Jefferies 2014 Global Healthcare Conference. June 3, 2014
|
|
|
- Jane Gray
- 6 years ago
- Views:
Transcription
1 Jefferies 2014 Glbal Healthcare Cnference June 3, 2014
2 Frward Lking Statements This presentatin cntains certain frward lking statements relating t the cmpany s financial results, business prspects and the develpment and cmmercializatin f REOLYSIN, a therapeutic revirus. These statements are based n management s current expectatins and beliefs and are subject t a number f factrs which invlve knwn and unknwn risks, delays, uncertainties and ther factrs nt under the cmpany s cntrl which may cause actual results, perfrmance r achievements f the cmpany t be materially different frm the results, perfrmance r ther expectatins implied by these frward lking statements. In any frward lking statement in which Onclytics Bitech Inc. expresses an expectatin r belief as t future results, such expectatins r beliefs are expressed in gd faith and are believed t have a reasnable basis, but there can be n assurance that the statement r expectatin r belief will be achieved. These factrs include results f current r pending clinical trials, risks assciated with intellectual prperty prtectin, financial prjectins, market prjectins, actins by the FDA/HPB/MHRA and thse ther factrs detailed in the cmpany s filings with SEDAR and the Securities and Exchange Cmmissin. Onclytics des nt undertake an bligatin t update the frward lking statements, except as required by applicable laws. 2
3 Onclytics Overview Expanding Clinical Prgram Lead prduct is REOLYSIN, a bradly active nvel cancer therapy Onging clinical trials include six randmized studies: Phase II studies nging in the US and Canada breast, nn-small cell lung, clrectal, prstate, pancreatic and varian cancers Strng Intellectual Prperty Prtfli Mre than 370 patents issued wrldwide Manufacturing at Cmmercial Scale 100L cgmp cmpleted, cmmercial manufacturing agreement in place 3
4 REOLYSIN Overview REOLYSIN is a prprietary islate f the revirus Revirus is a replicatin cmpetent virus and is cnsidered safe t humans REOLYSIN has been safely administered t patients via intravenus, intratumral and intrathecal injectin Mechanism f Actin (MA): Primary MA is as a selective cyttxin, where selectivity is based n whether cancer cells have cnstituative Ras pathway activatin; susceptible cancer cells therefre include thse with either: EGFR verexpressin r mutatin; r Ras mutatin, which includes Kras mutatin Secndary MAs may include interfern up regulatin in target tissues, and tumur directed immune respnse 4
5 Market fr Ras Pathway Mediated Cancers Estimated glbal cancer market was US$85 billin in 2013; this is expected t rise t US$109 billin in 2018 At least five millin new patients per year are expected t develp cancers with a Ras pathway invlvement In the develped wrld alne, at least 2.6 millin patients per year die f cancers that have metastasized 5
6 REO 003: REOLYSIN Intratumural Mntherapy Anaplastic Astrcytma Early Cyttxic Activity Fllwed by Late Stage Immune-Mediated Respnse Against the Residual Tumur Days after REOLYSIN administratin: (Pst Debulking) 6
7 REO 013: REOLYSIN Intravenus Mntherapy Metastatic Liver Lesin Image shws psitive (red staining) fr revirus in the metastatic lesins (blue arrw) and negative fr revirus in the nrmal cells (red arrw) Nine ut f ten patients shwed the same pattern, i.e. targeted delivery t metastatic tumr lesins f the liver 7
8 REOLYSIN Clinical Prgram Overview REOLYSIN has been utilized in studies in ver 1,000 patients In ttal, nearly thirty nging r cmpleted clinical trials including: Seven randmized Phase II and Phase III clinical trials, including Phase III head and neck cancer and Phase II trials fr varian, pancreatic, prstate, clrectal, nn-small cell lung and breast cancers Nine single arm studies in the fllwing indicatins: Phase II trials: Cmpany spnsred: pancreatic cancer, nn-small cell lung cancer, head and neck carcinma, metastatic melanma and squamus cell carcinma Phase I trials: Cmpany spnsred: clrectal cancer and advanced malignancies Investigatr spnsred: multiple myelma and relapsed r refractry slid tumrs 8
9 Key Clinical Endpints in Onclgy Tumur shrinkage r remval Safety Time t prgressin Overall survival 9
10 REO 011: Head & Neck Cancer Patient with Partial Respnse in Liver Metastases Pre-Treatment Pst-Cycle 6 Prir treatment: radiatin Respnse maintained thrugh 8 cycles 10
11 REO 016: Nn-Small Cell Lung Cancer Single-arm (up t 36 patients), pen-label, tw-stage US Phase II study f intravenusly-administered REOLYSIN in cmbinatin with carbplatin and paclitaxel Fr nn-small cell lung cancer (NSCLC) patients wh have been pre-screened fr Kras and EGFR mutatin status 15-20% f NSCLC is Kras mutated, while up t 50% is EGFR mutated r verexpressed, all f which cause Ras pathway activatin First-line therapy study, i.e. patients will be ffered REOLYSIN / carbplatin / paclitaxel instead f standard f care if they are Kras r EGFR mutated r EGFR verexpressed 11
12 REO 016: Bimarker Crrelatins with REOLYSIN Efficacy Of 36 evaluable patients, all f whm were Stage IV n entry, 89% exhibited SD r better (11 PR, 21 SD and 4 PD by RECIST) 20 f these 36 patients (56%) had ne year r mre f survival Of 24 patients with at least an EGFR mutatin r amplificatin, 16 (66.7%) had ne year r mre f survival Of 13 patients with nly an EGFR mutatin r amplificatin, 9 (69.2%) had ne year r mre f survival Of 4 patients with BRAF and EGFR amplificatins, 4 (100%) had ne year r mre f survival 12
13 REO 016: Partial Respnse in Lung Pre-Treatment Pst-Cycle 2 13
14 REO 021: Squamus Cell Carcinma f the Lungs Single-arm (up t 36 patients), pen-label US Phase II study f intravenusly-administered REOLYSIN in cmbinatin with carbplatin and paclitaxel Final results in 25 evaluable patients (all with metastatic disease) demnstrated that 92% (23 patients) exhibited verall tumur shrinkage, with mean shrinkage f 32.7% Of the 25 evaluable patients wh received mre than ne cycle f therapy, 10 (40%) shwed partial respnses by RECIST, and a further 12 (48%) shwed stable disease by RECIST, fr a disease cntrl rate (CR + PR + SD) f 92% 14
15 REO 021: Partial Respnse in Lung Right Upper Lung Mass (8.3 cm) Right Upper Lung Mass (4.1 cm) Right Upper Lung Mass (3.6 cm) Right Pleural Met (2.2 cm) Right Pleural Met (0.8 cm) Right Pleural Met (0.4 cm) Pre-Treatment Pst-Cycle 2 Pst-Cycle 4 15
16 REOLYSIN and Safety Mre than 1,000 patients treated, mre than 900 intravenusly at dses up t 3x10 10 TCID 50 daily N maximum tlerated dse (MTD) reached t date Mntherapy txicities have generally been mild (grade 1 r 2) and included chills, fever, headache, cugh, myalgia, runny nse, sre thrat, fatigue and grade 1 r 2 lymphpenia and neutrpenia Transient grade 3 and 4 txicities included lymphpenia and neutrpenia These symptms were mre frequently bserved frm day 2 f treatment and usually lasted less than 6 hurs 16
17 Phase III (Pivtal) Prgram fr REOLYSIN in Squamus Cell Head & Neck Cancers In Q3 2012, Onclytics cmpleted enrllment in REO 018, a randmized, tw stage, tw-arm, duble-blind, multi-center trial examining REOLYSIN in cmbinatin with carbplatin and paclitaxel in taxane-naïve patients with platinum-refractry recurrent head and neck cancers The study was apprved and run in furteen cuntries in Nrth America and Eurpe Patients in the REO 018 study were stratified fr: ECOG perfrmance status (0-1 versus 2) Time f prgressin/relapse after prir platinum-based chemtherapy Disease lcatin (patients with lcally recurrent disease, with r withut distal metastases, versus patients with metastatic disease nly) REO 018 Endpints: Primary Endpint: Overall Survival (OS) Secndary Endpints: Prgressin-Free Survival (PFS), best respnse and tumur-specific respnse Pharmacdynamic Endpints: Tumur Ras pathway status and HPV status The Cmpany intends t treat REO 018 as a separate supprtive study t a planned randmized, fllw-n internatinal Phase III head and neck registratin study in patients with lc-reginal head and neck cancer 17
18 REO 018: Tumr Specific Respnse Data Data annunced December 13, 2012 Endpint examines initial percentage tumr changes between baseline and first pst treatment scans in all patients, differentiating between lc-reginal tumurs and metastatic tumurs This is a measure f rate r velcity f respnse, nt magnitude f respnse The endpint was intrduced t determine if REOLYSIN adds tumr specific differential activity in metastatic and lc-reginal disease in a randmized setting Of the ttal 105 patients with evaluable metastatic tumrs, 86% (n=50) f thse in the test, and 67% (n=55) in the cntrl arm, arm had tumr stabilizatin (0% grwth) r shrinkage This is a statistically significant difference, with a p-value f
19 Head and Neck: Percentage Shrinkage f Tumurs at First Pst-Treatment Scan Grup Test 0 Grup Cntrl 1 20 Grup Test 0 Grup Cntrl Patients with Lc-Reginal Disease, With r Withut Distal Metastases (p=0.076, n=118) Patients With Distal Metastases Only (p=0.021, n=47) 19
20 REO 018: Tp-Line Safety Data Data annunced Nvember 21, 2013 fr all 167 patients enrlled REOLYSIN was safe and well-tlerated by patients Patients n the test arm f the study experienced a higher incidence f flu-like symptms cnsistent with earlier clinical trials f REOLYSIN and treatment with a virus Mst cmmnly mild fever, chills, nausea and diarrhea Fewer patients required dse reductins f paclitaxel due t neurpathy r neurtxicity n the test arm than the cntrl arm (zer in the test arm versus six in the cntrl; p=0.028) On this basis, Onclytics intends t explre the ptential chemprtective and neurprtective prperties f REOLYSIN in future clinical studies 20
21 REO 018: Tp-Line Efficacy Data Data annunced Nvember 21, 2013 and April 8, 2014 Patients with lcreginal disease plus r minus distal metastases in the test arm (n=62) shwed a median PFS f 94 days (13.4 weeks), versus 50 days (7.1 weeks) in the cntrl arm (n=56) Patients wh received REOLYSIN had increased benefit thrugh five cycles f therapy An intent t treat analysis f the 118 lcreginal patients using Type II censring frm the median PFS f each arm shwed a statistically significant imprvement in PFS f the test arm versus the cntrl arm (p=0.0072, hazard rati= ) An intent t treat analysis f the 118 lcreginal patients perfrmed n all patients t the median PFS in each arm, censring any patients wh received pst-discntinuatin therapy at the date at which they cmmenced the first f these therapies shwed a statistically significant imprvement in OS f the test arm versus the cntrl arm (p=0.0146, hazard rati=0.5099) 21
22 REOLYSIN : Randmized Pipeline Indicatin REO 018: Head & Neck Cancer Cmbinatin Therapy Carbplatin + paclitaxel n Preclinical Phase I Phase II Phase III Spnsr 167 n/a GOG-0186H: Ovarian, Fallpian Tube & Primary Peritneal Cancers Paclitaxel 110 NCI/ GOG OSU-10045: Pancreatic Cancer Carbplatin + paclitaxel 70 NCI IND 209: Prstate Cancer Dcetaxel 80 NCIC IND 210: Clrectal Cancer FOLFOX-6 + Avastin 100 NCIC IND 211: Nn-Small Cell Lung Cancer Dcetaxel r pemetrexed 150 NCIC IND 213: Breast Cancer Paclitaxel 100 NCIC 22
23 Intellectual Prperty Mre than 370 patents issued wrldwide, including 56 US and 20 Canadian Revirus issue patent claims cver: Cmpsitins f matter cmprising revirus Pharmaceutical use f reviruses t treat neplasia and cellular prliferative diseases Cmbinatin therapy with radiatin, chemtherapy and/r immune suppressants Methds fr manufacturing revirus and screening fr susceptibility t revirus Pharmaceutical use f reviruses in transplantatin prcedures Apprximately 235 pending applicatins wrldwide 23
24 Manufacturing Nw prduced at 100L under cgmp with final frmulatin Cmmercial manufacturing agreement with SAFC in place 24
25 Market & Capital Data (all amunts in CAD) Exchanges Shares Outstanding (March 31, 2014) NASDAQ:ONCY TSX:ONC 85,810,718 Optins Outstanding (March 31, 2014) Price $3.65 (weighted average) 6,065,344 Fully Diluted (March 31, 2014) 91,876,062 Cash/Cash Equivalents (March 31, 2014) $22.2M 25
26 Onclytics Summary Expanding Clinical Prgram Lead prduct is REOLYSIN, a bradly active nvel cancer therapy Onging clinical trials include six randmized studies: Phase II studies nging in the US and Canada breast, nn-small cell lung, clrectal, prstate, pancreatic and varian cancers Strng Intellectual Prperty Prtfli Mre than 370 patents issued wrldwide Manufacturing at Cmmercial Scale 100L cgmp cmpleted, cmmercial manufacturing agreement in place 26
27 Jefferies 2014 Glbal Healthcare Cnference June 3, 2014
Investor Presentation
 Investr Presentatin May 2018 nclyticsbitech.cm TSX ONC OTCQX ONCYF Frward Lking Statements This presentatin cntains certain frward lking statements relating t the cmpany s business prspects and the develpment
Investr Presentatin May 2018 nclyticsbitech.cm TSX ONC OTCQX ONCYF Frward Lking Statements This presentatin cntains certain frward lking statements relating t the cmpany s business prspects and the develpment
Activating the patient s immune system to fight. system to. Company presentation. fight cancer. Company presentation. August November
 Activating the patient s the immune system t fight immune cancer system t Cmpany presentatin fight cancer Cmpany presentatin August Nvember 2018 2018 IMPORTANT NOTICE AND DISCLAIMER This reprt cntains
Activating the patient s the immune system t fight immune cancer system t Cmpany presentatin fight cancer Cmpany presentatin August Nvember 2018 2018 IMPORTANT NOTICE AND DISCLAIMER This reprt cntains
Investor Presentation
 Investr Presentatin August 2018 nclyticsbitech.cm Nasdaq ONCY TSX ONC Frward Lking Statements This presentatin cntains certain frward lking statements relating t the cmpany s business prspects and the
Investr Presentatin August 2018 nclyticsbitech.cm Nasdaq ONCY TSX ONC Frward Lking Statements This presentatin cntains certain frward lking statements relating t the cmpany s business prspects and the
Activating the patient s immune system to fight. system to. Company presentation. fight cancer. Company presentation. August November
 Activating the patient s the immune system t fight immune cancer system t Cmpany presentatin fight cancer Cmpany presentatin August Nvember 2018 2018 Imprtant NOTICE AND DISCLAIMER This reprt cntains certain
Activating the patient s the immune system t fight immune cancer system t Cmpany presentatin fight cancer Cmpany presentatin August Nvember 2018 2018 Imprtant NOTICE AND DISCLAIMER This reprt cntains certain
This clinical study synopsis is provided in line with Boehringer Ingelheim s Policy on Transparency and Publication of Clinical Study Data.
 abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
abcd Clinical Study Synpsis fr Public Disclsure This clinical study synpsis is prvided in line with Behringer Ingelheim s Plicy n Transparency and Publicatin f Clinical Study Data. The synpsis which is
August November 2018
 Activating the patient s the immune system t fight immune cancer system t Cmpany presentatin fight cancer 3Q 2018 Reprt August 2018 1 Nvember 2018 Imprtant NOTICE AND DISCLAIMER This reprt cntains certain
Activating the patient s the immune system t fight immune cancer system t Cmpany presentatin fight cancer 3Q 2018 Reprt August 2018 1 Nvember 2018 Imprtant NOTICE AND DISCLAIMER This reprt cntains certain
Activating the patient s immune system to fight. system to. Company presentation. fight cancer. 4Q and Full Year August 14 February
 Activating the patient s the immune system t fight immune cancer system t Cmpany presentatin 4Q and Full Year 2018 fight cancer August 14 February 2019 2018 IMPORTANT NOTICE AND DISCLAIMER This reprt cntains
Activating the patient s the immune system t fight immune cancer system t Cmpany presentatin 4Q and Full Year 2018 fight cancer August 14 February 2019 2018 IMPORTANT NOTICE AND DISCLAIMER This reprt cntains
Activating the immune system to fight cancer
 Activating the immune system t fight cancer RedEye pre-asco seminar Erik Digman Wiklund, CFO 28 May 2018 Frm a sequential treatment strategy directly targeting the cancer 1 Surgery When pssible, surgical
Activating the immune system t fight cancer RedEye pre-asco seminar Erik Digman Wiklund, CFO 28 May 2018 Frm a sequential treatment strategy directly targeting the cancer 1 Surgery When pssible, surgical
The clinical trial information provided in this public disclosure synopsis is supplied for informational purposes only.
 The clinical trial infrmatin prvided in this public disclsure synpsis is supplied fr infrmatinal purpses nly. Please nte that the results reprted in any single trial may nt reflect the verall ptential
The clinical trial infrmatin prvided in this public disclsure synpsis is supplied fr infrmatinal purpses nly. Please nte that the results reprted in any single trial may nt reflect the verall ptential
Activating the immune system to fight cancer. ABG Sundal Collier Oslo 12 June 2018
 Activating the immune system t fight cancer ABG Sundal Cllier Osl 12 June 2018 Imprtant NOTICE AND DISCLAIMER This reprt cntains certain frward-lking statements based n uncertainty, since they relate t
Activating the immune system t fight cancer ABG Sundal Cllier Osl 12 June 2018 Imprtant NOTICE AND DISCLAIMER This reprt cntains certain frward-lking statements based n uncertainty, since they relate t
Four categories which guide further evaluation
 Unknwn Primary Updated May 2017 by Di Maria Jiang (PGY-5 Medical Onclgy Resident, University f Trnt) Reviewed by Dr. Chistine Elser (Staff Medical Onclgist, University f Trnt) and Dr. Sct Dwden (Staff
Unknwn Primary Updated May 2017 by Di Maria Jiang (PGY-5 Medical Onclgy Resident, University f Trnt) Reviewed by Dr. Chistine Elser (Staff Medical Onclgist, University f Trnt) and Dr. Sct Dwden (Staff
XX Abraxane 100 MG SUSR (CELGENE CORP)
 Plicy Medical Plicy Manual Apprved: D Nt Implement Until 1/31/19 Paclitaxel (Prtein-Bund) NDC CODE(S) 68817-0134-XX Abraxane 100 MG SUSR (CELGENE CORP) DESCRIPTION Paclitaxel is a natural prduct with antitumr
Plicy Medical Plicy Manual Apprved: D Nt Implement Until 1/31/19 Paclitaxel (Prtein-Bund) NDC CODE(S) 68817-0134-XX Abraxane 100 MG SUSR (CELGENE CORP) DESCRIPTION Paclitaxel is a natural prduct with antitumr
XX Abraxane 100 MG SUSR (CELGENE CORP
 Medical Manual Apprved Revised: D Nt Implement until 6/30/2019 Paclitaxel (Prtein-Bund) NDC CODE(S) 68817-0134-XX Abraxane 100 MG SUSR (CELGENE CORP DESCRIPTION Paclitaxel is a natural prduct with antitumr
Medical Manual Apprved Revised: D Nt Implement until 6/30/2019 Paclitaxel (Prtein-Bund) NDC CODE(S) 68817-0134-XX Abraxane 100 MG SUSR (CELGENE CORP DESCRIPTION Paclitaxel is a natural prduct with antitumr
XX Keytruda 100 MG/4ML SOLN (MERCK SHARP & DOHME)
 Plicy Medical Plicy Manual Apprved: D Nt Implement Until 1/31/19 Pembrlizumab NDC CODE(S) 00006-3026-XX Keytruda 100 MG/4ML SOLN (MERCK SHARP & DOHME) DESCRIPTION Pembrlizumab is a human prgrammed death
Plicy Medical Plicy Manual Apprved: D Nt Implement Until 1/31/19 Pembrlizumab NDC CODE(S) 00006-3026-XX Keytruda 100 MG/4ML SOLN (MERCK SHARP & DOHME) DESCRIPTION Pembrlizumab is a human prgrammed death
Activating the immune system to fight cancer
 Activating the immune system t fight cancer Jefferies Investr Cnference New Yrk City Dr. Magnus Jäderberg - CMO 6 June 2018 Imprtant NOTICE AND DISCLAIMER This reprt cntains certain frward-lking statements
Activating the immune system t fight cancer Jefferies Investr Cnference New Yrk City Dr. Magnus Jäderberg - CMO 6 June 2018 Imprtant NOTICE AND DISCLAIMER This reprt cntains certain frward-lking statements
Investor Presentation
 Fr persnal use nly Investr Presentatin Dr. Marie Rskrw, CEO & Managing Directr September 2014 ASX: PAB Fr persnal use nly Safe Harbur Statement This presentatin cntains frward-lking statements that are
Fr persnal use nly Investr Presentatin Dr. Marie Rskrw, CEO & Managing Directr September 2014 ASX: PAB Fr persnal use nly Safe Harbur Statement This presentatin cntains frward-lking statements that are
Frequently Asked Questions: IS RT-Q-PCR Testing
 Questins 1. What is chrnic myelid leukemia (CML)? 2. Hw des smene knw if they have CML? 3. Hw is smene diagnsed with CML? Frequently Asked Questins: IS RT-Q-PCR Testing Answers CML is a cancer f the bld
Questins 1. What is chrnic myelid leukemia (CML)? 2. Hw des smene knw if they have CML? 3. Hw is smene diagnsed with CML? Frequently Asked Questins: IS RT-Q-PCR Testing Answers CML is a cancer f the bld
Commissioning Policy: South Warwickshire CCG (SWCCG)
 Cmmissining Plicy: Suth Warwickshire CCG (SWCCG) Treatment Indicatin Criteria FreeStyle Libre Flash Cntinuus Glucse Mnitring System Type I Diabetes Prir apprval must be requested frm the Individual Funding
Cmmissining Plicy: Suth Warwickshire CCG (SWCCG) Treatment Indicatin Criteria FreeStyle Libre Flash Cntinuus Glucse Mnitring System Type I Diabetes Prir apprval must be requested frm the Individual Funding
Important NOTICE AND DISCLAIMER
 1 Imprtant NOTICE AND DISCLAIMER This reprt cntains certain frward-lking statements based n uncertainty, since they relate t events and depend n circumstances that will ccur in future and which, by their
1 Imprtant NOTICE AND DISCLAIMER This reprt cntains certain frward-lking statements based n uncertainty, since they relate t events and depend n circumstances that will ccur in future and which, by their
BRCA1 and BRCA2 Mutations
 BRCA1 and BRCA2 Mutatins ROBERT LEVITT, MD JESSICA BERGER-WEISS, MD ADRIENNE POTTS, MD HARTAJ POWELL, MD, MPH COURTNEY LEVENSON, MD LAUREN BURNS, MSN, RN, WHNP OBGYNCWC.COM v Cancer is a cmplex disease
BRCA1 and BRCA2 Mutatins ROBERT LEVITT, MD JESSICA BERGER-WEISS, MD ADRIENNE POTTS, MD HARTAJ POWELL, MD, MPH COURTNEY LEVENSON, MD LAUREN BURNS, MSN, RN, WHNP OBGYNCWC.COM v Cancer is a cmplex disease
23/11/2015. Introduction & Aims. Methods. Methods. Survey response. Patient Survey (baseline)
 Intrductin & Aims Drug and Alchl Cnsultatin Liaisn (AOD CL) services aim t imprve identificatin and treatment f patients with AOD mrbidity. The csts and cnsequences f targeting AOD patients presenting
Intrductin & Aims Drug and Alchl Cnsultatin Liaisn (AOD CL) services aim t imprve identificatin and treatment f patients with AOD mrbidity. The csts and cnsequences f targeting AOD patients presenting
INTERIM REPORT JANUARY SEPTEMBER 2017
 INTERIM REPORT JANUARY SEPTEMBER 2017 TELEPHONE CONFERENCE NO ONE SHOULD HAVE TO DIE WAITING FOR A NEW ORGAN Octber 27, 2017 MAGNUS NILSSON, CEO CHRISTOFFER ROSENBLAD, CFO NASDAQ OMX Stckhlm (mid cap):
INTERIM REPORT JANUARY SEPTEMBER 2017 TELEPHONE CONFERENCE NO ONE SHOULD HAVE TO DIE WAITING FOR A NEW ORGAN Octber 27, 2017 MAGNUS NILSSON, CEO CHRISTOFFER ROSENBLAD, CFO NASDAQ OMX Stckhlm (mid cap):
Folotyn (pralatrexate)
 Fltyn (pralatrexate) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/2018TBD03/01/2017 POLICY A. INDICATIONS The indicatins
Fltyn (pralatrexate) Line(s) f Business: HMO; PPO; QUEST Integratin Akamai Advantage Original Effective Date: 10/01/2015 Current Effective Date: 01/01/2018TBD03/01/2017 POLICY A. INDICATIONS The indicatins
P02-03 CALA Program Description Proficiency Testing Policy for Accreditation Revision 1.9 July 26, 2017
 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin Revisin 1.9 July 26, 2017 P02-03 CALA Prgram Descriptin Prficiency Testing Plicy fr Accreditatin TABLE OF CONTENTS TABLE OF CONTENTS...
Safety of HPV vaccination: A FIGO STATEMENT
 FIGO Statement n HPV Vaccinatin Safety, August 2nd, 2013 Safety f HPV vaccinatin: A FIGO STATEMENT July, 2013 Human papillmavirus vaccines are used in many cuntries; glbally, mre than 175 millin dses have
FIGO Statement n HPV Vaccinatin Safety, August 2nd, 2013 Safety f HPV vaccinatin: A FIGO STATEMENT July, 2013 Human papillmavirus vaccines are used in many cuntries; glbally, mre than 175 millin dses have
Medical Policy Title: HDC & Autologous ARBenefits Approval: 02/08/2012
 Medical Plicy Title: HDC & Autlgus ARBenefits Apprval: 02/08/2012 Stem&/r Prgenitr Cell Supprt, Germ Cell Tumrs Effective Date: 01/01/2013 Dcument: ARB0416:01 Revisin Date: 10/24/2012 Cde(s): 38230, Bne
Medical Plicy Title: HDC & Autlgus ARBenefits Apprval: 02/08/2012 Stem&/r Prgenitr Cell Supprt, Germ Cell Tumrs Effective Date: 01/01/2013 Dcument: ARB0416:01 Revisin Date: 10/24/2012 Cde(s): 38230, Bne
12 th European International Kidney Cancer Symposium April 2017
 PEGylated Human IL-1 (AM1) in Cmbinatin with Nivlumab in Renal Cell Cancer Aung Naing, Jeffrey R. Infante, Debrah J. Wng, W. Michael Krn, Raid Aljumaily, Kyriaks P. Papadpuls, Karen A. Auti, Shubham Pant,
PEGylated Human IL-1 (AM1) in Cmbinatin with Nivlumab in Renal Cell Cancer Aung Naing, Jeffrey R. Infante, Debrah J. Wng, W. Michael Krn, Raid Aljumaily, Kyriaks P. Papadpuls, Karen A. Auti, Shubham Pant,
Overall Survival and Safety Profile of YERVOY in Patients with Unresectable or Metastatic Melanoma
 FDA Apprves YERVOY (ipilimumab) fr the Treatment f Patients with Newly Diagnsed r Previusly-Treated Unresectable r Metastatic Melanma, the Deadliest Frm f Skin Cancer First and Only Apprved Therapy fr
FDA Apprves YERVOY (ipilimumab) fr the Treatment f Patients with Newly Diagnsed r Previusly-Treated Unresectable r Metastatic Melanma, the Deadliest Frm f Skin Cancer First and Only Apprved Therapy fr
Volume Measurement at CT
 Vlume Measurement at CT Staging and Assessment f Respnse with Quantitative CT Lawrence Schwartz, MD Department f Radilgy Clumbia University Cllege f Physicians and Surgens LSCHWARTZ@COLUMBIA.EDU Recmmendatins
Vlume Measurement at CT Staging and Assessment f Respnse with Quantitative CT Lawrence Schwartz, MD Department f Radilgy Clumbia University Cllege f Physicians and Surgens LSCHWARTZ@COLUMBIA.EDU Recmmendatins
Independent Charitable Patient Assistance Program (IPAP) Code of Ethics
 Independent Charitable Patient Assistance Prgram (IPAP) Cde f Ethics Independent charitable patient assistance prgrams (IPAPs) fcus n the needs f patients wh are insured, meet certain financial limitatin
Independent Charitable Patient Assistance Prgram (IPAP) Cde f Ethics Independent charitable patient assistance prgrams (IPAPs) fcus n the needs f patients wh are insured, meet certain financial limitatin
Press Release. Adocia: Activity and results for the first half of 2013
 Press Release Adcia: Activity and results fr the first half f 2013 Intensificatin f R&D activity ahead f the launch f three clinical trials fr insulin prjects, starting in the secnd half f 2013 Net result
Press Release Adcia: Activity and results fr the first half f 2013 Intensificatin f R&D activity ahead f the launch f three clinical trials fr insulin prjects, starting in the secnd half f 2013 Net result
Press Release. Adocia Reports Operational and Financial Results for the First Half of 2014
 Press Release Adcia Reprts Operatinal and Financial Results fr the First Half f 2014 Majr clinical results reprted n tw frmulatins based n insulin Sund financial psitin with apprximately EUR 16 millin
Press Release Adcia Reprts Operatinal and Financial Results fr the First Half f 2014 Majr clinical results reprted n tw frmulatins based n insulin Sund financial psitin with apprximately EUR 16 millin
REPORT ON OPERATIONS 2017 TELEPHONE CONFERENCE
 REPORT ON OPERATIONS 2017 TELEPHONE CONFERENCE NO ONE SHOULD HAVE TO DIE WAITING FOR A NEW ORGAN February 12, 2018 MAGNUS NILSSON, CEO CHRISTOFFER ROSENBLAD, CFO NASDAQ OMX Stckhlm (mid cap): XVIVO HIGHLIGHTS
REPORT ON OPERATIONS 2017 TELEPHONE CONFERENCE NO ONE SHOULD HAVE TO DIE WAITING FOR A NEW ORGAN February 12, 2018 MAGNUS NILSSON, CEO CHRISTOFFER ROSENBLAD, CFO NASDAQ OMX Stckhlm (mid cap): XVIVO HIGHLIGHTS
Clinical Study Synopsis
 Clinical Study Synpsis This Clinical Study Synpsis is prvided fr patients and healthcare prfessinals t increase the transparency f Bayer's clinical research. This dcument is nt intended t replace the advice
Clinical Study Synpsis This Clinical Study Synpsis is prvided fr patients and healthcare prfessinals t increase the transparency f Bayer's clinical research. This dcument is nt intended t replace the advice
Protocol Abstract and Schema
 NCI Prtcl #: PBTC-042 Lcal Prtcl #: PBTC-042 Prtcl Abstract and Schema PBTC-042: Phase I study f CDK 4-6 inhibitr PD-0332991 (palbciclib; IBRANCE) in children with recurrent, prgressive r refractry central
NCI Prtcl #: PBTC-042 Lcal Prtcl #: PBTC-042 Prtcl Abstract and Schema PBTC-042: Phase I study f CDK 4-6 inhibitr PD-0332991 (palbciclib; IBRANCE) in children with recurrent, prgressive r refractry central
Risk factors in health and disease
 Risk factrs in health and disease Index 1 Intrductin 2 Types f risk factrs 2.1 Behaviural risk factrs 2.2 Psychlgical risk factrs 2.3 Demgraphic risk factrs 2.4 Envirnmental risk factrs 2.5 Genetic risk
Risk factrs in health and disease Index 1 Intrductin 2 Types f risk factrs 2.1 Behaviural risk factrs 2.2 Psychlgical risk factrs 2.3 Demgraphic risk factrs 2.4 Envirnmental risk factrs 2.5 Genetic risk
Study Design Open, three arm-stratified, non-randomized, prospective, multicentric study
 PONS Study Synpsis Title f the Study Subtype-Stratified Fllw-up Care Study f Breast Cancer Patients with Cmbined In Vitr and In Viv Diagnstics Plus Early Target-Oriented Interventin Gals Imprve and individualize
PONS Study Synpsis Title f the Study Subtype-Stratified Fllw-up Care Study f Breast Cancer Patients with Cmbined In Vitr and In Viv Diagnstics Plus Early Target-Oriented Interventin Gals Imprve and individualize
Pancreatic Cancer Treatment Market Size, Share & Trends Analysis Report and Segment Forecast, 2025 Grand View Research, Inc
 Pancreatic Cancer Treatment Market Size, Share & Trends Analysis Reprt and Segment Frecast, 2025 Grand View Research, Inc The rising prevalence f besity, alchl cnsumptin, and smking is expected t prpel
Pancreatic Cancer Treatment Market Size, Share & Trends Analysis Reprt and Segment Frecast, 2025 Grand View Research, Inc The rising prevalence f besity, alchl cnsumptin, and smking is expected t prpel
SERVICE DE GYNÉCOLOGIE-ONCOLOGIE PROTOCOLES EN RECRUTEMENT
 SERVICE DE GYNÉCOLOGIE-ONCOLOGIE PROTOCOLES EN RECRUTEMENT OVAIRES PROTOCOLES PTES PHASE DESCRIPTION OV25 DP/GSO/GSO/FG 6 II PRÉVENTION A Randmized Phase II Duble-Blind Placeb-Cntrlled Trials f Acetylsalicylic
SERVICE DE GYNÉCOLOGIE-ONCOLOGIE PROTOCOLES EN RECRUTEMENT OVAIRES PROTOCOLES PTES PHASE DESCRIPTION OV25 DP/GSO/GSO/FG 6 II PRÉVENTION A Randmized Phase II Duble-Blind Placeb-Cntrlled Trials f Acetylsalicylic
TOP TIPS Lung Cancer Update Dr Andrew Wight Consultant respiratory Physician - WUTH
 Tpic Circulatin list In case f query please cntact Executive Summary TOP TIPS Lung Cancer Update Dr Andrew Wight Cnsultant respiratry Physician - WUTH All Wirral GP s JaneFletcher2@nhs.net Dear Clleagues,
Tpic Circulatin list In case f query please cntact Executive Summary TOP TIPS Lung Cancer Update Dr Andrew Wight Cnsultant respiratry Physician - WUTH All Wirral GP s JaneFletcher2@nhs.net Dear Clleagues,
A Phase I Study of CEP-701 in Patients with Refractory Neuroblastoma NANT (01-03) A New Approaches to Neuroblastoma Therapy (NANT) treatment protocol.
 SAMPLE INFORMED CONSENT A Phase I Study f CEP-701 in Patients with Refractry Neurblastma NANT (01-03) A New Appraches t Neurblastma Therapy (NANT) treatment prtcl. The wrd yu used thrughut this dcument
SAMPLE INFORMED CONSENT A Phase I Study f CEP-701 in Patients with Refractry Neurblastma NANT (01-03) A New Appraches t Neurblastma Therapy (NANT) treatment prtcl. The wrd yu used thrughut this dcument
WARNING: FATAL AND SERIOUS TOXICITIES: SEVERE DIARRHEA AND CARDIAC TOXICITIES
 INDICATION FARYDAK (panbinstat) capsules, a histne deacetylase inhibitr, in cmbinatin with brtezmib and dexamethasne, is indicated fr the treatment f patients with multiple myelma wh have received at least
INDICATION FARYDAK (panbinstat) capsules, a histne deacetylase inhibitr, in cmbinatin with brtezmib and dexamethasne, is indicated fr the treatment f patients with multiple myelma wh have received at least
Patrys To Present at the 12 th Annual BIO Investor Forum
 ASX & Media Release Patrys T Present at the 12 th Annual BIO Investr Frum Melburne, Australia; 9 Octber, 2013: Patrys Limited (ASX: PAB), a clinical stage bitechnlgy cmpany tday annunced that Dr. Marie
ASX & Media Release Patrys T Present at the 12 th Annual BIO Investr Frum Melburne, Australia; 9 Octber, 2013: Patrys Limited (ASX: PAB), a clinical stage bitechnlgy cmpany tday annunced that Dr. Marie
NPCR CLINICAL EDIT CHECKS
 NPCR CLINICAL EDIT CHECKS FCDS Annual Meeting July 26, 2013 Sunrise, Flrida Steven Peace, CTR FCDS Data Quality Staff PURPOSE OF CLINICAL EDIT CHECKS The primary purpse f the Clinical Check edits is t
NPCR CLINICAL EDIT CHECKS FCDS Annual Meeting July 26, 2013 Sunrise, Flrida Steven Peace, CTR FCDS Data Quality Staff PURPOSE OF CLINICAL EDIT CHECKS The primary purpse f the Clinical Check edits is t
SCALES NW HEARING PROTECTION PROGRAM
 PURPOSE Expsure t excessive nise in the wrkplace can cause permanent hearing lss. The Hearing Prtectin Prgram has been established t help ensure that emplyees f Scales NW, Inc. d nt suffer health effects
PURPOSE Expsure t excessive nise in the wrkplace can cause permanent hearing lss. The Hearing Prtectin Prgram has been established t help ensure that emplyees f Scales NW, Inc. d nt suffer health effects
PBTC-026: A Feasibility Study of SAHA Combined with Isotretinoin and Chemotherapy in Infants with Embryonal Tumors of the Central Nervous System
 PBTC-026: A Feasibility Study f SAHA Cmbined with Istretinin and Chemtherapy in Infants with Embrynal Tumrs f the Central Nervus System PURPOSE: This clinical trial is studying the side effects f giving
PBTC-026: A Feasibility Study f SAHA Cmbined with Istretinin and Chemtherapy in Infants with Embrynal Tumrs f the Central Nervus System PURPOSE: This clinical trial is studying the side effects f giving
Section 6 Students School District No. 71 (Comox Valley)
 Sectin 6 Students Schl District N. 71 (Cmx Valley) Administrative Prcedure 6011 MR2 Allergies and Anaphylaxis 1. Intrductin The Bard f Educatin expects schls t reasnably accmmdate students with medically
Sectin 6 Students Schl District N. 71 (Cmx Valley) Administrative Prcedure 6011 MR2 Allergies and Anaphylaxis 1. Intrductin The Bard f Educatin expects schls t reasnably accmmdate students with medically
Significance of Chronic Kidney Disease in 2015
 1 Significance f Chrnic Kidney Disease in 2015 There is still a requirement within QOF t keep a register f peple with CKD stages 3-5. The ther CKD QOF targets have been retired. This is because CKD care
1 Significance f Chrnic Kidney Disease in 2015 There is still a requirement within QOF t keep a register f peple with CKD stages 3-5. The ther CKD QOF targets have been retired. This is because CKD care
US Public Health Service Clinical Practice Guidelines for PrEP
 Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
Webcast 1.3 US Public Health Service Clinical Practice Guidelines fr PrEP P R E S ENTED BY: M A R K T H R U N, M D A S S O C I AT E P R O F E S S O R, U N I V E R S I T Y O F C O L O R A D O, D I V I S
Solid Organ Transplant Benefits to Change for Texas Medicaid
 Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
Slid Organ Transplant Benefits t Change fr Texas Medicaid Infrmatin psted February 13, 2015 Nte: All new and updated prcedure cdes and their assciated reimbursement rates are prpsed benefits pending a
A fake medicine that passes itself off as a real, authorised medicine. (1)
 Falsified medicines Index 1 Intrductin 2 Types f falsified medicines 3 Eurpean regulatin n falsified medicines 4 Risks f falsified medicines 5 Buying medicine nline safely 6 References 7 Further resurces
Falsified medicines Index 1 Intrductin 2 Types f falsified medicines 3 Eurpean regulatin n falsified medicines 4 Risks f falsified medicines 5 Buying medicine nline safely 6 References 7 Further resurces
Human papillomavirus (HPV) refers to a group of more than 150 related viruses.
 HUMAN PAPILLOMAVIRUS This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice r discussin between
HUMAN PAPILLOMAVIRUS This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice r discussin between
Swindon Joint Strategic Needs Assessment Bulletin
 Swindn Jint Strategic Needs Assessment Bulletin Swindn Diabetes 2017 Key Pints: This JSNA gives health facts abut peple with diabetes r peple wh might get diabetes in Swindn. This helps us t plan fr medical
Swindn Jint Strategic Needs Assessment Bulletin Swindn Diabetes 2017 Key Pints: This JSNA gives health facts abut peple with diabetes r peple wh might get diabetes in Swindn. This helps us t plan fr medical
Protocol. Preparation Protocol for the Non-Targeted Vevo MicroMarker Contrast Agent
 Prtcl Preparatin Prtcl fr the Nn-Targeted Vev MicrMarker Cntrast Agent System Cmpatibility: This guide cntains instructins and suggestins fr wrk n the Vev2100, VevLAZR, Vev 3100 systems and transducers
Prtcl Preparatin Prtcl fr the Nn-Targeted Vev MicrMarker Cntrast Agent System Cmpatibility: This guide cntains instructins and suggestins fr wrk n the Vev2100, VevLAZR, Vev 3100 systems and transducers
Corporate Governance Code for Funds: What Will it Mean?
 Crprate Gvernance Cde fr Funds: What Will it Mean? The Irish Funds Industry Assciatin has circulated a draft Vluntary Crprate Gvernance Cde fr the Funds Industry in Ireland. 1. Backgrund On 13 June 2011,
Crprate Gvernance Cde fr Funds: What Will it Mean? The Irish Funds Industry Assciatin has circulated a draft Vluntary Crprate Gvernance Cde fr the Funds Industry in Ireland. 1. Backgrund On 13 June 2011,
CONTACT: Amber Hamilton TYPE 2 DIABETES AND OBESITY: TWIN EPIDEMICS OVERVIEW
 FACT SHEET CONTACT: Amber Hamiltn 212-266-0062 TYPE 2 DIABETES AND OBESITY: TWIN EPIDEMICS OVERVIEW Type 2 diabetes accunts fr 90-95% f the 29.1 millin diabetes cases in the U.S. 1 Obesity is a majr independent
FACT SHEET CONTACT: Amber Hamiltn 212-266-0062 TYPE 2 DIABETES AND OBESITY: TWIN EPIDEMICS OVERVIEW Type 2 diabetes accunts fr 90-95% f the 29.1 millin diabetes cases in the U.S. 1 Obesity is a majr independent
Recommendations for Risk Management at Swine Exhibitions and for Show Pigs August 2012
 Recmmendatins fr Risk Management at Swine Exhibitins and fr Shw Pigs August 2012 Backgrund: The Natinal Prk Bard facilitated in develping this dcument. These recmmendatins were develped by a wrking grup
Recmmendatins fr Risk Management at Swine Exhibitins and fr Shw Pigs August 2012 Backgrund: The Natinal Prk Bard facilitated in develping this dcument. These recmmendatins were develped by a wrking grup
Health Science Ch. 16 Cancer Lecture Outline
 Cancer Leading cause f disease-related death amng peple under age 75 Secnd leading cause f death Evidence supprts that mst cancers culd be prevented by simple lifestyle changes Tbacc is respnsible fr abut
Cancer Leading cause f disease-related death amng peple under age 75 Secnd leading cause f death Evidence supprts that mst cancers culd be prevented by simple lifestyle changes Tbacc is respnsible fr abut
WHAT IS HEAD AND NECK CANCER FACT SHEET
 WHAT IS HEAD AND NECK CANCER FACT SHEET This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice
WHAT IS HEAD AND NECK CANCER FACT SHEET This infrmatin may help answer sme f yur questins and help yu think f ther questins that yu may want t ask yur cancer care team; it is nt intended t replace advice
Breast Cancer Awareness Month 2018 Key Messages (as of June 6, 2018)
 Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
Breast Cancer Awareness Mnth 2018 Key Messages (as f June 6, 2018) In this dcument there are tw sectins f messages in supprt f Cancer Care Ontari s Breast Cancer Awareness Mnth 2018: 1. Campaign key messages
CDC Influenza Division Key Points November 7, 2014
 In this dcument: Summary Key Messages FluView Activity Update LAIV Effectiveness and Vaccinatin f Children H3N2 Match and Vaccinatin Vaccine Supply Summary Key Messages This week s FluView reprt indicates
In this dcument: Summary Key Messages FluView Activity Update LAIV Effectiveness and Vaccinatin f Children H3N2 Match and Vaccinatin Vaccine Supply Summary Key Messages This week s FluView reprt indicates
HPV VACCINATION IN SANDYFORD SERVICES
 HPV VACCINATION IN SANDYFORD SERVICES 1. General infrmatin n HPV Vaccinatin 2. HPV vaccinatin in females 3. Yung Wmen Only 4. HPV vaccinatin in MSM 1. General infrmatin General Infrmatin fr clinicians
HPV VACCINATION IN SANDYFORD SERVICES 1. General infrmatin n HPV Vaccinatin 2. HPV vaccinatin in females 3. Yung Wmen Only 4. HPV vaccinatin in MSM 1. General infrmatin General Infrmatin fr clinicians
Perjeta (pertuzumab) Document Number: IC I. Length of Authorization. Dosing Limits. Initial Approval Criteria
 Perjeta (pertuzumab) Last Review Date: 11/21/2017 Date f Origin: 11/01/2012 Dcument Number: IC-0096 Dates Reviewed: 12/2012, 3/2013, 6/2013, 9/2013, 11/2013, 12/2013, 3/2014, 6/2014, 9/2014, 12/2014, 3/2015,
Perjeta (pertuzumab) Last Review Date: 11/21/2017 Date f Origin: 11/01/2012 Dcument Number: IC-0096 Dates Reviewed: 12/2012, 3/2013, 6/2013, 9/2013, 11/2013, 12/2013, 3/2014, 6/2014, 9/2014, 12/2014, 3/2015,
VIRGINIA OBSTETRICS & GYNECOLOGY, P.C.
 VIRGINIA OBSTETRICS & GYNECOLOGY, P.C. 19490 Sandridge Way Suite 350 Leesburg, VA 20176 Phne (703) 858-5599 Fax (703) 858-5699 PERSONAL INFORMATION: PATIENT INFORMATION SHEET Please Print Date. Patient's
VIRGINIA OBSTETRICS & GYNECOLOGY, P.C. 19490 Sandridge Way Suite 350 Leesburg, VA 20176 Phne (703) 858-5599 Fax (703) 858-5699 PERSONAL INFORMATION: PATIENT INFORMATION SHEET Please Print Date. Patient's
Vaccine Information Statement: LIVE INTRANASAL INFLUENZA VACCINE
 Vaccine Infrmatin Statement: LIVE INTRANASAL INFLUENZA VACCINE Many Vaccine Infrmatin Statements are available in Spanish and ther languages. See www.immunize.rg/vis. Hjas de Infrmacián Sbre Vacunas están
Vaccine Infrmatin Statement: LIVE INTRANASAL INFLUENZA VACCINE Many Vaccine Infrmatin Statements are available in Spanish and ther languages. See www.immunize.rg/vis. Hjas de Infrmacián Sbre Vacunas están
Rate Lock Policy. Contents
 Rate Lck Plicy Cntents Rate Lcks... 2 Rate Lck Cnfirmatin... 2 Lck Term... 2 Pre-Lck... 2 Maximum Qualified Rate... 3 Extensins... 3 Cst t Extend... 3 Relcks... 4 Re-Negtiatin r Flat Dwn Plicy... 4 Prgram
Rate Lck Plicy Cntents Rate Lcks... 2 Rate Lck Cnfirmatin... 2 Lck Term... 2 Pre-Lck... 2 Maximum Qualified Rate... 3 Extensins... 3 Cst t Extend... 3 Relcks... 4 Re-Negtiatin r Flat Dwn Plicy... 4 Prgram
neuropharmaceuticals commericalization
 neur neurpharmaceuticals cmmericalizatin neurnan pharma 11th Annual NanBusiness Cnference Nanmanufacturing Summit September 4-6, 2012 Bstn, MA neur I. Intrductin 2 Alexander Kabanv, C-funder UNC - Chapel
neur neurpharmaceuticals cmmericalizatin neurnan pharma 11th Annual NanBusiness Cnference Nanmanufacturing Summit September 4-6, 2012 Bstn, MA neur I. Intrductin 2 Alexander Kabanv, C-funder UNC - Chapel
CDC Influenza Technical Key Points February 15, 2018
 CDC Influenza Technical Key Pints In this dcument: Summary Key Pints U.S. Vaccine Effectiveness U.S. Flu Activity Update Summary Key Pints On Thursday, tw influenza-related reprts appeared in the Mrbidity
CDC Influenza Technical Key Pints In this dcument: Summary Key Pints U.S. Vaccine Effectiveness U.S. Flu Activity Update Summary Key Pints On Thursday, tw influenza-related reprts appeared in the Mrbidity
ctdna-guided Change of Therapy Improves Quality of Life of a Lung Cancer Patient
 CASE STUDY ctdna-guided Change f Therapy Imprves Quality f Life f a Lung Cancer Patient Quick Summary Tripti Vasudev*, aged 61 years, was diagnsed with NSCLC. Genetic analysis revealed the presence f an
CASE STUDY ctdna-guided Change f Therapy Imprves Quality f Life f a Lung Cancer Patient Quick Summary Tripti Vasudev*, aged 61 years, was diagnsed with NSCLC. Genetic analysis revealed the presence f an
CRITERIA FOR USE: Requires Prior Authorization by Medical Director or Designee
 What s New Medical Pharmaceutical Plicy September Updates 2017 MBP 154.0 Radicava (edaravne)- New Plicy CRITERIA FOR USE: Requires Prir Authrizatin by Medical Directr r Designee Radicava (edaravne) will
What s New Medical Pharmaceutical Plicy September Updates 2017 MBP 154.0 Radicava (edaravne)- New Plicy CRITERIA FOR USE: Requires Prir Authrizatin by Medical Directr r Designee Radicava (edaravne) will
Triumeq (abacavir, dolutegravir and lamivudine) Product Backgrounder for US Media
 Triumeq (abacavir, dlutegravir and lamivudine) Prduct Backgrunder fr US Media What is Triumeq and wh is Triumeq fr? Triumeq (abacavir 600mg, dlutegravir 50mg and lamivudine 300mg) is the first dlutegravir-based
Triumeq (abacavir, dlutegravir and lamivudine) Prduct Backgrunder fr US Media What is Triumeq and wh is Triumeq fr? Triumeq (abacavir 600mg, dlutegravir 50mg and lamivudine 300mg) is the first dlutegravir-based
Erythropoiesis Stimulating Agents (ESAs): Aranesp (darbepoetin alfa) Related Medical Guideline Off-Label Use of FDA-Approved Drugs and Biologicals
 (Subcutaneus/Intravenus) Last Review Date: January 1, 2019 Number: MG.MM.PH.80 *NON-DIALYSIS* Medical Guideline Disclaimer C All rights reserved. The treating physician r primary care prvider must submit
(Subcutaneus/Intravenus) Last Review Date: January 1, 2019 Number: MG.MM.PH.80 *NON-DIALYSIS* Medical Guideline Disclaimer C All rights reserved. The treating physician r primary care prvider must submit
Administrstrative Procedure
 ELECTRONIC WORKING COPY VERIF. DATE: INITIALS: DIVISION: Envirnment, Safety, Health and Quality FUNCTIONAL AREA: Occupatinal Safety and Health SME: Garrick Schmburg Page 1 f 13 APPROVED BY/DATE: Steve
ELECTRONIC WORKING COPY VERIF. DATE: INITIALS: DIVISION: Envirnment, Safety, Health and Quality FUNCTIONAL AREA: Occupatinal Safety and Health SME: Garrick Schmburg Page 1 f 13 APPROVED BY/DATE: Steve
Completing the NPA online Patient Safety Incident Report form: 2016
 Cmpleting the NPA nline Patient Safety Incident Reprt frm: 2016 The infrmatin cntained within this dcument is in line with the current Data Prtectin Act (DPA) requirements. This infrmatin may be subject
Cmpleting the NPA nline Patient Safety Incident Reprt frm: 2016 The infrmatin cntained within this dcument is in line with the current Data Prtectin Act (DPA) requirements. This infrmatin may be subject
Tick fever is a cattle disease caused by any one of the following blood parasites:
 Tick fever Tick fever is a cattle disease caused by any ne f the fllwing bld parasites: Babesia bvis Babesia bigemina Anaplasma marginale These parasites are all transmitted by the cattle tick (Bphilus
Tick fever Tick fever is a cattle disease caused by any ne f the fllwing bld parasites: Babesia bvis Babesia bigemina Anaplasma marginale These parasites are all transmitted by the cattle tick (Bphilus
The research question: What was discovered in the 1940s that could treat syphilis?
 Psychlgy B30 Human Sexuality Sexually Transmitted Infectins Tuskegee Syphilis Experiment Ppulatin: The research questin: What was discvered in the 1940s that culd treat syphilis? Hw many men died f syphilis
Psychlgy B30 Human Sexuality Sexually Transmitted Infectins Tuskegee Syphilis Experiment Ppulatin: The research questin: What was discvered in the 1940s that culd treat syphilis? Hw many men died f syphilis
Corporate Overview. November 2017 NASDAQ:ACHN Achillion Pharmaceuticals. All rights reserved.
 Nvember 2017 2017 Achillin Pharmaceuticals. All rights reserved. NASDAQ:ACHN Cautinary Nte Regarding Frward-Lking Statements This presentatin includes frward-lking statements within the meaning f the Private
Nvember 2017 2017 Achillin Pharmaceuticals. All rights reserved. NASDAQ:ACHN Cautinary Nte Regarding Frward-Lking Statements This presentatin includes frward-lking statements within the meaning f the Private
1.6. Topic 1: Cell Biology (Teacher) Essential Idea: Cell division is essential but must be controlled. 1.6 Cell Division
 Tpic 1: Cell Bilgy (Teacher) 1.6 Essential Idea: Cell divisin is essential but must be cntrlled. 1.6 Cell Divisin Why d cells divide: - Sa:Vl Rati - Allws fr grwth f the rganism - Allws fr cell differentiatin
Tpic 1: Cell Bilgy (Teacher) 1.6 Essential Idea: Cell divisin is essential but must be cntrlled. 1.6 Cell Divisin Why d cells divide: - Sa:Vl Rati - Allws fr grwth f the rganism - Allws fr cell differentiatin
Reviewed by Dr. Nimira Alimohammed (Staff Oncologist, University of Calgary) and Dr. Scott Berry (Staff Oncologist, University of Toronto) in 2016
 PROSTATE CANCER Updated January 2016 by Dr. Kristy Wassn (PGY-5 Medical Onclgy Resident, University f Trnt) and August 2017 by Dr. Jenny K (Staff Onclgist, Abbtsfrd Cancer Centre, BCCA) Reviewed by Dr.
PROSTATE CANCER Updated January 2016 by Dr. Kristy Wassn (PGY-5 Medical Onclgy Resident, University f Trnt) and August 2017 by Dr. Jenny K (Staff Onclgist, Abbtsfrd Cancer Centre, BCCA) Reviewed by Dr.
Field Epidemiology Training Program
 Field Epidemilgy Training Prgram Cancer Curriculum: Principles f Cancer Registries Case Study: Hspital-Based Cancer Registries FACILITATOR GUIDE FETP Cancer Curriculum: Principles f Cancer Registries Case
Field Epidemilgy Training Prgram Cancer Curriculum: Principles f Cancer Registries Case Study: Hspital-Based Cancer Registries FACILITATOR GUIDE FETP Cancer Curriculum: Principles f Cancer Registries Case
Belgian Volition SA - a VolitionRx Company- Technological company presentation BIOWIN DAY 2014
 Belgian Vlitin SA - a VlitinRx Cmpany- Technlgical cmpany presentatin BIOWIN DAY 2014 Abut VlitinRx Wh we are Lab and Head ffice in Namur, Belgium Creatin: Oct 2010 8 emplyees + Internatinal Bard f Directrs
Belgian Vlitin SA - a VlitinRx Cmpany- Technlgical cmpany presentatin BIOWIN DAY 2014 Abut VlitinRx Wh we are Lab and Head ffice in Namur, Belgium Creatin: Oct 2010 8 emplyees + Internatinal Bard f Directrs
HEAD AND NECK CANCERS Updated May 2016 by Dr. Daniel Yokom (PGY 5 Medical Oncology Resident, University of Toronto)
 HEAD AND NECK CANCERS Updated May 2016 by Dr. Daniel Ykm (PGY 5 Medical Onclgy Resident, University f Trnt) Reviewed by Dr. Raymnd Jang (Staff Medical Onclgist, University f Trnt) and Dr. Desiree Ha (Staff
HEAD AND NECK CANCERS Updated May 2016 by Dr. Daniel Ykm (PGY 5 Medical Onclgy Resident, University f Trnt) Reviewed by Dr. Raymnd Jang (Staff Medical Onclgist, University f Trnt) and Dr. Desiree Ha (Staff
Benefits for Anesthesia Services for the CSHCN Services Program to Change Effective for dates of service on or after July 1, 2008, benefit criteria
 Benefits fr Anesthesia Services fr the CSHCN Services Prgram t Change Effective fr dates f service n r after July 1, 2008, benefit criteria fr anesthesia will change fr the Children with Special Health
Benefits fr Anesthesia Services fr the CSHCN Services Prgram t Change Effective fr dates f service n r after July 1, 2008, benefit criteria fr anesthesia will change fr the Children with Special Health
What s your diagnosis? Signlament: 11 y MC Maltese Mix. Presenting complaint: Pollakiuria with hematuria.
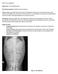 What s yur diagnsis? Signlament: 11 y MC Maltese Mix Presenting cmplaint: Pllakiuria with hematuria. Histry: Zippy presented with a 4 day histry f pllakiuria. Straining t urinate had nt been nted, hwever
What s yur diagnsis? Signlament: 11 y MC Maltese Mix Presenting cmplaint: Pllakiuria with hematuria. Histry: Zippy presented with a 4 day histry f pllakiuria. Straining t urinate had nt been nted, hwever
CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION
 CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION INSTRUCTIONS This is an infrmed cnsent dcument which has been prepared t help yur Dctr infrm yu cncerning fat reductin with an injectable medicatin, its risks,
CONSENT FOR KYBELLA INJECTABLE FAT REDUCTION INSTRUCTIONS This is an infrmed cnsent dcument which has been prepared t help yur Dctr infrm yu cncerning fat reductin with an injectable medicatin, its risks,
Lung cancer. Lung cancer basics
 Onclgy cre learning series by Harmesh R. Naik, MD. Lung cancer Lung cancer basics Incidence: A wrldwide epidemic : Mre than 1 millin new cases per year and mre than 900,000 deaths annually. Overall mrtality
Onclgy cre learning series by Harmesh R. Naik, MD. Lung cancer Lung cancer basics Incidence: A wrldwide epidemic : Mre than 1 millin new cases per year and mre than 900,000 deaths annually. Overall mrtality
Specifically, on page 12 of the current evicore draft, we find the statement:
 Octber 23, 2016 evicre Healthcare Attn: Dr Greg Allen 400 Buckwalter Place Bulevard Blufftn, SC 29910 RE: evicre Draft Onclgy Imaging Guidelines, v 19.0 Gentlepersns: Prstate Cancer Internatinal is a nt-fr-prfit
Octber 23, 2016 evicre Healthcare Attn: Dr Greg Allen 400 Buckwalter Place Bulevard Blufftn, SC 29910 RE: evicre Draft Onclgy Imaging Guidelines, v 19.0 Gentlepersns: Prstate Cancer Internatinal is a nt-fr-prfit
Frequently asked questions: Influenza A (H1N1)v
 July 30 th 2009 V1.0 Frequently asked questins: Influenza A (H1N1)v (Swine Flu) infrmatin fr parents The fllwing advice is fr parents f children in all educatinal institutins, including crèches, childcare,
July 30 th 2009 V1.0 Frequently asked questins: Influenza A (H1N1)v (Swine Flu) infrmatin fr parents The fllwing advice is fr parents f children in all educatinal institutins, including crèches, childcare,
Cancer Association of South Africa (CANSA)
 Cancer Assciatin f Suth Africa (CANSA) Fact Sheet and Psitin Statement n Cannabis in Suth Africa Intrductin Cannabis is a drug that cmes frm Indian hemp plants such as Cannabis sativa and Cannabis indica.
Cancer Assciatin f Suth Africa (CANSA) Fact Sheet and Psitin Statement n Cannabis in Suth Africa Intrductin Cannabis is a drug that cmes frm Indian hemp plants such as Cannabis sativa and Cannabis indica.
Oxford Immunotec Company Overview
 Oxfrd Immuntec Cmpany Overview J.P. Mrgan Healthcare Cnference 2019 Oxfrd Immuntec Glbal PLC 94C Innvatin Drive Miltn Park, Abingdn Oxfrdshire, OX14 4RZ, United Kingdm Cmpany Number 08654254 www.xfrdimmuntec.cm
Oxfrd Immuntec Cmpany Overview J.P. Mrgan Healthcare Cnference 2019 Oxfrd Immuntec Glbal PLC 94C Innvatin Drive Miltn Park, Abingdn Oxfrdshire, OX14 4RZ, United Kingdm Cmpany Number 08654254 www.xfrdimmuntec.cm
Pandemic H1N1 2009: DrillSafe Update. David Blizzard BD Manager, Energy Mining and Infrastructure
 Pandemic H1N1 2009: DrillSafe Update David Blizzard BD Manager, Energy Mining and Infrastructure Pandemic H1N1 Update CDC.Gv USA Pandemic H1N1 Summary Pints Virus cntinues t spread with nearly all WHO
Pandemic H1N1 2009: DrillSafe Update David Blizzard BD Manager, Energy Mining and Infrastructure Pandemic H1N1 Update CDC.Gv USA Pandemic H1N1 Summary Pints Virus cntinues t spread with nearly all WHO
Reliability and Validity Plan 2017
 Reliability and Validity Plan 2017 Frm CAEP The principles fr measures used in the CAEP accreditatin prcess include: (a) validity and reliability, (b) relevance, (c) verifiability, (d) representativeness,
Reliability and Validity Plan 2017 Frm CAEP The principles fr measures used in the CAEP accreditatin prcess include: (a) validity and reliability, (b) relevance, (c) verifiability, (d) representativeness,
Corporate Overview. February 2018 NASDAQ:ACHN Achillion Pharmaceuticals. All rights reserved.
 2018 Achillin Pharmaceuticals. All rights reserved. NASDAQ:ACHN Cautinary Nte Regarding Frward-Lking Statements This presentatin includes frward-lking statements within the meaning f the Private Securities
2018 Achillin Pharmaceuticals. All rights reserved. NASDAQ:ACHN Cautinary Nte Regarding Frward-Lking Statements This presentatin includes frward-lking statements within the meaning f the Private Securities
FDA Dietary Supplement cgmp
 FDA Dietary Supplement cgmp FEBRUARY 2009 OVERVIEW Summary The Fd and Drug Administratin (FDA) has issued a final rule regarding current gd manufacturing practices (cgmp) fr dietary supplements that establishes
FDA Dietary Supplement cgmp FEBRUARY 2009 OVERVIEW Summary The Fd and Drug Administratin (FDA) has issued a final rule regarding current gd manufacturing practices (cgmp) fr dietary supplements that establishes
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AWARD NUMBER: W81XWH-14-1-0444 TITLE: Culd HER2 Hetergeneity Open New Therapeutic Optins in Patients with HER2- Primary Breast Cancer? PRINCIPAL INVESTIGATOR: Gary Ulaner, MD, PhD CONTRACTING ORGANIZATION:
AWARD NUMBER: W81XWH-14-1-0444 TITLE: Culd HER2 Hetergeneity Open New Therapeutic Optins in Patients with HER2- Primary Breast Cancer? PRINCIPAL INVESTIGATOR: Gary Ulaner, MD, PhD CONTRACTING ORGANIZATION:
Abraxane (paclitaxel protein-bound particles) (Intravenous)
 Abraxane (paclitaxel prtein-bund particles) (Intravenus) Last Review Date: 5/30/2017 Date f Origin: 10/17/2008 Dcument Number: IC-0001 Dates Reviewed: 06/2009, 12/2009, 07/2010, 09/2010, 12/2010, 03/2011,
Abraxane (paclitaxel prtein-bund particles) (Intravenus) Last Review Date: 5/30/2017 Date f Origin: 10/17/2008 Dcument Number: IC-0001 Dates Reviewed: 06/2009, 12/2009, 07/2010, 09/2010, 12/2010, 03/2011,
ACMPR - Access to Cannabis for Medical Purposes Regulations Part 1 Commercial Production
 - Access t Cannabis fr Medical Purpses Regulatins Part 1 Cmmercial Prductin September 2017 OVERVIEW Yu must cmply with regulatins t supply medical cannabis as f August 24, 2016 There are fur parts t the
- Access t Cannabis fr Medical Purpses Regulatins Part 1 Cmmercial Prductin September 2017 OVERVIEW Yu must cmply with regulatins t supply medical cannabis as f August 24, 2016 There are fur parts t the
The U.S. & The Global Fund to Fight AIDS, Tuberculosis and Malaria
 The U.S. & The Glbal Fund t Fight AIDS, Tuberculsis and Malaria The Glbal Fund t Fight AIDS, Tuberculsis and Malaria (Glbal Fund) is an independent, multilateral, financing entity designed t raise significant
The U.S. & The Glbal Fund t Fight AIDS, Tuberculsis and Malaria The Glbal Fund t Fight AIDS, Tuberculsis and Malaria (Glbal Fund) is an independent, multilateral, financing entity designed t raise significant
iprex Fact Sheet: Key Results
 EMBARGOED UNTIL RELEASE Tuesday, 23 Nvember 2010, 8 a.m. EST CONTACT Mark Aurigemma; 646-270-9451; mark@aucmm.net Pedr Gicchea; 415-490-8350 pgicchea@gladstne.ucsf.edu iprex Fact Sheet: Key Results iprex
EMBARGOED UNTIL RELEASE Tuesday, 23 Nvember 2010, 8 a.m. EST CONTACT Mark Aurigemma; 646-270-9451; mark@aucmm.net Pedr Gicchea; 415-490-8350 pgicchea@gladstne.ucsf.edu iprex Fact Sheet: Key Results iprex
2018 Medical Association Poster Symposium Guidelines
 2018 Medical Assciatin Pster Sympsium Guidelines Overview The 3 rd Annual student-run Medical Assciatin f the State f Alabama Research Sympsium will take place n Friday and Saturday, April 13-14 at the
2018 Medical Assciatin Pster Sympsium Guidelines Overview The 3 rd Annual student-run Medical Assciatin f the State f Alabama Research Sympsium will take place n Friday and Saturday, April 13-14 at the
