Mitotic Chromosome Structure: Reproducibility of Folding and Symmetry between Sister Chromatids
|
|
|
- Derrick Berry
- 6 years ago
- Views:
Transcription
1 Biophysical Journal Volume 96 February Mitotic Chromosome Structure: Reproducibility of Folding and Symmetry between Sister Chromatids Yuri G. Strukov and A. S. Belmont * Department of Cell and Developmental Biology, and Biophysics Program, University of Illinois, Urbana-Champaign, Illinois ABSTRACT Mitotic chromosome structure and pathways of mitotic condensation remain unknown. The limited amount of structural data on mitotic chromosome structure makes it impossible to distinguish between several mutually conflicting models. Here we used a Chinese hamster ovary cell line with three different lac operator-tagged vector insertions distributed over an ~1 mm chromosome arm region to determine positioning reproducibility, long-range correlation in large-scale chromatin folding, and sister chromatid symmetry in minimally perturbed, metaphase chromosomes. The three-dimensional positions of these lac operator-tagged spots, stained with lac repressor, were measured in isolated metaphase chromosomes relative to the central chromatid axes labeled with antibodies to topoisomerase II. Longitudinal, but not axial, positioning of spots was reproducible but showed intrinsic variability, up to ~300 nm, between sister chromatids. Spot positions on the same chromatid were uncorrelated, and no correlation or symmetry between the positions of corresponding spots on sister chromatids was detectable, showing the absence of highly ordered, long-range chromatin folding over tens of mega-basepairs. Our observations are in agreement with the absence of any regular, reproducible helical, last level of chromosome folding, but remain consistent with any hierarchical folding model in which irregularity in folding exists at one or multiple levels. INTRODUCTION The large-scale structure of mitotic chromosomes and the mechanisms underlying chromosome condensation remain elusive after more than four decades of experimental efforts. Chromosomal proteins and protein complexes with enzymatic activities necessary for condensation and maintenance of chromosome structure have been identified and studied in vitro; however, their actual in vivo functions are still unclear (1 3). Similarly, from a structural point of view, the number of distinct levels of chromatin compaction involved in the transition between interphase and mitotic chromosomes remains unknown. The apparent irregularity of chromosome folding is one of the major challenges in deciphering chromosome structure. This irregularity makes it difficult to define, isolate, or study individual structural elements of chromosomes. Other factors complicating the analysis of chromosome structure include the extremely high compaction of chromatin within mitotic chromosomes and the sensitivity of native chromatin morphology to even slight changes in the ionic strength of the environment. A number of different models of mitotic chromosomes can be roughly divided into two, apparently mutually conflicting groups. Radial-loop type models are based largely on observations of mitotic chromosomes after extensive extraction of chromosomal proteins. This treatment is aimed at relaxation of tightly compacted chromatin to reveal its substructure. According to radial-loop models, loops of 30 nm chromatin fibers are attached to a nonhistone protein scaffold through DNA-protein interactions (4,5). The estimated size Submitted August 11, 2008, and accepted for publication October 21, *Correspondence: asbel@uiuc.edu of these loops measured by different methods and in different species varies between 20 and 150 kbp. Later modifications of the radial-loop model suggested that the scaffold of each chromatid is helically coiled rather than corresponding to a simple linear arrangement of the loop bases (6,7). Instead, hierarchical coiling models are derived from experiments designed to avoid, or at least minimize, perturbation of the native chromosome morphology. Hierarchical coiling models assume that the DNA molecule is either regularly or irregularly coiled into a hierarchy of distinct folding motifs, with each higher-level folding unit formed by coiling of a lower-level folding motif (8 11). It may be that both types of mitotic chromosome models are valid for reflecting different aspects of mitotic chromosome structure. However, neither model group explicitly addresses the more basic question of the DNA folding reproducibility within mitotic chromosomes. Here folding reproducibility refers to both comparisons of identical chromosomes isolated from different cells and comparisons of folding between sister chromatids of the same chromosome. The question of folding reproducibility is critical for ultimately understanding the underlying mechanisms of chromosome condensation. Chromosome-specific banding patterns along the longitudinal mitotic chromosome axis observed after certain treatments are one of the most prominent, reproducible features of mitotic chromosome structure (12 14). These banding patterns demonstrate reproducibility in the folding of DNA sequences with respect to position along the chromosome axis on a DNA sequence scale of several mega-basepairs (Mbp). How reproducible folding is on a smaller scale, and how reproducible positioning of specific DNA sequences is transverse to the chromosome axis remain unknown. Editor: Alberto Diaspro. Ó 2009 by the Biophysical Society /09/02/1617/12 $2.00 doi: /j.bpj
2 1618 Strukov and Belmont Besides the observed reproducible banding patterns, other experimental results have suggested the existence of additional levels of order within mitotic chromosomes. These may be related to chromosome banding patterns, or perhaps even the cause of these banding patterns, or they may be independent of these banding patterns. A helical structure of topoisomerase IIa axial staining, proposed to be part of a chromosome scaffold, was observed after partial extraction of histone H1 using a polyanioncontaining buffer (6). However, only ~1% of chromosomes showed regular helical coiling of scaffolds, with sister chromatids related by mirror symmetry, and most chromosomes instead formed misshapen halos. More recently it was suggested that this apparent helical coiling may reflect overcondensation of chromosomes in a small fraction of cells induced by prolonged exposure to mitotic inhibitors (15). It therefore remains unclear whether symmetry between sister chromatids is present in mitotic chromosomes with minimally perturbed morphology. Reproducible positioning of specific DNA sequences relative to the longitudinal axes of chromatids was suggested based on fluorescence in situ hybridization (FISH) experiments. The same peripheral or axial positioning in both prometaphase and metaphase chromosomes for several specific probes was described (16). This led to a model in which the transition between prophase and metaphase chromosomes involved progressive shortening of the chromosome axis and further condensation without significant reorganization of sequences transverse to the chromosome axis. However, the actual differences in sequence distribution observed in that study were not dramatically different from those expected for a random distribution. Moreover, interpretation of these results was further complicated by possible systematic experimental errors. The FISH procedure used in that study included harsh conditions of fixation and DNA denaturation, and clearly led to chromosome flattening, suggesting a significant perturbation of chromosome morphology that might have led to significant large-scale rearrangement of DNA sequences (16). In contrast, highly reproducible targeting of specific, geneamplified DNA sequences to the metaphase chromatid axes was previously observed in mammalian chromosomes, and no obvious regularity or symmetry was seen in the positions of these same spots in prophase or telophase chromosomes (17). These results suggested a more pronounced structural transition between prophase and metaphase. More recently, light and electron microscopy of artificial chromosome regions containing lac operator repeats suggested the existence of a nm folding subunit in metaphase chromosomes (18). Of interest, immunogold staining of these regions suggested a distinct lack of reproducibility in positioning transverse to the chromosome axis. These varied results prompted a reexamination of the question of reproducibility of DNA sequence folding within mitotic chromosomes using the lac operator/lac repressor system, which permits labeling of specific regions in minimally perturbed, formaldehyde-fixed chromosomes (19). Here we report results suggesting the absence of reproducibility in lateral positions of specific sequences in mitotic chromosomes as well as a lack of strict symmetry in DNA folding between sister chromatids. We show that even though condensation of sister chromatids is precisely spatially and temporally coordinated, they follow independent condensation pathways. These results are discussed in terms of models and mechanisms of mitotic condensation. MATERIALS AND METHODS Cell line and cell culture MC8_I Chinese hamster ovary (CHO) cells, a derivative of the DG44 cell line (20), were grown in F-12 (HAM) medium (Gibco BRL, Boston, MA) lacking thymidine and hypoxanthine and supplemented with 10% dialyzed fetal bovine serum (FBS; HyClone, Salt Lake City, UT) and 0.25 mm methotrexate (MTX; Molecular Probes, Eugene, OR). The C6 cell line (21) was created after transfection with a 15 kbp supercoiled plasmid psv2dhfr- 8$32 carrying a 256mer lac operator direct repeat (19). MC8_I CHO cells were isolated after gene amplification of the C6 cells. Isolation and immunofluorescence staining of mitotic chromosomes Isolation of mitotic chromosomes was carried out according to a previously published protocol with modifications (22). CHO cells in a 75 cm 2 flask, grown to confluency, were incubated for 2.5 h with media supplemented with 600 ng/ml nocodazole, which was then replaced with fresh media without nocadazole. After 15 min of incubation, mitotic cells were detached from the surface by gentle shaking of the flask, collected into a 50 ml conical tube, and sedimented at 500 g for 10 min at þ4 C. After hypotonic treatment with 75 mm KCl at 37 C for 10 min, the mitotic cells were centrifuged at 500 g and vortexed in a small volume of buffer PA (15 mm Tris- HCl, 0.2 mm spermine, 0.5 mm spermidine, 2 mm EDTA, 0.5 mm EGTA, 80 mm KCl, 20 mm NaCl, ph 7.2 at þ4 C) containing 0.1% digitonin. Isolated chromosomes were deposited on 12 mm round glass coverslips (#1.5; Fisher Scientific, Pittsburgh, PA) by centrifugation at 3000 g for 10 min at þ4 C and then fixed with 1.6% formaldehyde (Polyscience, Pittsburgh, PA) in PBS* for 10 min at room temperature (PBS* is PBS with 5 mm MgCl 2 and 0.1 mm EDTA; PBS (1 liter): 8.00 g NaCl, 2.16 g Na 2 HPO 4 *(7H 2 O), 0.20 g KCl, 0.20 g K 2 HPO 4 in deionized water, ph 7.4). Staining of fixed chromosomes with lac repressor was performed as described elsewhere (23). Staining for topoisomerase IIa was done for h at þ4 C in PBS supplemented with 0.5% BSA (Sigma, St. Louis, MO) using primary rabbit topoisomerase IIa antibody at 1:30 dilution (24). Texas Red-labeled secondary antibodies were used for detection of lac operator sequences, and fluorescein isothiocyanate (FITC)-labeled secondary antibodies were used for topoisomerase IIa. DNA was stained with either 0.2 mm/ml 4 0,6-diamidino-2-phenylindole (DAPI) or 0.5 mm/ml propidium iodide in PBS*. Microscopy and image processing Three-dimensional (3D) optical sections were collected with an Olympus IMT-2 inverted fluorescence microscope with an Olympus 60/NA 1.4 objective and a cooled Photometrics CCD camera. Voxel size was 74 nm in the x and y directions, and 100 nm along the optical axis. All images of chromosomes were corrected for the CCD dark current, variations in the microscope illumination field, and variations in the gain between individual pixels, as well as for mercury lamp brightness variations, and then
3 Chromosome Folding Reproducibility 1619 deconvolved as described previously (25) with calculated point spread function. Images were analyzed with Newvision (26) and ImageJ ( nih.gov/ij/). All quantitative analysis of images was done with MATLAB 6 (The MathWorks, Natick, MA). The scripts used in this work are available upon request. Chromatic aberration measurements and control of accuracy of measurements of z-coordinates were done using fluorescent, multicolored micro beads (diameter: 90 and 560 nm; Molecular Probes, Portland, OR) deposited on glass coverslips. Suspensions of 90 and 560 nm diameter micro beads were diluted, mixed together, and deposited on round glass coverslips (Corning, Corning, NY) at a density of beads of each size per square millimeter. The coverslips were air-dried and mounted with Prolong mounting medium (Molecular Probes). RESULTS Experimental system In this work we used MC8_I cells derived in our laboratory from DG44 CHO cells. DG44 cells are CHO cells that contain a double deletion of the DHFR gene, induced by x-ray irradiation (20). DG44 cells were transfected with the psv2dhfr-8.32 plasmid containing 256 copies of a 36 bp lac operator sequence (19). A stable cell line, C6, containing copies of the psv2dhfr-8.32 transgene inserted at a single chromosome site was selected (21). This C6 cell line was used for gene amplification, using methotrexate selection (19), to generate the MC8_I cell line. One of the longer chromosomes within MC8_I cells contains a single chromatid region with three nearby transgene array sites, creating three closely spaced pairs of spots within the two sister chromatids of the metaphase chromosome (Fig. 1, A C). Spots were located in the middle of the chromosome arm, away from both the centromere and telomere. A fourth spot is located on the opposite arm of the same chromosome. By light microscopy, the three lac operator-containing loci have the same appearance of diffraction limited spots of ~0.2 mm diameter. Variation between intensity levels in images of individual spots on the same chromosome was relatively small, which allowed us to treat individual spots as identical. These loci likely represent copies of the original C6 transgene insertion site, together with large stretches of flanking genomic CHO DNA generated by the gene amplification process. We found that MTX was necessary for maintenance of multiple inserts. If MTX was removed from the medium, cells with multiple spots were replaced by cells with a single pair of spots after ~20 cell passages. Chromosomes within intact mitotic cells are packed very densely, their orientation in three dimensions is random, and they are usually bent, which makes it difficult to follow chromatid axes. Therefore, for these experiments we used chromosomes isolated from mitotic cells and deposited by gentle centrifugation onto glass coverslips. Deposited on coverslips, the chromosomes appear straight and parallel to the glass surface (Fig. 1, A and B). The lac operator/lac repressor system was used for detection of transgene insertion sites (19), avoiding the harsh conditions used for the DNA denaturation required for FISH approaches and better preserving the 3D chromosome architecture. By examining the 3D locations of the three pairs of lac operator-containing insertion sites in MC8_I cells, we were able to compare both the reproducibility of DNA folding in different metaphase chromosomes and the reproducibility in folding within the two sister chromatids from the same metaphase chromosome. Chromatid axes were detected by immunostaining against topoisomerase IIa (Fig. 1, A C), which concentrates at the axial cores of isolated metaphase chromosomes (27 29). All chromosomes stained for lac op repeats showed six spots (three per sister chromatid) in this chromosome region, indicating that all inserts were detectable by antibody staining irrespective of their positions on mitotic chromosomes (Fig. 1, A C). Lac operator spots could be located at the periphery of chromosomes as well as in internal regions between sister chromatids (Fig. 1 B). Calibration and control of accuracy of measurements Spot sizes corresponded to the diffraction limit of the fluorescence microscope. However, the spot centers can be determined much more precisely. The signal/noise ratio (>30) and photon counts (>1200) in images of chromosomes were high enough to guarantee that centroid calculations gave accurate estimates of the centers of the spots (30,31). Separate excitation filters were used for DNA (DAPI) and topoisomerase IIa signals, but both were detected with a single 537/30 nm emission filter. A 625/30 nm emission filter was used for detection of lac operator sequences immunostained with Texas-Red-labeled antibodies. We found significant chromatic aberration shifts in the positions of multicolored beads imaged with two different emission filters (data not shown). Bead centroids were separated by Dx ¼ (20 5 7) nm, Dy ¼ (46 5 7) nm, Dz ¼ ( ) nm, or 0.3, 0.6, and 3.5 pixels, respectively. Chromatic aberration was corrected by adjusting the calculated coordinates of the centroids of lac operator spots and chromatid axis positions by the calculated mean values in all images. To estimate the accuracy of measurements of Z chromatic shift, suspensions of 90 and 560 nm diameter micro beads were air-dried on coverslips and mounted in an antifading agent. 3D stacks of optical sections were collected with 100 nm increments in the z-direction. Because of significant differences in the fluorescence intensity of the 90 and 560 nm beads, different exposures were used for different beads in the same field. The centroid of a fluorescent bead was defined as the center of intensity-weighted voxels. Images were thresholded such that levels below the threshold were set to zero and values above the threshold were unchanged. The measured centers of larger and smaller beads sitting close to each other on the coverslip surface were separated by nm (mean 5 SD) in the axial direction,
4 1620 Strukov and Belmont FIGURE 1 Visualization of longitudinal and axial positioning of closely spaced lac operator-containing vector inserts. Isolated mitotic chromosomes from the MC8_I clone show three nearby vector transgene sites (top chromosome arm), with a fourth transgene site on the opposite chromosome arm. Lac repressor and topoisomerase II staining labeled vector transgene sites and chromosome axes, respectively. (A): (a) Lac repressor staining of lac operator-containing vector insert sites; (b) anti-topoisomerase II antibody staining; (c) total DNA stained with DAPI; (d) merged lac repressor (cyan) and topoisomerase II (red) staining; (e) 3 enlargement from (d); (f) merged DAPI (yellow) and lac repressor signal (cyan); (g) 3 enlargement from f. (B): (a f) Six examples of isolated metaphase chromosomes. Subpanels (left to right): topoisomerase II immunostaining, lac repressor staining, merged (topoisomerase II red, lac repressor cyan), merged, 3 enlargement. (C): Two stereo pairs of metaphase chromosomes reconstructed from optical sections. Total DNA stained with DAPI (blue), anti-topoisomerase II (red), and lac repressor staining (green). (D) 2D model of a fragment of a chromosome with lac op inserts: lac op spots, green; sister chromatid axes, red. Bars: 1 mm. close to the predicted 230 nm separation. Variations in bead diameter, fluorescent labeling throughout the bead interior, and deformation of coverslip surfaces could also contribute to the observed discrepancy between predicted and measured values. Shifts due to chromatic aberration and relative z coordinates of the beads were highly reproducible as demonstrated by measurements done on the same samples several days later. The 3D positions of centers of lac operator-tagged spots within mitotic chromosomes were also calculated by measuring centroids of spots after thresholding. The shape of the microscope point spread function with poor resolution along the z axis made it difficult to extract 3D coordinates of the topoisomerase-labeled chromosome axes; therefore, 2D projections of the axes were used with the assumption that the axes of isolated chromosomes were parallel to the surface of coverslips. To find the axes of mitotic chromosomes, deconvolved optical sections with topoisomerase IIa immunostaining signal were projected and two maxima of intensity in each row of pixels perpendicular to chromatid axes (one per chromatid) were detected. These maxima of topoisomerase IIa immunostaining corresponded to the central regions of daughter chromatids. The curves corresponding to the central regions of chromatids were calculated by fitting a low-degree polynomial using the measured chromatid centers. The segments of the central curves, with positions and lengths limited by the coordinates of the spots
5 Chromosome Folding Reproducibility 1621 FIGURE 2 Quantitative analysis of an imaged CHO metaphase chromosome with lac operator inserts. (A) Anti-topo II immunostaining. (B) Lac op immunostaining. (C) Superimposition of A and B.(D)3enlarged region from C. (E) Extraction of geometrical parameters from D: (green and red) centers of extracted 3D coordinates of lac op spots of left and right chromatids, respectively; (blue and cyan) traces of centers of topo II signal for left and right chromatid axes, respectively; (black and pink) 4th degree polynomial and linear fits of axes traces, respectively. (F) Model of metaphase chromosome used for Monte Carlo simulation. The distance between a spot and the chromatid axis was distributed uniformly between 0 and R max ; angles q and q 0 were distributed uniformly between 0 and 2p. Spot positions in sister chromatids were assigned independently, modeling the absence of any correlation of spot positioning in sister chromatids. Bars: 1 mm. 1 and 3 (Fig. 1 D), were approximated by linear segments using a line fit (Fig. 2, A E). Longitudinal positions of inserts on daughter chromatids vary within an ~250 nm range The high correlation between sister chromatid lengths and perfect registration of chromosomal bands could result from physical connections established by cohesin complexes between sister chromatids during mitotic condensation (32). Cohesins do not disassociate from chromosomal arms completely until the metaphase-anaphase transition, although release of cohesins from chromosomal arms starts in prophase and precedes release from centromeric regions (33). Even though chromosomal bands, or large, multicopy vector inserts (18), appeared to have the same longitudinal positions in sister chromatids, a comparison of the longitudinal positions of the small inserts in the MC8_I cell line revealed variation in the spot positions between sister chromatids. The original images of 97 chromosomes with three pairs of spots were rotated so that the chromosomes were parallel to the x axis, with the origin set close to the telomere of the chromosome arm with the multiple spots (Fig. 1 D). For easier reference, the pairs of spots were numbered 1 (closest to the telomere) through 3 (closest to the centromere). Pairs 1 and 3 were separated longitudinally by mm (mean 5 SD; min 0.5, max 1.4), representing ~70 Mbp of the CHO genome (23). The length of the chromosomal arm containing the inserts is mm (mean 5 SD; min 1.0, max 9.9). Between sister chromatids there remains significant variation in the longitudinal distance, S, separating spots 1R and 3R, or 1L and 3L (Fig. 1 D), and S values for sister chromatids are weakly correlated (r 2 ¼ 0.33; Fig. 3 A). Distance S, as expected, is correlated with the length of the chromosome arm (Fig. 3 B), although this correlation is not strong (r 2 ¼ 0.48; Fig. 3 B). We next calculated relative differences between the longitudinal coordinates for a single pair of spots on sister chromatids of the same chromosome (D x for left and right chromatids; Fig. 1 D). The difference in longitudinal positions of sister spots varies approximately between 300 nm and 300 nm (Fig. 3 C). Averaging over all chromosomes, longitudinal separation between sister spots is nm (mean 5 SD, min 280 nm, max 320 nm). The average value of absolute separations for all three pairs together is nm (mean 5 SD, min 0, max 320). If the longitudinal distance, S, between spots 1 and 3 of the same sister is normalized to one for each chromatid for all chromosomes, then the distances between spots 1 and 2 (e.g., 1R to 2R) are on average over all chromatids 0.36 (min 0.12, max 0.59, SD 0.09). The histogram distribution of normalized distances between spots 1 and 2 (Fig. 3 D) supports the observation that longitudinal positions of specific sequences in mitotic chromosomes are reproduced with little variation. There is no correlation, however, between the longitudinal distances between spots 1 and 2 of the sister chromatids (r 2 ¼ 0.02). This is because the distance between spots 1 and 2 is comparable in length to the random deviation from average position, and the random deviations of individual spots are not correlated. These numbers could be explained by random positioning of spots within perfectly registered, bandlike ~300 nm thick fibers in sister chromatids. Of interest, nm is roughly the width of chromatin fibers previously observed in mitotic chromosomes (18). Even though DAPI staining produced banding patterns that are similar and registered in sister chromatids, longitudinal positions of the same specific, relatively short sequences of sister chromatids are not strictly fixed and correlated. It is interesting to note that patterns formed by the anti-topo II antibody staining are roughly symmetrical between sister chromatids in terms of large-scale longitudinal variation in labeling intensity and appear similar for different chromosomes, and probably have the same origin as the DAPI banding pattern (Fig. 1 B).
6 1622 Strukov and Belmont FIGURE 3 Longitudinal spot positions are reproducible but show significant variability. (A) Distances between spots 1 and 3 (Fig. 1 D) vary significantly between sister chromatids. Scatter plot where each spot corresponds to measured distances on the left and right chromatids for a particular metaphase chromosome. (B) Correlation between chromatid arm length versus longitudinal separation of spots 1 and 3, with plotted values representing average of measurements on sister chromatids. (C) Longitudinal coordinates of individual spots vary within ~300 nm. Histogram of differences between longitudinal coordinates of sister spots, e.g., 1L and 1R. (D) Histogram of distribution of longitudinal distances between spots 1 and 2 normalized by distance between spots 1 and 3. Vector inserts do not occupy fixed positions with respect to chromatid axes Relative to the longitudinal positions, the axial coordinates of the inserts showed a greater variability. Distances from the spot centers to the chromatid axes were measured in 2D projections of 3D stacks of optical sections. Spots found on the left of the corresponding chromatid axis were given a positive axial coordinate, and spots on the right side were given a negative coordinate. Spots did not have fixed and reproducible positions relative to the chromatid axes (Fig. 4, A and B, and Table 1). Histograms of lateral deviations from the axis for spot 1 of the left chromatid only (Fig. 4 A) and for all three pairs of both chromatids together (Fig. 4 B) show a slight bias away from the chromatid axis, with the average spot position pixels (~30 50 nm) away from the calculated chromatid axis (Table 1). This observation is confirmed by plotting lateral deviation from the axis of the spots of the left chromatid versus deviations for the spot of the right chromatid (Fig. 4 C): the data points are more abundant in the area of the plot with positive abscissa and negative ordinate. In other words, this means that the lac op spots, and possibly the rest of the DAPI-labeled DNA, are more likely to be found outside the metaphase axes than between them. Lateral positions of the sister spots are not correlated with each other (r 2 ¼ 0.08). One possible explanation for the observed randomness is that the imaged chromosomes represent a range of condensation levels or stages, and that more fixed locations would be observed within a given subset of chromosomes with similar degree of condensation. To test whether this is the case, the deviation of the projected spot position from the chromosome axis was plotted as a function of chromosome arm length for the top pair of spots of each chromosome (Fig. 4 D). The distribution of points in Fig. 4 D demonstrates that the lateral position of a spot and the length of the chromosomal arm are not correlated in 2D projections. Chromosomal arms of the same length show different spot patterns: the spots can occupy any axial position within a chromatid, both internal and external. This real data set was compared with Monte Carlo computer-generated data (Fig. 2 F) obtained using the following model: Longitudinal positions of the simulated spots were determined by the average of measured positions of spots from the real chromosomes. Spots were allowed to deviate randomly with a uniform distribution within a 300 nm range as suggested by the observed data. Specifically, the distribution of the relative difference between the longitudinal positions of sister spots has a shape close to a triangle centered at zero relative difference (Fig. 3, C and D). This triangle shape is consistent with the convolution of two independent rectangular probability distributions, which would be the consequence of a uniform distribution for each spot and no correlation for longitudinal position between sister chromatids. The longitudinal distance between spots 1 and 3 (e.g., 1R to 3R as in Fig. 1 D) varies significantly due to variations in
7 Chromosome Folding Reproducibility 1623 FIGURE 4 Lateral positions of individual spots relative to chromatid axis are nonreproducible, uncorrelated between sister chromatids, and independent of the degree of chromosome condensation. (A) Distance histogram for spot 1L to chromatid axis. Positive distances correspond to spots located on the left (exterior) of the chromatid axis, and negative distances correspond to spots on the right (interior) of the axis. (B) Histogram of axial positions for all spots. (C) Scatter plot showing comparison of lateral spot positions for sister chromatids: left spot (x axis) versus right spot (y axis). (D) Axial position versus length of chromosomal arm for the top pair of lac op spots of all chromosomes; (gray circles) right chromatids; (solid circles) left chromatids. (E) Scatter plots from 2D projections of actual (open circles) and simulated (black circles) chromosome measurements demonstrate comparable distributions for axial positions of lac operator spots. Mean values, SDs, and absence of correlation between sister chromatids were similar for experimental and simulated distributions. the length of chromosomal arms. A random stretching factor was used for simulated chromosomes to make distributions of their geometrical features close to the observed data. Distances from a spot to a chromatid axis were assumed to be uniformly distributed between 0 and R max, where R max was taken from experimental data, and axial angles q and q 0 TABLE 1 Mean distances (SD) from spots to the corresponding axes of chromatids in pixels in 2D projections (absolute distances to the axes and axial coordinates for each spot are shown) Spots Left chromatid Absolute distance, pixels Distance, pixels Right chromatid Absolute distance, pixels Distance, pixels First pair 1.46 (1.13) 0.54 (1.76) 1.57 (1.07) 0.40 (1.86) Second pair 1.71 (1.21) 0.74 (1.96) 1.61 (1.10) 0.72 (1.81) Third pair 1.63 (1.01) 0.59 (1.83) 1.53 (1.00) 0.72 (1.68) were assumed to be uniformly distributed between 0 and 2p. The model also assumed that the radial and axial positions of given spots were not influenced by the other spots on the chromosome (Fig. 2 F). Several hundred chromosomes were generated using Monte Carlo simulation according to this model. Chromosomes generated with the computer according to the described random folding model produced distributions of the measured geometrical features, such as absolute and relative longitudinal and lateral positions of sister spots or spots of the same chromatid similar to the imaged chromosomes in terms of mean values and SDs. For imaged and simulated chromosomes, the average distances from spots to the chromatid axis were and pixels, respectively (mean 5 SD). The similarity of distributions of lateral positions of lac op spots in 2D projections between imaged and simulated chromosomes is demonstrated in Fig. 4 E. This finding supports the idea
8 1624 Strukov and Belmont that chromosome condensation occurs through irregular folding, at least at higher levels of organization. Daughter chromatids compact independently The results described above suggest two possibilities. First, they could be the consequence of an irreproducible and therefore irregular folding of chromatin, at least at the higher levels of folding within mitotic chromatids. Alternatively, folding of chromatin within individual daughter chromatids could be very reproducible; however, the two daughter chromatids may be rotated relative to each other within the metaphase chromosome, explaining the data presented above. In addition, it is possible that shortening of chromatids during mitotic condensation is accompanied by rotation of chromatids around their axes by certain angles. This, in turn, could lead to changes of the lateral positions of spots relative to the chromatid axes in 2D projections when the chromosomes are compared at different stages of condensation. To test whether rotation was responsible for the significant variation in the lateral positions of the spots between sister chromatids, projections of the 3D spot locations or the three pairs of spots onto the YZ plane were examined. If condensation within chromatids followed the same pathways, then differences in relative positions of spots of different chromosomes would be due either to rotation of the chromatids during condensation relative to each other, or, alternatively, to the lack of a fixed orientation of one chromatid relative to the other. We found, however, that spot locations projected on the YZ plane are not related by rotation only. Some of them are in configurations that require not only rotation and stretching, but also mirror reflection to be superimposed with each other. Specifically, using the fact that there were three pairs of spots on the same chromosome, we sought to determine whether it is possible to superimpose the spots of two daughter chromatids by rotating one of the chromatids. The spots of one chromatid were rotated around the chromatid axis by an angle and translated in three dimensions to provide the best superimposition of the spots of one daughter chromatid with the other (i.e., the root mean-square (RMS) distance was a minimum). The same procedure was applied to chromosomes after a mirror reflection of one of the chromatids (right) relative to a plane bisecting the two daughter chromatids. The Optimization Toolbox of MATLAB was used to solve this optimization problem. The cost function, which was a sum of three squared distances between sister spots, was minimized over possible 2D or 3D translations of spots of one chromatid with respect to the other, and rotations of one of the chromatids around its axis, with the other chromatid being fixed. Optimization was done for both 2D projections (Fig. 5, A and B) and 3D data (Fig. 5, C and D) for real and Monte Carlo simulated data. For the 2D case, the RMS distance between sister spots in 2D projections was minimized through translation of the dots of the right chromatid relative to the left chromatid in X and Y. Calculations were repeated for 2D projections after the spots of the right chromatid were replaced with their mirror reflection with respect to the chromatid axis. Comparisons of distributions of the minimized RMS for all chromosomes have not revealed any preference for the original chromosomes or for chromosomes in which the right chromatid was substituted with its mirror reflection. Some sister chromatids show a configuration of sister spots that is close to translational symmetry, some chromatids look more mirror-symmetrical, and in a fraction of chromosomes the sister spots cannot be superimposed at all (Fig. 5, A and B). Similar calculations were done with the 3D data. RMS minimization between spots of sister chromatids in 3D was done through 3D translation and rotation of spots of the right chromatid around an axis parallel to its axis. The distribution of minimized distances was found to be similar for both original and mirror-reflected chromatids. No correlation between the inferred symmetry of chromatids and compaction, estimated by the length of chromosomal arms, was observed. For example, for 2D projections, the average lengths of chromosomal arms for all chromatids showing translational symmetry was mm (mean 5 SD) and mm (mean 5 SD) for mirror symmetryrelated chromatids. The minimized (through translation and rotation around chromatid axes) mean-square distances were similar for the original chromosomes and for chromosomes where one of the chromatids was mirror-reflected. The results obtained from computer-generated data (using the model summarized in Fig. 2 F) were similar to the results from the real data, suggesting that there is little correlation between positions of spots on daughter chromatids due to significant variations in lateral positions, and that chromatids condense independently of each other. DISCUSSION Our work was motivated by longstanding questions concerning mitotic chromosome condensation. Do specific sequences occupy fixed positions in mitotic chromosomes? Is there any correlation in the relative positions of sequences separated by distances of up to several tens of Mbp? And finally, is there any symmetry or reproducibility in the large-scale chromatin folding of daughter chromatids? Our first goal was to examine the reproducibility of chromatin folding within minimally perturbed isolated metaphase chromosomes. Although we cannot rule out some structural perturbation caused by the chromosome isolation conditions, our procedure exploited everything we have learned about buffer conditions and isolation protocols to minimize structural changes. Certainly our procedures are significantly improved relative to those used in all previous experiments using FISH procedures. Using cell lines containing several nearby vector inserts of small size, we show that the axial
9 Chromosome Folding Reproducibility 1625 FIGURE 5 Similar distributions of spot positions relative to chromatid axes for real and computer-generated data. Minimal RMS deviations in pixels for spots of sister chromatids were found through an optimization procedure between original chromosomes (abscissa) and chromosomes in which a mirror reflection of the right chromatid was performed (ordinate). The left chromatid was fixed. For the right chromatid, 2D translation and rotation of the projection was allowed for the 2D case (A and B), and 3D translation and rotation around the chromatid axis was allowed for the 3D case (C and D). (A) 2D projections, real data. (B) 2D projections, simulated data. (C) 3D real data. (D) 3D simulated data. positions of spots vary among different chromosomes and therefore are not reproducible. Our second goal was to study correlation between the large-scale structures of sister chromatids in a metaphase chromosome. We found that the large-scale folding patterns of daughter chromatids, as judged by distributions of vector inserts, are independent of each other and do not show translational or mirror symmetry. Similarly, we found no correlation between lateral positions of spots of the same chromatid. In the cell line used in this work, neighboring spots are separated by ~30 40 Mbp of DNA, which corresponds to several mitotic bands. These results argue against any long-range correlation in chromatin folding over tens of Mbp distances, as might be predicted in a regular, helical organization. Our results are inconsistent with simple, highly ordered helical models or more general hierarchical folding models for mitotic chromosome structure, which invoke a high degree of folding reproducibility across multiple levels of chromatin folding. However, they remain consistent with hierarchical chromosome folding models, in which largescale mitotic chromatin is formed through irregular successive compaction of lower-level chromatin fibers. Improvement of the detection technique Here we describe an experimental approach to study the largescale structure of mitotic chromosomes by investigating the distribution of specific sequences in isolated chromosomes with preserved large-scale structure. It is challenging to study the structure of mitotic chromosomes experimentally, for several reasons: the tight compaction of the chromatin, the susceptibility of the large-scale chromatin structure to changes in ionic conditions, and the physical limits of resolution of fluorescence microscopy. Extraction of chromosomes, a popular technique, is used to partially or completely decondense tightly packed mitotic chromatin in an attempt to decompose the chromosome into the structural elements that comprise the original chromosome, and hence enable visualization of those elements. The question remains, however, as to whether halos of extracted chromosomes are adequate simplifications of intact chromosomes and may be used to infer the original large-scale structure of chromatin before extraction. Experiments with extracted chromosomes have led to the radial-loop models of mitotic chromosomes. However, there is no evidence
10 1626 Strukov and Belmont from experiments on intact chromosomes that the loop-like structures are present. A hierarchically folded chromatin fiber that is held compact by certain chromosomal proteins, such as topoisomerase II and condensins, may turn, after extraction of histones and other soluble proteins, into an artificial set of loops attached to a resistant to extraction core or scaffold. These covalent or noncovalent complexes of DNA and scaffold proteins withstand extraction. The size of the loops may reflect the frequency of the sites at which DNA interacts with the scaffold proteins. It was hypothesized that the so-called SAR (Scaffold Associated Region) sequences may serve as such anchor DNA sequences responsible for interaction with scaffold proteins. Indeed, it has been shown that SAR sequences remain bound to interphase nuclear matrices after high salt extraction, and specifically and cooperatively interact with topoisomerase II. Large-scale structures are highly susceptible to changes in the ionic strength of the environment. This is why we made measurements on isolated, formaldehyde-fixed metaphase chromosomes with preserved morphology, at least at the level of light microscopy. The chromosomes were not subjected to any treatment that causes large-scale rearrangements of chromatin, including dehydration, unlike the FISH-based detection techniques used in previous studies (16,22), which included partial or complete extraction, fixation with methanol: acetic acid, and drying of chromosomes. It has been proposed that there could exist two distinct processes of mitotic condensation. The first stage is characterized by condensation of chromatin into prophase chromosomes. After the chromosomes split into two chromatids, the second stage is distinguished by extra coiling of chromatids (regular or irregular) (34). We cannot exclude the possibility that prolonged incubation of mitotic cells with colchicine induces further condensation of chromatids. This may have led to the mirror or other types of symmetry between sister chromatids observed in earlier studies (6,7,16), but did not occur in our experiments, which used shorter mitotic arrest protocols. Finally, resolution of conventional fluorescent light microscopy is limited by ~200 nm perpendicular to the optical axis, which makes it difficult to observe the fine structural elements of chromosomes. Electron microscopy has better resolution; however, it is more difficult and tedious to collect data and preserve unperturbed large-scale structure in samples for electron microscopy. Optical rather than physical serial sections may be used in fluorescence microscopy. Our methodology allowed us to measure accurately, with subpixel accuracy, the 2D positions of fluorescently labeled vector inserts. Resolution along the optical axis was lower due to the fundamental limitations of light microscopy. No strict order and reproducibility within mitotic chromosomes We found that individual spots do not occupy fixed reproducible lateral positions on chromatids. Lateral positions of individual spots may change within the entire width of a chromatid width, which is ~ nm, while longitudinal positions vary within only an ~300 nm, bandlike region. One explanation for this observation is that condensation of chromatids is not reproducible and does not follow one established scenario for each mitosis, and the large-scale chromatin structure is irregular. Alternatively, chromatids could rotate around their axes relative to each other during condensation by a random angle; then 2D projections of individual specific DNA sequences would not have fixed lateral positions even when each chromatid follows the same condensation pathway. However, measurements of the 3D positions of spots exclude the possibility that folding of chromatids is reproducible, as sister chromatids in different examples show neither mirror nor translational symmetry. An alternative explanation for the lack of positioning reproducibility is that the observed variability among chromosomes is the result of their being caught at different stages of condensation. Even though nocodazole treatment blocks the cell cycle at prometaphase, the degree of chromosome compaction might depend on the block duration. We therefore tested whether axial spot positioning might be a function of chromosome length, and therefore the degree of chromosome condensation, but no correlation was observed. Our observations of chromosome geometry fit into several different models of mitotic chromosomes, all involving irregularity of folding at one or multiple levels. Specifically, one possibility is that the nm large-scale chromatin fiber observed in earlier studies (8 10) is folded irregularly and nonreproducibly to form the final nm chromatid. Within the nm chromatid, specific sequences could occupy positions that are reproducible from mitosis to mitosis. The opposite possibility is that the nm fiber is coiled regularly in a larger mitotic chromatid, but that lower levels of chromatin folding within this nm fiber are irregularly folded. This second possibility would explain the longitudinal variability in spot positioning and at least some of the considerable axial variation. A third possibility is that irregularity of folding occurs at multiple levels of chromosome organization. Sequence-specific, reproducible banding patterns in isolated and mitotic chromosomes imply a certain degree of order as well as spatial correlation between daughter chromatids. Specific sequences are always found within the same bands/regions of mitotic chromosomes. It is possible that reproducibility is limited to the lower levels of chromatin compaction, and the higher levels observed during mitosis are more independent. We claim that at least for a fraction of chromatin, reproducibility of chromosome condensation is limited only to the longitudinal and not the lateral positioning of specific sequences, which is consistent with the phenomenon of chromosomal banding. Previous results suggest, however, that a subset of DNA sequences may be reproducibly targeted to specific regions of mitotic chromosomes (17).
11 Chromosome Folding Reproducibility 1627 Daughter chromatids are spatially not correlated with each other It has been known for a long time that linear dimensions of sister chromatids in mitosis are highly correlated, with the length, width, and banding patterns of chromatids of the same chromosome being the same at different stages of mitotic condensation. Previous studies have reported mirror symmetry between daughter chromatids (6,16). This implies precise coordination between chromatids due either to close bonds between chromatids mediated, for instance, by sister chromatid cohesion until their separation in anaphase or to a regular and reproducible condensation process. Here we argue that daughter chromatids do not possess symmetry and compact independently of each other. In previous work, we examined the chromosomal folding of band-sized chromosome regions formed by multicopy vector insertions carrying lac operator repeats (18). Anti-lac repressor immunogold staining was combined with electron microscopy to visualize these chromosome band-like structures by the vector insertions. These results showed that the minimal-size, lac operator-containing chromosome band was ~ nm wide, and that these band-like structures showed sharp borders within the same chromatid, and formed structures of similar shape, size, and width within sister chromatids, but that sister bands might not register exactly between sister chromatids. These results are consistent with the general idea that sequences are distributed throughout a nm band structure formed by the folding of a fiber of the same width as the band. The degree of misregistration of lac operator-containing bands in sister chromatids requires further analysis, but could explain at least part of the longitudinal variation in spot position we observed in this work. However, it seems that again an additional level of irreproducibility of folding must be invoked to explain the observed variation in axial positioning of sequences. Similarly, topoisomerase II distribution demonstrates distinctive substructure at the core regions of chromosome arms consisting of foci of different sizes connected in a linear manner along the chromosome length. Occasionally, a helical structure is suggested. However, sister chromatids show similar misregistration in the density of anti-topoisomerase II staining similar to that described above for the bands of lac operator-containing chromatin (Fig. 1). The apparent lack of symmetry in axial distributions of topoisomerase II also argues against strict spatial correlation between sister chromatids. The variable longitudinal distribution of topoisomerase II, as well as many other chromosomal proteins, may arise from the same underlying mechanism as the formation of chromosome banding patterns, which are related to DNA sequence content or protein distributions (e.g., cohesin-mediated linking of homologous sequences). If mitotic condensation of chromatids is spatially uncorrelated, then the interactions between daughter chromatids are not fixed and may break and reestablish frequently during mitotic condensation, or they are not necessarily sequence specific. Lack of symmetry between daughter chromatids in 2D projections may be due to both nonreproducible irregular folding of chromatids and/or rotational freedom of chromatids. It is interesting that in human tissue culture cells, residual cohesins have been localized between late metaphase chromatid axes (33). This suggests that at least for a small fraction of DNA sequences, either their positions are correlated between the sisters or cohesins link nonhomologous DNA fragments. We found that more lac op spots are found outside both sister chromatid axes, as marked by anti-topo II antibody staining, than between the axes. This interesting observation may reflect the fact that DNA in sister chromatids is not distributed axially uniformly around the axes at this stage of mitosis, probably due to residual cohesion links, with more DNA found at the periphery of metaphase chromosomes. It remains to be seen whether cohesins, which are always found between axes of sister chromatids, have a preference for certain regions of chromatin or DNA and influence distribution of DNA in mitotic chromosomes. Future directions Previous attempts to study large-scale mitotic chromosomes were hindered by the absence of methods for labeling of specific DNA sequences in morphologically intact chromosomes. A lac repressor/lac operator detection system allows labeling of chromosomes with preserved structure as well as observation of tagged chromatin regions in live cells in vivo. Further improvements in spatial resolution (and in the axial direction specifically) of fluorescent microscopes would allow investigators to measure the positions of spots and chromatid axes with higher accuracy to build 3D models of chromosomes at different stages of condensation. A closer look at positioning of different types of DNA sequences (such as active versus silent DNA, or early-replicating versus late-replicating) with respect to chromosomal cores could be also informative. Our results do not exclude the possibility that the lack of reproduced lateral positions of specific sequences in mitotic chromosomes comes from variations in angular positions (e.g., angles Q and Q 0 in Fig. 2 F) of sequences with fixed radial deviations away from chromatid axes. Observation of mitotic condensation in live cells, following temporal rearrangement of specific DNA sequences relative to each other during mitosis, could help to establish intermediate condensation steps separating interphase versus mitotic chromosome structure. Finally, more needs to be known about the nature of the forces driving mitotic chromatin condensation, and the roles of specific chromosomal proteins and their modifications in establishing these forces. We thank Dan Sullivan for providing generous advice and the antibody against topoisomerase IIa.
JCB. Visualization of early chromosome condensation: a hierarchical folding, axial glue model of chromosome structure. The Journal of Cell Biology
 JCB Published Online: 7 September, 2004 Supp Info: http://doi.org/10.1083/jcb.200406049 Downloaded from jcb.rupress.org on December 5, 2018 Article Visualization of early chromosome condensation: a hierarchical
JCB Published Online: 7 September, 2004 Supp Info: http://doi.org/10.1083/jcb.200406049 Downloaded from jcb.rupress.org on December 5, 2018 Article Visualization of early chromosome condensation: a hierarchical
Supplemental Information. 3D-CLEM Reveals that a Major Portion. of Mitotic Chromosomes Is Not Chromatin
 Molecular Cell, Volume 64 Supplemental Information 3D-CLEM Reveals that a Major Portion of Mitotic Chromosomes Is Not Chromatin Daniel G. Booth, Alison J. Beckett, Oscar Molina, Itaru Samejima, Hiroshi
Molecular Cell, Volume 64 Supplemental Information 3D-CLEM Reveals that a Major Portion of Mitotic Chromosomes Is Not Chromatin Daniel G. Booth, Alison J. Beckett, Oscar Molina, Itaru Samejima, Hiroshi
Cellular Reproduction, Part 1: Mitosis Lecture 10 Fall 2008
 Cell Theory 1 Cellular Reproduction, Part 1: Mitosis Lecture 10 Fall 2008 Cell theory: All organisms are made of cells All cells arise from preexisting cells How do new cells arise? Cell division the reproduction
Cell Theory 1 Cellular Reproduction, Part 1: Mitosis Lecture 10 Fall 2008 Cell theory: All organisms are made of cells All cells arise from preexisting cells How do new cells arise? Cell division the reproduction
Supplementary Figure 1. Mother centrioles can reduplicate while in the close association
 C1-GFP distance (nm) C1-GFP distance (nm) a arrested HeLa cell expressing C1-GFP and Plk1TD-RFP -3 s 1 2 3 4 5 6 7 8 9 11 12 13 14 16 17 18 19 2 21 22 23 24 26 27 28 29 3 b 9 8 7 6 5 4 3 2 arrested HeLa
C1-GFP distance (nm) C1-GFP distance (nm) a arrested HeLa cell expressing C1-GFP and Plk1TD-RFP -3 s 1 2 3 4 5 6 7 8 9 11 12 13 14 16 17 18 19 2 21 22 23 24 26 27 28 29 3 b 9 8 7 6 5 4 3 2 arrested HeLa
Mitotic Chromosome Condensation Andrew Belmont
 Mitotic Chromosome Condensation Prof. Department of Cell and Developmental Biology University of Illinois At Urbana-Champaign 1 Mitotic chromosome condensation: Still a mystery Goals for this lecture:
Mitotic Chromosome Condensation Prof. Department of Cell and Developmental Biology University of Illinois At Urbana-Champaign 1 Mitotic chromosome condensation: Still a mystery Goals for this lecture:
Imaging of Chromosomes at Nanometer-Scale Resolution, Using Soft X-Ray Microscope
 Imaging of Chromosomes at Nanometer-Scale Resolution, Using Soft X-Ray Microscope K. Takemoto, A. Yamamoto 1, I. Komura 2, K. Nakanishi 3, H. Namba 2 and H. Kihara Abstract In order to clarify the process
Imaging of Chromosomes at Nanometer-Scale Resolution, Using Soft X-Ray Microscope K. Takemoto, A. Yamamoto 1, I. Komura 2, K. Nakanishi 3, H. Namba 2 and H. Kihara Abstract In order to clarify the process
Mitosis in Onion Root Tip Cells
 Mitosis in Onion Root Tip Cells A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules, or chromosomes.
Mitosis in Onion Root Tip Cells A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules, or chromosomes.
Mitosis. AND Cell DiVISION
 Mitosis AND Cell DiVISION Cell Division Characteristic of living things: ability to reproduce their own kind. Cell division purpose: When unicellular organisms such as amoeba divide to form offspring reproduction
Mitosis AND Cell DiVISION Cell Division Characteristic of living things: ability to reproduce their own kind. Cell division purpose: When unicellular organisms such as amoeba divide to form offspring reproduction
Chapter 2. Mitosis and Meiosis
 Chapter 2. Mitosis and Meiosis Chromosome Theory of Heredity What structures within cells correspond to genes? The development of genetics took a major step forward by accepting the notion that the genes
Chapter 2. Mitosis and Meiosis Chromosome Theory of Heredity What structures within cells correspond to genes? The development of genetics took a major step forward by accepting the notion that the genes
Exercise 6. Procedure
 Exercise 6 Procedure Growing of root tips Select a few medium-sized onion bulbs. Carefully remove the dry roots present. Grow root tips by placing the bulbs on glass tubes (of about 3 4 cm. diameter) filled
Exercise 6 Procedure Growing of root tips Select a few medium-sized onion bulbs. Carefully remove the dry roots present. Grow root tips by placing the bulbs on glass tubes (of about 3 4 cm. diameter) filled
Mitosis in Onion Root Tip Cells
 Mitosis in Onion Root Tip Cells A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules, or chromosomes.
Mitosis in Onion Root Tip Cells A quick overview of cell division The genetic information of plants, animals and other eukaryotic organisms resides in several (or many) individual DNA molecules, or chromosomes.
Cell Cycle and Mitosis
 Cell Cycle and Mitosis Name Period A# THE CELL CYCLE The cell cycle, or cell-division cycle, is the series of events that take place in a eukaryotic cell between its formation and the moment it replicates
Cell Cycle and Mitosis Name Period A# THE CELL CYCLE The cell cycle, or cell-division cycle, is the series of events that take place in a eukaryotic cell between its formation and the moment it replicates
How Cells Divide. Chapter 10
 How Cells Divide Chapter 10 Bacterial Cell Division Bacteria divide by binary fission. -the single, circular bacterial chromosome is replicated -replication begins at the origin of replication and proceeds
How Cells Divide Chapter 10 Bacterial Cell Division Bacteria divide by binary fission. -the single, circular bacterial chromosome is replicated -replication begins at the origin of replication and proceeds
LAB EXPERIMENT 4: Mitosis in Onion Root Tip Cells
 Cell Biology Lecturer: Assist. Prof. Dr. Altijana Hromić-Jahjefendić Assistant: Furkan Enes Oflaz LAB EXPERIMENT 4: Mitosis in Onion Root Tip Cells Objective After completing this exercise, you should
Cell Biology Lecturer: Assist. Prof. Dr. Altijana Hromić-Jahjefendić Assistant: Furkan Enes Oflaz LAB EXPERIMENT 4: Mitosis in Onion Root Tip Cells Objective After completing this exercise, you should
The Process of Cell Division
 Lesson Overview 10.2 The Process of Cell Division THINK ABOUT IT What role does cell division play in your life? Does cell division stop when you are finished growing? Chromosomes What is the role of chromosomes
Lesson Overview 10.2 The Process of Cell Division THINK ABOUT IT What role does cell division play in your life? Does cell division stop when you are finished growing? Chromosomes What is the role of chromosomes
5.2. Mitosis and Cytokinesis. Chromosomes condense at the start of mitosis. Connecting
 5.2 Mitosis and Cytokinesis KEY CONCEPT Cells divide during mitosis and cytokinesis. MAIN IDEAS Chromosomes condense at the start of mitosis. Mitosis and cytokinesis produce two genetically identical daughter
5.2 Mitosis and Cytokinesis KEY CONCEPT Cells divide during mitosis and cytokinesis. MAIN IDEAS Chromosomes condense at the start of mitosis. Mitosis and cytokinesis produce two genetically identical daughter
The Cell Cycle. Chapter 10
 The Cell Cycle Chapter 10 Why Do Cells Divide? Unicellular 1. Reproduction Multicellular 1. Grow 2. Repair 3. Development/reproduction Types of Division Prokaryotic cells Binary fission = asexual reproduction
The Cell Cycle Chapter 10 Why Do Cells Divide? Unicellular 1. Reproduction Multicellular 1. Grow 2. Repair 3. Development/reproduction Types of Division Prokaryotic cells Binary fission = asexual reproduction
Chromosomes & Cell Division
 Chromosomes & Cell Division Cell Division The growth and splitting of cells into two new, identical cells called daughter cells. Mitosis Meiosis DNA replicates Parent cell Chromosomes separate Cell division
Chromosomes & Cell Division Cell Division The growth and splitting of cells into two new, identical cells called daughter cells. Mitosis Meiosis DNA replicates Parent cell Chromosomes separate Cell division
Chapter 8 The Cell Cycle
 What molecule stores your genetic information or determines everything about you? DNA a nucleic acid How are DNA molecules arranged in the nucleus? As you can see DNA is: Chapter 8 The Cell Cycle 1. Arranged
What molecule stores your genetic information or determines everything about you? DNA a nucleic acid How are DNA molecules arranged in the nucleus? As you can see DNA is: Chapter 8 The Cell Cycle 1. Arranged
Evidence of Activity-Specific, Radial Organization of Mitotic Chromosomes in Drosophila
 Evidence of Activity-Specific, Radial Organization of Mitotic Chromosomes in Drosophila Yuri G. Strukov 1,Tûba H. Sural 2,3, Mitzi I. Kuroda 2,3, John W. Sedat 1 * 1 Department of Biochemistry and Biophysics,
Evidence of Activity-Specific, Radial Organization of Mitotic Chromosomes in Drosophila Yuri G. Strukov 1,Tûba H. Sural 2,3, Mitzi I. Kuroda 2,3, John W. Sedat 1 * 1 Department of Biochemistry and Biophysics,
CYTOGENETICS Dr. Mary Ann Perle
 CYTOGENETICS Dr. Mary Ann Perle I) Mitosis and metaphase chromosomes A) Chromosomes are most fully condensed and clearly distinguishable during mitosis. B) Mitosis (M phase) takes 1 to 2 hrs and is divided
CYTOGENETICS Dr. Mary Ann Perle I) Mitosis and metaphase chromosomes A) Chromosomes are most fully condensed and clearly distinguishable during mitosis. B) Mitosis (M phase) takes 1 to 2 hrs and is divided
Histones- protein molecules that are used to fold and package DNA into chromosomes.
 Chromosome- a portion of the DNA in a cell, a chromosome is created when the DNA segment coils around histones then twists further to create a long twisted mass. Histones- protein molecules that are used
Chromosome- a portion of the DNA in a cell, a chromosome is created when the DNA segment coils around histones then twists further to create a long twisted mass. Histones- protein molecules that are used
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis
 Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Prokaryotes Have a Simpler Cell Cycle Cell division in prokaryotes takes place in two stages, which together make up a simple cell cycle 1. Copy
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Prokaryotes Have a Simpler Cell Cycle Cell division in prokaryotes takes place in two stages, which together make up a simple cell cycle 1. Copy
Name: Date: Block: 10-2 Cell Division Worksheet
 10-2 Cell Division Worksheet W hat do you think would happen if a cell were simple to split into two, without any advance preparation? Would each daughter cell have everything it needed to survive? Because
10-2 Cell Division Worksheet W hat do you think would happen if a cell were simple to split into two, without any advance preparation? Would each daughter cell have everything it needed to survive? Because
Chapter 8: Cellular Reproduction
 Chapter 8: Cellular Reproduction 1. The Cell Cycle 2. Mitosis 3. Meiosis 2 Types of Cell Division 2n 1n Mitosis: occurs in somatic cells (almost all cells of the body) generates cells identical to original
Chapter 8: Cellular Reproduction 1. The Cell Cycle 2. Mitosis 3. Meiosis 2 Types of Cell Division 2n 1n Mitosis: occurs in somatic cells (almost all cells of the body) generates cells identical to original
Mitosis & Meiosis. Diploid cells- (2n)- a cell that has 2 of each chromosome - 1 from mom, 1 from dad = 1 pair
 Mitosis & Meiosis Diploid cells- (2n)- a cell that has 2 of each chromosome - 1 from mom, 1 from dad = 1 pair The pair is called homologous chromosomes The homologous chromosomes contain the same gene
Mitosis & Meiosis Diploid cells- (2n)- a cell that has 2 of each chromosome - 1 from mom, 1 from dad = 1 pair The pair is called homologous chromosomes The homologous chromosomes contain the same gene
Biology 4A Laboratory MITOSIS Asexual Reproduction OBJECTIVE
 Biology 4A Laboratory MITOSIS Asexual Reproduction OBJECTIVE To study the cell cycle and understand how, when and why cells divide. To study and identify the major stages of cell division. To relate the
Biology 4A Laboratory MITOSIS Asexual Reproduction OBJECTIVE To study the cell cycle and understand how, when and why cells divide. To study and identify the major stages of cell division. To relate the
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis
 Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Chromosomes Chromosomes were first observed by the German embryologist Walther Fleming in 1882. Chromosome number varies among organisms most
Breaking Up is Hard to Do (At Least in Eukaryotes) Mitosis Chromosomes Chromosomes were first observed by the German embryologist Walther Fleming in 1882. Chromosome number varies among organisms most
5.2. Mitosis and Cytokinesis. Chromosomes condense at the start of mitosis.
 5.2 Mitosis and Cytokinesis VOCABULARY chromosome histone chromatin chromatid centromere prophase metaphase anaphase telophase Biochemistry As you will learn in the chapter From DNA to Proteins, a nucleotide
5.2 Mitosis and Cytokinesis VOCABULARY chromosome histone chromatin chromatid centromere prophase metaphase anaphase telophase Biochemistry As you will learn in the chapter From DNA to Proteins, a nucleotide
SDS-Assisted Protein Transport Through Solid-State Nanopores
 Supplementary Information for: SDS-Assisted Protein Transport Through Solid-State Nanopores Laura Restrepo-Pérez 1, Shalini John 2, Aleksei Aksimentiev 2 *, Chirlmin Joo 1 *, Cees Dekker 1 * 1 Department
Supplementary Information for: SDS-Assisted Protein Transport Through Solid-State Nanopores Laura Restrepo-Pérez 1, Shalini John 2, Aleksei Aksimentiev 2 *, Chirlmin Joo 1 *, Cees Dekker 1 * 1 Department
Cell Division. Learning Objectives: Introduction. Revised Fall 2018
 Revised Fall 2018 Cell Division Learning Objectives: 1. Define cell cycle and the ordered sequence of events in the cell cycle (Interphase and The divisional phase or M phase) 2. Explain the stages in
Revised Fall 2018 Cell Division Learning Objectives: 1. Define cell cycle and the ordered sequence of events in the cell cycle (Interphase and The divisional phase or M phase) 2. Explain the stages in
Developing Auto-focusing Microscopy For Ultra High- Throughput Biodosimetry
 Developing Auto-focusing Microscopy For Ultra High- Throughput Biodosimetry NINA BAHAR D R. G U Y G A R T Y, M E N T O R R A D I O L O G I C A L R E S E A R C H A C C E L E R A T O R F A C I L I T Y C
Developing Auto-focusing Microscopy For Ultra High- Throughput Biodosimetry NINA BAHAR D R. G U Y G A R T Y, M E N T O R R A D I O L O G I C A L R E S E A R C H A C C E L E R A T O R F A C I L I T Y C
10-2 Cell Division. Copyright Pearson Prentice Hall
 10-2 Cell Division Copyright Pearson Prentice Hall Cell Growth and Division In multicellular organisms, cell division makes new cells To replace old or damaged ones So organisms can grow In single-celled
10-2 Cell Division Copyright Pearson Prentice Hall Cell Growth and Division In multicellular organisms, cell division makes new cells To replace old or damaged ones So organisms can grow In single-celled
Bacterial cell. Origin of replication. Septum
 Bacterial cell Bacterial chromosome: Double-stranded DNA Origin of replication Septum 1 2 3 Chromosome Rosettes of Chromatin Loops Scaffold protein Chromatin Loop Solenoid Scaffold protein Chromatin loop
Bacterial cell Bacterial chromosome: Double-stranded DNA Origin of replication Septum 1 2 3 Chromosome Rosettes of Chromatin Loops Scaffold protein Chromatin Loop Solenoid Scaffold protein Chromatin loop
Mitosis. Mitosis with cancer and cloning
 Mitosis Mitosis with cancer and cloning Chromosome- a portion of the DNA in a cell, a chromosome is created when the DNA segment coils around histones then twists further to create a long twisted mass.
Mitosis Mitosis with cancer and cloning Chromosome- a portion of the DNA in a cell, a chromosome is created when the DNA segment coils around histones then twists further to create a long twisted mass.
Origin of replication. Septum
 Bacterial cell Bacterial chromosome: Double-stranded DNA Origin of replication Septum 1 2 3 Chromosome Rosettes of Chromatin Loops Chromatin Loop Solenoid Scaffold protein Scaffold protein Chromatin loop
Bacterial cell Bacterial chromosome: Double-stranded DNA Origin of replication Septum 1 2 3 Chromosome Rosettes of Chromatin Loops Chromatin Loop Solenoid Scaffold protein Scaffold protein Chromatin loop
BIOLOGY 111. CHAPTER 9: The Links in Life s Chain Genetics and Cell Division
 BIOLOGY 111 CHAPTER 9: The Links in Life s Chain Genetics and Cell Division The Links in Life s Chain: Genetics and Cell Division 9.1 An Introduction to Genetics 9.2 An Introduction to Cell Division 9.3
BIOLOGY 111 CHAPTER 9: The Links in Life s Chain Genetics and Cell Division The Links in Life s Chain: Genetics and Cell Division 9.1 An Introduction to Genetics 9.2 An Introduction to Cell Division 9.3
8.1 Cellular Transport
 Ch 8: Cellular Transport and the Cell Cycle 8.1 Cellular Transport Inside This Section... Osmosis Passive vs. Active Transport Transport of Large Particles What is Osmosis? Diffusion of water across a
Ch 8: Cellular Transport and the Cell Cycle 8.1 Cellular Transport Inside This Section... Osmosis Passive vs. Active Transport Transport of Large Particles What is Osmosis? Diffusion of water across a
Unit 6: Study Guide Cell Division. diploid gene allele interphase (G1, S, G2) prophase metaphase anaphase
 Unit 6: Study Guide Cell Division 1. Define: chromatin chromosome chromatid pair (sister chromatid) centromere spindle fibers haploid diploid gene allele interphase (G1, S, G2) prophase metaphase anaphase
Unit 6: Study Guide Cell Division 1. Define: chromatin chromosome chromatid pair (sister chromatid) centromere spindle fibers haploid diploid gene allele interphase (G1, S, G2) prophase metaphase anaphase
Figure S1. HP1α localizes to centromeres in mitosis and interacts with INCENP. (A&B) HeLa
 SUPPLEMENTARY FIGURES Figure S1. HP1α localizes to centromeres in mitosis and interacts with INCENP. (A&B) HeLa tet-on cells that stably express HP1α-CFP, HP1β-CFP, or HP1γ-CFP were monitored with livecell
SUPPLEMENTARY FIGURES Figure S1. HP1α localizes to centromeres in mitosis and interacts with INCENP. (A&B) HeLa tet-on cells that stably express HP1α-CFP, HP1β-CFP, or HP1γ-CFP were monitored with livecell
Mitosis and Cytokinesis
 B-2.6 Summarize the characteristics of the cell cycle: interphase (called G1, S, G2); the phases of mitosis (called prophase, metaphase, anaphase, and telophase); and plant and animal cytokinesis. The
B-2.6 Summarize the characteristics of the cell cycle: interphase (called G1, S, G2); the phases of mitosis (called prophase, metaphase, anaphase, and telophase); and plant and animal cytokinesis. The
Fig. 1. Simple columnar epithelial cells lining the small intestine.
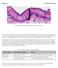 Mitosis Prelab Reading Fig. 1. Simple columnar epithelial cells lining the small intestine. The tall cells pictured in Fig. 1 form the lining of the small intestine in humans and other animals. These cells
Mitosis Prelab Reading Fig. 1. Simple columnar epithelial cells lining the small intestine. The tall cells pictured in Fig. 1 form the lining of the small intestine in humans and other animals. These cells
This colorized scanning electron micrograph is showing a white blood cell undergoing cell division.
 10 1 Cell Growth Why are cells small? This colorized scanning electron micrograph is showing a white blood cell undergoing cell division. This relates to cell theory because this is HOW all cells come
10 1 Cell Growth Why are cells small? This colorized scanning electron micrograph is showing a white blood cell undergoing cell division. This relates to cell theory because this is HOW all cells come
Genetics and Cellular Function
 Genetics and Cellular Function DNA replication and the cell cycle Mitosis Mitosis Mitosis: division of cells that results in daughter cells with the same the genetic information that the original cell
Genetics and Cellular Function DNA replication and the cell cycle Mitosis Mitosis Mitosis: division of cells that results in daughter cells with the same the genetic information that the original cell
Cell Division. Chromosome structure. Made of chromatin (mix of DNA and protein) Only visible during cell division
 Chromosome structure Made of chromatin (mix of DNA and protein) Only visible during cell division Chromosome structure The DNA in a cell is packed into an elaborate, multilevel system of coiling and folding.
Chromosome structure Made of chromatin (mix of DNA and protein) Only visible during cell division Chromosome structure The DNA in a cell is packed into an elaborate, multilevel system of coiling and folding.
The Cell Cycle and Cell Division
 Content Vocabulary Directions: On each line, write the term from the word bank that correctly replaces the underlined words in each sentence. NOTE: You may need to change a term to its plural form. cell
Content Vocabulary Directions: On each line, write the term from the word bank that correctly replaces the underlined words in each sentence. NOTE: You may need to change a term to its plural form. cell
U3.2.3: Eukaryotic chromosomes are linear DNA molecules associated with histone proteins. (Oxford Biology Course Companion page 151).
 Cell Division Study Guide U3.2.3: Eukaryotic chromosomes are linear DNA molecules associated with histone proteins. (Oxford Biology Course Companion page 151). 1. Describe the structure of eukaryotic DNA
Cell Division Study Guide U3.2.3: Eukaryotic chromosomes are linear DNA molecules associated with histone proteins. (Oxford Biology Course Companion page 151). 1. Describe the structure of eukaryotic DNA
Cell Growth and Division
 Cell Growth and Division CHAPTER 10 EQ Why do cells grow and divide? DID YOU KNOW THAT The adult human body produces roughly 2 TRILLION cells every day. WHY DO CELLS REPRODUCE? So that the organism can
Cell Growth and Division CHAPTER 10 EQ Why do cells grow and divide? DID YOU KNOW THAT The adult human body produces roughly 2 TRILLION cells every day. WHY DO CELLS REPRODUCE? So that the organism can
MITOSIS AND THE CELL CYCLE PowerPoint Notes
 1 Name: Date: MITOSIS AND THE CELL CYCLE PowerPoint Notes THE FUNCTIONS OF CELL DIVISION 1. Cell division is vital for all. living organisms This is the only process that can create. new cells 2. Cell
1 Name: Date: MITOSIS AND THE CELL CYCLE PowerPoint Notes THE FUNCTIONS OF CELL DIVISION 1. Cell division is vital for all. living organisms This is the only process that can create. new cells 2. Cell
Mitosis and Cellular Division. EQ: How do the cells in our body divide?
 Mitosis and Cellular Division EQ: How do the cells in our body divide? Cell division is the process by which cellular material is divided between two new daughter cells. 1 Mother Cell 2 Daughter cells.
Mitosis and Cellular Division EQ: How do the cells in our body divide? Cell division is the process by which cellular material is divided between two new daughter cells. 1 Mother Cell 2 Daughter cells.
The Cell Cycle and How Cells Divide
 The Cell Cycle and How Cells Divide 1 Phases of the Cell Cycle The cell cycle consists of Interphase normal cell activity The mitotic phase cell divsion INTERPHASE Growth G 1 (DNA synthesis) Growth G 2
The Cell Cycle and How Cells Divide 1 Phases of the Cell Cycle The cell cycle consists of Interphase normal cell activity The mitotic phase cell divsion INTERPHASE Growth G 1 (DNA synthesis) Growth G 2
Mitosis/Meiosis Simulation Activities
 Mitosis/Meiosis Simulation Activities In this simulation, you will demonstrate an understanding of mitosis, meiosis, segregation, independent assortment, and crossing over, all processes involved with
Mitosis/Meiosis Simulation Activities In this simulation, you will demonstrate an understanding of mitosis, meiosis, segregation, independent assortment, and crossing over, all processes involved with
Instructions for Use. APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests
 3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
3URGXFW,QIRUPDWLRQ Sigma TACS Annexin V Apoptosis Detection Kits Instructions for Use APO-AB Annexin V-Biotin Apoptosis Detection Kit 100 tests For Research Use Only. Not for use in diagnostic procedures.
The Cell Cycle MITOSIS
 The Cell Cycle MITOSIS Outcomes 1. Explain the events of the cell cycle Interphase Mitosis Prophase Metaphase Anaphase Telophase Cytokinesis 2. Use a simulation to demonstrate the behaviour of chromosomes
The Cell Cycle MITOSIS Outcomes 1. Explain the events of the cell cycle Interphase Mitosis Prophase Metaphase Anaphase Telophase Cytokinesis 2. Use a simulation to demonstrate the behaviour of chromosomes
Reproduction is a fundamental property of life. Cells are the fundamental unit of life. Reproduction occurs at the cellular level with one mother
 Cell Division ision Reproduction is a fundamental property of life. Cells are the fundamental unit of life. Reproduction occurs at the cellular level with one mother cell giving rise to two daughter cells.
Cell Division ision Reproduction is a fundamental property of life. Cells are the fundamental unit of life. Reproduction occurs at the cellular level with one mother cell giving rise to two daughter cells.
Efficient Measurement of Large Light Source Near-field Color and Luminance Distributions for Optical Design and Simulation
 Efficient Measurement of Large Light Source Near-field Color and Luminance Distributions for Optical Design and Simulation Hubert Kostal*, Douglas Kreysar, Ronald Rykowski Radiant Imaging, Inc., 22908
Efficient Measurement of Large Light Source Near-field Color and Luminance Distributions for Optical Design and Simulation Hubert Kostal*, Douglas Kreysar, Ronald Rykowski Radiant Imaging, Inc., 22908
Why do cells reproduce?
 Outline Cell Reproduction 1. Overview of Cell Reproduction 2. Cell Reproduction in Prokaryotes 3. Cell Reproduction in Eukaryotes 1. Chromosomes 2. Cell Cycle 3. Mitosis and Cytokinesis Examples of Cell
Outline Cell Reproduction 1. Overview of Cell Reproduction 2. Cell Reproduction in Prokaryotes 3. Cell Reproduction in Eukaryotes 1. Chromosomes 2. Cell Cycle 3. Mitosis and Cytokinesis Examples of Cell
Learning Objectives: Model the steps of mitosis in order to gain a better understanding of the process
 Mitosis On Goodness as a Final Cause: Since goodness is that which all things desire, it is clear that that goodness implies the aspect of an end....for a thing is perfect when it can reproduce its like,
Mitosis On Goodness as a Final Cause: Since goodness is that which all things desire, it is clear that that goodness implies the aspect of an end....for a thing is perfect when it can reproduce its like,
DAPI ASY1 DAPI/ASY1 DAPI RAD51 DAPI/RAD51. Supplementary Figure 1. Additional information on meiosis in R. pubera. a) The
 a % 10 Number of crossover per bivalent b 0 1 c DAPI/telomere 80 1 60 40 1 2 20 d 0 0 1 2 >=3 DAPI ASY1 DAPI/ASY1 e DAPI RAD51 DAPI/RAD51 Supplementary Figure 1. Additional information on meiosis in R.
a % 10 Number of crossover per bivalent b 0 1 c DAPI/telomere 80 1 60 40 1 2 20 d 0 0 1 2 >=3 DAPI ASY1 DAPI/ASY1 e DAPI RAD51 DAPI/RAD51 Supplementary Figure 1. Additional information on meiosis in R.
APGRU4L1 Chap 12 Extra Reading Cell Cycle and Mitosis
 APGRU4L1 Chap 12 Extra Reading Cell Cycle and Mitosis Dr. Ramesh Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008
APGRU4L1 Chap 12 Extra Reading Cell Cycle and Mitosis Dr. Ramesh Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008
10-2 Cell Division. Chromosomes
 Cell Division In eukaryotes, cell division occurs in two major stages. The first stage, division of the cell nucleus, is called mitosis. The second stage, division of the cell cytoplasm, is called cytokinesis.
Cell Division In eukaryotes, cell division occurs in two major stages. The first stage, division of the cell nucleus, is called mitosis. The second stage, division of the cell cytoplasm, is called cytokinesis.
A. Incorrect! All the cells have the same set of genes. (D)Because different types of cells have different types of transcriptional factors.
 Genetics - Problem Drill 21: Cytogenetics and Chromosomal Mutation No. 1 of 10 1. Why do some cells express one set of genes while other cells express a different set of genes during development? (A) Because
Genetics - Problem Drill 21: Cytogenetics and Chromosomal Mutation No. 1 of 10 1. Why do some cells express one set of genes while other cells express a different set of genes during development? (A) Because
Cell Cycle and Cell Division
 122 Cell Cycle and Cell Division 1. Meiosis I is reductional division. Meiosis II is equational division due to [1988] (a) pairing of homologous chromosomes (b) crossing over (c) separation of chromatids
122 Cell Cycle and Cell Division 1. Meiosis I is reductional division. Meiosis II is equational division due to [1988] (a) pairing of homologous chromosomes (b) crossing over (c) separation of chromatids
CELL CYCLE INTRODUCTION PART I ANIMAL CELL CYCLE INTERPHASE
 CELL CYCLE INTRODUCTION The nuclei in cells of eukaryotic organisms contain chromosomes with clusters of genes, discrete units of hereditary information consisting of double-stranded DNA. Structural proteins
CELL CYCLE INTRODUCTION The nuclei in cells of eukaryotic organisms contain chromosomes with clusters of genes, discrete units of hereditary information consisting of double-stranded DNA. Structural proteins
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division The Cell Cycle: Cell Growth, Cell Division 2007-2008 2007-2008 Getting from there to here Going from egg to baby. the original
Biology is the only subject in which multiplication is the same thing as division The Cell Cycle: Cell Growth, Cell Division 2007-2008 2007-2008 Getting from there to here Going from egg to baby. the original
Creating Identical Body Cells
 Creating Identical Body Cells 5.A Students will describe the stages of the cell cycle, including DNA replication and mitosis, and the importance of the cell cycle to the growth of organisms 5.D Students
Creating Identical Body Cells 5.A Students will describe the stages of the cell cycle, including DNA replication and mitosis, and the importance of the cell cycle to the growth of organisms 5.D Students
General Biology. Overview: The Key Roles of Cell Division. Unicellular organisms
 General Biology Course No: BNG2003 Credits: 3.00 8. The Cell Cycle Prof. Dr. Klaus Heese Overview: The Key Roles of Cell Division The continuity of life is based upon the reproduction of cells, or cell
General Biology Course No: BNG2003 Credits: 3.00 8. The Cell Cycle Prof. Dr. Klaus Heese Overview: The Key Roles of Cell Division The continuity of life is based upon the reproduction of cells, or cell
Cell Division. During interphase, a cell s DNA is in a loose form called. It condenses into tightly coiled structures called chromosomes during.
 Cell Division The is a cell s total DNA. Prokaryotes DNA is found mostly in a single called the and also in small circles called. Eukaryotes have several DNA double helices packaged into. During interphase,
Cell Division The is a cell s total DNA. Prokaryotes DNA is found mostly in a single called the and also in small circles called. Eukaryotes have several DNA double helices packaged into. During interphase,
The Cell Cycle. Chapter 12. Biology. Edited by Shawn Lester. Eighth Edition Neil Campbell and Jane Reece. PowerPoint Lecture Presentations for
 Chapter 12 The Cell Cycle Edited by Shawn Lester PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
Chapter 12 The Cell Cycle Edited by Shawn Lester PowerPoint Lecture Presentations for Biology Eighth Edition Neil Campbell and Jane Reece Lectures by Chris Romero, updated by Erin Barley with contributions
WHITE PAPER. Efficient Measurement of Large Light Source Near-Field Color and Luminance Distributions for Optical Design and Simulation
 Efficient Measurement of Large Light Source Near-Field Color and Luminance Distributions for Optical Design and Simulation Efficient Measurement of Large Light Source Near-Field Color and Luminance Distributions
Efficient Measurement of Large Light Source Near-Field Color and Luminance Distributions for Optical Design and Simulation Efficient Measurement of Large Light Source Near-Field Color and Luminance Distributions
The Chromosomes of a Frimpanzee: An Imaginary Animal
 The Chromosomes of a Frimpanzee: An Imaginary Animal Introduction By now, you have heard the terms chromosome, mitosis, and meiosis. You probably also know that chromosomes contain genetic information
The Chromosomes of a Frimpanzee: An Imaginary Animal Introduction By now, you have heard the terms chromosome, mitosis, and meiosis. You probably also know that chromosomes contain genetic information
PowerPoint Image Slideshow
 COLLEGE BIOLOGY PHYSICS Chapter 10 # Cell Chapter Reproduction Title PowerPoint Image Slideshow CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 12 The Cell Cycle 2014 Pearson
COLLEGE BIOLOGY PHYSICS Chapter 10 # Cell Chapter Reproduction Title PowerPoint Image Slideshow CAMPBELL BIOLOGY TENTH EDITION Reece Urry Cain Wasserman Minorsky Jackson 12 The Cell Cycle 2014 Pearson
10-2 Cell Division mitosis. cytokinesis. Chromosomes chromosomes Slide 1 of 38
 In eukaryotes, cell division occurs in two major stages. The first stage, division of the cell nucleus, is called mitosis. The second stage, division of the cell cytoplasm, is called cytokinesis. Chromosomes
In eukaryotes, cell division occurs in two major stages. The first stage, division of the cell nucleus, is called mitosis. The second stage, division of the cell cytoplasm, is called cytokinesis. Chromosomes
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
Vocabulary: cell division, centriole, centromere, chromatid, chromatin, chromosome, cytokinesis, DNA, interphase, mitosis
 Cell Division Answer Key Vocabulary: cell division, centriole, centromere, chromatid, chromatin, chromosome, cytokinesis, DNA, interphase, mitosis Prior Knowledge Questions (Do these BEFORE using the Gizmo.)
Cell Division Answer Key Vocabulary: cell division, centriole, centromere, chromatid, chromatin, chromosome, cytokinesis, DNA, interphase, mitosis Prior Knowledge Questions (Do these BEFORE using the Gizmo.)
Why do cells divide? Cells divide in order to make more cells they multiply in order to create a larger surface to volume ratio!!!
 Why do cells divide? Cells divide in order to make more cells they multiply in order to create a larger surface to volume ratio!!! Chromosomes Are made of chromatin: a mass of genetic material composed
Why do cells divide? Cells divide in order to make more cells they multiply in order to create a larger surface to volume ratio!!! Chromosomes Are made of chromatin: a mass of genetic material composed
Name. A.P. Biology Chapter 12 The Cell Cycle
 A.P. Biology Chapter 12 The Cell Cycle Name Living species MUST possess the ability to r if they are to flourish. The Cell Cycle follows the life of a cell from its o until its d. The Key Roles Of Cell
A.P. Biology Chapter 12 The Cell Cycle Name Living species MUST possess the ability to r if they are to flourish. The Cell Cycle follows the life of a cell from its o until its d. The Key Roles Of Cell
Chapter 10 Cell Growth and Division
 Chapter 10 Cell Growth and Division 10 1 Cell Growth 2 Limits to Cell Growth The larger a cell becomes, the more demands the cell places on its DNA. In addition, the cell has more trouble moving enough
Chapter 10 Cell Growth and Division 10 1 Cell Growth 2 Limits to Cell Growth The larger a cell becomes, the more demands the cell places on its DNA. In addition, the cell has more trouble moving enough
General Biology. Overview: The Key Roles of Cell Division The continuity of life is based upon the reproduction of cells, or cell division
 General Biology Course No: BNG2003" Credits: 3.00 " " " 8. The Cell Cycle Prof. Dr. Klaus Heese Overview: The Key Roles of Cell Division The continuity of life is based upon the reproduction of cells,
General Biology Course No: BNG2003" Credits: 3.00 " " " 8. The Cell Cycle Prof. Dr. Klaus Heese Overview: The Key Roles of Cell Division The continuity of life is based upon the reproduction of cells,
Cell Cycle and Mitosis Tutorial
 Cell Cycle and Mitosis Tutorial Get Started Interphase Prophase Metaphase Anaphase Telophase Take the Quiz What is the Cell Cycle? All cells go through a series of events in their life. Cells grow, divide,
Cell Cycle and Mitosis Tutorial Get Started Interphase Prophase Metaphase Anaphase Telophase Take the Quiz What is the Cell Cycle? All cells go through a series of events in their life. Cells grow, divide,
Cell Division Mitosis Notes
 Cell Division Mitosis Notes Cell Division process by which a cell divides into 2 new cells Why do cells need to divide? 1.Living things grow by producing more cells, NOT because each cell increases in
Cell Division Mitosis Notes Cell Division process by which a cell divides into 2 new cells Why do cells need to divide? 1.Living things grow by producing more cells, NOT because each cell increases in
meiosis asexual reproduction CHAPTER 9 & 10 The Cell Cycle, Meiosis & Sexual Life Cycles Sexual reproduction mitosis
 meiosis asexual reproduction CHAPTER 9 & 10 The Cell Cycle, Meiosis & Sexual Sexual reproduction Life Cycles mitosis Chromosomes Consists of a long DNA molecule (represents thousands of genes) Also consists
meiosis asexual reproduction CHAPTER 9 & 10 The Cell Cycle, Meiosis & Sexual Sexual reproduction Life Cycles mitosis Chromosomes Consists of a long DNA molecule (represents thousands of genes) Also consists
9/3/2009 DNA i DNA n euk euk yotes Organizatio Organ izatio n of o f gen ge e n tic Locati t on: In n ucleu e s material mater in e ial
 DNA in eukaryotes Organization of genetic material in eukaryotes Location: In nucleus In mitochondria DNA in eukaryotes Nuclear DNA: Long, linear molecules; Chromatin chromosomes; 10% of DNA in genes,
DNA in eukaryotes Organization of genetic material in eukaryotes Location: In nucleus In mitochondria DNA in eukaryotes Nuclear DNA: Long, linear molecules; Chromatin chromosomes; 10% of DNA in genes,
10-2 Cell Division. Slide 1 of 38. End Show. Copyright Pearson Prentice Hall
 1 of 38 Cell Division In eukaryotes, cell division occurs in two major stages. The first stage, division of the cell nucleus, is called mitosis. The second stage, division of the cell cytoplasm, is called
1 of 38 Cell Division In eukaryotes, cell division occurs in two major stages. The first stage, division of the cell nucleus, is called mitosis. The second stage, division of the cell cytoplasm, is called
Dako IT S ABOUT TIME. Interpretation Guide. Agilent Pathology Solutions. ALK, ROS1 and RET IQFISH probes (Dako Omnis) MET IQFISH probe (Dako Omnis)
 INTERPRETATION Dako Agilent Pathology Solutions IQFISH Interpretation Guide ALK, ROS1 and RET IQFISH probes (Dako Omnis) MET IQFISH probe (Dako Omnis) IT S ABOUT TIME For In Vitro Diagnostic Use ALK, ROS1,
INTERPRETATION Dako Agilent Pathology Solutions IQFISH Interpretation Guide ALK, ROS1 and RET IQFISH probes (Dako Omnis) MET IQFISH probe (Dako Omnis) IT S ABOUT TIME For In Vitro Diagnostic Use ALK, ROS1,
Prentice Hall Biology Slide 1 of 38
 Prentice Hall Biology 1 of 38 2 of 38 In eukaryotes, cell division occurs in two major stages. The first stage, division of the cell nucleus, is called mitosis. The second stage, division of the cell cytoplasm,
Prentice Hall Biology 1 of 38 2 of 38 In eukaryotes, cell division occurs in two major stages. The first stage, division of the cell nucleus, is called mitosis. The second stage, division of the cell cytoplasm,
Biology is the only subject in which multiplication is the same thing as division. AP Biology
 Biology is the only subject in which multiplication is the same thing as division Chapter 12. The Cell Cycle: Cell Growth, Cell Division Where it all began You started as a cell smaller than a period at
Biology is the only subject in which multiplication is the same thing as division Chapter 12. The Cell Cycle: Cell Growth, Cell Division Where it all began You started as a cell smaller than a period at
Organization of genetic material in eukaryotes
 Organization of genetic material in eukaryotes biologiemoleculara.usmf.md pass.: bmgu e.usmf.md 1 DNA in eukaryotes Location: In nucleus In mitochondria biologiemoleculara.usmf.md e.usmf.md pass.: bmgu
Organization of genetic material in eukaryotes biologiemoleculara.usmf.md pass.: bmgu e.usmf.md 1 DNA in eukaryotes Location: In nucleus In mitochondria biologiemoleculara.usmf.md e.usmf.md pass.: bmgu
Unit 4 Student Notes Cell Cycle
 Name Date Unit 4 Student Notes Cell Cycle B-2.6 Summarize the characteristics of the cell cycle: interphase (called G1, S, G2); the phases of mitosis (called prophase, metaphase, anaphase, and telophase);
Name Date Unit 4 Student Notes Cell Cycle B-2.6 Summarize the characteristics of the cell cycle: interphase (called G1, S, G2); the phases of mitosis (called prophase, metaphase, anaphase, and telophase);
Molecular Cell Biology - Problem Drill 22: The Mechanics of Cell Division
 Molecular Cell Biology - Problem Drill 22: The Mechanics of Cell Division Question No. 1 of 10 1. Which of the following statements about mitosis is correct? Question #1 (A) Mitosis involves the dividing
Molecular Cell Biology - Problem Drill 22: The Mechanics of Cell Division Question No. 1 of 10 1. Which of the following statements about mitosis is correct? Question #1 (A) Mitosis involves the dividing
Section Cell Growth. A. Limits to Cell Growth 1. DNA Overload 2. Exchanging Materials 3. Ratio of Surface Area to Volume 4.
 Getting Through Materials move through cells by diffusion. Oxygen and food move into cells, while waste products move out of cells. How does the size of a cell affect how efficiently materials get to all
Getting Through Materials move through cells by diffusion. Oxygen and food move into cells, while waste products move out of cells. How does the size of a cell affect how efficiently materials get to all
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division 2007-2008 Where it all began You started as a cell smaller than a
Almost every cell in the human body has an identical set of 46 chromosomes, produced through the process of mitosis.
 M I T O S I S Mitosis Mitosis is the type of cell division that occurs for growth (adding new cells) and repair (replacing old or damaged cells). It results in two daughter cells that have identical chromosomes
M I T O S I S Mitosis Mitosis is the type of cell division that occurs for growth (adding new cells) and repair (replacing old or damaged cells). It results in two daughter cells that have identical chromosomes
Chapter 10. Cell Cycle - Mitosis
 Chapter 10 Cell Cycle - Mitosis WHAT CELL REPRODUCTION ACCOMPLISHES Cell division plays important roles in the lives of organisms. Cell division replaces damaged or lost cells permits growth allows for
Chapter 10 Cell Cycle - Mitosis WHAT CELL REPRODUCTION ACCOMPLISHES Cell division plays important roles in the lives of organisms. Cell division replaces damaged or lost cells permits growth allows for
Cell Growth and Reproduction. Page 201
 Cell Growth and Reproduction Page 201 Describe cells that make up a multicellular organisms Comes in wide variety of shapes and sizes Name the cell with the largest diameter. Yolk of an ostrich egg Name
Cell Growth and Reproduction Page 201 Describe cells that make up a multicellular organisms Comes in wide variety of shapes and sizes Name the cell with the largest diameter. Yolk of an ostrich egg Name
Cell Division. Introduction. Chromatin, Chromosomes, and Chromatids, Oh My! The Cell Cycle
 Introduction Cell Division Just like a butterfly passes through different phases (such as caterpillar, chrysalis, and adult butterfly) there are a series of phases in a cell's life as it gets ready to
Introduction Cell Division Just like a butterfly passes through different phases (such as caterpillar, chrysalis, and adult butterfly) there are a series of phases in a cell's life as it gets ready to
MITOSIS AND THE CELL CYCLE PowerPoint Notes
 1 Name: Date: MITOSIS AND THE CELL CYCLE PowerPoint Notes THE FUNCTIONS OF CELL DIVISION 1. Cell division is vital for all. This is the process that can create. 2. Cell Division always involves main processes:
1 Name: Date: MITOSIS AND THE CELL CYCLE PowerPoint Notes THE FUNCTIONS OF CELL DIVISION 1. Cell division is vital for all. This is the process that can create. 2. Cell Division always involves main processes:
Biology is the only subject in which multiplication is the same thing as division
 Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division Ch. 10 Where it all began You started as a cell smaller than a period
Biology is the only subject in which multiplication is the same thing as division 2007-2008 The Cell Cycle: Cell Growth, Cell Division Ch. 10 Where it all began You started as a cell smaller than a period
Cell Division Mitosis Notes
 Cell Division Mitosis Notes Cell Division process by which a cell divides into 2 new cells Why do cells need to divide? 1.Living things grow by producing more cells, NOT because each cell increases in
Cell Division Mitosis Notes Cell Division process by which a cell divides into 2 new cells Why do cells need to divide? 1.Living things grow by producing more cells, NOT because each cell increases in
Chapter 8. The Cellular Basis of Reproduction and Inheritance. Lecture by Mary C. Colavito
 Chapter 8 The Cellular Basis of Reproduction and Inheritance PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education,
Chapter 8 The Cellular Basis of Reproduction and Inheritance PowerPoint Lectures for Biology: Concepts & Connections, Sixth Edition Campbell, Reece, Taylor, Simon, and Dickey Copyright 2009 Pearson Education,
