Clinical guideline Published: 21 April 2011 nice.org.uk/guidance/cg121
|
|
|
- Lynn Atkinson
- 6 years ago
- Views:
Transcription
1 Lung cancer: diagnosis and management Clinical guideline Published: 21 April 2011 nice.org.uk/guidance/cg121 NICE All rights reserved. Subject to Notice of rights (
2 Your responsibility The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals and practitioners are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or the people using their service. It is not mandatory to apply the recommendations, and the guideline does not override the responsibility to make decisions appropriate to the circumstances of the individual, in consultation with them and their families and carers or guardian. Local commissioners and providers of healthcare have a responsibility to enable the guideline to be applied when individual professionals and people using services wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with complying with those duties. Commissioners and providers have a responsibility to promote an environmentally sustainable health and care system and should assess and reduce the environmental impact of implementing NICE recommendations wherever possible. Page 2 of
3 Contents Introduction... 5 Patient-centred care... 7 Key priorities for implementation Guidance Access to services and referral Communication Diagnosis and staging Treatment Palliative interventions and supportive and palliative care Follow-up and patient perspectives Notes on the scope of the guidance Implementation Research recommendations Selection of patients with NSCLC for treatment with curative intent Effectiveness of surgery with or without multimodality treatment in N2 disease Pulmonary rehabilitation, optimisation of drug treatment and enhanced recovery programmes New regimens for radiotherapy with curative intent Imaging modalities for monitoring response and recurrent disease Other versions of this guideline Full guideline NICE pathway Information for the public Related NICE guidance Updating the guideline...35 Appendix A: The Guideline Development Group, National Collaborating Centre and NICE project team Guideline Development Group Page 3 of
4 2005 Guideline Development Group National Collaborating Centre for Cancer NICE project team Appendix B: The Guideline Review Panel Guideline Review Panel Guideline Review Panel Appendix C: The algorithms...42 Changes after publication...43 About this guideline...44 Page 4 of
5 This guideline replaces CG24. This guideline is partially replaced by NG12. This guideline is the basis of QS17. Introduction This guidance updates and replaces NICE clinical guideline 24 (published February 2005). There are more than 39,000 new cases of lung cancer in the UK each year and more than 35,000 people die from the condition; more than for breast cancer and colorectal cancer combined. Lung cancer is now the leading cause of cancer death in women. About 90% of lung cancers are caused by smoking. Now that fewer men smoke, lung cancer deaths in men have decreased by more than a quarter in the UK (a 27% reduction between 1971 and 2006). However, the number of women who smoke has risen and deaths from lung cancer in women have increased. Only about 5.5% of lung cancers are currently cured. Although the cure rate is rising slowly, the rate of improvement has been slower than for other common cancers. Outcomes in the UK are worse than those in some European countries and North America. There is evidence that outcomes vary within the UK, which among other factors may be explained by variations in the standard of care. This updated guideline provides recommendations for good practice in the diagnosis and treatment of non-small-cell (NSCLC) and small-cell lung cancer (SCLC). The guideline will assume that prescribers will use a drug's summary of product characteristics to inform decisions made with individual patients. Changes in this update New and updated recommendations are included on communication, diagnosis and staging, selection of patients with non-small-cell lung cancer (NSCLC) for treatment with curative intent, surgery with curative intent for NSCLC, smoking cessation, combination treatment for NSCLC, Page 5 of
6 treatment for small-cell lung cancer (SCLC), managing endobronchial obstruction, managing brain metastases, and follow-up and patient perspectives. Recommendations are marked as [2005], [2011] or [new 2011]. [2005] indicates that the evidence has not been updated and reviewed since [2011] indicates that the evidence has been reviewed but no changes have been made to the recommendation. [new 2011] indicates that the evidence has been reviewed and the recommendation has been added or updated. Since publication of NICE clinical guideline 24 in 2005, a number of new systemic therapies have been granted a marketing authorisation by the European Medicines Agency for use in people with NSCLC. NICE has published technology appraisals for pemetrexed, gefitinib and erlotinib. Other technology appraisals are in development. The NHS has also commissioned a review of first-line therapy for NSCLC through the NIHR Health Technology Assessment Programme. This review is due to be published in Page 6 of
7 Patient-centred care This guideline offers best practice advice on the care of adults with lung cancer. Treatment and care should take into account patients' needs and preferences. People with lung cancer should have the opportunity to make informed decisions about their care and treatment, in partnership with their healthcare professionals. If patients do not have the capacity to make decisions, healthcare professionals should follow the Department of Health's advice on consent and the code of practice that accompanies the Mental Capacity Act. In Wales, healthcare professionals should follow advice on consent from the Welsh Government. Good communication between healthcare professionals and patients is essential. It should be supported by evidence-based written information tailored to the patient's needs. Treatment and care, and the information patients are given about it, should be culturally appropriate. It should also be accessible to people with additional needs such as physical, sensory or learning disabilities, and to people who do not speak or read English. If the patient agrees, families and carers should have the opportunity to be involved in decisions about treatment and care. Families and carers should also be given the information and support they need. Page 7 of
8 Key priorities for implementation The following recommendations have been identified as priorities for implementation. The importance of early diagnosis The public needs to be better informed of the symptoms and signs that are characteristic of lung cancer, through coordinated campaigning to raise awareness. [2005] Communication Ensure that a lung cancer clinical nurse specialist is available at all stages of care to support patients and carers. [new 2011] Diagnosis and staging Choose investigations that give the most information about diagnosis and staging with the least risk to the patient. Think carefully before performing a test that gives only diagnostic pathology when information on staging is also needed to guide treatment. [new 2011] Offer PET-CT, or EBUS-guided TBNA, or EUS-guided FNA, or non-ultrasound-guided TBNA as the first test for patients with an intermediate probability of mediastinal malignancy (lymph nodes between 10 and 20 mm maximum short axis on CT) who are potentially suitable for treatment with curative intent. [new 2011] Surgery with curative intent for non-small-cell lung cancer Offer patients with NSCLC who are medically fit and suitable for treatment with curative intent, lobectomy (either open or thoracoscopic) as the treatment of first choice. For patients with borderline fitness and smaller tumours (T1a b, N0, M0), consider lung parenchymalsparing operations (segmentectomy or wedge resection) if a complete resection can be achieved. [new 2011] Radiotherapy with curative intent for non-small-cell lung cancer Radical radiotherapy is indicated for patients with stage I, II or III NSCLC who have good performance status (WHO 0, 1) and whose disease can be encompassed in a radiotherapy treatment volume without undue risk of normal tissue damage [1]. [2005] Combination treatment for non-small-cell lung cancer Page 8 of
9 Ensure all patients potentially suitable for multimodality treatment (surgery, radiotherapy and chemotherapy in any combination) are assessed by a thoracic oncologist and by a thoracic surgeon. [new 2011] Assessing patients with small-cell lung cancer Arrange for patients with small-cell lung cancer (SCLC) to have an assessment by a thoracic oncologist within 1 week of deciding to recommend treatment. [new 2011] Managing endobronchial obstruction Every cancer network should ensure that patients have rapid access to a team capable of providing interventional endobronchial treatments. [new 2011] Follow-up and patient perspectiveses Offer all patients an initial specialist follow-up appointment within 6 weeks of completing treatment to discuss ongoing care. Offer regular appointments thereafter, rather than relying on patients requesting appointments when they experience symptoms. [new 2011] [1] The GDG recognised that radiotherapy techniques have advanced considerably since the 2005 guideline and centres would reasonably wish to offer these techniques (including SBRT and 4-D planning) to patients. These treatments have the advantage of reducing the risk of damage to normal tissue (estimated by using measurements such as V20). Page 9 of
10 1 Guidance The following guidance is based on the best available evidence. The full guideline gives details of the methods and the evidence used to develop the guidance. Some of the abbreviations used in this guidance are defined below. EBUS endobronchial ultrasound EUS endoscopic ultrasound FNA fine needle aspiration TBNA transbronchial needle aspiration. 1.1 Access to services and referral The importance of early diagnosis The public needs to be better informed of the symptoms and signs that are characteristic of lung cancer, through coordinated campaigning to raise awareness. [2005] Referral and indications for chest radiography This recommendation has been replaced by recommendations in section 1.1 of the NICE guideline on suspected cancer This recommendation has been replaced by recommendations in section 1.1 of the NICE guideline on suspected cancer This recommendation has been replaced by recommendations in section 1.1 of the NICE guideline on suspected cancer This recommendation has been replaced by recommendations in section 1.1 of the NICE guideline on suspected cancer. Page 10 of
11 1.1.6 Where a chest X-ray has been requested in primary or secondary care and is incidentally suggestive of lung cancer, a second copy of the radiologist's report should be sent to a designated member of the lung cancer MDT, usually the chest physician. The MDT should have a mechanism in place to follow up these reports to enable the patient's GP to have a management plan in place. [2005] 1.2 Communication Find out what the patient knows about their condition without assuming a level of knowledge. Provide patients with the opportunity to discuss tests and treatment options in a private environment, with the support of carers, and time to make an informed choice. [new 2011] Ensure that a lung cancer clinical nurse specialist is available at all stages of care to support patients and carers. [new 2011] Offer accurate and easy-to-understand information to patients and their carers. Explain the tests and treatment options, including potential survival benefits, side effects and effect on symptoms. [new 2011] Consider tailor-made decision aids to help patients to: understand the probable outcomes of treatment options consider the personal value they place on benefits versus harms of treatment options feel supported in decision-making move through the steps towards making a decision take part in decisions about their healthcare. [new 2011] Offer patients a record of all discussions that have taken place with them and a copy of any correspondence with other healthcare professionals. Ensure all communications are worded in such a way to assist understanding. [new 2011] Respect the patient's choice if they do not wish to confront future issues. [new 2011] Page 11 of
12 1.2.7 Avoid giving patients unexpected bad news by letter. Only give unexpected bad news by phone in exceptional circumstances. [new 2011] Offer to discuss end-of-life care with the patient sensitively and when appropriate. Wherever possible, avoid leaving this discussion until the terminal stages of the illness. [new 2011] Document discussions with the patient about end-of-life care. In particular, document: specific concerns of the patient their understanding of their illness and its prognosis important values or personal goals for care their preferences for the types of care or treatment that may be beneficial in the future and their availability. [new 2011] Share information between healthcare professionals about: any problems the patient has the management plan what the patient has been told what the patient has understood (where possible) the involvement of other agencies any advance decision made by the patient. [new 2011] 1.3 Diagnosis and staging Effectiveness eness of diagnostic and staging investigations Sputum cytology is rarely indicated and should be reserved for the investigation of patients who have centrally placed nodules or masses and are unable to tolerate, or unwilling to undergo, bronchoscopy or other invasive tests. [2005] Page 12 of
13 1.3.2 Patients with known or suspected lung cancer should be offered a contrastenhanced chest CT scan to further the diagnosis and stage the disease. The scan should also include the liver and adrenals [2]. [2005] In the assessment of mediastinal and chest wall invasion: CT alone may not be reliable other techniques such as ultrasound should be considered where there is doubt surgical assessment may be necessary if there are no contraindications to resection. [2005] Ensure all patients potentially suitable for treatment with curative intent are offered PET-CT before treatment. [new 2011] Every cancer network should have a system of rapid access to PET-CT scanning for eligible patients. [2005] Magnetic resonance imaging (MRI) should not routinely be performed to assess the stage of the primary tumour (T-stage) in NSCLC. [2005] MRI should be performed, where necessary to assess the extent of disease, for patients with superior sulcus tumours. [2005] Offer EBUS-guided TBNA for biopsy of paratracheal and peri-bronchial intraparenchymal lung lesions. [new 2011] Every cancer network should have at least one centre with EBUS and/or EUS to ensure timely access. [new 2011] The local test performance of non-ultrasound-guided TBNA, EBUS and EUSguided FNA should be the subject of audit. [new 2011] Ensure adequate samples are taken without unacceptable risk to the patient to permit pathological diagnosis including tumour sub-typing and measurement of predictive markers. [new 2011] Sequence of investigations Page 13 of
14 Choose investigations that give the most information about diagnosis and staging with least risk to the patient. Think carefully before performing a test that gives only diagnostic pathology when information on staging is also needed to guide treatment. [new 2011] Chest CT should be performed before: an intended fibreoptic bronchoscopy any other biopsy procedure. [2005] Peripheral primary tumour Offer CT- or ultrasound-guided transthoracic needle biopsy to patients with peripheral lung lesions when treatment can be planned on the basis of this test. [new 2011] Biopsy any enlarged mediastinal nodes ( 10 mm maximum short axis on CT) or other lesions in preference to the primary lesion if determination of stage affects treatment [3]. [new 2011] Central primary tumour Offer fibreoptic bronchoscopy to patients with central lesions on CT where nodal staging does not influence treatment. Enlarged lymph nodes ( 10 mm maximum short axis on CT) may be simultaneously sampled with TBNA (nonultrasound-guided) if required for diagnosis. [new 2011] Mediastinal lymph node assessment Offer PET-CT as the preferred first test after CT showing a low probability of mediastinal malignancy (lymph nodes 10 mm maximum short axis on CT) for patients who are potentially suitable for treatment with curative intent. [new 2011] Offer PET-CT, or EBUS-guided TBNA, or EUS-guided FNA, or non-ultrasoundguided TBNA as the first test for patients with an intermediate probability of mediastinal malignancy (lymph nodes between 10 and 20 mm maximum short Page 14 of
15 axis on CT) who are potentially suitable for treatment with curative intent. [new 2011] Offer neck ultrasound with sampling of visible lymph nodes or non-ultrasoundguided TBNA to patients with a high probability of mediastinal malignancy (lymph nodes > 20 mm maximum short axis on CT). If neck ultrasound is negative, follow with non-ultrasound-guided TBNA, EBUS-guided TBNA or EUS-guided FNA. If non-ultrasound-guided TBNA is negative follow with EBUS-guided TBNA or EUS-guided FNA. [new 2011] Offer neck ultrasound with biopsy of visible lymph nodes to patients that have neck nodes detected by initial CT. If negative, follow with non-ultrasoundguided TBNA or EBUS-guided TBNA or EUS-guided FNA. [new 2011] Evaluate PET-CT-positive mediastinal nodes by mediastinal sampling (except when there is definite distant metastatic disease or a high probability that N2/ N3 disease is metastatic [for example, if there is a chain of lymph nodes with high 18 F-deoxyglucose uptake]). [new 2011] Consider combined EBUS and EUS for initial staging of the mediastinum as an alternative to surgical staging. [new 2011] Confirm negative results obtained by non-ultrasound-guided TBNA using EBUS-guided TBNA, EUS-guided FNA or surgical staging. [new 2011] Confirm negative results obtained by EBUS-guided TBNA and/or EUS-guided FNA using surgical staging if clinical suspicion of mediastinal malignancy is high. [new 2011] Stage M1b Confirm the presence of isolated distant metastases/synchronous tumours by biopsy or further imaging (for example, MRI or PET-CT) in patients being considered for treatment with curative intent. [new 2011] Consider MRI or CT of the head in patients selected for treatment with curative intent, especially in stage III disease. [new 2011] Page 15 of
16 Offer patients with features suggestive of intracranial pathology, CT of the head followed by MRI if normal, or MRI as an initial test. [new 2011] An X-ray should be performed in the first instance for patients with localised signs or symptoms of bone metastasis. If the results are negative or inconclusive, either a bone scan or an MRI scan should be offered. [2005] Avoid bone scintigraphy when PET-CT has not shown bone metastases. [new 2011] Organisational factors relevant to diagnosis and staging Patients who have lung cancer suitable for radical treatment or chemotherapy, or need radiotherapy or ablative treatment for relief of symptoms, should be treated without undue delay, according to the Welsh Assembly Government and Department of Health recommendations (within 31 days of the decision to treat and within 62 days of their urgent referral). [2005] Multidisciplinary teams All patients with a likely diagnosis of lung cancer should be referred to a member of a lung cancer MDT (usually a chest physician). [2005] The care of all patients with a working diagnosis of lung cancer should be discussed at a lung cancer MDT meeting. [2005] Rapid access lung clinics Rapid access clinics [4] should be provided where possible for the investigation of patients with suspected lung cancer, because they are associated with faster diagnosis and less patient anxiety. [2005] Cancer clinical nurse specialists All cancer units/centres should have one or more trained lung cancer clinical nurse specialists to see patients before and after diagnosis, to provide continuing support, and to facilitate communication between the secondary care team (including the MDT), the patient's GP, the community team and the Page 16 of
17 patient. Their role includes helping patients to access advice and support whenever they need it. [2005] 1.4 Treatment Smoking cessation Inform patients that smoking increases the risk of pulmonary complications after lung cancer surgery. [new 2011] Advise patients to stop smoking as soon as the diagnosis of lung cancer is suspected and tell them why this is important. [new 2011] Offer nicotine replacement therapy and other therapies to help patients to stop smoking in line with Smoking cessation services (NICE public health guidance 10) and Varenicline for smoking cessation (NICE technology appraisal guidance 123). [new 2011] Do not postpone surgery for lung cancer to allow patients to stop smoking. [new 2011] Selection of patients with non-small-cell lung cancer for treatment with curative intent Perioperative mortality When evaluating surgery as an option for patients with NSCLC, consider using a global risk score such as Thoracoscore to estimate the risk of death. Ensure the patient is aware of the risk before giving consent for surgery. [new 2011] Cardiovascular function Avoid surgery within 30 days of myocardial infarction. [new 2011] Seek a cardiology review in patients with an active cardiac condition, or three or more risk factors, or poor cardiac functional capacity. [new 2011] Offer surgery without further investigations to patients with two or fewer risk factors and good cardiac functional capacity. [new 2011] Page 17 of
18 1.4.9 Optimise any primary cardiac treatment and begin secondary prophylaxis for coronary disease as soon as possible. [new 2011] Continue anti-ischaemic treatment in the perioperative period, including aspirin, statins and beta-blockers. [new 2011] If a patient has a coronary stent, discuss perioperative anti-platelet treatment with a cardiologist. [new 2011] Consider revascularisation (percutaneous intervention or coronary artery bypass grafting) before surgery for patients with chronic stable angina and conventional indications for revascularisation. [new 2011] Lung function Perform spirometry in all patients being considered for treatment with curative intent. Measure T L CO if breathlessness is disproportionate or there is other lung pathology (for example, lung fibrosis). [new 2011] Offer patients surgery if they have an FEV 1 within normal limits and good exercise tolerance. [new 2011] Offer patients with predicted postoperative FEV 1 or T L CO below the recommended limit of 30% the option of undergoing surgery if they accept the risks of dyspnoea and associated complications. [new 2011] When considering surgery perform a segment count to predict postoperative lung function. [new 2011] Consider using shuttle walk testing (using a distance walked of more than 400 m as a cut-off for good function) to assess fitness of patients with moderate to high risk of postoperative dyspnoea. [new 2011] Consider cardiopulmonary exercise testing to measure VO 2 max and assess lung function in patients with moderate to high risk of postoperative dyspnoea, using more than 15 ml/kg/minute as a cut-off for good function. [new 2011] Assessment before radiotherapy with curative intent Page 18 of
19 A clinical oncologist specialising in thoracic oncology should determine suitability for radiotherapy with curative intent, taking into account performance status and comorbidities. [new 2011] Surgery with curative intent for non-small-cell lung cancer Offer patients with NSCLC who are medically fit and suitable for treatment with curative intent, lobectomy (either open or thoracoscopic) as the treatment of first choice. For patients with borderline fitness and smaller tumours (T1a b, N0, M0), consider lung parenchymal-sparing operations (segmentectomy or wedge resection) if a complete resection can be achieved. [new 2011] Offer more extensive surgery (bronchoangioplastic surgery, bilobectomy, pneumonectomy) only when needed to obtain clear margins. [new 2011] Perform hilar and mediastinal lymph node sampling or en bloc resection for all patients undergoing surgery with curative intent. [new 2011] For patients with T3 NSCLC with chest wall involvement who are undergoing surgery, complete resection of the tumour should be the aim by either extrapleural or en bloc chest wall resection. [2005] Radiotherapy with curative intent for non-small-cell lung cancer Radical radiotherapy is indicated for patients with stage I, II or III NSCLC who have good performance status (WHO 0, 1) and whose disease can be encompassed in a radiotherapy treatment volume without undue risk of normal tissue damage [5]. [2005] All patients should undergo pulmonary function tests (including lung volumes and transfer factor) before having radical radiotherapy for NSCLC. [2005] Patients who have poor lung function but are otherwise suitable for radical radiotherapy should still be offered radiotherapy, provided the volume of irradiated lung is small. [2005] Patients with stage I or II NSCLC who are medically inoperable but suitable for radical radiotherapy should be offered the CHART regimen. [2005] Page 19 of
20 Patients receiving radiotherapy with curative intent should be part of a national quality assurance programme [5]. [new 2011] Patients with stages IIIA or IIIB NSCLC who are eligible for radical radiotherapy and who cannot tolerate or do not wish to have chemoradiotherapy should be offered the CHART regimen. [2005] If CHART is not available, conventionally fractionated radiotherapy to a dose of Gy in fractions over 61/2 weeks or 55 Gy in 20 fractions over 4 weeks should be offered. [2005] Combination treatment for non-small-cell lung cancer Offer patients with stage I III NSCLC who are not suitable for surgery an assessment by a clinical oncologist specialising in thoracic oncology for radiotherapy with curative intent. [new 2011] Consider chemoradiotherapy for patients with stage II or III NSCLC who are not suitable for surgery. Balance potential benefit in survival with the risk of additional toxicities. [new 2011] Ensure all patients potentially suitable for multimodality treatment (surgery, radiotherapy and chemotherapy in any combination) are assessed by a thoracic oncologist and by a thoracic surgeon. [new 2011] Offer postoperative chemotherapy to patients with good performance status (WHO 0 or 1) and T1 3 N1 2 M0 NSCLC. [new 2011] Consider postoperative chemotherapy in patients with good performance status (WHO 0 or 1) and T2 3 N0 M0 NSCLC with tumours greater than 4 cm in diameter. [new 2011] Offer a cisplatin-based combination chemotherapy regimen for adjuvant chemotherapy. [new 2011] For patients with NSCLC who are suitable for surgery, do not offer neo-adjuvant chemotherapy outside a clinical trial. [new 2011] Page 20 of
21 Ensure eligible patients have the benefit of detailed discussion of the risks and benefits of adjuvant chemotherapy. [new 2011] Treat Pancoast tumours in the same way as other types of NSCLC. Offer multimodality therapy according to resectability, stage of the tumour and performance status of the patient. [new 2011] Chemotherapy for non-small-cell lung cancer Chemotherapy should be offered to patients with stage III or IV NSCLC and good performance status (WHO 0, 1 or a Karnofsky score of ), to improve survival, disease control and quality of life. [2005] Chemotherapy for advanced NSCLC should be a combination of a single thirdgeneration drug (docetaxel, gemcitabine, paclitaxel or vinorelbine) plus a platinum drug. Either carboplatin or cisplatin may be administered, taking account of their toxicities, efficacy and convenience. [2005] Patients who are unable to tolerate a platinum combination may be offered single-agent chemotherapy with a third-generation drug. [2005] Docetaxel monotherapy should be considered if second-line treatment is appropriate for patients with locally advanced or metastatic NSCLC in whom relapse has occurred after previous chemotherapy. [2005] Gefitinib Refer to Gefitinib for the first-line treatment of locally advanced or metastatic non-small-cell lung cancer (NICE technology appraisal guidance 192 [2010]). Pemetrexed Refer to Pemetrexed for the first-line treatment of non-small-cell lung cancer (NICE technology appraisal guidance 181 [2010]). Erlotinib Page 21 of
22 Refer to Erlotinib for the treatment of non-small-cell lung cancer (NICE technology appraisal guidance 162 [2008]). Assessing patients with small-cell lung cancer Arrange for patients with small-cell lung cancer (SCLC) to have an assessment by a thoracic oncologist within 1 week of deciding to recommend treatment. [new 2011] First-line treatment for limited-stage disease small-cell lung cancer 1.4. Offer patients with limited-stage disease SCLC (broadly corresponding to T1 4, N0 3, M0) four to six cycles of cisplatin-based combination chemotherapy. Consider substituting carboplatin in patients with impaired renal function, poor performance status (WHO 2 or more) or significant comorbidity. [new 2011] Offer concurrent chemoradiotherapy to patients with limited-stage disease SCLC (broadly corresponding to T1 4, N0 3, M0) and a WHO performance status of 0 or 1 if they present with disease that can be encompassed in a radical thoracic radiotherapy volume. Start the radiotherapy during the first or second cycle of chemotherapy. [new 2011] Offer sequential radical thoracic radiotherapy to patients with limited-stage disease SCLC (broadly corresponding to T1 4, N0 3, M0) who are unfit for concurrent chemoradiotherapy but who respond to chemotherapy. [new 2011] Surgical treatment for patients with small-cell lung cancer Consider surgery in patients with early-stage SCLC (T1 2a, N0, M0). [new 2011] First-line treatment for extensive-stage e-stage disease small-cell lung cancer Offer platinum-based combination chemotherapy to patients with extensivestage disease SCLC (broadly corresponding to T1 4, N0 3, M1a/b including cerebral metastases) if they are fit enough. [new 2011] Page 22 of
23 Assess the patient's condition before each cycle of chemotherapy for extensivestage disease SCLC (broadly corresponding to T1 4, N0 3, M1a/b) and offer up to a maximum of six cycles, depending on response and toxicity. [new 2011] For patients with extensive-stage disease SCLC, thoracic radiotherapy should be considered after chemotherapy if there has been a complete response at distant sites and at least a good partial response within the thorax. [new 2011] Maintenance treatment for small-cell lung cancer Offer maintenance treatment to patients with SCLC only in the context of a clinical trial. [new 2011] Prophylactic cranial irradiation in small-cell lung cancer Offer prophylactic cranial irradiation at a dose of 25 Gy in 10 fractions to patients with limited-stage disease SCLC and WHO performance status 2 or less, if their disease has not progressed on first-line treatment. [new 2011] Offer prophylactic cranial irradiation to patients with extensive-stage disease SCLC and WHO performance status 2 or less, if their disease has not progressed on first-line treatment. [new 2011] Second-line treatment for patients with small-cell lung cancer that has relapsed after first-line treatment Offer patients with SCLC that has relapsed after first-line treatment assessment by a thoracic oncologist. [new 2011] Inform patients whose disease has not responded to first-line treatment that there is very limited evidence that second-line chemotherapy will be of benefit. [new 2011] Offer patients with relapsed SCLC, who are suitable for chemotherapy, treatment with an anthracycline-containing regimen or further treatment with a platinum-based regimen to a maximum of six cycles. [new 2011] Page 23 of
24 Offer radiotherapy for palliation of local symptoms to patients with SCLC that has relapsed after first-line treatment. [new 2011] Topotecan Refer to Topotecan for the treatment of small-cell lung cancer (NICE technology appraisal guidance 184 [2009]). 1.5 Palliative interventions and supportive and palliative care Providing palliative care Supportive and palliative care of the patient should be provided by general and specialist palliative care providers in accordance with the NICE guidance Improving supportive and palliative care for adults with cancer. [2005] Patients who may benefit from specialist palliative care services should be identified and referred without delay. [2005] Palliative radiotherapy Patients who cannot be offered curative treatment, and are candidates for palliative radiotherapy, may either be observed until symptoms arise and then treated, or be treated with palliative radiotherapy immediately. [2005] Managing endobronchial obstruction When patients have large airway involvement, monitor (clinically and radiologically) for endobronchial obstruction to ensure treatment is offered early. [new 2011] Offer external beam radiotherapy and/or endobronchial debulking or stenting to patients with impending endobronchial obstruction. [new 2011] Every cancer network should ensure that patients have rapid access to a team capable of providing interventional endobronchial treatments. [new 2011] Other palliative treatments Page 24 of
25 1.5.7 Pleural aspiration or drainage should be performed in an attempt to relieve the symptoms of a pleural effusion. [2005] Patients who benefit symptomatically from aspiration or drainage of fluid should be offered talc pleurodesis for longer-term benefit. [2005] Non-drug interventions based on psychosocial support, breathing control and coping strategies should be considered for patients with breathlessness. [2005] Non-drug interventions for breathlessness should be delivered by a multidisciplinary group, coordinated by a professional with an interest in breathlessness and expertise in the techniques (for example, a nurse, physiotherapist or occupational therapist). Although this support may be provided in a breathlessness clinic, patients should have access to it in all care settings. [2005] Opioids, such as codeine or morphine, should be considered to reduce cough. [2005] Patients with troublesome hoarseness due to recurrent laryngeal nerve palsy should be referred to an ear, nose and throat specialist for advice. [2005] Patients who present with superior vena cava obstruction should be offered chemotherapy and radiotherapy according to the stage of disease and performance status. [2005] Stent insertion should be considered for the immediate relief of severe symptoms of superior vena caval obstruction or following failure of earlier treatment. [2005] Managing brain metastases Offer dexamethasone to patients with symptomatic brain metastases and reduce to the minimum necessary maintenance dose for symptomatic response. [new 2011] Consider palliative whole-brain radiotherapy for patients with symptomatic brain metastases with good performance status (WHO 0 or 1). [new 2011] Page 25 of
26 Hypercalcaemia, bone pain and pathological fractures For patients with bone metastasis requiring palliation and for whom standard analgesic treatments are inadequate, single-fraction radiotherapy should be administered. [2005] Managing other symptoms: weight loss, loss of appetite, difficulty swallowing, fatigue and depression Other symptoms, including weight loss, loss of appetite, depression and difficulty swallowing, should be managed by multidisciplinary groups that include supportive and palliative care professionals. [2005] 1.6 Follow-up and patient perspectives Offer all patients an initial specialist follow-up appointment within 6 weeks of completing treatment to discuss ongoing care. Offer regular appointments thereafter, rather than relying on patients requesting appointments when they experience symptoms. [new 2011] Offer protocol-driven follow-up led by a lung cancer clinical nurse specialist as an option for patients with a life expectancy of more than 3 months. [new 2011] Ensure that patients know how to contact the lung cancer clinical nurse specialist involved in their care between their scheduled hospital visits. [new 2011] The opinions and experiences of lung cancer patients and carers should be collected and used to improve the delivery of lung cancer services. Patients should receive feedback on any action taken as a result of such surveys. [2005] [2] This recommendation was outside the scope of the 2011 update but the GDG recognised that many centres include the lower neck when performing CT scans for the diagnosis of lung cancer. The GDG also recognised that contrast medium should only be given with caution to patients with known renal impairment. [3] Many patients with lung cancer will not be fit for treatment with curative intent. This needs to be taken into account when choosing diagnostic and staging investigations. Page 26 of
27 [4] These were previously known as early diagnosis clinics. [5] The GDG recognised that radiotherapy techniques have advanced considerably since the 2005 guideline and centres would reasonably wish to offer these techniques (including SBRT and 4-D planning) to patients. These treatments have the advantage of reducing the risk of damage to normal tissue (estimated by using measurements such as V20). Page 27 of
28 2 Notes on the scope of the guidance NICE guidelines are developed in accordance with a scope that defines what the guideline will and will not cover. Groups that are covered Adults (18 years and older) with newly diagnosed non-small-cell lung cancer (NSCLC). Adults with newly diagnosed small-cell lung cancer (SCLC). Adults with relapsed NSCLC. Adults with relapsed SCLC. Groups that are not covered Adults with mesothelioma. Adults with lung metastases arising from primary cancers originating outside the lung. Children (younger than 18) with lung cancer. Adults with rare lung tumours (for example, pulmonary blastoma). Adults with benign lung tumours (for example, bronchial adenoma). How this guideline was developedeloped NICE commissioned the National Collaborating Centre for Cancer to develop this guideline. The Centre established a Guideline Development Group (see appendix A), which reviewed the evidence and developed the recommendations. An independent Guideline Review Panel oversaw the development of the guideline (see appendix B). There is more information about how NICE clinical guidelines are developed on the NICE website, and in How NICE clinical guidelines are developed: an overview for stakeholders, the public and the NHS. Page 28 of
29 3 Implementation NICE has developed tools to help organisations implement this guidance. Page 29 of
30 4 Research recommendations The Guideline Development Group has made the following recommendations for research, based on its review of evidence, to improve NICE guidance and patient care in the future. 4.1 Selection of patients with NSCLC for treatment with curative intent Further studies should be performed into factors that predict successful outcome of treatment with curative intent. Studies should include fitness parameters and functional imaging. Why this is important Despite much research into factors that predict a successful outcome after treatment with curative intent it is still not clear how these relate to the patient with borderline fitness. To ensure that fitness assessment is robust, consistent and meaningful, the place of exercise testing, lung function testing and functional imaging should be clearly defined by appropriately designed trials. 4.2 Effectiveness of surgery with or without multimodality treatment in N2 disease Patients with non-bulky single zone N2 disease should be considered for trials of surgery with or without multimodality treatment. Outcomes should include mortality and 5-year survival. Why this is important A number of randomised controlled trials have been evaluated in this guideline that have shown that surgery, as part of multimodality treatment, does not worsen prognosis in patients with N2 disease. However, these studies did not distinguish between those patients who might intuitively benefit from surgery (a limited number of nodes involved and/or a single zone affected) and those with more extensive disease and potentially less favourable biology (many nodes involved and/or multiple zones affected). Further trials are needed to establish the role of surgeryin this heterogeneous group. 4.3 Pulmonary rehabilitation, optimisation of drug treatment and enhanced recovery programmes Research should be undertaken into the benefits of pulmonary rehabilitation, optimisation of drug treatment and enhanced recovery programmes before and after surgery. Outcomes should include Page 30 of
31 mortality, survival, pulmonary complications, pulmonary function and quality of life (including assessment by EQ-5D). Why this is important There is some evidence that pulmonary rehabilitation, optimisation of drug treatment and enhanced recovery programmes are effective in patients undergoing surgery for some conditions but none for patients undergoing surgery for lung cancer. Fitness for surgery and the ability of the patient to recover following surgery are key factors in the success of this treatment for lung cancer. The effectiveness of interventions to improve these factors should be evaluated. 4.4 New regimens for radiotherapy with curative intent Research should be considered into dose escalation in radiotherapy with curative intent, including stereotactic body irradiation (SBRT). Outcomes should include mortality, pulmonary complications, pulmonary function and validated quality of life measures (including assessment by EQ-5D). Why this is important There have been considerable technological advances in radiotherapy equipment that has allowed radiotherapy to be more accurately delivered to the tumour and hence less damaging to normal tissues. This has allowed new regimens to be developed, including SBRT, which have not been evaluated adequately for their efficacy and toxicity. 4.5 Imaging modalities for monitoring response and recurrent disease Randomised controlled trials should be conducted to examine the value of imaging modalities and other interventions in the monitoring of response and recurrent disease. Why this is important Patients with lung cancer have high recurrence rates even when treated with curative intent. It is not known whether imaging modalities and other interventions in the follow-up period can improve outcomes by detecting recurrence or relapse earlier. Therefore no firm recommendations can be made about their scheduling or use. This question should be addressed through properly designed clinical trials. Page 31 of
32 5 Other versions of this guideline 5.1 Full guideline The full guideline, The diagnosis and treatment of lung cancer (update of NICE clinical guideline 24), contains details of the methods and evidence used to develop the guideline. It is published by the National Collaborating Centre for Cancer. 5.2 NICE pathway The recommendations from this guideline have been incorporated into a NICE pathway. 5.3 Information for the public NICE has produced information for the public explaining this guideline. We encourage NHS and voluntary sector organisations to use text from this information in their own materials about lung cancer. Page 32 of
33 6 Related NICE guidance Published Gefitinib for the first-line treatment of locally advanced or metastatic non-small-cell lung cancer. NICE technology appraisal guidance 192 (2010) Pemetrexed for the maintenance treatment of non-small-cell lung cancer. NICE technology appraisal guidance 190 (2010) Percutaneous radiofrequency ablation for primary and secondary lung cancers. NICE interventional procedure guidance 372 (2010) Topotecan for the treatment of relapsed small-cell-lung cancer. NICE technology appraisal guidance 184 (2009) Pemetrexed for the first-line treatment of non-small cell-lung cancer. NICE technology appraisal guidance 181 (2009) Erlotinib for the treatment of non-small cell lung cancer. NICE technology appraisal guidance 162 (2008) Bevacizumab for the treatment of non-small-cell lung cancer (terminated appraisal). NICE technology appraisal 148 (2008) Endobronchial ultrasound-guided transbronchial needle aspiration for mediastinal masses. NICE interventional procedure guidance 254 (2008) Smoking cessation services. NICE public health guidance 10 (2008) Pemetrexed for the treatment of non-small-cell lung cancer. NICE technology appraisal guidance 124 (2007) Varenicline for smoking cessation. NICE technology appraisal guidance 123 (2007) Workplace interventions to promote smoking cessation. NICE public health guidance 5 (2007) Brief interventions and referral for smoking cessation. NICE public health guidance 1 (2006) Referral guidelines for suspected cancer. NICE clinical guideline 27 (2005) Cryotherapy for malignant endobronchial obstruction. NICE interventional procedure guidance 142 (2005) Page 33 of
34 Photodynamic therapy for localised inoperable endobronchial cancer. NICE interventional procedure guidance 137 (2005) Photodynamic therapy for advanced bronchial carcinoma. NICE interventional procedure guidance 87 (2004) Improving supportive and palliative care for adults with cancer. NICE cancer service guidance (2004) Under developmentelopment NICE is developing the following guidance (details available from our website): Erlotinib monotherapy for the maintenance treatment of non-small-cell lung cancer. NICE technology appraisal guidance. Publication date to be confirmed. Smoking cessation in secondary care. NICE public health guidance. Publication date to be confirmed. Page 34 of
35 7 Updating the guideline NICE clinical guidelines are updated so that recommendations take into account important new information. New evidence is checked 3 years after publication, and healthcare professionals and patients are asked for their views; we use this information to decide whether all or part of a guideline needs updating. If important new evidence is published at other times, we may decide to do a more rapid update of some recommendations. Please see our website for information about updating the guideline. Page 35 of
36 Appendix A: The Guideline Development elopment Group, National Collaboratingating Centre and NICE project team 2011 Guideline Development Group Mr Barrie White Neurosurgeon, Queens Medical Centre, Nottingham Professor Mark Baker Divisional Medical Director for Oncology and Surgery and Lead Cancer Clinician, Leeds Teaching Hospitals NHS Trust Dr David Baldwin Consultant Physician, Nottingham University Hospital NHS Trust Barry Attwood Patient and carer member Mr Sion Barnard Consultant Thoracic Surgeon, Freeman Hospital, Newcastle-upon-Tyne Dr Jeremy Braybrooke Consultant Medical Oncologist, University Hospitals Bristol NHS Foundation Trust Dr Paul Cane Consultant Histopathologist, Guys & St Thomas' NHS Foundation Trust, London Dr James Entwisle Consultant Radiologist, Glenfield Hospital, University Hospitals of Leicester NHS Trust Dr Jesme Fox Patient and carer member, The Roy Castle Lung Cancer Foundation Thomas Haswell Patient and carer member Mr Matthew Hatton Consultant Clinical Oncologist, Weston Park Hospital, Sheffield Page 36 of
37 Dana Knoyle Macmillan Lung Cancer Nurse Specialist, Prince Charles Hospital, Cwm Taf Health Board Dr Richard Neal Senior Lecturer in General Practice, North Wales Clinical School, Cardiff University Mr Richard Page Consultant Thoracic Surgeon, Liverpool Heart and Chest Hospital Bob Park Director, North East London Cancer Network Sue Pascoe Lung Cancer Clinical Nurse Specialist, Royal Cornwall Hospital Dr Michael Peake Consultant and Senior Lecturer in Respiratory Medicine, Glenfield Hospital, University Hospitals of Leicester NHS Trust Dr Robert Rintoul Consultant Respiratory Physician, Papworth Hospital NHS Foundation Trust, Cambridge Dr Abebaw Yohannes Reader in Physiotherapy, Manchester Metropolitan University Dr Andrew Wilcock Macmillan Clinical Reader in Palliative Medicine and Medical Oncology, University of Nottingham 2005 Guideline Development Group Dr Jesme Baird Director of Patient Care, The Roy Castle Lung Cancer Foundation Ms Caroline Belchamber Senior Oncology Physiotherapist, Poole Hospital, Dorset Dr David Bellamy GP, Bournemouth, Dorset Page 37 of
Lung cancer. The diagnosis and treatment of lung cancer. Issued: April NICE clinical guideline 121. guidance.nice.org.
 Lung cancer The diagnosis and treatment of lung cancer Issued: April 2011 NICE clinical guideline 121 guidance.nice.org.uk/cg121 NICE has accredited the process used by the Centre for Clinical Practice
Lung cancer The diagnosis and treatment of lung cancer Issued: April 2011 NICE clinical guideline 121 guidance.nice.org.uk/cg121 NICE has accredited the process used by the Centre for Clinical Practice
Lung cancer: the diagnosis and treatment of lung cancer (partial update of NICE clinical guideline 24)
 Lung cancer: the diagnosis and treatment of lung cancer (partial update of NICE clinical guideline 24) NICE guideline Draft for consultation, October 2010 If you wish to comment on this version of the
Lung cancer: the diagnosis and treatment of lung cancer (partial update of NICE clinical guideline 24) NICE guideline Draft for consultation, October 2010 If you wish to comment on this version of the
Lung Cancer Clinical Guidelines: Surgery
 Lung Cancer Clinical Guidelines: Surgery 1 Scope of guidelines All Trusts within Manchester Cancer are expected to follow this guideline. This guideline is relevant to: Adults (18 years and older) with
Lung Cancer Clinical Guidelines: Surgery 1 Scope of guidelines All Trusts within Manchester Cancer are expected to follow this guideline. This guideline is relevant to: Adults (18 years and older) with
NICE Quality Standards and COF
 NICE Quality Standards and COF David Baldwin Consultant Respiratory Physician NUH Hon Senior Lecturer Nottingham University Clinical lead NICE lung cancer GL Chair NICE QS Topic Expert Group Quality Standards
NICE Quality Standards and COF David Baldwin Consultant Respiratory Physician NUH Hon Senior Lecturer Nottingham University Clinical lead NICE lung cancer GL Chair NICE QS Topic Expert Group Quality Standards
National Optimal Lung Cancer Pathway
 National Optimal Lung Cancer Pathway This document was produced by the Lung Clinical Expert Group 2017 Document Title: National Optimal Lung Cancer Pathway and Implementation Guide Date of issue: August
National Optimal Lung Cancer Pathway This document was produced by the Lung Clinical Expert Group 2017 Document Title: National Optimal Lung Cancer Pathway and Implementation Guide Date of issue: August
Clinical guideline Published: 27 April 2011 nice.org.uk/guidance/cg122
 Ovarian cancer: recognition and initial management Clinical guideline Published: 27 April 2011 nice.org.uk/guidance/cg122 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Ovarian cancer: recognition and initial management Clinical guideline Published: 27 April 2011 nice.org.uk/guidance/cg122 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
National Optimal Lung Cancer Pathways. Dr Sadia Anwar Nottingham University Hospitals NHS Trust Clinical Lead for Lung Cancer
 National Optimal Lung Cancer Pathways Dr Sadia Anwar ttingham University Hospitals NHS Trust Clinical Lead for Lung Cancer Overview How NOLCP evolved How it relates to national guidance Pathways Implementation
National Optimal Lung Cancer Pathways Dr Sadia Anwar ttingham University Hospitals NHS Trust Clinical Lead for Lung Cancer Overview How NOLCP evolved How it relates to national guidance Pathways Implementation
Clinical guideline Published: 23 February 2009 nice.org.uk/guidance/cg81
 Advanced breast cancer: diagnosis and treatment Clinical guideline Published: 23 February 2009 nice.org.uk/guidance/cg81 NICE 20. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Advanced breast cancer: diagnosis and treatment Clinical guideline Published: 23 February 2009 nice.org.uk/guidance/cg81 NICE 20. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
North of Scotland Cancer Network Clinical Management Guideline for Non Small Cell Lung Cancer
 THIS DOCUMENT IS North of Scotland Cancer Network Clinical Management Guideline for Non Small Cell Lung Cancer [Based on WOSCAN NSCLC CMG with further extensive consultation within NOSCAN] UNCONTROLLED
THIS DOCUMENT IS North of Scotland Cancer Network Clinical Management Guideline for Non Small Cell Lung Cancer [Based on WOSCAN NSCLC CMG with further extensive consultation within NOSCAN] UNCONTROLLED
Clinical Advice to Cancer Alliances for the Commissioning of the whole Lung Cancer Pathway
 Clinical Advice to Cancer Alliances for the Commissioning of the whole Lung Cancer Pathway This document was produced by the Lung Cancer Clinical Expert August 2017 Document Title: Clinical Advice to Cancer
Clinical Advice to Cancer Alliances for the Commissioning of the whole Lung Cancer Pathway This document was produced by the Lung Cancer Clinical Expert August 2017 Document Title: Clinical Advice to Cancer
NICE guideline Published: 24 January 2018 nice.org.uk/guidance/ng83
 Oesophago-gastric cancer: assessment and management in adults NICE guideline Published: 24 January 18 nice.org.uk/guidance/ng83 NICE 18. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Oesophago-gastric cancer: assessment and management in adults NICE guideline Published: 24 January 18 nice.org.uk/guidance/ng83 NICE 18. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Lung Cancer Case Study
 Lung Cancer Case Study Presented by s GP Education Programme 2 Part One Initial presentation 60 year old lady, presents with a 6 week history of right sided chest pain. The pain is like a dull ache, but
Lung Cancer Case Study Presented by s GP Education Programme 2 Part One Initial presentation 60 year old lady, presents with a 6 week history of right sided chest pain. The pain is like a dull ache, but
Molly Boyd, MD Glenn Mills, MD Syed Jafri, MD 1/1/2010
 LSU HEALTH SCIENCES CENTER NSCLC Guidelines Feist-Weiller Cancer Center Molly Boyd, MD Glenn Mills, MD Syed Jafri, MD 1/1/2010 Initial Evaluation/Intervention: 1. Pathology Review 2. History and Physical
LSU HEALTH SCIENCES CENTER NSCLC Guidelines Feist-Weiller Cancer Center Molly Boyd, MD Glenn Mills, MD Syed Jafri, MD 1/1/2010 Initial Evaluation/Intervention: 1. Pathology Review 2. History and Physical
Slide 1. Slide 2. Slide 3. Investigation and management of lung cancer Robert Rintoul. Epidemiology. Risk factors/aetiology
 Slide 1 Investigation and management of lung cancer Robert Rintoul Department of Thoracic Oncology Papworth Hospital Slide 2 Epidemiology Second most common cancer in the UK (after breast). 38 000 new
Slide 1 Investigation and management of lung cancer Robert Rintoul Department of Thoracic Oncology Papworth Hospital Slide 2 Epidemiology Second most common cancer in the UK (after breast). 38 000 new
Endobronchial Ultrasound in the Diagnosis & Staging of Lung Cancer
 Endobronchial Ultrasound in the Diagnosis & Staging of Lung Cancer Dr Richard Booton PhD FRCP Lead Lung Cancer Clinician, Consultant Respiratory Physician & Speciality Director Manchester University NHS
Endobronchial Ultrasound in the Diagnosis & Staging of Lung Cancer Dr Richard Booton PhD FRCP Lead Lung Cancer Clinician, Consultant Respiratory Physician & Speciality Director Manchester University NHS
LCA Lung Clinical Forum. 21 st October 2014
 LCA Lung Clinical Forum 21 st October 2014 Welcome Dr Liz Sawicka Chair - LCA Lung Pathway Group Succession planning Dr Kate Haire Consultant in Public Health Medicine, LCA Commissioning Intentions for
LCA Lung Clinical Forum 21 st October 2014 Welcome Dr Liz Sawicka Chair - LCA Lung Pathway Group Succession planning Dr Kate Haire Consultant in Public Health Medicine, LCA Commissioning Intentions for
Clinical guideline Published: 23 May 2012 nice.org.uk/guidance/cg140
 Palliative care for adults: strong opioids for pain relief Clinical guideline Published: 23 May 12 nice.org.uk/guidance/cg140 NICE 18. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Palliative care for adults: strong opioids for pain relief Clinical guideline Published: 23 May 12 nice.org.uk/guidance/cg140 NICE 18. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Clinical guideline Published: 1 November 2011 nice.org.uk/guidance/cg131
 Colorectal cancer: diagnosis and management Clinical guideline Published: 1 November 2011 nice.org.uk/guidance/cg131 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Colorectal cancer: diagnosis and management Clinical guideline Published: 1 November 2011 nice.org.uk/guidance/cg131 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
National Breast Cancer Audit next steps. Martin Lee
 National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
National Breast Cancer Audit next steps Martin Lee National Cancer Audits Current Bowel Cancer Head & Neck Cancer Lung cancer Oesophagogastric cancer New Prostate Cancer - undergoing procurement Breast
Larry Tan, MD Thoracic Surgery, HSC. Community Cancer Care Educational Conference October 27, 2017
 Larry Tan, MD Thoracic Surgery, HSC Community Cancer Care Educational Conference October 27, 2017 To describe patient referral & triage for the patient with suspected lung cancer To describe the initial
Larry Tan, MD Thoracic Surgery, HSC Community Cancer Care Educational Conference October 27, 2017 To describe patient referral & triage for the patient with suspected lung cancer To describe the initial
GROUP 1: Peripheral tumour with normal hilar and mediastinum on staging CT with no disant metastases. Including: Excluding:
 GROUP 1: Including: Excluding: Peripheral tumour with normal hilar and mediastinum on staging CT with no disant metastases Solid pulmonary nodules 8mm diameter / 300mm3 volume and BROCK risk of malignancy
GROUP 1: Including: Excluding: Peripheral tumour with normal hilar and mediastinum on staging CT with no disant metastases Solid pulmonary nodules 8mm diameter / 300mm3 volume and BROCK risk of malignancy
NICE guideline Published: 10 February 2016 nice.org.uk/guidance/ng36
 Cancer of the upper aerodigestive e tract: assessment and management in people aged 16 and over NICE guideline Published: 10 February 2016 nice.org.uk/guidance/ng36 NICE 2018. All rights reserved. Subject
Cancer of the upper aerodigestive e tract: assessment and management in people aged 16 and over NICE guideline Published: 10 February 2016 nice.org.uk/guidance/ng36 NICE 2018. All rights reserved. Subject
Clinical Management Guideline for Small Cell Lung Cancer
 Diagnosis and Staging: Key Points 1. Ensure a CT scan that is
Diagnosis and Staging: Key Points 1. Ensure a CT scan that is
Single Technology Appraisal (STA)
 Single Technology Appraisal (STA) Durvalumab for maintenance treatment of locally advanced unresectable non-small cell lung cancer that has not progressed after platinum-based chemoradiation therapy Response
Single Technology Appraisal (STA) Durvalumab for maintenance treatment of locally advanced unresectable non-small cell lung cancer that has not progressed after platinum-based chemoradiation therapy Response
Interventional procedures guidance Published: 20 December 2017 nice.org.uk/guidance/ipg600
 Endobronchial valve insertion to reduce lung volume in emphysema Interventional procedures guidance Published: 20 December 2017 nice.org.uk/guidance/ipg600 Your responsibility This guidance represents
Endobronchial valve insertion to reduce lung volume in emphysema Interventional procedures guidance Published: 20 December 2017 nice.org.uk/guidance/ipg600 Your responsibility This guidance represents
Unstable angina and NSTEMI
 Issue date: March 2010 Unstable angina and NSTEMI The early management of unstable angina and non-st-segment-elevation myocardial infarction This guideline updates and replaces recommendations for the
Issue date: March 2010 Unstable angina and NSTEMI The early management of unstable angina and non-st-segment-elevation myocardial infarction This guideline updates and replaces recommendations for the
THORACIC MALIGNANCIES
 THORACIC MALIGNANCIES Summary for Malignant Malignancies. Lung Ca 1 Lung Cancer Non-Small Cell Lung Cancer Diagnostic Evaluation for Non-Small Lung Cancer 1. History and Physical examination. 2. CBCDE,
THORACIC MALIGNANCIES Summary for Malignant Malignancies. Lung Ca 1 Lung Cancer Non-Small Cell Lung Cancer Diagnostic Evaluation for Non-Small Lung Cancer 1. History and Physical examination. 2. CBCDE,
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 Ongoing care for adults with psychosis or schizophrenia bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly
Ongoing care for adults with psychosis or schizophrenia bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly
Clinical guideline Published: 23 July 2011 nice.org.uk/guidance/cg126
 Stable angina: management Clinical guideline Published: 23 July 2011 nice.org.uk/guidance/cg126 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Stable angina: management Clinical guideline Published: 23 July 2011 nice.org.uk/guidance/cg126 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Early and locally advanced non-small-cell lung cancer (NSCLC)
 Early and locally advanced non-small-cell lung cancer (NSCLC) ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up P. E. Postmus, K. M. Kerr, M. Oudkerk, S. Senan, D. A. Waller, J.
Early and locally advanced non-small-cell lung cancer (NSCLC) ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up P. E. Postmus, K. M. Kerr, M. Oudkerk, S. Senan, D. A. Waller, J.
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 Assessment and immediate management of suspected acute coronary syndrome bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They
Assessment and immediate management of suspected acute coronary syndrome bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Malignant melanoma: assessment and management of malignant melanoma 1.1 Short title Malignant Melanoma 2 The remit The Department
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Malignant melanoma: assessment and management of malignant melanoma 1.1 Short title Malignant Melanoma 2 The remit The Department
NICE guideline Published: 10 February 2016 nice.org.uk/guidance/ng36
 Cancer of the upper aerodigestive e tract: assessment and management in people aged 16 and over NICE guideline Published: 10 February 2016 nice.org.uk/guidance/ng36 NICE 2018. All rights reserved. Subject
Cancer of the upper aerodigestive e tract: assessment and management in people aged 16 and over NICE guideline Published: 10 February 2016 nice.org.uk/guidance/ng36 NICE 2018. All rights reserved. Subject
NICE BULLETIN Diagnosis & treatment of prostate cancer
 Diagnosis & treatment of prostate cancer NICE provided the content for this booklet which is independent of any company or product advertised Diagnosis and treatment of prostate cancer Introduction In
Diagnosis & treatment of prostate cancer NICE provided the content for this booklet which is independent of any company or product advertised Diagnosis and treatment of prostate cancer Introduction In
The diagnosis and treatment of lung cancer (update)
 Clinical Guideline The diagnosis and treatment of lung cancer (update) Full Guideline 0 0 This document is a partial update of NICE clinical guideline (published February 00) and will replace it. The sections
Clinical Guideline The diagnosis and treatment of lung cancer (update) Full Guideline 0 0 This document is a partial update of NICE clinical guideline (published February 00) and will replace it. The sections
Smoking cessation interventions and services
 National Institute for Health and Care Excellence Guideline version (Final) Smoking cessation interventions and services [E] Evidence reviews for advice NICE guideline NG92 Evidence reviews FINAL These
National Institute for Health and Care Excellence Guideline version (Final) Smoking cessation interventions and services [E] Evidence reviews for advice NICE guideline NG92 Evidence reviews FINAL These
Clinical guideline Published: 27 June 2012 nice.org.uk/guidance/cg143
 Sickle cell disease: managing acute painful episodes in hospital Clinical guideline Published: 27 June 2012 nice.org.uk/guidance/cg143 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Sickle cell disease: managing acute painful episodes in hospital Clinical guideline Published: 27 June 2012 nice.org.uk/guidance/cg143 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Technology appraisal guidance Published: 18 July 2018 nice.org.uk/guidance/ta531
 Pembrolizumab for untreated PD- L1-positive metastatic non-small-cell lung cancer Technology appraisal guidance Published: 18 July 2018 nice.org.uk/guidance/ta531 NICE 2018. All rights reserved. Subject
Pembrolizumab for untreated PD- L1-positive metastatic non-small-cell lung cancer Technology appraisal guidance Published: 18 July 2018 nice.org.uk/guidance/ta531 NICE 2018. All rights reserved. Subject
An Update: Lung Cancer
 An Update: Lung Cancer Andy Barlow Consultant in Respiratory Medicine Lead Clinician for Lung Cancer (West Herts Hospitals NHS Trust) Lead for EBUS-Harefield Hospital (RB&HFT) Summary Lung cancer epidemiology
An Update: Lung Cancer Andy Barlow Consultant in Respiratory Medicine Lead Clinician for Lung Cancer (West Herts Hospitals NHS Trust) Lead for EBUS-Harefield Hospital (RB&HFT) Summary Lung cancer epidemiology
Author(s) Approval date: 12/05/16. Committee. June Operational Date: Review: Version No. 1.1 Supercedes 1.0 Links to other policies
 Reference No: Title: Author(s) Ownership: Approval by: Operational Date: Systemic Anti-Cancer Therapy (SACT) Guidelines for Peritoneal Mesothelioma Professor Richard Wilson (Consultant/Chair in Cancer
Reference No: Title: Author(s) Ownership: Approval by: Operational Date: Systemic Anti-Cancer Therapy (SACT) Guidelines for Peritoneal Mesothelioma Professor Richard Wilson (Consultant/Chair in Cancer
and Strength of Recommendations
 ASTRO with ASCO Qualifying Statements in Bold Italics s patients with T1-2, N0 non-small cell lung cancer who are medically operable? 1A: Patients with stage I NSCLC should be evaluated by a thoracic surgeon,
ASTRO with ASCO Qualifying Statements in Bold Italics s patients with T1-2, N0 non-small cell lung cancer who are medically operable? 1A: Patients with stage I NSCLC should be evaluated by a thoracic surgeon,
Guidelines for the Management of Renal Cancer West Midlands Expert Advisory Group for Urological Cancer
 Guidelines for the Management of Renal Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Guidelines for the Management of Renal Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Adam J. Hansen, MD UHC Thoracic Surgery
 Adam J. Hansen, MD UHC Thoracic Surgery Sometimes seen on Chest X-ray (CXR) Common incidental findings on computed tomography (CT) chest and abdomen done for other reasons Most lung cancers discovered
Adam J. Hansen, MD UHC Thoracic Surgery Sometimes seen on Chest X-ray (CXR) Common incidental findings on computed tomography (CT) chest and abdomen done for other reasons Most lung cancers discovered
Lung cancer forms in tissues of the lung, usually in the cells lining air passages.
 Scan for mobile link. Lung Cancer Lung cancer usually forms in the tissue cells lining the air passages within the lungs. The two main types are small-cell lung cancer (usually found in cigarette smokers)
Scan for mobile link. Lung Cancer Lung cancer usually forms in the tissue cells lining the air passages within the lungs. The two main types are small-cell lung cancer (usually found in cigarette smokers)
Technology appraisal guidance Published: 24 August 2016 nice.org.uk/guidance/ta402
 Pemetrexed ed maintenance treatment for non-squamous non-small-cell lung cancer after pemetrexed ed and cisplatin Technology appraisal guidance Published: 24 August 2016 nice.org.uk/guidance/ta402 NICE
Pemetrexed ed maintenance treatment for non-squamous non-small-cell lung cancer after pemetrexed ed and cisplatin Technology appraisal guidance Published: 24 August 2016 nice.org.uk/guidance/ta402 NICE
Guidelines for the Management of Bladder Cancer West Midlands Expert Advisory Group for Urological Cancer
 Guidelines for the Management of Bladder Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
Guidelines for the Management of Bladder Cancer West Midlands Expert Advisory Group for Urological Cancer West Midlands Clinical Networks and Clinical Senate Coversheet for Network Expert Advisory Group
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
For personal use only. Not to be reproduced without permission of the editor
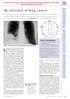 For personal use only. Not to be reproduced without permission of the editor (permissions@pharmj.org.uk) An overview of lung cancer November is Lung Cancer Awareness Month. With public smoking bans springing
For personal use only. Not to be reproduced without permission of the editor (permissions@pharmj.org.uk) An overview of lung cancer November is Lung Cancer Awareness Month. With public smoking bans springing
Faster Cancer Treatment Indicators: Use cases
 Faster Cancer Treatment Indicators: Use cases 2014 Date: October 2014 Version: Owner: Status: v01 Ministry of Health Cancer Services Final Citation: Ministry of Health. 2014. Faster Cancer Treatment Indicators:
Faster Cancer Treatment Indicators: Use cases 2014 Date: October 2014 Version: Owner: Status: v01 Ministry of Health Cancer Services Final Citation: Ministry of Health. 2014. Faster Cancer Treatment Indicators:
Clinical guideline Published: 23 March 2011 nice.org.uk/guidance/cg118
 Colorectal cancer prevention: ention: colonoscopic surveillance in adults with ulcerative colitis, Crohn's disease or adenomas Clinical guideline Published: March 2011 nice.org.uk/guidance/cg118 NICE 2018.
Colorectal cancer prevention: ention: colonoscopic surveillance in adults with ulcerative colitis, Crohn's disease or adenomas Clinical guideline Published: March 2011 nice.org.uk/guidance/cg118 NICE 2018.
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 Chronic fatigue syndrome myalgic encephalomyelitis elitis overview bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated
Chronic fatigue syndrome myalgic encephalomyelitis elitis overview bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated
Lung Cancer Imaging Guidelines: Integration with the Lung Cancer Diagnosis and Staging Clinical Pathway
 Lung Cancer Imaging Guidelines: Integration with the Lung Cancer Diagnosis and Staging Clinical Pathway Cancer Imaging Guidance L-1 Version 1 January 2014 Cancer Imaging Program Cancer Care Ontario Page
Lung Cancer Imaging Guidelines: Integration with the Lung Cancer Diagnosis and Staging Clinical Pathway Cancer Imaging Guidance L-1 Version 1 January 2014 Cancer Imaging Program Cancer Care Ontario Page
Bladder Cancer Guidelines
 Bladder Cancer Guidelines Agreed by Urology CSG: October 2011 Review Date: September 2013 Bladder Cancer 1. Referral Guidelines The following patients should be considered as potentially having bladder
Bladder Cancer Guidelines Agreed by Urology CSG: October 2011 Review Date: September 2013 Bladder Cancer 1. Referral Guidelines The following patients should be considered as potentially having bladder
Charles Mulligan, MD, FACS, FCCP 26 March 2015
 Charles Mulligan, MD, FACS, FCCP 26 March 2015 Review lung cancer statistics Review the risk factors Discuss presentation and staging Discuss treatment options and outcomes Discuss the status of screening
Charles Mulligan, MD, FACS, FCCP 26 March 2015 Review lung cancer statistics Review the risk factors Discuss presentation and staging Discuss treatment options and outcomes Discuss the status of screening
Surgical management of lung cancer
 Surgical management of lung cancer Nick Roubos FRACS Cardiothoracic Surgeon Box Hill Hospital, Epworth Eastern Thoracic Oncology Non Small Cell Lung Cancer (NSCLC) Small Cell Lung Cancer Mesothelioma Pulmonary
Surgical management of lung cancer Nick Roubos FRACS Cardiothoracic Surgeon Box Hill Hospital, Epworth Eastern Thoracic Oncology Non Small Cell Lung Cancer (NSCLC) Small Cell Lung Cancer Mesothelioma Pulmonary
Clinical guideline Published: 13 June 2012 nice.org.uk/guidance/cg141
 Acute upper gastrointestinal bleeding in over 16s: management Clinical guideline Published: June 2012 nice.org.uk/guidance/cg141 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Acute upper gastrointestinal bleeding in over 16s: management Clinical guideline Published: June 2012 nice.org.uk/guidance/cg141 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Technology appraisal guidance Published: 29 June 2011 nice.org.uk/guidance/ta227
 Erlotinib monotherapy for maintenance treatment of non-small-cell lung cancer Technology appraisal guidance Published: 29 June 2011 nice.org.uk/guidance/ta227 NICE 2018. All rights reserved. Subject to
Erlotinib monotherapy for maintenance treatment of non-small-cell lung cancer Technology appraisal guidance Published: 29 June 2011 nice.org.uk/guidance/ta227 NICE 2018. All rights reserved. Subject to
Erlotinib (Tarceva) for non small cell lung cancer advanced or metastatic maintenance monotherapy
 Erlotinib (Tarceva) for non small cell lung cancer advanced or metastatic maintenance monotherapy September 2008 This technology summary is based on information available at the time of research and a
Erlotinib (Tarceva) for non small cell lung cancer advanced or metastatic maintenance monotherapy September 2008 This technology summary is based on information available at the time of research and a
Bronchogenic Carcinoma
 A 55-year-old construction worker has smoked 2 packs of ciggarettes daily for the past 25 years. He notes swelling in his upper extremity & face, along with dilated veins in this region. What is the most
A 55-year-old construction worker has smoked 2 packs of ciggarettes daily for the past 25 years. He notes swelling in his upper extremity & face, along with dilated veins in this region. What is the most
Guideline for the Management of Vulval Cancer
 Version History Guideline for the Management of Vulval Cancer Version Date Brief Summary of Change Issued 2.0 20.02.08 Endorsed by the Governance Committee 2.1 19.11.10 Circulated at NSSG meeting 2.2 13.04.11
Version History Guideline for the Management of Vulval Cancer Version Date Brief Summary of Change Issued 2.0 20.02.08 Endorsed by the Governance Committee 2.1 19.11.10 Circulated at NSSG meeting 2.2 13.04.11
Technology appraisal guidance Published: 26 October 2016 nice.org.uk/guidance/ta416
 Osimertinib for treating locally advanced or metastatic EGFR T790M mutation- positive non-small-cell lung cancer Technology appraisal guidance Published: 26 October 2016 nice.org.uk/guidance/ta416 NICE
Osimertinib for treating locally advanced or metastatic EGFR T790M mutation- positive non-small-cell lung cancer Technology appraisal guidance Published: 26 October 2016 nice.org.uk/guidance/ta416 NICE
LUNG CANCER. Agnieszka Słowik, MD. Department of Oncology, University Hospital in Cracow Jagiellonian University
 LUNG CANCER Agnieszka Słowik, MD Department of Oncology, University Hospital in Cracow Jagiellonian University Epidemiology Most common malignancy worldwide Place of lung cancer among other malignancies
LUNG CANCER Agnieszka Słowik, MD Department of Oncology, University Hospital in Cracow Jagiellonian University Epidemiology Most common malignancy worldwide Place of lung cancer among other malignancies
North of Scotland Cancer Network Clinical Management Guideline for Mesothelioma
 THIS DOCUMENT IS North of Scotland Cancer Network Clinical Management Guideline for Mesothelioma [Based on WOSCAN SCLC CMG with further extensive consultation within NOSCAN] UNCONTROLLED WHEN PRINTED Document
THIS DOCUMENT IS North of Scotland Cancer Network Clinical Management Guideline for Mesothelioma [Based on WOSCAN SCLC CMG with further extensive consultation within NOSCAN] UNCONTROLLED WHEN PRINTED Document
Technology appraisal guidance Published: 28 September 2016 nice.org.uk/guidance/ta411
 Necitumumab for untreated advanced or metastatic squamous non-small-cell lung cancer Technology appraisal guidance Published: 28 September 2016 nice.org.uk/guidance/ta411 NICE 2017. All rights reserved.
Necitumumab for untreated advanced or metastatic squamous non-small-cell lung cancer Technology appraisal guidance Published: 28 September 2016 nice.org.uk/guidance/ta411 NICE 2017. All rights reserved.
Cancer of Unknown Primary (CUP) Protocol
 1 Department of Oncology. Cancer of Unknown Primary (CUP) Protocol Version: Document type: Document sponsor Designation Document author [ s] Designation[s] Approving committee / Group Ratified by: Date
1 Department of Oncology. Cancer of Unknown Primary (CUP) Protocol Version: Document type: Document sponsor Designation Document author [ s] Designation[s] Approving committee / Group Ratified by: Date
Specialised Services Policy: CP22. Stereotactic Radiosurgery
 Specialised Services Policy: CP22 Document Author: Assistant Director of Planning Executive Lead: Director of Planning ad Performance Approved by: Management Group Issue Date: 01 July 2015 Review Date:
Specialised Services Policy: CP22 Document Author: Assistant Director of Planning Executive Lead: Director of Planning ad Performance Approved by: Management Group Issue Date: 01 July 2015 Review Date:
Medical technologies guidance Published: 2 October 2018 nice.org.uk/guidance/mtg39
 ifuse for treating chronic sacroiliac joint pain Medical technologies guidance Published: 2 October 2018 nice.org.uk/guidance/mtg39 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
ifuse for treating chronic sacroiliac joint pain Medical technologies guidance Published: 2 October 2018 nice.org.uk/guidance/mtg39 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Health Board/Region: All-Wales
 Peer Review: Cancer Sub-site: Gynaecology Health Board/Region: All-Wales Cycle: Second Date of review: February 2018 This report describes the findings and themes observed by clinical review panels during
Peer Review: Cancer Sub-site: Gynaecology Health Board/Region: All-Wales Cycle: Second Date of review: February 2018 This report describes the findings and themes observed by clinical review panels during
NICE guideline Published: 6 July 2016 nice.org.uk/guidance/ng49
 Non-alcoholic fatty liver disease (NAFLD): assessment and management NICE guideline Published: 6 July 20 nice.org.uk/guidance/ng49 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Non-alcoholic fatty liver disease (NAFLD): assessment and management NICE guideline Published: 6 July 20 nice.org.uk/guidance/ng49 NICE 2017. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Appendix 10. Page 1 of 209
 Appendix 10 Lung cancer: the diagnosis and treatment of lung cancer (2005) including the original sections of the guideline that have been updated by the 2011 document. Page 1 of 209 Foreword Lung cancer
Appendix 10 Lung cancer: the diagnosis and treatment of lung cancer (2005) including the original sections of the guideline that have been updated by the 2011 document. Page 1 of 209 Foreword Lung cancer
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
The diagnosis and treatment of lung cancer
 The diagnosis and treatment of lung cancer NICE guideline Second draft for consultation, September 2004 If you wish to comment on the recommendations, please make your comments on the full version of the
The diagnosis and treatment of lung cancer NICE guideline Second draft for consultation, September 2004 If you wish to comment on the recommendations, please make your comments on the full version of the
Clinical guideline Published: 17 March 2008 nice.org.uk/guidance/cg64
 Prophylaxis against infective endocarditis: antimicrobial prophylaxis against infective e endocarditis in adults and children undergoing interventionalentional procedures Clinical guideline Published:
Prophylaxis against infective endocarditis: antimicrobial prophylaxis against infective e endocarditis in adults and children undergoing interventionalentional procedures Clinical guideline Published:
Low back pain and sciatica in over 16s NICE quality standard
 March 2017 Low back pain and sciatica in over 16s NICE quality standard Draft for consultation This quality standard covers the assessment and management of non-specific low back pain and sciatica in young
March 2017 Low back pain and sciatica in over 16s NICE quality standard Draft for consultation This quality standard covers the assessment and management of non-specific low back pain and sciatica in young
SIGN 137 Management of lung cancer. A national clinical guideline February Evidence
 SIGN 137 Management of lung cancer A national clinical guideline February 2014 Evidence KEY TO EVIDENCE STATEMENTS AND GRADES OF RECOMMENDATIONS LEVELS OF EVIDENCE 1 ++ High quality meta-analyses, systematic
SIGN 137 Management of lung cancer A national clinical guideline February 2014 Evidence KEY TO EVIDENCE STATEMENTS AND GRADES OF RECOMMENDATIONS LEVELS OF EVIDENCE 1 ++ High quality meta-analyses, systematic
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary and secondary
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary and secondary
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Lung cancer Surgery. 17 TH ESO-ESMO MASTERCLASS IN CLINICAL ONCOLOGY March, 2017 Berlin, Germany
 17 TH ESO-ESMO MASTERCLASS IN CLINICAL ONCOLOGY 24-29 March, 2017 Berlin, Germany Lung cancer Surgery Sven Hillinger MD, Thoracic Surgery, University Hospital Zurich Case 1 59 y, female, 40 py, incidental
17 TH ESO-ESMO MASTERCLASS IN CLINICAL ONCOLOGY 24-29 March, 2017 Berlin, Germany Lung cancer Surgery Sven Hillinger MD, Thoracic Surgery, University Hospital Zurich Case 1 59 y, female, 40 py, incidental
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: diagnosis and management of colorectal cancer 1.1 Short title Colorectal cancer 2 The remit The Department
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Colorectal cancer: diagnosis and management of colorectal cancer 1.1 Short title Colorectal cancer 2 The remit The Department
Technology appraisal guidance Published: 6 September 2017 nice.org.uk/guidance/ta476
 Paclitaxel as albumin-bound nanoparticles with gemcitabine for untreated metastatic pancreatic cancer Technology appraisal guidance Published: 6 September 2017 nice.org.uk/guidance/ta476 NICE 2018. All
Paclitaxel as albumin-bound nanoparticles with gemcitabine for untreated metastatic pancreatic cancer Technology appraisal guidance Published: 6 September 2017 nice.org.uk/guidance/ta476 NICE 2018. All
There are a number of national guidelines and performance standards which support the implementation of a straight to CT pathway.
 December 2015 CONTENTS Contents... 2 1 Introduction... 3 2 Case for Change... 3 3 Evidence... 3 3.1 National and regional policy... 3 3.2 Local audit... 4 4 Supporting Work Initiatives... 5 4.1 Identification
December 2015 CONTENTS Contents... 2 1 Introduction... 3 2 Case for Change... 3 3 Evidence... 3 3.1 National and regional policy... 3 3.2 Local audit... 4 4 Supporting Work Initiatives... 5 4.1 Identification
NICE guideline Published: 21 September 2016 nice.org.uk/guidance/ng56
 Multimorbidity: clinical assessment and management NICE guideline Published: 21 September 2016 nice.org.uk/guidance/ng56 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Multimorbidity: clinical assessment and management NICE guideline Published: 21 September 2016 nice.org.uk/guidance/ng56 NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and-conditions#notice-ofrights).
Endobronchial valve insertion to reduce lung volume in emphysema
 NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Endobronchial valve insertion to reduce lung volume in emphysema Emphysema is a chronic lung disease that
NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Interventional procedure consultation document Endobronchial valve insertion to reduce lung volume in emphysema Emphysema is a chronic lung disease that
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM)
 SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician GMCN ROYAL WOLVERHAMPTON HOSPITALS The Royal Wolverhampton Hospitals Trust Lung MDT (11-2C-1) - 2011/12 Dr Angela Morgan
SELF ASSESSMENT REPORT (MULTI-DISCIPLINARY TEAM) Network Trust MDT MDT Lead Clinician GMCN ROYAL WOLVERHAMPTON HOSPITALS The Royal Wolverhampton Hospitals Trust Lung MDT (11-2C-1) - 2011/12 Dr Angela Morgan
Technology appraisal guidance Published: 16 May 2018 nice.org.uk/guidance/ta520
 Atezolizumab for treating locally advanced or metastatic non-small-cell lung cancer after chemotherapy Technology appraisal guidance Published: 16 May 2018 nice.org.uk/guidance/ta520 NICE 2018. All rights
Atezolizumab for treating locally advanced or metastatic non-small-cell lung cancer after chemotherapy Technology appraisal guidance Published: 16 May 2018 nice.org.uk/guidance/ta520 NICE 2018. All rights
Ovarian cancer. Quick reference guide. Issue date: April The recognition and initial management of ovarian cancer
 Issue date: April 2011 Ovarian cancer The recognition and initial management of ovarian cancer Developed by the National Collaborating Centre for Cancer About this booklet This is aquick reference guide
Issue date: April 2011 Ovarian cancer The recognition and initial management of ovarian cancer Developed by the National Collaborating Centre for Cancer About this booklet This is aquick reference guide
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
bring together everything NICE says on a topic in an interactive flowchart. are interactive and designed to be used online. They are updated regularly as new NICE guidance is published. To view the latest
Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181)
 Putting NICE guidance into practice Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181) Published: July 2014 This costing report accompanies Lipid modification:
Putting NICE guidance into practice Costing report: Lipid modification Implementing the NICE guideline on lipid modification (CG181) Published: July 2014 This costing report accompanies Lipid modification:
NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE SCOPE
 DRAFT NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary
DRAFT NATIONAL INSTITUTE FOR HEALTH AND CLINICAL EXCELLENCE 1 Guideline title SCOPE Chronic obstructive pulmonary disease: the management of adults with chronic obstructive pulmonary disease in primary
Costing report: Bladder cancer
 Putting NICE guidance into practice Costing report: Bladder cancer Implementing the NICE guideline on bladder cancer (NG2) Published: February 2015 Updated September 2015 to update the unit cost of transurethral
Putting NICE guidance into practice Costing report: Bladder cancer Implementing the NICE guideline on bladder cancer (NG2) Published: February 2015 Updated September 2015 to update the unit cost of transurethral
Horizon Scanning Centre November Enobosarm (Ostarine) for cachexia in patients with advanced non-small cell lung cancer first line
 Horizon Scanning Centre November 2012 Enobosarm (Ostarine) for cachexia in patients with advanced non-small cell lung cancer first line SUMMARY NIHR HSC ID: 5206 This briefing is based on information available
Horizon Scanning Centre November 2012 Enobosarm (Ostarine) for cachexia in patients with advanced non-small cell lung cancer first line SUMMARY NIHR HSC ID: 5206 This briefing is based on information available
North of England Cancer Network. NECN Lung Cancer Clinical Guidelines
 North of England Cancer Network Lung Cancer Clinical Guidelines Lung NSSG on behalf of NECN Document Information Title: NECN Lung Cancer Clinical Guidelines Author: Lung NSSG Members Circulation List:
North of England Cancer Network Lung Cancer Clinical Guidelines Lung NSSG on behalf of NECN Document Information Title: NECN Lung Cancer Clinical Guidelines Author: Lung NSSG Members Circulation List:
Case Scenario 1. The patient agreed to a CT guided biopsy of the left upper lobe mass. This was performed and confirmed non-small cell carcinoma.
 Case Scenario 1 An 89 year old male patient presented with a progressive cough for approximately six weeks for which he received approximately three rounds of antibiotic therapy without response. A chest
Case Scenario 1 An 89 year old male patient presented with a progressive cough for approximately six weeks for which he received approximately three rounds of antibiotic therapy without response. A chest
Audit Report. Lung Cancer Quality Performance Indicators. Patients diagnosed April 2014 March Published: May 2016
 NORTH OF SCOTLAND PLANNING GROUP Lung Cancer Managed Clinical Network Audit Report Lung Cancer Quality Performance Indicators Patients diagnosed April 2014 March 2015 Published: May 2016 Mr Hardy Remmen
NORTH OF SCOTLAND PLANNING GROUP Lung Cancer Managed Clinical Network Audit Report Lung Cancer Quality Performance Indicators Patients diagnosed April 2014 March 2015 Published: May 2016 Mr Hardy Remmen
Delirium. Quick reference guide. Issue date: July Diagnosis, prevention and management
 Issue date: July 2010 Delirium Diagnosis, prevention and management Developed by the National Clinical Guideline Centre for Acute and Chronic Conditions About this booklet This is a quick reference guide
Issue date: July 2010 Delirium Diagnosis, prevention and management Developed by the National Clinical Guideline Centre for Acute and Chronic Conditions About this booklet This is a quick reference guide
2010 National Audit of Dementia (Care in General Hospitals) Guy's and St Thomas' NHS Foundation Trust
 Royal College of Psychiatrists 2010 National Audit of Dementia (Care in General Hospitals) Organisational checklist results and commentary for: Guy's and St Thomas' NHS Foundation Trust The 2010 national
Royal College of Psychiatrists 2010 National Audit of Dementia (Care in General Hospitals) Organisational checklist results and commentary for: Guy's and St Thomas' NHS Foundation Trust The 2010 national
They are updated regularly as new NICE guidance is published. To view the latest version of this NICE Pathway see:
 Step 3: GAD with marked functional impairment or that has not improved after step 2 interventionsentions bring together everything NICE says on a topic in an interactive flowchart. are interactive and
Step 3: GAD with marked functional impairment or that has not improved after step 2 interventionsentions bring together everything NICE says on a topic in an interactive flowchart. are interactive and
Technology appraisal guidance Published: 28 October 2009 nice.org.uk/guidance/ta183
 Topotecan for the treatment of recurrent and stage IVB cervical cancer Technology appraisal guidance Published: 28 October 2009 nice.org.uk/guidance/ta183 NICE 2018. All rights reserved. Subject to Notice
Topotecan for the treatment of recurrent and stage IVB cervical cancer Technology appraisal guidance Published: 28 October 2009 nice.org.uk/guidance/ta183 NICE 2018. All rights reserved. Subject to Notice
Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases
 Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases Date: April 2015 Date for review: April 2018 1. Principles The recognised specialist HPB MDT for Greater
Manchester Cancer Colorectal Pathway Board: Guidelines for management of colorectal hepatic metastases Date: April 2015 Date for review: April 2018 1. Principles The recognised specialist HPB MDT for Greater
National Horizon Scanning Centre. Erlotinib (Tarceva) in combination with bevacizumab for advanced or metastatic non-small cell lung cancer
 Erlotinib (Tarceva) in combination with bevacizumab for advanced or metastatic non-small cell lung cancer This technology summary is based on information available at the time of research and a limited
Erlotinib (Tarceva) in combination with bevacizumab for advanced or metastatic non-small cell lung cancer This technology summary is based on information available at the time of research and a limited
