FEP Medical Policy Manual
|
|
|
- Silvia Lucas
- 6 years ago
- Views:
Transcription
1 FEP Medical Policy Manual FEP Oncologic Applications of Positron Emission Tomography Scanning Effective Date: October 15, 2017 Related Policies: Miscellaneous (Noncardiac, Nononcologic) Applications of Positron Emission Tomography Cardiac Applications of Positron Emission Tomography Scanning Interim Positron Emission Tomography Scanning in Oncology to Detect Early Response During Treatment Oncologic Applications of Positron Emission Tomography Scanning Description Positron emission tomography (PET) scans are based on the use of positron-emitting radionuclide tracers coupled to organic molecules, such as glucose, ammonia, or water. The radionuclide tracers simultaneously emit 2 high-energy photons in opposite directions that can be simultaneously detected (referred to as coincidence detection) by a PET scanner, comprising multiple stationary detectors that encircle the area of interest. FDA REGULATORY STATUS The FDA website includes various PET-related documents. As of June 2016, the following radiopharmaceuticals have been granted FDA-approval, to be used with PET for carcinoma-related indications: Carbon-11 choline- Suspected prostate cancer recurrence based on elevated blood PSA after therapy and noninformative bone scintigraphy, CT, or MRI Fluorine- fluorodeoxyglucose- Suspected or existing diagnosis of cancer, all types Fluorine- fluciclovine- Suspected prostate cancer recurrence based on elevated blood PSA levels after treatment Gallium-68 dotatate- Localization of somatostatin receptor positive NETs in adult and pediatric patients POLICY STATEMENT All policy statements apply to both positron emission tomography (PET) scans and PET plus computed tomography (CT) scans, i.e., PET scans with or without PET/CT fusion. For the clinical situations indicated that may be considered medically necessary, this assumes that the results of the PET scan will influence treatment decisions. If the results will not influence treatment decisions, these situations would be considered not medically necessary. Original Policy Date: June 2012 Page: 1
2 Effective Policy Date: October 15, 2017 Page: 2 of 19 BONE CANCER PET scanning may be considered medically necessary in the staging or restaging of Ewing sarcoma and osteosarcoma. PET scanning is considered not medically necessary in the staging of chondrosarcoma. BRAIN CANCER PET scanning may be considered medically necessary in the staging or restaging of brain cancer. BREAST CANCER PET scanning may be considered medically necessary in the staging or restaging of breast cancer for the following application: Detecting locoregional or distant recurrence or metastasis (except axillary lymph nodes) when suspicion of disease is high and other imaging is inconclusive PET scanning is considered not medically necessary in the evaluation of breast cancer for all other applications, including but not limited to the following: Differential diagnosis in patients with suspicious breast lesions or an indeterminate or low suspicion finding on mammography Staging axillary lymph nodes Predicting pathologic response to neoadjuvant therapy for locally advanced disease CERVICAL CANCER PET scanning may be considered medically necessary in the initial staging of patients with locally advanced cervical cancer. PET scanning may be considered medically necessary in the evaluation of known or suspected recurrence. COLORECTAL CANCER PET scanning may be considered medically necessary as a technique for: Staging or restaging to detect and assess resectability of hepatic or extrahepatic metastases of colorectal cancer, and To evaluate a rising and persistently elevated carcinoembryonic antigen (CEA) level when standard imaging, including CT scan, is negative PET scanning is considered not medically necessary as: A technique to assess the presence of scarring versus local bowel recurrence in patients with previously resected colorectal cancer A technique contributing to radiotherapy treatment planning ENDOMETRIALCANCER PET scanning is considered medically necessary in the: Detection of lymph node metastases, and Assessment of endometrial cancer recurrence ESOPHAGEAL CANCER PET scanning may be considered medically necessary in the Staging of esophageal cancer, and Determining response to preoperative induction therapy
3 Effective Policy Date: October 15, 2017 Page: 3 of 19 PET scanning is considered not medically necessary in other aspects of the evaluation of esophageal cancer, including but not limited to the following applications: Detection of primary esophageal cancer. GASTRIC CANCER PET scanning may be considered medically necessary in the: Initial diagnosis and staging of gastric cancer, and Evaluation for recurrent gastric cancer after surgical resection, when other imaging modalities are inconclusive. HEAD AND NECK CANCER PET scanning may be considered medically necessary in the evaluation of head and neck cancer in the: Initial diagnosis of suspected cancer, Initial staging of disease, and restaging of residual or recurrent disease during follow-up, and Evaluation of response to treatment LUNG CANCER PET scanning may be considered medically necessary for any of the following applications: Patients with a solitary pulmonary nodule as a single scan technique (not dual-time) to distinguish between benign and malignant disease when prior CT scan and chest x-ray findings are inconclusive or discordant, As staging or restaging technique in those with known non-small-cell lung cancer, and To determine resectability for patients with a presumed solitary metastatic lesion from lung cancer PET scanning is considered not medically necessary in staging of small-cell lung cancer LYMPHOMA, INCLUDING HODGKIN DISEASE PET scanning may be considered medically necessary as a technique for staging lymphoma either during initial staging or for restaging at follow-up MELANOMA PET scanning may be considered medically necessary as a technique for assessing extranodal spread of malignant melanoma at initial staging or at restaging during follow-up treatment for advanced disease (stage III or IV). PET scanning is considered not medically necessary in managing stage 0, I, or II melanoma PET scanning is considered not medically necessary as a technique to detect regional lymph node metastases in patients with clinically localized melanoma who are candidates to undergo sentinel node biopsy MULTIPLE MYELOMA PET scanning is considered not medically necessary in all aspects of managing multiple myeloma NEUOENDOCRINE TUMORS PET scanning is considered not medically necessary in all aspects of managing neuroendocrine tumors OVARIAN CANCER PET scanning may be considered medically necessary in the evaluation of patients with signs and/or symptoms of suspected ovarian cancer recurrence (restaging) when standard imaging, including CT scan, is inconclusive
4 Effective Policy Date: October 15, 2017 Page: 4 of 19 PET scanning is considered not medically necessary in the initial evaluation of known or suspected ovarian cancer in all situations PANCREATIC CANCER PET scanning may be considered medically necessary in the initial diagnosis and staging of pancreatic cancer when other imaging and biopsy are inconclusive PET scanning is considered not medically necessary as a technique to evaluate other aspects of pancreatic cancer PENILE CANCER PET scanning is considered not medically necessary in all aspects of managing penile cancer PROSTATE CANCER PET scanning with 11C-choline may be medically necessary for evaluating response to primary treatment in prostate cancer. PET scanning with 68Gallium is considered not medically necessary in all aspects of managing prostate cancer PET scanning for all other indications in known or suspected prostate cancer is considered not medically necessary RENAL CELL CARCINOMA PET scanning is considered not medically necessary in all aspects of managing renal cancer SOFT TISSUE SARCOMA PET scanning is considered not medically necessary in evaluation of soft tissue sarcoma, including but not limited to the following applications: Distinguishing between benign lesions and malignant soft tissue sarcoma, Distinguishing between low-grade and high-grade soft tissue sarcoma, Detecting locoregional recurrence, Detecting distant metastasis, and Evaluating response to imatinib and other treatments for gastrointestinal stromal tumors TESTICULAR CANCER PET scanning may be considered medically necessary in evaluation of residual mass following chemotherapy of stage IIB and III seminomas. (The scan should be completed no sooner than 6 weeks after chemotherapy.) Except as noted above for seminoma, PET scanning is considered not medically necessary in evaluation of testicular cancer, including but not limited to the following applications: Initial staging of testicular cancer, Distinguishing between viable tumor and necrosis/fibrosis after treatment of testicular cancer, and Detection of recurrent disease after treatment of testicular cancer THYROID CANCER PET scanning may be considered medically necessary in the restaging of patients with differentiated thyroid cancer when thyroglobulin levels are elevated and whole-body iodine-131 imaging is negative.
5 Effective Policy Date: October 15, 2017 Page: 5 of 19 PET scanning is considered not medically necessary in the evaluation of known or suspected differentiated or poorly differentiated thyroid cancer in all other situations UNKNOWN PRIMARY PET scanning may be considered medically necessary in patients with an unknown primary who meet ALL of the following criteria: In patients with a single site of disease outside the cervical lymph nodes, and Patient is considering local or regional treatment for a single site of metastatic disease, and After a negative workup for an occult primary tumor, and PET scan will be used to rule out or detect additional sites of disease that would eliminate the rationale for local or regional treatment PET scanning is considered not medically necessary for other indications in patients with an unknown primary, including, but not limited to the following: As part of the initial workup of an unknown primary, and As part of the workup of patients with multiple sites of disease CANCER SURVEILLANCE PET scanning is considered not medically necessary when used as a surveillance tool for patients with cancer or with a history of cancer. A scan is considered surveillance if performed more than 6 months after completion of cancer therapy (12 months for lymphoma) in patients without objective signs or symptoms suggestive of cancer recurrence (see Policy Guidelines section). POLICY GUIDELINES PATIENT SELECTION As with any imaging technique, the medical necessity of positron emission tomography (PET) scanning depends in part on what imaging techniques are used before or after the PET scanning. Due to its expense, PET scanning is typically considered after other techniques, such as computed tomography (CT), magnetic resonance imaging (MRI), or ultrasonography, provide inconclusive or discordant results. In patients with melanoma or lymphoma, PET scanning may be considered an initial imaging technique. If so, the medical necessity of subsequent imaging during the same diagnostic evaluation is unclear. Thus, PET should be considered for the medically necessary indications above only when standard imaging (eg, CT, MRI) is inconclusive or not indicated. Patient selection criteria for PET scanning also may be complex. For example, it may be difficult to determine from claims data whether a PET scan in a patient with malignant melanoma is being done primarily to evaluate extranodal disease or regional lymph nodes. Similarly, it may be difficult to determine whether a PET scan in a patient with colorectal cancer is being performed to detect hepatic disease or evaluate local recurrence. Due to the complicated hierarchy of imaging options in patients with malignancy and complex patient selection criteria, a possible implementation strategy for this policy is its use for retrospective review, possibly focusing on cases with multiple imaging tests, including PET scans. Use of PET scanning for surveillance as described in the policy statement and policy rationale refers to the use of PET to detect disease in asymptomatic patients at various intervals. This is not the same as the use of PET for detecting recurrent disease in symptomatic patients; these applications of PET are considered within tumor-specific categories in the policy statements.
6 Effective Policy Date: October 15, 2017 Page: 6 of 19 BENEFIT APPLICATION Services, drugs, or supplies that are not medically necessary are not covered (See General Exclusion Section of brochure). RATIONALE Summary of Evidence Bone Sarcoma For individuals who have suspected or diagnosed bone sarcoma and in need of staging or restaging information who receive F-FDG-PET or F-FDG-PET/CT, the evidence includes systematic reviews and meta-analyses of many studies. Relevant outcomes are test accuracy and test validity. Pooled analyses have shown that PET or PET/CT can effectively diagnose and stage bone cancer. PET or PET/CT has high sensitivities and specificities in detecting metastases in bone and lymph nodes; however, the tests have low sensitivity in detecting lung metastases. Clinical guidelines include PET and CT to inform management decisions that may offer clinical benefit. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who are asymptomatic after completing bone sarcoma treatment who receive F-FDGPET or F-FDG-PET/CT, there is no evidence. Relevant outcomes are test accuracy and test validity. The evidence is insufficient to determine the effects of the technology on health outcomes. Brain Tumors For individuals who have diagnosed brain tumors and in need of staging or restaging information or who have suspected brain cancer or are asymptomatic after completing brain cancer treatment who receive F-FDG-PET, F-FET-PET, or 11C-methionine PET, the evidence includes several systematic reviews and meta-analyses. Relevant outcomes are test accuracy and test validity. Pooled analyses have shown that PET or PET/CT can be effective in distinguishing brain tumors from normal tissue. Indirect comparisons between the radiotracers 11C-methionine and F-FDG have shown that 11C-methionine may have better diagnostic performance. Clinical guidelines include PET to inform management decisions that may offer clinical benefit. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. Breast Cancer For individuals who have diagnosed breast cancer and inconclusive results from other imaging techniques who receive adjunctive F-FDG-PET or F-FDG-PET/CT for stating or restaging, the evidence includes meta-analyses. Relevant outcomes are test accuracy and test validity. While studies included in the meta-analyses report variability in estimates of sensitivity and specificity, F-FDG-PET or F-FDG- PET/CT may be helpful in situations in which standard staging results are equivocal or suspicious, particularly in patients with locally advanced or metastatic disease. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who have suspected or diagnosed breast cancer and in need of staging or restaging information who receive F-FDG-PET or F-FDG-PET/CT, the evidence includes a TEC Assessment, several systematic reviews, and meta-analyses. Relevant outcomes are test accuracy and test validity. There is no evidence supporting the use of PET in diagnosing breast cancer. The false-negative rates (5.5%-8.5%) using PET in patients with breast cancer can be considered unacceptable, given that breast biopsy can provide more definitive results. PET/CT may be considered for detection of metastases only when results from other imaging techniques are inconclusive. The evidence is insufficient to determine the effects of the technology on health outcomes.
7 Effective Policy Date: October 15, 2017 Page: 7 of 19 For individuals who are asymptomatic after completing breast cancer treatment who receive F-FDGPET or F-FDG-PET/CT, there is no evidence. Relevant outcomes are test accuracy and test validity. The evidence is insufficient to determine the effects of the technology on health outcomes. Cervical Cancer For individuals who have diagnosed cervical cancer and in need of staging or restaging information who receive F-FDG-PET or F-PET/CT, the evidence includes an Agency for Healthcare Research and Quality (AHRQ) report and a meta-analysis. Relevant outcomes are test accuracy and test validity. Pooled results show that PET can be used for staging or restaging and for detection of recurrent disease. Clinical guidelines include PET and CT to inform management decisions that may offer clinical benefit. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who have suspected cervical cancer or who are asymptomatic after completing cervical cancer treatment who receive F-FDG-PET or F-FDG-PET/CT, there is no evidence. Relevant outcomes are test accuracy and test validity. The evidence is insufficient to determine the effects of the technology on health outcomes. Colorectal Cancer For individuals who have diagnosed colorectal cancer and in need of staging or restaging information who receive F-FDG-PET or F-PET/CT, the evidence includes a TEC Assessment and several metaanalyses. Relevant outcomes are test accuracy and test validity. Several pooled analyses evaluating staging or restaging using PET or PET/CT resulted in wide ranges of sensitivities and specificities, from the low 60s to the high 90s. The evidence is insufficient to determine the effects of the technology on health outcomes. For individuals who have suspected colorectal cancer or who are asymptomatic after completing colorectal cancer treatment who receive F-FDG-PET or F-PET/CT, the evidence includes a TEC Assessment and meta-analysis. Relevant outcomes are test accuracy and test validity. A meta-analysis evaluating the diagnostic accuracy of PET or PET/CT showed a high sensitivity but low specificity. The evidence for the use of PET or PET/CT does not show a benefit over the use of contrast CT in patients with colorectal cancer. The evidence is insufficient to determine the effects of the technology on health outcomes. Endometrial Cancer For individuals who have diagnosed endometrial cancer in need of staging or restaging information or who are asymptomatic after completing endometrial cancer treatment who receive F-FDG-PET or FPET/CT, the evidence includes a systematic review and meta-analysis. Relevant outcomes are test accuracy and test validity. Pooled estimates from the meta-analysis showed high sensitivities and specificities for F-FDG-PET/CT in detecting lymph node metastases and endometrial cancer recurrence following treatment. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. Esophageal Cancer For individuals who have diagnosed esophageal cancer and in need of staging or restaging information who receive F-FDG-PET or F-PET/CT, the evidence includes several meta-analyses. Relevant outcomes are test accuracy and test validity. Pooled estimates have shown high sensitivities and specificities compared to other diagnostic imaging techniques. Clinical guidelines include PET and CT to inform management decisions that may offer clinical benefit. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome.
8 Effective Policy Date: October 15, 2017 Page: 8 of 19 For individuals who have suspected esophageal cancer or who are asymptomatic after completing esophageal cancer treatment who receive F-FDG-PET or F-FDG-PET/CT, the evidence includes metaanalyses. Relevant outcomes are test accuracy and test validity. Pooled analyses showed adequate sensitivities but low specificities. The evidence is insufficient to determine the effects of the technology on health outcomes. Gastric Cancer For individuals who have suspected or diagnosed gastric cancer and in need of staging or restaging information, who receive F-FDG-PET or F-PET/CT, the evidence includes several meta-analyses. Relevant outcomes are test accuracy and test validity. Pooled analyses, with sensitivities and specificities ranging from the high 70s to the high 80s, have shown that PET or PET/CT can inform staging or restaging of patients with gastric cancer. Clinical guidelines include PET/CT to inform management decisions that may offer clinical benefit. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who are asymptomatic after completing gastric cancer treatment who receive F-FDGPET or F-FDG-PET/CT, the evidence includes meta-analyses. Relevant outcomes are test accuracy and test validity. Pooled analyses showed low sensitivities and specificities. The evidence is insufficient to determine the effects of the technology on health outcomes. Head and Neck Cancer For individuals who have suspected or diagnosed head and neck cancer who need staging or restaging information who receive F-FDG-PET or F-PET/CT, the evidence includes a TEC Assessment and several meta-analyses. Relevant outcomes are test accuracy and test validity. In patients with head and neck cancers, PET and PET/CT are better able to detect local and metastatic disease compared with other imaging techniques. Evidence has also shown that F-FDG-PET/CT may be useful in predicting response to therapy. Two meta-analyses calculated the ability of F-FDG-PET or PET/CT to detect residual or recurrent disease during various stages of treatment and another meta-analysis calculated the ability of positive PET or PET/CT results to predict overall survival and event-free survival. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who are asymptomatic after completing head and neck cancer treatment who receive FFDG-PET or F-FDG-PET/CT, there is no evidence. Relevant outcomes are test accuracy and test validity. The evidence is insufficient to determine the effects of the technology on health outcomes. Non-Small-Cell Lung Cancer For individuals who have suspected non-small-cell lung cancer and inconclusive results from other imaging techniques or who have diagnosed non-small cell lung cancer and in need of staging or restaging information who receive F-FDG-PET or F-PET/CT, the evidence includes several meta-analyses. Relevant outcomes are test accuracy and test validity. Pooled analyses have shown that PET and PET/CT have better diagnostic performance compared with conventional imaging techniques. Clinical guidelines include PET/CT to inform management decisions that may offer clinical benefit. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who have suspected non-small-cell lung cancer or who are asymptomatic after completing non-small-cell lung cancer treatment who receive F-FDG-PET or F-FDG-PET/CT, there is no evidence. Relevant outcomes are test accuracy and test validity. The evidence is insufficient to determine the effects of the technology on health outcomes.
9 Effective Policy Date: October 15, 2017 Page: 9 of 19 Small-Cell Lung Cancer For individuals with diagnosed small-cell lung cancer and in need of staging or restaging information who receive F-FDG-PET or F-PET/CT, the evidence includes a systematic review and a meta-analysis. Relevant outcomes are test accuracy and test validity. While the quality of the studies was considered low, PET and PET/CT can be considered for staging or restaging in patients with small-cell lung cancer. Clinical guidelines include PET/CT to inform management decisions that may offer clinical benefit. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who have suspected small-cell lung cancer or who are asymptomatic after completing small-cell lung cancer treatment who receive F-FDG-PET or F-FDG-PET/CT, there is no evidence. Relevant outcomes are test accuracy and test validity. The evidence is insufficient to determine the effects of the technology on health outcomes. Hodgkin and Non-Hodgkin Lymphoma For individuals who have suspected or diagnosed Hodgkin and non-hodgkin lymphoma in need of staging or restaging information who receive F-FDG-PET or PET/CT, the evidence includes a TEC Assessment and several meta-analyses. Relevant outcomes are test accuracy and test validity. PET and PET/CT have been found to provide useful information in the management of Hodgkin and non-hodgkin lymphoma. The Deauville 5-point scale was developed based on PET results and can be used for staging and treatment response for patients with lymphoma. Clinical guidelines include PET/CT to inform management decisions that may offer clinical benefit. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who are asymptomatic after completing Hodgkin lymphoma treatment who receive FFDG-PET or F-FDG-PET/CT, there is no evidence. Relevant outcomes are test accuracy and test validity. The evidence is insufficient to determine the effects of the technology on health outcomes. Melanoma For individuals who have suspected or diagnosed stage I or II melanoma and in need of staging or restaging information who receive F-FDG-PET or F-PET/CT, the evidence includes a TEC Assessment. Relevant outcomes are test accuracy and test validity. Evidence has shown PET and PET/CT are not as beneficial as the reference standard (sentinel node biopsy) for assessing regional lymph nodes. The evidence is insufficient to determine the effects of the technology on health outcomes. For individuals who have diagnosed advanced melanoma (stage III or IV) and in need of staging for restaging information who receive F-FDG-PET or F-PET/CT, the evidence includes a TEC Assessment and a meta-analysis. Relevant outcomes are test accuracy and test validity. Evidence has shown PET and PET/CT can detect systemic metastases in patients with advanced melanoma. Clinical guidelines include PET/CT for staging or restaging stage III or IV disease and for surveillance. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who are asymptomatic after completing melanoma treatment who receive F-FDG-PET or F-FDG-PET/CT, the evidence includes retrospective and observational studies. Relevant outcomes are test accuracy and test validity. At the discretion of the physician, imaging surveillance can be considered every 3 to 12 months. Since recurrences usually occur within 3 years, screening asymptomatic patients beyond 3 to 5 years is not recommended. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome.
10 Effective Policy Date: October 15, 2017 Page: 10 of 19 Multiple Myeloma For individuals who have suspected or diagnosed multiple myeloma in need of staging or restaging information who receive F-FDG-PET or F-PET/CT, the evidence includes 2 systematic reviews. Relevant outcomes are test accuracy and test validity. The evidence did not compare PET or PET/CT with other modalities and, therefore, did not provide comparative effectiveness information. The evidence is insufficient to determine the effects of the technology on health outcomes. For individuals who are asymptomatic after completing multiple myeloma treatment who receive F- FDGPET or F-FDG-PET/CT, there is no evidence. Relevant outcomes are test accuracy and test validity. The evidence is insufficient to determine the effects of the technology on health outcomes. Neuroendocrine Tumors For individuals who have suspected or diagnosed neuroendocrine tumors and in need of staging or restaging information or who are asymptomatic after completing neuroendocrine tumor treatment who receive F-FDG-PET, F-PET/CT, 68Ga-PET, or 68Ga-PET/CT, the evidence includes 2 meta-analyses. Relevant outcomes are test accuracy and test validity. The evidence did not compare PET, PET/CT, Ga- PET, or Ga-PET/CT with other modalities and, therefore, did not provide comparative effectiveness information. The evidence is insufficient to determine the effects of the technology on health outcomes. Ovarian Cancer For individuals who have diagnosed ovarian cancer and in need of staging or restaging information who receive F-FDG-PET or F-PET/CT, the evidence includes an AHRQ systematic review and several meta-analyses. Relevant outcomes are test accuracy and test validity. Pooled sensitivities and specificities have supported the use of PET and PET/CT for the detection of recurrent ovarian cancer. Clinical guidelines include PET/CT to inform management decisions that may offer clinical benefit. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who have suspected ovarian cancer or who are asymptomatic after completing ovarian cancer treatment who receive F-FDG-PET or F-FDG-PET/CT, there is no evidence. Relevant outcomes are test accuracy and test validity. The evidence is insufficient to determine the effects of the technology on health outcomes. Pancreatic Cancer For individuals who have suspected or diagnosed pancreatic cancer and with inconclusive results from other imaging techniques who receive adjunctive F-FDG-PET or F-FDG-PET/CT for staging or restaging, the evidence includes a TEC Assessment and a systematic review. Relevant outcomes are test accuracy and test validity. The evidence has shown that PET and PET/CT do not have a high enough negative predictive value to surpass current standard decision thresholds. Therefore PET or PET/CT should only be considered if results from standard staging methods are inconclusive. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who have suspected or diagnosed pancreatic cancer and in need of staging or restaging information who receive F-FDG-PET or F-PET/CT, the evidence includes an AHRQ systematic review, a TEC Assessment, and a meta-analysis published after the review and assessment. Relevant outcomes are test accuracy and test validity. The evidence has shown that PET and PET/CT do not have a high enough negative predictive value to surpass current standard decision thresholds. Therefore PET or PET/CT should only be considered if results from standard staging methods are inconclusive. The evidence is insufficient to determine the effects of the technology on health outcomes.
11 Effective Policy Date: October 15, 2017 Page: 11 of 19 For individuals who are asymptomatic after completing pancreatic cancer treatment who receive F- FDGPET or F-FDG-PET/CT, there is no evidence. Relevant outcomes are test accuracy and test validity. The evidence is insufficient to determine the effects of the technology on health outcomes. Penile Cancer For individuals who have suspected or diagnosed penile cancer and in need of staging or restaging information who receive F-FDG-PET or F-PET/CT, the evidence includes a systematic review and a meta-analysis. Relevant outcomes are test accuracy and test validity. The evidence has shown that PET had a low sensitivity, and no comparisons were made with other modalities. The evidence is insufficient to determine the effects of the technology on health outcomes. For individuals who are asymptomatic after completing penile cancer treatment who receive F-FDG-PET or F-FDG-PET/CT, there is no evidence. Relevant outcomes are test accuracy and test validity. The evidence is insufficient to determine the effects of the technology on health outcomes. Prostate Cancer For individuals who have suspected or diagnosed prostate cancer and in need of staging or restaging information who receive 11C-choline PET or 11C-choline PET/CT, evidence includes several metaanalyses. Relevant outcomes are test accuracy and test validity. Meta-analyses have reported that the choice of radiotracer affects the sensitivity and specificity of the scans, with most evidence showing that the use of 11C-choline results in the highest sensitivities and specificities compared with F-FDG-PET and 11C-acetate. Of interest is a single study that investigated the use of PET/CT results to inform patient decisions on radiotherapy treatment plans. The study reported that 40% of the patients altered the extent of the treatment planned based on the PET/CT results. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who are asymptomatic after completing prostate cancer treatment who receive 11C-choline PET or 11C-choline PET/CT, there is no evidence. Relevant outcomes are test accuracy and test validity. The evidence is insufficient to determine the effects of the technology on health outcomes. For individuals who have suspected or diagnosed prostate cancer and in need of staging or restaging information who receive 68Ga-PET or 68Ga-PET/CT, the evidence includes a meta-analysis of small single-institution studies. Relevant outcomes are test accuracy and test validity. The evidence was limited, resulting in estimates with large confidence intervals. The evidence is insufficient to determine the effects of the technology on health outcomes. Renal Cell Carcinoma For individuals who are diagnosed renal cell carcinoma and in need of staging or restaging information who receive F-FDG-PET or F-PET/CT, the evidence includes a systematic review and meta-analysis. Relevant outcomes are test accuracy and test validity. The review concluded that PET has potential to detect metastatic or recurrent lesions in patients with renal cell cancer, but that additional prospective studies are needed. The evidence is insufficient to determine the effects of the technology on health outcomes. Soft Tissue Sarcoma For individuals who have diagnosed soft tissue sarcoma and in need of staging or restaging information who receive F-FDG-PET or F-PET/CT, the evidence includes an AHRQ systematic review. Another systematic review evaluated PET for assessing response to imatinib. Relevant outcomes are test accuracy and test validity. The review reported that PET had low diagnostic accuracy and there was a lack of studies comparing PET with alternative diagnostic modalities. The evidence is insufficient to determine the effects of the technology on health outcomes.
12 Effective Policy Date: October 15, 2017 Page: 12 of 19 For individuals with diagnosed soft tissue sarcoma and in need of rapid reading of activity following imatinib treatment who receive F-FDG-PET or F-PET/CT, the evidence includes a systematic review. Relevant outcomes are test accuracy and test validity. The review concluded that PET/CT can be used to monitor treatment response to imatinib, which can lead to individually adapted treatment strategies. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who have soft tissue sarcoma or who are asymptomatic after completing soft tissue sarcoma treatment who receive F-FDG-PET or F-FDG-PET/CT, the evidence includes a systematic review. Relevant outcomes are test accuracy and test validity. The review concluded that there was insufficient evidence on the use of PET for detection of loco-regional recurrence. The evidence is insufficient to determine the effects of the technology on health outcomes. Testicular Cancer For individuals with diagnosed testicular cancer in need of staging or restaging information who receive F-FDG-PET or F-PET/CT, the evidence includes an AHRQ systematic review and assessment. Relevant outcomes are test accuracy and test validity. Results have shown that PET or PET/CT can evaluate residual masses following chemotherapy for seminoma. Clinical guidelines include PET/CT to inform management decisions that may offer clinical benefit. There is no evidence supporting the use of PET or PET/CT in nonseminoma patients. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who have suspected testicular cancer or who are asymptomatic after completing testicular cancer treatment who receive F-FDG-PET or F-FDG-PET/CT, there is no evidence. Relevant outcomes are test accuracy and test validity. The evidence is insufficient to determine the effects of the technology on health outcomes. Thyroid Cancer For individuals with diagnosed thyroid cancer and in need of staging or restaging information who receive F-FDG-PET or F-PET/CT, the evidence includes systematic reviews and meta-analyses. Relevant outcomes are test accuracy and test validity. Pooled analyses have shown that PET or PET/CT can effectively detect recurrent differentiated thyroid cancer. Clinical guidelines include PET/CT to inform management decisions that may offer clinical benefit. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome. For individuals who have suspected thyroid cancer or who are asymptomatic after completing thyroid cancer treatment who receive F-FDG-PET or F-FDG-PET/CT, there is no evidence. Relevant outcomes are test accuracy and test validity. The evidence is insufficient to determine the effects of the technology on health outcomes. Unknown Primary and Single-Site Metastatic Disease For individuals with unknown primary and single-site metastatic disease who receive F-FDG-PET or FPET/CT, the evidence includes a TEC Assessment. Relevant outcomes are test accuracy and test validity. Studies reviewed in the Assessment showed that PET identified previously undetected metastases confirmed by biopsy. PET can contribute to the management of patients with unknown primary. Clinical guidelines include PET/CT to inform management decisions that may offer clinical benefit. The evidence is sufficient to determine that the technology results in a meaningful improvement in the net health outcome.
13 Effective Policy Date: October 15, 2017 Page: 13 of 19 SUPPLEMENTAL INFORMATION Not applicable. U.S. Preventive Services Task Force Recommendations Medicare National Coverage There is no national coverage determination (NCD). In the absence of an NCD, coverage decisions are left to the discretion of local Medicare carriers. REFERENCES 1. Food and Drug Administration. Positron Emission Tomography (PET). 2016; Accessed April 27, 2. CardinalHealth. FDA-Approved Radiopharmaceuticals. 2016; FDAApprovedRadiopharmaceuticalsandApprovedUses.pdf. Accessed April 17, 3. Blue Cross Blue Shield Association Technology Evaluation Center (TEC). FDG Positron Emission Tomography for Non-CNS Cancers. Technol Eval Cent Assess. 1997;12(3). 4. Seidenfeld J, Samson DJ, Bonnell CJ, et al. Management of small cell lung cancer. Evid Rep Technol Assess (Full Rep) Jul 2006(143): Blue Cross Blue Shield Association Technology Evaluation Center (TEC). FDG Positron Emission Tomography in Melanoma. Technol Eval Cent Assess. 1999;14(27). 6. Blue Cross Blue Shield Association Technology Evaluation Center (TEC). FDG Positron Emission Tomography in Lymphoma. Technol Eval Cent Assess. 1999;14(26). 7. Blue Cross Blue Shield Association Technology Evaluation Center (TEC). FDG Positron Emission Tomography in Colorectal Cancer. Technol Eval Cent Assess. 1999;14(25). 8. Blue Cross Blue Shield Association Technology Evaluation Center (TEC). FDG Positron Emission Tomography in Head and Neck Cancer. Technol Eval Cent Assess. 2000;15(4). 9. Blue Cross Blue Shield Association Technology Evaluation Center (TEC). FDG Positron Emission Tomography to Manage Patients with an Occult Primary Carcinoma and Metastasis outside the Cervical Lymph Nodes. Technol Eval Cent Assess. 2002;17(14). 10. Blue Cross Blue Shield Association Technology Evaluation Center (TEC). FDG Positron Emission Tomography for Evaluating Esophageal Cancer. Technol Eval Cent Assess. 2001;16(21). 11. Wallace MB, Nietert PJ, Earle C, et al. An analysis of multiple staging management strategies for carcinoma of the esophagus: computed tomography, endoscopic ultrasound, positron emission tomography, and thoracoscopy/laparoscopy. Ann Thorac Surg. Oct 2002;74(4): PMID Westerterp M, van Westreenen HL, Reitsma JB, et al. Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy--systematic review. Radiology. Sep 2005;236(3): PMID Blue Cross Blue Shield Association Technology Evaluation Center (TEC). FDG Positron Emission Tomography in Pancreatic Cancer. Technol Eval Cent Assess. 1999;14(28). 14. Matchar DB, Kulasingam SL, Havrilesky L, et al. Positron Emission Testing for Six Cancers (Brain, Cervical, Small Cell Lung, Ovarian, Pancreatic and Testicular) (Contract No ). Rockville, MD: Agency for Healthcare Research and Quality; Ioannidis JPA, Lau J. FDG-PET for the Diagnosis and Management of Soft Tissue Sarcoma (Contract No ). Rockville, MD: Agency for Healthcare Research and Quality; Blue Cross Blue Shield Association Technology Evaluation Center (TEC). Positron Emission Tomography in Breast Cancer. Technol Eval Cent Assess. 2001;16(5). 17. Blue Cross Blue Shield Association Technology Evaluation Center (TEC). FDG Positron Emission Tomography for Evaluating Breast Cancer. Technol Eval Cent Assess. 2003; (14).. Isasi CR, Moadel RM, Blaufox MD. A meta-analysis of FDG-PET for the evaluation of breast cancer recurrence and metastases. Breast Cancer Res Treat. Mar 2005;90(2): PMID Sloka JS, Hollett PD, Mathews M. Cost-effectiveness of positron emission tomography in breast cancer. Mol Imaging Biol. Sep-Oct 2005;7(5): PMID
14 Effective Policy Date: October 15, 2017 Page: 14 of Sloka JS, Hollett PD, Mathews M. A quantitative review of the use of FDG-PET in the axillary staging of breast cancer. Med Sci Monit. Mar 2007; 13(3):RA PMID Podoloff DA, Advani RH, Allred C, et al. NCCN task force report: positron emission tomography (PET)/computed tomography (CT) scanning in cancer. J Natl Compr Canc Netw. May 2007;5 Suppl 1(suppl 1):S1-22; quiz S PMID Podoloff DA, Ball DW, Ben-Josef E, et al. NCCN task force: clinical utility of PETin a variety of tumor types. J Natl Compr Canc Netw. Jun 2009;7 Suppl 2(suppl 2):S1-26. PMID Ospina MB, Horton J, Seida J, et al. Positron emission tomography for nine cancers (bladder, brain, cervical, kidney, ovarian, pancreatic, prostate, small cell lung, testicular). Technology Assessment Report Project ID: PETC1207. Agency for Healthcare Research and Quality, PMID Dunet V, Rossier C, Buck A, et al. Performance of F-fluoro-ethyl-tyrosine ( F-FET) PET for the differential diagnosis of primary brain tumor: a systematic review and Metaanalysis. J Nucl Med. Feb 2012;53(2): PMID Cheng X, Bao L, Xu Z, et al. (1)(8)F-FDG-PET and (1)(8)F-FDG-PET/CT in the detection of recurrent or metastatic medullary thyroid carcinoma: a systematic review and meta-analysis. J Med Imaging Radiat Oncol. Apr 2012; 56(2): PMID Cheng X, Li Y, Liu B, et al. F-FDG PET/CT and PET for evaluation of pathological response to neoadjuvant chemotherapy in breast cancer: a meta-analysis. Acta Radiol. Jul 2012; 53(6): PMID Gwynne S, Mukherjee S, Webster R, et al. Imaging for target volume delineation in rectal cancer radiotherapy--a systematic review. Clin Oncol (R Coll Radiol). Feb 2012;24(1): PMID Lu YY, Chen JH, Lin WY, et al. FDG PET or PET/CT for detecting intramedullary and extramedullary lesions in multiple myeloma: a systematic review and meta-analysis. Clin Nucl Med. Sep 2012;37(9): PMID Ruben JD, Ball DL. The efficacy of PET staging for small-cell lung cancer: a systematic review and cost analysis in the Australian setting. J Thorac Oncol. Jun 2012; 7(6): PMID Sadeghi R, Gholami H, Zakavi SR, et al. Accuracy of F-FDG PET/CT for diagnosing inguinal lymph node involvement in penile squamous cell carcinoma: systematic review and meta-analysis of the literature. Clin Nucl Med. May 2012; 37(5): PMID Treglia G, Castaldi P, Rindi G, et al. Diagnostic performance of Gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and gastroenteropancreatic neuroendocrine tumours: a meta-analysis. Endocrine. Aug 2012;42(1): PMID Treglia G, Cocciolillo F, de Waure C, et al. Diagnostic performance of F-dihydroxyphenylalanine positron emission tomography in patients with paraganglioma: a meta-analysis. Eur J Nucl Med Mol Imaging. Jul 2012; 39(7): PMID Treglia G, Mirk P, Stefanelli A, et al. F-Fluorodeoxyglucose positron emission tomography in evaluating treatment response to imatinib or other drugs in gastrointestinal stromal tumors: a systematic review. Clin Imaging. May-Jun 2012; 36(3): PMID van Lammeren-Venema D, Regelink JC, Riphagen, II, et al. (1)(8)F-fluoro-deoxyglucose positron emission tomography in assessment of myeloma-related bone disease: a systematic review. Cancer. Apr ; 1 (8): PMID Wu LM, Hu JN, Hua J, et al. F-fluorodeoxyglucose positron emission tomography to evaluate recurrent gastric cancer: a systematic review and meta-analysis. J Gastroenterol Hepatol. Mar 2012; 27(3): PMID Barger RL, Jr., Nandalur KR. Diagnostic performance of dual-time F-FDG PET in the diagnosis of pulmonary nodules: a meta-analysis. Acad Radiol. Feb 2012;19(2): PMID Liu F, Zhang Q, Zhu D, et al. Performance of positron emission tomography and positron emission tomography/computed tomography using fluorine- -fluorodeoxyglucose for the diagnosis, staging, and recurrence assessment of bone sarcoma: a systematic review and meta-analysis. Medicine (Baltimore). Sep 2015; 94(36):e1462. PMID Treglia G, Salsano M, Stefanelli A, et al. Diagnostic accuracy of (1)(8)F-FDG-PET and PET/CT in patients with Ewing sarcoma family tumours: a systematic review and a meta-analysis. Skeletal Radiol. Mar 2012; 41(3): PMID Völker T, Denecke T, Steffen I, et al. Positron emission tomography for staging of pediatric sarcoma patients: results of a prospective multicenter trial. J Clin Oncol. Dec ;25(34): PMID National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Bone Cancer Version 2. Accessed April 17,
15 Effective Policy Date: October 15, 2017 Page: 15 of Dunet V, Pomoni A, Hottinger A, et al. Performance of F-FET versus F-FDG-PET for the diagnosis and grading of brain tumors: systematic review and meta-analysis. Neuro Oncol. Mar 2016; (3): PMID Zhao C, Zhang Y, Wang J. A meta-analysis on the diagnostic performance of ( )F-FDG and (11)C-methionine PET for differentiating brain tumors. AJNR Am J Neuroradiol. Jun 2014;35(6): PMID Deng SM, Zhang B, Wu YW, et al. Detection of glioma recurrence by (1)(1)C-methionine positron emission tomography and dynamic susceptibility contrast-enhanced magnetic resonance imaging: a meta-analysis. Nucl Med Commun. Aug 2013;3 4(8): PMID National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Central Nervous System Cancers. Version Caldarella C, Treglia G, Giordano A. Diagnostic performance of dedicated positron emission mammography using fluorine- -fluorodeoxyglucose in women with suspicious breast lesions: a meta-analysis. Clin Breast Cancer. Aug 2014; 14(4): PMID Hong S, Li J, Wang S. FDG PET-CT for diagnosis of distant metastases in breast cancer patients. A metaanalysis. Surg Oncol. Jun 2013;22(2): PMID Rong J, Wang S, Ding Q, et al. Comparison of FDG PET-CT and bone scintigraphy for detection of bone metastases in breast cancer patients. A meta-analysis. Surg Oncol. Jun 2013; 22(2): PMID Xiao Y, Wang L, Jiang X, et al. Diagnostic efficacy of F-FDG-PET or PET/CT in breast cancer with suspected recurrence: a systematic review and meta-analysis. Nucl Med Commun. Nov 2016; 37(11): PMID Liu Q, Wang C, Li P, et al. The role of ( )F-FDG PET/CT and MRI in assessing pathological complete response to neoadjuvant chemotherapy in patients with breast cancer: a systematic review and meta-analysis. Biomed Res Int. 2016; 2016: PMID Sheikhbahaei S, Trahan TJ, Xiao J, et al. FDG-PET/CT and MRI for evaluation of pathologic response to neoadjuvant chemotherapy in patients with breast cancer: a meta-analysis of diagnostic accuracy studies. Oncologist. Aug 2016;21(8): PMID Wang Y, Zhang C, Liu J, et al. Is F-FDG PET accurate to predict neoadjuvant therapy response in breast cancer? A meta-analysis. Breast Cancer Res Treat. Jan 2012;131(2): PMID National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 2. Accessed April 11, 53. Yen TC, See LC, Chang TC, et al. Defining the priority of using F-FDG PET for recurrent cervical cancer. J Nucl Med. Oct 2004;45(10): PMID Chu Y, Zheng A, Wang F, et al. Diagnostic value of F-FDG-PET or PET-CT in recurrent cervical cancer: a systematic review and meta-analysis. Nucl Med Commun. Feb 2014; 35(2): PMID National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Cervical Cancer. Version Accessed April 11, 56. Li C, Lan X, Yuan H, et al. F-FDG PET predicts pathological response to preoperative chemoradiotherapy in patients with primary rectal cancer: a meta-analysis. Ann Nucl Med. Jun 2014;28(5): PMID Maffione AM, Chondrogiannis S, Capirci C, et al. Early prediction of response by (1)(8)F-FDG PET/CT during preoperative therapy in locally advanced rectal cancer: a systematic review. Eur J Surg Oncol. Oct 2014;40(10): PMID Memon S, Lynch AC, Akhurst T, et al. Systematic review of FDG-PET prediction of complete pathological response and survival in rectal cancer. Ann Surg Oncol. Oct 2014; 21(11): PMID Jones M, Hruby G, Solomon M, et al. The role of FDG-PET in the initial staging and response assessment of anal cancer: a systematic review and meta-analysis. Ann Surg Oncol. Oct 2015; 22(11): PMID Ye Y, Liu T, Lu L, et al. Pre-operative TNM staging of primary colorectal cancer by ( )F-FDG PET-CT or PET: a meta-analysis including 2283 patients. Int J Clin Exp Med. 2015; 8(11): PMID Rymer B, Curtis NJ, Siddiqui MR, et al. FDG PET/CT can assess the response of locally advanced rectal cancer to neoadjuvant chemoradiotherapy: evidence from meta-analysis and systematic review. Clin Nucl Med. May 2016; 41(5): PMID Yu T, Meng N, Chi D, et al. Value of ( )F-FDG PET/CT in detecting local recurrent colorectal cancer: a pooled analysis of 26 individual studies. Cell Biochem Biophys. Jun 2015; 72(2): PMID Maffione AM, Marzola MC, Capirci C, et al. Value of ( )F-FDG PET for predicting response to neoadjuvant therapy in rectal cancer: systematic review and meta-analysis. AJR Am J Roentgenol. Jun 2015; 204(6): PMID
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 PET Scanning: Oncologic Applications Page 1 of 88 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Positron Emission Tomography (PET) Scanning: Oncologic Applications
PET Scanning: Oncologic Applications Page 1 of 88 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: Positron Emission Tomography (PET) Scanning: Oncologic Applications
Original Policy Date
 MP 6.01.17 Oncologic Applications of PET Scanning Medical Policy Section Radiology Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature/12:2013 Return to Medical
MP 6.01.17 Oncologic Applications of PET Scanning Medical Policy Section Radiology Issue 12:2013 Original Policy Date 12:2013 Last Review Status/Date Reviewed with literature/12:2013 Return to Medical
Page: 1 of 29. For this policy, PET scanning is discussed for the following 4 applications in oncology:
 Emission Tomography Scanning Page: 1 of 29 Last Review Status/Date: June 2015 Description Positron emission tomography (PET) scans are based on the use of positron-emitting radionuclide tracers coupled
Emission Tomography Scanning Page: 1 of 29 Last Review Status/Date: June 2015 Description Positron emission tomography (PET) scans are based on the use of positron-emitting radionuclide tracers coupled
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 PET Scanning: Oncologic Applications Page 1 of 42 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See also: Positron Emission Tomography (PET) Scanning: Oncologic
PET Scanning: Oncologic Applications Page 1 of 42 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See also: Positron Emission Tomography (PET) Scanning: Oncologic
Oncologic Applications of PET Scanning
 6.01.26 Oncologic Applications of PET Scanning Section 6.0 Radiology Subsection Effective Date February 15, 2015 Original Policy Date January 26, 2009 Next Review Date December 2015 Description Positron
6.01.26 Oncologic Applications of PET Scanning Section 6.0 Radiology Subsection Effective Date February 15, 2015 Original Policy Date January 26, 2009 Next Review Date December 2015 Description Positron
Positron Emission Tomography (PET) for Oncologic Conditions Medical Necessity Criteria
 Positron Emission Tomography (PET) for Oncologic Conditions Medical Necessity Criteria Positron Emission Tomography (PET) is a minimally invasive diagnostic imaging procedure using an injected radionuclide
Positron Emission Tomography (PET) for Oncologic Conditions Medical Necessity Criteria Positron Emission Tomography (PET) is a minimally invasive diagnostic imaging procedure using an injected radionuclide
MEDICAL POLICY SUBJECT: POSITRON EMISSION TOMOGRAPHY (PET) ONCOLOGIC APPLICATIONS. POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY PAGE: 1 OF: 13 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
MEDICAL POLICY PAGE: 1 OF: 13 If the member's subscriber contract excludes coverage for a specific service it is not covered under that contract. In such cases, medical policy criteria are not applied.
Dr Sneha Shah Tata Memorial Hospital, Mumbai.
 Dr Sneha Shah Tata Memorial Hospital, Mumbai. Topics covered Lymphomas including Burkitts Pediatric solid tumors (non CNS) Musculoskeletal Ewings & osteosarcoma. Neuroblastomas Nasopharyngeal carcinomas
Dr Sneha Shah Tata Memorial Hospital, Mumbai. Topics covered Lymphomas including Burkitts Pediatric solid tumors (non CNS) Musculoskeletal Ewings & osteosarcoma. Neuroblastomas Nasopharyngeal carcinomas
Index. Surg Oncol Clin N Am 16 (2007) Note: Page numbers of article titles are in boldface type.
 Surg Oncol Clin N Am 16 (2007) 465 469 Index Note: Page numbers of article titles are in boldface type. A Adjuvant therapy, preoperative for gastric cancer, staging and, 339 B Breast cancer, metabolic
Surg Oncol Clin N Am 16 (2007) 465 469 Index Note: Page numbers of article titles are in boldface type. A Adjuvant therapy, preoperative for gastric cancer, staging and, 339 B Breast cancer, metabolic
POSITRON EMISSION TOMOGRAPHY (PET)
 Status Active Medical and Behavioral Health Policy Section: Radiology Policy Number: V-27 Effective Date: 08/27/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
Status Active Medical and Behavioral Health Policy Section: Radiology Policy Number: V-27 Effective Date: 08/27/2014 Blue Cross and Blue Shield of Minnesota medical policies do not imply that members should
MEDICAL POLICY SUBJECT: POSITRON EMISSION TOMOGRAPHY (PET) ONCOLOGIC APPLICATIONS. POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: POSITRON EMISSION PAGE: 1 OF: 16 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an
MEDICAL POLICY SUBJECT: POSITRON EMISSION PAGE: 1 OF: 16 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including an
PET IMAGING (POSITRON EMISSION TOMOGRAPY) FACT SHEET
 Positron Emission Tomography (PET) When calling Anthem (1-800-533-1120) or using the Point of Care authorization system for a Health Service Review, the following clinical information may be needed to
Positron Emission Tomography (PET) When calling Anthem (1-800-533-1120) or using the Point of Care authorization system for a Health Service Review, the following clinical information may be needed to
Subject: PET Scan With or Without CT Attenuation. Original Effective Date: 11/7/2017. Policy Number: MCR: 610. Revision Date(s): Review Date:
 Subject: PET Scan With or Without CT Attenuation Policy Number: MCR: 610 Revision Date(s): MHW Original Effective Date: 11/7/2017 Review Date: DISCLAIMER This Molina Clinical Review (MCR) is intended to
Subject: PET Scan With or Without CT Attenuation Policy Number: MCR: 610 Revision Date(s): MHW Original Effective Date: 11/7/2017 Review Date: DISCLAIMER This Molina Clinical Review (MCR) is intended to
Clinical indications for positron emission tomography
 Clinical indications for positron emission tomography Oncology applications Brain and spinal cord Parotid Suspected tumour recurrence when anatomical imaging is difficult or equivocal and management will
Clinical indications for positron emission tomography Oncology applications Brain and spinal cord Parotid Suspected tumour recurrence when anatomical imaging is difficult or equivocal and management will
performed to help sway the clinician in what the appropriate diagnosis is, which can substantially alter the treatment of management.
 Hello, I am Maura Polansky at the University of Texas MD Anderson Cancer Center. I am a Physician Assistant in the Department of Gastrointestinal Medical Oncology and the Program Director for Physician
Hello, I am Maura Polansky at the University of Texas MD Anderson Cancer Center. I am a Physician Assistant in the Department of Gastrointestinal Medical Oncology and the Program Director for Physician
Molecular Imaging and Cancer
 Molecular Imaging and Cancer Cancer causes one in every four deaths in the United States, second only to heart disease. According to the U.S. Department of Health and Human Services, more than 512,000
Molecular Imaging and Cancer Cancer causes one in every four deaths in the United States, second only to heart disease. According to the U.S. Department of Health and Human Services, more than 512,000
Colorectal Cancer and FDG PET/CT
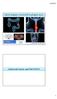 Hybrid imaging in colorectal & esophageal cancer Emmanuel Deshayes IAEA WorkShop, November 2017 Colorectal Cancer and FDG PET/CT 1 Clinical background Cancer of the colon and rectum is one of the most
Hybrid imaging in colorectal & esophageal cancer Emmanuel Deshayes IAEA WorkShop, November 2017 Colorectal Cancer and FDG PET/CT 1 Clinical background Cancer of the colon and rectum is one of the most
The Use of PET Scanning in Urologic Oncology
 The Use of PET Scanning in Urologic Oncology Dr Nicholas C. Buchan Uro-oncology Fellow 1 2 Aims To understand the basic concepts underlying PET scanning. Understand the emerging role of PET Scanning for
The Use of PET Scanning in Urologic Oncology Dr Nicholas C. Buchan Uro-oncology Fellow 1 2 Aims To understand the basic concepts underlying PET scanning. Understand the emerging role of PET Scanning for
Nuclear Medicine in Oncology
 Radiopharmaceuticals Nuclear Medicine in Oncology Practice Pharmaceutical Radionuc lide Function Tumor type Diphosphonates Tc-99m Osteoblast Bone tumor & metast. Ga-citrate Ga-67 Fe-analogue Bronchogenous
Radiopharmaceuticals Nuclear Medicine in Oncology Practice Pharmaceutical Radionuc lide Function Tumor type Diphosphonates Tc-99m Osteoblast Bone tumor & metast. Ga-citrate Ga-67 Fe-analogue Bronchogenous
Medical Affairs Policy
 Medical Affairs Policy Service: PET Scan (Positron Emission Tomography) PUM 250-0010-1712 Medical Policy Committee Approval 12/01/17 Effective Date 04/01/18 Prior Authorization Needed Yes Disclaimer: This
Medical Affairs Policy Service: PET Scan (Positron Emission Tomography) PUM 250-0010-1712 Medical Policy Committee Approval 12/01/17 Effective Date 04/01/18 Prior Authorization Needed Yes Disclaimer: This
PET/CT Frequently Asked Questions
 PET/CT Frequently Asked Questions General Q: Is FDG PET specific for cancer? A: No, it is a marker of metabolism. In general, any disease that causes increased metabolism can result in increased FDG uptake
PET/CT Frequently Asked Questions General Q: Is FDG PET specific for cancer? A: No, it is a marker of metabolism. In general, any disease that causes increased metabolism can result in increased FDG uptake
Medical Affairs Policy
 Medical Affairs Policy Service: PET Scan (Positron Emission Tomography) PUM 250-0010 Medical Policy Committee Approval 03/17/17 Effective Date 05/22/17 Prior Authorization Needed Yes Disclaimer: This policy
Medical Affairs Policy Service: PET Scan (Positron Emission Tomography) PUM 250-0010 Medical Policy Committee Approval 03/17/17 Effective Date 05/22/17 Prior Authorization Needed Yes Disclaimer: This policy
Lymphoma Cover Cover. Non-small cell lung Cover Cover. Ovary Cover Cover. Brain Cover Cover. Cervix Cover with exceptions * Cover
 POLICY: PG0450 ORIGINAL EFFECTIVE: 11/28/18 LAST REVIEW: MEDICAL POLICY Positron Emission Tomography (PET) Oncology Applications GUIDELINES This policy does not certify benefits or authorization of benefits,
POLICY: PG0450 ORIGINAL EFFECTIVE: 11/28/18 LAST REVIEW: MEDICAL POLICY Positron Emission Tomography (PET) Oncology Applications GUIDELINES This policy does not certify benefits or authorization of benefits,
NCCN Practice Guidelines Narrative Summary of Indications for FDG PET and PET/CT
 NCCN Practice Guidelines Narrative Summary of Indications for FDG PET and PET/CT NCCN guidelines were reviewed on 2/14/2016 for utilization of 18F-fluorodeoxyglucose (FDG) PET and PET/CT (available at:
NCCN Practice Guidelines Narrative Summary of Indications for FDG PET and PET/CT NCCN guidelines were reviewed on 2/14/2016 for utilization of 18F-fluorodeoxyglucose (FDG) PET and PET/CT (available at:
PET/CT in oncology. Positron emission tomography
 Clinical Medicine 2012, Vol 12, No 4: 368 72 PET/CT in oncology Fahim-Ul-Hassan, SpR Nuclear Medicine, Guy s Hospital, London; Gary J Cook, professor of Clinical PET, KCL Division of Imaging Sciences &
Clinical Medicine 2012, Vol 12, No 4: 368 72 PET/CT in oncology Fahim-Ul-Hassan, SpR Nuclear Medicine, Guy s Hospital, London; Gary J Cook, professor of Clinical PET, KCL Division of Imaging Sciences &
Appendix 1: Regional Lymph Node Stations for Staging Esophageal Cancer
 Appendix 1: Regional Lymph Node Stations for Staging Esophageal Cancer Locoregional (N stage) disease was redefined in the seventh edition of the AJCC Cancer Staging Manual as any periesophageal lymph
Appendix 1: Regional Lymph Node Stations for Staging Esophageal Cancer Locoregional (N stage) disease was redefined in the seventh edition of the AJCC Cancer Staging Manual as any periesophageal lymph
Los Angeles Radiological Society 62 nd Annual Midwinter Radiology Conference January 31, 2010
 Los Angeles Radiological Society 62 nd Annual Midwinter Radiology Conference January 31, 2010 Self Assessment Module on Nuclear Medicine and PET/CT Case Review FDG PET/CT IN LYMPHOMA AND MELANOMA Submitted
Los Angeles Radiological Society 62 nd Annual Midwinter Radiology Conference January 31, 2010 Self Assessment Module on Nuclear Medicine and PET/CT Case Review FDG PET/CT IN LYMPHOMA AND MELANOMA Submitted
Using PET/CT in Prostate Cancer
 Using PET/CT in Prostate Cancer Legal Disclaimer These materials were prepared in good faith by MITA as a service to the profession and are believed to be reliable based on current scientific literature.
Using PET/CT in Prostate Cancer Legal Disclaimer These materials were prepared in good faith by MITA as a service to the profession and are believed to be reliable based on current scientific literature.
F NaF PET/CT in the Evaluation of Skeletal Malignancy
 F NaF PET/CT in the Evaluation of Skeletal Malignancy Andrei Iagaru, MD September 26, 2013 School of of Medicine Ø Introduction Ø F NaF PET/CT in Primary Bone Cancers Ø F NaF PET/CT in Bone Metastases
F NaF PET/CT in the Evaluation of Skeletal Malignancy Andrei Iagaru, MD September 26, 2013 School of of Medicine Ø Introduction Ø F NaF PET/CT in Primary Bone Cancers Ø F NaF PET/CT in Bone Metastases
PET imaging of cancer metabolism is commonly performed with F18
 PCRI Insights, August 2012, Vol. 15: No. 3 Carbon-11-Acetate PET/CT Imaging in Prostate Cancer Fabio Almeida, M.D. Medical Director, Arizona Molecular Imaging Center - Phoenix PET imaging of cancer metabolism
PCRI Insights, August 2012, Vol. 15: No. 3 Carbon-11-Acetate PET/CT Imaging in Prostate Cancer Fabio Almeida, M.D. Medical Director, Arizona Molecular Imaging Center - Phoenix PET imaging of cancer metabolism
The Importance of PET/CT in Human Health. Homer A. Macapinlac, M.D.
 The Importance of PET/CT in Human Health Homer A. Macapinlac, M.D. Learning objectives Provide an overview of the impact of PET/CT imaging on the management of patients and its impact on health care expenditures.
The Importance of PET/CT in Human Health Homer A. Macapinlac, M.D. Learning objectives Provide an overview of the impact of PET/CT imaging on the management of patients and its impact on health care expenditures.
CT PET SCANNING for GIT Malignancies A clinician s perspective
 CT PET SCANNING for GIT Malignancies A clinician s perspective Damon Bizos Head, Surgical Gastroenterology Charlotte Maxeke Johannesburg Academic Hospital Case presentation 54 year old with recent onset
CT PET SCANNING for GIT Malignancies A clinician s perspective Damon Bizos Head, Surgical Gastroenterology Charlotte Maxeke Johannesburg Academic Hospital Case presentation 54 year old with recent onset
PET/CT in breast cancer staging
 PET/CT in breast cancer staging Anni Morsing Consultant, PhD, DMSc Rigshospitalet 1 18F- FDG PET/CT for breastcancer staging Where is the clinical impact? To which women should 18F- FDG PET/CT be offered?
PET/CT in breast cancer staging Anni Morsing Consultant, PhD, DMSc Rigshospitalet 1 18F- FDG PET/CT for breastcancer staging Where is the clinical impact? To which women should 18F- FDG PET/CT be offered?
PET/MR:Techniques, Indications and Applications
 PET/MR:Techniques, Indications and Applications Franz Wolfgang Hirsch Professor and Head of the Department of Pediatric Radiology University Hospital Leipzig / Germany Children s Hospital University Leipzig
PET/MR:Techniques, Indications and Applications Franz Wolfgang Hirsch Professor and Head of the Department of Pediatric Radiology University Hospital Leipzig / Germany Children s Hospital University Leipzig
Original Date: September 1997 PET SCANS (for FDG or Dotatate)
 National Imaging Associates, Inc. Clinical guidelines Original Date: September 1997 PET SCANS (for FDG or Dotatate) Page 1 of 9 CPT Codes: Last Review Date: August 2017 78811 - Limited area e.g. Chest,
National Imaging Associates, Inc. Clinical guidelines Original Date: September 1997 PET SCANS (for FDG or Dotatate) Page 1 of 9 CPT Codes: Last Review Date: August 2017 78811 - Limited area e.g. Chest,
DEPARTMENT OF ONCOLOGY ELECTIVE
 DEPARTMENT OF ONCOLOGY ELECTIVE 2015-2016 www.uwo.ca/oncology Oncology Elective Program Administrator: Ms. Kimberly Trudgeon Room A4-901C (Admin) LHSC London Regional Cancer Centre (Victoria Campus) Phone:
DEPARTMENT OF ONCOLOGY ELECTIVE 2015-2016 www.uwo.ca/oncology Oncology Elective Program Administrator: Ms. Kimberly Trudgeon Room A4-901C (Admin) LHSC London Regional Cancer Centre (Victoria Campus) Phone:
PET-MRI in malignant bone tumours. Lars Stegger Department of Nuclear Medicine University Hospital Münster, Germany
 PET-MRI in malignant bone tumours Lars Stegger Department of Nuclear Medicine University Hospital Münster, Germany Content From PET to PET/MRI General considerations Bone metastases Primary bone tumours
PET-MRI in malignant bone tumours Lars Stegger Department of Nuclear Medicine University Hospital Münster, Germany Content From PET to PET/MRI General considerations Bone metastases Primary bone tumours
Recommendations for cross-sectional imaging in cancer management, Second edition
 www.rcr.ac.uk Recommendations for cross-sectional imaging in cancer management, Second edition Carcinoma of unknown primary origin (CUP) Faculty of Clinical Radiology www.rcr.ac.uk Contents Carcinoma of
www.rcr.ac.uk Recommendations for cross-sectional imaging in cancer management, Second edition Carcinoma of unknown primary origin (CUP) Faculty of Clinical Radiology www.rcr.ac.uk Contents Carcinoma of
PET CT for Staging Lung Cancer
 PET CT for Staging Lung Cancer Rohit Kochhar Consultant Radiologist Disclosures Neither I nor my immediate family members have financial relationships with commercial organizations that may have a direct
PET CT for Staging Lung Cancer Rohit Kochhar Consultant Radiologist Disclosures Neither I nor my immediate family members have financial relationships with commercial organizations that may have a direct
Medical Policy An independent licensee of the Blue Cross Blue Shield Association
 CAE of Malignancy with MRI of the Breast Page 1 of 9 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See also: Computer-Aided Evaluation of Malignancy with Magnetic
CAE of Malignancy with MRI of the Breast Page 1 of 9 Medical Policy An independent licensee of the Blue Cross Blue Shield Association Title: See also: Computer-Aided Evaluation of Malignancy with Magnetic
FDG-PET/CT in Gynaecologic Cancers
 Friday, August 31, 2012 Session 6, 9:00-9:30 FDG-PET/CT in Gynaecologic Cancers (Uterine) cervical cancer Endometrial cancer & Uterine sarcomas Ovarian cancer Little mermaid (Edvard Eriksen 1913) honoring
Friday, August 31, 2012 Session 6, 9:00-9:30 FDG-PET/CT in Gynaecologic Cancers (Uterine) cervical cancer Endometrial cancer & Uterine sarcomas Ovarian cancer Little mermaid (Edvard Eriksen 1913) honoring
Esophageal Cancer. What is the value of performing PET scan routinely for staging of esophageal cancers
 Esophageal Cancer What is the value of performing PET scan routinely for staging of esophageal cancers What is the sensitivity and specificity of PET scan for metastatic lesions When should PET scan be
Esophageal Cancer What is the value of performing PET scan routinely for staging of esophageal cancers What is the sensitivity and specificity of PET scan for metastatic lesions When should PET scan be
Cancers of unknown primary : Knowing the unknown. Prof. Ahmed Hossain Professor of Medicine SSMC
 Cancers of unknown primary : Knowing the unknown Prof. Ahmed Hossain Professor of Medicine SSMC Definition Cancers of unknown primary site (CUPs) Represent a heterogeneous group of metastatic tumours,
Cancers of unknown primary : Knowing the unknown Prof. Ahmed Hossain Professor of Medicine SSMC Definition Cancers of unknown primary site (CUPs) Represent a heterogeneous group of metastatic tumours,
Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association
 PET Scanning in Oncology to Detect Page 1 of 9 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: Positron Emission Tomography (PET) Scanning: In Oncology to Detect
PET Scanning in Oncology to Detect Page 1 of 9 Medical Policy An Independent Licensee of the Blue Cross and Blue Shield Association Title: Positron Emission Tomography (PET) Scanning: In Oncology to Detect
FDG PET/CT STAGING OF LUNG CANCER. Dr Shakher Ramdave
 FDG PET/CT STAGING OF LUNG CANCER Dr Shakher Ramdave FDG PET/CT STAGING OF LUNG CANCER FDG PET/CT is used in all patients with lung cancer who are considered for curative treatment to exclude occult disease.
FDG PET/CT STAGING OF LUNG CANCER Dr Shakher Ramdave FDG PET/CT STAGING OF LUNG CANCER FDG PET/CT is used in all patients with lung cancer who are considered for curative treatment to exclude occult disease.
STRADA DEGLI ALBERONI 11// TURIN ITALY. Full time employee as a freelancer contract since
 E UROPEAN CURRICULUM VITAE FORMAT PERSONAL INFORMATION Name VINCENZO ARENA Address STRADA DEGLI ALBERONI 11//9 10133 - TURIN ITALY Telephone 011 3160158 Fax Mobile E-mail 011 3160828 +39 338 2941141 vincenzo.arena@affidea.it
E UROPEAN CURRICULUM VITAE FORMAT PERSONAL INFORMATION Name VINCENZO ARENA Address STRADA DEGLI ALBERONI 11//9 10133 - TURIN ITALY Telephone 011 3160158 Fax Mobile E-mail 011 3160828 +39 338 2941141 vincenzo.arena@affidea.it
JOHNS HOPKINS HEALTHCARE
 Page 1 of 18 ACTION: New Policy Effective Date: 06/06/2003 Revising Review Dates: 10/22/03, 10/22/04, Superseding 10/21/05, 05/30/06, 10/13/06, 03/03/08, Archiving 03/02/09, 06/04/10, 08/23/11, 03/07/14,
Page 1 of 18 ACTION: New Policy Effective Date: 06/06/2003 Revising Review Dates: 10/22/03, 10/22/04, Superseding 10/21/05, 05/30/06, 10/13/06, 03/03/08, Archiving 03/02/09, 06/04/10, 08/23/11, 03/07/14,
COLORECTAL CARCINOMA
 QUICK REFERENCE FOR HEALTHCARE PROVIDERS MANAGEMENT OF COLORECTAL CARCINOMA Ministry of Health Malaysia Malaysian Society of Colorectal Surgeons Malaysian Society of Gastroenterology & Hepatology Malaysian
QUICK REFERENCE FOR HEALTHCARE PROVIDERS MANAGEMENT OF COLORECTAL CARCINOMA Ministry of Health Malaysia Malaysian Society of Colorectal Surgeons Malaysian Society of Gastroenterology & Hepatology Malaysian
FACULTY MEMBERSHIP APPLICATION Tulane Cancer Center
 FACULTY MEMBERSHIP APPLICATION Tulane Cancer Center 1430 Tulane Ave., Box SL-68, New Orleans, LA 70112-2699 J. Bennett Johnston Building, Mezzanine (Floor 1A), Suite A102 (504) 988-6060, fax (504) 988-6077,
FACULTY MEMBERSHIP APPLICATION Tulane Cancer Center 1430 Tulane Ave., Box SL-68, New Orleans, LA 70112-2699 J. Bennett Johnston Building, Mezzanine (Floor 1A), Suite A102 (504) 988-6060, fax (504) 988-6077,
Bone PET/MRI : Diagnostic yield in bone metastases and malignant primitive bone tumors
 Bone PET/MRI : Diagnostic yield in bone metastases and malignant primitive bone tumors Lars Stegger, Benjamin Noto Department of Nuclear Medicine University Hospital Münster, Germany Content From PET to
Bone PET/MRI : Diagnostic yield in bone metastases and malignant primitive bone tumors Lars Stegger, Benjamin Noto Department of Nuclear Medicine University Hospital Münster, Germany Content From PET to
BRACHYTHERAPY FOR TREATMENT OF MISCELLANEOUS CANCERS
 BRACHYTHERAPY FOR TREATMENT OF MISCELLANEOUS CANCERS Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
BRACHYTHERAPY FOR TREATMENT OF MISCELLANEOUS CANCERS Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan.
Positron emission tomography/computer tomography in the evaluation of head and neck cancer treatment
 Positron emission tomography/computer tomography in the evaluation of head and neck cancer treatment Severina Šedienė 1, Ilona Kulakienė 1, Viktoras Rudžianskas 2 1 Lithuanian University of Health Sciences,
Positron emission tomography/computer tomography in the evaluation of head and neck cancer treatment Severina Šedienė 1, Ilona Kulakienė 1, Viktoras Rudžianskas 2 1 Lithuanian University of Health Sciences,
An Introduction to PET Imaging in Oncology
 January 2002 An Introduction to PET Imaging in Oncology Janet McLaren, Harvard Medical School Year III Basics of PET Principle of Physiologic Imaging: Allows in vivo visualization of structures by their
January 2002 An Introduction to PET Imaging in Oncology Janet McLaren, Harvard Medical School Year III Basics of PET Principle of Physiologic Imaging: Allows in vivo visualization of structures by their
PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES. U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease
 PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease Refer back to original requester with this paperwork and review previous
PATHWAY FOR INVESTIGATION OF ADULTS PRESENTING WITH ASCITES U/S Abdo/pelvis shows ascites without obvious evidence of 1 liver disease Refer back to original requester with this paperwork and review previous
PET/CT in lung cancer
 PET/CT in lung cancer Andrei Šamarin North Estonia Medical Centre 3 rd Baltic Congress of Radiology 08.10.2010 Imaging in lung cancer Why do we need PET/CT? CT is routine imaging modality for staging of
PET/CT in lung cancer Andrei Šamarin North Estonia Medical Centre 3 rd Baltic Congress of Radiology 08.10.2010 Imaging in lung cancer Why do we need PET/CT? CT is routine imaging modality for staging of
Contents. 3 Pneumology Introduction Positron Emission Tomography: Past and Present 1. 2 Fundamentals. xxx
 xxx IX Contents 1 Introduction Positron Emission Tomography: Past and Present 1 1.1 Survey.......................... 1 Physical and Biochemical Fundamentals.... 2 PET in National and International Medical
xxx IX Contents 1 Introduction Positron Emission Tomography: Past and Present 1 1.1 Survey.......................... 1 Physical and Biochemical Fundamentals.... 2 PET in National and International Medical
Nuclear medicine in oncology. 1. Diagnosis 2. Therapy
 Nuclear medicine in oncology 1. Diagnosis 2. Therapy Diagnosis - Conventional methods - Nonspecific radiopharmaceuticals cumulating in tumours - Specific radiopharmaceuticals (receptor- and immunoscintigraphy)
Nuclear medicine in oncology 1. Diagnosis 2. Therapy Diagnosis - Conventional methods - Nonspecific radiopharmaceuticals cumulating in tumours - Specific radiopharmaceuticals (receptor- and immunoscintigraphy)
Phillip J. Koo, MD Division Chief of Diagnostic Imaging Banner MD Anderson Cancer Center, USA
 ADVANCED PET IMAGING IN PROSTATE CANCER Phillip J. Koo, MD Division Chief of Diagnostic Imaging Banner MD Anderson Cancer Center, USA PET, positron-emission tomography DISCLAIMER Please note: The views
ADVANCED PET IMAGING IN PROSTATE CANCER Phillip J. Koo, MD Division Chief of Diagnostic Imaging Banner MD Anderson Cancer Center, USA PET, positron-emission tomography DISCLAIMER Please note: The views
New Visions in PET: Surgical Decision Making and PET/CT
 New Visions in PET: Surgical Decision Making and PET/CT Stanley J. Goldsmith, MD Director, Nuclear Medicine Professor, Radiology & Medicine New York Presbyterian Hospital- Weill Cornell Medical Center
New Visions in PET: Surgical Decision Making and PET/CT Stanley J. Goldsmith, MD Director, Nuclear Medicine Professor, Radiology & Medicine New York Presbyterian Hospital- Weill Cornell Medical Center
Prostate Cancer Basics: Background Information for Outreach Activities with Oncologists, Urologists and Surgeons
 Prostate Cancer Basics: Background Information for Outreach Activities with Oncologists, Urologists and Surgeons Legal Disclaimer These materials were prepared in good faith by MITA as a service to the
Prostate Cancer Basics: Background Information for Outreach Activities with Oncologists, Urologists and Surgeons Legal Disclaimer These materials were prepared in good faith by MITA as a service to the
Value of true whole-body FDG- PET/CT scanning protocol in oncology and optimization of its use based on primary malignancy
 Value of true whole-body FDG- PET/CT scanning protocol in oncology and optimization of its use based on primary malignancy Ronnie Sebro MD, Ph.D Carina Mari Aparici MD, Miguel Hernandez Pampaloni MD, PhD
Value of true whole-body FDG- PET/CT scanning protocol in oncology and optimization of its use based on primary malignancy Ronnie Sebro MD, Ph.D Carina Mari Aparici MD, Miguel Hernandez Pampaloni MD, PhD
Breast Cancer Imaging
 Breast Cancer Imaging I. Policy University Health Alliance (UHA) will cover breast imaging when such services meet the medical criteria guidelines (subject to limitations and exclusions) indicated below.
Breast Cancer Imaging I. Policy University Health Alliance (UHA) will cover breast imaging when such services meet the medical criteria guidelines (subject to limitations and exclusions) indicated below.
Assessment of renal cell carcinoma by two PET tracer : dual-time-point C-11 methionine and F-18 fluorodeoxyglucose
 Assessment of renal cell carcinoma by two PET tracer : dual-time-point C-11 methionine and F-18 fluorodeoxyglucose Poster No.: C-0805 Congress: ECR 2015 Type: Scientific Exhibit Authors: S. Ito, K. Kato,
Assessment of renal cell carcinoma by two PET tracer : dual-time-point C-11 methionine and F-18 fluorodeoxyglucose Poster No.: C-0805 Congress: ECR 2015 Type: Scientific Exhibit Authors: S. Ito, K. Kato,
Management of Neck Metastasis from Unknown Primary
 Management of Neck Metastasis from Unknown Primary.. Definition Histologic evidence of malignancy in the cervical lymph node (s) with no apparent primary site of original tumour Diagnosis after a thorough
Management of Neck Metastasis from Unknown Primary.. Definition Histologic evidence of malignancy in the cervical lymph node (s) with no apparent primary site of original tumour Diagnosis after a thorough
Positron Emission Tomography in Lung Cancer
 May 19, 2003 Positron Emission Tomography in Lung Cancer Andrew Wang, HMS III Patient DD 53 y/o gentleman presented with worsening dyspnea on exertion for the past two months 30 pack-year smoking Hx and
May 19, 2003 Positron Emission Tomography in Lung Cancer Andrew Wang, HMS III Patient DD 53 y/o gentleman presented with worsening dyspnea on exertion for the past two months 30 pack-year smoking Hx and
Joint Comments on Positron Emission Tomography (NaF-18) to Identify Bone Metastasis of Cancer (CAG-00065R1)
 July 2, 2009 Tamara Syrek Jensen, J.D. Acting Director, Coverage and Analysis Group Centers for Medicare & Medicaid Services 7500 Security Blvd., Mail Stop C1-09-06 Baltimore, MD 21244 Re: Joint Comments
July 2, 2009 Tamara Syrek Jensen, J.D. Acting Director, Coverage and Analysis Group Centers for Medicare & Medicaid Services 7500 Security Blvd., Mail Stop C1-09-06 Baltimore, MD 21244 Re: Joint Comments
Bone and CT Scans Are Complementary for Diagnoses of Bone Metastases in Breast Cancer When PET Scans Findings Are Equivocal: A Case Report
 Bone and CT Scans Are Complementary for Diagnoses of Bone Metastases in Breast Cancer When Scans Findings Are Equivocal: A Case Report Yuk-Wah Tsang 1, Jyh-Gang Leu 2, Yen-Kung Chen 3, Kwan-Hwa Chi 1,4
Bone and CT Scans Are Complementary for Diagnoses of Bone Metastases in Breast Cancer When Scans Findings Are Equivocal: A Case Report Yuk-Wah Tsang 1, Jyh-Gang Leu 2, Yen-Kung Chen 3, Kwan-Hwa Chi 1,4
Prof. Dr. NAGUI M. ABDELWAHAB,M.D.; MARYSE Y. AWADALLAH, M.D. AYA M. BASSAM, Ms.C.
 Role of Whole-body Diffusion MR in Detection of Metastatic lesions Prof. Dr. NAGUI M. ABDELWAHAB,M.D.; MARYSE Y. AWADALLAH, M.D. AYA M. BASSAM, Ms.C. Cancer is a potentially life-threatening disease,
Role of Whole-body Diffusion MR in Detection of Metastatic lesions Prof. Dr. NAGUI M. ABDELWAHAB,M.D.; MARYSE Y. AWADALLAH, M.D. AYA M. BASSAM, Ms.C. Cancer is a potentially life-threatening disease,
Cancer. Description. Section: Surgery Effective Date: October 15, 2016 Subsection: Original Policy Date: September 9, 2011 Subject:
 Subject: Saturation Biopsy for Diagnosis, Last Review Status/Date: September 2016 Page: 1 of 9 Saturation Biopsy for Diagnosis, Description Saturation biopsy of the prostate, in which more cores are obtained
Subject: Saturation Biopsy for Diagnosis, Last Review Status/Date: September 2016 Page: 1 of 9 Saturation Biopsy for Diagnosis, Description Saturation biopsy of the prostate, in which more cores are obtained
M etastatic disease influences patient management; Whole body PET/CT for initial staging of choroidal melanoma SCIENTIFIC REPORT
 1270 SCIENTIFIC REPORT Whole body PET/CT for initial staging of choroidal P T Finger, M Kurli, S Reddy, L B Tena, A C Pavlick... Aim: To investigate the value of whole body positron emission tomography/computed
1270 SCIENTIFIC REPORT Whole body PET/CT for initial staging of choroidal P T Finger, M Kurli, S Reddy, L B Tena, A C Pavlick... Aim: To investigate the value of whole body positron emission tomography/computed
The role of PET/CT imaging in penile cancer
 Review Article The role of PET/CT imaging in penile cancer Sarah R. Ottenhof 1, Erik Vegt 2 1 Department of Urology, Netherlands Cancer Institute, Amsterdam, the Netherlands; 2 Department of Nuclear Medicine,
Review Article The role of PET/CT imaging in penile cancer Sarah R. Ottenhof 1, Erik Vegt 2 1 Department of Urology, Netherlands Cancer Institute, Amsterdam, the Netherlands; 2 Department of Nuclear Medicine,
Tumour Markers. For these reasons, only a handful of tumour markers are commonly used by most doctors.
 Tumour Markers What are Tumour Markers? Tumour markers are substances that can be found in the body when cancer is present. They are usually found in the blood or urine. They can be products of cancer
Tumour Markers What are Tumour Markers? Tumour markers are substances that can be found in the body when cancer is present. They are usually found in the blood or urine. They can be products of cancer
ASPS Recommended Insurance Coverage Criteria for Third- Party Payers
 ASPS Recommended Insurance Coverage Criteria for Third- Party Payers Breast Implant Associated Anaplastic Large Cell Lymphoma BACKGROUND Anaplastic Large Cell Lymphoma (ALCL) is a rare type of cancer of
ASPS Recommended Insurance Coverage Criteria for Third- Party Payers Breast Implant Associated Anaplastic Large Cell Lymphoma BACKGROUND Anaplastic Large Cell Lymphoma (ALCL) is a rare type of cancer of
Table 1. Some other positron tracers with applications in oncology.
 CME: CLINICAL PRACTICE AND ITS BASIS Nuclear Medicine Edited by Peter J Ell FMedSci, University Chair and Director, Institute of Nuclear Medicine, UCL; Clinical Director, University College London Hospitals
CME: CLINICAL PRACTICE AND ITS BASIS Nuclear Medicine Edited by Peter J Ell FMedSci, University Chair and Director, Institute of Nuclear Medicine, UCL; Clinical Director, University College London Hospitals
SELF-ASSESSMENT MODULE REFERENCE SPR 2018 Oncologic Imaging Course Adrenal Tumors November 10, :00 12:10 p.m.
 SELF-ASSESSMENT MODULE REFERENCE SPR 2018 Oncologic Imaging Course Adrenal Tumors November 10, 2018 10:00 12:10 p.m. Staging Susan E. Sharp, MD 1. In the International Neuroblastoma Risk Group Staging
SELF-ASSESSMENT MODULE REFERENCE SPR 2018 Oncologic Imaging Course Adrenal Tumors November 10, 2018 10:00 12:10 p.m. Staging Susan E. Sharp, MD 1. In the International Neuroblastoma Risk Group Staging
Understanding Biological Activity to Inform Drug Development
 National Cancer Policy Forum Understanding Biological Activity to Inform Drug Development December 12, 2016 Wolfgang Weber Molecular Imaging and Therapy Service Department of Radiology RECIST Response
National Cancer Policy Forum Understanding Biological Activity to Inform Drug Development December 12, 2016 Wolfgang Weber Molecular Imaging and Therapy Service Department of Radiology RECIST Response
Monitoring Patients Undergoing Cancer Therapy. By Timothy K. Egan
 F E A T U R E By Timothy K. Egan Before placement in a computed tomography scanner, a patient is fitted with an immobilization device. Immobilization ensures that the same area of the patient is scanned
F E A T U R E By Timothy K. Egan Before placement in a computed tomography scanner, a patient is fitted with an immobilization device. Immobilization ensures that the same area of the patient is scanned
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: October 15, 2018 Related Policies: None Intraoperative Radiotherapy Description Intraoperative radiotherapy (IORT) is delivered directly to exposed tissues during
FEP Medical Policy Manual Effective Date: October 15, 2018 Related Policies: None Intraoperative Radiotherapy Description Intraoperative radiotherapy (IORT) is delivered directly to exposed tissues during
Title: What is the role of pre-operative PET/PET-CT in the management of patients with
 Title: What is the role of pre-operative PET/PET-CT in the management of patients with potentially resectable colorectal cancer liver metastasis? Pablo E. Serrano, Julian F. Daza, Natalie M. Solis June
Title: What is the role of pre-operative PET/PET-CT in the management of patients with potentially resectable colorectal cancer liver metastasis? Pablo E. Serrano, Julian F. Daza, Natalie M. Solis June
PET-CT versus MRI in the identification of hepatic metastases from colorectal carcinoma: An evidence based review of the current literature.
 PET-CT versus MRI in the identification of hepatic metastases from colorectal carcinoma: An evidence based review of the current literature. Poster No.: C-1275 Congress: ECR 2017 Type: Scientific Exhibit
PET-CT versus MRI in the identification of hepatic metastases from colorectal carcinoma: An evidence based review of the current literature. Poster No.: C-1275 Congress: ECR 2017 Type: Scientific Exhibit
Nuclear Medicine and PET. D. J. McMahon rev cewood
 Nuclear Medicine and PET D. J. McMahon 150504 rev cewood 2018-02-15 Key Points Nuclear Medicine and PET: Imaging: Understand how Nuc Med & PET differ from Radiography & CT by the source of radiation. Be
Nuclear Medicine and PET D. J. McMahon 150504 rev cewood 2018-02-15 Key Points Nuclear Medicine and PET: Imaging: Understand how Nuc Med & PET differ from Radiography & CT by the source of radiation. Be
Positron emission tomography Medicare Services Advisory Committee
 Positron emission tomography Medicare Services Advisory Committee Authors' objectives To assess the effectiveness of positron emission tomography (PET), the report addressed the following (truncated) six
Positron emission tomography Medicare Services Advisory Committee Authors' objectives To assess the effectiveness of positron emission tomography (PET), the report addressed the following (truncated) six
MEDICAL POLICY SUBJECT: MAMMOGRAPHY: COMPUTER- AIDED DETECTION (CAD) POLICY NUMBER: CATEGORY: Technology Assessment
 MEDICAL POLICY SUBJECT: MAMMOGRAPHY: COMPUTER- PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
MEDICAL POLICY SUBJECT: MAMMOGRAPHY: COMPUTER- PAGE: 1 OF: 5 If a product excludes coverage for a service, it is not covered, and medical policy criteria do not apply. If a commercial product, including
Introduction Pediatric malignancies Changing trends & Radiation burden Radiation exposure from PET/CT Image gently PET & CT modification - PET/CT
 Introduction Pediatric malignancies Changing trends & Radiation burden Radiation exposure from PET/CT Image gently PET & CT modification - PET/CT protocols Tips Leukaemia / lymphoma: ~ 35% acute lymphoblastic
Introduction Pediatric malignancies Changing trends & Radiation burden Radiation exposure from PET/CT Image gently PET & CT modification - PET/CT protocols Tips Leukaemia / lymphoma: ~ 35% acute lymphoblastic
Research Article Prevalence of Clinically Significant Extraosseous Findings on Unenhanced CT Portions of 18 F-Fluoride PET/CT Bone Scans
 The Scientific World Journal Volume 2012, Article ID 979867, 5 pages doi:10.1100/2012/979867 The cientificworldjournal Research Article Prevalence of Clinically Significant Extraosseous Findings on Unenhanced
The Scientific World Journal Volume 2012, Article ID 979867, 5 pages doi:10.1100/2012/979867 The cientificworldjournal Research Article Prevalence of Clinically Significant Extraosseous Findings on Unenhanced
ANZUP SURVEILLANCE RECOMMENDATIONS FOR METASTATIC TESTICULAR CANCER POST-CHEMOTHERAPY
 ANZUP SURVEILLANCE RECOMMENDATIONS FOR METASTATIC TESTICULAR CANCER POST-CHEMOTHERAPY Note: These surveillance recommendations are provided as recommendations only. Clinicians should take into account
ANZUP SURVEILLANCE RECOMMENDATIONS FOR METASTATIC TESTICULAR CANCER POST-CHEMOTHERAPY Note: These surveillance recommendations are provided as recommendations only. Clinicians should take into account
Radiological Manifestations of Metastatic Melanoma
 Radiological Manifestations of Metastatic Melanoma Lia Hojman, Universidad de Chile Year VII Our patient: Clinical Presentation A 24 year old male. Former smoker. 8 months ago, he suffered a burn in his
Radiological Manifestations of Metastatic Melanoma Lia Hojman, Universidad de Chile Year VII Our patient: Clinical Presentation A 24 year old male. Former smoker. 8 months ago, he suffered a burn in his
FEP Medical Policy Manual
 FEP Medical Policy Manual Effective Date: July 15, 2018 Related Policies: None Genetic Testing for PTEN Hamartoma Tumor Syndrome Description The PTEN hamartoma tumor syndrome (PHTS) includes several syndromes
FEP Medical Policy Manual Effective Date: July 15, 2018 Related Policies: None Genetic Testing for PTEN Hamartoma Tumor Syndrome Description The PTEN hamartoma tumor syndrome (PHTS) includes several syndromes
PSMA PET SCANNING AND THERANOSTICS IN PROSTATE CANCER KEVIN TRACEY, MD, FRCPC PRECISION DIAGNSOTIC IMAGING REGIONAL PET/CT CENTRE
 PSMA PET SCANNING AND THERANOSTICS IN PROSTATE CANCER KEVIN TRACEY, MD, FRCPC PRECISION DIAGNSOTIC IMAGING REGIONAL PET/CT CENTRE DISCLOSURES/CONFLICTS NONE OBJECTIVES Understand current diagnostic role
PSMA PET SCANNING AND THERANOSTICS IN PROSTATE CANCER KEVIN TRACEY, MD, FRCPC PRECISION DIAGNSOTIC IMAGING REGIONAL PET/CT CENTRE DISCLOSURES/CONFLICTS NONE OBJECTIVES Understand current diagnostic role
POSITRON EMISSION TOMOGRAPHY (PET) & COMBINED PET/CT SCANS
 POSITRON EMISSION TOMOGRAPHY (PET) & COMBINED PET/CT SCANS Protocol: RAD023 Effective Date: November 1, 2017 Table of Contents Page COMMERCIAL & MEDICAID COVERAGE RATIONALE... 1 MEDICARE COVERAGE RATIONALE...
POSITRON EMISSION TOMOGRAPHY (PET) & COMBINED PET/CT SCANS Protocol: RAD023 Effective Date: November 1, 2017 Table of Contents Page COMMERCIAL & MEDICAID COVERAGE RATIONALE... 1 MEDICARE COVERAGE RATIONALE...
Cancer of Unknown Primary (CUP)
 Cancer of Unknown Primary (CUP) Pathways and Guidelines V1.0 London Cancer September 2013 The following pathways and guidelines document has been compiled by the London Cancer CUP technical subgroup and
Cancer of Unknown Primary (CUP) Pathways and Guidelines V1.0 London Cancer September 2013 The following pathways and guidelines document has been compiled by the London Cancer CUP technical subgroup and
Triage of Limited Versus Extensive Disease on 18 F-FDG PET/CT Scan in Small Cell lung Cancer
 Triage of Limited Versus Extensive Disease on F-FDG PET/CT Scan in Small Cell lung Cancer Riaz Saima 1*, Bashir Humayun 1, Niazi Imran Khalid 2 1 Department of Nuclear Medicine, Shaukat Khanum Memorial
Triage of Limited Versus Extensive Disease on F-FDG PET/CT Scan in Small Cell lung Cancer Riaz Saima 1*, Bashir Humayun 1, Niazi Imran Khalid 2 1 Department of Nuclear Medicine, Shaukat Khanum Memorial
MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND (TRUS)
 MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND 06/16/05, 05/18/06, 03/15/07, 02/21/08 PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under
MEDICAL POLICY SUBJECT: TRANSRECTAL ULTRASOUND 06/16/05, 05/18/06, 03/15/07, 02/21/08 PAGE: 1 OF: 5 If the member's subscriber contract excludes coverage for a specific service it is not covered under
Medical Policy. MP Ingestible ph and Pressure Capsule
 Medical Policy BCBSA Ref. Policy: 2.01.81 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Medicine Related Policies 2.01.20 Esophageal ph Monitoring 6.01.33 Wireless Capsule Endoscopy as a
Medical Policy BCBSA Ref. Policy: 2.01.81 Last Review: 11/15/2018 Effective Date: 11/15/2018 Section: Medicine Related Policies 2.01.20 Esophageal ph Monitoring 6.01.33 Wireless Capsule Endoscopy as a
The Role of PET / CT in Lung Cancer Staging
 July 2004 The Role of PET / CT in Lung Cancer Staging Vlad Vinarsky, Harvard Medical School Year IV Patient AM HPI: 81 yo F p/w hemoptysis x 1 month LLL lesion on CXR, not responsive to Abx 35 pack-year
July 2004 The Role of PET / CT in Lung Cancer Staging Vlad Vinarsky, Harvard Medical School Year IV Patient AM HPI: 81 yo F p/w hemoptysis x 1 month LLL lesion on CXR, not responsive to Abx 35 pack-year
PET/CT Value: Rocky Mountain Cancer Centers
 PET/CT Value: Rocky Mountain Cancer Centers Glenn Balasky Executive Director Rocky Mountain Cancer Centers glenn.balasky@usoncology.com CANM/CAMRT Joint Conference March 22, 2018 Vancouver, British Columbia
PET/CT Value: Rocky Mountain Cancer Centers Glenn Balasky Executive Director Rocky Mountain Cancer Centers glenn.balasky@usoncology.com CANM/CAMRT Joint Conference March 22, 2018 Vancouver, British Columbia
Scintimammography and Gamma Imaging of the Breast and Axilla
 Scintimammography and Gamma Imaging of the Breast and Axilla Policy Number: 6.01.18 Last Review: 9/2017 Origination: 9/2006 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC)
Scintimammography and Gamma Imaging of the Breast and Axilla Policy Number: 6.01.18 Last Review: 9/2017 Origination: 9/2006 Next Review: 9/2018 Policy Blue Cross and Blue Shield of Kansas City (Blue KC)
Physician Follow-Up and Guideline Adherence in Post- Treatment Surveillance of Colorectal Cancer
 Physician Follow-Up and Guideline Adherence in Post- Treatment Surveillance of Colorectal Cancer Gabriela M. Vargas, MD Kristin M. Sheffield, PhD, Abhishek Parmar, MD, Yimei Han, MS, Kimberly M. Brown,
Physician Follow-Up and Guideline Adherence in Post- Treatment Surveillance of Colorectal Cancer Gabriela M. Vargas, MD Kristin M. Sheffield, PhD, Abhishek Parmar, MD, Yimei Han, MS, Kimberly M. Brown,
Noninvasive Differential Diagnosis of Pulmonary Nodules Using the Standardized Uptake Value Index
 doi: 10.5761/atcs.oa.14-00241 Original Article Noninvasive Differential Diagnosis of Pulmonary Nodules Using the Standardized Uptake Value Index Satoshi Shiono, MD, 1 Naoki Yanagawa, MD, 2 Masami Abiko,
doi: 10.5761/atcs.oa.14-00241 Original Article Noninvasive Differential Diagnosis of Pulmonary Nodules Using the Standardized Uptake Value Index Satoshi Shiono, MD, 1 Naoki Yanagawa, MD, 2 Masami Abiko,
Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services. Cancer of Unknown Primary Network Site Specific Group. Clinical Guidelines
 Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services Cancer of Unknown Primary Network Site Specific Group Revision due: April 2019 Page 1 of 11 VERSION CONTROL THIS IS A CONTROLLED DOCUMENT.
Somerset, Wiltshire, Avon and Gloucestershire (SWAG) Cancer Services Cancer of Unknown Primary Network Site Specific Group Revision due: April 2019 Page 1 of 11 VERSION CONTROL THIS IS A CONTROLLED DOCUMENT.
