michael T. Tetzlaff MD, PhD Associate Professor Departments of Pathology (Dermatopathology) and Translational
Staging for Residents, Nurses, and Multidisciplinary Health Care Team
 Staging for Residents, Nurses, and Multidisciplinary Health Care Team Donna M. Gress, RHIT, CTR Validating science. Improving patient care. Learning Objectives Introduce the concept and history of stage
Staging for Residents, Nurses, and Multidisciplinary Health Care Team Donna M. Gress, RHIT, CTR Validating science. Improving patient care. Learning Objectives Introduce the concept and history of stage
Case Scenario 1 Worksheet. Primary Site C44.4 Morphology 8743/3 Laterality 0 Stage/ Prognostic Factors
 CASE SCENARIO 1 9/10/13 HISTORY: Patient is a 67-year-old white male and presents with lesion located 4-5cm above his right ear. The lesion has been present for years. No lymphadenopathy. 9/10/13 anterior
CASE SCENARIO 1 9/10/13 HISTORY: Patient is a 67-year-old white male and presents with lesion located 4-5cm above his right ear. The lesion has been present for years. No lymphadenopathy. 9/10/13 anterior
Uterine Cervix. Protocol applies to all invasive carcinomas of the cervix.
 Uterine Cervix Protocol applies to all invasive carcinomas of the cervix. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
Uterine Cervix Protocol applies to all invasive carcinomas of the cervix. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition and FIGO 2001 Annual Report Procedures Cytology (No Accompanying
Rebecca Vogel, PGY-4 March 5, 2012
 Rebecca Vogel, PGY-4 March 5, 2012 Historical Perspective Changes In The Staging System Studies That Started The Talk Where We Go From Here Cutaneous melanoma has become an increasingly growing problem,
Rebecca Vogel, PGY-4 March 5, 2012 Historical Perspective Changes In The Staging System Studies That Started The Talk Where We Go From Here Cutaneous melanoma has become an increasingly growing problem,
Thyroid Gland. Protocol applies to all malignant tumors of the thyroid gland, except lymphomas.
 Thyroid Gland Protocol applies to all malignant tumors of the thyroid gland, except lymphomas. Procedures Cytology (No Accompanying Checklist) Partial Thyroidectomy Total Thyroidectomy With/Without Lymph
Thyroid Gland Protocol applies to all malignant tumors of the thyroid gland, except lymphomas. Procedures Cytology (No Accompanying Checklist) Partial Thyroidectomy Total Thyroidectomy With/Without Lymph
6. Cervical Lymph Nodes and Unknown Primary Tumors of the Head and Neck
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
3/23/2017. Disclosure of Relevant Financial Relationships. Pathologic Staging Updates in Breast Cancer. Pathologic Staging Updates Breast Cancer
 Pathologic Staging Updates in Breast Cancer Disclosure of Relevant Financial Relationships USCAP requires that all planners (Education Committee) in a position to influence or control the content of CME
Pathologic Staging Updates in Breast Cancer Disclosure of Relevant Financial Relationships USCAP requires that all planners (Education Committee) in a position to influence or control the content of CME
8th Edition of the TNM Classification for Lung Cancer. Proposed by the IASLC
 8th Edition of the TNM Classification for Lung Cancer Proposed by the IASLC Introduction Stage classification - provides consistency in nomenclature - improves understanding of anatomic extent of tumour
8th Edition of the TNM Classification for Lung Cancer Proposed by the IASLC Introduction Stage classification - provides consistency in nomenclature - improves understanding of anatomic extent of tumour
B REAST STAGING FORM. PATHOLOGIC Extent of disease through completion of definitive surgery. CLINICAL Extent of disease before any treatment
 B REAST STAGING FORM Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery (DCIS) (LCIS) (Paget s) mi a b c a b c d TUMOR SIZE: S TAGE
B REAST STAGING FORM Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery (DCIS) (LCIS) (Paget s) mi a b c a b c d TUMOR SIZE: S TAGE
ACRIN 6666 Therapeutic Surgery Form
 S1 ACRIN 6666 Therapeutic Surgery Form 6666 Instructions: Complete a separate S1 form for each separate area of each breast excised with the intent to treat a cancer (e.g. each lumpectomy or mastectomy).
S1 ACRIN 6666 Therapeutic Surgery Form 6666 Instructions: Complete a separate S1 form for each separate area of each breast excised with the intent to treat a cancer (e.g. each lumpectomy or mastectomy).
Radiotherapy and Conservative Surgery For Merkel Cell Carcinoma - The British Columbia Cancer Agency Experience
 Radiotherapy and Conservative Surgery For Merkel Cell Carcinoma - The British Columbia Cancer Agency Experience Poster No.: RO-0003 Congress: RANZCR FRO 2012 Type: Scientific Exhibit Authors: C. Harrington,
Radiotherapy and Conservative Surgery For Merkel Cell Carcinoma - The British Columbia Cancer Agency Experience Poster No.: RO-0003 Congress: RANZCR FRO 2012 Type: Scientific Exhibit Authors: C. Harrington,
This form may provide more data elements than required for collection by standard setters such as NCI SEER, CDC NPCR, and CoC NCDB.
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
5/8/2014. AJCC Stage Introduction and General Rules. Acknowledgements* Introduction. Melissa Pearson, CTR North Carolina Central Cancer Registry
 AJCC Stage Introduction and General Rules Linda Mulvihill Public Health Advisor NCRA Annual Meeting May 2014 National Center for Chronic Disease Prevention and Health Promotion Division of Cancer Prevention
AJCC Stage Introduction and General Rules Linda Mulvihill Public Health Advisor NCRA Annual Meeting May 2014 National Center for Chronic Disease Prevention and Health Promotion Division of Cancer Prevention
Patient age and cutaneous malignant melanoma: Elderly patients are likely to have more aggressive histological features and poorer survival
 MOLECULAR AND CLINICAL ONCOLOGY 7: 1083-1088, 2017 Patient age and cutaneous malignant melanoma: Elderly patients are likely to have more aggressive histological features and poorer survival FARUK TAS
MOLECULAR AND CLINICAL ONCOLOGY 7: 1083-1088, 2017 Patient age and cutaneous malignant melanoma: Elderly patients are likely to have more aggressive histological features and poorer survival FARUK TAS
Protocol for the Examination of Specimens From Patients With Squamous Cell Carcinoma of the Skin
 Protocol for the Examination of Specimens From Patients With Squamous Cell Carcinoma of the Skin Protocol applies to invasive squamous cell carcinomas of the skin. Squamous cell carcinomas of the eyelid,
Protocol for the Examination of Specimens From Patients With Squamous Cell Carcinoma of the Skin Protocol applies to invasive squamous cell carcinomas of the skin. Squamous cell carcinomas of the eyelid,
Disclosures. Outline. What IS tumor budding?? Tumor Budding in Colorectal Carcinoma: What, Why, and How. I have nothing to disclose
 Tumor Budding in Colorectal Carcinoma: What, Why, and How Disclosures I have nothing to disclose Soo-Jin Cho, MD, PhD Assistant Professor UCSF Dept of Pathology Current Issues in Anatomic Pathology 2017
Tumor Budding in Colorectal Carcinoma: What, Why, and How Disclosures I have nothing to disclose Soo-Jin Cho, MD, PhD Assistant Professor UCSF Dept of Pathology Current Issues in Anatomic Pathology 2017
B REAST STAGING FORM. PATHOLOGIC Extent of disease through completion of definitive surgery. CLINICAL Extent of disease before any treatment
 B REAST STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery (DCIS) (LCIS) (Paget s) mi c a b c d TUMOR SIZE:
B REAST STAGING FORM CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery (DCIS) (LCIS) (Paget s) mi c a b c d TUMOR SIZE:
CAP Cancer Protocol and ecc Summary of Changes for August 2014 Thyroid Agile Release
 CAP Cancer Protocol and ecc Summary of Changes for August 2014 Thyroid Agile Release 2 REVISION HISTORY Date Author / Editor Comments 5/19/2014 Jaleh Mirza Created the document 8/12/2014 Samantha Spencer/Jaleh
CAP Cancer Protocol and ecc Summary of Changes for August 2014 Thyroid Agile Release 2 REVISION HISTORY Date Author / Editor Comments 5/19/2014 Jaleh Mirza Created the document 8/12/2014 Samantha Spencer/Jaleh
M ALIGNANT MELANOMA OF THE UVEA STAGING FORM
 CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX T0 a a a d b c d a TUMOR SIZE: S TAGE C ATEGORY D EFINITIONS LATERALITY:
CLINICAL Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX T0 a a a d b c d a TUMOR SIZE: S TAGE C ATEGORY D EFINITIONS LATERALITY:
INTRODUCTION TO CANCER STAGING
 INTRODUCTION TO CANCER STAGING Patravoot Vatanasapt, MD Dept. Otorhinolaryngology Khon Kaen Cancer Registry Faculty of Medicine Khon Kaen University THAILAND Staging is the attempt to assess the size
INTRODUCTION TO CANCER STAGING Patravoot Vatanasapt, MD Dept. Otorhinolaryngology Khon Kaen Cancer Registry Faculty of Medicine Khon Kaen University THAILAND Staging is the attempt to assess the size
Melanoma Case Scenario 1
 Melanoma Case Scenario 1 History and physical 11/5/16 Patient is a single, 48-year-old male in good health who presented to his primary physician for a yearly physical exam during which a 3.4 x 2.8 x 1.5
Melanoma Case Scenario 1 History and physical 11/5/16 Patient is a single, 48-year-old male in good health who presented to his primary physician for a yearly physical exam during which a 3.4 x 2.8 x 1.5
Interactive Staging Bee
 Interactive Staging Bee ROBIN BILLET, MA, CTR GA/SC REGIONAL CONFERENCE NOVEMBER 6, 2018? Clinical Staging includes any information obtained about the extent of cancer obtained before initiation of treatment
Interactive Staging Bee ROBIN BILLET, MA, CTR GA/SC REGIONAL CONFERENCE NOVEMBER 6, 2018? Clinical Staging includes any information obtained about the extent of cancer obtained before initiation of treatment
Update on staging colorectal carcinoma, the 8 th edition AJCC. General overview of staging. When is staging required? 11/1/2017
 Update on staging colorectal carcinoma, the 8 th edition AJCC Dale C. Snover, MD November 3, 2017 General overview of staging Reason for uniform staging Requirements to use AJCC manual and/or CAP protocols
Update on staging colorectal carcinoma, the 8 th edition AJCC Dale C. Snover, MD November 3, 2017 General overview of staging Reason for uniform staging Requirements to use AJCC manual and/or CAP protocols
Pancreas (Exocrine) Protocol applies to all carcinomas of the exocrine pancreas.
 Pancreas (Exocrine) Protocol applies to all carcinomas of the exocrine pancreas. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist)
Pancreas (Exocrine) Protocol applies to all carcinomas of the exocrine pancreas. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist)
Melanoma Case Scenario 1
 Melanoma Case Scenario 1 History and physical 11/5/16 Patient is a single, 48-year-old male in good health who presented to his primary physician for a yearly physical exam during which a 3.4 x 2.8 x 1.5
Melanoma Case Scenario 1 History and physical 11/5/16 Patient is a single, 48-year-old male in good health who presented to his primary physician for a yearly physical exam during which a 3.4 x 2.8 x 1.5
Protocol for the Examination of Specimens From Patients With Thymic Tumors
 Protocol for the Examination of Specimens From Patients With Thymic Tumors Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual For accreditation
Protocol for the Examination of Specimens From Patients With Thymic Tumors Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual For accreditation
Major Rule Changes. Donna M. Gress, RHIT, CTR Technical Editor, AJCC Cancer Staging Manual First Author, Chapter 1: Principles of Cancer Staging
 AJCC 8 th Edition Staging Major Rule Changes Donna M. Gress, RHIT, CTR Technical Editor, AJCC Cancer Staging Manual First Author, Chapter 1: Principles of Cancer Staging Validating science. Improving patient
AJCC 8 th Edition Staging Major Rule Changes Donna M. Gress, RHIT, CTR Technical Editor, AJCC Cancer Staging Manual First Author, Chapter 1: Principles of Cancer Staging Validating science. Improving patient
Greater Manchester and Cheshire HPB Unit Guidelines for the Assessment & Management of Hepatobiliary and Pancreatic Disease Chapter 14
 Greater Manchester and Cheshire HPB Unit Guidelines for the Assessment & Management of Hepatobiliary and Pancreatic Disease Chapter 14 Contents 14. Neuroendocrine Tumours 161 14.1. Diagnostic algorithm
Greater Manchester and Cheshire HPB Unit Guidelines for the Assessment & Management of Hepatobiliary and Pancreatic Disease Chapter 14 Contents 14. Neuroendocrine Tumours 161 14.1. Diagnostic algorithm
This form may provide more data elements than required for collection by standard setters such as NCI SEER, CDC NPCR, and CoC NCDB.
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
Malignant tumors of melanocytes : Part 3. Deba P Sarma, MD., Omaha
 Malignant tumors of melanocytes : Part 3 Deba P Sarma, MD., Omaha Let s go over one case of melanoma using the following worksheet. Of the various essential information that needs to be included in the
Malignant tumors of melanocytes : Part 3 Deba P Sarma, MD., Omaha Let s go over one case of melanoma using the following worksheet. Of the various essential information that needs to be included in the
AJCC 8 Implementation January 1, 2018 Melanoma of the Skin. Suraj Venna
 AJCC 8 Implementation January 1, 2018 Melanoma of the Skin Suraj Venna Personalized Medicine AJCC 8 th Edition This Time It s Personal Traditional AJCC (TNM) population-based analyses of large databases
AJCC 8 Implementation January 1, 2018 Melanoma of the Skin Suraj Venna Personalized Medicine AJCC 8 th Edition This Time It s Personal Traditional AJCC (TNM) population-based analyses of large databases
Completing the Puzzle AJCC TNM Staging Breast. Nicole Catlett, CTR 2017 Kentucky Cancer Registry Fall Conference, September 21 & 22, 2017
 Completing the Puzzle AJCC TNM Staging Breast Nicole Catlett, CTR 2017 Kentucky Cancer Registry Fall Conference, September 21 & 22, 2017 OBJECTIVES Understanding of Breast TNM staging Identify clinical
Completing the Puzzle AJCC TNM Staging Breast Nicole Catlett, CTR 2017 Kentucky Cancer Registry Fall Conference, September 21 & 22, 2017 OBJECTIVES Understanding of Breast TNM staging Identify clinical
NAACCR Webinar Series 1
 Collecting Cancer Data: Melanoma 2013 2014 NAACCR Webinar Series April 3, 2014 Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching
Collecting Cancer Data: Melanoma 2013 2014 NAACCR Webinar Series April 3, 2014 Q&A Please submit all questions concerning webinar content through the Q&A panel. Reminder: If you have participants watching
Protocol for the Examination of Specimens From Patients With Invasive Carcinoma of the Breast
 Protocol for the Examination of Specimens From Patients With Invasive Carcinoma of the Breast Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual
Protocol for the Examination of Specimens From Patients With Invasive Carcinoma of the Breast Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC Staging Manual
Small Intestine. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition
 Small Intestine Protocol applies to all invasive carcinomas of the small intestine, including those with focal endocrine differentiation. Excludes carcinoid tumors, lymphomas, and stromal tumors (sarcomas).
Small Intestine Protocol applies to all invasive carcinomas of the small intestine, including those with focal endocrine differentiation. Excludes carcinoid tumors, lymphomas, and stromal tumors (sarcomas).
VULVAR CARCINOMA. Page 1 of 5
 VULVAR CARCINOMA EXAMPLE OF A VULVAR CARCINOMA USING PROPOSED TEMPLATE Case: Invasive squamous cell carcinoma arising in D-VIN Tumor in left labia major Left partial vaginectomy and sentinel lymph node
VULVAR CARCINOMA EXAMPLE OF A VULVAR CARCINOMA USING PROPOSED TEMPLATE Case: Invasive squamous cell carcinoma arising in D-VIN Tumor in left labia major Left partial vaginectomy and sentinel lymph node
Retinoblastoma. Protocol applies to retinoblastoma only.
 Retinoblastoma Protocol applies to retinoblastoma only. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist) Biopsy (No Accompanying
Retinoblastoma Protocol applies to retinoblastoma only. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist) Biopsy (No Accompanying
11/21/13 CEA: 1.7 WNL
 Case Scenario 1 A 70 year-old white male presented to his primary care physician with a recent history of rectal bleeding. He was referred for imaging and a colonoscopy and was found to have adenocarcinoma.
Case Scenario 1 A 70 year-old white male presented to his primary care physician with a recent history of rectal bleeding. He was referred for imaging and a colonoscopy and was found to have adenocarcinoma.
Protocol for the Examination of Resection Specimens From Patients With Invasive Carcinoma of the Breast
 Protocol for the Examination of Specimens From Patients With Invasive Carcinoma of the Breast Version: Breast Invasive 4.2.0.0 Protocol Posting Date: February 2019 CAP Laboratory Accreditation Program
Protocol for the Examination of Specimens From Patients With Invasive Carcinoma of the Breast Version: Breast Invasive 4.2.0.0 Protocol Posting Date: February 2019 CAP Laboratory Accreditation Program
Uveal Melanoma. Protocol applies to malignant melanoma of the uvea.
 Uveal Melanoma Protocol applies to malignant melanoma of the uvea. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist) Biopsy (No Accompanying
Uveal Melanoma Protocol applies to malignant melanoma of the uvea. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6 th edition Procedures Cytology (No Accompanying Checklist) Biopsy (No Accompanying
Protocol for the Examination of Specimens From Patients With Melanoma of the Skin
 Protocol for the Examination of Specimens From Patients With Melanoma of the Skin Protocol applies to melanoma of cutaneous surfaces only. Based on AJCC/UICC TNM, 7th edition Protocol web posting date:
Protocol for the Examination of Specimens From Patients With Melanoma of the Skin Protocol applies to melanoma of cutaneous surfaces only. Based on AJCC/UICC TNM, 7th edition Protocol web posting date:
WHAT DOES THE PATHOLOGY REPORT MEAN?
 Melanoma WHAT IS MELANOMA? Melanoma is a type of cancer that affects cells called melanocytes. These cells are found mainly in skin but also in the lining of other areas such as nose and rectum, and also
Melanoma WHAT IS MELANOMA? Melanoma is a type of cancer that affects cells called melanocytes. These cells are found mainly in skin but also in the lining of other areas such as nose and rectum, and also
S1.04 Principal clinician. G1.01 Comments. G2.01 *Specimen dimensions (prostate) S2.02 *Seminal vesicles
 Prostate Cancer Histopathology Reporting Proforma (Radical Prostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family name Given name(s) Date of birth Sex Male
Prostate Cancer Histopathology Reporting Proforma (Radical Prostatectomy) Includes the International Collaboration on Cancer reporting dataset denoted by * Family name Given name(s) Date of birth Sex Male
Seventh Edition Staging 2017 Breast
 Seventh Edition Staging 2017 Breast Donna M. Gress, RHIT, CTR Validating science. Improving patient care. No materials in this presentation may be repurposed in print or online without the express written
Seventh Edition Staging 2017 Breast Donna M. Gress, RHIT, CTR Validating science. Improving patient care. No materials in this presentation may be repurposed in print or online without the express written
Ritu Nayar, MD Professor and Vice Chair of Pathology Northwestern University, Feinberg School of Medicine Chicago, IL
 Ritu Nayar, MD Professor and Vice Chair of Pathology Northwestern University, Feinberg School of Medicine Chicago, IL email: r-nayar@northwestern.edu Nothing to disclose College of American Pathologists
Ritu Nayar, MD Professor and Vice Chair of Pathology Northwestern University, Feinberg School of Medicine Chicago, IL email: r-nayar@northwestern.edu Nothing to disclose College of American Pathologists
Testis. Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies.
 Testis Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies. Protocol revision date: January 2005 Based on AJCC/UICC
Testis Protocol applies to all malignant germ cell and malignant sex cord-stromal tumors of the testis, exclusive of paratesticular malignancies. Protocol revision date: January 2005 Based on AJCC/UICC
NAACCR Webinar Series 1
 Collecting Cancer Data: Skin Malignancies 2/4/2010 NAACCR 2009 2010 Webinar Series Questions Please use the Q&A panel to submit your questions Send questions to All Panelist Collecting Cancer Data: Skin
Collecting Cancer Data: Skin Malignancies 2/4/2010 NAACCR 2009 2010 Webinar Series Questions Please use the Q&A panel to submit your questions Send questions to All Panelist Collecting Cancer Data: Skin
Protocol for the Examination of Specimens From Patients With Primary Gestational Trophoblastic Malignancy
 Protocol for the Examination of Specimens From Patients With Primary Gestational Trophoblastic Malignancy Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC
Protocol for the Examination of Specimens From Patients With Primary Gestational Trophoblastic Malignancy Version: Protocol Posting Date: June 2017 Includes ptnm requirements from the 8 th Edition, AJCC
Sentinel Node Alphabet Soup: MSLT-1, DeCOG-SLT, MSLT-2, UNC
 Sentinel Node Alphabet Soup: MSLT-1, DeCOG-SLT, MSLT-2, UNC David W. Ollila MD James and Jesse Millis Professor of Surgery University of North Carolina, Chapel Hill Disclosures: None July 15, 2018 AJCC
Sentinel Node Alphabet Soup: MSLT-1, DeCOG-SLT, MSLT-2, UNC David W. Ollila MD James and Jesse Millis Professor of Surgery University of North Carolina, Chapel Hill Disclosures: None July 15, 2018 AJCC
Merkel Cell Carcinoma
 NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) NCCN Evidence Blocks Version 2.2018 June 15, 2018 NCCN.org Continue Version 2.2018, 06/15/18 National Comprehensive Cancer Network, Inc.
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) NCCN Evidence Blocks Version 2.2018 June 15, 2018 NCCN.org Continue Version 2.2018, 06/15/18 National Comprehensive Cancer Network, Inc.
Colon and Rectum. Protocol revision date: January 2005 Based on AJCC/UICC TNM, 6th edition
 Colon and Rectum Protocol applies to all invasive carcinomas of the colon and rectum. Carcinoid tumors, lymphomas, sarcomas, and tumors of the vermiform appendix are excluded. Protocol revision date: January
Colon and Rectum Protocol applies to all invasive carcinomas of the colon and rectum. Carcinoid tumors, lymphomas, sarcomas, and tumors of the vermiform appendix are excluded. Protocol revision date: January
Merkel Cell Carcinoma
 NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) NCCN Evidence Blocks Version 1.2018 October 6, 2017 NCCN.org Continue *Christopher K. Bichakjian, MD/Chair ϖ University of Michigan Comprehensive
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ) NCCN Evidence Blocks Version 1.2018 October 6, 2017 NCCN.org Continue *Christopher K. Bichakjian, MD/Chair ϖ University of Michigan Comprehensive
Desmoplastic Melanoma: Surgical Management and Adjuvant Therapy
 Desmoplastic Melanoma: Surgical Management and Adjuvant Therapy Dale Han, MD Assistant Professor Department of Surgery Section of Surgical Oncology No disclosures Background Desmoplastic melanoma (DM)
Desmoplastic Melanoma: Surgical Management and Adjuvant Therapy Dale Han, MD Assistant Professor Department of Surgery Section of Surgical Oncology No disclosures Background Desmoplastic melanoma (DM)
Carcinoma of the Renal Pelvis and Ureter Histopathology
 Carcinoma of the Renal Pelvis and Ureter Histopathology Reporting Proforma (NEPHROURETERECTOMY AND URETERECTOMY) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
Carcinoma of the Renal Pelvis and Ureter Histopathology Reporting Proforma (NEPHROURETERECTOMY AND URETERECTOMY) Includes the International Collaboration on Cancer reporting dataset denoted by * Family
Registrar s Guide to Chapter 1, AJCC Seventh Edition. Overview. Learning Objectives. Describe intent and purpose of AJCC staging
 Registrar s Guide to Donna M. Gress, RHIT, CTR Validating science. Improving patient care. This presentation was supported by the Cooperative Agreement Number DP13-1310 from The Centers for Disease Control
Registrar s Guide to Donna M. Gress, RHIT, CTR Validating science. Improving patient care. This presentation was supported by the Cooperative Agreement Number DP13-1310 from The Centers for Disease Control
The New AJCC: 8 th Edition and Beyond. 8th Edition AJCC Melanoma Staging System. Jeffrey E. Gershenwald, MD, FACS. AJCC Physician to Physician
 AJCC Physician to Physician 8th Edition AJCC Melanoma Staging System Jeffrey E. Gershenwald, MD, FACS Dr. John M. Skibber Professor, Department of Surgical Oncology Professor, Department of Cancer Biology
AJCC Physician to Physician 8th Edition AJCC Melanoma Staging System Jeffrey E. Gershenwald, MD, FACS Dr. John M. Skibber Professor, Department of Surgical Oncology Professor, Department of Cancer Biology
Protocol for the Examination of Specimens from Patients with Invasive Carcinoma of the Breast
 Protocol for the Examination of Specimens from Patients with Invasive Carcinoma of the Breast Protocol applies to all invasive carcinomas of the breast, including ductal carcinoma in situ (DCIS) with microinvasion.
Protocol for the Examination of Specimens from Patients with Invasive Carcinoma of the Breast Protocol applies to all invasive carcinomas of the breast, including ductal carcinoma in situ (DCIS) with microinvasion.
Gastric Cancer Histopathology Reporting Proforma
 Gastric Cancer Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given name(s) Date of birth Sex Male Female Intersex/indeterminate
Gastric Cancer Histopathology Reporting Proforma Mandatory questions (i.e. protocol standards) are in bold (e.g. S1.01). S1.01 Identification Family name Given name(s) Date of birth Sex Male Female Intersex/indeterminate
Exercise 15: CSv2 Data Item Coding Instructions ANSWERS
 Exercise 15: CSv2 Data Item Coding Instructions ANSWERS CS Tumor Size Tumor size is the diameter of the tumor, not the depth or thickness of the tumor. Chest x-ray shows 3.5 cm mass; the pathology report
Exercise 15: CSv2 Data Item Coding Instructions ANSWERS CS Tumor Size Tumor size is the diameter of the tumor, not the depth or thickness of the tumor. Chest x-ray shows 3.5 cm mass; the pathology report
Disclosures. SLNB for Melanoma 25/02/2014 SENTINEL LYMPH NODE BIOPSY FOR MELANOMA: CURRENT GUIDELINES AND THEIR CLINICAL APPLICATION
 8 th Canadian Melanoma Conference February 22, 2014 Rimrock Resort Hotel, Banff, Alberta SENTINEL LYMPH NODE BIOPSY FOR MELANOMA: CURRENT GUIDELINES AND THEIR CLINICAL APPLICATION Christopher Bichakjian,
8 th Canadian Melanoma Conference February 22, 2014 Rimrock Resort Hotel, Banff, Alberta SENTINEL LYMPH NODE BIOPSY FOR MELANOMA: CURRENT GUIDELINES AND THEIR CLINICAL APPLICATION Christopher Bichakjian,
Procedures Needle Biopsy Transurethral Prostatic Resection Suprapubic or Retropubic Enucleation (Subtotal Prostatectomy) Radical Prostatectomy
 Prostate Gland Protocol applies to invasive carcinomas of the prostate gland. Protocol web posting date: July 2006 Protocol effective date: April 2007 Based on AJCC/UICC TNM, 6 th edition Procedures Needle
Prostate Gland Protocol applies to invasive carcinomas of the prostate gland. Protocol web posting date: July 2006 Protocol effective date: April 2007 Based on AJCC/UICC TNM, 6 th edition Procedures Needle
NUMERATOR: Reports that include the pt category, the pn category and the histologic grade
 Quality ID #100 (NQF 0392): Colorectal Cancer Resection Pathology Reporting: pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes) with Histologic Grade National Quality Strategy Domain: Effective
Quality ID #100 (NQF 0392): Colorectal Cancer Resection Pathology Reporting: pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes) with Histologic Grade National Quality Strategy Domain: Effective
You Are Going to Cut How Much Skin? Locoregional Surgical Treatment. Justin Rivard MD, MSc, FRCSC September 21, 2018
 You Are Going to Cut How Much Skin? Locoregional Surgical Treatment Justin Rivard MD, MSc, FRCSC September 21, 2018 Presenter Disclosure Faculty/Speaker: Justin Rivard Relationships with financial sponsors:
You Are Going to Cut How Much Skin? Locoregional Surgical Treatment Justin Rivard MD, MSc, FRCSC September 21, 2018 Presenter Disclosure Faculty/Speaker: Justin Rivard Relationships with financial sponsors:
Kidney Case 1 SURGICAL PATHOLOGY REPORT
 Kidney Case 1 Surgical Pathology Report February 9, 2007 Clinical History: This 45 year old woman was found to have a left renal mass. CT urography with reconstruction revealed a 2 cm medial mass which
Kidney Case 1 Surgical Pathology Report February 9, 2007 Clinical History: This 45 year old woman was found to have a left renal mass. CT urography with reconstruction revealed a 2 cm medial mass which
L ARYNX S TAGING F ORM
 CLI N I CA L Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX T0 Tis a b L ARYNX S TAGING F ORM LATERALITY: TUMOR SIZE: left
CLI N I CA L Extent of disease before any treatment y clinical staging completed after neoadjuvant therapy but before subsequent surgery TX T0 Tis a b L ARYNX S TAGING F ORM LATERALITY: TUMOR SIZE: left
You Want ME to Stage that Case???
 You Want ME to Stage that Case??? Jayne Holubowsky, CTR, Director, Virginia Cancer Registry 2 nd DelMarVa-DC Regional Conference October 11, 2018 What s New in the AJCC 8 th Edition Objectives Explain
You Want ME to Stage that Case??? Jayne Holubowsky, CTR, Director, Virginia Cancer Registry 2 nd DelMarVa-DC Regional Conference October 11, 2018 What s New in the AJCC 8 th Edition Objectives Explain
NUMERATOR: Reports that include the pt category, the pn category and the histologic grade
 Quality ID #100 (NQF 0392): Colorectal Cancer Resection Pathology Reporting: pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes) with Histologic Grade National Quality Strategy Domain: Effective
Quality ID #100 (NQF 0392): Colorectal Cancer Resection Pathology Reporting: pt Category (Primary Tumor) and pn Category (Regional Lymph Nodes) with Histologic Grade National Quality Strategy Domain: Effective
Melanoma of the Skin INTRODUCTION SUMMARY OF CHANGES
 24 Melanoma of the Skin C44.0 Skin of lip, NOS C44.1 Eyelid C44.2 External ear C44.3 Skin of other and unspecified parts of face C44.4 Skin of scalp and neck C44.5 Skin of trunk C44.6 Skin of upper limb
24 Melanoma of the Skin C44.0 Skin of lip, NOS C44.1 Eyelid C44.2 External ear C44.3 Skin of other and unspecified parts of face C44.4 Skin of scalp and neck C44.5 Skin of trunk C44.6 Skin of upper limb
Collaborative Stage for TNM 7 - Revised 07/14/2009 [ Schema ]
![Collaborative Stage for TNM 7 - Revised 07/14/2009 [ Schema ] Collaborative Stage for TNM 7 - Revised 07/14/2009 [ Schema ]](/thumbs/71/65936251.jpg) MelanomaSkin CS Tumor Size Collaborative Stage for TNM 7 - Revised 07/14/2009 [ Schema ] Code 000 No mass/tumor found Description 001-988 001-988 millimeters (code exact size in millimeters) 989 989 millimeters
MelanomaSkin CS Tumor Size Collaborative Stage for TNM 7 - Revised 07/14/2009 [ Schema ] Code 000 No mass/tumor found Description 001-988 001-988 millimeters (code exact size in millimeters) 989 989 millimeters
3/23/2017. Disclaimer. Common Changes in TNM Staging. Overview. Overview. Understanding Terminology. Overview
 Common Changes in TNM Staging Understanding the General Rules of Cancer Staging Thomas P. Baker, MD FCAP March 5, 2017 Disclaimer The identification of specific products or scientific instrumentation is
Common Changes in TNM Staging Understanding the General Rules of Cancer Staging Thomas P. Baker, MD FCAP March 5, 2017 Disclaimer The identification of specific products or scientific instrumentation is
10. HPV-Mediated (p16+) Oropharyngeal Cancer
 1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
1 Terms of Use The cancer staging form is a specific document in the patient record; it is not a substitute for documentation of history, physical examination, and staging evaluation, or for documenting
Reporting of Cancer Stage Information by Acute Care Hospitals in Ontario
 Reporting of Cancer Stage Information by Acute Care Hospitals in Ontario Forward This document is an accompanying reference to Ontario s staging policy entitled Guidelines for Staging Patients with Cancer
Reporting of Cancer Stage Information by Acute Care Hospitals in Ontario Forward This document is an accompanying reference to Ontario s staging policy entitled Guidelines for Staging Patients with Cancer
Springer Healthcare. Staging and Diagnosing Cutaneous Melanoma. Concise Reference. Dirk Schadendorf, Corinna Kochs, Elisabeth Livingstone
 Concise Reference Staging and Diagnosing Cutaneous Melanoma Dirk Schadendorf, Corinna Kochs, Elisabeth Livingstone Extracted from Handbook of Cutaneous Melanoma: A Guide to Diagnosis and Treatment Published
Concise Reference Staging and Diagnosing Cutaneous Melanoma Dirk Schadendorf, Corinna Kochs, Elisabeth Livingstone Extracted from Handbook of Cutaneous Melanoma: A Guide to Diagnosis and Treatment Published
Melanoma of the Skin
 24 Melanoma of the Skin C44.0 Skin of lip, NOS C44.1 Eyelid C44.2 External ear C44.3 Skin of other and unspecified parts of face C44.4 Skin of scalp and neck C44.5 Skin of trunk C44. Skin of upper limb
24 Melanoma of the Skin C44.0 Skin of lip, NOS C44.1 Eyelid C44.2 External ear C44.3 Skin of other and unspecified parts of face C44.4 Skin of scalp and neck C44.5 Skin of trunk C44. Skin of upper limb
Melanoma Quality Reporting
 Melanoma Quality Reporting September 1, 2013 December 31, 2016 Laurence McCahill, MD Surgical Oncologist Metro Health Surgical Oncology Metro Health Professional Building 2122 Health Drive SW Wyoming,
Melanoma Quality Reporting September 1, 2013 December 31, 2016 Laurence McCahill, MD Surgical Oncologist Metro Health Surgical Oncology Metro Health Professional Building 2122 Health Drive SW Wyoming,
Collaborative Stage Site-Specific Instructions - BREAST
 Slide 1 Collaborative Stage Site-Specific Instructions - BREAST In this presentation, we are going to review the CS Data Items and coding instructions for the breast primary site. Slide 2 Reading Assignments
Slide 1 Collaborative Stage Site-Specific Instructions - BREAST In this presentation, we are going to review the CS Data Items and coding instructions for the breast primary site. Slide 2 Reading Assignments
Gastric Cancer Staging AJCC eighth edition. Duncan McLeod Westmead Hospital, NSW
 Gastric Cancer Staging AJCC eighth edition Duncan McLeod Westmead Hospital, NSW Summary of changes New clinical stage prognostic groups, ctnm Postneoadjuvant therapy pathologic stage groupings, yptnm -
Gastric Cancer Staging AJCC eighth edition Duncan McLeod Westmead Hospital, NSW Summary of changes New clinical stage prognostic groups, ctnm Postneoadjuvant therapy pathologic stage groupings, yptnm -
Enrollment Form: Pancreas
 Tissue Source Site (TSS) Name: TSS Identifier: _ TSS Unique Patient #: Completed By: Completion Date (MM/DD/YYYY): Form Notes: An Enrollment Form should be completed for each TCGA qualified case upon qualification
Tissue Source Site (TSS) Name: TSS Identifier: _ TSS Unique Patient #: Completed By: Completion Date (MM/DD/YYYY): Form Notes: An Enrollment Form should be completed for each TCGA qualified case upon qualification
